Abstract
Despite considerable advances in the treatment of lymphoma, the prognosis of patients with relapsed and/or refractory disease continues to be poor; thus, a continued need exists for the development of novel approaches and therapies. Epigenetic dysregulation might drive and/or promote tumorigenesis in various types of malignancies and is prevalent in both B cell and T cell lymphomas. Over the past decade, a large number of epigenetic-modifying agents have been developed and introduced into the clinical management of patients with haematological malignancies. In this Review, we provide a concise overview of the most promising epigenetic therapies for the treatment of lymphomas, including inhibitors of histone deacetylases (HDACs), DNA methyltransferases (DNMTs), enhancer of zeste homologue 2 (EZH2), bromodomain and extra-terminal domain proteins (BETs), protein arginine N-methyltransferases (PRMTs) and isocitrate dehydrogenases (IDHs), and highlight the most promising future directions of research in this area.
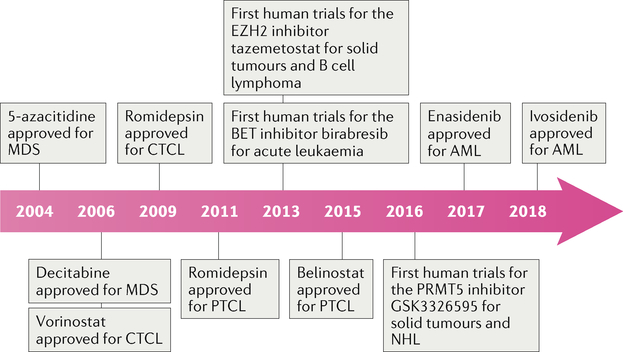
Epigenomics describes the regulation of transcription and translation of genetic information independent of mutations. The prototypical examples of such mechanisms include DNA and histone modification through the transfer of acetyl or methyl groups, which can lead to the silencing of tumour-suppressor genes and/or the overexpression of proto-oncogenes. Epigenetic dysregulation has been shown to have a major role in the pathogenesis of most haematological malignancies, although the use of drugs designed to target epigenetic mechanisms in clinical practice has, thus far, mainly been limited to patients with acute myeloid leukaemia (AML), myelodysplastic syndrome (MDS), multiple myeloma or T cell lymphoma (TCL). FDA-approved epigenetic-modulating agents include the histone deacetylase (HDAC) inhibitors vorinostat (2006), romidepsin (2009), panobinostat (2015) and belinostat (2015), the DNA methyltransferase (DNMT) inhibitors azacitidine (2004) and decitabine (2006) and the isocitrate dehydrogenase (IDH) inhibitors enasidenib (2017) and ivosidenib (2018) (FIG. 1). Research conducted over the past decade has demonstrated a high frequency of somatic mutations in genes that encode epigenetic enzymes in B cell lymphomas1–5. Additionally, patterns of aberrant DNA methylation have been observed in diffuse large B cell lymphoma (DLBCL) and are associated with different clinical outcomes in specific epigenetic subgroups6. Consequently, epigenetic dysregulation has been exploited for the development of novel agents.
Fig. 1 |. Timeline of FDA approvals of epigenetic-modulating therapies for patients with lymphomas.
AML, acute myeloid leukaemia; BET, bromodomain and extra-terminal domain protein; CTCL, cutaneous T cell lymphoma; EZH2, enhancer of zeste homologue 2; MDS, myelodysplastic syndrome; PRMT5, protein arginine N-methyltransferase 5; PTCL, peripheral T cell lymphoma; NHL, non-Hodgkin lymphoma.
In this Review, we describe the most promising epigenetic-modifying agents currently under investigation for various types of lymphoma. We include drugs that are already approved by the FDA, those that are currently in clinical trials and agents that are currently in the preclinical setting but are likely to soon be translated into first-in-human and/or proof-of-concept trials (Box 1). The various classes of agent include inhibitors of HDACs, DNMTs, enhancer of zeste homologue 2 (EZH2), bromodomain and extra-terminal domain proteins (BETs), protein arginine N-methyltransferases (PRMTs) and IDHs.
Box 1 |. Proposed epigenetic-based treatments by lymphoma subtypes.
-
Follicular lymphoma
HDAC inhibitors
EZH2 inhibitors
-
Diffuse large B cell lymphoma
EZH2 inhibitors
HDAC inhibitor plus a BCL-2 inhibitor
-
Hodgkin lymphoma
HDAC inhibitor plus an immune-checkpoint inhibitor
-
Cutaneous T cell lymphoma
HDAC inhibitors
-
Peripheral T cell lymphoma
Azacitidine plus CHOP (specifically AITL)
AITL, angioimmunoblastic T cell lymphoma; CHOP cyclophosphamide, doxorubicin, vincristine and prednisone; EZH2, enhancer of zeste homologue 2; HDAC, histone deacetylase.
HDAC inhibitors
Histone and non-histone protein acetylation is regulated through the opposing functions of histone acetyl-transferases (HATs) and HDACs. Human HDACs are classified into four major classes on the basis of sequence homology to yeast proteins and shared cellular localization and function: class I (HDAC1, HDAC2, HDAC3 and HDAC8); class II (HDAC4, HDAC5, HDAC6, HDAC7, HDAC9 and HDAC10), class III (NAD-dependent protein deacetylase sirtuin 1 (SIRT1)-SIRT7); and class IV (HDAC11)7. Acetylation typically increases gene expression by inducing an open chromatin state that improves the ability of various transcription factors to bind to DNA, as well as by enhancing the activity of transcriptional activators (such as p53) or impairing the function of transcriptional repressors (such as BCL-6)8,9 (FIG. 2a).
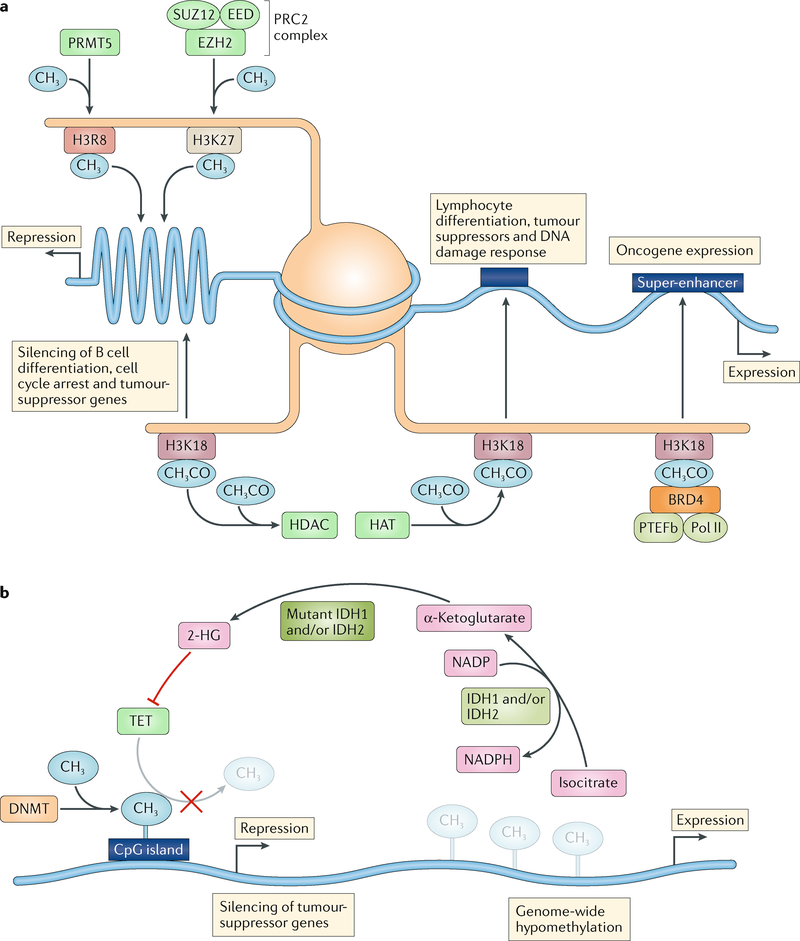
Fig. 2 |. Mechanisms of action of common epigenetic enzymes.
a | Histone acetylation is regulated by the interplay of histone acetyltransferases (HATs) and histone deacetylases (HDACs). HATs catalyse the transfer of acetyl groups (CH3CO) from acetyl-CoA onto lysine groups on histone tails (such as histone H3 lysine 18 (H3K18)). HDACs catalyse the removal of acetyl groups. The net effect of acetylation is typically an open chromatin state leading to increased expression of genes controlling lymphocyte differentiation, tumour suppression and DNA damage repair. Reduced levels of acetylation might be caused by upregulation of HDACs or loss of function of HATs. Bromodomain proteins (such as BRD4) bind to acetylated lysine groups and, through the recruitment of positive transcription elongation factor b (PTEFb) and RNA polymerase II (Pol II), are able to bind to super-enhancers, resulting in expression of oncogenes. Enhancer of zeste homologue 2 (EZH2) associates with the subunits EED and SUZ12 to form polycomb repressive complex 2 (PRC2). This complex then catalyses the transfer of methyl groups (CH3) to lysine groups on histone tails (such as H3K27), which silences genes that usually regulate B cell differentiation. Protein arginine N-methyltransferases (PRMTs) catalyse the methylation of arginine groups on histone tails (such as H3R8), leading to the development of a closed chromatin state and thus silencing genes with a key role in cell cycle regulation. b | DNA methyltransferases (DNMTs) catalyse the transfer of methyl groups to cytosine nucleotides. Methylation of CpG islands within gene promoters leads to the silencing of tumour-suppressor genes and might be caused either by upregulation of DNMTs or loss of function of one or more members of the methylcytosine dioxygenase (TET) family of proteins. Isocitrate dehydrogenase (IDH) enzymes catalyse the synthesis of α-ketoglutarate using the cofactor NADP. Mutations in IDH1 or IDH2 produce the oncometabolite 2-hydroxyglutarate (2-HG), which inhibits TET proteins. Genome-wide hypomethylation occurs by spontaneous or enzyme-driven deamination of cytosine residues, causing DNA to become unstable.
Multiple findings suggest that histone acetylation has a key role in lymphoid malignancies and particularly in germinal centre-derived tumours. Loss-of-function mutations in genes encoding proteins with established roles in histone acetylation, such as CREBBP and, less frequently, EP300, are commonly observed in DLBCL (~25% of patients) and in follicular lymphoma (FL; 60% of patients) but can also be found in small subsets of patients with other types of lymphoma2,5 (BOX 2). Somatic mutations in CREBBP result in impaired p53 activation while also promoting the oncogenic effects of BCL-6 (REF5); additionally, loss-of-function mutations of CREBBP also result in repression of genes that are important for exit from the germinal centres, including most genes regulated by BCL-6 (REFS10,11). Among these target genes, silencing of those involved in MHC class II-mediated antigen presentation might have important roles such as disruption of immune surveillance11,12. Other acetyltransferases implicated in lymphoma include TIP60 (also known as KAT5), which accelerates the development of MYC-driven tumours when deleted in mice and is expressed at lower levels in a small fraction of FLs and DLBCLs13; other genes involved in epigenetic regulation are infrequently mutated. Data from translational studies have confirmed the activity of HDAC inhibitors either alone or in combination with hypomethylating agents, targeted therapies or chemotherapy in a range of haematological malignancies including acute lymphoblastic leukaemia (ALL), cutaneous T cell lymphoma (CTCL), DLBCL, Hodgkin lymphoma (HL) and Burkitt lymphoma (BL)14–20. Furthermore, inhibition of HDAC6 has been shown to upregulate CD20 and enhances the efficacy of anti-CD20 monoclonal antibodies, such as rituximab21. Collectively, these data suggest that deregulation of either HDACs or HATs is an important mechanism of lymphomagenesis and thus an attractive target for pharmacological inhibition.
