Abstract
Islet transplantation is a promising long-term, compliance-free, complication-preventing treatment for type 1 diabetes. However, islet transplantation is currently limited to a narrow set of patients due to the shortage of donor islets and side effects from immunosuppression. Encapsulating cells in an immunoisolating membrane can allow for their transplantation without the need for immunosuppression. Alternatively, “open” systems may improve islet health and function by allowing vascular ingrowth at clinically attractive sites. Many processes that enable graft success in both approaches occur at the nanoscale level—in this review we thus consider nanotechnology in cell replacement therapies for type 1 diabetes. A variety of biomaterial-based strategies at the nanometer range have emerged to promote immune-isolation or modulation, proangiogenic, or insulinotropic effects. Additionally, coating islets within nano-thin polymer films has burgeoned as an islet protection modality. Materials approaches that utilize nanoscale features manipulate biology at the molecular scale, offering unique solutions to the enduring challenges of islet transplantation.
Keywords: islet transplantation, biomaterials, polymers
1. Introduction
Type 1 diabetes (T1D) is a chronic metabolic disease where affected individuals are unable to produce a sufficient amount of insulin and consequently lack glycemic control [1]. Although the pathophysiology of T1D is widely heterogeneous and incompletely understood, it is generally caused by the gradual autoimmune destruction of insulin-secreting β-cells, which are found within clusters known as islets of Langerhans in the pancreas [2]. T1D has historically been treated by frequent blood glucose (BG) measurements and insulin injections; more recently, continuous glucose monitoring systems and insulin pumps have become available for patients as well [3]. Despite improvements in diabetes care, these therapies require constant patient attention, which can cause a high degree of psychological stress. Moreover, available treatments do not achieve optimally regulated BG homeostasis, resulting in life-threatening complications such as vascular disorders [4], nerve damage [5], and episodes of hypoglycemic unawareness [6]. The development of a technology that can provide physiologic BG control without the requirement of frequent patient intervention would thus substantially improve the lives of type 1 diabetics.
Two strategies have emerged to address these shortcomings in diabetes care: closed-loop insulin delivery systems (i.e. glucose-responsive insulin pumps, also referred to as artificial pancreases), and β-cell replacement therapies (i.e. bioartificial pancreases). The commercial closed-loop product MiniMed 670G (Medtronic) represents the first of such technologies to enter the market [7]. Despite remarkable advances and reduced patient burden achieved by this device, glucose control still requires patient interaction. β-cell replacement therapies, where primary animal [8] or human [9] islets or stem cell-derived islet-like clusters [10] are transplanted into diabetic patients to replace those destroyed by autoimmune attack, have the potential to provide long-term, stress-free euglycemia restoration.
Allogeneic islet transplantation into the liver via the portal vein was established by the Edmonton protocol in 2000, with 50–70% of recipients attaining insulin independence [9, 11]. However, enduring systemic immunosuppression is a compulsory adjunct to the surgical procedure to deter an immune attack on the transplanted tissue; thus, the application of islet transplantation is limited to the most severely impacted patients [11]. Two materials-based strategies have been developed in parallel to improve upon the limitations of the Edmonton protocol: islet immunoisolation and “open” islet transplantation systems.
Encapsulating islets within a semipermeable immunoisolating material or device which maintains the free passage of nutrients, oxygen, and insulin to and from the encapsulated cells, while preventing direct immune contact, promises to greatly expand the scope of islet transplantation by, in most cases, obviating the need for systemic immunosuppression [12–18]. Graft function is dependent on the timely interchange of nutrients and insulin between the transplanted tissue and the host bloodstream. However, diffusion distances are increased by the addition of the semipermeable barrier, exacerbating the problem of oxygen and nutrient delivery to the already poorly serviced layers of the cell clusters. As the islet isolation procedure dissociates islets from the vasculature, this nutrient exchange is limited to slow, passive diffusion [19]. Further, many accessible transplantation sites suitable for encapsulated islet transplantation, such as the intraperitoneal and subcutaneous space, are poorly oxygenated [20, 21]. Given the high respiratory rate of islet tissue [22], and the detrimental effects of hypoxia on β-cell insulin secretion [23, 24], low oxygen levels impair islet survival and limit graft function. Overall, achieving acceptable mass transport is a major consideration for transplanted immunoisolated islets.
Alternatively, “open” systems do not employ an immune barrier and seek to improve β-cell replacement therapies by modulating the transplantation site. Although not physically immunoisolating, “open” systems can permit host interaction, deliver therapeutics, and provide mechanical support at clinically attractive sites. Therapeutics can be delivered to modulate the immune system, attract blood vessel and neural investment, and act directly on islets to support function. The reestablishment of the islet vasculature theoretically provides the most efficient means to confer adequate bidirectional mass transport. Because material and cell engineering may one day be able to establish immune tolerance to transplanted cells, “open” systems may become the leading cell transplant modality. Nanotechnology plays a key role in the advancement of material design and methods to engineer cells, producing enabling technologies for either encapsulation or “open” systems for cell replacement therapies.
Nanoscale considerations permeate all aspects of cellular replacement therapies. Insulin, for example, has a hydrodynamic diameter of approximately 2 nm [25] or 3.5 nm [26] for the monomer or hexamer respectively, whereas the cellular length scale is roughly microns. Thus, the pore size of an immune barrier must consistently be between these values to provide necessary selective permeability. Consequently, nuanced approaches have been developed to achieve tight nanoscale control of material pore size. Implanted materials also dependably induce a foreign body reaction (FBR) driven by the host innate immune system, resulting in the formation of a fibrotic capsule surrounding the implant [27]. The FBR can potentiate chronic inflammation at the graft site, and the deposited collagenous sheath can further occlude the essential bidirectional mass transport required for cell survival and diabetes correction [28]. The recipient immune system can also mount an attack against the antigens of the therapeutic cells through a wide variety of methods, some of which can be ameliorated by an immunoisolating membrane. These focal challenges currently limit the application of materials-assisted islet transplantation.
This manuscript reviews the literature at the interface of nanotechnology and islet replacement therapies, although many of the principles explored herein are applicable to the broader practice of tissue engineering and regenerative medicine. It will begin by reviewing the various strategies where nanotechnology has been and could be applied to overcome the fundamental challenges of islet replacement therapies, including mitigating the immune response, ensuring acceptable mass transport, and revascularizing the graft. It will then examine nanoscale engineering techniques applied to translatable macro-scale devices. Finally, it will survey techniques and outcomes of cellular nanoencapsulation technologies and provide an evaluation of their merit in the broader context of islet transplantation.
2. Nanotechnology in Materials-Assisted Islet Replacement Therapy
Molecular scale physical and cellular processes often determine the fate of islet transplantation outcomes. It is unsurprising then that nanotechnological innovations have been applied to biomaterials-assisted cell transplantation to overcome its major limitations. Broadly, encapsulation devices must limit the immune response and provide adequate mass transfer to and from the encapsulated cells. The latter requirement may be accomplished by ensuring adequate nutrient transport across an isolating membrane, or by introducing vasculature near the graft. On the other hand, open systems seek to reintegrate transplanted islets with the host vasculature as soon as possible to minimize cellular loss. The application of nanotechnology to manipulate the immune response, ensure islet survival, and integrate the transplanted matter with the host vasculature is reviewed below.
2.1. Nanotechnology in Immune Isolation and Manipulation
Danger signals in the body are created in response to the introduction and continued presence of an implant [29–31], which stimulates the immune system. The attenuation of fibrotic growth is a key goal for encapsulation device success. The FBR begins with nonspecific protein adsorption, the recognition of which induces a cellular response of the innate immune system resulting in inflammation and the formation of a fibrotic capsule around the implant [27]. It has been shown that macrophages largely regulate the FBR [32], to the extent that mice with dysfunctional macrophages were not observed to have fibrosis [33]. Macrophages localized at the graft site can polarize into classically activated pro-inflammatory (M1) or alternatively activated anti-inflammatory (M2) phenotypes as characterized by their interleukin (IL) and matrix metalloprotease (MMP) secretion profiles [34–37]. Although thorough characterizations have suggested that this bimodal framework is a simplification and that a range of subtypes exist [38, 39], manipulating macrophage polarization towards the M2 phenotype is generally recognized as a means to mitigate the FBR [40]. Neutrophils are another type of immune cell that contribute to the cellular response to a foreign body following implantation. They exacerbate the inflammatory response by secreting both chemokines and cytokines and synthesizing neutrophil extracellular traps (NETs) atop foreign implants [41]. Physiologically, NETs trap and kill pathogens for phagocytosis [42, 43]. It has been found that neutrophil numbers increase by 30–500-fold in mouse peritoneal exudate cells in reaction to insertion of implanted materials when compared to those receiving saline [41]. Of the adaptive immune system, regulatory T-cells (Tregs) play an important role in establishing immune tolerance to implanted biomaterials by secreting a set of anti-inflammatory cytokines [44]. Each of these components of the multifaceted immune response represent an engineering target to mitigate the effects of the FBR.
Both material and cellular components of the graft contribute to the immune reaction. Modulation of the immune system in cellular transplantation systems is differentiated from some forms of immunosuppression in that it does not result in the broad ablation of a cell type or eliminate immune responses to life-threatening infections. Cellular engineering strategies have been developed to reduce the immunogenicity of the transplanted tissue. Genome editing to match (donor to recipient) or eliminate human leukocyte antigen (HLA) genes has been suggested as a promising approach to improve the immune compatibility of stem cell-derived β-cells [45]. The safety of xenogeneic tissue has been improved by gene editing strategies as well [46, 47]. Islet-like clusters differentiated from human T1D patient-specific induced pluripotent stem cells have been shown to have similar function to those derived from healthy patients, which may represent a source of autologous tissue with lower immunogenicity than allogeneic tissue [48]. Alternatively, islet transplantation in immune-privileged sites (e.g. the testis [49], brain [50], thymus [51, 52], and anterior chamber of the eye [53]), has been explored, but these sites are generally undesirable for large-volume transplantation. This section will continue with a focus on nanoscale materials strategies to mitigate the immune response.
