Abstract
Periostin (POSTN), a secretory matricellular matrix protein, plays a multitude of biologic functions. Various splice variants of POSTN have been described; however, their expression pattern and functional implications are not completely understood. This study was undertaken to decipher the differential expression pattern of POSTN and its splice variants in various tissues and cell types. We show that POSTN was more highly expressed in anterior cruciate ligament (ACL) remnants compared with articular cartilage at the cellular and tissue level. Isoforms 1 and 8 were highly expressed only in articular chondrocytes, suggesting their splice-specific regulation in chondrocytes. To discern the role of total POSTN and full-length human POSTN isoform 1 (hPOSTN-001), we stably transfected human chondrosarcoma 1 (hCh-1) cell line with hPOSTN-001 using a pcDNA3.1–hPOSTN-001 construct. RNA-sequencing analysis of hCh-1 cells identified differentially expressed genes with a known role in chondrocyte function and osteoarthritis. Similar expression of a subset of candidate genes was revealed in ACL progenitor cells and chondrocytes as well as in ACL progenitor cells in which POSTN activity was altered by overexpression and by small interfering RNA gene knockdown. Cells expressing total POSTN, not isoform 1, exhibited increased cell adhesion potential. These findings suggest an important role for POSTN in the knee.—Cai, L., Brophy, R. H., Tycksen, E. D., Duan, X., Nunley, R. M., Rai, M. F. Distinct expression pattern of periostin splice variants in chondrocytes and ligament progenitor cells.
Keywords: cartilage, anterior cruciate ligament, periostin isoforms, RNA-seq, osteoarthritis
Periostin (POSTN), also called osteoblast-specific factor 2, is a 93-kDa secretory matricellular matrix protein and a member of the novel vitamin K–dependent γ-carboxylated protein family (1). POSTN was first cloned from the mouse MC3T3-E1 osteoblast-like cells and shares homology with the insect protein fasciclin 1 (2). It functions as a cell adhesion molecule for preosteoblasts and is thought to be involved in osteoblast recruitment, attachment, and spreading. POSTN is encoded by a gene located on chromosome 13 (13q13.3) in human (3) and is highly conserved between mouse and human. Structurally, POSTN contains 1 typical N terminus, followed by a cysteine-rich domain, a 4-fold repeat structure of about 140 aa, and 1 C-terminal hydrophilic domain. Alternative splicing exclusively affects the C-terminal region (3–5), which is devoid of known protein domains and appears to be intrinsically disordered. In rat, mouse, and human, exons 17–22 are of a symmetrical nature and have similar lengths. Furthermore, these exons share remarkable sequence similarity at the DNA level and are alternatively spliced. Various combinations of 3 of these 6 exons depict 8 alternative splicing variants resulting in 8 protein-coding isoforms. There is yet another noncoding ninth isoform. The functional effects of alternative splicing are therefore difficult to predict, although the C-terminal region is thought to regulate cell-matrix interactions through binding of additional extracellular matrix proteins such as collagen, fibronectin, and tenascin-C. On a transcriptional scale, alternative splicing could give rise to POSTN variants in a tissue-, development-, or disease-dependent manner, the functional impact of which is not well understood (6).
POSTN is frequently overexpressed in some cancers (7), and its isoforms generally exhibit tissue-specific expression (3–5, 8, 9). Isoform 1 is expressed predominantly in osteosarcoma, as well as breast, ovary, testes, urinary bladder, and heart tissues. Isoform 2 is expressed in placenta and normal bladder tissues. Isoform 3 has been detected in ovarian carcinoma and normal adult kidneys as well as in adipose, colon, lymph, prostate, and bladder cells. Isoform 4 is expressed in normal and cancerous bladder tissues. Isoform 5 is expressed in normal and cancerous bladder, normal adult kidney, and the thyroid tissues. Isoform 6 is detected in renal tissues. Isoform 8 is mainly detected in renal cell carcinoma (10–13).
POSTN is also expressed in collagen-rich fibrous connective tissues and has been implicated in collagen fibrillogenesis (14). In the musculoskeletal system, POSTN is expressed in periosteum, bone, chondrocytes of developing bone (3, 15), osteoarthritic cartilage (16, 17), articular chondrocytes (16), anterior cruciate ligament (ACL) (18, 19), osteoarthritic meniscus (20), muscles, and periodontal ligaments (21, 22). However, there is limited information on the isoform-specific expression of POSTN in musculoskeletal tissues. All known POSTN splice variants are protein coding and therefore have potential functional roles, but understanding of their functional implications remains fragmented. This knowledge gap led us to test the expression patterns of all known POSTN transcript variants in ACL and cartilage at tissue and cellular levels. These analyses are a first step toward understanding the role of POSTN splice variants, particularly isoform 1, in the musculoskeletal system.
MATERIALS AND METHODS
Patients and specimen collection
The Institutional Review Board of Washington University in St. Louis, MO, USA, approved this study (approval 201104119). All patients provided written and signed informed consent prior to participation in the study. Articular cartilage specimens were obtained from patients undergoing total knee replacement surgery at the study institution. Undamaged fragments of articular cartilage were carefully collected from the tibial surface. Every effort was exercised to avoid inclusion of subchondral bone in cartilage samples. Similarly, ACL tear remnants were collected from patients during arthroscopic ACL reconstruction surgery at the study institution. We did not pool samples from different patients and therefore presented independent data points from each patient separately. Specimens were brought to the lab in sterile containers containing saline solution for cell isolation or RNAlater solution (Thermo Fisher Scientific, Waltham, MA, USA) for RNA preparation. Prior to RNA preparation, cartilage and ACL tissues were homogenized in Trizol reagent (Thermo Fisher Scientific) using the Polytron System (Kinematica, Luzem, Switzerland). Lysed tissues and cells were stored in Trizol reagent at −80°C until used for RNA isolation. Table 1 lists characteristics of patients and specifies experiments for which tissues or cells were used.
TABLE 1.
Characteristics of patients used for sample collection for various experiments
| Patient ID | Age (yr) | Sex | Medical condition | Procedure | Tissue | Material | Experiment | Data in |
|---|---|---|---|---|---|---|---|---|
| P12-006 | 21 | Female | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| P12-009 | 28 | Male | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| P12-011 | 26 | Male | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| P12-018 | 14 | Female | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| P12-022 | 19 | Female | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| P12-024 | 32 | Male | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| P12-003 | 15 | Female | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| P12-012 | 32 | Male | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| P12-021 | 39 | Male | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| P12-025 | 33 | Male | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| P12-029 | 18 | Male | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| P12-032 | 40 | Female | ACLT | ACL-R | ACL | Tissues | Real-time PCR | Fig. 1 |
| S1-2M | 59 | Female | OA | TKA | Cartilage | Tissues | Real-time PCR | Fig. 1 |
| S1-3M | 52 | Male | OA | TKA | Cartilage | Tissues | Real-time PCR | Fig. 1 |
| S1-8M | 57 | Female | OA | TKA | Cartilage | Tissues | Real-time PCR | Fig. 1 |
| S1-9M | 66 | Female | OA | TKA | Cartilage | Tissues | Real-time PCR | Fig. 1 |
| S1-10M | 71 | Female | OA | TKA | Cartilage | Tissues | Real-time PCR | Fig. 1 |
| S1-11M | 65 | Male | OA | TKA | Cartilage | Tissues | Real-time PCR | Fig. 1 |
| S1-14M | 56 | Female | OA | TKA | Cartilage | Tissues | Real-time PCR | Fig. 1 |
| S1-16M | 61 | Male | OA | TKA | Cartilage | Tissues | Real-time PCR | Fig. 1 |
| P15-035 | 40 | Female | ACLT | ACL-R | ACL | Cells | Immunostaining | Fig. 2 |
| P15-036 | 37 | Male | ACLT | ACL-R | ACL | Cells | Immunostaining | Fig. 2 |
| P15-037 | 51 | Female | ACLT | ACL-R | ACL | Cells | Immunostaining | Fig. 2 |
| CS244 | 65 | Male | OA | TKA | Cartilage | Cells | Immunostaining | Fig. 2 |
| CS245 | 52 | Female | OA | TKA | Cartilage | Cells | Immunostaining | Fig. 2 |
| CS246 | 60 | Female | OA | TKA | Cartilage | Cells | Immunostaining | Fig. 2 |
| P15-004 | 19 | Female | ACLT | ACL-R | ACL | Cells | Real-time PCR | Fig. 3 |
| P15-005 | 14 | Male | ACLT | ACL-R | ACL | Cells | Real-time PCR | Fig. 3 |
| P15-006 | 32 | Male | ACLT | ACL-R | ACL | Cells | Real-time PCR | Fig. 3 |
| P15-007 | 33 | Male | ACLT | ACL-R | ACL | Cells | Real-time PCR | Fig. 3 |
| P15-009 | 63 | Female | ACLT | ACL-R | ACL | Cells | Real-time PCR | Fig. 3 |
| P15-011 | 18 | Male | ACLT | ACL-R | ACL | Cells | Real-time PCR | Fig. 3 |
| CS-203 | 63 | Male | OA | TKA | Cartilage | Cells | Real-time PCR | Fig. 3 |
| CS-204 | 64 | Female | OA | TKA | Cartilage | Cells | Real-time PCR | Fig. 3 |
| CS-67 | 67 | Female | OA | TKA | Cartilage | Cells | Real-time PCR | Fig. 3 |
| CS-76 | 81 | Female | OA | TKA | Cartilage | Cells | Real-time PCR | Fig. 3 |
| CS-139 | 53 | Female | OA | TKA | Cartilage | Cells | Real-time PCR | Fig. 3 |
| CS-140 | 60 | Male | OA | TKA | Cartilage | Cells | Real-time PCR | Fig. 3 |
| CS-195 | 62 | Male | OA | TKA | Cartilage | Cells | Real-time PCR | Fig. 3 |
| CS-201 | 63 | Female | OA | TKA | Cartilage | Cells | Real-time PCR | Fig. 3 |
| CS-202 | 64 | Female | OA | TKA | Cartilage | Cells | Real-time PCR | Fig. 3 |
| P15-051 | 16 | Male | ACLT | ACL-R | ACL | Cells | Cell adhesion assay | Fig. 8 |
| P15-053 | 17 | Female | ACLT | ACL-R | ACL | Cells | Cell adhesion assay | Fig. 8 |
| P15-058 | 23 | Male | ACLT | ACL-R | ACL | Cells | Cell adhesion assay | Fig. 8 |
| CS271 | 69 | Female | OA | TKA | Cartilage | Cells | Cell adhesion assay | Fig. 8 |
| CS272 | 79 | Male | OA | TKA | Cartilage | Cells | Cell adhesion assay | Fig. 8 |
| CS273 | 54 | Male | OA | TKA | Cartilage | Cells | Cell adhesion assay | Fig. 8 |
| P15-030 | 15 | Female | ACLT | ACL-R | ACL | Cells | RNA-seq validation | Fig. 9 |
| P15-041 | 38 | Male | ACLT | ACL-R | ACL | Cells | RNA-seq validation | Fig. 9 |
| P15-044 | 15 | Male | ACLT | ACL-R | ACL | Cells | RNA-seq validation | Fig. 9 |
| P15-047 | 26 | Female | ACLT | ACL-R | ACL | Cells | RNA-seq validation | Fig. 9 |
| P15-048 | 44 | Male | ACLT | ACL-R | ACL | Cells | RNA-seq validation | Fig. 9 |
| CS67 | 67 | Female | OA | TKA | Cartilage | Cells | RNA-seq validation | Fig. 9 |
| CS76 | 81 | Female | OA | TKA | Cartilage | Cells | RNA-seq validation | Fig. 9 |
| CS139 | 53 | Female | OA | TKA | Cartilage | Cells | RNA-seq validation | Fig. 9 |
| CS140 | 60 | Male | OA | TKA | Cartilage | Cells | RNA-seq validation | Fig. 9 |
| CS195 | 62 | Male | OA | TKA | Cartilage | Cells | RNA-seq validation | Fig. 9 |
| CS201 | 63 | Female | OA | TKA | Cartilage | Cells | RNA-seq validation | Fig. 9 |
| P15-057 | 20 | Female | ACLT | ACL-R | ACL | Cells | Isoform 1 overexpression | Fig. 10 |
| P15-058 | 23 | Male | ACLT | ACL-R | ACL | Cells | Isoform 1 overexpression | Fig. 10 |
| P15-059 | 19 | Male | ACLT | ACL-R | ACL | Cells | Isoform 1 overexpression | Fig. 10 |
| P15-051 | 16 | Male | ACLT | ACL-R | ACL | Cells | POSTN siRNA | Fig. 11 |
| P15-053 | 17 | Female | ACLT | ACL-R | ACL | Cells | POSTN siRNA | Fig. 11 |
| P15-056 | 50 | Female | ACLT | ACL-R | ACL | Cells | POSTN siRNA | Fig. 11 |
| P15-061 | 48 | Male | ACLT | ACL-R | ACL | Cells | POSTN siRNA | Fig. 11 |
| P15-062 | 41 | Female | ACLT | ACL-R | ACL | Cells | POSTN siRNA | Fig. 11 |
ACLT, ACL tear; ACL-R, ACL reconstruction; TKA, total knee arthroplasty.
Cell culture
Primary chondrocytes
Chondrocytes were isolated via enzymatic digestion as previously described (16). Briefly, cartilage fragments were collected in DMEM and nutrient mixture F-12 (DMEM-F12; Thermo Fisher Scientific) containing 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific) and 2% penicillin and streptomycin (10,000 U/ml; Thermo Fisher Scientific). Tissue fragments were digested using an enzyme cocktail containing 0.025% collagenase P (1.5 U/mg; Roche, Basel, Switzerland) and 0.025% pronase (7 U/mg; Roche) in complete DMEM-F12 in a spinner flask. After incubation at 37°C for overnight, the digest was filtered through a 70 µm–pore size cell strainer and centrifuged at 1500 rpm for 5 min. The pellet was washed with calcium- and magnesium-free HBSS (Thermo Fisher Scientific) and suspended in complete DMEM-F12 supplemented with 50 mg/L l-ascorbic acid. Cells were seeded in 6-well plates at a density of 0.5 × 105 cells/cm2. The plates were maintained at 37°C and 5% CO2 with 95% humidity (standard cell culture conditions). Once 80% confluence was reached, the cells were trypsinized using 0.125% trypsin-EDTA (Thermo Fisher Scientific) and collected in Trizol reagent. Data obtained from cells in the first passage were used for this study.
ACL progenitor cells
ACL tear remnants were washed with sterile PBS (Thermo Fisher Scientific), further diced in 3–4 mm3 pieces and placed in 10-cm dishes containing DMEM-F12 supplemented with 10% FBS and 1% penicillin and streptomycin. Cells egressing from ACL explants were continued for 3 wk. After explant cultures were established, the original pieces of the ACL remnants were removed. The plates were maintained at standard culture conditions. At about 80% confluence, cells were trypsinized and collected into complete growth medium. Cells at passage 1 were used for the subsequent studies.
