Abstract
We are interested in developing a vaccine that prevents genital herpes. Adjuvants have a major impact on vaccine immunogenicity. We compared two adjuvants, an experimental Merck Sharp & Dohme lipid nanoparticle (LNP) adjuvant, LNP-2, with CpG oligonucleotide combined with alum for immunogenicity in mice when administered with herpes simplex virus type 2 (HSV-2) glycoproteins C, D and E (gC2, gD2, gE2). The immunogens are intended to produce neutralizing antibodies to gC2 and gD2, antibodies to gD2 and gE2 that block cell-to-cell spread, and antibodies to gE2 and gC2 that block immune evasion from antibody and complement, respectively. Overall, CpG/alum was better at producing serum and vaginal IgG binding antibodies, neutralizing antibodies, antibodies that block virus spread from cell-to-cell, and antibodies that block immune evasion domains on gC2. We used a novel high throughput biosensor assay to further assess differences in immunogenicity by mapping antibody responses to seven crucial epitopes on gD2 involved in virus entry or cell-to-cell spread. We found striking differences between CpG/alum and LNP-2. Mice immunized with gD2 CpG/alum produced higher titers of antibodies than LNP-2 to six of seven crucial epitopes and produced antibodies to more crucial epitopes than LNP-2. Measuring epitope-specific antibodies helped to define mechanisms by which CpG/alum outperformed LNP-2 and is a valuable technique to compare adjuvants.
Keywords: Adjuvants, herpes simplex virus, vaccines, epitopes, antibodies, biosensor
1. Introduction
Vaccines to prevent infectious diseases are among the most important public health advances in the past century. The development of new vaccines and improvements in existing vaccines will depend in part on advances in adjuvant technology. Adjuvants have a major impact on reducing the amount of subunit antigen required to produce an immune response while increasing potency [1–5]. Relatively few adjuvants have been approved for use in humans despite the multitude of adjuvants that are in development [6]. Licensed adjuvants include aluminum-based adjuvants (alum), monophosphoryl lipid A (MPL), QS-21, CpG, MF59, and some adjuvant combinations including MPL and alum (referred to as ASO4), and MPL and QS-21 (referred to as ASO1B) [1, 7–10]. Preclinical immunogenicity studies are important to evaluate both efficacy and safety of new adjuvants [11, 12].
CpG oligodeoxynucleotide is an agonist of Toll-like receptor 9 (TLR9) and is a potent inducer of antigen-specific B and T cells [13–15]. CpG was recently approved for use in humans as an adjuvant with hepatitis B surface antigen (HBSAg) [8]. Alum has been widely used as a vaccine adjuvant for approximately 90 years and is a robust inducer of antibodies [1]. In preclinical studies in guinea pigs, we evaluated CpG and alum as an adjuvant for an HSV-2 subunit antigen vaccine containing glycoproteins C and D (gC2 and gD2) [16]. Subsequently, the Friedman laboratory added glycoprotein E (gE2) to gC2 and gD2 with CpG/alum as the adjuvant in a trivalent vaccine [17, 18]. The current study was designed to compare antibody responses using CpG/alum with those using a novel Merck Sharp & Dohme (MSD) lipid nanoparticle (LNP) adjuvant, LNP-2. We focused on antibody responses because antibodies correlate with protection for most successful prophylactic vaccines [19, 20].
LNPs are novel vehicles used to deliver nucleic acids, such as short interfering RNA (siRNA) or modified mRNA and they may also have immune modulating properties [21–25]. In the context of nucleic acid delivery, LNPs are primarily used to effect cellular uptake and endosomal escape of large molecular weight and highly charged cargo, such as siRNA or to deliver TLR9 agonists, such as CpG [22, 26–28]. In these capacities, the focus is on reducing immune stimulating properties of LNPs. LNPs are also potential adjuvants that enhance immunity when co-administered with subunit antigens [29–32]. Some appealing features of LNPs are their ability to form particles that are typically <100nm that are taken up by dendritic cells, and their capacity to induce strong immune responses [33]. Swaminathan et al. evaluated a novel LNP formulation (LNP-6) in C57BL/6 and BALB/c mice comparing LNP-6 alone, a synthetic TLR9 agonist immune-modulatory oligonucleotides (IMO) IMO-2125 alone, or both in combination to enhance immune responses to HBSAg and ovalbumin [28]. The results indicated that LNP-6 alone stimulated potent antibody responses to HBSAg and ovalbumin that was predominantly TH2, and that combining LNP with IMO-2125 shifted the response to TH1. LNP-6 alone stimulated CD4+ and CD8+ antigen-specific T cell responses that were further enhanced by adding IMO-2125. In addition, LNP-6 induced strong B- and T-cell responses when co-administered with tetravalent Dengue virus envelope antigens in rodents and non-human primates [34].
We evaluated whether immunization of mice with CpG/alum or a new LNP formulation, LNP-2, produced antibodies to gC2 and gD2 that neutralized virus, antibodies to gD2 and gE2 that blocked cell-to-cell spread, and antibodies to gE2 and gC2 that blocked immune evasion from antibody and complement [18, 35]. In addition, we used high throughput biosensor technology to assess antibody responses to crucial gD2 epitopes involved in virus entry and cell-to-cell spread. The results of the high throughput biosensor technology helped define the mechanisms by which CpG/alum outperformed LNP-2.
2. Materials and methods
2.1. Murine ethics statements
Female C57BL/6 mice studies were performed in accordance with protocol No. 805187 approved by The Institutional Animal Care and Use Committee of the University of Pennsylvania. The protocol followed recommendations in the Institute for Laboratory Animals Research’s “Guide for the Care and Use of Laboratory Animals.” At the end of the experiment, mice were deeply anesthetized with ketamine and xylazine and terminally bled by cardiac puncture. Animals were observed until breathing and heartbeat were no longer detected. These methods are consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
2.2. Immunizations
The subunit antigens in this study were purified from baculovirus-infected sf9 cells and consist of bac-gC2(426t), bac-gD2(306t) and bac-gE2(24–405t) [17]. Seven groups of C57BL/6 mice with 10 animals per group were immunized three times at 2-week intervals with 50μl into each hind limb quadriceps containing: 1) Antigen alone: all three subunit antigens (gC2, gD2 and gE2) were used at 5μg each and mixed with PBS instead of an adjuvant; 2) gC2 LNP-2: LNP-2 adjuvant was used at 125μg mixed with 5μg gC2; 3) gD2 LNP-2: 125μg LNP-2 was mixed 5μg gD2; 4) gE2 LNP-2: 125μg LNP-2 was mixed with 5μg gE2; 5) gC2 CpG: 50μg CpG and 125μg alum were mixed with 5μg gC2; 6) gD2 CpG: 50μg CpG and 125μg alum were mixed with 5μg gD2; and 7) gE2 CpG: 50μg CpG and 125μg alum were mixed with 5μg gE2. Two weeks after the third immunization, all mice were terminally bled. This study was designed to assess immunogenicity.