Box 2 |. Biomarker-driven selection of epigenetic therapies for lymphoma.
-
HDAC inhibitors
Loss of CREBBP or EP300 (HATs) and TET2 mutations
-
DNMT inhibitors
Genome-wide or promoter (such as DAPK1) méthylation status and TET2 mutations
-
EZH2 inhibitors
EZH2 mutations and loss of INI1 or SMARCA4
-
BET inhibitors
MYC overexpression or translocation
-
PRMT5 inhibitors
PRMT5 overexpression and wild-type TP53
-
IDH inhibitors
IDH1 or IDH2 mutations
BET, bromodomain and extra-terminal domain protein; DNMT, DNA methyltransferase; EZH2, enhancer of zeste homologue 2; HAT, histone acetyltransferase; HDAC, histone deacetylase; IDH, isocitrate dehydrogenase; PRMT5, protein arginine N-methyltransferase 5.
Five HDAC inhibitors are currently approved by the FDA22–26 (FIG. 1). As the initial HDAC inhibitor approved for TCL, vorinostat has been tested in patients with relapsed non-Hodgkin lymphoma (NHL), including FL, marginal zone lymphoma (MZL) and mantle cell lymphoma (MCL). In general, this agent has shown modest levels of single-agent activity, with the exception of FL, in which an overall response rate (ORR) of47–49% and a complete response (CR) rate of 23% was observed27,28. In addition to its established role in myeloma, panobinostat has shown promise in HL (27% ORR), DLBCL (17.1% ORR) and CTCL (17.3% ORR)29–31. Other HDAC inhibitors currently in development have shown signs of activity in lymphomas, including abexinostat and quisinostat (broad-spectrum HDAC inhibitors), mocetinostat and entinostat (both of which inhibit HDAC1, HDAC2, HDAC3 and HDAC11) and chidamide (which inhibits HDAC1, HDAC2, HDAC3 and HDAC10). Response rates range between 12% and 56% depending on both the drug and the lymphoma subtype, with the most robust responses seen with abexinostat in FL (56% ORR) and chidamide in peripheral T cell lymphoma (PTCL) (28% ORR and 14% CR rate) (TABLE 1; Supplementary Table 1). Treatment with HDAC inhibitors is frequently associated with thrombocytopenia (in 80–90% of patients), fatigue (in 30–50%) and gastrointestinal toxicities (in 40–60%), with exact frequencies depending on the specific agent32–36. Whether or not the presence of mutations in genes encoding HAT enzymes serves as a reliable biomarker of response to HDAC inhibitors currently remains to be seen. In a phase II trial exploring the safety and efficacy of panobinostat in patients with relapsed DLBCL, mutations in myocyte-specific enhancer factor 2B (MEF2B) were detected in 15.4% of patients and were associated with an improved response to therapy. However, these findings are based on data from only 6 patients harbouring the MEF2B mutation out of a small study population of 39 patients. Furthermore, data on copy number variations were not included in the analysis. Lastly, no association was determined between mutations in CREBBP or EP300 and response to therapy37.
Table 1 |.
Summary of trials involving single-agent HDAC inhibitors
| Agent | n | Lymphoma subtype | Outcomes | Common grade ≥3 adverse events (% of patients) | Year | Refs |
|---|---|---|---|---|---|---|
| Panobinostat | 35 | DLBCL | ORR 17.1% and CRR 11.4% | Thrombocytopenia (83); neutropenia (34); diarrhoea (11) | 2018 | 30 |
| Panobinostat | 139 | CTCL | ORR 17.3% | Thrombocytopenia (23); neutropenia (13); diarrhoea (6) | 2013 | 31 |
| Panobinostat | 129 | HL | ORR 27%, CRR 4% and median PFS 6.1 months | Thrombocytopenia (79); neutropenia (21); anaemia (21) | 2012 | 29 |
| Vorinostat | 56 | FL, NHL and MCL | FL: ORR 49% and median PFS 20 months; NHL: ORR 43%; MCL: ORR 0% | In 80% in total, including thrombocytopenia (48); neutropenia (41); leukopenia (12.5) | 2014 | 27 |
| Vorinostat | 35 | FL and MZL | FL: ORR 47% and CRR 23%; MZL: ORR 22% | Thrombocytopenia (29); neutropenia (17); lymphopenia (14) | 2011 | 28 |
| Abexinostat | 100 | NHL and CLL | ORR 28%; FL: ORR 56% | In 82% in total, including thrombocytopenia (80); neutropenia (27); anaemia (12) | 2017 | 32 |
| Mocetinostat | 72 | DLBCL and FL | DLBCL: ORR 18.9% and CBR 54.1%; FL: ORR 11.5% and CBR 73.1% | In 57% in total, including fatigue (23); neutropenia (15); thrombocytopenia (12) | 2017 | 34 |
| BeLinostat | 22 | Aggressive BCL | ORR 10.5% | Lymphopenia (10); all other adverse effects occurred in 5% or fewer patients | 2016 | 152 |
| Entinostat | 49 | HL | ORR 12%, CBR 24%, median PFS 5.5 months and OS 25.1 months | Thrombocytopenia (63); anaemia (47); neutropenia (41) | 2016 | 33 |
| Quisinostat | 26 | MF | ORR 24% and median PFS 5.1 months | Hypertension (4); fatigue (4); pruritus (4) | 2016 | 35 |
| Chidamide | 79 | PTCL | ORR 28%, CRR 14%, median PFS 2.1 months and median OS 21.4 months | Thrombocytopenia (22); leukopenia (13); neutropenia (11) | 2015 | 36 |
All trials included in this table were phase II designs. BCL, B cell lymphoma; CBR, clinical benefit rate; CLL, chronic lymphocytic leukaemia; CRR, complete response rate; CTCL, cutaneous T cell lymphoma; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; HDAC, histone deacetylase; HL, Hodgkin lymphoma; MCL, mantle cell lymphoma; MF, mycosis fungoides and/or Sezary syndrome; MZL, marginal zone lymphoma; NHL, non-Hodgkin lymphoma; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PTCL, peripheral T cell lymphoma.
Novel HDAC inhibitor-based combination regimens are being evaluated in patients with a variety of lymphoid malignancies (TABLE 2). HDAC inhibitors seem to have synergistic effects when used in combination with anti-CD20 monoclonal antibodies, proteasome inhibitors or immunomodulatory agents37–40. In a report published in November 2017, romidepsin combined with the antimetabolite pralatrexate demonstrated impressive levels of activity in patients with PTCL, with a 71% ORR, as well as a 33% ORR when TCLs were excluded from the analysis41. Also of note, the novel first-in-class dual HDAC and PI3K inhibitor CUDC-907 has shown exceptional levels of activity (55% ORR) and tolerability (grade ≥3 adverse events in 43%) in patients with either transformed or de novo DLBCL, as well as in those with other lymphoma subtypes (57% ORR) in a phase I first-in-human trial42. As a result of these promising initial observations, this agent is currently being investigated further in a phase II cohort of adult patients with DLBCL and a phase I trial involving paediatric patients with lymphomas (NCT02674750 and NCT02909777).
Table 2 |.
Summary of trials involving HDAC inhibitors in combination with other agents
| Drug | Trial phase (n) | Lymphoma subtype | Outcomes | Common grade ≥3 adverse events (% of patients) | Year | Refs |
|---|---|---|---|---|---|---|
| Vorinostat + R-ICE | I (29) | AH lymphomas | ORR 70% and CRR 30% | In 86% in total, including hypophosphataemia (41); hypokalaemia (34); febrile neutropenia (28) | 2013 | 44 |
| Vorinostat + bexarotene | I (23) | CTCL | ORR 17.3% and CBR 30.4% | Hypertriglyceridemia (13); neutropenia (13); nausea (4) | 2012 | 153 |
| Vorinostat + Gem/Bu/Mel + HDT-ASCT | I/II (78) | DLBCL, HL and TCL | 25-month EFS 61.5% and 25-month OS 73% | Mucositis (38); hyperbilirubinemia (15); rash (8) | 2015 | 46 |
| Vorinostat + R-CHOP | I/II (72) | DLBCL | 2-year PFS 73% and 2-year OS 86% | Neutropenia (63); febrile neutropenia (38); anaemia (36) | 2018 | 43 |
| Vorinostat + rituximab | II (28) | FL, MZL and MCL | ORR 46% and median PFS 29.2 months | Fatigue (32); lymphopenia (25); thrombocytopenia (17) | 2015 | 38 |
| Romidepsin + pralatrexate | I (29) | NHL | ORR 58%; PTCL: ORR 71% | Thrombocytopenia (28); anaemia (24); febrile neutropenia (14) | 2018 | 41 |
| Romidepsin + CHOP | I (35) | PTCL | CRR51% | Neutropenia (89); thrombocytopenia (78); anaemia (43) | 2015 | 47 |
| Romidepsin + ICE | I (17) | PTCL | ORR 78% and CRR 64% | Thrombocytopenia (95); neutropenia (84); anaemia (73) | 2015 | 48 |
| Romidepsin + [enalidomide | I/II (21) | TCL | PTCL: ORR 50%; CTCL: ORR 56% | NR | 2014 | 40 |
| Panobinostat + everolimus | I (30) | FL, TCL, MCL and HL | ORR 33%; HL: ORR 43% and CRR 15% | Thrombocytopenia (63); neutropenia (47); anaemia (30) | 2013 | 154 |
| Panobinostat + R-ICE | I/II (23) | HL | CRR 82% | Anaemia (100); thrombocytopenia (100); neutropenia (64) | 2018 | 45 |
| Panobinostat + rituximab | II (40) | DLBCL | ORR 28% and median DOR 14.5 months | Thrombocytopenia (68); neutropenia (32); vomiting (11) | 2016 | 37 |
| Panobinostat + bortezomib | II (23) | PTCL | ORR 43% and CRR 22% | Thrombocytopenia (74); neutropenia (43); diarrhoea (22) | 2015 | 39 |
| CUDC-907 | I (44) | DLBCL, tFL and HL | SD in 57%; DLBCL: ORR 55% | In 43% in total, including thrombocytopenia (20); neutropenia (9) | 2016 | 42 |
CBR, clinical benefit rate; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; CRR, complete response rate; CTCL, cutaneous T cell lymphoma; DLBCL, diffuse large B cell lymphoma; DOR, duration of response; EFS, event-free survival; FL, follicular lymphoma; Gem/Bu/Mel, gemcitabine, busulfan and melphalan; HDAC, histone deacetylase; HDT-ASCT, high-dose therapy-autologous stem cell transplantation; HL, Hodgkin lymphoma; ICE, ifosfamide, carboplatin and etoposide; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NHL, non-Hodgkin lymphoma; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PTCL, peripheral T cell lymphoma; R-ICE, rituximab, ifosfamide, carboplatin and etoposide; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; SD, stable disease; TCL, T cell lymphoma; tFL, transformed follicular lymphoma.
