Abstract
Background:
Many studies have documented lower likelihood of live birth with increasing body mass index (BMI) among women undergoing assisted reproductive technologies (ART), but few have examined the association with waist circumference (WC), an anthropometric measure that allows assessment of central adiposity.
Objective(s):
To examine the relation between baseline WC and infertility treatment outcomes among women undergoing treatment with ART.
Study Design:
We followed 264 women who underwent 445 ART cycles for infertility treatment at the Massachusetts General Hospital between 2010 and 2017. WC was assessed at enrollment. We used cluster-weighted generalized estimating equation models to estimate the probability of live birth by tertiles of waist circumference (<77, 77-86, >86 cm), while accounting for multiple treatment cycles per woman and adjusting for age, race, smoking, infertility diagnosis, day 3 follicle-stimulating hormone, BMI, and height.
Results:
Mean (SD) WC and BMI were 83.6 (12.6) cm and 24.1 (4.3) kg/m2, respectively WC and BMI were positively correlated (r=0.69, p<0.0001). WC was inversely related to the probability of live birth after adjusting for BMI and other confounders. The multivariable adjusted probability of live birth (95% confidence interval) for women in increasing tertiles of WC were 53% (42-65%), 42% (32-53%), and 38% (28-50%) (p, trend=0.04). When women were classified in joint categories of BMI and WC, women with a BMI ≥ 25kg/m2 and a WC ≥ 77cm had the lowest live birth rate (38% (27-50%)), while women with a BMI between 18.5 and 25kg/m2 and a WC < 77cm had the highest (54% (42-66%)). The results were similar using different WC cut-off values.
Conclusion(s):
WC was inversely related to the probability of live birth among women undergoing assisted reproductive technology independently of BMI.
Keywords: assisted reproductive technology, IVF, ICSI, in vitro fertilization, intracytoplasmic sperm injection, waist circumference, body mass index
Introduction
Obesity has become a major public health problem as its prevalence has reached epidemic proportions. Between 2007 and 2016, the prevalence of obesity and severe obesity among women in the United States increased from 35.4% to 41.1% and from 7.3% to 9.7%, respectively.1 Obesity has been identified as a risk factor for subfertility, especially with ovulation disorders.2, 3 Several studies have shown the increased risk of delayed time to pregnancy among obese women.4–9 Obesity also predicts a lower probability of live birth among women undergoing ART.10, 11 The most updated systematic review and meta-analysis concluded that both overweight (BMI ≥ 25 kg/m2) and obese (BMI ≥ 30 kg/m2) women have a significant lower live birth rate, compared with women with a normal BMI (18.5–24.9 kg/m2).12
A known limitation of BMI as a marker of adiposity is that, despite being highly correlated to fat mass13 it is also positively related to lean mass.14, 15 Hence, BMI can misclassify muscular individuals as obese, although misclassification of individuals with high adiposity as “normal” is a more frequent problem.16 Women presenting to fertility centers tend to have a lower BMI than women of reproductive age in the general population and thus misclassification may occur more often in studies conducted in fertility centers than in other settings,1, 11 but the extent of this issue is unknown. Waist circumference (WC) is an anthropometric measure that is not only highly correlated to fat mass but is also used allow for classification of central obesity.17 In fact, WC can predict risk of multiple chronic conditions when considered alone or in combination with BMI.18–24 However, few studies have examined the association of WC with fertility or outcomes of infertility treatment. To address this gap, we examined the relation between WC and ART outcomes, when considered alone or in combination with BMI, in an ongoing prospective cohort study of women in couples presenting for infertility evaluation and treatment.
Material and methods
Study population
This study consisted of a subset of women enrolled in the Environment and Reproductive Health (EARTH) Study: an ongoing prospective cohort started in 2004 to explore environmental and lifestyle determinants of fertility among couples presenting for evaluation or treatment of infertility to the Massachusetts General Hospital (MGH) Fertility Center (Boston, MA). Women age 18 to 45 years planning to use their own gametes for ART were eligible. Women remained eligible to participate if their physician later determined that using donor oocytes or embryos was clinically necessary. Among women referred to the study, approximately 60% of those approached by the research staff enrolled in the study.