2.3. Formulation of LNP-2 and CpG/alum adjuvants
LNP-2 was prepared by rapid precipitation, as previously described [27, 28]. The lipid components of LNP-2 include (2S)-1-({6-[(3β))-cholest-5-en-3-yloxy]hexyl}oxy)-N,N,- dimethyl1–3-[(9Z)-octadec-9-en-1yloxyl]propan-2-amine, distearoylphosphatidylcholine, cholesterol, and poly(ethylene glycol)2000-dimyristoylglycerol in a molar ratio of 58:30:10:2, respectively. The antigens were co-mixed with 125μg LNP-2 or 50μg CpG oligonucleotide TCCATGACGTTCCTGACGTT (Coley Pharmaceutical) and 125μg alum (Alhydrogel; Accurate Chemical and Scientific Corp.) immediately before injection into mice. The antigens and adjuvants were diluted in a final volume of 100μl and animals were immunized with 50μl in each hind limb.
2.4. Antibody assays
All antibody studies were performed using similar concentrations of serum or purified IgG when comparing CpG/alum with LNP-2.
2.4.1. IgG ELISA assays on sera or vaginal wash fluids
Vaginal wash fluids were collected just prior to euthanizing the mice. 20μl of PBS was introduced into the vaginal cavity and immediately retrieved using a micropipette. This procedure was repeated and the fluids pooled for each mouse. Purified bac-gC2(426t), bac-gD2(306t) or bac-gE2(24–405t) were added to 96-well High Binding Costar microtiter plates (Corning Incorporated, Corning, NY). Sera were added at a 1:1000 dilution in PBS 0.05% Tween 20 (PBST) or vaginal wash fluids were added at a 1:200 dilution and bound IgG was detected [18, 36].
ELISA assays to detect IgG1 and IgG2a isotypes:
The assays were performed using 100ng of each glycoprotein antigen. Mouse serum was added at a 1:1000 dilution and bound IgG was detected using rat anti-mouse IgG1 or IgG2a (BD Pharmingen) at 1:2000.
2.4.2. Neutralizing antibody assays
Serum was incubated at 56°C for 30 min to inactivate complement. Serial serum dilutions starting at 1:20 were incubated with 100 PFU of HSV-2 strain MS and plaques counted in Vero cells [16]. The endpoint neutralization titer was considered the dilution that reduced plaque numbers by ≥50% compared with PBS controls.
2.4.3. gC2 blocking assay measured by ELISA
Wells of a 96-well High Binding Costar microtiter plate were coated with 200ng/well of purified C3b in sodium bicarbonate binding buffer (pH 8.5) for 1 h at RT, overnight at 4°C, and blocked for 2 h at RT with 5% (wt/vol) nonfat milk in PBST [18]. 10μl of serum from each mouse in the group was pooled and the IgG was purified in a Protein G Spin Plate (Thermo Scientific, Pierce Biotechnology). Pooled sera was used rather than evaluating each serum separately because insufficient serum was available to purify IgG from individual animals. Purified pooled murine IgG at 50μg/ml was incubated with 50ng gC2(426t) for 1 h at 37°C and added to C3b-coated wells for 1 h. Bound gC2 was detected with rabbit anti-gC2 serum. The OD 405nm reading obtained with antigen alone was set at a relative value of 1 and the OD readings in the other groups were adjusted in comparison with the antigen alone group. This adjustment enabled us to compare C3b binding values obtained in assays run on different days.
2.4.4. gE2 blocking assays
IgG that blocked the interaction of gE2 with IgG Fc was evaluated by a capture ELISA [35]. Human IgG from an HSV-1/HSV-2 seronegative subject was used to coat ELISA wells at 1μg IgG/well. Murine IgG was purified and pooled as above, and 50μg/ml was incubated with 400ng gE2(24–405t) for 1 h at 37°C and added to IgG coated wells for 1 h. Bound gE2 was detected with rabbit anti-gE2 serum. The OD 405nm reading obtained with antigen alone was set at a relative value of 1, as noted for C3b binding.
2.4.5. Cell-to-cell spread assays
Cell-to-cell spread assays were performed by infecting Vero cells with approximately 100 PFU of HSV-2 for 1 h. Cells were acid washed with citrate buffer pH 3.0 for 1 min followed by several washes with media pH 7.2. A 1:40 dilution of mouse sera was added for 4 h at 37°C, then removed and followed immediately by adding a 1:40 dilution of mouse serum in a methylcellulose overlay to prevent cell-free virus from spreading in the supernatant fluids [17]. Plaque size was determined at 72 h using an inverted light microscope fitted with an eyepiece micrometer [37]. Plaque size was measured for plaques in one quadrant of the plate (approximately 25 plaques), with measurements extending into additional quadrants if fewer than 25 plaques were present in the initial quadrant. This assay is designed to measure antibodies that block cell-to-cell spread without requiring the antibodies to block virus entry as measured in the neutralizing antibody assay.
2.4.6. Antibody responses to crucial gD2 epitopes
Assays were performed using the Carterra Microfluidics Continuous Flow Microspotter surface plasmon resonance imaging (CFM/SPRi) system [35]. Briefly, 21 gD2 MAbs representing seven communities/subcommunities were amine-coupled to a CDM200M sensor chip (XanTec GmbH) in a 96-spot format that was placed in the SPR imager (IBIS MX96), blocked with ethanolamine, and primed with running buffer (PBS-0.01% Tween 20). Antibody competitions were performed by incubating 75ng of soluble gD2(285t) with 4μl of mouse serum in 200μl total volume and flowing the gD2:mouse serum mix across the gD2 MAbs plated on the biosensor chip. After each flow through of the gD2(285t):mouse serum mix, the chip surface was regenerated using 10mM glycine pH 2.0. Every 10th cycle gD2 without mouse serum was flowed across the chip and the RU value was used to reset the background binding of gD2 alone for comparison with the gD2:mouse serum mix. The blocking activity of the mouse serum was calculated for each MAb as a percentage using the formula: [1-(RU gD2(285t) + mouse serum)/(RU gD(285t) alone)]*100%. We used gD2(285t) for gD2 epitope mapping studies despite immunizing mice with gD2(306t) based on MAbs in one community (MAb 77S) binding better to gD2(285t) than to gD2(306t) [38].
