Abstract
The processes that define mammalian physiology evolved millions of years ago in response to ancient signaling molecules, most of which were acquired by ingestion and digestion. In this way, evolution inextricably linked diet to all major physiological systems including the nervous system. The importance of diet in neurological development is well documented, although the mechanisms by which diet-derived signaling molecules (DSMs) affect cognition are poorly understood. Studies on the positive impact of nutritive and non-nutritive bioactive molecules on brain function are encouraging but lack the statistical power needed to demonstrate strong positive associations. Establishing associations between DSMs and cognitive functions like mood, memory and learning are made even more difficult by the lack of robust phenotypic markers that can be used to accurately and reproducibly measure the effects of DSMs. Lastly, it is now apparent that processes like neurogenesis and neuroplasticity are embedded within layers of interlocked signaling pathways and gene regulatory networks. Within these interdependent pathways and networks, the various transducers of DSMs are used combinatorially to produce those emergent adaptive gene expression responses needed for stimulus-induced neurogenesis and neuroplasticity. Taken together, it appears that cognition is encoded genomically and modified by epigenetics and epitranscriptomics to produce complex transcriptional programs that are exquisitely sensitive to signaling molecules from the environment. Models for how DSMs mediate the interplay between the environment and various neuronal processes are discussed in the context of the food–brain axis.
Subject terms: Neuroscience, Systems biology, Dendritic excitability
Introduction
Recent reports of adult hippocampal neurogenesis (AHN) in small animal models has stimulated interest in understanding the molecular mechanisms underlying this process and in particular, those genomic and environmental factors that influence neurogenesis in humans.1–4 The prospect that adult humans can improve neurological processes such as mood, emotion and cognition with food and lifestyle modification is intriguing. Research results on the role of physical activity,5–7 mastication,8–10 and cognitive training11 in promoting neurogenesis and cognitive performance are encouraging in that they may provide simple, noninvasive interventions for improving neurological health. The results of studies on the role of caloric restriction12 and food with all its diet-derived signaling molecules (DSMs) (e.g., macronutrients, micronutrients, biotransformed nutrients, phytochemicals, anti-nutrients and xenobiotics) are less clear but still promising.12–14 The problem of weak significance levels in studies on the impact of food and lifestyle on cognition was recently highlighted in a systematic evidence review entitled “Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment and Clinical Alzheimer’s-Type Dementia commissioned by the NASEM/NIA.”15 For this comprehensive meta-analysis, 263 eligible publications were chosen from a total 9448 references involving 13 different interventions. Of these 13 interventions, only four (physical activity, raloxifene—a selective estrogen receptor modulator, B vitamins and cognitive training) were associated with delaying or preventing age-related cognitive decline, albeit at low to moderate levels of statistical significance. The review mentioned several factors explaining the low statistical correlations between these interventions and cognitive health. These factors included, but were not limited to, large variations in study design, subject populations, protocols, as well as low adherence and high attrition rates. The lack of clear clinical endpoints, including different cognitive assessment tools, also confounded the interpretation of results and contributed to inconsistent study results. The NASEM/NIA review was less a critique of the role of food and DSMs on neurogenesis and cognition than it was a call for more, larger and better-designed studies including those involving multimodalities or “best packages” of interventions. The review also suggested that research on dietary interventions, including supplementation with B vitamins, should be a high priority.15
While the challenges of interpreting large numbers of dietary interventions in the aggregate are many, there remains a large body of literature describing the important and positive relationship between food and neurological development and function in young children16–20 and the elderly.21–25 In terms of the impact of diet on early brain development in children, Prado and Dewey18 concluded that undernourished children are at risk of not reaching their full developmental potential in cognitive, motor, and socio-emotional abilities. In many instances, however, these abilities can be restored if nutritional rehabilitation occurs early in brain development, including intrauterine brain growth. In the case of the aging brain, many molecular and cellular processes, including neural stem cell development, are altered in response to aging. Poor diet is known to cause age-related changes in the systemic environment that increases the risk of both chronic disease and cognitive decline among the elderly.23 The purpose of this review is to focus attention on the molecular mechanisms that transduce DSMs into the nonrandom, DNA-encoded, emergent adaptive transcription programs needed for neurogenesis and stimulus-induced neuronal, and synaptic, plasticity. Because of space limitations, we are unable to discuss in any detail the importance of epigenetics and the human microbiome on neurogenesis and cognitive function. Instead, we refer the reader to the following reviews on neuroepigenetics1,4,26,27 and the gut–brain axis.28,29
The evolution of food–brain interactions
The wonders of the modern human brain can be traced to its humble beginnings. Starting with a brain of approximately 470 ml in the Hominini,30 the human brain has grown to about 1350 ml over the past 2 million years.31 The near tripling in the size of the human brain is the result of many factors not the least of which are the external inputs of energy and the molecular building blocks provided by macronutrients (e.g., proteins, carbohydrates and lipids). Although macronutrients are essential for energy and the assembly of neural and non-neural tissues, it is also likely that the micronutrients, biotransformed nutrients, phytochemicals and even anti-nutrients and xenobiotics (i.e., DSMs), triggered the plethora of molecular processes required for the growth, development and differentiation of the modern brain and all its parts. When stimuli from DSMs are integrated neuronally and subjected to the pressures of natural selection, cellular responses can emerge that produce higher-order cognition that is adaptive, sustainable and knowledge generating. This is almost assured considering the extensive feedforward/feedback regulatory controls at play in the brain as discussed later.
Since AHN was first discovered in the mammalian brain by Altman and Das,32 it was often considered a phylogenetic reversion, away from lifelong neurogenesis, in favor of neurological stability within the complexity of the brain.33 We now know that AHN occurs throughout the animal kingdom and while humans have fewer neurogenic zones than fish, within these neurogenic zones, substantial and highly functional neurons can be produced. This suggests that at least for the mammalian dentate gyrus, evolution has moved toward neurogenic plasticity rather than away from it.33 This idea is supported by recent studies using imaging connectomics34 and graph theory showing that normal brain maturation, from infancy to adulthood, involves significant co-evolution and integration of structural (neurons and glial cells) and functional (cognitive processes) networks.35 We propose that this co-evolution of neuronal and synaptic plasticity is supported, if not driven, by the constant interplay between the brain and external stimuli from food. This concept is consistent with the ecological intelligence hypothesis for primate brain evolution.36 According to this hypothesis, “foraging cognition” involving spatial memory, value-based decision making and inhibitory control creates those dynamic feedforward and feedback interactions that are adaptive and lead to higher-quality foods, more productive food sources and larger brains.36,37 We believe the principles of the food–brain axis described below, complement and extend the ecological intelligence hypothesis by connecting the nutritional environment with neurological structures and processes through well-studied signaling pathways and gene regulatory networks.
The food–brain axis
In recent years, it has become apparent that all physiological, metabolic and genetic processes and systems are interconnected and interdependent to some degree. Examples include the inflammation–immunity axis,38 the hypothalamus–pituitary–gonadal axis39 and the gut–brain axis.28,29 In each case, an axis suggests diverse regulatory lineages, orchestrating control and outcomes over other critical processes. This makes all the systems involved highly sensitive to extracellular signals including those from the environment. The food–brain axis represents more than just connectivity and relatedness between what we eat and how our brain grows and functions; it illustrates dynamic interdependencies between food and neurological processes. This semi-quantitative interpretation of “axis” should enable researchers to categorize, quantify and predict neurological changes as a function of food quality and/or quantity.
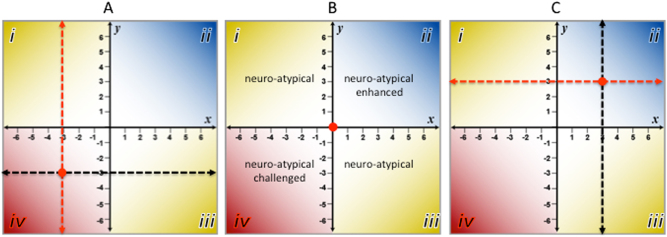
Here, the food–brain axis is defined as a horizontal line of independent variables thought to be causative (e.g., food) that transects a vertical line of dependent variable thought to be the effects (e.g., neurogenesis, neuroplasticity and neuropathologies). Figure 1 shows three hypothetical examples (a, b and c) depicting the consequences of changing the quantity and/or quality of the food from poor (e.g., -3) to good (e.g., +3) on the X-axis, and its effect on neural growth, differentiation and function on the Y-axis. In addition to being an informative method for displaying the dynamic relationship between diet and brain structure and function, the food–brain axis organizes these interactions into four quadrants (i.e., i–iv). Figure 1b shows the nominal or neuro-typical condition for food–brain interactions whereas Fig. 1a-iv shows a neuro-atypical/challenged condition in which neurological processes are dysfunctional, degenerative and pathological as a result of poor diet. Conversely, Fig. 1c-ii illustrates a neuro-atypical/enhanced condition in which neurological processes and structures are enhanced in response to dietary inputs that are higher in quality and/or quantity.
Fig. 1.