Clinical trials designed to investigate the added benefit of HDAC inhibitors in combination with standard chemotherapy regimens, as part of either frontline or salvage therapy, in larger cohorts of patients have had mixed results. For example, vorinostat was found to cause high rates of febrile neutropenia and sepsis when combined with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) in 72 patients with newly diagnosed advanced-stage DLBCL and fell short of the predefined efficacy end points (2-year progression-free survival (PFS) and overall survival (OS) of 73% and 86%, respectively)43. Studies involving smaller cohorts of patients receiving vorinostat or panobinostat combined with rituximab, ifosfamide, carboplatin and etoposide (R-ICE) have demonstrated a 70% ORR (30% had CRs) across all types of lymphoma and an 82% CR rate in patients with HL44,45. However, the combination therapy in the latter study was associated with considerable levels of haematological toxicities, including grade 4 neutropenia (in 55% of patients) and thrombocytopenia (in 100%)45. Vorinostat has also been tested in combination with gemcitabine, busulfan and melphalan as part of the conditioning regimen before autologous stem cell transplantation (ASCT). This combination was found to be safe and highly active, with 25-month event-free survival (EFS) and OS values of 61.5% and 73%, respectively, in patients with DLBCL46. This regimen, combined with lenalidomide, is currently being investigated in a phase I trial involving patients with non-germinal centre B cell (GCB)-like DLBCL (NCT02589145). Romidepsin has also been combined with chemotherapy in patients with T cell lymphomas. Combinations include with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) in a phase I trial involving 37 patients with previously untreated PTCL and with ifosfamide, carboplatin and etoposide (ICE) in a phase I trial involving 17 patients with relapsed PTCL. Both trials seem to suggest safety, tolerability and clinical benefit from this combination; thus, randomized studies with larger cohorts of patients are warranted47,48. Other clinical trials combining HDAC inhibitors with chemotherapy are currently ongoing (Supplementary Table 1).
DNMT inhibitors
DNMTs catalyse the transfer of methyl groups onto cytosine nucleotides within CpG sequences in DNA strands, resulting in changes in conformation that prevent the binding of transcription factors and thus silence the expression of methylated genes. DNMTs include DNMT1, which maintains pre-existing methylation patterns, and DNMT3A and DNMT3B, which establish new sites of methylation49 (FIG. 2b). In general, the DNA of nonmalignant cells is heavily methylated, with the exception of CpG islands, which are typically located near gene promoters. The traditional model linking DNA methylation with oncogenesis describes the hypermethylation of promoters controlling tumour-suppressor genes and genome-wide hypomethylation leading to genomic instability6. Indeed, the presence of specific DNA methylation patterns is associated with tumorigenesis as well as prognosis across a variety of malignancies. Specific genes that have been found to be hypermethylated and subsequently lead to lymphomagenesis include TP63 in chronic lymphocytic leukaemia (CLL), DAPK1 in FL, miR-34A in DLBCL, CDKN2B in MCL, P16 (also known as CDKN2A) in TCL and genes regulated by components of the polycomb repressive complex 2 (PRC2) in BL50–55. In DLBCL, the disruption of DNA methylation patterns is associated with unfavourable clinical outcomes and is potentially more prognostically relevant than the International Prognostic Index score6,56,57. Differences in DNA methylation signatures also characterize molecular subtypes of DLBCL (for example, GCB and non-GCB)58,59. Heterogeneous DNA methylation has been shown to correlate with reduced time to first treatment and unfavourable markers such as unmutated immunoglobulin heavy chain variable region (IGHV) and TP53 deletions in patients with CLL60.
Unlike in myeloid malignancies, alterations in DNA methylation in patients with B cell lymphomas are not known to be caused by specific mutations in DNMTs or other genes that encode enzymes that regulate methylation or demethylation (such as IDH or TET). Although the precise mechanisms remain unclear, altered DNA methylation has been suggested to, in part, be achieved through the actions of the mutagenic enzyme activation-induced cytidine deaminase, which catalyses the deamination of methylated cytosine nucleotides61. This enzyme is expressed specifically in GCBs and induces somatic hypermutation and class switching of the immunoglobulin loci61. In addition, DNMT3B can be overexpressed in DLBCL and in BL (in as many as 86% of BLs) and might be regulated by microRNAs; expression of this enzyme might even correlate with adverse outcomes62,63.
The DNMT inhibitors (or hypomethylators) azacitidine and decitabine represent the first class of epigenetic-modulating drugs to be approved by the FDA. These drugs are pyrimidine nucleoside analogues of cytosine that are able to become incorporated into DNA and form covalent bonds between the 5-azacytosine ring and the DNMT enzyme, thus causing irreversible DNMT inactivation64. Logically, DNMT inhibitors would seem likely to function by reversing the silencing of tumour-suppressor genes, although the precise mechanism of action of DNMT inhibitors is poorly understood. However, data published in the past few years indicate that the antitumour effects of these agents might be mediated by stimulation of the immune tumour microenvironment. For example, in non-lymphoma models, DNMT inhibitors have been shown to cause reactivation of endogenous retroviruses, leading to upregulation of the viral defence pathway and a type I interferon response65. Data from other studies have also demonstrated increased expression of tumour-associated antigens, such as MAGE and other cancer-testis antigens, and activation of the IFNα-IFNβ signalling pathway, leading to improved immune recognition66,67.
Both azacitidine and decitabine were initially approved for MDS (FIG. 1), although both agents are also commonly used as off-label treatments for AML mainly in elderly patients (often arbitrarily defined as those ≥60 years of age) or in those who are not fit to receive intensive remission induction therapy, as maintenance following induction and for relapsed and/or refractory disease per National Comprehensive Cancer Network (NCCN) guidelines68. In fact, both agents have been shown to improve survival compared with conventional induction therapy in elderly patients, likely owing to the high risk of treatment-related toxicities and the typically unfavourable prognosis of this patient population69–72.
The potential for expanding the role of azacitidine and/or decitabine beyond myeloid malignancies has led to the initiation of trials designed to investigate the effects of these agents across various lymphoid malignancies and in novel combinations64,73–80 (TABLE 3). The outcomes of studies testing these agents as monotherapies in patients with CLL, NHL or HL have been disappointing, with limited levels of clinical benefit observed73–75. Furthermore, no strong evidence is available from clinical trials to suggest that DNA methylation status is a reliable biomarker that is predictive of response to DNMT inhibitors. However, DNMT inhibitors have been shown to render chemotherapy-resistant DLBCLs sensitive to the chemotherapies that form the CHOP regimen both in laboratory experiments and in serial biopsy samples from patients with DLBCL enrolled in a phase I clinical trial64. Furthermore, the sequential administration of decitabine followed by doxorubicin to mouse xenograft models of lymphoma has been shown to result in a substantial reduction in tumour burden64. Building upon preclinical data, similar to HDAC inhibitors, the efficacy of DNMT inhibitors in combination with standard first-line R-CHOP has been explored, and these trials have demonstrated impressive response rates with few added toxicities compared with standard R-CHOP alone64,76. Azacitidine has also been combined with chemotherapy as a second-line therapy and in patients who are eligible for high-dose therapy (TABLE 3), and this approach merits further study77,78. In conclusion, the future role of DNMT inhibitors in patients with lymphoma is uncertain, although these agents could potentially still have value if used in combination with other regimens or therapies. In particular, a strong scientific rationale exists for the combination of DNMT inhibitors with HDAC inhibitors because both hypermethylated DNA and under-acetylated histones are associated with closed chromatin states that repress gene expression through independent mechanisms. Both therapies can cause substantial toxicities at high doses but also retain their chromatin-modifying properties at lower doses; therefore, further investigations of the performance of this combination at various dosages and treatment durations should be conducted.
Table 3 |.
Summary of trials involving DNMT inhibitors
| Agents | Trial phase (n) | Lymphoma subtype | Outcomes | Common grade ≥3 adverse events (% of patients) | Year | Refs or ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|
| Azacitidine + R-CHOP | I (33) | DLBCL, FL (grade 3b) and tFL | ORR 97% and CRR 85% | In 64% in total, including neutropenia (58); febrile neutropenia (21) | 2017 | 76 |
| Azacitidine + romidepsin | II (30) | HL, NHL and TCL | NHL: ORR 28% and CRR 16%; PTCL: ORR 83% and CRR 50% | NR | 2017 | 79 |
| Azacitidine+vorinostat + HDT-ASCT | I (60) | DLBCL, HL and TCL | DLBCL: EFS 65% and OS 77%; HL: EFS 76% and OS 95%; TCL: EFS 88% and OS 88% (all at a median follow-up duration of 15 months) | Neutropenic fever (100); mucositis (32); elevated transaminase levels (25) | 2016 | 78 |
| Azacitidine + R-CHOP | I (12) | DLBCL | CRR 92% | Neutropenia (100); neutropenic fever (33) | 2013 | 64 |
| Azacitidine | II (9) | CLL | ORR 0% | NR | 2013 | 73 |
| Azacitidine + R-CV | I (10) | DLBCL, FL,HL, SLL and CTCL | ORR 20%, CRR 10% and CBR40% | Neutropenia (50); thrombocytopenia (40); anaemia (20) | 2011 | 77 |
| Decitabine | I (20) | CLL and NHL | ORR 0% | Thrombocytopenia (55); neutropenia (45); febrile neutropenia (35) | 2010 | 74 |
| Decitabine + R-CHOP | I/II | DLBCL | Study ongoing | - | 2016 | NCT02951728 |
| Azacitidine + avelumab + utomilumab | Ib | DLBCL | Study ongoing | - | 2016 | NCT02951156 |
CBR, clinical benefit rate; CLL, chronic lymphocytic leukaemia; CRR, complete response rate; CTCL, cutaneous T cell lymphoma; DLBCL, diffuse large B cell lymphoma; DNMT, DNA methyltransferase; EFS, event-free survival; FL, follicular lymphoma; HDT-ASCT, high-dose therapy-autologous stem cell transplantation; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; NR, not reported; ORR, overall response rate; OS, overall survival; PTCL, peripheral T cell lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; R-CV, rituximab, cyclophosphamide and vincristine; SLL, small lymphocytic leukaemia; TCL, T cell lymphoma; tFL, transformed follicular lymphoma.
EZH2 inhibitors
EZH2 is a component of PRC2, along with EED and SUZ12. EZH2 is the enzymatic subunit that catalyses the methylation of Lys27 of histone H3 (H3K27) with as many as three methyl groups. EZH2 has an essential role in the GCBs that give rise to FL and DLBCL, as documented in mouse models in which deletion of this gene completely abrogated the formation of germinal centres. This effect is mediated by the EZH2-mediated silencing of genes encoding cell cycle checkpoint proteins and those involved in plasma cell differentiation81–83 (FIG. 2 a).
