Abstract
Aldo‐keto reductase family 1 member B10 (AKR1B10) is a secretory protein overexpressed in hepatocellular carcinoma (HCC). We aimed to evaluate AKR1B10 as a serum marker for detection of HCC. Herein, we conducted a cohort study that consecutively enrolled 1,244 participants from three independent hospitals, including HCC, healthy controls (HCs), benign liver tumors (BLTs), chronic hepatitis B (CHB), and liver cirrhosis (LC). Serum AKR1B10 was tested by time‐resolved fluorescent assays. Data were plotted for receiver operating characteristic (ROC) curve analyses. Alpha‐fetoprotein (AFP) was analyzed for comparison. An exploratory discovery cohort demonstrated that serum AKR1B10 increased in patients with HCC (1,567.3 ± 292.6 pg/mL; n = 69) compared with HCs (85.7 ± 10.9 pg/mL; n = 66; P < 0.0001). A training cohort of 519 participants yielded an optimal diagnostic cutoff of serum AKR1B10 at 267.9 pg/mL. When ROC curve was plotted for HCC versus all controls (HC + BLT + CHB + LC), serum AKR1B10 had diagnostic parameters of the area under the curve (AUC) 0.896, sensitivity 72.7%, and specificity 95.7%, which were better than AFP with AUC 0.816, sensitivity 65.1%, and specificity 88.9%. Impressively, AKR1B10 showed promising diagnostic potential in early‐stage HCC and AFP‐negative HCC. Combination of AKR1B10 with AFP increased diagnostic accuracy for HCC compared with AKR1B10 or AFP alone. A validation cohort of 522 participants confirmed these findings. An independent cohort of 68 patients with HCC who were followed up showed that serum AKR1B10 dramatically decreased 1 day after operation and was nearly back to normal 3 days after operation. Conclusion: AKR1B10 is a potent serum marker for detection of HCC and early‐stage HCC, with better diagnostic performance than AFP.
Abbreviations
- ACHXSM CSU
Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University
- AFP
alpha‐fetoprotein
- AKR1B10
aldo‐keto reductase family 1 member B10
- AUC
area under the curve
- BCLC
Barcelona Clinic Liver Center
- BLT
benign liver tumor
- CHB
chronic hepatitis B
- CT
computed tomography
- HC
healthy control
- HCC
hepatocellular carcinoma
- LC
liver cirrhosis
- MRI
magnetic resonance imaging
- ROC
receiver operating characteristic
Hepatocellular carcinoma (HCC) is a highly lethal malignancy with a 5‐year survival rate of <5% in patients with an unresectable disease.1, 2, 3 Chronic hepatitis B (CHB) or C infection and cirrhosis are the leading risk factors. Approximately 80%‐90% of HCC cases occur in the setting of cirrhosis, and the risk of developing HCC is 15‐20 times higher in hepatitis B–infected persons than in uninfected populations.4, 5 Other risk factors of HCC include obesity, diabetes, aflatoxin, alcohol, and nonalcoholic fatty liver diseases.3
Radical hepatectomy is a prime curative option for HCC at early stage, but the early‐stage HCC is usually asymptomatic, and only 20% of diagnosed HCC cases are surgically resectable.6 A revolutionary improvement in HCC diagnosis, particularly in early diagnosis of HCC, is needed. Current diagnostic tools of HCC include blood tests for liver function and tumor markers, imaging, and biopsies, but none have convincing diagnostic value for screening or early diagnosis of HCC.7 Blood tests for HCC markers are important tools in disease management. Identified HCC markers include alpha‐fetoprotein (AFP), Lens culinaris agglutinin A‐reactive fraction of α‐fetoprotein (AFP‐L3, an isoform of AFP), and des‐gamma‐carboxy prothrombin (DCP). AFP is a serum glycoprotein clinically used as a marker of HCC for more than 50 years, but sensitivity and specificity of AFP as a serum marker for HCC diagnosis are limited. Tumors in organs derived from the same endodermal lining as the hepatic diverticulum may display an increased serum AFP, such as in stomach, pancreatic, and biliary cancers. Pregnancy and nonseminomatous germ‐cell carcinomas also raise serum AFP. In addition, AFP is increased in approximately 20% and 40% of patients with chronic hepatitis and cirrhosis, respectively, and may fluctuate with inflammatory activity; therefore, positive predictive value of AFP is low at 9%‐32%.8, 9 At a cutoff of >400 ng/mL, specificity of AFP for HCC is close to 100%, but sensitivity falls to 45%; if a cutoff point is set at 20 ng/mL, sensitivity rises to 78.9%, but specificity declines to 78.1%.10 AFP at ~10‐200 ng/mL is a clinical dilemma for HCC diagnosis. AFP‐L3 is an isoform of AFP,11 and DCP is an abnormal form of prothrombin12, 13; neither is included in diagnostic criteria or recommended for screening of HCC by the American Association for the Study of Liver Diseases or the European Association for Study of the Liver. Herein, we report a large‐scale multicenter study of aldo‐keto reductase family 1 member B10 (AKR1B10) as a serum marker for detection of HCC.
AKR1B10 is a secretory protein up‐regulated in HCC.14, 15, 16 AKR1B10 can eliminate cytotoxic and carcinogenic α and β‐unsaturated carbonyl compounds17, 18, 19, 20, 21 and regulate de novo fatty acid/lipid synthesis.22, 23, 24 In normal tissues, AKR1B10 is specifically expressed in the colon and small intestine, where it is secreted into the lumen.14, 15 AKR1B10 is overexpressed in HCC, being a potential marker.25, 26, 27 This multicenter study demonstrated that AKR1B10 is a potent serum marker for detection of HCC, including early‐stage HCC and AFP‐negative HCC.
Patients and Methods
Study Design and Participants
This study enrolled a total of 1,244 participants, including HCC, healthy controls (HCs), benign liver tumors (BLTs), CHB, and liver cirrhosis (LC) (Fig. 1). An exploratory discovery cohort consisted of 66 HCs and 69 patients with HCC enrolled between January 2015 and April 2015 at the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University (ACHXSM CSU), Changsha, China. In the training phase, 519 participants comprising 209 HCC, 203 HCs, 57 BLTs, 10 CHB, and 40 LC were recruited at ACHXSM CSU from May 2015 to September 2016. A validation cohort of 522 participants, including 204 HCC, 208 HCs, 50 BLTs, 22 CHB, and 38 LC, was enrolled from multicenters, including ACHXSM CSU; Hunan Provincial People's Hospital at Changsha, China; and the First Affiliated Hospital, Nanhua University School of Medicine at Hengyang, China, from October 2016 to June 2017. An independent cohort of 68 patients with HCC, from whom serums were collected before operation and 1 day and 3 days after operation, was recruited to observe dynamic changes of serum AKR1B10 by surgical removal of primary tumors. This study was approved by the ethics committee of each center and conformed to ethical guidelines of the 1975 Declaration of Helsinki. Informed consent was obtained from participants according to the committees’ guidelines.
