Abstract
Magnetoencephalography (MEG) is a non-invasive neuroimaging technique that provides whole-head measures of neural activity with millisecond temporal resolution. Over the last three decades, MEG has been used for assessing brain activity, most commonly in adults. MEG has been used less often to examine neural function during early development, in large part due to the fact that infant whole-head MEG systems have only recently been developed. In this review, an overview of infant MEG studies is provided, focusing on the period from birth to three years. The advantages of MEG for measuring neural activity in infants are highlighted (See Box 1), including the ability to assess activity in brain (source) space rather than sensor space, thus allowing direct assessment of neural generator activity. Recent advances in MEG hardware and source analysis are also discussed. As the review indicates, efforts in this area demonstrate that MEG is a promising technology for studying the infant brain. As a noninvasive technology, with emerging hardware providing the necessary sensitivity, an expected deliverable is the capability for longitudinal infant MEG studies evaluating the developmental trajectory (maturation) of neural activity. It is expected that departures from neuro-typical trajectories will offer early detection and prognosis insights in infants and toddlers at-risk for neurodevelopmental disorders, thus paving the way for early targeted interventions.
Keywords: MEG, Infant, Development, Auditory, Somatosensory, Visual
1. Introduction
Magnetoencephalography (MEG) non-invasively measures electromagnetic neural activity, with the temporal resolution of MEG limited only by the data acquisition rate, and thus allowing real-time (sub-millisecond) assessment of brain neural activity. MEG, in combination with structural magnetic resonance imaging (sMRI), provides good spatial resolution of brain activity via source localization, especially for cortical activity (Miller et al., 2007). For this reason, MEG has become a popular method for basic and clinical research in children and adults (Gaetz et al., 2014; Stufflebeam et al., 2009) as well as a clinical tool contributing to the presurgical workup of patients with epilepsy (Gaetz et al., 2014; Lewine and Orrison, 1995; Schwartz et al., 2010). Despite its potential for contributing to our understanding of early brain development, the use of MEG with infants is still limited. The purpose of this review is to summarize the existing infant MEG literature and describe how recent advances in hardware and analysis methods will facilitate future research. We begin by outlining advantages of MEG for functional neuroimaging research studying cognitive and perceptual processes in awake infants. The existing infant MEG literature is then summarized, first describing the hardware and then reviewing published auditory, somatosensory, visual and resting-state infant studies. Finally, future directions are discussed, with a focus on the use of MEG to better understand infant brain maturation in typical as well as clinical infant and toddler populations (see Box 2).
Box 2. Future Directions in Infant MEG Research.
Longitudinal studies examining the maturation of neural activity
MEG and structural MRI studies examining associations between brain function and brain structure
Distributed source modeling to measure activity in brain areas outside primary/secondary eloquent cortex
Examining whole-brain functional connectivity in resting-state and during cognitive tasks
Continued development of whole-head pediatric MEG systems with features such as flexible helmet size or ‘wearable’ systems
1.1. Why electrophysiology in infants?
Although the infant MEG literature is relatively small, its electrical counterpart electroencephalography (EEG) has been an important clinical and research tool for many decades. As detailed in this issue, the EEG literature has demonstrated the utility of electrophysiological techniques for characterizing the maturation of neural activity in infants and toddlers. For instance, EEG studies have demonstrated rapid change in the morphology and latency of auditory evoked potentials (AEPs) over the first months and years of life (Barnet, 1975; Ohlrich et al., 1978; Kurtzberg et al., 1984). Similar visual (Ellingson et al., 1972; Taylor and McCulloch, 1992; Webb et al., 2011; Jones et al., 2015) and somato-sensory (Boor and Goebel, 2000; Desmedt et al., 1976) EEG studies have been conducted. Although the majority of infant EEG research has focused on time-domain responses (e.g., averaged evoked responses), some work has employed time-frequency analyses, examining the trial-to-trial pattern of neural oscillatory activity across a range of frequencies from low-frequency delta (1–3 Hz) and theta (3–6 Hz) to alpha (6–9 Hz), beta (12–30 Hz), and gamma activity (30–50 Hz) (for reviews, see (Bell, 1998; Bell and Cuevas, 2012; Saby et al., 2016)). Note that the frequency “bands” in infant electrophysiology do not match entirely with the canonical adult ranges due to developmental changes in peak frequencies, with this trajectory itself being of potential diagnostic/prognostic utility.
1.2. Why MEG?
Functional magnetic resonance imaging (fMRI) and EEG have been widely used to measure brain activity in younger populations. However, fMRI is challenging on a practical level due to the loud noises associated with MRI as well as the need for the infant to remain motionless during the scan. As a result, infant fMRI studies are typically performed during natural sleep to examine resting-state networks (Cao et al., 2017; Smyser et al., 2011), and thus with limited ability to directly study brain functions during tasks (e.g., face-recognition or language processes).
Compared to fMRI, EEG and MEG are silent and less restrictive, providing an environment better suited for examining sensory, cognitive and social brain processes in awake infants (although the challenges of complex cognitive paradigms and behavioral responses remain). Although EEG has been instrumental in advancing our understanding of brain activity in infants, EEG analyses are typically limited to grand averages of sensor-level responses across subjects, with sensor-based analyses providing limited information regarding the spatial aspects of brain activation and often hiding real within- and between-subject effects (Edgar et al., 2003, 2017). For example, many early auditory EEG studies with infants reported data obtained solely from midline EEG sensors (e.g., Cz) and thus were unable to separately examine left and right hemisphere activity, an analysis strategy of concern given that many studies show different rates of maturation for the left and right hemisphere (e.g. (Edgar et al., 2015a; Edgar et al., 2015b; Edgar et al., 2015c),). More recent infant EEG studies have utilized high-density EEG caps, providing whole-head coverage and the capability to study hemispheric differences, given that appropriate analysis strategies are adopted (e.g. surface Laplacians). Nonetheless, as described below, source localization for EEG remains more challenging than MEG.
Compared to EEG, MEG is less sensitive to conductivity differences between the brain, cerebral spinal fluid, skull, and scalp, and thus, for source localization, MEG is often preferred (Hämäläinen et al., 1993). For source localization with infants, MEG offers an additional advantage in that it is much less sensitive to distortions of the volume current caused by incompletely developed (i.e., open) fontanels and sutures and thus, inaccurate modeling of path conductivity, and thus inaccurate estimates of neural generators (Lew et al., 2013). Furthermore, for measuring cortical auditory activity, MEG is often preferred as the superior temporal gyrus (STG) auditory generators are favorably positioned to provide distinct measures of left and right STG activity given MEG’s preferential sensitivity to superficial tangentially-oriented neural currents and thus spatially-separated left and right auditory neuromagnetic fields (Edgar et al., 2003), even in infants (Paetau et al., 1995; Huotilainen et al., 2008). Given hemispheric differences in the rate of auditory cortex maturation (Edgar et al., 2013, 2014a), electrophysiology analysis methods that do not distinguish left and right auditory cortex activity can fail to show true brain maturation changes (Edgar et al., 2017).
Despite its advantages, like all neuroimaging techniques, MEG has limitations. For instance, MEG is mainly sensitive to the currents flowing tangential to the surface of the scalp (Williamson and Kaufman, 1990), and thus sulcal brain activity is ‘highlighted’. EEG, in contrast, is sensitive to both radial and tangential currents. Furthermore, depth sensitivity (for both EEG and MEG) is limited by the inverse square law and is thus critically dependent on the distance between source and detectors. Whereas the distance between the brain and recording sensors is inevitably further for MEG than scalp-placed EEG electrodes, the recent development of smaller-scale MEG hardware reduces the distance between brain sources and the MEG sensors, improving the intrinsic sensitivity of these systems for infant and young child use compared to adult helmet sensor arrays. Nonetheless, MEG source estimation is usually used to resolve neuronal activity 0.5–2 cm in the cerebral cortex (Dale and Sereno, 1993; Dale et al., 2000; Gramfort et al., 2013; Ou et al., 2009) with the MEG signal tending to emphasize activity from superficial cortical regions over activity from deeper structures (this may not always be a disadvantage as it provides a method of intrinsically reducing sensor-level contamination from distant brain sources). However, it is of note that with adequate experimental design and data analyses, MEG studies have demonstrated the ability to record activity from deeper brain structures. For example, auditory brainstem responses (ABRs), clinically recorded by EEG to study the integrity of auditory pathways, can be measured using MEG (Coffey et al., 2016; Parkkonen et al., 2009).
Given the above EEG and MEG differences, it is often recommended to record MEG and EEG simultaneously to obtain a more comprehensive representation of brain activity, with several adult studies reporting more accurate source estimates for combined MEG + EEG than either modality alone (Parkkonen et al., 2009; Molins et al., 2008; Sharon et al., 2007). A recent modeling study also suggests that the addition of EEG data would be beneficial for MEG source localization in infants (Lew et al., 2013), with recent studies demonstrating the feasibility of whole-head MEG + EEG recording in infants (Bakhireva et al., 2015; Stephen et al.,2018). It is noted, however, that EEG þ MEG studies are more difficult to conduct than using EEG or MEG alone from a practical implementation point of view.
2. MEG systems
Previous papers have described MEG technology and hardware (infant and adult) and readers with an interest in these topics are directed to such papers (Hämäläinen et al., 1993; Roberts et al., 2014; Okada et al., 2006, 2016). Text in this section focuses on general differences between infant and adult MEG systems as well as the evolution of infant MEG systems and the opportunities infant MEG systems afford.
Many existing infant studies have used adult MEG systems due to their more widespread availability (Table 1). A limitation of this approach, however, is the fixed dimension of the adult MEG helmet, resulting in a large distance between active neural circuits of the brain and the MEG detectors in infants and young children (the “small head” problem). As the strength of the magnetic field associated with neural activity decays rapidly (1/distance2) (Hämäläinen et al., 1993), the use of adult MEG systems results in a less than optimal, and sometimes even inadequate, signal-to-noise ratio for infant MEG recordings (Gaetz et al., 2008).
Table 1.
Summary of infant MEG studies by focus of research.
| Paper | Age(s) Studied | Stimuli | System |
|---|---|---|---|
| Auditory | |||
| Paetau et al., 1995 | 0.3–15 years | Tones, non-words | dc-SQUID |
| Lengle et al., 2001 | Fetuses, neonates | Tones | Magnes |
| Huotilainen et al., 2003 | Neonates | Tones | Elekta |
| Cheour et al., 2004 | Neonates | Tones | Elekta |
| Lutter et al., 2004 | 1.5–8.5 weeks | Tones | Magnes |
| Holst et al., 2005 | Fetuses, neonates | Tones | SARA |
| Draganova et al., 2005 | Fetuses, neonates | Tones | SARA |
| Sambeth et al., 2006 | Neonates | Tones | Elekta |
| Lutter et al., 2006 | 0–6 months | Tones | Magnes |
| Draganova et al., 2007 | Fetuses, neonates | Tones | SARA |
| Govindan et al., 2008 | Fetuses, neonates | Tones | SARA |
| Sambeth et al., 2009 | Neonates | Tones | Elekta |
| Sheridan et al., 2010b | Fetuses, neonates | Tones | SARA |
| Muenssinger et al., 2013b | Fetuses, neonates | Tones | SARA II |
| Schleger et al., 2014 | Fetuses, neonates | Tones | SARA |
| Edgar et al., 2015b | 6–59 months | Tones | Artemis 123 |
| Stephen et al., 2017 | 6–68 months | Tones |
BabySQUID Elekta |
| Speech/Language | |||
| Kujala et al., 2004 | Neonates | Vowels | Elekta |
| Pihko et al., 2004a | Neonates | Syllables | Elekta |
| Imada et al., 2006 | Neonates, 6 & 12 months | Tones, chords, syllables | Elekta |
| Sambeth et al., 2008 | Neonates | Singing, speech | Elekta |
| Travis et al., 2011 | 12–18 months | Words, pictures | Elekta |
| Bosseler et al., 2013 | 6 & 12 months | Native, non-native syllables | Elekta |
| Kuhl et al., 2014 | 7–12 months | Native, non-native syllables | Elekta |
| Hartkopf et al., 2016 | Fetuses, 0–3 months | Syllables | SARA II |
| Zhao and Kuhl, 2016 | 9 months | Music, non-words | Elekta |
| Ferjan Ramirez et al., 2017 | 11 months | English & Spanish syllables | Elekta |
| Somatosensory/Motor | |||
| Gondo et al., 2001 | 6–21 months | Tactile | Magnes (Nevalainen et al., 2008b) |
| Pihko et al., 2004b | Neonates | Tactile | Elekta |
| Pihko et al., 2005 | Neonates | Tactile, median nerve | Elekta |
| Lauronen et al., 2006 | Neonates, 6 months, adults | Tactile, median nerve | Elekta |
| Nevalainen et al., 2008a | Neonates | Tactile | Elekta |
| Nevalainen et al., 2008b | Term & preterm neonates (tested at term age) | Tactile | Elekta |
| Pihko et al., 2009 | Neonates, 6–18 months | Tactile | Elekta |
| Neonates | Tactile | Elekta | |
| Nevalainen etal., 2012 | |||
| Rahkonen et al., 2013 | Term & preterm neonates (tested at term age) | Tactile | Elekta |
| Nevalainen etal., 2015 | Term & preterm neonates (tested at term age) | Tactile | Elekta |
| Berchicci et al., 2011 | 11–47 weeks, 2–5 years, adults | Squeezing a pipette | BabySQUID |
| Berchicci et al., 2015 | 11–47 weeks, 2–5 years, adults | Squeezing a pipette | BabySQUID |
| Meltzoff et al., 2018 | 7 months | Tactile | Elekta |
| Visual | |||
| Sheridan et al., 2008 | Fetuses, neonates | Light flashes | SARA |
| Matuz et al., 2012 | Fetuses, 2–5 weeks | Light flashes, tones | SARA |
| Multisensory | |||
| Pihko et al., 2011 | Neonates | Tactile, vowels | Elekta |
| Kivisto et al., 2015 | Neonates | Tactile, vowels | Elekta |
| Spontaneous/Resting | |||
| Haddad et al., 2006 | Neonates | Spontaneous (sleep, awake) | SARA |
| Lutter et al., 2006 | 0–6 months | Spontaneous (sleep) | Magnes |
| Wakai and Lutter, 2016 | 1–6 months | Spontaneous (sleep) | Magnes |
| Sanjuan et al., 2016 | 6 months | Spontaneous (awake) | Elekta |
| Stephen et al., 2018 | 6 months | Rest & squeezing a pipette | Elekta |
| Methods/Technical Reports | |||
| Okada et al., 2006 | 7 months | Spontaneous, tactile | BabySQUID |
| Johnson et al., 2010 | 4 years | Broadband noise | KIT |
| Hirata et al., 2014 | Not specified | Mother-child interactions | KIT |
| Roberts et al., 2014 | 14,18, & 48 months | Spontaneous, tones | Artemis 123 |
| Okada et al., 2016 | 3 years | Spontaneous, tones | BabyMEG |
Dedicated pediatric MEG systems are highlighted in bold text.
