Abstract
Enhanced recovery after surgery (ERAS) aims to improve perioperative care, hasten recovery to the normal physiological state and shorten length of stay (LoS). There is evidence that ERAS programmes following elective caesarean section (ELCS) confer benefit through faster return to physiological state and reduced LoS for mother and baby. Baseline audit of ELCS in 2013 revealed a mean LoS of 3 days. We piloted an ERAS discharge pathway promoting day 2 discharge, which rose from 5.0% to 40.2%. 19.2% of women went home on day 1. Many women fed back that they would prefer day 1 discharge. We hypothesised that a day 1 discharge pathway for low-risk women could benefit both women and services at our maternity unit. From October 2015, we developed a ‘fast-track pathway’ (FTP) using a Plan-Do-Study-Act approach. Between October 2015 and April 2016, we prospectively audited clinical outcomes, LoS and maternal satisfaction from all women placed on the FTP. We held regular multidisciplinary team meetings to allow contemporaneous analysis. Satisfaction was analysed by Likert scale at postoperative surveys. Women were identified in antenatal clinic after meeting predefined low-risk criteria. 27.3% of women (n=131/479) delivering by ELCS entered the FTP. 76.2% of women on the FTP were discharged on day 1. Mean LoS fell to 1.31 days. 94.2% of women who established breast feeding at day 1 were still breast feeding at 7 days. Overall satisfaction at day 7 was 4.71 on a 5-point Likert scale. 73.1% of women reported good pain control. Additional financial savings are estimated at £99 886 annually. There were no related cases of readmission. Day 1 discharge after ELCS is safe and acceptable in carefully selected, low-risk women and has high satisfaction. There may be resultant financial savings and improved flow through a maternity unit with no detected adverse effect on breast feeding, maternal morbidity or postnatal readmissions.
Keywords: obstetrics, quality improvement, elective caesarean section, enhanced recovery after surgery, fast-track surgery, patient satisfaction
Problem
Enhanced recovery after surgery (ERAS) pathways can reduce length of stay (LoS) without compromising quality of care,1 which is increasingly important in the context of increasing bed occupancy in the National Health Service (NHS) and calls for efficiency savings. ERAS initiatives refer to a post-surgical pathway which aims to expedite return to the normal physiological state, improving patient outcomes and shortening LoS.2 3 ERAS programmes have become increasingly popular for elective surgery in a range of surgical specialties, including gynaecology.4–7 However, implementation in obstetrics has been slower despite increasing numbers of caesarean sections performed every year in the UK. In 2015–2016, 80 737 elective caesarean sections (ELCSs) were performed in England; this represents an increase from 10.7% to 14.7% of all NHS deliveries since 2005–2006.8
Chelsea and Westminster Hospital is a high-volume London hospital with approximately 6750 deliveries/year (2016/2017) and an ELCS rate of 20.5% (2016). Delivery numbers and ELCS rates have steadily increased since 2013 (2013—14.7%, 2014—15.2%, 2015—18.8%). Since 2013, we have been made aware of problems with flow of women from the operating theatre to recovery and subsequently to the postnatal ward. This resulted in insufficient patient beds and delayed starting times for subsequent ELCS lists, with postponement of delivery for some women.
Background
In 2012, the National Institute for Health and Care Excellence (NICE) evaluated current evidence and issued guidance that women delivering by uncomplicated ELCS should be offered discharge after 24 hours with home follow-up.9 There are additional barriers to early discharge in obstetrics when compared with other surgical specialties including maternal acceptability, breastfeeding establishment, and concerns over neonatal safety.10 11
In a 2013 UK survey of lead obstetric anaesthetists, 3 of 158 units reported implementation of ERAS programmes, 5 units were undergoing implementation and 2 units were considering a programme. Moreover, 152/158 (96%) supported the concept of enhanced recovery for elective obstetric surgery although 36% expressed they would like more evidence of benefit.12 We were unable to find any study of obstetrician acceptability for ERAS. Investigation of maternal acceptability and additional barriers to implementation were also lacking and warrant further evaluation.13 14
A review of clinical protocols for ERAS after ELCS found five different clinical protocols with 25 clinical components.14 Programmes were highly variable with only three components common to all protocols: early oral intake, early mobilisation and removal of urinary catheter.
The concept of fast-track pathways (FTPs) facilitating day 1 discharge following low-risk ELCS has more recently been promoted as a safe and desirable option following success in other specialties.15 Two studies have evaluated day 1 discharge and suggested that it is safe for low-risk women with no increase in adverse outcomes, maternal or infant readmissions.16 17 There is also some evidence that early discharge after caesarean may improve maternal–neonatal bonding and maternal satisfaction.18
In summary, despite NICE guidance and good evidence of safety, uptake of ERAS after ELCS has been slow. FTPs are even less common. It has been identified that there is a need for quality improvement reports which detail the processes and barriers to implementation of ERAS and fast-track surgery in obstetrics.14 Investigation of maternal satisfaction with such pathways is lacking19 and warranted,20 21 especially given the additional maternal–neonatal factors to be considered in obstetrics.
Measurement
This quality improvement project aimed to increase the number of women being discharged on day 1 following ELCS, using a FTP. The primary outcome of day 1 discharge was defined as any discharge before 2359 hours on the day following surgery.
