Abstract
OBJECTIVE
To investigate the association between intakes of n-6 polyunsaturated fatty acids (PUFAs) and type 2 diabetes risk in three prospective cohort studies of U.S. men and women.
RESEARCH DESIGN AND METHODS
We followed 83,648 women from the Nurses’ Health Study (NHS) (1980–2012), 88,610 women from NHSII (1991–2013), and 41,771 men from the Health Professionals Follow-Up Study (HPFS) (1986–2012). Dietary data were collected every 2–4 years by using validated food-frequency questionnaires. Self-reported incident diabetes, identified biennially, was confirmed by using a validated supplementary questionnaire.
RESULTS
During 4.93 million person-years of follow-up, 18,442 type 2 diabetes cases were documented. Dietary n-6 PUFAs accounted for 4.4–6.8% of total energy, on average, and consisted primarily of linoleic acid (LA) (≥98%). In multivariate-adjusted models, hazard ratios (95% CIs) of type 2 diabetes risk comparing extreme n-6 PUFA quintiles (highest vs. lowest) were 0.91 (0.85, 0.96) (Ptrend = 0.002) for total n-6 PUFAs and 0.92 (0.87, 0.98) (Ptrend = 0.01) for LA. In an isocaloric substitution model, diabetes risk was 14% (95% CI 5%, 21%) (P = 0.002) lower when LA isocalorically replaced saturated fats (5% of energy), 17% (95% CI 9%, 24%) (P < 0.001) lower for trans fats (2% of energy), or 9% (95% CI 17%, 0.1%) (P = 0.047) lower for carbohydrates (5% of energy). Replacing n-3 PUFAs or monounsaturated fats with LA was not significantly associated with type 2 diabetes risk.
CONCLUSIONS
Our study provides additional evidence that LA intake is inversely associated with risk of type 2 diabetes, especially when replacing saturated fatty acids, trans fats, or carbohydrates.
Introduction
Polyunsaturated fatty acids (PUFAs) in the American diet are mostly n-6 PUFAs, particularly linoleic acid (LA) (1). Given the compelling evidence supporting the benefits of dietary n-6 PUFAs in coronary heart disease, LA is recommended as a healthy energy source for maintaining long-term health (2). However, the effects of n-6 PUFAs on type 2 diabetes risk remain unclear (3).
To date, large clinical trials that examine the effects of n-6 PUFA intake on type 2 diabetes risk are lacking, and findings from prospective cohort studies are mixed (4–12). Most early investigations assessed diet only once and did not capture potential changes in food composition over time. In addition, the association between dietary n-6 PUFAs and type 2 diabetes risk has not been evaluated explicitly with respect to other macronutrients in an isocaloric context (i.e., by a substitution model) (13). Recent studies focusing on fatty acid biomarkers showed that proportions of LA in blood or adipose tissue were independently associated with lower risk for type 2 diabetes (14,15). Given the modest correlations between n-6 PUFA biomarkers and intake (16), however, the extent to which these associations can be ascribed to the intake of specific fatty acids is debatable.
In this study, we investigated the association between intake of n-6 PUFAs, including LA and arachidonic acid (AA), and type 2 diabetes risk in three large prospective cohort studies of American men and women. Specifically, we estimated type 2 diabetes risk when LA replaces other macronutrients, especially saturated fatty acids (SFAs), trans fats, and carbohydrates, in an isocaloric substitution model.
Research Design and Methods
Study Populations
The Nurses’ Health Study (NHS), NHSII, and Health Professionals Follow-Up Study (HPFS) are ongoing prospective cohort studies. The NHS includes 121,700 female registered nurses who were aged 30–55 years when enrolled in 1976 (17); the NHSII includes 116,671 female registered nurses who were aged 25–44 years when enrolled in 1989; and the HPFS consists of 51,529 male health professionals who were aged 40–75 years when enrolled in 1986. Participants in all studies have been followed through questionnaires, mailed biennially, in order to collect and update information on lifestyles, health-related behaviors, and medical histories. The institutional review boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health approved the study protocol. The completion of self-administered questionnaires was considered to imply informed consent.
Among participants who completed baseline food frequency questionnaires (FFQs) (NHS in 1980, n = 92,468; NHSII in 1991, n = 97,605; and HPFS in 1986, n = 51,530), we excluded individuals if they 1) reported a diagnosis of diabetes, cardiovascular disease, or cancer at baseline (5,313 participants in NHS, 5,130 in NHSII, and 6,926 in HPFS); 2) had daily energy intake outside of a normal range (<500 or >3,500 kcal/day for NHS and <800 or >4,200 kcal/day for the HPFS) or missing dietary fat data (402 participants in NHS, 2,164 in NHSII, and 1,282 in HPFS); 3) had a missing date for diagnosis of type 2 diabetes (2,206 participants in NHS, 1,025 in NHSII, and 402 in HPFS); or 4) completed only the baseline questionnaire or were missing age at baseline (900 participants in NHS, 676 in NHSII, and 1,149 in HPFS). This left 83,647 participants from NHS, 88,610 from NHSII, and 41,771 from HPFS for the current analysis.
Ascertainment of Diet and n-6 PUFA Intake
In 1980, 1984, and 1986, and every 4 years thereafter, NHS participants completed a validated FFQ to assess their habitual diet over the preceding year. A similar FFQ has been sent to NHSII and HPFS participants every 4 years since 1991 and 1986, respectively (18). The questionnaires inquire about how often, on average, participants had consumed specific foods and the types of fats, oils, and margarines they used during cooking and at the table in the previous year. Nutrient intake was calculated on the basis of U.S. Department of Agriculture and Harvard University food composition databases, which have been updated over time to include new food items and reflect changes in food composition. Multiple validation studies have demonstrated the validity of FFQ assessments of dietary fats (19–21). In the most recent validation study using NHS participants, deattenuated Spearman correlation coefficients of energy-adjusted nutrient intake assessments by FFQs versus multiple 7-day diet records were 0.55 (P < 0.001) for LA and 0.52 (P < 0.001) for AA.
