Summary
Lon, an ATP‐dependent protease in bacteria, influences diverse cellular processes by degrading damaged, misfolded and short‐lived regulatory proteins. In this study, we characterized the effects of lon mutation and determined the molecular mechanisms underlying Lon‐mediated virulence regulation in Erwinia amylovora, an enterobacterial pathogen of apple. Erwinia amylovora depends on the type III secretion system (T3SS) and the exopolysaccharide (EPS) amylovoran to cause disease. Our results showed that mutation of the lon gene led to the overproduction of amylovoran, increased T3SS gene expression and the non‐motile phenotype. Western blot analyses showed that mutation in lon directly affected the accumulation and stability of HrpS/HrpA and RcsA. Mutation in lon also indirectly influenced the expression of flhD, hrpS and csrB through the accumulation of the RcsA/RcsB proteins, which bind to the promoter of these genes. In addition, lon expression is under the control of CsrA, possibly at both the transcriptional and post‐transcriptional levels. Although mutation in csrA abolished both T3SS and amylovoran production, deletion of the lon gene in the csrA mutant only rescued amylovoran production, but not T3SS. These results suggest that CsrA might positively control both T3SS and amylovoran production partly by suppressing Lon, whereas CsrA may also play a critical role in T3SS by affecting unknown targets.
Keywords: CsrA/RsmA, csrB/rsmB, GacS/GacA, HrpS/HrpR, post‐translational regulation, RcsA/RcsB, T3SS
Introduction
Erwinia amylovora, an enterobacterial plant pathogen, causes fire blight disease of apples and pears in more than 50 countries around the world. The type III secretion system (T3SS) and the exopolysaccharide (EPS) amylovoran are two major pathogenicity factors of the pathogen (Khan et al., 2012; Zhao, 2014). The T3SS in E. amylovora is encoded by the hypersensitive response and pathogenicity (hrp) island. It has been demonstrated that expression of the hrp‐T3SS genes is activated by the master regulator HrpL, a member of the exocytoplasmic functions (ECF) subfamily of sigma factors (McNally et al., 2012; Wei and Beer, 1995). In turn, hrpL transcription is positively regulated by alternative sigma factor 54 (RpoN), its modulation protein YhbH, bacterial enhancer‐binding protein (bEBP) HrpS and integration host factor IHF (Ancona et al., 2014; Lee and Zhao, 2016). HrpS, a member of the NtrC family, activates RpoN‐dependent transcription by mediating the isomerization of the RpoN–RNA polymerase (RNAP) complex, whereas the nucleoid‐associated protein IHF enables the interaction between HrpS and RpoN (Bush and Dixon, 2012; Lee and Zhao, 2016; Lee et al., 2016). Moreover, the RpoN–HrpL alternative sigma factor cascade is further activated by linear nucleotide second messengers (p)ppGpp, which are also essential for T3SS gene expression and virulence under nutrient stress conditions (Ancona et al., 2015b).
In addition, amylovoran plays an important role in virulence, biofilm formation and survival of the bacterium (Koczan et al., 2009; Sjulin and Beer, 1978). Genome‐wide screening of two‐component systems (TCSs) identified major regulators of amylovoran production in E. amylovora (Zhao et al., 2009a). Among them, the enterobacterial‐specific Rcs phosphorelay system is essential for pathogenicity and amylovoran production (Ancona et al., 2015a; Bereswill and Geider, 1997; Bernhard et al., 1990; Wang et al., 2009, 2012). The Rcs system is an unusual complex TCS, comprising three core proteins RcsBCD and one auxiliary protein RcsA without the phosphorylation site (Bernhard et al., 1990; Gottesman et al., 1985; Majdalani and Gottesman, 2005; Wehland et al., 1999). The RcsB homodimer or RcsA/RcsB heterodimer binds to the conserved RcsAB box to regulate gene expression, including amsG, the first gene of the amylovoran biosynthetic operon, and flhD in E. amylovora (Ancona et al., 2015a; Bernhard et al., 1993; Wehland et al., 1999). Furthermore, the GacS/GacA (GrrS/GrrA and BarA/UvrY) system, a widely distributed TCS in Gammaproteobacteria, negatively regulates amylovoran biosynthesis and T3SS in E. amylovora (Li et al., 2014). It has been shown recently that negative regulation of virulence by GacS/GacA in E. amylovora acts through the non‐coding small regulatory RNA (sRNA) csrB, which binds to and neutralizes the positive effect of the RNA‐binding protein CsrA on T3SS gene expression and amylovoran production, indicating a critical role of CsrA in E. amylovora virulence (Ancona et al., 2016). However, the targets of CsrA remain unknown in E. amylovora.
Lon is a highly conserved cytosolic protease belonging to the AAA+ superfamily of ATPase, and acts as a major player in general protein quality control by degrading damaged or misfolded proteins (Chung and Goldberg, 1981). The proteolytic activity of Lon also contributes to the post‐translational regulation of functional proteins. To recognize potentially deleterious proteins, Lon tends to bind a cluster of aromatic and non‐polar residues, which are embedded in most native proteins (Gur and Sauer, 2008). As one well‐characterized Lon substrate, the RcsA protein level is generally maintained low by HN‐S‐mediated transcriptional repression and Lon‐dependent degradation (Sledjeski and Gottesman, 1995; Torres‐Cabassa and Gottesman, 1987). Increased stability of RcsA and its associated overproduction of EPS in the lon mutant of some enterobacterial species lead to mucoid colonies (Gottesman et al., 1985; Lai et al., 2003). Lon also controls the SulA protein level, which inhibits cell division as part of the SOS response under DNA damage‐inducing conditions (Huisman and D'Ari, 1981). Failure to remove accumulated SulA in the lon mutant strain blocks cell division, leading to the irradiation sensitivity phenotype (Gottesman et al., 1981; Mizusawa and Gottesman, 1983).
Furthermore, Lon has been implicated in the regulation of the T3SS in several important Gram‐negative bacteria (Bretz et al., 2002; Jackson et al., 2004; Takaya et al., 2005). In Yersinia pestis, Lon positively regulates the T3SS by degrading YmoA, a small histone‐like protein that suppresses T3SS gene expression (Jackson et al., 2004). In Pseudomonas syringae, Lon acts as a negative regulator of the T3SS by degrading HrpR and effector proteins. HrpR, a homologue of HrpS, forms a heterohexamer with HrpS and is maintained low by Lon under non‐inductive conditions (Bretz et al., 2002). Once HrpL‐dependent T3SS gene expression is activated, Lon affects the stability of effector proteins, thereby modulating the secretion rate (Losada and Hutcheson, 2005). In E. amylovora, Lon is involved in EPS regulation and UV tolerance, but is not required for the infection of apple seedlings (Eastgate et al., 1995). However, the effect of lon mutation on E. amylovora virulence and its underlying molecular mechanisms has not been fully characterized.
The purpose of this study was to characterize the effect of lon mutation in E. amylovora, to identify potential targets of Lon and to determine the molecular mechanisms underlying Lon‐mediated virulence regulation. Our results showed that mutation of the lon gene led to amylovoran overproduction, increased T3SS gene expression and the non‐motile phenotype by direct targeting of RcsA and HrpS/HrpA, and indirectly by affecting the expression of the flhD, hrpS and csrB sRNA genes through the accumulation of the RcsA/RcsB proteins. Moreover, mutation of the csrA gene led to the up‐regulation of lon expression, suggesting that positive regulation of T3SS and amylovoran production by CsrA could occur partly through suppression of lon expression.
Results
Characterization of the Ea1189 lon mutant in E. amylovora
It has been reported previously that E. amylovora Lon is involved in EPS production, but is not required for the infection of apple seedlings (Eastgate et al., 1995). In this study, we generated an insertional mutant strain with a defect in the lon gene in the background of the wild‐type (WT) strain Ea1189 (Table 1). Consistent with previous reports, the Ea1189 lon mutant exhibited a mucoid colony on growth medium (Fig. S1A, see Supporting Information) and produced about 10 times more amylovoran than that of the WT strain, which could be partially complemented (Fig. 1A). Transcript levels of rcsA (a rate‐limiting regulatory gene) and amsG (the first gene in the ams operon for amylovoran biosynthesis) were about five‐ and 40‐fold higher, respectively, in the lon mutant (Fig. 1B). The Ea1189 lon mutant induced a typical hypersensitive response (HR) lesion on tobacco (data not shown) and was as pathogenic as WT on immature pears, although the disease progress was similar or slightly faster in the mutant at 4 days post‐inoculation (Fig. 1C). We also found that expression of the T3SS regulatory (hrpL) and effector (hrpA) genes was about six‐fold higher in the lon mutant relative to the WT (Fig. 1D). These results indicate that Lon suppresses the expression of the genes required for T3SS and amylovoran in E. amylovora, but is not indispensable for its virulence.
Table 1.
