Summary
The wheat Lr34res allele, coding for an ATP‐binding cassette transporter, confers durable resistance against multiple fungal pathogens. The Lr34sus allele, differing from Lr34res by two critical nucleotide polymorphisms, is found in susceptible wheat cultivars. Lr34res is functionally transferrable as a transgene into all major cereals, including rice, barley, maize, and sorghum.
Here, we used transcriptomics, physiology, genetics, and in vitro and in vivo transport assays to study the molecular function of Lr34.
We report that Lr34res results in a constitutive induction of transcripts reminiscent of an abscisic acid (ABA)‐regulated response in transgenic rice. Lr34‐expressing rice was altered in biological processes that are controlled by this phytohormone, including dehydration tolerance, transpiration and seedling growth. In planta seedling and in vitro yeast accumulation assays revealed that both LR34res and LR34sus act as ABA transporters. However, whereas the LR34res protein was detected in planta the LR34sus version was not, suggesting a post‐transcriptional regulatory mechanism.
Our results identify ABA as a substrate of the LR34 ABC transporter. We conclude that LR34res‐mediated ABA redistribution has a major effect on the transcriptional response and physiology of Lr34res‐expressing plants and that ABA is a candidate molecule that contributes to Lr34res‐mediated disease resistance.
Keywords: abscisic acid (ABA), cereal crops, durable disease resistance, fungal pathogen, LR34 ABC transporter
Introduction
Achieving durable field resistance against fungal pathogens is a priority of most cereal breeding programs. On average, > 10% of the global crop production is lost to diseases and pests (Chakraborty & Newton, 2011). For example, it has been estimated that 5.47 million tons of wheat (Triticum aestivum), worth US$979 million, are lost annually to the wheat stripe rust disease alone (Beddow et al., 2015). In the wheat gene pool, three genes were identified that confer durable adult plant resistance against multiple fungal diseases (Ellis et al., 2014). These genes were named Lr34 (= Yr18/Sr57/Pm38), Lr46 (= Yr29/Sr58/Pm39) and Lr67 (= Yr46/Sr55/Pm46). Their expression results in partial resistance against all races of the fungal wheat pathogens causing leaf rust (Puccinia triticina), stripe rust (Puccinia striiformis f.sp. tritici), stem rust (Puccinia graminis f.sp. tritici) and powdery mildew (Blumeria graminis f.sp. tritici). Also, these genes cause leaf tip necrosis (LTN), a senescence‐like process that mainly develops in flag leaves of adult wheat plants (Singh, 1992; Krattinger et al., 2009). The partial resistance does not involve a hypersensitive response or callose deposition but is characterized by a reduced fungal growth rate, a phenotype that is also referred to as ‘slow‐rusting’ or ‘slow‐mildewing’ (Rubiales & Niks, 1995; Risk et al., 2012). Hence, the resistance conferred by Lr34, Lr46, and Lr67 is different from most other disease resistance mechanisms that often result in complete but race‐specific resistance linked to hypersensitive response (Dodds & Rathjen, 2010). The Lr34 gene has been used in wheat breeding for more than a century, and no pathogen adaptation has been recorded so far. Because of its durability and broad‐spectrum specificity, Lr34 became one of the most frequently used disease resistance genes in wheat breeding. The Lr34 resistance and LTN are conferred by a single gene encoding a full‐size ATP‐binding cassette (ABC) transporter (Krattinger et al., 2009; Risk et al., 2012). All resistant wheat cultivars carry the same Lr34 allele (Lr34res) that evolved from an ancestral, susceptible allele (Lr34sus) after wheat domestication by two gain‐of‐function mutations (Krattinger et al., 2013). Lr34res is functionally transferrable into all major cereals as a transgene, including barley (Hordeum vulgare), rice (Oryza sativa), maize (Zea mays), and sorghum (Sorghum bicolor) (Risk et al., 2013; Krattinger et al., 2016; Sucher et al., 2016; Schnippenkoetter et al., 2017; Boni et al., 2018). In these cereal species, Lr34res resulted in enhanced resistance against various biotrophic or hemi‐biotrophic fungal pathogens, as well as in the development of LTN. In contrast to wheat, where the Lr34res‐mediated phenotype only develops in adult plants, disease resistance and LTN were already visible at seedling stage in some transgenic lines. In barley, for example, Lr34res conferred partial resistance against barley leaf rust (Puccinia hordei) and barley powdery mildew (B. graminis f.sp. hordei) (Risk et al., 2013; Boni et al., 2018), whereas in rice the expression of Lr34res resulted in resistance against the fungal rice blast pathogen (Magnaporthe oryzae) in seedlings and adult plants (Krattinger et al., 2016). The similarity of the Lr34res‐mediated phenotype across different cereal species suggests a conserved molecular mechanism and implies that the LR34res substrate is common to all cereals.
ABC transporters comprise a large gene family in plants. For example, the full‐size ABCG transporter subfamily, to which Lr34 belongs, has 20 members in rice. ABC transporters manage the active transport of various molecules across biological membranes, including heavy metals, lipids, glucosinolates, and phytohormones (Hwang et al., 2016). One of the major challenges of unraveling the molecular function of this protein family is that a single ABC transporter can have multiple, structurally unrelated substrates (Lu et al., 2015).
Here, we used transcriptomics analyses in rice as a starting point to elucidate the molecular function of the durable disease resistance mediated by Lr34res. We show that both the LR34res and LR34sus protein versions are able to transport ABA in an in vitro yeast assay, whereas only LR34res resulted in changed ABA fluxes in planta. Both protein versions were present at equal amounts in yeast, whereas only LR34res was detected in planta, suggesting a regulatory mechanism on the post‐transcriptional level.
Materials and Methods
Plant materials and growth conditions
Rice plants were grown in a growth cabinet at 28°C : 24°C, day : night, 75% humidity, and 12 h photoperiod with 600 μmol m−2 s−1 light or in a glasshouse. Plants were grown in soil (c. 1 l per pot) in a nutrient solution consisting of 0.1% Sequestrene® Rapid (Syngenta, Dielsdorf, Switzerland) and 0.2% Wuxal fertilizer (Syngenta). Wheat and barley plants were grown in a standard glasshouse.
Pathogen strains
Magnaporthe oryzae isolate FR13 was grown on oatmeal agar (50 g l−1 oat flakes, 2 g l−1 yeast extract, 10 g l−1 rice starch and 15 g l−1 agar) at room temperature in the dark. The fungus was transferred to white light/blue light (Philips TL‐D 15W BLB) for three additional days to enhance sporulation. Rice blast conidia were then harvested from plates by rinsing with sterile distilled water (H2O) and raking with a spatula. Spores were filtered through three layers of gauze and suspended to a final density of (1–2.5) × 105 conidia ml−1. The powdery mildew (B. graminis f.sp. hordei) isolate K1 was propagated on living plants, and inoculations were performed by shaking the spores from a pot containing c. 25 sporulating plants.
RNA sequencing
Two transgenic rice lines that had been previously described were used for the RNA sequencing (RNAseq; Krattinger et al., 2016). Both lines showed partial rice blast resistance at seedling and adult plant stages. Seedling leaves and wild‐type leaves were harvested when the plants were c. 1 month old and the fourth leaf was fully expanded. The youngest, fourth leaf was used for RNA extraction. For adult plants, the upper half of flag leaves was harvested c. 2 wk after anthesis. Flag leaves of lines 5 and 16 showed c. 0.5 cm and c. 2 cm of LTN, respectively. Three biological replicates were used per line and time point. Total RNA was extracted using the SV Total RNA Isolation System (Promega). Library preparation and sequencing were done at GATC Biotech, Konstanz, Germany. Between 25.8 million and 50 million 50 bp single‐end reads were produced per sample on a HiSeq 2500 sequencing system (Illumina). Reads were aligned to the O. sativa ‘Nipponbare’ reference genome (MSU7, rice.plantbiology.msu.edu) with tophat v.1.2 (Trapnell et al., 2010) allowing up to 10 alignments per read (‐g 10) and the options ‐a 8 ‐m 1 ‐i 50 ‐I 2000 ‐F 0.2. Count tables were generated with rcount (Schmid & Grossniklaus, 2015) as described previously (Schmid et al., 2012) but using the read length as allocation distance for calculating the weights of the reads with multiple alignments. Variation in gene expression was analyzed with a general linear model in R with the package edger (Robinson et al., 2010) according to a crossed factorial design with three explanatory factors GENOTYPE (transgenic or sister line), TISSUE (flag leaf or seedling leaf), and LINE (plant line number 5 or 16). Genes differentially expressed between specific conditions were identified with pairwise comparisons using tagwise dispersion estimates and Benjamini–Hochberg multiple testing corrections. Genes with an adjusted P‐value (false discovery rate) < 0.05 and a minimal log2(fold change) = 2 were considered to be differentially expressed. In total, we detected sequence reads corresponding to 18 990 rice genes. To define an ‘Lr34res‐responsive core gene set’ we considered genes that were differentially expressed more than four‐fold (log2 > 2) in at least three of the four conditions (lines 5 and 16; seedling and adult plants). In addition, we removed genes with low read numbers (< 50 reads) and genes annotated as transposons.
The meta‐analysis in Genevestigator (Hruz et al., 2008) was performed using the condition search tool ‘perturbations’. An Lr34res‐responsive core gene was classified as pathogen inducible and/or drought inducible if it was responsive to the respective stress in at least two samples with a fold change of log2 > 2 according to the ‘perturbations’ tool. Validation of RNAseq data through semi‐quantitative reverse transcription PCR (RT‐PCR) or quantitative RT‐PCR (RT‐qPCR) was done on leaves of the Lr34res‐expressing rice line 19, a line that was not used for the RNAseq experiment. RNA was extracted from 42‐d‐old plants of lines 19 and 19sib using the SV Total RNA Isolation System (Promega). Complementary DNA (cDNA) was synthesized from 1 μg of RNA using the i‐Script™ cDNA Synthesis kit (Bio‐Rad). RT‐qPCR was performed on a CFX96 Touch™ Real‐Time PCR Detection system (Bio‐Rad) with the Kapa SYBR® FAST qPCR Master Mix (Kapa Biosystems). One reaction included 5 μl KAPA Master Mix, 4 μl of 1 : 20 diluted cDNA template and 500 nM primers. The sequences of primers used in this study are listed in Supporting Information Table S1. UBC1 or the sucrose synthase‐1 (OsRSs1) genes were used as reference genes (Wang et al., 1992; Krattinger et al., 2016).
