Abstract
Cellular metabolism maintains the life of cells, allowing energy production required for building cellular constituents and maintaining homeostasis under constantly changing external environments. Neuronal cells maintain their structure and function for the entire life of organisms and the loss of neurons, with limited neurogenesis in adults, directly causes loss of complexity in the neuronal networks. The nervous system organizes the neurons by placing cell bodies containing nuclei of similar types of neurons in discrete regions. Accordingly, axons must travel great distances to connect different types of neurons and peripheral organs. The enormous surface area of neurons makes them high-energy demanding to keep their membrane potential. Distal axon survival is dependent on axonal transport that is another energy demanding process. All of these factors make metabolic stress a potential risk factor for neuronal death and neuronal degeneration often associated with metabolic diseases. This review discusses recent findings on metabolic dysregulations under neuronal degeneration and pathways protecting neurons in these conditions.
Keywords: neurodegeneration, metabolism, axon, nicotinamide adenine dinucleotide, axon degeneration
Introduction
Genetic and epidemiological evidence indicates the role of abnormal metabolism in neurodegenerative diseases. It is known that many neurological disorders are directly caused by mutations in enzymes and transporters of the metabolite, i.e., small molecules essential for cellular metabolism. Growing evidence also suggests the close association of metabolic defects and neuronal degeneration, e.g. type 2 diabetes and Alzheimer’s disease. In addition, age dependent decline of cellular metabolism coincides with neurodegenerative diseases including Alzheimer’s and Parkinson’s disease. Deficits in cellular metabolism result in the failure of energy production, biomolecule synthesis (amino acids, nucleotides, fatty acids, lipids), ROS regulation, or proteostasis: ultimately affecting all type of cells in the body. This review will primarily focus on the metabolic consequences in the neuronal cell under neurodegenerative conditions.
1. The link between neuronal degeneration and metabolic defects
The nervous system is a high-energy demanding network of cells that consumes about 15% of the total energy expenditure in the human body. The dysregulation of cellular energy metabolism is the primary risk factor for various neurodegenerative disorders (Camandola and Mattson, 2017; Procaccini et al., 2016). Cellular metabolic regulators collect nutritional information and initiate signals that adapt cellular metabolism for a constantly changing environment. These metabolic regulators including mTOR, PPARγ, and AMPK, are essential for neuronal development and homeostasis. Dysregulated cellular metabolism reflected by alterations of signaling in and out of metabolic regulators are common in neurodegenerative diseases.
1.1. Metabolic dysregulation in neurodegenerative conditions
mTOR signaling
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that is regulated by the cellular nutrient and growth factor levels (Saxton and Sabatini, 2017; Sengupta et al., 2010). mTOR is the core component of two distinct protein complexes, mTORC1 and mTORC2, which regulate diverse cellular functions including protein synthesis, immune response, cell growth, proliferation, and autophagy. mTORC1 is activated by growth factors and nutrients and inhibited by energetic stresses. FKBP12 suppresses mTORC1 complex formation and downstream kinase cascades upon binding to rapamycin, an FDA-approved antibiotic and immunosuppressant drug. mTORC2 is far less sensitive to rapamycin and activates AKT signaling that in turn promotes cell survival, proliferation and also various metabolic pathways. Growing evidence shows that neurodegenerative disorders including Alzheimer’s (AD), Parkinson’s (PD), and Huntington’s (HD) disease exhibit dysregulated mTOR signaling. Thus, targeting mTOR signaling for neurodegenerative treatment is an emerging topic (Bové et al., 2011; Cai et al., 2015).
Among diverse sets of cellular processes controlled by mTOR, autophagy, a conserved catabolic process that degrades cellular components (Ohsumi, 2014), plays important roles in neurodegeneration (Menzies et al., 2017). Protein misfolding and aggregate formation are a common feature of various age-associated neurodegenerative diseases. Accumulation of misfolded protein is toxic to cells and autophagy dysregulation is implicated in this process. Rapamycin inhibits mTORC1 and stimulates autophagy by increasing autophagosome formation and lysosome-mediated clearance of autophagosomes. It is possible that rapamycin promotes clearance or suppression of misfolded protein aggregates and improves cognitive function. Consistent with this idea, long-term administration of rapamycin in AD model mice PDAPP overexpressing human APP containing V717F (Indiana) mutation or genetic ablation of one copy of mTOR in CNS neurons of AD model mice Tg2576 overexpressing human APP containing the double mutation KM670/671NL (Swedish) mutation showed improved cognitive function, increased autophagy, and reduced Aβ42, a proteolytic cleavage product of APP forming aggregate (Caccamo et al., 2014; Spilman et al., 2010). Rapamycin also depletes the PrP amyloid plaque deposition with increased autophagy in Gerstmann–Sträussler–Scheinker disease (GSS) model mice (Cortes et al., 2012). In addition, genetic reduction of S6K1, a downstream target of mTOR, leads to the reduced transcription of β-site APP cleaving enzyme 1 (BACE-1) and tau thereby reduces Aβ production and phosphorylated tau in AD mice Tg2576 (Caccamo et al., 2015). Similarly, neuronal mTOR hyperactivation causes various neurological defects depending on the age of the animal (Kassai et al., 2014). Autophagy and BACE1 transcription regulation through mTOR signaling likely play an important role in AD and the mechanism that overactivates mTOR under disease conditions will be a key to understand the pathogenesis of AD.
Parkinson’s disease (PD) is characterized by α-synuclein aggregates, mitochondrial damage, and apoptosis in substantia nigra dopaminergic neurons. Mitophagy is an mTOR regulated cellular defense mechanism that induces clearance of damaged mitochondria without releasing proapoptotic factors. In healthy mitochondria, PINK1 shuttled from the cytosol is kept low by mitochondria protease mediated PINK1 c- terminal cleavage, which releases PINK1 from mitochondria. However, mitochondria dysfunction and subsequent membrane potential loss slow PINK1 proteolysis and cause the accumulation of PINK1 on the mitochondria outer membrane. Mitochondria tethered PINK1 phosphorylates PARKIN and stimulates recruitment of PARKIN to mitochondria and activates PARKIN’s E3 ligase activity that promotes mitophagy. Mitophagy dysfunction by mutations in PINK1 and PARKIN causes familial PD (Pickrell and Youle, 2015). Rapamycin prevents mitophagy dysfunction and the neurodegenerative phenotype in PD model mice harboring the PARKIN mutation (Siddiqui et al., 2015). In addition, the role of autophagy in the clearance of α-synuclein aggregates is implicated using an in vitro cell model (Webb et al., 2003). These results suggest the beneficial effect of restoring the mTOR pathway in the treatment of PD. Stress-induced protein DDIT4 (REDD1 or RTP801) is induced in the postmortem human PD brain as well as cellular or mouse PD models. DDIT4 inhibits AKT pro-survival signaling perhaps through mTORC2 (Malagelada et al., 2008). However, DDIT4 also binds 14–3-3, TSC2 inhibitor, and promotes TSC2 mediated mTORC1 inhibition that is known to protect neurons. Whether the role of DDIT4 is protective or harmful in different stages of PD still needs to be determined.
The mTOR inhibitor, rapamycin or its analogue CCI-779, improves behavior tasks and reduces aggregate formation in fly or mouse Huntington’s disease (HD) models (Ravikumar et al., 2004). Huntingtin (Htt) interacts with Rheb, a small GTPase protein that stimulates mTORC1 activity and induces mTOR signaling (Pryor et al., 2014). The activation of mTORC1 by the deletion of one copy of TSC1, a negative regulator of mTORC1, accelerates the onset of motor deficits and premature death (Pryor et al., 2014). Another study showed that the activation of mTORC1 by the expression of constitutively active Rheb alleviates striatal atrophy as well as other metabolic defects in HD disease model mice (J. H. Lee et al., 2015). Interestingly, the striatal autophagy signal is increased in the case of Rheb activation and Htt aggregates are reduced after Rheb mediated mTORC1 activation in the striatum. These results show activation of mTOR signaling has a biphasic effect on HD pathophysiology. Although further studies are required, it is clear that cellular metabolism modulates HD pathophysiology through mTOR signaling.
Diabetes is known as a potential risk factor for Alzheimer’s disease, with abnormalities in insulin signaling reported in AD brains from human patients and model mice (Frölich et al., 1998; Orr et al., 2014). Brain insulin signal dysregulation (hyper phosphorylation of IRS1 and downstream kinases PI3K and AKT) and Aβ burden in AD model mice Tg2576 expressing mutant APP (Swedish) are suppressed by the removal of one copy of mTOR (Caccamo et al., 2018). The administration of a high sucrose diet to AD model mice 3xTg expressing mutant APP (KM670/671NL), Tau (P301L), and PSEN1 (M146V) exaggerates the dysregulation of brain insulin signaling and accumulation of Aβ and phosphorylated Tau. Interestingly, the sucrose-mediated effects are mTOR dependent and administration of rapamycin blocks the effects of sucrose (Orr et al., 2014). In contrast, brain hypometabolism due to low glucose levels is common in Alzheimer’s disease patients. Accordingly, the selective expression of glucose transporter GLUT1 in neurons rescued the life span, behavior, and pathology of Aβ42 expressing AD Drosophila by increasing unfolded protein response (Niccoli et al., 2016). Metformin that stimulates glucose transport mimic the GLUT1-mediated rescue effects. These results indicate that high glucose stimulates or suppresses AD pathology depending on the context. It seems that the balance between glucose stimulation and mTOR activity is important or the effect of glucose may differ in different models and disease stages. Further studies are required to fully evaluate the role of glucose homeostasis in AD brains.
PPARγ signaling
Peroxisome proliferator-activated receptor (PPAR) is a ligand activated nuclear hormone receptor. PPAR regulates cellular metabolic processes including inflammation signaling, mitochondria function, and lipid metabolism, which are pathways often dysregulated in neurodegeneration. PPAR is activated by a diverse set of fatty acids and forms heterodimers with the retinoid X receptors (RXRs), which then bind to PPAR response elements (PPRE) to modulate gene expression (Desvergne et al., n.d.). There are three subtypes of PPAR, α, β/δ, and γ, and all subtypes are expressed in the brain (Warden et al., 2016). PPARγ regulates various pathways that are linked to insulin sensitivity, blood glucose, lipid metabolism, antioxidants, and inflammation. Interestingly, PPARγ depletion from CNS neurons attenuates high-fat, diet-induced weight gain by reducing food intake and increasing energy expenditure (Lu et al., 2011), suggesting that PPARγ is one of the key molecules connecting the nervous system to systemic metabolism.
The involvement of PPAR signaling in neuronal degeneration is indicated by experiments showing beneficial effects of PPARγ activation in neurodegenerative models (Corona and Duchen, 2016). Conditional depletion of PPARγ in neurons results in increased brain damage and oxidative stress after middle cerebral artery occlusion (Zhao et al., 2009). Cultured PPARγ KO neurons are more susceptible to oxidative glucose deprivation compared with wild type and pharmacological activation of PPARγ suppresses neuronal death. PPARγ activation induces the expression of antioxidant genes including catalase, SOD1, and GST, suggesting PPARγ-mediated neuronal protection is, at least partially, dependent on the enhancement of antioxidant pathways (Zhao et al., 2009). PPARγ agonists are also neuroprotective in traumatic brain injury model (Qi et al., 2010).
