Abstract
Objective
To investigate whether the genetic burden of type 2 diabetes modifies the association between the quality of dietary fat and the incidence of type 2 diabetes.
Design
Individual participant data meta-analysis.
Data sources
Eligible prospective cohort studies were systematically sourced from studies published between January 1970 and February 2017 through electronic searches in major medical databases (Medline, Embase, and Scopus) and discussion with investigators.
Review methods
Data from cohort studies or multicohort consortia with available genome-wide genetic data and information about the quality of dietary fat and the incidence of type 2 diabetes in participants of European descent was sought. Prospective cohorts that had accrued five or more years of follow-up were included. The type 2 diabetes genetic risk profile was characterized by a 68-variant polygenic risk score weighted by published effect sizes. Diet was recorded by using validated cohort-specific dietary assessment tools. Outcome measures were summary adjusted hazard ratios of incident type 2 diabetes for polygenic risk score, isocaloric replacement of carbohydrate (refined starch and sugars) with types of fat, and the interaction of types of fat with polygenic risk score.
Results
Of 102 305 participants from 15 prospective cohort studies, 20 015 type 2 diabetes cases were documented after a median follow-up of 12 years (interquartile range 9.4-14.2). The hazard ratio of type 2 diabetes per increment of 10 risk alleles in the polygenic risk score was 1.64 (95% confidence interval 1.54 to 1.75, I2=7.1%, τ2=0.003). The increase of polyunsaturated fat and total omega 6 polyunsaturated fat intake in place of carbohydrate was associated with a lower risk of type 2 diabetes, with hazard ratios of 0.90 (0.82 to 0.98, I2=18.0%, τ2=0.006; per 5% of energy) and 0.99 (0.97 to 1.00, I2=58.8%, τ2=0.001; per increment of 1 g/d), respectively. Increasing monounsaturated fat in place of carbohydrate was associated with a higher risk of type 2 diabetes (hazard ratio 1.10, 95% confidence interval 1.01 to 1.19, I2=25.9%, τ2=0.006; per 5% of energy). Evidence of small study effects was detected for the overall association of polyunsaturated fat with the risk of type 2 diabetes, but not for the omega 6 polyunsaturated fat and monounsaturated fat associations. Significant interactions between dietary fat and polygenic risk score on the risk of type 2 diabetes (P>0.05 for interaction) were not observed.
Conclusions
These data indicate that genetic burden and the quality of dietary fat are each associated with the incidence of type 2 diabetes. The findings do not support tailoring recommendations on the quality of dietary fat to individual type 2 diabetes genetic risk profiles for the primary prevention of type 2 diabetes, and suggest that dietary fat is associated with the risk of type 2 diabetes across the spectrum of type 2 diabetes genetic risk.
Introduction
Diabetes is regarded as one of the most serious public health challenges of the 21st century, affecting the health of an estimated 8.8% people in 2017, which is projected to increase to 9.9% by 2045.1 The individual risk of type 2 diabetes reflects the interplay between lifestyle and dietary factors acting on a backdrop of genetic predisposition.2
Currently, 243 genetic loci are associated with the risk of type 2 diabetes in successive waves of large-scale genetic association studies.3 4 5 6 These risk alleles, when aggregated into a polygenic risk score, can provide a continuous measure of the genetic risk and are predictive of incident type 2 diabetes.3 7 However, the extent to which the risk of the disease conferred by the presence of type 2 diabetes risk-increasing alleles can be modified by dietary factors is unclear.8 9 A previous prospective study suggested that genetic predisposition could interact with a Western dietary pattern to determine the risk of type 2 diabetes in men,8 but no interaction was detected between a type 2 diabetes polygenic risk score and a Mediterranean diet score on the development of type 2 diabetes in the InterAct study.9 Integrating genetic and environmental information represents one of the greatest challenges facing the implementation of precision medicine in metabolic diseases.2 10 11
Recommendations aimed at improving dietary quality are an essential part of preventing and treating type 2 diabetes, as reflected in current dietary guidelines. These guidelines recommend dietary patterns rich in unsaturated fatty acids, polyunsaturated fats in particular, and limited in saturated fat and sugars to reduce the risk of type 2 diabetes.12 13 However, major gaps in the evidence for the relation between the quality of dietary fat and the risk of type 2 diabetes exist, including uncertainty on the benefits of increasing monounsaturated fat in place of carbohydrate and uncertainty on the effects of saturated fat.12 13 14 15 16 To promote appropriate dietary patterns, it is essential to know whether individual nutrients and food groups affect chronic diseases such as type 2 diabetes.17
In this study, we undertook an individual participant data meta-analysis to investigate the association of the quality of dietary fat with the incidence of type 2 diabetes and to evaluate whether the presence of known type 2 diabetes risk-increasing alleles modify the association between subtypes of fat and the risk of type 2 diabetes.
Methods
Study design, search strategy, and selection criteria
We elaborated an analysis plan, including harmonized definitions of exposures, outcomes, and covariates for combining individual participant data from prospective cohort studies or multicohort consortia. Our main research question was to investigate whether the presence of known type 2 diabetes risk-increasing alleles modifies the association between the quality of dietary fat and the risk of type 2 diabetes. The prespecified analysis plan is provided in appendix 1. This article follows Preferred Reporting Items for Systematic reviews and Meta-Analysis of Individual Participant Data (PRISMA-IPD) reporting guidelines (appendix 2).18
We sought data from large prospective cohort studies or large multicohort consortia through a systematic literature search and discussion with investigators (appendix 3). Two independent researchers conducted the electronic searches in Medline, Embase, and Scopus from studies published between January 1970 and February 2017 with the terms “diet” or “dietary fat” or “fat quality” or “fat intake,” or “genetics” or “genotype” or “gene,” or “diabetes,” or “cohort” or “prospective,” and a combination of the words “risk,” “relative,” “hazard,” or “ratio.” There were no language restrictions. Prospective cohort studies were eligible if they had genome-wide genetic data and information about the quality of dietary fat and the incidence of type 2 diabetes; included more than 500 patients of European ancestry, not selected on the basis of having any previous chronic disease; recruited adults (aged ≥20 and ≤80 at baseline); and had accrued five years or more of median follow-up that allowed time dependent analyses. On identification of eligible cohorts, lead investigators from each contributing cohort were asked to participate in a standardized individual participant data meta-analysis of the quality of dietary fat, genetic risk, and the incidence of type 2 diabetes by March 2017. Before data analysis, candidate cohorts were requested to provide information on type 2 diabetes risk-increasing variants and descriptive statistics of total and subtypes of dietary fat. Within participating cohorts, we excluded patients of non-European ancestry, patients with prevalent diabetes, patients who reported implausible baseline energy intake (<500 or >4500 kcal/d; 1 kcal = 4.18 kJ = 0.00418 MJ), patients who had missing genetic or quality of dietary fat data, or patients aged under 20 and over 80 at baseline (appendix 1). The rationale for excluding patients who reported implausible energy intake was based on potential confounding or bias associated with extreme energy intake. We also excluded patients who had missing genetic information as we are unable to quantify genetic risk in the absence of genetic data. To limit bias owing to effects of pre-existing disease on dietary intake (such as, reverse causality), the primary analysis was restricted to patients without specific known chronic diseases at baseline (such as, cancer or cardiovascular disease). Institutional Review Board or oversight committees, or both, approved the study in each participating cohort and all patients provided written informed consent.
Study outcome
The primary outcome for this analysis was incidence of type 2 diabetes. The details of the methods used for defining endpoint adjudication in each prospective cohort are described in appendix 4. In brief, ascertainment of type 2 diabetes was defined by fasting or non-fasting glucose determinations, treatment with either insulin or a hypoglycemic agent at the follow-up examinations, or by reviewing multiple sources of evidence, including linkage to primary care registers and secondary care registers, hospital admissions, and mortality data.
