Abstract
Objective:
The choroid plexus (CP) is an important physiological barrier and produces cerebrospinal fluid, neurotrophic, angiogenic, and inflammatory factors involved in brain development. CP abnormalities have also been implicated in both schizophrenia and bipolar disorder. A CP transcriptomic analysis in schizophrenia identified an upregulation of immune/inflammatory genes that correlated with peripheral inflammatory markers. Our goal was to examine 1) CP volume across psychotic probands, their first degree and Axis-II A relatives, 2) CP familiality, and 3) CP covariance with clinical, cognitive, brain, and peripheral marker measures.
Method:
CP volume was quantified using FreeSurfer 6 in psychosis probands, their first degree and Axis-IIA relatives, and healthy subjects organized by DSM-IV-TR diagnosis (n=1,451). Analyte, structural connectivity and genotype data were collected from a subset of subjects.
Results:
CP volume was significantly larger in probands compared to controls, first-degree relatives had significant intermediate CP volume between probands and controls, and total CP volume was significantly heritable. Larger CP volume was associated with worse cognition, smaller total gray matter and amygdala volume, larger lateral ventricle volume, and lower structural connectivity in probands. We also found associations between larger CP volume and higher levels of IL6 in probands.
Conclusion:
Our findings suggest the involvement of the CP across the psychosis spectrum with a potential pathophysiological mechanism involving the neuro-immune axis, which functions in maintaining brain homeostasis and interacting with the peripheral immune and inflammatory system. The CP may be an important target for future research.
Introduction
The choroid plexus (CP) has been largely ignored in clinical neuroscience, but recent evidence suggests that it plays several important roles in brain homeostasis. The CP is located in the lateral, third and fourth ventricles and produces cerebrospinal fluid (CSF). The CP consists of an epithelial cell monolayer which forms a physical barrier called the blood–CSF barrier, and a stromal compartment containing permeable fenestrated blood vessels, as well as immune, pluripotent, hematopoietic, neuronal progenitor and telocyte cells (1,2). The choroid plexus epithelium limits the paracellular passage via tight junctions and allows intercellular communication through gap junctions (3). Transport across the blood–CSF barrier is tightly controlled involving both active and passive transport, which plays an important role in cell division, migration, differentiation and synaptogenesis during early brain development (4,5). The blood–CSF barrier can function as an enzymatic barrier with the ability to conjugate drugs and other proteins assisting in elimination and providing protection against oxidative stress (6). Also, the blood–CSF barrier is an immunological barrier with resident immune cells that can modulate leukocyte migration, produce pro-inflammatory cytokines, expresses major histocompatibility complex molecules and molecules for leukocyte adhesion (6). Lastly, the lateral ventricle CP secretes chemokines, lymphokines, growth factors, carriers, and remodelers that promote the proliferation of neural stem cells (1,7–9), and the CP epithelium transcriptome specifically functions in molecular transport, neurological disease processes, immunological functions, ciliary movement, hematopoiesis and angiogenesis (10). While our understanding of the role of the CP in brain homeostasis is growing, much less is known about its role in psychotic disorders, despite the intersection between CP function and the neurodevelopmental, neurodegenerative, and neuro-immune hypotheses in psychosis pathophysiology.
CP abnormalities have been discussed as a possible pathological feature in schizophrenia and bipolar disorder beginning with Morowoka and Kitabayashi in 1921, where morphological changes in choroid plexus epithelium (smaller and longer cells), vascular endothelium degeneration, large cystic formations, and cellular fat depositions in CP epithelium cells were identified in schizophrenia, while evidence of CP epithelium hypersecretion and degeneration of vascular epithelium and connective tissue were found in bipolar mania as compared to healthy controls (11,12). However, despite these findings the role of the CP in psychosis has been largely ignored. More recently, case reports have identified an association between worse cognition (memory difficulties and inattention), mood (depression, anhedonia, lethargy, isolation, and suicidal ideation), and psychosis (persecutory delusions, ideas of reference, auditory hallucinations, hostility, blunted affect, poverty of speech, emotional/social withdrawal and motor retardation) with fourth ventricle CP papilloma or third ventricle tumor, which resulted in full symptom remission after tumor resection (13–15). Moreover, cross-sectional studies using CT brain imaging have identified an association between the presence of CP calcification and schizophrenia neurobiology (16–19), but no brain MRI studies examining CP volume in schizophrenia exist to date. Previous, imaging studies have identified an association between increased CP calcification size and greater hallucination intensity, worse formal thought disorder, regional brain volume reductions (frontal, temporal, parietal, hippocampal, and cerebellar), and larger ventricular brain ratio at the frontal horns, but less is known regarding negative symptoms or cognitive dysfunction (16,18,19). In schizophrenia, a recent transcriptomic analysis of the CP found that a co-expression module of immune and inflammation genes was upregulated and this module was significantly associated with disease status and positively correlated with C-reactive protein (CRP), cortisol, and pro-inflammatory cytokines in the serum and frontal cortex of the same individuals (20). Therefore, these findings suggest that the increased size of the CP might be involved in psychosis phenomenology (positive, negative, and cognitive symptoms), neurobiology (regional volumetric reductions), and neuro-immune axis connection, but more research is needed to further our understanding of the role of the CP across the psychosis spectrum.