Dehydration stress and leaf transpiration experiments
For adult plants of lines 8 and 8sib, one plant per pot was grown in nutrient solution for 33 d. Then, pots were removed from nutrient solution and leaf rolling was used as a visual sign of dehydration stress (Bunnag & Pongthai, 2013). Plants of line 8 did not show LTN when the dehydration experiments were conducted. Transpiration rate and stomatal conductance on leaves of whole plants were measured using an LI‐6400 portable photosynthesis system with a 6400‐11 narrow leaf chamber (Li‐Cor, Lincoln, NE, USA). Measurements were done on 4‐wk‐old plants using a flow rate of 200 μmol s−1, a CO2 concentration of 380 μmol mol−1, and a light intensity of 2000 μmol m−2 s−1. Transpiration measurements on detached leaves were done as described previously (Mittelheuser & Van Steveninck, 1969). Briefly, flag leaves of booting tillers were detached and the FW of each leaf was determined. Lr34res‐expressing rice leaves did not show LTN at the time of harvesting. Leaves were incubated with their cut ends in 5 ml Eppendorf tubes containing either 5 ml H2O, 5 ml of a 10 μM ABA ((±)‐ABA; Sigma Aldrich) or 10 μM jasmonic acid (JA) solution ((±)‐JA; Sigma Aldrich). Methanol (MeOH) was used to prepare the hormone stock solutions, and equal amounts of 100% MeOH were added to the H2O control. Tubes were weighed after 0 and 24 h and transpiration was calculated as H2O uptake per gram leaf FW. Values were corrected for H2O loss by evaporation.
ABA germination experiments
Early seedling establishment was determined as described previously (Zhao et al., 2015). In brief, rice seeds were dehusked and sterilized with 70% ethanol and 1.25% sodium hypochlorite (NaOCl). Rice caryopses were then washed five times with double‐distilled H2O and plated on Petri dishes containing ½ Murashige and Skoog medium with or without 5 μM ABA. The ABA stock solution was prepared in MeOH, and equivalent amounts of 100% MeOH were added to the control plates. Plates were then placed upright at room temperature in the dark, and seedling growth was evaluated after 7 d. The optimal ABA concentration of 5 μM was determined in an initial experiment with an ABA concentration gradient.
Tritiated‐ABA accumulation assay in rice seedlings
Rice seeds were dehusked and sterilized with 70% ethanol and 1% NaOCl. Rice caryopses were then cold‐treated in sterile H2O in the dark at 4°C for 24 h and subsequently shifted to continuous light at 30°C for 40–72 h until all germinated seedlings reached the same growth stage. For the accumulation experiment, germinated seedlings were incubated in 300 μl of a bathing solution (10 mM potassium chloride, 10 mM Mes, pH 6.05) containing 1.5 nM tritiated ABA (3H‐ABA, ART 1186; American Radiolabeled Chemicals, St Louis, MO, USA) or 1 : 5000 (v/v) diluted tritiated JA (3H‐JA, ART 2152; American Radiolabeled Chemicals) for 30 min at 30°C under white light. After incubation, seedlings were washed three times with 500 μl ice‐cold bathing solution and radioactivity was determined with a liquid scintillation counter.
Heterologous expression of Lr34 in yeast and ABA loading assays
Full‐length cDNA of Lr34res was amplified from the binary vector p6U:cLr34res (Risk et al., 2013) and a hemagglutinin (HA)‐tag was introduced at the 3′ end of the cDNA using primer C‐HALr34 (5′‐GGG CGG CCG CTT AAG CGT AAT CTG GAA CAT CGT ATG GGT ACC TCT TCT GGA AAT TAA G‐3′). Amplification was performed with Herculase II Fusion DNA polymerase (Agilent Technologies, Santa Clara, CA, USA). The PCR fragment was cloned into the NotI site of the Escherichia coli yeast shuttle vector pNEV‐N (Sauer & Stolz, 1994), resulting in pNEV:cLr34res‐HA. For the generation of the Lr34sus‐HA construct, pNEV:cLr34res‐HA and pGY:Lr34sus were subsequently digested with NruI and BstEII to exchange the polymorphic sites between Lr34res and Lr34sus. Resulting fragments were re‐ligated using the TaKaRa Ligation Kit LONG (TaKaRa Bio Co., Otsu, Japan), The constructs were then transformed into yeast strains W303 (Ralser et al., 2012) and YMM12 (Alejandro et al., 2012) using electroporation and selected on uracil‐minus minimal media. 3H‐ABA loading tests were performed as described previously with minor modifications (Kang et al., 2015). In brief, yeast cells were precultured in minimum salt–glucose medium in the absence of uracil (SG‐URA) at pH 5.5 overnight and resuspended in fresh SG‐URA media at OD600 = 0.2. Cultured cells were harvested by centrifugation at the mid‐log phase (up to OD600 = 1.0) after 4–8 h incubation. Pellets were washed twice using cold SG‐URA medium (pH 6.3) and then resuspended in same medium at an OD600 of 10. 3H‐ABA (4.5 nM, 7.4 kBq, 1.63 TBq mmol−1) and 45 nM unlabeled ABA were added to the cell suspension and gently mixed. For measurements, 100 μl of cell suspension were filtered through nitrocellulose membranes and the cells remaining on the filter were washed twice with 2 ml of ice‐cold media at each time point. The radioactivity on the filter was determined using a liquid scintillation counter. For ABA accumulation assays in the presence of ABC transporter inhibitors, yeast cells were preincubated with the respective inhibitor for 20 min. Then, they were incubated for 10 min in the same medium with radiolabeled ABA as already described. For ABA accumulation assays in the presence of various unlabeled chemicals, yeast cells were incubated with 3 μM of the respective chemical for 10 min in the same medium with 50 nM radiolabeled and unlabeled ABA mixture as already described.
Cloning and transformation of genomic HA‐Lr34res and HA‐Lr34sus alleles into barley
The N‐terminal HA‐tag was added to the genomic Lr34res and Lr34sus constructs by homologous recombination in yeast. To do so, the binary plasmid p6U (DNA Cloning Service, Hamburg, Germany) was digested with EcoRI (New England Biolabs, Ipswich, MA, USA) and the Leu2 cassette was amplified on the template plasmid pRS305 using primers dst236 (5′‐CTC CAC GAA AAT ATC CGA ACG CAG CAA GAT TGG GTC CTT TTC ATC ACG TGC‐3′) and dst237 (5′‐TGC CCA GGC AAG ACC GAG ATG CAC CGC GAT GCG GCC GCC ACC GCG GT‐3′). This created a yeast compatible vector, called p6Uyeast. The p6Uyeast vector was linearized using SfiI, and different PCR products (Table S1) based on p6U::Lr34res (Risk et al., 2013) were transformed into yeast strain RGSY for homologous recombination. The p6Uyeast plasmids with the different alleles were used for Agrobacterium‐mediated stable transformation of barley cv Golden Promise as described previously (Hensel et al., 2008, 2009).
Protein extraction, sodium dodecyl sulfate polyacrylamide gel electrophoresis, and Western blot
Total protein extracts were obtained by grinding barley leaves in liquid nitrogen (N) using a mortar and pestle. Then, 3 ml of extraction buffer (100 mM sodium chloride, 50 mM Tris hydrochloride pH 8, 25 mM sucrose, 5 mM EDTA, 10% glycerol, 1% Triton X‐100, 5 mM dithiothreitol and one tablet of cOmplete™ protease inhibitor cocktail (Sigma‐Aldrich) per 10 ml extraction buffer) was added per 1 g of leaf material. Soluble proteins were separated from debris by two centrifugation steps at 15 000 g for 20 min and 10 min. Enrichment of the membrane fraction was achieved by ultracentrifugation (100 000 g) using an Optima Xpn 100 ultracentrifuge (Beckman Coulter, Brea, CA, USA). Protein concentration measurement was done by Bradford assay using Protein Assay Dye Reagent Concentrate (Bio‐Rad) and a Spectra Max 190 spectrometer (Bucher Biotec, Basel, Switzerland). Equal protein amounts (24 μg) were separated on a 6.5% acrylamide/bis‐acrylamide gel (Bio‐Rad) and blotted to a nitrocellulose membrane (Amersham™ Protran™ 0.2 μm NC; GE Healthcare Life Sciences, Marlborough, MA, USA) using the Mini‐Protean II system (Bio‐Rad). Blots were incubated with 1 : 1000 rat monoclonal antibody (Anti‐HA‐Peroxidase High Affinity; Roche). Signals were detected using the WesternBright™ Quantum kit (Advansta, San Jose, CA, USA) and quantified with a Fusion FX6‐XT‐820.EPI camera and the evolutioncap software (Vilber Lourmat/Witec AG, Sursee, Switzerland).
Analysis of ABA‐deficient crosses
The Lr34res‐expressing line 19 in the genetic background of ‘Nipponbare’ (Krattinger et al., 2016) was crossed with the OsABA8ox1 overexpressing line 27‐3 and the corresponding wild‐type ‘Toyohikari’ (Mega et al., 2015).
