Abstract
CC-chemokine receptor 5 (CCR5) is the principal coreceptor for macrophage-tropic strains of human immunodeficiency virus type 1 (HIV-1). We have generated a set of anti-CCR5 monoclonal antibodies and characterized them in terms of epitope recognition, competition with chemokine binding, receptor activation and trafficking, and coreceptor activity. MC-4, MC-5, and MC-7 mapped to the amino-terminal domain, MC-1 to the second extracellular loop, and MC-6 to a conformational epitope covering multiple extracellular domains. MC-1 and MC-6 inhibited regulated on activation normal T cell expressed and secreted (RANTES), macrophage inflammatory polypeptide-1β, and Env binding, whereas MC-5 inhibited macrophage inflammatory polypeptide-1β and Env but not RANTES binding. MC-6 induced signaling in different functional assays, suggesting that this monoclonal antibody stabilizes an active conformation of CCR5. Flow cytometry and real-time confocal microscopy showed that MC-1 promoted strong CCR5 endocytosis. MC-1 but not its monovalent isoforms induced an increase in the transfer of energy between CCR5 molecules. Also, its monovalent isoforms bound efficiently, but did not internalize the receptor. In contrast, MC-4 did not prevent RANTES binding or subsequent signaling, but inhibited its ability to promote CCR5 internalization. These results suggest the existence of multiple active conformations of CCR5 and indicate that CCR5 oligomers are involved in an internalization process that is distinct from that induced by the receptor's agonists.
INTRODUCTION
Chemokines constitute a large family of proteins that regulate leukocyte recruitment to sites of inflammation and coordinate their trafficking throughout the body. They mediate these functions through the binding and activation of seven transmembrane domain G protein-coupled receptors (GPCRs) specifically expressed by various populations of leukocytes (Baggiolini, 1998; Murphy et al., 2000). CC-chemokine receptor 5 (CCR5) is a functional receptor for the inflammatory CC-chemokines macrophage inflammatory polypeptide (MIP)-1α, MIP-1β, regulated on activation normal T cell expressed and secreted (RANTES), monocyte chemotactic protein (MCP)-2, and MCP-4 (Samson et al., 1996a; Murphy et al., 2000). It is expressed on memory T lymphocytes, macrophages, dendritic cells, thymocytes, hematopoietic progenitors, and other cell populations (Murphy et al., 2000). CCR5 is thought to be involved in the recruitment of leukocytes in a growing number of inflammatory diseases, such as rheumatoid arthritis, multiple sclerosis, and asthma, and it plays a major role in acquired immunodeficiency syndrome pathogenesis (Berger et al., 1999; Gerard and Rollins, 2001). Cellular entry of human immunodeficiency virus (HIV) is initiated by the interaction between the gp120 subunit of the viral Env glycoprotein, CD4, and a coreceptor that belongs to the chemokine receptor family. CCR5 is the principal coreceptor for macrophage tropic (M-tropic or R5) HIV strains, which are responsible for viral transmission and predominate during the asymptomatic phase of the disease. The essential role of CCR5 in HIV pathogenesis was demonstrated by the strong resistance toward HIV infection of individuals homozygous for a nonfunctional allele (CCR5Δ32) of the coreceptor gene (Liu et al., 1996; Samson et al., 1996b). These individuals have no obvious pathological phenotype, making CCR5 an attractive candidate for therapeutic intervention. Agents proposed as potential viral entry blockers include chemokines or chemokine analogs, monoclonal antibodies (mAbs), and chemical antagonists (Moore and Stevenson, 2000). The first described inhibitors of CCR5 coreceptor function were its natural ligands MIP-1α, MIP-1β, and RANTES (Cocchi et al., 1995). Synthetic derivatives of natural chemokines, such as amino-oxypentane (AOP)-RANTES were later shown to display enhanced HIV suppressive activities (Simmons et al., 1997). Two complementary mechanisms have been proposed to account for the ability of chemokines to inhibit HIV infection. First, chemokines compete for gp120 binding (Trkola et al., 1996). Second, agonists induce CCR5 internalization, resulting in a prolonged reduction of the coreceptor number at the cell surface (Alkhatib et al., 1997; Amara et al., 1997; Mack et al., 1998), a parameter found to be critical for HIV infection (Wu et al., 1997a). The relative contribution of these two mechanisms remains to be clarified in each case. The pronounced antiviral activity of AOP-RANTES has been attributed to the efficient induction of endocytosis and the inability of CCR5 to reaccumulate on the cell surface after removal of the ligand (Mack et al., 1998; Signoret et al., 2000). It has also been suggested that CCR5 endocytosis induced by antibodies may account for HIV resistance of exposed-uninfected individuals (Lopalco et al., 2000).
Monoclonal antibodies blocking chemokine and gp120 binding to CCR5 have been described (Wu et al., 1997b; Hill et al., 1998; Lee et al., 1999; Olson et al., 1999), as well as a first set of chemical CCR5 antagonists (Baba et al., 1999) that are presently regarded as candidate therapeutic inhibitors of viral entry. However, from a theoretical point of view, compounds able to inhibit gp120 binding and promote efficient and prolonged internalization of CCR5, without triggering the intracellular signaling cascades, would constitute ideal antiviral agents. Monoclonal antibodies interacting with various epitopes of a receptor constitute interesting tools to study the relationship between stabilization of an “active” receptor conformation and the various consequences of this activation (G protein coupling, internalization). Moreover, the availability of dimeric or monomeric isoforms of the same monoclonal allows for determination of the contribution of receptor dimerization in the studied processes.
In this study, we have characterized a novel set of anti-CCR5 mAbs and have determined their functional properties. Their epitopes were characterized by using a large panel of chimeric and mutant receptors. We correlated these findings with the ability of the mAbs to inhibit chemokine and/or gp120 binding, to modulate activation of intracellular cascades, and to influence receptor trafficking.
MATERIALS AND METHODS
Generation of Monoclonal Antibodies against CCR5
BALB/c mice were immunized at 4-wk intervals by at least six intraperitoneal injections of Chinese hamster ovary (CHO) cells stably transfected with CCR5. Four days after the last injection, the spleens were removed and the splenocytes fused with P3X63-Ag8 plasmocytoma cells. Culture supernatants were screened by flow cytometry on CHO cells expressing CCR5, or CXCR4 as control. In addition to MC-1 (Mack et al., 1998) and MC-5 (Mack et al., 2000), three monoclonal antibodies (MC-4, MC-6, and MC-7) were obtained that specifically recognize CCR5 and do not cross-react with CHO cells overexpressing CCR1–4 or CXCR4. None of the mAbs stained CCR5-deficient (CCR5Δ32/Δ32) peripheral blood mononuclear cells. Apart from the clone MC-5 (IgG-2a), all other clones were of IgG-1 isotype.
Generation of Monovalent mAb Isoforms
A plasmid encoding an MC-1 single-chain fragment (ScFv-MC-1) was performed by reverse transcription on total RNA extracted from the αCCR5 hybridoma MC-1 with random hexamers and the SuperScript reverse transcriptase (Invitrogen, Paisley, United Kingdom). The light and heavy variable domains were cloned by polymerase chain reaction amplification with Pfu polymerase (Orlandi et al., 1989). As described previously, the two domains were joined by a linker coding for (Gly4Ser1)3 and a C-terminal tail encoding six histidines was attached to facilitate purification. The single-chain fragment was expressed in the periplasmic space of Escherichia coli and purified by Ni-NTA (QIAGEN S.A., Courtaboeuf, France) as described (Mack et al., 1995).
The F(ab) fragments were obtained by digesting MC-1, MC-4, and MC-6 mAbs (2 mg/ml each), respectively, for 2 h with 0.02 mg/ml, for 4 h with 0.02 mg/ml, and for 2 h with 0.1 mg/ml papain (Sigma, St. Louis, MO) in phosphate-buffered saline (PBS) containing 20 mM EDTA and 20 mM cysteine (Sigma). After stopping the reaction with 30 mM iodocetamide, the antibodies were dialyzed overnight against PBS. Fc fragments and undigested mAbs were removed with Protein A-Sepharose CL-4B (Pharmacia, Freiburg, Germany). Aliquots of each digestion were checked by SDS-PAGE and Coomassie blue staining. By using fluorescein isothiocyanate (FITC)-labeled secondary antibodies specific for F(ab) fragments (Jackson Immunoresearch, West Grove, PA), similar mean channel fluorescence values were obtained with F(ab) fragments and half-equimolar amounts of the parental monoclonal antibodies, indicating that the binding properties were not affected by the digestion procedure.
CCR5 Constructs
All CCR5 constructs used in this study have been previously described (Lee et al., 1999; Samson et al., 1997; Blanpain et al., 1999a, 2000, 2001). The constructs were sequenced and subcloned into the bicistronic expression vector pEFIN3 for the generation of stable cell lines as previously described (Samson et al., 1997).
Cell Culture and Expression of Mutant Receptors in CHO-K1 Cells
CHO-K1 cells stably expressing apoaequorin, Gα16 and wild-type or mutant CCR5 receptor (Blanpain et al., 1999b) were cultured in Ham's F-12 medium supplemented with 10% fetal calf serum (Invitrogen), 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen), 250 μg/ml Zeocin (Invitrogen), and 400 μg/ml G418 (Invitrogen).
Fluorescence-activated Cell Sorting (FACS) Analysis
Binding affinities of the different mAbs for CCR5 were determined by flow cytometry. CCR5-expressing CHO-K1 cells were incubated with mAbs for 30 min on ice, washed, stained with different anti-mouse IgG secondary antibodies conjugated with phycoerythrin (Sigma), FITC (Sigma), or Texas Red (Jackson Immunoresearch), and analyzed on a FACScan (BD Biosciences, Aalst, Belgium). The binding parameters were determined by nonlinear regression using the PRISM software (GraphPad Software, San Diego, CA)
Epitopes were mapped by flow cytometry with a panel of ∼70 CHO-K1 cell lines stably expressing chimeric and point mutant receptors (Samson et al., 1997; Lee et al., 1999; Blanpain et al., 1999a, 2000). Cells were incubated for 30 min on ice with saturating concentrations of anti-CCR5 mAbs, washed, and stained with polyethylene (PE)-conjugated anti-mouse Ig antibody (Sigma). CHO-K1 cells expressing CCR2b were used as negative control.