Process measures were developed to understand factors impacting on success of day 1 discharge:
Time entering operating theatre (percentage entering before 14:00).
Pain control scores at day 1.
Removal of intravenous cannula and urinary catheter within 6 hours of leaving the operating theatre.
Percentage of discharge medications prepared on operation day.
Time of medical review at day 1.
Breastfeeding rates at day 1.
Balancing measures were also collected to identify potential risks and adverse effects of the interventions:
Maternal satisfaction scores and qualitative feedback on days 1 and 7.
Staff feedback.
Breastfeeding rates at day 7.
Pain control at day 7.
Readmission rates at day 7.
Historical audit data from 2013 (16 women) provided us with baseline data for some of these measures:
16/16 of women entered the operating theatre before 14:00.
Mean of 3.00 for pain control at day 1 (on 5-point Likert scale where 1 is poor control and 5 is excellent).
Median time of catheter removal was 22.5 hours (IQR 2.25 hour).
Median time of intravenous cannula removal was 27 hours (IQR 5.5 hours).
Following initial pilot of the ERAS pathway in February 2013, the following measures were obtained from 30 women:
Median time of catheter removal was 6 hours (IQR 2 hours)—11/30 (36.7%) had their catheters removed within 6 hours.
Median time of intravenous cannula removal was 6 hours (IQR 1 hour)—29/30 (30%) had their cannulae removed within 6 hours.
ERAS was then formally introduced in 2014. Baseline data were available from retrospective audit of 896 women who delivered by ELCS between June 2014 and 2015.
Mean LoS was 3.25 days (SD 0.45)—40.6% (364/896) stayed three or more days, 40.2% (360/896) 2 days and 19.2% (172/896) were discharged on day 1.
Breastfeeding rate at day one 98%.
An audit of all ELCS over 2 weeks in November 2015 (n=15) revealed
100% (15/15) entered theatre before 14:00.
0/12 (0%) had their catheters removed within 6 hours—3/15 missing.
Cannulae removal within 6 hours—15/15 data missing.
1/15 (6.7%) had discharge medications prepared on day 1.
Design
FTP was developed to specifically facilitate day 1 discharge for low-risk women and prospectively evaluate process and balancing measures. This incorporated published guidance from enhanced recovery principles2 22 23 with stakeholder consultation in a multidisciplinary team (MDT) approach. The post-ELCS FTP protocol included
Encouraging fluid intake as early as feasible after the operation.
Mobilisation within 12 hours.
Removal of venous cannula and urinary catheter within 6 hours.
Discharge medications being ordered on postoperative day 0.
Postoperative full blood count before 10:00 on postoperative day 1.
MDT discharge review before 14:00 on postoperative day 1.
Contemporaneous recording of measures.
From audit data available after introduction of ERAS in 2014, it was apparent that the existence of the ERAS pathway was not sufficient to ensure changes in practice. Preoperative education has been identified as important in enhanced recovery after surgery.24 Thus, the new FTP also included a preoperative education session delivered by a midwife and physiotherapist during the pre-assessment clinic (PAC). Leaflets and digital information (via a smartphone app25) were provided on the benefits of FTP and preoperative and postoperative optimisation of health.
At each PAC, a senior midwife identified women who fulfilled criteria for FTP (online supplement 1). These women were planned to be first on each operating list, in order to facilitate timely recovery and discharge for women through pre-planning.
bmjoq-2018-000465supp001.pdf (307.1KB, pdf)
As part of this project, we trained a dedicated recovery nurse as fast-track champion. Her work as champion included facilitation of FTP, audit and feedback to maternity unit leads. To facilitate this, she worked a Monday–Friday shift pattern matching ELCS lists, while also assisting the recovery unit nursing lead on overseeing the recovery and high-dependency care provided for all obstetric patients. As part of her existing job plan to allow for professional development, she had an audit day once a month, which became dedicated to FTP audit. These changed to address continuity of care and sustainability of the pathway,26 without requiring any additional resource or cost. Other MDT members included the recovery unit nurse lead, theatre scrub team, pre-assessment midwife, obstetric anaesthetic consultant and trainee, obstetric consultant and trainee, and maternity unit pharmacist.
Women were required to stay for a minimum of 24 hours after ELCS, but FTP women were supported to leave as close to 24 hours post-surgery as clinically appropriate. For safety and practical reasons, women were not discharged between 00:00 and 7:00.
The anaesthetic technique was left to the discretion of the individual anaesthetist. In most cases, this was a spinal anaesthetic containing hyperbaric bupivacaine 0.5% and diamorphine (300 µg). If a combined spinal and epidural technique was used, the spinal component was the same as for the single shot spinal. At completion of ELCS, women received rectal diclofenac, unless contraindicated, and were prescribed standard postoperative analgesia of regular oral paracetamol, dihydrocodeine and ibuprofen with additional oral morphine as required.
Women who were discharged on FTP were surveyed by the recovery nurse on day 1 (face-to-face) and day 7 by telephone, using a proforma (online supplement 2). Following discharge, women were seen by a community midwife within 48 hours. In this way, we hoped to identify any adverse effects related to day 1 discharge.
bmjoq-2018-000465supp002.pdf (245.3KB, pdf)
To assess the financial implications of the project, we planned meetings with hospital coders and maternity unit managers.