Bacterial strains and plasmids used in this study.
| Strains, plasmids | Description | Reference, source |
|---|---|---|
| Erwinia amylovora | ||
| Ea1189 | Wild‐type, isolated from apple | Wang et al. (2009) |
| Δlon | lon::Cm; CmR‐insertional mutant of lon of Ea1189 | This study |
| ΔrcsA | rcsA::Cm; CmR‐insertional mutant of rcsA of Ea1189 | Ancona et al. (2014) |
| ΔrcsB | rcsB::Km; KmR‐insertional mutant of rcsB of Ea1189 | Wang et al. (2009) |
| Δlon/rcsA | rcsA::Cm, lon::Km; KmR‐insertional mutant of lon of ΔrcsA | This study |
| Δlon/rcsB | rcsB::Km, lon::Cm; CmR‐insertional mutant of lon of ΔrcsB | This study |
| ΔcsrA | csrA::Cm; CmR‐insertional mutant of csrA of Ea1189 | Ancona et al. (2016) |
| ΔcsrB | csrB::Cm; CmR‐insertional mutant of csrB of Ea1189 | Ancona et al. (2016) |
| Δlon/csrA | csrA::Km, lon::Cm; KmR‐insertional mutant of csrA of Δlon | This study |
| Escherichia coli | ||
| DH10B | F– mcrA Δ(mrr‐hsdRMS‐mcrBC) Φ80lacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara leu) 7697 galU galK rpsL nupG λ– | Invitrogen (Carlsbad, CA, USA) |
| Plasmids | ||
| pKD46 | ApR, PBAD gam bet exo pSC101 oriTS | Datsenko and Wanner (2000) |
| pKD32 | CmR, FRT cat FRT tL3 oriR6Kγ bla rgnB | Datsenko and Wanner (2000) |
| pKD13 | KmR, FRT kan FRT tL3 oriR6Kγ bla rgnB | Datsenko and Wanner (2000) |
| pWSK29 | ApR, cloning vector, low copy number | Wang and Kushner (1991) |
| pLon | 3077‐bp DNA fragment containing promoter sequence of lon gene in pWSK29 | This study |
| pHrpS‐His6 | 1537‐bp DNA fragment containing promoter sequence of hrpS gene and C‐terminal His‐tag in pWSK29 | This study |
| pHrpA‐His6 | 803‐bp DNA fragment containing promoter sequence of hrpA gene and C‐terminal His‐tag in pWSK29 | Ancona et al. (2015b) |
| pRcsA‐His6 | 1058‐bp DNA fragment containing promoter sequence of rcsA gene and C‐terminal His‐tag in pWSK29 | This study |
| pRcsB‐His6 | 1142‐bp DNA fragment containing promoter sequence of rcsB gene and C‐terminal His‐tag in pWSK29 | This study |
| pLon‐His6 | 2877‐bp DNA fragment containing promoter sequence of lon gene and C‐terminal His‐tag in pWSK29 | This study |
Figure 1.
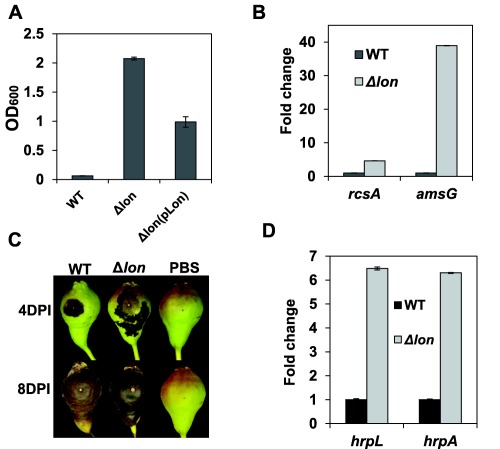
Characterization of the lon mutant. (A) Amylovoran production of the wild‐type (WT), the lon mutant and its complementation strain grown in MBMA medium for 24 h at 28 °C. (B) Relative gene expression of the rcsA and amsG genes in the lon mutant compared with the WT grown in MBMA medium for 18 h at 28 °C. (C) Disease symptoms caused by the WT and the lon mutant on immature pear fruits at 4 and 8 days post‐inoculation (DPI). (D) Relative gene expression of the hrpL and hrpA genes in the lon mutant compared with the WT grown in hrp‐inducing medium (HMM) for 6 h at 18 °C. The optical densities at 600 nm (OD600) (A) and the relative fold change (B, D) are the means of three replicates, and similar results were obtained from repeated experiments. A representative of three independent experiments is presented for the virulence assay (C). Error bars indicate standard deviation.
Lon negatively regulates the T3SS by targeting HrpS and HrpA
Previous studies in P. syringae have shown that Lon negatively regulates the T3SS by targeting HrpR and effector proteins, but not HrpS (Bretz et al., 2002; Losada and Hutcheson, 2005). Erwinia amylovora only contains HrpS, which shares about 40%–44% amino acid identity with HrpR and HrpS of P. syringae, respectively. We examined the abundance and stability of HrpS and HrpA proteins in the WT and lon mutant in hrp‐inducing medium (HMM) using Western blot. The lon mutant accumulated about three‐ and two‐fold more HrpS and HrpA protein, respectively, than that in the WT (Fig. 2A,B). The half‐life of HrpS in vivo was increased to more than 45 min in the lon mutant from less than 15 min in the WT (Fig. 2C), and the half‐life of HrpA was also increased from about 15 min to more than 45 min in the absence of Lon (Fig. 2D). These results indicate that HrpS and HrpA are directly targeted by Lon‐dependent degradation.
Figure 2.
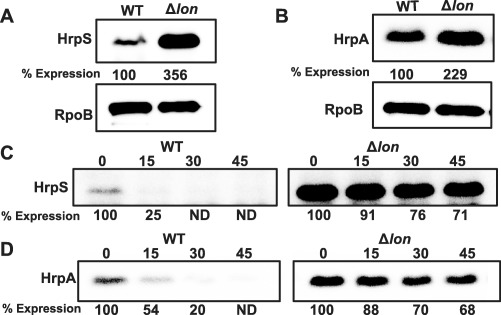
Lon‐dependent degradation of HrpS/HrpA regulates type III secretion system (T3SS) gene expression in Erwinia amylovora. Abundance of HrpS‐His6 (A) and HrpA‐His6 (B) proteins in the wild‐type (WT) and lon mutant strains grown in hrp‐inducing medium (HMM) for 6 h at 18 °C; the abundance of the RpoB protein was used as a loading control. Half‐life of HrpS‐His6 (C) and HrpA‐His6 (D) proteins in the WT and lon mutant strains. For protein stability test, cells were grown in HMM for 6 h at 18 °C. Translation was stopped by the addition of tetracycline, and samples were taken at the indicated time points (min) at the top. The relative protein abundance at the bottom of each lane was calculated using ImageJ software. Cropped gel blots and percentage expression values are representatives of three independent experiments. ND, not detected.
Lon negatively regulates amylovoran by targeting RcsA, but not RcsB
In enterobacteria, previous reports have shown that Lon negatively regulates EPS biosynthesis by the degradation of RcsA, an auxiliary protein of the Rcs system (Gottesman et al., 1985; Torres‐Cabassa and Gottesman, 1987). Therefore, we examined the abundance and stability of RcsA, as well as RcsB, in the WT and lon mutant grown in MBMA medium using Western blot. As expected, the RcsA protein was 2.4‐fold more abundant and exhibited a longer half‐life (>45 min) in the lon mutant (Fig. 3A,C), indicating that RcsA is also negatively regulated by Lon‐dependent degradation in E. amylovora. Interestingly, the RcsB protein was 1.6‐fold more abundant in the lon mutant, but RcsB protein stability was not significantly affected (Fig. 3B,D), suggesting that RcsB might not be directly targeted by Lon, but its expression might be subject to feedback regulation of the Rcs system.
Figure 3.
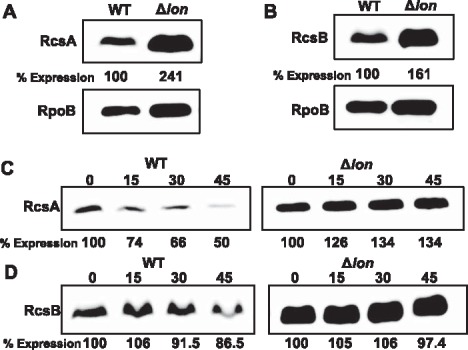
Lon‐dependent degradation of RcsA regulates amylovoran production in Erwinia amylovora. Abundance of RcsA‐His6 (A) and RcsB‐His6 (B) proteins in the wild‐type (WT) and lon mutant strains grown in MBMA medium for 24 h at 28 °C; the abundance of the RpoB protein was used as a loading control. Half‐life of RcsA‐His6 (C) and RcsB‐His6 (D) proteins in the WT and lon mutant strains. For protein stability test, cells were grown in MBMA medium for 24 h at 28 °C. Translation was stopped by the addition of tetracycline, and samples were taken at the indicated time points (min) at the top. Relative protein abundance at the bottom of each lane was calculated using ImageJ software. Cropped gel blots and percentage expression values are representatives of three independent experiments.
RcsA/RcsB accumulation suppresses motility and flhD transcription in the lon mutant
In addition, we found that the lon mutant was non‐motile (Fig. 4A). The diameter of the WT on the motility plate was 9 mm at 24 h and 29.6 mm at 48 h, whereas no circular movement was observed in the lon mutant, which remained at about 6 mm (Fig. 4B). Complementation of the lon mutant restored the motile phenotype (Fig. 4A), indicating that Lon is essential for motility in E. amylovora.
Figure 4.
