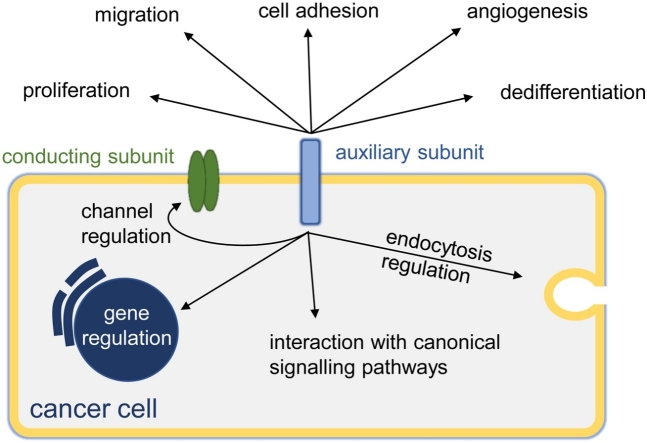
Graphical abstract
Abbreviations: BK, large-conductance calcium-activated potassium channel; CaCC, calcium-activated chloride channel; CAM, cell-adhesion molecule; CLC, voltage-gated chloride channel; CLCA, chloride channel accessory; DREAM, downstream regulatory element antagonistic modulator; GIRK, G-protein inwardly rectifying potassium channel; KChIP, potassium channel interacting protein; Kir, inwardly-rectifying potassium channel; SUR, sulfonylurea receptor; VGCC, voltage-gated calcium channel; VGKC, voltage-gated potassium channel; VGSC, voltage-gated sodium channel
Keywords: Auxiliary subunit, Cancer, Calcium channel, Chloride channel, Potassium channel, Sodium channel
Highlights
-
•
Ion channels consist of conducting and non-conducting (auxiliary) subunits.
-
•
Auxiliary subunits regulate ion conductance and have non-conducting roles.
-
•
Ion channels control diverse cellular processes and are aberrantly expressed in cancer.
-
•
Auxiliary subunits play major roles in cancer cells, including regulating adhesion, migration, invasion and gene expression.
Abstract
Several superfamilies of plasma membrane channels which regulate transmembrane ion flux have also been shown to regulate a multitude of cellular processes, including proliferation and migration. Ion channels are typically multimeric complexes consisting of conducting subunits and auxiliary, non-conducting subunits. Auxiliary subunits modulate the function of conducting subunits and have putative non-conducting roles, further expanding the repertoire of cellular processes governed by ion channel complexes to processes such as transcellular adhesion and gene transcription. Given this expansive influence of ion channels on cellular behaviour it is perhaps no surprise that aberrant ion channel expression is a common occurrence in cancer. This review will focus on the conducting and non-conducting roles of the auxiliary subunits of various Ca2+, K+, Na+ and Cl− channels and the burgeoning evidence linking such auxiliary subunits to cancer. Several subunits are upregulated (e.g. Cavβ, Cavγ) and downregulated (e.g. Kvβ) in cancer, while other subunits have been functionally implicated as oncogenes (e.g. Navβ1, Cavα2δ1) and tumour suppressor genes (e.g. CLCA2, KCNE2, BKγ1) based on in vivo studies. The strengthening link between ion channel auxiliary subunits and cancer has exposed these subunits as potential biomarkers and therapeutic targets. However further mechanistic understanding is required into how these subunits contribute to tumour progression before their therapeutic potential can be fully realised.
1. Introduction
Ion channels are heteromeric membrane protein complexes which permit transmembrane ion conduction. Several ion channels, e.g. K+ channels and voltage-gated Na+ channels (VGSCs), are notable for regulating membrane potential in excitable cells [1], but an expanding repertoire of other cellular processes, such as proliferation, differentiation [2], cell volume control and migration [3,4], are also known to be influenced by ion channels. Owing to their extensive impact on cellular function, it is no surprise that ion channel dysregulation is a common characteristic in cancer [5]. Ion channels are often multimeric, with ion-conducting subunits accompanied by non-conducting auxiliary subunits [6]. Auxiliary subunit-mediated modulation of the conducting subunit is well established but increasing evidence has unveiled a multitude of non-conducting roles for these proteins as well [[7], [8], [9], [10], [11], [12], [13], [14]]. An emerging field has focused on investigating auxiliary subunits in cancer, which, like the conducting subunits, are often aberrantly expressed and could represent novel therapeutic targets. In this review, we dissect the conducting and non-conducting roles of the auxiliary subunits of Ca2+, K+, Na+ and Cl− channels and the growing evidence supporting a link to cancer.
2. Ca2+ channels
Ca2+ channels regulate a multitude of cellular processes; accordingly, much research has focused on various Ca2+ channels in cancer, including voltage-gated Ca2+ channels (VGCCs) [15], STIM and Orai [16], and TRP channels [17]. In terms of Ca2+ channel auxiliary subunits however, only VGCC auxiliary subunits have received notable attention thus far. VGCCs are transmembrane complexes responsible for the inward Ca2+ current seen in excitable cells following depolarisation, however VGCCs are also expressed in other non-excitable cell types, e.g. osteoblasts and osteoclasts [18,19]. VGCCs are composed of a Ca2+-conducting α1 subunit (Cav1-3.x) associated with multiple auxiliary subunits (α2δ1-4, β1-4, γ1-8), with the exception of Cav3.x, which can form a T-type Ca2+ channel in the absence of an associated auxiliary subunit (Fig. 1) [20]. A Cav1/2 subunit is joined at the membrane by an α2δ-, β-, and potentially a γ-subunit, although γ-subunits are not always precipitated with Cavα [21]. Cavα1 subunits have an oncogenic influence in cancer [15]. Research into Cav auxiliary subunits in cancer is a growing field, but it appears Cav auxiliary subunits have both oncogenic and tumour-suppressive effects.
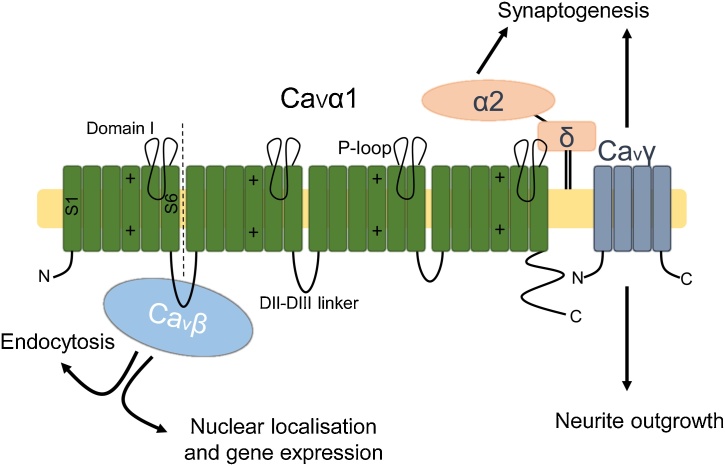
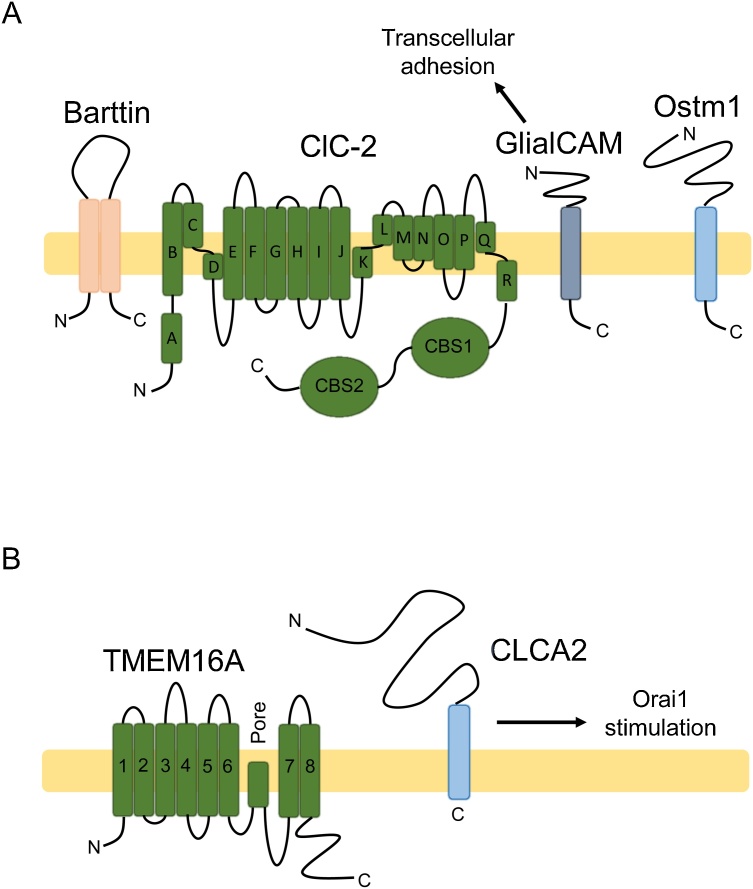
Fig. 1.
Voltage-gated Ca2+ channel auxiliary subunits. Voltage-gated Ca2+ channels (VGCCs) are composed of a conducting α1 subunit accompanied and functionally modulated by Cavβ, α2δ and Cavγ subunits [20]. α1 consists of four domains (domains I-IV), each consisting of six segments (S1-S6). The voltage-sensing domain is found within S4 of each domain and the pore consists of the P-loop found between S5-6 of each domain. Cavβ modulates Ca2+ influx via binding the DI-DII linker of α1. Cavβs are also involved in regulating gene expression and endocytosis [22,[36], [37], [38],40,44]. α2δ subunits are extracellular proteins that remain associated to the membrane via a GPI-anchor [54]. α2δ subunits are involved in synaptogenesis [65]. Cavγ subunits are four-pass transmembrane proteins also involved in cervical ganglion neurite outgrowth and synaptogenesis [108,109].
2.1. CaVβ
The VGCC β-subunits are cytoplasmic proteins that interact with the α1 DI-DII intracellular linker region [[22], [23], [24]]. β-subunit binding enhances membrane expression of α1 subunits [25,26], however the mechanism by which this occurs has not yet been elucidated. It is thought that β-subunit binding prevents ER retention and the subsequent degradation of Cav2.2, resulting in a higher proportion of Cav2.2 at the plasma membrane [25,27]. However, membrane targeting of the DI-DII linker of Cav2.2 via an inserted palmitoylation motif still results in ER retention and degradation, leading to the hypothesis that Cavβ subunits are required for correct folding, and thus membrane insertion, of functional α1 subunits [28]. The impact on electrophysiological properties of α1 subunits by Cavβs is complex. In general, Cavβs increase current density and regulate activation/inactivation kinetics. For instance, disruption of the Cavβ3-CaV2.2 interaction by a small molecule inhibitor results in a decrease in current density and a depolarised shift in the voltage threshold of activation and inactivation [29]. In comparison, Cavβ2 enhances the current density more than Cavβ3, potentially through increased membrane expression as Cavβ2a, unlike Cavβ3, contains a palmitoylation site [30]. Additionally, forced membrane localisation of Cavβ3 using the N-terminal Lyn sequence enhanced the current density relative to WT- Cavβ3 [30]. The complexity arises in the differential sensitivity to PIP2-mediated modulation of different Cavβs [30,31], competition for α1-binding between Cavβ subunits [32], the spectrum of functionally-distinct Cavβ splice variants [33,34], and the opposing impacts on α1-function by the different domains within the Cavβ protein [35].
Cavβs are functional independent of direct α1 association. All Cavβs demonstrate nucleus localisation, Cavβ4 particularly within nucleoli, and gene expression regulation [[36], [37], [38], [39]]. All Cavβs also contain a Src homology 3 domain capable of regulating endocytosis via interaction with dynamin and can interact with small GTPases [40,41]. Cavβs show subunit-specific function as well, for instance Cavβ1 is expressed in muscle progenitor cells (MPCs) earlier than Cav1.1, where it regulates proliferation and directly suppresses myogenin expression. Accordingly, Cavβ1 knockout mice demonstrate impaired muscle development [36,42]. Similarly, Cavβ2 is required for ventricle cell proliferation and heart development in zebrafish, although pharmacological VGCC inhibition caused a similar phenotype, suggesting Cavβ2 may be functioning in an α1-dependent manner [43]. Cavβ2 is also required for depolarisation-induced c-Fos and meCP2 activation, which intriguingly was shown to be independent of Ca2+ influx [37]. Cavβ4 regulates cell proliferation in vitro [44], downregulates Wnt signalling via sequestration of the Wnt pathway effector TCF4 [39], and regulates gene expression via various interacting partners [45,46]. Interestingly, the nuclear localisation of Cavβ4 was inhibited when co-expressed with Cav1.1 and only upon depolarisation and the presence of extracellular Ca2+ did Cavβ4 interact with its nuclear signalling partner, B56δ [45].
Owing to its role in driving cellular functions such as proliferation and migration, it is perhaps no surprise that CaVα1 expression is increased in various cancers [[47], [48], [49]]. However, much research has also been dedicated to evaluating the involvement of Cav auxiliary subunits in cancer. Cavβ1 expression is upregulated in colon cancer [50], Cavβ2 mutations are seen in bladder cancer [51] and increased Cavβ3 expression is observed in patients with recurrent non-small cell lung tumours compared to recurrence-free patients [52]. Furthermore, expression of Cavβ1 and Cavβ3 are included in proposed high-risk gene signatures that correlate with decreased patient survival in colon and recurring non-small cell lung cancer [50,52]. However, the aforementioned studies are largely limited to statistical observations based on tissue sequencing data that identified altered Cavβ RNA expression as a high-risk prognostic marker [[50], [51], [52]]. Chen et al. (2016) offered additional pathophysiological justification for increased Cavβ2 expression in cancer, by observing an enrichment in mutations of genes, including CACNB2 which encodes Cavβ2, involved in NCAM-mediated neurite outgrowth [51].
2.2. α2δ
The CaV α2δ subunit has a unique structure compared to other auxiliary subunits. The translated polypeptide is proteolytically cleaved into two separate proteins, α2 and δ, which remain coupled by a disulphide bond [53]. The α2 segment is extracellular while the δ-subunit remains associated with the membrane via a GPI-anchor [54]. α2δ and CaVβ subunits can both induce surface expression of α1, but also function synergistically to maximise α1 surface expression and Ca2+ current [26,55,56]. Preventing proteolytic cleavage of the α2δ1 proprotein reduces both Cav2.2 surface expression and presynaptic Ca2+ influx in hippocampal neurons [57] and site-directed mutagenesis of either cysteine residue involved in the disulphide interaction, which results in a dissociation of α2, reduces the whole-cell Ca2+ current [53]. Similarly, digestion of the GPI anchor of α2δ3, by prokaryotic phosphatidylinositol-phospholipase C, results in a release of the α2δ from the membrane and a decreased Ca2+ current [54]. Both these results suggest an intact α2δ subunit is required at the membrane to induce and sustain the α2δ-mediated regulation of α1 subunits. In addition to its role in trafficking, α2δ has been proposed to stabilise α1 at the membrane by reducing internalisation and in targeting α1 to detergent-resistant membranes [54,58]. Phenotypes of α2δ knockout mice have been very informative, both α2δ1 and α2δ3 have thus been implicated in neuropathic pain, with α2δ1-overexpressing mice demonstrating hyperalgesia [59] and α2δ3 -knockout mice demonstrating an enhanced insensitivity to pain [60]. Mice deficient in α2δ2, the isoform found overwhelmingly in cerebellar Purkinje neurons, present with seizures and ataxia [61]. Gabapentin, used in the treatment of epilepsy and neuropathic pain, preferentially binds to α2δ1/2 and lowers α2δ surface expression, demonstrating that the α2δ auxiliary subunit is a druggable target [[62], [63], [64]]. All α2δ subunits are involved in synaptogenesis, but potentially through different mechanisms [65]. α2δ1 promotes cortical synaptogenesis, independently of Ca2+ influx, through binding to secreted astrocytic thrombospondin in the postsynaptic membrane and promoting actin remodelling via Rac-1 [66], whereas loss of α2δ4 causes impaired retinal synaptogenesis, which correlates with a decrease in presynaptic Cav1.4 [67,68].
More is known about the involvement of α2δ subunits in cancer compared to the other Cav auxiliary subunits. Increased α2δ1 expression occurs in both ovarian and hepatocellular tumour-initiating cells and correlates with decreased overall survival and a shorter progression-free survival in clinical ovarian samples [[69], [70], [71]]. Zhao et al. developed a monoclonal antibody against α2δ1, 1B50-1 [71]. Sorting of a 1B50-1-positive subpopulation of Hep-11 cells, a hepatocellular carcinoma (HCC) cell line, resulted in a subset of cells that initiated tumour formation in all implanted mice, whereas the 1B50-1-negative subpopulation failed to form any tumours. Furthermore, 62/86 of HCC samples were 1B50-1-positive compared to 0/6 normal tissue samples. in vivo experimentation demonstrated that administering 1B50-1 reduced tumour volume following implantation of two HCC cell lines and increased survival, especially when co-administered with doxorubicin, compared to doxorubicin or 1B50-1 alone. Lastly, in vitro work in the same study demonstrated α2δ1 to be involved in maintaining cell viability and spheroid formation, via increasing Ca2+ influx through L-type and N-type Ca2+ channels and MAPK signalling [71]. In non-small cell lung cancer cells, α2δ1 expression confers radioresistance in vitro, by enhancing the DNA repair response, and chemoresistance in vivo, potentially through MAPK signalling [72,73]. In addition, various miRNAs that are downregulated in cancer target α2δ1 expression, including hsa-miR-208a-3p and hsa-miR-1207-5p in medulloblastoma [74], and miR-107 in chronic myeloid leukaemia (CML) [75]. Overexpressing miR-107 promotes differentiation in CML cell lines, which is reversed when expression of α2δ1 is restored [75].
The involvement of α2δ2 in cancer is complex, as α2δ2 can be both oncogenic and tumour suppressive [76,77]. α2δ2 was initially identified as a potential tumour suppressor gene as it is encoded by CACNA2D2, which is absent in the 3p21.3 chromosomal deletion commonly observed in lung and breast cancer [78]. Similarly, CACNA2D2 is deleted in cervical carcinoma [79], is commonly methylated in head and neck squamous cell carcinoma [80], is downregulated in lung squamous cell carcinoma via miR-205 [81], and its expression correlates with improved survival in patients with lung adenocarcinoma [82]. Functionally, in vitro experiments using various non-small cell lung cancer cell lines have demonstrated that overexpression of α2δ2 induces apoptosis via mitochondrial cytochrome-c release and subsequent caspase activation [77]. In contrast, α2δ2 overexpression occurs in prostate tumours [76] and in insulin-secreting pancreatic adenomas, where elevated intracellular Ca2+ is known to stimulate β-cell proliferation [83]. Furthermore, α2δ2 overexpression in prostate cancer cells induces tumourigenesis and angiogenesis in mice, which is treatable by administering the α2δ2 inhibitor, gabapentin [76].
Conversely, α2δ3 is considered a tumour suppressor gene, as downregulation or deletion is seen in nasopharyngeal cancer [84], breast cancer [85], oesophageal squamous cell carcinoma [86,87], gastric cancer [88,89], lung cancer [90] and cholangiocarcinoma [91]. Mice implanted with cancer cells overexpressing α2δ3 show a decreased tumour volume, compared to implanted control cells, in nasopharyngeal cancer [84], oesophageal cancer [87] and glioma [92] models. The consensus mechanism points towards an inhibition of motility and invasion by α2δ3, and induction of apoptosis through an increase in intracellular Ca2+, leading to mitochondria-induced apoptosis [84,87,92].
