This randomized clinical trial examines the futility of studying anodal transcranial direct current stimulation vs sham stimulation as an adjunctive intervention during speech therapy to improve speech production (naming) for older adults with long-term poststroke aphasia.
Key Points
Question
For individuals with long-term poststroke aphasia undergoing speech therapy, is it futile to conduct further research to evaluate the treatment efficacy of brain stimulation (anodal transcranial direct current stimulation [tDCS]) as an adjunctive intervention to improve speech production (naming)?
Findings
This randomized clinical trial used a futility design to test adjunctive anodal tDCS during speech therapy among 74 patients with long-term aphasia who received 3 weeks of therapy coupled with either anodal tDCS or sham tDCS. The magnitude of pretreatment to posttreatment improvement using anodal tDCS compared with sham did not find evidence that further investigation of anodal tDCS was futile.
Meaning
Anodal tDCS during speech therapy should be further assessed for treatment of patients with aphasia.
Abstract
Importance
Aphasia is a debilitating language disorder for which behavioral speech therapy is the most efficient treatment, but therapy outcomes are variable and full recovery is not always achieved. It remains unclear if adjunctive brain stimulation (anodal transcranial direct current stimulation [A-tDCS]) applied during aphasia therapy can improve outcomes.
Objective
To examine the futility of studying A-tDCS as an adjunctive intervention during speech therapy to improve speech production (naming) for individuals with long-term poststroke aphasia.
Design, Setting, and Participants
Double-blinded, prospective randomized clinical trial using a futility design to test adjunctive A-tDCS during speech therapy. The setting was an outpatient clinic. Enrollment of individuals began in August 2012 and was completed in March 2017, and the duration of follow-up was 6 months. Analyses began in April 2017. The study recruited from a volunteer sample, and 89 patients were screened. Patients with long-term (>6 months) aphasia due to 1 previous left hemisphere stroke were enrolled. In comparing A-tDCS and sham tDCS, patients were matched based on site (University of South Carolina or Medical University of South Carolina), baseline age, type of aphasia, and aphasia severity.
Interventions
Outpatient speech therapy for 3 weeks (15 sessions, 45 minutes each) combined with either A-tDCS vs sham tDCS applied to preserved left temporal lobe regions.
Main Outcomes and Measures
The primary outcome was the ability to name common objects, assessed twice before and after therapy.
Results
A total of 74 patients were enrolled. Participants had a mean (SD) age of 60 (10) years, had 15 (2) years of education, and were 44 (40) months from stroke onset. There were 52 men (70%) and 62 non-Hispanic white individuals (84%). Most were retired or not employed (59 [80%]). Broca aphasia was the most common aphasia type (39 [52.7%]). The adjusted mean (SE) change from pretreatment baseline in correct naming was 13.9 (2.4) words (95% CI, 9.0-18.7) for A-tDCS and 8.2 (2.2) words (95% CI, 3.8-12.6) for sham tDCS, with mean (SE) A-tDCS difference of 5.7 (3.3) words (95% CI, −0.9 to 12.3), indicating a relative 70% increase in correct naming for A-tDCS relative to sham. The futility hypothesis P value was .90, indicating failure to reject the null hypothesis and, therefore, providing no evidence that further study of A-tDCS is futile. No serious adverse events were associated with A-tDCS.
Conclusions and Relevance
Our findings provide motivation to proceed with another trial to study the effect of A-tDCS on the outcome of aphasia treatment in individuals poststroke. Anodal tDCS during speech therapy is feasible and potentially transformative for aphasia treatment and should be further studied.
Trial Registration
ClinicalTrials.gov Identifier: NCT01686373
Introduction
The National Institute on Deafness and Other Communication Disorders estimates that at least 1 million people experience poststroke aphasia in the United States.1 Considerable evidence suggests behavioral aphasia treatment is effective in improving communication and quality of life in individuals with long-term aphasia.2,3,4 Nevertheless, even with therapy, aphasia recovery is often minimal.5
During the past decade, several pilot studies have indicated adjunctive transcranial direct current stimulation (tDCS) may improve the effects of aphasia treatment.6,7,8,9 Transcranial direct current stimulation is a noninvasive method that uses an electrical current (1-2 mA) typically induced between 2 electrodes placed on the scalp. The specific neural mechanism underlying tDCS modulation is not completely understood, but anodal tDCS (A-tDCS) has been shown to generally enhance cortical activity, whereas cathodal stimulation usually has the opposite effect.10
Based on promising pilot data,6,7 we carried out a double-blinded randomized clinical trial to test whether further investigation of the efficacy of adjunctive A-tDCS combined with aphasia therapy to manage long-term poststroke aphasia is futile. We used a futility design in which the null hypothesis assumed a benefit of A-tDCS compared with sham tDCS (S-tDCS), and the alternative hypothesis assumed no difference between A-tDCS and S-tDCS.11,12,13 Instead of demonstrating efficacy, the futility design permits the identification of treatments that do not warrant further investigation, demonstrating a lack of superiority. Treatments for which a lack of superiority cannot be demonstrated are then suitable candidates for further investigation with traditional superiority trial designs.