Mutations in EZH2 are highly prevalent in patients with B cell lymphomas and occur specifically in GCB-DLBCLs and FLs, with an incidence of approximately 15–20% in both tumour types3,84. The vast majority of these genetic lesions involve point mutations resulting in substitution of tyrosine 641 (Y641; either Y641F, Y641N, Y641S or Y641H) within the histone methyl-transferase domain of EZH2. These alterations lead to a gain-of-function effect whereby the mutant form of this protein enables more efficient trimethylation of H3K27 (REF85). The consequence of these mutations is the aberrant and permanent silencing of the same cell cycle checkpoint and plasma cell differentiation genes that EZH2 represses in nonmalignant cells of the germinal centre82–86. In vivo, expression of the gain-of-function mutant allele in GCBs synergizes with BCL-2 deregulation and accelerates the development of lymphomas82, thus providing a rationale for the development of drugs designed to inhibit EZH2. In preclinical studies, EZH2 inhibitors have been shown to induce proliferation arrest, differentiation and eventual apoptosis of DLBCL cells over the course of several days. These effects are more rapid in DLBCL cells that harbour EZH2 mutations but also occur in EZH2-wild-type DLBCL cells82. These findings have also been demonstrated in mice, in which the EZH2 inhibitor tazemetostat enabled dose-dependent inhibition of tumour growth in both EZH2-mutant (Y646F) and EZH2-wild-type B cell NHL xenografts associated with a loss of H3K27 trimethylation87,88. This general dependency that goes beyond cells harbouring EZH2 mutations reflects the essential role that EZH2 has in the survival of the GCBs from which these lymphomas arise. In FL, SESTRIN1 (encoded by the tumour-suppressor gene SESN1, located within chromosome 6q) seems to be an important target of EZH2 because cells characterized by 6q deletions are rendered less susceptible to EZH2 inhibitors89. In contrast to B cell lymphomas, EZH2 acts as a tumour suppressor in T cell malignancies, which manifest loss-of-function mutations of EZH2 and genes encoding other components of PRC2 (REF90). In particular, genes encoding components of PRC2 have an especially high rate of deletions or sequence mutations in early T cell precursor ALL91. Homozygous inactivation of EHZ2 in mouse models of leukaemia was found to accelerate the progression of early T cell precursor ALL, in part through activation of the STAT3 pathway92.
EZH2 inhibitors first entered clinical trials in 2013 (FIG. 1). Results from these initial trials have since been reported for tazemetostat, valemetostat and GSK2816126 and generally show encouraging preliminary results93–95 (TABLE 4). The largest study with data available thus far is a phase II trial involving 165 patients with relapsed and/or refractory DLBCL or FL. Patients were stratified by EZH2 mutation status, and results reveal ORRs of 40% and 63% in patients with EZH2+ DLBCL and FL, respectively. By contrast, ORRs were 18% and 28% in those with EZH2− DLBCL and FL, respectively. Grade ≥3 adverse events were reported in 18% of patients, and the most common toxicities across all grades were nausea, thrombocytopenia, cough, diarrhoea, fatigue and weakness96. In an updated report presented at the Congress of the European Haematology Association (EHA) of 82 evaluable patients with FL (as of 1 May 2018), an ORR of 71% with CRs in 11% of patients was reported in patients with EZH2+ disease. Again, a modest ORR (33%) was observed in patients with EZH2− disease97. On the basis of the above evidence, a role clearly exists for EZH2 mutation status in predicting a response to therapy in both DLBCL and FL (BOX 2). However, because some patients with EZH2-wild-type disease also respond, albeit to a lesser extent than those with mutant EZH2, more selective biomarkers of responsiveness continue to be needed. To this end, an analysis of mutations in genes encoding INI1 (also known as SMARCB1) and SMARCA4 (components of the SWI/SNF complex) was conducted in a cohort of patients with solid tumours receiving tazemetostat. Interestingly, patients whose tumours had a loss of INI1 and SMARCA4 were found to have a better response than those with wild-type disease, most likely owing to higher levels of oncogenic addiction to EZH2 in these tumours93. Moving forward, further investigations of the predictive value of this biomarker might be warranted in patients with lymphoma.
Table 4 |.
Summary of trials involving EZH2 inhibitors
| Drug | Trial phase (n) | Lymphoma subtype | Outcomes | Common grade ≥3 adverse events (% of patients) | Year | Refs or ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|
| Tazemetostat | I (21) | B cell NHL | ORR 38% and CR 14% | In 9% in total, including thrombocytopenia (3); neutropenia (3) | 2018 | 93 |
| Tazemetostat | II (165) | DLBCL and FL | ORR 40% in EZH2-mutant DLBCL and 18% in EZH2-wild-type DLBCL; ORR 63% in EZH2-mutant FL and 28% in EZH2--wild-type FL | In 18% in total | 2017 | 96 |
| Valemetostat | I (15) | NHL | ORR 53% and CBR 86%; TCL: 80% ORR | NR | 2017 | 95 |
| GSK2816126 | I (14) | NHL | DLBCL: 1 PR; FL: 1 SD | NR | 2016 | 94 |
| Tazemetostat + R-CHOP | Ib/II | DLBCL | Study ongoing | - | 2016 | NCT02889523 |
| Tazemetostat | II | B cell NHL | Study ongoing | - | 2016 | NCT02875548 |
| MAK683 | I/II | DLBCL | Study ongoing | - | 2016 | NCT02900651 |
| CPI-1205 | I | Lymphomas | Study ongoing | - | 2015 | NCT02395601 |
| Tazemetostat + atezolizumab | I | DLBCL | Study ongoing | - | 2014 | NCT02220842 |
CBR, clinical benefit rate; CR, complete response; DLBCL, diffuse large B cell lymphoma; EZH2, enhancer of zeste homologue 2; FL, follicular lymphoma; NHL, non-Hodgkin lymphoma; NR, not reported; ORR, overall response rate; PR, partial response; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; SD, stable disease; TCL, T cell lymphoma.
Dual inhibition of EZH1 and EZH2 might lead to greater suppression of H3K27 trimethylation and provide higher levels of anti-lymphoma activity than inhibition of EZH2 alone98. This discovery has led to the development of valemetostat, which has indeed shown good levels of activity across a range of B cell and T cell NHL subtypes (53% ORR and 86% clinical benefit rate (CBR)) in a phase I trial involving 15 patients. Of particular note, an 80% ORR was reported in patients with T cell lymphomas95. Given the encouraging clinical activity and tolerability of this class of drugs, tazemetostat is being investigated in various patient populations and drug combinations such as with chemotherapy (NCT02889523) or immunotherapy (NCT02220842). Furthermore, two phase I pharmacokinetic studies designed to evaluate drug metabolism (drug interactions and gastric pH effects) and the effects of different formulations (such as intravenous or oral administration) (NCT03010982 and NCT03028103) are currently recruiting patients. Of note, as of April 2018, the FDA has placed a partial hold on enrolment in clinical trials investigating the effects of tazemetostat owing to a case of secondary T cell lymphoma that developed in a paediatric patient with sarcoma who had been on tazemetostat for 15 months and had a partial response.
BET inhibitors
Bromodomains are protein motifs that recognize and bind to acetylated lysine moieties located on histone tails. BETs consist of two amino-terminal tandem bromodomains and an extra-terminal (non-bromodomain) region. The BET family includes BRD2, BRD3 and BRD4 and bromodomain testis-specific protein99. Upon binding to acetylated lysine groups, BETs induce gene expression either directly by recruiting transcription factors to DNA and initiating transcription or indirectly by interacting with gene super-enhancers (non-coding regions of DNA that bind to transcription factors and activate nearby target genes controlling cellular identity) (FIG. 2a). Thus, BETs contribute to the development and progression of malignancies by both activating and potentiating the expression of key oncogenes100.
Although BET mutations or translocations are rare, BETs can be overexpressed101. Consequently, BET inhibition has been shown to be effective in preclinical studies across multiple types of cancers, including breast, neuroendocrine, ovarian and haematological malignancies as well as in rhabdomyosarcoma and glioma102–106. In B cell lymphoid malignancies, BETs might activate the MYC oncogene and the BCL2 anti-apoptotic signalling pathways. This suggestion arises from the observation that the binding of BRD4 to super-enhancers such as those that regulate MYC is inhibited in myeloma cells exposed to the BET inhibitor JQ1, resulting in a selective decrease in the expression of this oncogene107. MYC signalling and other oncogenes associated with super-enhancers seem to be preferentially sensitive to BET inhibition; thus, MYC expression could potentially serve as a biomarker in future clinical trials. In patients with double-hit DLBCL, resistance to venetoclax can be overcome by administration of the BET inhibitor CPI-203, likely owing to downregulation of BCL-2-like protein (BFL1)108. In patients with CLL, high concentrations of BRD4 have been found to be localized to the sites of super-enhancers known to regulate the transcription of several genes in the B cell receptor signalling pathway, as well as that of genes with oncogenic effects in CLL including TCL1, miR-21, miR-155, IL4R and IL21R. BET inhibition was then shown to decrease the expression of many of these genes as well as to reduce the tumour burden in mouse models of CLL101. BET proteolysis-targeting chimaeras (PROTACs), which induce the degradation of BETs through the targeted sequestration of ubiquitin ligases, have been shown to work analogously to BET inhibitors in preclinical models of MCL and might even be more effective. BET PROTACs induce apoptosis in ibrutinib-resistant MCL cells and demonstrated improved survival when compared with BET inhibition with OTX015 in mouse models109. BET inhibitors are also active in CTCL, Epstein-Barr virus (EBV)-associated lymphoproliferative disease and primary effusion lymphoma and have been shown to decrease the rate of tumour growth and disease progression in mouse xenograft models110–112. JQ1 has also been shown to prolong the survival duration of mouse xenograft models of MYC-driven lymphoma, including those that were resistant to etoposide and those carrying TP53 deletions113.
Birabresib (also known as OTX-015 or MK-8628) was the first BET inhibitor to enter clinical trials, which happened in 2013 (FIG. 1). The first results, from a phase I trial of 45 patients with relapsed and/or refractory lymphoma or myeloma were reported in 2016 and, most notably, revealed a 47% CBR among 17 evaluable patients with DLBCL; however, only 4 patients with indolent lymphomas had any clinical benefit, and none of the 12 patients with myeloma responded to treatment. The most common adverse events were cytopenias, specifically thrombocytopenia, in virtually all patients including grade ≥3 thrombocytopenia in 58%, anaemia in 91%, neutropenia in 51%, diarrhoea in 47%, fatigue in 27% and nausea in 24%. From this study, a recommended phase II dose and schedule of 80 mg daily for 14 days on, 7 days off was successfully established114. CPI-0610 has also been tested in a phase I trial involving 44 patients with relapsed and/or refractory lymphoma (mainly DLBCL, FL or HL), and 12 patients in this study had clinical benefit, including CRs in 2 patients with DLBCL115. The third phase I study with published data available was designed to investigate the effects of INCB057643 in patients with solid or haematological malignancies, including four patients with FL and one patient with DLBCL. In this trial, one patient (with FL) had a CR, and two patients had stable disease116. A phase I trial investigating the effects of BAY1238097 in eight patients with various advanced-stage malignancies revealed only negative outcomes: all patients either had disease progression or serious adverse events. No maximum tolerated or recommended phase II dose was established for this drug, and the trial was terminated prematurely owing to both safety concerns and a lack of efficacy117. Several phase I trials designed to test BET inhibitors, including molibresib, CC-90010 and INCB054329, are currently ongoing for patients with various advanced-stage malignancies (NCT01943851, NCT03220347 and NCT02431260). At the time of an interim analysis of the phase I/II trial investigating the safety and efficacy of INCB054329, four patients with lymphoma had been treated, although the outcomes of these patients are yet to be reported118. In December 2018, data from 27 patients with various subtypes of NHL treated with molibresib were presented at the American Society of Hematology (ASH) annual meeting. The ORR among the entire cohort was 18.5%, with one patient with DLBCL having a sustained complete remission after receiving treatment for 54 weeks. Also of note, three out of six patients with T cell lymphomas had a PR119 (TABLE 5).
