Abstract
Background
Nutritional interventions to prevent stunting of infants and young children are most often applied in rural areas in low‐ and middle‐income countries (LMIC). Few interventions are focused on urban slums. The literature needs a systematic assessment, as infants and children living in slums are at high risk of stunting. Urban slums are complex environments in terms of biological, social, and political variables and the outcomes of nutritional interventions need to be assessed in relation to these variables. For the purposes of this review, we followed the UN‐Habitat 2004 definitions for low‐income informal settlements or slums as lacking one or more indicators of basic services or infrastructure.
Objectives
To assess the impact of nutritional interventions to reduce stunting in infants and children under five years old in urban slums from LMIC and the effect of nutritional interventions on other nutritional (wasting and underweight) and non‐nutritional outcomes (socioeconomic, health and developmental) in addition to stunting.
Search methods
The review used a sensitive search strategy of electronic databases, bibliographies of articles, conference proceedings, websites, grey literature, and contact with experts and authors published from 1990. We searched 32 databases, in English and non‐English languages (MEDLINE, CENTRAL, Web of Science, Ovid MEDLINE, etc). We performed the initial literature search from November 2015 to January 2016, and conducted top up searches in March 2017 and in August 2018.
Selection criteria
Research designs included randomised (including cluster‐randomised) trials, quasi‐randomised trials, non‐randomised controlled trials, controlled before‐and‐after studies, pre‐ and postintervention, interrupted time series (ITS), and historically controlled studies among infants and children from LMIC, from birth to 59 months, living in urban slums. The interventions included were nutrition‐specific or maternal education. The primary outcomes were length or height expressed in cm or length‐for‐age (LFA)/height‐for‐age (HFA) z‐scores, and birth weight in grams or presence/absence of low birth weight (LBW).
Data collection and analysis
We screened and then retrieved titles and abstracts as full text if potentially eligible for inclusion. Working independently, one review author screened all titles and abstracts and extracted data on the selected population, intervention, comparison, and outcome parameters and two other authors assessed half each. We calculated mean selection difference (MD) and 95% confidence intervals (CI). We performed intervention‐level meta‐analyses to estimate pooled measures of effect, or narrative synthesis when meta‐analyses were not possible. We used P less than 0.05 to assess statistical significance and intervention outcomes were also considered for their biological/health importance. Where effect sizes were small and statistically insignificant, we concluded there was 'unclear effect'.
Main results
The systematic review included 15 studies, of which 14 were randomised controlled trials (RCTs). The interventions took place in recognised slums or poor urban or periurban areas. The study locations were mainly Bangladesh, India, and Peru. The participants included 9261 infants and children and 3664 pregnant women. There were no dietary intervention studies. All the studies identified were nutrient supplementation and educational interventions. The interventions included zinc supplementation in pregnant women (three studies), micronutrient or macronutrient supplementation in children (eight studies), nutrition education for pregnant women (two studies), and nutrition systems strengthening targeting children (two studies) intervention. Six interventions were adapted to the urban context and seven targeted household, community, or 'service delivery' via systems strengthening. The primary review outcomes were available from seven studies for LFA/HFA, four for LBW, and nine for length.
The studies had overall high risk of bias for 11 studies and only four RCTs had moderate risk of bias. Overall, the evidence was complex to report, with a wide range of outcome measures reported. Consequently, only eight study findings were reported in meta‐analyses and seven in a narrative form. The certainty of evidence was very low to moderate overall. None of the studies reported differential impacts of interventions relevant to equity issues.
Zinc supplementation of pregnant women on LBW or length (versus supplementation without zinc or placebo) (three RCTs)
There was no evidence of an effect on LBW (MD –36.13 g, 95% CI –83.61 to 11.35), with moderate‐certainty evidence, or no evidence of an effect or unclear effect on length with low‐ to moderate‐certainty evidence.
Micronutrient or macronutrient supplementation in children (versus no intervention or placebo) (eight RCTs)
There was no evidence of an effect or unclear effect of nutrient supplementation of children on HFA for studies in the meta‐analysis with low‐certainty evidence (MD –0.02, 95% CI –0.06 to 0.02), and inconclusive effect on length for studies reported in a narrative form with very low‐ to moderate‐certainty evidence.
Nutrition education for pregnant women (versus standard care or no intervention) (two RCTs)
There was a positive impact on LBW of education interventions in pregnant women, with low‐certainty evidence (MD 478.44g, 95% CI 423.55 to 533.32).
Nutrition systems strengthening interventions targeting children (compared with no intervention, standard care) (one RCT and one controlled before‐and‐after study)
There were inconclusive results on HFA, with very low‐ to low‐certainty evidence, and a positive influence on length at 18 months, with low‐certainty evidence.
Authors' conclusions
All the nutritional interventions reviewed had the potential to decrease stunting, based on evidence from outside of slum contexts; however, there was no evidence of an effect of the interventions included in this review (very low‐ to moderate‐certainty evidence). Challenges linked to urban slum programming (high mobility, lack of social services, and high loss of follow‐up) should be taken into account when nutrition‐specific interventions are proposed to address LBW and stunting in such environments. More evidence is needed of the effects of multi‐sectorial interventions, combining nutrition‐specific and sensitive methods and programmes, as well as the effects of 'up‐stream' practices and policies of governmental, non‐governmental organisations, and the business sector on nutrition‐related outcomes such as stunting.
Keywords: Child, Preschool; Humans; Infant; ; Poverty Areas; Urban Population; Bangladesh; Case-Control Studies; Controlled Before-After Studies; Diet, Healthy; Dietary Supplements; Growth Disorders; Growth Disorders/prevention & control; India; Micronutrients; Micronutrients/administration & dosage; Mothers; Mothers/education; Nutrients; Nutrients/administration & dosage; Nutrition Therapy; Nutrition Therapy/methods; Peru; Pregnant People; Randomized Controlled Trials as Topic; Thinness; Thinness/diet therapy; Wasting Syndrome; Wasting Syndrome/diet therapy; Zinc; Zinc/administration & dosage
Plain language summary
Effects of nutritional interventions to increase nutritional status in children living in urban slums in low‐ and middle‐income countries
UN‐Habitat estimates that there are at least one billion people living in urban slums, that is, places in cities without adequate access to health care, clean water, and sanitation. For this review, we defined low‐income informal settlements or slums as lacking one or more indicators of basic services or infrastructure. More than 90% of these slums are in low‐ and middle‐income nations and the residents are usually living in poverty, with little food security. One consequence of an inadequate diet is growth stunting, that is, very short stature for age. Stunting is associated with greater susceptibility to infection, cognitive (memory and thinking skills) and behavioural problems, and lower adult work performance and earnings. About 25% of children living in urban settings in low‐ and middle‐income countries are stunted. In slum areas, this figure is higher. For example, in Dhaka, Bangladesh it is 48%, and in Pune, India it is 59% of children under five years old.
Nutritional methods (interventions) to improve infant and young children's growth have not been comprehensively or systematically assessed for urban slums. We included 15 studies in the review, involving 9261 children less than five years old and 3664 pregnant women. About 73% of children were less than one year old. The interventions provided maternal education; nutrient supplementation of mothers, infants, and children; improving nutrition systems; or a combination of these but not dietary modification. The reliability of the studies was very low to moderate overall because studies were not designed to cope with research problems linked to urban slum communities, such as high mobility and high loss of participants to follow‐up. This meant that the effectiveness of the intervention could not be properly assessed at later dates.
We assessed the effect of interventions taking both statistical and clinical significance into account. Where intervention outcomes were statistically insignificant, we conclude there was 'unclear effect'.
There was no effect of giving mothers nutrient supplementation on birth weight and length, there were inconclusive results for nutrient supplementation in infants and children on improving children's height or stunting status, there was a positive impact on birth weight of maternal education interventions where there was a positive difference in birth weight of 478 g in infants exposed to the intervention, and inconclusive results of improving health systems that support nutrition on children's stunting status and a positive effect on height. There were no reported side effects from these interventions.
The review showed the need to better understand urban slum environments and their people as evidence showed that interventions included in this review were successful in other locations outside of urban poor areas. More evidence is needed of the effects of multi‐sectorial interventions, combining nutrition‐specific and sensitive methods and programmes, as well as the effects of 'up‐stream' practices and policies of governmental, non‐governmental organisations (NGOs), and the business sector to improve low birth weight and stunting in poor urban environments.
Summary of findings
Summary of findings for the main comparison. Zinc supplementation in pregnant women versus supplementation without zinc or placebo to reduce stunting in children (low birth weight, length at birth and at 12 months).
| Zinc supplementation in pregnant women versus supplementation without zinc or placebo to reduce stunting in children (low birth weight, length at birth and at 12 months) | ||||
|
Patient or population: pregnant women Settings: poor urban slums Intervention: zinc supplementation Comparison: supplementation without zinc or placebo | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Length (cm) at birth |
No evidence of an effect: MD –0.13 (–0.36 to 0.10) | 1337 (2) | ⊕⊕⊕⊝ Moderatea | Caulfield 1999; Osendarp 2000 |
| Length (cm) at 12 months | Unclear effect: 0.13, SD 0.16 (longitudinal regression modelling, adjusted for age; age quadratic; age–treatment interaction; sex; sex–treatment interaction; birth anthropometric measure; maternal anthropometry; primiparity; breastfeeding; complementary feeding in previous months; diarrhoea morbidity; and hygiene and sanitation index) | 237 (1) | ⊕⊕⊝⊝ Lowb | Iannotti 2008 |
| Low birth weight (g) | No evidence of an effect: MD –36.13 (–83.61 to 11.35) | 1367 (2) | ⊕⊕⊕⊝ Moderatec | Caulfield 1999; Osendarp 2000 |
|
CI: confidence interval; MD: mean difference; SD: standard deviation. GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
aThe overall risk of bias was high for Iannotti 2014. We downgraded two levels for indirectness of evidence (geographical coverage) and precision. Refer to Appendix 14 for more details. bThe overall risk of bias was high for Caulfield 1999 and moderate for Osendarp 2000. We downgraded one level for indirectness of evidence (geographical coverage). Refer to Appendix 14 for more details. cThe overall risk of bias was high for Caulfield 1999 and moderate for Osendarp 2000. We downgraded one level for indirectness of evidence (geographical coverage). Refer to Appendix 14 for more details.
Summary of findings 2. Micronutrient or macronutrient supplementation interventions in children versus no intervention, or placebo to reduce stunting (height‐for‐age, length velocity, and length at 12 months).
| Micronutrient or macronutrient supplementation interventions in children versus no intervention, or placebo to reduce stunting (HFA, length velocity, and length at 12 months) | ||||
|
Patient or population: children under 5 years old Settings: poor urban slums Intervention: micronutrient or macronutrient supplementation Comparison: no intervention or placebo | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Height‐for‐age (z‐score) | No evidence of an effect: MD –0.02 (–0.06 to 0.02) | 2601 (3) | ⊕⊕⊝⊝ Lowa | Iannotti 2014; Oelofse 2003; Taneja 2010 |
|
Height‐for‐age (z‐score) Length gain (6 months) |
Unclear effect: change in height‐for‐age z‐score and length were not significantly different among the groups. | 653 (1) | ⊕⊕⊝⊝ Lowb | Rahman 2002 |
|
Height‐for‐age (%) at 18 months, 21 months Length gain |
Unclear effect | 324 (1) | ⊕⊕⊕⊝ Moderatec | Radhakrishna 2013 |
| Length velocity (change in cm since start of supplementation) | Unclear effect: controlling for initial anthropometric status, sex, and age at the beginning of supplementation, and for additional covariates (feeding practices, maternal characteristics, socioeconomic variables, and initial presupplementation morbidity rates) Unclear effect: baseline to 2 months, baseline to 4 months, and baseline to 6 months. |
315 (1) | ⊕⊕⊝⊝ Lowd | Begin 2008 |
| Length velocity (cm/month) | Effect: MD 0.22 (0.02 to 0.43) | 75 (1) | ⊕⊝⊝⊝ Very lowe | Moursi 2003 |
| Length (cm) at 12 months | Effect: MD 2.3 (no CI provided) | 100 (1) | ⊕⊕⊝⊝ Lowf | Sur 2003 |
|
CI: confidence interval; MD: mean difference. GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
aThe overall risk of bias was high for Oelofse 2003 and Iannotti 2014, and moderate for Taneja 2010. We downgraded two levels for bias and inconsistency. Refer to Appendix 14 for more details. Therefore, the overall GRADE was low. bThe overall risk of bias was high. We downgraded two levels for bias and indirectness of evidence (geographical coverage). Refer to Appendix 14 for more details. Therefore, the overall GRADE was low. cThe overall risk of bias was moderate and there was no evidence that bias had significantly influenced the results of the intervention. We downgraded one level for indirectness of evidence (geographical coverage). Refer to Appendix 14 for more details. Therefore, the overall GRADE was moderate. dThe overall risk of bias was high. We downgraded two levels for bias and indirectness of evidence (geographical coverage). Refer to Appendix 14 for more details. Therefore, the overall GRADE was low. eThe overall risk of bias was high. We downgraded three levels for bias, indirectness of evidence (geographical coverage), and precision, Refer to Appendix 14 for more details. Therefore, the overall GRADE was very low. fThe overall risk of bias was moderate. We downgraded two levels for indirectness of evidence (geographical coverage) and precision. Refer to Appendix 14 for more details. Therefore, the overall GRADE was low.
Summary of findings 3. Nutrition education intervention for pregnant women versus standard care or no intervention, to reduce stunting in children (low birth weight).
| Nutrition education intervention for pregnant women versus standard care or no intervention, to reduce stunting in children (low birth weight) | ||||
|
Patient or population: pregnant women Settings: poor urban slums Intervention: nutrition education Comparison: standard care or no intervention | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Nutrition education versus standard care or no intervention | ||||
| Low birth weight (g) | Effect: MD 478.44 (423.55 to 533.32) | 415 (2) | ⊕⊕⊝⊝ Lowa | Akter 2012; Jahan 2014 |
|
CI: confidence interval; MD: mean difference. GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
1The overall risk of bias was high for both studies. We downgraded two levels for bias and indirectness of evidence (geographical coverage). Refer to Appendix 14 for more details. Therefore, the overall GRADE was low.
Summary of findings 4. Nutrition systems strengthening interventions targeting children compared with no intervention or standard care to reduce stunting (height‐for‐age, length at 18 months).
| Nutrition systems strengthening interventions targeting children compared with no intervention or standard care to reduce stunting (height‐for‐age, length at 18 months) | ||||
|
Patient or population: children under 5 years old Settings: poor urban slums Intervention: nutrition systems strengthening Comparison: no intervention or standard care | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Nutrition support vs no intervention | ||||
| Height‐for‐age (z‐score) at 18 months | Effect: MD 0.386 (0.209 to 0.562) unadjusted difference; MD 0.272 (0.099 to 0.445) adjusted for socioeconomic status, hygiene score, and birth weight variables | 377 (1) | ⊕⊕⊝⊝ Lowa | Penny 2005 |
| Height‐for‐age (z‐score) | Unclear effect: intervention (2013 boys –1.33, 2013 girls 1.41; 2011 boys –1.69, 2011 girls –1.46) control (2013 boys –1.27, 2013 girls 1.28; 2011 boys –1.65, 2011 girls –1.49) | 999 (1) | ⊕⊝⊝⊝ Very lowb | Pridmore 2014 |
| Length (cm) at 18 months | Effect: MD 1.068 (0.488 to 1.648) unadjusted difference; MD 0.714 (0.146 to 1.282) adjusted for socioeconomic status, hygiene score, and birth weight variables | 377 (1) | ⊕⊕⊝⊝ Lowc | Penny 2005 |
|
CI: confidence interval; MD: mean difference. GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
aThe overall risk of bias was high and, as it was a systems strengthening intervention, it was not possible to blind allocation. We downgraded two levels for bias and indirectness of evidence (geographical coverage). Refer to Appendix 14 for more details. Therefore, the overall GRADE was low. bThe overall risk of bias was high because this was not a randomised controlled trial (downgraded one level). We started GRADE assessment at low because this is not an RCT. We downgraded two levels for bias and indirectness of evidence (geographical coverage). Refer to Appendix 14 for more details. Therefore, the overall GRADE was very low. cThe risk of bias was high and as it was a systems strengthening intervention it was not possible to blind allocation. We downgraded two levels for bias and indirectness of evidence (geographical coverage). Refer to Appendix 14 for more details. Therefore, the overall GRADE was low.
Background
In 2012, approximately 33% of urban residents in low‐ and middle‐income countries (LMIC) lived in slums (United Nations 2012), and the most recent estimate suggests that by 2030 slum populations of LMIC are expected to reach two billion people (UN‐Habitat 2003). For the purposes of this review, we follow the UN‐Habitat 2004 definition for low‐income informal settlements or slums as lacking one or more indicators of basic services or infrastructure including improved water and sanitation facilities, security of tenure, durability of housing, and sufficient living area. Urban settings can be defined by administrative boundaries, a threshold of population size, or population density among other criteria which can vary from country to country. Periurban setting is an area between consolidated urban and rural regional (UNICEF 2012). Every day, more than 100,000 people move to slums in LMICs. Nearly 1.5 billion people currently live in urban slums without adequate access to health care, clean water, and sanitation (BRC 2012; UN‐Habitat 2017). Evidence shows that children living in slums are more likely to experience undernutrition, including stunting, than children living elsewhere in the city (Awasthi 2003; Ezeh 2017; Ghosh 2004; Haddad 1999; Hussain 1999; Lilford 2017; Menon 2001; Pryer 2002; Ruel 1999; Unger 2013). While efforts towards reduction of stunting have succeeded globally (Lundeen 2014), and in Ethiopia and in Mahrastrata state, India (Haddad 2014), in Africa and South Asia (e.g. Pakistan, Congo, Senegal, Sierra Leone), stunting rates have unfortunately remained largely static (Bhutta 2013; Development Initiatives 2017). Achieving 2025 World Health Organization (WHO) global health targets to reduce stunting by 40% in children aged under five years will depend on continuous efforts to prevent stunting within slums.
Low height‐for‐age (HFA) or stunting reflects a failure to reach a minimal stature associated with current and future healthy development and is a key indicator of chronic undernutrition. Stunted children have impaired growth with permanent consequences in their adult life, and face a high risk of morbidity and mortality (Black 2008; Dewey 2011; Grantham‐McGregor 2007; McDonald 2013; Victora 2008). Stunting is associated with greater susceptibility to infection and other diseases, with cognitive and behavioural deficits, with poor school performance, and lower adult work performance and earnings. Stunting in infancy and childhood is one of the primary factors that recycles poverty into future generations. Data available for the year 2016 from the WHO for 199 LMICs found that, on average, 25.24% of all children living in urban areas are stunted. Estimates for stunting in slum areas in cities are not available, but are likely to be higher. One study conducted in an urban slum area of Dhaka, Bangladesh published in 2018 reports that 48% of children were stunted at 24 months of age (Islam 2018), and another study. in Pune, India, conducted in 2012, reported that 58.7% of all infants and children under five years old were stunted (Mamulwar 2014). The WHO has set a target to reduce stunting globally by 40% by 2025, which requires a 3.9% reduction per annum. If this target could be met and extended to 2030 at the same rate of reduction then the number of stunted children would reduce to 86 million by 2030. This target is unlikely to be realised given that the current trend towards the 2025 target suggests that there will still be 127 million stunted children rather than the WHO target of 100 million (Development Initiatives 2018). Reducing stunting requires context‐relevant interventions that work for these populations.
Description of the condition
Stunting reflects chronic undernutrition during the most critical periods of growth and development in early life. Stunting in children can be assessed by physical growth performance through anthropometry. Growth faltering happens mostly from three months to 18 to 24 months of age (Victora 2010). The prevalence of stunting increases very rapidly between 12 and 24 months of age (40% to 54%), continues increasing until 36 months of age (58%), and then remains fairly stable until five years of age (55%) (Bhutta 2013).
Diagnosis and causes
Stunting is defined as the percentage of children aged 0 to 59 months whose HFA is below minus two standard deviations for moderate and minus three standard deviations for severe stunting from the median of the 2006 WHO Child Growth Standards (UNICEF 2013).
The causes of stunting are multi‐sectorial and multifactorial, as shown in Figure 1, and are classified as immediate (individual level), intermediate (individual/household level), and underlying (maternal, household, and regional characteristics). Common immediate causes of stunting among infants and young children are intrauterine growth retardation (IUGR), inadequate nutrition after the recommended period of exclusive breastfeeding, and frequent infections during early life (Frongillo 1999; Shrimpton 2001; Victora 2010). Figure 1 shows a model by Fenske 2013, which conceptualised the causes of stunting in India. It used regression analysis to model the effects of determinants for stunting. Although this conceptual framework considers infections as intermediate causes, we considered them in this review as immediate based on the work by Bhutta 2013; Black 2013; Frongillo 1999; Shrimpton 2001; and Victora 2010. Driving these immediate causes are intermediate and underlying causes including food security; childcare practices; maternal education; access to health services;, and water, hygiene, and sanitation conditions. Ultimately, these factors are embedded in the larger political, economic, social, and cultural environment (Bogin 2014). In Fenske's model, child age and sex were considered non‐modifiable risk factors, with household wealth and maternal education as modifiable, and showing the largest effects on stunting (Fenske 2013).
1.
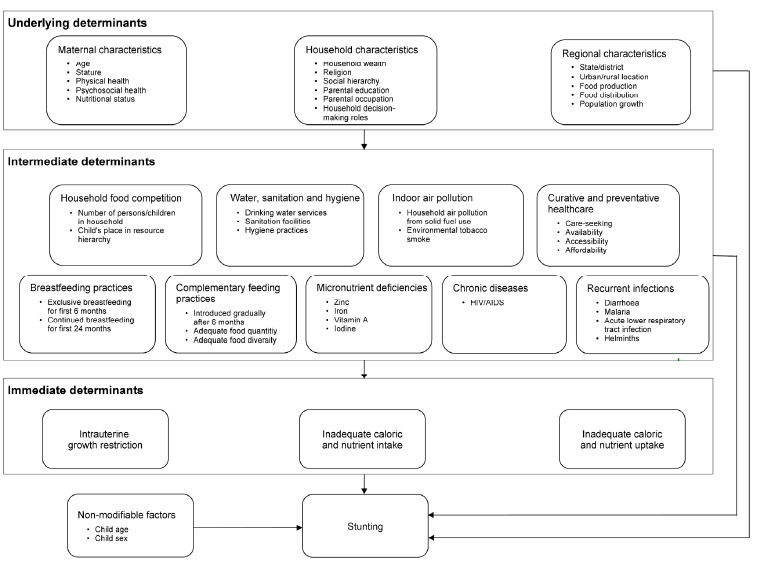
Conceptual framework of stunting (source: Fenske 2013)
Children are typically screened as stunted in the first two years of life (Victora 2010). However, the process for a child becoming stunted is determined by the cumulative effects that span across generations. Even before the child is conceived, if his/her mother has previously experienced nutritional insults, this can have detrimental impacts on her children (Victora 2010). This relates to the intergenerational influence hypothesis that malnutrition of the mother during her foetal and early postnatal development has health consequences for her offspring, especially low birth weight (LBW) and obesity (Barker 1990; Barker 1995; Bogin 2007; Drake 2004; Gluckman 2004; Kuzawa 2005; Kuzawa 2007; Varela‐Silva 2009). For the child, the process of becoming stunted may start in utero if the pregnant mother has nutrient deficiencies, infections, or other insults (Dewey 2011).
To explore the root causes of children's undernutrition in the context specific to poor urban settings, we conducted a scoping review that also assessed the impact of risk factors on children's undernutrition (Goudet 2017). This scoping review found that the mother's education was the most reported factor associated with a child's stunting, followed by the child's age, the child's gender, household income, family size, and the child's morbidity status. These findings were similar to those reported by Fenske 2013. In urban settings, the mother's education may be even more important for nutritional status than in other contexts as educational attainment can be linked to the ability of mothers to make choices in caring practices (Unger 2013). Education is also associated with income and income is important in influencing food choices and diversity available, meaning that education has two potential leveraging systems. In terms of the age of the child, the reported age groups with the highest prevalence of stunting were: 36 to 47 months (Olack 2011), and 48 to 60 months (Alam 2011). The study by Alam excluded those under 24 months and focused on 24‐ to 60‐month olds (Alam 2011). Analysis by gender showed that boys were more at risk than girls. Low household income was identified as a risk factor and is also well known to be an underlying cause of stunting. In urban settings, the dependence on cash flow aggravates the importance of household income. On family size, there were conflicting results with two studies finding that living in a small family was a predictor of stunting (Mian 2002; Veiga 2010), while three studies found the opposite (Neervoort 2013; Shit 2012; Singh 2011). Finally, in terms of morbidity, diarrhoea was the most reported type of illness associated with stunting.
Consequences of stunting
The vicious cycle of undernutrition and disease means that stunted children are more likely to become sick due to their immunodeficiency status and sick children are more likely to become stunted due to poor nutrient absorption (UNICEF 2013). A severely stunted child faces a 5.5 times higher risk of dying than a non‐stunted child (McDonald 2013). In terms of disability and mortality burden, stunting in children 36 months or older contributes to about 9.4 million disability‐adjusted life years (Bhutta 2013). In the long term, stunting in children may affect adult size, intellectual ability, school achievement, school performance, economic productivity, and reproductive ability, and may increase the risk of metabolic disorders and cardiovascular disease (Black 2008; Dewey 2011; Grantham‐McGregor 2007; Victora 2008). The fact that stunted children are likely to develop obesity and other chronic diseases in their adult life places them at even greater risk in transitional countries experiencing increasing urbanisation and shifts in diet and lifestyle. The consequences of nutritional transition in urban settings create economic and social challenges in many LMICs where stunting is prevalent, especially among poorer population groups (UNICEF 2013). This nutritional transition will contribute to the intergenerational malnutrition cycle with the youngest generation born to obese or overweight low‐income mothers being at higher risk of being malnourished (Varela‐Silva 2012).
A window of opportunity to prevent long‐lasting consequences of stunting exists in the first 1000 days of a child's life (the first two years of a child's life and the nine months of life in their mother's uterus) (Bhutta 2008; UNICEF 2013; Victora 2008). Long‐term consequences of stunting can be averted or minimised in adult life if it is prevented within this timeframe (Bhutta 2008; UNICEF 2013; Victora 2008). There is a limited opportunity for catch‐up growth during adolescence because stunted children often experience a delay in skeletal maturation, lengthening the total period of time for growth in height (Dewey 2011; Martorell 1994). Even so, the height deficits experienced by the age of seven years are often greater than any possibility for growth recovery during adolescence (Bogin 1992).
Description of the intervention
Reductions in stunting can be achieved through evidence‐based interventions. In the Lancet series (2008) on maternal and child undernutrition, there was clear evidence for a set of interventions that were successful in promoting children's health (Bhutta 2008). Combining and scaling up 10 of these proven nutrition‐specific interventions (the ones in yellow and orange in Figure 2) to 90% coverage could reduce stunting by 20%, which represents 33.5 million fewer stunted children (Bhutta 2013; Fenske 2013; Milman 2005; Remans 2011).
2.
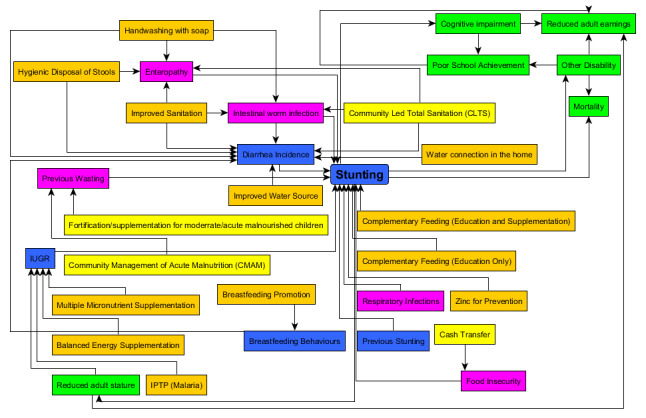
Logic model showing direct linkages between stunting risk factors, intervention and mortality/disability (the model is inspired by LIST and purple and yellow boxes were added based on new evidence gathered in this review) (LIST 2014). Blue, or purple (new) are risk factors, orange or yellow (new) are interventions, green are consequences of stunting.
Ruel 2013 categorised these interventions as follows.
Nutrition‐specific interventions address the immediate determinants of foetal and child nutrition and development; adequate food and nutrient intake, feeding, caring and parenting practices, and low burden of infectious diseases.
Examples of nutrition‐specific interventions include: nutrition intervention targeting adolescents, and women at preconception, and during pregnancy; maternal dietary or micronutrient supplementation; promotion of optimum breastfeeding; complementary feeding and responsive feeding practices and stimulation; dietary supplementation; diversification and micronutrient supplementation or fortification for children; treatment of severe acute malnutrition; disease prevention and management; nutrition in emergencies.
Nutrition‐sensitive interventions address the underlying determinants of foetal and child nutrition and development: food security; adequate caring resources at the maternal, household and community levels; and access to health services and a safe and hygienic environment; and incorporate specific nutrition goals and actions. Nutrition‐sensitive programmes can serve as delivery platforms for nutrition‐specific interventions, potentially increasing their scale, coverage, and effectiveness.
Examples of nutrition‐sensitive interventions include: agriculture and food security; social safety nets; early child development initiatives; maternal mental health services; women's empowerment initiatives; child protection services; schooling; water, sanitation, and hygiene (WASH) initiatives; health and family planning services.
Specifically, to tackle the direct causes of stunting, recommended interventions should focus on improving nutrition and preventing related diseases (Figure 2; LIST 2014). The logical model in Figure 2 shows how interventions can tackle the immediate causes of stunting: diarrhoea/enteropathy/intestinal worm infections, IUGR, breastfeeding behaviours, respiratory infections, previous wasting, and previous stunting. This model has been designed based on the 'lives saved tool' for stunting and has integrated enteropathy, intestinal worm infections (Black 2013; Brown 2013; Keusch 2013; Keusch 2014; Lantagne 2014; Olofin 2013; Richard 2013), and previous wasting (Khara 2014), as additional risk factors based on the cited work (purple colour in Figure 2). The related interventions have been added in yellow colour. The model has also been modified to integrate the consequences of stunting (presented in green colour) and new risk factors identified in our scoping review and previous literature have been added (in purple colour). Micronutrient interventions for children include strategies for supplementation of vitamin A (in the neonatal period and late infancy), preventive zinc supplements, iron supplements for children in areas where malaria is not endemic (in malaria‐endemic areas, iron supplementation can increase the risk of mortality) (Yakoob 2011), and universal promotion of iodised salt (Black 2013). Improvement of complementary feeding could substantially reduce stunting and the related burden of disease (Imdad 2011). Strategies for achieving this in food‐secure populations include nutrition counselling, and in food‐insecure populations nutrition counselling, food supplements, conditional cash transfers, or a combination of these treatment interventions for acute malnutrition include community‐based management of acute malnutrition (CMAM) and fortification/supplementation for moderate acute malnourished (MAM) children. Interventions to reduce the risk of IUGR include intermittent preventive treatment of malaria during pregnancy, use of insecticide‐treated bed nets for pregnant women (Ishaque 2011), multiple micronutrient supplementation, and balanced energy protein supplementation for pregnant women who are food insecure (Imdad 2011). To reduce the risk of the effect of diarrhoea/enteropathy on stunting (Checkley 2008), interventions include WASH interventions (e.g. improved water sources, water in the home, improved sanitation, handwashing with soap, disposal of faeces, and community‐led total sanitation) (Cairncross 2004; Cairncross 2010), as well as promotion of optimal breastfeeding practices (Black 2013; Lamberti 2013). Cash transfer can have an impact on children's nutrition and can lead to a reduction in stunting in food‐insecure households because the cash enables households to buy better food and healthcare (Bangladesh) (Mascie‐Taylor 2010).
In the context of urban slums, our scoping review found that the interventions tackling children's stunting status were (Goudet 2017):
nutritional interventions (supplementation, micronutrient fortified food or complementary food, promotion of nutrition);
health interventions (Reproductive and Child Health (RCH) and immunisation, and performance pay related to improved provision and access to health services);
WASH interventions (sanitation programmes and community‐based handwashing programmes);
safety net programmes (conditional cash transfer) (Table 1).
Table 1. Findings from our scoping review (children under five years old, stunting as an outcome)
| Authors | Study title | Study location | Study design | Intervention type |
| Attanasio 2005 | The short‐term impact of a conditional cash subsidy on child health and nutrition in Colombia | Colombia | RCT | Safety net – conditional cash transfer with nutritional transfer |
| Berger 2008 | Malnutrition and morbidity among children not reached by the national vitamin A capsule programme in urban slum areas of Indonesia | Jakarta, Surabaya, Semarang, Makassar, and Padang, Indonesia | Cluster‐RCT | Nutrition – micronutrient supplementation (vitamin A) |
| Kiran 2011 | Influence of Reproductive and Child Health programme on nutritional status and immunisation status in urban slum children | India | Cross‐sectional study | Health – reproductive and child health (immunisation, antenatal care, skilled attendance during delivery, and treatment of common childhood illnesses) |
| Langford 2011 | Hand‐washing, subclinical infections, and growth: a longitudinal evaluation of an intervention in Nepali slums | Katmandu, Nepal | Non‐RCT | WASH – community‐based handwashing programme |
| Oelofse 2003 | Micronutrient deficiencies in South African infants and the effect of a micronutrient‐fortified complementary food on their nutritional status, growth and development | South Africa | RCT | Nutrition – micronutrient fortified complementary food |
| Semba 2011 | Consumption of micronutrient‐fortified milk and noodles is associated with lower risk of stunting in preschool‐aged children in Indonesia | Urban slums and non‐urban slum areas, Indonesia | Cluster‐RCT | Nutrition – micronutrient fortified milk and fortified noodles |
| Waihenya 1996 | Maternal nutritional knowledge and the nutritional status of preschool children in a Nairobi slum | Kibera slum, Nairobi, Kenya | Cross‐sectional study | Nutrition – promotion of nutrition |
| RCT: randomised controlled trial; WASH: water, sanitation, and hygiene. | ||||
This systematic review focused on nutritional intervention only within the nutrition‐specific interventions, as this was the most reported type of intervention in the scoping review.
How the intervention might work
We created a conceptual model of how a nutritional intervention in urban settings might work (Figure 3). The model presents nutritional interventions that tackle determinants of stunting at the individual, household, community, and country level, as evidence has shown that these levels have an independent effect on children's health and nutritional status (Goudet 2011a; Goudet 2011b; Harpham 2009; Madise 1999; Milman 2005; Spears 2013; Unger 2013). Milman 2005 demonstrated that factors at country level (initial and change in immunisation rate, initial and change in safe water rate, initial female literacy rate, initial government consumption, initial income distribution, and the initial proportion of the economy devoted to agriculture) were independently associated with improvements in stunting. The study findings suggested that both interventions at country level and specific interventions at community/individual level were important. At the household level, determinants noted were socioeconomic status (SES), cultural and psychosocial factors that influenced behaviours and childcare practices, food security (access to healthy food), and access to public services. At the community level, the determinants include local governance (capacity and ability), legal and political structures, employment opportunities, markets, and willingness of the private sector to support nutrition goals. It is also key to determine the correct level of intervention to maximise programme effectiveness. For example, sanitation upgrades were more effective in promoting child health when implemented using a clustered method rather than at an individual household level in an urban context (Bangladesh) (Buttenheim 2007).
3.
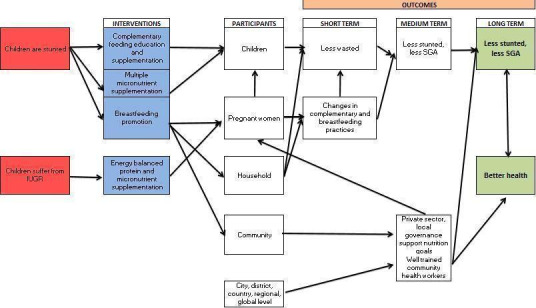
Logic model of nutritional intervention tackling stunting in an urban setting
Interventions that aim to change social factors at a household or community level can contribute towards an enabling environment for improved child nutrition (Pridmore 2007; Pridmore 2010). Promoting understanding of these factors and involving the community residents, community leaders, and community‐based organisations can encourage understanding of issues related to land tenure and people's rights in order to develop successful programmes (BRC 2012; Ghosh 2004). Approaches to delivering interventions can involve governmental or non‐governmental agencies undertaking broad‐scale programmes, or community‐based initiatives that use community resources internal to the slums (Ernst 2013). Both of these strategies may involve fundamental infrastructure changes and include improvements to housing structures, developing roadways, and access to water and sanitation, which have an impact on children's health. Interventions that work to effect more immediate change in health outcomes include improved access to quality health care and improving the quality of local schools and the training of community health workers (Ernst 2013). A notion of time has been integrated to reflect how to eliminate stunting long term. These interventions should be supplemented by improvements in the intermediate and underlying determinants of stunting by creating an enabling environment and a political will towards stunting reduction.
Why it is important to do this review
The review was informed by the findings from the authors' scoping review (Goudet 2017), which confirmed the value of undertaking a full systematic review. The results for the interventions, although limited (21 studies eligible with only 15 using stunting as an outcome), were useful in mapping the interventions in nutrition specific and sensitive (nutrition, health, water, sanitation, hygiene and safety net programmes to protect participants from poverty). We were able to extract enough information from most studies to show nutritional outcomes and to measure effectiveness. The scoping review helped to identify the appropriate Population, Intervention, Comparison and Outcome (PICO) parameters for this systematic review (Table 2). We concluded that it would be useful to conduct a full systematic review specific to nutritional interventions only, with a more detailed search strategy, to assess the quality of the studies and to conduct meta‐analyses to calculate and compare the effect of nutritional interventions on children's nutritional health. Specifically the reasons for conducting this review were existing review evidence was unable to analyse stunting in children aged less than five years in LMIC urban slums in a systematic manner and other information sources such as grey literature, nutrition technical websites, and programmes under implementation were not included in the existing significant relevant reviews (i.e. Bhutta 2008; Bhutta 2013). For this Cochrane systematic review, the interventions specificity targeted slum areas and stunting, and therefore this review differed from those previously published in the Lancet series because of its geographical focus. We also searched new sources of published studies and cover work since 2012 (the Lancet systematic review ended in 2011).