Nanomaterials-based immune system modulation may occur through the introduction of chemically-modified host-interfacing surfaces, or the local delivery or presentation of immunosuppressive drugs and biological agents (Fig. 1). Such technologies have also been reviewed previously [54]. Nanotechnological innovations in all these areas are advancing our ability to more precisely and specifically manipulate the immune system and are discussed below.
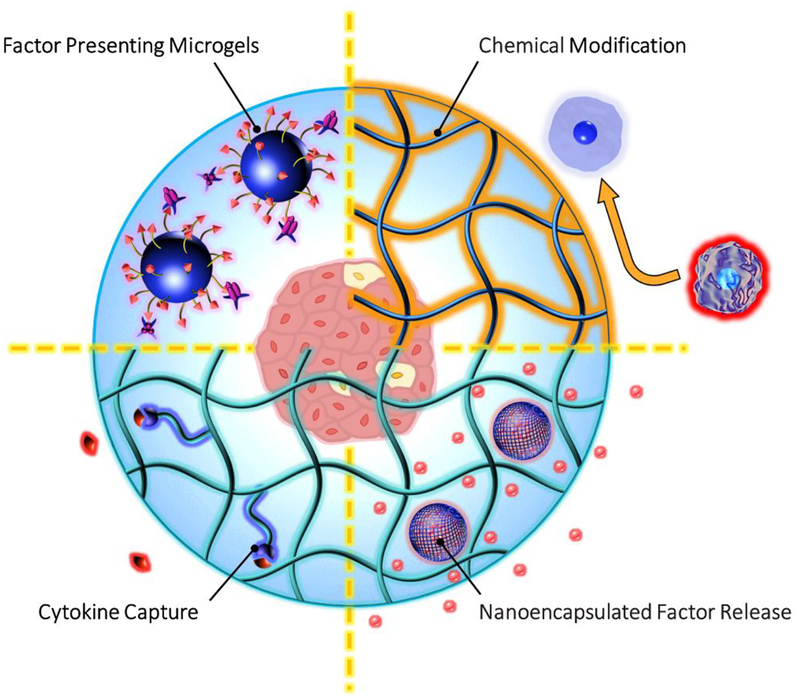
Fig. 1: Nanomaterial strategies to mitigate the immune response.
Chemical modification of host-interfacing polymers and encapsulants can provide specific functions including (starting top right, proceeding clockwise): tuning macrophage polarization by presentation of chemical structures including zwitterions, hydrophilic polymers, and polymer brushes; controlling factor (e.g. dexamethasone, curcumin, cyclosporine-A) release from nanoparticles; providing cytokine or factor (red) capture by peptide-recognition in functionalized hydrogels; enabling co-delivery of immunomodulatory molecules (e.g. biotin-streptavidin-FasL), not always requiring the use of an encapsulating hydrogel.
2.1.1. Chemical Modification
Maximizing material biocompatibility is a critical goal in many biomaterials applications, including biosensor development [55], orthopedic implants, [56] and regenerative medicine [57]. The FBR is generally considered to be initiated by the cellular recognition of denatured nonspecific proteins, which are spontaneously adsorbed on the material surface beginning within nanoseconds following implantation [58–60]. Although it has been suggested by Blaszykowski et al. that preventing the irreversible unfolding of select proteins at the biomaterial-host interface is the critical factor that endows a material with biocompatibility [61], a large body of research in low-fouling polymers has sought to comprehensively reduce protein adsorption altogether [62]. The magnitude of fibrosis in response to a material is dependent on several nanoscale biological, physical, and chemical processes. Accordingly, the rational design of materials at this scale can mitigate the fibrotic response.
Hydrophilic nanoscale polymer networks, which form hydrogels following crosslinking, are commonly employed in cell encapsulation systems due to their high water content and natural extracellular matrix (ECM) mimicry [63]. These properties make them suitable polymers for host-interfacing applications as well. Sodium alginate, an unbranched polysaccharide derived from brown seaweed [64], is likely the most widely used material for islet encapsulation as it is recognized as relatively biocompatible and can be gelled under mild conditions by complexation with divalent cations [65]. Further, the alginate matrix mesh size under gelation conditions used in cell encapsulation is in the nanometer range [66], permitting insulin and smaller molecule diffusion while barring the transport of larger molecules. Complexation with poly-l-lysine (PLL) provides a means by which to further tune this permeability [67, 68].
As alginate is derived from natural sources, polymer inconsistency and residual endotoxin levels remain concerns in its use [64]. Despite its presumed biocompatible characteristics, it has also been observed that alginate alone provokes some degree of fibrosis [69], possibly by macrophage surface recognition and activation [70]. A study by the Langer/Anderson lab found that triazole-modified alginates significantly lessened the FBR in immune-competent mice and nonhuman primates [21, 71]. Interesting nanoscale events seem to be responsible for success of these alginate analogues: the authors concluded that surface-localized triazole groups deterred macrophage recognition and hence fibrotic capsule deposition [71]. It was also noted that the lead triazole-modified group (Z1-Y15) was more hydrophilic than other high performing candidates [71], suggesting a positive relationship between hydrophilicity and biocompatibility. Providing evidence to support the hypothesis posited by Blaszykowski et al. (discussed above) [61], net protein adsorption was not correlated with the fibrotic predisposition of such polymers [71]. Intraperitoneal transplantation of human stem cell-derived islets encapsulated in 1.5 mm diameter Z1-Y15 modified alginate spheres restored euglycemia for 174 days in immune competent mice [72].
In terms of fouling proclivity alone, it has been suggested that the strength and degree of a hydration layer around the polymer may be negatively correlated with protein adsorption [73]. In addition, proteins, which often have surface-exposed charged residues, spontaneously bind to charged surfaces via electrostatic interactions [74]. These hypotheses may explain the success of several zwitterionic polymers, which bind to water strongly via ionic solvation and are net neutral in charge [75]. For example, zwitterionic poly(carboxybetaine methacrylate) (PCBMA) hydrogels synthesized by Zhang et al. substantially reduced the magnitude of fibrosis in comparison to poly(2-hydroxyethyl methacrylate) (PHEMA), when implanted subcutaneously for three months in mice [76]. Again, macrophages near the implant in PCBMA treated groups expressed more anti-inflammatory markers than those near PHEMA controls [76]. The zwitterionic phospholipid phosphorylcholine (PC), which mimics the surface of blood cells, has been found to have anti-fouling and anti-thrombotic characteristics as well [77, 78].
Polymer chain flexibility is also negatively correlated with the fibrotic response due to the effects of steric repulsion [75]. Thin polymer brushes have been designed to limit protein adsorption by this mechanism [62], though many of the applications from this line of research are beyond the scope of this review. In the context of cell encapsulation, poly(ethylene glycol) (PEG) has often been employed to this end (see Section 4.1). The examples provided above show that a variety of nanoscale chemical, physical, and biological properties of interfacing polymers modulate the immune response to a graft.
It is worth acknowledging that bulk material properties affect the fibrotic response as well. Several authors have reported a relationship between implant geometry and the FBR in immune competent animals [79–82]. An important finding for the practice of spherical islet microencapsulation is the observation that larger spheres (greater than 1.5 mm in diameter) resist fibrotic deposition to a greater degree than smaller spheres across a wide spectrum of biomaterials [79]. It has also been suggested that smooth-contoured implants induce a weaker FBR than those with rough edges [81]. Additionally, material stiffness has also been positively correlated with the magnitude of the FBR by several studies [76, 83]. Such findings emphasize that design considerations at length scales ranging from the nanometer to millimeter must be considered in FBR-resistant material design.
2.1.2. Immunosuppressive Drug Delivery
Several drugs have been developed for immunosuppression in organ transplantation, including glucocorticoids, non-steroidal anti-inflammatory drugs, and polyphenols [84]. Because many of these drugs have adverse side-effects when delivered systemically or chronically [84], local delivery can both reduce the effective dosage manifold and localize its effects in an effort to establish an anti-inflammatory microenvironment at the graft site. Expectedly, these strategies have been adopted in a variety of cell encapsulation systems via nanotechnology.
Cyclosporine-A (CsA) is a clinical immunosuppressive drug widely used to prevent transplant rejection by inhibiting T-cell proliferation [85]. Poly-lactide nanoparticles, where CsA is used as the initiator for lactide polymerization, have been explored as a method to increase the effectiveness of the drug. Similar dose-dependent suppression of T-cell proliferation and inflammatory cytokine production levels were observed in vitro with the nanoparticles [86]. Effectiveness in vivo was postulated to be dependent on transportation to draining lymph nodes by dendritic cells [86]. Similarly, ketoprofen release from slightly larger biodegradable capsules (5 and 20 μm mean diameter) reduced pericapsular overgrowth of alginate-based microcapsules transplanted in the peritoneal cavity of mice [87].
Combinations of drugs with islet coatings can be quite effective. The employment of 6-arm-PEG-catechol in tandem with tacrolimus, an immunosuppressive drug used in the Edmonton islet transplantation protocol [88], and anti-CD154 monoclonal antibody (mAb) (MR-1), which blocks CD4+ T-cell activation, provided murine transplant recipients with up to 50 days of normoglycemic levels [89]. Three different PEG layers and a systemically dosed anti-inflammatory drug cocktail including tacrolimus, rapamycin, MR-1, anti-CD19 mAb, and clodrosome significantly boosted median survival time of islet xenografts [90]. A separate study translated this layered surface camouflage approach to non-human primates, finding that such strategies increased post-transplantation survival to 150 days as opposed to roughly 5.5 days with untreated and unaltered islets, and roughly 77.5 days with islet and immunosuppressive drug combinations [91]. Functionalized nanocoatings, including those engineered for immunosuppression, are discussed in more detail in Section 4.3.
