Abstract
Osteoarthritis is a prevalent and debilitating disease that involves pathological contributions from numerous joint tissues and cells. The joint is a challenging arena for drug delivery, since the joint has poor bioavailability for systemically administered drugs and experiences rapid clearance of therapeutics after intra-articular injection. Moreover, each tissue within the joint presents unique barriers to drug localization. In this review, the various applications of nanotechnology to overcome these drug delivery limitations are investigated. Nanomaterials have reliably shown improvements to retention profiles of drugs within the joint space relative to injected free drugs. Additionally, nanomaterials have been modified through active and passive targeting strategies to facilitate interactions with and localization within specific joint tissues such as cartilage and synovium. Last, the limitations of drawing cross-study comparisons, the implications of synovial fluid, and the potential importance of multi-modal therapeutic strategies are discussed. As emerging, cell-specific disease modifying osteoarthritis drugs continue to be developed, the need for targeted nanomaterial delivery will likely become critical for effective clinical translation of therapeutics for osteoarthritis.
Keywords: osteoarthritis, drug delivery, nanoparticles, nanomaterials, intra-articular, tissue targeting, cartilage, synovium, joint retention
Graphical Abstract
1. INTRODUCTION
Osteoarthritis (OA) is one of the most common joint disorders, affecting 151 million people worldwide [1], and is growing in prevalence [2,3]. In the United States alone, OA imposes a financial burden of $185.5 billion annually [4]. This disease considerably reduces the quality of life for patients and is associated with numerous comorbidities [5–9]. Clinically available interventions are limited to palliative care, and there is currently no pharmacologic option that impacts disease pathogenesis. Direct intra-articular injections of drugs are commonly used to overcome the poor joint bioavailability observed with systemic administration. Unfortunately, rapid drug clearance from the joint remains a critical limitation in drug efficacy for a majority of therapeutic molecules. This concept has been observed across a variety of substances ranging from small molecular drugs to high molecular weight macromolecules, and across animal species, as highlighted in Table 1 and described extensively in other reviews [10,11]. Significant effort has focused on engineering drug delivery systems to sustain drug levels within the joint for prolonged periods of time. Moving forward, however, many drug targets and emerging therapies require not only prolonged release, but also tissue and cell specificity. The goal of this review is to highlight the design considerations for nano-scale drug carriers to achieve site-specific delivery within the joint. This review will cover how different features of joint cells and tissues can be exploited for drug targeting, the current technologies in preclinical stages, and considerations for furthering the field of targeted OA drug delivery. Here, therapeutic delivery is considered from the perspectives of whole joint retention as well as tissue-specific intra-articular localization.
Table 1.
Half-lives or residence times of select OA therapeutics after intra-articular administration illustrate the short dwell time in the joint.
1.1. Treating OA as a disease of the whole joint
Osteoarthritis is a disabling degenerative joint disease that ultimately leads to chronic pain and inflammation in joints. The hallmark characteristic of OA pathology is the destruction of cartilage, the avascular tissue that lines the articular surface of bones and allows for near-frictionless articulation during movement [18,19]. The etiology of OA varies between patients and is often unknown, but once initiated, OA progression is driven by a vicious cycle of pathological changes in multiple joint tissues [20]. In early stages of OA, the cartilage releases products of proteoglycan degradation and damage-associated molecular patterns (DAMPs) into the joint space [21]. These molecules activate immune cells in the synovium, the cell-dense tissue that lines the joint space, which respond by secreting pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α) [22]. These cellular signals cause chondrocytes, the sole cell type of cartilage, to accelerate the production of catabolic enzymes such as matrix metalloproteinases (MMPs)-1, −3, and −13, and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 and −5 (aggrecanases-1 and −2) [23,24]. A positive feedback loop is thus initiated as these proteinases degrade the cartilage matrix and release more DAMPs, thereby propagating inflammatory cascades in the synovium and sustaining chronic low-grade inflammation. Coupled with the inherent limited self-renewal capabilities of cartilage [25], this environment yields conditions in which cartilage extracellular matrix (ECM) breakdown outweighs ECM repair or synthesis. As disease progresses, this pathological cycle is sustained and ultimately results in cartilage degradation, subchondral bone outgrowth, synovitis, disruption to ligament molecular structures, meniscal damage, and alternation of joint mechanics [26].
Clinical presentation of OA is typified by joint pain, at which point OA progression has often reached end stages [27] when the disease is no longer believed to be reversible. Disease modification and prevention is possible at early stages of OA when the cartilage has suffered only proteoglycan loss; however, the subsequent breakdown of the collagen network marks the point at which the disease is considered irreversible [23,28]. Unfortunately, early stage interventions can be difficult to implement as stratification of OA remains a challenge. Clinically, disease stage is assessed radiographically, which is indirect and insensitive [29], and the numerous phenotypes of OA, with various etiologies, may present differently [30]. However, one opportunity for therapeutic intervention during early stages of disease is in cases of post-traumatic OA (PTOA), a phenotype of OA associated with a traumatic injury that is often experienced in younger, active, and military populations [31]. In this subset of OA patients, there is a clear event associated with OA initiation, and therefore identifies a window for therapeutic intervention before OA progression reaches irreversible stages. Knowledge gained from therapeutic investigations of PTOA interventions may be applicable to other phenotypes of OA, which may become increasingly relevant as biomarkers and imaging techniques to understand disease progression are developed for the clinic [29,32].
Regardless of the OA phenotype, the pathological signals associated with OA progression transpire among the various joint tissues including cartilage, synovium, subchondral bone, fat pad, and synovial fluid, resulting in extensive tissue cross-talk and a multifaceted disease progression [5,26,33–35]. Cartilage and synovium are especially key players in the early stages of OA when the disease is still considered reversible - strategically timed and localized delivery to these tissues may allow for effective prevention of OA [28,36]. Each of these tissues undergoes unique pathological changes and contributes differently to exacerbating the OA environment [37]. For example, synoviocytes are responsible for producing a majority of the inflammatory cytokines in the joint, while chondrocyte catabolism and production of MMPs propagate the OA cycle and make cartilage self-repair unfeasible [38]. These and countless other self-destructive conditions occur contemporaneously; as such, disease reversal depends on addressing multiple pathological sources. Toward this strategy of multimodal disease intervention, there is a need to understand how to direct specific drugs to unique tissues of the joint. This approach would allow for high efficiency drug delivery with limited off-target effects and multifactorial mediation of disease pathology [39].
1.2. Therapeutic administration routes for the joint
Guiding drug localization through targeting joint tissues or cells could efficiently deliver a drug to the intended site of action, reduce the dose required to elicit a therapeutic effect, reduce off-target effects, and increase residence time within the target tissue and whole joint. Such improvements to drug localization could not only improve clinical joint retention and therapeutic effects, but also enable promising disease modifying OA drugs (DMOADs) to be clinically translatable.
Clinical options for OA drug delivery predominantly include systemic administration or direct injection into the joint. Oral administration is common for nonsteroidal anti-inflammatory drugs (NSAIDs) and other pain management drugs because of the ease and frequency of administration. However, prolonged systemic exposure to such drugs puts patients at risk of serious gastrointestinal and cardiovascular complications [40,41]. Additionally, OA is commonly localized in specific joints such as the knees or hips [3]. As such, local delivery is a more direct method than systemic administration for achieving therapeutic doses and reduced exposure to other areas of the body.
Intra-articular injection is an advantageous alternative to systemic administration for drugs intended for joint tissues but carry a high risk of systemic toxicity or that have poor stability and joint bioavailability [10,42]. After intra-articular injection, therapeutics bypass the biophysical barriers associated with systemic joint entry and are in immediate contact with joint tissues [43]. Despite its advantages, there are risks associated with intra-articular injections including mild swelling [44], infection [45], and insufficient duration of drug residence. Localization and prolonged retention are critical for therapeutics to act efficiently with minimal injections, so improving these conditions after intra-articular injection has been an area of interest for DMOAD delivery [11]. Overall, intra articular injection is a desirable alternative for administration of drugs intended to act locally with joint tissues, and will be the primary delivery route discussed this review.
1.3. Implications of OA on joint clearance and tissue targeting
The progression of OA complicates drug delivery because it affects both whole joint retention as well as targeting opportunities for individual tissues. Clearance of molecules from the joint space is facilitated by drainage through the lymphatics and a mix of fenestrated and non-fenestrated (continuous) capillaries underlying the synovium surrounding the joint [46,47]. In general, molecules less than 10 kDa are considered to be cleared via the capillaries while larger macromolecules are thought to be cleared via the lymphatics [12], however this phenomenon has the potential to change with disease. In addition to an influx of immune cells such as lymphocytes and macrophages into the synovium; fibrosis, edema and fibrin accumulation are commonly observed on the synovial surface with disease progression [48,49]. Drainage of larger macromolecules such as proteins has been demonstrated to be enhanced with rheumatoid arthritis (RA), an inflammatory arthritis driven by systemic inflammation and autoimmune dysfunction [50,51], due to greater synovial lymph flow [52]. However, the extent to which OA pathogenesis influences the clearance route of injected materials is still in early stages of exploration.
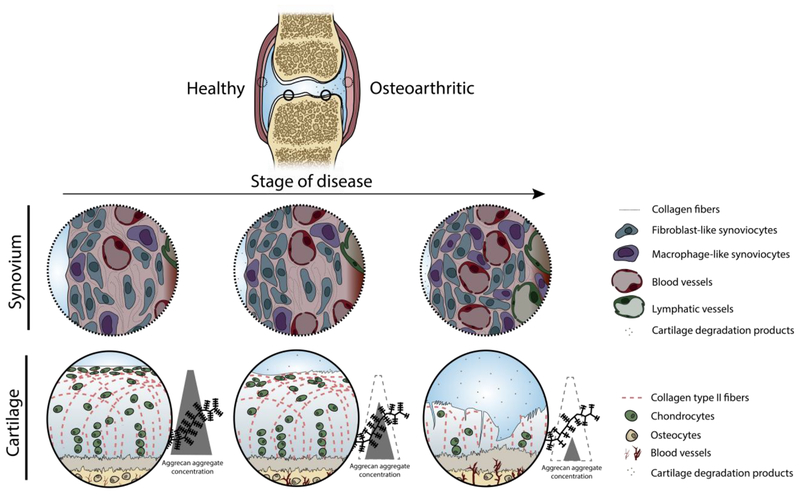
In addition to affecting joint clearance mechanisms, the progression of OA may affect tissue targeting within the joint as biochemical, structural, and compositional changes occur in the cartilage [53], synovium [54], subchondral bone [55], synovial fluid [56], and meniscal tissues [57]. Figure 1 highlights the structural and compositional changes that occur in the two main tissues targeted with injectable therapeutics: cartilage and synovium. Such structural and compositional changes have the potential to influence the fate of injected substances and are discussed in detail in later sections of this article.
Figure 1.
Structural and compositional changes to the joint during OA disease progression, highlighting the changes to the cartilage and synovium. Synovium undergoes marked immune cell infiltration, increased vascularization, and increased vascular fenestration. Cartilage breakdown begins at the articular surface with proteoglycan fragmentation and loss, surface fibrillation, and chondrocyte disorganization and clustering at the articular surface [58]. In late stages of disease, the cartilage has lost bulk tissue as proteoglycans are depleted, the collagen network degraded by MMPs, deep fibrillation and fissures have developed, and the remaining chondrocytes are unable to repair at a sufficient rate.
1.4. Improving joint drug delivery via nanotechnology
Nanomaterials can address the current challenges of intra-articular drug delivery. First, the size of nanomaterials can be engineered to clear more slowly than a free drug, thereby improving drug residence time and biodistribution in the joint. At the same time, nano-scale materials have the ability to penetrate ECM and cell barriers, thereby conferring the ability to release drugs intracellularly or within the tissue ECM [59]. These attributes have been harnessed in other disease applications, namely cancer and solid tumor targeting [60,61]. In the joint, cell and tissue penetration must be balanced with lymphatic clearance, as smaller materials can be removed from the joint more readily. Thus, careful design of nanomaterial size may offer better control over drug biodistribution and efficacy compared to macro-scale delivery systems.
The various forms of nanomaterials also provide opportunities to deliver different classes of drugs [62,63]. For example, lipid-based carriers are particularly suitable for loading hydrophobic drugs and have demonstrated loading efficiencies as high as 90% for corticosteroid delivery [64,65]. On the other hand, a range of protein-based, polar, hydrophobic, and hydrophilic drugs can be encapsulated in polymeric nanoparticles that have tunable degradation rates [66,67]. Emerging nanomaterial designs include those which are stimuli or environmentally responsive such that therapeutic release can be triggered upon an external stimulus or in response to disease-related changes in pH or oxidative stress [68,69]. Together, nanomaterials are capable of accommodating a range of drug classes that can serve as potential DMOADs, including enzyme inhibitors, cytokine inhibitors, growth factors, and subchondral bone regulators [70].
Considering that most DMOADs need to act at specific sites within the joint, there is growing interest in designing nanomaterial drug carriers to localize to those target tissues or cells. Herein, nanomaterial design is categorized as using active or passive targeting. Similar to nanomedicine terminology used in other fields such as cancer, passive targeting refers to the effect of a carrier’s inherent physiochemical properties, such as size, charge, and hydrophilicity, on biophysical interactions with proteins, cells, and tissues. Active targeting refers to particle modifications using specific biochemical moieties to elicit interactions with target cells, cellular components, or ECM molecules. This is often accomplished by conjugating ligands to the nanomaterial for receptors overexpressed on target diseased cells. For delivery to cartilage, the unique components of the ECM have been exploited as opportunities for both passive and active targeting. For example, nanomaterial systems have been engineered to possess a cationic surface charge to facilitate passive targeting to anionic joint tissues such as cartilage and synovial fluid. Strategies for active targeting to cartilage include binding peptides for collagen type II, while the synovium has been actively targeted by incorporating ligands specific to the synoviocytes, endothelial cells, and macrophages that are present in the synovium. It is important to note that delivery systems that exploit active targeting moieties must still leverage favorable physiochemical properties in order to effectively reach its target.
2. NANOMATERIALS FOR ENHANCING WHOLE JOINT RETENTION
Historically, the primary goal of engineered drug-carrying vehicles for intra-articular delivery was to provide prolonged whole joint retention, treating the entire joint as the target reservoir for injected drugs. This strategy of sequestering the drug for as long as possible within the joint may be appropriate for drugs that act on multiple tissue types in the joints. For example, interleukin-1 (IL-1) receptor contributes to the inflammatory environment and is present throughout the synovium, cartilage, other joint tissues [71]; an IL-1 receptor antagonist would therefore be appropriate if delivered indiscriminately throughout the joint as a method of reducing cytokine-induced damage.
The general approach of treating the entire intra-articular space as the drug depot might not be an effective strategy if drugs exhibit poor uptake in a tissue of interest. To date, most efforts to prolong whole joint retention and sustain drug release have focused on incorporating drugs into carriers too large to be rapidly cleared from the joint via capillaries (Table 2, Figure 2A–C) [72]. For example, large microparticles generally have slower joint clearance rates than nanomaterials [73] and tend to accumulate in the synovium. If such materials are used for drugs needed in the cartilage, the drug must diffuse from the synovium to the cartilage in efficacious concentrations, while substantial off-target drug uptake occurs. Therefore, for drugs injected into the intraarticular space, the therapeutic site of action should inform the nanomaterial design, including whether or not a targeting strategy is needed to localize the delivery vehicles to specific cells or tissues within the joint. In some cases, nanomaterial targeting is studied with the goal of improving overall joint retention of a drug in addition to tissue specific drug delivery (Table 2, Figure 2D). In such cases, the site of nanomaterial localization may be exploited as a reservoir for controlled drug release over time within the joint.
Table 2.
