Abstract
This study combined in vitro production of bovine blastocysts, multiple embryo transfer techniques, and a conceptus-endometrial explant co-culture system to test the hypothesis that bovine endometrium exposed to long vs. short day 15 conceptuses would exhibit a different transcriptome profile reflective of potential for successful pregnancy establishment. Bovine endometrial explants collected at the late luteal stage of the estrous cycle were cultured in RPMI medium for 6 h with nothing (control), 100 ng/mL recombinant ovine interferon tau (IFNT), a long day 15 conceptus, or a short day 15 conceptus. Transcriptional profiling of the endometrial explants found that exposure of endometrium to IFNT, long conceptuses, or short conceptuses altered (P < 0.05) expression of 491, 498, and 230 transcripts, respectively, compared to the control. Further analysis revealed three categories of differentially expressed genes (DEG): (i) commonly responsive to exposure to IFNT and conceptuses, irrespective of size (n = 223); (ii) commonly responsive to IFNT and long conceptuses only (n = 168); and genes induced by the presence of a conceptus but independent of IFNT (n = 108). Of those 108 genes, 101 were exclusively induced by long conceptuses and functional analysis revealed that regulation of molecular function, magnesium-ion transmembrane transport, and clathrin coat assembly were the principal gene ontologies associated with these DEG. In conclusion, bovine endometrium responds differently to age-matched conceptuses of varying size in both an IFNT-dependent and -independent manner, which may be reflective of the likelihood of successful pregnancy establishment.
Keywords: transcriptome profiling, endometrium explant, conceptus, cattle
Bovine endometrium responds differently in terms of its gene expression signature to age-matched conceptuses of varying size, in both an interferon-dependent and independent manner, which may be reflective of the likelihood of successful pregnancy establishment.
Introduction
Embryo mortality is a significant contributor to poor reproductive efficiency in dairy and beef cows. A significant proportion of embryonic loss in cattle, particularly lactating dairy cows, occurs during the first 2 weeks after conception, before maternal recognition of pregnancy, which occurs around day 16 [1–3]. Communication between the developing embryo and the mother is vital for the successful establishment and maintenance of pregnancy. Up to the blastocyst stage, the bovine embryo is relatively autonomous, as blastocysts can be produced in vitro in the absence of contact with the female reproductive tract and are capable of establishing pregnancy after transfer to a synchronous uterus. During the preimplantation period, the hatched bovine blastocyst undergoes significant morphological changes, passing sequentially from a spherical- to ovoid-, then tubular- and finally filamentous-shaped structure that primarily involves proliferation of the conceptus trophectoderm cells. During this time, the elongating conceptus secretes interferon tau (IFNT), the maternal pregnancy recognition signal in ruminants [4, 5]. In contrast to pre-hatching development, elongation is predominantly maternally driven, dependent on substances in the uterine lumen fluid (or histotroph) [6]; blastocysts do not elongate in vitro [7] and the absence of uterine glands in vivo results in failure of blastocysts to elongate following embryo transfer [8].
Spatial and temporal changes of the endometrial transcriptome and histotroph composition are necessary to establish uterine receptivity to implantation and, in turn, are pivotal to the likelihood of successful pregnancy in cattle. Those modifications are primarily regulated by progesterone (P4) and conceptus-derived IFNT, which prevents development of the endometrial luteolytic mechanism [6, 9]. The role of P4 in uterine receptivity is unequivocal [10–12]. Low circulating P4 concentrations in the first week after ovulation, as frequently occurs in high-producing lactating dairy cows, are associated with under-developed conceptuses [13] with an altered transcriptomic signature [14] and a low likelihood of establishing pregnancy [15, 16]. On the other hand, elevated concentrations of circulating P4 in the period immediately after conception have been associated with advanced conceptus elongation [17], increased IFNT production [18], and greater pregnancy rates in cattle and sheep [19–21]. Despite the definitive association between P4 and conceptus elongation, significant natural variation in age-matched in vivo- and in vitro-derived conceptuses occurs, even amongst conceptuses developing in the same uterus [22–24]. This would suggest that part of the ability to elongate is intrinsic to the embryo and may be related to oocyte and/or blastocyst quality.
Conceptus length on a given day in the period around pregnancy recognition is thought to be indicative of its quality and the likelihood of establishing and maintaining a pregnancy [14], although this has yet to be definitively established. While significant differences in the transcriptomes of long and short day 15 conceptuses have been reported [14], the interaction between such divergent conceptuses and the endometrium has not been described. Previous data have indicated that the endometrium can act as a sensor of embryo quality and that it responds differently in terms of its gene expression signature to embryos of varying developmental competency [25–27]. We hypothesized that bovine endometrium exposed to long vs. short day 15 conceptuses would exhibit a different transcriptome profile reflective of potential for successful pregnancy establishment. The specific objectives of this study were to characterize the variation in conceptus size on day 15 following transfer of blastocysts on day 7 to determine effects of long and short day 15 conceptuses on the endometrial transcriptome and to identify IFNT-dependent and -independent responses of the endometrium.
Material and methods
All experimental procedures involving animals were sanctioned by the Animal Research Ethics Committee of University College Dublin and were licensed by the Health Products Regulatory Authority, Ireland, in accordance with Statutory Instrument No. 543 of 2012 under Directive 2010/63/EU on the Protection of Animals used for Scientific Purposes.
Experimental animals and procedures
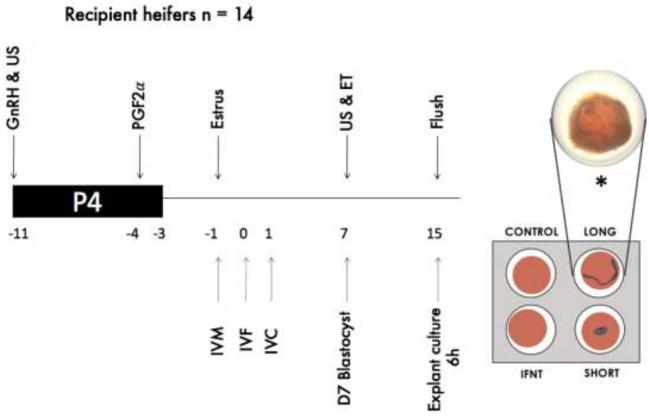
Charolais- or Limousin-cross heifers (n = 56; 21–32 months in age and 555–670 kg) were used to generate conceptuses used in this study. All animals were kept under identical conditions during the study (February–May 2016) and were fed a diet consisting of grass and maize silage supplemented with a standard beef ration. The experimental procedures are illustrated in Figure 1. Briefly, estrous cycles of all heifers were synchronized using an 8-day intravaginal P4 device (PRID, 1.55 g P4; Ceva Santé Animale, Libourne, France). On the day of PRID insertion, each heifer received a 2 mL intramuscular injection of an analog of gonadotrophin releasing hormone (GnRH; Ovarelin, Ceva Santé Animale, equivalent to 100 μg gonadorelin). One day before PRID removal, all heifers received a 5 mL intramuscular injection of an analog of prostaglandin F2 alpha (PGF2α; Enzaprost, Ceva Santé Animale, Libourne, equivalent to 25 mg Dinoprost) to induce luteolysis of the corpus luteum (CL). Only those heifers observed in standing estrus (day -1) were used as recipients of in vitro produced blastocysts (n = 14) on day 7 of the estrous cycle.
Figure 1.
Experimental design used to model the local dialogue between the endometrium and a long or short age-matched conceptus. US = ultrasound scanning; P4 = progesterone releasing device; GnRH = gonadotrophin-releasing hormone; PGF2α = prostaglandin F2α; ET = multiple embryo transfer (10 embryos per recipient); flush = conceptus recovery; IVM = in vitro maturation; IVF = in vitro fertilization; IVC = in vitro culture; explant culture 6 h = long and short conceptuses co-cultured on top of a uterine explant for 6 h; additional explants were cultured with media alone (control) or containing 100 ng/mL of interferon-tau (IFNT). Day 0 was considered the day of the estimated fertilization (28–32 h after starting estrus behavior). *Representative image of day 15 long conceptus individually placed on top of endometrial explant.
Day 0 was the presumptive day of fertilization (28–32 h after first standing to be mounted) [28–30]. All animals were ultrasound scanned using a portable ultrasound machine (Easi-Scan; BCF Technology Ltd, Bellshill, Scotland, UK) fitted with a 4.5 to 8.5-MHz linear array transducer before initiation of the synchronization protocol and on day 7 of the estrous cycle immediately before embryo transfer to recipients to assess CL location. On day 7, in vitro-derived embryos (n = 10; day 7 Code 1–2 according to the manual of the International Embryo Technology Society [31]) were pooled before being loaded into straws for transcervical transfer to the uterine horn ipsilateral to the ovary bearing the CL. Recipient heifers were slaughtered in a commercial abattoir on day 15 (8 days-post ET). Prior to slaughter, a plasma sample was taken from all recipients to measure progesterone by solid-phase radioimmunoassay using the Coat-A-Count radioimmunoassay (Siemens Healthcare Diagnostics Inc.). Sensitivity and coefficient of variance were 0.01 ng/mL and 4.3%, respectively. CL volume and weight were recorded at slaughter. Reproductive tracts were recovered and brought back to the laboratory within 2 h. Each uterine horn was gently flushed with 20 mL of sterile phosphate-buffered saline (PBS) containing 5% fetal calf serum (FCS). The number and dimension (length and width) of each recovered conceptus were recorded and they were then classified as ovoid, tubular, or filamentous based on shape as described previously [14, 32].
In vitro embryo production
Blastocysts were produced in vitro using methods described previously [33]. Briefly, immature cumulus–oocyte complexes (COCs) were recovered by aspirating follicles from the ovaries of heifers and cows slaughtered at a local abattoir, washed in PBS, and matured for 24 h in groups of 50 in 500 μL TCM-199 supplemented with 10% FCS and 10 ng/mL epidermal growth factor at 39°C under an atmosphere of 5% CO2 in air with maximum humidity. Matured COCs were inseminated with frozen-thawed bull sperm at a concentration of 1 × 106 sperm/mL. Semen from the same bull was used for production of all embryos. Gametes were co-incubated for 20 h at 39°C in an atmosphere of 5% CO2 in air with maximum humidity. Presumptive zygotes were denuded by gentle vortexing and cultured in synthetic oviduct fluid droplets (25 μL; 25 embryos per droplet) at 39°C in a humidified atmosphere with 5% CO2 and 5% O2 under mineral oil. Blastocysts for transfer to recipient heifers were removed from culture, pooled, and loaded into straws (10 embryos per straw) on day 7 (IVF = day 0).
