Abstract
Ubiquitous expression of GABA type A receptors (GABAAR) in the central nervous system establishes their central role in coordinating most aspects of neural function and development. Dysregulation of GABAergic neurotransmission manifests in a number of human health disorders and conditions that in certain cases can be alleviated by drugs targeting these receptors. Precise changes in the quantity or activity of GABAARs localized at the cell surface and at GABAergic postsynaptic sites directly impact the strength of inhibition. The molecular mechanisms constituting receptor trafficking to and from these compartments therefore dictate the efficacy of GABAAR function. Here we review the current understanding of how GABAARs traffic through biogenesis, plasma membrane transport, and degradation. Emphasis is placed on discussing novel GABAergic synaptic proteins, receptor and scaffolding posttranslational modifications, activity-dependent changes in GABAAR confinement, and neuropeptide and neurosteroid mediated changes. We further highlight modern techniques currently advancing the knowledge of GABAAR trafficking and clinically relevant neurodeve lopmental diseases connected to GABAergic dysfunction.
Keywords: GABAAR, trafficking, synapse, inhibition, plasticity
INTRODUCTION
GABAA receptors (GABAARs) are ligand-gated ionotropic chloride (Cl−) channels responsible for most fast inhibitory neurotransmission in the mature central nervous system (CNS). Ubiquitous expression supports their central role in regulating most aspects of CNS function. Clinically, these receptors are important drug targets for anti-convulsant, anxiolytic, and sedative-hypnotic agents including benzodiazepines (BZs), barbiturates, alcohol, certain anesthetics and neurosteroids. Underlying deficits in GABAergic neurotransmission occur in a wide variety of neurological disorders such as epilepsy, psychiatric disorders (anxiety [Mohler, 2012; Nuss, 2015], depression [Luscher et al., 2011b; Pehrson and Sanchez, 2015], post-traumatic stress disorder [Pitman et al., 2012; Moller et al., 2016; Trousselard et al., 2016; Bandelow et al., 2017]) and neurodevelopmental disorders including autism (Robertson et al., 2016; Chao et al., 2010; Coghlan et al., 2012), Fragile X (Lozano et al., 2015; Wang et al., 2015) and schizophrenia (Gonzalez-Burgos et al., 2010; Bristow et al., 2015; Wijtenburg et al., 2015). Importantly, pathophysiological events including seizures (Scharfman and Brooks-Kayal, 2014), ischemic stroke (Carmichael, 2012; Blicher et al., 2015; Wu and Sun, 2015), traumatic brain injury (Guerriero et al., 2015) and stress can cause adaptive changes in GABAAR neurotransmission, compromising GABAergic inhibition and further hampering recovery.
Binding of the neurotransmitter GABA induces GABAAR ion channel opening, C1− influx, and subsequent membrane hyperpolarization (Fig. 1A). These receptors are heteropentameric structures typically composed of two α (α1–6), two β (β1–3), and either a γ (γ1–3) or a δ subunit (Olsen and Sieghart, 2009). The most common GABAARs providing fast synaptic inhibition in the mature mammalian cerebral cortex contain α1β2γ2 subunits (Fig. 1A,B) (Sieghart, 2006). Despite the great diversity of GABAAR subunits (α1–6, βl–3, γ1–3, δ, ε, θ, π, ρl–3) and possible configurations, these receptors produce two types of currents: synaptic (phasic) and tonic. Presynaptic terminal release of GABA onto post-synaptically clustered GABAARs triggers fast, transient synaptic currents, while ambient “spill over” GABA generates a persistent tonic current via activation of high affinity, low conductance extrasynaptic receptors. GABAAR subunit composition therefore determines receptor cell surface localization, electrophysiological properties and drug sensitivities.
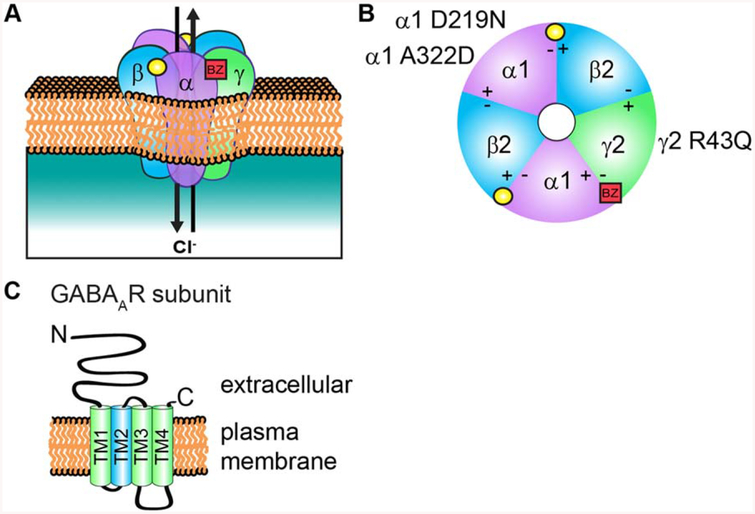
Figure 1.
GABAaR structure and subunit topology. (A) A representation of the heteropentameric GABAAR composed of αβγ subunits. Binding of the neurotransmitter GABA (yellow circle) at the αβ interface triggers ion channel opening and allows the rapid influx of Cl− and membrane hyper polarization in the mature nervous system. (B) Extracellular representation of the receptor showing all five subunits contributing to the central ion pore and the general binding sites of GABA (yellow circle) and BZs (red square). BZs bind at the interface of an α1/2/3/5 and y subunit. Also shown are specific mutations in a and γ2 subunits discussed in this review that contribute to epilepsy disorders by disrupting receptor assembly and trafficking. (C) All subunits have a common topology includ ing an extracellular N-terminal domain, short C-terminal tail, and four transmembrane domains (TM1–4). GABAAR subunit TM2 (blue) contributes to formation of the receptor ion channel pore, while the intracellular loop between TM3 and TM4 contain sites of phosphorylation and protein interactions that modulate channel function and/or trafficking. [Color figure can be viewed at wileyonlinelibrary.com]
Receptors containing α1–α3 subunits are primarily located at GABAergic synapses via direct association with gephyrin (Tretter et al., 2008, 20ll; Mukherjee et al., 2011), the key GABAergic and glycinergic postsynaptic scaffolding protein (Essrich et al., l998; Schweizer et al., 2003; Triller and Choquet, 2005; Jacob et al., 2008; Luscher et al., 2011a). The γ2 subunit is required for maintenance of most synaptic receptors and gephyrin (Essrich et al., l998; Schweizer et al., 2003). In addition, recent identification of the GABAAR regulatory Lhfpl (GARLH) family as putative GABAAR auxiliary subunits indicates the γ2 subunit is central in formation of a native tripartite complex between receptors, GARLH protein LH4 and the postsynaptic cell adhesion protein neuroligin 2 (NL2) (Yamasaki et al. 2017). Although predominantly clustered at synapses, γ2-containing receptors are also found at extrasynaptic sites and contribute to tonic current that is sensitive to positive allosteric modulation by the benzodiazepine drug class (Preno-sil et al., 2006). Receptors containing δ, a4 or a6 subunits do not interact with gephyrin, leading to their extrasynaptic localization and contribution to tonic benzodiazepine-insensitive GABAAR currents (Glykys and Mody, 2007a; Lee and Maguire, 2014). The a5 subunit can contribute to both modes of GABAAR signaling as it can directly interact with gephyrin synaptically (Brady and Jacob, 2015) and extrasynaptically with the actin binding protein radixin, a member of the ezrin/radixin/moesin (ERM) family (Loebrich et al., 2006). Although synaptic and extrasynaptic receptors are often described as distinct entities, single molecule tracking, electrophysiological, biochemical, and other imaging-based methods have revealed that receptors in both populations diffuse continually in the plasma membrane, with enhanced accumulation of synaptic receptors depending primarily on interactions between gephyrin and GABAARs (Fig. 2).
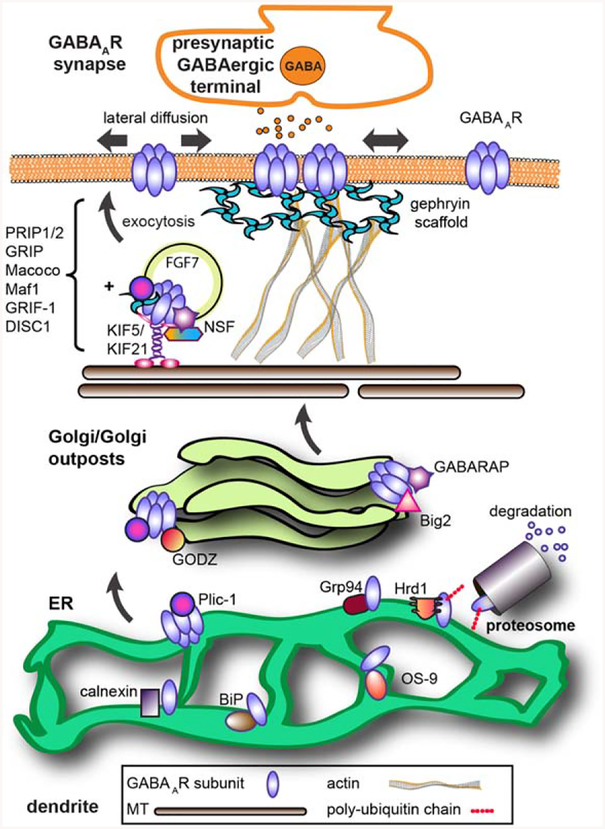
Figure 2.
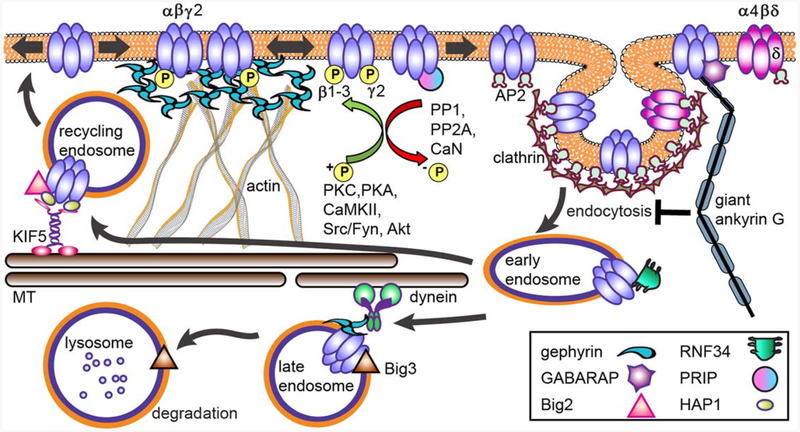
Forward trafficking of GABAARs. The process of GABAAR synthesis, assembly and forward trafficking is highly of misfolding regulated. Subunit assembly into pentameric recep tors in the ER involves ER chaperones (BiP, calnexin). Identification (Grp94, OS-9) leads to E3 ligase (Hrd1) ubiquitination of receptors and their destruc tion in the proteasome. Plic-1 inhibits proteosomal degrada tion of receptors, facilitating receptor ER exit to the Golgi. In the Golgi, palmitoylation of y subunits by the palmitoyl transferase GODZ is a key step in promoting forward traf ficking to the synapse. The GTP exchange factor Big2 increases receptor exit from the Golgi. A large number of GABAAR-associated proteins contribute to surface delivery of vesicles containing receptors from the Golgi. The kinesin KIF5 is the main microtubule-dependent motor transporting inhibitory synapse components (in addition to receptors this includes gephyrin, GABARAP, and FGF7) although recent work shows KIF21 contributes to extrasynaptic receptor delivery. Once inserted into the plasma membrane at extrasynaptic sites, receptors laterally diffuse until inter actions with the gephyrin scaffold enhance receptor synap tic accumulation. [Color figure can be viewed at wileyonlinelibrary.com]
In addition to their inhibitory function in the mature CNS, GABAARs are of fundamental importance in organization of newly forming circuits by promoting dendritic outgrowth and synaptogenesis. Synapses develop first with the emergence of excitatory GABAergic synaptic signals that drive the subsequent establishment of glutamatergic synapses, before GABA neurotransmission shifts to functioning as an inhibitory signal (Ben-Ari et al., 2007; Deng et al., 2007; Cellot and Cherubini, 2013). The polarity of GABAAR neurotransmission (inhibitory or excitatory) depends on the intracellular chloride concentration ([Cl−]i) set by the activity of two cationchloride transporters: Cl− importer Na-K-Cl− cotransporter (NKCC1) and neuronal Cl− extruder K +-Cl− cotransporter (KCC2). NKCC1 function predominates in early brain development generating a Cl− reversal potential more positive than the membrane potential (Vm), leading to Cl− efflux and depolarization when the GABAAR opens. As development proceeds, KCC2 expression increases, resulting in lower Cl− levels inside neurons and inhibitory GABAAR signaling via Cl− influx (Farrant and Kaila, 2007; Galanopoulou, 2008). Therefore, the functional outcome of altering GABAergic activity is highly dependent on chloride homeostasis. In addition, EGABA is similarly regulated in neural differentiation of adult born neurons in the hippocampus and olfactory bulb (reviewed in Lledo et al., 2006; Markwardt and Overstreet-Wadiche, 2008). Furthermore, pathological injury and disease states including seizures, ischemic stroke and traumatic brain injury can modulate EGABA (reviewed in Loscher et al., 2013).
In simplified terms, the strength of phasic and tonic GABAAR neurotransmission is determined by surface receptor levels, although receptor clustering at synapses by key scaffolding protein interactions is essential for phasic GABAAR signaling (Jacob et al., 2008; Luscher et al., 2011a). Here we discuss cellular mechanisms modulating GABAAR intracellular trafficking, surface levels, and localization during constitutive and abnormal conditions, thus providing key information about the dynamic calibration of GABAAR neurotransmission.
GABAAR ASSEMBLYAND FORWARD TRAFFICKING
GABAAR biogenesis is controlled via regulated assembly of subunits into heteropentamers within the endoplasmic reticulum (ER). Due to the diversity of receptors generated in recombinant systems during multiple independent subunit transfections, studies on assembly of concatenated subunit constructs have been a major route for determining receptor configuration. As viewed from outside the cell, the α1β2γ2 GABAAR subtype indicates a counterclockwise subunit configuration of γ-β-α-β-α (Fig. 1B) (Minier and Sigel, 2004; Baur et al., 2006). GABAAR studies using heterologous cells show that a β subunit is required for receptor cell surface expression, while the αβ interface that forms the GABA-binding site is required for functional responses. Although unlikely to occur in vivo, βl or ß3 homomeric channels form, while other individual subunit expression leads to ER retention (Krishek et al., 1996; Wooltorton et al., 1997). Co-expression of γ2 subunits with αβ leads to preferential assembly of αβγ2 receptors (Draguhn et al., 1990; Angelotti and Macdonald, 1993). Receptor complex formation begins with αβ heterodimer formation, with the N-terminal domains controlling this process. Several high-resolution structural studies of pentameric ligand-gated ion channels, including the recently solved human β3 GABAAR homopentamer informs our understanding of the receptor (Miller and Aricescu, 2014). Each individual subunit has a large N-terminal extracellular domain (ECD) followed by four transmembrane domains (TM1-TM4) and a short extracellular C terminus (Fig. 1C). The TM2 of each subunit contributes to the formation of the receptor ion channel pore, where the large intracellular loop between TM3 and TM4 is important for protein interactions and phosphorylation-dependent regulation. The ECD (ER luminal during receptor assembly) is comprised of an N-terminal α-helix followed by a core β sandwich of 10 β strands, with the GABA-binding site near the ECD midpoint. Studies of a α1β2γ recombinant receptors demonstrate that the N-terminal putative α helical region of the α1 subunit is critical for surface receptor expression, while deletion of the other subunits N-terminal extensions had minimal effects on surface expression (Wong et al., 2015). Rather, deletion of the β2 α-helix decreased GABA sensitivity and receptor desensitization, while γ2 N-terminal deletions reduced incorporation of γ2 in receptors. ER chaperones including calnexin and binding immunoglobulin protein (BiP/Grp78) contribute to receptor assembly (Fig. 2) (Connolly et al., 1996; Bradley et al., 2008), facilitating maturation of the α1 subunit (Di et al., 2013) in a glycan-dependent and independent manner. Recent findings suggest GABA can function as an intracellular chaperone for both recombinant (Eshaq et al., 2010) and endogenous receptors, as enhanced intracellular GABA levels promotes receptor forward trafficking and exocytosis in neurons (Wang et al., 2015). In support of this finding, immunogold labeling of rodent brain slices also identified GABA in the rough ER.
ERAD/Ubiquitination/Seizure Disorders
The exit of GABAARs from the ER is negatively regulated by constitutive ER-associated degradation (ERAD) (Gallagher et al., 2007; Saliba et al., 2007; Bradley et al., 2008). ERAD recognition of misfolded proteins leads to their dislocation from the ER membrane, ubiquitination via E3 ligases, and proteosomal degradation in the cytoplasm (Fig. 2). Chronic neuronal blockade via 24 h tetrodotoxin (TTX) treatment increases β3 subunit GABAAR ubiquitination and ERAD, leading to reduced receptor cell surface expression and decreased inhibition (Saliba et al., 2007). Conversely, enhanced neuronal activity diminished β3-subunit ubiquitination and improved receptor stability. Reduced calcium entry via voltagegated calcium channels (VGCC) also contributes to ubiquitination and degradation of receptor subunits (Saliba et al., 2009), while enhanced VGCC activity promotes β3 S383 phosphorylation and receptor insertion (Saliba et al., 2012), suggesting a mechanistic link to activity-dependent changes.
GABAAR subunit mutations that result in enhanced ERAD contribute to genetically determined epilepsies, or idiopathic generalized epilepsies (IGEs) (Fig. 1C). The α1 A322D GABAAR subunit mutation causes autosomal dominant juvenile myoclonic epilepsy (Cossette et al., 2002) while the γ2 R43Q mutation is linked to childhood absence epilepsy. Further studies of familial epilepsy have identified other γ2 mutants that impair receptor assembly and trafficking (Huang et al., 2014). ER luminal proteins glucose-regulated protein 94 (Grp94) and osteosarcoma amplified 9 (OS-9) recognize and convey misfolded α1 subunits to the E3 ligase Hrd1 (SYVN1 gene) for ubiquitination and ERAD in HEK 293 cells (Fig. 2) (Di et al., 2016). Repression of Grp94 promotes functional surface expression of the misfolded α1 A322D mutant subunit, while enhancing proteosomal degradation machinery reduces WT α1 subunit levels, reinforcing the emerging theme that ERAD is a key regulator of GABAAR cell surface levels. Changes in GABAAR ERAD may contribute to other neuronal disorders, as increased levels of Hrd1 and decreased α1 subunit protein levels were observed in middle frontal cortex of autism spectrum disorder (ASD) subjects (Crider et al., 2014). Similarly, the α1 D219N subunit mutant contributing to IGEs is ER retained (Lachance-Touchette et al., 2011). Treatment with the FDA approved drug and L-type Calcium Channel Blocker verapramil enhances recombinant α1D219Nβ2γ2 subunit assembly and ER chaperone calnexin assisted forward trafficking (Han et al., 2015), suggesting proteostasis regulation as a potential therapeutic in treatment of IGEs. An important negative regulator of GABAAR ERAD is Plic-1 (also known as ubiquilin 1, UBQLN1), a protein with a ubiquitin-like (UBL) proteosomal binding domain and ubiquitin-associated domains. Plic-1 increases stability of GABAAR in the ER by inhibiting α and β subunit ubiquitination and proteosomal degradation, thereby increasing receptor surface expression (Fig. 2) (Bedford et al., 2001; Saliba et al., 2008).
