ABSTRACT
Type 2 diabetes mellitus (T2DM) accounts for approximately 90% of all diabetic patients, and osteoporosis is one of the complications during T2DM process. ATP6V1H (V-type proton ATPase subunit H) displays crucial roles in inhibiting bone loss, but its role in osteogenic differentiation remains unknown. Therefore in this study, we aimed to explore the biological role of ATP6V1H in osteogenic differentiation. OM (osteogenic medium) and HG (high glucose and free fatty acids) were used to induce the MC3T3-E1 cells into osteogenic differentiation in a T2DM simulating environment. CCK8 assay was used to detect cell viability. Alizarin Red staining was used to detect the influence of ATP6V1H on osteogenic differentiation. ATP6V1H expression increased in OM-MC3T3-E1 cells, while decreased in OM+HG-MC3T3-E1 cells. ATP6V1H promoted osteogenic differentiation of OM+HG-MC3T3-E1 cells. Overexpression of ATP6V1H inhibited Akt/GSK3β signaling pathway, while knockdown of ATP6V1H promoted Akt/GSK3β signaling pathway. ATP6V1H overexpression promoted osteogenic differentiation of OM+HG-MC3T3-E1 cells. The role of ATP6V1H in osteogenic differentiation in a T2DM simulating environment involved in Akt/GSK3β signaling pathway. These data demonstrated that ATP6V1H could serve as a potential target for osteogenic differentiation in a T2DM simulating environment.
KEYWORDS: Akt/GSK3β, ATP6V1H, MC3T3-E1, Osteogenic Differentiation, T2DM
INTRODUCTION
Diabetes mellitus is a chronical metabolic disorder, and could be divided into type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM).1 T2DM is the most common type of diabetes, which accounts for more than 90% of all diabetes mellitus cases and usually develops in middle-aged adults.2,3
ATP6V1H (V-type proton ATPase subunit H) encodes the subunit H of V-type proton ATPase,4 and plays crucial roles in various biological processes, including a key role in regulating functions of osteoblastic cells.5 Through interacting with TGF-β receptor I and AP-2 complex, ATP6V1H regulates the proliferation and differentiation of bone marrow stromal cells.6 In zebrafish, ATP6V1H loss-of-function mutants led to severe reduced number of mature-calcified bone cells, demonstrating that ATP6V1H could regulate bone formation.5 In addition, ATP6V1H± knockout mice showed significantly decreased bone remodeling, bone matrix loss and impaired bone formation.7
During the process of T2DM, diabetic osteoporosis is a major complication, which results from variations in nutrition supplement, hormone mutation and alterations in the bone microenvironment.8,9 Diabetic osteoporosis could subsequently lead to bone loss, reduced bone mineral density, destructed bone microarchitecture and fractures.10 Moreover, high levels of glucose and free fatty acids have been reported to induce osteoblasts apoptosis and inhibit osteogenic differentiation of pre-osteoblastic cell line MC3T3-E1.11,12 To the best of our knowledge, to date, the role of ATP6V1H in diabetic osteoporosis still remains unknown. Therefore in this study, we aimed to detect the biological role of ATP6V1H in osteogenic differentiation.
In the present study, using the OM+HG to mimic T2DM in MC3T3-E1 cells, we found that ATP6V1H expression increased in OM-MC3T3-E1 cells, while decreased in OM+HG-MC3T3-E1 cells. ATP6V1H promotes osteogenic differentiation of OM+HG-MC3T3-E1 cells. Overexpression of ATP6V1H inhibits Akt/GSK3β signaling pathway, while knockdown of ATP6V1H promotes Akt/GSK3β signaling pathway. These data demonstrated that ATP6V1H could serve as the potential target for osteogenic differentiation in a T2DM simulating environment.
MATERIALS AND METHODS
Cell line and cell culture
Mouse pre-osteoblastic MC3T3-E1 cell line was purchased from ATCC (Manassas, USA). MC3T3-E1 cell line was cultured in α-MEM (Hyclone, UT, USA) medium along with 10% fetal bovine serum (FBS, Hyclone), 100 units/ml penicillin and 100 g/ml streptomycin.
To induce osteogenic differentiation, MC3T3-E1 cells were cultured in α-MEM with10% FBS, 20 mM beta-glycerophosphate and 100 mg/mL ascorbic acid for 14 days.
MC3T3-E1 cells were cultured in a mimic T2DM condition in osteogenic medium α-MEM along with 25 mM glucose (Sigma, MO, USA), 1 mM FFA (palmitic acid and oleic acid at a ratio of 1:2).12 MC3T3-E1 cells were maintained in a humidified incubator at 37°C with 5% (v/v) CO2.
CCK8 assay
CCK8 assay was used to detect cell viability according to the manufacturer’s instruction. Briefly, total of 2 × 103/well cells were cultured in 96-well plates at a final volume of 100 μl. The cells were cultured for 0, 7, 14 days in 5% (v/v) atmosphere of CO2 at 37°C incubator. Three parallel wells were carried out for each group. Cell viability assay performed each day using Cell Counting Kit-8 (Dojindo) by measuring OD450.