Table 5 |.
Summary of trials involving BET inhibitors
| Drug | Trial phase (n) | Lymphoma subtype | Outcomes | Common grade ≥3 adverse events (% of patients) | Year | Refs or ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|
| INCB057643 | I/II (5) | Advanced-stage malignancies (4 FL and 1 DLBCL) | FL: 1 CR and 2 SD | In 16% in total | 2017 | 116 |
| Birabresib | I (33) | DLBCL or indolent Lymphomas | DLBCL: CBR 47% | Thrombocytopenia (58); anaemia (27); neutropenia (22) | 2016 | 114 |
| INCB054329 | I (4) | Advanced-stage malignancies | Not reported for lymphoma | Thrombocytopenia (9); neutropenia (4) | 2017 | 118 |
| MoLibresib | I (27) | NHL | ORR 18.5%; TCL: ORR 50% | Thrombocytopenia (70); hyperbilirubinemia (7); anaemia (7) | 2018 | 119 |
| CPI-0610 | I (44) | DLBCL, FL and HL | 2 CR and 12 with clinical benefit | NR | 2015 | 115 |
| CC-90010 | I | NHL | Study ongoing | — | 2017 | NCT03220347 |
BET, bromodomain and extra-terminal domain protein; CBR, clinical benefit rate; CR, complete response; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; NR, not reported; ORR, overall response rate; SD, stable disease; TCL, T cell lymphoma.
PRMT inhibitors
PRMTs catalyse the monomethylation or dimethylation of arginine residues on histone and non-histone proteins. A total of nine human PRMTs are known to exist, although PRMT5 seems to be the most relevant to oncogenesis. PRMT5 is a type II PRMT that specifically catalyses the symmetrical dimethylation of arginine residues located on the H3 or H4 proteins, resulting in gene silencing99 (FIG. 2a).
No PRMT inhibitors have thus far received FDA approval, and the first clinical trial designed to investigate a PRMT5 inhibitor (GSK3326595) commenced in 2016 for patients with solid tumours or NHL (NCT02783300). Despite this lack of clinical evidence, a growing body of data from preclinical studies has demonstrated a potentially important role of this class of drug, specifically for the treatment of lymphoid malignancies. Activating mutations in PRMT5 have not been reported in patients with lymphoma, although PRMT5 is overexpressed in different subtypes and might potentially serve as a biomarker. For example, PRMT5 is highly expressed in EBV+ human lymphoma cells, in comparison with nonmalignant resting or wild-type B cells120. Furthermore, PRMT5 inhibition leads to the selective killing of EBV-transformed lymphoma cells and restoration of tumour-suppressor gene PTPRO function, which is typically repressed in EBV+ lymphoma cells120. In a correlative analysis of human DLBCL samples, high levels of PRMT5 expression were found to be associated with inferior outcomes121. In the same study, PRMT5-knockout mouse models of MYC+ lymphoma demonstrated improved disease-free survival durations. Furthermore, PRMT5-depleted human BL cells were shown to have decreased tumorigenic potential in mouse xenograft models. Given the observed interactions between PRMT5 and MYC, the authors concluded that targeting the PRMT5 signalling pathway might be most effective in patients with MYC-driven tumours121. PRMT5 might also enhance the activity of BCL-6 in DLBCL cells; for example, PRMT5 inhibitors have been shown to synergize with BCL-6 inhibitors in the killing of BCL-6-expressing DLBCL cells in vitro. Notably, PRMT5 is also required for formation of GCBs, which are the cell of origin in most DLBCLs122. PRMT5 overexpression has also been identified in MCL, and PRMT5 inhibition was shown to have cytotoxic effects on MCL cell lines that were associated with downregulation of the genes encoding cyclin D1 (CCND1) and the differentiation protein MCL1 (REFS123–124). Oral administration of the PRMT5 inhibitor EPZ015666 has been shown to induce dose-dependent reductions in tumour volume in mouse xenograft models of MCL125. PRMT5 might also have a role in the development of T cell lymphomas. Similar to EBV-transformed lymphoma, PRMT5 expression is upregulated in human T lymphotropic virus (HTLV)-transformed adult T cell leukaemia/lymphoma (ATLL), and PRMT5 inhibition was shown to have selective cytotoxic effects on HTLV+ lymphoma cells126. Overexpression of PRMT5 in ATLL seems to interact with oncogenic CCND1, MYC and NOTCH1 in driving lymphomagenesis and might also directly silence p53 (REF.127).
IDH inhibitors
IDH catalyses the oxidative decarboxylation of isocitrate to α-ketoglutarate as part of the tricarboxylic acid (or Krebs) cycle. Mutations in either IDH1 or IDH2 lead to the accumulation of 2-hydroxyglutarate (2-HG). This metabolite has been shown to inhibit several demethylation pathways, such as those driven by TET or Jumonji proteins, thus indirectly acting as an epigenetic regulator. As a result of 2-HG accumulation, aberrant DNA or histone methylation can occur128 (FIG. 2b). As a consequence of this aberrant regulation, IDH mutations can essentially be viewed as gain-of-function mutations and are potentially targetable.
Among lymphoid malignancies, dysregulation of the IDH epigenetic pathway has been best characterized in T cell lymphomas. Whole-exome sequencing of peripheral T cell lymphoma samples has demonstrated that approximately 30% of patients with angioimmunoblastic T cell lymphoma (AITL) have IDH2 mutations, although such mutations occur less frequently in other subtypes of PTCL129–131. Similar to AML, R172 is the most prevalent IDH mutation, is associated with having the highest levels of 2-HG compared with the other two hotspot mutations, IDH1R132 and IDH2R140 (REF132), and should be considered an important biomarker for the selection of patients to receive IDH inhibitors, potentially regardless of tumour histology. Interestingly, according to results published in the past few years, all cells harbouring IDH2 mutations are PD-1+ and are associated with downregulation of T helper 1 cell-like differentiation genes (such as STAT1 and IFNG)129–131. Furthermore, an analysis of gene expression signatures demonstrated increased methylation of the promoters that regulate T cell receptor signalling and T cell differentiation in IDH2R172K cell lines, illustrating a potential mechanism of lymphoma-genesis131. The IDH epigenetic signalling pathway might also have a role in CLL. In CLL samples obtained from 214 patients, leukaemic cells were found to have reduced levels of IDH2 expression, with overexpression of IDH1 compared with nonmalignant B cells that was associated with improved treatment-free survival133. No data are currently available from preclinical studies testing IDH inhibitors specifically in models of IDH-mutant lymphomas, such as AITL or CLL.
Both the IDH2 inhibitor enasidenib and, subsequently, the IDH1 inhibitor ivosidenib have been approved in the past 2 years for patients with relapsed and/or refractory AML who carry mutations in IDH2 or IDH1, respectively134,135 (FIG. 1). IDH inhibitors have the unique adverse effect of differentiation syndrome, which can manifest as dyspnoea, fever, pulmonary infiltrates, acute kidney injury, bone and joint pain, lymphadenopathy or rash. In the landmark trial of enasidenib, differentiation syndrome occurred in 11.7% of patients (7% were grade 3–4 in severity) and was managed effectively using systemic corticosteroids. The frequency of this adverse event was similar in patients receiving ivosidenib, of whom 10.6% of patients developed differentiation syndrome, including grade ≥3 differentiation syndrome in 5% of patients. Other common adverse events included hyperbilirubinaemia, anaemia and thrombocytopenia134–136. Of note, patients with acquired therapeutic resistance to enasidenib were found to have new point mutations at either Q316 or I319, both of which are associated with increased circulating 2-HG levels137.
Currently, no results are available from trials of IDH inhibitors in lymphoma. However, clinical trials investigating inhibitors of IDH2 (enasidenib), IDH1 (ivosidenib) and both IDH1 and IDH2 (vorasidenib) in patients with advanced-stage haematological malignancies are currently underway (NCT01915498, NCT02074839 and NCT02492737). Of particular note, enasidenib is currently being investigated in a phase I/II trial specifically for patients with AITL (NCT02273739) (Supplementary Table 2).
Future directions
Efforts to target epigenetic alterations in lymphomas have been successful in a number of disease subtypes, although ample opportunity to maximize the benefits of epigenetic-based therapies continues to exist. Overcoming inherent or acquired resistance to standard therapies is essential and is particularly important given that patients are living longer and are often being exposed to a growing number of lines of therapy. As discussed above, the efficacy of epigenetic-based monotherapies in lymphoid malignancies is limited. Combination therapy, however, appears to improve the efficacy of these agents substantially, as demonstrated in phase I and phase II trials investigating HDAC or DNMT inhibitors in conjunction with chemotherapy, small-molecule inhibitors or both (TABLES 1–3). This paradigm of using epigenetic-based combination therapies to overcome resistance to single agents by inhibiting cell signalling bypass pathways has been extensively tested in preclinical studies. For example, BET inhibitors have been combined with a multitude of small-molecule inhibitors including those targeting mTOR, BTK, PI3K, ATR, EZH2 and HDACs, in addition to lenalidomide138–142. Similarly, decitabine has been combined with inhibitors of BCL-2, JAK-STAT, AKT and BET143. Many of these combinations have demonstrated synergistic activity in lymphoma cell lines or mouse xenograft models; therefore, the next rational step would be translation into clinical trials in order to evaluate the safety and efficacy of these combinations.
The combination of epigenetic-modulating agents with immunotherapy provides perhaps the most exciting avenue for future research. Histone modification typically results in closed chromatin states at the MHC class II promoters, and in MCL and DLBCL cells this can be reversed by HDAC inhibition, thus enhancing antigen-specific immune recognition and activation144,145. In addition, DNMT inhibitors seem to increase sensitivity to immune-checkpoint inhibition146,147. These preclinical observations are beginning to be translated from the laboratory to the clinic as several early phase trials designed to investigate the combination of epigenetic-modulating agents with immune-checkpoint inhibition in patients with lymphoma, including entinostat plus pembrolizumab (NCT03179930), vorinostat plus pembrolizumab (NCT03150329), azacitidine plus avelumab and/or utomilumab (NCT02951156) and tazemetostat plus atezolizumab (NCT02220842). Preliminary results of these studies have not yet been reported; however, on the basis of a strong biological rationale and compelling preclinical data, combining epigenetic-modulating agents with immunotherapies should be further explored.
Investigations of the potential therapeutic value of epigenetic-modifying agents that target other proteins should continue to be pursued. For example, the histone acetyltransferase CREBBP and the lysine-specific histone methyltransferase KMT2D are the two most frequently altered genes in FL and DLBCL2,4,148,149. However, both are generally deleted or inactivated, thus acting either directly or indirectly as tumour-suppressor genes, interfering with terminal B cell differentiation and ultimately inducing lymphomagenesis11,148,149. Inherent challenges exist in developing agents designed to specifically target loss-of-function mutations, although modulating the downstream targets of such proteins or targeting compensatory signalling pathways that are activated in response to loss of function of the gene of interest might be possible. CREBBP-mutant lymphomas have been shown to be biologically addicted to HDAC3, suggesting that HDAC3-specific, or pan-HDAC inhibitors might be useful agents in these patients11. Additionally, because CREBBP loss is associated with downregulation of MHC class II and immune evasion, and given that this effect is counteracted by HDAC3 inhibitors, combination treatment with immune-stimulating therapies, such as immune-checkpoint inhibitors, could be considered11. Novel histone demethylase inhibitors might counteract the loss of methylation typically observed in KMT2D-inactivated tumours, and the effectiveness of this approach could also be enhanced by combination with immunotherapy148,149.