Type 2 diabetes polygenic risk score
A total of 88 of the known 89 genome-wide associated type 2 diabetes risk-increasing variants reported at the time of the analysis were selected for this analysis (DUSP9 was excluded owing to its location on the X chromosome).3 4 Sixty eight out of the 88 type 2 diabetes risk-increasing variants were available in all participating cohorts either through direct genotyping or by imputation with sufficient imputation quality. Details regarding the variants included in the polygenic risk score, cohort-specific genotyping platform, imputation panels, and quality metrics are summarized in supplementary tables 1 and 2. Consistent with previous studies,7 19 20 we used a weighted method to calculate the polygenic risk score on the basis of the 68 type 2 diabetes variants. Each variant was coded with the expected number of type 2 diabetes risk-increasing alleles and weighted by its relative effect size (β coefficient) on type 2 diabetes. β coefficients were obtained from a previously published genome-wide association meta-analysis in 34 840 cases and 114 981 controls.3 Using these external weights, we calculated the polygenic risk score (PRS) for each individual in each cohort by using the equation: PRS = (β1 × SNP1 + β2 × SNP2 + … + β68 × SNP68) × (68/sum of the β coefficients). The polygenic risk score ranges from 0 to 136, with each unit corresponding to one average risk allele and higher scores indicating a higher genetic predisposition to type 2 diabetes.
Assessment of dietary fat quality
We assessed total and subtypes of fat by using validated cohort-specific semiquantitative food-frequency questionnaires or diet history (supplementary table 3). We expressed main dietary variables as percentages of energy intake from total and subtypes of fat, including polyunsaturated fat (omega 3 and omega 6 polyunsaturated fat), monounsaturated fat (comprising oleic acid, palmitoleic acid, gondoic acid, erucic acid, and nervonic acid), and saturated fat (including butyric acid, caproic acid, caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid, and stearic acid). Total omega 3 polyunsaturated fat (including dietary α-linoleic acid, eicosapentaenoic acid, and docosahexaenoic acid and excluding supplementation, n=13 cohorts), total omega 6 polyunsaturated fat (including linoleic acid and arachidonic acid and excluding supplementation, n=13 cohorts), and total trans fat (n=11 cohorts) were expressed as g/d. Because cumulative averages of dietary components yield more precise dietary intake estimates than baseline intakes alone,21 cohorts with repeated measurements of diet calculated the cumulative averages of dietary variables.
Assessment of covariates
Demographic, lifestyle, and clinical characteristics were assessed at baseline and during follow-up assessments by self report questionnaires or through clinical examination. Covariate definitions were harmonized across studies and are detailed in supplementary table 4. In brief, this information included: age (years, continuous), sex (men or women), body mass index (BMI, kg/m2, continuous), smoking (never, former, or current; if former was not reported then current v non-current smoker), physical activity (categories of metabolic equivalents, physical activity scores, or leisure-time physical activity, as defined by individual studies), family history of diabetes (yes or no), dyslipidemia (yes or no, defined based on treatment with lipid-lowering drugs or diagnosis of dyslipidemia), hypertension (yes or no, defined based on treatment with antihypertensive drugs or diagnosis of hypertension), and dietary factors including total energy intake (kcal/d, continuous), protein intake (% of energy intake, continuous), dietary fiber (g/d, continuous), magnesium intake (g/d, continuous), and alcohol intake (g/d, continuous).
Quality assessment of included cohort studies and integrity of individual participant data
We assessed the quality of the included cohort studies or large multicohort consortia and integrity of the individual participant data according to a prespecified protocol (appendix 1). Two independent researchers assessed the quality of participating cohorts by using the Newcastle-Ottawa Scale.22 We considered a study to have a low risk of bias if it scored as a such (good quality or fair quality) in all the following domains: population selection, comparability, and outcome. Individual participant data were obtained between August and September 2017. Individual participant data integrity and data quality control procedures comprised range and consistency checks on all individual participant data outputs to identify invalid, out of range, or disparate items. Errors, missing information, or inconsistencies were queried and rectified as necessary with input from each analyst between September and December 2017.
Data synthesis
We undertook a two stage individual participant data meta-analysis to obtain adjusted summary estimates of incident type 2 diabetes for polygenic risk score, isocaloric replacement of carbohydrate (referred to refined starch and sugars) with total or subtypes of fat, and the interaction of total or subtypes of fat with polygenic risk score.23
In each cohort (first stage of the two stage meta-analysis), we used Cox proportional hazards models to estimate the risk of type 2 diabetes comparing type 2 diabetes cases to all other patients without diabetes at the end of follow-up after exploring that the proportional hazards assumption was not violated. In studies with a case cohort design, all Cox proportional hazards models were Prentice weighted.24 We modeled polygenic risk score, total or subtypes of fat intake, and the interaction terms as continuous variables. We used polynomials to investigate the presence of a non-linear association, and found little evidence of a non-linear association when removing outliers in total or subtypes of fat intake variables. Therefore, we winsorized total or subtypes of fat intake variables (±4 SD from the mean). We adjusted the models to investigate the association between polygenic risk score and the risk of type 2 diabetes for age, sex, BMI, smoking, physical activity, parental history of type 2 diabetes, history of dyslipidemia and hypertension, and dietary factors. Additional cohort-specific adjustments, including multicenter recruitment site or family relatedness, were included as appropriate. We used nutrient replacement models to investigate the hazard ratios of type 2 diabetes by substituting a percentage of energy from total or subtypes of fat for equivalent energy from carbohydrate (referred to refined starch and sugars because dietary fiber was included in the models). These models were further adjusted for subtypes of fat specific to each statistical model (supplementary table 4). As dietary sources of monounsaturated fat might also contain high amounts of saturated fat,25 isocaloric replacement models for monounsaturated fat accounted for dietary saturated fat and total energy to allow for the interpretation of the hypothetical replacement of monounsaturated fat with carbohydrate, independent of other fatty acids, as described in earlier studies.26 27 28 29 30 Isocaloric substitution of fat from carbohydrate represents a realistic approach considering that total energy intake is primarily balanced between the amount of fat and carbohydrate and that calories from saturated fat tend to mainly replace low quality carbohydrate.27 31 In post hoc analyses, we further estimated the effects of increasing polyunsaturated fat intake in place of saturated fat.
To test the interaction between dietary fat subtypes and genetic risk on the risk of type 2 diabetes we used Cox proportional hazards models incorporating an interaction term between dietary fat variables and the polygenic risk score. Given the strong collinearity between the interaction term and both dietary fats variables and polygenic risk score, we centered these variables by using the combined mean value of all cohorts as recommended elsewhere.32 We conducted further analyses, not prespecified in the analysis plan, to investigate whether total or subtypes of fat mediate the association between genetic risk and the incidence of type 2 diabetes in additional models with and without adjustment for total and subtypes of fat. In each participating cohort, confounders with more than 5% of observations missing were handled by multiple imputation using chained equations.33 34 If less than 5% of values were missing, participants with the missing information were excluded from the analysis (appendix 1, supplementary table 5).
In the second stage of the individual participant data meta-analysis, summary statistics provided by each cohort were combined by inverse-variance weighted random effects meta-analyses (DerSimonian and Laird procedure).35 We used between study variance (τ2) to assess heterogeneity.36 We conducted additional meta-analyses iteratively excluding one cohort at a time to estimate robustness of the meta-analytic estimates. Small study effects owing to potential publication bias, poor methodological quality in smaller studies, artefactual associations, true heterogeneity, or chance were evaluated by using contour-enhanced funnel plots alongside visual examination and statistical tests for asymmetry (Debray’s test).37 In addition, we assessed the possibility of ascertainment bias by plotting adjusted estimates from each participating cohort study against the percentage of individuals with genetic information in each study. We further restricted all our analyses to cohorts classified as a low risk of bias and cohorts with repeated measurements of diet. Finally, we conducted additional analyses by using the joint meta-analysis method.38 In brief, the joint meta-analysis is a multivariate meta-analysis approach that simultaneously estimates the main genetic effects and interaction term contribution on the risk of type 2 diabetes.
We considered two sided α level of 0.05 for all analyses. Statistical analyses were carried out using SAS software, version 9.3 or R software, versions 3.1.0 and 3.2.0. Meta-analyses and meta regressions were conducted using the metafor package implemented in R, version 3.2.0 and METAL.39
Patient and public involvement
No patients were directly involved in designing the research question or in conducting the research. No patients were asked for advice on interpretation or writing up the results. There are no plans to involve patients or relevant patient community in dissemination at this moment.
