Abstract
Bisphosphonates are generally used to treat bone diseases, such as bone metastasis from cancer. There is evidence that, through the modification of the pharmacokinetics and biodistribution of bisphosphonates by formulating them into nanoparticles, they may be able to treat extraskeletal tumors. However, many previously reported bisphosphonate nanoparticle formulations show extensive premature release of bisphosphonates. Herein, using zoledronate (Zol), a third generation bisphosphonate, we developed a new Zol nanoparticle formulation (denoted as Zol-NPs) by encapsulating anionic lipid-coated Zol-calcium nanocomplexes into poly (lactic-co-glycolic) acid nanoparticles emulsified with octadecanoic acid-hydrazone-polyethylene glycol (2000), an acid-sensitive cleavable emulsifying agent. The resultant Zol-NPs, about 180 nm in hydrodynamic diameter, show very limited premature release of Zol (i.e. < 5% in 48 h in a simulated physiological condition) and enhanced cytotoxicity to both murine cancer cells and macrophages. In a mouse model with orthotopically transplanted mammary tumors, Zol-NPs significantly reduced the distribution of Zol in bones, but increased its distribution in tumors. Importantly, Zol-NPs also significantly inhibited tumor growth, whereas the equivalent dose of free Zol did not. This platform technology may be exploited to treat extraskeletal tumors with bisphosphonates.
Keywords: Bisphosphonate-metal complex, zoledronic acid, reverse microemulsion, PLGA nanoparticle, PEGshedding, biodistribution, extraskeletal tumor, treatment
Graphical Abstract
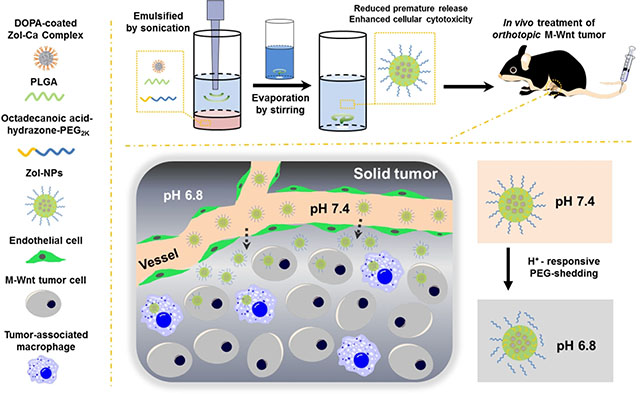
1. Introduction
Bisphosphonates are a class of compounds with a unique ‘P-C-P’ structure. Many bisphosphonates are indicated to treat bone diseases, including cancer bone metastasis.1–2 Data from clinical trials have shown that certain bisphosphonates can inhibit skeletal tumor growth, decrease skeletal tumor burden and relieve cancer-associated bone diseases such as bone pain and fracture.3–5
Recently, there is increasing interest in applying bisphosphonates to treat extraskeletal tumors.6–8 Unfortunately, because of the extensive renal clearance and binding of bisphosphonates to bones, intravenous injection of such compounds often results in a very limited distribution of them in extraskeletal tumors, and thus none or minimum activity against those tumors.9–11 Moreover, extensive bone absorption of bisphosphonates may induce severe side effects such as osteonecrosis of the jaw.12–13 To enable the use of bisphosphonates to treat extraskeletal tumors, various delivery systems and formulations have been developed and tested to modulate the pharmacokinetics and biodistribution of bisphosphonates. One of the earliest bisphosphonate delivery systems was developed based on liposomes such as liposomal clodronic acid (Clo) and liposomal zoledronate.14–16 Hydroxyapatite (HA)-based materials have also been used to deliver bisphosphonates due to the strong affinity between HA and bisphosphonates.17–18 Those delivery systems in general increase the cytotoxicity of bisphosphonates to tumor cells and/or enhance their antitumor activity in animal models, however, many of them have extensive premature release of bisphosphonates. For instance, Buiting and colleagues have found that over 70% of Clo were released from a liposomal Clo formulation within 3 h.19 Khajuria and colleagues reported that over 60% of Zol was released within 60 min from their Zol-loaded HA nanoparticles.20 Premature release of bisphosphonates from nanoparticles, especially in physiological conditions, can potentially render the bisphosphonate formulations less effective in modifying the biodistribution of bisphosphonates and their activity against extraskeletal tumors. Recently, new bisphosphonate delivery systems developed based on organic material-coated inorganic materials have shown reduced premature release. For instance, Zhang and Rosenholm have prepared polyethyleneimine (PEI)-functionalized mesoporous silica nanoparticles (MSNs) to load Zol and used a tethered lipid bilayer as the “gate-keeper” to control the release rate of Zol. 21–22 Our group previously developed Zol-calcium (Zol-Ca) nanocomplexes and coated them with a bilayer of lipids (denoted as Zol-Ca@bilipid), which showed a moderate premature release rate (i.e. ~30% of Zol within 10 h at a physiological pH).23–24 Salzano and colleagues have developed cationic lipid-coated Zol-containing calcium phosphate nanoparticles (named as PLCaPs) and tested their activity against several types of tumors in animal models.25–27 The authors do not report the release profile of Zol from the PLCaPs, however the coating of cationic lipids on the nanoparticles likely helps decrease the premature release of Zol from the calcium phosphate complexes.
Herein, we report a new bisphosphonate nanoparticle formulation consisted of biocompatible materials. Zol-Ca nanocomplexes were prepared following our previously reported method 23 and then encapsulated into nanoparticles prepared with poly (lactic-co-glycolic) acid (PLGA) and emulsified with octadecanoic acid-hydrazone-polyethylene glycol (PEG2k), an acid-sensitive cleavable emulsifying agent synthesized by conjugating octadecanoic acid hydrazide and PEG2k aldehyde.28 The resultant nanoparticles (i.e. Zol-NPs) were characterized, and the release of Zol from such NPs was evaluated in a simulated physiological condition. In cell culture, the Zol-NPs were significantly more cytotoxic than free Zol against tumor cells as well as macrophages. In a mouse model with orthotopically transplanted mammary tumors, the Zol-NPs significantly reduced Zol distribution in bones, increased its distribution in tumor tissues, and more importantly, more effectively inhibited tumor growth, as compared to free Zol.