Methods
Patients
The trial protocol is available in Supplement 1. Patients were enrolled from August 2012 to March 2017, and analyses began in April 2017. Patient inclusion criteria was single-event ischemic stroke in the left hemisphere, longer than 6 months poststroke, between the ages of 25 and 80 years, previously right-handed, aphasia as confirmed using the Western Aphasia Battery-Revised (WAB-R14), no magnetic resonance imaging (MRI) contraindications, and able to achieve at least 65% accuracy on a screening version of the aphasia treatment task (see details in the section titled Aphasia Treatment). The correlation between performance on the screening version of the aphasia treatment task and overall aphasia severity, measured as the Aphasia Quotient (AQ; a 100-point scale) on the WAB-R, was r = 0.27, P = .02. Exclusion criteria was history of brain surgery, seizures during the previous 12 months, sensitive scalp (per patient report), more than 80% naming accuracy on the Philadelphia Naming Test (PNT),15 and unable to overtly name at least 5 of 80 items during pretreatment functional MRI (fMRI) sessions. The study was approved by the institutional review boards at the University of South Carolina and the Medical University of South Carolina, where all data collection occurred. All participants provided written consent for study inclusion. An independent data safety monitoring board assessed safety and quality of the study.
Randomization and Blinding
Eligible individuals were randomized to either A-tDCS or S-tDCS coupled with a computerized behavioral treatment of anomia.16 The Biostatistics Core at the Data Coordination Unit (located at Medical University of South Carolina) programmed the randomization algorithm, which used the minimal sufficient balancing method to prevent imbalances in site, baseline age, aphasia type, and aphasia severity.17 Study participants and all members of the study team (the speech language pathologists [SLPs] who administered clinical testing and treatment, study coordinators, and principal and coinvestigators) were blinded to the intervention assignment.
Transcranial Direct Current Stimulation
Brain stimulation relied on a constant current stimulator (Phoresor II PM850; Iomed Inc) that provided 1 mA of A-tDCS stimulation induced between two 5 × 5 cm saline-soaked sponges (electrodes). The selection of 1 mA current was consistent with our previous pilot studies and our in-house data suggesting that 1 mA is less likely to induce scalp pain compared with 2 mA, a current strength also commonly used in the literature. The anode electrode was placed on the left scalp over a targeted cortical region and the cathode electrode was placed on the contralateral supraorbital frontal scalp region (above the right eyebrow). All participants completed 2 MRI sessions at baseline, which included T1- and T2-weighted structural MRI and a picture naming fMRI protocol. As our goal was to stimulate surviving eloquent tissue, the anodal electrode was placed over the temporal lobe region with the highest naming related activation on the fMRI (for more details on the fMRI setup, see the study by Fridriksson5). Each individual’s fMRI data were coregistered with their T1 scan, and a magnetic position tracker (Ascension Technology flock-of-birds) in combination with MRIreg (http://people.cas.sc.edu/rorden/mricro/mrireg/index.html) was used to coregister each individual's scalp coordinates with their T1 scan. Using this setup, the desired cortical region was located and demarcated on a latex cap worn by the patient. This cap was carefully fitted on the patient prior to the start of each tDCS administration to accurately position the anode electrode in the same area from one day to the next. Following positioning, the cap was removed and the electrodes were held in place with self-adhesive bandages. The scalp coordinates where the left hemisphere electrode was placed for each participant can be seen in eFigure in Supplement 2. The A-tDCS stimulation was started at the beginning of the behavioral treatment sessions and remained active during the first 20 minutes of the 45-minute treatment session. The 20-minute stimulation period was chosen based on our preliminary studies that suggested it was well tolerated by participants and was not associated with serious adverse events. Typically, participants in tDCS studies report itching or tingling sensation under the electrodes during the first 15 to 20 seconds of stimulation; however, this sensation is transient.10 To blind patients as to whether they were receiving active or sham tDCS, the same scalp sensation was induced during the start of the S-tDCS sessions when the tDCS stimulation was applied to the scalp for 30 seconds but then the current was gradually decreased over 15 seconds as the current was shunted to a load resistor. In-house hardware was used to mask treatment type (A-tDCS vs S-tDCS) for both patients as well as the SLPs. The described randomization scheme directed an independent technician to set the position of an internal switch on the sham controller. Neither the patient nor SLP was aware of the position and the SLP did not know which switch position (X or Y) was the sham position. Treatment type was encoded in the software so the SLP only needed to enter a patient and session number to start stimulation without knowing whether those specific numbers were assigned to A-tDCS or S-tDCS. Following each individual’s treatment, a technician validated whether the tDCS device was delivering anodal or sham stimulation.