Results
We identified 19 eligible prospective cohort studies or multicohort consortia, of which 15 provided individual participant data (appendix 3). A flow diagram of the study selection and individual participant data obtained is detailed in supplementary figure 1. Descriptions and baseline characteristics of included prospective cohort studies or multicohort consortia are detailed in table 1 and appendix 4. Overall, participating prospective cohort studies had a low risk of bias in population selection, comparability, and outcome domains (supplementary table 6). We did not encounter issues that we were unable to resolve with the individual participant data contributor during the individual participant data integrity check.
Table 1.
Prospective cohort studies that contributed to the meta-analysis of individual participant data on the quality of dietary fat and genetic risk of type 2 diabetes
| Participating cohorts | Abbreviation | Country | Baseline | Sample size | No (%) of type 2 diabetes incident cases | Years of follow-up |
|---|---|---|---|---|---|---|
| Atherosclerosis Risk in Communities Study | ARIC | USA | 1987-1989 | 6690 | 442 (6.6) | 6 |
| Bogalusa Heart Study | BHS | USA | 1998-2001 | 790 | 46 (6.6) | 5 |
| Cardiovascular Health Study | CHS | USA | 1989-1990 | 2813 | 258 (9.2) | 20 |
| Diet, Cancer and Health* | DCH | Denmark | 1993-1997 | 8788 | 3987 (40.1) | 12.5 |
| European Prospective Investigation of Cancer-InterAct* | EPIC-InterAct | Europe (10 countries) | 1991-1997 | 20 856 | 9257 (44.4) | 9.6 |
| Framingham Heart Study | FHS | USA | 1991-1996 | 6710 | 289 (4.3) | 12 |
| FINRISK Study | FINRISK | Finland | 2007 | 3822 | 172 (4.5) | 7.6 |
| Health 2000 | Health 2000 | Finland | 2000 | 1946 | 138 (7.1) | 12.4 |
| Health Professionals’ Follow-up Study | HPFS | USA | 1986 | 5587 | 939 (16.8) | 22 |
| Inter99 study | Inter99 | Denmark | 1999 | 5607 | 279 (5.5) | 11 |
| Malmö Diet and Cancer study | MDC-CC | USA | 1991-1994 | 3745 | 440 (11.9) | 16 |
| Multi-Ethnic Study of Atherosclerosis | MESA | USA | 2000-2002 | 1535 | 136 (8.9) | 9.1 |
| Nurses’ Health Study | NHS | USA | 1984 | 8832 | 1453 (17.1) | 28 |
| Rotterdam Study I | RS-I | The Netherlands | 1989 | 2509 | 324 (12.9) | 10.2 |
| Women's Genome Health Study | WGHS | USA | 1991 | 22 120 | 1855 (8.4) | 12 |
| Total | 102 350 | 20 015 (19.6) | 12 |
Prospective nested case control.
Of 102 350 participants included in the individual participant data meta-analysis, 20 015 type 2 diabetes cases were documented after a median follow-up of 12 years (interquartile range 9.4-14.2 years). Table 2 and supplementary table 7 show the data on participant’s dietary intake, genetic risk profile, and covariates. Type 2 diabetes genetic risk score mean was 70 (SD 5.6) units. Baseline mean intakes of total and subtypes of fat in percentage of energy were 32.8% (5.9) for total fat, 5.8% (1.6) for polyunsaturated fat, 11.9% (2.6) for monounsaturated fat, and 12% (2.8) for saturated fat. Mean intakes of total omega 3 polyunsaturated fat, omega 6 polyunsaturated fat, and trans fat were 1.5 g/d (0.6), 11.3 g/d (5.7), and 2.0 g/d (1.1), respectively.
Table 2.
Baseline characteristics of patients included in studies that contributed to the meta-analysis of individual participant data on the quality of dietary fat and genetic risk of type 2 diabetes. Values are means (SD)
| Cohort | Age (years) | PRS (units) | Total fat (%) | PUFA (%) | MUFA (%) | SFA (%) | Omega 3 PUFA (g/d) | Omega 6 PUFA (g/d) | Total trans fat (g/d) |
|---|---|---|---|---|---|---|---|---|---|
| ARIC | 53.9 (5.6) | 70.1 (5.6) | 33.2 (6.7) | 5.1 (1.5) | 12.7 (2.9) | 12.2 (3) | 0.8 (0.4) | 8.9 (5.3) | 3.0 (1.8) |
| BHS | 33.1 (4.9) | 67.1 (5.4) | 33.8 (4.9) | 6.9 (1.4) | 12.8 (1.9) | 11.2 (2.1) | NA | NA | NA |
| CHS | 72.3 (5.3) | 66.5 (5.4) | 32.2 (6) | 7.5 (2.2) | 11.6 (2.4) | 10.3 (2.2) | 2.2 (1.1) | 14.8 (6.7) | 3.8 (2.1) |
| DCH* | 56.3 (4.4) | 70.1 (5.6) | 32.4 (5.3) | 5.4 (1.4) | 11.8 (2.1) | 12.4 (2.7) | 2.7 (1.1) | 11.5 (5.8) | NA |
| EPIC-InterAct* | 53.8 (8.7) | 71.2 (5.7) | 34.8 (5.7) | 5.8 (1.8) | 13.6 (3.3) | 13.3 (3.4) | NA | NA | NA |
| FHS | 50.8 (13.4) | 68.6 (5.4) | 31.3 (5.6) | 6.0 (1.4) | 11.6 (2.4) | 10.8 (2.5) | 1.5 (0.6) | 11.8 (6.7) | 2.5 (1.1) |
| FINRISK | 48.4 (13.5) | 70.6 (5.5) | 31.2 (6.5) | 5.8 (1.6) | 11.5 (2.7) | 10.9 (2.9) | 1.3 (0.8) | 11.3 (5.6) | 2.6 (1.3) |
| Health 2000 | 51.6 (11.1) | 68.9 (5.5) | 36.9 (5.1) | 5.5 (1.2) | 11.9 (1.9) | 14.1 (2.6) | 2.8 (1.3) | 12.5 (5.8) | 1.1 (0.5) |
| HPFS | 54.2 (8.7) | 68.9 (5.6) | 32.4 (6.2) | 5.9 (1.6) | 12.4 (2.7) | 11.2 (2.7) | 1.4 (0.6) | 11.9 (5.4) | 2.9 (1.6) |
| Inter99 | 46.0 (7.8) | 70.2 (5.6) | 32.6 (7) | 5.5 (1.5) | 11.1 (2.8) | 12.7 (3.5) | 0.9 (0.5) | 11.4 (6.0) | 2.5 (1.2) |
| MDC-CC | 57.3 (6.0) | 69.7 (5.4) | 36.8 (6.1) | 5.9 (1.5) | 12.8 (2.2) | 15.7 (3.8) | 2.4 (0.9) | 12.5 (6.3) | N/A |
| MESA | 60.5 (9.4) | 70.7 (5.6) | 31.3 (6.9) | 6.1 (1.8) | 12.0 (2.8) | 10.5 (3.2) | 1.2 (0.6) | 10.7 (5.9) | 3.2 (2.2) |
| NHS | 51.7 (6.7) | 68.3 (5.8) | 34.8 (5.8) | 6.7 (1.8) | 12.7 (2.4) | 12.5 (2.6) | 1.5 (0.6) | 10.8 (4.6) | 3.8 (1.7) |
| RS-I | 65.3 (6.8) | 69.6 (5.6) | 36 (6.2) | 6.8 (2.7) | 12.3 (2.7) | 14.2 (3.1) | 1.1 (0.5) | 12.5 (6.1) | 2.1 (0.9) |
| WGHS | 54.6 (7.1) | 70.3 (5.5) | 30 (6.1) | 5.8 (1.5) | 11.2 (2.6) | 10.2 (2.5) | 1.4 (0.6) | 10.9 (5.8) | 2.3 (1.3) |
PRS=polygenic risk score; PUFA=polyunsaturated fat; MUFA=monounsaturated fat; SFA=saturated fat; NA=not available.
