Key Points
Question
What is the effect of vouchers to offset the co-payment costs for P2Y12 inhibitors on medication persistence and major adverse cardiovascular events (MACE) among patients with acute myocardial infarction?
Findings
In this cluster randomized trial conducted at 301 hospitals that enrolled 11 001 adult patients with acute myocardial infarction, provision of vouchers to offset the cost of medication co-payments for P2Y12 inhibitors significantly increased patient-reported medication persistence through 1 year (87.0% vs 83.8%), but there was no significant difference in 1-year MACE outcomes (hazard ratio, 1.07).
Meaning
Providing co-payment assistance for P2Y12 inhibitor medications after myocardial infarction increased persistence with a guideline-recommended therapy but did not improve clinical outcomes at 1 year.
Abstract
Importance
Despite guideline recommendations, many patients discontinue P2Y12 inhibitor therapy earlier than the recommended 1 year after myocardial infarction (MI), and higher-potency P2Y12 inhibitors are underutilized. Cost is frequently cited as an explanation for both of these observations.
Objective
To determine whether removing co-payment barriers increases P2Y12 inhibitor persistence and lowers risk of major adverse cardiovascular events (MACE).
Design, Setting, and Participants
Cluster randomized clinical trial among 301 hospitals enrolling adult patients with acute MI (June 5, 2015, through September 30, 2016); patients were followed up for 1 year after discharge (final date of follow-up was October 23, 2017), with blinded adjudication of MACE; choice of P2Y12 inhibitor was per clinician discretion.
Interventions
Hospitals randomized to the intervention (n = 131 [6436 patients]) provided patients with co-payment vouchers for clopidogrel or ticagrelor for 1 year (median voucher value for a 30-day supply, $137 [25th-75th percentile, $20-$339]). Hospitals randomized to usual care (n = 156 [4565 patients]) did not provide study vouchers.
Main Outcomes and Measures
Independent coprimary outcomes were patient-reported persistence with P2Y12 inhibitor (defined as continued treatment without gap in use ≥30 days) and MACE (death, recurrent MI, or stroke) at 1 year among patients discharged with a prescription for clopidogrel or ticagrelor.
Results
Among 11 001 enrolled patients (median age, 62 years; 3459 [31%] women), 10 102 patients were discharged with prescriptions for clopidogrel or ticagrelor (clopidogrel prescribed to 2317 [36.0%] in the intervention group and 2497 [54.7%] in the usual care group), 4393 of 6135 patients (72%) in the intervention group used the voucher, and follow-up data at 1 year were available for 10 802 patients (98.2%). Patient-reported persistence with P2Y12 inhibitors at 1 year was higher in the intervention group than in the control group (unadjusted rates, 5340/6135 [87.0%] vs 3324/3967 [83.8%], respectively; P < .001; adjusted difference, 2.3% [95% CI, 0.4% to 4.1%]; adjusted odds ratio, 1.19 [95% CI, 1.02 to 1.40]). There was no significant difference in MACE at 1 year between intervention and usual care groups (unadjusted cumulative incidence, 10.2% vs 10.6%; P = .65; adjusted difference, 0.66% [95% CI, −0.73% to 2.06%]; adjusted hazard ratio, 1.07 [95% CI, 0.93 to 1.25]).
Conclusions and Relevance
Among patients with MI, provision of vouchers to offset medication co-payments for P2Y12 inhibitors, compared with no vouchers, resulted in a 3.3% absolute increase in patient-reported persistence with P2Y12 inhibitors and no significant reduction in 1-year MACE outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT02406677
This cluster randomized clinical trial evaluated the effect of a voucher to offset medication co-payment costs on persistent P2Y12 inhibitor use and on risk of major adverse cardiovascular events (MACE) at 1 year among adult patients with myocardial infarction discharged with prescriptions for clopidogrel or ticagrelor.
Introduction
Antiplatelet therapy is a cornerstone of treatment for patients with myocardial infarction (MI).1,2,3 Both American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend 1 year of P2Y12 inhibitor therapy after MI and further state a preference for ticagrelor or prasugrel over clopidogrel for patients without a high risk of bleeding (European Society of Cardiology guidelines class I recommendation, American College of Cardiology/American Heart Association guidelines class IIa recommendation).4,5 Randomized trials have shown ticagrelor and prasugrel to further reduce major adverse cardiovascular events (MACE) when compared with clopidogrel.2,3 Nonetheless, among patients with MI in the United States, clopidogrel remains the most commonly prescribed P2Y12 inhibitor,6 and 30% to 60% of patients do not complete a 1-year course of P2Y12 inhibitor therapy.7,8 Premature cessation of P2Y12 inhibitor therapy has been associated with cardiovascular mortality and morbidity.9,10
Patients’ inability to afford medications is frequently cited as a barrier to prescription and persistence with evidence-based treatment for the prescribed period,11 even among insured patients.12,13 Generic clopidogrel was approved by the US Food and Drug Administration in 2012, whereas ticagrelor and prasugrel were nongeneric through October 2017 and often were associated with higher out-of-pocket costs.
The hypothesis for this study was that by removing co-payment barriers to both generic and nongeneric P2Y12 inhibitors, medication choice by clinicians would be more concordant with guideline recommendations, patients would be more likely to complete 1 year of treatment as recommended by guidelines, and, with improved selection and persistence with P2Y12 inhibitor therapy, the risk of MACE would decrease.
Methods
Study Design
The Affordability and Real-World Antiplatelet Treatment Effectiveness After Myocardial Infarction Study (ARTEMIS) was an investigator-initiated, pragmatic, multicenter, cluster randomized clinical trial. Details of the study design have been published,14 and the protocol and statistical analysis plan are available in Supplement 1 and Supplement 2. A cluster randomized design was selected to study the effect on clinician prescribing behavior and to avoid ascertainment bias, because patient-level randomization would likely lead to higher study withdrawal rates for patients randomized to no co-payment reduction.
All patients were aware of the randomization status of their treating hospital at the time of enrollment and provided written informed consent. The study was approved by the institutional review boards at Duke University and all participating sites.
Study Population
This study randomized 301 US hospitals that treated at least 50 patients with MI annually and had both clopidogrel and ticagrelor available for clinical use on their hospital formulary. Patients were eligible for inclusion if they were 18 years or older, hospitalized with ST-segment elevation MI or non–ST-segment elevation MI, treated with any P2Y12 inhibitor at the time of enrollment, and had any US-based commercial or government health insurance with prescription drug benefits. Consecutive patient enrollment was encouraged, excluding patients with prior intracranial hemorrhage, contraindication to P2Y12 inhibitor use, involvement in another study that specified the type and duration of P2Y12 inhibitor to be used within the next year, a life expectancy less than 1 year, or plans to move outside the United States.
Randomization and Intervention
Participating hospitals were randomized in a 1:1 ratio to intervention or usual care according to a computer-generated randomization list. Randomization was stratified with a block size of 4 by annual hospital MI volume and site-reported baseline proportion of ticagrelor use.
In both groups, P2Y12 inhibitor selection and treatment duration were determined by the clinical care team in accordance with local standards of care. For hospitals randomized to the intervention group, study personnel educated all clinicians with a pocket card describing the availability of a study voucher that provided co-payment reductions for clopidogrel or ticagrelor over the next 1 year. Before hospital discharge, enrolled patients were given a voucher card that could be used at any pharmacy to fill either a generic (clopidogrel) or brand-name (ticagrelor) P2Y12 inhibitor. Medication refills needed to be initiated by the patient at any local or mail-order pharmacy. The study provided no refill reminders and no other interventions to improve medication adherence.