Forward Trafficking
Once assembled, GABAARs undergo transport from the ER to the Golgi apparatus, followed by translocation to the plasma membrane. In the Golgi, the γ2 subunit is subject to palmitoylation by the Golgispecific DHHC zinc finger enzyme (GODZ), a process important for synaptic GABAAR maintenance and surface expression (Fig. 2) (Keller et al., 2004; Fang et al., 2006). The critical role of this enzyme in palmitoylating the γ2 subunit was validated in GODZ KO mice where loss of this protein resulted in reduced receptor clustering, innervation, and inhibitory strength (Kilpatrick et al., 2016). During Golgi forward transport, GABAARs are segregated into distinct vesicles from excitatory glutamatergic AMPA receptors (AMPARs), and are subsequently inserted at the cell surface via specialized RabGTPases and SNARE complexes SNAP23–syntaxin1A/B–VAMP2 and SNAP25–syntaxin1A/B–VAMP2, respectively (Gu et al., 2016). Proteins contributing to GABAAR trafficking from the Golgi to the plasma membrane include Big2 (brefeldin A-inhibited GDP/GTP exchange factor 2) (Charych et al., 2004), GABARAP (GABA receptor-associated protein) (Wang et al., 1999), GRIP (Glutamate receptor interacting protein) (Charych et al., 2004; Kittler et al., 2004), PRIP1/2 (phospholipase C-related catalyti-cally inactive proteins 1 and 2) (Kanematsu et al., 2002; Uji et al., 2002), GABAARs-interacting factor (GRIF-1) (Beck et al., 2002), Macoco (Smith et al., 2010), and N-ethylmaleimide-sensitive factor (NSF) (Fig. 2) (Goto et al., 2005). As an Arf guanine exchange factor (GEF), Big2 promotes membrane budding and vesicular trafficking from the Golgi, and can interact with the intracellular loop of all β subunits (Charych et al., 2004). Accordingly, coexpression of Big2 and the β3 subunit increased receptor surface levels in heterologous cells. Big2 has also been identified in recycling endosome membranes (Ishizaki et al., 2008; Boal and Stephens, 2010) and more recent work indicates it contributes to neuronal migration (Zhang et al., 2012, 2013), expanding the neuronal role of this protein. One of the first discovered GABAAR interacting proteins (Wang et al., 1999), GABARAP is a family member of the UBLs proteins which contain ubiquitin related sequences and control a variety of cellular processes (reviewed in van der Veen and Ploegh, 2012). The UBL GABARAP/GATE-16 subfamily contributes to autophagy and includes GABARAP, GABARAPL1, GATE-16 (Golgi-associated ATPase enhancer of 16 kDa) and GABARAPL3 in mammals (Shaid et al., 2013). Extensively studied (reviewed in Luscher et al., 2011a), GABARAP is concentrated in the Golgi and somatodendritic membrane, interacts with γ subunits and microtubules (Wang et al., 1999), and overexpression increases GABAAR surface levels (Leil et al., 2004). More recent work revealed GABARAP was essential for NMDA-induced increases in GABAAR synapses occurring via enhanced exocytosis (Marsden et al., 2007). However, GABARAP is not critical in regulation of surface receptor levels, as GABARAP knockout mice show a normal distribution of γ2-GABAARs and gephyrin, suggesting functional redundancy (O’Sullivan et al., 2005). With respect to GABAAR trafficking and general neuronal autophagic processes, GABARAP/GATE-16 protein family functions remain underexplored.
Multiple GABARAP interacting proteins are implicated in regulation of receptor trafficking or localization including the PDZ domain-containing GRIP, which promotes NMDA dependent GABAAR synaptic plasticity (Marsden et al., 2007) and glutamatergic AMPAR synaptic stabilization (Dong et al., 1997). A recent study identified missense gain of function mutations in PDZ 4–6 of the GRIP1 gene in autistic patients, resulting in enhanced AMPAR recycling and increased GluA2 surface levels (Mejias et al., 2011). Although GABAAR dysfunction is heavily implicated in autistic disorders, and GRIP1 binds to gephyrin via PDZ 4–6, GABAAR synaptic plasticity was not investigated in this study. Both PRIP1/2 and the NSF ATPase interact with GABAARs both indirectly via GABARAP and directly with GABAAR β subunits (Kanematsu et al., 2002; Terunuma et al., 2004; Goto et al., 2005; Mizo-kami et al., 2007). Interestingly, PRIP and γ2 bind to the same site of GABARAP, and PRIP1/2 knockout mice show a reduction in GABAAR expression and impaired γ2 subunit specific pharmacology including reduced benzodiazepine sensitivity and Zn2+ modulation (Kanematsu et al., 2002). NSF is a core component of SNARE-mediated fusion machinery and participates in regulating receptor cell surface trafficking (Chou et al., 2010). NSF also interacts with AMPAR, playing an essential role in receptor insertion, synaptic stabilization and endosomal recycling (reviewed in Anggono and Huganir, 2012), suggesting overlapping regulation of excitatory and inhibitory receptor trafficking.
Vesicular GABAAR transport from the trans-Golgi network to the plasma membrane relies on the microtubule-dependent molecular motor kinesin KIF5 family (KIF5A, KIF5B, KIF5C) (Fig. 2) (Twel-vetrees et al., 2010). KIF5A binds to GABARAP in vivo (KIF5B and KIF5C do not bind GABARAP), and contributes to GABAAR trafficking to the neuronal surface (Nakajima et al., 2012). Conditional knockout of KIF5A in mice results in deficits of GABAAR plasma membrane levels, seizures, and 75% lethality within 3 weeks (Nakajima et al., 2012). In addition to forward trafficking, KIF5 contributes to post-endocytic receptor transport via HAP1 (hun-tingtin-associated protein 1) (to be described further in the recycling section). FGF7 (fibroblast growth factor 7) is an inhibitory postsynaptic cell derived organizer of GABAergic presynaptic terminals (Ter-auchi et al., 2010) that requires gephyrin and KIF5-dependent transit for synapse targeting (Fig. 2). An additional kinesin family protein, KIF21B, was recently demonstrated to co-precipitate with the GABAAR γ2-subunit and colocalize at GABAergic synapses in neurons (Labonte et al., 2014). Knockdown of KIF21B by shRNA methods decreased receptor surface levels and the intensity of extrasynaptic γ2 clusters, but did not affect synaptic GABAARs concentration opposed to presynaptic terminals labeled with synaptic vesicle glycoprotein 2A.
Scaffolding proteins such as gephyrin are often used as adaptor proteins for transporting receptors to synapses (Kneussel, 2005). FGF7 and gephyrin traffic together via KIF5, strongly suggesting that many inhibitory synapse components including GABAAR, gephyrin, GABARAP and FGF7 may travel together in a complex. Another KIF5 interactor, GRIF-1/traf-ficking kinesin binding protein 2 (TRAK2) (Brickley et al., 2005), also associates with the GABAAR β2 subunit (Beck et al., 2002), although identification of direct interaction with receptors in the brain is lacking and the role of TRAK family kinesin adaptors in GABAAR trafficking remains unclear (reviewed in Stephenson, 2014). Finally, Maf1 and the Maf1interacting protein Macoco were shown to interact with β3 subunits via yeast two-hybrid assays, with Macoco overexpression increasing receptor surface expression (Fig. 2) (Smith et al., 2010). Although Maf1 was originally identified in yeast as a repressor of transcription (Pluta et al., 2001; Upadhya et al., 2002), further insight into Maf1 neuronal function has not occurred.
A recent interest has emerged in understanding the role of disrupted in schizophrenia (DISC1) protein in GABAAR trafficking, as genomic screenings have linked mutations of DISC1 to psychiatric illnesses (Devine et al., 2016). Overexpression of wild-type DISC1 leads to enhanced GABAAR surface expression and miniature inhibitory postsynaptic currents (mIPSCs) in a KIF5-dependent manner, while knockdown of this protein has the opposite effect (Fig. 2) (Wei et al., 2015). Furthermore, Saito et al. (2016) found postnatal knockdown of DISC1 in mouse prefrontal cortex disturbs dendritic development, reduces α2-containing GABAAR synaptic clustering, decreases mIPSC amplitude, and causes sensorimotor defects (Saito et al., 2016). This work also provided evidence that DISC1 specifically regulates α2-containing GABAARs in immature neurons, and pharmacological agents enhancing α2/α3-containing GABAAR channel function could reverse the behavioral defects seen in DISC1 mutant mice. Studies using DISC1 total knockout mice demonstrate a loss of GABAergic interneurons within the prefrontal cortex and loss of total cell density in the deep layers of the cortex (Umeda et al., 2016), as well as a reduction in neuropeptide-Y expressing neurons and their respective fiber length (Morosawa et al., 2017). Interestingly, GABA-induced NKCC1-dependent depolarization is critical for regulation of dendritic development during adult hippocampal neurogenesis via AKT-mTOR signaling (Kim et al., 2012). Moreover, human patients with schizophrenia exhibited a significant interaction between single nucleotide polymorphisms in DISC1 and the chloride-regulator NKCC1 (SLC12A2). These findings were later confirmed using functional MRI studies in two human subjects who were homozygous for both these risk alleles, identifying that hippocampal connectivity and responsiveness were diminished in a recognition memory task (Callicott et al., 2013). The evolving story of DISC1’s role in GABAAR trafficking and function in development and cognitive impairment represents an exciting area of research into the basis of certain psychiatric illnesses.
THE GABAERGIC SYNAPSE
Gephyrin
The confinement of GABAARs at synaptic sites is a key step in tuning the strength of phasic inhibition. The post-synaptic inhibitory scaffolding protein gephyrin is the main organizer of GABAAR synaptic localization and density (Jacob et al., 2005), as gephyrin knock out mice exhibit a robust loss of GABAAR clustering (Kneussel et al., 1999), although gephyrin-independent synaptic clustering does occur (Kneussel et al., 2001; Levi et al., 2004). Gephyrin is a highly conserved 93 kDa protein that is hypothesized to form multimeric complexes which associate with a number of cytoskeletal proteins (Giesemann et al., 2003), contributing to its scaffolding function (Tretter et al., 2012). The architecture of gephyrin scaffolding arises from the N terminal or G domain of gephyrin participating in dimer-dimer self-associations, while the C terminal or E domain forms trimer interactions, likely to create a hexagonal lattice (Fig. 3) (Kneussel and Betz, 2000; Saiyed et al., 2007). This structure tethers freely-diffusing receptors at synaptic sites through binding GABAAR a1, α2, α3, α5, β2, and β3 subunits (Tretter et al., 2008; Wu et al., 2012; Kowalczyk et al., 2013; Brady and Jacob, 2015). The GABAAR γ2 subunit also plays an important role in gephyrin-receptor attachment, as γ2-knockout mice demonstrate diminished clustering of gephyrin and GABAARs (Gunther et al., 1995; Essrich et al., 1998). Importantly, synaptic GABAAR clustering can occur independent of y2 in some cases (Kerti-Szigeti et al., 2014). Functionally, gephyrin acts to confine receptors undergoing diffusion at the cell surface membrane and limit their escape into the extrasynaptic space, a process that is influenced by neuronal activity (see section on activity-dependent plasticity of GABAAR synapses) and GABAAR specific drugs including the benzodiazepine, diazepam (Gouzer et al., 2014; Levi et al., 2015). Gephyrin’s scaffolding ability is influenced by extensive posttranslational modifications (Fig. 5). Mass spectrome try studies alone have revealed 22 sites of phosphory lation in gephyrin’s C domain and 1 additional threonine 324 site in the E-domain (Herweg and Schwarz, 2012; Kuhse et al., 2012; Tyagarajan et al., 2013). Yet, the exact role of gephyrin phosphoryla tion is complex and controversial, highlighted by the bidirectional effect of altering gephyrin phosphoryla tion at the serine (S) 270 site. Initial findings by Tyagarajan et al. (2011) identified phosphorylation of this site by Glycogen Synthase Kinase 3β to nega tively modulate gephyrin clustering, possibly due to enhanced Ca2+-dependent protease calpain−1 mediated degradation. Accordingly, overexpression of a phosphodeficient S270A gephyrin mutant enhanced both the amplitude and frequency of mIPSCs, sugges ting increased functional GABAAR clustering. It was later revealed that the S270 site was also a substrate for the proline-directed serine/threonine kinase, cyclin-dependent kinase 5 (CDK5) (Kuhse et al., 2012; Kalbouneh et al., 2014). Gephyrin synaptic clusters were found to be basally phosphorylated at Ser270 in a CDK5-dependent manner (Kuhse et al., 2012), with CDK5 knockdown or inhibition leading to loss of phosphorylated gephyrin clusters and postsynaptic γ2-containing GABAARs (Kalbouneh et al., 2014). To further complicate these findings, S270 is cross-regulated by phosphorylation of a neighboring S268 residue targeted by extracellular signalregulated kinase 1/2 (Tyagarajan et al., 2013) (role for ERK also described in Wuchter et al., 2012). This study suggested these serine residues control distinct gephyrin clustering dynamics including postsynaptic cluster size and number, again in conjunction with calpain activity. Expanding the role of gephyrin serine site regulation, phosphomutant studies and in vitro kinase assays indicate that increased phosphory lation of gephyrin on S305 (phosphopeptide mass spectrometry identification Tyagarajan et al., 2013) by the kinase CAMKII is required for activitydependent inhibitory plasticity (Flores et al., 2015). Considering the number of additional gephyrin phos phorylation sites identified in vivo and the challenges of gephyrin point mutant studies (overexpression concerns), continued multidisciplinary efforts are necessary to resolve the functional relevance of these modifications.
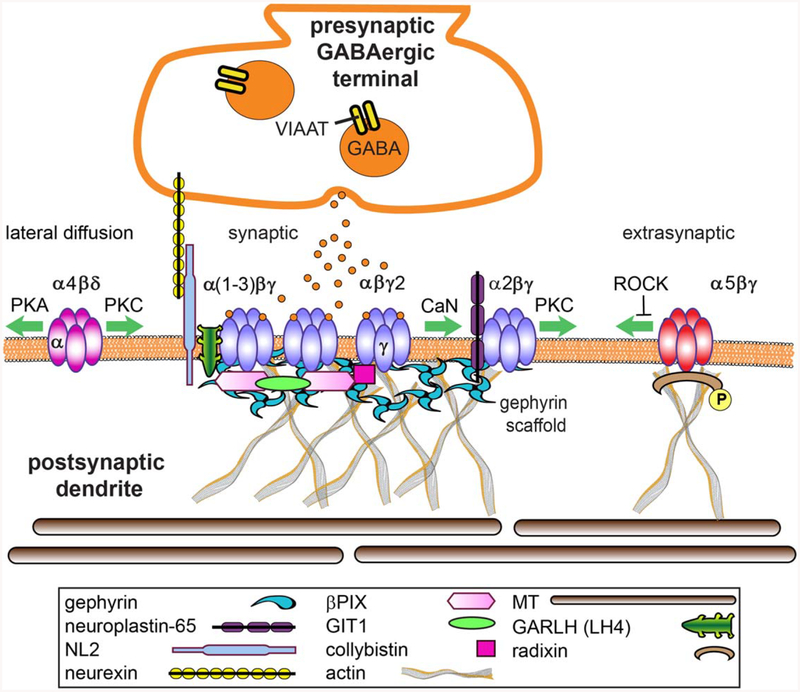
Figure 3.
GABAAR synapse structure. GABAARs composed of α(1–3)βγ subunits are largely syn-aptically localized via gephyrin interactions and contribute to phasic currents, whereas α(4 or 6)βd receptors are extrasynaptic and generate tonic current. α5βγ receptors are found in both locations due to binding with gephyrin at synapses and radixin extrasynaptically. Proteomics and other mod ern strategies have significantly enriched the complexity of the inhibitory synapse, however the functions of many new components have yet to be defined. Key synaptic adhesion, scaffold and sig naling proteins that mediate lateral diffusion of receptors in and out of the synapse are depicted and discussed in this review. [Color figure can be viewed at wileyonlinelibrary.com]
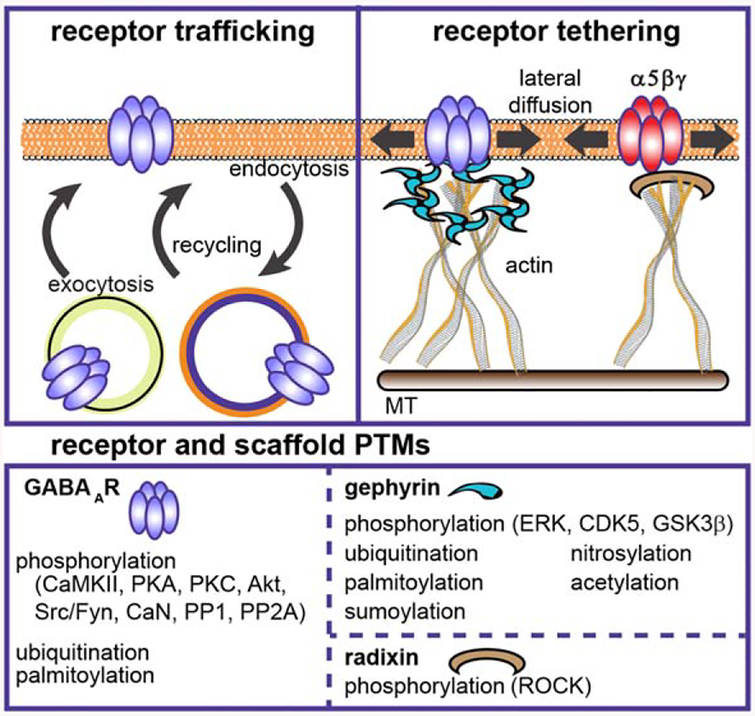
Figure 5.
GABAergic postsynaptic plasticity. Many forms of GABAAR plasticity rely on modulation of receptor traf ficking or tethering via scaffold interactions, including activity-dependent iLTP and iLTD. In addition to newly identified phosphorylation sites and signaling pathways, diverse post-translational modifications are surfacing as regulators of GABAAR trafficking processes, both at the level of receptors and scaffolding. [Color figure can be viewed at wileyonlinelibrary.com]
Other post-translational modifications including SUMOylation, acetylation, and palmitoylation are important regulators of gephyrin function. Gephyrin G- and E-domain lysine (K) residues can undergo SUMOylation and acetylation in vitro and in vivo (Ghosh et al., 2016). Ghosh et al. (2016) found conju gation of the small UBL modifier (SUMO)1 or SUMO2 to the K148 or K724 site, respectively. Overexpression of gephyrin with lysine-to-arginine mutations at these sites reduces gephyrin scaffolding and postsynaptic GABAAR strength as evidenced by reduced mIPSC in cultured neurons. Accordingly, gephyrin SUMOylation and clustering is regulated by the E3 SUMO-ligase protein inhibitor of activated STAT3 (PIAS3). PIAS3 modulation of gephyrin organization is negatively influenced by acetylation of gephyrin at a K666 site. Collectively, this work provides evidence of functional crosstalk between gephyrin phosphorylation, acetylation, and SUMOy-lation to dictate formation of postsynaptic clustering and to conversely dynamically alter gephyrin cluster size and density. Palmitoylation of gephyrin cysteine residues by the palmitoyl acyltransferse DHHC-12 also appears essential for gephyrin synaptic cluster ing, an enzymatic-process fine-tuned by changes in GABAergic activity (Dejanovic et al., 2014). Gephyrin further interacts with neuronal nitric oxide synthase and is subject to S-nitrosylation, a modifica tion that reduces gephyrin cluster size, while pharma cological inhibition of nitric oxide reverses this process and increases the number of cell surface syn aptic GABAARs (Dejanovic and Schwarz, 2014). How these collective post-translational modifications act in concert to regulate gephyrin scaffolding and clustering properties remains poorly understood.
A major contributor to gephyrin accumulation and stability at GABAergic synapses is the Dbl-like GEF, collybistin (Fig. 3). Collybistin is a RhoGEF expressed in three major isoforms (1–3) that functions as an important intermediary for gephyrin traf ficking to synapses (Harvey et al., 2004). Collybistin’s scaffolding role results from its direct interaction with the gephyrin E domain, the plasma membrane via a pleckstrin homology domain, and a Src-homology-domain—3 (SH3) in specific isoforms. The SH3 domain enzymatically activates the small GTPase CDC42, a known regulator of actin dynam ics. CDC42 directly interacts with the presynaptic scaffolding protein NL2 to facilitate gephyrin synap tic accumulation (Poulopoulos et al., 2009; Soykan et al., 2014) (see trans-synaptic components of GABAergic synapses section), a process negatively regulated by phosphorylation of NL2 by the prolinedirected kinase Pin1 (Antonelli et al., 2014). The SH3-domain further forms a direct contact with the GABAAR α2 subunit to strengthen gephyrin binding interactions, while the α3 subunit binds gephyrin independent of collybistin (Saiepour et al., 2010; Tretter et al., 2011), suggesting a cell or synapse specific role of collybistin in regulating gephyrin cluster ing and GABAAR scaffolding function. In support of this argument, collybistin knock-out mice demon strate selective loss of gephyrin and GABAAR clus tering in braiand compartment-specific regions, culminating in reduced GABAergic neurotransmis sion, anxiety, and impaired spatial learning (Papadopoulos et al., 2007). Alterations in cellular levels of phosphatidylinositol 3-phosphate have also been demonstrated to critically regulate gephyrin synaptic accumulation and GABAergic neurotransmission, likely via collybistin (Papadopoulos et al., 2017).