The M. oryzae leaf sheath assay was performed as described previously (Saitoh et al., 2012; Krattinger et al., 2016). In brief, c. 3 cm long leaf sheath segments of 5‐wk‐old plants were filled with a conidial spore suspension (1 × 105 spores ml−1) of the isolate FR13. The leaf sheaths were incubated in the dark for 28 h at room temperature in humid Petri dishes. The plant tissue was fixed and stained by boiling for 1 min in lactophenol–trypan blue (30 ml ethanol, 10 ml glycerol, 10 ml lactic acid, 10 mg trypan blue, and 10 ml distilled H2O). Subsequently, the samples were decolored with chloral hydrate (2.5 g of chloral hydrate in 1 ml double‐distilled H2O) for at least 30 min. The appressorial sites were analyzed with a Zeiss Axio Imager Z1 microscope (Carl Zeiss AG, Feldbach, Switzerland). Each invasive hypha was assigned to one of the four levels of invasive growth according to Saitoh et al. (2012): level 1, invasive hypha is shorter than 10 μm without branches; level 2, 10–20 μm long invasive hypha with zero to two branches; level 3, invasive hypha is longer than 20 μm and/or has more than two branches per cell; level 4, more than one cell is infected by the invasive hypha.
ABA concentration measurement
ABA concentrations of whole leaves were measured using the Phytodetek® ABA test kit (Agdia‐Biofords, Grigny, France) according to the manufacturer's instruction. Rice seedlings were grown for 3 wk in growth cabinets. The latest fully developed leaves were harvested and immediately frozen in liquid N. Leaves of three plants were pooled for one biological replicate. Leaves were cut into c. 2 cm segments and then lyophilized for 24 h. The tissue was ground in 2 ml Eppendorf tubes containing two 4 mm glass beads using a Retsch® M300 TissueLyser (Retsch GmbH, Haan, Germany) at 30 Hz for several minutes. ABA was extracted by adding 500 μl cold 80% MeOH to each sample and samples were mixed on an overhead shaker in the dark overnight. The extraction was repeated with 1 ml cold 80% MeOH. After the second extraction, samples were washed twice with 500 μl of cold 80% MeOH. Supernatants of the four extractions were pooled and reduced to c. 500 μl in a SpeedVac (Thermo Fisher Scientific, Waltham, MA, USA). Tris‐buffered saline (TBS, 1×) was then added up to 1 ml and samples were 1 : 10 diluted in 1× TBS for analysis.
Results
Lr34res induces transcripts reminiscent of an ABA‐regulated stress response in rice
Previous studies in wheat and barley revealed that Lr34res induces constitutive defense responses even in the absence of pathogen infection (Hulbert et al., 2007; Chauhan et al., 2015). RNAseq in transgenic rice that expressed Lr34res revealed an ‘Lr34res‐responsive core gene set’ consisting of 146 up‐ and 13 downregulated transcripts that were repeatedly detected in two independent transgenic events at seedling and adult plant stage compared with wild‐type plants (Table S2). In both transgenic events, rice blast resistance and LTN were present in seedlings and adult plants (Krattinger et al., 2016). Uninfected leaves were used for the transcriptomics analysis based on the previous observations that Lr34res‐based molecular mechanisms are constitutively activated. The rice plants used for the RNAseq experiment were grown under optimal conditions, and there were no signs of pathogen infection or abiotic stresses. Despite these stress‐free growth conditions, meta‐analyses revealed that most of the 159 differentially expressed core genes in rice were responsive to stresses (Hruz et al., 2008; Shaik & Ramakrishna, 2014). More precisely, many of these genes are inducible by both biotic and abiotic stresses and are therefore referred to as ‘multiple stress responsive genes’ (Figs 1a, S1). Some of the Lr34res‐responsive core genes have been reported to confer broad‐spectrum disease resistance or abiotic stress tolerance (Table S3). An example is BBTI4, a gene coding for a Bowman–Birk‐type bran trypsin inhibitor that conferred partial and broad‐spectrum resistance against the bacterial blight disease (Xanthomonas oryzae pv oryzae) when it was overexpressed in rice (Pang et al., 2013). BBTI4 transcript levels were c. 15‐fold induced in the presence of Lr34res (Fig. S1c). The chitinase gene RC24 (OsCHIT8) that was c. 90‐fold induced in Lr34res‐expressing rice plants (Fig. S1c) has been reported to confer field resistance to stripe rust in transgenic wheat (Huang et al., 2013). Other genes that were highly upregulated by Lr34res are known to confer tolerance to drought and salinity, such as OsLEA3‐1, OsRNS4, OsMIOX, or OsRCI2‐5 (Table S3; Xiao et al., 2007; Duan et al., 2012; Li et al., 2014; Zheng et al., 2014). In wheat, it has been previously observed that the Lr34res‐responsive genes were associated with ABA inducibility (Hulbert et al., 2007). We therefore compared the rice ‘Lr34res‐responsive core gene set’ with two transcriptomics studies performed after hormone treatments in rice and the model grass Brachypodium distachyon (Garg et al., 2012; Kakei et al., 2015). Similar to wheat, the core gene set of rice was more similar to an ABA‐regulated response than to salicylic acid (SA)‐ or JA‐regulated responses (Fig. 1b,c; Tables S4, S5). In summary, transcriptomics studies in wheat, barley, and rice point to a common mechanism of Lr34res that involves the constitutive induction of transcripts reminiscent of an ABA‐regulated stress response.
Figure 1.
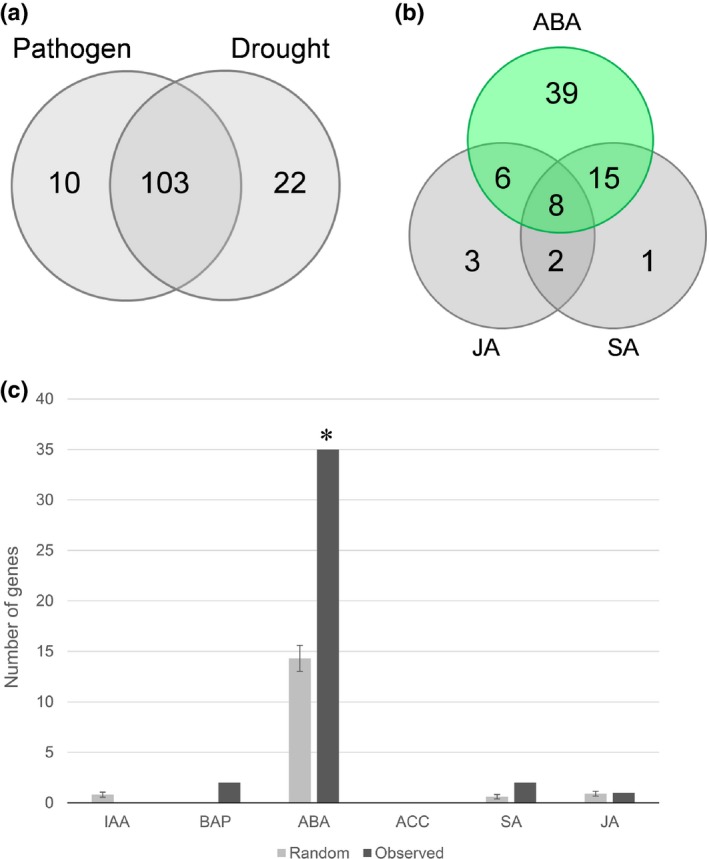
Lr34res constitutively induces transcripts reminiscent of an abscisic acid (ABA)‐regulated multiple stress response in rice. (a) Number of genes of the ‘Lr34res‐responsive core gene set’ that were responsive to pathogens and/or drought according to the gene expression database Genevestigator (Hruz et al., 2008). (b) Number of genes of the ‘Lr34res‐responsive core gene set’ that were responsive to ABA, salicylic acid (SA), and jasmonic acid (JA; Garg et al., 2012). (c) Comparison of gene numbers in the ‘Lr34res‐responsive core gene set’ (observed) to a randomly generated gene list. For the random data set, 159 rice genes (same number as ‘Lr34res‐responsive core gene set’) were randomly selected from the 18 990 rice genes detected in our transcriptomics study and their hormone responsiveness determined according to Garg et al. (2012). * denotes a statistical significance (P < 0.0001) based on a chi‐square test between the observed and the mean of 10 random data sets. Genes that were responsive to multiple hormones according to Garg et al. (2012) were not considered. Error bars represent ± SE. ACC, 1‐aminocyclopropane‐1‐carboxylic acid; BAP, benzylaminopurine; IAA, indole‐3‐acetic acid.
Lr34res‐expressing rice plants show alterations in several ABA‐regulated traits
We performed several physiological experiments to test for a correlation between the ‘Lr34res‐responsive core gene set’ and ABA‐mediated phenotypes. Krattinger et al. (2016) identified two basic types of Lr34res transgenic rice lines depending on their Lr34res expression patterns. One line (line 8) had low Lr34res expression levels and mainly developed LTN at adult plant stage, whereas the strong Lr34res‐expressing lines 5, 11, 16, and 19 developed LTN already at seedling stage. Rice blast resistance was observed in all lines in seedlings and adult plants. The strong Lr34res‐expressing lines are phenotypically very similar. Because of their reduced seed production, which is associated with reduced tiller number and sterility caused by the strong expression of Lr34res, these lines were used interchangeably for the various physiological experiments. For line 8, no negative impact on plant vigor was observed (Krattinger et al., 2016).
ABA regulates many biologically important processes, including stomatal closure and drought tolerance (Kuromori et al., 2014). The strong Lr34res‐expressing line 19 and the weak Lr34res‐expressing line 8 both showed increased dehydration tolerance in glasshouse conditions, which was linked to reduced transpiration rates and reduced stomatal conductance (Fig. 2a,b). The decreased transpiration rate of Lr34res‐expressing rice plants was phenocopied in detached wild‐type leaves by the addition of exogenous ABA (Fig. 2c). ABA is also a repressor of seed germination and early seedling growth (Zhao et al., 2015). Growth of Lr34res lines 16 and 19 on medium containing 5 μM ABA resulted in a c. 50% reduction of root length compared with sib lines (Fig. 2d–f), indicating hypersensitivity to exogenous ABA. In addition, LTN is associated with the upregulation of senescence‐associated genes (Krattinger et al., 2009; Risk et al., 2013), which is in line with the fact that ABA is a promoter of leaf senescence (Liang et al., 2014).