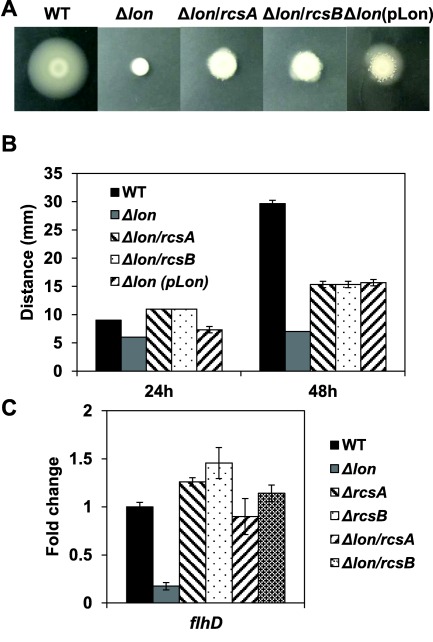
RcsA/RcsB accumulation suppresses motility and flhD transcription in the lon mutant. (A) Movement of the wild‐type (WT), lon, rcsA, rcsB, lon/rcsA and lon/rcsB mutants and the complementation strain of the lon mutant. Photographs were taken at 48 h post‐incubation on a motility agar plate. (B) The moving distance of the WT, lon, rcsA, rcsB, lon/rcsA and lon/rcsB mutants and the complementation strain of the lon mutant. The diameters of the circle around the inoculation site (mm) were measured at 24 and 48 h post‐inoculation. (C) Relative gene expression of flhD in the five mutant strains compared with the WT grown in MBMA medium for 18 h at 28 °C. The values of the relative fold change are the means of three replicates and the experiment was repeated three times with similar results. Error bars indicate standard deviation.
In order to identify suppressors of the lon mutant in controlling motility, we performed a transposon mutagenesis screening in the lon mutant background and obtained nine mutants with partially restored motility (Table 2). Among them, transposon insertion in the rcsA gene showed the strongest recovery in motility in the lon mutant (Table 2). In order to confirm this result, we constructed the lon/rcsA and lon/rcsB double deletion mutants. Interestingly, both the lon/rcsA and lon/rcsB double mutants partially recovered motility (Fig. 4B). Expression of the flhD gene, the master regulator of flagellar biosynthesis, was also recovered to the WT level in the lon/rcsA and lon/rcsB double mutants, but decreased five‐fold in the lon mutant (Fig. 4C). The transcript levels of the flhD gene increased slightly in the rcsA and rcsB single mutants, although they exhibited irregular and slightly decreased motility (Wang et al., 2009). These results indicate that the accumulation of RcsA/RcsB negatively regulates flhD transcription, and thus suppresses motility in the lon mutant. In addition, other regulators, such as the RNA chaperone Hfq and cholera toxin transcription activator YqeI, also contribute to the suppression of motility in the lon mutant, but the functional relevance in these situations was not determined in this study (Table 2).
Table 2.
Tn5 mutants with recovered motility in the lon mutant background.
| Accession | Gene | Description | Motility (24 h, mm) | Motility (48 h, mm) |
|---|---|---|---|---|
| EAM_0994 | lon | Lon protease | 6 | 7 |
| EAM_1482 | rcsA | Colanic acid capsular biosynthesis activation protein A | 11.7 ± 0.58 | 16.3 ± 0.58 |
| EAM_0427 | bspA | Mechanosensitive ion channel protein | 6.7 ± 0.58 | 11.7 ± 0.58 |
| EAM_0436 | hfq | RNA chaperone | 8.7 ± 0.58 | 14 |
| EAM_3422 | yqeI | Cholera toxin transcription activator | 7.3 ± 0.58 | 12.3 ± 0.58 |
| EAM_3014 | Hypothetical protein | 6 | 10.7 ± 0.58 | |
| EAM_2896 | hrpI | Type III secretion protein | 5 | 9.3 ± 0.58 |
| EAM_2178 | udk | Uridine kinase | 5 | 8 |
| EAM_0009 | Hypothetical protein | 5 | 8 | |
| EAM_0609 | Acetyltransferase | 5.8 ± 0.58 | 9 |
Expression of hrpS is transcriptionally activated by RcsA/RcsB
A previous microarray study has shown that RcsB is required for full T3SS gene expression, but the mechanism remains uncertain (Wang et al., 2012). Bioinformatic analyses of promoters found a potential RcsAB box (TAGGA‐N4‐TCTTA) located 350 bp upstream of the hrpS start site. Indeed, hrpS gene expression was down‐regulated in both rcsA and rcsB mutants in HMM, whereas it was up‐regulated by about two‐fold in the lon mutant (Fig. 5A). Deletion of either rcsA or rcsB in the lon mutant diminished this up‐regulation of hrpS gene expression in the lon mutant, suggesting that hrpS gene expression is transcriptionally activated by RcsA/RcsB. Binding of RcsA/RcsB to the hrpS upstream sequence was assessed by electrophoretic mobility shift assay (EMSA), as described previously (Ancona et al., 2015a). A distinct band shift of the hrpS DNA probe was observed with RcsB and RcsA/RcsB proteins, but not with the RcsA protein alone (Fig. 5B). Western blot analyses also showed that the abundance of RcsA and RcsB proteins was increased in the lon mutant grown in HMM when compared with the WT (Fig. 5C). These results indicate that the accumulation of RcsA/RcsB proteins leads to the up‐regulation of hrpS transcription in the lon mutant.
Figure 5.
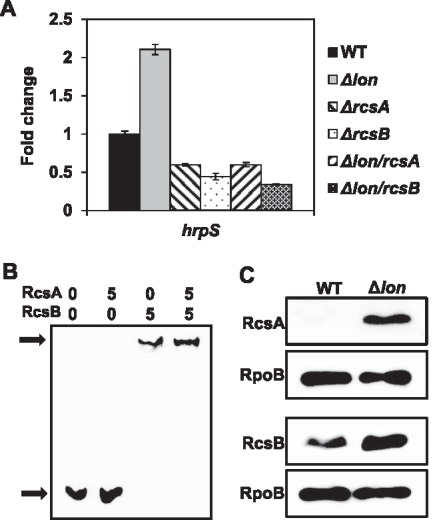
Lon‐dependent degradation of RcsA regulates the expression of hrpS in Erwinia amylovora. (A) Relative gene expression of the hrpS gene in the wild‐type (WT), lon, rcsA, rcsB, lon/rcsA and lon/rcsB mutants compared with the WT grown in hrp‐inducing medium (HMM) for 6 h at 18 °C. The values of the relative fold change are the means of three replicates and the experiment was repeated three times with similar results. Error bars indicate standard deviation. (B) Electrophoretic mobility shift assay for a 58‐bp fragment of the hrpS upstream region and RcsA/RcsB proteins. Black arrows at the bottom and top indicate the free probe and the protein–DNA complex, respectively. The concentration of RcsA and RcsB (pmol) is indicated above each lane. (C) Abundance of RcsA‐His6 and RcsB‐His6 proteins in the lon mutant strains compared with the WT grown in HMM for 6 h at 18 °C. The abundance of the RpoB protein was used as a loading control. Cropped gel blots are representatives of three independent experiments.
Expression of the csrB sRNA is suppressed by RcsA/RcsB accumulation in the lon mutant
The virulence gene expression of the lon mutant observed in this study was very similar to that reported for the gacS/gacA and csrB mutants in E. amylovora (Ancona et al., 2016; Li et al., 2014). We hypothesized that a connection between Lon and the GacS/GacA‐Csr regulatory system exists. It has also been proposed that the Rcs system negatively regulates the expression of rsmB, a homologue of csrB sRNA, possibly by direct binding to its upstream sequence in Pectobacterium (Andresen et al., 2010). Bioinformatic analysis of the csrB gene indeed found a potential RcsA/RcsB box (TACGA‐N4‐TCTTA), which is located 172 bp upstream of the start site and is close to the GacA‐binding site (Fig. 6A) (Lee and Zhao, 2016). A shifted band of the csrB DNA probe was observed with RcsB and RcsA/RcsB proteins, but not with RcsA alone (Fig. 6B). Transcript levels of csrB decreased about five‐fold in the lon mutant, but increased 1.5‐fold in the rcsA and rcsB mutants in MBMA medium (Fig. 6C). Deletion of the rcsA/rcsB gene in the lon mutant restored csrB expression to the WT level (Fig. 6C). However, csrA transcript levels were slightly increased in the five mutants tested relative to the WT (Fig. 6C). Similar expression patterns for both csrA and csrB were observed in these mutants grown in HMM (Fig. S1B). These results indicate that RcsA/RcsB accumulation in the lon mutant leads to the suppression of csrB transcription.
Figure 6.