To address this issue, infant studies using adult MEG systems often position the infant’s head in the MEG helmet so that the brain area of interest is as close to the sensors as possible. This is achieved by placing foam padding inside the MEG helmet to push the infants’ head to one side of the helmet, or by placing the scanner in a supine position, thus allowing the investigator to rest one side of the infants’ head directly on the helmet’s inner surface (see Fig. 1B and Pihko et al. (2009)). With either of these methods, if recordings from both hemispheres are desired, then the infant must be repositioned and the study repeated. Although this approach allows researchers to obtain information from both hemispheres, observed hemispheric differences may be due to confounding variables such as vigilance and/or habituation effects (Kujala et al., 2004). This approach also doubles measurement time and severely impacts whole-brain distributed source localization as well as functional connectivity analyses.
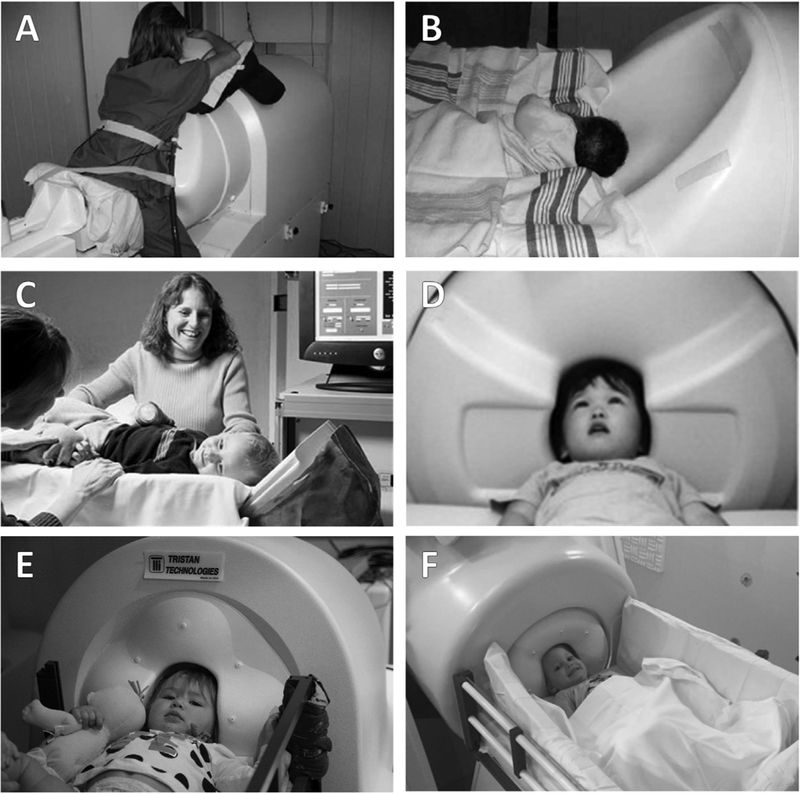
Fig. 1.
Photos of existing pediatric MEG systems. (A) SARA system (VSM Med Tech Ltd.) being used for fetal MEG recording. (B) SARA system with cradle attachment adapted for infant recording (both images from Holst et al., 2005). (C) BabySQUID system with partial head coverage (Tristan Technologies Inc.) (D) KIT 151-channel whole-head system (Model PQ1151R; Yokogawa/KIT; image adapted from Kikuchi et al., 2013). (E) Artemis 123 whole-head system (Tristan Technologies Inc.). (F) Baby-MEG 375-channel whole-head system (Tristan Technologies Inc.).
Whereas most studies employing an adult MEG system have attempted to position infants’ heads against one side of the sensor array, others have successfully obtained whole-head infant MEG data with the infant’s head placed in the center of an adult MEG helmet (Bosseler et al., 2013; Kuhl et al., 2014; Zhao and Kuhl, 2016; Travis et al., 2011), with continuous head tracking and head movement compensation employed to correct for head motion (Taulu et al., 2004; Larson and Taulu, 2017). However, due to the distance between the infants’ head and the helmet, the signal-to-noise ratio is reduced. As a result, source localization accuracy may be compromised, thus limiting research studies or clinical examinations examining activity from deeper brain regions or from weaker generators.
2.1. Customized Infant and Young Child MEG Systems
To address the above limitations, MEG systems customized for children have been developed (Roberts et al., 2014; Okada et al., 2006, 2016; Johnson et al., 2010). These systems are designed to reduce the distance between the infant’s brain and the MEG detectors, therefore providing greater sensitivity and spatial resolution when measuring infant brain activity (Okada et al., 2006). In addition to the distance between the infant’s head and the helmet, another issue with many adult MEG systems is the distance between the helmet surface and the MEG sensors (the “inside the dewar” problem), with most adult systems including an additional displacement of the MEG sensors from the helmet inner surface (~1.5–2 cm). Ultimately, the distance between brain-source and MEG detection-coil (thus brain-to-helmet + helmet-to-coil distances) greatly impacts the accurate detection, characterization, and localization of neural activity in infants. To this end, a few of the dedicated infant systems employ a coil-in-vacuum configuration that allows placement of the detection-coils (sensors) as close as 6 mm from the helmet surface, thereby providing a substantial increase in brain signal amplitude at the sensor level than a MEG system with sensors immersed in liquid helium and with sensors constrained to be a greater distance from the helmet surface.
The first specialized infant and fetal MEG system was the SQUID Array for Reproductive Assessment (SARA; VSM MedTech Ltd., Port Coquitlam, Canada), a system designed to record brain activity from fetuses as well as neonates and infants. The original SARA had 151 first order gradiometers arranged in a concave array ‘holding’ the pregnant woman’s abdomen (the system was recently upgraded to 156 sensors; SARA II). For measuring fetal activity, an ultrasound is performed before or after MEG acquisition to estimate the location of the fetus inside the mother’s abdomen (Fig. 1a). SARA can also be used to record brain activity from infants by attaching a cradle to the system and resting the infant’s head on the ‘helmet’ surface (see Fig. 1b). Due to the shape of the sensor array, to achieve whole-head coverage, at least three recordings must be performed, one for each hemisphere and one for the vertex. As described below, studies using SARA have followed infants longitudinally from the fetal stage to infancy.
Shortly after SARA, BabySQUID (Tristan Technologies Inc., San Diego, California, US) was designed for use with neonates and infants (Fig. 1c). The BabySQUID system, originally installed at the University of New Mexico, consists of 76 first-order axial gradiometers and five reference magnetometers (for technical details, see Okada et al. (2006)). BabySQUID uses the coil-in-vacuum design to reduce distance between the sensors and scalp. The helmet, which does not attempt to provide whole-head coverage, was based on a standard reference for the head size of babies, and has an ellipsoidal shape with a radius of curvature of 7.5 cm along the coronal section and 10 cm along the sagittal section. BabySQUID is equipped with a head-localization system (IR camera) to measure head position throughout the scan.
Although the development of these early pediatric systems represented a significant advance, both the SARA and the BabySQUID permitted recording from only one hemisphere at a time. Later MEG systems addressed this issue by developing whole-head infant systems. In 2009, the Kanazawa Institute of Technology (KIT) designed the first whole-head infant and young child MEG system (Johnson et al., 2010) (Fig. 1d). The shape of the helmet is similar to that of adult systems, albeit with a smaller circumference (53.4 cm) designed to fit >90% of 5-year--old boys and >95% of 5-year-old girls (based on normative data of Caucasian children in the US). The original KIT system consisted of 64 channels (Model PQ1064R-N2m) and has since been upgraded to 151 channels (Model PQ1151R). Although described as an infant and child MEG system, to date, most studies using the KIT system have focused on children pre-school age (~3 years) and older (e.g. (He et al., 2014; Yoshimura et al., 2013; Remijn et al., 2014),).
Two other whole-head infant MEG systems have been recently developed. The Artemis 123™ (Tristan Technologies Inc., San Diego, California, US) was designed for use with children from birth to 3 years of age (Roberts et al., 2014). This system, located at the Children’s Hospital of Philadelphia (Fig. 1e), has 123 sensors (first-order axial gradiometers) and a helmet circumference of 50 cm, which corresponds to the median head circumference of a 3-year-old in the US. Similar to the BabySQUID, the Artemis 123 employs a coil-in-vacuum sensor configuration to minimize the distance between the helmet surface and sensors (6–9 mm). Artemis 123 is equipped with continuous head tracking as well as EEG amplifiers to allow for simultaneous MEG-EEG recordings.
The most recently developed infant MEG system is BabyMEG (Tristan Technologies Inc., San Diego, California, US), located at Boston Children’s Hospital (Fig. 1f). BabyMEG incorporates a high-density array of 375 sensors (all magnetometers) distributed across two levels, the first level consisting of 270 sensors and the second level with 105 sensors (Okada et al., 2016). Helmet circumference is 52 cm in order to fit children up to 4 years of age. As with BabySQUID and Artemis 123, Baby-MEG incorporates a coil-in-vacuum system, with the sensor-to-scalp distance ranging 6–11 mm. BabyMEG also includes continuous head-tracking and a closed-cycle helium recycler, thus eliminating the costs and labor associated with helium transfer.
Finally, although not exclusively for infants, it is of note that progress is being made in the development of a portable MEG system using optically-pumped magnetometers (OPMs) (Boto et al., 2017, 2018). OPMs (previously referred to as atomic magnetometers) measure the transmission of laser light through a vapor of spin-polarized atoms (typically rubidium), providing a highly sensitive measure of the local magnetic field. Using commercialized single-unit OPMs, multi-channel arrays of OPMs can be mounted on a 3D-printed head-cast (Boto et al., 2017, 2018), leading to a wearable OPM device (Boto et al., 2018). In addition to allowing free and natural movement, wearable OPM devices can be optimized to the subject’s head size, thus resulting in minimal distance between the detectors and the scalp. Wearable OPM devices also provide more accurate sensor position and orientation, thus allowing more precise source imaging (Boto et al., 2017, 2018). There are, however, technical difficulties to overcome before OPM systems can be used in infants. For example, in its current form, the wearable OPM device is too physically cumbersome for use with infants and young children. In addition, the current design covers the face and may be uncomfortable for infants. The use of OPMs in infant research would greatly benefit from development of a more compact device that is similar to the size and fit of an EEG cap. Furthermore, current OPM systems require extensive ambient magnetic field nulling (to approximately the nT level), currently achievable over a limited (~40 cm) spatial extent. Progress in this domain will enhance opportunities for utilization.