2.3. CaVγ
The interaction between CaVγ-subunits and α1 subunits is less well understood. Cavγ-subunits were originally identified following immunoprecipitation of the skeletal muscle 1,4-dihydropyridine (DHP) receptor (later known as L-type VGCCs), which yielded γ1 as a binding partner [93,94]. Following the discovery of CaVγ1, seven more Cavγ-subunits were identified by homology studies [[95], [96], [97], [98]]. Cavγ2 and Cavγ3 have been shown to associate with Cav2.1 [99], Cavγ2-4 to Cav2.2 [99] and Cavγ6 to Cav3.1 [100]. Using cryo-electron microscopy, the γ-subunit was predicted to interact with the Cav1.1 voltage-sensing domain (S4) of domain IV [24]. However, the α1-γ coupling remains contentious as more recent efforts failed to precipitate a Cavγ-subunit with Cav2. Further, Cavγ2 can regulate Cav2.2 indirectly, suggesting a direct coupling may not be necessary for Cavγ-induced channel modulation [21,101]. Cavγ-subunit mRNA is expressed in skeletal muscle (γ1,6,7) and brain (γ2-8) as well as other tissues such as kidney, liver, colon, testis and lung [98]. Functionally, Cavγ-subunits negatively regulate VGCC-mediated Ca2+ influx by decreasing channel expression and current amplitude [102], hyperpolarising the voltage threshold of inactivation, accelerating channel inactivation [103], and increasing the time taken for recovery from inactivation [96]. Cavγ-induced regulation of Ca2+ influx observed at the cellular level is supported by the Stargazer mouse mutant, which lacks Cavγ2 and presents with ataxia and absence seizures [104]. Interestingly, a subclass of Cavγ-subunits, γ2/3/4/5/8 (known as transmembrane AMPA receptor regulatory proteins [TARPs]), which localise to the brain [105], interact with ionotropic AMPA receptors and induce membrane localisation [106,107]. Other functions of γ-subunits include Cavγ7-induced neurite outgrowth in superior cervical ganglion neurons [108] and Cavγ2-induced synaptogenesis [109].
Aberrant Cavγ expression is seen in various cancers, including increased Cavγ1 in early progressing human epidermal growth factor-positive (HER2+) metastatic breast cancer [110], increased Cavγ4 in bladder squamous cell carcinoma [111] and increased Cavγ7 in leiomyoma via downregulation of miR-197 [112]. Furthermore, a prediction algorithm using a dataset of 1.7 million cancer mutations identified Cavγ3 as a putative oncogene [113]. Similar to Cavβ, the functional role of Cavγ in cancer is not yet clear. However, a Cavγ4 mutation appears in a cluster of mutations involved in MAPK signalling [111], suggesting a possible role in regulation of mitogenesis.
In summary, although Cavα1 subunits have an oncogenic role [15], it is not yet clear whether Cav auxiliary subunits function through Cavα1 or have secondary functions in cancer, or both. Given that Cavβ and Cavγ are both oncogenic but have antagonistic effects on α1 function, and Cavα2δ can be oncogenic or tumour suppressive, it would seem that the involvement of auxiliary subunit-mediated Ca2+ influx in cancer is tumour type/stage-specific, dependent on the expression profile of other subunits, or subordinate to a secondary function of the auxiliary subunit. Cav auxiliary subunits have functions, potentially α1-independent, that could contribute to oncogenesis and tumour progression. All Cavβs regulate gene expression and interact with small GTPases [[36], [37], [38],40,41,44]. Cavβ1 and Cavβ2 are also essential for maintaining proliferation and cellular plasticity during development [36,43]. The TARP family of Cavγs induce AMPA receptor membrane trafficking [107], a receptor with an emerging involvement in cancer [114,115], and Cavγ4 and Cavγ7 induce transcellular adhesion and neurite outgrowth respectively [108,109]. α2δ1 is also involved in transcellular adhesion [66]. Furthermore, increased Ca2+ conductance potentially underpins both the oncogenic function of α2δ1 and α2δ2 [71,83] and the tumour suppressive function of α2δ2 and α2δ3 [77,92].
3. K+ channels
K+ channels represent an extensive superfamily of channels, many of which have been implicated in regulating key elements of tumour progression [[116], [117], [118]]. Here, we focus on the function and involvement in cancer of the auxiliary subunits of the voltage-gated K+ channel (VGKC), BK channel and Kir channel complexes (Fig. 2A-C). VGKC α-subunits represent a diverse family of forty K+-conducting proteins, Kv1-12.x, which conduct an outward K+ current in response to depolarisation of the membrane potential. Three classes of VGKC auxiliary subunits have been identified: Kvβ1-3, KChIP1-4, and KCNE1-5 which canonically interact with Kv1, KV4, and Kv7.1 respectively [[119], [120], [121], [122]], although Kvβs and KCNEs interact with other VGKC α-subunits and KVβs also interact with TRPV1 and K2P2.1 [[123], [124], [125], [126]]. The activity of Kv1 [116,127], Kv4 [128], and Kv7.1 [129] is upregulated in various cancers. However, the expression pattern of VGKC auxiliary subunits in cancer is more complex.
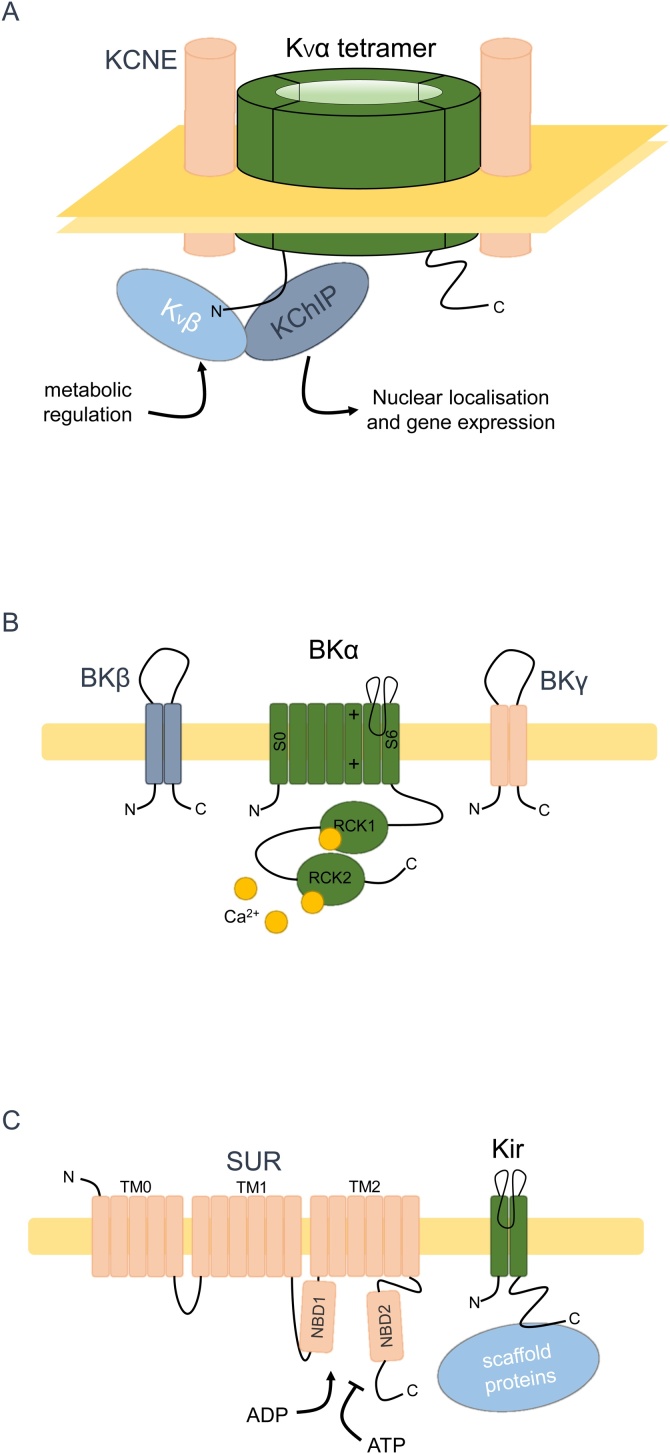
Fig. 2.
K+ channel auxiliary subunits. (A) Voltage-gated K+ channels (VGKCs). The conducting subunit, Kvα forms tetramers within the membrane that are accompanied and functionally modulated by four Kvβs (for Kv1), four KChIPs (for Kv4) or two KCNEs (Kv7.1) [[119], [120], [121], [122]]. The function of Kvβ is modulated by pyridine nucleotides [143]. KChIPs are involved in regulating gene expression [173]. (B) Large conductance Ca2+-activated K+ (BK) channels. BK channels consist of a K+-conducting, seven-pass (S0-S6) membrane protein subunit (BKα/Slo) accompanied and modulated by dual-pass BKβ and BKγ [182,187]. S0 of BKα is required for interaction with BKβ, S4 is involved in voltage-sensing, the pore region is formed by the linker of S5-6 and an enlarged C-terminus containing two RCK (regulator of conductance of K+) domains sense intracellular Ca2+ [330]. (C) Inwardly rectifying K+ (Kir) channels. Tetrameric Kir6 subunits, containing the K+-conducting pore, are functionally regulated at the membrane by 17-pass SUR subunits (1:1 stoichiometry), which confer ATP-sensitivity onto Kir6 via NBDs (nucleotide binding domains) [196]. Kir1-4 can be bound and modulated by various C-terminal binding proteins [331].
3.1. Kvβ
Kvβ subunits are cytoplasmic proteins, which form homo- or heterotetramers [130] that are involved in trafficking of Kv1 and Kv4.3 to the cell surface [[131], [132], [133]]. Additionally, Kvβ2 is involved in targeted axonal trafficking of Kv1.2 and Kvβ1 differentially regulates the Kv composition in ventricular myocytes [134,135]. Kvβ1 and Kvβ3 modulate VGKC α-subunits via an N-terminal ball domain, which permits rapid inactivation of delayed-rectifying Kv1 α-subunits [136,137]. Kvβ1 also slows deactivation, accelerates slow inactivation and hyperpolarises activation of Kv1.2 [138]. Kvβ2 lacks the ability to inactivate delayed-rectifying Kv1 channels, but does hyperpolarise channel activation [139]. Kvβ1 and Kvβ2 are both expressed in developing rat heart and skeletal muscle and during induced myogenesis of L6E9 cells [140]. Furthermore, deletion of Kvβ1 results in aberrant cardiac electrical activity and cardiac hypertrophy in female mice [141]. Kvβ2 deletion leads to reduced Kv1.5 surface expression in coronary arterial myocytes and a reduction in total skeletal muscle volume, potentially mediated through downregulation of Pax7 and upregulation of NEDD4 [133,142]. Interestingly, Kvβs are part of the aldo-keto reductase (AKR) superfamily owing to their C-terminal AKR domain. The AKR domain allows for binding and functional modulation by pyridine nucleotides (NAD and NADP). NADP+ inhibits KVβ1- and KVβ3-mediated inactivation of Kv1.5 as well as inhibiting Kvβ2-mediated hyperpolarisation of Kv1.5 activation [143,144].
Evidence suggests that Kvβs are downregulated in cancer. Kvβ1 is downregulated in malignant thyroid carcinomas relative to benign thyroid adenomas [145,146]. The gene encoding Kvβ2 is the most significant site of methylation in non-functional (non-hormone secreting) pituitary adenoma compared to functional (hormone-secreting) adenomas and is one of the genes ablated in the common 1p36.3 chromosome deletion seen in neuroblastoma [147,148]. Methylation of the promoter of the gene encoding Kvβ3 is seen in oral squamous cell cancers relative to adjacent normal tissue [149]. Together, these data suggest Kvβs are tumour suppressor genes, but in depth in vitro and in vivo characterisation of Kvβ in cancer is still currently lacking.
3.2. KCNE
KCNEs are single-pass transmembrane proteins that interact primarily with Kv7; two KCNEs interact with tetrameric Kv7 [150]. In vitro studies document a range of effects of KCNEs on Kv7.1. For example, KCNE1 and KCNE3 both increase surface expression and current density, while KCNE4 and KCNE5 have no effect on current density [151]. KCNE2 and KCNE3 interaction with Kv7.1 produces voltage-insensitive channels and all KCNEs depolarise the activation voltage of Kv7, with KCNE4 and KCNE5 depolarising activation to a non-physiological membrane potential [151]. KV7.1 has a well-established role in cardiac rhythm and in regulating osmotic and salt transport across gastrointestinal, cochlear and renal epithelia; this is reflected in Kcne1 knockout mice demonstrating atypical QT intervals, hair cell degeneration, impaired renal fluid, glucose and electrolyte uptake, and faecal Na+ and K+ wasting [[152], [153], [154], [155]]. Furthermore, mutations in KCNE1 underlie Long QT Syndrome 5 and Jervis and Lange-Nielsen syndrome, a disorder characterised by deafness and cardiac arrhythmia [156,157].
With regard to cancer, KCNE1-3 are expressed in uterine cancer cell lines, in which they influence proliferation [158] and a 5-fold and 3-fold upregulation of KCNE3 and KCNE4 respectively has been reported in gliobastoma datasets [159]. Paradoxical to the upregulation of KCNE1 in uterine cancer cell lines, KCNE1 overexpression in an astroglioma cell line (U87-MG) induces apoptosis and KCNE1 is one of the four genes deleted in the 21q22.12 microdeletion which causes a predisposition to acute myelogenous leukaemia [160,161]. The apoptotic influence of KCNE1 in U87-MG cells is proposed to occur through canonical K+ efflux through Kv7.1, inducing decreased cytoplasmic K+, a known apoptotic trigger [160,162], whereas KCNE1 induces uterine cancer cell proliferation via modulation of HERG channels [158,163]. HERG channels induce proliferation in a range of cell lines and HERG channel inhibition decreases MAPK phosphorylation and c-fos expression in MDA-MB-435S cells [164]. Out of all the Kv auxiliary subunits however, KCNE2 has the most established link to cancer. KCNE2 downregulation is observed in gastric cancer tissue and gastric cancer cell lines, correlates with gastritis cystica profunda development (preneoplastic condition characterised by large gastric cysts) and is a risk factor in gastric cancer stratification [[165], [166], [167]]. Furthermore, Kcne2 knockout mice display a 6-fold increase in stomach size, an upregulation of Ki67 and Cyclin D1 in gastric mucosa, an increase in the metaplastic marker TFF2, pyloric adenomas and neoplastic invasion compared to wild-type mice [168]. Overexpression of KCNE2 in the SGC7901 gastric cancer cell line reduces proliferation and significantly reduces xenograft tumour volume compared to parental SGC7901 cells [167].
KCNE2-Kv7.1 complexes, in the apical membrane of non-excitable gastric parietal cells, are essential for maintaining acidification of the stomach, as KCNE2 transforms Kv7.1 to a constitutively open channel that is potentiated by extracellular H+ [169]. Luminal K+ released by KCNE2-Kv7.1 is then recycled back into the parietal cell, in exchange for H+, via the H+/K+ ATPase, resulting in gastric acidification [169,170]. Kcne1 knockout mice demonstrate reduced H+ secretion, reduced gastric acidification, gastric hyperplasia and atypical Kv7.1 localisation [170]. However, it is not yet known whether KCNE2 downregulation contributes to gastric cancer progression through a failure to acidify the lumen of the stomach or via its role in regulating tumour cell proliferation.
3.3. KChIP
Ca2+-sensing Kv channel interacting proteins (KChIPs) are involved in KV4 channel modulation. KChIPs increase surface channel density, hyperpolarise the voltage of activation, slow inactivation and accelerate the recovery from inactivation [119,171]. KChIPs were identified by a yeast 2-hybrid screen searching for interaction partners with Kv4.2/3 N-termini [119]. Interestingly, KChIP3 was already known as calsenilin/downstream regulatory element antagonistic modulator (DREAM). KChIP3/DREAM plays a key role in differentiation and apoptosis independently of K+ channels [172]. DREAM binds upstream genetic elements (DRE sites) as a tetramer and represses transcription of the downstream gene until upon Ca2+ stimulation, DREAM tetramers dissociate from DNA allowing gene transcription [173]. Despite KChIP3 being the first Ca2+-sensing transcriptional repressor identified, the other KChIPs are also capable of DRE-site binding [174]. DREAM expression is required for maintenance of human embryonic stem cell pluripotency; DREAM knockdown by siRNA results in an increase in apoptosis and spontaneous differentiation [172]. Potentially independent of its nuclear role, DREAM expression induces Ca2+-mediated apoptosis possibly through sequestration of hexokinase I from mitochondria [175,176]. Additionally, DREAM expression induces process outgrowth in pheochromocytoma PC12 cells by RhoA inactivation and induces thrombus formation in anucleate platelets via PI3K stimulation [177,178]. There is currently limited evidence of a role for KChIPs in cancer. However, one study identified KChIP4 gene disruption in a renal cancer cell chromosomal break [179]. In addition, KChIP1 upregulation and KChIP3 downregulation have been shown in glioblastoma multiforme, with KChIP2 upregulation correlating with decreased survival for glioblastoma patients [180]. The involvement of KChIP3/DREAM in regulating differentiation, apoptosis, transcellular adhesion and process outgrowth suggests cancer-expressed or downregulated KChIPs could be a worthwhile subject of further study.
3.4. BK channels
Large conductance Ca2+-activated K+ (BK) channels are seven membrane-pass K+ channels that conduct a particularly large outward K+ current synergistically in response to membrane depolarisation and a rise in intracellular Ca2+ ([Ca2+]i) [181]. BK channels can be stimulated by depolarisation or increased [Ca2+]i alone, however the required membrane potential (V1/2 = 168 mV at [Ca2+]i = 0) or [Ca2+]i (EC50 ≥10 μM at resting membrane potential) are out of physiological range [182]. BK channels are expressed in most tissues and are involved in a range of functions, such as learning and memory [183], pain modulation [184] and blood pressure regulation [185]. BK channels are upregulated in glioblastoma primary cells and promote proliferation and invasion [117,186]. BK channel function is modulated by two groups of auxiliary subunits- BKβ1-4 and BKγ1-4, both double-pass membrane proteins. BKβ1 and BKβ2 increase Ca2+ sensitivity [187], BKβ2 hyperpolarises and accelerates channel activation [188], BKβ3 depolarises channel activation [188] and BKβ4 hyperpolarises channel activation whilst simultaneously inhibiting channel opening at low [Ca2+]i but enhancing activation at high [Ca2+]i [189]. BKγ subunits hyperpolarise BK channel activation [190]. BKγ1 hyperpolarises channel activation to such an extent (−140 mV in LNCaP prostate cancer cells) that BK channels open without the need for increased [Ca2+]i at resting membrane potentials [182].
Despite the extensive involvement of BK channels in a range of physiological processes, the link between BK channel auxiliary subunits and cancer is still very tentative, with thus far only BKγ1 implicated. There are conflicting reports on the involvement of BKγ1 (also known as LRRC26 and CAPC) in cancer. BKγ1 is upregulated in the MDA-MB-456 breast cancer cell line and in metastatic secondary breast cancer tumours compared to the primary tumour of a single patient [191]. BKγ1 is also upregulated in many breast and prostate cancer cell lines and breast, prostate, colon and pancreatic samples [192,193]. However, BKγ1 is frequently methylated in triple-negative breast cancer specimens and cell lines and siRNA knockdown of BKγ1 in the triple-negative HCC70 breast cancer cell line enhances anchorage-independent growth, invasion, migration, and NF-κB activity [194]. Similarly, knockdown of BKγ1 expression enhances anchorage-independent growth in LNCaP cells and overexpression of BKγ1 in the triple-negative MDA-MB-231 breast cancer cell line downregulates NF-κB activity and inhibits tumourigenesis and metastasis in nude mice [195]. Furthermore, BKγ1 expression is lowest in poorly differentiated and highly invasive prostate and breast cancer lines [195]. Thus, BKγ1 appears to have oncogenic and tumour-suppressive function depending on the cancer type. At this stage, the mechanism by which BKγ1 performs these functions in cancer cells is unclear. BK channels may thus perform multiple functions in cancer cells, dependent on, or independent of, BKγ1.
3.5. Kir channels
Inwardly-rectifying K+ (Kir) channels are double pass membrane proteins which form tetramers in the membrane [196]. Kir channels lack a voltage sensor domain. IKir is instead dictated by the electrochemical gradient and an increasing intracellular blocking of the pore when the membrane potential (Em) > EK, resulting in an inward IK when Em < EK and an outward IK when Em > Ek, which is progressively blocked as Em rises [197]. Kir channels are therefore important for maintenance of the hyperpolarised resting membrane potential and regulating activity in excitable cells, such as vascular smooth muscle [198], central neurons [199] and cardiomyocytes [200]. Subfamilies of Kir channels exist that are ATP-sensitive (KATP channels; Kir6.x) and G-protein gated (G-protein inwardly rectifying K+ channels- GIRKs; Kir3.x) [201,202]. KATP channels are inhibited by ATP/stimulated by ADP. They function as metabolic sensors, for instance in smooth muscle where KATP channels regulate vascular tone [203]. GIRKs facilitate G-protein-mediated inhibitory neurotransmitter signalling, such as GABA signalling [204,205].