Human chondrosarcoma cell line
In-house human chondrosarcoma 1 (hCh-1) cells (23) were cultured in Roswell Park Memorial Institute medium 1640 (Thermo Fisher Scientific), supplemented with 10% FBS and 2% penicillin and streptomycin. Cells were maintained at standard culture conditions, and the medium was replenished every 2 d.
Generation of stable expression clones
To stably transfect human POSTN isoform 1 (hPOSTN-001) in hCh-1 cells, we used recombinant DNA technology to prepare and characterize the plasmid, its transfection into the cells, and selection of stable hPOSTN-001–expressing clones as described below.
pcDNA3.1–hPOSTN-001 plasmid construction
hPOSTN-001 cDNA sequence was obtained from the National Center for Biotechnology Information (NCBI, NM_006475.2). The insert was chemically synthesized and inserted into BamHI and EcoRI (Thermo Fisher Scientific) sites of eukaryotic expression vector pcDNA3.1(+) plasmid including a neomycin-resistant gene (Thermo Fisher Scientific). Successful cloning of the insert into the vector was confirmed by restriction endonucleases, namely, HindIII and XhoI (Thermo Fisher Scientific), as well as by sequencing. The sequencing reads were matched with the corresponding reference sequence using Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi). The forward and reverse reads were more precisely aligned with the reference sequence using ClustalW (http://www.clustal.org/clustal2/). High copy plasmid DNA from Escherichia coli (E. coli) transformants was prepared with a plasmid mini kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions and quantified using a NanoDrop ND1000 spectrophotometer. A QiaQuick Gel Extraction Kit (Qiagen) was used to recover DNA fragments from agarose gels. Competent E. coli strain preparation, ligation of DNA, and all molecular methods were performed using standard molecular biology techniques. To make transfection-ready constructs, we undertook a restriction digestion on pcDNA3.1–hPOSTN-001 to obtain the expression fragment with the vector using SalI and on pcDNA3.1(+) to obtain empty vector using SalI.
Transfection of hCh-1 cells
Prior to transfection, hCh-1 cells were digested with 0.125% trypsin-EDTA solution and counted using a cell counter. Cell suspension was seeded in 6-well plates at a density of 0.8 × 106 cells per well and cultured overnight in an incubator set at 37°C with 5% CO2. The cells were then transfected for 24 h after plating with pcDNA3.1(+) or pcDNA3.1–hPOSTN-001 using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. Briefly, 2 μg of DNA [pcDNA3.1(+) vector control or pcDNA3.1–hPOSTN-001] and 5 μl Lipofectamine 2000 were diluted in 200 μl Opti-MEM (Thermo Fisher Scientific) and incubated at room temperature for 5 min. The diluted DNA and Lipofectamine were combined and incubated for another 5 min at room temperature prior to their dropwise addition to cells. Cells were maintained at 37°C in an incubator containing 5% CO2 for 24 h prior to additional experiments.
Selection of clones
Positive clones were selected by geneticin (G418; Thermo Fisher Scientific) at a concentration of 400 mg/L and then confirmed by genome PCR. Medium containing G418 was changed daily for the first 7 d, then every other day until selection of stable clones. After 14 d, surviving clones were picked from culture when resistant cells continued to grow into visible clones. Drug-resistant clones were picked and trypsinized in a 96-well plate and were dispersed by gentle agitation and transferred to 96-well culture plates. Cells transfected with pcDNA3.1(+) empty vector were used as vehicle-transfected control (vehicle). The cell line transfected with pcDNA3.1–hPOSTN-001 was designated hPOSTN-001, whereas nontransfected cells were treated as control.
Validation of overexpression in clones
In order to validate the stable clones overexpressing hPOSTN-001, cells were evaluated at both the DNA and RNA levels. First, genomic DNA was isolated using SDS-based lysis buffer and proteinase K (50 mM EDTA pH 8.0, 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 10% SDS, 1 mg/ml proteinase K) and incubated at 55°C overnight. Briefly, to each sample, 2 volumes of 100% ethanol were added, and tubes were gently inverted until appearance of precipitate. The contents were then centrifuged for 15 min at 14,000 rpm; ethanol was poured off and replaced with 1 ml of fresh 70% ethanol to repeat as above. Finally, DNA was resuspended in 100 μl of Tris-EDTA buffer. Using 5′-ACCAAGGCCCAAATGTCTGT-3′ (forward) and 5′-GGAAGCCACTTTGTCTCCCA-3′ (reverse) primers, hPOSTN-001 cDNA was amplified by PCR.
RNA preparation and DNase I treatment
Total RNA samples were prepared from the homogenized tissues and lysed cells using a combination of the Trizol:chloroform method as previously described (24) and resuspended in diethyl pyrocarbonate–treated water. A total of 250 ng of RNA was treated with DNase I (1 U/μl; Thermo Fisher Scientific) to remove traces of double-stranded genomic DNA. Briefly, to an RNase-free 0.5-ml microfuge tube containing 250 ng RNA, the following reagents were added: 1 μl 10× DNase I reaction buffer; 1 μl DNase I, amplification grade; and 1 U/μl diethyl pyrocarbonate–treated water to final volume of 10 μl. The microfuge tube was incubated for 15 min at room temperature. Finally, DNase I was inactivated by the addition of 1 μl of 25 mM EDTA solution (pH 8.0), and the reaction was terminated by heating at 65°C for 10 min.
First strand cDNA synthesis
DNase I-treated RNA was reverse transcribed with a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific) to synthesize single-stranded cDNA. Briefly, 10 μl of 2× reverse transcription master mix was added to RNA sample followed by quick spinning to mix the contents. Samples were loaded on a thermal cycler at 25°C for 10 min, then 37°C for 2 h, and finally reaction was terminated at 85°C for 5 min. cDNA was stored at −80°C until use.
Real-time PCR
Real-time PCR was performed for validation of POSTN and its 8 transcript variants using standard methods (24). We used 20 μl of reaction mixture containing 10 μl of Sybr Green PCR master mix (Thermo Fisher Scientific), 1 µl cDNA, and 200 nM of custom-designed gene- and isoform-specific primers (Table 2). Primers for real-time PCR were designed using NCBI’s Primer BLAST and obtained from Thermo Fisher Scientific. Real-time PCR was performed on a 7500 fast real-time PCR system (Thermo Fisher Scientific) to detect the gene expression level. The amplification was performed as follows: initial denaturation at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 14 s, and annealing and extension at 60°C for 60 s. All reactions were performed in technical duplicates. The relative expression levels of the genes were normalized to cyclophilin A and calculated using the 2−ΔΔCt method.
TABLE 2.
Characteristics of primers
| Symbol | Accession # | Primer sequence, 5′–3′ |
Location | Amplicon size (bp) | |
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| POSTN | NM_006475.2 | CAACGCAGCGCTATTCTGAC | CCAAGTTGTCCCAAGCCTCA | 453–554 | 101 |
| POSTN-001 | NM_006475.2 | TGAAGGCAGTCTTCAGCCTA | GTGACCTTGGTGACCTCTTC | 2173–2481 | 309 |
| POSTN-002 | NM_001135934.1 | CCCGTGACTGTCTATAAGCC | GTGACCTTGGTGACCTCTTC | 2111–2310 | 200 |
| POSTN-003 | NM_001135935.1 | CCGTGACTGTCTATAGACCC | TCCTCACGGGTGTGTCTTCT | 2111–2399 | 288 |
| POSTN-004 | NM_001135936.1 | CCCGTGACTGTCTATAAGCC | TCCTCACGGGTGTGTCTTCT | 2111–2309 | 199 |
| POSTN-005 | NM_001286665.1 | CCGTGACTGTCTATAGACCC | GTGACCTTGGTGACCTCTTC | 2112–2400 | 289 |
| POSTN-006 | NM_001286666.1 | CCGTGACTGTCTATAGTCCTG | ATTTGGTGACCTTGGTGACC | 2112–2225 | 114 |
| POSTN-007 | NM_001286667.1 | CCGTGACTGTCTATAGTCCTG | TCCTCACGGGTGTGTCTTCT | 2112–2219 | 108 |
| POSTN-008 | NM_001330517.1 | TGAAGGCAGTCTTCAGCCTA | TCCTCACGGGTGTGTCTTCT | 2173–2480 | 308 |
| MARCKS | NM_002356.6 | CCAGTTCTCCAAGACCGCAG | TCTCCTGTCCGTTCGCTTTG | 420–517 | 98 |
| HIST1H3I | NM_003533.2 | GCTACCAGAAGTCGACCGAG | GGAAGCGCAGATCGGTCTTA | 167–262 | 96 |
| DMD | NM_004006.2 | GAAACTGCCAAGCATCAGGC | GCCTTTTGCAACTCGACCAG | 10,157–10,291 | 135 |
| ZNF714 | NM_182515.3 | AAGCAAGACCCGATCACCAG | TTGCTCTGGCCAAAGGTCTC | 439–561 | 123 |
| ZNF91 | NM_003430.3 | ACTTTTGGCCAGAGCAGAGC | GTGCACCTTACACTCATCCAC | 484–611 | 128 |
| MAL2 | NM_052886.2 | CCTTCGTCTGCCTGGAGATTC | GCCCAGAAAGAGGAGCGAAA | 215–354 | 140 |
| CNTN1 | NM_001843.3 | TACGGGATGGTCAGAAGCAC | GGGTCACAGAGAAGCACCA | 585–706 | 122 |
| DLEC1 | NM_007337.3 | AGTGTTGGCAGGTTCTGCAT | TCAAACACCGAAGGCAGGAT | 1537–1655 | 119 |
| MPZL2 | NM_005797.3 | CAGGTGAAGAACCCACCTGA | GATCATCAGTGCACAGGCAG | 753–881 | 129 |
| DACH2 | NM_053281.3 | AATGGGACCGAATCAGAGCC | GTGCAGCAAATGGAGACTGC | 900–996 | 97 |
| GJB2 | NM_004004.5 | GGCTCACCGTCCTCTTCATT | GTAGCACACGTTCTTGCAGC | 286–410 | 125 |
| LRP1B | NM_018557.2 | GCTGACACCACCAGTTTCCT | CCACAGCAATGCCCTCTACA | 2689–2791 | 103 |
| HSD17B3 | NM_000197.1 | GGCGATGGAATTGGGAAAGC | CTGTGGCAATGGCCTCTAGT | 217–319 | 103 |
| ZIC3 | NM_003413.3 | GCCTATCAAGCAGGAGCTGT | TCATGCATGGTGCTGAAGGT | 1240–1335 | 96 |
| PARVA | NM_018222.5 | GTGGCCATCTTACACCTGCT | TGGATTTGCCGAGACTGGAG | 639–769 | 131 |
| HIST1H2BE | NM_003523.2 | ACCTCCAGGGAGATCCAGAC | GGTGTACTTGGTAACGGCCT | 271–369 | 99 |
| TSPYL5 | NM_033512.2 | TCAGGTCATAGCTGGTGGGA | TATCCATGCTGCCTTCCGTC | 584–714 | 131 |
| PCDHB5 | NM_015669.4 | ATCACTGTCACCGACATGGG | GGGTGTAGGAGGTTTGGGTG | 1487–1598 | 112 |
| CMKLR1 | NM_001142343.1 | GGTCTGGTTCCTCAACCTGG | GCTGATCTTGCACATGGCTG | 802–922 | 121 |
| TRPM3 | NM_020952.5 | CGCTCGCAGCCAGATCTTTA | AACGGTGCATGCTTACTCCA | 1297–1433 | 137 |
| DRD2 | NM_000795.3 | GGGTCACCGTCATGATCTCC | GGTTGGCAATGATGCACTCG | 687–794 | 108 |
| OLFM3 | NM_001288821.1 | CCAAAGAGTGCTGAGCTTGG | AAGCACCAAATCGGGTTCCA | 707–828 | 122 |
| ITGA8 | NM_003638.2 | ACTTGCCAGGTTCCAGACTC | TGTGTTTGCAATGCTCTGGC | 2080–2169 | 90 |
| LUZP2 | NM_001009909.3 | CTAAGCTTCCAGATGCAGCG | TTGAGAGTCACAGGCAGTGG | 1008–1129 | 122 |
| RIPPLY3 | NM_018962.2 | CAGAGACCAGGGCATCAACC | TTGGATGAGGAGCACTTGCC | 666–779 | 114 |
| PPIA | NM_021130.4 | TCTGCACTGCCAAGACTGAG | TGGTCTTGCCATTCCTGGAC | 430–546 | 117 |
Purification of PCR products and sequencing of isoforms
RNA isolated from cells was subjected to DNase I treatment followed by first strand cDNA synthesis. Semiquantitative PCR was performed using isoform-specific primers. PCR product was run on 2% agarose gel. Amplified DNA bands were excised from gel and purified with QiaQuick Gel Extraction Kit (Qiagen). The purified PCR products were then sequenced in an automated DNA sequencer, and the sequence data were analyzed using BLAST from the NCBI. To further confirm the identity of amplified products, we aligned the sequences to GenBank (https://www.ncbi.nlm.nih.gov/genbank/).
Immunofluorescence and confocal microscopy
We performed immunofluorescence for POSTN using human chondrocytes and ACL-derived cells. In a separate experiment, we also used hCh-1 cells: control cells (nontransfected), vehicle cells (transfected with empty vector), and hPOSTN-001 cells (transfected with pcDNA3.1–hPOSTN-001). Briefly, 1 × 105 cells were cultured onto each well of an 8-well chamber slide for 48 h. Following culture, cell medium was removed, and cells were washed (3 times) with 1× PBS for 5 min. Cells were then fixed with freshly prepared 4% paraformaldehyde solution for 15 min at room temperature. This was followed by washing 3 times in 1× PBS. Then cells were treated with 0.1% PBS-Tween for 15 min followed by washing 3 times with 1× PBS for 3 min each. Blocking was performed with the use of 10% normal goat serum (NGS) at room temperature. After 1 h of incubation, blocking buffer was removed, and rabbit anti-POSTN primary antibody (ab14041; Abcam, Cambridge, MA, USA) diluted 1:100 in 2% NGS was applied to the chamber slides. Slides were incubated overnight at 4°C. The next day, slides were washed 3 times with 1× PBS for 5 min. Slides were then incubated at room temperature for 2 h with the following secondary antibodies diluted in 2% NGS: Texas red phalloidin (T7471, 1:500; Thermo Fisher Scientific) and goat anti-rabbit 488 (ab150077, 1:200; Abcam). Slides were finally washed 3 times in 1× PBS for 3 min. Afterwards, slides were mounted with Fluoro-Gel II mounting medium (17985-50; Electron Microscopy Sciences, Hatfield, PA, USA) and sealed with fingernail polish. Images were acquired with either a Zeiss LSM880 confocal laser scanning microscope with Airyscan (for hCh-1 cells; Carl Zeiss, Oberkochen, Germany) or a Leica TCS SPEII confocal microscope (for ACL-derived cells and chondrocytes; Leica Microsystems, Buffalo Grove, IL, USA) depending on the availability of the equipment.