3. Review of existing studies
In this section, we survey MEG publications involving infants. For this review, “infants” were considered to be 0–36 months of age. A PubMed search was conducted (last search May 22, 2018) using a combination of the following keywords: “magnetoencephalography” or “MEG”, AND “infant” or “neonate”, or “development”. Publications not otherwise identified in PubMed were also included. Papers were excluded if they were not written in English, purely clinical in nature (e.g., application of MEG for identifying seizure activity), or focused exclusively on fetal MEG (for reviews of fetal MEG, see (Anderson and Thomason, 2013; Lowery et al., 2006; Sheridan et al., 2010a)). Table 1 lists papers meeting these criteria. The following paragraphs highlight studies most relevant to a key issue of this review – direct examination of neural generator activity, thus obtaining measures in source rather than sensor space.
3.1. Studies of auditory processes to simple stimuli (tones)
EEG studies have described the development of the auditory system across the lifespan. Using EEG, Sharma et al. (1997) noted that although the development of the peripheral auditory system is completed in early childhood (Brown et al., 2000; Eggermont et al., 1991; Ponton et al., 1992), the central auditory pathways exhibit anatomical and physiologic changes through early adulthood (Kraus et al., 1985; Huttenlocher, 1979), with a progressive decrease in the latency of auditory responses from infancy to late adolescence. In another demonstration, cross-sectional findings from Barnet et al. (Barnet, 1975) showed that over a 3-year period (from 10 days to 37 months of age) the latency decrease for the auditory P2 response was 75 ms and for the auditory N2 response was 215 ms. Longitudinal studies examining auditory responses in infants showed similar latency changes (Ohlrich et al., 1978; Novak et al., 1989; Kushnerenko et al., 2002; Choudhury and Benasich, 2011). A limitation of the above studies - the majority conducted in the 1970s and 80s – is that brain activity was typically measured only from midline EEG sensors.
MEG studies have extended these findings by tracking the development of primary sensory auditory responses before and after birth (using the SARA fetal MEG system). As shown in Table 1, several studies have utilized the SARA to examine auditory responses in fetuses and very young infants, with these studies focusing on developmental changes in the latency of auditory evoked response. These studies have reported a steady decrease of P2m (magnetic analogous of auditory P2 response) latency as a function of gestational age (Lengle et al., 2001; Draganova et al., 2005, 2007; Holst et al., 2005; Govindan et al., 2008; Hartkopf et al., 2016; Sheridan et al., 2008), as well as a larger percentage of detected auditory components in neonates than fetuses. Such studies are unique to MEG, as comparable EEG studies would be impractical due to the sensitivity of the EEG signal to distortion by amniotic fluid and layers of skin and muscle. Of course, without the knowledge of the fetal head position at the time of the recording, it is not possible to separately examine left and right auditory cortex activity. Immediately after birth, other studies have shown that the latencies of P2m response are stable for the first 8 weeks of life (using 37-channel Magnes (4D Neuroimaging, Inc)), and then rapidly change between 40 and 54 weeks (from 250 ms to 150 ms) (Lutter et al., 2006).
Studies with older infants and children have shown that the latency of the auditory evoked field (AEF) continues to decrease for several years after birth, albeit at a slower rate than over the first few months of life (Edgar et al., 2015b; Stephen et al., 2017), and with whole-head MEG studies examining activity in source space and separately examining left and right auditory cortex activity (Yoshimura et al., 2012, 2013). As an example, Edgar et al., 2015b, 2015d used the infant MEG Artemis 123™ to examine left and right auditory cortex neural activity in infants and young children 6– to 59-months. Cross-sectional analyses showed the expected decrease in the latency of the P2m response as a function of age, with Edgar et al. (2015b) showing a latency decrease of ~0.6 ms/month observed for P2m (i.e., 7.2 ms/year).
3.2. Studies of sound change-detection
In addition to assessing basic auditory encoding processes, MEG studies have examined the ability of infants to detect deviations from regularities in the sound environment by comparing responses to ‘standard’ (frequently repeated) and ‘deviant’ auditory stimuli (Huotilainen et al., 2008). These studies focused on a neural marker of change detection called the mismatch negativity (MMN, or its magnetic counterpart MMNm or MMF), which is determined by subtracting the response to standard stimuli from the response to deviant stimuli (Naatanen et al., 1978). Another endogenous response to deviant sounds is called late discriminative negativity (LDN or LDNm), a response that occurs 300 ms–600 ms after stimulus onset (Draganova et al., 2005, 2007; Cheour et al., 2004; Huotilainen et al., 2003). The sources of MMNm and LDNm have been reported to be different (Huotilainen et al., 2003), suggesting that multiple cortical areas are involved in change detection. Examining mismatch responses in fetuses, Draganova et al. (2007) showed that auditory mismatch responses to changes in sound frequency can be detected in utero as early as 28-weeks gestational age. Other studies have reported MMNm responses in fetuses during the third trimester of pregnancy (Sheridan et al., 2008; Muenssinger et al., 2013a, 2013b), providing further evidence that auditory discrimination begins before birth. Studies evaluating MMNm in the newborn’s brain (Cheour et al., 2004; Huotilainen et al., 2003; Sambeth et al., 2006, 2009) have focused on the morphology of MMNm responses and the ability of neonates to detect different types of deviant sounds using modified oddball paradigms. For example, Sambeth et al. (2006) reported two deflections in the MMNm response, one at ~345 ms and a second at ~615 ms. They found stronger responses to novel (natural sounds) stimuli compared to deviant (tone) stimuli (with both being stronger than responses to the standard (tone) token) for the earlier MMNm, but no difference between novel and deviant stimuli for the later MMNm, suggesting these components have different neural mechanisms of auditory change detection and discrimination. Sambeth and colleagues later used multiple deviants (frequency, duration, intensity, and gap) in one oddball paradigm and found that neonates are able to detect up to four types of deviances within a sound stream (Sambeth et al., 2009).
In addition to using simple auditory stimuli (e.g., tones) to study an infants’ ability to detect change, an increasing number of MEG studies have investigated infant MMNm using stimuli with language components. Studies in this area have focused on establishing the feasibility of using MEG to localize speech processes in infants, examining MMNm responses to changes in vowel quality (Kujala et al., 2004) (e.g., [a] vs.[i]) and syllables (Pihko et al., 2004a). Pihko et al. (2004a) further noted the amplitude of the responses were higher in quiet than in active sleep, indicating the importance of monitoring sleep stages when recording responses to speech sounds from sleeping infants.
3.3. Studies of speech and language processing
Most of the MEG studies examining how language is processed during the first year of life have focused on localizing language-related brain regions (Kuhl et al., 2014; Zhao and Kuhl, 2016; Travis et al., 2011; Ferjan Ramirez et al., 2017; Imada et al., 2006). These studies have used distributed source modeling approaches – minimum norm estimation (MNE; 25) or standardized low resolution electromagnetic tomography (sLORETA (Pascual-Marqui, 2002);)– to characterize patterns of brain activation beyond primary sensory (in this case auditory) cortex in infants. For instance, Imada et al. (2006) showed that passive listening to speech sounds activates not only auditory cortex but also areas involved in speech production, specifically Broca’s area, by 6 months of age. Kuhl and colleagues (Kuhl et al., 2014) also found that passive listening to speech sounds activates motor areas (Broca’s area, cerebellum) in infants, with the magnitude of activation of these areas being sensitive to the native versus non-native nature of these sounds by 11–12 months. In another study by the same group, Ferjan Ramírez and colleagues (Ferjan Ramirez et al., 2017) reported that Spanish-English bilingual but not English monolingual 11-month-old infants activated prefrontal and orbitofrontal areas during passive listening of speech sounds. These results are consistent with a growing literature suggesting that dual language exposure in infancy may support the development of executive function skills (Kovacs and Mehler, 2009a, 2009b; Estes and Hay, 2015).
Another way to examine speech and language processing in infants is to study activity to speech stimuli in specific frequency bands via time-frequency analysis. Bosseler et al. (2013) examined activity in the theta band (4–8 Hz) at the sensor level during passive listening of native and non-native speech sounds in 6- and 12-month-old infants. Although no differences in theta power were observed for native and non-native syllables in the younger infants, the older infants showed larger theta power for native syllables, interpreted as reflecting more attention to native stimuli. A limitation of this study, noted by the authors, was that individual infant MRIs were not obtained and thus distributed source modeling of theta activity was not attempted.
Looking to the future, studies are needed that examine associations between brain neural function and brain structure to better understand the mechanisms that support auditory cortex maturation and the development of language. Studies examining auditory encoding processes in older children provide support for this line of research. For example, in an MEG + MRI study with older children (age 6–16 years), Roberts et al. (2013) showed in typically developing children that greater acoustic radiation white-matter fractional anisotropy was associated with earlier M50 auditory latencies, and with a loss of this function-structure association in children with autism spectrum disorder (ASD). Given significantly delayed M50 latencies in the children with ASD, these findings suggested that group differences in the maturation of myelination early in life may contribute to auditory latency delays in ASD. Although, as previously noted, maturation of auditory M50 responses has been demonstrated using MEG in infants and young children (see Fig. 3 and Edgar et al. (2015b)), acquisition of motion-free diffusion tensor imaging in this age group presents challenges. Nonetheless, many investigators use hypotheses generated in the multimodal developmental trajectories of older children to help account for the electrophysiological measures obtained in younger children. Future, multimodal studies in infants will provide a more direct understanding of the maturation of brain activity in typically developing populations as well as in infants with neurodevelopmental disorders.
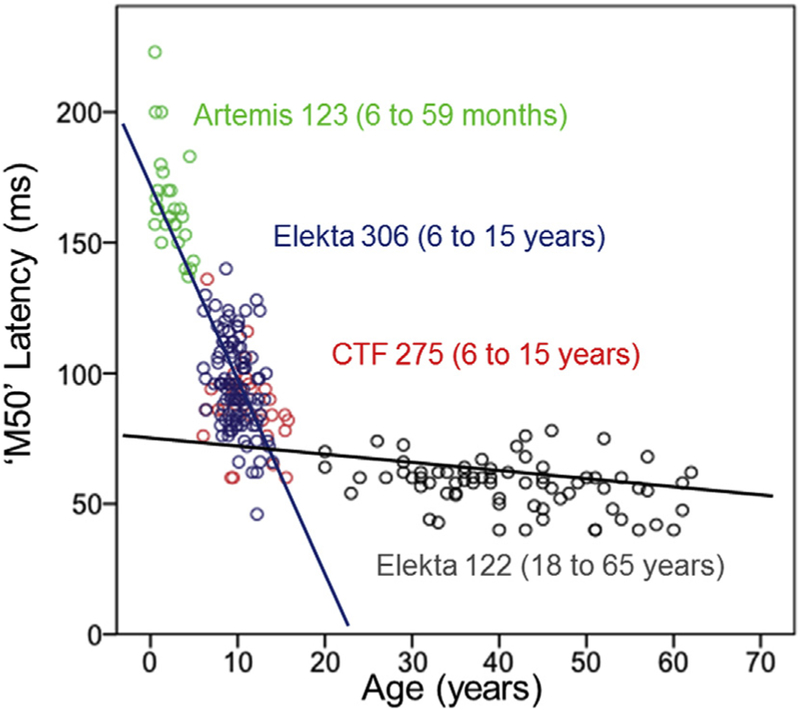
Fig. 3.
Scatterplot of auditory M50 latencies from 6-month-old to 65-year-old subjects. The analogies between infant and child and adult morphologies in auditory evoked fields permit assessment of the lifespan trajectory of component latencies. This response is robustly observed across different hardware platforms with findings suggesting a continuous shortening of “M50” latency from infancy to adulthood. The absence of discontinuity indicates the potential to track a component latency across the lifespan despite differing hardware, offering potential for multicenter implementations.
3.4. Studies of somatosensory processes
Although studies with adults commonly use electrical stimulation (e.g., median or tibial nerve) to characterize primary/secondary somatosensory cortex activity, studies with infants typically employ pneumatic tactile stimuli as these stimuli are painless and also allow resolution of cortical receptive fields of individual digits. In addition, pneumatic stimuli do not produce the stimulus-related artifacts that accompany electrical stimulation, with such artifacts particularly large in infants due to the close proximity of the stimulating electrodes to the MEG sensors.