For endocytosis experiments, 5 × 105 CHO cells expressing wtCCR5 were incubated for 30 min at 37°C with chemokines or mAbs. Cells were then placed on ice, incubated with 15 μg of MC-1 or MC-4 or their respective F(ab) fragments for 1 h, washed with cold PBS, and stained with FITC-conjugated anti-mouse Ig antibody (F313; DAKO, Hamburg, Germany). Cells were washed and analyzed on a FACSCalibur (BD Biosciences). For experiments with ScFv-MC-1, cells were incubated for 45 min with 3 μg/ml ScFv-MC-1, on ice or 37°C as indicated. Cells were washed twice with cold PBS and incubated with 4 μg/ml anti-His antibody (Dianova, Hamburg, Germany) for 45 min, on ice or at 37°C as indicated. Cells were washed again with cold PBS and stained with PE-labeled rabbit antimouse F(ab)2 (DAKO), and analyzed on a FACSCalibur with CellQuest software (BD Biosciences).
For inhibition of chemokine-induced CCR5 endocytosis by mAbs, CHO cells expressing CCR5 were incubated on ice with medium or MC-4 or MC-4 F(ab) for 30 min. Cells were then incubated with RANTES or AOP-RANTES for 30 min at 37°C. The cells were washed, incubated with 15 μg/ml MC-4 or MC-4 F(ab) followed by FITC-conjugated anti-mouse F(ab) Ig antibody (DAKO).
Binding Assays
Competition binding assays were performed with CCR5 expressing CHO-K1 cells as described (Blanpain et al., 1999a), by using 0.08 nM 125I-MIP-1β or 125I-RANTES (2000 Ci/mmol; Amersham plc, Little Chalfont, Buckinghamshire, United Kingdom) as tracer, variable concentrations of mAbs, and 40,000 cells. Total binding was measured in the absence of competitor and nonspecific binding was measured in the presence of a 100-fold excess of unlabeled chemokine. Soluble JRFL gp120 was iodinated as described (Lee et al., 1999). Env binding assays were performed on CCR5-expressing CHO-K1 cells with 125I-JRFL gp120 as tracer, in the presence of 100 nM sCD4 and competitors. Total binding was measured in the absence of competitor and nonspecific binding was measured in the presence of a 100-fold excess of unlabeled gp120.
Aequorin-based Functional Assay
The functional response to chemokines and monoclonals was analyzed with an aequorin-based assay as described (Blanpain et al., 1999b). Inhibition of chemokine signaling by anti-CCR5 mAbs was analyzed with the same assay. mAbs were incubated for 30 min at room temperature with 50,000 CHO-K1 cells expressing CCR5 then RANTES or MIP-1β (1 nM final concentration) was added to the cell suspension, and the luminescence was recorded for 30 s in a luminometer. Stimulation (100%) was defined as the response to 1 nM chemokine in the absence of mAbs, and 0% as the luminescence in the absence of ligand. A chemokine dose-response curve was used as positive control in each experiment.
Guanosine-5′-O-(3-thio)triphosphate (GTPγS) Binding Assay
Membranes of CHO-K1 cells expressing 20 μg/ml CCR5, prepared from cells treated or not with 100 ng/ml pertussis toxin for 18 h, were incubated in 20 mM HEPES pH 7.4, 100 mM NaCl, 3 mM MgCl2, 3 μM GDP, and 10 μg/ml saponin, with different concentrations of RANTES or mAbs, in 96-well microplates (Basic FlashPlates; PerkinElmer Life Sciences, Boston, MA), for 15 min at room temperature. After addition of 0.1 nM [35S]GTPγS (Amersham plc), the microplates were shaken for 1 min and incubated further for 30 min at 30°C. The incubation was stopped by centrifugation of the plates for 10 min at 800 × g and 4°C, and aspiration of the supernatant. Microplates were counted in a TopCount (Packard Instrument, Meriden, CT) for 1 min/well. Neither RANTES nor mAbs effected [35S]GTPγS binding to membranes of CHO-K1 cells expressing other related (CCR8) or unrelated (CRF2) GPCRs. Functional parameters were determined with the PRISM software (GraphPad Software) by using nonlinear regression applied to a sigmoidal dose-response model.
Inhibition of cAMP Accumulation
Inhibition of cAMP accumulation by chemokines and monoclonals was performed on CCR5-expressing cells spread on Petri dishes (25,000 cells/well) containing cultured overnight. Cells were preincubated for 15 min in Krebs-Ringer-HEPES buffer and 1 mM 3-isobutyl-1-methylxanthine (Calbiochem, San Diego, CA), and then incubated for 20 min in the same medium supplemented with 5 μM forskolin and variable concentrations of RANTES or 10 μg/ml mAbs. The cAMP content was measured by enzyme-linked immunosorbent assay (cAMP-screen, CS100; Tropix, Bedford, MA) according to the procedure specified by the manufacturer.
In Vivo Cellular Assays for Receptor Trafficking and Oligomerization
For confocal microscopy in living cells, clonal cell lines expressing CCR5-green fluorescent protein (GFP) were seeded on 22-mm round glass coverslips, and grown for 18 h. Coverslips were rinsed in DMEM/F-12 and placed in the observation chamber (maintained at 37°C) of an MRC 1024 confocal microscope (Bio-Rad, Hercules, CA) fitted on an Axiovert 100 inverted microscope (Zeiss, Welwyn Garden City, United Kingdom) equipped with a Plan-Neofluar 40×/1.3 oil immersion objective (Zeiss). The 488-nm excitation beam of an Argon-Krypton laser and a 522–532-nm band-pass emission filter were used for viewing enhanced green fluorescent protein (EGFP). The 568-nm excitation beam and a 605–632-nm band-pass emission filter were used for viewing transferrin AlexaFluor 564. The beam power was kept below 10% of maximal power to reduce photobleaching and phototoxicity. Pinhole was set to generate 1-μm-thick optical sections. Fields of interest (512 × 512 pixels) were selected visually. Data were sequentially collected for each fluorochrome (approximate collection time 4 s), every 3 min for the indicated time. Cells expressing CCR5-GFP were incubated with 50 μg/ml AlexaFluor-Transferrin (Molecular Probes, Eugene, OR) alone or together with ligands for 15–45 min, and washed three times before viewing.
For induction of CCR5-GFP endocytosis, 10 μg/ml mAbs or 100 nM RANTES were added to the cells, and images were acquired every 3 min for 45 min. For ScFv-MC-1 experiments, cells were first incubated for 45 min with ScFv-MC-1, washed three times, incubated with anti-His (4 μg/ml) for 30 min, and images were collected every 3 min. No endocytosis was seen with control IgG or anti-His antibody. For inhibition of CCR5-GFP endocytosis, the cells were first incubated with 10 μg/ml mAbs for 45 min then with 100 nM RANTES, and frames were acquired every 3 min for 45 min.
For determining the endocytic pathways, CCR5-GFP–expressing cells were incubated with 100 ng/ml pertussis toxin (Sigma) for 18 h, 0.45 M sucrose for 1 h, 2.5 μg/ml filipin III (Sigma) for 45 min, or 10 mM β-methyl-cyclodextrine (Sigma) for 45 min at 37°C, and then tested for endocytosis in DMEM/F-12 containing the inhibitor.
Arrestin translocation assays were performed by transfecting 100 ng of β-arrestin 2-EGFP (gift of Mark Scott, ICGM, Paris, France) into CCR5-expressing cells. The day after, cells were analyzed by confocal microscopy as described above after addition of RANTES or mAbs.
The bioluminescence resonance energy transfer (BRET) assay was performed as described by Angers et al. (2000). Briefly, humanized Renilla luciferase (Packard Instrument) and the yellow variant of GFP (CLONTECH) were fused to the last C-terminal residue of CCR5 and expressed in human embryonic kidney 293 cells. Fusion proteins were expressed at the plasma membrane and were internalized upon agonist stimulation (as determined by FACS analysis). In stable clones expressing either wild-type CCR5 or the fusion proteins RANTES and MIP-1β resulted in the inhibition of forskolin-induced cAMP production. Antibody-promoted changes of BRET ratio were calculated by subtracting the basal BRET ratio, measured in the absence of antibodies, from the BRET ratios observed in the presence of the indicated antibodies. The details of the application of the BRET assay to CCR5 will be described elsewhere (Issafras, Bouvier, and Nerullo, unpublished data).
RESULTS
Generation and Epitope Mapping of Anti-CCR5 mAbs
Mice were immunized with CHO cells expressing human CCR5. Five CCR5-specific mAbs (MC-1, MC-4, MC-5, MC-6, and MC-7) were isolated and further characterized. Saturation binding experiments were conducted using flow cytometry. All mAbs bound CCR5 with high affinity, with Kd values of 0.54 ± 0.25 (MC-1), 0.61 ± 0.24 (MC-4), 0.35 ± 0.21 (MC-5), and 1.18 ± 0.28 μg/ml (MC-6; our unpublished data). All mAbs stained CCR5 on monocytic and lymphocytic populations of freshly isolated human peripheral blood mononuclear cells, similarly to the reference antibody 2D7 (our unpublished data).