2. Materials and Methods
2.1. Reagents
Zoledronate monohydrate and Alexa Fluor 647-labeled Zol (Zol-AF647) were from TCI America (Portland, OR) and BioVinc LLC (Los Angeles, CA), respectively. Calcium chloride, sodium hydrate, sodium dodecyl sulfate (SDS), sodium hydrogen phosphate, sodium dihydrogen phosphate, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), formic acid, polyoxyethylene (5) nonylphenylether (NP-5), and PLGA with molecular weights ranging from 4,000 to 15,000 (average of 9,500) were from Sigma-Aldrich (St. Louis, MO). The 1,2-dioleoyl-sn-glycero-3-phosphate monosodium (DOPA) was from Avanti Polar Lipid, Inc. (Alabaster, AL). Chloroform, cyclohexane, ethanol and dimethyl sulfoxide were from Thermo Fisher Scientific Co. (Waltham, MA). Two types of emulsifying agents, acid-sensitive octadecanoic acid-hydrazone-PEG2K and acid-insensitive octadecanoic acid-amide-PEG2K, were synthesized and purified as we reported previously.28–29
2.2. Cells and animals
M-Wnt mamary tumor cells were originally isolated from MMTV-Wnt-1 transgenic mice,30–31 and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL of streptomycin at 37°C, 5% CO2. J774A.1 murine macrophage cells were from the ATCC (Manassas, VA) and cultured in DMEM containing 10% FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin at 37°C, 5% CO2. Cell culture media, reagents, and phosphate-buffered saline (PBS, 10 mM, pH 7.4) were from Invitrogen (Carlsbad, CA).
Female C57BL/6 mice (6–8 weeks) were from Charles River Laboratories (Wilmington, MA). The animal protocol was approved by the IACUC at The University of Texas at Austin. An orthotopic mammary tumor model was established in mice by injecting M-Wnt tumor cells (3 × 105 cells/mouse) subcutaneously into the 4th mammary fat pad.
2.3. Preparation of Zol-Ca complex-based nanoparticle (denoted as Zol-NPs)
The spherical DOPA-coated Zol-Ca nanocomplexes were prepared using a reverse microemulsion (RM) method as previously described.23 The concentration of Zol in complexes in suspension was determined using ionic exchange high performance liquid chromatography (IE-HLPC).23 To prepare Zol-NPs, the DOPA-coated Zol-Ca nanocomplexes, PLGA and the acid-sensitive octadecanoic acid-hydrazon-PEG2K or acid-insensitive octadecanoic acid-amide-PEG2K were suspended in 0.7 mL CHCl3 in a glass vial. Thereafter, 6 mL of water was added and the vial was placed into an ice bath. Probe sonication was used to induce the formation of emulsions, and then magnetic stirring was applied to the emulsions to allow the evaporation of CHCl3. After 3 h of evaporation, the suspension was treated under vacuum by a rotary evaporator to remove residual CHCl3, followed by passing through a 1.2 μm nylon filter. The resultant suspension was centrifuged at 13,000 × g for 15 min to collect Zol-NPs. After washing twice, the Zol-NPs obtained were re-suspended in an aqueous solution for further use. Additionally, to prepare fluorescein isothiocyanate (FITC)-labeled Zol-NPs, 5% of PLGA was replaced by FITC-labeled PLGA synthesized as previously described.28
2.4. Characterization of Zol-NPs
The Zol-NPs prepared with an input weight ratio of 0.5 mg DOPA-coated Zol-Ca nanocomplexes, 2.5 mg PLGA, and 2 mg octadecanoic acid-hydrazon-PEG2K were used in the following experiments. The morphology of the Zol-NPs was characterized using transmission electron microscopy (TEM) as previously described.23–24 To determine the Zol concentration in the Zol-NPs in water suspension, a certain volume of such suspension was mixed with THF and formic acid (final concentration of 7.5% and 2.5%, respectively) to break the Zol-NPs and induce the release of Zol. After centrifugation (16,000 × g, 10 min), Zol concentration in the supernatant was determined by IE-HPLC, and the original concentration was calculated accordingly. The drug-loading efficiency and the recovery efficiency were measured as previously described.23–24 The hydrodynamic size and zeta potential of the Zol-NPs were measured using a Zetasizer Nano ZS (Malvern, Westborough, MA). The stability of the Zol-NPs was studied by evaluating the particle size after they were incubated in culture medium with or without 10% FBS for 16 h.
2.5. In vitro release of Zol from Zol-NPs
The release of Zol from the Zol-NPs was determined as previously described,23–24 using a 2-mL dialysis tube (MWCO 50 KDa) with 1 mL Zol-NPs in water suspension (i.e. 0.6 mg of Zol) placed inside and 9 mL of sodium phosphate buffer (PB, 10 mM, pH 7.4) as the release medium. As a control, the diffusion of free Zol across the dialysis membrane was also studied.
2.6. Confirmation of acid-sensitive shedding of PEG from the Zol-NPs
To confirm the acid-sensitive shedding of PEG from the surface of Zol-NPs, such NPs were pre-incubated in 0.25 M PB with different pH values (5.0, 6.8 or 7.4) at 37°C or 4°C. After 16 h of incubation, the NPs were centrifuged (15,000 × g, 20 min), resuspended in water and treated with a Lugol’s solution for iodide staining of PEG and estimate the amount of PEG remaining on the surface of the nanoparticles.28 After 15 min of reaction, the absorption values at 450 nm were measured.
To further confirm the acid-sensitive shedding of PEG from the Zol-NPs, FITC-labeled Zol-NPs were pre-incubated in 0.25 M PB with pH values of 6.8 and 7.4 at 37°C or 4°C. After 16 h of incubation, the NPs were centrifuged, washed with PBS twice, and re-suspended in cell culture medium. J774A.1 cells (24-well plate, 1 × 105 cells per well) were incubated with such pre-treated FITC-labeled Zol-NPs for 1 h. Thereafter, cells were washed twice with cold PBS and lysed with a 1% SDS solution. The fluorescence intensity of the cell lysates was measured to determine the binding and/or uptake of the nanoparticles by the macrophages. The excitation wavelength was 485 nm, and the emission wavelength was 528 nm. As a control, a similar Zol nanoparticle formulation prepared using the acid-insensitive octadecanoic acid-amide-PEG2K was also included in this experiment.
2.7. In vitro cytotoxicity assay
A standard MTT assay was used to determine the cytotoxicity of Zol-NPs to M-Wnt and J774A.1 cells.23–24 Cells were seeded in 96-well plates (5 × 103 cells per well) and incubated for 24 h, followed by incubation with Zol-NPs, free Zol, or the corresponding Zol-free NPs as various concentrations for 48 h. The IC50 values were calculated using the Prism software (GraphPad, La Jolla, CA.).