Somatosensory evoked fields (SEFs) in newborns have been described in a series of studies in which tactile stimulation was applied to the fingertip of sleeping infants positioned against one side of a conventional adult-sized MEG helmet (Pihko et al., 2009; Lauronen et al., 2006; Nevalainen et al., 2008a, 2012; Pihko and Lauronen, 2004). These studies have observed a broad U-shaped deflection around 60 ms, with source modeling of this component revealing an anteriorly-oriented dipolar source in the contralateral primary somatosensory cortex (SI). The simplicity of the newborn response contrasts with the SEF in adults, in adults characterized by two deflections from SI – an initial component with an anteriorly-oriented dipolar source (i.e., a relatively focal neural generator) and a second and notably more prominent component with a posteriorly-oriented dipolar source. The reason for the absence of an analogous posteriorly-oriented component in newborns is not clear. One possibility is that this component relies on GABAergic inhibitory processes, which are still maturing in infants (Nevalainen et al., 2014). It also possible that the cortico-cortical connections necessary for producing adult-like responses are absent or undeveloped in newborns.
In addition to describing early response from SI, several newborn studies have capitalized on the spatial resolution afforded by MEG to localize and measure activity from secondary somatosensory cortex (SII). These studies have suggested that the SII response, which peaks at around 200 ms in newborns, may be valuable as a prognostic tool for predicting outcomes in infants at risk for neurodevelopmental problems such as those born prematurely (Nevalainen et al., 2015; Rahkonen et al., 2013) or with prenatal drug exposure (Kivisto et al., 2015).
Most MEG studies examining somatosensory processing in infants have focused on newborns, although a few papers have considered SEFs in older infants (Pihko et al., 2009; Gondo et al., 2001; Meltzoff et al., 2018). In recent work, Meltzoff et al. (2018) examined SEFs to tactile stimulation of the hands and the feet of 7-month-old infants. They observed two main components in the SEF response, peaking around 100 ms and 250 ms, with earlier (~55 ms) activation observed in a subset of participants. Source modeling of these components suggested that they originated in SI, with the sources for the hand more lateral than the foot, findings in accordance with the somatotopic organization of the somatosensory cortex. In a second study from the same group, distributed source localization analyses showed that these same areas were also activated when infants were merely observing another person’s hands and feet being touched. In addition to examining evoked responses, Meltzoff et al. (2018) also reported enhanced power in the beta band (12–18 Hz) in hand and foot regions of the somatosensory cortex during the observation of hand and foot touch, respectively.
Few other studies with infants have considered event-related changes in sensorimotor brain rhythms, although examination of post-stimulus changes in oscillatory rhythms is common in MEG studies of somatosensory and motor functions in older children and adults (Gaetz and Cheyne, 2006; Kurz et al., 2014; Jones et al., 2010). Across two studies with infants 11–47 weeks of age, Berchicci and colleagues examined suppression of the sensorimotor mu rhythm during a prehension task (squeezing a pipette) relative to rest (Nevalainen et al., 2014, 2015). These studies focused on developmental changes in mu-rhythm peak frequency as well as in the functional connectivity and organization of the sensorimotor network. Analyses for these two studies, however, were limited to sensor space given that anatomical information was not available for individual participants.
3.5. Studies of visual processes
Whereas infant visual responses to simple (e.g., checkerboard) or social (e.g., face) stimuli have been studied using EEG (Halit et al., 2003, 2004; Siper et al., 2016; de Haan et al., 2003; de Haan and Nelson, 1999), there are only a few studies using MEG to study primary visual evoked fields (VEFs) or evoked responses to face stimuli in young children. To our knowledge, only two studies have examined habituation of visual response in fetuses and newborns using a train of light flashes (Sheridan et al., 2008; Matuz et al., 2012). These studies showed decreased neonatal VEFs to successive light flashes only when the light flashes were followed by a long break (inter-stimulus interval) or a deviant stimulus (e.g., sound). Although neuroimaging studies examining visual processes or socio-cognitive processes using visual stimuli (e.g., face) have been done in pre-school or school-age children (see He et al. (2014) and review in Taylor et al. (2012)), MEG studies examining how infants process social visual stimuli have not yet been conducted.
3.6. Studies of spontaneous MEG
Whereas most MEG infant studies have examined neural activity in response to a sensory event, a few studies have examined spontaneous, ongoing brain activity. Early work focused on demonstrating the feasibility of recording and characterizing brain rhythms in sleeping and waking infants using MEG (Lutter et al., 2006; Haddad et al., 2006). More recent studies have utilized spontaneous brain recordings to address novel questions regarding brain development in the first weeks and months of life. In one such study, Wakai et al. (Wakai and Lutter, 2016) analyzed MEG recordings from sleeping infants to elucidate the role of slow rhythms in the formation of sleep spindles. In other work, Sanjuan and colleagues (Sanjuan et al., 2016) documented a positive correlation between left anterior theta power and maternal perinatal post-traumatic stress disorder (PTSD) severity, suggesting delayed cortical maturation in infants born to mothers with PTSD. Stephen and colleagues (Stephen et al., 2018) recently examined the utility of MEG for assessing brain function in infants prenatally exposed to alcohol. They found broadband increased spectral amplitude in the resting MEG of these infants, most prominent in left anterior and posterior temporal regions. Furthermore, the extent of spectral amplitude was correlated with an estimate of the number of drinks during pregnancy, suggesting that broadband spectral amplitude may be a useful marker of prenatal alcohol exposure. Of note, some of these studies focused on sensor-level data due the absence of MRIs from individual participants. Recent advances in MEG analysis, particularly in the use of age-matched MRI templates in place of individuals MRIs (Akiyama et al., 2013), will facilitate analyses of spontaneous brain activity in source space, thereby providing greater spatial information as well as allowing assessment of resting-state functional connectivity.
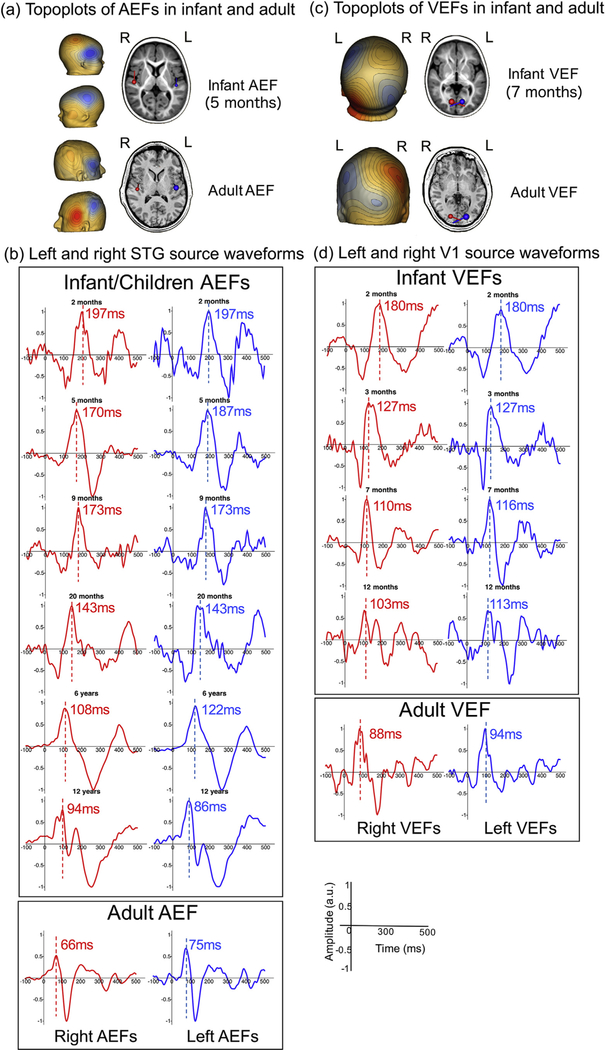
3.7. Examples of auditory and visual evoked responses in infants, school-age children, and adults
Fig. 2 shows evoked fields from primary auditory and visual areas in infants, school-age children, and adults. Data from infants were obtained using the Artemis 123 and data from school-age children and adults were obtained using a 275-channel CTF system. Fig. 2(a) and (b) shows auditory evoked fields (AEFs) from left and right STG to 500 Hz tones and their topoplots. Fig. 2(c) and (d) shows visual evoked fields (VEFs) from primary visual cortex (V1) and their topoplots to reversed checkerboard stimuli. Fig. 2 (b) shows in infants (2–20 months old) the presence of P2m (likely analogous to the adult auditory M50) responses in left and right auditory cortices, with the P2m chosen based on dipole orientation and magnetic field topography. In school-age children (6 and 12 years old) and adult participants, analogous M50 as well as M100 responses are observed bilaterally. P2m (M50) responses were observed to occur earlier in time as a function of age. This pattern is consistent with Edgar et al. (2015b) who showed earlier P2m latencies in older compared to young children (ages 6 months to 5 years). For VEFs, the first negative deflection, equivalent to the visual N1, was identified based on dipole orientation in primary visual cortex (Fig. 2 (c)). The infant equivalent of the adult visual N1 peaked at 180 ms at 2 months, with visual N1 latencies observed to be similar at 6 months, 12 months, and in adults (~100–120 ms). This pattern is consistent with the EEG literature, which has shown rapid maturation of the visual N1 response within the first 4 months of life (see 122). The above data also mirror findings reported in the literature that have indicated that auditory and visual systems mature at different rates (Edgar et al., 2015b; Lippe et al., 2009).
Fig. 2.
Examples of (a) Topoplots of auditory evoked fields (AEFs) from a representative 5-month-old infant and from an adult, (b) Source waveforms from left and right STG (c) Topoplots of visual evoked fiExamples of (a) Topoplots of auditory evoked fields (AEFs) from a representative 5-month-old infant and from an adult, (b) Source waveforms from left and right STG (c) Topoplots of visual evoked temis 123 whole-head system (Tristan rphological analogies can be seen in the infant, young children, and adult waveforms, although for auditory responses the component latencies (marked with dash lines) have strikingly longer latencies in infants. For example, the “infant M50” occurs at 197 ms for a 2-month-old infant, and the M50 latencies decrease to 108 ms in a representative 6-year-old child, and 66 ms in a representative adult. By contrast VEF latencies appear to mature faster toward the adult values compared to AEF.
4. Discussion and future directions
Research examining brain neural activity in infants using EEG is sizeable (reviewed in this issue), and studies examining patterns of brain blood flow in infants using fMRI are increasingly prominent (also reviewed in this issue). As detailed above, MEG offers a complementary and non-overlapping approach for understanding functional brain activity in infants. Here we discuss future directions for infant MEG.
Although progress has been made utilizing MEG to study the maturation of primary/secondary sensory cortices across fetal and infant development, more studies are needed. Such studies will likely increasingly use time-frequency analyses to examine changes to specific properties of infant neural circuits such as maturational changes in the trial-to-trial similarity in the phase of neural oscillatory activity to repeated presentations of the same stimuli (inter-trial coherence; ITC) or increases/decreases in neural activity with respect to a pre-stimulus baseline (event-related spectral perturbation; ERSP) (Makeig, 1993). Time-frequency analyses also allow researchers to separate the recorded signal into different frequency bands, with oscillatory activity within specific frequency bands one of the most promising candidate mechanisms associated with information processing (Basar et al., 2001; Klimesch et al., 2005; Ho et al., 2008). Frequency specific oscillations and phase-coupling are also essential mechanisms for coordinating activity from different brain regions and facilitate interactions between local and global brain areas during cognitive tasks (Buzsaki and Draguhn, 2004; Vidaurre et al., 2018). For example, whereas cortical oscillations in 30–50 Hz gamma band and networks of interconnected inhibitory interneurons (Whittington et al., 1995) are thought to reflect early states of sensory perception (Pantev et al., 1991), later beta-band (14–30 Hz) activity is thought to be associated with encoding the sensory percept (Kopell et al., 2000; Traub et al., 1999).
Although time-frequency analyses are of interest when examining brain activity in regions of a priori interest (such as left and right auditory cortex when examining auditory encoding processes), future infant MEG studies will likely apply distributed source localization to examine activity throughout the brain, and thus allowing measurement of local and long-range connectivity. Compared to connectivity analyses performed in sensor space, source-space functional connectivity analyses allow more direct interpretation of study findings and also avoid problems associated with computing functional connectivity measures at the sensor level. For instance, given that coherence (a measure of functional connectivity) between two (MEG and EEG) sensors is strongly related to the distance between the sensors (e.g., inflated by volume conduction effects at short distances), functional connectivity analyses at the sensor level must adjust functional connectivity measures to remove variability due to inter-electrode distances. As an example, as shown in one EEG coherence study in children, inter-electrode distance was found to account for over 50% of the variance in connectivity estimates (Barry et al., 2005). By examining connectivity in source, rather than sensor space, such problems are mitigated.