Table 2. Parameters informed by the scoping review
| Parameters | Scoping review | Recommendations for Cochrane Review |
| Type of studies | Included 12 RCTs, 33 cross‐sectional studies, 1 case study, and 11 cohort studies | We included randomised (including cluster‐randomised) and quasi‐randomised trials with either individual or cluster randomisation, and non‐RCTs, controlled before‐and‐after studies (case control or repeated measures), ITS, and historically controlled studies. |
| Population | More than half of the studies (51%) focused on children under 5 years of age | We focused on infants and children under 5 years old. Research showed that it is key to intervene in children's stunting as early as possible in a child's life (fetus up to 24 months old). As only 19% of the studies focused on children under 2 years old, the under 5 years range was preferred. |
| Intervention | All of the interventions were nutrition‐specific or nutrition‐sensitive with nutritional intervention being the most dominant type (76%): school feeding, supplementation/fortification, and nutrition promotion. The other interventions were health (14%), water sanitation and hygiene (9%), and programmes that provided a safety net to individuals and families to protect them from poverty (1%). Only 71% of interventions were assessed as effective. | As nutritional intervention was the most reported type, we limited the parameter to this category. |
| Comparison | The comparison groups were control, no control, no intervention, or rural areas | We excluded comparisons with rural areas as we considered that it would not help us to draw conclusions in terms of programmatic implications. These studies were mostly nutritional surveillance programmes with children randomly sampled at 1 time point. Consequently, the intervention duration and the change in anthropometric measurements were not taken into consideration. We included comparison with the same intervention combined with other components if the 2 areas were urban. This can show the added benefits of a combined intervention; for example, a complementary feeding education intervention versus a complementary feeding education intervention + nutrition promotion. |
| Outcome | Stunting, underweight and wasting using, height‐for‐age, weight‐for‐age, and weight‐for‐height z‐scores or prevalence or mean using NCHS, WHO, and IAP growth standards or a combination of these. Definitions included:
|
We used stunting operationalised as height‐for‐age z‐score. We used the change in z‐score to compare the impacts of intervention between studies as the use of different growth standards (NCHS, WHO, and IAP) makes the outcomes difficult to compare. Indices, anthropometric measurements, and change in anthropometric measurement were included. We did not include measurement of micronutrient deficiencies as the literature was too limited (only 2 studies). |
| IAP: Indian Association of Paediatrics; ITS: interrupted time series; NCHS: National Center for Health Statistics; RCT: randomised controlled trial; WHO: World Health Organization. | ||
We also identified a range of systematic reviews that overlapped with this review. While evidence exists from these reviews, there is a need to identify the nutritional interventions that meet this review's PICO parameters and to present an overview of the interventions that work in urban settings to promote infant and children's nutrition. Thus, this review builds on, and complements the following reviews. In the Lancet series "The health of people who live in slums" by Lilford 2017, a systematic overview was conducted of reviews of determinants of health in slum settings and interventions that aimed to improve the health of people who live in slums. One of the key findings of that review was that health services should be proactive in providing immunisation and surveillance for childhood malnutrition. In the Turley 2013 review, the focus was on infrastructural interventions in slums and their health impact. There was a limited but consistent body of evidence to suggest that slum upgrading may have reduced the incidence of diarrhoeal diseases and water‐related expenditure. Three studies were identified under nutritional deficiencies in slum settings and would be relevant to the present review. Mori 2012 assessed zinc supplementation for improving pregnancy and infant outcome and included one study in urban slums. De‐Regil 2011 evaluated the effect of home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age including one study in an urban setting. Sguassero 2012 analysed community‐based supplementary feeding interventions for promoting the growth of children under five years of age in LMICs and the findings showed that this intervention had a negligible impact on child growth. However, this should be interpreted with caution due to the high heterogeneity of the studies and only one study in an urban slum was included. Hossain 2017 included interventions reducing stunting in LMICs, 14 studies, mostly rural and none in slums, demonstrated an impact with the successful interventions including a combination of political commitment, multi‐sectorial collaboration, community engagement, community‐based service delivery platforms and wider programme coverage and compliance. From the combined results of our scoping review, the systematic reviews, and the other existing evidence, we were able to draw conclusions by assessing the impact of nutritional interventions on stunting in the context of the urban slum environment.
Objectives
Primary objective
To assess the impact of nutritional interventions to reduce stunting in infants and children under five years old in urban slums from LMICs.
Secondary objective
To assess the effect of nutritional interventions on other nutritional (wasting and underweight) and non‐nutritional outcomes (socioeconomic, health and developmental) in addition to stunting.
Methods
Criteria for considering studies for this review
Types of studies
We included the following study designs in this review based on the criteria set down by the Cochrane Effective Practice and Organisation of Care (EPOC) Group.
Randomised (including cluster‐randomised) trials: any experimental design where stunted children were allocated to one or other of the interventions (e.g. micronutrient supplementation or complementary feeding education).
Quasi‐randomised trials with either individual or cluster‐randomisation: we included studies with at least two intervention sites and two comparator sites.
Non‐randomised controlled trials: we included studies with at least two intervention sites and two comparator sites
Controlled before‐and‐after (CBA) studies (cohort or cross‐sectional): the timing of the period of the study in both the intervention and comparator should have been comparable. Pre‐ and postintervention periods of measurement of both groups should have been the same. Both groups were comparable for key characteristics.
Interrupted time series (ITS) (according to EPOC standards): studies with a clearly defined time point when the intervention occurred; these studies must have had at least three data points, one before and two after the intervention began and with a control group in a different site with no intervention.
Historically controlled studies: studies with repeated measures made in stunted children at each time point and with a control group in a different site with no intervention.
Types of participants
We included children from LMIC, aged from birth to 59 months, living in urban slums.
For the purposes of this review we follow the UN‐Habitat 2004 definitions for low‐income informal settlements or slums as lacking one or more of the following:
access to improved water (adequate quantities of water that was affordable and available without excessive physical effort and time);
access to improved sanitation (access to an excreta disposal system, in the form of a private or public toilet, shared with a reasonable number of people);
security of tenure (evidence of documentation that can be used as proof of secure tenure status, or for protection from forced evictions)
durability of housing (permanent and adequate structure in a non‐hazardous location, protecting its inhabitants from the extremes of climatic conditions such as rain, heat, cold, or humidity);
sufficient living area (not more than three people sharing the same room).
We included studies that specified the location of the intervention as a slum, assuming that this met the UN‐Habitat definition criteria. We also included studies that did not specify the location as being a slum but did provide detailed description of the location enabling us to classify it as a slum based on the UN‐Habitat definition criteria. We included studies conducted in urban slums or semi/periurban slums, or both. We included studies conducted in urban areas considered as deprived by taking into account the overall poverty level of the inhabitants.
We considered LMICs, defined as those with a gross national income (GNI) per capita, calculated using the World Bank Atlas method (datahelpdesk.worldbank.org/knowledgebase/articles/378832):
for low‐income countries: a GNI per capita of USD 1045 or less in 2013;
for middle‐income countries: a GNI per capita of more than USD 1045 but less than USD 12,746 (World Bank 2014).
Types of interventions
Our intention from the start of the review process was to include, analyse, and present findings from many, but not all, types of nutritional interventions. Based on the scoping review discussed in Table 1, we considered the following nutritional interventions for this review.
Nutritional interventions (e.g. counselling in feeding practices, maternal dietary or micronutrient supplementation; promotion of optimum breastfeeding; complementary feeding and responsive feeding practices and stimulation; dietary supplementation; diversification and micronutrient supplementation or fortification for children).
Comparator: controls included treatment, intervention, or placebo.
Combined approach programmes (e.g. zinc supplementation plus home‐based nutrition counselling intervention) only if the other cointerventions were the same in both the intervention and comparison groups.
Interventions at an individual or community level (slum).
We excluded the following interventions.
Treatment interventions for severe and moderate acute malnutrition as opposed to chronic malnutrition if implemented as a single intervention. Such excluded studies included CMAM for severe acute malnourished children (SAM), and inpatient treatment of SAM children or fortified food for MAM children. As wasting is considered a risk factor for stunting, we included interventions to reduce wasting only if the nature of the intervention could have positive impact on linear growth.
Comparisons with rural areas as explained in Table 1.
Types of outcome measures
We included studies reporting on primary outcomes and studies reporting on primary and secondary outcomes. We did not include studies reporting on secondary outcomes only.
Primary outcomes
-
Stunting as measured by anthropometry (Table 3).
Height expressed in centimetres or height‐for‐age (HFA) z‐score.
Low birth weight (LBW; as birth length is not usually available, birth weight serves as a proxy for small size at birth, itself a proxy for inadequate foetal nutrition and growth).
We compared these measures in terms of:
height gain during the intervention;
change in malnutrition indices (HFA z‐score below –2 standard deviations or –3 standard deviations, or both) during the intervention;
change in z‐score during the intervention.
We included studies using IAP, WHO growth standards, and NCHS references as explained in Table 1. The nutritional outcomes were followed up postintervention. We did not limit the follow‐up period as interventions to manage stunting can have effects that span a lifetime.
Table 3. Definition and explanation of anthropometric indicators, height‐for‐age, low birth weight, weight‐for‐age, weight‐for‐height, mid‐upper arm circumference, and triceps skinfold thickness (UNICEF 2013)
| HFA: HFA z‐score measures linear growth. A child who is < –2 SD from the median of the WHO Child Growth Standards in terms of HFA is considered short for his/her age, or stunted. This condition reflects the cumulative effect of chronic malnutrition. If a child is < –3 SD from the median of the WHO Child Growth Standards, then he/she is considered to be severely stunted. Stunting often reflects a failure to receive adequate nutrition over a long period of time and is worsened by recurrent and chronic illness. Therefore, HFA reflects the long‐term effects of malnutrition in a population and does not vary appreciably according to recent dietary intake. |
| Birth weight or LBW: birth weight is the body mass of a baby at its birth. It represents the growth of all tissues of the body and is the most commonly used indicator of the adequacy of antenatal growth. Inadequate birth weight may be caused by deficiencies or excess of some nutrients, infections, congenital anomalies, adverse maternal behaviour (e.g. smoking, drug use, heavy physical labour), and by variation in gestation length. LBW is defined as a weight < 2500 g at birth for a pregnancy of 37–42 weeks' gestation. Some studies reported differences in mean birth weight between control and intervention groups. Other studies presented LBW as a dichotomous variable (yes/no). |
| WFH: WFH z‐score describes current nutritional status. A child who is < –2 SD from the median of the WHO Child Growth Standards for WFH is considered to be too thin for his/her height, or wasted. This condition reflects acute or recent nutritional deficit. As with stunting, wasting is considered severe if the child is < –3 SD below the reference median or by an MUAC < 115 mm with or without nutritional oedema. In the presence of bilateral pitting oedema, the term kwashiorkor is used. Severe wasting is closely linked to mortality risk. |
| WFA: WFA z‐score is a composite index of WFH and HFA. Thus, it does not distinguish between acute malnutrition (wasting) and chronic malnutrition (stunting). A child can be underweight for age because he/she is stunted, because he/she is wasted, or both. Children whose WFA is < –2 SD from the median of the WHO Child Growth Standards are classified as underweight. Children whose WFA is < –3 SD from the median of the WHO Child Growth Standards are considered severely underweight. WFA is a good overall indicator of a population's nutritional health. |
| MUAC: measures the muscle mass of the upper arm. A flexible measuring tape is wrapped around the mid‐upper arm (between the shoulder and elbow) to measure its circumference. MUAC should be measured to the nearest 0.1 cm. MUAC is a rapid and effective predictor of risk of death in children aged 6–59 months and is increasingly being used to assess adult nutritional status. The cutoffs are: well nourished ≥ 135 mm, at risk of malnutrition 125–134 mm, moderate acute malnutrition 115–124 mm, and severe acute malnutrition < 115 mm. |
| Triceps skinfold thickness: is used to estimate body fat, measured on the right arm halfway between the olecranon process of the elbow and the acromial process of the scapula. Reference values have been published for school children from the US or Europe but not specifically for LMIC. |
| HFA: height‐for‐age; LBW: low birth weight; MUAC: mid‐upper‐arm circumference; SD: standard deviation; WFA: weight‐for‐age; WFH: weight‐for‐height; WHO: World Health Organization. |
Secondary outcomes
The secondary outcomes were prioritised as nutritional outcomes first and non‐nutritional outcomes second.
-
Nutritional outcomes as measured by anthropometry (Table 3).
Weight expressed in kilograms or WFA z‐score.
Weight and height combined and expressed in WFH z‐score.
MUAC, triceps skinfold thickness expressed in millimetres.
We compared these measures in terms of:
height or weight gain during the intervention;
change in malnutrition indices (WFA and WFH below –2 standard deviations or –3 standard deviations, or both) during the intervention.
-
Non‐nutritional outcomes such as health, socioeconomic, and developmental:
health measured by diarrhoea, acute respiratory infection, measures of physical well‐being (e.g. Harvard Step Test), death;
socioeconomic, measured by at least one of the following: household income; household assets; households above or below poverty threshold; employment and occupation. Developmental (cognitive, mental and motor skill) as defined by trialists (e.g. the Bayley Scales of Infant Development Bayley Mental Development Index, Bayley Psychomotor Development Index, Stanford‐Binet Test, DENVER II Developmental Screening Test)
any potential negative or positive effects associated with the intervention, such as increased undernutrition/diarrhoea or improved nutritional status in the siblings.
Search methods for identification of studies
We used a sensitive search strategy for electronic bibliographic databases, bibliographies of included articles, and grey literature sources. The search strategy for PubMed was validated at protocol stage by Cochrane Public Health (CPH). In 2018, a CPH information specialist reviewed and validated a new search strategy for PubMed and CENTRAL. We created a Technical Advisory Group (TAG) whose members were experts in the fields of urban health, nutrition, and vulnerabilities. The TAG was responsible for providing guidance, identifying additional published and unpublished references, ensuring that evidence‐based recommendations were disseminated widely, and, where possible, implemented. Specifically for this systematic review, the TAG acted as a review advisory group as detailed by CPH ( https://ph.cochrane.org/other‐contributors ) (see section Review Advisory Group members). We conducted the search in English but used search terms in French, Spanish, and any other languages for slum‐specific terms. The search included all publications from January 1990 up to March 2017. An updated search in August 2018 was limited to optimised search strategies of PubMed and CENTRAL because analysis of previously identified studies revealed that other databases did not contribute to the retrieval of relevant published studies. Findings from publications before 1990 may be out of date in the very rapid changing environment of the slums.
We narrowed down our review to the results using our PICO inclusion criteria (children under five years old in urban slums in LMICs). We focused on nutritional outcomes and searched additional nutrition‐specific databases and sources of literature including grey literature, nutrition technical websites, and websites of NGO with a strong expertise in nutrition. As these other sources of literature were not included in the previous reviews (Bhutta 2008; Bhutta 2013), we expected to identify new studies. We also contacted implementing organisations that may have unpublished studies from their programmes in urban slums, which was not done for the previous reviews.
Electronic searches
We specifically designed the search depending on the database requirements. We used free‐text terms and, where available, controlled vocabulary (e.g. MeSH) in the database searches (Table 4). The complete search strategies and search terms are documented in the Appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12).
Table 4. Databases selected for review
| Database | URL Links | Date of last search |
| Cochrane Central Register of Studies (CENTRAL) | www.thecochranelibrary.com/view/0/index.html | August 2018 |
| PubMed | www.ncbi.nlm.nih.gov/ | August 2018 |
| Web of Science | login.webofknowledge.com/ (Web of Science core collections) | March 2017 |
| Ovid MEDLINE | ovidsp.uk.ovid.com/ | March 2017 |
| Biosis Citation Index | login.webofknowledge.com/ | March 2017 |
| MEDLINE | login.webofknowledge.com/ | March 2017 |
| IBECS (English) | ibecs.isciii.es/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=IBECS&lang=i&form=F | March 2017 |
| WORLDCAT (OCLC) | www.oclc.org/en‐UK/home.html | March 2017 |
| CINAHL (EBSCO) | www.ebscohost.com/academic/cinahl‐plus‐with‐full‐text | March 2017 |
| Popline | www.popline.org/ | March 2017 |
| BIBLIOMAP | eppi.ioe.ac.uk/webdatabases/Intro.aspx?ID=7 | March 2017 |
| ZETOC | zetoc.jisc.ac.uk/ | March 2017 |
| WHO International Clinical Trials Registry Platform | www.who.int/ictrp/en/ | March 2017 |
| MetaRegister of Controlled Trials (mRCT) | www.isrctn.com/page/mrct | March 2017 |
| UNSCN | unscn.org/en/home/ | March 2017 |
| African Index Medicus | indexmedicus.afro.who.int/cgi‐bin/wxis.exe/iah/ | March 2017 |
| ClinicalTrials.gov | www.clinicaltrials.gov/ | March 2017 |
| Global Health Library | www.globalhealthlibrary.net/php/index.php | March 2017 |
| WHOLIS – the WHO Library Information System | dosei.who.int/uhtbin/cgisirsi/Thu+Jul++5+16:26:22+MEST+2012/0/49 | March 2017 |
| Health Management ProQuest | search.proquest.com/advanced | March 2017 |
| Google Scholar | scholar.google.co.uk/ | March 2017 |
| Loughborough University Catalogue plus | www.lboro.ac.uk/library/ | March 2017 |
| Grey literature report | www.greylit.org/library/search | March 2017 |
| Virtual health library | www.bireme.br/php/index.php?lang=en | March 2017 |
| Index Medicus for South‐East Asia Region (IMSEAR) | www.who.int/library/databases/searo/en/ | March 2017 |
| Virtual Health Sciences Library (VHSL) | www.emro.who.int/HIS/VHSL/ | March 2017 |
| 3ie impact | www.3ieimpact.org/en/ | March 2017 |
| eLENA e‐Library of Evidence for Nutrition Actions | www.who.int/elena/en/ | March 2017 |
| Global database on the Implementation of Nutrition Action (GINA) | extranet.who.int/nutrition/gina/ | March 2017 |
| Nutrition Landscape Information System (NLIS) | apps.who.int/nutrition/landscape/search.aspx | March 2017 |
| Urban humanitarian response portal | www.urban‐response.org/ | March 2017 |
| African Population Health Research Centre (APHRC) | aphrc.org/publications/ | March 2017 |
We handsearched reference lists of eligible studies for any additional relevant articles. We contacted subject experts and study authors and asked them to provide additional information and further relevant references.
We performed the initial literature handsearch from November 2015 to January 2016. We conducted a top‐up handsearch in March 2017 and identified zero new eligible studies. We conducted a top‐up search in August 2018 using CENTRAL and PubMed and databases only and identified four eligible studies.
For unpublished and ongoing studies, we contacted a list of experts and researchers working in the field. The list included experts working in the organisations and international groups reported below. We also searched their websites.
UN agencies: WHO Department of Child and Adolescent Health and Development; the United Nations Children's Fund (UNICEF); the World Food Program (WFP); the World Bank (WB); the United Nations Standing Committee on Nutrition (UNSCN); the United Nations Refugee Agency (UNHCR).
Technical bodies (nutrition): the Food and Nutrition Technical Assistance Project (FANTA‐2); the Emergency Nutrition Network (ENN); the International Malnutrition Task Force (IMTF); the Humanitarian Practice Network (HPN); the CMAM Forum; the Global Nutrition Cluster (GNC); the Global Alliance for Improved Nutrition (GAIN); Helen Keller International (HKI).
Technical bodies (urban slums): UN‐HABITAT; Slum Dwellers International (SDI); Cities Alliances.
Academic institutions: Centers for Disease Control and Prevention (CDC); the International Centre for Diarrhoeal Disease Research (ICDDR); the Institute of Child Health London (ICH); the London School of Hygiene and Tropical Medicine (LSHTM); the Institute of Tropical Medicine (ITP) Antwerp, Belgium; Jameel Poverty Action Lab (J‐PAL); International Initiate for Impact Evaluation (3ie).
NGOs and related websites: Save the Children (STC); Doctors without Borders (MSF); Valid International; Concern Worldwide; Action Against Hunger (ACF); CARE; NutritionWorks; Medecins du Monde (MDM); Oxfam; Red Cross movement; WorldVision; BRAC; Plan; Family Health International; Global Communities; ALNAP; Reliefweb; Coordination Sud.
National departments for international development and non‐institutional donors: USAID; UK Department for International Development (DFID); Swedish International Cooperation Development Agency (SIDA); Danish International Development Agency (DANIDA); French agency for International Development (AFD); Comic Relief.
Conference proceedings and others
Nutrition: Field Exchange: The Emergency Nutrition Network Magazine, International Nutrition Congress; International Conference on Nutrition, Nutrition and Nurture.
LMIC: African Nutritional Epidemiology Conference (ANEC).
Human/anthropological biology/nutrition/urban health: journals for which articles are not included in the databases searched.
Public health conferences (e.g. American Public Health Association; European Public Health Association).
Global: International Conference on Urban Health, World Congress of Epidemiology.
Reference lists
We checked the reference lists of all the eligible studies.
Data collection and analysis
Selection of studies
We screened titles and abstracts of studies for inclusion and then retrieved the full text of potentially eligible studies for screening. We independently applied the inclusion criteria to those retrieved publications. One review author (SG) screened all titles and abstracts, and two review authors (PG and BB) assessed half each. We discussed any disagreements on study inclusion to reach consensus. When this was not possible, we consulted a fourth review author (NM). We sought further information from the authors where papers contained insufficient information to make a decision about eligibility. We included reasons for non‐selection of the studies screened for inclusion. We used section 1 – general information and 2 – study eligibility of the prestandardised data extraction form adapted from the CPH Group's Data Extraction and Assessment Template (Appendix 13) to capture information from all screened studies in an excel spreadsheet. We recorded necessary information about inclusion decisions in order to design a PRISMA flow chart and a Characteristics of excluded studies table. We used Refworks as our reference management software.
Data extraction and management
We obtained data from the included studies using a standardised data extraction form in Excel (Appendix 13), which was tested and adapted before use. Sections 3 to 8 of the Excel form are presented as text to illustrate the categories of data extracted. The captured data in Excel were then transferred to Review Manager 5 (Review Manager 2014). All authors (SG, PG, BB, and NM) independently did this for half of the studies each (two authors did the same half) and the entries were compared and checked by SG. We then cross‐checked the data. We discussed any differences between the two data extraction sheets to reach a consensus or consult a third author (PG, BB, or NM) if a consensus was impossible to reach. We contacted study authors to obtain any missing information or to clarify unclear data by obtaining the original report.
Section 3 to 8 of the standardised data extraction form (Appendix 13) extracted data related to the following categories from all the included studies. For the complete text form, refer to Appendix 13, the pre‐standardised data extraction form:
-
Section 3: study details.
Aims.
Location.
Delivery: community‐based/primary health care/secondary health care/direct.
Funding source, budget, implementing partner; design, integration within existing government health.
Setting: delivered in humanitarian crisis/disaster or development; characteristics, squatter settlement, legal, dilapidated and change in living conditions (improving or worsening).
Duration of intervention.
Sample size and unit of randomisation.
-
Section 4: participants.
Population: children data (age, sex), socioeconomic data, baseline anthropometry.
Comparison group: children data (age, sex), socioeconomic data, baseline anthropometry.
-
Section 5: intervention plus cointervention group/comparison group.
Classification of the intervention.
Context: food security, slum size, location, exposure to flooding, eviction, fire.
-
Intervention type and components:
type: micronutrient supplementation, complementary feeding.
Section 6: outcomes.
We extracted data pertaining to the primary and secondary outcomes defined earlier. For secondary outcomes, we included any of the prioritised outcomes (nutritional or non‐nutritional).
-
Section 7: results.
We extracted data from each type of study design (e.g. RCT, CBA, etc.) that we included in this review.
Other information.
Recommendations: we collected data on authors' potential recommendations based on the study results.
Limitations: we collected data on study limitations.
-
Section 8: 'Risk of bias' assessment.
We extracted data on risk of bias using the Cochrane EPOC Group's guidance for assessing risk of bias for studies with a separate control group (RCTs, controlled clinical trials (CCTs), CBAs), to assess observational study designs, and for ITS studies. Risk of bias was assessed at the study level as specified below.
Assessment of impact on equity
We addressed aspects highlighted by the PROGRESS framework on inequality issues using the prestandardised form (O'Neill 2014; Ueffing 2009) (Appendix 13). We collected categories of disadvantaged groups for place, race, occupation, gender, religion, education, and socioeconomic aspects.
Assessment of risk of bias in included studies
We assessed risk of bias at the study level. We carried out risk of bias assessment by capturing the information based on the standard criteria described by the Cochrane EPOC Group (EPOC 2013), using section 8 of the prestandardised form (Appendix 13), and the principles of EPOC (Cochrane 2017) to assess risk of bias for three added domains; similarity of outcome measures at baseline, similarity of baseline characteristics, and protection against contamination. All authors (SG, PG, BB, NM) independently assessed the risks of bias of half of the included studies (two authors did the same half) and the entries were compared and checked by SG for seven domains and for the three added domains one review author (SG) assessed the risks of bias and two review authors (NM and BB) independently assessed the bias of half of the included studies each. Where there were disagreements the authors discussed the differences and then reassessed until reaching agreement.
For included studies, the risk of bias assessment was based on the following domains.
Sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessors.
Incomplete outcome data.
Selective outcome reporting.
Similarity of outcome measures at baseline.
Similarity of baseline characteristics.
Protection against contamination.
Other sources of bias.
When information was not sufficient to assess the risk of bias related to sampling, allocation, and reporting for primary outcomes, we contacted the study authors and requested further details. We used a table to record the certainty assessment of each study with a summary statement.
Overall risk of bias
For each study, we assessed the overall level of risk of bias by combining risk of bias for each of the domains. We considered studies to be of low risk of bias if they were assessed as having no domain at high risk of bias and fewer than two unclear risk of bias. We considered studies to be of moderate risk of bias if they were assessed with one domain at high risk of bias and fewer than three risk of bias or no domain at high risk of bias and less than four unclear risk of bias. We considered studies to be of high risk of bias if they were assessed with more than one domain at high risk of bias or more than three unclear risk of bias.
Risk of bias for included studies was documented in the 'Risk of bias' table for each study in the Characteristics of included studies table. We also summarised results in a 'Risk of bias graph' and a 'Risk of bias' summary.
Assessment of the evidence using GRADE framework
The four review authors analysed the certainty of evidence for the primary outcome (length, HFA, and LBW) and secondary outcome (weight, WFA, WFH, and MUAC) using the GRADE approach (Guyatt 2008). GRADE is the system of rating certainty of evidence and grading the strength of recommendations in systematic reviews (Guyatt 2010; Guyatt 2011). Using GRADE, the certainty of the evidence is based on a set of items that increase or decrease the certainty of evidence. We classified the certainty as high, moderate, low, or very low. The use of GRADE allowed us to systematically and transparently grade certainty based on the following factors.
Factors decreasing certainty of evidence:
study limitations;
inconsistency of results;
indirectness of evidence;
imprecision;
publication bias;
plausible confounding, which would reduce a demonstrated effect.
Factors increasing certainty of evidence:
large magnitude of effect;
dose–response gradient.
Based on these criteria, we graded each outcome grouping as one of the following.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: any estimate of effect is uncertain.
We created 'Summary of findings' tables for this assessment.
Measures of treatment effect
We registered and reported measures of effect in the same way that the study's authors reported them. We interpreted a non‐statistically significant effect as unclear effect. We standardised measures of effect as mean differences (MD) in natural units or using a standardised scale to allow for comparisons across studies with 95% confidence intervals (CI). For continuous data, we presented the results as MD if outcomes were measured in the same way between trials. We used the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods using Hedges' (adjusted) g. We did not combine change‐based estimates (e.g. comparing change rates or change scores) with absolute differences or analysis of covariance‐adjusted differences in these analyses. For dichotomous data, we used risk ratios (RRs) with 95% CIs.
Unit of analysis issues
Cluster‐randomised trials
We included cluster‐randomised trials. If the findings were reported at the individual level, we reported the method used to take into account clustering. In case the clustering effect was not taken into account, we had planned to adjust the sample size to allow for comparison with a sample size of individuals. However, in the present review, we were unable to do so due to lack of data in the present review. When possible, we planned to calculate the intracluster correlation coefficient (ICC) as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), and we planned to reanalyse the data. When the data were not available, we planned to estimate the ICC from another source or from the literature and we planned to report this. We planned also to conduct sensitivity analyses to examine the impact of variation in the ICC. In all cases, we noted the approach taken. No method was used to account for clustering in the lone cluster randomised trial included due to insufficient information and information on ICC was provided as in the original study.
Multiple time points
We used the time points reported by the studies. Due to heterogeneity in this reporting, we were unable to group outcomes measured at similar points or at similar age points (e.g. children at birth, one year old and three years old). When outcomes were measured at multiple time points, we used a mean effect size to avoid dependence problems due to data heterogeneity. When possible we used a single measure that was closest to a one‐year follow‐up for our primary outcomes.
Dealing with missing data
In case of missing data, we contacted the study's authors by email when contact details were available. If the data could not be found, we noted this in the study's form and in the 'Risk of bias' table. We excluded the study from the meta‐analysis if it was impossible to obtain the requested information. If following contact with a study's authors there were no additional data available, and if we thought that the missing data may have introduced a serious bias, then we used sensitivity analysis to explore the impact of including such studies in the overall assessment of results.
Assessment of heterogeneity
We considered heterogeneity by examining the study design, participants, setting, intervention duration, and age group. If studies reporting the primary outcome were sufficiently similar, we conducted a meta‐analysis. When meta‐analysis could not be conducted, we reported the results in a narrative way. We assessed statistical heterogeneity in each meta‐analysis using the T², I², and Chi² statistics to estimate the percentage of variability that was due to heterogeneity rather than to sampling error or chance and graphically with a forest plot (Review Manager 2014). We considered an I² value greater than 50% to indicate substantial heterogeneity and we considered it statistically significant if the P value for the Chi² test was less than 0.1. We created forest plots and I² calculations using Review Manager 5 (Review Manager 2014). We noted the result of these statistical tests in the text. Where meta‐analysis was undertaken, we examined forest plots visually for heterogeneity.
We assessed issues of clinical and methodological heterogeneity in a narrative way detailing relevant study‐specific characteristics.
Methods: study design, group assignment, outcome assessment, adjustment for confounders.
Population: setting, age.
Intervention: components, duration.
Context: urban slum/periurban slum, baseline mortality and morbidity.
Delivery: primary, secondary or community‐based, approach (lay counsellors (e.g. community health workers and peer counsellors) versus professional counselling; personalised versus group intervention).
Assessment of reporting biases
We assessed the risk of publication bias qualitatively based on the characteristics of the included studies. We were unable to use a funnel plot to investigate the risk of publication bias by intervention type and outcome measure and thus we were unable to visually examine the funnel plot for asymmetry. When a study's authors were contacted and there were no additional data available, and if we thought that these missing data may have introduced a serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Data synthesis
We conducted meta‐analyses to obtain an overall estimate of the effect of an intervention when more than one study examined similar interventions using similar outcomes; more than one study was conducted in similar populations and measured similar outcomes; the study used a robust method such as an RCT; or a combination of these. We then used a fixed‐effect analysis for combining data. If there was statistical heterogeneity, we used a random‐effects analysis to produce an overall summary. We carried out statistical analysis using Review Manager 5 (Review Manager 2014).
We carried out a narrative synthesis of the results, grouping our findings by the type of nutritional intervention, study population (pregnant women; infant and young children), and outcome measured. The narrative synthesis evaluation was based on direction of effect.
Subgroup analysis and investigation of heterogeneity
We conducted meta‐analyses to provide an estimate of one type of intervention/component on stunting in children. We were able to conduct the analysis if the interventions shared similar methods and outcome measures. If the study design varied between studies, we favoured studies with low risk of bias to conduct the statistical analysis.
We planned to conduct the subgroup analyses sharing the same characteristics based on:
age of the children (younger or older than 24 months);
nutritional status at baseline (stunting or not);
location (Asia, Africa, Latin America);
duration of the intervention (less than or more than 12 months);
intervention component (nutrition counselling, fortification, etc.);
intervention design (single, combined);
source of funding.
Due to insufficient studies sharing the same characteristics, we were unable to perform these analyses.
Sensitivity analysis
We carried out sensitivity analysis to examine the effects of removing studies at high risk of bias. We identified those studies in the assessment with a high or unclear risk of bias.
We conducted comparative analysis to test for sensitivity of the results of the review by determining whether results differed when studies at high risk of bias were excluded.
We planned to conduct comparative analysis to test for sensitivity of the results of the review by:
comparing results if we included studies that may have been excluded because only the abstract could be found (where some data and results were provided in the abstract) as no studies were included with abstract only;
comparing results if we included studies that may have been excluded due to the age range of participants (e.g. a study may have included preschool‐aged children as well as school‐aged children) as studies including preschool aged and over five years old children were not selected;
comparing results that may have been excluded due to potentially confounding cointerventions (e.g. the cointervention was only implemented in the intervention group and not in the control group);
determining whether results differed when studies at high risk of bias were excluded.
We were unable to carry out sensitivity analysis to examine the effects of funding source on findings as funding source information was scarce.
'Summary of findings' tables
We included 'Summary of findings' tables for the primary outcomes and secondary outcomes, including the number of participants and studies for each outcome, a summary of the intervention effect, and measure of the certainty of the body of evidence according to the GRADE Working Group (Guyatt 2011) and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b; Section 8.5 and Chapter 12).
Technical Advisory Group
Components of the protocol were discussed during two meetings (1 April 2014, 29 September 2014) with our TAG. TAG members were academics with a recognised expertise in urban health, nutrition, or vulnerabilities. They provided comments to ensure that the review met its intended goal of assessing the effectiveness of nutritional interventions in a systematic and comprehensive way and that the review appropriately informed research and programmes.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies tables.
Results of the search
The search strategy identified 1807 references for possible inclusion; 197 of which were duplicates. We excluded 1622 records as out of scope. The main reasons for excluding the studies were the location (not in an LMIC, not in a city), the participants (not children), or the intervention (not nutritional). None of the studies were translated. We contacted 10 authors for additional information; four were contacted and came back and provided clarification on the methods used to finalise the risk of bias analysis. For the others, either the email was not valid or there was no contact information available. Figure 4 depicts the process for assessing and selecting the studies.
4.

Study flow diagram.
A total of 29 studies met the initial selection criteria. We included 15 of these studies (Akter 2012; Begin 2008; Caulfield 1999; Iannotti 2008; Iannotti 2014; Jahan 2014; Moursi 2003; Oelofse 2003; Osendarp 2000; Penny 2005; Pridmore 2014; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010; overview of the studies included in Table 5), and excluded 14 based on full‐text revision (Agustina 2013; Akeredolu 2014; Choudhury 2016; Effendy 2015; Iannotti 2013; Kæstel 2005; Kikafunda 1998; Krebs 2011; Mitter 2012; Poudel 2004; Saran 2002; Semba 2011; Soofi 2013; Tomlinson 2016). All of these studies were in English so no translation was required.
Table 5. Overview of studies included in synthesis
| Author year, country of conduct, setting | Study design | Overall risk of bias | Other key detail of intervention related to urban settings | Level of factors tackled | Population (sample size: intervention/ control) | Outcome (method of synthesis) | Time point of measurement |
| Intervention category: zinc supplementation in pregnant women vs supplementation without zinc or placebo | |||||||
| Caulfield 1999, Peru, slums of Lima | RCT | High | High compliance of supplementation | Individual, service delivery | Pregnant women and newborns (IG: 488, CG: 469) | Length (MA), LBW (MA), MUAC (MA) | Birth up to 7 days after birth |
| Iannotti 2008, Peru, slums of Lima | RCT | High | None | Individual | Pregnant women and newborns (IG: 273, CG: 273) | Length (NS), weight (NS), MUAC (NS), diarrhoea (NS) | At birth and monthly from month 1 to month 12 |
| Osendarp 2000, Bangladesh, selected areas of Dhaka city slums | RCT | Moderate | None | Individual | Pregnant women and newborns (IG: 194, CG: 216) | Length (MA), LBW (MA), MUAC (MA) | Baseline, 7 and 8 months' gestation, birth |
| Intervention category: micronutrient or macronutrient supplementation in children vs no intervention or placebo | |||||||
| Begin 2008, Guatemala, low‐income areas of Guatemala city | RCT | High | None | Individual | Children aged 6–7 months (IG: 254, CG: 61) | MUAC (NS), length (NS), diarrhoea (NS) | Monthly |
| Iannotti 2014, Haiti, poorest communities in the second largest city | RCT | High | Integrated in an urban community health programme | Individual, community, service delivery | Children aged 6–11 months (IG: 159, CG: 156) | HFA (MA), WFA (MA) | 6 monthly visits for participants recruited between 6 and 11 months plus follow‐up 6 months after the end of the study |
| Moursi 2003, Congo Brazzaville, a borough in Brazzaville | RCT | High | None | Individual | Children aged 4.5 months (IG: 37, CG: 38) | Height (NS), WFA (NS), WFH (MA), infant and young children practices and dietary intake (NS), rate of ill days, incidence of diseases (NS) | 10, 16, 24, and 32 weeks of age grouped in 10–15, 16–23, 24–31, and 16– 31 weeks of age |
| Oelofse 2003, South Africa, urban disadvantaged black community | RCT | High | None | Individual | Children aged 6–12 months) (IG: 16, CG: 14) | HFA (MA), WFA (NS), WFH (NS) | 6 and 12 months |
| Radhakrishna 2013, India, low‐income urban communities in South India | RCT | Moderate | Integrated in a community centre | Individual, community, service delivery | Children aged 4–18 months (IG: 163, CG: 161) | HFA (NS), height (NS), weight gain (NS), WFH (NS), skinfold thickness (triceps, subscapular) (NS) | Every 3 months |
| Rahman 2002, Bangladesh, slums in Dhaka | RCT | High | None | individual | Children aged 12–35 months (IG1: 165, IG2: 157, IG3: 171, CG: 161) | Height (NS), HFA (NS), weight (NS), WFA (NS), WFH (NS) | At enrolment and after 3 and 6 months |
| Sur 2003, India, slum of Kolkata | RCT | Moderate | None | Individual | LBW newborns (IG: 50, CG: 50) | Height (NS), WFA (NS), diarrhoeal episodes (NS) | Monthly for 1 year |
| Taneja 2010, India, a slum in New Delhi | RCT | Moderate | None | Individual | Children aged 6–30 months (IG: 1093, CG: 1133) | HFA (MA), WFA (MA), WFH (MA), diarrhoea (MA) | At enrolment and 4 months later |
| Intervention category: nutrition education for pregnant women vs standard care or no intervention | |||||||
| Akter 2012, Bangladesh, poor urban areas | RCT | High | Adapted to poor urban settings (demonstration of affordable nutritious meal and free access to maternity health care) | Individual, community, service delivery | Pregnant women (IG: 57, CG: 58) | LBW (MA), weight at birth (NS) | Monthly |
| Jahan 2014, Bangladesh, Dhaka city | RCT | High | Low‐cost and short‐term intervention that can cover a large number of population | Individual, service delivery | Pregnant women and newborns (IG: 150, CG: 150) | LBW (MA), initiation rate of breastfeeding (NS) | Monthly from months 6 to 9 of pregnancy, birth, and 1 month post partum |
| Intervention category: nutrition systems strengthening | |||||||
| Penny 2005, Peru, Trujillo | Cluster RCT | High | Facility based and ensured that activities of the intervention enhanced existing activities and were sustainable | Individual, community, service delivery | Children aged 0–18 months (IG: 187, CG: 190) | HFA (NS), height (NS), WFA (NS), WFH (NS) | At birth, 3, 6, 9, 12, 15, and 18 months |
| Pridmore 2014, Kenya, poor areas of Mombasa | Non‐randomised controlled trial (control group before‐after and case‐control) | High | Tackling urban‐specific social, economic, and environmental factors operating at local, municipal, provincial, and central levels | Individual, household, community, service delivery, city level | Children aged 24–59 months (IG: 999, CG: 999) | HFA (NS) | 2 times within 1 year |
| CG: control group; HFA: height‐for‐age; IG: intervention group; LBW: low birth weight; MA: meta‐analysis; MUAC: mid‐upper‐arm circumference; NS: narrative synthesis; RCT: randomised controlled trial; WFA: weight‐for‐age; WFH: weight‐for‐height. | |||||||
All the included studies contributed data in this review; eight for meta‐analysis and seven for narrative analysis.