Less investigated means of gephyrin regulation have also emerged. The axon growth regulating pro tein growth-associated protein 43 (GAP43) was recently found to play an important role in gephyrin aggregation state and GABAAR clustering in devel oping neurons (Wang et al., 2015). GAP43-association with gephyrin is enhanced during conditions of reduced PKC and neuronal activity in devel oping cortical neurons, resulting in gephyrin misfolding. RNA level regulation of gephyrin scaf folding function by the non-octamer-containing, POU-domain DNA-binding protein (NONO), is implicated in certain patients with intellectual dis ability (Mircsof et al., 2015). NONO participates in neuronal RNA transport complexes (Kanai et al., 2004), its concentration at synapses is activity-dependent (Zhang et al., 2012), and one-third of its target transcripts are synaptic proteins (Mircsof et al., 2015). NONO-deficient mice demonstrate gross aber rations in dendrite morphology, gephyrin scaffolding, reductions in GABAAR α2-subunit levels, and pheno typic and behavioral changes suggesting syndromic intellectual disability (Mircsof et al., 2015).
Diverse GABAAR subtype- and region-specific synapse constituents highlight the complexity of GABAergic neurotransmission. For example, the cell adhesion molecule (CAM) neuroplastin-65 (Fig. 3), which is prominently expressed during synaptogenesis (Watson et al., 2006), is localized at α1 and α2-subunit containing GABAAR synapses, but not α3 (Sarto-Jackson et al., 2012) (much like collybistin). shRNA knockdown of neuroplastin-65 reduces α2 synaptic levels in vitro, while in vivo knockout models demonstrate an enhanced α1/α2 subunit ratio at GABAergic synapses without a change in gephyrin levels opposed to presynaptic terminals positive for the vesicular inhibitory amino acid transporter (VIAAT), suggesting neuroplastin-65 has a selective regulatory role in α2 GABAAR accumulation (Sarto-Jackson et al., 2012; Herrera-Molina et al., 2014). The CAM neurofascin facilitates developmental clus tering of gephyrin on the axon hillock and is critical for axo-axonic GABAergic synapse clustering (Bur-karth et al., 2007; Kriebel et al., 2011). Accordingly, knockdown of neurofascin in the basolateral amyg dala of mice results in reduced GABAergic input at the axon initial segment (AIS), impaired synaptic plasticity and excitability, and hindered fear extinc tion in behavioral testing (Saha et al., 2017). It is important to note that most GABAAR clusters along the AIS contain a α2 subunit (Nusser et al., 1996; Brunig et al., 2002; Muir and Kittler, 2014; Gao and Heldt, 2016). Interestingly, in vivo viral expression of a chimera construct containing the TM3-TM4 region of a1 and the α2 intracellular gephyrin binding loop selectively disrupts α2-GABAAR clustering periso-matically, but not at the AIS in the frontal cortex of mice (Hines et al., 2013). Expression of this domi nant negative chimera, by interfering with endoge nous α2 and gephyrin interaction, led to the replacement of somatic clusters of α2 with α1, reduced γ-power, and working memory deficits in mice. These findings illuminate an interesting ques tion about the differences in composition of inhibi tory synapses at the AIS versus the soma and why interference with α2-gephyrin binding has divergent effects. The unique nature of axo-axonic synaptic plasticity is highlighted by optogenetic excitation studies in hippocampal neurons demonstrating physi cal relocation of the AIS but not repositioning of axo axonic GABAergic synapses (Wefelmeyer et al., 2015).
Trans-Synaptic Components of GABAergic Synapses
One of the most well characterized trans-synaptic interactions crucial for GABAAR synapse develop ment is that of neuroligins and neurexins (Fig. 3) (Varoqueaux et al., 2006). Neurexins are found presynaptically and induce differentiation of GABAergic and glutamatergic post-synaptic densi ties during maturation and plasticity (Graf et al., 2004; Krueger et al., 2012), although overexpression of neurexins leads to reduced GABAergic neurotrans mission (Zhang et al., 2010). The calsyntenins are another adhesion family governing synapse forma tion, with calsyntenin-3 identified as an a-neurexin interactor (Pettem et al., 2013; Lu et al., 2014) that contributes to inhibitory synapse development and neurotransmission (Pettem et al., 2013; Um et al., 2014). Dystroglycan (DG), a core component of the dystrophin-glycoprotein complex, is an additional α- neurexin interactor that regulates GABAAR synaptic scaffolding and plasticity (Levi et al., 2002; Graf et al., 2004; Pribiag et al., 2014). Studies using conditional deletion of Dag1 (DG) in pyramidal cells show diminished innervation of CCK-positive interneurons while parvalbumin positive basket cell terminals are unaffected, indicating a cell-type and synapse-specific trans-synaptic function (Friih et al., 2016; Panzanelli et al., 2017). Postsynaptically, different neuroligins (NL1–4) are found at either glutamatergic or GABAergic synapses and play important scaffold ing and receptor recruitment roles. GABAergic syn apses primarily rely on NL2 for synapse integrity, and when expressed with recombinant GABAARs in HEK cells, NL2 supports formation of functional GABAergic synapses in neuron co-culture systems (Dong et al., 2007). Moreover, NL2 is critical for GABAergic synapse formation and coding in the ret ina (Hoon et al., 2009), while enhanced expression of this protein in cerebellar granule cells can accelerate GABAAR synapse development (Fu and Vicini, 2009; Brown et al., 2014) and strengthen inhibitory synaptic function in hippocampal neurons (Chubykin et al., 2007). Despite these observations, functional GABAAR synapses can also form in HEK-neuron co culture systems independent of NL2 (Fuchs et al., 2013), a process dependent on the N-terminal ECDs of GABAAR subunits (Brown et al., 2016). Intrigu ingly, NL1–3 triple knockout mice suggest loss of these proteins does not alter the total number of syn apses occurring in hippocampal, neocortical, or brainstem neurons, but results in a profound reduc tion in spontaneous IPSC and a minor decrease in glutamatergic activity in brainstem slices, ultimately resulting in an enhanced excitatory/inhibitory ratio and respiratory failure (Varoqueaux et al., 2006). The number of asymmetric and symmetric synapses is unchanged in the hippocampus of NL2 lacking mice as measured by electron microscopy, yet VIAAT pos itive puncta and IPSC amplitude are reduced and an anxiety phenotype is seen (Chubykin et al., 2007;Blundell et al., 2009). The anxiety phenotype in NL2 KO mice has been suggested to result from reduced perisomatic inhibitory synapses in the basal amyg dala and hyperactivity of projection neurons in this region (Babaev et al., 2016). Furthermore, selective knockout of NL2 in the medial prefrontal cortex of mice has been shown to reduce synaptic inhibition after 6–7 weeks and produce cognitive impairment phenotypes (Liang et al., 2015).
The recent discovery of the NL2 interacting GARLH family proteins proposed to function as GABAAR auxiliary subunits, more specifically lipoma HMGIC fusion partner-like 3 and 4 (LH3 and LH4), further advances our knowledge of GABAAR clustering and regulation (Fig. 3) (Yamasaki et al., 2017). Auxiliary subunits previously described for other ligand-gated ion channels, including AMPA and kainate glutamate receptors, influence channel characteristics or receptor localization (Jackson and Nicoll, 2011; Yan and Tomita, 2012). Yamasaki et al. (2017) demonstrated native GABAAR com plexes contain LH4 in the cerebellum and hippocam pus, with this protein likely acting as a bridge between synaptic GABAAR γ2-subunits and NL2 to provide synaptic anchoring properties. Accordingly, knockdown of LH4 in vitro and in vivo results in a dramatic reduction of γ2-synaptic clustering and inhibitory neurotransmission (Yamasaki et al., 2017). The evolutionarily conserved extracellular synaptic organizing protein, MADD-4/Ce-punctin, also is a recently discovered neuroligin binding partner and a critical mediator of GABAergic synapse formation in Caenorhabditis elegans (C. elegans) (Maro et al., 2015; Tu et al., 2015). The evolving knowledge of newly identified and additional unknown GABAergic trans-synaptic proteins may prove highly insightful to human health conditions and disorders.
GABAAR stability at synapses is also influenced by actin cytoskeletal interactions occurring between gephyrin and the microfilament system (Giesemann et al., 2003; Bausen et al., 2006). Recent work identified GABAAR direct regulation via an actinremodeling pathway originating at the signaling scaf fold protein G protein-coupled receptor kinaseinteracting protein 1 (GIT1) and the GEF βPix (Fig. 3) (Smith et al., 2014). GIT1 and βPix form native complexes with GABAAR subunits, and mediate downstream activity of the Rho family GTPase Rac1 and the effector p21-activating kinase to pro mote receptor synaptic stability. Our current overall understanding of the proteins involved in inhibitory synapse stability and clustering remains incomplete, as highlighted by two recent in vivo inhibitory synapse proteomic screenings using α2-pHluorin (pH-sensitive GFP) tagged subunit knock-in mice (Nakamura et al., 20l6) or viral expression of inhibi tory fusion proteins including gephyrin (Uezu et al., 20l6). These mass spectrometry methods revealed l40 (Uezu et al., 20l6) and l49 (Nakamura et al., 20l6) novel protein components of GABAAR/inhibitory synapses spanning multiple trafficking, stability, and regulatory pathways. Collectively, these studies further validated molecular interactions between GABAAR intracellular loops and the metabotropic glutamate receptor subunit mGluR5, the Dbl family GEF Ephexin, the metabotropic GABA B receptor (GABABR) auxiliary subunit KCTDl2, and initiated characterization of a novel inhibitory synaptic regula tor inhibitory synaptic protein l (InSynl). Future investigations will need to dissect the exact roles of these identified proteins in GABAAR regulation and function.
Extrasynaptic GABAARs
Although synaptic receptors participate in phasic inhibition, extrasynaptic GABAARs are responsible for setting the inhibitory tone of a neuron through the generation of a constant tonic current. Receptors composed of α4βδ or α6βδ subunits are found extrasynaptically and respond to low concentrations of ambient or “spillover” GABA (Fig. 3) (Saxena and Macdonald, l996; Haas and Macdonald, l999; Ke et al., 2000; Bianchi et al., 200l; Brown et al., 2002; Terpstra et al., 2002). Although hippocampal tonic currents are largely generated by synaptic spillover (Glykys and Mody, 2007b), other GABA sources include astrocytes (Liu et al., 2000; Kozlov et al., 2006), and neurogliaform cells (Olah et al., 2009). Furthermore, extrasynaptic GABAARs can be sponta neously open in the absence of GABA (Wlodarczyk et al., 20l3). GABAARs incorporating the α5-subunit with βγ2 also represent a large pool of extrasynaptic GABAergic signaling (Brunig et al., 2002; Crestani et al., 2002), although this receptor subtype can be found clustered both synaptically (Serwanski et al., 2006; Zarnowska et al., 2009; Brady and Jacob, 20l5) and extrasynaptically (Loebrich et al., 2006). Scaffolding of α5βγ2 GABAARs extrasynaptically occurs due to interaction with the ERM family pro tein radixin (Fig. 3) (Loebrich et al., 2006), whereas α5 was recently shown to interact with gephyrin at synaptic sites (Brady and Jacob, 20l5). Radixin acts in a phospho-dependent manner to scaffold α5βγ2 receptors to the actin cytoskeleton ultimately reducing diffusion rates and concentrating channel activity away from axon terminals (Hausrat et al., 20l5). Bidirectional control of radixin phosphorylation state by the RhoA GTP- and Rho-kinase (ROCK) dependent pathway is contingent on GABAergic versus glutamatergic activity (Fig. 3). Application of GABA favors radixin phosphorylation and retention of α5-GABAARs extrasynaptically, while AMPA treatment leads to dephosphorylation and increased percentage of α5-subunit receptors found synaptically (Hausrat et al., 20l5). Additional GABAAR subtypes are also subject to synaptic/extrasynaptic exchange following manipulations of the excitatory/inhibitory balance and/or kinase signaling. Gerrow and Triller (20l4) identified GABABR activity increases α2-GABAARs diffusion from synapses via PKC activity, allowing α5-GABAAR synaptic accumulation due to available synaptic binding slots (Gerrow and Triller, 20l4). PKC-activation also promotes synaptic accumulation of α4-containing GABAARs (Carlson et al., 20l6), while PKA-activation shifts α4-receptors extrasynap tically (Fig. 3) (Carlson et al., 20l6). These findings highlight the dynamic nature of GABAAR synaptic/extrasynaptic exchange and the diverse mechanisms to fine-tune GABAergic neurotransmission.
GABAAR INTERNALIZATION, RECYCLING, AND LYSOSOMAL DEGRADATION
GABAAR Internalization
Regulated internalization of cell surface receptors is a universal cellular response to moderate signaling and function. GABAAR internalization occurs through clathrin-mediated endocytosis dependent on dynamin (the GTPase that is responsible for fission of endocytic vesicles from the plasma membrane) and binding of the adaptor protein AP2 to specific GABAAR subunits (Fig. 4) (Kittler et al., 2000), although clathrin-independent endocytosis occurs in heterologous cells and in C. elegans (Cinar and Barnes, 200l; Rowland et al., 2006). The interaction of AP2 with GABAARs is partly regulated by PKA and PKC mediated-phosphorylation of serine residues within a highly basic ten amino acid sequence motif in the intracellular loop of the β subunits (S409 in β1, S4l0 in β2, S408/409 in β3), with increased phosphorylation reducing AP2 and GABAAR interaction and endocytosis (Fig. 4) (McDonald et al., l998; Brandon et al., 2002, 2003; Kittler et al., 2005; Smith et al., 2008). Two additional motifs in the β-subunit appear important for AP2 interactions: (l) a dileucine motif is critical for receptor internalization in HEK cells (Herring et al., 2005) and (2) three argi nine residues (405RRR407) within the β3-subunit intracellular domain are important for AP2-stabilization of receptors at dendritic endocytic zones (Smith et al., 2012). Accordingly, benzodiazepine resistance in epileptic mice undergoing sustained seizures is linked to reductions in PKC phosphoryla tion of β subunits and loss of surface receptors (including benzodiazepine-sensitive γ2 GABAARs) (Terunuma et al., 2008). Moreover, S408/409D phosphomimetic mutants were recently shown to block oxygen-glucose deprivation (OGD) induced receptor internalization and reduce cell death in cultured hip pocampal neurons (Mele et al., 2014). The impor tance of phosphoregulation of these residues was revealed by S408/409A homozygous mice (the S/A mutation reduces AP2 interaction, mimicking phos phorylation; Jacob et al., 2009), which exhibit increased phasic but decreased tonic inhibition, and demonstrate the core phenotypes of ASDs (Vien et al., 2015). Moreover, a common model of fragile X syndrome and ASDs, the Fmr1 KO mouse, demon strates enhanced S408/409 phosphorylation, further providing evidence for deregulation of these sites in disease (Vien et al., 2015). How specific PKC isoforms participate in phosphoregulation of GABAAR surface levels and internalization is still unclear. For instance, ethanol induced internalization of α1-containing GABAARs is PKCγ dependent (Kumar et al., 2010), while PKCε reduces GABAAR sensitivity to ethanol and BZs by acting at a γ2 S327 residue (Qi et al., 2007).
Figure 4.
GABAAR endocytosis, recycling and degradation. GABAARs primarily undergo clathrin-dependent endocytosis. β, γ, and δ subunits interact with the clathrin-adaptor protein 2 (AP2) complex. Phosphorylation of AP2-interaction motifs within receptor subunits increases cell-surface receptor levels and enhances GABAAR neurotransmission. Giant ankyrin G, in addition to its scaffold function, negatively regulates GABAAR endocytosis. After internalization, clathrin-coated vesicles fuse with early endosomes, allowing for subsequent receptor recycling or targeting for degradation in lysosomes. HAP1 interaction with β subunits promotes receptor recycling. Ubiq uitination of GABAAR contributes to lysosomal targeting, with the ubiquitin E3 ligase RNF34 directly interacting with the γ2 subunit. In contrast to the Arf GEF Big2 found on recycling endo-somes membranes, Big3 is colocalized with neuronal lysosomes and promotes receptor degrada tion. [Color figure can be viewed at wileyonlinelibrary.com]
The intracellular loop of the γ2 subunit also con tains two AP2 interactions domains, a 12 basic amino acid region similar to the β-subunits and a classical YGYECL motif (Smith et al., 2008). The Tyr 365/367 within the YGYECL motif are targets of Fyn and other Src family kinases (Fig. 4) (Brandon et al., 2001; Jurd et al., 2010), and phosphorylation at these sites reduce AP2 binding (Kittler et al., 2008). Tyro sine to phenylalanine mutations inhibits AP2 binding to the γ2 subunit, and heterozygous Y365/7F knock-in mice demonstrate surface and synaptic accumula tion of GABAARs and spatial memory deficits (Tret-ter et al., 2009). Importantly, homozygous Y365/7F knock-in mice are developmentally lethal, highlight ing the importance of these residues in regulating GABAAR activity. BDNF-induced phosphorylation of the γ2 subunit Y365/7 residues was recently impli cated as a promoter of receptor surface expression during hippocampal neurogenesis (Vithlani et al., 2013). The authors found heterozygous Y365/7F mice demonstrated an antidepressant-like phenotype and enhanced neurogenesis that could not be further enhanced by BDNF. Interestingly, Y365/7F mice also exhibit sex-specific alterations in both dentate gyrus granule cells (Kretschmannova et al., 2013) and thalamic relay neurons of the dorsal lateral genic ulate nucleus (Nani et al., 2013), where female mice have increased α4 and δ subunit levels and associated increased tonic inhibition, changes not seen in male mice. Alpha subunit composition may also play a role in regulating endocytosis, as heterozygous α1 subunit deletion mice, which develop absence epi lepsy, exhibit reduced clathrin-mediated endocytosis of α1-containing GABAARs and an increase in the relative fraction of residual a1 at the cell surface (Zhou et al., 2013). Neurons from these mice have reduced mIPSCs, altered current kinetics and respon siveness to BZs, possibly due to changes in receptor α1/α3 ratios and β2 subunit proportions. Giant ankryin-G, a protein typically characterized to be important for AIS assembly and scaffolding (Bennett and Lorenzo, 2013), was recently found to be a novel regulator of somatodendritic GABAAR synaptic sta bilization (Fig. 4) (Tseng et al., 2015). Mitigation of GABAAR internalization by giant ankryin-G was found to be dependent on interaction with GABARAP via a neuronal specific exon-coding sequence at extrasynaptic sites, with knockdown of giant ankyrin-G leading to enhanced GABAAR endocytosis, while overexpression reduced it.
With respect to extrasynaptic receptors, the intracellular domain of the δ subunit also binds to the μ2 subunit of AP2 (Fig. 4) (Kittler et al., 2005). Mutation of two AP2 interacting motifs, a YxxΦ motif and an atypical R/K-rich motif, abolish association of GST-δ-intracellular domain fusion protein with the μ2 subunit of AP2 by pull-down assay (Gonzalez et al., 2012). Interestingly, 5–10 min ethanol expo sure enhances β subunit S408/409 phosphorylation, while simultaneously increasing association of the AP2 μ2-subunit with δ-containing GABAARs, sug gesting receptor composition dependent AP2 recruit ment, consistent with rapid ethanol induced impairment in α4βδ GABAARs seen in vivo and in cultured neurons (Gonzalez et al., 2012). Chronic exposure to the benzodiazepine site antagonist fluma-zenil, recently identified as a negative modulator at α4βδ GABAARs, also enhances endocytosis and deg radation of α4βδ recombinant receptors via a clathrin-dependent pathway in heterologous cells (Kuver and Smith, 2016).