Aphasia Treatment
The aphasia treatment was performed through a computerized task that involved matching pictures depicting common objects with words that were heard (via headphones) and seen (the face of the speaker below the nose is shown on the computer screen).7,16 Patients were instructed to press a green response button if the picture and spoken word matched and a red response button if they did not. Incorrect matches included a semantic foil, a phonological, or an unrelated word. Half of the pairs represented a correct match. Immediate feedback was provided following each response, and task accuracy was displayed on the computer screen at the end of each session to allow patients to monitor their progress. A total of 160 low-, medium-, and high-frequency words not included on the PNT were targeted in the computerized treatment task. Most participants completed treatment in clinics, whereas a few received treatment at their place of residence.
Procedures
The initial screening visit occurred over 2 days. Participants underwent a medical history and comprehensive neurologic, language, and cognitive testing using the following tests: National Institutes of Health Stroke Scale,18 WAB-R, the Boston Naming Test–Second Edition,19 the Pyramids and Palm Trees Test,20 the Apraxia of Speech Rating Scale,21 and the matrix reasoning subtest of the Wechsler Adult Intelligence Scale, Third Edition.22 Consistent with what is a typical dose of outpatient therapy for long-term aphasia in the United States,23 both study arms received 3 weeks of the computerized anomia treatment (15 sessions within 21 days, 45-minute sessions). Patients were assessed at the end of each treatment session for adverse events, vital signs, and discomfort ratings (for potential scalp sensations associated with tDCS) using the Wong-Baker FACES Pain Rating Scale.24 Treatment fidelity was monitored through periodic observations of assessment and treatment sessions by the principal investigator (J.F.) and the lead clinician in charge of the study.
Outcomes
The primary end point was the change in the number of correctly named common objects at 1 week posttreatment, measured using a portion (Naming 80) of the trained items from the treatment plus the PNT. Only some treatment items were selected to decrease assessment time at each time. The PNT is commonly used in research studies to assess anomia and includes 175 pictures depicting mid-frequency to high-frequency nouns, which patients are instructed to name 1 item at a time. Naming accuracy was scored based on PNT scoring guidelines.15
The pretreatment to posttreatment change was computed as the difference between the mean of the 2 pretreatment assessments and the mean of the 2 posttreatment sessions. Secondary outcomes included change in the number of correctly named items at 4 and 24 weeks posttreatment.
Statistical Analysis
The primary null hypothesis assumed A-tDCS would lead to at least a 1.5-item greater improvement in correct naming compared with S-tDCS. The alternative hypothesis assumed no difference between the 2 conditions. The statistical hypotheses were H0: μA − μS ≥ 1.5 vs HA: μA − μS <1.5, in which μA was the expected change (pretreatment and 1-week posttreatment) in the number of correctly named items in the A-tDCS group and μS was the expected change in the S-tDCS group. If the null hypothesis was rejected at a 1-sided significance level of .10, then A-tDCS would be unlikely to be effective for aphasia management, and further study of A-tDCS would be considered futile.
In preliminary studies with 5 treatment sessions, the mean difference between the A-tDCS and S-tDCS groups in naming accuracy was 2.5 words (change from baseline; pooled SD 2.6), and the S-tDCS group mean change was 4.0.6,7 To estimate the sample size, we assumed the mean change from baseline to 1-week posttesting for the A-tDCS group under the null hypothesis of nonfutility to be μA = μS + 1.5 = 4 + 1.5 = 5.5. Under these assumptions (H0: μA = 5.5 and HA: μA = 4) with 33 individuals per group, a 2-sample t test with a .10 1-sided significance level will have 85% power to reject the null hypothesis that the A-tDCS treatment is 1.5 points better than S-tDCS and declare futility when the A-tDCS treatment comes from a distribution with mean change of 4 (assuming the pooled SD is 2.6). Assuming a dropout rate of 5%, the required sample size was inflated from 33 to 37 per group to account for the effect of the dropouts in the intent-to-treat analysis using an inflation factor.25
The primary analysis was an intent-to-treat analysis and was adjusted for enrolling site and baseline aphasia severity measured as the AQ from the WAB-R. Missing data (for 1 patient) were imputed using multiple imputation, assuming a monotone missing mechanism, missing at random, and used 10 imputed data sets (SAS PROC MI and MIANALYZE). All analyses were conducted in SAS version 9.3 (SAS Institute Inc).