Certain Kir channels are regulated by auxiliary subunits. Kir6 binds sufonylurea receptors (SUR) 1 or 2 in an octameric conformation (tetrameric Kir6 plus tetrameric SUR) to form a KATP channel [196]. Channel assembly is required before KATP is released from the endoplasmic reticulum [206]. SUR subunits impart differential sensitivity to ADP/ATP and are the binding target of sulfonylureas, a common form of treatment for type 2 diabetes mellitus [207,208]. SUR1 is overexpressed in cerebral metastases where it decreases vascular permeability [209]. Resveratrol binds to and inhibits SUR1, inducing apoptosis in HEK293 cells, suggesting a potential pro-survival function of SUR1 [210]. SUR2B expression is present in leiomyoma and metastatic breast cancer cells and glibenclamide, a sulfonylurea targeting SUR proteins, inhibits proliferation in these cells [211,212]. SUR2 expression, along with Kir6.2, is upregulated in cervical cancer biopsies [213]. In addition, the effectiveness of glibenclamide at inhibiting proliferation correlates with the Kir6.2 expression of the cell line tested, suggesting proliferation is dependent on SUR and Kir6.2 activity [213]. Glibenclamide also inhibits proliferation in MDA-MB-231 breast cancer cells, inducing G0/G1 cell cycle arrest through an upregulation of P27 and reduction of cyclin E [212]. Treatment of MDA-MD-231 cells with the KATP channel opener, minoxidil, conversely induces proliferation, suggesting K+ influx underlies KATP-regulated proliferation [212]. Glibenclamide treatment also prevents tumour growth in vivo in Sprague-Dawley rats treated with N-nitroso-N-methylurea [214]. Furthermore, in insulinoma, a pancreatic β-cell cancer characterised by insulin release, which is regulated by KATP channels, SUR1 expression is increased [215]. In summary, SUR subunits appear to play an oncogenic role in a Kir-dependent manner.
4. Na+ channels
There is a growing body of evidence supporting a role for Na+ channels in regulating various aspects of cancer progression [216,217]. With regard to auxiliary subunits, however, only those of the VGSC have been characterised to date and will therefore be the focus of this section (Fig. 3).
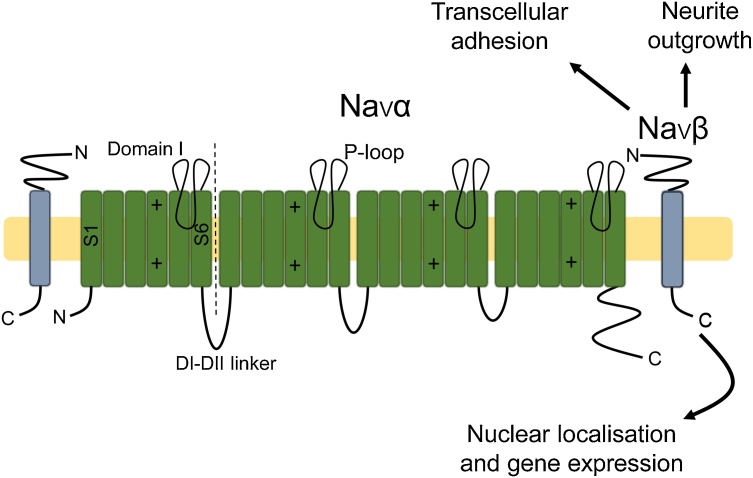
Fig. 3.
Voltage-gated Na+ channel auxiliary subunits. Voltage-gated Na+ channels (VGSCs) contain a conducting Navα subunit and auxiliary Navβ subunits. Navα consists of four domains (domains I-IV), each containing six segments (S1-S6). The voltage-sensing domain is found within S4 of each domain and the pore consists of the P-loop found between S5-6 of each domain. Navβs function as cell adhesion molecules via an extracellular immunoglobulin domain [238,239,332]. Navβs also induce neurite outgrowth and migration [245] and the intracellular domain of Navβ2 has putative transcription regulation function [248].
4.1. Voltage-gated Na+ channels
VGSCs conduct an inward Na+ current in response to membrane depolarisation [218]. VGSCs are composed of a pore-forming α-subunit (Nav1.1–1.9) and auxiliary β-subunits (Navβ1-Navβ4). Navβs are single pass transmembrane glycoproteins that bind Navα covalently, in the case of Navβ2 and Navβ4 [219,220], or non-covalently, in the case of Navβ1 and Navβ3 [[221], [222], [223]]. INa is responsible for propagation of action potentials and mutations in Navβs underlie certain types of epilepsy [224] and cardiac arrhythmia [225]. Navβ1-3 traffic Navα to the cell surface [[226], [227], [228]] and all Navβs increase INa [[229], [230], [231]]. Navβs induce other changes in Navα gating kinetics, including accelerated recovery from inactivation [232,233] and accelerated inactivation [230,234]. Navβs can both positively and negatively shift the voltage of activation [235,236] and inactivation [222,226], possibly dependent on endogenous expression of Nav subunits and other Nav-interacting proteins in the experimental system used. Navβs are also cell adhesion molecules, owing to the presence of an extracellular immunoglobulin loop [[237], [238], [239], [240]], which permits NaVβ-mediated neurite outgrowth [[241], [242], [243], [244]]. NaVβ1 plays an important role in regulating neuronal migration in CNS development, particularly in the cerebellum [14,245], and NaVβ2 promotes dendritic expansion during hippocampal development via a Navα-independent mechanism [243]. NaVβ subunits are also substrates for proteolytic processing by secretases [246,247] and evidence suggests that the cleaved intracellular domain of NaVβ2 shuttles to the nucleus to regulate expression of α-subunit genes [248].
Emerging evidence suggests that Navβs play diverse functional roles in cancer. Navβ1 is upregulated in breast cancer samples and is more highly expressed in strongly metastatic, compared to weakly metastatic, prostate cancer cell lines [249,250]. Overexpression of Navβ1 in the MDA-MB-231 breast cancer cell line promotes primary tumour growth and metastasis to multiple organs when grafted into mice, compared to parental MDA-MB-231 cells [249]. The Navβ1-induced increase in primary and secondary tumour growth was accompanied by a decrease in apoptotic cleaved caspase-3 staining, no change in proliferative Ki67 staining, and an increase in endothelial CD31 staining, suggesting increased apoptotic resistance and vascularisation underlie the oncogenic influence of Navβ1 [249]. In vitro, MDA-MB-231-Navβ1 cells demonstrate increased cell-cell adhesion, VGSC-mediated Na+ current and neurite-like process outgrowth, which is reversible by inhibiting INa [249,251]. Interestingly, MDA-MB-231-Navβ1 cells show decreased in vitro motility and proliferation compared to MDA-MB-231 cells and knockdown of endogenous Navβ1 in the MCF-7 breast cancer cell line increases cell migration [251]. Similarly, Navβ1 is also expressed in cervical cancer cells where it inhibits motility [252]. Furthermore, treatment of mouse melanoma B16F10 cells with the anti-cancer polymethoxyflavone, casticin, inhibits cell migration and invasion and causes a concomitant genomic upregulation of SCN1B (encoding for Navβ1) [253]. Navβ1 therefore appears to have a negative influence on cell behaviour in vitro and potentially induces tumour growth and metastasis through an increase in apoptotic resistance and transcellular adhesion.
Navβ2 also appears to be oncogenic. Navβ2 expression is increased in strongly metastatic prostate cancer cell lines relative to weakly metastatic cell lines [254]. Perineural invasion is common in invasive prostate cancer, and LNCaP prostate cancer cells overexpressing Navβ2 demonstrate an increased association with ex vivo murine spinal cord axons and an increase in migration, invasion and growth [254,255]. Despite the invasion-promoting behaviour of Navβ2 in vitro, overexpression of Navβ2 in LNCaP cells inhibits tumour growth, compared to LNCaP cells, when implanted into mice, suggesting the functional contribution of Navβ2 might be site or stage-specific during cancer progression [255].
Unlike Navβ1 and Navβ2, Navβ3 and Navβ4 are considered tumour-suppressive. SCN3B (encoding for Navβ3) expression is strongly upregulated by p53 following DNA damage and Navβ3 expression induces apoptosis and suppresses colony formation in osteosarcoma and glioblastoma cell lines [256]. Navβ4 expression is downregulated in thyroid and high-grade breast cancer and is associated with favourable survival [231,257]. Downregulation of Navβ4 in MDA-MB-231 breast cancer cells with shRNA increases primary tumour growth and metastasis in xenograft mice models, relative to MDA-MB-231 cells overexpressing Navβ4 [231]. Furthermore, loss of Navβ4 increases Navα-independent RhoA-mediated cancer cell migration and invasion [231]. Navβ4 also suppresses invasion in cervical cancer cells [252]. Navβs are structurally very similar and generally have a broadly comparable effect increasing INa, so it is intriguing that Navβ1 and Navβ2 are oncogenic, whereas Navβ3 and Navβ4 are tumour-suppressive. Additionally, both Navβ1 and Navβ4 were investigated using the same breast cancer cell, MDA-MB-231, so the endogenous VGSC subunit expression accompanying the Navβ-subunit is comparable [231,249]. Both Navβ1 and Navβ4 inhibit cell migration in vitro and induce neurite outgrowth in developing neurons, thus it is unclear where the functional discrepancy between the two proteins lies [231,241,251,258].
5. Cl− channels
Cl− channels are a family of relatively poorly understood proteins that facilitate transmembrane Cl− transport. Cl− concentration is highest intracellularly and ECl ˜-30 to −60 mV, so channels conduct an outward Cl− current at resting membrane potentials that can reverse on depolarisation, although inwardly and outwardly rectifying Cl− channels have been identified [13]. Cl− channels are involved in regulating a range of bodily functions, including renal salt retention [259], synaptic inhibition [260], skeletal muscle contraction [261], smooth muscle tone [262] and sperm motility [263]. Various subfamilies of Cl− exist, but only the voltage-gated Cl− channel (CLC) and Ca2+-sensitive Cl− channel (CaCC) subfamilies possess auxiliary subunits with a robust link to cancer (Fig. 4A, B).
Fig. 4.
Cl− channel auxiliary subunits. (A) CLCs are a subfamily of voltage-sensitive Cl− channels and transporters found at the plasma membrane and internal membranes [13]. Barttin modulates ClC-K, GlialCAM modulates ClC-2 and Ostm1 modulates the intracellular ClC-7 transporter [264,266,267]. CLCs are composed of eighteen helical domains and two C-terminal cystathionine-β-synthase (CBS) domains which facilitate dimerization [333]. Depicted is the plasma membrane ClC-2 which interacts with single-pass GlialCAM, the only ClC auxiliary subunit implicated in cancer [264]. GlialCAM can also function as a cell adhesion molecule [268]. (B) Two separate CaCC conducting subunits exist- TMEM16 and Bestrophin. Depicted is eight-pass TMEM16 A which is modulated directly by secreted CLCA1 and indirectly by single-pass CLCA2 [303,305]. CLCA2 stimulates Ca2+ store replenishment by interacting with Orai1 and STIM1 [305].
5.1. Voltage-gated Cl− channels
CLCs represent a range of cell surface Cl− channels (ClC-1,2,K) and intracellular Cl− exchangers (ClC-3-7). Some CLCs are regulated by auxiliary subunits; ClC-2 by GlialCAM [264,265], ClC-7 by Ostm1 [266], and ClC-K by Barttin [267]. GlialCAM targets ClC-2 to cell-cell junctions, increases Cl− current (ICl), accelerates ICl activation, and abolishes ClC-2 inward rectification and pH sensitivity [264]. GlialCAM also functions as a cell adhesion molecule via an extracellular immunoglobulin domain [268,269]. ClC-7 is an intracellular, electrogenic H+/Cl− exchanger involved in lysosomal acidification [270]. Interestingly, ClC-7 regulates the trafficking and expression of its auxiliary subunit, Ostm1 [266,271]. Nevertheless, Ostm1 is required to activate ClC-7 function [270]. Barttin traffics ClC-K to the cell surface, resulting in increased ICl, and abolishes the voltage-dependence of ClC-K [[272], [273], [274]]. Mutations in the gene encoding Barttin are the cause of Bartter syndrome type IV, characterised by hypokalaemia, blood alkalosis and hypotension [275,276]. Knockin mice with the disease-causing Barttin mutation R8L present with reduced plasma membrane Barttin-ClC-K complexes and transepithelial Cl− transport is impaired in the loop of Henle [277].
GlialCAM (also called HepaCAM) was identified as a putative tumour suppressor gene that is silenced in hepatocellular carcinoma [278]. GlialCAM downregulation is observed in liver, bladder, prostate, kidney, breast, uterus, colon, stomach, and rectal cancer biopsies [269,[278], [279], [280], [281], [282]]. Functionally, when GlialCAM is expressed in the liver carcinoma cell line HepG2, cell motility and adhesion are increased, colony formation is reduced, and proliferation is reduced [278]. Similarly, when expressed in MCF-7 breast cancer cells, GlialCAM increases cell motility and adhesion, decreases proliferation, and induces p53-mediated cellular senescence [279,283]. GlialCAM inhibits proliferation and β-catenin signalling in bladder carcinoma cells [284,285]. Furthermore, in renal carcinoma cells, GlialCAM decreases proliferation, induces cell cycle arrest, and stimulates c-Myc degradation [286]. GlialCAM expression is also sufficient for reducing Notch-mediated invasion and migration in prostate cancer cells [282]. Lastly, GlialCAM stabilises connexin-43 at cell-cell gap junctions [287], connexin-43 being a potential tumour suppressor itself [288,289]. In summary, GlialCAM has a strong anti-proliferative influence when expressed in cancer cells, which could underpin its role as a tumour suppressor.
5.2. Ca2+-sensitive Cl− channels
Four single membrane-pass auxiliary subunits of CaCCs have been identified (known as Ca2+-activated Cl− channel regulator or Cl− channel accessory [CLCA]1-4) [290,291]. Interestingly, the molecular identities of the conducting subunits were only discovered later and termed Best1-4 and TMEM16 [[292], [293], [294], [295]]. CaCCs demonstrate voltage-dependence at steady-state, which is abolished following an increase in [Ca2+]i [296]. Increased [Ca2+]i also increases ICl and accelerates current onset [296]. CaCCs are expressed in epithelia and excitable tissues, where they regulate excitability [297], smooth muscle contraction [298] and fluid secretion [299]. Expression of CLCA1 and CLCA2 in HEK293 cells induces an enlarged and outwardly-rectifying ICaCC [290,300]. More recent work has demonstrated that the secreted N-terminus of CLCA1, produced following autoproteolysis, is sufficient to stabilise TMEM16 A at the membrane, increasing ICaCC [[301], [302], [303]]. CLCA1 contains an intrinsic metalloprotease domain in the N-terminus that is thought to be responsible for autoproteolysis and regulating mucus turnover in the colon [304]. Despite CLCA2 enlarging ICaCC, CLCA2 does not interact directly with TMEM16 or Best1 [305]. Instead, CLCA2 interacts directly with store-operated Ca2+ channels, Orai1 and STIM-1, stimulating ER Ca2+ replenishment following cytosolic depletion [305].
CLCAs have a well-documented tumour-suppressive role [[306], [307], [308]]. CLCA1 is downregulated in colorectal and pancreatic cancer specimens [306,[309], [310], [311]]. CLCA1 knockdown induces proliferation and inhibits differentiation of caco-2 colorectal cancer cells [311]. Furthermore, CLCA1 overexpression inhibits Wnt signalling and colorectal tumour growth and metastasis in vivo [306]. CLCA2 expression is also decreased in high-grade nasopharyngeal, colorectal, lymphoid and breast cancer specimens compared to low grade samples [307,[312], [313], [314]]. Expression of CLCA2 decreases nasopharyngeal and breast tumourigenesis in vivo [307,312,315]. Similarly, CLCA2 depletion increases the number of circulating prostate tumour cells in mice [316]. At a cellular level, CLCA2 inhibits Wnt signalling [317], decreases invasion [315], inhibits proliferation [312], induces transcellular adhesion [316], inhibits epithelial-to-mesenchymal transition [312,316], induces differentiation [316,318], inhibits focal adhesion kinase [312,319] and induces p53-mediated cellular senescence [320]. The ability of CLCA2 to inhibit cancer cell migration appears to be ICl independent, as inhibiting ICl has a further anti-migratory effect in cells expressing CLCA2 as well as having an anti-migratory effect in cells not expressing CLCA2 [312]. Ramena et al. observed CLCA2 at cell-cell junctions, interacting with EVA1/ZO-1 or β-catenin [317]. Sequestration of β-catenin at the plasma membrane was therefore suggested as a mechanism for CLCA2-induced inhibition of epithelial-to-mesenchymal transition. CLCA4 expression is decreased in bladder, hepatocellular and breast cancer specimens compared to adjacent normal tissue [308,321,322]. CLCA4 expression also decreases tumourigenicity in mice [321]. Furthermore, CLCA4 depletion induces epithelial-to-mesenchymal transition via PI3K/Akt signalling [308,322]. Despite the abundance of evidence implicating CLCAs as tumour suppressor genes, CLCAs have also been implicated in induction of lung colonization in vivo via adhesive interactions between endothelial CLCA and β4 integrin expressed on circulating cancer cells [323,324]. Similarly, increased CLCA2 expression is seen in circulating lung adenocarcinoma cells and ovarian cancer cell aggregates [325,326], suggesting CLCAs may potentially be tumour suppressors on the one hand, and metastasis-promoting on the other.
6. Conclusion
Many ion channel auxiliary subunits are upregulated, e.g. Cavβs, or downregulated, e.g. Kvβs, in tumours and thus may represent novel cancer biomarkers. in vitro and in vivo experimentation has further implicated various auxiliary subunits in tumour formation and progression, such as Navβ1 and α2δ1 (Fig. 5). However, others, e.g. CLCAs, NaVβ3/4, may function as tumour suppressors. Clearly, it is important from a treatment perspective to understand the mechanistic function of ion channel auxiliary subunits, including the extent that they contribute to cancer progression through potentiating ion conductance or via non-conducting signalling. For example, α2δ1- and α2δ2-induced Ca2+ influx may promote hepatocellular carcinoma cell sphere formation and pancreatic adenoma proliferation respectively [71,83]. Other examples include NaVα-dependent, NaVβ1-mediated process outgrowth and the extent of glibenaclamide-induced inhibition of SUR2-mediated cancer cell proliferation correlating with the mRNA expression of Kir6.2 [213,249]. Validating the contribution of ion conductance to the oncogenic function of these auxiliary subunits would provide a potential therapeutic target, as many ion channel inhibitors are already in clinical use and could be repurposed [[327], [328], [329]]. On the other hand, numerous auxiliary subunits many regulate cancer progression via non-conducting roles, e.g. regulation of transcription, proliferation and differentiation by Cavβ1 and KChIP3 [36,172]. Various auxiliary subunits also function as adhesion molecules in cancer cells, e.g. GlialCAM, CLCAs and Navβs [254,278,316]. Further work is required to fully delineate the diverse functional contributions of these subunits to carcinogenesis, tumour progression and metastasis, and understand their potential as novel therapeutic targets.
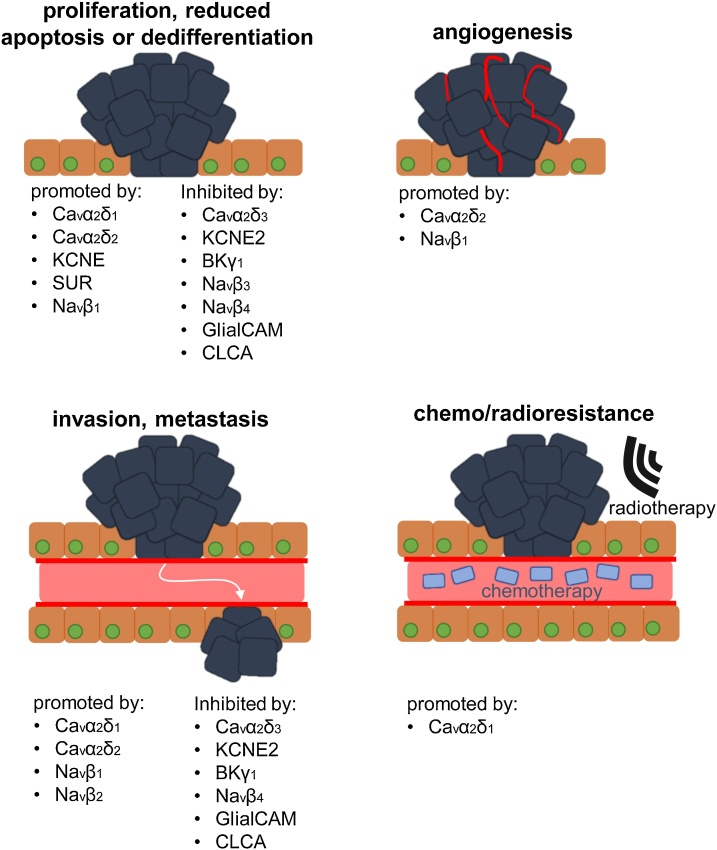
Fig. 5.