Four quadrants of the Food-Brain Axis. Three examples of the Food-Brain Axis where the X-axis represents increasing quantity and/or quality of dietary inputs (independent variables) and the Y-axis represent the changes in neuronal growth, development and cognitive function (dependent variables) from poor (-6) to optimal (+6) states. The transect points for the XY axes are represented by the red dots
The neuro-atypical quadrants A-i and C-iii pose interesting questions about food–brain interactions. In the case of quadrant A-i (neuro-atypical/enhanced) one might surmise that brain development and function (as defined by measures of intelligence) may be more dependent on robust genetic factors and age than on the quantity and/or quality of food intake. For example, it is known that the genetic contribution to human intelligence is approximately 80% for adults with additive genetic variance contributed by selective mating based on similar phenotypes.40 Therefore, if prenatal and early postnatal nutrition are adequate (e.g., nursing), the impact of nutritional deficiency later in life may have little or no measurable effect on cognitive performance. For quadrant C-iii, strong genetic determinants like Fragile X syndrome, Huntington’s disease, PKU as well as traumatic brain injury (TBI; Box 1) come into play. These are conditions that make neurological structures and processes refractory to the benefits of abundant, higher-quality dietary inputs. PKU, an autosomal recessive metabolic disorder, causes a toxic accumulation of dietary phenylalanine in the brain. If undiagnosed and left untreated, PKU can cause serious cognitive impairment, behavioral and mental disorders as well as seizures, regardless of food quality and/or quality. However, nutritional intervention with a low phenylalanine diet supplemented with large neutral amino acids, vitamin D and B12 can prevent or mitigate the physical, neurological and developmental problems associated with PKU.41 Therefore, interpreting the impact of food quality and/or quantity (x-axis) in quadrant A-i, age must be taking into account while in quadrant C-iii, early diagnosis of deleterious genetic factors or structural damage to brain tissues are key factors for consideration.
Food and Traumatic Brain Injury (TBI).
The Centers for Disease Control and Prevention defines TBI as an injury that disrupts normal brain function.42 Injury includes bumps, blows or jolts to the head, penetrating trauma and explosive blasts. Even mild TBI can trigger neurodegenerative disorders.42–44 Most clinical studies typically monitor nutritional deficits as a consequence of TBI and only a handful of studies focus on diet as a factor in recovery.43,45 It should be noted that, morbidity and long-term mortality from TBI are associated with a decline in digestive function as a result of either multiple organ dysfunction or systemic inflammatory response syndromes.46 Animal studies are consistent with these clinical findings motivating the search for a more mechanistic approach to this question.46 Primary TBI (pTBI) is characterized by insult to neuronal membrane and vasculature. Vascular damage triggers secondary TBI (sTBI) involving a cascade of events starting with ischemia, production of ROS, further damage to neuronal membranes, increased intracellular Ca2+, endoplasmic reticulum stress and ultimately apoptosis and autophagy.47 The events associated with pTBI are so sudden that the impact of nutritional supplementation is difficult to assess. However, those dietary supplements thought to reduce the effects of sTBI are vitamin D with progesterone, antioxidant vitamins C and E, zinc, magnesium and the omega-3 fatty acid, docosahexaenoic acid (DHA).44,48 Interest in DHA is increasing because of evidence from animal studies suggesting that DHA has both preventive and therapeutic benefits against trauma and stroke.5 Among the mechanisms associated with DHA consumption or administration are 1) a reduction of neuronal and white matter loss, 2) decrease of proinflammatory cytokines, BBB leakage, matrix metalloproteinase activity and 3) regulation of microglia activation.5 Other food-based supplements frequently considered beneficial are glutamine, choline, creatinine, curcumin, flavonoids, phenolic acids, quercetin and resveratrol.43,45,49–51 Ketones metabolically derived from medium chain triglycerides or the high-fat ketogenic diet are also being considered for treating cognitive impairment present in many brain and metabolic disorders such as Alzheimer’s disease, trauma and the metabolic syndrome. Ketone bodies such as β-hydroxybutyrate can serve as energy substrates that compensate for deficits in brain glucose utilization, as seen in people with mild cognitive impairment, Alzheimer’s disease and insulin resistance.52,53
DSMs and the brain
The impact of food on brain development and function has been extensively reviewed, although these reviews are often more descriptive than mechanistic in nature.15,54,55 They emphasize the neuroprotective aspects of nutrition but seldom discuss the mechanistic role of DSMs in AHN and cognition. Some of the first to systematically review research on the mechanistic effects of DSMs on the brain were Gomez–Pinilla55 and Zheng and Berthoud.56 A list of some of the dietary factors discussed in these reviews is shown in Table 1. While not an exhaustive list, Table 1 underscores the multifunctional character of DSMs that range from sources of energy and building blocks for cellular structures to chemical signals that trigger gene regulatory cascades that control transcriptional programs in the brain. One of the best examples of a multifunctional DSM is the long-chain polyunsaturated fatty acid (PUFA), DHA, which can serve as a nutrient, transcription regulator, immuno-modulator and neurotransmitter.
Table 1.
Diet-derived signaling molecules (DSM) that supports brain function
| DSM | Function in the Brain | References |
|---|---|---|
| Choline | A macronutrient important for normal brain development, nerve function; a precursor of acetylcholine which promotes cognitive flexibility and adaptive behavior in response to new and unexpected environmental circumstances | 55,156 |
| D-Glucose | Biotransformed from more complex sugars and carbohydrates; D-glucose provides the energy needs of the brain in the form of ATP; enhances cognitive function and neuroprotective for AD | 78,107 |
| Folate | Required for metabolism of 5-MTHF and homocysteine; deficiency in 5-MTHF is associated treatment-refractory depression while overproduction homocysteine is associated with neuropsychiatric disorders; folate is also a precursor for the methyl-donor, SAM, which is required to epigenetic modification of DNA and chromatin | 21,55,131,136–138,149 |
| Omega-3 fatty acids (EPA, DHA, ALA) | Neuroprotective against AD; reduces the levels of AD biomarkers (β-amyloid plaque and neurofibrillary tangles) in cerebral spinal fluid; DHA has been implicated reducing severity of depression and bipolar disorder | 56–59,73,147,148, 157,158 |
| Plant polyphenols | Neuroprotective for AD and Parkinson’s disease; neurotrophic and associated with enhanced neuronal survival and promotes neuronal differentiation in vitro; helps maintain metabolic homeostasis which has a protective effect on membranes; involved in histone deacetylation | 13,43,45,49,54,55 |
| Vitamin A | Antioxidant; prevents cognitive decline; perinatal deficiency correlated with increased risk of schizophrenia; promotes neuronal differentiation of neuronal stem cells | 21,55,159 |
| Vitamin B3 (niacin) | Transactivation of a PI3K/Akt signaling cascade to prevent/reduce brain damage from stroke; neuroprotective for Parkinson’s disease | 156,160 |
| Vitamin B6 (pyridoxine) | Coenzyme for the biosynthesis of neurotransmitters; required for metabolism of homocysteine which is implicated in the development of psychiatric disorders including depression | 21,161 |
| Vitamin B12 | Essential for brain development, neuronal myelination and cognitive function including mood; methyl-donor for methionine and SAM, the latter serving as the methyl-donor for epigenetic modification of DNA and chromatin | 15,131,162 |
| Vitamin C | Neuroprotective against oxidative damage in the brain; higher intake associated with lower AD | 21,55,163 |
| Vitamin D | Neuroprotective against oxidative damage; deficiency correlated with greater risk of schizophrenia and multiple sclerosis | 55,164 |
| Vitamin E | Antioxidant; prevents membrane oxidation DHA peroxidation; slows cognitive decline and the advancement of AD | 55,165 |
Table 1 lists twelve well-characterized DSMs and their purported and demonstrated impact on neurological function. Not shown are various non-dietary plant compounds (e.g., forskolin, huprazine A, ginko) and minerals (i.e., Ca, Cu, Fe, Se, Zn) known, or thought to be involved in preserving or stimulating cognition in humans and/or laboratory animals. Table adapted from Gomez-Padilla55
Reduced brain or circulating DHA concentration has been implicated in depression, bipolar disorder and attention deficit (AD) disorder.57–59 However, intervention studies with long-chain omega-3 PUFAs have yielded mixed results.5,57–60 One recent meta-analysis, however, suggested an overall beneficial effect for EPA in major depressive disorder patients, especially at high doses.61 Interestingly, the beneficial effects of EPA were also observed in subjects taking antidepressants. Whether the beneficial effects of high EPA dosage together with antidepressants are additive or synergistic can have significant therapeutic implications and thus requires further study. Most studies designed to assess the benefits of omega-3 PUFAs on children with AD disorder are inconclusive. Another recent study, however, showed significant improvement in working memory for children with attention deficit hyperactive disorder supplemented with EPA62 (see Box 2).
For DSMs to impact various neurological structures and functions in ways that produce neurogenesis, synaptic plasticity and adaptive behaviors, there must be an efficient communication system allowing dietary stimuli to be delivered to the brain from the gut. These connections are provide by the 400–600 million neurons in the human enteric system77 that creates a virtual information highway through which DSMs can communicate critical chemical information from the environment to the brain.78 Alternatively, oxygen and nutrients in peripheral blood can be delivered to the brain via the middle cerebral arteries and their fenestrated capillaries to support hippocampal6 and hypothalamic functions.79 These communication channels permit dietary inputs to be more than just fuel and building blocks for the brain, but also a means for delivering important chemical signals from the extracellular environment to the neuron where they are continually integrated into those signaling pathways and neuronal activity needed for metabolic homeostasis, cognition and overall health.55,56,79 Box 3, Box 4.
