Abstract
Post-translational acetylation of lysine residues has emerged as a key regulatory mechanism in all eukaryotic organisms. Originally discovered in 1963 as a unique modification of histones, acetylation marks are now found on thousands of nonhistone proteins located in virtually every cellular compartment. Here we summarize key findings in the field of protein acetylation over the past 20 years with a focus on recent discoveries in nuclear, cytoplasmic, and mitochondrial compartments. Collectively, these findings have elevated protein acetylation as a major post-translational modification, underscoring its physiological relevance in gene regulation, cell signaling, metabolism, and disease.
Graphical Abstract
1. INTRODUCTION
During the lifetime of a protein there are many points at which an acetyl group may be added to influence function. As early as during its translation, a protein may be N-terminally acetylated to preserve its stability, interactions, or subcellular localization.1 N-Terminal acetylation is a major covalent modification occurring on eukaryotic proteins, with >80% of human proteins bearing an acetyl group at the α-amino position of the first amino acid. Once a protein is properly localized, acetylation of key lysine residues can occur enzymatically or spontaneously to influence its intermolecular interactions, enzymatic functions, localization, and eventual degradation. Post-translational acetylation of lysine residues will be the primary focus of the current review.
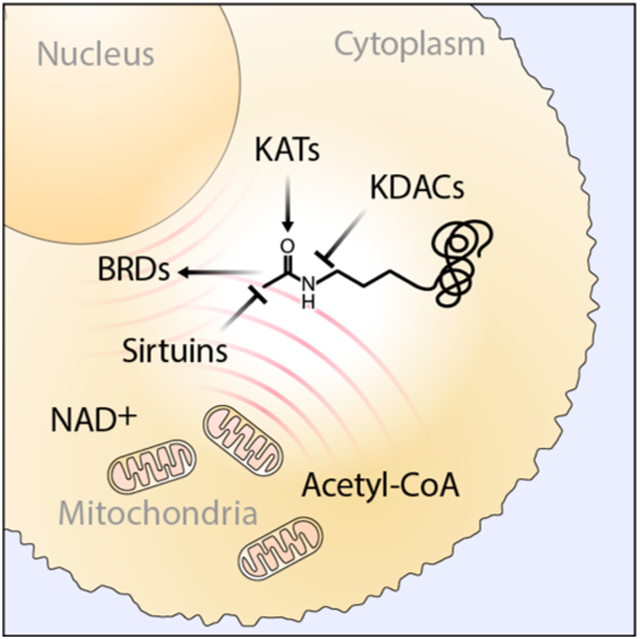
Lysine acetylation describes the transfer of an acetyl group from acetyl-coenzyme A (acetyl-CoA) to the primary amine in the ε-position of the lysine side chain within a protein, a process that leads to neutralization of the position’s positive electrostatic charge. Acetylation can occur nonenzymatically; however, in most known cases, the level of acetylation results from the balance of opposing enzymatic activities. Marks are “written” by lysine acetyltransferases (KATs) and “erased” by lysine deacetylases (KDACs). Acetylated lysine residues, amidst their many functions, can be functionally interpreted by a third group of proteins, the so-called “readers”, which harbor specific acetyl–lysine binding domains, most prominently bromodomains. The dynamic interplay between the writers, erasers, and readers of acetylation regulates critical epigenomic and metabolic processes, in addition to other major cellular functions.
Historically, investigators have focused on acetylation in the nucleus, where this mark regulates histone biology and transcription.2-5 Advances in mass spectrometric technologies have since revealed relevant targets of acetylation in nearly all intracellular compartments.6,7 Compartmentalization of cellular proteins and nutrients is essential for cell specialization and function. As such, cellular acetylation is driven by the localization of enzymes, metabolites, and cofactors required to balance acetylation and deacetylation levels. Importantly, mitochondria have emerged as organelles in which acetylation is more prominent than phosphorylation8 and plays a key role in integrating metabolic cues with the bioenergetic equilibrium of the cell.
In this review, we give an overview of the chemistry and biology underlying protein lysine acetylation in mammals, review recent developments in the understanding of lysine acetylation, and provide examples of its function and regulation in distinct cellular compartments.
2. CHEMISTRY OF REVERSIBLE LYSINE ACETYLATION
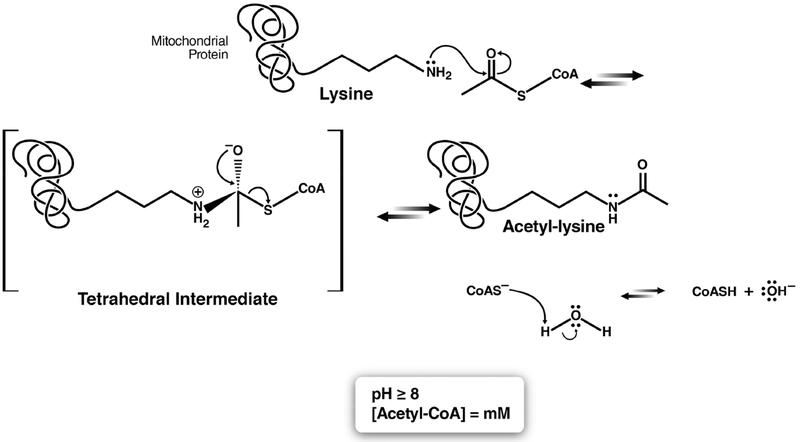
The transfer of the acetyl group from acetyl-CoA to the ε-primary amine of a lysine residue can occur spontaneously or enzymatically. In mitochondria, acetylation is regulated in part by chemical, nonenzymatic mechanisms due to the high pH and high local acetyl-CoA concentrations within this compartment.9 The mechanism of nonenzymatic acetylation proceeds first via deprotonation of the lysine primary amine by naturally occurring hydroxide ions, followed by attack of the acetyl-CoA terminal carbonyl by the nucleophilic amine. A putative tetrahedral intermediate is transiently formed and decomposes into the reaction products acetyl-lysine, coenzyme A, and hydroxide (Figure 1).10
Figure 1.
Proposed reaction mechanism of spontaneous acetylation in the mitochondria.10
2.1. Lysine Acetyltransferases
The human proteome contains 21 putative KATs that catalyze lysine acetylation (Table 1). The best characterized have been catalogued into three major families based on homology to yeast proteins but also on structural and biochemical features of catalysis: (1) GCN5-related N-acetyltransferases (GNAT), (2) the p300/CREB-binding protein (p300/CBP), (3) and the MOZ, Ybf2, Sas2, and Tip60 (MYST) family. A number of other proteins have acetyltransferase activity, such as TBP-associated factor 250kd (TAFII250 (KAT4)), αTubulin acetyltransferase (αTAT1), circadian locomoter output cycles protein kaput CLOCK (KAT13D), and nuclear receptor coactivator-1 (NCoA-1), but do not belong to any of the major acetyltransferase families.
Table 1.
Putative Lysine Acetyltransferases (KATs) and Their Common Aliases Listed with the Subcellular Localization, Crystal Structures (if Available), and UniProt ID
| PROTEIN NAME |
ALIASES | SUBFAMILY | LOCALIZATION | STRUCTURES AVAILABLE | UNIPROT ID |
REFERENCES |
|---|---|---|---|---|---|---|
| αTAT1 | MEC17 | N/A | Cytoplasmic | KAT Domain | Q5SQ10 | 17-20 |
| KAT1 | HAT1 | GNAT | Nuclear, Cytoplasmic | KAT Domain | O14929 | 21-24 |
| KAT2A | GCN5 | Nuclear | KAT Domain, Bromodomain | Q92830 | 25-29 | |
| KAT2B | PCAF | Nuclear | KAT Domain, Bromodomain | Q92831 | 27, 30-33 | |
| ATF2 | CREB2 | Nuclear, Mitochondrial | DNA Binding Domain | P15336 | 34-37 | |
| KAT3A | CBP | P300/CBP | Nuclear, Cytoplasmic | Bromodomain | Q92793 | 27, 38-41 |
| KAT3B | p300 | Nuclear, Cytoplasmic | KAT Domain, Bromodomain | Q09472 | 27, 42-45 | |
| KAT4 | TAF1, TAFII250 | TAFII250 | Nuclear | Complete Protein | P21675 | 27, 46-49 |
| KAT5 | TIP60 | MYST | Nuclear, Cytoplasmic | KAT Domain (PDB: 2OU2) | Q92993 | 50-52 |
| KAT6A | MYST3, MOZ | Nuclear | KAT Domain, PhD Finger | Q92794 | 53-55 | |
| KAT6B | MYST4, MORF | Nuclear | N/A | Q8WYB5 | 56, 57 | |
| KAT7 | MYST2, HBO1 | Nuclear | N/A | O95251 | 58-61 | |
| KAT8 | MYST1, MOF | Nuclear, Mitochondrial | KAT Domain | Q9H7Z6 | 62-66 | |
| KAT9 | ELP3 | ELP3 | Nuclear, Cytoplasmic | N/A | Q9H9T3 | 67, 68 |
| GCN5L1 | BLOS1 | N/A | Cytoplasmic, Mitochondrial | N/A | P78537 | 69, 70 |
| KAT12 | GTF3C4 | N/A | Nuclear | N/A | Q9UKN8 | 71 |
| KAT13A | NCoA-1, SRC1 | SRCs | Nuclear, Cytoplasmic | NR Binding Domain | Q15788 | 72, 73 |
| KAT13B | NCoA-3, TRAM1 | Nuclear,Cytoplasmic, Exosome | NR Binding Domain | Q9Y6Q9 | 74, 75 | |
| KAT13C | NCoA-2, TIF2, SRC3 | Nuclear, Cytoplasmic | NR Binding Domain | Q15596 | 73, 76, 77 | |
| KAT13D | CLOCK | Nuclear, Cytoplasmic | DNA Bindinq Domain | O15516 | 78-82 | |
| KAT14 | CSR2B | N/A | Nuclear, Cytoplasmic | N/A | Q9H8E8 | 83 |
The first cloned mammalian acetyltransferase was the GCN5 homologue PCAF (KAT2B). In this study, Nakatani and colleagues reported conserved sequence homology between PCAF and the GCN5 genes in yeast and human. The authors performed in vitro acetylation assays using recombinant proteins to demonstrate that PCAF (KAT2B) can acetylate whole nucleosomes while the function of human GCN5 (KAT2A) was limited to free histones.11 Using similar assays, the enzymatic activity was demonstrated for CBP/p300 (KAT3A/B),12 TAFII250 (KAT4),13 TIP60 (KAT5),14 and NCoA-1 (KAT13A).15,16
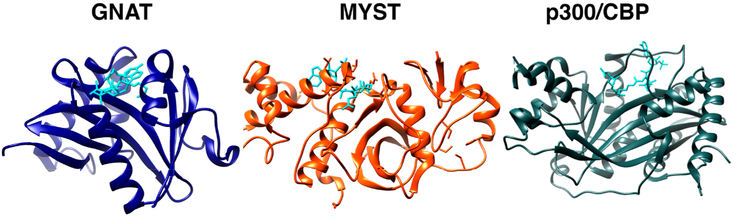
Despite considerable divergence in primary sequence, KATs from distinct families exhibit structurally homologous acetyl-CoA binding regions, which generally adopt a globular α/β fold (Figure 2). Regions flanking the central acetyl-CoA-binding cleft are not generally conserved, and they may serve to guide substrate specific activities.84 Among the KAT subfamilies, three prevailing mechanisms have been identified. GNAT family members use an active site glutamate to deprotonate the lysine ε-amine, enabling nucleophilic attack of the acetyl-CoA carbonyl, followed by formation of a transient tetrahedral intermediate and its subsequent collapse into acetyl-lysine and coenzyme A (Figure 3).85 The same mechanism has been proposed for KATs of the MYST family.86 A two-step mechanism involving an active site acetyl-cysteine intermediate was originally proposed for MYST enzymes.87 However, mutagenizing this cysteine residue does not affect enzymatic activity within the context of a preassembled ternary complex.86 Mutagenesis of an active site glutamate, however, ablates activity without reducing levels of autoacetylation.62,88 Collectively, these data suggest that the active site glutamate plays a particularly significant role for MYST family catalysis. However, acetyl-cysteine intermediates may still be relevant depending on cellular context for MYST family members with still undefined mechanisms.
Figure 2.
Structures of catalytic KAT domains from GNAT (human GCN5, blue, PDB: 1Z4R), MYST (human MOZ, orange, PDB: 2RC4), and KAT3A/B(CBP/p300) (human KAT3B(p300), gray, PDB: 3BIY) families. Acetyl-CoA is shown in cyan. Images rendered in Chimera (UCSF).
Figure 3.
Proposed reaction mechanism for GNAT family KATs.85
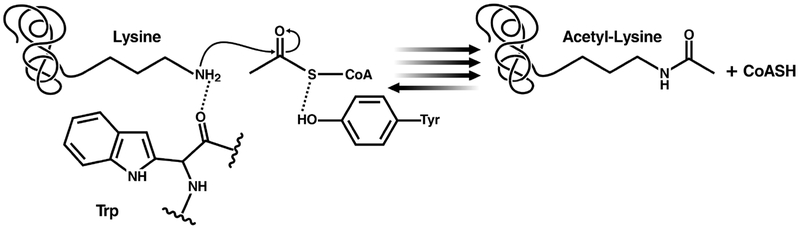
The mechanism utilized by p300/CBP family members is categorized as a “hit and run” (Theorell–Chance) mechanism. It is ordered and rapid, and the ternary complex formed is kinetically irrelevant for catalysis.89 Instead of an active site basic residue, aromatic residues lining a shallow catalytic pocket steer the lysine substrate and allow for nucleophilic attack of acetyl-CoA by lowering its pKa.90 A tyrosine residue then acts as an acid to protonate the sulfhydryl of CoA, leaving as reaction products acetyl-lysine and CoA (Figure 4). This may partially explain the relative substrate promiscuity observed for p300.91 The mechanisms used by several KATs [i.e., KAT13D (CLOCK), KAT13A (SRC1), KAT13B (SRC3), KAT4 (TAF1), KAT9 (ELP3), and KAT12 (GTF3C4), among others] have not been formally investigated.
Figure 4.
Proposed reaction mechanism for p300 family KATs.89
The enzymatic activity of HAT proteins may vary depending on the cellular microenvironment. For example, the substrate specificity and therefore the catalytic activity of KAT2A/B (GCN5/PCAF) may be influenced by accessory proteins within this complex that help target the acetyltransferase to its substrates, thus enhancing activity. For example, using immunoprecipitation followed by gel filtration chromatography, KAT2A/B (GCN5/PCAF) can be separated from a large macromolecular structure consisting of the TBP-free-TAF complex (TFTC) and the SPT3-TAF9-GCN5-acetyltransferase (STAGA) module.92-94 These complexes are large, up to 2 megadaltons, and likely vary in composition across the genome to transduce highly specific stimuli.95
Recent studies have identified two putative mitochondrial KAT enzymes, suggesting that acetylation in the mitochondria can be enzymatically triggered which raises interesting questions about the catalytic mechanisms of these proteins. GCN5-like protein 1 (GCN5L1) was proposed to act as a mitochondrial KAT and a counter-regulator to SIRT3, a mitochondrial lysine deacetylase. Notably, robust in vitro acetylation required the presence of additional mitochondrial factors, suggesting that GCN5L1 activity may not be direct.70 In addition, KAT8 (MOF) localizes specifically to mitochondria in HeLa cells and its catalytic activity is required for appropriate mitochondrial gene expression.65 However, it remains unclear whether KAT8 (MOF) enzymatic activity regulates mitochondrial protein acetylation. It is important to note that environmental conditions in mitochondria are unique. Investigators must consider the especially oxidative conditions when assessing the potential catalytic mechanisms for mitochondrial KATs.
Autoacetylation is an important mechanism of HAT enzymatic regulation. In 2004, Cole and colleagues identified a cluster of key lysine residues within an activation loop motif of KAT3B (p300) that must be acetylated in order for the enzyme to have robust catalytic activity.96,97 In this model, the activation loop regulates KAT3B (p300) activity by competing with substrates for the active site. Upon hyperacetylation, the activation loop is displaced, allowing for substrates to interact with the active site.98,99 Active site autoacetylation appears to be a conserved process as RTT109, a yeast acetyltransferase, autoacetylates its active site at K290 to increase its affinity for acetyl-CoA.100 Similar to KAT3B (p300), KAT8 (MOF) also requires autoacetylation for its activity, shifting the structure of the protein to allow for better substrate binding and catalytic activity in vitro and in vivo.62 In contrast, KAT13D (CLOCK) acetylates its dimerization partner BMAL1, a modification that facilitates the assembly of a CRY1-CLOCK-BMAL1 complex and suppresses its activity in a negative feedback loop essential for circadian rhythmicity.101
2.2. Lysine Deacetylases and Sirtuins
The reversible nature of lysine acetylation is essential to its function in the regulation of critical cellular processes. The possible existence of enzymatic deacetylation was first suggested in 1978 when it was observed that n-butyrate treatment induced the differentiation of Friend erythroleukemic cells into hemoglobin-synthesizing normoblast-like cells, a phenotype that correlated with strong histone hyperacetylation.102 This early work characterizing n-butyrate and Trapoxin103,104 as KDAC inhibitors paved the way for Schreiber and colleagues to purify the first KDAC from bovine calf thymus lysates using a Trapoxin based affinity matrix.105 Following this, and in rapid succession, KDACs 2–11 were discovered through sequence homology analyses to yeast deacetylases.106-112
At the same time, the silent information regulator (Sir) protein family, known to suppress gene expression at telomeres and rDNA,113,114 gained attention as potential deacetylase enzymes. Mutation of Sir proteins in yeast induced hyperacetylation of histones.115 In 1999, Frye and colleagues identified five human cDNAs with sequence homology to the yeast Sir2 gene, and shortly after, Sir2 was identified as an NAD+ dependent histone deacetylase.116,117 The family known as Sirtuins was completed using a phylogenetic classification scheme identifying the last two members, SIRT6 and SIRT7.118
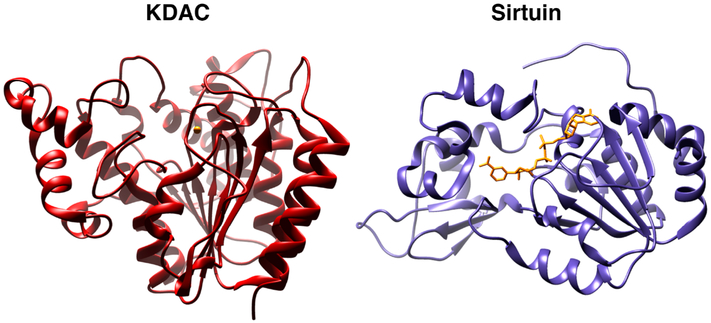
KDACs and sirtuin proteins are mechanistically and structurally distinct (Figure 5). They are formally categorized into four distinct enzyme classes based on structural homology with yeast transcriptional repressors and unique catalytic mechanisms.119,120 (Table 2) Class I, II, and IV enzymes are Zn2+-dependent and are comprised by KDACs 1–11. Class I enzymes (KDAC1, 2, 3, 8) localize mainly to the nucleus, while class II (KDAC4–7, 9, 10) and Class IV (KDAC11) enzymes generally shuttle between the nucleus and cytoplasm. The Sirtuin proteins 1–7 encompass the class III enzymes and are described in the text below (Table 3). Similar to KATs, KDACs are also often found in large, macromolecular complexes that function primarily in gene repression. For example CoREST, NuRD, and Sin3 complexes harbor a catalytic core composed of a KDAC1:KDAC2 dimer, and the NCoR complex contains KDAC3.121,122
Figure 5.
Structures of catalytic KDAC domains from KDAC (human KDAC2, red, PDB: 4LXZ) and Sirtuin (human SIRT1, purple, PDB: 4I5I families). KDAC zinc and Sirtuin NAD are shown in yellow. Images rendered in Chimera (UCSF).
Table 2.
Zn2+ Dependent Lysine Deacetylases (KDACs) Listed with Subcellular Localization, Relevant Crystal Structures (if Available), Common Inhibitors, and UniProt ID
| PROTEIN NAME |
SUBFAMILY | LOCALIZATION | STRUCTURES AVAILABLE |
INHIBITORS | UNIPROT ID |
REFERENCES |
|---|---|---|---|---|---|---|
| KDAC1 | I | Nuclear, Cytoplasmic | Complete Protein | Panobinostat, Vorinostat, Romidepsin | Q13547 | 123-125 |
| KDAC2 | Nuclear, Cytoplasmic | Deacetylase Domain | Panobinostat, Vorinostat, Romidepsin | Q92769 | 126-128 | |
| KDAC3 | Nuclear, Cytoplasmic | Deacetylase Domain | Panobinostat, Vorinostat | O15379 | 128-130 | |
| KDAC8 | Nuclear, Cytoplasmic | Complete Protein | Panobinostat, Vorinostat, PCI-34051 | Q9BY41 | 131-136 | |
| KDAC4 | IIA | Nuclear, Cytoplasmic | Deacetylase Domain | Panobinostat, Romidepsin | P56524 | 137-141 |
| KDAC5 | Nuclear, Cytoplasmic | N/A | Panobinostat | Q9UQL6 | 142-144 | |
| KDAC7 | Nuclear, Cytoplasmic, Mitochondrial | Deacetylase Domain | Panobinostat | Q8WUI4 | 145-150 | |
| KDAC9 | Nuclear, Cytoplasmic | N/A | Panobinostat, Vorinostat | Q9UKV0 | 151, 152 | |
| KDAC6 | IIB | Nuclear, Cytoplasmic | Deacetylase Domain | Panobinostat, Vorinostat, Romidepsin, Tubastatin A |
Q9UBN7 | 112, 153 |
| KDAC10 | Nuclear, Cytoplasmic | N/A | Panobinostat | Q969S8 | 111, 154 | |
| KDAC11 | IV | Nuclear, Cytoplasmic | N/A | N/A | Q96DB2 | 110, 155 |
Table 3.
NAD+ Dependent Sirtuin Deacetylases Listed with Subcellular Localization, Relevant Crystal Structures (if Available), Common Inhibitors, and UniProt ID
| PROTEIN NAME | LOCALIZATION | STRUCTURES AVAILABLE | INHIBITORS | UNIPROT ID | REFERENCES |
|---|---|---|---|---|---|
| SIRT1 | Nuclear, Cytoplasmic | Deacetylase Domain | EX-527, Nicotinamide | Q96EB6 | 168-175 |
| SIRT2 | Nuclear, Cytoplasmic | Complete Protein | EX-527, Nicotinamide | Q8IXJ6 | 175-181 |
| SIRT3 | Mitochondrial | Deacetylase Domain | EX-527, Nicotinamide | Q9NTG7 | 175, 180, 182-186 |
| SIRT5 | Mitochondrial, Nuclear, Cytoplasmic | Deacetylase Domain | Nicotinamide, Suramin | Q9NXA8 | 186-191 |
| SIRT6 | Nuclear | Complete Protein | N/A | Q8N6T7 | 164, 192-195 |
| SIRT7 | Nuclear | N/A | N/A | Q9NRC8 | 196, 197 |
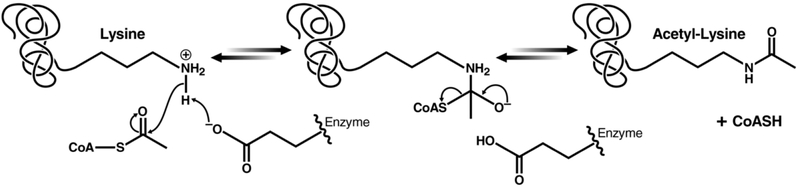
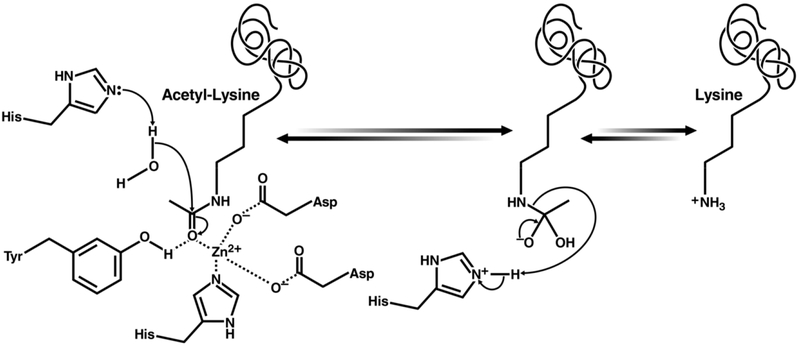
Mechanistic insight into KDAC catalysis derives from studies of HDLP, a deacetylase homologue from the bacterium Aquifex aeolicus.156 Like HDLP, KDACs utilize an active site histidine to deprotonate a critical water molecule, enabling nucleophilic attack of the acetyl group carbonyl (Figure 6). Decomposition of the oxyanionic tetrahedral intermediate releases acetate and the deacetylated lysine as reaction products. The divalent cation (Zn2+) is important for positioning and polarizing a catalytic water molecule, and it is positioned itself by aspartic acid and histidine residues of a classical catalytic triad (charge-relay network). This Zn2+ is a critical target of inhibitors of the class I, II, and IV KDACs, which mainly function via chelation.
Figure 6.
Proposed reaction mechanism for class I, II, and IV KDACs.157
Class III KDACs function independently of an active site metal and, instead, rely on nicotinamide adenine dinucleotide (NAD+) as a cofactor for catalytic activity.158 Of the seven sirtuins in mammals, only SIRT1, 2, and 3 have robust lysine deacetylase activity. More limited deacetylase activity has been reported for SIRT5, SIRT6, and SIRT7, while SIRT4 has no reported deacetylase activity (Table 3).159-163 SIRT6 and SIRT7 localize primarily to the nucleus, SIRT1 and SIRT2 shuttle between the nucleus and cytoplasm, and SIRT3 is a bona fide mitochondrial matrix protein.164 Unlike class I, II, and IV KDACs, sirtuins are not found in large repressive macromolecular complexes. However, certain binding partners regulate their enzymatic activity. For example, the active regulator of sirtuin (AROS) has been shown to stimulate SIRT1-mediated deacetylation of p53,165 while deleted in breast cancer 1 (DBC1) negatively impacts SIRT1 activity.166,167
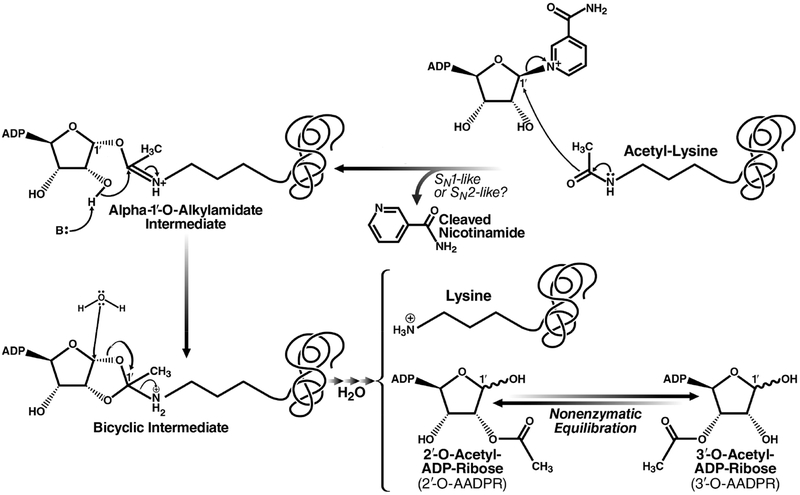
The sirtuin reaction mechanism proceeds by nucleophilic addition of acetyl oxygen to the anomeric (C1′) carbon of the nicotinamide ribose via SN1, concerted SN2, or dissociative SN2-like mechanisms, resulting in the formation of a C1′-O-alkylamidate intermediate (Figure 7). Next, a histidine residue extracts an electron from the 2′-hydroxyl group of the NAD+ ribose, which then attacks the C1′-O-alkylamidate carbon, generating a bicyclic intermediate. A base deprotonates a water molecule, enabling its attack of the bicyclic intermediate. Collapse of the bicyclic intermediate generates the deacetylated lysine and O-acetyl-ADP-ribose.198 Sirtuins likely also have weak ADP ribosyltransferase activity via incomplete catalysis through this described mechanism. ADP ribosyltransferase activity has been formally reported for SIRT4 and SIRT6.199 The mitochondrial SIRT5 enzyme exhibits broad deacylase activity, accepting malonyl- and succinyl-lysine substrates.188,191,200 The biological function of this distinct activity is not yet clear.
Figure 7.
Proposed reaction mechanism for class III KDACs/sirtuins. Reprinted with permission from ref 201. Copyright 2010 The Royal Society of Chemistry.
2.3. Acetyl–Lysine Binding Modules
An important function of lysine acetylation is the generation of novel recognition surfaces for the binding of proteins harboring “reader” domains specific for the post-translationally modified residue. The best-characterized reader module of acetyl-lysines is a structurally conserved protein domain called the bromodomain. The first reference to the bromodomain is traced to the characterization of the Drosophila gene brahma (brm), a regulator of homeotic genes now known to be a core catalytic component of SWI/SNF chromatin remodelers.202 The conserved structural motif discovered in the brm gene was termed a bromodomain, yet it is etymologically distinct from elemental bromine. Apart from the observation of its frequent occurrence in transcriptional regulators, the bromodomain was relatively uncharacterized from the time of its discovery in 1992 202 to the determination of its structure by Zhou and colleagues in 1999.33 NMR studies of the KAT2B (PCAF) bromodomain revealed that this domain binds acetyl-lysine residues on histones and described the structural details of this interaction.
The bromodomain is approximately 110 amino acids in length, and there are 61 distinct bromodomains encoded by 46 proteins (Table 4). The bromodomain is conserved from yeast to humans and are encoded in an increasing number of factors during eukaryotic evolution.203 In mammals, bromodomains can be divided into several distinct subfamilies based mostly on structural homology.204,205 While most bromodomain-containing proteins encode one bromodomain, up to six bromodomains have been documented in a single protein (Polybromo-1). The so-called bromo- and extraterminal (ET) domain-containing (BET) proteins encode a characteristic double bromodomain motif and are implicated in recruiting the positive transcription elongation factor b (P-TEFb) and other factors to signal inducible genes, including those regulated by the transcription factor c-myc in several cancers.206-208 Nearly all bromodomain-containing proteins are nuclear factors that bind chromatin to regulate its structure and function. They function mostly as transcriptional coactivators (i.e., KAT3B (p300), BRD4), but repressive functions of certain bromodomain-containing proteins are also known (i.e., BAZ2A, ZYMND11). Remarkably, many nuclear KATs harbor bromodomains. The KAT2A (GCN5) bromodomain is important for chromatin remodeling209 and regulation of sequential histone acetylation events.210 A recent structural analysis of the core catalytic domain of KAT3B (p300) showed an assembled configuration of the bromodomain and of PHD, RING, and KAT domains with the RING domain positioned over the KAT domain substrate-binding pocket, providing insight into how chromatin–substrate targeting and KAT regulation might be linked.42
Table 4.
Bromodomain Containing Proteinsa
| PROTEIN NAME | BROMODOMAINS | LOCALIZATION | BROMODOMAIN STRUCTURE | UNIPROT ID | REFERENCES |
|---|---|---|---|---|---|
| BPTF | 1 | Nuclear, Cytoplasmic | Yes | Q12830 | 27, 211, 212 |
| KAT3A (CBP) | 1 | Nuclear, Cytoplasmic | Yes | Q92793 | 27 |
| KAT3B (p300) | 1 | Nuclear, Cytoplasmic | Yes | Q09472 | 27 |
| BRWD1 | 2 | Nuclear, Cytoplasmic | 1 of 2 | Q9NSI6 | 27 |
| PHIP | 2 | Nuclear, Cytoplasmic | 1 of 2 | Q8WWQ0 | 27, 213, 214 |
| BRPF1 | 1 | Nuclear, Cytoplasmic | Yes | P55201 | 56, 215-219 |
| TRIM24 | 1 | Nuclear, Cytoplasmic | Yes | O15164 | 220, 221 |
| SP100 | 1 | Nuclear, Cytoplasmic | Yes (4PTB) | P23497 | 222, 223 |
| KAP1 | 1 | Nuclear, Cytoplasmic | Yes | Q13263 | 224-228 |
| ZMYND11 | 1 | Nuclear, Cytoplasmic | Yes | Q15326 | 229, 230 |
| KAT2A (GCN5) | 1 | Nuclear | Yes | Q92830 | 27 |
| KAT2B (PCAF) | 1 | Nuclear | Yes | Q92831 | 27 |
| CECR2 | 1 | Nuclear | Yes | Q9BXF3 | 27 |
| BRDT | 2 | Nuclear | 2 of 2 | Q58F21 | 27, 231-235 |
| BRD4 | 2 | Nuclear | 2 of 2 | O60885 | 27, 46, 236-239 |
| BRD3 | 2 | Nuclear | 2 of 2 | Q15059 | 27, 240 |
| BRD2 | 2 | Nuclear | 2 of 2 | P25440 | 241-244 |
| BAZ1A | 1 | Nuclear | No | Q9NRL2 | 245, 246 |
| BRD8B | 2 | Nuclear | No | Q9H039 | 247 |
| BAZ1B | 1 | Nuclear | No | Q9UIG0 | 248, 249 |
| BRD9 | 1 | Nuclear | Yes | Q9H8M2 | 27, 46, 250-252 |
| BRD7 | 1 | Nuclear | Yes | Q9NPI1 | 250, 253 |
| BRPF3 | 1 | Nuclear | No | Q9ULD4 | 59, 254, 255 |
| BRD1 | 1 | Nuclear | Yes | 095696 | 27, 256 |
| ATAD2B | 1 | Nuclear | Yes | Q9ULI0 | 27, 257, 258 |
| TRIM33 | 1 | Nuclear | Yes | Q9UPN9 | 259-262 |
| SP110 | 1 | Nuclear | No | Q9HB58 | 263, 264 |
| SP140 | 1 | Nuclear | No | Q13342 | 265, 266 |
| SP140L | 1 | Nuclear | No | Q9H930 | 267 |
| BAZ2B | 1 | Nuclear | Yes | Q9UIF8 | 268, 269 |
| BAZ2A | 1 | Nuclear | Yes | Q9UIF9 | 270, 271 |
| KMT2A | 1 | Nuclear | Yes | Q03164 | 272-275 |
| TAF1L | 1 | Nuclear | Yes | Q8IZX4 | 27, 237 |
| TAF1 | 2 | Nuclear | 2 of 2 | P21675 | 27, 46, 48, 49, 276, 277 |
| ZMYND8 | 1 | Nuclear | Yes | Q9ULU4 | 278-280 |
| PBRM1 | 6 | Nuclear | 6 of 6 | Q86U86 | 27, 281, 282 |
| BRG1 | 1 | Nuclear | Yes | P51532 | 27, 283-285 |
| SMARCA2 | 1 | Nuclear | Yes (5DKC) | P51531 | 286 |
| BRWD3 | 2 | Nuclear, Extracellular | No | Q6RI45 | 287 |
| ATAD2 | 1 | Nuclear, Exosome | Yes | Q6PL18 | 27, 288-291 |
| ASH1L | 1 | Nuclear,Tight Junctions | Yes | Q9NR48 | 27, 292, 293 |
Proteins are organized according to their observed subcellular localization. UniProt IDs refer to human proteins. References correspond to protein localization and relevant crystal structures drawn from mouse and human data.