We held monthly MDT meetings to discuss perceived problems and successes with stakeholders. Quantitative and qualitative data were fed back and where issues arose, interventions were planned. Quality improvement outcomes were reported on a 6-monthly basis at maternal morbidity meetings.
Strategy
Plan-Do-Study-Act (PDSA) cycles were used to analyse, assess and improve implementation of the FTP.
PDSA cycle 1: antenatal preparation of women
Plan—Feedback from women indicated they required antenatal preparation in order to increase acceptability and prepare for day 1 discharge.
Do—We set up an antenatal education programme to be delivered during PACs. During initial set-up, an obstetrician attended the antenatal education session on a daily basis to address any concerns from midwifery staff or women.
Study—Feedback from women indicated that the antenatal education was useful in preparing them for fast-track discharge.
After introduction of the antenatal education programme, we found a gradual increase in the number of women accepting FTP: 14 women in October 2015, 17 women in November 2015, 24 women in December 2015 and 25 women in January 2016.
Act—We realised that staff also required FTP education in order for us to identify suitable women and to provide these women with the antenatal education package. We prepared a staff education video, which was presented and circulated to all maternity staff. Trainees, consultants and midwives were all targeted in engagement events. Stickers were introduced to put on the women’s notes, so they could be easily identified as being on the FTP on 31 January 2018. We also introduced education sessions on the first postnatal day as a refresher. Following these changes, we had 17 women accepting the FTP in February 2016 and 34 in March 2016.
PDSA cycle 2: development of the FTP checklist
Plan—Success rates in late December 2015 decreased. Staff fed back to the team that a checklist for women might help staff comply with FTP standards.
Do—We introduced a checklist of FTP standards, which was completed by recovery nurses. We included that medications should be prepared by the operating team immediately after surgery. We allocated a dedicated fast-track nurse champion who worked 9:00–16:00 Monday to Friday, who took responsibility for adherence to fast-track protocols and service evaluation.
Study—We observed an increase in the number of women having catheters and cannulae removed within the FTP targets. Pre-checklist introduction, intravenous cannulae targets were met by 59%, and this rose to 65%. For urinary catheters, removal rates within targets rose from 49% to 62%. Pharmacists expressed concern with the practice of early preparation of discharge medications and were initially reluctant. After MDT meetings with the lead pharmacist, concerns were addressed and discharge medication preparation on day 0 rose from 1% (1/15) to 80% (51/64). When the fast-track nurse was present, FTP was successful in 72% of cases; this dropped to 38% in the following month where she was absent.
Act—We have continued with the FTP checklist and the dedicated FTP nurse champion in recovery. Resistance from staff was approached by presenting interim results of FTP at departmental meetings, including maternal feedback, which was overwhelmingly positive.
PDSA cycle 3: improvement of flow of women after ELCS
Plan—Process mapping revealed that women sometimes experienced extended stays in recovery due to lack of postnatal beds and that this hindered the FTP. Furthermore, the postnatal ward was a busy area with a high workload and staff would find it difficult to identify and prioritise the women on FTP.
Do—We identified a space next to the recovery bay where FTP women could be cared for postnatally until the time of discharge. This space was also an antenatal induction of labour bay.
Study—Caring for FTP women separately from the postnatal ward was well received by recovery staff as they were able to provide continuity of care between recovery and the new FTP bay. Feedback from women was mixed. Some women did not like being in the same bay as antenatal women, who were sometimes in discomfort from early labour. As the programme expanded, the bay was not big enough to accommodate all the FTP women.
Act—We had to abandon this idea and women were moved to the postnatal ward after recovery. Stickers on the notes helped postnatal staff identify FTP women and we are currently developing an electronic record flag.
PDSA cycle 4: improvement of postoperative pain relief
Plan—Our surveys of maternal experience revealed that some felt they were asked to mobilise too early. We aimed to optimise postoperative pain control to enhance maternal experience and facilitate day 1 discharge.
Do—We engaged with anaesthetists to discuss optimal analgesia intraoperatively and the regime for enhanced recovery after ELCS. We identified a lead anaesthetist for FTP. The MDT agreed that oral morphine elixir could be given as required in the first 24 hours after ELCS and that women could be discharged if more than 2 hours had elapsed since the last dose.
Study—We analysed pain scores and maternal satisfaction with early mobilisation. Results in both areas were good. Satisfaction with early mobilisation and pain at day 1 was 4.81 (SD 0.49); 87% reported a score of 5.00 for excellent pain relief. Experience of pain control reported at day 7 was 4.61; 72% of women responded a 5.00 (online supplement 3).
bmjoq-2018-000465supp003.pdf (369.1KB, pdf)
Act—The multimodal pain control package we devised has been adopted. We are now considering adding patient-administered pain relief27 for simple analgesia and making physiotherapy advice videos available electronically via the dedicated smartphone app.