2.8. In vivo biodistribution of Zol-NPs in a mouse model with orthotopic mammary tumor
The biodistribution of Zol in the Zol-NPs in major organs, bones, and tumor tissues was evaluated in C57BL/6 mice with orthotopically transplanted M-Wnt tumors. Mice were randomized into different groups to receive Zol-NPs, free Zol, or sterile PBS by intravenous injection. The dose of Zol (containing 2% of Zol-AF647) was 2 μg/mouse. Mice (n = 3–6) were euthanized 24 h after the injection, and the right hind leg bone, tumor, and major organs were harvested and then imaged with an IVIS Spectrum Imaging System (Caliper Life Sciences, Waltham, MA) (Ex = 640 nm, Em = 680 nm). The fluorescence intensity values were used to estimate Zol distribution. Data reported are the values normalized based on the PBS group (i.e. the value of the Zol group minus the corresponding value from the PBS group).
2.9. In vivo antitumor activity of Zol-NPs
The Zol-NPs’ ability to inhibit tumor growth was evaluated in M-Wnt tumor-bearing C57BL/6 mice (n = 5–6). Eight days after tumor cell injection, mice were randomized to receive Zol-NPs, free Zol, Zol-free NPs or PBS by intravenous injection. The day of tumor cell inoculation was set as day 0, and the two injections of Zol were on days 8 and 25 (or where mentioned). The dose of Zol was 3 μg per injection per mouse. Tumor size and mouse body weight were measured every two or three days. Mice were euthanized 22 days after the first injection of the Zol-NPs (i.e. day 30) to harvest tumor tissues, which were then processed for H&E staining.
2.10. Statistical analysis
Data analyses for two groups or more than two groups were carried out using Student’s t-test (unpaired) or one-way ANOVA (no pairing) followed by Fisher’s least significant test, respectively, using the GraphPad Prism software. A p value of < 0.05 is considered significant.
3. Results and discussion
3.1. Preparation and characterization of Zol-NPs
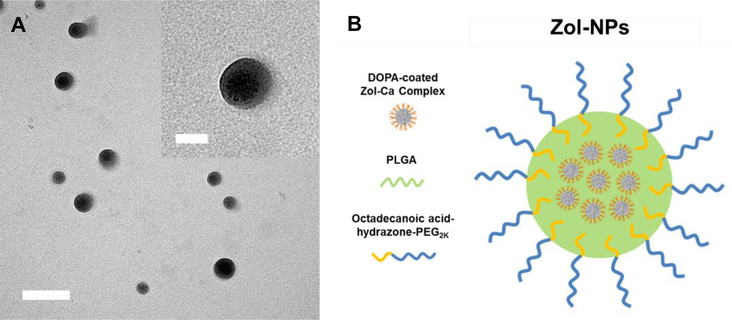
The Zol-NPs were prepared by emulsifying the DOPA-coated Zol-Ca nanocomplexes into PLGA nanoparticles using our previously synthesized acid-sensitive octadecanoic acid-hydrazone-PEG2K as the emulsifying agent.28 The input weight ratio of components was optimized to 0.5 mg of DOPA-coated ZolCa complexes, 2.5 mg of PLGA, and 2 mg of octadecanoic acid-hydrazone-PEG2K. Shown in Fig. 1A are representative TEM images of the resultant Zol-NPs, which are spherical and around 50–100 nm in diameter. In every Zol-nanoparticle, there is at least one smaller particle (Fig. 1A, inset), which is likely the DOPA-coated Zol-Ca nanocomplex.23 The loading efficiency of Zol in the Zol-NPs and the recovery efficiency of Zol were determined to be ~1.4% and ~32%, respectively. Dynamic light scattering showed that the hydrodynamic size (intensity distribution) and zeta potential of the Zol-NPs were 178.1 ± 1.2 nm and −28.2 ± 0.8 mV, respectively. Shown in Fig. 1B is a schematic of the hypothetical structure of the Zol-NPs, with the DOPA-coated Zol-Ca nanocomplexes embedded in the PLGA nanoparticles and the octadecanoic acid-hydrazone-PEG2K molecules inserted on the surface of the nanoparticles using the octadecanoic group as an anchor.
Fig. 1.
(A) A representative TEM micrograph of Zol-NPs (bar = 200 nm). Inset is an enlarged micrograph showing multiple small particles in a single Zol-NP (bar = 50 nm). (B) A schematic showing the hypothetical structure of a Zol-NP, with DOPA-coated Zol-Ca complexes embedded in the PLGA nanoparticles prepared with the acid-sensitive octadecanoic acid-hydrazone-PEG2K as an emulsifying agent.
3.2. In vitro release of Zol from Zol-NPs and the stability of Zol-NPs
Fig. 2A shows the release profile of Zol from the Zol-NPs in PB (10 mM, pH 7.4) at 37 °C. There was not an apparent burst release of Zol from the Zol-NPs, and only less than 5% of Zol was released after 48 h, demonstrating a significantly reduced premature release of Zol, as compared to our previously reported Zol-Ca@bilipid NPs (i.e. over 45% Zol was released in 48 h).24 The reduction of premature release of Zol from Zol-NPs is expected to be beneficial for the in vivo applications of Zol and further supports the hypothetical structure of Zol-NPs, i.e. the DOPA-coated Zol-Ca complexes are embedded in the PLGA cores of Zol-NPs. The release of compounds incorporated in PLGA nanoparticles is generally by diffusion of the compounds through the polymer and is facilitated by the hydrolysis and/or erosion of the PLGA.32 In polymeric PLGA nanoparticles, it is relatively difficult for water molecules to diffuse across the PLGA layer and contact the DOPA-coated Zol-Ca nanocomplexes embedded in the PLGA to induce the dissociation of Zol-Ca complexes and the release of Zol. In contrast, the lipid layer that is used to wrap the DOPA-coated Zol-Ca nanocomplexes in our previously reported Zol-Ca@bilipid nanoparticles is in a more active dynamic state. Water molecules can readily diffused through the lipid layer to induce the breakage of the Zol-Ca complexes, enabling the rapid release of Zol from the ZolCa@bilipid NPs.24
Fig. 2.
(A) The in vitro release profile of Zol from Zol-NPs in 10 mM sodium phosphate buffer, pH 7.4. As a control, the diffusion of free Zol across the dialysis membrane was also measured. (B) Stability of Zol-NPs in cell culture medium with or without 10% FBS. After incubation for 2 or 16 h, the hydrodynamic size of Zol-NPs was measured. For both A and B, data are mean ± S.D. (n = 3).