WC assessments were introduced to the study in 2010. At study entry, participants underwent an anthropometric assessment by trained study staff. Waist circumference was measured using a Gullick II Plus Measuring Tape and recorded to the nearest millimeter. Participants were asked to stand, discover their abdomen and hold their shirt or examination gown above their abdomen with their crossed arms. Study staff then placed the measuring tape at the level of the umbilicus on a plane parallel to the floor and applying pressure based on the tape’s pressure indicator. All participants completed a staff-administered brief questionnaire aimed at collecting basic demographic, medical and lifestyle information. Participants were also asked to allow investigators access to their medical records, and to complete a detailed questionnaire addressing lifestyle and medical history at home. Women were then followed during their in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles for clinical outcomes until a live birth was achieved or discontinuation of treatment.
The current analysis included 264 women with available WC measurements who underwent 445 ART cycles between 2010 and 2017. The minimum and maximum number of ART cycles contributed per woman were one (N=142) and five (N=10). This study was approved by the Institutional Review Boards of the MGH and the Harvard T.H. Chan School of Public Health. All participants provided written informed consent after the study procedures were explained by study staff.
Clinical management and assessment of outcomes
Trained study staff abstracted clinical information from the patients’ electronic medical records. Details of patient clinical management have been described elsewhere.10 In brief, for fresh ART cycles, patients underwent one of three stimulation protocols as clinically indicated: 1) luteal-phase gonadotropin-releasing hormone (GnRH) agonist protocol; 2) follicular-phase GnRH-agonist/Flare protocol; or 3) GnRH-antagonist protocol. Patients were monitored by clinical staff during gonadotropin stimulation for serum estradiol (E2), follicle size and counts, and endometrial thickness, and administered human chorionic gonadotropin (hCG) approximately 35 hours before the scheduled oocyte retrieval to induce oocyte maturation. Embryologists classified oocytes as germinal vesicle (GV), metaphase I (MI), metaphase II (MII), or degenerated, and then determined fertilization rate as the number of oocytes with 2 pronuclei divided by the number of MII oocytes at 17 to 20 hours after either conventional IVF or intracytoplasmic sperm injection (ICSI). For cryo-thaw or donor-egg recipient cycles, patients underwent endometrial preparation protocols as clinically indicated. Early ART end points referred to those preceding embryo transfer, including markers of ovarian responses to stimulation (peak E2 levels, endometrial thickness, MII and total oocytes), and fertilization rate. Clinical outcomes per initiated ART cycle were assessed, including the probabilities of implantation (a serum β-hCG level >6mIU/mL, measured approximately 17 days after oocyte retrieval), clinical pregnancy (presence of intrauterine gestational sac[s] on transvaginal ultrasonography at 6 weeks), and live birth (the birth of a neonate on or after 24 weeks of gestation). The denominator for all clinical outcomes was the total number of ART cycles started.