Alkaline phosphatase (ALP) activity assay
ALP Diethanolamine Activity Kit (Sigma, USA) was used to detect ALP activity according to the manufacturer’s protocol. Briefly, the cells were lysed by RIPA buffer (Sigma, USA) and then incubated with p-nitrophenol phosphate at 37°C. One hour later, 2 M NaOH was added to stop the reaction. The 405 nm absorbance was detected using a microplate reader (SpectraMAX Plus, Sunnyvale, CA).
Alizarin red staining
MC3T3-E1 cells were first washed PBS, followed by fixing at room temperature in 95% ethanol for 10 min. Subsequently, the cells were washed using sterilized water, and incubated with 0.1% Alizarin Red at 37°C for 30 min. The mineralized nodules were stained as orange and red bodies, which were further dissolved by 10% cetylpyridinium chloride. The 562 nm absorbance was detected using spectrophotometer (Bio-Rad, Hercules, CA, USA).
Plasmid transfection
The MC3T3-E1 cells were transfected using the ATP6V1H overexpression plasmid (p-ATP6V1H), si-ATP6V1H, and the negative control plasmid (p-NC) using Lipofectamine 2000 according to the manufacturer’s instruction (Invitrogen, California, USA). Plasmid transfection was performed before osteogenic induction. The sequence of si-ATP6V1H is 5ʹ-GUCGAUUCCUGCGAGUGAAdTdT-3ʹ.
RNA isolation and quantitative real-time PCR (qRT-PCR)
RNA was isolated from MC3T3-E1 cells using TRIzol reagent (Invitrogen, CA) according to the manufacturer’s protocol. cDNA was synthesized using PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). The primers used for detecting the mRNA expression levels are as follows: Runx2: forward 5ʹ- ACA CAC CTC CTC CTA CAC CA-3ʹ, reverse 5ʹ- CTT GTC CTG TGT TCC CAT TC-3ʹ; Col1α1: forward 5ʹ- GGG GCA AGA CAG TCA TCG AA −3ʹ, reverse 5ʹ- GAG GGA ACC AGA TTG GGG TG −3ʹ. ATP6V1H: forward 5ʹ- CAG GTT CGC TAC AAT GCT CT −3ʹ, reverse 5ʹ- TCT GAG GCT GCT CTG ACT GT −3ʹ. GAPDH: forward 5ʹ- TCA CCA CCA TGG AGA AGG C − 3ʹ, reverse 5ʹ- GCT AAG CAG TTG GTG GTG CA −3ʹ. The GAPDH mRNA level was used for normalization. The relative expression levels of Runx2, Col1α1 and ATP6V1H were measured by 2 –ΔΔCT method in reference to day zero values.
Western blot
MC3T3-E1 cells were lysed using RIPA buffer (sigma, USA) containing 1 mM phenylmethanesulfonyl fluoride (Sigma, USA). The lysates were electrophoresed in 10% SDS-PAGE gel. Subsequently, the lysates were transferred to polyvinylidene fluoride membranes (Invitrogen, Carlsbad, CA, USA), which were then blocked using 5% milk at room temperature. The membranes were incubated with primary antibodies at 4°C overnight. The primary antibodies were as follows: mouse polyclonal anti-Runx2 (1:500) (Sigma, St. Louis, USA), rabbit polyclonal anti-Col1α1 (1:500) (Sigma), rabbit polyclonal anti-ATH6V1H (1:500) (Sigma), rabbit polyclonal anti-SPC-1 (1:500) (Sigma), rabbit polyclonal anti-p-Akt (1:500) (Sigma), rabbit polyclonal anti-Akt (1:1,000) (Santa Cruz, Dallas, USA), rabbit polyclonal anti-p-GSK3β (1:300) (Sangon, Shanghai, China), rabbit polyclonal anti-GSK3β (1:300) (Sangon, Shanghai, China), and rabbit monoclonal anti-β-actin (1:1,000) (Cell Signaling Technology, Boston, USA). Then, the membranes were incubated with HRP conjugated secondary antibody for 2 h at room temperature. The signals were detected using a chemiluminescence reagent kit (Millipore, MA, USA). Image-Pro Plus 6.0 software was used to analyze the relative protein levels.
Statistical analysis
All data analyzed as the mean ± SD. All data are from three separate experiments. The statistical significance was determined using Student’s t-test. * P < .01, compared with the control group; # P < .01, compared with OM group. P < .05 was considered significant.
RESULTS
T2DM model was successfully established in MC3T3-E1 cells
To induce osteogenic differentiation, we cultured MC3T3-E1 cells using osteogenic medium (OM). Moreover, 25 mM glucose (high glucose, HG) and 1 mM FFA (palmitic acid and oleic acid at a ratio of 1:2) were added into osteogenic medium (OM+HG-FFA) to establish the T2DM model in MC3T3-E1 cells. As shown in Fig.1A, the cell viability of MC3T3-E1 cells showed almost no difference in the OM group as compared with control group, while were significantly inhibited in OM+HG-FFA group after 7 and 14 days treatments. HG-FFA treatment significantly decreased OM induced ALP activity (Fig.1B). After 14 days of differentiation, the mRNA and protein levels of runt-related transcription factor 2 (Runx2) significantly increased in OM group as compared with the control group (14 days without differentiation), while significantly decreased after HG-FFA treatment (Fig.1C-1E). In addition, the mRNA and protein levels of collagen type I α1 (Col1α1) significantly decreased after HG-FFA treatment as compared with OM group (Fig.1F-1H). These data demonstrate that we successfully established T2DM model in MC3T3-E1 cells.
