Abstract
Background
Respiratory syncytial virus (RSV) is one of the main viral causes of lower respiratory tract illness (LRTI), especially in young children. RSV vaccines, including maternal and infant vaccines, are under development; however, more epidemiological studies are needed to develop effective vaccination strategies.
Objectives
To estimate detailed age‐specific incidence rates and severity of RSV‐associated LRTI (RSV‐LRTI) using data from a community‐based prospective cohort study in the Philippines.
Patients/Methods
Cohort children who visited health facilities due to acute respiratory symptoms were identified, and nasopharyngeal swabs were collected to detect RSV. The severity of RSV‐LRTI was assessed using the severity definition proposed by the World Health Organization. Risk factors for developing RSV‐LRTI and contribution of SpO2 measurement were also evaluated.
Results
A total of 395 RSV episodes which occurred in children aged 2‐59 months were categorised as 183 RSV‐LRTI, 72 as severe RSV‐LRTI and 29 as very severe RSV‐LRTI. Children aged 3‐5 months had the highest incidence rate of RSV‐LRTI, at 207.4 per 1000 child‐years (95% CI: 149.0‐279.5). Younger age group, place of living and low educational level of caregivers were associated with developing RSV‐LRTI. Clinical manifestations had low levels of agreement with hypoxaemia as measured by pulse oximeter.
Conclusion
The highest burden of RSV was observed in young infants aged 3‐5 months, whereas the burden was also high in those aged 12‐20 months. Future vaccination strategies should consider the protection of older children, especially those aged one year, as well as young infants.
Keywords: cohort study, lower respiratory tract illness, pulse oximetry, respiratory syncytial virus
1. INTRODUCTION
Human respiratory syncytial virus (RSV), recently renamed as Human orthopneumovirus belonging to the Pneumoviridae family Orthopneumovirus genus,1 is one of the most important viral pathogens which causes lower respiratory tract illness (LRTI) including bronchiolitis and pneumonia, especially in infants and young children.2, 3 Respiratory syncytial virus is divided into two subgroups, A and B, based on antigenic and sequence variations. The predominant subgroup and proportion of each subgroup vary between epidemics.4 A recent global estimate showed that the incidence of RSV‐associated acute lower respiratory infection was 33.1 million, with 3.2 million hospital admissions and 59 600 in‐hospital deaths in children younger than 5 years old in 2015.5 Despite the burden of this disease, there are no commercially available vaccines and the monoclonal antibody is only a specific measure taken to prevent severe disease in high‐risk infants.6, 7 The incidence rate (IR) of RSV‐associated lower respiratory illness (RSV‐LRTI) is higher in the first 6 months of life compared with older age groups, and the illness is generally more severe in young infants.5, 8 For these reasons, it is believed that natural maternal immunity is not enough to prevent severe RSV‐LRTI in early infancy. Different strategies for vaccination, including maternal vaccination and infant vaccination, have been proposed to prevent severe infection.9, 10 However, effective implementation of these strategies depends on understanding the epidemiological patterns of severe infections, including age‐specific incidences.
Respiratory syncytial virus infections are associated with various levels of respiratory illnesses, from cold‐like mild illness to very severe and even life‐threatening LRTI. To evaluate the disease burden and effectiveness of vaccines or other interventions, it is necessary to define the severity of RSV infection.11 To identify severe LRTI with hypoxaemia, percutaneous arterial oxygen saturation (SpO2) measurement by a pulse oximeter has been shown to be effective. Studies have reported that the recognition of hypoxaemia using a pulse oximeter improved the management of children with LRTI.12, 13 It was also shown that one of the most important indicators for severe RSV‐LRTI was an SpO2 level of less than 90%.14 Recently, the World Health Organization (WHO) expert group proposed that the measurement of SpO2 should be included in the severity definition for RSV‐LRTI.11
Several prospective cohort studies have been conducted in low‐ and middle‐income countries to define age‐specific IRs.15, 16, 17, 18, 19, 20, 21, 22 However, to date, few studies have reported the IR of RSV‐LRTI using standard definitions of severity. Hence, in this study, we analysed the data from a community‐based prospective cohort study of children younger than five years old to understand in detail the age‐specific incidence of RSV‐LRTI, using the definitions proposed by the WHO expert group.11 We examined possible risk factors for developing RSV‐LRTI and severe RSV‐LRTI and compared clinical manifestations between severe and non‐severe cases using data from a community‐based prospective cohort study in the Philippines. Further, we evaluated the level of concordance between the severity of the LRTI definitions and the classification based on the integrated management of childhood illness (IMCI).23
2. MATERIALS AND METHODS
2.1. Study design
In the previously described prospective cohort study,24, 25 a cohort of 4012 children younger than 5 years old was followed in two municipalities, Kawayan and Caibiran, on a main island of Biliran province in the Philippines, from March 2014 to June 2016. From this larger data set, we focused on respiratory tract illness (RTI) episodes identified at health facilities over a time window of exactly 2 years, between April 2014 and March 2016. Cohort children were identified by household visits. Demographic and socioeconomic information were collected using standard questionnaire forms by the study nurses, after obtaining written informed consent for participation from parents or guardians. Newborn infants were also recruited for the cohort. Duration of exclusive breastfeeding was checked with the caregivers through a retrospective interview. Study nurses recommended the caregivers to take their children to health facilities when children had difficulty breathing or chest indrawing. Those children who visited health facilities due to respiratory symptoms were evaluated by study nurses or a physician.24, 25 SpO2 was measured using pulse oximetry, the equipment for which (PalmSat® 2500A, Nonin Medical Inc., Minnesota, USA) was provided by the study. The study was approved by the Institutional Review Board of the Research Institute for Tropical Medicine and the Ethics Committee of Tohoku University Graduate School of Medicine.
2.2. Definition of RSV‐associated RTI
An RTI was defined as a respiratory episode with cough and/or difficult breathing. A new episode was identified if the onset of the new RTI episode was at least 7 days from the previous episode. An episode of RSV‐associated RTI (RSV‐RTI) was defined based on positive laboratory results for RSV. If there were two RSV‐positive samples within a month, the episode with the latter positive sample was considered as prolonged shedding, except if the RSV subgroups or the partial G gene sequences were different from each other.
2.3. Classification of RTI severity
We categorised RTIs into LRTI, severe LRTI and very severe LRTI based on the severity definitions proposed by the WHO expert group (Supporting Information Table S1).11, 26 Briefly, LRTI was defined as RTI with fast breathing (≥50 breaths/min in children aged 2‐11 months, ≥40 breaths/min in children aged 12‐59 months) or SpO2 < 95%; severe LRTI as LRTI with chest indrawing or SpO2 < 93% and very severe LRTI as LRTI with inability to feed, sleeping most of the time, difficult to wake or SpO2 < 90%. When RTI occurred in children aged 0‐1 month, the severity could not be defined based on the WHO definitions mentioned above.11, 26 Therefore, for this age group, we categorised RTI with ≥60 breaths/min or SpO2 < 95% as LRTI and RTI with chest indrawing or SpO2 < 93% as severe LRTI. We also classified RSV‐associated respiratory disease using Integrated Management of Child Illness (IMCI), both with and without the revision proposed in 2014.26 In this revision, children with pneumonia having chest indrawing without danger signs were classified as “pneumonia” (non‐severe disease). Hospitalisation of the children with severe respiratory disease was indicated by local treating physician.
2.4. Viral detection and defining RSV subgroups
Nasopharyngeal swabs were collected from RTI for RSV testing as described previously,27 including real‐time polymerase chain reaction (PCR) to screen for RSV,28, 29 conventional PCR for subgrouping and sequencing of the second variable region of the G gene (RSV‐A: 342 bp and RSV‐B: 330 bp).30, 31 Testing for RSV and other viruses, including rhinovirus, enterovirus, human metapneumovirus, human adenovirus, parainfluenza virus and influenza virus, was conducted using primers described in Supporting Information Table S2. Details of the procedure were described elsewhere.24 We did not exclude RSV‐RTI with other viruses from the analysis.
