Abstract
Introduction
Countries aspiring to universal health coverage view close-to-community (CTC) providers as a low-cost means of increasing coverage. However, due to lack of coordination and unreliable funding, the quality of large-scale CTC healthcare provision is highly variable and routine data about service quality are not trustworthy. Quality improvement (QI) approaches are a means of addressing these issues, yet neither the costs nor the budget impact of integrating QI approaches into CTC programme costs have been assessed.
Methods
This paper examines the costs and budget impact of integrating QI into existing CTC health programmes in five countries (Ethiopia, Indonesia, Kenya, Malawi, Mozambique) between 2015 and 2017. The intervention involved: (1) QI team formation; (2) Phased training interspersed with supportive supervision; which resulted in (3) QI teams independently collecting and analysing data to conduct QI interventions. Project costs were collected using an ingredients approach from a health systems perspective. Based on project costs, costs of local adoption of the intervention were modelled under three implementation scenarios.
Results
Annualised economic unit costs ranged from $62 in Mozambique to $254 in Ethiopia per CTC provider supervised, driven by the context, type of community health model and the intensity of the intervention. The budget impact of Ministry-led QI for community health is estimated at 0.53% or less of the general government expenditure on health in all countries (and below 0.03% in three of the five countries).
Conclusion
CTC provision is a key component of healthcare delivery in many settings, so QI has huge potential impact. The impact is difficult to establish conclusively, but as a first step we have provided evidence to assess affordability of QI for community health. Further research is needed to assess whether QI can achieve the level of benefits that would justify the required investment.
Keywords: health systems, health economics, health policy, public health, health services research
Key questions.
What is already known?
The quality of close-to-community (CTC) healthcare services is highly variable and routine programme data are of poor quality.
Quality of care provided by CTC providers can be improved through quality improvement (QI) approaches and measures.
Stakeholders perceive QI approaches to be an additional and diversionary cost in resource-limited settings.
What are the new findings?
Across the countries studied, capital costs of training are similar across implementation scenarios and represent a large proportion of the total cost of implementing QI approaches.
Recurrent economic costs of QI per CTC provider range from $54 in Mozambique to $233 in Ethiopia, driven by costs of staff and volunteer time.
The budget impact of national-scale QI for CTC programmes ranges from 0.03% to 0.58% of general government expenditure on health.
What do the new findings imply?
Sustaining recurrent costs of QI for CTC programs is likely affordable within budget constraints if capital costs of training are supported.
Systematic measuring of the benefits of QI on processes and outcomes should be a routine part of policy and practice to underpin investment decisions.
Introduction
Many governments struggling to achieve universal health coverage (UHC) in resource-poor settings are considering expanding healthcare coverage at low cost through the use of close-to-community (CTC) providers of healthcare.1–6 Composed of a wide range of typologies, CTC providers are lay health workers with relevant training for their responsibilities. They include: community health volunteers, community health (extension) workers, nutrition counsellors and traditional birth attendants, among others.1 7 CTC providers deliver a range of preventive, promotive and curative healthcare services at community level depending on context and policy5 8 9 and have been found to be effective in expanding service coverage in certain contexts and clinical areas.10 11 However, CTC providers face numerous challenges working at the interface between communities and health systems due to factors such as: working remotely (where it can be difficult to maintain standards), lower literacy rates, higher attrition rates, less education and fewer support structures than other professional, formal cadres of healthcare workers more closely linked to the formal health sector.9 12 Additionally, efforts to consider quality at the health system or global level continue to leave out CTC providers and ignore the potential contribution of the community level to health system goals.13–15
Despite the perception that CTC provision of care is ‘cheap’, economic evaluation of the work of CTC providers and programmes is complex due to a unique combination of challenges. First, costing involving this cadre is complicated by its composition of primarily part-time and/or volunteer workers (who may pay out-of-pocket costs that are difficult to measure for food or transport to support the effectiveness of the programmes).16 Second, drawing generalisable conclusions is also difficult as the responsibilities, training, supervision and remuneration of CTC providers between (and even within) countries vary widely.7 17 These challenges are not unique to CTC programmes, but this is an area where challenges are particularly numerous and acute. Additionally, cost-effectiveness studies rely on causal, proximal clinical outcomes to an intervention and high-quality data.18 19 With community health, however, the long-term benefits of the primarily preventive and promotive services provided by CTC health workers are challenging to measure and to attribute20–22 and the quality of the data on both costs and benefits are questionable.23–26 Few studies and models to date have taken this complexity sufficiently into account to collect real life data on the full set of services, focusing instead on a limited set of services and/or heavily on modelling.18 27 28
Policy makers are beginning to question whether CTC providers can achieve equitable service quality at low cost.29 Evidence is growing for systematically incorporating quality improvement (QI) approaches into community health programmes in low-income and middle-income countries, especially in maternal and child health.30–34 These community-level approaches appear to have been successful in terms of improving the quality and equity of services, but there is limited information about costs or cost-effectiveness of implementation.32 34 This lack of financial data acts as a barrier to decision makers, who may perceive the financial and time costs of incorporating QI approaches to be high when compared with the urgency of further expanding coverage while under pressure to show progress towards UHC.35 36 We set out to examine the costs of integrating QI approaches in community health programmes at a mid-level of administration in Ethiopia, Indonesia, Kenya, Malawi, Mozambique—five countries with established community health programmes addressing maternal and/or child health among other priorities at CTC level through preventive and promotive care (table 1). This study is a first, essential step towards assessing the cost-effectiveness of this approach.
Table 1.
Intervention sites for quality improvement (QI) capacity development intervention47 48 80–84
| Country Region |
Administrative unit (district or equivalent) |
QI teams | Setting | Catchment population | CTC providers | # of CTC providers supervised | Focus of CTC programme | Policy ratio of CTC providers to population |
|
Ethiopia Southern Nations, Nationalities and Peoples’ Region, Sidama Zone |
Shebedino woreda* | 1 Community QI team at woreda* level 9 Community QI teams covering health centre catchments: Abela health centre Galuko-hirreye health centre Gebre-kirstos health centre Mero kawado health centre Telamo health centre Fura health centre Dobe toga health centre Morocho negasha health centre Dulacha health centre |
Rural, medium-remote | 244 489 | Health extension workers | 68 | Preventive, curative, family planning |
2:5000 |
|
Indonesia Cianjur district |
Cianjur district | 4 Community QI teams covering three puskesmas† Cikalongkulon Pukesmas† Ciranjang 1 Pukesmas† Ciranjang 2 Pukesmas† Gekbrong Pukesmas† |
Suburban, medium-remote | 188 323 | Puskesmas midwives | 47 | Maternal health (including delivery) | ~1:5000–6000 general population (but serve women) |
|
Kenya Nairobi county |
Embakasi West subcounty Kasarani subcounty Lang’ata subcounty |
3 Community QI teams at subcounty 9 Community QI teams covering community units: Maili Saba Unit Bangladesh Unit Southlands Unit Raila Unit Gitari Marigu Unit Housing Development Dept Unit City Carton Unit Matopeni Unit Ribakia Unit |
Urban, non-remote | 737 460 | Community health volunteers Community health extension workers |
1530 | Preventive | 1:500 1:2500 |
| Malawi | Mchinji, Salima districts |
2 Community QI teams at district level | Rural, remote | 213 206 | Health surveillance assistants | 121 | Preventive, curative |
1:1000 |
|
Mozambique Maputo province |
Manhiça, Moamba districts |
2 Community QI teams at district level | Rural, non-remote | 214 388 | Agentes polivalentes elementares | 68 | Preventive, curative |
~1:500–2000 |
*Woreda is the Amharic word for district, at the level below Zone in the Ethiopian health system.