2.5. Statistical analysis
The two-tailed Mann-Whitney test was used to determine statistical significance comparing two groups. The two-tailed Fisher’s exact test was used to evaluate proportional differences between variables. A two-tailed, one-sample t-test adjusted for a hypothetical value of 1 was used to compare two groups when one group showed no variability. Results were considered significant at p<0.05.
3. Results
HSV-2 gC2, gD2, or gE2 subunit antigen was mixed with LNP-2 or CpG/alum and used to immunize 10 mice/group three times at two-week intervals (total six groups). An additional group of 10 mice served as a control and contained all three immunogens, gC2, gD2 and gE2 in PBS without an adjuvant. Two weeks after the third immunization animals were terminally bled and vaginal wash fluids obtained for antibody studies.
3.1. Serum and vaginal mucosal antibody responses
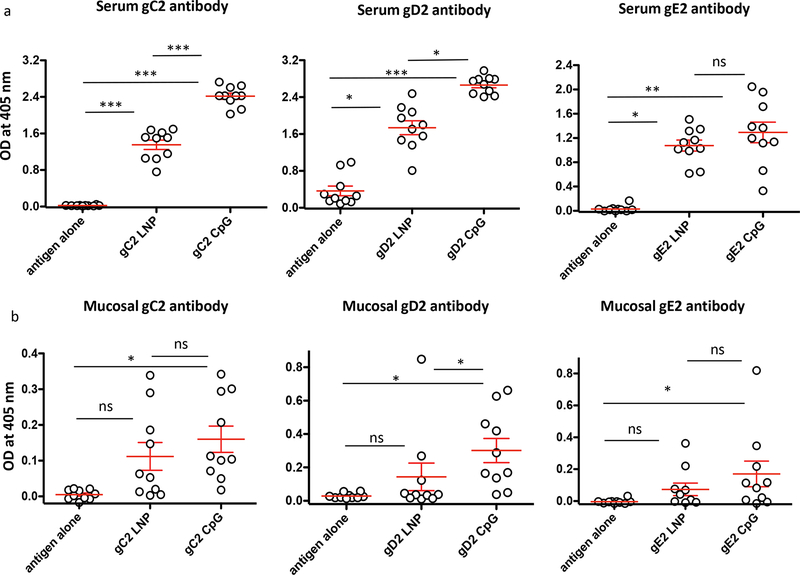
Sera were evaluated at a 1:1000 dilution. CpG/alum produced significantly higher IgG titers than LNP-2 to gC2 and gD2. The titers were also higher to gE2; however, the differences did not reach statistical significance (Fig 1a). Statistically significant differences were detected between groups that were immunized with antigen alone or with either LNP-2 or CpG/alum (Fig 1a). Mucosal IgG titers were performed at a 1:200 dilution of vaginal wash fluids. CpG/alum produced significantly higher vaginal IgG titers to gD2. Titers were also higher to gC2 and gE2, but did not reach statistical significance (Fig 1b). Compared to antigen alone, CpG/alum produced significantly higher mucosal antibody titers to each of the three antigens, while LNP-2 did not (Fig 1b). We conclude that CpG/alum outperformed LNP-2.
Figure 1. Serum and vaginal wash fluid IgG ELISA titers.
(a) Sera and (b) vaginal wash fluids were tested for IgG binding to gC2, gD2, or gE2. Each individual mouse is shown. Error bars represent SEM. Note that the Y-axis scale varies in these figures. *, p<0.05; **, p<0.01; ***, p<0.001; ns, not significant. P values were determined by the two-tailed Mann-Whitney test.
3.2. Serum TH1 and TH2 responses
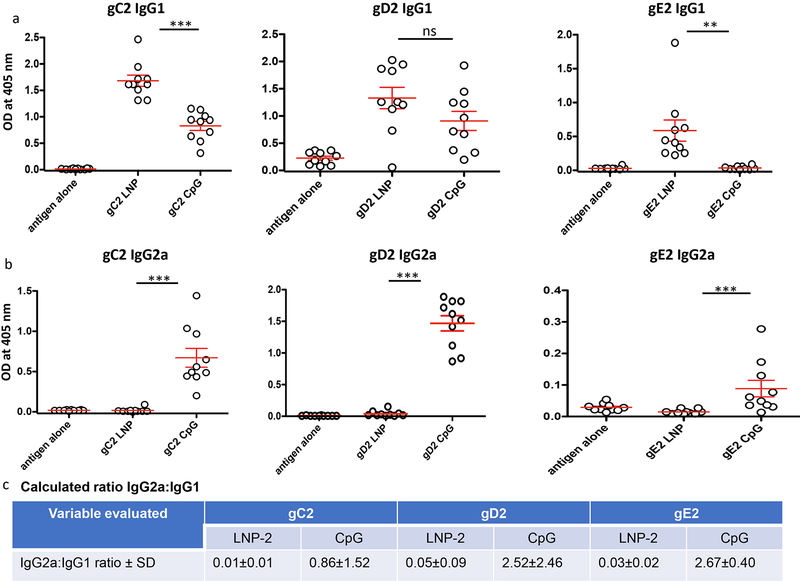
We evaluated the serum IgG2a and IgG1 antigen-specific responses to gC2, gD2 and gE2. The ratio of IgG2a to IgG1 is a marker of a TH1 (pro-inflammatory, cell mediated) or TH2 (anti-inflammatory, humoral) immune response. A ratio of IgG2a:IgG1 of >2.0 suggests a predominant TH1 response, while a ratio of <0.5 represents a predominant TH2 response. Ratios between 0.5 and 2.0 are considered balanced TH1:TH2 response [39]. HSV-2 gC2 LNP-2, gD2 LNP-2, and gE2 LNP-2 produced antigen-specific IgG1 but very little IgG2a (Figs 2a, b) consistent with a TH2 response (Fig 2c). In contrast, CpG/alum produced a more balanced IgG2a:IgG1 response (Figs 2 a–c). The optimum response has not been determined for an HSV-2 subunit antigen vaccine, although a balanced response is considered desirable [40].
Figure 2. Serum IgG1 and IgG2a titers.
Serum was diluted 1:1000 and tested by ELISA for (a) IgG1 or (b) IgG2a binding to gC2, gD2 and gE2. Each individual mouse is shown. Error bars represent SEM. Note that the Y-axis scale varies in these figures. **, p<0.01; ***, p<0.001; ns, not significant. P values were determined by the two-tailed Mann-Whitney test. (c) Table showing the calculated ratio of IgG2a:IgG1 based on results shown in (a-b).