Statistical analysis
Participants were categorized by tertiles of WC measured in centimeter (cm). The associations of baseline personal and reproductive characteristics with WC were evaluated using Kruskal-Wallis tests for continuous variables and Chi-square or Fisher’s Exact tests for categorical variables. We used cluster-weighted generalized estimating equations models25 to evaluate the associations of WC with ART outcomes, while accounting for correlation among multiple treatment cycles per woman and the unbalanced design. A normal distribution and identity link were specified for continuous outcomes (peak E2 levels, endometrial thickness, and day 3 FSH levels); a Poisson distribution and log link for count outcomes (number of total and mature oocytes retrieved); and a log-binomial distribution for proportions (fertilization and clinical outcomes). Tests for linear trend across tertiles of WC were conducted using the median WC in each tertile as a continuous variable in the regression models. Primary analyses were performed using measured WC (divided into tertiles) and included terms for WC-adjusted BMI (BMI residual). Confounding was evaluated based on previous knowledge and taking into consideration the associations between WC with baseline characteristics and the probability of live birth. Multivariable adjusted models were included terms for age (continuous, years), race/ethnicity (white vs. non-white), smoking (ever vs. never), infertility diagnosis (male factor, female factor, unexplained), day 3 FSH levels (IU/L), BMI (continuous, kg/m2) and height (continuous, cm). Population marginal means were used to present population averages adjusted for the covariates (at the mean level for continuous variables and at a value weighted according to their frequencies for categorical variables) in the model.26 We also assessed the relation of WC and ART using the World Health Organization (WHO) suggested cut-off values for WC (80 and 88 cm)27 rather than distribution based cutoffs. Because WC and BMI tend to be highly correlated, we calculated BMI-adjusted WC values (residuals of the regression of WC on BMI) and WC-adjusted BMI values (residuals of the regression of BMI on WC) to allow for the computation of more efficient estimates in multivariable models.17 We performed sensitivity analyses to evaluate the robustness of the findings and modeling assumptions, which included modeling WC as a restricted cubic spline and adjusting models with different strategies. We tested whether the association between WC and ART outcomes differed between fresh and cryo cycles or by primary infertility diagnosis by introducing cross product terms to the multivariable adjusted models. When there was a suggestive interaction effect (p-value <0.10), stratified analysis was further conducted. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
The study population included 264 women who underwent 445 ART cycles of which 158 (35.5%) resulted in a live birth. Mean (SD) age, WC and BMI of participants were 34.9 (4.2) years, 83.6 (12.6) cm and 24.1 (4.3) kg/m2, respectively. The majority of women were white (80%), had a college degree (99%), and had never smoked (76%). Forty-one percent of women reported at least one prior pregnancy. The most common initial primary infertility diagnosis was unexplained infertility (46%). WC was positively related to BMI (r=0.69, p<0.0001), but not to other personal or reproductive characteristics (Table 1).
Table 1.
Demographic and baseline reproductive characteristics of study participants (N=264 women)
| Characteristics | All participants | Waist circumference, (cm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Range, cm | 61 - 158 | Tertile 1 (<77.0) | Tertile 2 (77.0-86.0) | Tertile 3 (>86.0) | P value* | ||||
| Waist circumference (cm), mean (SD) | 83.6 | (12.6) | 71.7 | (4.0) | 81.0 | (3.0) | 97.3 | (10.6) | |
| Number of participants | 264 | 85 | 88 | 91 | |||||
| Personal characteristics | |||||||||
| Age (years), mean (SD) | 34.9 | (4.2) | 34 .6 | (4.3) | 34 .5 | (4.3) | 35 .4 | (4. 1) | 0.17 |
| BMI (kg/m2), mean (SD) | 24.1 | (4.3) | 21.5 | (3.5) | 22.8 | (2.4) | 27.8 | (3.9) | <.0001 |
| Height (cm), mean (SD) | 165.6 | (6.8) | 164.6 | (6.5) | 166.1 | (7.0) | 166.0 | (6.7) | 0.32 |
| Smoker, N (%) | |||||||||
| Never | 201 | (76) | 66 | (78) | 67 | (76) | 68 | (75) | 0.74 |
| Past | 59 | (22) | 19 | (22) | 19 | (22) | 21 | (23) | |
| Current | 4 | (2) | 0 | (0) | 2 | (2) | 2 | (2) | |
| White, N (%) | 212 | (80) | 64 | (75) | 69 | (78) | 79 | (87) | 0.13 |
| College degree or higher, N (%) | 261 | (99) | 83 | (98) | 87 | (99) | 91 | (100) | 0.34 |
| Reproductive characteristics | |||||||||
| Initial primary infertility diagnosis, N (%) | |||||||||
| Male factor | 71 | (27) | 21 | (25) | 28 | (32) | 22 | (24) | 0.39 |
| Female factor | 71 | (27) | 23 | (27) | 18 | (20) | 30 | (33) | |
| Unexplained | 122 | (46) | 41 | (48) | 42 | (48) | 39 | (43) | |
| Initial treatment protocol, N (%) | |||||||||
| Antagonist | 41 | (16) | 11 | (13) | 16 | (18) | 14 | (15) | 0.75 |
| Flare | 30 | (11) | 9 | (10) | 7 | (8) | 14 | (15) | |
| Luteal phase agonist | 180 | (68) | 60 | (71) | 61 | (69) | 59 | (65) | |
| Endometrial preparation (donor/cyro cyles) | 13 | (5) | 5 | (6) | 4 | (5) | 4 | (5) | |
| Ever pregnant, N (%) | 109 | (41) | 32 | (38) | 37 | (42) | 40 | (44) | 0.59 |
| Type of fertilization procedure, N (%)a | 115 | (48) | 34 | (45) | 42 | (51) | 39 | (48) | 0.80 |
| In vitro fertilization | |||||||||
| Intracytoplasmic sperm injection | 125 | (52) | 41 | (55) | 41 | (49) | 43 | (52) | |
| Day three FSH (IU/L), mean (SD) | 7.21 | (2.23) | 7.48 | (2.48) | 7.07 | (2.02) | 7.09 | (2.19) | 0.36 |
Values are means (SD) or N (%) and are standardized to the age distribution of the study population.