Figure 2.
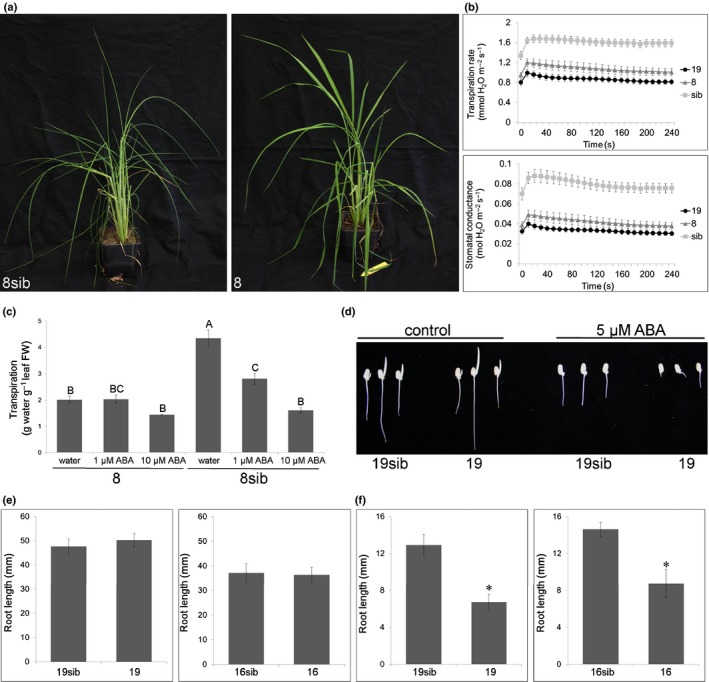
Lr34res‐expressing rice plants show alterations in abscisic acid (ABA)‐regulated processes. (a) Representative examples of line 8sib and the low‐expressing Lr34res line 8 after 4 d of dehydration stress. The leaf rolling in line 8sib is a sign of dehydration stress. (b) Transpiration rate and stomatal conductance measured on leaves of 4‐wk‐old plants. n = 5–15 biological replicates, mean ± SE. (c) Transpiration rate in detached flag leaves of Lr34res line 8 and 8sib incubated in water, 1 μM ABA, or 10 μM ABA. Letters indicate treatments with similar transpiration rates (P > 0.05, Tukey's honest significance test, n = 5 biological replicates). (d) Representative example of early seedling establishment of rice lines 19 and 19sib on ½ Murashige and Skoog (½MS) medium with and without ABA. The three examples per line and treatment were selected to reflect the entire range of root lengths observed. (e, f) Root length of Lr34res‐containing seedlings compared with their respective sibs on ½MS medium with no ABA (e) and 5 μM ABA (f) after 7 d. N = 3, n = 41–64 biological replicates, mean ± SE, *, P < 0.01 compared with sib (Student's t‐test).
Lr34res acts as an ABA transporter
Our analyses revealed that ABA‐regulated genes and physiological processes are altered in Lr34res‐expressing rice plants. We therefore tested for an effect of LR34res on ABA fluxes in rice seedlings incubated in radiolabeled 3H‐ABA. Both Lr34res‐expressing lines 11 and 19 accumulated significantly more ABA than their respective sibs after 30 min. In contrast, a homozygous rice line expressing Lr34sus did not show differences in ABA accumulation (Fig. 3a,b). The Lr34sus line 131 was chosen from several Lr34sus‐expressing events because it showed Lr34 expression levels similar to the strong Lr34res‐expressing line 19 both in coleoptiles of young seedlings and in leaves of 4‐wk‐old plants (Fig. S2a–d). However, line 131 was susceptible to rice blast, did not develop LTN (Fig. 3a) and there was no induction of Lr34res‐responsive genes (Fig. S2b). Increased ABA accumulation was thus specific to Lr34res‐expressing rice lines, which correlates with the induction of ABA‐regulated genes, the physiological alterations, and disease resistance. Lr34res expression levels in coleoptiles of seedlings were c. 70‐fold lower than in leaves of 6‐wk‐old plants. Hence, the changes in ABA fluxes observed in Lr34res‐expressing rice seedlings occur at a very early growth stage and at very low Lr34res expression levels. As controls, nongerminated caryopses of lines 11 and 19 were incubated in 3H‐ABA and young seedlings in 3H‐JA. In both cases, we did not observe differences between Lr34res‐expressing lines and sibs (Fig. S3a,b).
Figure 3.
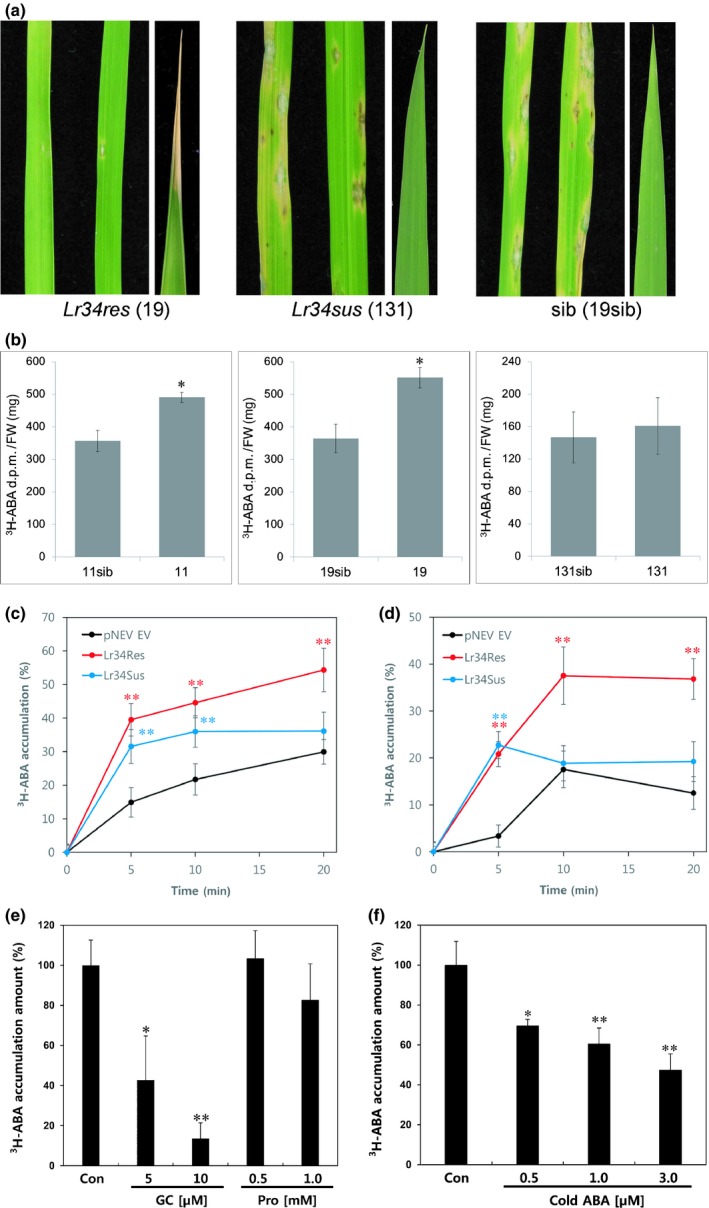
LR34 transports abscisic acid (ABA). (a) Rice blast resistance and leaf tip necrosis in 4‐wk‐old plants of Lr34res‐expressing line 19, Lr34sus‐expressing line 131 and 19sib. (b) Tritiated abscisic acid (3H‐ABA) accumulation (disintegrations per minute, d.p.m.) in rice seedlings of lines 11, 19, and 131 compared with respective sib lines. N = 3, n = 13–23, mean ± SEM. *, P < 0.01 compared with sib (Student's t‐test). (c) Relative 3H‐ABA accumulation in yeast strain W303 expressing Lr34res or Lr34sus. Mean ± SE, **, P < 0.01 compared with empty vector (EV) control. The graphs show averaged results of three independent experiments. (d) Relative 3H‐ABA accumulation in yeast strain YMM12 expressing Lr34res or Lr34sus. Mean ± SE, **, P < 0.01 compared with EV control. The graphs show averaged results of three independent experiments. (e) Relative 3H‐ABA accumulation in yeast strain YMM12 after preincubation with the ABC transporter inhibitor glibenclamide (GC) and the ABCC‐specific inhibitor probenecid (Pro). The control (Con) without inhibitor was set to 100%, mean ± SE. *, P < 0.05, **, P < 0.01. (f) Relative 3H‐ABA accumulation (50 nM) in yeast strain YMM12 in the absence (Con) or presence of cold ABA. The control (Con) without inhibitor was set to 100%, mean ± SEM. *, P < 0.05, **, P < 0.01.
To obtain additional evidence for LR34‐mediated ABA fluxes, we expressed the Lr34res and Lr34sus cDNAs in yeast strains W303 and YMM12. The latter carries loss‐of‐function mutations in eight ABC transporter genes. Beside the W303 wild‐type strain, YMM12 was chosen to reduce a possible effect of endogenous ABC transporters on the uptake experiments. In both yeast strains, we consistently found increased ABA uptake within 5–20 min when Lr34res was expressed compared with the empty vector control (Fig. 3c,d). In YMM12, a saturation was observed after 10 min, whereas relative ABA accumulation continued to increase in the W303 yeast strain up to 20 min. Surprisingly, we also observed a significantly higher ABA accumulation for Lr34sus at certain time points. The ABC transporter inhibitor glibenclamide but not the ABCC subfamily‐specific inhibitor probenecid blocked ABA accumulation by the ABCG transporter LR34 (Fig. 3e). Addition of increasing concentrations of cold ABA competed with the labeled ABA and resulted in a decrease of measurable ABA uptake (Fig. 3f). These experiments demonstrate that both the LR34res and LR34sus protein versions result in increased ABA uptake in yeast, whereas LR34res, but not LR34sus, changed ABA fluxes in plants. Similar to rice, the addition of SA and methyl jasmonate did not compete for ABA uptake (Fig. S3c). On the other hand, addition of the diterpene alcohol sclareol resulted in a c. 40% reduction of 3H‐ABA uptake (Fig. S3d), which might indicate competition for substrate binding. Sclareol was tested because it had been identified as a substrate of several ABCG transporters (Jasinski et al., 2001; van den Brule et al., 2002; Hwang et al., 2016).