Taken together, we sought to investigate 1) CP volume across psychotic disorder probands, their first-degree and Axis IIA relatives, and healthy subjects, 2) CP familiality between probands and their first-degree relatives, and 3) CP covariance with clinical, brain, and peripheral marker measures using data from the cross-sectional Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium. We hypothesize that CP volume is increased in psychosis and is heritable, and that increased CP volume is associated with worse psychosis symptoms and cognition, correlated with regional brain atrophy, and is related to increased levels of peripheral inflammatory markers.
Methods
Participants
A subset of participants for whom FreeSurfer analyses could be completed were included from the B-SNIP multisite study that included Harvard University, Wayne State University, Maryland Psychiatric Research Center, University of Chicago, University of Texas Southwestern Medical Center at Dallas and Institute of Living/Yale University. The study protocol was approved by the institutional review board at each local site. Probands with diagnoses of schizophrenia (SZP), schizoaffective disorder (SADP), or psychotic bipolar I disorder (BPP), at least one of each proband’s first-degree relatives, and healthy control (NC) subjects (21) were recruited and provided written consent (see supplementary methods for inclusion criteria).
The Structured Clinical Interview for DSM-IV (SCID-IV) was administered to all subjects and a consensus diagnosis was made using clinical interviews, medical records and psychiatric histories. Relatives were assessed with the Structured Interview for DSM-IV Personality Disorders (SIDP-IV) to evaluate personality traits specifically relevant to the psychosis spectrum, represented by the cluster A personality disorders (21). Average daily chlorpromazine (CPZ) equivalents were generated and antipsychotics were categorized into first generation and second generation antipsychotic classes (Table 1). Probands were administered the Positive and Negative Symptom Scale (PANSS) and Montgomery-Asberg Depression Rating Scale (MADRS). Cognition was evaluated using the Brief Assessment of Cognition (BACS) battery (22) and all scores were adjusted for age and sex and z-transformed to a range of ±4.0. The Birchwood Psychosocial Function Scale (SFS) was used to capture social functioning in all groups. For group sample size by clinical measure see ST1.
Table 1:
Sample Characteristics of the Comparisons between Probands, First Degree Relatives and Control Subjects
| NC (n=333) | Probands (n=554) | Relatives (n=522) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | N | N | X2 | p-value | ||||
| Sex (Male/Female)a | 149/184 | 267/287 | 158/364 | 39.3 | <0.001 | |||
| Race (AA/CA/OT)a | 88/215/30 | 206/313/35 | 146/350/26 | 24.5 | <0.001 | |||
| Antipsychotic class (FGA/SGA/none) | - | 48/406/90 | - | - | - | |||
| Mean | SD | Mean | SD | Mean | SD | F | p-value | |
| Age (years)a | 37.0 | 12.4 | 35.5 | 12.5 | 40.8 | 15.6 | 13.6 | <0.001 |
| Duration of Illness (years) | - | - | 17.9 | 12.2 | - | - | - | - |
| Age of onset (years) | - | - | 17.6 | 7.7 | - | - | - | - |
| PANSS Total Scores | - | - | 62.9 | 16.9 | - | - | - | - |
| Daily CPZ equivalents | - | - | 446.9 | 390.5 | - | - | - | - |
| ICV (mm3) | 1,431,124.5 | 191,440.9 | 143,5747.3 | 184,991.4 | 1,406,251.9 | 179,218.8 | 2.58 | 0.055 |
Notes: NC, normal controls; SD, standard deviation; AA, African American; OT, other; CA, Caucasian; NC, healthy control; FGA, first generation antipsychotics; SGA, second generation antipsychotic; CPZ, chlorpromazine; PANSS, Positive and Negative Symptoms Scale; ICV, intracranial volume
, p<0.05.
Structural MRI
Subjects were scanned at all six sites and T1 MPRAGE images were processed using Freesurer 6.0 (see supplementary methods for imaging protocol). Forty-seven participants were removed for scanner inhomogeneity and motion that interfered with segmentation. Participants with greater than four standard deviations (n=6) and between the third and fourth standard deviation (n=19) were removed and winsorised to the third standard deviation, respectively. To examine the relationship between CP volume and the dysconnectivity hypothesis in psychosis, we ran an exploratory analysis of diffusion weighted images to extract the average tissue fractional anisotropy (FAt) in a subset of subjects (Probands, n=131; and Relatives, n=140, see supplementary methods). Healthy control subjects were not included in this analysis due to the small sample size (n=42).
Since few studies have published on CP volume using FreeSurfer, two raters (PL and OL) established reliability using manual ratings for the scans. The CP for 24 raw scans (4 per site) were randomly and blindly manually rated for small, medium and large volumes. The raters had a high intra-class correlation coefficient for their ratings (ICC=0.79) and moderate Spearman correlations with FreeSurfer volume (r=0.44, p <0.01).