Nucleic acid-based regulation of the food–brain axis
Central to our understanding of how the food–brain Axis functions is the premise that homeostasis and the adaptive behaviors associated with cognition are the result of the complex interplay between signal transduction and transcriptional programming in the neuron. Fortunately, evidence is mounting to support the notion that the brain’s cognitive and non-cognitive functions are encoded genomically80,81 and subject to epigenetic1,82–84 and epitranscriptomic4 modification. In the case of epigenetics, a recent study demonstrated that transient activation of mature neuronal circuits can up-regulate and down-regulate transcription, particular for early genes like Arc, c-Fos and Jun-b, by dynamic modification of chromatin accessibility (i.e., chromatin condensation or de-condensation).27 The authors of this study concluded that activity-induced reshaping of the transcriptome plays an important role in regulating synaptic plasticity, cognitive function and neurological disorders. Other studies show that activity-induced epitranscriptomics (i.e., post-transcriptional RNA editing and RNA methylation of coding and non-coding RNAs) in the brain can produce experience-dependent plasticity leading to learning and memory.4,26,85
One of the challenges to understanding a nucleic acid-based model for cognition is the temporal scales that can span several orders of magnitude between stimulus sensing and experience-dependent neuronal plasticity. For example, the time to elicit an electrophysiological signal from a neuron is in the range of microseconds, while the initiation of transcription and translation of RNA can span minutes to hours. On the other hand, the time required for learning and durable memory might take days to years.85 This temporal discordance makes the trajectory between stimulus sensing and neuronal plasticity non-linear and difficult to understand.
One possible way to resolve these manifold differences in timing between sensing and response is to view them in the context of molecular processes that affect the rate and magnitude of each step along the trajectory. These molecular processes include epigenetic and epitranscriptomics modifications of chromatin, DNA and RNA (e.g., altered RNA structure, half-life, localization and ligand affinity and, methylation of histones, DNA and RNA85–87) and homeostatic scaling.86,88 All of these processes help create the diversity of transcription programs required for proper neuronal function. In the case of homeostatic scaling, both the proteome88 and transcriptome86 are altered to adjust synaptic strength up or down in response to changes in inputs. Deficiencies in homeostatic scaling are associated with neurological disorders such as autism spectrum disorder, epilepsy, Parkinson’s and schizophrenia and underscore the need for tight control over network activity for proper neuronal function.88 Interestingly, many of the transcription factors (e.g., CREB, Elk1, SRF), kinases (e.g., CaMK, CDK5, MAPK) and growth factors (e.g., BDNF) associated with homeostatic scaling are also components of pathways (e.g., ERK) that crosstalk with neurogenesis signaling (Fig. 2, Transactivation of Signaling Pathways, Supplemental Table 1 A comparison of signal transduction pathway relative of signaling for glucose signaling).86 In terms of the potential impact of inputs like DSMs on homeostatic scaling, expression of BDNF, a neurotrophic factor involved in neurogenesis, memory and learning, can be triggered by a variety of polyphenolics compounds found in plant-based foods (Table 2).
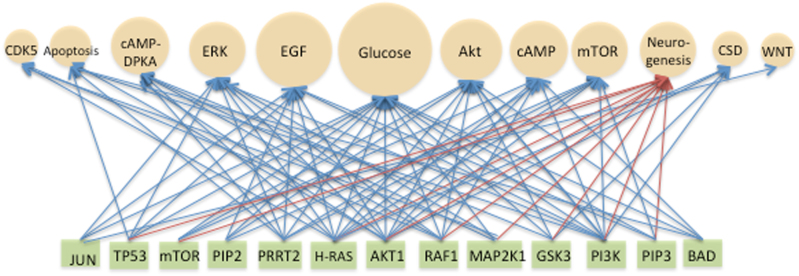
Fig. 2.
Transactivation of Signaling Pathways. A bipartite network illustrating the potential for transactivation (i.e., crosstalk) between 12 signaling pathways (beige spheres) and 13 signaling proteins (green rectangles). The glucose signaling pathway was used as a reference for weighting the other 11 signaling pathways in terms of their percent similarity with proteins involved in glucose signaling (sphere size approximates percent relatedness). The 13 signaling proteins were those common to glucose signaling and to at least three of the other signaling pathways (e.g., JUN is common to three pathways while AKT1 is common to ten pathways). Signaling proteins common to neurogenesis signaling are indicated by red arrows. The source of the signaling pathways and proteins was Pathways Online SABiosciences. For the neurogenesis signaling pathway, several sources were used.56, 152–155 A list of signaling pathways and proteins used to construct this network is provided in Supplemental Table 1. The selection of pathways and proteins was for illustrative purposes and not intended to be exhaustive. Abbreviations: Akt, serine/threonine kinase; cAMP, cyclic adenosine monophosphate; cAMP-DPKA, cyclic adenosine monophosphate-dependent protein kinase A; CSD, cytoskeletal dynamics; CDK5, cyclin-dependent kinase 5; EGF, epidermal growth factor; ERK, extracellular signal‐regulated kinase; mTOR, mammalian target of rapamycin; Wnt, wingless-integration site 1
Table 2.
Neurotrophic polyphenolic signaling molecules
| Compound | Model | Pathway | Neurotrophic factors | Function | References |
|---|---|---|---|---|---|
| Astilbin | Mouse | Erk, Akt | BDNF | Antidepressant-like effects | 166 |
| Butein | Mouse | Erk, CREB | BDNF | Cognitive Enhancement | 167 |
| CAPE | Mouse | Nrf2/ARE | BDNF | Protective of dopaminergic neurons | 168 |
| Curcumin | Rat | Akt/GSk-3β | BDNF | Reduced β-amyloid-induced cognitive impairment | 169 |
| Fisetin | Mouse | Erk, CREB | BDNF | Cognitive enhancement | 167 |
| Resveratrol | Rat | Erk, CREB | BDNF | Antidepressant-like effects | 170,171 |
| Rosmarinic acid | Rat | Erk | BDNF | Antidepressant-like effects | 172 |
Table 2 shows seven naturally occurring plant-based polyphenolic compounds and their impact on six different physiologically relevant signaling pathways and their neurotrophic effects on BDNF. Also, shown are the experimental animal models used to demonstrate these effects. (Adapted from Moosavi et al.13). Molecular weights (in kD) for these compounds are as follows: astilbin, 0.450; butein, 0.272; CAPE, 0.284; curcumin, 0.368; fisetin, 0.286; resveratrol, 0.228; rosmarinic acid, 0.360
CAPE caffeic acid phenethyl ester, BDNF brain-derived neurotrophic factor
Over the past decade, the toolbox for regulating gene expression in the mammalian brain has expanded to include long-noncoding RNAs,89 enhancer RNA90 and long-lived circular RNAs.91 These regulatory RNAs also have the potential to be modified by epigenetics and/or epitranscriptomics to regulate neuronal behaviors, synaptic scaling, plasticity and ultimately cognition.
The dynamics of the food–brain axis can also be bidirectional in that food intake can regulate the expression of genes involved in memory, learning and adaptive behaviors, while adaptive behaviors, learning and memory can regulate gene expression to change food intake. This neuronal bidirectionality is best illustrated by the peptide hormones leptin, ghrelin, insulin and nesfatin-1 that use nutrient sensing to regulate satiety, hunger and food reward signals in the brain.92,93 When expressed or injected into rat brains, for example, nesfatin-1 can create anorexia in rats by inhibiting food intake, modulating the excitability of glucose sensitive neurons, and acting on the melanocortin system to enhance UCP1 expression in brown adipose tissue.93 It is the bidirectional nature of activity-dependent changes in neuronal function that holds promise for dietary interventions designed to create those adaptive and positive self-managing behaviors that contribute to good mental health.
Lastly, in addition to epigenetics, epitranscriptomics and noncoding RNAs, many of the more traditional mechanisms come into play when discussing regulating gene expression in the brain. These mechanisms include negative and positive feedforward/feedback control94,95 of neuronal gene expression including the use of the same regulatory schemes that evolved over millions of years to support the normal growth and development of all physiological systems. These regulatory schemes include nutrient sensing, signal transduction and those cis-acting (e.g., promoters, terminators, and enhancers) and trans-acting (e.g., activators, repressors, coactivators and corepressors) elements required for transcriptional control in eukaryotes.96
Nutrient sensing
The ability for cells to discern and respond to fluctuations in extracellular signals is required of all living cells and is the starting point for the homeostatic/allostatic control of all physiological systems. As previously discussed, nutrient availability is a strong selective pressure for shaping the evolution of most cellular processes.36,97 Pathways that detect extracellular and intracellular changes in levels of nutrients, biotransformed nutrients (e.g., glucose, vitamin D, vitamin A), energy and hormones, activate signaling pathways to compensate for these fluctuations and reestablish homeostasis. When food is abundant, nutrient-sensing pathways stimulate energy storage and anabolic processes while nutritional scarcity triggers homeostatic mechanisms that mobilize glucose and lipid stores97,98 by processes like autophagy.97,99 The glucose transport protein, GLUT2, is a good example of an evolutionarily conserved nutrient sensing mechanism. As a plasma membrane glycoprotein encoded by the SLC2A2 gene in humans,79 GLUT2 can also be found in the brains of invertebrates like Drosophila melanogaster. 100,101 GLUT2 facilitates bidirectional glucose transport expressed in the pancreas and hypothalamus and because of its low affinity for glucose, it is considered an excellent glucosensor in astrocytes and neurons.102
From glucose sensing to neurogenesis
Biological systems are parsimonious in terms of achieving adaptive responses to changes in the environment. As mentioned above, the brain draws upon efficient and well-developed molecular processes like nutrient sensing, transactivation of signaling pathways (i.e., signaling crosstalk) and stimulus-specific combinatorial gene regulation to support the neural growth, differentiation and experience-dependent neuronal plasticity.56,103,104 To see how transactivation of signaling is achieved one need only compare the components of various signaling pathways to a critically important pathway such as glucose signaling (Fig. 2).