Figure 1.
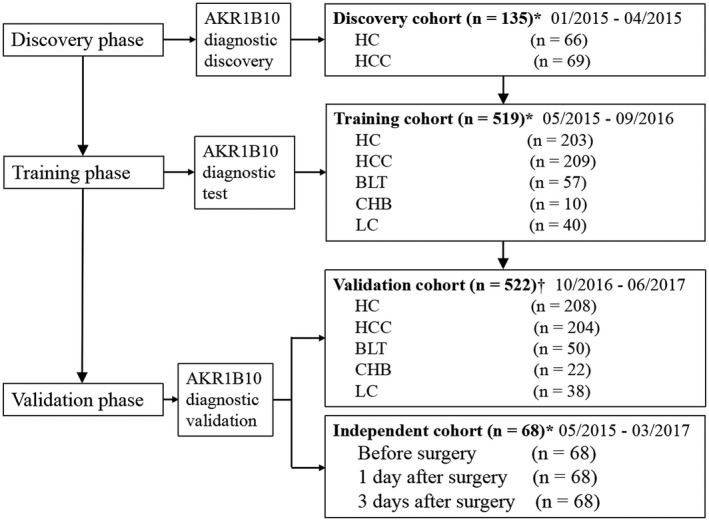
Study flow. A total of 135 participants, including 69 HCC and 66 healthy controls, were enrolled as an exploratory discovery cohort for evaluation of potential of serum AKR1B10 as a diagnostic marker of HCC. A training cohort was then designed to test the diagnostic value of AKR1B10 for discrimination of HCC from healthy controls and other liver diseases, followed by a validation cohort to verify the findings in the training cohort. An independent cohort of 68 surgical patients were recruited and followed up for serum AKR1B10 changes after operation. *Participants from the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University at Changsha, China. †Participants from the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University at Changsha, China; Hunan Provincial People's Hospital at Changsha, China; and the First Affiliated Hospital of University of South China at Hengyang, China.
Eligibility and exclusion criteria of subjects are listed in Supporting Table S1. Briefly, HCC was diagnosed based on ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) and AFP serology and confirmed by histopathology according to guidelines of the American Association for the Study of Liver Diseases.28 HCC at Barcelona Clinic Liver Cancer (BCLC) stages 0 and A was regarded as early‐stage HCC.29 All patients with HCC were newly diagnosed and treatment naive; patients who had undergone systemic or local anticancer therapy before serum collection and patients who had a history of other tumors were excluded. HCs were subjects who visited the hospital for annual physical examination, had normal liver biochemistry, and were serologically negative of hepatitis viruses. All HCs had no history of liver and gastrointestinal diseases and malignancies. BLT includes hepatic hemangioma, focal nodular hyperplasia, and hepatic adenoma diagnosed by ultrasound, CT, or MRI and histopathology. Diagnostic criteria of CHB were hepatitis B surface antigen–positive for more than 6 months and elevation of serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT).30 Diagnosis of LC was based on a history of CHB infection, confirmed by biopsy or two imaging technologies, i.e., hepatic ultrasound with CT or MRI. To limit the possible presence of early‐stage HCC clinically unrecognized in cirrhosis, patients with cirrhosis with <20 years of chronic hepatitis history and in compensated phase of the disease were preferred. Characteristics of the included and excluded subjects in each center are summarized in Supporting Tables S2 and S3. Detailed information of patients with HCC enrolled in both training and validation cohorts are summarized in Supporting Table S4; no statistically significant clinical differences existed in patients with HCC between these two cohorts.
Serum Sample Collections
A standard operating procedure for collections of serum specimens was developed and used by all centers to minimize potential bias. In brief, blood (2 mL) was collected in a plain VACUTAINER tube containing no anticoagulant and allowed to clot at room temperature for 30 minutes, followed by centrifugation at 1,500g for 10 minutes at 4°C to remove clots. Supernatants (serum) were immediately transferred into clean polypropylene tubes (200 µL per tube) using a Pasteur pipette, encoded with a number, and stored or transported at ‐80°C. Serum samples that were hemolyzed, icteric, or lipemic were excluded.
Serum AKR1B10 Measurement
AKR1B10 protein in serum was measured by a time‐resolved fluorescent kit (Light of Life Biotechnology Ltd., China). In brief, samples (100 μL/well) were added in duplicates. Plates were incubated at 37°C for 1 hour, washed five times with phosphate‐buffered saline (PBS) Tween (PBST; 0.05% Tween‐20 in PBS), and then incubated at 37°C for 1 hour with 100 μL/well of biotin‐labeled detection antibody diluted at 1:500 with antibody diluent. After being washed five times with PBST, plates were incubated at 37°C for 30 minutes with 100 μL/well of streptavidin‐Eu3+ conjugates diluted at 1:5,000 with antibody diluent. Specific binding was detected with 100 μL/well of enhancement solution with gentle mix for 5 minutes. Fluorescence was measured at excitation wavelength of 340 nm, emission wavelength of 615 nm, delay time of 0.40 ms, window time of 0.40 ms, and cycling time of 1.0 ms.
Statistical Analysis
Analyses were performed using SPSS 19.0 software (IBM, Armonk, NY) and MedCal 15.2.2 (Ostend, Belgium). Receiver operating characteristic (ROC) curves were applied to evaluate sensitivity, specificity, and respective areas under the curves (AUCs) with 95% confidence interval. The optimal cutoff value for diagnosis was determined by maximizing the sum of sensitivity and specificity and minimizing distance of the cutoff value to the top left corner of the ROC curve. Binary logistic regression was used to estimate function of the combination of AKR1B10 with AFP, and the values of the functions were used as one marker and subjected to ROC analysis. ROC curves were compared statistically as reported.31 AKR1B10 levels between HCC and controls were tested by unpaired t tests. Association between serum AKR1B10 concentrations and clinicopathological characteristics was analyzed with a chi‐square test. AKR1B10 levels in serum collected before and after surgical resection of HCC were analyzed by one‐way repeated measures analysis of variance. A P value of <0.05 is considered statistically significant.
Results
Serum AKR1B10 Concentrations and Diagnostic Potential in HCC
AKR1B10 is a secretory protein overexpressed in HCC, thus being a potential serum marker.14, 15, 25 To test this idea, we enrolled an exploratory discovery cohort of 135 participants, including 66 HCs and 69 patients with HCC. Results showed that AKR1B10 increased in patients with HCC up to 1,567.3 ± 292.6 pg/mL versus 85.7 ± 10.9 pg/mL in HCs (Fig. 2A; P < 0.0001). AFP was measured in parallel as comparison (Fig. 2B). ROC analyses demonstrated the potential of AKR1B10 as a diagnostic marker of HCC, and combination of AKR1B10 with AFP may increase the diagnostic accuracy of HCC (Fig. 2C).