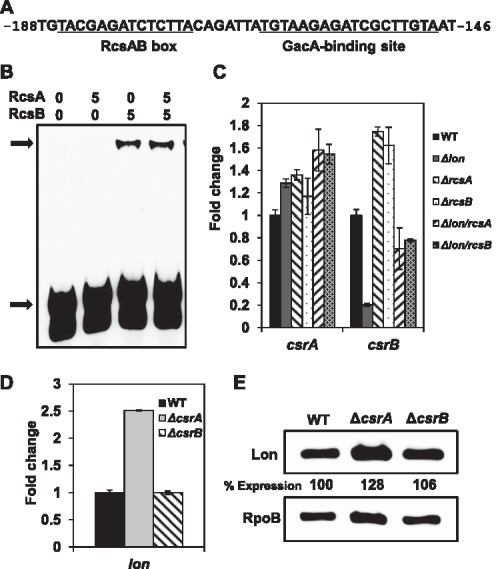
RcsA/RcsB accumulation suppresses csrB sRNA expression and the effect of csrA mutation on the lon gene. (A) The consensus RcsA/RcsB box and GacA‐binding site on the csrB upstream region. Numbers refer to the nucleotide position relative to the start site of the csrB gene. (B) Electrophoretic mobility shift assay for a 58‐bp fragment of the csrB upstream region and RcsA/RcsB proteins. Black arrows at the bottom and top indicate the free probe and the protein–DNA complex, respectively. The concentration of RcsA and RcsB (pmol) is indicated above each lane. (C) Relative gene expression of csrA and csrB in the lon, rcsA, rcsB, lon/rcsA and lon/rcsB mutants compared with the wild‐type (WT) grown in MBMA medium for 18 h at 28 °C. (D) Relative expression of lon in the csrA and csrB mutants compared with the WT grown in MBMA medium for 18 h at 28 °C. The values of the relative fold change are the means of three replicates and the experiment was repeated twice with similar results. Error bars indicate standard deviation. (E) Abundance of Lon‐His6 protein in the csrA and csrB mutant strains compared with the WT grown in MBMA for 18 h at 28 °C. The abundance of the RpoB protein was used as a loading control. Relative protein abundance at the bottom of each lane was calculated using ImageJ software. Cropped gel blots and percentage expression values are representatives of three independent experiments.
Transcription of lon is suppressed by CsrA
Given that mutations in lon and csrA caused opposite virulence gene expression patterns in E. amylovora (Ancona et al., 2016), we further hypothesized that increased Lon activity might contribute to the decrease in T3SS and amylovoran production observed in the csrA mutant. Transcript levels of lon in the csrA mutant were about 2.5‐fold up‐regulated in both MBMA and HMM growth conditions, whereas no significant changes were observed in the csrB mutant (Figs 6D and S1C). However, Western blot analyses showed slightly increased Lon protein levels in the csrA mutant, but not in the csrB mutant (Figs 6E and S1D). These results suggest that CsrA might mainly regulate the expression of lon at the transcriptional level, but the possibility that CsrA post‐transcriptionally affects lon translation could not be excluded.
To determine the effects of the increased Lon levels on the csrA mutant, we generated a lon/csrA double deletion mutant. The lon/csrA mutant still failed to cause disease on immature pears (Fig. 7A), but exhibited significantly increased amylovoran production (Fig. 7B). Western blot analyses revealed that the lon/csrA mutant showed slightly increased HrpS protein expression when compared with the csrA mutant, but HrpA proteins were barely detected in both mutants (Fig. 7C,D). These results suggest that CsrA might positively control both T3SS and amylovoran production partly by suppressing Lon, whereas CsrA may also play a critical role in T3SS by affecting unknown targets.
Figure 7.
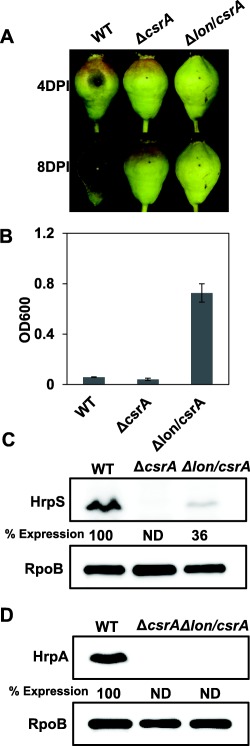
Effect of lon mutation in the csrA mutant. (A) Disease symptoms caused by the wild‐type (WT), csrA and lon/csrA mutant strains on immature pear fruits at 4 and 8 days post‐inoculation (DPI). (B) Amylovoran production of the WT, csrA and lon/csrA mutant strains grown in MBMA medium for 24 h at 28 °C. The optical densities at 600 nm (OD600) are the means of three replicates and the experiment was repeated three times with similar results. Error bars indicate standard deviation. (C) Abundance of the HrpS‐His6 protein in the csrA and lon/csrA mutant strains compared with the WT grown in hrp‐inducing medium (HMM) for 6 h at 18 °C. (D) Abundance of the HrpA‐His6 protein in the csrA and lon/csrA mutant strains compared with the WT grown in HMM for 6 h at 18 °C. The abundance of the RpoB protein was used as a loading control. The relative protein abundance at the bottom of each lane was calculated using ImageJ software. Cropped gel blots and percentage expression values are representatives of three independent experiments. ND, not detected.
Discussion
In bacteria, the abundance and quality of functional proteins are constantly monitored to meet the physiological needs of the cell by robust and highly selective post‐translational degradation and modification. As one of the major ATP‐dependent proteases in bacteria, Lon has been found to influence diverse cellular processes. In this study, we not only corroborated that Lon directly degrades RcsA, but also demonstrated that HrpA and HrpS, an activator of RpoN‐dependent transcription of the T3SS, are direct targets of Lon in E. amylovora. We further provided evidence that the accumulation of RcsA/RcsB proteins in the lon mutant represses motility by inhibiting flhD expression, and promotes EPS production and T3SS gene expression by suppressing csrB sRNA expression and activating hrpS expression. Moreover, we documented that the expression of lon is under the control of CsrA, possibly at both the transcriptional and post‐transcriptional levels. These results are novel and further suggest that CsrA contributes to the activation of both T3SS and amylovoran production partly by suppressing Lon.
The complex enterobacterial‐specific Rcs system was originally identified as a primary activator of EPS biosynthesis in Escherichia coli (Gottesman et al., 1985; Majdalani and Gottesman, 2005). It is also well established that Lon negatively regulates the Rcs system by targeting RcsA, and thus inhibits EPS overproduction (Torres‐Cabassa and Gottesman, 1987). We confirmed that Lon also directly degrades RcsA in E. amylovora, the only plant pathogen known to require a functional Rcs system for its pathogenesis (Ancona et al., 2015a; Wang et al., 2009, 2012; Zhao et al., 2009a). Here, we also provided novel insights into the role of the Rcs system in the regulation of E. amylovora virulence through the characterization of the lon mutant, which accumulates higher levels of RcsA/RcsB proteins.
Previous studies have reported that the Rcs system also negatively regulates motility through transcriptional repression of the flagellum master regulator flhDC (Francez‐Charlot et al., 2003; Wang et al., 2007). In E. coli, the rcsB mutant is hypermotile, whereas the rcsA mutant exhibits a WT level of motility (Francez‐Charlot et al., 2003; Fredericks et al., 2006). RcsA has been shown to affect E. coli motility when expressed at high levels (Fredericks et al., 2006). The rcsB mutant of Proteus mirabilis also shows increased flhDC expression and motility (Clemmer and Rather, 2007). However, in Salmonella, RcsA is not involved in the regulation of flhDC expression, and thus mutation in lon has no effect on flagellum formation and motility (Takaya et al., 2002; Wang et al., 2007). In contrast, the rcsB mutant of E. amylovora is less motile than the WT despite increased flhDC promoter activity (Wang et al., 2009; Zhao et al., 2009a). Over‐expression of RcsBD56E, a phosphorylation mimic variant, significantly reduces motility in E. amylovora (Ancona et al., 2015a). This is consistent with our current observations that the accumulation of RcsA/RcsB in the lon mutant leads to decreased flhD expression and motility, suggesting that the Rcs system in E. amylovora indeed acts as a negative regulator of motility, and this is dependent on its expression level.
HrpS in E. amylovora and HrpR/HrpS in P. syringae are bEBPs, critical for the activation of RpoN‐dependent hrpL gene expression (Hutcheson et al., 2001; Lee et al., 2016; Wei et al., 2000). In general, bEBP is regulated through its N‐terminal regulatory domain that interacts with various signal transduction intermediates, including phosphoryl groups, ligands and anti‐activator proteins (Bush and Dixon, 2012). However, HrpR and HrpS in P. syringae and HrpS in E. amylovora lack this regulatory domain. In P. syringae, HrpR is subject to Lon‐dependent degradation, whereas HrpS activity is suppressed by direct interaction with HrpV, which further interacts with a chaperone‐like protein HrpG to relieve the suppression (Bretz et al., 2002; Jovanovic et al., 2011; Ortiz‐Martín et al., 2010; Preston et al., 1998; Wei et al., 2005). In E. amylovora, HrpG and HrpV form a stable heterodimeric complex in vitro, suggesting that a similar regulation mechanism may exist (Gazi et al., 2015). In this study, we demonstrated that HrpS is regulated at least at two levels. Despite the low amino acid identity with P. syringae HrpR and HrpS, E. amylovora HrpS is a direct Lon substrate, and hrpS gene expression is under positive regulation of the Rcs system. This is consistent with previous microarray analysis of the rcsB mutant, which showed that the Rcs system, especially RcsB, is required for full T3SS gene expression (Wang et al., 2012).