2.5. Statistical methods
Proportion of RSV subgroups by severe levels was compared using chi‐squared test. Agreement of severity classification between IMCI26 and the RSV‐LRTI severe case definition and agreement of SpO2 < 93% and having other clinical manifestations were measured using Cohen's kappa statistic. Age‐specific IRs were calculated for every three‐month age group, except for children aged <3 months. These IRs were calculated separately for 0‐1 month (as undefined RSV‐RTI) and 2 months old. The IRs of each age group were calculated as the sum of new episodes divided by the sum of the child‐years obtained for each age group. Poisson regression model was used to calculate 95% confidential interval (CI) of IRs. To evaluate the risk factors associated with developing RSV‐LRTI, we calculated hazard ratios (HRs) for total RSV‐LRTI (including RSV‐LRTI, severe and very severe RSV‐LRTI) and total severe RSV‐LRTI (severe LRTI and very severe LRTI) using the Cox proportional hazard model. Adjusted HRs (aHRs) were calculated by adjusting for age, sex and place of living (municipality) and other factors with P < 0.1 in the univariate analysis. Socioeconomic status was assessed using the Simple Poverty Scorecard® Poverty‐Assessment Tool Philippines.32 Extreme poverty was defined as a score <30 points. The hazard ratio for exclusive breastfeeding was calculated separately for children <6 months. The frequencies of the clinical manifestations were compared between children with severe (severe RSV‐LRTI and very severe RSV‐LRTI) and non‐severe (RSV‐RTI and RSV‐LRTI) cases, using logistic regression models. Adjusted odds ratios (aORs) were calculated by adjusting for age, sex and place of living. The cox proportional hazard regression models and the logistic regression models were performed using R version 3.4.4,33 with the survival package.34
3. RESULTS
3.1. Categorisation of RSV‐RTI episodes
The first RSV epidemic was observed from May 2014 to January 2015 with the RSV mainly being subgroup A, and the second epidemic was observed from October 2015 to January 2016, with the RSV mainly being subgroup B.27 This analysis included 3817 children, yielding 4629 child‐years of follow‐up from April 2014 to March 2016. During this period, 2916 RTI episodes were identified at the health facilities, among which, 408 were classified as RSV‐RTI (Figure 1).
Figure 1.
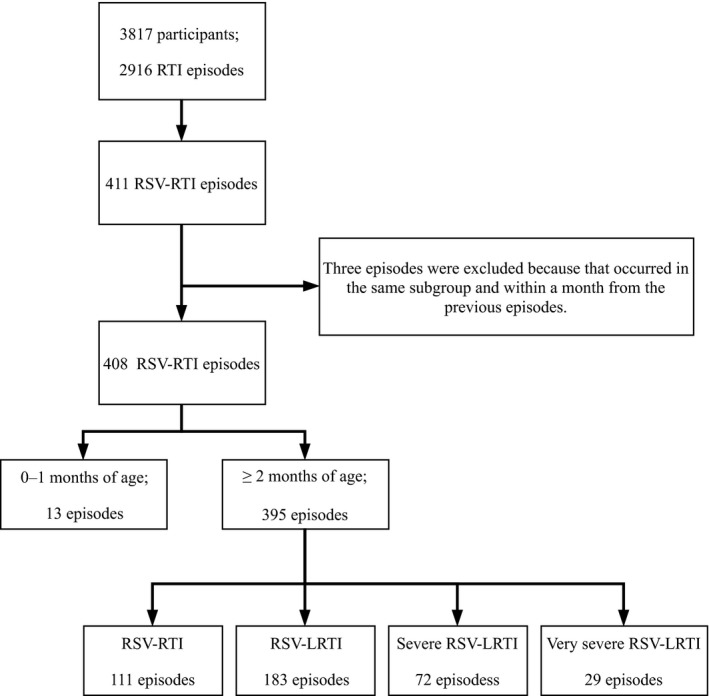
Study participants and severity assessment for respiratory syncytial virus‐associated respiratory tract illness (RSV‐RTI) in Biliran cohort study, Philippines, from April 2014 to March 2016. LRTI, lower respiratory tract illness
Among 408 RSV‐RTI episodes, 395 occurred in children aged 2 months or older, and 284 (71.9%) of those were placed into the RSV‐LRTI categories, including 183 RSV‐LRTI, 72 severe RSV‐LRTI and 29 very severe RSV‐LRTI (Figure 1). Six per cent of total RSV‐LRTI, 31% (31/101) of total severe RSV‐RLTI and 62% (18/29) of the very severe RSV‐LRTI episodes were identified by SpO2 only (Supporting Information Figure S1). Among total severe RSV‐LRTI (n = 101), 26 had SpO2 measured after starting oxygen treatment and at least 24% (24/101) had SpO2 < 90%, but only three were hospitalised. The proportion of RSV‐associated hospitalisation was 7.1% (28/395). Of these (n = 28), SpO2 data were available for 12 children and 25% (3/12) of those had SpO2 < 90%. There were 13 undefined RSV‐RTI episodes in children aged 0‐1 month, of which eight (61.5%) were hospitalised, all with chest indrawing, and three (23.1%) had SpO2 < 93% (Supporting Information Table S3). None of the 408 RSV‐RTI episodes was fatal.
3.2. Comparison between classification systems of severity
Among the 395 cases of children aged 2 months or older, using the RSV‐LRTI severity definition, the median age of children with RSV‐RTI was inversely associated with severity (Table 1). However, 71% (51/72) of the severe RSV‐LRTI and 51% (15/29) of the very severe RSV‐LRTI episodes occurred in children aged one year or older. The number of total RSV‐RTI in the municipality of Caibiran was twice that of Kawayan. In RSV‐associated hospitalisation, 96.4% (27/28) were classified as severe or very severe RSV‐LRTI. One hospitalised child had chest indrawing and inability to feed; however, this child did not have either fast breathing or SpO2 < 95%; therefore, this case was not classified as RSV‐LRTI.
Table 1.
Characteristics and distributions of RSV‐RTI episodes based on the RSV‐LRTI severity definition in children aged 2 to 59 mo
| Characteristics | (1) | (2) | (3) | (4) | (2) + (3) + (4) | (3) + (4) | (1) + (2) + (3) + (4) |
|---|---|---|---|---|---|---|---|
| RSV‐RTI (n = 111) | RSV‐LRTI (n = 183) | Severe RSV‐LRTI (n = 72) | Very severe RSV‐RLTI (n = 29) | Total RSV‐LRTI (n = 284) | Total severe RSV‐LRTI (n = 101) | Total (n = 395) | |
| Age in months, median [IQR] | 25 [11, 42] | 19 [11, 30] | 16 [10, 23] | 12 [6, 17] | 18 [10, 27] | 15 [9, 22] | 19 [10, 31] |
| Age in months, n (%) | |||||||
| 2‐5 | 14 (13) | 30 (16) | 10 (14) | 6 (21) | 46 (16) | 16 (16) | 60 (15) |
| 6‐11 | 19 (17) | 17 (9) | 11 (15) | 8 (28) | 36 (13) | 19 (19) | 55 (14) |
| 12‐23 | 21 (19) | 70 (38) | 36 (50) | 12 (41) | 118 (42) | 48 (48) | 139 (35) |
| 24‐35 | 21 (19) | 32 (17) | 4 (6) | 3 (10) | 39 (14) | 7 (7) | 60 (15) |
| 36‐59 | 36 (32) | 34 (19) | 11 (15) | 0 (0) | 45 (16) | 11 (11) | 81 (20) |
| Sex, n (%) | |||||||
| Male | 51 (46) | 90 (49) | 41 (57) | 20 (69) | 151 (53) | 61 (60) | 202 (51) |
| Female | 60 (54) | 93 (51) | 31 (43) | 9 (31) | 133 (47) | 40 (40) | 193 (49) |
| Place of living (municipality), n (%) | |||||||
| Caibiran | 67 (60) | 132 (72) | 48 (67) | 16 (55) | 196 (69) | 64 (63) | 263 (66) |
| Kawayan | 44 (40) | 51 (28) | 24 (33) | 13 (45) | 88 (31) | 37 (37) | 134 (34) |
| Health facility, n (%) | |||||||
| Outpatient | 110 (99) | 183 (100) | 55 (76) | 19 (66) | 257 (90) | 74 (73) | 367 (93) |
| Hospitalisation | 1 (1) | 0 (0) | 17 (24) | 10 (34) | 27 (10) | 27 (27) | 28 (7) |
| IMCI classification, n (%) | |||||||
| Cough and cold | 109 (98) | 7 (4) | 4 (6) | 3 (10) | 14 (5) | 7 (7) | 123 (31) |
| Pneumonia | 0 (0) | 173 (94) | 15 (21) | 9 (31) | 197 (69) | 24 (24) | 197 (69) |