RNA-sequencing
RNA-sequencing library preparation
We used 3 groups of hCh-1 cells for RNA-sequencing (RNA-seq) analysis: control cells (nontransfected), vehicle cells (transfected with empty vector), and hPOSTN-001 cells (transfected with pcDNA3.1–hPOSTN-001). A total of 1 μg of RNA from each sample (in technical triplicates) was prepared with the Ribo-Zero Gold Kit (Illumina, San Diego, CA, USA) according to the manufacturer’s protocol, indexed, pooled, and sequenced on a single lane as single-end 50 bp on an Illumina HiSeq 3000 for a target depth of 19 million reads per sample. Basecalls and demultiplexing were performed with Illumina’s bcl2fastq2 software. RNA-seq reads were then aligned to the Ensembl (https://ensembl.org/index.html) v.76 top-level assembly with spliced transcripts alignment to a reference (STAR) software v.2.0.4b (25). Gene counts were derived from the number of uniquely aligned unambiguous reads by featureCounts in the Subread software package v.1.4.5 (26). Sequencing performance was assessed for the total number of aligned reads, total number of uniquely aligned reads, features detected, alignment/sampling bias, and gene coverage with RSeQC, an RNA-seq quality control package, v.2.3 (27).
RNA-seq data analysis
All gene counts were imported into the R/Bioconductor package empirical analysis of digital gene expression in R (EdgeR; https://bioconductor.org/packages/release/bioc/html/edgeR.html), and trimmed mean of M-value normalization size factors were calculated to adjust for samples differences in library size (28, 29). Ribosomal genes and genes not expressed in at least 3 samples at >1 counts per million were excluded from further analysis. The trimmed mean of M-value size factors and the matrix of counts were then imported into the R/Bioconductor package linear models for microarray data (limma) (30, 31). Weighted likelihoods, based on the observed mean-variance relationship of every gene and sample, were then calculated for all samples with the R/Bioconductor voomWithQualityWeights function, and the data were fitted to a generalized linear model. Differential expression analysis was then performed to analyze for differences among 3 different groups (hPOSTN-001, vehicle, and control). The results were filtered for only those genes with Benjamini-Hochberg false discovery rate (FDR) adjusted P values ≤0.05.
Gene ontology analysis
For each contrast extracted with limma, global perturbations in known gene ontology (GO) terms were detected using the R/Bioconductor generally applicable gene set enrichment (GAGE) package to test for changes in expression of the reported log2 fold changes reported by limma in each term vs. the background log2 fold changes of all genes found outside the respective term (32). The R/Bioconductor packages heatmap3 (33) and Pathview (34) were used to illustrate changes across groups of samples for each GO term with a Benjamini-Hochberg FDR adjusted P value ≤0.05.
Validation of RNA-seq results by real-time PCR
To validate the expression profiles obtained by RNA-seq, real-time PCR was performed on differentially expressed genes as previously described using primers shown in Table 2.
Analysis of cell adhesion
The adhesion of hCh-1 cells (control, vehicle, and hPOSTN-001), ACL progenitor cells, and chondrocytes to a fibronectin-coated microplate was analyzed upon fluorogenic labeling by dye calcein acetoxymethyl ester using the Vybrant cell adhesion assay kit (Thermo Fisher Scientific) according to the recommended protocol. Briefly, cells were digested with 0.125% trypsin-EDTA, washed twice with 1× PBS, and resuspended in serum-free medium at 5 × 106 cells/ml. After, cells were labeled by incubation with 5 µl of calcein acetoxymethyl ester (1 mM) per 1 ml of culture medium at a final concentration of 5 µM. After 30 min of incubation at 37°C, cells were washed twice and resuspended in serum-free medium at 5 × 106 cells/ml. We added 100 µl of the calcein-labeled cells to microplate coated with fibronectin (CWP001; R&D Systems, Minneapolis, MN, USA). Cell attachment was allowed to proceed for 60 min at 37°C. Nonadherent calcein-labeled cells were removed by careful washing 4 times with prewarmed serum-free medium. Prewarmed culture medium was added to each well, and plates were swirled gently and finally inverted and blot dried. Next, 200 µl of PBS was dispensed to each well, and fluorescence was measured using a fluorescein filter set at 494 nm (absorbance) and 517 nm (emission) with the use of the Synergy H11 plate reader (BioTek Instruments, Winooski, VT, USA). The percentage of adhesion was determined by dividing the corrected (background subtracted) fluorescence of adherent cells by the total corrected fluorescence of cells added to each microplate. The fluorescence of control hCh-1 cells and ACL progenitor cells was designated 100%.
Validation of the role of total POSTN and hPOSTN-001 in ACL progenitor cells and chondrocytes
To validate the role of overexpression of total POSTN in case of control and vehicle cells, and the role of overexpression of hPOSTN-001 in the case of hPOSTN-001 and control cells, we determined the expression of the following genes in ACL progenitor cells and chondrocytes using real-time PCR as above: MARCKS, HIST1H3I, MAL2, DLEC1, LRP1B, and TSPYL5.
Transient overexpression of hPOSTN-001 in ACL progenitor cells
We transiently transfected ACL progenitor cells to test the effect of hPOSTN-001 on selected genes identified from RNA-seq analysis. Briefly, cells were collected from culture dish by digestion with 0.125% trypsin-EDTA solution and counted using a cell counter. Cell suspension was seeded in 12-well plates at a density of 2 × 105 cells per well and cultured overnight in an incubator set at 37°C with 5% CO2. After aspirating medium and washing with PBS, cells were transfected with pcDNA3.1–hPOSTN-001 using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. Briefly, 1 μg of DNA and 3 μl Lipofectamine 2000 were diluted in 50 μl Opti-MEM (Thermo Fisher Scientific) separately and incubated at room temperature for 5 min. The diluted DNA and Lipofectamine were combined and incubated for another 5 min at room temperature prior to their dropwise addition to cells. After 15 h of culture, the culture medium was replenished with fresh medium. Cells were further cultured for 48 h. Afterwards, RNA was isolated from these cells and analyzed for mRNA expression using real-time PCR. Nontransfected cells were used as control, and mock transfected cells were used to determine any possible nonspecific effects that might be caused by transfection reagent or process.
POSTN knockdown in ACL progenitor cells
To examine if knockdown of POSTN has an effect on gene expression, we silenced the expression of POSTN from human ACL progenitor cells using human POSTN small interfering RNA (siRNA) as previously described (16). Prior to transfection, ACL progenitor cells were digested with 0.125% trypsin-EDTA solution and counted using a cell counter. Cell suspension was seeded in 12-well plates at a density of 2 × 105 cells per well and cultured overnight in an incubator set at 37°C with 5% CO2. Cells were transfected with 2.5 nM of POSTN siRNA oligonucleotides (S20889; Thermo Fisher Scientific) and TransIT-siQuest transfection reagents (MOR2114; Mirus, Madison, WI, USA). After 15 h of culture, the medium was changed, and the cells were maintained at 37°C in an incubator containing 5% CO2 for 48 h. After, cells were collected in Trizol reagent for RNA isolation followed by real-time PCR. Nontransfected cells were used as control, and mock transfected cells were used to determine any possible nonspecific effects that might be caused by the transfection reagent or process.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). Real-time PCR data was analyzed by the Mann-Whitney U test or the Kruskal-Wallis test with Dunn’s multiple comparison. Cell adhesion data was analyzed by Student’s t test and 1-way ANOVA. Results are expressed as means ± sem unless indicated otherwise. A value of P < 0.05 was considered to indicate a statistically significant difference. All experiments were conducted using biologic and technical replicates.
RESULTS
Construction of the pcDNA3.1–hPOSTN-001 plasmid
Plasmid map (Supplemental Fig. S1A) indicates the successful integration of hPOSTN-001 cDNA sites in the pcDNA3.1(+) vector. Restriction digestion of pcDNA3.1–hPOSTN-001 confirmed a band with 2500-bp size as detected through 1% agarose gel electrophoresis (Supplemental Fig. S1B). After cleaving with HindIII and XhoI enzymes, the 2500-bp sized band was also detected on the agarose gels from 2 positive recombinant plasmids (Supplemental Fig. S1B). The results from the sequencing of pcDNA3.1–hPOSTN-001 and alignment to GenBank data revealed that the cloned hPOSTN-001 gene sequence was correct. Digestion of pcDNA3.1–hPOSTN-001 with SalI resulted in 2 fragments: a 5.8-kbp fragment (which included 2.5 kbp hPOSTN-001 and 3.3 kbp vector) and a 2.1-kbp fragment (Supplemental Fig. S1B). Similarly, digestion of pcDNA3.1(+) by SalI yielded a 3.3-kbp fragment of empty vector (Supplemental Fig. S1B).
During our attempt to establish clones of hCh-1 cells stably transfected with pcDNA3.1–hPOSTN-001, we selected 13 drug-resistant clones. The hPOSTN-001 cDNA was successfully inserted into genomic DNA of 6 clones, out of which 4 clones showed overexpression of hPOSTN-001. Genomic PCR of 2 pcDNA3.1–hPOSTN-001 clones revealed that the the pcDNA3.1–hPOSTN-001 insert had successful integration with genomic DNA in these clones as demonstrated by the 779-bp PCR product (Supplemental Fig. S1C). Only 1 clone (clone 11) that showed highest expression of hPOSTN-001 by PCR was selected for subsequent experiments.
Confirmation of transcript variants by PCR and sequencing
Prior to assessing the expression pattern of the 8 known POSTN splice variants (Supplemental Fig. S2A) in tissues and cells, we confirmed the amplicon size by agarose gel electrophoresis. We found that each amplicon was of the exact molecular size as expected by the sequence-specific primers (Supplemental Fig. S2B). DNA sequencing of purified PCR products further confirmed the specificity of each isoform (Supplemental Fig. S3). Some of the characteristics of isoforms are summarized in Table 3.
TABLE 3.
Characteristics of POSTN splice variants
| Isoform name | Accession # (mRNA/protein) | mRNA (bp) | CDS (bp) | Protein (aa) | Exon skipped |
|---|---|---|---|---|---|
| POSTN-001 | NM_006475.2/NP_006466.2 | 3390 | 2511 | 836 | None |
| POSTN-002 | NM_001135934.1/NP_001129406.1 | 3219 | 2340 | 779 | 17, 18 |
| POSTN-003 | NM_001135935.1/NP_001129407.1 | 3225 | 2346 | 781 | 17, 21 |
| POSTN-004 | NM_001135936.1/NP_001129408.1 | 3135 | 2256 | 751 | 17, 18, 21 |
| POSTN-005 | NM_001286665.1/NP_001273594.1 | 3309 | 2430 | 809 | 17 |
| POSTN-006 | NM_001286666.1/NP_001273595.1 | 3129 | 2250 | 749 | 17, 18, 19 |
| POSTN-007 | NM_001286667.1/NP_001273596.1 | 3045 | 2166 | 721 | 17, 18, 19, 21 |
| POSTN-008 | NM_001330517.1/NP_001317446.1 | 3306 | 2427 | 808 | 21 |
CDS, coding sequence.
Expression pattern of POSTN mRNA and its splice variants in ACL and cartilage tissues
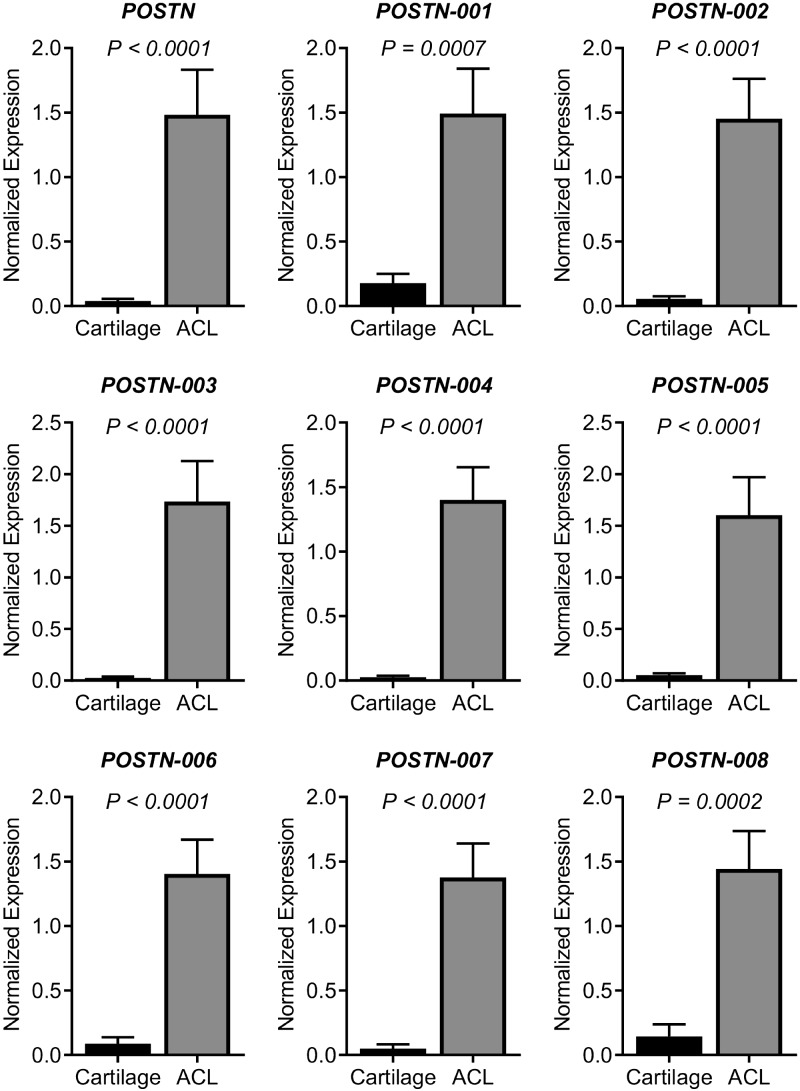
Real-time PCR data revealed that expression of POSTN was significantly (P < 0.0001) higher in ACL remnants than in articular cartilage. All POSTN isoforms (1–8) were also significantly higher in ACL tear remnants than in articular cartilage (Fig. 1).
Figure 1.
Expression pattern of POSTN and its splice variants in cartilage and ACL tissues. To determine whether POSTN and its splice variants are differentially expressed, we compared the expressions profile between cartilage (n = 8) and ACL remnants (n = 12). We observed that POSTN and all 8 transcript variants were significantly more highly expressed in ACL tissue than cartilage.