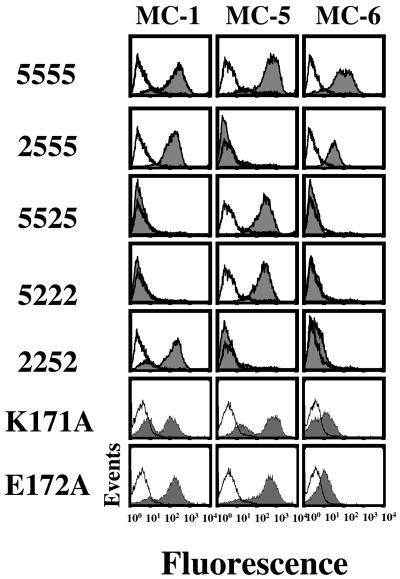
The contribution of extracellular domains of CCR5 to the epitopes was determined by testing a set of CHO-K1 cell lines stably expressing CCR5-CCR2b chimeras in FACS analysis. Two previously mapped mAbs (3A9 and 2D7) were used as controls (Wu et al., 1997b). As shown in Table 1 and Figure 1, MC-4, MC-5, MC-7, and 3A9 recognize epitopes located within the amino-terminal domain of CCR5. MC-1 and 2D7 are specific for the second extracellular loop (ECL2) of CCR5 (Figure 1). MC-6 requires multiple CCR5 domains for recognition, including ECL1, ECL2, and the amino-terminal domain (Figure 1).
Table 1.
Epitope mapping of anti-CCR5 mAbs
| 2D7 | 3A9 | MC-1 | MC-4 | MC-5 | MC-6 | MC-7 | |
|---|---|---|---|---|---|---|---|
| CCR5-CCR2b chimera | |||||||
| 5555 | + | + | + | + | + | + | + |
| 2222 | − | − | − | − | − | − | − |
| 5222 | − | + | − | + | + | − | + |
| 2555 | + | − | + | − | − | (+) | − |
| 5255 | + | + | + | + | + | − | + |
| 5525 | − | + | − | + | + | − | + |
| 2252 | + | − | + | − | − | − | − |
| 2225 | − | − | − | − | − | − | − |
| ECL2 | N-ter | ECL2 | N-ter | N-ter | MD | N-ter | |
| CCR5 amino-terminal mutants | |||||||
| CCR5 | + | + | + | + | + | + | + |
| Δ2 | ND | + | + | ND | (+) | + | ND |
| Δ2–3 | ND | + | + | ND | − | + | ND |
| Δ2–4 | ND | + | + | ND | − | + | ND |
| Δ2–5 | − | + | + | + | − | + | (+) |
| Δ2–9 | ND | + | + | + | − | + | − |
| Δ2–13 | ND | + | + | (+) | − | + | − |
| Δ2–17 | ND | + | + | − | − | + | − |
| D2A | ND | + | + | + | + | + | + |
| Y3A | ND | + | + | + | + | + | + |
| Q4A | ND | + | + | + | + | + | + |
| V5A | ND | + | + | + | + | + | + |
| S6A | ND | + | + | + | + | + | + |
| S7A | ND | + | + | + | + | + | − |
| P8A | ND | + | + | + | + | + | − |
| I9A | ND | + | + | + | + | + | + |
| Y10A | ND | + | + | + | + | + | − |
| D11A | ND | + | + | + | + | + | − |
| I12A | ND | + | + | + | + | + | + |
| N13A | ND | + | + | + | + | + | + |
| E18A | ND | + | + | + | + | + | + |
| CCR5 second extracellular loop mutants | |||||||
| 5555 | + | + | + | + | + | + | + |
| 55(25)5 | − | + | − | + | + | − | + |
| 55(52)5 | + | + | + | + | + | − | + |
| 22(25)5 | − | − | − | − | − | − | − |
| 22(52)5 | + | − | + | − | − | − | − |
| K171A | − | ND | + | + | + | − | ND |
| E172A | − | ND | + | + | + | − | ND |
ND, not determined.
Figure 1.
Immunostaining of CCR5-CCR2b chimeras and CCR5 point mutants with anti-CCR5 mAbs. CCR5-CCR2b chimeras are coded according to the origin of the N-terminal domain (first digit), and of the three extracellular loops (last three digits): 5555 and 2222 represent CCR5 and CCR2b, respectively; 2555 represents a chimeric receptor containing the amino-terminal domain of CCR2b and the three extracellular loops of CCR5. CCR5 point mutants are designated by the nature of their amino acid substitution. CHO-K1 cells stably expressing these constructs were stained with saturating concentrations of the different anti-CCR5 mAbs and phycoerythrin-conjugated anti-mouse IgG, and analyzed by FACS. Fluorescence histograms are shown together with the background fluorescence obtained for cells expressing CCR2b.
Specific residues involved in the epitopes of MC-4, MC-5, and MC-7 were determined with amino-terminal truncations and alanine substitution mutants of CCR5 (Table 1). The first three residues of CCR5 are essential for the MC-5 epitope and MC-5 was the only mAb able to recognize CCR5 in Western blotting, suggesting that its epitope is linear (Oppermann et al., 1999; Mack et al., 2000). Alanine scanning mutants demonstrated that the MC-7 epitope includes S7-P8 and Y10-D11 (Table 1). The MC-4 epitope was mapped more distally in the CCR5 amino terminus (residues 14–17 and E18). MC-1 and MC-6, like 2D7, were not affected by mutations in CCR5 N terminus (Table 1).
Chimeras and point mutants involving ECL2 were used to specify the epitopes of MC-1 and MC-6 (Table 1). MC-1 and 2D7 recognized the first part of ECL2. MC-6 binding was affected by replacement of both the first and the second halves of ECL2, and by the point substitutions K171A and E172A (but not R168A) in the first part of ECL2 (Figure 1 and Table 1). Other point mutations known to affect the conformation of the extracellular domains of CCR5, such as C178R (that disrupts the disulfide bond linking ECL1 and ECL2; Blanpain et al., 1999b, 2000), reduced or prevented binding of MC-1, MC-6, and 2D7, but had little effects on other mAbs. The other point mutants tested had no effects on mAb recognition.
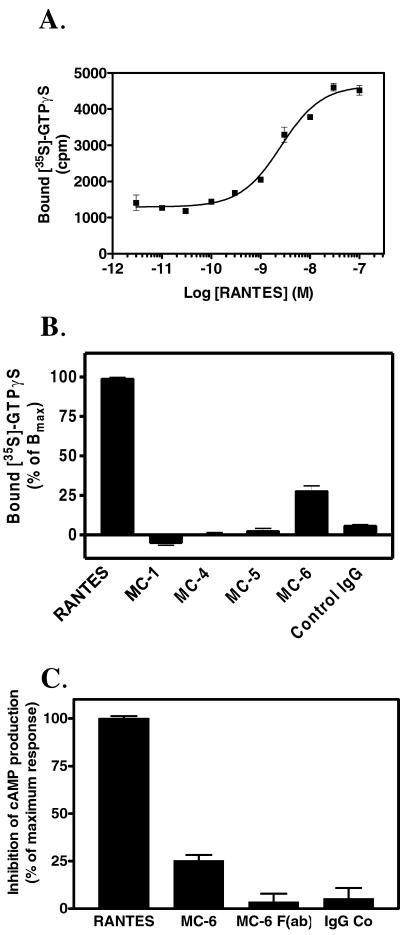
MC-6 Binding to CCR5 Promotes G protein Activation but not Calcium Mobilization
Using CCR5-expressing CHO cells, we examined the ability of the different mAbs (at the concentration of 10 μg/ml) to promote [35S]GTPγS binding to membranes. RANTES promoted a marked and dose-dependent increase in [35S]GTPγS binding, with an EC50 value of 2.3 nM (Figure 2A). Among the mAbs tested, only MC-6, but not its F(ab) fragment, induced a significant increase in GTPγS binding to membranes of CCR5-expressing cells (Figure 2B; our unpublished data). This effect was blocked by pertussis toxin pretreatment (our unpublished data). Noteworthy, MC-1 had no effect in this assay, although it promoted receptor internalization, as shown below. Neither RANTES nor MC-1 nor any other mAb increased GTPγS binding to membranes of cells expressing other receptors (our unpublished data). MC-6, but not its F(ab) fragment, was also able to inhibit cAMP accumulation in CCR5-expressing CHO-K1 cells with an efficacy similar to that found in the GTPγS assay (Figure 2C).
Figure 2.
CCR5 activation by the multidomain mAb MC-6. Effect of different concentrations of RANTES (A) and anti-CCR5 mAbs at 10 μg/ml (B) on the binding of [35S]GTPγS to membranes of CHO-K1 cells expressing human CCR5. Data are presented as raw cpm values (A) or normalized (B) to basal (0%) and maximal [35S]GTPγS binding in response to RANTES (100%). These experiments were repeated four times with similar results. All data points were analyzed in triplicates (error bars: SEM). (C) Inhibition of forskolin-stimulated cAMP accumulation. CHO-K1 cells expressing CCR5 were incubated for 20 min with 5 μM forskolin and RANTES or the various mAbs, and cAMP was measured by enzyme-linked immunosorbent assay. The results were normalized for basal (0%, in the presence of forskolin only) and maximal response (100%, cAMP levels obtained with a saturating concentration of RANTES). The experiments were performed in triplicate (error bars: SEM), and the figure represents a typical experiment out of two performed independently.
We also investigated whether the mAbs could induce intracellular calcium release by using an aequorin-based assay. None of the mAbs, including MC-6, were able to promote calcium signaling in CCR5-expressing cells, whereas RANTES induced a robust response characterized by an EC50 value of 2.5 nM (our unpublished data).