Conceptuses-explant co-culture
To obtain endometrium for explant culture, reproductive tracts were collected postmortem from animals slaughtered in the late luteal phase of the estrous cycle based on ovarian morphology [34] as previously described [35]. Immediately after collection, tracts were placed on ice and transported to the laboratory within approximately 1 h. Once in the laboratory, the ipsilateral uterine horn was dissected from the remainder of the tract, washed with 1% PBS (Gibco) containing (v/v) 1% 100 X antibiotic-antimycotic (ABAM; Gibco), and sprayed with 70% ethanol. Next, endometrium from the intercaruncular areas of the middle third of the horn was isolated as described previously [36]. Briefly, the endometrium was exposed by opening the uterine horn longitudinally along the anti-mesometrial side, and the uterine luminal surface was washed with 1% PBS containing 1% ABAM. Then, an 8 mm biopsy punch was used to obtain a portion of the intercaruncular endometrium and myometrium. Sterile scissors were then used to completely remove the myometrium from the intercaruncular endometrium biopsy. After collection, the endometrial explants (50–80 mg) were washed in conical tubes containing 25 mL of Hank balanced salt solution (HBSS; Gibco) containing 1% ABAM. The media was poured off, and explants were washed twice more in 25 mL of HBSS without ABAM before placing them upright individually in culture wells (4-well plate with 15 mm diameter x 11 mm deep) containing 1 mL of Roswell Park Memorial Institute (RPMI) medium (Gibco) medium plus 1% ABAM.
Culture wells with endometrial explants placed epithelial side up were incubated in 5% CO2 and air at 38.8°C for 4 h. The media was aspirated and then replaced with 1 mL of pre-warmed media (RPMI with 1% ABAM). Then, explants were cultured in RPMI for 6 h in 5% CO2 and air at 38.8°C with either (i) nothing (control; n = 6); (ii) 100 ng/mL recombinant ovine IFNT (provided by G. Charpigny, INRA, France; n = 6); (iii) a long day 15 conceptus (n = 7; 25.4 ± 5.7 mm, mean length ± SE); or (iv) a short day 15 conceptus (n = 6; 1.8 ± 0.3 mm) (Figure 1). In order to minimize variation, explants from the same uterus were used across all treatments in a given replicate. After incubation, conceptuses were removed from the top of the endometrial explant and both the conceptuses and the explants were individually snap-frozen in liquid nitrogen and stored at −80°C for RNA extraction. The sex of the conceptus was determined as previously described [35].
Explant RNA extraction and RNA sequencing
A total of 25 samples were sequenced (control: 6; IFNT: 6; long conceptus: 7; short conceptus: 6). Total RNA was isolated from frozen explant endometrium (∼40 mg) by homogenizing in 1 mL of TRIzol reagent and a Qiagen RNeasy Mini Kit (Qiagen) as described previously [35]. RNA quantity and quality were determined using an Agilent Bioanalyzer (Agilent Technology). To eliminate DNA contamination, samples were treated with DNase as previously described. RNA concentrations and integrity were determined by quantitative high-sensitivity RNA analysis on the Fragment Analyzer instrument (Catalog # DNF-472, Advanced Analytical Technologies, Inc., Ankeny, IA). RNA library preparation and sequencing was conducted by the University of Missouri DNA Core facility as previously described [37]. Sequencing was performed on an Illumina NextSeq 500 sequencer to a depth of 30–40 million raw reads per sample.
Statistical analysis
Raw sequences (fastq) were subjected to quality control using windowed adaptive quality trimming approach implemented in fqtrim (https://ccb.jhu.edu/software/fqtrim/). The quality reads were then mapped to the bovine reference genome UMD3.1 using the Hisat2 mapper (https://ccb.jhu.edu/software/hisat2/) [38]. The Ensembl gene annotation along with the read alignment files was used to quantify reads that were mapped to each gene using the FeatureCounts tool [39]. Differential expression analysis between sample groups was performed by robustly fitting the expression data to a generalized linear model using the EdgeR-robust method [40].
Gene ontology (GO) terms were downloaded from Ensembl, and representation analysis was done using REVIGO, a freely available web server that takes all GO terms associated with a given gene set and reduces it to a smaller subset of GO terms by removing redundant terms (that relate to similar function). The final set of GO terms is then visualized in semantic similarity-based scatter plots. This means that the x and y coordinates represent semantic values from using an eigenvalue decomposition of the terms' pairwise distance matrix so that more semantically similar GO terms are also closer in the plot [41].
Results
Conceptus recovery
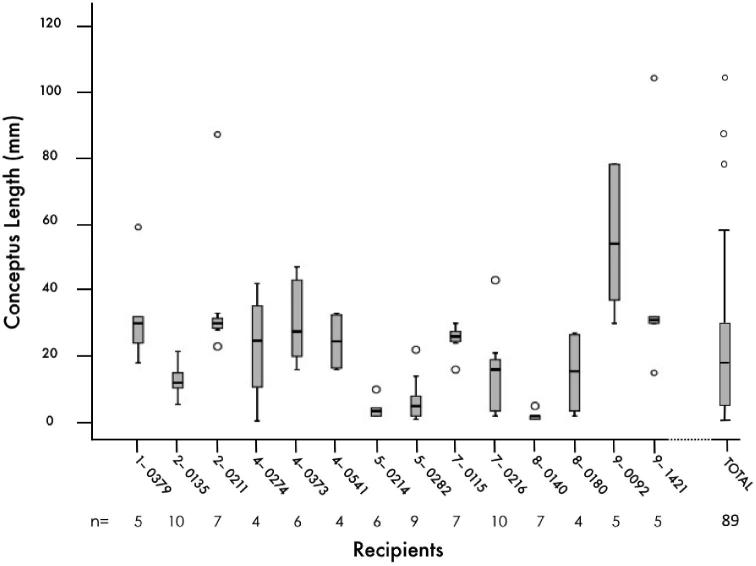
Mean (±SE) CL volume and weight on day 15 were 5.7 ± 0.4 cm3 and 5.6 ± 0.3 g, respectively. Mean progesterone concentration on day 15 was 6.7 ± 0.2 ng/mL. Day 15 conceptuses were recovered from all 14 recipient heifers following the transfer (10 per recipient) of day 7 in vitro-produced blastocysts. Overall, conceptus recovery rate was 63.6% (89/140). The mean length of all recovered conceptuses was 21.3 ± 2.1 mm with a range of 0.5 to 104 mm in length (Figure 2). Conceptus morphology was also heterogeneous with 18% ovoid (16/89), 16% tubular (14/89), and 66% filamentous (59/89). Within a replicate, single long (25.4 ± 5.7 mm, n = 7) or short (ovoid, 1.8 ± 0.3 mm, n = 6) conceptuses recovered from the same recipient were individually co-cultured on top of an endometrial explant for 6 h (Figure 1). Details regarding length, morphology, and sex of the individual conceptuses co-cultured are summarized in Table 1.
Figure 2.
Box plots showing the variation in the conceptus length within recipient on day 15 of pregnancy. Each box plot represents the length (mm) of the recovered conceptuses from each individual recipient, identified by its tag number. The box plot on the right represents the length of all recovered conceptuses (n = 89) from all recipients enrolled in this study (n = 14). Note that “o” represents the outliers.
Table 1.
Summary of day 15 conceptuses (long and short) co-cultured for 6 h on bovine endometrial explants (Expl).
| Conceptus classification | Expl 1 | Expl 2 | Expl 3 | Expl 4 | Expl 5 | Expl 6 | Expl 7 | Length* (mm) |
|---|---|---|---|---|---|---|---|---|
| Long | ||||||||
| Sex | Male | Female | Male | Female | Male | Male | Male | 25.4 ± 5.7 |
| Length | 14.0 mm | 8.0 mm | 21.0 mm | 19.0 mm | 33.0 mm | 54.0 mm | 29.0 mm | |
| Short | ||||||||
| Sex | Female | Male | Female | Male | Male | Male | 1.8 ± 0.3 | |
| Length | 2.0 mm | 2.0 mm | 2.0 mm | 2.5 mm | 0.5 mm | 2.0 mm |
*Mean ± standard error.
Transcriptome analysis
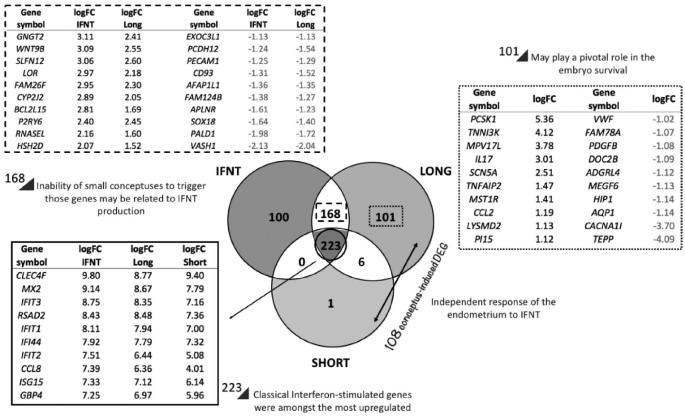
Compared to the control, culture of endometrial explants with either IFNT, long conceptuses, or short conceptuses altered (false discovery rate, FDR P < 0.05) expression of 491, 498, and 230 genes, respectively (Supplemental Tables S1–3). As illustrated in Figure 3, 223 transcripts were commonly regulated by conceptuses and IFNT; these included established classical interferon-stimulated genes (ISGs) such as ISG15 and radical S-adenosyl methionine domain containing 2 (RSAD2). In addition, 168 transcripts were common between IFNT- and long conceptus-treated endometrium. However, only 6 of 108 conceptus-induced DEG were shared between short and long conceptuses. Further, 101 transcripts were exclusively regulated by long conceptuses, while only one gene (StAR-related lipid transfer domain containing 9, STARD9) was exclusive to short conceptuses.
Figure 3.
Venn diagram illustrating the number of differentially expressed genes (DEG) in bovine endometrial explants exposed to recombinant ovine interferon-tau (IFNT), a bovine day 15 long conceptus, or a bovine day 15 short conceptus compared to control endometrium. The solid lined box lists the top 10 most upregulated transcripts common to explants cultured with IFNT- or a conceptus compared to control endometrium. The dashed lined box lists the top 10 most upregulated (left) and downregulated (right) transcripts common to IFNT and long conceptus groups. The dotted lined box lists the top 10 most upregulated (left) and downregulated (right) transcripts exclusively regulated by long conceptuses.