Lastly, noninvasive liquid biopsies, such as cell-free DNA (cfDNA) and circulating tumour cells (CTCs), are increasingly being studied and used for the purpose of detection of minimal residual disease, as well as for the genetic profiling of a known malignancy. These modalities can be valuable in patients with most types of solid tumour as well as in those with lymphomas given the limited sensitivity of imaging and the invasiveness and inconvenience of obtaining tissue biopsy samples. Mutations in genes with established roles in epigenetic regulation such as TET2, DNMT3A and IDH2 have been found to be 83% concordant between cfDNA and tissue biopsy samples in patients with AITL150. Furthermore, an assay for the analysis of DNA methylation patterns in CTCs has been developed for both prostate and breast cancer cells and has been shown to be both feasible and clinically useful151. In summary, these novel techniques show considerable potential and should be further explored in patients with lymphoma to aid in the diagnosis, monitoring, surveillance and prognostication of this disease.
Conclusions
Over the past 10 years, more than 60 manuscripts and abstracts have been published reporting results from clinical trials investigating the safety and/or efficacy of epigenetic-modifying agents in patients with lymphoma, and almost half of these were published in the past 2 years. In addition, more than 50 ongoing clinical trials are currently registered at ClinicalTrials.gov. Given the large number of drugs currently undergoing clinical investigation, in addition to those already approved by the FDA, rational prioritization of the development and selection of these therapies will be extremely important in helping move the field forward. The large body of evidence presented in this Review demonstrates an important clinical role for the epigenetic-modifying drugs across the spectrum of lymphoid malignancies. As HDAC and DNMT inhibitors are the oldest of these drugs and have already been implemented into routine use for patients with certain types of lymphomas and leukaemias, they are, unsurprisingly, also the most extensively studied at this time. As a result, the process of investigating these agents in new contexts and in novel combinations is generally faster and more efficient than the same process for newer classes of drugs. However, the future is remarkably promising for newer epigenetic agents (such as EZH2, BET, PRMT5 and IDH inhibitors), and most of these drugs have only transitioned from the preclinical to the clinical arena within the past 5 years. With the first preliminary results from trials involving these agents beginning to be reported, we are entering a very exciting era for the field of epigenetics in lymphoma.
Supplementary Material
Key points.
Epigenetic-modifying drugs are routinely used in acute myeloid leukaemia, myelodysplastic syndrome and T cell lymphomas, but their role in other malignancies, including B cell lymphomas, has not yet been established.
B cell lymphomas typically have a high frequency of somatic mutations in genes encoding enzymes with a role in epigenetic modifications.
In addition to expanding the role of histone deacetylase and DNA methyltransferase inhibitors for new indications, novel classes of agents are also being investigated for lymphoma, including enhancer of zeste homologue 2 (EZH2), bromodomain and extra-terminal domain protein (BET), isocitrate dehydrogenase (IDH) and protein arginine N-methyltransferase 5 (PRMT5) inhibitors.
The selection and rational prioritization of epigenetic agents are important for both designing future studies and choosing the most appropriate agents for patients in clinical practice.
Potential future research directions include investigating novel combinations, exploring the therapeutic role of targeting new epigenetic pathways and discovering new biomarkers to guide patient selection.
Acknowledgements
The authors gratefully acknowledge financial support from the US National Institutes of Health (NIH) (NIH P50 CA192937 (MSK Lymphoma SPORE) to L.P, A.M. and A.Y and NIH 2R01-CA172492-06A1 to L.P.); the Leukaemia and Lymphoma Society of America SCOR grant 7014-17 (to A.Y.); the US National Cancer Institute, Cancer Center Support Grant P30 CA008748 (to A.Y.); and the Cycle for Survival and the Marie-Josee and Henry R. Kravis Center for Molecular Oncology.
Competing interests
L.P. receives research support from Sanofi. A.M. has received consultancy fees and research support from Janssen. A.Y. has received honoraria and/or consultancy fees from Abbvie, Biopath, Curis, Epizyme, Janssen, Merck, Roche, Takeda and Xynomic and has received research support from Bristol-Myers Squibb, Curis, Janssen, Merck, Roche and Syndax. The other authors declare no competing interests.
Footnotes
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41571-019-0190-8.
References
- 1.Lunning MA Mutation of chromatin modifiers; an emerging hallmark of germinal center B cell lymphoma. Blood Cancer J. 5, e361 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morin RD et al. Frequent mutation of histonemodifying genes in non-Hodgkin lymphoma. Nature 476, 298–303 (2011).This paper demonstrates that somatic mutations in genes encoding histone-modifying enzymes, including KMT2D and MEF2B, are frequent in DLBCL and FL.
- 3.Morin RD et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B cell lymphomas of germinal-cell origin. Nat. Genet 42, 181–184 (2010).This paper demonstrates that somatic mutations of EZH2 are frequent in GCB-like DLBCL and FL.
- 4.Pasqualucci L et al. Analysis of the coding genome of diffuse large B cell lymphoma. Nat. Genet 43, 830–836 (2011).This study reveals that the coding genome of DLBCL typically contains more than 30 gene alterations per patient, including a high frequency of KMT2D mutations.
- 5.Pasqualucci L et al. Inactivating mutations of acetyltransferase genes in B cell lymphoma. Nature 471, 189–195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambwe N et al. Variability in DNA methylation defines novel epigenetic subgroups of DLBCL associated with different clinical outcomes. Blood 123, 1699–1708 (2014).This study demonstrates an association between degree of DNA methylation variability and patient survival outcomes in DLBCL.
- 7.McClure JJ et al. Advances and challenges of HDAC inhibitors in cancer therapeutics. Adv. Cancer Res 138, 183–211 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Gu W & Roeder RG Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90, 595–606 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Bereshchenko OR et al. Acetylation inactivates the transcriptional repressor BCL6. Nat. Genet 32, 606–613 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Zhang J et al. The CREBBP acetyltransferase is a haploinsufficient tumor suppressor in B cell lymphoma. Cancer Discov. 7, 322–337 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Y et al. CREBBP inactivation promotes the development of HDAC3-dependent lymphomas. Cancer Discov. 7, 38–53 (2017).This paper demonstrates that somatic mutations in CREBBP result in unopposed deacetylation by HDAC3, leading to development of germinal cell-derived lymphomas in mice.
- 12.Green MR et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc. Natl Acad. Sci. USA 112, 1116–1125 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorrini C et al. Tip60 is a haplo-insufficient tumour suppressor required for an oncogene-induced DNA damage response. Nature 448, 1063–1067 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Castro PG et al. The HDAC inhibitor panobinostat (LBH589) exerts in vivo anti-leukaemic activity against MLL-rearranged acute lymphoblastic leukaemia and involves the RNF20/RNF40/WAC-H2B ubiquitination axis. Leukemia 32, 323–331 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Rozati S et al. Romidepsin and azacitidine synergize in their epigenetic modulatory effects to induce apoptosis in CTCL. Clin. Cancer Res 22, 2020–2031 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Ageberg M et al. The histone deacetylase inhibitor valproic acid sensitizes diffuse large B cell lymphoma cell lines to CHOP-induced cell death. Am. J. Transl Res 5, 170–183 (2013). [PMC free article] [PubMed] [Google Scholar]
- 17.Buglio D et al. Vorinostat inhibits STAT6-mediated Th2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood 112, 1424–1433 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein JM et al. The histone deacetylase inhibitor LBH589 (panobinostat) modulates the crosstalk of lymphocytes with Hodgkin lymphoma cell lines. PLOS ONE. 8, e79502 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kewitz S et al. Histone deacetylase inhibition restores cisplatin sensitivity of Hodgkin’s lymphoma cells. Leuk. Res 36, 773–778 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Kretzner L et al. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-myc, hTERT, and microRNA levels. Cancer Res. 71, 3912–3920 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bobrowicz M et al. HDAC6 inhibition upregulates CD20 levels and increases the efficacy of anti-CD20 monoclonal antibodies. Blood 130, 1628–1638 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Olsen EA et al. Phase IIB multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T cell lymphoma. J. Clin. Oncol 25, 3109–3115 (2007).This paper presents results from a phase II trial of the HDAC inhibitor vorinostat, leading to the first FDA approval of an HDAC inhibitor.
- 23.Whittaker SJ et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T cell lymphoma. J. Clin. Oncol 28, 4485–4491 (2010).This paper presents results from the phase II trial of the HDAC inhibitor romidepsin, leading to its approval for CTCL.
- 24.Coiffier B et al. Results from a pivotal, open-label, phase II study of romidepsin relapsed or refractory peripheral T cell lymphoma after prior systemic therapy. J. Clin. Oncol 30, 631–636 (2012).This paper presents results from the phase II trial of the HDAC inhibitor romidepsin, leading to its approval for PTCL.
- 25.O’Connor OA et al. Belinostat in patients with relapsed or refractory peripheral T cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J. Clin. Oncol 33, 2492–2499 (2015).This paper presents results from the phase II trial of the HDAC inhibitor belinostat, leading to its approval for PTCL.
- 26.San-Miguel JF et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma: a multicentre, randomized, double-blind phase 3 trial. Lancet Oncol. 15, 1195–1206 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Ogura M et al. A multicentre phase II study of vorinostat in patients with relapsed or refractory indolent B cell non-Hodgkin lymphoma and mantle cell lymphoma. Br. J. Haematol 165, 768–776 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirschbaum M et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-hodgkin’s lymphoma and mantle cell lymphoma. J. Clin. Oncol 29, 1198–1203 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younes A et al. Panobinostat in patients with relapsed/refractory hodgkin’s lymphoma after autologous stem-cell transplantation: results of a phase II study. J. Clin. Oncol 30, 2197–2203 (2012).This paper presents results from a large clinical trial of panobinostat in patients with heavily pretreated HL who relapsed or were refractory to autologous transplant.