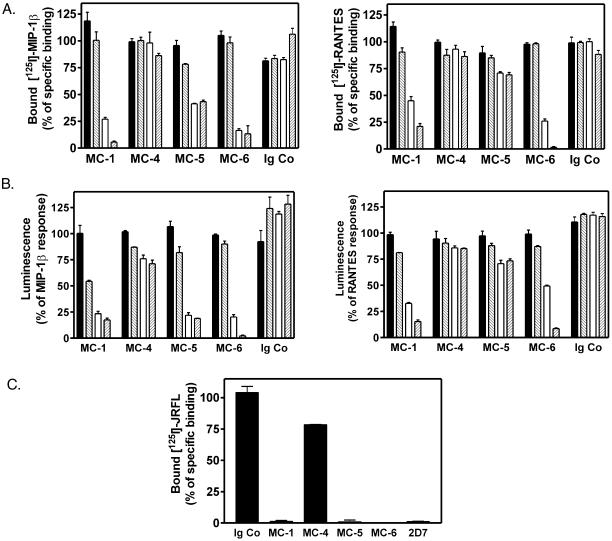
mAbs Differently Antagonize the Function of RANTES, MIP-1β, and gp120 onto CCR5
We next investigated the ability of the mAbs to compete for 125I-MIP-1β or 125I-RANTES binding to CCR5. As shown in Figure 3A, MC-1 and MC-6 inhibited both MIP-1β and RANTES binding with an IC50 value <1 μg/ml. MC-4 and control IgG did not compete for chemokine binding. MC-5 competed partially for MIP-1β binding but competed very poorly for RANTES binding up to concentrations of 10 μg/ml. We further tested whether these mAbs prevent CCR5 activation by chemokines. In agreement with the binding data, MC-1, MC-5, and MC-6 inhibited calcium mobilization induced by 1 nM MIP-1β, in a concentration-dependent manner (Figure 3B). MC-1 and MC-6, but not MC-5, inhibited calcium signaling in response to 1 nM RANTES. MC-4 and control IgG had no effect.
Figure 3.
Inhibition of MIP-1β, RANTES, and HIV Env binding and receptor activation. (A) Different concentrations of anti-CCR5 mAbs (▪, 0 μg/ml; ▧, 0.1 μg/ml; □, 1 μg/ml; ▨, 10 μg/ml) were tested for their ability to compete with binding of 125I-MIP-1β or 125I-RANTES to CHO cells expressing CCR5. Results were normalized for nonspecific binding (0%) and specific binding in the absence of competitor (100%). (B) mAb-mediated inhibition of functional responses to MIP-1β and RANTES in cell lines coexpressing CCR5 and apoaequorin. Cells were preincubated for 30 min with mAbs then MIP-1β or 1 nM RANTES was added, and luminescence was recorded for 30 s. Results were normalized for the chemokine response in the absence of antibodies (100%) and the basal luminescence of the cells (0%). (C) Influence of mAbs on the binding of 125I-gp120 derived from the macrophage-tropic HIV-1 strain JRFL to CCR5-expressing CHO-K1 cells, in the presence of 100nM sCD4. Results were normalized for nonspecific binding (0%) and specific binding in the absence of competitor (100%). All experiments were repeated at least twice with similar results. All points were performed in triplicates (error bars: SEM).
We and others have shown that chemokines and HIV Env bind overlapping but distinct CCR5 domains (Rucker et al., 1996; Wu et al., 1997b; Farzan et al., 1998; Blanpain et al., 1999a). We therefore tested the ability of our mAbs to compete for the binding of 125I-JRFL-gp120 to CCR5. MC-1, MC-5, MC-6, and the reference mAb 2D7 efficiently inhibited JRFL-gp120 binding in the presence of soluble CD4 (Figure 3C). Neither control isotype IgG nor MC-4 inhibited Env binding up to concentrations of 10 μg/ml.
Bivalent, but not Monovalent MC-1, Promotes CCR5 Endocytosis
The ability of our mAbs to induce CCR5 internalization in CHO-K1 cells was investigated. As shown in Figure 4A, MC-1 induced a dose-dependent down-modulation of the cell surface receptor. As measured by FACS analysis, 5 μg/ml MC-1 induced a 50% decrease of cell surface CCR5, similar to the level of down-modulation obtained with 100 nM RANTES (Figure 4, A and B). No significant endocytosis was seen when cells were incubated on ice with MC-1 (our unpublished data). Incubation of cells at 37°C with MC-4 or MC-5 had no effect (Figure 4A; our unpublished data). Induction of internalization by MC-1 was also found in lymphocyte and monocyte populations expressing CCR5 naturally. Given the lower expression level of the receptor in these cells, the phenomenon was however less demonstrative than in CHO cells (our unpublished data).
Figure 4.
MC-1 mAb induces CCR5 endocytosis in CHO cells. CHO cells expressing CCR5 were incubated with MC-1 (▪]), MC-4 (□) (A), or RANTES (▪) (B) for 30 min at 37°C. Cell surface CCR5 was detected by flow cytometry with a saturating concentration of the same mAbs or MC-4 for RANTES-induced CCR5 down-modulation. Results were normalized for the fluorescence of unstimulated cells (100%) and for the background fluorescence (0%). All experiments were repeated at least twice with similar results.
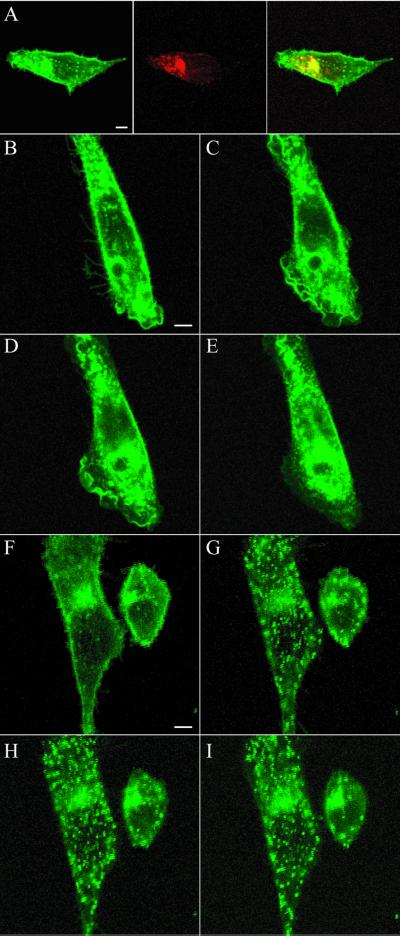
Because MC-1 promoted CCR5 endocytosis without triggering detectable signaling, we investigated whether MC-1 or RANTES could induce different routes of receptor endocytosis. CHO-K1 cells expressing high levels of a CCR5-GFP chimeric protein were generated. This chimeric receptor was activated by chemokines with a concentration-response curve similar to wtCCR5 (our unpublished data). In the absence of ligands, cells expressing CCR5-GFP showed intense fluorescence at the cell surface, although a significant proportion of the fusion protein was localized intracellularly (Figure 5A). This fraction of labeling likely corresponds partly to biosynthetic compartments, such as endoplasmic reticulum and Golgi complex, but also to endosomal compartments. Indeed, the early endosome marker AlexaFluor 594-Transferrin colocalized with intracellular CCR5-GFP, even in the absence of ligands (Figure 5A), and recycled efficiently from this endosomal compartment to the cell surface (our unpublished data). Addition of 100 nM RANTES to these cells induced a profound subcellular redistribution of the fluorescence (Figure 5, B–E, and Video 1). Shortly after agonist addition, a local enhancement of membrane-associated fluorescence was observed, possibly resulting from receptor clustering. Ruffling of membranes was also apparent as a consequence of receptor signaling and cytoskeletal rearrangements. After 10–15 min, CCR5-GFP was internalized into endocytic vesicles that progressively fused with larger endosomal compartments. In contrast to endocytosis mediated by RANTES, the subcellular redistribution of CCR5-GFP induced by MC-1 occurred in a very different manner in terms of morphology and time course (Figure 5, F–J, and Video 2). Immediately after 10 μg/ml MC-1 addition, enhancement of cell surface fluorescence was observed, but soon thereafter, a large number of small intracellular vesicles was formed, which did not fuse further with endosomes (Figure 5G). MC-5, MC-6, and isotype control IgG were unable to induce CCR5-GFP endocytosis (our unpublished data).
Figure 5.
Down-modulation of cell surface CCR5-GFP by RANTES and MC-1. (A) CHO-K1 cells expressing CCR5-GFP (green) were incubated with AlexaFluor-Transferrin (red) for 30 min, washed twice with media, and analyzed by confocal microscopy for colocalization (yellow). Dynamics of CCR5-GFP was measured after stimulation of the cells by RANTES or MC-1 mAb. The cells were exposed to 100 nM RANTES or 10 μg/ml MC-1 in DMEM/F-12 medium at 37°C, under confocal microscopy. Images were acquired every 3 min for 45 min. CCR5-GFP distribution is shown 0, 15, 30 and 45 min after addition of RANTES (B–E) or MC-1 (F–I). This session is representative of at least five similar experiments. Bar, 5 μm. Videos of the experiments described in this figure are available in the online version of this article.
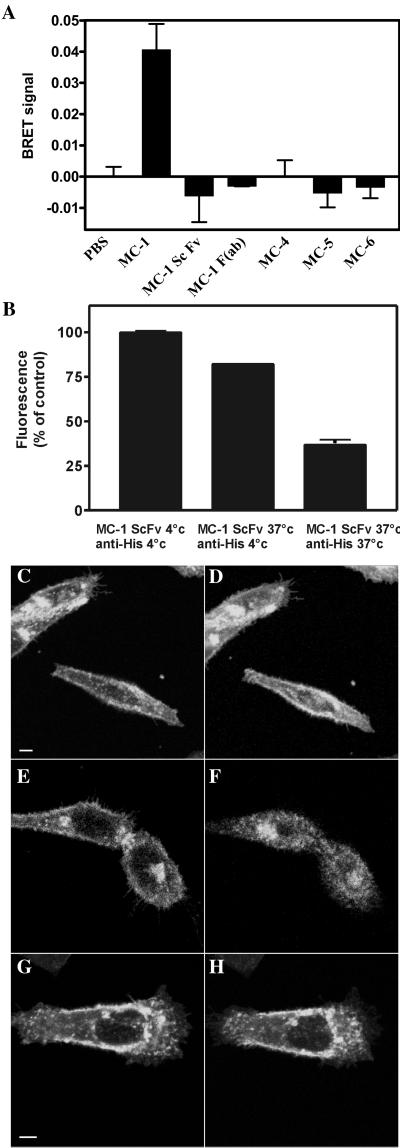
To investigate further in vivo the involvement of oligomers in the endocytosis process mediated by MC-1, we took advantage of a new biophysical assay based on BRET, which allows to monitor the interaction between two fusion proteins (Angers et al., 2000). Addition of 10 μg/ml MC-1 produced a robust increase of the BRET signal (Figure 6A) in 293T cells coexpressing CCR5-luc and CCR5-YFP, with kinetics similar to that found for CCR5-GFP endocytosis (our unpublished data). Neither MC-4, MC-5, MC-6 (Figure 6A), nor control IgG (our unpublished data) induced changes in the BRET signal. These results indicate that MC-1 induces a profound change in the physical proximity between two or more CCR5 molecules.