At the time of each medication fill or refill, the co-payment was charged to the study, resulting in zero out-of-pocket cost for the patient. For Medicare- or Medicaid-insured patients who cannot receive co-payment assistance, the voucher covered the entire cost of clopidogrel or ticagrelor prescriptions. The median voucher value was $137 (25th-75th percentile, $20-$339) for a 30-day medication supply. From the patient perspective, regardless of insurance type, the voucher allowed patients to fill prescriptions for clopidogrel or ticagrelor without out-of-pocket co-payment. Voucher support was limited to replacing lost or nonfunctioning cards and contacting pharmacies if a patient reported that the voucher was not honored.
Although the voucher did not apply to co-payment costs for prasugrel, study enrollment was open to all eligible patients with MI, and clinicians within both study groups were free to prescribe prasugrel to enrolled patients. This was based on the perception that if only patients treated with clopidogrel or ticagrelor were eligible for enrollment, trial participation would incentivize a bias in treatment selection. Intervention group patients discharged with a prescription for prasugrel did not receive a study co-payment voucher, even if they later switched to ticagrelor or clopidogrel. Among hospitals randomized to usual care, no co-payment cards were provided. Patients in both groups were not precluded from using any commercially available co-payment voucher.
The first patient was enrolled on June 5, 2015. In November 2015, the steering committee was alerted to a widening divergence in enrollment between groups attributed to differential patient incentive to enroll. At that time, 199 of the planned 300 hospitals had been randomized, with 1372 and 898 patients enrolled in the intervention and usual care groups, respectively. For sites remaining to be recruited, the steering committee changed the randomization schema from 1:1 to 2:1 usual care vs intervention, effective November 16, 2015. No outcomes data were analyzed before this decision. Patient enrollment was completed on September 30, 2016.
Study End Points
The independent coprimary end points were persistence of P2Y12 inhibitor therapy and MACE at 1 year. We hypothesized that the co-payment intervention could help patients continue a guideline-recommended therapy longer and allow clinicians to prescribe the treatment they perceived the patient’s risk profile warranted, regardless of the patient’s ability to afford that medication. Through these mechanisms, a co-payment reduction intervention could potentially reduce MACE. Medication persistence and MACE were prioritized as independent coprimary outcomes because improving patient medication-taking and clinical outcomes were deemed most clinically meaningful.14
Medication persistence refers to the continuation of treatment for the prescribed duration.15 The primary definition of persistence was continued P2Y12 inhibitor use without a gap of 30 days or longer as reported by the patient during interviews conducted at 3, 6, 9, and 12 months after index MI discharge. During these interviews, patients were asked to list their current medications (interviewers were unaware of treatment group assignment unless a patient volunteered that information). If a P2Y12 inhibitor was not included, then patients were asked the date of discontinuation. If a P2Y12 inhibitor was included, patients were asked if their P2Y12 inhibitor had been temporarily discontinued for any period since the previous study contact. Switches in P2Y12 inhibitor drug were considered persistent unless a gap of 30 days or longer occurred. For example, a patient previously treated with ticagrelor but switched to prasugrel without a gap in treatment was considered persistent, whereas a patient treated with ticagrelor but switched to clopidogrel after a 31-day hiatus would be considered nonpersistent. MACE was the composite of all-cause death, recurrent MI, or stroke.14 Hospital bills and medical records were collected; MACE events were independently adjudicated by physicians at the coordinating center who were blinded to randomized status.
Prespecified secondary outcomes included (1) P2Y12 inhibitor type prescribed at index MI discharge; (2) 1-year P2Y12 inhibitor persistence measured by pharmacy fills; (3) 1-year P2Y12 inhibitor use measured by serum drug levels; (4) individual components of MACE (death, MI, stroke, cardiovascular death); and (5) bleeding. Additional secondary outcomes (persistence with initial P2Y12 inhibitor prescribed at hospital discharge, death or nonpersistence, health care utilization and cost, unplanned revascularization, MACE plus unplanned revascularization at 1 year) are not reported in this article.
P2Y12 inhibitor type prescribed at index discharge was abstracted from the medical record by the enrolling site. Because patient self-report may overestimate medication use, patients were linked to their pharmacy fill records via an anonymous unique identifier to assess medication persistence. Symphony Health pharmacy data contain more than 10 billion adjudicated prescription claims for more than 220 million patients in the United States.16 Patients with linked pharmacy fill records had at least 1 fill of any medication in the Symphony Health system during the 1-year follow-up. Persistence was defined, in a manner similar to patient self-report, as continuous fill without a gap in supply of 30 days or longer from the last dispensed pill.
In a serum drug level substudy, approximately 250 patients who provided consent at enrollment were randomly selected at each of 3, 6, 9, and 12 months after index MI discharge to undergo phlebotomy for measurement of serum drug metabolite levels for clopidogrel, prasugrel, or ticagrelor. The randomization process oversampled to ensure an adequate number of samples in case patients had died or had withdrawn by the time of the blood draw. Phlebotomy kits were assigned to 1071 randomly selected patients, and blood samples were processed in the core laboratory at Quest Diagnostics. Bleeding was defined using the Bleeding Academic Research Consortium bleeding definition; type 2 or higher bleeding (ie, bleeding that requires diagnostic studies, hospitalization, or treatment by a health care professional) was reported.17
Statistical Analysis
The overall study sample size of 300 sites and 11 000 participants was selected assuming a control group event rate of 12% to detect an 18% relative reduction (2.16% absolute difference) in MACE, with an intracluster correlation (ICC) of 0.01 and 80% to 85% power.13,14 For the coprimary patient-reported persistence end point, this sample size was expected to provide 85% to 90% power to detect an absolute 4% difference between groups under the same cluster-randomized assumptions. This 4% difference was selected based on a prior randomized trial (the Post-Myocardial Infarction Free Rx Event and Economic Evaluation [MI-FREEE] trial) in which co-payment reduction increased medication use by 4% to 6% when measured by pharmacy fills and lowered MACE risk in secondary analysis. The expected patient-reported medication persistence at 1 year was 70% in the control group.13 Therefore, this study hypothesized that a 4% increase in persistence, coupled with increased use of higher-potency P2Y12 inhibitors, would lead to a clinically important difference (ie, lower risk of MACE). Coprimary end points were powered independently with a 2-sided type I error of .05, without adjusting for multiple comparisons.
Primary Analyses
The primary analyses analyzed patients according to the randomization group, regardless of whether the intervention group patient used the study voucher. The primary analysis was performed in patients discharged with a prescription for clopidogrel or ticagrelor, since these patients would potentially benefit from study voucher use. For persistence, the last observation carried forward method was used for patients who died before 1 year or had missing 1-year P2Y12 inhibitor treatment status. For MACE, follow-up time was censored on the withdrawal date.