Local delivery of compounds may offer possibilities for combination with nanoscale materials. Co-encapsulating pentoxifylline with islets in alginate microcapsules, for example, decreased IL-2 levels in an in vitro model [92]. Dexamethasone and curcumin were identified to have the greatest mitigation of host immune response following subcutaneous biomaterial injections when compared with 14 other small molecule anti-inflammatory drugs [93]. Likewise, this same group showed improved glycemic control and reduction of fibrotic overgrowth in diabetic mice as a result of combining curcumin with rat islet microcapsules [93]. Dexamethasone released from a macroporous polydimethylsiloxane (PDMS) device improved transplant engraftment by initiating M2 macrophage polarization as well [94].
Likely working through a different mechanism in addition to a delivery route, nano-curcumin (a micelle formulation that increases oral bioactivity) administration to type 2 diabetic human subjects significantly decreased their HbA1c, fasting BG, triglyceride, and body mass index levels as opposed to those treated with a placebo [95]. Exosomes, a kind of naturally derived nanoparticle, for the delivery of curcumin have also been reviewed by Oskouie et al. [96]. Dexamethasone alleviated fibrosis when included in the preparation of electrospun fibers as well [37]. Several successful studies noted above involving the local delivery of dexamethasone suggest it may improve clinical outcomes. Nanoscale materials such as nanoparticles, exosomes, and micelles, as well as macro-materials that release drugs through nanoscale phenomena, are well-suited technologies for localized drug delivery. Their integration in islet transplantation platforms will continue to improve the state of the science.
2.1.3. Biological Modulation and Manipulation
Biological factors and cells can also modulate the immune response with a greater degree of specificity than immunosuppressive drugs. Introducing biological factors into materials with nanoscale techniques permits spatiotemporal control of their presentation and release in cell delivery systems. Both immobilization and controlled delivery techniques have been explored in this effort and are discussed below.
PEG hydrogels were functionalized to adsorb monocyte chemotactic protein (MCP)-1, a chemokine that recruits inflammatory cells, via tethered affinity peptides [97]. Encapsulating β-cells in these functionalized hydrogels substantially decreased MCP-1 secretion from encapsulated MIN-6 cells following stimulation with interferon (IFN)-γ, IL-1β and tumor necrosis factor (TNF)-α [97]. This group also functionalized a PEG hydrogel with an IL-1 receptor recognition peptide which conferred protection against IFN-γ, IL-1β, and TNF-α [98]. A similar structure developed by Su et al. protected MIN-6 cells from cytokine and β-cell-specific T lymphocyte destruction in vitro [99]. The prevention of cytokine-islet interactions within the immune barrier may prevent critical destructive events with encapsulated tissue.
Similar to immunosuppressive small molecule delivery, biological agents may be delivered from nanoscale structures [100]. Several studies presented the design of anti-CD4-coated poly(lactic-co-glycolic acid) (PLGA)-based nanoparticles for the targeted delivery of leukemia inhibitory factor (LIF) to T-cells, which stimulated their differentiation into Tregs [101, 102]. Nanoparticle-encapsulated LIF attached to PEG-coated islets increased the cure rate in full major histocompatibility complex (MHC) mismatch diabetic recipient mice over PEG coating alone [103]. In a multifaceted approach, integration of transforming growth factor (TGF)-β, IL-2, and rapamycin into nanoparticle systems successfully induced FoxP3+ Tregs in vitro in mouse and human cells [104]. Unutmaz et al. were able to differentiate inflammatory cytokine-resistant stable and functional CD25+ FoxP3+ regulatory cells from human CD4+ cells in vitro, providing more insight into this possible treatment pathway [105].
The importance of Tregs in establishing immune tolerance is also evidenced by outcomes upon their co-delivery with islet grafts. Co-localized Tregs in an abdominal fat pad site, transplanted via poly(lactide-co-glycolide) (PLG)–scaffolds, established normal glycemic levels in non-obese diabetic (NOD) mice [106]. The authors suggested that systemic tolerance was induced, as a second transplant into the kidney capsule was not rejected when the scaffold-assisted islets were removed [106]. Other accessory cells derived from immune-privileged sites have also been shown to improve graft function when simultaneously transplanted with islets [107–109]. These cellular co-delivery strategies may provide advantages over other methods due to the wide range of cytokines secreted by these supporting cells, though regulatory hurdles to using more cell types in a therapy do exist.
In an effort to find potent, but potentially simpler avenues for immunomodulation, factors can also be expressed or presented on the islet surface to impart function directly at the islet-host interface. Grafts were enhanced with the addition of Jagged-1 (JAG-1) on PEG-conjugated islet surfaces, leading to an increase in Treg levels and a shift towards an anti-inflammatory cytokine signature [110]. Fas ligand (FasL), a T-cell apoptotic factor [111], and CXCL12, a T-cell chemorepellent [112], have been expressed and presented on the surface of islets, delivering signals to approaching T-cells [113, 114]. Remarkably, an immune-tolerant microenvironment at the graft site was established by presenting FasL on the surface of microparticles that were co-transplanted with unencapsulated islets [115]. Attachment to biomaterial beads further increased the translatability of this approach as it requires less processing of the islets and can be shelf stable [115]. Interestingly, temporary systemic rapamycin delivery extended the survival of the FasL microgel islet grafts [115], suggesting efficacy of combinatorial strategies to maximize graft tolerance. Combining both peptide-MHC multimer and anti-Fas mAb onto microparticles also achieved allogeneic histocompatibility [116]. Likewise, adding anti-Fas mAb to encapsulated islet PEG hydrogel surfaces induced T-cell apoptosis [117]. These studies represent promising potential for diabetic immunomodulation utilizing apoptotic interventions.
Conversely, preventing apoptosis of the islet cells themselves may improve transplant success. Notably, islet allografts were shielded from rejection and 70% of NOD mice did not develop autoimmune diabetes when an adeno-associated virus expressing X-linked inhibitor of apoptosis (XIAP) was administered [118]. This approach differs from those discussed earlier in that the goal was to reinforce islets with resistance to stressors rather than manipulate the immune response itself.
The developing understanding of the progression of events in the immune response against implanted biomaterials and tissues allows researchers to target specific pathways and events to manipulate courses of action. The versatility and precision bestowed by nanotechnology provide a means by which to exploit this knowledge.
2.2. Nanotechnology in Improving Islet Survival and Graft Function
In addition to avoiding a deleterious host response, encapsulated islets must receive an adequate nutrient supply and positive extracellular cues in order to survive and function in vivo. Further, insulin must be delivered to the bloodstream in a timely manner for the graft to provide a therapeutic function. Immunoisolated islets, rendered avascular during isolation, must accomplish adequate mass transport by diffusion across the immune-excluding membrane [19]. Within common transplantation sites, dissolved oxygen levels are several orders of magnitude lower than other nutrients (e.g. glucose) [20, 21]. Notably, such constraints are likely even more limiting following the formation of the fibrotic capsule [119]. Consequently, improving oxygen delivery to encapsulated tissues has been extensively studied; relaxing the requirement for immunosuppression, islets may also be vascularized to improve bidirectional mass transport. Nanotechnological innovations for each of these paradigms are discussed below.
2.2.1. Oxygen Delivery
Inadequate islet oxygenation was suspected as a major challenge when necrotic cores were observed in islets cultured at atmospheric oxygen levels (21%) in nutrient-rich media [120, 121]. Evidence mounted in support of this notion when this phenomenon was observed in explanted microencapsulated islets even in the absence of a fibrotic response [122]. Subsequent mathematical modeling and observations revealed that the high respiratory rate, low physiological oxygen levels in common transplantation sites, and poor diffusional capacity of oxygen limited oxygen transport to the cells at the cluster center leading to islet core necrosis [20, 22, 119, 123–128]. Even at oxygen levels above a critical threshold needed for survival, modestly reducing islet oxygenation significantly impairs the insulin secretory capacity of the cell cluster [23, 24, 129]. A variety of strategies have henceforth been developed to improve islet oxygenation during culture and transplantation (Fig. 2). Several of those which utilize nanoscale engineering are discussed here.
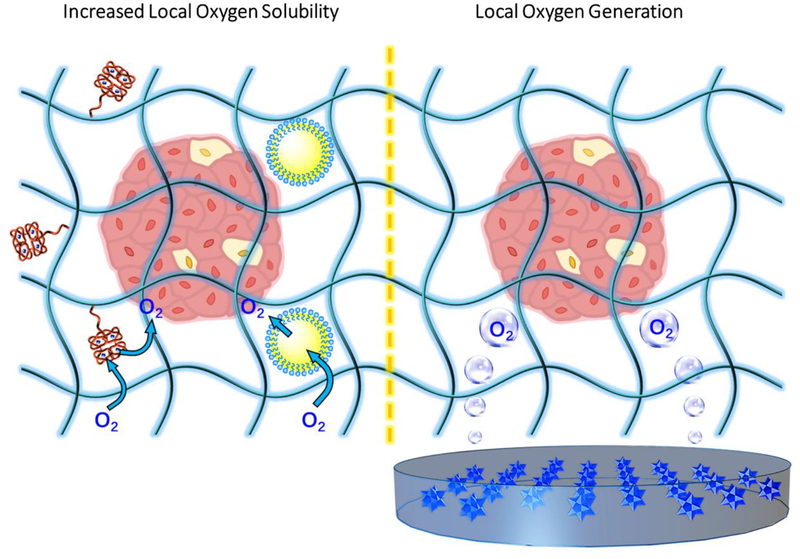
Fig. 2: Nanotechnology for improved oxygenation.