It has been proposed that type III effector proteins in the cytoplasm are maintained in an unfolded state to pass through the narrow secretion machinery, and are generally associated with specific chaperone(s) to prevent premature folding and aggregation (Page and Parsot, 2002; Stebbins and Galán, 2001). Meanwhile, effector proteins may also be exposed to Lon degradation, as features of unfolded proteins, such as hydrophobic peptides, can be easily recognized by Lon (Gur and Sauer, 2008). In P. syringae, Lon affects the stability of at least eight type III effector proteins, which becomes rate limiting for effector secretion (Losada and Hutcheson, 2005). The Hrp pilus of P. syringae and E. amylovora consists of HrpA subunits and extends to the plant cell by the addition of HrpA at the distal end (Jin and He, 2001; Li et al., 2002). We showed here that HrpA stability is greatly enhanced in the lon mutant, suggesting that the HrpA protein is subject to Lon degradation, but we could not rule out the possibility that other T3SS proteins might also be targeted by Lon. Therefore, Lon, as a negative regulator of the T3SS in E. amylovora, could also function at multiple stages.
The widely distributed GacS/GacA system is a conserved global dual regulatory system, which specifically activates the expression of the csrB/rsmB sRNAs and antagonizes the activity of the CsrA/RsmA proteins (Lapouge et al., 2008; Li et al., 2014; Romeo et al., 2013; Vakulskas et al., 2015). At the transcriptional level, the expression of csrB also requires IHF, ppGpp and DksA in E. coli and Salmonella enterica (Edwards et al., 2011; Martínez et al., 2014; Zere et al., 2015). In E. amylovora, the expression of csrB is positively mediated by GacS/GacA and IHF (Lee and Zhao, 2016). Here, we have provided evidence that the accumulation of RcsA/RcsB in the lon mutant inhibits csrB expression, suggesting that Lon indirectly activates csrB expression.
However, CsrA positively activates T3SS and amylovoran production in E. amylovora, but the molecular mechanisms of CsrA regulation remain enigmatic (Ancona et al., 2016). CsrA generally binds to GGA motifs in the 5′ untranslated region of target transcripts and affects the translation rate or stability of target mRNAs either positively or negatively (Vakulskas et al., 2015). CsrA also promotes premature transcription termination by altering the Rho‐dependent transcript structure in E. coli, such as pgaA mRNA, which encodes a polysaccharide adhesin export protein (Figueroa‐Bossi et al., 2014). Recent studies have shown that lon mRNA could be co‐purified with CsrA protein in E. coli, and (sequencing by cross‐linking immunoprecipitation) CLIP‐seq data from Salmonella have shown that CsrA binds to the coding region of lon mRNA, which is conserved in E. amylovora (Edwards et al., 2011; Holmqvist et al., 2016). Our results suggest that Lon is possibly under the control of CsrA at both the transcriptional and post‐transcriptional levels. It is reasonable to speculate that Lon might be a direct target of CsrA, as deletion of lon in the csrA mutant background fully restores amylovoran production. However, although CsrA may indirectly promote HrpS stability and hrpS gene expression by suppressing Lon, deletion of lon in the csrA mutant background fails to restore the T3SS and virulence, suggesting that CsrA may also target other unknown regulators in addition to Lon. Therefore, further studies are needed to determine the molecular targets of CsrA in the regulation of the T3SS in E. amylovora.
Based on our results, it is tempting to speculate that Lon‐mediated suppression of RcsA and HrpS/HrpA activities could effectively block E. amylovora pathogenesis, allowing the bacteria to utilize cellular resources in other processes under non‐pathogenic conditions. Increased motility might also enable the bacteria to reach infection sites. Several studies have shown that environmental stimuli, such as phosphate molecules, can regulate Lon activity. Inorganic phosphate (polyP) forms a complex with Lon and promotes the degradation of ribosomal proteins under nutrient starvation (Kuroda et al., 2001). Cardiolipin and lipopolysaccharide (LPS), found in inner and outer membranes, respectively, in Gram‐negative bacteria, directly bind to Lon through their phosphate groups and inhibit Lon activity (Minami et al., 2011; Sugiyama et al., 2013). The Lon activity in vitro can also be inhibited by polyP, cyclic adenosine monophosphate (cAMP), (p)ppGpp and c‐di‐GMP (Osbourne et al., 2014). In E. amylovora, (p)ppGpp and c‐di‐GMP have been shown to positively regulate T3SS and amylovoran production, respectively, suggesting potential involvement of the nucleotide second messengers in the post‐translational regulation of virulence (Ancona et al., 2015b; Edmunds et al., 2013), which warrants further investigation.
In summary, we propose the following working model for Lon‐mediated virulence regulation, and its interaction with Rcs and Gac‐Csr regulatory systems, in E. amylovora (Fig. 8). Lon broadly impacts E. amylovora virulence by the negative regulation of amylovoran production and T3SS, and its positive effect on motility. These could mainly be achieved through the direct targeting of major regulators of amylovoran (RcsA) and the T3SS (HrpS/HrpA) and, indirectly, through suppression of the Rcs system. Over‐activation of the Rcs system inhibits flagellar formation and csrB sRNA expression, and activates T3SS (hrpS) expression. However, CsrA protein positively regulates both amylovoran and T3SS, partly by the suppression of Lon activity and of other unknown regulators. The balance of CsrA and Lon activities is further monitored by the regulatory circuit between the Rcs and Gac‐Csr systems. Future research should focus on understanding the molecular mechanisms underlying the regulation of Lon by CsrA and the identification of other CsrA and Lon targets.
Figure 8.
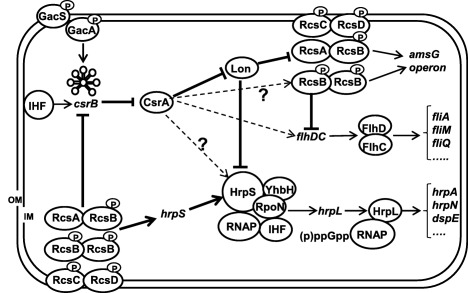
A working model illustrating Lon‐mediated virulence regulation and its interaction with Rcs and Gac‐Csr regulatory systems in Erwinia amylovora. This model is based on findings obtained in this study as well as those reported in previous studies (Ancona et al., 2014; 2015a, 2015b, 2016; Lee and Zhao, 2016; Lee et al., 2016; Li et al., 2014; Wang et al., 2009, 2012; Zhao et al., 2009a). FlhD/C, master regulator of flagellar formation; HrpL, an exocytoplasmic functions (ECF) sigma factor and master regulator of the type III secretion system (T3SS); HrpS, σ54‐dependent enhancer‐binding protein; IHF, integration host factor; RpoN, σ54 alternative sigma factor; YhbH, σ54 modulation protein (ribosome‐associated protein); RNAP, RNA polymerase; (p)ppGpp, guanosine tetraphosphate and guanosine pentaphosphate; GacS/GacA and RcsA/B/C/D, two‐component regulatory systems; csrB, small non‐coding regulatory RNA; CsrA, RNA‐binding protein; OM, outer membrane; IM, inner membrane; P, phosphorylation. Symbols: ↓, positive effect; ⊥, negative effect; dashed line with/without ?, unknown mechanism.
Experimental Procedures
Bacterial strains and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. Erwinia amylovora and Escherichia coli strains were routinely grown in Luria‐Bertani (LB) broth. For T3SS gene expression, HMM [1 g (NH4)2SO4, 0.246 g MgCl2.6H2O, 0.1 g NaCl, 8.708 g K2HPO4, 6.804 g KH2PO4], supplemented with 10 mm galactose as carbon source, was used (Ancona et al., 2014). For amylovoran production, MBMA minimal medium [3 g KH2PO4, 7 g K2HPO4, 1 g (NH4)2SO4, 2 mL glycerol, 0.5 g citric acid, 0.03 g MgSO4], supplemented with 1% sorbitol, was used (Wang et al., 2009). Antibiotics were used at the following concentrations when appropriate: 100 μg/mL ampicillin (Ap), 50 μg/mL kanamaycin (Km) and 10 μg/mL chloramphenicol (Cm). Primer sequences used for mutant construction, mutant confirmation, quantitative reverse transcription real‐time polymerase chain reaction (qRT‐PCR), inverse PCR, cloning and EMSA in this study are listed in Table S1 (see Supporting Information).
Mutant generation by λ‐Red recombinase cloning
As described previously, E. amylovora Ea1189 mutant strains were generated using the λ phage recombinase method (Zhao et al., 2009b). Briefly, competent cells were prepared from overnight cultures of E. amylovora strains carrying pKD46, which were subcultured to exponential phase [optical density at 600 nm (OD600) = 0.8] in LB containing 0.1% arabinose. Recombination DNA fragments, which contained a CmR or KmR gene with their own promoter flanked with a 50‐nucleotide homology from the target genes, were generated by PCR using plasmids pDK32 or pKD13 as template and transformed into competent cells by electroporation. The resulting mutants were selected on LB plates with appropriate antibiotics, and confirmed by PCR. In the corresponding mutant strains, the coding regions of the target genes were deleted, except for the first and last 50 nucleotides. Double mutant strains were generated using single mutants as a background strain as indicated.
Virulence, amylovoran production and motility assays
Virulence assay on immature Bartlett pear fruits (Pyrus communis L. cv. Bartlett) was performed as described previously (Ancona et al., 2014, 2016; Wang et al., 2009). Briefly, bacterial inoculum was prepared from overnight cultures and resuspended in phosphate‐buffered saline (PBS) to OD600 = 0.1 and then diluted 100 times [approximately 106 colony‐forming units (CFU)/mL]. Surface‐sterilized immature pears were air dried, pricked with a sterile needle, inoculated with 2 μL of cell suspensions and incubated in a humidified chamber at 28 °C. Symptoms were recorded at 4 and 8 days post‐inoculation. Pears were assayed in triplicate for each strain, and the experiments were repeated three times.