Results
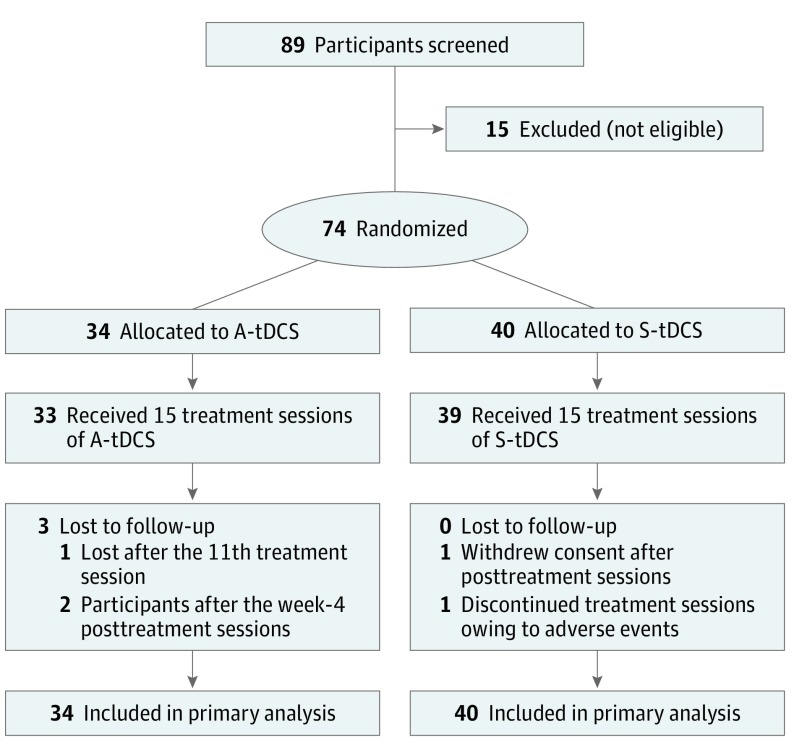
Between August 2012 and April 2017, 89 patients were screened, and 74 patients (83%) were enrolled (Figure 1). Thirty-four individuals (41%) were randomized to receive A-tDCS, and 40 (48%) were randomized to receive S-tDCS. The last individual was randomized during April 2017. On May 25, 2017, the study database was partially locked, up to and including the 1-week posttreatment visits. Once all follow-up visits were completed, the database was locked on November 8, 2017. One individual withdrew consent after completing posttreatment assessments, and 1 individual was lost to follow-up after the 11th treatment session. Therefore, the primary outcome was missing for only 1 individual.
Figure 1. Participant Flow Diagram.
A-tDCS indicates anodal transcranial direct current stimulation; S-tDCS, sham transcranial direct current stimulation.
Two individuals who had hemorrhagic stroke rather than ischemic stroke were erroneously enrolled, both in the S-tDCS group. As this was an intent-to-treat trial, their data were included in the primary analyses. Baseline demographic and clinical characteristics were similar between the 2 treatment arms (Table 1). The enrolled individuals had a mean (SD) age of 60 (10) years, had 15 (2) years of education, and were 44 (40) months from stroke onset. There were 52 men (70%) and 62 non-Hispanic white individuals (84%). Most were retired or not employed (59 [80%]). Broca aphasia was the most common aphasia type (39 [52.7%]). Several of the clinical characteristics were somewhat higher on average at baseline in the A-tDCS group, although not statistically significantly different from the S-tDCS group. eTable in Supplement 2 compares the distribution of aphasia types and severity in the current trial with a large national cohort of patients with long-term aphasia (AphasiaBank26). Overall, the current trial included more severe aphasia than the AphasiaBank cohort as indicated by a lower WAB-R AQ and has greater rate of Broca aphasia and fewer participants with anomic aphasia. To ensure proper blinding, each patient and clinician was asked to guess the stimulation type at the end of their treatment phase. Patients’ guessing accuracy was 47.9% and clinicians’ guessing accuracy was 54.2%, meaning that each group’s accuracy was essentially at chance guessing. All but 1 participant improved on the treatment task as suggested by greater task accuracy on the last treatment session compared with the first treatment session (overall mean [SD] change in accuracy was 10.3 [7.9]), suggesting that patients were actively participating in the aphasia therapy and were compliant with the task.