We calculated cumulative averages of intake of nutrients or foods based on valid assessments from baseline to the end of follow-up in order to better represent long-term diet. To reduce the possibility of reverse causation bias, we stopped updating dietary information if a participant reported a diagnosis of cardiovascular disease or cancer. Nutrient intakes were adjusted for total energy by using the residual method.
Ascertainment of Incident Type 2 Diabetes
Participants who reported a diagnosis of diabetes were mailed a supplementary questionnaire regarding diagnosis date, symptoms, diagnostic tests, and hypoglycemic therapy. The diagnosis of type 2 diabetes was considered confirmed if at least one of the following was reported on the supplementary questionnaire, according to the National Diabetes Data Group criteria (22): one or more classic symptoms (excessive thirst, polyuria or frequent urination, weight loss, hunger) plus fasting plasma glucose ≥7.8 mmol/L or random plasma glucose ≥11.1 mmol/L; two or more elevated plasma glucose concentrations on different occasions (fasting glucose ≥7.8 mmol/L, random plasma glucose ≥11.1 mmol/L, plasma glucose ≥11.1 mmol/L after ≥2 h as shown by an oral glucose tolerance test, or all three) in the absence of symptoms; or treatment with hypoglycemic medication. The diagnostic criteria changed in June 1998, and fasting plasma glucose of 7.0 mmol/L (instead of 7.8 mmol/L) was considered to be the threshold for the diagnosis of diabetes according to the American Diabetes Association criteria. In validation studies, 61 of 62 randomly selected cases of type 2 diabetes (98%) in NHS—a number that was confirmed by using the supplementary questionnaire—were reconfirmed after an endocrinologist blinded to the disease status of patients reviewed medical records (23); in the HPFS, 57 of 59 cases (97%) were reconfirmed (24).
Statistical Analysis
Macronutrients were analyzed as percentages of energy by dividing the energy from a specific macronutrient by total energy intake. Spearman correlation coefficients among dietary fatty acids were calculated by using data assessed at baseline, the midpoint of follow-up, and the last FFQ before the end of follow-up. Person-years of follow-up for each participant were calculated from the return date of the baseline questionnaire to the date when the participant received a diagnosis of type 2 diabetes, the date of their death, or the end of follow-up, whichever occurred first. Hazard ratios (HRs) and 95% CIs of incident type 2 diabetes were estimated by using a time-dependent Cox proportional hazards regression model in each cohort, with follow-up duration as the time scale. Regression models were stratified jointly by age in months and calendar year in order to better control for their confounding and possible interactions; they were further adjusted for ethnicity, family history of diabetes, menopausal status and hormone use after menopause (NHS and NHSII participants only), oral contraceptive use (NHSII participants only), multivitamin use, smoking status, alcohol intake, physical activity, baseline hypertension, baseline hypercholesterolemia, updated BMI, total energy intake, and intake of fruits and vegetables. We further adjusted for percentages of energy from total fats, trans fats, cis-monounsaturated fatty acids (cis-MUFAs), and other PUFAs to estimate the main associations of n-6 PUFAs with type 2 diabetes compared with SFAs. We tested for linear trend by modeling the median value of n-6 PUFAs in each category as a continuous variable. We used the likelihood ratio test to examine proportional hazards assumption by fitting a model that included interaction terms between n-6 PUFAs and duration of follow-up; the assumption was unlikely to be violated (P > 0.05 for all tests).
We estimated type 2 diabetes risk when LA isocalorically replaces energy from SFAs, trans fats, cis-MUFAs, or carbohydrates. Dietary covariates included total calories, total fats (for the fat-fat substitution models only), AA, and all other macronutrients except the one being replaced. In such models, regression coefficients for LA can be interpreted as the estimated effect of isocalorically substituting LA for the specific nutrient while holding constant the intake of total energy and other macronutrients. We analyzed the three cohorts separately, and then we pooled the results in a fixed-effect model when the P value for heterogeneity was >0.05.
We performed several sensitivity analyses to examine the robustness of findings from the substitution analyses: 1) adjusting for hypertension and hypercholesterolemia diagnosed during follow-up, 2) using the averages of nutrient intake from the two most recent FFQ assessments for each 4-year follow-up period, or 3) using baseline dietary data only. Statistical analyses were performed by using SAS 9.4 (SAS Institute, Cary, NC). All P values were two-sided; statistical significance was defined as P < 0.05.
Results
Baseline characteristics of study participants are presented in Table 1. Those with higher total n-6 PUFA intake were more likely to be Caucasian but less likely to smoke, to drink alcohol, to engage in physical activities, or to use multivitamins. NHS and HPFS participants who consumed more n-6 PUFAs were younger and less likely to have hypertension, whereas NHSII participants who consumed more n-6 PUFAs were older and heavier. In terms of dietary factors, participants with higher n-6 PUFA intake also consumed more MUFAs and trans fats, and fewer carbohydrates, proteins, fruits, and vegetables.
Table 1.