As reviewed elsewhere in this issue, structural MRI and functional MRI studies are examining the maturation of brain structure and the maturation of blood-flow patterns in infants and young children (Levman et al., 2017). As detailed in Stiles and Jernigan (2010), additional knowledge of structural brain development in infants is also based on histological studies from postmortem materials of fetuses, infants and children (Kostovic and Vasung, 2009; Vasung et al., 2016; Huang et al., 2006, 2009) and data acquired in other species (e.g., rodents or monkeys (Kostovic and Goldman-Rakic, 1983). Cross-sectional and longitudinal MEG studies are now needed to further understand the maturation of neural functional activity in infants. A focus on neural activity is of interest as brain development during embryonic and early fetal periods primarily concerns the migration of neurons to the correct location, followed by events that establish a regional identity for each neuron. Brain development during the late fetal period through infancy involves specification and refinement, a process achieved by establishing primary sensorimotor neural networks via the formation of local and long-range connections between neurons (Kolb and Fantie, 1997). It is highly likely that multimodal studies capitalizing on the complementary strengths of MRI/MRS and MEG or EEG will yield valuable insights into brain maturation. Research into maturation of neural activity across infant development is particularly important, given that it is a peak period of neural organization that contributes to both normal variation and the onset of mental illness. It is hoped that via MEG functional brain markers that predict future brain function and neurocognitive outcomes will be identified, with these functional markers eventually used to identify children at risk for neurodevelopmental disorders.
The primary/secondary auditory cortex studies reviewed above (Kurtzberg et al., 1984; Edgar et al., 2015b; Lowery et al., 2006; Sheridan et al., 2010b) provide Examples of the potential of cross-sectional and longitudinal MEG studies to establish the developmental trajectory of brain function from infancy to adulthood. In some cases, it is likely that direct comparisons can be made between infant and adult responses. As an example, Fig. 3 plots infant, child and adult M50 auditory latencies across infant (Edgar et al., 2015b), young child and adolescent (Edgar et al., 2015a, 2015c) and adults studies (Smith et al., 2010), with Fig. 3 scatterplot supporting the claim that the infant P2m auditory component slowly ‘becomes’ the adult M50, and with M50 latency changing very little after age 18 years. The above provides an example of an auditory response that the literature, so far, suggests can be tracked across the human lifespan and also suggesting the robustness of auditory latency measures to differences in scanner platform.
Not all responses, however, appear throughout the lifespan. For example, the first negative auditory component identified in infants is often labeled the N2 (EEG) or N2m (MEG), with the literature suggesting that the N2m component does not develop into the adult M100 auditory response. For example, Edgar et al. (2015b) showed that N2m latency did not change as a function of age in children 6 months and older, a finding consistent with several previous studies (Kushnerenko et al., 2002; Choudhury and Benasich, 2011; Onishi and Davis, 1969; Tanguay et al., 1973), and consistent with the observations that the adult N100/M100 is not robustly observed until early adolescence (Edgar et al., 2014a; Ponton et al., 2000, 2002). The above provides an example of an auditory response that studies so far suggest is not easily tracked across the human lifespan.
As detailed in this review, most infant studies have examined primary sensory activity. Future infant MEG studies will likely extend this line of research via examining activity throughout the brain. In general, whereas primary sensory activity is usually well modeled by an equivalent current dipole (ECD) model, examination of activity outside primary sensory cortices will likely involve distributed source modeling given that the active brain areas are often not known a priori.
Regarding source localization, whereas the MEG inverse model depends on tissue magnetic permeability (which does not differ much between tissues), the EEG inverse model depends on tissue electrical conductivities which differ by a factor of ~30 between brain tissue, skull and scalp. Consequently, imprecision in the knowledge of tissue properties has less influence on MEG than EEG source modeling, making MEG less dependent on the use of realistic head models (e.g., BEM or finite element method (FEM) (Lew et al., 2013; Stenroos et al., 2014). In any case, researchers are developing age-specific infant MRI templates (Akiyama et al., 2013), thus allowing researchers to calculate BEM using tessellated brain surface meshes from skull-stripped age-specific MRI templates (e.g., neonate (2–4 weeks), 3-months, 6 months, 9-months, 12-months, 18-months, 24-months) in large-scale infant imaging studies (i.e., Baby Connectome Project (Howell et al., 2018)). Several open-source MEG analysis packages provide the ability to compute forward solutions using realistic head models (e.g., OpenMEEG BEM in Brainstorm (Gramfort et al., 2010) or MNE Python).
An example of the utility of distributed source modeling involves the use of infant MEG to study face processing. Orienting to face stimuli is a skill that is essential to the development of social and language skills (Dawson et al., 2004; Baron-Cohen et al., 1997) early in life, with failure to detect or respond to faces an early predictor of ASD (Association AP, 2000). Although EEG studies have identified atypical face-sensitive neural responses (e.g., N290 and P400) in high-risk ASD infants via examining EEG sensor activity (McCleery et al., 2009; Webb et al., 2006), MEG may be preferred in studying higher-level social processes in infants given that face processing involves activation in a widespread network that encompasses visual, limbic, temporal and prefrontal regions (Haxby et al., 1999; Leung et al., 2015; Fusar-Poli et al., 2009; Vuilleumier and Pourtois, 2007), and given that neuroimaging research reveals abnormal activity in non-fusiform face areas in individuals with ASD (Schultz et al., 2000; Pierce et al., 2001).
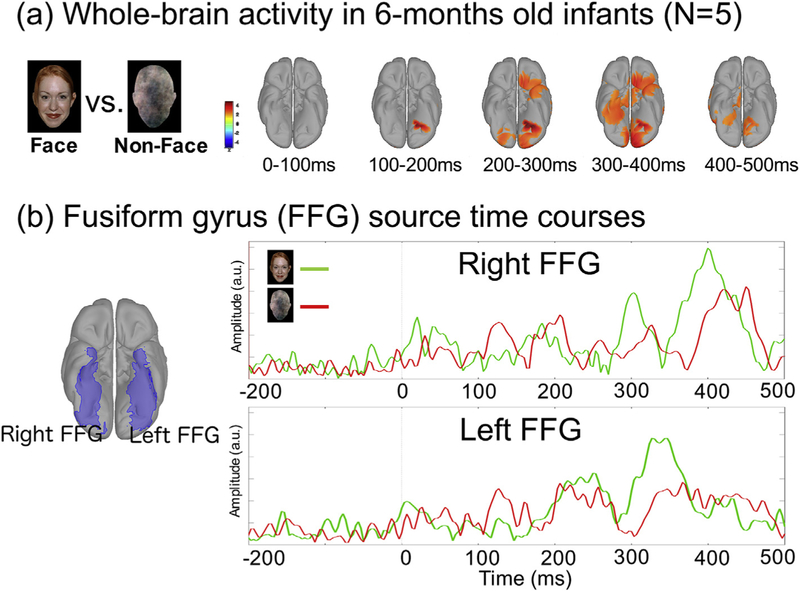
Fig. 4 shows infant whole-brain activity associated with face and non-face stimuli measured using an infant MEG system (Artemis 123™) in five 6-months-old infants. Given that face-processing networks contain multiple nodes (Haxby et al., 1999; Leung et al., 2015; Fusar-Poli et al., 2009; Vuilleumier and Pourtois, 2007), distributed source modeling (dSPM (Dale et al., 2000)) was used to estimate activity throughout the brain (using age-matched MRI templates (http://neuroimage.usc.edu/forums/showthread.php?2123-Atlas-for-1-year-old-babies (Li et al., 2015; Shi et al., 2011);). The dSPM maps in Fig. 4 (a) shows the difference of brain activity between face and non-face stimuli from 0 to 500 ms after stimulus onset. Greater activity in fusiform gyrus and visual cortex was observed in face than non-face conditions between 200 and 400 ms post-stimulus. Fig. 4(b) shows the averaged time courses of fusiform gyrus activity for face and non-face conditions. Consistent with the dSPM maps shown in Fig. 2(a), face stimuli elicited stronger neural activity than non-face stimuli around 300 ms and 400 ms in right fusiform gyrus. The face versus non-face contrast indicates stronger face than non-face activity in multiple brain regions across time, including V1, fusiform gyrus, and temporal and frontal regions (Fig. 4 (a)). The observed hemisphere differences in activation are also of interest, perhaps suggesting hemispheric differences in maturation. In our ongoing studies, infant MEG data are acquired using this task in typically developing infants as well as in toddlers recently diagnosed with ASD to identify possible face processing deficits in toddlers with ASD.
Fig. 4.
An example of (a) Face versus non-face contrast of whole-brain activity in five 6-month-old infants, and (b) source waveforms from left and right fusiform gyrus (FFG) to face and non-face stimuli. Greater activity (indicated in red) was observed in response to face versus non-face stimuli in FFG (200–400 ms post-stimulus), V1, temporal pole and frontal areas. FFG source waveforms showed that right FFG activity in response to face stimuli peaked at ~300 ms and ~400 ms, and left FFG activity to face stimuli peaked at ~350 ms.
Finally, of great interest are multimodal studies examining associations between infant brain neural function and brain structure. Development of the cerebral cortex is often divided into two major phases. The first encompasses the formation of cortical neurons and their assembly into a laminar structure, a process that is complete by ~18 weeks gestational age (Sidman and Rakic, 1973). The second major phase, and of interest for infant brain studies, is the elaboration of cortical connections: myelination of white-matter tracks, and the establishment of local neural circuits via the growth of axons and of dendritic branches. Although these events begin during the 2nd trimester of gestation, most cortical growth is postnatal. Indeed, at birth, the volume of the infant’s cortex is only 1/3 size of the adult brain (Huttenlocher et al., 1994; Huttenlocher and Dabholkar, 1997; Lyall et al., 2015). The subsequent increase in brain size results largely from the growth of neurons and their connections (Conel, 1939–1963; Schade, 1961). These developmental events are of interest, as these changes occur at the time of emergence of cortical sensory functions. Multimodal brain imaging studies examining young children and adolescents provide evidence that brain development involves straightforward brain structure-function mechanistic relations (Roberts et al., 2013; Edgar et al., 2014b, 2015e; Berman et al., 2016). The recent availability of whole-head infant MEG and high-resolution multi-band diffusion and structural MRI place us at an important time in the history of developmental neurobiology, with noninvasive neuro-imaging techniques ideally positioning us to begin developing, testing, and refining models of brain structure-function relations.
Box 1. Advantages of MEG.
Excellent temporal (millisecond) and spatial resolution
Silent and minimal preparation time for studying brain activity in awake infants
Reference free with minimal distortion of magnetic fields with respect to the different conductivity of brain compartments (CSF, scalp, skull).
Head movement compensation methods correct for moderate head motion, rendering MEG suitable for use with awake infants
Accurate source analyses are possible with age-appropriate MRI templates, mitigating the need for individual MRIs
Acknowledgements
This research was supported by grants from the National Institute of Mental Health (R01 DC008871 to Dr. Timothy Roberts, R01 MH107506 to Dr. J. Christopher Edgar, K01 MH108822 to Dr. Yuhan Chen), the National Institute of Child Health and Human Development (R01 HD093776 to Dr. J. Christopher Edgar), and the institutional IDDRC (U54 HD086984, especially the Neuroimaging and Neurocircuitry Core, Director Dr. Roberts). The authors would like to thank the subjects who enrolled in the reviewed studies and Michelle Slinger, John Dell, Rachel Golembski, Peter Lam, and Matt Ku, who helped with data collection. Dr. Roberts gratefully acknowledges the Oberkircher family for the Oberkircher Family Chair in Pediatric Radiology.