FIGURE 1.
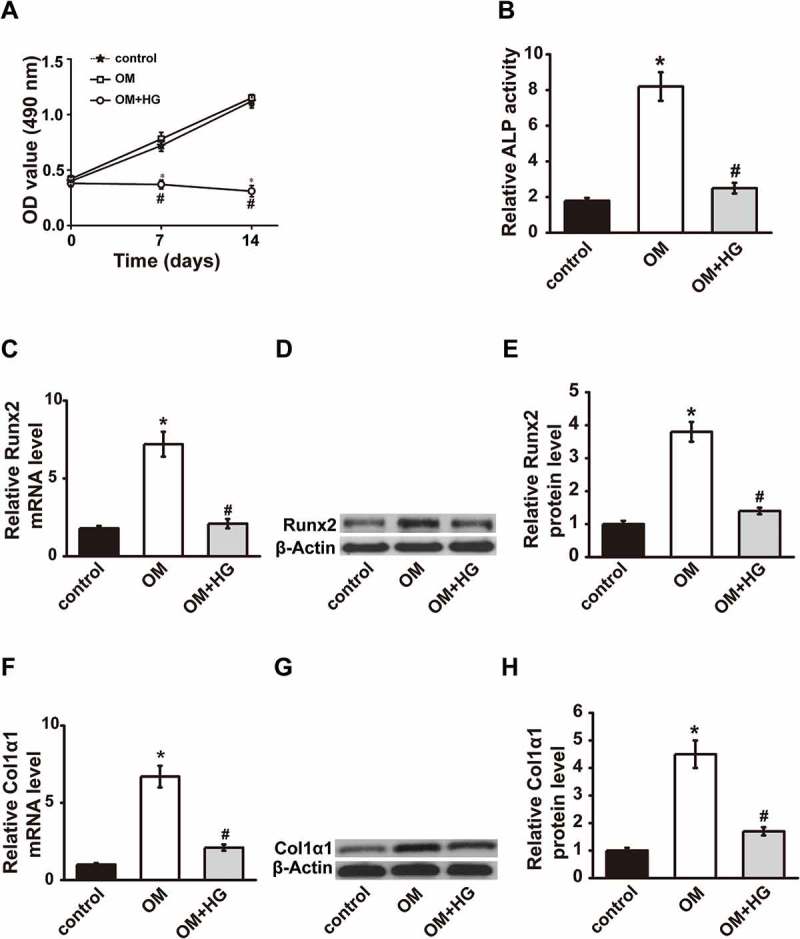
T2DM model was established in MC3T3-E1 cells. (A) Cell viability was measured by CCK-8 assay 0, 7 and 14 days after combined treatments with OM and HG. ALP activity (B), Runx2 expression (C-E) and Col1α1 expression (F-H) were measured 14 days after OM and HG treatment. OM, osteogenic medium; HG, high glucose-free fatty acids; ALP, alkaline phosphatase; Runx2, runt-related transcription factor 2; Col1α1, collagen type I α 1. Control, MC3T3-E1 cells cultured in α-MEM with 10% FBS for 14 days. * P < .01, compared with the control group (growth medium); # P < .01, compared with OM group.
ATP6V1H expression decreased in T2DM model
We next detected the role of ATP6V1H in diabetic osteoporosis using the established T2DM model. In MC3T3-E1 cells, 5 days after osteogenic differentiation by OM, ATP6V1H mRNA and protein levels showed no difference in the OM group when compared with the control group. However, 14 days after osteogenic differentiation by OM, ATP6V1H mRNA and protein levels significantly increased as compared with the control group (Fig. 2A-2C). In T2DM model (OM+HG-FFA), ATP6V1H mRNA and protein levels significantly decreased as compared with the OM group (Fig. 2D-2F). These data demonstrated that HG-FFA treatment markedly down-regulated ATP6V1H expression compared with the OM group, and more importantly inhibited osteogenic differentiation.
FIGURE 2.
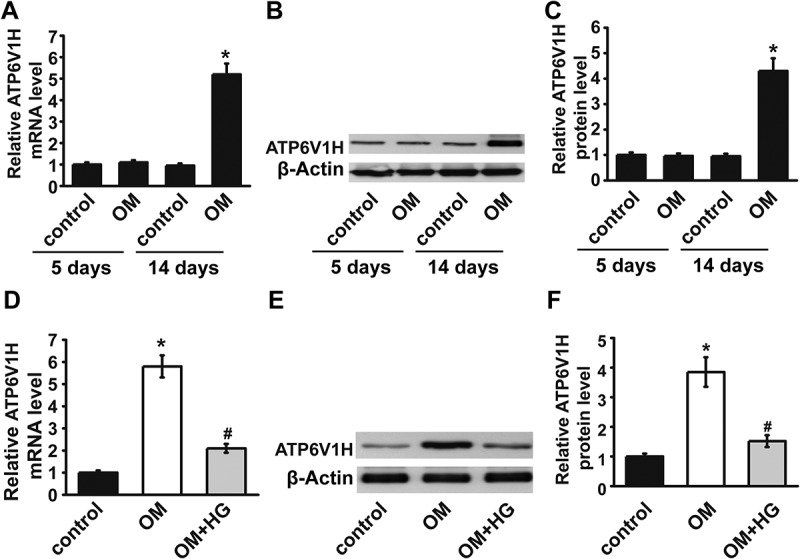
The expression of ATP6V1H induced by OM was decreased after HG treatment. (A-C) The mRNA and protein expression levels of ATP6V1H significantly increased 14 days after OM treatment. (D-F) HG treatment rescued the upregulation of ATP6V1H induced by OM at day 14. OM, osteogenic medium; HG, high glucose-free fatty acids. Control, MC3T3-E1 cells cultured in α-MEM with 10% FBS for 14 days. * P < .01, compared with the control group; # P < .01, compared with OM group.