A number of noxious stimuli trigger GABAAR endocytosis including seizure models (Goodkin et al., 2005, 2008; Naylor et al., 2005), OGD conditions (Arancibia-Carcamo et al., 2009), and prolonged ago nist application (Chaumont et al., 2013). Protein phosphatases (Fig. 4) have an important role in regu lating receptor endocytosis under these conditions. For example, inhibition of the calcium-sensitive phosphatase calcineurin (CaN) by FK506 or serine/threonine protein phosphatase 1 (PP1) and 2A (PP2A) by okadaic acid reverses reduction of surface γ2-subunit containing GABAARs and mIPSC ampli tude induced by status epilepticus treatments in slice preparations (Joshi et al., 2015). NMDA receptor mediated calcium entry and CaN activation was further shown to decrease surface α2-containing recep tors during in vitro epileptiform activity by live-cell imaging techniques (Eckel et al., 2015). Activation of the transient receptor potential cation channel sub family V member 1 also appears to cause GABAAR endocytosis in the dentate gyrus of rodents dependent on calcium influx, CaN, and dynamin-activity (Cha vez et al., 2014). In addition, inflammation and release of the proinflammatory cytokine tumor necro sis factor-α (TNFα) stimulates GABAAR internaliza tion (α1/2/5, β3, γ2) in a CaN-independent pathway in cultured hippocampal neurons (Pribiag and Stellwagen, 2013). Activation of TNF receptor 1 by TNFα initiates a p38 MAPK/PI3K/PP1 and dynamin GTPase pathway prompting dephosphorylation of S408/409 on the β3 subunit, reduction of mIPSC amplitude, and diminished gephyrin synaptic clustering. Conversely, activation of p38 MAPK by the cytokine interleukin−1β increases surface expression of the α5-subunit, augmenting tonic current and reducing excitatory long-term potentiation, a process involved in inflammation-induced memory deficits in the hippocampus (Wang et al., 2012). Moreover, this work found TNFα exposure did not significantly alter tonic current. These opposing findings suggest non-p38 MAPK downstream effectors activated by TNFα and interleukin−1β, respectively, may influence activity and recruitment of proteins like PP1. Recent evi dence has also recognized the amyloid β (Aβ) peptide, most commonly associated with the patho genesis of Alzhimer’s disease, as a stimulator of GABAAR endocytosis (Ulrich, 2015). Acute applica tion of the Aβ 1–42 peptide fragment decreased GABAergic current in somatosensory cortical slices and this effect was reversed by inhibiting dynamin. The specificity of GABAAR subtypes undergoing Aβ treatment induced internalization remains to be identified.
GABAAR Recycling
Surface biotinylation assays suggest the majority of constitutively internalized GABAARs rapidly recycle back to the cell surface (70% in 1 h), while significant degradation occurs over longer time scales (6 h) (Kittler et al., 2004). The recycling of GABAARs is partly mediated by HAP1 direct association with the β subunits (Fig. 4) (Kittler et al., 2004; Twelvetrees et al., 2010). HAP1 functions as a kinesin adaptor and localizes to early endosomes containing GABAAR, and thus overexpression of HAP1 pro motes receptor surface levels and reverses intracellu lar accumulation of receptors by constitutive (Kittler et al., 2004) and OGD-induced endocytosis (Mele et al., 2017). Mutation of the HAP1 protein resulting in polyglutamine expansion, as seen in Huntington’s Disease, results in dysregulated transport of GABAARs to the cell surface and compromised inhibitory neurotransmission (Twelvetrees et al., 2010). Moreover, Huntingtin protein and HAP1 have recently been identified as a key regulator of autopha gosome transport in neurons (Wong and Holzbaur, 2014). The recycling activity of HAP1 likely arises from its association with the kinesin KIF5 (Fig. 4). Purified complexes of β3-GABAAR/KIF5 and HAP− 1/KIF5 are readily immunoprecipitated from rat brain tissue, while acute blockade of KIF5 reduces GABAAR synaptic levels and signaling strength (Twelvetrees et al., 2010). The integral membrane protein CAML (calcium-modulating cyclophilin ligand) has also been implicated in GABAAR for ward trafficking and recycling via interaction with the γ2 subunit cytoplasmic and fourth transmembrane domain regions (Yuan et al., 2008). CAML-deficient neurons have reduced recycling of internalized GABAARs and decreased GABAergic neurotrans mission in electrophysiological recordings.
Lysosomal Degradation
GABAARs undergoing constitutive internalization from the cell surface may be subjected to lysosomalmediated degradation (Fig. 4), a process blocked by the lysosomal proteolytic inhibitor leupeptin (Kittler et al., 2004). Acute leupeptin treatment also increases the size, number, and strength of GABAergic synap ses in cortical slices (Arancibia-Carcamo et al.,2009). Certain stimuli can also accelerate degradation of surface GABAARs. For instance, cultured hippo campal neurons undergo enhanced lysosomalmediated degradation of α2-containing receptors in response to benzodiazepine treatment (Jacob et al., 2012). Ubiquitination of 7 lysine residues within the intracellular loop of the γ2 subunit plays a key role in GABAAR lysosomal targeting (Arancibia-Carcamo et al., 2009). Mutation of these lysines to arginine (K7R) reduced colocalization of receptors at late endosomes, made receptors impervious to leupeptin treatment in heterologous cells, and blocked an OGD-induced loss of surface receptor clusters (Ara-ncibia-Carcamo et al., 2009). The ubiquitin E3-ligase, ring finger protein 34 (RNF34), directly inter acts with the intracellular loop of γ2, co-immunoprecipitates with this subunit from brain extracts, and can be found colocalized at GABAergic synapses (Fig. 4) (Jin et al., 2014). Overexpression of RNF34 enhances the rate of γ2-GABAAR degrada tion and reduces GABAAR synaptic cluster size and strength. Interestingly, expression of the γ2 K7R mutant in these experiments did not reverse RNF34-induced degradation of GABAARs in co-transfected HEK293 cells, but mutation of additional lysine resi dues in a K8R, K9R, and K10R mutant did. More over, RNF34 mediated ubiquitination appears to contribute to both proteasomal and lysosomal degra dation of GABAARs in these cells. The ARF GEF, Brefeldin A-inhibited guanine nucleotide-exchange protein 3 (BIG3), may also be important for lyso somal trafficking of GABAARs (Fig. 4). BIG3 is pri marily expressed in pancreatic islets and the brain, and is found colocalized with lysosomes in neurons. BIG3 KO mice demonstrate increased GABAAR syn aptic size and current, suggesting this protein is involved in negative regulation of GABAAR levels (Liu et al., 2016). The exact role of specific GABAAR subunits, associated E3-ligases and ubiquitination patterns, and lysosomal trafficking proteins that regulate the transition of surface receptors to lysosomes is an important area of future research.
ACTIVITY-DEPENDENT PLASTICITY OF GABAAR SYNAPSES
Synapse plasticity refers to strengthening or weaken ing of individual synapses in response to changes in stimuli at a local or system level. Two decades of research have uncovered various forms of short and long term plasticity of GABAergic neurotransmission in different brain regions, with underlying cellular and molecular mechanisms being identified both preand post-synaptically (reviewed in (Flores and Méndez, 2014)). Persistent changes in synaptic efficacy are generally referred to as long-term potentiation (LTP) and long-term depression (LTD). Traditionally this described excitatory synapse plastic ity that produced a respective increase or decrease in synapse strength. However, with growing awareness of GABAergic synapse plasticity, these terms now refer to changes in the gain of either synapse type (for GABAergic synapses, iLTD and iLTP). Although changes in presynaptic efficacy of inhibitory synapses are well characterized and an important part of plastic ity (reviewed in Castillo et al., 2011; Castillo, 2012), here we will focus on postsynaptic cell-based plasticity mechanisms related to GABAAR trafficking.
GABAAR postsynaptic plasticity is encompassed by changes in channel function, receptor number or clustering/lateral diffusion, and altered chloride homeostasis (changing the GABA reversal potential/EGABA) (Fig. 5). Tonic inhibition generated by extrasynaptic receptors is also dynamic, with acute and chronic stress inducing changes in cell surface trafficking and subunit specific expression (reviewed in Maguire, 2014). Receptor phosphorylation state is a key means for altering channel function or receptor trafficking via activation of protein kinases (including PKC, PKA, CaMKII, and Src) or phosphatases (CaN, PP1, PP2A) (Fig. 5) (reviewed in Nakamura et al., 2015). Investigation of GABAergic postsynaptic plasticity has largely focused on excitation driven changes. Early plasticity studies identified that intra cellular application of CaMKII (Wang et al., 1995) and experimental epilepsy kindling models (Nusser et al., 1998) elevated GABAAR surface levels and potentiated the inhibitory response. Later studies revealed that moderate NMDA activation of hippo campal neurons promote CaMKIIα translocation to inhibitory synapses and CaMKIIα-dependent inser tion of GABAARs with concomitant phosphatase mediated AMPAR (GluR1) removal (Marsden et al., 2007, 2010). Aside from NMDAR stimulation, enhanced VGCC activity promotes CaMKII phos phorylation of the β3 subunit S383 residue and receptor insertion (Fig. 4) (Saliba et al., 2012), suggesting multiple routes for calcium signaling to enhance GABAAR surface levels. In addition, a quantum dot single-particle tracking study showed that NMDAinduced iLTP required CaMKII phosphorylation of β3 S383 to reduce GABAAR lateral diffusion and enhance recruitment of the synaptic scaffold protein gephyrin (Petrini et al., 2014). More recently, this experimental approach revealed additional gluta mateand calcium-evoked plasticity of GABAergic synapses. Low glutamate levels stimulate mGluRdriven calcium store release via PKC and IP3 recep tors and stabilization of surface GABAARs, while robust NMDAR activation promoted CaN phosphatase activity and destabilization of postsynaptic GABAAR (Bannai et al., 2015). This is consistent with earlier findings where glutamate application pro motes CaMKIIα translocation to excitatory synapses, enhanced AMPAR surface levels, and reduced plasma membrane GABAAR levels (Marsden et al., 2007, 2010). Glutamate, high levels of neuronal activity, or strong NMDAR activation also reduces plasma membrane GABAaR cluster size, stimulates receptor lateral mobility, and decreases mIPSC amplitude via CaN (Bannai et al., 2009) and dephos phorylation of the γ2 subunit S327 residue (Fig. 3) (Muir et al., 2010). These molecular studies in neuro nal culture are consistent with earlier slice and in vivo studies showing LTD of GABAergic inhibition via NMDAR activation requires CaN (Lu et al., 2000) and the γ2 subunit (Wang et al., 2003). Similar mechanisms for iLTD and iLTP that rely on GABAAR trafficking are supported by studies in other brain regions such as the deep cerebellar nuclei (Morishita and Sastry, 1996; Ouardouz and Sastry, 2000). Together these plasticity studies show that glutamate receptor activation and calcium-sensitive signaling pathways can generate either iLTP or iLTD. Moderate or ambient glutamate signaling promotes kinase activity that stabilizes GABAARs, while strong stimulation leads to CaN phosphatase activation and receptor diffusion. Ultimately, glutamatergic activity dictates bidirectional modulation of GABAergic postsynaptic strength and receptor clustering.
Structural reorganization of gephyrin scaffolding is now emerging as a key mechanism of rapid inhibitory synaptic plasticity with changes occurring on a minute timescale (Specht et al., 2013). Gephyrin posttranslational modifications (described earlier) such as phosphorylation and ubiquitination are likely to be central in regulating synapse architecture. A recent organotypic slice culture study of gephyrin dynamics identified that activity patterns promoting NMDAR LTP (carbachol treatment or theta burst stimulation) increase gephyrin cluster size and formation in a CaMKII-dependent manner (Flores et al., 2015). Fur thermore this gephyrin plasticity was associated with enhanced phosphorylation of gephyrin S305, a CaMKII phospho site identified by in vitro kinase assay.
The critical role of CaMKII in GABAAR synaptic plasticity is also seen in other brain regions. For example, rebound potentiation (RP), a cerebellar form of iLTP that follows Purkinje cell (PC, principal output neurons of cerebellum) depolarization, requires CaMKII activation (Kano et al., 1996) and increased association between the γ2 subunit and GABARAP (Kawaguchi and Hirano, 2007). RP relies on CaMKII recruitment of β2 containing receptors to the cell surface and occurs only on basket cell interneuron-PC soma synapses and not on stellate interneurons-distal PC dendrite synapses (He et al., 2015). This synapse specific potentiation by CaMKII is consistent with its ability to selectively increase β2-containing GABAARs and mIPSC amplitude (Houston et al., 2008). Together this indicates two levels of selectivity based on input and subunit com position, as β2 containing synapses are also expressed on dendrites.
At the other end of the neuronal activity spectrum, early studies using chronic activity blockade (24–48 h TTX treatment) resulted in homeostatic preand post-synaptic GABAergic plasticity with decreased GABAAR synaptic clusters, mIPSC amplitude and frequency (Kilman et al., 2002). In vivo chronic sen sory deprivation via whisker trimming decreases GABAAR number and weakens inhibitory synapses (Micheva and Beaulieu, 1995; Fuchs and Salazar, 1998; Gainey et al., 2016). In contrast, whisker train ing promotes GABAergic synaptogenesis on den dritic spines, while not on dendritic shafts (Jasinska et al., 2010). Analogous findings in the visual cortex (Chen et al., 2012) indicate that a pool of dynamic GABAergic spine synapses support changes to net work activity levels. These chronic in vivo protocols reveal how neuronal adaptation operates across a continuum of GABAAR synaptic plasticity, where reductions in activity lead to loss of inhibition, while learning or heightened neuronal activity strengthens existing GABAergic synapse or initiates new synapse formation. However, some sensory modulation can occur in an inverse fashion, as brief monocular depri vation potentiates iLTP (Maffei et al., 2006), concur rent with increasing gephyrin and GABAAR perisomatic accumulation (Petrini et al., 2014). Other visual cortex studies indicate that GABAAR endocy tosis contributes to LTD occurring with repetitive fir ing, while slow membrane oscillations, comparable to activity during sleep state, promote iLTP via exocytosis (Kurotani et al., 2008). Similarly, acquisition of a fear response is accompanied by surface GABAAR decreases (Chhatwal et al., 2005) and extinction of fear requires GABAAR insertion and GABARAP-receptor interaction (Lin et al., 2009). Parallel homeostatic changes are observed at excit atory synapses of interneurons, where increased net work activity also promotes interneuron excitability and mEPSC amplitude. However, we currently are not aware of studies examining homeostatic plasticity of GABAergic synapses on inhibitory interneurons. Collectively, GABAergic synapse plasticity and rewiring is fundamental for sensory experience adap tation, with functional changes in inhibition matching structural changes.
Homeostatic changes in GABAAR currents also occur through shifts in chloride homeostasis and GABA reversal potential (EGABA). Studies in youn ger neurons with depolarizing and excitatory GABA show network activity blockade of voltage-gated sodium (Na) channels results in an increase in ampli tude of mPSCs via chloride accumulation and a depo larizing shift in the GABA reversal potential (Gonzalez-Islas et al., 2010; Lindsly et al., 2014). During development, regulated changes in intracellu lar chloride levels control receptor subunit composi tion in cerebellar neurons, tuning inhibition (Succol et al., 2012). Specifically, increased KCC2 levels and activity lead to a decrease in α3 subunits, while α1 and δ subunit expression increases. It remains to be shown whether changes in chloride homeostasis in the adult brain regulate subunit expression and recep tor composition in a similar fashion. KCC2 overex pression can switch the GABA response polarity to inhibition in young neurons (Lee et al., 2005), and conversely, downregulation of KCC2 surface levels by glutamate application abolished normal hyperpolarizing GABAergic inhibition in mature neurons (Lee et al., 2011). Interestingly, NL2 precedes KCC2 expression in development, and NL2 appears to promote KCC2 expression and the GABA switch from excitatory to inhibitory signaling as well as main tain GABA inhibition in mature neurons (Sun et al., 2013). Aberrant chloride homeostasis is an important contributor to the pathology of neuronal injury (reviewed in De Koninck, 2007; Kaila et al., 2014) including spinal cord injury, neuropathic pain ische mia, and traumatic brain injury. Furthermore, pertur bation or loss of the normal developmental GABA shift occurs in mouse models of neurodevelopmental disorders including Down syndrome (He et al., 2014; Contractor et al., 2015; Deidda et al., 2015).
In summary, plasticity provokes many questions from the synapse to cell to circuit level. Clearly, due to the large number of overlapping trafficking regula tors, protein localization to excitatory or inhibitory synapses within a neuron is central to achieve specif icity and avoid producing overall synonymous changes at both synapse types. What are the distinct mechanisms occurring in pyramidal cells, interneur ons and interneuron subtypes? How is cell regionspecific plasticity achieved? To what degree does synapse-specific plasticity modulate circuit output? At the organism level, how do experience, drug expo sure or natural stimuli produce discrete patterns to appropriately modify inhibitory neurotransmission? A key step in achieving this local or populationbased plasticity may be through modulating GABAAR synapse development and trafficking by endogenous neuropeptides and neurosteroids.
NEUROPEPTIDE AND NEUROSTEROID MODULATION
Brain-Derived Neurotrophic Factor
The brain-derived neurotrophic factor (BDNF) protein plays a critical role in neurogenesis, neuroplas ticity, and neuroprotection. Knockout of the cognate BDNF target tropomyosin receptor kinase B (TrkB) disrupts GABAergic synaptogenesis in the cerebel lum (Rico et al., 2002). Furthermore, single-cell con ditional deletion of BDNF in the cortex causes a decrease in inhibitory postsynaptic currents and the number of GABAergic synapses onto pyramidal neu rons lacking BDNF (Kohara et al., 2007). The spe cific localization of BDNF/TrkB signaling is critical for this signaling pathway’s role in synaptogenesis, as genetic ablation of BDNF-expressing cells in the internal granular layer of the cerebellum did not affect GABAergic synapse maturation, while selec tive deletion of BDNF from glutamatergic axons aris ing from distant precerebellar nuclei/spinal cord mossy fibers did (Chen et al., 2016). During neuronal development of cultured cerebrocortical neurons, excitatory, depolarizing GABAAR activity regulates a positive feedback loop by controlling secretion of BDNF, activation of TrkB and phosphatidylinositol 3-kinase (PI3K) and PKC activity, and reduction in receptor internalization (Porcher et al., 2011). Additionally, immature cultured hippocampal neurons demonstrated enhanced expression of gephyrin, gephyrin-α1 interaction, and GABAAR association at synapses following exogenous BDNF application (Gonzalez, 2014). Contrasting with BDNF enhance ment of GABAergic signaling early in development, acute exposure of BDNF in mature cultured hippo campal neurons enhanced receptors at the cell surface within 5 min, but decreased GABAAR current, func tion, and surface levels around 30–60 min (Brunig et al., 2001; Jovanovic et al., 2004). Accordingly, enhanced phosphorylation of the β3 subunit S408/409 sites by PKC occurred during initial BDNF treat ment, while longer exposure caused recruitment of protein phosphatase 2A (PP2A), β3 dephosphoryla tion, and endocytosis (Jovanovic et al., 2004). In a similar mature hippocampal paradigm, 15 min BDNF treatment led to a reduction in mIPSC amplitudes and long-lasting reduction of GABAAR levels up to 12 h (Brunig et al., 2001). In agreement with these find ings, hippocampal and amygdala neurons treated for 5–20 min with BDNF had a reduction in a1-GABAAR surface levels in vitro (Mou et al., 2011). Gephyrin levels are also dynamically altered in the amygdala following BDNF, where 20 min exposure decreased the gephyrin pool, while a more chronic treatment lead to an increase (Mou et al., 2013).
In contrast, 2 h BDNF treatment in intact acute prefrontal cortical slices from adult mice enhances phosphorylation of the γ2-GABAAR subunit Y365/367 residues and causes long term stabilization of receptor surface levels (Vithlani et al., 2013). Inter estingly, hippocampal neurons derived from PRIP1/2 knockout mice demonstrate enhanced β3-phosphorylation and GABAergic current strength in response to BDNF. PRIP is required for the recruit ment of the phosphatase PP2A to GABAAR β- subunits (Fig. 4) (Kanematsu et al., 2006). Thus, BDNF treatment may be favoring PKC activity and phosphorylation of β subunits, an effect that would deter internalization, but can be offset by phosphatase activity. Additionally, BDNF regulates GABAAR subunit expression at the gene level in response to seizure paradigms in vitro and in vivo (Lund et al., 2008). BDNF acts to downregulate α1 subunit expression following pilocarpine-induced status epilepticus via a JAK/STAT signaling mechanism (Lund et al., 2008), while enhancing α4 subunit levels via an early growth response factor 3 (Egr3)/MAPKdependent pathway (Roberts et al., 2005, 2006).