Increases in dissolved oxygen tension can support cell survival as well as glucose responsive function. Local solubility facilitated by hemoglobin-conjugated (red) hydrogels or perfluorocarbon (PFC, yellow) emulsions (left); and positioning of islets near an oxygen-generating source that may be comprised of metal peroxide particulates (right). Depicted oxygen represents idealized dissolved oxygen flow.
Many approaches improve oxygen delivery by increasing oxygen solubility near the islet. Perfluorocarbons (PFCs) are fluorinated oils which dissolve high levels of oxygen and have a low oxygen affinity and thus are efficient oxygen delivery vehicles [130]. Islet culture within PFC-supplemented media was shown to improve insulin secretion [131], providing a strategy to reduce cell loss prior to transplantation. Preoxygenation of a PFC emulsion-enriched media, stabilized in particulates at roughly 80 nm in diameter using egg yolk-derived lipid surfactants, protected islets from hypoxia as evidenced by reduced expression of apoptotic markers and morphological analysis [132]. Further, incorporating a PFC emulsion in barium alginate microspheres improved islet health after several days in hypoxic culture, although some cytotoxicity to the surfactant was observed [133]. Medical grade silicones such as PDMS also have a high oxygen carrying capacity [134] and desirable properties (e.g. biocompatibility and flexibility) for certain biomedical applications [135]. In the context of islet encapsulation, their use in larger devices as a scaffolding material may provide dual functionality as mechanical reinforcement and a material for improved oxygen solubility. For example, a PDMS-containing polyurethane nanomat was integrated into an islet macroencapsulation device to this end [136, 137].
Similar to cytokine-quenching hydrogels, nanoscale matrix modification with biological agents may improve islet oxygenation as well. An islet encapsulating hemoglobin-conjugated hydrogel significantly improved graft outcomes in diabetic mice following intraperitoneal transplantation [138, 139]. The authors suggested that in addition to its oxygen carrying capacity, the ability of hemoglobin to scavenge damaging reactive oxygen species contributed to graft success. Hemoglobin has also been attached to polymer nanoparticles to facilitate oxygen transport [140, 141], though, to our knowledge, this technology has not yet been applied to β-cell replacement therapies.
Oxygen generating materials comprised of inorganic peroxide particulates can provide an additional source of oxygen to cells during culture and transplantation. Such compounds generally generate oxygen by reacting with water to produce hydrogen peroxide, which then decomposes into water and oxygen [142]. Manganese oxide nanoparticles were demonstrated to both produce oxygen and catalyze hydrogen peroxide decomposition during in vitro culture with murine insulinoma cells [143]. Furthermore, the co-encapsulation of islets with calcium peroxide particulates in alginate capsules improved islet health during in vitro culture [144]. The Stabler group integrated calcium peroxide particulates within a PDMS scaffold [145, 146]. PDMS significantly impeded water transport to the particulates due to its hydrophobicity, which prolonged the duration of oxygen generation for over one month and improved the survival of cells encapsulated in a hydrogel surrounding the scaffold under hypoxic conditions [145]. Further, the culture of islets on the oxygen generating scaffold improved graft outcomes following transplantation [146]. The calcium peroxide particles used in the above studies were larger than nanoscale. Their reference in this section was nonetheless pertinent given that smaller particles (which have been previously fabricated [147]) may generate more oxygen per weight [148]. To the authors’ knowledge, the application of such technology to generate oxygen in an encapsulation structure in vivo has not been reported.
Many strategies for achieving adequate oxygenation or resistance to oxygen-related stressors, such as electrolysis [149], genetic engineering [150–152], preconditioning [153, 154], in situ generation by photosynthetic algae [155, 156], and exogenous oxygen injections [157] have been explored but are beyond the scope of this review. Readers are directed to several excellent reviews on this subject [20, 158]. Ongoing nanotechnological considerations related to oxygen transport will continue to improve oxygen delivery in islet replacement therapies.
2.2.2. Matrix Mimicry and Vascularization
Survival and engraftment of islets following transplantation is low [159], stressing the supply of available transplantable tissue, and necessitating larger transplant volumes. In addition to increasing local oxygen levels, islet survival has been improved by a number of techniques including matrix mimicry, growth factor supplementation, and vascularization (Fig. 3). A given technique may have a primary target but often also has parallel effects. For instance, recruiting vasculature can improve the supply of nutrients and waste product removal, but productive crosstalk between endothelial cells and islets also occurs. Similarly, ECM produced by endothelial cells further support islet health. The ECM is a natural material with nanoscale properties which provides cells with receptor binding ligands (e.g. integrins) and mechanical signals (e.g. stiffness, tensile strength, density, etc.). Thus, mimicking the ECM requires consideration of nanoscale materials features.
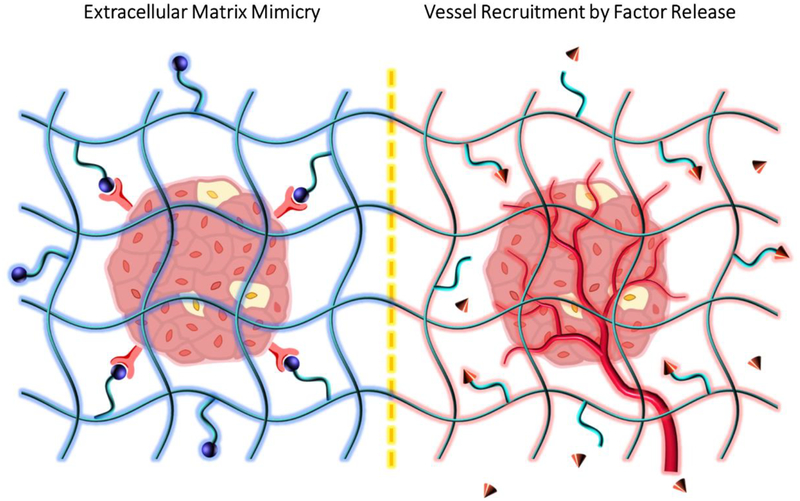
Fig. 3: Islet health improved by nanoscale materials engineering.
Providing environmental support that mimics the native pancreatic environment can increase islet survival and function. Peptide-functionalized hydrogels (e.g. RGD, blue spheres) for ECM mimicry (left, cell surface receptors shown in red); and factor (e.g. VEGF, red cones) release by degradation of labile bonds stimulates vessel recruitment (right).
Evidence suggests that the ECM is partially responsible for the maintenance of cell arrangement in pancreatic islets. When removed during the isolation procedure, cell arrangements (i.e. the relative locations of β- and α-cells) change but are then capable of moving back toward the native arrangement following transplantation or embedding in Matrigel [160], presumably due to sensing matrix ligands. Matrix proteins that may support islet function include collagen I, collagen III [161], collagen IV, fibronectin, and laminin. Matrigel or other cell derived matrices, which may include many of these proteins, can improve islet health, though translatability due to complexity and batch-to-batch variation is a concern. Therefore, reduction and simplification are important to prepare for regulatory approval.
Decellularized ECM has made it to the clinic for certain applications, such as dermal reconstruction, despite its inherent complexity. Several methods have been explored that utilize the pancreas as tissue source including repopulating decellularized pancreas [162–165], culturing islets on decellularized pancreas [166], and differentiating stem cells into β-cells using β-cell line produced matrix [167]. Techniques have also included other tissue sources and methods such as processing decellularized ECM into hydrogels [168, 169] and culturing islets on decellularized liver [170]. Pancreatic or liver matrices have been used inside an encapsulation device [171], and construction of a bilayer transplantation device was accomplished from porcine pericardium and tendon [172]. Further, Peloso et al. showed that human pancreas retained tolerogenic properties and bound factors following decellularization [173]. Chaimov et al. utilized decellularized pancreas ECM as a matrix to form capsules, where an alginate capsule was temporarily used and is then removed following ECM crosslinking [174]. In trying to simplify the decellularized matrix approach, a controlled number of matrix components can be used.
Collagens are an important part of the ECM in nearly every tissue. Collagen has been used in modules that increase vascularization for a therapeutic cell cargo [175]. Yap et al. selected collagen IV modified scaffolds for in vivo studies after in vitro comparison against fibronectin and laminin, showing that collagen IV modification improved the function of implanted islets and increased their vascularity [176]. In addition, Salvay et al. found that collagen IV outperformed fibronectin and laminin-322 in vivo as a coating for microporous scaffolds supporting islet transplantation in alternative sites [177]. Research has shown that islets receive signals from collagen through α1, α3 and β1 integrins [178, 179]. Another way to mimic the natural islet matrix is to blend an ECM protein with a scaffold material. Marchioli et al., for example, blended gelatin with alginate to make a 3-dimensional (3D) printable matrix that maintained the viability of INS1E β-cells [180]. In a study that obtained super-resolution microscopy images of primary β-cells, Phelps et al. demonstrated that collagen IV or laminin facilitated the attachment of dissociated primary islet cells (human or rat respectively), provided a neuronal culture media was used [181]. Stephens et al. transplanted islets into the subcutaneous space in an oligomeric collagen gel, which retained more naturally occurring crosslinks than monomeric collagen; better cure rates and even some allogeneic islet transplantation engraftment was observed [182]. Bernard et al. also investigated coating 1 μm melamine beads with various ECM proteins [183]. Single matrix components do not completely recapitulate the in vivo microenvironment but do simplify the process and can have positive functional outcomes.