Figure 2.
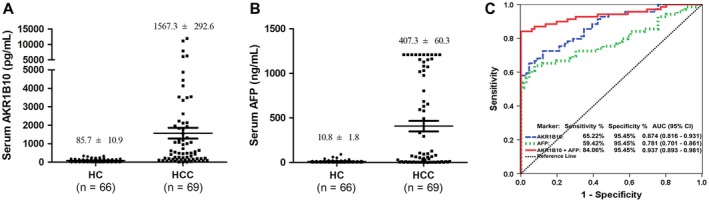
Exploratory data from discovery cohort: AKR1B10 as a potential serum marker for detection of HCC, alone or in combination with AFP. (A) Serum AKR1B10 levels in HCC and HC. (B) Serum AFP levels in HCC and HC. (C) ROC curve analyses for diagnostic potential of AKR1B10, AFP, and combination of AKR1B10 and AFP. Abbreviation: CI, confidence interval.
We then designed a two‐phase study to explore and confirm the diagnostic value of AKR1B10 in HCC. In the training phase, we enrolled 519 subjects, including HCC, HCs, BLTs, CHB, and LC. As shown in Fig. 3A, serum AKR1B10 in patients with HCC increased to 1,769.5 ± 171.0 pg/mL versus 80.6 ± 5.0 pg/mL in HCs (P < 0.0001). Serum AKR1B10 was mildly increased in some patients with LC and CHB (Fig. 3A). Serum AFP increased in patients with HCC and some patients with CHBs and LC (Fig. 3A, right). An independent validation cohort (n = 522) confirmed these training cohort findings, in which serum AKR1B10 was high at 1,546.2 ± 153.2 pg/mL in patients with HCC versus 89.8 ± 5.3 pg/mL in HCs (Fig. 3B; P < 0.0001). In both training and validation cohorts, serum AKR1B10 concentrations displayed a similar distribution (Supporting Table S5). The serum level of AKR1B10 in patients with HCC was associated with AFP, AST, ALT, and tumor size (P < 0.05) but not with tumor number, vascular invasion, and tumor‐node‐metastasis stages (Supporting Table S6). Together, data indicate that serum AKR1B10 is greatly increased in patients with HCC, being a potential diagnostic marker.
Figure 3.
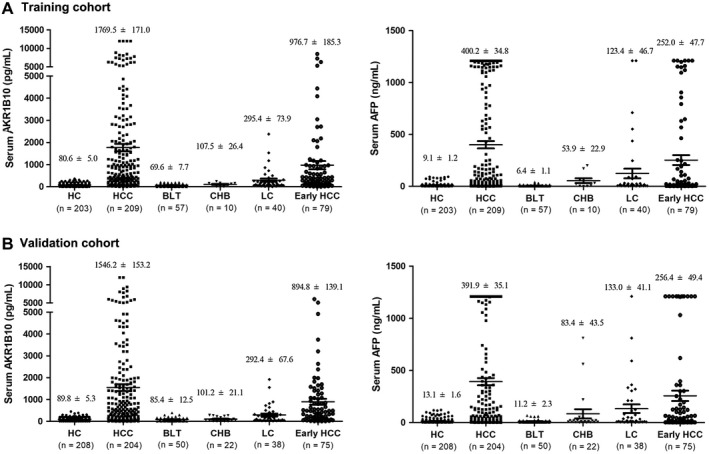
Serum AKR1B10 and AFP levels in training and validation cohorts. (A) Training cohort: AKR1B10 (left); AFP (right). (B) Validation cohort: AKR1B10 (left); AFP (right).
Diagnostic Performance of AKR1B10 in HCC
We then assessed the diagnostic value of serum AKR1B10 in HCC. We plotted ROC curves for AKR1B10 with HCC versus all different control groups in the training cohort. When the ROC curve for AKR1B10 was plotted with HCC versus all controls (HC + BLT + CHB + LC), the optimal diagnostic cutoff value of serum AKR1B10 was at 267.9 pg/mL, which yielded AUC 0.896, sensitivity 72.7%, and specificity 95.7%. The optimal diagnostic cutoff value of AFP was at 20.2 ng/mL, which yielded AUC 0.816, sensitivity 65.1%, and specificity 88.9% (Fig. 4A, left; Table 1; P = 0.0003), suggesting that AKR1B10 has better diagnostic performance. ROC curve data for AKR1B10 in HCC versus high‐risk controls (CHB + LC) are shown in Fig. 4A (middle) and Table 1 (P < 0.0001), and ROC curve data for AKR1B10 in HCC versus HCs, BLTs, CHB, or LC alone are presented in Supporting Figs. S1‐S4 and Supporting Table S7. Details are omitted here. In the LC analysis, we further plotted HCC with or without cirrhosis versus LC (Supporting Fig. S5; Supporting Table S8). All data indicate that AKR1B10 is a preferable serum marker for detection of HCC when compared with AFP.
Figure 4.
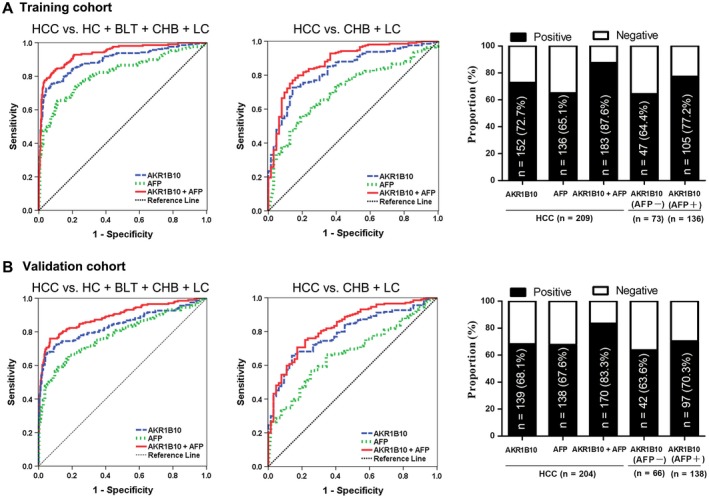
Serum AKR1B10 for detection of HCC. (A) Training cohort: ROC curves for HCC versus all controls (left); ROC curves for HCC versus high‐risk controls (CHB + LC) (middle); positive rates for AKR1B10, AFP, or both in HCC and for AKR1B10 by AFP status (right). (B) Validation cohort: ROC curves for HCC versus all controls (left); ROC curves for HCC versus high‐risk controls (CHB + LC) (middle); positive rates for AKR1B10, AFP, or both in HCC and for AKR1B10 by AFP status (right).