Prospective nested case control.
Associations of genetic risk and quality of dietary fat with incidence of type 2 diabetes
Figure 1 shows that the hazard ratio of type 2 diabetes was 1.64 (95% confidence interval 1.54 to 1.75, P<0.001, I2=7.1%, τ2=0.003) per increment of 10 risk alleles in polygenic risk score after adjusting cohort estimates for demographics, lifestyle, and clinical characteristics. In meta-analyses for the association between the quality of dietary fat and the risk of type 2 diabetes, the increase of polyunsaturated fat or total omega 6 polyunsaturated fat in place of carbohydrate (refined starch and sugars) was associated with a lower risk of type 2 diabetes, with adjusted hazard ratios of 0.90 (0.82 to 0.98, P=0.02, I2=18.0%, τ2=0.006; per 5% of energy; fig 2) and 0.99 (0.97 to 1.00, P=0.05, I2=58.8%, τ2=0.001; per increment of 1 g/d; fig 3), respectively. The increase of monounsaturated fat in place of carbohydrate was associated with a higher risk of type 2 diabetes (hazard ratio 1.10, 95% confidence interval 1.01 to 1.19, P=0.04, I2=25.9%, τ2=0.006; per increment of 5% of energy; fig 4). Isocaloric replacement of carbohydrate with total fat, saturated fat, total omega 3 polyunsaturated fat, and trans fat yielded non-significant associations with adjusted hazard ratios of 0.98 (95% confidence interval 0.94 to 1.02, P=0.26, I2=61.0%, τ2=0.003; per increment of 5% of energy; fig 5), 0.95 (0.90 to 1.00, P=0.07, I2=0.0%, τ2=0.0; per increment of 5% of energy; fig 6), 0.95 (0.89 to 1.02, P=0.17, I2=30.9%, τ2=0.005; per increment of 1 g/d; fig 7), and 0.98 (0.94 to 1.01, P=0.21, I2=2.4%, τ2=0.001; per increment of 1 g/d; fig 8), respectively.
Fig 1.
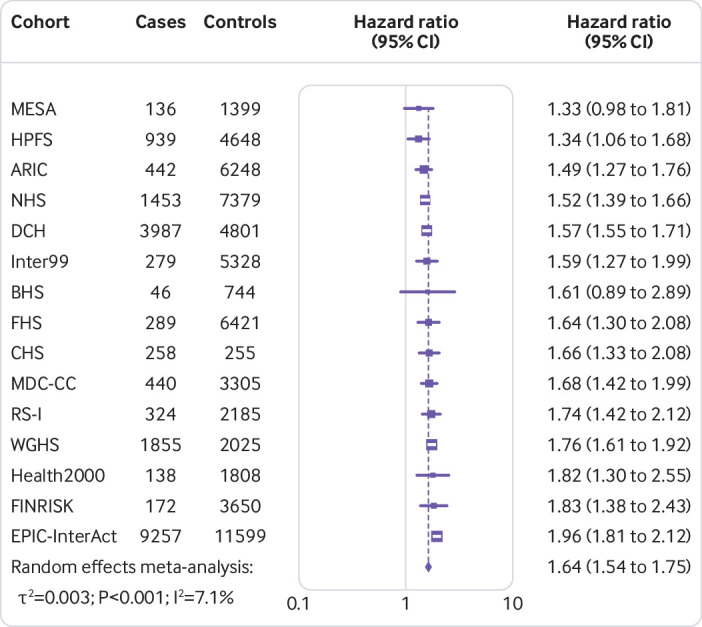
Combined risk of type 2 diabetes per increment of 10 risk alleles in the polygenic risk score
Fig 2.
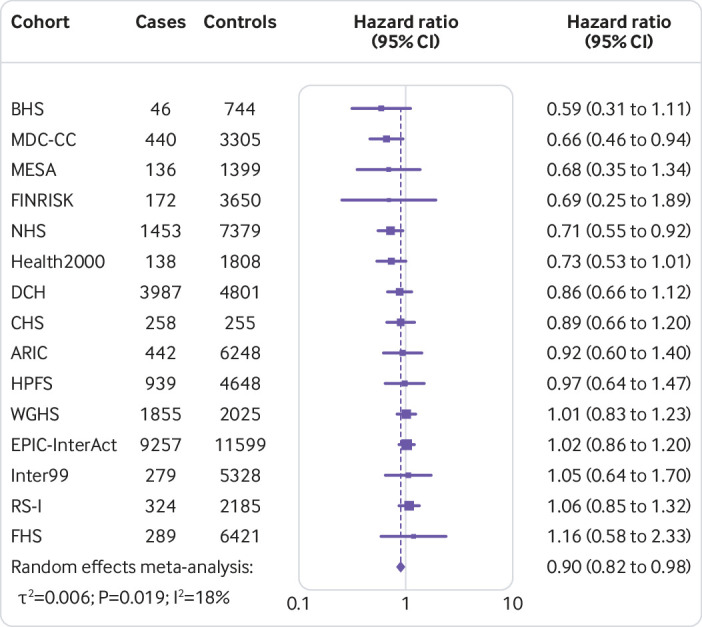
Risk of type 2 diabetes associated with isocaloric replacement (5% energy) of carbohydrate with polyunsaturated fat
Fig 3.
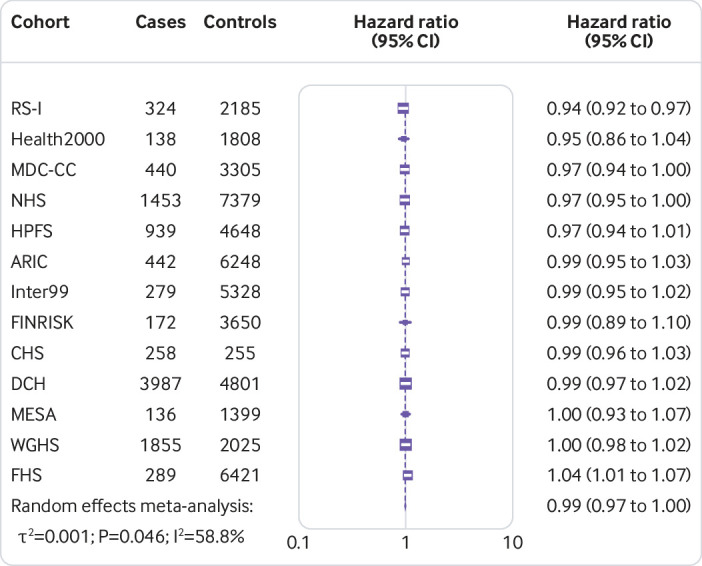
Risk of type 2 diabetes associated with isocaloric replacement (1 g/d) of carbohydrate with total omega 6 polyunsaturated fat
Fig 4.
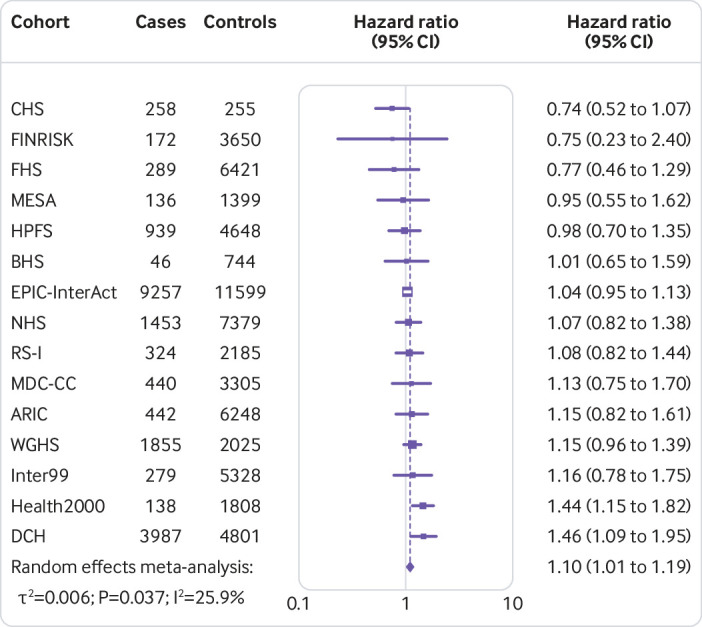
Risk of type 2 diabetes associated with isocaloric replacement (5% energy) of carbohydrate with monounsaturated fat
Fig 5.