ATP6V1H overexpression facilitates osteogenic differentiation
To further detect the role of ATP6V1H in osteogenic differentiation, we overexpressed ATP6V1H in MC3T3-E1 cells using p-ATP6V1H, while MC3T3-E1 cells transfected with p-NC serve as a negative control. The mRNA and protein levels of ATP6V1H in MC3T3-E1 cells significantly increased after ATP6V1H overexpression (OM+HG-FFA+p-ATP6V1H) as compared with negative control (OM+HG-FFA+p-NC) (Fig.3A-3C), confirming the successful overexpression of ATP6V1H in MC3T3-E1 cells. Runx2 and Col1α1 expression levels increased after osteogenic differentiation, while decreased in T2DM model (OM+HG-FFA+p-NC). In T2DM model, ATP6V1H overexpression (OM+HG-FFA+p-ATP6V1H) recovered mRNA and protein levels of Runx2 (Fig.3D-3F) and Col1α1 (Fig.3G-3I) in T2DM model as compared with negative control (OM+HG-FFA+p-NC). ATP6V1H overexpression also recovered ALP activity which was inhibited in T2DM model (Fig. 3J). There are more mineralized nodules when ATP6V1H was overexpressed (Fig. 3K), accompanied by a significant increase in absorbance (Fig. 3L). These results demonstrated that ATP6V1H overexpression facilitates osteogenic differentiation.
FIGURE 3.
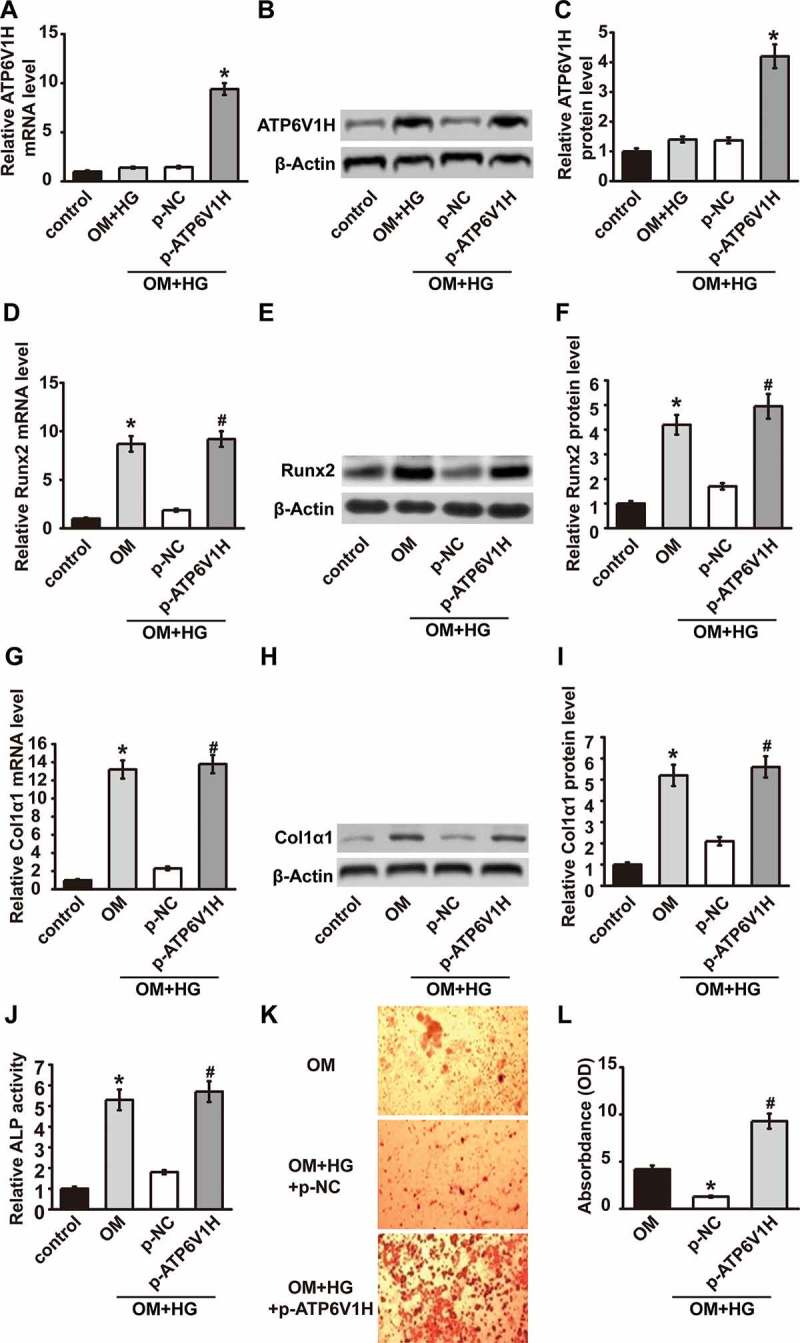
ATP6V1H overexpression facilitates osteogenic differentiation. (A-C) ATP6V1H was overexpressed by transfection of plasmid ATP6V1H. (D-F) Runx2 expression was restored by ATP6V1H compared to combined treatments with OM and HG. (G-I) Col1α1 expression was restored by ATP6V1H compared to combined treatments with OM and HG. (J) ALP activity ALP was restored by ATP6V1H compared to combined treatments with OM and HG. (K) The Alizarin Red staining was performed to determine the mineralized degree and (L) the absorbance showed quantified results. OM, osteogenic medium; HG, high glucose-free fatty acids; p-NC, negative control plasmid; p-ATP6V1H, ATP6V1H plasmid. *P < .01, compared with the control group; #P < .01, compared with OM group.