3.3. Antibodies that block immune evasion domains on gC2 and gE2
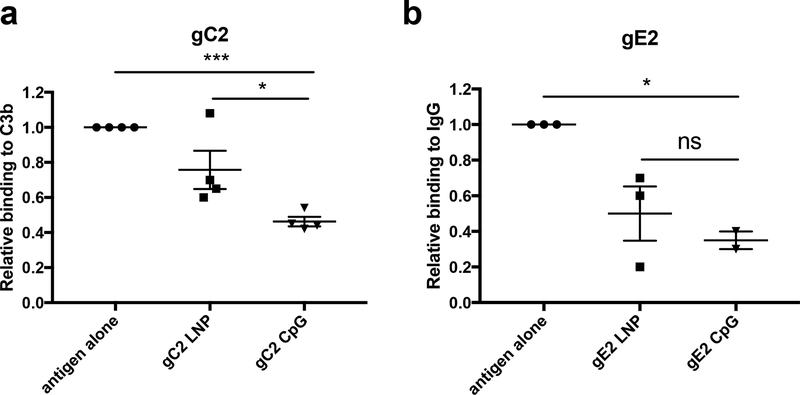
Antibodies to gC2 and gE2 produced by immunization block immune evasion domains on gC2 that bind complement component C3b and on gE2 that bind the Fc domain of IgG [16–18, 41]. Blocking immune evasion domains is part of our rationale for including gC2 and gE2 in the trivalent subunit antigen vaccine. Sera were evaluated from mice immunized with antigen alone, gC2 LNP-2, or gC2 CpG/alum. IgG was purified from 100μl of pooled sera using 10μl from each of 10 mice/group. Antibodies produced by gC2 CpG/alum immunization blocked C3b binding to gC2 significantly better than antibodies produced by antigen alone or by gC2 LNP-2 (Fig 3a).
Figure 3. Blocking C3b binding to gC2 and IgG Fc binding to gE2.
Sera were pooled from 10 mice per group, IgG was purified and 50μg/ml was tested for (a) blocking gC2 binding to C3b or (b) blocking gE2 binding to IgG Fc. Results shown are the mean and SEM of 4 determinations for (a) and 2–3 for (b). P values comparing LNP with CpG were performed using the two-tailed Mann-Whitney test, while P values comparing antigen alone with LNP or CpG were calculated using a two-tailed one-sample t-test adjusted for a hypothetical value of 1. *, p<0.05; ***, p<0.001; ns, not significant.
Similar studies were performed to compare gE2 LNP-2 and gE2 CpG/alum. Differences were not significant comparing gE2 LNP-2 and gE2 CpG/alum for blocking IgG Fc binding to gE2, although gE2 CpG, but not gE2 LNP-2 blocked IgG Fc binding better than antigen alone (Fig 3b). We conclude that CpG/alum outperformed LNP-2 in blocking gC2 immune evasion domains, and although differences between CpG/alum and LNP-2 were not significant for blocking gE2 immune evasion domains, the results indicate that CpG/alum was more effective.
3.4. Antibodies that block HSV-2 cell-to-cell spread
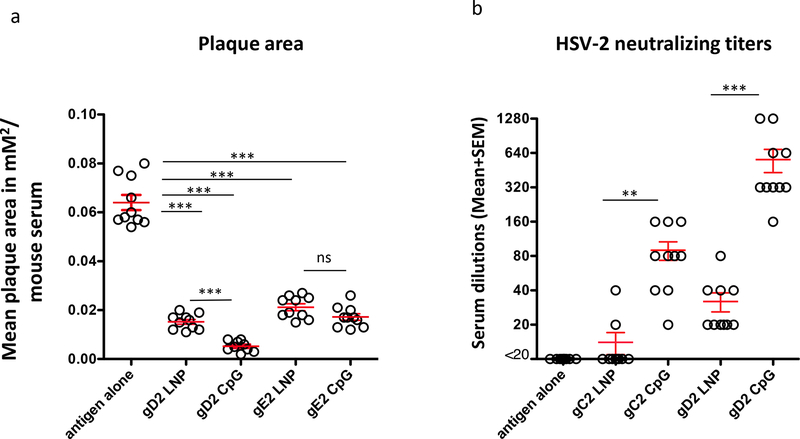
Both gD2 and gE2 promote virus spread from cell-to-cell, and antibodies to each glycoprotein block cell-to-cell spread resulting in small plaques [17, 35, 42–44]. Virus was mixed with a 1:40 dilution of serum from each mouse. Plaque size was determined for 25 plaques per serum and plotted as the average plaque size for each animal (Fig 4a). The smallest plaques were detected in the gD2 CpG/alum group. These plaques were significantly smaller than those in the gD2 LNP-2 group. No significant differences were detected comparing gE2 LNP-2 with gE2 CpG/alum, although the CpG group had smaller plaques than the LNP group (p=0.075). Each group immunized with LNP-2 or CpG/alum had smaller plaques than the antigen alone group (Fig 4a).
Figure 4. Plaque size and serum neutralizing antibody titers.
(a) Plaque size as a marker of cell-to-cell spread: The average plaque size of approximately 25 plaques was plotted for each mouse serum. ***, p<0.001; ns, not significant. (b) Neutralizing antibody titers in gC2- and gD2-immunized mice: Sera from mice (n=10/group) immunized with antigen alone without adjuvant, gC2 LNP-2, gC2 CpG/alum, gD2 LNP-2 and gD2 CpG/alum were tested for neutralizing activity in the absence of complement. Each individual mouse is shown. **, p<0.01; ***, p<0.001. For (a-b), error bars represent SEM, and P values were determined by the two-tailed Mann-Whitney test.
3.5. Neutralizing antibody responses
Antibodies to gC2 or gD2 neutralize virus [18, 35]. We compared neutralizing antibody responses to gC2 or gD2 in the absence of complement. Neutralizing titers produced by gD2 or gC2 CpG/alum were significantly higher than gD2 or gC2 LNP-2 (Fig 4b). We conclude that the adjuvant has a major impact on the neutralizing activity of gC2 and gD2 antigens.