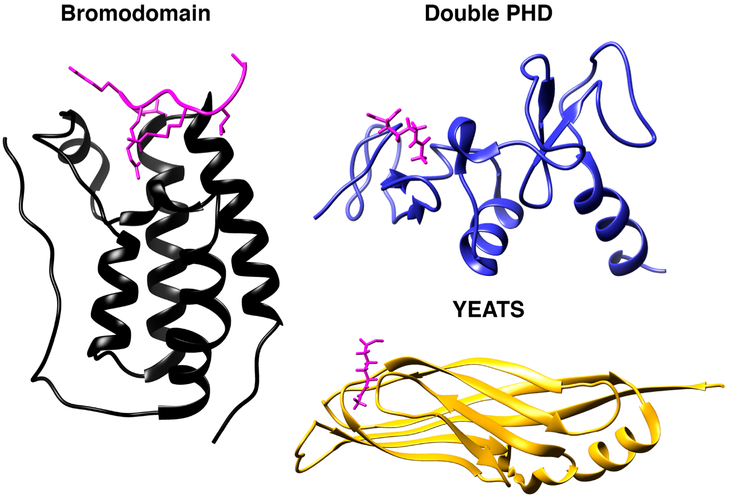
The bromodomain structure is well characterized, with >400 high-resolution X-ray crystal structures available and near complete structural coverage across the protein family. The domain is composed of four left-handed α-helices (αZ, αA, αB, and αC) connected by two loops (ZA and BC loops, Figure 8).33 This structure forms a hydrophobic cavity that serves as the acetyl-lysine recognition site. A hydrogen bond mediated by a conserved bromodomain asparagine residue and the acetyl-lysine carbonyl serves as the ligand recognition mechanism. Tyrosine residues lining the bromodomain cleft also play a significant role in ligand positioning via π–π stacking and hydrogen bond formation with critical water molecules. Helical regions of bromodomains are moderately conserved, but the length and sequence of the loop regions vary considerably. Some bromodomains cooperatively bind multiply acetylated peptides, such as the testis-specific BET protein BRDT.235 Others are controlled by post-translational modifications on nearby proteins. In vitro, the bromodomain:acetyl-lysine interaction is relatively weak (Kd = low micromolar). In vivo, the combined affinities of adjacent or proximal protein domains (i.e., helicase, SAND, distinct bromodomain) may modulate specificities and/or strength of binding.
Figure 8.
Structures of acetylation reader domains: Bromodomain (human BRD4, black, PDB: 3UVW), double PHD (human DPF3, blue, PDB: 2KWJ), and YEATS (human AF9, yellow, PDB: 4TMP). Acetyl-lysine ligands shown in pink. Images rendered in Chimera (UCSF).
Several other protein domains have been reported to accept acetyl-lysine residues as ligands. The plant homeodomain (PHD) finger domain is generally recognized as a methyl-lysine reader domain, but when present in tandem in the protein DPF3b, it binds acetylated lysine residues on histone H3 and H4 molecules (Figure 8).294 The tandem PHD:acetyl-lysine binding mode is mechanistically distinct from that of the bromodomain, utilizing aspartic acid within the first PHD domain to form a hydrogen bond with the acetyl amide of the ligand. Interestingly, this aspartic acid also serves to recognize N-terminally acetylated peptides in addition to acetyl-lysine residues. Notably, proteins other than DPF3b encode tandem PHD domains, such as the CHD4 chromatin remodeler and KAT6A (MOZ), both of which have been shown to bind acetylated histones.295,296
The YEATS domain also recognizes acetyl-lysine residues.297 YEATS domains are present in five human proteins (YEATS2, ENL, AF9, TFIIF, and GAS41). AF9 and ENL are both components of the so-called superelongation complex (SEC), a multimeric complex containing P-TEFb, AFF1/AFF4 scaffolds, and the ELL1/ELL4 elongation factors.298 Structurally, the YEATS domain adopts an Immunoglobulin fold (Figure 8), and its interaction with acetyl-lysine is mediated by several hydrogen bonds in addition to aromatic residues important for ligand positioning.298 Acetylated H3K9 is a ligand for the AF9 YEATS domain, and the ENL YEATS domain exhibits a preference for acetylated H3K27, although ENL correlates genome-wide with both acetylated H3K9 and H3K27 in acute myeloid leukemia (AML) cells.299 The two other YEATS domain-containing proteins, GAS41 and YEATS2, belong to chromatin-remodeling complexes. The AF9 YEATS domain has an expanded binding repertoire of acyl-lysine marks, and it can also accommodate modifications, such as crotonylation.300 Importantly, translocations between genes encoding ENL/AF9 and MLL methyltransferase occur frequently, and the resultant fusion proteins are oncogenic drivers.301 Specifically, the ENL YEATS domain is required for tethering the SEC to enforce oncogenic gene expression programs in AML.299
While the bromodomain and YEATS and tandem PHD domains specifically recognize acetyl-lysine residues, readers have recently been found that specifically bind unmodified lysine residues. The SET protein functions through its acidic-domain to bind the C-terminus of the transcription factor p53 only when p53 is not acetylated. The function appears to be conserved, as proteins with similar domains, such as VPRBP, DAXX, and PELP1, also bound preferentially to nonacetylated p53. In addition, the SET acidic-domain recognizes non-acetylated lysine-rich domains of histone H3, KU70, and FOXO1, suggesting broad implications for this mechanism of recognition.302
3. WIDENING SCOPE OF PROTEIN ACETYLATION
In 1997, over three decades after the discovery of acetylation on histones and tubulin, the transcription factor p53 was identified as a nonhistone KAT substrate.303 By 2000, 10 more nuclear proteins and transcription factors were found to be substrates of acetylation, leading to speculation that acetylation may rival phosphorylation as a post-translational modification.304 Six years later, the first acetylome screen identified 388 acetylation sites in 195 proteins, more acetylation sites than were identified in the previous 40 years.305 Since then, more than 155 systems-wide acetylome studies have revealed the existence of thousands of acetylation sites on many cellular proteins, connecting lysine acetylation to virtually every cellular function and most biological outcomes (Figure 9). Mass spectrometry analyses of acetylation have been conducted in a wide variety of species ranging from Gram-positive306,307 and -negative bacteria,308 to budding yeast,309 to plants,310-312 to eukaryotic human pathogens,313,314 rodents, and humans. These have provided valuable insight into the stoichiometry and dynamics of lysine acetylation, as well as interactions with other PTMs.
Figure 9.
Acetylome studies reveal the scope of biological functions regulated by acetylation in mammalian cells.
Proteomic-based studies generally rely on an enrichment step in which pan-acetyl lysine antibodies are used to purify acetylated proteins from trypsin-digested lysates.305,315 Notably, the use of an antibody raised against a single antigen can conceivably bias which proteins are purified from lysates, suggesting that most current studies only capture a subset of cellular acetylation sites.316,317 Stable isotope labeling of amino acids in culture (SILAC)318 and a label-free approach319 have been used to assess the dynamics of acetylated protein stoichiometry. These studies have revealed that in mammalian cells individual acetylation sites appear conserved across species but not across tissue types.4,5 A high degree of overlap is observed in human, rat, and mouse liver tissues, yet little overlap exists between rat liver and rat heart.320,321 Acetylation occurs in regions with defined secondary structure, such as α-helices and β-sheets, unlike phosphorylation.305,315
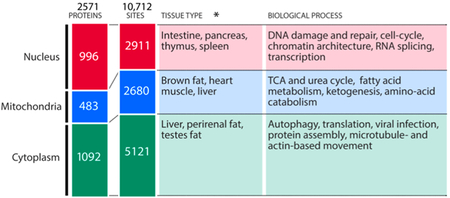
Nuclear protein acetylation levels are high in tissues with actively dividing cells and in tumors. Many acetylation sites are found on proteins related to DNA damage, cell-cycle control, and transcription (Box 1).305,322,315 Mitochondrial acetylation is primarily found on proteins related to cellular metabolic processes and is enriched in highly metabolically active tissues such as brown fat, heart, and liver, and it likely plays a role in other tissue types depending on their metabolic activity and capacity to respond to insulin.319-321 Cytoplasmic acetylation has been relatively understudied despite the fact that tubulin was the second protein discovered to be acetylated.323-325 Notably, it is difficult to exclusively study cytoplasmic acetylation because cellular fractionation methods are imperfect, and many proteins tend to shuttle between the cytoplasm and other subcellular compartments. With these caveats in mind, cytoplasmic acetylation is observed predominantly in liver, peri-renal, and testis fat, tissues with high cellular concentrations of acetyl-CoA.73
Box 1. Acetylated proteins identified in proteomic studies: compartmentalization, tissue enrichment (*in rodent), and related biological processes as described in refs 315, 319, and 320.
Several groups have taken genetic approaches to probe KAT-or sirtuin-specific acetylation sites. Examples include KAT2A/KAT2B knockdown studied in HeLa cells,326 KAT13D-(CLOCK) knockout studied in mouse liver tissues,327 SIRT1 knockout studied in mouse embryonic fibroblasts328 or liver tissues,329 and SIRT3 knockout studies in mouse liver tissues319,330 (Box 2).
Box 2. Genetic approaches to identify acetylation sites, tissue type, and biological process as described in refs 319, 326, 327, 328, 329, and 330.
| ENZYME |
Δ ACETYLATED PROTEINS∣SITES |
TISSUE OR CELL TYPE |
BIOLOGICAL PROCESS |
|---|---|---|---|
| KAT2A KAT2B |
398∣1569 | HeLa cells | Chromatin organization, Pol II transcription elongation, histone lysine methylation, cell cycle, actin-mediated cell contraction |
| KAT13D |
179∣306 |
Mouse liver |
Glycolysis, TCA cycle, amino acid metabolism, fatty acid metabolism |
| SIRT1 | 1800∣4623 114 ∣ 807 |
MEFs Mouse liver |
DNA repair, signaling, transcription factor assembly, transcription elongation, spliceosome assembly, fatty acid metabolism, TCA cycle, small molecule metabolism |
| SIRT3 | 1791 ∣ 306 | Mouse liver Mitochondria |
Fatty acid metabolism, TCA cycle, amino acid catabolism, ketone body metabolism, electron transport chain |
The first integrative studies provided evidence for coordinated regulation of PTMs.7 For example, one study noted coordination between acetylation and phosphorylation in the nucleus upon DNA damage, but most changes in phosphorylation occurred in the cytoplasm.322 Surveys of acetylation and succinylation sites found substantial overlap between both acylation sites in mitochondria, suggesting potential competition between these modifications.331,332 Environmental cues, such as caloric restriction, microbiome components, and viral infection, and drugs, such as KDAC inhibitors and aspirin, also affect global acetylation levels 318,333-336
4. NUCLEAR ACETYLATION REGULATES GENE EXPRESSION
4.1. Histone Acetylation
When nucleosomal histones are assembled with DNA, each subunit displays an N-terminal tail that can be post-translationally modified. Core nucleosomes are composed of pairs of histones H2A, H2B, H3, and H4; variants of these histones are important in chromatin regulation and gene expression. Histone H1 and its accompanying variants are regarded as “linker” histones as they connect core nucleosomes into denser 30 nm fibers.337 Acetylation sites have been observed on all histone subunits, including linker histones, and the occur in both the tail and globular domains (Summarized in Box 3). Acetylation occurs abundantly in the tail domains while acetylation in the globular domain appears less abundant.338 Tail acetylation sites are evenly spaced among the nucleosomal histone subunits and possess some functional redundancy.339 Acetylation sites are well conserved, in contrast to methylation, where species-specific differences exist.340 Together, the specific array of histone modifications, known as the “histone code”, may serve as a highly dynamic regulatory system for gene expression control in mammalian cells.
Box 3. Histone lysine acetylation sites and their domain location341.
| HISTONE |
LYSINE MODIFICATION |
|
|---|---|---|
| Tail Domain |
Globular Domain |
|
| H2A | K5,K9, K13, K15 | K36, K118 |
| H2B | K5, K11, K12, K15, K16, K23, K24 | K46, K57, K120 |
| H3 | K4, K9, K14, K18, K23, K27 | K36, K37, K56, K64, K79, K112, K115, K112 |
| H4 | K5, K8, K12, K16, K20, K31 | K77, K79, K91 |
| H1 | *K16, K33 | K45, K63, K74, K89, K96, K105, K167 |
Each histone has additional isoforms not listed in this table.
Histone H1 N-terminal domain is structurally distinct from tail domains found in histone H2-4
Acetylation of key lysine residues is generally thought to disrupt the electrostatic interactions between the phospho-diester backbones of DNA and lysine-rich nucleosomes to expose DNA to transcription machinery.342 Key advances in the analysis of histone acetylation come from the use of electron transfer dissociation and electron capture dissociation mass spectrometry.343,344 These methods allow for the analysis of long histone peptides (>20 a.a.) and therefore the identification of multiple modifications on individual histone proteins. Using this and other methodologies, several comprehensive studies have observed the combinatorial patterns of histone modifications on each subunit.338,345-348 These analyses are essential to determine whether specific modifications are compatible on the same histone at the same time, potentially identifying important rules by the histone code.
The best-studied acetylation sites are found on histone H3 and H4, but acetylation of H2A and H2B tails has also been correlated with increased transcriptional activity.349,350 Histone 3 lysine acetylation has been observed on 14 residues, six of which are located on the tail region and eight in the globular domain.341 H4 is acetylated at nine lysines, six in the tail region and three in the globular domain.341 In addition, lysine acetylation has been observed on H2A tails at four sites,338,351,352 and the globular domain at two sites Box 3.353 Turnover of histone acetylation is unequal at different sites.351 While acetylation of histone tails generally has a fast turnover (<30 min), with the exception of H3K4, H2AK13, and H2AK15, nearly all globular domain modifications were more stable with a half-life greater than 2 h.351
Histone H1 is highly modified and was first identified to be acetylated in vivo in 20 04.354,355 Multiple proteomic approaches have identified H1 acetylation sites at 11 lysine residues (K16, K33, K45, K63, K74, K89, K96, K105, K167, K168, K190), albeit at low frequency.191,338,348,356-358 Given its role in DNA condensation, histone H1 was originally thought to act primarily as a suppressor of gene expression, but its function is now understood to be more nuanced.359 For example, H1.4K34ac is detected in distal and proximal promoter regions of highly transcribed genes in induced pluripotent stem cells and cancer cancer cell lines, induced pluripotent stem cells (iPSCs), and testicular germ cell tumors.358 In addition, an inverse relationship between the presence of H1 on chromatin and acetylation marks on H3 and H4 has been described.360,361
4.2. Transcription Factor Acetylation
Notably, nuclear lysine acetylation is not restricted to histones but is also found on numerous transcription factors including p53, NF-κB, and STAT3. Mechanistically, acetylation modulates transcription factor activity at multiple steps. These include inducing nuclear translocation or protein stabilization, sterically preventing ubiquitination, modifying molecular complex composition, and facilitating chromatin binding specificities. Proteomics studies have identified many known acetylation sites on transcription factors, of which we only list those with additional functional studies (Table 5). We illustrate these phenomena using well-characterized case studies below (Figure 10).
Table 5.
Selection of Acetylated Transcription Factors and Their Writers and Erasers
| PROTEIN NAME |
ACETYL-LYSINE | WRITER | ERASER | UNIPROT ID |
REFS |
|---|---|---|---|---|---|
| AR | K630, K632, K633 | KAT2B, KAT3A, KAT3B, KAT5 | KDAC1, KDAC7 | P10275 | 362-365 |
| ATM | K3016 | KAT5 | Unknown | Q13315 | 52, 366 |
| BCL6 | K379 | KAT3B | Unknown | P41182 | 367 |
| BMAL1 | K537 | KAT13D | SIRT1 | 000327 | 101, 368 |
| CDK9 | K44, K48 | KAT2A, KAT2B, KAT3B | KDAC3, SIRT2 | P50750 | 369-372 |
| ChREBP | K672 | KAT3B | SIRT1 | Q9NP71 | 373, 374 |
| CREB | K91, K94, K136 | KAT3A, KAT3B | SIRT1, KDAC8 | P16220 | 375, 376 |
| CRTC2 | K628 | KAT3A, KAT3B | SIRT1 | Q53ET0 | 377 |
| CycT1 | K380, K386, K390, K404 | KAT3B | KDAC1, KDAC3 | 060563 | 315, 378, 379 |
| E2F1 | K117, K120, K125 | KAT2A, KAT2B, KAT3B | HDAC1 | Q01094 | 380-382 |
| EKLF | K270, K284 | KAT3A, KAT3B | Unknown | Q13351 | 383, 384 |
| ERα | K226, K268, K299, K302, K303 | KAT3A | SIRT1 | P03372 | 385-387 |
| Foxo1 | K242, K245, K248, K262, K265, K274 | KAT3B | SIRT1, SIRT2, SIRT3 | Q12278 | 379-393 |
| Foxp3 | K31, K327, K263, K268 | KAT3B, KAT5 | KDAC7, KDAC9, SIRT1 | Q9BPZS1 | 50, 394-396 |
| FXR | K217 | KAT3B | SIRT1 | Q96RI1 | 397, 398 |
| GABPB1 | K69, K340, K369 | KAT3B | SIRT7 | Q06547 | 399 |
| GATA1 | K158, K246, K252,K312 | KAT3A, KAT3B | KDAC5 | P15976 | 400-403 |
| HIF1α | K10, K11, K12, K19, K21, K709 | KAT2B, KAT3B | KDAC1, KDAC4, SIRT1, SIRT2 | Q16665 | 404-408 |
| HIF2α | K385, K685, K741 | KAT3A | SIRT1 | Q99814 | 409, 410 |
| HMG17 | K2 | KAT2B | Unknown | P05204 | 411 |
| IFNαR2 | K399 | KAT3A | Unknown | P48551 | 412 |
| Myc | K143, K148, K157, K275, K317, K323, K371 | KAT2B, KAT3B, KAT5 | SIRT1, SIRT2 | P01106 | 315, 413-416 |
| Notch 1 | K1764, K1770, K1771, K1772, K1785, K1935, K2050, K2068, K2146, K2147, K2150, K2154, K2161, K2164 | KAT2B, KAT3B | SIRT1 | P46531 | 417 |
| p53 | K120, K321, K373, K381, K382 | KAT6A, KAT3B | KDAC1, SIRT1, SIRT2 | P04637 | 315,418-422 |
| PAF53 | K373 | KAT2B | SIRT7 | Q9GZS1 | 423 |
| PRLR | K277, K339, K412, K456, K466, K472, K505, K514, K517, K526, K533, K536, K590, K601 | KAT3A | KDAC6, SIRT2 | P15471 | 424 |
| Pygo2 | K11, K43, K44, K47 | KAT3A, KAT3B | Unknown | Q9BRQ0 | 425 |
| RelA | K122, K123, K218, K221, K310, K314, K315 | KAT2B, KAT3A, KAT3B | KDAC3, SIRT1, SIRT2 | Q04206 | 426-430 |
| Rb | K873, K874 | PCAF | SIRT1 | P06400 | 431 |
| RORy | K69, K81, K99, K112 | KAT3B | KDAC1, SIRT1 | P51449 | 432, 433 |
| RPB1 | K1888, K1909, K1916, K1923, K1937, K1958, K1972, K1986 | KAT3B | Unknown | P24928 | 434, 435 |
| SMAD7 | K64, K70 | KAT3B | KDAC1, KDAC3, KDAC5, KDAC6 | O15105 | 436, 437 |
| Sp1 | K703 | KAT3B | Unknown | P08047 | 315, 438 |
| Sp3 | K551 | KAT3B | Unknown | Q02447 | 439-441 |
| SREBPIc | K289, K309 | KAT3B | SIRT1 | P36956 | 442 |
| STAT2 | K390 | KAT3A | Unknown | P52630 | 412, 443 |
| STAT3 | K49, K87, K685 | KAT3A, KAT3B | KDAC1, KDAC2, KDAC3 | P40763 | 444-446 |
| STAT5b | K359, K694, K696, K701 | KAT3A | SIRT2, KDAC6 | P51692 | 424, 447 |
| HIV-1 Tat | K28, K50, K51 | KAT2A, KAT2B, KAT3B | SIRT1 | P04608, P04610 | 448-451 |
| UBF1 | K352 | KAT3A | Unknown | P17480 | 452 |
| YY1 | K173, K174, K178, K179, K180, K181 | KAT2B, KAT3B | KDAC1, KDAC2 | P25490 | 453 |
Figure 10.
Mechanisms driving acetylation dependent regulation of transcription factors.
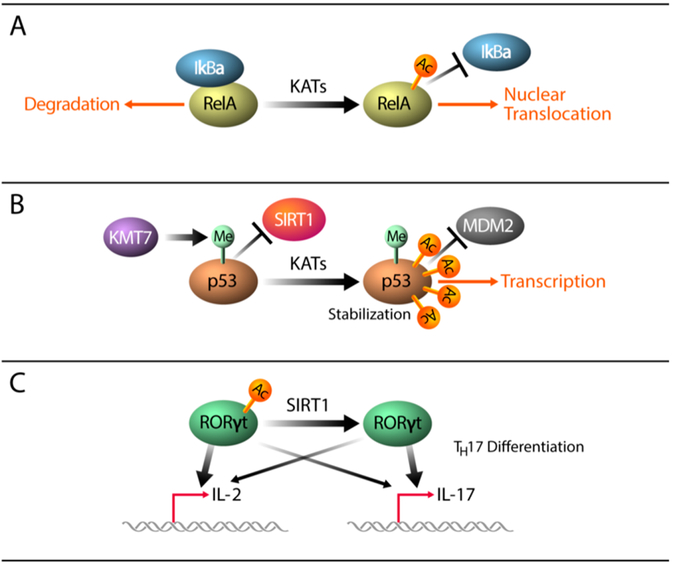
Several excellent reviews have documented the functions of transcription factor acetylation.6,454-456 Here we exemplify a few key principles (Figure 10). In the case of transcription factors such as NF-κB and STAT3 that are cytoplasmic when inactive, signaling begins with an extracellular stimulus that leads to a cascade of PTMs resulting in changes in dimer structures and translocation from the cytoplasm to the nucleus.457,458 STAT3 activation is marked by specific phosphorylation and acetylation events that allow for dimerization and subsequent nuclear localization.459 While phosphorylation is thought to be dominant for dimerization and DNA binding, several studies suggest a phosphorylation-independent mechanism of dimerization.460,461 Chin and colleagues demonstrate that acetylation at K685 by KAT3A/B (CBP/p300) induces homodimerization and nuclear translocation of STAT proteins.462 RelA is a subunit of NF-κB generally sequestered in the cytoplasm through its interaction with its negative regulator IκBα. Upon cell stimulation, RelA is acetylated by KAT3A/B (CBP/p300) at several residues. Acetylation at K221 disrupts the RelA-IκBα interaction, allowing for nuclear translocation and increased DNA binding (Figure 10(A).427,463
Changes in acetylation can also induce changes in protein stability, as is the case for the p53 transcription factor. Acetylation can directly compete with ubiquitination at distinct lysine residues.464 It can also mediate structural changes to prevent ubiquitination by sterically hindering interaction with ubiquitin ligases.464,465 At homeostasis, p53 is maintained at low levels in the nucleus primarily through ubiquitin-mediated proteolysis.466 Upon DNA damage, p53 becomes highly acetylated at its carboxy-terminal domain (CTD), preventing MDM2-mediated ubiquitination and degradation.467,468 Acetylation of the p53 CTD is catalyzed primarily by KAT3B (p300), but KAT6A (MOZ) also acetylates p53 at K120 and K382.418,467 Crosstalk exists between factor acetylation and other PTMs, notably lysine methylation. For example, lysine methyltransferase 7 (KMT7, SET7/9)-mediated monomethylation at K372 promotes acetylation and stabilization of p53 by disrupting its interaction with the deacetylase SIRT1 (Figure 10B).469-472 A similar phenomenon has been reported for RelA acetylation at K310 which prevents KMT7-mediated monomethylation at K314 and K315, thus stabilizing the protein.473 Furthermore, acetylation of p53 is recognized by KAT3A/B (CPB/p300) and KAT4 bromodomains to facilitate acetylation of histone H3 and histone H4 at p53 response genes, which induces cell-cycle arrest or apoptosis.474-476
Acetylation can further influence the DNA binding affinity and promoter specificity of transcription factors such as the T cell lineage master regulators RAR-related orphan receptor gamma (RORγ) and Forkhead box O proteins (FoxO). RORγ is acetylated by KAT3B(p300) and deacetylated by KDAC1 and SIRT1 at K69, K81, K99, and K112, with the latter activating DNA binding of this lineage-determining transcription factor.432,433 Deacetylation of RORγ increases transcription of the interleukin-17 (IL-17) gene but decreases activation of IL-2 (Figure 10C).433 Similarly, acetylation of FoxO proteins by KAT3B (p300) facilitates dissociation from promoters of genes such as p27 and MnSOD, a process that is reversible upon SIRT1, SIRT2, or SIRT3 overexpression.477 Interestingly, acetylation of the Forkhead box P3 protein (FOXP3) enhances the stability and function of the transcription factor, a master regulator of regulatory T cell identity.478 FOXP3 acetylation is regulated by a balance between KAT3B (p300) and KAT5 (TIP60) acetylation and KDAC7, KDAC9, and SIRT1 deacetylation at K31, K263, and K274.50,394,395,479 Of note, acetylation of KAT5 (TIP60) by KAT3B (p300) increases its ability to acetylate FOXP3, highlighting the multiple layers of KAT cooperation required for appropriate signaling and regulatory T cell function. 395,396,480
4.3. Acetylation of the Basal Transcription Machinery
In addition to transcription factors, a growing number of factors associated with the RNA polymerase II complex are acetylated. Basal transcription factor acetylation has been studied in less detail, and these data are summarized here. Choudhary et al. list eight TBP-associated proteins (TAFs) that are acetylated in the nucleus.315 These proteins compose the basal transcription factor TFIID and contribute to transcription initiation of the RNA polymerase II complex.481 The function of TAF acetylation in the TFIID complex remains largely unknown, but acetylation of TAF(I)68, the second largest subunit of the TATA box-binding protein-containing factor TIF-IB/SL1, enhances binding to rDNA and was linked to enhanced RNA polymerase I transcription.482 A more recent study highlighted the importance of CBP in RNA Pol II regulation at promoters in Drosophila. In this system, CBP was present at the promoters of nearly all expressed genes and was found to play a role in promoter-proximal pausing, especially at genes with CBP and GAF co-occupancy.483
Acetylation of the positive transcription factor b (P-TEFb) is studied in detail.369,371,378 P-TEFb is composed of a cyclin T subunit, and the cyclin-dependent kinase CDK9. When assembled in an active elongation complex, is critical to phosphorylate negative elongation factors and the C-terminal domain (CTD) of the largest RNA polymerase II subunit at serine 2, activating transcription elongation by the polymerase complex.484,485 P-TEFb is stored predominantly in the nucleoplasm in a ribonucleoprotein complex (7SK RNP) but is released and activated upon increased transcriptional demand.486,487 This release is caused in part by acetylation of four sites in cyclin T1 (K380, K386, K390, K404), which dissociates acetylated cyclin T1/CDK9 from the 7SK RNP and activates CDK9 activity on negative elongation factors and the polymerase CTD.378 CDK9 also is subject to acetylation at two lysine residues (K44, K48).369,371 K48 acetylation disrupts ATP binding and inhibits CDK9 kinase activity directly,371,488 while K44 acetylation activates P-TEFb activity. Additional cellular elongation factors found to be acetylated by mass spectrometry include FACT members SUP16H and SSRP1, but also the RTF1 subunit of the PAF1 complex as well as SUB1 and the CTD phosphatase FCP1,315 but these marks have not yet been studied functionally.
The CTD of RNA polymerase II is acetylated. The CTD is a long and flexible domain structure composed of heptapeptide repeats with the consensus sequence YSPTSPS, which is conserved across eukaryotes. Interestingly, the CTD has expanded in metazoans to include a C-terminal region of heptad repeats that are less strictly aligned with the consensus sequence. In mammals, this region contains eight heptad repeats where the serine in position 7 is replaced with a lysine.435 Acetylation of these lysine residues is mediated by KAT3B(p300) and is enriched downstream of transcription start sites in actively transcribed genes, linking this modification to polymerase pausing.434 Accordingly, activation of signal-induced genes is inhibited when lysines are mutated to arginines in the CTD. However, the acetylated RNA polymerase II is not only found on signal-induced genes but also on many actively transcribed genes, implicating additional functions for CTD acetylation in transcription.434 Notably, both RNA polymerase I and III subunits are also acetylated.315 PAF53, a regulatory subunit of RNA polymerase I, is acetylated by KAT3A(CBP) at K373.423 PAF53 acetylation is maintained at low levels by SIRT7, which facilitates robust rRNA transcription. Induction of stress by glucose deprivation suppresses SIRT7 activity, leading to hyperacetylation of PAF53 and repression of rRNA transcription.423
5. PROTEIN STABILITY AND AGGREGATION IN THE CYTOPLASM
5.1. Tubulin and HSP90 Are Regulated by KDAC6
Lysine acetylation in the cytoplasm is historically a very “old” concept, as tubulin was the first nonhistone acetylation substrate identified.323-325,489 Tubulin forms microtubules, a major structural element in the cytoplasm, composed of α/β tubulin dimers.490-492 Acetylation of α-tubulin occurs on the luminal side of microtubules at K40 and is catalyzed predominantly by α-tubulin acetyltransferase αTAT1, a non-canonical KAT homologous to zebrafish or C. elegans MEC17.493-495 It is unclear whether acetylation is a cause or a consequence of tubulin stability, although this modification is generally considered a marker of protein stability. αTAT1 overexpression destabilizes microtubules; however, this is mainly attributed to enhanced αTAT1-tubulin interactions and not considered a consequence of increased acetyltransferase activity.495 Tubulin is deacetylated by KDAC6 112 and SIRT2.161 KDAC6 is the major tubulin deacetylase, and KDAC6 overexpression increases the chemotactic motility of murine fibroblasts, possibly due to tubulin destabilization.112 SIRT2 plays an important role in tubulin deacetylation in response to macrophage and NLRP3 inflammasome activation161,496 and also regulates tubulin acetylation on mitotic spindles.497
HSP90 gained considerable attention due to its potential as a therapeutic target in hematologic malignancies.498 HSP90 exists in two major isoforms: HSP90α, which is stress-inducible and tightly regulated, and HSP90β, which is constitutively expressed.498 HSP90 acetylation is detected on up to 22 distinct residues on HSP90α, and 5 distinct residues HSP90β.315,498,499 KDAC6 deacetylates HSP90, influencing glucocorticoid receptor (GR) or mineralocorticoid receptor (MR) signaling.500,501 KDAC1 may also influence HSP90 acetylation,502 though the KATs responsible remain elusive. Acetylation predominantly occurs on the middle domain of HSP90, where it regulates intermolecular interactions and chaperone activity.501
5.2. Tau and Alzheimer’s Disease
Acetylation also regulates microtubule-associated proteins (MAPs) with Tau as a prominent example.503,504 Tau is highly expressed in neurons, and mutations in Tau serve as important markers for dementia and Alzheimer’s disease.505 These mutations are linked to microtubule-binding repeats causing neurological defects associated with the disruption of Tau–microtubule interactions. Tau aggregation produces paired helical filaments seen in neurofibrillary tangles present in the brains of individuals afflicted with neurodegeneration.506
Acetylation was identified on more than a dozen lysine residues in Tau using in vitro, cell-based, and mass spectrometric assays.507-509 Acetylation is a common feature across MAP family members as the microtubule-binding domains of MAP2 and MAP4 are also acetylated.510 Tau, like MAP2 and 4 proteins, possesses intrinsic acetyltransferase activity.510-512 In addition, several KATs have been identified to modify Tau, including KAT3A/B(CBP/p300) and KAT2B-(PCAF).507,508 Deacetylases that target Tau include SIRT1, SIRT2, and KDAC6 with robust activities by SIRT1 and KDAC6.508,513,514
Several acetylation sites on Tau are well characterized.515 These include K274, K280, and K281. Acetylation of these sites reduces Tau interaction with microtubules by interfering with functions of the microtubule-interacting domain.507,516 Acetylation of K274 and K281 leads to mislocalization of Tau, while K280 acetylation promotes Tau aggregation.507,517 Acetylation of a distinct site, K174, slows cellular turnover of Tau and contributes to cognitive defects in mouse models of Alzheimer’s disease. Notably, acetylation of specific RXGS motifs in Tau inhibit phosphorylation and aggregation of the protein, indicating opposing effects of different acetylation sites in Tau on neurogenerative pathogenesis.518 These sites are also targeted by distinct KDACs: RXGS motifs are preferentially deacetylated by KDAC6, while SIRT1 targets K174, K274, K280, and K281.508,518 As Tau is decorated with many post-translational modifications, including lysine methylation and ubiquitination, these modifications can competitively inhibit Tau acetylation in vitro and in vivo.509,519,520
6. MITOCHONDRIA
6.1. Mitochondrial Acetylation Regulates Cell Metabolism
Acetylation is widespread in mitochondrial proteins: 1/3 of mitochondrial proteins are acetylated,521 and many proteins carry multiple acetylated lysines.305,315 Mitochondrial acetylation is strongly conserved from Drosophila to humans.522 Not surprisingly, acetylated proteins are involved in major functions of mitochondria (e.g., TCA cycle, oxidative phosphorylation, β-oxidation of lipids, amino acid metabolism, carbohydrate metabolism, nucleotide metabolism, and the urea cycle).523,524 Mitochondrial metabolism results from high concentrations of acetyl-CoA from aerobic catabolism of pyruvate, β-oxidation of long-chain fatty acids, and decarboxylation of malonyl-CoA.525
Three of the seven class III deacetylases (SIRT3, 4, and 5) are mitochondrial.526 SIRT3 has robust NAD+-dependent protein deacetylase activity, and mice lacking SIRT3 show significant hyperacetylation of mitochondrial proteins,527 while mice lacking SIRT4 or SIRT5 do not. Proteins that become hyperacetylated in the absence of SIRT3 control the shift to a fasting metabolism when the source of energy switches from glucose to lipids and amino acids. Thus, SIRT3 is linked to the energy status of the cell,528-532 and it is expressed at the highest levels in metabolically active tissues (e.g., liver, kidney, and heart).533,534 SIRT3 expression is also increased in glucose-poor, fasting states, including calorie restriction in liver and kidney.535-539
An important unresolved question regarding mitochondrial protein acetylation is the mechanism of acetylation itself. Is a mitochondrial KAT required? Mitochondria contain high concentrations of acetyl-CoA in millimolar amounts,540 and therefore, a nonenzymatic mechanism could account for the high level of mitochondrial protein acetylation.541 Indeed, increased mitochondrial protein acetylation is associated with physiological conditions that result in higher levels of acetyl-CoA (e.g., fasting, calorie restriction, high-fat diet, and ethanol intoxication).535,542-545
Three mitochondrial KATs have been reported. One is GCN5L1, which is homologous to a prokaryotic acetyltransferase.70 Mitochondrial protein acetylation is lower when the enzyme is lacking and increased when it is overexpressed.546 The second is the nuclear MYST family acetyltransferase KAT8 (MOF). It controls nuclear and mitochondrial respiratory genes by regulating oxidative phosphorylation.65 KAT8 (MOF) is important in tissues that are energetically demanding. For example, conditional knockouts of this gene result in hypertrophic cardiomyopathy and cardiac failure in mouse. However, the function of KAT8 (MOF) mediated mitochondrial acetylation in these cell types is not yet clear. Third, is acetyl-CoA acetyltransferase 1 (ACAT1), a regulator of the pyruvate dehydrogenase complex in mitochondria. ACAT1 was reported to influence acetylation of two mitochondrial proteins: PDHA1 and PDP1.547 ACAT1 knockdown led to a decrease in acetylation of PDHA1 and PDP1, inhibiting their function and leading to changes in glucose homeostasis that could contribute to the Warburg effect. It is critical to note that none of the studies of mitochondrial KATs use in vitro methodologies to show that acetylation of mitochondrial substrates is direct. This leaves a possibility that GCN5L1, KAT8 (MOF), or ACAT1 may modulate mitochondrial Acetyl-CoA levels or pH, influencing the efficiency of spontaneous acetylation in this cellular compartment.
SIRT3 is also important to the respiratory chain. Mice without SIRT3 use 10% less O2 and make 50% less ATP than wild-type mice.534,548 SIRT3 deacetylates and activates mitochondrial respiratory chain complexes (e.g., NDUFA9 (complex I)534 and SDHA (complex II))549,550 and regulates ATP synthase.551
6.2. Metabolic Targets of SIRT3
SIRT3 is a key enzyme in metabolism, necessary for efficient fatty acids utilization in the liver and for utilization of lipid-derived acetate and ketone bodies in peripheral tissues during fasting. The first identified target of SIRT3 is acetyl-CoA synthetase 2, which generates acetyl-CoA from acetate in extrahepatic tissues during fasting.552,553 During fasting, acetate is made by the liver from acetyl-CoA and can be used as energy by other tissues.554 SIRT3 regulates fatty acid oxidation by deacetylation and activation of long chain acyl-CoA dehydrogenase during fasting.535 β-Oxidation intermediates (e.g., long chain fatty acids) accumulate in mice that lack SIRT3.535 SIRT3 also regulates ketone body production by deacetylating and activation of 3-hydroxy-3-methylglutaryl-CoA synthase 2, a key step in the synthesis of ketone bodies.