From Kruskal-Wallis test for continuous variables and Chi-square tests for categorical variables or Fisher when cells < 5 individuals.
24 women were not included due to not reaching fertilization.
WC was inversely associated with estradiol (E2) trigger levels. The adjusted mean (95% confidence interval (CI)) E2 trigger levels for women in increasing tertiles of WC were 2319 (2116-2521), 2118 (1936-2301), and 2011 (1838-2185) pmol/L (p, trend = 0.01) (Table 2). However, we did not observe associations of WC with endometrial thickness, day 3 FSH levels, oocyte yield or fertilization rate (Table 2).
Table 2.
Association between waist circumference and intermediate assisted reproductive technology (ART) outcomes
| Tertile of waist circumference, cm |
||||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted means (95% CI)a | Cycles | Tertile 1 (<77.0 cm) | Tertile 2 (77.0-86.0 cm) | Tertile 3 (>86.0 cm) | P trend | |||
| Endometrial thickness, mm | 422 | 10.4 | (9.6-11.1) | 10.4 | (9.8-11.0) | 9.9 | (9.3-10.5) | 0.17 |
| E2 trigger levels, pmol/L | 353 | 2319 | (2116-2521) | 2118 | (1936-2301) | 2011* | (1838-2185) | 0.01 |
| Day 3 FSH, IU/Lb | 445 | 7.59 | (6.98-8.20) | 7.23 | (6.76-7.70) | 7.05 | (6.54-7.56) | 0.11 |
| Total oocyte yield, n | 350 | 11.8 | (10.5-13.2) | 10.9 | (9.6-12.3) | 11.8 | (10.5-13.2) | 0.84 |
| M2 oocyte yield, n | 350 | 9.6 | (8.5-10.9) | 9.1 | (8.0-10.4) | 9.7 | (8.7-10.8) | 0.78 |
| Fertilization, rate | 349 | 0.71 | (0.64-0.76) | 0.74 | (0.69-0.79) | 0.72 | (0.67-0.77) | 0.81 |
All models were adjusted for age, race (white vs. non-white), smoking (ever vs. never), infertility diagnosis (male factor, female factor, unexplained), day 3 FSH, BMI, and height.
Not adjusted for day 3 FSH.
p<0.05 compared with tertile 1
WC was inversely related to the probabilities of implantation, clinical pregnancy and live birth per initiated treatment cycle (Figure 1). The probabilities of implantation, clinical pregnancy and live birth for women in the highest category of WC were, respectively, 14%, 13% and 15% lower than that of women in the lowest category of WC (Figure 1). Results did not change after excluding height from all the models. A similar pattern was observed when the analyses were conducted using the WHO suggested cut-off values27 for WC (Table S1), when WC was modeled as a continuous variable allowing for non-linear relations (Figure S1-S3), and in sensitivity analyses employing different approaches to account for the collinearity between WC and BMI (Table S2).