3.6. Antibodies that block crucial gD2 epitopes involved in neutralization or cell-to-cell spread
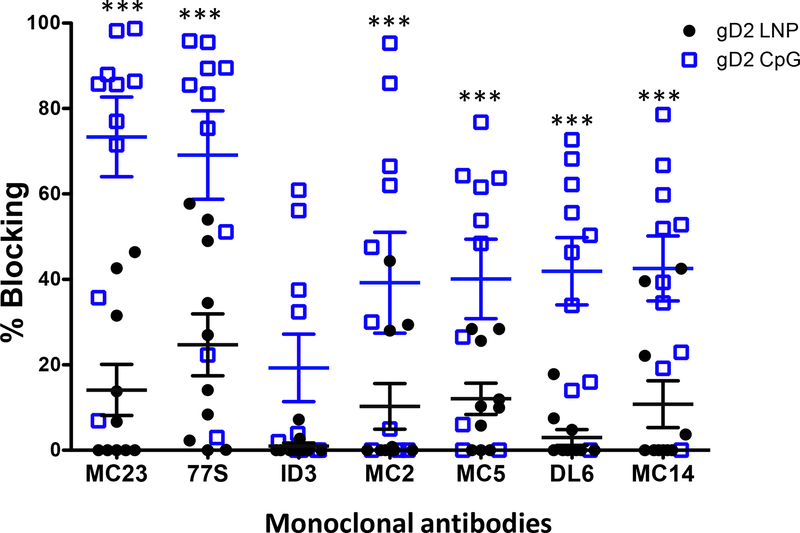
We reported that protection against intravaginal HSV-2 infection in guinea pigs immunized with gD2 CpG/alum correlated with antibodies that bind to crucial gD2 epitopes involved in virus attachment to HVEM and nectin-1 receptors, activation of downstream entry molecules gH2/gL2, and cell-to-cell spread [35]. The greater the number of crucial epitopes bound, the better the protection was against genital herpes. The assay to measure epitope-specific antibodies uses a high throughput biosensor platform in which monoclonal antibodies (MAbs) are coupled to a biosensor chip. HSV-2 gD2 antigen is incubated with mouse serum and the gD2/serum mix is floated over the MAbs on the biosensor chip. Binding of gD2 to a MAb on the biosensor chip indicates that the mouse serum did not contain antibody to the epitope recognized by the MAb, while decreased or no binding of gD2 indicates that antibody was produced and competitively inhibited gD2 binding to the MAb [38, 45]. The higher the titer of the antibody and/or the higher the avidity, the greater the percent blocking of gD2 binding to the MAb on the biosensor chip.
We evaluated 21 MAbs that are grouped into seven communities/subcommunities [35, 38]. Results are shown for prototype MAbs from each of these seven communities/subcommunities (Fig 5). The prototype MAbs are representative of the results obtained with all 21 MAbs. Prototype MAbs include MAb MC23 that blocks entry by the nectin-1 receptor, MAb 77S that blocks entry by both the nectin-1 and HVEM receptors, MAb 1D3 that blocks entry by the HVEM receptor, MAbs MC2 and MC5 that are hypothesized to block gD2 activation of gH2/gL2, and MAbs DL6 and MC14 that block cell-to-cell spread [35, 38, 46–48]. More mice immunized with CpG/alum than LNP-2 produced antibodies to each of the crucial epitopes identified by the seven prototype MAbs, and the titers of antibodies were higher in mice immunized with CpG/alum based on greater blocking of gD2 binding to the MAbs (Fig 5). CpG/alum significantly outperformed LNP-2 for blocking gD2 binding to 6 of 7 prototype antibodies. The only exception was 1D3 where differences favored CpG/alum but did not reach statistical significance (p=0.10).
Figure 5. Antibodies in mouse immune serum that bind to crucial gD2 epitopes and block gD2 binding to MAbs.
Blocking gD2 binding to seven prototype MAbs on a biosensor chip by each mouse serum. Sera were tested once. Error bars represent SEM. ***, p<0.001. P values were calculated by the two-tailed Mann-Whitney test.
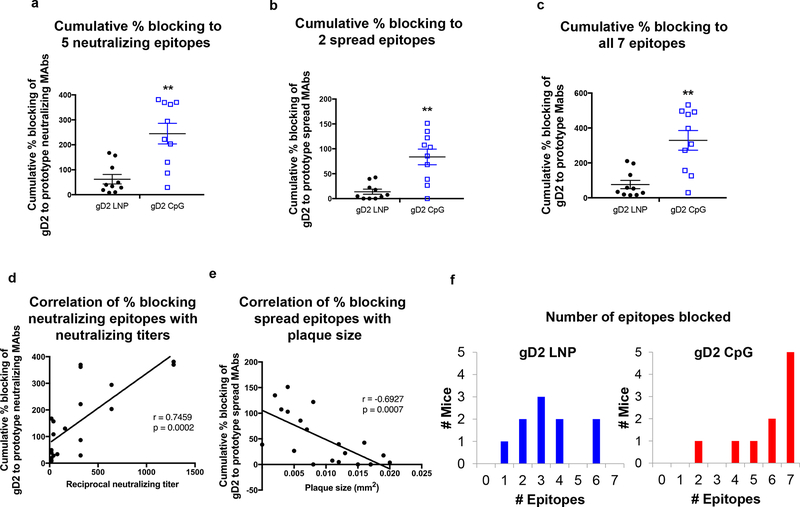
We calculated the cumulative percent blocking by each mouse serum of gD2 binding to five neutralizing epitopes represented by MAbs MC23, 77S, 1D3, MC2 and MC5 (Fig 6a), or to two cell-to-cell spread epitopes identified by MAbs DL6 and MC14 (Fig 6b), or to all seven crucial epitopes (Fig 6c) [35]. CpG/alum significantly outperformed LNP-2 in each analysis. We assessed the correlation between gD2 antibodies that block neutralizing epitopes and neutralizing titers (Fig 6d) or gD2 antibodies that block cell-to-cell spread epitopes and plaque size (Fig 6e). Neutralizing antibody titers correlated with the cumulative percent blocking of gD2 binding to MAbs that recognize neutralizing epitopes (p=0.0002) (Fig 6d), while plaque size correlated with the cumulative percent blocking of gD2 binding to MAbs that recognize cell-to-cell spread epitopes (p=0.0007) (Fig 6e). We evaluated the number of crucial epitopes blocked by each mouse in the gD2 LNP-2 group or the gD2 CpG/alum group (Fig 6f). The mean number of epitopes blocked per mouse was significantly higher in the gD2 CpG/alum group at 5.8 ± 1.7 epitopes compared with gD2 LNP-2 that produced antibodies that blocked 3.4 ± 1.6 epitopes (p=0.008) (Fig 6f). We conclude that antibodies produced to gD2 epitopes involved in virus entry or cell-to-cell spread help to define the mechanisms by which CpG/alum outperformed LNP-2 in producing gD2 neutralizing antibodies and small plaques.
Figure 6. Blocking gD2 binding by prototype MAbs in each community and subcommunity.