References
- Akiyama LF, Richards TR, Imada T, Dager SR, Wroblewski L, Kuhl PK, 2013. Age-specific average head template for typically developing 6-month-old infants. PLoS One 8 e73821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AL, Thomason ME, 2013. Functional plasticity before the cradle: a review of neural functional imaging in the human fetus. Neurosci. Biobehav. Rev 37, 2220–2232. [DOI] [PubMed] [Google Scholar]
- Association AP, 2000. Diagnostic and Statistical Manual of Mental Disorders, 4 ed. American Psychiatric Association, Washington, DC. [Google Scholar]
- Bakhireva LN, Lowe JR, Gutierrez HL, Stephen JM, 2015. Ethanol, neurodevelopment, infant and child Health (ENRICH) prospective cohort: study design considerations. Adv Pediatr Res 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnet AB, 1975. Auditory evoked potentials during sleep in normal children from ten days to three years of age. Electroencephalogr. Clin. Neurophysiol 39, 29–41. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Baldwin DA, Crowson M, 1997. Do children with autism use the speaker’s direction of gaze strategy to crack the code of language? Child Dev. 68, 48–57. [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M, 2005. Adjusting EEG coherence for inter-electrode distance effects: an exploration in normal children. Int. J. Psychophysiol 55, 313–321. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M, 2001. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int. J. Psychophysiol 39, 241–248. [DOI] [PubMed] [Google Scholar]
- Bell MA, 1998. The ontogeny of the EEG during infancy and childhood: implications for cognitive development In: Berlin, Garreau B (Ed.), Neuroimaging in Child Neuropsychiatric Disorders. Springer-Verlag, pp. 97–111. [Google Scholar]
- Bell MA, Cuevas K, 2012. Using EEG to study cognitive development: issues and practices. J. Cognit. Dev 13, 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchicci M, Zhang T, Romero L, Peters A, Annett R, Teuscher U, Bertollo M, Okada Y, Stephen J, Comani S, 2011. Development of mu rhythm in infants and preschool children. Dev. Neurosci 33, 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchicci M, Tamburro G, Comani S, 2015. The intrahemispheric functional properties of the developing sensorimotor cortex are influenced by maturation. Front. Hum. Neurosci 9, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JI, Edgar JC, Blaskey L, Kuschner ES, Levy SE, Ku M, Dell J,Roberts TP, 2016. Multimodal diffusion-MRI and MEG assessment of auditory and language system development in autism spectrum disorder. Front. Neuroanat 10, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boor R, Goebel B, 2000. Maturation of near-field and far-field somatosensory evoked potentials after median nerve stimulation in children under 4 years of age. Clin. Neurophysiol 111, 1070–1081. [DOI] [PubMed] [Google Scholar]
- Bosseler AN, Taulu S, Pihko E, Makela JP, Imada T, Ahonen A, Kuhl PK, 2013. Theta brain rhythms index perceptual narrowing in infant speech perception. Front. Psychol 4, 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boto E, Meyer SS, Shah V, Alem O, Knappe S, Kruger P, Fromhold TM, Lim M, Glover PM, Morris PG, Bowtell R, Barnes GR, Brookes MJ, 2017. A new generation of magnetoencephalography: room temperature measurements using optically-pumped magnetometers. Neuroimage 149, 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boto E, Holmes N, Leggett J, Roberts G, Shah V, Meyer SS, Munoz LD,Mullinger KJ, Tierney TM, Bestmann S, Barnes GR, Bowtell R, Brookes MJ, 2018. Moving magnetoencephalography towards real-world applications with a wearable system. Nature 555, 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DK, Bowman DM, Kimberley BP, 2000. The effects of maturation and stimulus parameters on the optimal f(2)/f(1) ratio of the 2f(1)-f(2) distortion product otoacoustic emission in neonates(1). Hear. Res 145, 17–24. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A, 2004. Neuronal oscillations in cortical networks. Science 304,1926–1929. [DOI] [PubMed] [Google Scholar]
- Cao M, He Y, Dai Z, Liao X, Jeon T, Ouyang M, Chalak L, Bi Y, Rollins N, Dong Q, Huang H, 2017. Early development of functional network segregation revealed by connectomic analysis of the preterm human brain. Cerebr. Cortex 27, 1949–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheour M, Imada T, Taulu S, Ahonen A, Salonen J, Kuhl P, 2004Magnetoencephalography is feasible for infant assessment of auditory discrimination. Exp. Neurol 190 (Suppl. 1), S44–S51. [DOI] [PubMed] [Google Scholar]
- Choudhury N, Benasich AA, 2011. Maturation of auditory evoked potentials from 6 to48 months: prediction to 3 and 4 year language and cognitive abilities. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 122, 320–338. [DOI] [PubMed] [Google Scholar]
- Coffey EB, Herholz SC, Chepesiuk AM, Baillet S, Zatorre RJ, 2016. Cortical contributions to the auditory frequency-following response revealed by MEG. Nat. Commun 7, 11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conel J, 1939–1963. The Postnatal Development of the Human Cerebral Cortex. HarvardUniversity Press, Cambridge, MA. [Google Scholar]
- Dale AM, Sereno MI, 1993. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J. Cognit. Neurosci 5, 162–176. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E, 2000. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron 26, 55–67. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J, 2004. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev. Psychol 40, 271–283. [DOI] [PubMed] [Google Scholar]
- de Haan M, Nelson CA, 1999. Brain activity differentiates face and object processing in 6-month-old infants. Dev. Psychol 35, 1113–1121. [DOI] [PubMed] [Google Scholar]
- de Haan M, Johnson MH, Halit H, 2003. Development of face-sensitive event-related potentials during infancy: a review. Int. J. Psychophysiol 51, 45–58. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Brunko E, Debecker J, 1976. Maturation of the somatosensory evoked potentials in normal infants and children, with special reference to the early N1 component. Electroencephalogr. Clin. Neurophysiol 40, 43–58. [DOI] [PubMed] [Google Scholar]
- Draganova R, Eswaran H, Murphy P, Huotilainen M, Lowery C, Preissl H, 2005Sound frequency change detection in fetuses and newborns, a magnetoencephalographic study. Neuroimage 28, 354–361. [DOI] [PubMed] [Google Scholar]
- Draganova R, Eswaran H, Murphy P, Lowery C, Preissl H, 2007. Serial magnetoencephalographic study of fetal and newborn auditory discriminative evoked responses. Early Hum. Dev 83, 199–207. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Huang MX, Weisend MP, Sherwood A, Miller GA, Adler LE,Canive JM, 2003. Interpreting abnormality: an EEG and MEG study of P50 and the auditory paired-stimulus paradigm. Biol. Psychol 65, 1–20. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Khan SY, Blaskey L, Chow VY, Rey M, Gaetz W, Cannon KM, Monroe JF, Cornew L, Qasmieh S, Liu S, Welsh JP, Levy SE, Roberts TP, 2015. Neruomagnetic noise predicts evoked-response delays and core language deficits in autism spectrum disorders. J. Autism Dev. Disord 45 (2), 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Lanza MR, Daina AB, Monroe JF, Khan SY, Blaskey L, Cannon KM, Jenkins J 3rd, Qasmieh S, Levy SE, Roberts TP, 2014. Missing and delayed auditory responses in young and older children with autism spectrum disorders. Front. Hum. Neurosci 8, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Chen YH, Lanza M, Howell B, Chow VY, Heiken K, Liu S,Wootton C, Hunter MA, Huang M, Miller GA, Canive JM, 2014. Cortical thickness as a contributor to abnormal oscillations in schizophrenia? Neuroimage Clin 4, 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Fisk Iv CL, Berman JI, Chudnovskaya D, Liu S, Pandey J,Herrington JD, Port RG, Schultz RT, Roberts TP, 2015. Auditory encoding abnormalities in children with autism spectrum disorder suggest delayed development of auditory cortex. Mol. Autism 6, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Murray R, Kuschner ES, Pratt K, Paulson DN, Dell J, Golembski R, Lam P, Bloy L, Gaetz W, Roberts TP, 2015. The maturation of auditory responses in infants and young children: a cross-sectional study from 6 to 59 months. Front. Neuroanat 9, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Khan SY, Blaskey L, Chow VY, Rey M, Gaetz W, Cannon KM,Monroe JF, Cornew L, Qasmieh S, Liu S, Welsh JP, Levy SE, Roberts TP, 2015. Neuromagnetic oscillations predict evoked-response latency delays and core language deficits in autism spectrum disorders. J. Autism Dev. Disord 45, 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Murray RE, Kuschner E, Pratt K, Paulson D, Dell J, Golembski R, Lam P, Bloy L, Gaetz W, Roberts TPL, 2015. The maturation of auditory responses in infants and young children: a cross-sectional study from 6 to 59 months. Front. Neuroanat [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Heiken K, Chen YH, Herrington JD, Chow V, Liu S, Bloy L, Huang M, Pandey J, Cannon KM, Qasmieh S, Levy SE, Schultz RT, Roberts TP, 2015. Resting-state alpha in autism spectrum disorder and alpha associations with thalamic volume. J. Autism Dev. Disord 45, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Fisk Iv CL, Chen YH, Stone-Howell B, Hunter MA, Huang M, Bustillo JR, Canive JM, Miller GA, 2017. By our bootstraps: comparing methods for measuring auditory 40 Hz steady-state neural activity. Psychophysiology 54, 1110–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW, Coupland SG, Winkelaar R, 1991. Maturation of the traveling-wave delay in the human cochlea. J. Acoust. Soc. Am 90, 288–298. [DOI] [PubMed] [Google Scholar]
- Ellingson RJ, Lathrop GH, Nelson B, Danahy T, 1972. Visual evoked potentials of infants. Rev. Electroencephalogr. Neurophysiol. Clin 2, 395–400. [DOI] [PubMed] [Google Scholar]
- Estes KG, Hay JF, 2015. Flexibility in bilingual infants’ word learning. Child Dev 86,1371–1385. [DOI] [PubMed] [Google Scholar]
- Ferjan Ramirez N, Ramirez RR, Clarke M, Taulu S, Kuhl PK, 2017. Speech discrimination in 11-month-old bilingual and monolingual infants: a magnetoencephalography study. Dev Sci 20. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P, 2009. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci 34, 418–432. [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Cheyne D, 2006. Localization of sensorimotor cortical rhythms induced by tactile stimulation using spatially filtered MEG. Neuroimage 30, 899–908. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Otsubo H, Pang EW, 2008. Magnetoencephalography for clinical pediatrics: the effect of head positioning on measurement of somatosensory-evoked fields. Clin. Neurophysiol 119, 1923–1933. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Gordon RS, Papadelis C, Fujiwara H, Rose DF, Edgar JC,Schwartz ES, Roberts TPL, 2014. Magnetoencephalography for clinical pediatrics: recent advances in hardware, methods, and clinical applications. J. Pediatr. Epilepsy 4, 139–155. [Google Scholar]
- Gondo K, Tobimatsu S, Kira R, Tokunaga Y, Yamamoto T, Hara T, 2001A magnetoencephalographic study on development of the somatosensory cortex in infants. Neuroreport 12, 3227–3231. [DOI] [PubMed] [Google Scholar]
- Govindan RB, Wilson JD, Preissl H, Murphy P, Lowery CL, Eswaran H, 2008. An objective assessment of fetal and neonatal auditory evoked responses. Neuroimage 43, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Papadopoulo T, Olivi E, Clerc M, 2010. OpenMEEG: opensource software for quasistatic bioelectromagnetics. Biomed. Eng. Online 9, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Strohmeier D, Haueisen J, Hamalainen MS, Kowalski M, 2013. Time-frequency mixed-norm estimates: sparse M/EEG imaging with non-stationary source activations. Neuroimage 70, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad N, Shihabuddin B, Preissl H, Holst M, Lowery CL, Eswaran H, 2006. Magnetoencephalography in healthy neonates. Clin. Neurophysiol 117, 289–294. [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, Johnson MH, 2003. Cortical specialisation for face processing: face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage 19, 1180–1193. [DOI] [PubMed] [Google Scholar]
- Halit H, Csibra G, Volein A, Johnson MH, 2004. Face-sensitive cortical processing in early infancy. JCPP (J. Child Psychol. Psychiatry) 45, 1228–1234. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV, 1993Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys 65, 413–497. [Google Scholar]
- Hartkopf J, Schleger F, Weiss M, Hertrich I, Kiefer-Schmidt I, Preissl H,Muenssinger J, 2016. Neuromagnetic signatures of syllable processing in fetuses and infants provide no evidence for habituation. Early Hum. Dev 100, 61–66. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Clark VP, Schouten JL, Hoffman EA, Martin A, 1999. The effect of face inversion on activity in human neural systems for face and object perception. Neuron 22, 189–199. [DOI] [PubMed] [Google Scholar]
