Abstract
Kinesins and myosins are motor proteins that can move actively along microtubules and actin filaments, respectively. Plants have evolved a unique set of motors that function as regulators and organizers of the cytoskeleton and as drivers of long-distance transport of various cellular components. Recent progress has established the full complement of motors encoded in plant genomes and has revealed valuable insights into the cellular functions of many kinesin and myosin isoforms. Interestingly, several of the motors were found to functionally connect the two cytoskeletal systems and thereby to coordinate their activities. In this review, we discuss the available genetic, cell biological, and biochemical data for each of the plant kinesin and myosin families from the context of their subcellular mechanism of action as well as their physiological function in the whole plant. We particularly emphasize work that illustrates mechanisms by which kinesins and myosins coordinate the activities of the cytoskeletal system.
Keywords: cytoskeleton, microtubules, actin filaments, cytoplasmic streaming, cell division, cell growth
INTRODUCTION
One of the major inventions of eukaryotic cells was the evolution of cytoskeletal motors that convert the chemical energy stored in ATP into a mechanical force (150). Combined with the increasing complexity of cytoskeletal arrays of actin filaments and microtubules, this active transport enabled eukaryotic cells to attain much larger cell sizes and more sophisticated cell shapes. Cytoskeletal motors are generally grouped on the basis of the type of filament they associate with: Kinesins and dyneins generate force along microtubules, whereas myosins do so along actin filaments. This review presents an overview of our current understanding of the mechanisms and functions of kinesin and myosin motors, which are the only cytoskeletal motors found in spermatophytes. Although most research so far has focused on one or the other motor type, it has become increasingly clear that the two cytoskeletal motor systems influence each other’s behavior and control cellular function in a concerted fashion.
INTRODUCTION TO MOTOR PROTEINS
Plants encode a large number of motor proteins that can be grouped into different families on the basis of their protein domain composition. Although some of the motors found in plants have direct homologs in animals or other eukaryotes, a number of motor families have evolved specifically in plants and are not found in other clades. In this section, we discuss the composition of the plant cell motor pool and outline what we know about the basic enzymatic functions of plant motors.
Plants Encode a Unique Complement of Motor Proteins
Angiosperm genomes contain anywhere from 41 to 61 kinesin genes and 7 to 27 myosin genes, meaning that, relative to animals, plants contain fewer myosins and more kinesins (81, 105, 108). The reason for this difference is not clear, but the various contractile and sensory processes that are unique to animals may have required gene duplication and functional diversification of myosins that were not needed in plants. In contrast, land plants construct several unique microtubule arrays and also lack dynein, which catalyzes the bulk of minus-end-directed transport in animals. Together, these conditions may have driven the increase in kinesin numbers in plants, particularly the increase in the kinesin-14 family, which is predicted to mediate minus-end-directed transport (see the section titled Motors and Cargo Transport, below).
At a very general level, all plant motors consist of two (or more) heavy chains that generate mechanical force and several light chains that stabilize, and/or regulate the activity of, the heavy chains. The heavy chains typically consist of four protein domains (Figure 1a). First, a motor domain provides the propulsive force by translating the energy released from ATP hydrolysis into a conformational change. Second, a neck domain acts as a lever arm to translate the small movement within the motor domain into a much larger step. Third, a dimerization domain allows two or more motors to function as a single unit. Finally, a cargo-binding domain couples the motor to other cellular components that are moved by the motor. Variations on this general scheme allow for different functional specialization of the different motor families.
Figure 1:
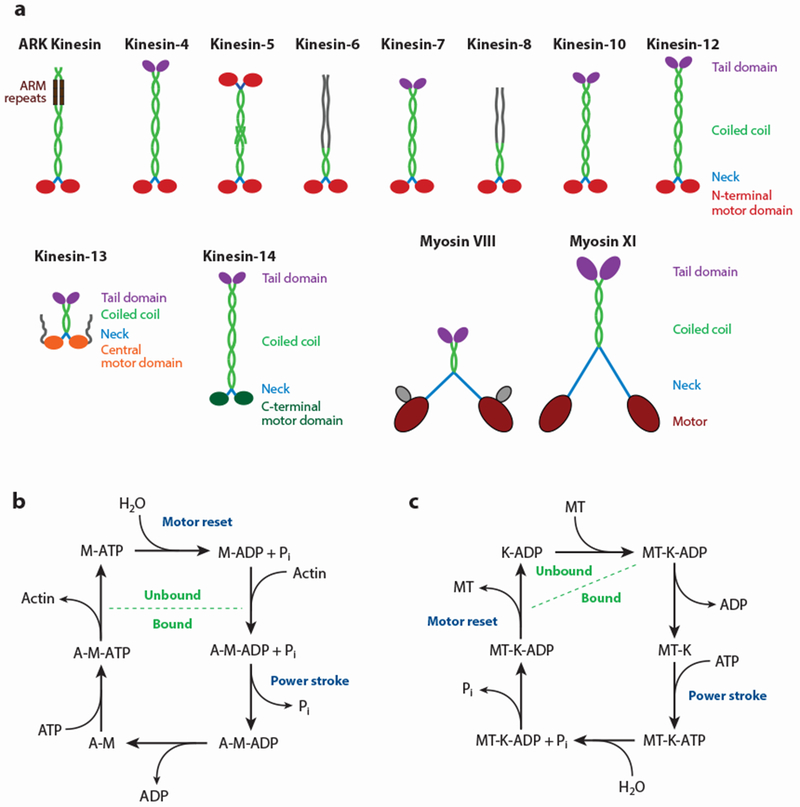
Domain organization and ATPase cycle of kinesin and myosin motors. (a) The heavy chain of motor proteins consists of a globular motor domain, which binds ATP and the respective cytoskeletal filament. All plant myosins and most plant kinesins contain the motor domain at the N terminus of the protein. In the case of the kinesin-13 and kinesin-14 families the motor domain is in the middle and at the C terminus of the protein, respectively. The neck domain serves as a lever arm. The coiled-coil domains function as dimerization interfaces, and the globular tail domain binds to cargo. Abbreviation: ARM repeats, armadillo-like repeats. Myosin VIII in angiosperms has an N-terminal extension, MyTH8, of unknown function (gray). (b) ATP hydrolysis cycle of myosin motors. Myosin (M) has a low affinity for actin filaments (A) when bound to ATP. The power stroke occurs upon release of inorganic phosphate (Pi) after ATP hydrolysis. (c) ATP hydrolysis cycle of kinesin motors. Kinesin (K) has its lowest affinity for microtubules (MTs) when bound to ADP. The power stroke occurs upon ATP binding.
Myosin motors form two families in plants.
Myosin proteins are found in almost all eukaryotes and are grouped into separate families on the basis of sequence similarity within their motor domain. These myosin families are traditionally referred to as classes and are labeled with roman numerals (16). Interestingly, this grouping is also reflected in the overall protein domain composition of the different classes, suggesting that motor domains and other functional domains coevolved (59). Of the more than 30 classes of myosins found in eukaryotes (88, 119), all plants from chlorophytes to angiosperms encode only two: myosin VIII and myosin XI. Whereas genomes of algae and early-branching terrestrial plants up to Selaginella (Lycopodiopsida, or clubmosses) contain only a single type of both myosins VIII and XI, sometimes with several closely related paralogs, there has been a wide range of duplications and sequence divergence in angiosperms, suggesting increasing functional specialization in more recently evolving plants. Specifically, angiosperms encode two types of myosin VIII and at least five types of myosin XI, with additional genome duplications leading to a total of eight myosin XI types in eudicots (81). The paucity of genome information from Polypodiopsida (ferns) and gymnosperms does not allow for any inferences about subfunctionalization of myosin genes prior to the emergence of angiosperms.
For the remainder of this discussion, we use the systematic gene nomenclature that emerged from the recent comprehensive analysis of 67 complete plant genomes (81) but mention the original gene names in parentheses (55, 56, 104, 106).
Myosin XI is similar to myosin V in animals and fungi.
Class XI myosins have a similar domain organization as class V myosins in animals and fungi (55). This similarity is clearly apparent in the length of the neck region and the C-terminal globular cargo-binding domain. For both classes of myosins, the neck typically consists of six IQ motifs, and the globular tails in both cases are formed by the DIL domain, which is named after the mouse myosin V Dilute (MyoVa) protein (69). Homology modeling and experimental validation of subdomain interactions suggest that the myosin XI DIL domain can assume a conformation similar to that of myosin V despite low levels of sequence conservation (67). Interestingly, the weak conservation of amino acid residues in the DIL domain is concentrated in buried residues (67), suggesting that selection pressure acted on preservation of protein folding while the surface of this domain was free to evolve new interactions with other proteins (see the section titled Motors and Cargo Transport, below). The presence of a DIL domain in both myosins V and XI strongly suggests that this is an ancient type of motor that existed before the divergence of opisthokonts (animals, fungi, amoeba) and bikonts (plants and many protists) (107, 119).
Myosin XI motors function as dimers that can walk processively along actin filaments (145), and these myosins are generally assumed to drive cytoplasmic streaming. This processive walking is facilitated by the relatively long neck region because it allows for steps of 35-nm length, which matches the half-helical repeat of actin filaments. This correspondence of myosin step size and actin structure allows the myosin to remain on the same side of an actin filament without the need to spiral around it. Motor dimerization is mediated by the coiled-coil domain, as demonstrated for Arabidopsis Myo11F (MYA1). In this case, the interaction between monomers is remarkably weak and can be detected only in the presence of the cargo-binding domain (68). This finding suggests that motor dimerization and cargo binding are coordinated processes, which ensures that processive motor dimers are formed only when bound to the surface of an organelle (68).
Myosin VIII is unique to plants.
Class VIII myosins emerged early in plant evolution and are unique to this lineage. This group of myosins typically has a neck region consisting of only three or four IQ motifs and a much shorter coiled-coil region than does myosin XI. The C-terminal tails show some sequence conservation, most notably a double tryptophan motif near the C terminus (81). The function of this motif is not known. At the N terminus, myosin VIII genes of terrestrial plants (Embryophyta) contain a conserved extension (MyTH8) of unknown function.
There are relatively few studies of myosin VIII motors in flowering plants. The Myo8C (ATM1) protein of Arabidopsis has been localized with specific antibodies to the cell periphery, cell plates, newly formed cross-walls, and plasmodesmata (106). These localizations were confirmed with full-length fusions to GFP under control of the native promoter in both wild-type and myo8c mutant plants (40, 151). In addition, truncated myosin VIII tail constructs were found to localize to small intracellular structures that seemed to align on actin filaments (40) and that may represent endosomes (34, 115). While these localizations may suggest specific functions of myosin VIII in angiosperms, genetic evidence for any of these functions is still lacking. Recent progress in understanding myosin VIII function in Physcomitrella is described below (see the sections titled Motors and Cytoskeletal Organization and Motor Proteins and Whole Plant Physiology, below).
Kinesin motor inventory in plants is distinct from that in animals.
Kinesins belong to a large superfamily and are classified into 14 families on the basis of the amino acid sequence of the motor domains (63). In the plant kingdom, some of these families are absent in certain lineages, whereas others have expanded substantially (108). For example, the kinesin-2 and kinesin-9 families, which are involved in flagellar assembly and motility, are prominently absent in angiosperms because angiosperms lack flagella, but these two families are present in the chlorophyte alga Chlamydomonas reinhardtii and the bryophyte Physcomitrella patens, which possess motile flagella as part of their life cycles (108, 122). In contrast, the kinesin-3 and kinesin-11 families appear to be totally absent in plants (108, 122). Moreover, the kinesin-14 family is vastly overrepresented in plants compared to animals. Both P. patens and angiosperms contain a large number of kinesin-14s, probably to perform functions related to microtubule organization and cargo transport that are accomplished by dynein in animal cells (169) (see the sections titled Motors and Cytoskeletal Organization and Motors and Cargo Transport, below). The kinesin-7 and kinesin-12 families have also expanded in plants and appear to have taken on new functions compared with their animal versions. Whereas animal kinesin-7s are involved in kinetochore capture and chromosome movement during mitosis, the Arabidopsis NACK1 and NACK2 kinesin-7s are involved in phragmoplast expansion during cytokinesis (84, 141). In addition, the Arabidopsis MKRP1 and MKRP2 kinesin-7s contain a mitochondrial targeting sequence and are thought to function within mitochondria (50). Similarly, unlike animal kinesin-12s that contribute to spindle assembly and organelle transport, the Arabidopsis PAKRP1 and PAKRP2 kinesin-12s and their Physcomitrella orthologs, KINID1a and KINID1b, are involved in the organization of phragmoplast microtubules (43, 44, 65, 94). Other plant kinesin-12s such as the Arabidopsis POK1 and POK2 contribute to the function of the preprophase band (67, 70, 134). Therefore, the localization and function of plant kinesins cannot be readily inferred from their animal counterparts. For the remainder of this review, we follow the standardized kinesin nomenclature but also provide the original gene name to avoid confusion.
Enzymatic Activity of Motor Proteins Translates Chemical Energy into Mechanical Force
The fundamental function of both kinesin and myosin motor proteins is the generation of mechanical force along cytoskeletal elements. Recent progress has revealed new insights into the basic mechanochemistry of these motors, which we discuss below.
The ATP hydrolysis cycle drives conformational changes in the motor domain.
Both myosins and kinesins are typical P-loop NTPases that share structural similarities and the basic hydrolytic mechanism between each other and with G proteins (149). Within the two motor families, the enzymatic cores are highly conserved with respect to their homologs in animals and fungi. As such, the basic hydrolytic cycle and the ensuing conformational change can be easily projected onto plant motors. Crucially, the ATPase cycle controls the strength of filament binding of the myosin and kinesin motor domains, thus providing a mechanism for the motors to cycle between bound and unbound states. Interestingly, binding and release occur at different points of the ATP hydrolysis cycle for the two motor families (Figure 1b,c). The conformational changes during nucleotide binding, hydrolysis, and release are used to drive the movement of the motor along the cytoskeleton.