Nanomaterials developed with active and passive targeting modalities improve joint pharmacokinetics and whole-joint retention.
| Nanomaterial type | Tissue targeting modality | Physical properties | Animal model | Major findings | Ref. |
|---|---|---|---|---|---|
| Dextran, fluorescently labeled | None | 10kDa (~1.4nm)[a]
500kDa (~5.2nm)[a] |
Rat (healthy and MMT OA model) | Smaller 10kDa molecules escaped joint more rapidly than larger 500kDa molecules. Larger molecules cleared more slowly in OA knees than healthy knees, while smaller molecules performed similarly in OA and healthy knees. | [78] |
| Carbon nanotube (PEGylated) | None | 20 × 100nm (tube) | Mouse (healthy and DMM OA model) | Significantly improved retention rate relative to free fluorophore for 14 days in both models. | [74] |
| Poly(D, L)-lactide nanoparticle, dye loaded | None | 300nm (spherical) | Mice (healthy and inflamed) | Nanoparticles leaked out of healthy and inflamed knees. Injection with hyaluronic acid slowed release from the joints. | [84] |
| Triblock self-assembly nanoparticle for protein tethering | None | 270nm ± 5nm (spherical) | Rat (healthy) | Significantly improved retention rate relative to free IL-1 receptor antagonist for 14 days. | [75] |
| Polymeric self-assembly nanoparticle for protein encapsulation | None | 500nm & 900nm (spherical) | Rat (healthy) | Significantly improved joint retention for 14 days, with free protein cleared the fastest and the 900nm particles clearing the slowest. | [76] |
| Kartogenin-conjugated chitosan nanoparticles | None | 150nm ± 39nm (spherical) −11.84mV ± 1.2mV |
Rat (ACLT OA model) | Nanoparticles detectable in the joint at 24 days post injection, with the same retention and disease modifying effect as micro-scale particles of the same makeup. | [85] |
| Thermoresponsive pluronic-chitosan-kartogenin nanoparticle | None | 650nm at 4°C 395nm at 37°C |
Rat (ACLT and DMM OA model) | Joint elimination half-life was 115.2 ± 2.2 hr with cold treatment and 109.3 ± 3.6 hr without cold treatment. | [86] |
| Globular protein (Avidin) | Passive (positive charge for cartilage) | ~7nm +20mV |
Rat (healthy) | At 24 hours, significantly more Avidin (cationic) than Neutravidin (neutral) in remained various joint tissues. At 7 days, Avidin is mostly cleared from joint tissues. | [81] |
| PAMAM dendrimer, PEGylated to control surface charge, with IGF-1 conjugated | Passive (positive charge for cartilage) | “Gen 4” 14kDa (~4.5nm), 64 NH2 groups per molecule (less cationic) | Rat (healthy) | Whole joint retention was the greatest for more-charged “Gen 6” dendrimer, with joint half lives of 0.41 days for free IGF-1, 1.08 days for “Gen 4” dendrimers, and 4.21 days for “Gen 6” dendrimers. | [82] |
| “Gen 6” 58kDa (~6.7nm), 256 NH2 groups per molecule (more cationic) | |||||
| Cationic nanoparticle comprised of dextran proprionate and Eudragit RL100, fluorescently tagged | Passive (positive zeta potential for synovial fluid) | 100nm – 150nm (PDI 0.1 – 0.4) Positive zeta potential previously confirmed (data not shown) |
Rat (healthy) | With and without intra-articular injection of exogenous hyaluronate, cationic nanoparticles had significantly improved whole joint retention (74% of signal after 7 days) relative to free tetrapeptide (23% of signal after 2 days). | [80] |
| Cationic nanoparticle comprised of PLGA, PVA, and Eudragit RL, loaded with DiR dye | Passive (positive zeta potential for synovial fluid) | 170.1nm ± 6.0nm (PDI 0.111 ± 0.0006) +21.3mV ± 2.4mV |
Mouse (healthy) | Significantly longer joint retention was observed for the nanoparticles (50% remaining after 28 days) relative to free dye (30% remaining after 3 days) | [79] |
| DOTAM derivative with collagen type II targeting peptide | Active (targeting peptide, cartilage) | N/A | Mouse (healthy) | Knee retention up to 200 hr enhanced by targeting peptide, where increasing the sites of peptide conjugation increases joint retention. | [77] |
[a] Diameter in nm approximated using the equation reported by Erickson [87] as Minimum radius = 0.066 *[mass in Daltons]⅓
AIA = antigen-induced arthritis; ACLT = anterior cruciate ligament transection; DIR = 1,1′-dioctadecyl-3,3,3′,3′ tetramethylindotricarbocyanine iodide; DOTAM = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid amide; DMM = destabilization of the medial meniscus; DAP = dorsal air pouch; IGF-1 = insulin-like growth factor 1; IL-1 = interleukin-1; PAMAM = polyamidoamine; PDI = polydispersity index; PEG = polyethylene glycol; PLGA = poly (lactic co-glycolic acid); PVA = polyvinyl alcohol; MMT = medial meniscus transection
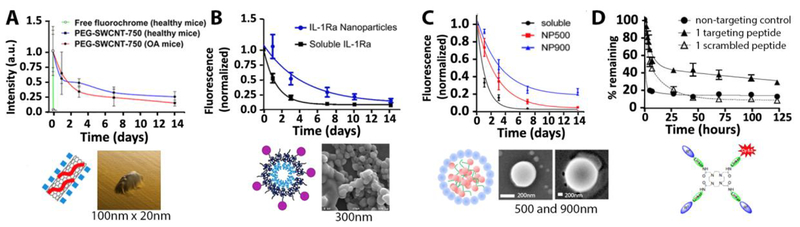
Figure 2.
Whole joint retention profiles across different nanomaterials (above) with a variety of structures, compositions, sizes, and shapes (below). In all cases, retention is presented as fluorescence intensity normalized the signal intensity immediately after injection. (A-C) Studies that include a soluble fluorophore show that joint retention is prolonged by nanomaterials within two weeks. (D) Incorporating a collagen type II binding peptide for cartilage targeting also slowed whole joint release. (A) PEG-based nano-tube [74]1, (B) tri-block polymeric nanoparticle [75]2, (C) self-assembling polymeric nanoparticle [76]3, (D) DOTAM carrier molecule [77]4. Figures reprinted with permission (see footnotes). DOTAM = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid amide; IL-1Ra = Interluekin-1 receptor antagonist; PEG-SWCNT-750 = polyethylene glycol single-walled carbon nanotubes (with a 750nm emitting fluorochrome); NP500 or NP900 = Nanoparticle of 500nm or 900nm.
2.1. Nanomaterial properties for whole-joint retention
Across the literature, the use of nanomaterials has consistently demonstrated improved joint retention relative to free drugs alone (Table 2, Figure 2), with free drugs typically residing only a few days and many nanomaterials lasting over a week in the joint. However, it remains difficult to extract structure-function relationships regarding the influence of unique particle properties on whole joint release kinetics, as few direct comparisons of nanomaterials have been conducted within a single study. Moreover, the trend of prolonged retention compared to free drug is true across a broad spectrum of nanomaterial properties. In fact, enhanced retention has been observed in nanomaterials ranging from 4.5nm dendrimers to 900nm polymeric nanoparticles (Table 2, Figure 2C). So, while no single set of design parameters is uniformly superior for joint retention, nanomaterials offer clear advantages over free drug injections for prolonged retention in the joint.
Moreover, the structural and physiological changes associated with OA progression appear to influence the fate of nanomaterials based on their size. In fact, a recent article following 10kDa and 500kDa dextran molecules showed that the larger dextrans cleared more slowly in medial meniscus transection-induced OA knees relative to healthy knees, while smaller dextrans performed similarly in both healthy and diseased conditions [78]. These findings highlight that nanomaterial retention is influenced not only by the nanomaterial size, but also the physiological environment in which it is administered.
2.2. Targeting techniques to improve whole-joint retention
Tissue-specific active and passive targeting modalities have also been employed to improve residence time in the joint space by facilitating immobilization within specific joint tissues. For example, the synovial fluid has been passively targeted as a method to improve whole joint residence. In such drug delivery platforms, nanomaterials were designed to ionically cross-link with endogenous, and in some cases exogenous, hyaluronic acid in the synovial fluid [79,80]. In both of these cases, nanomaterials were decorated with cationic Eudragit to form micrometer-sized gels through ionic interactions with the hyaluronic acid polysaccharides in the intra-articular space after injection. The signal from one such Eudragit nanoparticle-hyaluronate system was detectable even after four weeks post injection, whereas free dye reached near-zero fluorescence signal after two weeks in vivo, illustrating a successful application of tissue targeting as a method to prolong whole joint retention [79].
Nanomaterial targeting to cartilage has also been exploited to prolong whole joint retention. This strategy includes drug delivery platforms designed to passively accumulate in anionic cartilage ECM [81,82] and specifically interact with biomolecules such as collagen type II [77,83], to facilitate immobilization within cartilage and therefore prolong overall joint retention. For example, a collagen II-binding peptide [83] has been conjugated to a DOTAM (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid amide)-based molecular drug delivery platform that, after intra-articular injection, showed significantly longer joint retention than with a scrambled peptide [77] (Figure 1D). Additionally, increasing the number of targeting peptides within a single DOTAM molecule further prolonged joint residence and, after eight days post injection, approximately 50% of the targeted molecules were still within the joint via fluorescent tracking. A similar trend was observed with a passive, electrostatic-based targeting strategy for cartilage whereby dendrimers with more targeting potential (more cationic surface charge) had longer joint retention profiles, likely due to accumulation in the anionic cartilage ECM [82]. In this case, dendrimer charge was increased by modulating the number of primary anime groups per dendrimer from 64 to 256, and the resulting joint half-life increased from 1.08 days to 4.21 days respectively. Both dendrimer formulations showed full-thickness cartilage penetration 6 days after injection via multiphoton microscopy. Such studies show that tissue targeting techniques may improve both localization to specific tissue compartments and, accordingly, prolong whole joint retention. Therefore, considering DMOADs with unique therapeutic targets, nanomaterial systems are being tuned to direct the drug cargo to specific tissues and cells within the joint, highlighted in the following review sections.
3. NANOMATERIAL DELIVERY FOR CARTILAGE
3.1. Cartilage as a tissue target for intra-articular drug delivery
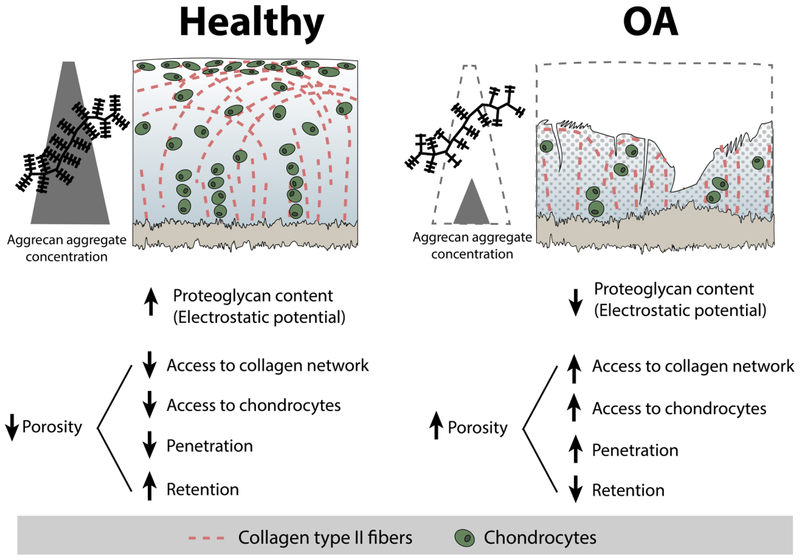
When considering intra-articular drug delivery, cartilage can be an important site for therapeutic intervention. Cartilage is one of the first tissues in the joint to deteriorate, the products of which stimulate the inflammatory environment that perpetuates the disease [53]. Additionally, protection and/or regeneration of eroded cartilage extracellular matrix is critical to restoring mechanical and metabolic balance in the joint. Accordingly, cartilage is a target site for therapeutic delivery to which nanomaterials have been designed to enhance payload delivery (Table 3). As cartilage undergoes OA progression, cartilage experiences proteoglycan depletion, catabolic changes to cell expression, tissue erosion and collagen deterioration, and bone invasion. These structural and compositional changes to the matrix create a “moving target” for targeting systems, as illustrated in Figure 3.
Table 3.
Nanomaterials designed for drug delivery to cartilage and chondrocytes
| Nanomaterial type | Targeting modality | Physical properties | Animal model | Major findings | Ref. |
|---|---|---|---|---|---|
| PAMAM dendrimer, PEGylated to control surface charge, with IGF-1 conjugated | Passive (positive charge for cartilage) | “Gen 4” 14kDa (~4.5nm), 64 NH2 groups per molecule (less cationic) | Ex vivo bovine cartilage (healthy) | The more charged “Gen 6” dendrimer transported more slowly into cartilage (6 days vs. 2 days for “Gen 4” for full thickness distribution), but to a greater extent (nearly twice as much “Gen 6” compared to “Gen 4” after 6 days). | [82] |
| “Gen 6” 58kDa (~6.7nm), 256 NH2 groups per molecule (more cationic) | |||||
| Globular protein (Avidin) | Passive (positive charge for cartilage) | ~7nm +20mV |
Ex vivo bovine explants (healthy and trypsin treated) | Positively charged Avidin has stronger interactions with cartilage than the neutral form of the protein. | [88] |
| Triblock self-assembly nanoparticle | Passive | 300nm | In vivo rat (healthy) | Nanoparticles penetrated the full-thickness of cartilage in vivo, shown by fluorescence microscopy. | [75] |
| Micelle ± CPP | Passive (naked) | 15nm | Ex vivo bovine explants (healthy, IL-1α, or trypsin treated) | CPP modification increased association with chondrocytes. Only smaller micelles could diffuse through the cartilage (liposomes were trapped in the superficial zone). | [89] |
| Active (CPP) | 106nm | ||||
| Liposome ± CPP | Passive (naked) | 138nm | |||
| Active (CPP) | 397nm | ||||
| Peptidic siRNA carrier (CPP-siRNA complex, coated with albumin) | Active (contains a CPP) | ~55nm | Ex vivo human explants (healthy and OA) | After incubating tissue for 48hrs with the carrier, the carrier was present throughout the cartilage, primarily accumulated intracellularly and aggregated in the superficial zone. Signal was detectable in chondrocyte lacunae after 14–21 days in culture. | [90] |
| Poly(propylene sulphide) nanoparticle with collagen type II peptide | Active (targeting peptide for collagen II) | 38nm | In vivo mouse (healthy) | The 38nm targeted particles are immobilized within the tissue with a 71-fold greater accumulation than scrambled controls at 48hrs. For targeted particles, 38nm particles had 14.9-fold more accumulation than 96nm particles in the cartilage matrix. | [83] |
| 96nm | |||||
| −Peptide: −3mV ± 9mV +Peptide: +18mV± 3.5mV | |||||
| Liposome with an anti-collagen II antibody | Active (antibody for collagen II) | 150–250nm | In vivo guinea pig (spontaneous OA model), intravenous injection | Antibody-enhanced liposomes qualitatively showed selective binding to OA cartilage. Liposomes without the targeting antibody did not show significant binding to cartilage. | [91] |
| Liposome with an anti-collagen II antibody | Active (antibody for collagen II) | 100–300nm | In vivo mouse (DMM OA model), retroorbital injection | Amount of nanomaterial increased proportionately with disease severity via fluorescence tracking. | [92] |
| Hyaluronic acid-coated bovine serum albumin nanoparticles | Active (binding to chondrocyte CD44) | 108.1nm ± 5.9nm −21.1mV± 3.2mV |
In vitro chondrocytes in monolayer | Hyaluronic acid coating statistically improved chondrocyte uptake of the loaded drug through active transport processes. | [93] |
| Hyaluronic acid-coated Polylactide (PLA) nanoparticles | Active (binding to chondrocyte CD44) | 650nm ± 40nm | In vitro chondrocytes and synoviocytes in monolayer | Hyaluronic acid coating improved uptake into chondrocytes relative to poly vinyl alcohol (passive strategy). | [94] |
CPP = cell penetrating peptide (nonspecific to chondrocytes); DMM = destabilization of the medial meniscus; IGF-1 = insulin-like growth factor 1; IL-1α = interleukin 1 alpha; PAMAM = polyamidoamine; PEG = polyethylene glycol
Figure 3.