In amino acid metabolism, SIRT3 regulates the aminotransferase that forms glutamine by transferring an α-amino to α-ketoglutarate. Another enzyme, glutamate dehydrogenase (GLUD1), regenerates α-ketoglutarate from glutamate and releases nitrogen as ammonia in the urea cycle.525 SIRT3 accelerates the urea cycle by activating ornithine trans-carbamoylase (OTC). Humans with urea cycle disorders and mice without SIRT3 have similar metabolic profiles, including increased levels of serum ornithine and reduced levels of citrulline.537
Other pathological conditions exhibit lower levels of SIRT3. Tumors often have reduced levels of SIRT3. As a result, glucose use is enhanced because of increased levels of reactive oxygen species (ROS) that activate hypoxia-inducible factor 1 alpha (HIF1α), which, in turn, activates glycolytic genes.549,555,556 SIRT3 also deacetylates and activates isocitrate dehydrogenase 2 and increases ROS levels as a byproduct of oxidative phosphorylation.557 SIRT3 deacetylates and activates the ROS-scavenging enzyme manganese superoxide dismutase to reduce oxidative damage in the liver.558-560 Mice without SIRT3 show greater oxidative stress,558 particularly on a high-fat diet,543 and have higher ROS levels than normal under calorie restriction.557
7. THERAPEUTIC TARGETING OF LYSINE ACETYLATION
7.1. KDAC Inhibitors
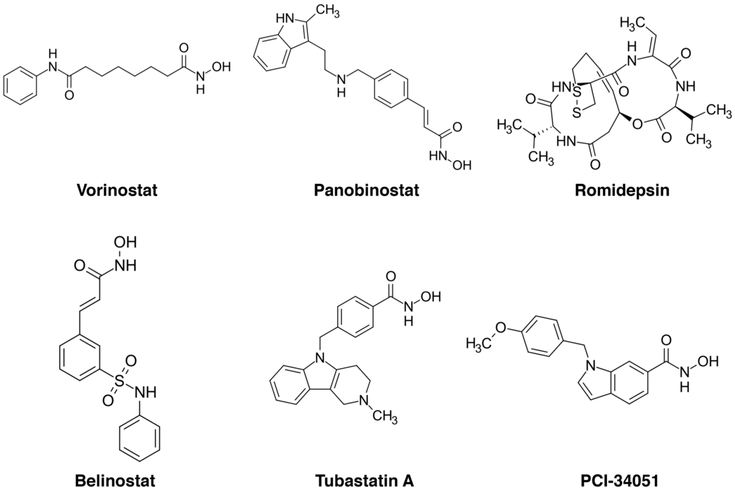
The manipulation of lysine acetylation using small molecules now known to be KDAC inhibitors was instrumental in the discovery of this modification. N-Butyrate was known to control gene expression and to induce differentiation of acute erythroid leukemia cells.102,561,562 Trichostatin A and tetrapeptide trapoxin are potent KDAC inhibitors.103,563,564 Suberoylanilide hydroxamic acid (SAHA) induces terminal differentiation and apoptosis in transformed cells and inhibits KDAC1 and KDAC3.565 SAHA (also known as Vorinostat) was approved by the Food and Drug Administration (FDA) to treat cutaneous T cell lymphoma. The antiepileptic drug valproic acid also inhibits KDACs566 and is in clinical trials for various indications. Other KDAC inhibitors are approved by the FDA (some of which are displayed in Figure 11), while others remain in clinical development.567,568
Figure 11.
Selected chemical structures of KDAC inhibitors.
Several hypotheses may explain the mechanisms of action of KDAC inhibitors. These small molecules might induce DNA damage and cell cycle interruption, cause ROS to accumulate, or activate apoptotic pathways.569 Most likely, in some way, these small molecules encourage apoptosis or hinder proliferation.570 Hyper-acetylation from small-molecule KDAC inhibitors has been observed at the tumor suppressor gene CDKN1A571 and in reactivation of latent HIV.572 Thus, acetylation-mediated transcriptional disruptions might explain the effects of KDAC inhibition on cellular proliferation and other phenotypes.
KDAC inhibitors targeting class I/II/IV enzymes generally chelate the divalent metal ion required for catalysis, although not all inhibitors exploit this mechanism.149 Available small molecules mostly target class I and II KDACs with limited selectivity for individual KDACs.573 However, emergent small molecules are active against a more restricted range of KDACs. Preclinical examples include specific inhibition of the cytoplasmic KDAC6 by Tubastatin A and of KDAC8 by PCI-34051.574,575 Importantly, the subset of differentially acetylated proteins differs depending on the KDAC inhibitor used.335
7.2. Sirtuin Modulators
SIRT1 is an attractive target for modulation given early connections between Sir2 and replicative lifespan in yeast.528,576 Indeed, as discussed, sirtuin activity closely ties key metabolic and epigenomic processes. However, specific targeting of sirtuins, while exciting, has proven difficult. Adding to this challenge, initial clinical studies with sirtuin activators have been inconsistent. While it is clear that sirtuin genetic deletion results in large changes in acetylation substrates and gross chromosomal abnormalities that lead to DNA damage,351,569 more extensive work is required to understand this family of genes and their therapeutic potential.
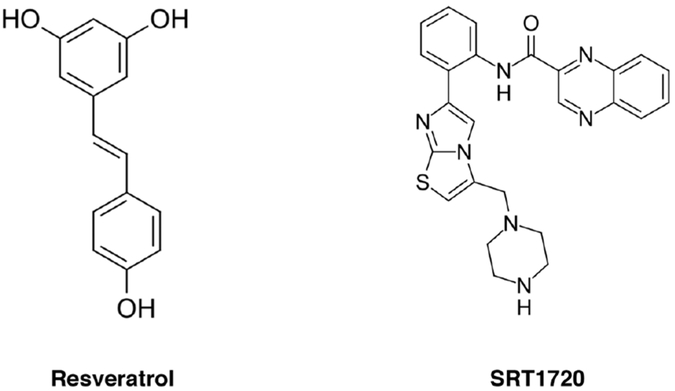
Polyphenolic compounds, namely the phytochemical resveratrol, were originally shown to activate sirtuin activity by enhancing cofactor and substrate binding via engagement of the SIRT1 N-terminus.577,578 These polyphenols lack potency in sirtuin binding, have low retention times in humans, and likely have considerable off-target effects.579,580 More recent high-throughput screening methodologies uncovered other SIRT1 activators, such as SRT1720, with interesting biological effects that lead to extended lifespan and improved health in mice and some efficacy against xenografted tumor growth models.581-583
Controversy has erupted about the action of resveratrol and SRT1720 (Figure 12). Two studies demonstrated in vitro that resveratrol-mediated SIRT1 activation required the presence of a fluorophore conjugated to substrate peptides,584,585 an observation that was supported by structural data.174 In vivo, resveratrol induces hypoacetylation for a subset of non-fluorophore labeled peptides, but also induces hypteracetylation of other substrates while leaving a large proportion of genes unchanged.586,587 These contradictory effects in global protein acetylation could be due to off target effects, such as inhibition of SIRT3 or activation of SIRT5.187 Importantly, they also may be explained by significant sequence specificities of resveratrol-mediated SIRT1 activation due to allosteric mechanisms.
Figure 12.
Selected chemical structures of sirtuin activators.
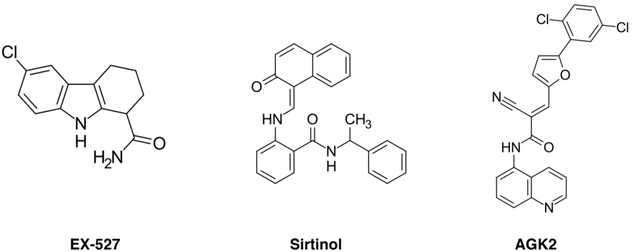
A number of specific inhibitors of sirtuin activity have been identified (Figure 13). Examples include indols, such as EX-527 targeting SIRT1, along with compounds such as sirtinol and tenovin derivatives.175,588-590 A growing number of SIRT2-specific inhibitors have been tested, including AGK2,591-593 SirReal inhibitors,594 and most recently 33i.595 Due to the high degree of conservation among sirtuin active sites, not surprisingly, several inhibitors bind to two or more sirtuins: cambinol with an IC50 ~ 60 μM for SIRT1 and SIRT2,596 salermide targeting SIRT1 and SIRT2,597,598 and suramin that inhibits SIRT5 but also strongly inhibits SIRT2 and SIRT1.190,599 Inhibitors that target SIRT3 are also being tested.600,601 Not much is known about the mechanism behind sirtuin inhibitors; however, in some cases, they likely function by interfering with NAD+ engagement.175
Figure 13.
Selected chemical structures of sirtuin inhibitors.
7.3. KAT Inhibitors
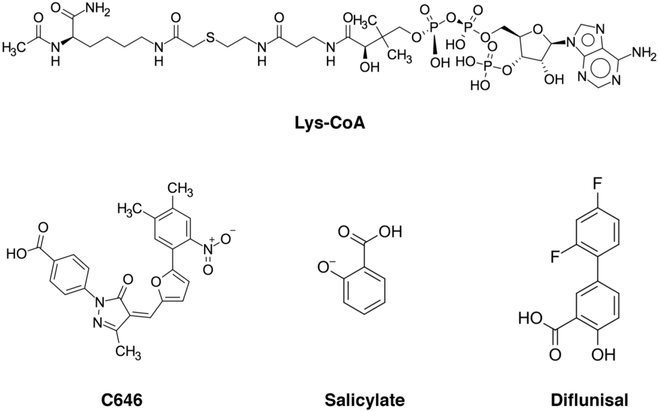
KAT3A/B(CBP/p300) has emerged as a potential therapeutic target for respiratory diseases, HIV infection, metabolic diseases, and cancer.602 However, the relatively shallow substrate-binding site in p300 is a challenging drug target, and most compounds to date target the acetyl-CoA binding site in the enzyme.603 Early KAT inhibitors include several phytochemicals, such as curcumin,604 garcinol,605 and anacardic acid.606 Chemical inhibitors were originally developed as bisubstrate acetyl-CoA mimics,607 pioneered by the Cole laboratory, and later replaced by smaller, more selective synthetic compounds, such as C646.608 C646 is a pyrazolonefuran (Figure 14) that was discovered via virtual ligand screening. It efficiently reduces histone acetylation levels within cells and displays cytotoxic properties toward certain cancer cells.608 Notably, a recent study characterized A-485, the most potent and specific p300 inhibitor identified to date.609 A-485 was found to be 1000-fold more potent than other cell permeable HAT inhibitors, including C646, and highly specific to the KA3A/B(CBP/p300) BHC (bromodomain HAT-C/H3) domains. A-485 was also found to suppress proliferation in 61 cancer cell lines with an EC50 < 2 μM, indicating the compound may have some therapeutic potential, especially against hematological malignancies and prostate cancer.609 Importantly, further characterization of the potential off target effect and studies in preclinical animal models are likely necessary prior to moving forward in any clinical setting.
Figure 14.
Selected chemical structures of KAT inhibitors.
Another study used naturally occurring acyl-CoA derivatives conjugated to biotin to affinity-purify KATs. Palmitoyl-CoA was recovered and found to inhibit GCN5 (KAT2A). This metabolite, among other acyl-CoA derivatives, was also able to bind PCAF (KAT2B) and MOF (KAT8) and modestly reduce levels of histone acetylation, underscoring that Acyl-CoA cofactors may act as endogenous regulators of lysine acetyltransferase activities.610 Interestingly, some long chain fatty acid metabolites such as myristic acid (required to produce myristoyl-CoA) have also been reported to activate deacylation activity in sirtuins, especially SIRT6.611
Salicylate inhibits KAT3A/B(CBP/p300) acetyltransferase activity by directly competing with acetyl-CoA and down-regulates the specific acetylation of histones and nonhistone proteins in cells.612 Furthermore, diflunisal, an FDA-approved drug containing a salicylic acid substructure, inhibited KAT3A/B(CBP/p300) more potently than salicylate. Both drugs are orally bioavailable and inhibited p300-dependent myelogenous leukemic cell growth in vitro and in vivo, pointing to a potential new clinical application. In addition, p300-induced Tau acetylation was inhibited by salicylate or its derivative salsalate, which enhanced Tau turnover and reduced Tau level.613 In a mouse model of Alzheimer’s disease, administration of salsalate after disease onset rescued Tau-induced memory deficits and prevented hippocampal atrophy, underscoring the clinical potential of KAT inhibitors in Alzheimer’s disease.
7.4. Bromodomain Inhibitors
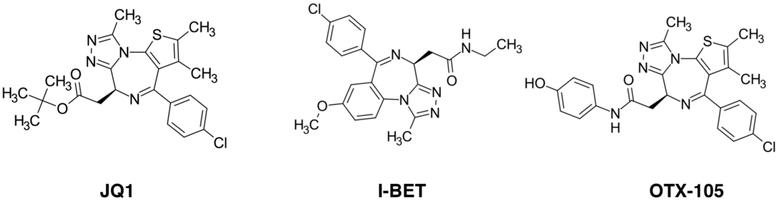
Small-molecule inhibition of bromodomains is the most recent advancement in efforts to pharmacologically target the protein acetylation network. Rather than disrupting enzymatic catalysis, these compounds target protein:protein interactions by inhibiting bromodomain recognition of its acetyl-lysine residue-containing ligand. The first bromodomain drug discovery attempts were described in the HIV field targeting the interaction of the acetylated form of the viral transactivator Tat (acK50) with the bromodomain of KAT2B/PCAF, a critical step in transcription from the integrated HIV provirus.614-616 The structure-based approach led to the discovery of a class of N1-aryl-propane-1,3-diamine compounds that selectively inhibited the acTat:PCAF interaction, albeit with relatively low potency. Also, the intracellular introduction of acetylated histone H4 peptides induced dissociation of BRD4 from chromatin and reduced cell growth.617 A year later, a patent from Mitsubishi Pharmaceuticals indicated that thienodiazepines bind BRD4 bromodomains.618 This patent report spurred the discovery of a lead compound, JQ1, with therapeutic activity against a rare squamous epithelial cancer called the NUT midline carcinoma.619 The NUT midline carcinoma is cytogenetically defined by a translocation of the BRD4 gene that results in an in-frame fusion with the nuclear protein in testis (NUT), a tissue-specific acetyltransferase.620
At the same time as the initial report of JQ1, the laboratory of Alexander Tarakhovsky in collaboration with GlaxoSmithKline reported the discovery of I-BET, a synthetic compound mimicking acetylated histones and disrupting chromatin complexes responsible for expression of inflammatory genes in activated macrophages, thus conferring protection against lipopolysaccharide-induced endotoxic shock and bacteria-induced sepsis.621 Interestingly, BET inhibitors also support immunotherapeutic applications by suppressing expression of Programmed Cell Death Protein Ligand 1 (PDL1),622 which increases cytotoxic T-cell activity and limits tumor progression in mice. Since the characterization of BET inhibitors and their preclinical application in cancer and immunology disease models, their potential utility in modulating male fertility,233 neurocognitive function,623 cardiovascular disease,624 and viral infections625 has been described.
JQ1, I-BET, and related compounds are powerful inhibitors of both bromodomains of the BET protein BRD4, with similar activity also against bromodomains of BRD2, BRD3 and the testis-specific BET protein BRDT.619 They function primarily by competing with acetyl-lysine binding by forming a hydrogen bond with a critical asparagine residue that otherwise engages the acetyl-lysine. The pharmacophore is a methyltriazole that is common to most available BET inhibitors (Figure 15). Recently, second generation BET inhibitors have been described, including bivalent compounds that target both BET bromodomains and achieve potency orders of magnitude above that of JQ1.236 Phthalimide-conjugated BET inhibitors that function as heterobifunctional small molecules have also been reported, which direct BET proteins to E3 ligase activity of cereblon, allowing for rapid and exquisitely specific destruction of BET proteins within the cell.626
Figure 15.
Selected chemical structures of BET inhibitors.
More than 20 early clinical trials are in process with BET inhibitors.619,627 Their focus is primarily on the treatment of various hematological malignancies, as BET proteins are coactivators of several critical oncogenes, including MYC,206 in addition to important regulators of cell proliferation and fate, such as MYB,628 BCL2, and FOSL1.629 Transcriptional disruption of these genes is linked to antineoplastic phenotypes observed under BET inhibition, likely operating via local removal of BRD4 and associated transcription factors (i.e., P-TEFb) from acetylated chromatin or acetylated transcription factors (i.e., TWIST,630 GATA-1,622,631 and ERG628) in addition to indirect effects on transcription and the cell cycle. Several BET inhibitor trials have completed Phase I or reported tolerability and partial clinical outcomes.632,633 Thus far, BET inhibitors appear well-tolerated with dose-limiting side effects such as diarrhea, fatigue, and reversible thrombocytopenia.634
As BET inhibitors are rapidly advancing into clinical trials, inhibitors of non-BET bromodomains are also being developed.269,635 Current non-BET bromodomain inhibitors have been described mainly for bromodomains of acetyl-transferases (i.e., p300/CBP) and chromatin remodeling components (i.e., BRD7, BRG1). Most non-BET targeting small molecules are at the stage of being chemical probes,636 and it has emerged that druggability varies among individual bromodomains.
8. CONCLUSIONS AND PERSPECTIVES
Lysine acetylation has moved from being a specialized mark on histones to a critical modification controlling cell fate, proliferation, and metabolism. The modification causes a change in the electrostatic charge of its cognate lysine residue, recruits reader proteins, and is tightly linked to fluctuations in key cellular metabolites, such as NAD+ and acetyl-CoA. In respective cellular compartments, lysine acetylation regulates diverse molecular outcomes, such as gene-specific chromatin processes, enzymatic regulation, protein multimerization, localization, and stability. Reader protein domains, including the bromodomain, tandem PHD, YEATS, and acidic domains, have evolved to specifically bind to acetylated or nonacetylated lysine residues, thus coordinating the acetylation response. Our understanding of other acylation marks is rapidly evolving; examples include lysine crotonylaton, succinylation, and malonylation, with shared enzymes that place and remove the marks, such as KAT3A/B and sirtuins, respectively.637,638 Pharmacological targeting of lysine acetylation is an established and briskly advancing field, starting from KDAC inhibitors, moving to sirtuin activators, and now including KAT and bromodomain inhibitors. The effects and mechanisms underlying these compounds are still being uncovered, and future studies must consider the role of newer acylation marks in drug action.
Other open questions concern the issue how partitioning of critical metabolites contributes to the function of lysine acetylation in distinct cellular milieus. A considerable degree of diversity of nonhistone acetylation has emerged in metazoans, especially in mammals. This could be due to a more discrete compartmentalization of acetyl-CoA in lower eukaryotes. In yeast, acetyl-CoA is 20–30-fold enriched in mitochondria as compared to other cellular compartments.639 In this context, acetyl-CoA does not permeate past the mitochondrial membrane and allows for distinction between acetyl-CoA as a metabolic intermediate and a cofactor for lysine acetylation. In mammals, this distinction is not as clear, and the question how other acyl group donors such as succinyl-CoA or malonyl-CoA compartmentalize remains yet unexplored. The opening of the lysine acetylation field to nutrition, exercise, and aging as well as its growing influence on disease pathogenesis and treatment of cancer, neurodegeneration, and HIV is exciting and signals far-reaching significance. Lysine acetylation may be key to the understanding of how such processes are molecularly defined. In the future, lysine acetylation and its directed intervention hold promise and are aimed at significantly improving health- and lifespan in humans.
ACKNOWLEDGMENTS
We thank John Carroll for assistance with graphics, Gary Howard for editorial support, and Veronica Fonseca for administrative support. We are thankful to members of the Ott lab for helpful discussions and to Ryan K. Quinn for important feedback on figures. We are grateful for funding from the UCSF Discovery Fellows Program and the American Society for Microbiology Robert D. Watkins Fellowship (to I.A.) and funding from the AmfAR Institute for HIV Cure Research and the NIH (to M.O., R01AI083139-06, R01DA043142, and 1DP1DA038043; to E.V., 5R24 DK085610-06 and R21AG051111). The authors declare no competing financial interest.
Biography
Ibraheem Ali began his graduate work in 2013 at University of California, San Francisco, where he joined Dr. Melanie Ott’s lab to study the role of HIV-1 Tat protein modifications in viral transcription. He has been recognized for his scientific outreach, in addition to his research, by the UCSF Discovery Fellowship and the American Society for Microbiology, Robert D. Watkins Graduate Research Fellowship. Ibraheem graduated from California State University, Fresno with a B.S. in Biology. His general research interests include regulation of viral latency and eukaryotic transcription.
Ryan Conrad is currently a postdoctoral researcher in the laboratory of Melanie Ott at the Gladstone Institutes. He recently received his Ph.D. from the University of California, San Francisco, where his doctoral research focused on bromodomain-containing proteins and their versatile functions in regulating HIV-1 transcription and latency. Ryan graduated from Vassar College in 2011 with degrees in Biochemistry and French & Francophone Studies. His general research interests include transcriptional regulation, chromatin biology, and the pharmacological targeting of epigenetic phenomena.
Eric Verdin, M.D., is the President and Chief Executive Officer of The Buck Institute for Research on Aging. A native of Belgium, Dr. Verdin received his Doctorate of Medicine (M.D.) from the University of Liege and additional clinical and research training at Harvard Medical School. He has held faculty positions at the University of Brussels, the National Institutes of Health (NIH), and the Picower Institute for Medical Research. His main interests include the molecular virology of HIV and novel approaches to eradicate HIV infection. Dr. Verdin’s laboratory also focuses on a family of proteins—called histone deacetylases—and their role in the aging process and the immune system.
Melanie Ott, M.D.-Ph.D., is a Senior Investigator at the Gladstone Institutes and Professor of Medicine at the University of California, San Francisco. Melanie obtained an M.D. degree from the University of Frankfurt/M. in Germany and a Ph.D. from the Picower Graduate School of Molecular Medicine in New York. Before coming to Gladstone, she held a junior faculty position at the German Cancer Research Center (DKFZ) in Heidelberg. Dr. Ott’s research focuses on how viral pathogens interact with host cells at the molecular level. Special emphasis lies on the regulation of HIV transcription and the role of reversible factor acetylation.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Aksnes H; Drazic A; Marie M; Arnesen T First Things First: Vital Protein Marks by N-Terminal Acetyltransferases. Trends Biochem. Sci 2016, 41, 746–60. [DOI] [PubMed] [Google Scholar]
- (2).Struhl K Histone Acetylation and Transcriptional Regulatory Mechanisms. Genes Dev. 1998, 12, 599–606. [DOI] [PubMed] [Google Scholar]
- (3).Grunstein M Histone Acetylation in Chromatin Structure and Transcription. Nature 1997, 389, 349–52. [DOI] [PubMed] [Google Scholar]
- (4).Strahl BD; Allis CD The Language of Covalent Histone Modifications. Nature 2000, 403, 41–5. [DOI] [PubMed] [Google Scholar]
- (5).Turner BM Histone Acetylation and an Epigenetic Code. BioEssays 2000, 22, 836–45. [DOI] [PubMed] [Google Scholar]
- (6).Choudhary C; Weinert BT; Nishida Y; Verdin E; Mann M The Growing Landscape of Lysine Acetylation Links Metabolism and Cell Signalling. Nat. Rev. Mol. Cell Biol 2014, 15, 536–50. [DOI] [PubMed] [Google Scholar]
- (7).Duan G; Walther D The Roles of Post-Translational Modifications in the Context of Protein Interaction Networks. PLoS Comput. Biol 2015, 11, e1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Gnad F; Forner F; Zielinska DF; Birney E; Gunawardena J; Mann M Evolutionary Constraints of Phosphorylation in Eukaryotes, Prokaryotes, and Mitochondria. Mol. Cell. Proteomics 2010, 9, 2642–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Santo-Domingo J; Demaurex N Perspectives On: Sgp Symposium on Mitochondrial Physiology and Medicine: The Renaissance of Mitochondrial Ph. J. Gen. Physiol 2012, 139, 415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wagner GR; Payne RM Widespread and Enzyme-Independent Nepsilon-Acetylation and Nepsilon-Succinylation of Proteins in the Chemical Conditions of the Mitochondrial Matrix. J. Biol. Chem 2013, 288, 29036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Yang XJ; Ogryzko VV; Nishikawa J; Howard BH; Nakatani Y A P300/Cbp-Associated Factor That Competes with the Adenoviral Oncoprotein E1a. Nature 1996, 382, 319–24. [DOI] [PubMed] [Google Scholar]
- (12).Ogryzko VV; Schiltz RL; Russanova V; Howard BH; Nakatani Y The Transcriptional Coactivators P300 and Cbp Are Histone Acetyltransferases. Cell 1996, 87, 953–9. [DOI] [PubMed] [Google Scholar]
- (13).Mizzen CA; Yang XJ; Kokubo T; Brownell JE; Bannister AJ; Owen-Hughes T; Workman J; Wang L; Berger SL; Kouzarides T; Nakatani Y; Allis CD The Taf(Ii)250 Subunit of Tfiid Has Histone Acetyltransferase Activity. Cell 1996, 87, 1261–70. [DOI] [PubMed] [Google Scholar]
- (14).Yamamoto T; Horikoshi M Novel Substrate Specificity of the Histone Acetyltransferase Activity of Hiv-1-Tat Interactive Protein Tip60. J. Biol. Chem 1997, 272, 30595–8. [DOI] [PubMed] [Google Scholar]
- (15).Chen H; Lin RJ; Schiltz RL; Chakravarti D; Nash A; Nagy L; Privalsky ML; Nakatani Y; Evans RM Nuclear Receptor Coactivator Actr Is a Novel Histone Acetyltransferase and Forms a Multimeric Activation Complex with P/Caf and Cbp/P300. Cell 1997, 90, 569–80. [DOI] [PubMed] [Google Scholar]
- (16).Spencer TE; Jenster G; Burcin MM; Allis CD; Zhou J; Mizzen CA; McKenna NJ; Onate SA; Tsai SY; Tsai MJ; O’Malley BW Steroid Receptor Coactivator-1 Is a Histone Acetyltransferase. Nature 1997, 389, 194–8. [DOI] [PubMed] [Google Scholar]
- (17).Taschner M; Vetter M; Lorentzen E Atomic Resolution Structure of Human Alpha-Tubulin Acetyltransferase Bound to Acetyl-Coa. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 19649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Friedmann DR; Aguilar A; Fan J; Nachury MV; Marmorstein R Structure of the Alpha-Tubulin Acetyltransferase, Alphatat1, and Implications for Tubulin-Specific Acetylation. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 19655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Davenport AM; Collins LN; Chiu H; Minor PJ; Sternberg PW; Hoelz A Structural and Functional Characterization of the Alpha-Tubulin Acetyltransferase Mec-17. J. Mol. Biol 2014, 426, 2605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Szyk A; Deaconescu AM; Spector J; Goodman B; Valenstein ML; Ziolkowska NE; Kormendi V; Grigorieff N; Roll-Mecak A Molecular Basis for Age-Dependent Microtubule Acetylation by Tubulin Acetyltransferase. Cell 2014, 157, 1405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Wu H; Moshkina N; Min J; Zeng H; Joshua J; Zhou MM; Plotnikov AN Structural Basis for Substrate Specificity and Catalysis of Human Histone Acetyltransferase 1. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 8925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yang X; Li L; Liang J; Shi L; Yang J; Yi X; Zhang D; Han X; Yu N; Shang Y Histone Acetyltransferase 1 Promotes Homologous Recombination in DNA Repair by Facilitating Histone Turnover. J. Biol. Chem 2013, 288, 18271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Verreault A; Kaufman PD; Kobayashi R; Stillman B Nucleosomal DNA Regulates the Core-Histone-Binding Subunit of the Human Hat1 Acetyltransferase. Curr. Biol 1998, 8, 96–108. [DOI] [PubMed] [Google Scholar]
- (24).Chang L; Loranger SS; Mizzen C; Ernst SG; Allis CD; Annunziato AT Histones in Transit: Cytosolic Histone Complexes and Diacetylation of H4 During Nucleosome Assembly in Human Cells. Biochemistry 1997, 36, 469–80. [DOI] [PubMed] [Google Scholar]
- (25).Schuetz A; Bernstein G; Dong A; Antoshenko T; Wu H; Loppnau P; Bochkarev A; Plotnikov AN Crystal Structure of a Binary Complex between Human Gcn5 Histone Acetyltransferase Domain and Acetyl Coenzyme A. Proteins: Struct., Funct., Genet 2007, 68, 403–7. [DOI] [PubMed] [Google Scholar]
- (26).Ringel AE; Wolberger C Structural Basis for Acyl-Group Discrimination by Human Gcn5l2. Acta Crystallogr. D Struct Biol 2016, 72, 841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Filippakopoulos P; Picaud S; Mangos M; Keates T; Lambert JP; Barsyte-Lovejoy D; Felletar I; Volkmer R; Muller S; Pawson T; Gingras AC; Arrowsmith CH; Knapp S Histone Recognition and Large-Scale Structural Analysis of the Human Bromodomain Family. Cell 2012, 149, 214–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Martinez E; Palhan VB; Tjernberg A; Lymar ES; Gamper AM; Kundu TK; Chait BT; Roeder RG Human Staga Complex Is a Chromatin-Acetylating Transcription Coactivator That Interacts with Pre-Mrna Splicing and DNA Damage-Binding Factors in Vivo. Mol. Cell. Biol 2001, 21, 6782–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Hudson BP; Martinez-Yamout MA; Dyson HJ; Wright PE Solution Structure and Acetyl-Lysine Binding Activity of the Gcn5 Bromodomain. J. Mol. Biol 2000, 304, 355–70. [DOI] [PubMed] [Google Scholar]
- (30).Clements A; Rojas JR; Trievel RC; Wang L; Berger SL; Marmorstein R Crystal Structure of the Histone Acetyltransferase Domain of the Human Pcaf Transcriptional Regulator Bound to Coenzyme A. EMBO J. 1999, 18, 3521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Shi S; Lin J; Cai Y; Yu J; Hong H; Ji K; Downey JS; Lu X; Chen R; Han J; Han A Dimeric Structure of P300/Cbp Associated Factor. BMC Struct. Biol 2014, 14, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Zeng L; Zhang Q; Gerona-Navarro G; Moshkina N; Zhou MM Structural Basis of Site-Specific Histone Recognition by the Bromodomains of Human Coactivators Pcaf and Cbp/P300. Structure 2008, 16, 643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Dhalluin C; Carlson JE; Zeng L; He C; Aggarwal AK; Zhou MM Structure and Ligand of a Histone Acetyltransferase Bromodomain. Nature 1999, 399, 491–6. [DOI] [PubMed] [Google Scholar]
- (34).Panne D; Maniatis T; Harrison SC Crystal Structure of Atf-2/C-Jun and Irf-3 Bound to the Interferon-Beta Enhancer. EMBO J. 2004, 23, 4384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Laughlin JD; Nwachukwu JC; Figuera-Losada M; Cherry L; Nettles KW; LoGrasso PV Structural Mechanisms of Allostery and Autoinhibition in Jnk Family Kinases. Structure 2012, 20, 2174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lau E; Kluger H; Varsano T; Lee K; Scheffler I; Rimm DL; Ideker T; Ronai ZA Pkcepsilon Promotes Oncogenic Functions of Atf2 in the Nucleus While Blocking Its Apoptotic Function at Mitochondria. Cell 2012, 148, 543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wang L; Payton R; Dai W; Lu L Hyperosmotic Stress-Induced Atf-2 Activation through Polo-Like Kinase 3 in Human Corneal Epithelial Cells. J. Biol. Chem 2011, 286, 1951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Iioka T; Furukawa K; Yamaguchi A; Shindo H; Yamashita S; Tsukazaki T P300/Cbp Acts as a Coactivator to Cartilage Homeoprotein-1 (Cart1), Paired-Like Homeoprotein, through Acetylation of the Conserved Lysine Residue Adjacent to the Homeodomain. J. Bone Miner. Res 2003, 18, 1419–29. [DOI] [PubMed] [Google Scholar]
- (39).Popp TA; Tallant C; Rogers C; Fedorov O; Brennan PE; Muller S; Knapp S; Bracher F Development of Selective Cbp/P300 Benzoxazepine Bromodomain Inhibitors. J. Med. Chem 2016, 59, 8889–8912. [DOI] [PubMed] [Google Scholar]
- (40).Crawford TD; Romero FA; Lai KW; Tsui V; Taylor AM; de Leon Boenig G; Noland CL; Murray J; Ly J; Choo EF; Hunsaker TL; Chan EW; Merchant M; Kharbanda S; Gascoigne KE; Kaufman S; Beresini MH; Liao J; Liu W; Chen KX; Chen Z; Conery AR; Cote A; Jayaram H; Jiang Y; Kiefer JR; Kleinheinz T; Li Y; Maher J; Pardo E; Poy F; Spillane KL; Wang F; Wang J; Wei X; Xu Z; Xu Z; Yen I; Zawadzke L; Zhu X; Bellon S; Cummings R; Cochran AG; Albrecht BK; Magnuson S Discovery of a Potent and Selective in Vivo Probe (Gne-272) for the Bromodomains of Cbp/Ep300. J. Med. Chem 2016, 59, 10549–10563. [DOI] [PubMed] [Google Scholar]
- (41).Pradhan A; Liu Y The Calcium-Responsive Transactivator Recruits Creb Binding Protein to Nuclear Bodies. Neurosci. Lett 2004, 370, 191–5. [DOI] [PubMed] [Google Scholar]
- (42).Delvecchio M; Gaucher J; Aguilar-Gurrieri C; Ortega E; Panne D Structure of the P300 Catalytic Core and Implications for Chromatin Targeting and Hat Regulation. Nat. Struct. Mol. Biol 2013, 20, 1040–6. [DOI] [PubMed] [Google Scholar]
- (43).Kaczmarska Z; Ortega E; Goudarzi A; Huang H; Kim S; Marquez JA; Zhao Y; Khochbin S; Panne D Structure of P300 in Complex with Acyl-Coa Variants. Nat. Chem. Biol 2017, 13, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Curtis AM; Seo SB; Westgate EJ; Rudic RD; Smyth EM; Chakravarti D; FitzGerald GA; McNamara P Histone Acetyltransferase-Dependent Chromatin Remodeling and the Vascular Clock. J. Biol. Chem 2004, 279, 7091–7. [DOI] [PubMed] [Google Scholar]