Figure 6.
Receptor oligomerization are involved in MC-1-induced CCR5 endocytosis. (A) 293T cells expressing CCR5-luc and CCR5-YFP were incubated with coelenterazine, and the luminescence and the fluorescence signals were quantified before and after the addition of the indicated mAb. The BRET ratio was defined as [(emission at 485 nm ± 20)/(emission at 530 nm ± 25)] − [(emission at 485 nm ± 20)/(emission at 530 nm ± 25)] for CCR5-luc expressed alone in the same experiments. Variation in BRET signal compared with baseline (addition of buffer alone) after 10 min of incubation with antibodies is displayed. All experiments were repeated at least twice with similar results. All points were performed in triplicates (error bars: SEM). (B) CHO cells expressing CCR5 were incubated with 3 μg/ml ScFv-MC-1 for 45 min at 4 or 37°C, washed twice with cold PBS, and incubated with anti-His at 4 or 37°C as indicated. Surface expression of CCR5 was measured by flow cytometry with PE-labeled anti-mouse antibody. Fluorescence was normalized for cells incubated with ScFv MC-1 and anti-His both at 4°C (100%), and for background fluorescence (0%). (C–H) Confocal microscopy. Cells expressing CCR5-GFP were incubated with 10 μg/ml ScFv-MC-1 at 37°C and are shown before (C) and 45 min after the incubation (D). Cells were then washed three times with buffer (E) and incubated with an anti-polyhistidine antibody (anti-His, 4 μg/ml) for 45 min (F). Videos of the experiment described in this figure are available in the online version of this article. Anti-His antibody alone did not result in CCR5-GFP endocytosis after 45 min of observation (G and H). All experiments were at least repeated twice with similar results.
We also investigated the functional properties of monovalent versions of MC-1 by flow cytometry and confocal microscopy. Both a single chain fragment of MC-1 (ScFv-MC-1) tagged with poly-histidine and a purified F(ab) fragment of MC-1 continued to bind CCR5 with high affinity (our unpublished data). CCR5 internalization promoted by ScFv-MC-1 was quantified by FACS analysis. CCR5 surface expression of cells incubated on ice with both ScFv-MC-1 and anti-His mAb was used as control (100% fluorescence). As shown in Figure 6B, the incubation of cells with ScFv-MC-1 at 37°C followed by anti-His on ice did not significantly reduce surface expression of CCR5, indicating that monovalent MC-1 could not induce CCR5 down-modulation. However, when scFv-MC-1 and anti-His mAb were both incubated at 37°C, CCR5 surface fluorescence decreased by 50%, indicating that cross-linking of scFv-MC-1 can restore the effect of the parental divalent antibody. Confocal microscopy was used to visualize the endocytosis of CCR5 with various combinations of the monovalent ScFv-MC-1 and cross-linking secondary antibodies. Neither monovalent ScFv-MC-1 nor MC-1 F(ab) was able to induce significant internalization of CCR5-GFP (Figure 6, C and D; our unpublished data). We then induced cross-linkage of the ScFv-MC-1 with anti-His mAb, after a 45-min preincubation with 10 μg/ml ScFv-MC-1 alone. This partially restored the effect on CCR5 endocytosis (Figure 6, E and F, and Video 3), whereas anti-His alone had no effect (Figure 6, G and H). In addition, the two monovalent isoforms of MC-1, MC-1 F(ab) and MC-1 ScFv, did not induce significant changes in the BRET signal (Figure 6A). Taken together, these results demonstrate that the bridging property of divalent antibodies is necessary for inducing CCR5 internalization.
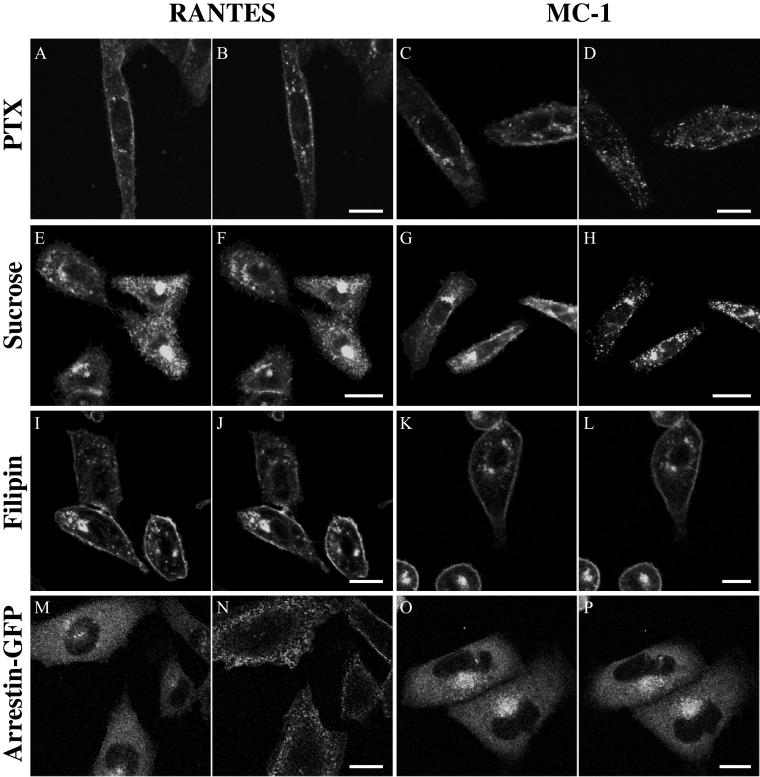
MC-1 Induces CCR5 Endocytosis via a Clathrin-independent but Caveolae-dependent Pathway
To investigate the mechanisms implicated in the endocytosis induced by MC-1, we treated cells with drugs blocking different pathways of GPCR internalization. As shown in Figure 7, A and B, pertussis toxin did not prevent endocytosis induced by MC-1, whereas it inhibited strongly the endocytosis mediated by RANTES (Figure 7, C and D). Hypertonic sucrose severely decreased RANTES-induced internalization (Figure 7, E and F), whereas this treatment had no effect on the ability of MC-1 to promote CCR5 endocytosis (Figure 7, G and H). Two cholesterol-depleting agents, β-methyl-cyclodextrine and filipin, inhibited almost completely the endocytosis mediated by both RANTES and MC-1 (Figure 7, I–L; our unpublished data). We also used a living cells assay to study the recruitment dependence of the β-arrestin after RANTES or MC-1 binding to CCR5. RANTES induced, within a minute, a robust translocation of β-arrestin-GFP from the cytosol to the plasma membrane in CCR5-expressing cells (Figure 7, M and N, and Video 4). In contrast, no subcellular redistribution of β-arrestin-GFP was seen after the addition of MC-1 (Figure 7, O and P, and Video 5). These results demonstrate that MC-1 promotes CCR5 endocytosis via an arrestin- and clathrin-independent pathway, but that this pathway is highly dependent on the integrity of cholesterol-rich microdomains.
Figure 7.
MC-1 induces CCR5 endocytosis by an arrestin and clathrin-independent, caveolae-dependent pathway. (A–L). CHO-K1 cells expressing CCR5-GFP were incubated for 18 h with 100 ng/ml pertussis toxin 100 (A–D) or for 1 h with 0.45 M sucrose (E–H), or or 2.5 μg/ml filipin (I–L) for 1 h at 37°C. Cells were then stimulated with 100 nM RANTES (A, B, E, F, I, and J) or 10 μg/ml MC-1 (C, D, G, H, K, and L), and the dynamics of CCR5-GFP was recorded by confocal microscopy. Responses are shown before (A, C, E, G, I, and K) and 30 min after (B, D, F, H, J, and L) ligand addition. (M–P). CHO-K1 cells stably expressing CCR5 were transfected with β-arrestin-GFP; 24 h after transfection, subcellular redistribution of β-arrestin-GFP was analyzed by confocal microscopy. The cells were exposed to 100 nM RANTES (M and N) or 10 μg/ml MC-1 (O and P). Images were acquired every 15 s for 10 min. Responses are shown before (M and O), and 2 min (N) or 10 min (P) after ligand addition. Videos of the dynamics of β-arrestin-GFP trafficking in response to RANTES stimulation, as described in this figure, are available in the online version of this article. All experiments were repeated at least twice with similar results. Bar, 20 μm.