Although early depolarizing GABAAR signaling promotes neuronal maturation (Ben-Ari et al., 20l2), growing evidence suggests that BDNF signaling can promote transient excitatory (or diminished inhibi tory) GABAAR signaling following noxious stimuli or injury. Adult cats who receive BNDF treatment following a unilateral vestibular neurectomy, a proce dure reported to enhance reactive cell proliferation in the vestibular nucleus and recovery following CNS injury (Tighilet et al., 2007), demonstrate accelerated functional recovery associated with decreased KCC2 and increased GABAAR levels (Dutheil et al., 20l6). A BDNF/TrkB-dependent depolarizing shift in EGABA also occurs pre-synaptically following nerve injury in the spinal cord that reduces presynaptic inhibition and contributes to the initiation of neuro pathic pain (Chen et al., 20l4).
Importantly, the non-cleaved BDNF precursor, proBDNF, binds a separate receptor known as p75NTR, and was found to stimulate GABAAR β3 subunit dephosphorylation via the RhoA-ROCK-PTEN path way. As a result, GABAAR internalization was enhanced, surface and total levels were reduced, and GABAergic neurotransmission was dampened (Rif-fault et al., 20l4). These findings were confirmed by in vivo work using P0 rats given intracerebroventricular injections of tPA-Stop, an inhibitor of plasminogen causing accumulation of proBDNF, producing decreased β3 subunit phosphorylation, and reduced mIPSC amplitude and frequency. Neuronal expression of a non-maturable version of proBDNF abolished the excitatory to inhibitory shift of GABA and reduced KCC2 levels in the cortex (Riffault et al., 20l6). Simi larly, interfering with early depolarizing GABA in newborn mice using the NKCCl-blocker bumetanide prolonged the critical period of postnatal development in the visual cortex and led to long-term reductions in inhibitory tone and BDNF expression without a change in visual function (Deidda et al., 20l5). Accordingly, co-treatment of the BDNF mimetic drug 7,8-dihydroxyflavonone reversed the deficiencies in plasticity induced by bumetanide treatment. Over all, the experimental and brain-region dependent responses to BDNF and its precursors highlight the specific and diverse role of this protein in regulation of GABAAR function and plasticity.
Insulin
Circulating hormones involved in basic metabolic and energy balance are important for neuronal development and stability. The major role of insulin in regulating GABAAR synaptic (Wan et al., l997) and surface levels (Mielke and Wang, 2005) is well described in a previous review (Luscher et al., 20lla). In summary, insulin acts to promote rapid forward trafficking of newly synthesized GABAARs from the ER and Golgi via an—AKT/PRIP depen dent pathway and GABAAR β subunit phosphoryla tion (S409 in βl, S4l0 in β2, S408/409 in β3) (Fig. 4) (Wang et al., 2003; Xu et al., 2006; Fujii et al., 20l0). A PI3K pathway is also involved in increased GABAAR phospholipid interactions following insulin exposure (Vetiska et al., 2007). A role for aberrant insulin signaling and GABAAR trafficking in ASD was recently described for individuals carrying the MET tyrosine kinase receptor risk allele (Lo et al., 20l6). Conditional mutant mice expressing kinase-dead Met restricted to hippocampal and cortical regions demonstrate an enhanced excitatory/inhibi tory ratio, loss of postsynaptic inhibition, and resis tance to insulin-induced enhancements in the strength of GABAergic neurotransmission.
Neurosteroids and Oxytocin
Neurosteroids act at most GABAAR subtypes and can both enhance or inhibit GABAergic neurotransmis sion. 3a-hydroxy A-ring reduced metabolites of pro gesterone, deoxycorticosterone, and testosterone are positive allosteric modulators of specific GABAARs (notably, allopregnanolone or 3α,5α-tetrahydroprogesterone [THP] and tetrahydro-deoxycorticosterone [THDOC]). In contrast, 3β-OH pregnane steroids and pregnenolone sulfate are GABAAR antagonists. Neu rosteroids which enhance receptor activation have binding sites at the α subunit and the β-α subunit interface, while the δ subunit does not affect initial binding but enhances the efficacy of potentiation (Hosie et al., 2009). For instance, THP and THDOC shift GABA activation from partial to full agonism at δ-containing receptors (Wohlfarth et al., 2002; Bianchi and Macdonald, 2003; Zheleznova et al., 2008). The δ subunit is important for the physiological response to neurosteroids, as mice deficient of this subunit have attenuated responsiveness to the behav ioral and GABAAR modulatory effects of these agents (Mihalek et al., l999; Boehm et al., 2006; Shen et al., 20l0).
Surface expression of α4βδ GABAARs is highly plastic in response to neuroactive steroids including THP (Shen et al., 2005), THDOC (Stell et al., 2003; Comenencia-Ortiz et al., 20l4), progesterone and its metabolite allopregnanolone. Several in vivo studies show steroids can robustly enhance total levels of the α4 and δ subunit (Gulinello et al., 2001; Hsu et al., 2003; Maguire and Mody, 2007). Accordingly, marked changes in circulating levels of neuroactive steroids that occur during the estrous cycle (Lovick et al., 2005; Maguire et al., 2005; Carver et al., 2014), puberty (Shen et al., 2007), pregnancy (Sanna et al., 2009), and post-partum (Maguire and Mody, 2008) ultimately fine-tune GABAAR inhibitory neu rotransmission (Maguire and Mody, 2009). Additional alterations of neurosteroids have been identified in seizure susceptibility (Tan et al., 2015; Joshi et al., 2017), social isolation behavior in mice (Agis-Balboa et al., 2007), and in the cerebrospinal fluid of humans with depression (Romeo et al., 1998) and post-traumatic stress disorders (Rasmusson et al., 2006). Localization of receptors and the release site of endogenous neurosteroids may also impact func tional neurosteroid effects. For instance, mice sub jected to temporal lobe epileptic seizures show increased synaptic α4 levels and insensitivity to allopregnanolone in mIPSC slice recordings of dentate granule cells (Sun et al., 2007). It is unclear if this change in responsiveness to neurosteroids is strictly due to α4 receptor synaptic association or a broader byproduct of the dramatic changes in cellular func tion and toxicity incurred by seizure events. The local production of neurosteroids also has an important developmental role in fine-tuning thalamic neuron transitions from relatively slow to fast phasic signal ing (Brown et al., 2015).
Neurosteroids act to enhance extrasynaptic recep tor surface levels by influencing the phosphorylation status of GABAAR subunits. For example, THDOC exposure enhances PKC-mediated phosphorylation of the α4 subunit S443 site leading to receptor inser tion and increased tonic current (Abramian et al., 2010, 2014), while also enhancing PKC-mediated phosphorylation of β3 subunit S408/409 residues (Adams et al., 2015). Acute hippocampal slices treated with allopregnolone or the synthetic neuroste roid SGE-516 also demonstrate enhanced S408/409 phosphorylation and increased total β3 subunit sur face levels, although these two agents had distinct effects on spontaneous IPSC amplitude (Modgil et al., 2017). Modifying PKA and PKC activity in HEK293 cells alters the sensitivity of both synaptic α1β3γ and extrasynaptic α4β3δ responsiveness to THDOC potentiation (Adams et al., 2015). Alanine mutation of β3 S408/409 sites diminished potentia tion of synaptic receptors to THDOC, while triple mutant α4S443Aβ3S408A,S409Aδ receptors were required to yield the same insensitivity to changes in broad spectrum kinase inhibition by staurosporine, suggesting phosphorylation of both subunits is required for neurosteroid regulation of extrasynaptic receptors (Adams et al., 2015). Kindling and broad activation of PKC by the phorbol ester PMA also reduces responsiveness to THDOC in temporal lobe rat slices (Kia et al., 2011), suggesting overlapping mechanisms of action at GABAARs. Neurosteroids may also play a role in the ethanol-induced increase in α4 subunit levels (Shen et al., 2012) and intake behavior (Rewal et al., 2009), possibly explaining high sensitivity to THDOC during alcohol with drawal (Devaud et al., 1996).
The neuropeptide oxytocin, commonly associated with childbirth and breastfeeding, also enhanced pro tein expression of extrasynaptic GABAAR α4 and δ subunits in a mixed neuronal/astrocyte primary cell preparation (Kaneko et al., 2016). This effect of oxyto cin increased responsiveness to THIP in electrophysiological recordings and provided neuroprotective effects against oxygen-glucose deprivation treatment. Interestingly, the oxytocin receptor (Oxtr) has also been implicated as a regulator of the excitatory to inhibitory switch of GABAAR function by controlling KCC2 activity (Leonzino et al., 2016). Developmental upregulation of KCC2 function and surface stability through phosphorylation is carefully regulated by Oxtr signaling, and thus mice lacking Oxtr have reduced phospho-KCC2, enhanced excitatory/inhibitory ratio, and seizure susceptibility. Collectively, neurosteroid and neuropeptide signaling dynamically fine-tune GABAAR activity, but their mechanistic role in regu lating homeostatic GABAAR trafficking and expres sion remains to be fully elucidated.
TECHNICAL ADVANCEMENTS AND FUTURE OUTLOOK
Considerable scientific progress has revealed a net work of molecular mechanisms underlying GABAer-gic plasticity (Fig. 5). Yet, advancement of new and existing methods is promoting major inroads for our understanding of GABAAR signaling and trafficking. Recent proteomic strategies have vastly expanded the protein repertoire of GABAergic synapses to nearly 200 proteins by targeted purification of GABAARs, NL2, or gephyrin (collybistin and InSyn1 to a lesser extent) and their respective interactomes (Kang et al., 2014; Loh et al., 2016; Nakamura et al., 2016; Uezu et al., 2016). Microscopy-based approaches encom pass the fastest growing means of discovery in vitro and in vivo, including optogenetically controlled GABAARs (Lin et al., 2014, 2015), two-photon based GABA photolysis (Oh et al., 2016), single-particle-tracking of receptor diffusion (Petrini and Barberis, 2014), quantitative super-resolution imaging of gephyrin (Pennacchietti et al., 2017) and gephyrin recombinant antibody-like proteins (Gross et al., 2013, 2016) or fluorescent super-binding peptides (Maric et al., 2017). Additionally, proximity ligation assays allow for researchers to detect native protein interactions utilizing widely-available primary anti bodies (Smith et al., 2014; Tseng et al., 2015), while emerging single-molecule fluorescence resonance energy transfer methods (smFRET) have yet to be applied. When considering these advancements in parallel with the rise of modern clinical genomics like human exome sequencing (Myers and Mefford, 2015; Yuan et al., 2015; Wang et al., 2017), the dis covery rate and resolution of GABAergic mediators applicable to human health and disease is primed to accelerate exponentially.
Acknowledgments
Contract grant sponsor: NIH/NIGMS; contract grant number:
T32 GM08424—Institutional Research Training Group.
LITERATURE CITED
- Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, et al. 2014. Neurosteroids pro mote phosphorylation and membrane insertion of extrasy-naptic GABAA receptors. Proc Natl Acad Sci U S A 111: 7132–7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. 2010. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibi tion. J Biol Chem 285:41795–41805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Thomas P, Smart TG. 2015. Modulation of neurosteroid potentiation by protein kinases at synaptic- and extrasynaptic-type GABAA receptors. Neuropharmacology 88:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. 2007. Down-regulation of neurosteroid bio synthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci U S A 104:18736–18741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotti TP, Macdonald RL. 1993. Assembly of GABAA receptor subunits: alpha 1 beta 1 and alpha 1 beta 1 gamma 2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci 13:1429–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggono V, Huganir RL. 2012. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol 22:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli R, Pizzarelli R, Pedroni A, Fritschy JM, Del Sal G, Cherubini E, Zacchi P. 2014. Pin1-dependent signal ling negatively affects GABAergic transmission by mod ulating neuroligin2/gephyrin interaction. Nat Commun 5: 5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia-Carcamo IL, Yuen EY, Muir J, Lumb MJ, Michels G, Saliba RS, Smart TG, et al. 2009. Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition. Proc Natl Acad Sci USA 106:17552–17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaev O, Botta P, Meyer E, Muller C, Ehrenreich H, Brose N, Luthi A, et al. 2016. Neuroligin 2 deletion alters inhibitory synapse function and anxiety-associated neuro nal activation in the amygdala. Neuropharmacology 100: 56–65. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Baldwin D, Abelli M, Bolea-Alamanac B, Bourin M, Chamberlain SR, Cinosi E, et al. 2017. Biolog ical markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophys iology and neurocognition. World J Biol Psychiatry 18: 162–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H, Levi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, et al. 2009. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffu sion dynamics. Neuron 62:670–682. [DOI] [PubMed] [Google Scholar]
- Bannai H, Niwa F, Sherwood MW, Shrivastava AN, Arizono M, Miyamoto A, Sugiura K, et al. 2015. Bidirec tional control of synaptic GABAAR clustering by gluta mate and calcium. Cell Rep 13:2768–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R, Minier F, Sigel E. 2006. A GABA(A) receptor of defined subunit composition and positioning: concatena tion of five subunits. FEBS Lett 580:1616–1620. [DOI] [PubMed] [Google Scholar]
- Bausen M, Fuhrmann JC, Betz H, O’Sullivan GA. 2006. The state of the actin cytoskeleton determines its associa tion with gephyrin: role of ena/VASP family members. Mol Cell Neurosci 31:376–386. [DOI] [PubMed] [Google Scholar]
- Beck M, Brickley K, Wilkinson HL, Sharma S, Smith M, Chazot PL, Pollard S, et al. 2002. Identification, molecu lar cloning, and characterization of a novel GABAA receptor-associated protein, GRIF-1. J Biol Chem 277: 30079–30090. [DOI] [PubMed] [Google Scholar]
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, et al. 2001. GABA(A) receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci 4:908–916. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. 2007. GABA: A Pioneer Transmitter That Excites Immature Neurons and Generates Primitive Oscillations. Physiol Rev 87:1215–1284. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. 2012. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 18:467–486. [DOI] [PubMed] [Google Scholar]
- Bennett V, Lorenzo DN. 2013. Spectrin- and ankyrin-based membrane domains and the evolution of vertebrates. Curr Top Membr 72:1–37. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. 2001. Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAa receptors. J Neurosci 21:1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. 2003. Neurosteroids shift par tial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci 23: 10934–10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blicher JU, Near J, Næss-Schmidt E, Stagg CJ, Johansen-Berg H, Nielsen JF, Østergaard L, et al. 2015. GABA lev els are decreased after stroke and GABA changes during rehabilitation correlate with motor improvement. Neuro-rehabil Neural Repair 29:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, Sudhof TC, et al. 2009. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion mole cule neuroligin 2. Genes Brain Behav 8:114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boal F, Stephens DJ. 2010. Specific functions of BIG1 and BIG2 in endomembrane organization. PLoS One 5: e9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL 2nd, Homanics GE, Blednov YA, Harris RA. 2006. delta-Subunit containing GABAA receptor knock out mice are less sensitive to the actions of 4,5,6,7-tetra-hydroisoxazolo-[5,4-c]pyridin-3-ol. Eur J Pharmacol 541: 158–162. [DOI] [PubMed] [Google Scholar]
- Bradley CA, Taghibiglou C, Collingridge GL, Wang YT. 2008. Mechanisms involved in the reduction of GABAA receptor alpha1-subunit expression caused by the epi lepsy mutation A322D in the trafficking-competent receptor. J Biol Chem 283:22043–22050. [DOI] [PubMed] [Google Scholar]
- Brady ML, Jacob TC. 2015. Synaptic localization of alpha5 GABA (A) receptors via gephyrin interaction regulates dendritic outgrowth and spine maturation. Dev Neurobiol 75:1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon N, Jovanovic J, Moss S. 2002. Multiple roles of protein kinases in the modulation of gamma-aminobutyric acid(A) receptor function and cell surface expression. Pharmacol Ther 94:113–122. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Hill J, Smart TG, Moss SJ. 2001. Constitutive tyrosine phosphorylation of the GABA(A) receptor gamma 2 subunit in rat brain. Neuropharmacology 41:745–752. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Colledge M, Kittler JT, Brandon JM, Scott JD, Moss SJ. 2003. A-kinase anchor ing protein 79/150 facilitates the phosphorylation of GABA(A) receptors by cAMP-dependent protein kinase via selective interaction with receptor beta subunits. Mol Cell Neurosci 22:87–97. [DOI] [PubMed] [Google Scholar]
- Brickley K, Smith MJ, Beck M, Stephenson FA. 2005. GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J Biol Chem 280:14723–14732. [DOI] [PubMed] [Google Scholar]
- Bristow GC, Bostrom JA, Haroutunian V, Sodhi MS. 2015. Sex differences in GABAergic gene expression occur in the anterior cingulate cortex in schizophrenia. Schizophr Res 167:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AR, Herd MB, Belelli D, Lambert JJ. 2015. Devel opmentally regulated neurosteroid synthesis enhances GABAergic neurotransmission in mouse thalamocortical neurones. J Physiol 593:267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LE, Fuchs C, Nicholson MW, Stephenson FA, Thomson AM, Jovanovic JN. 2014. Inhibitory synapse formation in a co-culture model incorporating GABAer-gic medium spiny neurons and HEK293 cells stably expressing GABAA receptors. J Vis Exp 93:e52115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LE, Nicholson MW, Arama JE, Mercer A, Thomson AM, Jovanovic JN. 2016. gamma-Aminobutyric Acid Type A (GABAA) receptor subunits play a direct structural role in synaptic contact formation via their N-terminal extracellular domains. J Biol Chem 291: 13926–13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. 2002. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol 136:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM. 2001. BDNF reduces miniature inhibitory postsynap-tic currents by rapid downregulation of GABA(A) recep tor surface expression. Eur J Neurosci 13:1320–1328. [DOI] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. 2002. Intact sort ing, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol 443:43–55. [DOI] [PubMed] [Google Scholar]
- Burkarth N, Kriebel M, Kranz EU, Volkmer H. 2007. Neu-rofascin regulates the formation of gephyrin clusters and their subsequent translocation to the axon hillock of hip pocampal neurons. Mol Cell Neurosci 36:59–70. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Feighery EL, Mattay VS, White MG, Chen Q, Baranger DA, Berman KF, et al. 2013. DISC1 and SLC12A2 interaction affects human hippocampal func tion and connectivity. J Clin Invest 123:2961–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Bohnsack JP, Morrow AL. 2016. Ethanol regu lation of synaptic GABAA alpha4 receptors is prevented by protein kinase A activation. J Pharmacol Exp Ther 357:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Bohnsack JP, Patel V, Morrow AL. 2016. Reg ulation of extrasynaptic GABAA alpha4 receptors by ethanol-induced protein kinase A, but not protein kinase C activation in cultured rat cerebral cortical neurons. J Pharmacol Exp Ther 356:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. 2012. Brain excitability in stroke: the Yin and Yang of stroke progression. Arch Neurol 69:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Wu X, Gangisetty O, Reddy DS. 2014. Perimenstrual-like hormonal regulation of extrasynaptic delta-containing GABAA receptors mediating tonic inhi bition and neurosteroid sensitivity. J Neurosci 34:14181–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE. 2012. Presynaptic LTP and LTD of excitatory and inhibitory synapses. Cold Spring Harbor Perspect Biol 4:a005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Chiu CQ, Carroll RC. 2011. Long-term synap tic plasticity at inhibitory synapses. Curr Opin Neurobiol 21:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellot G, Cherubini E. 2013. Functional role of ambient GABA in refining neuronal circuits early in postnatal development. Front Neural Circuits 7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, et al. 2010. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phe notypes. Nature 468:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Yu W, Li R, Serwanski DR, Miralles CP, Li X, Yang BY, et al. 2004. A four PDZ domain-containing splice variant form of GRIP1 is localized in GABAergic and glutamatergic synapses in the brain. J Biol Chem 279:38978–38990. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. 2004. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem 90:173–189. [DOI] [PubMed] [Google Scholar]