- (45).Lill NL; Grossman SR; Ginsberg D; DeCaprio J; Livingston DM Binding and Modulation of P53 by P300/Cbp Coactivators. Nature 1997, 387, 823–7. [DOI] [PubMed] [Google Scholar]
- (46).Crawford TD; Tsui V; Flynn EM; Wang S; Taylor AM; Cote A; Audia JE; Beresini MH; Burdick DJ; Cummings R; Dakin LA; Duplessis M; Good AC; Hewitt MC; Huang HR; Jayaram H; Kiefer JR; Jiang Y; Murray J; Nasveschuk CG; Pardo E; Poy F; Romero FA; Tang Y; Wang J; Xu Z; Zawadzke LE; Zhu X; Albrecht BK; Magnuson SR; Bellon S; Cochran AG Diving into the Water: Inducible Binding Conformations for Brd4, Taf1(2), Brd9, and Cecr2 Bromodomains. J. Med. Chem 2016, 59, 5391–402. [DOI] [PubMed] [Google Scholar]
- (47).Wang H; Curran EC; Hinds TR; Wang EH; Zheng N Crystal Structure of a Taf1-Taf7 Complex in Human Transcription Factor Iid Reveals a Promoter Binding Module. Cell Res. 2014, 24, 1433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Louder RK; He Y; Lopez-Blanco JR; Fang J; Chacon P; Nogales E Corrigendum: Structure of Promoter-Bound Tfiid and Model of Human Pre-Initiation Complex Assembly. Nature 2016, 536, 112. [DOI] [PubMed] [Google Scholar]
- (49).Flynn EM; Huang OW; Poy F; Oppikofer M; Bellon SF; Tang Y; Cochran AG A Subset of Human Bromodomains Recognizes Butyryllysine and Crotonyllysine Histone Peptide Modifications. Structure 2015, 23, 1801–14. [DOI] [PubMed] [Google Scholar]
- (50).Li B; Samanta A; Song X; Iacono KT; Bembas K; Tao R; Basu S; Riley JL; Hancock WW; Shen Y; Saouaf SJ; Greene MI Foxp3 Interactions with Histone Acetyltransferase and Class Ii Histone Deacetylases Are Required for Repression. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 4571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Yi J; Huang X; Yang Y; Zhu WG; Gu W; Luo J Regulation of Histone Acetyltransferase Tip60 Function by Histone Deacetylase 3. J. Biol. Chem 2014, 289, 33878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Sun Y; Jiang X; Chen S; Fernandes N; Price BD A Role for the Tip60 Histone Acetyltransferase in the Acetylation and Activation of Atm. Proc. Natl. Acad. Sci. U. S. A 2005, 102, 13182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Holbert MA; Sikorski T; Carten J; Snowflack D; Hodawadekar S; Marmorstein R The Human Monocytic Leukemia Zinc Finger Histone Acetyltransferase Domain Contains DNA-Binding Activity Implicated in Chromatin Targeting. J. Biol. Chem 2007, 282, 36603–13. [DOI] [PubMed] [Google Scholar]
- (54).Xiong X; Panchenko T; Yang S; Zhao S; Yan P; Zhang W; Xie W; Li Y; Zhao Y; Allis CD; Li H Selective Recognition of Histone Crotonylation by Double Phd Fingers of Moz and Dpf2. Nat. Chem. Biol 2016, 12, 1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Dreveny I; Deeves SE; Fulton J; Yue B; Messmer M; Bhattacharya A; Collins HM; Heery DM The Double Phd Finger Domain of Moz/Myst3 Induces Alpha-Helical Structure of the Histone H3 Tail to Facilitate Acetylation and Methylation Sampling and Modification. Nucleic Acids Res. 2014, 42, 822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ullah M; Pelletier N; Xiao L; Zhao SP; Wang K; Degerny C; Tahmasebi S; Cayrou C; Doyon Y; Goh SL; Champagne N; Cote J; Yang XJ Molecular Architecture of Quartet Moz/Morf Histone Acetyltransferase Complexes. Mol. Cell. Biol 2008, 28, 6828–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Ali M; Yan K; Lalonde ME; Degerny C; Rothbart SB; Strahl BD; Cote J; Yang XJ; Kutateladze TG Tandem Phd Fingers of Morf/Moz Acetyltransferases Display Selectivity for Acetylated Histone H3 and Are Required for the Association with Chromatin. J. Mol. Biol 2012, 424, 328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Burke TW; Cook JG; Asano M; Nevins JR Replication Factors Mcm2 and Orc1 Interact with the Histone Acetyltransferase Hbo1. J. Biol. Chem 2001, 276, 15397–408. [DOI] [PubMed] [Google Scholar]
- (59).Doyon Y; Cayrou C; Ullah M; Landry AJ; Cote V; Selleck W; Lane WS; Tan S; Yang XJ; Cote J Ing Tumor Suppressor Proteins Are Critical Regulators of Chromatin Acetylation Required for Genome Expression and Perpetuation. Mol. Cell 2006, 21, 51–64. [DOI] [PubMed] [Google Scholar]
- (60).Havasi A; Haegele JA; Gall JM; Blackmon S; Ichimura T; Bonegio RG; Panchenko MV Histone Acetyl Transferase (Hat) Hbo1 and Jade1 in Epithelial Cell Regeneration. Am. J. Pathol 2013, 182, 152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Siriwardana NS; Meyer R; Havasi A; Dominguez I; Panchenko MV Cell Cycle-Dependent Chromatin Shuttling of Hbo1-Jade1 Histone Acetyl Transferase (Hat) Complex. Cell Cycle 2014, 13, 1885–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Yuan H; Rossetto D; Mellert H; Dang W; Srinivasan M; Johnson J; Hodawadekar S; Ding EC; Speicher K; Abshiru N; Perry R; Wu J; Yang C; Zheng YG; Speicher DW; Thibault P; Verreault A; Johnson FB; Berger SL; Sternglanz R; McMahon SB; Cote J; Marmorstein R Myst Protein Acetyltransferase Activity Requires Active Site Lysine Autoacetylation. EMBO J 2012, 31, 58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).McCullough CE; Song S; Shin MH; Johnson FB; Marmorstein R Structural and Functional Role of Acetyltransferase Hmof K274 Autoacetylation. J. Biol. Chem 2016, 291, 18190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Sun B; Guo S; Tang Q; Li C; Zeng R; Xiong Z; Zhong C; Ding J Regulation of the Histone Acetyltransferase Activity of Hmof Via Autoacetylation of Lys274. Cell Res. 2011, 21, 1262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Chatterjee A; Seyfferth J; Lucci J; Gilsbach R; Preissl S; Bottinger L; Martensson CU; Panhale A; Stehle T; Kretz O; Sahyoun AH; Avilov S; Eimer S; Hein L; Pfanner N; Becker T; Akhtar A Mof Acetyl Transferase Regulates Transcription and Respiration in Mitochondria. Cell 2016, 167, 722–738 (e23).. [DOI] [PubMed] [Google Scholar]
- (66).Cai Y; Jin J; Swanson SK; Cole MD; Choi SH; Florens L; Washburn MP; Conaway JW; Conaway RC Subunit Composition and Substrate Specificity of a Mof-Containing Histone Acetyltransferase Distinct from the Male-Specific Lethal (Msl) Complex. J. Biol. Chem 2010, 285, 4268–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Creppe C; Malinouskaya L; Volvert ML; Gillard M; Close P; Malaise O; Laguesse S; Cornez I; Rahmouni S; Ormenese S; Belachew S; Malgrange B; Chapelle JP; Siebenlist U; Moonen G; Chariot A; Nguyen L Elongator Controls the Migration and Differentiation of Cortical Neurons through Acetylation of Alpha-Tubulin. Cell 2009, 136, 551–64. [DOI] [PubMed] [Google Scholar]
- (68).Pokholok DK; Hannett NM; Young RA Exchange of Rna Polymerase Ii Initiation and Elongation Factors During Gene Expression in Vivo. Mol. Cell 2002, 9, 799–809. [DOI] [PubMed] [Google Scholar]
- (69).Pu J; Schindler C; Jia R; Jarnik M; Backlund P; Bonifacino JS Borc, a Multisubunit Complex That Regulates Lysosome Positioning. Dev. Cell 2015, 33, 176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Scott I; Webster BR; Li JH; Sack MN Identification of a Molecular Component of the Mitochondrial Acetyltransferase Programme: A Novel Role for Gcn5l1. Biochem. J 2012, 443, 655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Hsieh YJ; Kundu TK; Wang Z; Kovelman R; Roeder RG The Tfiiic90 Subunit of Tfiiic Interacts with Multiple Components of the Rna Polymerase Iii Machinery and Contains a Histone-Specific Acetyltransferase Activity. Mol. Cell. Biol 1999, 19, 7697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Laganiere J; Deblois G; Giguere V Functional Genomics Identifies a Mechanism for Estrogen Activation of the Retinoic Acid Receptor Alpha1 Gene in Breast Cancer Cells. Mol. Endocrinol 2005, 19, 1584–92. [DOI] [PubMed] [Google Scholar]
- (73).Flajollet S; Lefebvre B; Cudejko C; Staels B; Lefebvre P The Core Component of the Mammalian Swi/Snf Complex Smarcd3/Baf60c Is a Coactivator for the Nuclear Retinoic Acid Receptor. Mol. Cell. Endocrinol 2007, 270, 23–32. [DOI] [PubMed] [Google Scholar]
- (74).Kang D; Oh S; Ahn SM; Lee BH; Moon MH Proteomic Analysis of Exosomes from Human Neural Stem Cells by Flow Field-Flow Fractionation and Nanoflow Liquid Chromatography-Tandem Mass Spectrometry. J. Proteome Res 2008, 7, 3475–80. [DOI] [PubMed] [Google Scholar]
- (75).Oda Y; Ishikawa MH; Hawker NP; Yun QC; Bikle DD Differential Role of Two Vdr Coactivators, Drip205 and Src-3, in Keratinocyte Proliferation and Differentiation. J. Steroid Biochem. Mol. Biol 2007, 103, 776–80. [DOI] [PubMed] [Google Scholar]
- (76).Atkins GB; Hu X; Guenther MG; Rachez C; Freedman LP; Lazar MA Coactivators for the Orphan Nuclear Receptor Roralpha. Mol. Endocrinol 1999, 13, 1550–7. [DOI] [PubMed] [Google Scholar]
- (77).Kruse SW; Suino-Powell K; Zhou XE; Kretschman JE; Reynolds R; Vonrhein C; Xu Y; Wang L; Tsai SY; Tsai MJ; Xu HE Identification of Coup-Tfii Orphan Nuclear Receptor as a Retinoic Acid-Activated Receptor. PLoS Biol. 2008, 6, e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Wang Z; Wu Y; Li L; Su XD Intermolecular Recognition Revealed by the Complex Structure of Human Clock-Bmal1 Basic Helix-Loop-Helix Domains with E-Box DNA. Cell Res. 2013, 23, 213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Lee C; Etchegaray JP; Cagampang FR; Loudon AS; Reppert SM Posttranslational Mechanisms Regulate the Mammalian Circadian Clock. Cell 2001, 107, 855–67. [DOI] [PubMed] [Google Scholar]
- (80).Kwon I; Lee J; Chang SH; Jung NC; Lee BJ; Son GH; Kim K; Lee KH Bmal1 Shuttling Controls Transactivation and Degradation of the Clock/Bmal1 Heterodimer. Mol. Cell. Biol 2006, 26, 7318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Zhao WN; Malinin N; Yang FC; Staknis D; Gekakis N; Maier B; Reischl S; Kramer A; Weitz CJ Cipc Is a Mammalian Circadian Clock Protein without Invertebrate Homologues. Nat. Cell Biol 2007, 9, 268–75. [DOI] [PubMed] [Google Scholar]
- (82).Kondratov RV; Chernov MV; Kondratova AA; Gorbacheva VY; Gudkov AV; Antoch MP Bmal1-Dependent Circadian Oscillation of Nuclear Clock: Posttranslational Events Induced by Dimerization of Transcriptional Activators of the Mammalian Clock System. Genes Dev. 2003, 17, 1921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Guelman S; Kozuka K; Mao Y; Pham V; Solloway MJ; Wang J; Wu J; Lill JR; Zha J The Double-Histone-Acetyltransferase Complex Atac Is Essential for Mammalian Development. Mol. Cell. Biol 2009, 29, 1176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Berndsen CE; Denu JM Catalysis and Substrate Selection by Histone/Protein Lysine Acetyltransferases. Curr. Opin. Struct. Biol 2008, 18, 682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Jiang J; Lu J; Lu D; Liang Z; Li L; Ouyang S; Kong X; Jiang H; Shen B; Luo C Investigation of the Acetylation Mechanism by Gcn5 Histone Acetyltransferase. PLoS One 2012, 7, e36660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Berndsen CE; Albaugh BN; Tan S; Denu JM Catalytic Mechanism of a Myst Family Histone Acetyltransferase. Biochemistry 2007, 46, 623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Yan Y; Harper S; Speicher DW; Marmorstein R The Catalytic Mechanism of the Esa1 Histone Acetyltransferase Involves a Self-Acetylated Intermediate. Nat. Struct. Biol 2002, 9, 862–9. [DOI] [PubMed] [Google Scholar]
- (88).Yan Y; Barlev NA; Haley RH; Berger SL; Marmorstein R Crystal Structure of Yeast Esa1 Suggests a Unified Mechanism for Catalysis and Substrate Binding by Histone Acetyltransferases. Mol. Cell 2000, 6, 1195–205. [DOI] [PubMed] [Google Scholar]
- (89).Liu X; Wang L; Zhao K; Thompson PR; Hwang Y; Marmorstein R; Cole PA The Structural Basis of Protein Acetylation by the P300/Cbp Transcriptional Coactivator. Nature 2008, 451, 846–50. [DOI] [PubMed] [Google Scholar]
- (90).Zhang X; Ouyang S; Kong X; Liang Z; Lu J; Zhu K; Zhao D; Zheng M; Jiang H; Liu X; Marmorstein R; Luo C Catalytic Mechanism of Histone Acetyltransferase P300: From the Proton Transfer to Acetylation Reaction. J. Phys. Chem. B 2014, 118, 2009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Dancy BM; Cole PA Protein Lysine Acetylation by P300/Cbp. Chem. Rev 2015, 115, 2419–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Ogryzko VV; Kotani T; Zhang X; Schiltz RL; Howard T; Yang XJ; Howard BH; Qin J; Nakatani Y Histone-Like Tafs within the Pcaf Histone Acetylase Complex. Cell 1998, 94, 35–44. [DOI] [PubMed] [Google Scholar]
- (93).Martinez E; Kundu TK; Fu J; Roeder RG A Human Spt3-Tafii31-Gcn5-L Acetylase Complex Distinct from Transcription Factor Iid. J. Biol. Chem 1998, 273, 23781–5. [DOI] [PubMed] [Google Scholar]
- (94).Demeny MA; Soutoglou E; Nagy Z; Scheer E; Janoshazi A; Richardot M; Argentini M; Kessler P; Tora L Identification of a Small Taf Complex and Its Role in the Assembly of Taf-Containing Complexes. PLoS One 2007, 2, e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Nagy Z; Tora L Distinct Gcn5/Pcaf-Containing Complexes Function as Co-Activators and Are Involved in Transcription Factor and Global Histone Acetylation. Oncogene 2007, 26, 5341–57. [DOI] [PubMed] [Google Scholar]
- (96).Karanam B; Jiang L; Wang L; Kelleher NL; Cole PA Kinetic and Mass Spectrometric Analysis of P300 Histone Acetyltransferase Domain Autoacetylation. J. Biol. Chem 2006, 281, 40292–301. [DOI] [PubMed] [Google Scholar]
- (97).Thompson PR; Wang D; Wang L; Fulco M; Pediconi N; Zhang D; An W; Ge Q; Roeder RG; Wong J; Levrero M; Sartorelli V; Cotter RJ; Cole PA Regulation of the P300 Hat Domain Via a Novel Activation Loop. Nat. Struct. Mol. Biol 2004, 11, 308–15. [DOI] [PubMed] [Google Scholar]
- (98).Karukurichi KR; Wang L; Uzasci L; Manlandro CM; Wang Q; Cole PA Analysis of P300/Cbp Histone Acetyltransferase Regulation Using Circular Permutation and Semisynthesis. J. Am. Chem. Soc 2010, 132, 1222–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Karanam B; Wang L; Wang D; Liu X; Marmorstein R; Cotter R; Cole PA Multiple Roles for Acetylation in the Interaction of P300 Hat with Atf-2. Biochemistry 2007, 46, 8207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Albaugh BN; Arnold KM; Lee S; Denu JM Autoacetylation of the Histone Acetyltransferase Rtt109. J. Biol. Chem 2011, 286, 24694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Hirayama J; Sahar S; Grimaldi B; Tamaru T; Takamatsu K; Nakahata Y; Sassone-Corsi P Clock-Mediated Acetylation of Bmal1 Controls Circadian Function. Nature 2007, 450, 1086–90. [DOI] [PubMed] [Google Scholar]
- (102).Riggs MG; Whittaker RG; Neumann JR; Ingram VM N-Butyrate Causes Histone Modification in Hela and Friend Erythroleukaemia Cells. Nature 1977, 268, 462–4. [DOI] [PubMed] [Google Scholar]
- (103).Kijima M; Yoshida M; Sugita K; Horinouchi S; Beppu T Trapoxin, an Antitumor Cyclic Tetrapeptide, Is an Irreversible Inhibitor of Mammalian Histone Deacetylase. J. Biol. Chem 1993, 268, 22429–35. [PubMed] [Google Scholar]
- (104).Yoshida M; Horinouchi S; Beppu T Trichostatin a and Trapoxin: Novel Chemical Probes for the Role of Histone Acetylation in Chromatin Structure and Function. BioEssays 1995, 17, 423–30. [DOI] [PubMed] [Google Scholar]
- (105).Taunton J; Hassig CA; Schreiber SL A Mammalian Histone Deacetylase Related to the Yeast Transcriptional Regulator Rpd3p. Science 1996, 272, 408–11. [DOI] [PubMed] [Google Scholar]
- (106).Yang WM; Yao YL; Sun JM; Davie JR; Seto E Isolation and Characterization of Cdnas Corresponding to an Additional Member of the Human Histone Deacetylase Gene Family. J. Biol. Chem 1997, 272, 28001–7. [DOI] [PubMed] [Google Scholar]
- (107).Zeng Y; Tang CM; Yao YL; Yang WM; Seto E Cloning and Characterization of the Mouse Histone Deacetylase-2 Gene. J. Biol. Chem 1998, 273, 28921–30. [DOI] [PubMed] [Google Scholar]
- (108).Emiliani S; Fischle W; Van Lint C; Al-Abed Y; Verdin E Characterization of a Human Rpd3 Ortholog, Hdac3. Proc. Natl. Acad. Sci. U. S. A 1998, 95, 2795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Fischle W; Emiliani S; Hendzel MJ; Nagase T; Nomura N; Voelter W; Verdin E A New Family of Human Histone Deacetylases Related to Saccharomyces Cerevisiae Hda1p. J. Biol. Chem 1999, 274, 11713–20. [DOI] [PubMed] [Google Scholar]
- (110).Gao L; Cueto MA; Asselbergs F; Atadja P Cloning and Functional Characterization of Hdac11, a Novel Member of the Human Histone Deacetylase Family. J. Biol. Chem 2002, 277, 25748–55. [DOI] [PubMed] [Google Scholar]
- (111).Guardiola AR; Yao TP Molecular Cloning and Characterization of a Novel Histone Deacetylase Hdac10. J. Biol. Chem 2002, 277, 3350–6. [DOI] [PubMed] [Google Scholar]
- (112).Hubbert C; Guardiola A; Shao R; Kawaguchi Y; Ito A; Nixon A; Yoshida M; Wang XF; Yao TP Hdac6 Is a Microtubule-Associated Deacetylase. Nature 2002, 417, 455–8. [DOI] [PubMed] [Google Scholar]
- (113).Fritze CE; Verschueren K; Strich R; Easton Esposito R Direct Evidence for Sir2Modulation of Chromatin Structure in Yeast Rdna. EMBO J. 1997, 16, 6495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Gotta M; Strahl-Bolsinger S; Renauld H; Laroche T; Kennedy BK; Grunstein M; Gasser SM Localization of Sir2p: The Nucleolus as a Compartment for Silent Information Regulators. EMBO J. 1997, 16, 3243–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Braunstein M; Rose AB; Holmes SG; Allis CD; Broach JR Transcriptional Silencing in Yeast Is Associated with Reduced Nucleosome Acetylation. Genes Dev. 1993, 7, 592–604. [DOI] [PubMed] [Google Scholar]
- (116).Frye RA Characterization of Five Human Cdnas with Homology to the Yeast Sir2 Gene: Sir2-Like Proteins (Sirtuins) Metabolize Nad and May Have Protein Adp-Ribosyltransferase Activity. Biochem. Biophys. Res. Commun 1999, 260, 273–9. [DOI] [PubMed] [Google Scholar]
- (117).Imai S; Armstrong CM; Kaeberlein M; Guarente L Transcriptional Silencing and Longevity Protein Sir2 Is an Nad-Dependent Histone Deacetylase. Nature 2000, 403, 795–800. [DOI] [PubMed] [Google Scholar]
- (118).Frye RA Phylogenetic Classification of Prokaryotic and Eukaryotic Sir2-Like Proteins. Biochem. Biophys. Res. Commun 2000, 273, 793–8. [DOI] [PubMed] [Google Scholar]
- (119).Seto E; Yoshida M Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harbor Perspect. Biol 2014, 6, a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Delcuve GP; Khan DH; Davie JR Roles of Histone Deacetylases in Epigenetic Regulation: Emerging Paradigms from Studies with Inhibitors. Clin. Epigenet 2012, 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Kelly RD; Cowley SM The Physiological Roles of Histone Deacetylase (Hdac) 1 and 2: Complex Co-Stars with Multiple Leading Parts. Biochem. Soc. Trans 2013, 41, 741–9. [DOI] [PubMed] [Google Scholar]
- (122).Watson PJ; Fairall L; Schwabe JW Nuclear Hormone Receptor Co-Repressors: Structure and Function. Mol. Cell. Endocrinol 2012, 348, 440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Millard CJ; Watson PJ; Celardo I; Gordiyenko Y; Cowley SM; Robinson CV; Fairall L; Schwabe JW Class I Hdacs Share a Common Mechanism of Regulation by Inositol Phosphates. Mol. Cell 2013, 51, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Watson PJ; Millard CJ; Riley AM; Robertson NS; Wright LC; Godage HY; Cowley SM; Jamieson AG; Potter BV; Schwabe JW Insights into the Activation Mechanism of Class I Hdac Complexes by Inositol Phosphates. Nat. Commun 2016, 7, 11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Cai RL; Yan-Neale Y; Cueto MA; Xu H; Cohen D Hdac1, a Histone Deacetylase, Forms a Complex with Hus1 and Rad9, Two G2/M Checkpoint Rad Proteins. J. Biol. Chem 2000, 275, 27909–16. [DOI] [PubMed] [Google Scholar]
- (126).Liu J; Xu D; Wang H; Zhang Y; Chang Y; Zhang J; Wang J; Li C; Liu H; Zhao M; Lin C; Zhan Q; Huang C; Qian H The Subcellular Distribution and Function of Mta1 in Cancer Differentiation. Oncotarget 2014, 5, 5153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Bressi JC; Jennings AJ; Skene R; Wu Y; Melkus R; De Jong R; O’Connell S; Grimshaw CE; Navre M; Gangloff AR Exploration of the Hdac2 Foot Pocket: Synthesis and Sar of Substituted N-(2-Aminophenyl)Benzamides. Bioorg. Med. Chem. Lett 2010, 20, 3142–5. [DOI] [PubMed] [Google Scholar]
- (128).Lauffer BE; Mintzer R; Fong R; Mukund S; Tam C; Zilberleyb I; Flicke B; Ritscher A; Fedorowicz G; Vallero R; Ortwine DF; Gunzner J; Modrusan Z; Neumann L; Koth CM; Lupardus PJ; Kaminker JS; Heise CE; Steiner P Histone Deacetylase (Hdac) Inhibitor Kinetic Rate Constants Correlate with Cellular Histone Acetylation but Not Transcription and Cell Viability. J. Biol. Chem 2013, 288, 26926–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Chini CC; Escande C; Nin V; Chini EN Hdac3 Is Negatively Regulated by the Nuclear Protein Dbc1. J. Biol. Chem 2010, 285, 40830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Watson PJ; Fairall L; Santos GM; Schwabe JW Structure of Hdac3 Bound to Co-Repressor and Inositol Tetraphosphate. Nature 2012, 481, 335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Decroos C; Christianson DW Design, Synthesis, and Evaluation of Polyamine Deacetylase Inhibitors, and High-Resolution Crystal Structures of Their Complexes with Acetylpolyamine Amidohydrolase. Biochemistry 2015, 54, 4692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Decroos C; Clausen DJ; Haines BE; Wiest O; Williams RM; Christianson DW Variable Active Site Loop Conformations Accommodate the Binding of Macrocyclic Largazole Analogues to Hdac8. Biochemistry 2015, 54, 2126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Somoza JR; Skene RJ; Katz BA; Mol C; Ho JD; Jennings AJ; Luong C; Arvai A; Buggy JJ; Chi E; Tang J; Sang BC; Verner E; Wynands R; Leahy EM; Dougan DR; Snell G; Navre M; Knuth MW; Swanson RV; McRee DE; Tari LW Structural Snapshots of Human Hdac8 Provide Insights into the Class I Histone Deacetylases. Structure 2004, 12, 1325–34. [DOI] [PubMed] [Google Scholar]
- (134).Vannini A; Volpari C; Gallinari P; Jones P; Mattu M; Carfi A; De Francesco R; Steinkuhler C; Di Marco S Substrate Binding to Histone Deacetylases as Shown by the Crystal Structure of the Hdac8-Substrate Complex. EMBO Rep. 2007, 8, 879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Whitehead L; Dobler MR; Radetich B; Zhu Y; Atadja PW; Claiborne T; Grob JE; McRiner A; Pancost MR; Patnaik A; Shao W; Shultz M; Tichkule R; Tommasi RA; Vash B; Wang P; Stams T Human Hdac Isoform Selectivity Achieved Via Exploitation of the Acetate Release Channel with Structurally Unique Small Molecule Inhibitors. Bioorg. Med. Chem 2011, 19, 4626–34. [DOI] [PubMed] [Google Scholar]
- (136).Dowling DP; Gantt SL; Gattis SG; Fierke CA; Christianson DW Structural Studies of Human Histone Deacetylase 8 and Its Site-Specific Variants Complexed with Substrate and Inhibitors. Biochemistry 2008, 47, 13554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Burli RW; Luckhurst CA; Aziz O; Matthews KL; Yates D; Lyons KA; Beconi M; McAllister G; Breccia P; Stott AJ; Penrose SD; Wall M; Lamers M; Leonard P; Muller I; Richardson CM; Jarvis R; Stones L; Hughes S; Wishart G; Haughan AF; O’Connell C; Mead T; McNeil H; Vann J; Mangette J; Maillard M; Beaumont V; Munoz-Sanjuan I; Dominguez C Design, Synthesis, and Biological Evaluation of Potent and Selective Class Iia Histone Deacetylase (Hdac) Inhibitors as a Potential Therapy for Huntington’s Disease. J. Med. Chem 2013, 56, 9934–54. [DOI] [PubMed] [Google Scholar]
- (138).Bottomley MJ; Lo Surdo P; Di Giovine P; Cirillo A; Scarpelli R; Ferrigno F; Jones P; Neddermann P; De Francesco R; Steinkuhler C; Gallinari P; Carfi A Structural and Functional Analysis of the Human Hdac4 Catalytic Domain Reveals a Regulatory Structural Zinc-Binding Domain. J. Biol. Chem 2008, 283, 26694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Liu Y; Randall WR; Schneider MF Activity-Dependent and -Independent Nuclear Fluxes of Hdac4Mediated by Different Kinases in Adult Skeletal Muscle. J. Cell Biol 2005, 168, 887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Wang AH; Kruhlak MJ; Wu J; Bertos NR; Vezmar M; Posner BI; Bazett-Jones DP; Yang XJ Regulation of Histone Deacetylase 4 by Binding of 14–3-3 Proteins. Mol. Cell. Biol 2000, 20, 6904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Cohen TJ; Choi MC; Kapur M; Lira VA; Yan Z; Yao TP Hdac4 Regulates Muscle Fiber Type-Specific Gene Expression Programs. Mol. Cells 2015, 38, 343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Grozinger CM; Schreiber SL Regulation of Histone Deacetylase 4 and 5 and Transcriptional Activity by 14–3-3-Dependent Cellular Localization. Proc. Natl. Acad. Sci. U. S. A 2000, 97, 7835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).McGee SL; van Denderen BJ; Howlett KF; Mollica J; Schertzer JD; Kemp BE; Hargreaves M Amp-Activated Protein Kinase Regulates Glut4 Transcription by Phosphorylating Histone Deacetylase 5. Diabetes 2008, 57, 860–7. [DOI] [PubMed] [Google Scholar]
- (144).McKinsey TA; Zhang CL; Lu J; Olson EN Signal-Dependent Nuclear Export of a Histone Deacetylase Regulates Muscle Differentiation. Nature 2000, 408, 106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Dequiedt F; Kasler H; Fischle W; Kiermer V; Weinstein M; Herndier BG; Verdin E Hdac7, a Thymus-Specific Class Ii Histone Deacetylase, Regulates Nur77 Transcription and Tcr-Mediated Apoptosis. Immunity 2003, 18, 687–98. [DOI] [PubMed] [Google Scholar]
- (146).Parra M; Kasler H; McKinsey TA; Olson EN; Verdin E Protein Kinase D1 Phosphorylates Hdac7 and Induces Its Nuclear Export after T-Cell Receptor Activation. J. Biol. Chem 2005, 280, 13762–70. [DOI] [PubMed] [Google Scholar]
- (147).Navarro MN; Goebel J; Feijoo-Carnero C; Morrice N; Cantrell DA Phosphoproteomic Analysis Reveals an Intrinsic Pathway for the Regulation of Histone Deacetylase 7 That Controls the Function of Cytotoxic T Lymphocytes. Nat. Immunol 2011, 12, 352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Bakin RE; Jung MO Cytoplasmic Sequestration of Hdac7 from Mitochondrial and Nuclear Compartments Upon Initiation of Apoptosis. J. Biol. Chem 2004, 279, 51218–25. [DOI] [PubMed] [Google Scholar]
- (149).Lobera M; Madauss KP; Pohlhaus DT; Wright QG; Trocha M; Schmidt DR; Baloglu E; Trump RP; Head MS; Hofmann GA; Murray-Thompson M; Schwartz B; Chakravorty S; Wu Z; Mander PK; Kruidenier L; Reid RA; Burkhart W; Turunen BJ; Rong JX; Wagner C; Moyer MB; Wells C; Hong X; Moore JT; Williams JD; Soler D; Ghosh S; Nolan MA Selective Class Iia Histone Deacetylase Inhibition Via a Non-chelating Zinc-Binding Group. Nat. Chem. Biol 2013, 9, 319–25. [DOI] [PubMed] [Google Scholar]
- (150).Schuetz A; Min J; Allali-Hassani A; Schapira M; Shuen M; Loppnau P; Mazitschek R; Kwiatkowski NP; Lewis TA; Maglathin RL; McLean TH; Bochkarev A; Plotnikov AN; Vedadi M; Arrowsmith CH Human Hdac7 Harbors a Class Iia Histone Deacetylase-Specific Zinc Binding Motif and Cryptic Deacetylase Activity. J. Biol. Chem 2008, 283, 11355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Zhou X; Marks PA; Rifkind RA; Richon VM Cloning and Characterization of a Histone Deacetylase, Hdac9. Proc. Natl. Acad. Sci. U. S. A 2001, 98, 10572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Mahlknecht U; Schnittger S; Will J; Cicek N; Hoelzer D Chromosomal Organization and Localization of the Human Histone Deacetylase 9 Gene (Hdac9). Biochem. Biophys. Res. Commun 2002, 293, 182–91. [DOI] [PubMed] [Google Scholar]
- (153).Hai Y; Christianson DW Histone Deacetylase 6 Structure and Molecular Basis of Catalysis and Inhibition. Nat. Chem. Biol 2016, 12, 741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Tong JJ; Liu J; Bertos NR; Yang XJ Identification of Hdac10, a Novel Class Ii Human Histone Deacetylase Containing a Leucine-Rich Domain. Nucleic Acids Res. 2002, 30, 1114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Liu H; Hu Q; Kaufman A; D’Ercole AJ; Ye P Developmental Expression of Histone Deacetylase 11 in the Murine Brain. J. Neurosci. Res 2008, 86, 537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Finnin MS; Donigian JR; Cohen A; Richon VM; Rifkind RA; Marks PA; Breslow R; Pavletich NP Structures of a Histone Deacetylase Homologue Bound to the Tsa and Saha Inhibitors. Nature 1999, 401, 188–93. [DOI] [PubMed] [Google Scholar]
- (157).Newkirk TL; Bowers AA; Williams RM Discovery, Biological Activity, Synthesis and Potential Therapeutic Utility of Naturally Occurring Histone Deacetylase Inhibitors. Nat. Prod. Rep 2009, 26, 1293–320. [DOI] [PubMed] [Google Scholar]
- (158).Katsyuba E; Auwerx J Modulating Nad(+) Metabolism, from Bench to Bedside. EMBO J. 2017, 36, 2670–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Laurent G; German NJ; Saha AK; de Boer VC; Davies M; Koves TR; Dephoure N; Fischer F; Boanca G; Vaitheesvaran B; Lovitch SB; Sharpe AH; Kurland IJ; Steegborn C; Gygi SP; Muoio DM; Ruderman NB; Haigis MC Sirt4 Coordinates the Balance between Lipid Synthesis and Catabolism by Repressing Malonyl Coa Decarboxylase. Mol. Cell 2013, 50, 686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Nakagawa T; Lomb DJ; Haigis MC; Guarente L Sirt5 Deacetylates Carbamoyl Phosphate Synthetase 1 and Regulates the Urea Cycle. Cell 2009, 137, 560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).North BJ; Marshall BL; Borra MT; Denu JM; Verdin E The Human Sir2 Ortholog, Sirt2, Is an Nad+-Dependent Tubulin Deacetylase. Mol. Cell 2003, 11, 437–44. [DOI] [PubMed] [Google Scholar]
- (162).Liszt G; Ford E; Kurtev M; Guarente L Mouse Sir2 Homolog Sirt6 Is a Nuclear Adp-Ribosyltransferase. J. Biol. Chem 2005, 280, 21313–20. [DOI] [PubMed] [Google Scholar]
- (163).Ford E; Voit R; Liszt G; Magin C; Grummt I; Guarente L Mammalian Sir2 Homolog Sirt7 Is an Activator of Rna Polymerase I Transcription. Genes Dev. 2006, 20, 1075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Michishita E; Park JY; Burneskis JM; Barrett JC; Horikawa I Evolutionarily Conserved and Nonconserved Cellular Localizations and Functions of Human Sirt Proteins. Mol. Biol. Cell 2005, 16, 4623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Kim EJ; Kho JH; Kang MR; Um SJ Active Regulator of Sirt1 Cooperates with Sirt1 and Facilitates Suppression of P53 Activity. Mol. Cell 2007, 28, 277–90. [DOI] [PubMed] [Google Scholar]
- (166).Zhao W; Kruse JP; Tang Y; Jung SY; Qin J; Gu W Negative Regulation of the Deacetylase Sirt1 by Dbc1. Nature 2008, 451, 587–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Kim JE; Chen J; Lou Z Dbc1 Is a Negative Regulator of Sirt1. Nature 2008, 451, 583–6. [DOI] [PubMed] [Google Scholar]
- (168).Langley E; Pearson M; Faretta M; Bauer UM; Frye RA; Minucci S; Pelicci PG; Kouzarides T Human Sir2 Deacetylates P53 and Antagonizes Pml/P53-Induced Cellular Senescence. EMBO J. 2002, 21, 2383–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Vaziri H; Dessain SK; Ng Eaton E; Imai SI; Frye RA; Pandita TK; Guarente L; Weinberg RA Hsir2(Sirt1) Functions as an Nad-Dependent P53 Deacetylase. Cell 2001, 107, 149–59. [DOI] [PubMed] [Google Scholar]