By combining two motor domains into a functional unit via a dimerization domain, the domains’ activities can be coupled for a continuous binding and unbinding cycle that allows the motor to repeatedly step along the cytoskeletal filament in a hand-over-hand mechanism. This continuous or processive movement is possible only if at least one motor is bound to the filament at any moment in time. Put another way, the motor must remain in a bound state for more than half of the total enzymatic cycle (tbound/ttotal > 0.5). This parameter is termed duty ratio and can be used to identify processive motors.
The small conformational change in the motor domain that drives movement is termed the power stroke. An α-helical neck region that extends from the motor domain converts this small change into a much larger displacement, thereby producing the characteristic step for any given type of motor. Larger step sizes translate into faster movements, which explains why the fastest myosins have long neck regions. In the case of myosins, the neck region is bound and stabilized by light chains that are typically calmodulin or calmodulin-like proteins. These light chains also introduce a mechanism to regulate the motor’s activity by sensing calcium. At high calcium concentrations, single calmodulin chains can dissociate from the neck, thereby decreasing the step size and slowing down movements (144).
Movement of motors depends on turnover rates, duty cycle, and processivity.
Plant kinesins vary greatly in speed and processivity, but limited information is available about the structural determinants of these motile properties. Experiments with animal kinesins identified the length of the neck linker to be a major determinant of processivity in vitro (120, 121). Recently, structure-function analysis of the neck linker and neck coiled-coil domains of the Arabidopsis FRA1 kinesin-4 showed that both the charge and length of these domains affect processivity of the motor domain in vitro (31). However, genetic and live-imaging experiments showed that the motility of these FRA1 mutants can differ dramatically in vivo, sometimes even showing results opposite to the in vitro results (31). Therefore, in vitro structure-function experiments should be coupled with in vivo cell biological analyses to identify physiologically relevant determinants of kinesin motility. The number of kinesins on a cargo also greatly affects the processivity of transport, as exemplified by the P. patens KCBP-b kinesin-14 (51, 167). Whereas individual KCBP-b motors are nonprocessive, multiple KCBP-b motors on a cargo lead to processive motility, likely by providing multiple microtubule binding sites that keep the cargo attached to the microtubule track between ATPase cycles of the individual motors.
As plant myosin XI motors are the fastest known myosins, considerable work has been done to study their underlying mechanochemistry. Myosin XI motors drive cytoplasmic streaming that can reach speeds of 14 μm/s in angiosperms (126) and 60 μm/s in charophycean algae (53) (see the section titled Motors and Cargo Transport, below). In addition, purified myosin XI from Chara is able to propel actin filaments in vitro at 60 μm/s (42). These high speeds are made possible by a very fast ATP hydrolysis rate of almost 400 s−1, which is driven primarily by the rapid release of ADP at the end of the reaction (48). Given that tight myosin-actin binding is lost when ATP enters the binding pocket in the motor domain, this fast ADP release also results in a very low duty ratio of <0.3 (48). Thus, Chara myosin XI is a very fast, but not processive, motor, and the high speeds of cytoplasmic streaming found in this species are possible only because of the simultaneous action of many motors along an array of parallel actin filaments (161).
The situation is different for angiosperm myosins, which have a much higher duty ratio [e.g., 0.7 for Myo11F (MYA1) (37) and 0.9 for Myo8C (ATM1) (40) of Arabidopsis] and are therefore processive motors. Although some of the motors still reach high velocities [~7 μm/s for a Myo11B of Nicotiana tabacum (145)], others have slower hydrolysis cycles [~3.2 μm/s for Myo11F (MYA1) of Arabidopsis thaliana (37)], suggesting that some of the speed variation found for organelle movements (see the section titled Motors and Cargo Transport, below) may be attributable to the action of different motors. Recently, it was found that Myo11G (XI-I) of Arabidopsis, which is found predominantly on the nuclear envelope (5, 140), is a very slow motor, supporting speeds of only 0.25 μm/s (41). This motor is thus in the same category as Myo8C (ATM1), which has been proposed to function more as a tension sensor or generator than as a motor for long-distance transport of cargo (40). This recently uncovered variation in enzymatic characteristics of myosin XI isoforms suggests that these motors have evolved to perform different specialized functions, possibly analogous to the wide range of myosin classes found in animals (88). It will be interesting to correlate the different enzymatic parameters such as actin affinity and ADP release rates with structural features of the individual motor domains (23, 49) to enable at least a rough prediction of the motor properties of uncharacterized myosins.
MOTORS AND CYTOSKELETAL ORGANIZATION
The microtubule and actin cytoskeleton together orchestrate critical cellular processes such as membrane trafficking, signaling, morphogenesis, and division. The functional versatility of these cytoskeletal systems depends on their ability to dynamically organize into structurally distinct arrays. Here, we discuss the mechanisms by which kinesins and myosins contribute to the formation, maintenance, and function of cytoskeletal arrays (Table 1). Because actin and microtubule systems often appear to work in a coordinated manner, we highlight how motor proteins mediate interaction between these two cytoskeletal systems, either indirectly through intermediary proteins or directly through binding to both microtubules and actin filaments.
Table 1.
Functions of plant motor proteins in different cytoskeletal arrays
| Cytoskeletal array | Motor protein | Known or proposed function | Reference(s) |
|---|---|---|---|
| Interphase cytoskeleton in diffusely growing cells | Kinesins | ||
| KCBP (kinesin-14) | Cross-links microtubules and/or microtubules and actin filaments | 142 | |
| KRP125c (kinesin-5) | Cross-links microtubules | 8 | |
| Kinesin-13A | Depolymerizes microtubules | 86 | |
| Kin7 (kinesin-7) | Promotes microtubule polymerization | 80 | |
| KCH (kinesin-14) | Transports actin filaments along microtubules | 158 | |
| FRA1 (kinesin-4) | Promotes secretion of noncellulosic cell wall material | 58, 170 | |
| Myosins | |||
| Myo11B2, Myo11E, Myo11F (myosin XI) | Transport organelles; deform and bundle actin filaments | 14, 100, 101 | |
| Interphase cytoskeleton in tip-growing cells | Kinesins | ||
| ARK1 (orphan kinesin) | Promotes catastrophe of endoplasmic microtubules | 25 | |
| KINID1a and KINID1b (kinesin-12) | Focus microtubule plus ends at the growing tip | 44 | |
| Myosins | |||
| Myosin XI | Delivers regulators of actin polymerization to the growing tip (Physcomitrella patens) | 30 | |
| Myosin XI | Transports organelles; dynamic actin rearrangement and organization | 72, 96, 100 | |
| Preprophase band (PPB) | Kinesins | ||
| POK1 and POK2 (kinesin-12) | Retain markers of the cortical division site | 70, 134 | |
| ARK3 (orphan kinesin) | Contributes to PPB formation or maintenance | 62, 73 | |
| KCBP (kinesin-14) | Unknown function at the PPB site | 12 | |
| Myosins | |||
| Myosin VIII | Unknown function at the PPB site | 162 | |
| Spindle apparatus | Kinesins | ||
| KRP125c (kinesin-5) | Cross-links and stabilizes interpolar microtubules | 8, 76 | |
| ATK1 (kinesin-14) | Translocates microtubules to the spindle poles | 15, 75 | |
| ATK5 (kinesin-14) | Cross-links and organizes microtubules at the spindle midzone and poles | 2, 3 | |
| Phragmoplast | Kinesins | ||
| PAKRP1 and PAKRP2 (kinesin-12) | Stabilize antiparallel microtubules at the phragmoplast midzone (Arabidopsis thaliana) | 65, 94 | |
| KINID1a and KINID1b (kinesin-12) | Stabilize antiparallel microtubules at the phragmoplast midzone (P. patens) | 43, 44 | |
| NACK1 (HIK) and NACK2 (TES) (kinesin-7) | Promote disassembly of the phragmoplast midzone | 84, 141 | |
| Kin4-Ia and Kin4-Ic (kinesin-4) | Inhibit microtubule growth at the phragmoplast midzone (P. patens) | 21 | |
| KCBP (kinesin-14) | Pulls on peripheral microtubules to orient the phragmoplast | 12 | |
| Myosins | |||
| Myosin VIII | Incorporates peripheral microtubules into the phragmoplast; pulls on actin filaments to orient the phragmoplast | 162 | |
Motor Proteins Regulate Cytoskeletal Dynamics and Organization During Cell Growth
The spatial organization of the microtubule and actin cytoskeleton during interphase determines the growth and shape of cells (Figure 2a). Typically, the microtubule cytoskeleton is thought to drive diffuse cell growth by orienting the deposition of cellulose microfibrils (138), whereas the actin cytoskeleton is considered to drive tip cell growth by mediating the transport of secretory vesicles to the growth site (109). However, accumulating evidence discussed below indicates that both cytoskeletal systems are important for diffuse cell growth and tip cell growth, perhaps by increasing the fidelity or efficiency of the cellular activities required for these growth processes.
Figure 2:
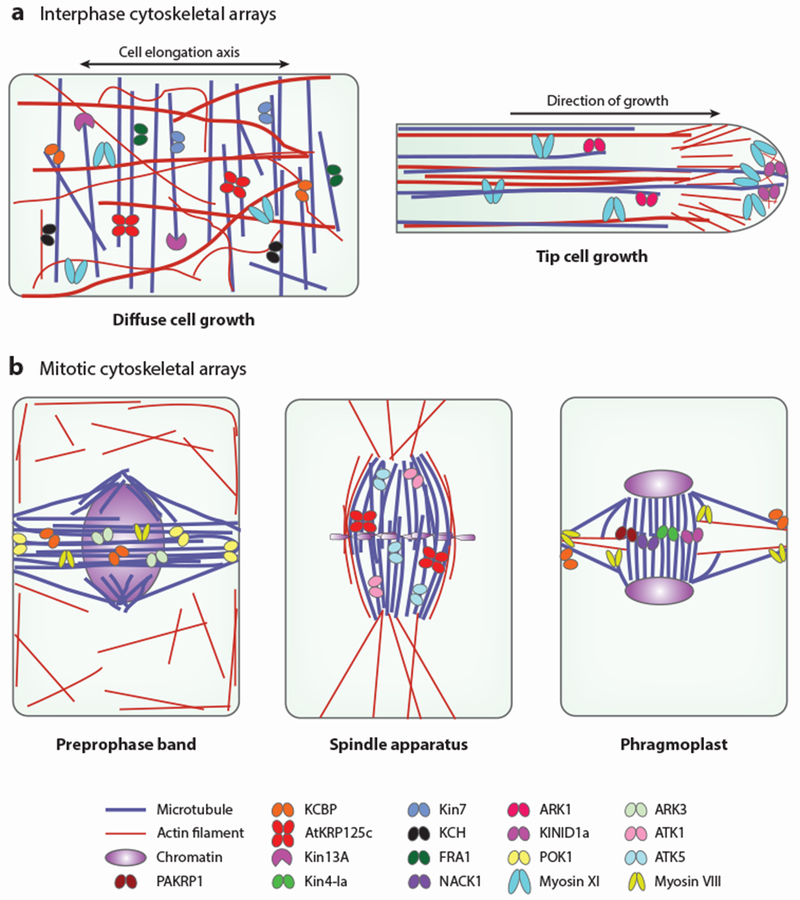
Distribution of kinesin and myosin motors to different cytoskeletal arrays. Microtubule (purple) and actin filament (red) organization is shown during interphase (a) and mitosis (b). The tip-growing cell in panel a represents an angiosperm root hair or pollen tube or Physcomitrella patens protonemata. The localization of kinesin and myosin motors that are known to contribute to the organization and/or the function of these cytoskeletal arrays is shown. In cases for which multiple kinesin or myosin isoforms are known to show similar localization and function redundantly, only one isoform is shown for clarity. Details on the molecular function of the individual motors are described in the text and are summarized in Table 1.
Multiple kinesins differentially regulate cortical microtubule organization in diffusely growing cells.
Several kinesin families affect the spatial organization of cortical microtubules through a variety of mechanisms. The plant-specific KCBP (a kinesin-14 member) is thought to contribute to the organization of cortical microtubule rings in trichomes by cross-linking microtubules. KCBP contains two microtubule-binding domains—the C-terminal motor domain and the N-terminal tail encompassing a MyTH4 (Myosin Tail Homology 4) domain (142)—and bundles microtubules in vitro (54). Deletion of the N-terminal domain rendered KCBP nonfunctional, supporting a role for microtubule cross-linking by KCBP (142). Interestingly, KCBP also binds to actin filaments in vitro via an N-terminal FERM domain (142), thus raising the possibility that KCBP directly affects actin filament organization and physically connects microtubules and actin filaments. However, the significance of the FERM domain remains unclear because structure-function analyses indicated that it is dispensable (142).
Destabilization of microtubules is another mechanism employed by kinesins to affect cortical microtubule organization. The best studied example in plants is kinesin-13A, which promotes microtubule depolymerization. Elegant studies of differentiating xylem vessels showed that kinesin-13A is specifically recruited to regions of cell wall pit formation via a ROP GTPase signaling pathway; once at these regions, kinesin-13A begins to depolymerize cortical microtubules, thus preventing secondary cell wall deposition at these sites (86). Whereas kinesin-13A depolymerizes microtubules in vitro, in vivo this activity requires interaction with another microtubule-binding protein termed MIDD1 (86), which is brought to the plasma membrane by activated ROP11 (85, 87). MIDD1 is thought to be needed to target kinesin-13A to microtubules because kinesin-13A lacks the lysine-rich neck region that contributes to microtubule binding in animals. Taken together, the ROP11-MIDD1-kinesin-13A pathway is an excellent case study of how cells spatially control kinesin activity.
In contrast to kinesin-13A, the kinesin-7 AtKin7 functions as a microtubule stabilizer by boosting microtubule rescue and reducing microtubule catastrophe (80). Like many kinesins, AtKin7 is normally autoinhibited, presumably to prevent indiscriminate stabilization of microtubules. Activation of AtKin7 involves a novel mechanism through binding of the AtESP separase to the tail domain of AtKin7, again highlighting the need to spatiotemporally regulate kinesin activity in cells (32, 66).