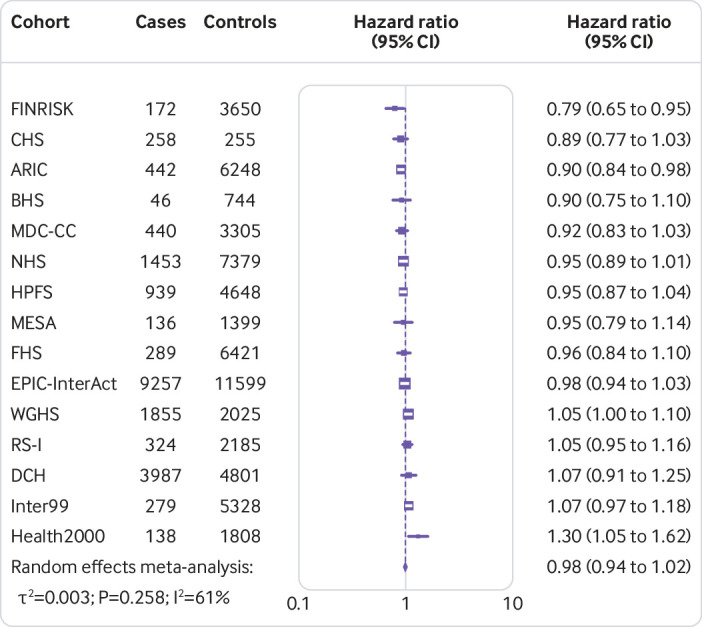
Risk of type 2 diabetes associated with isocaloric replacement (5% energy) of carbohydrate with total fat
Fig 6.
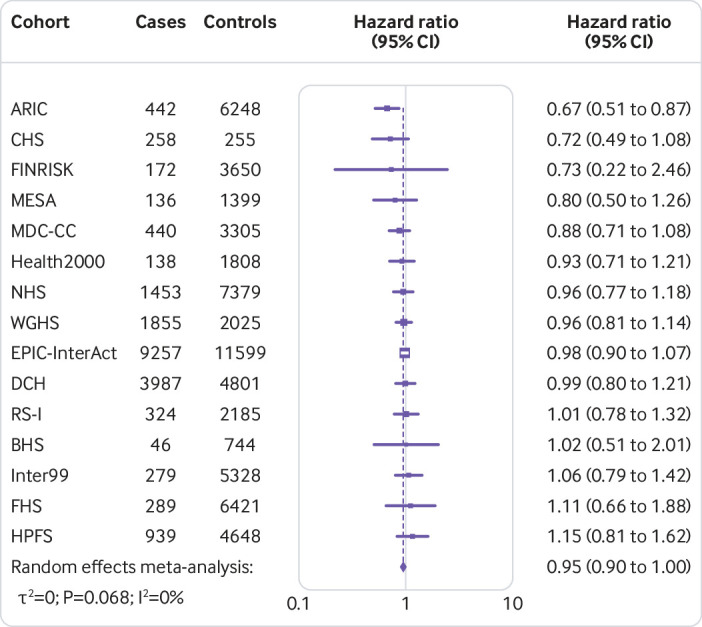
Risk of type 2 diabetes associated with isocaloric replacement (5% energy) of carbohydrate with saturated fat
Fig 7.
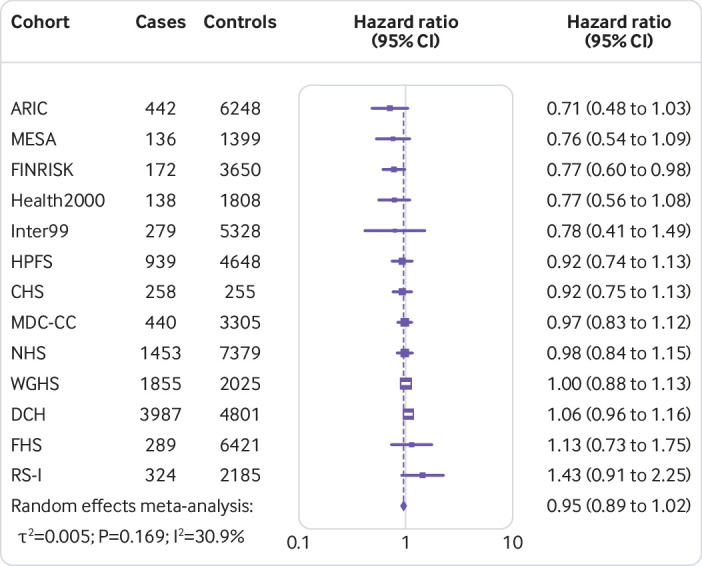
Risk of type 2 diabetes associated with isocaloric replacement (1 g/d) of carbohydrate with total omega 3 polyunsaturated fat
Fig 8.
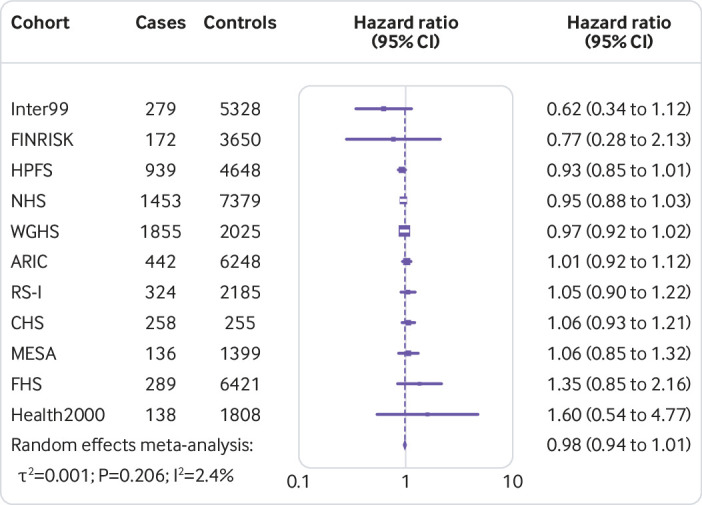
Risk of type 2 diabetes associated with isocaloric replacement (1 g/d) of carbohydrate with total trans fat
The association of total omega 6 polyunsaturated fat with the risk of type 2 diabetes showed moderate heterogeneity, however, subsequent sensitivity analyses testing for single cohort-driven effects did not reveal evidence of cohort dominant bias (supplementary fig 2). We found visual and statistical suggestive evidence of small study effects in the contour-enhanced funnel plots for the individual participant data meta-analysis of the overall association of polyunsaturated fat with the risk of type 2 diabetes (Debray’s test P=0.05), but not for the omega 6 polyunsaturated fat and monounsaturated fat association (Debray’s test P=0.70 and P=0.64, respectively, supplementary fig 3). Evidence of small study effects was likely owing to reporting bias given that presumed missing studies were mainly allocated in areas of non-significance.40 We did not detect evidence of ascertainment bias in our meta-analytic estimates (supplementary fig 4).
Sensitivity analyses for the association between the quality of dietary fat and the risk of type 2 diabetes in cohorts classified as a low risk of bias (n=12) showed consistent findings with the primary analysis except for the association between omega 6 polyunsaturated fat and the risk of type 2 diabetes (hazard ratio 0.98, 95% confidence interval 0.97 to 1.00, P=0.07, I2=65.5%, τ2 <0.001; per increment of 1 g/d; supplementary fig 5). Further analyses including only cohorts with repeated measurements of diet (n=8) showed similar findings, except for the association of monounsaturated fat and omega 6 polyunsaturated fat, with adjusted hazard ratios of 1.10 (95% confidence interval 0.97 to 1.25, P=0.12, I2=0.0%, τ2=0.0; per increment of 5% of energy) and 0.99 (0.98 to 1.01, P=0.42, I2=48.2%, τ2 <0.001; per increment of 1 g/d), respectively (supplementary fig 6). Isocaloric replacement of saturated fat with polyunsaturated fat was associated with a lower risk of type 2 diabetes, with an adjusted hazard ratio of 0.91 (0.85 to 0.98, P=0.02, I2=47.2%, τ2=0.02; per increment of 5% of energy; supplementary fig 7).