In addition, we studied the release profiles of bisphosphonates from other recently reported bisphosphonate delivery systems. Wang and colleagues reported a similar nanoparticle formulation (i.e. cationic lipid-coated Zol-Ca complexes) that has limited premature release.33 However, the release study was conducted in 100 mM phosphate buffer,34 and there is evidence that high concentrations of HPO42− anions may reduce the dissociation rate of the Zol-Ca complexes.24 Although the PLCaP NPs developed by Salzano and colleagues have been used to in several animal studies, the in vitro release profiles of bisphosphonates from them cannot be found.25–27 The PEI-functionalized MSNs with a tethered lipid bilayer developed by Zhang and Rosenholm have shown a controllable release profile for Zol, and the mechanism is attributed to the adsorption of Zol by cationic PEI and the ‘gate-keeper’ effect of the lipid bilayer.21–22 As mentioned above, a reduction of premature release of Zol is expected to be beneficial for the in vivo applications of Zol.
Fig. 2B shows the hydrodynamic size of Zol-NPs after they were incubated in cell culture medium with or without 10% FBS at 37°C for up to 16 h. There is not any significant change in the hydrodynamic size of Zol-NPs, indicating that the Zol-NPs will not likely aggregate after intravenous injection.
3.3. Confirmation of acid-sensitive shedding of PEG from the Zol-NPs
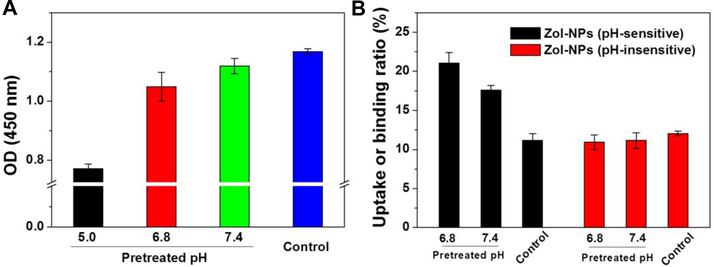
Shown in Fig. 3A are Lugol’s iodide-staining results of Zol-NPs after they were pre-incubated at different pH values for 16 h to facilitate PEG shedding. It is clear that the pre-incubation at a lower pH solution, especially pH 5.0, resulted in a significantly reduced residual amount of PEG on the surface of the Zol-NPs, which may be attributed to the relatively higher concentration of H+ in pH 5.0 that facilitates the hydrolysis of hydrazone bond in the octadecanoic acid-hydrazone-PEG2K molecules. Since it is known that PEGylation of nanoparticles significantly reduces their uptake and/or binding by cells in culture,35–36 we used such assay to further confirm the acid-sensitive sheddable PEGylation of our Zol-NPs by studying the effect of pre-incubation of the Zol-NPs at different pH values (i.e. pH 6.8 versus 7.4) on the binding and/or uptake of the Zol-NPs by J774A.1 macrophages. In this experiment, 5% of PLGA was replaced by FITC-conjugated PLGA to make the as-prepared Zol-NPs fluorescent. As shown in Fig. 3B, pre-incubation of the Zol-NPs for 16 h, especially in pH 6.8, significantly promoted the binding and/or uptake of them by the J774A.1 cells. In contrast, subjecting the control Zol-NPs (TEM images in Fig. S1), which were prepared with the acid-insensitive octadecanoic acid-amide-PEG2K as an emulsifying agent in the same pre-incubation condition, did not significantly influence the binding and/or uptake of them by the J774A.1 cells. Taken together, data in Fig. 3 show that the shedding of PEG from the Zol-NPs is more sensitive in an acidic condition.
Fig. 3.
Confirmation of acid-sensitive sheddable PEGylation of the Zol-NPs and its influence on the cellular uptake and/or binding of such Zol-NPs by macrophages in culture. (A) Shedding of PEG from the Zol-NPs after incubation at different pH values as measured using an iodide staining method. Zol-NPs in suspension were pretreated in 0.25 M PB at pH 5.0, 6.8 or 7.4 at 37 °C for 16 h. The NPs were then reacted with a Lugol’s solution. Zol-NPs pretreated in 0.25 M PB with pH value of 7.4 at 4 °C for 16 h were used as a control. Data are mean ± S.D. (n = 3, * p < 0.05). (B) Uptake and/or binding of Zol-NPs by J774A.1 murine macrophages. Fluorescently-labeled Zol-NPs (with 5% of PLGA labeled with FITC) were treated in 0.25 M PB, pH 6.8 or 7.4, at 37°C for 16 h. Thereafter, such pretreated Zol-NPs were collected and incubated with J774A.1 cells for 1 h. Fluorescence intensity of the cell lysate was measured. Zol-NPs pretreated in 0.25 M PB with pH value of 7.4 at 4°C for 16 h were used as a control. For a comparison, Zol-NPs prepared with the acid-insensitive octadecanoic acid-amide-PEG2K were also included. Data are mean ± S.D. (n = 4, * p < 0.05).
3.4. In vitro antiproliferative activity of Zol-NPs
The antiproliferative activity of the Zol-NPs was evaluated in M-Wnt tumor cells and J774A.1 macrophages and compared to that of free Zol. As shown in Fig. 4, the Zol-NPs are significantly more effective than free Zol in inhibiting the growth and proliferation of the M-Wnt mouse mammary tumor cells (Fig. 4A) as well as the J774A.1 macrophages (Fig. 4B). The relative IC50 value of the Zol-NPs was calculated to be ~0.38 μg/mL in the M-Wnt tumor cells and ~0.13 μg/mL in the J774A.1 macrophages. As a comparison, the IC50 values of free Zol against both cell lines are greater than 1 μg/mL, the highest concentration tested. We speculate that the significantly improved antiproliferative activity of the Zol-NPs, relative to free Zol, may be attributed to the increased uptake of Zol by both cell lines, because data from previous studies demonstrated that incorporating bisphosphonates into nanoparticles increases their uptake by tumor cells, as compared to free bisphosphonates.37–38 For example, Liu et al. reported forcing bisphosphonates to kill cancer cells by formulating them with nanoscale coordination polymers.39 Nonetheless, it is clear that encapsulating bisphosphonates such as Zol into nanoparticles renders them more effective against not only tumor cells, but also macrophages. Being more cytotoxic to macrophages is expected to be beneficial because tumor-associated macrophages (TAMs) can account for up to 50% of solid tumor mass, especially in breast tumors.40–42 Data from previous studies showed that TAMs are one of the major targets of bisphosphoantes.43–44 TAMs are innate immune cells recruited into tumors and they develop immunesuppressive functions within tumor microenviroment.45–47 Clinical evidence shows that high density of TAMs is correlated with poorer prognosis and higher risk of metastasis, and TAM depletion is an effective strategy to improve the effects of tumor therapeutical agents.48–50
Fig. 4.
In vitro cytotoxicity of Zol-NPs to M-Wnt murine mammary cancer cells (A) and J774A.1 murine macrophages (B) after 48 h of incubation. Free Zol and Zol-free NPs prepared without the Zol-Ca complexes were included as controls. Data are mean ± S.D. (n = 6, * p < 0.05).