Age-standardized participant characteristics at baseline according to quintiles of total n-6 PUFA intake
| NHS, 1980 |
NHSII, 1991 |
HPFS, 1986 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 16,729) | Q3 (n = 16,730) | Q5 (n = 16,729) | Q1 (n = 17,722) | Q3 (n = 17,724) | Q5 (n = 17,722) | Q1 (n = 8,354) | Q3 (n = 8,355) | Q5 (n = 8,354) | |
| n-6 PUFAs, % energy | 2.55 ± 0.42 | 4.17 ± 0.20 | 6.70 ± 1.25 | 3.31 ± 0.42 | 4.76 ± 0.18 | 6.91 ± 1.13 | 3.42 ± 0.49 | 5.14 ± 0.20 | 7.65 ± 1.34 |
| Age, years | 47.6 ± 7.1 | 45.8 ± 7.2 | 45.0 ± 7.1 | 35.7 ± 4.7 | 36.1 ± 4.6 | 36.6 ± 4.6 | 54.6 ± 9.7 | 52.7 ± 9.5 | 52.6 ± 9.2 |
| Caucasian race | 97 | 97 | 98 | 94 | 96 | 97 | 94 | 95 | 96 |
| Alcohol intake, g/day | 9.4 ± 14.1 | 5.9 ± 9.5 | 4.7 ± 7.9 | 3.7 ± 7.9 | 3.1 ± 5.7 | 2.7 ± 5.0 | 15.3 ± 20.1 | 11.1 ± 14.2 | 8.4 ± 11.7 |
| Current smoking | 32 | 27 | 28 | 13 | 12 | 12 | 11 | 10 | 9 |
| Physical activity, MET h/week | 15.9 ± 23.6 | 14.0 ± 18.4 | 12.6 ± 18.5 | 25.0 ± 32.0 | 20.5 ± 26.3 | 18.2 ± 24.0 | 23.7 ± 32.0 | 21.1 ± 29.3 | 19.5 ± 26.6 |
| BMI, kg/m2 | 24.1 ± 4.2 | 24.4 ± 4.3 | 24.3 ± 4.6 | 24.0 ± 4.9 | 24.5 ± 5.2 | 24.9 ± 5.6 | 24.8 ± 4.8 | 25.0 ± 4.9 | 24.9 ± 4.8 |
| Family history of diabetes | 24 | 25 | 25 | 16 | 16 | 16 | 18 | 19 | 18 |
| Multivitamin use | 36 | 34 | 32 | 48 | 44 | 40 | 43 | 42 | 41 |
| Any use of hormone after menopause | 15 | 15 | 15 | 3 | 3 | 3 | – | – | – |
| Current use of oral contraceptives | – | – | – | 11 | 10 | 11 | – | – | – |
| Hypertension | 17 | 15 | 14 | 6 | 6 | 6 | 20 | 19 | 18 |
| Hypercholesterolemia | 5 | 5 | 5 | 14 | 15 | 14 | 10 | 10 | 11 |
| Total energy, kcal | 1,535 ± 499 | 1,590 ± 489 | 1,559 ± 514 | 1,758 ± 540 | 1,802 ± 555 | 1,779 ± 549 | 1,955 ± 619 | 2,011 ± 614 | 2,032 ± 643 |
| Total fats, % energy | 34.3 ± 8.9 | 38.7 ± 7.0 | 43.2 ± 6.7 | 25.4 ± 5.2 | 30.4 ± 4.4 | 35.2 ± 4.8 | 25.3 ± 6.3 | 30.6 ± 4.7 | 35.6 ± 5.1 |
| PUFAs, % energy | 3.06 ± 0.46 | 4.72 ± 0.23 | 7.31 ± 1.27 | 3.80 ± 0.47 | 5.34 ± 0.23 | 7.68 ± 1.26 | 4.34 ± 1.01 | 5.77 ± 0.74 | 7.94 ± 1.44 |
| n-3 PUFAs, % energy | 0.52 ± 0.11 | 0.56 ± 0.09 | 0.61 ± 0.13 | 0.48 ± 0.15 | 0.58 ± 0.13 | 0.77 ± 0.22 | 0.52 ± 0.19 | 0.63 ± 0.16 | 0.79 ± 0.25 |
| LA, % energy | 2.46 ± 0.42 | 4.08 ± 0.20 | 6.61 ± 1.25 | 3.24 ± 0.42 | 4.68 ± 0.18 | 6.83 ± 1.13 | 3.35 ± 0.49 | 5.05 ± 0.21 | 7.57 ± 1.34 |
| AA, mg/day | 136 ± 60 | 154 ± 64 | 153 ± 70 | 148 ± 780 | 163 ± 78 | 162 ± 81 | 156 ± 74 | 177 ± 76 | 178 ± 83 |
| Trans fats, % energy | 1.62 ± 0.47 | 2.22 ± 0.54 | 2.87 ± 0.85 | 1.31 ± 0.50 | 1.67 ± 0.57 | 1.89 ± 0.65 | 1.02 ± 0.45 | 1.31 ± 0.48 | 1.42 ± 0.54 |
| SFAs, % energy | 15.4 ± 4.3 | 15.7 ± 3.4 | 15.5 ± 3.2 | 10.3 ± 2.7 | 11.3 ± 2.3 | 11.9 ± 2.2 | 10.2 ± 3.4 | 11.2 ± 2.6 | 11.5 ± 2.4 |
| Cis-MUFAs, % energy | 14.2 ± 4.3 | 16.1 ± 3.5 | 17.5 ± 3.3 | 10.0 ± 2.3 | 12.1 ± 2.0 | 13.7 ± 2.2 | 10.1 ± 2.7 | 12.3 ± 2.1 | 14.3 ± 2.5 |
| Protein, % energy | 19.9 ± 4.5 | 19.2 ± 3.7 | 18.0 ± 3.5 | 19.5 ± 4.0 | 19.5 ± 3.3 | 18.7 ± 3.3 | 18.5 ± 3.7 | 18.6 ± 3.3 | 18.1 ± 3.2 |
| Carbohydrates, % energy | 39.8 ± 10.8 | 38.8 ± 8.7 | 38.3 ± 8.5 | 54.1 ± 8.3 | 49.6 ± 6.7 | 46.2 ± 6.6 | 50.3 ± 10.2 | 46.6 ± 7.5 | 43.9 ± 7.5 |
| Fruits and vegetables, servings/day | 4.63 ± 2.44 | 4.03 ± 1.90 | 3.39 ± 1.69 | 4.71 ± 3.16 | 4.32 ± 2.55 | 4.06 ± 2.41 | 5.99 ± 3.29 | 5.34 ± 2.63 | 4.97 ± 2.44 |
| Cereal fiber, g/day | 2.11 ± 1.51 | 2.50 ± 1.47 | 2.74 ± 1.53 | 5.53 ± 3.50 | 5.66 ± 2.95 | 5.62 ± 2.94 | 5.97 ± 4.16 | 5.80 ± 3.65 | 5.68 ± 3.82 |
Data are mean (SD) or percentage and are standardized to the age distribution of the study population.