IFNT-dependent endometrial response to the conceptus
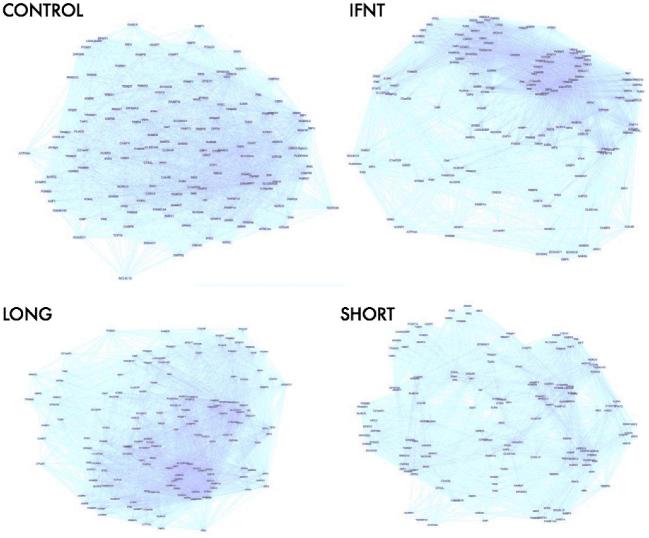
A total of 223 common transcripts were upregulated in both conceptus- and IFNT-treated explants compared to control explants (Figure 3; Supplemental Table S4). Known ISGs were amongst the most upregulated transcripts in all groups. Moreover, logFC in gene expression of ISGs was similar across the three groups (see Table 2). The top 20 most upregulated endometrial transcripts in response to conceptuses and IFNT are listed in Table 2. Principal component analyses showed clear separation of the three treated groups (IFNT, small conceptus, and long conceptus) from the control (Figure 4). To further compare the expression pattern of these 223 genes within each sample group, we calculated mutual information (MI) of variation between gene expression in each group. MI measures the information content that two variables share: a numerical value ranging from 0 to 1 depending on, intuitively, how much knowing one variable would predict variability of the other [42]. The pairwise MI between genes was used to generate maximum relevance minimum redundancy networks [43]. The networks showed differential interaction of genes in IFNT- and conceptus-treated endometrium compared to controls (Figure 5).
Table 2.
Gene ID, gene symbol, gene name, and logarithm of fold change (logFC) of top 20 upregulated transcripts differentially expressed genes (DEG) in bovine endometrial explants commonly responsive to exposure to IFNT and conceptuses, irrespective of size (n = 223).
| Gene ID | Gene symbol | Gene name | logFC IFNT | logFC Long | logFC Short |
|---|---|---|---|---|---|
| ENSBTAG00000004560 | CLEC4F | C-type lectin domain family 4 member F | 9.80 | 8.77 | 9.40 |
| ENSBTAG00000008471 | MX2 | MX dynamin like GTPase 2 | 9.14 | 8.67 | 7.79 |
| ENSBTAG00000009768 | IFIT3 | interferon induced protein with tetratricopeptide repeats 3 | 8.75 | 8.35 | 7.16 |
| ENSBTAG00000016061 | RSAD2 | radical S-adenosyl methionine domain containing 2 | 8.43 | 8.48 | 7.36 |
| ENSBTAG00000007881 | IFIT1 | interferon induced protein with tetratricopeptide repeats 1 | 8.11 | 7.94 | 7.00 |
| ENSBTAG00000034349 | IFI44 | interferon induced protein 44 | 7.92 | 7.79 | 7.32 |
| ENSBTAG00000034918 | IFIT2 | interferon induced protein with tetratricopeptide repeats 2 | 7.51 | 6.44 | 5.08 |
| ENSBTAG00000014113 | CCL8 | C-C motif chemokine ligand 8 | 7.39 | 6.36 | 4.01 |
| ENSBTAG00000014707 | ISG15 | ISG15 ubiquitin-like modifier | 7.33 | 7.12 | 6.14 |
| ENSBTAG00000002416 | n.a. | n.a. | 7.25 | 6.97 | 5.96 |
| ENSBTAG00000030932 | IFI44L | interferon induced protein 44 like | 7.21 | 7.03 | 6.48 |
| ENSBTAG00000012894 | SAMD9 | sterile alpha motif domain containing 9 | 7.07 | 7.15 | 6.25 |
| ENSBTAG00000014628 | OAS2 | 2′-5′-oligoadenylate synthase 2 | 6.99 | 6.60 | 6.03 |
| ENSBTAG00000038233 | n.a. | n.a. | 6.98 | 6.93 | 5.09 |
| ENSBTAG00000045588 | n.a. | n.a. | 6.74 | 6.50 | 5.66 |
| ENSBTAG00000012335 | UBA7 | ubiquitin like modifier activating enzyme 7 | 6.70 | 6.61 | 5.93 |
| ENSBTAG00000037634 | n.a. | n.a. | 6.59 | 6.00 | 4.93 |
| ENSBTAG00000038938 | n.a. | n.a. | 6.55 | 6.22 | 4.99 |
| ENSBTAG00000005603 | CXCL11 | C-X-C motif chemokine ligand 11 | 6.53 | 6.06 | 3.33 |
| ENSBTAG00000001725 | CXCL10 | C-X-C motif chemokine ligand 10 | 6.38 | 6.41 | 5.76 |
Refer to Figure 3. For full list of DEGs, see Supplemental Table S4.
n.a. Not applicable. Uncharacterized protein.
Figure 4.
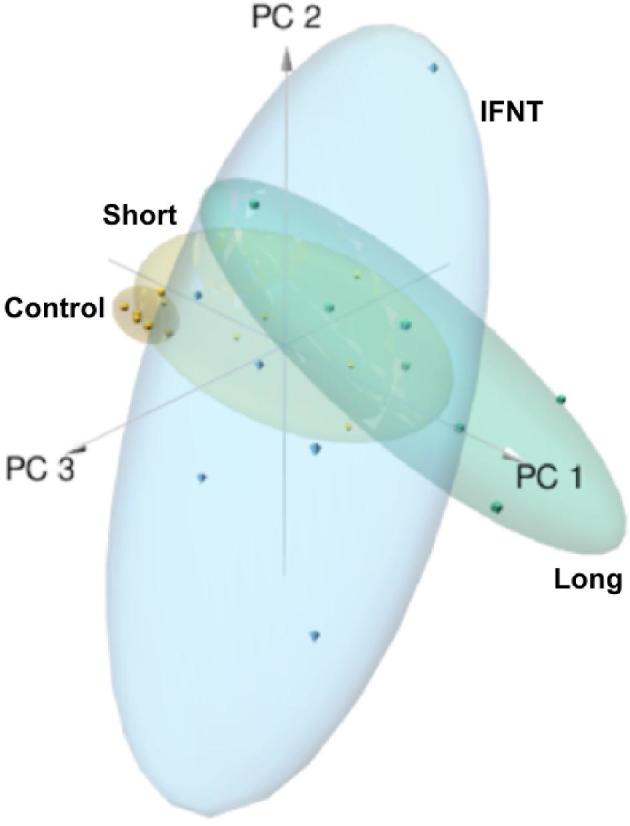
Principal component analysis illustrating endometrial gene expression between uterine explants exposed to recombinant ovine interferon-tau, a bovine day 15 long conceptus, a bovine day 15 short conceptus, or medium alone (control).
Figure 5.
Maximum relevance minimum redundancy network analysis of differentially expressed genes common to interferon-tau (IFNT), long conceptus, and short conceptus treatments compared to the control (n = 223). Note the differential clustering of commonly differentially expressed genes between treatments.
The most significant GO terms associated with predicted biological functions of genes differentially expressed in IFNT and conceptus treatments compared to the control were cellular process (GO: 000987), metabolic process (GO: 0008152), response to stimulus (GO: 0050896), and reproduction (GO: 0000003) (see Supplemental Table S5 for GO analysis).
A direct comparison of endometrial transcriptomes (RNA-Seq) following culture with long or short conceptuses revealed nine DEGs within the set of genes commonly differentially expressed in the three groups (IFNT, long, and short) compared to control, which were differentially expressed in response to conceptus size. Expression of C-X-C motif chemokine ligand 11 (CXCL11; LogFC 2.78), CCL8 (LogFC 2.42), tryptophanyl-tRNA synthetase (WARS; LogFC 1.55), GBP6 (LogFC 1.45), CD274 molecule (CD274; LogFC 1.39), GBP1 (LogFC 1.18), Mab-21 domain containing 1 (MB21D1; LogFC 1.03), cytidine and dCMP deaminase domain containing 1 (CDADC1; LogFC 0.96), and F-box protein 33 (FBXO33; LogFC 0.90) was higher in endometrium exposed to long compared to short conceptuses.
IFNT-dependent endometrial response to conceptuses of different sizes
A list of all genes commonly responsive to IFNT and long conceptuses compared to control (n = 168) is illustrated in Supplemental Table S6. The top 20 most upregulated and the 15 downregulated endometrial transcripts in response to long conceptuses and IFNT are listed in Table 3. Functional analysis of this gene set showed that they are overrepresented by metabolism, cell–cell adhesion, receptor-mediated endocytosis, mitochondrion organization, S-adenosylmethionine biosynthesis, determination of ventral identity, and glucocorticoid-mediated signaling pathway functions mainly.
Table 3.