In addition, PPARγ agonist genistein promotes Aβ clearance through increased expression of APOE in AD model mice APPswe/PS1dE9 (or AβPP/PS1) expressing mutant APP (KM670/671NL) and pathogenic PSEN1 lacking exon 9 (Bonet-Costa et al., 2016). PPARγ activity is also regulated by the recruitment of cofactors including PGC1α. Ectopic expression of PGC1α suppresses the expression of BACE1, reduces Aβ production, and improves spatial and recognition memory in AD model mice expressing pathogenic APP (Katsouri et al., 2016). PGC1α expression is shown to decrease in the AD patient brain, suggesting the role of PGC1α in AD pathogenesis (Katsouri et al., 2011)
AMPK signaling
AMP-activated protein kinase (AMPK) is an energy-sensing enzyme that is activated by reduced energy availability reflected by an increase of AMP:ATP ratio (Burkewitz et al., 2014). Activated AMPK suppresses ATP consumption and increases ATP generation via fatty acid oxidation. AMPK is a heterotrimer consisting of a catalytic α subunit and a regulatory β and γ subunit. AMPK is activated by the phosphorylation of the α subunit Thr172 by upstream kinases LKB1, CaMKKβ, and Tak1. AMPK kinase activity is allosterically activated, to a much lesser extent than Thr172 phosphorylation, by the binding of AMP to the γ subunit. However, this AMP binding facilitates the LKB1 mediated α subunit Thr172 phosphorylation. AMPK regulates numerous metabolic pathways including mTOR, which is inhibited by the phosphorylation of TSC2 and raptor (Gwinn et al., 2008; Inoki et al., 2003). AMPK is a potential therapeutic target for diseases involving metabolic dysregulation.
The AMPK pathway is dysregulated under neurodegenerative conditions including Parkinson’s, Huntington’s, and Alzheimer’s disease. Extracellular Aβ oligomer formation and intracellular accumulation of hyperphosphorylated tau are two prominent features of Alzheimer’s and other related dementia. Aβ42 oligomers induce AMPK activation (pT172 AMPK) depending on the CAMKK2 in cultured hippocampal neurons. This CAMKK2-AMPK signaling induces tau phosphorylation (S262), causing synaptic toxicity in AD model mice (APP/SWE, IND) (Mairet-Coello et al., 2013). Deletion of AMPKα2, the most abundant AMPK catalytic subunit, reduces the phosphorylation of endogenous tau in wild type mice and induces significant reduction of insoluble and MC1 positive tau (a form of pathological tau found in an early phase of AD) in AD model mice expressing Tau P301S (Domise et al., 2016). These results suggest the important role of AMPK mediated tau phosphorylation in AD pathogenesis.
Calorie restriction (CR) promotes lifespan by slowing the progression of age-associated diseases including neurodegeneration (Bayliss et al., 2016; Gräff et al., 2013). Genetic and pharmacological evidence supports the strong connection between CR and AMPK activation (Cantó and Auwerx, 2011). While a high-fat/cholesterol diet regimen upregulates BASE1 expression, CR reduces BACE1 transcription via the activation of the AMPK-SIRT1-PGC-1 pathway (R. Wang et al., 2013). Similarly, the cognitive deficits and neuroinflammation associated with altered APP processing are observed in wild type mice after eight weeks of a high fat/high cholesterol diet regimen that inactivates AMPK (Thirumangalakudi et al., 2008).
Overactivation of AMPK is reported in the HD patient brain and HD model mice. AMPK activation by AICAR facilitates Htt aggregate formation and neuronal death by nuclear translocation of AMPKα1 (Ju et al., 2011). Mechanistically, AMPKα1 is activated and translocated into the striatal neuron nucleus in HD model mice and suppresses Bcl2 expression, thereby promoting neuronal cell death.
Overactivation of AMPK is also observed in Lewy body disease (Jiang et al., 2013). Increased lactate, likely due to the mitochondrial dysfunction that is a hallmark of PD, is known to activate AMPK and may contribute to the initial AMPK activation process (Ross et al., 2010). Activated AMPK then phosphorylates α-synuclein S129 and induces PIKE-L, a small GTPase that negatively regulates AMPK to bind to α-synuclein. This leads to the sequestration of PIKE-L into α-synuclein containing Lewy bodies, hinders PIKE-L from AMPK inhibition, and further activates AMPK with a feed forward loop manner (Kang et al., 2017). Overactivation of AMPK results in FOXO3a translocation into the nucleus and increases the expression of proapoptotic Bim and cleaved caspase3 in the hypoxia-ischemia rat brain damage model (D. Li et al., 2017).
1.2. NAD+ metabolism and neurodegeneration
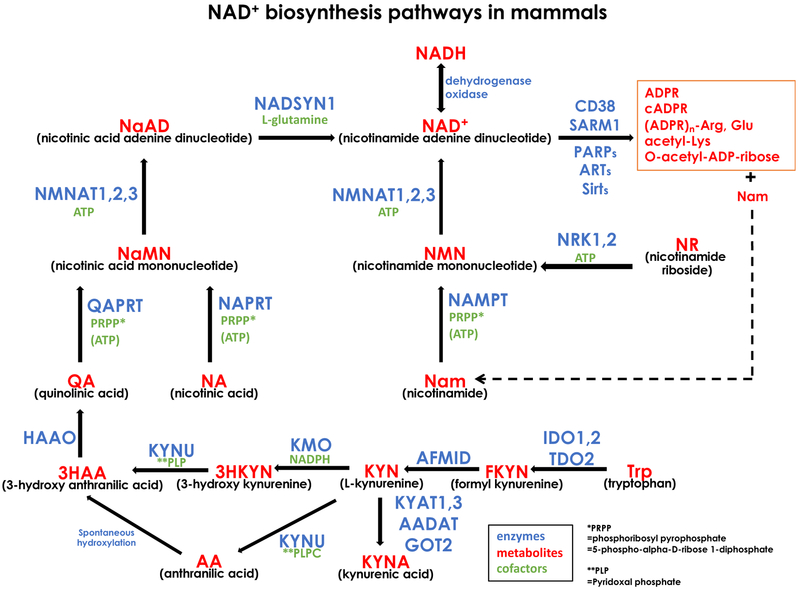
Nicotinamide adenine dinucleotide (NAD+), its reduced form NADH, and its phosphorylated forms are central regulators of cell metabolism by serving as cofactors for glycolysis, oxidative phosphorylation, TCA cycle, redox reactions, and protein modifications including acetylation and ADP-ribosylation. Mammalian NAD+ synthesis pathways consist of multiple enzymes and substrates (Fig.1). Vitamin B3 (nicotinamide (Nam) or nicotinic acid (NA)) is the primary precursor of NAD+ synthesis in mammalian cells. Nam is converted to nicotinamide mononucleotide (NMN) by NAMPT and NA is converted to nicotinic acid mononucleotide (NaMN) by NaPRT, respectively. There are three mammalian enzymes, NMNAT1, 2, and 3, that convert NMN to NAD+ or NaMN to nicotinic acid adenine dinucleotide (NaAD). NMNAT1, 2, and 3 localize to the nucleus, Golgi/cytosol, and mitochondria, respectively, suggesting discrete regulation of NAD+ metabolism in different cellular compartments (Berger et al., 2005; Gilley and Coleman, 2010; Ryu, 2018). NAD+ synthase produces NAD+ from NaAD. Nrk1 and 2 provide an alternative route to synthesize NMN from nicotinamide riboside (NR). NaMN is also produced from tryptophan (Trp) via a de novo NAD+ synthesis pathway consisting of multiple enzymes. The availability and usage of NAD+ precursors including tryptophan (Trp), Nam, NA, NMN, NaMN, and NR vary depending on the type of cells. While the interconversion between NAD+ and NADH is a major reaction in the redox pathway, NAD+ is consumed by different classes of enzymes including deacetylase, ADP ribosylase, and NAD+ glycohydrorase. The half-life of NAD+ estimated by using isotope labelled NAD+ precursors is about one hour in Hela derivative cells, 15 minutes to 15 hours in various moue tissues, and seven hours in cultured neurons, indicating the active nature of NAD+ consuming enzymes (Ijichi et al., 1966; Liu et al., n.d.; Rechsteiner et al., 1976; Sasaki et al., 2016).
Figure1. NAD+ biosynthesis pathways.
A summary of mammalian NAD+ synthesis and catabolism. Official gene symbols of each enzyme, metabolites and their common abbreviated names, and cofactors necessary for enzymes are summarized.
NAD+ metabolism is impaired in various neurological disorders including Alzheimer’s, Parkinson’s, and retinal degeneration diseases (Cantó et al., 2015; Fang et al., 2017; J. B. Lin et al., 2016; Verdin, 2015) and neuronal injury accelerates NAD+ consumption (see detail in section 2.2). CD38 is a constitutively active NAD+ glycohydrolase that mainly produces ADPR and Nam but also has the capability to produce cADPR and NaADP (Graeff et al., 2009; W. K. Lin et al., 2017). Mice deficient in CD38 show elevated NAD+ in various tissues including the brain and show social behavior deficits (Jin et al., 2007; Young et al., 2006). Recent studies show that the depletion of CD38 reduces the number of Aβ plaque and both soluble and insoluble forms of Aβ in AD model mice APP.PS expressing mutant APP (KM670/671NL) and pathogenic PSEN1 lacking exon 9 (Blacher et al., 2015). In these mice, α-, β-, and γ-secretase activity is significantly reduced without altering their expression levels. Increased NAD+, a CD38 inhibitor, or a cADPR antagonist reduced Aβ production in cultured neurons from APP, suggesting that CD38 enzymatic activity and the product cADPR likely modulate APP processing. CD38 also modulates the recruitment of microglia and macrophage in the hippocampus of APP/PS mice. Consistent with the reduced Aβ burden and microglia/macrophage accumulation, APP.PS:CD38−/− mice showed significant improvement in special learning memory and cortical synaptophysin levels compared with APP.PS mice.
The tryptophan catabolic route and the kynurenine pathway produces the NAD+ precursor, quinolinic acid, by using multiple enzymes (Fig.1). Some of the kynurenine pathway metabolites have neuromodulation activities. Quinolinic acid (QA) is a NMDA agonist and kynurenic acid (KYNA) is an antagonist for excitatory amino acid receptors including NMDA, AMPA, kainic acid, and glutamate receptors. Metabolites in the kynurenine pathways are altered in AD, PD, and HD patient brains. KYNA in the serum and red blood cells are decreased in AD patients (Hartai et al., 2007). The reduction in KNA and KYNA and the increase in 3-hydroxy kynurenine (3HKYN) are observed in various regions of the PD brain (Ogawa et al., 1992). QA and 3HKYN are increased in the HD brain (Guidetti et al., 2004). The inhibition of two kynurenine pathway enzymes, TDO, a rate-limiter of QA synthesis, and KMO, ameliorates neurodegenerative phenotypes including neuronal death, motor function, and life span in Drosophila AD (Aβ42), PD(α-Syn), and HD (Htt93Q) models (Breda et al., 2016; Campesan et al., 2011). Although the underlying mechanism is not clear, pharmacological inhibition of TDO also provides robust neuroprotection in Drosophila neurodegeneration models (Campesan et al., 2011).