The availability of tissue targets within cartilage changes during disease progression. The alterations to proteoglycan content, collagen matrix, cells, and porosity create a “moving target” for nanomaterials, suggesting some cartilage targeting strategies may depend on the condition of cartilage. Here, penetration refers to the depth to or ability with which a nanomaterial can enter the cartilage ECM, and retention refers to the amount of time a nanomaterial resides within the matrix once it has gotten into the ECM.
It is important to note that ex vivo and in vitro studies have focused on the effects of nanomaterial properties on targeting and localization to cartilage, but uptake into other “off-target” tissues in the joint is often not reported. Therefore, these findings demonstrate the influence of the material properties on cartilage associations, but do not necessarily validate tissue-specific targeting. Further studies may be needed to determine the degree to which off-target associations occur and the efficiency of cartilage-specific localization when in the presence of all joint tissues.
3.2. Passive targeting strategies for cartilage
To enhance passive accumulation of nanomaterials in cartilage, engineering efforts have focused on tuning particle size and charge. Size is an important design criterion for targeting to cartilage primarily because of the tissue’s uniquely dense ECM, therefore size is a critical determinant of a carrier’s ability to penetrate through the tissue depth [95]. Cartilage porosity is influenced by the packing and arrangement of all of its components, including the collagen network, chondrocytes, and proteoglycans. The mesh size of the collagen II network, which makes up 60% of the dry weight of cartilage [96], is approximately 50nm - 200nm [34,74,88,95,97]. This collagen network is densely filled with aggrecan molecules which are 200–350nm long [98] with ~4.5–15nm between glycosaminoglycan side chains [83,98–100]. Together, cartilage is commonly described as having a pore size between 6–11nm [95,96,101,102]. The porosity has been shown to increase at greater tissue depth [100] and with disease-induced degeneration [102].
Despite the generally accepted physical parameters for cartilage matrix network structure and porosity, discrepancies exist in the literature regarding the threshold size of nanomaterials that allows for and prohibits matrix penetration (Table 4). Ex vivo studies so far have shown that nanomaterials up to ~55nm have been able to penetrate through the full thickness of cartilage [90]. In an in vivo rat study, complete cartilage penetration was observed with a 300nm particle in cryosectioned samples three days post injection [75]. Despite the success of these relatively large nanomaterials, other studies have found that nanomaterials as small as 6nm (67kDa) get trapped in the superficial zone and unsuccessfully penetrate healthy cartilage [103]. These thresholds of nanomaterial size for cartilage penetration appear to change in diseased cartilage. In multiple studies, nanomaterials that were unable to enter healthy cartilage were able to penetrate damaged cartilage, including 6nm, 8nm, 15nm, and 55nm systems. Ex vivo, particles of 138nm were unable to penetrate damaged tissues [89], suggesting that the threshold penetration size for OA cartilage may exist between approximately 55–140nm.
Table 4.
Size exclusion of nanomaterials in cartilage as a function of nanomaterial diameter and cartilage integrity. Note that observation of full thickness penetration may be influenced by experimental conditions including incubation time or bath conditions (surface area of cartilage exposure, temperature, etc.), which varies across ex vivo studies.
| Nanomaterial type | Cartilage tissue source | Size characterization Technique [a] | Healthy tissue | Damaged/Diseased tissue | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Reported size of full-thickness cartilage penetration | Reported size of partial or no cartilage penetration | Cartilage disease model | Reported size of full-thickness cartilage penetration | Reported size of partial or no cartilage penetration | ||||
| Cationic dendrimer | Ex vivo bovine and in vivo rat | MW | 14kDa (4.5nm) and 58kDa (6.7nm) | N/A | N/A | N/A | N/A | [82] |
| Gd-labeled proteins | Ex vivo bovine | MW | 45kDa (~5nm)[b] | 67kDa (~6nm)[b] | Trypsin | 67kDa (~6nm)[b][c] | N/A | [103] |
| Antibody fragments | Ex vivo bovine | MW | 48kDa (~5nm)[b] | 150kDa (~8nm)[b] | 14-day cytokine | 150kDa (~8nm)[b] | N/A | [111,112] |
| Globular protein | Ex vivo bovine | MW | 7nm | 15nm | Trypsin | 15nm | N/A | [81,88] |
| Micelle or liposome | Ex vivo bovine | DLS | 15nm | 138nm | Trypsin | 15nm | 138nm | [89] |
| 7 day IL-1α | 15nm | 138nm | ||||||
| Polymeric nanoparticle | In vivo mouse | DLS | 38nm | 96nm | N/A | N/A | N/A | [83] |
| Albumin-coated peptidic siRNA carrier | Ex vivo human | AFM (TEM) | ~55nm | N/A | Human OA | ~55nm | N/A | [90] |
| Polymeric nanoparticle | Ex vivo bovine | DLS (SEM) | N/A | 260–290nm (112–123nm) | Collagenase | N/A | 260–290nm (112–123nm) | [110] |
| Triblock nanoparticle | In vivo rat | DLS (SEM) | 300nm | N/A | N/A | N/A | N/A | [75] |
[a] Diameter reported by respective authors unless otherwise specified. AFM = atomic force microscopy; DLS = dynamic light scattering; MW = estimated from molecular weight; SEM = scanning electron microscopy; TEM = transmission electron microscopy. Parenthesis indicates that a technique was used to confirm the primary characterization technique (listed first), but may not have necessarily been used to quantify nanomaterial size.
[b] Diameter in nm approximated using the equation reported by Erickson [87] as Minimum radius = 0.066 * [mass in Daltons]⅓
IL-1α = Interleukin 1 alpha, at 5ng/mL
Considering the conflicting data in studies of healthy cartilage, the ability for materials to enter the matrix may be influenced by material properties beyond nanomaterial diameter alone. Other nanomaterial properties such as hydrophilicity and shape may influence the mobility through the cartilage ECM and past the collagen meshwork. Additionally, the observation of ex vivo full thickness cartilage penetration may be influenced by experimental conditions such as incubation time, temperature, and the surface area of cartilage exposure. Nevertheless, it may be reasonable in some cases to increase the size, and hence payload capacity, of a delivery system at the expense of tissue penetration depth. For example, drug loaded carriers that localize to the superficial cartilage zones may still be able release drugs that then penetrate the deeper zones. Indeed, the earliest stages of cartilage degeneration occur in the superficial zone, and therefore higher drug concentrations may be desirable in this anatomic region [53]. A potential superficial zone target was recently illuminated in a study of epidermal growth factor receptor (EGFR), a cell surface receptor that was found to maintain superficial chondrocyte number, cartilage lubrication, collagen organization, and cartilage mechanical strength [104]. Delivery of agents that restore or promote EGFR signaling has been proposed as a therapeutic strategy for protecting cartilage at early stages of OA, and represents an opportunity in which delivery specifically to the superficial zone chondrocytes would be advantageous. This concept underscores the value of coupling a therapeutic with a vehicle that provides localization and drug release relevant to site of action and disease mechanism.
Intentional control over nanomaterial zeta potential has also been explored as a passive targeting strategy for cartilage. The dense network of sulfated proteoglycans gives cartilage a bulk anionic charge [105,106], corresponding to a proteoglycan content or fixed charge density of −158mM to −182mM for human articular cartilage [107]. Therefore, the tissue electrostatics serve as a potential passive localization approach for drug delivery to cartilage. Avidin, a positively charged globular protein of approximately +20mV [108], has demonstrated improved penetration and residence in ex vivo cartilage relative to its neutral form, NeutrAvidin [88]. Tantalum oxide (Ta2O5) nanoparticles for cartilage imaging have also demonstrated the influence of zeta potential on cartilage interactions. In these studies, +7.6mV ammonium-functionalized nanoparticles fully distributed throughout ex vivo murine cartilage while neutral phosphonate and anionic carboxylate particles interacted weakly with ex vivo murine cartilage, accumulating only in the superficial zone with a slow uptake [109].
Reliance on zeta potential as the targeting strategy in nanomaterial design has limitations, as the charge density of cartilage is altered as disease progresses. Indeed, one of the first signs of cartilage pathology in OA i s the loss of proteoglycans [53], the highly sulfated and negatively charged molecules that contribute to the charge density of cartilage. Additionally, nanomaterial zeta potential can change in the presence of synovial fluid, sometimes masking cationic charge through the adsorption of anionic synovial fluid components [110]. While zeta potential may be an engineering criterion for improved cartilage interactions ex vivo, this design approach is likely not uniformly advantageous across all stages of disease or joint conditions.
3.3. Active targeting to cartilage
Active targeting strategies for cartilage have primarily exploited binding modalities for the collagen network and chondrocytes. Type II collagen is present throughout the tissue depth at varying concentrations and orientations and makes up 90% to 95% of the collagen network in cartilage [53]. Under disease conditions, these protein fibers become exposed to the synovial cavity as the proteoglycans are released and the tissue surface degrades. In 2008 a six amino acid peptide (WYRGRL) was identified for its affinity for binding to collagen type II; this peptide then could be conjugated to nanoparticles for targeting articular cartilage [83]. While both un-targeted and targeted poly(propylene sulphide) nanoparticles entered the cartilage matrix, nanoparticles with the peptide experienced a 71-fold greater accumulation in ex vivo cartilage relative to those with a scrambled peptide.
Antibodies for collagen type II [113,114] have also been incorporated in nanomaterial systems to increase interactions with cartilage [91,115]. For example, a 150–200nm fluorescent liposome decorated with collagen type II antibodies were formulated for theranostic applications in OA. After intravenous injection, these particles migrate into the joint but do not bind to cartilage unless the collagen II network is exposed, namely i n conditions of tissue degradation [91]. Additionally, similar particles were shown via fluorescence to accumulate in the knee in a manner proportional to OA severity in vivo [92]. Accordingly, accumulation of such particle systems could be used to both spatially identify and localize therapeutics to regions of cartilage damage.
Chondrocytes, the cell type solely responsible for the maintenance and production of cartilage extracellular matrix, have also served as a target for enhanced OA therapeutic delivery. Numerous current or potential OA drugs act specifically on chondrocytes, including chondroprotective agents [116], nucleic acids [117,118], growth factors [119–121], and matrix metalloprotease inhibitors [122–124]. Therefore, for these therapies to be efficacious, the drug or drug carriers must reach the cellular destination and reside in that location for a suitable timeframe and concentration [125]. In one study, nanomaterial uptake into chondrocytes was enhanced with a cell penetrating peptide, which yielded approximately 4-times greater micelle and liposome association with chondrocytes in vitro relative to untargeted carriers [89]. Adsorption of hyaluronic acid onto nanomaterials has also been used in vitro to enhance interactions with chondrocyte via binding to CD44, which is present on chondrocyte surfaces [93]. The ability to penetrate the matrix and deliver a payload to chondrocytes is important as groups investigate emerging therapeutics including RNA interference, CRISPR/Cas9, and small molecular drugs which need to act intracellularly to produce a therapeutic effect [126–130]. Cartilage-targeted nanomaterials will be key enabling technologies for these promising new therapies. However, cartilage-targeting vehicles are still in early stages of investigation and the therapeutic benefits of such strategies have yet to be determined in large animal models or humans.
4. NANOMATERIAL DELIVERY TO THE SYNOVIUM
4.1. Synovium as a tissue target for intra-articular drug delivery
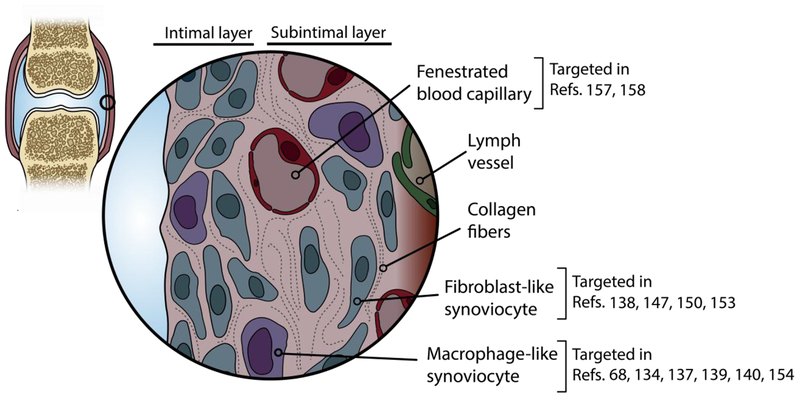
To address the multifaceted disease progression in OA, nanomaterials are being designed to target not only cartilage, but also other joint tissues such as the synovium. The synovium, a thin heterogeneous connective tissue that lines diarthrodial joints, is a prime target in an OA joint because of its role in inflammation. The synovium is also the site at which transport of molecules into and out of the joint occurs - intercellular gaps of approximately 0.1 – 5.5μm exist at the synovial surface, and a rich network of fenestrated and continuous capillaries are found just beneath the surface [131]. While OA has not traditionally been classified as an inflammatory disease, more recently, attention has shifted toward recognizing synovitis as a potential target for treatment [35,54]. Currently, anti-inflammatory therapies such as corticosteroids and antagonists to inflammatory cytokines are being prescribed to patients diagnosed with OA but have been unable to modify pathogenesis of the whole joint disease significantly due to toxicity or transient effects after intra-articular injection in clinical trials [11]. Hence, more efficient delivery systems of therapeutics for the synovium need to be explored. Nanomaterials designed for delivery to the synovium typically target one of the synoviocyte types: Type A synovial macrophages, a minority population in normal joints that increases drastically in inflammatory conditions and produces pro-inflammatory mediators, or Type B fibroblast-like cells, the dominant population in normal synovium that produces ECM components of synovial fluid and synovial membrane (Figure 4). To date, many nanoscale advancements for synoviocyte targeting have been accomplished in the field of rheumatoid arthritis (RA), a classical inflammatory arthropathy. Moving forward, we may be able to exploit knowledge gained in the RA field to improve carrier design for synoviocyte targeting in OA applications. The different types of nano-scale particles that have been investigated to target the OA and RA synovium are summarized for passive and active targeting strategies Tables 5 and 6, respectively.
Figure 4.
Synovium is a cellularly dense and heterogeneous tissue that contributes to OA pathology. Accordingly, many groups have engineered nanomaterial systems to deliver drugs to this tissue, using both nonspecific and cell-targeting vehicles.
Table 5.