As a nutrient, none is more important than glucose in terms of signaling changes in the extracellular environment and fueling cellular processes with ATP synthesis. In the brain, the energy generated from ATP hydrolysis is essential for sustaining ongoing neuronal activities as well as “housekeeping” functions (e.g., buffering ions, recycling neurotransmitters, and brain phospholipid remodeling that accounts for 20% of ATP consumption) in both the resting and awake brain.105,106 According to Zhu et al,107 the brain produces approximately 5.7 kg of ATP daily, which is equivalent to the complete oxidative combustion of 56 g of glucose in a single day. This provides 77% of the total energy needs of the cortical gray matter alone.107
As can be seen in Fig. 2, the potential for crosstalk between different signaling pathways is considerable. This suggests that any DSM, with even modest affinity for one of the signaling proteins in this limited list, has the potential to transactivate other signaling pathways to create interlocking pathways capable of linking neurogenesis to nutrient sensing. By using transactivation to interlock signaling pathways, even small fluctuations in the concentration of any component of a pathway can produce rapid, nonlinear and dynamical shifts in pathway outcomes.
Polyphenolics: non-nutritive DSMs
While glucose may be the most essential of the DSMs, other macronutrients like amino acids108 and lipids109 can also activate signaling pathways. However, it is the non-nutritive bioactive compounds like plant-based phenolic acids that intrigue researchers as potential dietary interventions to support neurological health. The main reason for this interest is the excellent pharmacokinetic properties of many plant polyphenolics including their ability to cross the blood brain barrier (BBB).54,110,111 For example, a study using a noninvasive, localized BBB-opening technique in vivo, showed that molecules in the 3 to 70 kD could be easily delivered trans-BBB.111 Many polyphenolics fall in the range of 0.2 to 0.5 kD (Table 2). In another recent review of the consumption levels and neuroprotective properties of 17 phenolic acids, it was reported that the daily consumption of these compounds varied from country to country and by sex, with the highest consumption level among Europeans being 1786 mg/day for Danish men.54 Furthermore, ellagic acid, commonly found in many fruit and vegetable, was recently found to improve brain injury outcomes in rats and increase proliferation of neural stem cells through the Wnt/β-catenin signaling pathway.112 Other studies suggest that the antioxidant properties of polyphenolics can be neuroprotective for AD113 and modulate hippocampal neurogenesis.114 The mechanism for how this is accomplished is unclear, however, as shown in Table 2, expression of the neurotrophic factor BDNF can be induced by five different polyphenolics through one pathway (e.g., ERK) or by seven different compounds through six different pathways. That many of these pathways and their signaling proteins overlap with factors involved in neurogenesis signaling114 (Fig. 2), is good evidence in support of combinatorial crosstalk and its role in expressing a critical factor (i.e., brain-derived neurotrophic factor (BDNF) vital to learning, memory and higher order thinking.114
Food–brain axis as a complex system
As discussed above, signaling molecules from the environment produce adaptive experience-dependent behaviors that make cognition a complex system. Complex systems in biology consist of many components with some degree of connectedness and interdependency. Guided by external input from the environment and a small set of rules (e.g., combinatorial and feedforward/feedback gene regulation and homeostasis) complex systems are capable of producing emergent adaptive behaviors125,126 that are not observed in random networks. Furthermore, these adaptive behaviors emerge dynamically and do not adhere to linear, dose-dependent or deterministic kinetics. Fortunately, these behaviors are stochastic and therefore, they can be described probabilistically.125 One of the best descriptions of a complex biological system is that of Gregor et al,127 who, in discussing the “Onset of Collective Behavior in Social Amoebae,” described signaling as a dynamical process capable of self-organizing the behavior of individual cells into synchronous pules of cAMP synthesis in a population of cells. This has led some to posit that complexity is a fundamental property of nature that guides growth and behavior of all living cells.128
Essential to producing emergent adaptive behaviors by neurons is the requirement for large amounts of informational input (i.e., DSMs) from the environment126,129 and a “decision space” where inputs are turned into outputs (i.e., solutions). Because of the large number of decisions that must take place in a complex system like the brain, input must be abundant and diverse. In the context of the food–brain axis, this means that dietary input should be rich in non-nutritive bioactive molecules (e.g., polyphenolics) as well as nutritionally dense macronutrients (e.g., carbohydrates, amino acids, omega-3 PUFAs). As shown in Fig. 1, changing the quality and/or quantity of dietary input can change the set point in the food–brain Axis to produce experience-dependent changes in the brain that are either advantageous or deleterious to cognitive health.
PUFAs and the Brain.
Phospholipids make up 99% of the brain’s esterified fatty acids. Approximately 20% of fatty acids bound to phospholipids consist of the long-chain polyunsaturated fatty acids (PUFAs) arachidonic acid (AA, 20:4n-6) and DHA (22:6n-3), which preferentially incorporate to the stereospecifically-numbered (sn-2) position of the phospholipid molecule.63,64 AA and DHA account for approximately 87 and 68% of total brain PUFAs in rats and humans, respectively, reflecting selective mechanisms for incorporating and retaining them within brain membrane phospholipids.63,64 Synthesis of AA and DHA in the brain is very low (<1%),65,66 so they must be obtained from circulating AA and DHA derived directly from the diet or synthesized in the liver from dietary linoleic (LA, 18:2n-6) and alpha-linolenic acid (ALA, 18:3n-6), respectively.67,68 AA and DHA passively cross the BBB in their unesterified form.69 A recent study showed that the incorporation rate of unesterified DHA by the brain exceeds that of esterified DHA by 10-fold, confirming that the unesterified plasma pool is the main source of DHA (and other fatty acids) to the brain.70 In the brain, AA and DHA regulate transcription and neuroreceptor-coupled signaling71,72 and serve as precursors to bioactive lipid mediators that modulate immunity.73,74 The brain may be more vulnerable to low DHA than AA, because LA, the dietary precursor to AA, exceeds ALA in food by approximately 10-fold.75,76
Transactivation, feedback loops and complexity.
While reports on the ability of DSMs to impact neural development and function are many, it is more challenging to identify the signaling proteins through which their influence is exerted. This is due in part to the complexity of signal transduction networks regulating neuronal identity and activity. Two of the best examples of this complexity are the transcription factors CREB and c-Fos, which play essential roles in long-term modulation of neuronal activity.115 A series of signaling pathways including cAMP/PKA, Ras/ERK, Ca++/CaMK, and PI3K/Akt converge to regulate CREB and c-Fos gene expression.115,116 This combinatorial control allows neurons to make long-term changes in their activity profile in response to both membrane depolarization and hormonal inputs. However, recent work in other cell types has revealed that this core set of pathways is also integrated with metabolic signaling. The Ras/ERK cascade is mutually antagonistic with AMPK, a kinase that becomes active when cellular ATP levels fall, through both regulatory phosphorylation117,118 and through mutual interactions with the scaffold protein KSR2.119 Similarly, PI3K/Akt signaling is tightly intertwined through both positive and negative feedbacks with the mTOR kinase complexes,120 which are highly sensitive to amino acid availability.121 Beyond these interconnections, this network can be expanded to include many more components and feedback loops.122 Thus, macronutrient availability presumably influences the decision of neurons to activate CREB and c-Fos, and thereby alter their activity profile. This implication has yet to be explored in detail but an emerging body of research suggests that DSMs do indeed impact the CREB and c-Fos signaling networks in the neuron.123,124 The elaborate feedback structures of these networks create a formidable barrier to understanding signaling in the neuron and necessitate a systems-level approach to unambiguously identify the molecular mode of action for DSMs.
1-Carbon metabolism and neurological function.