Involvement of ion channel auxiliary subunits in different stages of tumour progression. A number of different ion channel auxiliary subunits are up- or down-regulated in cancer cells promoting proliferation, reducing apoptosis and differentiation. Other auxiliary subunits have been shown to regulate angiogenesis, invasion, and metastasis, thus promoting tumour progression. Finally, ion channel auxiliary subunits may also play a role in chemo/radioresistance, underscoring the potential importance of these proteins in relation to therapeutic intervention.
Conflicts of interest statement
The authors declare that they have no conflicts of interest.
Acknowledgement
This work was supported by BBSRC Doctoral Training Partnership in “Mechanistic Biology and its Strategic Application” Grant BB/M011151/1.
References
- 1.Hille B. 2nd ed. Sinauer Associates Inc.; Sunderland (Massachusetts): 1992. Ionic Channels of Excitable Membranes. [Google Scholar]
- 2.Blackiston D.J., McLaughlin K.A., Levin M. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8:3527–3536. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdul Kadir L., Stacey M., Barrett-Jolley R. Emerging roles of the membrane potential: action beyond the action potential. Front. Physiol. 2018;9:1661. doi: 10.3389/fphys.2018.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwab A., Fabian A., Hanley P.J., Stock C. Role of ion channels and transporters in cell migration. Physiol. Rev. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- 5.Prevarskaya N., Skryma R., Shuba Y. Ion channels in cancer: are cancer hallmarks oncochannelopathies? Physiol. Rev. 2018;98:559–621. doi: 10.1152/physrev.00044.2016. [DOI] [PubMed] [Google Scholar]
- 6.Kaczmarek L.K. Non-conducting functions of voltage-gated ion channels. Nat. Rev. Neurosci. 2006;7:761–771. doi: 10.1038/nrn1988. [DOI] [PubMed] [Google Scholar]
- 7.Pongs O., Schwarz J.R. Ancillary subunits associated with voltage-dependent K+ channels. Physiol. Rev. 2010;90:755–796. doi: 10.1152/physrev.00020.2009. [DOI] [PubMed] [Google Scholar]
- 8.Li Q., Yan J. Modulation of BK channel function by auxiliary Beta and gamma subunits. Int. Rev. Neurobiol. 2016;128:51–90. doi: 10.1016/bs.irn.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 10.Bouza A.A., Isom L.L. Handbook of Experimental Pharmacology. 2017. Voltage-gated sodium channel beta subunits and their related diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolphin A.C. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J. Physiol. 2016;594:5369–5390. doi: 10.1113/JP272262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black J.L., 3rd The voltage-gated calcium channel gamma subunits: a review of the literature. J. Bioenerg. Biomembr. 2003;35:649–660. doi: 10.1023/b:jobb.0000008029.22650.c5. [DOI] [PubMed] [Google Scholar]
- 13.Duran C., Thompson C.H., Xiao Q., Hartzell H.C. Chloride channels: often enigmatic, rarely predictable. Annu. Rev. Physiol. 2010;72:95–121. doi: 10.1146/annurev-physiol-021909-135811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel F., Brackenbury W.J. Dual roles of voltage-gated sodium channels in development and cancer. Int. J. Dev. Biol. 2015 doi: 10.1387/ijdb.150171wb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchanan P.J., McCloskey K.D. CaV channels and cancer: canonical functions indicate benefits of repurposed drugs as cancer therapeutics. Eur. Biophys. J. 2016;45:621–633. doi: 10.1007/s00249-016-1144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mo P., Yang S. The store-operated calcium channels in cancer metastasis: from cell migration, invasion to metastatic colonization. Front. Biosci. (Landmark edition) 2018;23:1241–1256. doi: 10.2741/4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapovalov G., Ritaine A., Skryma R., Prevarskaya N. Role of TRP ion channels in cancer and tumorigenesis. Semin. Immunopathol. 2016;38:357–369. doi: 10.1007/s00281-015-0525-1. [DOI] [PubMed] [Google Scholar]
- 18.Liu L., Li H., Cui Y., Li R., Meng F., Ye Z., Zhang X. Calcium channel opening rather than the release of ATP causes the apoptosis of osteoblasts induced by overloaded mechanical stimulation. Cell. Physiol. Biochem. 2017;42:441–454. doi: 10.1159/000477592. [DOI] [PubMed] [Google Scholar]
- 19.Grossinger E.M., Kang M., Bouchareychas L., Sarin R., Haudenschild D.R., Borodinsky L.N., Adamopoulos I.E. Ca(2+)-Dependent regulation of NFATc1 via KCa3.1 in inflammatory osteoclastogenesis. J. Immunol. 2018;200:749–757. doi: 10.4049/jimmunol.1701170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catterall W.A., Perez-Reyes E., Snutch T.P., Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 21.Muller C.S., Haupt A., Bildl W., Schindler J., Knaus H.G., Meissner M., Rammner B., Striessnig J., Flockerzi V., Fakler B., Schulte U. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14950–14957. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T.P., Campbell K.P. Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 23.Van Petegem F., Clark K.A., Chatelain F.C., Minor D.L., Jr Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J., Yan Z., Li Z., Yan C., Lu S., Dong M., Yan N. Structure of the voltage-gated calcium channel Cav1.1 complex. Science. 2015;350 doi: 10.1126/science.aad2395. [DOI] [PubMed] [Google Scholar]
- 25.Altier C., Garcia-Caballero A., Simms B., You H., Chen L., Walcher J., Tedford H.W., Hermosilla T., Zamponi G.W. The Cavbeta subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat. Neurosci. 2011;14:173–180. doi: 10.1038/nn.2712. [DOI] [PubMed] [Google Scholar]
- 26.Cassidy J.S., Ferron L., Kadurin I., Pratt W.S., Dolphin A.C. Functional exofacially tagged N-type calcium channels elucidate the interaction with auxiliary alpha2delta-1 subunits. Proc. Natl. Acad. Sci. U. S. A. 2014;111:8979–8984. doi: 10.1073/pnas.1403731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waithe D., Ferron L., Page K.M., Chaggar K., Dolphin A.C. Beta-subunits promote the expression of Ca(V)2.2 channels by reducing their proteasomal degradation. J. Biol. Chem. 2011;286:9598–9611. doi: 10.1074/jbc.M110.195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page K.M., Rothwell S.W., Dolphin A.C. The CaVbeta subunit protects the I-II loop of the voltage-gated calcium channel CaV2.2 from proteasomal degradation but not oligoubiquitination. J. Biol. Chem. 2016;291:20402–20416. doi: 10.1074/jbc.M116.737270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X., Liu D., Zhou D., Si Y., Xu D., Stamatkin C.W., Ghozayel M.K., Ripsch M.S., Obukhov A.G., White F.A., Meroueh S.O. Small-molecule CaValpha1CaVbeta antagonist suppresses neuronal voltage-gated calcium-channel trafficking. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E10566–e10575. doi: 10.1073/pnas.1813157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh B.C., Kim D.I., Falkenburger B.H., Hille B. Membrane-localized beta-subunits alter the PIP2 regulation of high-voltage activated Ca2+ channels. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3161–3166. doi: 10.1073/pnas.1121434109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park C.G., Park Y., Suh B.C. The HOOK region of voltage-gated Ca2+ channel beta subunits senses and transmits PIP2 signals to the gate. J. Gen. Physiol. 2017;149:261–276. doi: 10.1085/jgp.201611677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeon J.H., Park C.G., Hille B., Suh B.C. Translocatable voltage-gated Ca(2+) channel beta subunits in alpha1-beta complexes reveal competitive replacement yet no spontaneous dissociation. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E9934–e9943. doi: 10.1073/pnas.1809762115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi S.X., Mittman S., Colecraft H.M. Distinctive modulatory effects of five human auxiliary beta2 subunit splice variants on L-type calcium channel gating. Biophys. J. 2003;84:3007–3021. doi: 10.1016/S0006-3495(03)70027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etemad S., Obermair G.J., Bindreither D., Benedetti A., Stanika R., Di Biase V., Burtscher V., Koschak A., Kofler R., Geley S., Wille A., Lusser A., Flockerzi V., Flucher B.E. Differential neuronal targeting of a new and two known calcium channel beta4 subunit splice variants correlates with their regulation of gene expression. J. Neurosci. 2014;34:1446–1461. doi: 10.1523/JNEUROSCI.3935-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park C.G., Suh B.C. The HOOK region of beta subunits controls gating of voltage-gated Ca(2+) channels by electrostatically interacting with plasma membrane. Channels Austin (Austin) 2017;11:467–475. doi: 10.1080/19336950.2017.1335841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor J., Pereyra A., Zhang T., Messi M.L., Wang Z.M., Herenu C., Kuan P.F., Delbono O. The Cavbeta1a subunit regulates gene expression and suppresses myogenin in muscle progenitor cells. J. Cell Biol. 2014;205:829–846. doi: 10.1083/jcb.201403021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Servili E., Trus M., Maayan D. Atlas, beta-Subunit of the voltage-gated Ca(2+) channel Cav1.2 drives signaling to the nucleus via H-Ras. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E8624–e8633. doi: 10.1073/pnas.1805380115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Yamada Y., Fan M., Bangaru S.D., Lin B., Yang J. The beta subunit of voltage-gated Ca2+ channels interacts with and regulates the activity of a novel isoform of Pax6. J. Biol. Chem. 2010;285:2527–2536. doi: 10.1074/jbc.M109.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rima M., Daghsni M., Lopez A., Fajloun Z., Lefrancois L., Dunach M., Mori Y., Merle P., Bruses J.L., De Waard M., Ronjat M. Down-regulation of the Wnt/beta-catenin signaling pathway by Cacnb4. Mol. Biol. Cell. 2017;28:3699–3708. doi: 10.1091/mbc.E17-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beguin P., Nagashima K., Gonoi T., Shibasaki T., Takahashi K., Kashima Y., Ozaki N., Geering K., Iwanaga T., Seino S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Gutierrez G., Miranda-Laferte E., Neely A., Hidalgo P. The Src homology 3 domain of the beta-subunit of voltage-gated calcium channels promotes endocytosis via dynamin interaction. J. Biol. Chem. 2007;282:2156–2162. doi: 10.1074/jbc.M609071200. [DOI] [PubMed] [Google Scholar]
- 42.Schuster-Gossler K., Cordes R., Gossler A. Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proc. Natl. Acad. Sci. U. S. A. 2007;104:537–542. doi: 10.1073/pnas.0608281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chernyavskaya Y., Ebert A.M., Milligan E., Garrity D.M. Voltage-gated calcium channel CACNB2 (beta2.1) protein is required in the heart for control of cell proliferation and heart tube integrity. Dev. Dyn. 2012;241:648–662. doi: 10.1002/dvdy.23746. [DOI] [PubMed] [Google Scholar]
- 44.Rima M., Daghsni M., De Waard S., Gaborit N., Fajloun Z., Ronjat M., Mori Y., Bruses J.L., De Waard M. The beta4 subunit of the voltage-gated calcium channel (Cacnb4) regulates the rate of cell proliferation in Chinese Hamster ovary cells. Int. J. Biochem. Cell Biol. 2017;89:57–70. doi: 10.1016/j.biocel.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 45.Tadmouri A., Kiyonaka S., Barbado M., Rousset M., Fablet K., Sawamura S., Bahembera E., Pernet-Gallay K., Arnoult C., Miki T., Sadoul K., Gory-Faure S., Lambrecht C., Lesage F., Akiyama S., Khochbin S., Baulande S., Janssens V., Andrieux A., Dolmetsch R., Ronjat M., Mori Y., De Waard M. Cacnb4 directly couples electrical activity to gene expression, a process defective in juvenile epilepsy. EMBO J. 2012;31:3730–3744. doi: 10.1038/emboj.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ronjat M., Kiyonaka S., Barbado M., De Waard M., Mori Y. Nuclear life of the voltage-gated Cacnb4 subunit and its role in gene transcription regulation. Channels (Austin, Tex.) 2013;7:119–125. doi: 10.4161/chan.23895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang C.Y., Lai M.D., Phan N.N., Sun Z., Lin Y.C. Meta-analysis of public microarray datasets reveals voltage-gated calcium gene signatures in clinical Cancer patients. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X., Wang W., Zhang S., Wang X., Tang Z., Gu J., Li J., Huang J. CACNA1B (Cav2.2) overexpression and its association with clinicopathologic characteristics and unfavorable prognosis in non-small cell lung Cancer. Dis. Mark. 2017;2017 doi: 10.1155/2017/6136401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suo A., Childers A., D’Silva A., Petersen L.F., Otsuka S., Dean M., Li H., Enwere E.K., Pohorelic B., Klimowicz A., Souza I.A., Hamid J., Zamponi G.W., Bebb D. Cav3.1 overexpression is associated with negative characteristics and prognosis in non-small cell lung cancer. Oncotarget. 2018;9:8573–8583. doi: 10.18632/oncotarget.24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao P., He M., Zhang C., Geng C. Integrated analysis of gene expression signatures associated with colon cancer from three datasets. Gene. 2018;654:95–102. doi: 10.1016/j.gene.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Chen M., Rothman N., Ye Y., Gu J., Scheet P.A., Huang M., Chang D.W., Dinney C.P., Silverman D.T., Figueroa J.D., Chanock S.J., Wu X. Pathway analysis of bladder cancer genome-wide association study identifies novel pathways involved in bladder cancer development. Genes Cancer. 2016;7:229–239. doi: 10.18632/genesandcancer.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitra R., Lee J., Jo J., Milani M., McClintick J.N., Edenberg H.J., Kesler K.A., Rieger K.M., Badve S., Cummings O.W., Mohiuddin A., Thomas D.G., Luo X., Juliar B.E., Li L., Mesaros C., Blair I.A., Srirangam A., Kratzke R.A., McDonald C.J., Kim J., Potter D.A. Prediction of postoperative recurrence-free survival in non-small cell lung cancer by using an internationally validated gene expression model. Clin. Cancer Res. 2011;17:2934–2946. doi: 10.1158/1078-0432.CCR-10-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calderon-Rivera A., Andrade A., Hernandez-Hernandez O., Gonzalez-Ramirez R., Sandoval A., Rivera M., Gomora J.C., Felix R. Identification of a disulfide bridge essential for structure and function of the voltage-gated Ca(2+) channel alpha(2)delta-1 auxiliary subunit. Cell Calcium. 2012;51:22–30. doi: 10.1016/j.ceca.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies A., Kadurin I., Alvarez-Laviada A., Douglas L., Nieto-Rostro M., Bauer C.S., Pratt W.S., Dolphin A.C. The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc. Natl. Acad. Sci. U. S. A. 2010;107:1654–1659. doi: 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shistik E., Ivanina T., Puri T., Hosey M., Dascal N. Ca2+ current enhancement by alpha 2/delta and beta subunits in Xenopus oocytes: contribution of changes in channel gating and alpha 1 protein level. J. Physiol. 1995;489(Pt 1):55–62. doi: 10.1113/jphysiol.1995.sp021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nieto-Rostro M., Ramgoolam K., Pratt W.S., Kulik A., Dolphin A.C. Ablation of alpha2delta-1 inhibits cell-surface trafficking of endogenous N-type calcium channels in the pain pathway in vivo. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E12043–e12052. doi: 10.1073/pnas.1811212115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadurin I., Ferron L., Rothwell S.W., Meyer J.O., Douglas L.R., Bauer C.S., Lana B., Margas W., Alexopoulos O., Nieto-Rostro M., Pratt W.S., Dolphin A.C. Proteolytic maturation of alpha2delta represents a checkpoint for activation and neuronal trafficking of latent calcium channels. eLife. 2016;5 doi: 10.7554/eLife.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernstein G.M., Jones O.T. Kinetics of internalization and degradation of N-type voltage-gated calcium channels: role of the alpha2/delta subunit. Cell Calcium. 2007;41:27–40. doi: 10.1016/j.ceca.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 59.Li C.Y., Zhang X.L., Matthews E.A., Li K.W., Kurwa A., Boroujerdi A., Gross J., Gold M.S., Dickenson A.H., Feng G., Luo Z.D. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neely G.G., Hess A., Costigan M., Keene A.C., Goulas S., Langeslag M., Griffin R.S., Belfer I., Dai F., Smith S.B., Diatchenko L., Gupta V., Xia C.P., Amann S., Kreitz S., Heindl-Erdmann C., Wolz S., Ly C.V., Arora S., Sarangi R., Dan D., Novatchkova M., Rosenzweig M., Gibson D.G., Truong D., Schramek D., Zoranovic T., Cronin S.J., Angjeli B., Brune K., Dietzl G., Maixner W., Meixner A., Thomas W., Pospisilik J.A., Alenius M., Kress M., Subramaniam S., Garrity P.A., Bellen H.J., Woolf C.J., Penninger J.M. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell. 2010;143:628–638. doi: 10.1016/j.cell.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barclay J., Balaguero N., Mione M., Ackerman S.L., Letts V.A., Brodbeck J., Canti C., Meir A., Page K.M., Kusumi K., Perez-Reyes E., Lander E.S., Frankel W.N., Gardiner R.M., Dolphin A.C., Rees M. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Field M.J., Cox P.J., Stott E., Melrose H., Offord J., Su T.Z., Bramwell S., Corradini L., England S., Winks J., Kinloch R.A., Hendrich J., Dolphin A.C., Webb T., Williams D. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran-Van-Minh A., Dolphin A.C. The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J. Neurosci. 2010;30:12856–12867. doi: 10.1523/JNEUROSCI.2700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lotarski S., Hain H., Peterson J., Galvin S., Strenkowski B., Donevan S., Offord J. Anticonvulsant activity of pregabalin in the maximal electroshock-induced seizure assay in alpha2delta1 (R217A) and alpha2delta2 (R279A) mouse mutants. Epilepsy Res. 2014;108:833–842. doi: 10.1016/j.eplepsyres.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Eroglu C., Allen N.J., Susman M.W., O’Rourke N.A., Park C.Y., Ozkan E., Chakraborty C., Mulinyawe S.B., Annis D.S., Huberman A.D., Green E.M., Lawler J., Dolmetsch R., Garcia K.C., Smith S.J., Luo Z.D., Rosenthal A., Mosher D.F., Barres B.A. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Risher W.C., Kim N., Koh S., Choi J.E., Mitev P., Spence E.F., Pilaz L.J., Wang D., Feng G., Silver D.L., Soderling S.H., Yin H.H., Eroglu C. Thrombospondin receptor alpha2delta-1 promotes synaptogenesis and spinogenesis via postsynaptic Rac1. J. Cell Biol. 2018;217:3747–3765. doi: 10.1083/jcb.201802057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kerov V., Laird J.G., Joiner M.L., Knecht S., Soh D., Hagen J., Gardner S.H., Gutierrez W., Yoshimatsu T., Bhattarai S., Puthussery T., Artemyev N.O., Drack A.V., Wong R.O., Baker S.A., Lee A. alpha2delta-4 is required for the molecular and structural organization of rod and cone photoreceptor synapses. J. Neurosci. 2018;38:6145–6160. doi: 10.1523/JNEUROSCI.3818-16.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y., Fehlhaber K.E., Sarria I., Cao Y., Ingram N.T., Guerrero-Given D., Throesch B., Baldwin K., Kamasawa N., Ohtsuka T., Sampath A.P., Martemyanov K.A. The auxiliary calcium channel subunit alpha2delta4 is required for axonal elaboration, synaptic transmission, and wiring of rod photoreceptors. Neuron. 2017;93:1359–1374. doi: 10.1016/j.neuron.2017.02.021. e1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amhimmid Badr S., Waheeb Fahmi M., Mahmoud Nomir M., Mohammad El-Shishtawy M. Calcium channel alpha2delta1 subunit as a novel biomarker for diagnosis of hepatocellular carcinoma. Cancer Biol. Med. 2018;15:52–60. doi: 10.20892/j.issn.2095-3941.2017.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu D., Holm R., Goscinski M.A., Trope C.G., Nesland J.M., Suo Z. Prognostic and clinicopathological significance of Cacna2d1 expression in epithelial ovarian cancers: a retrospective study. Am. J. Cancer Res. 2016;6:2088–2097. [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao W., Wang L., Han H., Jin K., Lin N., Guo T., Chen Y., Cheng H., Lu F., Fang W., Wang Y., Xing B., Zhang Z. 1B50-1, a mAb raised against recurrent tumor cells, targets liver tumor-initiating cells by binding to the calcium channel alpha2delta1 subunit. Cancer Cell. 2013;23:541–556. doi: 10.1016/j.ccr.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 72.Sui X., Geng J.H., Li Y.H., Zhu G.Y., Wang W.H. Calcium channel alpha2delta1 subunit (CACNA2D1) enhances radioresistance in cancer stem-like cells in non-small cell lung cancer cell lines. Cancer Manag. Res. 2018;10:5009–5018. doi: 10.2147/CMAR.S176084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu J., Wang S., Zhao W., Duan J., Wang Z., Chen H., Tian Y., Wang D., Zhao J., An T., Bai H., Wu M., Wang J. Mechanistic exploration of cancer stem cell marker voltage-dependent calcium channel alpha2delta1 subunit-mediated chemotherapy resistance in small-cell lung cancer. Clin. Cancer Res. 2018;24:2148–2158. doi: 10.1158/1078-0432.CCR-17-1932. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y., Li L., Liang P., Zhai X., Li Y., Zhou Y. Differential expression of microRNAs in medulloblastoma and the potential functional consequences. Turk. Neurosurg. 2018;28:179–185. doi: 10.5137/1019-5149.JTN.19379-16.2. [DOI] [PubMed] [Google Scholar]