Figure 1:
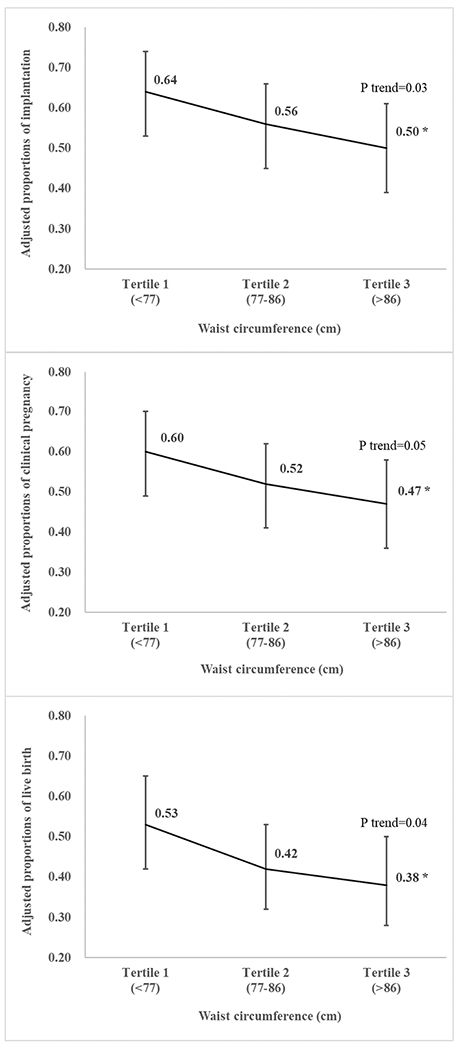
Adjusted probabilities (95% confidence intervals) of implantation, clinical pregnancy and live birth per initiated treatment cycle across tertiles of waist circumference among women undergoing infertility treatment with ART. All models were adjusted for age, race (white vs. non-white), smoking (ever vs. never), infertility diagnosis (male factor, female factor, unexplained), day 3 FSH, BMI, and height. *: p<0.05 compared with tertile 1.
We then classified women in joint categories of WC and BMI. Women with BMI≥25 kg/m2 and WC≥77 cm had the lowest probability (95% CI) of live birth per cycle started (38% (27-50%)), whereas women with a BMI between 18.5 and 25 kg/m2 and WC<77 cm had the highest probability of live birth (54% (42-66%)) (Figure 2). Overweight women with higher WC always had the lowest probability of live birth (Table S3). In contrast, overweight women with lower WC had chance of treatment success similar to those with normal BMI. The results were similar using different WC cut-off values (Table S3).
Figure 2:
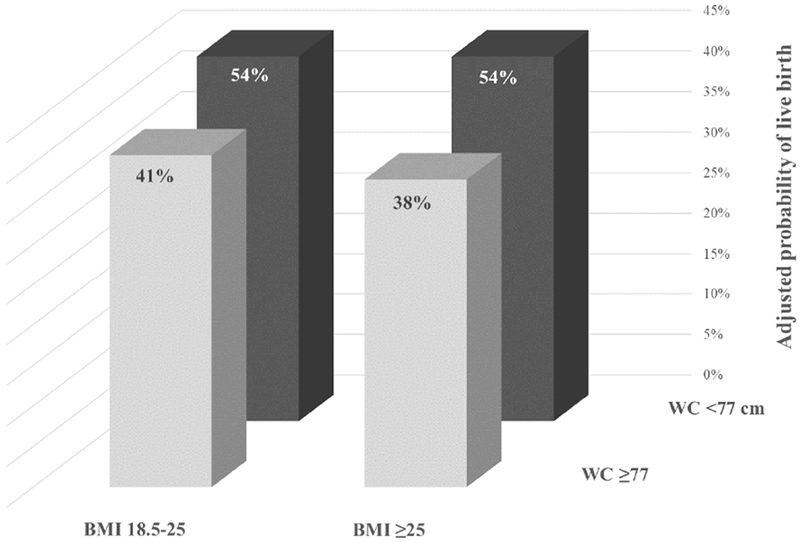
Adjusted probability of live birth in joint categories of BMI (18.5-25 or ≥25 kg/m2) and waist circumference (<77 or ≥77 cm) for women undergoing infertility treatment with ART. All models were adjusted for age, race (white vs. non-white), smoking, infertility diagnosis (male factor, female factor, unexplained), day 3 FSH, and height.