- Chaumont S, Andre C, Perrais D, Boue-Grabot E, Taly A, Garret M. 2013. Agonist-dependent endocytosis of gamma-aminobutyric acid type A (GABAA) receptors revealed by a gamma2(R43Q) epilepsy mutation. J Biol Chem 288:28254–28265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez AE, Hernández VM, Rodenas-Ruano A, Chan CS, Castillo PE. 2014. Compartment-specific modulation of GABAergic synaptic transmission by TRPV1 channels in the dentate gyrus. J Neurosci 34:16621–16629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AI, Zang K, Masliah E, Reichardt LF. 2016. Gluta-matergic axon-derived BDNF controls GABAergic syn aptic differentiation in the cerebellum. Sci Rep 6: 20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Villa KL, Cha JW, So PT, Kubota Y, Nedivi E. 2012. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JT, Guo D, Campanelli D, Frattini F, Mayer F, Zhou L, Kuner R, et al. 2014. Presynaptic GABAergic inhibi tion regulated by BDNF contributes to neuropathic pain induction. Nat Commun 5:5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. 2005. Reg ulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci 25:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WH, Wang D, McMahon T, Qi ZH, Song M, Zhang C, Shokat KM, et al. 2010. GABAA receptor trafficking is regulated by protein kinase C(epsilon) and the N-ethylmaleimide-sensitive factor. J Neurosci 30:13955–13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. 2007. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54:919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar H, Barnes EM Jr. 2001. Clathrin-independent endo-cytosis of GABA(A) receptors in HEK 293 cells. Biochemistry 40:14030–14036. [DOI] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. 2012. GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci Biobehav Rev 36:2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comenencia-Ortiz E, Moss SJ, Davies PA. 2014. Phosphor ylation of GABAA receptors influences receptor traffick ing and neurosteroid actions. Psychopharmacology (Berl) 231:3453–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. 1996. Assembly and cell surface expression of hetero-meric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem 271:89–96. [DOI] [PubMed] [Google Scholar]
- Contractor A, Klyachko VA, Portera-Cailliau C. 2015. Altered neuronal and circuit excitability in Fragile X Syn drome. Neuron 87:699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossette P, Liu L, Brisebois K, Dong H, Lortie A, Vanasse M, Saint-Hilaire JM, et al. 2002. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet 31:184–189. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, et al. 2002. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A 99:8980–8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider A, Pandya CD, Peter D, Ahmed AO, Pillai A. 2014. Ubiquitin-proteasome dependent degradation of GABAAalpha1 in autism spectrum disorder. Mol Autism 5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y 2007. Altered chloride homeostasis in neu rological disorders: a new target. Curr Opin Pharmacol 7: 93–99. [DOI] [PubMed] [Google Scholar]
- Deidda G, Allegra M, Cerri C, Naskar S, Bony G, Zunino G, Bozzi Y, et al. 2015. Early depolarizing GABA con trols critical-period plasticity in the rat visual cortex. Nat Neurosci 18:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejanovic B, Schwarz G. 2014. Neuronal nitric oxide synthase-dependent S-nitrosylation of gephyrin regulates gephyrin clustering at GABAergic synapses. J Neurosci 34:7763–7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejanovic B, Semtner M, Ebert S, Lamkemeyer T, Neuser F, Luscher B, Meier JC, et al. 2014. Palmitoylation of gephyrin controls receptor clustering and plasticity of GABAergic synapses. PLoS Biol 12:e1001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Yao J, Fang C, Dong N, Luscher B, Chen G. 2007. Sequential postsynaptic maturation governs the temporal order of GABAergic and glutamatergic synaptogenesis in rat embryonic cultures. J Neurosci 27:10860–10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. 1996. Sensi tization of gamma-aminobutyric acidA receptors to neu roactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther 278:510–517. [PubMed] [Google Scholar]
- Devine MJ, Norkett R, Kittler JT. 2016. DISC1 is a coordinator of intracellular trafficking to shape neuronal devel opment and connectivity. J Physiol 594:5459–5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di XJ, Han DY, Wang YJ, Chance MR, Mu TW. 2013. SAHA enhances Proteostasis of epilepsy-associated alpha1(A322D)beta2gamma2 GABA(A) receptors. Chem Biol 20:1456–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di XJ, Wang YJ, Han DY, Fu YL, Duerfeldt AS, Blagg BS, Mu TW. 2016. Grp94 protein delivers gamma-aminobutyric acid type A (GABAA) receptors to Hrd1 protein-mediated endoplasmic reticulum-associated deg radation. J Biol Chem 291:9526–9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. 1997. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 386:279–284. [DOI] [PubMed] [Google Scholar]
- Dong N, Qi J, Chen G. 2007. Molecular reconstitution of functional GABAergic synapses with expression of neuroligin-2 and GABAA receptors. Mol Cell Neurosci 35:14–23. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. 1990. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn21. Neuron 5:781–788. [DOI] [PubMed] [Google Scholar]
- Dutheil S, Watabe I, Sadlaoud K, Tonetto A, Tighilet B. 2016. BDNF signaling promotes vestibular compensation by increasing neurogenesis and remodeling the expression of potassium-chloride cotransporter KCC2 and GABAA receptor in the vestibular nuclei. J Neurosci 36:6199–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel R, Szulc B, Walker MC, Kittler JT. 2015. Activation of calcineurin underlies altered trafficking of alpha2 sub unit containing GABAA receptors during prolonged epi leptiform activity. Neuropharmacology 88:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshaq RS, Stahl LD, Stone R 2nd, Smith SS, Robinson LC, Leidenheimer NJ. 2010. GABA acts as a ligand chaper one in the early secretory pathway to promote cell surface expression of GABAA receptors. Brain Res 1346:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. 1998. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci 1:563–571. [DOI] [PubMed] [Google Scholar]
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. 2006. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci 26:12758–12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Kaila K. 2007. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res 160:59–87. [DOI] [PubMed] [Google Scholar]
- Flores CE, Mendez P. 2014. Shaping inhibition: activity dependent structural plasticity of GABAergic synapses. Front Cell Neurosci 8:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CE, Nikonenko I, Mendez P, Fritschy JM, Tyagarajan SK, Muller D. 2015. Activity-dependent inhibitory synapse remodeling through gephyrin phos phorylation. Proc Natl Acad Sci U S A 112:E65–E72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Früh S, Romanos J, Panzanelli P, Bürgisser D, Tyagarajan SK, Campbell KP, Santello M, et al. 2016. Neuronal dys-troglycan is necessary for formation and maintenance of functional CCK-positive basket cell terminals on pyrami dal cells. J Neurosci 36:10296–10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Vicini S. 2009. Neuroligin-2 accelerates GABAergic synapse maturation in cerebellar granule cells. Mol Cell Neurosci 42:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs C, Abitbol K, Burden JJ, Mercer A, Brown L, Iball J, Anne Stephenson F, et al. 2013. GABA(A) receptors can initiate the formation of functional inhibitory GABAergic synapses. Eur J Neurosci 38:3146–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs JL, Salazar E. 1998. Effects of whisker trimming on GABAA receptor binding in the barrel cortex of develop ing and adult rats. J Comp Neurol 395:209–216. [PubMed] [Google Scholar]
- Fujii M, Kanematsu T, Ishibashi H, Fukami K, Takenawa T, Nakayama KI, Moss SJ, et al. 2010. Phospholipase C-related but catalytically inactive protein is required for insulin-induced cell surface expression of gamma-aminobutyric acid type A receptors. J Biol Chem 285:4837–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainey MA, Wolfe R, Pourzia O, Feldman DE. 2016. Whisker deprivation drives two phases of inhibitory syn apse weakening in layer 4 of rat somatosensory cortex. PLoS One 11:e0148227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. 2008. GABA(A) receptors in normal development and seizures: friends or foes? Curr Neuropharmacol 6:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MJ, Ding L, Maheshwari A, Macdonald RL. 2007. The GABAA receptor alpha1 subunit epilepsy mutation A322D inhibits transmembrane helix formation and causes proteasomal degradation. Proc Natl Acad Sci US A 104:12999–13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Heldt SA. 2016. Enrichment of GABAA receptor alpha-subunits on the axonal initial segment shows regional differences. Front Cell Neurosci 10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrow K, Triller A. 2014. GABAA receptor subunit com position and competition at synapses are tuned by GABAB receptor activity. Mol Cell Neurosci 60:97–107. [DOI] [PubMed] [Google Scholar]
- Ghosh H, Auguadri L, Battaglia S, Simone Thirouin Z, Zemoura K, Messner S, Acuna MA, et al. 2016. Several posttranslational modifications act in concert to regulate gephyrin scaffolding and GABAergic transmission. Nat Commun 7:13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesemann T, Schwarz G, Nawrotzki R, Berhorster K, Rothkegel M, Schluter K, Schrader N, et al. 2003. Com plex formation between the postsynaptic scaffolding pro tein gephyrin, profilin, and Mena: a possible link to the microfilament system. J Neurosci 23:8330–8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. 2007a. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron 56:763–770. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. 2007b. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol 582:1163–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. 2010. Alter ations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep 12:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Chub N, Garcia-Bereguiain MA, Wenner P. 2010. GABAergic synaptic scaling in embryonic moto neurons is mediated by a shift in the chloride reversal potential. J Neurosci 30:13016–13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Moss SJ, Olsen RW. 2012. Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellu lar domain of delta-containing GABAA receptors. J Neurosci 32:17874–17881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MI. 2014. Brain-derived neurotrophic factor pro motes gephyrin protein expression and GABAA receptor clustering in immature cultured hippocampal cells. Neu-rochem Int 72:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. 2008. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci 28:2527–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. 2005. Status epilepticus increases the intracellular accumulation of GABAA receptors. JNeurosci 25:5511–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Terunuma M, Kanematsu T, Misumi Y, Moss SJ, Hirata M. 2005. Direct interaction of N-ethylmaleimide-sensitive factor with GABA(A) receptor beta subunits. Mol Cell Neurosci 30:197–206. [DOI] [PubMed] [Google Scholar]
- Gouzer G, Specht CG, Allain L, Shinoe T, Triller A. 2014. Benzodiazepine-dependent stabilization of GABA(A) receptors at synapses. Mol Cell Neurosci 63:101–113. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. 2004. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119: 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GG, Junge JA, Mora RJ, Kwon HB, Olson CA, Takahashi TT, Liman ER, et al. 2013. Recombinant probes for visualizing endogenous synaptic proteins in living neurons. Neuron 78:971–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GG, Straub C, Perez-Sanchez J, Dempsey WP, Junge JA, Roberts RW, Trinh LA, et al. 2016. An E3-ligase-based method for ablating inhibitory synapses. Nat Meth 13:673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Chiu S-L, Liu B, Wu P-H, Delannoy M, Lin D-T, Wirtz D, et al. 2016. Differential vesicular sorting of AMPA and GABAA receptors. Proc Natl Acad Sci U S A 113:E922–E931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero RM, Giza CC, Rotenberg A. 2015. Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep 15:27–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. 2001. Short-term exposure to a neuroactive steroid increases alpha4 GABA(A) receptor subunit levels in association with increased anxiety in the female rat. Brain Res 910:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, et al. 1995. Benzodiazepine–insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A 92:7749–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KF, Macdonald RL. 1999. GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic proper ties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol 514:27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DY, Guan BJ, Wang YJ, Hatzoglou M, Mu TW. 2015. L-type calcium channel blockers enhance trafficking and function of epilepsy-associated alpha1(D219N) subunits of GABA(A) receptors. ACS Chem Biol 10:2135–2148. [DOI] [PubMed] [Google Scholar]
- Harvey K, Duguid IC, Alldred MJ, Beatty SE, Ward H, Keep NH, Lingenfelter SE, et al. 2004. The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J Neurosci 24:5816–5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausrat TJ, Muhia M, Gerrow K, Thomas P, Hirdes W, Tsukita S, Heisler FF, et al. 2015. Radixin regulates synap tic GABAA receptor density and is essential for reversal learning and short-term memory. Nat Commun 6:6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Duguid I, Clark B, Panzanelli P, Patel B, Thomas P, Fritschy JM, Smart TG. 2015. Interneuron- and GABA(A) receptor-specific inhibitory synaptic plasticity in cerebellar Purkinje cells. Nat Commun 6:7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Nomura T, Xu J, Contractor A. 2014. The develop mental switch in GABA polarity is delayed in fragile X mice. J Neurosci 34:446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Molina R, Sarto-Jackson I, Montenegro-Venegas C, Heine M, Smalla KH, Seidenbecher CI, Beesley PW, et al. 2014. Structure of excitatory synapses and GABAA receptor localization at inhibitory synapses are regulated by neuroplastin-65. J Biol Chem 289:8973–8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Dillon GH, Leidenheimer NJ. 2005. PKC modulation of GABAA receptor endocy-tosis and function is inhibited by mutation of a dileucine motif within the receptor beta 2 subunit. Neuropharmacology 48:181–194. [DOI] [PubMed] [Google Scholar]
- Herweg J, Schwarz G. 2012. Splice-specific glycine recep tor binding, folding, and phosphorylation of the scaffold ing protein gephyrin. J Biol Chem 287:12645–12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines RM, Hines DJ, Houston CM, Mukherjee J, Haydon PG, Tretter V, Smart TG, et al. 2013. Disrupting the clus tering of GABAA receptor alpha2 subunits in the frontal cortex leads to reduced gamma-power and cognitive defi cits. Proc Natl Acad Sci U S A 110:16628–16633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon M, Bauer G, Fritschy JM, Moser T, Falkenburger BH, Varoqueaux F. 2009. Neuroligin 2 controls the matu ration of GABAergic synapses and information process ing in the retina. J Neurosci 29:8039–8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. 2009. Con served site for neurosteroid modulation of GABA A receptors. Neuropharmacology 56:149–154. [DOI] [PubMed] [Google Scholar]
- Houston CM, Hosie AM, Smart TG. 2008. Distinct regula tion of beta2 and beta3 subunit-containing cerebellar syn aptic GABAA receptors by calcium/calmodulin-dependent protein kinase II. J Neurosci 28:7574–7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, Waldeck R, Faber DS, Smith SS. 2003. Neuroste roid effects on GABAergic synaptic plasticity in hippo campus. J Neurophysiol 89:1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Hernandez CC, Hu N, Macdonald RL. 2014. Three epilepsy-associated GABRG2 missense mutations at the gamma+/beta-interface disrupt GABAA receptor assembly and trafficking by similar mechanisms but to different extents. Neurobiol Dis 68:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki R, Shin HW, Mitsuhashi H, Nakayama K. 2008. Redundant roles of BIG2 and BIG1, guanine-nucleotide exchange factors for ADP-ribosylation factors in mem brane traffic between the trans-Golgi network and endo-somes. Mol Biol Cell 19:2650–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. 2011. The expanding social net work of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron 70: 178–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. 2005. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci 25:10469–10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Michels G, Silayeva L, Haydon J, Succol F, Moss SJ. 2012. Benzodiazepine treatment induces subtype-specific changes in GABA(A) receptor traffick ing and decreases synaptic inhibition. Proc Natl Acad Sci US A 109:18595–18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. 2008. GABAA receptor traf ficking and its role in the dynamic modulation of neuro nal inhibition. Nat Rev Neurosci 9:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Wan Q, Vithlani M, Saliba RS, Succol F, Pangalos MN, Moss SJ. 2009. GABA(A) receptor mem brane trafficking regulates spine maturity. Proc Natl Acad Sci U S A 106:12500–12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska M, Siucinska E, Cybulska-Klosowicz A, Pyza E, Furness DN, Kossut M, Glazewski S. 2010. Rapid, learning-induced inhibitory synaptogenesis in murine barrel field. JNeurosci 30:1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Chiou TT, Serwanski DR, Miralles CP, Pinal N, De Blas AL. 2014. Ring finger protein 34 (RNF34) interacts with and promotes gamma-aminobutyric acid type-A receptor degradation via ubiquitination of the gamma2 subunit. J Biol Chem 289:29420–29436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Rajasekaran K, Hawk KM, Brar J, Ross BM, Tran CA, Chester SJ, et al. 2015. Phosphatase inhibition pre vents the activity-dependent trafficking of GABAA receptors during status epilepticus in the young animal. Epilepsia 56:l355–l365. [DOI] [PubMed] [Google Scholar]
- Joshi S, Rajasekaran K, Williamson J, Kapur J. 2017. Neu rosteroid-sensitive delta-GABAA receptors: A role in epileptogenesis?. Epilepsia 58:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. 2004. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci 24:522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, Tretter V, Walker J, Brandon NJ, Moss SJ. 2010. Fyn kinase contributes to tyrosine phosphorylation of the GABA(A) receptor gamma2 subunit. Mol Cell Neurosci 44:l29–l34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. 2014. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 15:637–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbouneh H, Schlicksupp A, Kirsch J, Kuhse J. 2014. Cyclin-dependent kinase 5 is involved in the phosphory lation of gephyrin and clustering of GABAA receptors at inhibitory synapses of hippocampal neurons. PLoS One 9:e104256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Dohmae N, Hirokawa N. 2004. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron 43:513–525. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Pappas C, Tajiri N, Borlongan CV. 2016. Oxy tocin modulates GABAAR subunits to confer neuropro tection in stroke in vitro. Sci Rep 6:35659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Jang IS, Yamaguchi T, Nagahama H, Yoshimura K, Hidaka K, Matsuda M, et al. 2002. Role of the PLC-related, catalytically inactive protein pl30 in GABA(A) receptor function. Embo J 21:1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Yasunaga A, Mizoguchi Y, Kuratani A, Kittler JT, Jovanovic JN, Takenaka K, et al. 2006. Modu lation of GABA(A) receptor phosphorylation and mem brane trafficking by phospholipase C-related inactive protein/protein phosphatase l and 2A signaling complex underlying brain-derived neurotrophic factor-dependent regulation of GABAergic inhibition. J Biol Chem 281: 22180–22189. [DOI] [PubMed] [Google Scholar]
- Kang Y, Ge Y, Cassidy RM, Lam V, Luo L, Moon K-M, Lewis R, et al. 2014. A combined transgenic proteomic analysis and regulated trafficking of neuroligin-2. J Biol Chem 289:29350–29364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Kano M, Fukunaga K, Konnerth A. 1996. Ca(21)-induced rebound potentiation of gamma-aminobutyric acid-mediated currents requires activation of Ca21/calmodulin-dependent kinase II. Proc Natl Acad Sci USA 93:1335l–13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi SY, Hirano T. 2007. Sustained structural change of GABA(A) receptor-associated protein under lies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. J Neurosci 27:6788–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Cohen BM, Bang JY, Yang M, Renshaw PF. 2000. Assessment of GABA concentration in human brain using two-dimensional proton magnetic resonance spec troscopy. Psychiatry Res 100:169–178. [DOI] [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B. 2004. The gamma2 subu nit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci 24:588l–589l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerti-Szigeti K, Nusser Z, Eyre MD. 2014. Synaptic GABAA receptor clustering without the gamma2 subunit. 34:10219–10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia A, Ribeiro F, Nelson R, Gavrilovici C, Ferguson SS, Poulter MO. 2011. Kindling alters neurosteroid-induced modulation of phasic and tonic GABAA receptormediated currents: role of phosphorylation. J Neurochem 116:l043–l056. [DOI] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. 2002. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) recep tors clustered at neocortical synapses. J Neurosci 22: l328–l337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick CL, Murakami S, Feng M, Wu X, Lal R, Chen G, Du K, Luscher B. 2016. Dissociation of golgi-associated DHHC-type zinc finger protein (GODZ)- and sertoli cell gene with a zinc finger domain-β (SERZ-β)- mediated palmitoylation by loss of function analyses in knockout mice. J Biol Chem 291:2737l–27386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J, Feighery E, et al. 2012. Interplay between DISCl and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell 148:1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Arancibia-Carcamo IL, Moss SJ. 2004. Associa tion of GRIPl with a GABA(A) receptor associated pro tein suggests a role for GRIPl at inhibitory synapses. Biochem Pharmacol 68:l649–l654. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, et al. 2005. Phos-pho-dependent binding of the clathrin AP2 adaptor com plex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci U S A 102:14871–14876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Kukhtina V, Vahedi-Faridi A, Gu Z, Tretter V, Smith KR, et al. 2008. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 com plex to a YECL motif in the GABAA receptor gamma2 subunit. Proc Natl Acad Sci USA 105:3616–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. 2000. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci 20:7972–7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. 2004. Huntingtin-associated protein l regulates inhibitory synaptic transmission by modulat ing gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci U S A 101:12736–12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M. 2005. Postsynaptic scaffold proteins at non-synaptic sites. EMBO Rep 6:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Betz H. 2000. Clustering of inhibitory neuro transmitter receptors at developing postsynaptic sites: the membrane activation model. Trends Neurosci 23:429–435. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Gasnier B, Feng G, Sanes JR, Betz H. 2001. Gephyrin-independent clustering of post-synaptic GABA(A) receptor subtypes. Mol Cell Neurosci 17:973–982. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. 1999. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci 19: 9289–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. 2007. A local reduction in cortical GABAer-gic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci 27:7234–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk S, Winkelmann A, Smolinsky B, Forstera B, Neundorf I, Schwarz G, Meier JC. 2013. Direct binding of GABAA receptor beta2 and beta3 subunits to gephyrin. Eur J Neurosci 37:544–554. [DOI] [PubMed] [Google Scholar]