†Puskesmas is the Bahasa word for community health facility.
CTC, close-to-community.
Methods
We nested this costing within REACHOUT, a consortium of research partners in community health conducting an implementation research study addressing the feasibility and effectiveness of QI at community level.37 While the CTC providers’ typology and responsibilities varied across the countries, we used a common approach to QI team establishment and training. Based on actual project costs, we have then taken a scenario planning approach to assess the costs and budget impact of a long-term Ministry of Health (MoH)-led adoption of this approach by public sector staff in each setting. We report (in 2017USD): total and annualised economic costs per country; total and average annual financial costs of the intervention per country; for the MoH-led adoption, we report the same and add the unit economic and financial costs of intervention per: catchment population, CTC provider, QI team trained and administrative area. We also report the budget impact of national scale-up of MoH-led QI.
The intervention
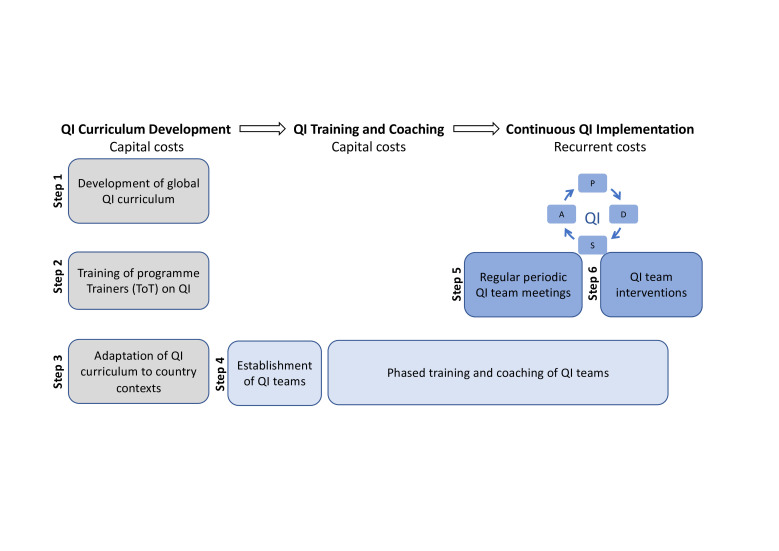
QI capacity development efforts were guided by a common approach across the study countries, as shown in figure 1. In all settings, after curriculum development and adaptation of the training materials, QI teams made up of CTC providers, supervisors and health facility staff (average eight people) were established. In Kenya and Ethiopia, project team and MoH partners decided in step 3 to form QI teams at both the community and the district levels. These teams were trained in three phases to conduct QI for community health using Plan-Do-Study-Act (PDSA) cycles. PDSA approaches are characterised by local selection, prioritisation and action on quality problems identified from local data.38–42 Training content included: standards for quality in community health, quality assurance and QI concepts, community health information systems, supportive supervision, and so on. The three phases of training and exchange (implemented over 9–12 months) were interspersed with periods of implementation of QI by the teams, involving team meetings and interventions to improve quality supported by mentorship from supervisors, with the expectation that implementation could be continued indefinitely in what is termed ‘continuous QI’. Examples of QI priorities tackled include: improving timeliness of reporting by CTC providers; improving follow-up of pregnant women referred for antenatal care; reducing rates of unskilled delivery. These priorities were selected and improvement was measured by the teams using local community health information systems and data.
Figure 1.
Common approach to capacity development for quality improvement (QI) for close-to-community (CTC) healthcare across countries.
Study site selection
The common approach to QI in community health was implemented with 21 QI teams in 11 administrative areas of the five countries serving a total of 1.6 million people in their catchment areas. In each country, study sites were selected for the QI intervention in collaboration with stakeholders from the community and MoH building on earlier supportive supervision interventions for the CTC programme (see table 1). Further description of the CTC provider typologies in each of the study sites including selection, training and responsibilities can be found in Supplementary File 1.43
bmjgh-2019-001390supp001.pdf (101.4KB, pdf)
Costing approach
The costing took a health systems perspective, taking into account health system resource and time costs (we differentiate that from health system costs, as CTC providers may not be salaried individuals whose time is explicitly valued by the health system).44 45 Specifically, we collected and report both economic and financial costs of the intervention, as well as the budget impact of national scale-up based on the financial costs only. Financial costs refer to outlay of money; economic costs encompass financial costs and opportunity costs of time, even where people are already salaried or are volunteers and their time is ‘free’. An ingredients approach was used to assess the costs of each phase of the intervention in the following categories: staff time (encompasses volunteer time), lodging/ transport, communication, venue, refreshment, stationery.46 In our model, costs incurred during the training are treated as capital costs while the QI implementation represents recurrent costs of the intervention. The useful life of the training is taken as 4 years (ie, all participating staff would receive full retraining in year 5). Details of specific cost adjustments made at each of the steps of the intervention when calculating country costs can be found in Supplementary File 2.
bmjgh-2019-001390supp002.pdf (47.4KB, pdf)
Data on the actual costs in local currency of QI capacity development and functioning were collected retrospectively (March–July 2017) from country research teams using a combination of structured questionnaire on activities and a spreadsheet for unit resource costs (Supplementary Files 3 and 4). Project costings for consumables were calculated by multiplying units of resources consumed by market rates in May 2017. For other categories, that is, salaries, venue, transport, communication, actual project expenses incurred were used. Data were provided by implementation and finance team members from each REACHOUT country partner institution and validity of data was confirmed through back-checking financial reporting and audited information. Salaries for the public sector staff involved in intervention activities were obtained from public documents referenced here; where not available they were estimated from available data.47–52 Where available, actual value of employment benefits were used. Where not available, an assumption of 15% of salary was applied. We excluded outcome-related costs, for example, costs averted due to improved health, as outside the scope of the study.
bmjgh-2019-001390supp003.pdf (55.4KB, pdf)
bmjgh-2019-001390supp004.pdf (45.8KB, pdf)
Annual costs are reported in 2017USD and exchange rates from May 2017 were used.53 For details of cost adjustments made at each of the steps of the intervention when calculating country costs, see Supplementary File 2. (NB country costs cannot be added together to compute the actual total project cost due to these adjustments). A discount rate of 3% was applied to future costs; because inflation was only relevant to the development (sunk) costs, this is not accounted for in the model. Data were input and managed in Microsoft Excel V.15.32.