Table 1. Baseline Demographics and Clinical Characteristicsa.
| Variable | Mean (SD) | |
|---|---|---|
| A-tDCS (n = 34) | S-tDCS (n = 40) | |
| Age, y | 60 (11) | 60 (10) |
| Men, No. (%) | 24 (70.5) | 28 (70) |
| Non-Hispanic white, No. (%) | 27 (79.4) | 35 (88) |
| Education, y | 15 (3) | 14 (2) |
| Time since stroke onset, mo | 44 (45) | 40 (35) |
| Picture word matching screen accuracy, % | 76 (13) | 73 (14) |
| WAB-R aphasia quotient | 60 (19) | 56 (20) |
| BNT total No. correct | 22 (19) | 17 (16) |
| PPTT total | 46 (4) | 46 (4) |
| Matrix reasoning-WAIS III | 12 (6) | 11 (5) |
| ASRSb | 3 (2) | 3 (2) |
| NIH Stroke Scale scoreb | 5 (3) | 5 (3) |
| PNT correct | 62 (45) | 55 (41) |
| Naming 80 correct | 21 (18) | 16 (16) |
| History, No. (%) | ||
| Diabetes | 5 (14.7) | 6 (15) |
| Depression | 4 (11.7) | 9 (22.5) |
| Aphasia type, No. (%) | ||
| Global | 1 (2.9) | 2 (5.0) |
| Broca aphasia | 18 (52.9) | 21 (52.5) |
| Transcortical motor | 1 (2.9) | 0 (0.0) |
| Wernicke aphasia | 3 (8.8) | 2 (5.0) |
| Transcortical sensory | 0 (0.0) | 0 (0.0) |
| Conduction | 6 (17.6) | 9 (22.5) |
| Anomic | 5 (14.7) | 6 (15.0) |
Abbreviations: ASRS, Apraxia of Speech Rating Scale; A-tDCS, anodal transcranial direct current stimulation; BNT, Boston Naming Test; NIH, National Institutes of Health; PNT, Philadelphia Naming Test; PPTT, Pyramids and Palm Trees Test; S-tDCS, sham transcranial direct current stimulation; WAB-R, Western Aphasia Battery-Revised; WAIS III, Wechsler Adult Intelligence Scale.
No statistically significant differences between groups were detected at baseline (P > .05).
Higher scores indicate that they were worse.
Table 2 demonstrates the results from the primary analysis, which is based on the intent-to-treat sample (n = 74). The P value of .90 indicates a failure to reject the null hypothesis, and there is no evidence that further investigation of A-tDCS would be futile as an adjunctive treatment for poststroke aphasia. Baseline aphasia severity (AQ) was correlated with the overall improvement in naming at 1 week posttreatment (Pearson ρ = 0.29, P = .01). The adjusted mean (SE) 1-week posttreatment change was an increase in 13.9 (2.4) (95% CI, 9.0-18.7) items correctly named for the A-tDCS group and 8.2 (2.2) (95% CI, 3.8-12.6) for the S-tDCS group (mean [SE] difference of 5.7 [3.3]; 95% CI, −0.9 to 12.3; Figure 2). The results of an unadjusted, completers-only analysis excluding the 2 ineligible patients with hemorrhagic stroke (n = 71) of the primary outcome were consistent with the primary analysis of the intent-to-treat sample (test of H0: μA − μS ≥ 1.5; t statistic, 1.35; 1-sided P = .91). Because of an imbalance at baseline on the primary outcome, a sensitivity analysis was conducted and the primary outcome was adjusted for baseline PNT + Naming 80 score, treatment site, and baseline AQ; the results were consistent with the primary analysis (test of H0: μA − μS ≥ 1.5; t statistic, 1.2; 1-sided P = .89). At 4 weeks posttreatment, the adjusted mean (SE) change from baseline in correct naming was an increase in 16.8 (2.8) correctly named (95% CI, 11.3-22.4) for A-tDCS and 9.4 (2.5) (95% CI,4.4-14.5) for S-tDCS (intent-to-treat sample, adjusted for site and baseline aphasia severity) (test of μA−μS ≥ 1.5, 1-sided P = .94). At 24 weeks posttreatment, the adjusted mean (SE) change from baseline in correct naming was 14.9 (3.7) (95% CI, 8.8-21.1) for A-tDCS and 7.1 (3.3) (95% CI, 1.59-12.0) for S-tDCS (intent-to-treat sample, adjusted for site and baseline AQ) (test of H0: μA−μS ≥ 1.5, 1-sided P = .90).