Table 1.
Results of AKR1B10 in Differentiating HCC or Early‐Stage HCC From All Controls (HCs + BLTs + CHB + LC) or High‐Risk Controls (CHB + LC)
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive LR | Negative LR | AUC (95% CI) | P Value | |
|---|---|---|---|---|---|---|---|---|
| Training Cohort | ||||||||
| HCC vs. HC + BLT + CHB + LC | ||||||||
| AKR1B10 | 72.7 | 95.7 | 91.6 | 84.4 | 16.78 | 0.29 | 0.896 (0.867‐0.921) | 0.0003* |
| AFP | 65.1 | 88.9 | 79.1 | 79.7 | 5.84 | 0.39 | 0.816 (0.781‐0.848) | <0.0001† |
| AKR1B10 + AFP | 84.2 | 90.1 | 84.6 | 89.8 | 8.50 | 0.18 | 0.937 (0.913‐0.956) | 0.0001‡ |
| HCC vs. CHB + LC | ||||||||
| AKR1B10 | 72.7 | 85.7 | 94.6 | 48.6 | 5.09 | 0.32 | 0.834 (0.785‐0.876) | <0.0001* |
| AFP | 55.0 | 81.0 | 90.6 | 35.2 | 2.89 | 0.56 | 0.712 (0.654‐0.765) | <0.0001† |
| AKR1B10 + AFP | 77.0 | 85.7 | 94.7 | 52.9 | 5.39 | 0.27 | 0.877 (0.832‐0.913) | 0.0002‡ |
| Early‐stage HCC vs. HC + BLT + CHB + LC | ||||||||
| AKR1B10 | 64.6 | 92.3 | 67.1 | 91.4 | 8.34 | 0.38 | 0.831 (0.790‐0.866) | 0.0102* |
| AFP | 59.5 | 77.7 | 39.5 | 88.7 | 2.67 | 0.52 | 0.719 (0.672‐0.762) | <0.0001† |
| AKR1B10 + AFP | 84.8 | 79.3 | 50.0 | 95.5 | 4.09 | 0.19 | 0.885 (0.850‐0.915) | 0.0090‡ |
| Early‐stage HCC vs. CHB + LC | ||||||||
| AKR1B10 | 60.8 | 85.7 | 84.2 | 63.5 | 4.25 | 0.46 | 0.751 (0.672‐0.820) | 0.0009* |
| AFP | 32.9 | 87.3 | 76.5 | 50.9 | 2.59 | 0.77 | 0.595 (0.510‐0.677) | <0.0001† |
| AKR1B10 + AFP | 62.0 | 87.3 | 86.0 | 64.7 | 4.88 | 0.43 | 0.802 (0.727‐0.864) | 0.0140‡ |
| Validation Cohort | ||||||||
| HCC vs. HC + BLT + CHB + LC | ||||||||
| AKR1B10 | 72.1 | 90.7 | 83.1 | 83.6 | 7.71 | 0.31 | 0.840 (0.806‐0.871) | 0.0375* |
| AFP | 67.6 | 80.7 | 69.0 | 79.8 | 3.51 | 0.40 | 0.789 (0.751‐0.823) | <0.0001† |
| AKR1B10 + AFP | 77.0 | 93.5 | 88.2 | 86.5 | 11.80 | 0.25 | 0.894 (0.864‐0.919) | 0.0001‡ |
| HCC vs. CHB + LC | ||||||||
| AKR1B10 | 72.1 | 90.4 | 82.6 | 83.6 | 7.48 | 0.31 | 0.840 (0.806‐0.871) | <0.0001* |
| AFP | 66.2 | 82.0 | 69.9 | 79.3 | 3.67 | 0.41 | 0.781 (0.743‐0.815) | <0.0001† |
| AKR1B10 + AFP | 76.0 | 93.2 | 87.6 | 86.0 | 11.12 | 0.26 | 0.890 (0.860‐0.916) | 0.0025‡ |
| Early‐stage HCC vs. HC + BLT + CHB + LC | ||||||||
| AKR1B10 | 60.8 | 85.7 | 84.2 | 63.5 | 4.25 | 0.46 | 0.751 (0.672‐0.820) | 0.0462* |
| AFP | 32.9 | 87.3 | 76.5 | 50.9 | 2.59 | 0.77 | 0.595 (0.510‐0.677) | 0.0398† |
| AKR1B10 + AFP | 62.0 | 87.3 | 84.10 | 93.1 | 4.88 | 0.43 | 0.802 (0.727‐0.864) | 0.1111‡ |
| Early‐stage HCC vs. CHB + LC | ||||||||
| AKR1B10 | 61.3 | 85.9 | 83.6 | 65.5 | 4.36 | 0.45 | 0.758 (0.678‐0.827) | <0.0001* |
| AFP | 60.0 | 65.6 | 67.2 | 58.3 | 1.75 | 0.61 | 0.590 (0.503‐0.673) | <0.0001† |
| AKR1B10 + AFP | 61.3 | 85.9 | 83.6 | 65.5 | 4.36 | 0.45 | 0.757 (0.677‐0.826) | 0.6722‡ |
AKR1B10 vs. AFP.
AKR1B10 + AFP vs. AFP.
AKR1B10 + AFP vs. AKR1B10.
Abbreviations: CI, confidence interval; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
We further evaluated the diagnostic value of AKR1B10 in combination with AFP. In 209 patients with HCC in the training cohort, AKR1B10 was positive in 152 (72.7%) cases, and AFP was positive in 136 (65.1%) cases; a total of 183 (87.6%) cases were positive in AKR1B10, AFP, or both (Fig. 4A, right). ROC curve analyses showed that combination of AKR1B10 with AFP improved diagnostic performance for HCC compared with AKR1B10 or AFP alone (Fig. 4A; Table 1; P < 0.05; Supporting Figs. S1‐S5; Supporting Tables S7 and S8).
Using the same cutoff value of AKR1B10 in the training cohort, we analyzed 522 participants in a validation cohort. As shown in Fig. 4B, Table 1, Supporting Figs. S1‐S5, and Supporting Tables S7 and S8, AKR1B10 demonstrated similar diagnostic performance for HCC, and combination of AKR1B10 with AFP increased the diagnostic accuracy. We further plotted ROC curves for AKR1B10 in all participants from three cohorts, and similar diagnostic performance was yielded (Supporting Fig. S6A, Supporting Table S9). Together, our results suggest that AKR1B10 is a potent serum marker for detection of HCC.