Included studies
Study design
The eligible studies were 13 RCTs (Akter 2012; Begin 2008; Caulfield 1999; Iannotti 2008; Iannotti 2014; Jahan 2014; Moursi 2003; Oelofse 2003; Osendarp 2000; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010), one cluster RCT (Penny 2005), and one non‐RCT using intervention versus control group CBA and case‐control study design (Pridmore 2014). For the cluster RCT, we were unable to calculate the ICC due to the lack of information in the study and we were unable to find external estimates obtained from similar studies to recalculate (Penny 2005).
Location of studies
The most common locations were Bangladesh (27%; Akter 2012; Jahan 2014; Osendarp 2000; Rahman 2002), India (20%; Radhakrishna 2013; Sur 2003; Taneja 2010), and Peru (20%; Caulfield 1999; Iannotti 2008; Penny 2005), with the other locations being Haiti, Guatemala, South Africa, Congo, and Kenya.
Slum setting
More than half of the studies were conducted solely in slum settings (Caulfield 1999; Iannotti 2014; Osendarp 2000; Pridmore 2014; Rahman 2002; Sur 2003; Taneja 2010), with the rest conducted in poor urban or periurban areas. None of the studies mentioned comparing their classification of a slum with the UN‐Habitat 2004 definition. Nevertheless in 10 studies (Begin 2008; Caulfield 1999; Iannotti 2008; Iannotti 2014; Osendarp 2000; Penny 2005; Pridmore 2014; Rahman 2002; Sur 2003; Taneja 2010), the settings were presented in a way which matched some of the components of the definition.
The areas were described as impoverished shantytown (Caulfield 1999; Iannotti 2008), informal settlements (Pridmore 2014), or as communities having low SES (Penny 2005; Sur 2003). Housing was described as non‐durable, with flood exposure, and poor access to sanitation (Penny 2005; Sur 2003; shared and unhygienic in Rahman 2002), with high density and poor facilities lacking an adequate water source, paved streets, street lighting, and gas supply. In Rahman 2002, 82% had only one small room and one‐third of the households had access to supplies of cooking gas which were usually shared and about two‐thirds of the households had electricity and almost all households had access to safe drinking water through either pipes or tube wells. Children were described as anaemic and with growth faltering (Begin 2008; Iannotti 2014).
Intervention
There were no dietary intervention studies, that is, there were no interventions in which unprepared foods or prepared meals were given to mothers. All the studies identified were nutrient supplementation and educational interventions. Interventions included zinc supplementation in pregnant women and micronutrient or macronutrient supplementation in children (73%; Begin 2008; Caulfield 1999; Iannotti 2008; Iannotti 2014; Moursi 2003; Oelofse 2003; Osendarp 2000; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010), nutrition education for pregnant women (13%; Akter 2012; Jahan 2014) and nutrition systems strengthening (13%; Penny 2005; Pridmore 2014).
The intervention duration (which was not necessarily the supplementation duration) was on average 1.2 years with a minimum of 0.03 years and a maximum of 3.5 years. Only one study stated the cost of the intervention was GBP 400,000 (Pridmore 2014).
Investigators, trainers, or field workers delivered 53% of the interventions at the participants homes (Akter 2012; Begin 2008; Moursi 2003; Oelofse 2003; Pridmore 2014; Rahman 2002; Sur 2003; Taneja 2010), and delivered 40% at health facilities during antenatal visits or other (Caulfield 1999; Iannotti 2008; Iannotti 2014; Jahan 2014; Penny 2005), or clinic at the community centre (Radhakrishna 2013), and one delivered the intervention at home and at hospital (Osendarp 2000).
Micronutrient or macronutrient supplementation in children and pregnant women (11 studies)
Within the supplementation interventions, 20% supplemented pregnant women (Caulfield 1999; Iannotti 2008; Osendarp 2000), and explored the impact of the supplementation on newborn health outcomes. The others targeted children (80%; Begin 2008; Iannotti 2014; Moursi 2003; Oelofse 2003; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010). Fifty‐four percent supplemented zinc only (Caulfield 1999; Iannotti 2008; Osendarp 2000; Radhakrishna 2013; Sur 2003), 18% zinc and vitamin A (Rahman 2002; Taneja 2010), 1% bovine serum concentrate (BSC) with supplemental micronutrients (Begin 2008), and 27% fortified food (Iannotti 2014; Moursi 2003; Oelofse 2003).
Vitamins and mineral composition
The micronutrients supplemented were vitamins A, B, C, D, and E; and minerals zinc sulphate, ferrous sulphate, folic acid, calcium, copper, iodine, magnesium, manganese, phosphorus, potassium, and selenium. All interventions included zinc (Caulfield 1999; Iannotti 2008; Iannotti 2014; Oelofse 2003; Osendarp 2000; Radhakrishna 2013; Sur 2003; Taneja 2010), four interventions ferrous sulphate (Caulfield 1999; Iannotti 2008; Iannotti 2014; Oelofse 2003), and two studies used vitamin A and folic acid (Iannotti 2014; Oelofse 2003).
The information reported for vitamin and mineral supplementation was as follows:
Begin 2008: 13 vitamins and minerals (folic acid 35 μg, iron 10 mg, zinc 5 mg, vitamin A, thiamine, riboflavin, niacin, vitamin B6, vitamin B12, vitamin C, iodine, and selenium);
Caulfield 1999: iron 60 mg (as ferrous sulphate) and 250 mg folate (folic acid), with or without an additional zinc 15 mg (as zinc sulphate);
Iannotti 2008: zinc sulphate 15 mg, ferrous sulphate 60 mg, and folic acid 250 μg, or ferrous sulphate 60 mg and folic acid 250 μg;
Iannotti 2014: lipid‐based nutrient supplement (LNS) 20 g/day provided 108 kcal, protein, fat, and 19 vitamins and minerals (80 μg folic acid, iron 9 mg, zinc 4 mg, vitamin A, thiamine, riboflavin, niacin, pantothenic acid, vitamin B6, B12, vitamin C, calcium, copper, iodine, magnesium, manganese, phosphorus, potassium, selenium);
Oelofse 2003: 60g of dry product provided 1304 kj and 19 vitamins and minerals (folic acid 17.6 μg, iron 8 mg, zinc 5.6 mg, vitamin A, vitamin C, thiamine, riboflavin, niacin, calcium, vitamin D, vitamin E, biotin, pantothenic acid, vitamin B12, vitamin B6, phosphorus, iodine, potassium, sodium, chloride);
Osendarp 2000: elemental zinc 30 mg/day;
Radhakrishna 2013: zinc 5 mg plus riboflavin 0.5 mg/day;
Rahman 2002 zinc 20 mg/day for 14 days or vitamin A 60,000 retinol equivalents, or zinc plus vitamin A;
Sur 2003: elemental zinc 5 mg as zinc sulphate in vitamin B complex‐based syrup;
Taneja 2010: elemental zinc 10 mg to infants and 20 mg to older children for four months and at enrolment, all children also received a single dose of vitamin A (104.7 μmol for infants and 209.4 μmol for older children).
Fortified food composition
The fortified food content of different interventions was variable:
Iannotti 2014: LNS provided 108 kcal and other nutrients including vitamin A, vitamin B12, iron, and zinc at 80% of the recommended amounts;
Oelofse 2003: the quantity prescribed during the intervention was 60 g/day of dry cereal and would ensure consumption of 100% of recommended daily allowance (RDA) for vitamin A, 80% for iron, and more than 100% for zinc;
Moursi 2003: addition of amylase (33.7 mg per 100 g of dry matter) contributed in similar amounts to the total energy density of gruels in each group: 75 kJ per 100 g of gruel in the intervention group and 63 kJ per 100 g of gruel in the control group;
Begin 2008: a mix of BSC, with or without supplemental micronutrients and maize flour.
Target group
Supplementation interventions targeting pregnant women
Three studies were supplementation of micronutrients to pregnant women; zinc sulphate, ferrous sulphate, and folic acid (Caulfield 1999; Iannotti 2008) and zinc only (Osendarp 2000). In Caulfield 1999, women in the intervention group received a daily supplement containing iron 60 mg (as ferrous sulphate) and folate 250 mg (folic acid) and zinc 15 mg (zinc sulphate), while the women in control group received iron and folate only. In Iannotti 2008, pregnant women in the intervention group received zinc sulphate 15 mg, ferrous sulphate 60 mg, and folic acid 250 μg and in the control group received no zinc, but ferrous sulphate 60 mg and folic acid 250 μg. In Osendarp 2000, the amount of zinc given was based on twice the recommended daily intake for zinc during the last two trimesters of pregnancy in the intervention group versus a non‐nutritive placebo in the control group. Supplementation of pregnant women started between 10 and 24 weeks' gestation and continued through four weeks after delivery in Iannotti 2008, between 10 and 24 weeks' gestation only in Caulfield 1999, and between 12 and 16 weeks' gestation until delivery in Osendarp 2000.
Supplementation interventions targeting children
Eight studies included supplementation of macro/micronutrient targeting children (Begin 2008; Iannotti 2014; Moursi 2003; Oelofse 2003; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010). For supplementation, in Iannotti 2014, the LNS provided 108 kcal of energy and other nutrients including vitamin A, vitamin B12, iron, and zinc. In Begin 2008, maltodextrins (corn syrup solids), sugar, flavouring agents, whey protein concentrate (WPC) or BSC, and either a vitamin/mineral supplement or additional maize flour. In Oelofse 2003, the intervention group received a micronutrient‐fortified complementary food throughout the six‐month period while the control group did not receive any additional complementary food, but continued their normal diet. In Moursi 2003, the intervention group received a maize/soy‐based flour with an industrial amylase and the control group received a similar flour without amylase. The mothers in each group were shown individually on one occasion what amounts of flour and water to use in order to obtain gruels of similar consistency to the ones they were used to preparing. In Taneja 2010, in the intervention area, infants (aged 6 to 11 months) received a daily dose of elemental zinc 10 mg as zinc gluconate, and older (aged 12 to 35 months) children received a daily dose of 20 mg. At enrolment, all these children received a dose of vitamin A (104.7 μmol to infants and 209.4 μmol to older children). The children in the control group did not receive vitamin A or zinc.
Supplementation duration of children varied; fortified food lasted seven months in Iannotti 2014; dietary supplements daily for eight months in Begin 2008; micronutrient‐fortified complementary food throughout the six‐month period in Oelofse 2003; four months of zinc supplementation in Taneja 2010; the distribution of flours with and without added amylase started at 18 weeks of age, after having made sure that the parents had already spontaneously introduced complementary foods, and continued for 14 weeks in Moursi 2003; daily doses (five days a week) from the day of enrolment (usually within seven days of birth) to the age of one completed year in Sur 2003.
Nutrition education for pregnant women (two studies)
Two studies included nutrition education and a complementary food recipe demonstration for pregnant women (Akter 2012; Jahan 2014). In Akter 2012, the intervention group received nutrition education twice in the first month and once per month for the next two months before delivery and the control group received routine hospital advice on food intake, immunisation, personal hygiene, and breastfeeding. In Jahan 2014, women in the intervention group attended monthly education sessions at the clinic for three months including the nutritional value of food, the importance of exclusive breastfeeding, establishing an adequate diet during pregnancy and lactation, cooking practices for optimum retention of nutrients, and awareness about food taboos relating to pregnancy and infant feeding. A demonstration was provided on cooking a highly nutritious local dish that could be made with affordable, readily available ingredients.
Nutrition system strengthening (two studies)
Penny 2005, in the intervention area, promoted the quality of nutrition services (counselling) in the health facilities and into existing child‐oriented national programmes (immunisation, monitoring of growth and development, and management of acute respiratory infections and diarrhoea). Pridmore 2014, created an enabling environment for nutrition by establishing a multi‐sectorial nutrition working group and by working with stakeholders to plan, act, and evaluate small‐scale inter‐sectorial, co‐ordinated interventions.
Determinants of stunting tackled at individual, household, community, and country level in selected studies
The logic model presents interventions that tackle the determinants of stunting at individual, household, community, and country level (Figure 3). Eight of the selected studies addressed determinants only at the individual level (pregnant women or children) (Begin 2008; Iannotti 2008; Moursi 2003; Oelofse 2003; Osendarp 2000; Rahman 2002; Sur 2003; Taneja 2010). The following interventions deployed strategies at household level; Pridmore 2014, and at community level; Akter 2012; Iannotti 2014; Penny 2005; Pridmore 2014; Radhakrishna 2013, and at regional and country level (Pridmore 2014), as well as at the individual level. Additionally, the following studies included a service delivery component to improve access to and utilisation of the programs: Akter 2012; Caulfield 1999; Iannotti 2014; Jahan 2014; Penny 2005; Pridmore 2014; Radhakrishna 2013.
Urban specificity
The logic model (Figure 3) and the Background section presented different approaches that interventions in urban settings might take to tackle determinants at individual, household, and community levels. This urban adaptation or specificity includes three approaches: 1. interventions that aim to change social factors at a household or community level; 2. interventions undertaking a community‐based initiative using community resources internal to the slums; and 3. interventions that work to effect immediate change in health outcomes (improved access to health care, improving the quality of schools).
The studies that underwent an adaptation to fit within these three approaches included the following:
Caulfield 1999: chose the targeted area for the high compliance for supplementation in the targeted areas fitting under approach 3;
Iannotti 2014: integrated the intervention in a community‐based programme or an existing service delivery centre fitting under approach 2 and 3;
Akter 2012: used activities to increase access to health and tackle socioeconomic determinants meeting approach 1 and 3;
Jahan 2014: tackled socioeconomic determinants meeting approach 1;
Pridmore 2014: the interventions aimed to tackle urban specific socioeconomic and environmental factors operating at local, municipality, provincial, and central levels under approach 1, 2, and 3;
Penny 2005: facilitated health access in a sustainable way using approach 3.
Participants
Across all studies, children's ages ranged from birth to 59 months with a mean of 11.4 months. Seventy three percent of the studies focused on children less than one year old. Some studies reported that 33% were newborns (Akter 2012; Caulfield 1999; Iannotti 2008; Jahan 2014; Radhakrishna 2013). Other studies reported that 27% were under one year old (Begin 2008; Iannotti 2014; Moursi 2003; Oelofse 2003). The mean participant sample size was 617 and ranged from 60 (Oelofse 2003) to 2482 (Taneja 2010).
The nutritional status of participants at baseline differed. Four studies focused on healthy children and excluded children with MAM, SAM, LBW, or with congenital abnormalities (Begin 2008; Iannotti 2014; Moursi 2003; Oelofse 2003), whereas in the other three, there were no inclusion criteria based on child nutritional status (Penny 2005; Pridmore 2014; Taneja 2010). Sur 2003 selected LBW infants, and in Begin 2008, more than half of the infants and children in the survey area had anaemia based on previous studies, but there was no mention whether this was the case for participants in the sample.
Unit of randomisation
Two studies considered the mother–infant dyad as the unit of analysis (Iannotti 2008; Jahan 2014), one the health facilities (Penny 2005), one the household (Pridmore 2014), while the rest used individual infant/child or the mother as the unit of analysis (Akter 2012; Begin 2008; Caulfield 1999; Iannotti 2014; Moursi 2003; Oelofse 2003; Osendarp 2000; Sur 2003; Taneja 2010).
Outcomes
The primary outcome measures were HFA (seven studies; Iannotti 2014; Oelofse 2003; Penny 2005; Pridmore 2014; Radhakrishna 2013; Taneja 2010; Rahman 2002); LBW (four studies; Akter 2012; Caulfield 1999; Jahan 2014; Osendarp 2000); length in nine studies; at infants' birth (Caulfield 1999; Osendarp 2000) and others (Begin 2008; Penny 2005; Radhakrishna 2013; Rahman 2002; Sur 2003), and length velocity or change in length measurement during specific period (two studies; Iannotti 2008; Moursi 2003). The outcome data were presented using HFA and length‐for‐age z‐scores, or prevalence of LBW or MD of length measurement (velocity) or mean birth length in centimetres or mean birth weight in kilogram, or a combination of these. The secondary nutritional outcomes were mainly WFA (six studies; Iannotti 2014; Oelofse 2003; Penny 2005; Penny 2005; Taneja 2010; Rahman 2002), WFH (five studies; Moursi 2003; Oelofse 2003; Radhakrishna 2013; Rahman 2002; Taneja 2010), difference in weight (one study; Moursi 2003), and MUAC at infants' birth (two studies; Caulfield 1999; Osendarp 2000). The growth references/standards used for z‐score calculation were based on WHO for three studies, NCHS for the older studies, or both.
Timing of anthropometric measurements differed among studies.
Akter 2012: at the time of the infant's birth and for the mothers at 6 to 9 months of pregnancy.
Begin 2008: monthly anthropometrics but outcomes reported on in the paper: at baseline (6 to 7 months) and 2, 4, and 6 months post baseline for growth outcomes.
Caulfield 1999: at baseline, 7 and 8 months' gestation, and birth.
Iannotti 2008; at birth and monthly from month 1 to month 12.
Iannotti 2014: 6‐monthly visits for participants recruited between 6 and 11 months plus a follow‐up 6 months after the end of the study.
Jahan 2014: on a monthly basis from months 6 to 9 of pregnancy, birth and 1‐month postpartum.
Moursi 2003: every 7th day.
Oelofse 2003: 10, 16, 24, and 32 weeks of age grouped in 10 to 15, 16 to 23, 24 to 31, and 16 to 31 weeks of age.
Osendarp 2000: at baseline, 7 months' gestation and monthly until 8 months' gestation, and at birth within 72 hours of birth.
Penny 2005: at birth, 3, 6, 9, 12, 15, and 18 months.
Pridmore 2014: two times in July 2011 and in June 2013.
Sur 2003: monthly for one year.
Taneja 2010: at birth or up to seven days after birth.
The secondary outcomes were all health related, diarrhoea and morbidity. Other non‐nutritional outcomes were psychomotor tests, pregnancy duration, and mother's blood pressure during gestation.
Excluded studies
We excluded 13 studies because of the age limit (children in the sample were older than 60 months; Agustina 2013; Mitter 2012; Tomlinson 2016); because the review primary outcomes were not included (Effendy 2015; Iannotti 2013); because the study was in an urban area but not necessarily in a slum or poor urban area (Akeredolu 2014; Kæstel 2005; Kikafunda 1998); because the studies were cross‐sectional without an intervention (Krebs 2011; Semba 2011); because the studies were non‐randomised with only one intervention site (Choudhury 2016; Poudel 2004; Saran 2002); or because the findings were not disaggregated for urban areas (Soofi 2013). For further details, see the Characteristics of excluded studies table.
Risk of bias in included studies
See the 'Risk of bias' tables included in the Characteristics of included studies table for an assessment of the risk of bias for each included trial and Figure 5 and Figure 6 for an overall summary of the risk of bias of all included trials.
5.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
6.
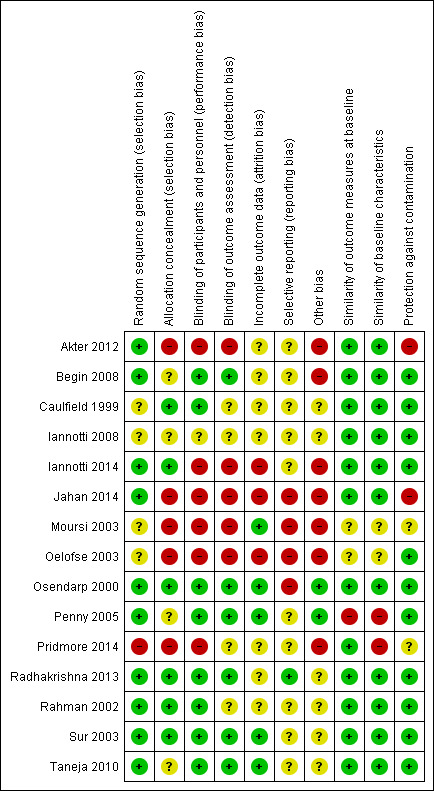
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
None of the studies was assessed of overall low risk of bias according to our pre‐established criteria. Four trials were of overall moderate risk of bias (Osendarp 2000; Radhakrishna 2013; Sur 2003; Taneja 2010), while all the other included studies were of high risk of bias.
In Table 5, we presented the overall risk of bias of the evidence for each study.
Sequence generation (selection bias)
We assessed 10 trials at low risk of selection bias (Akter 2012; Begin 2008; Iannotti 2014; Jahan 2014; Osendarp 2000; Penny 2005; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010). Four studies did not provide sufficient information to evaluate the risk and were rated at unclear of selection bias (Caulfield 1999; Iannotti 2008; Moursi 2003; Oelofse 2003), and one study was at high risk of selection bias as there was no randomisation (Pridmore 2014).
Allocation
We assessed six trials as having adequate methods for concealing the allocation sequence before and until assignment (Caulfield 1999; Iannotti 2014; Osendarp 2000; Radhakrishna 2013; Rahman 2002; Sur 2003). Five studies were assessed as high risk of bias as allocation concealment was not done (Akter 2012), the education intervention could not be concealed (Jahan 2014), no information was provided (Moursi 2003), there was no mention of concealing the participants to the group they were allocated to (Oelofse 2003), or not done (Pridmore 2014). In four studies, the risk was unclear, because the information provided was insufficient (Begin 2008; Iannotti 2008; Penny 2005; Taneja 2010).
Blinding
Risk of blinding of participants and personnel was low in eight studies (Begin 2008; Caulfield 1999; Osendarp 2000; Penny 2005; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010). This assessment was high in six studies: Akter 2012 because it was not possible to blind the providers of the intervention; there was insufficient information supplied to allow judgement on this outcome (Iannotti 2014; Moursi 2003; Oelofse 2003); because it was an education intervention it was not possible to blind the providers of the intervention (Jahan 2014); blinding was not undertaken in Pridmore 2014. Risk of blinding of participants and personnel was unclear in Iannotti 2008.
Detection bias was low in six studies (Begin 2008; Osendarp 2000; Penny 2005; Radhakrishna 2013; Sur 2003; Taneja 2010). Risk of detection bias was high in five studies; in Akter 2012, due to the nature of the intervention; in Iannotti 2014, because it was not possible to fully blind allocation; in Jahan 2014, there was no mention in the paper that the outcome was blinded during assessment and it would have been difficult to do this given that this was an education intervention; and Moursi 2003 and Oelofse 2003 provided no information. Four studies were at unclear risk of detection bias (Caulfield 1999; Iannotti 2008; Pridmore 2014; Rahman 2002).
Incomplete outcome data
Five studies were at low risk of attrition bias either because there was no loss to follow‐up (Sur 2003), or because there were similar rates of loss to follow‐up in the control and intervention groups (Moursi 2003; Osendarp 2000; Penny 2005; Sur 2003; Taneja 2010). It was unclear in seven studies as there was no analysis of attrition effect (Akter 2012; Begin 2008; Caulfield 1999; Iannotti 2008; Rahman 2002), not enough information about the participants that left the study (Akter 2012), or there were no specific analyses presented to look at a comparison between those who started the intervention and those who completed, making it impossible to assess the overall risk of attrition (Radhakrishna 2013). In the other studies, the risk was high because the loss to follow‐up was high and there was insufficient analysis of the characteristics of the leavers (Iannotti 2014; Jahan 2014; Oelofse 2003).
Selective reporting
Only one study was at low risk of reporting bias (Radhakrishna 2013). The protocol showed that the primary outcomes identified in the protocol were reported upon. In 10 studies, there was unclear risk of selective reporting as we could not assess whether all the outcomes were reported (Akter 2012; Begin 2008; Caulfield 1999; Iannotti 2008; Iannotti 2014; Penny 2005; Pridmore 2014; Rahman 2002; Sur 2003; Taneja 2010). In four studies, the risk was high as there was insufficient reporting on all of the outcomes (Jahan 2014; Moursi 2003; Oelofse 2003; Osendarp 2000).
Other potential sources of bias
We assessed two studies at low risk of other bias (Osendarp 2000; Penny 2005); six studies at unclear risk of other bias (Caulfield 1999; Iannotti 2008; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010); and seven studies at high risk of other bias (Akter 2012; Begin 2008; Iannotti 2014; Jahan 2014; Moursi 2003; Oelofse 2003; Pridmore 2014), due to reasons highlighted in the Characteristics of included studies table.
Similarity of outcome measures at baseline
Twelve studies assessed children or maternal outcomes at baseline with no differences or small differences, hence the risk of bias for this domain was low (Akter 2012; Begin 2008; Caulfield 1999; Iannotti 2008; Iannotti 2014; Jahan 2014; Osendarp 2000; Penny 2005; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010). The risk for two studies was unclear as there was insufficient information (Moursi 2003; Oelofse 2003). One study was at high risk as neither the design nor the analysis controlled for selection bias (Pridmore 2014).
Similarity of baseline characteristics
Eleven studies assessed children or maternal characteristics at baseline with no differences or small differences, hence the risk of bias for this domain was low (Akter 2012; Begin 2008; Caulfield 1999; Iannotti 2008; Iannotti 2014; Jahan 2014; Osendarp 2000; Penny 2005; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010). The risk was unclear for two studies as there was insufficient information for assessment (Moursi 2003; Oelofse 2003). One study was at high risk as there were no data and the authors reported that at baseline the two areas were quite different socioeconomically and in other characteristics (Pridmore 2014). One study was at high risk as there were some differences on baseline characteristics in the control group compared to the intervention group (Penny 2005).
Protection against contamination
In 11 studies, it was unlikely that the control group received the intervention as it was a supplementation intervention with randomisation of participants (Begin 2008; Caulfield 1999; Iannotti 2008; Iannotti 2014; Oelofse 2003; Osendarp 2000; Penny 2005; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010). In two studies, the risk was unclear as there was insufficient information for assessment (Moursi 2003; Pridmore 2014). In two studies, risk was high because local investigators were in communication with participants in both the intervention and control groups (Akter 2012), and it was possible that the control group could have received information in the second study (Jahan 2014) and cross‐communication was likely.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
See: Table 1; Table 2; Table 3; and Table 4.
We organised the summary results by intervention type and by primary and secondary outcomes. See the Data and analyses section for detailed results on the primary and secondary outcomes.
Zinc supplementation in pregnant women versus supplementation without zinc or placebo
Three trials, comprised of 3149 pregnant women and 2062 newborns compared birth weight outcomes of supplemented pregnant women versus control (Caulfield 1999; Iannotti 2008; Osendarp 2000). Table 6 used visual representations to indicate reported effect direction per study and per primary and secondary outcomes. Meta‐analysis were conducted for RCTs only.
Table 6. Summary of effect direction for nutritional and non‐nutritional outcomes from included studies for maternal interventions
| Author year, design | Overall risk of bias | Height | HFA | LBW | MUAC | Weight | WFA | WFH | Diarrhoea |
| Caulfield 1999, RCT | High | = | NR | = | = | NR | NR | NR | NR |
| Iannotti 2008, RCT | High | = | = | NR | ++ | ++ | NR | NR | = |
| Osendarp 2000, RCT | Moderate | = | NR | = | = | NR | NR | NR | NR |
| Effect direction: ++: positive health impact; –: negative health impact; =: unclear effect (a non‐statistically significant effect was interpreted as unclear effect). HFA: height‐for‐age; LBW: low birth weight; MUAC: mid‐upper‐arm circumference; NR: not reported; RCT: randomised controlled trial; WFA: weight‐for‐age; WFH: weight‐for‐height. | |||||||||
Primary outcomes
Height or height‐for‐age
Meta‐analysis
Meta‐analysis conducted on data from the two RCTs including pregnant women identified no evidence of an effect of supplementation in pregnant women on newborn length (MD –0.13 cm, 95% CI –0.36 to 0.10; I² = 0%; studies = 2; participants = 1337; moderate‐certainty evidence; Analysis 1.1; Figure 7; Table 1; Caulfield 1999; Osendarp 2000).
1.1. Analysis.
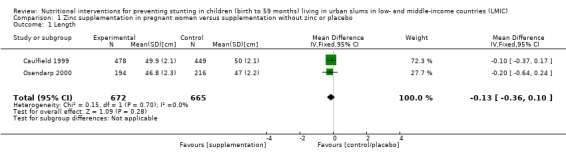
Comparison 1 Zinc supplementation in pregnant women versus supplementation without zinc or placebo, Outcome 1 Length.
7.
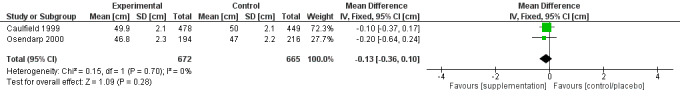
Forest plot of comparison: 1. Zinc supplementation in pregnant women versus supplementation without zinc or placebo, outcome: 1.1 Length [cm].
Narrative synthesis
In Iannotti 2008, change in mean length from birth to 12 months for the zinc group versus control group showed a negative difference in the first 5 and 11 months. The MDs in length were –0.12 cm at birth (262 infants in zinc group, 260 infants in control group) and +0.16 cm at 12 months (115 infants in zinc group, 122 infants in control group). In regression modelling for longitudinal analysis in the original study, there was no statistically significant treatment differences in length after adjustment for covariates (infant and maternal biological factors, age, socioeconomic and environmental conditions, and infant morbidities and diet) and showed an unclear effect of the intervention on length at 12 months (MD 0.13, SD 0.16; P = 0.403; low‐certainty evidence; Table 1).
Low birth weight
Meta‐analysis
Meta‐analysis conducted only on data from the two RCTs including pregnant women identified no evidence of an effect of supplementation in pregnant women on newborn LBW (continuous variable) (MD –36.13 g, 95% CI –83.61 to 11.35; I² = 0%; studies = 2; participants = 1367; moderate‐certainty evidence; Analysis 1.2; Figure 8; Table 1; Caulfield 1999; Osendarp 2000).
1.2. Analysis.
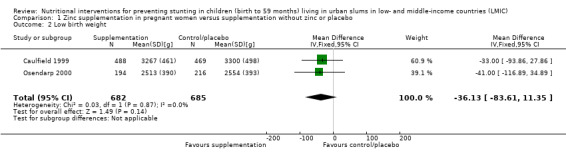
Comparison 1 Zinc supplementation in pregnant women versus supplementation without zinc or placebo, Outcome 2 Low birth weight.
8.
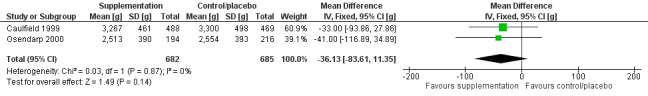
Forest plot of comparison: 1. Zinc supplementation in pregnant women versus supplementation without zinc or placebo, outcome: 1.2 Low birth weight [g].
Secondary outcomes
Weight
Narrative synthesis
The results from Iannotti 2008 concluded that infants born to mothers antenatally supplemented with zinc had significantly (P < 0.05) larger mean bodyweight starting in month 4 and continuing to month 12. In longitudinal regression modelling, antenatal zinc was associated with greater weight at 12 months (by 0.58 kg, SD 0.12; P < 0.001), after adjustment for a range of covariates (infant and maternal biological factors, age, socioeconomic and environmental conditions, and infant morbidities and diet).
Mid‐upper‐arm circumference
Meta‐analysis
Meta‐analysis conducted on data from the two RCTs including pregnant women identified no evidence of an effect of supplementation on children's MUAC (MD 0.01 mm, 95% CI –0.13 to 0.14; I² = 37%; studies = 2; participants = 1264; Analysis 1.3; Figure 9; Caulfield 1999; Osendarp 2000).
1.3. Analysis.
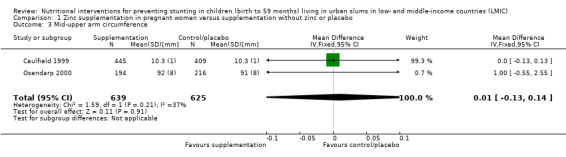
Comparison 1 Zinc supplementation in pregnant women versus supplementation without zinc or placebo, Outcome 3 Mid‐upper arm circumference.
9.
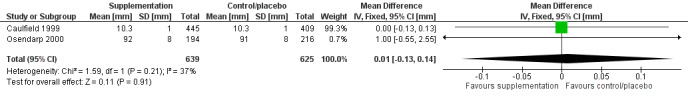
Forest plot of comparison: 1. Zinc supplementation in pregnant women versus supplementation without zinc or placebo, outcome: 1.3 Mid‐upper arm circumference [mm].
Narrative synthesis
The results from Iannotti 2008 concluded that infants born to mothers antenatally supplemented with zinc did not have significantly larger MUAC in month 12. In longitudinal regression modelling, antenatal zinc was not associated with greater MUAC at 12 months (0.09 cm, SD 0.09; P = 0.294), after adjustment for a range of covariates (infant and maternal biological factors, age, socioeconomic and environmental conditions, and infant morbidities and diet.
Other secondary outcomes
Diarrhoea
Narrative synthesis
In Iannotti 2008, there was no statistically significant difference in the prevalence of diarrhoea by treatment group.
Micronutrient or macronutrient supplementation in children versus no intervention or placebo
Eight studies, including 4598 infants and children, compared the length or HFA of children receiving supplementation versus no supplementation or no intervention (Begin 2008; Iannotti 2014; Moursi 2003; Oelofse 2003; Radhakrishna 2013; Rahman 2002; Sur 2003; Taneja 2010). Table 7 used visual representations to indicate reported effect direction per study and per primary and secondary outcomes.
Table 7. Summary of effect direction for nutritional and non‐nutritional outcomes from included studies for supplementation in children intervention
| Author year, design | Overall risk of bias | Height | HFA | MUAC | Weight | WFA | WFH | Diarrhoea |
Feeding practices dietary intake |
Rate of ill days | Incidence of diseases | Skinfolds |
| Begin 2008, RCT | High | = | NR | = | NR | NR | NR | = | NR | NR | NR | NR |
| Iannotti 2014, RCT | High | NR | = | NR | NR | = | NR | NR | NR | NR | NR | NR |
| Moursi 2003, RCT | High | ++ | NR | NR | NR | = | = | NR | = | = | = | NR |
| Oelofse 2003, RCT | High | NR | = | NR | NR | = | = | NR | NR | NR | NR | NR |
| Radhakrishna 2013, RCT | Moderate | = | = | NR | = | = | = | NR | NR | NR | NR | ++ |
| Rahman 2002, RCT | High | = | = | NR | = | = | = | NR | NR | NR | NR | NR |
| Sur 2003, RCT | Moderate | ++ | NR | NR | NR | ++ | NR | ++ | NR | NR | NR | NR |
| Taneja 2010, RCT | Moderate | NR | = | NR | NR | = | = | = | NR | NR | NR | NR |
| Effect direction: ++: positive health impact; –: negative health impact; =: unclear effect (a non‐statistically significant effect was interpreted as unclear effect). HFA: height‐for‐age; MUAC: mid‐upper‐arm circumference; NR: not reported; RCT: randomised controlled trial; WFA: weight‐for‐age; WFH: weight‐for‐height. | ||||||||||||
Primary outcomes
Height or height‐for‐age
Meta‐analysis
Meta‐analysis conducted on data from the three RCTs including infants and children aged under 60 months identified no evidence of an effect of supplementation on LFA/HFA (MD –0.02 z‐score, 95% CI –0.06 to 0.02; I² = 0%; studies = 3; participants = 2601; low‐certainty evidence; Analysis 2.1; Figure 10; Table 2; Iannotti 2014; Oelofse 2003; Taneja 2010).
2.1. Analysis.
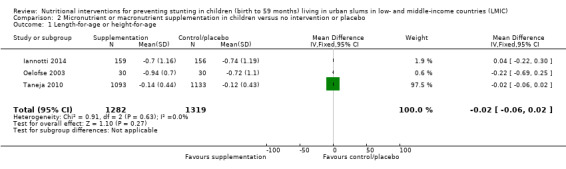
Comparison 2 Micronutrient or macronutrient supplementation in children versus no intervention or placebo, Outcome 1 Length‐for‐age or height‐for‐age.
10.
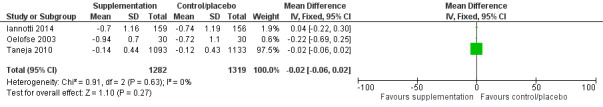
Forest plot of comparison: 2. Micronutrient or macronutrient supplementation in children versus no intervention or placebo, outcome: 2.1 Length‐for‐age or height‐for‐age.
Narrative synthesis
In Sur 2003, there was evidence of an effect of zinc supplementation during pregnancy in infants on length with low‐certainty evidence (Table 2), but only at age 12 months (MD 2.3 cm; 100 infants) and not at months 1 to 11. This was calculated based on the length at birth (supplemented: 46.4 cm, control: 46.4 cm) plus the difference in length gain to 12 months (supplemented: +23.7 cm, control: +21.4 cm).
In Moursi 2003, there was evidence of an effect of consumption of amylase‐containing gruels on the length velocity of Congolese infants with very low‐certainty evidence (Table 2); the difference in length velocity (at 16 to 31 months) intervention versus control was +0.22 cm per month (P = 0.04) adjusted for growth that preceded the specified time interval and morbidity during the same time interval.
In Begin 2008, there was no significant difference by treatment group in the mean change in final length after eight months' supplementation controlling for initial anthropometric status, sex, and age at the beginning of supplementation with low‐certainty evidence (Table 2).
In Rahman 2002, there was an unclear effect on HFA z‐score and length at six months with low‐certainty evidence (Table 2). Gains in length during the follow‐up period were not significantly different among the four groups.
In Radhakrishna 2013, there was an unclear effect on the prevalence of HFA at 18 and 24 months and in length in the intervention versus control group with moderate‐certainty evidence (Table 2). The gain in length from 6 to 18 months was 12.4 cm in the intervention group versus 12.6 cm in the control group.