Table 2. Primary Outcome: Change in Correct Naming on Philadelphia Naming Test and 80 Trained Items at 1-Week Posttreatment Period.
| Variable | Mean (95% CI) | H0: μA−μS ≥ 1.5 t Statistic | P Value (1-sided) |
|---|---|---|---|
| Intent-to-Treat Sample, Adjusted Means | |||
| A-tDCS (n = 34) | 13.9 (9.0 to 18.7) | 1.27a | .896 |
| S-tDCS (n = 40) | 8.2 (3.8 to 12.6) | ||
| Difference | 5.7 (−0.9 to 12.3) | ||
| Completers Only Sample, Unadjusted Means | |||
| A-tDCS (n = 33) | 14.0 (7.7 to 20.4) | 1.35b | .909 |
| S-tDCS (n = 38) | 7.8 (4.3 to 11.4) | ||
| Difference | 6.2 (−0.7 to 13.2) |
Abbreviations: A-tDCS, anodal transcranial direct current stimulation; NA, not applicable; S-tDCS, sham transcranial direct current stimulation.
Adjusted for site and baseline aphasia quotient.
Unadjusted.
Figure 2. Mean (SE) Change in Correct Naming by Treatment Group.
A-tDCS indicates anodal transcranial direct current stimulation; S-tDCS, sham transcranial direct current stimulation.
The treatment sessions were well tolerated. There were 2 enrolled individuals (3%) who did not receive all 15 treatment sessions (1 A-tDCS individual and 1 S-tDCS individual). The A-tDCS individual dropped out after treatment session 11. The S-tDCS individual experienced a seizure during the course of the trial, and treatment sessions were subsequently discontinued. Importantly, the individual who experienced the seizure was in the S-tDCS group, thus receiving sham stimulation.
There were 8 mild, nonserious adverse events (Table 3), and there were no statistically significant differences between treatment groups for number of adverse events. Two individuals (6%) in the A-tDCS group experienced transient scalp redness/irritation (erythema) compared with none in the S-tDCS group. On the Wong-Baker FACES Pain Rating Scale, most often individuals reported no hurt: 94% (n = 476) in A-tDCS vs 86% (n = 511) in S-tDCS. The highest pain rating reported was 3 (indicating “hurts even more”), which was reported 4 times by 2 individuals (3%), both in the S-tDCS group. Vital signs were similar between groups for all treatment sessions.
Table 3. Adverse Eventsa.
| Adverse Event | Treatment, No. (%) of Patients | |
|---|---|---|
| A-tDCS (n = 34) | S-tDCS (n = 40) | |
| Headache | 0 (0) | 2 (5) |
| Dizziness | 1 (3) | 2 (5) |
| Erythema | 2 (6) | 0 (0) |
| Convulsion | 0 (0) | 1 (2.5) |
| Hypertension | 0 (0) | 1 (2.5) |
Abbreviations: A-tDCS, anodal transcranial direct current stimulation; S-tDCS, sham transcranial direct current stimulation.
No statistically significant differences between treatment groups were detected (Fisher exact test, 2-sided P > .20).
Discussion
This study found no evidence that further study of adjunctive A-tDCS would be futile when combined with behavioral aphasia treatment. Given that we failed to reject the null hypothesis, that A-tDCS results in better treatment outcome than S-tDCS, the results suggest a larger trial may be warranted to further evaluate the effects of A-tDCS on aphasia treatment. The current results, along with our previous smaller pilot studies,6,7 lend support to the underlying scientific hypothesis that adjunctive A-tDCS improves the outcomes of long-term aphasia treatment among individuals with poststroke aphasia, although further research is needed to test this definitively.
Breitensten and colleagues2 found that baseline stroke severity was associated with aphasia treatment outcome in which patients with more severe aphasia were less likely to respond. In the current trial, stroke severity, as assessed by the National Institutes of Health Stroke Scale, and the distribution of aphasia types, was comparable across the 2 study arms. However, there were several numerical differences in aphasia severity at baseline, all of which were not statistically significant, but the A-tDCS group was nominally better at baseline. However, the difference in the primary outcome remained even after adjusting for baseline differences in aphasia severity; thus, the observed difference is unlikely to be due to differences in baseline status.