3.5. In vivo distribution of Zol in a mouse model with orthotopical M-Wnt tumor
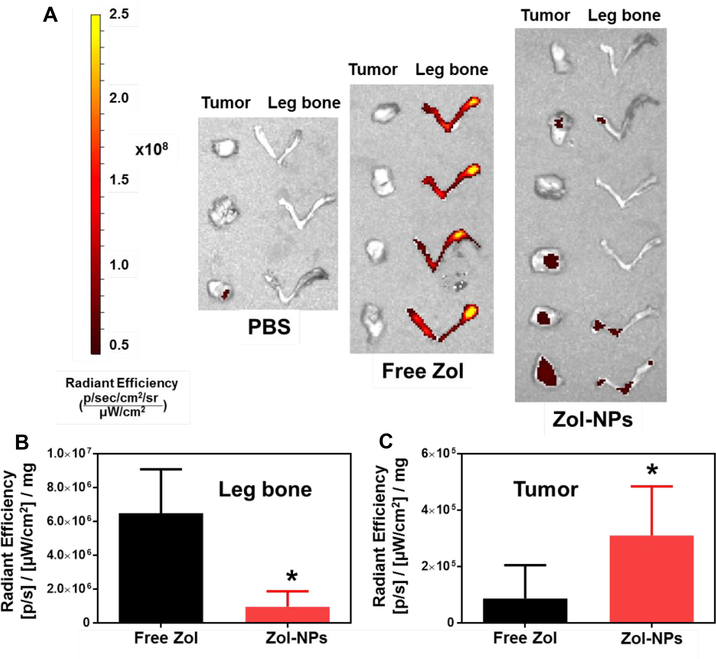
To understand the extent to which encapsulating Zol into our Zol-NPs affects its biodistribution, the distribution of Zol in M-Wnt tumor-bearing C57BL/6 mice after intravenous injection of Zol-NPs were evaluated and compared to that of free Zol. The Zol-NPs used in this experiment were labeled fluorescently by including 2% (w/w) of Zol-AF647. Data from previous studies showed that the in vivo distribution of Zol-AF647 is very similar to that of Zol.51–52 Mice were euthanized 24 h after i.v. injection of Zol-NPs, Zol or PBS to harvest tumor tissues, right hind leg bones, and major organs. The contents of Zol in these samples were determined based on the relavent fluorescent intensity in them (Figs. S2 – S4). Fig 5A shows representative fluorescent images of tumors and leg bones of mice from different groups. It is clear that the fluorescence intensities in the leg bones of mice that were injected with the Zol-NPs are significantly lower than that in mice that were injected with free Zol (Fig. 5A and 5B). However, the fluorescence intensities in the tumor tissues in mice that were injected with the Zol-NPs are significantly higher than in mice that were injected with free Zol (i.e. ~4-fold) (Fig. 5A and 5C). It is likely that the minimum premature release of Zol from Zol-NPs, as suggested by data in Fig. 2A, resulted in a reduction in its absorption in the bones, and the nanoparticle nature of the Zol-NPs provided the Zol encapsulated in them the opportunity to distribute or accumulate into tumor tissues due to the enhanced permeation and retention (EPR) phenomenon.53–54 Moreover, since it is well known that nanoparticles are readily captured by the mononuclear phagocyte system,55 we also measured the fluorescence intensity values of liver and spleen of each mouse (Figure S5). The fluorescence intensity in the livers of mice that were injected with the Zol-NPs were 2.2-fold higher than that in mice that were injected with the free Zol, but the difference is not significant. In the spleen, the fluorescence intensity in the Zol-NPs group is significant higher than that in the free Zol group, but neither of these two groups is significantly different from the PBS group. Finally, the ratio of fluorescence intensity values in tumor vs. in liver in mice injected with Zol-NPs is about 1/3, as compared to ~1/5 in mice injected with free Zol, suggesting that Zol-NPs also increased the relative distribution of Zol in tumor tissues.
Fig. 5.
In vivo distribution of Zol-NPs in C57BL/6 mice with orthotopic M-Wnt tumors. M-Wnt tumors and rear leg bones were collected 24 h after mice were injected (i.v.) with Zol-NPs, free Zol, or PBS. (A) Fluorescent images of orthotopic M-Wnt tumors and leg bones collected 24 h after i.v. injection of PBS, free Zol, or Zol NPs (2% of Zol was labeled with AF647). (B-C) Relative mean fluorescence intensity of AF647-labeled Zol in leg bones (B) and M-Wnt tumors (C), respectively, as normalized by the weight of tumors and leg bones. For B and C, data are mean ± S.D. (n = 3 – 6, * p < 0.05 vs. Free Zol).
3.6. In vivo antitumor activity of Zol-NPs
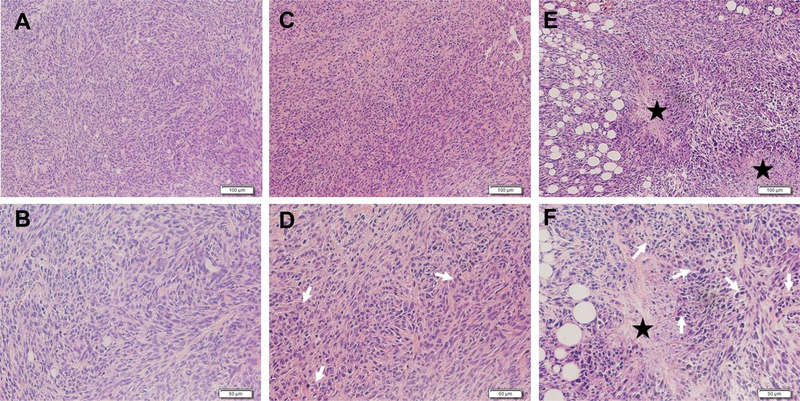
To identify the extent to which encapsulating Zol in our Zol-NPs affects its activity against extraskeletal tumors, we evaluated the antitumor ability of our Zol-NPs in a mouse model with orthotopical M-Wnt mammary tumor. The day of tumor cell implantation was recorded as day 0, and the Zol-NPs were injected on days 8 and 25. Shown in Fig. 6A are the growth curves of M-Wnt tumors in mice treated with Zol-NPs, free Zol, or PBS. At the dose tested, free Zol did not show any significant activity as compared to PBS as a negative control (Fig. 6A). However, our Zol-NPs significantly inhibited the tumor growth (Fig. 6A). The relative body weight changes of mice during the treatments are shown in Fig. S6. Neither Zol-NPs nor free Zol at the dosing regimen significantly affect the body weight of the mice, as compared to PBS. Data from a separate study show that the Zol-free NPs (i.v. injected on days 7 and 10 after tumor cell injection) do not have any activity against M-Wnt tumors (Fig. 6B). Shown in Fig. 7 are representative H&E images of M-Wnt tumors in mice treated with Zol, free or in our Zol-NPs. Tumors in mice that were injected with PBS (Fig. 7 A–B) or free Zol (Fig. 7 C–D) showed a high density of tumor cells, while tumors in mice treated with Zol-NPs have more cells in apoptosis and necrosis (Fig. 7 E–F).