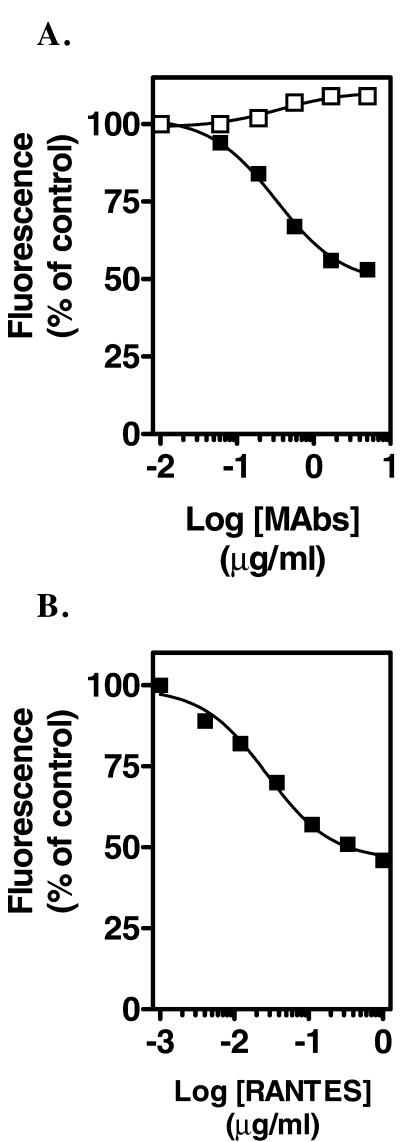
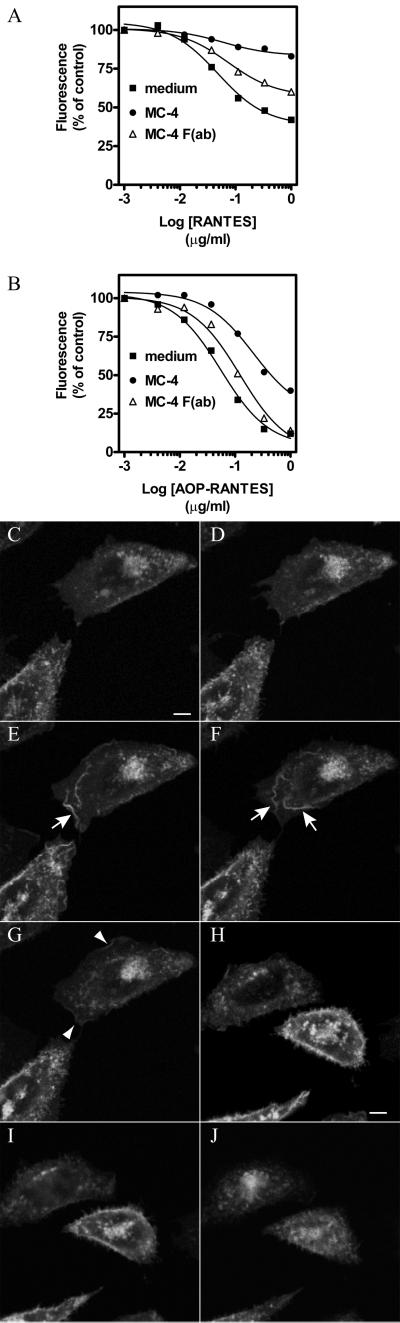
MC-4 Inhibits CCR5 Endocytosis
We also investigated whether mAbs could inhibit CCR5 internalization mediated by chemokines. Cells were preincubated with mAbs then with chemokines at 37°C, and surface expression of CCR5 was measured by flow cytometry. MC-4 inhibited RANTES-induced CCR5 endocytosis by >75% (Figure 8A). As described, AOP-RANTES was more potent than RANTES in mediating CCR5 internalization (Figure 8B). In the presence of 10 μg/ml MC-4, a 10-fold higher concentration of AOP-RANTES was required to induce 50% receptor down-modulation (Figure 8B). MC-4 F(ab) also inhibited CCR5 endocytosis although less efficiently than bivalent antibodies (Figure 8, A and B). Real-time confocal microscopy was used to study the effect of MC-4 on the dynamics of CCR5-GFP trafficking. As described above, MC-4 alone had no effect on receptor localization (Figure 8, C and D). When cells were stimulated with 100 nM RANTES in the presence of MC-4, enhancement of membrane fluorescence and ruffling were observed (Figure 8, E–G, and Video 6), as in the absence of MC-4. However, no subsequent internalization occurred, and membrane ruffling could be observed during the 45-min observation period (Figure 8G). Isotype control IgG had no effect on CCR5-GFP localization (Figure 8, H and I) or RANTES-induced internalization (Figure 8J).
Figure 8.
MC-4 inhibits CCR5 endocytosis mediated by chemokines. (A and B) CHO-K1 cells expressing CCR5 were preincubated with medium (▪), 10 μg/ml MC-4 (●), or MC-4 F(ab) (▵) for 30 min on ice and then incubated with RANTES (A) or AOP-RANTES (B) for 30 min at 37°C. Surface expression of CCR5 was measured by flow cytometry with the antibody MC-4 or MC-4 F(ab) (15 μg/ml, on ice) and FITC-conjugated secondary antibodies. Fluorescence was normalized as 100% in the absence of chemokines and 0% for background fluorescence. All experiments were repeated at least three times with similar results. Cells expressing CCR5-GFP are shown before (C), and 30 min (D) after MC-4 addition (10 μg/ml) at 37°C. Cells were then stimulated with 100 nM RANTES, and the subsequent dynamics of CCR5-GFP was recorded every 3 min by confocal microscopy. Responses are shown 15 (E), 30 (F), and 45 min (H) after RANTES addition. Arrows point to agonist-stimulated receptor patches. Arrowheads point to remaining cell surface CCR5-GFP after 45 min of RANTES stimulation. This session is representative of at least four similar experiments. Videos of the dynamics of CCR5-GFP trafficking in response to RANTES stimulation, as described in this figure, are available in the online version of this article. CCR5-GFP–expressing cells did not show differences in cell surface receptor levels before (H) and 30 min after control Ig addition (I), and did not inhibit the ability of RANTES to induce CCR5 endocytosis after 45 min of observation (J).
DISCUSSION
mAbs Recognizing the Second Extracellular Loop of CCR5 Antagonize Chemokine Binding
We have characterized five anti-CCR5 mAbs in terms of epitope mapping, inhibition of HIV Env binding and chemokine function, and investigated their ability to activate CCR5 and modulate its intracellular trafficking. On the basis of their recognition of various CCR5-CCR2b chimeras, these mAbs could be classified into three groups. The epitopes of MC-4, MC-5, and MC-7 are located in the amino-terminal domain, that of MC-1 within ECL2, whereas that of MC-6 involves multiple extracellular domains. MC-5 recognizes a linear epitope including the first two residues, that of MC-7 includes Y10 and D11. These are clearly two dominant epitopes, recognized also by a number of other mAbs (Wu et al., 1997b; Hill et al., 1998; Lee et al., 1999; Olson et al., 1999). ECL2, the longest extracellular loop of CCR5, also contains dominant epitopes. Both MC-1 and 2D7 mapped to the first part of ECL2 but recognize different epitopes. MC-6 binding, like most multidomain mAbs, was highly dependent on K171 and E172.
Numerous studies have highlighted the importance of CCR5 amino terminus and ECL2 to chemokine binding and HIV coreceptor function (Rucker et al., 1996; Samson et al., 1997; Farzan et al., 1998). ECL2 is particularly important for chemokine binding and selectivity, whereas the N terminus plays the dominant role for the gp120-CCR5 interaction. In line with these observations, mAbs such as 539, 531, and 2D7, recognizing ECL2 epitopes inhibit efficiently chemokine binding and signaling, as well as HIV entry (Lee et al., 1999). Our present results are consistent with these previous observations. Both the ECL2 mAb MC-1 and the multidomain mAb MC-6, which rely on ECL2 among other domains, efficiently inhibited binding of RANTES, MIP-1β, and Env to CCR5, as well as the functional response to chemokines. The N-terminal mAb MC-5 inhibited binding of MIP-1β and gp120, but had no effect on RANTES binding and signaling, whereas MC-4 had no effect. The differential inhibition of MIP-1β and RANTES function by MC-5 provides further evidence that CCR5 ligands may use different extracellular residues for high-affinity binding.
Partial Activation of CCR5 by a Conformation-sensitive Multidomain mAb
Chemokine receptors are coupled to heterotrimeric G proteins belonging to both pertussis toxin-sensitive Gαi and pertussis toxin-insensitive Gαq families. They regulate in turn a number of intracellular cascades, including inhibition of cAMP production, intracellular Ca2+ release, and activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinases. These cascades mediate the biological effects of chemokines, such as chemotaxis and/or increase of integrin adhesiveness (Sanchez-Madrid and del Pozo, 1999). Activation of GPCRs upon ligand binding is believed to involve the reorganization of the transmembrane helix bundle, unmasking intracellular domains that interact with G proteins, and triggering their activation (Wess, 1997). Like chemokines, mAbs might also, by binding to CCR5, modify the equilibrium between its inactive and active states, and either trigger signaling cascades, or prevent their activation.
mAbs directed at GPCRs and displaying agonist activities are rare, and were not reported so far for CCR5. However, an anti-CCR2b mAb was shown to activate this receptor in B cells (Frade et al., 1997). Our mAbs were tested in three functional assays. We showed that the multidomain mAb MC-6 induced CCR5 activation in GTPγS and cAMP accumulation assays. MC-6 was however unable to promote intracellular calcium release, whereas chemokines were equally efficient in all three assays. This discrepancy suggests that MC-6 and chemokines stabilize different active conformations of CCR5, coupling differently to intracellular signaling cascades. Differential coupling of a receptor to G proteins according to ligands has been proposed for CXCR2 (Hall et al., 1999). The conformational epitope of MC-6 includes multiple CCR5 domains involved in chemokine binding, which might explain why MC-6 promotes at least some of the conformational changes required for receptor activation. The absence of modification of BRET signal after bivalent MC-6 addition would suggest that the oligomerization state of the receptor is not modified in this process. Because the MC-6 F(ab) fragment has no signaling properties, it remains however possible that bivalent monoclonals modify the conformation of preexisting dimers without changing the distance between the BRET donor (luciferase) and the BRET acceptor (yellow GFP).
mAb-induced Internalization of CCR5 Oligomers Involves an Arrestin-independent Caveolae-dependent Pathway
A molecule that would trigger efficient CCR5 internalization without activating intracellular signaling cascades would have obvious advantages in terms of therapeutic usefulness as an HIV entry blocker. MC-1, although unable to activate the receptor in a number of bioassays, induced CCR5-GFP internalization. The redistribution dynamics was however strikingly different to that promoted by chemokines, because CCR5-GFP-containing vesicles formed more rapidly but did not fuse with each other nor with larger endosomal structures. On the other hand, CCR5 activation by the MC-6 mAb did not induce CCR5-GFP endocytosis, demonstrating that G protein activation and receptor internalization can be totally dissociated. In the classical model of receptor desensitization, binding of a ligand to its receptor leads to G protein activation, after the recruitment of regulatory proteins that mediate phosphorylation of the receptor (G protein-coupled receptor kinases), inhibition of signaling (arrestins), and ultimately its clathrin-mediated endocytosis (adaptor-related protein complex AP-2, dynamin) (Lefkowitz, 1998). However, recent experimental evidence has shown that receptor activation and internalization can be dissociated. First, a number of mutant receptors are unable to signal, but internalize normally, whereas other mutants do not internalize despite normal signaling capacity (Cheung et al., 1990; Hunyady et al., 1994; Bennett et al., 2000). Second, morphine, an agonist of the μ-opioid receptor, does not induce endocytosis, whereas the receptor is internalized well in response to other ligands (Keith et al., 1996). An antagonist of the CCK receptor mediating endocytosis has also been described (Roettger et al., 1997). Finally, constitutive activity of GPCR mutants does not necessarily result in enhanced basal phosphorylation and internalization (Mhaouty-Kodja et al., 1999; Thomas et al., 2000). These observations led to the suggestion that receptors may exist in multiple active (and inactive) conformational states, each corresponding to a specific range of functional properties (Thomas et al., 2000). In this context, it is conceivable that different mAbs may stabilize preferentially one of these usually transient conformations. MC-6 would stabilize a signaling status of CCR5, whereas MC-1 stabilizes a conformation triggering internalization.