(a) Cumulative percent blocking of gD2 binding by each mouse serum to (a) five prototype MAbs that bind to neutralizing epitopes, (b) two prototype MAbs that bind to cell-to-cell spread epitopes, and (c) all seven prototype MAbs that bind to neutralizing or cell-to-cell spread epitopes. Error bars in (a–c) represent SEM. (d) Correlation of neutralizing antibody titers of mice immunized with gD2 LNP-2 (n=10) or gD2 CpG/alum (n=10) with the cumulative percent blocking of antibodies produced to the five neutralizing epitopes. (e) Correlation of plaque size of mice immunized with gD2 LNP-2 (n=10) or gD2 CpG/alum (n=10) with the cumulative percent blocking of antibodies produced to the two cell-to-cell spread epitopes. (f) The number of epitopes blocked by mice in the gD2 LNP-2 or gD2 CpG/alum group. **, p<0.01; P values for (a-c) were calculated by the two-tailed Mann-Whitney test, (d–e) were calculated using Spearman’s correlation, and (f) by the two-tailed Fisher’s exact test (p=0.008).
4. Discussion
Measuring epitope-specific antibody responses is a powerful approach to assess adjuvants. An impressive difference emerged when we evaluated antibody responses to seven crucial gD2 epitopes involved in virus entry and cell-to-cell spread. CpG/alum produced significantly higher titers of antibodies that blocked six of seven crucial gD2 epitopes. Four of these epitopes are conformational, including those recognized by MAbs MC23, 77S, MC2 and MC5, while three are linear, 1D3, DL6, and MC14 [35]. Antibody responses to both conformational and linear epitopes were reduced in the LNP-2-immunized animals, suggesting that disrupting gD2 conformation is not sufficient to explain the reduced antibody responses [35, 38].
Different mechanisms of action of the adjuvants likely explain the greater breadth of epitope responses produced by CpG/alum. CpG is a TLR9 agonist while alum triggers the NLRP3 pathway [13–15, 49]. The combination generally induces a balanced TH1:TH2 response through a MyD88/IL-10 dependent pathway [50]. LNP-based adjuvants typically result in TH2-type immunity [28]. Our internal investigations and published evidence suggest that cationic nanoparticles/nanocarriers like LNP-2 may activate the TLR2 or TLR4 pathways [51, 52]. Our results suggest that the TLR9 and NLRP3 pathways stimulated by CpG and alum function as more potent adjuvants for the gC2, gD2 and gE2 antigens compared to TLR2 and/or TLR4 pathways that may potentially be activated by LNP-2. Although not measured here, it seems likely that CpG/alum induced more potent CD4 T helper cell responses than LNP-2 to account for enhanced antibody responses.
It is possible that the antibody responses to CpG/alum or LNP-2 may vary depending on whether the antigens are produced in baculovirus, as in our studies, or in mammalian cells because different glycosylation patterns may impact immune responses [53]. It is also possible that different concentrations or dosing intervals for LNP-2 may have yielded higher antibody titers. The LNP-2 dose of 125ug was chosen based on the our published studies and work in-progress using LNP-based vaccine formulations. In those studies, 125μg of LNP produced significant antibody responses against HBSAg, ovalbumin, and Dengue envelope antigens [28, 32, 34]. The majority of the murine vaccine studies conducted by the authors with LNP-based adjuvants used a two- or three-dose regimen separated by two weeks. The murine studies by the Friedman laboratory with CpG/alum and HSV-2 subunit proteins used a three-dose regimen, two weeks apart [16, 17]. We chose 125ug of LNP-2 adjuvant and a three- dose regimen every two weeks as a reasonable strategy for head-to-head comparisons between LNP-2 and CpG/alum adjuvants. LNP-2 was chosen as an adjuvant for this study (rather than our previously evaluated LNP-6 adjuvant) because LNP-2 and LNP-6 demonstrated comparable adjuvant properties in our internal unpublished murine studies. LNP-2 has not been evaluated as extensively as LNP-6. CpG/alum was superior to LNP-2 in this study; however, LNP formulations may vary widely from one another and it is possible that other LNP adjuvants may have different modes-of-action with divergent effects on antibody responses.
We previously reported that the more crucial gD2 epitopes blocked, the better the protection was against intravaginal challenge in gD2-immunized guinea pigs [35]. We also performed antibody passive transfer studies in mice to demonstrate that antibodies to each of seven crucial epitopes protected against subsequent genital challenge with HSV-2 [35]. The current study was not designed to compare protection after genital challenge. Based on our prior challenge results in guinea pigs and mice, the significant differences in epitope responses comparing LNP-2 and CpG/alum are likely to translate into differences in protection.
We have now evaluated epitope-specific antibody responses in mice and guinea pigs immunized with the same gD2 subunit antigen, bac-gD2(306t) with CpG/alum as adjuvants [35]. Nine of ten (90%) mice produced antibodies that competed with MAb MC14 that blocks cell-to-cell spread, compared with 3/24 (13%) guinea pigs, and 7/10 (70%) mice produced antibodies that competed with MC2 that is proposed to interfere with the interaction between gD2 and gH2/gL2, compared with 8/25 (32%) guinea pigs [35, 48]. The mean number of epitopes blocked in mice was 5.8 ± 1.7 compared with 4.2 ± 1.3 in guinea pigs (p=0.007 by Mann-Whitney) [35]. We hypothesize that the more potent epitope-specific responses noted in mice may help explain why gD2 immunized mice appear to be easier to protect against genital herpes than gD2 immunized guinea pigs [17, 18]. C57BL/6 mice are inbred while Hartley strain guinea pigs are outbred, which may, in part explain the species differences that have emerged. Future studies using outbred mice or inbred guinea pigs may help to clarify the impact of genetics on epitope responses.
We previously reported epitope-specific antibody responses in subjects that were enrolled in the Herpevac Trial for Women [45, 54]. These women received an experimental GSK gD2 vaccine administered with MPL/alum. The human studies are not directly comparable to the mice and guinea pig studies in that a 281 amino acid fragment of gD2 was used in the human studies compared with 306 amino acids in mice and guinea pigs, and MPL/alum was the adjuvant in the human studies compared with CpG/alum in mice and guinea pigs. All the epitopes evaluated in the animal and human studies are included in the 281 and 306 gD2 amino acid fragments used for the human and animal vaccine trials. Antibodies were produced to a mean of 2.9 epitopes in the human studies compared to 5.8 in mice and 4.2 in guinea pigs [35]. The poor response to crucial epitopes in immunized humans may explain why humans are difficult to protect using an HSV-2 vaccine, particularly if these species differences are reproduced using identical antigens and adjuvants in humans and outbred animals.