Scenario planning and sensitivity analysis
Based on project costing, we present three scenarios for adoption of the intervention in each country, which we term ‘MoH-led QI’. These scenarios assume the interventions were to be repeated across the same administrative area and population as the project-led approach. Specifically, we present the economic costs of MoH-led QI per administrative area of the intervention (table 1) by step of the intervention (figure 1). Where multiple levels of QI teams were involved (ie, in Kenya and Ethiopia), we have included costs for both and described this as increased intensity of intervention.
All scenarios for MoH-led QI involved the following modifications to the project costs: (1) dropping all development costs as sunk costs incurred by REACHOUT (steps 1–3); (2) health system staff acting as trainers (step 4); and (3) periodic mentorship at quarterly QI team meetings (step 5). Deterministic sensitivity analyses were conducted around ‘best’ case and ‘worst’ case scenarios for MoH-led QI, based on the level of involvement required of project staff in the scale-up and the frequency of QI team meetings and interventions (Supplementary File 5 for details).
bmjgh-2019-001390supp005.pdf (43.3KB, pdf)
Budget impact analysis
Budget impact analysis was conducted by comparing the financial costs of MoH-led QI, scaled up linearly to national level based on the total number of administrative areas in the country, with the annual general government expenditure on health (GGHE). GGHE was chosen as a comparator for the budget impact analysis for two reasons: first, financing for community QI is unlikely to be a repurposing of community/preventive care budgets. In part, this is due to the reliance on unpaid or low-paid staff in current community/preventive care budgets, making this a misleading comparison (in addition to the variability in pay levels for CTC providers between contexts). Also, what is proposed is a systemic change to the health system, given how CTC providers are used (across a broad spectrum of health areas) and could be supported by general government funding. The argument is for government investment, so need to compare with GGHE. Second, as community/preventive care budgets are often not earmarked in externally available documents, using these as the basis of budget impact analysis would require us to estimate a percentage of GGHE rather than relying on empirical data. Specific analyses for each health system or even budget-holding unit with more granular data would still be required for ultimate financing decisions—this analysis is indicative of broader trends in investment in community health systems and quality across systems.
GGHE data were obtained from the National Health Accounts database (on 6 October 2017)54 and inflated from 2014USD (the most recent year to have complete data) to 2017USD,55 assuming no change in expenditure over these 3 years as GGHE as a portion of total government expenditure has remained constant for some time. We have not included salaries of public sector staff as financial costs in the budget impact analysis because no additional staff were hired to conduct the QI activities.
Ethical approval
Country research activities described herein were governed under national approvals; details available in Supplementary File 6.
bmjgh-2019-001390supp006.pdf (40.2KB, pdf)
Patient and public involvement
Co-development of research questions in the wider REACHOUT sproject was done with relevant government counterparts and community health stakeholders in each country; patients were not directly involved in any way. Results will be disseminated to participants through technical working groups in each country as relevant.
Results
Total costs of project-led QI intervention
The economic costs of developing the intervention, establishing and training 29 QI teams, and mentoring those teams through one completed QI cycle were incurred across the 11 administrative areas in the five countries as part of the REACHOUT project. These ranged from $11 351.32 (Mozambique) to $333 589.89 (Kenya) and show the full costs of the dedicated technical project teams, curriculum development and training. When aggregated across countries, costs of conducting the three phases of training made up about 70% of the total costs and were driven largely by people-time and by the intensive, phased nature of the training. Training costs varied widely between the five countries and were greatest in Kenya at $267 111 (where the highest number (12) of teams were trained), and were least in Indonesia at $3868, where the project team limited costs of this phase through use of available public sector venues. The total recurrent costs of implementation across countries (incurred in QI team meetings and QI interventions) were similar to development costs in year 1 (15%–16% of the total costs).
Total costs of MoH adoption of QI intervention
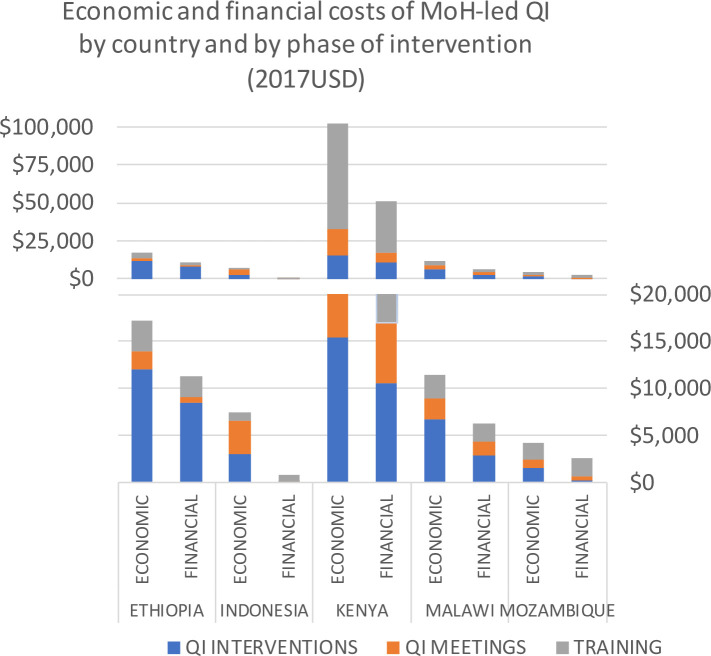
When MoH-led adoption of the QI approach is modelled for the same sites, the economic costs per administrative district are less than the project-incurred costs in each country, showing that unit costs of the intervention were higher for the project than those that would be faced by local decision makers. The annualised economic costs range from $4250.07 in Mozambique to $102 339.98 in Kenya (see table 2 for details of country costs). In sites where teams deliberately selected or prioritised QI problems that could be solved at low cost without additional project funding, the capital costs of training (incurred in year 1) represent a larger percentage of the total spend. Ethiopian and Malawian project teams provided additional external ‘project funds’ to the QI teams when developing QI interventions (to cover items such as venue for refresher training of CTC providers, transportation for QI team to visit field facility sites and test new tools), which increased implementation costs. The average annual financial costs are lower in all sites than the annualised economic costs, as expected (figure 2). This is because a QI approach to community health requires an ongoing investment of time from existing staff in the form of trainings and meetings.
Table 2.