The use of monovalent and bivalent forms of MC-1 allowed us to investigate whether this internalization-prone conformation involves oligomeric structures. The two monovalent versions of MC-1 continued to bind specifically CCR5 but could no longer mediate internalization of the receptor, whereas cross-linking by a secondary antibody partially rescued the ability of the single-chain Fv fragment to induce receptor endocytosis. This partial restoration might be due to the fact that cross-linking of the monovalent forms of MC-1 by anti-His antibodies does not necessarily restore the original geometry of the bivalent monoclonal. Moreover, BRET analysis demonstrated that bivalent MC-1, but not its monovalent forms, modifies the interaction between CCR5 polypeptides.
For many membrane receptor families, such as growth factor or cytokine receptors, dimerization is necessary both for signal transduction and endocytosis. Although GPCRs were until recently believed to operate as monomers, experimental evidence now suggests that homo- or heterodimerization occurs, and is sometimes necessary for normal receptor trafficking and function (Hebert and Bouvier, 1998). Using biophysical approaches, several groups have observed constitutive homo- or heterodimerization of GPCRs in living cells (Angers et al., 2000; Overton and Blumer, 2000; Rocheville et al., 2000a,b; McVey et al., 2001; Kroeger et al., 2001). For some, but not all receptors, a change in the energy transfer level was observed after ligand addition. As stated in these studies, modifications of fluorescent resonance energy transfer or BRET signals do not allow to distinguish clearly between the de novo association of receptor subunits, and the conformation change of preexisting dimers, modifying the relative distance between the two probes. Homodimerization (α-factor receptor) and heterodimerization of receptors (dopamine D2 and somatostatin SST5, SST1, and SST5, β2 adrenergic and opioid OP1, bradykinin B2, and angiotensin AT1) have been shown to affect their signaling and internalization properties (AbdAlla et al., 2000; Yesilaltay and Jenness, 2000; Overton and Blumer, 2000; Roche-ville et al., 2000a,b; Jordan et al., 2001).
CCR5 homodimerization has been described, as well as heterodimerization with CCR2b (Benkirane et al., 1997; Mellado et al., 1999; Rodriguez-Frade et al., 1999). However, whether this process is regulated by ligand binding or necessary for any of the CCR5 functions has not been conclusively demonstrated so far. We provide herein the evidence that bridging CCR5 polypeptides by bivalent antibodies is necessary for the induction of internalization by MC-1, and modification of energy transfer in BRET assays. These data demonstrate the involvement of oligomers in the MC-1–induced internalization process. However, they do not allow discrimination between the de novo association of monomeric CCR5, aggregation of preexisting CCR5 dimers, or structural reorganization of these constitutive dimers.
MC-1–mediated CCR5 internalization involves a pathway independent of arrestin and clathrin, but dependent on cholesterol-rich caveolae. Two recent studies reported that CCR5 could be found in membrane raft microdomains and this subcellular localization may contribute to the ability of CCR5 to mediate chemotaxis and to support HIV infection (Manes et al., 1999, 2000). The pathway of MC-1–induced endocytosis contrasts with the internalization process promoted by chemokine agonists, which is arrestin and clathrin dependent. Whether the dimerization or oligomerization state of CCR5 is also involved in the chemokine-induced internalization pathway will require further investigation.
To the best of our knowledge, MC-1 is the first mAb able to promote endocytosis of a native G protein-coupled receptor. Internalization of tagged receptors (human muscarinic M1 and thyrotropin-releasing hormone receptors) has however been reported after incubation with antibodies directed against the tags (Petrou et al., 1997; Tolbert and Lameh, 1998).
mAb Binding to CCR5 N Terminus Prevents Internalization without Preventing Chemokine-induced Signaling
MC-4 was able to potently and specifically inhibit endocytosis mediated by RANTES and AOP-RANTES, without preventing intracellular signaling. Instead, persistent ruffling of the plasma membrane was seen in confocal microscopy, demonstrating prolonged activation of the receptor. Recently, a mAb (CCR5-02), mapping to the same N-terminal epitope of CCR5 as MC-4 was found to inhibit HIV infection. This effect was attributed to receptor dimerization (Vila-Coro et al., 2000). Interestingly, antibodies directed against the amino-terminal domain of the bradykinin B2 receptor, which is involved in dimerization, have been shown to reduce receptor internalization induced by bradykinin (AbdAlla et al., 1999). The inhibition of CCR5 endocytosis by MC-4 is probably not related to the state of CCR5 oligomerization because MC-4 did not induce changes in BRET signal, and its monovalent version was also able to inhibit CCR5 endocytosis. Therefore, this effect probably results from the stabilization of a particular conformation of the receptor.
CONCLUSION
We have characterized five mAbs that recognize CCR5 expressed on primary cells. They map to distinct epitopes, including the N-terminal segment (MC-4, MC-5, MC-7), the second extracellular loop (MC-1), or both (and other) domains (MC-6). Many of these antibodies exhibit functional properties (partial activation of CCR5 signaling pathways, stimulation of internalization without signaling, inhibition of internalization without impairing signaling) that together suggest the existence of multiple active conformation states of CCR5. The differential properties of monovalent and divalent forms of the MC-1 antibody on both endocytosis and bioluminescence energy transfer also indicate that the endocytic pathway activated by this antibody involves CCR5 oligomers. Finally, some of the monoclonal antibodies have interesting properties that might be used in different fields. The MC-1 mAb, which competes for gp120 binding and promotes efficient internalization of the receptor without triggering intracellular signaling, might constitute the basis for the development of anti-HIV therapeutic agents. Other mAbs, which appear to stabilize active conformations or dimers, might also be useful as tools to purify and crystallize active states of CCR5 for structural studies.
Supplementary Material
ACKNOWLEDGMENTS
Expert technical assistance was provided by M.J. Simons, H. Nguyen Tran, M.E. Decobecq, and T. Rupp. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 464), the Actions de Recherche Concertées of the Communauté Française de Belgique, the French Agence Nationale de Recherche sur le SIDA, the Center de Recherche Interuniversitaire en Vaccinologie, the Belgian program on Interuniversity Poles of attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, the BIOMED and BIOTECH program of the European Community (grants BIO4-CT98-0543 and BMH4-CT98-2343), the Fonds de la Recherche Scientifique Médicale of Belgium, Télévie and the Fondation Médicale Reine Elisabeth to M.P. The scientific responsibility is assumed by the authors. C.B. is Aspirant, and J.M.V. is Chercheur Qualifié, of the Belgian Fonds National de la Recherche Scientifique. V.W. is recipient of a First fellowship of the Région Wallonne. We thank Amanda Proudfoot for kindly providing RANTES, Robin Offord and Brigitte Dufour for the synthesis of AOP-RANTES, Mark Scott for providing β-arrestin-2-GFP, and the AIDS Research and Reference Reagent Program for providing soluble CD4 and the 2D7 anti-CCR5 mAb.
Footnotes
Online version of this article contains video material for Figures 5–8. Online version available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–03-0129. Article and publication date are at www.molbiolcell.org/cgi/10.1091/mbc.01–03-0129.