To determine the difference between LR34res and LR34sus in planta, we used barley plants that were transformed with Lr34 constructs containing an N‐terminal HA tag (HA‐Lr34). Lr34 expression was driven by the native wheat promoter (Risk et al., 2013). T1 plants of the two independent Lr34res‐expressing events HA‐Lr34res_1 and HA‐Lr34res_12 were resistant to barley powdery mildew and developed LTN, indicating that the HA tag does not interfere with the function of the LR34res protein (Fig. 4a). Similar to the Lr34sus‐expressing rice line 131, two HA‐Lr34sus events (HA‐Lr34sus_1 and HA‐Lr34sus_6) were susceptible to barley powdery mildew and did not develop LTN, despite having similar or even higher Lr34 expression levels than the two HA‐Lr34res events (Fig. 4a,b). Interestingly, the HA‐LR34res protein, but not the HA‐LR34sus protein, was detectable by Western blot in extracts from barley leaves (Fig. 4c). On the other hand, both protein versions were present in yeast at similar amounts (Fig. 4d). These data indicate that both LR34 protein versions transport ABA and that the difference between LR34res and LR34sus in planta is regulated on the protein level through a post‐transcriptional or post‐translational mechanism. Whether the intermediate ABA uptake of LR34sus in yeast is caused by a reduced transport activity or slightly lower protein amounts could not be determined.
Figure 4.
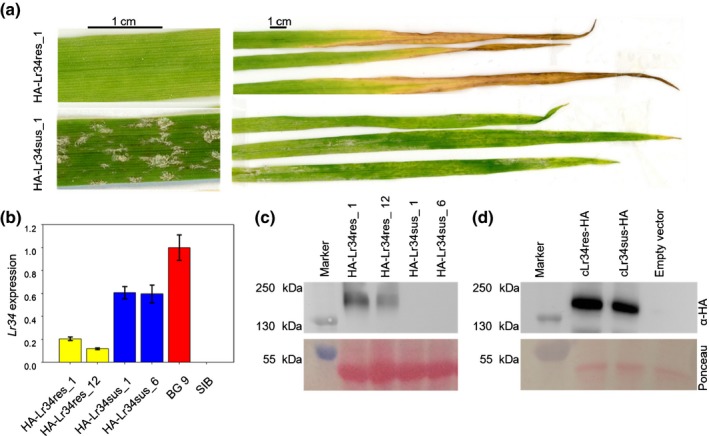
LR34res and LR34sus show differences in protein amounts in planta. (a) Barley leaves of the hemagglutinin (HA)‐Lr34res_1 and HA‐Lr34sus_1 events inoculated with barley powdery mildew (Blumeria graminis f.sp. hordei) 7 d post inoculation. Leaf tip necrosis developed in leaves expressing HA‐Lr34res but not HA‐Lr34sus. (b) Relative expression levels of transgenic lines expressing HA‐LR34res or HA‐Lr34sus. Expression values were normalized to GAPDH and set in relation to the Lr34res‐expressing control line BG9 (Risk et al., 2013). Leaves of 14‐d‐old plants were used for the quantitative reverse transcription PCR experiments. Eight T1 plants that were PCR positive for the respective construct were pooled. n = 3 technical replicates, mean ± SEM. (c, d) Western blot of extracts generated from barley leaves (c) and yeast cells (d). The PageRuler™ Plus Prestained Protein Ladder 10–250 kDa (Thermo Fisher Scientific) was used. The predicted molecular weight of LR34res is c. 160 kDa.
Expressing Lr34res in an ABA‐deficient background enhances the Lr34res phenotype
To test for a genetic link between ABA and the Lr34res‐mediated phenotype, Lr34res‐expressing rice plants of line 19 (in the genetic background of ‘Nipponbare’) were crossed to plants that overexpress the ABA catabolizing enzyme ABA 8′‐hydroxylase (OsABA8ox1). Plants of the OsABA8ox1 overexpressing line E0082::OsABA8ox1 27‐3 (27‐3) had approximately four‐fold lower ABA levels and showed an ABA‐deficient phenotype compared with the respective wild‐type (‘Toyohikari’) plants (Mega et al., 2015). The phenotypic response of the F1 plants was unexpected. Although we expected a weakening of the Lr34res‐mediated phenotype in an ABA‐deficient background, F1 plants that expressed Lr34res and overexpressed OsABA8ox1 (‘19 × 27‐3’) showed an enhanced Lr34res phenotype compared with F1 plants that resulted from a cross of line 19 with ‘Toyohikari’ (‘19 × Toyohikari’). This was most noticeable by longer LTN and an even stronger induction of Lr34res‐responsive genes (Fig. 5a,b). Also, ‘19 × 27‐3’ F1 plants were more resistant to M. oryzae than ‘19 × Toyohikari’ plants were (Fig. 5c). It has been found, however, that reduced ABA levels per se can increase resistance against M. oryzae in rice (Ton et al., 2009; De Vleesschauwer et al., 2013). F1 plants resulting from the cross ‘19sib × 27‐3’ indeed showed a certain increase in disease resistance compared with ‘19sib × Toyohikari’. The level of disease resistance in the ‘19 × 27‐3’ plants, however, was significantly higher than in the ‘19sib × 27‐3’ plants.
Figure 5.
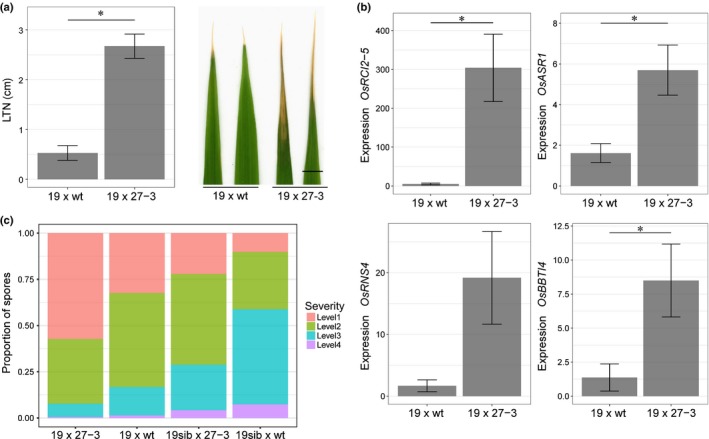
Expression of Lr34res in an abscisic acid (ABA)‐deficient rice background enhances the Lr34res‐mediated phenotype. (a) Leaf tip necrosis (LTN) on the penultimate leaf of 4‐wk‐old plants. Bar, 0.5 cm. n = 15–18 biological replicates; * indicates a significant difference with P < 0.001 (Student's t‐test). (b) Expression levels of the four Lr34res‐responsive core genes BBTI4, OsRCI2‐5, OsASR1, and OsRNS4. Expression levels were normalized to the ubiquitin‐conjugating enzyme UBC1, n = 4 biological replicates, mean ± SE; * indicates a significant difference at P < 0.05 (Student's t‐test). (c) Classification of biotrophic, invasive Magnaporthe oryzae growth on rice leaf sheaths 28 h after inoculation (Saitoh et al., 2012; Krattinger et al., 2016). Levels 1–4 correspond to different lengths of invasive hyphae, with level 1 being the shortest. The y‐axis shows the proportion of appressorial penetration sites that belong to the different infection levels. Six biological replicates were averaged for each cross and at least 45 penetration sites were evaluated for each replicate. A statistical analysis of the disease resistance is presented in Supporting Information Fig. S5 and Table S6.
Discussion
Here, we show that the phytohormone ABA is a substrate of LR34. Our in planta and in vitro uptake experiments are in agreement with the induction of ABA‐regulated genes and the ABA‐mediated physiological changes caused by the expression of Lr34res in rice. The results of the OsABA8ox1 overexpressing lines further provide a genetic link between Lr34res‐mediated phenotypes and ABA, as LTN and Lr34res‐induced transcripts were strongly induced in the ABA‐deficient background. The exact molecular basis of this result needs to be determined in the future. It is likely that cell‐type or tissue‐specific differences in local ABA concentrations mediated by LR34res result in the more pronounced LTN and stronger induction of Lr34res‐responsive genes even when overall ABA concentrations are reduced. The increased disease resistance in the ‘19 × 27‐3’ plants could be the result of a direct or indirect action of ABA redistribution. In a direct mode of resistance induction, LR34res‐mediated ABA redistribution would cause increased disease resistance, possibly through the upregulation of the Lr34res induced stress response. Some of the genes induced by Lr34res, including BBTI4 and RC24, have already been shown to confer broad‐spectrum disease resistance (Huang et al., 2013; Pang et al., 2013). It is thus possible that the sum of the Lr34res‐induced genes creates a more hostile environment for pathogens, resulting in the characteristic, quantitative reduction of pathogen growth rates. Interestingly, an RNAseq study focusing on pathogens revealed no transcriptional response of P. triticina and B. graminis f.sp. hordei to the reduction of pathogen growth caused by the presence of Lr34res in wheat and barley, respectively (Sucher et al., 2017). It is also possible that reduced local ABA concentrations in certain cells contribute to the Lr34res‐mediated disease resistance. On the other hand, an indirect mode of resistance activation could be the result of an additive effect of the reduced ABA levels and the transport of another substrate by LR34res. It is characteristic for ABC transporters that they can have multiple, structurally unrelated substrates (Jasinski et al., 2003). For example, the diterpene sclareol reduced the uptake of labeled ABA in Lr34res‐expressing yeast, indicating that sclareol might also be an LR34res substrate. Sclareol has been identified as an antifungal substance that serves as a putative substrate for ABC transporters (van den Brule et al., 2002). However, sclareol is not likely to be the physiological substrate of LR34, because it is not synthesized in monocot plants. Although the extent and time point of LTN development depends on the environment, a genetic uncoupling of Lr34res‐mediated LTN and disease resistance has never been observed. All the Lr34res loss‐of‐function mutants identified so far lost both LTN and disease resistance (Krattinger et al., 2009; Spielmeyer et al., 2013). We thus consider it likely that the Lr34res‐mediated disease resistance and LTN are caused by the same pathway involving an identical substrate. We therefore conclude that ABA at least partially explains the Lr34res‐mediated disease resistance.