- 30.Zaja F et al. Single-agent panobinostat for relapsed/refractory diffuse large B cell lymphoma: clinical outcome and correlation with genomic data. A phase 2 study of the Fondazione Italiana Linfomi. Leuk. Lymphoma 59, 2904–2910 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Duvic M et al. Panobinostat activity in both bexarotene-exposed and naïve patients with refractory cutaneous T cell lymphoma: results of a phase II trial. Eur. J. Cancer 49, 386–394 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Ribrag V et al. Safety and efficacy of abexinostat, a pan-histone deacetylase inhibitor, in non-Hodgkin lymphoma and chronic lymphocytic leukemia: results of a phase II study. Haematologica 102, 903–909 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batlevi C et al. ENGAGE-501: phase II study of entinostat (SNDX-275) in relapsed and refractory Hodgkin lymphoma. Haematologica 101,968–975 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batlevi C et al. A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br. J. Haematol 178, 434–441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Child F et al. Phase II multicentre trial of oral quisinostat, a histone deacetylase inhibitor, in patients with previously treated stage IB-IVA myclosis fungoides/Sezary syndrome. Br J. Dermatol 175, 80–88 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Shi Y et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T cell lymphoma. Ann. Oncol 26, 1766–1771 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Assouline S et al. Phase 2 study of panobinostat with or without rituximab in relapsed diffuse large B cell lymphoma. Blood 128, 185–194 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen R et al. A phase II study of vorinostat and rituximab for treatment of newly diagnosed and relapsed/refractory indolent non-Hodgkin lymphoma. Haematologica 100, 357–362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan D et al. Panobinostat in combination with bortezomib in patients with relapsed or refractory peripheral T cell lymphoma: an open-label, multicentre phase 2 trial. Lancet Haematol. 2, e326–e333 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Lunning MA et al. A phase I/II trial of the combination of romidepsin and lenalidomide in patients with relapsed/refractory lymphoma and myeloma: phase I results. J. Clin. Oncol 32 (Suppl. 15), 8582 (2014). [Google Scholar]
- 41.Amengual JE et al. A phase I study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T cell lymphoma. Blood 131, 397–407 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Younes A et al. Safety, tolerability, and preliminary activity of CUDC-907, a first-in-class, oral, dual inhibitor of HDAC and PI3K in patients with relapsed or refractory lymphoma or multiple myeloma: an open-label, dose-escalation, phase I trial. Lancet Oncol. 17, 622–631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perksy DO et al. A phase I/II trial of vorinostat (SAHA) in combination with rituximab-CHOP in patients with newly diagnosed advanced stage diffuse large B cell lymphoma (DLBCL): SWOG S0806. Am. J. Hematol 93, 486–493 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Budde LE et al. A phase I study of pulse high-dose vorinostat (V) plus rituximab (R), ifosphamide, carboplatin, and etoposide (ICE) in patients with relapsed lymphoma. Br. J. Haematol 161, 183–191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu B et al. Phase-I and randomized phase-II trial of panobinostat in combination with ICE (ifosfamide, carboplatin, etoposide) in relapsed or refractory classical Hodgkin lymphoma. Leuk. Lymphoma 59, 863–870 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Nieto Y et al. Vorinostat combined with high-dose gemcitabine, busulfan, and melphalan with autologous stem cell transplantation in patients with refractory lymphomas. Biol. Blood Marrow Transplant 21, 1914–1920 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupuis J et al. Combination of romidepsin with cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated patients with peripheral T cell lymphoma: a non-randomised, phase Ib/2 study. Lancet Haematol. 2, e160–e165 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Chihara D et al. High response rate of romidepsin in combination with ICE (ifosfamide, carboplatin, and etoposide) in patients with relapsed or refractory peripheral T cell lymphoma: updates of phase I trial. Blood 126, 3987 (2015). [Google Scholar]
- 49.Pfister SX & Ashowrth A Marked for death: targeting epigenetic changes in cancer. Nat. Rev. Drug Discov 16, 241–263 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Humphries LA et al. Pro-apoptotic TP53 homolog TAp63 is repressed via epigenetic silencing and B cell receptor signaling in chronic lymphocytic leukemia. Br. J. Haematol 163, 590–602 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giachelia M et al. Quantification of DAPK1 promoter methylation in bone marrow and peripheral blood as a follicular lymphoma biomarker. J. Mol. Diag 16, 467–476 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Asmar F et al. Diffuse large B cell lymphoma with combined TP53 mutation and MIR34A methylation: another “double hit” lymphoma with very poor outcome? Oncotarget 5, 1912–1925 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flinders C et al. Epigenetic changes mediated by polycomb repressive complex 2 and E2a are associated with drug resistance in a mouse model of lymphoma. Genome Med. 8, 54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leshchenko V et al. Genomewide DNA methylation analysis reveals novel targets for drug development in mantle cell lymphoma. Blood 116, 1025–1034 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagasawa T et al. Multi-gene epigenetic silencing of tumor suppressor genes in T cell lymphoma cells; delayed expression of the p16 protein upon reversal of the silencing. Leuk. Res 30, 303–312 (2006). [DOI] [PubMed] [Google Scholar]
- 56.De S et al. Aberration in DNA methylation in B cell lymphomas has a complex origin and increases with disease severity. PLOS Genet. 9, 1003137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wedge E et al. Global hypomethylation is an independent prognostic factor in diffuse large B cell lymphoma. Am. J. Hematol. 92, 689–694 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Shaknovich R et al. DNA methylation signatures define molecular subtypes of diffuse large B cell lymphoma. Blood 116, e81–e89 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alizadeh A et al. Distinct types of diffuse large B cell lymphoma identified by gene expression profiling. Nature 403, 503–511 (2000).This seminal paper describes two molecularly distinct types of DLBCL, which are identified as GCB-like and activated B cell-like.
- 60.Oakes C et al. Evolution of DNA methylation is linked to genetic aberrations in chronic lymphocytic leukemia. Cancer Discov. 4, 348–361 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teater M et al. AICDA drives epigenetic heterogeneity and accelerates germinal center-derived lymphomagenesis. Nat. Commun 9, 222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amara K et al. DNA methyltransferase DNMT3b protein overexpression as a prognostic factor in patients with diffuse large B cell lymphomas. Cancer Sci. 101, 1722–1730 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robaina M et al. Deregulation of DNMT1, DNMT3B and miR-29s in Burkitt lymphoma suggests novel contribution for disease pathogenesis. Exp. Mol. Pathol 98, 200–207 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Clozel T et al. Mechanism-based epigenetic chemosensitization therapy of diffuse large B cell lymphoma. Cancer Discov. 3, 1002–1019 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiappinelli K et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 162, 974–986 (2015).This study demonstrates a mechanism of action of DNMT inhibitors that occurs through upregulation of immune signalling through a viral defence pathway.
- 66.Guo Z et al. De novo induction of a cancer/testis antigen by 5-aza-2′-deoxycytidine augments adoptive immunotherapy in a murine tumor model. Cancer Res. 66, 1105–1113 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Topper M et al. Epigenetic therapy ties MYC depletion to reversing immune evasion and treating lung cancer. Cell 171, 1284–1300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.National Comprehensive Cancer Network. Acute myeloid leukemia. NCCN; https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf (2019). [Google Scholar]
- 69.Silverman LR et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J. Clin. Oncol 20, 2429–2440 (2002).This study demonstrates improved survival with azacitidine compared with best supportive care in patients with MDS, leading to its FDa approval.
- 70.Kantarjian H et al. Decitabine improves patient outcomes in myelodysplastic syndromes. Cancer 106, 1794–1780 (2006).This study demonstrates improved time to AML transformation with decitabine compared with best supportive care in patients with MDS, leading to its FDA approval.
- 71.Fenaux P et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol 28, 562–569 (2010). [DOI] [PubMed] [Google Scholar]
- 72.Kantarjian HM et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol 30, 2670–2677 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malik A et al. Azacitidine in fludarabine-refractory chronic lymphocytic leukemia: a phase II study. Clin. Lymphoma Myeloma Leuk 13, 292–295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blum KA et al. Phase I trial of low dose decitabine targeting DNA hypermethylation in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma: dose-limiting myelosuppression without evidence of DNA hypomethylation. Br. J. Haematol 150, 189–195 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan H et al. Low-dose decitabine-based chemoimmunotherapy for patients with refractory advanced solid tumors: a phase I/II report. J. Immunol. Res 2014, 371087 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin P et al. A phase I, open-label, multicenter trial of oral azacitidine (cc-486) plus R-CHOP in patients with high-risk, previously untreated diffuse large B cell lymphoma, grade 3B follicular lymphoma or transformed lymphoma. Blood 130, 192 (2017).28455282 [Google Scholar]
- 77.Moss JJ et al. A phase I study of the combination of azactidine, cyclophosphamide, vincristine, and rituximab in relapsed and refractory lymphoma. Blood 118, 1624 (2011). [Google Scholar]
- 78.Nieto Y et al. Double epigenetic modulation of high-dose chemotherapy with azacitidine and vorinostat for patients with refractory or poor-risk relapsed lymphoma. Cancer 122, 2680–2688 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Falchi L et al. A phase I/2 study of oral 5-azacitidine and romidepsin in patients with lymphoid malignancies reveals promising activity in heavily pretreated peripheral T cell lymphoma (PTCL). Blood 130, 1515 (2017). [Google Scholar]
- 80.Pera B et al. Combinatorial epigenetic therapy in diffuse large B cell lymphoma pre-clinical models and patients. Clin. Epigenetics 8, 79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beguelin W et al. EZH2 enables germinal centre formation through epigenetic silencing of CDKN1A and an Rb-E2F1 feedback loop. Nat. Commun 8, 877 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beguelin W et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 23, 677–692 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caganova M et al. Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. J. Clin. Invest 123, 5009–5022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bodor C et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood 122, 3165–3168 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sneeringer CJ et al. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B cell lymphomas. Proc. Natl Acad. Sci. USA 107, 20980–20985 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beguelin W et al. EZH2 and BCL6 cooperate to assemble CBX8-BCOR complex to repress bivalent promoters, mediate germinal center formation and lymphomagenesis. Cancer Cell 30, 197–213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knutson S et al. Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-hodgkin lymphoma. Mol. Cancer Ther 13, 842–854 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Brach D et al. EZH2 inhibition by tazemetostat results in altered dependency on B cell activation signaling in DLBCL. Mol. Cancer Ther 16, 2586–2597 (2017). [DOI] [PubMed] [Google Scholar]
- 89.Oricchio E et al. Genetic and epigenetic inactivation of SESTRIN1 controls mTORC1 and response to EZH2 inhibition in follicular lymphoma. Sci. Transl Med 9, 9969 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ntziachristos P et al. Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat. Med 18, 298–301 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J et al. The genetic basis of early T cell precursor acute lymphoblastic leukemia. Nature 481, 157–163 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Danis E et al. EZH2 controls an early hematopoietic program and growth and survival signaling in early T cell precursor acute lymphoblastic leukemia. Cell Rep. 14, 1953–1965 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Italiano A et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 19, 649–659 (2018).This is the first published study reporting results from the first-in-human phase I trial of the EZH2 inhibitor tazemetostat.