- 75.Ruan J., Liu X., Xiong X., Zhang C., Li J., Zheng H., Huang C., Shi Q., Weng Y. miR107 promotes the erythroid differentiation of leukemia cells via the downregulation of Cacna2d1. Mol. Med. Rep. 2015;11:1334–1339. doi: 10.3892/mmr.2014.2865. [DOI] [PubMed] [Google Scholar]
- 76.Warnier M., Roudbaraki M., Derouiche S., Delcourt P., Bokhobza A., Prevarskaya N., Mariot P. CACNA2D2 promotes tumorigenesis by stimulating cell proliferation and angiogenesis. Oncogene. 2015;34:5383–5394. doi: 10.1038/onc.2014.467. [DOI] [PubMed] [Google Scholar]
- 77.Carboni G.L., Gao B., Nishizaki M., Xu K., Minna J.D., Roth J.A., Ji L. CACNA2D2-mediated apoptosis in NSCLC cells is associated with alterations of the intracellular calcium signaling and disruption of mitochondria membrane integrity. Oncogene. 2003;22:615–626. doi: 10.1038/sj.onc.1206134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lerman M.I., Minna J.D. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res. 2000;60:6116–6133. [PubMed] [Google Scholar]
- 79.Mitra S., Mazumder Indra D., Basu P.S., Mondal R.K., Roy A., Roychoudhury S., Panda C.K. Alterations of RASSF1A in premalignant cervical lesions: clinical and prognostic significance. Mol. Carcinog. 2012;51:723–733. doi: 10.1002/mc.20837. [DOI] [PubMed] [Google Scholar]
- 80.Ghosh S., Ghosh A., Maiti G.P., Alam N., Roy A., Roy B., Roychoudhury S., Panda C.K. Alterations of 3p21.31 tumor suppressor genes in head and neck squamous cell carcinoma: correlation with progression and prognosis. Int. J. Cancer. 2008;123:2594–2604. doi: 10.1002/ijc.23834. [DOI] [PubMed] [Google Scholar]
- 81.Huang W., Jin Y., Yuan Y., Bai C., Wu Y., Zhu H., Lu S. Validation and target gene screening of hsa-miR-205 in lung squamous cell carcinoma. Chin. Med. J. 2014;127:272–278. [PubMed] [Google Scholar]
- 82.Lindskog C., Fagerberg L., Hallstrom B., Edlund K., Hellwig B., Rahnenfuhrer J., Kampf C., Uhlen M., Ponten F., Micke P. The lung-specific proteome defined by integration of transcriptomics and antibody-based profiling. FASEB J. 2014;28:5184–5196. doi: 10.1096/fj.14-254862. [DOI] [PubMed] [Google Scholar]
- 83.Cromer M.K., Choi M., Nelson-Williams C., Fonseca A.L., Kunstman J.W., Korah R.M., Overton J.D., Mane S., Kenney B., Malchoff C.D., Stalberg P., Akerstrom G., Westin G., Hellman P., Carling T., Bjorklund P., Lifton R.P. Neomorphic effects of recurrent somatic mutations in Yin Yang 1 in insulin-producing adenomas. Proc. Natl. Acad. Sci. U. S. A. 2015;112:4062–4067. doi: 10.1073/pnas.1503696112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong A.M., Kong K.L., Chen L., Liu M., Wong A.M., Zhu C., Tsang J.W., Guan X.Y. Characterization of CACNA2D3 as a putative tumor suppressor gene in the development and progression of nasopharyngeal carcinoma. Int. J. Cancer. 2013;133:2284–2295. doi: 10.1002/ijc.28252. [DOI] [PubMed] [Google Scholar]
- 85.Palmieri C., Rudraraju B., Monteverde M., Lattanzio L., Gojis O., Brizio R., Garrone O., Merlano M., Syed N., Lo Nigro C., Crook T. Methylation of the calcium channel regulatory subunit alpha2delta-3 (CACNA2D3) predicts site-specific relapse in oestrogen receptor-positive primary breast carcinomas. Br. J. Cancer. 2012;107:375–381. doi: 10.1038/bjc.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qin Y.R., Fu L., Sham P.C., Kwong D.L., Zhu C.L., Chu K.K., Li Y., Guan X.Y. Single-nucleotide polymorphism-mass array reveals commonly deleted regions at 3p22 and 3p14.2 associate with poor clinical outcome in esophageal squamous cell carcinoma. Int. J. Cancer. 2008;123:826–830. doi: 10.1002/ijc.23577. [DOI] [PubMed] [Google Scholar]
- 87.Li Y., Zhu C.L., Nie C.J., Li J.C., Zeng T.T., Zhou J., Chen J., Chen K., Fu L., Liu H., Qin Y., Guan X.Y. Investigation of tumor suppressing function of CACNA2D3 in esophageal squamous cell carcinoma. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wanajo A., Sasaki A., Nagasaki H., Shimada S., Otsubo T., Owaki S., Shimizu Y., Eishi Y., Kojima K., Nakajima Y., Kawano T., Yuasa Y., Akiyama Y. Methylation of the calcium channel-related gene, CACNA2D3, is frequent and a poor prognostic factor in gastric cancer. Gastroenterology. 2008;135:580–590. doi: 10.1053/j.gastro.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 89.Yuasa Y., Nagasaki H., Akiyama Y., Hashimoto Y., Takizawa T., Kojima K., Kawano T., Sugihara K., Imai K., Nakachi K. DNA methylation status is inversely correlated with green tea intake and physical activity in gastric cancer patients. Int. J. Cancer. 2009;124:2677–2682. doi: 10.1002/ijc.24231. [DOI] [PubMed] [Google Scholar]
- 90.Tai A.L., Mak W., Ng P.K., Chua D.T., Ng M.Y., Fu L., Chu K.K., Fang Y., Qiang Song Y., Chen M., Zhang M., Sham P.C., Guan X.Y. High-throughput loss-of-heterozygosity study of chromosome 3p in lung cancer using single-nucleotide polymorphism markers. Cancer Res. 2006;66:4133–4138. doi: 10.1158/0008-5472.CAN-05-2775. [DOI] [PubMed] [Google Scholar]
- 91.You H.L., Huang W.T., Liu T.T., Weng S.W., Eng H.L. Mutations of candidate tumor suppressor genes at chromosome 3p in intrahepatic cholangiocarcinoma. Exp. Mol. Pathol. 2017;103:249–254. doi: 10.1016/j.yexmp.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 92.Jin Y., Cui D., Ren J., Wang K., Zeng T., Gao L. CACNA2D3 is downregulated in gliomas and functions as a tumor suppressor. Mol. Carcinog. 2017;56:945–959. doi: 10.1002/mc.22548. [DOI] [PubMed] [Google Scholar]
- 93.Glossmann H., Striessnig J., Hymel L., Schindler H. Purified L-type calcium channels: only one single polypeptide (alpha 1-subunit) carries the drug receptor domains and is regulated by protein kinases. Biomed. Biochim. Acta. 1987;46:S351–356. [PubMed] [Google Scholar]
- 94.Campbell K.P., Sharp A.H., Leung A.T. 32,000-Dalton subunit of the 1,4-dihydropyridine receptor. Ann. N. Y. Acad. Sci. 1989;560:251–257. doi: 10.1111/j.1749-6632.1989.tb24102.x. [DOI] [PubMed] [Google Scholar]
- 95.Letts V.A., Felix R., Biddlecome G.H., Arikkath J., Mahaffey C.L., Valenzuela A., Bartlett F.S., 2nd, Mori Y., Campbell K.P., Frankel W.N. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat. Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 96.Klugbauer N., Dai S., Specht V., Lacinova L., Marais E., Bohn G., Hofmann F. A family of gamma-like calcium channel subunits. FEBS Lett. 2000;470:189–197. doi: 10.1016/s0014-5793(00)01306-5. [DOI] [PubMed] [Google Scholar]
- 97.Chu P.J., Robertson H.M., Best P.M. Calcium channel gamma subunits provide insights into the evolution of this gene family. Gene. 2001;280:37–48. doi: 10.1016/s0378-1119(01)00738-7. [DOI] [PubMed] [Google Scholar]
- 98.Burgess D.L., Gefrides L.A., Foreman P.J., Noebels J.L. A cluster of three novel Ca2+ channel gamma subunit genes on chromosome 19q13.4: evolution and expression profile of the gamma subunit gene family. Genomics. 2001;71:339–350. doi: 10.1006/geno.2000.6440. [DOI] [PubMed] [Google Scholar]
- 99.Sharp A.H., Black J.L., 3rd, Dubel S.J., Sundarraj S., Shen J.P., Yunker A.M., Copeland T.D., McEnery M.W. Biochemical and anatomical evidence for specialized voltage-dependent calcium channel gamma isoform expression in the epileptic and ataxic mouse, stargazer. Neuroscience. 2001;105:599–617. doi: 10.1016/s0306-4522(01)00220-2. [DOI] [PubMed] [Google Scholar]
- 100.Lin Z., Witschas K., Garcia T., Chen R.S., Hansen J.P., Sellers Z.M., Kuzmenkina E., Herzig S., Best P.M. A critical GxxxA motif in the gamma6 calcium channel subunit mediates its inhibitory effect on Cav3.1 calcium current. J. Physiol. 2008;586:5349–5366. doi: 10.1113/jphysiol.2008.159111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tselnicker I., Tsemakhovich V.A., Dessauer C.W., Dascal N. Stargazin modulates neuronal voltage-dependent Ca(2+) channel Ca(v)2.2 by a Gbetagamma-dependent mechanism. J. Biol. Chem. 2010;285:20462–20471. doi: 10.1074/jbc.M110.121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferron L., Davies A., Page K.M., Cox D.J., Leroy J., Waithe D., Butcher A.J., Sellaturay P., Bolsover S., Pratt W.S., Moss F.J., Dolphin A.C. The stargazin-related protein gamma 7 interacts with the mRNA-binding protein heterogeneous nuclear ribonucleoprotein A2 and regulates the stability of specific mRNAs, including CaV2.2. J. Neurosci. 2008;28:10604–10617. doi: 10.1523/JNEUROSCI.2709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eberst R., Dai S., Klugbauer N., Hofmann F. Identification and functional characterization of a calcium channel gamma subunit. Pflugers Arch. 1997;433:633–637. doi: 10.1007/s004240050324. [DOI] [PubMed] [Google Scholar]
- 104.Leitch B., Shevtsova O., Guevremont D., Williams J. Loss of calcium channels in the cerebellum of the ataxic and epileptic stargazer mutant mouse. Brain Res. 2009;1279:156–167. doi: 10.1016/j.brainres.2009.04.051. [DOI] [PubMed] [Google Scholar]
- 105.Moss F.J., Dolphin A.C., Clare J.J. Human neuronal stargazin-like proteins, gamma2, gamma3 and gamma4; an investigation of their specific localization in human brain and their influence on CaV2.1 voltage-dependent calcium channels expressed in Xenopus oocytes. BMC Neurosci. 2003;4:23. doi: 10.1186/1471-2202-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zheng C.Y., Chang K., Suh Y.H., Roche K.W. TARP gamma-8 glycosylation regulates the surface expression of AMPA receptors. Biochem. J. 2015;465:471–477. doi: 10.1042/BJ20140806. [DOI] [PubMed] [Google Scholar]
- 107.Riva I., Eibl C., Volkmer R., Carbone A.L., Plested A.J. Control of AMPA receptor activity by the extracellular loops of auxiliary proteins. eLife. 2017;6 doi: 10.7554/eLife.28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Waithe D., Ferron L., Dolphin A.C. Stargazin-related protein gamma(7) is associated with signalling endosomes in superior cervical ganglion neurons and modulates neurite outgrowth. J. Cell. Sci. 2011;124:2049–2057. doi: 10.1242/jcs.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Louros S.R., Caldeira G.L., Carvalho A.L. Stargazin dephosphorylation mediates homeostatic synaptic downscaling of excitatory synapses. Front. Mol. Neurosci. 2018;11:328. doi: 10.3389/fnmol.2018.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Omarini C., Bettelli S., Caprera C., Manfredini S., Caggia F., Guaitoli G., Moscetti L., Toss A., Cortesi L., Kaleci S., Maiorana A., Cascinu S., Conte P.F., Piacentini F. Clinical and molecular predictors of long-term response in HER2 positive metastatic breast cancer patients. Cancer Biol. Ther. 2018;19:879–886. doi: 10.1080/15384047.2018.1480287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X., Zhang M., Hou Y., Xu L., Li W., Zou Z., Liu C., Xu A., Wu S. Single-cell analyses of transcriptional heterogeneity in squamous cell carcinoma of urinary bladder. Oncotarget. 2016;7:66069–66076. doi: 10.18632/oncotarget.11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ling J., Wu X., Fu Z., Tan J., Xu Q. Systematic analysis of gene expression pattern in has-miR-197 over-expressed human uterine leiomyoma cells. Biomed. Pharmacother. 2015;75:226–233. doi: 10.1016/j.biopha.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 113.Kumar R.D., Searleman A.C., Swamidass S.J., Griffith O.L., Bose R. Statistically identifying tumor suppressors and oncogenes from pan-cancer genome-sequencing data. Bioinformatics. 2015;31:3561–3568. doi: 10.1093/bioinformatics/btv430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kitaura H., Sonoda M., Teramoto S., Shirozu H., Shimizu H., Kimura T., Masuda H., Ito Y., Takahashi H., Kwak S., Kameyama S., Kakita A. Ca(2+) -permeable AMPA receptors associated with epileptogenesis of hypothalamic hamartoma. Epilepsia. 2017;58:e59–e63. doi: 10.1111/epi.13700. [DOI] [PubMed] [Google Scholar]
- 115.Ruiz D.S., Luksch H., Sifringer M., Temme A., Staufner C., Rzeski W., Marzahn J., Grabarska A., Ikonomidou C., Stepulak A. AMPA receptor antagonist CFM-2 decreases survivin expression in Cancer cells. Anticancer Agents Med. Chem. 2018;18:591–596. doi: 10.2174/1871520618666180228123406. [DOI] [PubMed] [Google Scholar]
- 116.Aissaoui D., Mlayah-Bellalouna S., Jebali J., Abdelkafi-Koubaa Z., Souid S., Moslah W., Othman H., Luis J., ElAyeb M., Marrakchi N., Essafi-Benkhadir K., Srairi-Abid N. Functional role of Kv1.1 and Kv1.3 channels in the neoplastic progression steps of three cancer cell lines, elucidated by scorpion peptides. Int. J. Biol. Macromol. 2018;111:1146–1155. doi: 10.1016/j.ijbiomac.2018.01.144. [DOI] [PubMed] [Google Scholar]
- 117.Rosa P., Sforna L., Carlomagno S., Mangino G., Miscusi M., Pessia M., Franciolini F., Calogero A., Catacuzzeno L. Overexpression of large-conductance calcium-activated potassium channels in human glioblastoma stem-like cells and their role in cell migration. J. Cell. Physiol. 2017;232:2478–2488. doi: 10.1002/jcp.25592. [DOI] [PubMed] [Google Scholar]
- 118.Thuringer D., Chanteloup G., Boucher J., Pernet N., Boudesco C., Jego G., Chatelier A., Bois P., Gobbo J., Cronier L., Solary E., Garrido C. Modulation of the inwardly rectifying potassium channel Kir4.1 by the pro-invasive miR-5096 in glioblastoma cells. Oncotarget. 2017;8:37681–37693. doi: 10.18632/oncotarget.16949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.An W.F., Bowlby M.R., Betty M., Cao J., Ling H.P., Mendoza G., Hinson J.W., Mattsson K.I., Strassle B.W., Trimmer J.S., Rhodes K.J. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 120.Chen H., Kim L.A., Rajan S., Xu S., Goldstein S.A. Charybdotoxin binding in the I(Ks) pore demonstrates two MinK subunits in each channel complex. Neuron. 2003;40:15–23. doi: 10.1016/s0896-6273(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 121.Rhodes K.J., Strassle B.W., Monaghan M.M., Bekele-Arcuri Z., Matos M.F., Trimmer J.S. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J. Neurosci. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bahring R., Vardanyan V., Pongs O. Differential modulation of Kv1 channel-mediated currents by co-expression of Kvbeta3 subunit in a mammalian cell-line. Mol. Membr. Biol. 2004;21:19–25. doi: 10.1080/09687680310001597749. [DOI] [PubMed] [Google Scholar]
- 123.Bavassano C., Marvaldi L., Langeslag M., Sarg B., Lindner H., Klimaschewski L., Kress M., Ferrer-Montiel A., Knaus H.G. Identification of voltage-gated K(+) channel beta 2 (Kvbeta2) subunit as a novel interaction partner of the pain transducer Transient Receptor Potential Vanilloid 1 channel (TRPV1) Biochim. Biophys. Acta. 2013;1833:3166–3175. doi: 10.1016/j.bbamcr.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 124.Kisselbach J., Schweizer P.A., Gerstberger R., Becker R., Katus H.A., Thomas D. Enhancement of K2P2.1 (TREK1) background currents expressed in Xenopus oocytes by voltage-gated K+ channel beta subunits. Life Sci. 2012;91:377–383. doi: 10.1016/j.lfs.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 125.Wang L., Takimoto K., Levitan E.S. Differential association of the auxiliary subunit Kvbeta2 with Kv1.4 and Kv4.3 K+ channels. FEBS Lett. 2003;547:162–164. doi: 10.1016/s0014-5793(03)00705-1. [DOI] [PubMed] [Google Scholar]
- 126.Lewis A., McCrossan Z.A., Abbott G.W. MinK, MiRP1, and MiRP2 diversify Kv3.1 and Kv3.2 potassium channel gating. J. Biol. Chem. 2004;279:7884–7892. doi: 10.1074/jbc.M310501200. [DOI] [PubMed] [Google Scholar]
- 127.Wu J., Chen Z., Liu Q., Zeng W., Wu X., Lin B. Silencing of Kv1.5 gene inhibits proliferation and induces apoptosis of osteosarcoma cells. Int. J. Mol. Sci. 2015;16:26914–26926. doi: 10.3390/ijms161126002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim H.J., Jang S.H., Jeong Y.A., Ryu P.D., Kim D.Y., Lee S.Y. Involvement of Kv4.1 K(+) channels in gastric cancer cell proliferation. Biol. Pharm. Bull. 2010;33:1754–1757. doi: 10.1248/bpb.33.1754. [DOI] [PubMed] [Google Scholar]
- 129.Shimizu T., Fujii T., Takahashi Y., Takahashi Y., Suzuki T., Ukai M., Tauchi K., Horikawa N., Tsukada K., Sakai H. Up-regulation of Kv7.1 channels in thromboxane A2-induced colonic cancer cell proliferation. Pflugers Arch. 2014;466:541–548. doi: 10.1007/s00424-013-1341-x. [DOI] [PubMed] [Google Scholar]
- 130.Gulbis J.M., Zhou M., Mann S., MacKinnon R. Structure of the cytoplasmic beta subunit-T1 assembly of voltage-dependent K+ channels. Science. 2000;289:123–127. doi: 10.1126/science.289.5476.123. [DOI] [PubMed] [Google Scholar]
- 131.Yang E.K., Alvira M.R., Levitan E.S., Takimoto K. Kvbeta subunits increase expression of Kv4.3 channels by interacting with their C termini. J. Biol. Chem. 2001;276:4839–4844. doi: 10.1074/jbc.M004768200. [DOI] [PubMed] [Google Scholar]