Secondary outcomes
Weight or weight‐for‐age
Meta‐analysis
Meta‐analysis conducted on data from four RCTs including children aged less than 60 months identified no evidence of an effect of micronutrient supplementation in children on WFA (MD 0.04 z‐score, 95% CI –0.01 to 0.10; I² = 15%; studies = 4; participants = 2646; Analysis 2.2; Figure 11; Iannotti 2014; Moursi 2003; Oelofse 2003; Taneja 2010).
2.2. Analysis.
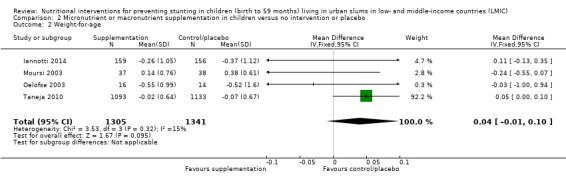
Comparison 2 Micronutrient or macronutrient supplementation in children versus no intervention or placebo, Outcome 2 Weight‐for‐age.
11.
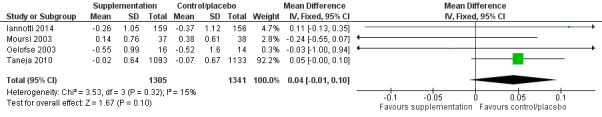
Forest plot of comparison: 2. Micronutrient or macronutrient supplementation in children versus no intervention or placebo, outcome: 2.2 WFA.
Narrative synthesis
Sur 2003 was not included in the meta‐analysis because the children were selected with LBW while in the four other RCTs they were not. The findings in Sur 2003 showed an effect of supplementation on LBW infants WFA z‐score but this was only significant at one year of age (supplemented –1.45, SD 0.95 versus control –2.17, SD 0.90; participants = 100).
In Rahman 2002, there was an unclear effect on WFA z‐score and weight at six months. Gains in weight during follow‐up period were not significantly different among the four groups.
In Radhakrishna 2013, there was no difference in weight gain and in the distribution of underweight between the zinc and placebo groups at 18, 21, and 24 months.
Weight‐for‐height
Meta‐analysis
Meta‐analysis conducted on data from three RCTs including children aged under five years identified no evidence of an effect of supplementation in children on WFH (MD 0.04 z‐score, 95% CI –0.01 to 0.09; I² = 46%; studies = 3; participants = 2331; Analysis 2.3; Figure 12; Moursi 2003; Oelofse 2003; Taneja 2010).
2.3. Analysis.
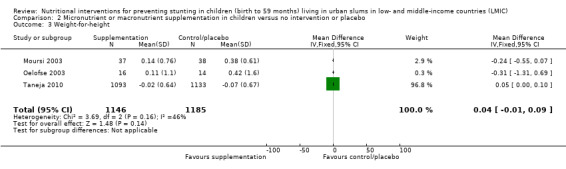
Comparison 2 Micronutrient or macronutrient supplementation in children versus no intervention or placebo, Outcome 3 Weight‐for‐height.
12.
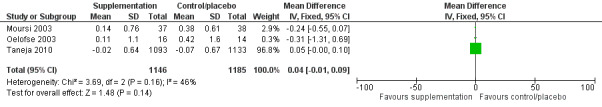
Forest plot of comparison: 2. Micronutrient or macronutrient supplementation in children versus no intervention or placebo, outcome: 2.3 WFH.
Narrative synthesis
In Rahman 2002, there was an unclear effect on WFH z‐score.
In Radhakrishna 2013, there was an unclear effect on the prevalence of WFH at 18, 21, and 24 months in intervention versus control group.
Mid‐upper arm circumference
Narrative synthesis
In Begin 2008, there was no change in MUAC in the different groups during the supplementation period.
Skinfolds
Narrative synthesis
In Radhakrishna 2013, skinfold thicknesses showed a significant increase in subscapular skinfold (SSF) and triceps skinfold (TSF) at 18, 21, and 24 months when compared to baseline. At 18 and 21 months of age, the SSF was significantly higher in the intervention group (mean 0.331 cm, 95% CI 0.049 to 0.613) compared to the control group (mean 0.318 cm, 95% CI 0.025 to 0.611). Similarly, at 21 and 24 months, the TSF was significantly higher in the intervention group (mean 0.425 cm, 95% CI 0.095 to 0.755) compared to placebo group (mean 0.389 cm, 95% CI 0.047 to 0.731).
Other secondary outcomes
Infant and young children practices and dietary intake
Narrative synthesis
In Moursi 2003, breastfeeding prevalence at 24 weeks was not statistically different between groups (100% in the intervention group and 92% in the control group). Comparisons for breastfeeding frequency and duration, and dietary intake between the groups at 24 weeks of age showed no statistically significant differences.
Morbidity, diarrhoea
Narrative synthesis
In Begin 2008, the mean prevalence of diarrhoea (ranged from 10.4% to 13.5%) did not differ significantly by treatment group, either before or after controlling for sex, age at initiation of supplementation, initial diarrhoea rates during the presupplementation observation period, breastfeeding practices, maternal characteristics, initial plasma zinc and serum ferritin concentrations, and socioeconomic variables. In Moursi 2003, there was also no significant difference in the percentage of days ill and the incidence of diseases between the intervention and control groups during all time intervals with the exception of respiratory illness between 16 and 23 weeks of age and 16 to 31 weeks of age for which the percentage of days ill with cough or rhinitis and their incidence were significantly higher in the intervention group. Taneja 2010 did not report on the impact of the intervention on diarrhoea incidence. In Sur 2003, the infants in the supplemented group had 66 diarrhoeal episodes giving an incidence of 1.36 episodes per child per year of observation, compared with 89 episodes in the control group with an incidence of 1.93 episodes per child per year of observation. The difference was statistically significant (RR 1.4, 95% CI 1.02 to 2.00; P < 0.03), showing a percentage reduction of 29%.
Education for pregnant women versus standard care or no intervention
Two studies including 415 pregnant women and newborns compared the birth weight outcomes after nutritional education versus a control group (Akter 2012; Jahan 2014). Table 8 used visual representations to indicate reported effect direction per study and per primary and secondary outcomes.
Table 8. Summary of effect direction for nutritional and non‐nutritional outcomes from included studies for education intervention
| Author year, design | Overall risk of bias | LBW | Initiation of breastfeeding |
| Akter 2012, RCT | High | ++ | NR |
| Jahan 2014, RCT | High | ++ | ++ |
| Effect direction: ++: positive health impact, –: negative health impact, =: unclear effect (a non‐statistically significant effect was interpreted as unclear effect). LBW: low birth weight; NR: not reported; RCT: randomised controlled trial. | |||
Primary outcomes
Low birth weight
Meta‐analysis
Meta‐analysis conducted using data from the two RCTs including children aged under five years identified evidence of an effect of nutrition education in pregnant women on newborn LBW (continuous variable) (MD 478.44 g, 95% CI 423.55 to 533.32; I² = 0%; studies = 2; participants = 415; low‐certainty evidence; Analysis 3.1; Figure 13; Table 3; Akter 2012; Jahan 2014).
3.1. Analysis.
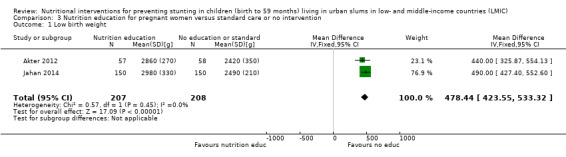
Comparison 3 Nutrition education for pregnant women versus standard care or no intervention, Outcome 1 Low birth weight.
13.
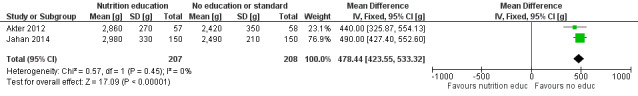
Forest plot of comparison: 3. Nutrition education for pregnant women versus standard care or no intervention, outcome: 3.1 LBW (g).
Other secondary outcomes
Narrative synthesis
In Jahan 2014, the intervention had an impact on the rate of initiation of breastfeeding within one hour of 52% higher favouring the intervention group (86.0% in the intervention group versus 56.7% in the control group; P < 0.001).
System strengthening
Two studies including infants and children compared the length or HFA of children receiving nutrition support (raising the profile of nutrition in the health facilities for Penny 2005, and intersectorial actions to change the social determinants of malnutrition in Pridmore 2014 (1809 infants including 810 infants in 2011 and 999 infants in 2013) versus no intervention. In Penny 2005, nutritional education started from the infant's birth (377 newborns). Table 9 used visual representations to indicate reported effect direction per study and per primary and secondary outcomes.
Table 9. Summary of effect direction on nutritional and non‐nutritional outcomes from included studies for system strengthening intervention
| Authors year, design | Overall risk of bias | Height | HFA | WFA | WFH |
| Penny 2005, cluster RCT | High | ++ | ++ | = | = |
| Pridmore 2014, non‐randomised controlled trial (control group before‐after and case‐control) | High | NR | ++ | NR | NR |
| Effect direction: ++: positive health impact; –: negative health impact; =: unclear effect (a non‐statistically significant effect was interpreted as unclear effect). HFA: height‐for‐age; NR: not reported; RCT: randomised controlled trial; WFA: weight‐for‐age; WFH: weight‐for‐height. | |||||
Primary outcomes
Height or height‐for‐age
Narrative synthesis
In Pridmore 2014, there was a decrease in the mean HFA between the end of the intervention compared to the start in both intervention and control group with very low certainty of evidence (Table 4). But the findings showed that the decrease was more important in the control group and that the nutrition support intervention did not influence HFA at the end of the intervention (female HFA mean z‐score intervention –1.41, SD 1.28, 256 infants versus control –1.28, SD 1.31, 255 infants). The findings of Pridmore 2014 suggested that boys benefited more from the interventions than girls (a decrease of 6.1 percentage points in the male intervention group versus an increase of 1.3% percentage points in the female intervention group). Comparisons with the control group suggested that other factors were at play such as negative changes in employment, food security, income, and population change as both male and female groups registered a decrease in stunting (–7.2 percentage points for male and –11.4 percentage points for female). These might have impacted more the intervention group compared with the control group.
In Penny 2005, there was a small effect on LFA at 18 months (unadjusted MD in z‐score: 0.386, 95% CI 0.209 to 0.562; participants = 377; P < 0.0001; adjusted for SES, hygiene score, and birth weight variables MD in z‐score: 0.272, 95% CI 0.099 to 0.445; P = 0.002; low‐certainty evidence; Table 4). While in Penny 2005, there was an impact on HFA with low‐certainty evidence (Table 4), it was important to note that the MD in length was small (at 18 months: mean length: 79.36 cm, SD 2.74 in the intervention group versus 78.29 cm, SD 2.66 in the control group; MD 1.07 cm). While statistically significant, there may not have been any practical clinical effect. Moreover, in Penny 2005, both intervention and control groups continued to decline in LFA z‐score through 18 months of age.
In Penny 2005, there was an effect in length at 18 months (unadjusted MD: 1.068 cm, 95% CI 0.488 to 1.648; P < 0.0003; adjusted difference MD: 0.714, 95% CI 0.146 to 1.282; P = 0.014 adjusted for SES hygiene score, and birth weight variables) with moderate‐certainty evidence (Table 4).
Secondary outcomes
Weight‐for‐age
Narrative synthesis
In Penny 2005, there was an effect of the nutrition support intervention on infant's and children's underweight status at 18 months (unadjusted MD z‐score 0.285, 95% CI 0.099 to 0.471; participants = 377; P = 0.003; adjusted MD z‐score 0.194, 95% 0.008 to 0.38; P = 0.041 after adjustment for SES, hygiene score, and birth weight variables and after application of the random‐effects model in recognition of the cluster design).
Weight‐for‐height
Narrative synthesis
In Penny 2005, there was an unclear effect of the nutrition support intervention on infant's and children's wasting status at 18 months (unadjusted MD z‐score 0.091, 95% CI –0.089 to 0.271; participants = 377; P = 0.319; adjusted MD z‐score 0.048, 95% CI –0.139 to 0.237; P = 0.609 after adjustment for SES, hygiene score, and birth weight variables and after application of the random‐effects model in recognition of the cluster design).
Subgroup analyses
We were unable to conduct the subgroup analyses for the following reasons.
For the age of the children (younger or older than 24 months): there were insufficient studies sharing the same characteristics.
For nutritional status at baseline (stunting or not): there was only one study which included children with LBW at baseline (Sur 2003).
For the location (Asia, Africa, Latin America): there were insufficient studies sharing the same characteristics.
For duration of the intervention (less than or more than 12 months): there were insufficient studies sharing the same characteristics.
For the intervention component (nutrition counselling, fortification, etc.): this was done as the main analysis.
For intervention design (single, combined): all studies reported nutritional intervention only.
For the source of funding: there was only one study reporting on funding and source of funding.
We intended to add a subgroup analysis of the interventions tackling factors at individual, household, and community level versus the interventions tackling factors at the individual level only. We were unable to conduct this due to the insufficient number of studies reporting on the same outcomes. Likewise, for interventions that took an urban approach, only one subgroup analysis was possible with Akter 2012 and Jahan 2014 (Table 3). All the other interventions which were adapted to the urban context could not be grouped to perform a subgroup analysis due to the difference in outcomes reported.
Sensitivity analysis
The sensitivity analysis to examine the effects of removing studies with overall high risk of bias from the meta‐analyses (Akter 2012; Caulfield 1999; Iannotti 2014; Jahan 2014; Moursi 2003; Oelofse 2003) resulted in only one study for zinc supplementation in pregnant women versus supplementation without zinc or placebo and only one study for micronutrient or macronutrient supplementation in children versus no intervention or placebo. In both case, there were similar findings on effect by excluding studies at high risk of bias.
We were unable to conduct the planned comparative analysis to test for sensitivity of the results of the review for the following reasons.
No studies were included with abstract only.
No studies including preschool aged and over five years old children were included.
No studies were excluded due to potentially confounding cointerventions.
Funding source information was provided for only one study.
Equity
We were unable to assess equity adequately as only one of the studies provided results disaggregated by the PROGRESS variables (Pridmore 2014). As all studies were conducted in poor urban areas or slums, the target population fall in the low SES class. Nevertheless, as each country and slum setting was very different, and there was insufficient information to characterise further the population studied, equity was impossible to assess.
Discussion
Summary of main results
Our systematic review included 15 studies conducted in LMIC, of which 14 were RCTs. The interventions took place in impoverished shantytowns, informal settlements, communities with low SES, exposed to flooding, and with poor access to sanitation. Half of the sites were in recognised slum settings and the other half in poor urban or periurban areas. The study locations were mainly Peru, Bangladesh, and India. These studies included nutrition outcome data for 9261 infants and children, and 3664 pregnant women. Seventy three percent of the studies included infants less than one year old which means that the evidence was limited to address issues of the whole target population. The interventions included; zinc supplementation in pregnant women (three studies), micronutrient or macronutrient supplementation in children (eight studies), nutrition education for pregnant women (two studies), and nutrition systems strengthening targeting children (two studies).
There were no interventions in which unprepared foods were just given to mothers or prepared meals supplied.
Six interventions out of the 15 employed an urban approach as depicted in Figure 3 aiming to: to change social factors at a household or community level; undertake community‐based initiative using community resources internal to the slums; and work to effect immediate change in health outcomes (improved access to health care, improving the quality of schools). Eight studies tackled determinants of malnutrition at the individual level only while the other seven studies used approaches that targeted household, community, and for some 'service delivery' to increase access to health services via systems strengthening. The intervention duration (which was not necessarily the supplementation duration) was short (on average 1.2 years) with 53% of the interventions delivered at the participants' homes by investigators, trainers, or field workers, and the others using a mix of deliveries at health facilities during antenatal visits, daycare centres, home, and hospital.
Eleven studies had overall high risk of bias and only four trials had moderate risk of bias. Overall, the evidence was complex to report with a wide range of outcome measures. Consequently, we reported only eight study findings in meta‐analyses and seven in a narrative form. The certainty of evidence was very low to moderate. Primary outcomes included LBW; length at birth, at 12 and 18 months; HFA; and growth velocity. Findings on zinc supplementation interventions in pregnant women showed no effect on LBW and length with low‐ to moderate‐certainty evidence (Table 1). Findings on micronutrient or macronutrient supplementation in children showed inconclusive results on HFA and length with very low‐ to moderate‐certainty evidence (Table 2). Findings on nutrition education for pregnant women interventions showed a positive impact on LBW at infant's birth with low‐certainty evidence (Table 3). Findings on nutrition system strengthening were demonstrative of inconclusive results on HFA with very low‐ to low‐certainty evidence and a positive influence on length at 18 months (based on only one study) with low‐certainty evidence (Table 4). Secondary outcomes included WFA, WFH, weight, and MUAC. Findings on zinc supplementation interventions in pregnant women showed a positive effect on weight (one study) and no evidence of an effect on MUAC. Findings on micronutrient or macronutrient supplementation in children showed inconclusive results on WFA, and no evidence of an effect on WFH and MUAC. Findings on nutrition systems strengthening were demonstrative of a positive impact on WFA and no evidence of an effect on WFH at 18 months (based on only one study). A key limitation of conclusions on secondary outcomes was that studies reporting these outcomes were excluded when primary outcomes were not included. Subgroup and equity analysis were not performed due to the lack of studies sharing similar methods and outcomes, and lack of disaggregated data related to PROGRESS. Sensitivity analysis was only performed to assess the impact of removing the six studies with high risk of bias and showed similar findings. No study reported any adverse effects.
Overall completeness and applicability of evidence
Slums and poor urban areas are known to be complex settings in which to conduct research and interventions due to a range of factors, including the population's mobility; the informal nature of the slums, which are often characterised by lack of basic and social services; temporary nature of housing structures; and exposure to floods. It is possible that these factors have mediated negatively the impact of the interventions on children's stunting. The complexity of the urban environment needs to be recognised when designing interventions aimed at improving infant and child nutrition in slums. For example, if important challenges such as access (physical, social, and security) to the programme centre are taken into account at the design phase, interventions might offer more convincing impact. The urban environment was mostly not taken account of in the design stage of the studies reviewed, which is likely one component of the unsuccessful interventions in the slum environment (Lilford 2017; SPHERE 2017). The fact that the setting was a poor urban one was only taken into account, in part, in the design of the Akter 2012 and Jahan 2014 studies, which used community clinics and provided low‐cost solutions. Iannotti 2014 recommended a community‐based rally post as locations temporarily established in the communities. In other studies, such as in Penny 2005, food insecurity and well‐functioning facilities were limiting factors for the intervention.
All of the nutritional intervention types included in this review have the potential to decrease stunting and previous evidence demonstrated the impact of maternal supplementation, macro/micronutrient supplementation in children, nutrition education, and support and counselling on nutritional outcomes in children (Bhutta 2008; Bhutta 2013; De‐Regil 2012; Haider 2017; Hossain 2017; Lassi 2013; Mori 2012; Sguassero 2012), but not specifically in poor urban environments. The adaptation or the absence of adaptation that the included interventions underwent before being implemented in the poor urban setting, and their resulting approaches, plus the challenges faced in a poor urban environment are therefore key for designing successful interventions to improve infant and child nutrition in slums. The urban SPHERE guidance on using the Sphere Standards in urban settings help in taking into account the urban complexity in programming interventions (SPHERE 2017). These urban guidelines aim to fill a gap in current guidance on how to implement humanitarian standards in urban contexts and will complement the existing SPHERE guidelines (not specific to urban areas) which are a set of minimum standards in the core areas of humanitarian assistance. Thus, in presenting the completeness and the applicability of evidence reviewed here, the authors extracted key elements in SPHERE that were recommended for urban customisation and commented on their presence or absence for interventions assessed successful.
Overall, there was evidence suggesting that the interventions could not improve HFA. The small positive effect for length of infants and young children from nutrition promotion and micro/macronutrient supplementation interventions in poor urban settings was tempered by the negative findings for HFA z‐scores, which is a more robust standardised measured by both gender and age. The limited evidence gathered demonstrated that the interventions targeting pregnant women had the potential to improve infants' birth weight but not length at birth. For LBW, the interventions that had an impact were nutrition education versus standard care or no intervention in Akter 2012 and Jahan 2014 with moderate‐certainty evidence. Both interventions used a printed manual in addition to any nutrient supplementation. The manual included information on food security, caring practices and disease control, pregnancy‐related personal hygiene, the need for increased food intake, early initiation of breastfeeding, and exclusive breastfeeding. Nutrition education in both interventions was conducted in the outpatient areas of clinics for one hour over a three‐month period. In addition to advice and counselling provided covering nutrition‐specific and ‐sensitive topics, there was a practical demonstration of an optimal nutritional value fortified food made for the mothers (in addition to their normal diet) with locally available foods. The costs of the fortified foods were low (about 3% of family income in Jahan 2014). These interventions had a positive impact on reduction of LBW, which is key to stop the intergenerational cycle of malnutrition.
The studies by Akter 2012 and Jahan 2014 included messages about the preparation of appropriate low‐cost complementary food. Penny 2005 aimed to raise the profile of nutrition into existing child‐oriented national programmes in health facilities and was integrated into existing national programmes. Additionally, Penny 2005 included training of health staff and a scheme of accreditation measured the health service compliance. Even so, in both control and intervention groups HFA z‐score continued to decline. This casts doubt on the long‐term effectiveness of the intervention. One of the success factors in these studies might be that some of the recommendations required only a very small monetary contribution by mothers. An RCT currently underway by Kimani‐Murage 2013, which hopes to extend the impact of similar interventions by using a community‐based approach, will be able to add to these findings. This RCT is following the growth of infants from birth to one year of age and hopes to demonstrate whether the interventions have a long‐term positive impact on stunting.
Regarding micro‐ or macronutrient supplementation interventions, individual studies did show small effects, but the pooled effect showed no impact on primary outcomes across the three studies (Iannotti 2014; Moursi 2003; Sur 2003). The modalities of supplementation (duration, timing, composition, children's age) varied between interventions and resulted in high heterogeneity and difficulty in assessing the overall effect. Supplementation in control groups may have been beneficial (Iannotti 2008), decreasing the impact of the intervention and showing the importance of nutritional deficiencies in poor urban communities. Taneja 2010 noted that the period of supplementation should be longer and started with younger children. In the interventions with small positive effects, follow‐up in the form of home visits (57% of all of the intervention were delivered at the home of the participant) or centre visits were done (Iannotti 2014; Moursi 2003; Sur 2003).
The review identified gaps in evidence with limitations in the number of studies, location, children's ages, approaches undertaken, follow‐up duration, and equity. For the nutrition counselling interventions targeting pregnant women, and for nutrition promotion and support interventions targeting infants and young children, only two studies were included in each with moderate‐ and low‐certainty evidence. Geographically, most of the studies were conducted in Peru, Bangladesh, and India suggesting a gap in the evidence from other regions of the world. In terms of age, 73% of the studies focused on children less than one year old, which leaves a gap in the evidence for older children. As the first 1000 days of life (from conception to two years of age) are known to be critical in determining growth performance, this evidence gap is crucial.
Most of the interventions were supplementation (60%; Begin 2008; Caulfield 1999; Iannotti 2008; Iannotti 2014; Moursi 2003; Oelofse 2003; Osendarp 2000; Sur 2003; Taneja 2010), and mono‐sectorial interventions (interventions that are not combined with nutrition‐sensitive interventions). While nutrition education interventions in pregnant women have the potential to improve birth weight, it would have been useful to have interventions comparing nutrition education only with nutrition education and nutrition‐sensitive interventions (e.g. WASH). In Goudet 2017's scoping review on the interventions tackling malnutrition in urban areas, interventions combined different types of approach. The majority had health/nutrition promotion in addition to the intervention itself. The scoping review included more interventions than in the present review as the nutrition‐specific and ‐sensitive interventions were included. Pridmore 2014 recommended a holistic approach to comprehensively address undernutrition with specific attention due to the susceptibility of slums to economic and other shocks. For most of the interventions, there was a lack of follow‐up. This is an important consideration for future studies in order to measure effect detection, as interventions can have a lag effect on growth and time is needed to capture this. We could not assess if the impact at birth on improved birth weights was sustained and resulted in higher HFA z‐score at age two years because there was no evidence. Only in Iannotti 2008 and Sur 2003 were nutritional outcomes after one year measured, and Iannotti 2014 and Oelofse 2003, measured outcomes after six months. The other studies made anthropometric measurements only during the supplementation period itself. This could be explained by the difficulty of following up a very mobile population, but is also likely a consequence of the length of time sponsors were willing to fund such interventions.
There were high losses to follow‐up and dropout rates (20%) in four studies due to the urban nature of the population and its high mobility (Iannotti 2014; Jahan 2014; Oelofse 2003; Osendarp 2000). We were unable to measure equity, which is another gap because gender, income, and education are crucial determinants that often lead to inequalities in health outcomes. Only one study reported gender, education, and socioeconomic variables, but they did not have disaggregated data to enable us to look at inequalities.
Quality of the evidence
We assessed none of the studies to have low overall risk of bias, four studies had moderate overall risk of bias (Osendarp 2000; Radhakrishna 2013; Sur 2003; Taneja 2010), and the rest had high overall risk of bias. The overall certainty of evidence was very low to moderate (Table 1; Table 2; Table 3; Table 4). We downgraded certainty mainly for high risk of bias of the studies, indirectness (zinc only supplementation and geographic limitation), and imprecision (small sample size). Overall, we had no evidence to suggest that there was publication bias as studies with no effect were published and these would likely be more difficult to publish than studies showing an effect.
Regarding the impact on LBW, length at birth and at 18 months, and HFA at 18 months, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Regarding the other primary outcomes, certainty of evidence was very low certainty or low. For some studies, such as HFA change under nutrition systems strengthening, we were very uncertain about the estimate. It is possible that higher‐quality research will improve confidence in the estimate of effect. To better capture the intervention impact in the future, standardised approaches should be employed in studies to enable repeatability in the effect estimate. Furthermore, it would be interesting to compare how approaches tackling different levels of determinants versus approaches tackling determinants at the individual level compare in terms of impact as community factors have been proven to be important determinants in the urban context. Additionally, it would be useful to compare the impact of interventions using just a nutritional intervention versus nutritional intervention and WASH as performed in WASH trials (the WASH trials were conducted in rural areas and showed limited impact; the same trials should be conducted to assess whether urban settings change the causal pathway). There is one large RCT of this type which could contribute to more evidence (Kimani‐Murage 2013).
Potential biases in the review process
In this review, we aimed to meet the highest standards recommended in the Cochrane Handbook for Systematic Reviews of Interventions and to minimise bias with review authors alternatively and independently checking judgement and analyses. For most studies, protocols were not available thus making bias in reporting impossible to assess in some cases (Akter 2012; Iannotti 2008). We recognised possible uncertainty from unidentified studies in the search impact.
Agreements and disagreements with other studies or reviews
This is the first review to look at the impact of nutritional interventions on child nutritional status in LMICs in the context of poor urban settings thus making direct comparison of findings impossible. Nevertheless comparisons can be drawn with reviews exploring the impact of nutritional interventions in infants and young children in poor‐ and middle‐income countries (Bhutta 2008; Bhutta 2013; De‐Regil 2012; Hossain 2017; Lassi 2013; Mori 2012; Sguassero 2012).
The nutritional interventions included in our review: micronutrient supplementation in pregnant women, micro‐ or macronutrient supplementation in children, nutrition counselling, support, and health system strengthening have all been assessed as proven interventions elsewhere, mostly in rural contexts (Bhutta 2013). In contrast, the studies included in the present review did not have an impact on most of the primary outcomes, or if the studies had an impact the certainty of evidence was very low to moderate, casting doubts on the results. We can hypothesise that the interventions which did not have an effect failed to take into account the complexity of operating in urban settings. Only very few studies included a specific design to fit the slums or poor urban settings and even if they did so they did not show impact, possibly due to poor methods employed. Slums and poor urban areas are not necessarily homogeneous places and future research should include variables and methods to allow for disaggregation of covariates to assess impact according to SES, gender, and other key determinants of malnutrition.
Two previous reviews of maternal supplementation interventions with zinc, folic acid, or iron reported only negative results (Lassi 2013; Mori 2012). Two other reviews found a positive impact of these supplements on birth weight (Haider 2017; Peña‐Rosas 2015). The Bhutta 2013 review including Lassi 2013, Peña‐Rosas 2015, and an earlier review of Haider 2017, support the potential replacement of iron–folate supplements in pregnancy with multiple micronutrient supplements in populations at risk as a successful intervention to reduce LBW. The evidence gathered here could sustain the hypothesis that the lack of effect could be related to the intervention modality of single nutrient supplementation versus multiple nutrient supplementation. Maternal multiple micronutrient supplementation interventions in urban poor settings should be tested to confirm an effect on birth weight and birth length. Previous reviews relating to supplementation in infants and young children confirm our findings of no effect on HFA (De‐Regil 2011; Sguassero 2012). The De‐Regil 2011 review found limited evidence that supplementation (home fortification with micronutrient powder) had no effect on growth. However, that review found reduced anaemia and iron deficiency in infants and young children when compared with no intervention or placebo. The Sguassero 2012 review also concluded that supplementary feeding had a negligible impact on child growth but also noted that the results should be interpreted with caution because the studies included in the review were clinically diverse. Beyond the differences in modalities in the studies presented, one key factor in urban settings that might prevent improved growth and optimal absorption of micronutrients is the lack of access to optimal hygiene, such as inadequate water and sanitation facilities, leading to diarrhoeal morbidity and environmental enteropathy in infants and young children, which compromise skeletal growth.
For nutrition education and counselling, the systematic review of Hossain 2017 on interventions reducing stunting in LMICs found that such interventions had the potential to reduce stunting in children. They noted that the successful interventions included a combination of political commitment, multi‐sectorial collaboration, community engagement, community‐based service delivery platforms, and wider programme coverage and compliance. While in Bhutta 2013, strategies for breastfeeding promotion had a small effect on stunting. This does not concur with the evidence gathered in this review as the impact found was only on birth weight and not on HFA. One reason could be that families were food insecure and thus knowledge could not translate into action except if the solution was low cost as demonstrated in Akter 2012 and Jahan 2014.
Other previous reviews have explored other types of intervention (e.g. health, infrastructure) and their impact on health outcomes in urban settings (Goudet 2017; Lilford 2017; Turley 2013). In the Lancet series 'The health of people who live in slums' by Lilford 2017, a systematic overview was conducted of determinants of health in slum settings and interventions that aim to improve the health of people who live in slums. One of the key findings of that review was that health services should be proactive in providing immunisation and surveillance for childhood malnutrition. In the Turley 2013 review, the focus was on infrastructural interventions in slums and their health impact and it found limited evidence of an impact of slum upgrading on health. None of the interventions included had another sectorial component and thus we were not able to carry out subgroup analysis comparing interventions, for example, nutrition‐only interventions and interventions with other non‐nutritional components. We recommend that further research explore the impact of multi‐sectorial approaches on stunting versus nutrition‐only, preferably using methods of trial designed specifically for urban areas.
Authors' conclusions
Implications for practice.
This review is focused on supplemental interventions only, because our scoping review identified few nutrition‐sensitive interventions in slum environments on which to base a review. This review provides no evidence that supplemental interventions can increase height‐for‐age in urban slums. It also provides extremely limited evidence, from nutrition promotion targeting pregnant women, that interventions can decrease low birth weight in poor urban slum environments.
As noted in Lilford 2017, cost and economy of scale are key criteria in urban slum settings and as such, community‐based nutrition promotion and counselling from the third trimester of pregnancy, and nutrition support to health facilities, hold potential for improved weight at birth at a relatively low cost for the families and the public health system. There is not enough information on the cost of nutritional interventions and how these could be provided systematically to poor urban communities in a way that would make them successful. Economic data on the intervention cost, and related costs such as hospitalisation, should be captured in the future to inform budget decisions at policy level.
Creative approaches combining multi‐sectorial components, innovative targeting, and long‐term follow‐up are needed to increase the effectiveness. As noted in Lilford 2017, the specificity of the poor urban setting provides the potential for economies of scale due to the neighbourhood effect and for increasing returns to investments to create a healthy environment. This specificity should be used and maximised. Challenges linked to urban slum programming (high mobility, lack of social services, and high loss of follow‐up) should be taken into account and ways for urban slum adaptation should be explored further if nutrition‐specific interventions are to improve low birth weight and stunting in such environments. Longer‐term interventions might be a solution to address urban mobility challenges and to ensure continuity of services during the first three to five years of life, when human growth is most amenable to stunting prevention.
Our review demonstrates that we still do not have enough evidence of multi‐sectorial interventions, combining nutrition‐specific and sensitive methods and programmes. Neither do we have enough information about 'up‐stream' practices and policies of governmental, non‐governmental organisations and business, and commercial organisations with interests and ownership of urban slum environments
Implications for research.
This review has identified extremely limited rigorous evidence evaluating the effect of nutritional interventions on stunting in under five‐year‐old children living in slums and poor urban settings. This is an under‐researched topic as only a small number of studies (15) met the inclusion criteria. The certainty of the evidence is very low to moderate due to the lack of representativeness in nutritional interventions (mainly supplementation), duration of follow‐up, children's age (less than one year), short duration, and limited geographic coverage. These deficits leave us with significant research gaps. One high‐quality trial is currently ongoing and will, hopefully, begin to fill these gaps (Kimani‐Murage 2013). To gain more evidence, the authors advocate the systematic inclusion of urban slums in nutrition‐sensitive and ‐specific interventions, as well as in national nutrition monitoring systems such as demographic and health surveys, in large enough sample sizes to describe the nutrition status of the urban poor. This will require better preparation of researchers. For example, in agreement with Lilford 2017, there is an urgent need for research training for nutrition in the 'slum health' context, which could be developed and become an academic speciality.
In line with the proposed SPHERE 2017 guidelines, programmes to implement methodological adaptations to the urban environment have to be incorporated in research to maximise the chance of showing that interventions proven to be successful in rural contexts may also be successful in complex and challenging urban environments. Although UN Habitat has a definition for an urban slum, local definitions of slums used in research are very heterogeneous, which weakens comparability.
We are in complete agreement with Subramanian 2016, who argue cogently that to‐date the international community has promoted failed policies for unintegrated, single‐factorial interventions toward stunting. Instead, they propose the use of a, "…support‐led policy approach with a focus on integrated and structural factors…" to address the problems of inadequate nutrition, WASH, and education; high exposure to infection; and insecurities of food, employment, and housing. This new approach, supported strongly by the present systematic review, requires the measurement and understanding of social, economic, and political structural factors 'upstream' from the urban slum (e.g. governmental and private business actions). These upstream factors and forces need to be incorporated if nutrition‐specific or maternal education interventions are to improve low birth weight and stunting in poor urban environments.
What's new
| Date | Event | Description |
|---|---|---|
| 9 October 2019 | Amended | PLS ‐ third paragraph, first sentence amended for clarity |
Acknowledgements
Sophie Goudet was a beneficiary of an AXA Research Fund postdoctoral grant.
We would like to thank the staff at the editorial office of the Cochrane Public Health Group (CPHG) for editorial assessment of the review.
We would like to thank the TAG members for their contribution to this review, Prof Shams El Arifee, Dr Elizabeth Kimani‐Murage, Elena Lucchi, Prof CG Nicholas Mascie‐Taylor, Prof Rachel Musoke, Dr David Osrin, Prof Pat Pridmore, Prof Sabina Faiz Rashid, and Morwenna Sullivan.