Amylovoran production was determined using the cetylpyrimidinium chloride (CPC) method, as described previously (Bellemann et al., 1994; Zhao et al., 2009a). Briefly, overnight‐grown cultures were re‐inoculated into 5 mL of MBMA medium to OD600 = 0.2. After 24 h of incubation at 28 °C with shaking, 1 mL of each culture was centrifuged at 4500 g for 10 min, and 50 μL of 50 mg/mL CPC were added to the supernatant. After 10 min of incubation at room temperature, the amylovoran concentration was quantified by measuring OD600 turbidity and normalized to a cell density of 1.0. Each experiment was performed in triplicate and repeated three times.
Motility was performed on motility agar plates (10 g tryptone, 5 g NaCl and 2.5 g agar per litre), as described previously (Zhao et al., 2009a). Diameters were measured at 24 and 48 h post‐inoculation, and each experiment was performed in triplicate and repeated three times.
qRT‐PCR
For in vitro gene expression of T3SS and amylovoran production, RNA was isolated from cultures in HMM for 6 h at 18 °C, and in MBMA medium for 18 h at 28 °C, respectively (Ancona et al., 2016; Wang et al., 2012). To avoid RNA degradation, 4 mL of RNA protect reagent (Qiagen, Hilden, Germany) was added to 2 mL of bacterial cell cultures and the cells were harvested by centrifugation. RNA was extracted using an RNeasy® mini kit (Qiagen) following the manufacturer's instructions, and DNase I treatment was performed with a TURBO DNA‐free kit (Ambion, Austin, TX, USA). RNA was quantified using a Nano‐Drop ND100 spectrophotometer (Nano‐Drop Technologies, Wilmington, DE, USA).
Reverse transcription was performed using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. Power SYBR® Green PCR master mix (Applied Biosystems, Foster City, CA, USA) with appropriate primers (Table S1) was mixed with cDNAs of selected genes, and qRT‐PCR was performed using the StepOnePlus Real‐Time PCR system (Applied Biosystems) under the following conditions: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The dissociation curve was measured after the program was completed, and the relative gene expression was calculated with the relative quantification (ΔΔCt) method using the rpoD gene as an endogenous control. The experiment was repeated at least twice.
Western blot
Western blot was performed as described previously (Ancona et al., 2015b, 2016). The genomic DNA regions containing the promoter and coding sequences of the hrpS, hrpA, rcsA, rcsB and lon genes with a 6‐His tag at the C‐terminus were cloned into pWSK29. Genes were oriented opposite to vector promoters, and the expression of genes was driven only by their native promoters. The resulting plasmids were sequenced at the University of Illinois at Urbana‐Champaign core sequencing facility and transformed by electroporation into the WT and mutants. For Western blot, equal amounts of E. amylovora cells grown in HMM at 18 °C for 6 h or MBMA medium at 28 °C for 24 h were collected. To test protein stability, tetracycline was added to cell cultures with a final concentration of 50 μg/mL, and equal amounts of cells were collected at different time points. Cell lysates were resolved by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). After blocking with 5% milk in PBS, membranes were probed with 1.0 μg/mL rabbit anti‐His antibodies (GenScript, Piscataway, NJ, USA) or rabbit anti‐RNA polymerase beta (E. coli RpoB) antibodies (Abcam, Cambridge, MA, USA) diluted 1 : 2000, followed by horseradish peroxidase‐linked anti‐rabbit IgG antibodies (Amersham Bioscience, Uppsala, Sweden) diluted 1 : 10 000. Immunoblots were developed using enhanced chemiluminescence reagents (Pierce, Rockford, IL, USA) and visualized using an ImageQuant LAS 4010 CCD camera (GE Healthcare, South Plainfield, NJ, USA). The experiment was performed at least three times.
Transposon mutagenesis and screening for the motile mutants
The EZ::T5™ <KAN‐2> Tnp Transposome™ kit (Epicentre, Madison, WI, USA) was used for random mutagenesis following the manufacturer's instructions. Briefly, to prepare the competent cells, an overnight culture of the lon mutant was subcultured to exponential phase (OD600 = 0.8) in LB. Cells were then harvested, washed with cold sterile water and transformed with 1 μL of the EZ‐Tn5 <Kan> Tnp transposome by electroporation. After 3 h of incubation at 28 °C, transformants were plated on LB with Km. A total of 1045 colonies was picked and stored at −20 °C. For screening, the WT, lon mutant and Tn5 mutant strains were grown overnight in LB in 96‐well microwell plates, and 2 μL of each culture were directly inoculated on the motility agar plate. After 24 and 48 h of incubation at 28 °C, the mutants with restored motility were selected and the motility of the mutants was re‐examined as described above.
Inverse PCR was performed to determine the transposon insertion site as described previously with a few modifications (Martin and Mohn, 1999). Genomic DNA of overnight cell cultures was isolated using a MasterPure™ complete DNA & RNA purification kit (Epicentre), digested with the restriction enzyme (PstI or PvuI) and re‐ligated using T4 DNA ligase. For PCRs, DNA samples treated with PstI were amplified with the primer pair KAN‐2 FP‐1/KAN‐2 RP‐1, whereas DNA samples treated with PvuI were amplified with the primer pair KAN‐2 FP1/PvuI‐right or KAN‐2 RP1/PvuI‐left (Table S1). The PCR products were gel purified and sequenced at the University of Illinois at Urbana‐Champaign core sequencing facility. The flanking sequences of the transposon insertion site were analysed using blast search at the National Center for Biotechnology Information (NCBI).
EMSA
EMSAs for RcsA/RcsB binding to the upstream regions of the rsmB sRNA and the hrpS gene were performed as described previously (Ancona et al., 2015a; Lee and Zhao, 2016). Briefly, complementary oligonucleotides (Table S1) were 3′ biotinylated using the biotin 3′ end DNA labelling kit (Pierce) and annealed before use. Reaction volumes of 10 μL with 20 fmol of labelled oligonucleotides were incubated with 5 pmol of either or both RcsA/RcsB proteins in 1 × binding buffer, 50 ng/μL Poly(dI·dC), 0.5 mm MgCl2, 0.1% Nonidet P‐40, 0.05 mg/mL bovine serum albumin and 5% glycerol. Reactions were incubated for 20 min at room temperature, mixed with 2.5 μL of 5 × loading buffer and resolved into a 6% native polyacrylamide gel in 0.5 × Tris/Borate/EDTA; 0.089M Tris, 0.089M borate, 2 mM EDTA (TBE) buffer. The resolved reactions were transferred to a positively charged nylon membrane and UV cross‐linked. The chemiluminescent signals were developed using the lightshift chemiluminescent EMSA kit (Pierce) and visualized using an ImageQuant LAS 4010 charge‐coupled device (CCD) camera (GE Healthcare). The experiment was repeated at least twice.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 (A) Mucoid colony morphology of the lon mutant. The wild‐type (WT) and lon mutant strains were grown in Luria‐Bertani (LB) at 28 °C. Photographs were taken at 48 h after incubation. RcsA/RcsB accumulation suppresses csrB sRNA expression and the effect of csrA mutation on the lon gene. (B) Relative gene expression of csrA and csrB in the lon mutant compared with WT grown in hrp‐inducing medium (HMM) for 6 h at 18 °C. (C) Relative expression of lon in the csrA and csrB mutants compared with WT grown in HMM for 6 h at 18 °C. The values of the relative fold change were the means of three replicates and the experiment was repeated twice with similar results. Error bars indicate standard deviation. (D) Abundance of the Lon‐His6 protein in the csrA and csrB mutant strains compared with WT grown in HMM for 6 h at 18 °C. The abundance of the RpoB protein was used as a loading control. Relative protein abundance at the bottom of each lane was calculated using ImageJ software. Cropped gel blots and percentage expression values are representatives of three independent experiments.
Table S1 Primers used in this study.
Acknowledgements
This project was supported by the Agriculture and Food Research Initiative Competitive Grants Program Grant no. 2016–67013‐24812 from the US Department of Agriculture National Institute of Food and Agriculture. The authors have no conflicts of interest to declare.