Perturbations in 1-carbon (1C) metabolic pathways significantly affect brain health and neurological functions. In a rat hippocampal cell line, folate deficiency was shown to cause differentiation-associated apoptosis, homocysteinylation and subsequent aggregation of neuronal proteins that contributes to alterations of differentiation and plasticity.130 Folate/vitamin B12 deficiency during gestation and lactation impairs cerebellar synapsin expression through a deregulation of ER-α//Src tyrosine kinase pathway in rats.131 In humans, the accelerated rate of brain atrophy in the elderly with mild cognitive impairment can be alleviated with vitamin B-complex supplementation.132 Interventions with folinate, a folate coenzyme, helps stabilize treatment for schizophrenia associated with folate receptor autoantibodies.133 Maternal dietary choline regulates development of the cerebral cortex in the offspring mice.134 while excess methionine inhibits neural tube closure in mouse embryos.135 Key enzymes in 1C metabolism are also closely related to brain health and neuro-functions. One example is the enzyme glycine N-methyltransferase, (GNMT) which plays critical roles in folate dependent reactions,136 methyl group homeostasis,137 and cellular defense against DNA damage.138 It is plausible that this enzyme is also important for proper neuronal function. In fact, enhanced expression of GNMT in cortical mixed neuron-glial cultures culture has been shown to be neuroprotective.139 In GNMT knockout mice, alterations in the adenosylmethionine pathway impair neurogenesis and contribute to cognitive decline.140 Mutations in genes encoding the glycine cleavage system also predispose mice and humans to neural tube defects.141
Conclusion
Studies on the impact of food on neurological health are advancing our understanding of the dynamic interactions of the food–brain axis. This is important as the proportion of elderly (65 and older) in the global population with AD is expected to reach 70 million by the year 2030.142,143 It is not surprising that many individuals are anticipating the development of preventions, treatments and cures for neurodegenerative and neuropsychiatric diseases based on novel therapeutics and dietary interventions involving the foods we eat daily. This heightened expectation is fostered by research (predominantly involving small laboratory animals) demonstrating that many foods contain compounds that are neuroprotective, antipsychotic and anti-depressive (Tables 1, 2). It should be stressed, however, that mental and physical health are tightly linked and both can be strongly influenced by genetics and environmental factors such as physical activity and nutrition. In the case of AD, several genetic factors have been identified including the ε4 allele of the APOE gene, which is a strong risk indicator for both AD and coronary artery disease.144,145 Both diseases are also associated with sedentary lifestyle and obesity.143,146 Therefore, the expectation that there will be the nutritional equivalent of a “magic bullet” to ensure normal cognitive function into old age may be premature. Currently, PUFAs like DHA and EPA are being used in psychotherapy for AD,73 depression147 and personality disorders.148 Although PUFA responsiveness in all of these studies was slight to modest, the results are encouraging. Another recent study showed that a cerebral folate deficiency involving 5-MTHF is likely responsible for treatment-refractory depression and that subjects receiving sapropterin, a tetrahydrobiopterin analog, showed marked improvement.149
Finally, strategies for enhancing cognition and therapies for treating neuropathologies that include evidence-based nutrition should become more common as we learn more about the food–brain axis. This will require a multiscale, top-down approach that includes diverse data sets that span different locations, scales (e.g., tissues, cells and molecules) and time points to reveal the underlying connectivity, interdependencies and adaptiveness of biological systems like the brain.150,151 From nutrient to nucleotide to neuron, all scales must be analyzed vertically and orthogonally to fully understand the molecular basis of cognition in all its forms and dysfunctions. Accomplishing this daunting task will require collaboration and communication across multiple disciplines like food science, nutrition, genomics, molecular biology, neuroscience and informatics. Such transdisciplinary approaches are not only vital to understanding food–brain axis but other complex biological systems as well. Recognizing the importance of DSMs in signaling those transcription programs needed for neurogenesis and activity-dependent changes in neuronal functions is an important first step.
Electronic supplementary material
Acknowledgements
The authors want to thank Taekyung (T-K) Kim, University of Texas, Southwestern Medical Center and Darshan Kelly, ARS-USDA Western Human Nutrition Research Center (NIFA Project Number CA-D-MCB-5896-H) for their helpful advice and encouragement. We are also indebted to Peter C. Wainwright, former interim dean of the UC Davis College of Biological Sciences for his encouragement and support of the Neuro-Nutrition Workgroup from June 2016 to June 2017. Finally, we want to acknowledge the President’s Council of Advisors on Science and Technology (PCAST) for the enthusiasm they expressed for this topic at its September 28, 2015 meeting in Washington D.C.
Author contributions
All authors contributed equally in developing the material for this article and for critically reviewing the manuscript and approval of the final draft. R.L.R. conceived the theme of the article based on his participation in the PCAST meeting of September 28, 2015.
Competing Interests
The authors declare that they have no competing financial interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies the paper on the npj Science of Food website (10.1038/s41538-017-0002-4).
References
- 1.Gaudi S, Guffanti G, Fallon J, Macciardi F. Epigenetic mechanisms and associated brain circuits in the regulation of positive emotions: A role for transposable elements. J. Comp. Neurol. 2016;524:2944–2954. doi: 10.1002/cne.24046. [DOI] [PubMed] [Google Scholar]
- 2.Overall RW, Walker TL, Fischer TJ, Brandt MD, Kempermann G. Different mechanisms must be considered to explain the increase in hippocampal neural precursor cell proliferation by physical activity. Front. Neurosci. 2016;10:362. doi: 10.3389/fnins.2016.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu. Rev. Cell Dev. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 4.Yao B, et al. Epigenetic mechanisms in neurogenesis. Nat. Rev. Neurosci. 2016;17:537–549. doi: 10.1038/nrn.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun, G. Y. et al. Docosahexaenoic acid (DHA): an essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fatty Acids (2017) (in press). [DOI] [PMC free article] [PubMed]
- 6.Alfini AJ, et al. Hippocampal and cerebral blood flow after exercise cessation in master athletes. Front. Aging Neurosci. 2016;8:184. doi: 10.3389/fnagi.2016.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saez de Asteasu ML, Martinez-Velilla N, Zambom-Ferraresi F, Casas-Herrero A, Izquierdo M. Role of physical exercise on cognitive function in healthy older adults: a systematic review of randomized clinical trials. Ageing. Res. Rev. 2017;37:117–134. doi: 10.1016/j.arr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Smith N, Miquel-Kergoat S, Thuret S. The impact of mastication on cognition: evidence for intervention and the role of adult hippocampal neurogenesis. J. Nutr. Health Aging. 2015;3:115–123. [Google Scholar]
- 9.Utsugi C, Miyazono S, Osada K, Matsuda M, Kashiwayanagi M. Impaired mastication reduced newly generated neurons at the accessory olfactory bulb and pheromonal responses in mice. Arch. Oral Biol. 2014;59:1272–1278. doi: 10.1016/j.archoralbio.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Tada A, Miura H. Association between mastication and cognitive status: a systematic review. Arch. Gerontol. Geriatr. 2017;70:44–53. doi: 10.1016/j.archger.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Jacoby N, Ahissar M. What does it take to show that a cognitive training procedure is useful? A critical evaluation. Prog. Brain Res. 2013;207:121–140. doi: 10.1016/B978-0-444-63327-9.00004-7. [DOI] [PubMed] [Google Scholar]
- 12.Hornsby AK, et al. Short-term calorie restriction enhances adult hippocampal neurogenesis and remote fear memory in a Ghsr-dependent manner. Psychoneuroendocrinology. 2016;63:198–207. doi: 10.1016/j.psyneuen.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moosavi F, Hosseini R, Saso L, Firuzi O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2016;10:23–42. doi: 10.2147/DDDT.S96936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utsugi C, et al. Hard-diet feeding recovers neurogenesis in the subventricular zone and olfactory functions of mice impaired by soft-diet feeding. PLoS One. 2014;9:e97309. doi: 10.1371/journal.pone.0097309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kane, R. L. et al. United States Department of Health and Human Services Agency for Healthcare Research and Quality: Comparative Effectiveness Review No. 188. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096221/ (2017).
- 16.Black MM, et al. Early childhood development coming of age: science through the life course. Lancet. 2016;389:77–90. doi: 10.1016/S0140-6736(16)31389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurliyana AR, Mohd Shariff Z, Mohd Taib MN, Gan WY, Tan KA. Early nutrition, growth and cognitive development of infants from birth to 2 years in Malaysia: a study protocol. BMC Pediatr. 2016;16:160. doi: 10.1186/s12887-016-0700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr. Rev. 2014;72:267–284. doi: 10.1111/nure.12102. [DOI] [PubMed] [Google Scholar]
- 19.Georgieff MK, Brunette KE, Tran PV. Early life nutrition and neural plasticity. Dev. Psychopathol. 2015;27:411–423. doi: 10.1017/S0954579415000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Val-Laillet D, et al. A maternal Western diet during gestation and lactation modifies offspring’s microbiota activity, blood lipid levels, cognitive responses, and hippocampal neurogenesis in Yucatan pigs. FASEB J. 2017;31:2037–2049. doi: 10.1096/fj.201601015R. [DOI] [PubMed] [Google Scholar]
- 21.Lim SY, et al. Nutritional factors affecting mental health. Clin. Nutr. Res. 2016;5:143–152. doi: 10.7762/cnr.2016.5.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schattin A, Baur K, Stutz J, Wolf P, de Bruin ED. Effects of physical exercise combined with nutritional supplements on aging brain related structures and functions: a systematic review. Front. Aging Neurosci. 2016;8:161. doi: 10.3389/fnagi.2016.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lucia C, Murphy T, Thuret S. Emerging molecular pathways governing dietary regulation of neural stem cells during aging. Front. Physiol. 2017;8:17. doi: 10.3389/fphys.2017.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris MC, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11:1007–1014. doi: 10.1016/j.jalz.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naninck EF, et al. Early micronutrient supplementation protects against early stress-induced cognitive impairments. FASEB J. 2017;31:505–518. doi: 10.1096/fj.201600834R. [DOI] [PubMed] [Google Scholar]
- 26.Marshall, P. & Bredy, T. W. Cognitive neuroepigenetics: the next evolution in our understanding of the molecular mechanisms underlying learning and memory? NPJ Sci. Learn. http://doi:10.1038/npjscilearn.2016.14 (2016). [DOI] [PMC free article] [PubMed]
- 27.Su Y, et al. Neuronal activity modifies the chromatin accessibility landscape in the adult brain. Nat. Neurosci. 2017;20:476–483. doi: 10.1038/nn.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandhu KV, et al. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl. Res. 2016;179:223–244. doi: 10.1016/j.trsl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Soty M, Gautier-Stein A, Rajas F, Mithieux G. Gut-brain glucose signaling in energy homeostasis. Cell Metab. 2017;25:1231–1242. doi: 10.1016/j.cmet.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 30.Kimbel WH, Villmoare B. From Australopithecus to Homo: the transition that wasn’t. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;371:20150248. doi: 10.1098/rstb.2015.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofman MA. Evolution of the human brain: when bigger is better. Front. Neuroanat. 2014;8:15. doi: 10.3389/fnana.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 33.Kempermann G. Adult neurogenesis: an evolutionary perspective. CSH Perspect Biol. 2016;8:a018986. doi: 10.1101/cshperspect.a018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sporns O, Tononi G, Kotter R. The human connectome: a structural description of the human brain. PLoS Comput. Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao M, Huang H, Peng Y, Dong Q, He Y. Toward developmental connectomics of the human brain. Front. Neuroanat. 2016;10:25. doi: 10.3389/fnana.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosati AG. Foraging cognition: reviving the ecological intelligence hypothesis. Trends Cogn. Sci. 2017;21:691–702. doi: 10.1016/j.tics.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 37.DeCasien AR, Williams SA, Higham JP. Primate brain size is predicted by diet but not sociality. Nat. Ecol. Evol. 2017;1:112. doi: 10.1038/s41559-017-0112. [DOI] [PubMed] [Google Scholar]
- 38.Koelwyn GJ, Wennerberg E, Demaria S, Jones LW. Exercise in regulation of inflammation-immune axis function in cancer initiation and progression. Oncology. 2015;29:908–920. [PMC free article] [PubMed] [Google Scholar]
- 39.Plant TM. 60 years of neuroendocrinology: the hypothalamo-pituitary-gonadal axis. J. Endocrinol. 2015;226:T41–T54. doi: 10.1530/JOE-15-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plomin R, Deary IJ. Genetics and intelligence differences: five special findings. Mol. Psychiatry. 2015;20:98–108. doi: 10.1038/mp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Hafid N, Christodoulou J. Phenylketonuria: a review of current and future treatments. Transl. Pediatr. 2015;4:304–317. doi: 10.3978/j.issn.2224-4336.2015.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frieden, T. R., Houry, D. & Baldwin, G. The Report to Congress on Traumatic Brain Injury in theUnited States: Epidemiology and Rehabilitation. Centers for Disease Control and Prevention & National Center for Injury Prevention and Control, Atlanta, GA,https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html (2016).