POSTN protein in ACL cells and chondrocytes
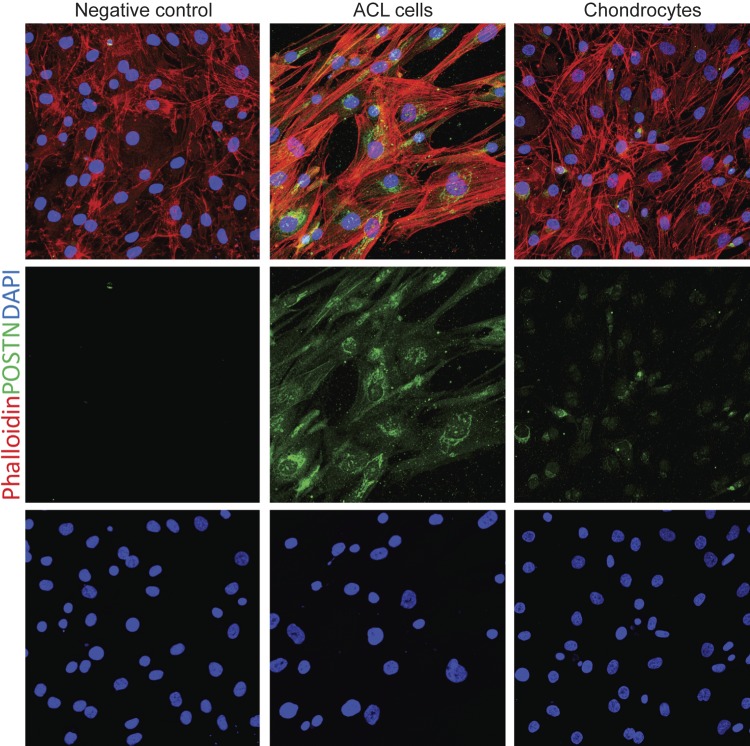
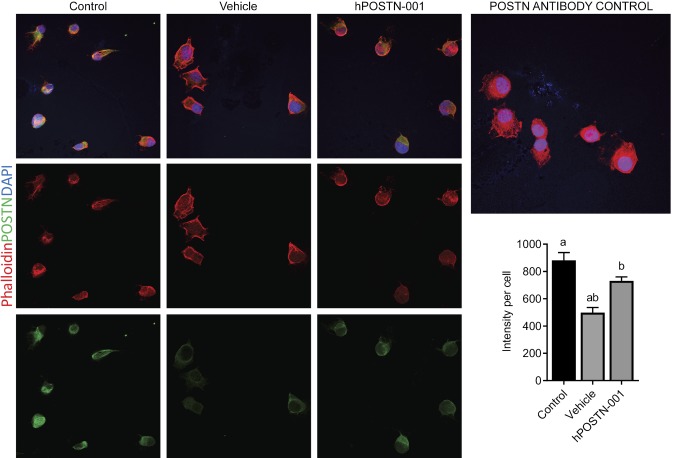
Immunostaining of human ACL progenitor cells and chondrocytes (3 biologic replicates) revealed that POSTN was highly present in ACL cells, whereas chondrocytes showed faint signal for POSTN (Fig. 2).
Figure 2.
POSTN immunostaining in ACL cells and chondrocytes. To confirm and compare POSTN protein in ACL progenitor cells and chondrocytes, we performed the immunostaining for POSTN. Immunostaining of human ACL progenitor cells (n = 3) and chondrocytes (n = 3) revealed that POSTN was highly present in ACL cells, whereas chondrocytes showed faint signal for POSTN. Phalloidin (red-orange) was used to visualize F-actin, DAPI (blue) staining showed nuclei, and POSTN was shown by green staining. For POSTN antibody control, we used chondrocytes as shown in the left panel. Magnification value, ×400.
Expression pattern of POSTN and its transcript variants in cells
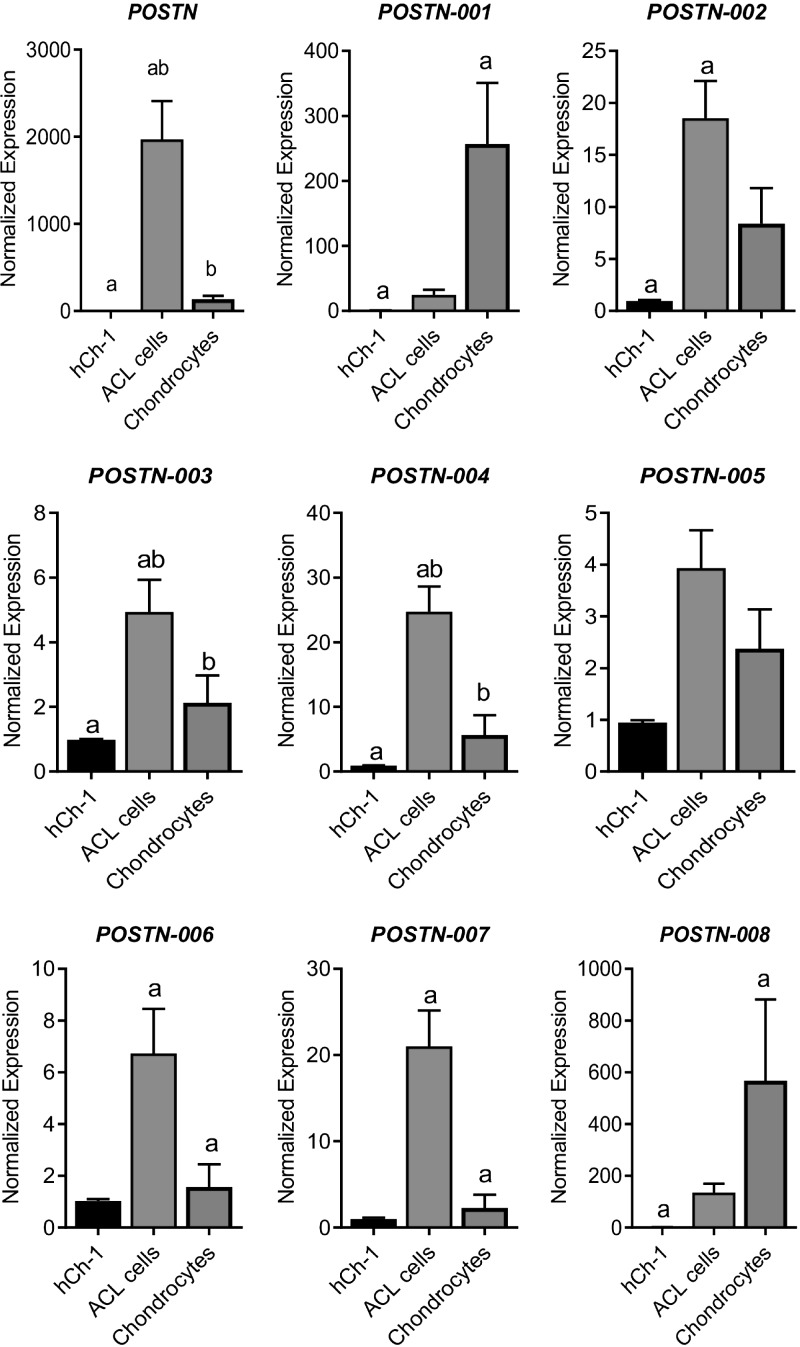
Next, we tested the expression of POSTN and its all known transcript variants in ACL cells, chondrocytes, and an hCh-1 cell line (Fig. 3). Our data showed that total POSTN was significantly higher in ACL cells compared with both hCh-1 cells and chondrocytes. In addition, we observed that various isoforms exhibited different expression patterns. Isoforms 1 and 8 showed a similar pattern: both were significantly higher in chondrocytes compared with hCh-1 cells. Isoform 2 was significantly higher in ACL cells compared with hCh-1 cells. Isoforms 3 and 4 showed a similar pattern to that of total POSTN: both were significantly higher in ACL cells compared with both hCh-1 cells and chondrocytes. Isoform 5 did not show any significant difference in any comparison tested. Lastly, isoforms 6 and 7 showed a similar pattern: both were significantly higher in ACL cells than chondrocytes.
Figure 3.
Expression pattern of POSTN and its splice variants in hCh-1 cells, chondrocytes, and ACL progenitor cells. To determine whether POSTN and its splice variants are differentially expressed, we compared the expressions profile in hCh-1 cells (n = 3), articular chondrocytes (n = 9), and ACL progenitor cells (n = 6). We observed that POSTN mRNA was significantly higher in ACL progenitor cells compared with both hCh-1 cells and chondrocytes. Expression of splice variants showed 3 patterns: those highly expressed in chondrocytes (isoforms 1 and 8), those highly expressed in ACL progenitor cells (isoforms 2, 3, 4, 6 and 7), and those that showed no significant difference (isoform 5). Similar lowercase letters (a, b) in each graph represent statistical significance from each other at P < 0.05.
Effect of POSTN overexpression on isoform expression
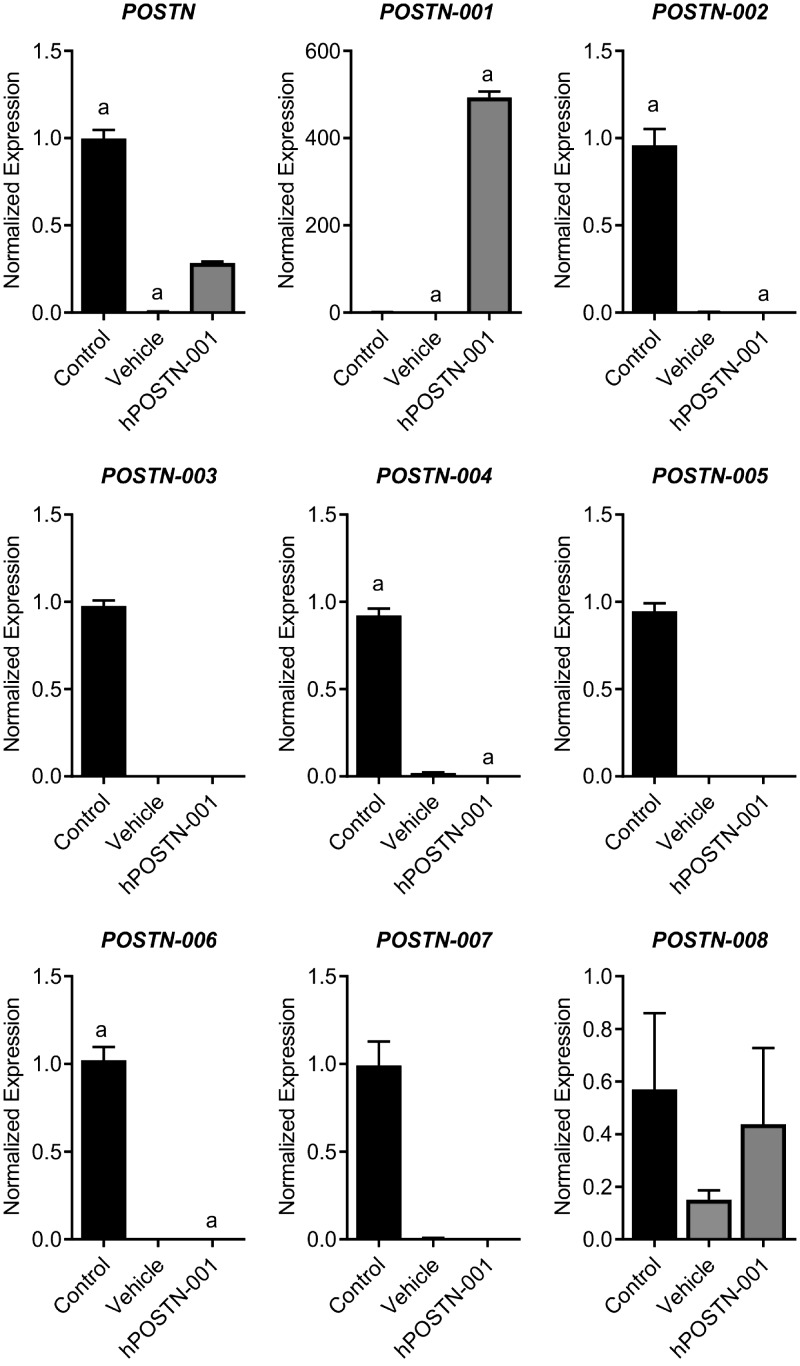
Because the real-time PCR data confirmed that hCh-1 cells lacked both total POSTN mRNA and isoform 1, we transfected these cells using a sequence-specific plasmid of full-length isoform 1. After stable transfection and drug (neomycin) selection for ∼2 wk, the expression of isoform 1 was exclusively present in hCh-1 cells transfected with pcDNA3.1-hPOSTN-001 plasmid and absent in vehicle and control cells (Fig. 4). There was no effect of this transfection on other isoforms. Immunofluorescence analysis of total POSTN showed increased signals in control cells and hPOSTN-001 cells compared to vehicle cells as shown in Fig. 5.
Figure 4.
Expression pattern of POSTN and its splice variants in hCh-1 cells stably transfected clones. To determine whether POSTN and its splice variants are differentially expressed, we compared the expression profile of nontransfected hCh-1 cells (control, n = 3), vehicle cells (vehicle, n = 3), and cells transfected with pcDNA3.1–hPOSTN-001 (hPOSTN-001, n = 3). We observed that POSTN mRNA was significantly higher in control compared with vehicle cells. POSTN isoform 1 was significantly higher on hPOSTN-001 cells compared with control and vehicle cells. Lowercase letters (a) in each graph represent statistical significance from each other at P < 0.05.
Figure 5.
POSTN immunostaining in hCh-1 cells. To detect POSTN protein in hCh-1 nontransfected cells (control, n = 3), vehicle-transfected cells (vehicle, n = 3), and cells transfected with pcDNA3.1-hPOSTN-001 (hPOSTN-001, n = 3), cells were subjected to immunofluorescence. Phalloidin (red-orange) was used to visualize F-actin, DAPI (blue) staining showed nuclei, and POSTN was shown by green staining. Magnification value, ×400. Quantification showed that control and hPOSTN-001 cells had significantly higher levels of POSTN signals than vehicle cells. These data reflected the same pattern as shown by real-time PCR. Similar lowercase letters (a, b) in each graph represent statistical significance from each other at P < 0.05.