Plasma analyte assays
Blood samples from the Chicago site were obtained from 107 psychosis probands (BPP, n=48; SADP, n=23; SZP, n=36) and 58 healthy controls at baseline, batched, spun at 4°C, divided into 300μl aliquots, and stored at −80°C. The plasma concentrations of inflammatory/ neurotrophic (IL-1β, IL2, IL4, IL6, IL8, IL10, IL12, IL12p70, IFNγ, TNFα, CRP, sFlt-1, VEGF, TGFβ) markers were determined using MESO SCALE DISCOVERY’S (MSD) MULTI-ARRAY® Technology. These analytes were selected based on their implication in schizophrenia and bipolar disorder (23–29). See supplementary methods for analyte quality control. See ST1 for a breakdown of the number of subjects assayed by analyte and by group.
Statistical Analysis
Descriptive statistics were computed for demographic variables across groups and differences were examined using χ2 test or analysis of variance (ANOVA). Pairwise contrasts using analysis of covariance (ANCOVA) to assess CP volume were run on probands, first-degree relatives (Relatives), Axis II Cluster A family members (Axis IIAR) and NC. An exploratory analysis of diagnostic (SZP, SADP, and BPP) groups compared to NC was performed to determine if a particular group was driving the effect.
Effect sizes were calculated using Cohen’s d. Partial correlations for clinical, cognitive, analyte and neuroimaging measures were analyzed using Spearman (non-normal distribution for several variables). Calculated p-values were false discovery rate (FDR) corrected for multiple comparisons (q-value) and this was performed for each hypothesis tested, as well as for exploratory analyses. All data were adjusted for age, sex, race and site. Imaging data were additionally adjusted for total intracranial volume (ICV). To examine whether CP volume is determined by total lateral ventricle volume and/or total gray matter volume we performed CP group comparisons using total lateral ventricle volume and total lateral ventricle volume plus total gray matter volume instead of ICV as a covariate to regress out the effects of cortical atrophy and ventricular enlargement. Analyte data were additionally adjusted for storage days. All statistical analyses were performed using R (version 3.4.3).
Familiality for total CP volume was quantified in 340 probands and 464 of their first-degree relatives using Sequential Oligogenic Linkage Analysis Routines (SOLAR) version 8.1.4 with a maximum likelihood method. Significance was determined by comparing a model explaining phenotypic variation by family membership to a model assuming family membership has no variation. Familiality estimates were calculated using a polygenic model and CP volume was covaried for age, sex, race, site, and ICV.
Results
Demographics
Our final sample included 554 probands (diagnostic groups: SZP, n=223; SADP, n=141; BPP, n=190), 522 first-degree relatives, 42 Axis IIAR, and 334 NC (Table 1, ST5). The probands, family members and NC were matched for ICV but significantly differed for age, sex, and race (Table 1). The diagnostic groups showed no differences for age, but were significantly (p<0.001) different for sex, race, duration of illness, age of onset and ICV (ST2). PANSS total score were in the moderate range (>58 and ≤75) for the proband group (Table 1). PANSS total score were in the mild (≤58) and moderate range for the diagnostic groups, and they were significantly different between the three diagnostic groups (ST2) (30). Daily CPZ equivalents were not significantly different between the three diagnostic groups (ST2). Many of the probands were on second generation antipsychotics (n=406), fewer on first generation (n=48), none were taking both first and second-generation antipsychotics, and the remaining weren’t taking any antipsychotic medications (n=90) (Table 1).
Choroid plexus volumetric differences
Probands had significantly larger bilateral CP volume compared to NC (q<0.001, Cohen’s d=0.335 and 0.301 for left and right, respectively, Figure 1A, ST3 and ST4) and these results remained significant after covarying for lateral ventricle volume (q<0.05, Cohen’s d=0.193 and 0.162 for left and right respectively) or lateral ventricle volume plus total gray matter volume (q<0.05, Cohen’s d=0.188 and 0.181 for left and right respectively). Probands had significantly greater bilateral CP volume compared to first-degree relatives (Figure 1A, left CP q=<0.001, Cohen’s d=0.226; right CP q<0.001, Cohen’s d=0.205) but these results were no longer significant after covarying for lateral ventricle volume with or without total gray matter volume (ST4). There were no significant CP volume differences between first-degree relatives and NC (Figure 1A)., Probands showed a non-significant increase in the left CP volume (Cohen’s d=0.254) compared to Axis IIAR. while Axis IIAR demonstrated a non-significant bilateral enlargement (Cohen’s d=0.09–0.25) compared to NC (ST5).
Figure 1.
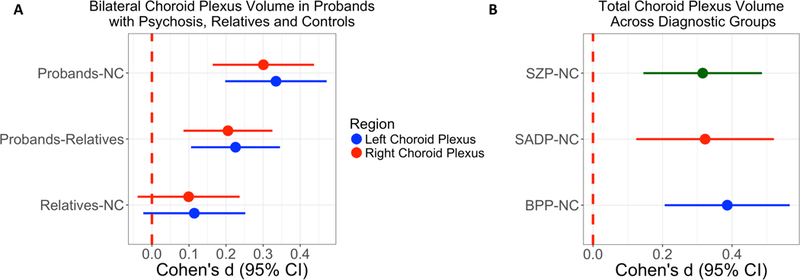
Effect sizes for choroid plexus volume comparisons. 1A) Pairwise contrasts between probands, first-degree relatives, and healthy controls (NC) for bilateral choroid plexus volume. 1B) Comparisons of total choroid plexus volume for schizophrenia (SZP), schizoaffective (SADP), and psychotic bipolar I disorder (BPP) compared to NC. Dot indicates Cohen’s d and lines indicate confidence intervals (CI). Cohen’s d estimates were adjusted for age, sex, race, site, and intracranial volume.