As shown in Table 2, the primary dietary n-6 PUFA was LA (≥98%); AA intake contributed only 2% of total n-6 PUFAs and 0.07–0.09% of total energy. LA intake ranged from 4.3% of total energy (NHS in 1980) to 6.7% during the follow-up (NHSII in 2011). The Spearman correlation coefficients between LA and trans fats were between 0.29 (HPFS in 1986) and 0.60 (NHS in 1980) in early follow-up cycles and were substantially attenuated in later cycles (r between 0.05 [NHSII in 2011] and −0.20 [HPFS in 2010]). Top food sources of LA included items containing plant oils, margarines (before the year 2000), and nuts, whereas AA mainly came from animal products such as poultry, fish, red meat, and eggs (Supplementary Table 1).
Table 2.
Spearman correlations among specific dietary fats at baseline, midpoint, and end of follow-up
| n-6 PUFAs | LA | AA | n-6 PUFAs | LA | AA | n-6 PUFAs | LA | AA | |
|---|---|---|---|---|---|---|---|---|---|
| NHS, 1980 |
NHSII, 1991 |
HPFS, 1986 |
|||||||
| % Energy, mean ± SD | 4.37 ± 1.54 | 4.28 ± 1.54 | 0.09 ± 0.03 | 4.92 ± 1.34 | 4.84 ± 1.34 | 0.08 ± 0.04 | 5.31 ± 1.58 | 5.23 ± 1.58 | 0.08 ± 0.03 |
| n-3 PUFAs | 0.28* | 0.28* | 0.31* | 0.58* | 0.57* | 0.26* | 0.47* | 0.46* | 0.32* |
| SFAs | 0.003 | <0.001 | 0.18* | 0.23* | 0.24* | −0.02* | 0.18* | 0.18* | 0.06* |
| Trans fats | 0.60* | 0.60* | −0.06* | 0.34* | 0.35* | −0.09* | 0.29* | 0.29* | −0.09* |
| MUFAs | 0.29* | 0.29* | 0.31* | 0.53* | 0.53* | 0.06* | 0.52* | 0.52* | 0.15* |
| NHS, 1994 | NHSII, 1999 | HPFS, 1998 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % Energy, mean ± SD | 4.77 ± 1.42 | 4.70 ± 1.41 | 0.07 ± 0.03 | 5.55 ± 1.62 | 5.49 ± 1.62 | 0.06 ± 0.03 | 5.46 ± 1.53 | 5.39 ± 1.52 | 0.07 ± 0.03 |
| n-3 PUFAs | 0.47* | 0.47* | 0.22* | 0.67* | 0.67* | 0.21* | 0.44* | 0.44* | 0.25* |
| SFAs | 0.27* | 0.27* | 0.17* | 0.34* | 0.33* | 0.22* | 0.31* | 0.31* | 0.14* |
| Trans fats | 0.34* | 0.34* | 0.02* | 0.30* | 0.29* | 0.13* | 0.31* | 0.32* | −0.004 |
| MUFAs | 0.52* | 0.51* | 0.20* | 0.53* | 0.53* | 0.25* | 0.55* | 0.55* | 0.16* |
| NHS, 2010 | NHSII, 2011 | HPFS, 2010 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % Energy, mean ± SD | 6.44 ± 2.04 | 6.36 ± 2.03 | 0.08 ± 0.04 | 6.76 ± 1.85 | 6.67 ± 1.85 | 0.09 ± 0.05 | 6.28 ± 1.95 | 6.19 ± 1.95 | 0.09 ± 0.05 |
| n-3 PUFAs | 0.65* | 0.64* | 0.34* | 0.55* | 0.54* | 0.31* | 0.60* | 0.59* | 0.42* |
| SFAs | 0.12* | 0.12* | −0.05* | 0.11* | 0.11* | −0.03* | 0.19* | 0.19* | 0.001 |
| Trans fats | −0.16* | −0.15* | −0.18* | 0.05* | 0.05* | −0.08* | −0.21* | −0.20* | −0.22* |
| MUFAs | 0.46* | 0.46* | 0.14* | 0.56* | 0.56* | 0.16* | 0.51* | 0.51* | 0.12* |
*P < 0.001.
Total n-6 PUFAs were associated with a higher risk for type 2 diabetes in the age-adjusted model, but the associations were greatly attenuated after controlling for established type 2 diabetes risk factors, including BMI (Table 3, model 2). After further adjusting for total fats, MUFAs, trans fats, and n-3 PUFAs (Table 3, model 3), we observed an inverse association between n-6 PUFA intake and type 2 diabetes risk: HRs (95% CIs) for low to high quintiles were 1 (reference), 0.93 (0.88, 0.97), 0.95 (0.91, 1.00), 0.92 (0.87, 0.97), and 0.91 (0.85, 0.96) (Ptrend = 0.001). The association between LA intake and type 2 diabetes risk in model 3 was similar after controlling for other fats: HRs (95% CIs) were 1 (reference), 0.94 (0.89, 0.98), 0.95 (0.89, 1.00), 0.93 (0.88, 0.98), and 0.92 (0.87, 0.98) (Ptrend = 0.005) for low to high quintiles of LA intake.
Table 3.