Gene ID, gene symbol, gene name, and logarithm of fold change (logFC) of top 20 most upregulated and the 15 downregulated commonly differentially expressed genes (DEG) in bovine endometrial explants commonly responsive to IFNT and long conceptuses only (n = 168).
| Gene ID | Gene symbol | Gene name | logFC IFNT | logFC Long | |
|---|---|---|---|---|---|
| Highest expression | |||||
| ENSBTAG00000047325 | GNGT2 | G protein subunit gamma transducin 2 | 3.11 | 2.41 | |
| ENSBTAG00000002664 | WNT9B | Wnt family member 9B | 3.09 | 2.55 | |
| ENSBTAG00000025219 | n.a. | n.a. | 3.06 | 2.60 | |
| ENSBTAG00000032997 | LOR | LOR protein; Uncharacterized protein | 2.97 | 2.18 | |
| ENSBTAG00000006549 | FAM26F | family with sequence similarity 26 member F | 2.95 | 2.30 | |
| ENSBTAG00000010033 | n.a. | n.a. | 2.89 | 2.05 | |
| ENSBTAG00000012419 | BCL2L15 | BCL2 like 15 | 2.81 | 1.69 | |
| ENSBTAG00000038737 | P2RY6 | pyrimidinergic receptor P2Y6 | 2.40 | 2.45 | |
| ENSBTAG00000009091 | RNASEL | ribonuclease L | 2.16 | 1.60 | |
| ENSBTAG00000038154 | HSH2D | hematopoietic SH2 domain containing | 2.07 | 1.52 | |
| ENSBTAG00000038494 | n.a. | n.a. | 2.01 | 1.43 | |
| ENSBTAG00000002767 | GNB4 | G protein subunit beta 4 | 2.01 | 1.81 | |
| ENSBTAG00000018418 | TMEM74B | transmembrane protein 74B | 2.01 | 1.56 | |
| ENSBTAG00000019741 | C3AR1 | complement component 3a receptor 1 | 1.90 | 1.46 | |
| ENSBTAG00000006256 | PTPRO | protein tyrosine phosphatase, receptor type O | 1.89 | 1.37 | |
| ENSBTAG00000011543 | PDCD1 | programmed cell death 1 | 1.87 | 1.67 | |
| ENSBTAG00000015978 | ANXA1 | annexin A1 | 1.85 | 1.76 | |
| ENSBTAG00000017704 | ABCG2 | ATP binding cassette subfamily G member 2 (Junior blood group) | 1.78 | 1.18 | |
| ENSBTAG00000033702 | STARD8 | StAR related lipid transfer domain containing 8 | 1.77 | 1.67 | |
| ENSBTAG00000011936 | ATP8B4 | ATPase phospholipid transporting 8B4 (putative) | 1.70 | 1.57 | |
| Lowest expression | |||||
| ENSBTAG00000001174 | MRPL15 | mitochondrial ribosomal protein L15 | −0.77 | −0.62 | |
| ENSBTAG00000014058 | LDB2 | LIM domain binding 2 | −0.80 | −0.92 | |
| ENSBTAG00000002699 | KIT | KIT proto-oncogene receptor tyrosine kinase | −0.88 | −0.90 | |
| ENSBTAG00000003312 | CHST15 | carbohydrate sulfotransferase 15 | −0.97 | −1.11 | |
| ENSBTAG00000006606 | FAM101B | family with sequence similarity 101 member B | −1.00 | −0.82 | |
| ENSBTAG00000012035 | EXOC3L1 | exocyst complex component 3 like 1 | −1.13 | −1.13 | |
| ENSBTAG00000008437 | PCDH12 | protocadherin 12 | −1.24 | −1.54 | |
| ENSBTAG00000012066 | PECAM1 | platelet and endothelial cell adhesion molecule 1 | −1.25 | −1.29 | |
| ENSBTAG00000004207 | CD93 | CD93 molecule | −1.31 | −1.52 | |
| ENSBTAG00000019948 | AFAP1L1 | actin filament associated protein 1 like 1 | −1.36 | −1.35 | |
| ENSBTAG00000038700 | FAM124B | family with sequence similarity 124 member B | −1.38 | −1.27 | |
| ENSBTAG00000027516 | APLNR | apelin receptor | −1.61 | −1.23 | |
| ENSBTAG00000011662 | SOX18 | transcription factor SOX-18 | −1.64 | −1.40 | |
| ENSBTAG00000008583 | PALD1 | paladin | −1.98 | −1.72 | |
| ENSBTAG00000008735 | VASH1 | vasohibin 1 | −2.13 | −2.04 |
Refer to Figure 3. For full list of DEGs, see Supplemental Table S6.
IFNT-independent endometrial response to conceptuses of different sizes
Day 15 conceptuses modified the expression of 108 genes in endometrial explants that were not differentially expressed in response to IFNT compared to controls (Figure 3). Therefore, these genes were considered potential conceptus-induced and IFNT-independent regulated genes. Cell-matrix adhesion, regulation of molecular function, ventricular septum development, and magnesium ion transmembrane transport were the principal GO terms associated with this set of genes.
Only 6 of 108 conceptuses-induced DEGs were shared between long and short groups. Of those, leucine-rich repeat transmembrane neuronal 2 (LRRTM2; logFC 4.14 and 4.42, logFC in Long and Short vs. Control, respectively), MUC4 (logFC 4.24 and 4.55), C4AL (logFC 1.89 and 1.63), and tRNA methyltransferase 13 homolog (TRMT13; logFC 1.25 and 1.01) genes were exclusively upregulated in the endometrium of explants co-cultured with conceptuses, independently of IFNT and regardless the conceptuses size. Moreover, xylosyltransferase 1 (XYLT1; logFC −0.62 and −1.01) and leucine zipper protein 1 (LUZP1; logFC −0.95 and −0.91) genes were downregulated in the endometrium by day 15 conceptuses.
Interestingly, 101 endometrial transcripts were exclusively regulated by long conceptuses (Supplemental Table S7), 64 and 37 of which were up- and downregulated, respectively (Figure 3). Those endometrial genes showing either the highest or the lowest fold change expression in response to long conceptuses are listed in Table 4. Functional analysis revealed that regulation of molecular function, visual perception, magnesium ion transmembrane transport, regulation of cytokinesis, clathrin coat assembly, and beta-amyloid metabolism were the principal GO terms associated with these DEGs.
Table 4.
Gene ID, gene symbol, gene name, and logarithm of fold change (logFC) of top 20 unique differentially expressed genes (DEG) in bovine endometrial explants co-cultured with long conceptuses compared to control explants (n = 101).
| Gene ID | Gene symbol | Gene name | logFC Long | |
|---|---|---|---|---|
| Highest expression | ||||
| ENSBTAG00000020843 | PCSK1 | proprotein convertase subtilisin/kexin type 1 | 5.36 | |
| ENSBTAG00000000965 | TNNI3K | Bos taurus TNNI3 interacting kinase (TNNI3K), mRNA. | 4.12 | |
| ENSBTAG00000047520 | MPV17L | Mpv17-like protein | 3.78 | |
| ENSBTAG00000002150 | IL17 | Interleukin-17A | 3.02 | |
| ENSBTAG00000009155 | SCN5A | sodium channel protein type 5 subunit alpha | 2.51 | |
| ENSBTAG00000035995 | TNFAIP2 | TNF alpha induced protein 2 | 1.47 | |
| ENSBTAG00000015046 | MST1R | macrophage stimulating 1 receptor | 1.41 | |
| ENSBTAG00000037811 | CCL2 | C-C motif chemokine ligand 2 | 1.19 | |
| ENSBTAG00000017683 | LYSMD2 | LysM domain containing 2 | 1.13 | |
| ENSBTAG00000019587 | PI15 | peptidase inhibitor 15 | 1.12 | |
| ENSBTAG00000010987 | NF-kappa-B inhibitor zeta | 1.12 | ||
| ENSBTAG00000013163 | ADAM33 | ADAM metallopeptidase domain 33 | 1.11 | |
| ENSBTAG00000007921 | DAPP1 | dual adaptor of phosphotyrosine and 3-phosphoinositides 1 | 1.10 | |
| ENSBTAG00000006366 | NFATC4 | nuclear factor of activated T-cells 4 | 1.10 | |
| ENSBTAG00000011115 | CH25H | cholesterol 25-hydroxylase | 1.06 | |
| ENSBTAG00000009891 | PPP4R4 | protein phosphatase 4 regulatory subunit 4 | 1.06 | |
| ENSBTAG00000021568 | VWCE | von Willebrand factor C and EGF domains | 1.05 | |
| ENSBTAG00000013225 | NBN | nibrin | 0.98 | |
| ENSBTAG00000004386 | SOCS1 | suppressor of cytokine signaling 1 | 0.95 | |
| ENSBTAG00000008690 | n.a. | n.a. | 0.95 | |
| Lowest expression | ||||
| ENSBTAG00000019124 | EIF4EBP2 | eukaryotic translation initiation factor 4E binding protein 2 | −0.81 | |
| ENSBTAG00000002914 | GALNT18 | polypeptide N-acetylgalactosaminyltransferase 18 | −0.82 | |
| ENSBTAG00000031658 | SEMA6B | semaphorin 6B | −0.86 | |
| ENSBTAG00000007206 | STRIP2 | striatin interacting protein 2 | −0.89 | |
| ENSBTAG00000015177 | PRSS23 | protease, serine 23 | −0.90 | |
| ENSBTAG00000019049 | AATK | apoptosis associated tyrosine kinase | −0.90 | |
| ENSBTAG00000020854 | BCL6B | B-cell CLL/lymphoma 6B | −0.90 | |
| ENSBTAG00000015108 | USHBP1 | USH1 protein network component harmonin binding protein 1 | −0.93 | |
| ENSBTAG00000035836 | COLGALT2 | collagen beta(1-O)galactosyltransferase 2 | −0.98 | |
| ENSBTAG00000020717 | CXorf36 | chromosome X open reading frame 36 | −1.01 | |
| ENSBTAG00000012265 | VWF | von Willebrand factor precursor | −1.02 | |
| ENSBTAG00000004542 | FAM78A | family with sequence similarity 78 member A | −1.08 | |
| ENSBTAG00000021697 | PDGFB | platelet derived growth factor subunit B | −1.08 | |
| ENSBTAG00000025140 | DOC2B | double C2 domain beta | −1.09 | |
| ENSBTAG00000019733 | ADGRL4 | adhesion G protein-coupled receptor L4 | −1.12 | |
| ENSBTAG00000020839 | MEGF6 | multiple EGF like domains 6 | −1.13 | |
| ENSBTAG00000000781 | HIP1 | huntingtin interacting protein 1 | −1.14 | |
| ENSBTAG00000000745 | n.a. | n.a. | −1.37 | |
| ENSBTAG00000045610 | CACNA1I | calcium voltage-gated channel subunit alpha1 I | −3.70 | |
| ENSBTAG00000012785 | TEPP | testis, prostate and placenta expressed | −4.09 |
Refer to Figure 3. For full list of DEG see Supplemental Table S7. Positive and negative values refer to up- or downregulation of gene expression, respectively.
Discussion
The endometrium can act as a sensor of embryo quality, responding differently in terms of its gene expression signature to embryos of varying quality [25–27]. Although the uterine milieu is critical for the survival and growth of the conceptus, an appropriate uterine environment may not be sufficient to ensure pregnancy maintenance if the quality of the embryo is intrinsically compromised. The underlying factors that regulate conceptus–maternal crosstalk between normal (long) and presumed lagging (short) conceptuses and the endometrium are unknown. The current study combined in vitro production of bovine blastocysts, multiple embryo transfer, and conceptus-endometrial explant co-culture to investigate the response of the endometrium to age-matched conceptuses of different sizes collected from the same uterine environment. The main findings were that: (i) day 15 conceptuses vary significantly in length even when derived from the same uterine environment; and (ii) the endometrium responds in an IFNT-dependent and independent manner to conceptuses of different sizes which likely reflects the ability to successfully establish pregnancy.