Diagnostic Performance of AKR1B10 in Early‐stage HCC
Diagnosis of HCC at early stage is the key for patient survival but has long been a clinical issue. In this study, we further evaluated the potential of AKR1B10 as a serum marker for detection of early‐stage HCC. In the training cohort, 79 patients with HCC were diagnosed at early stage (BCLC stages 0 and A). Serum AKR1B10 was at a lower level in patients with early‐stage HCC than in those with late‐stage HCC (Fig. 3) but diagnostically informative. AKR1B10 was positive in 60.8% of patients with early‐stage HCC versus AFP at 48.1% (Fig. 5A, right). ROC curve analyses indicated that AKR1B10 showed a promising diagnostic value. In ROC curve plotted for AKR1B10 with early‐stage HCC versus all controls (HCs + BLTs + CHB + LC), diagnostic parameters were AUC 0.831, sensitivity 64.6%, and specificity 92.3% versus AFP at AUC 0.719, sensitivity 59.5%, and specificity 77.7% (Fig. 5A, Table 1; P = 0.0102). Similar results were obtained in ROC curve analyses with early‐stage HCC versus high‐risk controls (CHBs + LC) (Fig. 5A, Table 1; P = 0.0009) and early‐stage HCC versus HCs, BLTs, CHB, or LC alone (Supporting Figs. S1‐S4; Supporting Tables S7 and S8). These results were confirmed in the validation cohort with 75 early‐stage HCC (Fig. 5B, Table 1; P < 0.05; Supporting Figs. S1‐S4, Supporting Tables S7 and S8). Further analyses with total early‐stage HCC (n = 154) from all cohorts also gained similar results (Supporting Fig. S6B, Supporting Table S9). Combination of AKR1B10 with AFP also increased the diagnostic accuracy for early‐stage HCC compared with AKR1B10 or AFP alone (Fig. 5, Table 1; P < 0.05; Supporting Figs. S1‐S4, Supporting Tables S7 and S8). Together, our results suggest that serum AKR1B10 is a potent marker for detection of early‐stage HCC with better accuracy than AFP.
Figure 5.
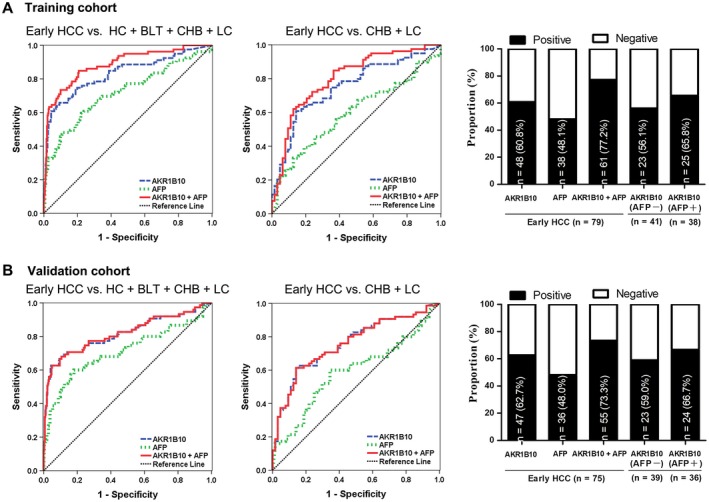
Serum AKR1B10 in detection of early‐stage HCC. (A) Training cohort: ROC curves for early‐stage HCC versus all controls (left); ROC curves for early‐stage HCC versus high‐risk controls (CHB + LC) (middle); positive rates for AKR1B10, AFP, or both in HCC and for AKR1B10 by AFP status (right). (B) Validation cohort: ROC curves for early‐stage HCC versus all controls (left); ROC curves for early‐stage HCC versus high‐risk controls (CHB + LC) (middle); positive rates for AKR1B10, AFP, or both in HCC and for AKR1B10 by AFP status (right).
Serum AKR1B10 in Detection of AFP‐negative HCC
AFP is negative in more than one third of HCC. To effectively detect AFP‐negative HCC, we thus investigated the performance of AKR1B10 in AFP‐negative patients. In the training cohort, 73 cases were AFP negative, in which 47 (64.4%) cases were AKR1B10 positive (Fig. 4A, right). As shown in Supporting Fig. S7 and Table 2, AKR1B10 demonstrated the value in detection of AFP‐positive HCC, but more importantly, AKR1B10 exhibited diagnostic value in AFP‐negative HCC. ROC analysis for AKR1B10 in AFP‐negative HCC versus all controls (HCs + BLTs + CHB + LC) demonstrated a diagnostic value with AUC 0.891, sensitivity 71.2%, and specificity 92.6% (Table 2). These findings were confirmed in the validation cohort with 66 AFP‐negative HCC (Fig. 4B; Supporting Fig. S7; Table 2). Interestingly, in AFP‐negative early‐stage HCC (n = 80), similar diagnostic performance was observed in ROC curve analyses (Supporting Fig. S8; Table 2). These results suggest the privilege of AKR1B10 in detection of AFP‐negative HCC and AFP‐negative early‐stage HCC.
Table 2.