Important immune responses to evaluate in Phase I human trials with gC2, gD2 and gE2 antigens include serum and mucosa IgG antibodies, serum neutralizing antibodies, antibodies that block cell-to-cell spread, antibodies that block immune evasion domains, and antibodies to crucial epitopes. We intend to expand the analysis of epitope-specific antibody responses to include epitopes on gC2 and gE2. This expansion requires a large panel of gC2 and gE2 MAbs that can be arranged into communities, and then determining the crucial gC2 and gE2 functions blocked by the MAbs [35, 38]. Information learned about epitope-specific antibody responses will then be used to identify gaps in immune responses to crucial epitopes. For example, our current results indicate that gC2 CpG/alum produced higher titers of antibodies that blocked C3b binding than gC2 LNP-2. We will better understand the deficiencies in the gC2 LNP-2 antibody profile once we are able to measure antibody responses to crucial gC2 epitopes that bind C3b. One method to avoid gaps in antibody responses is to modify epitopes that are weakly immunogenic. Our current study suggests that another approach to avoid gaps is to modify adjuvants.
5. Conclusions
Antibody responses to crucial gD2 epitopes involved in virus entry and cell-to-cell spread helped explain differences between gD2 CpG/alum and gD2 LNP-2 in producing neutralizing antibodies and small plaques. Epitope mapping is a powerful new tool to compare antibody responses produced by different adjuvants.
Acknowledgments
We thank John Lambris, Department of Pathology at the University of Pennsylvania for providing C3b and Sarah Ratcliffe and Pamela Shaw from the Center for AIDS Research Biostatistical Core at the University of Pennsylvania for assistance with the biostatistical analysis. We also thank Dai Wang and Lan Zhang from Merck & Co. Inc., for their thoughtful comments on the manuscript.
Funding statement
This work was supported by the National Institutes of Health grants number R21AI105959 (Awasthi and Friedman co-PI), RO1AI104854 (Friedman PI) and RO1AI18289 (Cohen PI). The funding source had no role in the study design, collection of data, analysis or interpretation of data, in writing the manuscript, or in the decision to submit the article for publication.
Abbreviations
- HSV-2
herpes simplex virus type 2
- gC2, gD2, gE2
HSV-2 glycoproteins C, D or E
- LNP
lipid nanoparticle
- MPL
monophosphoryl lipid A
- HBSAg
hepatitis B surface antigen
- TLR9
toll-like receptor 9
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
☐ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
☒ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
GS, JSS, MEG, AJB and ASE are employees and stockholders of Merck & Co., Kenilworth, NJ, USA. BB and NTD are employees of Carterra Inc. and have a financial interest in the company. SA, GHC and HMF have patent applications for HSV vaccines. LMH and TMC have no competing interests.
References
- [1].Kool M, Fierens K, Lambrecht BN. Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Microbiol. 2012;61:927–34. [DOI] [PubMed] [Google Scholar]
- [2].Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–608. [DOI] [PubMed] [Google Scholar]
- [4].O’Hagan DT, Fox CB. New generation adjuvants--from empiricism to rational design. Vaccine. 2015;33 Suppl 2:B14–20. [DOI] [PubMed] [Google Scholar]
- [5].Fox CB, Haensler J. An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev Vaccines. 2013;12:747–58. [DOI] [PubMed] [Google Scholar]
- [6].Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin Immunol. 2018. [DOI] [PubMed] [Google Scholar]
- [7].Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–32. [DOI] [PubMed] [Google Scholar]
- [8].Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the Advisory Committee on Immunization Practices for Use of a Hepatitis B Vaccine with a Novel Adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kensil CR, Kammer R. QS-21: a water-soluble triterpene glycoside adjuvant. Expert Opin Investig Drugs. 1998;7:1475–82. [DOI] [PubMed] [Google Scholar]
- [10].Lecrenier N, Beukelaers P, Colindres R, Curran D, De Kesel C, De Saegher JP, et al. Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Rev Vaccines. 2018;17:619–34. [DOI] [PubMed] [Google Scholar]
- [11].Batista-Duharte A, Martinez DT, Carlos IZ. Efficacy and safety of immunological adjuvants. Where is the cut-off? Biomed Pharmacother. 2018;105:616–24. [DOI] [PubMed] [Google Scholar]
- [12].Sulczewski FB, Liszbinski RB, Romao PRT, Rodrigues Junior LC. Nanoparticle vaccines against viral infections. Arch Virol. 2018. [DOI] [PubMed] [Google Scholar]
- [13].Klinman DM, Klaschik S, Sato T, Tross D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv Drug Deliv Rev. 2009;61:248–55. [DOI] [PubMed] [Google Scholar]
- [14].Tross D, Klinman DM. Effect of CpG oligonucleotides on vaccine-induced B cell memory. Journal of immunology. 2008;181:5785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wilson HL, Dar A, Napper SK, Marianela Lopez A, Babiuk LA, Mutwiri GK. Immune mechanisms and therapeutic potential of CpG oligodeoxynucleotides. Int Rev Immunol. 2006;25:183–213. [DOI] [PubMed] [Google Scholar]
- [16].Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, et al. Immunization with a Vaccine Combining Herpes Simplex Virus 2 (HSV-2) Glycoprotein C (gC) and gD Subunits Improves the Protection of Dorsal Root Ganglia in Mice and Reduces the Frequency of Recurrent Vaginal Shedding of HSV-2 DNA in Guinea Pigs Compared to Immunization with gD Alone. J Virol. 2011;85:10472–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Awasthi S, Huang J, Shaw C, Friedman HM. Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes. J Virol. 2014;88:8421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Awasthi S, Hook LM, Shaw CE, Pahar B, Stagray JA, Liu D, et al. An HSV-2 Trivalent Vaccine Is Immunogenic in Rhesus Macaques and Highly Efficacious in Guinea Pigs. PLoS Pathog. 2017;13:e1006141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Iwasaki A Exploiting Mucosal Immunity for Antiviral Vaccines. Annual review of immunology. 2016;34:575–608. [DOI] [PubMed] [Google Scholar]
- [20].Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, et al. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis. 2014;209:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lin Q, Chen J, Zhang Z, Zheng G. Lipid-based nanoparticles in the systemic delivery of siRNA. Nanomedicine (Lond). 2014;9:105–20. [DOI] [PubMed] [Google Scholar]