Results
Discharge rates
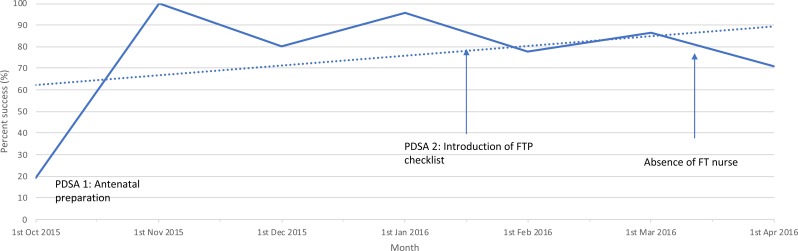
A total of 131 women entered the FTP between October 2015 and March 2016. Also, 30 of 131 women were subsequently excluded within 6 hours postoperatively by the operating surgeon or paediatrician due to a perioperative contraindication to ERAS (figure 1). Overall, 76.2% (77/101) of women who remained on the FTP were discharged on day 1. This represents an overall total of 38.0% of the 310 low-risk ELCS performed in the same period. The run chart (figure 2) describes monthly success rates. Increases in success can be seen following interventions of PDSA cycles 1 and 2. Mean duration of admission was 1.31 days (SD 0.80). This compares with a mean of 3.25 days (SD 0.45) (19.2% day 1 discharge) programme at baseline (table 1).
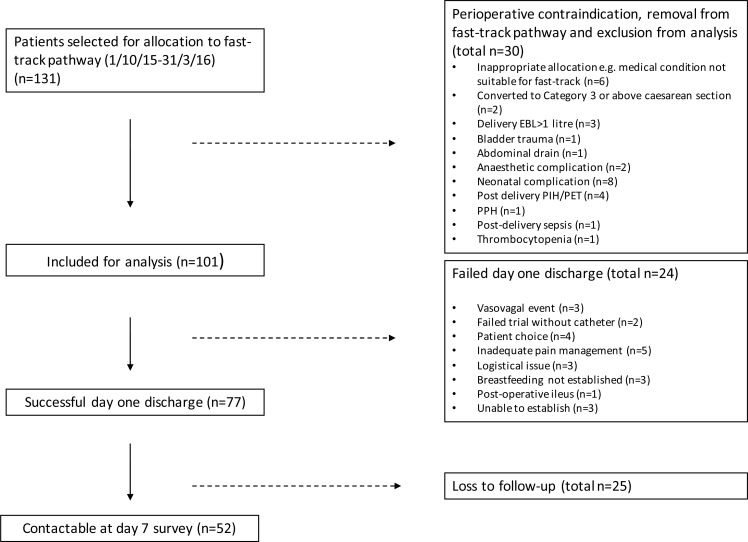
Figure 1.
Study profile. EBL, estimated blood loss; PET, pre-eclamptic toxaemia; PIH, pregnancy-induced hypertension; PPH, postpartum haemorrhage. Patients who were booked for a category 4 elective caesarean at Chelsea and Westminster Hospital were preoperatively assessed for allocation to fast-track pathway between 1 October 2015 and 31 March 2016. 131 of women were randomly invited and agreed to participate; all other women were allocated to the standard care enhanced recovery pathway. 30 women were removed from the pathway by the operating obstetrician immediately after surgery due to a maternal or neonatal perioperative contraindication. 24 women were unable to go home at day 1 due to reasons described. 77 women complete successful day 1 discharge and were followed up. 52 of 77 women were contactable at day 7.
Figure 2.
Run chart: day 1 discharge success rate on fast-track protocol during quality improvement project. FT, fast-track; FTP, fast-track protocol; PDSA, Plan-Do-Study-Act cycle. Run chart to demonstrate trends in day 1 discharge success rate over time period of 1 October 2015 to 1 April 2016. Raw data (solid line), average fit (dotted line), interventions and events are demonstrated (arrows).
Table 1.
Process measures at baseline (including introduction of an enhanced recovery programme) vs after introduction of a fast-track pathway
| Process measures | Baseline (2013–2014) |
Fast-track pathway (2015–2016) |
| Mean length of stay (SD) | 3.25 (0.45) | 1.31 (0.80) |
| % ELCS day 1 discharge | 19.2 (172/896) | 38.0 (118/310) |
| Entered theatre before 14:00 % (n/N) | 100 (16/16) | 93.1 (94/101) |
| Mean satisfaction day 1 pain control* (SD) | 3.00 | 4.81 (0.49) |
| Catheter removed within 6 hours % (n/N) | 36.7 (11/30) | 64.9 (50/77) |
| Cannulae removal within 6 hours % (n/N) | 30.0 (9/30) | 62.3 (48/77) |
| Median time of catheter removal (hours) (IQR) | 22.5 (2.25) | 6 (1.2) |
| Median time of intravenous cannula removal (hours) (IQR) | 27 (5.5) | 6 (0.8) |
| Discharge medications prepared by 6 hours | 6.7 (1/15) | 78.2 (79/101) |
| Breastfeeding uptake rate at day 1 % (n/N) | 98.0 (878/896) | 97.0 (98/101) |
| Reviewed by community midwife within 48 hours | NA | 96.2 (50/52)‡ |
| Readmission rate | 1.1 (59/5500)† | 3.9 (3/77) |
| Breast feeding at 7 days | NA | 94.2 (49/52) |
*5-point Likert scale.
†Background rate for all deliveries per annum.
‡52 women contactable at 7 days.