| Severe pneumonia or severe disease | 2 (2) | 3 (2) | 53 (74) | 17 (59) | 73 (26) | 70 (69) | 75 (26) |
| Revised IMCI classification, n (%) | |||||||
| Cough and cold | 109 (98) | 7 (4) | 4 (6) | 3 (10) | 14 (5) | 7 (7) | 123 (31) |
| Pneumonia | 0 (0) | 173 (94) | 68 (94) | 15 (52) | 256 (90) | 83 (82) | 256 (65) |
| Severe pneumonia or severe disease | 2 (2) | 3 (2) | 0 (0) | 11 (38) | 14 (5) | 11 (11) | 16 (4) |
| RSV subgroup | |||||||
| RSV‐A | 38 (34) | 42 (23) | 27 (38) | 8 (28) | 77 (27) | 35 (35) | 115 (29) |
| RSV‐B | 70 (63) | 131 (72) | 43 (60) | 17 (59) | 191 (67) | 60 (59) | 261 (66) |
| Undefined | 3 (3) | 10 (5) | 2 (3) | 4 (14) | 16 (6) | 6 (6) | 19 (5) |
| Co‐detected other viruses, n (%) | |||||||
| Human adenovirus | 1 (1) | 0 (0) | 2 (3) | 0 (0) | 2 (1) | 2 (2) | 3 (1) |
| Rhinovirus | 6 (5) | 13 (7) | 8 (11) | 3 (10) | 24 (8) | 11 (11) | 30 (7) |
| Enterovirus | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 1 (0) |
| Human metapneumovirus | 0 (0) | 2 (1) | 0 (0) | 1 (3) | 3 (1) | 1 (1) | 3 (1) |
| Parainfluenza virus | 2 (2) | 2 (1) | 0 (0) | 0 (0) | 2 (1) | 0 (0) | 4 (1) |
| Influenza virus | 2 (2) | 3 (2) | 0 (0) | 0 (0) | 3 (1) | 0 (0) | 5 (1) |
IMCI, integrated management of childhood illness; IQR, interquartile range; LRTI, lower respiratory tract illness; RSV, respiratory syncytial virus; RTI, respiratory tract illness.
Percentages are rounded off to integers. Total RSV‐LRTI consists of RSV‐LRTI, severe RSV‐LRTI and very severe RSV‐LRTI.
Comparing between RSV‐RTI severity definitions and IMCI classification, 98.2% (109/111) of RSV‐RTI episodes were classified as “cough or cold” by IMCI, 94.5% (173/183) of RSV‐LRTI episodes were classified as “pneumonia” and 58.6% (17/29) of very severe RSV‐LRTI cases as “severe pneumonia or very severe disease” (Table 1). The overall agreement between non‐severe (cough and cold/pneumonia; RSV‐RTI/RSV‐LRTI) and severe (severe pneumonia or severe disease; severe RSV‐LRTI/very severe RSV‐LRTI) classifications was 91.0%, and the kappa statistic was 0.74. When we applied the revised IMCI,26 which excluded having chest indrawing from the criteria of severe disease, none of the severe RSV‐LRTI episodes was classified as “severe pneumonia or severe disease” by IMCI (0/72) and 52% (15/29) of the very severe RSV‐LRTI cases were classified as “pneumonia” by IMCI.
Within these 395 episodes, the subgroup of RSV was identified in 376 episodes. The proportion of severe or very severe episodes was higher in the subgroup A compared with the subgroup B without statistical significance (43.8% vs 29.9%, P = 0.06). Other viruses were also detected in 11.4% (46/395) of the episodes. Rhinovirus was the most frequently co‐detected virus (30/46) across all severity categories of RSV‐RTI (Table 1).
3.3. Age‐specific IRs of RSV‐LRTI
The overall IRs of total RSV‐LRTI and total severe RSV‐LRTI were 62.1 and 22.1 per 1000 child‐years in children aged 2‐59 months, respectively (Figure 2, Supporting Information Table S4). Incidence rates for children aged 2‐23 months were 124.0 and 51.5 per 1000 child‐years for total RSV‐LRTI and total severe RSV‐LRTI, respectively, and these IRs were significantly higher than those of children aged 24‐59 months (P < 0.001). Children aged 3‐5 months had the highest IRs for total RSV‐LRTI (207.4 per 1000 child‐years) and total severe RSV‐LRTI (74.5 per 1000 child‐years). The IRs of total RSV‐LRTI and total severe RSV‐LRTI declined in children aged 6‐8 months compared with children aged 3‐5 months (P = 0.001 and P = 0.125, respectively). Total RSV‐LRTI increased again in children aged 12‐14 months compared with children aged 9‐11 months without statistical significance (P = 0.07). After 20 months old, IRs declined with age. The same trends were seen when IRs were calculated by RSV subgroup, municipality or epidemics (Supporting Information Figure S2). When we considered RSV‐RTI with ≥60 breaths/min or SpO2 < 95% as LRTI and RSV‐RTI with chest indrawing or SpO2 < 93% as severe LRTI for children aged 0‐1 month, 8 out of 13 were severe RSV‐LRTI and the overall incidence rate of total RSV‐LRTI and that of total severe RSV‐LRTI were 63.1 and 23.5 per 1000 child‐years in children aged 0‐59 months, respectively.
Figure 2.
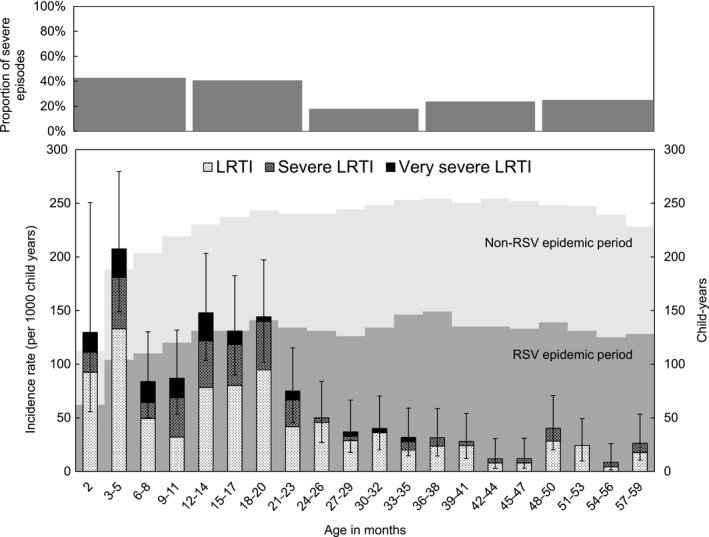
Age‐specific incidence rates of RSV‐LRTI and distributions of followed up child‐years in children aged less than 5 y in Biliran cohort study from April 2014 to March 2016. LRTI, lower respiratory tract illness; RSV, respiratory syncytial virus. The proportion of severe episodes was calculated as proportion of the total severe RSV‐LRTI episodes among total RSV‐LRTI by every 1 y of age. Incidence rates were calculated for 2 mo of age and every 3 mo of ages from 3 to 59 mo of ages. Child‐years were calculated for every 3 mo of ages. The child‐years during RSV epidemic period is shown as dark grey in background, and light grey shows child‐years out of RSV epidemic period. The epidemic periods were defined as May 2014‐January 2015 and October 2015‐January 2016 during the study period of April 2014‐March 2016. Whiskers show 95% confidence interval of incidence rate for whole RSV‐LRTI in each age group
Fast breathing is one of the criteria for LRTI, and the cut‐off points differ by age (50 breaths/min in children aged 2‐11 months and 40 breaths/min in children aged 12‐59 months). To exclude the influence of age on the criteria for fast breathing, we calculated age‐specific IRs of RSV‐RTI with chest indrawing or SpO2 < 93% independently without using fast breathing. Incidence rates of RSV‐RTI episodes with chest indrawing and SpO2 < 93% were still high in those aged 12‐20 months, and particularly IR in 12‐14 months was higher than those in 6‐8 months for both chest indrawing and SpO2 < 93%. (Supporting Information Figure S3). The proportion of the total severe RSV‐LRTI episodes among total RSV‐LRTI was 42.7% (35/82) in the first year of life (2‐11 months) with the highest proportion in 9‐11 months (Figure 2). The proportion did not decline in the second year of life (40.7%, 48/118, P = 0.777) and decreased significantly only in the third year of life (17.9%, 7/39, P = 0.010).