Effect of hPOSTN-001 overexpression on global gene expression
RNA-seq
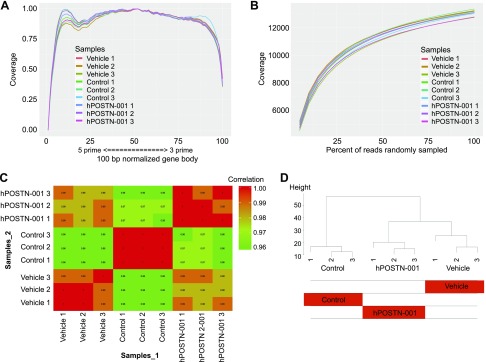
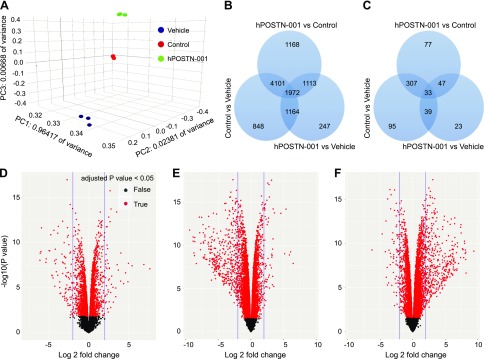
We subjected cDNAs from pcDNA3.1–POSTN-001–transfected (hPOSTN-001), vehicle-transfected (vehicle), and nontransfected (control) cells to RNA-seq. The known junction saturation (Fig. 6A) and read distribution over known gene models (Fig. 6B) were quantified with RSeQC. Performance of the samples was assessed with Spearman correlations (Fig. 6C) and hierarchical clustering (Fig. 6D). A mean of 19,172,669 50-bp single-end reads per sample were aligned to the Ensembl Genome Reference Consortium human genome reference sequence 38.76. Our data showed that 11,434 genes out of 14,506 genes detected were significantly (unadjusted P value ≤0.05) differentially expressed between the 3 groups. Out of these, 10,613 passed FDR of 0.05 at any fold change. As shown in Fig. 7A, 247 unique genes were expressed between hPOSTN-001 and vehicle (Table 4 and Supplemental Table S1), 1168 between hPOSTN-001 and control (Table 5 and Supplemental Table S2), and 848 between control and vehicle (Table 6 and Supplemental Table S3) with FDR ≤ 0.05. At 2-fold change and FDR ≤ 0.05, 23 unique genes were expressed between hPOSTN-001 and vehicle, 77 between hPOSTN-001 and control, and 95 between control and vehicle (Fig. 7B, C).
Figure 6.
RNA-seq analysis. A) End bias plot showing the alignment of all reads across a theoretical 100-bp normalized gene body. All samples (n = 3 per group) showed excellent coverage from 3′ to 5′, indicating that sampling of exons for isoform quantification should be uniform and robust. B) Junction saturation plot illustrating the random sampling of reads from all aligned BAM files for each sample in 5% intervals, where the total number of reads spanning known exon-exon junctions are summed for each interval and plotted to find the point of junction saturation. A plateau for each sample at or near 100% sampling indicates that each sample was sequenced to near complete junction saturation and that any further sequencing would only increase sequencing depth for each detected gene rather than discover new genes not yet detected. C) Spearman correlation matrix of limma voomWithQualityWeights transformed expression values. D) Hierarchical clustering of the limma voomWithQualityWeights transformed expression values; the heatmap of model coefficients shows that all samples cluster as would be expected according to transfection status.
Figure 7.
RNA-seq quantitative data. A) Three-dimensional principle components analysis illustrating that 96.4% of all variance can be accounted for in the first principle component and that all samples cluster by their respective transfection status. B, C) Venn diagrams showing number of differentially expressed transcripts in each comparison and the overlaps between any 2 or more comparisons. The numbers of transcripts shown in overlapping areas illustrate the number of transcripts common to 2 or more comparisons. All numbers are given using ANOVA at P < 0.05. The numbers of significantly differentially expressed transcripts at any fold change (B) or at ≥2-fold (C) are depicted in the form of Venn diagrams. D–F) Volcano plot of all genes expressed at >1 count per million in all samples: hPOSTN-001 vs. vehicle (D), hPOSTN-001 vs. control (E), and control vs. vehicle (F). The observed log2 fold change is on the x axis and the unadjusted P value converted to the –log10 scale is on the y axis. All genes with Benjamini-Hochberg adjusted P values ≤0.05 are highlighted in red.
TABLE 4.
Gene transcripts differentially expressed between hPOSTN-001 and vehicle cells
| Gene symbol | Gene name | P | FDR | Fold change |
|---|---|---|---|---|
| Gene transcripts elevated in hPOSTN-001 cells compared with vehicle cells | ||||
| MOV10L1 | Mov10 RISC complex RNA helicase–like 1 | <0.001 | <0.001 | 205.52 |
| HSD17B3 | Hydroxysteroid 17-β dehydrogenase 3 | <0.001 | <0.001 | 44.18 |
| CMKLR1 | Chemerin chemokine-like receptor 1 | <0.001 | <0.001 | 32.04 |
| POSTN | Periostin | <0.001 | <0.001 | 30.78 |
| TRPM3 | Transient receptor potential cation channel subfamily M member 3 | <0.001 | <0.001 | 25.00 |
| DRD2 | Dopamine receptor D2 | <0.001 | <0.001 | 21.12 |
| BRINP1 | BMP/retinoic acid inducible neural specific 1 | <0.001 | <0.001 | 16.07 |
| MASP1 | Mannan binding lectin serine peptidase 1 | <0.001 | <0.001 | 13.18 |
| TMEM119 | Transmembrane protein 119 | <0.001 | <0.001 | 11.86 |
| ALPL | Alkaline phosphatase, liver/bone/kidney | <0.001 | <0.001 | 11.38 |
| GRIK3 | Glutamate ionotropic receptor kainate type subunit 3 | <0.001 | 0.001 | 10.54 |
| CDH4 | Cadherin 4 | <0.001 | <0.001 | 8.19 |
| PCDH19 | Protocadherin 19 | <0.001 | <0.001 | 7.41 |
| COL6A3 | Collagen type VI α 3 chain | <0.001 | <0.001 | 6.95 |
| KRT18 | Keratin 18 | 0.002 | 0.009 | 6.79 |
| FAM180A | Family with sequence similarity 180 member A | <0.001 | <0.001 | 6.71 |
| PCSK2 | Proprotein convertase subtilisin/kexin type 2 | <0.001 | <0.001 | 6.41 |
| PDE4B | Phosphodiesterase 4B | <0.001 | <0.001 | 6.41 |
| CALN1 | Calneuron 1 | <0.001 | <0.001 | 6.03 |
| HS3ST1 | Heparan sulfate-glucosamine 3-sulfotransferase 1 | <0.001 | <0.001 | 6.03 |
| Gene transcripts repressed in hPOSTN-001 cells compared with vehicle cells | ||||
| RIPPLY3 | Ripply transcriptional repressor 3 | <0.001 | <0.001 | −70.45 |
| LUZP2 | Leucine zipper protein 2 | <0.001 | <0.001 | −68.84 |
| ITGA8 | Integrin subunit α 8 | <0.001 | <0.001 | −64.64 |
| CNTN1 | Contactin 1 | <0.001 | <0.001 | −61.46 |
| OLFM3 | Olfactomedin 3 | <0.001 | <0.001 | −41.78 |
| MAP10 | Microtubule associated protein 10 | <0.001 | <0.001 | −40.75 |
| EVA1A | Eva-1 homolog A, regulator of programmed cell death | <0.001 | <0.001 | −31.50 |
| SOSTDC1 | Sclerostin domain containing 1 | <0.001 | <0.001 | −30.16 |
| MUM1L1 | MUM1-like 1 | <0.001 | <0.001 | −27.39 |
| RGS4 | Regulator of G-protein signaling 4 | <0.001 | <0.001 | −24.82 |
| PABPC4L | Poly(A) binding protein cytoplasmic 4–like | <0.001 | <0.001 | −24.19 |
| SEMA3D | Semaphorin 3D | <0.001 | <0.001 | −22.57 |
| PIEZO2 | Piezo type mechanosensitive ion channel component 2 | <0.001 | <0.001 | −20.91 |
| EPHA6 | EPH receptor A6 | <0.001 | <0.001 | −19.66 |
| DMBX1 | Diencephalon/mesencephalon homeobox 1 | <0.001 | <0.001 | −19.30 |
| TBX15 | T-box 15 | <0.001 | <0.001 | −18.45 |
| PEX5L | Peroxisomal biogenesis factor 5–like | <0.001 | <0.001 | −18.16 |
| SLC38A4 | Solute carrier family 38 member 4 | <0.001 | <0.001 | −17.28 |
| PIK3C2G | Phosphatidylinositol-4-phosphate 3-kinase catalytic subunit type 2 γ | <0.001 | <0.001 | −16.84 |
| RERGL | RERG-like | <0.001 | <0.001 | −14.88 |
Only protein-coding transcripts are shown.
TABLE 5.
Gene transcripts differentially expressed between hPOSTN-001 and control cells
| Gene symbol | Gene name | P | FDR | Fold change |
|---|---|---|---|---|
| Gene transcripts elevated in hPOSTN-001 cells compared with control cells | ||||
| MOV10L1 | Mov10 RISC complex RNA helicase–like 1 | <0.001 | <0.001 | 90.80 |
| GJB2 | Gap junction protein β 2 | <0.001 | <0.001 | 60.18 |
| LRP1B | LDL receptor related protein 1B | <0.001 | <0.001 | 42.41 |
| HSD17B3 | Hydroxysteroid 17-β dehydrogenase 3 | <0.001 | <0.001 | 36.43 |
| ZIC3 | Zic family member 3 | <0.001 | <0.001 | 24.05 |
| DCLK1 | Doublecortin-like kinase 1 | <0.001 | <0.001 | 12.20 |
| APCDD1 | APC down-regulated 1 | <0.001 | <0.001 | 10.57 |
| RSPO3 | R-spondin 3 | <0.001 | <0.001 | 10.47 |
| CTNNA3 | Catenin α 3 | <0.001 | <0.001 | 10.09 |
| DACH2 | Dachshund family transcription factor 2 | <0.001 | <0.001 | 9.84 |
| MPZL2 | Myelin protein zero-like 2 | <0.001 | <0.001 | 9.48 |
| CMKLR1 | Chemerin chemokine-like receptor 1 | <0.001 | <0.001 | 9.17 |
| APLNR | Apelin receptor | <0.001 | <0.001 | 8.99 |
| TRPM3 | Transient receptor potential cation channel subfamily M member 3 | <0.001 | <0.001 | 8.81 |
| LRRC17 | Leucine-rich repeat containing 17 | <0.001 | <0.001 | 7.22 |
| SYT4 | Synaptotagmin 4 | <0.001 | <0.001 | 7.17 |
| SOX21 | SRY-box 21 | <0.001 | <0.001 | 7.07 |
| PCSK2 | Proprotein convertase subtilisin/kexin type 2 | <0.001 | <0.001 | 6.83 |
| PCDH10 | Protocadherin 10 | <0.001 | <0.001 | 6.50 |
| HS3ST1 | Heparan sulfate-glucosamine 3-sulfotransferase 1 | <0.001 | <0.001 | 6.18 |
| Gene transcripts repressed in hPOSTN-001 cells compared with control cells | ||||
| PCDHB5 | Protocadherin β 5 | <0.001 | <0.001 | −667.80 |
| MARCKS | Myristoylated alanine-rich PKC substrate | <0.001 | <0.001 | −464.80 |
| TSPYL5 | TSPY-like 5 | <0.001 | <0.001 | −461.22 |
| HIST1H2BE | Histone cluster 1 H2B family member e | <0.001 | <0.001 | −423.59 |
| PARVA | Parvin α | <0.001 | <0.001 | −401.67 |
| MYL1 | Myosin light chain 1 | <0.001 | <0.001 | −390.57 |
| H2AFJ | H2A histone family member J | <0.001 | <0.001 | −387.34 |
| HIST1H3I | Histone cluster 1 [3H] family member i | <0.001 | <0.001 | −331.21 |
| TGM3 | Transglutaminase 3 | <0.001 | <0.001 | −238.97 |
| PIK3C2G | Phosphatidylinositol-4-phosphate 3-kinase catalytic subunit type 2 γ | <0.001 | <0.001 | −229.54 |
| ZXDA | Zinc finger X-linked duplicated A | <0.001 | <0.001 | −211.53 |
| CDH19 | Cadherin 19 | <0.001 | <0.001 | −203.42 |
| MELTF | Melanotransferrin | <0.001 | <0.001 | −186.80 |
| TMEM47 | Transmembrane protein 47 | <0.001 | <0.001 | −162.18 |
| ARMCX2 | Armadillo repeat containing X-linked 2 | <0.001 | <0.001 | −158.24 |
| MAP10 | Microtubule associated protein 10 | <0.001 | <0.001 | −157.43 |
| COL22A1 | Collagen type XXII α 1 chain | <0.001 | <0.001 | −156.85 |
| HCK | HCK proto-oncogene, Src family tyrosine kinase | <0.001 | <0.001 | −151.10 |
| COL1A1 | Collagen type I α 1 chain | <0.001 | <0.001 | −146.07 |
| TOMM40L | Translocase of outer mitochondrial membrane 40–like | <0.001 | <0.001 | −139.23 |
Only protein-coding transcripts are shown.
TABLE 6.
Gene transcripts differentially expressed between control and vehicle cells
| Gene symbol | Gene name | P | FDR | Fold change |
|---|---|---|---|---|
| Gene transcripts elevated in control cells compared with vehicle cells | ||||
| MARCKS | Myristoylated alanine-rich PKC substrate | <0.001 | <0.001 | 686.06 |
| HIST1H3I | Histone cluster 1 [3H] family member i | <0.001 | <0.001 | 299.91 |
| DMD | Dystrophin | <0.001 | <0.001 | 292.68 |
| ZNF714 | Zinc finger protein 714 | <0.001 | <0.001 | 287.84 |
| ZNF91 | Zinc finger protein 91 | <0.001 | <0.001 | 275.43 |
| MYL1 | Myosin light chain 1 | <0.001 | <0.001 | 271.62 |
| H2AFJ | H2A histone family member J | <0.001 | <0.001 | 255.43 |
| TSPYL5 | TSPY-like 5 | <0.001 | <0.001 | 250.62 |
| ZXDA | Zinc finger X-linked duplicated A | <0.001 | <0.001 | 231.36 |
| IBSP | Integrin binding sialoprotein | <0.001 | <0.001 | 217.27 |
| HCK | HCK proto-oncogene, Src family tyrosine kinase | <0.001 | <0.001 | 205.73 |
| TMEM47 | Transmembrane protein 47 | <0.001 | <0.001 | 178.78 |
| MELTF | Melanotransferrin | <0.001 | <0.001 | 176.23 |
| ZNF429 | Zinc finger protein 429 | <0.001 | <0.001 | 169.58 |
| TMEM119 | Transmembrane protein 119 | <0.001 | <0.001 | 168.70 |
| ALDH1A1 | Aldehyde dehydrogenase 1 family member A | <0.001 | <0.001 | 134.53 |
| NDNF | Neuron derived neurotrophic factor | <0.001 | <0.001 | 120.87 |
| CDCA7L | Cell division cycle associated 7–like | <0.001 | <0.001 | 120.67 |
| ARMCX2 | Armadillo repeat containing X-linked 2 | <0.001 | <0.001 | 114.66 |
| LPCAT2 | Lysophosphatidylcholine acyltransferase 2 | <0.001 | <0.001 | 112.10 |
| Gene transcripts repressed in control cells compared with vehicle cells | ||||
| DACH2 | Dachshund family transcription factor 2 | <0.001 | <0.001 | −76.22 |
| MPZL2 | Myelin protein zero–like 2 | <0.001 | <0.001 | −25.64 |
| DLEC1 | Deleted in lung and esophageal cancer 1 | <0.001 | <0.001 | −18.35 |
| CNTN1 | Contactin 1 | <0.001 | <0.001 | −17.02 |
| MAL2 | Mal, T-cell differentiation protein 2 (gene/pseudogene) | <0.001 | <0.001 | −16.55 |
| PCDH10 | Protocadherin 10 | <0.001 | <0.001 | −14.19 |
| GJB2 | Gap junction protein β 2 | <0.001 | <0.001 | −12.89 |
| CSRNP3 | Cysteine and serine-rich nuclear protein 3 | <0.001 | <0.001 | −12.38 |
| BHLHE22 | Basic helix-loop-helix family member e22 | <0.001 | <0.001 | −11.18 |
| APCDD1 | APC down-regulated 1 | <0.001 | <0.001 | −10.39 |
| SCN1A | Sodium voltage-gated channel α subunit 1 | <0.001 | <0.001 | −10.03 |
| NRCAM | Neuronal cell adhesion molecule | <0.001 | <0.001 | −9.92 |
| PTPRC | Protein tyrosine phosphatase, receptor type C | <0.001 | <0.001 | −9.22 |
| EPYC | Epiphycan | <0.001 | <0.001 | −8.75 |
| MUM1L1 | MUM1-like 1 | <0.001 | <0.001 | −8.25 |
| EPHA6 | EPH receptor A6 | <0.001 | <0.001 | −8.09 |
| RAI2 | Retinoic acid induced 2 | <0.001 | <0.001 | −7.61 |
| NXPH2 | Neurexophilin 2 | <0.001 | <0.001 | −7.46 |
| LRP1B | LDL receptor related protein 1B | <0.001 | <0.001 | −7.45 |
| GPR50 | GPCR 50 | <0.001 | <0.001 | −7.36 |
Only protein-coding transcripts are shown.