Conceptus length in the preimplantation period has been suggested to be indicative of quality and the likelihood of establishing and maintaining a pregnancy [14, 44]. However, this has not been firmly established. Numerous studies have suggested that a failure in the mechanisms involved in conceptus elongation and maternal pregnancy recognition may be the cause of early pregnancy loss in subfertile and infertile heifers up to and including implantation [37, 45]. Interestingly, despite the clear association between P4 concentrations and conceptus elongation [13, 17, 46], significant variation in size exists between conceptuses recovered on the same day regardless of embryo source (in vivo or in vitro) [22, 24] or P4 concentration [23]. In the present study, significant variation in day 15 conceptus length and morphology was also observed within recipient (Figure 2), despite the fact that embryos were produced in vitro under the same conditions until the blastocyst stage and were of similar morphological quality at the time of transfer on day 7. While it is well accepted that elongation is a maternally driven process, the fact that such embryos were exposed to a common uterine environment would suggest that, at least, part of the ability to elongate is intrinsic to the embryo and may have origins in follicle/oocyte quality. In the pig, asynchrony of trophectoderm elongation is also evident amongst conceptuses growing in a common uterine environment, and rapid progression through this phase has been associated with conceptus competency [47].
The transfer of multiple embryos into the same uterus allows characterization of the variation in conceptus length within a common uterine environment. While this may not be reflective of the physiological conditions in cattle where, typically, a single embryo is present, we are not aware of any evidence that the early development of up to 10 embryos in the uterus is compromised compared to a single embryo. This is supported by the fact that the variation in conceptus length and morphology observed was similar to that reported by Ribeiro et al. [48], who reported the length of recovered conceptuses (n = 160) on day 15 after artificial insemination of nonsuperstimulated dairy cows.
In cattle, maternal recognition of pregnancy and prevention of luteolysis is dependent on conceptus-derived IFNT [49]. Appropriate communication between the developing embryo and the mother is vital for the successful establishment and maintenance of pregnancy. We and others have previously used an endometrial explant co-culture system to elucidate this fine dialogue by examining changes in endometrial gene expression induced by blastocysts [50], an elongating conceptus [36], or conceptus secretory proteins [51]. Due to the maintenance of normal cellular and extracellular architecture in endometrial explants [36], some of the limitations of traditional cell culture can be overcome; for example, uterine explants allow the communication between resident populations of endometrial cells which cannot be achieved with current 2D and 3D cell culture technologies. We hypothesized that differences in endometrial response to long and short conceptuses could be either dependent or independent of IFNT. The following discussion focuses on three categories of DEG compared to the control endometrium: (i) genes commonly responsive to exposure to IFNT and conceptuses, irrespective of size (n = 223); (ii) genes commonly responsive to IFNT and long conceptuses only (n = 168), and (iii) genes induced by the presence of a conceptus but independent of IFNT (n = 108).
IFNT-dependent endometrial response to the conceptus
RNA-Seq analysis revealed 223 DEGs common to IFNT- and conceptus-exposed explants compared to control explants, all of which were upregulated. This is not surprising given that the maternal pregnancy recognition signal is conceptus-derived IFNT. The conceptus trophectoderm expresses IFNT as early as the blastocyst stage of development and secretes detectable quantities of IFNT when cultured in vitro [52, 53]. However, major effects on endometrial gene expression in vivo are not detectable until day 15–16 of pregnancy [54, 55]. Consistent with our observations, Forde et al. [56] found that, of genes upregulated in pregnant endometria, MX2, bone marrow stromal cell antigen 2 (BST2), RSAD2, ISG15, 2′,5′- oligoadenylate synthetase 1, 40/46 kDa (OAS1X), ubiquitin-specific peptidase 18 (USP18), interferon-induced protein 44 (IFI44), sterile alpha motif domain containing 9 (SAMD9), transcript variant 2, mRNA, and interferon-induced protein with tetratricopeptide repeats 2 (IFIT2) were among the most highly expressed. Moreover, in the latter study, the expression of BST2, IFI44, RSAD2, SAMD9, and USP18 also increased in the intercaruncular regions of the endometrium exposed for 2 h to ovine recombinant IFNT. Similar data have been reported from comparison of pregnant and cyclic heifers on day 15 and 18 [52]. These commonalities between our ex vivo findings and those derived from in vivo studies reinforce the validity of the present model.
Given the large number of IFNT-induced genes common to all three groups, one could hypothesize that differences in the ability to trigger the main signal for maternal recognition of pregnancy (dependent on IFNT) between long or short conceptuses may not fully explain putative differences in developmental competence. However, the expression network analysis indicated that, although those transcripts were commonly regulated by long and short conceptuses, they cluster differently in the network topology, reflecting a variation in the response of the endometrium to conceptuses of different sizes that could also be associated with lower production of IFNT by short conceptuses [57].
There were 100 genes uniquely differentially expressed in IFNT-treated endometrium (i.e. not induced by either a long or short conceptus). These may possibly be explained by the concentration of IFNT used (100 ng/mL), which may be higher than that produced by the conceptuses in the 6 h of culture. The concentration of IFNT used was based on RIA measurements made from (i) uterine fluids and (ii) from the in vitro production of elongated sheep conceptuses and used previously in endometrial cell cultures [58]. In addition, we have previously reported a positive correlation between conceptus length and IFNT production [57]. Based on those data, a conceptus of 20 mm length produced approximately 10 000 IU/mL (=100 ng/mL) IFNT in 24 h, while a conceptus of 2 mm produced approximately 10 ng/mL. Thus, it is likely that after 6 h co-culture, the amount of IFNT in the conceptus-treated explants was less than 100 ng/mL. The fact that recombinant ovine IFNT was used may also be a contributory factor.
Threshold concentrations of IFNT required for pregnancy maintenance, below which pregnancy will not be maintained, are still not known [59]; however, the effect is likely acute given that pregnancy can be established even when embryos are transferred just before luteolysis [22, 60]. While studies in the mid-eighties involving the transfer of trophoblastic vesicles at the time of embryo transfer were based on the hypothesis that the additional IFNT produced by the trophoblast vesicles would augment the conceptus-derived product and improve embryo survival [61], other studies have shown that advanced conceptuses, which produce large quantities of IFNT, may not always be associated with improved pregnancy rates [46].
Direct comparison of endometrial transcriptomes induced by long and short conceptuses identified nine genes that were differentially expressed in response to conceptus size. Long conceptuses increased the expression of CXCL11, CCL8, WARS, GBP1, GBP6, CD274, MB21D1, CDADC1, and FBXO33 in the endometrium compared to short conceptuses. CXCL11, a chemokine strongly stimulated in a dose-dependent manner by IFN-gamma (type I IFN), from endometrial epithelial cells stimulates migration of trophoblast cells and T cells in women [62]. In addition, CXCL11 stimulated proliferation of endometrial stromal cells and apoptosis of endometrial epithelial cells in the same study. This process is of particular importance during implantation in species with invasive placentation such as primates and rodents [63]. However, despite the relatively noninvasive synepitheliochorial placentation in ruminants, the endometrial stroma also undergoes structural changes and angiogenesis [64, 65]. Consistent with our results, a recent study found mRNA expression of CCL8 higher in the bovine endometrium on both day 15 and 18 of pregnancy [66]. Moreover, in that study, stimulation with IFNT increased CCL8, which in turn decreased both the expression of prostaglandin-endoperoxide synthase 2 (PTGS2) and OXTR in endometrial cell culture. Therefore, CCL8 could contribute to inhibition of endometrial PGF2α production, as it is known that oxytocin stimulates PG production by activating PTGS2 in endometrial cells [67]. WARS is a member of aminoacyl-tRNA synthetase family that catalyzes the aminoacylation of tRNAtrp with tryptophan, a critical step in cellular protein synthesis, and is stimulated by IFN-gamma [68]. In a previous study from our laboratory, WARS was one of the 27 DEGs on day 16 as part of the early endometrial response to the conceptus and may represent an early endometrial marker of a viable conceptus [56]. In addition, others have also shown that WARS mRNA or protein expression increased in the endometrium of pregnant cows on day 17 [69] and pregnant ewes on day 16 [70]. Other transcripts differentially expressed between long and short conceptuses in the current study were guanylate binding proteins (GBP), which belong to a family of GTPases. GBPs regulate inhibition of proliferation, invasion of endothelial cells, and cell survival [69]. As in this study, GBP1 and GBP6 were upregulated in the endometrium of pregnant cows in the preimplantation period in other studies [69, 71]. Schnoor et al. [72] found that GBP1 is regulated by IFN-gamma and their findings suggested that the functional significance of GBP upregulation is to protect cells against proinflammatory cytokine induced apoptosis. CD274 (also known as programmed cell death ligand 1, PD-L1) is suggested to promote and enhance induced regulatory T cells [73]. In agreement with our results, Vasudevan et al. [74] observed that the abundance of mRNA of CD274 was 18-fold greater in pregnant compared to cyclic endometrium in dairy heifers on day 17, followed by a reduction on day 20. Moreover, inhibition or absence of expression of CD274 during pregnancy in mice led to increased maternal rejection in allogeneic, but not syngeneic, pregnancies [75], highlighting its role in immune modulation during early pregnancy. All these nine DEGs were common to IFNT, and no differences in their expression between IFNT and long conceptuses were found. Moreover, as has been shown above, some of these transcripts are stimulated in a dose-dependent manner by type I IFNs. Therefore, differences in expression level of those transcripts between long and short conceptuses could be due to differences in IFNT production by such embryos [57]. These findings suggest that conceptus-derived IFNT induces uterine receptivity by stimulating the endometrium to produce chemokines, cytokine, transcription factors, etc. which may induce inhibition of endometrial PGF2α, migration of trophoblast cell and T cells, proliferation of endometrial stromal cells, and apoptosis of endometrial epithelial cells. The regulation of this cellular movement by factors such as chemokines may promote the establishment of immunological environments suitable for conceptus implantation and subsequent development [76].
IFNT-dependent endometrial response to conceptuses of different sizes
Another interesting group of DEGs are those transcripts that were common to IFNT and long conceptuses (n = 168) as they represent genes that are induced in the endometrium, presumably by a threshold level of IFNT which short conceptuses are unable to induce. Functional analysis of this gene set showed that they are mainly overrepresented by metabolism, cell-cell adhesion, receptor-mediated endocytosis, mitochondrion organization, S-adenosylmethionine biosynthesis, determination of ventral identity, and glucocorticoid-mediated signaling pathway functions. Nutrients, including amino acids and lipids, are important components of maternally derived secretions that are crucial for embryonic survival before implantation [77, 78]. For that reason, it is not surprising that maternal metabolism pathways change during early pregnancy [79]. Our results suggest that those metabolic pathways could be regulated by conceptuses of different sizes via IFNT possibly because of the increased energy demands of long conceptuses. This is consistent with recent studies in which the top biological processes enriched with DEG between long and short conceptuses were related to metabolism and biosynthesis [14, 48].