- (170).Nasrin N; Kaushik VK; Fortier E; Wall D; Pearson KJ; de Cabo R; Bordone L Jnk1 Phosphorylates Sirt1 and Promotes Its Enzymatic Activity. PLoS One 2009, 4, e8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Zhao X; Allison D; Condon B; Zhang F; Gheyi T; Zhang A; Ashok S; Russell M; MacEwan I; Qian Y; Jamison JA; Luz JG The 2.5 a Crystal Structure of the Sirt1 Catalytic Domain Bound to Nicotinamide Adenine Dinucleotide (Nad+) and an Indole (Ex527 Analogue) Reveals a Novel Mechanism of Histone Deacetylase Inhibition. J. Med. Chem 2013, 56, 963–9. [DOI] [PubMed] [Google Scholar]
- (172).Davenport AM; Huber FM; Hoelz A Structural and Functional Analysis of Human Sirt1. J. Mol. Biol 2014, 426, 526–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Dai H; Case AW; Riera TV; Considine T; Lee JE; Hamuro Y; Zhao H; Jiang Y; Sweitzer SM; Pietrak B; Schwartz B; Blum CA; Disch JS; Caldwell R; Szczepankiewicz B; Oalmann C; Yee Ng P; White BH; Casaubon R; Narayan R; Koppetsch K; Bourbonais F; Wu B; Wang J; Qian D; Jiang F; Mao C; Wang M; Hu E; Wu JC; Perni RB; Vlasuk GP; Ellis JL Crystallographic Structure of a Small Molecule Sirt1 Activator-Enzyme Complex. Nat. Commun 2015, 6, 7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Cao D; Wang M; Qiu X; Liu D; Jiang H; Yang N; Xu RM Structural Basis for Allosteric, Substrate-Dependent Stimulation of Sirt1 Activity by Resveratrol. Genes Dev. 2015, 29, 1316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Gertz M; Fischer F; Nguyen GT; Lakshminarasimhan M; Schutkowski M; Weyand M; Steegborn C Ex-527 Inhibits Sirtuins by Exploiting Their Unique Nad+-Dependent Deacetylation Mechanism. Proc. Natl. Acad. Sci. U. S. A 2013, 110, E2772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Rack JG; VanLinden MR; Lutter T; Aasland R; Ziegler M Constitutive Nuclear Localization of an Alternatively Spliced Sirtuin-2 Isoform. J. Mol. Biol 2014, 426, 1677–91. [DOI] [PubMed] [Google Scholar]
- (177).Finnin MS; Donigian JR; Pavletich NP Structure of the Histone Deacetylase Sirt2. Nat. Struct. Biol 2001, 8, 621–5. [DOI] [PubMed] [Google Scholar]
- (178).Moniot S; Schutkowski M; Steegborn C Crystal Structure Analysis of Human Sirt2 and Its Adp-Ribose Complex. J. Struct. Biol 2013, 182, 136–43. [DOI] [PubMed] [Google Scholar]
- (179).Feldman JL; Dittenhafer-Reed KE; Kudo N; Thelen JN; Ito A; Yoshida M; Denu JM Kinetic and Structural Basis for Acyl-Group Selectivity and Nad(+) Dependence in Sirtuin-Catalyzed Deacylation. Biochemistry 2015, 54, 3037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Rumpf T; Gerhardt S; Einsle O; Jung M Seeding for Sirtuins: Microseed Matrix Seeding to Obtain Crystals of Human Sirt3 and Sirt2 Suitable for Soaking. Acta Crystallogr., Sect. F: Struct. Biol. Commun 2015, 71, 1498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Knyphausen P; de Boor S; Kuhlmann N; Scislowski L; Extra A; Baldus L; Schacherl M; Baumann U; Neundorf I; Lammers M Insights into Lysine Deacetylation of Natively Folded Substrate Proteins by Sirtuins. J. Biol. Chem 2016, 291, 14677–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Schwer B; North BJ; Frye RA; Ott M; Verdin E The Human Silent Information Regulator (Sir)2 Homologue Hsirt3 Is a Mitochondrial Nicotinamide Adenine Dinucleotide-Dependent Deacetylase. J. Cell Biol 2002, 158, 647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Jin L; Wei W; Jiang Y; Peng H; Cai J; Mao C; Dai H; Choy W; Bemis JE; Jirousek MR; Milne JC; Westphal CH; Perni RB Crystal Structures of Human Sirt3 Displaying Substrate-Induced Conformational Changes. J. Biol. Chem 2009, 284, 24394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Gai W; Li H; Jiang H; Long Y; Liu D Crystal Structures of Sirt3 Reveal That the Alpha2-Alpha3 Loop and Alpha3-Helix Affect the Interaction with Long-Chain Acyl Lysine. FEBS Lett. 2016, 590, 3019–28. [DOI] [PubMed] [Google Scholar]
- (185).Disch JS; Evindar G; Chiu CH; Blum CA; Dai H; Jin L; Schuman E; Lind KE; Belyanskaya SL; Deng J; Coppo F; Aquilani L; Graybill TL; Cuozzo JW; Lavu S; Mao C; Vlasuk GP; Perni RB Discovery of Thieno[3,2-D]Pyrimidine-6-Carboxamides as Potent Inhibitors of Sirt1, Sirt2, and Sirt3. J. Med. Chem 2013, 56, 3666–79. [DOI] [PubMed] [Google Scholar]
- (186).Szczepankiewicz BG; Dai H; Koppetsch KJ; Qian D; Jiang F; Mao C; Perni RB Synthesis of Carba-Nad and the Structures of Its Ternary Complexes with Sirt3 and Sirt5. J. Org. Chem 2012, 77, 7319–29. [DOI] [PubMed] [Google Scholar]
- (187).Gertz M; Nguyen GT; Fischer F; Suenkel B; Schlicker C; Franzel B; Tomaschewski J; Aladini F; Becker C; Wolters D; Steegborn C A Molecular Mechanism for Direct Sirtuin Activation by Resveratrol. PLoS One 2012, 7, e49761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Du J; Zhou Y; Su X; Yu JJ; Khan S; Jiang H; Kim J; Woo J; Kim JH; Choi BH; He B; Chen W; Zhang S; Cerione RA; Auwerx J; Hao Q; Lin H Sirt5 Is a Nad-Dependent Protein Lysine Demalonylase and Desuccinylase. Science 2011, 334, 806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Matsushita N; Yonashiro R; Ogata Y; Sugiura A; Nagashima S; Fukuda T; Inatome R; Yanagi S Distinct Regulation of Mitochondrial Localization and Stability of Two Human Sirt5 Isoforms. Genes Cells 2011, 16, 190–202. [DOI] [PubMed] [Google Scholar]
- (190).Schuetz A; Min J; Antoshenko T; Wang CL; Allali-Hassani A; Dong A; Loppnau P; Vedadi M; Bochkarev A; Sternglanz R; Plotnikov AN Structural Basis of Inhibition of the Human Nad+-Dependent Deacetylase Sirt5 by Suramin. Structure 2007, 15, 377–89. [DOI] [PubMed] [Google Scholar]
- (191).Park J; Chen Y; Tishkoff DX; Peng C; Tan M; Dai L; Xie Z; Zhang Y; Zwaans BM; Skinner ME; Lombard DB; Zhao Y Sirt5-Mediated Lysine Desuccinylation Impacts Diverse Metabolic Pathways. Mol. Cell 2013, 50, 919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Michishita E; McCord RA; Berber E; Kioi M; Padilla-Nash H; Damian M; Cheung P; Kusumoto R; Kawahara TL; Barrett JC; Chang HY; Bohr VA; Ried T; Gozani O; Chua KF Sirt6 Is a Histone H3 Lysine 9 Deacetylase That Modulates Telomeric Chromatin. Nature 2008, 452, 492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Kawahara TL; Michishita E; Adler AS; Damian M; Berber E; Lin M; McCord RA; Ongaigui KC; Boxer LD; Chang HY; Chua KF Sirt6 Links Histone H3 Lysine 9 Deacetylation to Nf-Kappab-Dependent Gene Expression and Organismal Life Span. Cell 2009, 136, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Pan PW; Feldman JL; Devries MK; Dong A; Edwards AM; Denu JM Structure and Biochemical Functions of Sirt6. J. Biol. Chem 2011, 286, 14575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).You W; Rotili D; Li TM; Kambach C; Meleshin M; Schutkowski M; Chua KF; Mai A; Steegborn C Structural Basis of Sirtuin 6 Activation by Synthetic Small Molecules. Angew. Chem., Int. Ed 2017, 56, 1007–1011. [DOI] [PubMed] [Google Scholar]
- (196).Kiran S; Chatterjee N; Singh S; Kaul SC; Wadhwa R; Ramakrishna G Intracellular Distribution of Human Sirt7 and Mapping of the Nuclear/Nucleolar Localization Signal. FEBS J. 2013, 280, 3451–66. [DOI] [PubMed] [Google Scholar]
- (197).Singh S; Kumar PU; Thakur S; Kiran S; Sen B; Sharma S; Rao VV; Poongothai AR; Ramakrishna G Expression/Localization Patterns of Sirtuins (Sirt1, Sirt2, and Sirt7) During Progression of Cervical Cancer and Effects of Sirtuin Inhibitors on Growth of Cervical Cancer Cells. Tumor Biol. 2015, 36, 6159–71. [DOI] [PubMed] [Google Scholar]
- (198).Sauve AA; Schramm VL Sir2 Regulation by Nicotinamide Results from Switching between Base Exchange and Deacetylation Chemistry. Biochemistry 2003, 42, 9249–56. [DOI] [PubMed] [Google Scholar]
- (199).Haigis MC; Mostoslavsky R; Haigis KM; Fahie K; Christodoulou DC; Murphy AJ; Valenzuela DM; Yancopoulos GD; Karow M; Blander G; Wolberger C; Prolla TA; Weindruch R; Alt FW; Guarente L Sirt4 Inhibits Glutamate Dehydrogenase and Opposes the Effects of Calorie Restriction in Pancreatic Beta Cells. Cell 2006, 126, 941–54. [DOI] [PubMed] [Google Scholar]
- (200).Rardin MJ; He W; Nishida Y; Newman JC; Carrico C; Danielson SR; Guo A; Gut P; Sahu AK; Li B; Uppala R; Fitch M; Riiff T; Zhu L; Zhou J; Mulhern D; Stevens RD; Ilkayeva OR; Newgard CB; Jacobson MP; Hellerstein M; Goetzman ES; Gibson BW; Verdin E Sirt5 Regulates the Mitochondrial Lysine Succinylome and Metabolic Networks. Cell Metab. 2013, 18, 920–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Hirsch BM; Zheng W Sirtuin Mechanism and Inhibition: Explored with N(Epsilon)-Acetyl-Lysine Analogs. Mol. BioSyst 2011, 7, 16–28. [DOI] [PubMed] [Google Scholar]
- (202).Tamkun JW; Deuring R; Scott MP; Kissinger M; Pattatucci AM; Kaufman TC; Kennison JA Brahma: A Regulator of Drosophila Homeotic Genes Structurally Related to the Yeast Transcriptional Activator Snf2/Swi2. Cell 1992, 68, 561–72. [DOI] [PubMed] [Google Scholar]
- (203).Haynes SR; Dollard C; Winston F; Beck S; Trowsdale J; Dawid IB The Bromodomain: A Conserved Sequence Found in Human, Drosophila and Yeast Proteins. Nucleic Acids Res. 1992, 20, 2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (204).Fujisawa T; Filippakopoulos P Functions of Bromodomain-Containing Proteins and Their Roles in Homeostasis and Cancer. Nat. Rev. Mol. Cell Biol 2017, 18, 246. [DOI] [PubMed] [Google Scholar]
- (205).Sanchez R; Zhou MM The Role of Human Bromodomains in Chromatin Biology and Gene Transcription. Curr. Opin Drug Discov Devel 2009, 12, 659–65. [PMC free article] [PubMed] [Google Scholar]
- (206).Delmore JE; Issa GC; Lemieux ME; Rahl PB; Shi J; Jacobs HM; Kastritis E; Gilpatrick T; Paranal RM; Qi J; Chesi M; Schinzel AC; McKeown MR; Heffernan TP; Vakoc CR; Bergsagel PL; Ghobrial IM; Richardson PG; Young RA; Hahn WC; Anderson KC; Kung AL; Bradner JE; Mitsiades CS Bet Bromodomain Inhibition as a Therapeutic Strategy to Target C-Myc. Cell 2011, 146, 904–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Ott CJ; Kopp N; Bird L; Paranal RM; Qi J; Bowman T; Rodig SJ; Kung AL; Bradner JE; Weinstock DM Bet Bromodomain Inhibition Targets Both C-Myc and Il7r in High-Risk Acute Lymphoblastic Leukemia. Blood 2012, 120, 2843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Mertz JA; Conery AR; Bryant BM; Sandy P; Balasubramanian S; Mele DA; Bergeron L; Sims RJ 3rd Targeting Myc Dependence in Cancer by Inhibiting Bet Bromodomains. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 16669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Syntichaki P; Topalidou I; Thireos G The Gcn5 Bromodomain Co-Ordinates Nucleosome Remodelling. Nature 2000, 404, 414–7. [DOI] [PubMed] [Google Scholar]
- (210).Cieniewicz AM; Moreland L; Ringel AE; Mackintosh SG; Raman A; Gilbert TM; Wolberger C; Tackett AJ; Taverna SD The Bromodomain of Gcn5 Regulates Site Specificity of Lysine Acetylation on Histone H3. Mol. Cell. Proteomics 2014, 13, 2896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Li H; Fischle W; Wang W; Duncan EM; Liang L; Murakami-Ishibe S; Allis CD; Patel DJ Structural Basis for Lower Lysine Methylation State-Specific Readout by Mbt Repeats of L3mbtl1 and an Engineered Phd Finger. Mol. Cell 2007, 28, 677–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Ruthenburg AJ; Li H; Milne TA; Dewell S; McGinty RK; Yuen M; Ueberheide B; Dou Y; Muir TW; Patel DJ; Allis CD Recognition of a Mononucleosomal Histone Modification Pattern by Bptf Via Multivalent Interactions. Cell 2011, 145, 692–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).De Semir D; Nosrati M; Bezrookove V; Dar AA; Federman S; Bienvenu G; Venna S; Rangel J; Climent J; Meyer Tamguney TM; Thummala S; Tong S; Leong SP; Haqq C; Billings P; Miller JR 3rd; Sagebiel RW; Debs R; Kashani-Sabet M Pleckstrin Homology Domain-Interacting Protein (Phip) as a Marker and Mediator of Melanoma Metastasis. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 7067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).Cox OB; Collins P; Monteiro O; Talon R; Bradley A; Fedorov O; Amin J; Marsden B; Spencer J; von Delft F; Brennan P A Poised Fragment Library Enables Rapid Synthetic Expansion Yielding the First Reported Inhibitors of Phip(2), an Atypical Bromodomain. Chem. Sci 2016, 7, 2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Lubula MY; Eckenroth BE; Carlson S; Poplawski A; Chruszcz M; Glass KC Structural Insights into Recognition of Acetylated Histone Ligands by the Brpf1 Bromodomain. FEBS Lett. 2014, 588, 3844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (216).Bamborough P; Barnett HA; Becher I; Bird MJ; Chung CW; Craggs PD; Demont EH; Diallo H; Fallon DJ; Gordon LJ; Grandi P; Hobbs CI; Hooper-Greenhill E; Jones EJ; Law RP; Le Gall A; Lugo D; Michon AM; Mitchell DJ; Prinjha RK; Sheppard RJ; Watson AJ; Watson RJ Gsk6853, a Chemical Probe for Inhibition of the Brpf1 Bromodomain. ACS Med. Chem. Lett 2016, 7, 552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (217).Zhu J; Caflisch A Twenty Crystal Structures of Bromodomain and Phd Finger Containing Protein 1 (Brpf1)/Ligand Complexes Reveal Conserved Binding Motifs and Rare Interactions. J. Med. Chem 2016, 59, 5555–61. [DOI] [PubMed] [Google Scholar]
- (218).Demont EH; Bamborough P; Chung CW; Craggs PD; Fallon D; Gordon LJ; Grandi P; Hobbs CI; Hussain J; Jones EJ; Le Gall A; Michon AM; Mitchell DJ; Prinjha RK; Roberts AD; Sheppard RJ; Watson RJ 1,3-Dimethyl Benzimidazolones Are Potent, Selective Inhibitors of the Brpf1 Bromodomain. ACS Med. Chem. Lett 2014, 5, 1190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Unzue A; Zhao H; Lolli G; Dong J; Zhu J; Zechner M; Dolbois A; Caflisch A; Nevado C The “Gatekeeper” Residue Influences the Mode of Binding of Acetyl Indoles to Bromodomains. J. Med. Chem 2016, 59, 3087–97. [DOI] [PubMed] [Google Scholar]
- (220).Tsai WW; Wang Z; Yiu TT; Akdemir KC; Xia W; Winter S; Tsai CY; Shi X; Schwarzer D; Plunkett W; Aronow B; Gozani O; Fischle W; Hung MC; Patel DJ; Barton MC Trim24 Links a Non-Canonical Histone Signature to Breast Cancer. Nature 2010, 468, 927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Palmer WS; Poncet-Montange G; Liu G; Petrocchi A; Reyna N; Subramanian G; Theroff J; Yau A; Kost-Alimova M; Bardenhagen JP; Leo E; Shepard HE; Tieu TN; Shi X; Zhan Y; Zhao S; Barton MC; Draetta G; Toniatti C; Jones P; Geck Do M; Andersen JN Structure-Guided Design of Iacs-9571, a Selective High-Affinity Dual Trim24-Brpf1 Bromodomain Inhibitor. J. Med. Chem 2016, 59, 1440–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Seeler JS; Marchio A; Losson R; Desterro JM; Hay RT; Chambon P; Dejean A Common Properties of Nuclear Body Protein Sp100 and Tif1alpha Chromatin Factor: Role of Sumo Modification. Mol. Cell. Biol 2001, 21, 3314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (223).Milovic-Holm K; Krieghoff E; Jensen K; Will H; Hofmann TG Flash Links the Cd95 Signaling Pathway to the Cell Nucleus and Nuclear Bodies. EMBO J. 2007, 26, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Moosmann P; Georgiev O; Le Douarin B; Bourquin JP; Schaffner W Transcriptional Repression by Ring Finger Protein Tif1 Beta That Interacts with the Krab Repressor Domain of Kox1. Nucleic Acids Res. 1996, 24, 4859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (225).Ryan RF; Schultz DC; Ayyanathan K; Singh PB; Friedman JR; Fredericks WJ; Rauscher FJ 3rd Kap-1 Corepressor Protein Interacts and Colocalizes with Heterochromatic and Euchromatic Hp1 Proteins: A Potential Role for Kruppel-Associated Box-Zinc Finger Proteins in Heterochromatin-Mediated Gene Silencing. Mol. Cell. Biol 1999, 19, 4366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (226).Iyengar S; Ivanov AV; Jin VX; Rauscher FJ 3rd; Farnham PJ Functional Analysis of Kap1 Genomic Recruitment. Mol. Cell. Biol 2011, 31, 1833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (227).Wang W; Cai J; Wu Y; Hu L; Chen Z; Hu J; Chen Z; Li W; Guo M; Huang Z Novel Activity of Krab Domain That Functions to Reinforce Nuclear Localization of Krab-Containing Zinc Finger Proteins by Interacting with Kap1. Cell. Mol. Life Sci 2013, 70, 3947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (228).Zeng L; Yap KL; Ivanov AV; Wang X; Mujtaba S; Plotnikova O; Rauscher FJ 3rd; Zhou MM Structural Insights into Human Kap1 Phd Finger-Bromodomain and Its Role in Gene Silencing. Nat. Struct. Mol. Biol 2008, 15, 626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (229).Harter MR; Liu CD; Shen CL; Gonzalez-Hurtado E; Zhang ZM; Xu M; Martinez E; Peng CW; Song J Bs69/Zmynd11 C-Terminal Domains Bind and Inhibit Ebna2. PLoS Pathog. 2016, 12, e1005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (230).Wang J; Qin S; Li F; Li S; Zhang W; Peng J; Zhang Z; Gong Q; Wu J; Shi Y Crystal Structure of Human Bs69 Bromo-Znf-Pwwp Reveals Its Role in H3k36me3 Nucleosome Binding. Cell Res. 2014, 24, 890–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (231).Steilmann C; Cavalcanti MC; Bartkuhn M; Pons-Kuhnemann J; Schuppe HC; Weidner W; Steger K; Paradowska A The Interaction of Modified Histones with the Bromodomain Testis-Specific (Brdt) Gene and Its Mrna Level in Sperm of Fertile Donors and Subfertile Men. Reproduction 2010, 140, 435–43. [DOI] [PubMed] [Google Scholar]
- (232).Fukazawa H; Masumi A The Conserved 12-Amino Acid Stretch in the Inter-Bromodomain Region of Bet Family Proteins Functions as a Nuclear Localization Signal. Biol. Pharm. Bull 2012, 35, 2064–8. [DOI] [PubMed] [Google Scholar]
- (233).Matzuk MM; McKeown MR; Filippakopoulos P; Li Q; Ma L; Agno JE; Lemieux ME; Picaud S; Yu RN; Qi J; Knapp S; Bradner JE Small-Molecule Inhibition of Brdt for Male Contraception. Cell 2012, 150, 673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (234).Martin MP; Olesen SH; Georg GI; Schonbrunn E Cyclin-Dependent Kinase Inhibitor Dinaciclib Interacts with the Acetyl-Lysine Recognition Site of Bromodomains. ACS Chem. Biol 2013, 8, 2360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (235).Moriniere J; Rousseaux S; Steuerwald U; Soler-Lopez M; Curtet S; Vitte AL; Govin J; Gaucher J; Sadoul K; Hart DJ; Krijgsveld J; Khochbin S; Muller CW; Petosa C Cooperative Binding of Two Acetylation Marks on a Histone Tail by a Single Bromodomain. Nature 2009, 461, 664–8. [DOI] [PubMed] [Google Scholar]
- (236).Tanaka M; Roberts JM; Seo HS; Souza A; Paulk J; Scott TG; DeAngelo SL; Dhe-Paganon S; Bradner JE Design and Characterization of Bivalent Bet Inhibitors. Nat. Chem. Biol 2016, 12, 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (237).Picaud S; Leonards K; Lambert JP; Dovey O; Wells C; Fedorov O; Monteiro O; Fujisawa T; Wang CY; Lingard H; Tallant C; Nikbin N; Guetzoyan L; Ingham R; Ley SV; Brennan P; Muller S; Samsonova A; Gingras AC; Schwaller J; Vassiliou G; Knapp S; Filippakopoulos P Promiscuous Targeting of Bromodomains by Bromosporine Identifies Bet Proteins as Master Regulators of Primary Transcription Response in Leukemia. Sci. Adv 2016, 2, e1600760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (238).Albrecht BK; Gehling VS; Hewitt MC; Vaswani RG; Cote A; Leblanc Y; Nasveschuk CG; Bellon S; Bergeron L; Campbell R; Cantone N; Cooper MR; Cummings RT; Jayaram H; Joshi S; Mertz JA; Neiss A; Normant E; O’Meara M; Pardo E; Poy F; Sandy P; Supko J; Sims RJ 3rd; Harmange JC; Taylor AM; Audia JE Identification of a Benzoisoxazoloazepine Inhibitor (Cpi-0610) of the Bromodomain and Extra-Terminal (Bet) Family as a Candidate for Human Clinical Trials. J. Med. Chem 2016, 59, 1330–9. [DOI] [PubMed] [Google Scholar]
- (239).Ran X; Zhao Y; Liu L; Bai L; Yang CY; Zhou B; Meagher JL; Chinnaswamy K; Stuckey JA; Wang S Structure-Based Design of Gamma-Carboline Analogues as Potent and Specific Bet Bromodomain Inhibitors. J. Med. Chem 2015, 58, 4927–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (240).Nilsson LM; Green LC; Muralidharan SV; Demir D; Welin M; Bhadury J; Logan DT; Walse B; Nilsson JA Cancer Differentiating Agent Hexamethylene Bisacetamide Inhibits Bet Bromodomain Proteins. Cancer Res. 2016, 76, 2376–83. [DOI] [PubMed] [Google Scholar]
- (241).Tripathi S; Mathur S; Deshmukh P; Manjula R; Padmanabhan B A Novel Phenanthridionone Based Scaffold as a Potential Inhibitor of the Brd2 Bromodomain: Crystal Structure of the Complex. PLoS One 2016, 11, e0156344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Baud MG; Lin-Shiao E; Zengerle M; Tallant C; Ciulli A New Synthetic Routes to Triazolo-Benzodiazepine Analogues: Expanding the Scope of the Bump-and-Hole Approach for Selective Bromo and Extra-Terminal (Bet) Bromodomain Inhibition. J. Med. Chem 2016, 59, 1492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (243).Bamborough P; Diallo H; Goodacre JD; Gordon L; Lewis A; Seal JT; Wilson DM; Woodrow MD; Chung CW Fragment-Based Discovery of Bromodomain Inhibitors Part 2: Optimization of Phenylisoxazole Sulfonamides. J. Med. Chem 2012, 55, 587–96. [DOI] [PubMed] [Google Scholar]
- (244).Gosmini R; Nguyen VL; Toum J; Simon C; Brusq JM; Krysa G; Mirguet O; Riou-Eymard AM; Boursier EV; Trottet L; Bamborough P; Clark H; Chung CW; Cutler L; Demont EH; Kaur R; Lewis AJ; Schilling MB; Soden PE; Taylor S; Walker AL; Walker MD; Prinjha RK; Nicodeme E The Discovery of I-Bet726 (Gsk1324726a), a Potent Tetrahydroquinoline Apoa1 up-Regulator and Selective Bet Bromodomain Inhibitor. J. Med. Chem 2014, 57, 8111–31. [DOI] [PubMed] [Google Scholar]
- (245).Jones MH; Hamana N; Nezu J; Shimane M A Novel Family of Bromodomain Genes. Genomics 2000, 63, 40–5. [DOI] [PubMed] [Google Scholar]
- (246).Poot RA; Dellaire G; Hulsmann BB; Grimaldi MA; Corona DF; Becker PB; Bickmore WA; Varga-Weisz PD Huchrac, a Human Iswi Chromatin Remodelling Complex Contains Hacf1 and Two Novel Histone-Fold Proteins. EMBO J. 2000, 19, 3377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (247).Couture JP; Nolet G; Beaulieu E; Blouin R; Gevry N The P400/Brd8 Chromatin Remodeling Complex Promotes Adipogenesis by Incorporating Histone Variant H2a.Z at Ppargamma Target Genes. Endocrinology 2012, 153, 5796–808. [DOI] [PubMed] [Google Scholar]
- (248).Bozhenok L; Wade PA; Varga-Weisz P Wstf-Iswi Chromatin Remodeling Complex Targets Heterochromatic Replication Foci. EMBO J. 2002, 21, 2231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (249).Poot RA; Bozhenok L; van den Berg DL; Steffensen S; Ferreira F; Grimaldi M; Gilbert N; Ferreira J; Varga-Weisz PD The Williams Syndrome Transcription Factor Interacts with Pcna to Target Chromatin Remodelling by Iswi to Replication Foci. Nat. Cell Biol 2004, 6, 1236–44. [DOI] [PubMed] [Google Scholar]
- (250).Theodoulou NH; Bamborough P; Bannister AJ; Becher I; Bit RA; Che KH; Chung CW; Dittmann A; Drewes G; Drewry DH; Gordon L; Grandi P; Leveridge M; Lindon M; Michon AM; Molnar J; Robson SC; Tomkinson NC; Kouzarides T; Prinjha RK; Humphreys PG Discovery of I-Brd9, a Selective Cell Active Chemical Probe for Bromodomain Containing Protein 9 Inhibition. J. Med. Chem 2016, 59, 1425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (251).Wang N; Li F; Bao H; Li J; Wu J; Ruan K Nmr Fragment Screening Hit Induces Plasticity of Brd7/9 Bromodomains. ChemBioChem 2016, 17, 1456–63. [DOI] [PubMed] [Google Scholar]
- (252).Martin LJ; Koegl M; Bader G; Cockcroft XL; Fedorov O; Fiegen D; Gerstberger T; Hofmann MH; Hohmann AF; Kessler D; Knapp S; Knesl P; Kornigg S; Muller S; Nar H; Rogers C; Rumpel K; Schaaf O; Steurer S; Tallant C; Vakoc CR; Zeeb M; Zoephel A; Pearson M; Boehmelt G; McConnell D Structure-Based Design of an in Vivo Active Selective Brd9 Inhibitor. J. Med. Chem 2016, 59, 4462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Sun H; Liu J; Zhang J; Shen W; Huang H; Xu C; Dai H; Wu J; Shi Y Solution Structure of Brd7 Bromodomain and Its Interaction with Acetylated Peptides from Histone H3 and H4. Biochem. Biophys. Res. Commun 2007, 358, 435–41. [DOI] [PubMed] [Google Scholar]
- (254).Yan K; You L; Degerny C; Ghorbani M; Liu X; Chen L; Li L; Miao D; Yang XJ The Chromatin Regulator Brpf3 Preferentially Activates the Hbo1 Acetyltransferase but Is Dispensable for Mouse Development and Survival. J. Biol. Chem 2016, 291, 2647–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (255).Feng Y; Vlassis A; Roques C; Lalonde ME; Gonzalez-Aguilera C; Lambert JP; Lee SB; Zhao X; Alabert C; Johansen JV; Paquet E; Yang XJ; Gingras AC; Cote J; Groth A Brpf3-Hbo1 Regulates Replication Origin Activation and Histone H3k14 Acetylation. EMBO J. 2016, 35, 176–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (256).Qin S; Jin L; Zhang J; Liu L; Ji P; Wu M; Wu J; Shi Y Recognition of Unmodified Histone H3 by the First Phd Finger of Bromodomain-Phd Finger Protein 2 Provides Insights into the Regulation of Histone Acetyltransferases Monocytic Leukemic Zinc-Finger Protein (Moz) and Moz-Related Factor (Morf). J. Biol. Chem 2011, 286, 36944–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (257).Alabert C; Bukowski-Wills JC; Lee SB; Kustatscher G; Nakamura K; de Lima Alves F; Menard P; Mejlvang J; Rappsilber J; Groth A Nascent Chromatin Capture Proteomics Determines Chromatin Dynamics During DNA Replication and Identifies Unknown Fork Components. Nat. Cell Biol 2014, 16, 281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (258).Leachman NT; Brellier F; Ferralli J; Chiquet-Ehrismann R; Tucker RP Atad2b Is a Phylogenetically Conserved Nuclear Protein Expressed During Neuronal Differentiation and Tumorigenesis. Dev. Growth Differ. 2010, 52, 747–55. [DOI] [PubMed] [Google Scholar]
- (259).He W; Dorn DC; Erdjument-Bromage H; Tempst P; Moore MA; Massague J Hematopoiesis Controlled by Distinct Tif1gamma and Smad4 Branches of the Tgfbeta Pathway. Cell 2006, 125, 929–41. [DOI] [PubMed] [Google Scholar]
- (260).Dupont S; Mamidi A; Cordenonsi M; Montagner M; Zacchigna L; Adorno M; Martello G; Stinchfield MJ; Soligo S; Morsut L; Inui M; Moro S; Modena N; Argenton F; Newfeld SJ; Piccolo S Fam/Usp9x, a Deubiquitinating Enzyme Essential for Tgfbeta Signaling, Controls Smad4Monoubiquitination. Cell 2009, 136, 123–35. [DOI] [PubMed] [Google Scholar]
- (261).Shi X; Mihaylova VT; Kuruvilla L; Chen F; Viviano S; Baldassarre M; Sperandio D; Martinez R; Yue P; Bates JG; Breckenridge DG; Schlessinger J; Turk BE; Calderwood DA Loss of Trim33 Causes Resistance to Bet Bromodomain Inhibitors through Myc- and Tgf-Beta-Dependent Mechanisms. Proc. Natl. Acad. Sci. U. S. A 2016, 113, E4558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).Gallouet AS; Ferri F; Petit V; Parcelier A; Lewandowski D; Gault N; Barroca V; Le Gras S; Soler E; Grosveld F; Davidson I; Romeo PH Macrophage Production and Activation Are Dependent on Trim33. Oncotarget 2017, 8, 5111–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (263).Bloch DB; Nakajima A; Gulick T; Chiche JD; Orth D; de La Monte SM; Bloch KD Sp110 Localizes to the Pml-Sp100 Nuclear Body and May Function as a Nuclear Hormone Receptor Transcriptional Coactivator. Mol. Cell. Biol 2000, 20, 6138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (264).Roscioli T; Cliffe ST; Bloch DB; Bell CG; Mullan G; Taylor PJ; Sarris M; Wang J; Donald JA; Kirk EP; Ziegler JB; Salzer U; McDonald GB; Wong M; Lindeman R; Buckley MF Mutations in the Gene Encoding the Pml Nuclear Body Protein Sp110 Are Associated with Immunodeficiency and Hepatic Veno-Occlusive Disease. Nat. Genet 2006, 38, 620–2. [DOI] [PubMed] [Google Scholar]
- (265).Dent AL; Yewdell J; Puvion-Dutilleul F; Koken MH; de The H; Staudt LM Lysp100-Associated Nuclear Domains (Lands): Description of a New Class of Subnuclear Structures and Their Relationship to Pml Nuclear Bodies. Blood 1996, 88, 1423–6. [PubMed] [Google Scholar]
- (266).Bloch DB; de la Monte SM; Guigaouri P; Filippov A; Bloch KD Identification and Characterization of a Leukocyte-Specific Component of the Nuclear Body. J. Biol. Chem 1996, 271, 29198–204. [DOI] [PubMed] [Google Scholar]
- (267).Saare M; Hamarik U; Venta R; Panarina M; Zucchelli C; Pihlap M; Remm A; Kisand K; Toots U; Moll K; Salupere R; Musco G; Uibo R; Peterson P Sp140l, an Evolutionarily Recent Member of the Sp100 Family, Is an Autoantigen in Primary Biliary Cirrhosis. J. Immunol. Res 2015, 2015, 526518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (268).Ferguson FM; Fedorov O; Chaikuad A; Philpott M; Muniz JR; Felletar I; von Delft F; Heightman T; Knapp S; Abell C; Ciulli A Targeting Low-Druggability Bromodomains: Fragment Based Screening and Inhibitor Design against the Baz2b Bromodomain. J. Med. Chem 2013, 56, 10183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (269).Drouin L; McGrath S; Vidler LR; Chaikuad A; Monteiro O; Tallant C; Philpott M; Rogers C; Fedorov O; Liu M; Akhtar W; Hayes A; Raynaud F; Muller S; Knapp S; Hoelder S Structure Enabled Design of Baz2-Icr, a Chemical Probe Targeting the Bromodomains of Baz2a and Baz2b. J. Med. Chem 2015, 58, 2553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (270).Tallant C; Valentini E; Fedorov O; Overvoorde L; Ferguson FM; Filippakopoulos P; Svergun DI; Knapp S; Ciulli A Molecular Basis of Histone Tail Recognition by Human Tip5 Phd Finger and Bromodomain of the Chromatin Remodeling Complex Norc. Structure 2015, 23, 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (271).Gu L; Frommel SC; Oakes CC; Simon R; Grupp K; Gerig CY; Bar D; Robinson MD; Baer C; Weiss M; Gu Z; Schapira M; Kuner R; Sultmann H; Provenzano M; Cancer, I. P. o. E. O. P.; Yaspo ML; Brors B; Korbel J; Schlomm T; Sauter G; Eils R; Plass C; Santoro R Baz2a (Tip5) Is Involved in Epigenetic Alterations in Prostate Cancer and Its Overexpression Predicts Disease Recurrence. Nat. Genet 2015, 47, 22–30. [DOI] [PubMed] [Google Scholar]
- (272).Hsieh JJ; Ernst P; Erdjument-Bromage H; Tempst P; Korsmeyer SJ Proteolytic Cleavage of Mll Generates a Complex of N- and C-Terminal Fragments That Confers Protein Stability and Subnuclear Localization. Mol. Cell. Biol 2003, 23, 186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (273).Wang Z; Song J; Milne TA; Wang GG; Li H; Allis CD; Patel DJ Pro Isomerization in Mll1 Phd3-Bromo Cassette Connects H3k4me Readout to Cyp33 and Hdac-Mediated Repression. Cell 2010, 141, 1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (274).Fair K; Anderson M; Bulanova E; Mi H; Tropschug M; Diaz MO Protein Interactions of the Mll Phd Fingers Modulate Mll Target Gene Regulation in Human Cells. Mol. Cell. Biol 2001, 21, 3589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (275).Gue M; Sun JS; Boudier T Simultaneous Localization of Mll, Af4 and Enl Genes in Interphase Nuclei by 3d-Fish: Mll Translocation Revisited. BMC Cancer 2006, 6, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Jacobson RH; Ladurner AG; King DS; Tjian R Structure and Function of a Human Tafii250 Double Bromodomain Module. Science 2000, 288, 1422–5. [DOI] [PubMed] [Google Scholar]