Passively targeted nanomaterials for drug delivery to synovium or synoviocytes
| Nanomaterial type | Targeting Modality | Physical properties | Animal model | Major findings | Ref. |
|---|---|---|---|---|---|
| Gold nanoparticles (no drug) | Passive | 5nm – 52nm | Ex vivo porcine synovium | Effective tissue permeation was only achieved with the smallest particles (5nm). Exposure to pro-inflammatory factors did not affect permeation. | [143] |
| Chloroquine loaded solid lipid nanoparticle | Passive | 113.6nm | In vivo rat (CFA RA model) | TNF-α levels were significantly reduced when chloroquine was loaded into a solid lipid nanoparticle versus free suspension of chloroquine. | [141] |
| Brucine loaded PLGA nanoparticles in PLGA microparticles | Passive | 12.38nm | In vivo rat (air-pouch inflammatory model) | Burst release of brucine was slowed and particles stayed in the articular cavity for significant time. | [132] |
| Dendritic polyglycerol sulfate (no drug) | Passive | 3nm | Ex vivo human cells (articular chondrocytes and synovial fibroblasts) | Cells treated with NPs did not show any change in the synthesis of pro-inflammatory cytokines (TNF-α and IL-6) and a greater expression of antiinflammatory cytokines (IL-10). | [150] |
| Thiolated glycol chitosan nanoparticles encapsulating polymerized Notch 1 targeting siRNA | Passive | 200nm | In vitro murine macrophages In vivo mice (CIA RA model) | In vitro Notch-1 inhibition of siRNA-NPs in murine macrophage cell was confirmed. siRNA-NPs exhibited higher targeting efficiency in the arthritic joints of CIA mice. | [134] |
| Chitosan-graft-PEI nanoparticles complexed with plasmid enhanced green fluorescent protein | Passive (gene delivery) | 100nm–300nm | In vitro rabbit synoviocytes | NPs were able to carry plasmid DNA inside synoviocytes where the DNA was detected entering the cell nuclei. | [151] |
| Melittin-Derived Cationic Amphipathic Nanocomplexes combined with siRNA targeting the p65 subunit of NF-κB | Passive (gene delivery) | 55nm | In vivo mice (CAIA RA model) | Administration of p5RHH-p65 siRNA nanocomplexes decreased inflammatory cytokine expression and cellular influx into the joints, protected against bone erosions, and preserved cartilage integrity. | [152] |
| Multiwall Carbon Nanotubes (no drug) | Passive | 60nm diameter 10μm length |
In vitro human FLS In vitro RAW 264.7 cells In vivo rats (healthy) |
MWCNTs led to formation of granulation tissues within adipose tissues at higher concentrations in vivo. With RAW 264.7 cells, the MWCNTs increased the TNF-α, MCP-1 and RANTES induced inflammatory responses in a dose dependent manner while decrease MIP-1α. With HFLS, they decreased secretion of IL-6 and MCP-1. | [153] |
| Carbon nanotubes coated with PEG and DEX | Passive | 180nm (DEX-PEG-coated CNT) −18mV |
In vitro human FLS | DEX and PEG coated CNTs were able to decrease released of inflammatory cytokines (IL-1β, TNF-α and IL-6) and MMP3 at both a gene and protein expression level at a lower concentration than free DEX in vitro with FLS. | [138] |
| N-trimethyl chitosan-polysilicon acid nanoparticles coated with decoy oligodeoxynucleotides | Passive (gene delivery) | Without methotrexate: 159nm, +23mV With methotrexate: 184nm, +33mV |
In vitro human synovial sarcoma cells In vitro primary RA synovial fibroblasts |
Nanoparticles decorated with decoy oligodeoxynucleotides specific to transcription factor NFkB resulted in significant decreases in inflammatory mediators (IL-6 and IL-8). | [147] |
| Clodronate liposomes | Passive | 120nm –160nm | Human patients with RA | A single IA dose of clodronate liposomes significantly reduced the number of CD68-positive macrophages and the expression of ICAM-1 and VCAM-1 in the synovial lining. | [140] |
| Superparamagnetic iron oxide nanoparticles (no drug) | Passive (Stimuli Responsive - magnetic) | Not Reported | In vivo sheep (healthy) | Intraarticular and periarticular injection of the particles led to uptake in the synovium, with increased local concentration when an extracorporeal magnet was applied. | [148] |
| DEX-HPMA copolymer conjugate | Passive (Stimuli Responsive - pH sensitivity) | 73kDa (~2.8nm)[a] | In vivo rats (AIA RA model) | The conjugate has greater anti-inflammatory effects compared with systemically administered free DEX. This differential effect of the conjugate was related to its selective accumulation, potential macrophage-mediated retention, and pH-sensitive drug release in arthritic joints. | [68] |
| Mineralized nanoparticles composed of PEGylated hyaluronic acid as the hydrophilic shell, 5β-cholanic acid as the hydrophobic core, and calcium phosphate as the pH-responsive mineral and loaded with methotrexate | Passive (Stimuli Responsive - pH sensitivity) | 218nm–265nm | In vivo rats (CIA RA model) | The mineralized nanoparticles revealed pH-dependent demineralization followed by acceleration of methotrexate release into the cytosol. | [69] |
| PLGA gold / iron/ gold half-shell nanoparticles conjugated with RGD and loaded with methotrexate | Passive (Stimuli Responsive - NIR and magnetic) | 135nm | In vivo mice (CIA RA model) | When combined with consecutive NIR irradiation and external magnetic field application, these nanoparticles provide enhanced therapeutic effects. | [133] |
[a] Diameter in nm approximated using the equation reported by Erickson [87] as Minimum radius = 0.066 * [mass in Daltons]⅓
AIA = Adjuvant-induced Arthritis; CAIA = Collagen Antibody-Induced Arthritis; CIA = Collagen Induced Arthritis; CFA = Complete Freund’s Adjuvant; CNT = Carbon Nanotube; DEX = dexamethasone; FLS = Fibroblast-Like Synoviocytes; HFLS = Human Fibroblast-Like Synoviocytes; HPMA = N-(2-hydroxypropyl)methacrylamide; IA = Intra-articular; ICAM-1 = Intercellular Adhesion Molecule 1; IL = Interleukin; MCP-1 = Monocyte Chemoattractant Protein 1; MIP1α = Macrophage Inflammatory Protein 1 Alpha; MMP = Matrix Metalloproteinase; MWCNT = Multiwall Carbon Nanotubes; NFkB = nuclear factor kappa-light-chain-enhancer of activated B cells; NIR = Near infrared; PEI = Polyethylenimine; PEG = poly (ethylene glycol); PLGA = poly(lactic-co-glycolic acid); RA = Rheumatoid Arthritis; RANTES = Regulated on activation, normal T cell expressed and secreted; RAW 264.7 = Macrophage-like cell line derived from tumors induced in male BALB/c mice by the Abelson murine leukemia virus; RGD = arginine-glycine aspartic acid; SLNs = Solid Lipid Nanoparticles; TNF-α = Tumor Necrosis Factor Alpha; VCAM-1 = vascular cell adhesion molecule 1.
Table 6.
Active-targeting nanomaterials for drug delivery to synovium or synoviocytes
| Nanomaterial type | Targeting modality | Physical properties | Animal model | Major findings | Ref. |
|---|---|---|---|---|---|
| Alginate nanoparticles decorated with tuftsin peptide and loaded with IL-10 plasmid DNA | Active (surface receptor) | ~300nm | In vivo rat (adjuvant-arthritis model) | Targeted alginate nanoparticles loaded with IL-10 plasmid DNA can efficiently re-polarize macrophages from an M1 to an M2 state. | [137] |
| Self-assembling block copolymer with protein-tethering moiety (IL-1Ra) | Active (IL-1 surface receptors) | 270nm ± 5nm |
In vitro rabbit synoviocytes In vivo rat (healthy) |
IL-1Ra-tethered particles bound to synoviocytes via the IL-1 receptor and significantly increased whole joint retention of IL-1Ra relative to soluble IL-Ra. | [75] |
| Micelle carriers of camptothecin, surface modified by vasoactive intestinal peptide | Active (surface receptors) | Not reported | In vivo rat (CIA RA model) | Single subcutaneous injections of the micelles led to mitigated inflammation in joint if CIA mice up to ~4.5 weeks after induction. | [139] |
| RGD peptide–exposing long circulating PEG liposomes loaded with dexamethasone and targeted to αvβ3 integrins expressed on angiogenic VECs | Active (surface receptors) | 100nm |
In vitro HUVECs In vivo rats (AIA RA model) |
In vivo, increased targeting of radiolabeled RGD-PEG-L to areas of LPS-induced inflammation in rats was observed. | [158] |
| αvβ3-targeted fumagillin nanoparticles | Active (surface receptors) | 250nm | In vivo rats (K/BxN model of RA) | Synovial tissues from animals treated with targeted fumagillin nanoparticles showed a significant decrease in inflammation and angiogenesis, and preserved proteoglycan integrity. | [157] |
| Nanocarrier composed of lipids, PEG-PLGA forming a hydrophilic shell, folic acid around the hydrophilic shell as a targeting ligand, and poly(cyclohexane-1,4-diylacetone dimethylene ketal) (PCADK) and PLGA as a hydrophobic core and loaded with methotrexate | Active (surface receptors) | 133.6 – 208.5nm (varied based on composition of components) |
In vitro RAW 264.7 cells In vivo rats (AIA RA model) |
Folate targeted particles demonstrated superior uptake in RAW 264.7 cells than untargeted cells. A smaller paw size and decrease swelling was observed in the AIA model of rat when injected with the targeted particles than the untargeted particles. | [156] |
| PLGA nanoparticles coated either with macrophage-derived microvesicle proteins or red blood cell membrane proteins and loaded with tacrolimus | Active (surface receptors) | 130nm |
In vitro HUVECs In vivo rats (CIA RA model) |
In vitro binding of the microvesicle coated particles was greater than controls. The microvesicle coated particles also showed greater therapeutic impact in CIA model than controls. | [159] |
AIA = Antigen-induced arthritis; CIA = Collagen Induced Arthritis; HUVEC = Human umbilical vein endothelial cells; IL-1Ra = Interleukin 1 Receptor Antagonist; LPS = Lipopolysaccharide; MPCM = Mouse peritoneal cavity macrophages; PEG = poly (ethylene glycol); PLGA = poly (lactic co-glycolic acid); RA = Rheumatoid Arthritis; RAW 264.7 = Macrophage-like cell line derived from tumors induced in male BALB/c mice by the Abelson murine leukemia virus; RGD = arginine-glycine-aspartic acid; VEC = Vascular Endothelial Cells.
4.2. Passive Targeting to the Synovium
Nanomaterials designed for delivery to the synovium have been engineered in sizes ranging from 5nm to 300nm and with a range of materials including synthetic polymers [132,133], polysaccharides [134–137], carbon-nanotubes [138], micelles [139], liposomes [140], and lipids [141]. When targeting synoviocyte-acting drugs to the synovium, both the size and composition of nanomaterials are critical factors to control the degree of tissue penetration and transport between the synovium and the synovial fluid. For instance, fat-soluble small molecules can diffuse through and between synovial cells more readily than hydrophilic molecules of similar sizes, while water and small solutes are able to move readily from plasma to synovial fluid through the synovium [142]. In 2013, gold nanoparticles were used to demonstrate size selectivity in ex vivo porcine synovium, with only 5nm particles effectively permeating the entire thickness of the tissues [143]. The transport of larger particles (>250nm) is limited by the microvascular endothelium and the interstitial spaces [72]. As a result, most large particles tend to naturally accumulative in the synovium. This innate passive accumulation of particles leads to prolonged synovium retention of nanomedicines, even for those systems not intentionally designed for synovium targeting. The application of passive nanomaterial accumulation as a method to improve therapeutic delivery has been exploited in a variety of disease arenas including cancer, atherosclerosis and inflammatory bowel disease [144].
Synovial macrophages are a prime target for OA therapeutics due to their inherent phagocytic capacity and role in OA. These synovial cells from the monocyte and macrophage lineage have been passively targeted using nano- and micro-materials ranging from 3nm to 6μm (Table 5). Specifically, particles possessing diameters of 2μm to 3μm exhibited maximal phagocytosis and attachment in macrophages [145]. On the other hand, sub-micron sized nanoparticles have been shown to not only translocate to the deep underlying tissues of the synovium, but also to be phagocytosed. In contrast, particles closer to 25μm were simply covered by multinucleated giant cells and not phagocytosed [73]. In the case of non-phagocytosable particles, therapeutic delivery relies on the release and diffusion of the drug from the particle into the surrounding tissue and ultimately to receptor binding. However, for drugs that bind to intracellular receptors or targets, exhibit poor diffusion through the tissue or poor partitioning into cell membranes, or have adverse off-target effects, internalization of the drug carrier by the macrophages may be advantageous. Hence, research groups have taken advantage of the phagocytic capacity of macrophages when implementing therapeutics aimed at depleting these inflammatory cells by designing their carriers to be within phagocytosable size ranges. For instance, bisphosphonates such as clodronate have been investigated since the 1990s in 200nm liposomal formulations for phagocytosis by Type A synovial macrophages to induce apoptosis by inhibiting the binding of transcription factors such as Nuclear Factor-kappa B (NFkB) to DNA [140]. However, more recent studies have shown that the indiscriminate depletion of macrophages in the synovium might not necessarily attenuate severity of arthritis and that macrophages are in themselves vital for modulating homeostasis in the joint [146]. Therefore, as further investigation into the role of different macrophage phenotypes continues, improvements in drug targeting and vehicles for modulating synovial inflammation may emerge.
Gene delivery targets are also being explored with passive nanomaterials to target inflammation-associated pathways in which resident cells of the synovium are involved. Activated Type A synovial macrophages present a unique opportunity to target the overall reduction of cyclic inflammation in an OA environment. For example, thiolated glycol chitosan nanoparticles have been used to encapsulate small interfering RNA (siRNA) targeting the Notch1 pathway, one that has been implicated in the polarization of macrophages towards the pro-inflammatory phenotype [134]. These nanomaterials have successfully inhibited this inflammation-related gene in murine macrophage cell lines and in mice with collagen-induced arthritis (CIA), a common RA animal model, demonstrating overall inhibition of limb inflammation, bone erosion, and cartilage damage [134]. Nanoparticles made from N-trimethyl chitosan-polysialic acid have been coated with decoy oligodeoxynucleotides specific to the transcription factor NFkB, a pivotal mediator in inflammatory pathways. These nanoparticles have been delivered to synovial cell lines in vitro, resulting in significant decreases in inflammatory cytokines IL-6 and IL-8 [147]. In this manner, more recent studies are exploring the implications of manipulating gene expression of synoviocytes on the overall OA disease progression.
Stimuli responsive nanomaterials for arthritic conditions are also being investigated to target the synovium as an approach to increase efficiency of drug delivery and decrease the doses that are required to achieve therapeutic impact. For instance, superparamagnetic iron oxide nanoparticles have demonstrated greater retention and uptake by synoviocytes in vivo in the presence of extracorporeal magnets [148]. By incorporating gold into drug-loaded magnetic nanoparticles, near-infrared irradiation has been used in conjunction with an external magnetic field to generate local heat at the site of inflammation in the synovium, permitting an accelerated release of the encapsulated therapeutic [133]. Furthermore, acidic extracellular pH in the inflamed synovium has also been used as a stimuli to increase responsiveness of nanomaterials. Acidosis is a common feature of arthritic joints due to increased metabolic activity and insufficient vascular supply [149]. Several groups have taken advantage of this feature by designing nanomaterials using pH-responsive ionizable materials that undergo changes in conformation or stability in response to changes in environmental pH and hence demonstrate superior acidosis-mediated drug release [68,69].
4.3. Active Targeting to the Synovium
Modification of nanomaterials with active targeting moieties has led to greater therapeutic impact by the nanomaterials on synoviocytes than by non-targeted vehicles. The receptors presented on macrophages have been leveraged to increase uptake of therapeutics to this cell population in the synovium. For example, IL-10 plasmid DNA loaded alginate nanoparticles have been decorated with tuftsin, a peptide that interacts with macrophages and promotes phagocytosis by binding with Fc and neuropilin-1 receptors on macrophages [137]. The proportion of synovial macrophages displaying M2 anti-inflammatory markers increased from 46% to 66% when rats were treated with tuftsin decorated IL-10 nanoparticles in an adjuvant induced RA rat model. Folate receptor β (FRβ), demonstrated to be specifically expressed on activated macrophages in inflamed joints, has also been used to target and deliver therapeutics to the synovium [154–156]. In one such study, nanocarriers were composed of lipids, polyethylene glycol-poly(lactic-co-glycolic acid) (PEG-PLGA) to form a hydrophilic shell, and folic acid conjugated to the hydrophilic shell as a targeting ligand for FRβs on activated macrophages, and poly(cyclohexane-1,4-diylacetone dimethylene ketal) (PCADK) and PLGA as a hydrophobic core [156]. These carriers were loaded with methotrexate, a common disease-modifying anti-rheumatic drug, and used to compare the therapeutic impact of the targeted carrier against the untargeted carrier in an adjuvant-induced arthritis rat model. Serum levels of pro-inflammatory cytokines (TNF-α and IL-6), paw swelling, and erythema were reduced significantly in the rats that were injected with targeted particles compared to non-targeted particles. The overexpression of vasoactive intestinal peptide (VIP) receptors on macrophages and over-proliferating synoviocytes has also been leveraged for synovial targeting. For example, micelles loaded with camptothecin, a topoisomerase inhibitor, have been decorated with VIPs. When compared to untargeted micelles and the therapeutic alone, this targeting strategy led to significantly smaller paw thickness and clinical arthritis score of CIA mice [139].