- 43.Committee on Nutrition, Trauma, and the Brain; Food and Nutrition Board; Institute of Medicine of the National Academies. Nutrition and Traumatic Brain Injury: Improving Acute and Subacute Health Outcomes in Military Personnel (The National Academies Press, Cambridge, MA, 2011). [PubMed]
- 44.Lucke-Wold BP, et al. Supplements, nutrition, and alternative therapies for the treatment of traumatic brain injury. Nutr. Neurosci. 2016;5:1–13. doi: 10.1080/1028415X.2016.1236174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bistrian BR, Askew W, Erdman JW, Oria MP. Nutrition and traumatic brain injury: a perspective from the Institute of Medicine report. JPEN J. Parenter. Enteral. Nutr. 2011;35:556–559. doi: 10.1177/0148607111416122. [DOI] [PubMed] [Google Scholar]
- 46.Ma, E. L. et al. Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav. Immun. (2017) (in the press). [DOI] [PMC free article] [PubMed]
- 47.Luo CL, et al. Autophagy is involved in traumatic brain injury-induced cell death and contributes to functional outcome deficits in mice. Neuroscience. 2011;184:54–63. doi: 10.1016/j.neuroscience.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence DW, Sharma B. A review of the neuroprotective role of vitamin D in traumatic brain injury with implications for supplementation post-concussion. Brain Inj. 2016;30:960–968. doi: 10.3109/02699052.2016.1147081. [DOI] [PubMed] [Google Scholar]
- 49.DeLegge MH, Smoke A. Neurodegeneration and inflammation. Nutr. Clin. Pract. 2008;23:35–41. doi: 10.1177/011542650802300135. [DOI] [PubMed] [Google Scholar]
- 50.Li X, et al. Protective effects of quercetin on mitochondrial biogenesis in experimental traumatic brain injury via the Nrf2 signaling pathway. PLoS One. 2016;11:e0164237. doi: 10.1371/journal.pone.0164237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scrimgeour AG, Condlin ML. Nutritional treatment for traumatic brain injury. J Neurotrauma. 2014;31:989–999. doi: 10.1089/neu.2013.3234. [DOI] [PubMed] [Google Scholar]
- 52.Castellano CA, et al. Regional brain glucose hypometabolism in young women with polycystic ovary syndrome: Possible link to mild insulin resistance. PLoS One. 2015;10:e0144116. doi: 10.1371/journal.pone.0144116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Courchesne-Loyer A, et al. Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: a dual tracer quantitative positron emission tomography study. J. Cerebr. Blood F Met. 2017;37:2485–2493. doi: 10.1177/0271678X16669366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szwajgier, D., Borowiec, K. & Pustelniak, K. The neuroprotective effects of phenolic acids: molecular mechanism of action. Nutrients. 10.3390/nu9050477 (2017). [DOI] [PMC free article] [PubMed]
- 55.Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat. Rev. Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng H, Berthoud HR. Neural systems controlling the drive to eat: mind versus metabolism. Physiology. 2008;23:75–83. doi: 10.1152/physiol.00047.2007. [DOI] [PubMed] [Google Scholar]
- 57.Cunnane SC, Chouinard-Watkins R, Castellano CA, Barberger-Gateau P. Docosahexaenoic acid homeostasis, brain aging and Alzheimer’s disease: can we reconcile the evidence? Prostaglandins Leukot. Essent. Fatty Acids. 2013;88:61–70. doi: 10.1016/j.plefa.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 58.McNamara RK, et al. Lower docosahexaenoic acid concentrations in the postmortem prefrontal cortex of adult depressed suicide victims compared with controls without cardiovascular disease. J. Psychiatr. Res. 2013;47:1187–1191. doi: 10.1016/j.jpsychires.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McNamara RK, Welge JA. Meta-analysis of erythrocyte polyunsaturated fatty acid biostatus in bipolar disorder. Bipolar Disord. 2016;18:300–306. doi: 10.1111/bdi.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saunders EF, et al. Omega-3 and omega-6 polyunsaturated fatty acids in bipolar disorder: A review of biomarker and treatment studies. J. Clin. Psychiatr. 2016;77:e1301–e1308. doi: 10.4088/JCP.15r09925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mocking RJ, et al. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatr. 2016;6:e756. doi: 10.1038/tp.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Widenhorn-Muller K, Schwanda S, Scholz E, Spitzer M, Bode H. Effect of supplementation with long-chain omega-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): a randomized placebo-controlled intervention trial. Prostaglandins Leukot. Essent. Fatty Acids. 2014;91:49–60. doi: 10.1016/j.plefa.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Taha AY, et al. Altered lipid concentrations of liver, heart and plasma but not brain in HIV-1 transgenic rats. Prostaglandins Leukot. Essent. Fatty Acids. 2012;87:91–101. doi: 10.1016/j.plefa.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taha AY, Cheon Y, Ma K, Rapoport SI, Rao JS. Altered fatty acid concentrations in prefrontal cortex of schizophrenic patients. J. Psychiatr. Res. 2013;47:636–643. doi: 10.1016/j.jpsychires.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeMar JC, et al. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim. Biophys. Acta. 2006;1761:1050–1059. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 66.DeMar JC, Ma K, Chang L, Bell JM, Rapoport SI. -AlphaLinolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J. Neurochem. 2005;94:1063–1076. doi: 10.1111/j.1471-4159.2005.03258.x. [DOI] [PubMed] [Google Scholar]
- 67.Domenichiello AF, Kitson AP, Bazinet RP. Is docosahexaenoic acid synthesis from alpha-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res. 2015;59:54–66. doi: 10.1016/j.plipres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Domenichiello AF, et al. The effect of linoleic acid on the whole body synthesis rates of polyunsaturated fatty acids from alpha-linolenic acid and linoleic acid in free-living rats. J. Nutr. Biochem. 2016;30:167–176. doi: 10.1016/j.jnutbio.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Chen CT, Green JT, Orr SK, Bazinet RP. Regulation of brain polyunsaturated fatty acid uptake and turnover. Prostaglandins Leukot. Essent. Fatty Acids. 2008;79:85–91. doi: 10.1016/j.plefa.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Chen CT, et al. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci. Rep. 2015;5:15791. doi: 10.1038/srep15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orr SK, et al. Unesterified docosahexaenoic acid is protective in neuroinflammation. J. Neurochem. 2013;127:378–393. doi: 10.1111/jnc.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramadan E, et al. Chronic valproate treatment blocks D2-like receptor-mediated brain signaling via arachidonic acid in rats. Neuropharmacology. 2011;61:1256–1264. doi: 10.1016/j.neuropharm.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Devassy JG, Leng S, Gabbs M, Monirujjaman M, Aukema HM. Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv. Nutr. 2016;7:905–916. doi: 10.3945/an.116.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trepanier MO, Hopperton KE, Orr SK, Bazinet RP. N-3 polyunsaturated fatty acids in animal models with neuroinflammation: an update. Eur. J. Pharmacol. 2016;785:187–206. doi: 10.1016/j.ejphar.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 75.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson GH, Keast DR, Kris-Etherton PM. Dietary modeling shows that the substitution of canola oil for fats commonly used in the United States would increase compliance with dietary recommendations for fatty acids. J. Am. Diet Assoc. 2007;107:1726–1734. doi: 10.1016/j.jada.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 77.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 78.Zuker CS. Food for the brain. Cell. 2015;161:9–11. doi: 10.1016/j.cell.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 79.Leloup C, et al. Glucose and hypothalamic astrocytes: more than a fueling role? Neuroscience. 2016;323:110–120. doi: 10.1016/j.neuroscience.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 80.Mattick JS. The central role of RNA in human development and cognition. FEBS Lett. 2011;585:1600–1616. doi: 10.1016/j.febslet.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Mattick JS, Mehler MF. RNA editing, DNA recoding and the evolution of human cognition. Trends Neurosci. 2008;31:227–233. doi: 10.1016/j.tins.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Meaney MJ, Ferguson-Smith AC. Epigenetic regulation of the neural transcriptome: the meaning of the marks. Nat. Neurosci. 2010;13:1313–1318. doi: 10.1038/nn1110-1313. [DOI] [PubMed] [Google Scholar]