- 132.Shi G., Nakahira K., Hammond S., Rhodes K.J., Schechter L.E., Trimmer J.S. Beta subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- 133.Nystoriak M.A., Zhang D., Jagatheesan G., Bhatnagar A. Heteromeric complexes of aldo-keto reductase auxiliary KVbeta subunits (AKR6A) regulate sarcolemmal localization of KV1.5 in coronary arterial myocytes. Chem. Biol. Interact. 2017;276:210–217. doi: 10.1016/j.cbi.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gu C., Zhou W., Puthenveedu M.A., Xu M., Jan Y.N., Jan L.Y. The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron. 2006;52:803–816. doi: 10.1016/j.neuron.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 135.Aimond F., Kwak S.P., Rhodes K.J., Nerbonne J.M. Accessory Kvbeta1 subunits differentially modulate the functional expression of voltage-gated K+ channels in mouse ventricular myocytes. Circ. Res. 2005;96:451–458. doi: 10.1161/01.RES.0000156890.25876.63. [DOI] [PubMed] [Google Scholar]
- 136.Rettig J., Heinemann S.H., Wunder F., Lorra C., Parcej D.N., Dolly J.O., Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- 137.Leicher T., Bahring R., Isbrandt D., Pongs O. Coexpression of the KCNA3B gene product with Kv1.5 leads to a novel A-type potassium channel. J. Biol. Chem. 1998;273:35095–35101. doi: 10.1074/jbc.273.52.35095. [DOI] [PubMed] [Google Scholar]
- 138.Peters C.J., Vaid M., Horne A.J., Fedida D., Accili E.A. The molecular basis for the actions of KVbeta1.2 on the opening and closing of the KV1.2 delayed rectifier channel. Channels (Austin, Tex.) 2009;3:314–322. doi: 10.4161/chan.3.5.9558. [DOI] [PubMed] [Google Scholar]
- 139.Heinemann S.H., Rettig J., Graack H.R., Pongs O. Functional characterization of Kv channel beta-subunits from rat brain. J. Physiol. 1996;493(Pt 3):625–633. doi: 10.1113/jphysiol.1996.sp021409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grande M., Suarez E., Vicente R., Canto C., Coma M., Tamkun M.M., Zorzano A., Guma A., Felipe A. Voltage-dependent K+ channel beta subunits in muscle: differential regulation during postnatal development and myogenesis. J. Cell. Physiol. 2003;195:187–193. doi: 10.1002/jcp.10203. [DOI] [PubMed] [Google Scholar]
- 141.Tur J., Chapalamadugu K.C., Padawer T., Badole S.L., Kilfoil P.J., 2nd, Bhatnagar A., Tipparaju S.M. Deletion of Kvbeta1.1 subunit leads to electrical and haemodynamic changes causing cardiac hypertrophy in female murine hearts. Exp. Physiol. 2016;101:494–508. doi: 10.1113/EP085405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chapalamadugu K.C., Tur J., Badole S.L., Kukreja R.C., Brotto M., Tipparaju S.M. Physiological role of Kvbeta2 (AKR6) in murine skeletal muscle growth and regulation. Acta Physiol. Oxf. (Oxf) 2018;224 doi: 10.1111/apha.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tipparaju S.M., Saxena N., Liu S.Q., Kumar R., Bhatnagar A. Differential regulation of voltage-gated K+ channels by oxidized and reduced pyridine nucleotide coenzymes, American journal of physiology. Cell Physiol. 2005;288:C366–376. doi: 10.1152/ajpcell.00354.2004. [DOI] [PubMed] [Google Scholar]
- 144.Tipparaju S.M., Li X.P., Kilfoil P.J., Xue B., Uversky V.N., Bhatnagar A., Barski O.A. Interactions between the C-terminus of Kv1.5 and Kvbeta regulate pyridine nucleotide-dependent changes in channel gating. Pflugers Arch. 2012;463:799–818. doi: 10.1007/s00424-012-1093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Borup R., Rossing M., Henao R., Yamamoto Y., Krogdahl A., Godballe C., Winther O., Kiss K., Christensen L., Hogdall E., Bennedbaek F., Nielsen F.C. Molecular signatures of thyroid follicular neoplasia. Endocr. Relat. Cancer. 2010;17:691–708. doi: 10.1677/ERC-09-0288. [DOI] [PubMed] [Google Scholar]
- 146.Pfeifer A., Wojtas B., Oczko-Wojciechowska M., Kukulska A., Czarniecka A., Eszlinger M., Musholt T., Stokowy T., Swierniak M., Stobiecka E., Rusinek D., Tyszkiewicz T., Kowal M., Jarzab M., Hauptmann S., Lange D., Paschke R., Jarzab B. Molecular differential diagnosis of follicular thyroid carcinoma and adenoma based on gene expression profiling by using formalin-fixed paraffin-embedded tissues. BMC Med. Genomics. 2013;6:38. doi: 10.1186/1755-8794-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ling C., Pease M., Shi L., Punj V., Shiroishi M.S., Commins D., Weisenberger D.J., Wang K., Zada G. A pilot genome-scale profiling of DNA methylation in sporadic pituitary macroadenomas: association with tumor invasion and histopathological subtype. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.White P.S., Thompson P.M., Gotoh T., Okawa E.R., Igarashi J., Kok M., Winter C., Gregory S.G., Hogarty M.D., Maris J.M., Brodeur G.M. Definition and characterization of a region of 1p36.3 consistently deleted in neuroblastoma. Oncogene. 2005;24:2684–2694. doi: 10.1038/sj.onc.1208306. [DOI] [PubMed] [Google Scholar]
- 149.Towle R., Truong D., Hogg K., Robinson W.P., Poh C.F., Garnis C. Global analysis of DNA methylation changes during progression of oral cancer. Oral Oncol. 2013;49:1033–1042. doi: 10.1016/j.oraloncology.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 150.Morin T.J., Kobertz W.R. Counting membrane-embedded KCNE beta-subunits in functioning K+ channel complexes. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1478–1482. doi: 10.1073/pnas.0710366105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bendahhou S., Marionneau C., Haurogne K., Larroque M.M., Derand R., Szuts V., Escande D., Demolombe S., Barhanin J. In vitro molecular interactions and distribution of KCNE family with KCNQ1 in the human heart. Cardiovasc. Res. 2005;67:529–538. doi: 10.1016/j.cardiores.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 152.Drici M.D., Arrighi I., Chouabe C., Mann J.R., Lazdunski M., Romey G., Barhanin J. Involvement of IsK-associated K+ channel in heart rate control of repolarization in a murine engineered model of Jervell and Lange-Nielsen syndrome. Circ. Res. 1998;83:95–102. doi: 10.1161/01.res.83.1.95. [DOI] [PubMed] [Google Scholar]
- 153.Vetter D.E., Mann J.R., Wangemann P., Liu J., McLaughlin K.J., Lesage F., Marcus D.C., Lazdunski M., Heinemann S.F., Barhanin J. Inner ear defects induced by null mutation of the isk gene. Neuron. 1996;17:1251–1264. doi: 10.1016/s0896-6273(00)80255-x. [DOI] [PubMed] [Google Scholar]
- 154.Vallon V., Grahammer F., Richter K., Bleich M., Lang F., Barhanin J., Volkl H., Warth R. Role of KCNE1-dependent K+ fluxes in mouse proximal tubule. J. Am. Soc. Nephrol. 2001;12:2003–2011. doi: 10.1681/ASN.V12102003. [DOI] [PubMed] [Google Scholar]
- 155.Arrighi I., Bloch-Faure M., Grahammer F., Bleich M., Warth R., Mengual R., Drici M.D., Barhanin J., Meneton P. Altered potassium balance and aldosterone secretion in a mouse model of human congenital long QT syndrome. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8792–8797. doi: 10.1073/pnas.141233398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bianchi L., Shen Z., Dennis A.T., Priori S.G., Napolitano C., Ronchetti E., Bryskin R., Schwartz P.J., Brown A.M. Cellular dysfunction of LQT5-minK mutants: abnormalities of IKs, IKr and trafficking in long QT syndrome. Hum. Mol. Genet. 1999;8:1499–1507. doi: 10.1093/hmg/8.8.1499. [DOI] [PubMed] [Google Scholar]
- 157.Tyson J., Tranebjaerg L., Bellman S., Wren C., Taylor J.F., Bathen J., Aslaksen B., Sorland S.J., Lund O., Malcolm S., Pembrey M., Bhattacharya S., Bitner-Glindzicz M. IsK and KvLQT1: mutation in either of the two subunits of the slow component of the delayed rectifier potassium channel can cause Jervell and Lange-Nielsen syndrome. Hum. Mol. Genet. 1997;6:2179–2185. doi: 10.1093/hmg/6.12.2179. [DOI] [PubMed] [Google Scholar]
- 158.Suzuki T., Takimoto K. Selective expression of HERG and Kv2 channels influences proliferation of uterine cancer cells. Int. J. Oncol. 2004;25:153–159. [PubMed] [Google Scholar]
- 159.Biasiotta A., D’Arcangelo D., Passarelli F., Nicodemi E.M., Facchiano A. Ion channels expression and function are strongly modified in solid tumors and vascular malformations. J. Transl. Med. 2016;14:285. doi: 10.1186/s12967-016-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stathopoulos A., Melas C., Attali B., Blum D., Levivier M., Brotchi J., Velu T., Tenenbaum L. Overexpression of mouse IsK protein fused to green fluorescent protein induces apoptosis of human astroglioma cells. Neurol. Res. 2007;29:628–631. doi: 10.1179/016164107X166326. [DOI] [PubMed] [Google Scholar]
- 161.Shinawi M., Erez A., Shardy D.L., Lee B., Naeem R., Weissenberger G., Chinault A.C., Cheung S.W., Plon S.E. Syndromic thrombocytopenia and predisposition to acute myelogenous leukemia caused by constitutional microdeletions on chromosome 21q. Blood. 2008;112:1042–1047. doi: 10.1182/blood-2008-01-135970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maeno E., Ishizaki Y., Kanaseki T., Hazama A., Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Du C., El Harchi A., Zhang H., Hancox J.C. Modification by KCNE1 variants of the hERG potassium channel response to premature stimulation and to pharmacological inhibition. Physiol. Rep. 2013;1 doi: 10.1002/phy2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Afrasiabi E., Hietamaki M., Viitanen T., Sukumaran P., Bergelin N., Tornquist K. Expression and significance of HERG (KCNH2) potassium channels in the regulation of MDA-MB-435S melanoma cell proliferation and migration. Cell. Signal. 2010;22:57–64. doi: 10.1016/j.cellsig.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 165.Li X., Cai H., Zheng W., Tong M., Li H., Ao L., Li J., Hong G., Li M., Guan Q., Yang S., Yang D., Lin X., Guo Z. An individualized prognostic signature for gastric cancer patients treated with 5-Fluorouracil-based chemotherapy and distinct multi-omics characteristics of prognostic groups. Oncotarget. 2016;7:8743–8755. doi: 10.18632/oncotarget.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kuwahara N., Kitazawa R., Fujiishi K., Nagai Y., Haraguchi R., Kitazawa S. Gastric adenocarcinoma arising in gastritis cystica profunda presenting with selective loss of KCNE2 expression. World J. Gastroenterol. 2013;19:1314–1317. doi: 10.3748/wjg.v19.i8.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yanglin P., Lina Z., Zhiguo L., Na L., Haifeng J., Guoyun Z., Jie L., Jun W., Tao L., Li S., Taidong Q., Jianhong W., Daiming F. KCNE2, a down-regulated gene identified by in silico analysis, suppressed proliferation of gastric cancer cells. Cancer Lett. 2007;246:129–138. doi: 10.1016/j.canlet.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 168.Roepke T.K., Purtell K., King E.C., La Perle K.M., Lerner D.J., Abbott G.W. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Heitzmann D., Grahammer F., von Hahn T., Schmitt-Graff A., Romeo E., Nitschke R., Gerlach U., Lang H.J., Verrey F., Barhanin J., Warth R. Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells. J. Physiol. 2004;561:547–557. doi: 10.1113/jphysiol.2004.075168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Roepke T.K., Anantharam A., Kirchhoff P., Busque S.M., Young J.B., Geibel J.P., Lerner D.J., Abbott G.W. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J. Biol. Chem. 2006;281:23740–23747. doi: 10.1074/jbc.M604155200. [DOI] [PubMed] [Google Scholar]
- 171.Holmqvist M.H., Cao J., Hernandez-Pineda R., Jacobson M.D., Carroll K.I., Sung M.A., Betty M., Ge P., Gilbride K.J., Brown M.E., Jurman M.E., Lawson D., Silos-Santiago I., Xie Y., Covarrubias M., Rhodes K.J., Distefano P.S., An W.F. Elimination of fast inactivation in Kv4 A-type potassium channels by an auxiliary subunit domain. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1035–1040. doi: 10.1073/pnas.022509299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fontan-Lozano A., Capilla-Gonzalez V., Aguilera Y., Mellado N., Carrion A.M., Soria B., Hmadcha A. Impact of transient down-regulation of DREAM in human embryonic stem cell pluripotency: the role of DREAM in the maintenance of hESCs. Stem Cell Res. 2016;16:568–578. doi: 10.1016/j.scr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 173.Carrion A.M., Link W.A., Ledo F., Mellstrom B., Naranjo J.R. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- 174.Link W.A., Ledo F., Torres B., Palczewska M., Madsen T.M., Savignac M., Albar J.P., Mellstrom B., Naranjo J.R. Day-night changes in downstream regulatory element antagonist modulator/potassium channel interacting protein activity contribute to circadian gene expression in pineal gland. J. Neurosci. 2004;24:5346–5355. doi: 10.1523/JNEUROSCI.1460-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Craig T.A., Ramachandran P.L., Bergen H.R., 3rd, Podratz J.L., Windebank A.J., Kumar R. The regulation of apoptosis by the downstream regulatory element antagonist modulator/potassium channel interacting protein 3 (DREAM/KChIP3) through interactions with hexokinase I. Biochem. Biophys. Res. Commun. 2013;433:508–512. doi: 10.1016/j.bbrc.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jo D.G., Kim M.J., Choi Y.H., Kim I.K., Song Y.H., Woo H.N., Chung C.W., Jung Y.K. Pro-apoptotic function of calsenilin/DREAM/KChIP3. FASEB J. 2001;15:589–591. doi: 10.1096/fj.00-0541fje. [DOI] [PubMed] [Google Scholar]
- 177.Kim H.J., Lee W.H., Kim M.J., Shin S., Jang B., Park J.B., Wasco W., Buxbaum J.D., Kim Y.S., Choi E.K. Calsenilin, a presenilin interactor, regulates RhoA signaling and neurite outgrowth. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim K., Tseng A., Barazia A., Italiano J.E., Cho J. DREAM plays an important role in platelet activation and thrombogenesis. Blood. 2017;129:209–225. doi: 10.1182/blood-2016-07-724419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bonne A., Vreede L., Kuiper R.P., Bodmer D., Jansen C., Eleveld M., van Erp F., Arkesteijn G., Hoogerbrugge N., van Ravenswaaij C., Schoenmakers E.F., Geurts van Kessel A. Mapping of constitutional translocation breakpoints in renal cell cancer patients: identification of KCNIP4 as a candidate gene. Cancer Genet. Cytogenet. 2007;179:11–18. doi: 10.1016/j.cancergencyto.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 180.Neant I., Haiech J., Kilhoffer M.C., Aulestia F.J., Moreau M., Leclerc C. Ca(2+)-Dependent transcriptional repressors KCNIP and regulation of prognosis genes in glioblastoma. Front. Mol. Neurosci. 2018;11:472. doi: 10.3389/fnmol.2018.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Magleby K.L. Gating mechanism of BK (Slo1) channels: so near, yet so far. J. Gen. Physiol. 2003;121:81–96. doi: 10.1085/jgp.20028721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yan J., Aldrich R.W. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature. 2010;466:513–516. doi: 10.1038/nature09162. [DOI] [PubMed] [Google Scholar]
- 183.Typlt M., Mirkowski M., Azzopardi E., Ruettiger L., Ruth P., Schmid S. Mice with deficient BK channel function show impaired prepulse inhibition and spatial learning, but normal working and spatial reference memory. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cao X.H., Chen S.R., Li L., Pan H.L. Nerve injury increases brain-derived neurotrophic factor levels to suppress BK channel activity in primary sensory neurons. J. Neurochem. 2012;121:944–953. doi: 10.1111/j.1471-4159.2012.07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Brenner R., Perez G.J., Bonev A.D., Eckman D.M., Kosek J.C., Wiler S.W., Patterson A.J., Nelson M.T., Aldrich R.W. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 186.Goda A.A., Siddique A.B., Mohyeldin M., Ayoub N.M., El Sayed K.A. The Maxi-K (BK) channel antagonist penitrem a as a novel breast cancer-targeted therapeutic. Mar. Drugs. 2018;16 doi: 10.3390/md16050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Orio P., Latorre R. Differential effects of beta 1 and beta 2 subunits on BK channel activity. J. Gen. Physiol. 2005;125:395–411. doi: 10.1085/jgp.200409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Uebele V.N., Lagrutta A., Wade T., Figueroa D.J., Liu Y., McKenna E., Austin C.P., Bennett P.B., Swanson R. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J. Biol. Chem. 2000;275:23211–23218. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- 189.Wang B., Rothberg B.S., Brenner R. Mechanism of beta4 subunit modulation of BK channels. J. Gen. Physiol. 2006;127:449–465. doi: 10.1085/jgp.200509436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yan J., Aldrich R.W. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc. Natl. Acad. Sci. U. S. A. 2012;109:7917–7922. doi: 10.1073/pnas.1205435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Khatun A., Shimozawa M., Kito H., Kawaguchi M., Fujimoto M., Ri M., Kajikuri J., Niwa S., Fujii M., Ohya S. Transcriptional repression and protein degradation of the Ca(2+)-Activated K(+) channel KCa1.1 by androgen receptor inhibition in human breast Cancer cells. Front. Physiol. 2018;9:312. doi: 10.3389/fphys.2018.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Egland K.A., Liu X.F., Squires S., Nagata S., Man Y.G., Bera T.K., Onda M., Vincent J.J., Strausberg R.L., Lee B., Pastan I. High expression of a cytokeratin-associated protein in many cancers. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5929–5934. doi: 10.1073/pnas.0601296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Anaganti S., Hansen J.K., Ha D., Hahn Y., Chertov O., Pastan I., Bera T.K. Non-AUG translational initiation of a short CAPC transcript generating protein isoform. Biochem. Biophys. Res. Commun. 2009;380:508–513. doi: 10.1016/j.bbrc.2009.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Miyagawa Y., Matsushita Y., Suzuki H., Komatsu M., Yoshimaru T., Kimura R., Yanai A., Honda J., Tangoku A., Sasa M., Miyoshi Y., Katagiri T. Frequent downregulation of LRRC26 by epigenetic alterations is involved in the malignant progression of triple-negative breast cancer. Int. J. Oncol. 2018 doi: 10.3892/ijo.2018.4301. [DOI] [PubMed] [Google Scholar]
- 195.Liu X.F., Xiang L., Zhang Y., Becker K.G., Bera T.K., Pastan I. CAPC negatively regulates NF-kappaB activation and suppresses tumor growth and metastasis. Oncogene. 2012;31:1673–1682. doi: 10.1038/onc.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dorschner H., Brekardin E., Uhde I., Schwanstecher C., Schwanstecher M. Stoichiometry of sulfonylurea-induced ATP-sensitive potassium channel closure. Mol. Pharmacol. 1999;55:1060–1066. doi: 10.1124/mol.55.6.1060. [DOI] [PubMed] [Google Scholar]
- 197.Hagiwara S., Miyazaki S., Rosenthal N.P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J. Gen. Physiol. 1976;67:621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sancho M., Gao Y., Hald B.O., Yin H., Boulton M., Steven D., MacDougall K., Parrent A., Pickering J.G., Welsh D.G. An assessment of KIR channel function in human cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2019 doi: 10.1152/ajpheart.00022.2019. [DOI] [PubMed] [Google Scholar]
- 199.Amarillo Y., Tissone A.I., Mato G., Nadal M.S. Inward rectifier potassium current IKir promotes intrinsic pacemaker activity of thalamocortical neurons. J. Neurophysiol. 2018;119:2358–2372. doi: 10.1152/jn.00867.2017. [DOI] [PubMed] [Google Scholar]