Financial and economic costs of Ministry of Health-led quality improvement for community health in each country (2017USD)
| Country | Financial costs | Unit annual financial costs per: | Economic costs | Unit annualised economic cost per: | ||||||||||||
| Capital costs of training | Annual recurrent costs | Average annual cost | Administrative area | QI team trained | QI team member | CTC provider supervised | Capita | Capital costs of training | Annual recurrent costs | Annualised cost | Administrative area | QI team trained | QI team member | CTC provider supervised | Capita | |
| Ethiopia | 8509.25 | 9034.92 | 11 324.13 | 11 324.13 | 1258.24 | 179.75 | 166.53 | 0.05 | 12 326.54 | 13 959.67 | 17 275.84 | 17 275.84 | 1919.54 | 274.22 | 254.06 | 0.07 |
| Indonesia | 2008.98 | 62.15 | 602.62 | 200.87 | 150.65 | 20.78 | 12.82 | 0.00 | 3371.14 | 6536.81 | 7443.74 | 2481.25 | 1860.94 | 256.68 | 158.38 | 0.03 |
| Kenya | 84 853.87 | 16 938.84 | 39 766.82 | 13 255.61 | 3313.90 | 414.24 | 25.99 | 0.05 | 259 211.01 | 32 605.21 | 102 339.98 | 34 113.33 | 8528.33 | 1066.04 | 66.89 | 0.14 |
| Malawi | 4878.76 | 4316.62 | 5629.14 | 2814.57 | 2814.57 | 201.04 | 46.52 | 0.03 | 9210.99 | 8932.05 | 11 410.06 | 5705.03 | 5705.03 | 407.50 | 94.30 | 0.05 |
| Mozambique | 4963.79 | 588.03 | 1923.42 | 961.71 | 961.71 | 83.63 | 28.29 | 0.01 | 7001.19 | 2366.56 | 4250.07 | 2125.03 | 2125.03 | 184.79 | 62.50 | 0.02 |
CTC, close-to community; QI, quality improvement.
Figure 2.
Annualised economic costs and average annual financial costs of Ministry of Health (MoH)-led quality improvement (QI) (by country and by step; 2017USD).
The overall costs of MoH-led QI show high intercountry variability (figure 2), in part due to the differences in the sites (table 1) in terms of geography, population density and the wage differential. In Kenya and Ethiopia where two levels of QI teams were formed, the impact on cost is demonstrated in a high resource-level difference in both sites, as well as a high unit cost per CTC provider supervised in Ethiopia and a high unit cost per QI team member trained in Kenya.
Based on the scenarios described in Supplementary File 5, active adoption (ie, greater ownership by public sector staff in training and more frequent QI interventions) drove up the annualised economic costs in each country by 7%–21% while more passive adoption led to decreased costs of 67%–92% of the base case values, with the greatest variability observed in Indonesia and Malawi (Supplementary File 7). Training, which is a relatively static cost across scenarios, represented a smaller proportion of the costs in these two sites, increasing sensitivity to the different levels of activity in the intervention phase.
bmjgh-2019-001390supp007.pdf (175.4KB, pdf)
Unit costs of MoH-led QI for community health
As shown in table 2, the costs of MoH-led QI per capita are between <0.01–0.5 (financial) and 0.02–0.14 (economic). The annualised economic costs per administrative area are between $2125 (Mozambique) and $34 113 (Kenya). Despite that variation, the annualised economic costs per CTC provider supervised are much closer, ranging from $62 (Mozambique) to $254 (Ethiopia). Mozambique presents the lowest economic costs overall and economic unit costs in all cases except per QI team trained, for which unit costs in Indonesia and Ethiopia are lowest (table 2). The average financial cost per CTC provider supervised ranges from $12 in Indonesia to $166 in Ethiopia.
In both Ethiopia and Kenya, the intensity of the intervention was much greater, involving formation of two levels: district-equivalent QI teams and community QI teams. Ethiopia was the most expensive site in which to embed the intervention across the key indicators of cost per CTC provider supervised. The number of health extension workers in Shebedino woreda is almost equivalent to the number of QI team members, so these unit costs appear very close. The Ethiopian costs are dominated by the cost-heavy intervention that was chosen by the woreda (district) community QI team, which was a 4-day refresher training exercise. In Nairobi, the site of the Kenyan intervention and the other outlier due to cost, high density of both CTC providers and population make it appear high cost at the administrative unit level, yet more affordable at these more granular unit levels (table 2). Mozambique had the least expensive intervention in terms of absolute costs and this remained true across all indicators. Indonesia, as the only Asian context, was the least expensive site to conduct the intervention financially, showing similar cost structures and constraints despite very different geography and health system structures.
Budget impact of MoH-led QI for community health
Annual government spending on health ranged from $15–16 per capita in Ethiopia and Malawi to $49 per capita in Kenya, whereas the annualised financial costs of MoH-led QI is between <$0.01 to $0.05 per capita. Based on scaling up the average annual financial costs of the intervention per administrative district to nationwide coverage, the budget impact of MoH-led QI for community health represents less than 0.53% of the GGHE in all countries. The impact of MoH-led QI on annual government budgets varies somewhat by these levels of health expenditure, as Ethiopia has the lowest GGHE and the highest costs, so it shows the greatest budget impact, though still low (at 0.53%). In Kenya, the other study site that implemented ‘two-level’ community QI, budget impact of national-scale community QI is 0.16% of GGHE, and in the three other study countries the budget impact is 0.03% or less of GGHE. Also relevant to budgeting is the fact that the financial outlay would be greatest in year 1, when the training occurs, with low recurrent financial outlay; after annualisation this variation is masked.
Discussion
Summary of findings
We found that the economic costs of integrating QI approaches into community health range from $62 to $254 per CTC provider, with the most expensive unit cost incurred in Ethiopia. Collecting costs was a complicated exercise across the countries and intercountry variability was high. The largest component of costs of our phased training model were capital costs of capacity development generated in the training portion of the intervention, out of which the biggest cost driver was the time of existing public sector staff. In sites reporting high financial outlays, these were driven in part by the selection of venues and trainers, as well as general higher cost of living particularly in Nairobi. Greater intensity of the intervention (ie, two levels of QI teams; more teams per administrative area) was correlated to higher costs, both economic and financial. In Ethiopia, Kenya and Malawi, QI interventions drove up costs as teams were provided additional financing to use for interventions rather than working within existing resources. Across settings, national scale-up of the approach would have a budget impact of between 0.02% and 0.03% (in Indonesia, Malawi, Mozambique) up to 0.16% (Kenya) and 0.53% (Ethiopia) relative to the GGHE.