ATP6V1H overexpression inhibited Akt/GSK3β signaling pathway
Previous study has showed the important role of Akt/GSK3β pathway in osteogenic differentiation.13 We next explored whether the role of ATP6V1H in osteogenic differentiation is achieved through Akt/GSK3β pathway. ATP6V1H overexpression in MC3T3-E1 cells was first confirmed by western blot (Fig. 4A-4B). ATP6V1H overexpression significantly inhibited p-Akt and p-GSK3β expression levels in MC3T3-E1 cells (Fig. 4C-4D). These data demonstrated that ATP6V1H overexpression downregulates the Akt/GSK3β signaling pathway in MC3T3-E1 cells.
FIGURE 4.
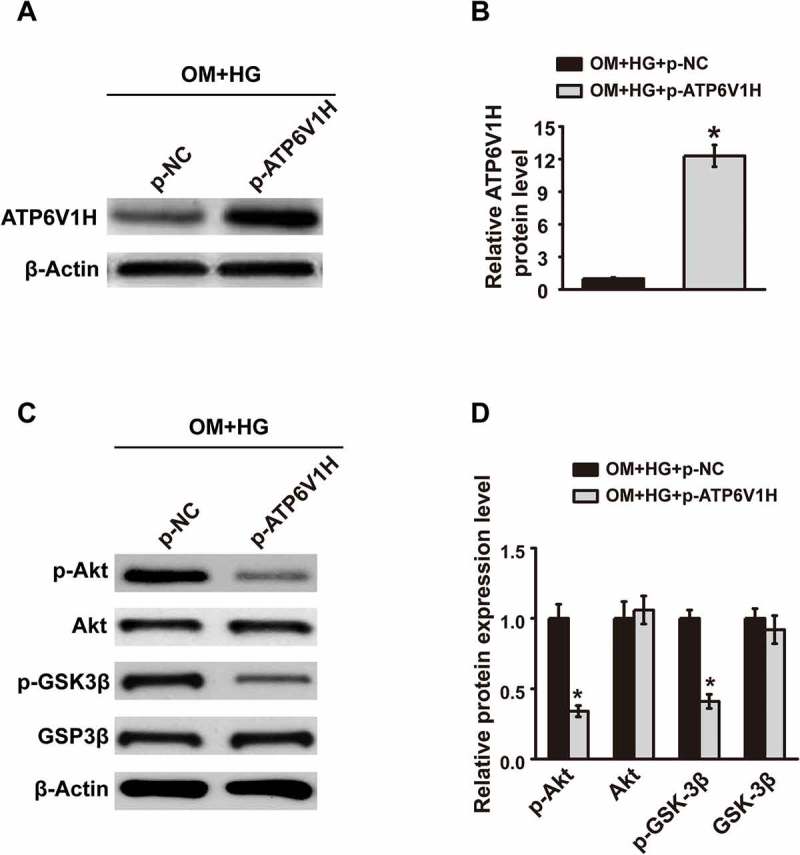
ATP6V1H downregulates the Akt/GSK3β signaling pathway in MC3T3-E1 cells. (A) The expression of ATP6V1H was detected by western blotting of protein samples from high glucose cultures of MC3T3-E1 cells transfected with negative control plasmid or ATP6V1H plasmid. (B) Quantitative assay of ATP6V1H western blot was performed by Image J software. (C) The expression of p-Akt, Akt, p-GSK3β and GSK3β was detected by western blotting of protein samples from high glucose cultures of MC3T3-E1 cells transfected with negative control plasmid or ATP6V1H plasmid. (D) Quantitative assay was performed by Image J software. OM, osteogenic medium; HG, high glucose-free fatty acids; p-NC, negative control plasmid; p-ATP6V1H, ATP6V1H plasmid; Akt, Serine/Threonine Kinase 1; GSK-3β, Glycogen Synthase Kinase 3β. *P < .01, compared with OM+HG+p-NC group.