Imbalances in patient enrollment were expected to occur in this cluster randomized clinical trial18; therefore, the primary analysis for persistence was prespecified as a logistic regression model, with parameters estimated using generalized estimating equations to account for within-hospital clustering. Outcomes models adjusted for patient and hospital characteristics, including a propensity score that estimated assignment to the intervention group using a logistic regression model containing 50 covariates prospectively selected by the steering committee based on clinical relevance (eTables 1 and 2 in Supplement 3). All covariates were included in the model regardless of whether there were statistically significant differences between randomized groups. The functional form and possible transformations of the propensity score to be included in the outcome model were assessed. After data collection was complete, the balance of covariates between intervention and usual care groups was assessed using standardized differences.19 Data on patient race were collected because prior studies suggested that patients of nonwhite race were less likely to be prescribed higher-potency P2Y12 inhibitors6 and that racial differences in medication nonadherence likely mediate disparities in cardiovascular morbidity and mortality20; race was retrospectively abstracted from the medical record.
Cluster heterogeneity was quantified using ICCs calculated from unadjusted and adjusted models; ICCs were compared across all hospitals and also by treatment group. Because of the change in randomization ratio, timing of hospital randomization was added to the adjustment model, as reflected in the statistical analysis plan. For MACE, the primary analysis used a Cox proportional hazards model accounting for within-hospital clustering with robust standard errors and adjusting for the same patient and hospital characteristics (eTables 1 and 2 in Supplement 3). The proportional hazards assumption was assessed graphically by plotting log[−log(survival)] vs log of time and tested by including a time-dependent covariate for intervention by log of time in the model. The proportional hazards assumption was not violated (P = .46).
Secondary Analyses
Prespecified secondary analyses included (1) comparing the coprimary outcomes among all enrolled patients, regardless of discharge P2Y12 inhibitor selection; (2) comparing medication persistence using pharmacy fill data and serum drug metabolite levels; and (3) assessing the coprimary outcomes among the as-treated population, defined as intervention group patients who used the study voucher at least once during the 1 year after index MI discharge. For all of these comparisons, regression models were performed with parameters estimated using generalized estimating equations and adjusted for the same patient and hospital characteristics described above.
Prespecified Sensitivity Analyses
Sensitivity analyses using different adjustment methodologies were performed. First, analyses for medication persistence and MACE were repeated using the same patient and hospital characteristics described above but accounting for within-hospital clustering using random intercepts for hospitals instead of generalized estimating equations. Second, comparisons of the coprimary outcomes were repeated in a propensity-matched study population. Within each of the 4 randomization strata (baseline percent ticagrelor use and annual MI volume) and by time of randomization (2:1 vs 1:1), we matched intervention group patients to usual care group patients based on the logit of the propensity score, with a caliper width of 0.2 times the standard deviation.21 After propensity matching, absolute standardized differences for all covariates in eTable 3 in Supplement 3 were less than 0.10. Then we fit a logistic regression or Cox regression model stratified by match pair to estimate the effect on persistence and MACE, respectively.
Post Hoc Analyses
The post hoc comparison of the proportion of patients who declined study participation was analyzed using logistic regression with generalized estimating equations accounting for clustering by site. Unadjusted and adjusted differences in the coprimary outcomes between treatment groups were calculated using Stata 15; risk differences were calculated using regression risk analysis, with standard errors and 95% confidence intervals calculated using the Delta method.22,23,24
Because voucher use implied at least 1 drug fill, a post hoc as-treated analysis compared intervention group patients who used the voucher at least once vs usual care group patients who filled the drug prescription at least once. As a sensitivity analysis, missing persistence data were imputed using full conditional specification method for 10 imputed data sets. Logistic regression models with generalized estimating equations were fit for each data set and combined the results; imputation used all above variables along with MACE, time to MACE, and P2Y12 inhibitor status at each interview.
Significance thresholds were not adjusted for multiple comparisons; all secondary and post hoc analyses were exploratory. All statistical analyses, except as noted, were performed using SAS statistical software version 9.4 (SAS Institute Inc). All statistical tests were 2-sided, with a significance level of .05.
Results
Study Population
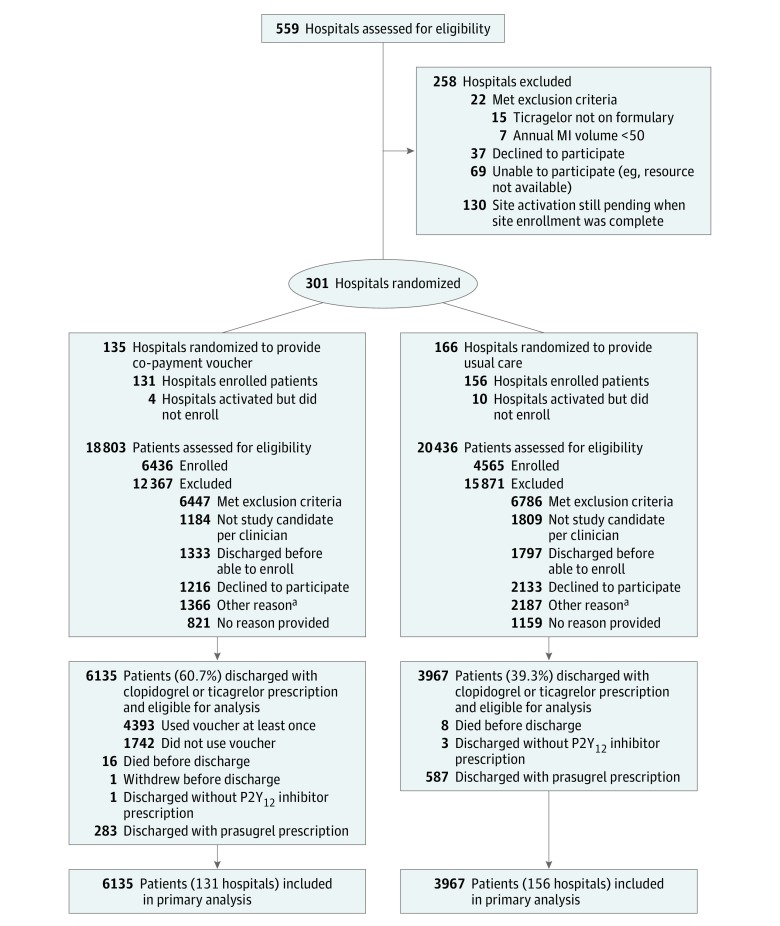
A total of 301 hospitals were randomized, 135 to the intervention group and 166 to usual care (Figure 1). Between June 5, 2015, and September 30, 2016, a total of 26 006 patients were screened and met study inclusion and exclusion criteria, from which 11 001 patients (42%) were enrolled. Reasons for nonenrollment included patient not deemed a study candidate per the clinician (n = 2993 [12%]), patient discharged before able to enroll (n = 3130 [12%]), patient declined (n = 3349 [13%]), other (n = 3553 [14%]), and no reason provided (n = 1980 [8%]). Intervention group sites enrolled a median of 38 patients per site, whereas usual care group sites enrolled a median of 20 patients per site. Among patients deemed by the clinician to be eligible for enrollment, patient declines were more common among usual care than intervention sites (29.3% vs 25.7%, P = .004).
Figure 1. Patient Recruitment and Flow Through the Study.
aSubclassifications of “other” were not collected.
Hospital characteristics such as size, teaching status, annual MI volume, and pre-enrollment ticagrelor use were not significantly different between randomized groups (eTable 3 in Supplement 3). Baseline characteristics of patients stratified by randomized groups are reported in Table 1; these characteristics were well-balanced between randomized groups except for race (standardized difference, 0.11). Seventeen percent of patients reported not filling a medication prescription in the prior year because of inability to afford the medication.