Since greater CP volume was observed bilaterally in probands, subsequent analyses assessed total CP volume by summating the corresponding left and right CP volumes. All three diagnostic groups (SZP, SADP and BPP) displayed greater total CP volume compared to NC (q≤0.001, Cohen’s d = 0.315 to 0.386 for diagnostic groups, Figure 1B, ST4). When including lateral ventricle volume as a covariate instead of ICV, then SZP and BPP (Cohen’s d = 0.192, 0.252, respectively, ST4) remained significantly larger than NC (q<0.05) while SADP did not. A similar pattern was noted when including lateral ventricle and total gray mater volume as covariates instead of ICV (SZ, Cohen’s d = 0.198; BPP, Cohen’s d = 0.259, see ST4) and this was significantly (q<0.05) different from controls.
Total CP volume was found to be moderately heritable (Log likelihood = −5039.68, h2R is 0.297, standard error = 0.087, p<0.001, kurtosis 0.570).
CP volume correlations with clinical variables
PANSS positive total score and negative total score, age of onset and daily chlorpromazine equivalents displayed no significant relationships in probands (ST6). To address the dopamine and serotonin link to CP (31,32), we explored the relationship of antipsychotics that are predominantly dopamine antagonism (first-generation antipsychotics) versus serotonin-dopamine antagonists (second-generation antipsychotics) on CP volume. There was no significant relationship (p=0.25, F=1.38, df=2) between first-generation (CP volume mean=1089.4 and SD=304.2), second-generation (CP volume mean=1141.9 and SD=327.6), and no antipsychotic use (CP volume mean=1097.4 and SD=329.5) in our sample. SFS total was not significantly related to CP volume in the probands, first-degree relatives or NC (Figure 2A, ST7), as well as Axis IIAR (ST5).
Figure 2.
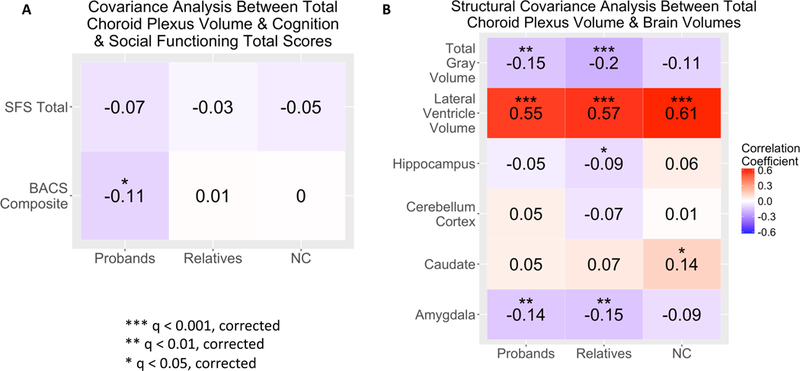
Choroid plexus volume correlations with clinical and brain structural measures in probands, first-degree relatives and healthy controls (NC). 2A) Partial correlations between choroid plexus volume and cognition (BACS composite score) and functioning (SFS total). 2B) Partial correlations between choroid plexus volume and total gray matter, lateral ventricle, hippocampus, cerebellum cortex, caudate and amygdala volume. Partial correlations were adjusted for age, sex, race, site. Imaging data was additionally adjusted for total intracranial volume. FDR corrected q-values.
CP volume correlations with Cognition
Larger CP volume had a significant (r=−0.11, q<0.05), relationship with worse BACS composite scores in probands, but not in family members or NC (Figure 2A, ST7). For subcategories within the BACS, probands showed significant associations between greater CP volume and lower performance in verbal fluency (r=−0.132, q<0.05) and symbol coding (r=−0.109, q<0.05), but not for other subdomains or in NC, relatives (SF1, ST7) and Axis IIAR (ST5).
CP volume correlations with brain structure
Larger CP volume significantly (q<0.001) correlated with larger lateral ventricle volume in all three groups (Figure 2B, ST8). Lower total gray matter volume displayed a significant (q<0.01) relationship with larger CP volume in probands and first-degree relatives. Smaller amygdala volume had a significant (q<0.01), relationship with bigger CP volume in probands and relatives. First degree relatives demonstrated a relationship between larger CP volume and smaller hippocampal volume (q<0.05). NC showed a significant (q<0.05) correlation between higher CP volume and higher caudate volume (ST8). Axis IIAR had significant (q<0.05) positive relationships between larger CP volume and bigger lateral ventricle or larger caudate volume (ST5). No significant relationships were observed between CP volume and cerebellum cortex volume.
In examining the relationship between CP volume and brain structural connectivity, it was observed that in a subset of our neuroimaging sample that increased CP volume was significantly negatively associated with global FAt (p=0.012, Figure 3) in probands and this same relationship was not seen in relatives.
Figure 3.