3.4. Risk factor analysis for developing total RSV‐LRTI and total severe RSV‐LRTI
In the univariate analysis, younger age group, place of living and educational level of caregiver were significantly associated with developing both total RSV‐LRTI and total severe RSV‐LRTI (Table 2). Birth order and having other children aged <6 years in the same household were significantly associated with developing total severe RSV‐LRTI. After the adjustment, younger age group was still significantly associated with developing both total RSV‐LRTI (range of aHR: 1.7‐8.5) and total severe RSV‐LRTI (range of aHR: 7.5‐11.3) compared with the age group of 36‐59 months (Table 2). Living in the municipality of Caibiran was significantly associated with both total RSV‐LRTI (aHR: 2.5, 95% CI: 2.0‐3.2, P < 0.001) and total severe RSV‐LRTI (aHR: 1.9, 95% CI: 1.3‐2.8, P = 0.002). Low educational level of caregiver (≤10 years of education) was also a significant risk of developing total RSV‐LRTI (range of aHR: 1.5‐1.7). The aHR was lower in the group with exclusive breastfeeding than those with non‐exclusive breastfeeding, but this difference was not statistically significant (aHR: 0.2, 95% CI: 0.0‐1.2, P = 0.09). The other factors we examined were not significantly associated with developing RSV‐LRTI.
Table 2.
Hazard ratios of risk factors for developing total RSV‐LRTI and total severe RSV‐LRTI in children aged 2‐59 mo
| Risk factors | Child‐years (n = 4571) | Total RSV‐LRTI (n = 284) | Total severe RSV‐LRTI (n = 101) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IR | HR | 95% CI | aHRa | 95% CI | n | IR | HR | 95% CI | aHRb | 95% CI | ||
| Age in months | |||||||||||||
| 2‐5 | 242 | 46 | 190.1 | 8.3** | 5.6‐12.4 | 8.5** | 5.7‐12.7 | 16 | 66.1 | 11.8** | 5.5‐25.4 | 11.3** | 5.2‐24.3 |
| 6‐11 | 421 | 36 | 85.5 | 3.7** | 2.4‐5.8 | 3.8** | 2.5‐5.9 | 19 | 45.1 | 8.1** | 3.9‐16.9 | 7.5** | 3.6‐16.0 |
| 12‐23 | 950 | 118 | 124.2 | 5.4** | 3.9‐7.6 | 5.4** | 3.8‐7.5 | 48 | 50.5 | 9.1** | 4.7‐17.4 | 8.3** | 4.3‐16.1 |
| 24‐35 | 986 | 39 | 39.6 | 1.7** | 1.1‐2.7 | 1.7** | 1.1‐2.7 | 7 | 7.1 | 1.3 | 0.5‐3.3 | 1.2 | 0.5‐3.2 |
| 36‐59 | 1972 | 45 | 22.8 | Ref | Ref | 11 | 5.6 | Ref | Ref | ||||
| Sex | |||||||||||||
| Male | 2395 | 150 | 62.6 | Ref | Ref | 60 | 25.1 | Ref | Ref | ||||
| Female | 2177 | 134 | 61.6 | 1.0 | 0.8‐1.2 | 1.0 | 0.8‐1.3 | 41 | 18.8 | 0.8 | 0.5‐1.1 | 0.8 | 0.5‐1.2 |
| Place of living (municipality) | |||||||||||||
| Caibiran | 2168 | 196 | 90.4 | 2.5** | 1.9‐3.2 | 2.5** | 2.0‐3.2 | 64 | 29.5 | 1.9** | 1.3‐2.9 | 1.9** | 1.3‐2.8 |
| Kawayan | 2404 | 88 | 36.6 | Ref | Ref | 37 | 15.4 | Ref | Ref | ||||
| Gestational age | |||||||||||||
| ≥36 wk | 4145 | 268 | 64.7 | Ref | Ref | 97 | 23.4 | Ref | Ref | ||||
| <36 wk | 51 | 3 | 58.8 | 0.9 | 0.3‐2.7 | 1.0 | 0.3‐3.0 | 2 | 39.2 | 1.7 | 0.4‐6.5 | 1.8 | 0.4‐7.2 |
| Unknown | 375 | 13 | 34.7 | 0.5** | 0.3‐0.9 | 0.8 | 0.4‐1.5 | 2 | 5.3 | 0.2** | 0.1‐0.9 | 0.4 | 0.1‐1.8 |
| Birthweight (g) | |||||||||||||
| ≥ 2500 | 3053 | 204 | 66.8 | Ref | Ref | 71 | 23.3 | Ref | Ref | ||||
| <2500 | 518 | 30 | 57.9 | 0.9 | 0.6‐1.3 | 0.9 | 0.6‐1.4 | 12 | 23.2 | 1.0 | 0.5‐1.8 | 1.1 | 0.6‐2.0 |
| Unknown | 1000 | 50 | 50.0 | 0.7* | 0.5‐1.0 | 1.0 | 0.7‐1.4 | 18 | 18.0 | 0.8 | 0.5‐1.3 | 1.3 | 0.8‐2.3 |
| No. of family member (persons) | |||||||||||||
| <7 | 2385 | 139 | 58.3 | Ref | Ref | 51 | 21.4 | Ref | Ref | ||||
| ≥7 | 2187 | 145 | 66.3 | 1.1 | 0.9‐1.4 | 1.1 | 0.8‐1.3 | 50 | 22.9 | 1.1 | 0.7‐1.6 | 0.8 | 0.5‐1.2 |
| Having smoker in the same HH | |||||||||||||
| No | 1916 | 122 | 63.7 | Ref | Ref | 46 | 24.0 | Ref | Ref | ||||
| Yes | 2656 | 162 | 61.0 | 1.0 | 0.8‐1.2 | 0.9 | 0.7‐1.1 | 55 | 20.7 | 0.9 | 0.6‐1.3 | 0.7 | 0.5‐1.1 |
| Birth order | |||||||||||||
| 1st | 1489 | 89 | 59.8 | Ref | Ref | 27 | 18.1 | Ref | Ref | ||||
| 2nd or 3rd | 1821 | 105 | 57.7 | 1.0 | 0.7‐1.3 | 0.9 | 0.7‐1.2 | 36 | 19.8 | 1.1 | 0.7‐1.8 | 1.1 | 0.6‐1.9 |
| >3rd | 1262 | 90 | 71.3 | 1.2 | 0.9‐1.6 | 1.0 | 0.7‐1.3 | 38 | 30.1 | 1.7** | 1.0‐2.7 | 1.5 | 0.9‐2.7 |
| Socioeconomic status (points) | |||||||||||||
| ≥30 | 2309 | 139 | 60.2 | Ref | Ref | 49 | 21.2 | Ref | Ref | ||||
| <30 | 2262 | 145 | 64.1 | 1.1 | 0.8‐1.3 | 1.0 | 0.8‐1.3 | 52 | 23.0 | 1.1 | 0.7‐1.6 | 0.9 | 0.6‐1.4 |
| Educational level of caregiver (y) | |||||||||||||
| ≤6 | 1237 | 68 | 55.0 | 1.3 | 0.8‐1.9 | 1.5** | 1.0‐2.3 | 25 | 20.2 | 1.4 | 0.7‐2.8 | 1.3 | 0.6‐2.8 |
| 7‐9 | 1320 | 91 | 68.9 | 1.6** | 1.1‐2.3 | 1.7** | 1.2‐2.5 | 25 | 18.9 | 1.3 | 0.7‐2.6 | 1.2 | 0.6‐2.4 |
| 10 | 1172 | 88 | 75.1 | 1.7** | 1.2‐2.5 | 1.7** | 1.2‐2.5 | 39 | 33.3 | 2.3** | 1.2‐4.4 | 2.1** | 1.1‐4.1 |
| ≥11 | 843 | 37 | 43.9 | Ref | Ref | 12 | 14.2 | Ref | Ref | ||||
| Educational level of father (y) | |||||||||||||
| ≤6 | 1342 | 79 | 58.9 | 0.9 | 0.6‐1.3 | 0.8 | 0.5‐1.1 | 37 | 27.6 | 2.4* | 1.0‐6.1 | 2.2 | 0.8‐5.7 |
| 7‐9 | 748 | 49 | 65.5 | 1.0 | 0.6‐1.5 | 0.8 | 0.5‐1.3 | 16 | 21.4 | 1.9 | 0.7‐5.0 | 1.7 | 0.6‐4.8 |