Improved targeting of nanomaterials to the synovium has been achieved by exploiting receptors overexpressed on endothelial cells in the inflamed synovium. For example, the increased presence of angiogenic endothelial cells in an inflamed synovium corresponds to an overexpression of αvβ3 integrins, a receptor for vitronectin, on these cells. This led to a study in which a perfluorocarbon nanoparticle was decorated with peptidomimetic vitronectin antagonists that bind overexpressed αvβ3 integrins on the inflamed synovium and deliver their therapeutic load of fumagillin, an angiogenesis inhibiting substance [157]. The functionalization of the nanoparticle surface with vitronectin antagonists led to greater therapeutic efficacy in arthritic animals. Specifically, the targeted fumagillin-loaded particles yielded a lower disease score and paw thickness in the K/BxN transgenic murine model of inflammatory arthritis than in targeted particles without therapeutics. In yet another study, a polyethylene glycol liposome was designed with cyclic conformations of peptides containing arginylglycylaspartic acid (RGD), a binding peptide motif responsible for integrin engagement by cells. These peptides were demonstrated to be specific ligands for αvβ3 integrins and hence increased targeting of the drug delivery vehicle to vascular endothelial cells in the inflamed synovium. This study demonstrated a 3-fold increased localization of the RGD-decorated particles to the site of inflammation in a rat model of lipopolysaccharide (LPS)-induced inflammation than both particles with no peptide and with a scrambled peptide (RAD). By encapsulating a corticosteroid, dexamethasone, into this targeted liposome and injecting them intravenously, a greater anti-inflammatory effect in a rat model of experimental arthritis was exhibited [158]. Also as a method of exploiting cell adhesive mechanisms, nanoparticles have been coated with macrophage-derived microvesicles that mimic the adhesion characteristics of macrophages in the synovium or the pannus of inflamed tissue [159]. Through proteomic analysis and in vitro characterization with human umbilical vein cells (HUVECs), the microvesicles were determined to target intercellular adhesion molecule 1 (ICAM-1) or P-selectins. These carriers were loaded with tacrolimus, an immunomodulator that inhibits T-cell activation, and injected into collagen-induced arthritis mouse model. The macrophage-derived microvesicle-coated nanoparticles demonstrated significantly decreased bone erosion and paw swelling when compared to free drug controls, untargeted controls, and red blood cell membrane coated nanoparticle controls. Taken together, numerous examples of actively targeted nanocarriers have increased the efficiency of therapeutics over untargeted nanocarriers, underscoring the therapeutic advantages of targeting.
5. ADDITIONAL CONSIDERATIONS AND LIMITATIONS
5.1. Multi-modal combination therapies
Given that OA is a multifactorial disease, it is likely important to target various pathologic tissues in the osteoarthritic joint when delivering disease modifying therapies, yet very few nanomaterial systems have been used in this manner. Delivery of multiple therapeutics has been implemented in a thermoresponsive system that was loaded with both kartogenin, a chondroprotective drug, and diclofenac, an non-steroidal anti-inflammatory drug NSAID [86]. While these drugs were not intentionally directed to different tissue depots within the joint, this system demonstrated that nanomaterials are capable of multi-modal therapeutic delivery in the joint. As tissue targeting delivery strategies continue to be developed, opportunities will be created to enhance multi-modal pharmacological treatments for OA through intentional drug delivery to specific drug action sites within the joint.
5.2. The influence of synovial fluid on intra-articular nanomaterials
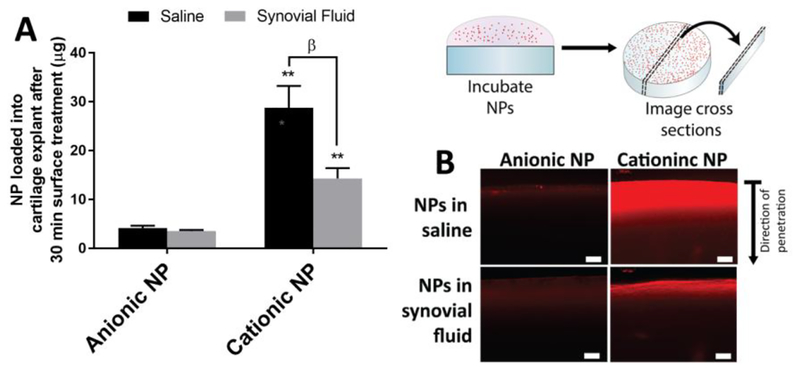
Synovial fluid, the viscous non-Newtonian fluid within synovial joints, is not only essential for shock absorption and lubrication within the joint but also for modulating the transport of various molecules to different components of the joint. Synovial fluid is a mixture of a protein-rich ultrafiltrate of plasma and hyaluronan synthesized by synoviocytes [142]. As discussed previously, nanomaterials have been designed for intentional entrapment in the synovial fluid to sustain the joint residence of therapeutics [79,80]. However, the interaction between nanomaterials and synovial fluid may be disadvantageous in other tissue targeting strategies. For example, synovial fluid has been shown to increase the size and reverse the charge of cationic particles, which negatively influenced the particle loading into cartilage (Figure 5) [110]. In cell uptake studies, incubation of nanoparticles with synovial fluid impacted nanoparticle internalization by synoviocytes [110]. These changes are hypothesized to occur through adsorption of synovial fluid components on the particle surfaces to create a corona. Protein corona formation on the particles could mask targeting ligands incorporated onto the particle surface, and may also influence their trafficking to cells and tissue within the joint. It is conceivable that nanoparticles could be engineered to control the adsorption of specific synovial fluid components that favor interactions with target cells or tissues. Accordingly, the role of synovial fluid on particle properties and performance is an important area of investigation to more fully understand the state of nanomaterials as they exist in the intra-articular space.
Figure 5.
Synovial fluid impacts the ability of nanomaterials to enter the cartilage matrix. Here, nanomaterials were composed of a poly(lactide-co-glycolide) core with polyvinyl alcohol and/or didodecyldiamonium bromide to create an anionic or cationic surface charge, respectively. (A) When the articular surface of bovine cartilage explants were exposed to such particles in a suspension of saline or synovial fluid, the amount of cationic particles significantly reduced but anionic particles remained unchanged. (B) Fluorescence of the particles shows that penetration was reduced for cationic particles when synovial fluid was included. NPs = nanoparticles; ** p < 0.01 (anionic vs. cationic NPs); β p < 0.01 (saline vs synovial fluid). [110] Adapted with permission from Molecular Pharmaceutics. Copyright 2017 American Chemical Society.
5.3. Limitations of cross-study comparisons
It is important to understand how nanomaterial properties influence joint interactions in order to best design delivery vehicles for intentional localization within the joint. The conditions under which materials are characterized can impact the property measurements, and this is particularly important when considering how properties will likely differ between the biological environment and controlled laboratory settings. For example, dynamic light scattering (DLS) is commonly employed to measure size and zeta potential of nanomaterials, a technique for which the mathematical outcome and data interpretation are strongly influenced by experimental conditions such as sample diluent, temperature, concentration, and/or mathematical weighting [160]. However, these values are not often described within the intra-articular drug delivery literature. Additionally, the polydispersity of nanomaterial systems is often not reported. This factor is also important, as it may influence joint tissue localization and functional outcome; systems with multi-model size distributions may have differently sized nanomaterial populations that accumulate in different regions of the joint. Without sufficient characterization and detailed reporting, the ability to draw comparisons between studies is limited [160,161].
Cross-study comparisons are also confounded by the differences in tissue sourcing and models in which nanomaterials are assessed. In vivo, intra-articular injection of nanomaterials is most commonly studied in small rodents, primarily in rat and mouse and occasionally in rabbit and guinea pig. However, ex vivo and in vitro experiments regarding cartilage interactions are conducted almost exclusively on bovine cartilage. Cartilage thickness ranges from ~100μm for rats [162], ~1–1.6mm for bovine calves [163], and ~2mm for humans [164] depending on the measurement site. Material transport into cartilage has been found to be proportional to the square of cartilage thickness [165], and therefore nanomaterial associations with cartilage may vary considerably across tissue sources and species. Translation of findings across experiments is also relevant for the synovium, where nanomaterial interfaces are primarily studied in vitro in two dimensional cell culture and in vivo in small mammals. As drug delivery systems progress across preclinical studies to clinical trials, the potential limitations in scalability and translatability of these models should be considered.
Investigations of nanomaterials for the joint are also conducted across a variety of in vivo OA models and stages of disease progression. The physiologic factors that drive joint clearance and biodistribution change in the presence of disease, partially because of heightened capillary and lymphatic flow [11] and structural changes to tissue extracellular matrix [166]. Moreover, these pathophysiologic alterations can vary across different animal models of OA [167,168], suggesting that the nanomaterial pharmacokinetics and biodistribution for a single material may differ when studied in different OA models. Nevertheless, the various models present opportunities to evaluate different phenotypes of disease and therefore different therapeutic strategies. For example, post-traumatic OA models such as surgical ligament and meniscal transection, tibial fracture, and non-invasive ligament rupture can be used to study early stage drug interventions when the disease is still considered reversible, which has clinically relevant implications [169–171]. Moving forward, to successfully translate nanomaterials to the clinic, it will be important to understand how nanomaterial fate changes after injection at specific stages of disease, especially as the fields of early disease diagnosis by biomarker and imaging continue to advance [172–174].
To improve the advancement and comparison across studies in the field of nanomedicine for the joint, we can look to initiatives in cancer and other disease arenas. For example, a Cancer Nanomedicine Repository has been established as an open-source database to collect and make available open-access datasets related to nanoparticles for tumor delivery [175,176]. Initiatives like this may be beneficial for monitoring the progress of delivery to the joint, comparing across animal models, help inform decisions about nanomaterial design for specific joint targets, and highlight the need for uniform characterization and reporting across the field.
5.4. Biocompatibility of nanotechnology for the joint
While nanomaterials have made progress as a potential platform for intra-articular drug administration, these materials have inherent risks and limitations. By virtue of their size, nanomaterials have the capability to pass through biological barriers and exert changes at the sub-cellular level, thereby possessing the potential to induce toxic effects [177]. Additionally, nanomaterials may cause adverse effects via unintentional payload delivery to off-target sites, which is a potential risk in the joint where numerous tissues exist in close proximity [39]. Accordingly, as nanomaterials are developed for the joint, it is critical that toxicity is evaluated on the target cells as well as in off-target sites before proceeding to clinical trials [178]. While no adverse effects or toxicity-related complications have been reported for nanomaterials discussed in this review, researchers should continue to monitor for potential cytotoxicity after cell uptake or ECM accumulation in target and off target tissues. The effects of nanoparticle accumulation in cartilage, for example, on ECM mechanics, nutrient transport, and lubrication should be evaluated for any transient or long-term consequences. The ability of nanomaterials to degrade or erode in the joint is an important design parameter, and has primarily driven by the desired intra-articular drug release profile. Non-degradable wear particles from permanent orthopedic implants have been shown to stimulate macrophage infiltration and bone loss [179]. Consequently, the long-term fate of non-resorbable or slowly resorbing nanomaterials for drug delivery within the joint and their impact on inflammation, tissue abrasion, and other adverse effects warrants further investigation.
6. CONCLUSIONS AND PERSPECTIVE
OA is a prevalent and debilitating disease that is hallmarked by the degradation of articular cartilage and a cascading inflammatory response that perpetuates the disease. After intra-articular injection, the efficacy of drugs is hindered by the rapid clearance from the joint space, which ultimately decreases the time and opportunity for the therapeutics to interact with the diseased tissue and cells. Numerous nano-scale drug delivery systems have been implemented to address these limitations in whole-joint retention and localization within specific target sites. Depending on the therapeutic of interest, common targets in the joint include the cartilage, synovium, and the cells within those tissues.
Clear trends exist indicating that the implementation of a nanomaterial system has significant improvements to joint retention relative to injecting naked therapeutics. To further improve localization to specific joint targets, nanomaterials have been engineered with modalities that have improved associations with cartilage and synovial cells, via passive and active design approaches. Moving forward, incorporating the knowledge of the various nanomaterials may yield efficient multi-modal therapeutic delivery systems; this approach may continue to push us closer toward a comprehensive therapeutic strategy for halting OA progression. In addition, these nanomaterials can be investigated for drug delivery to other joint tissues including fat pad, meniscus, ligaments, and subchondral bone, all of which uniquely contribute to and are affected by OA progression.
While much progress had been made toward improving joint and tissue retention in preclinical investigations through the development of nanomaterials, opportunities for improvement still exist. Challenges include clarifying structure-function relationships between nanomaterial properties and joint tissue interactions at various stages of disease. Additionally, the role of synovial fluid on drug pharmacodynamics, particle properties, and potential masking effects is only in early stages of investigation and should be explored further to better understand how to overcome, or possibly leverage, these interferences if needed. Nonetheless, nanomedicine for the joint has made considerable progress and shows great promise as an implementable method for sustained and efficient drug delivery for OA. Moving forward, as site-specific drugs are developed as mono- and multi-modal DMOADs, the ability to intentionally direct drugs and drug carriers to specific compartments within the joint will become a critical step toward clinical translation. Targeted nanomaterials serve as a promising platform to localize these emerging therapeutics with sufficient bioavailability, reduced off target effects, and overall improved therapeutic efficacy.
Statement of Significance.
Improving drug delivery to the joint is a pressing clinical need. Over 27 million Americans live with osteoarthritis, and this figure is continuously expanding. Numerous drugs have been investigated but failed in clinical trials, likely related to poor bioavailability to target cells. This article comprehensively reviews the advances in nano-scale delivery vehicles designed to overcome the delivery barriers in the joint. This is the first review to analyze active and passive targeting strategies systematically for different target sites while also delineating between tissue homing and whole joint retention. By bringing together the lessons learned across numerous nano-scale platforms, researchers may be able to hone future carrier designs, allowing emerging therapeutics to perform with clinically relevant efficacy and disease modifying potential.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the National Institutes of Health (NIH) National Center for Advancing Translational Sciences under award number UL1 TR001427. This material has also been funded through the NIH, National Institute of Arthritis and Musculoskeletal and Skin Diseases, R01 AR071335–01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of the Drug Delivery for Musculoskeletal Applications Special Issue, edited by Robert S. Hastings and Professor Johnna S. Temenoff.
DISCLOSURES
The authors do not have any conflicts of interest to disclose.
1Adapted with permission from ACS NANO. Copyright 2014 American Chemical Society.
2 Reprinted from Biomaterials, 33, Whitmire et al., Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins, 7665–7675, Copyright 2012, with permission from Elsevier. Reprinted from The Lancet, 33, Whitmire et al., Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins, 7665–7675, Copyright 2012, with permission from Elsevier.
3Reprinted from Materials Views, 3, Singh et al., Nanoengineered Particles for Enhanced Intra-Articular Retention and Delivery of Proteins, 1562–1567, Copyright 2014, with permission from Elsevier
4Adapted with permission from Bioconjugate Chemistry. Copyright 2015, American Chemical Society.