The Lr34res allele spontaneously evolved after wheat domestication, most likely in a field of ancient farmers (Kolmer et al., 2008; Krattinger et al., 2013), and so far has not been found in wild progenitors of wheat. It is possible that the more ancient Lr34sus allele plays a role in ABA‐regulated stress responses and that tight regulation through post‐transcriptional or post‐translational regulation ensures its inactivation in stress‐free conditions. The spontaneous sequence changes that led to Lr34res might have interrupted this regulatory mechanism, which resulted in a constitutive defense response even in the absence of pathogen infection or abiotic stresses. Transgenic rice and barley plants with high Lr34res expression levels had a severe reduction in plant vigor and yield, most likely caused by the increased energy demand of the constitutive defense response. In wheat, it has been shown that the endogenous Lr34res allele can result in a slight yield penalty in high‐input farming systems with full fungicide control, whereas this allele had a beneficial effect in low‐input farming systems (Singh & HuertaEspino, 1997; Johnston et al., 2017). The increased energy demand could also explain why Lr34res was only maintained in domesticated wheat grown in agricultural ecosystems.
Several ABCG transporters in the model plant Arabidopsis thaliana can act as ABA transporters (Kang et al., 2010, 2015; Kuromori et al., 2010). For example, ABCG40 is an ABA importer expressed mainly in guard cells (Kang et al., 2010), whereas ABCG25 acts as an ABA exporter that is localized to the vascular tissue, the site of ABA production (Kuromori et al., 2010). These Arabidopsis ABCG transporters were identified in mutation screens for alterations in ABA‐regulated phenotypes. LR34, on the other hand, is the first example of an agronomically relevant transporter protein that is involved in ABA redistribution.
Role of ABA in disease resistance
ABA has a multifaceted role in disease resistance and it can act as a repressor or enhancer of disease resistance depending on the pathogen, the developmental stage of the plant, and the type of affected tissue (Ton et al., 2009; De Vleesschauwer et al., 2013). For example, exogenous application of ABA increased resistance against the fungal brown spot disease of rice (De Vleesschauwer et al., 2010), whereas spraying of ABA on rice and barley leaves resulted in increased susceptibility against the blast pathogen M. oryzae (Ulferts et al., 2015). It has also been shown that M. oryzae produces and secretes ABA during the infection process to induce susceptibility, which resulted in increased ABA contents in rice leaves 24 h after infection (Cao et al., 2016). The ABA‐induced susceptibility to rice blast is thus linked to increased leaf ABA concentrations. In contrast, Lr34res did not result in an increase of whole‐leaf ABA contents (Fig. S4). The Lr34res‐induced disease resistance is thus likely to be caused by ABA‐redistribution in the leaf, which might result in leaf areas/tissues with increased ABA concentrations, although ABA contents might be reduced in other leaf tissues. The repressive effect of ABA on disease resistance mainly occurs through its antagonistic effect on the SA signaling pathway, which plays an important role in the defense response against pathogens. In rice, exogenous application of ABA resulted in reduced transcript levels of OsWRKY45 and OsNPR1, two important co‐factors of the SA‐dependent defense response. Downregulation of these two genes was associated with increased susceptibility to pathogens (Jiang et al., 2010; Xu et al., 2013; Ueno et al., 2015). OsWRKY45 and OsNPR1, however, were not differentially expressed in the Lr34res RNAseq data. This indicates that the Lr34res‐mediated ABA redistribution has no antagonistic effect on the SA pathway and that the Lr34res‐mediated disease resistance might be independent of SA‐mediated defense signaling, OsWRKY45, and OsNPR1. A recent study also reported that SA‐independent modulation of the ABA signaling pathway through the rice NAC transcription factor ONAC066 regulated disease resistance (Liu et al., 2018).
A model for durable multipathogen disease resistance in cereals
Recently, the wheat multipathogen resistance gene Lr67 was found to encode a hexose transporter (Moore et al., 2015). Similar to Lr34res, the resistant Lr67res allele evolved after wheat domestication as a result of spontaneous sequence changes, and only the susceptible Lr67sus allele was found in wild, diploid wheat progenitors. Lr67res and Lr34res both result in slow‐rusting and slow‐mildewing responses and the development of LTN. Hence, this type of durable broad‐spectrum disease resistance emerged multiple times as results of spontaneous mutations in different transporter genes. Whereas the LR67sus protein had a high affinity for glucose in yeast uptake experiments, glucose uptake was abolished by the resistant hexose transporter version. LR67res exerted a dominant negative effect on homoeologous copies of the hexose transporter. Moore et al. (2015) hypothesized that the dominant negative action of LR67res might increase the hexose : sucrose ratio in the leaf apoplastic space, which induces a sugar‐mediated signaling response resulting in hostile growth conditions for pathogenic fungi. Interestingly, there is a lot of crosstalk between sugar and ABA signaling (Eveland & Jackson, 2012). For example, several sugar signaling‐insensitive mutants were affected in components of ABA signaling (Arroyo et al., 2003). Maruyama et al. (2014) reported that the glyoxylate cycle, whose key enzymes isocitrate lyase and malate synthase were strongly induced by Lr34res, may be involved in glucose accumulation in response to drought stress in rice. A link between ABA and sugar signaling was also reported in grape (Vitis vinifera), where the two hexose transporters VvHT1 and VvHT5 are regulated by ABA. VvHT1 contains multiple ABA response elements in its promoter region, and ABA plays an important role in the transcriptional regulation of VvHT1 during infection with biotrophic fungal and oomycete pathogens (Hayes et al., 2010). Similarly, the expression of VvHT5 is controlled by the ASR protein VvMSA that binds to the VvHT5 promoter region (Cakir et al., 2003). Interestingly, the closest rice homologue of VvMSA is ASR1 (Perez‐Diaz et al., 2014), which is among the Lr34res‐responsive core gene set (Table S2).
Given the phenotypic similarity of Lr34res‐ and Lr67res‐mediated disease resistance, we hypothesize that the two genes trigger similar resistance mechanisms initiated either by ABA or sugar signaling, respectively. Sugars and ABA are basic molecules found in all plant species. This can explain why Lr34res‐mediated resistance works in different species against many fungal pathogens.
Accession numbers
The raw RNAseq files were deposited at the Sequence Read Archive at the National Centre for Biotechnology Information with BioProject accession PRJNA317706 (SRR3348352–RR3348377).
Author contributions
SGK, JK, EM and BK designed the research; SGK, JK, SB, RB, HC, LLS, EW and JS performed the research; GH and JK produced transgenic barley lines; SGK, JK, MDR, and MWS analyzed the data; SGK, JK, SB, RB, EM and BK wrote the manuscript. SGK, JK, SB and RB contributed equally to this work.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Lr34res induces a multiple stress response in rice.
Fig. S2 Characterization of the Lr34sus rice line 131.
Fig. S3 LR34 changes ABA fluxes.
Fig. S4 ABA concentrations in whole leaves of 3‐wk‐old plants of Lr34res containing lines 19 and 16 compared to sib lines.
Fig. S5 Cluster analysis of the leaf sheath infection assay.
Table S1 Primer sequences used in this study.
Table S2 Lr34res‐responsive core gene set consisting of 146 up‐regulated and 13 down‐regulated genes.
Table S3 Lr34res‐responsive core genes with a reported function in abiotic or biotic stress tolerance.
Table S4 Comparison of the ‘Lr34res‐responsive core gene set’ to a microarray study of 7‐d‐old rice seedlings incubated in solutions of 100 μM abscisic acid (ABA), 100 μM salicylic acid (SA), 100 μM jasmonic acid (JA), 50 μM benzyl aminopurine (BAP; cytokinin), 50 μM indole‐3‐acetic‐acid (IAA; auxin) or 100 μM 1‐aminocyclopropane‐1‐carboxylic acid (ACC; ethylene derivative).
Table S5 Comparison of the ‘Lr34res‐responsive core gene set’ to an RNAseq analysis performed in Brachypodium seedlings incubated in 10 μM abscisic acid (ABA), 100 μM salicylic acid (SA), 30 μM methyl jasmonate (MJ), 1 μM cytokinin (CK) or 10 μM indole‐3‐acetic acid (IAA; auxin).
Table S6 P‐values of the generalized linear analysis based on the leaf sheath assay.
Acknowledgements
We are grateful to Professor Yutaka Sato from the NARO Hokkaido Agricultural Research Center, Japan, for providing seeds of the OsABA8ox1 overexpressing line 27‐3. We would also like to thank Professor Kazuo Shinozaki from the Riken Center for Sustainable Resource Science for providing valuable comments on the manuscript, Gabriele Buchmann for producing the p6Uyeast vector, Dr Tina Jordan for producing the Lr34 yeast cDNA constructs, Sabine Sommerfeld for excellent technical assistance with the barley transformations, and Professor Anne C. Roulin for assistance with statistical analyses. This work was supported by an Advanced Investigator grant from the European Research Council (ERC‐2009‐AdG 249996, Durable resistance) and by the Swiss National Science Foundation grant 310030_163260. SGK and JK received support from an Ambizione fellowship of the Swiss National Science Foundation.