Appendices
Appendix 1. MEDLINE search strategy (PubMed)
MEDLINE (PubMed) 1173 results
| Topics | Search | Query |
| A. Urban Slum | #1 | Search "Poverty Areas"[Mesh] OR "Socioeconomic Factors"[Mesh:NoExp] OR "Poverty"[Mesh] |
| #2 | Search urban* [tiab] OR slum* [tiab] OR shant* [tiab] OR ghetto* [tiab] OR shack* [tiab] OR bidonville* [tiab] OR squat* [tiab] OR "informal settlement" [tiab] OR "informal urban settlement" [tiab] OR barrada [tiab] OR "barrio baja" [tiab] OR "barrio pobre" [tiab] OR taudi* [tiab] OR "irregular settlement" [tiab] OR "informal housing" [tiab] OR favela* [tiab] OR "irregular settlement" [tiab] OR basti* [tiab] | |
| #3 | Search (#1 OR #2) | |
| B. LMIC | #4 | Search "Developing Countries"[Mesh] |
| #5 | Search developing countr*[tiab] OR middle income countr*[tiab] OR low income countr*[tiab] OR LMIC[tiab] | |
| C. Low income economies | #6 | Search afghan* [tiab] OR Benin* [tiab] OR Burkin* [tiab] OR Burundi* [tiab] OR Central African Republic* [tiab] OR Chad [tiab] OR chadian [tiab] OR Comoros [tiab] OR Comorian [tiab] OR Congo* [tiab] OR Eritrea* [tiab] OR Ethiopia* [tiab] OR Gambia* [tiab] OR Guinea* [tiab] OR Haiti* [tiab] OR North Korea* [tiab] OR Democratic People's Republic of Korea [tiab] OR Liberia* [tiab] OR Madagascar [tiab] OR Malagasy* [tiab] OR Malawi* [tiab] OR Mali* [tiab] OR Mozambiqu* [tiab] OR Nepal* [tiab] OR Niger* [tiab] OR Rwanda* [tiab] OR Senegal* [tiab] OR Sierra Leon* [tiab] OR Somalia* [tiab] OR South Sudan* [tiab] OR Tanzania* [tiab] OR Togo* [tiab] OR Uganda* [tiab] OR Zimbabw* [tiab] |
| D. lower middle income economies | #7 | Search Angola* [tiab] OR Armenia* [tiab] OR Bangladesh* [tiab] OR Bhutan* [tiab] OR Bolivia* [tiab] OR Cabo Verd* [tiab] OR Cambodia* [tiab] OR Cameroon* [tiab] OR Congo* [tiab] OR Cote d'Ivoir* [tiab] OR Djibout* [tiab] OR Egypt* [tiab] OR Salvador* [tiab] OR Georgia* [tiab] OR Ghana* [tiab] OR Guatemala* [tiab] OR Hondura* [tiab] OR India* [tiab] OR Indonesia* [tiab] OR Jordan* [tiab] OR Kenya* [tiab] OR Kiribati [tiab] OR Kosov* [tiab] OR Kyrgyz* [tiab] OR Laos [tiab] OR laotian [tiab] OR Lesoth* [tiab] OR Mauritania* [tiab] OR Micronesia* [tiab] OR Moldov* [tiab] OR Mongolia* [tiab] OR Morocc* [tiab] OR Myanmar* [tiab] OR burmese [tiab] OR Nicaragua* [tiab] OR Nigeria* [tiab] OR Pakistan* [tiab] OR Papua* [tiab] OR Philippin* [tiab] OR Sao Tome* [tiab] OR Solomon Island* [tiab] OR Sri Lank* [tiab] OR Sudan* [tiab] OR Swazi* [tiab] OR Syria* [tiab] OR Tajik* [tiab] OR Timor* [tiab] OR Tunisia* [tiab] OR Ukrain* [tiab] OR Uzbek* [tiab] OR Vanuatu* [tiab] OR Vietnam* [tiab] OR West Bank [tiab] OR Gaza [tiab] OR palestina* [tiab] OR Yemen* [tiab] OR Zambia* [tiab] |
| E. upper‐middle‐income economies | #8 | Search Albania* [tiab] OR Algeria* [tiab] OR Samoa* [tiab] OR Argentina* [tiab] OR Azerbaijan* [tiab] OR Belarus* [tiab] OR Belize' [tiab] OR Bosnia* [tiab] OR Herzegovina* [tiab] OR Botswana* [tiab] OR Brazil* [tiab] OR Bulgaria* [tiab] OR chinese [tiab] OR China [tiab] OR Colombia* [tiab] OR Costa Rica* [tiab] OR Croatia* [tiab] OR Cuba* [tiab] OR Dominica* [tiab] OR Guinea* [tiab] OR Ecuador* [tiab] OR Fiji* [tiab] OR Gabon* [tiab] OR Grenada* [tiab] OR Guyana* [tiab] OR Iran* [tiab] OR Iraq* [tiab] OR Jamaica* [tiab] OR Kazakh* [tiab] OR Lebanon [tiab] OR lebanese [tiab] OR Libya* [tiab] OR Macedonia* [tiab] OR Malaysia* [tiab] OR Maldiv* [tiab] OR Marshall Island* [tiab] OR Mauriti* [tiab] OR Mexic* [tiab] OR Montenegr* [tiab] OR Namibia* [tiab] OR Nauru* [tiab] OR Panama* [tiab] OR Paraguay* [tiab] OR Peru* [tiab] OR Romania* [tiab] OR Russia* [tiab] OR Samoa* [tiab] OR Serbia* [tiab] OR South Africa* [tiab] OR Lucia* [tiab] OR St Vincent [tiab] OR Grenadin* [tiab] OR Suriname* [tiab] OR Thailand [tiab] OR thai [tiab] OR Tonga* [tiab] OR Turkey [tiab] OR turkish [tiab] OR Turkmen* [tiab] OR Tuvalu* [tiab] OR Venezuela* [tiab] |
| F. all LMIC terms | #9 | Search (#4 OR #5 OR #6 OR #7 OR #8) |
| #10 | Search (#3 AND #9) | |
| I. children | #11 | Search "Infant"[Mesh] OR "Child, Preschool"[Mesh] |
| #12 | Search child* [tiab] OR infant* [tiab] OR baby [tiab] OR toddler* [tiab] OR babies [tiab] OR preschool* [tiab] OR newborn* [tiab] OR neonat* [tiab] | |
| #13 | Search (#11 OR #12) | |
| #14 | Search (#10 AND #13) | |
| nutrition interventions | #16 | Search nutrition intervention* [tiab] OR nutrition program* [tiab] OR nutrition counsel* [tiab] OR nutrition advice* [tiab] OR nutrition educat* [tiab] OR nutritional intervention* [tiab] OR nutritional program* [tiab] OR nutritional counsel* [tiab] OR nutritional advice* [tiab] OR nutritional educat* [tiab] OR diet intervention* [tiab] OR diet program* [tiab] OR diet counsel* [tiab] OR diet advice* [tiab] OR diet educat* [tiab] OR dietary intervention* [tiab] OR dietary program* [tiab] OR dietary counsel* [tiab] OR dietary advice* [tiab] OR dietary educat* [tiab] OR food intervention* [tiab] OR food program* [tiab] OR food counsel* [tiab] OR food advice* [tiab] OR food educat* [tiab] OR feeding intervention* [tiab] OR feeding program* [tiab] OR feeding counsel* [tiab] OR feeding advice* [tiab] OR feeding educat* [tiab] |
| #15 | Search "Dietary Supplements"[Mesh] OR "Infant Food"[Mesh] OR "Child Nutritional Physiological Phenomena"[Mesh] OR "Maternal Nutritional Physiological Phenomena"[Mesh] OR "Feeding Behavior"[Mesh:NoExp] OR "Food, Fortified"[Mesh] | |
| #17 | Search supplements[tiab] OR supplementation[tiab] | |
| #18 | Search (#16 OR #15 OR #17) | |
| #19 | Search (#14 AND #18) | |
| J. Study designs: RCT, CCT, IST, CBA, historical controlled studies | #20 | Search "Controlled Clinical Trial" [Publication Type] OR "Randomized Controlled Trial" [Publication Type] OR "Comparative Study" [Publication Type] OR "Interrupted Time Series Analysis"[Mesh] OR "Controlled Before‐After Studies"[Mesh] OR "Historically Controlled Study"[Mesh] |
| #21 | Search random*[tiab] OR (controlled[tiab] AND (trial[tiab] OR study[tiab] OR cohort[tiab] OR longitudinal[tiab])) OR cross‐sectional[tiab] OR interrupted time series[tiab] OR "before and after study" [tiab] OR before‐after study [tiab] OR cross‐sequential[tiab] OR control group*[tiab] OR matched control*[tiab] OR matched cohort*[tiab] | |
| #22 | Search (#20 OR #21) | |
| #23 | Search (#19 AND #22) |
Appendix 2. CENTRAL search strategy
| Topic | Search | Query |
| A. Urban Slum | #1 | [mh "Poverty Areas"] |
| #2 | [mh ^"Socioeconomic Factors"] | |
| #3 | [mh Poverty] | |
| #4 | urban*:ti,ab,kw or slum*:ti,ab,kw or shant*:ti,ab,kw or ghetto*:ti,ab,kw or shack*:ti,ab,kw or bidonville*:ti,ab,kw or squat*:ti,ab,kw or "informal settlement":ti,ab,kw or "informal urban settlement":ti,ab,kw or barrada:ti,ab,kw or "barrio baja":ti,ab,kw or "barrio pobre":ti,ab,kw or taudi*:ti,ab,kw or "irregular settlement":ti,ab,kw or "informal housing":ti,ab,kw or favela*:ti,ab,kw or "irregular settlement":ti,ab,kw or basti*:ti,ab,kw | |
| #5 | {or #1‐#4} | |
| B. LMIC | #6 | [mh "Developing Countries"] |
| #7 | developing countr*:ti,ab,kw or "middle income countr*":ti,ab,kw or "low income countr*":ti,ab,kw or LMIC:ti,ab,kw | |
| #8 | afghan*:ti,ab,kw or Benin*:ti,ab,kw or Burkin*:ti,ab,kw or Burundi*:ti,ab,kw or "Central African Republic*":ti,ab,kw or Chad:ti,ab,kw or chadian:ti,ab,kw or Comoros:ti,ab,kw or Comorian:ti,ab,kw or Congo*:ti,ab,kw or Eritrea*:ti,ab,kw or Ethiopia*:ti,ab,kw or Gambia*:ti,ab,kw or Guinea*:ti,ab,kw or Haiti*:ti,ab,kw or "North Korea*":ti,ab,kw or "Democratic People's Republic of Korea":ti,ab,kw or Liberia*:ti,ab,kw or Madagascar:ti,ab,kw or Malagasy*:ti,ab,kw or Malawi*:ti,ab,kw or Mali*:ti,ab,kw or Mozambiqu*:ti,ab,kw or Nepal*:ti,ab,kw or Niger*:ti,ab,kw or Rwanda*:ti,ab,kw or Senegal*:ti,ab,kw or "Sierra Leon*":ti,ab,kw or Somalia*:ti,ab,kw or "South Sudan*":ti,ab,kw or Tanzania*:ti,ab,kw or Togo*:ti,ab,kw or Uganda*:ti,ab,kw or Zimbabw*:ti,ab,kw | |
| #9 | angola*:ti,ab,kw or Armenia*:ti,ab,kw or Bangladesh*:ti,ab,kw or Bhutan*:ti,ab,kw or Bolivia*:ti,ab,kw or "Cabo Verd*":ti,ab,kw or Cambodia*:ti,ab,kw or Cameroon*:ti,ab,kw or Congo*:ti,ab,kw or "Cote d'Ivoir*":ti,ab,kw or Djibout*:ti,ab,kw or Egypt*:ti,ab,kw or Salvador*:ti,ab,kw or Georgia*:ti,ab,kw or Ghana*:ti,ab,kw or Guatemala*:ti,ab,kw or Hondura*:ti,ab,kw or India*:ti,ab,kw or Indonesia*:ti,ab,kw or Jordan*:ti,ab,kw or Kenya*:ti,ab,kw or Kiribati:ti,ab,kw or Kosov*:ti,ab,kw or Kyrgyz*:ti,ab,kw or Laos:ti,ab,kw or laotian:ti,ab,kw or Lesoth*:ti,ab,kw or Mauritania*:ti,ab,kw or Micronesia*:ti,ab,kw or Moldov*:ti,ab,kw or Mongolia*:ti,ab,kw or Morocc*:ti,ab,kw or Myanmar*:ti,ab,kw or burmese:ti,ab,kw or Nicaragua*:ti,ab,kw or Nigeria*:ti,ab,kw or Pakistan*:ti,ab,kw or Papua* :ti,ab,kw or Philippin*:ti,ab,kw or "Sao Tome*":ti,ab,kw or "Solomon Island*":ti,ab,kw or "Sri Lank*":ti,ab,kw or Sudan*:ti,ab,kw or Swazi*:ti,ab,kw or Syria*:ti,ab,kw or Tajik*:ti,ab,kw or Timor*:ti,ab,kw or Tunisia*:ti,ab,kw or Ukrain*:ti,ab,kw or Uzbek*:ti,ab,kw or Vanuatu*:ti,ab,kw or Vietnam*:ti,ab,kw or "West Bank":ti,ab,kw or Gaza:ti,ab,kw or palestina*:ti,ab,kw or Yemen*:ti,ab,kw or Zambia*:ti,ab,kw | |
| #10 | Albania*:ti,ab,kw or Algeria*:ti,ab,kw or Samoa*:ti,ab,kw or Argentina*:ti,ab,kw or Azerbaijan*:ti,ab,kw or Belarus*:ti,ab,kw or Belize:ti,ab,kw or Bosnia*:ti,ab,kw or Herzegovina*:ti,ab,kw or Botswana*:ti,ab,kw or Brazil*:ti,ab,kw or Bulgaria*:ti,ab,kw or chinese:ti,ab,kw or China:ti,ab,kw or Colombia*:ti,ab,kw or Costa Rica*:ti,ab,kw or Croatia*:ti,ab,kw or Cuba*:ti,ab,kw or Dominica*:ti,ab,kw or Guinea*:ti,ab,kw or Ecuador*:ti,ab,kw or Fiji*:ti,ab,kw or Gabon*:ti,ab,kw or Grenada*:ti,ab,kw or Guyana*:ti,ab,kw or Iran*:ti,ab,kw or Iraq*:ti,ab,kw or Jamaica*:ti,ab,kw or Kazakh*:ti,ab,kw or Lebanon:ti,ab,kw or lebanese:ti,ab,kw or Libya*:ti,ab,kw or Macedonia*:ti,ab,kw or Malaysia*:ti,ab,kw or Maldiv*:ti,ab,kw or "Marshall Island*":ti,ab,kw or Mauriti*:ti,ab,kw or Mexic*:ti,ab,kw or Montenegr*:ti,ab,kw or Namibia*:ti,ab,kw or Nauru*:ti,ab,kw or Panama*:ti,ab,kw or Paraguay*:ti,ab,kw or Peru*:ti,ab,kw or Romania*:ti,ab,kw or Russia*:ti,ab,kw or Samoa*:ti,ab,kw or Serbia*:ti,ab,kw or "South Africa*":ti,ab,kw or Lucia*:ti,ab,kw or "St Vincent":ti,ab,kw or Grenadin*:ti,ab,kw or Suriname*:ti,ab,kw or Thailand:ti,ab,kw or thai:ti,ab,kw or Tonga*:ti,ab,kw or Turkey:ti,ab,kw or turkish:ti,ab,kw or Turkmen*:ti,ab,kw or Tuvalu*:ti,ab,kw or Venezuela*:ti,ab,kw | |
| #11 | {or #6‐#10} | |
| A+B | #12 | #5 and #11 |
| C. Children | #13 | [mh Infant] or [mh "Child, Preschool"] |
| #14 | child*:ti,ab,kw or infant*:ti,ab,kw or baby:ti,ab,kw or toddler*:ti,ab,kw or babies:ti,ab,kw or preschool*:ti,ab,kw or newborn*:ti,ab,kw or neonat*:ti,ab,kw | |
| #15 | #13 or #14 | |
| A+B+C | #16 | #12 and #15 |
| D. Nutrition | #17 | (nutrition* or diet* or feeding or food) near/3 (intervention* or program* or advice or counsel* or educat*):ti,ab,kw |
| #18 | [mh "Dietary Supplements"] or [mh "Infant Food"] or [mh "Child Nutritional Physiological Phenomena"] or [mh "Maternal Nutritional Physiological Phenomena"] or [mh ^"Feeding Behavior"] or [mh "Food, Fortified"] | |
| #19 | supplements:ti,ab,kw or supplementation:ti,ab,kw | |
| #20 | {or #17‐#19} | |
| A+B+C+D | #21 | #16 and #20 |
| Limited to Central | #22 | #21 in Trials |
Appendix 3. Web of Science search strategy
| Topic | Search | Query |
| A. Urban Slum | #1 | urban OR slum* OR shant* OR ghetto* OR shack* OR bidonville* OR bustee* OR bostee* OR bosti* OR squat* OR "informal settlement" OR "informal urban settlement" OR barrada OR "barrio baja" OR "barrio pobre" OR taudi* OR "irregular settlement" OR "informal housing" OR favela OR "irregular settlement" OR basti* |
| B. Children | #2 | child* OR infant OR baby OR toddler OR babies OR kid OR preschool OR "under 5 year" OR newborn OR neonat* OR girl OR boy OR bambinio OR enfant OR bébé OR "under five year" NOT adolescent |
| C. Nutrition | #3 | nutrition OR undernourish* OR malnutrition OR undernutrition OR wasting OR stunting OR stunted OR wasted OR kwashiorkor OR SAM OR GAM OR MAM OR "growth falter" OR "low birth weight" OR marasmus OR thin OR emaciated OR "nutritional status" OR nutriti* OR malnutrition OR "body mass index" OR BMI OR "short stature" OR "weight‐for‐age" OR "height‐for‐age" OR MUAC OR "mid upper arm circumference" OR anthropometry OR "skinfold thickness" OR starvation OR underweight OR malnourishment OR "dietary deficiency" OR hunger OR "food deprived" OR "dietary energy requirement" OR vitamin* OR micronutrient |
| D. Design | #4 | RCT OR "randomized controlled trial" OR "randomised |
| A+B+C+D | #5 | Refined by: COUNTRIES/TERRITORIES: ( ZAMBIA OR INDONESIA OR INDIA OR ETHIOPIA OR MADAGASCAR OR BRAZIL OR SENEGAL OR MALAWI OR HAITI OR CHILE OR BANGLADESH OR SOUTH AFRICA OR COSTA RICA OR GUINEA BISSAU OR COLOMBIA OR PAKISTAN OR GUATEMALA OR CAMEROON OR IRAN OR ARGENTINA OR BENIN OR KENYA OR TANZANIA OR ZIMBABWE OR ECUADOR OR YEMEN OR UGANDA OR URUGUAY OR MALI OR NIGERIA OR RWANDA OR MEXICO OR LAOS OR VIETNAM OR NEPAL OR GUINEA OR TURKEY OR GHANA OR EGYPT OR GAMBIA OR CONGO OR CAMBODIA OR ZAIRE OR VENEZUELA OR BURKINA FASO OR PERU OR TUNISIA OR SYRIA OR BOLIVIA OR THAILAND OR SUDAN OR SRI LANKA OR ARMENIA OR MALAYSIA OR AFGHANISTAN ) AND [excluding] RESEARCH AREAS: ( UROLOGY NEPHROLOGY OR OPHTHALMOLOGY OR CARDIOVASCULAR SYSTEM CARDIOLOGY OR PHARMACOLOGY PHARMACY OR ENDOCRINOLOGY METABOLISM OR NURSING OR SPORT SCIENCES OR REHABILITATION OR IMMUNOLOGY OR PSYCHOLOGY OR ENGINEERING OR DENTISTRY ORAL SURGERY MEDICINE OR ALLERGY OR VIROLOGY ) AND LANGUAGES: ( ENGLISH ) |
Appendix 4. Ovid MEDLINE search strategy
| Topic | Search | Query |
| A. Urban Slum | #1 | urban OR slum* OR shant* OR ghetto* OR shack* OR bidonville* OR bustee* OR bostee* OR bosti* OR squate OR (informal adj settlement) OR (informal adj urban adj settlement) OR barrada OR (barrio adj baja) OR (barrio adj pobre) OR taudi* OR (irregular adj settlement) OR (informal adj housing) OR favela OR (irregular adj settlement) OR basti*).ti,ab. |
| B. Children | #2 | child* OR infant OR baby OR toddler OR babies OR kid OR preschool OR newborn OR neonat* OR girl OR boy OR bambinio OR enfant OR bebe OR (five adj year)).ti,ab. |
| C. Nutrition | #3 | nutrition OR undernourish* OR malnutrition OR undernutrition OR wasting OR stunting OR stunted OR wasted OR kwashiorkor OR SAM OR GAM OR MAM OR (growth adj falter) OR (low adj birth adj weight) OR marasmus OR thin OR emaciated OR (nutritional adj status) OR nutriti* OR malnutrition OR (body adj mass adj index) OR BMI OR (short adj stature) OR (weight adj age) OR (height adj age) OR MUAC OR (mid adj upper adj arm adj circumference) OR anthropometry OR (skinfold adj thickness) OR starvation OR underweight OR malnourishment OR (dietary adj deficiency) OR hunger OR (food adj deprived) OR (dietary adj energy adj requirement) OR vitamin* OR micronutrient).ti,ab. |
| D. Design | #4 | ((RCT OR (randomized adj controlled adj trial) OR (randomised adj controlled adj trial) OR (randomized adj control adj trial) OR (randomised adj control adj trial) OR (quasi adj randomised) OR (quasi adj randomized) OR (non adj randomised adj controlled adj trial) OR (non adj randomized adj controlled adj trial) OR (non adj randomised adj control adj trial) OR (non adj randomized adj control adj trial) OR (historically adj controlled adj study) OR (interrupted adj time adj series) OR (systematic adj review) OR (cohort adj study) OR (cross adj sectional adj study) OR (longitudinal adj study) OR (cross adj sequential adj study) OR (meta adj analysis) OR (literature adj review)).ti,ab. |
| A+B+C+D | #5 |
Appendix 5. Biosis Citation Index
| Topic | Search | Query |
| A. Urban Slum | #1 | urban OR slum* OR shant* OR ghetto* OR shack* OR bidonville* OR bustee* OR bostee* OR bosti* OR squat* OR "informal settlement" OR "informal urban settlement" OR barrada OR "barrio baja" OR "barrio pobre" OR taudi* OR "irregular settlement" OR "informal housing" OR favela OR "irregular settlement" OR basti* |
| B. Children | #2 | child* OR infant OR baby OR toddler OR babies OR kid OR preschool OR "under 5 year" OR newborn OR neonat* OR girl OR boy OR bambinio OR enfant OR bébé OR "under five year" NOT adolescent |
| C. Nutrition | #3 | nutrition OR undernourish* OR malnutrition OR undernutrition OR wasting OR stunting OR stunted OR wasted OR kwashiorkor OR SAM OR GAM OR MAM OR "growth falter" OR "low birth weight" OR marasmus OR thin OR emaciated OR "nutritional status" OR nutriti* OR malnutrition OR "body mass index" OR BMI OR "short stature" OR "weight‐for‐age" OR "height‐for‐age" OR MUAC OR "mid upper arm circumference" OR anthropometry OR "skinfold thickness" OR starvation OR underweight OR malnourishment OR "dietary deficiency" OR hunger OR "food deprived" OR "dietary energy requirement" OR vitamin* OR micronutrient |
| D. Design | #4 | RCT OR "randomized controlled trial" OR "randomised controlled trial" OR "randomized control trial" OR "randomised control trial" OR "quasi randomised" OR "quasi randomized" OR "non randomised controlled trial" OR "non randomized controlled trial" OR "non randomised control trial" OR "non randomized control trial" OR "historically controlled study" OR "interrupted time series" OR "before and after study" OR "systematic review" OR "cohort study" OR "cross‐sectional study" OR "longitudinal study" OR "cross‐sequential study" OR "meta analysis" OR "literature review" |
| A+B+C+D | #5 |
Appendix 6. IBECS search strategy
| Topic | Search | Query |
| A. Urban Slum | #1 | urban OR slum$ OR shant$ OR ghetto$ OR shack$ OR bidonville$ OR bustee$ OR bostee$ OR bosti$ OR squat$ OR informal settlement OR informal urban settlement OR barrada OR barrio baja OR barrio pobre OR taudi$ OR irregular settlement OR informal housing OR favela OR irregular settlement OR basti$ |
| B. Children | #2 | child$ OR infant OR baby OR toddler OR babies OR kid OR preschool OR under 5 year OR newborn OR neonat$ OR girl OR boy OR bambinio OR enfant OR bébé OR under five year AND NOT adolescent |
| C. Nutrition | #3 | nutrition OR undernourish$ OR malnutrition OR undernutrition OR wasting OR stunting OR stunted OR wasted OR kwashiorkor OR SAM OR GAM OR MAM OR growth falter OR low birth weight OR marasmus OR thin OR emaciated OR nutritional status OR nutriti$ OR malnutrition OR body mass index OR BMI OR short stature OR weight‐for‐age OR height‐for‐age OR MUAC OR mid upper arm circumference OR anthropometry OR skinfold thickness OR starvation OR underweight OR malnourishment OR dietary deficiency OR hunger OR food deprived OR dietary energy requirement OR vitamin$ OR micronutrient |
| A+B+C | #4 |
Appendix 7. CINAHL search strategy
| Topic | Search | Query |
| A. Urban Slum (all fields and title) | #1 | urban OR slum* OR shant* OR ghetto* OR shack* OR bidonville* OR bustee* OR bostee* OR bosti* OR squat* OR "informal settlement" OR "informal urban settlement" OR barrada OR "barrio baja" OR "barrio pobre" OR taudi* OR "irregular settlement" OR "informal housing" OR favela OR "irregular settlement" OR basti* |
| B. Location (all fields and title) | #2 | "developing countries" OR "poverty areas" OR Africa OR "South America" OR "Asia" |
| C. Nutrition (all fields and title) | #3 | nutrition OR undernourish* OR malnutrition OR undernutrition OR wasting OR stunting OR stunted OR wasted OR kwashiorkor OR SAM OR GAM OR MAM OR "growth falter" OR "low birth weight" OR marasmus OR thin OR emaciated OR "nutritional status" OR nutriti* OR malnutrition OR "body mass index" OR BMI OR "short stature" OR "weight‐for‐age" OR "height‐for‐age" OR MUAC OR "mid upper arm circumference" OR anthropometry OR "skinfold thickness" OR starvation OR underweight OR malnourishment OR "dietary deficiency" OR hunger OR "food deprived" OR "dietary energy requirement" OR vitamin* OR micronutrient |
| D. Design | #4 | RCT OR "randomized controlled trial" OR "randomised controlled trial" OR "randomized control trial" OR "randomised control trial" OR "quasi randomised" OR "quasi randomized" OR "non randomised controlled trial" OR "non randomized controlled trial" OR "non randomised control trial" OR "non randomized control trial" OR "historically controlled study" OR "interrupted time series" OR "before and after study" OR "systematic review" OR "cohort study" OR "cross‐sectional study" OR "longitudinal study" OR "cross‐sequential study" OR "meta analysis" OR "literature review" |
Appendix 8. POPLINE search strategy
| Topic | ID | Search |
| A. Urban Slum (all fields and title) | #1 | Urban in all fields and title urban OR slum* OR shant* OR ghetto* OR shack* OR bidonville* OR bustee* OR bostee* OR bosti* OR squat* OR "informal settlement" OR "informal urban settlement" OR barrada OR "barrio baja" OR "barrio pobre" OR taudi* OR "irregular settlement" OR "informal housing" OR favela OR "irregular settlement" OR basti* |
| B. Children (all fields) | #2 | Child child* OR infant OR baby OR toddler OR babies OR kid OR preschool OR "under 5 year" OR newborn OR neonat* OR girl OR boy OR bambinio OR enfant OR bébé OR "under five year" NOT adolescent |
| C. Nutrition (all fields and title) | #3 | nutrition OR undernourish* OR malnutrition OR undernutrition OR wasting OR stunting OR stunted OR wasted OR kwashiorkor OR SAM OR GAM OR MAM OR "growth falter" OR "low birth weight" OR marasmus OR thin OR emaciated OR "nutritional status" OR nutriti* OR malnutrition OR "body mass index" OR BMI OR "short stature" OR "weight‐for‐age" OR "height‐for‐age" OR MUAC OR "mid upper arm circumference" OR anthropometry OR "skinfold thickness" OR starvation OR underweight OR malnourishment OR "dietary deficiency" OR hunger OR "food deprived" OR "dietary energy requirement" OR vitamin* OR micronutrient |
| D. Design (all fields) | #4 | RCT OR "randomized controlled trial" OR "randomised controlled trial" OR "randomized control trial" OR "randomised control trial" OR "quasi randomised" OR "quasi randomized" OR "non randomised controlled trial" OR "non randomized controlled trial" OR "non randomised control trial" OR "non randomized control trial" OR "historically controlled study" OR "interrupted time series" OR "before and after study" OR "systematic review" OR "cohort study" OR "cross‐sectional study" OR "longitudinal study" OR "cross‐sequential study" OR "meta analysis" OR "literature review" |
| E. Region | #5 | |
| A+B+C+D+E | #6 |
Appendix 9. BIBLIOMAP search strategy
| Topic | Search | Query |
| A. Urban Slum | #1 | urban OR slum* OR shant* OR ghetto* OR shack* OR bidonville* OR bustee* OR bostee* OR bosti* OR squat* OR "informal settlement" OR "informal urban settlement" OR barrada OR "barrio baja" OR "barrio pobre" OR taudi* OR "irregular settlement" OR "informal housing" OR favela OR "irregular settlement" OR basti* |
| B. Children | #2 | child* OR infant OR baby OR toddler OR babies OR kid OR preschool OR "under 5 year" OR newborn OR neonat* OR girl OR boy OR bambinio OR enfant OR bébé OR "under five year" |
| C. Nutrition | #3 | nutrition OR undernourish* OR malnutrition OR undernutrition OR wasting OR stunting OR stunted OR wasted OR kwashiorkor OR SAM OR GAM OR MAM OR "growth falter" OR "low birth weight" OR marasmus OR thin OR emaciated OR "nutritional status" OR nutriti* OR malnutrition OR "body mass index" OR BMI OR "short stature" OR "weight‐for‐age" OR "height‐for‐age" OR MUAC OR "mid upper arm circumference" OR anthropometry OR "skinfold thickness" OR starvation OR underweight OR malnourishment OR "dietary deficiency" OR hunger OR "food deprived" OR "dietary energy requirement" OR vitamin* OR micronutrient |
| D. Region | #4 | RCT OR "randomized controlled trial" OR "randomised controlled trial" OR "randomized control trial" OR "randomised control trial" OR "quasi randomised" OR "quasi randomized" OR "non randomised controlled trial" OR "non randomized controlled trial" OR "non randomised control trial" OR "non randomized control trial" OR "historically controlled study" OR "interrupted time series" OR "before and after study" OR "systematic review" OR "cohort study" OR "cross‐sectional study" OR "longitudinal study" OR "cross‐sequential study" OR "meta analysis" OR "literature review" |
| A+B+C+D | #5 |
Appendix 10. UNSCN search strategy
| Topic | Search | Query |
| A. Urban Slum | #1 | urban OR slum OR shanty OR ghetto OR shack OR bidonville OR bustee OR bostee OR bosti OR squat OR informal settlement OR informal urban settlement OR barrada OR barrio baja OR barrio pobre OR taudi OR irregular settlement OR informal housing OR favela OR irregular settlement OR basti |
| B. Children | #2 | child OR infant OR baby OR toddler |
| C. Nutrition | #3 | nutrition OR undernourished OR malnutrition OR undernutrition OR wasting OR stunting OR stunted OR wasted OR kwashiorkor OR SAM OR GAM OR MAM OR growth falter OR low birth weight OR marasmus OR thin OR emaciated OR nutritional status OR nutrition OR malnutrition OR body mass index OR BMI OR short stature OR weight‐for‐age OR height‐for‐age OR MUAC OR mid upper arm circumference OR anthropometry OR skinfold thickness OR starvation OR underweight OR malnourishment OR dietary deficiency OR hunger OR food deprived OR dietary energy requirement OR vitamin OR micronutrient |
| A+B+C | #4 |
Appendix 11. African Index Medicus
| Topic | Search | Query |
| A. Urban Slum | #1 | urban OR slum OR shanty OR ghetto OR shack OR bidonville OR bustee OR bostee OR bosti OR squat OR informal settlement OR informal urban settlement OR barrada OR barrio baja OR barrio pobre OR taudi OR irregular settlement OR informal housing OR favela OR irregular settlement OR basti |
| B. Children | #2 | child OR infant OR baby OR toddler |
| C. Nutrition | #3 | nutrition OR undernourished OR malnutrition OR undernutrition OR wasting OR stunting OR stunted OR wasted OR kwashiorkor OR SAM OR GAM OR MAM OR growth falter OR low birth weight OR marasmus OR thin OR emaciated OR nutritional status OR nutrition OR malnutrition OR body mass index OR BMI OR short stature OR weight‐for‐age OR height‐for‐age OR MUAC OR mid upper arm circumference OR anthropometry OR skinfold thickness OR starvation OR underweight OR malnourishment OR dietary deficiency OR hunger OR food deprived OR dietary energy requirement OR vitamin OR micronutrient |
| A+B+C | #4 |
Appendix 12. WHOLIS search strategy
| Topic | Search | Query |
| A. Urban Slum | #1 | urban OR slum OR shanty OR ghetto OR shack OR bidonville OR bustee OR bostee OR bosti OR squat OR informal settlement OR informal urban settlement OR barrada OR barrio baja OR barrio pobre OR taudi OR irregular settlement OR informal housing OR favela |
| B. Nutrition | #2 | nutrition OR undernourished OR malnutrition OR undernutrition OR wasting OR stunting |
| A+B | #3 |
Appendix 13. Data extraction prestandardised form
Pre‐standardised form –nutrition‐specific and sensitive interventions for preventing stunting in children (0 to 5 years) living in urban slums
| Study ID: | Report ID: | Date form completed: |
| First author: | Year of study: | Data extractor: |
| Citation: | ||
1. General Information
| Publication type Journal Article c Abstract c Other (specify e.g. book chapter)___________________ | |
| Country of study: | |
| Funding source of study: | Potential conflict of interest from funding? Y / N / unclear |
2. Study Eligibility
| Study Characteristics | Page/ Para/ Figure # | |||
| Type of study | c Randomised Controlled Trial (RCT) c Cluster Randomised Controlled Trial (cluster RCT) |
c Controlled Before and After (CBA) study · Contemporaneous data collection · Comparable control site · At least 2 x intervention and 2 x control clusters |
||
| c Interrupted Time Series (ITS) · At least 3 time points before and 3 after the intervention · Clearly defined intervention point |
c Non‐randomised controlled trials | |||
| c Historically controlled studies | ||||
| c Quasi randomised |
Does the study design meet the criteria for inclusion? Yes c No c Exclude Unclear c |
|||
| Description in text: | ||||
| Participants | Describe the participants included: Children from low and middle income countries, from birth to five years old living in urban slums in low and middle income countries (LMIC). | |||
| Are participants defined as a group having specific social or cultural characteristics? | Yes c No c Unclear c Details: |
|||
| How is the geographic boundary defined? | Details: Specific location (e.g. state / country): |
|||
| Do the participants meet the criteria for inclusion? | Yes c No c Exclude Unclear c | |||
| Types of intervention | Strategies included in the intervention | ||||
| Focus of the intervention | |||||
| Does the intervention meet the criteria for inclusion? | Yes c No c Exclude Unclear c | ||||
| Duration of intervention | Start date: | Stop date: | Intervention duration: | ||
| Is the duration of intervention adequate for inclusion? | Yes c No c Exclude Unclear c | ||||
| Types of outcome measures | List outcomes: | ||||
| Outcome measured at a population level or individual level? | Details: | ||||
| Do the outcome measures meet the criteria for inclusion? | Yes c No c Exclude Unclear c | ||||
Summary of Assessment for Inclusion
| Include in review c Exclude from review c | |
| Independently assessed, and then compared? Yes c No c | Differences resolved Yes c No c |
| Request further details? Yes c No c | Contact details of authors: |
| Notes: | |
DO NOT PROCEED IF PAPER EXCLUDED FROM REVIEW
3. Study details
| Study intention | Descriptions as stated in the report/paper | Page/ Para/ Figure # |
| Aim of intervention | What was the problem that this intervention was designed to address? | |
| Aim of study | What was the study designed to assess? Are these clearly stated? | |
| Location of study |
Where was the study conducted? ‐ Urban slums: ‐ Peri urban slums: ‐ Country: ‐ City: ‐ Slum: |
|
| Equity pointer: Social context of the study | e.g. was study conducted in a particular setting that might target/exclude specific population s? See also Inclusion/exclusion criteria under Methods, below. | |
| Start and end date of the study | Identify which elements of planning of the intervention should be included | |
| Total study duration | ||
| Delivery | Specify if either community based / primary health care / secondary health care / direct | |
| Funding: | Funding source, budget, implementing partner; design, integration within existing government health | |
| Setting | whether delivered in humanitarian crisis / disaster or development; including origin of slum, defining characteristics, whether squatter settlement or legal but dilapidated, and whether conditions were improving or worsening |
| Methods | Descriptions as stated in the report/paper | Page/ Para/ Figure # |
| Method/s of recruitment of participants (How were potential participants approached and invited to participate? Where were participants recruited from? Does this differ from the intervention setting?) |
||
| Inclusion/exclusion criteria for participation in study | ||
| Representativeness of sample: Are participants in the study likely to be representative of the target population? | ||
| Total number of intervention groups | ||
| Assumed risk estimate (e.g. baseline or population risk noted in Background) |
References: | |
| Sample size calculation: What assumptions were made? Were these assumptions appropriate? |
(Yes/No/Unclear) | |
| What was the unit of randomisation? Allocation by individuals or cluster/groups |
||
| What was the unit of analysis? Is this the same as the unit of randomisation? |
(Yes/No/Unclear) | |
| Statistical methods used and appropriateness of these methods | (Check with your statistician if unsure about appropriateness) |
4. Participants
|
Participants Include if relevant |
Include information for each group (i.e. intervention and controls) under study | Page/ Para/ Figure # | ||
| · What percentage of selected individuals agreed to participate? | ||||
| · Total number randomised (or total pop. at start of study for NRCTs) | ||||
| · Number allocated to each intervention group (no. of individuals) | ||||
| · For cluster trials, number of clusters, number of people per cluster | ||||
| · Where there any significant baseline imbalances? | Yes c No c Unclear c Details: |
|||
| · Number and reason for (and sociodemographic differences of) withdrawals and exclusions for each intervention group | ||||
| · Were patients who entered the study adequately accounted for? | ||||
| · What percentage of patients completed the study? | ||||
| · What percentage of participants received the allocated intervention or exposure of interest? | ||||
| · Is the analysis performed by intervention allocation status (intention to treat) rather than the actual intervention received? Have any attempts been made to impute missing data? | ||||
| · Age (median, mean and range if possible) | ||||
| · Sex | ||||
| · Race/Ethnicity | ||||
| · Principal health problem (incl. stage of illness) | ||||
| · Diagnostic criteria | ||||
| · Co‐morbidity | ||||
| · Other socio‐demographics (e.g. also consider possible proxies for these e.g. low baseline nutritional status) | ||||
| · PROGRESS categories reported at baseline (indicate letters of those reported: Place of residence, race, occupation, gender, religion, education, SES, social capital) | ||||
| Subgroups | Enter a description of any participant subgroups from this paper to be analysed in the review. | |||
5. Intervention Group 1 (copy and paste table for each Intervention group)
| Group name: | (State brief name for this intervention group.) | Page/ Para/ Figure # |
| Details of intervention or control condition (Include if relevant in sufficient detail for replication) | ||
| · Intervention component (supplementation, fortification,…) | ||
| · Theoretical basis (include key references) | ||
| · Content (list the strategies intended and delivered) | ||
| · Did the intervention include strategies to address diversity/disadvantage? | Enter a description of any relevant strategies | |
| · Delivery (e.g. Stages (sequential or simultaneous), timing, frequency, duration, intensity, fidelity – process indicators) | ||
| · Providers (who, number, education/training in intervention delivery, ethnicity etc. if potentially relevant to acceptance and uptake by participants | ||
| · Co‐interventions | ||
| Duration of intervention | ||
| Duration of follow‐up | ||
| Was sustainability discussed by the authors? Was is a consideration in study development? | ||
| Economic variables i.e. costs of the intervention, and changes in other (e.g. health care) costs as result of interventionª | Yes c List in Outcome section if appropriate No c Unclear c Details: |
|
| Other economic information (from a societal, non‐healthcare view – e.g. lost wages, time) | Yes c No c Details: |
|
| Resource requirements to replicate intervention (e.g. staff numbers, hours of implementation, equipment?) | ||
| Subgroups | Enter a description of any intervention subgroups from this report to be analysed in the review. | |
| What are the moderators/mediators of changes stated in the study? | ||
| Do the authors describe any political or organisational context? | List relevant dot points | |
| Were any partnerships referred to? | List these as dot points | |
| Was a process evaluation conducted? | What components were included in the process evaluation? (e.g. dose, frequency, consistency, implemented as intended etc) | |
| Control/comparison (what information is provided about what the control or comparison group received?) | Enter a description of what was provided for the control group, if applicable | |
6. Outcomes
(This table is set up for 2 outcome measure to save spaces, copy and paste table as often as required)
| Question | Outcome 1 | Page/ Para/ Figure # | Outcome 2 | Page/ Para/ Figure # |
| Is there an analytic framework applied (e.g. logic model, conceptual framework)? | ||||
| Outcome definition (with diagnostic criteria if relevant) | ||||
| Type of outcome: Is this a modifiable variable (Community level, neighbourhood level, individual level) or desired health outcome | ||||
| Time points measured | ||||
| Time points reported | ||||
| Is there adequate latency for the outcome to be observed? | ||||
| Is the measure repeated on the same individuals or redrawn from the population / community for each time point? | ||||
| Unit of measurement (if relevant) | ||||
| For scales – upper and lower limits and indicate whether high or low score is good | ||||
| How is the measure applied? Telephone survey, mail survey, in person by trained assessor, routinely collected data, other | ||||
| How is the outcome reported? Self or study assessor | ||||
| Is this outcome/tool validated? | ||||
| …And has it been used as validated? | ||||
| Is it a reliable outcome measure? | ||||
| Is there adequate power for this outcome? | ||||
| Were PROGRESS categories analysed by outcome? Indicate the letters of those that outcomes were analysed by (place of residence, race, occupation, gender, religion, education, SES, social capital) |
7. Results
Copy and paste the appropriate table for each outcome and subgroup at each time point, including baseline
For RCT/CCT
Dichotomous outcome page/para/fig
| Comparison | |||||
| Outcome | |||||
| Subgroup | |||||
| Time point | |||||
| Results | Intervention | Comparison | |||
| Events | No. participants | Events | No. participants | ||
| No. of missing participants and reasons | |||||
| Any other results reported | |||||
| Reanalysis required? (specify ‐ (e.g. correlation adjustment) |
|||||
| Reanalysis possible? | yes/no/unclear | ||||
| Reanalysed results | |||||
For RCT/CCT
Continuous outcome page/para/fig
| Comparison | |||||||
| Outcome | |||||||
| Subgroup | |||||||
| Time point | |||||||
| Post‐intervention or change from baseline? | |||||||
| Results | Intervention | Comparison | |||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | ||
| No. missing participants and reasons | |||||||
| Any other results reported | |||||||
| Reanalysis required? (specify) | |||||||
| Reanalysis possible? | yes/no/unclear | ||||||
| Reanalysed results | |||||||
For RCT/CCT
Generic inverse variance method
Page/para/figure
| Comparison | |||||
| Outcome | |||||
| Subgroup | |||||
| Time point | |||||
| Results | Effect estimate | SE (or other variance) | Intervention no. | Control no. | |
| No. missing participants and reasons | |||||
| Any other results reported | |||||
| Reanalysis required? (specify) | |||||
| Reanalysis possible? | yes/no/unclear | ||||
| Reanalysed results | |||||
For quasi RCT
Page/para/figure
| Comparison | |||||
| Outcome | |||||
| Subgroup | |||||
| Time point | |||||
| Results | Effect estimate | SE (or other variance) | Intervention no. | Control no. | |
| No. missing participants and reasons | |||||
| Any other results reported | |||||
| Reanalysis required? (specify) | |||||
| Reanalysis possible? | yes/no/unclear | ||||
| Reanalysed results | |||||
For non RCT
Page/para/figure
| Comparison | |||||
| Outcome | |||||
| Subgroup | |||||
| Time point | |||||
| Results | Effect estimate | SE (or other variance) | Intervention no. | Control no. | |
| No. missing participants and reasons | |||||
| Any other results reported | |||||
| Reanalysis required? (specify) | |||||
| Reanalysis possible? | yes/no/unclear | ||||
| Reanalysed results | |||||
For CBA
Page/para/fig
| Comparison | |||
| Assignment | How were control and treatment groups selected?? Is there likely to be an effect if these were the opposite way? | ||
| Contemporaneous data collection? | |||
| Outcome | |||
| Subgroup | |||
| Time point | |||
| Post‐intervention or change from baseline? | |||
| Intervention | Comparison | ||
| No. participants measured |
|||
| No. missing participants and reasons | |||
| Baseline result (with variance measure) | |||
| Post‐intervention results (with variance measure) | |||
| Change (Post – baseline) (with variance measure) | |||
| Difference in change (intervention – control) (with variance measure) | |||
| Any other results reported | |||
| Reanalysis required? (specify) | |||
| Reanalysis possible? | yes/no/unclear | ||
| Reanalysed results | |||
For ITS
Generic inverse variance method Page/para/fig
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Length of time points measured | ||||||
| Snapshot or interval measured | ||||||
| No. participants measured | ||||||
| No. missing participants and reasons | ||||||
| Pre‐intervention | Post‐intervention | |||||
| No. of time points measured | ||||||
| Mean value (with variance measure) | ||||||
| Difference in means (post – pre) | ||||||
| Percent relative change | ||||||
| Result reported by authors (with variance measure) | ||||||
| Reanalysis required? (specify) | ||||||
| Reanalysis possible? | yes/no/unclear | |||||
| Individual time point results | ||||||
| Read from figure? | yes/no | |||||
| Reanalysed results | Change in level | SE | Change in slope | SE | ||
For historically controlled studies
Page/para/fig
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Length of time points measured | ||||||
| Snapshot or interval measured | ||||||
| No. participants measured | ||||||
| No. missing participants and reasons | ||||||
| Pre‐intervention | Post‐intervention | |||||
| No. of time points measured | ||||||
| Mean value (with variance measure) | ||||||
| Difference in means (post – pre) | ||||||
| Percent relative change | ||||||
| Result reported by authors (with variance measure) | ||||||
| Reanalysis required? (specify) | ||||||
| Reanalysis possible? | yes/no/unclear | |||||
| Individual time point results | ||||||
| Read from figure? | yes/no | |||||
| Reanalysed results | Change in level | SE | Change in slope | SE | ||
Other relevant information
| Were outcomes relating to harms/unintended effects of the intervention described? Include any data for these in the outcomes tables above | |||
| Potential for author conflict i.e. evidence that author or data collectors would benefit if results favoured the intervention under study or the control | |||
| Key conclusions of the study authors | |||
| Could the inclusion of this study potentially bias the generalisability of the review? Equity pointer: Remember to consider whether disadvantaged populations may have been excluded from the study. | |||
| Is there potential for differences in relative effects between advantaged and disadvantaged populations? (e.g. are children from lower income families less likely to wear bicycle helmets) | |||
| Are interventions likely to be aimed at the disadvantaged? (e.g. school meals aimed at poor children). | |||
| Issues affecting directness (Note any aspects of population, intervention, etc. that affect this study's direct applicability to the review question) |
|||
| Recommendations | |||
| Limitations | |||
| References to other relevant studies | |||
| Additional notes by review authors | |||
| Correspondence required for further study information (from whom, what and when) | |||
8. Risk of bias assessment
| Domain | Review authors'judgement* | Description | Page/ Para/ Figure # |
| Was the allocation sequence adequately generated? | Yes / No / Unclear | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. | |
| Was allocation adequately concealed? | Yes / No / Unclear | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | |
| Were baseline outcome measurements similar? | Yes/No/Unclear | Note whether baseline outcome measurements were reported and whether there were any important differences between groups. If there were important differences between groups, note whether appropriate adjusted analysis was performed to account for this. | |
| Were baseline characteristics similar? | Yes/No/Unclear | Note whether baseline characteristics were reported and whether there were any important differences between groups. | |
|
Were incomplete outcome data adequately addressed? Assessments should be made for each main outcome (or class of outcomes). |
Yes / No / Unclear | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | |
|
Was knowledge of the allocated intervention adequately prevented during the study? Separate assessments should be made for relevant groups of people involved in the study i.e. participants, outcome assessors, investigators, data assessors etc |
Yes / No / Unclear |
Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective, or whether blinding was appropriate. · Participants – yes, no, unclear [record supporting statement from study]. · Investigators – yes, no, unclear [record supporting statement from study]. · Outcomes assessors – yes, no, unclear [record supporting statement from study]. Data assessors – yes, no, unclear [record supporting statement from study]. |
|
| Was the study adequately protected against contamination? | Yes/No/Unclear | State whether and how the possibility of contamination was minimised by the study design/implementation. | |
|
Are reports of the study free of suggestion of selective outcome reporting? Assessments should be made for each main outcome (or class of outcomes). |
Yes / No / Unclear | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | |
|
Other sources of bias · |
Yes / No / Unclear | State any important concerns about bias not addressed in the other domains in the tool. | |
| ITS: Was the intervention independent of other changes? | Yes/No/Unclear | Describe whether or not the intervention occurred independently of other changes over time and whether or not the outcomes may have been influenced by other confounding variables/historic events during the study period. | |
| ITS: Was the shape of the intervention effect pre‐specified? | Yes/No/Unclear | State whether or not the point of analysis was the point of intervention. If not, describe whether a rationale for the shape of the intervention effect was given by the study authors. | |
| ITS: Was the intervention unlikely to affect data collection? | Yes/No/Unclear | Describe whether or not the intervention was likely to affect data collection and what the potential impact might have been. | |
|
ITS: Was knowledge of the allocated interventions adequately prevented during the study? Separate assessments should be made for relevant groups of people involved in the study i.e. participants, outcome assessors, investigators, data assessors etc |
Yes/No/Unclear |
Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective, or whether blinding was appropriate. · Participants – yes, no, unclear [record supporting statement from study]. · Investigators – yes, no, unclear [record supporting statement from study]. · Outcomes assessors – yes, no, unclear [record supporting statement from study]. Data assessors – yes, no, unclear [record supporting statement from study]. |
|
|
ITS: Was incomplete outcome data adequately addressed? Assessments should be made for each main outcome (or class of outcomes). |
Yes/No/Unclear | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | |
| ITS: Was the study free from selective reporting? | Yes/No/Unclear | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | |
| ITS: Was the study free from other risks of bias? | Yes/No/Unclear | State any important concerns about bias not addressed in the other domains in the tool. |
* Note: For each section above 'Yes' indicates a 'low risk of bias'; 'No' indicates a 'high risk of bias'; 'Unclear' indicates an 'uncertain risk of bias'. When entering the data into RevMan, the options to choose from will be 'Low', 'High' and 'Unclear'
9. Results
Comparison:
Outcome:
Subcategory:
| Treatment group: | Control group: | ||
| Observed (n) | total (N) | observed (n) | total (N) |
| Treatment group: | Control group: | |
| Total randomised | ||
| excluded* | ||
| Observed | ||
| lost to follow up* |
*Reasons for loss/exclusion:
Subcategory:
| Treatment group: | Control group: | ||
| Observed (n) | total (N) | observed (n) | total (N) |
| Treatment group: | Control group: | |
| Total randomised | ||
| excluded* | ||
| Observed | ||
| lost to follow up* |
*Reasons for loss/exclusion
♠ Costs associated with the intervention can be linked with provider or participant outcomes in an economic evaluation (depends on the type of economic evaluation)
Appendix 14. Summary of findings – notes
Summary of findings table 1
aStarted GRADE at high certainty because evidence was based on a randomised controlled trial (RCT). The risk of bias registered mostly as low or unclear risk with an overall risk of bias assessed high for Iannotti 2008. The decision was made not to downgrade on the unclear evidence as the outcome being assessed was an objective measure rather than subjectively assessed and there was no other evidence of bias in the papers. There was only one study so it was not possible to assess inconsistency. No downgrading for consistency. For indirectness the evidence only existed for zinc supplementation and we set out to look at micronutrient supplementation. The geographical coverage of the study was limited to one country and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we only identified one study in Latin America. For this reason, the evidence was downgraded one level to moderate. For precision, the sample size was relatively small (237 participants). The confidence interval (CI) covered a difference in length from unclear effect to 0.44 cm difference, which was a very small difference at the top end of the CI. The small sample size accounted for a small amount of imprecision and led to a downgrading of one level to low. For publication bias, we found only one study, which showed unclear effect, which suggested studies without an effect were published and these would likely be more difficult to publish than studies showing an effect. We had no evidence to suggest that there was publication bias and, therefore, no downgrading was recommended. There was no evidence to upgrade the evidence. Therefore, the overall GRADE suggested was low.