- (277).Akai Y; Adachi N; Hayashi Y; Eitoku M; Sano N; Natsume R; Kudo N; Tanokura M; Senda T; Horikoshi M Structure of the Histone Chaperone Cia/Asf1-Double Bromodomain Complex Linking Histone Modifications and Site-Specific Histone Eviction. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 8153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (278).Li N; Li Y; Lv J; Zheng X; Wen H; Shen H; Zhu G; Chen TY; Dhar SS; Kan PY; Wang Z; Shiekhattar R; Shi X; Lan F; Chen K; Li W; Li H; Lee MG Zmynd8 Reads the Dual Histone Mark H3k4me1-H3k14ac to Antagonize the Expression of Metastasis-Linked Genes. Mol. Cell 2016, 63, 470–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (279).Savitsky P; Krojer T; Fujisawa T; Lambert JP; Picaud S; Wang CY; Shanle EK; Krajewski K; Friedrichsen H; Kanapin A; Goding C; Schapira M; Samsonova A; Strahl BD; Gingras AC; Filippakopoulos P Multivalent Histone and DNA Engagement by a Phd/Brd/Pwwp Triple Reader Cassette Recruits Zmynd8 to K14ac-Rich Chromatin. Cell Rep 2016, 17, 2724–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (280).Gong F; Chiu LY; Cox B; Aymard F; Clouaire T; Leung JW; Cammarata M; Perez M; Agarwal P; Brodbelt JS; Legube G; Miller KM Screen Identifies Bromodomain Protein Zmynd8 in Chromatin Recognition of Transcription-Associated DNA Damage That Promotes Homologous Recombination. Genes Dev. 2015, 29, 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (281).Sutherell CL; Tallant C; Monteiro OP; Yapp C; Fuchs JE; Fedorov O; Siejka P; Muller S; Knapp S; Brenton JD; Brennan PE; Ley SV Identification and Development of 2,3-Dihydropyrrolo[1,2-a]Quinazolin-5(1h)-One Inhibitors Targeting Bromodomains within the Switch/Sucrose Nonfermenting Complex. J. Med. Chem 2016, 59, 5095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (282).Pawlowski R; Muhl SM; Sulser T; Krek W; Moch H; Schraml P Loss of Pbrm1 Expression Is Associated with Renal Cell Carcinoma Progression. Int. J. Cancer 2013, 132, E11–7. [DOI] [PubMed] [Google Scholar]
- (283).Sanchez-Tillo E; Lazaro A; Torrent R; Cuatrecasas M; Vaquero EC; Castells A; Engel P; Postigo A Zeb1 Represses E-Cadherin and Induces an Emt by Recruiting the Swi/Snf Chromatin-Remodeling Protein Brg1. Oncogene 2010, 29, 3490–500. [DOI] [PubMed] [Google Scholar]
- (284).Singh M; Popowicz GM; Krajewski M; Holak TA Structural Ramification for Acetyl-Lysine Recognition by the Bromodomain of Human Brg1 Protein, a Central Atpase of the Swi/Snf Remodeling Complex. ChemBioChem 2007, 8, 1308–16. [DOI] [PubMed] [Google Scholar]
- (285).Lolli G; Caflisch A High-Throughput Fragment Docking into the Baz2b Bromodomain: Efficient in Silico Screening for X-Ray Crystallography. ACS Chem. Biol 2016, 11, 800–7. [DOI] [PubMed] [Google Scholar]
- (286).Kowenz-Leutz E; Pless O; Dittmar G; Knoblich M; Leutz A Crosstalk between C/Ebpbeta Phosphorylation, Arginine Methylation, and Swi/Snf/Mediator Implies an Indexing Transcription Factor Code. EMBO J. 2010, 29, 1105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (287).Suh EJ; Kabir MH; Kang UB; Lee JW; Yu J; Noh DY; Lee C Comparative Profiling of Plasma Proteome from Breast Cancer Patients Reveals Thrombospondin-1 and Brwd3 as Serological Biomarkers. Exp. Mol. Med 2012, 44, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (288).Zou JX; Revenko AS; Li LB; Gemo AT; Chen HW Ancca, an Estrogen-Regulated Aaa+ Atpase Coactivator for Eralpha, Is Required for Coregulator Occupancy and Chromatin Modification. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 18067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (289).Gonzales PA; Pisitkun T; Hoffert JD; Tchapyjnikov D; Star RA; Kleta R; Wang NS; Knepper MA Large-Scale Proteomics and Phosphoproteomics of Urinary Exosomes. J. Am. Soc. Nephrol 2009, 20, 363–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (290).Chaikuad A; Petros AM; Fedorov O; Xu J; Knapp S Structure-Based Approaches Towards Identification of Fragments for the Low-Druggability Atad2 Bromodomain. MedChemComm 2014, 5, 1843–1848. [Google Scholar]
- (291).Poncet-Montange G; Zhan Y; Bardenhagen JP; Petrocchi A; Leo E; Shi X; Lee G. R. t.; Leonard PG; Geck Do MK; Cardozo MG; Andersen JN; Palmer WS; Jones P; Ladbury JE Observed Bromodomain Flexibility Reveals Histone Peptide- and Small Molecule Ligand-Compatible Forms of Atad2. Biochem. J 2015, 466, 337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (292).Nakamura T; Blechman J; Tada S; Rozovskaia T; Itoyama T; Bullrich F; Mazo A; Croce CM; Geiger B; Canaani E Huash1 Protein, a Putative Transcription Factor Encoded by a Human Homologue of the Drosophila Ash1 Gene, Localizes to Both Nuclei and Cell-Cell Tight Junctions. Proc. Natl. Acad. Sci. U. S. A 2000, 97, 7284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (293).Gregory GD; Vakoc CR; Rozovskaia T; Zheng X; Patel S; Nakamura T; Canaani E; Blobel GA Mammalian Ash1l Is a Histone Methyltransferase That Occupies the Transcribed Region of Active Genes. Mol. Cell. Biol 2007, 27, 8466–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (294).Zeng L; Zhang Q; Li S; Plotnikov AN; Walsh MJ; Zhou MM Mechanism and Regulation of Acetylated Histone Binding by the Tandem Phd Finger of Dpf3b. Nature 2010, 466, 258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (295).Musselman CA; Ramirez J; Sims JK; Mansfield RE; Oliver SS; Denu JM; Mackay JP; Wade PA; Hagman J; Kutateladze TG Bivalent Recognition of Nucleosomes by the Tandem Phd Fingers of the Chd4 Atpase Is Required for Chd4-Mediated Repression. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (296).Qiu Y; Liu L; Zhao C; Han C; Li F; Zhang J; Wang Y; Li G; Mei Y; Wu M; Wu J; Shi Y Combinatorial Readout of Unmodified H3r2 and Acetylated H3k14 by the Tandem Phd Finger of Moz Reveals a Regulatory Mechanism for Hoxa9 Transcription. Genes Dev 2012, 26, 1376–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (297).Li Y; Wen H; Xi Y; Tanaka K; Wang H; Peng D; Ren Y; Jin Q; Dent SY; Li W; Li H; Shi X Af9 Yeats Domain Links Histone Acetylation to Dot1l-Mediated H3k79 Methylation. Cell 2014, 159, 558–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (298).Luo Z; Lin C; Shilatifard A The Super Elongation Complex (Sec) Family in Transcriptional Control. Nat. Rev. Mol. Cell Biol 2012, 13, 543–7. [DOI] [PubMed] [Google Scholar]
- (299).Wan L; Wen H; Li Y; Lyu J; Xi Y; Hoshii T; Joseph JK; Wang X; Loh YE; Erb MA; Souza AL; Bradner JE; Shen L; Li W; Li H; Allis CD; Armstrong SA; Shi X Enl Links Histone Acetylation to Oncogenic Gene Expression in Acute Myeloid Leukaemia. Nature 2017, 543, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (300).Li Y; Sabari BR; Panchenko T; Wen H; Zhao D; Guan H; Wan L; Huang H; Tang Z; Zhao Y; Roeder RG; Shi X; Allis CD; Li H Molecular Coupling of Histone Crotonylation and Active Transcription by Af9 Yeats Domain. Mol. Cell 2016, 62, 181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (301).Krivtsov AV; Armstrong SA Mll Translocations, Histone Modifications and Leukaemia Stem-Cell Development. Nat. Rev. Cancer 2007, 7, 823–33. [DOI] [PubMed] [Google Scholar]
- (302).Wang D; Kon N; Lasso G; Jiang L; Leng W; Zhu WG; Qin J; Honig B; Gu W Acetylation-Regulated Interaction between P53 and Set Reveals a Widespread Regulatory Mode. Nature 2016, 538, 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (303).Gu W; Roeder RG Activation of P53 Sequence-Specific DNA Binding by Acetylation of the P53 C-Terminal Domain. Cell 1997, 90, 595–606. [DOI] [PubMed] [Google Scholar]
- (304).Kouzarides T Acetylation: A Regulatory Modification to Rival Phosphorylation? EMBO J. 2000, 19, 1176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (305).Kim SC; Sprung R; Chen Y; Xu Y; Ball H; Pei J; Cheng T; Kho Y; Xiao H; Xiao L; Grishin NV; White M; Yang XJ; Zhao Y Substrate and Functional Diversity of Lysine Acetylation Revealed by a Proteomics Survey. Mol. Cell 2006, 23, 607–18. [DOI] [PubMed] [Google Scholar]
- (306).Liu L; Wang G; Song L; Lv B; Liang W Acetylome Analysis Reveals the Involvement of Lysine Acetylation in Biosynthesis of Antibiotics in Bacillus Amyloliquefaciens. Sci. Rep 2016, 6, 20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (307).Kosono S; Tamura M; Suzuki S; Kawamura Y; Yoshida A; Nishiyama M; Yoshida M Changes in the Acetylome and Succinylome of Bacillus Subtilis in Response to Carbon Source. PLoS One 2015, 10, e0131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (308).Weinert BT; Satpathy S; Hansen BK; Lyon D; Jensen LJ; Choudhary C Accurate Quantification of Site-Specific Acetylation Stoichiometry Reveals the Impact of Sirtuin Deacetylase Cobb on the E. Coli Acetylome. Mol. Cell. Proteomics 2017, 16, 759. 10.1074/mcp.M117.067587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (309).Downey M; Johnson JR; Davey NE; Newton BW; Johnson TL; Galaang S; Seller CA; Krogan N; Toczyski DP Acetylome Profiling Reveals Overlap in the Regulation of Diverse Processes by Sirtuins, Gcn5, and Esa1. Mol. Cell. Proteomics 2015, 14, 162–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (310).Konig AC; Hartl M; Boersema PJ; Mann M; Finkemeier I The Mitochondrial Lysine Acetylome of Arabidopsis. Mitochondrion 2014, 19 (Pt B), 252–60. [DOI] [PubMed] [Google Scholar]
- (311).Smith-Hammond CL; Hoyos E; Miernyk JA The Pea Seedling Mitochondrial Nepsilon-Lysine Acetylome. Mitochondrion 2014, 19 (Pt B), 154–65. [DOI] [PubMed] [Google Scholar]
- (312).Smith-Hammond CL; Swatek KN; Johnston ML; Thelen JJ; Miernyk JA Initial Description of the Developing Soybean Seed Protein Lys-N(Epsilon)-Acetylome. J. Proteomics 2014, 96, 56–66. [DOI] [PubMed] [Google Scholar]
- (313).Xue B; Jeffers V; Sullivan WJ; Uversky VN Protein Intrinsic Disorder in the Acetylome of Intracellular and Extracellular Toxoplasma Gondii. Mol. BioSyst 2013, 9, 645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (314).Miao J; Lawrence M; Jeffers V; Zhao F; Parker D; Ge Y; Sullivan WJ Jr.; Cui L Extensive Lysine Acetylation Occurs in Evolutionarily Conserved Metabolic Pathways and Parasite-Specific Functions During Plasmodium Falciparum Intraerythrocytic Development. Mol. Microbiol 2013, 89, 660–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (315).Choudhary C; Kumar C; Gnad F; Nielsen ML; Rehman M; Walther TC; Olsen JV; Mann M Lysine Acetylation Targets Protein Complexes and Co-Regulates Major Cellular Functions. Science 2009, 325, 834–40. [DOI] [PubMed] [Google Scholar]
- (316).Kim SY; Sim CK; Zhang Q; Tang H; Brunmeir R; Pan H; Karnani N; Han W; Zhang K; Xu F An Alternative Strategy for Pan-Acetyl-Lysine Antibody Generation. PLoS One 2016, 11, e0162528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (317).Svinkina T; Gu H; Silva JC; Mertins P; Qiao J; Fereshetian S; Jaffe JD; Kuhn E; Udeshi ND; Carr SA Deep, Quantitative Coverage of the Lysine Acetylome Using Novel Anti-Acetyl-Lysine Antibodies and an Optimized Proteomic Workflow. Mol. Cell. Proteomics 2015, 14, 2429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (318).Zhu X; Liu X; Cheng Z; Zhu J; Xu L; Wang F; Qi W; Yan J; Liu N; Sun Z; Liu H; Peng X; Hao Y; Zheng N; Wu Q Quantitative Analysis of Global Proteome and Lysine Acetylome Reveal the Differential Impacts of Vpa and Saha on Hl60 Cells. Sci. Rep 2016, 6, 19926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (319).Rardin MJ; Newman JC; Held JM; Cusack MP; Sorensen DJ; Li B; Schilling B; Mooney SD; Kahn CR; Verdin E; Gibson BW Label-Free Quantitative Proteomics of the Lysine Acetylome in Mitochondria Identifies Substrates of Sirt3 in Metabolic Pathways. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 6601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (320).Lundby A; Lage K; Weinert BT; Bekker-Jensen DB; Secher A; Skovgaard T; Kelstrup CD; Dmytriyev A; Choudhary C; Lundby C; Olsen JV Proteomic Analysis of Lysine Acetylation Sites in Rat Tissues Reveals Organ Specificity and Subcellular Patterns. Cell Rep. 2012, 2, 419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (321).Bing Y; Zhaobao W Effects of Ginkgo Biloba Extract on Free Radical Metabolism of Liver in Mice During Endurance Exercise. Afr. J. Tradit., Complementary Altern. Med 2010, 7, 291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (322).Beli P; Lukashchuk N; Wagner SA; Weinert BT; Olsen JV; Baskcomb L; Mann M; Jackson SP; Choudhary C Proteomic Investigations Reveal a Role for Rna Processing Factor Thrap3 in the DNA Damage Response. Mol. Cell 2012, 46, 212–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (323).Piperno G; Fuller MT Monoclonal Antibodies Specific for an Acetylated Form of Alpha-Tubulin Recognize the Antigen in Cilia and Flagella from a Variety of Organisms. J. Cell Biol 1985, 101, 2085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (324).Piperno G; LeDizet M; Chang XJ Microtubules Containing Acetylated Alpha-Tubulin in Mammalian Cells in Culture. J. Cell Biol 1987, 104, 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (325).L’Hernault SW; Rosenbaum JL Chlamydomonas Alpha-Tubulin Is Posttranslationally Modified in the Flagella During Flagellar Assembly. J. Cell Biol 1983, 97, 258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (326).Fournier M; Orpinell M; Grauffel C; Scheer E; Garnier JM; Ye T; Chavant V; Joint M; Esashi F; Dejaegere A; Gonczy P; Tora L Kat2a/Kat2b-Targeted Acetylome Reveals a Role for Plk4 Acetylation in Preventing Centrosome Amplification. Nat. Commun 2016, 7, 13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (327).Masri S; Patel VR; Eckel-Mahan KL; Peleg S; Forne I; Ladurner AG; Baldi P; Imhof A; Sassone-Corsi P Circadian Acetylome Reveals Regulation of Mitochondrial Metabolic Pathways. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 3339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (328).Simon GM; Cheng J; Gordon JI Quantitative Assessment of the Impact of the Gut Microbiota on Lysine Epsilon-Acetylation of Host Proteins Using Gnotobiotic Mice. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 11133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (329).Kim SY; Sim CK; Tang H; Han W; Zhang K; Xu F Acetylome Analysis Identifies Sirt1 Targets in Mrna-Processing and Chromatin-Remodeling in Mouse Liver. PLoS One 2015, 10, e0140619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Sol EM; Wagner SA; Weinert BT; Kumar A; Kim HS; Deng CX; Choudhary C Proteomic Investigations of Lysine Acetylation Identify Diverse Substrates of Mitochondrial Deacetylase Sirt3. PLoS One 2012, 7, e50545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (331).Weinert BT; Scholz C; Wagner SA; Iesmantavicius V; Su D; Daniel JA; Choudhary C Lysine Succinylation Is a Frequently Occurring Modification in Prokaryotes and Eukaryotes and Extensively Overlaps with Acetylation. Cell Rep. 2013, 4, 842–51. [DOI] [PubMed] [Google Scholar]
- (332).Gu S; Liu Y; Zhu B; Ding K; Yao TP; Chen F; Zhan L; Xu P; Ehrlich M; Liang T; Lin X; Feng XH Loss of Alpha-Tubulin Acetylation Is Associated with Tgf-Beta-Induced Epithelial-Mesenchymal Transition. J. Biol. Chem 2016, 291, 5396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (333).Zhong T; Ren F; Huang CS; Zou WY; Yang Y; Pan YD; Sun B; Wang E; Guo QL Swimming Exercise Ameliorates Neurocognitive Impairment Induced by Neonatal Exposure to Isoflurane and Enhances Hippocampal Histone Acetylation in Mice. Neuroscience 2016, 316, 378–88. [DOI] [PubMed] [Google Scholar]
- (334).Tatham MH; Cole C; Scullion P; Wilkie R; Westwood NJ; Stark LA; Hay RT A Proteomic Approach to Analyze the Aspirin-Mediated Lysine Acetylome. Mol. Cell. Proteomics 2017, 16, 310–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (335).Scholz C; Weinert BT; Wagner SA; Beli P; Miyake Y; Qi J; Jensen LJ; Streicher W; McCarthy AR; Westwood NJ; Lain S; Cox J; Matthias P; Mann M; Bradner JE; Choudhary C Acetylation Site Specificities of Lysine Deacetylase Inhibitors in Human Cells. Nat. Biotechnol 2015, 33, 415–23. [DOI] [PubMed] [Google Scholar]
- (336).Liu X; Liu S; Bode L; Liu C; Zhang L; Wang X; Li D; Lei Y; Peng X; Cheng Z; Xie P Persistent Human Borna Disease Virus Infection Modifies the Acetylome of Human Oligodendroglia Cells Towards Higher Energy and Transporter Levels. Virology 2015, 485, 58–78. [DOI] [PubMed] [Google Scholar]
- (337).Robinson PJ; Rhodes D Structure of the ‘30 Nm’ Chromatin Fibre: A Key Role for the Linker Histone. Curr. Opin. Struct. Biol 2006, 16, 336–43. [DOI] [PubMed] [Google Scholar]
- (338).Tweedie-Cullen RY; Brunner AM; Grossmann J; Mohanna S; Sichau D; Nanni P; Panse C; Mansuy IM Identification of Combinatorial Patterns of Post-Translational Modifications on Individual Histones in the Mouse Brain. PLoS One 2012, 7, e36980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (339).Ma XJ; Wu J; Altheim BA; Schultz MC; Grunstein M Deposition-Related Sites K5/K12 in Histone H4 Are Not Required for Nucleosome Deposition in Yeast. Proc. Natl. Acad. Sci. U. S. A 1998, 95, 6693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (340).Garcia BA; Hake SB; Diaz RL; Kauer M; Morris SA; Recht J; Shabanowitz J; Mishra N; Strahl BD; Allis CD; Hunt DF Organismal Differences in Post-Translational Modifications in Histones H3 and H4. J. Biol. Chem 2007, 282, 7641–55. [DOI] [PubMed] [Google Scholar]
- (341).Huang H; Lin S; Garcia BA; Zhao Y Quantitative Proteomic Analysis of Histone Modifications. Chem. Rev 2015, 115, 2376–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (342).Luger K; Mader AW; Richmond RK; Sargent DF; Richmond TJ Crystal Structure of the Nucleosome Core Particle at 2.8 a Resolution. Nature 1997, 389, 251–60. [DOI] [PubMed] [Google Scholar]
- (343).Udeshi ND; Compton PD; Shabanowitz J; Hunt DF; Rose KL Methods for Analyzing Peptides and Proteins on a Chromatographic Timescale by Electron-Transfer Dissociation Mass Spectrometry. Nat. Protoc 2008, 3, 1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (344).Syka JE; Coon JJ; Schroeder MJ; Shabanowitz J; Hunt DF Peptide and Protein Sequence Analysis by Electron Transfer Dissociation Mass Spectrometry. Proc. Natl. Acad. Sci. U. S. A 2004, 101, 9528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (345).Garcia BA; Thomas CE; Kelleher NL; Mizzen CA Tissue-Specific Expression and Post-Translational Modification of Histone H3 Variants. J. Proteome Res 2008, 7, 4225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (346).Garcia BA; Pesavento JJ; Mizzen CA; Kelleher NL Pervasive Combinatorial Modification of Histone H3 in Human Cells. Nat. Methods 2007, 4, 487–9. [DOI] [PubMed] [Google Scholar]
- (347).Phanstiel D; Brumbaugh J; Berggren WT; Conard K; Feng X; Levenstein ME; McAlister GC; Thomson JA; Coon JJ Mass Spectrometry Identifies and Quantifies 74 Unique Histone H4 Isoforms in Differentiating Human Embryonic Stem Cells. Proc. Natl. Acad. Sci. U. S. A 2008, 105, 4093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (348).Tan M; Luo H; Lee S; Jin F; Yang JS; Montellier E; Buchou T; Cheng Z; Rousseaux S; Rajagopal N; Lu Z; Ye Z; Zhu Q; Wysocka J; Ye Y; Khochbin S; Ren B; Zhao Y Identification of 67 Histone Marks and Histone Lysine Crotonylation as a New Type of Histone Modification. Cell 2011, 146, 1016–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (349).Gatta R; Mantovani R Nf-Y Affects Histone Acetylation and H2a.Z Deposition in Cell Cycle Promoters. Epigenetics 2011, 6, 526–34. [DOI] [PubMed] [Google Scholar]
- (350).Puerta C; Hernandez F; Lopez-Alarcon L; Palacian E Acetylation of Histone H2a.H2b Dimers Facilitates Transcription. Biochem. Biophys. Res. Commun 1995, 210, 409–16. [DOI] [PubMed] [Google Scholar]
- (351).Zheng Y; Thomas PM; Kelleher NL Measurement of Acetylation Turnover at Distinct Lysines in Human Histones Identifies Long-Lived Acetylation Sites. Nat. Commun 2013, 4, 2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (352).Poplawski A; Hu K; Lee W; Natesan S; Peng D; Carlson S; Shi X; Balaz S; Markley JL; Glass KC Molecular Insights into the Recognition of N-Terminal Histone Modifications by the Brpf1 Bromodomain. J. Mol. Biol 2014, 426, 1661–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (353).Chen Y; Zhao W; Yang JS; Cheng Z; Luo H; Lu Z; Tan M; Gu W; Zhao Y Quantitative Acetylome Analysis Reveals the Roles of Sirt1 in Regulating Diverse Substrates and Cellular Pathways. Mol. Cell. Proteomics 2012, 11, 1048–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (354).Garcia BA; Busby SA; Barber CM; Shabanowitz J; Allis CD; Hunt DF Characterization of Phosphorylation Sites on Histone H1 Isoforms by Tandem Mass Spectrometry. J. Proteome Res 2004, 3, 1219–27. [DOI] [PubMed] [Google Scholar]
- (355).Vaquero A; Scher M; Lee D; Erdjument-Bromage H; Tempst P; Reinberg D Human Sirt1 Interacts with Histone H1 and Promotes Formation of Facultative Heterochromatin. Mol. Cell 2004, 16, 93–105. [DOI] [PubMed] [Google Scholar]
- (356).Wisniewski JR; Zougman A; Kruger S; Mann M Mass Spectrometric Mapping of Linker Histone H1 Variants Reveals Multiple Acetylations, Methylations, and Phosphorylation as Well as Differences between Cell Culture and Tissue. Mol. Cell. Proteomics 2007, 6, 72–87. [DOI] [PubMed] [Google Scholar]
- (357).Singh SK; Maeda K; Eid MM; Almofty SA; Ono M; Pham P; Goodman MF; Sakaguchi N Ganp Regulates Recruitment of Aid to Immunoglobulin Variable Regions by Modulating Transcription and Nucleosome Occupancy. Nat. Commun 2013, 4, 1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (358).Kamieniarz K; Izzo A; Dundr M; Tropberger P; Ozretic L; Kirfel J; Scheer E; Tropel P; Wisniewski JR; Tora L; Viville S; Buettner R; Schneider R A Dual Role of Linker Histone H1.4 Lys 34 Acetylation in Transcriptional Activation. Genes Dev. 2012, 26, 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (359).Happel N; Doenecke D Histone H1 and Its Isoforms: Contribution to Chromatin Structure and Function. Gene 2009, 431, 1–12. [DOI] [PubMed] [Google Scholar]
- (360).Sun J; Wei HM; Xu J; Chang JF; Yang Z; Ren X; Lv WW; Liu LP; Pan LX; Wang X; Qiao HH; Zhu B; Ji JY; Yan D; Xie T; Sun FL; Ni JQ Histone H1-Mediated Epigenetic Regulation Controls Germline Stem Cell Self-Renewal by Modulating H4k16 Acetylation. Nat. Commun 2015, 6, 8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (361).Bernier M; Luo Y; Nwokelo KC; Goodwin M; Dreher SJ; Zhang P; Parthun MR; Fondufe-Mittendorf Y; Ottesen JJ; Poirier MG Linker Histone H1 and H3k56 Acetylation Are Antagonistic Regulators of Nucleosome Dynamics. Nat. Commun 2015, 6, 10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (362).Karvonen U; Janne OA; Palvimo JJ Androgen Receptor Regulates Nuclear Trafficking and Nuclear Domain Residency of Corepressor Hdac7 in a Ligand-Dependent Fashion. Exp. Cell Res 2006, 312, 3165–83. [DOI] [PubMed] [Google Scholar]
- (363).Fu M; Wang C; Reutens AT; Wang J; Angeletti RH; Siconolfi-Baez L; Ogryzko V; Avantaggiati ML; Pestell RG P300 and P300/Camp-Response Element-Binding Protein-Associated Factor Acetylate the Androgen Receptor at Sites Governing Hormone-Dependent Transactivation. J. Biol. Chem 2000, 275, 20853–60. [DOI] [PubMed] [Google Scholar]
- (364).Gaughan L; Logan IR; Cook S; Neal DE; Robson CN Tip60 and Histone Deacetylase 1 Regulate Androgen Receptor Activity through Changes to the Acetylation Status of the Receptor. J. Biol. Chem 2002, 277, 25904–13. [DOI] [PubMed] [Google Scholar]
- (365).Fu M; Rao M; Wu K; Wang C; Zhang X; Hessien M; Yeung YG; Gioeli D; Weber MJ; Pestell RG The Androgen Receptor Acetylation Site Regulates Camp and Akt but Not Erk-Induced Activity. J. Biol. Chem 2004, 279, 29436–49. [DOI] [PubMed] [Google Scholar]
- (366).Sun Y; Xu Y; Roy K; Price BD DNA Damage-Induced Acetylation of Lysine 3016 of Atm Activates Atm Kinase Activity. Mol. Cell. Biol 2007, 27, 8502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (367).Bereshchenko OR; Gu W; Dalla-Favera R Acetylation Inactivates the Transcriptional Repressor Bcl6. Nat. Genet 2002, 32, 606–13. [DOI] [PubMed] [Google Scholar]
- (368).Nakahata Y; Kaluzova M; Grimaldi B; Sahar S; Hirayama J; Chen D; Guarente LP; Sassone-Corsi P The Nad+-Dependent Deacetylase Sirt1Modulates Clock-Mediated Chromatin Remodeling and Circadian Control. Cell 2008, 134, 329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (369).Fu J; Yoon HG; Qin J; Wong J Regulation of P-Tefb Elongation Complex Activity by Cdk9 Acetylation. Mol. Cell. Biol 2007, 27, 4641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (370).Zhang H; Park SH; Pantazides BG; Karpiuk O; Warren MD; Hardy CW; Duong DM; Park SJ; Kim HS; Vassilopoulos A; Seyfried NT; Johnsen SA; Gius D; Yu DS Sirt2 Directs the Replication Stress Response through Cdk9 Deacetylation. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 13546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (371).Sabo A; Lusic M; Cereseto A; Giacca M Acetylation of Conserved Lysines in the Catalytic Core of Cyclin-Dependent Kinase 9 Inhibits Kinase Activity and Regulates Transcription. Mol. Cell. Biol 2008, 28, 2201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (372).Jang MK; Mochizuki K; Zhou M; Jeong HS; Brady JN; Ozato K The Bromodomain Protein Brd4 Is a Positive Regulatory Component of P-Tefb and Stimulates Rna Polymerase Ii-Dependent Transcription. Mol. Cell 2005, 19, 523–34. [DOI] [PubMed] [Google Scholar]
- (373).Bricambert J; Miranda J; Benhamed F; Girard J; Postic C; Dentin R Salt-Inducible Kinase 2 Links Transcriptional Coactivator P300 Phosphorylation to the Prevention of Chrebp-Dependent Hepatic Steatosis in Mice. J. Clin. Invest 2010, 120, 4316–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (374).Marmier S; Dentin R; Daujat-Chavanieu M; Guillou H; Bertrand-Michel J; Gerbal-Chaloin S; Girard J; Lotersztajn S; Postic C Novel Role for Carbohydrate Responsive Element Binding Protein in the Control of Ethanol Metabolism and Susceptibility to Binge Drinking. Hepatology 2015, 62, 1086–100. [DOI] [PubMed] [Google Scholar]
- (375).Lu Q; Hutchins AE; Doyle CM; Lundblad JR; Kwok RP Acetylation of Camp-Responsive Element-Binding Protein (Creb) by Creb-Binding Protein Enhances Creb-Dependent Transcription. J. Biol. Chem 2003, 278, 15727–34. [DOI] [PubMed] [Google Scholar]
- (376).Paz JC; Park S; Phillips N; Matsumura S; Tsai WW; Kasper L; Brindle PK; Zhang G; Zhou MM; Wright PE; Montminy M Combinatorial Regulation of a Signal-Dependent Activator by Phosphorylation and Acetylation. Proc. Natl. Acad. Sci. U. S. A 2014, 111, 17116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (377).Liu Y; Dentin R; Chen D; Hedrick S; Ravnskjaer K; Schenk S; Milne J; Meyers DJ; Cole P; Yates J 3rd; Olefsky J; Guarente L; Montminy M A Fasting Inducible Switch Modulates Gluconeogenesis Via Activator/Coactivator Exchange. Nature 2008, 456, 269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (378).Cho S; Schroeder S; Kaehlcke K; Kwon HS; Pedal A; Herker E; Schnoelzer M; Ott M Acetylation of Cyclin T1 Regulates the Equilibrium between Active and Inactive P-Tefb in Cells. EMBO J. 2009, 28, 1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (379).Safronova OS; Nakahama K; Morita I Acute Hypoxia Affects P-Tefb through Hdac3 and Hexim1-Dependent Mechanism to Promote Gene-Specific Transcriptional Repression. Nucleic Acids Res. 2014, 42, 8954–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (380).Wang C; Rauscher FJ 3rd; Cress WD; Chen J Regulation of E2f1 Function by the Nuclear Corepressor Kap1. J. Biol. Chem 2007, 282, 29902–9. [DOI] [PubMed] [Google Scholar]
- (381).Martinez-Balbas MA; Bauer UM; Nielsen SJ; Brehm A; Kouzarides T Regulation of E2f1 Activity by Acetylation. EMBO J. 2000, 19, 662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (382).Zhao Y; Tan J; Zhuang L; Jiang X; Liu ET; Yu Q Inhibitors of Histone Deacetylases Target the Rb-E2f1 Pathway for Apoptosis Induction through Activation of Proapoptotic Protein Bim. Proc. Natl. Acad. Sci. U. S. A 2005, 102, 16090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (383).Zhang W; Bieker JJ Acetylation and Modulation of Erythroid Kruppel-Like Factor (Eklf) Activity by Interaction with Histone Acetyltransferases. Proc. Natl. Acad. Sci. U. S. A 1998, 95, 9855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (384).Chen X; Bieker JJ Unanticipated Repression Function Linked to Erythroid Kruppel-Like Factor. Mol. Cell. Biol 2001, 21, 3118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (385).Kim MY; Woo EM; Chong YT; Homenko DR; Kraus WL Acetylation of Estrogen Receptor Alpha by P300 at Lysines 266 and 268 Enhances the Deoxyribonucleic Acid Binding and Transactivation Activities of the Receptor. Mol. Endocrinol 2006, 20, 1479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (386).Ma Y; Fan S; Hu C; Meng Q; Fuqua SA; Pestell RG; Tomita YA; Rosen EM Brca1 Regulates Acetylation and Ubiquitination of Estrogen Receptor-Alpha. Mol. Endocrinol. 2010, 24, 76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (387).Wang C; Fu M; Angeletti RH; Siconolfi-Baez L; Reutens AT; Albanese C; Lisanti MP; Katzenellenbogen BS; Kato S; Hopp T; Fuqua SA; Lopez GN; Kushner PJ; Pestell RG Direct Acetylation of the Estrogen Receptor Alpha Hinge Region by P300 Regulates Transactivation and Hormone Sensitivity. J. Biol. Chem 2001, 276, 18375–83. [DOI] [PubMed] [Google Scholar]
- (388).Zhao Y; Yang J; Liao W; Liu X; Zhang H; Wang S; Wang D; Feng J; Yu L; Zhu WG Cytosolic Foxo1 Is Essential for the Induction of Autophagy and Tumour Suppressor Activity. Nat. Cell Biol 2010, 12, 665–75. [DOI] [PubMed] [Google Scholar]
- (389).Jing E; Gesta S; Kahn CR Sirt2 Regulates Adipocyte Differentiation through Foxo1 Acetylation/Deacetylation. Cell Metab. 2007, 6, 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (390).Hariharan N; Maejima Y; Nakae J; Paik J; Depinho RA; Sadoshima J Deacetylation of Foxo by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ. Res 2010, 107, 1470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (391).Tsai KL; Sun YJ; Huang CY; Yang JY; Hung MC; Hsiao CD Crystal Structure of the Human Foxo3a-Dbd/DNA Complex Suggests the Effects of Post-Translational Modification. Nucleic Acids Res. 2007, 35, 6984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (392).Frescas D; Valenti L; Accili D Nuclear Trapping of the Forkhead Transcription Factor Foxo1 Via Sirt-Dependent Deacetylation Promotes Expression of Glucogenetic Genes. J. Biol. Chem 2005, 280, 20589–95. [DOI] [PubMed] [Google Scholar]
- (393).Dansen TB; Smits LM; van Triest MH; de Keizer PL; van Leenen D; Koerkamp MG; Szypowska A; Meppelink A; Brenkman AB; Yodoi J; Holstege FC; Burgering BM Redox-Sensitive Cysteines Bridge P300/Cbp-Mediated Acetylation and Foxo4 Activity. Nat. Chem. Biol 2009, 5, 664–72. [DOI] [PubMed] [Google Scholar]