Associations between total n-6 PUFAs and LA and type 2 diabetes risk in NHS, NHSII, and HPFS*
| Quintiles of fatty acid intake (% energy) |
P for trend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| n-6 PUFAs | ||||||
| NHS | ||||||
| Median (range) | 2.62 (0.66, 3.09) | 3.47 (3.09, 3.82) | 4.16 (3.82, 4.53) | 4.95 (4.53, 5.49) | 6.32 (5.49, 20.8) | |
| Cases/person-years | 1,800/447,983 | 1,780/448,767 | 1,922/448,995 | 1,882/448,948 | 1,991/448,694 | |
| Model 1 | 1 | 1.01 (0.94, 1.07) | 1.10 (1.03, 1.17) | 1.08 (1.01, 1.15) | 1.16 (1.08, 1.23) | <0.001 |
| Model 2 | 1 | 0.96 (0.90, 1.03) | 1.02 (0.95, 1.09) | 0.97 (0.90, 1.03) | 1.01 (0.95, 1.08) | 0.71 |
| Model 3 | 1 | 0.95 (0.89, 1.02) | 1.00 (0.93, 1.07) | 0.94 (0.87, 1.01) | 0.97 (0.90, 1.06) | 0.53 |
| NHSII | ||||||
| Median (range) | 3.41 (0.87, 3.84) | 4.17 (3.84, 4.46) | 4.76 (4.46, 5.08) | 5.43 (5.08, 5.88) | 6.60 (5.88, 24.77) | |
| Cases/person-years | 953/355,884 | 996/357,336 | 1,061/358,269 | 1,189/358,491 | 1,261/358,163 | |
| Model 1 | 1 | 1.04 (0.95, 1.13) | 1.09 (0.99, 1.19) | 1.19 (1.09, 1.30) | 1.22 (1.12, 1.33) | <0.001 |
| Model 2 | 1 | 0.96 (0.88, 1.05) | 0.95 (0.86, 1.03) | 0.99 (0.91, 1.08) | 0.98 (0.90, 1.07) | >0.99 |
| Model 3 | 1 | 0.93 (0.85, 1.02) | 0.91 (0.82, 1.00) | 0.94 (0.85, 1.04) | 0.91 (0.80, 1.02) | 0.21 |
| HPFS | ||||||
| Median (range) | 3.53 (0.94, 4.04) | 4.43 (4.04, 4.79) | 5.13 (4.79, 5.50) | 5.91 (5.50, 6.43) | 7.24 (6.43, 21.1) | |
| Cases/person-years | 691/179,560 | 688/181,090 | 756/181,399 | 743/181,415 | 729/180,777 | |
| Model 1 | 1 | 1.00 (0.90, 1.11) | 1.12 (1.01, 1.24) | 1.09 (0.98, 1.21) | 1.07 (0.96, 1.19) | 0.11 |
| Model 2 | 1 | 0.94 (0.84, 1.04) | 1.03 (0.92, 1.14) | 0.98 (0.88, 1.09) | 0.97 (0.87, 1.08) | 0.83 |
| Model 3 | 1 | 0.86 (0.77, 0.96) | 0.90 (0.80, 1.01) | 0.82 (0.73, 0.92) | 0.74 (0.65, 0.85) | <0.001 |
| Pooled† | ||||||
| Model 2 | 1 | 0.96 (0.91, 1.00) | 1.00 (0.95, 1.05) | 0.98 (0.93, 1.02) | 0.99 (0.95, 1.04) | 0.87 |
| Model 3 | 1 | 0.93 (0.88, 0.97) | 0.95 (0.91, 1.00) | 0.92 (0.87, 0.97) | 0.91 (0.85, 0.96) | 0.001 |
| LA | ||||||
| NHS | ||||||
| Median (range) | 2.54 (0.65, 3.01) | 3.39 (3.01, 3.73) | 4.07 (3.73, 4.44) | 4.86 (4.44, 5.40) | 6.23 (5.40, 20.8) | |
| Cases/person-years | 1,800/447,964 | 1,792/448,793 | 1,907/448,907 | 1,898/449,043 | 1,978/448,681 | |
| Model 1 | 1 | 1.01 (0.95, 1.08) | 1.09 (1.02, 1.16) | 1.09 (1.02, 1.16) | 1.15 (1.08, 1.22) | <0.001 |
| Model 2 | 1 | 0.97 (0.91, 1.04) | 1.02 (0.95, 1.08) | 0.98 (0.92, 1.05) | 1.01 (0.95, 1.08) | 0.65 |
| Model 3 | 1 | 0.96 (0.89, 1.02) | 0.99 (0.93, 1.07) | 0.96 (0.89, 1.03) | 0.98 (0.91, 1.06) | 0.70 |
| NHSII | ||||||
| Median (range) | 3.33 (0.86, 3.76) | 4.08 (3.76, 4.38) | 4.68 (4.38, 4.99) | 5.35 (4.99, 5.80) | 6.51 (5.80, 24.7) | |
| Cases/person-years | 957/355,840 | 1,012/357,413 | 1,057/358,191 | 1,175/358,537 | 1,259/358,161 | |
| Model 1 | 1 | 1.05 (0.96, 1.14) | 1.08 (0.99, 1.18) | 1.17 (1.08, 1.28) | 1.22 (1.12, 1.33) | <0.001 |
| Model 2 | 1 | 0.97 (0.89, 1.07) | 0.94 (0.86, 1.03) | 0.99 (0.91, 1.08) | 0.98 (0.90, 1.07) | 0.93 |
| Model 3 | 1 | 0.95 (0.87, 1.04) | 0.91 (0.82, 1.00) | 0.94 (0.85, 1.04) | 0.93 (0.82, 1.05) | 0.33 |
| HPFS | ||||||
| Median (range) | 3.45 (0.77, 3.96) | 4.35 (3.96, 4.71) | 5.05 (4.71, 5.42) | 5.83 (5.42, 6.35) | 7.16 (6.35, 21.0) | |
| Cases/person-years | 698/179,551 | 694/181,094 | 741/181,452 | 745/181,349 | 729/180,794 | |
| Model 1 | 1 | 1.00 (0.90, 1.11) | 1.08 (0.97, 1.20) | 1.08 (0.97, 1.20) | 1.06 (0.95, 1.17) | 0.15 |
| Model 2 | 1 | 0.94 (0.84, 1.05) | 1.00 (0.90, 1.11) | 0.98 (0.88, 1.09) | 0.97 (0.87, 1.08) | 0.80 |
| Model 3 | 1 | 0.87 (0.78, 0.97) | 0.88 (0.79, 0.99) | 0.83 (0.74, 0.94) | 0.77 (0.67, 0.88) | <0.001 |
| Pooled | ||||||
| Model 2 | 1 | 0.97 (0.92, 1.01) | 0.99 (0.94, 1.04) | 0.98 (0.94, 1.03) | 0.99 (0.95, 1.04) | 0.87 |
| Model 3 | 1 | 0.94 (0.89, 0.98) | 0.95 (0.90, 1.00) | 0.93 (0.88, 0.98) | 0.92 (0.87, 0.98) | 0.005 |
*HRs and 95% CIs were calculated through the use of a Cox proportional hazards model. Model 1 was adjusted for age. Model 2 was adjusted for the model 1 variable as well as ethnicity (Caucasian, African American, Asian, or other ethnicity), smoking status (never, former, current [1–14, 15–24, or ≥25 cigarettes/day], or missing), alcohol intake ( 0.0, 0.1–4.9, 5.0–14.9, or >15.0 g/day in women and 0.0, 0.1–4.9, 5.0–29.9, or >30.0 g/day in men, or missing), family history of diabetes (yes/no), menopausal status and postmenopausal hormone use (premenopause, postmenopause [never, former, or current hormone use], or missing; for women only), physical activity (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27.0 MET h/week or missing), multivitamin use (yes/no), baseline hypertension, baseline hypercholesterolemia, updated BMI (<23.0, 23.0–24.9, 25.0–29.9, 30.0–34.9, or >35.0 kg/m2 or missing), total energy intake, and intake of fruits and vegetables. Model 3 was adjusted for the model 1 and 2 variables as well as total fats, trans fats, MUFAs, and other PUFAs.