The KCH family of kinesin-14s is a prime example of a bifunctional protein that can directly link microtubules and actin filaments. Early electron microscopy studies showed close association between microtubules and actin filaments (reviewed in Reference 18), suggesting a functional interaction between them. Recent live-imaging studies confirmed this finding, and drug-washout experiments further revealed a reciprocal dependency with regard to the efficient and appropriate reformation of cortical microtubules and actin filaments (113). KCH kinesins bind to microtubules and actin filaments in vitro and colocalize with microtubules and actin filaments in vivo (13, 27, 102, 148, 165). In addition, these kinesins move actively along cortical microtubules (57), indicating that they may transport actin filaments along microtubules. Importantly, recent in vitro experiments showed that a rice KCH member, OsKCH1, transports actin filaments along microtubules in vitro (158). Curiously, the velocity of transport varied greatly depending on the orientation of the actin filaments with respect to that of the microtubules. This bias may arise due to low torsional flexibility in OsKCH1 (158), perhaps because of a tetrameric stalk configuration that may occur in this kinesin (118). The next key step is to examine whether microtubule-based transport of actin filaments is compromised in kch mutants and, if so, whether this decreased ability to transport actin filaments affects the organization and function of the microtubule and actin arrays.
Myosin XI motors increase filament dynamics and affect actin organization in diffusely growing cells.
Myosin motors in plants are best known for their effect on organelle movement and cytoplasmic streaming (see the section titled Motors and Cargo Transport, below), but they also have a marked effect on the actin filaments that serve as their tracks. This effect is evident in triple and quadruple myosin XI knockouts (myo11b2 myo11e myo11f and myo11b2 myo11e myo11f myo11g) in which the typically thick longitudinal bundles of actin filaments of leaf midvein epidermis cells are replaced with thinner, more skewed actin bundles (101). The loss of longitudinal actin bundles was confirmed in quantitative studies that revealed an increase in the average angle between actin filaments and the long axis of epidermal cells of several tissues in both double (myo11b2 myo11e) and triple (myo11b2 myo11e myo11f) mutants (14, 147). This skewing of actin filaments was accompanied by increased disorder as measured by a decrease in parallelness in aboveground tissues (14, 147).
The effect of myosin XI on actin filament organization may result from the direct action of mechanical forces by the motors on their tracks. For example, repeated movement of organelles along an actin filament network may eventually lead to parallel orientation and bundling of these filaments (146). This model is supported by the observation that global actin dynamics are reduced in hypocotyl and root epidermal cells of a triple myo11b2 myo11e myo11f mutant (14). Furthermore, direct observation of the behavior of individual cortical actin filaments (129) showed a marked decrease in filament buckling and straightening, a drop in severing frequency, and an increase in the lifetime and length of these filaments when Myo11B2, Myo11E, and Myo11F were absent (14). These results demonstrate that the force generated by myosin XI motors is sufficient to deform individual and bundled actin filaments and may explain the altered global actin filament dynamics in Arabidopsis myosin mutants.
Kinesins and myosins affect the dynamics and organization of microtubule and actin filament ends in tip-growing cells.
Studies of root hairs in Arabidopsis and of caulonemal cells in Physcomitrella have uncovered a role for kinesins in regulating microtubule behavior in tip-growing cells. In the Arabidopsis ark1-1 mutant, root hairs have more endoplasmic (but not cortical) microtubules and grow in a wavy manner (111). ARK1 is an ungrouped plant-specific kinesin, and follow-up studies showed that it localizes to growing microtubule plus ends and triggers their catastrophe (25). How ARK1 induces microtubule catastrophe remains to be studied, but the mechanism does not involve the C-terminal armadillo repeat domain (ARM), on the basis of biochemical studies using truncated ARK1 proteins (24).
In Physcomitrella, the KINID1a and KINID1b kinesin-12 family members also localize to growing microtubule plus ends and are proposed to coalesce them to form a focused microtubule spot at the center of the apical dome in growing protonemata (44). Curiously, myosin XI and actin filaments also converge into an apical spot in these cells, with myosin XI accumulation at the growing tip preceding that of actin (30). In fact, myosin XI is required to set up the initial cell polarization and the focusing of actin filaments to a single spot for tip growth (155). It is tempting to speculate that myosins and kinesins mediate a functional connection between actin filaments and microtubules in these cells, similar to the situation in mouse melanocytes and budding yeast, where adaptor proteins connect myosin V to growing microtubule plus ends to orient melanocyte movement and the mitotic spindle, respectively (9, 164). Both KINID1a and KINID1b contain an SxIP motif that binds to EB1 in animals (46), raising the possibility that EB1 mediates their plus-end tracking activity. It will be interesting to determine whether KINID1a and KINID1b also bind to myosin XI, either directly or indirectly, and to test whether such an interaction provides a mechanism to focus myosin XI at the growing tip. Focusing of myosin XI might in turn lead to the delivery of regulators of actin polymerization at this spot, thus resulting in a positive feedback loop that would reinforce the polarization of the cell toward one spot (30). Conversely, a KINID–EB1–myosin XI complex might also work to converge growing microtubule tips into a single spot. Live imaging of these proteins along with experiments using drugs to specifically disrupt the microtubule or actin cytoskeleton should shed light on the functional relationship between these proteins and the resulting cytoskeletal structures
It is not clear whether myosin XI motors perform equivalent functions in tip-growing cells of angiosperms. Although Myo11E (XIK) of Arabidopsis also primarily localizes to the region of actin accumulation in the tip of growing root hairs (96, 98), it also affects global actin filament dynamics (96), unlike myosin XI in Physcomitrella (155). In contrast, the nearly identical Myo11C1 and Myo11C2 isoforms in Arabidopsis pollen are needed for normal filament bundling (72), and genetic redundancy in angiosperms may mask the central role of myosin XI in actin organization in these organisms.
The Organization and Function of Mitotic Microtubule Arrays Depend on Kinesins
During mitosis, the microtubule cytoskeleton is restructured in a stereotypical sequence to segregate the nuclear and cytoplasmic contents (Figure 2b). Each of the major mitotic microtubule arrays—the preprophase band (PPB), spindle apparatus, and phragmoplast—in turn undergoes dynamic reorganization to perform its functions. Many of the key structural transitions and their functions rely on kinesins, as evidenced by the finding that approximately one-third of Arabidopsis kinesins and approximately one-half of Physcomitrella kinesins are thought to contribute to mitosis (76, 153).
Kinesins are essential for marking the cortical division site defined by the preprophase band.
The PPB, a transient structure that is formed during prophase, forecasts the cell division site and enhances the fidelity of division plane orientation (79, 117). How the PPB influences events that occur in its absence during cytokinesis is a central question in plant cell biology. The kinesin-12 family members POK1 and POK2 have emerged as core components of the machinery that marks the PPB site. These kinesins localize to the PPB in a microtubule-dependent manner and persist at the cortical division site after PPB disassembly through an unknown mechanism (70). At the cortical division site, these kinesins retain the cortical division site markers TAN and RanGAP1, which localize to the PPB independently of POK1 during prophase (70, 157, 166). Recently, POK1 was also found to recruit pleckstrin homology GAPs (PHGAPs) to the cortical division site during metaphase (134). These GAPs interact with multiple ROPs and therefore have the potential to regulate cytoskeletal dynamics and membrane trafficking during cytokinesis. Interestingly, Myo8A of Physcomitrella also accumulates at the cortical division site in tobacco BY-2 cells and, curiously, in moss, which does not form a PPB (162). The recruitment of multiple proteins to the cortical division site at different stages of mitosis may reflect a maturation process that makes the site competent for phragmoplast guidance and fusion. Alternatively, at least some of them may function redundantly, as evidenced by the weaker phenotypes of the tan1 and phgap mutants relative to the pok1 pok2 double mutant (70, 134). This interpretation is also compatible with the stronger phenotype of myosin VIII knockouts in Physcomitrella (162) relative to Arabidopsis (139), because moss lacks PPBs and possibly also cortical division site markers such as TAN that could provide redundant pathways to orient the cell plate in angiosperms.
Many other kinesins also localize to the PPB and/or to the PPB-defined cortical division site. One of them is the ungrouped kinesin ARK3 (AtKINUa), which accumulates at the PPB, most notably in stomatal meristemoid cells (62, 73). Targeted downregulation of ARK3 in the stomatal lineage leads to clustering of stomata that is associated with misoriented cell division planes (62). The function of ARK3 at the PPB is still unclear, but the finding that ARK3 degrades rapidly during PPB disassembly following nuclear envelope breakdown (73) suggests that it may contribute to PPB formation and/or maintenance, either directly or through other proteins that may interact with its ARM domain. KCBP is another kinesin that localizes to the PPB and persists at the cortical division site through mitosis (12). Retention of KCBP at this site depends on its N-terminal MyTH4-FERM domain, which may directly interact with plasma membrane lipids on the basis of the finding that the Physcomitrella KCBP ortholog binds to liposomes (167).
Morphology of the spindle apparatus depends on plus- and minus-end-directed kinesins.
Spindle morphology and dynamics depend on forces generated by kinesins that act in the central overlap zone. Plus-end-directed kinesins typically generate poleward forces that elongate the spindle, whereas minus-end-directed kinesins mostly generate inward forces that shorten the spindle. The tetrameric kinesin-5 motors are one of the major plus-end-directed kinesins in the spindle. The Arabidopsis kinesin-5 AtKRP125c localizes to the spindle, and conditional loss-of-function mutants show monopolar or splayed spindles (8), consistent with their predicted role in cross-linking and stabilizing antiparallel microtubules in the spindle midzone. The Physcomitrella kinesin-5s also localize to the spindle, most conspicuously toward the spindle poles, where parallel microtubules dominate (76). The motility and directionality of the various plant kinesin-5s need to be determined to begin to make sense of how they might affect microtubule organization in different regions of the spindle.
ATK1 and ATK5 are two minus-end-directed kinesin-14s that localize to the spindle apparatus. In the atk1-1 mutant, both mitotic and male meiotic spindles appear multipolar (15, 75). This defect correlates with reduced microtubule accumulation at spindle poles, suggesting that ATK1 is needed to translocate microtubules to the spindle pole. ATK1 is a nonprocessive kinesin (74), and the finding that ATK1 function is dosage dependent is consistent with multiple ATK1 molecules being needed for effective microtubule translocation (75). The lack of spindle bipolarity in atk1-1 leads to abnormal chromosome segregation during meiosis and to reduced male fertility, whereas in mitosis this defect is corrected by anaphase, perhaps due to functional compensation by other minus-end-directed kinesins such as ATK5. Mitotic spindles in the atk5-1 mutant are indeed abnormally broad (3), supporting this notion. However, the microtubule plus-end tracking activity of ATK5 and the conspicuous localization to the spindle midzone indicate additional roles for ATK5 (3). In vitro experiments in which ATK5 captured and coaligned microtubules in both parallel and antiparallel orientations (2) suggest that ATK5 may contribute to the formation of both the spindle midzone and poles. Multiple mechanisms for spindle pole formation may be particularly important in plants because of the absence of centrosomes and dynein, which perform these functions in animal cells.
Both kinesins and myosins are involved in the formation, expansion, and guidance of the phragmoplast.
The phragmoplast is the plant cytokinetic apparatus. It arises from microtubules derived from the anaphase spindle midzone and appears as a disk-shaped structure consisting of two antiparallel sets of microtubules. As the phragmoplast expands outward, the central microtubules are disassembled to generate a ringlike structure that ultimately inserts at the cortical site defined by the PPB. The task of the phragmoplast is to spatially direct vesicles containing cell wall material to construct the cell plate that physically divides the daughter cells (see the section titled Motors and Cargo Transport, below). Kinesins contribute to the antiparallel microtubule organization of the phragmoplast, which is particularly important for its structure and function. Kinesin-12 members in both Arabidopsis (PAKRP1 and PAKRP2) and Physcomitrella (KINID1a and KINID1b) localize to the phragmoplast midzone and are needed for stable antiparallel microtubule organization (43, 44, 65, 94). As the phragmoplast matures, this stable midzone disintegrates to enable phragmoplast expansion through a mechanism that involves kinase pathways. The best studied pathway involves the kinesin-7s NACK1 and NACK2 (HIK and TES), which initiate a MAP kinase cascade (84, 141) that ends up phosphorylating microtubule-bundling MAP65 proteins (114), thus destabilizing the phragmoplast midzone and promoting microtubule turnover. In pollen, an additional pathway involves interaction between the kinase TIO (Two-In-One) and kinesin-12A and kinesin-12B (89). Because interaction with kinesins is reduced in active-site mutations of TIO (89), disassembly of the phragmoplast core likely involves phosphorylation of kinesin-12s and possibly other substrates. Recent work on Physcomitrella kinesin-4s has underscored the importance of the extent of microtubule overlap in the phragmoplast midzone for correct cell plate formation. In cells lacking Kin4-Ia and Kin4-Ic, microtubules overlap more extensively at the phragmoplast midzone, and this defect correlates with a reduced phragmoplast expansion rate and abnormally thick and malformed cell plates (21). The N-terminal domain of Kin4-1c inhibits microtubule growth in vitro (21), suggesting that these kinesins keep the region of microtubule overlap short by regulating microtubule polymerization.
An exciting recent development was the discovery that myosin VIII and actin filaments are involved in phragmoplast guidance, providing another example of a functional interaction between microtubule and actin cytoskeletal systems. During anaphase, Physcomitrella Myo8A localizes to the cortical division site and to the plus ends of microtubules at the leading edge of the phragmoplast (162). In addition, actin filaments extend between the phragmoplast and the cortical division site and facilitate the incorporation of peripheral microtubules into the growing edge of the phragmoplast (162). It is thought that these actin filaments are stabilized with their minus ends at the cortical division site by Myo8A, which would be consistent with the slow action of Myo8C from Arabidopsis (40), which also steers the phragmoplast. In this model, these actin filaments could steer the phragmoplast to the cortical division site and also function as tracks for the Myo8A motors at the plus ends of the phragmoplast peripheral microtubules to guide these microtubules into the expanding phragmoplast (162). It is not known how the different localizations of myosin at the plasma membrane and microtubule plus ends are achieved. In Arabidopsis, the kinesin-14 KCBP at the division site has been proposed to facilitate phragmoplast guidance by pulling on microtubules at the growing phragmoplast edge (12), although this mechanism remains to be experimentally verified. Given the importance of phragmoplast guidance to cytokinesis and the fact that both microtubules and actin filaments have been observed to extend from the phragmoplast to the cell division site, it will be interesting to determine whether the microtubule and actin cytoskeletal systems provide redundant mechanisms to ensure accurate phragmoplast guidance. Alternatively, angiosperms and bryophytes may have evolved to use different systems for phragmoplast guidance.