Sustainability of the approach
Sustaining QI approaches (or ‘MoH-led QI’) for community health will depend on financial commitment to take on recurrent costs by the subnational administrative units and national decision makers. In Malawi, Kenya and Indonesia, study countries with some decentralisation of health financing allocation decisions, the district (or equivalent administrative) level management has indicated a commitment to allocate funds to cover the recurrent costs for the year following the end of the project-led intervention. This financial commitment would likely come from the general health budget rather than the community health or preventive care budget, which is misleading in its size—it relies on unpaid or underpaid staff, the specifics of which varies by country, as well as heavy external financing. Because this is a system-wide change to the health system, given that CTC providers are used across a broad range of health areas and are a cadre of human resources for health, the argument for government investment is beyond the community budget to the GGHE. Given the wide range of services offered and benefits of high-quality CTC care, a societal perspective might be optimal,3 12 56 57 but benefits are beyond the scope of this study.
Despite the limited budget impact of this intervention, workload may be a challenge to the recurrent time costs. Time is a non-financial outlay, which is positive for the inclusion of the approach into local budgets going forward, although it may present challenges related to workload of mid-level health systems management staff. A reduction in meeting frequency may be feasible after the initial intensive start-up/mentorship phase of implementation to reduce recurrent time costs as well as financial costs; in the base case, we used a quarterly frequency to reflect this (rather than the original monthly design). However, as Greenhalgh et al write, diffusion of effective innovations in high-functioning health service delivery organisations is a notorious challenge,58 so it is likely to be a greater challenge where resources are limited.
The project-led intervention has been conducted on a pilot scale in each country, so it is not known whether these unit costs are similar at scale or whether economies of scale or scope might be achieved.59 The use of budget impact analysis was an attempt to address affordability at scale.60
In looking at affordability of scale-up, the costs of the phased training and mentorship intrinsic to the intervention design as described are higher than a traditional one-off workshop training. Reduced costs for training might also be achieved by inclusion of the QI material into in-service training for CTC providers and supervisors. Another option is a one-time external investment to cover training costs that would then be sustained by leveraging domestic cofinancing for the recurrent costs.
Benefits of the QI approach can be difficult to capture
For policy makers and donors to be convinced by costing data, they must first be convinced of the benefits of what is being costed, and this has created a challenge for QI approaches generally. We have not presented data on the individual improvements achieved by the 29 improvement teams included in our study, which are similar to those observed by other community QI projects from several settings in sub-Saharan Africa.31–34 61–65 Immediate process outcomes of the QI approach we used included: improved supervision and integration of the community health programme to the health system, consensus building across levels of the health system on priority problems and improved data quality on critical health service areas—all of which have been shown to support improved performance of CTC providers.5 9 43 66 67 The health impacts of integrating QI are harder to attribute due to the complex, iterative and locally driven nature of the approach. Measuring and attributing the downstream benefits of a service delivery intervention that are intrinsically valuable to a decision maker or population is challenging.20 68–72 Adding to the challenges of potential confounding, in ‘Step Six’ of the intervention (figure 1), QI teams have the freedom to design and test QI interventions to address locally relevant problems they select (in contrast to having a standard QI intervention imposed by higher-level or external stakeholders). These have greater potential to directly affect and yield benefits in priority health areas. However, this freedom or choice makes it challenging to evaluate outcomes systematically across intervention sites, as they are likely to be yielded in different health areas depending on the QI intervention selected by each QI team.
Community health services are often a low priority for domestic investment in health systems despite being shown to be cost-effective.18 19 27 73 The interventions that are funded out of the health budget are more often those that are most visible (facilities, ambulances) or urgent and curative (tertiary care) that can show immediate impact and benefit to the politician, rather than those with longer-term population-wide benefits like community health and preventive services.74 Where funded, the focus of investment in community health has been on increasing coverage towards UHC with limited emphasis on quality. Here we show that with a small additional investment, coverage of the population by CTC providers can potentially be transformed into meaningful coverage through improved performance and stronger linkages to higher-level healthcare services and providers.
For countries where this QI approach has been piloted through the REACHOUT project, the policy implications of affordability need to be contextualised beyond what is presented in the budget impact analysis here. Sub-national ‘use cases’ for adoption of this QI approach are being developed jointly with national policy makers. These cases will bring out multiple feasible locally relevant scenarios for adoption and scale-up of the approach, considering current staffing ratios, strategy development and budget cycles. Following on from discussions of affordability, assessment of whether QI for community health is a good investment requires a quantification of the benefits yielded by the intervention coupled with this cost analysis. To assess cost-effectiveness and relevance to UHC, further data on benefits derived from the intervention are required as well as an assessment of the reach of those benefits on the target population. Further, a qualitative exploration of decision space for the various funders of community health and their values in terms of benefits is planned to supplement the findings of this study, building on the abovementioned work by McCollum et al.74
Strengths and limitations of the study
Having robust, primary cost data collected and compared across countries and specifically looking at quality of care is very valuable, given the global focus on quality under UHC.12 13 15 75 76 At the same time, a major limitation of this (any) intercountry analysis is the differences in contexts. Variations in health systems, administrative units, CTC provider tasks and typology (Supplementary File 1) were easier to identify and describe than aspects of hierarchy, expectations of training allowances, donor and project fatigue, but these less tangible aspects also affect the design and cost of getting a QI approach for community health to work. Nevertheless, findings around affordability and cost drivers were robust across contexts. We emphasised contextualisation of the intervention to each country, encouraging country teams to adapt while maintaining fidelity to the intervention design within a given set of restrictions.77–79 In step 3 (figure 1), the intervention explicitly asked teams to adapt the global curriculum as appropriate to their context, bringing in local trainers and approaches as well as modifying the composition of the QI teams to best reflect existing health system structure, management and reporting lines. This is most clearly exemplified by the varied intensity of the intervention in Ethiopia and Kenya as compared with the other three settings, in addition to minor modifications due to variations in health system structures and supervisory approaches.
Significant challenges were faced in three of the five countries to estimate the costs of participation of public sector staff (as trainees and facilitators) due to sensitivity around salary data. In Malawi, public sector salaries were not publicly available and we received confidential estimates from multiple sources in addition to the limited public reference data. In Indonesia, the range of salaries within each tier is wide, reflecting the years of service of the individual more strongly than their level of responsibility. In Kenya, public sector expenses for participation in trainings were split into several categories (per diem, dinner allowance, workshop sitting allowance, local transport allowance). These were additional to the costs of mobilisation (referring to the phoning and follow-up with supervisees to ensure attendance) and facilitation but not applicable to all, making the actual costs of participation in training difficult to calculate but possible to estimate. In contrast, in Ethiopia and Mozambique public sector staff salaries are publicly available and presented no difficulty. The sensitivity around salary information reflects both transparency by the government and cultural values related to money and privacy.