REFERENCES
- [1].Benders KEM, van Weeren PR, Badylak SF, Saris DBF, Dhert WJA, Malda J, Extracellular matrix scaffolds for cartilage and bone regeneration, Trends Biotechnol. 31 (2013) 169–176. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- [2].Hootman JM, Helmick CG, Projections of US prevalence of arthritis and associated activity limitations, Arthritis Rheum. 54 (2006) 226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- [3].Lawrence RC, Estimates of the Prevalence of Arthritis and Other Rheumatic Conditions in the United States, Part II, Arthritis Rheum. 58 (2008) 26–35. doi: 10.1002/art.23176.Estimates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA, Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data, Arthritis Rheum. 60 (2009) 3546–3553. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- [5].Bijlsma JWJ, Berenbaum F, Lafeber FPJG, Osteoarthritis: An update with relevance for clinical practice, Lancet. 377 (2011) 2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- [6].Calvet J, Orellana C, Larrosa M, Navarro N, Chillarón JJ, Gratacós J, Calvet J, Orellana C, Larrosa M, Navarro N, Chillarón JJ, Galisteo C, High prevalence of cardiovascular co-morbidities in patients with symptomatic knee or hand osteoarthritis, Scand. J. Rheumatol 9742 (2015). doi: 10.3109/03009742.2015.1054875. [DOI] [PubMed] [Google Scholar]
- [7].van Dijk GM, Veenhof C, Schellevis F, Hulsmans H, Bakker JP, Arwert H, Dekker JH, Lankhorst GJ, Dekker J, Comorbidity, limitations in activities and pain in patients with osteoarthritis of the hip or knee, BMC Musculoskelet. Disord. 9 (2008) 95. doi: 10.1186/1471-2474-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wise BL, Niu J, Zhang Y, Wang N, Jordan JM, Choy E, Hunter DJ, Psychological factors and their relation to osteoarthritis pain, Osteoarthr. Cartil. 18 (2010) 883–887. doi: 10.1016/j.joca.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lin EHB, Katon W, Williams JW, Kroenke K, Hunkeler E, Harpole L, Hoffing M, Della Penna R, Langston C, Effect of Improving Depression Care on Pain and Functional Outcomes, JAMA. 290 (2003). doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- [10].Larsen C, Østergaard J, Larsen SW, Jensen H, Jacobsen S, Lindegaard C, Andersen PH, Intra-articular depot formulation principles: Role in the management of postoperative pain and arthritic disorders, J. Pharm. Sci 97 (2008) 4622–4654. doi: 10.1002/jps.21346. [DOI] [PubMed] [Google Scholar]
- [11].Evans C, Kraus V, Setton L, Progress in intra-articular therapy, Rheumatol, Nat Rev. 10 (2015) 11–22. doi: 10.1038/nrrheum.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gerwin N, Hops C, Lucke A, Intraarticular drug delivery in osteoarthritis, Adv. Drug Deliv. Rev 58 (2006) 226–242. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- [13].Owen S, Francis H, Roberts M, Disappearance kinetics of solutes from synovial fluid after in tra-articular injection, Br. J. Clin. Pharmacol 38 (1994) 349–355. doi: 10.1111/j.1365-2125.1994.tb04365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schumacher HR, Aspiration and injection therapies for joints, Arthritis Rheum. 49 (2003) 413–20. doi: 10.1002/art.11056. [DOI] [PubMed] [Google Scholar]
- [15].Chevalier X, Goupille P, Beaulieu a D., Burch FX, Bensen WG, Conrozier T, Loeuille D, Kivitz a J., Silver D, Appleton BE, Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study, Arthritis Rheum. 61 (2009) 344–52. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- [16].Vugmeyster Y, Wang Q, Xu X, Harrold J, Daugusta D, Li J, Zollner R, Flannery CR, Rivera-Bermúdez MA, Disposition of human recombinant lubricin in naive rats and in a rat model of post-traumatic arthritis after intra-articular or intravenous administration, AAPS J 14 (2012) 97–104. doi: 10.1208/s12248-011-9315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Larsen NE, Dursema HD, Pollak CT, Skrabut EM, Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models, J. Biomed. Mater. Res. - Part B Appl. Biomater 100 B (2012) 457–462. doi: 10.1002/jbm.b.31971. [DOI] [PubMed] [Google Scholar]
- [18].Ulrich-Vinther M, Maloney MD, Schwarz EM, Rosier R, O’Keefe RJ, Articular cartilage biology., J. Am. Acad. Orthop. Surg 11 (n.d.) 421–30. [DOI] [PubMed] [Google Scholar]
- [19].Jahn S, Seror J, Klein J, Lubrication of Articular Cartilage, Annu. Rev. Biomed. Eng 18 (2016) 235–258. doi: 10.1063/PT.3.3898. [DOI] [PubMed] [Google Scholar]
- [20].Jiménez G, Cobo-Molinos J, Antich C, López-Ruiz E, Osteoarthritis: Trauma vs Disease, in: Adv. Exp. Med. Biol, 2018: pp. 63–83. doi: 10.1007/978-3-319-76735-2_3. [DOI] [PubMed] [Google Scholar]
- [21].Rosenberg JH, Rai V, Dilisio MF, Agrawal DK, Damage-associated molecular patterns in the pathogenesis of osteoarthritis: potentially novel therapeutic targets., Mol. Cell. Biochem. 434 (2017) 171–179. doi: 10.1007/s11010-017-3047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang T, He C, Pro-inflammatory cytokines: The link between obesity and osteoarthritis, Cytokine Growth Factor Rev. 44 (2018) 38–50. doi: 10.1016/j.cytogfr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- [23].Bondeson J, Wainwright S, Hughes C, Caterson B, The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review., Clin. Exp. Rheumatol 26 (n.d.) 139–45. [PubMed] [Google Scholar]
- [24].Verma P, Dalal K, ADAMTS-4 and ADAMTS-5: Key enzymes in osteoarthritis, J. Cell. Biochem 112 (2011) 3507–3514. doi: 10.1002/jcb.23298. [DOI] [PubMed] [Google Scholar]
- [25].V Medvedeva E, Grebenik EA, Gornostaeva SN, Telpuhov VI, V Lychagin A, Timashev PS, Chagin AS, Repair of Damaged Articular Cartilage: Current Approaches and Future Directions., Int. J. Mol. Sci 19 (2018). doi: 10.3390/ijms19082366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Loeser RF, Goldring SR, Scanzello CR, Goldring MB, Osteoarthritis: A disease of the joint as an organ, Arthritis Rheum. 64 (2012) 1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pan F, Jones G, Clinical Perspective on Pain and Pain Phenotypes in Osteoarthritis, Curr. Rheumatol. Rep 20 (2018) 79. doi: 10.1007/s11926-018-0796-3. [DOI] [PubMed] [Google Scholar]
- [28].Mort JS, Billington CJ, Articular cartilage and changes in arthritis: matrix degradation, Arthritis Res 3 (2001) 337. doi: 10.1186/ar325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mobasheri A, Bay-Jensen A-C, Gualillo O, Larkin J, Levesque MC, Henrotin Y, Soluble biochemical markers of osteoarthritis: Are we close to using them in clinical practice?, Best Pract. Res. Clin. Rheumatol 31 (2017) 705–720. doi: 10.1016/j.berh.2018.07.002. [DOI] [PubMed] [Google Scholar]
- [30].Deveza LA, Loeser RF, Is osteoarthritis one disease or a collection of many?, Rheumatology. 57 (2018) iv34–iv42. doi: 10.1093/rheumatology/kex417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Carbone A, Rodeo S, A review of current understanding of post-traumatic osteoarthritis resulting from sports injuries, J. Orthop. Res (2016). http://doi.wiley.com/10.1002/jor.23341. [DOI] [PubMed] [Google Scholar]
- [32].Hafezi-Nejad N, Demehri S, Guermazi A, Carrino JA, Osteoarthritis year in review 2017: updates on imaging advancements, Osteoarthr. Cartil. 26 (2018) 341–349. doi: 10.1016/j.joca.2018.01.007. [DOI] [PubMed] [Google Scholar]
- [33].Goldring MB, Update on the biology of the chondrocyte and new approaches to treating cartilage diseases, Best Pract. Res. Clin. Rheumatol 20 (2006) 1003–1025. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- [34].Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ, Cartilage in normal and osteoarthritis conditions, Best Pract. Res. Clin. Rheumatol 22 (2008) 351–384. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [35].Berenbaum F, Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!), Osteoarthr. Cartil 21 (2013) 16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- [36].Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, Go ldring SR, Jones G, Teichtahl AJ, Pelletier JP, Osteoarthritis, Nat. Rev. Dis. Prim. 2 (2016). doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- [37].Silawal S, Triebel J, Bertsch T, Schulze-Tanzil G, Osteoarthritis and the Complement Cascade, Clin. Med. Insights Arthritis Musculoskelet. Disord 11 (2018) 117954411775143. doi: 10.1177/1179544117751430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lopes EBP, Filiberti A, Husain SA, Humphrey MB, Immune Contributions to Osteoarthritis, Curr. Osteoporos. Rep 15 (2017) 593–600. doi: 10.1007/s11914-017-0411-y. [DOI] [PubMed] [Google Scholar]
- [39].De Jong WH, a Borm PJ, Drug delivery and nanoparticles: applications and hazards, Int. J. Nanomedicine 3 (2008) 133–149. doi: 10.2147/IJN.S596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bijlsma JWJ, Berenbaum F, Lafeber FPJG, Osteoarthritis: An update with relevance for clinical practice, Lancet. 377 (2011) 2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- [41].Laev SS, Salakhutdinov NF, Anti-arthritic agents: Progress and potential, Bioorg. Med. Chem 23 (2015) 3059–3080. doi: 10.1016/J.BMC.2015.05.010. [DOI] [PubMed] [Google Scholar]
- [42].Holyoak DT, Tian YF, van der Meulen MCH, Singh A, Osteoarthritis: Pathology, Mouse Models, and Nanoparticle Injectable Systems for Targeted Treatment, Ann. Biomed. Eng 44 (2016) 2062–2075. doi: 10.1007/s10439-016-1600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wehling P, Evans C, Wehling J, Maixner W, Effectiveness of intra-articular therapies in osteoarthritis: a literature review., Ther. Adv. Musculoskelet. Dis 9 (2017) 183–196. doi: 10.1177/1759720X17712695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen AL, Desai P, Adler EM, Di Cesare PE, Granulomatous inflammation after Hylan G-F 20 viscosupplementation of the knee : a report of six cases., J. Bone Joint Surg. Am 84-A (2002) 1142–7. [PubMed] [Google Scholar]
- [45].Charalambous CP, Tryfonidis M, Sadiq S, Hirst P, Paul A, Septic arthritis following intra-articular steroid injection of the knee - a survey of current practice regarding antiseptic technique used during intra articular steroid injection of the knee, Clin. Rheumatol 22 (2003) 386–390. doi: 10.1007/s10067-003-0757-7. [DOI] [PubMed] [Google Scholar]
- [46].Weinberg JB, Pippen AMM, Greenberg CS, Extravascular fibrin formation and dissolution in synovial tissue of patients with osteoarthritis and rheumatoid arthritis, Arthritis Rheum. 34 (1991) 996–1005. doi: 10.1002/art.1780340809. [DOI] [PubMed] [Google Scholar]
- [47].de Lange-Brokaar BJE, Ioan-Facsinay A, van Osch GJVM, Zuurmond AM, Schoones J, Toes REM, Huizinga TWJ, Kloppenburg M, Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review, Osteoarthr. Cartil 20 (2012) 1484–1499. doi: 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- [48].Freemont AJ, The Pathophysiology of Cartilage and Synovium, Br. J. Rheumatol 35 (1996) 10–13. [DOI] [PubMed] [Google Scholar]
- [49].Goldie I, The Synovial Microvascular Derangement in Rheumatoid Arthritis and Osteoarthritis, Acta Orthop 40 (1969) 751–764. doi: 10.3109/17453676908989539. [DOI] [PubMed] [Google Scholar]
- [50].Scott DL, Wolfe F, Huizinga TW, Rheumatoid arthritis, Lancet. 376 (2010) 1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- [51].Senthelal S, Thomas MA, Arthritis, StatPearls Publishing, 2018. http://www.ncbi.nlm.nih.gov/pubmed/30085534 (accessed December 14, 2018). [PubMed] [Google Scholar]
- [52].Wallis WJ, Simkin PA, Nelp WB, Protein traffic in human synovial effusions, Arthritis Rheum. 30 (1987) 57–63. doi: 10.1002/art.1780300108. [DOI] [PubMed] [Google Scholar]
- [53].Buckwalter JA, Mankin HJ, Grodzinsky AJ, Articular cartilageOS Instr. Course Lect 54 (2005) 465–480. doi: 10.1136/ard.51.9.1028-a. [DOI] [PubMed] [Google Scholar]
- [54].Mathiessen A, Conaghan PG, Synovitis in osteoarthritis: current understanding with therapeutic implications, Arthritis Res. Ther 19 (2017) 18. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stewart HL, Kawcak CE, The Importance of Subchondral Bone in the Pathophysiology of Osteoarthritis, Front. Vet. Sci 5 (2018) 178. doi: 10.3389/fvets.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL, A systems biology approach to synovial joint lubrication in health, injury, and disease, Wiley Interdiscip. Rev. Syst. Biol. Med 4 (2012) 15–37. doi: 10.1002/wsbm.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tsujii A, Nakamura N, Horibe S, Age-related changes in the knee meniscus, Knee. 24 (2017) 1262–1270. doi: 10.1016/J.KNEE.2017.08.001. [DOI] [PubMed] [Google Scholar]
- [58].Bay-Jensenn A-C, Hoegh-Madsen S, Dam E, Henriksen K, Sondergaard BC, Pastoureau P, Which elements are involved in reversible and irreversible cartilage degradation in osteoarthritis?, Rheumatol Int. 30 (2010) 435–442. doi: 10.1007/s00296-009-1183-1. [DOI] [PubMed] [Google Scholar]
- [59].Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y, Cellular Uptake, Intracellular Trafficking, and Cytotoxicity of Nanomaterials, Small. 7 (2011) 1322–1337. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]
- [60].Mendes M, Sousa JJ, Pais A, Vitorino C, Mendes M, Sousa JJ, Pais A, Vitorino C, Targete d Theranostic Nanoparticles for Brain Tumor Treatment, Pharmaceutics. 10 (2018) 181. doi: 10.3390/pharmaceutics10040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK, Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size, Cancer Res. 55 (1995) 3752–6. http://www.ncbi.nlm.nih.gov/pubmed/7641188 (accessed December 11, 2018). [PubMed] [Google Scholar]
- [62].Ferrari M, Onuoha SC, Pitzalis C, Trojan horses and guided missiles: targeted therapies in the war on arthritis, Nat. Rev. Rheumatol (2015) 1–10. doi: 10.1038/nrrheum.2015.17. [DOI] [PubMed] [Google Scholar]
- [63].Kavanaugh TE, Werfel TA, Cho H, Hasty KA, Duvall CL, Particle-based technologies for osteoarthritis detection and therapy, (2016) 132–147. doi: 10.1007/s13346-015-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].de Silva P, Hazleman M, Thomas B, Wraight D, Liposomes in arthritis: a new approach, Lancet (London, England). 1 (1979) 1320–2. http://www.ncbi.nlm.nih.gov/pubmed/87780 (accessed April 4, 2016). [DOI] [PubMed] [Google Scholar]
- [65].Bonanomi MH, Velvart M, Stimpel M, Fehr K, Weder HG, Studies of pharmacokinetics and therapeutic effects of glucocorticoids entrapped in liposomes after intraarticular application in healthy rabbits and in rabbits with antigen-induced arthritis, Rheumatol. Int 7 (1987) 203–212. [DOI] [PubMed] [Google Scholar]