†Study estimates from three cohorts were pooled by using a fixed effects model.
Intake of AA was positively associated with type 2 diabetes risk in the age-adjusted model (Supplementary Table 2), and the associations were attenuated after adjusting for established type 2 diabetes risk factors and other dietary fats. We further adjusted for major sources of AAs to explore whether these factors could explain the associations. When comparing extreme AA quintiles (low vs. high), HRs (95% CIs) were 1.22 (1.16, 1.29) after controlling for red meat, 1.23 (1.17, 1.30) for processed meat, 1.24 (1.17, 1.31) for poultry, and 1.35 (1.26, 1.44) for fish.
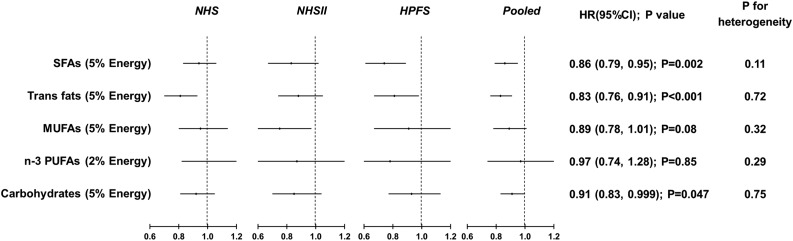
Figure 1 presents estimated type 2 diabetes risk when LA was modeled to specifically replace other macronutrients. Isocalorically replacing energy from SFAs with that from LA (5% of energy) was associated with a 14% (HR 0.86 [95% CI 0.79, 0.95]) lower type 2 diabetes risk, and replacing trans fats with LA (2% of energy) was associated with a 17% (HR 0.83 [95% CI 0.76, 0.91]) lower risk. Type 2 diabetes risk was not significantly different when substituting energy from LA for that from MUFAs (HR 0.89 [95% CI 0.78, 1.01]; 5% of total energy) or total n-3 PUFAs (HR 0.97 [95% CI 0.74, 1.28]; 2% of total energy). When LA replaced 5% of energy from carbohydrates, the HR of type 2 diabetes was 0.91 (95% CI 0.83, 0.999) (P = 0.047). We observed no significant between-study heterogeneity in the substitution analyses (all P for heterogeneity >0.10).
Figure 1.
HRs for type 2 diabetes, with LA substituting for energy from other macronutrients. HRs were calculated in Cox proportional hazards model after adjusting for age, ethnicity (Caucasian, African American, Asian, and other ethnicity), smoking status (never, former, current [1–14, 15–24, or ≥25 cigarettes/day], or missing), alcohol intake (0.0, 0.1–4.9, 5.0–14.9, and >15.0 g/day in women; 0.0, 0.1–4.9, 5.0–29.9, and >30.0 g/day in men; or missing), family history of diabetes (yes/no), menopausal status and hormone use after menopause (premenopause, postmenopause hormone use [never, former, or current], or missing; for women only), physical activity (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, and ≥27.0 MET h/week or missing), multivitamin use (yes/no), baseline hypertension, baseline hypercholesterolemia, updated BMI (<23.0, 23.0–24.9, 25.0–29.9, 30–34.9, and >35.0 kg/m2 or missing), total energy intake, and intake of fruits and vegetables. For fat-fat substitution, we further adjusted for other fats and total fats; for carbohydrate substitution, we further adjusted for energy from protein. Study estimates from the three cohorts were pooled by using a fixed-effects model. Black dots indicate point estimates; the horizontal lines represent the 95% CIs; and the vertical dashed lines represent the reference lines for an HR of 1.
In sensitivity analyses, findings from the substitution analyses were largely similar after further adjusting for incident hypertension and hypercholesterolemia (Supplementary Table 3), or when using the two most recent dietary assessments in order to measure long-term diet (Supplementary Table 4). The associations were attenuated, however, when only baseline dietary data, rather than all follow-up dietary data, were used (Supplementary Table 5).
Conclusions
In three cohort studies of U.S. men and women, we found that higher LA intake was associated with lower type 2 diabetes risk, especially when LA was modeled to isocalorically substitute for SFAs, trans fats, or carbohydrates. Accounting for <2% of total n-6 PUFAs, AA was associated with a higher type 2 diabetes risk. These associations were independent of established and potential risk factors of type 2 diabetes and remained in sensitivity analyses.