In summary, both kinesin and myosin motors play a major role in regulating the dynamics and organization of the microtubule and actin cytoskeleton. In some cases, the effect of the motor proteins seems to form a positive feedback loop that reinforces the function of the cytoskeleton and motors (for example, myosin XI’s effect on actin bundling), whereas in other cases they generate negative feedbacks that dampen a response (for example, kinesin-13A’s disassembly of microtubules). Although these functions often seem to be limited to the filament that serves as a track for the respective motor, there is also increasing evidence for a kinesin effect on actin filaments and for a myosin effect on microtubules. Future research will have to establish the regulatory mechanisms that coordinate these processes.
MOTORS AND CARGO TRANSPORT
Molecular motors exert their function by moving cellular components, their cargo, along cytoskeletal filaments. These movements encompass the broad, seemingly undirected movements of cytoplasmic streaming, as well as more directed movements such as delivery of secretory vesicles to sites of growth and transport to the forming cell plate during cell division. This section discusses our current understanding of the motors and mechanisms involved in these long-distance movements.
Cytoplasmic Streaming Depends on the Acto-Myosin System
A classic example of long-distance transport in plant cells is cytoplasmic streaming, which was first described for charophycean algae in the eighteenth century by Bonaventura Corti (19). These movements are easily detectable as the rapid motions of refractive particles inside the cell and are found in most cells of angiosperms. Despite many descriptions of cytoplasmic streaming over the years, the precise nature of the movements is still not fully resolved. Use of fluorescent markers has firmly established that individual organelles move during cytoplasmic streaming (11, 17, 110, 152); however, it is still not clear to what extent bulk flow of cytoplasm (26) occurs in cells other than those of Chara. Most studies have focused on movement of organelles, for which speeds of up to 14 μm/s have been reported (126). These organelles frequently alter their behavior between fast, directional travel and slow, locally confined movements reminiscent of Brownian motion (see, for example, 83). Inhibitor treatments revealed that these movements are actin dependent, which suggested that myosins, as actin-based motors, drive cytoplasmic streaming (124). This conjecture was ultimately confirmed by reverse genetic approaches in the model species A. thaliana, in which loss of single or multiple myosin XI–coding genes resulted in reduced movement speeds of different organelles (72, 100, 101, 103, 147).
Specificity of Myosin Motors as Determined by Mutant Analysis Appears to Be Low
The effect of the myosin knockout mutants was unexpectedly nonspecific, with a single mutation in myo11e (xik) resulting in slower movements of several organelles (Golgi stacks, peroxisomes, mitochondria, and the ER) (100, 147). In addition, none of the affected organelles stopped completely upon loss of a single myosin isoform and were instead affected by mutations in different myosin genes. For example, peroxisomes were slower in both myo11b2 (mya2) and myo11e mutants (100). These results suggested broad, nonspecific activities of myosin motors, with overlapping functions for several organelles. By contrast, loss of the nearly identical pollen-specific myosins Myo11C1 (XIC) and Myo11C2 (XIE) resulted in dramatic reduction in peroxisome and Golgi stack movements, whereas secretory vesicles labeled with YFP-RabA4d showed nearly normal motility near the tips of growing pollen tubes (72). Thus, secretory vesicles are being propelled by myosins other than Myo11C1 and Myo11C2, suggesting more specificity of myosin-organelle interactions in certain cases. At this time, it is not clear how these sometimes conflicting results about specificity and redundancy within the myosin XI motor family can be resolved with a simple, coherent explanation. In fact, at least three different models have been proposed to explain the observations (reviewed in 33) (Figure 3a).
Figure 3:
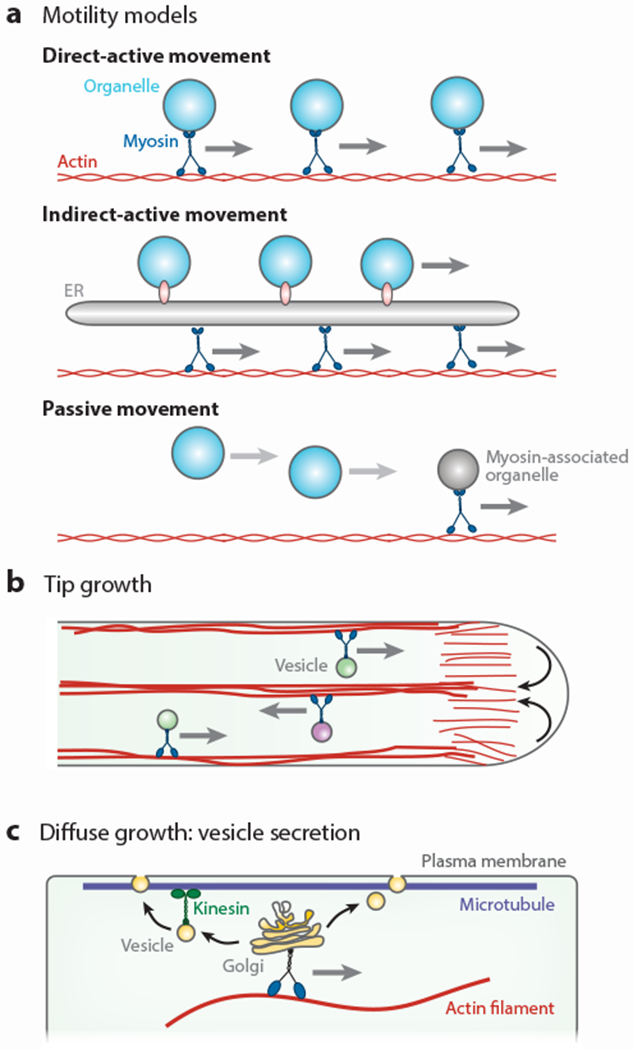
Transport of organelles by myosin motors. (a) Three motility models attempt to explain organelle motility. Direct-active movement assumes that every organelle (cyan) requires binding of a myosin motor to its surface to achieve motility. Indirect-active movement assumes that only the endoplasmic reticulum (ER) (gray) associates with myosin motors and that all other organelles bind to the ER to achieve movement. Passive movement assumes that most organelles do not bind to myosin motors but get dragged along in a hydrodynamic flow generated by other, myosin-associated organelles (gray circle). (b) Myosin motors transport organelles and secretory vesicles to the tip of root hairs and pollen tubes. The polarity of cortical and internal actin filaments (red) leads to reverse fountain streaming. Small secretory vesicles can enter the actin-free zone in the apical dome, where they passively move with bulk flow (black arrows). (c) Secretory vesicle movement during diffuse growth. Myosin motors move Golgi stacks and trans-Golgi networks along actin filaments (red). Secretory vesicles associate with cortical microtubules (purple), possibly with the help of kinesin motors, before they fuse with the plasma membrane (gray line).
In the first model, every organelle associates with its own motor to drive active movements, although several organelles may share the same motor isoform, in analogy to what has been found in yeast and mammalian cells (39). In the second model, only the ER binds myosins at its surface, whereas other organelles attach to the ER to achieve motility in an indirect way (131). In the third model, the active movements of a few organelles with motors on their surface generate a hydrodynamic flow that then leads to the passive movement of other organelles (97). The currently available data do not favor one of these three models, and localization and protein-protein interaction studies that aimed to identify the cargo of myosin motors seem to support or contradict each of the models.
Cargo Identification by Fluorescently Tagged Myosin XI Tails Suggests Indirect Action
In principle, the cargo of a given myosin XI isoform should be labeled by a fluorescently tagged C-terminal globular tail region of that motor protein because this domain functions as the organellebinding domain (67). Transient expression of fluorescently labeled globular tail constructs with or without the coiled-coil domain revealed various localizations of the different isoforms, ranging from discrete puncta that colocalized with specific organelle markers to broad, seemingly diffuse labeling of the cytoplasm (5, 67, 116, 128). These results suggest that different myosin isoforms associate with different organelles and therefore carry different cargo. Curiously, these apparent myosin cargos did not always match the organelles that showed reduced speeds in the corresponding myosin mutant. In addition, overexpression of some of the myosin XI tail constructs exerted a dominant-negative effect on the movement of a variety of organelles without localizing to them (4, 5, 35, 127, 128), making the interpretation of these experiments difficult (154). The creation of functional full-length Myo11E fusions that complemented the myo11e mutant phenotype also did not resolve this puzzle, because the fusion protein formed small, highly motile spots that accumulated near the growing root hair tip but did not colocalize with established organelle markers (96, 98). Taken together, these results suggest that Myo11E and probably other myosins exert their effect on organelle motility at least partially indirectly.
Protein-Protein Interactions Identify a Novel Class of Myosin-Binding Proteins on Endomembranes
A second, independent approach to identifying myosin XI cargo is to screen for proteins that can attach to the cargo-binding globular tail domain of the myosin. Yeast two-hybrid screens resulted in the identification of a novel family of proteins that are defined by the presence of the domain of unknown function 593 (DUF593) that can bind to myosin XI globular tails with a low level of isoform specificity (61, 99, 133). This large family of 16 members in Arabidopsis and 14 members in rice, termed myosin-binding proteins or MyoB, shows remarkable variability in size and domain organization, with some proteins containing coiled-coil domains or transmembrane domains (45, 99). For a few MyoB proteins, their subcellular localization has been determined with fluorescent protein fusions, although the function of these fusion proteins has not been tested in complementation assays. In most cases, the MyoB-GFP fusions localized to small punctate structures that moved rapidly through the cytoplasm and that appeared to colocalize with Myo11E (61, 99). This likely involvement of MyoB proteins in myosin-driven motions was further underscored by the genetic interaction between myosin xi and MyoB mutants that showed a strong enhancement of otherwise weak phenotypes (61, 99). A further clue for the involvement of MyoB proteins in organelle motility comes from the overexpression of isolated DUF593 that presumably cannot associate with organelles. In these cells, a variety of organelles showed slower movements, suggesting that the DUF593 blocked myosin XI binding to their normal cargo (97).
So far, the identity of the small structures labeled by GFP-tagged MyoB proteins is, with two exceptions, unknown. A family member in Zea mays encoded by the Floury1 gene is localized to the ER of endosperm cells, where it plays an undefined role in protein body formation (45). Another MyoB family member termed RISAP is localized to the trans-Golgi network (TGN) in a subapical domain of growing pollen tubes, where it interacts with the small GTPase Rac5 (133). Overexpression of RISAP leads to defects in pollen tube organization and secretion (133). Although the MyoB proteins provide a mechanism that allows myosin XI motors to attach to some target organelles, the structures labeled by GFP-MyoB constructs so far do not match any of the organelles typically measured for cytoplasmic streaming, an observation that is consistent with an indirect effect of myosins on organelle motility. Alternatively, attachment of myosin to moving organelles may involve other mechanisms, such as the recently discovered adaptor proteins of the MadA and MadB families (61).
Myosins Can Bind To and Move RNA Processing Bodies
A recent discovery suggests that myosin XI motors can also directly move structures that are not defined by a limiting membrane. RNA processing bodies (P-bodies) are ribonucleoprotein complexes that function in translational repression of mRNAs, gene silencing, and mRNA degradation (7). P-bodies can move through plant cells in patterns that are similar to those of conventional, membrane-bounded organelles (38), and these directed movements are reduced in myosin xi mutants (128). To test for the potential involvement of myosin in these movements, the interaction between several myosin XI tail constructs and a central component of P-bodies, DECAPPING PROTEIN1 (DCP1), was examined with a yeast two-hybrid assay, pulldown of bacterially expressed proteins, and bimolecular fluorescence complementation in tobacco leaves (132). Interestingly, this interaction appears to extend across large phylogenetic distances, as mouse and yeast myosin V tails can also interact with Arabidopsis DCP1 (132). This finding is highly remarkable, as most of the conserved residues between myosin V and XI globular tails are buried deep inside the folded domain and very few amino acid residues on the surface are conserved (67). Importantly, this discovery indicates that active cytoplasmic streaming may involve many more cellular components than the large organelles commonly used as markers.
Taken together, the evidence presents a complex picture of myosin action during cytoplasmic streaming. Although there is mounting evidence for direct interaction of myosins with cargo by specific adaptor proteins, which would support a direct-active transport process, there are also a number of instances in which the mutant phenotype and localization data point to a more indirect action of the motors. It remains to be seen whether these complexities can be explained by an indirect-active transport model (131) or by a passive flux (97). An additional complication is the feedback of myosin XI activity on actin dynamics and organization (see the section titled Motors and Cytoskeletal Organization, above) (14, 96, 101), because organelle motility depends on the organization of actin filaments, particularly the availability and organization of F-actin bundles (1).
Interphase Nuclear Motions Are Driven by a Myosin Motor on the Nuclear Surface
In contrast to the above examples, Myo11G (XI-I) has a direct effect on nuclear shape and motility. Loss-of-function mutations in Myo11G were identified by their rounded nuclei, which differed in shape from the elongated nuclei found in wild-type cells (140). Nuclear movements are greatly reduced in the mutant, and this scenario is consistent with data from expression of tagged-tail or full-length Myo11G sequences that displayed localization to the nuclear envelope (5, 140). Association of Myo11G with the cytoplasmic side of the nuclear envelope depends on the WPP domain–interacting tail-anchored proteins WIT1 and WIT2, because these proteins interact directly with Myo11G and wit1 wit2 double mutants phenocopy the myo11g (xi-i; kaku1) mutant in root and mesophyll cells (140). Thus, the data on Myo11G and nuclear motion in vegetative cells support a cohesive picture that is supported by protein-protein interactions, cellular localization, and mutant phenotype. By contrast, loss of both WIT paralogs in pollen tubes resulted in altered nuclear motions in pollen tubes, whereas loss of Myo11G did not affect nuclear motions (168), suggesting possible redundancy among myosin paralogs in this cell type. Interestingly, Myo11G may also perform additional functions, as tagged constructs associate with different smaller organelles (5), particularly in wit1 wit2 double mutants (140). In addition, the maize homolog Opaque1 appears to localize to the ER, where it functions during protein body formation (159). Finally, Myo11G was found to interact with MyoB proteins (61, 99) and DCP1 (132), further underlining possible secondary functions of Myo11G. In this context, rice encodes three different Myo11G isoforms, which may indicate broader functional specialization of this myosin XI subtype in grasses (81).