ATP6V1H DOWN-REGULATION PROMOTED AKT/GSK3β SIGNALING PATHWAY
We next detected the influence of ATP6V1H down-regulation on the Akt/GSK3β signaling pathway. ATP6V1H down-regulation in MC3T3-E1 cells was first confirmed by western blot (Fig. 5A-5B). The down-regulation of ATP6V1H in MC3T3-E1 cells significantly promoted p-Akt and p-GSK3β expression levels in MC3T3-E1 cells (Fig. 5C-5D). These data demonstrated that ATP6V1H down-regulation promoted the Akt/GSK3β signaling pathway in MC3T3-E1 cells.
FIGURE 5.
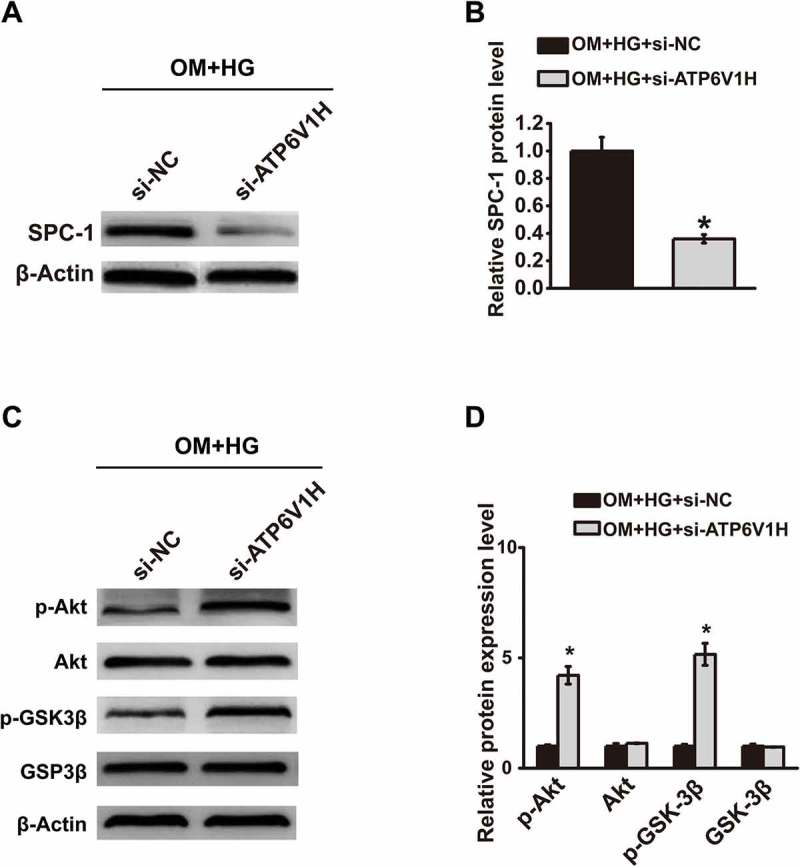
Knockdown of ATP6V1H upregulates the Akt/GSK3β signaling pathway in MC3T3-E1 cells. (A) The expression of ATP6V1H was detected by western blotting of protein samples from high glucose cultures of MC3T3-E1 cells transfected with si-NC or si-ATP6V1H. (B) Quantitative assay of ATP6V1H western blot was performed by Image J software. (C) The expression of p-Akt, Akt, p-GSK3β and GSK3β was detected by western blotting of protein samples from high glucose cultures of MC3T3-E1 cells transfected with si-NC or si-ATP6V1H. (D) Quantitative assay was performed by Image J software. OM, osteogenic medium; HG, high glucose-free fatty acids; sh-NC, negative control siRNA; si-ATP6V1H, ATP6V1H siRNA; Akt, Serine/Threonine Kinase 1; GSK-3β, Glycogen Synthase Kinase 3 beta. *P < .01, compared with OM+HG+si-NC group.
DISCUSSION
In the present study, we explored the role of ATP6V1H in osteogenic differentiation in MC3T3-E1 Cells. We found that ATP6V1H expression increased in OM-MC3T3-E1 cells, while decreased in OM+HG-MC3T3-E1 cells. ATP6V1H promotes osteogenic differentiation of OM+HG-MC3T3-E1 cells. Overexpression of ATP6V1H inhibits Akt/GSK3β signaling pathway, while knockdown of ATP6V1H promotes Akt/GSK3β signaling pathway. These data demonstrated that ATP6V1H could serve as the potential target for osteogenic differentiation in a T2DM simulating environment. To the best of our knowledge, this is the first evidence showing the role of ATP6V1H in osteogenic differentiation of T2DM model.