Sensitivity analyses showed that the relation between WC and ART outcomes did not differ between fresh and cryo cycles or by primary infertility diagnosis. Further adjusting for male partner BMI and WC in the subset of women for whom these data were available did not change the findings either (data not shown). Moreover, while graphic evaluation of the relation between WC and ART outcomes suggested non-linear relations (Figure S1-S3), formal tests for non-linearity showed that non-linear models not are superior to linear models in describing the relation between WC and ART outcomes in this study population.
Discussion
We examined the associations of baseline WC with embryological and clinical outcomes in a cohort of women undergoing ART. WC was associated with lower E2 trigger levels and, more importantly, lower probability of implantation, clinical pregnancy and live birth per treatment cycle independently of BMI and other potential confounders. When women were jointly classified in categories of BMI and WC, overweight women with higher WC had the lowest probabilities of live birth. In contrast, overweight women with lower WC had probabilities of live birth similar to those with normal BMI. These data suggest that WC provides information regarding a woman’s chances of treatment success with ART above and beyond her BMI.
To our knowledge, only two previous studies have evaluated the relation between WC and outcomes of infertility treatments. Hansen and colleagues conducted a secondary data analysis from a randomized, multicenter clinical trial in women undergoing intrauterine insemination (IUI), which found that neither BMI nor WC were associated to the probability of live birth.28 Additionally, Moran and colleagues evaluated the association of WC and ART outcomes in a pilot weight loss study among obese women presenting for infertility treatment. They found that decreases in WC was a better predictor of higher probability of live birth than changes in BMI, which did not predict ART outcomes.29 Waist circumference has also been related to lower fecundability independently of BMI in cohorts of pregnancy planners without a history of infertility.30, 31 Moreover, visceral fat area was related to response to ovulation induction with clomiphene citrate among women with PCOS independently of BMI.32 Furthermore, emerging evidence suggests that WC may be a risk factor for pregnancy loss.33, 34 Collectively, these data suggest that abdominal obesity, may impact fertility above and beyond global measures of adiposity, which has been the main focus of the literature on the relation between adiposity and fertility.
The observed association between WC and ART outcomes may reflect a true biological effect. WC has been linked to insulin resistance and chronic inflammation. Insulin resistance is an important pathogenic factor in common metabolic disorders and plays a key pathogenic role in the infertility.35 WC has been reported as an independent predictor of insulin resistance after controlling for BMI,36 as well as a great predictor of insulin sensitivity.37 Moreover, WC was related to chronic inflammation,38, 39 which can further affect ovulation and steroidogenesis.40–42 Whether the observed relation is mediated through these or other mechanisms deserves further evaluation.
Strengths of our study include its prospective design with no loss to follow-up and robust assessment of intermediate and clinical outcomes, all of which aid in the interpretation of the findings. BMI and WC were all measured by trained study staff, which minimized variability of the measurements. The sample size of the study population allowed us to evaluate clinically relevant outcomes with sufficient statistical power, including live birth. The main limitation of the study is the relatively small size of groups of women jointly classified according to their BMI and WC which resulted in stratum sizes as small as 13 treatment cycles in some analyses. While the results were robust regardless of cutoff values used in these joint models, additional larger cohort studies are warranted. In addition, WC was only measured once at enrollment, and thus any changes in WC during ART treatment were not captured by our study.
In conclusion, we found an association of high WC with lower probability of live birth among women undergoing infertility treatment with ART. This association was independent of BMI, was consistent across different cut-off points of WC employed in the analysis and, in stratified analyses, was more pronounced for women with high WC and high BMI. These results suggest that central adiposity may be important for human fertility above and beyond overall adiposity.