bStarted GRADE at high certainty because evidence was based on RCTs. The overall risk of bias was moderate for Osendarp 2000 and high for Caulfield 1999. The decision was made not to downgrade on the unclear evidence as the outcome being assessed was an objective measure rather than subjectively assessed and there was no other evidence of bias in the papers. There were only two studies so it was difficult to assess inconsistency statistically. However, a narrative comparison showed very similar findings across the two studies. No downgrading for consistency. For indirectness, the evidence only existed for zinc supplementation and we set out to look at micronutrient supplementation. The geographical coverage of the studies was also limited to two countries and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we had only identified studies in Asia and Latin America and no studies from Africa. For this reason, the evidence was downgraded one level to moderate. For precision, the sample size was adequate (greater than 400) and the CI largely covered an area of no effect, with even the upper end of the CI only stretching into a small difference of 11.35 g in birth weight. No downgrading for precision. For publication bias, only two studies existed and neither had a small sample size. Both showed unclear effect, which suggested studies without an effect are being published and these would likely be more difficult to publish than studies showing an effect. We had no evidence to suggest that there was publication bias and, therefore, no downgrading is recommended. There was no evidence to upgrade the evidence. Therefore, the overall GRADE suggested was moderate.
cStarted GRADE at high certainty because evidence was based on RCTs. The overall risk of bias was moderate for Osendarp 2000 and high for Caulfield 1999. The decision was made not to downgrade on the unclear evidence as the outcome being assessed was an objective measure rather than subjectively assessed and there was no other evidence of bias in the papers. There were only two studies so it was difficult to assess inconsistency statistically. However, a narrative comparison showed very similar findings across the two studies. No downgrading for consistency. For indirectness, the evidence only existed for zinc supplementation and we set out to look at micronutrient supplementation. The geographical coverage of the studies was also limited to two countries and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we only identified studies in Asia and Latin America and no studies from Africa. For this reason, the evidence was downgraded one level to moderate. For precision, the sample size was adequate (greater than 400) and the CI largely covered an area of no effect, with even the upper end of the CI only stretching into a small difference of 0.11 cm (within measurement error of devices measuring height so very small) in birth length. No downgrading for precision. For publication bias only two studies existed and neither had a small sample size. Both showed unclear effect, which suggested studies without an effect were being published and these would likely be more difficult to publish than studies showing an effect. We had no evidence to suggest that there has publication bias and, therefore, no downgrading was recommended. There was no evidence to upgrade the evidence. Therefore, the overall GRADE suggested was moderate.
Summary of findings table 2
aStarted GRADE at high certainty because evidence was based on RCTs. The overall risk of bias was high for Oelofse 2003, and Iannotti 2014, and moderate for Taneja 2010. Given that the majority of the evidence came from high‐risk studies with some RCT evidence from a lower bias risk study, it was decided to downgrade for bias one grade to moderate. There were only three studies so it was difficult to assess inconsistency statistically, although the I² statistic of 30% suggested heterogeneity was not important. A narrative comparison showed very similar findings for Oelofse 2003 and Taneja 2010 (unclear effect), with Iannotti 2014 showing a small difference (0.13 z‐scores, standard error 0.05) between the control group and the group supplemented for six months (only after age was controlled for). This difference in the findings led to some uncertainty in the findings and resulted in a further one level decrease of the evidence to low. For indirectness, the evidence existed across regions and for different types of supplementation composition. There was no reason to downgrade for indirectness. For precision, the sample size was adequate (greater than 400) and the CI largely covered an area of unclear effect, with even the upper end of the CI only stretching into a small difference of 0.03 z‐scores in height‐for‐age. No downgrading for precision. For publication bias, only three studies existed and none had a small sample size. Two of the three studies showed unclear effect, which suggested studies without an effect were being published and these would likely be more difficult to publish than studies showing an effect. We had no evidence to suggest that there was publication bias and, therefore, no downgrading was recommended. There was no evidence to upgrade the evidence. Therefore, the overall GRADE suggested was low.
bStarted GRADE at high certainty because evidence was based on an RCT. The overall risk of bias was high for Rahman 2002. The decision was made to downgrade based on bias. There was only one study so it was not possible to assess inconsistency. No downgrading for consistency. For indirectness, the evidence only existed for one country (Bangladesh) and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we had only identified one study in Asia. We also only had information on the types of supplement used in this study, which did not cover all types of supplementation available. For these reasons, the evidence was downgraded one level to moderate. For precision, the sample size was reasonable (653 participants) and larger than required to achieve adequate power for the growth outcomes as other outcomes examined needed larger sample sizes. The CIs could be calculated from the information reported in the paper and they were not large suggesting that precision was not affected by the sample size of the study. Therefore, we decided not to downgrade the score for precision. For publication bias, we found only one study, which showed unclear effect (suggesting no effect studies were published) and we had no evidence to suggest that there was publication bias. Therefore, no downgrading was recommended for publication bias. There was no evidence to upgrade the evidence. Therefore, the overall GRADE suggested was low.
cStarted GRADE at high certainty because evidence was based on an RCT. The overall risk of bias was moderate for Radhakrishna 2013. The decision was, therefore, made not to downgrade based on bias. There was only one study so it was not possible to assess inconsistency. No downgrading for consistency. For indirectness, the evidence only existed for one country (India) and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we only identified one study in Asia. We also only had information on the types of supplement used in this study, which did not cover all types of supplementation available. For these reasons, the evidence was downgraded one level to moderate. For precision, the sample size was reasonable (324 participants) and adequate to achieve power for the outcome under consideration. The CIs were calculated in the paper and were small (within 0.4 z‐scores) suggesting that precision was not affected by the sample size of the study. Therefore, we decided not to downgrade the score for precision. For publication bias, we found only one study, which showed unclear effect (suggesting unclear effect studies were published) and we had no evidence to suggest that there was publication bias. Therefore, no downgrading was recommended for publication bias. There was no evidence to upgrade the evidence. Therefore, the overall GRADE suggested was moderate.
dStarted GRADE at high certainty because evidence was based on an RCT. The overall risk of bias was high for Begin 2008. The study author reported that consumption of the bovine supplement was 12% lower than other supplements and this was likely to have affected results. The decision was made to downgrade bias one level to moderate based on this information. There was only one study so it was not possible to assess inconsistency. No downgrading for consistency. For indirectness, the evidence only existed for one country (Guatemala) and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we only identified one study in Latin America. We also only had information on the types of supplement used in this study, which did not cover all types of supplementation available. For these reasons, the evidence was downgraded one level to low. For precision, the sample size was relatively small (315 participants). The CIs for the velocity estimates were small, which suggested the small sample size was likely not a significant factor in affecting the estimates. Therefore, no downgrading of the evidence for precision. For publication bias, we found only one study, which showed unclear effect, which suggested studies without an effect were published and these would likely be more difficult to publish than studies showing an effect. We had no evidence to suggest that there was publication bias and, therefore, no downgrading was recommended. There was no evidence to upgrade the evidence. Therefore, the overall GRADE suggested was low.
eStarted GRADE at high certainty because evidence was based on an RCT. The overall risk of bias was high for Moursi 2003. The decision was made to downgrade bias one level to moderate based on the fact that there could be allocation bias and reporting of outcome bias from this study. There was only one study so it was not possible to assess inconsistency. No downgrading for consistency. For indirectness, the evidence only existed for one country (Congo) and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we only identified one study in Africa. We also only had information on the types of supplement used in this study, which did not cover all types of supplementation available. For these reasons, the evidence was downgraded one level to low. For precision, the sample size was relatively small (75 participants). The significant difference observed only became significant after controlling for many factors in the analysis and the original sample size calculation did not take account the need for this multiple adjustment. Therefore, we decided to downgrade by one level for precision to very low. For publication bias, we found only one study, which showed a small effect when controlling for other factors. We had no evidence to suggest that there was publication bias and, therefore, no downgrading was recommended. There was no evidence to upgrade the evidence. Therefore, the overall GRADE suggested was very low.
fStarted GRADE at high certainty because evidence was based on an RCT. The overall risk of bias was moderate for Sur 2003 and there was no evidence that bias was significantly influenced the results of the intervention. The decision was, therefore, made not to downgrade based on bias. There was only one study so it was not possible to assess inconsistency. No downgrading for consistency. For indirectness, evidence only existed for one country (India) and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we only identified one study in Asia. We also only had information on the types of supplement used in this study, which did not cover all types of supplementation available. For these reasons, the evidence was downgraded one level to moderate. For precision, the sample size was relatively small (100 participants) and the paper reported no CIs, which means it is difficult to see how the sample size affected precision. Therefore, we decided to downgrade by one level for precision to low. For publication bias, we found only one study and we had no evidence to suggest that there was publication bias. Therefore, no downgrading was recommended for publication bias. There was no evidence to upgrade the evidence. Therefore, the overall GRADE suggested was low.
Summary of findings table 3
aStarted GRADE at high certainty because evidence was based on RCTs. The overall risk of bias was high risk for both studies (Akter 2012; Jahan 2014). In Akter 2012, the same team delivered the intervention as assessed the outcome and in Jahan 2014, there was no accounting in the analysis for a clinic level effect when the intervention was delivered at the clinic level. Jahan 2014 also lost 20% of participants and there was no analysis of the potential effects of this on the findings. The decision was, therefore, made to downgrade by one level because of the risk of bias in both studies to moderate. There were only two studies so it was difficult to assess inconsistency statistically. However, a narrative comparison showed very similar findings across the two studies for birth weight effects. No downgrading for consistency. The geographical coverage of the studies was limited to Bangladesh and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we only identified studies in Asia and no studies from Africa or Latin America. For this reason, the evidence was downgraded one level to low. For precision, the sample size was adequate (greater than 400) and the CI largely covered an area of an effect being present. No downgrading for precision. For publication bias, only two studies existed and neither had a very small sample size. We had no evidence to suggest that there was publication bias and, therefore, no downgrading was recommended. There was no evidence to upgrade the evidence. Therefore, the overall GRADE suggested was low.
Summary of findings table 4
aStarted GRADE at high certainty because evidence was based on an RCT. The overall risk of bias was high for Penny 2005. The decision was, therefore, made to downgrade based on bias. There was only one study so it was not possible to assess inconsistency. No downgrading for consistency. For indirectness, the evidence only existed for one country (Peru) and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we only identified one study in Latin America. We also only had information on the type of nutrition system strengthening used in this study, which did not cover all types of intervention available. For these reasons, the evidence was downgraded one level to moderate. For precision, the sample size was fairly small (377 participants) although approaching the 400 recommended for continuous outcomes. The CIs were also fairly narrow and did not cross the no effect category. They tightened further on adjustment for other factors. Therefore, we decided not to downgrade for precision. For publication bias, we found only one study and we had no evidence to suggest that there was publication bias. Therefore, no downgrading was recommended for publication bias. There was no evidence to upgrade the evidence as the effect size was not large. Therefore, the overall GRADE suggested was low.
bStarted GRADE at low certainty because evidence was not based on an RCT. The overall risk of bias was high for Pridmore 2014. There were no additional biases identified beyond the effect of this not being an RCT so no further downgrading was made for risk of bias. There was only one study so it was not possible to assess inconsistency. No downgrading for consistency. For indirectness, evidence only existed for one country (Kenya) and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we only identified one study in Africa. We also only had information on the type of nutrition system strengthening used in this study, which did not cover all types of intervention available. For these reasons, the evidence was downgraded one level to very low. For precision, the sample size was adequate and CIs were not wide. Therefore, we decided not to downgrade for precision. For publication bias, we found only one study and we had no evidence to suggest that there was publication bias. Therefore, no downgrading was recommended for publication bias. There was no evidence to upgrade the evidence as the effect size was not large. Therefore, the overall GRADE suggested was very low.
cStarted GRADE at high certainty because evidence was based on an RCT. The overall risk of bias was high for Penny 2005. The decision was, therefore, made to downgrade based on bias. There was only one study so it was not possible to assess inconsistency. No downgrading for consistency. For indirectness, the evidence only existed for one country (Peru) and it was unclear whether context would change study findings. Our research question pertained to low‐ and middle‐income countries and we only identified one study in Latin America. We also only had information on the type of nutrition system strengthening used in this study, which did not cover all types of intervention available. For these reasons, the evidence was downgraded one level to moderate. For precision, the sample size was fairly small (377 participants) although approaching the 400 recommended for continuous outcomes. The CIs were also fairly narrow and did not cross the no effect category. They tightened further on adjustment for other factors. Therefore, we decided not to downgrade for precision. For publication bias, we found only one study and we had no evidence to suggest that there was publication bias. Therefore, no downgrading was recommended for publication bias. There was no evidence to upgrade the evidence as the effect size was not large. Therefore, the overall GRADE suggested was moderate.
Data and analyses
Comparison 1. Zinc supplementation in pregnant women versus supplementation without zinc or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length | 2 | 1337 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.36, 0.10] |
| 2 Low birth weight | 2 | 1367 | Mean Difference (IV, Fixed, 95% CI) | ‐36.13 [‐83.61, 11.35] |
| 3 Mid‐upper arm circumference | 2 | 1264 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.13, 0.14] |
Comparison 2. Micronutrient or macronutrient supplementation in children versus no intervention or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length‐for‐age or height‐for‐age | 3 | 2601 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 2 Weight‐for‐age | 4 | 2646 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.01, 0.10] |
| 3 Weight‐for‐height | 3 | 2331 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.01, 0.09] |
Comparison 3. Nutrition education for pregnant women versus standard care or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Low birth weight | 2 | 415 | Mean Difference (IV, Fixed, 95% CI) | 478.44 [423.55, 533.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akter 2012.
| Methods | RCT Sample size: 115 Inclusion criteria: women who were 7 months pregnant who agreed to participate full‐time and gave informed consent. Exclusion criteria: not able to comply full‐time with the intervention |
|
| Participants | Pregnant women and newborn Age: newborn Country: Bangladesh Setting: poor urban areas in Dhaka city |
|
| Interventions |
Type: nutrition education and complementary food recipe demonstration. A manual was developed to provide nutrition education to women. Topics included food security, caring practices, and disease control. Trained investigator used the manual and provided explanation of health benefits to motivate the women. Nutrition education included promotion of increased meals during pregnancy (from 3 to 5 times), food hygiene, rest, and optimal infant breastfeeding practices. An inexpensive nutritious meal called khichuri made from local available food was explained (650 kcal). IEC materials (flip charts) used to demonstrate. Urban specificity: free maternity health care Level of factors tackled: individual, service delivery Delivery: women attending the Maternal and Child Training Institute which provides maternity care for a nominal fee or free of charge to poor women. Duration (years): 0.25 Comparison: IG: nutrition education in group of 6–8 participants twice in the first month and once a month for the next 2 months before delivery CG: routine hospital advice on food intake, immunisation, personal hygiene, and breastfeeding Measurement: women weighed monthly up to delivery, newborn infants' birth weights measured within 24 hours after delivery, and breastfeeding practices observed 1 month after delivery. PROGRESS at baseline: place of residence, SES, age, monthly family income, educational level, occupation of women and their husbands data were collected but nutritional status data were not disaggregated by any of them. |
|
| Outcomes | LBW, weight at birth | |
| Notes | No funding information Impact of the intervention: IG: mean birth weight 2.86 kg (SD 0.27) CG: mean birth weight 2.42 kg (SD 0.35) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used to allocate women to IG or CG. |
| Allocation concealment (selection bias) | High risk | As this was an education intervention it was not possible to conceal allocation to the providers of the intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As this was an education intervention it was not possible to blind the providers of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | As this was an education intervention it was not possible to blind the providers of the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appeared that nobody left the study, but this was not totally clear in the paper. |
| Selective reporting (reporting bias) | Unclear risk | Authors did not address this risk directly. |
| Other bias | High risk | The same team delivered the intervention and assessed the impact of the intervention. Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Low risk | Maternal nutrition outcomes were measured prior to the intervention, and no important differences were present across study groups (only measured maternal nutrition outcomes). |
| Similarity of baseline characteristics | Low risk | Baseline characteristics of the study and control providers reported and similar in both groups. |
| Protection against contamination | High risk | Local investigators were in communication with participants in both IG and CG and cross‐communication was likely. |
Begin 2008.
| Methods | RCT with 4 arms Sample size: 315 Inclusion criteria: infants aged < 5 months not meeting the exclusion criteria. Exclusion criteria: severely malnourished, evidence of congenital abnormalities; parents planned to move from the study community within the next few months; or parents did not consent to participate |
|
| Participants | Children Age: 6–7 months Country: Guatemala Setting: low‐income neighbourhoods ('asentamientos') on the outskirts of Guatemala City. Previous research indicated high level of anaemia in infants (defined as altitude‐corrected haemoglobin < 10.3 g/dL, corresponding to a measured haemoglobin < 11.0 g/dL) at 6 months of age. |
|
| Interventions |
Type: supplementation of maize flour, maltodextrins (corn syrup solids), sugar, flavouring agents, WPC or BSC, and either a vitamin/mineral supplement or additional maize flour. Vitamin and mineral supplement included vitamins A, B1, B2, B3, B6, B12, C, and D3; folic acid; iron; zinc; iodine; and selenium. These contained 1 US recommended dietary allowances for a 1‐year‐old child except for calcium and phosphorus (0.3 RDA). Children received 1 of 4 maize‐based dietary supplements daily for 8 months. Fields workers visited homes daily and added water to the mix which was fed to the child by spoon. The field worker observed and recorded the amount consumed by the child. All children enrolled received daily supplements of iron 10 mg from 4–5 months of age before starting the intervention. Urban specificity: none Level of factors tackled: individual Delivery: delivered by field workers in the household Duration (years): 0.7 Comparison: IG1: BSC IG3: WPC+MMN IG4: BSC+MMN CG: WPC Measurement: monthly anthropometric measurement. The outcomes reported in the paper: baseline (6–7 months) and 2, 4, and 6 months postbaseline for growth outcomes. PROGRESS at baseline: gender, maternal education, SES variables, and age but nutritional status data were not disaggregated. |
|
| Outcomes | LFA, MUAC, diarrhoea | |
| Notes | No funding information Impact of the intervention: paper only gave these values at baseline. See Table 3 for this information. Change in length and weight data included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to 1 of the 12‐letter codes, using a block randomisation scheme, with block length of 12. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure not fully reported in paper. Additional information provided by the author: the supplements were produced and shipped from the producer in Iowa. The study statistician had developed a 12‐letter code and each container of supplements had a letter marked on it. As there were 4 products, each group had 3 different letters assigned. Only the statistician and the manufacturer knew which letters corresponded to which product. Each child was randomly assigned to 1 of the 12‐letter codes by the field supervisor, using a block randomisation scheme prepared by the statistician. The field supervisor and field workers who distributed the supplements, the data collectors for all study outcomes, and the study participants were all blind to the treatment group assignment. The statistician was not involved in the field implementation, but she did participate in the downstream data analyses, working initially with data masked to group identity. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Paper described a double‐blind approach but did not give any information about how this was adhered to or ensured during the study. Additional information provided by corresponding author: the supplements were produced and shipped from the producer in Iowa. The study statistician had developed a 12‐letter code and each container of supplements had a letter marked on it. As there were 4 products, each group had 3 different letters assigned. Only the statistician and the manufacturer knew which letters corresponded to which product. Each child was randomly assigned to 1 of the 12‐letter codes by the field supervisor, using a block randomisation scheme prepared by the statistician. The field supervisor and field workers who distributed the supplements, the data collectors for all study outcomes, and the study participants were all blind to the treatment group assignment. The statistician was not involved in the field implementation, but she did participate in the downstream data analyses, working initially with data masked to group identity. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Baseline anthropometric and biochemical assessments were completed before treatments were initiated. The identity of the individual treatments remained blinded until all data collection, laboratory assays, and preliminary analyses of data were completed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 49% did not complete the trial but there did not appear to be differences in dropouts across the groups on measured parameters. Given the high level of dropout, it is impossible to say for sure what was the impact of the incomplete outcome data. Additional information provided: there was a high dropout rate; but the dropout rate did not differ by study group. The article also stated that the interpretation of the group‐wise comparisons did not differ when shorter periods of observation were considered (i.e. when there were fewer dropouts). The treatment groups were similar at baseline, the dropout rates did not differ by study group, and those who left the study early did not differ significantly from those who remained with regard to their baseline characteristics. The reasons for exclusion and attrition were reported. |
| Selective reporting (reporting bias) | Unclear risk | Authors did not address this risk directly, but all outcomes described in methods were reported. |
| Other bias | High risk | Duration was too long and probably intruded on family life. Infants who were given BSC consumed 12% less of the amount offered than infants on other treatments. The lower amount consumed could have affected growth. Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Low risk | Children's nutrition outcomes were measured prior to the intervention, and there were no important differences across study groups. |
| Similarity of baseline characteristics | Low risk | Baseline characteristics of the study and control providers were reported and similar in the IG and CG. |
| Protection against contamination | Low risk | It is unlikely that the CG received the intervention as it was a supplementation intervention with randomisation of participants. No risk of communication between investigators for different types of treatment vs control. The identity of the individual treatments remained blinded until all data collection, laboratory assays, and preliminary analyses of data were completed. |
Caulfield 1999.
| Methods | RCT Sample size: 1295 mothers, 957 newborns Inclusion criteria: mothers having uncomplicated pregnancy, carrying a singleton foetus and living in coastal Peru for ≥ 6 months before becoming pregnant Exclusion criteria: none |
|
| Participants | Pregnant women and newborns Age: at birth Country: Peru Setting: in Villa El Salvador, an impoverished shantytown in Lima, Peru. Pregnant women are reported to consume 7 mg/day of zinc of low‐to‐moderate bioavailability (Sacco 1999) and have lower serum and urinary zinc concentrations during pregnancy than seen in more zinc‐replete populations (Caulfield (a) 1999). |
|
| Interventions |
Type: supplementation of zinc The supplements were distributed monthly during antenatal visits and women were recommended to take 1 tablet every day between meals together with a juice rich in ascorbic acid, lemonade, or water. Compliance was monitored monthly during the visit and biweekly by health workers during home visits. They observed the number of tablets remaining in each blister pack. Urban specificity: compliance of supplementation high Level of factors tackled: individual, service delivery Delivery: via antenatal care distribution system and outreach services health workers who interviewed women in their homes Duration (years): 2 Comparison: Upon entry into antenatal care between 10 and 24 weeks' gestation, women were randomly assigned within parity (nullipara or multipara) and week of gestation at enrolment (< 17 weeks vs ≥ 17 weeks) strata. IG: pregnant women received a daily supplement containing iron 60 mg (as ferrous sulphate) and folate 250 mg (folic acid), with an additional zinc 15 mg (as zinc sulphate) beginning at 10–24 weeks' gestation CG: pregnant women received a daily supplement containing iron 60 mg (as ferrous sulphate) and folate 250 mg (folic acid) beginning at 10–24 weeks' gestation Measurement: at birth up to 7 days after birth PROGRESS at baseline: SES collected via interviews, data were collected but nutritional status data were not disaggregated by any of them. |
|
| Outcomes | LBW, MUAC | |
| Notes | No funding information Impact of the intervention: IG: birth weight 3267 g, SD 461 CG: birth weight 3300 g, SD 498 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated that participants were 'randomly assigned' to IG vs CG, but did not describe the randomisation method. Quote: "Upon entry into prenatal care between 10 and 24 week gestation, women were randomly assigned within parity (nullipara or multipara) and week of gestation at enrolment (<17 week vs. >=17 week) strata, to receive a daily supplement containing 60 mg iron (as ferrous sulfate) and 250 mg folate (folic acid), with or without an additional 15 mg zinc (as zinc sulfate)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Neither the health personnel nor the investigators had knowledge of the coding scheme until analyses of these data were largely complete." This seems to protect against allocation bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Supplements all had the same brick colour and shape. Produced by a local pharmaceutical company (Instituto Quimiotera´pico, SA, Lima, Peru) and distributed in coded blister packages. Tablets distributed monthly during antenatal visits with the recommendation to take 1 tablet every day, between meals, together with an available juice rich in ascorbic acid, lemonade, or water. Neither the health personnel nor the investigators had knowledge of the coding scheme until analyses of these data were largely complete. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Neither the health personnel nor the investigators had knowledge of the coding scheme until analyses of these data were largely complete." The meaning of 'largely complete' was not clear and left open possibilities for assessment bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20% of the possible sample did not participate or complete the study. This percentage may be large enough to bias results. Quote: "Of these women, 18 (1%) were found to live in another community and therefore not eligible to participate, 92 women (7%) declined to participate after discussing it with their husband or other family members, 71 (5%) moved out of the study area, 30 (2%) miscarried, and 58 (4%) left the study for other reasons. Further, 10 women (1%) were subsequently determined to have twin pregnancies or to have developed complications of pregnancy, and were no longer eligible for the study." |
| Selective reporting (reporting bias) | Unclear risk | Authors did not address this risk directly, but all outcomes described in methods were reported. |
| Other bias | Unclear risk | Authors did not describe only the 'strengths' of the study and did not describe any 'weaknesses' or 'limitations'. Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Low risk | Maternal nutrition outcomes were measured prior to the intervention, and there were no important differences across study groups (only measured maternal nutritional outcomes). |
| Similarity of baseline characteristics | Low risk | Baseline characteristics of the study and control providers were reported and slightly different. Nevertheless, maternal characteristics were taken into account in the analysis. |
| Protection against contamination | Low risk | It is unlikely that the CG received the intervention as it was a supplementation intervention with randomisation of participants. Investigators did not know the content of the supplements so could not communicate about intervention or control with each other or with participants. |
Iannotti 2008.
| Methods | RCT Sample size: 546 Inclusion criteria: For mothers: uncomplicated pregnancy, carrying a singleton foetus and living in coastal Peru for ≥ 6 months before becoming pregnant. For infant: healthy and residing in the study area. Exclusion criteria: For the original study: high‐risk pregnancies, multiple pregnancy, or not eligible for vaginal delivery. For this follow‐on study: additional exclusion criteria were an unhealthy infant (not defined in the paper) and must have stayed residing in the study area and remained willing to participate in the study. |
|
| Participants | Pregnant women and newborns Age: at birth Country: Peru Setting: urban shantytown in Lima, Peru. Pregnant women reported to consume zinc 7 mg/day of low‐to‐moderate bioavailability (Sacco 1999), and have lower serum and urinary zinc concentrations during pregnancy than seen in more zinc‐replete populations (Caulfield 1999). |
|
| Interventions |
Type: maternal zinc supplementation during pregnancy Compliance was recorded in home visits by fieldworker during pregnancy until delivery. Urban specificity: none Level of factors tackled: individual Delivery: during monthly antenatal visits Duration (years): 2 Comparison: IG: zinc sulphate 15 mg, ferrous sulphate 60 mg, and folic acid 250 μg (zinc group) CG: ferrous sulphate 60 mg and folic acid 250 μg Supplementation began in both groups between 10 and 24 weeks' gestation (mean 15.6 weeks, SD 4.6) and continued through 4 weeks after delivery. Measurement: at birth and monthly from month 1 to month 12 PROGRESS at baseline: SES and demographic characteristics data were collected but nutritional status data were not disaggregated by any of them. |
|
| Outcomes | LBW, LFA, diarrhoea | |
| Notes | No funding information Impact of the intervention: IG: difference in means presented CG: difference in means presented |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper described that groups were randomly allocated but did not report how. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure not fully reported in this paper or the original Caulfield (a) 1999 paper referred to in this paper. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to allow judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to allow judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Approximately 40% attrition and no specific analyses presented to compare those who stayed and those who left the study, although differences between the CG and IG in sociodemographic characteristics were the same as for the original study, i.e. households with electricity were significantly (P = 0.05) higher in the CG than in the IG. This would likely reduce an effect of zinc on growth as television ownership indicates improved SES. Therefore, it is unlikely that there is a problem with incomplete outcome data but there was insufficient information to fully assess this. |
| Selective reporting (reporting bias) | Unclear risk | Authors did directly address this risk, but all outcomes described in methods were reported. |
| Other bias | Unclear risk | The authors described some limitations that may have introduced bias. Quote: "This study was limited by the fact that we considered morbidity and dietary surveillance data primarily from the second half of infancy." Quote: "in recognition of the health and growth benefits that may be attributed to iron and folic acid in the supplements in both treatment and control groups, there may have been some attenuation of outcome effects in the present study." Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Low risk | Performance or patient outcomes were measured prior to the intervention, and there were no important differences across study groups. |
| Similarity of baseline characteristics | Low risk | Baseline characteristics of the study and control providers were reported and similar except electricity was found to be significantly higher in the CG than in the zinc group (P = 0.05). |
| Protection against contamination | Low risk | Unlikely that the CG received the intervention as it was a supplementation intervention. The same investigator measured all participants and was blinded to IG and CG. |
Iannotti 2014.