Molecular function of differentially expressed transcripts
Genes that were distinct between any 2 groups were used for GO enrichment. We focused on the differences in the molecular functions significantly enriched in each comparison. We found that genes elevated in control cells compared with vehicle cells represented sulfur compound binding, transmembrane receptor activity, extracellular matrix binding, cell adhesion binding, proteoglycan binding, and growth factor binding. Genes repressed in control cells were mainly enriched for catalytic activity and enhancer sequence-specific DNA binding (Supplemental Fig. S4A). Comparison between hPOSTN-001 and vehicle cells revealed that genes elevated in the former were enriched for cell adhesion molecule, cadherin binding, and calcium ion binding and structural molecular integrity, whereas genes repressed in the former were enriched for neuropeptide receptor activity and SH3/SH2 adaptor activity (Supplemental Fig. S4B). Genes elevated in hPOSTN-001 cells compared with control cells were enriched for catalytic activity and protein transferase and ligase activity. In contrast, genes repressed in hPOSTN-001 cells were enriched for a number of molecular function terms such as sulfur compound binding, transmembrane receptor activity, growth factor binding, and collagen binding (Supplemental Fig. S4C).
Validation by real-time PCR
We observed that all 25 tested genes showed a similar gene expression pattern as that of observed by RNA-seq analysis (Table 7). The real-time PCR–derived fold changes were very similar to the RNA-seq fold changes reported from the limma with voom analysis. All genes tested by real-time PCR were significantly differentially regulated in both the analysis of the RNA-seq results via limma with voom as well as the GraphPad Mann-Whitney U tests performed on the real-time PCR results. These observations indicate a strong concordance and reliability of the RNA-seq findings.
TABLE 7.
Validation of RNA-seq data using real-time PCR for selected genes
| Gene symbol | Control vs. vehicle |
hPOSTN-001
vs. control |
hPOSTN-001
vs. vehicle |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RNA-seq |
Real-time PCR |
RNA-seq |
Real-time PCR |
RNA-seq |
Real-time PCR |
|||||||
| Fold change | P | Fold change | P | Fold change | P | Fold change | P | Fold change | P | Fold change | P | |
| MARCKS | 686.06 | <0.001 | ∞ | — | −464.80 | <0.001 | ∞ | — | n.s. | — | 0.00 | — |
| HIST1H3I | 299.91 | <0.001 | 1489.74 | <0.001 | −331.21 | <0.001 | −1950.95 | <0.001 | n.s. | — | −1.31 | 0.128 |
| DMD | 292.68 | <0.001 | 185.85 | <0.001 | −87.37 | <0.001 | −179.32 | <0.001 | n.s. | — | 1.04 | 0.943 |
| ZNF714 | 287.84 | <0.001 | 693.09 | <0.001 | −107.52 | <0.001 | −901.34 | <0.001 | n.s. | — | −1.30 | 0.531 |
| ZNF91 | 275.43 | <0.001 | 4.77 | <0.001 | −74.55 | <0.001 | −5.11 | <0.001 | n.s. | — | −1.07 | 0.475 |
| MAL2 | −16.55 | <0.001 | −13.83 | <0.001 | n.s. | — | 1.76 | 0.001 | −12.60 | <0.001 | −7.88 | <0.001 |
| CNTN1 | −17.02 | <0.001 | −15.52 | <0.001 | −3.61 | <0.001 | −3.42 | <0.001 | −61.46 | <0.001 | −53.02 | <0.001 |
| DLEC1 | −18.35 | <0.001 | −337.95 | 0.001 | 2.47 | 0.002 | 26.16 | 0.002 | −7.43 | <0.001 | −12.92 | 0.001 |
| MPZL2 | −25.64 | <0.001 | ∞ | — | 9.48 | <0.001 | ∞ | — | −2.71 | <0.001 | −6.10 | 0.001 |
| DACH2 | −76.22 | <0.001 | −77.45 | <0.001 | 9.84 | <0.001 | 6.35 | <0.001 | −7.75 | <0.001 | −12.21 | <0.001 |
| GJB2 | −12.89 | <0.001 | −10.09 | <0.001 | 60.18 | <0.001 | 65.11 | <0.001 | 4.67 | <0.001 | 6.45 | <0.001 |
| LRP1B | −7.45 | <0.001 | −7.19 | <0.001 | 42.41 | <0.001 | 46.62 | <0.001 | 5.70 | <0.001 | 6.48 | <0.001 |
| HSD17B3 | n.s. | — | 1.11 | 0.553 | 36.43 | <0.001 | −1.04 | 0.718 | 44.18 | <0.001 | 1.07 | 0.690 |
| ZIC3 | −5.20 | <0.001 | −6.64 | <0.001 | 24.05 | <0.001 | 29.75 | <0.001 | 4.63 | <0.001 | 4.48 | 0.000 |
| PARVA | 80.56 | <0.001 | 3044.90 | <0.001 | −401.67 | <0.001 | −3490.57 | <0.001 | −4.99 | 0.011 | −1.15 | 0.777 |
| HIST1H2BE | 108.21 | <0.001 | −1.63 | 0.005 | −423.59 | <0.001 | 1.18 | 0.135 | n.s. | — | −1.39 | 0.005 |
| TSPYL5 | 250.62 | <0.001 | 136,573.66 | 0.006 | −461.22 | <0.001 | −61,464.31 | 0.006 | n.s. | — | 2.22 | 0.222 |
| PCDHB5 | 94.91 | <0.001 | 107.79 | <0.001 | −667.80 | <0.001 | −120.79 | <0.001 | −7.04 | <0.001 | −1.12 | 0.195 |
| CMKLR1 | 3.49 | 0.002 | 4.49 | <0.001 | 9.17 | <0.001 | 11.63 | <0.001 | 32.04 | <0.001 | 52.22 | <0.001 |
| TRPM3 | 2.84 | 0.015 | −1.51 | 0.558 | 8.81 | <0.001 | 17.71 | <0.001 | 25.00 | <0.001 | 11.75 | <0.001 |
| DRD2 | 12.71 | <0.001 | 16.03 | <0.001 | 1.66 | 0.006 | 1.50 | 0.008 | 21.12 | <0.001 | 24.12 | <0.001 |
| OLFM3 | n.s. | — | 1.27 | 0.077 | −50.24 | <0.001 | −336.41 | <0.001 | −41.78 | <0.001 | −265.75 | <0.001 |
| ITGA8 | −1.82 | <0.001 | −1.44 | 0.005 | −35.53 | <0.001 | −101.14 | <0.001 | −64.64 | <0.001 | −145.75 | <0.001 |
| LUZP2 | −3.57 | <0.001 | −6.12 | <0.001 | −19.31 | <0.001 | −5.42 | <0.001 | −68.84 | <0.001 | −33.15 | <0.001 |
| RIPPLY3 | n.s. | — | −1.13 | 0.286 | −52.75 | <0.001 | −61.36 | <0.001 | −70.45 | <0.001 | −69.33 | <0.001 |
n.s., not significant.
Effect of POSTN and hPOSTN-001 on cell adhesion
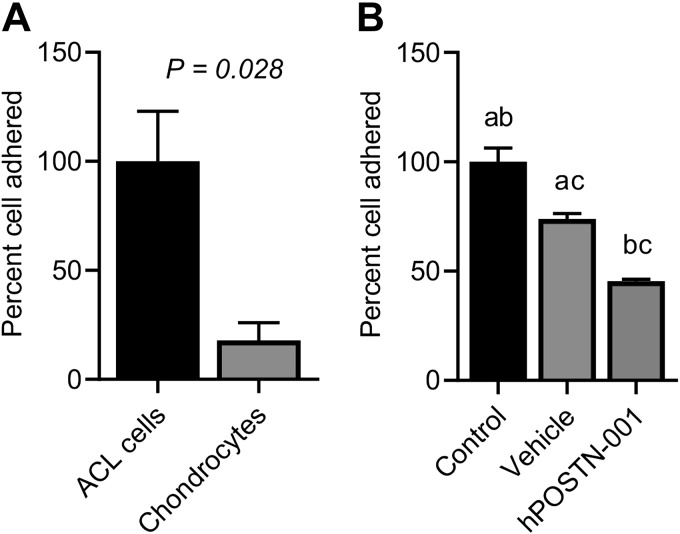
Quantitative cell adhesion assays showed different adhesion capacity of cells depending on the expression of total POSTN or hPOSTN-001 (Fig. 8). We observed that cells that highly expressed total POSTN (i.e., ACL progenitor cells), had significantly higher adhesion potential compared with chondrocytes that expressed lower total POSTN (Fig. 8A). Similarly, hCh-1 cells that had higher expression of total POSTN also had higher degree of cell adhesion compared with vehicle- and hPOSTN-001 cells, both of which had low expression of total POSTN (Fig. 8B). In addition, we found that cells that had higher expression of isoform 1 had lower cell adhesion. Specifically, chondrocytes that showed higher expression of isoform 1 had significantly low cell adhesion than ACL progenitor cells. Moreover, hPOSTN-001 cells expressing high levels of isoform 1 showed low percentage of adherent cells.
Figure 8.
Effect of POSTN or hPOSTN-001 on cell adhesion. Effect of total POSTN or hPOSTN-001 was examined on cell adhesion by Vybrant cell adhesion assay kit. A) We found that ACL progenitor cells (n = 3) that had higher expression of total POSTN compared with chondrocytes (n = 3) showed more cell adhesion potential. B) Similarly, control hCh-1 cells (n = 3) that had higher expression of total POSTN compared with vehicle (n = 3) and hPOSTN-001 trasfected cells (n = 3) also had significantly higher cell adhesion. Similar lowercase letters (a, b, c) in each graph represent statistical significance from each other at P < 0.05.
Expression of selected genes in ACL progenitor cells and chondrocytes
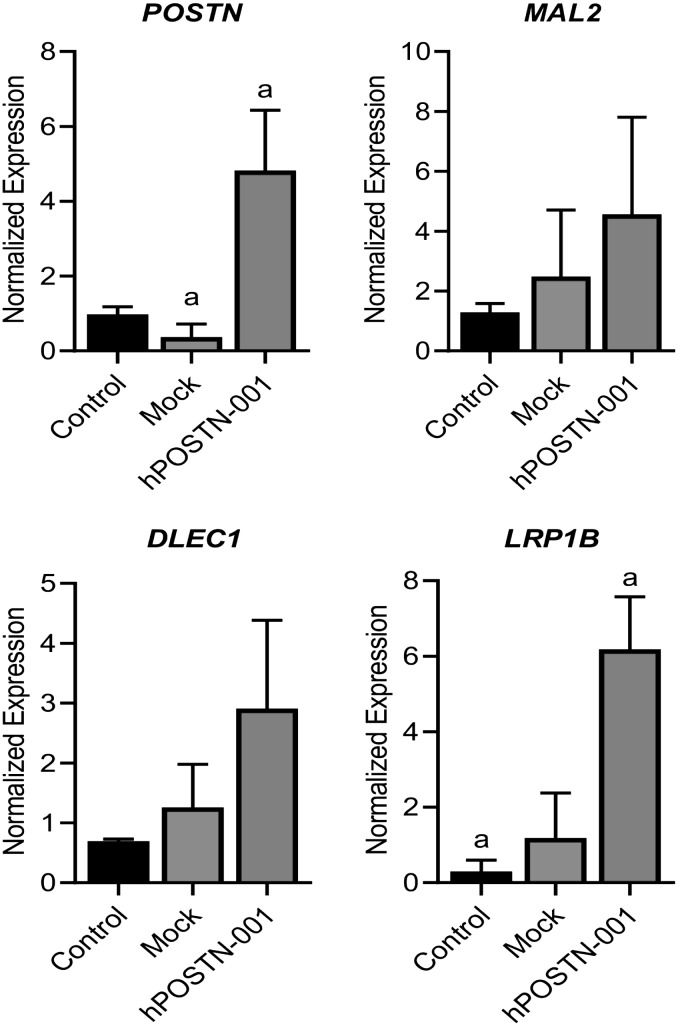
To get some insights into the role of total POSTN and hPOSTN-001 on genes chosen from RNA-seq analysis, we found that MARCKS and HIST1H3I were expressed at a higher level in ACL progenitor cells than in chondrocytes, whereas MAL2 and DLEC1 were expressed at a higher level in chondrocytes. Similarly, we found that TSPYL5 showed higher expression in ACL progenitor cells, whereas LRP1B showed higher expression in chondrocytes (Fig. 9).
Figure 9.
Real-time PCR of selected genes in ACL progenitor cells and chondrocytes. To get some insights into the role of total POSTN and POSTN isoform 1 on selected genes from RNA-seq analysis, we found that MARCKS, HIST1H3I, and PCDHB5 were more highly expressed in ACL progenitor cells (n = 5) than in chondrocytes (n = 6) and that MAL2, DLEC1, and LRP1B were significantly more highly expressed in chondrocytes than in ACL progenitor cells. Lowercase letters (a) in each graph represent statistical significance from each other at P < 0.05.