REFERENCES
- AbdAlla S, Lother H, Quitterer U. A.T1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407:94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- AbdAlla S, Zaki E, Lother H, Quitterer U. Involvement of the amino terminus of the B(2) receptor in agonist-induced receptor dimerization. J Biol Chem. 1999;274:26079–26084. doi: 10.1074/jbc.274.37.26079. [DOI] [PubMed] [Google Scholar]
- Alkhatib G, Locati M, Kennedy PE, Murphy PM, Berger EA. H.IV-1 coreceptor activity of C.C.R5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology. 1997;234:340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier JL, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor C.X.C.R4 contributes to inhibition of H.IV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M. Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (B.R.E.T) Proc Natl Acad Sci USA. 2000;97:3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, et al. A. small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Benkirane M, Jin DY, Chun RF, Koup RA, Jeang KT. Mechanism of transdominant inhibition of C.C.R5-mediated H.IV-1 infection by ccr5′4432. J Biol Chem. 1997;272:30603–30606. doi: 10.1074/jbc.272.49.30603. [DOI] [PubMed] [Google Scholar]
- Bennett TA, Maestas DC, Prossnitz ER. Arrestin binding to the G protein-coupled N-formyl peptide receptor is regulated by the conserved “DRY” sequence. J Biol Chem. 2000;275:24590–24594. doi: 10.1074/jbc.C000314200. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Blanpain C, et al. Multiple charged and aromatic residues in CCR5 amino-terminal domain are involved in high affinity binding of both chemokines and HIV-1 Env protein. J Biol Chem. 1999a;274:34719–34727. doi: 10.1074/jbc.274.49.34719. [DOI] [PubMed] [Google Scholar]
- Blanpain C, et al. Extracellular cysteines of CCR5 are required for chemokine binding, but dispensable for HIV-1 coreceptor activity. J Biol Chem. 1999b;274:18902–18908. doi: 10.1074/jbc.274.27.18902. [DOI] [PubMed] [Google Scholar]
- Blanpain C, et al. Multiple nonfunctional alleles of CCR5 are frequent in various human populations. Blood. 2000;96:1638–1645. [PubMed] [Google Scholar]
- Blanpain C, et al. Palmitoylation of CCR5 is critical for receptor trafficking and efficient activation of intracellular signaling pathways. J Biol Chem. 2001;276:23795–23804. doi: 10.1074/jbc.M100583200. [DOI] [PubMed] [Google Scholar]
- Cheung AH, Dixon RA, Hill WS, Sigal IS, Strader CD. Separation of the structural requirements for agonist-promoted activation and sequestration of the beta-adrenergic receptor. Mol Pharmacol. 1990;37:775–779. [PubMed] [Google Scholar]
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T. cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Farzan M, et al. A. tyrosine-rich region in the N terminus of CCR5 is important for human immunodeficiency virus type 1 entry and mediates an association between gp120 and CCR5. J Virol. 1998;72:1160–1164. doi: 10.1128/jvi.72.2.1160-1164.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Mellado M, del Real G, Gutierrez-Ramos JC, Lind P, Martinez A. Characterization of the C.C.R2 chemokine receptor: functional CCR2 receptor expression in B. cells. J Immunol. 1997;159:5576–5584. [PubMed] [Google Scholar]
- Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- Hall DA, Beresford IJ, Browning C, Giles H. Signaling by CXC-chemokine receptors 1 and 2 expressed in CHO cells: a comparison of calcium mobilization, inhibition of adenylyl cyclase and stimulation of GTPγS binding induced by IL-8 and GROα. Br J Pharmacol. 1999;126:810–818. doi: 10.1038/sj.bjp.0702329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert TE, Bouvier M. Structural and functional aspects of G. protein-coupled receptor oligomerization. Biochem Cell Biol. 1998;76:1–11. doi: 10.1139/bcb-76-1-1. [DOI] [PubMed] [Google Scholar]
- Hill CM, et al. The amino terminus of human C.C.R5 is required for its function as a receptor for diverse human and simian immunodeficiency virus envelope glycoproteins. Virology. 1998;248:357–371. doi: 10.1006/viro.1998.9283. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Baukal AJ, Balla T, Catt KJ. Independence of type I angiotensin II receptor endocytosis from G protein coupling and signal transduction. J Biol Chem. 1994;269:24798–24804. [PubMed] [Google Scholar]
- Jordan BA, Trapaidze N, Gomez I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with β2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci USA. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Kroeger KM, Hanyaloglu AC, Seeber RM, Miles LE, Eidne KA. Constitutive and agonist-dependent homo-oligomerization of the thyrotropin-releasing hormone receptor. Detection in living cells using bioluminescence resonance energy transfer. J Biol Chem. 2001;276:12736–12743. doi: 10.1074/jbc.M011311200. [DOI] [PubMed] [Google Scholar]
- Lee B, et al. Epitope mapping of C.C.R5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–9626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Lopalco L, Barassi C, Pastori C, Longhi R, Burastero SE, Tambussi G, Mazzotta F, Lazzarin A, Clerici M, Siccardi AG. CCR5-reactive antibodies in seronegative partners of HIV-seropositive individuals down-modulate surface CCR5 in vivo and neutralize the infectivity of R5 strains of HIV-1 in vitro. J Immunol. 2000;164:3426–3433. doi: 10.4049/jimmunol.164.6.3426. [DOI] [PubMed] [Google Scholar]
- Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, Cihak J, Plachy J, Stangassinger M, Erfle V, Schlondorff D. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6:769–775. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- Mack M, Riethmuller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci USA. 1995;92:7021–7025. doi: 10.1073/pnas.92.15.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M, et al. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes S, del Real G, Lacalle RA, Gomez-Mouton C, Sanchez-Palomino S, Delgado R, Alcami J, Mira E, Martinez-A. C. Membranes raft microdomains mediate lateral assemblies required for H.IV-1 infection. EMBO Rep. 2000;1:190–196. doi: 10.1093/embo-reports/kvd025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes S, Mira E, Gomez-Mouton C, Lacalle RA, Keller P, Labrador JP, Martinez A. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 1999;18:6211–6220. doi: 10.1093/emboj/18.22.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey M, Ramsay D, Kellett E, Rees S, Wilson S, Pope AJ, Milligan G. Monitoring receptor oligomerization using time-resolved fluorescence resonance energy transfer and bioluminescence resonance energy transfer. The human delta-opioid receptor displays constitutive oligomerization at the cell surface, which is not regulated by receptor occupancy. J Biol Chem. 2001;276:14092–14099. doi: 10.1074/jbc.M008902200. [DOI] [PubMed] [Google Scholar]
- Mellado M, Rodriguez-Frade JM, Vila-Coro AJ, de Ana AM, Martinez A. Chemokine control of HIV-1 infection. Nature. 1999;400:723–724. doi: 10.1038/23382. [DOI] [PubMed] [Google Scholar]
- Mhaouty-Kodja S, Barak LS, Scheer A, Abuin L, Diviani D, Caron MG, Cotecchia S. Constitutively active alpha-1b adrenergic receptor mutants display different phosphorylation and internalization features. Mol Pharmacol. 1999;55:339–347. doi: 10.1124/mol.55.2.339. [DOI] [PubMed] [Google Scholar]
- Moore JP, Stevenson M. New Targets for inhibitor of HIV-1 replication. Nat Rev Mol Cell Biol. 2000;1:40–49. doi: 10.1038/35036060. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International Union of Pharmacology XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Olson WC, et al. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and C.C-chemokine activity by monoclonal antibodies to CCR5. J Virol. 1999;73:4145–4155. doi: 10.1128/jvi.73.5.4145-4155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann M, Mack M, Proudfoot AE, Olbrich H. Differential effects of CC chemokines on CC chemokine receptor 5 (CCR5) phosphorylation and identification of phosphorylation sites on the CCR5 carboxyl terminus. J Biol Chem. 1999;274:8875–8885. doi: 10.1074/jbc.274.13.8875. [DOI] [PubMed] [Google Scholar]
- Orlandi R, Gussow DH, Jones PT, Winter G. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86:3833–3837. doi: 10.1073/pnas.86.10.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton MC, Blumer KJ. G-protein-coupled receptors function as oligomers in vivo. Curr Biol. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- Petrou C, Chen L, Tashjian AHJ. A receptor-G protein coupling-independent step in the internalization of the thyrotropin-releasing hormone receptor. J Biol Chem. 1997;272:2326–2333. doi: 10.1074/jbc.272.4.2326. [DOI] [PubMed] [Google Scholar]
- Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000a;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- Rocheville M, Lange DC, Kumar U, Sasi R, Patel RC, Patel YC. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J Biol Chem. 2000b;275:7862–7869. doi: 10.1074/jbc.275.11.7862. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Frade JM, Vila-Coro AJ, Martin A, Nieto M, Sanchez-Madrid F, Proudfoot AE, Wells TN, Martinez A, Mellado M. Similarities and differences in RANTES- and (AOP)-RANTES-triggered signals: implications for chemotaxis. J Cell Biol. 1999;144:755–765. doi: 10.1083/jcb.144.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roettger BF, Ghanekar D, Rao R, Toledo C, Yingling J, Pinon D, Miller LJ. Antagonist-stimulated internalization of the G protein-coupled cholecystokinin receptor. Mol Pharmacol. 1997;51:357–362. [PubMed] [Google Scholar]
- Rucker J, et al. Regions in beta-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell. 1996;87:437–446. doi: 10.1016/s0092-8674(00)81364-1. [DOI] [PubMed] [Google Scholar]
- Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996a;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- Samson M, LaRosa G, Libert F, Paindavoine P, Detheux M, Vassart G, Parmentier M. The second extracellular loop of CCR5 is the major determinant of ligand specificity. J Biol Chem. 1997;272:24934–24941. doi: 10.1074/jbc.272.40.24934. [DOI] [PubMed] [Google Scholar]
- Samson M, et al. Resistance to H.IV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996b;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoret N, Pelchen-Matthews A, Mack M, Proudfoot AE, Marsh M. Endocytosis and recycling of the HIV coreceptor CCR5. J Cell Biol. 2000;151:1281–1294. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Clapham PR, Picard L, Offord RE, Rosenkilde MM, Schwartz TW, Buser R, Wells TNC, Proudfoot AE. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- Thomas WG, Qian H, Chang CS, Karnik S. Agonist-induced phosphorylation of the angiotensin II (AT(1A)) receptor requires generation of a conformation that is distinct from the inositol phosphate-signaling state. J Biol Chem. 2000;275:2893–2900. doi: 10.1074/jbc.275.4.2893. [DOI] [PubMed] [Google Scholar]
- Tolbert LM, Lameh J. Antibody to epitope tag induces internalization of human muscarinic subtype 1 receptor. J Neurochem. 1998;70:113–119. doi: 10.1046/j.1471-4159.1998.70010113.x. [DOI] [PubMed] [Google Scholar]
- Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- Vila-Coro AJ, Mellado M, Martin DA, Lucas P, del Real G, Martinez A, Rodriguez-Frade JM. HIV-1 infection through the CCR5 receptor is blocked by receptor dimerization. Proc Natl Acad Sci USA. 2000;97:3388–3393. doi: 10.1073/pnas.050457797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]
- Wu L, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997a;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, et al. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997b;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilaltay A, Jenness DD. Homo-oligomeric complexes of the yeast alpha-factor pheromone receptor are functional units of endocytosis. Mol Biol Cell. 2000;11:2873–2884. doi: 10.1091/mbc.11.9.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.