| Methods | RCT Sample size: 589 Inclusion criteria: infants aged 6–11 months, in good health, a singleton birth, not severely malnourished, household was not receiving other food aid, and residence within the intervention catchment area Exclusion criteria: infants with fever, congenital health condition, or peanut allergy, multiple births, severely malnourished infants with a WFA z‐score < –3 SDs, and infants living in families already receiving food aid |
|
| Participants | Children Age: 6–11 months Country: Haiti Setting: Fort Saint Michel in the second largest city in Haiti after Port‐au‐Prince. The area is a low‐lying, flood‐prone, and densely populated area. Study community lived in the poorest communal section of the city, Petite Anse, with a population of 80,000 people. During the rainy season, the area is flood prone due to the topography and lack of waste and drain‐water management infrastructure. Much of the housing was described as poor; unfinished; and lacking in sanitation, water, and public power. |
|
| Interventions |
Type: supplementation (LNS, micronutrient supplementation vitamin A, vitamin B12, iron, and zinc). The LNS (Nutributter; Edesia: Global Nutrition Solutions) contained peanuts, sugar, soybean oil, non‐fat milk powder, whey, maltodextrin, a vitamin and mineral complex, and the emulsifier lecithin. 1 sachet of LNS provided 108 kcal/day; this is approximately 33% to 50% of the required energy needed for breastfeeding infants 6–11 months of age. Simple messages were provided to the IG: offer the child one‐half of the LNS sachet in the morning and the other half in the afternoon; wash hands and the sachet before giving it to the child; keep the sachet in a clean and covered container; the sachet intends to provide key vitamins and minerals and should not be replaced by other foods. All groups received messages related to optimal complementary feeding (diversity of foods) and hygiene (wash hands before feeding). Compliance data were collected via interviews about LNS consumption, adherence, and acceptability. All children benefited from the integrated management of childhood illness of a well‐baby services (vaccinations, vitamin A supplementation, minimal nutrition education, growth monitoring and referrals of SAM to hospital) of the MPHP. Urban specificity: in Fort Saint Michel Health Center catchment area, the LNS was tested for potential integration in the package of well‐baby services of the MSPP. The MSPP integrated package of well‐baby services was provided at clinics or rally posts at temporarily established locations in communities. Level of factors tackled: individual, community, service delivery Duration (years): 1.6 Comparison: IG1: 3‐month LNS provided 108 kcal and other nutrients including vitamin A, vitamin B12, iron, and zinc at 80% of the recommended amounts. IG2: 6‐month LNS provided 108 kcal and other nutrients including vitamin A, vitamin B12, iron, and zinc at 80% of the recommended amounts. CG: no supplement Measurement: 6 monthly visits for participants recruited between 6 and 11 months plus a follow‐up 6 months after the end of the study. PROGRESS at baseline: trial was open to all mothers with children under 1 year old in the community, data were collected but nutritional status data were not disaggregated by any of them. |
|
| Outcomes | WFA, LFA | |
| Notes | No funding information. Impact of the intervention: IG: for the 3‐month LNS group: at baseline mean LFA z‐score –0.49, SD 1.13 and mean WFA z‐score –0.26, SD 1.15 and endline (visit 6) mean LFA z‐score –0.74, SD 1.19 and mean WFA z‐score –0.34, SD 1.20. For the 6‐month LNS IG: at baseline mean LFA z‐score –0.39, SD 1.20 and mean WFA z‐score –0.21, SD 1.06 and endline (visit 6) mean LFA z‐score –0.70, SD 1.16 and mean WFA z‐score –0.26, SD 1.05 CG: at baseline mean LFA z‐score –0.45, SD 1.29 and mean WFA z‐score –0.22, SD 1.15 and endline (visit 6) mean LFA z‐score –0.74, SD 1.19 and mean WFA z‐score –0.37, SD 1.12 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment carried out through an allocation‐concealment mechanism whereby sealed paper forms that blinded group assignments were drawn from a container by mothers. |
| Allocation concealment (selection bias) | Low risk | Assignment was described as an allocation concealment mechanism but not details are provided as to how concealment was maintained and at what time it was broken during the study. Additional information provided by author: risk for selection bias was very low. All mother–baby dyads from the urban catchment area Cap Haitien were recruited. The study team used multiple different channels to identify and access mothers with infants 6–11 months including health records, community health workers, and household visits throughout communities. No eligible mothers declined to participate. Random assignment to group was completely blinded, with mothers drawing sealed forms from a small container. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Insufficient information supplied to allow judgement on this outcome. Additional information provided by author. Mothers/carers were not blinded to allocation assignment because there was no 'placebo' food used in this study. Thus, mothers in the IG received the actual Nutributter sachets and CG did not. Thus, there was the potential for reporting bias. Enumerators collecting data were blinded to allocation assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unclear in the paper whether there was blinding of outcome assessment. Further details provided by the author suggested it was not possible to fully blind allocation because the sachets were different for IG and CG and there is possibility that enumerators could remember assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were differences by age between groups and age could plausibly affect the growth outcomes studied. However, no data were presented on the outcomes by age to judge the potential effect of age on the outcome, although age was adjusted for analyses and it was this adjustment that makes the intervention effect significant. Author provided additional information. There was likely attrition bias in what we observed in the pattern of losses to follow‐up but only the outcome results for the 3‐month LNS group compared to the CG. The study was designed a priori to examine the efficacy of Nutributter for 6 months compared to control, and Nutributter for 3 months compared to control. The attrition bias, therefore, should not have affected the Nutributter for 6 months compared to control, only the latter Nutributter for 3 months compared to control. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol to establish this risk but all outcomes described in methods were reported and a comprehensive range of anthropometric outcomes presented. |
| Other bias | High risk | At baseline, the 6‐month LNS group had an older mean age and this variable was significant in changing significance of key outcome variables in the study. Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Low risk | Performance or patient outcomes were measured prior to the intervention, and there were no important differences across study groups with the exception of an older mean age in the 6‐month LNS group compared with that of children in 3‐month LNS group and CGs. |
| Similarity of baseline characteristics | Low risk | Baseline characteristics of the study and control providers were reported and similar in the IGs and CG. |
| Protection against contamination | Low risk | It is unlikely that the CG received the intervention as it was a supplementation intervention with randomisation of participants. The same investigators measured participants of the IGs and CG. |
Jahan 2014.
| Methods | RCT Sample size: 300 Inclusion criteria: women without complications and special requirements and a gestational age of 24 weeks Exclusion criteria: women with complications and special requirements |
|
| Participants | Pregnant women and newborns Age: at birth Country: Bangladesh Setting: urban poor women in Dhaka City |
|
| Interventions |
Type: nutrition education and complementary food recipe demonstration for pregnant women in their 3rd trimester of pregnancy. Education lasted for 1 hour and was provided in a way to promote behaviour change. Session included information on the nutritional value of food, importance of exclusively breastfeeding, an adequate diet during pregnancy and breastfeeding, improved cooking practices and awareness raising related to food taboos related to pregnancy and infant feeding, personal hygiene, rest during daytime, and accessing antenatal care services. A highly nutritious local and affordable called Khichuri (762 kcal and 21 g of protein) was demonstrated. The low cost (USD 0.22) per day made it affordable. The investigators were trained on topics related to the intervention for 3 weeks. A manual was developed including food security, caring practices, and disease control. Manuals, leaflets, and flip charts were used to deliver the information. The education was provided in the outpatient areas of clinics to groups of 6–8 women plus any accompanying family members for 1 hour over 3 months. Urban specificity: low cost and short‐term intervention that covered a large number of population (education instead of supplementation which is more expensive) Level of factors tackled: individual, service delivery Delivery: by trained investigators in the outpatient areas of clinics (the government Maternal and Child Health Training Institute, Azimpur, and the Marie Stopes Clinic, Bashbari, Dhaka) Duration (years): 0.3 Comparison IG: monthly education sessions at the clinic for 3 months giving advice. Each education session lasted for 1 hour, and the first session was preceded by an initial 2‐hour interview to obtain baseline information with demonstration for making the Khichuri, a highly nutritious local dish that can be made with affordable, readily available ingredients. CG: routine services from the health facilities Measurement: monthly from 6–9 months of pregnancy, birth, and 1‐month postpartum PROGRESS at baseline: age, education, and income but these data were not used to disaggregate nutritional status. |
|
| Outcomes | LBW, weight at birth | |
| Notes | No funding information Impact of the intervention: IG: baseline maternal bodyweight (mean 56.57 kg, SD 4.86), maternal bodyweight 9 months (mean 65.41 kg, SD 4.54), birth weight of newborn (mean 2.98 kg, SD 0.33), % LBW (mean 3%, SD 2.7%) CG: baseline maternal bodyweight (mean 56.8 kg, SD 2.49), maternal bodyweight 9 months (mean 62.20 kg, SD 3.28), birth weight of newborn (mean 2.49 kg, SD 0.21), % LBW (mean 67%, SD 44.7%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomised number table used. |
| Allocation concealment (selection bias) | High risk | Education intervention that could not be concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Education intervention so it was not possible to blind the providers of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention in the paper that the outcome was blinded during assessment and it would have been hard to do this given that this was an education intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% attrition and completers were not different on basic sociodemographic characteristics to the starters. However, no analysis of attrition affects were undertaken between the CG and IG. |
| Selective reporting (reporting bias) | High risk | No published protocol to establish selective reporting. |
| Other bias | High risk | 20% attrition rate. The intervention took part in group sessions at clinics but there was no account for clinic level biases through a random‐effects term. Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Low risk | Maternal nutrition outcomes were measured prior to the intervention, and no important differences were present across study groups (only maternal nutrition outcomes were measured). |
| Similarity of baseline characteristics | Low risk | Baseline characteristics of the study and control providers were reported and similar in both groups. |
| Protection against contamination | High risk | Unclear whether the CG could have received the intervention. Both investigators and participants would know what was happening in IG and CG. |
Moursi 2003.
| Methods | RCT Sample size: 75 Inclusion criteria: 10 weeks old at start of study, singletons; birth weight > 2500 g; brought up and breastfed by their mothers at home; weight‐for‐length z‐score > –2 at 16 weeks; not hospitalised for serious illnesses; no malformations; the mother or carer should not have intended to be absent for more than 1 week during the study; and parents had to consent to the presence of a trained assistant in their house for 24 hours when the child reached the age of 16 and 24 weeks and also accept weekly visits for collection of morbidity data. Exclusion criteria: violation of any of the inclusion criteria. |
|
| Participants | Children Age: 4.5 months Country: Congo Brazzaville Setting: in the borough of Poto‐Poto in Brazzaville where the prevalence of stunting of children 2 years old was high (15.5%) |
|
| Interventions |
Type: supplementation of a maize/soy‐based flour that contained amylase. Mothers were shown in each group in 1 single demonstration how to prepare the gruel of similar consistency to the ones they were used to preparing. Urban specificity: none Level of factors tackled: individual Delivery: investigators Duration (years): 0.7 Comparison: IG: maize/soy‐based flour that contained amylase CG: similar flour that did not contain amylase Measurement: 10, 16, 24, and 32 weeks of age grouped in 10–15, 16–23, 24–31, and 16–31 weeks of age. Consumption of complementary foods was assessed at the age of 24 weeks using a 24‐hour observed weighed food record. PROGRESS at baseline: SES data collected but nutritional status data not disaggregated by any of them. |
|
| Outcomes | Length velocity (cm/month), WFA, WFH, infant and young children practices and dietary intake, rate of ill days, incidence of diseases | |
| Notes | No funding information Impact of the intervention: IG: 16–31 weeks of age: 1.88, SD 0.07; 24–31 weeks of age: 1.85, SD 0.12 CG: 16–31 weeks of age: 1.66, SD 0.07; 24–31 weeks of age: 1.34, SD 0.13 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper stated that infants were randomly assigned on an individual basis to the CG or IG but no information was given on how that random allocation was achieved. |
| Allocation concealment (selection bias) | High risk | No information given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided regarding any blinding of participants or personnel to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information provided regarding any blinding of the outcome assessment. Dietary intake could be subject to bias if not blinded and this is a key outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 80 infants initially participated in the study but 5 (3 in the IG and 2 in the CG) dropped out after the age of 16 weeks. Of those who dropped out, 4 did so because their parents moved away and 1 child died. |
| Selective reporting (reporting bias) | High risk | No published protocol to establish this risk and the methods were not clear on what the specific measures of outcome to be assessed would be. |
| Other bias | High risk | Likely that all participants feed unobserved. There was only a single point of assessment on 1 day of dietary intake. There was no high‐quality monitoring of ongoing compliance with use of the gruels beyond 1 × 24‐hour assessment. The study was funded by the Institute de Recherche pour le Developpement (IRD or ex‐ORSTROM). Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Unclear risk | Insufficient information to assess this risk. |
| Similarity of baseline characteristics | Unclear risk | Insufficient information to assess this risk. |
| Protection against contamination | Unclear risk | Insufficient information to assess this risk. |
Oelofse 2003.
| Methods | RCT Sample size: 60 Inclusion criteria: infants aged 6 months enrolled at the clinic with birth weight > 2.5 kg and no congenital abnormalities Exclusion criteria: birth weight < 2.5 kg or born with congenital abnormalities |
|
| Participants | Children Age: 6–12 months Country: South Africa Setting: urban disadvantaged black community, Kayamandi, in the Western Cape, South Africa (12,000 inhabitants). Community has low SES indicated by type of housing, possession of household appliances, and access to basic amenities; low education or no formal education and most of the inhabitants worked in the industries in the city or as domestic workers in private homes. Infants were in the care of grandparents or other family members. |
|
| Interventions |
Type: supplementation micronutrient and fortified complementary food. Demonstrations were provided on how to prepare the porridge. A measuring spoon was provided to ensure the correct amount of porridge to be consumed. During home visits, the research assistants deliver batches of cereals and checked the consumption by the infant. Urban specificity: none Level of factors tackled: individual Delivery: community based (delivered in the home). At intervals of 1 week the research assistants paid home visits to deliver the next batch of infant cereal to the IG. These visits were also used to check cereal consumption by the infant. Duration (years): 1.3 Comparison: IG: micronutrient‐fortified complementary food throughout the 6‐month period. the quantity prescribed for use was 60 g/day of dry cereal and would ensure consumption of 100% of RDA for vitamin A, 80% for iron, and > 100% for zinc. CG: no complementary food, but continued with normal diet Measurement: time points results reported for in the paper were 6 months (x3) and 12 months (x3). PROGRESS at baseline: none |
|
| Outcomes | WFA, HFA, WFH | |
| Notes | No funding information Impact of the intervention: IG: baseline serum retinol experimental (mean 30.5, SD 7.4) and 12 months (mean 26.8, SD 5.8), total iron experimental baseline (mean 10.6, SD 4.4) and 12 months (mean 8.0, SD 3.2), haemoglobin experimental baseline (mean 10.8, SD 1.0) and 12 months (mean 10.8, SD 0.9), zinc experimental baseline (mean 79.3, SD 12.1) and 12 months (mean 85.0, SD 9.1), HAZ experimental baseline (mean –0.68, SD 1.35) and 12 months (mean –0.94, SD 0.70), WFA z‐score experimental baseline (mean 0.71, SD 1.10) and 12 months (mean –0.55, SD 0.99), WFH z‐score experimental baseline (mean 1.58, SD 1.10) and 12 months (mean 0.11 SD 1.10) CG: baseline serum retinol control baseline (mean 28.8, SD 6.6) and 12 months (mean 21.4, SD 5.7), total iron control (mean 9.6, SD 4.0) and 12 months (mean 6.5, SD 3.9), haemoglobin control (mean 10.3, SD 1.0) and 12 months (mean 21.4, SD 5.7), zinc control (mean 69.1, SD 15.8) and 12 months (mean 73.6, SD 12.1), HAZ control (mean –0.57, SD 0.87) and 12 months (mean ‐0.72, SD 1.1), and WFA z‐score control (mean 0.46, SD 1.21) and 12 months (mean ‐0.52, SD 1.6), HAZ control (mean –0.57, SD 0.87) and 12 months (mean –0.72, SD 1.10), WFH z‐score control (mean 1.11, SD 1.10) and 12 months (mean 0.42, SD 1.60) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each infant randomly allocated to either IG or CG. The paper did not explain how the randomisation process worked. |
| Allocation concealment (selection bias) | High risk | No mention of concealing to participants the group they were allocated to. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding in the paper. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention in the paper of blinding the outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 50% of participants did not complete the trial and there was no analysis of characteristics of those who remained and those who dropped out between the CG and IG. Attrition was slightly higher in the IG. |
| Selective reporting (reporting bias) | High risk | No published protocol to establish this risk. |
| Other bias | High risk | Serum zinc levels differed significantly at baseline between the CG and IG. Unclear if participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Unclear risk | Children outcomes were not measured prior to the intervention. |
| Similarity of baseline characteristics | Unclear risk | Baseline characteristics of the study and control providers not reported. |
| Protection against contamination | Low risk | Unlikely that the CG received the intervention as it was a supplementation intervention. |
Osendarp 2000.
| Methods | RCT Sample size: 559 Inclusion criteria: mothers planned to remain at or near their residences in Dhaka for the delivery, did not have an established medical risk for reduced or excessive birth weight, and provided informed consent. Exclusion criteria: any violation of inclusion criteria |
|
| Participants | Pregnant women and newborns Age: at birth Country: Bangladesh Setting: selected areas of Dhaka city slums |
|
| Interventions |
Type: supplementation of zinc Health workers provided 1‐week supply of supplements/placebo at a time. Women were instructed to consume 1 tablet daily between meals and not together with other vitamin or mineral supplements. Compliance was assessed by counting the remaining tablets in each strip during home visit. Urban specificity: none Level of factors tackled: individual Delivery: health workers provided weekly to the houses of women a 1‐week supply of zinc or placebo tablets weekly. Duration (years): 0.3 Comparison: IG: zinc amount based on twice the recommended daily intake for zinc during the last 2 trimesters of pregnancy, assuming low or moderate bioavailability, and was used previously in pregnant women without reports of adverse effects. The zinc content of the zinc tablets (zinc 31.0 mg/tablet; range 28.6–32.6; 20 tablets) CG: placebo tablets Measurement: baseline, 7 and 8 months' gestation, birth. Serum zinc concentrations, haemoglobin concentrations, and blood pressure assessed at baseline and again at 7 months' gestation during visits to the ICDDR, B Clinical Research and Service Centre. Information on dietary intake was collected at baseline and anthropometric measurements were made monthly from baseline until 8 months' gestation during home visits. Gestational age assessment, birth weight measurements, and infant anthropometric measurements were performed by trained physicians within 72 hours of birth. PROGRESS at baseline: SES and reproductive history of women. Categories for SES were developed using an index for urban populations on the basis of ownership of household durable goods. However, nutritional status data were not disaggregated by any of these variables. |
|
| Outcomes | Birth weight | |
| Notes | No funding information Impact of the intervention: IG: birth weight: 2513 g, SD 390; length: 46.8 cm, SD 2.3 CG: birth weight: 2554 g, SD 393; length: 47.0 cm, SD 2.2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation achieved by computer‐generated random‐letter assignment, and the codes remained unknown to both investigators and participants until study was completed. |
| Allocation concealment (selection bias) | Low risk | Randomisation achieved by computer‐generated random‐letter assignment, and the codes remained unknown to both investigators and participants until study was completed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was a cellulose tablet indistinguishable from the zinc supplement in both appearance and taste. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Codes for whether a participant was in the IG or IG remained unknown until the study was completed. Outcomes were objective measures that would be unlikely to be affected by knowing the assigned group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 559 women enrolled, 113 (20.2%) were lost to follow‐up before delivery (55 (20.4%) in the IG and 58 (20.0%) in the CG). As anticipated for this highly mobile population and despite the restrictions at enrolment, most losses to follow‐up (60) were due to out‐migration during the course of the study or to women leaving the area to deliver in their home villages. There were no differences in reasons for women being lost to follow‐up between the 2 groups. |
| Selective reporting (reporting bias) | High risk | No published protocol to establish this risk. |
| Other bias | Low risk | No other bias identified. Authors did not mention any competing effects of other interventions. |
| Similarity of outcome measures at baseline | Low risk | Maternal nutrition outcomes were measured prior to the intervention, and there were no important differences across study groups (only maternal nutritional outcomes were measured). |
| Similarity of baseline characteristics | Low risk | Baseline characteristics of the study and control providers were reported and similar across groups. |
| Protection against contamination | Low risk | Unlikely that the CG received the intervention as it was a supplementation intervention with randomisation of participants. |
Penny 2005.
| Methods | RCT Sample size: 377 Inclusion criteria: health facilities serving communities in periurban areas Exclusion criteria: hospitals and health centres with a characteristic not found in any other facility (i.e. they could not be paired for randomisation) |
|
| Participants | Children Age: birth to 18 months Country: Peru Setting: in Trujillo, a city located 400 km north of Lima (population 600,000). Inhabitants had low and insecure income, poor housing, and lack of essential services. Various nutritious foods were assessed to be available. Acute malnutrition was reported as uncommon while anaemia was common in children in these areas. |
|
| Interventions |
Type: improvement of the quality of nutrition counselling through training and provision of simple, standardised, age‐appropriate messages to be used at all points of contact with young children in the facility. Urban specificity: the project was facility based and ensured that activities of the intervention enhanced existing activities and were sustainable. Level of factors tackled: individual, community, service delivery Delivery: government health facility (community hospitals offering maternal and perinatal specialist services; health centres with medical staff always in attendance; and health centres with more limited services). Duration (years): 2 Comparison: IG: in 6 health facilities, the intervention aimed to raise the profile of nutrition and to integrate nutrition services into existing child‐oriented national programmes such as immunisation, monitoring of growth and development, and management of acute respiratory infections and diarrhoea. The project aimed to enhance the quality of nutrition counselling through training and provision of simple, standardised, age‐appropriate messages to be used at all points of contact with young children in the facility. Materials available in health facilities were adapted for the study and provided as flip charts and single‐page recipe fliers. 3 key messages were designed and disseminated among all staff in the facilities that had any contact with carers of young children. An accreditation scheme was used as a mechanism for institutional change. Accreditation was by local health professionals and by project workers and was based on the satisfaction of previously defined criteria that measured the health service compliance with the intervention. It was done by a review of health‐facility records, observation of contact with patients, interviews with carers of young children on leaving the facility, and by a few home visits to carers who had visited the facility in the preceding 2 weeks. CG: no intervention in 6 other health facilities Measurement: at birth, 3, 6, 9, 12, 15, and 18 months PROGRESS at baseline: housing, education, SES information collected at baseline but not used to disaggregate nutritional status and to assess equity. |
|
| Outcomes | WFA, LFA | |
| Notes | No funding information Impact of the intervention: IG: at 18 months: mean length 79.36 cm, SD 2.74; mean weight 10.77 kg, SD 1.16) CG: at 18 months: mean length 78.29cm, SD 2.66; mean weight 10.48Kg, SD 1.02) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin flipped in front of healthcare facilities being randomly allocated in pairs. |
| Allocation concealment (selection bias) | Unclear risk | Because this was an education intervention it was not possible to conceal allocation to the providers of the intervention. However, families were not told whether they were in the IG or CG. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to the group they were allocated to. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data were collected by field workers who were not involved in the delivery of the intervention. The outcome measures of WFA z‐score and LFA z‐score are objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition low and reasons for attrition similar across groups, with numbers leaving the study similar in both groups or at a level that would not over turn the effect size observed. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol to establish this risk but all outcomes described in methods were reported. |
| Other bias | Low risk | Biases could have arisen from reported behaviours and information on nutrient intake. However, effect on LFA and WFA was likely to be low. Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | High risk | Children's outcomes were measured prior to the intervention, and some differences were present across study groups. |
| Similarity of baseline characteristics | High risk | Baseline characteristics of the study and control providers were reported and some differences were reported in the CG compared to the IG. |
| Protection against contamination | Low risk | Allocation was done randomly and it was unlikely that the CG received the intervention. |
Pridmore 2014.
| Methods | Controlled before‐after and case‐control study design Sample size: 1809 Inclusion criteria: children aged 24–59 months Exclusion criteria: not reported |
|
| Participants | Children Age: 24–59 months Country: Kenya Setting: urban poor areas of Mombasa |
|
| Interventions |
Type: action research process including nutrition working groups, multifactorial co‐ordination and actions in intervention areas to improve nutrition, food security and living conditions, to expand their income‐generating activities; to strengthen psychosocial support; and help prevent domestic violence and mitigate its impact. Actions were focused towards self‐help/community groups. Urban specificity: tackling urban specific social, economic, and environmental factors operating at local, municipal, provincial and central levels. These included education, income, working conditions, housing, neighbourhood and community conditions, status of women, and level of social inclusion. These determinants were assessed to impact child nutrition through influencing access to nutritious foods, childcare practices, and access to basic services. Level of factors tackled: individual, household, community, service delivery, city level Delivery: community groups, service delivery stakeholders, city, and regional governance Duration (years): 3.5 Comparison: IG: multi‐sectorial nutrition working group, participatory action research, strengthening of the group members capacity building to work together to plan, act, and evaluate small‐scale intersectorial, co‐ordinated interventions aiming to tackle the social determinants of malnutrition (supply of fresh vegetable from urban farming, and improved level of sanitation and waste disposal) CG: no intervention Measurement: July 2011, the second in June 2013 PROGRESS at baseline: gender was collected and used. |
|
| Outcomes | HFA, WFH, WFA | |
| Notes | Funding: GBP 400,000 Impact of the intervention: IG: 2013 boys mean stunting –1.33, girls mean stunting 1.41. 2011 boys mean stunting –1.69, women mean stunting –1.46 CG: 2013 boys mean stunting –1.27, women mean stunting 1.28. 2011 boys mean stunting –1.65, women mean stunting –1.49 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised study. |
| Allocation concealment (selection bias) | High risk | Non‐randomised study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐randomised study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol to establish this risk but all outcomes described in methods were reported. |
| Other bias | High risk | Sample sizes may have been too small for power. Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Low risk | Information provided show no statistically significant difference between control and intervention in Kenya. |
| Similarity of baseline characteristics | High risk | No data provided but authors admitted that at baseline, the 2 areas were quite different socioeconomically and in other characteristics. |
| Protection against contamination | Unclear risk | Insufficient information to assess this risk. |
Radhakrishna 2013.
| Methods | RCT Sample size: 324 infants Inclusion criteria: term healthy infants who would stay in the study area until the child attained 2 years of age Exclusion criteria: preterm deliveries (gestational age < 37 weeks), LBW (< 2500 g) and infants with congenital abnormalities (neural tube defects, congenital heart disease, cleft palate, and cleft lip) or birth asphyxia |
|
| Participants | Infants Age: birth Country: India Setting: low‐income urban communities (population of around 25,000 located in the Secunderabad city of the South India) |
|
| Interventions |
Type: zinc supplementation in infants aged 4–18 months. Mothers were counselled on strategies to overcome problems with supplement adherence. Clinic conducted at community centre for study children and treat illnesses (respiratory tract infections, dysentery). Every month, the project staff, responsible for distributing the supplements in the field, collected empty bottles, and provided fresh supplements as per participant identification number and codes. Urban specificity: none Level of factors tackled: individual, service delivery, community Delivery: community groups, service delivery stakeholders, city, and regional governance Duration (years): 1.2 Comparison: IG: zinc 5 mg + riboflavin 0.5 mg/day CG: riboflavin 0.5 mg/day Measurement: weight and length measured at enrolment and again after 3 and 6 months PROGRESS at baseline: maternal age, father's age, number of family members, number of antenatal visits |
|
| Outcomes | Length, HFA, weight at 18 months, WFH, skinfold thickness (triceps, sub scapular) | |
| Notes | Funding: no information provided Impact of the intervention: IG: HFA at 18 months 61.6%, 21 months 39%, 24 months 29.5%; WFH at 18 months 6.5%, 21 months 11.8%, 24 months 14.3% CG: HFA at 18 months 61.9%, 21 months 25.1%, 24 months 25.2%; WFH at 18 months 10.1%, 21 months 19.1%, 24 months 24.6% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was computer‐generated and administered by a separate scientist to the study team. |
| Allocation concealment (selection bias) | Low risk | Intervention was concealed Quote: "The zinc and placebo were prepared and supplied by Biological Evans Limited, in a syrup base, which were of similar colour, consistency and flavour; in two sets of identical looking bottles, labelled 1 and 2." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was a robust system for minimising performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was a robust system for minimising detection bias (see text under 'Blinding of participants and personnel (performance bias)' for evidence). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was a reasonably low rate of attrition (163 infants began in the IG and 9 were lost to follow‐up; 161 began in the CG and 13 were lost to follow‐up) and so it is unlikely to have had great effect on the results. Nevertheless, there was no intention‐to‐treat analysis so it was not possible to fully assess the effect of the attrition. |
| Selective reporting (reporting bias) | Low risk | The study protocol identified the primary outcomes to be reported upon; these were reported in the paper. |
| Other bias | Unclear risk | None identified. Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Low risk | No statistically significant difference in the outcome measures at baseline. |
| Similarity of baseline characteristics | Low risk | No statistically significant difference in the characteristics between the CG and IG. |
| Protection against contamination | Low risk | Community‐based supplementation study where mothers were given bottles to administer at home. Risk of mother sharing bottles was low. |
Rahman 2002.
| Methods | RCT Sample size: 653 Inclusion criteria: children aged 12–35 months of either sex who had not received any vitamin A supplementation within the past 4 months Exclusion criteria: children with severe malnutrition (WFA < 60% of the National Center for Health Statistics median), with signs or symptoms of vitamin A or zinc deficiency; or with any systemic illness such as diarrhoea, respiratory infection, fever, or any other illness that warranted medical intervention at the time of enrolment |
|
| Participants | Children Age: 12–35 months Country: Bangladesh Setting: urban slums in the older part of Dhaka |
|
| Interventions |
Type: zinc and vitamin A supplementation after 3 and 6 months At enrolment, a health assistant fed the child syrup and demonstrated to the mother how to administrate the syrup at home in the morning after breakfast. Mothers were asked to keep the bottle. After 7 days, the health assistant visited the participants' homes and asked whether the child liked taking the syrup or not. On day 14, health assistant during the home visit gave vitamin A or placebo. Urban specificity: none Level of factors tackled: individual Delivery: community groups, service delivery stakeholders, city, and regional governance Duration (years): 0.5 Comparison: IG1: zinc 20 mg/day for 14 days and a placebo capsule on day 14 IG2: 5 mL placebo syrup/d for 14 d and vitamin A 60,000 retinol equivalents (200,000 IU) on day 14 IG3: 5 mL (1 tsp) zinc syrup containing 20 mg elemental Zn/d for 14 d and a 60 000‐RE vitamin A capsule on day 14 CG: 5 mL placebo syrup/d for 14 d and a placebo capsule on day 14 . Measurement: weight and length measured at enrolment and after 3 and 6 months PROGRESS at baseline: age, gender, breastfeeding, bodyweight at admission, mother's education, income |
|
| Outcomes | Height, weight, WFA, WFH, HFA | |
| Notes | Funding: no information provided Impact of the intervention: Gains in weight and length during follow‐up period were not significantly different among the 4 groups. IG1: HFA change 0.10, SD 0.51. Length gain during 6 months 4.29, SD 1.54. 57% of children did not have a change or decrease in WFA z‐scores. IG2: HFA change 0.05, SD 0.47. Length gain during 6 months 4.33, SD 1.41. 46% of children did not have a change or decrease in WFA z‐scores. IG3: HFA change 0.02, SD 0.49. Length gain during 6 months 4.12, SD 1.29. 50% of children did not have a change or decrease in WFA z‐scores. CG: HFA change 0.06, SD 0.50. Length gain during 6 months 4.25, SD 1.40. 54% of children did not have a change or decrease in WFA z‐scores. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study used "permuted blocks of random numbers." |
| Allocation concealment (selection bias) | Low risk | Intervention was concealed Quote: "A local pharmaceutical company (ACME Laboratories Ltd, Dhaka, Bangladesh) prepared the study syrups (zinc and placebo), which were supplied in identical 50‐mL bottles. The vitamin A and placebo capsules looked identical." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Paper described the study as double blind with only 1 person not involved in the study knowing about which group a participant belonged to. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether the assessment team were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether the assessment team were blinded. |
| Selective reporting (reporting bias) | Unclear risk | No protocol existed to make a fair assessment of reporting bias. |
| Other bias | Unclear risk | Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Low risk | No significant difference in outcome measures at baseline after adjusting for covariates such as age and sex. |
| Similarity of baseline characteristics | Low risk | No statistically significant difference in characteristics at baseline |
| Protection against contamination | Low risk | Community‐based supplementation study where mothers were given bottles to administer at home. Risk of mother sharing bottles was low. |
Sur 2003.
| Methods | RCT Sample size: 100 Inclusion criteria: not having major birth defects or congenital deformities and willing to participate. Exclusion criteria: major birth defects or congenital deformities and unwillingness to participate. |
|
| Participants | LBW newborns Age: at birth Country: India Setting: Tiljala slum of eastern Kolkata. People had low SES, being literate for 70%, and with low level of sanitation. |
|
| Interventions |
Type: zinc supplementation Mothers were shown individually how to administer the syrup and were advised to give their child the daily dose. Regular field visits were conducted by health workers to observe infant feeding practices (breastfeeding) and assessment of nutritional status. Urban specificity: none Level of factors tackled: individual Delivery: community based Duration (years): 1.9 Comparison: IG: 1 mL daily dose (5 days a week) elemental zinc 5 mg as zinc sulphate in vitamin B complex‐based syrup from day of enrolment (within 7 days of birth) to 1 year of age. CG: daily (5 days a week) an identical placebo of 1 mL of vitamin from day of enrolment (within 7 days of birth) to 1 year of age. Measurement: monthly for 1 year PROGRESS at baseline: demographic and SES data were collected but nutritional status data were not disaggregated by any of them. |
|
| Outcomes | Length, weight, WFA, number of days ill with diarrhoea | |
| Notes | No funding information Impact of the intervention: IG: difference at 1 year between final height and birth height: 23.7cm; WFA –1.45 (SD 0.95); weight: 6084 g; % days ill exclusive breastfed 3.7%; % days ill post breastfed 6.6% CG: difference at 1 year between final height and birth height: 21.4cm; WFA –2.17 ( ± 0.90); weight: 5280 g; % days ill exclusive breastfed 4.0%; % days ill post breastfed 10.2% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedules prepared through computerised programs of simple random numbers. |
| Allocation concealment (selection bias) | Low risk | Numbers were allotted to either IG or CG and then arranged serially. The children were assigned sequential serial numbers, and preparations were distributed accordingly. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The syrups administered to the study children in the 2 groups were prepared by Messrs. Greenco & Co Pvt. Ltd, a Kolkata‐based drug‐manufacturing company. Blinded at all preanalysis stages. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The codes of the 2 groups were kept confidential and sealed with a person unrelated to the trial and opened only after analysis of the outcome variables of the groups had been completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol to establish this risk but all outcomes described in methods were reported. |
| Other bias | Unclear risk | Authors stated that 97/100 births were institutional deliveries but it was unclear whether they deliberately sampled the children from health facilities (in which case results may not be generalisable to other types of births in the area) or whether the high percentage of institutional births was because most births are institutional deliveries. Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Low risk | Outcomes were measured, reported, and were similar at baseline. |
| Similarity of baseline characteristics | Low risk | Baseline characteristics of the study and control providers were reported and similar, There was no statistically significant difference in the baseline characteristics between CG and IG. |
| Protection against contamination | Low risk | This was a community‐based supplementation study where mothers were given bottles to administer at home. Risk of mother sharing bottles was low. |
Taneja 2010.
| Methods | RCT Sample size: 2482 Inclusion criteria: children aged 6–30 months Exclusion criteria: children from families intending to move out of the study area, requiring hospitalisation on the day of enrolment, having received vitamin A within the previous 2 months, or who refused to participate |
|
| Participants | Children Age: 6–30 months Country: India Setting: an urban slum of Dakshinpuri in New Delhi, India (15,000 dwellings and 75,000 inhabitants) |
|
| Interventions |
Type: supplementation of vitamin A and zinc At enrolment, all children also received a single dose of vitamin A (104.7 μmol for infants and 209.4 μmol for older children). Weight and length were measured at enrolment and 4 months later. Weekly visits were conducted by field workers to ascertain morbidity in the previous 7 days. Change in length, weight, LFA z‐scores, and weight‐for‐length z‐scores after 4 months of supplementation were assessed. Urban specificity: none Level of factors tackled: individual Duration (years): 0.3 Delivery: weekly visits by field workers at the participants house Comparison: IG: daily zinc supplementation administered at home CG: daily placebo supplementation administered at home Measurement: weight and length were measured at enrolment and 4 months later. Morbidity was measured every 7th day. PROGRESS at baseline: none |
|
| Outcomes | WFA, LFA/HFA, WFH, diarrhoea | |
| Notes | No effect in any of the subgroups defined for age, income, gender, zinc levels in the crude analysis nor after adjusting for age, gender, income, breastfeeding status, and baseline anthropometric status. No funding information Impact of the intervention: IG: mean change: LFA –0.14, SD 0.44; 0.12 cm less (95% CI –0.02 to 0.26) CG: mean change: LFA –0.12, SD 0.43; 3.55 cm change in length |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation scheme (in blocks of 8) generated off‐site by a statistician at Statens Serum Institute, Copenhagen, Denmark, who was not otherwise involved with the study, using SAS software (version 8.1; SAS Institute). |
| Allocation concealment (selection bias) | Unclear risk | Eligible children were individually allocated to zinc or placebo groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Zinc and placebo syrups were similar in appearance, taste, and packaging; and were prepared, packaged, and labelled with a unique identification number according to the randomisation scheme by GK Pharma Aps, Koge, Denmark in unbreakable bottles. The supplies for each child (6 bottles, 1 for each month and 2 extra in case of loss) were packed in a labelled plastic bag before the commencement of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation was by a person in Denmark not related to the study. Preparation of the syrups (zinc or placebo) was done before the commencement of the study and each child's supplies were packed in a labelled plastic bag using a unique identification number according to the randomisation scheme. The zinc and placebo syrups bottles were identical in appearance. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Measurements could not be obtained in 51 children who refused participation after enrolment, 184 who left the study area before completion of follow‐up, and 3 who died. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol to establish this risk, but all outcomes described in methods were reported. |
| Other bias | Unclear risk | Unclear if the participants benefited from other ongoing interventions during the study period. |
| Similarity of outcome measures at baseline | Low risk | Performance or patient outcomes were measured prior to the intervention, and there were no important differences across study groups (only maternal nutrition outcomes were measured). |
| Similarity of baseline characteristics | Low risk | Baseline characteristics of the study and control providers were reported and similar in both groups. |
| Protection against contamination | Low risk | Allocation was done randomly and it was unlikely that the CG received the intervention. Supplementation was administered at home by mothers. |
BSC: bovine serum concentrate; CG: control group; HFA: height‐for‐age; IEC: information, education, and communication; IG: intervention group; LBW: low birth weight; LFA: length‐for‐age; LNS: lipid‐based nutrient supplement; MMN: micronutrient mineral; MSPP: Ministry of Public Health and Population; MUAC: mid‐upper‐arm circumference; RCT: randomised controlled trial; RDA: recommended daily allowance; SAM: severe acute malnourished; SD: standard deviation; SES: socioeconomic status; WFA: weight‐for‐age; WFH: weight‐for‐height; WPC: whey protein concentrate.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agustina 2013 | Participants: children aged 1–6 years. We could not exclude the ≥ 5 years old from the analysis. |
| Akeredolu 2014 | Sample representative of urban area but not necessarily poor or slum area. In the text: 2 general hospitals (representing Urban and Rural areas in Lagos state) were selected. No email address for author. Based on information, excluded as not representative of poor urban area. |
| Choudhury 2016 | Study design did not meet the review PICO criteria. |
| Effendy 2015 | Outcomes are WFA and WFH. Height not measured. Study did not meet the review inclusion PICO criteria. |
| Iannotti 2013 | Conference abstract. Contacted author. Primary outcomes were not included. Study did not meet the review inclusion PICO criteria. |
| Kikafunda 1998 | Unclear from the text if the location was an urban slum. We contacted the author but received no response. |
| Krebs 2011 | Not an intervention but a cross‐sectional study. |
| Kæstel 2005 | Unclear from the text if the location was an urban slum. We contacted the author but received no response. |
| Mitter 2012 | Children's age did not meet the review PICO inclusion criteria. |
| Poudel 2004 | Study design did not meet the review PICO criteria. |
| Saran 2002 | Study design did not meet the review PICO criteria. |
| Semba 2011 | Not an intervention but a cross‐sectional study. |
| Soofi 2013 | Cluster urban and rural were used for sampling but were not used for the analysis. |
| Tomlinson 2016 | Children aged 0–6 years old. We could not exclude the ≥ 5 years from the analysis. |
PICO: Population, Intervention, Comparison and Outcome; WFA: weight‐for‐age; WFH: weight‐for‐height.