- Kozlov AS, Angulo MC, Audinat E, Charpak S. 2006. Target cell-specific modulation of neuronal activity by astro cytes. Proc Natl Acad Sci U S A 103:10058–10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmannova K, Hines RM, Revilla-Sanchez R, Terunuma M, Tretter V, Jurd R, Kelz MB, et al. 2013. Enhanced tonic inhibition influences the hypnotic and amnestic actions of the intravenous anesthetics etomidate and propofol. J Neurosci 33:7264–7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M, Metzger J, Trinks S, Chugh D, Harvey RJ, Harvey K, Volkmer H. 2011. The cell adhesion molecule neurofascin stabilizes axo-axonic GABAergic terminals at the axon initial segment. J Biol Chem 286:24385–24393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Moss SJ, Smart TG. 1996. Homomeric beta 1 gamma-aminobutyric acid A receptor-ion channels: eval uation of pharmacological and physiological properties. Mol Pharmacol 49:494–504. [PubMed] [Google Scholar]
- Krueger DD, Tuffy LP, Papadopoulos T, Brose N. 2012. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr Opin Neurobiol 22:412–422. [DOI] [PubMed] [Google Scholar]
- Kuhse J, Kalbouneh H, Schlicksupp A, Mukusch S, Nawrotzki R, Kirsch J. 2012. Phosphorylation of gephyrin in hippocampal neurons by cyclin-dependent kinase CDK5 at Ser-270 is dependent on collybistin. J Biol Chem 287:30952–30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Suryanarayanan A, Boyd KN, Comerford CE, Lai MA, Ren Q, Morrow AL. 2010. Ethanol reduces GABAA alpha1 subunit receptor surface expression by a protein kinase Cgamma-dependent mechanism in cultured cerebral cortical neurons. Mol Pharmacol 77:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotani T, Yamada K, Yoshimura Y, Crair MC, Komatsu Y. 2008. State-dependent bidirectional modification of somatic inhibition in neocortical pyramidal cells. Neuron 57:905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuver A, Smith SS. 2016. Flumazenil decreases surface expression of alpha4beta2delta GABAA receptors by increasing the rate of receptor internalization. Brain Res Bull 120:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte D, Thies E, Kneussel M. 2014. The kinesin KIF21B participates in the cell surface delivery of gamma2 subunit-containing GABAA receptors. Eur J Cell Biol 93:338–346. [DOI] [PubMed] [Google Scholar]
- Lachance-Touchette P, Brown P, Meloche C, Kinirons P, Lapointe L, Lacasse H, Lortie A, et al. 2011. Novel alpha1 and gamma2 GABAA receptor subunit mutations in families with idiopathic generalized epilepsy. Eur J Neurosci 34:237–249. [DOI] [PubMed] [Google Scholar]
- Lee H, Chen CX, Liu YJ, Aizenman E, Kandler K. 2005. KCC2 expression in immature rat cortical neurons is suf ficient to switch the polarity of GABA responses. Eur J Neurosci 21:2593–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Deeb TZ, Walker JA, Davies PA, Moss SJ. 2011. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat Neurosci 14:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V, Maguire J. 2014. The impact of tonic GABA(A) receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front Neural Circuits 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leil TA, Chen ZW, Chang CS, Olsen RW. 2004. GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J Neurosci 24:11429–11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonzino M, Busnelli M, Antonucci F, Verderio C, Mazzanti M, Chini B. 2016. The Timing of the Excitatory-to-Inhibitory GABA Switch Is Regulated by the Oxytocin Receptor via KCC2. Cell Rep [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Grady RM, Henry MD, Campbell KP, Sanes JR, Craig AM. 2002. Dystroglycan is selectively associated with inhibitory GABAergic synapses but is dispensable for their differentiation. J Neurosci 22:4274–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Le Roux N, Eugene E, Poncer JC. 2015. Benzodiaz epine ligands rapidly influence GABAA receptor diffu sion and clustering at hippocampal inhibitory synapses. Neuropharmacology 88:199–208. [DOI] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. 2004. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippo campal neurons. J Neurosci 24:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Xu W, Hsu YT, Yee AX, Chen L, Sudhof TC. 2015. Conditional neuroligin-2 knockout in adult medial prefron-tal cortex links chronic changes in synaptic inhibition to cognitive impairments. Mol Psychiatry 20:850–859. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. 2009. Block of gamma-aminobutyric acid-A receptor insertion in the amygdala impairs extinction of conditioned fear. Biol Psychiatry 66:665–673. [DOI] [PubMed] [Google Scholar]
- Lin WC, Davenport CM, Mourot A, Vytla D, Smith CM, Medeiros KA, Chambers JJ, et al. 2014. Engineering a light-regulated GABAA receptor for optical control of neural inhibition. ACS Chem Biol 9:1414–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WC, Tsai MC, Davenport CM, Smith CM, Veit J, Wilson NM, Adesnik H, et al. 2015. A Comprehensive Optogenetic Pharmacology Toolkit for In Vivo Control of GABA(A) Receptors and Synaptic Inhibition. Neuron 88:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsly C, Gonzalez-Islas C, Wenner P. 2014. Activity blockade and GABAA receptor blockade produce synaptic scaling through chloride accumulation in embryonic spinal motoneurons and interneurons. PLoS One 9:e94559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QY, Schaffner AE, Chang YH, Maric D, Barker JL. 2000. Persistent activation of GABA(A) receptor∕Cl(−) channels by astrocyte-derived GABA in cultured embryonic rat hippocampal neurons. J Neurophysiol 84:1392–1403. [DOI] [PubMed] [Google Scholar]
- Liu T, Li H, Hong W, Han W. 2016. Brefeldin A-inhibited guanine nucleotide exchange protein 3 is localized in lysosomes and regulates GABA signaling in hippocampal neurons. JNeurochem 139:748–756. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. 2006. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 7:179–193. [DOI] [PubMed] [Google Scholar]
- Lo FS, Erzurumlu RS, Powell EM. 2016. Insulin-indepen dent GABAA receptor-mediated response in the barrel cortex of mice with impaired met activity. J Neurosci 36: 3691–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebrich S, Bahring R, Katsuno T, Tsukita S, Kneussel M. 2006. Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton. Embo J 25:987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KH, Stawski PS, Draycott AS, Udeshi ND, Lehrman EK, Wilton DK, Svinkina T, et al. 2016. Proteomic analy sis of unbounded cellular compartments: synaptic clefts. Cell 166:1295–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Puskarjov M, Kaila K. 2013. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology 69:62–74. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Griffiths JL, Dunn SM, Martin IL. 2005. Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience 131:397–405. [DOI] [PubMed] [Google Scholar]
- Lozano R, Martinez-Cerdeno V, Hagerman RJ. 2015. Advances in the understanding of the gabaergic neurobi ology of FMR1 expanded alleles leading to targeted treat ments for fragile X spectrum disorder. Curr Pharm Des 21:4972–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER, Roder J. 2000. Calci-neurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron 26:197–205. [DOI] [PubMed] [Google Scholar]
- Lu Z, Wang Y, Chen F, Tong H, Reddy MV, Luo L, Seshadrinathan S, et al. 2014. Calsyntenin-3 molecular architecture and interaction with neurexin 1alpha. J Biol Chem 289:34530–34542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR. 2008. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal 1:ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. 2011a. GABAA recep tor trafficking-mediated plasticity of inhibitory synapses. Neuron 70:385–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. 2011b. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 16:383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. 2006. Potentiation of cortical inhibition by visual deprivation. Nature 443:81–84. [DOI] [PubMed] [Google Scholar]
- Maguire J 2014. Stress-induced plasticity of GABAergic inhibition. Front Cell Neurosci 8:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. 2007. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovar ian cycle and stress. J Neurosci 27:2155–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. 2008. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron 59:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. 2009. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology 34 Suppl 1:S84–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. 2005. Ovar ian cycle-linked changes in GABA(A) receptors mediat ing tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8:797–804. [DOI] [PubMed] [Google Scholar]
- Maric HM, Hausrat TJ, Neubert F, Dalby NO, Doose S, Sauer M, Kneussel M, Stromgaard K. 2017. Gephyrin-binding peptides visualize postsynaptic sites and modu late neurotransmission. Nat Chem Biol 13:153–160. [DOI] [PubMed] [Google Scholar]
- Markwardt S, Overstreet-Wadiche L. 2008. GABAergic signalling to adult-generated neurons. J Physiol 586: 3745–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro GS, Gao S, Olechwier AM, Hung WL, Liu M, Ozkan E, Zhen M, et al. 2015. MADD-4/punctin and neurexin organize C. elegans GABAergic postsynapses through neuroligin. Neuron 86:1420–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden KC, Beattie JB, Friedenthal J, Carroll RC. 2007. NMDA Receptor Activation Potentiates Inhibitory Trans mission through GABA Receptor-Associated Protein-Dependent Exocytosis of GABA(A) Receptors. J Neuro sci 27:14326–14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden KC, Shemesh A, Bayer KU, Carroll RC. 2010. Selective translocation of Ca21/calmodulin protein kinase IIalpha (CaMKIIalpha) to inhibitory synapses. Proc Natl Acad Sci U S A 107:20559–20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. 1998. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci 1:23–28. [DOI] [PubMed] [Google Scholar]
- Mejias R, Adamczyk A, Anggono V, Niranjan T, Thomas GM, Sharma K, Skinner C, et al. 2011. Gain-of-function glutamate receptor interacting protein 1 variants alter GluA2 recycling and surface distribution in patients with autism. Proc Natl Acad Sci U S A 108:4920–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele M, Aspromonte MC, Duarte CB. 2017. Downregula-tion of GABAA receptor recycling mediated by HAP1 contributes to neuronal death in in vitro brain ischemia. Mol Neurobiol 54:45–57. [DOI] [PubMed] [Google Scholar]
- Mele M, Ribeiro L, Inacio AR, Wieloch T, Duarte CB. 2014. GABA(A) receptor dephosphorylation followed by internalization is coupled to neuronal death in in vitro ischemia. Neurobiol Dis 65:220–232. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. 1995. Neonatal sensory depriva tion induces selective changes in the quantitative distribution of GABA-immunoreactive neurons in the rat barrel field cortex. J Comp Neurol 361:574–584. [DOI] [PubMed] [Google Scholar]
- Mielke JG, Wang YT. 2005. Insulin exerts neuroprotection by counteracting the decrease in cell-surface GABA receptors following oxygen-glucose deprivation in cul tured cortical neurons. J Neurochem 92:103–113. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, et al. 1999. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A 96:12905–12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PS, Aricescu AR. 2014. Crystal structure of a human GABAA receptor. Nature 512:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minier F, Sigel E. 2004. Techniques: Use of concatenated subunits for the study of ligand-gated ion channels. Trends Pharmacol Sci 25:499–503. [DOI] [PubMed] [Google Scholar]
- Mircsof D, Langouet M, Rio M, Moutton S, Siquier-Pernet K, Bole-Feysot C, Cagnard N, et al. 2015. Mutations in NONO lead to syndromic intellectual disability and inhibitory synaptic defects. Nat Neurosci 18:1731–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami A, Kanematsu T, Ishibashi H, Yamaguchi T, Tanida I, Takenaka K, Nakayama KI, et al. 2007. Phospholipase C-related inactive protein is involved in trafficking of gamma2 subunit-containing GABA(A) receptors to the cell surface. J Neurosci 27:1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modgil A, Parakala ML, Ackley MA, Doherty JJ, Moss SJ, Davies PA. 2017. Endogenous and synthetic neuroactive steroids evoke sustained increases in the efficacy of GABAergic inhibition via a protein kinase C-dependent mechanism. Neuropharmacology 113:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H 2012. The GABA system in anxiety and depres sion and its therapeutic potential. Neuropharmacology 62:42–53. [DOI] [PubMed] [Google Scholar]
- Möller AT, Bäckström T, Nyberg S, Sondergaard HP, Helstrom L. 2016. Women with PTSD have a changed sensitivity to GABA-A receptor active substances. Psy chopharmacology 233:2025–2033. [DOI] [PubMed] [Google Scholar]
- Morishita W, Sastry BR. 1996. Postsynaptic mechanisms underlying long-term depression of GABAergic transmis sion in neurons of the deep cerebellar nuclei. J Neurophysiol 76:59–68. [DOI] [PubMed] [Google Scholar]
- Morosawa S, Iritani S, Fujishiro H, Sekiguchi H, Torii Y, Habuchi C, Kuroda K, et al. 2017. Neuropeptide Y neuro nal network dysfunction in the frontal lobe of a genetic mouse model of schizophrenia. Neuropeptides [DOI] [PubMed] [Google Scholar]
- Mou L, Dias BG, Gosnell H, Ressler KJ. 2013. Gephyrin plays a key role in BDNF-dependent regulation of amyg dala surface GABAARs. Neuroscience 255:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou L, Heldt SA, Ressler KJ. 2011. Rapid brain-derived neurotrophic factor-dependent sequestration of amygdala and hippocampal GABA(A) receptors via different tyro sine receptor kinase B-mediated phosphorylation path ways. Neuroscience 176:72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir J, Arancibia-Carcamo IL, MacAskill AF, Smith KR, Griffin LD, Kittler JT. 2010. NMDA receptors regulate GABAA receptor lateral mobility and cluster ing at inhibitory synapses through serine 327 on the gamma2 subunit. Proc Natl Acad Sci USA 107: 16679–16684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir J, Kittler JT. 2014. Plasticity of GABAA receptor dif fusion dynamics at the axon initial segment. Front Cell Neurosci 8:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, et al. 2011. The resi dence time of GABAARs at inhibitory synapses is deter mined by direct binding of the receptor alpha1 subunit to gephyrin. J Neurosci 31:14677–14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CT, Mefford HC. 2015. Advancing epilepsy genetics in the genomic era. Genome Med 7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Yin X, Takei Y, Seog DH, Homma N, Hirokawa N. 2012. Molecular motor KIF5A is essential for GABA(A) receptor transport, and KIF5A deletion causes epilepsy. Neuron 76:945–961. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Darnieder LM, Deeb TZ, Moss SJ. 2015. Regulation of GABAARs by phosphorylation. Adv Pharmacol 72:97–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Morrow DH, Modgil A, Huyghe D, Deeb TZ, Lumb MJ, Davies PA, et al. 2016. Proteomic characteriza tion of inhibitory synapses using a novel pHluorin-tagged GABAAR alpha2 subunit knock-in mouse. J Biol Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nani F, Bright DP, Revilla-Sanchez R, Tretter V, Moss SJ, Smart TG. 2013. Tyrosine phosphorylation of GABAA receptor gamma2-subunit regulates tonic and phasic inhi bition in the thalamus. J Neurosci 33:12718–12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor DE, Liu H, Wasterlain CG. 2005. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 25:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss P 2015. Anxiety disorders and GABA neurotransmis sion: a disturbance of modulation. Neuropsychiatric Dis ease and Treatment 11:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Hajos N, Somogyi P, Mody I. 1998. Increased number of synaptic GABA(A) receptors underlies poten tiation at hippocampal inhibitory synapses. Nature 395: 172–177. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. 1996. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A 93:11939–11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan GA, Kneussel M, Elazar Z, Betz H. 2005. GABARAP is not essential for GABA receptor targeting to the synapse. Eur J Neurosci 22:2644–2648. [DOI] [PubMed] [Google Scholar]
- Oh WC, Lutzu S, Castillo PE, Kwon HB. 2016. De novo synaptogenesis induced by GABA in the developing mouse cortex. Science 353:1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. 2009. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461:1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. 2009. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuro pharmacology 56:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Sastry BR. 2000. Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cere bellar nuclei. J Neurophysiol 84:1414–1421. [DOI] [PubMed] [Google Scholar]
- Panzanelli P, Früuh S, Fritschy J-M. 2017. Differential role of GABAA receptors and neuroligin 2 for perisomatic GABAergic synapse formation in the hippocampus. Brain Structure and Function [DOI] [PubMed] [Google Scholar]
- Papadopoulos T, Korte M, Eulenburg V, Kubota H, Retiounskaia M, Harvey RJ, Harvey K, et al. 2007. Impaired GABAergic transmission and altered hippocam pal synaptic plasticity in collybistin-deficient mice. Embo j 26:3888–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos T, Rhee HJ, Subramanian D, Paraskevopoulou F, Mueller R, Schultz C, Brose N, et al. 2017. Endosomal phosphatidylinositol 3-phosphate pro motes gephyrin clustering and GABAergic neurotrans mission at inhibitory postsynapses. J Biol Chem 292: 1160–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson AL, Sanchez C. 2015. Altered y-aminobutyric acid neurotransmission in major depressive disorder: a critical review of the supporting evidence and the influence of serotonergic antidepressants. Drug Design, Development and Therapy 9:603–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchietti F, Vascon S, Nieus T, Rosillo C, Das S, Tyagarajan S, Diaspro A, et al. 2017. Nanoscale molecu lar reorganization of the inhibitory postsynaptic density is a determinant of GABAergic synaptic potentiation. The Journal of Neuroscience [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Barberis A. 2014. Diffusion dynamics of syn aptic molecules during inhibitory postsynaptic plasticity. Front Cell Neurosci 8:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Ravasenga T, Hausrat TJ, Iurilli G, Olcese U, Racine V, Sibarita J-B, et al. 2014. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat Commun 5: 10.1038/ncomms4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettem KL, Yokomaku D, Luo L, Linhoff MW, Prasad T, Connor SA, Siddiqui TJ, et al. 2013. The specific alpha-neurexin interactor calsyntenin-3 promotes excitatory and inhibitory synapse development. Neuron 80:113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, et al. 2012. Biological studies of posttraumatic stress disorder. Nat Rev Neurosci 13:769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta K, Lefebvre O, Martin NC, Smagowicz WJ, Stanford DR, Ellis SR, Hopper AK, et al. 2001. Maf1p, a negative effector of RNA polymerase III in Saccharomyces cerevi-siae. Mol Cell Biol 21:5031–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcher C, Hatchett C, Longbottom RE, McAinch K, Sihra TS, Moss SJ, Thomson AM, et al. 2011. Positive feed back regulation between gamma-aminobutyric acid type A (GABA(A)) receptor signaling and brain-derived neu rotrophic factor (BDNF) release in developing neurons. J Biol Chem 286:21667–21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, et al. 2009. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron 63: 628–642. [DOI] [PubMed] [Google Scholar]
- Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy J-M, Vogt KE. 2006. Specific Subtypes of GABA A receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol 96: 846–857. [DOI] [PubMed] [Google Scholar]
- Pribiag H, Peng H, Shah WA, Stellwagen D, Carbonetto S. 2014. Dystroglycan mediates homeostatic synaptic plas ticity at GABAergic synapses. Proc Natl Acad Sci USA 111:6810–6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribiag H, Stellwagen D. 2013. TNF-alpha downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABA(A) receptors. J Neurosci 33:15879–15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, Chou WH, et al. 2007. Protein kinase C epsilon regulates gamma-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phos phorylation of gamma2 subunits. J Biol Chem 282: 33052–33063. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, et al. 2006. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry 60:704–713. [DOI] [PubMed] [Google Scholar]