- [22].Chen Z, Luo B, Cai TQ, Thankappan A, Xu Y, Wu W, et al. Proof-of-concept Studies for siRNA-mediated Gene Silencing for Coagulation Factors in Rat and Rabbit. Mol Ther Nucleic Acids. 2015;4:e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pardi N, Hogan MJ, Naradikian MS, Parkhouse K, Cain DW, Jones L, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xu Y, Ou M, Keough E, Roberts J, Koeplinger K, Lyman M, et al. Quantitation of physiological and biochemical barriers to siRNA liver delivery via lipid nanoparticle platform. Mol Pharm. 2014;11:1424–34. [DOI] [PubMed] [Google Scholar]
- [27].Gindy ME, Feuston B, Glass A, Arrington L, Haas RM, Schariter J, et al. Stabilization of Ostwald ripening in low molecular weight amino lipid nanoparticles for systemic delivery of siRNA therapeutics. Mol Pharm. 2014;11:4143–53. [DOI] [PubMed] [Google Scholar]
- [28].Swaminathan G, Thoryk EA, Cox KS, Meschino S, Dubey SA, Vora KA, et al. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine. 2016;34:110–9. [DOI] [PubMed] [Google Scholar]
- [29].Bershteyn A, Hanson MC, Crespo MP, Moon JJ, Li AV, Suh H, et al. Robust IgG responses to nanograms of antigen using a biomimetic lipid-coated particle vaccine. J Control Release. 2012;157:354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Moyle PM, Hartas J, Henningham A, Batzloff MR, Good MF, Toth I. An efficient, chemically-defined semisynthetic lipid-adjuvanted nanoparticulate vaccine development system. Nanomedicine. 2013;9:935–44. [DOI] [PubMed] [Google Scholar]
- [31].du Plessis LH, Marais EB, Mohammed F, Kotze AF. Applications of lipid based formulation technologies in the delivery of biotechnology-based therapeutics. Curr Pharm Biotechnol. 2014;15:659–72. [DOI] [PubMed] [Google Scholar]
- [32].Thoryk EA, Swaminathan G, Meschino S, Cox KS, Gindy M, Casimiro DR, et al. Co-Administration of Lipid Nanoparticles and Sub-Unit Vaccine Antigens Is Required for Increase in Antigen-Specific Immune Responses in Mice. Vaccines (Basel). 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Skwarczynski M, Toth I. Recent advances in peptide-based subunit nanovaccines. Nanomedicine (Lond). 2014;9:2657–69. [DOI] [PubMed] [Google Scholar]
- [34].Swaminathan G, Thoryk EA, Cox KS, Smith JS, Wolf JJ, Gindy ME, et al. A Tetravalent Sub-unit Dengue Vaccine Formulated with Ionizable Cationic Lipid Nanoparticle induces Significant Immune Responses in Rodents and Non-Human Primates. Sci Rep. 2016;6:34215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hook LM, Cairns TM, Awasthi S, Brooks BD, Ditto NT, Eisenberg RJ, et al. Vaccine-induced antibodies to herpes simplex virus glycoprotein D epitopes involved in virus entry and cell-to-cell spread correlate with protection against genital disease in guinea pigs. PLoS Pathog. 2018;14:e1007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Awasthi S, Balliet JW, Flynn JA, Lubinski JM, Shaw CE, DiStefano DJ, et al. Protection provided by a herpes simplex virus 2 (HSV-2) glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1-seropositive guinea pigs. J Virol. 2014;88:2000–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McGraw HM, Friedman HM. Herpes simplex virus type 1 glycoprotein E mediates retrograde spread from epithelial cells to neurites. J Virol. 2009;83:4791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cairns TM, Ditto NT, Lou H, Brooks BD, Atanasiu D, Eisenberg RJ, et al. Global sensing of the antigenic structure of herpes simplex virus gD using high-throughput array-based SPR imaging. PLoS Pathog. 2017;13:e1006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology. 1989;7:145–73. [DOI] [PubMed] [Google Scholar]
- [40].Skoberne M, Cardin R, Lee A, Kazimirova A, Zielinski V, Garvie D, et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in Guinea pigs. J Virol. 2013;87:3930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hook LM, Awasthi S, Dubin J, Flechtner J, Long D, Friedman HM. A trivalent gC2/gD2/gE2 vaccine for herpes simplex virus generates antibody responses that block immune evasion domains on gC2 better than natural infection. Vaccine. 2019;37:664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cocchi F, Menotti L, Dubreuil P, Lopez M, Campadelli-Fiume G. Cell-to-cell spread of wild-type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin1 (PRR1/HveC/HIgR) and nectin2 (PRR2/HveB). J Virol. 2000;74:3909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Huber MT, Wisner TW, Hegde NR, Goldsmith KA, Rauch DA, Roller RJ, et al. Herpes simplex virus with highly reduced gD levels can efficiently enter and spread between human keratinocytes. J Virol. 2001;75:10309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang F, Zumbrun EE, Huang J, Si H, Makaroun L, Friedman HM. Herpes simplex virus type 2 glycoprotein E is required for efficient virus spread from epithelial cells to neurons and for targeting viral proteins from the neuron cell body into axons. Virology. 2010;405:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Whitbeck JC, Huang ZY, Cairns TM, Gallagher JR, Lou H, Ponce-de-Leon M, et al. Repertoire of epitopes recognized by serum IgG from humans vaccinated with herpes simplex virus type 2 glycoprotein D. J Virol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. Herpes virus fusion and entry: a story with many characters. Viruses. 2012;4:800–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nicola AV, Ponce de Leon M, Xu R, Hou W, Whitbeck JC, Krummenacher C, et al. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J Virol. 1998;72:3595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Atanasiu D, Saw WT, Lazear E, Whitbeck JC, Cairns TM, Lou H, et al. Using antibodies and mutants to localize the presumptive gH/gL binding site on HSV gD. J Virol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mirotti L, Alberca Custodio RW, Gomes E, Rammauro F, de Araujo EF, Garcia Calich VL, et al. CpG-ODN Shapes Alum Adjuvant Activity Signaling via MyD88 and IL-10. Frontiers in immunology. 2017;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lonez C, Irvine KL, Pizzuto M, Schmidt BI, Gay NJ, Ruysschaert JM, et al. Critical residues involved in Toll-like receptor 4 activation by cationic lipid nanocarriers are not located at the lipopolysaccharide-binding interface. Cell Mol Life Sci. 2015;72:3971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lonez C, Bessodes M, Scherman D, Vandenbranden M, Escriou V, Ruysschaert JM. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomedicine. 2014;10:775–82. [DOI] [PubMed] [Google Scholar]
- [53].Nelson CS, Herold BC, Permar SR. A new era in cytomegalovirus vaccinology: considerations for rational design of next-generation vaccines to prevent congenital cytomegalovirus infection. NPJ Vaccines. 2018;3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]