Characteristics of ongoing studies [ordered by study ID]
Kimani‐Murage 2013.
| Trial name or title | Effectiveness of personalised, home‐based nutritional counselling on infant feeding practices, morbidity and nutritional outcomes among infants in Nairobi slums: study protocol for a cluster randomised controlled trial |
| Methods | cRCT |
| Participants | Pregnant women and their child |
| Interventions | Home‐based intervention on infant feeding practices, nutrition, and health. The mothers will receive regular, personalised, home‐based counselling by trained community health workers on MIYCN. Regular assessment of knowledge, attitudes, and practices on MIYCN will be done, coupled with assessments of nutritional status of the mother–child dyads and diarrhoea morbidity for the children. |
| Outcomes | Nutritional status, diarrhoea |
| Starting date | March 2012 to February 2015 |
| Contact information | Elizabeth Kimani; email: ekimani@aphrc.org |
| Notes | A paper entitled "Potential effectiveness of Community Health Strategy to promote exclusive breastfeeding in urban poor settings in Nairobi, Kenya: a quasi‐experimental study" was published using data from this trial. Nutritional outcomes were not included. We have been in contact with the author and discussed the impact of the intervention on stunting. To‐date, there is no publication that can be included in this review. |
cRCT: cluster randomised controlled trial; MIYCN: maternal, infant, and young child nutrition.
Differences between protocol and review
There were some differences between the protocol and the review. These were as follows:
in subgroup analysis and investigation of heterogeneity, we were unable to conduct subanalysis as planned due to the lack of data.
in sensitivity analysis, we were unable to conduct analyses as planned, except those related to bias.
Contributions of authors
SG conducted all of the searches.
All authors contributed to reviewing the retrieved results and interpretation.
SG drafted the review.
All authors contributed to its finalisation by providing comments and editorial revisions.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
SG: none.
BB: none.
NM: none.
PG: none.
Edited (no change to conclusions)
References
References to studies included in this review
Akter 2012 {published data only}
- Akter SM, Roy SK, Thakur SK, Sultana M, Khatun W, Rahman R, et al. Effects of third trimester counseling on pregnancy weight gain, birthweight, and breastfeeding among urban poor women in Bangladesh. Food and Nutrition Bulletin 2012;33(3):194‐201. [DOI] [PubMed] [Google Scholar]
Begin 2008 {published data only}
- Begin F, Santizo MC, Peerson JM, Torun B, Brown KH. Effects of bovine serum concentrate, with or without supplemental micronutrients, on the growth, morbidity, and micronutrient status of young children in a low‐income, peri‐urban Guatemalan community. European Journal of Clinical Nutrition 2008;62(1):39‐50. [DOI] [PubMed] [Google Scholar]
Caulfield 1999 {published data only}
- Caulfield LE, Zavaleta N, Figueroa A, Leon Z. Maternal zinc supplementation does not affect size at birth or pregnancy duration in Peru. Journal of Nutrition 1999;129(8):1563‐8. [DOI] [PubMed] [Google Scholar]
Iannotti 2008 {published data only}
- Iannotti L, Zavaleta N, Leon Z, Shankar AH, Caulfield LE. Maternal zinc supplementation and growth in Peruvian infants. American Journal of Clinical Nutrition 2008;88(1):154‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iannotti 2014 {published data only}
- Iannotti LL, Dulience SJ, Green J, Joseph S, François J, Anténor ML, et al. Linear growth increased in young children in an urban slum of Haiti: a randomized controlled trial of a lipid‐based nutrient supplement.#. American Journal of Clinical Nutrition 2014;99(1):198‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jahan 2014 {published data only}
- Jahan K, Roy SK, Mihrshahi S, Sultana N, Khatoon S, Roy H, et al. Short‐term nutrition education reduces low birthweight and improves pregnancy outcomes among urban poor women in Bangladesh. Food and Nutrition Bulletin 2014;35(4):414‐21. [DOI] [PubMed] [Google Scholar]
Moursi 2003 {published data only}
- Moursi M, Mbemba F, Trèche S. Does the consumption of amylase‐containing gruels impact on the energy intake and growth of Congolese infants?. Public Health Nutrition 2003;6(3):249‐57. [DOI] [PubMed] [Google Scholar]
Oelofse 2003 {published data only}
- Oelofse A, Raaij JM, Benade AJ, Dhansay MA, Tolboom JJ, Hautvast JG. The effect of a micronutrient‐fortified complementary food on micronutrient status, growth and development of 6‐to 12‐month‐old disadvantaged urban South African infants. International Journal of Food Sciences and Nutrition 2003;54(5):399‐407. [DOI] [PubMed] [Google Scholar]
Osendarp 2000 {published data only}
- Osendarp SJ, Raaij JM, Arifeen SE, Wahed MA, Baqui AH, Fuchs GJ. A randomized, placebo‐controlled trial of the effect of zinc supplementation during pregnancy on pregnancy outcome in Bangladeshi urban poor. American Journal of Clinical Nutrition 2000;71(1):114‐9. [DOI] [PubMed] [Google Scholar]
Penny 2005 {published data only}
- Penny ME, Creed‐Kanashiro HM, Robert RC, Narro MR, Caulfield LE, Black RE. Effectiveness of an educational intervention delivered through the health services to improve nutrition in young children: a cluster‐randomised controlled trial. Lancet 2005;365(9474):1863‐72. [DOI] [PubMed] [Google Scholar]
Pridmore 2014 {published data only}
- Pridmore P, McCowan T, Carr‐Hill R, Amuyunzu‐Nyamongo M, Lang'o D, Charnes G, et al. Economic and Social Coucil. End of award report. Healthy Urbanisation: tackling child malnutrition through intervening to change the social determinants of health in informal settlements and slums. London (UK): Institute of Education, University of London 2014:23.
Radhakrishna 2013 {published data only}
- Radhakrishna KV, Hemalatha R, Geddam JB, Kumar PA, Balakrishna N, Shatrugna V. Effectiveness of zinc supplementation to full term normal infants: a community based double blind, randomized, controlled, clinical trial. PloS One 2013;8(5):61486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2002 {published data only}
- Rahman MM, Tofail F, Wahed MA, Fuchs GJ, Baqui AH, Alvarez JO. Short‐term supplementation with zinc and vitamin A has no significant effect on the growth of undernourished Bangladeshi children. American Journal of Clinical Nutrition 2002;75(1):87‐91. [DOI] [PubMed] [Google Scholar]
Sur 2003 {published data only}
- Sur D, Gupta DN, Mondal SK, Ghosh S, Manna B, Rajendran K, et al. Impact of zinc supplementation on diarrheal morbidity and growth pattern of low birth weight infants in Kolkata, India: a randomized, double‐blind, placebo‐controlled, community‐based study. Pediatrics 2003;112(6):1327‐32. [DOI] [PubMed] [Google Scholar]
Taneja 2010 {published data only}
- Taneja S, Strand TA, Sommerfelt H, Bahl R, Bhandari N. Zinc supplementation for four months does not affect growth in young north Indian children. Journal of Nutrition 2010;140(3):630‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agustina 2013 {published data only}
- Agustina R, Bovee‐Oudenhoven IM, Lukito W, Fahmida U, Rest O, Zimmermann MB, et al. Probiotics Lactobacillus reuteri DSM 17938 and Lactobacillus casei CRL 431 modestly increase growth, but not iron and zinc status, among Indonesian children aged 1–6 years. Journal of Nutrition 2013;143(7):1184‐93. [DOI] [PubMed] [Google Scholar]
Akeredolu 2014 {published data only}
- Akeredolu IA, Osisanya JO, Okafor JC, Seriki‐Mosadolorun J. Pregnancy outcomes of women in Lagos state: Is nutrition education responsible?. Pakistan Journal of Nutrition 2014;13(1):7. [Google Scholar]
Choudhury 2016 {published data only}
- Choudhury N, Bromage S, Alam MA, Shamsir Ahmed AM, Munirul Islam M, Iqbal Hossain M, et al. Intervention study shows suboptimal growth among children receiving a food supplement for five months in a slum in Bangladesh. Acta Paediatrica 2016;105(10):464‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Effendy 2015 {published data only}
- Effendy DS, Wirjatmadi B, Adriani M, Tosepu R. The influence of supplementary feeding by local food and 123 milk toward increasing the nutritional status of 12‐24 months children with undernutrition status in southeast Sulawesi province, Indonesia. International Journal of Research in Medical Sciences 2015;3(10):2704‐10. [DOI: ] [Google Scholar]
Iannotti 2013 {published data only}
- Iannotti L, Jean‐Louis S, Lesorogol C, Green J, Nickerson N. The effect of nutributter supplementation on complementary feeding practices among young children in an urban slum of Haiti. Annals of Nutrition & Metabolism 2013;63:1105. [DOI: ] [Google Scholar]
Kæstel 2005 {published data only}
- Kæstel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: a randomised, controlled trial in Guinea‐Bissau. European Journal of Clinical Nutrition 2005;59(9):1081‐9. [DOI] [PubMed] [Google Scholar]
Kikafunda 1998 {published data only}
- Kikafunda JK, Walker AF, Allan EF, Tumwine JK. Effect of zinc supplementation on growth and body composition of Ugandan preschool children: a randomized, controlled, intervention trial. American Journal of Clinical Nutrition 1998;68(6):1261‐6. [DOI] [PubMed] [Google Scholar]
Krebs 2011 {published data only}
- Krebs NF, Mazariegos M, Tshefu A, Bose C, Sami N, Chomba E, et al. Meat consumption is associated with less stunting among toddlers in four diverse low‐income settings. Food and nutrition bulletin 2011;32(3):185‐91. [PUBMED: 22073791] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitter 2012 {published data only}
- Mitter SS, Oriá RB, Kvalsund MP, Pamplona P, Joventino ES, Mota R, et al. Apolipoprotein E4 influences growth and cognitive responses to micronutrient supplementation in shantytown children from northeast Brazil. Clinics 2012;67(1):11‐8. [PUBMED: 22073791] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poudel 2004 {published data only}
- Poudel KC, Nakahara S, Okumura J, Wakai S. Day‐care centre supplementary feeding effects on child nutrition in urban slum areas of Nepal. Journal of Tropical Pediatrics 2004;50(2):116‐9. [DOI] [PubMed] [Google Scholar]
Saran 2002 {published data only}
- Saran S, Gopalan S, Krishna TP. Use of fermented foods to combat stunting and failure to thrive. Nutrition 2002;18(5):393‐6. [DOI] [PubMed] [Google Scholar]
Semba 2011 {published data only}
- Semba RD, Moench‐Pfanner R, Sun K, Pee S, Akhter N, Rah JH, et al. Consumption of micronutrient‐fortified milk and noodles is associated with lower risk of stunting in preschool‐aged children in Indonesia. Food and Nutrition Bulletin 2011;32(4):347‐53. [PUBMED: 22590968] [DOI] [PubMed] [Google Scholar]
Soofi 2013 {published data only}
- Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, et al. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster‐randomised trial. Lancet 2013;382(9886):29‐40. [DOI: 10.1016/S0140-6736(13)60437-7] [DOI] [PubMed] [Google Scholar]
Tomlinson 2016 {published data only}
- Tomlinson M, Hartley M, Roux IML, Rotheram‐Borus MJ. The Philani Mentor Mothers Intervention: neighbourhood wide impact on child growth in Cape Town's peri‐urban settlements. Vulnerable Children and Youth Studies 2016;11(3):211‐20. [DOI: 10.1080/17450128.2016.1214770] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Kimani‐Murage 2013 {published data only}
- Effectiveness of personalised, home‐based nutritional counselling on infant feeding practices, morbidity and nutritional outcomes among infants in Nairobi slums: study protocol for a cluster randomised controlled trial. Ongoing study March 2012 to February 2015. [DOI] [PMC free article] [PubMed]
Additional references
Alam 2011
- Alam A, Hakim M, Rouf MA, Haque MO, Ali ME, Sarker ZI. Nutritional status of urban slum children below five years: assessment by anthropometric measurements with special reference to socioeconomic status. Journal of Food, Agriculture & Environment 2011;9(2):85‐90. [Google Scholar]
Attanasio 2005
- Attanasio O, Gómez LC, Heredia P, Vera‐Hernández M. The short‐term impact of a conditional cash subsidy on child health and nutrition in Colombia, 2015. www.ifs.org.uk/edepo/rs_fam03.pdf (accessed prior to 1 June 2019).
Awasthi 2003
- Awasthi S. Environmental health project determinants of childhood mortality and morbidity in urban slums in India. Indian Pediatrics 2003;40:1145‐61. [PubMed] [Google Scholar]
Barker 1990
- Barker D. The fetal and infant origins of adult disease. BMJ 1990;301:1111‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barker 1995
- Barker D. Fetal origins of coronary heart disease. BMJ 1995;311:171‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2008
- Berger SG, Pee S, Bloem MW, Halati S, Semba RD. Malnutrition and morbidity among children not reached by the national vitamin A capsule programme in urban slum areas of Indonesia. Public Health 2008;122(4):371‐8. [DOI: 10.1016/j.puhe.2007.08.003] [DOI] [PubMed] [Google Scholar]
Bhutta 2008
- Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371(9610):417‐40. [DOI] [PubMed] [Google Scholar]
Bhutta 2013
- Bhutta Z, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence‐based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet 2013;382(9890):452‐77. [DOI] [PubMed] [Google Scholar]
Black 2008
- Black RE, Allen LH, Bhutta Z, Caulfield LE, Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. [DOI] [PubMed] [Google Scholar]
Black 2013
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 2013;382(9890):427‐51. [DOI] [PubMed] [Google Scholar]
Bogin 1992
- Bogin B, Wall M, MacVean RB. Longitudinal analysis of adolescent growth of ladino and Mayan school children in Guatemala: effects of environment and sex. American Journal of Physical Anthropology 1992;89:447‐57. [DOI] [PubMed] [Google Scholar]
Bogin 2007
- Bogin B, Varela‐Silva M, Rios L. Life history trade‐offs in human growth: adaptation or pathology?. American Journal of Human Biology 2007;19:631‐42. [DOI] [PubMed] [Google Scholar]
Bogin 2014
- Bogin B, Azcorra H, Wilson H, Vázquez‐Vázquez A, Avila‐Escalante ML, Castillo‐Burguete MT, et al. Globalization and children's diets: the case of Maya of Mexico and Central America. Anthropological Review 2014;77(1):11‐32. [Google Scholar]
BRC 2012
- British Red Cross. Learning from the City: British Red Cross Urban Learning Project Scoping Study. London (UK): British Red Cross, 2012. [www.alnap.org/node/10698.aspx] [Google Scholar]
Brown 2013
- Brown J, Cairncross S, Ensink JH. Water, sanitation, hygiene and enteric infections in children. Archives of Disease in Childhood 2013;98(8):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buttenheim 2007
- Buttenheim A. Hygienic latrine use and child wasting in urban Bangladesh. Center for Population Research 2007; Vol. 22, issue 7. [papers.ccpr.ucla.edu/papers/PWP‐CCPR‐2007‐003/PWP‐CCPR‐2007‐022/PWP‐CCPR‐2007‐022.pdf]
Cairncross 2004
- Cairncross S, Valdmanis V. Water supply, sanitation, and hygiene promotion. Disease Control Priorities Project, Disease Control Priorities Project Working Paper. Washington (DC): World Bank, 2004. [Google Scholar]
Cairncross 2010
- Cairncross S, Hunt C, Boisson S, Bostoen K, Curtis V, Fung IC, et al. Water, sanitation and hygiene for the prevention of diarrhoea. International Journal of Epidemiology 2010;39(1):i193‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caulfield (a) 1999
- Caulfield LE, Zavaleta N, Figueroa, A. Adding zinc to prenatal iron and folate supplements improves maternal and neonatal zinc status in a Peruvian population. Am. J. Clin. Nutr 1999;69:1257–1263. [DOI] [PubMed] [Google Scholar]
Checkley 2008
- Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, et al. Multi‐country analysis of the effects of diarrhoea on childhood stunting. International Journal of Epidemiology 2008;37(4):816‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane 2017
- Cochrane Effective Practice, Organisation of Care. Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors, 2017. epoc.cochrane.org/resources/epoc‐resources‐review‐authors (accessed prior to 1 June 2019).
De‐Regil 2011
- De‐Regil LM, Suchdev PS, Vist GE, Walleser S, Peña‐Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008959.pub2] [DOI] [PubMed] [Google Scholar]
De‐Regil 2012
- De‐Regil LM, Jefferds MED, Peña‐Rosas JP. Point‐of‐use fortification of foods with micronutrient powders containing iron in children of preschool and school age. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009666] [DOI] [PMC free article] [PubMed] [Google Scholar]
Development Initiatives 2017
- Development Initiatives. Global nutrition report 2017: nourishing the SDGs. Bristol (UK): Development Initiatives, 2017. [Google Scholar]
Development Initiatives 2018
- Development Initiatives. 2018 Global nutrition report: shining a light to spur action on nutrition. Bristol (UK): Development Initiatives, 2018. [Google Scholar]
Dewey 2011
- Dewey KG, Begum K. Long‐term consequences of stunting in early life. Maternal and Child Nutrition 2011;7:5‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drake 2004
- Drake A, Walker B. The intergenerational effects of fetal programming: non‐genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. Journal of Endocrinology 2004;180:1‐16. [DOI] [PubMed] [Google Scholar]
EPOC 2013
- Cochrane Effective Practice and Organisation of Care (EPOC) Group. EPOC‐specific resources for review authors, 2013. epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed prior to 1 June 2019).
Ernst 2013
- Ernst KC, Phillips BS. Slums are not places for children to live. Advances in Pediatrics 2013;60(1):53‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ezeh 2017
- Ezeh A, Oyebode O, Satterthwaite D, Chen Y, Ndugwa R, Sartori J, et al. The history, geography, and sociology of slums and the health problems of people who live in slums. Lancet 2017;389:547‐58. [DOI: ] [DOI] [PubMed] [Google Scholar]
Fenske 2013
- Fenske N, Burns J, Hothorn T, Rehfuess EA. Understanding child stunting in India: a comprehensive analysis of socio‐economic, nutritional and environmental determinants using additive quantile regression. PloS One 2013;8(11):e78692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frongillo 1999
- Frongillo EA Jr. Introduction. Journal of Nutrition 1999;129(2):529S‐30S. [DOI] [PubMed] [Google Scholar]
Ghosh 2004
- Ghosh S, Shah D. Nutritional problems in urban slum children. Indian Pediatrics 2004;41(7):682‐96. [PubMed] [Google Scholar]
Gluckman 2004
- Gluckman P, Hanson M. Living with the past: evolution, development, and patterns of disease. Science 2004;305:1733‐6. [DOI] [PubMed] [Google Scholar]
Goudet 2011a
- Goudet SM, Griffiths P, Bogin B, Selim NS. Impact of flooding on feeding practices of infants and young children in Dhaka, Bangladesh Slums: what are the coping strategies?. Maternal & Child Nutrition 2011;7(2):198‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goudet 2011b
- Goudet SM, Faiz S, Bogin BA, Griffiths PL. Pregnant women's and community health workers' perceptions of root causes of malnutrition among infants and young children in the slums of Dhaka, Bangladesh. American Journal of Public Health 2011;101(7):1225‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goudet 2017
- Goudet SM, Bogin B, Madise N, Griffiths P. Interventions to tackle malnutrition and its risk factors in children living in slums – a scoping review. Annals of Human Biology 2017;44(1):1‐10. [DOI: 10.1080/03014460.2016.1205660] [DOI] [PubMed] [Google Scholar]
Grantham‐McGregor 2007
- Grantham‐McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B, International Child Development Steering Group. Developmental potential in the first 5 years for children in developing countries. Lancet 2007;369(9555):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed.) 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2010
- Guyatt G, Akl EA, Hirsh J, Kearon C, Crowther M, Gutterman D, et al. The vexing problem of guidelines and conflict of interest: a potential solution. Annals of Internal Medicine 2010;152:738‐41. [DOI: 10.7326/0003-4819-152-11-201006010-00254] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. Rating the quality of evidence – study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [DOI] [PubMed] [Google Scholar]
Haddad 1999
- Haddad L, Ruel MT, Garrett JL. Are urban poverty and undernutrition growing? Some newly assembled evidence. World Development 1999;27(11):1891‐904. [Google Scholar]
Haddad 2014
- Haddad L, Nisbett N, Barnett I, Valli E. Maharashtra's child stunting declines: what is driving them? Findings of a multidisciplinary analysis. Brighton (UK): Institute of Development Studies; 2014.
Haider 2017
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD004905.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harpham 2009
- Harpham T. Urban health in developing countries: what do we know and where do we go?. Health & Place 2009;15(1):107‐16. [DOI: 10.1016/j.healthplace.2008.03.004] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hossain 2017
- Hossain M, Choudhury N, Adib Binte Abdullah K, Mondal P, Jackson AA, et al. Evidence‐based approaches to childhood stunting in low and middle income countries: a systematic review. Archives of Disease in Childhood 2017;102(10):903‐9. [DOI: 10.1136/archdischild-2016-311050] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hussain 1999
- Hussain A, Ali K, Kvale G. Determinants of mortality among children in the urban slums of Dhaka city, Bangladesh. Tropical Medicine & International Health 1999;4(11):758. [DOI] [PubMed] [Google Scholar]
Imdad 2011
- Imdad A, Bhutta ZA. Effect of balanced protein energy supplementation during pregnancy on birth outcomes. BMC Public Health 2011;11(3):S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishaque 2011
- Ishaque S, Yakoob MY, Imdad A, Goldenberg RL, Eisele TP, Bhutta ZA. Effectiveness of interventions to screen and manage infections during pregnancy on reducing stillbirths: a review. BMC Public Health 2011;11(3):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Islam 2018
- Islam MM, Sanin KI, Mahfuz M, Ahmed AS, Mondal D, Haque R, et al. Risk factors of stunting among children living in an urban slum of Bangladesh: findings of a prospective cohort study. BMC public health 2018;18(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keusch 2013
- Keusch GT, Rosenberg IH, Denno DM, Duggan C, Guerrant RL, Lavery JV, et al. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low‐and middle‐income countries. Food & Nutrition Bulletin 2013;34(3):357‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keusch 2014
- Keusch GT, Denno DM, Black RE, Duggan C, Guerrant RL, Lavery JV, et al. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clinical Infectious Diseases 2014;59 Suppl 4:S207‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khara 2014
- Khara T, Dolan C. The relationship between wasting and stunting, policy, programming and research implications, 2014. www.ennonline.net/attachments/1862/WAST_140714.pdf (accessed prior to 1 June 2019).
Kiran 2011
- Kiran B, Banapurmath CR. Influence of RCH programme on nutritional status and immunization status in urban slum children. International Journal of Current Biological and Medical Science 2011;1(4):143‐6. [Google Scholar]
Kuzawa 2005
- Kuzawa C, Pike I. Introduction. Fetal origins of developmental plasticity. American Journal of Human Biology 2005;17:1‐4. [DOI] [PubMed] [Google Scholar]
Kuzawa 2007
- Kuzawa C. Developmental origins of life history: growth, productivity, and reproduction. American Journal of Human Biology 2007;19:654‐61. [DOI] [PubMed] [Google Scholar]
Lamberti 2013
- Lamberti LM, Zakarija‐Grković I, Fischer Walker CL, Theodoratou E, Nair H, Campbell H, et al. Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: a systematic literature review and meta‐analysis. BMC Public Health 2013;13 Suppl 3:S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langford 2011
- Langford R, Lunn P, Panter‐Brick C. Hand‐washing, subclinical infections, and growth: a longitudinal evaluation of an intervention in Nepali slums. American Journal of Human Biology 2011;23(5):621‐9. [DOI: 10.1002/ajhb.21189] [DOI] [PubMed] [Google Scholar]
Lantagne 2014
- Lantagne D. WASH factors contributing to malnutrition, and interventions to reduce their impact. ENN Technical Nutrition Meeting; 2014 Oct 7‐9; Oxford (UK).
Lassi 2013
- Lassi ZS, Salam RA, Haider BA, Bhutta ZA. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD006896.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lilford 2017
- Lilford RJ, Oyebode O, Satterthwaite D, Melendez‐Torres GJ, Chen YF, Mberu B, et al. Improving the health and welfare of people who live in slums. Lancet 2017;389:559‐70. [DOI: ] [DOI] [PubMed] [Google Scholar]
LIST 2014
- LIST. Lives Saved Tool, 2014. list.cherg.org/ (accessed 20 October 2014).
Lundeen 2014
- Lundeen EA, Stein AD, Adair LS, Behrman JR, Bhargava SK, Dearden KA, et al. Height‐for‐age z scores increase despite increasing height deficits among children in 5 developing countries. American Journal of Clinical Nutrition 2014;100(3):821‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madise 1999
- Madise NJ, Matthews Z, Margetts B. Heterogeneity of child nutritional status between households: a comparison of six sub‐Saharan African countries. Population Studies 1999;53(3):331‐43. [Google Scholar]
Mamulwar 2014
- Mamulwar MS, Rathod HK, Jethani S, Dhone A, Bakshi T, Lanjewar B, et al. Nutritional status of under‐five children in urban slums of Pune. International Journal of Medicine and Public Health 2014;4(3):247‐52. [Google Scholar]
Martorell 1994
- Martorell R, Khan LK, Schroeder DG. Reversibility of stunting: epidemiological findings in children from developing countries. European Journal of Clinical Nutrition 1994;48:S45‐57. [PubMed] [Google Scholar]
Mascie‐Taylor 2010
- Mascie‐Taylor CG, Marks MK, Goto R, Islam R. Impact of a cash‐for‐work programme on food consumption and nutrition among women and children facing food insecurity in rural Bangladesh. Bulletin of the World Health Organization 2010;88(11):854‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2013
- McDonald CM, Olofin I, Flaxman S, Fawzi WW, Spiegelman D, Caulfield LE, et al. The effect of multiple anthropometric deficits on child mortality: meta‐analysis of individual data in 10 prospective studies from developing countries. American Journal of Clinical Nutrition 2013;97(4):896‐901. [DOI] [PubMed] [Google Scholar]
Menon 2001
- Menon P, Ruel MT, Morris SS. Socio‐economic differentials in child stunting are consistently larger in urban than in rural areas. Food and Nutrition Bulletin 2001;21(3):282‐9. [Google Scholar]
Mian 2002
- Mian RM, Ali M, Ferroni PA, Underwood P. The nutritional status of school‐aged children in an urban squatter settlement in Pakistan. Pakistan Journal of Nutrition 2002;1(3):121‐3. [Google Scholar]
Milman 2005
- Milman A, Frongillo EA, Onis M, Hwang JY. Differential improvement among countries in child stunting is associated with long‐term development and specific interventions. Journal of Nutrition 2005;135(6):1415‐22. [DOI] [PubMed] [Google Scholar]
Mori 2012
- Mori R, Ota E, Middleton P, Tobe‐Gai R, Mahomed K, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD000230.pub4] [DOI] [PubMed] [Google Scholar]
Neervoort 2013
- Neervoort F, Rosenstiel I, Bongers K, Demetriades M, Shacola M, Wolffers I. Effect of a school feeding programme on nutritional status and anaemia in an urban slum: a preliminary evaluation in Kenya. Journal of Tropical Pediatrics 2013;59(3):165‐74. [DOI] [PubMed] [Google Scholar]
O'Neill 2014
- O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. Journal of Clinical Epidemiology 2014;37(1):56‐64. [DOI: ] [DOI] [PubMed] [Google Scholar]
Olack 2011
- Olack B, Burke H, Cosmas L, Bamrah S, Dooling K, Feikin DR, et al. Nutritional status of under‐five children living in an informal urban settlement in Nairobi, Kenya. Journal of Health, Population, and Nutrition 2011;29(4):357‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olofin 2013
- Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, et al. Associations of suboptimal growth with all‐cause and cause‐specific mortality in children under five years: a pooled analysis of ten prospective studies. PloS One 2013;8(5):e64636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peña‐Rosas 2015
- Peña‐Rosas JP, De‐Regil LM, Garcia‐Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD004736.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pridmore 2007
- Pridmore P, Thomas L, Havemann K, Sapag J, Wood L. Social capital and healthy urbanization in a globalized world. Journal of Urban Health 2007;84(3 Suppl):i130‐43. [DOI: 10.1007/s11524-007-9172-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pridmore 2010
- Pridmore P. Identifying and Tackling the Social Determinants of Child Malnutrition in Urban Informal Settlements and Slums: a Cross National Review of the Evidence for Action (NICK). London (UK): Institute of Education, University of London, 2010. [Google Scholar]
Pryer 2002
- Pryer J, Rogers S, Normand C, Rahman A. Livelihoods, nutrition and health in Dhaka slums. Public Health Nutrition 2002;5(5):613‐8. [DOI] [PubMed] [Google Scholar]
Remans 2011
- Remans R, Pronyk PM, Fanzo JC, Chen J, Palm CA, Nemser B, et al. Millennium Villages Study Group. Multisector intervention to accelerate reductions in child stunting: an observational study from 9 sub‐Saharan African countries. American Journal of Clinical Nutrition 2011;94(6):1632‐42. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richard 2013
- Richard SA, Black RE, Gilman RH, Guerrant RL, Kang G, Lanata CF, et al. Childhood Malnutrition and Infection Network. Diarrhea in early childhood: short‐term association with weight and long‐term association with length. American Journal of Epidemiology 2013;178(7):1129‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruel 1999
- Ruel MT, Haddad L, Garrett JL. Some urban facts of life: implications for research and policy. World Development 1999;27(11):1917‐38. [Google Scholar]
Ruel 2013
- Ruel MT, Alderman H, Maternal and Child Nutrition Study Group. Nutrition‐sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition?. Lancet 2013;382(9891):536‐51. [DOI] [PubMed] [Google Scholar]
Sacco 1999
- Sacco LM, Caulfield LE, Zavaleta N, Retamozo L. Usual mineral intakes of Peruvian women during pregnancy. FASEB 1999;J 13(abs. A250). [DOI] [PubMed] [Google Scholar]
Sguassero 2012
- Sguassero Y, Onis M, Bonotti AM, Carroli G. Community‐based supplementary feeding for promoting the growth of children under five years of age in low and middle income countries. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD005039.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shit 2012
- Shit S, Taraphdar P, Mukhopadhyay DK, Sinhababu A, Biswas AB. Assessment of nutritional status by composite index for anthropometric failure: a study among slum children in Bankura, West Bengal. Indian Journal of Public Health 2012;56(4):305‐7. [DOI] [PubMed] [Google Scholar]
Shrimpton 2001
- Shrimpton R, Victora CG, Onis M, Lima RC, Blössner M, Clugston G. Worldwide timing of growth faltering: implications for nutritional interventions. Pediatrics 2001;107(5):E75. [DOI] [PubMed] [Google Scholar]
Singh 2011
- Singh P. Performance pay and information: reducing child malnutrition in urban slums: MPRA Paper No. 29403, 2011. mpra.ub.uni‐muenchen.de/29403/ (accessed prior to 1 June 2019). [29403]
Spears 2013
- Spears D. How much international variation in child height can sanitation explain? Policy Research working paper ; no. WPS 6351. Washington, DC: World Bank, 2013. documents.worldbank.org/curated/en/449651468191643600/How‐much‐international‐variation‐in‐child‐height‐can‐sanitation‐explain (accessed prior to 1 June 2019).
SPHERE 2017
- SPHERE. Using the sphere standards in urban settings, 2017. www.spherestandards.org/wp‐content/uploads/using‐the‐sphere‐standards‐in‐urban‐settings.pdf (accessed prior to 1 June 2019).
Subramanian 2016
- Subramanian SV, Mejía‐Guevara I, Krishna A. Rethinking policy perspectives on childhood stunting: time to formulate a structural and multifactorial strategy. Maternal & Child Nutrition 2016;12:219‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turley 2013
- Turley R, Saith R, Bhan N, Rehfuess E, Carter B. Slum upgrading strategies involving physical environment and infrastructure interventions and their effects on health and socio‐economic outcomes. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010067.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ueffing 2009
- Ueffing E, Tugwell P, Welch V, Petticrew M, Kristjansson E, for the Cochrane Health Equity Field. Equity checklist for systematic review authors. Version 2009‐05‐28, 2009. www.equity.cochrane.org/files/equitychecklist.pdf (accessed 16 October 2014).
UN‐Habitat 2003
- UN‐Habitat. The challenge of the slums. Global Report on Human Settlements, London (UK): Earthscan Publications Ltd, 2003.
UN‐Habitat 2004
- Un‐Habitat. The challenge of slums: global report on human settlements 2003. Management of Environmental Quality: an International Journal 2004;15(3):337‐8. [Google Scholar]
UN‐Habitat 2017
- UN‐Habitat. Slumc Almanac 2015 2016 Tracking Improvement in the Lives of Slum Dwellers. Technical Report. Nairobi GPO (Kenya): UN‐Habitat, 2017. [Google Scholar]
Unger 2013
- Unger A. Children's health in slum settings. Archives of Disease in Childhood 2013;98(10):799‐805. [DOI] [PubMed] [Google Scholar]
UNICEF 2012
- UNICEF. The state of the world's children 2012: children in an urban world. New York (NY): UNICEF, 2012. [Google Scholar]
UNICEF 2013
- UNICEF. Improving Child Nutrition. The achievable imperative for global progress. New York (NY): UNICEF, 2013. [Google Scholar]
United Nations 2012
- United Nations. The Millennium Development Goals Report 2012. United Nations Report, 2012. www.un.org/millenniumgoals/pdf/MDG%20Report%202012.pdf. United Nations, (accessed prior to 1 June 2019).
Varela‐Silva 2009
- Varela‐Silva MI, Azcorra H, Dickinson F, Bogin B, Frisancho AR. Influence of maternal stature, pregnancy age, and infant birth weight on growth during childhood in Yucatan, Mexico: a test of the intergenerational effects hypothesis. American Journal of Human Biology 2009;21(5):657‐63. [DOI] [PubMed] [Google Scholar]
Varela‐Silva 2012
- Varela‐Silva MI, Dickinson F, Wilson H, Azcorra H, Griffiths PL, Bogin B. The nutritional dual‐burden in developing countries – how is it assessed and what are the health implications?. Collegium Antropologicum 2012;36(1):39‐45. [PubMed] [Google Scholar]
Veiga 2010
- Veiga GR, Ferreira HS, Sawaya AL, Calado J, Florêncio TM. Dyslipidaemia and undernutrition in children from impoverished areas of Maceió, state of Alagoas, Brazil. International Journal of Environmental Research and Public Health 2010;7(12):4139‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Victora 2008
- Victora C, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371(9609):340‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Victora 2010
- Victora CG, Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 2010;125(3):e473‐80. [DOI] [PubMed] [Google Scholar]
Waihenya 1996
- Waihenya EW, Kogi‐Makau W, Muita JWG. Maternal nutritional knowledge and the nutritional status of preschool children in a Nairobi slum. East African Medical Journal 1996;73:419‐23. [PubMed] [Google Scholar]
World Bank 2014
- World Bank. Data. Countries and lending groups. data.worldbank.org/about/country‐and‐lending‐groups (accessed 22 October 2014).
Yakoob 2011
- Yakoob MY, Theodoratou E, Jabeen A, Imdad A, Eisele TP, Ferguson J, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011;11(Suppl 3):S23. [DOI] [PMC free article] [PubMed] [Google Scholar]