Fig. 6.
In vivo antitumor activity of the Zol-NPs in orthotopic M-Wnt tumor-bearing C57BL/6 mice. The growth curves of M-Wnt tumors in mice treated with (A) PBS, free Zol, Zol NPs, or (B) Zol-free blank NPs. Arrows indicate the days on which mice received treatments. Data are mean ± S.D. (*p < 0.05, vs. PBS or free Zol).
Fig.7.
Representative H&E images of M-Wnt tumors in C57BL/6 mice i.v. injected with PBS (A-B), free Zol (C-D), and Zol-NPs (E-F). Tumor tissues are represented by two different magnifications (100 × (top), 200 × (bottom)). The scale bars in the 100 × images represent 100 μm, and those in the 200 × images represent 50 μm. White arrows represent apoptotic cells, and stars represent necrotic areas.
Taken together, our Zol-NPs, at a well-tolerated dosing regimen, significantly increased the antitumor activity of Zol against extraskeletal tumors, likely because they favorably modified the distribution of the Zol in the tumor-bearing mice, i.e. increased its distribution in tumor tissues, while decreased its absorption in bones (Fig. 5). Previously, Junankar and colleagues showed that a fluorescent bisphosphonate was detected in 4T1 mammary tumors in mice after intravenous injection, but it bound to small, granular microcalcifications that were primarily taken up by TAMs, not tumor cells.51 Encapsulating Zol into our Zol-NPs, with minimum premature release, likely not only increased the distribution of Zol in tumor tissues in mice by taking advantage of the EPR effect, but also enabled the Zol to enter both tumor cells and TAMs due to their uptake of the Zol-NPs. The acid-sensitive sheddable PEGylation of the Zol-NPs likely had also helped the cellular uptake of such NPs in the slightly acidic microenvironment in tumor tissues. These properties, in combination with the improved cytotoxicity of the Zol-NPs against tumor cells and macrophages as shown in Fig. 4, are likely responsible for the enhanced antitumor activity of the Zol-NPs, relative to free Zol as shown in Fig. 6. Of course, a portion of the Zol in the Zol-NPs was likely released from the Zol-NPs before the Zol-NPs reached tumor tissues. In the tumor tissues, some Zol may have also released before the Zol-NPs were taken up by TAMs or tumor cells. The Zol released from the Zol-NPs before the nanoparticles were taken up by TAMs or tumor cells may be taken up by TAMs by the mechanism similar to the uptake of the intravenously injected free bisphosphonates as previously reported.51
4. Conclusion
We report a bisphosphonate delivery system with minimum premature release. Using Zol as an example, we incorporated DOPA-coated Zol-Ca nanocomplexes into nanoparticles prepared with PLGA. The resultant Zol-NPs significantly reduced the distribution of Zol in bones and increased its accumulation in extraskeletal tumors in mice with orthotopically implanted mammary tumors. More importantly, the Zol-NPs significantly inhibited orthotopic mammary tumor growth in a mouse model, whereas the equivalent dose of free Zol did not. This platform technology may be exploited to more effectively treat extraskeletal tumors with bisphosphonates.
Supplementary Material
Acknowledgments
This work was supported in part by the U.S. National Institutes of Health (CA135274 to Z.C. & SDH) and the Alfred and Dorothy Mannino Fellowship in Pharmacy at UT Austin (to Z.C.). S.A.V. was supported in part by the Becas-Chile Scholarship from the Government of Chile. R.F.A. was supported in part by a scholarship from the King Saud University. S.H. was supported in part by UT Austin College of Pharmacy.
References
- (1).Russell R; Watts N; Ebetino F; Rogers M Mechanisms of Action of Bisphosphonates: Similarities and Differences and Their Potential Influence on Clinical Efficacy. Osteoporos. Int 2008, 19, 733–759. [DOI] [PubMed] [Google Scholar]
- (2).Drake MT; Clarke BL; Khosla S In Bisphosphonates: Mechanism of Action and Role in Clinical Practice, Mayo Clin. Proc., Elsevier: 2008; 1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ripamonti C; Maniezzo M; Campa T; Fagnoni E; Brunelli C; Saibene G; Bareggi C; Ascani L; Cislaghi E Decreased Occurrence of Osteonecrosis of the Jaw after Implementation of Dental Preventive Measures in Solid Tumour Patients with Bone Metastases Treated with Bisphosphonates. The Experience of the National Cancer Institute of Milan. Ann. Oncol 2008, 20, 137–145. [DOI] [PubMed] [Google Scholar]
- (4).Jimenez‐Andrade JM; Mantyh WG; Bloom AP; Ferng AS; Geffre CP; Mantyh PW Bone Cancer Pain. Ann. N. Y. Acad. Sci 2010, 1198, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Guise TA Antitumor Effects of Bisphosphonates: Promising Preclinical Evidence. Cancer Treat. Rev 2008, 34, S19–S24. [DOI] [PubMed] [Google Scholar]
- (6).Hillner BE; Ingle JN; Chlebowski RT; Gralow J; Yee GC; Janjan NA; Cauley JA; Blumenstein BA; Albain KS; Lipton A American Society of Clinical Oncology 2003 Update on the Role of Bisphosphonates and Bone Health Issues in Women with Breast Cancer. J. Clin. Oncol 2003, 21, 4042–4057. [DOI] [PubMed] [Google Scholar]
- (7).Oster G; Lamerato L; Glass AG; Richert-Boe KE; Lopez A; Chung K; Richhariya A; Dodge T; Wolff GG; Balakumaran A Use of Intravenous Bisphosphonates in Patients with Breast, Lung, or Prostate Cancer and Metastases to Bone: A 15-Year Study in Two Large Us Health Systems. Support. Care Cancer 2014, 22, 1363–1373. [DOI] [PubMed] [Google Scholar]