ELCS, elective caesarean section; N, total number; NA, not available; n, number.
The most commonly recorded reasons for failed day 1 discharge on the FTP were inadequate pain relief, women declining day 1 discharge and breast feeding not being established (figure 1). Two per cent of women had urinary retention after removal of catheter at 6 hours postoperatively.
Furthermore, 93.1% (94/101) women entered theatre before 14:00 versus 100% (16/16) at baseline; 78.2% had discharge prescriptions ordered from pharmacy before 6 hours’ post-surgery, and this compared with <1% before fast-track.
The large majority of data were recorded prospectively. Missing values were identified retrospectively through investigation of hospital records where possible. Recording of urinary catheter and cannulae removal and preparation of discharge prescriptions had some missing data as it could not reliably be elicited retrospectively. Introducing the FTP checklist in January 2016 increased 6-hour cannulae removal from 59% (October–December) to 65% (January–March) and decreased proportion of missing values from 33% to 13%. Catheter removal rose from 49% to 62%; missing values declined from 5.4% to <1%.
Readmission and breastfeeding rates
In total, 67.5% of women were contactable after discharge; 96.0% of these women were seen by a community midwife within 48 hours as per routine community care. Also, 15.4% of women reported visiting a health professional within 7 days of discharge for minor ailments for mother or baby. Three women (3.9%) were readmitted to a hospital, with diagnoses of urosepsis (day 4), unspecified viral illness (day 8) and maternal bradycardia (day 5). The background postnatal readmission rate for all deliveries in our unit in 2016/2017 was 1% (59/5500). The three cases were reviewed by two independent obstetricians; no readmissions were classified as being related to the pathway. The FTP had minimal impact on the success of breast feeding, 3 of 101 women were not discharged on day 1 as they stayed to establish breast feeding, while the remaining 98 women successfully established breast feeding by day 1 (comparing with a 99% breastfeeding uptake rate for all women in the unit). In all 49 women where 7-day follow-up data were available, 100% (49/49) were still breast feeding at day 7.
Maternal satisfaction
There were 63 and 52 women who completed satisfaction surveys at days 1 and 7, respectively. Moreover, 95.2% (60/63) of women scored a satisfaction of 4 or 5 at day 1 on the 5-point Likert scale. At day 7, overall satisfaction with the pathway was 4.75.
Qualitative data were coded into positive, neutral and negative (online supplement 4). Comments were overwhelmingly positive and useful for continual motivation of maternity unit staff.
bmjoq-2018-000465supp004.pdf (99.7KB, pdf)
Using coded thematic analysis, three main themes were highlighted as common threads: staff care, environment and processes.
Staff care
Nineteen women specifically mentioned the positive attention they received from staff in their feedback, allowing them to mobilise early and achieve a day 1 discharge. Patient 80 stated that she “Preferred to mobilise early so this was good”. Patient 41 thought, “The theatre staff and recovery staff were amazing”.
Three women specifically reported feeling poorly supported on mobilising. Patient 41 “Felt mobilising was too early and struggled to get to the toilet”. Five women expressed that they would have appreciated more support with breast feeding, one patient stating she was “Left to it… to express and breastfeed overnight” (patient 126).
Environment
After 6 hours, women were co-located with antenatal women, patient 37 commenting that she was “Very happy to be in the elective bay as I didn’t like the postnatal ward last time”. This was reflected by others who felt that this allowed efficient and greater continuity of care. In contrast, patient 41 complained that she “Felt disappointed in being put in an antenatal area as felt it wasn’t a nice atmosphere”. This view was shared by others, who felt being close to the antenatal patients hampered their recovery, especially when near to women in early labour.
Processes
Two women specifically expressed that preoperative information was useful, patient two stating that the pathway had “Surpassed expectations… (with) clear plan and goals”. Five women specifically commented that they felt pressured by the timing of the pathway. Patient 91 stated that: “Discharge home with a toddler at 24 hours was difficult and not acceptable, even with the support from my husband and mother”. Three women specifically commented on the positive experience of leaving on day 1. Patient 12 stated that her experience was “Better than expected… a great experience… better than the first”, and an additional two women stated that the pathway was an improvement from their previous caesarean section.