Premitotic Nuclear Migration Depends on Kinesins
Correct nuclear positioning is a prerequisite for successful mitosis. In animals, this task requires the minus-end-directed microtubule motor cytoplasmic dynein (see, for example, References 6 and 47). In angiosperms, KCH kinesins appear to have taken on this function in the absence of dynein. The expression of KCH kinesins increases during mitosis, and they localize predominantly to poles of the nucleus (27, 28, 57). Loss of and overexpression of KCH kinesins leads to faster and slower premitotic nuclear migration, respectively (28). Notably, whereas KCH kinesins move actively along cortical microtubules during interphase, the perinuclear pool of KCH kinesins is nonmotile, perhaps due to strong interaction with the actin meshwork surrounding the nucleus (57). These findings raise the intriguing possibility that cortical KCH translocates actin filaments along microtubules, whereas perinuclear KCH is anchored to actin and hence translocates microtubules instead. Direct observation of the motility of microtubules and actin filaments at these locations is needed to test this hypothesis, which may shed light on how KCH kinesins mediate nuclear migration.
Postmitotic Nuclear Motions in Moss Require the Kinesin-14 KCBP-b
In apical caulonemal cells of Physcomitrella, daughter nuclei move toward the center of the cell in a microtubule-dependent manner (77). Targeted gene deletion studies revealed that this movement requires the kinesin-14 KCBP-b (167). Fluorescently tagged, endogenous KCBP-b localized around the presumptive daughter nuclei after anaphase, which coincides with the onset of nuclear movement in the apical cells (167). Nuclear movement is likely driven by a cluster of KCBP-b dimers; this action is necessary and sufficient for microtubule minus-end-directed processive motility and long-distance liposome transport in vitro (51, 167). In addition, the ATK kinesin-14s are involved in the minus-end-directed movement of daughter microtubules along the mother microtubule (167). Together, these findings support the idea that certain kinesin-14s perform minus-end-directed cargo transport in the absence of dynein in plants.
Light-Driven Chloroplast Movement Depends on Kinesins
The fluence rate and direction of light determine chloroplast positioning in plants. Analysis of loss-of-function mutants in Arabidopsis and tobacco showed that these chloroplast movements do not depend on myosin XIs (reviewed in 136). Instead, they depend on the formation of short actin filaments, termed chloroplast-actin filaments, around the chloroplasts (52). Counterintuitively, this process requires the kinesin-14s KAC1 and KAC2 in both Arabidopsis and Physcomitrella (123, 137). Unlike conventional kinesin-14s, KAC1 and KAC2 do not bind to microtubules and are also unlikely to exert mechanical force because key residues required for ATPase activity are altered in them (137). Curiously, the C-terminal domain of KAC1 binds to F-actin (137), but the in vivo significance of this interaction and how it might contribute to chloroplast movement remain to be determined. In Physcomitrella, the KCBP-b kinesin also contributes to the postanaphase chloroplast movement in apical caulonemal cells. In kcbp mutants, chloroplasts accumulate at the cell apex rather than being uniformly distributed, consistent with a reduction in minus-end-directed motility (167). However, because chloroplast localization of KCBP-b has not been shown, it remains to be seen whether KCBP-b directly or indirectly contributes to this chloroplast movement.
Secretory Vesicle Delivery in Tip-Growing Cells Is Actin Dependent
Cellular growth in plants requires the addition of membrane lipids and cell wall material that are transported to the plasma membrane by secretory vesicles that originate at the TGN. Delivery of these secretory vesicles to sites of growth is most clearly evident in tip-growing cells, where all growth is restricted to the apical dome of a tubular cell (Figure 3b). Growth of pollen tubes, root hairs, and moss protonemata depends completely on actin-based processes, as cytochalasin or latrunculin treatments disrupt growth at low concentrations (10, 78, 156). On the basis of these observations, it was expected that myosin motors are necessary for the delivery of secretory vesicles to the growing tip. Indeed, myosin mutants have reduced tip growth rates as well as reduced organelle motility (72, 91, 96, 100). In root hairs of the myo11e mutant, FRAP (fluorescence recovery after photobleaching) analysis revealed that secretory vesicles labeled with SCAMP2-YFP had a slower turnover at the root hair tip (98), suggesting that myosin activity is required for vesicle delivery. By contrast, loss of Myo11C1 and Myo11C2 from pollen reduced only movements of Golgi stacks and peroxisomes in the shank, whereas secretory vesicles labeled with YFP-RabA4d still accumulated normally in the slow-growing pollen tube tip (72). The movement pattern of these vesicles in the tip followed the normal reverse fountain streaming (60), suggesting that they are propelled by another pollen myosin (72). Unexpected conclusions from these observations are that the simple accumulation of vesicles is not sufficient to ensure rapid growth and that myosin motors can affect tip growth in other ways. One possibility may be a direct effect on actin organization or dynamics (as discussed above in the section titled Motors and Cytoskeletal Organization), which may then secondarily affect secretion and cell growth by as-yet-unknown mechanisms.
Long-Distance Transport of Cellulose Synthase Complexes Occurs Along Actin Filaments
Cellulose synthase complexes (CSCs) are a crucial component of the secretory vesicles that mediate growth in diffusely growing cells (95). Conveniently, fluorescently tagged cellulose synthase enzymes can be detected in the Golgi stacks and followed all the way to the plasma membrane, where their insertion can be monitored by an initial static phase that precedes their steady movement during cellulose synthesis (20, 36). This unusual feature of these plasma membrane proteins has allowed for a detailed analysis of the transport modalities employed by CSCs. Specifically, CSC distribution over the surface of hypocotyl cells depends on actin filaments and, by extension, on myosin motors (20, 36). Without actin-based long-distance transport, CSCs still reached the plasma membrane, but at a reduced rate (112), and they were limited to the vicinity of the (now static) Golgi stacks (36). These results are consistent with reduced growth rates of higher-order myosin XI mutants that have lost three or four motor genes and show reduced cell expansion (92, 101). Thus, the acto-myosin system is not essential for delivery of CSC-containing secretory vesicles to the plasma membrane, but it plays a major role in ensuring the rapid delivery and even distribution of these vesicles over the cell surface (Figure 3c). It will be necessary to test mutants in myosin genes to get a more detailed understanding of the mechanisms mediating this distribution. Complementary experiments with MyoB genes such as RISAP that encode TGN-localized myosin-binding proteins (133) may yield additional insights.
Microtubules Aid in Positioning of Secretory Vesicles for Fusion with the Plasma Membrane
Interestingly, moving Golgi stacks tend to stop preferentially over cortical microtubules (20, 160), an observation that was later confirmed for other organelles as well (38). These temporary stops occasionally coincided with the insertion of CSCs into the plasma membrane (20, 112), which is consistent with a model predicting that pauses in Golgi movements are conducive for exchange of transport vesicles with nearby organelles (83). It is not known why Golgi stacks (or other organelles) would stop at microtubules, but it is tempting to speculate that Golgi-localized kinesin motors (71) function as anchors that arrest Golgi stacks at cortical microtubules. In addition to these seemingly direct deliveries from Golgi stacks to the plasma membrane, CSCs were also delivered independently of Golgi stack stops (112). Importantly, these CSC insertions often appeared in association with microtubules, suggesting a guidance function for these cytoskeletal elements (20, 36). In fact, intermediary transport compartments of CSCs (termed MASC or SmaCC) can track along microtubules, sometimes at high rates. It is not known whether these movements involve kinesin motors or whether they are driven by growing and shrinking microtubules, but they do not involve the kinesin-4 encoded by the FRAGILE F1BER1 (FRA1) gene (58, 170). Nevertheless, this motor plays an important role in cell wall deposition, as fra1 mutants have thinner walls and reduced deposition rates of pectin and possibly other cell wall polysaccharides (170). The function of FRA1 requires abundant processive motility (31), but whether FRA1 transports secretory vesicles containing cell wall material remains to be determined. These results demonstrate that, although microtubules and kinesins may not be essential for bulk organelle transport, they play an important role in guiding the secretory process and ensuring the efficient delivery of secretory products (Figure 3c).
Kinesins Are Thought to Deliver Post-Golgi Vesicles to the Cell Plate During Cell Division
As the function of the phragmoplast is to direct the formation of the cell plate, kinesin-mediated transport of vesicles containing cell wall material to the midzone of the phragmoplast is to be expected. Despite the fundamental importance of this process, we know surprisingly little about which kinesins perform this task. The kinesin-10 AtPAKRP2 remains the best candidate to date. AtPAKRP2 shows punctate localization around the phragmoplast and increased concentration at the phragmoplast midzone, where microtubule plus ends overlap (64). Although AtPAKRP2 has been shown to bind to microtubules in an ATP-dependent manner in vitro (64), whether it can support processive plus-end-directed transport remains to be experimentally demonstrated. AtPAKRP2 is enriched in microsome fractions, and brefeldin A treatment resulted in loss of AtPAKRP2 signal at the phragmoplast (64). Together, these data provide indirect evidence for vesicle transport by AtPAKRP2. Cell biological and genetic experiments are needed to identify the cargo and function of this kinesin.
In summary, acto-myosin based movements are necessary for the long-distance delivery of secretory vesicles for growth, but the detailed function of myosins in this process is still not resolved. Delivery of secretory vesicles during tip growth and CSC complexes during diffuse growth represent good experimental systems in which to investigate this function. The role of kinesins during growth will be more difficult to assess because their local-level transport activity can be easily masked by the much faster (> 10-fold) myosin-driven bulk transport and because the function of kinesins seems to be more to increase the efficiency of a process that does not completely depend on them. Cytokinesis may prove to be a better system to study kinesin-mediated vesicle transport because cell plate formation depends on the delivery of vesicles along the phragmoplast microtubules.
MOTOR PROTEINS AND WHOLE PLANT PHYSIOLOGY
The subcellular activities of cytoskeletal motors can affect not only the functioning of individual cells but also the growth and development of the entire plant. Although there are still relatively few examples for such global effects of intracellular motor proteins, it is illustrative to examine these cases because they can reveal new insights into how cellular behavior influences the physiology of the whole plant.
Myosin Motors Are Required for Normal Development in Moss
Myosin VIII motors play an important role in defining plant architecture in P. patens. Loss of all five myosin VIII genes encoded in this moss led to viable plants that exhibited slower growth, reduced gametophyte size, curved protonemata, and enhanced branching (163). Interestingly, these mutants also displayed accelerated development and generated gametophores much sooner than wild-type plants. Curiously, most morphological defects could be rescued by low concentrations of auxin, although the mutants were at the same time hypersensitive to cytokinin (163). At face value, these results suggest that myosin VIII is involved in maintaining proper hormonal balance. It remains to be seen whether hormone levels, distribution, or perception is indeed altered in the mutant or whether the loss of myosin VIII activity modifies moss development independently of these hormones. In contrast to the case for moss, disruption of all four myosin VIII genes in Arabidopsis did not result in significant changes in growth of the plants (139).
Myosin Activity Is Required for Rapid Cell Expansion and for Plant Growth
Several myosin mutants result in smaller plants. In the case of class VIII myosins, this was most pronounced in higher-order mutants in Physcomitrella, but small changes in growth could be detected in single-gene knockouts (163). This observation suggests redundant function of the individual myosin VIII paralogs in moss. For myosin XI in angiosperms, a similar redundant function has been proposed on the basis of the observation that double, triple, quadruple, quintuple, and sextuple mutants in Arabidopsis displayed progressively more stunting (92, 93, 101, 103), whereas myosin XI single mutants affected only tip-growing cells (91, 100) or distorted trichome development (91). The more dramatic inhibition of organelle movements in triple and quadruple mutants correlated with reduced cell elongation in a variety of tissues, and reduced elongation in turn resulted in decreased plant sizes (101).
The correlation between speed of cytoplasmic streaming, cell size, and plant height was further strengthened in a recent study that directly manipulated organelle movement speeds. In this case, the motor domain of Myo11B2 (MYA2) in Arabidopsis was replaced with the motor domain of the high-speed Chara myosin XI (48, 49) (see the section titled Introduction to Motor Proteins, above). The plants expressing this faster myosin had faster cytoplasmic streaming, larger cells, and taller inflorescence stems (143). In a parallel experiment, the motor domain of the slow myosin V from humans was introduced in a similar way. These plants had slower cytoplasmic streaming, and such slower streaming correlated with smaller cell sizes and shorter inflorescence height (143). It remains to be established why both faster and slower motors appear to be dominant over the other endogenous motors that are still present in the plant. Nevertheless, the replacement of motor domains provides an elegant demonstration that myosin-driven cytoplasmic streaming can quantitatively affect growth and may function as a rheostat that fine-tunes growth rates for particular environmental conditions.
It is currently not clear how myosin activity contributes to cell and ultimately plant growth. Cytoplasmic streaming caused by myosin motors may lead to a faster and more even distribution of metabolites, which in turn may result in more efficient biosynthetic pathways and ultimately faster growth (125). Another possibility is that myosin activity leads to the quicker delivery of secretory vesicles to places of growth, allowing for faster growth (20, 36, 112). In addition, myosin activity affects actin filament dynamics (14, 96) and organization (72, 101, 147), which may also impact growth rates by altering secretory efficiency.
Kinesins Affect Plant Growth by Regulating Anisotropic Cell Expansion and Cell Division
Many kinesin mutants show growth defects because they affect the organization and/or function of the interphase or mitotic microtubule arrays, which in turn compromise the genetically determined programs of cell division and expansion that underlie plant growth and development. For example, the Arabidopsis rsw7 (radially swollen7) mutant, which contains a temperature-sensitive mutation in the kinesin-5 AtKRP125c, shows abnormal cortical microtubule organization at the restrictive temperature, which explains the swollen root and reduced growth anisotropy phenotype of this mutant (8). Similarly, the shorter organs of kinesin-13 knockout mutants (22, 29) are indicative of a defect in cortical microtubule organization. Because kinesin-13 depolymerizes microtubules, the reduced growth phenotype of these mutants may be due to abnormally high microtubule stability. Interestingly, moderate reduction of kinesin-13 levels unexpectedly leads to larger cells, likely because of compensatory cell expansion due to activation of the Theseus-mediated cell wall integrity pathway (29). In contrast, the fra1 mutant does not affect cortical microtubule dynamics and organization but still shows stunted growth and brittle inflorescence stems because the cell wall deposition function of the cortical microtubule array is compromised in this mutant (58, 170).