| 10 | 502 | 25 | 49.8 | 0.8 | 0.4‐1.3 | 0.6* | 0.4‐1.0 | 8 | 15.9 | 1.4 | 0.5‐4.2 | 1.2 | 0.4‐3.6 |
| ≥11 | 437 | 29 | 66.4 | Ref | Ref | 5 | 11.4 | Ref | Ref | ||||
| Unknown | 1543 | 102 | 66.1 | 1.0 | 0.7‐1.5 | 0.9 | 0.6‐1.3 | 35 | 22.7 | 2.0 | 0.8‐5.0 | 2.0 | 0.7‐5.3 |
| House wall material | |||||||||||||
| Light material | 1436 | 99 | 68.9 | Ref | Ref | 38 | 26.5 | Ref | Ref | ||||
| Strong material | 3136 | 185 | 59.0 | 0.9 | 0.7‐1.1 | 1.0 | 0.8‐1.3 | 63 | 20.1 | 0.8 | 0.5‐1.1 | 0.9 | 0.6‐1.3 |
| Kitchen location | |||||||||||||
| Outside of household | 1583 | 99 | 62.5 | Ref | Ref | 38 | 24.0 | Ref | Ref | ||||
| Inside of household | 2988 | 185 | 61.9 | 1.0 | 0.8‐1.3 | 0.9 | 0.7‐1.2 | 63 | 21.1 | 0.9 | 0.6‐1.3 | 0.8 | 0.6‐1.2 |
| Fuel type for cooking | |||||||||||||
| Electricity, LPG or kerosene | 308 | 17 | 55.2 | Ref | Ref | 5 | 16.2 | Ref | Ref | ||||
| Solid fuel | 4263 | 267 | 62.6 | 1.1 | 0.7‐1.9 | 1.0 | 0.6‐1.8 | 96 | 22.5 | 1.4 | 0.6‐3.4 | 1.1 | 0.4‐2.9 |
| Treatment of drinking water | |||||||||||||
| Nothing | 2070 | 136 | 65.7 | Ref | Ref | 44 | 21.3 | Ref | Ref | ||||
| Boil or bleach | 809 | 50 | 61.8 | 0.9 | 0.7‐1.3 | 1.1 | 0.8‐1.4 | 22 | 27.2 | 1.3 | 0.8‐2.1 | 1.3 | 0.8‐2.2 |
| Buying filtrated water | 1675 | 98 | 58.5 | 0.9 | 0.7‐1.2 | 1.0 | 0.8‐1.3 | 35 | 20.9 | 1.0 | 0.6‐1.5 | 1.0 | 0.7‐1.6 |
| Others | 17 | 0 | 0.0 | NA | NA | 0 | 0.0 | NA | NA | ||||
| Owning private toilet | |||||||||||||
| No | 1983 | 115 | 58.0 | Ref | Ref | 40 | 20.2 | Ref | Ref | ||||
| Yes | 2589 | 169 | 65.3 | 1.1 | 0.9‐1.4 | 1.3* | 1.0‐1.6 | 61 | 23.6 | 1.2 | 0.8‐1.7 | 1.4 | 0.9‐2.1 |
| # of other children <6 yr in HH | |||||||||||||
| None | 1575 | 87 | 55.2 | Ref | Ref | 28 | 17.8 | Ref | Ref | ||||
| 1 | 1843 | 133 | 72.2 | 1.3* | 1.0‐1.7 | 1.1 | 0.9‐1.5 | 53 | 28.8 | 1.6** | 1.0‐2.5 | 1.3 | 0.8‐2.2 |
| ≥ 2 | 1154 | 64 | 55.5 | 1.0 | 0.7‐1.4 | 0.8 | 0.6‐1.1 | 20 | 17.3 | 1.0 | 0.6‐1.7 | 0.7 | 0.4‐1.3 |
| # of other children 6‐14 yr in HH | |||||||||||||
| None | 1702 | 104 | 61.1 | Ref | Ref | 35 | 20.6 | Ref | Ref | ||||
| 1‐2 | 2006 | 116 | 57.8 | 0.9 | 0.7‐1.2 | 1.0 | 0.8‐1.3 | 42 | 20.9 | 1.0 | 0.7‐1.6 | 1.0 | 0.6‐1.6 |
| ≥ 3 | 864 | 64 | 74.1 | 1.2 | 0.9‐1.7 | 1.2 | 0.9‐1.6 | 24 | 27.8 | 1.4 | 0.8‐2.2 | 1.1 | 0.6‐2.1 |
| Breastfeeding statusc | |||||||||||||
| Non‐exclusive breastfeeding | 47 | 9 | 191.5 | Ref | Ref | 6 | 127.7 | Ref | Ref | ||||
| Exclusive breastfeeding | 70 | 8 | 114.3 | 0.6 | 0.2‐1.6 | 0.6 | 0.2‐1.6 | 2 | 28.6 | 0.2* | 0.0‐1.1 | 0.2* | 0.0‐1.2 |
| Unknown | 126 | 29 | 230.2 | 1.2 | 0.6‐2.6 | 1.1 | 0.5‐2.3 | 8 | 63.5 | 0.5 | 0.2‐1.5 | 0.5 | 0.2‐1.3 |
aHR, adjusted hazard ratio; HH, household; HR, hazard ratio; IR, incidence rate; LRTI, lower respiratory tract illness; RSV, respiratory syncytial virus.
Total RSV‐LRTI consists of RSV‐LRTI, severe RSV‐LRTI and very severe RSV‐LRTI. Total severe RSV‐LRTI consists of severe RSV‐LRTI and very severe RSV‐LRTI. Caregiver is the person that takes care of the child, knows best about the child and makes decisions for the best interest of the child. Solid fuel contains wood, charcoal, paper, etc
Adjusted for age group, sex, place of living, gestational age, birthweight, educational level of caregiver and number of other children <6 y in household.
Adjusted for age group, sex, place of living, gestational age, birth order, educational level of caregiver and father and number of other children <6 y in household.
Hazard ratio for breastfeeding status was adjusted for age group, sex, place of living and educational level of caregiver and calculated using subset data for 2‒6 y old.
P < 0.1.
P < 0.05.
3.5. Clinical signs and symptoms associated with severe and very severe LRTI in RSV‐RTI
In the comparison of clinical signs and symptoms between severe (severe RSV‐LRTI and very severe RSV‐LRTI) and non‐severe (RSV‐RTI and RSV‐LRTI) cases, having decreased breath sounds (aOR: 8.6, 95% CI: 1.9‐60.4), wheezing (aOR: 3.1, 95% CI: 1.9‐5.2), rales (aOR: 6.2, 95% CI: 3.5‐11.9), alar flaring (aOR: 26.7, 95% CI: 11.1‐75.3), axillary temperature ≥38°C (aOR: 2.0, 95% CI: 1.2‐3.4) and tachycardia (aOR: 2.0, 95% CI: 1.2‐3.3) were all significantly associated with severe cases after adjusting for age group, gender and place of living (Table 3). Grunting, decreasing breath sound and alar flaring had high specificity of more than 97%. The positive predictive value for severe cases was high for alar flaring (85.7%), whereas the negative predictive value was high for wheezing (80.7%) and alar flaring (81.5%). Although co‐detection of rhinovirus was not significantly associated with severe cases (aOR: 1.4, 95% CI: 0.6‐3.1, P = 0.390), the specificity for severe cases was as high as 93.5%. Decreasing breath sounds, wheezing, rale, alar flaring and chest indrawing were significantly associated with SpO2 < 93% (P < 0.05), without high levels of agreement (kappa statistic: 0.1‐0.3) (Table 3).