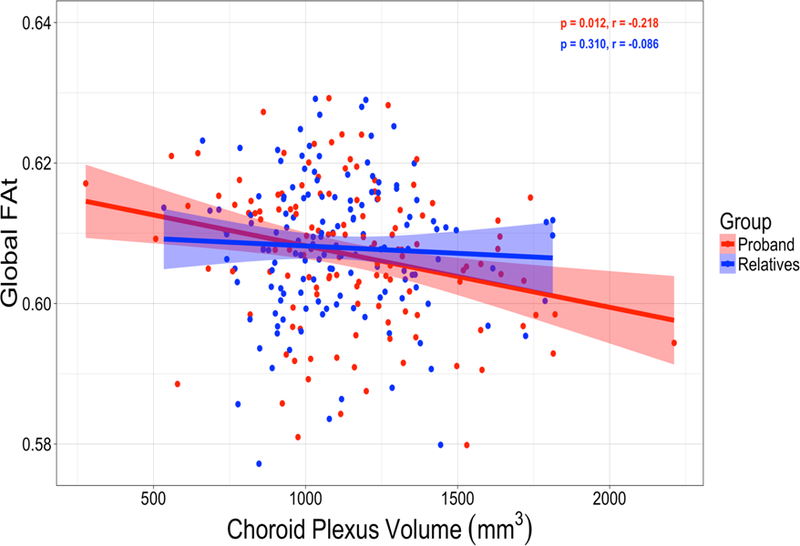
Choroid plexus volume correlations with free water corrected global fractional anisotropy (FAt) in probands and relatives. Partial correlations were adjusted for age, sex, race, site. Choroid plexus volume was additionally adjusted for total intracranial volume.
CP volume correlations with analytes
Elevations in IL6 was associated (r=0.23, p=0.02, uncorrected) with larger CP volume in probands, but not controls (Figure 4A and 4B, ST9). To test the effect of clinical condition we examined diagnostic group differences and found that greater levels of IL6 (r=0.31, p=0.04) and IL10 (r=0.36, p=0.01) were associated with larger CP volume in the BPP group, while greater IL4 levels were associated with smaller CP volume (r=−0.30, p=0.04) in controls (ST9). No other significant correlations were observed between CP volume and remaining analytes in the proband, diagnostic or NC groups. To address whether IL6 levels were associated with symptom severity, we performed a Spearman correlation between IL6 levels and PANSS total score or MADRS total score and no relationship was observed (r=0.059, p=0.542 and r=0.021, p=0.833, respectively).
Figure 4.
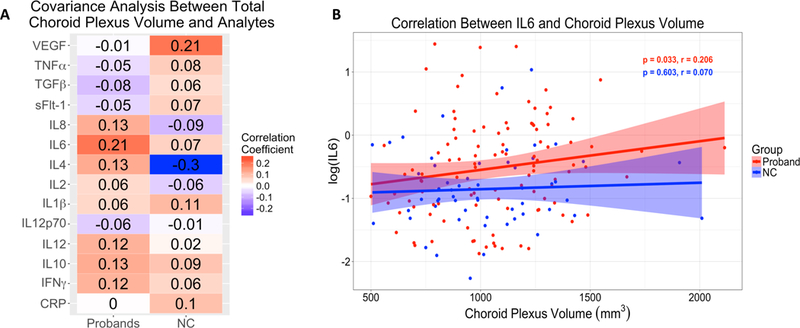
Choroid plexus volume partial correlations with analyte measures in probands and healthy controls (NC). 3A) Partial correlations between choroid plexus volume and analytes. 3B) Partial correlation between choroid plexus volume and log(IL6) in probands and NC. Partial correlations were adjusted for age, sex, and race and p-values FDR corrected for multiple comparisons. Imaging data was additionally adjusted for total intracranial volume and analyte data was additionally adjusted for sample storage days.
Discussion
In summary, we found that 1) bilateral CP volume was significantly larger in probands compared to controls and was independent of diagnostic category, 2) first-degree relatives had significant intermediate bilateral CP enlargement between probands and controls and 3) total CP volume was significantly heritable. We also demonstrated associations between larger CP volume and worse composite cognitive score, which was primarily driven by poorer performance in verbal fluency and digit symbol coding in probands. We did not observe any associations between CP size and positive or negative symptoms of psychosis or social functioning. Similarly, we established that greater CP volume was related to smaller total gray matter and amygdala volume, larger lateral ventricle volume, and lower structural connectivity in probands. Additionally, we uncovered an important association between larger CP volume and higher levels of IL6 in probands. Finally, we identified a candidate SNP (rs112819629) on chromosome 3p14.2 that was associated with total CP volume. Our results support our hypothesis that CP enlargement exists across psychotic disorders and that it is related to clinical and neuroimaging phenotypes observed in psychosis, which include cognitive impairment, brain atrophy, brain structural dysconnectivity, and increased inflammation.
Our finding of greater CP volume in psychosis suggests that the CP may be a common trait shared across the psychosis spectrum. Since this is the first brain structural MRI study examining CP volume in psychotic disorders, estimates of CP structural alterations do not exist. However, postmortem studies in schizophrenia and bipolar disorder have described morphological changes in the CP, such as smaller and longer epithelial cells, large cystic formations, epithelial cellular fat depositions, and epithelial hypersecretion, which would suggest that CP enlargement is a pathological feature of psychosis (11). Additionally, evidence supporting this hypothesis has come from observations of CP enlargement due to tumor formation or calcifications, which have shown that increased CP size is associated with worse symptoms of cognition, mood, and psychosis, as well as a pattern of regional brain atrophy similar to schizophrenia (13–19).