Financial implications
Consultation with hospital coders, finance departments and analysis of obstetric payments revealed the cost of planned LSCS of £3279/per delivery for day 1 discharge, £3616/per delivery for day 2 discharge and £3786/per delivery for day 3 discharge. Costs involved in the pathway set-up were estimated to be minimal. Allocation of a fast-track nurse was performed by adjustment of staff rostering and given 1 day/month ringfenced for audit work. Other clinical staff received no additional allocated time or payments. Resultantly, a minimum cost-saving of £337 per mother is estimated from day 1 discharge. The FTP increased day 1 discharge from 19.2% to 76.2% within the pathway. Moreover, 38.0% of women undergoing low-risk ELCS joined the FTP pathway; if this level of uptake could be sustained and assuming a 76.2% success rate, with 1000 ELCS per year, the overall financial savings to the hospital are estimated at a minimum of £99 886 per year. These numbers are gross estimates as in-depth cost-effectiveness analysis was outside the scope of this project; however, ERAS is consistently reported to demonstrate considerable savings, including after gynaecological surgery.28
Sustainability
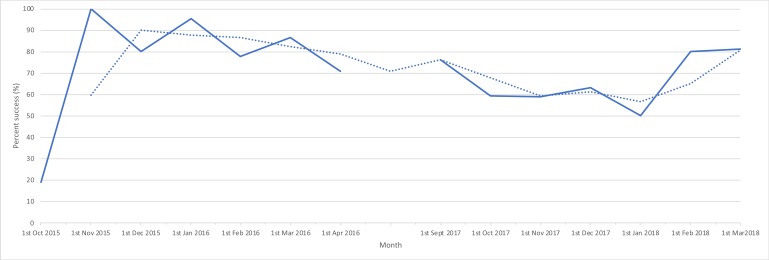
We have continued to collect data on the primary outcome of day 1 discharge to assess sustainability, along with maternal satisfaction to assess quality. Despite some month-to-month variability, success rates were maintained over time, with 81% success in the last time measure of February 2018 (figure 3). Uptake also increased with 280 women on the FTP in 2017/2018 versus 224 in 2015/2016.
Figure 3.
Run chart: day 1 discharge success rate on fast-track protocol during sustainability re-audit. Run chart to demonstrate trends in day 1 discharge success rate over time period 1 September 2017 to 1 March 2018, in comparison with quality improvement project period of 1 October 2015 to 1 April 2016. Raw data (solid line) and average fit (dotted line).
Lessons and limitations
Limitations
As historical audit data of good numbers were available, these were used in place of prospectively collected baseline measures. The data collection tool moving forward was designed specifically for prospective data collection. A limitation of this approach is that we were not always able to directly compare prospective measures with baseline measures.
The project was long, with three different stages of implementation, occurring over 3 years. There was therefore changes in staff over time. The fast-track team continued to meet regularly, allowing ongoing training of new fast-track recovery champions and good handover of data collection tools to new members.
It is a limitation that although we set a primary outcome measure, we did not set a SMART aim. The most appropriate SMART aim would have been to evaluate the proportion of day 1 discharge for women delivering by low-risk ELCS. Retrospective analysis revealed that this rose from 19.2% before FTP introduction to 38.0% at the end of 6 months. This is higher than the previously published rate of 25.2% by Wrench et al.17
A limitation of the data is that we only surveyed maternal satisfaction for women who achieved day 1 discharge on the FTP. Women who started on the FTP who did not manage to achieve day 1 discharge may have provided important insights, and these were not collected. Loss-to-follow-up and recall bias may have also affected reporting of satisfaction.
Recruitment to fast-track was slower than initially anticipated. We addressed this by increasing antenatal and staff engagement in PDSA 1 and through regular MDT and maternity unit meetings. However, we noticed that staff engagement required continual engagement for sustainability. For example, in the 2017/2018 re-audit, FTP success decreased in January 2018. Through contemporaneous data audit and monthly MDT, we were able to run a stakeholder engagement and reminder campaign regarding FTP protocols and a resultant increase in success was seen the following month. We have seen excellent maternal satisfaction with fast-track and believe that the answer to future sustainability is through antenatal education on benefits of ERAS and empowering women to request for fast-track surgery themselves, driving patient-centred care. This principle is the focus of our ongoing quality improvement.
This is a pathway designed for low-risk women. It is not appropriate for women with anticipated antenatal or perinatal issues. The facilities and layout of the maternity unit are also important when considering generalisability. It is optimal to have two theatres running concurrently so that emergency work does not disrupt elective work. We noticed that delays on time of surgery secondary to emergency cases could disrupt elective cases. If women entered recovery after 16:00, they were removed from the FTP, as removal of catheter and mobilisation overnight was not appropriate. Lastly, allocation of a fast-track nurse champion was found to be very important; in our unit, the authors believe there is evidence that this time investment was outweighed by financial and quality of care benefits. However, the effects, including on nursing workload, would need to be evaluated in each unit.29
To minimise loss-to-follow-up bias, we examined hospital records for all uncontactable women to detect readmission. However, we were not able to detect readmission to a different hospital unless informed by the woman.
Lessons learned
We did not involve team members with a Quality Improvement (QI) background from the start of this project. Hence, some of our PDSA cycles could have benefited from multiple measurements over time. We learnt about QI methodology as we carried out the project and would recommend that teams should have training or expertise in QI so that structured QI methodology could be followed from the outset.
We also found that some of our interventions were difficult to implement due to resistance from staff. Following ongoing engagement events and continued reporting of positive results, more staff were convinced of the benefits of FTP. This has also been observed in ERAS after major abdominal surgery.30 We learnt that engagement of front-line staff, and involvement in change ideas from the outset, is crucial to the success of any change intervention.
Conclusions
We demonstrate that an enhanced recovery FTP can accomplish day 1 discharge for low-risk women after caesarean delivery, with high levels of maternal satisfaction. Also, 76.2% of low-risk women delivering by ELCS achieved this aim in the FTP, representing 38.0% of all eligible women. Our analysis showed that the pathway was safe, with no adverse effects on pain control, breast feeding, maternal morbidity or hospital readmission. However, we did find that a flexible approach with careful preoperative selection and preparation of women was necessary. Selection of low-risk women with good social support systems is crucial for safety and success, and although only a small number of women declined day 1 discharge, it was not acceptable to every woman.