- (8).Rosen LS; Gordon D; Tchekmedyian S; Yanagihara R; Hirsh V; Krzakowski M; Pawlicki M; de Souza P; Zheng M; Urbanowitz G Zoledronic Acid Versus Placebo in the Treatment of Skeletal Metastases in Patients with Lung Cancer and Other Solid Tumors: A Phase Iii, Double-Blind, Randomized Trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J. Clin. Oncol 2003, 21, 3150–3157. [DOI] [PubMed] [Google Scholar]
- (9).Kimmel D Mechanism of Action, Pharmacokinetic and Pharmacodynamic Profile, and Clinical Applications of Nitrogen-Containing Bisphosphonates. J. Dent. Res 2007, 86, 1022–1033. [DOI] [PubMed] [Google Scholar]
- (10).Lin J Bisphosphonates: A Review of Their Pharmacokinetic Properties. Bone 1996, 18, 75–85. [DOI] [PubMed] [Google Scholar]
- (11).Weiss HM; Pfaar U; Schweitzer A; Wiegand H; Skerjanec A; Schran H Biodistribution and Plasma Protein Binding of Zoledronic Acid. Drug Metab. Disposition 2008, 36, 2043–2049. [DOI] [PubMed] [Google Scholar]
- (12).Woo S-B; Hellstein JW; Kalmar JR Systematic Review: Bisphosphonates and Osteonecrosis of the Jaws. Ann. Intern. Med 2006, 144, 753–761. [DOI] [PubMed] [Google Scholar]
- (13).Ruggiero SL; Mehrotra B; Rosenberg TJ; Engroff SL Osteonecrosis of the Jaws Associated with the Use of Bisphosphonates: A Review of 63 Cases. J. Oral Maxillofac. Surg 2004, 62, 527–534. [DOI] [PubMed] [Google Scholar]
- (14).Marra M; Salzano G; Leonetti C; Tassone P; Scarsella M; Zappavigna S; Calimeri T; Franco R; Liguori G; Cigliana G Nanotechnologies to Use Bisphosphonates as Potent Anticancer Agents: The Effects of Zoledronic Acid Encapsulated into Liposomes. Nanomed. Nanotechnol. Biol. Med 2011, 7, 955–964. [DOI] [PubMed] [Google Scholar]
- (15).Shmeeda H; Amitay Y; Tzemach D; Gorin J; Gabizon A Liposome Encapsulation of Zoledronic Acid Results in Major Changes in Tissue Distribution and Increase in Toxicity. J. Control. Release 2013, 167, 265–275. [DOI] [PubMed] [Google Scholar]
- (16).Zeisberger S; Odermatt B; Marty C; Zehnder-Fjällman A; Ballmer-Hofer K; Schwendener R Clodronate-Liposome-Mediated Depletion of Tumour-Associated Macrophages: A New and Highly Effective Antiangiogenic Therapy Approach. Br. J. Cancer 2006, 95, 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hengst V; Oussoren C; Kissel T; Storm G Bone Targeting Potential of Bisphosphonate-Targeted Liposomes: Preparation, Characterization and Hydroxyapatite Binding in Vitro. Int. J. Pharm 2007, 331, 224–227. [DOI] [PubMed] [Google Scholar]
- (18).Nancollas G; Tang R; Phipps R; Henneman Z; Gulde S; Wu W; Mangood A; Russell R; Ebetino F Novel Insights into Actions of Bisphosphonates on Bone: Differences in Interactions with Hydroxyapatite. Bone 2006, 38, 617–627. [DOI] [PubMed] [Google Scholar]
- (19).Buiting AM; Zhou F; Bakker JA; Van Rooijen N; Huang L Biodistribution of Clodronate and Liposomes Used in the Liposome Mediated Macrophage ‘Suicide’approach. J. Immunol. Methods 1996, 192, 55–62. [DOI] [PubMed] [Google Scholar]
- (20).Khajuria DK; Razdan R; Mahapatra DR Development, in Vitro and in Vivo Characterization of Zoledronic Acid Functionalized Hydroxyapatite Nanoparticle Based Formulation for Treatment of Osteoporosis in Animal Model. Eur. J. Pharm. Sci 2015, 66, 173–183. [DOI] [PubMed] [Google Scholar]
- (21).Liu J; Karaman DŞ; Zhang J; Rosenholm JM; Guo X; Cai K Nir Light-Activated Dual-Modality Cancer Therapy Mediated by Photochemical Internalization of Porous Nanocarriers with Tethered Lipid Bilayers. J. Mater. Chem. B 2017, 5, 8289–8298. [DOI] [PubMed] [Google Scholar]
- (22).Desai D; Zhang J; Sandholm J; Lehtimäki J; Grönroos T; Tuomela J; Rosenholm JM Lipid Bilayer-Gated Mesoporous Silica Nanocarriers for Tumor-Targeted Delivery of Zoledronic Acid in Vivo. Mol. Pharm 2017, 14, 3218–3227. [DOI] [PubMed] [Google Scholar]
- (23).Li X; Naguib YW; Valdes S; Hufnagel S; Cui Z Reverse Microemulsion-Based Synthesis of (Bis) Phosphonate–Metal Materials with Controllable Physical Properties: An Example Using Zoledronic Acid–Calcium Complexes. ACS Appl. Mater. Interfaces 2017, 9, 14478–14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Li X; Naguib YW; Cui Z In Vivo Distribution of Zoledronic Acid in a Bisphosphonate-Metal Complex-Based Nanoparticle Formulation Synthesized by a Reverse Microemulsion Method. Int. J. Pharm 2017, 526, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Salzano G; Marra M; Porru M; Zappavigna S; Abbruzzese A; La Rotonda M; Leonetti C; Caraglia M; De Rosa G Self-Assembly Nanoparticles for the Delivery of Bisphosphonates into Tumors. Int. J. Pharm 2011, 403, 292–297. [DOI] [PubMed] [Google Scholar]
- (26).Marra M; Salzano G; Leonetti C; Porru M; Franco R; Zappavigna S; Liguori G; Botti G; Chieffi P; Lamberti M New Self-Assembly Nanoparticles and Stealth Liposomes for the Delivery of Zoledronic Acid: A Comparative Study. Biotechnol. Adv 2012, 30, 302–309. [DOI] [PubMed] [Google Scholar]
- (27).Salzano G; Zappavigna S; Luce A; D’Onofrio N; Balestrieri M; Grimaldi A; Lusa S; Ingrosso D; Artuso S; Porru M Transferrin-Targeted Nanoparticles Containing Zoledronic Acid as a Potential Tool to Inhibit Glioblastoma Growth. J. Biomed. Nanotechnol 2016, 12, 811–830. [DOI] [PubMed] [Google Scholar]