While we observed an increase in CP volume in psychosis, this information was not sufficient to enhance our understanding of the pathophysiological role of CP enlargement in psychosis, which could be hypothesized to include a neurodevelopmental, neurodegenerative, neuro-immune or the effect of antipsychotic medications. In this study, we ruled out the effect of comorbidity of medication, such as antipsychotic dosage or antipsychotic class on CP volume and our finding was consistent with preclinical studies showing that infusion with dopamine or serotonin increases CP blood flow, and that dopamine receptor blockade reduces flow, but that it doesn’t necessarily change the CP volume (31,32). From a neurodevelopmental and neurodegenerative standpoint, we were not able to determine whether our CP volume finding was due to hypersecretion, decreased absorption, or other morphological changes due to limitations in structural brain imaging, which could explain the well-known finding of ventricular enlargement and/or cortical atrophy. However, we were able to show that CP volume was both independently and significantly increased in probands compared to controls after controlling for lateral ventricle volume or lateral ventricle volume plus total gray matter volume. Lateral ventricle enlargement and cortical atrophy is a well-established finding in schizophrenia and bipolar disorder, and our findings suggest that the CP is a more specific determinant of psychosis proneness and that it may contribute to lateral ventricle enlargement through a currently unknown mechanism. It remains to be determined whether CP enlargement is a cause or consequence of psychosis and this question could not be fully ascertained with our cross-sectional study. Thus, our findings suggest that CP alterations may be involved in psychosis and it would be important for future studies to identify whether the CP plays a pathogenic role, is governed by ventricular enlargement via cortical atrophy, is progressively increasing over the course of the illness, and whether it is specific to psychosis by examining non-psychotic bipolar I disorder or major depressive disorder groups.
Another important pathophysiologic mechanism involves the neuro-immune connection and its role in brain homeostasis. Thus, we examined the relationship between serum markers and CP volume, since the CP links peripheral signaling (inflammatory, angiogenic, and neurotrophic) to CNS molecular processes, which may contribute to the development and progression of psychotic disorders (20,26,33,34). We found that increased IL6 levels were significantly associated with larger CP volume. This finding is of interest since the CP expresses the receptor for IL6 (35) and in the blood IL6 has pleiotropic activity which induces the synthesis of acute phase proteins (e.g CRP), activates the acquired immune response by stimulating antibody production and effector T-cell development, and can promote the differentiation/proliferation of nonimmune cells (36). It is important to note that IL6 elevations are strongly implicated in schizophrenia and bipolar pathophysiology (25) and that cortical gray matter reductions (assessed in post mortem samples) in schizophrenia is exaggerated in patients with high expression of inflammatory cytokines (37). In further support of this hypothesis, a schizophrenia postmortem CP transcriptome study identified an immune/inflammation module that significantly positively correlated with serum CRP levels, which is downstream from IL6 (20). Lastly, a preliminary study by Zhou et al 2015 showed that CP volume was increased in patients with chronic regional pain syndrome compared to controls and they postulated that this finding was due to inflammatory processes (38). While this novel finding may suggest that CP enlargement is due to inflammatory pathways in any disorder rather than being specific to psychosis, we believe that their small sample size and lateralization of their findings, suggest that more work is needed before concluding that CP enlargement is specific to inflammatory activation in any disorder. Thus, IL6 and CRP may play a role in in stress-immune related pathways leading to immune activation and vascular dysfunction that can have detrimental CNS effects (25). The positive relationship between CP volume and IL6 provides further support that the CP links the peripheral and CNS environments. However, additional work is needed to better understand the significance of peripheral immune and inflammatory markers on CP structural alterations.
The association between the CP neuro-immune axis and our brain structural and cognitive findings are further supported by our covariance analysis. Studies have shown that increased levels of inflammation are associated with reductions in cortical gray matter volume (37). Additionally, neuroinflammation has been associated with white matter pathology and it may contribute to structural and functional dysconnectivity in psychosis (39). Lastly, animal studies have demonstrated that CD4+ T cells in the CP are responsible for cognitive functions such as spatial learning and memory, and that these CD4+ T cells accumulate during mental stress (40). Taken together, the association between higher levels of IL6 and increased CP volume, could explain the association between greater CP volume and total gray matter thinning, amygdala atrophy, ventricular enlargement, and lower structural connectivity, and these changes may culminate in worse cognition. This axis might be triggered by mental stress, which would active a neuro-immune cascade with significant downstream effects on cognition and brain structure.
A discussion of the familiality findings can be found in the supplemental discussion material.
To our knowledge, this is the first report of alterations in CP volumes across the psychosis spectrum. A major strength of this study is an automated parcellation approach with high intra-class correlations between raters (PL and OL) and moderate correlations with FreeSurfer CP volume. Another strength is the large, clinically well-characterized sample of patients across the psychosis spectrum. However, the subsample was not large enough to detect robust relationships with peripheral makers and genome-wide associations. Nevertheless, our results provide the first study examining the role of CP structure in psychotic disorders which can inform future studies.