- [66].Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V, PLGA-based nanoparticles: An overview of biomedical applications, J. Control. Release 161 (2012) 505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- [67].Makadia HK, Siegel SJ, Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier, Polym. 3 (2011) 1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wang D, Miller SC, Liu XM, Anderson B, Wang XS, Goldring SR, Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis, Arthritis Res. Ther 9 (2007) 1–9. doi: 10.1186/ar2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Alam MM, Han HS, Sung S, Kang JH, Sa KH, Al Faruque H, Hong J, Nam EJ, Kim IS, Park JH, Kang YM, Endogenous inspired biomineral-installed hyaluronan nanoparticles as pH-responsive carrier of methotrexate for rheumatoid arthritis, J. Control. Release 252 (2017) 62–72. doi: 10.1016/j.jconrel.2017.03.012. [DOI] [PubMed] [Google Scholar]
- [70].Janssen M, Mihov G, Welting T, Thies J, Emans P, Drugs and Polymers for Delivery Systems in OA Joints: Clinical Needs and Opportunities, (2014) 799–819. doi: 10.3390/polym6030799. [DOI] [Google Scholar]
- [71].Daheshia M, Yao JQ, The interleukin 1β pathway in the pathogenesis of osteoarthritis, J. Rheumatol 35 (2008) 2306–2312. doi: 10.3899/jrheum.080346. [DOI] [PubMed] [Google Scholar]
- [72].Edwards SHR, Intra-articular drug delivery: The challenge to extend drug residence time within the joint, Vet. J 190 (2011) 15–21. doi: 10.1016/j.tvjl.2010.09.019. [DOI] [PubMed] [Google Scholar]
- [73].Horisawa E, Kubota K, Tuboi I, Sato K, Yamamoto H, Takeuchi H, Kawashima Y, Size-Dependency of DLLactide Glycolide Copolymer Particulates for intra-Articular Delivery System on Phagocytosis in Rat Synovium, Pharm. Res 19 (2002) 132–139. [DOI] [PubMed] [Google Scholar]
- [74].Sacchetti C, Liu-bryan R, Magrini A, Rosato N, Bottini N, Bottini M, Polyethylene-Glycol-Modified Single-Walled Carbon Nanotubes for Intra-Articular Delivery to Chondrocytes, (2014) 12280–12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Whitmire RE, Scott Wilson D, Singh A, Levenston ME, Murthy N, García AJ, Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins, Biomaterials. 33 (2012) 7665–7675. doi: 10.1016/j.biomaterials.2012.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Singh A, Agarwal R, Diaz-Ruiz CA, Willett NJ, Wang P, Lee LA, Wang Q, Guldberg RE, Garcia AJ, Nanoengineered particles for enhanced intra-articular retention and delivery of proteins, Adv. Healthc. Mater 3 (2014) 1562–1567. doi: 10.1002/adhm.201400051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hu HY, Lim NH, Ding-Pfennigdorff D, Saas J, Wendt KU, Ritzeler O, Nagase H, Plettenburg O, Schultz C, Nazare M, DOTAM Derivatives as Active Cartilage-Targeting Drug Carriers for the Treatment of Osteoarthritis, Bioconjug. Chem 26 (2015) 383–388. doi: 10.1021/bc500557s. [DOI] [PubMed] [Google Scholar]
- [78].Mwangi TK, Berke IM, Nieves EH, Bell RD, Adams SB, Setton LA, Intra-articular clearance of labeled dextrans from naive and arthritic rat knee joints, J. Control. Release 283 (2018) 76–83. doi: 10.1016/j.jconrel.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kim SR, Ho MJ, Lee E, Lee JW, Choi YW, Kang MJ, Cationic PLGA/eudragit RL nanoparticles for increasing retention time in synovial cavity after intra-articular injection in knee joint, Int. J. Nanomedicine 10 (2015) 5263–5271. doi: 10.2147/IJN.S88363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Morgen M, Tung D, Boras B, Miller W, Malfait AM, Tortorella M, Nanoparticles for improved local retention after intra-articular injection into the knee joint, Pharm. Res 30 (2013) 257–268. doi: 10.1007/s11095-012-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bajpayee AG, Scheu M, Grodzinsky AJ, Porter RM, Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints, J. Orthop. Res 32 (2014) 1044–1051. doi: 10.1002/jor.22630. [DOI] [PubMed] [Google Scholar]
- [82].Geiger BC, Wang S, Padera RF, Grodzinsky AJ, Hammond PT, Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis, Sci. Transl. Med 10 (2018) eaat8800. doi: 10.1126/scitranslmed.aat8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rothenfluh D, Bermudez H, O’Neil CP, Hubbell J, Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage, Nat. Mater 7 (2008) 248–254. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- [84].Pradal J, Maudens P, Gabay C, Seemayer CA, Jordan O, Allémann E, Effect of particle size on the biodistribution of nano- and microparticles following intra-articular injection in mice, Int. J. Pharm 498 (2016) 119–129. doi: 10.1016/j.ijpharm.2015.12.015. [DOI] [PubMed] [Google Scholar]
- [85].Kang ML, Ko JY, Kim JE, Il Im G, Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration, Biomaterials. 35 (2014) 9984–9994. doi: 10.1016/j.biomaterials.2014.08.042. [DOI] [PubMed] [Google Scholar]
- [86].Kang ML, Kim JE, Il Im G, Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis, Acta Biomater. 39 (2016) 65–78. doi: 10.1016/j.actbio.2016.05.005. [DOI] [PubMed] [Google Scholar]
- [87].Erickson HP, Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy, Biol. Proced. Online 11 (2009) 32–51. doi: 10.1007/s12575-009-9008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bajpayee AG, Wong CR, Bawendi MG, Frank EH, Grodzinsky AJ, Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis, Biomaterials. 35 (2014) 538–549. doi: 10.1016/j.biomaterials.2013.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Elsaid KA, Ferreira L, Truong T, Liang A, Machan J, D’Souza GG, Pharmaceutical nanocarrier association with chondrocytes and cartilage explants: Influence of surface modification and extracellular matrix depletion, Osteoarthr. Cartil 21 (2013) 377–384. 10.1016/j.joca.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yan H, Duan X, Pan H, Holguin N, Rai MF, Akk A, Springer LE, Wickline SA, Sandell LJ, Pham CTN, Suppression of NF-κB activity via nanoparticle-based siRNA delivery alters early cartilage res ponses to injury, Proc. Natl. Acad. Sci 113 (2016) E6199–E6208. doi: 10.1073/pnas.1608245113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Cho H, Stuart JM, Magid R, Danila DC, Hunsaker T, Pinkhassik E, Hasty KA, Theranostic immunoliposomes for osteoarthritis, Nanomedicine Nanotechnology, Biol. Med 10 (2014) 619–627. doi: 10.1016/j.nano.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cho H, Kim BJ, Park S-H, Hasty KA, Min B-H, Noninvasive visualization of early osteoarthritic cartilage using targeted nanosomes in a destabilization of the medial meniscus mouse model, Int. J. Nanomedicine 13 (2018) 1215–1224. doi: 10.2147/IJN.S149375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chen Z, Chen J, Wu L, Li W, Chen J, Cheng H, Pan J, Cai B, Hyaluronic acid-coated bovine serum albumin nanoparticles loaded with brucine as selective nanovectors for intra-articular injection, Int. J. Nanomedicine 8 (2013) 3843–3853. doi: 10.2147/IJN.S50721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Laroui H, Grossin L, Léonard M, Stoltz JF, Gillet P, Netter P, Dellacherie E, Hyaluronate-covered nanoparticles for the therapeutic targeting of cartilage, Biomacromolecules. 8 (2007) 3879–3885. doi: 10.1021/bm700836y. [DOI] [PubMed] [Google Scholar]
- [95].Didomenico CD, Lintz M, Bonassar LJ, Molecular transport in articular cartilage - What have we learned from the past 50 years?, Nat. Rev. Rheumatol 14 (2018) 393–403. doi: 10.1038/s41584-018-0033-5. [DOI] [PubMed] [Google Scholar]
- [96].Sophia Fox AJ, Bedi A, Rodeo SA, The Basic Science of Articular Cartilage: Structure, Composition, and Function, Sport. Heal. A Multidiscip. Approach 1 (2009) 461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hall BK, Newman S, Cartilage: molecular aspects, CRC Press, Boston, 1991. https://www.crcpress.com/Cartilage-Molecular-Aspects/Hall-Newman/p/book/9780849388170 (accessed November 27, 2018). [Google Scholar]
- [98].Ng L, Grodzinsky AJ, Patwari P, Sandy J, Plaas A, Ortiz C, Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy, J. Struct. Biol 143 (2003) 242–257. doi: 10.1016/j.jsb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- [99].Levick JR, Flow through interstitium and other fibrous matrices, Q. J. Exp. Physiol 72 (1987) 409–437. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- [100].Torzilli PA, Arduino JM, Gregory JD, Bansal M, Effect of proteoglycan removal on solute mobility in articular cartilage, J. Biomech 30 (1997) 895–902. [DOI] [PubMed] [Google Scholar]
- [101].Nieminen HJ, Salmi A, Karppinen P, Haeggstrom E, Hacking SA, The potential utility of high-intensity ultrasound to treat osteoarthritis, Osteoarthr. Cartil 22 (2014) 1784–1799. doi: 10.1016/j.joca.2014.07.025. [DOI] [PubMed] [Google Scholar]
- [102].Majda D, Bhattarai A, Riikonen J, Napruszewska BD, Zimowska M, Michalik-Zym A, Töyräs J, Lehto V-P, New approach for determining cartilage pore size distribution: NaCl-thermoporometry, Microporous Mesoporous Mater. 241 (2017) 238–245. doi: 10.1016/J.MICROMESO.2017.01.005. [DOI] [Google Scholar]
- [103].Foy BD, Blake J, Diffusion of Paramagnetically Labeled Proteins in Cartilage: Enhancement of the 1-D NMR Imaging Technique, J. Magn. Reson 148 (2001) 126–134. doi: 10.1006/jmre.2000.2216. [DOI] [PubMed] [Google Scholar]
- [104].Jia H, Ma X, Tong W, Doyran B, Sun Z, Wang L, Zhang X, Zhou Y, Badar F, Chandra A, Lu XL, Xia Y, Han L, Enomoto-Iwamoto M, Qin L, EGFR signaling is critical for maintaining the superficial layer of articular cartilage and preventing osteoarthritis initiation, PNAS. 113 (2016) 14360–14365. doi: 10.1073/pnas.1608938113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Palmer AW, Guldberg RE, Levenston ME, Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography, Proc. Natl. Acad. Sci 103 (2006) 19255–19260. doi: 10.1073/pnas.0606406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Stanescu R, Leibovich SJ, The negative charge of articular cartilage surfaces, J. Bone Jt. Surg. - Ser. A 64 (1982) 388–398. doi: 10.2106/00004623-198264030-00009. [DOI] [PubMed] [Google Scholar]
- [107].Shapiro EM, Borthakur A, Gougoutas A, Reddy R, 23NA MRI accurately measures fixed charge density in articular cartilage, Magn. Reson. Med 47 (2002) 284–291. doi: 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bajpayee AG, Quadir MA, Hammond PT, Grodzinsky AJ, Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term, Osteoarthr. Cartil 24 (2016) 71–81. doi: 10.1016/j.joca.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Freedman JD, Lusic H, Snyder BD, Grinstaff MW, Tantalum oxide nanoparticles for the imaging of articular cartilage using X-ray computed tomography: Visualization of ex vivo/in vivo murine tibia and ex vivo human index finger cartilage, Angew. Chemie - Int. Ed 53 (2014) 8406–8410. doi: 10.1002/anie.201404519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Brown S, Pistiner J, Adjei I, Sharma B, Nanoparticle properties for delivery to cartilage: the implications of disease state, synovial fluid, and off-target uptake, Mol. Pharm (2017). doi: 10.1021/acs.molpharmaceut.7b00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Byun S, Sinskey YL, Lu YCS, Ort T, Kavalkovich K, Sivakumar P, Hunziker EB, Frank EH, Grodzinsky AJ, Transport of anti-il-6 antigen binding fragments into cartilage and the effects of injury, Arch. Biochem. Biophys 532 (2013) 15–22. doi: 10.1016/j.abb.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Maroudas A, Transport of solutes through cartilage: permeability to large molecules, J. Anat 122 (1976) 335–347. [PMC free article] [PubMed] [Google Scholar]
- [113].Noyori K, Koshino T, Takagi T, Okamoto R, Jasin HE, Binding characteristics of antitype II collagen antibody to the surface of diseased human cartilage as a probe for tissue damage, J. Rheumatol 21 (1994) 293–6. http://www.ncbi.nlm.nih.gov/pubmed/8182639 (accessed September 13, 2017). [PubMed] [Google Scholar]
- [114].Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM, Induction of arthritis with monoclonal antibodies to collagen, J. Immunol 148 (1992) 2103–8. [PubMed] [Google Scholar]
- [115].Cho H, Pinkhassik E, David V, Stuart JM, Hasty KA, Detection of early cartilage damage using targeted nanosomes in a post-traumatic osteoarthritis mouse model, Nanomedicine Nanotechnology, Biol. Med 11 (2015) 939–946. doi: 10.1016/j.nano.2015.01.011. [DOI] [PubMed] [Google Scholar]
- [116].Jain A, Mishra SK, Vuddanda PR, Singh SK, Singh R, Singh S, Targeting of diacerein loaded lipid nanoparticles to intra-articular cartilage using chondroitin sulfate as homing carrier for treatment of osteoarthritis in rats, Nanomedicine Nanotechnology, Biol. Med 10 (2014) 1031–1040. doi: 10.1016/j.nano.2014.01.008. [DOI] [PubMed] [Google Scholar]
- [117].Te Boekhorst BCM, Jensen LB, Colombo S, Varkouhi AK, Schiffelers RM, Lammers T, Storm G, Nielsen HM, Strijkers GJ, Foged C, Nicolay K, MRI-assessed therapeutic effects of locally administered PLGA nanoparticles loaded with anti-inflammatory siRNA in a murine arthritis model, J. Control. Release 161 (2012) 772–780. doi: 10.1016/j.jconrel.2012.05.004. [DOI] [PubMed] [Google Scholar]
- [118].Ha CW, Cho JJ, Elmallah RK, Cherian JJ, Kim TW, Lee MC, Mont MA, A Multicenter, Single-Blind, Phase IIa Clinical Trial to Evaluate the Efficacy and Safety of a Cell-Mediated Gene Therapy in Degenerative Knee Arthritis Patients, Hum. Gene Ther. Clin. Dev 26 (2015) 125–130. doi: 10.1089/humc.2014.145. [DOI] [PubMed] [Google Scholar]
- [119].Li Y, Wang Y, Chubinskaya S, Schoeberl B, Florine E, Kopesky P, Grodzinsky AJ, Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: Relevance to post-traumatic osteoarthritis, Osteoarthr. Cartil 23 (2015) 266–274. doi: 10.1016/j.joca.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Augustyniak E, Trzeciak T, Richter M, Kaczmarczyk J, Suchorska W, The role of growth factors in stem cell-directed chondrogenesis: a real hope for damaged cartilage regeneration, Int. Orthop (2014). doi: 10.1007/s00264-014-2619-0. [DOI] [PubMed] [Google Scholar]
- [121].Cassiede P, Dennis JE, Ma F, a I. Caplan, Osteochondrogenic potential of marrow mesenchymal progenitor cells exposed to TGF-beta 1 or PDGF-BB as assayed in vivo and in vitro, J. Bone Miner. Res 11 (1996) 1264–1273. doi: 10.1002/jbmr.5650110911. [DOI] [PubMed] [Google Scholar]
- [122].da Costa BR, Nüesch E, Reichenbach S, Jüni P, Rutjes AWS, Doxycycline for osteoarthritis of the knee or hip, Cochrane Database Syst. Rev. 11 (2012) CD007323. doi: 10.1002/14651858.CD007323.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Liu J, Khalil RA, Matrix Metalloproteinase Inhibitors as Investigational and Therapeutic Tools in Unrestrained Tissue Remodeling and Pathological Disorders, in: Prog. Mol. Biol. Transl. Sci, 2017: pp. 355–420. doi: 10.1016/bs.pmbts.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Yang C-Y, Chanalaris A, Troeberg L, ADAMTS and ADAM metalloproteinases in osteoarthritis - looking beyond the ‘usual suspects,’ Osteoarthr. Cartil 25 (2017) 1000–1009. doi: 10.1016/j.joca.2017.02.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Bajpayee AG, Grodzinsky AJ, Cartilage-targeting drug delivery: can electrostatic interactions help?, Nat. Rev. Rheumatol 13 (2017) 183–193. doi: 10.1038/nrrheum.2016.210. [DOI] [PubMed] [Google Scholar]