Conclusion
CTC providers are a key component of healthcare provision in many settings. QI for community health has the possibility of bringing CTC providers more definitively under the umbrella of human resources for health, better aligning community interests with the health system’s work. By integrating QI into community health services, policy makers hope to ensure the quality of the services delivered is being measured and improved (where required), leading to increased demand-side confidence in and utilisation of these services. As a first step towards assessing whether QI for CTC healthcare services is affordable, we have provided a detailed breakdown of the costs of community-level QI. Further research is needed to assess whether this type of intervention can achieve the level of benefits required to justify this investment, as decision makers work towards the domestic and global goals of universal access to high-quality healthcare services.
Acknowledgments
The authors thank Robinson Karuga, LVCT Health, Rosalind McCollum, finance officers in all participating institutions and the REACHOUT family.
Footnotes
Handling editor: Seye Abimbola
Twitter: @kumeghan
Contributors: Study design and measures: MBK, JJM, MT. Data collection, analysis, interpretation of results: MBK, AZK, SN, RL, MMA, KRC. Drafting of manuscript: MBK, JJM, EB, MT. Comments and revision of manuscript: all authors.
Funding: The study presented in this paper is part of the REACHOUT programme. This programme has received funding from the European Union Seventh Framework Programme ([FP7/2007-2013] [FP7/2007-2011]) under grant agreement number 306090. This publication reflects only the authors’ views, and the European Union is not liable for any use that may be made of the information contained herein. EB is funded by a Trust Core Grant awarded to the KEMRI-Wellcome Trust Research Program (#092654)
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: Institutional review was conducted and approval obtained from the Liverpool School of Tropical Medicine under protocol 14.007.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available upon reasonable request.
References
- 1.Olaniran A, Smith H, Unkels R, et al. . Who is a community health worker? – a systematic review of definitions. Glob Health Action 2017;10 10.1080/16549716.2017.1272223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider H, Okello D, Lehmann U. The global pendulum swing towards community health workers in low- and middle-income countries: a scoping review of trends, geographical distribution and programmatic orientations, 2005 to 2014. Hum Resour Health 2016;14 10.1186/s12960-016-0163-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider H, Lehmann U, et al. . From community health workers to community health systems: time to widen the horizon? Health Systems & Reform 2016;2:112–8. 10.1080/23288604.2016.1166307 [DOI] [PubMed] [Google Scholar]
- 4.Perry HB, Zulliger R, Rogers MM. Community health workers in low-, middle-, and high-income countries: an overview of their history, recent evolution, and current effectiveness. Annu Rev Public Health 2014;35:399–421. 10.1146/annurev-publhealth-032013-182354 [DOI] [PubMed] [Google Scholar]
- 5.Kok MC, Dieleman M, Taegtmeyer M, et al. . Which intervention design factors influence performance of community health workers in low- and middle-income countries? A systematic review. Health Policy Plan. 2015;30:1207–27. 10.1093/heapol/czu126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Conference on Primary Health Care Declaration of Alma-Ata. Available: http://www.who.int/publications/almaata_declaration_en.pdf [Accessed 5 Jun 2017].
- 7.Lewin S, Munabi-Babigumira S, Glenton C, et al. . Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst Rev. 2010;94 10.1002/14651858.CD004015.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kok MC, Kane SS, Tulloch O, et al. . How does context influence performance of community health workers in low- and middle-income countries? Evidence from the literature. Heal Res Policy Syst 2015;13 10.1186/s12961-015-0001-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kok MC, Broerse JEW, Theobald S, et al. . Performance of community health workers : situating their intermediary position within complex adaptive health systems. Hum Resour Health 2017:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kok MC, Kea AZ, Datiko DG, et al. . A qualitative assessment of health extension workers’ relationships with the community and health sector in Ethiopia: opportunities for enhancing maternal health performance. Hum Resour Health 2015;13 10.1186/s12960-015-0077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilmore B, McAuliffe E. Effectiveness of community health workers delivering preventive interventions for maternal and child health in low- and middle-income countries: a systematic review. BMC Public Health 2013;13 10.1186/1471-2458-13-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cometto G, Ford N, Pfaffman-Zambruni J, et al. . Health policy and system support to optimise community health worker programmes: an abridged who guideline. Lancet Glob Health 2018;6:e1397–404. 10.1016/S2214-109X(18)30482-0 [DOI] [PubMed] [Google Scholar]
- 13.Kruk ME, Gage AD, Arsenault C, et al. . The Lancet global health Commission high-quality health systems in the sustainable development goals era: time for a revolution 2018:1–57. [DOI] [PMC free article] [PubMed]
- 14.World Health Organization WHO guidelines on health policy and system support to optimize community health worker programmes, 2018. Available: http://apps.who.int/iris/bitstream/handle/10665/275474/9789241550369-eng.pdf?ua=1 [Accessed 29 Oct 2018]. [PubMed]
- 15.WHO, OECD, The World Bank Delivering quality health services: a global imperative for universal health coverage, 2018. Available: http://apps.who.int/bookorders [Accessed 6 Sep 2018].
- 16.Nyirenda L, Namakhoma I, Chikaphupha K. Report on the context analysis of close-to-community providers in Malawi. Reach Trust, 2014. [Google Scholar]
- 17.Kumar MB. Access to healthcare through community health workers in East and Southern Africa, 2014. [Google Scholar]
- 18.McPake B, Edoka I, Witter S, et al. . Cost-effectiveness of community-based practitioner programmes in Ethiopia, Indonesia and Kenya. Bull World Health Organ 2015;93:631–9. 10.2471/BLT.14.144899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaughan K, Kok MC, Witter S, et al. . Costs and cost-effectiveness of community health workers: evidence from a literature review. Hum Resour Health 2015;13 10.1186/s12960-015-0070-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lilford RJ, Chilton PJ, Hemming K, et al. . Evaluating policy and service interventions: framework to guide selection and interpretation of study end points. BMJ 2010;341:c4413 10.1136/bmj.c4413 [DOI] [PubMed] [Google Scholar]
- 21.Goals SD. The cost-effectiveness of close-to-community health programmes: What do we know and where are the gaps ? Hum Resour Health 2015;13. [Google Scholar]
- 22.Nkonki L, Tugendhaft A, Hofman K. A systematic review of economic evaluations of CHW interventions aimed at improving child health outcomes. Hum Resour Health 2017;15:1–19. 10.1186/s12960-017-0192-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otieno CF, Kaseje D, Ochieng’ BM, et al. . Reliability of community health worker collected data for planning and policy in a peri-urban area of Kisumu, Kenya. J Community Health 2012;37:48–53. 10.1007/s10900-011-9414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Admon AJ, Bazile J, Makungwa H, et al. . Assessing and improving data quality from community health workers: a successful intervention in Neno, Malawi. Public Heal action 2013;3:56–9. 10.5588/pha.12.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yourkavitch J, Zalisk K, Prosnitz D, et al. . How do we know? An assessment of integrated community case management data quality in four districts of Malawi. Health Policy Plan 2016;31:1162–71. 10.1093/heapol/czw047 [DOI] [PubMed] [Google Scholar]
- 26.Rambu Ngana F, Myers BA, Belton S. Health reporting system in two subdistricts in eastern Indonesia: highlighting the role of village midwives. Midwifery 2012;28:809–15. 10.1016/j.midw.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 27.Taylor C, Griffiths F, Lilford R. Affordability of comprehensive community health worker programmes in rural sub-Saharan Africa. BMJ Glob Health 2017;2:e000391 10.1136/bmjgh-2017-000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strengthening primary health care through CHWs: investment case and financing recommendations, 2015. Available: http://www.healthenvoy.org/wp-content/uploads/2015/07/CHW-Financing-FINAL-July-15-2015.pdf [Accessed 13 Jun 2017].