Recent studies show that the administration of NAD+ precursors that promote NAD+ synthesis modulate the progression of neurodegenerative diseases. For example, systemic administration of nicotinamide riboside (NR) significantly improves cognitive function in AD model mice Tg2576 expressing mutant APP (KM670/671NL) (B. Gong et al., 2013). NR treatment increases the NAD+ level in cerebral cortex and the expression of PGC1α whose expression is known to be reduced in AD patient brains (Katsouri et al., 2011). Ectopic expression of PGC1α suppressed the expression of β-site APP cleaving enzyme 1 (BACE1), the main enzyme involved in Aβ generation, and reduced Aβ deposition which then improved spatial and recognition memory in AD model mice APP23 expressing APP (KM670/671NL) (Katsouri et al., 2016). Accordingly, NR administration reduces BACE1 expression levels in a PGC1α dependent manner. Mechanistically, PGC1α promotes BACE1 degradation through ubiquitin proteasomal systems and improves Aβ pathology and cognitive function (B. Gong et al., 2013). NR also increases PGC1α associated energy metabolism genes including citrate synthase, aconitase, and pyruvate dehydrogenase kinase, and this may partially contribute to the improvement of cognitive function in AD mice (B. Gong et al., 2013). Genotoxic stresses also have a significant impact on AD disease progression. Introducing the heterozygosity of polymerase β, the primary base-excision repair enzyme, into 3xTgAD mice exacerbates AD disease phenotypes. NR administration significantly reduces Tau phosphorylation in 3xTgAD/Polβ mice brain without altering Aβ production or plaque formation, also suggesting the beneficial effect of NR towards Tau burden (Hou et al., 2018). The protection of mice from noise-induced hearing loss by systemic NR mediated SIRT3 activation suggests the beneficial effect of NR for acute neuronal injury as well (Brown et al., 2014).
Administration of nicotinamide mononucleotide (NMN), an alternative NAD+ precursor found in various tissues, also suppressed cognitive impairment in Alzheimer’s disease model mice APPswe/PS1dE9 (or AβPP/PS1) expressing mutant APP (KM670/671NL) and pathogenic PSEN1 lacking exon 9 (Yao et al., 2017). The elevations of p-JNK and Aβ in the hippocampus and cerebral cortex of AD mice are markedly reduced with NMN administration. NMN increases non-pathogenic α-secretase cleavage of APP and inhibits the APP Thr668 phosphorylation that promotes the β-secretase cleavage of APP and Aβ accumulation in AD mice. NMN administrated mice also attenuated inflammation and synaptic loss in AD brain (Yao et al., 2017). NMN improved mitochondrial bioenergetics and dynamics in the same AD mouse brain expressing mutant APP (KM670/671NL) and pathogenic PSEN1 lacking exon 9 (Long et al., 2015). Using a rat AD model generated by an intracerebroventricular Aβ oligomer infusion, NMN showed sustained improvement in cognitive function (X. Wang et al., 2016). NMN attenuated neuronal cell death and inhibition of LTP, and restored levels in NAD+ and ATP in the Aβ oligomer-treated hippocampal slices.
NMNAT2 is dominantly expressed in neuronal tissues and is an essential NAD+ biosynthesis enzyme for the peripheral nervous systems homeostasis (Hicks et al., 2012). NMNAT2 is also important for brain function, as higher NMNAT2 mRNA levels are associated with better cognitive performance and lower AD pathology in an aged human brain (Ali et al., 2016). NMNAT2 expression is positively regulated by the binding of phosphorylated cAMP-response element binding protein (pCREB) to CRE sites in the NMNAT2 promoter. The expression of pCREB and recruitment of pCREB to CRE are both suppressed and NMNAT2 is down regulated in the hippocampus and cortex of AD mice Tg4510 expressing pathogenic human Tau (P301L) (Ljungberg et al., 2012). Overexpression of NMNAT2 or NMNAT1 but not NMNAT3 significantly reduces AD related phenotypes in Tg4510. The NMNAT2:HSP90 complex reduced hyperphosphorylated Tau in the Tg4510 hippocampus while total Tau levels remained unchanged (Ali et al., 2016). The overexpression of NMNAT2 also reduced hyperphosphorylated Tau by restoring the activity of PP2A, the most active Tau phosphatase whose activity is reduced in the human AD brain and the AD mouse brain (Cheng et al., 2013; C. X. Gong et al., 1995).
NMNAT1 overexpression preserved cortical neuron functional connectivity and decreased the accumulation of detergent-insoluble tau in the cerebral cortex of AD model mice expressing pathogenic Tau (P301S) (Musiek et al., 2016). Although the detailed mechanism is still obscure, NMNAT improved AD related neuropathology by reducing the accumulation of hyperphospohrylated or detergent insoluble Tau.
DLK (MAP3K12) is a MAPKKK that promotes various injury-stimulated pathways in axon degeneration, neuronal apoptosis, or axonal regeneration (Miller et al., 2009; Pozniak et al., 2013; Shin et al., 2012; Yang et al., 2015). Genetic or pharmacological inhibition of DLK improved neuronal survival, synaptic integrity and cognitive functions in neurodegenerative models including Alzheimer’s disease and amyotrophic lateral sclerosis (ALS) (Le Pichon et al., 2017). Interestingly, DLK signaling promotes NMNAT2 turnover, a critical determinant of axonal homeostasis. The inhibition of DLK and downstream kinases, MKK4/7 and JNK, elevate the NMNAT2 level (Walker et al., 2017a). These results suggest the inhibition of DLK likely increases the neuronal NMNAT2 that may facilitate axonal stability and reduce accumulation of hyperphosphorylated Tau in AD brain.
Tau hyperacetylation is implicated in neurodegeneration and cognitive decline in the AD brain. Depletion of SIRT1 exacerbates premature mortality, synapse loss, and behavioral deficits in AD model mice (tauP301S) with increased acetylation at Lys174 of Tau in post synaptic density enriched brain lysate (Min et al., 2018). Ectopic expression of SIRT1 in the hippocampus reduces the acetylated Tau and attenuates the spread of Tau pathology. SIRT3 is markedly decreased in the AD brain and consequently acetylated P53 at Lys 320 is increased. Mitochondrial P53 negatively regulates the expression of ND2 and ND4, subunits of respiratory chain complex1, by occupying the P53 binding element in the mitochondrial DNA. SIRT3 overexpression restores ND2 and ND4 expression and mitochondrial oxygen consumption in cultured cortical neurons (J. Lee et al., 2018). SIRT3 overexpressing mice are also resistant to noise-induced hearing loss (Brown et al., 2014). SIRT6 expression is reduced in AD patients and (a) neuron specific SIRT6 deletion in mice brains resulted in DNA damage, neuronal cell death, and hyperphosphorylation of Tau due to increased GSK3α/β, a major Tau phosphorylating enzyme (Kaluski et al., 2017). Inhibition of SIRT2 is neuroprotective for the Drosophila HD model and reduces polyglutamine toxicity in cultured striatal neurons through downregulation of sterol biosynthesis genes (Luthi-Carter et al., 2010; Outeiro et al., 2007). In another study, SIRT2 overexpression is protective and pharmacological inhibition of SIRT2 is harmful for SHSY5Y cells treated with rotenone or diquat, suggesting a context dependent role of SIRT2 for neuronal homeostasis (Singh et al., 2017). A deficiency of SIRT5, mitochondria matrix sirutuin, increases kainic acid induced seizures, hippocampal neuronal degeneration, and mortality rates (F. Li and Liu, 2016). Similarly, SIRT4 deficient mice show a worsened seizure phenotype after kainic acid treatment without altering the mortality rate or percent of animals that responded. However, these defects are mainly due to the reduction of astrocyte glutamate transporter expression (Shih et al., 2014). These results indicate the important roles of sirtuins in neuronal homeostasis and studies to investigate the function of sirtuins in different types of cells and different types of diseases will provide further insight into the roles of these important NAD+ dependent enzymes.
While hyperactivation of MAP kinase signaling is detrimental to the nervous system, hypoactivity of MAP kinase signaling also causes neuronal dysfunction. JNK is constitutively phosphorylated and plays important roles in the maintenance of the adult nervous system. Deletion of neuron specific isoform JNK3 caused dysregulation of circadian rhythm behavior (Yoshitane et al., 2012). Depletion in the neuron of MKK7, an upstream kinase of JNK, resulted in age-dependent hypoactivity, dysregulated circadian behavior, and motor neuron axon degeneration with increased APP in the nervous system (Yamasaki et al., 2017). Recent studies suggest a link between neurodegeneration and the mechanism causing disruptions of the circadian clock (Musiek and Holtzman, 2016; Panda, 2016). One of the important functions of the circadian clock is to synchronize the body’s metabolism and adjust it to the rhythmic behavior of day and night cycles. The circadian clock is also known to regulate NAD+ metabolism through the NAD+ rate limiting enzyme NAMPT and NAD+ dependent deacetylase SIRT1 (Nakahata et al., 2009), suggesting the role of NAD+ metabolism in neurodegeneration induced by the dysregulation of the circadian clock.
2. Axonal homeostasis and neuronal degeneration
Degeneration of nervous systems is initiated by vascular change, neuroinflammation, glial dysfunction, or neurons themselves, and results in the loss of neuronal connections due to the death of the neuron and/or axon degeneration. The estimated cumulative length of axons is 149,000 to 176,000 km, about four times the Earth’s circumference, in human brain white matter at the age of 20 (Marner et al., 2003). This enormous stretch of the axon is generated during development and maintained throughout life. Since axons are highly vulnerable and poorly regenerated, the total length of axons in the white matter is reduced by 10% each decade after 20 years old with a much smaller decline in gray matter, resulting in the reduced complexity of the neuronal network in the aged brain (Marner et al., 2003). Axonal loss is also observed in various neurological disorders including Alzheimer’s, Parkinson’s, ALS, and genetic or acquired sensory neuropathies. While the axon is the highly important component of neuronal degeneration, the mechanism of axonal degeneration during aging and age-associated neuronal degeneration is not fully understood.
2.1. Axonal degeneration
The axon is an essential component of nervous system, establishing a complex network of neurons and target cells. The distal part of the axon is far from the cell body and axonal transport supplies the majority of components required for its homeostasis (Maday et al., 2014). ATP necessary for fast axonal transport is supplied by the glycolytic enzymes carried on its own cargo (Zala et al., 2013). Axons also have the capability to translate proteins locally to respond to acute environmental changes using mRNAs transported through the axon (Cioni et al., 2018). Defects in axonal transport or the physical disruption of an axon cuts its lifeline and causes degeneration of the axon distal to the affected site.
Axonal loss is at least partially secondary from the loss of neuronal soma (dying forward). However, growing evidence shows additional mechanisms of axon dismantling during development, aging, and neuronal degeneration (Luo and O’Leary, 2005). A dying back axon degeneration, observed in developmental nervous systems or neurodegenerative conditions, and Wallerian degeneration extensively studied using a traumatic axonal injury model, are two different types of axon degeneration that also share some features (Yaron and Schuldiner, 2016). Understanding the molecular mechanisms underlying these different types of axonal degeneration is an emerging topic.
Axonal degeneration is observed in various neurodegenerative conditions including Alzheimer’s, Parkinson’s, and Huntington’s disease. A subset of disease shows a relatively early phase of axon degeneration, suggesting its role in the pathogenesis of these diseases. Axonal dystrophy within the regions occupied by senile plaques, extracellular deposits of amyloid β, are observed in both early and late stages of brains from Alzheimer’s patients (Benes et al., 1991). Long-term in vivo imaging of axonal segments near Aβ plaques in the Alzheimer’s disease model mice APPswe/PS1dE9 revealed the dynamic nature of axonal dystrophies (Blazquez-Llorca et al., 2017). The lifetime of dystrophic structure is about 80 days. Dystrophic structures disappear followed by axonal degeneration; the loss of whole axons or one side of axon in the microscopic field. That the volume of dystrophic structure increases and decreases during its life suggests the existence of a balancing mechanism of disease progression and protection.
Mice expressing human LRRK2(R1441G) BAC transgene, a model of the most common form of familial Parkinson’s disease, showed a reduction in the number of TH-positive dendrites and the appearance of dystrophic neurites without SN dopamine neuron loss (Y. Li et al., 2009). A later study identified that the signs of axonopathy were preceding the abnormality in the TH-positive dendrites (Tagliaferro et al., 2015). Additional evidence also supports the early axonal phenotypes in Parkinson’s disease (Decressac et al., 2012; Kordower et al., 2013; Nuber et al., 2013). In Huntington’s disease model mice (HdhQ140/Q140), axonal swelling dramatically increases before the loss of cell bodies, dendritesa, and synapses (Marangoni et al., 2014).