Another method to simplify the presentation of ECM ligands is to functionalize scaffolds with specific peptide binding sites. Peptide amphiphile matrices can incorporate functional proteins. Lim et al. incorporated ECM binding peptides IKLLI, IKVAV, YIGSR, and RGDS, all of which can be found in laminin. Results showed that YIGSR and RGDS increased glucose responsiveness and related gene expression profiles better than IKLLI and IKVAV [184]. Lin and Anseth combined peptide presentation with fusion proteins. A PEG hydrogel framework was used to show that with presentation of EphA5-Fc receptor, ephrinA5-Fc ligand, and RGD, the individual cell density required to produce functional cells could be reduced by a factor of 10 for a MIN-6 cell line and primary dissociated islets [185]. Li et al. utilized EphA5-Fc and ephrinA5-Fc, but in this case the fusion proteins were attached to the surface of cell-sized beads constructed of PEG and PLL with a coating of decellularized pepsin digested pancreas ECM. Single cells were free to aggregate and showed responsiveness in vitro using this system [186].
Rather than functionalize hydrogels with ECM peptides, Chen et al. covered nanofiber scaffolds with β-cell membranes. Glucose-stimulated insulin secretion (GSIS) increased steadily over a 7-day period on these fibers, while it declined in uncoated fibers and coverslip controls [187]. It may also be useful to note that 3D culture environments for islets generally support islet health to a greater degree than 2-dimensional ones [188]. As a possible method to alleviate the concerns with variability and batch-to-batch variations in natural ECM proteins, it is feasible to use expression systems to synthesize analogs directly [189], perhaps reducing the concerns with material batch control. Overall, ECM derived from natural sources or synthetically constructed can be a powerful approach to improve islet viability.
Bioactive or soluble molecules bind to the ECM physiologically, offering another strategy to improve the survival of islets. Glucagon-like peptide-1 (GLP-1), an incretin hormone secreted by the digestive tract L-cells in response to food intake [190], is an insulinotropic agent [191], implicated in stimulating insulin secretion [192], promoting β-cell proliferation [193], and inhibiting β-cell apoptosis [194]. PEG hydrogels functionalized with GLP-1 protected β-cells from cytokine induced apoptosis [195]. Similarly, insulin-like growth factor (IGF)-2 has been found to promote the survival [196] and differentiation [197] of islets. Collagen microwell scaffolds were used as a natural reservoir for IGF-2 [198]. Some positive results on viability and insulin secretion have also been found with combinations of RGDS, GLP-1, IKVAV and MSCs combined in a PEG hydrogel [199]. Treatment of islets with growth hormone-releasing hormone agonist JI-36 can also improve transplantation results [200]. ECM mimicry and factor presentation are powerful techniques to address limitations surrounding islet transplantation.
Vascularization is one of the major limiting factors in attempts to replace or regenerate defective tissues. Because cells of most tissues are near a vascular supply (with the exception of some tissues such as cartilage [201, 202]), the thickness of an engineered tissue is severely limited without vascular intervention. The pancreatic islet is particularly sensitive to a lack of blood flow considering that the intra-islet capillaries are highly specialized and provide much of the ECM scaffolding that supports cells [203]. Furthermore, many of the complications arising from hyperglycemia are related to the microvasculature [204], making a diabetic transplant recipient a challenging environment in which to grow a mature vasculature. Several nanotechnological efforts to induce vascularization in islet grafts are discussed here. The reader is also referred to several excellent reviews on this subject [205–209].
A few broad categories of vascularization strategies utilize nanotechnology: surface roughness, porosity, factor release, and cellular tethering. Metal as well as polymer surface roughness tends to positively correlate with increases in vasculature [210, 211]. The development of the Theracyte device, discussed in Section 3, is an example of this approach. Bruker et al. established that pore size, independent of membrane material, could affect the number and proximity of vessels near the membrane [212]. Larger pores through the bulk of a device can act as effective scaffolding for vasculature [213]. These same materials can act as vehicles for therapeutic factors, in addition to providing a material shape that stimulates vascular growth. Nanocoatings (discussed in Section 4) are often functionalized for inducing blood vessel growth. For instance, immobilized vascular endothelial growth factor (VEGF) can be released from heparin, a cationic glycosaminoglycan, on the islet surface [214]. Factors can also be released in a variety of ways. VEGF, for example, has been released from a PEG hydrogel [215–217], a collagen gel [218], PLG scaffolds [219], chitosan or PLGA [220], and a planar membrane diffusion chamber [221]. Platelet-derived growth factor (PDGF)-BB has been delivered through a fibronectin fragment in a macroporous scaffold [222], while fibroblast growth factor (FGF)-2 has been delivered from gelatin microspheres [223], and from heparin-binding peptide amphiphiles [224]. FGF-1, on the other hand, has been delivered from a layer in an alginate capsule [144].
Finally, cells that form or support blood vessels can be included using nanotechnological approaches such as tethering [214]. When the goal is to vascularize the transplanted islets, it is presently not possible to impose full immune protection with an encapsulation membrane. Thus, when an immunoisolating membrane is part of the treatment system, the goal is instead to induce vasculature growth as close as possible to the device. Every approach outlined in this review is affected by the degree of vascularization, just as it is affected by other aspects of the transplantation process including the immune system, tissue quality, and material compatibility.
3. Nanotechnology in Macroscopic Islet Delivery Devices
Macroencapsulation refers to using implantable devices that are capable of housing large volumes of therapeutic cells. These devices often have a planar or cylindrical design and have several advantages. Due to their large capacity, sometimes only a single device is required to provide a curative dose using hundreds of thousands of islet equivalents. An ability to locate the entire graft following a period in the recipient is a benefit of a macro-device as compared to microencapsulated islets, where random dispersal from the original graft site can be observed. Larger devices must be transplanted into sites that can accommodate the implant volume and device shape, such as the subcutaneous or intraperitoneal locations. While that is a limitation in the versatility of macro-scale cell delivery devices, lower surgical complications, as compared to intraportal infusion, in those larger sites may balance that concern [12, 14, 225]. In addition, islets may respond to glucose better when in proximity with each other due to improved inter-islet synchronization [226], though competition for oxygen and nutrients ultimately imposes an upper constraint on the maximum supportable islet density [124, 227–230].
Macroencapsulation research was initiated by Dr. Bisceglie in 1933, who inserted insulinoma tissue into selectively-permeable membranes to determine the role of vascularization absence on transplanted tissue [231]. Algire et al. are credited with developing the first extravascular macrocapsule, initially intending to understand mechanisms of tissue rejection and cellular overgrowth. In this research, a nitrocellulose-based planar capsule was used to investigate the immune response to non-pancreatic cells, demonstrating improved cell viability of encapsulated cells and highlighting the role of humoral versus cell mediated immunity [232–235]. Encapsulation membranes can prevent cell contact with the protected cargo by operating in the micrometer range, while preventing transport of molecular mediators of immunity requires nanoscale material features. Some mediators of immunity are near the same length scale as insulin, requiring another method such as capturing or selective adsorption to a material to prevent their infiltration (discussed in Section 2.1.3).
As discussed above, the selectively permeable membranes that define the device boundaries are often comprised of nanoporous materials that exclude based on size. Nanotechnological interventions may be used to bestow devices with additional properties as well. Selected examples of macroscopic devices, which exhibit nanoscale features connected to a functional outcome, are discussed in this section (Fig. 4). Here the devices selected for discussion are divided into two broad categories: those that are designed to prevent immune cell infiltration (Fig. 4B) and those that support the engraftment of islets without strict immunoisolation (Fig. 4A).
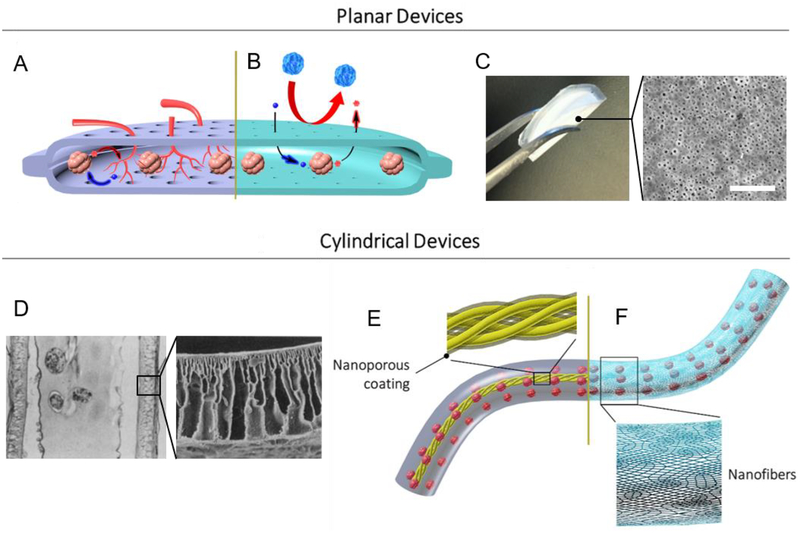
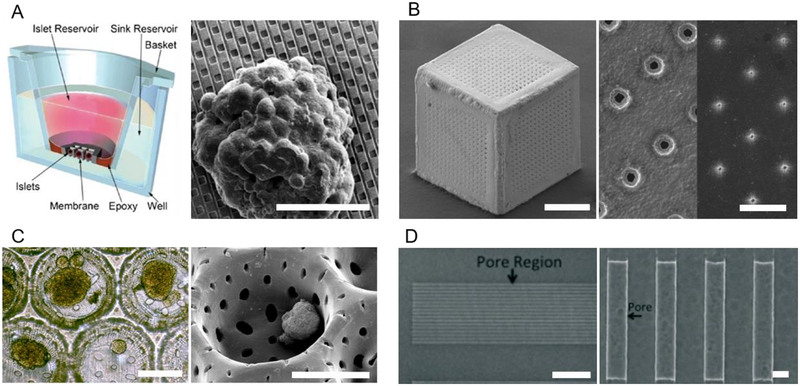
Fig. 4: Nanotechnology in Macroscopic Encapsulation and Cell Delivery Devices.