- 83.Meagher RB. ‘Memory and molecular turnover,’ 30 years after inception. Epigenetics Chromatin. 2014;7:37. doi: 10.1186/1756-8935-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Descalzi G, et al. Epigenetic mechanisms of chronic pain. Trends Neurosci. 2015;38:237–246. doi: 10.1016/j.tins.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nainar S, Marshall PR, Tyler CR, Spitale RC, Bredy TW. Evolving insights into RNA modifications and their functional diversity in the brain. Nat. Neurosci. 2016;19:1292–1298. doi: 10.1038/nn.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schaukowitch K, et al. An intrinsic transcriptional program underlying synaptic scaling during activity suppression. Cell Rep. 2017;18:1512–1526. doi: 10.1016/j.celrep.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baker-Andresen D, Ratnu VS, Bredy TW. Dynamic DNA methylation: a prime candidate for genomic metaplasticity and behavioral adaptation. Trends Neurosci. 2013;36:3–13. doi: 10.1016/j.tins.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 88.Sweatt JD. Dynamic DNA methylation controls glutamate receptor trafficking and synaptic scaling. J. Neurochem. 2016;137:312–330. doi: 10.1111/jnc.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.D’Haene E, et al. Identification of long non-coding RNAs involved in neuronal development and intellectual disability. Sci Rep. 2016;6:28396. doi: 10.1038/srep28396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim TK, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hollins SL, Cairns MJ. MicroRNA: Small RNA mediators of the brains genomic response to environmental stress. Prog. Neurobiol. 2016;143:61–81. doi: 10.1016/j.pneurobio.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 92.Murray S, Tulloch A, Gold MS, Avena NM. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat. Rev. Endocrinol. 2014;10:540–552. doi: 10.1038/nrendo.2014.91. [DOI] [PubMed] [Google Scholar]
- 93.Yuan JH, et al. Nesfatin-1 in the lateral parabrachial nucleus inhibits food intake, modulates excitability of glucosensing neurons, and enhances UCP1 expression in brown adipose tissue. Front. Physiol. 2017;8:235. doi: 10.3389/fphys.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mapelli L, Pagani M, Garrido JA, D’Angelo E. Integrated plasticity at inhibitory and excitatory synapses in the cerebellar circuit. Front. Cell Neurosci. 2015;9:169. doi: 10.3389/fncel.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Radhakrishnan B, Alwin Prem Anand A. Role of miRNA-9 in BrainDevelopment. J. Exp. Neurosci. 2016;10:101–120. doi: 10.4137/JEN.S32843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith NC, Matthews JM. Mechanisms of DNA-binding specificity and functional gene regulation by transcription factors. Curr. Opin. Struct. Biol. 2016;38:68–74. doi: 10.1016/j.sbi.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 97.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu F, Xu Y, Liu F. Hypothalamic roles of mTOR complex I: integration of nutrient and hormone signals to regulate energy homeostasis. Am. J. Physiol. Endocrinol. Metabol. 2016;310:E994–E1002. doi: 10.1152/ajpendo.00121.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maiese K. Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural Regen. Res. 2015;10:518–528. doi: 10.4103/1673-5374.155427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Limmer S, Weiler A, Volkenhoff A, Babatz F, Klambt C. The Drosophila blood-brain barrier: development and function of a glial endothelium. Front Neurosci. 2014;8:365. doi: 10.3389/fnins.2014.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miyamoto T, Wright G, Amrein H. Nutrient sensors. Curr. Biol. 2013;23:R369–R373. doi: 10.1016/j.cub.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leloup C, et al. Glucose transporter 2 (GLUT 2): expression in specific brain nuclei. Brain Res. 1994;638:221–226. doi: 10.1016/0006-8993(94)90653-X. [DOI] [PubMed] [Google Scholar]
- 103.Joo JY, Schaukowitch K, Farbiak L, Kilaru G, Kim TK. Stimulus-specific combinatorial functionality of neuronal c-fos enhancers. Nat. Neurosci. 2016;19:75–83. doi: 10.1038/nn.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wainwright PE, Colombo J. Nutrition and the development of cognitive functions: Interpretation of behavioral studies in animals and human infants. Am. J. Clin. Nutr. 2006;84:961–970. doi: 10.1093/ajcn/84.5.961. [DOI] [PubMed] [Google Scholar]
- 105.Du F, et al. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc. Natl. Acad. Sci. USA. 2008;105:6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Purdon AD, Rosenberger TA, Shetty HU, Rapoport SI. Energy consumption by phospholipid metabolism in mammalian brain. Neurochem. Res. 2002;27:1641–1647. doi: 10.1023/A:1021635027211. [DOI] [PubMed] [Google Scholar]
- 107.Zhu XH, et al. Quantitative imaging of energy expenditure in human brain. Neuroimage. 2012;60:2107–2117. doi: 10.1016/j.neuroimage.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Greenhaff PL, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am. J. Physiol. Endocrinol. Metabol. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 110.Andres-Lacueva C, et al. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2005;8:111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- 111.Choi JJ, Wang S, Tung YS, Morrison B, Konofagou EE. Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med. Biol. 2010;36:58–67. doi: 10.1016/j.ultrasmedbio.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu, Q. S. et al. Ellagic acid improves endogenous neural stem cells proliferation and neurorestoration through Wnt/beta-catenin signaling in vivo and in vitro. Mol. Nutr. Food Res.10.1002/mnfr.201600587 (2017). [DOI] [PubMed]
- 113.Choi DY, Lee YJ, Hong JT, Lee HJ. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012;87:144–153. doi: 10.1016/j.brainresbull.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 114.Ogle WO, Speisman RB, Ormerod BK. Potential of treating age-related depression and cognitive decline with nutraceutical approaches: a mini-review. Gerontology. 2013;59:23–31. doi: 10.1159/000342208. [DOI] [PubMed] [Google Scholar]
- 115.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chung L. A brief introduction to the transduction of neural activity into Fos signal. Dev. Reprod. 2015;19:61–67. doi: 10.12717/DR.2015.19.2.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shen CH, et al. Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Mol. Cell. 2013;52:161–172. doi: 10.1016/j.molcel.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zheng B, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol. Cell. 2009;33:237–247. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Costanzo-Garvey DL, et al. KSR2 is an essential regulator of AMP kinase, energy expenditure, and insulin sensitivity. Cell Metab. 2009;10:366–378. doi: 10.1016/j.cmet.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rodrik-Outmezguine VS, et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1:248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Bio. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caron E, et al. A comprehensive map of the mTOR signaling network. Mol. Syst. Biol. 2010;6:453. doi: 10.1038/msb.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reznikov LR, Pasumarthi RK, Fadel JR. Caffeine elicits c-Fos expression in horizontal diagonal band cholinergic neurons. Neuroreport. 2009;20:1609–1612. doi: 10.1097/WNR.0b013e3283329c3a. [DOI] [PubMed] [Google Scholar]
- 124.Wu YC, Wang YJ, Tseng GF. Ascorbic acid and alpha-tocopherol supplement starting prenatally enhances the resistance of nucleus tractus solitarius neurons to hypobaric hypoxic challenge. Brain Struct. Funct. 2011;216:105–122. doi: 10.1007/s00429-010-0300-y. [DOI] [PubMed] [Google Scholar]
- 125.Coveney PV, Fowler PW. Modelling biological complexity: a physical scientist’s perspective. J. R. Soc. Interface. 2005;2:267–280. doi: 10.1098/rsif.2005.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mazzocchi F. Complexity in biology. Exceeding the limits of reductionism and determinism using complexity theory. EMBO Rep. 2008;9:10–14. doi: 10.1038/sj.embor.7401147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gregor T, Fujimoto K, Masaki N, Sawai S. The onset of collective behavior in social amoebae. Science. 2010;328:1021–1025. doi: 10.1126/science.1183415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prindle A, Hasty J. Biochemistry. Stochastic emergence of groupthink. Science. 2010;328:987–988. doi: 10.1126/science.1190372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Perret N, Longo G. Reductionist perspectives and the notion of information. Prog. Biophys. Mol. Biol. 2016;122:11–15. doi: 10.1016/j.pbiomolbio.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 130.Akchiche N, et al. Homocysteinylation of neuronal proteins contributes to folate deficiency-associated alterations of differentiation, vesicular transport, and plasticity in hippocampal neuronal cells. FASEB J. 2012;26:3980–3992. doi: 10.1096/fj.12-205757. [DOI] [PubMed] [Google Scholar]
- 131.Pourie G, et al. Folate- and vitamin B12-deficient diet during gestation and lactation alters cerebellar synapsin expression via impaired influence of estrogen nuclear receptor alpha. FASEB J. 2015;29:3713–3725. doi: 10.1096/fj.14-264267. [DOI] [PubMed] [Google Scholar]