Effect of hPOSTN-001 overexpression on selected genes
As described above, mRNA expression of 3 genes, namely, MAL2, DLEC1, and LRP1B, was significantly suppressed in ACL progenitor cells that lacked expression of hPOSTN-001. To validate the effect of this isoform on expression of the aforementioned genes, ACL progenitor cells were transiently transfected with pcDNA3.1-hPOSTN-001. We observed that overexpression of hPOSTN-001 resulted in up-regulation of hPOSTN-001 and the other 3 genes of interest (MAL2, DLEC1, and LRP1B) as expected (Fig. 10).
Figure 10.
Effect of hPOSTN-001 overexpression on selected genes in ACL progenitor cells. To validate if the effect of hPOSTN-001 on 3 genes identified from RNA-seq analysis, we performed overexpression of POSTN isoform 1 in ACL progenitor cells (n = 3). We observed that overexpression of hPOSTN-001 in ACL progenitor cells resulted in up-regulation of mRNA expression of 3 genes, namely, MAL2, DLEC1, and LRP1B. Lowercase letters (a) in each graph represent statistical significance from each other at P < 0.05.
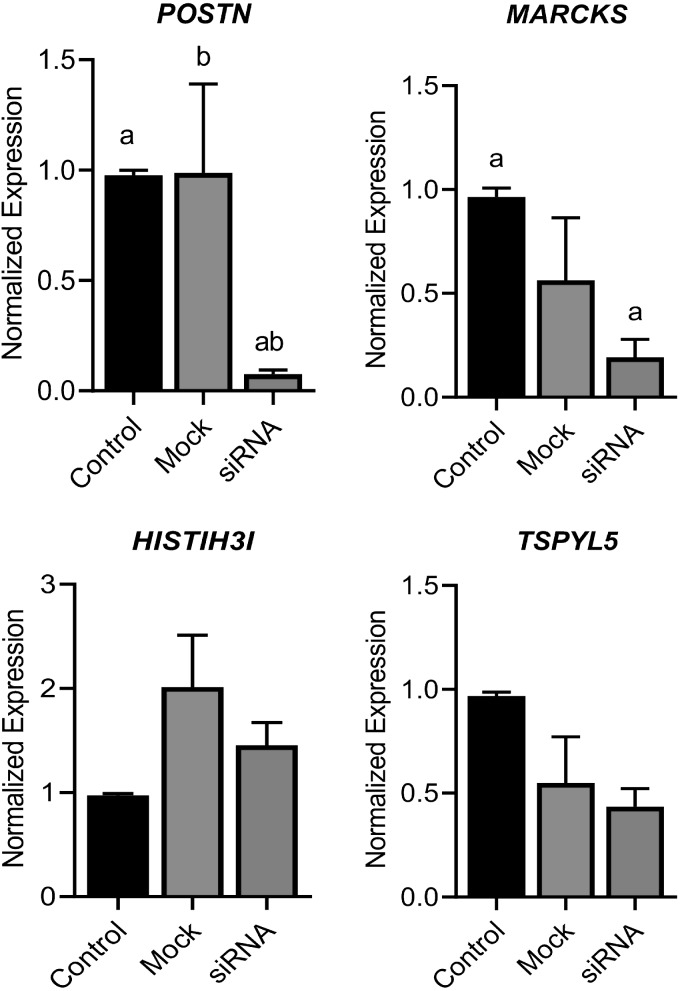
Effect of POSTN ablation on selected genes
Expression of 3 genes (MARCKS, HIST1H3I, and TSPYL5) was higher in ACL progenitor cells compared with chondrocytes. Because POSTN is overall higher in ACL progenitor cells, we knocked down its expression by siRNA and found that the expression of POSTN and other all 3 genes tested was significantly decreased in ACL progenitor cells lacking POSTN (Fig. 11).
Figure 11.
Effect of POSTN ablation on selected genes. To validate if the effect of POSTN on 3 genes identified from RNA-seq analysis, we performed POSTN knockdown in ACL progenitor cells (n = 5). Our data showed that knockdown of total POSTN from ACL progenitor cells resulted in suppression of these 3 genes: MARCKS, HIST1H3I, and TSPYL5. Similar lowercase letters (a, b) in each graph represent statistical significance from each other at P < 0.05.
DISCUSSION
In this study, we showed that ACL tissues had significantly higher expression of total POSTN compared with articular cartilage. This is the first study to compare the expression of POSTN between these 2 distinct knee joint tissues. Although studies have reported the expression of POSTN in cartilage (16, 17, 35) and available literature suggests that POSTN is a component of fibrous tissues such as ligaments, including ACL (36–38), our findings support the notion that POSTN is highly expressed in ACL tissues and that its levels are low in articular cartilage. Indeed, these expression levels are higher in osteoarthritis (OA) chondrocytes than in normal chondrocytes (16, 35). Interestingly, all 8 POSTN splice variants were significantly higher in ACL tissues than in the articular cartilage. Although the expression of total POSTN was also significantly higher in ACL progenitor cells compared with chondrocytes, there was a differential expression of different splice variants between these types of cells. Isoforms 1 and 8 were significantly highly expressed in chondrocytes, whereas others were mainly expressed in ACL progenitor cells. These findings suggest that different splice variants may have partially overlapping functions in different tissues and extracted cells. Moreover, the distinct expression patterns of isoforms 1 and 8 compared with the other splice variants suggest that these splice variants have a unique role in chondrocytes compared with the other transcript variants with more distinct functions in ACL cells.
The hCh-1 cells, which are chondrosarcoma cells, showed negligible expression levels for POSTN and its splice variants compared with both ACL cells and chondrocytes. Although there are reports that POSTN expression is elevated in metastatic tissues (39–41), some studies have reported its higher expression in 1 type of metastasis than others. For example, lower levels of POSTN expression were found in osteochondroma than in osteosarcoma (42). Given the significantly subtle levels of POSTN in these cells, we transfected hCh-1 cells with pcDNA3.1-hPOSTN-001 to enhance the expression of hPOSTN-001. Our data confirmed that clones stably transfected with pcDNA3.1-hPOSTN-001 indeed express higher levels of isoform 1. However, nontransfected control cells had significantly higher expression of total POSTN. RNA-seq analysis revealed a number of differentially expressed genes. The expression pattern of genes differentially expressed between control cells and vehicle cells was due to the total POSTN. However, changes in expression pattern for gene transcripts differentially expressed between hPOSTN-001 cells and vehicle cells were exclusively due to overexpression of POSTN isoform 1. Likewise, the expression pattern of genes differentially expressed between hPOSTN-001 cells and control cells could be due to isoform pattern (e.g., hPOSTN-001 cells have isoform 1 being highly overexpressed, whereas control cells have high expression of all isoforms).
Genes highly expressed in control cells (expressing high total POSTN) compared with vehicle cells were enriched for cell adhesion molecule binding, cadherin binding, calcium ion binding, structural molecule activity, and structural constituent of ribosomes. These findings are in line with many other studies that reported increase in cell adhesion following overexpression of POSTN. Overexpression of POSTN in a mouse osteoblast precursor cell line (MC3T3-E1) resulted in increased adhesion of these cells (43). Because cell adhesion is also mediated by E-cadherin, knockdown of the latter results in impaired cell adhesion (44). The effect of overexpression of POSTN on E-cadherin is cell dependent. For instance, POSTN stimulated E-cadherin expression in bladder cancer cells but suppressed it in prostate cancer cells (45). Although there are no reports of POSTN overexpression in hCh-1, our study is the first to suggest that genes related to cadherin binding are up-regulated in cells expressing POSTN. Calcium ion binding has also been recognized as a player in cell adhesion in conjunction with POSTN overexpression (46). Apart from its role in cell adhesion, POSTN has also been implicated in structural molecule activity (46).
In an attempt to dissect out the impact of hPOSTN-001 on global gene expression profiles, we compared the hPOSTN-001 cells and vehicle cells, because the former had significantly higher expression of isoform 1. Overall, genes highly expressed in hPOSTN-001 cells compared with vehicle cells were enriched for catalytic activity, ubiquitin protein ligase and transferase activity, and chromatin binding. Although the role of these molecular functions in the current study is not entirely clear, some molecular functions were repressed in the absence of isoform 1 in these cells, including sulfur compound binding, transmembrane receptor activity, growth factor binding, collagen binding, extracellular matrix structural constituent, and fibronectin binding. This may suggest that a lack of isoform 1 results in a negative impact on the structural constituents of a tissue. This is further supported by the results from hPOSTN-001 cells and control cells. In the transfected cells that have high expression of hPOSTN-001, genes were enriched for the same process as listed above. Further distinguishing the role of hPOSTN-001 from other isoforms, we observed that isoforms 2–7 were generally high in the nontransfected cells and that the molecular functions in this group included the same catalytic activity. These observations suggest that isoform 1 has a distinct role compared with total POSTN, expression of which is mainly driven by all other isoforms.
The transcripts that were highly differentially expressed, because of either overexpression of total POSTN (comparison of vehicle and nontransfected control cells) or overexpression of hPOSTN-001 (comparison of hPOSTN-001 cells and nontransfected control cells), suggest a distinction between isoform 1 and the other isoforms. Additional evidence for their distinct role comes from the comparison of 6 differentially expressed genes between ACL-derived cells that had higher expression of total POSTN and chondrocytes that had higher expression of isoform 1 (and isoform 8). Specifically, MARCKS, HIST1H3I, and TSPYL5 were down-regulated, whereas MAL2, DLEC1, and LRP1B were up-regulated in chondrocytes compared with ACL-derived cells. TSPYL5 (47), MAL2 (48), and LRP1B (49) are associated with tumorigenesis, whereas HIST1H3I plays a role in epigenetic regulation (50). MARCKS and DLEC1 have previously been associated with chondrocyte function and OA.
The role of MARCKS in chondrocyte function has just begun to emerge. MARCKS binds to calmodulin, actin, and synapsin. Phosphorylation of MARCKS has been shown to be increased in chondrocytes after IL-1 and oncostatin M, treatment suggesting a role in OA (51). A recent systematic analysis of transcriptomic profile of chondrocytes in osteoarthritic knee has shown that OA chondrocytes produce MARCKS (52). In contrast, DLEC1 has been shown to repress the expression of NF-κB signaling and regulate cell proliferation (53). Aberrant NF-κB activation has been implicated in the setting of inflammation (54) and is known to be associated with initiation of OA (55). Taken together, lower expression of MARCKS and higher expression of DLEC1 in chondrocytes suggest that cells lacking total POSTN appear to have less osteoarthritic phenotype, perhaps through the suppression of NF-κB signaling (35) compared with ACL-derived cells that express higher levels of total POSTN. These observations are in line with a previous report of higher expression of total POSTN in OA cartilage (16, 17, 35) and further support a distinct role of total POSTN compared with hPOSTN-001.
Although isoform 8 was not significantly different between control cells and hPOSTN-001 cells, it was more highly expressed in chondrocytes than in ACL-derived cells. We were unable to distinguish the role or roles of isoform 1 and 8, although it is plausible and perhaps likely that 1 or both play an important role or roles in chondrocytes. It remains elusive if these isoforms work in concert and whether or not they function in a cell autonomous way. Interestingly, isoform 1 and 8 are the only 2 isoforms in which exon 17 is not spliced. Future investigation to discern the role of these 2 isoforms within chondrocytes and between chondrocytes and ACL-derived cells could help answer these questions.
Previously, we (16) and others (56, 57) have shown that POSTN affects cell migration. In the current study, we show that cells expressing total POSTN exhibit heightened adhesion more than cells lacking POSTN or cells overexpressing isoform 1. As we know, POSTN was originally isolated from MC3T3-E1 osteoblast cells, where it was found to modulate cell adhesion (2), our data confirm that role in different cell types. Cell adhesion is an important process that facilitates adherence of cells with other cells or with their substrata. Adhesion of cells to the extracellular matrix regulates cell shape, proliferation, and intracellular signaling, as well as differentiation, and is therefore important in maintaining normal tissue function (58). Our findings, also for the first time, demonstrate that it is total POSTN, not isoform 1 alone, that influences cell adhesion. Although we cannot differentiate the function of isoforms 1 and 8 on cell adhesion in chondrocytes, it is tempting to believe that similar to isoform 1, isoform 8 also has no effect on cell adhesion, because both of these isoforms were high in chondrocytes that exhibited low cell adhesion.
Limitations of this study include the use of ACL progenitor cells instead of ACL fibroblasts to compare with chondrocytes. The available sample from ACL remnants was small, and we were unable to get the required number of cells via enzymatic digestion. Therefore, we began to culture these explants over a 3-wk time period and obtained a sufficient number of cells that crawled out of the tissues to complete this work. Moreover, ACL and articular cartilage tissues were obtained from patients undergoing ACL reconstruction surgery and total knee replacement, respectively. Healthy cartilage and ACL tissues typically are not accessible, tissues from cadavers are not available within a reasonable timeframe, and complete clinical history is usually lacking. RNA quality and cell yield are generally low given the time between death and processing of samples. We did not perform RNA-seq analysis on chondrocytes and ACL progenitor cells, although we did measure some key genes identified through RNA-seq analysis of hCh-1 cells. Cells were not transfected with total POSTN, because this would require either transfection of the cells with a large (about 36 kbp) genomic DNA or transfection of all 8 plasmids (corresponding to individual isoforms) at the same time, thus making it practically impossible to control the ratio of isoforms. We did not study the effect of POSTN knockdown on the isoform expression because we did not know a priori which specific isoforms were expressed in these cells, so we chose to transfect the cells with a full-length transcript (i.e., isoform 1). Furthermore, the siRNA available would knock down all isoforms, and there is no sequence to remove only 1 isoform from the cells. Finally, we were unable to distinguish the unique expression pattern of splice variants 1 and 8 with the use of RNA-seq because of their high degree of similarity and the limitations of single-end 50-bp sequencing reads to distinguish between repetitive elements shared across exons of the same gene that often define the differences between isoforms. As sequencing technology continues to improve and costs come down, we believe longer reads that span repetitive elements as well as splice junctions will ameliorate this shortcoming in follow-up studies.
Despite these limitations, we demonstrated differential expression of POSTN splice variants between cartilage and ACL tissues. Specifically, expression of isoforms 1 and 8 is predominant in chondrocytes, whereas the other isoforms are more abundant in ACL cells. In addition, total POSTN had a different impact on gene expression and cell function (adhesion) than isoform 1. More study is needed to understand if these splice variants have the capacity to interact with specific binding partners or induce specific signaling. The specific effects of POSTN isoform 1 overexpression suggest a complex regulation of this isoform in human chondrocytes. Further studies to assess the function and the ligand or binding partners of each splice variant are needed to better understand the complex role of POSTN in these specific tissues and the musculoskeletal system overall.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Washington University Genome Technology Access Center (GTAC) for assistance with RNA-seq analysis. This study was supported by research funds provided by the Department of Orthopaedic Surgery, Washington University. Dr. M. F. Rai is supported through the Pathway to Independence Award (R00-AR-064837) from the U.S. National Institutes of Health (NIH), National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NIAMS. The authors declare no conflicts of interest.