- (28).Zhu S; Niu M; O’Mary H; Cui Z Targeting of Tumor-Associated Macrophages Made Possible by Peg-Sheddable, Mannose-Modified Nanoparticles. Mol. Pharm 2013, 10, 3525–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Zhu S; Lansakara-P DS; Li X; Cui Z Lysosomal Delivery of a Lipophilic Gemcitabine Prodrug Using Novel Acid-Sensitive Micelles Improved Its Antitumor Activity. Bioconj. Chem 2012, 23, 966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Dunlap SM; Chiao LJ; Nogueira L; Usary J; Perou CM; Varticovski L; Hursting SD Dietary Energy Balance Modulates Epithelial-to-Mesenchymal Transition and Tumor Progression in Murine Claudin-Low and Basal-Like Mammary Tumor Models. Cancer Prev. Res 2012, 5, 930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Niu M; Valdes S; Naguib YW; Hursting SD; Cui Z Tumor-Associated Macrophage-Mediated Targeted Therapy of Triple-Negative Breast Cancer. Mol. Pharm 2016, 13, 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Makadia HK; Siegel SJ Poly Lactic-Co-Glycolic Acid (Plga) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Au KM; Satterlee A; Min Y; Tian X; Kim YS; Caster JM; Zhang L; Zhang T; Huang L; Wang AZ Folate-Targeted Ph-Responsive Calcium Zoledronate Nanoscale Metal-Organic Frameworks: Turning a Bone Antiresorptive Agent into an Anticancer Therapeutic. Biomaterials 2016, 82, 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hugentobler S; Morris D; Sreenan J; Diskin M Ion Concentrations in Oviduct and Uterine Fluid and Blood Serum During the Estrous Cycle in the Bovine. Theriogenology 2007, 68, 538–548. [DOI] [PubMed] [Google Scholar]
- (35).Suk JS; Xu Q; Kim N; Hanes J; Ensign LM Pegylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Del. Rev 2016, 99, 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Ferrari R; Lupi M; Colombo C; Morbidelli M; D’Incalci M; Moscatelli D Investigation of Size, Surface Charge, Pegylation Degree and Concentration on the Cellular Uptake of Polymer Nanoparticles. Colloids Surf. B. Biointerfaces 2014, 123, 639–647. [DOI] [PubMed] [Google Scholar]
- (37).Mohanraj V; Chen Y Nanoparticles-a Review. Tropical journal of pharmaceutical research 2006, 5, 561–573. [Google Scholar]
- (38).Cho K; Wang X; Nie S; Shin DM Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res 2008, 14, 1310–1316. [DOI] [PubMed] [Google Scholar]
- (39).Liu D; Kramer SA; Huxford-Phillips RC; Wang S; Della Rocca J; Lin W Coercing Bisphosphonates to Kill Cancer Cells with Nanoscale Coordination Polymers. Chem. Commun 2012, 48, 2668–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Solinas G; Germano G; Mantovani A; Allavena P Tumor-Associated Macrophages (Tam) as Major Players of the Cancer-Related Inflammation. J. Leukoc. Biol 2009, 86, 1065–1073. [DOI] [PubMed] [Google Scholar]
- (41).Luo Y; Zhou H; Krueger J; Kaplan C; Lee S-H; Dolman C; Markowitz D; Wu W; Liu C; Reisfeld RA Targeting Tumor-Associated Macrophages as a Novel Strategy against Breast Cancer. J. Clin. Invest 2006, 116, 2132–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Laoui D; Movahedi K; Van Overmeire E; Van den Bossche J; Schouppe E; Mommer C; Nikolaou A; Morias Y; De Baetselier P; Van Ginderachter JA Tumor-Associated Macrophages in Breast Cancer: Distinct Subsets, Distinct Functions. Int. J. Dev. Biol 2011, 55, 861–867. [DOI] [PubMed] [Google Scholar]
- (43).Rogers TL; Holen I Tumour Macrophages as Potential Targets of Bisphosphonates. Journal of translational medicine 2011, 9, 117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Gnant M; Clézardin P Direct and Indirect Anticancer Activity of Bisphosphonates: A Brief Review of Published Literature. Cancer treatment reviews 2012, 38, 407–415. [DOI] [PubMed] [Google Scholar]
- (45).Mantovani A; Bottazzi B; Colotta F; Sozzani S; Ruco L The Origin and Function of Tumor-Associated Macrophages. Immunol. Today 1992, 13, 265–270. [DOI] [PubMed] [Google Scholar]
- (46).Pollard JW Tumour-Educated Macrophages Promote Tumour Progression and Metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [DOI] [PubMed] [Google Scholar]
- (47).Condeelis J; Pollard JW Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis. Cell 2006, 124, 263–266. [DOI] [PubMed] [Google Scholar]
- (48).Medrek C; Pontén F; Jirström K; Leandersson K The Presence of Tumor Associated Macrophages in Tumor Stroma as a Prognostic Marker for Breast Cancer Patients. BMC Cancer 2012, 12, 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Yuan Z-Y; Luo R-Z; Peng R-J; Wang S-S; Xue C High Infiltration of Tumor-Associated Macrophages in Triple-Negative Breast Cancer Is Associated with a Higher Risk of Distant Metastasis. Onco Targets Ther. 2014, 7, 1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Zhang W; Zhu X-D; Sun H-C; Xiong Y-Q; Zhuang P-Y; Xu H-X; Kong L-Q; Wang L; Wu W-Z; Tang Z-Y Depletion of Tumor-Associated Macrophages Enhances the Effect of Sorafenib in Metastatic Liver Cancer Models by Antimetastatic and Antiangiogenic Effects. Clin. Cancer Res 2010, 16, 3420–3430. [DOI] [PubMed] [Google Scholar]
- (51).Junankar S; Shay G; Jurczyluk J; Ali N; Down J; Pocock N; Parker A; Nguyen A; Sun S; Kashemirov B Real-Time Intravital Imaging Establishes Tumor-Associated Macrophages as the Extraskeletal Target of Bisphosphonate Action in Cancer. Cancer Discov. 2015, 5, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Sun S; Błażewska KM; Kadina AP; Kashemirov BA; Duan X; Triffitt JT; Dunford JE; Russell RGG; Ebetino FH; Roelofs AJ Fluorescent Bisphosphonate and Carboxyphosphonate Probes: A Versatile Imaging Toolkit for Applications in Bone Biology and Biomedicine. Bioconj. Chem 2015, 27, 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Zhang L; Gu F; Chan J; Wang A; Langer R; Farokhzad O Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther 2008, 83, 761–769. [DOI] [PubMed] [Google Scholar]
- (54).Torchilin V Tumor Delivery of Macromolecular Drugs Based on the Epr Effect. Adv. Drug Del. Rev 2011, 63, 131–135. [DOI] [PubMed] [Google Scholar]
- (55).Storm G; Belliot SO; Daemen T; Lasic DD Surface Modification of Nanoparticles to Oppose Uptake by the Mononuclear Phagocyte System. Adv. Drug Del. Rev 1995, 17, 31–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.