Interaction between polygenic risk score and quality of dietary fat on the risk of type 2 diabetes
Next, we evaluated whether the presence of known type 2 diabetes risk-increasing alleles modified the association between the quality of dietary fat and the risk of type 2 diabetes. In meta-analyses of the interaction estimates between polygenic risk score and dietary fat subtypes on the risk of type 2 diabetes, we did not find evidence of significant interactions (P>0.05 for interaction; table 3, supplementary fig 8). Results were similar when the analysis was restricted to studies classified as a low risk of bias (supplementary table 8) or when only cohorts with repeated measurements of diet were included (supplementary table 9). Similarly, in the multivariate meta-analysis, we observed that the signal was mainly driven by the main genetic effects, with a marginal effect of the interaction term (supplementary table 10). In additional analysis to investigate whether total or subtypes of fat mediate the association between genetic risk and incidence of type 2 diabetes, we observed that any mediation effect was likely to be minimal (supplementary table 11).
Table 3.
Interaction between total and subtypes of fat intake and polygenic risk score on the risk of type 2 diabetes
| Dietary factor | β interaction (SE) | P value* | Direction of interaction in included studies† | τ2‡ | Sample size |
|---|---|---|---|---|---|
| Total fat, % energy | 0.019 (0.015) | 0.20 | +−+++−++–+– | 0 | 102 350 |
| PUFA, % energy | 0.049 (0.061) | 0.43 | –++++++++−+–+ | 0.003 | 102 350 |
| MUFA, % energy | 0.040 (0.032) | 0.22 | +−+++−−++−+− | 0 | 102 350 |
| SFA, % energy | −0.017 (0.031) | 0.58 | +−+−−+−+− | 0 | 102 350 |
| omega 3 PUFA, g/d | 0.025 (0.030) | 0.40 | −?+−?−+++−+++ | 0.002 | 80 704 |
| omega 6 PUFA, g/d | 0.007 (0.005) | 0.13 | −?++?−+++−+−+ | 0 | 80 704 |
| Total trans fat, g/d | 0.009 (0.017) | 0.56 | −?−??−+−?−+++ | 0 | 68 171 |
For interaction.
For each dietary factor the combined interaction P value, heterogeneity and sample size are shown. Direction of interaction represents the sign of the β in each cohort (Cohorts presented in alphabetical order: ARIC, BHS, CHS, DCH, EPIC-InterAct, FHS, FINRISK, Health 2000, HPFS, Inter99, MDC-CC, MESA, NHS, RS-1, WGHS).
Between study variance (τ2) was used to assess heterogeneity.
PUFA=polyunsaturated fat; MUFA=monounsaturated fat; SE=standard error; SFA=saturated fat; ?=not available.
Discussion
In the present individual participant data meta-analysis including 102 350 participants of European descent and free of diabetes at baseline from 15 prospective cohort studies or multicohort consortia, we have generated data on the potential interplay between genetic and dietary factors on the risk of type 2 diabetes. Our results suggest that a polygenic risk score computed from known type 2 diabetes risk-increasing alleles as well as dietary unsaturated fats were both associated with the risk of type 2 diabetes, with no evidence of meaningful interactions between genetic risk profile and the quality of dietary fat on the incidence of type 2 diabetes.
Results in relation to other studies
Currently, major worldwide organizations recommend dietary patterns rich in unsaturated fatty acids (both monounsaturated fat and polyunsaturated fat) and limit the intake of saturated fat and sugars to reduce the risk of type 2 diabetes.12 13 Our findings suggest that, regardless of genetic risk, consuming more polyunsaturated fat in place of refined starch and sugars is associated with a lower risk of type 2 diabetes, whereas consuming more monounsaturated fat in place of carbohydrate is associated with a higher risk of type 2 diabetes. These observations are in agreement with evidence on intermediate glycemic phenotypes, suggesting that only energy intake substitution with polyunsaturated fat, but not monounsaturated fat, was associated with favorable glycemic levels.16 Our results expand these observations to the risk of type 2 diabetes and support public health efforts that emphasize the consumption of foods rich in polyunsaturated fat.
The positive association between monounsaturated fat and the risk of type 2 diabetes is likely to reflect the fact that in populations from North America and non-Mediterranean European countries, which constitute the present analysis, animal products are the main sources of monounsaturated fat. Therefore, the expected benefits of monounsaturated fat on the risk of type 2 diabetes might be attenuated depending on the food matrix in which they are present,41 42 as recently documented for the association between different types of monounsaturated fat and coronary artery disease risk.25 Furthermore, the lack of association when replacing carbohydrate with saturated fat on the incidence of type 2 diabetes is consistent with previous studies for both incident type 2 diabetes and coronary artery disease.15 30 43 The present study, including the largest series to date of incident type 2 diabetes cases, extended follow-up, and detailed quality of dietary fat information, provides further evidence for the primary prevention of type 2 diabetes.
The results of the present analysis are in agreement with recent evidence that showed no appreciable interactions between dietary components and isolated type 2 diabetes risk-increasing alleles.44 In addition, the Diabetes Prevention Program showed that an intensive lifestyle modification is effective for the prevention of type 2 diabetes regardless of genetic risk.45 Our findings also concur with recent evidence in coronary artery disease, suggesting that both genetics and lifestyle factors are independently associated with the risk of coronary artery disease without evidence of interactions.46 In contrast, increasing evidence has shown that certain dietary or lifestyle factors such as unhealthy dietary patterns, sugar sweetened drinks, fried foods, and physical inactivity might interact with genetic susceptibility to elevated BMI on the risk of developing obesity.20 47 48 49 However, in lifestyle intervention trials for obesity little or no evidence of such interactions has emerged.50 51 Such findings support the notion that if interactions between genetic risk and dietary or lifestyle factors exist, their effects are likely to be small or undetected by conventional approaches.52 Taken together, these findings support lifestyle or dietary interventions for the prevention of type 2 diabetes to be deployed across all gradients of genetic risk in the population, as genetic burden does not seem to impede their effectiveness.
Factors that may have limited the ability to detect interactions include the use of a polygenic risk score for overall known type 2 diabetes genetic risk. In our study, the polygenic risk score is enriched for regulatory variants in transcription factor binding sites and enhancers active in pancreatic islets or adipose tissue, therefore reflecting multiple biological pathways.4 Thus, the score might not be able to uncover potential interactions that are driven by specific modulations of biological pathways or mechanisms. For example, genetic variants related to fat metabolism, such as PPARG or FADS, could be more biologically relevant for interaction analyses. However, further restraining the number of genetic variants will explain a smaller proportion of the phenotype, therefore limiting the clinical value of potential interactions. Another important factor for the lack of interactions is because dietary sources of fat involve a heterogeneous blend of nutrients and food matrices.53 Thus, the potential interaction between subtypes of fat and the genetic risk might be attenuated, depending on the food matrix in which subtypes of fat are present. Moreover, the design and implementation of new studies, such as genotype-based recall clinical trials intended for the investigation of genetic and lifestyle interactions, with more accurate objective measurement of diet are required to better understand the interplay between genetic and lifestyle factors on the risk of type 2 diabetes.54
Strengths and limitations
The strengths of this study include the larger number of incident type 2 diabetes cases, the harmonized covariate definitions and analysis plan among participating cohorts, the well validated measures of dietary factors and disease ascertainment, and the consistency of our findings in sensitivity analyses including only cohorts at low risk of bias or cohorts with repeated measurements of diet. This is the first large long term individual participant data meta-analysis to investigate whether the quality of dietary fat interacts with type 2 diabetes genetic burden on the development of type 2 diabetes.