Existing studies have reported inconsistent results regarding the relation between LA intake and diabetes risk. In a previous analysis of HPFS, dietary LA showed inverse associations with type 2 diabetes risk only among younger and leaner participants (4), whereas several other studies found a null association between baseline LA intake and long-term diabetes risk (5–8). In our study, we found consistent inverse associations between n-6 PUFAs and risk for type 2 diabetes, especially when we modeled the effects of substituting LA for other macronutrients. Of note, the interrelationships between PUFAs and other macronutrients such as trans fat changed over time in the cohorts, probably because of changes in the amounts of these macronutrients in some foods during the extended follow-up (25,26). To account for these changes, we derived cumulative means from repeated assessments of diet and used other strategies to minimize reverse causation bias. Overall, the consistent findings in the three cohorts of men and women suggest that important heterogeneity by gender is unlikely.
This analysis suggests that LA, as the dominant PUFA in the diet, could be a healthy source of energy for preventing type 2 diabetes when compared with SFAs, trans fats, and carbohydrates. Our data extend earlier findings from the NHS that isocalorically substituting total PUFAs for SFAs, total carbohydrates, and particularly trans fats was associated with a lower risk for type 2 diabetes. Likewise, replacing SFAs or carbohydrates with total PUFAs (not including long-chain marine n-3 PUFAs) was associated with a lower risk for type 2 diabetes in the Iowa Women’s Health Study. In an intervention study, replacing SFAs or carbohydrates with total PUFAs led to lower hemoglobin A1c, improved HOMA of insulin resistance, and a better acute insulin response (27). Abundant evidence supports the notions that SFAs and trans fats should be replaced by cis-unsaturated fats in order to improve blood lipid profile (28) and that replacing SFAs and refined carbohydrates with PUFAs and MUFAs may lead to a lower risk for coronary heart disease (29). This and our earlier study collectively indicate that the quality of dietary fats is not only an important determinant of cardiovascular disease risk but also of type 2 diabetes risk (30).
Potential mechanisms linking LA intake and type 2 diabetes have not been fully elucidated, but several possible explanations have been proposed. For example, incorporating unsaturated fats may improve cell membrane fluidity and functions, such as GLUT translocation, insulin receptor binding and affinity, cell signaling, and ion permeability, that collectively improve insulin sensitivity (31). LA might also affect the balance between fat oxidation and synthesis by regulating related gene expression (such as SREBP1) (32), which could explain the reduced hepatic fat contents of obese participants consuming a diet containing a large amount of LA during a 10-week intervention (33). A high-LA diet may reduce abdominal fat, which is an established risk factor for type 2 diabetes (34). On the other hand, intake of SFAs, trans fats, and refined carbohydrates may deteriorate insulin sensitivity and promote inflammation, both of which predispose to the onset of type 2 diabetes (26,35,36).
The positive association between AA intake and type 2 diabetes risk is consistent with previous findings from the E3N (Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale) study but not with those of the Melbourne Collaborative Cohort Study (MCCS). Consumption of AA is very low (mean <200 mg/day, <0.15% energy) when compared with that of other fatty acids. Thus, we must be cautious when interpreting these results. AA is consumed primarily through animal-based foods, including red meat, processed meat, fish, and poultry, which do not share common associations with type 2 diabetes risk (37). In our analysis, further adjustment for intakes of red meat, processed meat, fish, and poultry did not significantly change the results. AA is known to be a precursor of proinflammatory eicosanoids that might promote the pathogenesis of type 2 diabetes, although anti-inflammatory eicosanoids derived from AA have also been found (38). In studies focusing on circulating fatty acids as biomarkers, AA showed an inverse or a null association with type 2 diabetes risk (14,15). Tissue levels of AA are, however, tightly regulated and do not properly reflect its intake. Two intervention studies found that AA supplementation up to 1.5 g/day did not change platelet AA contents, immune functions, or inflammatory markers among healthy participants (39). Further studies are needed in order to replicate our findings and assess the potential effect of AA intake on diabetes risk.
Strengths of our study include the large sample size, long follow-up duration, and high follow-up rate. Although errors are inevitable when measuring diet, they are more likely to be nondifferential and might bias true associations toward the null, given the prospective study design. A limitation of our study is that participants were exclusively health professionals and primarily Caucasian, limiting the generalizability of the findings to other populations. In addition, we cannot exclude the existence of undiagnosed type 2 diabetes cases, although these would be identified independently of the dietary assessments and thus would be more likely to attenuate true associations of interest. Finally, we cannot exclude the role of residual or unmeasured confounding by other dietary and lifestyle factors in this observational study.
In conclusion, a high intake of LA is associated with a lower risk for type 2 diabetes among U.S. men and women, particularly when LA isocalorically replaces SFAs, trans fats, or carbohydrates. Although evidence is needed from intervention studies in order to substantiate the observed associations, our findings suggest that increasing dietary LA at the expense of unhealthy fats and carbohydrates might facilitate the prevention of type 2 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the participants for their dedication and contribution to the research.
Funding. This study was funded by National Institutes of Health research grants CA186107, CA176726, CA167552, P30 DK46200, DK58845, and DK112940-01. Q.S. was supported by the National Institutes of Health (grant nos. ES021372, ES022981, and HL035464). G.Z. is supported by a postdoctoral fellowship funded by Unilever Research and Development, the 100 Talents Program of the Chinese Academy of Sciences, the National Key Research and Development Program of China, The People’s Republic of China Ministry of Science and Technology (project no. 2018YFC604404), and the Key Deployment Project of the Chinese Academy of Sciences (ZDBS-SSW-DQC-01).
The funders had no role in designing the study; in collecting, analyzing, and interpreting data; in writing the report; or in deciding to submit the manuscript for publication.