Supplementary Material
acknowledgments:
This research was supported in part by grants MH-077851 (Dr. Tamminga), MH-078113 (Dr. Keshavan), MH-077945 (Dr. Pearlson), MH-077852 (Dr. Thaker), and MH-077862 (Dr. Sweeney) from the National Institute of Mental Health, grant SCDMH82101008006 (Dr. Keshavan) from the Commonwealth Research Center, grant UL1TR000114 (Dr. Bishop) from the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number, and the Livingston grant (Dr. Lizano) from Harvard Medical School.
The authors thank the families at each site who took part in this study, and the many scientific participants at each site who contributed to this project.
Footnotes
Previous presentation:
Oral Presentation at American Neuropsychiatric Association, Boston, MA, March 21–24, 2018
Location of work: Paulo Lizano MD, PhD, Department of Psychiatry, Beth Israel Deaconess Medical Center, 330 Brookline Ave KS253, Boston, MA 02215, USA
Disclosures
There are no conflicts of interest or competing interests to report for any of the listed authors.
Supplementary Figure 1: Choroid plexus volume correlations with BACS subscale scores in probands, first-degree relatives and healthy controls (NC). Partial correlations were adjusted for age, sex, race, site. Imaging data was additionally adjusted for total intracranial volume. FDR corrected q-values.
References
- 1.Falcão AM, Marques F, Novais A, Sousa N, Palha JA, Sousa JC. The path from the choroid plexus to the subventricular zone: go with the flow! Front Cell Neurosci. Frontiers; 2012;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roballo KCS, Gonçalves NJN, Pieri NCG, Souza AF, Andrade AFC, Ambrósio CE. Regulation of neural stem cells by choroid plexus cells population. Neurosci Lett 2016. July 28;626:35–41. [DOI] [PubMed] [Google Scholar]
- 3.De Bock M, Vandenbroucke RE, Decrock E, Culot M, Cecchelli R, Leybaert L. A new angle on blood-CNS interfaces: a role for connexins? FEBS Lett Wiley-Blackwell; 2014. April 17;588(8):1259–70. [DOI] [PubMed] [Google Scholar]
- 4.Saunders NR, Daneman R, Dziegielewska KM, Liddelow SA. Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol Aspects Med 2013. April;34(2–3):742–52. [DOI] [PubMed] [Google Scholar]
- 5.Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. BioMed Central; 2014;11(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortazavi MM, Griessenauer CJ, Adeeb N, Deep A, Shahripour R Bavarsad, Shahripour RB, et al. The choroid plexus: a comprehensive review of its history, anatomy, function, histology, embryology, and surgical considerations. Childs Nerv Syst. Springer Berlin Heidelberg; 2014. February;30(2):205–14. [DOI] [PubMed] [Google Scholar]
- 7.Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, Doetsch F. Age-Dependent Niche Signals from the Choroid Plexus Regulate Adult Neural Stem Cells. Cell Stem Cell 2016. November 3;19(5):643–52. [DOI] [PubMed] [Google Scholar]
- 8.Chiaretti A, Antonelli A, Genovese O, Pezzotti P, Rocco CD, Viola L, et al. Nerve growth factor and doublecortin expression correlates with improved outcome in children with severe traumatic brain injury. J Trauma. The Journal of Trauma: Injury, Infection, and Critical Care; 2008. July;65(1):80–5. [DOI] [PubMed] [Google Scholar]
- 9.Walter HJ, Berry M, Hill DJ, Cwyfan-Hughes S, Holly JM, Logan A. Distinct sites of insulin-like growth factor (IGF)-II expression and localization in lesioned rat brain: possible roles of IGF binding proteins (IGFBPs) in the mediation of IGF-II activity. Endocrinology 1999. January;140(1):520–32. [DOI] [PubMed] [Google Scholar]
- 10.Janssen SF, Gorgels TG, Ten JB Brink, Jansonius NM, Bergen AA. Gene expression-based comparison of the human secretory neuroepithelia of the brain choroid plexus and the ocular ciliary body: potential implications for glaucoma. Fluids Barriers CNS. BioMed Central; 2014. January 29;11(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.TAFT AE. A NOTE ON THE PATHOLOGY OF THE CHOROID PLEXUS IN GENERAL PARALYSIS. Arch NeurPsych American Medical Association; 1922. February 1;7(2):177–82. [Google Scholar]
- 12.DE SKE-R, 1921. THE CHOROID PLEXUS IN ORGANIC DISEASES OF THE BRAIN AND OF SCHIZOPHRENIA. 21AD
- 13.Arasappa R, Danivas V, Venkatasubramanian G. Choroid plexus papilloma presenting as schizophrenia: a case report. J Neuropsychiatry Clin Neurosci American Psychiatric AssociationArlington, VA; 2013;25(1):E26–7. [DOI] [PubMed] [Google Scholar]
- 14.Carson BS, Weingart JD, Guarnieri M, Fisher PG. Third ventricular choroid plexus papilloma with psychosis. Case report. J Neurosurg 4 ed. Journal of Neurosurgery Publishing Group; 1997. July;87(1):103–5. [DOI] [PubMed] [Google Scholar]
- 15.Gupta R, Bansal K, Lahan V, Kumar R, Agrawal S. Fourth ventricl choroid plexus papilloma (CPP) presenting as apathy and depression. Delhi Psychiatry Journal 2013. May 14;16:1–3. [Google Scholar]