Supplementary Material
AJOG at a Glance:
A. Why was this study conducted?
Many studies have identified overweight and obesity as risk factors for infertility and failed infertility treatment. However, the role abdominal obesity independent of overall adiposity, as measured by BMI, has received little attention.
B. What are the key findings?
The probability of achieving a live birth as a result of ART decreased with increasing waist circumference independently of BMI. Overweight women with a high waist circumference had the lowest probability of live birth whereas overweight women with low waist circumference had probabilities of live birth similar to those of women with normal BMI.
C. What does this study add to what is already known?
Waist circumference provides information regarding a woman’s chances of infertility treatment success with ART above and beyond her BMI.
Acknowledgment(s)
The authors acknowledge all members of the EARTH Study team, including research staff Ramace Dadd and Myra Keller, and physicians and staff at Massachusetts General Hospital fertility center. We are also grateful to all study participants.
Funding
Grants P30ES000002, R01ES009718, R01ES022955 and P30DK046200 from the National Institutes of Health. Dr. Li was supported by the Ministry of Science and Technology, Taiwan (MOST 106-2917-I-564-066).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Condensation:
Waist circumference is inversely related to live birth rates in infertility treatment with ART independently of BMI.
References
- 1.Hales CM,Fryar CD,Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. Jama 2018;319:1723–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A Obesity and reproductive disorders in women. Human reproduction update 2003;9:359–72. [DOI] [PubMed] [Google Scholar]
- 3.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstetrics and gynecology 2007;110:1050–8. [DOI] [PubMed] [Google Scholar]
- 4.Zaadstra BM, Seidell JC, Van Noord PA, et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ (Clinical research ed) 1993;306:484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology (Cambridge, Mass) 1999;10:422–8. [DOI] [PubMed] [Google Scholar]
- 6.Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L Body mass index and delayed conception: a European Multicenter Study on Infertility and Subfecundity. American journal of epidemiology 2000;151:1072–9. [DOI] [PubMed] [Google Scholar]
- 7.Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertility and sterility 2004;81:384–92. [DOI] [PubMed] [Google Scholar]
- 8.Nohr EA, Vaeth M, Rasmussen S, Ramlau-Hansen CH, Olsen J Waiting time to pregnancy according to maternal birthweight and prepregnancy BMI. Human reproduction (Oxford, England) 2009;24:226–32. [DOI] [PubMed] [Google Scholar]
- 9.Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Human reproduction (Oxford, England) 2010;25:253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavarro JE, Ehrlich S, Colaci DS, et al. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertility and sterility 2012;98:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luke B, Brown MB, Stern JE, et al. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Human Reproduction 2010;26:245–52. [DOI] [PubMed] [Google Scholar]
- 12.Supramaniam PR, Mittal M, Mcveigh E, Lim LN. The correlation between raised body mass index and assisted reproductive treatment outcomes: a systematic review and meta-analysis of the evidence. Reproductive health 2018;15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Q, Van Dam RM, Spiegelman D, Heymsfield SB, Willett WC, Hu FB. Comparison of dual-energy x-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. American journal of epidemiology 2010;172:1442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? American journal of epidemiology 1996;143:228–39. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher JC, Yalamanchili V, Smith LM. The effect of vitamin D supplementation on serum 25OHD in thin and obese women. The Journal of steroid biochemistry and molecular biology 2013;136:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark MK,Dillon JS. BMI misclassification, leptin, C-reactive protein, and interleukin-6 in young women with differing levels of lean and fat mass. Obesity research & clinical practice 2011;5:e85–e92. [DOI] [PubMed] [Google Scholar]
- 17.Hu F Obesity epidemiology. Oxford University Press; Number of pages. [Google Scholar]
- 18.Janssen I,Katzmarzyk PT, Ross R Waist circumference and not body mass index explains obesity-related health risk. The American journal of clinical nutrition 2004;79:379–84. [DOI] [PubMed] [Google Scholar]