- Rewal M, Jurd R, Gill TM, He DY, Ron D, Janak PH. 2009. Alpha4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J Neurosci 29:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Xu B, Reichardt LF. 2002. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci 5:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffault B, Kourdougli N, Dumon C, Ferrand N, Buhler E, Schaller F, Chambon C, Rivera C, Gaiarsa JL, Porcher C. 2016. Pro-brain-derived neurotrophic factor (proBDNF)-mediated p75NTR activation promotes depolarizing actions of GABA and increases susceptibility to epileptic seizures. Cereb Cortex [AQ] [DOI] [PubMed] [Google Scholar]
- Riffault B, Medina I, Dumon C. 2014. Pro-brain-derived neurotrophic factor inhibits GABAergic neurotransmis sion by activating endocytosis and repression of GABAA receptors. 34:13516–13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. 2006. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) con trols the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. J Biol Chem 281:29431–29435. [DOI] [PubMed] [Google Scholar]
- Roberts DS, Raol YH, Bandyopadhyay S, Lund IV, Budreck EC, Passini MA, Wolfe JH, et al. 2005. Egr3 stimulation of GABRA4 promoter activity as a mecha nism for seizure-induced up-regulation of GABA(A) receptor alpha4 subunit expression. Proc Natl Acad Sci U S A 102:11894–11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CE, Ratai EM, Kanwisher N. 2016. Reduced GABAergic action in the autistic brain. Curr Biol 26:80–85. [DOI] [PubMed] [Google Scholar]
- Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, et al. Rupprecht R. 1998. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry 155:910–913. [DOI] [PubMed] [Google Scholar]
- Rowland AM, Richmond JE, Olsen JG, Hall DH, Bamber BA. 2006. Presynaptic terminals independently regulate synaptic clustering and autophagy of GABAA receptors in Caenorhabditis elegans. J Neurosci 26:1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha R, Knapp S, Chakraborty D, Horovitz O, Albrecht A, Kriebel M, Kaphzan H, Ehrlich I, Volkmer H, Richter-Levin G. 2017. GABAergic synapses at the axon initial segment of basolateral amygdala projection neurons mod ulate fear extinction. Neuropsychopharmacology 42:473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiepour L, Fuchs C, Patrizi A, Sassoe-Pognetto M, Harvey RJ, Harvey K. 2010. Complex role of collybistin and gephyrin in GABAA receptor clustering. J Biol Chem 285:29623–29631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Taniguchi Y, Rannals MD, Merfeld EB, Ballinger MD, Koga M, Ohtani Y, et al. 2016. Early postnatal GABAA receptor modulation reverses deficits in neuro nal maturation in a conditional neurodevelopmental mouse model of DISC1. Mol Psychiatry 21:1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiyed T, Paarmann I, Schmitt B, Haeger S, Sola M, Schmalzing G, Weissenhorn W, et al. 2007. Molecular basis of gephyrin clustering at inhibitory synapses: role of G- and E-domain interactions. J Biol Chem 282:5625–5632. [DOI] [PubMed] [Google Scholar]
- Saliba RS, Gu Z, Yan Z, Moss SJ. 2009. Blocking L-type voltage-gated Ca21 channels with dihydropyridines reduces gamma-aminobutyric acid type A receptor expression and synaptic inhibition. J Biol Chem 284: 32544–32550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba RS, Kretschmannova K, Moss SJ. 2012. Activity-dependent phosphorylation of GABAA receptors regu lates receptor insertion and tonic current. Embo J 31: 2937–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba RS, Michels G, Jacob TC, Pangalos MN, Moss SJ. 2007. Activity-dependent ubiquitination of GABA(A) receptors regulates their accumulation at synaptic sites. J Neurosci 27:13341–13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba RS, Pangalos M, Moss SJ. 2008. The ubiquitin-like protein Plic-1 enhances the membrane insertion of GABAA receptors by increasing their stability within the endoplasmic reticulum. J Biol Chem 283:18538–18544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, et al. 2009. Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci 29:1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarto-Jackson I, Milenkovic I, Smalla KH, Gundelfinger ED, Kaehne T, Herrera-Molina R, Thomas S, et al. 2012. The cell adhesion molecule neuroplastin-65 is a novel interaction partner of gamma-aminobutyric acid type A receptors. J Biol Chem 287:14201–14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. 1996. Properties of putative cerebellar gamma-aminobutyric acid A receptor iso forms. Mol Pharmacol 49:567–579. [PubMed] [Google Scholar]
- Scharfman HE, Brooks-Kayal AR. 2014. Is plasticity of gabaergic mechanisms relavant to epileptogenesis? Adv Exp Med Biol 813:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Luscher B. 2003. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci 24:442–450. [DOI] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. 2006. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol 499:458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaid S, Brandts CH, Serve H, Dikic I. 2013. Ubiquitina tion and selective autophagy. Cell Death Differ 20:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, et al. 2007. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat Neurosci 10:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. 2005. Short-term steroid treatment increases delta GABAA receptor subu nit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology 49:573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. 2010. A critical role for alpha4beta-delta GABAA receptors in shaping learning deficits at puberty in mice. Science 327:1515–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Lindemeyer AK, Gonzalez C, Shao XM, Spigelman I, Olsen RW, Liang J. 2012. Dihydromyricetin as a novel anti-alcohol intoxication medication. J Neurosci 32:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W 2006. Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol 54:231–263. [DOI] [PubMed] [Google Scholar]
- Smith KR, Davenport EC, Wei J, Li X, Pathania M, Vaccaro V, Yan Z, et al. 2014. GIT1 and betaPIX are essential for GABA(A) receptor synaptic stability and inhibitory neurotransmission. Cell Rep 9:298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, McAinsh K, Chen G, Arancibia-Carcamo IL, Haucke V, Yan Z, Moss SJ, et al. 2008. Regulation of inhibitory synaptic transmission by a conserved atypical interaction of GABA(A) receptor beta- and gamma-subunits with the clathrin AP2 adaptor. Neuropharmacology 55:844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Muir J, Rao Y, Browarski M, Gruenig MC, Sheehan DF, Haucke V, et al. 2012. Stabilization of GABA(A) receptors at endocytic zones is mediated by an AP2 binding motif within the GABA(A) receptor beta3 subunit. J Neurosci 32:2485–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Oliver PL, Lumb MJ, Arancibia-Carcamo IL, Revilla-Sanchez R, Brandon NJ, Moss SJ, et al. 2010. Identification and characterisation of a Maf1/Macoco pro tein complex that interacts with GABAA receptors in neurons. Mol Cell Neurosci 44:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soykan T, Schneeberger D, Tria G, Buechner C, Bader N, Svergun D, Tessmer I, et al. 2014. A conformational switch in collybistin determines the differentiation of inhibitory postsynapses. Embo J 33:2113–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht Christian G, Izeddin I, Rodriguez Pamela C, El Beheiry M, Rostaing P, Darzacq X, Dahan M, et al. 2013. Quantitative nanoscopy of inhibitory synapses: counting gephyrin molecules and receptor binding sites. Neuron 79:308–321. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. 2003. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A 100:14439–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson FA. 2014. Revisiting the TRAK family of pro teins as mediators of GABAA receptor trafficking. Neu-rochemRes 39:992–996. [DOI] [PubMed] [Google Scholar]
- Succol F, Fiumelli H, Benfenati F, Cancedda L, Barberis A. 2012. Intracellular chloride concentration influences the GABAA receptor subunit composition. Nat Commun 3:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Erisir A, Kapur J. 2007. Dimin ished neurosteroid sensitivity of synaptic inhibition and altered location of the alpha4 subunit of GABA(A) recep tors in an animal model of epilepsy. J Neurosci 27: l2641–12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Zhang L, Chen G. 2013. An unexpected role of neuroligin-2 in regulating KCC2 and GABA functional switch. Mol Brain 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C, Shard C, Ranieri E, Hynes K, Pham DH, Leach D, Buchanan G, et al. 2015. Mutations of protocadherin l9 in female epilepsy (PCDHl9-FE) lead to allopregnano-lone deficiency. Hum Mol Genet 24:5250–5259. [DOI] [PubMed] [Google Scholar]
- Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. 2010. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature 465:783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra M, Ugurbil K, Gruetter R. 2002. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magn Reson Med 47: 1009–1012. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Jang IS, Ha SH, Kittler JT, Kanematsu T, Jovanovic JN, Nakayama KI, et al. 2004. GABAA recep tor phospho-dependent modulation is regulated by phos-pholipase C-related inactive protein type l, a novel protein phosphatase l anchoring protein. J Neurosci 24: 7074–7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, et al. 2008. Deficits in phosphor ylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci 28:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighilet B, Brezun JM, Sylvie GD, Gaubert C, Lacour M. 2007. New neurons in the vestibular nuclei complex after unilateral vestibular neurectomy in the adult cat. Eur J Neurosci 25:47–58. [DOI] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy J-M, Pangalos MN, Moss SJ. 2008. The clustering of GABAA receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha2 subunits to gephyrin. J Neurosci 28:1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Kerschner B, Milenkovic I, Ramsden SL, Ramerstorfer J, Saiepour L, Maric H-M, et al. 20ll. Molecular basis of the y-aminobutyric acid A receptor a3 subunit interaction with the clustering protein gephyrin. J Biol Chem 286:37702–37711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Mukherjee J, Maric HM, Schindelin H, Sieghart W, Moss SJ. 2012. Gephyrin, the enigmatic organizer at GABAergic synapses. Front Cell Neurosci 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Revilla-Sanchez R, Houston C, Terunuma M, Havekes R, Florian C, Jurd R, et al. 2009. Deficits in spa tial memory correlate with modified (gammaj-aminobu-tyric acid type A receptor tyrosine phosphorylation in the hippocampus. Proc Natl Acad Sci U S A 106:20039–20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A, Choquet D. 2005. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move!. Trends Neurosci 28:133–139. [DOI] [PubMed] [Google Scholar]
- Trousselard M, Lefebvre B, Caillet L, Andruetan Y, de Montleau F, Denis J, Canini F. 2016. Is plasma GABA level a biomarker of Post-Traumatic Stress Disorder (PTSD) severity? A preliminary study. Psychiatry Res 241:273–279. [DOI] [PubMed] [Google Scholar]
- Tseng WC, Jenkins PM, Tanaka M, Mooney R, Bennett V. 2015. Giant ankyrin-G stabilizes somatodendritic GABAer gic synapses through opposing endocytosis of GABAA receptors. Proc Natl Acad Sci U S A 112:1214–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Pinan-Lucarre B, Ji T, Jospin M, Bessereau JL. 2015. C. elegans punctin clusters GABA(A) receptors via neu-roligin binding and UNC-40/DCC recruitment . Neuron 86:1407–1419. [DOI] [PubMed] [Google Scholar]
- Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, et al. 2010. Delivery of GABAARs to synapses is mediated by HAPl-KIF5 and disrupted by mutant huntingtin. Neuron 65:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan SK, Ghosh H, Yevenes GE, Imanishi SY, Zeilhofer HU, Gerrits B, Fritschy JM. 2013. Extracellular signal-regulated kinase and glycogen synthase kinase 3beta regulate gephyrin postsynaptic aggregation and GABAergic synaptic function in a calpain-dependent mechanism. J Biol Chem 288:9634–9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan SK, Ghosh H, Yevenes GE, Nikonenko I, Ebeling C, Schwerdel C, Sidler C, Zeilhofer HU, Gerrits B, Muller D, Fritschy JM. 2011. Regulation of GABAer gic synapse formation and plasticity by GSK3beta-depen-dent phosphorylation of gephyrin. Proc Natl Acad Sci USA 108:379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezu A, Kanak DJ, Bradshaw TWA, Soderblom EJ, Catavero CM, Burette AC, Weinberg RJ, et al. 2016. Identification of an elaborate complex mediating postsyn-aptic inhibition. Science 353:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uji A, Matsuda M, Kukita T, Maeda K, Kanematsu T, Hirata M. 2002. Molecules interacting with PRIP-2, a novel Ins(l,4,5)P3 binding protein type 2: Comparison with PRIP-l. Life Sci 72:443–453. [DOI] [PubMed] [Google Scholar]
- Ulrich D 2015. Amyloid-beta impairs synaptic inhibition via GABA(A) receptor endocytosis. J Neurosci 35:9205–9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, Pramanik G, Ko JS, Song MY, Lee D, Kim H, Park KS, et al. 2014. Calsyntenins function as synapto-genic adhesion molecules in concert with neurexins. Cell Rep 6:1096–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda K, Iritani S, Fujishiro H, Sekiguchi H, Torii Y, Habuchi C, Kuroda K, et al. 2016. Immunohistochemical evaluation of the GABAergic neuronal system in the pre-frontal cortex of a DISCl knockout mouse model of schizophrenia. Synapse 70:508–518. [DOI] [PubMed] [Google Scholar]
- Upadhya R, Lee J, Willis IM. 2002. Maf1 is an essential mediator of diverse signals that repress RNA polymerase III transcription. Mol Cell 10:1489–1494. [DOI] [PubMed] [Google Scholar]
- van der Veen AG, Ploegh HL. 2012. Ubiquitin-like pro teins. Annu Rev Biochem 81:323–357. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, et al. 2006. Neuroli-gins determine synapse maturation and function. Neuron 51:741–754. [DOI] [PubMed] [Google Scholar]
- Vetiska SM, Ahmadian G, Ju W, Liu L, Wymann MP, Wang YT. 2007. GABAA receptor-associated phosphoino-sitide 3-kinase is required for insulin-induced recruitment of postsynaptic GABAA receptors. Neuropharmacology 52:146–155. [DOI] [PubMed] [Google Scholar]
- Vien TN, Modgil A, Abramian AM, Jurd R, Walker J, Brandon NJ, Terunuma M, et al. 2015. Compromising the phosphodependent regulation of the GABAAR beta3 subunit reproduces the core phenotypes of autism spec trum disorders. Proc Natl Acad Sci U S A 112:14805–14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithlani M, Hines RM, Zhong P, Terunuma M, Hines DJ, Revilla-Sanchez R, Jurd R, et al. 2013. The ability of BDNF to modify neurogenesis and depressive-like behaviors is dependent upon phosphorylation of tyrosine residues 365/367 in the GABA(A)-receptor gamma2 sub unit. J Neurosci 33:15567–15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, et al. 1997. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature 388:686–690. [DOI] [PubMed] [Google Scholar]
- Wang CY, Lin HC, Song YP, Hsu YT, Lin SY, Hsu PC, Lin CH, et al. 2015. Protein kinase C-dependent growth-associated protein 43 phosphorylation regulates gephyrin aggregation at developing GABAergic synapses. Mol Cell Biol 35:1712–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Zurek AA, Lecker I, Yu J, Abramian AM, Avramescu S, Davies PA, et al. 2012. Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABAA receptors. Cell Rep 2:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. 1999. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature 397: 69–72. [DOI] [PubMed] [Google Scholar]
- Wang H, Pati S, Pozzo-Miller L, Doering LC. 2015. Tar geted pharmacological treatment of autism spectrum dis orders: fragile X and Rett syndromes. Front Cell Neurosci 9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu S, Haditsch U, Tu W, Cochrane K, Ahmadian G, Tran L, et al. 2003. Interaction of calcineurin and type-A GABA receptor gamma 2 subunits produces long term depression at CA1 inhibitory synapses. J Neurosci 23:826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Eshaq RS, Meshul CK, Moore C, Hood RL, Leidenheimer NJ. 2015. Neuronal gamma-aminobutyric acid (GABA) type A receptors undergo cognate ligand chaperoning in the endoplasmic reticulum by endogenous GABA. Front Cell Neurosci 9:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, et al. 2003. Control of synaptic strength, a novel function of Akt. Neuron 38:915–928. [DOI] [PubMed] [Google Scholar]
- Wang RA, Cheng G, Kolaj M, Randic M. 1995. Alpha-sub-unit of calcium/calmodulin-dependent protein kinase II enhances gamma-aminobutyric acid and inhibitory syn aptic responses of rat neurons in vitro. J Neurophysiol 73: 2099–2106. [DOI] [PubMed] [Google Scholar]
- Wang Y, Du X, Bin R, Yu S, Xia Z, Zheng G, Zhong J, et al. 2017. Corrigendum: genetic variants identified from epilepsy of unknown etiology in chinese children by tar geted exome sequencing. Sci Rep 7:46520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RE, Desesso JM, Hurtt ME, Cappon GD. 2006. Postnatal growth and morphological development of the brain: a species comparison. Birth Defects Res B Dev Reprod Toxicol 77:471–484. [DOI] [PubMed] [Google Scholar]
- Wefelmeyer W, Cattaert D, Burrone J. 2015. Activity-dependent mismatch between axo-axonic synapses and the axon initial segment controls neuronal output. Proc Natl Acad Sci U S A 112:9757–9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Graziane NM, Gu Z, Yan Z. 2015. DISC1 protein regulates gamma-aminobutyric acid, type A (GABAA) receptor trafficking and inhibitory synaptic transmission in cortical neurons. J Biol Chem 290:27680–27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijtenburg SA, Yang S, Fischer BA, Rowland LM. 2015. In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci Biobehav Rev 51:276–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk AI, Sylantyev S, Herd MB, Kersante F, Lambert JJ, Rusakov DA, Linthorst AC, et al. 2013. GABA-independent GABAA receptor openings maintain tonic currents. J Neurosci 33:3905–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. 2002. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci 22: 1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LW, Tae HS, Cromer BA. 2015. Assembly, traffick ing and function of alpha1beta2gamma2 GABAA recep tors are regulated by N-terminal regions, in a subunit-specific manner. JNeurochem 134:819–832. [DOI] [PubMed] [Google Scholar]
- Wong YC, Holzbaur EL. 2014. The regulation of autopha gosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradation. J Neurosci 34:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Moss SJ, Smart TG. 1997. Pharmacologi cal and physiological characterization of murine homo meric beta3 GABA(A) receptors. Eur J Neurosci 9: 2225–2235. [DOI] [PubMed] [Google Scholar]
- Wu C, Sun D. 2015. GABA receptors in brain develop ment, function, and injury. Metab Brain Dis 30:367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wu Z, Ning G, Guo Y, Ali R, Macdonald RL, De Blas AL, et al. 2012. gamma-Aminobutyric acid type A (GABAA) receptor alpha subunits play a direct role in synaptic versus extrasynaptic targeting. J Biol Chem 287: 27417–27430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuchter J, Beuter S, Treindl F, Herrmann T, Zeck G, Templin MF, Volkmer H. 2012. A comprehensive small interfering RNA screen identifies signaling pathways required for gephyrin clustering. J Neurosci 32:14821–14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, et al. 2006. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 3:47–58. [DOI] [PubMed] [Google Scholar]
- Yamasaki T, Hoyos-Ramirez E, Martenson JS, Morimoto-Tomita M, Tomita S. 2017. GARLH Family Proteins Sta bilize GABAA Receptors at Synapses. Neuron 93:1138–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Tomita S. 2012. Defined criteria for auxiliary subu nits of glutamate receptors. J Physiol 590:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Low CM, Moody OA, Jenkins A, Traynelis SF. 2015. Ionotropic GABA and glutamate receptor muta tions and human neurologic diseases. Mol Pharmacol 88: 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Yao J, Norris D, Tran DD, Bram RJ, Chen G, Luscher B. 2008. Calcium-modulating cyclophilin ligand regulates membrane trafficking of postsynaptic GABA(A) receptors. Mol Cell Neurosci 38:277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowska ED, Keist R, Rudolph U, Pearce RA. 2009. GABAA receptor alpha5 subunits contribute to GABAA,slow synaptic inhibition in mouse hippocampus. J Neurophysiol 101:1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Atasoy D, Arac D, Yang X, Fucillo MV, Robison AJ, Ko J, et al. 2010. Neurexins physically and functionally interact with GABA(A) receptors. Neuron 66:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Neubert TA, Jordan BA. 2012. RNA binding pro teins accumulate at the postsynaptic density with synaptic activity. J Neurosci 32:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Neal J, Lian G, Hu J, Lu J, Sheen V. 2013. Fila-min A regulates neuronal migration through brefeldin A-inhibited guanine exchange factor 2-dependent Arf1 acti vation. J Neurosci 33:15735–15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Neal J, Lian G, Shi B, Ferland RJ, Sheen V. 2012. Brefeldin A-inhibited guanine exchange factor 2 regu lates filamin A phosphorylation and neuronal migration. J Neurosci 32:12619–12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznova N, Sedelnikova A, Weiss DS. 2008. alpha1-beta2delta, a silent GABAA receptor: recruitment by tracazolate and neurosteroids. Br J Pharmacol 153: 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Huang Z, Ding L, Deel ME, Arain FM, Murray CR, Patel RS, et al. 2013. Altered cortical GABAA receptor composition, physiology, and endocytosis in a mouse model of a human genetic absence epilepsy syn drome. J Biol Chem 288:21458–21472. [DOI] [PMC free article] [PubMed] [Google Scholar]