Table 1. Baseline Characteristics of Enrolled Patients Stratified by Randomized Group.
| Characteristic | No. (%) | |
|---|---|---|
| Intervention (n = 6436) | Usual Care (n = 4565) | |
| Demographic | ||
| Age, median (25th-75th percentile), y | 62 (54-70) | 62 (54-69) |
| Sex | ||
| Male | 4417 (68.6) | 3125 (68.5) |
| Female | 2019 (31.4) | 1440 (31.5) |
| Race | ||
| White | 5772 (89.7) | 3933 (86.2) |
| African American | 564 (8.8) | 533 (11.7) |
| Asian | 117 (1.8) | 102 (2.2) |
| American Indian or Alaskan Native | 61 (1.0) | 66 (1.5) |
| Native Hawaiian or Pacific Islander | 36 (0.6) | 20 (0.4) |
| Private insurance | 4061 (63.1) | 2986 (65.4) |
| College or higher education | 3057 (47.5) | 2359 (51.7) |
| Employed | 3036 (47.2) | 2111 (46.2) |
| Household income, $ | ||
| ≤10 000 | 437 (6.8) | 281 (6.2) |
| 10 001 to 20 000 | 644 (10.0) | 405 (8.9) |
| 20 001 to 30 000 | 668 (10.4) | 433 (9.5) |
| 30 001 to 50 000 | 906 (14.1) | 657 (14.4) |
| 50 001 to 70 000 | 708 (11.0) | 492 (10.8) |
| 70 001 to 100 000 | 626 (9.7) | 436 (9.6) |
| 100 001 to 150 000 | 398 (6.2) | 335 (7.3) |
| ≥150 001 | 259 (4.0) | 205 (4.5) |
| Patient preferred not to answer | 1790 (27.8) | 1321 (28.9) |
| Medical History | ||
| Prior MI | 1263 (19.6) | 973 (21.3) |
| Prior CABG surgery | 681 (10.6) | 518 (11.6) |
| Prior stroke/TIA | 390 (6.1) | 317 (6.9) |
| Peripheral arterial disease | 376 (5.8) | 316 (6.9) |
| Diabetes | 2019 (31.4) | 1555 (34.1) |
| Hypertension | 4332 (67.3) | 3243 (71.0) |
| Current or recent smoker | 2167 (33.7) | 1522 (33.3) |
| Admission Characteristics | ||
| STEMI | 2997 (46.6) | 2110 (46.2) |
| Weight, median (25th-75th percentile), kg | 89 (77-103) | 89 (77-104) |
| Home aspirin use | 2711 (42.1) | 1995 (43.7) |
| Home P2Y12 inhibitor use | 840 (13.1) | 741 (16.2) |
| Clopidogrel | 682 (10.6) | 613 (13.4) |
| Ticagrelor | 104 (1.6) | 75 (1.6) |
| Prasugrel | 54 (0.8) | 52 (1.1) |
| Creatinine clearance, median (25th-75th percentile), mL/min | 71 (53-90) | 70 (53-87) |
| Hemoglobin, median (25th-75th percentile), g/dL | 13.1 (11.7-14.3) | 13.1 (11.6-14.2) |
| Multivessel disease | 3029 (47.1) | 2050 (44.9) |
| Coronary Treatment | ||
| PCI | 5816 (90.4) | 4051 (88.7) |
| CABG surgery | 95 (1.5) | 64 (1.4) |
Abbreviations: CABG, coronary artery bypass graft; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation MI; TIA, transient ischemic attack.
By 1 year, the lost-to-follow-up rate was 1.8% overall, consisting of 95 patients (1.5%) in the intervention group and 104 patients (2.3%) in the usual care group. Among patients lost to follow-up, the median time to loss to follow-up was 140 days (25th-95th percentile, 91-226).
Among 11 001 enrolled patients, 24 died before discharge from the index MI, 1 patient withdrew before discharge, and 4 patients were enrolled and discharged home without a P2Y12 inhibitor (Figure 1). At discharge, clopidogrel was prescribed to 2317 patients (36.0%) in the intervention group and 2497 patients (54.7%) in the usual care group.
Among the 10 102 patients discharged alive with a prescription for either clopidogrel or ticagrelor (the primary analysis population), 6135 patients (60.7%) were in the intervention group and 3967 (39.3%) were in the usual care group. Patient characteristics of this population are reported in eTable 4 in Supplement 3 and again demonstrate good balance between randomized groups except for race (standardized difference, 0.11).
Coprimary Outcomes: Postdischarge P2Y12 Inhibitor Persistence and MACE
The coprimary outcomes of patient-reported P2Y12 inhibitor persistence and MACE were examined among the 10 102 patients discharged alive and receiving either clopidogrel or ticagrelor. Within 1 year after discharge, 85.8% of patients reported persistence with P2Y12 inhibitor therapy. Patients in the intervention group were more likely to report persistence with P2Y12 inhibitor therapy than patients in the usual care group (87.0% vs 83.8%, P < .001; observed increase, 3.3% [95% CI, 1.0% to 5.5%]). The intervention significantly increased persistence after adjusting for differences in patient characteristics between groups, with an adjusted odds ratio (OR) of 1.19 (95% CI, 1.02 to 1.40) (Table 2).
Table 2. Persistence With P2Y12 Inhibitor Therapy by 1 Yeara.
| Analysis | No./Total (%) | Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Usual Care | Observed Difference, % (95% CI) | OR (95% CI) | P Value | Adjusted Difference, % (95% CI) | OR (95% CI) | P Value | |
| Primary Outcome (Patient-Reported P2Y12 Inhibitor Persistence) | ||||||||
| Intention-to-treat | 5340/6135 (87.0) | 3324/3967 (83.8) | 3.3 (1.0-5.5) | 1.31 (1.12-1.54) | .001 | 2.3 (0.4-4.1) | 1.19 (1.02-1.40) | .03 |
| As-treated | 3956/4393 (90.1) | 3324/3967 (83.8) | 6.3 (4.1-8.4) | 1.79 (1.51-2.12) | <.001 | 4.6 (2.7-6.5) | 1.53 (1.29-1.82) | <.001 |
| Secondary Outcomes | ||||||||
| Pharmacy fill–based P2Y12 inhibitor persistence | ||||||||
| Intention-to-treat | 2820/5109 (55.2) | 1511/3264 (46.3) | 8.9 (4.6-13.2) | 1.56 (1.37-1.77) | <.001 | 7.9 (4.4-11.4) | 1.47 (1.29-1.66) | <.001 |
| As-treated | 2216/3705 (59.8) | 1511/3264 (46.3) | 13.5 (8.7-18.3) | 1.94 (1.70-2.21) | <.001 | 11.8 (7.7-15.8) | 1.78 (1.55-2.03) | <.001 |
| Detectable P2Y12 inhibitor drug level in randomly selected blood draws | ||||||||
| Intention-to-treat | 569/620 (91.8) | 284/324 (87.7) | 4.1 (0.1-8.2) | 1.56 (1.02-2.36) | .04 | 5.7 (0.9-10.6) | 1.86 (1.16-2.98) | .01 |
| As-treated | 493/530 (93.0) | 284/324 (87.7) | 5.4 (1.2-9.5) | 1.84 (1.16-2.93) | .01 | 7.3 (2.3-12.2) | 2.30 (1.38-3.84) | .001 |
Abbreviation: OR, odds ratio.