- 132.Smith AD, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One. 2010;5:e12244. doi: 10.1371/journal.pone.0012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramaekers VT, et al. Folinic acid treatment for schizophrenia associated with folate receptor autoantibodies. Mol. Genet. Metabol. 2014;113:307–314. doi: 10.1016/j.ymgme.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 134.Wang Y, Surzenko N, Friday WB, Zeisel SH. Maternal dietary intake of choline in mice regulates development of the cerebral cortex in the offspring. FASEB J. 2016;30:1566–1578. doi: 10.1096/fj.15-282426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dunlevy LP, Burren KA, Chitty LS, Copp AJ, Greene ND. Excess methionine suppresses the methylation cycle and inhibits neural tube closure in mouse embryos. FEBS Lett. 2006;580:2803–2807. doi: 10.1016/j.febslet.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 136.Wang YC, Chen YM, Lin YJ, Liu SP, Chiang EP. GNMT expression increases hepatic folate contents and folate-dependent methionine synthase-mediated homocysteine remethylation. Mol. Med. 2011;17:486–494. doi: 10.2119/molmed.2010.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang YC, Tang FY, Chen SY, Chen YM, Chiang EP. Glycine-N methyltransferase expression in HepG2 cells is involved in methyl group homeostasis by regulating transmethylation kinetics and DNA methylation. J. Nutr. 2011;141:777–782. doi: 10.3945/jn.110.135954. [DOI] [PubMed] [Google Scholar]
- 138.Wang YC, et al. A novel role of the tumor suppressor GNMT in cellular defense against DNA damage. Int. J. Cancer. 2014;134:799–810. doi: 10.1002/ijc.28420. [DOI] [PubMed] [Google Scholar]
- 139.Tsai MJ, et al. Enhanced expression of glycine N-methyltransferase by adenovirus-mediated gene transfer in CNS culture is neuroprotective. Ann. N. Y. Acad. Sci. 2010;1199:194–203. doi: 10.1111/j.1749-6632.2009.05169.x. [DOI] [PubMed] [Google Scholar]
- 140.Carrasco M, et al. Glycine N-methyltransferase expression in the hippocampus and its role in neurogenesis and cognitive performance. Hippocampus. 2014;24:840–852. doi: 10.1002/hipo.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Narisawa A, et al. Mutations in genes encoding the glycine cleavage system predispose to neural tube defects in mice and humans. Hum. Mol. Genet. 2012;21:1496–1503. doi: 10.1093/hmg/ddr585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Burkewitz K, Weir HJ, Mair WB. AMPK as a Pro-longevity Target. Exs. 2016;107:227–256. doi: 10.1007/978-3-319-43589-3_10. [DOI] [PubMed] [Google Scholar]
- 143.Michel JP. Is it possible to delay or prevent age-related cognitive decline? Korean J Fam Med. 2016;37:263–266. doi: 10.4082/kjfm.2016.37.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Agrawal M, Biswas A. Molecular diagnostics of neurodegenerative disorders. Front. Mol. Biosci. 2015;2:54. doi: 10.3389/fmolb.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grady C. The cognitive neuroscience of ageing. Nat. Rev. Neurosci. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nash MS, Kressler J. Model programs to address obesity and cardiometabolic disease: Interventions for suboptimal nutrition and sedentary lifestyles. Arch. Phys. Med. Rehab. 2016;97:S238–S246. doi: 10.1016/j.apmr.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 147.Deacon G, Kettle C, Hayes D, Dennis C, Tucci J. Omega 3 polyunsaturated fatty acids and the treatment of depression. Crit. Rev. Food Sci. 2017;57:212–223. doi: 10.1080/10408398.2013.876959. [DOI] [PubMed] [Google Scholar]
- 148.Bozzatello, P., Brignolo, E., De Grandi, E. & Bellino, S. Supplementation with omega-3 fatty acids in psychiatric disorders: a review of literature data. J. Clin. Med.10.3390/jcm5080069 (2016). [DOI] [PMC free article] [PubMed]
- 149.Pan LA, et al. Neurometabolic disorders: Potentially treatable abnormalities in patients with treatment-refractory depression and suicidal behavior. Am. J. Psychiatr. 2016;174:42–50. doi: 10.1176/appi.ajp.2016.15111500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kidd BA, Peters LA, Schadt EE, Dudley JT. Unifying immunology with informatics and multiscale biology. Nat. Immunol. 2014;15:118–127. doi: 10.1038/ni.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pezzulo, G. & Levin, M. Top-down models in biology: explanation and control of complex living systems above the molecular level. J. R. Soc. Interface. 10.1098/rsif.2016.0555 (2016). [DOI] [PMC free article] [PubMed]
- 152.Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta. 2013;1830:2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. CSH Perspect. Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Manns M, et al. Role of neuronal ras activity in adult hippocampal neurogenesis and cognition. Front. Neurosci. 2011;5:18. doi: 10.3389/fnins.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wakade C, Chong R, Bradley E, Thomas B, Morgan J. Upregulation of GPR109A in Parkinson’s disease. PLoS One. 2014;9:e109818. doi: 10.1371/journal.pone.0109818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Athanasopoulos D, Karagiannis G, Tsolaki M. Recent findings in Alzheimer disease and nutrition focusing on epigenetics. Adv. Nutr. 2016;7:917–927. doi: 10.3945/an.116.012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.DeGiorgio CM, Taha AY. Omega-3 fatty acids in epilepsy: animal models and human clinical trials. Expert. Rev. Neurother. 2016;16:1141–1145. doi: 10.1080/14737175.2016.1226135. [DOI] [PubMed] [Google Scholar]
- 159.Park SY, et al. Vitamin-mineral supplement use patterns in elderly Koreans: Data from the 2007-2008 Korean national health and nutrition examination survey. Korean J. Fam. Med. 2016;37:123–129. doi: 10.4082/kjfm.2016.37.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun H, et al. Niacin activates the PI3K/Akt cascade via PKC- and EGFR-transactivation-dependent pathways through hydroxyl-carboxylic acid receptor 2. PLoS One. 2014;9:e112310. doi: 10.1371/journal.pone.0112310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bhatia P, Singh N. Homocysteine excess: delineating the possible mechanism of neurotoxicity and depression. Fundam. Clin. Pharmacol. 2015;29:522–528. doi: 10.1111/fcp.12145. [DOI] [PubMed] [Google Scholar]
- 162.Venkatramanan S, Armata IE, Strupp BJ, Finkelstein JL. Vitamin B-12 and cognition in children. Adv. Nutr. 2016;7:879–888. doi: 10.3945/an.115.012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009;46:719–730. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wrzosek M, et al. Vitamin D and the central nervous system. Pharmacol. Rep. 2013;65:271–278. doi: 10.1016/S1734-1140(13)71003-X. [DOI] [PubMed] [Google Scholar]
- 165.Ibrahim BS, et al. Beneficial effects of vitamin C treatment on pregnant rats exposed to formaldehyde: reversal of immunosuppression in the offspring. Toxicol. Appl. Pharmacol. 2016;300:77–81. doi: 10.1016/j.taap.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 166.Lv QQ, et al. Antidepressant activity of astilbin: involvement of monoaminergic neurotransmitters and BDNF signal pathway. Biol. Pharm. Bull. 2014;37:987–995. doi: 10.1248/bpb.b13-00968. [DOI] [PubMed] [Google Scholar]
- 167.Cho N, et al. Cognitive-enhancing effects of Rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food Chem. Toxicol. 2013;58:355–361. doi: 10.1016/j.fct.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 168.Kurauchi Y, Hisatsune A, Isohama Y, Mishima S, Katsuki H. Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving haem oxygenase-1 and brain-derived neurotrophic factor. Br. J. Pharmacol. 2012;166:1151–1168. doi: 10.1111/j.1476-5381.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hoppe JB, et al. Free and nanoencapsulated curcumin suppress beta-amyloid-induced cognitive impairments in rats: involvement of BDNF and Akt/GSK-3beta signaling pathway. Neurobiol. Learn. Mem. 2013;106:134–144. doi: 10.1016/j.nlm.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 170.Zhang X, Huang G, Liu H, Chang H, Wilson JX. Folic acid enhances Notch signaling, hippocampal neurogenesis, and cognitive function in a rat model of cerebral ischemia. Nutr. Neurosci. 2012;15:55–61. doi: 10.1179/1476830511Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 171.Liu D, et al. Resveratrol reverses the effects of chronic unpredictable mild stress on behavior, serum corticosterone levels and BDNF expression in rats. Behav. Brain Res. 2014;264:9–16. doi: 10.1016/j.bbr.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 172.Jin X, Liu P, Yang F, Zhang YH, Miao D. Rosmarinic acid ameliorates depressive-like behaviors in a rat model of CUS and Up-regulates BDNF levels in the hippocampus and hippocampal-derived astrocytes. Neurochem. Res. 2013;38:1828–1837. doi: 10.1007/s11064-013-1088-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.