Not surprisingly, kinesins that are required for mitosis and meiosis affect plant growth and fertility. The inability to form bipolar spindles in the atk1-1 mutant results in chromosome segregation defects during meiosis and, consequently, reduced male fertility (15). Likewise, defective phragmoplast guidance and expansion also affect plant growth and development. In the pok1 pok2 double mutant, phragmoplasts and therefore cell plates are frequently misoriented, leading to grossly irregular cell patterns and miniature plants (82). In the ark3 mutant, misoriented cell division planes in the stomatal lineage also produce abnormal cell patterns in the form of stomatal clustering (62). Cell plate expansion defects in hik-1/tes-1 (nack1/nack2) double mutants lead to incomplete cytokinesis in somatic cells as well as in male and female gametophytes, resulting in abnormal seedlings, embryos, and pollen grains (90, 135, 141). Together, these examples highlight how the action of kinesins at the cellular level can scale to affect organ and whole plant morphology.
Myosin XI and Kinesin-7 Triple Mutants Display Slower Gravitropic Bending
The role of myosin motors and cytoplasmic streaming in plant growth suggests that tropic responses may be affected in myosin mutants. A comprehensive screen of myosin XI single and several double and triple mutants revealed that the myo11b2 myo11e myo11f (mya2 xik mya1) triple mutant had significantly reduced gravitropic bending, a reduced growth rate, and a stiffer inflorescence stem relative to wild type (139). Interestingly, the sedimentation of amyloplasts also appeared delayed in the triple mutant (139), suggesting that the impaired gravitropic response is not caused solely by slower growth. The mechanisms by which amyloplast sedimentation is affected by these myosins are not known, because actin organization appears to be normal in the triple mutant (139).
Recently, the kin7 triple mutant was also reported to exhibit slower gravitropic bending. Polar localization of the auxin efflux carrier PIN2 is altered in this mutant, providing a plausible explanation for the reduced gravitropism (80). However, the mechanism for PIN2 mislocalization in this mutant remains unknown, and it remains to be determined whether the microtubule polymerization activity of kinesin-7 plays a role in such mislocalization.
Myosin XI Affects Organ Straightening After Gravitropic Bending
Once stems or roots reach nearly vertical orientation during gravitropic responses, they begin to straighten to prevent overshooting of the target orientation (130). Interestingly, this straightening response is defective in another Arabidopsis myosin double mutant, myo11e myo11h (xik xif) (93). These mutants show normal gravitropic (and phototropic) responses, but bending does not stop in time, and as a result, the inflorescence stem shows dampened oscillations around vertical before settling on a stable growth direction. This behavior becomes even more evident on a clinostat where gravistimulated wild-type stems spontaneously straighten whereas myo11e myo11h stems continue to bend (93). The defect in organ straightening also occurs under normal growth conditions, as can be seen by the kinked growth of myo11e myo11h inflorescence stems (93). It is not clear how Myo11E and Myo11H myosins contribute to this response, but the limited expression of Myo11H in the thin layer of fiber cells within the vascular system (93) indicates that these cells play a crucial role in mediating organ straightening. Cytoplasmic streaming in young fiber cells is significantly slower in the double mutant, but other cellular defects, e.g., actin organization, have not been described so far. It will be interesting to see whether these myosin motors play a role in stem curvature sensing or in the response pathway that leads to straightening.
In summary, an increasing number of whole plant growth processes can be traced back to the function of cytoskeletal motors. Whereas some of these effects can be attributed to specific motor isoforms, others appear to be carried out collectively by a group of seemingly redundant motors. In many cases, the mechanistic link between motor activity within cells and whole plant response still has to be established.
OUTLOOK
The recent years have seen major advances in our understanding of kinesin and myosin motor function in plants. The cellular function of a number of motor isoforms has been identified, but progress has been unevenly distributed and often limited to the model species A. thaliana and P. patens (Table 1). Future research should take advantage of new genetic tools such as CRISPR/Cas9 to create multiple mutants or to endogenously tag motors. In addition, in vivo super-resolution microscopy combined with in vitro single-molecule experiments will be necessary to perform correlative structure-function studies to better understand the basic functioning of the motors and how they affect cellular processes. Recent discoveries of cross talk between cytoskeletal elements suggest that the effect of motors on the so-called other cytoskeletal network should be examined more carefully. Meeting these challenges will require a combination of biochemical, genetic, cell biological, and modeling approaches.
ACKNOWLEDGMENTS
Research in the Nebenführ lab is funded by National Science Foundation award MCB-1715794. Research in the Dixit lab is funded by National Science Foundation CAREER award MCB-1453726; the Center for Engineering Mechanobiology, a National Science Foundation Science and Technology Center, under award CMMI-1548571; and National Institutes of Health grant R01 GM114678.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Akkerman M, Overdijk EJR, Schel JHN, Emons AMC, Ketelaar T. 2011. Golgi body motility in the plant cortex correlates with actin cytoskeleton organization. Plant Cell Physiol. 52:1844–55 [DOI] [PubMed] [Google Scholar]
- 2.Ambrose JC, Cyr R. 2007. The kinesin ATK5 functions in early spindle assembly in Arabidopsis. Plant Cell 19:226–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrose JC, Li W, Marcus A, Ma H, Cyr R. 2005. A minus-end-directed kinesin with plus-end tracking protein activity is involved in spindle morphogenesis. Mol. Biol. Cell 16:1584–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avisar D, Abu-Abied M, Belausov E, Sadot E. 2012. Myosin XIK is a major player in cytoplasm dynamics and is regulated by two amino acids in its tail. J. Exp. Bot 63:241–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avisar D, Abu-Abied M, Belausov E, Sadot E, Hawes C, Sparkes IA. 2009. A comparative study of the involvement of 17 Arabidopsis myosin family members on the motility of Golgi and other organelles. Plant Physiol. 150:700–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baffet AD, Hu DJ, Vallee RB. 2015. Cdk1 activates pre-mitotic nuclear envelope dynein recruitment and apical nuclear migration in neural stem cells. Dev. Cell 33:703–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey-Serres J, Sorenson R, Juntawong P. 2009. Getting the message across: cytoplasmic ribonucleoprotein complexes. Trends Plant Sci. 14:443–53 [DOI] [PubMed] [Google Scholar]
- 8.Bannigan A, Scheible WR, Lukowitz W, Fagerstrom C, Wadsworth P, et al. 2007. A conserved role for kinesin-5 in plant mitosis. J. Cell Sci 120:2819–27 [DOI] [PubMed] [Google Scholar]
- 9.Beach DL, Thibodeaux J, Maddox P, Yeh E, Bloom K. 2000. The role of the proteins Kar9 and Myo2 in orienting the mitotic spindle of budding yeast. Curr. Biol 10:1497–506 [DOI] [PubMed] [Google Scholar]
- 10.Bibeau JP, Kingsley JL, Furt F, Tüzel E, Vidali L. 2017. F-actin mediated focusing of vesicles at the cell tip is essential for polarized growth. Plant Physiol. 176:352–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C. 1998. Stacks on tracks: The plant Golgi apparatus traffics on an actin/ER network. Plant J. 15:441–47 [DOI] [PubMed] [Google Scholar]
- 12.Buschmann H, Dols J, Kopischke S, Pena EJ, Andrade-Navarro MA, et al. 2015. Arabidopsis KCBP interacts with AIR9 but stays in the cortical division zone throughout mitosis via its MyTH4-FERM domain. J. Cell Sci 128:2033–46 [DOI] [PubMed] [Google Scholar]
- 13.Buschmann H, Green P, Sambade A, Doonan JH, Lloyd CW.2011. Cytoskeletal dynamics in interphase, mitosis and cytokinesis analysed through Agrobacterium-mediated transient transformation of tobacco BY-2 cells. New Phytol. 190:258–67 [DOI] [PubMed] [Google Scholar]
- 14.Cai C, Henty-Ridilla JL, Szymanski DB, Staiger CJ. 2014. Arabidopsis myosin XI: A motor rules the tracks. Plant Physiol. 166:1359–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Marcus A, Li W, Hu Y, Calzada JP, et al. 2002. The Arabidopsis ATK1 gene is required for spindle morphogenesis in male meiosis. Development 129:2401–9 [DOI] [PubMed] [Google Scholar]
- 16.Cheney RE, Riley MA, Mooseker MS. 1993. Phylogenetic analysis of the myosin superfamily. Cell Motil. Cytoskelet 24:215–23 [DOI] [PubMed] [Google Scholar]
- 17.Collings DA, Harper JDI, Marc J, Overall RL, Mullen RT. 2002. Life in the fast lane: actin-based motility of plant peroxisomes. Can. J. Bot 80:430–41 [Google Scholar]
- 18.Collings DA, Wasteneys GO. 2005. Actin microfilament and microtubule distribution patterns in the expanding root of Arabidopsis thaliana. Can. J. Bot 83:579–90 [Google Scholar]
- 19.Corti B 1774. Osservazioni Microscopiche Sulla Tremella e Sulla Circolazione del Fluido in una Pianta Acquajuola. Lucca, Italy: Appresso Giuseppe Rocchi [Google Scholar]
- 20.Crowell EF, Bischoff V, Desprea T, Rolland A, Stierhof Y-D, et al. 2009. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 21:1141–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Keijzer J, Kieft H, Ketelaar T, Goshima G, Janson ME. 2017. Shortening of microtubule overlap regions defines membrane delivery sites during plant cytokinesis. Curr. Biol 27:514–20 [DOI] [PubMed] [Google Scholar]
- 22.Deng ZY, Liu LT, Li T, Yan S, Kuang BJ, et al. 2015. OsKinesin-13A is an active microtubule depolymerase involved in glume length regulation via affecting cell elongation. Sci. Rep 5:9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diensthuber RP, Tominaga M, Preller M, Hartmann FK, Orii H, et al. 2015. Kinetic mechanism of Nicotiana tabacum myosin-11 defines a new type of a processive motor. FASEB J. 29:81–94 [DOI] [PubMed] [Google Scholar]
- 24.Eng RC, Halat LS, Livingston SJ, Sakai T, Motose H, Wasteneys GO. 2017. The ARM domain of ARMADILLO-REPEAT KINESIN 1 is not required for microtubule catastrophe but can negatively regulate NIMA-RELATED KINASE 6 in Arabidopsis thaliana. Plant Cell Physiol. 58:1350–63 [DOI] [PubMed] [Google Scholar]
- 25.Eng RC, Wasteneys GO. 2014. The microtubule plus-end tracking protein ARMADILLO-REPEAT KINESIN1 promotes microtubule catastrophe in Arabidopsis. Plant Cell 26:3372–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esseling-Ozdoba A, Houtman D, van Lammeren AAM, Eiser E, Emons AMC. 2008. Hydrodynamic flow in the cytoplasm of plant cells. J. Microsc 231:274–83 [DOI] [PubMed] [Google Scholar]
- 27.Frey N, Klotz J, Nick P. 2009. Dynamic bridges—a calponin-domain kinesin from rice links actin filaments and microtubules in both cycling and non-cycling cells. Plant Cell Physiol. 50:1493–506 [DOI] [PubMed] [Google Scholar]
- 28.Frey N, Klotz J, Nick P. 2010. A kinesin with calponin-homology domain is involved in premitotic nuclear migration. J. Exp. Bot 61:3423–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujikura U, Elsaesser L, Breuninger H, Sanchez-Rodriguez C, Ivakov A, et al. 2014. Atkinesin-13A modulates cell-wall synthesis and cell expansion in Arabidopsis thaliana via the THESEUS1 pathway. PLOS Genet. 10:e1004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furt F, Liu Y-C, Bibeau JP, Tüzel E, Vidali L. 2013. Apical myosin XI anticipates F-actin during polarized growth of Physcomitrella patens cells. Plant J. 73:417–28 [DOI] [PubMed] [Google Scholar]
- 31.Ganguly A, DeMott L, Dixit R. 2017. The Arabidopsis kinesin-4, FRA1, requires a high level of processive motility to function correctly. J. Cell Sci 130:1232–38 [DOI] [PubMed] [Google Scholar]
- 32.Ganguly A, Dixit R. 2013. Mechanisms for regulation of plant kinesins. Curr. Opin. Plant Biol 16:704–9 [DOI] [PubMed] [Google Scholar]
- 33.Geitmann A, Nebenführ A. 2015. Navigating the plant cell: intracellular transport logistics in the green kingdom. Mol. Biol. Cell 26:3373–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golomb L, Abu-Abied M, Belausov E, Sadot E. 2008. Different subcellular localizations and functions of Arabidopsis myosin VIII. BMC Plant Biol. 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffing LR, Gao HT, Sparkes IA. 2014. ER network dynamics are differentially controlled by myosins XI-K, XI-C, XI-E, XI-I, XI-1, and XI-2. Front. Plant Sci 5:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutierrez R, Lindeboom JJ, Paredez AR, Emons AMC, Ehrhardt DW. 2009. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat. Cell Biol 11:797–806 [DOI] [PubMed] [Google Scholar]
- 37.Hachikubo Y, Ito K, Schiefelbein JW, Manstein DJ, Yamamoto K. 2007. Enzymatic activity and motility of recombinant Arabidopsis myosin XI, MYA1. Plant Cell Physiol. 48:886–91 [DOI] [PubMed] [Google Scholar]
- 38.Hamada T, Tominaga M, Fukaya T, Nakamura M, Nakano A, et al. 2012. RNA processing bodies, peroxisomes, Golgi bodies, mitochondria, and endoplasmic reticulum tubule junctions frequently pause at cortical microtubules. Plant Cell Physiol. 53:699–798 [DOI] [PubMed] [Google Scholar]