Many studies suggest the presymptomatic appearance of axon terminal defects in ALS model mice (Dadon-Nachum et al., 2010). Three-dimensional live imaging of nerves using stimulated raman scattering microscopy revealed significant axonal degeneration in the peripheral nerves preceding the physiological motor dysfunctions in SOD1 mutant ALS model mice (Tian et al., 2016). However, the study of familial ALS associated with TDP-43 containing inclusions suggests the corticofugal spreading of disease in which anterograde axonal transport of pathogenic molecules spreads the disease, suggesting that disease onset is before axonal degeneration (Braak et al., 2013). The studies that test the efficacy of axonal protection on SOD1 mutant ALS models are not successful (Fischer et al., 2005; Velde et al., 2004). Further studies are required to elucidate the roles of axon degeneration in the pathogenesis of ALS.
Traumatic brain injury (TBI) is a leading cause of death and disability and 130 patients die every day in the United States from injuries that include TBI. TBI causes post-injury pathologies affecting memory, sensation, and emotion, and raises the risk for neurological disorders such as Alzheimer’s and Parkinson’s disease (Blennow et al., 2016; Prins et al., 2013). While the mechanism behind these brain pathologies is not well understood, it is known that TBI induces severe metabolic burden characterized by reduced cerebral blood flow, impairment of glucose uptake, and NAD+ reduction. These metabolic defects likely cause post-TBI energetic crisis followed by various TBI related pathologies. TBI is also known to induce axonal degeneration that continues years after injury in humans (Johnson et al., 2013). In the model mice brain, severe axonal degeneration is observed at 48 hours post TBI, and the depletion of SARM1 or induction of the Wlds transgene (see details in section 2.2) completely abolished the axon degeneration (Henninger et al., 2016; Yin et al., 2016). TBI induced metabolic dysfunctions in the brain, the reduction of total choline and n-acethylaspartate, are also attenuated in the absence of SARM1 (Henninger et al., 2016). Importantly, blocking axon degeneration attenuates post TBI symptoms including cognitive, motor, and visual deficits in these mice, suggesting the primary roles of axonal degeneration in post TBI pathologies.
It is clear that axon degeneration is an important component of neurodegenerative diseases, especially in its early phases. Axon degeneration may serve as an early disease marker and also may be a therapeutic target of neurodegenerative diseases. To evaluate the therapeutic potential of axonal protection and to develop treatments, further studies on the signal that initiate axon degeneration in different neurodegenerative diseases are warranted.
2.2. Axonal degeneration and NAD+ metabolism
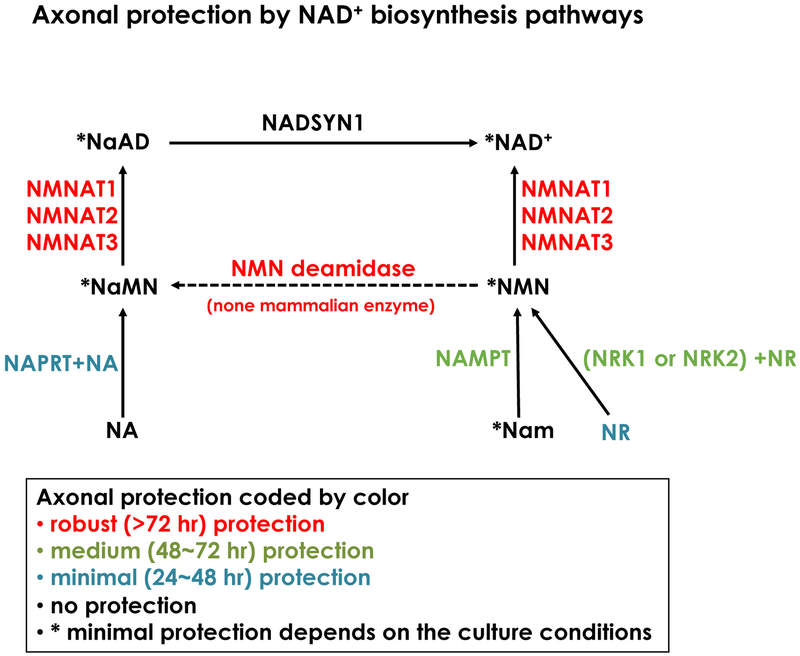
Prior to current histopathological evidences of axon degeneration in neurodegenerative diseases, Augustus Waller first described the importance of axon degeneration by analyzing the destruction of frog hypoglossal nerves upon axotomy. The seminal finding of the mouse harboring mutant gene, Wallerian degeneration slow (Wlds), whose transected nerves remain intact more than 14 days, later led to the novel concept that axon degeneration is an active regulated process. Wlds is composed of the N-terminal portion of Ufd2a and full length NMNAT1. Many excellent reviews on the topics of axon degeneration are published elsewhere (Adalbert and Coleman, 2013; Coleman and Freeman, 2010; Conforti et al., 2014; Geden and Deshmukh, 2016; Gerdts et al., 2016; Neukomm and Freeman, 2014; Salvadores et al., 2017; J. Wang and He, 2009). In these series of studies, many molecules and pathways are identified in the axon degeneration signaling cascade and the NAD+ biosynthesis pathway is one of the most important pathways modulating axon homeostasis (Fig.2). The major findings of axon degeneration signaling related to NAD+ metabolisms are: 1) NMNAT and its NAD+ synthesis activity are necessary and sufficient components of axonal protection by Wlds (Araki et al., 2004); 2) NMNAT2 is a labile axon maintenance factor and its degradation results in axon degeneration (Gilley and Coleman, 2010; Hicks et al., 2012); 3) SARM1 is a primary axon executor, degrading NAD+ with intrinsic and stimulus dependent NADase activity by the TIR domain (Essuman et al., 2017; Gerdts et al., 2016; Osterloh et al., 2012). These studies placed NAD+ metabolism as a central regulator of the axon degeneration signaling pathway.
Figure2. Axonal protection mediated by NAD+ biosynthesis pathways.
A summary of the effect of NAD+ biosynthesis pathways on injury induced axon degeneration using cultured DRG neurons. Official gene symbols of each enzyme, metabolites and their combinations are shown with the duration of axonal protection that is represented by colors indicated in the figure. Data is summarized from previous publications (Araki et al., 2004; Sasaki et al., 2016; 2006; J. Wang et al., 2005).
Remarkably, increasing axonal NMNAT and blocking the NMNAT2-SARM1 pathway that promotes axon degeneration after axotomy also attenuates axon degeneration in a diverse set of neurodegenerative disease models in vivo including TBI (Henninger et al., 2016; Yin et al., 2016; Ziogas and Koliatsos, 2018), Glaucoma (Williams et al., 2017; Zhu et al., 2013), peripheral neuropathy (Geisler et al., 2016; Turkiew et al., 2017; M. S. Wang et al., 2002), CMT (Meyer zu Horste et al., 2011; Samsam et al., 2003), motor neuron degeneration (Ferri et al., 2003), Ischemic injury (Verghese et al., 2011), gracile axonal dystrophy (Mi et al., 2005), and so on (Conforti et al., 2014). These results suggest that the manipulation of NAD+ biosynthesis is a potential therapeutic target.
Axonal injury induces rapid NAD+ decline preceding the loss of ATP and morphological degeneration (Gerdts et al., 2016; J. Wang et al., 2005). NAD+ is consumed by a variety of enzymes including CD38, Sirtuins, PARPs, and ART, and inhibition of these NAD+ consumers may delay axon degeneration. However, depletion of CD38 and PARP1, two major NAD+ consuming enzymes, did not alter axon degeneration profiles both in vitro and in vivo (Sasaki et al., 2009). Similarly, forced NAD+ depletion induced rapid axon degeneration, suggesting NAD+ depletion is sufficient to induce axon degeneration. We found that SARM1 is responsible for injury induced NAD+ depletion, however, the actual enzyme that degrades NAD+ was not identified (Gerdts et al., 2015). Much to our surprise, our recent efforts to identify the molecules responsible for injury induced NAD+ degradation revealed that the Toll/interleukin-1 receptor (TIR) domain of SARM1 is a NADase domain (Essuman et al., 2017). Interestingly, exogenous expression of NMNAT1 is able to block injury induced SARM1 NADase activity through an unknown mechanism (Sasaki et al., 2016). The next question is how NAD+ depletion leads to axonal degeneration. Since ATP decline is followed by NAD+ degradation, one can speculate that the failure of ATP dependent processes including axonal transport, ion homeostasis, and cytoskeletal organization directly leads to axon destruction. However, a recent report indicates the existence of additional axon degeneration signaling mediated by a Drosophila protein called axunded (axed) downstream of multiple axon injury insults (Neukomm et al., 2017). Axed, BTB (bric-à-brac, tramtrack, broad complex) and a BACK (BTB and C-terminal Kelch) containing protein, is required for a broad range of axonal degenerations after injury, SARM activation, axonal NMNAT depletion, and a combination of SARM activation and NMNAT depletion (Neukomm et al., 2017). Further studies are required to understand axon degeneration signals downstream of SARM and axed.
The next question is how SARM1 NADase activity is regulated. Upon axonal injury, NMNAT2 in the distal axons sharply declined due to its short half-life and lack of supplies from the cell bodies (Gilley and Coleman, 2010). One possibility is that the loss of NMNAT2 in the axon leads to the activation of SARM1 NADase activity. NMN, the precursor of NAD+, increases after axotomy due to the loss of its consuming enzyme NMNAT2 in the axon and it is suggested that the elevation of NMN promotes axon degeneration (Di Stefano et al., 2015). The expression of NMNAT in the axon rescues the NMN increase by compelling NMNAT2 loss. Similarly, exogenous expression of E. coli NMN deamidase, which reduces cellular NMN level by converting NMN to NaMN, provides strong axonal protection that is equivalent to NMNAT1 expression (Di Stefano et al., 2015). Similar to the injury-induced axon degeneration, depletion of NMNAT2 in mice caused reduced axon stability leading to insufficient innervation of phrenic nerves on the diaphragm muscle after birth (Hicks et al., 2012). Importantly, both SARM1 KO and NMN deamidase transgenic mice rescue NMNAT2 KO lethality and axonal phenotypes (Gilley et al., 2017; Hicks et al., 2012). This strong genetic evidence supports the idea that NMN accumulation is a trigger of axon degeneration dependent on SARM1 (Di Stefano et al., 2015). However, high NMN in the axon after NAMPT expression or exogenous application of NR to NRK1 expressing neurons did not cause axon degeneration (Sasaki et al., 2016). This suggests the presence of alternative mechanisms other than a simple NMN increase as a trigger of SARM1 activation.
Recent reports showed a positive correlation of NMNAT2 mRNA expression with the global cognition score in an aged cohort (Ali et al., 2016). Reduced NAD+ and NMNAT2 expression are also observed in the spinal cord of motor neuron disease model mice (Röderer et al., 2018), suggesting the importance of NMNAT2 in neuronal homeostasis. The NMNAT2 level in the distal tip of axons is predicted to be extremely low when considering the length of peripheral axons (1 meter), speed of axonal transport (0.04 meters/day), and the short half-life of NMNAT2 (24 hours). It is important to understand the mechanism of distal axon homeostasis under low NMNAT2 levels and axon degeneration signaling upon NMNAT2 loss.