Sustainability
We learnt that contemporaneous outcome data collection, MDT meetings and maternal satisfaction analysis are highly valuable for sustainability, as they allow ongoing QI and staff motivation. Allocation of a fast-track team, and most importantly the fast-track nurse champion, were highly important for project ownership. This should be prioritised by other implementers. We aim to expand the pathway to more low-risk women by adding a prompt to the WHO sign-out checklist (“is this woman suitable for FTP”). We have set a SMART aim to increase the proportion of day 1 discharge for all low-risk ELCS, from 38.0% to 50.0%, over 12 months. This will require maintenance of high success rates but also increased recruitment to the FTP. We aim to achieve this through improved engagement of women antenatally, allowing maternal request to drive service sustainability.
Acknowledgments
We are extremely grateful to the dedicated work of recovery nurses Christina Chrysanthopoulou, Ana Calvo and Sandra Oliva for their commitment to this project and provision of enhanced recovery services, along with all staff in the C&W maternity unit, without whom this project would not have been possible.
Footnotes
Presented at: Historical audit data from 2013 were presented as a conference poster and published in conference proceedings (Halder S, Onwere C, Brennan C, et al. PA.07 Enhanced recovery programme for elective caesarean section: Abstract PA.07. Arch Dis Child Fetal Neonatal Ed 2014;99:A19.1–A19. doi:10.1136/archdischild-2014-306576.52). Interim audit results were presented as conference abstract and published in conference proceedings (Lever S, Chidimma Kanu, Jennifer Hanrahan et al. 24-hour discharge after elective caesarean section: a prospective audit. BJOG: Top scoring abstracts of the RCOG World Congress. 2016;126(S2)pp. 213).
Contributors: NS planned the study. SH conducted the 2013 enhanced recovery project. SJL, WD, CK, SH, CC, JH and NS designed the fast-track pathway in 2015. SL, JH, WD, SO, NS and SH designed the data collection tools, conducted data collection and performed data analysis. SL, CK, JH and NS led monthly multidisciplinary team meetings. CC designed and oversaw the anaesthetic pathway. SL, CK, SO, WD and NS drafted the manuscript. All authors contributed to the interim drafts and reviewed the final manuscript.
Funding: This project was part of a service evaluation. Service evaluation was approved by the NHS trust audit department and no further ethical approval deemed to be required. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Paton F, Chambers D, Wilson P, et al. Initiatives to reduce length of stay in acute hospital settings: a rapid synthesis of evidence relating to enhanced recovery programmes. Health Services and Delivery Research 2014;2:1–118. 10.3310/hsdr02210 [DOI] [PubMed] [Google Scholar]
- 2.Lucas DN, Gough KL. Enhanced recovery in obstetrics--a new frontier? Int J Obstet Anesth 2013;22:92–5. 10.1016/j.ijoa.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 3.Nicholson A, Lowe MC, Parker J, et al. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 2014;101:172–88. 10.1002/bjs.9394 [DOI] [PubMed] [Google Scholar]
- 4.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606–17. 10.1093/bja/78.5.606 [DOI] [PubMed] [Google Scholar]
- 5.de Groot JJA, Ament SMC, Maessen JMC, et al. Enhanced recovery pathways in abdominal gynecologic surgery: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2016;95:382–95. 10.1111/aogs.12831 [DOI] [PubMed] [Google Scholar]
- 6.Chapman JS, Roddy E, Ueda S, et al. Enhanced recovery pathways for improving outcomes after minimally invasive gynecologic oncology surgery. Obstet Gynecol 2016;128:138–44. 10.1097/AOG.0000000000001466 [DOI] [PubMed] [Google Scholar]
- 7.Miralpeix E, Nick AM, Meyer LA, et al. A call for new standard of care in perioperative Gynecologic oncology practice: impact of enhanced recovery after surgery (ERAS) programs. Gynecol Oncol 2016;141:371–8. 10.1016/j.ygyno.2016.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Secondary Care Analysis Team Hospital maternity activity 2015–2016 summary report. NHS digital, 2016. Available: http://content.digital.nhs.uk/searchcatalogue?productid=23494&q=%22nhs+maternity+statistics%22&topics=0%2fHospital+care&sort=Relevance&size=10&page=1#top
- 9.National Institute for Health and Care Excellence Cesearean Section—NICE Clinical Guideline 132, 2009. Available: https://www.evidence.nhs.uk/document?ci=http%3a%2f%2farms.evidence.nhs.uk%2fresources%2fqipp%2f29459%2fattachment&returnUrl=Search%3fq%3denhanced%2brecovery%2bfor%2belective%2bsurgery&q=enhanced+recovery+for+elective+surgery
- 10.Benhamou D, Kfoury T. Enhanced recovery after caesarean delivery: potent analgesia and adequate practice patterns are at the heart of successful management. Anaesthesia Critical Care & Pain Medicine 2016;35:373–5. 10.1016/j.accpm.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 11.Prior E, Santhakumaran S, Gale C, et al. Breastfeeding after cesarean delivery: a systematic review and meta-analysis of world literature. Am J Clin Nutr 2012;95:1113–35. 10.3945/ajcn.111.030254 [DOI] [PubMed] [Google Scholar]