- 39.Hammer JA, Sellers JR. 2012. Walking to work: roles for class V myosins as cargo transporters. Nat. Rev. Mol. Cell Biol 13:13–26 [DOI] [PubMed] [Google Scholar]
- 40.Haraguchi T, Tominaga M, Matsumoto R, Sato K, Nakano A, et al. 2014. Molecular characterization and subcellular localization of Arabidopsis class VIII myosin, ATM1. J. Biol. Chem 289:12343–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haraguchi T, Tominaga M, Nakano A, Yamamoto K, Ito K. 2016. Myosin XI-I is mechanically and enzymatically unique among class-XI myosins in Arabidopsis. Plant Cell Physiol. 57:1732–43 [DOI] [PubMed] [Google Scholar]
- 42.Higashi-Fujime S, Ishikawa R, Iwasawa H, Kagami O, Kurimoto E, et al. 1995. The fastest actin-based motor protein from green algae, Chara, and its distinct mode of interaction with actin. FEBS Lett. 375:151–54 [DOI] [PubMed] [Google Scholar]
- 43.Hiwatashi Y, Obara M, Sato Y, Fujita T, Murata T, Hasebe M. 2008. Kinesins are indispensable for interdigitation of phragmoplast microtubules in the moss Physcomitrella patens. Plant Cell 20:3094–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiwatashi Y, Sato Y, Doonan JH. 2014. Kinesins have a dual function in organizing microtubules during both tip growth and cytokinesis in Physcomitrella patens. Plant Cell 26:1256–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holding DR, Otegui MS, Li B, Meeley RB, Dam T, et al. 2007. The maize Floury1 gene encodes a novel endoplasmic reticulum protein involved in zein protein body formation. Plant Cell 19:2569–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, et al. 2009. An EB1-binding motif acts as a microtubule tip localization signal. Cell 138:366–76 [DOI] [PubMed] [Google Scholar]
- 47.Hu DJ, Baffet AD, Nayak T, Akhmanova A, Doye V, Vallee RB. 2013. Dynein recruitment to nuclear pores activates apical nuclear migration and mitotic entry in brain progenitor cells. Cell 154:1300–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito K, Ikebe M, Kashiyama T, Mogami T, Kon T, Yamamoto K. 2007. Kinetic mechanism of the fastest motor protein, Chara myosin. J. Biol. Chem 282:19534–45 [DOI] [PubMed] [Google Scholar]
- 49.Ito K, Yamaguchi Y, Yanase K, Ichikawa Y, Yamamoto K. 2009. Unique charge distribution in surface loops confers high velocity on the fast motor protein Chara myosin. PNAS 106:21585–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itoh R, Fujiwara M, Yoshida S. 2001. Kinesin-related proteins with a mitochondrial targeting signal. Plant Physiol. 127:724–26 [PMC free article] [PubMed] [Google Scholar]
- 51.Jonsson E, Yamada M, Vale RD, Goshima G. 2015. Clustering of a kinesin-14 motor enables processive retrograde microtubule-based transport in plants. Nat. Plants 1:15087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, et al. 2009. Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. PNAS 106:13106–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamiya N, Kuroda K. 1956. Velocity distribution of the protoplasmic streaming in Nitella cells. Bot. Mag. Tokyo 69:544–54 [Google Scholar]
- 54.Kao YL, Deavours BE, Phelps KK, Walker RA, Reddy AS. 2000. Bundling of microtubules by motor and tail domains of a kinesin-like calmodulin-binding protein from Arabidopsis: regulation by Ca2+/calmodulin. Biochem. Biophys. Res. Commun 267:201–7 [DOI] [PubMed] [Google Scholar]
- 55.Kinkema M, Schiefelbein J. 1994. A myosin from a higher plant has structural similarities to class V myosins. J. Mol. Biol 239:591–97 [DOI] [PubMed] [Google Scholar]
- 56.Kinkema M, Wang H, Schiefelbein J. 1994. Molecular analysis of the myosin gene family in Arabidopsis. Plant Mol. Biol 16:1139–53 [DOI] [PubMed] [Google Scholar]
- 57.Klotz J, Nick P. 2012. A novel actin-microtubule cross-linking kinesin, NtKCH, functions in cell expansion and division. New Phytol. 193:576–89 [DOI] [PubMed] [Google Scholar]
- 58.Kong Z, Ioki M, Braybrook S, Li S, Ye ZH, et al. 2015. Kinesin-4 functions in vesicular transport on cortical microtubules and regulates cell wall mechanics during cell elongation in plants. Mol. Plant 8:1011–23 [DOI] [PubMed] [Google Scholar]
- 59.Korn ED. 2000. Coevolution of head, neck, and tail domains ofmyosin heavy chains. PNAS 97:12559–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kroeger JH, Bou Daher F, Grant M, Geitmann A. 2009. Microfilament orientation constrains vesicle flow and spatial distribution in growing pollen tubes. Biophys. J 97:1822–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurth EG, Peremyslov VV, Turner HL, Makarova KS, Iranzo J, et al. 2017. Myosin-driven transport network in plants. PNAS 114:1385–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lau OS, Davies KA, Chang J, Adrian J, Rowe MH, et al. 2014. Direct roles of SPEECHLESS in the specification of stomatal self-renewing cells. Science 345:1605–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, et al. 2004. A standardized kinesin nomenclature. J. Cell Biol 167:19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee YR, Giang HM, Liu B. 2001. A novel plant kinesin-related protein specifically associates with the phragmoplast organelles. Plant Cell 13:2427–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee YR, Liu B. 2000. Identification of a phragmoplast-associated kinesin-related protein in higher plants. Curr. Biol 10:797–800 [DOI] [PubMed] [Google Scholar]
- 66.Lee YR, Qiu W, Liu B. 2015. Kinesin motors in plants: from subcellular dynamics to motility regulation. Curr. Opin. Plant Biol 28:120–26 [DOI] [PubMed] [Google Scholar]
- 67.Li J-F, Nebenführ A. 2007. Organelle targeting of myosin XI is mediated independently by two globular tail subdomains. J. Biol. Chem 282:20593–602 [DOI] [PubMed] [Google Scholar]
- 68.Li J-F, Nebenführ A. 2008. Inter-dependence of dimerization and organelle binding in myosin XI. Plant J. 55:478–90 [DOI] [PubMed] [Google Scholar]
- 69.Li J-F, Nebenführ A. 2008. The tail that wags the dog: The globular tail domain defines the function of myosin V/XI. Traffic 9:290–98 [DOI] [PubMed] [Google Scholar]
- 70.Lipka E, Gadeyne A, Stockle D, Zimmermann S, De Jaeger G, et al. 2014. The phragmoplast-orienting kinesin-12 class proteins translate the positional information of the preprophase band to establish the cortical division zone in Arabidopsis thaliana. Plant Cell 26:2617–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu L, Lee Y-RJ, Pan R, Maloof JN, Liu B. 2005. An internal motor kinesin is associated with the Golgi apparatus and plays a role in trichome morphogenesis in Arabidopsis. Mol. Biol. Cell 16:811–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madison SL, Buchanan ML, Glass JD, McClain TF, Park E, Nebenführ A. 2015. Class XI myosins move specific organelles in pollen tubes and are required for normal fertility and pollen tube growth in Arabidopsis. Plant Physiol. 169:1946–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malcos JL, Cyr RJ. 2011. An ungrouped plant kinesin accumulates at the preprophase band in a cell cycle–dependent manner. Cytoskeleton 68:247–58 [DOI] [PubMed] [Google Scholar]
- 74.Marcus AI, Ambrose JC, Blickley L, Hancock WO, Cyr RJ. 2002. Arabidopsis thaliana protein, ATK1, is a minus-end directed kinesin that exhibits non-processive movement. Cell Motil. Cytoskelet 52:144–50 [DOI] [PubMed] [Google Scholar]
- 75.Marcus AI, Li W, Ma H, Cyr RJ. 2003. A kinesin mutant with an atypical bipolar spindle undergoes normal mitosis. Mol. Biol. Cell 14:1717–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miki T, Naito H, Nishina M, Goshima G. 2014. Endogenous localizome identifies 43 mitotic kinesins in a plant cell. PNAS 111:E1053–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miki T, Nishina M, Goshima G. 2015. RNAi screening identifies the armadillo repeat–containing kinesins responsible for microtubule-dependent nuclear positioning in Physcomitrella patens. Plant Cell Physiol. 56:737–49 [DOI] [PubMed] [Google Scholar]
- 78.Miller DD, deRuijter NCA, Bisseling T, Emons AMC. 1999. The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 17:141–54 [Google Scholar]
- 79.Mineyuki Y 1999. The preprophase band of microtubules: its function as a cytokinetic apparatus in higher plants. Int. Rev. Cytol 187:1–49 [Google Scholar]
- 80.Moschou PN, Gutierrez-Beltran E, Bozhkov PV, Smertenko A. 2016. Separase promotes microtubule polymerization by activating CENP-E-related kinesin Kin7. Dev. Cell 37:350–61 [DOI] [PubMed] [Google Scholar]
- 81.Mühlhausen S, Kollmar M. 2013. Whole genome duplication events in plant evolution reconstructed and predicted using myosin motor proteins. BMC Evol. Biol 13:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muller S, Han S, Smith LG. 2006. Two kinesins are involved in the spatial control of cytokinesis in Arabidopsis thaliana. Curr. Biol 16:888–94 [DOI] [PubMed] [Google Scholar]
- 83.Nebenführ A, Gallagher L, Dunahay TG, Frohlick JA, Masurkiewicz AM, et al. 1999. Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol. 121:1127–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishihama R, Soyano T, Ishikawa M, Araki S, Tanaka H, et al. 2002. Expansion of the cell plate in plant cytokinesis requires a kinesin-like protein/MAPKKK complex. Cell 109:87–99 [DOI] [PubMed] [Google Scholar]
- 85.Oda Y, Fukuda H. 2012. Initiation of cell wall pattern by a Rho- and microtubule-driven symmetry breaking. Science 337:1333–36 [DOI] [PubMed] [Google Scholar]
- 86.Oda Y, Fukuda H. 2013. Rho of plantGTPase signaling regulates the behavior of Arabidopsis kinesin-13A to establish secondary cell wall patterns. Plant Cell 25:4439–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oda Y, Iida Y, Kondo Y, Fukuda H. 2010. Wood cell-wall structure requires local 2D-microtubule disassembly by a novel plasma membrane–anchored protein. Curr. Biol 20:1197–202 [DOI] [PubMed] [Google Scholar]
- 88.Odronitz F, Kollmar M. 2007. Drawing the tree of eukaryotic life based on the analysis of 2,269 manually annotated myosins from 328 species. Genome Biol. 8:R196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oh SA, Allen T, Kim GJ, Sidorova A, Borg M, et al. 2012. Arabidopsis Fused kinase and the Kinesin-12 subfamily constitute a signalling module required for phragmoplast expansion. Plant J. 72:308–19 [DOI] [PubMed] [Google Scholar]
- 90.Oh SA, Bourdon V, Das ‘Pal M, Dickinson H, Twell D. 2008. Arabidopsis kinesins HINKEL and TETRASPORE act redundantly to control cell plate expansion during cytokinesis in the male gametophyte. Mol. Plant 1:794–99 [DOI] [PubMed] [Google Scholar]
- 91.Ojangu E-L, Järve K, Paves H, Truve E. 2007. Arabidopsis thaliana myosin XIK is involved in root hair as well as trichome morphogenesis on stems and leaves. Protoplasma 230:193–202 [DOI] [PubMed] [Google Scholar]
- 92.Ojangu E-L, Tanner K, Pata P, Järve K, Holweg CL, et al. 2012. Myosins XI-K, XI-1, and XI-2 are required for development of pavement cells, trichomes, and stigmatic papillae in Arabidopsis. BMC Plant Biol. 12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okamoto K, Ueda H, Shimada T, Tamura K, Kato T, et al. 2015. Regulation of organ straightening and plant posture by an actin-myosin XI cytoskeleton. Nat. Plants 1:15031. [DOI] [PubMed] [Google Scholar]
- 94.Pan R, Lee YR, Liu B. 2004. Localization of two homologous Arabidopsis kinesin-related proteins in the phragmoplast. Planta 220:156–64 [DOI] [PubMed] [Google Scholar]
- 95.Paredez AR, Somerville CR, Ehrhardt DW. 2006. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312:1491–95 [DOI] [PubMed] [Google Scholar]
- 96.Park E, Nebenführ A. 2013. Myosin XIK of Arabidopsis thaliana accumulates at the root hair tip and is required for fast root hair growth. PLOS ONE 8:e76745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peremyslov VV, Cole RA, Fowler JE, Dolja VV. 2015. Myosin-powered membrane compartment drives cytoplasmic streaming, cell expansion and plant development. PLOS ONE 10:e0139331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peremyslov VV, Klocko AL, Fowler JE, Dolja VV. 2012. Arabidopsis myosin XI-K localizes to the motile endomembrane vesicles associated with F-actin. Front. Plant Sci 3:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peremyslov VV, Morgun EA, Kurth EG, Makarova KS, Koonin EV, Dolja VV. 2013. Identification of myosin XI receptors in Arabidopsis defines a distinct class of transport vesicles. Plant Cell 25:3039–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peremyslov VV, Prokhnevsky AI, Avisar D, Dolja VV. 2008. Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis thaliana. Plant Physiol. 146:1109–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peremyslov VV, Prokhnevsky AI, Dolja VV. 2010. Class XI myosins are required for development, cell expansion, and F-actin organization in Arabidopsis. Plant Cell 22:1883–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Preuss ML, Kovar DR, Lee YR, Staiger CJ, Delmer DP, Liu B. 2004. A plant-specific kinesin binds to actin microfilaments and interacts with cortical microtubules in cotton fibers. Plant Physiol. 136:3945–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prokhnevsky AI, Peremyslov VV, Dolja VV. 2008. Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation, and organelle motility. PNAS 105:19744–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reddy ANS, Day IS. 2001. Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol. 2:research0024.1–0024.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reddy AS, Day IS. 2001. Kinesins in the Arabidopsis genome: a comparative analysis among eukaryotes. BMC Genom. 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reichelt S, Knight AE, Hodge TP, Baluska F, Samaj J, et al. 1999. Characterization of the unconventional myosin VIII in plant cells and its localization at the post-cytokinetic cell wall. Plant J. 19:555–67 [DOI] [PubMed] [Google Scholar]