- (394).Kwon HS; Lim HW; Wu J; Schnolzer M; Verdin E; Ott M Three Novel Acetylation Sites in the Foxp3 Transcription Factor Regulate the Suppressive Activity of Regulatory T Cells. J. Immunol 2012, 188, 2712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (395).Liu Y; Wang L; Predina J; Han R; Beier UH; Wang LC; Kapoor V; Bhatti TR; Akimova T; Singhal S; Brindle PK; Cole PA; Albelda SM; Hancock WW Inhibition of P300 Impairs Foxp3(+) T Regulatory Cell Function and Promotes Antitumor Immunity. Nat. Med 2013, 19, 1173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (396).Xiao Y; Nagai Y; Deng G; Ohtani T; Zhu Z; Zhou Z; Zhang H; Ji MQ; Lough JW; Samanta A; Hancock WW; Greene MI Dynamic Interactions between Tip60 and P300 Regulate Foxp3 Function through a Structural Switch Defined by a Single Lysine on Tip60. Cell Rep. 2014, 7, 1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (397).Kemper JK; Xiao Z; Ponugoti B; Miao J; Fang S; Kanamaluru D; Tsang S; Wu SY; Chiang CM; Veenstra TD Fxr Acetylation Is Normally Dynamically Regulated by P300 and Sirt1 but Constitutively Elevated in Metabolic Disease States. Cell Metab. 2009, 10, 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (398).Kim DH; Xiao Z; Kwon S; Sun X; Ryerson D; Tkac D; Ma P; Wu SY; Chiang CM; Zhou E; Xu HE; Palvimo JJ; Chen LF; Kemper B; Kemper JK A Dysregulated Acetyl/Sumo Switch of Fxr Promotes Hepatic Inflammation in Obesity. EMBO J. 2015, 34, 184–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (399).Ryu D; Jo YS; Lo Sasso G; Stein S; Zhang H; Perino A; Lee JU; Zeviani M; Romand R; Hottiger MO; Schoonjans K; Auwerx J A Sirt7-Dependent Acetylation Switch of Gabpbeta1 Controls Mitochondrial Function. Cell Metab. 2014, 20, 856–69. [DOI] [PubMed] [Google Scholar]
- (400).Boyes J; Byfield P; Nakatani Y; Ogryzko V Regulation of Activity of the Transcription Factor Gata-1 by Acetylation. Nature 1998, 396, 594–8. [DOI] [PubMed] [Google Scholar]
- (401).Hung HL; Lau J; Kim AY; Weiss MJ; Blobel GA Creb-Binding Protein Acetylates Hematopoietic Transcription Factor Gata-1 at Functionally Important Sites. Mol. Cell. Biol 1999, 19, 3496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (402).Watamoto K; Towatari M; Ozawa Y; Miyata Y; Okamoto M; Abe A; Naoe T; Saito H Altered Interaction of Hdac5 with Gata-1 During Mel Cell Differentiation. Oncogene 2003, 22, 9176–84. [DOI] [PubMed] [Google Scholar]
- (403).Delehanty LL; Bullock GC; Goldfarb AN Protein Kinase D-Hdac5 Signaling Regulates Erythropoiesis and Contributes to Erythropoietin Cross-Talk with Gata1. Blood 2012, 120, 4219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (404).Geng H; Liu Q; Xue C; David LL; Beer TM; Thomas GV; Dai MS; Qian DZ Hif1alpha Protein Stability Is Increased by Acetylation at Lysine 709. J. Biol. Chem 2012, 287, 35496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (405).Geng H; Harvey CT; Pittsenbarger J; Liu Q; Beer TM; Xue C; Qian DZ Hdac4 Protein Regulates Hif1alpha Protein Lysine Acetylation and Cancer Cell Response to Hypoxia. J. Biol. Chem 2011, 286, 38095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (406).Lim JH; Lee YM; Chun YS; Chen J; Kim JE; Park JW Sirtuin 1 Modulates Cellular Responses to Hypoxia by Deacetylating Hypoxia-Inducible Factor 1alpha. Mol. Cell 2010, 38, 864–78. [DOI] [PubMed] [Google Scholar]
- (407).Xenaki G; Ontikatze T; Rajendran R; Stratford IJ; Dive C; Krstic-Demonacos M; Demonacos C Pcaf Is an Hif-1alpha Cofactor That Regulates P53 Transcriptional Activity in Hypoxia. Oncogene 2008, 27, 5785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (408).Seo KS; Park JH; Heo JY; Jing K; Han J; Min KN; Kim C; Koh GY; Lim K; Kang GY; Uee Lee J; Yim YH; Shong M; Kwak TH; Kweon GR Sirt2 Regulates Tumour Hypoxia Response by Promoting Hif-1alpha Hydroxylation. Oncogene 2015, 34, 1354–62. [DOI] [PubMed] [Google Scholar]
- (409).Chen R; Xu M; Hogg RT; Li J; Little B; Gerard RD; Garcia JA The Acetylase/Deacetylase Couple Creb-Binding Protein/Sirtuin 1 Controls Hypoxia-Inducible Factor 2 Signaling. J. Biol. Chem 2012, 287, 30800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (410).Dioum EM; Chen R; Alexander MS; Zhang Q; Hogg RT; Gerard RD; Garcia JA Regulation of Hypoxia-Inducible Factor 2alpha Signaling by the Stress-Responsive Deacetylase Sirtuin 1. Science 2009, 324, 1289–93. [DOI] [PubMed] [Google Scholar]
- (411).Herrera JE; Sakaguchi K; Bergel M; Trieschmann L; Nakatani Y; Bustin M Specific Acetylation of Chromosomal Protein Hmg-17 by Pcaf Alters Its Interaction with Nucleosomes. Mol. Cell. Biol 1999, 19, 3466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (412).Tang X; Gao JS; Guan YJ; McLane KE; Yuan ZL; Ramratnam B; Chin YE Acetylation-Dependent Signal Transduction for Type I Interferon Receptor. Cell 2007, 131, 93–105. [DOI] [PubMed] [Google Scholar]
- (413).Zhang K; Faiola F; Martinez E Six Lysine Residues on C-Myc Are Direct Substrates for Acetylation by P300. Biochem. Biophys. Res. Commun 2005, 336, 274–80. [DOI] [PubMed] [Google Scholar]
- (414).Patel JH; Du Y; Ard PG; Phillips C; Carella B; Chen CJ; Rakowski C; Chatterjee C; Lieberman PM; Lane WS; Blobel GA; McMahon SB The C-Myc Oncoprotein Is a Substrate of the Acetyltransferases Hgcn5/Pcaf and Tip60. Mol. Cell. Biol 2004, 24, 10826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (415).Faiola F; Liu X; Lo S; Pan S; Zhang K; Lymar E; Farina A; Martinez E Dual Regulation of C-Myc by P300 Via Acetylation-Dependent Control of Myc Protein Turnover and Coactivation of Myc-Induced Transcription. Mol. Cell. Biol 2005, 25, 10220–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (416).Liu PY; Xu N; Malyukova A; Scarlett CJ; Sun YT; Zhang XD; Ling D; Su SP; Nelson C; Chang DK; Koach J; Tee AE; Haber M; Norris MD; Toon C; Rooman I; Xue C; Cheung BB; Kumar S; Marshall GM; Biankin AV; Liu T The Histone Deacetylase Sirt2 Stabilizes Myc Oncoproteins. Cell Death Differ. 2013, 20, 503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (417).Guarani V; Deflorian G; Franco CA; Kruger M; Phng LK; Bentley K; Toussaint L; Dequiedt F; Mostoslavsky R; Schmidt MH; Zimmermann B; Brandes RP; Mione M; Westphal CH; Braun T; Zeiher AM; Gerhardt H; Dimmeler S; Potente M Acetylation-Dependent Regulation of Endothelial Notch Signalling by the Sirt1 Deacetylase. Nature 2011, 473, 234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (418).Rokudai S; Laptenko O; Arnal SM; Taya Y; Kitabayashi I; Prives C Moz Increases P53 Acetylation and Premature Senescence through Its Complex Formation with Pml. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 3895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (419).Wang YH; Tsay YG; Tan BC; Lo WY; Lee SC Identification and Characterization of a Novel P300-Mediated P53 Acetylation Site, Lysine 305. J. Biol. Chem 2003, 278, 25568–76. [DOI] [PubMed] [Google Scholar]
- (420).Drost J; Mantovani F; Tocco F; Elkon R; Comel A; Holstege H; Kerkhoven R; Jonkers J; Voorhoeve PM; Agami R; Del Sal G Brd7 Is a Candidate Tumour Suppressor Gene Required for P53 Function. Nat. Cell Biol 2010, 12, 380–9. [DOI] [PubMed] [Google Scholar]
- (421).Abraham J; Kelly J; Thibault P; Benchimol S Post-Translational Modification of P53 Protein in Response to Ionizing Radiation Analyzed by Mass Spectrometry. J. Mol. Biol 2000, 295, 853–64. [DOI] [PubMed] [Google Scholar]
- (422).Hofmann TG; Möller A; Sirma H; Zentgraf H; Taya Y; Droge W; Will H; Schmitz ML Regulation of P53 Activity by Its Interaction with Homeodomain-Interacting Protein Kinase-2. Nat. Cell Biol 2002, 4, 1–10. [DOI] [PubMed] [Google Scholar]
- (423).Chen S; Seiler J; Santiago-Reichelt M; Felbel K; Grummt I; Voit R Repression of Rna Polymerase I Upon Stress Is Caused by Inhibition of Rna-Dependent Deacetylation of Paf53 by Sirt7. Mol. Cell 2013, 52, 303–13. [DOI] [PubMed] [Google Scholar]
- (424).Ma L; Gao JS; Guan Y; Shi X; Zhang H; Ayrapetov MK; Zhang Z; Xu L; Hyun YM; Kim M; Zhuang S; Chin YE Acetylation Modulates Prolactin Receptor Dimerization. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 19314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (425).Andrews PG; Kao KR Wnt/Beta-Catenin-Dependent Acetylation of Pygo2 by Cbp/P300 Histone Acetyltransferase Family Members. Biochem. J 2016, 473, 4193–4203. [DOI] [PubMed] [Google Scholar]
- (426).Kiernan R; Bres V; Ng RW; Coudart MP; El Messaoudi S; Sardet C; Jin DY; Emiliani S; Benkirane M Post-Activation Turn-Off of Nf-Kappa B-Dependent Transcription Is Regulated by Acetylation of P65. J. Biol. Chem 2003, 278, 2758–66. [DOI] [PubMed] [Google Scholar]
- (427).Chen LF; Mu Y; Greene WC Acetylation of Rela at Discrete Sites Regulates Distinct Nuclear Functions of Nf-Kappab. EMBO J 2002, 21, 6539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (428).Huang B; Yang XD; Zhou MM; Ozato K; Chen LF Brd4 Coactivates Transcriptional Activation of Nf-Kappab Via Specific Binding to Acetylated Rela. Mol. Cell. Biol 2009, 29, 1375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (429).Buerki C; Rothgiesser KM; Valovka T; Owen HR; Rehrauer H; Fey M; Lane WS; Hottiger MO Functional Relevance of Novel P300-Mediated Lysine 314 and 315 Acetylation of Rela/P65. Nucleic Acids Res 2008, 36, 1665–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (430).Yeung F; Hoberg JE; Ramsey CS; Keller MD; Jones DR; Frye RA; Mayo MW Modulation of Nf-Kappab-Dependent Transcription and Cell Survival by the Sirt1 Deacetylase. EMBO J 2004, 23, 2369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (431).Pickard A; Wong PP; McCance DJ Acetylation of Rb by Pcaf Is Required for Nuclear Localization and Keratinocyte Differentiation. J. Cell Sci 2010, 123, 3718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (432).Wu Q; Nie J; Gao Y; Xu P; Sun Q; Yang J; Han L; Chen Z; Wang X; Lv L; Tsun A; Shen J; Li B Reciprocal Regulation of Rorgammat Acetylation and Function by P300 and Hdac1. Sci. Rep 2015, 5, 16355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (433).Lim HW; Kang SG; Ryu JK; Schilling B; Fei M; Lee IS; Kehasse A; Shirakawa K; Yokoyama M; Schnolzer M; Kasler HG; Kwon HS; Gibson BW; Sato H; Akassoglou K; Xiao C; Littman DR; Ott M; Verdin E Sirt1 Deacetylates Rorgammat and Enhances Th17 Cell Generation. J. Exp. Med 2015, 212, 607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (434).Schroder S; Herker E; Itzen F; He D; Thomas S; Gilchrist DA; Kaehlcke K; Cho S; Pollard KS; Capra JA; Schnolzer M; Cole PA; Geyer M; Bruneau BG; Adelman K; Ott M Acetylation of Rna Polymerase Ii Regulates Growth-Factor-Induced Gene Transcription in Mammalian Cells. Mol. Cell 2013, 52, 314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (435).Simonti CN; Pollard KS; Schroder S; He D; Bruneau BG; Ott M; Capra JA Evolution of Lysine Acetylation in the Rna Polymerase Ii C-Terminal Domain. BMC Evol. Biol 2015, 15, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (436).Gronroos E; Hellman U; Heldin CH; Ericsson J Control of Smad7 Stability by Competition between Acetylation and Ubiquitination. Mol. Cell 2002, 10, 483–93. [DOI] [PubMed] [Google Scholar]
- (437).Simonsson M; Heldin CH; Ericsson J; Gronroos E The Balance between Acetylation and Deacetylation Controls Smad7 Stability. J. Biol. Chem 2005, 280, 21797–803. [DOI] [PubMed] [Google Scholar]
- (438).Hung JJ; Wang YT; Chang WC Sp1 Deacetylation Induced by Phorbol Ester Recruits P300 to Activate 12(S)-Lipoxygenase Gene Transcription. Mol. Cell. Biol 2006, 26, 1770–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (439).Braun H; Koop R; Ertmer A; Nacht S; Suske G Transcription Factor Sp3 Is Regulated by Acetylation. Nucleic Acids Res 2001, 29, 4994–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (440).Ehlting C; Haussinger D; Bode JG Sp3 Is Involved in the Regulation of Socs3 Gene Expression. Biochem. J 2005, 387, 737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (441).Ammanamanchi S; Freeman JW; Brattain MG Acetylated Sp3 Is a Transcriptional Activator. J. Biol. Chem 2003, 278, 35775–80. [DOI] [PubMed] [Google Scholar]
- (442).Ponugoti B; Kim DH; Xiao Z; Smith Z; Miao J; Zang M; Wu SY; Chiang CM; Veenstra TD; Kemper JK Sirt1 Deacetylates and Inhibits Srebp-1c Activity in Regulation of Hepatic Lipid Metabolism. J. Biol. Chem 2010, 285, 33959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (443).Paulson M; Pisharody S; Pan L; Guadagno S; Mui AL; Levy DE Stat Protein Transactivation Domains Recruit P300/Cbp through Widely Divergent Sequences. J. Biol. Chem 1999, 274, 25343–9. [DOI] [PubMed] [Google Scholar]
- (444).Wang R; Cherukuri P; Luo J Activation of Stat3 Sequence-Specific DNA Binding and Transcription by P300/Creb-Binding Protein-Mediated Acetylation. J. Biol. Chem 2005, 280, 11528–34. [DOI] [PubMed] [Google Scholar]
- (445).Yuan ZL; Guan YJ; Chatterjee D; Chin YE Stat3 Dimerization Regulated by Reversible Acetylation of a Single Lysine Residue. Science 2005, 307, 269–73. [DOI] [PubMed] [Google Scholar]
- (446).Ray S; Boldogh I; Brasier AR Stat3 Nh2-Terminal Acetylation Is Activated by the Hepatic Acute-Phase Response and Required for Il-6 Induction of Angiotensinogen. Gastroenterology 2005, 129, 1616–32. [DOI] [PubMed] [Google Scholar]
- (447).Kosan C; Ginter T; Heinzel T; Kramer OH Stat5 Acetylation: Mechanisms and Consequences for Immunological Control and Leukemogenesis. JAKSTAT 2013, 2, e26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (448).Kiernan RE; Vanhulle C; Schiltz L; Adam E; Xiao H; Maudoux F; Calomme C; Burny A; Nakatani Y; Jeang KT; Benkirane M; Van Lint C Hiv-1 Tat Transcriptional Activity Is Regulated by Acetylation. EMBO J. 1999, 18, 6106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (449).Deng L; de la Fuente C; Fu P; Wang L; Donnelly R; Wade JD; Lambert P; Li H; Lee CG; Kashanchi F Acetylation of Hiv-1 Tat by Cbp/P300 Increases Transcription of Integrated Hiv-1 Genome and Enhances Binding to Core Histones. Virology 2000, 277, 278–95. [DOI] [PubMed] [Google Scholar]
- (450).Col E; Caron C; Seigneurin-Berny D; Gracia J; Favier A; Khochbin S The Histone Acetyltransferase, Hgcn5, Interacts with and Acetylates the Hiv Transactivator, Tat. J. Biol. Chem 2001, 276, 28179–84. [DOI] [PubMed] [Google Scholar]
- (451).Pagans S; Pedal A; North BJ; Kaehlcke K; Marshall BL; Dorr A; Hetzer-Egger C; Henklein P; Frye R; McBurney MW; Hruby H; Jung M; Verdin E; Ott M Sirt1 Regulates Hiv Transcription Via Tat Deacetylation. PLoS Biol. 2005, 3, e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (452).Lee J; Hwang YJ; Boo JH; Han D; Kwon OK; Todorova K; Kowall NW; Kim Y; Ryu H Dysregulation of Upstream Binding Factor-1 Acetylation at K352 Is Linked to Impaired Ribosomal DNA Transcription in Huntington’s Disease. Cell Death Differ. 2011, 18, 1726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (453).Yao YL; Yang WM; Seto E Regulation of Transcription Factor Yy1 by Acetylation and Deacetylation. Mol. Cell. Biol 2001, 21, 5979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (454).Thiagarajan D; Vedantham S; Ananthakrishnan R; Schmidt AM; Ramasamy R Mechanisms of Transcription Factor Acetylation and Consequences in Hearts. Biochim. Biophys. Acta, Mol. Basis Dis 2016, 1862, 2221–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (455).Wang Z; Yu T; Huang P Post-Translational Modifications of Foxo Family Proteins (Review). Mol. Med. Rep 2016, 14, 4931–4941. [DOI] [PubMed] [Google Scholar]
- (456).Park JM; Jo SH; Kim MY; Kim TH; Ahn YH Role of Transcription Factor Acetylation in the Regulation of Metabolic Homeostasis. Protein Cell 2015, 6, 804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (457).Greene WC; Chen LF Regulation of Nf-Kappab Action by Reversible Acetylation. Novartis Found. Symp 2004, 259, 208–17 (discussion 218-25). [PubMed] [Google Scholar]
- (458).Zhuang S Regulation of Stat Signaling by Acetylation. Cell. Signalling 2013, 25, 1924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (459).Avalle L; Camporeale A; Camperi A; Poli V Stat3 in Cancer: A Double Edged Sword. Cytokine+ 2017, 98, 42. 10.1016/j.cyto.2017.03.018 [DOI] [PubMed] [Google Scholar]
- (460).Braunstein J; Brutsaert S; Olson R; Schindler C Stats Dimerize in the Absence of Phosphorylation. J. Biol. Chem 2003, 278, 34133–40. [DOI] [PubMed] [Google Scholar]
- (461).Kumar A; Commane M; Flickinger TW; Horvath CM; Stark GR Defective Tnf-Alpha-Induced Apoptosis in Stat1-Null Cells Due to Low Constitutive Levels of Caspases. Science 1997, 278, 1630–2. [DOI] [PubMed] [Google Scholar]
- (462).Xu YS; Liang JJ; Wang Y; Zhao XJ; Xu L; Xu YY; Zou QC; Zhang JM; Tu CE; Cui YG; Sun WH; Huang C; Yang JH; Chin YE Stat3 Undergoes Acetylation-Dependent Mitochondrial Translocation to Regulate Pyruvate Metabolism. Sci. Rep 2016, 6, 39517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (463).Pejanovic N; Hochrainer K; Liu T; Aerne BL; Soares MP; Anrather J Regulation of Nuclear Factor Kappab (Nf-Kappab) Transcriptional Activity Via P65 Acetylation by the Chaperonin Containing Tcp1 (Cct). PLoS One 2012, 7, e42020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (464).Caron C; Boyault C; Khochbin S Regulatory Cross-Talk between Lysine Acetylation and Ubiquitination: Role in the Control of Protein Stability. BioEssays 2005, 27, 408–15. [DOI] [PubMed] [Google Scholar]
- (465).Goel A; Janknecht R Acetylation-Mediated Transcriptional Activation of the Ets Protein Er81 by P300, P/Caf, and Her2/Neu. Mol. Cell. Biol 2003, 23, 6243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (466).Brooks CL; Gu W The Impact of Acetylation and Deacetylation on the P53 Pathway. Protein Cell 2011, 2, 456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (467).Rodriguez MS; Desterro JM; Lain S; Lane DP; Hay RT Multiple C-Terminal Lysine Residues Target P53 for Ubiquitin-Proteasome-Mediated Degradation. Mol. Cell. Biol 2000, 20, 8458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (468).Luo J; Su F; Chen D; Shiloh A; Gu W Deacetylation of P53 Modulates Its Effect on Cell Growth and Apoptosis. Nature 2000, 408, 377–81. [DOI] [PubMed] [Google Scholar]
- (469).Liu X; Wang D; Zhao Y; Tu B; Zheng Z; Wang L; Wang H; Gu W; Roeder RG; Zhu WG Methyltransferase Set7/9 Regulates P53 Activity by Interacting with Sirtuin 1 (Sirt1). Proc. Natl. Acad. Sci. U. S. A 2011, 108, 1925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (470).Huang J; Perez-Burgos L; Placek BJ; Sengupta R; Richter M; Dorsey JA; Kubicek S; Opravil S; Jenuwein T; Berger SL Repression of P53 Activity by Smyd2-Mediated Methylation. Nature 2006, 444, 629–32. [DOI] [PubMed] [Google Scholar]
- (471).Son MJ; Kim WK; Park A; Oh KJ; Kim JH; Han BS; Kim IC; Chi SW; Park SG; Lee SC; Bae KH Set7/9, a Methyltransferase, Regulates the Thermogenic Program During Brown Adipocyte Differentiation through the Modulation of P53 Acetylation. Mol. Cell. Endocrinol 2016, 431, 46–53. [DOI] [PubMed] [Google Scholar]
- (472).Ivanov GS; Ivanova T; Kurash J; Ivanov A; Chuikov S; Gizatullin F; Herrera-Medina EM; Rauscher F 3rd; Reinberg D; Barlev NA Methylation-Acetylation Interplay Activates P53 in Response to DNA Damage. Mol. Cell. Biol 2007, 27, 6756–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (473).Yang XD; Tajkhorshid E; Chen LF Functional Interplay between Acetylation and Methylation of the Rela Subunit of Nf-Kappab. Mol. Cell. Biol 2010, 30, 2170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (474).Barlev NA; Liu L; Chehab NH; Mansfield K; Harris KG; Halazonetis TD; Berger SL Acetylation of P53 Activates Transcription through Recruitment of Coactivators/Histone Acetyl-transferases. Mol. Cell 2001, 8, 1243–54. [DOI] [PubMed] [Google Scholar]
- (475).Li AG; Piluso LG; Cai X; Gadd BJ; Ladurner AG; Liu X An Acetylation Switch in P53 Mediates Holo-Tfiid Recruitment. Mol. Cell 2007, 28, 408–21. [DOI] [PubMed] [Google Scholar]
- (476).Wu Y; Lin JC; Piluso LG; Dhahbi JM; Bobadilla S; Spindler SR; Liu X Phosphorylation of P53 by Taf1 Inactivates P53-Dependent Transcription in the DNA Damage Response. Mol. Cell 2014, 53, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (477).Daitoku H; Sakamaki J; Fukamizu A Regulation of Foxo Transcription Factors by Acetylation and Protein-Protein Interactions. Biochim. Biophys. Acta, Mol. Cell Res 2011, 1813, 1954–60. [DOI] [PubMed] [Google Scholar]
- (478).Sakaguchi S; Miyara M; Costantino CM; Hafler DA Foxp3+ Regulatory T Cells in the Human Immune System. Nat. Rev. Immunol 2010, 10, 490–500. [DOI] [PubMed] [Google Scholar]
- (479).van Loosdregt J; Brunen D; Fleskens V; Pals CE; Lam EW; Coffer PJ Rapid Temporal Control of Foxp3 Protein Degradation by Sirtuin-1. PLoS One 2011, 6, e19047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (480).Samanta A; Li B; Song X; Bembas K; Zhang G; Katsumata M; Saouaf SJ; Wang Q; Hancock WW; Shen Y; Greene MI Tgf-Beta and Il-6 Signals Modulate Chromatin Binding and Promoter Occupancy by Acetylated Foxp3. Proc. Natl. Acad. Sci. U. S. A 2008, 105, 14023–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (481).Bieniossek C; Papai G; Schaffitzel C; Garzoni F; Chaillet M; Scheer E; Papadopoulos P; Tora L; Schultz P; Berger I The Architecture of Human General Transcription Factor Tfiid Core Complex. Nature 2013, 493, 699–702. [DOI] [PubMed] [Google Scholar]
- (482).Muth V; Nadaud S; Grummt I; Voit R Acetylation of Taf(I)68, a Subunit of Tif-Ib/Sl1, Activates Rna Polymerase I Transcription. EMBO J. 2001, 20, 1353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (483).Boija A; Mahat DB; Zare A; Holmqvist PH; Philip P; Meyers DJ; Cole PA; Lis JT; Stenberg P; Mannervik M Cbp Regulates Recruitment and Release of Promoter-Proximal Rna Polymerase Ii. Mol. Cell 2017, 68, 491–503 (e5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (484).Hsin JP; Manley JL The Rna Polymerase Ii Ctd Coordinates Transcription and Rna Processing. Genes Dev. 2012, 26, 2119–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (485).Meinhart A; Kamenski T; Hoeppner S; Baumli S; Cramer P A Structural Perspective of Ctd Function. Genes Dev. 2005, 19, 1401–15. [DOI] [PubMed] [Google Scholar]
- (486).Zhou Q; Li T; Price DH Rna Polymerase Ii Elongation Control. Annu. Rev. Biochem 2012, 81, 119–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (487).McNamara RP; Bacon CW; D’Orso I Transcription Elongation Control by the 7sk Snrnp Complex: Releasing the Pause. Cell Cycle 2016, 15, 2115–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (488).Cho S; Schroeder S; Ott M Cycling through Transcription: Posttranslational Modifications of P-Tefb Regulate Transcription Elongation. Cell Cycle 2010, 9, 1697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (489).L’Hernault SW; Rosenbaum JL Chlamydomonas Alpha-Tubulin Is Posttranslationally Modified by Acetylation on the Epsilon-Amino Group of a Lysine. Biochemistry 1985, 24, 473–8. [DOI] [PubMed] [Google Scholar]
- (490).Howes SC; Alushin GM; Shida T; Nachury MV; Nogales E Effects of Tubulin Acetylation and Tubulin Acetyltransferase Binding on Microtubule Structure. Mol. Biol. Cell 2014, 25, 257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (491).Tilney LG; Bryan J; Bush DJ; Fujiwara K; Mooseker MS; Murphy DB; Snyder DH Microtubules: Evidence for 13 Protofilaments. J. Cell Biol 1973, 59, 267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (492).Burton PR; Hinkley RE; Pierson GB Tannic Acid-Stained Microtubules with 12, 13, and 15 Protofilaments. J. Cell Biol 1975, 65, 227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (493).Shida T; Cueva JG; Xu Z; Goodman MB; Nachury MV The Major Alpha-Tubulin K40 Acetyltransferase Alphatat1 Promotes Rapid Ciliogenesis and Efficient Mechanosensation. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 21517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (494).Akella JS; Wloga D; Kim J; Starostina NG; Lyons-Abbott S; Morrissette NS; Dougan ST; Kipreos ET; Gaertig J Mec-17 Is an Alpha-Tubulin Acetyltransferase. Nature 2010, 467, 218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (495).Kalebic N; Sorrentino S; Perlas E; Bolasco G; Martinez C; Heppenstall PA Alphatat1 Is the Major Alpha-Tubulin Acetyltransferase in Mice. Nat. Commun 2013, 4, 1962. [DOI] [PubMed] [Google Scholar]
- (496).Misawa T; Takahama M; Kozaki T; Lee H; Zou J; Saitoh T; Akira S Microtubule-Driven Spatial Arrangement of Mitochondria Promotes Activation of the Nlrp3 Inflammasome. Nat. Immunol 2013, 14, 454–60. [DOI] [PubMed] [Google Scholar]
- (497).Nagai T; Ikeda M; Chiba S; Kanno S; Mizuno K Furry Promotes Acetylation of Microtubules in the Mitotic Spindle by Inhibition of Sirt2 Tubulin Deacetylase. J. Cell Sci 2013, 126, 4369–80. [DOI] [PubMed] [Google Scholar]
- (498).Kramer OH; Mahboobi S; Sellmer A Drugging the Hdac6-Hsp90 Interplay in Malignant Cells. Trends Pharmacol. Sci 2014, 35, 501–9. [DOI] [PubMed] [Google Scholar]
- (499).Scroggins BT; Robzyk K; Wang D; Marcu MG; Tsutsumi S; Beebe K; Cotter RJ; Felts S; Toft D; Karnitz L; Rosen N; Neckers L An Acetylation Site in the Middle Domain of Hsp90 Regulates Chaperone Function. Mol. Cell 2007, 25, 151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (500).Kovacs JJ; Murphy PJ; Gaillard S; Zhao X; Wu JT; Nicchitta CV; Yoshida M; Toft DO; Pratt WB; Yao TP Hdac6 Regulates Hsp90 Acetylation and Chaperone-Dependent Activation of Glucocorticoid Receptor. Mol. Cell 2005, 18, 601–7. [DOI] [PubMed] [Google Scholar]
- (501).Jimenez-Canino R; Lorenzo-Diaz F; Jaisser F; Farman N; Giraldez T; Alvarez de la Rosa D Histone Deacetylase 6-Controlled Hsp90 Acetylation Significantly Alters Mineralocorticoid Receptor Subcellular Dynamics but Not Its Transcriptional Activity. Endocrinology 2016, 157, 2515–32. [DOI] [PubMed] [Google Scholar]
- (502).Nishioka C; Ikezoe T; Yang J; Takeuchi S; Koeffler HP; Yokoyama A Ms-275, a Novel Histone Deacetylase Inhibitor with Selectivity against Hdac1, Induces Degradation of Flt3 Via Inhibition of Chaperone Function of Heat Shock Protein 90 in Aml Cells. Leuk. Res 2008, 32, 1382–92. [DOI] [PubMed] [Google Scholar]
- (503).Weingarten MD; Lockwood AH; Hwo SY; Kirschner MW A Protein Factor Essential for Microtubule Assembly. Proc. Natl. Acad. Sci. U. S. A 1975, 72, 1858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (504).Witman GB; Cleveland DW; Weingarten MD; Kirschner MW Tubulin Requires Tau for Growth onto Microtubule Initiating Sites. Proc. Natl. Acad. Sci. U. S. A 1976, 73, 4070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (505).Wang Y; Mandelkow E Tau in Physiology and Pathology. Nat. Rev. Neurosci 2016, 17, 22–35. [DOI] [PubMed] [Google Scholar]
- (506).Goedert M; Jakes R Mutations Causing Neurodegenerative Tauopathies. Biochim. Biophys. Acta, Mol. Basis Dis 2005, 1739, 240–50. [DOI] [PubMed] [Google Scholar]
- (507).Cohen TJ; Guo JL; Hurtado DE; Kwong LK; Mills IP; Trojanowski JQ; Lee VM The Acetylation of Tau Inhibits Its Function and Promotes Pathological Tau Aggregation. Nat. Commun 2011, 2, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (508).Min SW; Cho SH; Zhou Y; Schroeder S; Haroutunian V; Seeley WW; Huang EJ; Shen Y; Masliah E; Mukherjee C; Meyers D; Cole PA; Ott M; Gan L Acetylation of Tau Inhibits Its Degradation and Contributes to Tauopathy. Neuron 2010, 67, 953–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (509).Morris M; Knudsen GM; Maeda S; Trinidad JC; Ioanoviciu A; Burlingame AL; Mucke L Tau Post-Translational Modifications in Wild-Type and Human Amyloid Precursor Protein Transgenic Mice. Nat. Neurosci 2015, 18, 1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (510).Hwang AW; Trzeciakiewicz H; Friedmann D; Yuan CX; Marmorstein R; Lee VM; Cohen TJ Conserved Lysine Acetylation within the Microtubule-Binding Domain Regulates Map2/Tau Family Members. PLoS One 2016, 11, e0168913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (511).Cohen TJ; Constance BH; Hwang AW; James M; Yuan CX Intrinsic Tau Acetylation Is Coupled to Auto-Proteolytic Tau Fragmentation. PLoS One 2016, 11, e0158470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (512).Cohen TJ; Friedmann D; Hwang AW; Marmorstein R; Lee VM The Microtubule-Associated Tau Protein Has Intrinsic Acetyltransferase Activity. Nat. Struct. Mol. Biol 2013, 20, 756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (513).Noack M; Leyk J; Richter-Landsberg C Hdac6 Inhibition Results in Tau Acetylation and Modulates Tau Phosphorylation and Degradation in Oligodendrocytes. Glia 2014, 62, 535–47. [DOI] [PubMed] [Google Scholar]
- (514).Xiong Y; Zhao K; Wu J; Xu Z; Jin S; Zhang YQ Hdac6Mutations Rescue Human Tau-Induced Microtubule Defects in Drosophila. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 4604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (515).Tracy TE; Gan L Acetylated Tau in Alzheimer’s Disease: An Instigator of Synaptic Dysfunction Underlying Memory Loss: Increased Levels of Acetylated Tau Blocks the Postsynaptic Signaling Required for Plasticity and Promotes Memory Deficits Associated with Tauopathy. BioEssays 2017, 39, 1600224. 10.1002/bies.201600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (516).Sohn PD; Tracy TE; Son HI; Zhou Y; Leite RE; Miller BL; Seeley WW; Grinberg LT; Gan L Acetylated Tau Destabilizes the Cytoskeleton in the Axon Initial Segment and Is Mislocalized to the Somatodendritic Compartment. Mol. Neurodegener 2016, 11, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (517).Tracy TE; Sohn PD; Minami SS; Wang C; Min SW; Li Y; Zhou Y; Le D; Lo I; Ponnusamy R; Cong X; Schilling B; Ellerby LM; Huganir RL; Gan L Acetylated Tau Obstructs Kibra-Mediated Signaling in Synaptic Plasticity and Promotes Tauopathy-Related Memory Loss. Neuron 2016, 90, 245–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (518).Cook C; Carlomagno Y; Gendron TF; Dunmore J; Scheffel K; Stetler C; Davis M; Dickson D; Jarpe M; DeTure M; Petrucelli L Acetylation of the Kxgs Motifs in Tau Is a Critical Determinant in Modulation of Tau Aggregation and Clearance. Hum. Mol. Genet 2014, 23, 104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (519).Thomas SN; Funk KE; Wan Y; Liao Z; Davies P; Kuret J; Yang AJ Dual Modification of Alzheimer’s Disease Phf-Tau Protein by Lysine Methylation and Ubiquitylation: A Mass Spectrometry Approach. Acta Neuropathol. 2012, 123, 105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (520).Funk KE; Thomas SN; Schafer KN; Cooper GL; Liao Z; Clark DJ; Yang AJ; Kuret J Lysine Methylation Is an Endogenous Post-Translational Modification of Tau Protein in Human Brain and a Modulator of Aggregation Propensity. Biochem. J 2014, 462, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (521).Anderson KA; Hirschey MD Mitochondrial Protein Acetylation Regulates Metabolism. Essays Biochem. 2012, 52, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (522).Weinert BT; Wagner SA; Horn H; Henriksen P; Liu WR; Olsen JV; Jensen LJ; Choudhary C Proteome-Wide Mapping of the Drosophila Acetylome Demonstrates a High Degree of Conservation of Lysine Acetylation. Sci. Signaling 2011, 4, ra48. [DOI] [PubMed] [Google Scholar]
- (523).Wang Q; Zhang Y; Yang C; Xiong H; Lin Y; Yao J; Li H; Xie L; Zhao W; Yao Y; Ning Z-B; Zeng R; Xiong Y; Guan K-L; Zhao S; Zhao G-P Acetylation of Metabolic Enzymes Coordinates Carbon Source Utilization and Metabolic Flux. Science 2010, 327, 1004–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (524).Zhao S; Xu W; Jiang W; Yu W; Lin Y; Zhang T; Yao J; Zhou L; Zeng Y; Li H; Li Y; Shi J; An W; Hancock SM; He F; Qin L; Chin J; Yang P; Chen X; Lei Q; Xiong Y; Guan K-L Regulation of Cellular Metabolism by Protein Lysine Acetylation. Science 2010, 327, 1000–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (525).Berg JM; Tymoczko JL; Stryer L Biochemistry, 7th ed.; W.H. Freeman: New York, 2012; pp xxxii, 1054; 132 pp. [Google Scholar]