References
- Ancona, V. , Li, W. and Zhao, Y. (2014) Alternative sigma factor RpoN and its modulation protein YhbH are indispensable for Erwinia amylovora virulence. Mol. Plant Pathol. 15, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancona, V. , Chatnaparat, T. and Zhao, Y. (2015a) Conserved aspartate and lysine residues of RcsB are required for amylovoran biosynthesis, virulence, and DNA binding in Erwinia amylovora . Mol. Genet. Genomics, 290, 1265–1276. [DOI] [PubMed] [Google Scholar]
- Ancona, V. , Lee, J.H. , Chatnaparat, T. , Oh, J. , Hong, J.I. and Zhao, Y. (2015b) The bacterial alarmone (p)ppGpp activates the type III secretion system in Erwinia amylovora . J. Bacteriol. 197, 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancona, V. , Lee, J.H. and Zhao, Y. (2016) The RNA‐binding protein CsrA plays a central role in positively regulating virulence factors in Erwinia amylovora . Sci. Rep. 6, 37 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen, L. , Sala, E. , Koiv, V. and Mäe, A. (2010) A role for the Rcs phosphorelay in regulating expression of plant cell wall degrading enzymes in Pectobacterium carotovorum subsp. carotovorum . Microbiology, 156, 1323–1334. [DOI] [PubMed] [Google Scholar]
- Bellemann, P. , Bereswill, S. , Berger, S. and Geider, K. (1994) Visualization of capsule formation by Erwinia amylovora and assays to determine amylovoran synthesis. Int. J. Biol. Macromol. 16, 290–296. [DOI] [PubMed] [Google Scholar]
- Bereswill, S. and Geider, K. (1997) Characterization of the rcsB gene from Erwinia amylovora and its influence on exopolysaccharide synthesis and virulence of the fire blight pathogen. J. Bacteriol. 179, 1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard, F. , Poetter, K. , Geider, K. and Coplin, D.L. (1990) The rcsA gene from Erwinia amylovora: identification, nucleotide sequence, and regulation. Mol. Plant–Microbe Interact. 3, 429–437. [DOI] [PubMed] [Google Scholar]
- Bernhard, F. , Coplin, D.L. and Geider, K. (1993) A gene cluster for amylovoran synthesis in Erwinia amylovora: characterization and relationship to cps genes in Erwinia stewartii . Mol. Gen. Genet. 239, 158–168. [DOI] [PubMed] [Google Scholar]
- Bretz, J. , Losada, L. , Lisboa, K. and Hutcheson, S.W. (2002) Lon protease functions as a negative regulator of type III protein secretion in Pseudomonas syringae . Mol. Microbiol. 45, 397–409. [DOI] [PubMed] [Google Scholar]
- Bush, M. and Dixon, R. (2012) The role of bacterial enhancer binding proteins as specialized activators of σ54‐dependent transcription. Microbiol. Mol. Biol. Rev. 76, 497–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, C.H. and Goldberg, A.L. (1981) The product of the lon (capR) gene in Escherichia coli is the ATP‐dependent protease, protease La. Proc. Natl. Acad. Sci. USA, 78, 4931–4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmer, K.M. and Rather, P.N. (2007) Regulation of flhDC expression in Proteus mirabilis . Res. Microbiol. 158, 295–302. [DOI] [PubMed] [Google Scholar]
- Datsenko, K.A. and Wanner, B.L. (2000) One‐step inactivation of chromosomal genes in Escherichia coli K‐12 using PCR products. Proc. Natl. Acad. Sci. USA, 97, 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastgate, J.A. , Taylor, N. , Coleman, M.J. , Healy, B. , Thompson, L. and Roberts, I.S. (1995) Cloning, expression, and characterization of the lon gene of Erwinia amylovora: evidence for a heat shock response. J. Bacteriol. 177, 932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds, A.C. , Castiblanco, L.F. , Sundin, G.W. and Waters, C.M. (2013) Cyclic Di‐GMP modulates the disease progression of Erwinia amylovora . J. Bacteriol. 195, 2155–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, A.N. , Patterson‐Fortin, L.M. , Vakulskas, C.A. , Mercante, J.W. , Potrykus, K. , Vinella, D. , Camacho, M. , Fields, J.A. , Thompson, S.A. , Georgellis, D. , Cashel, M. , Babitzke, P. and Romeo, T. (2011) Circuitry linking the Csr and stringent response global regulatory systems. Mol. Microbiol. 80, 1561–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa‐Bossi, N. , Schwartz, A. , Guillemardet, B. , D'heygère, F. , Bossi, L. and Boudvillain, M. (2014) RNA remodeling by bacterial global regulator CsrA promotes Rho‐dependent transcription termination. Gene Dev. 28, 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francez‐Charlot, A. , Laugel, B. , Van Gemert, A. , Dubarry, N. , Wiorowski, F. , Castanié‐Cornet, M.P. , Gutierrez, C. and Cam, K. (2003) RcsCDB His‐Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli . Mol. Microbiol. 49, 823–832. [DOI] [PubMed] [Google Scholar]
- Fredericks, C.E. , Shibata, S. , Aizawa, S.I. , Reimann, S.A. and Wolfe, A.J. (2006) Acetyl phosphate‐sensitive regulation of flagellar biogenesis and capsular biosynthesis depends on the Rcs phosphorelay. Mol. Microbiol. 61, 734–747. [DOI] [PubMed] [Google Scholar]
- Gazi, A.D. , Charova, S. , Aivaliotis, M. , Panopoulos, N.J. and Kokkinidis, M. (2015) HrpG and HrpV proteins from the Type III secretion system of Erwinia amylovora form a stable heterodimer. FEMS Microbiol. Lett. 362, 1–8. [DOI] [PubMed] [Google Scholar]
- Gottesman, S. , Halpern, E. and Trisler, P. (1981) Role of sulA and sulB in filamentation by lon mutants of Escherichia coli K‐12. J. Bacteriol. 148, 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman, S. , Trisler, P. and Torres‐Cabassa, A. (1985) Regulation of capsular polysaccharide synthesis in Escherichia coli K‐12: characterization of three regulatory genes. J. Bacteriol. 162, 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur, E. and Sauer, R.T. (2008) Recognition of misfolded proteins by Lon, a AAA+ protease. Genes Dev. 22, 2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist, E. , Wright, P.R. , Li, L. , Bischler, T. , Barquist, L. , Reinhardt, R. , Backofen, R. and Vogel, J. (2016) Global RNA recognition patterns of post‐transcriptional regulators Hfq and CsrA revealed by UV crosslinking in vivo . EMBO J. 35, 991–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman, O. and D'Ari, R. (1981) An inducible DNA replication–cell division coupling mechanism in E. coli . Nature, 290, 797–799. [DOI] [PubMed] [Google Scholar]
- Hutcheson, S.W. , Bretz, J. , Sussan, T. , Jin, S. and Pak, K. (2001) Enhancer‐binding proteins HrpR and HrpS interact to regulate hrp‐encoded type III protein secretion in Pseudomonas syringae strains. J. Bacteriol. 183, 5589–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, M.W. , Silva‐Herzog, E. and Plano, G.V. (2004) The ATP‐dependent ClpXP and Lon proteases regulate expression of the Yersinia pestis type III secretion system via regulated proteolysis of YmoA, a small histone‐like protein. Mol. Microbiol. 54, 1364–1378. [DOI] [PubMed] [Google Scholar]
- Jin, Q. and He, S.Y. (2001) Role of the Hrp pilus in type III protein secretion in Pseudomonas syringae . Science, 294, 2556–2558. [DOI] [PubMed] [Google Scholar]
- Jovanovic, M. , James, E.H. , Burrows, P.C. , Rego, F.G. , Buck, M. and Schumacher, J. (2011) Regulation of the co‐evolved HrpR and HrpS AAA+ proteins required for Pseudomonas syringae pathogenicity. Nat. Commun. 2, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M.A. , Zhao, Y.F. and Korban, S.S. (2012) Molecular mechanisms of pathogenesis and resistance to the bacterial pathogen Erwinia amylovora, causal agent of fire blight disease in Rosaceae. Plant Mol. Biol. Rep. 30, 247–260. [Google Scholar]
- Koczan, J.M. , McGrath, M.J. , Zhao, Y. and Sundin, G.W. (2009) Contribution of Erwinia amylovora exopolysaccharides amylovoran and levan to biofilm formation: implications in pathogenicity. Phytopathology, 99, 1237–1244. [DOI] [PubMed] [Google Scholar]
- Kuroda, A. , Nomura, K. , Ohtomo, R. , Kato, J. , Ikeda, T. , Takiguchi, N. , Ohtake, H. and Kornberg, A. (2001) Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli . Science, 293, 705–708. [DOI] [PubMed] [Google Scholar]
- Lai, Y.C. , Peng, H.L. and Chang, H.Y. (2003) RmpA2, an activator of capsule biosynthesis in Klebsiella pneumoniae CG43, regulates K2 cps gene expression at the transcriptional level. J. Bacteriol. 185, 788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge, K. , Schubert, M. , Allain, F.H.T. and Haas, D. (2008) Gac/Rsm signal transduction pathway of γ‐proteobacteria: from RNA recognition to regulation of social behaviour. Mol. Microbiol. 67, 241–253. [DOI] [PubMed] [Google Scholar]
- Lee, J.H. and Zhao, Y. (2016) Integration host factor is required for RpoN‐dependent hrpL gene expression and controls motility by positively regulating rsmB sRNA in Erwinia amylovora . Phytopathology, 106, 29–36. [DOI] [PubMed] [Google Scholar]
- Lee, J.H. , Sundin, G.W. and Zhao, Y. (2016) Identification of the HrpS binding site in the hrpL promoter and effect of the RpoN binding site of HrpS on the regulation of the type III secretion system in Erwinia amylovora . Mol. Plant Pathol. 17, 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C.M. , Brown, I. , Mansfield, J. , Stevens, C. , Boureau, T. , Romantschuk, M. and Taira, S. (2002) The Hrp pilus of Pseudomonas syringae elongates from its tip and acts as a conduit for translocation of the effector protein HrpZ. EMBO J. 21, 1909–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Ancona, V. and Zhao, Y. (2014) Co‐regulation of polysaccharide production, motility, and expression of type III secretion genes by EnvZ/OmpR and GrrS/GrrA systems in Erwinia amylovora . Mol. Genet. Genomics, 289, 63–75. [DOI] [PubMed] [Google Scholar]