Naming was chosen as the primary outcome because anomia is present in all types of aphasia regardless of severity, and naming is commonly targeted in aphasia treatment to improve word retrieval and speech production. Although naming is not synonymous with speech production, naming impairment is directly associated with poor quality of life in patients with aphasia.27 Yet, other pilot studies have also suggested adjunctive A-tDCS during aphasia treatment can result in greater improvements in functional communication abilities.28 Whereas the standard of care for aphasia is behavioral speech therapy,29 a minimal clinically important difference in naming accuracy has not been established for English-speaking patients. Specifically, it is not clear what amount of improvement in language processing patients would consider as enhancement of daily functioning, although we believe that even 1 to 2 words’ improvement could be meaningful to some patients who have very limited speech output. At all 3 times posttreatment, the change from baseline in A-tDCS was nearly twice as large as that of the S-tDCS group, an effect that is likely to be meaningful. Nevertheless, based on the current data, we cannot assume the treatment effect demonstrated here would generalize to functional communication abilities.
The treatment task used here emphasizes lexical-semantic processing and was selected because it has been shown to improve naming in persons with aphasia.6,7,16 Most importantly, it enabled controlling of equal treatment time and intensity across the 2 study arms. There are other forms of aphasia therapy that are probably equally or more effective for improving naming, and the purpose of the current trial was not to confirm the effectiveness of aphasia therapy but to assess the adjuvant benefit of A-tDCS when combined with a proven form of aphasia therapy. Based on first principles, we can see no reason why the effect of A-tDCS should be treatment-type–specific suggesting that an effect of A-tDCS would likely generalize to other kinds of aphasia treatment approaches. However, this may need to be verified in future studies. It is also important that future studies assess factors such as the length of the aphasia treatment session in relation to the optimal tDCS duration and intensity as we do not know whether and how much the current results are specific to the current study protocol.
Given that the total number of A-tDCS sessions administrated here was more than 500, the rate of adverse events shown in this study would have to be considered very low. Erythema on the scalp occurred exclusively in the A-tDCS group (6% of individuals) but was mild and resolved within 1 to 2 days. These data suggest A-tDCS administered at 1 mA for 20 minutes is safe and has minimal adverse effects. A recent Cochrane review of tDCS studies in aphasia also reported no serious adverse events for any studies included in the review.30
Limitations
The current study was not powered for superiority analyses to compare A-tDCS with S-tDCS. Therefore, we cannot conclude that A-tDCS is effective for boosting the effect of aphasia even though the magnitude of naming improvement was numerically greater with A-tDCS compared with S-tDCS. It is also a limitation that we cannot definitively determine that the naming improvements translate to improvements in quality of life.
Conclusions
The results reported here suggest that adjunctive A-tDCS is worthy of further study in a randomized clinical trial as an option to enhance the effect of behavioral treatment of aphasia in stroke. They provide the necessary basis to inform a definitive trial to assess A-tDCS as a treatment option for aphasia.
Trial protocol.
eFigure. Coordinates for Anodal Electrode Placements on the Left Scalp for All Participants
eTable. Distribution of Aphasia Types and Severity in the Current Trial Compared to a Large National Cohort of Chronic Patients (AphasiaBank)
References
- 1.NIDCD National Institute on Deafness and Other Communication Disorders (NIDCD) fact sheet: aphasia. https://www.nidcd.nih.gov/sites/default/files/Documents/health/voice/FactSheetAphasia.pdf. Accessed July 11, 2018.