Results of AKR1B10 in Differentiating AFP‐Negative or AFP‐Positive HCC From All Controls (HCs + BLTs + CHB + LC) or High‐Risk Controls (CHB + LC)
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive LR | Negative LR | AUC (95% CI) | |
|---|---|---|---|---|---|---|---|
| Training Cohort | |||||||
| AFP‐negative HCC and early‐stage HCC | |||||||
| HCC vs. HC + BLT + CHB + LC | 71.2 | 92.6 | 68.4 | 93.4 | 9.59 | 0.31 | 0.891 (0.856‐0.920) |
| HCC vs. CHB + LC | 65.8 | 85.7 | 82.8 | 68.4 | 4.6 | 0.40 | 0.819 (0.744‐0.880) |
| Early‐stage HCC vs. HC + BLT + CHB + LC | 63.4 | 90.7 | 46.4 | 95.1 | 6.83 | 0.40 | 0.839 (0.797‐0.875) |
| Early‐stage HCC vs. CHB + LC | 61.0 | 81.0 | 67.6 | 76.1 | 3.20 | 0.48 | 0.754 (0.660‐0.833) |
| AFP‐positive HCC and early‐stage HCC | |||||||
| HCC vs. HC + BLT + CHB + LC | 76.5 | 95.7 | 88.1 | 90.6 | 17.64 | 0.25 | 0.899 (0.868‐0.925) |
| HCC vs. CHB + LC | 76.5 | 85.7 | 92.0 | 62.8 | 5.35 | 0.27 | 0.842 (0.784‐0.890) |
| Early‐stage HCC vs. HC + BLT + CHB + LC | 65.8 | 95.7 | 64.1 | 96.0 | 15.18 | 0.36 | 0.822 (0.778‐0.860) |
| Early‐stage HCC vs. CHB + LC | 65.8 | 85.7 | 73.5 | 80.6 | 4.61 | 0.40 | 0.748 (0.652‐0.829) |
| Validation Cohort | |||||||
| AFP‐negative HCC and early‐stage HCC | |||||||
| HCC vs. HC + BLT + CHB + LC | 68.2 | 91.0 | 60.8 | 93.3 | 7.57 | 0.35 | 0.805 (0.762‐0.843) |
| HCC vs. CHB + LC | 50.0 | 85.9 | 78.6 | 62.5 | 3.56 | 0.58 | 0.725 (0.640‐0.800) |
| Early‐stage HCC vs. HC + BLT + CHB + LC | 68.0 | 91.0 | 37.0 | 97.3 | 7.55 | 0.35 | 0.788 (0.741‐0.830) |
| Early‐stage HCC vs. CHB + LC | 64.0 | 85.9 | 64.0 | 85.9 | 4.55 | 0.42 | 0.742 (0.639‐0.829) |
| AFP‐positive HCC and early‐stage HCC | |||||||
| HCC vs. HC + BLT + CHB + LC | 73.9 | 90.7 | 77.3 | 89.0 | 7.93 | 0.29 | 0.858 (0.823‐0.888) |
| HCC vs. CHB + LC | 66.7 | 85.9 | 91.1 | 54.5 | 4.74 | 0.39 | 0.808 (0.747‐0.860) |
| Early‐stage HCC vs. HC + BLT + CHB + LC | 78.0 | 91.3 | 58.2 | 96.4 | 8.97 | 0.24 | 0.883 (0.846‐0.914) |
| Early‐stage HCC vs. CHB + LC | 62.0 | 84.4 | 75.6 | 74.0 | 3.97 | 0.45 | 0.766 (0.677‐0.840) |
Abbreviations: CI, confidence interval; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
Serum AKR1B10 was Associated with Tumor Size and Disease Stage and Decreased After Surgical Removal of Primary Tumors
Clinicopathological data indicated that AKR1B10 concentrations in serum associated with tumor size (Supporting Table S6; P = 0.020). We thus investigated the effect of tumor size on serum AKR1B10 level in more detail. As shown in Supporting Fig. S9, serum AKR1B10 level was associated with HCC size, particularly in ≥5‐cm HCC. Positive rate of serum AKR1B10 was also higher in patients with ≥5‐cm HCC than in those with ≤5‐cm HCC. Furthermore, we analyzed the serum AKR1B10 levels in patients with HCC at different BCLC stages, and data showed that AKR1B10 concentrations in serum positively associated with disease stages (Supporting Fig. S9).
We further investigated dynamic changes of serum AKR1B10 after surgical removal of primary tumors in liver. Paired serums were procured from 68 patients with HCC right before operation and at 1 day and 3 days after operation. As shown in Fig. 6, AKR1B10 in serum was dramatically decreased within 1 day after surgical removal of HCC and nearly back to normal 3 days after operation, suggesting specificity of AKR1B10 to HCC.
Figure 6.
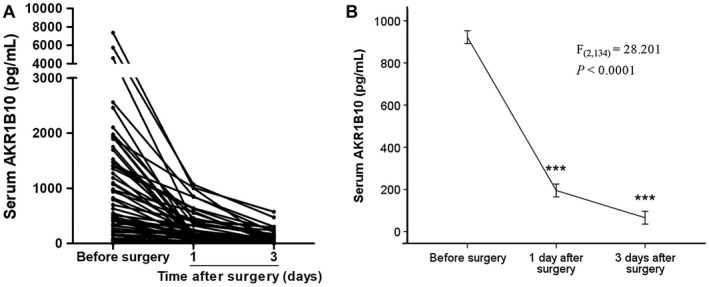
Changes of serum AKR1B10 concentrations by surgical resection of HCC mass. (A) Tendency chart of AKR1B10 concentrations in serum of patients with HCC before surgical resection and at 1 day and 3 days after operation. (B) One‐way repeated measures analysis of variance to evaluate AKR1B10 concentrations in serum collected before and after surgical resection of HCC. AKR1B10 levels decreased significantly after operation (P < 0.0001).
Discussion
A prevalent serum marker would improve clinical management of HCC. Secretory AKR1B10 protein is overexpressed in HCC,14, 25 and studies on surgical specimens and clinicopathological data indicated its potential as a marker for early‐stage HCC,32, 33 prognosis,27, 34, 35 BLT discrimination,26 and stratification of HCC risk in liver hepatitis B virus and hepatitis C virus infections.36, 37, 38, 39 These studies of AKR1B10 in HCC pave the road for this large‐scale multicenter serological study for detection of HCC, including early‐stage HCC and AFP‐negative HCC. This large‐scale study with 1,244 participants, including HCC, HC, BLT, CHB, and LC subjects, validated AKR1B10 as a potent serum marker for detection of HCC with better diagnostic performance than AFP, particularly in early‐stage HCC. AKR1B10 also demonstrated diagnostic value in AFP‐negative HCC, and combination of AKR1B10 with AFP increased the diagnostic accuracy for HCC and early‐stage HCC compared with AKR1B10 or AFP alone.
Pathogenesis of HCC is highly heterogeneous, and multiple etiological factors, such as chronic hepatitis virus infection and cirrhosis, are involved in the development and progression of HCC. Therefore, in addition to BLTs, patients with CHB and cirrhosis were enrolled as high‐risk controls. Because early, undetectable HCC may occur in advanced (decompensated) cirrhosis, special attention was paid to the enrollment of patients with cirrhosis. In this study, we preferred to enroll patients with LC with <20 years of chronic hepatitis history and in compensated phase of the disease. Our data showed that AKR1B10 increased in some patients with CHB and LC, but its level was markedly lower than in HCC. ROC curve analyses, plotted with HCC versus all controls (HCs + BLTs + CHB + LC) or HCC versus high‐risk controls (CHB + LC), all proved the value of AKR1B10 in discrimination of HCC from CHB and/or LC. Specificity of AKR1B10 as a diagnostic marker of HCC was further confirmed by an independent cohort of 68 surgical patients who were followed up, in whom serum AKR1B10 dramatically decreased 1 day after operation and was nearly back to normal 3 days after operation. It was noted that a few cases exhibited dramatic decrease of serum AKR1B10 after operation but still remained at a level higher than normal after 3 days of operation. This may be ascribed to micrometastasis of HCC in liver or distant organ(s) that was not clinically detected or due to expression and secretion of AKR1B10 in cirrhotic nodules in the liver that remained after operation. It would be of significance to follow up these cases. Nevertheless, the dramatic decrease of serum AKR1B10 concentrations after surgical resection of HCC masses indicates its specificity to primary tumors in the liver. This may also imply a potential use of AKR1B10 for evaluation of tumor burden, metastasis, and/or recurrence of HCC. Like literature reports,40, 41 AFP increased in patients with HCC recruited in this study, but AKR1B10 showed much better diagnostic accuracy for HCC than AFP.