Contributor Information
Simon G. Krattinger, Email: simon.krattinger@kaust.edu.sa.
Beat Keller, Email: bkeller@botinst.uzh.ch.
References
- Alejandro S, Lee Y, Tohge T, Sudre D, Osorio S, Park J, Bovet L, Lee Y, Geldner N, Fernie AR et al 2012. AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Current Biology 22: 1207–1212. [DOI] [PubMed] [Google Scholar]
- Arroyo A, Bossi F, Finkelstein RR, Leon P. 2003. Three genes that affect sugar sensing (Abscisic Acid Insensitive 4, Abscisic Acid Insensitive 5, and Constitutive Triple Response 1) are differentially regulated by glucose in Arabidopsis. Plant Physiology 133: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddow JM, Pardey PG, Chai Y, Hurley TM, Kriticos DJ, Braun HJ, Park RF, Cuddy WS, Yonow T. 2015. Research investment implications of shifts in the global geography of wheat stripe rust. Nature Plants 1: e15132. [DOI] [PubMed] [Google Scholar]
- Boni R, Chauhan H, Hensel G, Roulin A, Sucher J, Kumlehn J, Brunner S, Krattinger SG, Keller B. 2018. Pathogen‐inducible Ta‐Lr34res expression in heterologous barley confers disease resistance without negative pleiotropic effects. Plant Biotechnology Journal 16: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnag S, Pongthai P. 2013. Selection of rice (Oryza sativa L.) cultivars tolerant to drought stress at the vegetative stage under field conditions. American Journal of Plant Sciences 4: 1701–1708. [Google Scholar]
- Cakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R. 2003. A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell 15: 2165–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JD, Yang C, Li LJ, Jiang L, Wu Y, Wu CW, Bu QY, Xia GX, Liu XY, Luo YM et al 2016. Rice plasma membrane proteomics reveals Magnaporthe oryzae promotes susceptibility by sequential activation of host hormone signaling pathways. Molecular Plant–Microbe Interactions 29: 902–913. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Newton AC. 2011. Climate change, plant diseases and food security: an overview. Plant Pathology 60: 2–14. [Google Scholar]
- Chauhan H, Boni R, Bucher R, Kuhn B, Buchmann G, Sucher J, Selter LL, Hensel G, Kumlehn J, Bigler L et al 2015. The wheat resistance gene Lr34 results in the constitutive induction of multiple defense pathways in transgenic barley. The Plant Journal 84: 202–215. [DOI] [PubMed] [Google Scholar]
- De Vleesschauwer D, Gheysen G, Hofte M. 2013. Hormone defense networking in rice: tales from a different world. Trends in Plant Science 18: 555–565. [DOI] [PubMed] [Google Scholar]
- De Vleesschauwer D, Yang YN, Cruz CV, Hofte M. 2010. Abscisic acid‐induced resistance against the brown spot pathogen Cochliobolus miyabeanus in rice involves MAP kinase‐mediated repression of ethylene signaling. Plant Physiology 152: 2036–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Rathjen JP. 2010. Plant immunity: towards an integrated view of plant‐pathogen interactions. Nature Reviews Genetics 11: 539–548. [DOI] [PubMed] [Google Scholar]
- Duan JZ, Zhang MH, Zhang HL, Xiong HY, Liu PL, Ali J, Li JJ, Li Z. 2012. OsMIOX, a myo‐inositol oxygenase gene, improves drought tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). Plant Science 196: 143–151. [DOI] [PubMed] [Google Scholar]
- Ellis JG, Lagudah ES, Spielmeyer W, Dodds PN. 2014. The past, present and future of breeding rust resistant wheat. Frontiers in Plant Science 5: 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveland AL, Jackson DP. 2012. Sugars, signalling, and plant development. Journal of Experimental Botany 63: 3367–3377. [DOI] [PubMed] [Google Scholar]
- Garg R, Tyagi AK, Jain M. 2012. Microarray analysis reveals overlapping and specific transcriptional responses to different plant hormones in rice. Plant Signaling & Behavior 7: 951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MA, Feechan A, Dry IB. 2010. Involvement of abscisic acid in the coordinated regulation of a stress‐inducible hexose transporter (VvHT5) and a cell wall invertase in grapevine in response to biotrophic fungal infection. Plant Physiology 153: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel G, Kastner C, Oleszczuk S, Riechen J, Kumlehn J. 2009. Agrobacterium‐mediated gene transfer to cereal crop plants: current protocols for barley, wheat, triticale, and maize. International Journal of Plant Genomics 2009: e835608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel G, Valkov V, Middlefell‐Williams J, Kumlehn J. 2008. Efficient generation of transgenic barley: the way forward to modulate plant–microbe interactions. Journal of Plant Physiology 165: 71–82. [DOI] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. 2008. Genevestigator v3: a reference expression database for the meta‐analysis of transcriptomes. Advances in Bioinformatics 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wang J, Du Z, Zhang C, Li L, Xu ZQ. 2013. Enhanced resistance to stripe rust disease in transgenic wheat expressing the rice chitinase gene RC24 . Transgenic Research 22: 939–947. [DOI] [PubMed] [Google Scholar]
- Hulbert SH, Bai J, Fellers JP, Pacheco MG, Bowden RL. 2007. Gene expression patterns in near isogenic lines for wheat rust resistance gene Lr34/Yr18 . Phytopathology 97: 1083–1093. [DOI] [PubMed] [Google Scholar]
- Hwang JU, Song WY, Hong D, Ko D, Yamaoka Y, Jang S, Yim S, Lee E, Khare D, Kim K et al 2016. Plant ABC transporters enable many unique aspects of a terrestrial plant's lifestyle. Molecular Plant 9: 338–355. [DOI] [PubMed] [Google Scholar]
- Jasinski M, Ducos E, Martinoia E, Boutry M. 2003. The ATP‐binding cassette transporters: structure, function, and gene family comparison between rice and Arabidopsis. Plant Physiology 131: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski M, Stukkens Y, Degand H, Purnelle B, Marchand‐Brynaert J, Boutry M. 2001. A plant plasma membrane ATP binding cassette‐type transporter is involved in antifungal terpenoid secretion. Plant Cell 13: 1095–1107. [PMC free article] [PubMed] [Google Scholar]
- Jiang CJ, Shimono M, Sugano S, Kojima M, Yazawa K, Yoshida R, Inoue H, Hayashi N, Sakakibara H, Takatsuji H. 2010. Abscisic acid interacts antagonistically with salicylic acid signaling pathway in rice–Magnaporthe grisea interaction. Molecular Plant–Microbe Interactions 23: 791–798. [DOI] [PubMed] [Google Scholar]
- Johnston PA, Munro C, Butler RC, Browne J, Gibbs A, Shorter S. 2017. The future of Lr34 in modern, high‐input wheat breeding programs. Crop Science 57: 671–680. [Google Scholar]
- Kakei Y, Mochida K, Sakurai T, Yoshida T, Shinozaki K, Shimada Y. 2015. Transcriptome analysis of hormone‐induced gene expression in Brachypodium distachyon . Scientific Reports 5: e14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Hwang JU, Lee M, Kim YY, Assmann SM, Martinoia E, Lee Y. 2010. PDR‐type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proceedings of the National Academy of Sciences, USA 107: 2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Yim S, Choi H, Kim A, Lee KP, Lopez‐Molina L, Martinoia E, Lee Y. 2015. Abscisic acid transporters cooperate to control seed germination. Nature Communications 6: e8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmer JA, Singh RP, Garvin DF, Viccars L, William HM, Huerta‐Espino J, Ogbonnaya FC, Raman H, Orford S, Bariana HS et al 2008. Analysis of the Lr34/Yr18 rust resistance region in wheat germplasm. Crop Science 48: 1841–1852. [Google Scholar]
- Krattinger SG, Jordan DR, Mace ES, Raghavan C, Luo MC, Keller B, Lagudah ES. 2013. Recent emergence of the wheat Lr34 multi‐pathogen resistance: insights from haplotype analysis in wheat, rice, sorghum and Aegilops tauschii . Theoretical and Applied Genetics 126: 663–672. [DOI] [PubMed] [Google Scholar]
- Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta‐Espino J, McFadden H, Bossolini E, Selter LL, Keller B. 2009. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323: 1360–1363. [DOI] [PubMed] [Google Scholar]
- Krattinger SG, Sucher J, Selter LL, Chauhan H, Zhou B, Tang M, Upadhyaya NM, Mieulet D, Guiderdoni E, Weidenbach D et al 2016. The wheat durable, multipathogen resistance gene Lr34 confers partial blast resistance in rice. Plant Biotechnololgy Journal 14: 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromori T, Miyaji T, Yabuuchi H, Shimizu H, Sugimoto E, Kamiya A, Moriyama Y, Shinozaki K. 2010. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proceedings of the National Academy of Sciences, USA 107: 2361–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromori T, Sugimoto E, Shinozaki K. 2014. Intertissue signal transfer of abscisic acid from vascular cells to guard cells. Plant Physiology 164: 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li N, Song SF, Li YX, Xia XJ, Fu XQ, Chen GH, Deng HF. 2014. Cloning and characterization of the drought‐resistance OsRCI2‐5 gene in rice (Oryza sativa L.). Genetics and Molecular Research 13: 4022–4035. [DOI] [PubMed] [Google Scholar]
- Liang CZ, Wang YQ, Zhu YN, Tang JY, Hu B, Liu LC, Ou SJ, Wu HK, Sun XH, Chu JF et al 2014. OsNAP connects abscisic acid and leaf senescence by fine‐tuning abscisic acid biosynthesis and directly targeting senescence‐associated genes in rice. Proceedings of the National Academy of Sciences, USA 111: 10013–10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Yan S, Huang W, Yang J, Dong J, Zhang S, Zhao J, Yang T, Mao X, Zhu X et al 2018. NAC transcription factor ONAC066 positively regulates disease resistance by suppressing the ABA signaling pathway in rice. Plant Molecular Biology 98: 289–302. [DOI] [PubMed] [Google Scholar]