Glossary
- ACL
anterior cruciate ligament
- BLAST
Basic Local Alignment Search Tool
- FBS
fetal bovine serum
- FDR
false discovery rate
- GAGE
generally applicable gene set enrichment
- GO
gene ontology
- hCh-1
human chondrosarcoma 1
- hPOSTN-001
human POSTN isoform 1
- limma
linear models for microarray data
- NCBI
National Center for Biotechnology Information
- NGS
normal goat serum
- OA
osteoarthritis
- POSTN
periostin
- RNA-seq
RNA-sequencing
- siRNA
small interfering RNA
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting and revision of the manuscript, and all authors approved the final version to be published; Dr. M. F. Rai had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; L. Cai, R. H. Brophy, and M. F. Rai contributed to study conception and design; L. Cai, R. H. Brophy, E. D. Tycksen, X. Duan, R. M. Nunley, and M. F. Rai contributed to acquisition of data; and L. Cai, R. H. Brophy, E. D. Tycksen, X. Duan, and M. F. Rai contributed to analysis and interpretation of data.
REFERENCES
- 1.Coutu D. L., Wu J. H., Monette A., Rivard G. E., Blostein M. D., Galipeau J. (2008) Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J. Biol. Chem. 283, 17991–18001 [DOI] [PubMed] [Google Scholar]
- 2.Takeshita S., Kikuno R., Tezuka K., Amann E. (1993) Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 294, 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litvin J., Selim A. H., Montgomery M. O., Lehmann K., Rico M. C., Devlin H., Bednarik D. P., Safadi F. F. (2004) Expression and function of periostin-isoforms in bone. J. Cell. Biochem. 92, 1044–1061 [DOI] [PubMed] [Google Scholar]
- 4.Kim C. J., Isono T., Tambe Y., Chano T., Okabe H., Okada Y., Inoue H. (2008) Role of alternative splicing of periostin in human bladder carcinogenesis. Int. J. Oncol. 32, 161–169 [PubMed] [Google Scholar]
- 5.Horiuchi K., Amizuka N., Takeshita S., Takamatsu H., Katsuura M., Ozawa H., Toyama Y., Bonewald L. F., Kudo A. (1999) Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J. Bone Miner. Res. 14, 1239–1249 [DOI] [PubMed] [Google Scholar]
- 6.Hoersch S., Andrade-Navarro M. A. (2010) Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evol. Biol. 10, 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilman G., Mattiussi M., Brasseur F., van Baren N., Decottignies A. (2007) Human periostin gene expression in normal tissues, tumors and melanoma: evidences for periostin production by both stromal and melanoma cells. Mol. Cancer 6, 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimazaki M., Nakamura K., Kii I., Kashima T., Amizuka N., Li M., Saito M., Fukuda K., Nishiyama T., Kitajima S., Saga Y., Fukayama M., Sata M., Kudo A. (2008) Periostin is essential for cardiac healing after acute myocardial infarction. J. Exp. Med. 205, 295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu S., Barbe M. F., Liu C., Hadjiargyrou M., Popoff S. N., Rani S., Safadi F. F., Litvin J. (2009) Periostin-like-factor in osteogenesis. J. Cell. Physiol. 218, 584–592 [DOI] [PubMed] [Google Scholar]
- 10.Morra L., Rechsteiner M., Casagrande S., Duc Luu V., Santimaria R., Diener P. A., Sulser T., Kristiansen G., Schraml P., Moch H., Soltermann A. (2011) Relevance of periostin splice variants in renal cell carcinoma. Am. J. Pathol. 179, 1513–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai Y., Nakamura M., Zhou G., Li Y., Liu Z., Ozaki T., Mori I., Kakudo K. (2010) Novel isoforms of periostin expressed in the human thyroid. Jpn. Clin. Med. 1, 13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viloria K., Hill N. J. (2016) Embracing the complexity of matricellular proteins: the functional and clinical significance of splice variation. Biomol. Concepts 7, 117–132 [DOI] [PubMed] [Google Scholar]
- 13.Giusca S. E., Amalinei C., Lozneanu L., Ciobanu Apostol D., Andriescu E. C., Scripcariu A., Balan R., Avadanei E. R., Căruntu I. D. (2017) Heterogeneous periostin expression in different histological variants of papillary thyroid carcinoma. BioMed Res. Int. 2017, 8701386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norris R. A., Damon B., Mironov V., Kasyanov V., Ramamurthi A., Moreno-Rodriguez R., Trusk T., Potts J. D., Goodwin R. L., Davis J., Hoffman S., Wen X., Sugi Y., Kern C. B., Mjaatvedt C. H., Turner D. K., Oka T., Conway S. J., Molkentin J. D., Forgacs G., Markwald R. R. (2007) Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell. Biochem. 101, 695–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu S., Barbe M. F., Amin N., Rani S., Popoff S. N., Safadi F. F., Litvin J. (2008) Immunolocalization of Periostin-like factor and Periostin during embryogenesis. J. Histochem. Cytochem. 56, 329–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinzei N., Brophy R. H., Duan X., Cai L., Nunley R. M., Sandell L. J., Rai M. F. (2018) Molecular influence of anterior cruciate ligament tear remnants on chondrocytes: a biologic connection between injury and osteoarthritis. Osteoarthritis Cartilage 26, 588–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chijimatsu R., Kunugiza Y., Taniyama Y., Nakamura N., Tomita T., Yoshikawa H. (2015) Expression and pathological effects of periostin in human osteoarthritis cartilage. BMC Musculoskelet. Disord. 16, 215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rai M. F., Tycksen E. D., Sandell L. J., Brophy R. H. (2018) Advantages of RNA-seq compared to RNA microarrays for transcriptome profiling of anterior cruciate ligament tears. J. Orthop. Res. 36, 484–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brophy R. H., Tycksen E. D., Sandell L. J., Rai M. F. (2016) Changes in transcriptome-wide gene expression of anterior cruciate ligament tears based on time from injury. Am. J. Sports Med. 44, 2064–2075 [DOI] [PubMed] [Google Scholar]
- 20.Brophy R. H., Zhang B., Cai L., Wright R. W., Sandell L. J., Rai M. F. (2018) Transcriptome comparison of meniscus from patients with and without osteoarthritis. Osteoarthritis Cartilage 26, 422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozdemir C., Akpulat U., Sharafi P., Yıldız Y., Onbaşılar I., Kocaefe C. (2014) Periostin is temporally expressed as an extracellular matrix component in skeletal muscle regeneration and differentiation. Gene 553, 130–139 [DOI] [PubMed] [Google Scholar]
- 22.Rios H. F., Ma D., Xie Y., Giannobile W. V., Bonewald L. F., Conway S. J., Feng J. Q. (2008) Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J. Periodontol. 79, 1480–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chansky H., Robbins J. R., Cha S., Raskind W. H., Conrad E. U., Sandell L. J. (1998) Expression of cartilage extracellular matrix and potential regulatory genes in a new human chondrosarcoma cell line. J. Orthop. Res. 16, 521–530 [DOI] [PubMed] [Google Scholar]
- 24.Brophy R. H., Rai M. F., Zhang Z., Torgomyan A., Sandell L. J. (2012) Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J. Bone Joint Surg. Am. 94, 385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T. R. (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao Y., Smyth G. K., Shi W. (2014) featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Wang S., Li W. (2012) RSeQC: quality control of RNA-seq experiments. Bioinformatics 28, 2184–2185 [DOI] [PubMed] [Google Scholar]
- 28.Robinson M. D., McCarthy D. J., Smyth G. K. (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy D. J., Chen Y., Smyth G. K. (2012) Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie M. E., Phipson B., Wu D., Hu Y., Law C. W., Shi W., Smyth G. K. (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R., Holik A. Z., Su S., Jansz N., Chen K., Leong H. S., Blewitt M. E., Asselin-Labat M. L., Smyth G. K., Ritchie M. E. (2015) Why weight? Modelling sample and observational level variability improves power in RNA-seq analyses. Nucleic Acids Res. 43, e97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo W., Friedman M. S., Shedden K., Hankenson K. D., Woolf P. J. (2009) GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics 10, 161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao S., Guo Y., Sheng Q., Shyr Y. (2014) Advanced heat map and clustering analysis using heatmap3. BioMed Res. Int. 2014, 986048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo W., Brouwer C. (2013) Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 29, 1830–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attur M., Yang Q., Shimada K., Tachida Y., Nagase H., Mignatti P., Statman L., Palmer G., Kirsch T., Beier F., Abramson S. B. (2015) Elevated expression of periostin in human osteoarthritic cartilage and its potential role in matrix degradation via matrix metalloproteinase-13. FASEB J. 29, 4107–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamberlain C. S., Duenwald-Kuehl S. E., Okotie G., Brounts S. H., Baer G. S., Vanderby R. (2013) Temporal healing in rat achilles tendon: ultrasound correlations. Ann. Biomed. Eng. 41, 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Little D., Thompson J. W., Dubois L. G., Ruch D. S., Moseley M. A., Guilak F. (2014) Proteomic differences between male and female anterior cruciate ligament and patellar tendon. PLoS One 9, e96526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shih B., Brown J. J., Armstrong D. J., Lindau T., Bayat A. (2009) Differential gene expression analysis of subcutaneous fat, fascia, and skin overlying a Dupuytren’s disease nodule in comparison to control tissue. Hand (N. Y.) 4, 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismail R. S., Baldwin R. L., Fang J., Browning D., Karlan B. Y., Gasson J. C., Chang D. D. (2000) Differential gene expression between normal and tumor-derived ovarian epithelial cells. Cancer Res. 60, 6744–6749 [PubMed] [Google Scholar]
- 40.Wang W., Sun Q. K., He Y. F., Ma D. C., Xie M. R., Ji C. S., Hu B. (2014) Overexpression of periostin is significantly correlated to the tumor angiogenesis and poor prognosis in patients with esophageal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 7, 593–601 [PMC free article] [PubMed] [Google Scholar]
- 41.Bao S., Ouyang G., Bai X., Huang Z., Ma C., Liu M., Shao R., Anderson R. M., Rich J. N., Wang X. F. (2004) Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 5, 329–339 [DOI] [PubMed] [Google Scholar]
- 42.Hu F., Wang W., Zhou H. C., Shang X. F. (2014) High expression of periostin is dramatically associated with metastatic potential and poor prognosis of patients with osteosarcoma. World J. Surg. Oncol. 12, 287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cobo T., Viloria C. G., Solares L., Fontanil T., González-Chamorro E., De Carlos F., Cobo J., Cal S., Obaya A. J. (2016) Role of periostin in adhesion and migration of bone remodeling cells. PLoS One 11, e0147837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Valle I., Rudloff S., Carles A., Li Y., Liszewska E., Vogt R., Kemler R. (2013) E-cadherin is required for the proper activation of the Lifr/Gp130 signaling pathway in mouse embryonic stem cells. Development 140, 1684–1692 [DOI] [PubMed] [Google Scholar]
- 45.Kim C. J., Sakamoto K., Tambe Y., Inoue H. (2011) Opposite regulation of epithelial-to-mesenchymal transition and cell invasiveness by periostin between prostate and bladder cancer cells. Int. J. Oncol. 38, 1759–1766 [DOI] [PubMed] [Google Scholar]
- 46.Wei Y. C., Yang S. F., Chang S. L., Chen T. J., Lee S. W., Chen H. S., Lin L. C., Li C. F. (2018) Periostin overexpression is associated with worse prognosis in nasopharyngeal carcinoma from endemic area: a cohort study. OncoTargets Ther. 11, 3205–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S. R., Bryan J. N., Esebua M., Amos-Landgraf J., May T. J. (2017) Testis specific Y-like 5: gene expression, methylation and implications for drug sensitivity in prostate carcinoma. BMC Cancer 17, 158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Byrne J. A., Maleki S., Hardy J. R., Gloss B. S., Murali R., Scurry J. P., Fanayan S., Emmanuel C., Hacker N. F., Sutherland R. L., Defazio A., O’Brien P. M. (2010) MAL2 and tumor protein D52 (TPD52) are frequently overexpressed in ovarian carcinoma, but differentially associated with histological subtype and patient outcome. BMC Cancer 10, 497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu C. X., Li Y., Obermoeller-McCormick L. M., Schwartz A. L., Bu G. (2001) The putative tumor suppressor LRP1B, a novel member of the low density lipoprotein (LDL) receptor family, exhibits both overlapping and distinct properties with the LDL receptor-related protein. J. Biol. Chem. 276, 28889–28896 [DOI] [PubMed] [Google Scholar]
- 50.Buczkowicz P., Hawkins C. (2015) Pathology, molecular genetics, and epigenetics of diffuse intrinsic pontine glioma. Front. Oncol. 5, 147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Litherland G. J., Elias M. S., Hui W., Macdonald C. D., Catterall J. B., Barter M. J., Farren M. J., Jefferson M., Rowan A. D. (2010) Protein kinase C isoforms zeta and iota mediate collagenase expression and cartilage destruction via STAT3- and ERK-dependent c-fos induction. J. Biol. Chem. 285, 22414–22425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y. J., Chang W. A., Wu L. Y., Hsu Y. L., Chen C. H., Kuo P. L. (2018) Systematic analysis of transcriptomic profile of chondrocytes in osteoarthritic knee using next-generation sequencing and bioinformatics. J. Clin. Med. 7, 535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L., Zhang Q., Li L., Wang Z., Ying J., Fan Y., He Q., Lv T., Han W., Li J., Yang Y., Xu B., Wang L., Liu Q., Sun Y., Guo Y., Tao Q., Jin J. (2015) DLEC1, a 3p tumor suppressor, represses NF-κB signaling and is methylated in prostate cancer. J. Mol. Med. (Berl.) 93, 691–701 [DOI] [PubMed] [Google Scholar]
- 54.Clohisy J. C., Roy B. C., Biondo C., Frazier E., Willis D., Teitelbaum S. L., Abu-Amer Y. (2003) Direct inhibition of NF-kappa B blocks bone erosion associated with inflammatory arthritis. J. Immunol. 171, 5547–5553 [DOI] [PubMed] [Google Scholar]
- 55.Roman-Blas J. A., Jimenez S. A. (2006) NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage 14, 839–848 [DOI] [PubMed] [Google Scholar]
- 56.Conway S. J., Izuhara K., Kudo Y., Litvin J., Markwald R., Ouyang G., Arron J. R., Holweg C. T., Kudo A. (2014) The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 71, 1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Padial-Molina M., Volk S. L., Rios H. F. (2014) Periostin increases migration and proliferation of human periodontal ligament fibroblasts challenged by tumor necrosis factor -α and Porphyromonas gingivalis lipopolysaccharides. J. Periodontal Res. 49, 405–414 [DOI] [PubMed] [Google Scholar]
- 58.Lock J. G., Wehrle-Haller B., Strömblad S. (2008) Cell-matrix adhesion complexes: master control machinery of cell migration. Semin. Cancer Biol. 18, 65–76 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.