The prodegenerative role of MAP kinase signaling cascade is first shown using MAPKKK DLK deficient mice and identified JNK as a downstream target of DLK (Miller et al., 2009). Later MKK4/7 was identified as primary MAPKKs linking DLK (MAPKKK) and JNK(MAPK) in axonal ATP depletion that lead to axonal degeneration after traumatic injury (Yang et al., 2015). It is important to determine the role of DLK-MKK4/7- JNK signaling in the SARM1 dependent axon degeneration cascade. While the role of MKK4/7 is found downstream of SARM1 using direct activation of SARM1 by TIR dimerization (Yang et al., 2015), MKK4/7 inhibition did not block the NAD+ loss induced by TIR dimerization (Walker et al., 2017b). It seems that MKK4/7 is upstream of SARM1 judging by the NAD+ degradation that is a direct readout of SARM1 activation. Strong genetic evidence supports that NMNAT2 loss induced axonal degeneration is SARM1 dependent. It is shown that MKK4/7 or JNK is required for the first turnover of NMNAT2 in the axon and inhibition of this signaling increases the axonal NMNAT2 (Walker et al., 2017b). Thus, the DLK-MKK-JNK pathway likely promotes the turnover of axon survival factor after injury, leading to SARM1 activation.
AKT phosphorylate MKK4 Ser78 antagonizes the prodegenerative Ser257/Thr261 phosphorylation of MKK4 that stimulates JNK phosphorylation and axonal degeneration after traumatic injury (Yang et al., 2015). However, the AKT mediated survival signal is diminished with unknown mechanisms and axonal degeneration proceeds in normal cells. Independent studies showed that the overexpression of AKT significantly delays axon degeneration, supporting the roles of AKT for axonal survival (Wakatsuki et al., 2011). While former studies showed the inhibition of MKK and ATP depletion as downstream, the latter showed that the inhibition of GSK3β and microtubule reorganization by AKT is responsible for axonal protection. Consistently, GSK3α/β promotes Tau hyper phosphorylation and neuronal degeneration, and suppression of GSK3β provides axonal protection (Gerdts et al., 2011; Kaluski et al., 2017). Further studies on the mechanism that links MAP kinase signaling and neuronal metabolism after injury will be required.
3. Conclusions
Growing evidence indicates important roles of cellular metabolism in pathogenesis and progression of neurodegeneration. Studies of signaling pathways related to cardinal metabolic regulators including mTOR, PPARγ, and AMPK provide valuable information on cellular metabolic statuses in different stages of the disease. Analysis of metabolites and pathways in synthesis and consumption of NAD+, the central metabolic regulator, also convey cellular energetic status and activity of various NAD+ dependent enzymes that is crucial for neuronal homeostasis. Mechanisms that activate or inhibit these metabolic nodes in the course of neurodegeneration are not fully understood and will be important in future studies.
Metabolic genes are often universal across many types of cells. It should be noted that metabolic changes in non-neuronal cells including glial, immune, and vasculature cells must be taken into account to evaluate metabolic effects for pathogenesis and progression of neuronal degeneration. Inhibition of a metabolic enzyme may exert biphasic consequences in different types of cells.
While many pathways and molecules altered in neurodegeneration are identified, the cause of neuronal degeneration is uncertain. Axonal degeneration is often seen in early phase neurodegeneration and, regardless of cause or consequence, the signaling that leads to axon degeneration will provide valuable information on the pathogenesis of neurodegenerative diseases. Recent studies show the central role of NAD+ metabolism for the trigger of axon degeneration, suggesting this universal metabolic regulator may serve as an important check point for neuronal homeostasis.
Highlights.
Dysregulations of cellular metabolism play important roles in neurodegeneration.
Axonal degeneration is a crucial component of neurodegeneration.
NAD+ metabolism governs axonal degeneration.
Acknowledgements
This work was supported by the National Institutes of Health (Grant RO1AG013730 (JM), RO1NS065053 and RO1NS087632 (JM and AD)), National Institute of General Medical Science (8 P41 GM103422) from the National Institutes of Health. The author thanks Jeffrey Milbrandt and Aaron DiAntonio for fruitful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The author may derive benefits from licensing agreement with ChromaDex and Disarm Therapeutics.
References
- Adalbert R, Coleman MP, 2013. Review: Axon pathology in age-related neurodegenerative disorders. Neuropathol. Appl. Neurobiol 39, 90–108. doi: 10.1111/j.1365-2990.2012.01308.x [DOI] [PubMed] [Google Scholar]
- Ali YO, Allen HM, Yu L, Li-Kroeger D, Bakhshizadehmahmoudi D, Hatcher A, McCabe C, Xu J, Bjorklund N, Taglialatela G, Bennett DA, De Jager PL, Shulman JM, Bellen HJ, Lu H-C, 2016. NMNAT2:HSP90 Complex Mediates Proteostasis in Proteinopathies. PLoS Biol. 14, e1002472. doi: 10.1371/journal.pbio.1002472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J, 2004. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013. doi: 10.1126/science.1098014 [DOI] [PubMed] [Google Scholar]
- Bayliss JA, Lemus MB, Stark R, Santos VV, Thompson A, Rees DJ, Galic S, Elsworth JD, Kemp BE, Davies JS, Andrews ZB, 2016. Ghrelin-AMPK Signaling Mediates the Neuroprotective Effects of Calorie Restriction in Parkinson’s Disease. J. Neurosci 36, 3049–3063. doi: 10.1523/JNEUROSCI.4373-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Farol PA, Majocha RE, Marotta CA, Bird ED, 1991. Evidence for axonal loss in regions occupied by senile plaques in Alzheimer cortex. Neuroscience 42, 651–660. doi: 10.1016/0306-4522(91)90034-L [DOI] [PubMed] [Google Scholar]
- Berger F, Lau C, Dahlmann M, Ziegler M, 2005. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem 280, 36334–36341. doi: 10.1074/jbc.M508660200 [DOI] [PubMed] [Google Scholar]
- Blacher E, Dadali T, Bespalko A, Haupenthal VJ, Grimm MOW, Hartmann T, Lund FE, Stein R, Levy A, 2015. Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model. Ann. Neurol 78, 88–103. doi: 10.1002/ana.24425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez-Llorca L, Valero-Freitag S, Rodrigues EF, Merchán-Pérez Á, Rodríguez JR, Dorostkar MM, DeFelipe J, Herms J, 2017. High plasticity of axonal pathology in Alzheimer’s disease mouse models. Acta Neuropathol Commun 5, 14. doi: 10.1186/s40478-017-0415-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM, Yaffe K, Zetterberg H, 2016. Traumatic brain injuries. Nature Reviews Disease Primers 2016 2 2, 16084. doi: 10.1038/nrdp.2016.84 [DOI] [PubMed] [Google Scholar]
- Bonet-Costa V, Herranz-Pérez V, Blanco-Gandía M, Mas-Bargues C, Inglés M, Garcia-Tarraga P, Rodriguez-Arias M, Miñarro J, Borras C, Garcia-Verdugo JM, Viña J, 2016. Clearing Amyloid-β through PPARγ/ApoE Activation by Genistein is a Treatment of Experimental Alzheimer’s Disease. J. Alzheimers Dis 51, 701–711. doi: 10.3233/JAD-151020 [DOI] [PubMed] [Google Scholar]
- Bové J, Martínez-Vicente M, Vila M, 2011. Fighting neurodegeneration with rapamycin: mechanistic insights. Nature Reviews Neuroscience 2011 12:8 12, 437–452. doi: 10.1038/nrn3068 [DOI] [PubMed] [Google Scholar]
- Braak H, Brettschneider J, Ludolph AC, Lee VM, Trojanowski JQ, Del Tredici K, 2013. Amyotrophic lateral sclerosis—a model of corticofugal axonal spread. Nature Reviews Neurology 2013 9:12 9, 708–714. doi: 10.1038/nrneurol.2013.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breda C, Sathyasaikumar KV, Idrissi SS, Notarangelo FM, Estranero JG, Moore GGL, Green EW, Kyriacou CP, Schwarcz R, Giorgini F, 2016. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. PNAS 113, 5435–5440. doi: 10.1073/pnas.1604453113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KD, Maqsood S, Huang J-Y, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, Jaffrey SR, 2014. Activation of SIRT3 by the NAD+ Precursor Nicotinamide Riboside Protects from Noise-Induced Hearing Loss. Cell Metab. 20, 1059–1068. doi: 10.1016/j.cmet.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkewitz K, Zhang Y, Mair WB, 2014. AMPK at the Nexus of Energetics and Aging. Cell Metab. 20, 10–25. doi: 10.1016/j.cmet.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Belfiore R, Oddo S, 2018. Genetically reducing mTOR signaling rescues central insulin dysregulation in a mouse model of Alzheimer’s disease. Neurobiol. Aging doi: 10.1016/j.neurobiolaging.2018.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Caccamo A, Branca C, Talboom JS, Shaw DM, Turner D, Ma L, Messina A, Huang Z, Wu J, Oddo S, 2015. Reducing Ribosomal Protein S6 Kinase 1 Expression Improves Spatial Memory and Synaptic Plasticity in a Mouse Model of Alzheimer’s Disease. J. Neurosci 35, 14042–14056. doi: 10.1523/JNEUROSCI.2781-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, De Pinto V, Messina A, Branca C, Oddo S, 2014. Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer’s disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J. Neurosci 34, 7988–7998. doi: 10.1523/JNEUROSCI.0777-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Chen G, He W, Xiao M, Yan L-J, 2015. Activation of mTOR: a culprit of Alzheimer’s disease? Neuropsychiatric Disease and Treatment 11, 1015–1030. doi: 10.2147/NDT.S75717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camandola S, Mattson MP, 2017. Brain metabolism in health, aging, and neurodegeneration. The EMBO Journal 36, 1474–1492. doi: 10.15252/embj.201695810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campesan S, Green EW, Breda C, Sathyasaikumar KV, Muchowski PJ, Schwarcz R, Kyriacou CP, Giorgini F, 2011. The Kynurenine Pathway Modulates Neurodegeneration in a Drosophila Model of Huntington’s Disease. Current Biology 21, 961–966. doi: 10.1016/j.cub.2011.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Auwerx J, 2011. Calorie Restriction: Is AMPK a Key Sensor and Effector? Physiology 26, 214–224. doi: 10.1152/physiol.00010.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Menzies KJ, Auwerx J, 2015. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 22, 31–53. doi: 10.1016/j.cmet.2015.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X-S, Zhao K-P, Jiang X, Du L-L, Li X-H, Ma Z-W, Yao J, Luo Y, Duan D-X, Wang J-Z, Zhou X-W, 2013. Nmnat2 attenuates Tau phosphorylation through activation of PP2A. J. Alzheimers Dis 36, 185–195. doi: 10.3233/JAD-122173 [DOI] [PubMed] [Google Scholar]
- Cioni J-M, Koppers M, Holt CE, 2018. Molecular control of local translation in axon development and maintenance. Current Opinion in Neurobiology 51, 86–94. doi: 10.1016/j.conb.2018.02.025 [DOI] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR, 2010. Wallerian Degeneration, WldS, and Nmnat. 10.1146/annurev-neuro-060909-153248 33, 245–267. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Gilley J, Coleman MP, 2014. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nature Reviews Neuroscience 2011 12:8 15, 394–409. doi: 10.1038/nrn3680 [DOI] [PubMed] [Google Scholar]