Table 3.
Adjusted odds ratios for comparison of clinical manifestations between severe and non‐severe RSV‐LRTI and agreement between clinical manifestation and SpO2 < 93%
| Clinical manifestation | Total | Non‐severe case | Severe case | aORa | 95% CI | P‐value | Sensitivity | Specificity | Agreement with SpO2 < 93% | |
|---|---|---|---|---|---|---|---|---|---|---|
| RSV‐RTI and RSV‐LRTI | Severe and very severe RSV‐RTI | |||||||||
| n = 395 | n = 294 (%) | n = 101 (%) | % | κ | ||||||
| Illness history | ||||||||||
| Convulsion | ||||||||||
| No | 391 | 290 (74.2) | 101 (25.8) | — | ||||||
| Yes | 4 | 4 (100) | 0 (0.0) | — | — | — | — | — | ||
| Pallor | ||||||||||
| No | 394 | 293 (74.4) | 101 (25.6) | — | ||||||
| Yes | 1 | 1 (100) | 0 (0.0) | — | — | — | — | — | ||
| Physical examination | ||||||||||
| Grunting | ||||||||||
| No | 380 | 287 (75.5) | 93 (24.5) | Ref | ||||||
| Yes | 15 | 7 (46.7) | 8 (53.3) | 2.9 | 1.0‐8.8 | 0.052 | 7.9% | 97.6% | 84.20% | 0.1 |
| Decreasing breath sound | ||||||||||
| No | 386 | 292 (75.6) | 94 (24.4) | Ref | ||||||
| Yes | 9 | 2 (22.2) | 7 (77.8) | 8.6 | 1.9‐60.4 | 0.01 | 6.9% | 99.3% | 85.30% | 0.1 |
| Wheezing sounds | ||||||||||
| No | 281 | 227 (80.8) | 54 (19.2) | Ref | ||||||
| Yes | 114 | 67 (58.8) | 47 (41.2) | 3.1 | 1.9‐5.2 | <0.001 | 46.5% | 77.1% | 71.90% | 0.2 |
| Rales | ||||||||||
| No | 174 | 159 (91.4) | 15 (8.6) | Ref | ||||||
| Yes | 221 | 135 (61.1) | 86 (38.9) | 6.2 | 3.5‐11.9 | <0.001 | 85.1% | 53.9% | 54.60% | 0.1 |
| Alar flaring | ||||||||||
| No | 353 | 288 (81.6) | 65 (18.4) | Ref | ||||||
| Yes | 42 | 6 (14.3) | 36 (85.7) | 26.7 | 11.1‐75.3 | <0.001 | 35.6% | 98.0% | 83.90% | 0.2 |
| Chest indrawing | ||||||||||
| No | 325 | 293 (90.2) | 32 (9.8) | |||||||
| Yes | 70 | 1 (1.4) | 69 (98.6) | 790.6 | 156.6‐14 558.7 | <0.001 | 68.30% | 99.70% | 82.80% | 0.3 |
| Central cyanosis | ||||||||||
| No | 395 | 288 (81.6) | 65 (25.6) | — | ||||||
| Yes | 0 | 6 (14.3) | 36 | — | — | — | — | — | ||
| Apnoea | ||||||||||
| No | 395 | 294 (74.4) | 101 (25.6) | — | ||||||
| Yes | 0 | — | — | — | — | — | — | |||
| Poor skin turgor | ||||||||||
| No | 395 | 294 (74.4) | 101 (25.6) | — | ||||||
| Yes | 0 | — | — | — | — | — | — | |||
| Vital signs | ||||||||||
| Axillary temperature | ||||||||||
| <38°C | 300 | 232 (77.3) | 68 (22.7) | Ref | ||||||
| ≥38°C | 95 | 62 (65.3) | 33 (34.7) | 2 | 1.2‐3.4 | 0.008 | 32.7% | 78.9% | 71.00% | 0.1 |
| Tachycardia | ||||||||||
| No | 302 | 233 (77.2) | 69 (22.8) | Ref | ||||||
| Yes | 93 | 61 (65.6) | 32 (34.4) | 2 | 1.2‐3.3 | 0.01 | 31.7% | 79.3% | 72.10% | 0.1 |
| Co‐detected virus | ||||||||||
| Rhinovirus | ||||||||||
| No | 365 | 275 (75.3) | 90 (24.7) | Ref | ||||||
| Yes | 30 | 19 (63.3) | 11 (36.7) | 1.4 | 0.6‐3.1 | 0.39 | 10.9% | 93.5% | 80.3 | 0 |
LRTI, lower respiratory tract illness; OR, odds ratio; RSV, respiratory syncytial virus; RTI, respiratory tract illness.
Non‐severe RSV‐RTI consists of RSV‐LRTI and RSV‐RTI. Tachycardia is defined by age: >160 pulse/min for younger than 12 mo, >150 pulses/min for 12‐35 mo and >140 pulse/min for 36‐59 mo.
Adjusted for age group, sex and municipality
4. DISCUSSION
Using data from a prospective cohort study of rural communities in the Philippines, we examined the incidence rates and severity of RSV‐LRTI in detail. We estimated the overall IR of total RSV‐LRTI to be 62.1 per 1000 child‐years in children aged 2‐59 months. These rates are comparable to recent global estimates for IRs in low‐ to upper‐middle‐income countries, which are in the range of 41‐94 per 1000 child‐years in children aged 0‐59 months (from limited data with different methodologies).5 Other community‐based cohort studies conducted in low‐ and middle‐income countries have reported higher IRs for total RSV‐LRTI of up to 71‐94 per 1000 child‐years in Nigeria, Kenya and Indonesia.15, 18, 20 Such differences may be due to the fact that these studies conducted active case findings of children with acute respiratory symptoms, whereas we analysed children who visited health facilities, potentially underestimating IRs.
We found that age‐specific IRs were significantly higher in children younger than 2 years old than in the older age group, and the same pattern was observed for total severe LRTI. Among those, the IRs of total RSV‐LRTI and total severe RSV‐LRTI were especially high in children aged 3‐5 months. Peak IRs found in previous similar studies were 3‐5 months in Nigeria, 0‐5 months in Kenya and 6‐8 months in Indonesia.15, 18, 20 In the Indonesian study, the IR was lower in children aged 3‐5 months than in those aged 6‐8 months, which may have been related to different factors such as the level of maternal antibodies35, 36 or breastfeeding practice,37 since young infants were likely to develop RSV‐associated bronchiolitis and pneumonia.38 Interestingly, we found that IRs dropped in children aged 6‐8 months and 9‐11 months, then increased again in children aged 12‐14, 15‐17 and 18‐20 months (Figure 2), in a pattern similar to previous studies.15, 18, 20 In hospital‐based studies, a majority of RSV‐associated severe infections are seen in infants aged <1 year, especially in young infants aged <6 months.19, 39 However, our study and other prospective cohort studies have identified similar bimodal peaks in IRs of total RSV‐LRTI, that is, young infants aged <6 months and children aged 12‐23 months. We suspected that the increased IRs in children aged 12‐20 months might be due to the change in the criteria for fast breathing at 12 months old. However, the age‐specific IRs of severe RSV‐LRTI defined by only chest indrawing or SpO2 < 93% showed similar patterns, suggesting a true increase in incidence in the second years of life. Age‐specific IRs might also be affected by the timing of RSV epidemics since observed child‐year for each age group did not cover whole epidemic periods. Therefore, we compared the proportion of epidemic period in total observed child‐years for each age group. We found not much difference in the proportions of epidemic periods between age groups. The age‐specific pattern of IRs for RSV‐LRTI has important implications for future intervention strategies including vaccination. While current vaccination strategies focus primarily on protecting young infants, if the IRs of RSV‐LRTI (especially the more severe cases) are also high in children 12‐23 months, vaccination strategies may need to consider including a booster dose before 12 months.