- 12.Aluri S, Wrench IJ. Enhanced recovery from obstetric surgery: a U.K. survey of practice. Int J Obstet Anesth 2014;23:157–60. 10.1016/j.ijoa.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 13.Brown S, Small R, Argus B, et al. Early postnatal discharge from hospital for healthy mothers and term infants. Cochrane Database Syst Rev 2002;111 10.1002/14651858.CD002958 [DOI] [PubMed] [Google Scholar]
- 14.Corso E, Hind D, Beever D, et al. Enhanced recovery after elective caesarean: a rapid review of clinical protocols, and an umbrella review of systematic reviews. BMC Pregnancy Childbirth 2017;17 10.1186/s12884-017-1265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spanjersberg WR, Reurings J, Keus F, et al. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev 2011;90 10.1002/14651858.CD007635.pub2 [DOI] [PubMed] [Google Scholar]
- 16.Tan PC, Norazilah MJ, Omar SZ. Hospital discharge on the first compared with the second day after a planned cesarean delivery: a randomized controlled trial. Obstet Gynecol 2012;120:1273–82. 10.1097/AOG.0b013e3182723a95 [DOI] [PubMed] [Google Scholar]
- 17.Wrench IJ, Allison A, Galimberti A, et al. Introduction of enhanced recovery for elective caesarean section enabling next day discharge: a tertiary centre experience. Int J Obstet Anesth 2015;24:124–30. 10.1016/j.ijoa.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 18.Laronche A, Popescu L, Benhamou D. An enhanced recovery programme after caesarean delivery increases maternal satisfaction and improves maternal-neonatal bonding: a case control study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2017;210:212–6. 10.1016/j.ejogrb.2016.12.034 [DOI] [PubMed] [Google Scholar]
- 19.Pearsall EA, Meghji Z, Pitzul KB, et al. A qualitative study to understand the barriers and enablers in implementing an enhanced recovery after surgery program. Ann Surg 2015;261:92–6. 10.1097/SLA.0000000000000604 [DOI] [PubMed] [Google Scholar]
- 20.Jones EL, Wainwright TW, Foster JD, et al. A systematic review of patient reported outcomes and patient experience in enhanced recovery after orthopaedic surgery. Annals 2014;96:89–94. 10.1308/003588414X13824511649571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernard H, Foss M. Patient experiences of enhanced recovery after surgery (ERAS). Br J Nurs 2014;23:100–6. 10.12968/bjon.2014.23.2.100 [DOI] [PubMed] [Google Scholar]
- 22.Ghoreishi J. Indwelling urinary catheters in cesarean delivery. Int J Gynaecol Obstet 2003;83:267–70. 10.1016/S0020-7292(03)00144-9 [DOI] [PubMed] [Google Scholar]
- 23.Modesitt SC, Sarosiek BM, Trowbridge ER, et al. Enhanced recovery implementation in major gynecologic surgeries: effect of care standardization. Obstet Gynecol 2016;128:457–66. [DOI] [PubMed] [Google Scholar]
- 24.Forsmo HM, Pfeffer F, Rasdal A, et al. Compliance with enhanced recovery after surgery criteria and preoperative and postoperative counselling reduces length of hospital stay in colorectal surgery: results of a randomized controlled trial. Colorectal Dis 2016;18:603–11. 10.1111/codi.13253 [DOI] [PubMed] [Google Scholar]
- 25.Chelsea and Westminster NHS Foundation Trust New Mum & Baby app launched [webpage], 2017. Available: https://www.chelwest.nhs.uk/about-us/news/news-archive/2017/new-mum-and-baby-app-launched [Accessed 1 Apr 2019].
- 26.Brady KM, Keller DS, Delaney CP. Successful implementation of an enhanced recovery pathway: the nurse’s role. Aorn J 2015;102:469–81. 10.1016/j.aorn.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 27.Montgomery R, McNamara SA. Multimodal pain management for enhanced recovery: reinforcing the shift from traditional pathways through nurse-led interventions. Aorn J 2016;104:S9–S16. 10.1016/j.aorn.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 28.Relph S, Bell A, Sivashanmugarajan V, et al. Cost effectiveness of enhanced recovery after surgery programme for vaginal hysterectomy: a comparison of pre and post-implementation expenditures. Int J Health Plann Mgmt 2014;29:399–406. 10.1002/hpm.2182 [DOI] [PubMed] [Google Scholar]
- 29.Hübner M, Addor V, Slieker J, et al. The impact of an enhanced recovery pathway on nursing workload: a retrospective cohort study. Int J Surg 2015;24:45–50. 10.1016/j.ijsu.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 30.Hughes M, Coolsen MME, Aahlin EK, et al. Attitudes of patients and care providers to enhanced recovery after surgery programs after major abdominal surgery. J Surg Res 2015;193:102–10. 10.1016/j.jss.2014.06.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjoq-2018-000465supp001.pdf (307.1KB, pdf)
bmjoq-2018-000465supp002.pdf (245.3KB, pdf)
bmjoq-2018-000465supp003.pdf (369.1KB, pdf)
bmjoq-2018-000465supp004.pdf (99.7KB, pdf)