- Corona JC, Duchen MR, 2016. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radical Biology and Medicine 100, 153–163. doi: 10.1016/j.freeradbiomed.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes CJ, Qin K, Cook J, Solanki A, Mastrianni JA, 2012. Rapamycin delays disease onset and prevents PrP plaque deposition in a mouse model of Gerstmann- Sträussler-Scheinker disease. J. Neurosci 32, 12396–12405. doi: 10.1523/JNEUROSCI.6189-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadon-Nachum M, Melamed E, Offen D, 2010. The “Dying-Back” Phenomenon of Motor Neurons in ALS. J Mol Neurosci 43, 470–477. doi: 10.1007/s12031-010-9467-1 [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Lundblad M, Weikop P, Björklund A, 2012. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of α-synuclein in midbrain dopamine neurons. Neurobiology of Disease 45, 939–953. doi: 10.1016/j.nbd.2011.12.013 [DOI] [PubMed] [Google Scholar]
- Desvergne B, reviews W.W.E., 1999, n.d. Peroxisome proliferator-activated receptors: nuclear control of metabolism. pdfs.semanticscholar.org. [DOI] [PubMed] [Google Scholar]
- Di Stefano M, Nascimento-Ferreira I, Orsomando G, Mori V, Gilley J, Brown R, Janeckova L, Vargas ME, Worrell LA, Loreto A, Tickle J, Patrick J, Webster JRM, Marangoni M, Carpi FM, Pucciarelli S, Rossi F, Meng W, Sagasti A, Ribchester RR, Magni G, Coleman MP, Conforti L, 2015. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death and Differentiation 2014 22:5 22, 731–742. doi: 10.1038/cdd.2014.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domise M, Didier S, Marinangeli C, Zhao H, Chandakkar P, Buée L, Viollet B, Davies P, Marambaud P, Vingtdeux V, 2016. AMP-activated protein kinase modulates tau phosphorylation and tau pathology in vivo. Scientific Reports 2016 6 6, 26758. doi: 10.1038/srep26758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J, 2017. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD+Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron 93, 1334–1343.e5. doi: 10.1016/j.neuron.2017.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, Bohr VA, 2017. NAD+ in Aging: Molecular Mechanisms and Translational Implications. Trends Mol Med 23, 899–916. doi: 10.1016/j.molmed.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri A, Sanes JR, Coleman MP, Cunningham JM, Kato AC, 2003. Inhibiting Axon Degeneration and Synapse Loss Attenuates Apoptosis and Disease Progression in a Mouse Model of Motoneuron Disease. Current Biology 13, 669–673. doi: 10.1016/S0960-9822(03)00206-9 [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Davis AA, Tennant P, Wang M, Coleman M, Asress S, Adalbert R, Alexander GM, Glass JD, 2005. The WldS gene modestly prolongs survival in the SOD1G93A fALS mouse. Neurobiology of Disease 19, 293–300. doi: 10.1016/j.nbd.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Frölich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Türk A, Hoyer S, Zöchling R, Boissl KW, Jellinger K, Riederer P, 1998. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm 105, 423–438. doi: 10.1007/s007020050068 [DOI] [PubMed] [Google Scholar]
- Geden MJ, Deshmukh M, 2016. Axon degeneration: context defines distinct pathways. Current Opinion in Neurobiology 39, 108–115. doi: 10.1016/j.conb.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Doan RA, Strickland A, Huang X, Milbrandt J, DiAntonio A, 2016. Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice. Brain 139, 3092–3108. doi: 10.1093/brain/aww251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J, 2015. SARM1 activation triggers axon degeneration locally via NAD⁺ destruction. Science 348, 453–457. doi: 10.1126/science.1258366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Sasaki Y, Vohra B, Marasa J, Milbrandt J, 2011. Image-based screening identifies novel roles for IkappaB kinase and glycogen synthase kinase 3 in axonal degeneration. J. Biol. Chem 286, 28011–28018. doi: 10.1074/jbc.M111.250472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Milbrandt J, DiAntonio A, 2016. Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron 89, 449–460. doi: 10.1016/j.neuron.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J, Coleman MP, 2010. Endogenous Nmnat2 Is an Essential Survival Factor for Maintenance of Healthy Axons. PLoS Biol. 8, e1000300. doi: 10.1371/journal.pbio.1000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J, Ribchester RR, Coleman MP, 2017. Sarm1 Deletion, but Not WldS, Confers Lifelong Rescue in a Mouse Model of Severe Axonopathy. Cell Reports 21, 10–16. doi: 10.1016/j.celrep.2017.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM, 2013. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol. Aging 34, 1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Shaikh S, Wang J-Z, Zaidi T, Iqbal IG, Iqbal K, 1995. Phosphatase Activity Toward Abnormally Phosphorylated τ: Decrease in Alzheimer Disease Brain. Journal of Neurochemistry 65, 732–738. doi: 10.1046/j.1471-4159.1995.65020732.x [DOI] [PubMed] [Google Scholar]
- Graeff R, Liu Q, Kriksunov IA, Kotaka M, Oppenheimer N, Hao Q, Lee HC, 2009. Mechanism of cyclizing NAD to cyclic ADP-ribose by ADP-ribosyl cyclase and CD38. J. Biol. Chem 284, 27629–27636. doi: 10.1074/jbc.M109.030965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J, Kahn M, Samiei A, Gao J, Ota KT, Rei D, Tsai L-H, 2013. A Dietary Regimen of Caloric Restriction or Pharmacological Activation of SIRT1 to Delay the Onset of Neurodegeneration. J. Neurosci 33, 8951–8960. doi: 10.1523/JNEUROSCI.5657-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti P, LUTHICARTER R, AUGOOD S, SCHWARCZ R, 2004. Neostriatal and cortical quinolinate levels are increased in early grade Huntington’s disease. Neurobiology of Disease 17, 455–461. doi: 10.1016/j.nbd.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ, 2008. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Molecular Cell 30, 214–226. doi: 10.1016/j.molcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartai Z, Juhász A, Rimanóczy Á, Janáky T, Donkó T, Dux L, Penke B, Tóth GK, Janka Z, Kálmán J, 2007. Decreased serum and red blood cell kynurenic acid levels in Alzheimer’s disease. Neurochemistry International 50, 308–313. doi: 10.1016/j.neuint.2006.08.012 [DOI] [PubMed] [Google Scholar]
- Henninger N, Bouley J, Sikoglu EM, An J, Moore CM, King JA, Bowser R, Freeman MR, Brown RH, 2016. Attenuated traumatic axonal injury and improved functional outcome after traumatic brain injury in mice lacking Sarm1. Brain 139, 1094–1105. doi: 10.1093/brain/aww001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks AN, Lorenzetti D, Gilley J, Lu B, Andersson K-E, Miligan C, Overbeek PA, Oppenheim R, Bishop CE, 2012. Nicotinamide Mononucleotide Adenylyltransferase 2 (Nmnat2) Regulates Axon Integrity in the Mouse Embryo. PLOS ONE 7, e47869. doi: 10.1371/journal.pone.0047869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, Zhang Y, Moritoh K, O’Connell JF, Baptiste BA, Stevnsner TV, Mattson MP, Bohr VA, 2018. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. PNAS 115, E1876–E1885. doi: 10.1073/pnas.1718819115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi H, Ichiyama A, Hayaishi O, 1966. Studies on the biosynthesis of nicotinamide adenine dinucleotide. 3. Comparative in vivo studies on nicotinic acid, nicotinamide, and quinolinic acid as precursors of nicotinamide adenine dinucleotide. J. Biol. Chem 241, 3701–3707. [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan K-L, 2003. TSC2 Mediates Cellular Energy Response to Control Cell Growth and Survival. Cell 115, 577–590. doi: 10.1016/S0092-8674(03)00929-2 [DOI] [PubMed] [Google Scholar]
- Jiang P, Gan M, Ebrahim AS, Castanedes-Casey M, Dickson DW, Yen S-HC, 2013. Adenosine monophosphate-activated protein kinase overactivation leads to accumulation of α-synuclein oligomers and decrease of neurites. Neurobiol. Aging 34, 1504–1515. doi: 10.1016/j.neurobiolaging.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Liu H-X, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H, 2007. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446, 41–45. doi: 10.1038/nature05526 [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH, 2013. Axonal pathology in traumatic brain injury. Experimental Neurology 246, 35–43. doi: 10.1016/j.expneurol.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T-C, Chen H-M, Lin J-T, Chang C-P, Chang W-C, Kang J-J, Sun C-P, Tao M-H, Tu P-H, Chang C, Dickson DW, Chern Y, 2011. Nuclear translocation of AMPK-α1 potentiates striatal neurodegeneration in Huntington’s disease. The Journal of Cell Biology 194, 209–227. doi: 10.1083/jcb.201105010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluski S, Portillo M, Besnard A, Stein D, Einav M, Zhong L, Ueberham U, Arendt T, Mostoslavsky R, Sahay A, Toiber D, 2017. Neuroprotective Functions for the Histone Deacetylase SIRT6. Cell Reports 18, 3052–3062. doi: 10.1016/j.celrep.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Zhang Z, Liu X, Manfredsson FP, He L, Iuvone PM, Cao X, Sun YE, Jin L, Ye K, 2017. α-Synuclein binds and sequesters PIKE-L into Lewy bodies, triggering dopaminergic cell death via AMPK hyperactivation. PNAS 114, 1183–1188. doi: 10.1073/pnas.1618627114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassai H, Sugaya Y, Noda S, Nakao K, Maeda T, Kano M, Aiba A, 2014. Selective Activation of mTORC1 Signaling Recapitulates Microcephaly, Tuberous Sclerosis, and Neurodegenerative Diseases. Cell Reports 7, 1626–1639. doi: 10.1016/j.celrep.2014.04.048 [DOI] [PubMed] [Google Scholar]
- Katsouri L, Lim YM, Blondrath K, Eleftheriadou I, Lombardero L, Birch AM, Mirzaei N, Irvine EE, Mazarakis ND, Sastre M, 2016. PPARγ-coactivator-1α gene transfer reduces neuronal loss and amyloid-β generation by reducing β- secretase in an Alzheimer’s disease model. PNAS 113, 12292–12297. doi: 10.1073/pnas.1606171113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouri L, Parr C, Bogdanovic N, Willem M, Sastre M, 2011. PPARγ co- activator-1α (PGC-1α) reduces amyloid-β generation through a PPARγ-dependent mechanism. J. Alzheimers Dis 25, 151–162. doi: 10.3233/JAD-2011-101356 [DOI] [PubMed] [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT, 2013. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136, 2419–2431. doi: 10.1093/brain/awt192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pichon CE, Meilandt WJ, Dominguez S, Solanoy H, Lin H, Ngu H, Gogineni A, Ghosh AS, Jiang Z, Lee S-H, Maloney J, Gandham VD, Pozniak CD, Wang B, Lee S, Siu M, Patel S, Modrusan Z, Liu X, Rudhard Y, Baca M, Gustafson A, Kaminker J, Carano RAD, Huang EJ, Foreman O, Weimer R, Scearce-Levie K, Lewcock JW, 2017. Loss of dual leucine zipper kinase signaling is protective in animal models of neurodegenerative disease. Science Translational Medicine 9, eaag0394. doi: 10.1126/scitranslmed.aag0394 [DOI] [PubMed] [Google Scholar]