- He W, Brock J, Johnson BW, 2014. Face-sensitive brain responses measured from a four-year-old child with a custom-sized child MEG system. J. Neurosci. Methods 222, 213–217. [DOI] [PubMed] [Google Scholar]
- Hirata M, Ikeda T, Kikuchi M, Kimura T, Hiraishi H, Yoshimura Y, Asada M, 2014. Hyperscanning MEG for understanding mother-child cerebral interactions. Front. Hum. Neurosci 8, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MH, Ombao H, Edgar JC, Canive JM, Miller GA, 2008. Time-frequency discriminant analysis of MEG signals. Neuroimage 40, 174–186. [DOI] [PubMed] [Google Scholar]
- Holst M, Eswaran H, Lowery C, Murphy P, Norton J, Preissl H, 2005. Development of auditory evoked fields in human fetuses and newborns: a longitudinal MEG study. Clin. Neurophysiol 116, 1949–1955. [DOI] [PubMed] [Google Scholar]
- Howell BR, Styner MA, Gao W, Yap PT, Wang L, Baluyot K, Yacoub E, Chen G, Potts T, Salzwedel A, Li G, Gilmore JH, Piven J, Smith JK, Shen D, Ugurbil K, Zhu H, Lin W, Elison JT, 2018. The UNC/UMN Baby Connectome Project (BCP): an overview of the study design and protocol development. Neuroimage 10.1016/j.neuroimage.2018.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards LJ, Yarowsky P,Donohue P, Graham E, van Zijl PC, Mori S, 2006. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage 33, 27–38. [DOI] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, Miller MI, Mori S, 2009. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J. Neurosci 29, 4263–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotilainen M, Kujala A, Hotakainen M, Shestakova A, Kushnerenko E,Parkkonen L, Fellman V, Naatanen R, 2003. Auditory magnetic responses of healthy newborns. Neuroreport 14, 1871–1875. [DOI] [PubMed] [Google Scholar]
- Huotilainen M, Shestakova A, Hukki J, 2008. Using magnetoencephalography in assessing auditory skills in infants and children. Int. J. Psychophysiol 68, 123–129. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, 1979. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 163, 195–205. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS, 1997. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol 387, 167–178. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, 1994. Synaptogenesis in human cerebral cortex In: Dawson G,Fisher KW (Eds.), Human Behavior and the Developing Brain. The Guilford Press, New York. [Google Scholar]
- Imada T, Zhang Y, Cheour M, Taulu S, Ahonen A, Kuhl PK, 2006. Infant speech perception activates Broca’s area: a developmental magnetoencephalography study. Neuroreport 17, 957–962. [DOI] [PubMed] [Google Scholar]
- Johnson BW, Crain S, Thornton R, Tesan G, Reid M, 2010. Measurement of brain function in pre-school children using a custom sized whole-head MEG sensor array. Clin. Neurophysiol 121, 340–349. [DOI] [PubMed] [Google Scholar]
- Jones SR, Kerr CE, Wan Q, Pritchett DL, Hamalainen M, Moore CI, 2010. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J. Neurosci 30, 13760–13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJ, Venema K, Lowy R, Earl RK, Webb SJ, 2015. Developmental changes in infant brain activity during naturalistic social experiences. Dev. Psychobiol 57, 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivisto K, Nevalainen P, Lauronen L, Tupola S, Pihko E, Kivitie-Kallio S, 2015. Somatosensory and auditory processing in opioid-exposed newborns with neonatal abstinence syndrome: a magnetoencephalographic approach. J. Matern. Fetal Neonatal Med 28, 2015–2019. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Sauseng P, 2005. The functional significance of theta and upper alpha oscillations. Exp. Psycholv 52, 99–108. [DOI] [PubMed] [Google Scholar]
- Kolb B, Fantie B, 1997. In: Reynolds CR, Flether-Janzen E (Eds.), Development of the Child’s Brain and Behavior Springer, Boston, MA. [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD, 2000. Gamma rhythms and beta rhythms have different synchronization properties. Proc. Natl. Acad. Sci. U. S. A 97, 1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I, Goldman-Rakic PS, 1983. Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J. Comp. Neurol 219, 431–447. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Vasung L, 2009. Insights from in vitro fetal magnetic resonance imaging of cerebral development. Semin. Perinatol 33, 220–233. [DOI] [PubMed] [Google Scholar]
- Kovacs AM, Mehler J, 2009. Cognitive gains in 7-month-old bilingual infants. Proc.Natl. Acad. Sci. U. S. A 106, 6556–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AM, Mehler J, 2009. Flexible learning of multiple speech structures in bilingual infants. Science 325, 611–612. [DOI] [PubMed] [Google Scholar]
- Kraus N, Smith DI, Reed NL, Stein LK, Cartee C, 1985. Auditory middle latency responses in children: effects of age and diagnostic category. Electroencephalogr. Clin. Neurophysiol 62, 343–351. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Ramirez RR, Bosseler A, Lin JF, Imada T, 2014. Infants’ brain responses to speech suggest analysis by synthesis. Proc. Natl. Acad. Sci. U. S. A 111, 11238–11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala A, Huotilainen M, Hotakainen M, Lennes M, Parkkonen L, Fellman V, Naatanen R, 2004. Speech-sound discrimination in neonates as measured with MEG. Neuroreport 15, 2089–2092. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D, Hilpert PL, Kreuzer JA, Vaughan HG Jr., 1984. Differential maturation of cortical auditory evoked potentials to speech sounds in normal fullterm and very low-birthweight infants. Dev. Med. Child Neurol 26, 466–475. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Becker KM, Heinrichs-Graham E, Wilson TW, 2014. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev. Med. Child Neurol 56, 1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnerenko E, Ceponiene R, Balan P, Fellman V, Huotilaine M, Naatane R, 2002Maturation of the auditory event-related potentials during the first year of life. Neuroreport 13, 47–51. [DOI] [PubMed] [Google Scholar]
- Larson E, Taulu S, 2017. The importance of properly compensating for head movements during MEG acquisition across different age groups. Brain Topogr 30, 172–181. [DOI] [PubMed] [Google Scholar]
- Lauronen L, Nevalainen P, Wikstrom H, Parkkonen L, Okada Y, Pihko E, 2006. Immaturity of somatosensory cortical processing in human newborns. Neuroimage 33, 195–203. [DOI] [PubMed] [Google Scholar]
- Lengle JM, Chen M, Wakai RT, 2001. Improved neuromagnetic detection of fetal and neonatal auditory evoked responses. Clin. Neurophysiol 112, 785–792. [DOI] [PubMed] [Google Scholar]
- Leung RC, Pang EW, Cassel D, Brian JA, Smith ML, Taylor MJ, 2015. Early neural activation during facial affect processing in adolescents with Autism Spectrum Disorder. Neuroimage Clin 7, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levman J, MacDonald P, Lim AR, Forgeron C, Takahashi E, 2017. A pediatric structural MRI analysis of healthy brain development from newborns to young adults. Hum. Brain Mapp 38, 5931–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew S, Sliva DD, Choe MS, Grant PE, Okada Y, Wolters CH, Hamalainen MS, 2013. Effects of sutures and fontanels on MEG and EEG source analysis in a realistic infant head model. Neuroimage 76, 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewine JD, Orrison WW Jr., 1995. Magnetic source imaging: basic principles and applications in neuroradiology. Acad. Radiol 2, 436–440. [DOI] [PubMed] [Google Scholar]
- Li Z, Park BK, Liu W, Zhang J, Reed MP, Rupp JD, Hoff CN, Hu J, 2015. A statistical skull geometry model for children 0–3 years old. PLoS One 10 e0127322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe S, Kovacevic N, McIntosh AR, 2009. Differential maturation of brain signal complexity in the human auditory and visual system. Front. Hum. Neurosci 3, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery CL, Eswaran H, Murphy P, Preissl H, 2006. Fetal magnetoencephalography. Semin. Fetal Neonatal Med 11, 430–436. [DOI] [PubMed] [Google Scholar]
- Lutter WJ, Wakai RT, Maier MM, Baryshnikov BV, 2004. MEG sleep pattern dependence of auditory evoked fields in young infants. Neurol Clin Neurophysiol 2004, 77. [PubMed] [Google Scholar]
- Lutter WJ, Maier M, Wakai RT, 2006. Development of MEG sleep patterns and magnetic auditory evoked responses during early infancy. Clin. Neurophysiol 117, 522–530. [DOI] [PubMed] [Google Scholar]
- Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, Hamer RM, Shen D,Gilmore JH, 2015. Dynamic development of regional cortical thickness and surface area in early childhood. Cerebr. Cortex 25, 2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, 1993. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr. Clin. Neurophysiol 86, 283–293. [DOI] [PubMed] [Google Scholar]
- Matuz T, Govindan RB, Preissl H, Siegel ER, Muenssinger J, Murphy P, Ware M, Lowery CL, Eswaran H, 2012. Habituation of visual evoked responses in neonates and fetuses: a MEG study. Dev Cogn Neurosci 2, 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery JP, Akshoomoff N, Dobkins KR, Carver LJ, 2009. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biol. Psychiatry 66, 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Ramirez RR, Saby JN, Larson E, Taulu S, Marshall PJ, 2018. Infant brain responses to felt and observed touch of hands and feet: an MEG study. Dev Sci 21 (5), e12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Elbert T, Sutton BP, Heller W, 2007. Innovative clinical assessment technologies: challenges and opportunities in neuroimaging. Psychol. Assess 19, 58–73. [DOI] [PubMed] [Google Scholar]
- Molins A, Stufflebeam SM, Brown EN, Hamalainen MS, 2008. Quantification of the benefit from integrating MEG and EEG data in minimum l2-norm estimation. Neuroimage 42, 1069–1077. [DOI] [PubMed] [Google Scholar]
- Muenssinger J, Matuz T, Schleger F, Draganova R, Weiss M, Kiefer-Schmidt I, Wacker-Gussmann A, Govindan RB, Lowery CL, Eswaran H, Preissl H, 2013. Sensitivity to auditory spectral width in the fetus and infant - an fMEG study. Front. Hum. Neurosci 7, 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenssinger J, Matuz T, Schleger F, Kiefer-Schmidt I, Goelz R, Wacker-Gussmann A, Birbaumer N, Preissl H, 2013. Auditory habituation in the fetus and neonate: an fMEG study. Dev. Sci 16, 287–295. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Gaillard AW, Mantysalo S, 1978. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol 42, 313–329. [DOI] [PubMed] [Google Scholar]
- Nevalainen P, Lauronen L, Sambeth A, Wikstrom H, Okada Y, Pihko E, 2008Somatosensory evoked magnetic fields from the primary and secondary somatosensory cortices in healthy newborns. Neuroimage 40, 738–745. [DOI] [PubMed] [Google Scholar]
- Nevalainen P, Pihko E, Metsaranta M, Andersson S, Autti T, Lauronen L, 2008Does very premature birth affect the functioning of the somatosensory cortex?–A magnetoencephalography study. Int. J. Psychophysiol 68, 85–93. [DOI] [PubMed] [Google Scholar]
- Nevalainen P, Pihko E, Metsaranta M, Sambeth A, Wikstrom H, Okada Y, Autti T, Lauronen L, 2012. Evoked magnetic fields from primary and secondary somatosensory cortices: a reliable tool for assessment of cortical processing in the neonatal period. Clin. Neurophysiol 123, 2377–2383. [DOI] [PubMed] [Google Scholar]
- Nevalainen P, Lauronen L, Pihko E, 2014. Development of human somatosensory cortical functions - what have we learned from magnetoencephalography: a review. Front. Hum. Neurosci 8, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen P, Rahkonen P, Pihko E, Lano A, Vanhatalo S, Andersson S, Autti T, Valanne L, Metsaranta M, Lauronen L, 2015. Evaluation of somatosensory cortical processing in extremely preterm infants at term with MEG and EEG. Clin. Neurophysiol 126, 275–283. [DOI] [PubMed] [Google Scholar]
- Novak GP, Kurtzberg D, Kreuzer JA, Vaughan HG Jr., 1989. Cortical responses to speech sounds and their formants in normal infants: maturational sequence and spatiotemporal analysis. Electroencephalogr. Clin. Neurophysiol 73, 295–305. [DOI] [PubMed] [Google Scholar]
- Ohlrich ES, Barnet AB, Weiss IP, Shanks BL, 1978. Auditory evoked potential development in early childhood: a longitudinal study. Electroencephalogr. Clin. Neurophysiol 44, 411–423. [DOI] [PubMed] [Google Scholar]
- Okada Y, Pratt K, Atwood C, Mascarenas A, Reineman R, Nurminen J, Paulson D,2006. BabySQUID: a mobile, high-resolution multichannel magnetoencephalography system for neonatal brain assessment. Rev. Sci. Instrum 77, 024301–024301. [Google Scholar]
- Okada Y, Hamalainen M, Pratt K, Mascarenas A, Miller P, Han M, Robles J, Cavallini A, Power B, Sieng K, Sun L, Lew S, Doshi C, Ahtam B, Dinh C, Esch L, Grant E, Nummenmaa A, Paulson D, 2016. BabyMEG: a whole-head pediatric magnetoencephalography system for human brain development research. Rev. Sci. Instrum 87, 094301. [DOI] [PubMed] [Google Scholar]
- Onishi S, Davis H, 1969. Auditory evoked responses in the sleeping infant. Electroencephalogr. Clin. Neurophysiol 26, 114. [PubMed] [Google Scholar]