(A) Cell permissive (“open” or non-immunoisolating) macroencapsulation devices (such as PEC Direct) allow blood vessel penetration through pores from the host, supporting islet survival and regulation of glucose. Insulin (red) and glucose (blue) shown. (B) Immunoisolating macroencapsulation device with pores on the nanoscale. Host immune cells are prevented from accessing the graft, while glucose and insulin must diffuse through the permselective membrane. (C) Digital image of a folded immune-protective planar device described by Chang et al. [249] (left); top-view scanning electron microscopy image of nanoporous immunoisolating PCL membrane (right; scale bar: 200 nm; adapted with permission from [249]). (D) Microscope image of stained cross section of cylindrical hollow fiber device described by Lacy et al. [257] (left; magnification: ~41×); scanning electron microscopy image of acrylic copolymer membrane (right; magnification: ~400×; adapted with permission from [257]). (E) Thread reinforced alginate tubes use a nanoporous coating (shown in transparent gray over yellow thread) to crosslink alginate from the inside, while (F) a cylindrical nanofiber mat provides mechanical support and can function as a cell penetration resistant membrane.
3.1. Cell Penetration Restrictive Devices
Immune cell migration through an immunoisolating membrane can threaten graft function. Additionally, escape of cells with pluripotent characteristics which avoided full differentiation into β-cells and have the potential to generate tumors [236, 237], poses a significant safety concern [10]. Thus, membranes that prevent cell penetration can dually facilitate graft performance and provide safety to the recipient in stem cell-derived insulin producing cell transplant schemes. Baxter Healthcare used a preliminary polytetrafluoroethylene (PTFE) device (data from which would later inform the TheraCyte device) in 1998 to implant allografts in the epididymal fat pad of mice for up to 12 weeks with initially promising results [238]. The TheraCyte device uses laminated PTFE membranes to address the balance of diffusive and vascularization requirements with exclusion. An inner immunoisolating PTFE membrane has a pore size of 450 nm, while the outer PTFE membrane has a pore size of 5 μm, designed to facilitate angiogenesis [212]. A polyester mesh on the outside of the device provides mechanical stability, collectively providing an example of engineering at the nanoscale up to the macroscale.
Multiple studies have demonstrated that prevascularization of the TheraCyte device is beneficial for graft survival. Prevascularization can significantly decrease the curative dose of islets needed to reverse diabetes [239, 240]. Studies have shown 2–3-fold growth of new blood vessels when VEGF infusion was provided [221] and stabilization of microcirculatory flow by approximately two weeks post implant [241]. In an allograft study, immune-competent rats did not destroy the Theracyte encapsulated islets despite being pre-immunized with allogeneic islets to stimulate a vigorous memory reaction [242]. Other studies have even demonstrated that human embryonic stem cells (hESCs) can be derived into pancreatic progenitors and subsequently mature into β-cells within the implanted TheraCyte device to reverse diabetes in mice [243]. Some positive results from bone-marrow derived insulin producing cells transplanted into dogs have also been recently reported [244].
The TheraCyte device has been one of the most widely investigated encapsulation platforms, perhaps only second to microcapsules. Companies such as ViaCyte are continuing to develop the concept [12]. ViaCyte uses a semipermeable membrane device, branded as “Encaptra,” to encapsulate pancreatic progenitor cells for in vivo differentiation (with or without immunoisolation in PEC-Encap and PEC-Direct respectively) [123, 245]. It is very effective in allowing pancreatic progenitor cells to develop into mature β-cells, co-expressing classic β-cell differentiation genes (e.g. PDX1, Nkx6.1) [246]. Results from a clinical trial that is currently recruiting will add to our knowledge regarding integrated encapsulation and stem-cell differentiation technologies [247] (clinical trial number NCT03163511).
The TheraCyte/ViaCyte device was one of the first devices to show the importance of using pore sizes at the micro and nano scale. Twenty nanometer pores were shown to be 80–100-fold less effective in facilitating the growth of useful vessels near the membrane compared to 5 μm pores, while nanoscale pores are required for immune effector exclusion [212, 248]. Despite pore sizes that are certainly large enough for oxygen to pass, the distance of diffusion through a liquid filled pore is a limiting factor.
More recently, a nanoporous encapsulation device was developed by Chang et al. involving the casting of polycaprolactone (PCL) around nano-templated sacrificial zinc-oxide rods (Fig. 4C) [249]. Uniform controlled diameter template rods (as low as 15 nm) were very easily dissolved with weak acid [250]. Nanoporous PCL thin film membranes, with pore sizes of 20 nm and 200 nm showed nonsignificant differences in glucose diffusion when directly compared to a 400 nm PTFE immunoisolating membrane. Experiments showed little in vivo fibrosis and function during in vitro GSIS [251]. The membrane protected the graft from antigen-specific T-cell priming while also demonstrating viability of stem cell derived β-cells, which successfully responded to glucose challenge even after 6 months [249]. The fact that PCL slowly biodegrades is a useful property for delivering agents but may not be able to provide long-term immune protection.
To address the lack of oxygen transport through immunoisolating membranes, the Beta-O2 device supplements oxygen supply with gaseous oxygen. A user-refillable gas module is attached to the islet containing module, separated by a Silon IPN (an interpenetrating network of PDMS and polyetherfluoroethylene) oxygen permeable membrane, with an outer 200 nm PTFE immunoisolation membrane. An early study demonstrated reversal of diabetes in streptozotocin (STZ)-induced diabetic rats until explant at 90 days [252]. The authors attributed excellent insulin kinetics in intravenous glucose tolerance test (ivGTT) and islet functionality, following explant, to hypervascularization and the increased oxygen supply. If extraneous oxygen was dosed through the subcutaneous port on schedule, reduced HbA1c and near normal ivGTT curves were possible [123, 157], in both allogeneic and xenogeneic grafts [123]. During clinical trials, nonsignificant reduction of HbA1c levels and transient circulating C-peptide levels were observed [253]. The difficulty of translation [12, 254], including patient satisfaction factors [253], is an important design consideration for future development. The use of supplemental technology (for oxygen or other nutrients) in conjunction with an immunoisolating macroscopic device could be a defining factor of a clinically successful device.
Alginate and other hydrogels have been a material of choice for encapsulation [245, 248, 255]. Low energy to deformation, and rupture from physical wear suggest that a supporting material with enhanced mechanical properties may be required to enable practical application. The Islet Sheet is reinforced with a polymer mesh to reduce alginate fracture from friction against tissues. The device was made by sequestering and crosslinking alginate solution with islets between two already highly crosslinked layers of alginate, which is used as a semipermeable membrane to guard the islets [256]. A key advantage of the Islet Sheet is the tunability of the outer alginate layer, which can, for example, completely eliminate IgG transport via size exclusion [256]. Euglycemia was achieved in pancreatectomized dogs for 84 days, with improving ivGTT results over time [247, 256]. However, the Islet Sheet is deformable due to the mechanical weakness of the hydrogel used for encapsulation, which caused the device in many cases to deform or fold upon itself.
In addition to planar geometries, cylindrical macrocapsule designs were also explored for islet encapsulation, which often incorporated nanotechnological properties for mechanical reinforcement and chemical permselectivity. In the early 1990’s, Lacy et al. described the fabrication of a cylindrical device comprising a heterogeneous acrylic copolymer shell with islets seeded within alginate in the core (Fig. 4D) [257]. Here, the outer acrylic copolymer shell was fabricated in a trabecular structure for mechanical strength and an inner membrane for immune exclusion [257]. More recently, the Thread-Reinforced-Alginate-Fiber-For-Islets-enCapsulation (TRAFFIC) was produced from nylon sutures coated with nanoporous, Ca2+-releasing poly(methyl methacrylate) (PMMA) (Fig. 4E) [258]. Islets suspended in alginate solution were then used to coat the modified suture [259]. The TRAFFIC device was shown to reduce rejection compared to unencapsulated islets (similar to alginate microcapsules) in 3-month rat-to-mouse experiments [258]. In addition, the device was scalable and completely retrievable as demonstrated in large animal studies. Despite reinforcement provided by the nylon fibers, there are still concerns of hydrogel breakage and cell escape. To overcome these concerns, the Nanofiber-Enabled Encapsulation Device (NEED) was developed (Fig. 4F). The NEED device takes advantage of nanoscale fluidic phenomena to strengthen the mechanical properties of alginate and other hydrogels for macroencapsulatory purposes while providing a robust physical barrier composed of nanofiber networks to prevent any potential cell escape [260–262]. Additionally, the diameter of the nanofibers, being roughly correlated to the pore size of the membrane, can be tuned [263–265] to prevent or permit cell migration through the membrane. Mass transfer in any alginate utilizing device can be complicated by a fibrotic capsule, a problem that may be mitigated by recently explored technologies (see Section 2.1.1).
Control of pore dimensions as a result of uniform and precise nanofabrication techniques characterizes the following devices (Fig. 5). Utilizing a microfabrication approach based on a sacrificial layer, the NanoGland is a silicon microfabricated membrane device that hosts islets inside individual compartments, while in relatively close proximity [266]. This could be beneficial because it allows islets to communicate with each other while preventing islet aggregation. Knowing that pore sizes ranging from 3.6 nm to 60 μm were feasibly produced, Sabek et al. included a characterization of diffusion in a wide spectrum of nano- and micro-channels (Fig. 5A) [267]. The authors determined that channels on the micron scale should be pursued in experiments due to diffusional limitations imposed by nano-sized pores [267]. It is important to note that the NanoGland’s nanopores were sandwiched between micropores in an L-shape configuration, which may have impeded passive diffusion by increasing path length and decreasing pore density [268].
Fig. 5: Pore Configurations in Cell-Seeded Substrates.