ATP6V1H is one of the V-type proton ATPase family members, which are crucial for intracellular compartment acidification of eukaryotic cells, protein degradation regulation, facilitating cellular function and development.14,15 About 26.7% (4 in 15) SNPs in ATP6V1H genes in 1625 Han Chinese were significantly involved in low mineral density of spine bone.7 Furthermore, bone remodeling decreased, while bone resorption increased in ATP6V1H ± mice.7 Mechanically, the pH value increased in intracellular osteoclasts, which inhibited TGF-β1 activation and osteoblast formation, while the bone mineralization showed no great difference in ATP6V1H ± mice as compared with ATP6V1H +/+ mice.7
ATP6V1H was first found to be associated with the T2DM in Muscle IGF-I receptor (IGF-IR)-lysine-arginine (MKR) mice when using isobaric tags for relative and absolute quantification (iTRAQ).16 In patients with T2DM, the expression levels if oxidative phosphorylation genes, such as ATP6V1H, NDUFA5, NDUFA10 and COX11 significantly decreased in pancreatic islets as compared with healthy donors using microarray analysis,17 suggesting that the decreased oxidative phosphorylation genes may result in a deficiency in insulin secretion. The research on T2DM in Mexican Americans showed that ATP6V1H expression levels in peripheral blood varied among different persons. However, ATP6V1H expression significantly decreased post T2DM development of as compared with pre-development, indicating that ATP6V1H is involved in the critical molecular mechanism which regulates T2DM development.18 In the present study, we established T2DM model in MC3T3-E1 cells and found ATP6V1H expression increased after osteogenic differentiation, while decreased in T2DM MC3T3-E1 Cells. ATP6V1H promotes osteogenic differentiation of T2DM MC3T3-E1 cells, indicating the important role of ATP6V1H in T2DM, which is consistent with previous studies.
Akt/GSK3β signaling pathway plays crucial roles in the various biological process.19,20 In osteogenic differentiation, all-trans-retinoic acid along with Wnt3A promoted osteogenic markers and potentiated AKT phosphorylation. In addition, pre-treatment with LY294002 inhibited the expression levels of osteogenic markers, suggesting that AKT/GSK3β signaling pathway was partially dependent in all-trans-retinoic acid-induced osteogenic differentiation.21 Runx2 promoted osteogenic-related genes expression levels, while administration of LY294002 abolished the effect, indicating that Runx2 promotes osteoblast differentiation via regulating AKT/GSK3β/pathway.22 In our study, we provided evidence that ATP6V1H overexpression inhibits Akt/GSK3β signaling pathway, while knockdown of ATP6V1H promotes Akt/GSK3β signaling pathway. These data demonstrated that ATP6V1H facilitates osteogenic differentiation in MC3T3-E1 cells via Akt/GSK3β signaling pathway.
The well-accepted method to stimulate T2DM in MC3T3-E1 cells using 25 mM glucose plus FFA (palmitic acid and oleic acid at a ratio of 1:2) was chosen in this study. However, it has been reported that oleic acid prevents palmitic acid-induced inflammation and insulin resistance in many cells.23 The steatosis extent in oleic acid-treated hepatocytes was higher than palmitic acid-treated, while palmitic acid displayed more apoptosis-inducing effect than oleic acid.24 In overweight adults, insulin sensitivity index decreased by 24% when fed with palmitic diet than oleic diet,25 indicating different sensitivity of oleic and palmitic acid to insulin resistance.
It is important to note that all the data from the study are from the established T2DM model using MC3T3-E1 cell lines, therefore other pre-osteoblastic cell lines should be used for further verification. Moreover, results of our current study have not been acquired from T2DM animal models or T2DM patients, which should be collected in future to further confirm the present in vitro results.
Taken together, we found ATP6V1H expression increased after osteogenic differentiation, while decreased in T2DM MC3T3-E1 cells. ATP6V1H promotes osteogenic differentiation of T2DM MC3T3-E1 cells. ATP6V1H overexpression inhibits Akt/GSK3β signaling pathway, while knockdown of ATP6V1H promotes Akt/GSK3β signaling pathway. These data demonstrated that ATP6V1H facilitates osteogenic differentiation in MC3T3-E1 cells via Akt/GSK3β signaling pathway.
ATP6V1H promotes osteogenic differentiation in MC3T3-E1 cells via Akt/GSK3β signaling pathway. These data indicate that ATP6V1H may serve as a potential target for clinical treatment of T2DM patients.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 81873993, No. 81572155); Municipal Human Resources Development Program for Outstanding Leaders in Medical Disciplines in Shanghai (No. 2018BR38); the Special Funds of the Key Clinical Research Project (No. DYZD201803); Shanghai Clinical Ability Construction of the Three Grade Hospital (No. SHDC12015904).