Diagnosis of early HCC is a sophisticated clinical issue. In this study, HCC at BCLC stages 0 and A was regarded as early‐stage HCC and assessed for diagnostic performance of AKR1B10 and AFP. Our data demonstrated the value of serum AKR1B10 for detection of early‐stage HCC and the better performance of AKR1B10 than AFP. The high sensitivity and specificity of AKR1B10 in detection of early‐stage HCC may benefit from its low basal level (cutoff at 267.9 pg/mL for AKR1B10 vs. 20.2 ng/mL for AFP), allowing for sensitively detecting a small change of the serum level induced by a small tumor. It is noteworthy that the patient pool of early‐stage HCC was relatively small, and an expanded study may be warranted to determine the value of AKR1B10 in detection of early‐stage HCC.
AKR1B10 was first identified in HCC14 and then characterized as a secretory protein.15, 16 In the past years, clinical histological studies of HCC have identified AKR1B10 as a marker for prognosis and risk stratification of HCC.27, 34, 36, 37 However, this current study is the first large‐scale multicenter evaluation of AKR1B10 as a serum marker for HCC detection. AKR1B10 expression and potential as a biomarker is also reported in other tumors, such as lung, breast, and pancreatic cancers.42, 43, 44 In lung cancer, AKR1B10 is up‐regulated in non–small cell lung carcinoma in smokers,42 and in breast cancer, AKR1B10 promotes cancer growth and progression by promoting lipogenesis and lipid messenger‐mediated signaling cascades and thus is a potential therapeutic target.43, 45
In conclusion, our study demonstrates that serum AKR1B10 can differentiate HCC from HCs, BLTs, and high‐HCC‐risk chronic hepatitis and cirrhosis with high accuracy. AKR1B10 also has privilege in detection of early‐stage HCC and AFP‐negative HCC. In either situation, AKR1B10 showed appreciable sensitivity and specificity compared with AFP. Combination of AKR1B10 with AFP increased the diagnostic accuracy of HCC and early‐stage HCC. In brief, AKR1B10 is a potent serum marker for detection of HCC and early‐stage HCC with better accuracy than AFP.
Supporting information
Acknowledgment
We thank Professor Wuxiang Shi and Dr. Zhimao Bai for their advice on data statistical analysis.
Supported by the National Natural Science Foundation of China (81360309, 81472465, 81572738, 81670268, and 81772842), the Natural Science Foundation of Guangxi (2015GXNSFEA139003), the Lijiang Scholar Award in Guilin, and the High Level of Innovation Team and Outstanding Scholars Program in Colleges and Universities in Guangxi.
Potential conflict of interest: Nothing to report.
Contributor Information
Jing Wang, Email: wangjing0081@hnszlyy.com.
Junfei Jin, Email: changliangzijin@163.com.
Deliang Cao, Email: deliangcao0062@hnszlyy.com.
References
Author names in bold designate shared co‐first authorship.
- 1. Sun VC, Sarna L. Symptom management in hepatocellular carcinoma. Clin J Oncol Nurs 2008;12:759‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ozakyol A. Global epidemiology of hepatocellular carcinoma (HCC epidemiology). J Gastrointest Cancer 2017;48:238‐240. [DOI] [PubMed] [Google Scholar]
- 3. Mittal S, El‐Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47(Suppl):S2‐S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi J, Zhu L, Liu S, Xie WF. A meta‐analysis of case‐control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer 2005;92:607‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med 1991;325:675‐680. [DOI] [PubMed] [Google Scholar]
- 6. Zhu AX. Hepatocellular carcinoma: Are we making progress? Cancer Invest 2003;21:418‐428. [DOI] [PubMed] [Google Scholar]
- 7. Bialecki ES, Di Bisceglie AM. Diagnosis of hepatocellular carcinoma. HPB (Oxford) 2005;7:26‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson PJ. The role of serum alpha‐fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis 2001;5:145‐159. [DOI] [PubMed] [Google Scholar]
- 9. Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 2002;122:1609‐1619. [DOI] [PubMed] [Google Scholar]
- 10. Gupta S, Bent S, Kohlwes J. Test characteristics of alpha‐fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med 2003;139:46‐50. [DOI] [PubMed] [Google Scholar]
- 11. Sterling RK, Jeffers L, Gordon F, Sherman M, Venook AP, Reddy KR, et al. Clinical utility of AFP‐L3% measurement in North American patients with HCV‐related cirrhosis. Am J Gastroenterol 2007;102:2196‐2205. [DOI] [PubMed] [Google Scholar]
- 12. Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, et al. Des‐gamma‐carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med 1984;310:1427‐1431. [DOI] [PubMed] [Google Scholar]
- 13. Weitz IC, Liebman HA. Des‐gamma‐carboxy (abnormal) prothrombin and hepatocellular carcinoma: A critical review. Hepatology 1993;18:990‐997. [DOI] [PubMed] [Google Scholar]
- 14. Cao D, Fan ST, Chung SS. Identification and characterization of a novel human aldose reductase‐like gene. J Biol Chem 1998;273:11429‐11435. [DOI] [PubMed] [Google Scholar]
- 15. Luo DX, Huang MC, Ma J, Gao Z, Liao DF, Cao D. Aldo‐keto reductase family 1, member B10 is secreted through a lysosome‐mediated non‐classical pathway. Biochem J 2011;438:71‐80. [DOI] [PubMed] [Google Scholar]
- 16. Luo D, Bu Y, Ma J, Rajput S, He Y, Cai G, et al. Heat shock protein 90‐alpha mediates aldo‐keto reductase 1B10 (AKR1B10) protein secretion through secretory lysosomes. J Biol Chem 2013;288:36733‐36740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhong L, Liu Z, Yan R, Johnson S, Zhao Y, Fang X, et al. Aldo‐keto reductase family 1 B10 protein detoxifies dietary and lipid‐derived alpha, beta‐unsaturated carbonyls at physiological levels. Biochem Biophys Res Commun 2009;387:245‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zu X, Yan R, Robbins S, Krishack PA, Liao DF, Cao D. Reduced 293T cell susceptibility to acrolein due to aldose reductase‐like‐1 protein expression. Toxicol Sci 2007;97:562‐568. [DOI] [PubMed] [Google Scholar]
- 19. Zu X, Yan R, Pan J, Zhong L, Cao Y, Ma J, et al. Aldo‐keto reductase 1B10 protects human colon cells from DNA damage induced by electrophilic carbonyl compounds. Mol Carcinog 2017;56:118‐129. [DOI] [PubMed] [Google Scholar]