Primary analysis used an intention-to-treat approach in which outcomes were compared regardless of whether the intervention group patient used the study voucher. Prespecified secondary analyses of the as-treated population excluded intervention group patients who never used the study voucher during the 1 year after index myocardial infarction discharge.
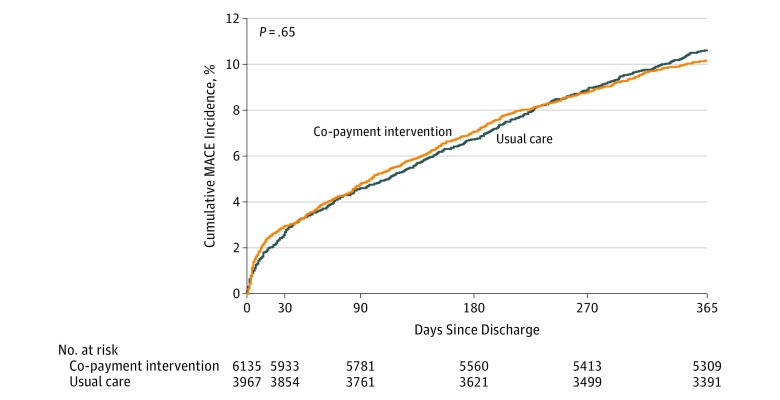
MACE occurred in 10.2% (95% CI, 9.4% to 10.9%) of patients in the intervention group and 10.6% (95% CI, 9.7% to 11.6%) of patients in the usual care group (observed difference, −0.5% [95% CI, −2.2% to 1.3%]) (Figure 2). After adjustment, there were no significant differences in MACE between the randomized groups (adjusted hazard ratio [HR], 1.07 [95% CI, 0.93 to 1.25]) (Table 3).
Figure 2. Cumulative Incidence Curves for Major Adverse Cardiovascular Events Among Patients Discharged With a Prescription for Clopidogrel or Ticagrelor.
Median duration of follow-up was 365 days (interquartile range, 0). The time-to-first MACE event up to 1 year after discharge was compared between intervention and control groups using a Cox proportional hazards model accounting for within-hospital clustering.
Table 3. Clinical Outcomes by 1 Yeara.
| Analysis | No. of Events (%)b | Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention (n = 6135) |
Usual Care (n = 3967) | Observed Difference, % (95% CI)c | HR (95% CI) | P Value | Adjusted Difference, % (95% CI)c | HR (95% CI) | P Value | |
| Primary Outcome (Death, MI, or Stroke) | ||||||||
| MACE | ||||||||
| Intention-to-treat | 614 (10.2) | 415 (10.6) | −0.5 (−2.2 to 1.3) | 0.96 (0.80 to 1.15) | .65 | 0.7 (−0.7 to 2.1) | 1.07 (0.93 to 1.25) | .35 |
| As-treated | 327 (7.5) | 415 (10.6) | −3.0 (−4.6 to −1.5) | 0.70 (0.58 to 0.84) | <.001 | −0.7 (−2.1 to 0.7) | 0.90 (0.76 to 1.08) | .25 |
| Secondary Outcomes | ||||||||
| Death | ||||||||
| Intention-to-treat | 237 (3.9) | 154 (3.9) | 0.0 (−1.0 to 1.0) | 1.00 (0.76 to 1.31) | .98 | 0.4 (−0.5 to 1.2) | 1.11 (0.88 to 1.39) | .39 |
| As-treated | 103 (2.3) | 154 (3.9) | −1.5 (−2.4 to −0.7) | 0.60 (0.44 to 0.81) | .001 | −0.6 (−1.4 to 0.2) | 0.82 (0.63 to 1.07) | .14 |
| Recurrent MI | ||||||||
| Intention-to-treat | 413 (6.9) | 280 (7.3) | −0.3 (−1.6 to 0.9) | 0.96 (0.79 to 1.15) | .64 | 0.5 (−0.7 to 1.6) | 1.08 (0.90 to 1.29) | .40 |
| As-treated | 235 (5.4) | 280 (7.3) | −1.7 (−2.9 to 0.6) | 0.74 (0.61 to 0.90) | .003 | 0.0 (−1.2 to 1.2) | 0.98 (0.80 to 1.20) | .83 |
| Stroke | ||||||||
| Intention-to-treat | 33 (0.8) | 19 (1.0) | −0.1 (−0.5 to 0.2) | 0.88 (0.59 to 1.31) | .53 | 0.0 (−0.3 to 0.3) | 0.98 (0.68 to 1.42) | .91 |
| As-treated | 29 (0.7) | 19 (1.0) | −0.3 (−0.6 to 0.1) | 0.71 (0.44 to 1.15) | .16 | −0.1 (−0.4 to 0.2) | 0.86 (0.57 to 1.31) | .48 |
| Cardiovascular death | ||||||||
| Intention-to-treat | 187 (3.1) | 117 (3.0) | 0.1 (−0.8 to 1.0) | 1.03 (0.77 to 1.38) | .82 | 0.5 (−0.2 to 1.2) | 1.18 (0.92 to 1.52) | .19 |
| As-treated | 80 (1.8) | 117 (3.0) | −1.1 (−1.9 to −0.4) | 0.61 (0.45 to 0.84) | .003 | −0.3 (−0.9 to 0.4) | 0.89 (0.66 to 1.21) | .46 |
| BARC type 2 or higher bleeding | ||||||||
| Intention-to-treat | 259 (4.4) | 157 (4.1) | 0.3 (−0.6 to 1.2) | 1.06 (0.87 to 1.30) | .56 | 0.6 (−0.3 to 1.5) | 1.15 (0.94 to 1.41) | .17 |
| As-treated | 184 (4.3) | 157 (4.1) | 0.1 (−0.8 to 1.0) | 1.00 (0.81 to 1.23) | .95 | 0.6 (−0.3 to 1.5) | 1.13 (0.91 to 1.40) | .26 |
Abbreviations: BARC, Bleeding Academic Research Consortium; HR, hazard ratio; MACE, major adverse cardiovascular events; MI, myocardial infarction.
Primary analysis used an intention-to-treat approach in which outcomes were compared regardless of whether the intervention group patient used the study voucher. Prespecified secondary analyses of the as-treated population excluded intervention group patients who never used the study voucher during the 1 year after index MI discharge.
Unadjusted Kaplan-Meier event rates.
Negative differences denote better outcomes for patients in the intervention group.
Secondary Outcomes
In the intervention group, 59.6% of patients were discharged with a prescription for ticagrelor and 4.4% with a prescription for prasugrel. In the usual care group, 32.4% of patients were discharged with a prescription for ticagrelor and 12.9% with a prescription for prasugrel (P < .001). Clinicians stated an intention to treat with P2Y12 inhibitor therapy for at least 1 year in 76.7% of patients in the intervention group and 57.8% of patients in the usual care group.