- Lu X, Dittgen J, Pislewska‐Bednarek M, Molina A, Schneider B, Svatos A, Doubsky J, Schneeberger K, Weigel D, Bednarek P et al 2015. Mutant allele‐specific uncoupling of PENETRATION3 functions reveals engagement of the ATP‐binding cassette transporter in distinct tryptophan metabolic pathways. Plant Physiology 168: 814–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Urano K, Yoshiwara K, Morishita Y, Sakurai N, Suzuki H, Kojima M, Sakakibara H, Shibata D, Saito K et al 2014. Integrated analysis of the effects of cold and dehydration on rice metabolites, phytohormones, and gene transcripts. Plant Physiology 164: 1759–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega R, Meguro‐Maoka A, Endo A, Shimosaka E, Murayama S, Nambara E, Seo M, Kanno Y, Abrams SR, Sato Y. 2015. Sustained low abscisic acid levels increase seedling vigor under cold stress in rice (Oryza sativa L.). Scientific Reports 5: e13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelheuser CJ, Van Steveninck RF. 1969. Stomatal closure and inhibition of transpiration induced by (RS)‐abscisic acid. Nature 221: 281–282. [Google Scholar]
- Moore JW, Herrera‐Foessel S, Lan CX, Schnippenkoetter W, Ayliffe M, Huerta‐Espino J, Lillemo M, Viccars L, Milne R, Periyannan S et al 2015. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nature Genetics 47: 1494–1498. [DOI] [PubMed] [Google Scholar]
- Pang ZQ, Zhou ZZ, Yin DD, Lv QM, Wang LX, Xu X, Wang J, Li XB, Zhao XF, Jiang GH et al 2013. Transgenic rice plants overexpressing BBTI4 confer partial but broad‐spectrum bacterial blight resistance. Journal of Plant Biology 56: 383–390. [Google Scholar]
- Perez‐Diaz J, Wu TM, Perez‐Diaz R, Ruiz‐Lara S, Hong CY, Casaretto JA. 2014. Organ‐ and stress‐specific expression of the ASR genes in rice. Plant Cell Reports 33: 61–73. [DOI] [PubMed] [Google Scholar]
- Ralser M, Kuhl H, Ralser M, Werber M, Lehrach H, Breitenbach M, Timmermann B. 2012. The Saccharomyces cerevisiae W303‐K6001 cross‐platform genome sequence: insights into ancestry and physiology of a laboratory mutt. Open Biology 2: e120093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risk JM, Selter LL, Chauhan H, Krattinger SG, Kumlehn J, Hensel G, Viccars LA, Richardson TM, Buesing G, Troller A et al 2013. The wheat Lr34 gene provides resistance against multiple fungal pathogens in barley. Plant Biotechnology Journal 11: 847–854. [DOI] [PubMed] [Google Scholar]
- Risk JM, Selter LL, Krattinger SG, Viccars LA, Richardson TM, Buesing G, Herren G, Lagudah ES, Keller B. 2012. Functional variability of the Lr34 durable resistance gene in transgenic wheat. Plant Biotechnology Journal 10: 477–487. [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubiales D, Niks RE. 1995. Characterization of Lr34, a major gene conferring nonhypersensitive resistance to wheat leaf rust. Plant Disease 79: 1208–1212. [Google Scholar]
- Saitoh H, Fujisawa S, Mitsuoka C, Ito A, Hirabuchi A, Ikeda K, Irieda H, Yoshino K, Yoshida K, Matsumura H et al 2012. Large‐scale gene disruption in Magnaporthe oryzae identifies MC69, a secreted protein required for infection by monocot and dicot fungal pathogens. PLos Pathogens 8: e1002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N, Stolz J. 1994. SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker's yeast and identification of the histidine‐tagged protein. The Plant Journal 6: 67–77. [DOI] [PubMed] [Google Scholar]
- Schmid MW, Grossniklaus U. 2015. rcount: simple and flexible RNA‐Seq read counting. Bioinformatics 31: 436–437. [DOI] [PubMed] [Google Scholar]
- Schmid MW, Schmidt A, Klostermeier UC, Barann M, Rosenstiel P, Grossniklaus U. 2012. A powerful method for transcriptional profiling of specific cell types in eukaryotes: laser‐assisted microdissection and RNA sequencing. PLoS ONE 7: e29685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnippenkoetter W, Lo C, Liu G, Dibley K, Chan WL, White J, Milne R, Zwart A, Kwong E, Keller B et al 2017. The wheat Lr34 multipathogen resistance gene confers resistance to anthracnose and rust in sorghum. Plant Biotechnology Journal 15: 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaik R, Ramakrishna W. 2014. Machine learning approaches distinguish multiple stress conditions using stress‐responsive genes and identify candidate genes for broad resistance in rice. Plant Physiology 164: 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RP. 1992. Association between gene Lr34 for leaf rust resistance and leaf tip necrosis in wheat. Crop Science 32: 874–878. [Google Scholar]
- Singh RP, Huerta‐Espino J. 1997. Effect of leaf rust resistance gene Lr34 on grain yield and agronomic traits of spring wheat. Crop Science 37: 390–395. [Google Scholar]
- Spielmeyer W, Mago R, Wellings C, Ayliffe M. 2013. Lr67 and Lr34 rust resistance genes have much in common – they confer broad spectrum resistance to multiple pathogens in wheat. BMC Plant Biology 13: e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher J, Boni R, Yang P, Rogowsky P, Buchner H, Kastner C, Kumlehn J, Krattinger SG, Keller B. 2016. The durable wheat disease resistance gene Lr34 confers common rust and northern corn leaf blight resistance in maize. Plant Biotechnology Journal 15: 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher J, Menardo F, Praz CR, Boni R, Krattinger SG, Keller B. 2017. Transcriptional profiling reveals no response of fungal pathogens to the durable, quantitative Lr34 disease resistance gene of wheat. Plant Pathology 67: 792–798. [Google Scholar]
- Ton J, Flors V, Mauch‐Mani B. 2009. The multifaceted role of ABA in disease resistance. Trends in Plant Science 14: 310–317. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. 2010. Transcript assembly and quantification by RNA‐Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology 28: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno Y, Yoshida R, Kishi‐Kaboshi M, Matsushita A, Jiang CJ, Goto S, Takahashi A, Hirochika H, Takatsuji H. 2015. Abiotic stresses antagonize the rice defence pathway through the tyrosine‐dephosphorylation of OsMPK6. PLoS Pathogens 11: e1005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulferts S, Delventhal R, Splivallo R, Karlovsky P, Schaffrath U. 2015. Abscisic acid negatively interferes with basal defence of barley against Magnaporthe oryzae . BMC Plant Biology 15: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brule S, Muller A, Fleming AJ, Smart CC. 2002. The ABC transporter SpTUR2 confers resistance to the antifungal diterpene sclareol. The Plant Journal 30: 649–662. [DOI] [PubMed] [Google Scholar]
- Wang MB, Boulter D, Gatehouse JA. 1992. A complete sequence of the rice sucrose synthase‐1 (RSs1) gene. Plant Molecular Biology 19: 881–885. [DOI] [PubMed] [Google Scholar]
- Xiao BZ, Huang YM, Tang N, Xiong LZ. 2007. Over‐expression of a LEA gene in rice improves drought resistance under the field conditions. Theoretical and Applied Genetics 115: 35–46. [DOI] [PubMed] [Google Scholar]
- Xu J, Audenaert K, Hofte M, De Vleesschauwer D. 2013. Abscisic acid promotes susceptibility to the rice leaf blight pathogen Xanthomonas oryzae pv oryzae by suppressing salicylic acid‐mediated defenses. PLoS ONE 8: e67413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Wu Y, He Y, Wang Y, Xiao J, Li L, Chen X, Xiong W. 2015. RopGEF2 is involved in ABA‐suppression of seed germination and post‐germination growth of Arabidopsis . The Plant Journal 84: 886–899. [DOI] [PubMed] [Google Scholar]
- Zheng J, Wang YY, He YA, Zhou JJ, Li YP, Liu QQ, Xie XZ. 2014. Overexpression of an S‐like ribonuclease gene, OsRNS4, confers enhanced tolerance to high salinity and hyposensitivity to phytochrome‐mediated light signals in rice. Plant Science 214: 99–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Lr34res induces a multiple stress response in rice.
Fig. S2 Characterization of the Lr34sus rice line 131.
Fig. S3 LR34 changes ABA fluxes.
Fig. S4 ABA concentrations in whole leaves of 3‐wk‐old plants of Lr34res containing lines 19 and 16 compared to sib lines.
Fig. S5 Cluster analysis of the leaf sheath infection assay.
Table S1 Primer sequences used in this study.
Table S2 Lr34res‐responsive core gene set consisting of 146 up‐regulated and 13 down‐regulated genes.
Table S3 Lr34res‐responsive core genes with a reported function in abiotic or biotic stress tolerance.
Table S4 Comparison of the ‘Lr34res‐responsive core gene set’ to a microarray study of 7‐d‐old rice seedlings incubated in solutions of 100 μM abscisic acid (ABA), 100 μM salicylic acid (SA), 100 μM jasmonic acid (JA), 50 μM benzyl aminopurine (BAP; cytokinin), 50 μM indole‐3‐acetic‐acid (IAA; auxin) or 100 μM 1‐aminocyclopropane‐1‐carboxylic acid (ACC; ethylene derivative).
Table S5 Comparison of the ‘Lr34res‐responsive core gene set’ to an RNAseq analysis performed in Brachypodium seedlings incubated in 10 μM abscisic acid (ABA), 100 μM salicylic acid (SA), 30 μM methyl jasmonate (MJ), 1 μM cytokinin (CK) or 10 μM indole‐3‐acetic acid (IAA; auxin).
Table S6 P‐values of the generalized linear analysis based on the leaf sheath assay.