IFNT secreted by long conceptuses may regulate the molecular dialogue between the conceptus and the endometrium by modulating the receptor-mediated endocytosis pathway as this is highly selective for absorption of macromolecular ligands such as peptide signaling growth factors, cytokines, hormones, or macromolecules involved in cell metabolism [80]. In response to this stimulus, mitochondria may adapt their structure and function [81] in order to support maximal ATP demands. In the current study, DEGs exclusively regulated by IFNT and long conceptuses were also overrepresented by the S-adenosylmethionine biosynthesis function. S-adenosylmethionine is an important metabolic intermediate in methionine [82] that inhibits the inflammatory reaction and oxidative stress and regulates the calcium (Ca2+) channel expression in the endometrium, attenuating uterine contraction in mice [83]. Interestingly, methionine sulfoxide reductase B3 (MSRB3) mRNA is highly expressed in long conceptuses [14]. The protein encoded by this gene catalyzes the reduction of methionine sulfoxide to methionine. As myometrium was removed from the explants, S-adenosylmethionine would be involved more likely in the regulation of inflammatory reaction and metabolism. Finally, it has been reported that cortisol is locally regulated in bovine endometrium throughout the estrous cycle and acts as a luteoprotective factor by selectively suppressing luteolytic PGF2α production without affecting basal PGE2 production (luteotropic) [84]. A previous study showed that mRNA expression of nuclear receptor subfamily 3 group C member 1 (NR3C1), a glucocorticoid receptor, was significantly higher in the bovine endometrium of pregnant compared to cyclic cows [85]. Moreover, IFNT modulated synthesis and secretion of cortisol in bovine endometrium [85]. In sheep, cortisol acts via NR3C1 to regulate endometrial functions during early pregnancy [86].
IFNT-independent endometrial response to conceptuses of different sizes
A previous study from our group described a group of proteins unique to the uterine luminal fluid of pregnant heifers on day 16 that were also produced by day 16 conceptuses following short-term culture in vitro [56]. It is reasonable to hypothesize that these conceptus-derived factors may stimulate more understated, localized gene expression changes in the endometrium during early pregnancy that are independent of IFNT. Studies identifying endometrial responses to these factors or to the early elongating conceptus, independent of IFNT, are limited and/or challenging to execute. Here using an ex vivo model, we identified that 2 out of 30 proteins secreted by a conceptus and detected in uterine luminal fluid of pregnant heifers (see [56]) are related with two DEGs uniquely regulated by long conceptuses. Heterogeneous nuclear ribonucleoprotein A2/B1 isoform A2 (HNRNPA2B1) and keratin type II cytoskeleton 75 (KRT25) produced by the conceptus may upregulate the expression of the genes topoisomerase (DNA) I (TOP1) and GABA type A receptor associated protein like 1 (GABARAPL1) in the endometrium of pregnant heifers and in the present study, respectively. DNA topoisomerases play key roles in mRNA transcription, DNA synthesis, DNA repair, and DNA recombination [87]. Conceptus–maternal interaction requires a continuous exchange of information and thus, the modulation of these processes, possibly by this enzyme, seems to play a fundamental role. However, to our knowledge, no information regarding the role of TOP1 in bovine endometrium is available in the literature. GABARAPL1, a member of GABARAP family, plays an important role in cell proliferation, invasion, and autophagic flux, as well as in mitochondrial homeostasis and cellular metabolic programs [88]. As has been mentioned along the discussion, most, if not all, of these processes are modulated in the endometrium exposed to a conceptus; however, the specific implication of GABARAPL1 during pregnancy needs further investigation.
Functional analysis revealed that regulation of molecular function, magnesium ion (Mg2+) transmembrane transport, regulation of cytokinesis, clathrin coat assembly, and beta-amyloid metabolism were the principal GO terms associated with IFNT-independent DEG in bovine endometrium. Several studies suggest that Mg2+ has significant impact on the metabolic state, which is mediated by its stimulatory effect on mitochondrial enzymes (reviewed by [89]). The effect of Mg2+ on energy metabolism partially interferes with the stimulatory effect of Ca2+ on energy metabolism and mitochondrial Ca2+ transport, causing inhibition of mitochondrial Ca2+ uptake [90]. In fact, several genes among the most downregulated in this set of DEGs are related to Ca2+ import and Ca2+ binding as calcium voltage-gated channel subunit alpha 1I (CACNA1I), multiple EGF-like domains 6 (MEGF6), adhesion 6 protein-coupled receptor L4 (ADGRL4), and double C2 domain beta (DOC2B). However, this elevation in the metabolism can cause mitochondrial oxidative stress. Mitochondrial inner membrane protein (MPV17L), one of the most upregulated transcripts in this genes set, has been reported to protect against reactive oxygen species-induced mitochondrial dysfunction [91]. This further suggests that long conceptus modulates the energy metabolism in the endometrium. Regarding beta-amyloid metabolism, the presence of excessive levels of cholesterol leads to increased production of beta-amyloid [92]. Metabolomic studies have demonstrated that lipids are available in the uterine fluid to be used by conceptus cells and the presence of an elongating conceptus causes changes in the lipid profile that may be associated with important biological events occurring during elongation [78]. Cholesterol is the precursor of glucocorticoids which its importance in the endometrium have previously been discussed above. Due to the intense molecule trafficking between long conceptuses and the endometrium, it is not surprising that clathrin coat assembly is one of the most overrepresented in the endometrium as clathrin-coated vesicles mediate the internalization of cargo molecules such as nutrients, hormones, and lipoproteins. Finally, several of the most upregulated transcripts exclusively regulated by long conceptuses [interleukin-17 (IL17), macrophage stimulating 1 receptor (MST1R), and chemokine C-C motif ligand 2 (CCL2)] are involved in inflammatory and immune response. In agreement with our result, a recent study has shown that expression of CCL2 was higher in the endometrium of day 15 of pregnant heifers compared with cyclic heifers [66]. Moreover, CCL2 decreased PGTS2 expression, suggesting that it may subsequently inhibit PGF2α production to establish pregnancy in cows. Interestingly, and as it occurs in the present study, those authors found that CCL2 expression was not affected by IFNT. Altogether, these findings reveal that there are factors other than IFNT that participate in the dialogue between the conceptus and the endometrium during the early pregnancy in cattle, and those factors are dependent on the conceptus development in terms of its length.
In conclusion, bovine endometrium responds differently in terms of its gene expression signature to age-matched long and short conceptuses in an IFNT-dependent and independent manner, which may be critical for embryo survival. In particular, short conceptuses failed to alter the expression of a large number of ISGs that were altered by both IFNT and long conceptuses, suggesting that insufficient IFNT production is a major contributory factor to lower survival of such conceptuses. Furthermore, the alteration of >100 endometrial transcripts uniquely by long conceptuses suggests that other aspects of maternal–embryo communication at this critical time are IFNT-independent.
Supplementary Material
Acknowledgments
The authors thank the farm staff at UCD Lyons Research Farm for their collaboration and patience. The authors acknowledge the excellent assistance of numerous colleagues and several PhD students for their assistance during embryo transfers and sample processing, especially Mary Wade, Dr John A. Browne and Dr Beatriz Fernández-Fuertes. Inmaculada Cuevas Gómez made enormous contribution to the elaboration of some figures and tables. Also, the authors thank Ceva Santé Animale for their collaboration.
Notes
All raw and processed data will be publicly available at the Gene Expression Omnibus (GEO) database.
Conference presentation. Presented in part at the 33rd Scientific meeting of the Association of Embryo Technology in Europe (AETE), Bath, UK, 2017.
Edited by Dr. Peter J. Hansen, PhD, University of Florida
Footnotes
Grant support. This work was funded by the Irish Department of Agriculture, Food and the Marine (Research Stimulus Fund, Grant 13S528) and Science Foundation Ireland (Grant 13/IA/1983) as well as NIH Grant R01HD072898.
Supplementary data
Supplemental Table S1. Differentially expressed genes in the bovine endometrium exposed to 100 ng/mL recombinant ovine IFNT compared to the control endometrium (>2 fold change, FDR P < 0.05).
Supplemental Table S2. Differentially expressed genes in the bovine endometrium exposed to long day 15 conceptuses compared to the control endometrium (>2 fold change, FDR P < 0.05).
Supplemental Table S3. Differentially expressed genes in the bovine endometrium exposed to short day 15 conceptuses compared to the control endometrium (>2 fold change, FDR P < 0.05).
Supplemental Table S4. Common genes differentially expressed in bovine endometrium explants co-cultured with 100 ng/mL of recombinant ovine interferon-tau (IFNT), Long day 15 conceptuses or short day 15 conceptus compared to control endometrium (>2 fold change, FDR P < 0.05).
Supplemental Table S5. Gene ontology (GO) analysis.
Supplemental Table S6. Common genes differentially expressed in bovine endometrium explants co-cultured with 100 ng/mL of recombinant ovine interferon-tau (IFNT) or long day 15 conceptus compared to control endometrium (>2 fold change, FDR P < 0.05).
Supplemental Table S7. Differentially expressed genes unique in bovine endometrium explants co-cultured with long day 15 conceptuses (>2 fold change, FDR P < 0.05).