- (526).He W; Newman JC; Wang MZ; Ho L; Verdin E Mitochondrial Sirtuins: Regulators of Protein Acylation and Metabolism. Trends Endocrinol. Metab 2012, 23, 467–76. [DOI] [PubMed] [Google Scholar]
- (527).Lombard DB; Alt FW; Cheng HL; Bunkenborg J; Streeper RS; Mostoslavsky R; Kim J; Yancopoulos G; Valenzuela D; Murphy A; Yang Y; Chen Y; Hirschey MD; Bronson RT; Haigis M; Guarente LP; Farese RV; Weissman S; Verdin E; Schwer B Mammalian Sir2 Homolog Sirt3 Regulates Global Mitochondrial Lysine Acetylation. Mol. Cell. Biol 2007, 27, 8807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (528).Lin SJ; Defossez PA; Guarente L Requirement of Nad and Sir2 for Life-Span Extension by Calorie Restriction in Saccharomyces Cerevisiae. Science 2000, 289, 2126–8. [DOI] [PubMed] [Google Scholar]
- (529).Lin SJ; Kaeberlein M; Andalis AA; Sturtz LA; Defossez PA; Culotta VC; Fink GR; Guarente L Calorie Restriction Extends Saccharomyces Cerevisiae Lifespan by Increasing Respiration. Nature 2002, 418, 344–8. [DOI] [PubMed] [Google Scholar]
- (530).Lin SJ; Ford E; Haigis M; Liszt G; Guarente L Calorie Restriction Extends Yeast Life Span by Lowering the Level of Nadh. Genes Dev 2004, 18, 12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (531).Bitterman KJ; Anderson RM; Cohen HY; Latorre-Esteves M; Sinclair DA Inhibition of Silencing and Accelerated Aging by Nicotinamide, a Putative Negative Regulator of Yeast Sir2 and Human Sirt1. J. Biol. Chem 2002, 277, 45099–107. [DOI] [PubMed] [Google Scholar]
- (532).Anderson RM; Bitterman KJ; Wood JG; Medvedik O; Sinclair DA Nicotinamide and Pnc1 Govern Lifespan Extension by Calorie Restriction in Saccharomyces Cerevisiae. Nature 2003, 423, 181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (533).Palacios OM; Carmona JJ; Michan S; Chen KY; Manabe Y; Iii JL; Goodyear LJ; Tong Q Diet and Exercise Signals Regulate Sirt3 and Activate Ampk and Pgc-1alpha in Skeletal Muscle. Aging 2009, 1, 771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (534).Ahn BH; Kim HS; Song S; Lee IH; Liu J; Vassilopoulos A; Deng CX; Finkel T A Role for the Mitochondrial Deacetylase Sirt3 in Regulating Energy Homeostasis. Proc. Natl. Acad. Sci. U. S. A 2008, 105, 14447–14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (535).Hirschey M; Shimazu T; Goetzman E; Jing E; Schwer B; Lombard D; Grueter C; Harris C; Biddinger S; Ilkayeva O; Stevens R; Li Y; Saha A; Ruderman N; Bain J; Newgard C; Farese R Jr.; Alt F; Kahn C; Verdin E Sirt3 Regulates Mitochondrial Fatty Acid Oxidation Via Reversible Enzyme Deacetylation. Nature 2010, 464, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (536).Caton PW; Holness MJ; Bishop-Bailey D; Sugden MC Pparalpha-Lxr as a Novel Metabolostatic Signalling Axis in Skeletal Muscle That Acts to Optimize Substrate Selection in Response to Nutrient Status. Biochem. J 2011, 437, 521–30. [DOI] [PubMed] [Google Scholar]
- (537).Hallows WC; Yu W; Smith BC; Devries MK; Ellinger JJ; Someya S; Shortreed MR; Prolla T; Markley JL; Smith LM; Zhao S; Guan KL; Denu JM Sirt3 Promotes the Urea Cycle and Fatty Acid Oxidation During Dietary Restriction. Mol. Cell 2011, 41, 139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (538).Tauriainen E; Luostarinen M; Martonen E; Finckenberg P; Kovalainen M; Huotari A; Herzig KH; Lecklin A; Mervaala E Distinct Effects of Calorie Restriction and Resveratrol on Diet-Induced Obesity and Fatty Liver Formation. J. Nutr. Metab 2011, 2011, 525094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (539).Alhazzazi TY; Kamarajan P; Joo N; Huang JY; Verdin E; D’Silva NJ; Kapila YL Sirtuin-3 (Sirt3), a Novel Potential Therapeutic Target for Oral Cancer. Cancer 2011, 117, 1670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (540).Garland PB; Shepherd D; Yates DW Steady-State Concentrations of Coenzyme a, Acetyl-Coenzyme a and Long-Chain Fatty Acyl-Coenzyme a in Rat-Liver Mitochondria Oxidizing Palmitate. Biochem. J 1965, 97, 587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (541).Paik WK; Pearson D; Lee HW; Kim S Nonenzymatic Acetylation of Histones with Acetyl-Coa. Biochim. Biophys. Acta, Nucleic Acids Protein Synth. 1970, 213, 513–22. [DOI] [PubMed] [Google Scholar]
- (542).Schwer B; Eckersdorff M; Li Y; Silva J; Fermin D; Kurtev M; Giallourakis C; Comb M; Alt F; Lombard D Calorie Restriction Alters Mitochondrial Protein Acetylation. Aging Cell 2009, 8, 604. 10.1111/j.1474-9726.2009.00503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- (543).Hirschey M; Aouizerat B; Jing E; Shimazu T; Grueter C; Collins A; Stevens R; Lam M; Muehlbauer M; Schwer B; Gao B; Bass N; Alt F; Deng C-X; Kakar S; Newgard C; Farese R Jr.; Kahn C; Verdin E Sirt3 Deficiency and Mitochondrial Protein Hyperacetylation Accelerate the Development of the Metabolic Syndrome. Mol. Cell 2011, 44, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (544).Fritz KS; Galligan JJ; Hirschey MD; Verdin E; Petersen DR Mitochondrial Acetylome Analysis in a Mouse Model of Alcohol-Induced Liver Injury Utilizing Sirt3 Knockout Mice. J. Proteome Res 2012, 11, 1633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (545).Picklo MJ Sr. Ethanol Intoxication Increases Hepatic N-Lysyl Protein Acetylation. Biochem. Biophys. Res. Commun 2008, 376, 615–9. [DOI] [PubMed] [Google Scholar]
- (546).Scott I; Webster BR; Chan CK; Okonkwo JU; Han K; Sack MN Gcn5-Like Protein 1 (Gcn5l1) Controls Mitochondrial Content through Coordinated Regulation of Mitochondrial Biogenesis and Mitophagy. J. Biol. Chem 2014, 289, 2864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (547).Fan J; Shan C; Kang HB; Elf S; Xie J; Tucker M; Gu TL; Aguiar M; Lonning S; Chen H; Mohammadi M; Britton LM; Garcia BA; Aleckovic M; Kang Y; Kaluz S; Devi N; Van Meir EG; Hitosugi T; Seo JH; Lonial S; Gaddh M; Arellano M; Khoury HJ; Khuri FR; Boggon TJ; Kang S; Chen J Tyr Phosphorylation of Pdp1 Toggles Recruitment between Acat1 and Sirt3 to Regulate the Pyruvate Dehydrogenase Complex. Mol. Cell 2014, 53, 534–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (548).Jing E; Emanuelli B; Hirschey MD; Boucher J; Lee KY; Lombard D; Verdin EM; Kahn CR Sirtuin-3 (Sirt3) Regulates Skeletal Muscle Metabolism and Insulin Signaling Via Altered Mitochondrial Oxidation and Reactive Oxygen Species Production. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 14608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (549).Finley LW; Carracedo A; Lee J; Souza A; Egia A; Zhang J; Teruya-Feldstein J; Moreira PI; Cardoso SM; Clish CB; Pandolfi PP; Haigis MC Sirt3 Opposes Reprogramming of Cancer Cell Metabolism through Hif1alpha Destabilization. Cancer Cell 2011, 19, 416–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (550).Cimen H; Han MJ; Yang Y; Tong Q; Koc H; Koc EC Regulation of Succinate Dehydrogenase Activity by Sirt3 in Mammalian Mitochondria. Biochemistry 2010, 49, 304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (551).Bao J; Scott I; Lu Z; Pang L; Dimond CC; Gius D; Sack MN Sirt3 Is Regulated by Nutrient Excess and Modulates Hepatic Susceptibility to Lipotoxicity. Free Radical Biol. Med 2010, 49, 1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (552).Hallows W; Lee S; Denu J Sirtuins Deacetylate and Activate Mammalian Acetyl-Coa Synthetases. Proc. Natl. Acad. Sci. U. S. A 2006, 103, 10230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (553).Schwer B; Bunkenborg J; Verdin RO; Andersen JS; Verdin E Reversible Lysine Acetylation Controls the Activity of the Mitochondrial Enzyme Acetyl-Coa Synthetase 2. Proc. Natl. Acad. Sci. U. S. A 2006, 103, 10224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (554).Shimazu T; Hirschey M; Hua L; Dittenhafer-Reed KE; Schwer B; Lombard D; Li Y; Bunkenborg J; Alt FW; Denu JM; Jacobson MP; Verdin E Sirt3 Deacetylates Mitochondrial 3-Hydroxy-3-Methylglutaryl Coa Synthase 2, Increases Its Enzymatic Activity and Regulates Ketone Body Production. Cell Metab 2010, 12, 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (555).Shulga N; Wilson-Smith R; Pastorino JG Sirtuin-3 Deacetylation of Cyclophilin D Induces Dissociation of Hexokinase Ii from the Mitochondria. J. Cell Sci 2010, 123, 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (556).Kim HS; Patel K; Muldoon-Jacobs K; Bisht KS; Aykin-Burns N; Pennington JD; van der Meer R; Nguyen P; Savage J; Owens KM; Vassilopoulos A; Ozden O; Park SH; Singh KK; Abdulkadir SA; Spitz DR; Deng CX; Gius D Sirt3 Is a Mitochondria-Localized Tumor Suppressor Required for Maintenance of Mitochondrial Integrity and Metabolism During Stress. Cancer Cell 2010, 17, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (557).Someya S; Yu W; Hallows WC; Xu J; Vann JM; Leeuwenburgh C; Tanokura M; Denu JM; Prolla TA Sirt3Mediates Reduction of Oxidative Damage and Prevention of Age-Related Hearing Loss under Caloric Restriction. Cell 2010, 143, 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (558).Qiu X; Brown K; Hirschey MD; Verdin E; Chen D Calorie Restriction Reduces Oxidative Stress by Sirt3-Mediated Sod2 Activation. Cell Metab. 2010, 12, 662–667. [DOI] [PubMed] [Google Scholar]
- (559).Tao R; Coleman MC; Pennington JD; Ozden O; Park S-H; Jiang H; Kim H-S; Flynn CR; Hill S; Hayes McDonald W; Olivier AK; Spitz DR; Gius D Sirt3-Mediated Deacetylation of Evolutionarily Conserved Lysine 122 Regulates Mnsod Activity in Response to Stress. Mol. Cell 2010, 40, 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (560).Chen Y; Zhang J; Lin Y; Lei Q; Guan KL; Zhao S; Xiong Y Tumour Suppressor Sirt3 Deacetylates and Activates Manganese Superoxide Dismutase to Scavenge Ros. EMBO Rep. 2011, 12, 534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (561).Candido EP; Reeves R; Davie JR Sodium Butyrate Inhibits Histone Deacetylation in Cultured Cells. Cell 1978, 14, 105–13. [DOI] [PubMed] [Google Scholar]
- (562).Vidali G; Boffa LC; Mann RS; Allfrey VG Reversible Effects of Na-Butyrate on Histone Acetylation. Biochem. Biophys. Res. Commun 1978, 82, 223–7. [DOI] [PubMed] [Google Scholar]
- (563).Yoshida M; Hoshikawa Y; Koseki K; Mori K; Beppu T Structural Specificity for Biological Activity of Trichostatin a, a Specific Inhibitor of Mammalian Cell Cycle with Potent Differentiation-Inducing Activity in Friend Leukemia Cells. J. Antibiot 1990, 43, 1101–6. [DOI] [PubMed] [Google Scholar]
- (564).Yoshida M; Kijima M; Akita M; Beppu T Potent and Specific Inhibition of Mammalian Histone Deacetylase Both in Vivo and in Vitro by Trichostatin A. J. Biol. Chem 1990, 265, 17174–9. [PubMed] [Google Scholar]
- (565).Richon VM; Emiliani S; Verdin E; Webb Y; Breslow R; Rifkind RA; Marks PA A Class of Hybrid Polar Inducers of Transformed Cell Differentiation Inhibits Histone Deacetylases. Proc. Natl. Acad. Sci. U. S. A 1998, 95, 3003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (566).Gottlicher M; Minucci S; Zhu P; Kramer OH; Schimpf A; Giavara S; Sleeman JP; Lo Coco F; Nervi C; Pelicci PG; Heinzel T Valproic Acid Defines a Novel Class of Hdac Inhibitors Inducing Differentiation of Transformed Cells. EMBO J. 2001, 20, 6969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (567).Mottamal M; Zheng S; Huang TL; Wang G Histone Deacetylase Inhibitors in Clinical Studies as Templates for New Anticancer Agents. Molecules 2015, 20, 3898–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (568).Subramanian S; Bates SE; Wright JJ; Espinoza-Delgado I; Piekarz RL Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals 2010, 3, 2751–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (569).Xu WS; Parmigiani RB; Marks PA Histone Deacetylase Inhibitors: Molecular Mechanisms of Action. Oncogene 2007, 26, 5541–52. [DOI] [PubMed] [Google Scholar]
- (570).Takai N; Desmond JC; Kumagai T; Gui D; Said JW; Whittaker S; Miyakawa I; Koeffler HP Histone Deacetylase Inhibitors Have a Profound Antigrowth Activity in Endometrial Cancer Cells. Clin. Cancer Res 2004, 10, 1141–9. [DOI] [PubMed] [Google Scholar]
- (571).Gui CY; Ngo L; Xu WS; Richon VM; Marks PA Histone Deacetylase (Hdac) Inhibitor Activation of P21waf1 Involves Changes in Promoter-Associated Proteins, Including Hdac1. Proc. Natl. Acad. Sci. U. S. A 2004, 101, 1241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (572).Lusic M; Marcello A; Cereseto A; Giacca M Regulation of Hiv-1 Gene Expression by Histone Acetylation and Factor Recruitment at the Ltr Promoter. EMBO J. 2003, 22, 6550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (573).Falkenberg KJ; Johnstone RW Histone Deacetylases and Their Inhibitors in Cancer, Neurological Diseases and Immune Disorders. Nat. Rev. Drug Discovery 2014, 13, 673–91. [DOI] [PubMed] [Google Scholar]
- (574).Butler KV; Kalin J; Brochier C; Vistoli G; Langley B; Kozikowski AP Rational Design and Simple Chemistry Yield a Superior, Neuroprotective Hdac6 Inhibitor, Tubastatin A. J. Am. Chem. Soc 2010, 132, 10842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (575).Balasubramanian S; Ramos J; Luo W; Sirisawad M; Verner E; Buggy JJ A Novel Histone Deacetylase 8 (Hdac8)-Specific Inhibitor Pci-34051 Induces Apoptosis in T-Cell Lymphomas. Leukemia 2008, 22, 1026–34. [DOI] [PubMed] [Google Scholar]
- (576).Kaeberlein M; McVey M; Guarente L The Sir2/3/4 Complex and Sir2 Alone Promote Longevity in Saccharomyces Cerevisiae by Two Different Mechanisms. Genes Dev. 1999, 13, 2570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (577).Howitz KT; Bitterman KJ; Cohen HY; Lamming DW; Lavu S; Wood JG; Zipkin RE; Chung P; Kisielewski A; Zhang LL; Scherer B; Sinclair DA Small Molecule Activators of Sirtuins Extend Saccharomyces Cerevisiae Lifespan. Nature 2003, 425, 191–6. [DOI] [PubMed] [Google Scholar]
- (578).Wood JG; Rogina B; Lavu S; Howitz K; Helfand SL; Tatar M; Sinclair D Sirtuin Activators Mimic Caloric Restriction and Delay Ageing in Metazoans. Nature 2004, 430, 686–9. [DOI] [PubMed] [Google Scholar]
- (579).Walle T; Hsieh F; DeLegge MH; Oatis JE Jr.; Walle UK High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos 2004, 32, 1377–82. [DOI] [PubMed] [Google Scholar]
- (580).Erdogan CS; Morup-Lendal M; Dalgaard LT; Vang O Sirtuin 1 Independent Effects of Resveratrol in Ins-1e Beta-Cells. Chem.-Biol. Interact 2017, 264, 52–60. [DOI] [PubMed] [Google Scholar]
- (581).Mercken EM; Mitchell SJ; Martin-Montalvo A; Minor RK; Almeida M; Gomes AP; Scheibye-Knudsen M; Palacios HH; Licata JJ; Zhang Y; Becker KG; Khraiwesh H; Gonzalez-Reyes JA; Villalba JM; Baur JA; Elliott P; Westphal C; Vlasuk GP; Ellis JL; Sinclair DA; Bernier M; de Cabo R Srt2104 Extends Survival of Male Mice on a Standard Diet and Preserves Bone and Muscle Mass. Aging Cell 2014, 13, 787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (582).Mitchell SJ; Martin-Montalvo A; Mercken EM; Palacios HH; Ward TM; Abulwerdi G; Minor RK; Vlasuk GP; Ellis JL; Sinclair DA; Dawson J; Allison DB; Zhang Y; Becker KG; Bernier M; de Cabo R The Sirt1 Activator Srt1720 Extends Lifespan and Improves Health of Mice Fed a Standard Diet. Cell Rep. 2014, 6, 836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (583).Chauhan D; Bandi M; Singh AV; Ray A; Raje N; Richardson P; Anderson KC Preclinical Evaluation of a Novel Sirt1Modulator Srt1720 in Multiple Myeloma Cells. Br. J. Haematol 2011, 155, 588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (584).Beher D; Wu J; Cumine S; Kim KW; Lu SC; Atangan L; Wang M Resveratrol Is Not a Direct Activator of Sirt1 Enzyme Activity. Chem. Biol. Drug Des 2009, 74, 619–24. [DOI] [PubMed] [Google Scholar]
- (585).Pacholec M; Bleasdale JE; Chrunyk B; Cunningham D; Flynn D; Garofalo RS; Griffith D; Griffor M; Loulakis P; Pabst B; Qiu X; Stockman B; Thanabal V; Varghese A; Ward J; Withka J; Ahn K Srt1720, Srt2183, Srt1460, and Resveratrol Are Not Direct Activators of Sirt1. J. Biol. Chem 2010, 285, 8340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (586).Hubbard BP; Gomes AP; Dai H; Li J; Case AW; Considine T; Riera TV; Lee JE; E SY; Lamming DW; Pentelute BL; Schuman ER; Stevens LA; Ling AJ; Armour SM; Michan S; Zhao H; Jiang Y; Sweitzer SM; Blum CA; Disch JS; Ng PY; Howitz KT; Rolo AP; Hamuro Y; Moss J; Perni RB; Ellis JL; Vlasuk GP; Sinclair DA Evidence for a Common Mechanism of Sirt1 Regulation by Allosteric Activators. Science 2013, 339, 1216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (587).Lakshminarasimhan M; Rauh D; Schutkowski M; Steegborn C Sirt1 Activation by Resveratrol Is Substrate Sequence-Selective. Aging 2013, 5, 151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (588).Napper AD; Hixon J; McDonagh T; Keavey K; Pons JF; Barker J; Yau WT; Amouzegh P; Flegg A; Hamelin E; Thomas RJ; Kates M; Jones S; Navia MA; Saunders JO; DiStefano PS; Curtis R Discovery of Indoles as Potent and Selective Inhibitors of the Deacetylase Sirt1. J. Med. Chem 2005, 48, 8045–54. [DOI] [PubMed] [Google Scholar]
- (589).Mai A; Massa S; Lavu S; Pezzi R; Simeoni S; Ragno R; Mariotti FR; Chiani F; Camilloni G; Sinclair DA Design, Synthesis, and Biological Evaluation of Sirtinol Analogues as Class Iii Histone/Protein Deacetylase (Sirtuin) Inhibitors. J. Med. Chem 2005, 48, 7789–95. [DOI] [PubMed] [Google Scholar]
- (590).McCarthy AR; Pirrie L; Hollick JJ; Ronseaux S; Campbell J; Higgins M; Staples OD; Tran F; Slawin AM; Lain S; Westwood NJ Synthesis and Biological Characterisation of Sirtuin Inhibitors Based on the Tenovins. Bioorg. Med. Chem 2012, 20, 1779–93. [DOI] [PubMed] [Google Scholar]
- (591).He X; Nie H; Hong Y; Sheng C; Xia W; Ying W Sirt2 Activity Is Required for the Survival of C6 Glioma Cells. Biochem. Biophys. Res. Commun 2012, 417, 468–72. [DOI] [PubMed] [Google Scholar]
- (592).Luthi-Carter R; Taylor DM; Pallos J; Lambert E; Amore A; Parker A; Moffitt H; Smith DL; Runne H; Gokce O; Kuhn A; Xiang Z; Maxwell MM; Reeves SA; Bates GP; Neri C; Thompson LM; Marsh JL; Kazantsev AG Sirt2 Inhibition Achieves Neuroprotection by Decreasing Sterol Biosynthesis. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 7927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (593).Riepsamen A; Wu L; Lau L; Listijono D; Ledger W; Sinclair DA; Homer H Correction: Nicotinamide Impairs Entry into and Exit from Meiosis I in Mouse Oocytes. PLoS One 2015, 10, e0130058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (594).Rumpf T; Schiedel M; Karaman B; Roessler C; North BJ; Lehotzky A; Olah J; Ladwein KI; Schmidtkunz K; Gajer M; Pannek M; Steegborn C; Sinclair DA; Gerhardt S; Ovadi J; Schutkowski M; Sippl W; Einsle O; Jung M Selective Sirt2 Inhibition by Ligand-Induced Rearrangement of the Active Site. Nat. Commun 2015, 6, 6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (595).Erburu M; Munoz-Cobo I; Diaz-Perdigon T; Mellini P; Suzuki T; Puerta E; Tordera RM Sirt2 Inhibition Modulate Glutamate and Serotonin Systems in the Prefrontal Cortex and Induces Antidepressant-Like Action. Neuropharmacology 2017, 117, 195–208. [DOI] [PubMed] [Google Scholar]
- (596).Heltweg B; Gatbonton T; Schuler AD; Posakony J; Li H; Goehle S; Kollipara R; Depinho RA; Gu Y; Simon JA; Bedalov A Antitumor Activity of a Small-Molecule Inhibitor of Human Silent Information Regulator 2 Enzymes. Cancer Res. 2006, 66, 4368–77. [DOI] [PubMed] [Google Scholar]
- (597).Lancelot J; Caby S; Dubois-Abdesselem F; Vanderstraete M; Trolet J; Oliveira G; Bracher F; Jung M; Pierce RJ Schistosoma Mansoni Sirtuins: Characterization and Potential as Chemotherapeutic Targets. PLoS Neglected Trop. Dis 2013, 7, e2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (598).Lara E; Mai A; Calvanese V; Altucci L; Lopez-Nieva P; Martinez-Chantar ML; Varela-Rey M; Rotili D; Nebbioso A; Ropero S; Montoya G; Oyarzabal J; Velasco S; Serrano M; Witt M; Villar-Garea A; Imhof A; Mato JM; Esteller M; Fraga MF Salermide, a Sirtuin Inhibitor with a Strong Cancer-Specific Proapoptotic Effect. Oncogene 2009, 28, 781–91. [DOI] [PubMed] [Google Scholar]
- (599).Cui H; Kamal Z; Ai T; Xu Y; More SS; Wilson DJ; Chen L Discovery of Potent and Selective Sirtuin 2 (Sirt2) Inhibitors Using a Fragment-Based Approach. J. Med. Chem 2014, 57, 8340–57. [DOI] [PubMed] [Google Scholar]
- (600).Salo HS; Laitinen T; Poso A; Jarho E; Lahtela-Kakkonen M Identification of Novel Sirt3 Inhibitor Scaffolds by Virtual Screening. Bioorg. Med. Chem. Lett 2013, 23, 2990–5. [DOI] [PubMed] [Google Scholar]
- (601).Alhazzazi TY; Kamarajan P; Xu Y; Ai T; Chen L; Verdin E; Kapila YL A Novel Sirtuin-3 Inhibitor, Lc-0296, Inhibits Cell Survival and Proliferation, and Promotes Apoptosis of Head and Neck Cancer Cells. Anticancer Res 2016, 36, 49–60. [PMC free article] [PubMed] [Google Scholar]
- (602).Dekker FJ; Haisma HJ Histone Acetyl Transferases as Emerging Drug Targets. Drug Discovery Today 2009, 14, 942–8. [DOI] [PubMed] [Google Scholar]
- (603).Marmorstein R Structure and Function of Histone Acetyltransferases. Cell. Mol. Life Sci 2001, 58, 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (604).Marcu MG; Jung YJ; Lee S; Chung EJ; Lee MJ; Trepel J; Neckers L Curcumin Is an Inhibitor of P300 Histone Acetylatransferase. Med. Chem 2006, 2, 169–74. [DOI] [PubMed] [Google Scholar]
- (605).Arif M; Pradhan SK; Thanuja GR; Vedamurthy BM; Agrawal S; Dasgupta D; Kundu TK Mechanism of P300 Specific Histone Acetyltransferase Inhibition by Small Molecules. J. Med. Chem 2009, 52, 267–77. [DOI] [PubMed] [Google Scholar]
- (606).Sun Y; Jiang X; Chen S; Price BD Inhibition of Histone Acetyltransferase Activity by Anacardic Acid Sensitizes Tumor Cells to Ionizing Radiation. FEBS Lett 2006, 580, 4353–6. [DOI] [PubMed] [Google Scholar]
- (607).Lau OD; Kundu TK; Soccio RE; Ait-Si-Ali S; Khalil EM; Vassilev A; Wolffe AP; Nakatani Y; Roeder RG; Cole PA Hats Off: Selective Synthetic Inhibitors of the Histone Acetyltrans-ferases P300 and Pcaf. Mol. Cell 2000, 5, 589–95. [DOI] [PubMed] [Google Scholar]
- (608).Gao XN; Lin J; Ning QY; Gao L; Yao YS; Zhou JH; Li YH; Wang LL; Yu L A Histone Acetyltransferase P300 Inhibitor C646 Induces Cell Cycle Arrest and Apoptosis Selectively in Aml1-Eto-Positive Aml Cells. PLoS One 2013, 8, e55481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (609).Lasko LM; Jakob CG; Edalji RP; Qiu W; Montgomery D; Digiammarino EL; Hansen TM; Risi RM; Frey R; Manaves V; Shaw B; Algire M; Hessler P; Lam LT; Uziel T; Faivre E; Ferguson D; Buchanan FG; Martin RL; Torrent M; Chiang GG; Karukurichi K; Langston JW; Weinert BT; Choudhary C; de Vries P; Van Drie JH; McElligott D; Kesicki E; Marmorstein R; Sun C; Cole PA; Rosenberg SH; Michaelides MR; Lai A; Bromberg KD Discovery of a Selective Catalytic P300/Cbp Inhibitor That Targets Lineage-Specific Tumours. Nature 2017, 550, 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (610).Montgomery DC; Sorum AW; Guasch L; Nicklaus MC; Meier JL Metabolic Regulation of Histone Acetyltransferases by Endogenous Acyl-Coa Cofactors. Chem. Biol 2015, 22, 1030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (611).Feldman JL; Baeza J; Denu JM Activation of the Protein Deacetylase Sirt6 by Long-Chain Fatty Acids and Widespread Deacylation by Mammalian Sirtuins. J. Biol. Chem 2013, 288, 31350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (612).Shirakawa K; Wang L; Man N; Maksimoska J; Sorum AW; Lim HW; Lee IS; Shimazu T; Newman JC; Schroder S; Ott M; Marmorstein R; Meier J; Nimer S; Verdin E Salicylate, Diflunisal and Their Metabolites Inhibit Cbp/P300 and Exhibit Anticancer Activity. eLife 2016, 5, 10.7554/eLife.11156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (613).Min SW; Chen X; Tracy TE; Li Y; Zhou Y; Wang C; Shirakawa K; Minami SS; Defensor E; Mok SA; Sohn PD; Schilling B; Cong X; Ellerby L; Gibson BW; Johnson J; Krogan N; Shamloo M; Gestwicki J; Masliah E; Verdin E; Gan L Critical Role of Acetylation in Tau-Mediated Neurodegeneration and Cognitive Deficits. Nat. Med 2015, 21, 1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (614).Zeng L; Li J; Muller M; Yan S; Mujtaba S; Pan C; Wang Z; Zhou MM Selective Small Molecules Blocking Hiv-1 Tat and Coactivator Pcaf Association. J. Am. Chem. Soc 2005, 127, 2376–7. [DOI] [PubMed] [Google Scholar]
- (615).Mujtaba S; He Y; Zeng L; Farooq A; Carlson JE; Ott M; Verdin E; Zhou MM Structural Basis of Lysine-Acetylated Hiv-1 Tat Recognition by Pcaf Bromodomain. Mol. Cell 2002, 9, 575–86. [DOI] [PubMed] [Google Scholar]
- (616).Dorr A; Kiermer V; Pedal A; Rackwitz HR; Henklein P; Schubert U; Zhou MM; Verdin E; Ott M Transcriptional Synergy between Tat and Pcaf Is Dependent on the Binding of Acetylated Tat to the Pcaf Bromodomain. EMBO J 2002, 21, 2715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (617).Nishiyama A; Mochizuki K; Mueller F; Karpova T; McNally JG; Ozato K Intracellular Delivery of Acetyl-Histone Peptides Inhibits Native Bromodomain-Chromatin Interactions and Impairs Mitotic Progression. FEBS Lett 2008, 582, 1501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (618).Miyoshi S, Ooike S, Iwata K, Hikawa H, Sugaraha K Antitumor Agent. Patent WO2009084693A1, 2009. [Google Scholar]
- (619).Filippakopoulos P; Qi J; Picaud S; Shen Y; Smith WB; Fedorov O; Morse EM; Keates T; Hickman TT; Felletar I; Philpott M; Munro S; McKeown MR; Wang Y; Christie AL; West N; Cameron MJ; Schwartz B; Heightman TD; La Thangue N; French CA; Wiest O; Kung AL; Knapp S; Bradner JE Selective Inhibition of Bet Bromodomains. Nature 2010, 468, 1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (620).Grayson AR; Walsh EM; Cameron MJ; Godec J; Ashworth T; Ambrose JM; Aserlind AB; Wang H; Evan GI; Kluk MJ; Bradner JE; Aster JC; French CA Myc, a Downstream Target of Brd-Nut, Is Necessary and Sufficient for the Blockade of Differentiation in Nut Midline Carcinoma. Oncogene 2014, 33, 1736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (621).Nicodeme E; Jeffrey KL; Schaefer U; Beinke S; Dewell S; Chung CW; Chandwani R; Marazzi I; Wilson P; Coste H; White J; Kirilovsky J; Rice CM; Lora JM; Prinjha RK; Lee K; Tarakhovsky A Suppression of Inflammation by a Synthetic Histone Mimic. Nature 2010, 468, 1119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (622).Zhu H; Bengsch F; Svoronos N; Rutkowski MR; Bitler BG; Allegrezza MJ; Yokoyama Y; Kossenkov AV; Bradner JE; Conejo-Garcia JR; Zhang R Bet Bromodomain Inhibition Promotes Anti-Tumor Immunity by Suppressing Pd-L1 Expression. Cell Rep 2016, 16, 2829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (623).Korb E; Herre M; Zucker-Scharff I; Darnell RB; Allis CD Bet Protein Brd4 Activates Transcription in Neurons and Bet Inhibitor Jq1 Blocks Memory in Mice. Nat. Neurosci 2015, 18, 1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (624).Anand P; Brown JD; Lin CY; Qi J; Zhang R; Artero PC; Alaiti MA; Bullard J; Alazem K; Margulies KB; Cappola TP; Lemieux M; Plutzky J; Bradner JE; Haldar SM Bet Bromodomains Mediate Transcriptional Pause Release in Heart Failure. Cell 2013, 154, 569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (625).Conrad RJ; Ott M Therapeutics Targeting Protein Acetylation Perturb Latency of Human Viruses. ACS Chem. Biol 2016, 11, 669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (626).Winter GE; Buckley DL; Paulk J; Roberts JM; Souza A; Dhe-Paganon S; Bradner JE Drug Development. Phthalimide Conjugation as a Strategy for in Vivo Target Protein Degradation. Science 2015, 348, 1376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (627).Liu Z; Wang P; Chen H; Wold EA; Tian B; Brasier AR; Zhou J Drug Discovery Targeting Bromodomain-Containing Protein 4. J. Med. Chem 2017, 60, 4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (628).Roe JS; Mercan F; Rivera K; Pappin DJ; Vakoc CR Bet Bromodomain Inhibition Suppresses the Function of Hema-topoietic Transcription Factors in Acute Myeloid Leukemia. Mol. Cell 2015, 58, 1028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (629).Lockwood WW; Zejnullahu K; Bradner JE; Varmus H Sensitivity of Human Lung Adenocarcinoma Cell Lines to Targeted Inhibition of Bet Epigenetic Signaling Proteins. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 19408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (630).Shi J; Wang Y; Zeng L; Wu Y; Deng J; Zhang Q; Lin Y; Li J; Kang T; Tao M; Rusinova E; Zhang G; Wang C; Zhu H; Yao J; Zeng YX; Evers BM; Zhou MM; Zhou BP Disrupting the Interaction of Brd4 with Diacetylated Twist Suppresses Tumorigenesis in Basal-Like Breast Cancer. Cancer Cell 2014, 25, 210–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (631).Lamonica JM; Deng W; Kadauke S; Campbell AE; Gamsjaeger R; Wang H; Cheng Y; Billin AN; Hardison RC; Mackay JP; Blobel GA Bromodomain Protein Brd3 Associates with Acetylated Gata1 to Promote Its Chromatin Occupancy at Erythroid Target Genes. Proc. Natl. Acad. Sci. U. S. A 2011, 108, E159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (632).Berthon C; Raffoux E; Thomas X; Vey N; Gomez-Roca C; Yee K; Taussig DC; Rezai K; Roumier C; Herait P; Kahatt C; Quesnel B; Michallet M; Recher C; Lokiec F; Preudhomme C; Dombret H Bromodomain Inhibitor Otx015 in Patients with Acute Leukaemia: A Dose-Escalation, Phase 1 Study. Lancet Haematol 2016, 3, e186–95. [DOI] [PubMed] [Google Scholar]
- (633).Odore E; Lokiec F; Cvitkovic E; Bekradda M; Herait P; Bourdel F; Kahatt C; Raffoux E; Stathis A; Thieblemont C; Quesnel B; Cunningham D; Riveiro ME; Rezai K Phase I Population Pharmacokinetic Assessment of the Oral Bromodomain Inhibitor Otx015 in Patients with Haematologic Malignancies. Clin. Pharmacokinet 2016, 55, 397–405. [DOI] [PubMed] [Google Scholar]
- (634).Theodoulou NH; Tomkinson NC; Prinjha RK; Humphreys PG Clinical Progress and Pharmacology of Small Molecule Bromodomain Inhibitors. Curr. Opin. Chem. Biol 2016, 33, 58–66. [DOI] [PubMed] [Google Scholar]
- (635).Chen P; Chaikuad A; Bamborough P; Bantscheff M; Bountra C; Chung CW; Fedorov O; Grandi P; Jung D; Lesniak R; Lindon M; Muller S; Philpott M; Prinjha R; Rogers C; Selenski C; Tallant C; Werner T; Willson TM; Knapp S; Drewry DH Discovery and Characterization of Gsk2801, a Selective Chemical Probe for the Bromodomains Baz2a and Baz2b. J. Med. Chem 2016, 59, 1410–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (636).Theodoulou NH; Tomkinson NC; Prinjha RK; Humphreys PG Progress in the Development of Non-Bet Bromodomain Chemical Probes. ChemMedChem 2016, 11, 477–87. [DOI] [PubMed] [Google Scholar]
- (637).Sabari BR; Tang Z; Huang H; Yong-Gonzalez V; Molina H; Kong HE; Dai L; Shimada M; Cross JR; Zhao Y; Roeder RG; Allis CD Intracellular Crotonyl-Coa Stimulates Transcription through P300-Catalyzed Histone Crotonylation. Mol. Cell 2015, 58, 203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (638).Hirschey MD; Zhao Y Metabolic Regulation by Lysine Malonylation, Succinylation, and Glutarylation. Mol. Cell. Proteomics 2015, 14, 2308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (639).Weinert BT; Iesmantavicius V; Moustafa T; Scholz C; Wagner SA; Magnes C; Zechner R; Choudhary C Acetylation Dynamics and Stoichiometry in Saccharomyces Cerevisiae. Mol. Syst. Biol 2014, 10, 716. [DOI] [PMC free article] [PubMed] [Google Scholar]