- 2.Breitenstein C, Grewe T, Flöel A, et al. ; FCET2EC study group . Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet. 2017;389(10078):1528-1538. doi: 10.1016/S0140-6736(17)30067-3 [DOI] [PubMed] [Google Scholar]
- 3.Brady MC, Kelly H, Godwin J, Enderby P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev. 2012;(5):CD000425. doi: 10.1002/14651858.CD000425.pub3 [DOI] [PubMed] [Google Scholar]
- 4.Brady MC, Kelly H, Godwin J, Enderby P, Campbell P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev. 2016;(6):CD000425. doi: 10.1002/14651858.CD000425.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridriksson J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J Neurosci. 2010;30(35):11558-11564. doi: 10.1523/JNEUROSCI.2227-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41(6):1229-1236. doi: 10.1161/STROKEAHA.109.576785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke. 2011;42(3):819-821. doi: 10.1161/STROKEAHA.110.600288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherney LR, Erickson RK, Small SL. Epidural cortical stimulation as adjunctive treatment for non-fluent aphasia: preliminary findings. J Neurol Neurosurg Psychiatry. 2010;81(9):1014-1021. doi: 10.1136/jnnp.2009.184036 [DOI] [PubMed] [Google Scholar]
- 9.Kang EK, Kim YK, Sohn HM, Cohen LG, Paik N-J. Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca’s homologue area. Restor Neurol Neurosci. 2011;29(3):141-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi: 10.1016/j.brs.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 11.Palesch YY, Tilley BC, Sackett DL, Johnston KC, Woolson R. Applying a phase II futility study design to therapeutic stroke trials. Stroke. 2005;36(11):2410-2414. doi: 10.1161/01.STR.0000185718.26377.07 [DOI] [PubMed] [Google Scholar]
- 12.Koch MW, Korngut L, Patry DG, et al. The promise of futility trials in neurological diseases. Nat Rev Neurol. 2015;11(5):300-305. doi: 10.1038/nrneurol.2015.34 [DOI] [PubMed] [Google Scholar]
- 13.Levin B. The futility study: progress over the last decade. Contemp Clin Trials. 2015;45(Pt A):69-75. doi: 10.1016/j.cct.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kertesz A. Western Aphasia Battery-Revised. San Antionio, TX: Pearson; 2007. [Google Scholar]
- 15.Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia naming test: scoring and rationale. Clin Aphasiol. 1996;24:121-134. [Google Scholar]
- 16.Fridriksson J, Baker JM, Whiteside J, et al. Treating visual speech perception to improve speech production in nonfluent aphasia. Stroke. 2009;40(3):853-858. doi: 10.1161/STROKEAHA.108.532499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao W, Hill MD, Palesch Y. Minimal sufficient balance-a new strategy to balance baseline covariates and preserve randomness of treatment allocation. Stat Methods Med Res. 2015;24(6):989-1002. doi: 10.1177/0962280212436447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864-870. doi: 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 19.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, PA: Lean & Febiger; 1983. [Google Scholar]
- 20.Howard D, Patterson K. The Pyramids and Palm Trees Test: A Test of Semantic Access From Words and Pictures. Cambridge, UK: Thames Valley Test Co; 1992. [Google Scholar]
- 21.Strand EA, Duffy JR, Clark HM, Josephs K. The Apraxia of Speech Rating Scale: a tool for diagnosis and description of apraxia of speech. J Commun Disord. 2014;51:43-50. doi: 10.1016/j.jcomdis.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale–Third Edition (WAIS–III). San Antonio, TX: Pearson; 1997. [Google Scholar]
- 23.Katz RC, Hallowell B, Code C, et al. A multinational comparison of aphasia management practices. Int J Lang Commun Disord. 2000;35(2):303-314. doi: 10.1080/136828200247205 [DOI] [PubMed] [Google Scholar]
- 24.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14(1):9-17. [PubMed] [Google Scholar]
- 25.Friedman L, Furberg C, DeMets D. Fundamentals of Clinical Trials. 2nd ed Littleton, MA: PSG Publishing Co, Inc; 1985. [Google Scholar]
- 26.Macwhinney B, Fromm D, Forbes M, Holland A. AphasiaBank: Methods for studying discourse. Aphasiology. 2011;25(11):1286-1307. doi: 10.1080/02687038.2011.589893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilari K, Needle JJ, Harrison KL. What are the important factors in health-related quality of life for people with aphasia? a systematic review. Arch Phys Med Rehabil. 2012;93(1 Suppl):S86-S95. doi: 10.1016/j.apmr.2011.05.028 [DOI] [PubMed] [Google Scholar]
- 28.Meinzer M, Darkow R, Lindenberg R, Flöel A. Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain. 2016;139(Pt 4):1152-1163. doi: 10.1093/brain/aww002 [DOI] [PubMed] [Google Scholar]
- 29.Fama ME, Turkeltaub PE. Treatment of poststroke aphasia: current practice and new directions. Semin Neurol. 2014;34(5):504-513. doi: 10.1055/s-0034-1396004 [DOI] [PubMed] [Google Scholar]
- 30.Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving aphasia in patients with aphasia after stroke. Cochrane Database Syst Rev. 2015;(5):CD009760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eFigure. Coordinates for Anodal Electrode Placements on the Left Scalp for All Participants
eTable. Distribution of Aphasia Types and Severity in the Current Trial Compared to a Large National Cohort of Chronic Patients (AphasiaBank)