In our study, 13 undefined RSV‐RTI episodes were identified in children aged <2 months due to unavailability of severity definitions.11, 26 Eight were hospitalised, and all of these had chest indrawing and three of them had SpO2 < 93%. Therefore, more than half of RSV‐RTI in children aged <2 months were considered to be severe. Incidence rates for those with chest drawing and SpO2 < 93% were highest in this age group, indicating the significant impact of RSV‐RTI in this age group. To reveal true burden and risk factors for this age group, further studies, particularly large‐scale birth cohort studies, should be conducted.
We found that younger age of children (range of aHRs for total RSV‐LRTI and total severe RSV‐LRTI: 1.7‐8.5 and 7.5‐11.3, respectively), living in Caibiran (aHRs for total RSV‐LRTI: 2.4, 95% CI: 1.9‐3.1 and for total severe RSV‐LRTI: 1.9, 95% CI: 1.3‐2.8) and lower educational level of caregivers (range of aHRs for total RSV‐LRTI: 1.5‐1.7) were significant risk factors for the development of total RSV‐LRTI and total severe RSV‐LRTI (Table 2). Caibiran had a larger epidemic of RSV, and particularly RSV‐B, in 2015.27 Although the reasons for such differences are unknown, epidemiological patterns of RSV epidemics including their size can vary among different locations and also between epidemics even in the same location. It is therefore important to conduct longitudinal studies in multiple locations to assess the true impact of RSV in communities. Low educational level of caregiver has been often cited as a risk factor for developing severe RSV infections,40, 41 as we found here. Other commonly identified risk factors, such as prematurity (OR: 2.0), low birthweight (OR: 1.9), being male (OR: 1.2), having siblings (OR: 1.6), household crowding (OR: 1.9) and non‐exclusive breastfeeding (OR: 2.2), identified in a meta‐analysis,42 were not significant risk factors in the present study. Prematurity and low birthweight were significantly associated with acute lower respiratory infection in case‐control studies conducted in the hospital settings with a large number of children with severe RSV‐LRTI in high‐income countries.42 In our study, which mainly included cases in primary care, we did not reach the statistical significance probably due to the insufficient number of children with low birthweight, prematurity and severe RSV‐LRTI. In other low‐ and middle‐income countries, only unpublished data of one case‐control study and one cohort study for prematurity and one cohort study for low birthweight were conducted and none of these studies showed prematurity or low birthweight as a significant risk factor for severe RSV‐LRTI.42
Children whose gestational age or birthweight was not reported by their mothers (categorised as “unknown”) were less likely to develop RSV‐LRTI compared with the reference group. There may be a recall bias that mothers who could not recall were more likely to have had children with normal weight and gestational age.
We included a relatively large number of children in our cohort study, but the number was still too small to assess all possible risk factors. Most other studies of risk factors have been conducted as case‐control studies, which are more suitable for evaluating risk factors that occur with low frequency, such as prematurity and low birthweight.42 In another prospective cohort study in Bohol, the Philippines, being male and having siblings were identified as significant risk factors,43 although there were some differences in the study designs. The Bohol study used RSV‐associated hospitalisation as an outcome and had twice as many participants as our study. On the other hand, a birth cohort study in Kilifi, Kenya, with fewer participants than ours,44 having 1‐2 siblings less than 6 years old living in the same household, was a statistically significant risk factor for developing total RSV‐LRTI. Thus, further studies are necessary to comprehensively evaluate various risk factors for developing severe RSV infections.
As a previous study showed,45 clinical manifestations, such as wheezing, rale, alar flaring and chest indrawing, were significantly associated with SpO2 < 93% in our study. However, these clinical manifestations had low level of agreement with SpO2. Predicting hypoxaemia by clinical manifestation seemed difficult because no sole clinical sign had high sensitivity and specificity as described elsewhere.45, 46 The combination of the clinical manifestations in Usen et al’s study in Gambia had 70% of sensitivity and 79% of specificity.45 Moreover, 32% of severe and 62% of very severe cases were only defined by SpO2 level without meeting other clinical criteria of the WHO RSV‐LRTI case definition. Therefore, it should be emphasised that the diagnostic value of SpO2 measurement increased as the level of severity increased. Some studies have shown that hypoxaemia is more prevalent in children with RSV‐LRTI compared with LRTI with other aetiology.47, 48 In our study, 19% (19/101) of the total severe RSV‐LRTI had SpO2 < 90%. However, only 16% (3/19) of those were actually hospitalised. Taken together, testing for hypoxaemia is crucial for evaluation of children with RSV‐associated RTI to identify severe cases. Home care for children with LRI should be considered with SpO2 measurement. However, setting a cut‐off point for application of the oxygen therapy and providing the necessary equipment are important to support health workers’ decision.
One limitation of our study was that we only included episodes observed in health facilities. Since health‐seeking behaviour can vary among age groups,49 we may have underestimated the IRs, particularly in older children who are less likely to visit health facilities than infants. Also, our study only included two sites from the same small island with two epidemics over two years, and thus, our results, including IRs, may not reflect the true burden. In addition, the study was not conducted as a birth cohort and most newborn infants were not recruited for the study immediately after birth. Therefore, observed child‐years in this age group were particularly small in infants aged less than 3 months.
5. CONCLUSION
Although young infants had the highest risk of developing severe and very severe RSV‐LRTI, there were also significant impacts on the second year of life. Future interventions, including vaccination, should consider children up to two years old as a population vulnerable to severe RSV‐LRTI. Integrated child care approaches should include the use of a pulse oximeter to evaluate the severity of LRTI, and adequate oxygen treatment should be given to severe LRTI with hypoxaemia.
Supporting information
ACKNOWLEDGEMENTS
We are grateful to Dr. Balasbas C and Dr. Plaza D, the medical health officers in the municipalities of Kawayan and Caibiran; Dr. Caneja J, a chief of Biliran Provincial Hospital; and Dr. Veloso E and the late Dr. Veneracion A, provincial health officers in that hospital, for their research cooperation. We would like to also thank the staff of the Tohoku‐RITM Collaborating Research Center on Emerging and Re‐emerging Infectious Diseases in Biliran and RITM; Ms. Estanislao‐Dueñas M, Ms. Abe S and Dr. Nakagawa E for administrative support; and Ms. Kishi M, Ms. Venturina M and staff of AFTM for technical support.
APPENDIX 1.
Members of the RSV working group in the Philippines are: Jhoys M. Landicho4, Mark Donald C. Reñosa4, Marianette T. Inobaya4, Portia P. Alday4, Amado Tandoc III4
Ueno F, Tamaki R, Saito M, et al; RSV Working Group in the Philippines . Age‐specific incidence rates and risk factors for respiratory syncytial virus‐associated lower respiratory tract illness in cohort children under 5 years old in the Philippines. Influenza Other Respi Viruses. 2019;13:339–353. 10.1111/irv.12639
See Appendix 1 for the members of the RSV Working Group in the Philippines.
Funding information
This work was supported by the Japan Initiative for Global Research Network on Infectious Diseases from the Japan Agency for Medical and Research and Development (AMED) (JP18fm0108013); the Science and Technology Research Partnership for Sustainable Development from AMED and Japan International Cooperation Agency (JP16jm0110001); and JSPS KAKENHI (grant number JP16H02642).
Contributor Information
Hitoshi Oshitani, Email: oshitanih@med.tohoku.ac.jp.