- 19.Lofgren I, Herron K, Zern T, et al. Waist circumference is a better predictor than body mass index of coronary heart disease risk in overweight premenopausal women. The Journal of nutrition 2004;134:1071–76. [DOI] [PubMed] [Google Scholar]
- 20.Savva S, Tornaritis M, Savva M, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. International journal of obesity 2000;24:1453. [DOI] [PubMed] [Google Scholar]
- 21.Ardern CI, Janssen I, Ross R, Katzmarzyk PT. Development of health-related waist circumference thresholds within BMI categories. Obesity research 2004;12:1094–103. [DOI] [PubMed] [Google Scholar]
- 22.Moore LL, Bradlee ML, Singer MR, et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 2004;28:559–67. [DOI] [PubMed] [Google Scholar]
- 23.Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clinic proceedings 2014;89:335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005;115:1623–30. [DOI] [PubMed] [Google Scholar]
- 25.Williamson JM,Datta S, Satten GA. Marginal analyses of clustered data when cluster size is informative. Biometrics 2003;59:36–42. [DOI] [PubMed] [Google Scholar]
- 26.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. The American Statistician 1980;34:216–21. [Google Scholar]
- 27.ORGANIZATION WH. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008. 2011. [Google Scholar]
- 28.Hansen KR, He ALW, Styer AK, et al. Predictors of pregnancy and live-birth in couples with unexplained infertility after ovarian stimulation–intrauterine insemination. Fertility and sterility 2016;105:1575–83. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran L, Tsagareli V, Norman R, Noakes M. Diet and IVF pilot study: short-term weight loss improves pregnancy rates in overweight/obese women undertaking IVF. The Australian & New Zealand journal of obstetrics & gynaecology 2011;51:455–9. [DOI] [PubMed] [Google Scholar]
- 30.Wise LA, Palmer JR, Rosenberg L. Body size and time-to-pregnancy in black women. Human reproduction (Oxford, England) 2013;28:2856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundaram R, Mumford SL, Buck Louis GM. Couples’ body composition and time-to-pregnancy. Human reproduction (Oxford, England) 2017;32:662–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellakwa HE, Sanad ZF, Hamza HA, Emara MA, Elsayed MA. Predictors of patient responses to ovulation induction with clomiphene citrate in patients with polycystic ovary syndrome experiencing infertility. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2016;133:59–63. [DOI] [PubMed] [Google Scholar]
- 33.Hahn KA, Hatch EE, Rothman KJ, et al. Body size and risk of spontaneous abortion among danish pregnancy planners. Paediatric and perinatal epidemiology 2014;28:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felisbino-Mendes MS, Matozinhos FP, Miranda JJ, Villamor E, Velasquez-Melendez G. Maternal obesity and fetal deaths: results from the Brazilian cross-sectional Demographic Health Survey, 2006. BMC pregnancy and childbirth 2014;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nestler JE, Stovall D, Akhter N, Iuorno MJ, Jakubowicz DJ. Strategies for the use of insulin-sensitizing drugs to treat infertility in women with polycystic ovary syndrome. Fertility and sterility 2002;77:209–15. [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. The Journal of pediatrics 2006;148:188–94. [DOI] [PubMed] [Google Scholar]
- 37.Wahrenberg H, Hertel K, Leijonhufvud BM, Persson LG, Toft E, Arner P. Use of waist circumference to predict insulin resistance: retrospective study. BMJ (Clinical research ed) 2005;330:1363–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ackermann D, Jones J, Barona J, et al. Waist circumference is positively correlated with markers of inflammation and negatively with adiponectin in women with metabolic syndrome. Nutrition research (New York, NY) 2011;31:197–204. [DOI] [PubMed] [Google Scholar]
- 39.Festa A, D’Agostino R JR., Williams K, et al. The relation of body fat mass and distribution to markers of chronic inflammation. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 2001;25:1407–15. [DOI] [PubMed] [Google Scholar]
- 40.Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. The Journal of clinical endocrinology and metabolism 2001;86:2453–5. [DOI] [PubMed] [Google Scholar]
- 41.Davis JS. Connecting Female Infertility to Obesity, Inflammation, and Materna Gut Dysbiosis. Endocrinology 2016;157:1725–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reproductive sciences (Thousand Oaks, Calif) 2009;16:216–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