- Lee J, Kim Y, Liu T, Hwang YJ, Hyeon SJ, Im H, Lee K, Alvarez VE, McKee AC, Um S-J, Hur M, Mook-Jung I, Kowall NW, Ryu H, 2018. SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease. Aging Cell 17, e12679. doi: 10.1111/acel.12679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Tecedor L, Chen YH, Monteys AM, Sowada MJ, Thompson LM, Davidson BL, 2015. Reinstating Aberrant mTORC1 Activity in Huntington’s Disease Mice Improves Disease Phenotypes. Neuron 85, 303–315. doi: 10.1016/j.neuron.2014.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Luo L, Xu M, Wu J, Chen L, Li J, Liu Z, Lu G, Wang Y, Qiao L, 2017. AMPK activates FOXO3a and promotes neuronal apoptosis in the developing rat brain during the early phase after hypoxia-ischemia. Brain Research Bulletin 132, 1–9. doi: 10.1016/j.brainresbull.2017.05.001 [DOI] [PubMed] [Google Scholar]
- Li F, Liu L, 2016. SIRT5 Deficiency Enhances Susceptibility to Kainate-Induced Seizures and Exacerbates Hippocampal Neurodegeneration not through Mitochondrial Antioxidant Enzyme SOD2. Front. Cell. Neurosci 10, 580. doi: 10.3389/fncel.2016.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, Beal MF, Burke RE, Li C, 2009. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat. Neurosci 12, 826–828. doi: 10.1038/nn.2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JB, Kubota S, Ban N, Yoshida M, Santeford A, Sene A, Nakamura R, Zapata N, Kubota M, Tsubota K, Yoshino J, Imai S-I, Apte RS, 2016. NAMPT-Mediated NAD+ Biosynthesis Is Essential for Vision In Mice. Cell Reports 17, 69–85. doi: 10.1016/j.celrep.2016.08.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WK, Bolton EL, Cortopassi WA, Wang Y, O’Brien F, Maciejewska M, Jacobson MP, Garnham C, Ruas M, Parrington J, Lei M, Sitsapesan R, Galione A, Terrar DA, 2017. Synthesis of the Ca2+-mobilizing messengers NAADP and cADPR by intracellular CD38 enzyme in the mouse heart: Role in β- adrenoceptor signaling. J. Biol. Chem 292, 13243–13257. doi: 10.1074/jbc.M117.789347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Su X, Quinn WJ III, Hui S, metabolism K.K.C., 2018, n.d. Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. cell.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg MC, Ali YO, Zhu J, Wu C-S, Oka K, Zhai RG, Lu H-C, 2012. CREB-activity and nmnat2 transcription are down-regulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum. Mol. Genet 21, 251–267. doi: 10.1093/hmg/ddr492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AN, Owens K, Schlappal AE, Kristian T, Fishman PS, Schuh RA, 2015. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol 15, 19. doi: 10.1186/s12883-015-0272-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, Nalbandian S, Fan W, Gayen JR, Mahata SK, Webster NJ, Schwartz MW, Olefsky JM, 2011. Brain PPAR-γ promotes obesity and is required for the insulin–sensitizing effect of thiazolidinediones. Nature Medicine 2011 17:5 17, 618–622. doi: 10.1038/nm.2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, O’Leary DDM, 2005. AXON RETRACTION AND DEGENERATION IN DEVELOPMENT AND DISEASE. 10.1146/annurev.neuro.28.061604.135632 28, 127–156. doi: [DOI] [PubMed] [Google Scholar]
- Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Moffitt H, Smith DL, Runne H, Gokce O, Kuhn A, Xiang Z, Maxwell MM, Reeves SA, Bates GP, Neri C, Thompson LM, Marsh JL, Kazantsev AG, 2010. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. PNAS 107, 7927–7932. doi: 10.1073/pnas.1002924107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur ELF, 2014. Axonal Transport: Cargo-Specific Mechanisms of Motility and Regulation. Neuron 84, 292–309. doi: 10.1016/j.neuron.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairet-Coello G, Courchet J, Pieraut S, Courchet V, Maximov A, Polleux F, 2013. The CAMKK2-AMPK Kinase Pathway Mediates the Synaptotoxic Effects of Aβ Oligomers through Tau Phosphorylation. Neuron 78, 94–108. doi: 10.1016/j.neuron.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada C, Jin ZH, Greene LA, 2008. RTP801 Is Induced in Parkinson’s Disease and Mediates Neuron Death by Inhibiting Akt Phosphorylation/Activation. J. Neurosci 28, 14363–14371. doi: 10.1523/JNEUROSCI.3928-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni M, Adalbert R, Janeckova L, Patrick J, Kohli J, Coleman MP, Conforti L, 2014. Age-related axonal swellings precede other neuropathological hallmarks in a knock-in mouse model of Huntington’s disease. Neurobiol. Aging 35, 2382–2393. doi: 10.1016/j.neurobiolaging.2014.04.024 [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B, 2003. Marked loss of myelinated nerve fibers in the human brain with age. Journal of Comparative Neurology 462, 144–152. doi: 10.1002/cne.10714 [DOI] [PubMed] [Google Scholar]
- Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Füllgrabe J, Jackson A, Jimenez Sanchez M, Karabiyik C, Licitra F, Lopez Ramirez A, Pavel M, Puri C, Renna M, Ricketts T, Schlotawa L, Vicinanza M, Won H, Zhu Y, Skidmore J, Rubinsztein DC, 2017. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron 93, 1015–1034. doi: 10.1016/j.neuron.2017.01.022 [DOI] [PubMed] [Google Scholar]
- Meyer zu Horste G, Miesbach TA, Muller JI, Fledrich R, Stassart RM, Kieseier BC, Coleman MP, Sereda MW, 2011. The Wlds transgene reduces axon loss in a Charcot-Marie-Tooth disease 1A rat model and nicotinamide delays post- traumatic axonal degeneration. Neurobiology of Disease 42, 1–8. doi: 10.1016/j.nbd.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Mi W, Beirowski B, Gillingwater TH, Adalbert R, Wagner D, Grumme D, Osaka H, Conforti L, Arnhold S, Addicks K, Wada K, Ribchester RR, Coleman MP, 2005. The slow Wallerian degeneration gene, WldS, inhibits axonal spheroid pathology in gracile axonal dystrophy mice. Brain 128, 405–416. doi: 10.1093/brain/awh368 [DOI] [PubMed] [Google Scholar]
- Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A, 2009. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat. Neurosci 12, 387–389. doi: 10.1038/nn.2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S-W, Sohn PD, Li Y, Devidze N, Johnson JR, Krogan NJ, Masliah E, Mok S-A, Gestwicki JE, Gan L, 2018. SIRT1 Deacetylates Tau and Reduces Pathogenic Tau Spread in a Mouse Model of Tauopathy. J. Neurosci 38, 3680–3688. doi: 10.1523/JNEUROSCI.2369-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM, 2016. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008. doi: 10.1126/science.aah4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Xiong DD, Patel T, Sasaki Y, Wang Y, Bauer AQ, Singh R, Finn SL, Culver JP, Milbrandt J, Holtzman DM, 2016. Nmnat1 protects neuronal function without altering phospho-tau pathology in a mouse model of tauopathy. Ann Clin Transl Neurol 3, 434–442. doi: 10.1002/acn3.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P, 2009. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science 324, 654–657. doi: 10.1126/science.1170803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neukomm LJ, Burdett TC, Seeds AM, Hampel S, Coutinho-Budd JC, Farley JE, Wong J, Karadeniz YB, Osterloh JM, Sheehan AE, Freeman MR, 2017. Axon Death Pathways Converge on Axundead to Promote Functional and Structural Axon Disassembly. Neuron 95, 78–91.e5. doi: 10.1016/j.neuron.2017.06.031 [DOI] [PubMed] [Google Scholar]
- Neukomm LJ, Freeman MR, 2014. Diverse cellular and molecular modes of axon degeneration. Trends Cell Biol. 24, 515–523. doi: 10.1016/j.tcb.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T, Cabecinha M, Tillmann A, Kerr F, Wong CT, Cardenes D, Vincent AJ, Bettedi L, Li L, Grönke S, Dols J, Partridge L, 2016. Increased Glucose Transport into Neurons Rescues Aβ Toxicity in Drosophila. Current Biology 26, 2291–2300. doi: 10.1016/j.cub.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber S, Harmuth F, Kohl Z, Adame A, Trejo M, Schönig K, Zimmermann F, Bauer C, Casadei N, Giel C, Calaminus C, Pichler BJ, Jensen PH, Müller CP, Amato D, Kornhuber J, Teismann P, Yamakado H, Takahashi R, Winkler J, Masliah E, Riess O, 2013. A progressive dopaminergic phenotype associated with neurotoxic conversion of α-synuclein in BAC-transgenic rats. Brain 136, 412–432. doi: 10.1093/brain/aws358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Matson WR, Beal MF, Myers RH, Bird ED, Milbury P, Saso S, 1992. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology 42, 1702–1702. doi: 10.1212/WNL.42.9.1702 [DOI] [PubMed] [Google Scholar]
- Ohsumi Y, 2014. Historical landmarks of autophagy research. Cell Research 2013 24:1 24, 9–23. doi: 10.1038/cr.2013.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr ME, Salinas A, Buffenstein R, Oddo S, 2014. Mammalian target of rapamycin hyperactivity mediates the detrimental effects of a high sucrose diet on Alzheimer’s disease pathology. Neurobiol. Aging 35, 1233–1242. doi: 10.1016/j.neurobiolaging.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou Y-J, Nathan C, Ding A, Brown RH, Conforti L, Coleman M, Tessier-Lavigne M, Züchner S, Freeman MR, 2012. dSarm/Sarm1 Is Required for Activation of an Injury-Induced Axon Death Pathway. Science 337, 481–484. doi: 10.1126/science.1223899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet J-C, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG, 2007. Sirtuin 2 Inhibitors Rescue α-Synuclein-Mediated Toxicity in Models of Parkinson’s Disease. Science 317, 516–519. doi: 10.1126/science.1143780 [DOI] [PubMed] [Google Scholar]
- Panda S, 2016. Circadian physiology of metabolism. Science 354, 1008–1015. doi: 10.1126/science.aah4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ, 2015. The Roles of PINK1, Parkin, and Mitochondrial Fidelity in Parkinson’s Disease. Neuron 85, 257–273. doi: 10.1016/j.neuron.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozniak CD, Ghosh AS, Gogineni A, Hanson JE, Lee S-H, Larson JL, Solanoy H, Bustos D, Li H, Ngu H, Jubb AM, Ayalon G, Wu J, Scearce-Levie K, Zhou Q, Weimer RM, Kirkpatrick DS, Lewcock JW, 2013. Dual leucine zipper kinase is required for excitotoxicity-induced neuronal degeneration. Journal of Experimental Medicine 210, 2553–2567. doi: 10.1084/jem.20122832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins M, Greco T, Alexander D, Giza CC, 2013. The pathophysiology of traumatic brain injury at a glance. Disease Models & Mechanisms 6, 1307–1315. doi: 10.1242/dmm.011585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procaccini C, Santopaolo M, Faicchia D, Colamatteo A, Formisano L, de Candia P, Galgani M, De Rosa V, Matarese G, 2016. Role of metabolism in neurodegenerative disorders. Metabolism - Clinical and Experimental 65, 1376–1390. doi: 10.1016/j.metabol.2016.05.018 [DOI] [PubMed] [Google Scholar]
- Pryor WM, Biagioli M, Shahani N, Swarnkar S, Huang W-C, Page DT, MacDonald ME, Subramaniam S, 2014. Huntingtin promotes mTORC1 signaling in the pathogenesis of Huntington’s disease. Sci. Signal 7, ra103–ra103. doi: 10.1126/scisignal.2005633 [DOI] [PubMed] [Google Scholar]
- Qi L, Jacob A, Wang P, Wu R, 2010. Peroxisome proliferator activated receptor-γ and traumatic brain injury. Int J Clin Exp Med 3, 283–292. [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC, 2004. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet 36, 585–595. doi: 10.1038/ng1362 [DOI] [PubMed] [Google Scholar]