Duality of Interest. G.Z. is supported by a postdoctoral fellowship funded by Unilever Research and Development. P.L.Z., A.J.W., and M.A. are employees of Unilever, a producer of food consumer products. F.B.H. has received research support from the California Walnut Commission and honoraria for lectures from Metagenics and Standard Process. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.Z. wrote the manuscript. G.Z., A.J.W., P.L.Z., F.B.H., and Q.S. conceived and designed the study, performed statistical analysis, and interpreted data. G.Z., G.L., W.C.W., A.J.W., M.A., P.L.Z., F.B.H., and Q.S. critically revised the manuscript and approved the final version. G.Z. and Q.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the EPI|Lifestyle 2018 Scientific Sessions of the American Heart Association, New Orleans, LA, 20–23 March 2018.
Footnotes
Clinical trial reg. nos. NCT00005152 and NCT00005182, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0412/-/DC1.
References
- 1.U.S. Department of Agriculture, Agricultural Research Service Nutrient Intakes from Food and Beverages: Mean Amounts Consumed per Individual, by Gender and Age, in the United States, 2013-2014. In What We Eat in America, NHANES 2013-2014. Hyattsville, MD, Centers for Disease Control and Prevention, National Center for Health Statistics, 2016 [Google Scholar]
- 2.Sacks FM, Lichtenstein AH, Wu JHY, et al.; American Heart Association . Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association [published correction appears in Circulation 2017;136:e195]. Circulation 2017;136:e1–e23 [DOI] [PubMed] [Google Scholar]
- 3.Schwab U, Lauritzen L, Tholstrup T, et al. . Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr Res 2014;58:25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–424 [DOI] [PubMed] [Google Scholar]
- 5.Dow C, Mangin M, Balkau B, et al. . Fatty acid consumption and incident type 2 diabetes: an 18-year follow-up in the female E3N (Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale) prospective cohort study. Br J Nutr 2016;116:1807–1815 [DOI] [PubMed] [Google Scholar]
- 6.Song Y, Manson JE, Buring JE, Liu S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: the Women’s Health Study. Diabetes Care 2004;27:2108–2115 [DOI] [PubMed] [Google Scholar]
- 7.Patel PS, Sharp SJ, Jansen E, et al. . Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr 2010;92:1214–1222 [DOI] [PubMed] [Google Scholar]
- 8.Kröger J, Zietemann V, Enzenbach C, et al. . Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2011;93:127–142 [DOI] [PubMed] [Google Scholar]
- 9.Hodge AM, English DR, O’Dea K, et al. . Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–197 [DOI] [PubMed] [Google Scholar]
- 10.Alhazmi A, Stojanovski E, McEvoy M, Garg ML. Macronutrient intake and type 2 diabetes risk in middle-aged Australian women. Results from the Australian Longitudinal Study on Women’s Health. Public Health Nutr 2014;17:1587–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer KA, Kushi LH, Jacobs DR Jr., Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care 2001;24:1528–1535 [DOI] [PubMed] [Google Scholar]
- 12.Salmerón J, Hu FB, Manson JE, et al. . Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr 2001;73:1019–1026 [DOI] [PubMed] [Google Scholar]
- 13.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(Suppl.):1220S–1228S; discussion 1229S–1231S [DOI] [PubMed] [Google Scholar]
- 14.Forouhi NG, Imamura F, Sharp SJ, et al. . Association of plasma phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med 2016;13:e1002094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JHY, Marklund M, Imamura F, et al.; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) . Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol 2017;5:965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan C, Spiegelman D, Rimm EB, et al. . Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185:570–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins DJ, Kendall CW, Vidgen E, et al. . High-protein diets in hyperlipidemia: effect of wheat gluten on serum lipids, uric acid, and renal function. Am J Clin Nutr 2001;74:57–63 [DOI] [PubMed] [Google Scholar]
- 18.Chiuve SE, Fung TT, Rimm EB, et al. . Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvini S, Hunter DJ, Sampson L, et al. . Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–867 [DOI] [PubMed] [Google Scholar]
- 20.Feskanich D, Rimm EB, Giovannucci EL, et al. . Reproducibility and validity of food intake mea-surements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–796 [DOI] [PubMed] [Google Scholar]
- 21.Garland M, Sacks FM, Colditz GA, et al. . The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr 1998;67:25–30 [DOI] [PubMed] [Google Scholar]
- 22.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 23.Manson JE, Rimm EB, Stampfer MJ, et al. . Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 24.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 25.Otite FO, Jacobson MF, Dahmubed A, Mozaffarian D. Trends in trans fatty acids reformulations of US supermarket and brand-name foods from 2007 through 2011. Prev Chronic Dis 2013;10:E85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtenstein AH. Dietary trans fatty acids and cardiovascular disease risk: past and present. Curr Atheroscler Rep 2014;16:433. [DOI] [PubMed] [Google Scholar]
- 27.Imamura F, Micha R, Wu JH, et al. . Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med 2016;13:e1002087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mensink RP. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis. Geneva, World Health Organization, 2016 [Google Scholar]
- 29.Li Y, Hruby A, Bernstein AM, et al. . Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol 2015;66:1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res 2009;48:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vessby B. Dietary fat and insulin action in humans. Br J Nutr 2000;83(Suppl. 1):S91–S96 [DOI] [PubMed] [Google Scholar]
- 32.Clarke SD. The multi-dimensional regulation of gene expression by fatty acids: polyunsaturated fats as nutrient sensors. Curr Opin Lipidol 2004;15:13–18 [DOI] [PubMed] [Google Scholar]
- 33.Bjermo H, Iggman D, Kullberg J, et al. . Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr 2012;95:1003–1012 [DOI] [PubMed] [Google Scholar]
- 34.Summers LK, Fielding BA, Bradshaw HA, et al. . Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 2002;45:369–377 [DOI] [PubMed] [Google Scholar]
- 35.Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol 2009;5:335–344 [DOI] [PubMed] [Google Scholar]
- 36.Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids 2010;45:893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014;383:1999–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med 2008;14:461–469 [DOI] [PubMed] [Google Scholar]
- 39.Calder PC. Dietary arachidonic acid: harmful, harmless or helpful? Br J Nutr 2007;98:451–453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.