(A) Schematic (left) of NanoGland device and scanning electron microscopy image (right) of islet seeded at the bottom of a sample well, rectangular nanopores in silicon substrate, fabricated by a sacrificial layer technique, shown in dark gray (adapted with permission from [267]; scale bar: 40 μm). (B) Scanning electron microscopy images of gold cell encapsulation device (left; scale bar: 200 μm), and close-up of pore structure (right; scale bar: 10 μm); pore size varied by modulating gold deposition time atop a substrate (adapted with permission from [273]). (C) Light-microscopy (left) and scanning electron microscopy (right) images of islets within poly(ethylene oxide terephthalate)/poly(butylene terephthalate) copolymer porous wells. Pores were forged by laser drilling (adapted with permission from [283]; scale bars: 200 μm). (D) Scanning electron microscopy images of nanoporous region of silicon nanopore membrane (left; scale bar: 20 μm), and close-up of rectangular pore structure (right; scale bar: 300 nm), fabricated by micro-electromechanical systems technology (adapted with permission from [296]).
While polymeric membranes have advantages centered around consistency, potential for modification, and easy fabrication [269], inadequate mechanical strength, broad pore size distributions, and potential degradability could compromise the graft [14]. In contrast, some proposed macrocapsules have utilized metals, such as alumina, which tend to be relatively bioinert [270]. Inorganic membrane development is novel in nature and has not been as extensively tested for macroencapsulation of islets. Flamme et al. demonstrated a simple two-step anodization etching process that can be used to create a device with large pore densities of 1010 pores per cm2 which selectively excluded immunoglobulin. The pore size was easily controlled (standard error of 2.35 nm) by anodization time and voltage [271]. Encapsulated MIN-6 cells were shown to be responsive to different glucose concentrations, while IgG diffusion was significantly diminished. Titania porous membranes have been fabricated, and also benefit from tight nanometer pore size distributions, but unfortunately were not mechanically robust [272]. In a composite approach, Randall et al. demonstrated that gold-coated self-folding porous membranes, made by lithographic patterning, could exhibit precisely controlled pore sizes based on the gold deposition duration (Fig. 5B). Cubes with 78 nm pores allowed insulin transport while preventing IgG diffusion [273]. These materials may prove to be highly valuable in the near future, due to their chemical and mechanical advantages compared to polymeric macroscopic encapsulation devices, and the nanoscale technologies that can create well-controlled pore size distributions for specific immunoisolation requirements.
3.2. Cell Permissive Devices
Immunoisolating devices have a high safety profile; however, the external barrier adds a diffusional delay which can impair graft function. Blood vessel growth into the device could significantly improve the engraftment and function of the therapeutic cells. As discussed in Section 3.1, ViaCyte is testing a cell permeable version of their device (PEC-Direct), which features macro-sized pores. Pores that are large enough to allow blood vessel ingrowth are above the nanoscale; however, some nanoscale phenomena and materials processing techniques can still be used to regulate these types of devices. In order to better understand these innovations, we also discuss some other influential macroencapsulation devices.
Sernova’s Cell Pouch device includes a focus on pre-vascularizing the device without a membrane that is strictly immunoisolating. A cylindrical device was implanted subcutaneously into the host for 4–5 weeks to allow for angiogenesis through the porous structure of the device followed by plug removal at the time of cell transplant. Successful reversal of diabetes in mice was reported in both subcutaneous and omental sites [274, 275]. Unfortunately, clinical data using multiple parallel chambers to provide sufficient volume for islets did not show signs of graft function, as shown by early loss of C-peptide [276]. A similar study utilized a nylon access catheter as the pre-implantation material to create a vascularized space that has no device once the islets are introduced. BG correction was observed in BALB/c and C57BL/6 mice, supporting the idea that a foreign body reaction can be productive if it is appropriately formed and vascularized [277]. Sernova is recruiting for a clinical trial to test the cylindrical device with removable center design (clinical trial number NCT03513939), promising to advance the understanding of subcutaneous transplantation.
Pore sizes that are shown to be resistant to cell penetration sometimes exhibit reduced diffusion kinetics. Examination of nanopore experimental data suggest that the channel architecture may play an important role since many alginate capsules (with average pore sizes near 5 nm [278]) can be functional for islets [279–282]. In addition, a large pore size distribution could compromise the immune barrier integrity of the device by facilitating cell penetration in certain areas [213]. Thus, some research seeks to create highly controlled macro-pores to fabricate cell permissive devices. Research from van Apeldoorn’s group has introduced pore formation in thin films combined with thermoformed wells for islets (Fig. 5C). After testing particulate leaching, casting on a pillared template and laser drilling, laser drilling was found to give the greatest control over pore dimensions, even following microthermoforming to create the wells for islets [283]. Islets in this device allowed blood vessel ingrowth and were able to restore murine glucose control.
Recently, researchers have investigated 3D printing as a method to create a mechanically robust structure for cell delivery [180]. 3D-printed poly(lactic acid) (PLA) scaffolds demonstrated in vitro functionality after four weeks, and supported differentiation of new insulin producing cells [284]. An advantage of using PLA is the potential polymer functionalization via covalent modification [284–287]. In continuation of this idea, 300 μm2 sized microwells were printed for individual islets to avoid clustering and hypoxia [288]. This device utilizes prevascularization before islet introduction. The nanoscale surface roughness of the material was modified by plasma etching and the device supported supplementation with a variety of factors including pro-vascularization factors [245, 288]. 3D printing can provide individual customization in size and shape as well as the ability to control features in the inner regions of the device that can be difficult to replicate with other fabrication techniques, thus more development is warranted.
3.3. Convection Enabled Devices
Macroscopic encapsulation and cell delivery devices for transplant are challenging to construct. Innovative ideas are still emerging to reach design goals. As discussed up to this point, macrodevices are prone to inadequate oxygenation and nutrient exchange as a result of larger reservoir volumes [255]. While synthetic polymers have low material batch-to-batch and formulation-to-formulation variations [289–291], and fabrication utilizing nanotechnology can improve on the limitations, there are still opportunities for improvement. When manipulating channels on the nanoscale, it is easier to create pores that significantly restrict diffusion of very small molecules (even if there is detectable passage); however, this can completely prevent overall device function.
Placing a device in direct contact with blood opens the possibility of high diffusion rates or convective transport. An exemplary configuration of an intravascular device is a hollow tube, embedded within a blood vessel where diffusion is the primary mode of transport across the membrane [12, 292]. On the other hand, ultrafiltration approaches utilize a connection to an artery for nutrient sensing and a shunt to a vein sets up convective flow through the device. Intravascular devices are accompanied by a high risk of thrombosis, and therefore require anticoagulation therapy (e.g. heparin). Multiple groups have shown that intravascular hollow fiber diffusion devices can reverse diabetes in mouse models and even support xenografts into dogs without immunosuppression [248, 293, 294]. However, risks, exemplified by a case of sudden death in canines due to acute complications, have tempered enthusiasm [12]. Nevertheless, Prochorov et al. reported encapsulated rabbit islets transplanted into human patients in nylon macrocapsules into either the femoral artery or the forearm cubital vein [295]. Diabetes was ameliorated in 14 of 19 diabetic recipients without immunosuppression, perhaps motivating future investigation.
The concept of ultrafiltration utilizing nanoscale technology has recently been recently reconsidered. Song et al. characterized a macrocapsule grafted similarly to an arterial-venous graft, utilizing the pressure difference between artery and vein to drive diffusion of glucose and nutrients in and insulin out [268, 296]. Using micro-electromechanical systems technology, slit nanopores 7 nm in width and 300 nm in depth were constructed with a linear path rather than a tortuous one (Fig. 5D). The device excluded TNF-α, IFN-γ, and IL-1β, while permitting passage of glucose and insulin. The pressure difference may have been responsible for overcoming the diffusive limitations of the nanopores [268]. While nanoscale features can be given to macroscale devices, it is also possible to protect transplanted islets with only nanoscale layers.
4. Nanoencapsulation
Within most immunoisolating macro- or micro-encapsulation devices, islets are separated from the host by the encapsulant by tens or hundreds of microns. However, several prevailing limitations of these approaches can be overcome by engineering semipermeable polymer barriers directly onto the islet surface at length scales well below the standard distance.
Spherical microcapsules, for example, typically feature diameters ranging from 250 μm to 1500 μm [16, 254, 297]; however, theoretical analyses of physiological oxygen transport suggest that the thickness of a spherical microcapsule should be controlled below 100 μm to avoid central islet hypoxia and necrosis in common transplantation sites [298]. Buchwald et al. also demonstrated that 1800 μm-diameter microspheres delay and reduce insulin secretion as a result of the large distance which must be traversed by passive diffusion [129]. An earlier study by Chicheportiche and Reach showed that capsule diameter as small as 350 μm may still impair insulin secretion [299]. Logically, nanoscale immune barriers can mitigate the effects of these diffusional limitations.
Graft void space (i.e. the transplant volume occupied by nonbiological material) also imposes several problems [19, 300]. Microencapsulated islets exceed the size that can be administered into the liver via the portal vein [301], whereas nanoencapsulated islets are compatible with transplantation into this clinically proven site [302]. In addition, many common droplet-generating techniques for islet microencapsulation often cannot ensure total islet coverage for each cell cluster, especially when capsule diameter is reduced. Islets protruding from the capsule surface are not immunoisolated and can thus initiate an immune cascade which targets the entire graft [303, 304]. Simply tuning the parameters of spherical microencapsulation techniques to produce smaller capsules is therefore not a viable strategy to minimize capsule size. While core-shell structures have been developed which may prevent islet protrusion [305–309], these structures do not address the diffusional problems discussed earlier, nor some challenges related to blood contact.
During intraportal islet transplantation, exposure to blood elicits an immediate blood-mediated inflammatory reaction (IBMIR) [310]. The IBMIR is characterized by thrombosis and complement pathway activation, which results in the acute loss of a substantial fraction of the graft [310–313], and suspected reduced function of the surviving cells [314]. Although nanoencapsulated islets are mainly considered for the long-term prevention of immune destruction, coatings can be functionalized to confer protection from the IBMIR [315], which we discuss further in this section.