Patient self-report may overestimate medication persistence; therefore, patients were linked to their pharmacy fill records to measure pharmacy fill–based persistence. Patients with at least 1 fill of any medication in Symphony Health pharmacy data during the 1 year after discharge constituted 82.8% of all patients alive and in the study at the time of discharge (n = 9087, of which 8373 were discharged with a prescription for clopidogrel or ticagrelor) and were similarly distributed between the intervention and usual care groups (83.2% vs 82.2%, P = .22). Among the subset of 8373 patients with linked pharmacy fill data, 51.7% were persistent to P2Y12 inhibitor treatment through 1 year. Pharmacy-defined persistence rates remained higher among patients in the intervention group than in the usual care group (55.2% vs 46.3%, P < .001; adjusted OR, 1.47 [95% CI, 1.29 to 1.66]).
In a substudy assessing serum drug metabolite levels, a total of 1006 patients had analyzable results, of which 944 were discharged with a prescription for clopidogrel or ticagrelor. Among these, 569 of 620 patients (91.8%) in the intervention group had detectable drug metabolite levels for at least 1 P2Y12 inhibitor at the time of blood draw, compared with 284 of 324 patients (87.7%) in the usual care group (Table 2).
Analyses of persistence in the overall enrolled population, including patients discharged with a prescription for prasugrel who were unable to benefit from the study voucher, were directionally consistent, although no longer statistically significant (eTable 6 in Supplement 3). MACE was not significantly different between randomized groups in the overall enrolled population, including patients initially discharged with a prescription for prasugrel (eTable 6 in Supplement 3). Individual components of MACE were also not significantly different between randomized groups (Table 3). Since MACE differences were not significant, comparisons of health care costs between groups were not performed.
Bleeding that required diagnostic studies, hospitalization, or treatment by a health care professional (Bleeding Academic Research Consortium type 2 or higher bleeding) occurred in 4.8% (95% CI, 4.3% to 5.4%) of patients in the intervention group and 4.6% (95% CI, 3.9% to 5.2%) of patients in the usual care group (unadjusted HR, 1.06 [95% CI, 0.87 to 1.30]; P = .56). After adjustment, there remained no significant difference in bleeding between the randomized groups (adjusted HR, 1.15 [95% CI, 0.94 to 1.41]) (Table 3), with similar results in sensitivity analyses (eTables 5 and 6 in Supplement 3).
In the 1 year postdischarge, 4393 of 6135 intervention group patients (72%) used the study voucher at least once. Patients who did not use the voucher more often were of nonwhite race, had lower levels of education, and had cardiovascular comorbidities (P < .01 for all) (eTable 7 in Supplement 3). Reasons for voucher nonuse were death or study withdrawal (n = 308 [18%]), no longer taking drug (n = 96 [6%]), no need for voucher with current insurance (n = 372 [21%]), pharmacy rejected voucher (n = 156 [9%]), lost voucher (n = 120 [7%]), forgot voucher (n = 76 [4%]), did not know how to use voucher (n = 46 [3%]), and no reason provided (n = 568 [33%]).
Within the intervention group, rates of medication persistence differed substantially between patients who did and did not use the co-payment voucher, whether measured by patient report (90.0% vs 79.4%, P < .001) or pharmacy fill supply (59.8% vs 43.0%, P < .001). Similarly, unadjusted MACE rates differed between patients with and without voucher use (7.49% vs 17.20%, P < .001) (eFigure in Supplement 3). As-treated comparisons of persistence showed results similar to those from the primary analysis, with larger differences in persistence between intervention and usual care groups (Table 2). The risk of MACE remained nonsignificantly different between randomized groups after multivariable adjustment (adjusted OR, 0.90 [95% CI, 0.76 to 1.08]). Similar results were seen in a post hoc analysis in which the intervention group patients who used a voucher (and therefore filled their prescription at least once) were compared with patients in the usual care group who filled their prescription at least once (eTable 8 in Supplement 3).
Sensitivity and Post Hoc Analyses
Sensitivity analyses for the primary outcomes using models with random intercepts for hospitals or among propensity-matched patients yielded results similar to the main overall results (eTable 5 in Supplement 3). Post hoc analyses of persistence showed an adjusted increase of 2.3% (95% CI, 0.4% to 4.1%). Post hoc comparisons of MACE remained statistically nonsignificant after adjustment (Table 3). A post hoc sensitivity analysis using multiple imputation to account for missing data on medication persistence (398 patients [3.9%] died before 1 year or had missing 1-year P2Y12 inhibitor treatment status) showed similar results compared with the last observation carried forward analysis (adjusted OR, 1.20 {95% CI, 1.02 to 1.41]).
Discussion
In this randomized trial involving 301 US hospitals and 11 001 patients with MI, a co-payment intervention increased patient-reported persistence with P2Y12 inhibitor therapy by 3.3% through 1 year but resulted in no significant reduction in MACE outcomes at 1 year.
Despite guideline recommendation, post-MI persistence with evidence-based medications has been suboptimal.13,25,26,27,28 Lower medication co-payments have been associated with greater evidence-based medication use.29,30,31 In the MI-FREEE trial, the elimination of co-payments increased medication adherence13 and led Aetna to launch a value-based insurance program reducing co-payments for all secondary prevention medications among insured beneficiaries with prior MI.32 Nevertheless, MI-FREEE involved a single payer, targeted generic medications with already low co-payments, and assessed an intervention that started a median of 49 days after MI, by which time adherence rates may have already declined substantially.27,33
In the ARTEMIS study, the co-payment intervention was started at the time of index MI discharge, and patients of all insurance types (including Medicare patients, who comprise the majority of post-MI patients but who are typically excluded from medication voucher use) were enrolled in the study. Participating study sites were diverse and representative of US hospitals treating patients with MI.34 With treatment choice and duration at the discretion of the treating clinician, a cluster randomized study design was selected to understand the effect of the intervention on access and adherence to guideline-recommended therapy from both patient and clinician perspectives.
For clinicians, eliminating co-payment differences between brand-name and generic P2Y12 inhibitors may affect prescribing practices and improve guideline adherence. Indeed, clinicians were more likely to state an intention to treat with P2Y12 inhibitor therapy for at least 1 year in the intervention group than the usual care group. Clinicians in the intervention group were also more likely to prescribe a guideline-preferred P2Y12 inhibitor. For patients, those in the intervention group were more likely to persist with P2Y12 inhibitor therapy through 1 year. As expected based on prior studies, patient self-reported persistence was much higher than pharmacy fill–defined persistence.35,36 Regardless of how persistence was defined, intervention group patients had higher medication persistence in both intention-to-treat and as-treated analyses.
Patient-reported persistence rates were 84% in the usual care group and 87% in the intervention group, with an observed increase of 3.3%. In contrast, pharmacy fill–based persistence rates were 46% and 55% for the usual care and intervention groups, respectively, resulting in an observed increase of 9%. These rates were similar to those observed in the MI-FREEE trial, which used pharmacy fill data to demonstrate a 4% to 6% absolute increase in medication use as a result of a co-payment intervention. A ceiling effect may have limited the intervention’s effect on patient-reported persistence. P2Y12 inhibitor medication adherence is already a focus of patient education, especially for patients with coronary stenting, for whom premature discontinuation may be life-threatening.9,10
Several P2Y12 inhibitor drug vouchers and financial assistance programs were also available during the study period.37,38 Although this study could not identify how many patients in the usual care group used these vouchers, this may have biased the study toward the null. Among patients in the intervention group, 28% never used the voucher provided. Some of these patients (n = 352) reported not using the voucher because they forgot to use it, lost it, or could not persuade the pharmacy to honor it. Few patients (n = 96) had early discontinuation of P2Y12 inhibitor use that obviated the need for voucher use. The majority of patients who did not use the voucher did not describe any barrier to use, suggesting that they did not need or chose not to use the provided voucher.