RSV Working Group in the Philippines:
Jhoys M. Landicho, Mark Donald C. Reñosa, Marianette T. Inobaya, Portia P. Alday, and Amado O. Tandoc, III
REFERENCES
- 1. Rima B, Collins P, Easton A, et al. ICTV virus taxonomy profile: Pneumoviridae. J Gen Virol. 2017;98:2912‐2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162:80‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev. 2014;35:519‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall CB, Walsh EE, Schnabel KC, et al. Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis. 1990;162:1283‐1290. [DOI] [PubMed] [Google Scholar]
- 5. Shi T, David AM, Katherine LO, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018;18:e295‐e311. [DOI] [PubMed] [Google Scholar]
- 7. Simões E, Bont L, Manzoni P, et al. Past, present and future approaches to the prevention and treatment of respiratory syncytial virus infection in children. Infectious Diseases and Therapy. 2018;7:87‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scheltema NM, Gentile A, Lucion F, et al. Global respiratory syncytial virus‐associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Global Health. 2017;5:e984‐e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scheltema NM, Kavelaars XM, Thorburn K, et al. Potential impact of maternal vaccination on life‐threatening respiratory syncytial virus infection during infancy. Vaccine. 2018;36:4693‐4700. [DOI] [PubMed] [Google Scholar]
- 10. Cromer D, van Hoek AJ, Newall AT, Pollard AJ, Jit M. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: a modelling and cost‐effectiveness analysis for England. Lancet Public Health. 2017;2:e367‐e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Modjarrad K, Giersing B, Kaslow DC, Smith PG, Moorthy VS.WHO RSV Vaccine Consultation Expert Group . WHO consultation on Respiratory Syncytial Virus Vaccine Development Report from a World Health Organization Meeting held on 23–24 March 2015. Vaccine. 2016;34:190‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duke T, Wandi F, Jonathan M, et al. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. Lancet. 2008;372:1328‐1333. [DOI] [PubMed] [Google Scholar]
- 13. McCollum ED, King C, Deula R, et al. Pulse oximetry for children with pneumonia treated as outpatients in rural Malawi. Bull World Health Organ. 2016;94:893‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atwell JE, Geoghegan S, Karron RA, Polack FP. Clinical predictors of critical lower respiratory tract illness due to respiratory syncytial virus in infants and children: data to inform case definitions for efficacy trials. J Infect Dis. 2016;214:1712‐1716. [DOI] [PubMed] [Google Scholar]
- 15. Simões E, Mutyara K, Soh S, Agustian D, Hibberd ML, Kartasasmita CB. The epidemiology of respiratory syncytial virus lower respiratory tract infections in children less than 5 years of age in Indonesia. Pediatr Infect Dis J. 2011;30:778. [DOI] [PubMed] [Google Scholar]
- 16. Anders KL, Nguyen HL, Nguyen NM, et al. Epidemiology and virology of acute respiratory infections during the first year of life: a birth cohort study in Vietnam. Pediatr Infect Dis J. 2015;34:361‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Homaira N, Luby SP, Petri WA, et al. Incidence of respiratory virus‐associated pneumonia in urban poor young children of Dhaka, Bangladesh, 2009–2011. PLoS ONE. 2012;7:e32056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nokes DJ, Okiro EA, Ngama M, et al. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi District, Kenya. Clin Infect Dis. 2008;46:50‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nokes DJ, Ngama M, Bett A, et al. Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin Infect Dis. 2009;49:1341‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robertson SE, Roca A, Alonso P, et al. Respiratory syncytial virus infection: denominator‐based studies in Indonesia, Mozambique, Nigeria and South Africa. Bull World Health Organ. 2004;82:914‐922. [PMC free article] [PubMed] [Google Scholar]
- 21. Broor S, Dawood FS, Pandey BG, et al. Rates of respiratory virus‐associated hospitalization in children aged <5 years in rural northern India. J Infect. 2014;68:281‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasan K, Jolly P, Marquis G, et al. Viral etiology of pneumonia in a cohort of newborns till 24 months of age in Rural Mirzapur, Bangladesh. Scand J Infect Dis. 2006;38:690‐695. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization, Newborn, Child and Adolescent Health Department of Maternal, World Health Organization . Revised WHO Classification and Treatment of Pneumonia in Children at Health Facilities: Evidence Summaries; 2014. [PubMed] [Google Scholar]
- 24. Furuse Y, Tamaki R, Okamoto M, et al. Association between preceding viral respiratory infection and subsequent respiratory illnesses among children: a prospective cohort study in the Philippines. J Infect Dis. 2018;219:197‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tamaki R, Tallo VL, Tan AG, et al. Comprehensive etiological and epidemiological study on acute respiratory infections in children: Providing evidence for the prevention and control of childhood pneumonia in the Philippines. J Disaster Res. 2018;13:740-750. [Google Scholar]
- 26. Karron RA, Black RE. Determining the burden of respiratory syncytial virus disease: the known and the unknown. Lancet. 2017;390:917‐918. [DOI] [PubMed] [Google Scholar]
- 27. Okamoto M, Dapat CP, Sandagon AMD, et al. Molecular characterization of respiratory syncytial virus in children with repeated infections with subgroup B in the Philippines. J Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonroy C, Vankeerberghen A, Boel A, Beenhouwer DH. Use of a multiplex real‐time PCR to study the incidence of human metapneumovirus and human respiratory syncytial virus infections during two winter seasons in a Belgian paediatric hospital. Clin Microbiol Infec. 2007;13:504‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malasao R, Okamoto M, Chaimongkol N, et al. Molecular characterization of human respiratory syncytial virus in the Philippines, 2012–2013. PLoS ONE. 2015;10:e0142192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sato M, Saito R, Sakai T, et al. Molecular epidemiology of respiratory syncytial virus infections among children with acute respiratory symptoms in a community over three seasons. J Clin Microbiol. 2005;43:36‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peret TC, Hall CB, Schnabel KC, Golub JA, Anderson LJ. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J Gen Virol. 1998;79(Pt 9):2221‐2229. [DOI] [PubMed] [Google Scholar]
- 32. Schreiner M. Simple Poverty Scorecard® Poverty‐Assessment Tool Philippines. https://dirp3.pids.gov.ph/ris/pjd/pidspjd07-2poverty.pdf. Accessed March 01, 2019.
- 33. R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Core Team; 2018. [Google Scholar]
- 34. Therneau TM. A package for survival analysis in S. 2015.
- 35. Stensballe LG, Ravn H, Kristensen K, Meakins T, Aaby P, Simoes E. Seasonal variation of maternally derived respiratory syncytial virus antibodies and association with infant hospitalizations for respiratory syncytial virus. J Pediatr. 2009;154:296‐298. [DOI] [PubMed] [Google Scholar]
- 36. Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low‐income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708‐715. [DOI] [PubMed] [Google Scholar]
- 37. Downham MA, Scott R, Sims DG, Webb JK, Gardner PS. Breast‐feeding protects against respiratory syncytial virus infections. Br Med J. 1976;2:274‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parrott Rh, Kim HW, Arrobio Jo, et al. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973;98:289‐300. [DOI] [PubMed] [Google Scholar]
- 39. Murray J, Bottle A, Sharland M, et al. Risk factors for hospital admission with RSV bronchiolitis in England: a population‐based birth cohort study. PLoS ONE. 2014;9:e89186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simoes E. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143:S118‐126. [DOI] [PubMed] [Google Scholar]
- 41. Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus‐associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133:1135‐1151. [DOI] [PubMed] [Google Scholar]
- 42. Shi T, Balsells E, Wastnedge E, et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta–analysis. Journal of Global Health. 2015;5:020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paynter S, Ware RS, Lucero MG, et al. Malnutrition: a risk factor for severe respiratory syncytial virus infection and hospitalization. Pediatr Infect Dis J. 2014;33:267. [DOI] [PubMed] [Google Scholar]
- 44. Okiro EA, Ngama M, Bett A, Cane PA, Medley GF, Nokes DJ. Factors associated with increased risk of progression to respiratory syncytial virus‐associated pneumonia in young Kenyan children*. Tropical Med Int Health. 2008;13:914‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Usen S, Weber M, Mulholland K, et al. Clinical predictors of hypoxaemia in Gambian children with acute lower respiratory tract infection: prospective cohort study. BMJ. 1999;318:86‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laman M, Ripa P, Vince J, Tefuarani N. Can clinical signs predict hypoxaemia in Papua New Guinean children with moderate and severe pneumonia? Ann Trop Paediatr. 2005;25:23‐27. [DOI] [PubMed] [Google Scholar]
- 47. Riccetto A, Ribeiro JD, Silva MT, Almeida RS, Arns CW, Baracat E. Respiratory syncytial virus (RSV) in infants hospitalized for acute lower respiratory tract disease: incidence and associated risks. Braz J Infect Dis. 2006;10:357‐361. [DOI] [PubMed] [Google Scholar]
- 48. Komurian‐Pradel F, Endtz H, Rakoto‐Andrianarivelo M, et al. Severity of pneumonia in under 5‐year‐old children from developing countries: a multicenter, prospective, observational study. Am J Trop Med Hyg. 2017;97:68‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taffa N, & Chepngeno G. Determinants of health care seeking for childhood illnesses in Nairobi slums. Trop Med Int Health. 2005;10:240-245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