References
- 1. Sartori R, Bastos MR, Wiltbank MC. Factors affecting fertilisation and early embryo quality in single- and superovulated dairy cattle. Reprod Fertil Dev 2010; 22(1):151–158. [DOI] [PubMed] [Google Scholar]
- 2. Wiltbank MC, Baez GM, Garcia-Guerra A, Toledo MZ, Monteiro PLJ, Melo LF, Ochoa JC, Santos JEP, Sartori R. Pivotal periods for pregnancy loss during the first trimester of gestation in lactating dairy cows. Theriogenology 2016; 86(1):239–253. [DOI] [PubMed] [Google Scholar]
- 3. Diskin MG, Morris DG. Embryonic and early foetal losses in cattle and other ruminants. Reprod Domest Anim 2008; 43(suppl 2):260–267. [DOI] [PubMed] [Google Scholar]
- 4. Roberts RM. 30 years on from the molecular cloning of interferon-tau. Reproduction 2017; 154(5):E1–E2. [DOI] [PubMed] [Google Scholar]
- 5. Bazer FW, Thatcher WW. Chronicling the discovery of interferon tau. Reproduction 2017; 154(5):F11–F20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forde N, Lonergan P. Transcriptomic analysis of the bovine endometrium: What is required to establish uterine receptivity to implantation in cattle? J Reprod Dev 2012; 58(2):189–195. [DOI] [PubMed] [Google Scholar]
- 7. Brandão DO, Maddox-Hyttel P, Løvendahl P, Rumpf R, Stringfellow D, Callesen H. Post hatching development: a novel system for extended in vitro culture of bovine embryos. Biol Reprod 2004; 71(6):2048–2055. [DOI] [PubMed] [Google Scholar]
- 8. Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002; 124:289–300. [PubMed] [Google Scholar]
- 9. Brooks K, Burns G, Spencer TE. Conceptus elongation in ruminants: roles of progesterone, prostaglandin, interferon tau and cortisol. J Anim Sci Biotechnol 2014; 5(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forde N, Carter F, Fair T, Crowe MA, Evans ACO, Spencer TE, Bazer FW, McBride R, Boland MP, O’Gaora P, Lonergan P, Roche JF. Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol Reprod 2009; 81(4):784–794. [DOI] [PubMed] [Google Scholar]
- 11. Lonergan P, Forde N, Spencer T. Role of progesterone in embryo development in cattle. Reprod Fertil Dev 2016; 28(2):66–74. [DOI] [PubMed] [Google Scholar]
- 12. Spencer TE, Forde N, Lonergan P. The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants. J Dairy Sci 2016; 99(7):5941–5950. [DOI] [PubMed] [Google Scholar]
- 13. Forde N, Mehta JP, Minten M, Crowe MA, Roche JF, Spencer TE, Lonergan P. Effects of low progesterone on the endometrial transcriptome in cattle. Biol Reprod 2012; 87(5):124. [DOI] [PubMed] [Google Scholar]
- 14. Barnwell CV, Farin PW, Ashwell CM, Farmer WT, Galphin SP, Farin CE. Differences in mRNA populations of short and long bovine conceptuses on Day 15 of gestation. Mol Reprod Dev 2016; 83(5):424–441. [DOI] [PubMed] [Google Scholar]
- 15. Diskin MG, Murphy JJ, Sreenan JM. Embryo survival in dairy cows managed under pastoral conditions. Anim Reprod Sci 2006; 96(3-4):297–311. [DOI] [PubMed] [Google Scholar]
- 16. Wiltbank MC, Souza AH, Carvalho PD, Cunha AP, Giordano JO, Fricke PM, Baez GM, Diskin MG. Physiological and practical effects of progesterone on reproduction in dairy cattle. Animal 2014; 8(s1):70–81. [DOI] [PubMed] [Google Scholar]
- 17. Carter F, Forde N, Duffy P, Wade M, Fair T, Crowe MA, Evans ACO, Kenny DA, Roche JF, Lonergan P. Effect of increasing progesterone concentration from day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod Fertil Dev 2008; 20(3):368–375. [DOI] [PubMed] [Google Scholar]
- 18. Mann GE, Lamming GE. Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction 2001; 121:175–180. [DOI] [PubMed] [Google Scholar]
- 19. Ashworth CJ, Sales DI, Wilmut I. Evidence of an association between the survival of embryos and the periovulatory plasma progesterone concentration in the ewe. Reproduction 1989; 87(1):23–32. [DOI] [PubMed] [Google Scholar]
- 20. McNeill RE, Diskin MG, Sreenan JM, Morris DG. Associations between milk progesterone concentration on different days and with embryo survival during the early luteal phase in dairy cows. Theriogenology 2006; 65(7):1435–1441. [DOI] [PubMed] [Google Scholar]
- 21. Stronge AJH, Sreenan JM, Diskin MG, Mee JF, Kenny DA, Morris DG. Post-insemination milk progesterone concentration and embryo survival in dairy cows. Theriogenology 2005; 64(5):1212–1224. [DOI] [PubMed] [Google Scholar]
- 22. Betteridge KJ, Eaglesome MD, Randall GC, Mitchell D. Collection, description and transfer of embryos from cattle 10-16 days after oestrus. Reproduction 1980; 59(1):205–216. [DOI] [PubMed] [Google Scholar]
- 23. Clemente M, de La Fuente J, Fair T, Al Naib A, Gutierrez-Adan A, Roche JF, Rizos D, Lonergan P. Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium? Reproduction 2009; 138(3):507–517. [DOI] [PubMed] [Google Scholar]
- 24. van Leeuwen J, Berg DK, Pfeffer PL. Morphological and gene expression changes in cattle embryos from hatched blastocyst to early gastrulation stages after transfer of in vitro produced embryos. PLoS One 2015; 10(6):e0129787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bauersachs S, Ulbrich SE, Zakhartchenko V, Minten M, Reichenbach M, Reichenbach H-D, Blum H, Spencer TE, Wolf E. The endometrium responds differently to cloned versus fertilized embryos. Proc Natl Acad Sci USA 2009; 106(14):5681–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macklon NS, Brosens JJ. The human endometrium as a sensor of embryo quality. Biol Reprod 2014; 91(4):98. [DOI] [PubMed] [Google Scholar]
- 27. Mansouri-Attia N, Sandra O, Aubert J, Degrelle S, Everts RE, Giraud-Delville C, Heyman Y, Galio L, Hue I, Yang X, Tian XC, Lewin HA et al.. Endometrium as an early sensor of in vitro embryo manipulation technologies. Proc Natl Acad Sci USA 2009; 106(14):5687–5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walker WL, Nebel RL, McGilliard ML. Time of ovulation relative to mounting activity in dairy cattle. J Dairy Sci 1996; 79(9):1555–1561. [DOI] [PubMed] [Google Scholar]
- 29. Valenza A, Giordano JO, Lopes G, Vincenti L, Amundson MC, Fricke PM. Assessment of an accelerometer system for detection of estrus and treatment with gonadotropin-releasing hormone at the time of insemination in lactating dairy cows. J Dairy Sci 2012; 95(12):7115–7127. [DOI] [PubMed] [Google Scholar]
- 30. Randi F, McDonald M, Duffy P, Kelly AK, Lonergan P. The relationship between external auditory canal temperature and onset of estrus and ovulation in beef heifers. Theriogenology 2018; 110:175–181. [DOI] [PubMed] [Google Scholar]
- 31. Stringfellow DA, Givens MD International Embryo Transfer Society . Manual of the International Embryo Transfer Society: A Procedural Guide and General Information for the Use of Embryo Transfer Technology Emphasizing Sanitary Procedures. Savory, Ill.: International Embryo Transfer Society; 2010. [Google Scholar]
- 32. Degrelle SA, Campion E, Cabau C, Piumi F, Reinaud P, Richard C, Renard J-P, Hue I. Molecular evidence for a critical period in mural trophoblast development in bovine blastocysts. Dev Biol 2005; 288(2):448–460. [DOI] [PubMed] [Google Scholar]
- 33. Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol Reprod Dev 2002; 61(2):234–248. [DOI] [PubMed] [Google Scholar]
- 34. Ireland JJ, Murphee RL, Coulson PB. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J Dairy Sci 1980; 63(1):155–160. [DOI] [PubMed] [Google Scholar]
- 35. Mathew DJ, Sánchez JM, Passaro C, Charpigny G, Susanta SK, Spencer TE, Lonergan P. Interferon tau-dependent and independent effects of the bovine conceptus on the endometrial transcriptome. Biol Reprod 2019; 100(2):365–380. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borges AM, Healey GD, Sheldon IM. Explants of intact endometrium to model bovine innate immunity and inflammation ex vivo. Am J Reprod Immunol 2012; 67:526–539. [DOI] [PubMed] [Google Scholar]
- 37. Moraes JGN, Behura SK, Geary TW, Hansen PJ, Neibergs HL, Spencer TE. Uterine influences on conceptus development in fertility-classified animals. Proc Natl Acad Sci USA 2018; 115:E1749–E1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 2015; 12:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014; 30:923–930. [DOI] [PubMed] [Google Scholar]
- 40. Zhou X, Lindsay H, Robinson MD. Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res 2014; 42:e91–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 2011; 6:e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cover TM, Thomas JA. Elements of Information Theory. John Wiley & Sons, New York, NY, 1991. [Google Scholar]
- 43. Ding C, Peng H. Minimum redundancy feature selection from microarray gene expression data. J Bioinform Comput Biol 2005; 3:185–205. [DOI] [PubMed] [Google Scholar]
- 44. Matsuyama S, Kojima T, Kato S, Kimura K. Relationship between quantity of IFNT estimated by IFN-stimulated gene expression in peripheral blood mononuclear cells and bovine embryonic mortality after AI or ET. Reprod Biol Endocrinol 2012; 10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McMillan WH, Donnison MJ. Understanding maternal contributions to fertility in recipient cattle: development of herds with contrasting pregnancy rates. Anim Reprod Sci 1999; 57:127–140. [DOI] [PubMed] [Google Scholar]
- 46. Randi F, Fernandez-Fuertes B, McDonald M, Forde N, Kelly AK, Bastos Amorin H, Muniz de Lima E, Morotti F, Marcondes Seneda M, Lonergan P. Asynchronous embryo transfer as a tool to understand embryo?uterine interaction in cattle: is a large conceptus a good thing? Reprod Fertil Dev 2016; 28:1999–2006. [DOI] [PubMed] [Google Scholar]
- 47. Blomberg LA, Schreier L, Li RW. Characteristics of peri-implantation porcine concepti population and maternal milieu influence the transcriptome profile. Mol Reprod Dev 2010; 77:978–989. [DOI] [PubMed] [Google Scholar]
- 48. Ribeiro ES, Greco LF, Bisinotto RS, Lima FS, Thatcher WW, Santos JE. Biology of preimplantation conceptus at the onset of elongation in dairy cows. Biol Reprod 2016; 94:97. [DOI] [PubMed] [Google Scholar]
- 49. Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction 2009; 138:195–209. [DOI] [PubMed] [Google Scholar]
- 50. Passaro C, Tutt D, Mathew DJ, Sanchez JM, Browne JA, Boe-Hansen G, Fair T, Lonergan P. Blastocyst-induced changes in the bovine endometrial transcriptome. Reproduction 2018; 156:219–229. [DOI] [PubMed] [Google Scholar]