- 94.Yap T et al. A phase I, open-label study of GSK2816126, an enhancer of zeste homolog 2(EZH2) inhibitor, in patients with relapsed/refractory diffuse large B cell lymphoma (DLBCL), trasnformed follicular lymphoma (tFL), other non-Hodgkin’s lymphomas (NHL), multiple myeloma (MM) and solid tumors [abstract]. J Clin. Oncol 34 (Suppl. 15), TPS2595 (2016). [Google Scholar]
- 95.Maruyama D et al. First-in-human study of the EZH½ dual inhibitor DS-3201b in patients with relapsed or refractory non-hodgkin lymphomas - preliminary results. Blood 130, 4070 (2017). [Google Scholar]
- 96.Morschhauser F et al. Interim report from a phase 2 multicenter study of tazemetostat, and EZH2 inhibitor, in patients with relapsed or refractory B cell non-hodgkin lymphomas. Hematol. Oncol 34, 24–25 (2017). [Google Scholar]
- 97.Morschhauser F et al. Interim update from a phase 2 multicenter study of tazemetostat, an EZH2 inhibitor, in patients with relapsed or refractory (R/R) follicular lymphoma (FL). Clin. Lymphoma Myeloma Leuk. 18 (Suppl. 1), 278–279 (2018). [Google Scholar]
- 98.Honma D et al. Novel orally bioavailable EZH½ dual inhibitors with greater antitumor efficacy than an EZH2 selective inhibitor. Cancer Sci. 108, 2069–2078 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shortt J et al. A chemical probe toolbox for dissecting the cancer epigenome. Nat. Rev Cancer 160, 160–182 (2017). [DOI] [PubMed] [Google Scholar]
- 100.Doroshow DB & LoRusso PM BET inhibitors: a novel epigenetic approach. Ann. Oncol 28, 1776–1787 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Ozer HG et al. BRD4 profiling identifies critical chronic lymphocytic leukemia oncogenic circuits and reveals sensitivity to PLX51107, a novel structurally distinct BET inhibitor. Cancer Discov. 8, 458–477 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vazquez R et al. The bromodomain inhibitor OTX015 (MK-8628) exerts anti-tumor activity in triple-negative breast cancer models as single agent and in combination with everolimus. Oncotarget 8, 7598–7613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L et al. BRD4 inhibitor IBET upregulates p27kip/cip protein stability in neuroendocrine tumor cells. Cancer Biol. Ther 18, 229–236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yokoyama Y et al. BET inhibitors suppress ALDH activity by targeting ALDH1A1 super-enhancer in ovarian cancer. Cancer Res. 76, 6320–6330 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Piunti A et al. Therapeutic targeting of polycomb and BET bromodomain proteins in diffuse intrinsic pontine gliomas. Nat. Med 23, 493–500 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gryder BE et al. PAX3-FOX01 establishes myogenic super enhancers and confers BET bromodomain vulnerability. Cancer Discov. 7, 884–899 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Loven J et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Esteve-Arenys A et al. The BET bromodomain inhibitor CPI203 overcomes resistance to ABT-199 (venetoclax) by downregulation of BFL-1/A1 in in vitro and in vivo models of MYC+/BCL2+ double hit lymphoma. Oncogene 37, 1830–1844 (2018). [DOI] [PubMed] [Google Scholar]
- 109.Sun B et al. BET protein proteolysis targeting chimera (PROTAC) exerts potent lethal activity against mantle cell lymphoma cells. Leukemia 32, 343–352 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Kohnken R et al. Diminished microRNA-29b level is associated with BRD4-mediated activation of oncogenes in cutaneous T cell lymphoma. Blood 131, 771–781 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.He A et al. JQ1 reduces Epstein-Barr virus-associated lymphoproliferative disease in mice without sustained oncogene repression. Leuk. Lymphoma 59, 1248–1251 (2017). [DOI] [PubMed] [Google Scholar]
- 112.Gopalakrishnan R et al. Immunomodulatory drugs target IKZF1-IRF4-MYC axis in primary effusion lymphoma in a cereblon-dependent manner and display synergistic cytotoxicity with BRD4 inhibitors. Oncogene 35, 1797–1810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hogg SJ et al. BET inhibition induces apoptosis in aggressive B cell lymphoma via epigenetic regulation of BCL-2 family members. Mol. Cancer Ther 15, 2030–2041 (2016). [DOI] [PubMed] [Google Scholar]
- 114.Amorim S et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Hematol. 3, e196–e204 (2016).This is the first published study reporting results from a phase I trial of the BET inhibitor OTX015.
- 115.Abramson JS et al. BET inhibitor CPI-0610 is well tolerated and induces responses in diffuse large B cell lymphoma and follicular lymphoma: preliminary analysis of an ongoing phase I study. Blood 126, 1491 (2015). [Google Scholar]
- 116.Forero-Torres A et al. Preliminary results from an ongoing phase I/II study of INCB057643, a bromodomain and extraterminal (BET) protein inhibitor, in patients with advanced malignancies. Blood 130, 4048 (2017). [Google Scholar]
- 117.Postel-Vinay S et al. First-in-human phase I dose escalation study of the bromodomain and extra-terminal motif (BET) inhibitor BAY 1238097 in subjects with advanced malignancies. Eur. J. Cancer 69 (Suppl. 1), 7–8 (2016). [Google Scholar]
- 118.Falchook G et al. Phase I/II study of INCB054329, a bromodomain and extraterminal (BET) protein inhibitor in patients with advanced malignancies [abstract]. Mol. Cancer Ther 17 (Suppl. 1), A093 (2018). [Google Scholar]
- 119.Dickinson M et al. A phase I study of molibresib (GSK525762), a selective bromodomain (BRD) and extra terminal protein (BET) inhibitor: results from part 1 of a phase I/II open label single agent study in subjects with non-hodgkin’s lymphoma (NHL) [abstract]. Blood 132, 1682 (2018). [Google Scholar]
- 120.Alinari L et al. Selective inhibition of protein arginine methyltransferase 5 blocks initiation and maintenance of B cell transformation. Blood 125, 2530–2543 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koh CM et al. MYC regulates the core pre-mRNA splicing machinery as an essential step in lymphomagenesis. Nature 523, 96–100 (2015). [DOI] [PubMed] [Google Scholar]
- 122.Lu X et al. PRMT5 interacts with BCL6 oncoprotein and is required for germinal center formation and lymphoma cell survival. Blood 132, 2026–2039 (2018).In this study, the authors show that PRMT5 interacts with BCL-6 in germinal centre formation and that concurrent targeting of both enzymes leads to synergistic killing of lymphoma cells.
- 123.Pal S et al. Low levels of miR-92b/96 induce PRMT5 translation and H3R8/H4R3 methylation in mantle cell lymphoma. EMBO J. 26, 3558–3569 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yan F et al. Developing a novel class of drug to inhibit protein arginine methyltransferase 5 (PRMT5) enzyme dysregulation in mantle cell lymphoma. Blood 118, 595 (2011). [Google Scholar]
- 125.Chan-Penebre E et al. A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat. Chem. Biol 11, 432–437 (2015).This paper presents preclinical data for a novel PRMT5 inhibitor in MCL cell lines and in MCL xenograft mouse models.
- 126.Panfil AR et al. PRMT5 is upregulated in HTLV-1- mediated T cell transformation and selective inhibition alters viral gene expression and infected cell survival. Viruses 8, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y et al. PRMT5 is required for lymphomagenesis triggered by multiple oncogenic drivers. Cancer Discov. 5, 288–303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Figueroa ME et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553–567 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nguyen TB et al. Identification of cell-type-specific mutations in nodal T cell lymphomas. Blood Cancer J. 7, e516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sakata-Yamagimoto M et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat. Genet. 46, 171–175 (2014). [DOI] [PubMed] [Google Scholar]
- 131.Wang C et al. IDH2 R172 mutations define a unique subgroup of patients with angioimmunoblastic T cell lymphoma. Blood 126, 1741–1752 (2015).This study demonstrates that IDH2R172 mutations define a unique phenotype in AITL with repression of genes involved with T cell receptor signalling and T cell differentiation, leading to lymphomagenesis.
- 132.Lemonnier F et al. The IDH2 R172K mutation associated with angioimmunoblastic T cell lymphoma produces 2HG in T cells and impacts lymphoid development. Proc. Natl Acad. Sci. USA 113, 15084–15089 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Van Damme M et al. Characterization of TET and IDH gene expression in chronic lymphocytic leukemia: comparison with normal B cells and prognostic significance. Clin. Epigenetics 8, 132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stein EM et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 136, 722–731 (2017).This paper presents results from the phase I/II trial of the IDH2 inhibitor enasidenib, leading to its FDA approval for IDH2-mutant AML.
- 135.DiNardo CD et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N. Engl. J. Med 378, 2386–2398 (2018).This paper presents results from the phase I trial of the IDH1 inhibitor ivosidenib, leading to its FDA approval for IDH1 -mutant AML.
- 136.Fathi AT et al. Differentiation syndrome associated with enasidenib, a selective inhibitor of mutant isocitrate dehydrogenase 2. JAMA Oncol. 4, 1106–1110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Intlekofer AM et al. Acquired resistance to IDH inhibition through trans or cis dimer-interface mutations. Nature 559, 125–129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boi M et al. The BET bromodomain inhibitor OTX015 affects pathogenetic pathways in preclinical B cell tumor models and synergizes with targeted drugs. Clin. Cancer Res 21, 1628–1638 (2015). [DOI] [PubMed] [Google Scholar]
- 139.Bernasconi E et al. Preclinical evaluation of the BET bromodomain inhibitor BAY1238097 for the treatment of lymphoma. Br. J. Haematol 178, 936–948 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Schaffer M et al. Identification of potential ibrutinib combinations in hematological malignancies using a combination high-throughput screen. Leuk. Lymphoma 59, 931–940 (2018). [DOI] [PubMed] [Google Scholar]
- 141.Gaudio E et al. Bromodomain inhibitor OTX015 (MK-8628) combined with targeted agents shows strong in vivo antitumor activity in lymphoma. Oncotarget 7, 58142–58147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Muralidharan SV et al. BET bromodomain inhibitors synergize with ATR inhibitors to induce DNA damage, apoptosis, senescence-associated secretory pathway and ER stress in MYC-induced lymphoma cells. Oncogene 35, 4689–4697 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Swerev TM et al. Activation of oncogenic pathways in classical Hodgkin lymphoma by decitabine: a rationale for combination with small molecular weight inhibitors. Int. J. Oncol 50, 555–566 (2017). [DOI] [PubMed] [Google Scholar]
- 144.Cycon KA et al. Histone deacetylase inhibitors activate CIITA and MHC class II antigen expression in diffuse large B cell lymphoma. Immunology 140, 259–272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tiper IV et al. Histone deacetylase inhibitors enhance CD1d-dependent NKT cell responses to lymphoma. Cancer Immunol. Immunother 65, 1411–1421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ghoneim HE et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 170, 142–157 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lai Q et al. Decitabine improves the efficiency of anti-PD-1 therapy via activating the response to IFN/ PD-L1 signal of lung cancer cells. Oncogene 37, 2302–2312 (2018). [DOI] [PubMed] [Google Scholar]
- 148.Zhang J et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat. Med 21, 1190–1198 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ortega-Molina A et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat. Med 21, 1199–1208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sakata-Yanagimoto M et al. Detection of the circulating tumor DNAs in angioimmunoblastic T cell lymphoma. Ann. Hematol 96, 1471–1475 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Pixberg CF et al. Analysis of DNA methylation in single circulating tumor cells. Oncogene 36, 3223–3231 (2017). [DOI] [PubMed] [Google Scholar]
- 152.Puvvada S et al. A phase II study of belinostat (PXD101) in relapsed and refractory aggressive B cell lymphomas: SWOG S0520. Leuk. Lymphoma 57, 2359–2369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dummer R et al. Vorinostat combined with bexarotene for treatment of cutaneous T cell lymphoma: in vitro and phase I clinical evidence supporting augmentation of retinoic acid receptor/ retinoid X receptor activation by histone deacetylase inhibition. Leuk. Lymphoma 53, 1501–1508 (2012). [DOI] [PubMed] [Google Scholar]
- 154.Oki Y et al. Phase I study of panobinostat plus everolimus in patients with relapsed or refractory lymphoma. Clin. Cancer Res 19, 6882–6890 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.