- 29.McCollum R, Gomez W, Theobald S, et al. . How equitable are community health worker programmes and which programme features influence equity of community health worker services? A systematic review. BMC Public Health 2016;16 10.1186/s12889-016-3043-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Twum-Danso NAY, Akanlu GB, Osafo E, et al. . A nationwide quality improvement project to accelerate Ghana's progress toward Millennium Development goal four: design and implementation progress. Int J Qual Heal Care 2012;24:601–11. 10.1093/intqhc/mzs060 [DOI] [PubMed] [Google Scholar]
- 31.Horwood CM, Youngleson MS, Moses E, et al. . Using adapted quality-improvement approaches to strengthen community-based health systems and improve care in high HIV-burden sub-Saharan African countries. AIDS 2015;29(Suppl 2):S155–S164. 10.1097/QAD.0000000000000716 [DOI] [PubMed] [Google Scholar]
- 32.Horwood C, Butler L, Barker P, et al. . A continuous quality improvement intervention to improve the effectiveness of community health workers providing care to mothers and children: a cluster randomised controlled trial in South Africa. Hum Resour Health 2017;15:1–11. 10.1186/s12960-017-0210-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tancred T, Mandu R, Hanson C, et al. . How people-centred health systems can reach the grassroots : experiences implementing community-level quality improvement in rural Tanzania and Uganda. Health Policy Plan 2014:1–13. [DOI] [PubMed] [Google Scholar]
- 34.Tancred T, Manzi F, Schellenberg J, et al. . Facilitators and barriers of community-level quality improvement for maternal and newborn health in Tanzania. Qual Health Res 2017;27:738–49. 10.1177/1049732316638831 [DOI] [PubMed] [Google Scholar]
- 35.Kruk ME, Larson E, Twum-Danso NAY. Time for a quality revolution in global health. Lancet Glob Health 2016;4:e594–6. 10.1016/S2214-109X(16)30131-0 [DOI] [PubMed] [Google Scholar]
- 36.Heiby J. The use of modern quality improvement approaches to strengthen African health systems: a 5-year agenda. Int J Qual Heal Care 2014;26:117–23. 10.1093/intqhc/mzt093 [DOI] [PubMed] [Google Scholar]
- 37.REACHOUT REACHOUT Consortium. Available: http://www.reachoutconsortium.org/ [Accessed 5 Jun 2017].
- 38.Berwick DM. The science of improvement. JAMA 2008;299 10.1001/jama.299.10.1182 [DOI] [PubMed] [Google Scholar]
- 39.Berwick DM. The question of improvement. JAMA 2012;307:2093–4. 10.1001/jama.2012.4146 [DOI] [PubMed] [Google Scholar]
- 40.Ramaswamy R, Reed J, Livesley N, et al. . Unpacking the black box of improvement. Int J Qual Heal Care 2018;30(suppl_1):15–19. 10.1093/intqhc/mzy009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varkey P, Reller MK, Resar RK. Basics of quality improvement in health care. Mayo Clin Proc 2007;82:735–9. 10.1016/S0025-6196(11)61194-4 [DOI] [PubMed] [Google Scholar]
- 42.Quality improvement made simple: what everyone should know about health Care quality improvement, 2013. Available: https://www.health.org.uk/sites/health/files/QualityImprovementMadeSimple.pdf [Accessed 19 Jun 2018].
- 43.Kok MC, Ormel H, Broerse JEW, et al. . Optimising the benefits of community health workers’ unique position between communities and the health sector: A comparative analysis of factors shaping relationships in four countries. Glob Public Health 2017;12:1404–32. 10.1080/17441692.2016.1174722 [DOI] [PubMed] [Google Scholar]
- 44.Russell LB, et al. The role of cost-effectiveness analysis in health and medicine. JAMA 1996;276 10.1001/jama.1996.03540140060028 [DOI] [PubMed] [Google Scholar]
- 45.Byford S, Raftery J. Perspectives in economic evaluation. BMJ 1998;316:1529–30. 10.1136/bmj.316.7143.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans DB, Edejer TT-T, Adam T, et al. . Methods to assess the costs and health effects of interventions for improving health in developing countries. BMJ 2005;331:1137–40. 10.1136/bmj.331.7525.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Government of the Republic of Malawi, Ministry of Health National community health strategy 2017-2022, 2017. Available: http://www.chwcentral.org/sites/default/files/National_Community_Health_Strategy_2017-2022%2BFINAL.pdf
- 48.Ministério da Saúde, Direcção Nacional deRecursos Humanos Plano Nacional de Desenvolvimento dos Recursos Humanos da Saúde, 2008. Available: http://www.misau.gov.mz/index.php/planos-estrategicos-orhs?download=110:plano-rh-portugues
- 49.Bowser D, Okunogbe A, Oliveras E, et al. . A cost-effectiveness analysis of community health workers in Mozambique. J Prim Care Community Health 2015;6:227–32. 10.1177/2150131915579653 [DOI] [PubMed] [Google Scholar]
- 50.Government of Mozambique M of E and F Salary table for state employees, 2018. [Google Scholar]
- 51.Davey S, Wilhelmsen S, Newbarnder W, et al. . Modelling the Cost of Community Health Services in Malawi : the Results of Piloting a New Planning and Costing Tool, 2016. [Google Scholar]
- 52.Government of Republic of Indonesia Perubahan ketujuh belas atas peraturan pemerintah nomor 7 tahun 1977 tentang peraturan gaji pegawai negeri sipil, 2015. Available: http://keuangan.unej.ac.id/wp-content/uploads/2015/06/PP-30-2015-Kenaikan-Gaji-PNS-2015-.pdf [Accessed 6 Nov 2018].
- 53.XE Currency Converter Live rates. Available: http://www.xe.com/currencyconverter/ [Accessed 15 Nov 2017].
- 54.World Health Organization Global Health expenditure database. Available: http://apps.who.int/nha/database/Select/Indicators/en [Accessed 5 Jun 2018].