- 16.Marinescu I, Udriştoiu I, Marinescu D. Choroid plexus calcification: clinical, neuroimaging and histopathological correlations in schizophrenia. Rom J Morphol Embryol 2013;54(2):365–9. [PubMed] [Google Scholar]
- 17.Sandyk R, Kay SR. Choroid plexus calcification: a biological marker of suicidality. Int J Neurosci 1991. March;57(1–2):95–7. [DOI] [PubMed] [Google Scholar]
- 18.Sandyk R Choroid plexus calcification as a possible marker of hallucinations in schizophrenia. Int J Neurosci 1993. July;71(1–4):87–92. [DOI] [PubMed] [Google Scholar]
- 19.Bersani G, Garavini A, Taddei I, Tanfani G, Pancheri P. Choroid plexus calcification as a possible clue of serotonin implication in schizophrenia. Neurosci Lett 1999. January 15;259(3):169–72. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Hwang Y, Lee D, Webster MJ. Transcriptome sequencing of the choroid plexus in schizophrenia. Transl Psychiatry Nature Publishing Group; 2016. November 29;6(11):e964–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry American Psychiatric AssociationArlington, VA; 2013. November;170(11):1263–74. [DOI] [PubMed] [Google Scholar]
- 22.Hill SK, Reilly JL, Keefe RSE, Gold JM, Bishop JR, Gershon ES, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry American Psychiatric AssociationArlington, VA; 2013. November;170(11):1275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckley PF, Mahadik S, Pillai A, Terry A. Neurotrophins and schizophrenia. Schizophr Res 2007. August;94(1–3):1–11. [DOI] [PubMed] [Google Scholar]
- 24.Lopes R, Soares R, Coelho R, Figueiredo-Braga M. Angiogenesis in the pathophysiology of schizophrenia - a comprehensive review and a conceptual hypothesis. Life Sci 2015. May 1;128:79–93. [DOI] [PubMed] [Google Scholar]
- 25.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry Nature Publishing Group; 2016. December;21(12):1696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dragunow M Meningeal and choroid plexus cells--novel drug targets for CNS disorders. Brain Res 2013. March 21;1501:32–55. [DOI] [PubMed] [Google Scholar]
- 27.Lizano PL, Keshavan MS, Tandon N, Mathew IT, Mothi SS, Montrose DM, et al. Angiogenic and immune signatures in plasma of young relatives at familial high-risk for psychosis and first-episode patients: A preliminary study. Schizophr Res 2016. January;170(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes BS, Steiner J, Molendijk ML, Dodd S, Nardin P, Gonçalves C- A, et al. C-reactive protein concentrations across the mood spectrum in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry 2016. December;3(12):1147–56. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes BS, Steiner J, Bernstein H- G, Dodd S, Pasco JA, Dean OM, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry Nature Publishing Group; 2016. April;21(4):554–64. [DOI] [PubMed] [Google Scholar]
- 30.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res 2005. November 15;79(2–3):231–8. [DOI] [PubMed] [Google Scholar]
- 31.Townsend JB, Ziedonis DM, Bryan RM, Brennan RW, Page RB. Choroid plexus blood flow: evidence for dopaminergic influence. Brain Res 1984. January 2;290(1):165–9. [DOI] [PubMed] [Google Scholar]
- 32.Faraci FM, Mayhan WG, Heistad DD. Effect of serotonin on blood flow to the choroid plexus. Brain Res 1989. January 23;478(1):121–6. [DOI] [PubMed] [Google Scholar]
- 33.Deczkowska A, Baruch K, Schwartz M. Type I/II Interferon Balance in the Regulation of Brain Physiology and Pathology. Trends Immunol 2016. March;37(3):181–92. [DOI] [PubMed] [Google Scholar]
- 34.Demeestere D, Libert C, Vandenbroucke RE. Therapeutic implications of the choroid plexus-cerebrospinal fluid interface in neuropsychiatric disorders. Brain Behav Immun 2015. November;50:1–13. [DOI] [PubMed] [Google Scholar]
- 35.Wang T, Wang B- R, Zhao H- Z, Kuang F, Fan J, Duan X- L, et al. Lipopolysaccharide up-regulates IL-6R alpha expression in cultured leptomeningeal cells via activation of ERK½ pathway. Neurochem Res 2008. September;33(9):1901–10. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014. September 4;6(10):a016295–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Catts VS, Sheedy D, McCrossin T, Kril JJ, Shannon Weickert C. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry Nature Publishing Group; 2016. December 13;6(12):e982–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou G, Hotta J, Lehtinen MK, Forss N, Hari R. Enlargement of choroid plexus in complex regional pain syndrome. Sci Rep Nature Publishing Group; 2015. September 21;5(1):14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 2015. January;161(1):102–12. [DOI] [PubMed] [Google Scholar]
- 40.Demeestere D, Libert C, Vandenbroucke RE. Therapeutic implications of the choroid plexus-cerebrospinal fluid interface in neuropsychiatric disorders. Brain Behav Immun 2015. November;50:1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