- Losada, L.C. and Hutcheson, S.W. (2005) Type III secretion chaperones of Pseudomonas syringae protect effectors from Lon‐associated degradation. Mol. Microbiol. 55, 941–953. [DOI] [PubMed] [Google Scholar]
- Majdalani, N. and Gottesman, S. (2005) The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 59, 379–405. [DOI] [PubMed] [Google Scholar]
- Martin, V.J. and Mohn, W.W. (1999) An alternative inverse PCR (IPCR) method to amplify DNA sequences flanking Tn5 transposon insertions. J. Microbiol. Methods, 35, 163–166. [DOI] [PubMed] [Google Scholar]
- Martínez, L.C. , Martínez‐Flores, I. , Salgado, H. , Fernández‐Mora, M. , Medina‐Rivera, A. , Puente, J.L. , Collado‐Vides, J. and Bustamante, V.H. (2014) In silico identification and experimental characterization of regulatory elements controlling the expression of the Salmonella csrB and csrC genes. J. Bacteriol. 196, 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally, R. , Toth, I.K. , Cock, P.J.A. , Pritchard, L. , Hedley, P.E. , Morris, J.A. , Zhao, Y.F. and Sundin, G.W. (2012) Genetic characterization of the HrpL regulon of the fire blight pathogen Erwinia amylovora reveals novel virulence factors. Mol. Plant Pathol. 13, 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami, N. , Yasuda, T. , Ishii, Y. , Fujimori, K. and Amano, F. (2011) Regulatory role of cardiolipin in the activity of an ATP‐dependent protease, Lon, from Escherichia coli . J. Biochem. 149, 519–527. [DOI] [PubMed] [Google Scholar]
- Mizusawa, S. and Gottesman, S. (1983) Protein degradation in Escherichia coli: the lon gene controls the stability of sulA protein. Proc. Natl. Acad. Sci. USA, 80, 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz‐Martín, I. , Thwaites, R. , Macho, A.P. , Mansfield, J.W. and Beuzón, C.R. (2010) Positive regulation of the Hrp type III secretion system in Pseudomonas syringae pv. phaseolicola . Mol. Plant–Microbe Interact. 23, 665–681. [DOI] [PubMed] [Google Scholar]
- Osbourne, D.O. , Soo, V.W. , Konieczny, I. and Wood, T.K. (2014) Polyphosphate, cyclic AMP, guanosine tetraphosphate, and c‐di‐GMP reduce in vitro Lon activity. Bioengineered, 5, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, A.L. and Parsot, C. (2002) Chaperones of the type III secretion pathway: jacks of all trades. Mol. Microbiol. 46, 1–11. [DOI] [PubMed] [Google Scholar]
- Preston, G. , Deng, W.L. , Huang, H.C. and Collmer, A. (1998) Negative regulation of hrp genes in Pseudomonas syringae by HrpV. J. Bacteriol. 180, 4532–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo, T. , Vakulsas, C.A. and Babitzke, P. (2013) Post‐transcriptional regulation on a global scale: form and function of Csr/Rsm systems. Envrion. Microbiol. 15, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjulin, T.M. and Beer, S.V. (1978) Mechanism of wilt induction by amylovoran in cotoneaster shoots and its relation to wilting of shoots infected by Erwinia amylovora . Phytopathology, 68, 89–94. [Google Scholar]
- Sledjeski, D. and Gottesman, S. (1995) A small RNA acts as an antisilencer of the H‐NS‐silenced rcsA gene of Escherichia coli . Proc. Natl. Acad. Sci. USA, 92, 2003–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins, C.E. and Galán, J.E. (2001) Maintenance of an unfolded polypeptide by a cognate chaperone in bacterial type III secretion. Nature, 414, 77–81. [DOI] [PubMed] [Google Scholar]
- Sugiyama, N. , Minami, N. , Ishii, Y. and Amano, F. (2013) Inhibition of Lon protease by bacterial lipopolysaccharide (LPS) though inhibition of ATPase. Adv. Biosci. Biotechnol. 4, 590–598. [Google Scholar]
- Takaya, A. , Tomoyasu, T. , Tokumitsu, A. , Morioka, M. and Yamamoto, T. (2002) The ATP‐dependent lon protease of Salmonella enterica serovar Typhimurium regulates invasion and expression of genes carried on Salmonella pathogenicity island 1. J. Bacteriol. 184, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaya, A. , Kubota, Y. , Isogai, E. and Yamamoto, T. (2005) Degradation of the HilC and HilD regulator proteins by ATP‐dependent Lon protease leads to downregulation of Salmonella pathogenicity island 1 gene expression. Mol. Microbiol. 55, 839–852. [DOI] [PubMed] [Google Scholar]
- Torres‐Cabassa, A.S. and Gottesman, S. (1987) Capsule synthesis in Escherichia coli K‐12 is regulated by proteolysis. J. Bacteriol. 169, 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakulskas, C.A. , Potts, A.H. , Babitzke, P. , Ahmer, B.M. and Romeo, T. (2015) Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol. Mol. Biol. Rev. 79, 193–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Korban, S.S. and Zhao, Y. (2009) The Rcs phosphorelay system is essential for pathogenicity in Erwinia amylovora . Mol. Plant Pathol. 10, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Qi, M. , Calla, B. , Korban, S.S. , Clough, S.J. , Cock, P.J. , Sundin, G.W. , Toth, I. and Zhao, Y.F. (2012) Genome‐wide identification of genes regulated by the Rcs phosphorelay system in Erwinia amylovora . Mol. Plant–Microbe Interact. 25, 6–17. [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Zhao, Y. , McClelland, M. and Harshey, R.M. (2007) The RcsCDB signaling system and swarming motility in Salmonella enterica serovar Typhimurium: dual regulation of flagellar and SPI‐2 virulence genes. J. Bacteriol. 189, 8447–8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R.F. and Kushner, S.R. (1991) Construction of versatile low‐copy‐number vectors for cloning, sequencing and gene expression in Escherichia coli . Gene, 100, 195–199. [PubMed] [Google Scholar]
- Wehland, M. , Kiecker, C. , Coplin, D.L. , Kelm, O. , Saenger, W. and Bernhard, F. (1999) Identification of an RcsA/RcsB recognition motif in the promoters of exopolysaccharide biosynthetic operons from Erwinia amylovora and Pantoea stewartii subspecies stewartii . J. Biol. Chem. 274, 3300–3307. [DOI] [PubMed] [Google Scholar]
- Wei, C.F. , Deng, W.L. and Huang, H.C. (2005) A chaperone‐like HrpG protein acts as a suppressor of HrpV in regulation of the Pseudomonas syringae pv. syringae type III secretion system. Mol. Microbiol. 57, 520–536. 15978082 [Google Scholar]
- Wei, Z.M. and Beer, S.V. (1995) hrpL activates Erwinia amylovora hrp gene transcription and is a member of the ECF subfamily of s factors. J. Bacteriol. 177, 6201–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Z. , Kim, J.F. and Beer, S.V. (2000) Regulation of hrp genes and type III protein secretion in Erwinia amylovora by HrpX/HrpY, a novel two‐component system, and HrpS. Mol. Plant–Microbe Interact. 13, 1251–1262. [DOI] [PubMed] [Google Scholar]
- Zere, T.R. , Vakulskas, C.A. , Leng, Y. , Pannuri, A. , Potts, A.H. , Dias, R. , Tang, D. , Kolaczkowski, B. , Georgellis, D. , Ahmer, B.M.M. and Romeo, T. (2015) Genomic targets and features of BarA‐UvrY (‐SirA) signal transduction systems. PLoS One, 10, e0145035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y.F. (2014) Genomics of Erwinia amylovora and related species associated with pome fruit trees In: Genomics of Plant‐Associated Bacteria (Gross D., Lichens‐Park A. and Kole C., eds). Berlin: Springer‐Verlag; 1–36. [Google Scholar]
- Zhao, Y. , Wang, D. , Nakka, S. , Sundin, G.W. and Korban, S.S. (2009a) Systems level analysis of two‐component signal transduction systems in Erwinia amylovora: role in virulence, regulation of amylovoran biosynthesis and swarming motility. BMC Genomics, 10, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Sundin, G.W. and Wang, D. (2009b) Construction and analysis of pathogenicity island deletion mutants of Erwinia amylovora . Can. J. Microbiol. 55, 457–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 (A) Mucoid colony morphology of the lon mutant. The wild‐type (WT) and lon mutant strains were grown in Luria‐Bertani (LB) at 28 °C. Photographs were taken at 48 h after incubation. RcsA/RcsB accumulation suppresses csrB sRNA expression and the effect of csrA mutation on the lon gene. (B) Relative gene expression of csrA and csrB in the lon mutant compared with WT grown in hrp‐inducing medium (HMM) for 6 h at 18 °C. (C) Relative expression of lon in the csrA and csrB mutants compared with WT grown in HMM for 6 h at 18 °C. The values of the relative fold change were the means of three replicates and the experiment was repeated twice with similar results. Error bars indicate standard deviation. (D) Abundance of the Lon‐His6 protein in the csrA and csrB mutant strains compared with WT grown in HMM for 6 h at 18 °C. The abundance of the RpoB protein was used as a loading control. Relative protein abundance at the bottom of each lane was calculated using ImageJ software. Cropped gel blots and percentage expression values are representatives of three independent experiments.
Table S1 Primers used in this study.