Our study has several potential limitations. First, the observational nature of the study design does not allow us to infer causality regarding the effect of changes in fat intake on the risk of type 2 diabetes.
Second, although every effort was made to maximize the validity of the study, minimize bias, and incorporate heterogeneity and uncertainty, the estimated hazard ratios of dietary components could be affected by measurement error and residual confounding. A possible reason for the lack of interaction between the polygenic risk score and quality of dietary fat on the risk of type 2 diabetes could be imprecision in dietary intake measurement. We used cumulative averages to yield more precise dietary intake estimates than baseline intakes alone,21 but the use of more objective measurements of dietary intake such as the use of smartphone applications, wearable technology, or dietary intake biomarkers, is necessary to accurately ascertain dietary intake and reduce self reported errors.55
Third, we designed the study to model isocaloric replacement of carbohydrate with total or subtypes of fat, and thus the study of other nutrients such as the amount and subtypes of protein and carbohydrates or the replacement of fat with other nutrients might yield different findings.
Fourth, expanding our genetic score to include recently discovered low frequency variants in relevant type 2 diabetes loci such as PPARG, SLC30A8, MTNR1B, G6PC2, or CCND2, or those yet undiscovered, could prove useful in subsequent analyses. However, previous studies have shown that the inclusion of newly discovered type 2 diabetes risk-increasing alleles minimally increased type 2 diabetes heritability estimates.5 In addition, the use of weights from case control cross-sectional studies could have diminished our chances to detect interactions, if genetic effect sizes differed markedly from those ascertained in longitudinal studies. However, weighted and unweighted polygenic risk scores tend to have a comparable performance for complex traits prediction,56 57 and the capability of a polygenic risk score based on case control weights to predict incident type 2 diabetes is consistent.6 7
Fifth, when multiple testing was taken into account using Bonferroni’s adjustment, only the polygenic risk score association retained significance.
Finally, the inclusion of only patients of European descent based on sample availability might preclude the generalizability of our findings to other populations. However, our findings supporting the quality of dietary fat recommendation for the prevention of type 2 diabetes to be deployed across all gradients of genetic risk in the population could be applicable to other non-European populations, though further targeted studies are required.
Conclusion and policy implications
After evaluating the type 2 diabetes genetic risk profiles and the quality of dietary fat among 102 350 patients without diabetes at baseline from 15 prospective cohort studies, our individual participant data meta-analysis provides evidence that genetic risk and quality of dietary fat are each associated with the incidence of type 2 diabetes. Findings from this study serve to provide useful clinical answers as we prepare for the eventual translation of genetic profiles into clinical and public health practice. Our findings support dietary or lifestyle interventions for the prevention of type 2 diabetes to be deployed across all gradients of genetic risk in the population, as genetic burden does not seem to impede their effectiveness.
What is already known on this topic
Type 2 diabetes is a complex disease driven by genetic and lifestyle factors
Dietary recommendations aiming to improve dietary quality are a critical part of type 2 diabetes prevention and treatment
What this study adds
Genetic risk and quality of dietary fat are each associated with the incidence of type 2 diabetes
Dietary or lifestyle interventions for type 2 diabetes should be deployed across all gradients of genetic risk in the population, as genetic burden does not seem to impede their effectiveness
Acknowledgments
We thank Bianca Porneala (Division of General Internal Medicine, Massachusetts General Hospital) and Mackenzie Senn (USDA/ARS Children's Nutrition Research Center, Baylor College of Medicine) for helping with statistical analysis.
Web Extra.
Extra material supplied by the author
Supplementary materials: Appendix 1 to 5, supplementary tables 1 to 11, and supplementary figure 1 to 8
Supplementary materials: Full author list and affiliations
CHARGE Consortium Nutrition Working Group members: Jordi Merino, Marta Guasch-Ferré, Christina Ellervik, Hassan S Dashti, Stephen J Sharp, Peitao Wu, Kim Overvad, Chloé Sarnowski, Mikko Kuokkanen, Rozenn N Lemaitre, Anne E Justice, Ulrika Ericson, Kim V E Braun, Yuvaraj Mahendran, Alexis C Frazier-Wood, Dianjianyi Sun, Audrey Y Chu, Toshiko Tanaka, Jian’an Luan, Jaeyoung Hong, Anne Tjønneland, Ming Ding, Annamari Lundqvist, Kenneth Mukamal, Rebecca Rohde, Christina-Alexandra Schulz, Oscar H Franco, Niels Grarup, Yii-Der Ida Chen, Lydia Bazzano, Paul W Franks, Julie E Buring, Claudia Langenberg, Ching-Ti Liu, Torben Hansen, Majken K Jensen, Katri Sääksjärvi, Bruce M Psaty, Kristin L Young, George Hindy, Camilla Helene Sandholt, Paul M Ridker, Jose M Ordovas, James B Meigs, Oluf Pedersen, Peter Kraft, Markus Perola, Kari E North, Marju Orho-Melander, Trudy Voortman, Ulla Toft, Jerome I Rotter, Lu Qi, Nita G Forouhi, Dariush Mozaffarian, Thorkild I A Sørensen, Meir J Stampfer, Satu Männistö, Elizabeth Selvin, Fumiaki Imamura, Veikko Salomaa, Frank B Hu, Nick J Wareham, Josée Dupuis, Caren E Smith, Tuomas O Kilpeläinen, Daniel I Chasman, and Jose C Florez.
Contributors: JM, MGF, FBH, DIC, and JCF conceived the study concept and design. JM, HSD, and CES undertook the literature searches and study selection. JM and HSD acquired individual participant data. JD and JCF provided method support. MGF performed the analysis for NHS and HPFS. CE performed the analysis for WGHS. SJS performed the analysis for InterAct. PW performed the analysis for FHS. MK performed the analysis for FINRISK and Health2000. RNL performed the analysis for CHS. AEJ performed the analysis for ARIC. UE performed the analysis for MDC-CC. KMVB performed the analysis for RS-I. YM performed the analysis for Inter99. AFW performed the analysis for MESA. DS performed the analysis for BHS. TOK performed the analysis for DCH. JM, HSD, PW, and CS cleaned and quality checked data and conducted the meta-analyses. All authors contributed to interpretation of data. JM, MGF, CE, HSD, CES, TOK, JCF wrote the first draft of the Manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. JM, MGF, CE, and HSD contributed equally as first authors. CES, TOK, DIC, and JCF contributed equally as senior authors. See the supplementary materials for the full author list and affiliations.
Funding: See appendix 5 for the supporting organizations.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work except for BMP, who serves on the data and safety monitoring board of a clinical trial funded by Zoll LifeCor and on the Steering Committee of the Yale Open Data Access Committee funded by Johnson and Johnson.
Ethical approval: Institutional review boards, or oversight committees, or both, approved the study in each participating cohort and all participants provided written informed consent.
Data sharing: Data, the statistical code, and technical processes are available from the author at jmerino@mgh.harvard.edu
The corresponding author (JM) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas 8th Edition. November 2017. http://www.diabetesatlas.org.
- 2. Franks PW, McCarthy MI. Exposing the exposures responsible for type 2 diabetes and obesity. Science 2016;354:69-73. 10.1126/science.aaf5094. [DOI] [PubMed] [Google Scholar]
- 3. Morris AP, Voight BF, Teslovich TM, et al. Wellcome Trust Case Control Consortium. Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators. Genetic Investigation of ANthropometric Traits (GIANT) Consortium. Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium. South Asian Type 2 Diabetes (SAT2D) Consortium. DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 2012;44:981-90. 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuchsberger C, Flannick J, Teslovich TM, et al. The genetic architecture of type 2 diabetes. Nature 2016;536:41-7. 10.1038/nature18642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scott RA, Scott LJ, Mägi R, et al. DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes 2017;66:2888-902. 10.2337/db16-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet 2018;50:1505-13. 10.1038/s41588-018-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vassy JL, Hivert M-F, Porneala B, et al. Polygenic type 2 diabetes prediction at the limit of common variant detection. Diabetes 2014;63:2172-82. 10.2337/db13-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qi L, Cornelis MC, Zhang C, van Dam RM, Hu FB. Genetic predisposition, Western dietary pattern, and the risk of type 2 diabetes in men. Am J Clin Nutr 2009;89:1453-8. 10.3945/ajcn.2008.27249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langenberg C, Sharp SJ, Franks PW, et al. Gene-lifestyle interaction and type 2 diabetes: the EPIC interact case-cohort study. PLoS Med 2014;11:e1001647. 10.1371/journal.pmed.1001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med 2015;372:2229-34. 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- 11. Ashley EA. Towards precision medicine. Nat Rev Genet 2016;17:507-22. 10.1038/nrg.2016.86. [DOI] [PubMed] [Google Scholar]
- 12. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2014;37(Suppl 1):S120-43. 10.2337/dc14-S120. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 8th Edition. December 2015. https://health.gov/dietaryguidelines/2015/guidelines/.