Despite increased selection of evidence-based medication and longer duration of evidence-based treatment, the intervention did not significantly improve clinical outcomes. This observation has several potential explanations. First, the intervention targeted a critical medication class, but only 1 of several evidence-based medications that improve post-MI outcomes.
Second, clinician-based treatment gaps cannot be excluded; only two-thirds of patients’ physicians stated an intention to treat with at least 1 year of P2Y12 inhibitor therapy, despite guideline recommendations. Prior studies have attributed a substantial proportion of early P2Y12 inhibitor discontinuation to clinician decision rather than patient self-discontinuation.7,9
Third, ticagrelor uptake was already high in the control group (32.6%), compared with recent US practice (16.7% in the National Cardiovascular Data Registry).6 Larger differences in medication persistence may be needed to drive detectable differences in clinical outcomes. These results suggest that multifactor interventions are likely needed to achieve population-level health benefits. Co-payment reductions may be considered part of broader-scale strategies to incentivize risk-based treatment selection and promote adherence to guideline-recommended therapies. Whether co-payment intervention as part of these multipronged strategies can improve patient outcomes merits further study. The increase in both use of higher-potency P2Y12 inhibitors and in overall P2Y12 inhibitor persistence did not result in higher bleeding rates requiring medical attention among patients in the intervention group.
This study used co-payment vouchers to provide co-payment reductions universally, regardless of patients’ health insurance provider, prescription benefit manager, and pharmacy type. However, 28% of patients in the intervention group never used the provided voucher. As-treated analyses are important in trials in which incomplete penetrance of the intervention is expected, but these analyses need to be interpreted cautiously. Patients who never used the vouchers were more likely to be unemployed, less educated, and have higher rates of nonpersistence and MACE outcomes than intervention group patients who used the voucher at least once. The study prospectively adjusted for 50 clinically relevant patient characteristics, but unmeasured confounding remains in the as-treated analyses. The study may ultimately have been underpowered to detect significant differences in MACE because of the large number of intervention group patients who never used the voucher.
Limitations
This study has several limitations. First, this was a co-payment intervention trial, so only patients with US-based health insurance with prescription coverage were included.
Second, pharmacy fill records could only be linked for 83% of enrolled patients because of the diverse pharmacies across the United States, and not all pharmacy fills may have been captured; however, secondary analyses showed consistent results between groups among the subpopulations with pharmacy fill data and serum drug metabolite levels.
Third, imbalances in enrollment and patient characteristics were expected in this cluster-randomized design. Enrollment was faster in intervention than usual care groups, and intervention group sites may have been less likely to approach a prasugrel-treated patient for study involvement. Patient-level randomization was infeasible, since it would have led to an unacceptably higher lost-to-follow-up rate for patients who were randomized to no co-payment reduction. Patient-level randomization would also preclude study of clinician prescribing behavior. The ideal study design to minimize bias would have provided vouchers covering all P2Y12 inhibitors and collected longitudinal data on all patients with MI, regardless of P2Y12 inhibitor treatment and without requiring individual informed consent; however, this study design is impossible. While multivariable adjustment and multiple sensitivity analyses were prespecified, unmeasured confounding remains.
Fourth, the overall lost-to-follow-up rate was low but slightly higher for patients in the usual care vs intervention group. By design, study patients, coordinators, and clinicians could not be masked to treatment assignment group, but site personnel were not involved in collecting persistence data, and MACE events were adjudicated independently, masked to randomized status.
Fifth, to operationalize co-payment reduction in a manner agnostic to payers and pharmacies, this study used vouchers that required patient activation and use; many of the reasons for voucher nonuse are also the barriers leading to medication nonadherence.39
Conclusions
Among patients with MI, provision of vouchers to offset medication co-payments for P2Y12 inhibitors, compared with no vouchers, resulted in a 3.3% absolute increase in patient-reported persistence with P2Y12 inhibitors at 1 year and no significant reduction in MACE outcomes at 1 year.
ARTEMIS Study Protocol
ARTEMIS Statistical Analysis Plan
eTable 1. Hospital Characteristics
eTable 2. List of Adjustment Variables for Primary Persistence and MACE Models
eTable 3. List of Variables Included in the Propensity Score Model
eTable 4. Baseline Patient Characteristics Among Patients Discharged Alive on Ticagrelor or Clopidogrel
eTable 5. Persistence and Clinical Outcomes Among the Primary Analysis Population Using 3 Modeling Methods
eTable 6. Persistence and Clinical Outcomes Among All Enrolled Patients, Including Patients Discharged on Prasugrel
eTable 7. Characteristics of Patients Who Did and Did Not Use the Study Voucher
eTable 8. Post Hoc As-Treated Analyses of Medication Persistence, MACE, and Major Bleeding
eFigure. Cumulative Incidence Curves for Major Adverse Cardiovascular Events Among Intervention Group Patients With and Without Voucher Use and Usual Care Group Patients
eAppendix. List of Enrolling Sites and Corresponding Principal Investigators (in Order of Number Enrolled)
Data Sharing Statement
References
- 1.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators . Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494-502. doi: 10.1056/NEJMoa010746 [DOI] [PubMed] [Google Scholar]
- 2.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Braunwald E, McCabe CH, et al. ; TRITON-TIMI 38 Investigators . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015. doi: 10.1056/NEJMoa0706482 [DOI] [PubMed] [Google Scholar]
- 4.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016;134(10):e123-e155. [DOI] [PubMed] [Google Scholar]
- 5.Roffi M, Patrono C, Collet JP, et al. ; ESC Scientific Document Group . 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267-315. doi: 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 6.Basra SS, Wang TY, Simon DN, et al. Ticagrelor use in acute myocardial infarction: insights from the National Cardiovascular Data Registry. J Am Heart Assoc. 2018;7(12):e008125. doi: 10.1161/JAHA.117.008125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fosbøl EL, Ju C, Anstrom KJ, et al. Early cessation of adenosine diphosphate receptor inhibitors among acute myocardial infarction patients treated with percutaneous coronary intervention: insights from the TRANSLATE-ACS study (Treatment With Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome). Circ Cardiovasc Interv. 2016;9(11):e003602. doi: 10.1161/CIRCINTERVENTIONS.115.003602 [DOI] [PubMed] [Google Scholar]
- 8.Czarny MJ, Nathan AS, Yeh RW, Mauri L. Adherence to dual antiplatelet therapy after coronary stenting: a systematic review. Clin Cardiol. 2014;37(8):505-513. doi: 10.1002/clc.22289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehran R, Baber U, Steg PG, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382(9906):1714-1722. doi: 10.1016/S0140-6736(13)61720-1 [DOI] [PubMed] [Google Scholar]
- 10.Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113(24):2803-2809. doi: 10.1161/CIRCULATIONAHA.106.618066 [DOI] [PubMed] [Google Scholar]
- 11.Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424. doi: 10.1016/j.ahj.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottorff MB, Nutescu EA, Spinler S. Antiplatelet therapy in patients with unstable angina and non–ST-segment-elevation myocardial infarction: findings from the CRUSADE national quality improvement initiative. Pharmacotherapy. 2007;27(8):1145-1162. doi: 10.1592/phco.27.8.1145 [DOI] [PubMed] [Google Scholar]
- 13.Choudhry NK, Avorn J, Glynn RJ, et al. ; Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI-FREEE) Trial . Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365(22):2088-2097. doi: 10.1056/NEJMsa1107913 [DOI] [PubMed] [Google Scholar]
- 14.Doll JA, Wang TY, Choudhry NK, et al. Rationale and design of the Affordability and Real-world Antiplatelet Treatment Effectiveness after Myocardial Infarction Study (ARTEMIS): a multicenter, cluster-randomized trial of P2Y12 receptor inhibitor copayment reduction after myocardial infarction. Am Heart J. 2016;177:33-41. doi: 10.1016/j.ahj.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 15.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44-47. doi: 10.1111/j.1524-4733.2007.00213.x [DOI] [PubMed] [Google Scholar]
- 16.Symphony Health Integrated Dataverse. Symphony Health website. http://symphonyhealth.com/product/idv/. Accessed October 25, 2018.