- 51. Gross TS, Plante C, Thatcher WW, Hansen PJ, Helmer SD, Putney DJ. Secretory proteins of the bovine conceptus alter endometrial prostaglandin and protein secretion in vitro. Biol Reprod 1988; 39:977–987. [DOI] [PubMed] [Google Scholar]
- 52. Kubisch HM, Larson MA, Roberts RM. Relationship between age of blastocyst formation and interferon-τ secretion by in vitro–derived bovine embryos. Mol Reprod Dev 1998; 49:254–260. [DOI] [PubMed] [Google Scholar]
- 53. Lonergan P, Rizos D, Kanka J, Nemcova L, Mbaye AM, Kingston M, Wade M, Duffy P, Boland MP. Temporal sensitivity of bovine embryos to culture environment after fertilization and the implications for blastocyst quality. Reproduction 2003; 126:337–346. [DOI] [PubMed] [Google Scholar]
- 54. Bauersachs S, Ulbrich SE, Reichenbach H-D, Reichenbach M, Büttner M, Meyer HHD, Spencer TE, Minten M, Sax G, Winter G, Wolf E. Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol Reprod 2012; 86:46. [DOI] [PubMed] [Google Scholar]
- 55. Forde N, Carter F, Spencer TE, Bazer FW, Sandra O, Mansouri-Attia N, Okumu LA, McGettigan PA, Mehta JP, McBride R, O’Gaora P, Roche JF et al.. Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biol Reprod 2011; 85:144–156. [DOI] [PubMed] [Google Scholar]
- 56. Forde N, Bazer FW, Spencer TE, Lonergan P. ‘Conceptualizing’ the endometrium: identification of conceptus-derived proteins during early pregnancy in cattle. Biol Reprod 2015; 92:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rizos D, Scully S, Kelly AK, Ealy AD, Moros R, Duffy P, Al Naib A, Forde N, Lonergan P. Effects of human chorionic gonadotrophin administration on day 5 after oestrus on corpus luteum characteristics, circulating progesterone and conceptus elongation in cattle. Reprod Fertil Dev 2012; 24:472–481. [DOI] [PubMed] [Google Scholar]
- 58. Mansouri-Attia N, Aubert J, Reinaud P, Giraud-Delville C, Taghouti G, Galio L, Everts RE, Degrelle S, Richard C, Hue I, Yang X, Tian XC et al.. Gene expression profiles of bovine caruncular and intercaruncular endometrium at implantation. Physiol Genomics 2009; 39:14–27. [DOI] [PubMed] [Google Scholar]
- 59. Forde N, Lonergan P. Interferon-tau and fertility in ruminants. Reproduction 2017; 154:F33–F43. [DOI] [PubMed] [Google Scholar]
- 60. Kimura K, Matsuyama S. Successful nonsurgical transfer of bovine elongating conceptuses and its application to sexing. J Reprod Dev 2014; 60:210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Heyman Y, Chesné P, Chupin D, Ménézo Y. Improvement of survival rate of frozen cattle blastocysts after transfer with trophoblastic vesicles. Theriogenology 1987; 27:477–484. [DOI] [PubMed] [Google Scholar]
- 62. Hirota Y, Osuga Y, Koga K, Yoshino O, Hirata T, Morimoto C, Harada M, Takemura Y, Nose E, Yano T, Tsutsumi O, Taketani Y. The expression and possible roles of chemokine CXCL11 and its receptor CXCR3 in the human endometrium. J Immunol 2006; 177:8813–8821. [DOI] [PubMed] [Google Scholar]
- 63. Galán A, O’Connor JE, Valbuena D, Herrer R, Remohí J, Pampfer S, Pellicer A, Simón C. The human blastocyst regulates endometrial epithelial apoptosis in embryonic adhesion. Biol Reprod 2000; 63:430–439. [DOI] [PubMed] [Google Scholar]
- 64. King GJ, Atkinson BA, Robertson HA. Development of the bovine placentome from days 20 to 29 of gestation. Reproduction 1980; 59:95–100. [DOI] [PubMed] [Google Scholar]
- 65. Reynolds LP, Redmer DA. Growth and microvascular development of the uterus during early pregnancy in ewes. Biol Reprod 1992; 47:698–708. [DOI] [PubMed] [Google Scholar]
- 66. Sakumoto R, Hayashi K-G, Fujii S, Kanahara H, Hosoe M, Furusawa T, Kizaki K. Possible roles of CC- and CXC-chemokines in regulating bovine endometrial function during early pregnancy. Int J Mol Sci 2017; 18: E742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Asselin E, Drolet P, Fortier MA. Cellular mechanisms involved during oxytocin-induced prostaglandin F2alpha production in endometrial epithelial cells in vitro: role of cyclooxygenase-2. Endocrinology 1997; 138:4798–4805. [DOI] [PubMed] [Google Scholar]
- 68. Fleckner J, Rasmussen HH, Justesen J. Human interferon gamma potently induces the synthesis of a 55-kDa protein (gamma 2) highly homologous to rabbit peptide chain release factor and bovine tryptophanyl-tRNA synthetase. Proc Natl Acad Sci USA 1991; 88:11520–11524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Walker CG, Meier S, Littlejohn MD, Lehnert K, Roche JR, Mitchell MD. Modulation of the maternal immune system by the pre-implantation embryo. BMC Genomics 2010; 11:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Al-Gubory KH, Arianmanesh M, Garrel C, Bhattacharya S, Cash P, Fowler PA. Proteomic analysis of the sheep caruncular and intercaruncular endometrium reveals changes in functional proteins crucial for the establishment of pregnancy. Reproduction 2014; 147:599–614. [DOI] [PubMed] [Google Scholar]
- 71. Klein C, Bauersachs S, Ulbrich SE, Einspanier R, Meyer HHD, Schmidt SEM, Reichenbach H-D, Vermehren M, Sinowatz F, Blum H, Wolf E. Monozygotic twin model reveals novel embryo-induced transcriptome changes of bovine endometrium in the preattachment period. Biol Reprod 2006; 74:253–264. [DOI] [PubMed] [Google Scholar]
- 72. Schnoor M, Betanzos A, Weber DA, Parkos CA. Guanylate-binding protein-1 is expressed at tight junctions of intestinal epithelial cells in response to interferon-γ and regulates barrier function through effects on apoptosis. Mucosal Immunol 2009; 2:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206:3015–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vasudevan S, Kamat MM, Pate JL, Ott TL. Changes in uterine immune regulatory factors and effects of pregnancy associated glycoproteins on uterine immune cells during early pregnancy in dairy heifers. 49th Annual Meeting of the Society for the Study of Reproduction 2016, San Diego. [Google Scholar]
- 75. Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med 2005; 202:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kitaya K, Nakayama T, Daikoku N, Fushiki S, Honjo H. Spatial and temporal expression of ligands for CXCR3 and CXCR4 in human endometrium. J Clin Endocrinol Metab 2004; 89:2470–2476. [DOI] [PubMed] [Google Scholar]
- 77. França MR, da Silva MIS, Pugliesi G, Van Hoeck V, Binelli M. Evidence of endometrial amino acid metabolism and transport modulation by peri-ovulatory endocrine profiles driving uterine receptivity. J Anim Sci Biotechnol 2017; 8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ribeiro ES, Santos JEP, Thatcher WW. Role of lipids on elongation of the preimplantation conceptus in ruminants. Reproduction 2016; 152:R115–R126. [DOI] [PubMed] [Google Scholar]
- 79. Guo YS, Tao JZ. Metabolomics and pathway analyses to characterize metabolic alterations in pregnant dairy cows on D 17 and D 45 after AI. Sci Rep 2018; 8:5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kornilova ES. Receptor-mediated endocytosis and cytoskeleton. Biochemistry 2014; 79:865–878. [DOI] [PubMed] [Google Scholar]
- 81. Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science 2012; 337:1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Struck A-W, Thompson ML, Wong LS, Micklefield J. S-adenosyl-methionine-dependent methyltransferases: highly versatile enzyme in biocatalysis, biosynthesis and other biotechnological applications. ChemBioChem 2012; 13:2642–2655. [DOI] [PubMed] [Google Scholar]
- 83. Ge J, Han T, Li X, Shan L, Zhang J, Hong Y, Xia Y, Wang J, Hou M. S-adenosyl methionine regulates calcium channels and inhibits uterine smooth muscle contraction in rats with infectious premature delivery through the transient receptor protein 3/protein kinase Cβ/C-kinase-activated protein phosphatase-1 inhibitor of 17 kDa signaling pathway. Exp Ther Med 2018; 16:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee H-Y, Acosta TJ, Tanikawa M, Sakumoto R, Komiyama J, Tasaki Y, Piskula M, Skarzynski DJ, Tetsuka M, Okuda K. The role of glucocorticoid in the regulation of prostaglandin biosynthesis in non-pregnant bovine endometrium. J Endocrinol 2007; 193:127–135. [DOI] [PubMed] [Google Scholar]
- 85. Majewska M, Lee HY, Tasaki Y, Acosta TJ, Szostek AZ, Siemieniuch M, Okuda K, Skarzynski DJ. Is cortisol a modulator of interferon tau action in the endometrium during early pregnancy in cattle? J Reprod Immunol 2012; 93:82–93. [DOI] [PubMed] [Google Scholar]
- 86. Simmons RM, Satterfield MC, Welsh TH, Bazer FW, Spencer TE. HSD11B1, HSD11B2, PTGS2, and NR3C1 expression in the peri-implantation ovine uterus: effects of pregnancy, progesterone, and interferon tau1. Biol Reprod 2010; 82:35–43. [DOI] [PubMed] [Google Scholar]
- 87. Cretaio E, Pattarello L, Fontebasso Y, Benedetti P, Losasso C. Human DNA topoisomerase IB: structure and functions. Ital J Biochem 2007; 56:91–102. [PubMed] [Google Scholar]
- 88. Boyer-Guittaut M, Poillet L, Liang Q, Bôle-Richard E, Ouyang X, Benavides GA, Chakrama F-Z, Fraichard A, Darley-Usmar VM, Despouy G, Jouvenot M, Delage-Mourroux R et al.. The role of GABARAPL1/GEC1 in autophagic flux and mitochondrial quality control in MDA-MB-436 breast cancer cells. Autophagy 2014; 10:986–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pilchova I, Klacanova K, Tatarkova Z, Kaplan P, Racay P. The involvement of Mg2+ in regulation of cellular and mitochondrial functions. Oxid Med Cell Longev. 2017; 2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Boelens AD, Pradhan RK, Blomeyer CA, Camara AKS, Dash RK, Stowe DF. Extra-matrix Mg2+ limits Ca2+ uptake and modulates Ca2+ uptake-independent respiration and redox state in cardiac isolated mitochondria. J Bioenerg Biomembr 2013; 45:203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Krick S, Shi S, Ju W, Faul C, Tsai S, Mundel P, Böttinger EP. Mpv17l protects against mitochondrial oxidative stress and apoptosis by activation of Omi/HtrA2 protease. Proc Natl Acad Sci USA 2008; 105:14106–14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yao Z-X, Papadopoulos V. Function of β-amyloid in cholesterol transport: a lead to neurotoxicity. FASEB J 2002; 16:1677–1679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.