- 14. Eckel RH, Jakicic JM, Ard JD, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2960-84. 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 15. de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 2015;351:h3978. 10.1136/bmj.h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imamura F, Micha R, Wu JHY, et al. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med 2016;13:e1002087. 10.1371/journal.pmed.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forouhi NG, Krauss RM, Taubes G, Willett W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ 2018;361:k2139. 10.1136/bmj.k2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stewart LA, Clarke M, Rovers M, et al. PRISMA-IPD Development Group Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA 2015;313:1657-65. 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 19. Meigs JB, Shrader P, Sullivan LM, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med 2008;359:2208-19. 10.1056/NEJMoa0804742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang T, Heianza Y, Sun D, et al. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene-diet interaction analysis in two prospective cohort studies. BMJ 2018;360:j5644. 10.1136/bmj.j5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531-40. 10.1093/oxfordjournals.aje.a009849 [DOI] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos MTP. The NewcastleOttawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinicalepidemiology/oxford.html.
- 23. Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340:c221. 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 24. Onland-Moret NC, van der A DL, van der Schouw YT, et al. Analysis of case-cohort data: a comparison of different methods. J Clin Epidemiol 2007;60:350-5. 10.1016/j.jclinepi.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 25. Zong G, Li Y, Sampson L, et al. Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am J Clin Nutr 2018;107:445-53. 10.1093/ajcn/nqx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jakobsen MU, O’Reilly EJ, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009;89:1425-32. 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Hruby A, Bernstein AM, et al. Saturated Fats Compared With Unsaturated Fats and Sources of Carbohydrates in Relation to Risk of Coronary Heart Disease: A Prospective Cohort Study. J Am Coll Cardiol 2015;66:1538-48. 10.1016/j.jacc.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guasch-Ferré M, Babio N, Martínez-González MA, et al. PREDIMED Study Investigators Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr 2015;102:1563-73. 10.3945/ajcn.115.116046. [DOI] [PubMed] [Google Scholar]
- 29. Guasch-Ferré M, Becerra-Tomás N, Ruiz-Canela M, et al. Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr 2017;105:723-35. 10.3945/ajcn.116.142034. [DOI] [PubMed] [Google Scholar]
- 30. Dehghan M, Mente A, Zhang X, et al. Prospective Urban Rural Epidemiology (PURE) study investigators Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050-62. 10.1016/S0140-6736(17)32252-3. [DOI] [PubMed] [Google Scholar]
- 31. Zong G, Li Y, Wanders AJ, et al. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: two prospective longitudinal cohort studies. BMJ 2016;355:i5796. 10.1136/bmj.i5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aschard H. A perspective on interaction effects in genetic association studies. Genet Epidemiol 2016;40:678-88. 10.1002/gepi.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393-2393. 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Resche-Rigon M, White IR, Bartlett JW, Peters SAE, Thompson SG, PROG-IMT Study Group Multiple imputation for handling systematically missing confounders in meta-analysis of individual participant data. Stat Med 2013;32:4890-905. 10.1002/sim.5894. [DOI] [PubMed] [Google Scholar]
- 35. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009;172:137-59. 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Debray TPA, Moons KGM, Riley RD. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: A comparison of new and existing tests. Res Synth Methods 2018;9:41-50. 10.1002/jrsm.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Manning AK, LaValley M, Liu C-T, et al. Meta-analysis of gene-environment interaction: joint estimation of SNP and SNP × environment regression coefficients. Genet Epidemiol 2011;35:11-8. 10.1002/gepi.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Center for Statistical Genetics. Metal - Meta Analysis Helper. http://csg.sph.umich.edu/abecasis/Metal/
- 40. Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 41. Praagman J, Beulens JW, Alssema M, et al. The association between dietary saturated fatty acids and ischemic heart disease depends on the type and source of fatty acid in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort. Am J Clin Nutr 2016;103:356-65. 10.3945/ajcn.115.122671. [DOI] [PubMed] [Google Scholar]
- 42. Satija A, Bhupathiraju SN, Spiegelman D, et al. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J Am Coll Cardiol 2017;70:411-22. 10.1016/j.jacc.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mozaffarian D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016;133:187-225. 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li SX, Imamura F, Ye Z, et al. Interaction between genes and macronutrient intake on the risk of developing type 2 diabetes: systematic review and findings from European Prospective Investigation into Cancer (EPIC)-InterAct. Am J Clin Nutr 2017;106:263-75. 10.3945/ajcn.116.150094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hivert M-F, Jablonski KA, Perreault L, et al. DIAGRAM Consortium. Diabetes Prevention Program Research Group Updated genetic score based on 34 confirmed type 2 diabetes Loci is associated with diabetes incidence and regression to normoglycemia in the diabetes prevention program. Diabetes 2011;60:1340-8. 10.2337/db10-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khera AV, Emdin CA, Drake I, et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N Engl J Med 2016;375:2349-58. 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kilpeläinen TO, Qi L, Brage S, et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med 2011;8:e1001116. 10.1371/journal.pmed.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qi Q, Chu AY, Kang JH, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med 2012;367:1387-96. 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qi Q, Chu AY, Kang JH, et al. Fried food consumption, genetic risk, and body mass index: gene-diet interaction analysis in three US cohort studies. BMJ 2014;348:g1610. 10.1136/bmj.g1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Papandonatos GD, Pan Q, Pajewski NM, et al. GIANT Consortium. Diabetes Prevention Program and the Look AHEAD Research Groups Genetic Predisposition to Weight Loss and Regain With Lifestyle Intervention: Analyses From the Diabetes Prevention Program and the Look AHEAD Randomized Controlled Trials. Diabetes 2015;64:4312-21. 10.2337/db15-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Livingstone KM, Celis-Morales C, Papandonatos GD, et al. FTO genotype and weight loss: systematic review and meta-analysis of 9563 individual participant data from eight randomised controlled trials. BMJ 2016;354:i4707. 10.1136/bmj.i4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Robinson MR, English G, Moser G, et al. LifeLines Cohort Study Genotype-covariate interaction effects and the heritability of adult body mass index. Nat Genet 2017;49:1174-81. 10.1038/ng.3912. [DOI] [PubMed] [Google Scholar]
- 53. Ericson U, Hellstrand S, Brunkwall L, et al. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am J Clin Nutr 2015;101:1065-80. 10.3945/ajcn.114.103010. [DOI] [PubMed] [Google Scholar]
- 54. Atabaki-Pasdar N, Ohlsson M, Shungin D, et al. Statistical power considerations in genotype-based recall randomized controlled trials. Sci Rep 2016;6:37307. 10.1038/srep37307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang DD, Hu FB. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol 2018;6:416-26. 10.1016/S2213-8587(18)30037-8. [DOI] [PubMed] [Google Scholar]
- 56. Janssens ACJW, Moonesinghe R, Yang Q, Steyerberg EW, van Duijn CM, Khoury MJ. The impact of genotype frequencies on the clinical validity of genomic profiling for predicting common chronic diseases. Genet Med 2007;9:528-35. 10.1097/GIM.0b013e31812eece0. [DOI] [PubMed] [Google Scholar]
- 57. Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet 2013;9:e1003348. 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials: Appendix 1 to 5, supplementary tables 1 to 11, and supplementary figure 1 to 8
Supplementary materials: Full author list and affiliations