- 20. Shen Y, Zhong L, Johnson S, Cao D. Human aldo‐keto reductases 1B1 and 1B10: A comparative study on their enzyme activity toward electrophilic carbonyl compounds. Chem Biol Interact 2011;191:192‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yan R, Zu X, Ma J, Liu Z, Adeyanju M, Cao D. Aldo‐keto reductase family 1 B10 gene silencing results in growth inhibition of colorectal cancer cells: implication for cancer intervention. Int J Cancer 2007;121:2301‐2306. [DOI] [PubMed] [Google Scholar]
- 22. Ma J, Yan R, Zu X, Cheng JM, Rao K, Liao DF, et al. Aldo‐keto reductase family 1 B10 affects fatty acid synthesis by regulating the stability of acetyl‐CoA carboxylase‐alpha in breast cancer cells. J Biol Chem 2008;283:3418‐3423. [DOI] [PubMed] [Google Scholar]
- 23. Wang C, Yan R, Luo D, Watabe K, Liao DF, Cao D. Aldo‐keto reductase family 1 member B10 promotes cell survival by regulating lipid synthesis and eliminating carbonyls. J Biol Chem 2009;284:26742‐26748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen Y, Ma J, Yan R, Ling H, Li X, Yang W, et al. Impaired self‐renewal and increased colitis and dysplastic lesions in colonic mucosa of AKR1B8‐deficient mice. Clin Cancer Res 2015;21:1466‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Z, Yan R, Al‐Salman A, Shen Y, Bu Y, Ma J, et al. Epidermal growth factor induces tumour marker AKR1B10 expression through activator protein‐1 signalling in hepatocellular carcinoma cells. Biochem J 2012;442:273‐282. [DOI] [PubMed] [Google Scholar]
- 26. Matkowskyj KA, Bai H, Liao J, Zhang W, Li H, Rao S, et al. Aldoketoreductase family 1B10 (AKR1B10) as a biomarker to distinguish hepatocellular carcinoma from benign liver lesions. Hum Pathol 2014;45:834‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmitz KJ, Sotiropoulos GC, Baba HA, Schmid KW, Muller D, Paul A, et al. AKR1B10 expression is associated with less aggressive hepatocellular carcinoma: A clinicopathological study of 168 cases. Liver Int 2011;31:810‐816. [DOI] [PubMed] [Google Scholar]
- 28. Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma. Hepatology 2005;42:1208‐1236. [DOI] [PubMed] [Google Scholar]
- 29. Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698‐711. [DOI] [PubMed] [Google Scholar]
- 30. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661‐662. [DOI] [PubMed] [Google Scholar]
- 31. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837‐845. [PubMed] [Google Scholar]
- 32. Torres‐Mena JE, Salazar‐Villegas KN, Sanchez‐Rodriguez R, Lopez‐Gabino B, Del Pozo‐Yauner L, Arellanes‐Robledo J, et al. Aldo‐keto reductases as early biomarkers of hepatocellular carcinoma: A comparison between animal models and human HCC. Dig Dis Sci 2018;63:934‐944. [DOI] [PubMed] [Google Scholar]
- 33. Tsuzura H, Genda T, Sato S, Murata A, Kanemitsu Y, Narita Y, et al. Expression of aldo‐keto reductase family 1 member b10 in the early stages of human hepatocarcinogenesis. Int J Mol Sci 2014;15:6556‐6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang YY, Qi LN, Zhong JH, Qin HG, Ye JZ, Lu SD, et al. High expression of AKR1B10 predicts low risk of early tumor recurrence in patients with hepatitis B virus‐related hepatocellular carcinoma. Sci Rep 2017;7:42199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sonohara F, Inokawa Y, Hishida M, Kanda M, Nishikawa Y, Yamada S, et al. Prognostic significance of AKR1B10 gene expression in hepatocellular carcinoma and surrounding non‐tumorous liver tissue. Oncol Lett 2016;12:4821‐4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mori M, Genda T, Ichida T, Murata A, Kamei M, Tsuzura H, et al. Aldo‐keto reductase family 1 member B10 is associated with hepatitis B virus‐related hepatocellular carcinoma risk. Hepatol Res 2017;47:E85‐E93. [DOI] [PubMed] [Google Scholar]
- 37. Murata A, Genda T, Ichida T, Amano N, Sato S, Tsuzura H, et al. Pretreatment AKR1B10 expression predicts the risk of hepatocellular carcinoma development after hepatitis C virus eradication. World J Gastroenterol 2016;22:7569‐7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sato S, Genda T, Ichida T, Murata A, Tsuzura H, Narita Y, et al. Impact of aldo‐keto reductase family 1 member B10 on the risk of hepatitis C virus‐related hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1315‐1322. [DOI] [PubMed] [Google Scholar]
- 39. Sato S, Genda T, Hirano K, Tsuzura H, Narita Y, Kanemitsu Y, et al. Up‐regulated aldo‐keto reductase family 1 member B10 in chronic hepatitis C: association with serum alpha‐fetoprotein and hepatocellular carcinoma. Liver Int 2012;32:1382‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Di Bisceglie AM, Hoofnagle JH. Elevations in serum alpha‐fetoprotein levels in patients with chronic hepatitis B. Cancer 1989;64:2117‐2120. [DOI] [PubMed] [Google Scholar]
- 41. Chung JW, Kim BH, Lee CS, Kim GH, Sohn HR, Min BY, et al. Optimizing surveillance performance of alpha‐fetoprotein by selection of proper target population in chronic hepatitis B. PLoS One 2016;11:e0168189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fukumoto S, Yamauchi N, Moriguchi H, Hippo Y, Watanabe A, Shibahara J, et al. Overexpression of the aldo‐keto reductase family protein AKR1B10 is highly correlated with smokers’ non‐small cell lung carcinomas. Clin Cancer Res 2005;11:1776‐1785. [DOI] [PubMed] [Google Scholar]
- 43. Ma J, Luo DX, Huang C, Shen Y, Bu Y, Markwell S, et al. AKR1B10 overexpression in breast cancer: association with tumor size, lymph node metastasis and patient survival and its potential as a novel serum marker. Int J Cancer 2012;131:E862‐E871. [DOI] [PubMed] [Google Scholar]
- 44. Chung YT, Matkowskyj KA, Li H, Bai H, Zhang W, Tsao MS, et al. Overexpression and oncogenic function of aldo‐keto reductase family 1B10 (AKR1B10) in pancreatic carcinoma. Mod Pathol 2012;25:758‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang C, Cao Z, Ma J, Shen Y, Bu Y, Khoshaba R, et al. AKR1B10 activates diacylglycerol (DAG) second messenger in breast cancer cells. Mol Carcinog 2018;57:1300‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