- 200.Ji Y., Takanari H., Qile M., Nalos L., Houtman M.J.C., Romunde F.L., Heukers R., van Bergen En Henegouwen P.M.P., Vos M.A., van der Heyden M.A.G. Class III antiarrhythmic drugs amiodarone and dronedarone impair KIR 2.1 backward trafficking. J. Cell. Mol. Med. 2017;21:2514–2523. doi: 10.1111/jcmm.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li C.G., Cui W.Y., Wang H. Sensitivity of KATP channels to cellular metabolic disorders and the underlying structural basis. Acta Pharmacol. Sin. 2016;37:134–142. doi: 10.1038/aps.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tabak G., Keren-Raifman T., Kahanovitch U., Dascal N. Mutual action by Ggamma and Gbeta for optimal activation of GIRK channels in a channel subunit-specific manner. Sci. Rep. 2019;9:508. doi: 10.1038/s41598-018-36833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liu X., Duan P., Hu X., Li R., Zhu Q. Altered KATP channel subunits expression and vascular reactivity in spontaneously hypertensive rats with age. J. Cardiovasc. Pharmacol. 2016;68:143–149. doi: 10.1097/FJC.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ostrovskaya O.I., Orlandi C., Fajardo-Serrano A., Young S.M., Jr., Lujan R., Martemyanov K.A. Inhibitory signaling to ion channels in hippocampal neurons is differentially regulated by alternative macromolecular complexes of RGS7. J. Neurosci. 2018;38:10002–10015. doi: 10.1523/JNEUROSCI.1378-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang W., Touhara K.K., Weir K., Bean B.P., MacKinnon R. Cooperative regulation by G proteins and Na(+) of neuronal GIRK2 K(+) channels. eLife. 2016;5 doi: 10.7554/eLife.15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zerangue N., Schwappach B., Jan Y.N., Jan L.Y. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 207.Masia R., Enkvetchakul D., Nichols C.G. Differential nucleotide regulation of KATP channels by SUR1 and SUR2A. J. Mol. Cell. Cardiol. 2005;39:491–501. doi: 10.1016/j.yjmcc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 208.Burke M.A., Mutharasan R.K., Ardehali H. The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the KATP channel. Circ. Res. 2008;102:164–176. doi: 10.1161/CIRCRESAHA.107.165324. [DOI] [PubMed] [Google Scholar]
- 209.Thompson E.M., Pishko G.L., Muldoon L.L., Neuwelt E.A. Inhibition of SUR1 decreases the vascular permeability of cerebral metastases. Neoplasia. 2013;15:535–543. doi: 10.1593/neo.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hambrock A., de Oliveira Franz C.B., Hiller S., Grenz A., Ackermann S., Schulze D.U., Drews G., Osswald H. Resveratrol binds to the sulfonylurea receptor (SUR) and induces apoptosis in a SUR subtype-specific manner. J. Biol. Chem. 2007;282:3347–3356. doi: 10.1074/jbc.M608216200. [DOI] [PubMed] [Google Scholar]
- 211.Park S.H., Ramachandran S., Kwon S.H., Cha S.D., Seo E.W., Bae I., Cho C., Song D.K. Upregulation of ATP-sensitive potassium channels for estrogen-mediated cell proliferation in human uterine leiomyoma cells. Gynecol. Endocrinol. 2008;24:250–256. doi: 10.1080/09513590801893315. [DOI] [PubMed] [Google Scholar]
- 212.Nunez M., Medina V., Cricco G., Croci M., Cocca C., Rivera E., Bergoc R., Martin G. Glibenclamide inhibits cell growth by inducing G0/G1 arrest in the human breast cancer cell line MDA-MB-231. BMC Pharmacol. Toxicol. 2013;14:6. doi: 10.1186/2050-6511-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vazquez-Sanchez A.Y., Hinojosa L.M., Parraguirre-Martinez S., Gonzalez A., Morales F., Montalvo G., Vera E., Hernandez-Gallegos E., Camacho J. Expression of KATP channels in human cervical cancer: potential tools for diagnosis and therapy. Oncol. Lett. 2018;15:6302–6308. doi: 10.3892/ol.2018.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cocca C., Martin G., Nunez M., Gutierrez A., Cricco G., Mohamad N., Medina V., Croci M., Crescenti E., Rivera E., Bergoc R. Effect of glibenclamide on N-nitroso-N-methylurea-induced mammary tumors in diabetic and nondiabetic rats. Oncol. Res. 2005;15:301–311. doi: 10.3727/096504005776404526. [DOI] [PubMed] [Google Scholar]
- 215.Li C.J., Zhou H.L., Li J., Yao H.T., Su R., Li W.P. Roles of sulfonylurea receptor 1 and multidrug resistance protein 1 in modulating insulin secretion in human insulinoma. HBPD INT. 2011;10:88–94. doi: 10.1016/s1499-3872(11)60013-1. [DOI] [PubMed] [Google Scholar]
- 216.Xu S., Liu C., Ma Y., Ji H.L., Li X. Potential roles of amiloride-sensitive sodium channels in Cancer development. Biomed Res. Int. 2016;2016 doi: 10.1155/2016/2190216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nelson M., Yang M., Millican-Slater R., Brackenbury W.J. Nav1.5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget. 2015;6:32914–32929. doi: 10.18632/oncotarget.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hodgkin A.L., Huxley A.F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 1952;116:449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Das S., Gilchrist J., Bosmans F., Van Petegem F. Binary architecture of the Nav1.2-beta2 signaling complex. Elife. 2016;5 doi: 10.7554/eLife.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yu F.H., Westenbroek R.E., Silos-Santiago I., McCormick K.A., Lawson D., Ge P., Ferriera H., Lilly J., DiStefano P.S., Catterall W.A., Scheuer T., Curtis R. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J. Neurosci. 2003;23:7577–7585. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu Q., Jin Y., Wang K., Meng X.X., Yang Y., Yang Z., Zhao Y.S., Zhao M.Y., Zhang J.H. Study of the residues involved in the binding of beta1 to beta3 subunits in the sodium channel. C. R. Biol. 2014;337:73–77. doi: 10.1016/j.crvi.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 222.Zhu W., Voelker T.L., Varga Z., Schubert A.R., Nerbonne J.M., Silva J.R. Mechanisms of noncovalent beta subunit regulation of NaV channel gating. J. Gen. Physiol. 2017 doi: 10.1085/jgp.201711802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hartshorne R.P., Messner D.J., Coppersmith J.C., Catterall W.A. The saxitoxin receptor of the sodium channel from rat brain. Evidence for two nonidentical beta subunits. J. Biol. Chem. 1982;257:13888–13891. [PubMed] [Google Scholar]
- 224.Meisler M.H., O’Brien J.E., Sharkey L.M. Sodium channel gene family: epilepsy mutations, gene interactions and modifier effects. J. Physiol. 2010;588:1841–1848. doi: 10.1113/jphysiol.2010.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lin X., O’Malley H., Chen C., Auerbach D., Foster M., Shekhar A., Zhang M., Coetzee W., Jalife J., Fishman G.I., Isom L., Delmar M. Scn1b deletion leads to increased tetrodotoxin-sensitive sodium current, altered intracellular calcium homeostasis and arrhythmias in murine hearts. J. Physiol. 2015;593:1389–1407. doi: 10.1113/jphysiol.2014.277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Meadows L., Malhotra J.D., Stetzer A., Isom L.L., Ragsdale D.S. The intracellular segment of the sodium channel beta 1 subunit is required for its efficient association with the channel alpha subunit. J. Neurochem. 2001;76:1871–1878. doi: 10.1046/j.1471-4159.2001.00192.x. [DOI] [PubMed] [Google Scholar]
- 227.Dulsat G., Palomeras S., Cortada E., Riuro H., Brugada R., Verges M. Trafficking and localisation to the plasma membrane of Nav 1.5 promoted by the beta2 subunit is defective due to a beta2 mutation associated with Brugada syndrome. Biol. Cell. 2017;109:273–291. doi: 10.1111/boc.201600085. [DOI] [PubMed] [Google Scholar]
- 228.Ishikawa T., Takahashi N., Ohno S., Sakurada H., Nakamura K., On Y.K., Park J.E., Makiyama T., Horie M., Arimura T., Makita N., Kimura A. Novel SCN3B mutation associated with brugada syndrome affects intracellular trafficking and function of Nav1.5. Circ. J. 2013;77:959–967. doi: 10.1253/circj.cj-12-0995. [DOI] [PubMed] [Google Scholar]
- 229.Fahmi A.I., Patel M., Stevens E.B., Fowden A.L., John J.E., 3rd, Lee K., Pinnock R., Morgan K., Jackson A.P., Vandenberg J.I. The sodium channel beta-subunit SCN3b modulates the kinetics of SCN5a and is expressed heterogeneously in sheep heart. J. Physiol. 2001;537:693–700. doi: 10.1111/j.1469-7793.2001.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Isom L.L., Ragsdale D.S., De Jongh K.S., Westenbroek R.E., Reber B.F., Scheuer T., Catterall W.A. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 1995;83:433–442. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 231.Bon E., Driffort V., Gradek F., Martinez-Caceres C., Anchelin M., Pelegrin P., Cayuela M.L., Marionneau-Lambot S., Oullier T., Guibon R., Fromont G., Gutierrez-Pajares J.L., Domingo I., Piver E., Moreau A., Burlaud-Gaillard J., Frank P.G., Chevalier S., Besson P., Roger S. SCN4B acts as a metastasis-suppressor gene preventing hyperactivation of cell migration in breast cancer. Nat. Commun. 2016;7:13648. doi: 10.1038/ncomms13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Laedermann C.J., Syam N., Pertin M., Decosterd I., Abriel H. beta1- and beta3- voltage-gated sodium channel subunits modulate cell surface expression and glycosylation of Nav1.7 in HEK293 cells. Front. Cell. Neurosci. 2013;7:137. doi: 10.3389/fncel.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Merrick E.C., Kalmar C.L., Snyder S.L., Cusdin F.S., Yu E.J., Sando J.J., Isakson B.E., Jackson A.P., Patel M.K. The importance of serine 161 in the sodium channel beta3 subunit for modulation of Na(V)1.2 gating. Pflugers Arch. 2010;460:743–753. doi: 10.1007/s00424-009-0739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Isom L.L., De Jongh K.S., Patton D.E., Reber B.F., Offord J., Charbonneau H., Walsh K., Goldin A.L., Catterall W.A. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science. 1992;256:839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- 235.Zhao J., O’Leary M.E., Chahine M. Regulation of Nav1.6 and Nav1.8 peripheral nerve Na+ channels by auxiliary beta-subunits. J. Neurophysiol. 2011;106:608–619. doi: 10.1152/jn.00107.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cusdin F.S., Nietlispach D., Maman J., Dale T.J., Powell A.J., Clare J.J., Jackson A.P. The sodium channel {beta}3-subunit induces multiphasic gating in NaV1.3 and affects fast inactivation via distinct intracellular regions. J. Biol. Chem. 2010;285:33404–33412. doi: 10.1074/jbc.M110.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.McEwen D.P., Isom L.L. Heterophilic interactions of sodium channel beta1 subunits with axonal and glial cell adhesion molecules. J. Biol. Chem. 2004;279:52744–52752. doi: 10.1074/jbc.M405990200. [DOI] [PubMed] [Google Scholar]
- 238.Malhotra J.D., Kazen-Gillespie K., Hortsch M., Isom L.L. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J. Biol. Chem. 2000;275:11383–11388. doi: 10.1074/jbc.275.15.11383. [DOI] [PubMed] [Google Scholar]
- 239.Srinivasan J., Schachner M., Catterall W.A. Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15753–15757. doi: 10.1073/pnas.95.26.15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ratcliffe C.F., Westenbroek R.E., Curtis R., Catterall W.A. Sodium channel beta1 and beta3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J. Cell Biol. 2001;154:427–434. doi: 10.1083/jcb.200102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Brackenbury W.J., Davis T.H., Chen C., Slat E.A., Detrow M.J., Dickendesher T.L., Ranscht B., Isom L.L. Voltage-gated Na+ channel beta1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo. J. Neurosci. 2008;28:3246–3256. doi: 10.1523/JNEUROSCI.5446-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Davis T.H., Chen C., Isom L.L. Sodium channel beta1 subunits promote neurite outgrowth in cerebellar granule neurons. J. Biol. Chem. 2004;279:51424–51432. doi: 10.1074/jbc.M410830200. [DOI] [PubMed] [Google Scholar]
- 243.Maschietto M., Girardi S., Dal Maschio M., Scorzeto M., Vassanelli S. Sodium channel beta2 subunit promotes filopodia-like processes and expansion of the dendritic tree in developing rat hippocampal neurons. Front. Cell. Neurosci. 2013;7:2. doi: 10.3389/fncel.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhou T.T., Zhang Z.W., Liu J., Zhang J.P., Jiao B.H. Glycosylation of the sodium channel beta4 subunit is developmentally regulated and involves in neuritic degeneration. Int. J. Biol. Sci. 2012;8:630–639. doi: 10.7150/ijbs.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Brackenbury W.J., Calhoun J.D., Chen C., Miyazaki H., Nukina N., Oyama F., Ranscht B., Isom L.L. Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2283–2288. doi: 10.1073/pnas.0909434107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kim D.Y., Mackenzie Ingano L.A., Carey B.W., Pettingell W.P., Kovacs D.M. Presenilin/gamma -secretase-mediated cleavage of the voltage-gated sodium channel beta 2 subunit regulates cell adhesion and migration. J. Biol. Chem. 2005;280:23251–23261. doi: 10.1074/jbc.M412938200. [DOI] [PubMed] [Google Scholar]
- 247.Wong H.K., Sakurai T., Oyama F., Kaneko K., Wada K., Miyazaki H., Kurosawa M., De Strooper B., Saftig P., Nukina N. Beta subunits of voltage-gated sodium channels are novel substrates of BACE1 and gamma -secretase. J. Biol. Chem. 2005;280:23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- 248.Kim D.Y., Carey B.W., Wang H., Ingano L.A., Binshtok A.M., Wertz M.H., Pettingell W.H., He P., Lee V.M., Woolf C.J., Kovacs D.M. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat. Cell Biol. 2007;9:755–764. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nelson M., Millican-Slater R., Forrest L.C., Brackenbury W.J. The sodium channel β1 subunit mediates outgrowth of neurite-like processes on breast cancer cells and promotes tumour growth and metastasis. Int. J. Cancer. 2014;135:2338–2351. doi: 10.1002/ijc.28890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Diss J.K.J., Fraser S.P., Walker M.M., Patel A., Latchman D.S., Djamgoz M.B.A. Beta-subunits of voltage-gated sodium channels in human prostate cancer: quantitative in vitro and in vivo analyses of mRNA expression. Prostate Cancer Prostatic Dis. 2008;11:325–333. doi: 10.1038/sj.pcan.4501012. [DOI] [PubMed] [Google Scholar]
- 251.Chioni A.M., Brackenbury W.J., Calhoun J.D., Isom L.L., Djamgoz M.B. A novel adhesion molecule in human breast cancer cells: voltage-gated Na+ channel beta1 subunit. Int. J. Biochem. Cell Biol. 2009;41:1216–1227. doi: 10.1016/j.biocel.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sanchez-Sandoval A.L., Gomora J.C. Contribution of voltage-gated sodium channel beta-subunits to cervical cancer cells metastatic behavior. Cancer Cell Int. 2019;19:35. doi: 10.1186/s12935-019-0757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shih Y.L., Chou H.M., Chou H.C., Lu H.F., Chu Y.L., Shang H.S., Chung J.G. Casticin impairs cell migration and invasion of mouse melanoma B16F10 cells via PI3K/AKT and NF-kappaB signaling pathways. Environ. Toxicol. 2017 doi: 10.1002/tox.22417. [DOI] [PubMed] [Google Scholar]
- 254.Jansson K.H., Castillo D.G., Morris J.W., Boggs M.E., Czymmek K.J., Adams E.L., Schramm L.P., Sikes R.A. Identification of beta-2 as a key cell adhesion molecule in PCa cell neurotropic behavior: a novel ex vivo and biophysical approach. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jansson K., Lynch J., Lepori-Bui N., Czymmek K., Duncan R., Sikes R. Overexpression of the VSSC-associated CAM, β-2, enhances LNCaP cell metastasis associated behavior. Prostate. 2012;72:1080–1092. doi: 10.1002/pros.21512. [DOI] [PubMed] [Google Scholar]
- 256.Adachi K., Toyota M., Sasaki Y., Yamashita T., Ishida S., Ohe-Toyota M., Maruyama R., Hinoda Y., Saito T., Imai K., Kudo R., Tokino T. Identification of SCN3B as a novel p53-inducible proapoptotic gene. Oncogene. 2004;23:7791–7798. doi: 10.1038/sj.onc.1208067. [DOI] [PubMed] [Google Scholar]
- 257.Gong Y., Yang J., Wu W., Liu F., Su A., Li Z., Zhu J., Wei T. Preserved SCN4B expression is an independent indicator of favorable recurrence-free survival in classical papillary thyroid cancer. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Miyazaki H., Oyama F., Wong H.K., Kaneko K., Sakurai T., Tamaoka A., Nukina N. BACE1 modulates filopodia-like protrusions induced by sodium channel beta4 subunit. Biochem. Biophys. Res. Commun. 2007;361:43–48. doi: 10.1016/j.bbrc.2007.06.170. [DOI] [PubMed] [Google Scholar]
- 259.Simon D.B., Bindra R.S., Mansfield T.A., Nelson-Williams C., Mendonca E., Stone R., Schurman S., Nayir A., Alpay H., Bakkaloglu A., Rodriguez-Soriano J., Morales J.M., Sanjad S.A., Taylor C.M., Pilz D., Brem A., Trachtman H., Griswold W., Richard G.A., John E., Lifton R.P. Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat. Genet. 1997;17:171–178. doi: 10.1038/ng1097-171. [DOI] [PubMed] [Google Scholar]
- 260.Kaneda M., Wakamori M., Akaike N. GABA-induced chloride current in rat isolated Purkinje cells. Am. J. Physiol. 1989;256:C1153–1159. doi: 10.1152/ajpcell.1989.256.6.C1153. [DOI] [PubMed] [Google Scholar]
- 261.Mankodi A., Takahashi M.P., Jiang H., Beck C.L., Bowers W.J., Moxley R.T., Cannon S.C., Thornton C.A. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell. 2002;10:35–44. doi: 10.1016/s1097-2765(02)00563-4. [DOI] [PubMed] [Google Scholar]
- 262.Danielsson J., Perez-Zoghbi J., Bernstein K., Barajas M.B., Zhang Y., Kumar S., Sharma P.K., Gallos G., Emala C.W. Antagonists of the TMEM16A calcium-activated chloride channel modulate airway smooth muscle tone and intracellular calcium. Anesthesiology. 2015;123:569–581. doi: 10.1097/ALN.0000000000000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Liu S.W., Li Y., Zou L.L., Guan Y.T., Peng S., Zheng L.X., Deng S.M., Zhu L.Y., Wang L.W., Chen L.X. Chloride channels are involved in sperm motility and are downregulated in spermatozoa from patients with asthenozoospermia. Asian J. Androl. 2017;19:418–424. doi: 10.4103/1008-682X.181816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jeworutzki E., Lopez-Hernandez T., Capdevila-Nortes X., Sirisi S., Bengtsson L., Montolio M., Zifarelli G., Arnedo T., Muller C.S., Schulte U., Nunes V., Martinez A., Jentsch T.J., Gasull X., Pusch M., Estevez R. GlialCAM, a protein defective in a leukodystrophy, serves as a ClC-2 Cl(-) channel auxiliary subunit. Neuron. 2012;73:951–961. doi: 10.1016/j.neuron.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sirisi S., Elorza-Vidal X., Arnedo T., Armand-Ugon M., Callejo G., Capdevila-Nortes X., Lopez-Hernandez T., Schulte U., Barrallo-Gimeno A., Nunes V., Gasull X., Estevez R. Depolarization causes the formation of a ternary complex between GlialCAM, MLC1 and ClC-2 in astrocytes: implications in megalencephalic leukoencephalopathy. Hum. Mol. Genet. 2017;26:2436–2450. doi: 10.1093/hmg/ddx134. [DOI] [PubMed] [Google Scholar]
- 266.Lange P.F., Wartosch L., Jentsch T.J., Fuhrmann J.C. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature. 2006;440:220–223. doi: 10.1038/nature04535. [DOI] [PubMed] [Google Scholar]