- Rechsteiner M, Hillyard D, Olivera BM, 1976. Turnover of nicotinamide adenine dinucleotide in cultures of human cells. Journal of Cellular Physiology 88, 207–217. doi: 10.1002/jcp.1040880210 [DOI] [PubMed] [Google Scholar]
- Ross JM, Öberg J, Brené S, Coppotelli G, Terzioglu M, Pernold K, Goiny M, Sitnikov R, Kehr J, Trifunovic A, Larsson N-G, Hoffer BJ, Olson L, 2010. High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. PNAS 107, 20087–20092. doi: 10.1073/pnas.1008189107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röderer P, Klatt L, John F, Theis V, Winklhofer KF, Theiss C, Matschke V, 2018. Increased ROS Level in Spinal Cord of Wobbler Mice due to Nmnat2 Downregulation. Molecular Neurobiology 27, 723–11. doi: 10.1007/s12035-018-0999-7 [DOI] [PubMed] [Google Scholar]
- Ryu KW, 2018. Metabolic regulation of transcription through compartmentalized NAD+ biosynthesis. Science 360 eaan5780. doi: 10.1126/science.aan5780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadores N, Sanhueza M, Manque P, Court FA, 2017. Axonal Degeneration during Aging and Its Functional Role in Neurodegenerative Disorders. Front. Neurosci 11, 90. doi: 10.3389/fnins.2017.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsam M, Mi W, Wessig C, Zielasek J, Toyka KV, Coleman MP, Martini R, 2003. The Wlds Mutation Delays Robust Loss of Motor and Sensory Axons in a Genetic Model for Myelin-Related Axonopathy. J. Neurosci 23, 2833–2839. doi: 10.1523/JNEUROSCI.23-07-02833.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Araki T, Milbrandt J, 2006. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J. Neurosci 26, 8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Nakagawa T, Mao X, DiAntonio A, Milbrandt J, 2016. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD+depletion. Elife 5, 1010. doi: 10.7554/eLife.19749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BPS, Lund FE, Milbrandt J, 2009. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J. Neurosci 29, 5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM, 2017. mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976. doi: 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Sabatini DM, 2010. Regulation of the mTOR Complex 1 Pathway by Nutrients, Growth Factors, and Stress. Molecular Cell 40, 310–322. doi: 10.1016/j.molcel.2010.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J, Liu L, Mason A, Higashimori H, Donmez G, 2014. Loss of SIRT4 decreases GLT-1-dependent glutamate uptake and increases sensitivity to kainic acid. Journal of Neurochemistry 131, 573–581. doi: 10.1111/jnc.12942 [DOI] [PubMed] [Google Scholar]
- Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A, 2012. Dual Leucine Zipper Kinase Is Required for Retrograde Injury Signaling and Axonal Regeneration. Neuron 74, 1015–1022. doi: 10.1016/j.neuron.2012.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A, Bhaumik D, Chinta SJ, Rane A, Rajagopalan S, Lieu CA, Lithgow GJ, Andersen JK, 2015. Mitochondrial Quality Control via the PGC1α-TFEB Signaling Pathway Is Compromised by Parkin Q311X Mutation But Independently Restored by Rapamycin. J. Neurosci 35, 12833–12844. doi: 10.1523/JNEUROSCI.0109-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Hanson PS, Morris CM, 2017. Sirtuin-2 Protects Neural Cells from Oxidative Stress and Is Elevated in Neurodegeneration. Parkinson’s Disease 2017, 1–17. doi: 10.1155/2017/2643587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V, 2010. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLOS ONE 5, e9979. doi: 10.1371/journal.pone.0009979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro P, Kareva T, Oo TF, Yarygina O, Kholodilov N, Burke RE, 2015. An early axonopathy in a hLRRK2(R1441G) transgenic model of Parkinson disease. Neurobiology of Disease 82, 359–371. doi: 10.1016/j.nbd.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumangalakudi L, Prakasam A, Zhang R, Nelson HB, Sambamurti K, Kindy MS, Bhat NR, 2008. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. Journal of Neurochemistry 106, 475–485. doi: 10.1111/j.1471-4159.2008.05415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, Yang W, Mordes DA, Wang J-Y, Salameh JS, Mok J, Chew J, Sharma A, Leno-Duran E, Suzuki-Uematsu S, Suzuki N, Han SS, Lu F-K, Ji M, Zhang R, Liu Y, Strominger J, Shneider NA, Petrucelli L, Xie XS, Eggan K, 2016. Monitoring peripheral nerve degeneration in ALS by label-free stimulated Raman scattering imaging. Nat Commun 7, 13283. doi: 10.1038/ncomms13283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkiew E, Falconer D, Reed N, Höke A, 2017. Deletion of Sarm1 gene is neuroprotective in two models of peripheral neuropathy. Journal of the peripheral nervous system : JPNS 22, 162–171. doi: 10.1111/jns.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velde CV, Garcia ML, Yin X, Trapp BD, Cleveland DW, 2004. The neuroprotective factor Wld<Superscript>s</Superscript> does not attenuate mutant SOD1-mediated motor neuron disease. Neuromol Med 5, 193–203. doi: 10.1385/NMM:5:3:193 [DOI] [PubMed] [Google Scholar]
- Verdin E, 2015. NAD+ in aging, metabolism, and neurodegeneration. Science 350, 1208–1213. doi: 10.1126/science.aac4854 [DOI] [PubMed] [Google Scholar]
- Verghese PB, Sasaki Y, Yang D, Stewart F, Sabar F, Finn MB, Wroge CM, Mennerick S, Neil JJ, Milbrandt J, Holtzman DM, 2011. Nicotinamide mononucleotide adenylyl transferase 1 protects against acute neurodegeneration in developing CNS by inhibiting excitotoxic-necrotic cell death. Proc. Natl. Acad. Sci. U.S.A 108, 19054–19059. doi: 10.1073/pnas.1107325108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki S, Saitoh F, Araki T, 2011. ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation. Nature Cell Biology 2011 13: 12 13, 1415–1423. doi: 10.1038/ncb2373 [DOI] [PubMed] [Google Scholar]
- Walker LJ, Summers DW, Sasaki Y, Brace EJ, Milbrandt J, DiAntonio A, 2017a. MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife 6, 545. doi: 10.7554/eLife.22540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LJ, Summers DW, Sasaki Y, Brace EJ, Milbrandt J, DiAntonio A, 2017b. MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife 6, 545. doi: 10.7554/eLife.22540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, He Z, 2009. NAD and axon degeneration: From the Wlds gene to neurochemistry. Cell Adhesion & Migration 3, 77–87. doi: 10.4161/cam.3.1.7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z, 2005. A local mechanism mediates NAD-dependent protection of axon degeneration. The Journal of Cell Biology 170, 349–355. doi: 10.1083/jcb.200504028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MS, Davis AA, Culver DG, Glass JD, 2002. Wlds mice are resistant to paclitaxel (taxol) neuropathy. Ann. Neurol 52, 442–447. doi: 10.1002/ana.10300 [DOI] [PubMed] [Google Scholar]
- Wang R, Li JJ, Diao S, Kwak Y-D, Liu L, Zhi L, Büeler H, Bhat NR, Williams RW, Park EA, Liao F-F, 2013. Metabolic Stress Modulates Alzheimer’s β- Secretase Gene Transcription via SIRT1-PPARγ-PGC-1 in Neurons. Cell Metab. 17, 685–694. doi: 10.1016/j.cmet.2013.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu X, Yang Y, Takata T, Sakurai T, 2016. Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 1643, 1–9. doi: 10.1016/j.brainres.2016.04.060 [DOI] [PubMed] [Google Scholar]
- Warden A, Truitt J, Merriman M, Ponomareva O, Jameson K, Ferguson LB, Mayfield RD, Harris RA, 2016. Localization of PPAR isotypes in the adult mouse and human brain. Scientific Reports 2016 6 6, 27618. doi: 10.1038/srep27618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC, 2003. Alpha-Synuclein is degraded by both autophagy and the proteasome. J. Biol. Chem 278, 25009–25013. doi: 10.1074/jbc.M300227200 [DOI] [PubMed] [Google Scholar]
- Williams PA, Harder JM, Foxworth NE, Cardozo BH, Cochran KE, John SWM, 2017. Nicotinamide and WLDS Act Together to Prevent Neurodegeneration in Glaucoma. Front. Neurosci 11, 271. doi: 10.3389/fnins.2017.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Deki-Arima N, Kaneko A, Miyamura N, Iwatsuki M, Matsuoka M, Fujimori-Tonou N, Okamoto-Uchida Y, Hirayama J, Marth JD, Yamanashi Y, Kawasaki H, Yamanaka K, Penninger JM, Shibata S, Nishina H, 2017. Age-dependent motor dysfunction due to neuron-specific disruption of stress-activated protein kinase MKK7. Scientific Reports 2016 6 7, 7348. doi: 10.1038/s41598-017-07845-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wu Z, Renier N, Simon DJ, Uryu K, Park DS, Greer PA, Tournier C, Davis RJ, Tessier-Lavigne M, 2015. Pathological Axonal Death through a MAPK Cascade that Triggers a Local Energy Deficit. Cell 160, 161–176. doi: 10.1016/j.cell.2014.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Yang W, Gao Z, Jia P, 2017. Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neurosci. Lett 647, 133–140. doi: 10.1016/j.neulet.2017.03.027 [DOI] [PubMed] [Google Scholar]
- Yaron A, Schuldiner O, 2016. Common and Divergent Mechanisms in Developmental Neuronal Remodeling and Dying Back Neurodegeneration. Current Biology 26, R628–R639. doi: 10.1016/j.cub.2016.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin TC, Voorhees JR, Genova RM, Davis KC, Madison AM, Britt JK, Cintrón-Pérez CJ, McDaniel L, Harper MM, Pieper AA, 2016. Acute Axonal Degeneration Drives Development of Cognitive, Motor, and Visual Deficits after Blast-Mediated Traumatic Brain Injury in Mice. eNeuro 3, ENEURO.0220–16.2016. doi: 10.1523/ENEURO.0220-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitane H, Honma S, Imamura K, Nakajima H, Nishide SY, Ono D, Kiyota H, Shinozaki N, Matsuki H, Wada N, Doi H, Hamada T, Honma KI, Fukada Y, 2012. JNK regulates the photic response of the mammalian circadian clock. EMBO reports 13, 455–461. doi: 10.1038/embor.2012.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GS, Choleris E, Lund FE, Kirkland JB, 2006. Decreased cADPR and increased NAD+ in the Cd38−/− mouse. Biochem. Biophys. Res. Commun 346, 188–192. doi: 10.1016/j.bbrc.2006.05.100 [DOI] [PubMed] [Google Scholar]
- Zala D, Hinckelmann M-V, Yu H, Lyra da Cunha MM, Liot G, Cordelières FP, Marco S, Saudou F, 2013. Vesicular Glycolysis Provides On-Board Energy for Fast Axonal Transport. Cell 152, 479–491. doi: 10.1016/j.cell.2012.12.029 [DOI] [PubMed] [Google Scholar]
- Zhao X, Strong R, Zhang J, Sun G, Tsien JZ, Cui Z, Grotta JC, Aronowski J, 2009. Neuronal PPARγ Deficiency Increases Susceptibility to Brain Damage after Cerebral Ischemia. J. Neurosci 29, 6186–6195. doi: 10.1523/JNEUROSCI.5857-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Sasaki Y, Milbrandt J, Gidday JM, 2013. Protection of mouse retinal ganglion cell axons and soma from glaucomatous and ischemic injury by cytoplasmic overexpression of Nmnat1. Invest. Ophthalmol. Vis. Sci 54, 25–36. doi: 10.1167/iovs.12-10861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziogas NK, Koliatsos VE, 2018. Primary Traumatic Axonopathy in Mice Subjected to Impact Acceleration: A Reappraisal of Pathology and Mechanisms with High- Resolution Anatomical Methods. J. Neurosci 38, 4031–4047. doi: 10.1523/JNEUROSCI.2343-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]