- Ou W, Hamalainen MS, Golland P, 2009. A distributed spatio-temporal EEG/MEG inverse solver. Neuroimage 44, 932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetau R, Ahonen A, Salonen O, Sams M, 1995. Auditory evoked magnetic fields to tones and pseudowords in healthy children and adults. J. Clin. Neurophysiol 12, 177–185. [DOI] [PubMed] [Google Scholar]
- Pantev C, Makeig S, Hoke M, Galambos R, Hampson S, Gallen C, 1991. Human auditory evoked gamma-band magnetic fields. Proc. Natl. Acad. Sci. U. S. A 88, 8996–9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkonen L, Fujiki N, Makela JP, 2009. Sources of auditory brainstem responses revisited: contribution by magnetoencephalography. Hum. Brain Mapp 30, 1772–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD, 2002. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol 24 (Suppl D), 5–12. [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E, 2001. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain 124, 2059–2073. [DOI] [PubMed] [Google Scholar]
- Pihko E, Lauronen L, 2004. Somatosensory processing in healthy newborns. Exp.Neurol 190 (Suppl. 1), S2–S7. [DOI] [PubMed] [Google Scholar]
- Pihko E, Sambeth A, Leppanen PH, Okada Y, Lauronen L, 2004. Auditory evoked magnetic fields to speech stimuli in newborns–effect of sleep stages. Neurol Clin Neurophysiol 2004, 6. [PubMed] [Google Scholar]
- Pihko E, Lauronen L, Wikstrom H, Taulu S, Nurminen J, Kivitie-Kallio S,Okada Y, 2004. Somatosensory evoked potentials and magnetic fields elicited by tactile stimulation of the hand during active and quiet sleep in newborns. Clin. Neurophysiol 115, 448–455. [DOI] [PubMed] [Google Scholar]
- Pihko E, Lauronen L, Wikstrom H, Parkkonen L, Okada Y, 2005. Somatosensory evoked magnetic fields to median nerve stimulation in newborns. Int. Congr 1278, 211–214. [Google Scholar]
- Pihko E, Nevalainen P, Stephen J, Okada Y, Lauronen L, 2009. Maturation of somatosensory cortical processing from birth to adulthood revealed by magnetoencephalography. Clin. Neurophysiol 120, 1552–1561. [DOI] [PubMed] [Google Scholar]
- Pihko E, Lauronen L, Kivisto K, Nevalainen P, 2011. Increasing the efficiency of neonatal MEG measurements by alternating auditory and tactile stimulation. Clin. Neurophysiol 122, 808–814. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Coupland SG, Winkelaar R, 1992. Frequency-specific maturation of the eighth nerve and brain-stem auditory pathway: evidence from derived auditory brain-stem responses (ABRs). J. Acoust. Soc. Am 91, 1576–1586. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M, 2000. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin. Neurophysiol 111, 220–236. [DOI] [PubMed] [Google Scholar]
- Ponton C, Eggermont JJ, Khosla D, Kwong B, Don M, 2002. Maturation of human central auditory system activity: separating auditory evoked potentials by dipole source modeling. Clin. Neurophysiol 113, 407–420. [DOI] [PubMed] [Google Scholar]
- Rahkonen P, Nevalainen P, Lauronen L, Pihko E, Lano A, Vanhatalo S,Pesonen AK, Heinonen K, Raikkonen K, Valanne L, Autti T, Andersson S, Metsaranta M, 2013. Cortical somatosensory processing measured by magnetoencephalography predicts neurodevelopment in extremely low-gestationalage infants. Pediatr. Res 73, 763–771. [DOI] [PubMed] [Google Scholar]
- Remijn GB, Kikuchi M, Shitamichi K, Ueno S, Yoshimura Y, Nagao K,Tsubokawa T, Kojima H, Higashida H, Minabe Y, 2014. Somatosensory evoked field in response to visuotactile stimulation in 3- to 4-year-old children. Front. Hum. Neurosci 8, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TP, Lanza MR, Dell J, Qasmieh S, Hines K, Blaskey L, Zarnow DM, Levy SE, Edgar JC, Berman JI, 2013. Maturational differences in thalamocortical white matter microstructure and auditory evoked response latencies in autism spectrum disorders. Brain Res 1537, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TP, Paulson DN, Hirschkoff E, Pratt K, Mascarenas A, Miller P, Han M, Caffrey J, Kincade C, Power B, Murray R, Chow V, Fisk C, Ku M, Chudnovskaya D, Dell J, Golembski R, Lam P, Blaskey L, Kuschner E, Bloy L, Gaetz W, Edgar JC, 2014. Artemis 123: development of a whole-head infant and young child MEG system. Front. Hum. Neurosci 8, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saby JN, Meltzoff AN, Marshall PJ, 2016. Beyond the N1: a review of late somatosensory evoked responses in human infants. Int. J. Psychophysiol 110, 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambeth A, Huotilainen M, Kushnerenko E, Fellman V, Pihko E, 2006. Newborns discriminate novel from harmonic sounds: a study using magnetoencephalography. Clin. Neurophysiol 117, 496–503. [DOI] [PubMed] [Google Scholar]
- Sambeth A, Ruohio K, Alku P, Fellman V, Huotilainen M, 2008. Sleeping newborns extract prosody from continuous speech. Clin. Neurophysiol 119, 332–341. [DOI] [PubMed] [Google Scholar]
- Sambeth A, Pakarinen S, Ruohio K, Fellman V, van Zuijen TL, Huotilainen M,2009. Change detection in newborns using a multiple deviant paradigm: a study using magnetoencephalography. Clin. Neurophysiol 120, 530–538. [DOI] [PubMed] [Google Scholar]
- Sanjuan PM, Poremba C, Flynn LR, Savich R, Annett RD, Stephen J, 2016Association between theta power in 6-month old infants at rest and maternal PTSD severity: a pilot study. Neurosci. Lett 630, 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade JP, 1961. Van GW. Structural organization of the human cerebral cortex. 1.Maturation of the middle frontal gyrus. Acta Anat 47, 74–111. [DOI] [PubMed] [Google Scholar]
- Schleger F, Landerl K, Muenssinger J, Draganova R, Reinl M, Kiefer-Schmidt I, Weiss M, Wacker-Gussmann A, Huotilainen M, Preissl H, 2014. Magnetoencephalographic signatures of numerosity discrimination in fetuses and neonates. Dev. Neuropsychol 39, 316–329. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F,Skudlarski P, Lacadie C, Cohen DJ, Gore JC, 2000. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch. Gen. Psychiatr 57, 331–340. [DOI] [PubMed] [Google Scholar]
- Schwartz ES, Edgar JC, Gaetz WC, Roberts TP, 2010. Magnetoencephalography.Pediatr Radiol 40, 50–58. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kraus N, McGee TJ, Nicol TG, 1997. Developmental changes in P1 andN1 central auditory responses elicited by consonant-vowel syllables. Electroencephalogr. Clin. Neurophysiol 104, 540–545. [DOI] [PubMed] [Google Scholar]
- Sharon D, Hamalainen MS, Tootell RB, Halgren E, Belliveau JW, 2007. The advantage of combining MEG and EEG: comparison to fMRI in focally stimulated visual cortex. Neuroimage 36, 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan CJ, Preissl H, Siegel ER, Murphy P, Ware M, Lowery CL, Eswaran H, 2008. Neonatal and fetal response decrement of evoked responses: a MEG study. Clin. Neurophysiol 119, 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan CJ, Matuz T, Draganova R, Eswaran H, Preissl H, 2010. Fetal magnetoencephalography - achievements and challenges in the study of prenatal and early postnatal brain responses: a review. Infant Child Dev 19, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C, Draganova R, Ware M, Murphy P, Govindan R, Siegel ER,Eswaran H, Preissl H, 2010. Early development of brain responses to rapidly presented auditory stimulation: a magnetoencephalographic study. Brain Dev 32, 642–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, Shen D, 2011. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One 6 e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Rakic P, 1973. Neuronal migration, with special reference to developing human brain: a review. Brain Res 62, 1–35. [DOI] [PubMed] [Google Scholar]
- Siper PM, Zemon V, Gordon J, George-Jones J, Lurie S, Zweifach J, Tavassoli T, Wang AT, Jamison J, Buxbaum JD, Kolevzon A, 2016. Rapid and objective assessment of neural function in autism spectrum disorder using transient visual evoked potentials. PLoS One 11 e0164422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Edgar JC, Huang M, Lu BY, Thoma RJ, Hanlon FM, McHaffie G,Jones AP, Paz RD, Miller GA, Canive JM, 2010. Cognitive abilities and 50- and 100-msec paired-click processes in schizophrenia. Am. J. Psychiatry 167, 1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Neil JJ, 2011. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage 56, 1437–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenroos M, Hunold A, Haueisen J, 2014. Comparison of three-shell and simplified volume conductor models in magnetoencephalography. Neuroimage 94, 337–348. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Hill DE, Peters A, Flynn L, Zhang T, Okada Y, 2017. Development of auditory evoked responses in normally developing preschool children and children with autism spectrum disorder. Dev. Neurosci 39, 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JM, Flynn L, Kabella D, Schendel M, Cano S, Savage DD, Rayburn W, Leeman LM, Lowe J, Bakhireva LN, 2018. Hypersynchrony in MEG spectral amplitude in prospectively-identified 6-month-old infants prenatally exposed to alcohol. Neuroimage Clin 17, 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL, 2010. The basics of brain development. Neuropsychol. Rev 20,327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stufflebeam SM, Tanaka N, Ahlfors SP, 2009. Clinical applications of magnetoencephalography. Hum. Brain Mapp 30, 1813–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguay PE, Lee JC, Ornitz EM, 1973. A detailed analysis of the auditory evoked response wave form in children during REM and stage 2 sleep. Electroencephalogr. Clin. Neurophysiol 35, 241–248. [DOI] [PubMed] [Google Scholar]
- Taulu S, Kajola M, Simola J, 2004. Suppression of interference and artifacts by the signal space separation method. Brain Topogr 16, 269–275. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, McCulloch DL, 1992. Visual evoked potentials in infants and children. J. Clin. Neurophysiol 9, 357–372. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Donner EJ, Pang EW, 2012. fMRI and MEG in the study of typical and atypical cognitive development. Neurophysiol. Clin 42, 19–25. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Buhl EH, Jefferys JG, Faulkner HJ, 1999. On the mechanism of the gamma–> beta frequency shift in neuronal oscillations induced in rat hippocampal slices by tetanic stimulation. J. Neurosci 19, 1088–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis KE, Leonard MK, Brown TT, Hagler DJ Jr., Curran M, Dale AM, Elman JL, Halgren E, 2011. Spatiotemporal neural dynamics of word understanding in 12- to 18-month-old-infants. Cerebr. Cortex 21, 1832–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L, Lepage C, Rados M, Pletikos M, Goldman JS, Richiardi J, Raguz M, Fischi-Gomez E, Karama S, Huppi PS, Evans AC, Kostovic I, 2016. Quantitative and qualitative analysis of transient fetal compartments during prenatal human brain development. Front. Neuroanat 10, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaurre D, Hunt LT, Quinn AJ, Hunt BAE, Brookes MJ, Nobre AC,Woolrich MW, 2018. Spontaneous cortical activity transiently organises into frequency specific phase-coupling networks. Nat. Commun 9, 2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G, 2007. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia 45, 174–194. [DOI] [PubMed] [Google Scholar]
- Wakai RT, Lutter WJ, 2016. Slow rhythms and sleep spindles in early infancy.Neurosci. Lett 630, 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Dawson G, Bernier R, Panagiotides H, 2006. ERP evidence of atypical face processing in young children with autism. J. Autism Dev. Disord 36, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJ, Merkle K, Venema K, Greenson J, Murias M, Dawson G, 2011. Developmental change in the ERP responses to familiar faces in toddlers with autism spectrum disorders versus typical development. Child Dev. 82, 1868–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG, 1995. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373, 612–615. [DOI] [PubMed] [Google Scholar]
- Williamson JS, Kaufman L, 1990. Auditory evoked magnetic fields and electric potentials—theory of neuromagnetic fields In: Grandori F, Hoke M, Romani GL (Eds.), Advances in Audiology. Karger, Basel, pp. 1–39. [Google Scholar]
- Yoshimura Y, Kikuchi M, Shitamichi K, Ueno S, Remijn GB, Haruta Y, Oi M,Munesue T, Tsubokawa T, Higashida H, Minabe Y, 2012. Language performance and auditory evoked fields in 2- to 5-year-old children. Eur. J. Neurosci 35, 644–650. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Kikuchi M, Shitamichi K, Ueno S, Munesue T, Ono Y, Tsubokawa T, Haruta Y, Oi M, Niida Y, Remijn GB, Takahashi T, Suzuki M, Higashida H, Minabe Y, 2013. Atypical brain lateralisation in the auditory cortex and language performance in 3- to 7-year-old children with high-functioning autism spectrum disorder: a child-customised magnetoencephalography (MEG) study. Mol. Autism 4, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao TC, Kuhl PK, 2016. Musical intervention enhances infants’ neural processing of temporal structure in music and speech. Proc. Natl. Acad. Sci. U. S. A 113, 5212–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]