- 107.Richards TA, Cavalier-Smith T. 2005. Myosin domain evolution and the primary divergence of eukaryotes. Nature 436:1113–18 [DOI] [PubMed] [Google Scholar]
- 108.Richardson DN, Simmons MP, Reddy AS. 2006. Comprehensive comparative analysis of kinesins in photosynthetic eukaryotes. BMC Genom. 7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rounds CM, Bezanilla M. 2013. Growth mechanisms in tip-growing plant cells. Annu. Rev. Plant Biol 64:243–65 [DOI] [PubMed] [Google Scholar]
- 110.Runions J, Brach T, Kühner S, Hawes C. 2006. Photoactivation of GFP reveals protein dynamics within the endoplasmic reticulum membrane. J. Exp. Bot 57:43–50 [DOI] [PubMed] [Google Scholar]
- 111.Sakai T, Honing H, Nishioka M, Uehara Y, Takahashi M, et al. 2008. Armadillo repeat–containing kinesins and a NIMA-related kinase are required for epidermal-cell morphogenesis in Arabidopsis. Plant J. 53:157–71 [DOI] [PubMed] [Google Scholar]
- 112.Sampathkumar A, Gutierrez R, McFarlane HE, Bringmann M, Lindeboom JJ, et al. 2013. Patterning and lifetime of plasma membrane–localized cellulose synthase is dependent on actin organization in Arabidopsis interphase cells. Plant Physiol. 162:675–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sampathkumar A, Lindeboom JJ, Debolt S, Gutierrez R, Ehrhardt DW, et al. 2011. Live cell imaging reveals structural associations between the actin and microtubule cytoskeleton in Arabidopsis. Plant Cell 23:2302–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sasabe M, Kosetsu K, Hidaka M, Murase A, Machida Y. 2011. Arabidopsis thaliana MAP65-1 and MAP65-2 function redundantly with MAP65-3/PLEIADE in cytokinesis downstream of MPK4. Plant Signal. Behav 6:743–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sattarzadeh A, Franzen R, Schmelzer E. 2008. The Arabidopsis class VIII myosin ATM2 is involved in endocytosis. Cell Motil. Cytoskelet 65:457–68 [DOI] [PubMed] [Google Scholar]
- 116.Sattarzadeh A, Schmelzer E, Hanson MR. 2011. Analysis of organelle targeting by DIL domains of the Arabidopsis myosin XI family. Front. Plant Sci 2:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schaefer E, Belcram K, Uyttewaal M, Duroc Y, Goussot M, et al. 2017. The preprophase band of microtubules controls the robustness of division orientation in plants. Science 356:186–89 [DOI] [PubMed] [Google Scholar]
- 118.Schneider R, Persson S. 2015. Connecting two arrays: the emerging role of actin-microtubule crosslinking motor proteins. Front. Plant Sci 6:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sebé-Pedrós A, Grau-Bové X, Richards TA, Ruiz-Trillo I. 2014. Evolution and classification ofmyosins, a paneukaryotic whole-genome approach. Genome Biol. Evol 6:290–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shastry S, Hancock WO. 2010. Neck linker length determines the degree of processivity in kinesin-1 and kinesin-2 motors. Curr. Biol 20:939–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shastry S, Hancock WO. 2011. Interhead tension determines processivity across diverse N-terminal kinesins. PNAS 108:16253–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen Z, Collatos AR, Bibeau JP, Furt F, Vidali L. 2012. Phylogenetic analysis of the Kinesin superfamily from Physcomitrella. Front. Plant Sci 3:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shen Z, Liu YC, Bibeau JP, Lemoi KP, Tuzel E, Vidali L. 2015. The kinesin-like proteins, KAC1/2, regulate actin dynamics underlying chloroplast light-avoidance in Physcomitrella patens. J. Integr. Plant Biol 57:106–19 [DOI] [PubMed] [Google Scholar]
- 124.Shimmen T 2007. The sliding theory of cytoplasmic streaming: fifty years of progress. J. Plant Res 120:31–43 [DOI] [PubMed] [Google Scholar]
- 125.Shimmen T, Yokota E. 1994. Physiological and biochemical aspects of cytoplasmic streaming. Int. Rev. Cytol 155:97–139 [Google Scholar]
- 126.Sieberer B, Emons AMC.2000. Cytoarchitecture and pattern of cytoplasmic streaming in the root hairs of Medicago truncatula during development and deformation by nodulation factors. Protoplasma 214:118–27 [Google Scholar]
- 127.Sparkes IA, Runions J, Hawes C, Griffing L. 2009. Movement and remodeling of the endoplasmic reticulum in nondividing cells of tobacco leaves. Plant Cell 21:3937–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sparkes IA, Teanby NA, Hawes C. 2008. Truncated myosin XI tail fusions inhibit peroxisome, Golgi, and mitochondrial movement in tobacco leaf epidermal cells: a genetic tool for the next generation. J. Exp. Bot 59:2499–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Staiger CJ, Sheahan MB, Khurana P, Wang X, McCurdy DW, Blanchoin L. 2009. Actin filament dynamics are dominated by rapid growth and severing activity in the Arabidopsis cortical array. J. Cell Biol 184:269–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stanković B, Volkmann D, Sack FD. 1998. Autotropism, automorphogenesis, and gravity. Physiol. Plant 102:328–35 [DOI] [PubMed] [Google Scholar]
- 131.Stefano G, Renna L, Brandizzi F. 2014. The endoplasmic reticulum exerts control over organelle streaming during cell expansion. J. Cell Sci 127:947–53 [DOI] [PubMed] [Google Scholar]
- 132.Steffens A, Jaegle B, Tresch A, Hülskamp M, Jakoby M. 2014. Processing-body movement in Arabidopsis depends on an interaction between myosins and DECAPPING PROTEIN1. Plant Physiol. 164:1879–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stephan O, Cottier S, Fahlén S, Montes-Rodriguez A, Sun J, et al. 2014. RISAP is a TGN-associated RAC5 effector regulating membrane traffic during polar cell growth in tobacco. Plant Cell 26:4426–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stockle D, Herrmann A, Lipka E, Lauster T, Gavidia R, et al. 2016. Putative RopGAPs impact division plane selection and interact with kinesin-12 POK1. Nat. Plants 2:16120. [DOI] [PubMed] [Google Scholar]
- 135.Strompen G, El Kasmi F, Richter S, Lukowitz W, Assaad FF, et al. 2002. The Arabidopsis HINKEL gene encodes a kinesin-related protein involved in cytokinesis and is expressed in a cell cycle–dependent manner. Curr. Biol 12:153–58 [DOI] [PubMed] [Google Scholar]
- 136.Suetsugu N, Dolja VV, Wada M. 2010. Why have chloroplasts developed a unique motility system? Plant Signal Behav. 5:1190–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suetsugu N, Yamada N, Kagawa T, Yonekura H, Uyeda TQ, et al. 2010. Two kinesin-like proteins mediate actin-based chloroplast movement in Arabidopsis thaliana. PNAS 107:8860–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Szymanski DB, Cosgrove DJ. 2009. Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr. Biol 19:R800–11 [DOI] [PubMed] [Google Scholar]
- 139.Talts K, Ilau B, Ojangu E-L, Tanner K, Peremyslov VV, et al. 2016. Arabidopsis myosins XI1, XI2, and XIK are crucial for gravity-induced bending of inflorescence stems. Front. Plant Sci 7:1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tamura K, Iwabuchi K, Fukao Y, Kondo M, Okamoto K, et al. 2013. Myosin XI-I links the nuclear membrane to the cytoskeleton to control nuclear movement and shape in Arabidopsis. Curr. Biol 23:1776–81 [DOI] [PubMed] [Google Scholar]
- 141.Tanaka H, Ishikawa M, Kitamura S, Takahashi Y, Soyano T, et al. 2004. The AtNACK1/HINKEL and STUD/TETRASPORE/AtNACK2 genes, which encode functionally redundant kinesins, are essential for cytokinesis in Arabidopsis. Genes Cells 9:1199–211 [DOI] [PubMed] [Google Scholar]
- 142.Tian J, Han L, Feng Z, Wang G, Liu W, et al. 2015. Orchestration of microtubules and the actin cytoskeleton in trichome cell shape determination by a plant-unique kinesin. eLife 4:e09351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tominaga M, Kimura A, Yokota E, Haraguchi T, Shimmen T, et al. 2013. Cytoplasmic streaming velocity as a plant size determinant. Dev. Cell 27:345–52 [DOI] [PubMed] [Google Scholar]
- 144.Tominaga M, Kojima H, Yokota E, Nakamori R, Anson M, et al. 2012. Calcium-induced mechanical change in the neck domain alters the activity of plant myosin XI. J. Biol. Chem 287:30711–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tominaga M, Kojima H, Yokota E, Orii H, Nakamori R, et al. 2003. Higher plant myosin XI moves processively on actin with 35 nm steps at high velocity. EMBO J. 22:1263–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ueda H, Tamura K, Hara-Nishimura I. 2015. Functions of plant-specific myosin XI: from intracellular motility to plant postures. Curr. Opin. Plant Biol 28:30–38 [DOI] [PubMed] [Google Scholar]
- 147.Ueda H, Yokota E, Kutsuna N, Shimada T, Tamura K, et al. 2010. Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. PNAS 107:6894–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Umezu N, Umeki N, Mitsui T, Kondo K, Maruta S. 2011. Characterization of a novel rice kinesin O12 with a calponin homology domain. J. Biochem 149:91–101 [DOI] [PubMed] [Google Scholar]
- 149.Vale RD. 1996. Switches, latches, and amplifiers: common themes of G proteins and molecular motors. J. Cell Biol 135:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vale RD. 2003. The molecular toolbox for intracellular transport. Cell 112:467–80 [DOI] [PubMed] [Google Scholar]
- 151.Van Damme D, Bouget FY, Van Poucke K, Inze D, Geelen D. 2004. Molecular dissection of plant cytokinesis and phragmoplast structure: a survey of GFP-tagged proteins. Plant J. 40:386–98 [DOI] [PubMed] [Google Scholar]
- 152.vanGestel K, Köhler RH, Verbelen J-P. 2002. Plant mitochondria move on F-actin, but their positioning in the cortical cytoplasm depends on both F-actin and microtubules. J. Exp. Bot 53:659–67 [DOI] [PubMed] [Google Scholar]
- 153.Vanstraelen M, Inze D, Geelen D. 2006. Mitosis-specific kinesins in Arabidopsis. Trends Plant Sci. 11:167–75 [DOI] [PubMed] [Google Scholar]
- 154.Vick JK, Nebenführ A. 2012. Putting on the breaks: regulating organelle movements in plant cells. J. Integr. Plant Biol 54:868–74 [DOI] [PubMed] [Google Scholar]
- 155.Vidali L, Burkart GM, Augustine RC, Kerdavid E, Tüzel E, Bezanilla M. 2010. Myosin XI is essential for tip growth in Physcomitrella patens. Plant Cell 22:1868–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vidali L, McKenna ST, Hepler PK. 2001. Actin polymerization is essential for pollen tube growth. Mol. Biol. Cell 12:2534–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Walker KL, Muller S, Moss D, Ehrhardt DW, Smith LG. 2007. Arabidopsis TANGLED identifies the division plane throughout mitosis and cytokinesis. Curr. Biol 17:1827–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Walter WJ, Machens I, Rafieian F, Diez S. 2015. The non-processive rice kinesin-14OsKCH1transports actin filaments along microtubules with two distinct velocities. Nat. Plants 1:15111. [DOI] [PubMed] [Google Scholar]
- 159.Wang G, Wang F, Wang G, Wang F, Zhang X, et al. 2012. Opaque1 encodes a myosin XI motor protein that is required for endoplasmic reticulum motility and protein body formation in maize endosperm. Plant Cell 24:3447–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wightman R, Turner SR. 2008. The roles of the cytoskeleton during cellulose deposition at the secondary wall. Plant J. 54:794–805 [DOI] [PubMed] [Google Scholar]
- 161.Woodhouse FG, Goldstein RE. 2013. Cytoplasmic streaming in plant cells emerges naturally by filament self-organization. PNAS 110:14132–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu S-Z, Bezanilla M. 2014. Myosin VIII associates with microtubule ends and together with actin plays a role in guiding plant cell division. eLife 3:e03498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu SZ, Ritchie JA, Pan AH, Quatrano RS, Bezanilla M. 2011. Myosin VIII regulates protonemal patterning and developmental timing in the moss Physcomitrella patens. Mol. Plant 4:909–21 [DOI] [PubMed] [Google Scholar]
- 164.Wu XS, Tsan GL, Hammer JA 3rd. 2005. Melanophilin and myosin Va track the microtubule plus end on EB1. J. Cell Biol 171:201–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu T, Qu Z, Yang X, Qin X, Xiong J, et al. 2009. A cotton kinesin GhKCH2 interacts with both microtubules and microfilaments. Biochem. J 421:171–80 [DOI] [PubMed] [Google Scholar]
- 166.Xu XM, Zhao Q, Rodrigo-Peiris T, Brkljacic J, He CS, et al. 2008. RanGAP1 is a continuous marker of the Arabidopsis cell division plane. PNAS 105:18637–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yamada M, Tanaka-Takiguchi Y, Hayashi M, Nishina M, Goshima G. 2017. Multiple kinesin-14 family members drive microtubule minus end–directed transport in plant cells. J. Cell Biol 216:1705–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou X, Meier I. 2014. Efficient plant male fertility depends on vegetative nuclear movement mediated by two families of plant outer nuclear membrane proteins. PNAS 111:11900–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu C, Dixit R. 2012. Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins. Protoplasma 249:887–99 [DOI] [PubMed] [Google Scholar]
- 170.Zhu C, Ganguly A, Baskin TI, McClosky DD, Anderson CT, et al. 2015. The Fragile Fiber1 kinesincontributes to cortical microtubule–mediated trafficking of cell wall components. Plant Physiol. 167:780–82 [DOI] [PMC free article] [PubMed] [Google Scholar]