- 55.OECD Inflation (CpI) (indicator). Available: https://www.oecd-ilibrary.org/economics/inflation-cpi/indicator/english_eee82e6e-en
- 56.Bärnighausen T, Bloom DE, Cafiero-Fonseca ET, et al. . Valuing vaccination. Proceedings of the National Academy of Sciences 2014;111:12313–9. 10.1073/pnas.1400475111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dahn B, Woldemariam AT, Perry H, et al. . Strengthening Primary Health Care through Community Health Workers : Investment Case and Financing Recommendations, 2015: 1–66. [Google Scholar]
- 58.Greenhalgh T, Robert G, Macfarlane F, et al. . Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004;82:581–629. 10.1111/j.0887-378X.2004.00325.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vassall A, Sweeney S, Kahn J, et al. . Reference case for estimating the costs of global health services and interventions, 2017. Available: https://ghcosting.org/pages/standards/reference_case [Accessed 8 Oct 2017].
- 60.Bilinski A, Neumann P, Cohen J, et al. . When cost-effective interventions are unaffordable: integrating cost-effectiveness and budget impact in priority setting for global health programs. PLoS Med 2017;14:e1002397 10.1371/journal.pmed.1002397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waiswa P, Manzi F, Mbaruku G, et al. . Effects of the EQUIP quasi-experimental study testing a collaborative quality improvement approach for maternal and newborn health care in Tanzania and Uganda. Implementation Sci 2017;12:1–18. 10.1186/s13012-017-0604-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lunsford SS, Fatta K, Stover KE, et al. . Supporting close-to-community providers through a community health system approach: case examples from Ethiopia and Tanzania. Hum Resour Health 2015;13 10.1186/s12960-015-0006-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stover KE, Tesfaye S, Frew AH, et al. . Building district-level capacity for continuous improvement in maternal and newborn health. J Midwifery Women’s Heal 2014;59:S91–100. 10.1111/jmwh.12164 [DOI] [PubMed] [Google Scholar]
- 64.Sibley LM, Tesfaye S, Fekadu Desta B, et al. . Improving maternal and newborn health care delivery in rural Amhara and Oromiya regions of Ethiopia through the maternal and newborn health in Ethiopia partnership. J Midwifery Women’s Heal 2014;59:S6–S20. 10.1111/jmwh.12147 [DOI] [PubMed] [Google Scholar]
- 65.Twum-danso NAY, Dasoberi IN, Amenga-etego IA, et al. . Using quality improvement methods to test and scale up a new national policy on early post-natal care in Ghana. Health Policy Plan 2013:1–11. [DOI] [PubMed] [Google Scholar]
- 66.Chikaphupha KR, Kok MC, Nyirenda L, et al. . Motivation of health surveillance assistants in Malawi: a qualitative study. Malawi Med J 2016;28 10.4314/mmj.v28i2.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ndima SD, Sidat M, Give C, et al. . Supervision of community health workers in Mozambique: a qualitative study of factors influencing motivation and programme implementation. Hum Resour Health 2015;13 10.1186/s12960-015-0063-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore GF, Audrey S, Barker M, et al. . Process evaluation of complex interventions: medical Research Council guidance. BMJ 2015;350 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown C, Lilford R. Evaluating service delivery interventions to enhance patient safety. BMJ 2008;337:a2764 10.1136/bmj.a2764 [DOI] [PubMed] [Google Scholar]
- 70.Renmans D, Holvoet N, Criel B. Combining Theory-Driven Evaluation and Causal Loop Diagramming for Opening the 'Black Box' of an Intervention in the Health Sector: A Case of Performance-Based Financing in Western Uganda. Int J Environ Res Public Health 2017;14 10.3390/ijerph14091007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenhalgh T, Papoutsi C. Studying complexity in health services research: desperately seeking an overdue paradigm shift. BMC Med 2018;16 10.1186/s12916-018-1089-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell NC, Murray E, Darbyshire J, et al. . Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455–9. 10.1136/bmj.39108.379965.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Black RE, Taylor CE, Arole S, et al. . Comprehensive review of the evidence regarding the effectiveness of community–based primary health care in improving maternal, neonatal and child health: 8. summary and recommendations of the expert panel. J Glob Health 2017;7 10.7189/jogh.07.010908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCollum R, Theobald S, Otiso L, et al. . Priority setting for health in the context of devolution in Kenya: implications for health equity and community-based primary care. Health Policy Plan 2018:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Janabi A, Al-Wahdani B, Ammar W, et al. . Bellagio Declaration on high-quality health systems: from a quality moment to a quality movement. Lancet Glob Health 2018;6:e1144–5. 10.1016/S2214-109X(18)30372-3 [DOI] [PubMed] [Google Scholar]
- 76.Ghebreyesus TA, Adhanom Ghebreyesus T. How could health care be anything other than high quality? Lancet Glob Heal 2018;6:e1140–1. 10.1016/S2214-109X(18)30394-2 [DOI] [PubMed] [Google Scholar]
- 77.Long KM, McDermott F, Meadows GN. Being pragmatic about healthcare complexity: our experiences applying complexity theory and pragmatism to health services research. BMC Med 2018;16 10.1186/s12916-018-1087-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pérez D, Van der Stuyft P, Zabala MC, et al. . A modified theoretical framework to assess implementation fidelity of adaptive public health interventions. Implement Sci 2016;11 10.1186/s13012-016-0457-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mignogna J, Martin LA, Harik J, et al. . "I had to somehow still be flexible": exploring adaptations during implementation of brief cognitive behavioral therapy in primary care. Implement Sci 2018;13 10.1186/s13012-018-0768-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang H, Tesfaye R, Ramana GN, et al. . Ethiopia health extension program: an institutionalized community approach for universal health coverage. Washington, D.C: The World Bank, 2016. [Google Scholar]
- 81.Ministry of Health R of I Decree of the Ministry of health of the Republic of Indonesia number 1529/MENKES/SK/X/2010, 2004. [Google Scholar]
- 82.Ministry of Health R of I Midwifery situation in Indonesia, 2014. Available: http://www.depkes.go.id/resources/download/pusdatin/infodatin/infodatin-bidan.pdf
- 83.Government of Kenya M of H Strategy for community health 2014-2019, 2014. Available: http://guidelines.health.go.ke:8000/media/STRATEGY_FOR_COMMUNITY_HEALTH_2014-2019.pdf [Accessed 6 Nov 2018].
- 84.MISAU Programa de Revitalização DOS Agentes Polivalentes Elementares, 2010: 37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2019-001390supp001.pdf (101.4KB, pdf)
bmjgh-2019-001390supp002.pdf (47.4KB, pdf)
bmjgh-2019-001390supp003.pdf (55.4KB, pdf)
bmjgh-2019-001390supp004.pdf (45.8KB, pdf)
bmjgh-2019-001390supp005.pdf (43.3KB, pdf)
bmjgh-2019-001390supp006.pdf (40.2KB, pdf)
bmjgh-2019-001390supp007.pdf (175.4KB, pdf)