- [126].Wang S, Wei X, Sun X, Chen C, Zhou J, Zhang G, Wu H, Guo B, Wei L, A novel therapeutic strategy for cartilage diseases based on lipid nanoparticle-RNAi delivery system, Int. J. Nanomedicine Volume 13 (2018) 617–631. doi: 10.2147/IJN.S142797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zheng S, Zhou H, Chen Z, Li Y, Zhou T, Lian C, Gao B, Su P, Xu C, Type III Transforming Growth Factor-β Receptor RNA Interference Enhances Transforming Growth Factor β3-Induced Chondrogenesis Signaling in Human Mesenchymal Stem Cells, Stem Cells Int 2018 (2018) 1–11. doi: 10.1155/2018/4180857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Yang B, Kang X, Xing Y, Dou C, Kang F, Li J, Quan Y, Dong S, Effect of microRNA-145 on IL-lβ-induced cartilage degradation in human chondrocytes, FEBS Lett. 588 (2014) 2344–2352. doi: 10.1016/j.febslet.2014.05.033. [DOI] [PubMed] [Google Scholar]
- [129].Hu Q, Ding B, Yan X, Peng L, Duan J, Yang S, Cheng L, Chen D, Polyethylene glycol modifi ed PAMAM dendrimer delivery of kartogenin to induce chondrogenic differentiation of mesenchymal stem cells, Nanomedicine Nanotechnology, Biol. Med 13 (2017) 2189–2198. doi: 10.1016/j.nano.2017.05.011. [DOI] [PubMed] [Google Scholar]
- [130].Wang CL, Peng JP, Chen XD, LncRNA-CIR promotes articular cartilage degeneration in osteoarthritis by regulating autophagy, Biochem. Biophys. Res. Commun 505 (2018) 692–698. doi: 10.1016/J.BBRC.2018.09.163. [DOI] [PubMed] [Google Scholar]
- [131].Knight AD, Levick JR, Morphometry of The Ultrastructure of the Blood-Joint Barrier in the Rabbit Knee, Q. J. Exp. Physiol (1984) 271–288. [DOI] [PubMed] [Google Scholar]
- [132].Chen Z, Liu D, Wang J, Wu L, Li W, Chen J, Cai B-C, Cheng H, Development of nanoparticles-inmicroparticles system for improved local retention after intra-articular injection, Drug Deliv. 21 (2014) 342–350. doi: 10.3109/10717544.2013.848495. [DOI] [PubMed] [Google Scholar]
- [133].Kim HJ, Lee SM, Park KH, Mun CH, Park YB, Yoo KH, Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis, Biomaterials. 61 (2015) 95–102. doi: 10.1016/j.biomaterials.2015.05.018. [DOI] [PubMed] [Google Scholar]
- [134].Kim MJ, Park JS, Lee SJ, Jang J, Park JS, Back SH, Bahn G, Park JH, Kang YM, Kim SH, Kwon IC, Jo DG, Kim K, Notch1 targeting siRNA delivery nanoparticles for rheumatoid arthritis therapy, J. Control. Release 216 (2015) 140–148. doi: 10.1016/j.jconrel.2015.08.025. [DOI] [PubMed] [Google Scholar]
- [135].Howard KA, Paludan SR, Behlke MA, Besenbacher F, Deleuran B, Kjems J, Chitosan/siRNA nanoparticle-mediated TNF-α knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model, Mol. Ther 17 (2009) 162–168. doi: 10.1038/mt.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Lu H, Dai Y, Lv L, Zhao H, Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis, PLoS One. 9 (2014). doi: 10.1371/journal.pone.0084703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Jain S, Tran TH, Amiji M, Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis, Biomaterials. 61 (2015) 162–177. doi: 10.1016/j.biomaterials.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Lee YK, Kim SW, Park JY, Kang WC, Kang YJ, Khang D, Suppression of human arthritis synovial fibroblasts inflammation using dexamethasone-carbon nanotubes via increasing caveolin-dependent endocytosis and recovering mitochondrial membrane potential, Int. J. Nanomedicine 12 (2017) 5761–5779. doi: 10.2147/IJN.S142122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Otilia HÖ May Yue Koo, Israel Rubinstein, Actively Targeted Low-Dose Camptothecin as a Safe, Long-Acting, Disease-Modifying Nanomedicine for Rheumatoid Arthritis, Pharm. Res 28 (2011) 776–787. doi: 10.1109/TMI.2012.2196707.Separate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Barrera P, Blom A, Van Lent PLEM, Van Bloois L, Beijnen JH, Van Rooijen N, De Waal Malefijt MC, Van De Putte LBA, Storm G, Van Den Berg WB, Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis, Arthritis Rheum. 43 (2000) 1951–1959. doi:. [DOI] [PubMed] [Google Scholar]
- [141].Bhalekar MR, Upadhaya PG, Madgulkar AR, Fabrication and efficacy evaluation of chloroquine nanoparticles in CFA-induced arthritic rats using TNF-α ELISA, Eur. J. Pharm. Sci 84 (2016) 1–8. doi: 10.1016/j.ejps.2016.01.009. [DOI] [PubMed] [Google Scholar]
- [142].Gary F, Budd R, Gabriel S, Mclnnes IB, O’Dell J, Mader R, Kelley’s Textbook of Rheumatology, chapter 102 “Proliferative Bone Diseases,” 9th ed., Saunders, 2012. doi: 10.1016/B978-1-4377-1738-9.00102-X. [DOI] [Google Scholar]
- [143].Labens R, Lascelles BDX, Charlton AN, Ferrero NR, Van Wettere AJ, Xia XR, Blikslager AT, Ex vivo effect of gold nanoparticles on porcine synovial membrane, Tissue Barriers. 1 (2013) e24314. doi: 10.4161/tisb.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Durymanov M, Kamaletdinova T, Lehmann SE, Reineke J, Exploiting passive nanomedicine accumulation at sites of enhanced vascular permeability for non-cancerous applications, J. Control. Release 261 (2017) 10–22. doi: 10.1016/j.jconrel.2017.06.013. [DOI] [PubMed] [Google Scholar]
- [145].Champion J, Walker A, Mitragotri S, Role of particle size in phagocytosis of polymeric microspheres, Pharm. Res 25 (2008) 1815–1821. doi: 10.1007/s11095-008-9562-y.Role. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wu CL, McNeill J, Goon K, Little D, Kimmerling K, Huebner J, Kraus V, Guilak F, Conditional Macrophage Depletion Increases Inflammation and Does Not Inhibit the Development of Osteoarthritis in Obese Macrophage Fas-Induced Apoptosis-Transgenic Mice, Arthritis Rheumatol. 69 (2017) 1772–1783. doi: 10.1002/art.40161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wardwell PR, Forstner MB, Bader RA, Investigation of the cytokine response to NF-kB decoy oligonucleotide coated polysaccharide based nanoparticles in rheumatoid arthritis in vitro m odels, Arthritis Res. Ther 17 (2015). doi: 10.1186/s13075-015-0824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Schulze K, Koch A, Schöpf B, Petri A, Steitz B, Chastellain M, Hofmann M, Hofmann H, Von Rechenberg B, Intraarticular application of superparamagnetic nanoparticles and their uptake by synovial membrane - an experimental study in sheep, J. Magn. Magn. Mater 293 (2005) 419–432. doi: 10.1016/j.jmmm.2005.02.075. [DOI] [Google Scholar]
- [149].Levick JR, Hypoxia and acidosis in chronic inflammatory arthritis; relation to vascular supply and dynamic effusion pressure., J. Rheumatol 17 (1990) 579–82. http://www.ncbi.nlm.nih.gov/pubmed/2359066 (accessed December 17, 2018). [PubMed] [Google Scholar]
- [150].Schneider T, Welker P, Haag R, Dernedde J, Hug T, Licha K, Kohl B, Arens S, Ertel W, Schulze-Tanzil G, Effects of dendritic polyglycerol sulfate on articular chondrocytes, Inflamm. Res 64 (2015) 917–928. doi: 10.1007/s00011-015-0875-0. [DOI] [PubMed] [Google Scholar]
- [151].Lam J, Lu S, Kasper FK, Mikos AG, Strategies for controlled delivery of biologics for cartilage repair, Adv. Drug Deliv. Rev 84 (2015) 123–134. doi: 10.1016/j.addr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Zhou HF, Yan H, Pan H, Hou KK, Akk A, Springer LE, Hu Y, Allen JS, Wickline SA, Pham CTN, Peptide-siRNA nanocomplexes targeting NF-κB subunit p65 suppress nascent experimental arthritis, J. Clin. Invest 124 (2014) 4363–4374. doi: 10.1172/JCI75673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Nomura H, Takanashi S, Tanaka M, Haniu H, Aoki K, Okamoto M, Kobayashi S, Takizawa T, Usui Y, Oishi A, Kato H, Saito N, Specific biological responses of the synovial membrane to carbon nanotubes, Sci. Rep 5 (2015) 1–11. doi: 10.1038/srep14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Thomas TP, Goonewardena SN, Majoros IJ, Kotlyar A, Cao Z, Leroueil PR, Baker JR, Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis, Arthritis Rheum. 63 (2011) 2671–2680. doi: 10.1002/art.30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Nogueira E, Gomes AC, Preto A, Cavaco-Paulo A, Folate-targeted nanoparticles for rheumatoid arthritis therapy, Nanomedicine Nanotechnology, Biol. Med 12 (2016) 1113–1126. doi: 10.1016/j.nano.2015.12.365. [DOI] [PubMed] [Google Scholar]
- [156].Zhao J, Zhao M, Yu C, Zhang X, Liu J, Cheng X, Lee RJ, Sun F, Teng L, Li Y, Multifunctional folate receptor-targeting and pH-responsive nanocarriers loaded with methotrexate for treatment of rheumatoid arthritis, Int. J. Nanomedicine 12 (2017) 6735–6746. doi: 10.2147/IJN.S140992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Zhou H, Chan HW, Wickline SA, Lanza GM, Pham CTN, αvβ3-Targeted nanotherapy suppresses inflammatory arthritis in mice, FASEB J. 23 (2009) 2978–2985. doi:09-129874 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Koning GA, Schiffelers RM, Wauben MHM, Kok RJ, Mastrobattista E, Molema G, Ten Hagen TLM, Storm G, Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate-containing RGD peptide liposomes inhibits experimental arthritis, Arthritis Rheum. 54 (2006) 1198–1208. doi: 10.1002/art.21719. [DOI] [PubMed] [Google Scholar]
- [159].Li R, He Y, Zhu Y, Jiang L, Zhang S, Qin J, Wu Q, Dai W, Shen S, Pang Z, Wang J, Expressway to Rheumatoid Arthritis by Macrophage-derived Microvesicle-Coated Nanoparticles, Nano Lett. (2018) acs.nanolett.8b03439. doi: 10.1021/acs.nanolett.8b03439. [DOI] [PubMed] [Google Scholar]
- [160].Bhattacharjee S, DLS and zeta potential - What they are and what they are not?, J. Control. Release 235 (2016) 337–351. doi: 10.1016/j.jconrel.2016.06.017. [DOI] [PubMed] [Google Scholar]
- [161].Baer DR, Engelhard MH, Johnson GE, Laskin J, Lai J, Mueller K, Munusamy P, Thevuthasan S, Wang H, Washton N, Elder A, Baisch BL, Karakoti A, Kuchibhatla SVNT, Moon D, Surface characterization of nanomaterials and nanoparticles: important needs and challenging opportunities, J. Vac. Sci. Technol. A 31 (2013) 50820. doi: 10.1116/1.4818423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Malda J, de Grauw JC, Benders KEM, Kik MJL, van de Lest CHA, Creemers LB, Dhert WJA, van Weeren PR, Of Mice, Men and Elephants: The Relation between Articular Cartilage Thickness and Body Mass, PLoS One. 8 (2013) 1–8. doi: 10.1371/journal.pone.0057683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].McLure SWD, Fisher J, Conaghan PG, Williams S, Regional cartilage properties of three quadruped tibiofemoral joints used in musculoskeletal research studies, Proc. Inst. Mech. Eng. Part H J. Eng. Med 226 (2012) 652–656. doi: 10.1177/0954411912447158. [DOI] [PubMed] [Google Scholar]
- [164].Frisbie DD, Cross MW, McIlwraith CW, A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee, Vet. Comp. Orthop. Traumatol 19 (2006) 142–6. [PubMed] [Google Scholar]
- [165].Bajpayee AG, Scheu M, Grodzinsky AJ, Porter RM, A rabbit model demonstrates the influence of cartilage thickness on intra-articular drug delivery and retention within cartilage, J. Orthop. Res 33 (2015) 660–667. doi: 10.1002/jor.22841. [DOI] [PubMed] [Google Scholar]
- [166].Horton WE, Bennion P, Yang L, Cellular, molecular, and matrix changes in cartilage during aging and osteoarthritis, J. Musculoskelet. Neuronal Interact 6 (2006) 379–381. [PubMed] [Google Scholar]
- [167].van der Kraan PMPM, Factors that influence outcome in experimental osteoarthritis, Osteoarthr. Cartil 25 (2016) 1–7. doi: 10.1016/j.joca.2016.09.005. [DOI] [PubMed] [Google Scholar]
- [168].Teeple E, Jay GD, Elsaid KA, Fleming BC, Animal Models of Osteoarthritis: Challenges of Model Selection and Analysis, AAPS J 15 (2013) 438–446. doi: 10.1208/s12248-013-9454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Christiansen BA, Guilak F, Lockwood KA, Olson SA, Pitsillides AA, Sandell LJ, Silva MJ, van der Meulen MCH, Haudenschild DR, Non-invasive mouse models of post-traumatic osteoarthritis, Osteoarthr. Cartil 23 (2015) 1627–1638. doi: 10.1016/j.joca.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Kuyinu EL, Narayanan G, Nair LS, Laurencin CT, Animal models of osteoarthritis: classification, update, and measurement of outcomes, J. Orthop. Surg. Res 11 (2016) 19. doi: 10.1186/s13018-016-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Lampropoulou-Adamidou K, Lelovas P, Karadimas EV, Liakou C, Triantafillopoulos IK, Dontas I, Papaioannou NA, Useful animal models for the research of osteoarthritis, Eur. J. Orthop. Surg. Traumatol 24 (2014) 263–271. doi: 10.1007/s00590-013-1205-2. [DOI] [PubMed] [Google Scholar]
- [172].Watt FE, Osteoarthritis biomarkers: year in review, Osteoarthr. Cartil 26 (2018) 312–318. doi: 10.1016/J.JOCA.2017.10.016. [DOI] [PubMed] [Google Scholar]
- [173].de Sousa EB, Dos Santos GC, Duarte MEL, Moura V, Aguiar DP, Metabolomics as a promising tool for early osteoarthritis diagnosis, Brazilian J. Med. Biol. Res 50 (2017). doi: 10.1590/1414-431X20176485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Mathiessen A, Cimmino MA, Hammer HB, Haugen IK, Iagnocco A, Conaghan PG, Imaging of osteoarthritis (OA): What is new?, Best Pract. Res. Clin. Rheumatol 30 (2016) 653–669. doi: 10.1016/j.berh.2016.09.007. [DOI] [PubMed] [Google Scholar]
- [175].Integrated Nanotechnology & Biomedical Sciences laboratory, Cancer Nanomedicine Repository, (n.d.). http://inbs.med.utoronto.ca/cgi-bin/Repository.cgi (accessed September 25, 2018). [Google Scholar]
- [176].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW, Analysis of nanoparticle delivery to tumours, Nat. Rev. Mater 1 (2016) 16014. doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- [177].Foroozandeh P, Aziz AA, Insight into Cellular Uptake and Intracellular Trafficking of Nanop a rticles, Nanoscale Res. Lett. 13 (2018) 339. doi: 10.1186/s11671-018-2728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Rubinstein I, Weinberg GL, Nanomedicines for chronic non-infectious arthritis: the clinician’s perspective, Nanomedicine Nanotechnology, Biol. Med 8 (2012) S77–S82. doi: 10.1016/J.NANO.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Lin T, Pajarinen J, Nabeshima A, Córdova LA, Loi F, Gibon E, Lu L, Nathan K, Jämsen E, Yao Z, Goodman SB, Orthopaedic wear particle-induced bone loss and exogenous macrophage infiltration is mitigated by local infusion of NF-κB decoy oligodeoxynucleotide., J. Biomed. Mater. Res. A 105 (2017) 3169–3175. doi: 10.1002/jbm.a.36169. [DOI] [PMC free article] [PubMed] [Google Scholar]