- 267.Estevez R., Boettger T., Stein V., Birkenhager R., Otto E., Hildebrandt F., Jentsch T.J. Barttin is a Cl- channel beta-subunit crucial for renal Cl- reabsorption and inner ear K+ secretion. Nature. 2001;414:558–561. doi: 10.1038/35107099. [DOI] [PubMed] [Google Scholar]
- 268.Favre-Kontula L., Rolland A., Bernasconi L., Karmirantzou M., Power C., Antonsson B., Boschert U. GlialCAM, an immunoglobulin-like cell adhesion molecule is expressed in glial cells of the central nervous system. Glia. 2008;56:633–645. doi: 10.1002/glia.20640. [DOI] [PubMed] [Google Scholar]
- 269.He Y., Wu X., Luo C., Wang L., Lin J. Functional significance of the hepaCAM gene in bladder cancer. BMC Cancer. 2010;10:83. doi: 10.1186/1471-2407-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Leisle L., Ludwig C.F., Wagner F.A., Jentsch T.J., Stauber T. ClC-7 is a slowly voltage-gated 2Cl(-)/1H(+)-exchanger and requires Ostm1 for transport activity. EMBO J. 2011;30:2140–2152. doi: 10.1038/emboj.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Stauber T., Jentsch T.J. Sorting motifs of the endosomal/lysosomal CLC chloride transporters. J. Biol. Chem. 2010;285:34537–34548. doi: 10.1074/jbc.M110.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Scholl U., Hebeisen S., Janssen A.G., Muller-Newen G., Alekov A., Fahlke C. Barttin modulates trafficking and function of ClC-K channels. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11411–11416. doi: 10.1073/pnas.0601631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.L’Hoste S., Diakov A., Andrini O., Genete M., Pinelli L., Grand T., Keck M., Paulais M., Beck L., Korbmacher C., Teulon J., Lourdel S. Characterization of the mouse ClC-K1/Barttin chloride channel. Biochim. Biophys. Acta. 2013;1828:2399–2409. doi: 10.1016/j.bbamem.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 274.Fischer M., Janssen A.G., Fahlke C. Barttin activates ClC-K channel function by modulating gating. J. Am. Soc. Nephrol. 2010;21:1281–1289. doi: 10.1681/ASN.2009121274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hebert S.C. Bartter syndrome. Curr. Opin. Nephrol. Hypertens. 2003;12:527–532. doi: 10.1097/00041552-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 276.Naesens M., Steels P., Verberckmoes R., Vanrenterghem Y., Kuypers D. Bartter’s and Gitelman’s syndromes: from gene to clinic. Nephron Physiol. 2004;96:65–78. doi: 10.1159/000076752. [DOI] [PubMed] [Google Scholar]
- 277.Nomura N., Tajima M., Sugawara N., Morimoto T., Kondo Y., Ohno M., Uchida K., Mutig K., Bachmann S., Soleimani M., Ohta E., Ohta A., Sohara E., Okado T., Rai T., Jentsch T.J., Sasaki S., Uchida S. Generation and analyses of R8L barttin knockin mouse, American journal of physiology. Ren. Physiol. 2011;301:F297–307. doi: 10.1152/ajprenal.00604.2010. [DOI] [PubMed] [Google Scholar]
- 278.Chung Moh M., Hoon Lee L., Shen S. Cloning and characterization of hepaCAM, a novel Ig-like cell adhesion molecule suppressed in human hepatocellular carcinoma. J. Hepatol. 2005;42:833–841. doi: 10.1016/j.jhep.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 279.Moh M.C., Zhang T., Lee L.H., Shen S. Expression of hepaCAM is downregulated in cancers and induces senescence-like growth arrest via a p53/p21-dependent pathway in human breast cancer cells. Carcinogenesis. 2008;29:2298–2305. doi: 10.1093/carcin/bgn226. [DOI] [PubMed] [Google Scholar]
- 280.Huang Z., Yang Q., Huang Z. Identification of critical genes and five prognostic biomarkers associated with colorectal Cancer. Med. Sci. Monit. 2018;24:4625–4633. doi: 10.12659/MSM.907224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tao J., Liu Q., Wu X., Xu X., Zhang Y., Wang Q., Luo C. Identification of hypermethylation in hepatocyte cell adhesion molecule gene promoter region in bladder carcinoma. Int. J. Med. Sci. 2013;10:1860–1867. doi: 10.7150/ijms.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Du Z., Li L., Sun W., Wang X., Zhang Y., Chen Z., Yuan M., Quan Z., Liu N., Hao Y., Li T., Wang J., Luo C., Wu X. HepaCAM inhibits the malignant behavior of castration-resistant prostate cancer cells by downregulating Notch signaling and PF-3084014 (a gamma-secretase inhibitor) partly reverses the resistance of refractory prostate cancer to docetaxel and enzalutamide in vitro. Int. J. Oncol. 2018;53:99–112. doi: 10.3892/ijo.2018.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Moh M.C., Zhang C., Luo C., Lee L.H., Shen S. Structural and functional analyses of a novel ig-like cell adhesion molecule, hepaCAM, in the human breast carcinoma MCF7 cells. J. Biol. Chem. 2005;280:27366–27374. doi: 10.1074/jbc.M500852200. [DOI] [PubMed] [Google Scholar]
- 284.Du H.F., Ou L.P., Lv C.K., Yang X., Song X.D., Fan Y.R., Wu X.H., Luo C.L. Expression of hepaCAM inhibits bladder cancer cell proliferation via a Wnt/beta-catenin-dependent pathway in vitro and in vivo. Cancer Biol. Ther. 2015;16:1502–1513. doi: 10.1080/15384047.2015.1071732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wang Q., Luo C., Wu X., Du H., Song X., Fan Y. hepaCAM and p-mTOR closely correlate in bladder transitional cell carcinoma and hepaCAM expression inhibits proliferation via an AMPK/mTOR dependent pathway in human bladder cancer cells. J. Urol. 2013;190:1912–1918. doi: 10.1016/j.juro.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 286.Zhang Q.L., Luo C.L., Wu X.H., Wang C.Y., Xu X., Zhang Y.Y., Liu Q., Shen S.L. HepaCAM induces G1 phase arrest and promotes c-Myc degradation in human renal cell carcinoma. J. Cell. Biochem. 2011;112:2910–2919. doi: 10.1002/jcb.23207. [DOI] [PubMed] [Google Scholar]
- 287.Wu M., Moh M.C., Schwarz H. HepaCAM associates with connexin 43 and enhances its localization in cellular junctions. Sci. Rep. 2016;6:36218. doi: 10.1038/srep36218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Xu N., Chen H.J., Chen S.H., Xue X.Y., Chen H., Zheng Q.S., Wei Y., Li X.D., Huang J.B., Cai H., Sun X.L. Reduced Connexin 43 expression is associated with tumor malignant behaviors and biochemical recurrence-free survival of prostate cancer. Oncotarget. 2016;7:67476–67484. doi: 10.18632/oncotarget.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Busby M., Hallett M.T., Plante I. The complex subtype-dependent role of connexin 43 (GJA1) in breast Cancer. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19030693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gruber A.D., Elble R.C., Ji H.L., Schreur K.D., Fuller C.M., Pauli B.U. Genomic cloning, molecular characterization, and functional analysis of human CLCA1, the first human member of the family of Ca2+-activated Cl- channel proteins. Genomics. 1998;54:200–214. doi: 10.1006/geno.1998.5562. [DOI] [PubMed] [Google Scholar]
- 291.Elble R.C., Walia V., Cheng H.C., Connon C.J., Mundhenk L., Gruber A.D., Pauli B.U. The putative chloride channel hCLCA2 has a single C-terminal transmembrane segment. J. Biol. Chem. 2006;281:29448–29454. doi: 10.1074/jbc.M605919200. [DOI] [PubMed] [Google Scholar]
- 292.Sun H., Tsunenari T., Yau K.W., Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc. Natl. Acad. Sci. U. S. A. 2002;99:4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Miller A.N., Vaisey G., Long S.B. Molecular mechanisms of gating in the calcium-activated chloride channel bestrophin. eLife. 2019;8 doi: 10.7554/eLife.43231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L.J. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science (New York, N.Y.) 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 295.Reichhart N., Schoberl S., Keckeis S., Alfaar A.S., Roubeix C., Cordes M., Crespo-Garcia S., Haeckel A., Kociok N., Fockler R., Fels G., Mataruga A., Rauh R., Milenkovic V.M., Zuhlke K., Klussmann E., Schellenberger E., Strauss O. Anoctamin-4 is a bona fide Ca(2+)-dependent non-selective cation channel. Sci. Rep. 2019;9:2257. doi: 10.1038/s41598-018-37287-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Boese S.H., Aziz O., Simmons N.L., Gray M.A. Kinetics and regulation of a Ca2+-activated Cl- conductance in mouse renal inner medullary collecting duct cells, American journal of physiology. Ren. Physiol. 2004;286:F682–692. doi: 10.1152/ajprenal.00123.2003. [DOI] [PubMed] [Google Scholar]
- 297.Salzer I., Gantumur E., Yousuf A., Boehm S. Control of sensory neuron excitability by serotonin involves 5HT2C receptors and Ca(2+)-activated chloride channels. Neuropharmacology. 2016;110:277–286. doi: 10.1016/j.neuropharm.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hannigan K.I., Griffin C.S., Large R.J., Sergeant G.P., Hollywood M.A., McHale N.G., Thornbury K.D. The role of Ca(2+)-activated Cl(-) current in tone generation in the rabbit corpus cavernosum, American journal of physiology. Cell physiology. 2017;313:C475–c486. doi: 10.1152/ajpcell.00025.2017. [DOI] [PubMed] [Google Scholar]
- 299.Catalan M.A., Kondo Y., Pena-Munzenmayer G., Jaramillo Y., Liu F., Choi S., Crandall E., Borok Z., Flodby P., Shull G.E., Melvin J.E. A fluid secretion pathway unmasked by acinar-specific Tmem16A gene ablation in the adult mouse salivary gland. Proc. Natl. Acad. Sci. U. S. A. 2015;112:2263–2268. doi: 10.1073/pnas.1415739112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gruber A.D., Schreur K.D., Ji H.L., Fuller C.M., Pauli B.U. Molecular cloning and transmembrane structure of hCLCA2 from human lung, trachea, and mammary gland. Am. J. Physiol. 1999;276:C1261–1270. doi: 10.1152/ajpcell.1999.276.6.C1261. [DOI] [PubMed] [Google Scholar]
- 301.Sala-Rabanal M., Yurtsever Z., Berry K.N., Nichols C.G., Brett T.J. Modulation of TMEM16A channel activity by the von Willebrand factor type A (VWA) domain of the calcium-activated chloride channel regulator 1 (CLCA1) J. Biol. Chem. 2017;292:9164–9174. doi: 10.1074/jbc.M117.788232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Sala-Rabanal M., Yurtsever Z., Nichols C.G., Brett T.J. Secreted CLCA1 modulates TMEM16A to activate Ca(2+)-dependent chloride currents in human cells. eLife. 2015;4 doi: 10.7554/eLife.05875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yurtsever Z., Sala-Rabanal M., Randolph D.T., Scheaffer S.M., Roswit W.T., Alevy Y.G., Patel A.C., Heier R.F., Romero A.G., Nichols C.G., Holtzman M.J., Brett T.J. Self-cleavage of human CLCA1 protein by a novel internal metalloprotease domain controls calcium-activated chloride channel activation. J. Biol. Chem. 2012;287:42138–42149. doi: 10.1074/jbc.M112.410282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Nystrom E.E.L., Birchenough G.M.H., van der Post S., Arike L., Gruber A.D., Hansson G.C., Johansson M.E.V. Calcium-activated chloride channel regulator 1 (CLCA1) controls mucus expansion in Colon by proteolytic activity. EBioMedicine. 2018;33:134–143. doi: 10.1016/j.ebiom.2018.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sharma A., Ramena G., Yin Y., Premkumar L., Elble R.C. CLCA2 is a positive regulator of store-operated calcium entry and TMEM16A. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Li X., Hu W., Zhou J., Huang Y., Peng J., Yuan Y., Yu J., Zheng S. CLCA1 suppresses colorectal cancer aggressiveness via inhibition of the Wnt/beta-catenin signaling pathway. Cell Commun. Signal. 2017;15:38. doi: 10.1186/s12964-017-0192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Li X., Cowell J.K., Sossey-Alaoui K. CLCA2 tumour suppressor gene in 1p31 is epigenetically regulated in breast cancer. Oncogene. 2004;23:1474–1480. doi: 10.1038/sj.onc.1207249. [DOI] [PubMed] [Google Scholar]
- 308.Yu Y., Walia V., Elble R.C. Loss of CLCA4 promotes epithelial-to-mesenchymal transition in breast cancer cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Yang B., Cao L., Liu J., Xu Y., Milne G., Chan W., Heys S.D., McCaig C.D., Pu J. Low expression of chloride channel accessory 1 predicts a poor prognosis in colorectal cancer. Cancer. 2015;121:1570–1580. doi: 10.1002/cncr.29235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hu D., Ansari D., Zhou Q., Sasor A., Hilmersson K.S., Bauden M., Jiang Y., Andersson R. Calcium-activated chloride channel regulator 1 as a prognostic biomarker in pancreatic ductal adenocarcinoma. BMC Cancer. 2018;18:1096. doi: 10.1186/s12885-018-5013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yang B., Cao L., Liu B., McCaig C.D., Pu J. The transition from proliferation to differentiation in colorectal cancer is regulated by the calcium activated chloride channel A1. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Qiang Y.Y., Li C.Z., Sun R., Zheng L.S., Peng L.X., Yang J.P., Meng D.F., Lang Y.H., Mei Y., Xie P., Xu L., Cao Y., Wei W.W., Cao L., Hu H., Yang Q., Luo D.H., Liang Y.Y., Huang B.J., Qian C.N. Along with its favorable prognostic role, CLCA2 inhibits growth and metastasis of nasopharyngeal carcinoma cells via inhibition of FAK/ERK signaling. J. Exp. Clin. Cancer Res. 2018;37:34. doi: 10.1186/s13046-018-0692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Bustin S.A., Li S.R., Dorudi S. Expression of the Ca2+-activated chloride channel genes CLCA1 and CLCA2 is downregulated in human colorectal cancer. DNA Cell Biol. 2001;20:331–338. doi: 10.1089/10445490152122442. [DOI] [PubMed] [Google Scholar]
- 314.Balakrishnan A., von Neuhoff N., Rudolph C., Kamphues K., Schraders M., Groenen P., van Krieken J.H., Callet-Bauchu E., Schlegelberger B., Steinemann D. Quantitative microsatellite analysis to delineate the commonly deleted region 1p22.3 in mantle cell lymphomas. Genes Chromosomes Cancer. 2006;45:883–892. doi: 10.1002/gcc.20352. [DOI] [PubMed] [Google Scholar]
- 315.Gruber A.D., Pauli B.U. Tumorigenicity of human breast cancer is associated with loss of the Ca2+-activated chloride channel CLCA2. Cancer Res. 1999;59:5488–5491. [PubMed] [Google Scholar]
- 316.Porretti J., Dalton G.N., Massillo C., Scalise G.D., Farre P.L., Elble R., Gerez E.N., Accialini P., Cabanillas A.M., Gardner K., De Luca P., De Siervi A. CLCA2 epigenetic regulation by CTBP1, HDACs, ZEB1, EP300 and miR-196b-5p impacts prostate cancer cell adhesion and EMT in metabolic syndrome disease. Int. J. Cancer. 2018;143:897–906. doi: 10.1002/ijc.31379. [DOI] [PubMed] [Google Scholar]
- 317.Ramena G., Yin Y., Yu Y., Walia V., Elble R.C. CLCA2 interactor EVA1 is required for mammary epithelial cell differentiation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Riker A.I., Enkemann S.A., Fodstad O., Liu S., Ren S., Morris C., Xi Y., Howell P., Metge B., Samant R.S., Shevde L.A., Li W., Eschrich S., Daud A., Ju J., Matta J. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med. Genomics. 2008;1:13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sasaki Y., Koyama R., Maruyama R., Hirano T., Tamura M., Sugisaka J., Suzuki H., Idogawa M., Shinomura Y., Tokino T. CLCA2, a target of the p53 family, negatively regulates cancer cell migration and invasion. Cancer Biol. Ther. 2012;13:1512–1521. doi: 10.4161/cbt.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Tanikawa C., Nakagawa H., Furukawa Y., Nakamura Y., Matsuda K. CLCA2 as a p53-inducible senescence mediator. Neoplasia. 2012;14:141–149. doi: 10.1593/neo.111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Hou T., Zhou L., Wang L., Kazobinka G., Zhang X., Chen Z. CLCA4 inhibits bladder cancer cell proliferation, migration, and invasion by suppressing the PI3K/AKT pathway. Oncotarget. 2017;8:93001–93013. doi: 10.18632/oncotarget.21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Liu Z., Chen M., Xie L.K., Liu T., Zou Z.W., Li Y., Chen P., Peng X., Ma C., Zhang W.J., Li P.D. CLCA4 inhibits cell proliferation and invasion of hepatocellular carcinoma by suppressing epithelial-mesenchymal transition via PI3K/AKT signaling. Aging. 2018;10:2570–2584. doi: 10.18632/aging.101571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Abdel-Ghany M., Cheng H.C., Elble R.C., Pauli B.U. The breast cancer beta 4 integrin and endothelial human CLCA2 mediate lung metastasis. J. Biol. Chem. 2001;276:25438–25446. doi: 10.1074/jbc.M100478200. [DOI] [PubMed] [Google Scholar]
- 324.Abdel-Ghany M., Cheng H.C., Elble R.C., Lin H., DiBiasio J., Pauli B.U. The interacting binding domains of the beta(4) integrin and calcium-activated chloride channels (CLCAs) in metastasis. J. Biol. Chem. 2003;278:49406–49416. doi: 10.1074/jbc.M309086200. [DOI] [PubMed] [Google Scholar]
- 325.Musrap N., Tuccitto A., Karagiannis G.S., Saraon P., Batruch I., Diamandis E.P. Comparative proteomics of ovarian Cancer aggregate formation reveals an increased expression of calcium-activated chloride channel regulator 1 (CLCA1) J. Biol. Chem. 2015;290:17218–17227. doi: 10.1074/jbc.M115.639773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Man Y., Cao J., Jin S., Xu G., Pan B., Shang L., Che D., Yu Q., Yu Y. Newly identified biomarkers for detecting circulating tumor cells in lung adenocarcinoma. Tohoku J. Exp. Med. 2014;234:29–40. doi: 10.1620/tjem.234.29. [DOI] [PubMed] [Google Scholar]
- 327.Fairhurst C., Martin F., Watt I., Doran T., Bland M., Brackenbury W.J. Sodium channel-inhibiting drugs and cancer survival: protocol for a cohort study using the CPRD primary care database. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Fairhurst C., Watt I., Martin F., Bland M., Brackenbury W.J. Exposure to sodium channel-inhibiting drugs and cancer survival: protocol for a cohort study using the QResearch primary care database. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-006604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Fairhurst C., Watt I., Martin F., Bland M., Brackenbury W.J. Sodium channel-inhibiting drugs and survival of breast, colon and prostate cancer: a population-based study. Sci. Rep. 2015;5:16758. doi: 10.1038/srep16758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Dopico A.M., Bukiya A.N., Singh A.K. Large conductance, calcium- and voltage-gated potassium (BK) channels: regulation by cholesterol. Pharmacol. Ther. 2012;135:133–150. doi: 10.1016/j.pharmthera.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Leonoudakis D., Conti L.R., Anderson S., Radeke C.M., McGuire L.M., Adams M.E., Froehner S.C., Yates J.R., 3rd, Vandenberg C.A. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)-associated proteins. J. Biol. Chem. 2004;279:22331–22346. doi: 10.1074/jbc.M400285200. [DOI] [PubMed] [Google Scholar]
- 332.McEwen D.P., Meadows L.S., Chen C., Thyagarajan V., Isom L.L. Sodium channel beta1 subunit-mediated modulation of Nav1.2 currents and cell surface density is dependent on interactions with contactin and ankyrin. J. Biol. Chem. 2004;279:16044–16049. doi: 10.1074/jbc.M400856200. [DOI] [PubMed] [Google Scholar]
- 333.Markovic S., Dutzler R. The structure of the cytoplasmic domain of the chloride channel ClC-Ka reveals a conserved interaction interface. Structure. 2007;15:715–725. doi: 10.1016/j.str.2007.04.013. [DOI] [PubMed] [Google Scholar]