- 17.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 18.Campbell MK, Piaggio G, Elbourne DR, Altman DG; CONSORT Group . Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. doi: 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferdinand KC, Yadav K, Nasser SA, et al. Disparities in hypertension and cardiovascular disease in blacks: the critical role of medication adherence. J Clin Hypertens (Greenwich). 2017;19(10):1015-1024. doi: 10.1111/jch.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51(1):171-184. doi: 10.1002/bimj.200810488 [DOI] [PubMed] [Google Scholar]
- 22.StataCorp Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 23.Kleinman LC, Norton EC. What’s the risk? a simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44(1):288-302. doi: 10.1111/j.1475-6773.2008.00900.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norton EC, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. Stata J. 2013;13(3):492-509. https://www.stata-journal.com/article.html?article=st0306. Accessed December 3, 2018. [Google Scholar]
- 25.Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117(8):1028-1036. doi: 10.1161/CIRCULATIONAHA.107.706820 [DOI] [PubMed] [Google Scholar]
- 26.Mathews R, Wang TY, Honeycutt E, et al. ; TRANSLATE-ACS Study Investigators . Persistence with secondary prevention medications after acute myocardial infarction: insights from the TRANSLATE-ACS study. Am Heart J. 2015;170(1):62-69. doi: 10.1016/j.ahj.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathews R, Wang W, Kaltenbach LA, et al. Hospital variation in adherence rates to secondary prevention medications and the implications on quality. Circulation. 2018;137(20):2128-2138. doi: 10.1161/CIRCULATIONAHA.117.029160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doll JA, Hellkamp AS, Goyal A, Sutton NR, Peterson ED, Wang TY. Treatment, outcomes, and adherence to medication regimens among dual Medicare-Medicaid-eligible adults with myocardial infarction. JAMA Cardiol. 2016;1(7):787-794. doi: 10.1001/jamacardio.2016.2724 [DOI] [PubMed] [Google Scholar]
- 29.Choudhry NK, Patrick AR, Antman EM, Avorn J, Shrank WH. Cost-effectiveness of providing full drug coverage to increase medication adherence in post-myocardial infarction Medicare beneficiaries. Circulation. 2008;117(10):1261-1268. doi: 10.1161/CIRCULATIONAHA.107.735605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chernew ME, Shah MR, Wegh A, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health Aff (Millwood). 2008;27(1):103-112. doi: 10.1377/hlthaff.27.1.103 [DOI] [PubMed] [Google Scholar]
- 31.Oberjé EJ, de Kinderen RJ, Evers SM, van Woerkum CM, de Bruin M. Cost effectiveness of medication adherence-enhancing interventions: a systematic review of trial-based economic evaluations. Pharmacoeconomics. 2013;31(12):1155-1168. doi: 10.1007/s40273-013-0108-8 [DOI] [PubMed] [Google Scholar]
- 32.Aetna Aetna launching value-based program that improves medication adherence, cost and outcomes for members who have suffered from heart attacks. Aetna website. https://news.aetna.com/news-releases/aetna-launching-value-based-program-that-improves-medication-adherence-cost-and-outcomes-for-members-who-have-suffered-from-heart-attacks. Published November 14, 2011. Accessed March 6, 2018.
- 33.Faridi KF, Peterson ED, McCoy LA, Thomas L, Enriquez J, Wang TY. Timing of first postdischarge follow-up and medication adherence after acute myocardial infarction. JAMA Cardiol. 2016;1(2):147-155. doi: 10.1001/jamacardio.2016.0001 [DOI] [PubMed] [Google Scholar]
- 34.Masoudi FA, Ponirakis A, Yeh RW, et al. Cardiovascular care facts: a report from the National Cardiovascular Data Registry: 2011. J Am Coll Cardiol. 2013;62(21):1931-1947. doi: 10.1016/j.jacc.2013.05.099 [DOI] [PubMed] [Google Scholar]
- 35.Krousel-Wood M, Holt E, Joyce C, et al. Differences in cardiovascular disease risk when antihypertensive medication adherence is assessed by pharmacy fill versus self-report: the Cohort Study of Medication Adherence among Older Adults (CoSMO). J Hypertens. 2015;33(2):412-420. doi: 10.1097/HJH.0000000000000382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly K, Grau-Sepulveda MV, Goldstein BA, et al. The agreement of patient-reported versus observed medication adherence in type 2 diabetes mellitus (T2DM). BMJ Open Diabetes Res Care. 2016;4(1):e000182. doi: 10.1136/bmjdrc-2015-000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eli Lilly and Co Program description: Lilly Cares. Lilly website. http://www.lillycares.com/aboutlillycares.aspx. Accessed September 21, 2018.
- 38.Brilinta website. https://www.brilinta.com. Accessed September 21, 2018.
- 39.Mathews R, Peterson ED, Honeycutt E, et al. Early medication nonadherence after acute myocardial infarction: insights into actionable opportunities from the TReatment with ADP receptor iNhibitorS: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) study. Circ Cardiovasc Qual Outcomes. 2015;8(4):347-356. doi: 10.1161/CIRCOUTCOMES.114.001223 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ARTEMIS Study Protocol
ARTEMIS Statistical Analysis Plan
eTable 1. Hospital Characteristics
eTable 2. List of Adjustment Variables for Primary Persistence and MACE Models
eTable 3. List of Variables Included in the Propensity Score Model
eTable 4. Baseline Patient Characteristics Among Patients Discharged Alive on Ticagrelor or Clopidogrel
eTable 5. Persistence and Clinical Outcomes Among the Primary Analysis Population Using 3 Modeling Methods
eTable 6. Persistence and Clinical Outcomes Among All Enrolled Patients, Including Patients Discharged on Prasugrel
eTable 7. Characteristics of Patients Who Did and Did Not Use the Study Voucher
eTable 8. Post Hoc As-Treated Analyses of Medication Persistence, MACE, and Major Bleeding
eFigure. Cumulative Incidence Curves for Major Adverse Cardiovascular Events Among Intervention Group Patients With and Without Voucher Use and Usual Care Group Patients
eAppendix. List of Enrolling Sites and Corresponding Principal Investigators (in Order of Number Enrolled)
Data Sharing Statement