Conflicts of Interest
Fusong Jiang, Haojie Shan, Chenhao Pan, Zubin Zhou, Keze Cui, Yuanliang Chen, Haibo Zhong, Zhibin Lin, Nan Wang, Liang Yan, Xiaowei Yu declares no conflict of interest.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Sherwin RS.. The prevention of type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2005;34:199–219. viii. doi: 10.1016/j.ecl.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Ratajczak R. Structure, function and regulation of the plant vacuolar H(+)-translocating ATPase. Biochim Biophys Acta. 2000;1465:17–36. doi: 10.1016/s0005-2736(00)00129-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Huang H, Zhao G, Yokoyama T, Vega H, Huang Y, Sood R, Bishop K, Maduro V, Accardi J, et al. ATP6V1H deficiency impairs bone development through activation of MMP9 and MMP13. PLoS Genet. 2017;13:e1006481. doi: 10.1371/journal.pgen.1006481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Yang S, Zhang Y, Ji D, Jin Z, Duan X. ATP6V1H regulates the growth and differentiation of bone marrow stromal cells. Biochem Biophys Res Commun. 2018;502:84–90. doi: 10.1016/j.bbrc.2018.05.124. [DOI] [PubMed] [Google Scholar]
- 7.Duan X, Liu J, Zheng X, Wang Z, Zhang Y, Hao Y, Yang T, Deng H. Deficiency of ATP6V1H causes bone loss by inhibiting bone resorption and bone formation through the tgf-beta1 pathway. Theranostics. 2016;6:2183–95. doi: 10.7150/thno.17140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong K, Qu B, Liao D, Liu D, Wang C, Zhou J, Pan X. MiR-132 regulates osteogenic differentiation via downregulating Sirtuin1 in a peroxisome proliferator-activated receptor beta/delta-dependent manner. Biochem Biophys Res Commun. 2016;478:260–67. doi: 10.1016/j.bbrc.2016.07.057. [DOI] [PubMed] [Google Scholar]
- 9.Zhang WL, Meng HZ, Yang RF, Yang MW, Sun GH, Liu JH, Shi P-X, Liu F, Yang B. Melatonin suppresses autophagy in type 2 diabetic osteoporosis. Oncotarget. 2016;7:52179–94. doi: 10.18632/oncotarget.10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blakytny R, Spraul M, Jude EB. Review: the diabetic bone: a cellular and molecular perspective. Int J Low Extrem Wounds. 2011;10:16–32. doi: 10.1177/1534734611400256. [DOI] [PubMed] [Google Scholar]
- 11.Feng Z, Deng H, Du J, Chen D, Jiang R, Liang X. Lentiviral-mediated RNAi targeting p38MAPK ameliorates high glucose-induced apoptosis in osteoblast MC3T3-E1 cell line. Indian J Exp Biol. 2011;49:94–104. [PubMed] [Google Scholar]
- 12.You L, Gu W, Chen L, Pan L, Chen J, Peng Y. MiR-378 overexpression attenuates high glucose-suppressed osteogenic differentiation through targeting CASP3 and activating PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 2014;7:7249–61. [PMC free article] [PubMed] [Google Scholar]
- 13.Feng J, Sun Q, Liu L, Xing D. Photoactivation of TAZ via Akt/GSK3beta signaling pathway promotes osteogenic differentiation. Int J Biochem Cell Biol. 2015;66:59–68. doi: 10.1016/j.biocel.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Muench SP, Trinick J, Harrison MA. Structural divergence of the rotary ATPases. Q Rev Biophys. 2011;44:311–56. doi: 10.1017/S0033583510000338. [DOI] [PubMed] [Google Scholar]
- 15.Zhao W, Zhang Y, Yang S, Hao Y, Wang Z, Duan X. Analysis of two transcript isoforms of vacuolar ATPase subunit H in mouse and zebrafish. Gene. 2018;638:66–75. doi: 10.1016/j.gene.2017.09.065. [DOI] [PubMed] [Google Scholar]
- 16.Lu H, Yang Y, Allister EM, Wijesekara N, Wheeler MB. The identification of potential factors associated with the development of type 2 diabetes: a quantitative proteomics approach. Mol Cell Proteomics: MCP. 2008;7:1434–51. doi: 10.1074/mcp.M700478-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsson AH, Yang BT, Hall E, Taneera J, Salehi A, Nitert MD, Ling C. Decreased expression of genes involved in oxidative phosphorylation in human pancreatic islets from patients with type 2 diabetes. Eur J Endocrinol. 2011;165:589–95. doi: 10.1530/EJE-11-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina MF, Qu H-Q, Rentfro AR, Nair S, Lu Y, Hanis CL, McCormick JB, Fisher-Hoch SP. Decreased expression of ATP6V1H in type 2 diabetes: a pilot report on the diabetes risk study in Mexican Americans. Biochem Biophys Res Commun. 2011;412:728–31. doi: 10.1016/j.bbrc.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–31. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Chen X, Hu Y, Wu J, Cao Q, Chen S, Gao Y. All-trans retinoic acid modulates Wnt3A-induced osteogenic differentiation of mesenchymal stem cells via activating the PI3K/AKT/GSK3beta signalling pathway. Mol Cell Endocrinol. 2016;422:243–53. doi: 10.1016/j.mce.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Hu Y, Yang L, Zhou J, Tang Y, Zheng L, Qin P. Runx2 alleviates high glucose-suppressed osteogenic differentiation via PI3K/AKT/GSK3beta/beta-catenin pathway. Cell Biol Int. 2017;41:822–32. doi: 10.1002/cbin.10779. [DOI] [PubMed] [Google Scholar]
- 23.Bray GA, Lovejoy JC, Smith SR, DeLany JP, Lefevre M, Hwang D, Ryan DH, York DA. The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. J Nutr. 2002;132:2488–91. doi: 10.1093/jn/132.9.2488. [DOI] [PubMed] [Google Scholar]
- 24.Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S, et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24:830–40. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 25.Lovejoy JC, Smith SR, Champagne CM, Most MM, Lefevre M, DeLany JP, Denkins YM, Rood JC, Veldhuis J, Bray GA. Effects of diets enriched in saturated (palmitic), monounsaturated (oleic), or trans (elaidic) fatty acids on insulin sensitivity and substrate oxidation in healthy adults. Diabetes Care. 2002;25:1283–88. doi: 10.2337/diacare.25.8.1283. [DOI] [PubMed] [Google Scholar]