Challenges associated with conventional microcapsules motivated the broad exploration of engineering nano-thin polymer films which conform to the islet surface (Fig. 6). While microcapsule fabrication is achieved by droplet-generating techniques such as electrospray [316–318], emulsion-based strategies [319, 320], submerged jet extrusion [321], or microfluidic systems [322, 323], nanoencapsulation methods typically feature direct polymer deposition on the islet surface. Many techniques have been developed from early approaches in employing PEG as a polymer coating; in addition, the layer-by-layer (LBL) method has been used to produce versatile islet polymer films. The techniques, merits, and function (Fig. 6A) of these approaches are discussed in this section.
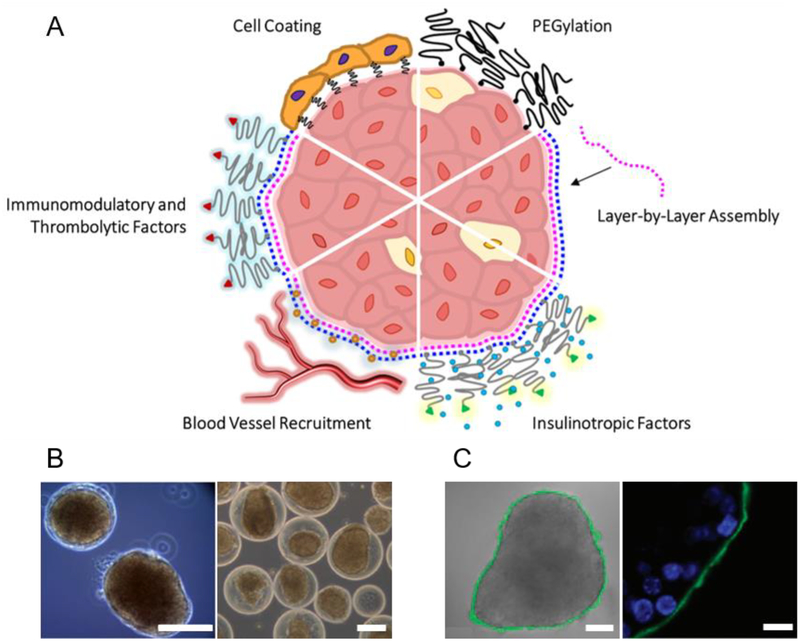
Fig. 6: Nanoencapsulation.
(A) Nano-thin coatings may be generated by (top right, proceeding clockwise): PEGylation; layer-by-layer (LBL) assembly of alternating polymer layers (e.g. polycations, pink, and polyanions, blue) deposited directly on the islet surface. Nano-coatings can be functionalized with (continuing clockwise): bioactive accessories such as insulinotropic agents including glucagon-like peptide (GLP)-1 (green; insulin shown as blue circle); blood vessel recruiting factors (e.g. heparin, orange circles); immunomodulatory and thrombolytic agents (red; e.g. soluble complement receptor (sCR)-1, thrombomodulin, urokinase, phosphorylcholine, heparin); or a cellular layer (e.g. endothelial cells, immunomodulatory cells). (B) Examples of conformal coating: phase contrast images of mouse islets conformally coated with a PEG-alginate composite gel (left) and rat islets conformally coated with a PEG-Matrigel composite gel by the method described in Manzoli et al. [339] (right; scale bars: 100 μm; adapted with permission from [339]). (C) Example of LBL nanocoating: brightfield image overlaid with confocal micrograph of 8-bilayer (PLL-g-PEG/fluorescein-labeled alginate, green) coating, fabrication described in Wilson et al. [400] (left; scale bar: 50 μm); confocal micrograph showing coating localized on peripheral islet extracellular surface (right; scale bar: 10 μm; adapted with permission from [400]).
4.1. PEG-Based Coatings
PEG and its derivatives are among the most widely applied biomaterials in tissue engineering [324–326]. PEG has many desirable properties for cell encapsulation, such as versatile and relatively mild gelation conditions [327], ECM mimicry [328], the ability to conceal surface antigens [329], and the ability to be functionalized with a wide variety of biological agents [324, 330]. It is also a suitable host-interfacing material because of its high hydrophilicity, which endows the polymer with low-fouling and low-opsonization characteristics [331, 332]. Several processes have been developed through which islets may be decorated with a thin coating of the polymer (i.e. PEGylated), including interfacial photopolymerization, covalent interactions, and non-covalent interactions.
4.1.1. PEGylation by Interfacial Polymerization
Early approaches developed in the 1990s by Hubbell’s group exploited the photopolymerizable properties of PEG diacrylate to conformally coat islets with the polymer [333–336]. Briefly, eosin Y, a photoinitiator, was adsorbed to the islet surface during incubation; the islets were then exposed to a PEG diacrylate precursor solution, and in situ crosslinking was facilitated outward from the islet surface by exposure to visible light and the addition of an accelerating agent [333]. The thickness of this conformal coating was tunable between several tens of microns by modulating various design parameters, such as laser exposure time and concentrations of the system’s components (e.g. eosin Y) [334]. By varying the concentration and molecular weight of precursor PEG diacrylate, the investigators were able to control the matrix pore size (and thus selective permeability) with sub-nanometer sensitivity to exclude larger immune components such as IgG, IgM, and C1q, while permitting passage of glucose, dissolved gases, smaller metabolites, and insulin [335]. Porcine islets encapsulated via this method were transplanted into the IP space of STZ-induced, immune-competent diabetic Sprague-Dawley rats [336]. The results indicated that islet viability was maintained in vivo for one month and xenoprotection was conferred by the conformal coating [336].
A modified version of this technology was patented (US Patent #6911227) and licensed by Novocell, now ViaCyte, wherein translation to larger animal models was pursued. A preclinical study in the subcutaneous space of diabetic baboons showed sustained islet function for 6 months (longer function was observed in some pilot studies in non-human primates as well) [12, 337], prompting a clinical trial in human patients. Following three successive subcutaneous implants of PEG-coated islets totaling roughly 50% of the islet dose expected to restore euglycemia, insulin dependence was significantly reduced, and response to an oral glucose tolerance test was improved. Notably, however, complete glycemic restoration was not achieved, and C-peptide levels were disappointingly low [12]. The investigators suggested that the highly fibrotic nature of the human diabetic subcutaneous space might inherently limit islet transplantation in this site [12]. Nevertheless, these early studies presented the robust exploration of a novel paradigm for islet encapsulation, which initiated a large body of research in this space in the proceeding decades.
More recently, microfluidic approaches have been developed to generate thin PEG coatings. Tomei et al. presented a method whereby islets, suspended in an aqueous phase with PEG, were extruded within an immiscible oil phase; flow through a conical constraint disrupted the aqueous jet, creating a thin coating on the islet surface [338]. A crosslinking agent was introduced into the PEG layer via diffusion through the oil phase, and gelation was induced by a pH change [338]. Importantly, this method achieved full islet coverage [338]. An optimized coating developed via this method (Fig. 6B) was tested in vivo in an allogeneic mouse model, where it significantly prolonged graft survival in comparison to naked islets in the absence of immunosuppression [339].
The mechanism of the early approaches relied on the diffusion of free radicals to propagate the crosslinking reaction, which raised concerns of cytotoxicity inflicted on the encapsulated cells. Further, these methods did not aim to achieve chemical attachment to the islet surface, and the encapsulation thickness could not be reduced below several microns. In the following sections, chemical polymer-islet association is discussed.
4.1.2. Covalent PEGylation
It was hypothesized by several investigators that covalent islet PEGylation could realize more mechanically robust and thinner polymer coatings. Native islets are surrounded by a peripheral protein-rich ECM, the thickness and composition of which varies considerably between species [340]. While this peripheral ECM is largely lost during the isolation procedure, a new matrix is gradually reestablished during culture [341]. Proteins within the ECM, as well as surface proteins on peripheral islet cells, provide conjugation sites for chemical modification.
Covalent PEGylation is often accomplished by using PEG terminated with an activated ester such as N-hydroxysuccinimide (NHS), which forms an amide bond with free amines on peripheral proteins. An early study pursued by Panza et al. assessed the treatment of islets with PEG-NHS and PEG-isocyanate whereby they confirmed the presence of the coating on the islet surface via fluorescent staining, suggested low cytotoxicity of the coating based on a metabolic assay, and observed similar responses to insulin secretagogue challenges in comparison to uncoated islets [342]. Contreras et al. successfully coated porcine islet surfaces with succinimide-activated PEG using membrane surface proteins on peripheral cells as a conjugation site [343], as porcine islets largely lack an ECM capsule [344]. A collection of studies using monomethoxy PEG (mPEG)-NHS show that raising polymer concentration and molecular weight may increase the immunoprotective properties of the coatings, but also increase their cytotoxicity [345–347]. Thus, these parameters must be optimized to address these competing requirements.
More recently, a heparin-functionalized, NHS-activated, star-shaped PEG (starPEG) polymer was developed to create a nanofilm on islet surfaces without adverse effects to islet health or secretagogue responsiveness, providing a versatile platform to functionalize such coatings with biological compounds [348]. Rengifo et al. introduced a biorthogonal scheme to coat islets with covalently-linked, stable starPEG and starPEG/alginate nano-films, using an NHS-based strategy to facilitate conjugation to the islet surface [349]. Over 50% of mice receiving strain-mismatched kidney-capsule allografts of islets coated with this method exhibited long-term diabetes correction, providing preliminary evidence that this strategy may have translational potential [349]. This group also showed that co-administration of a short-term immunosuppressant regimen improved graft outcomes of similarly encapsulated islets [350]. Continued studies will reveal the efficacy of covalent PEGylation for encapsulated islet transplantation.
4.1.3. PEGylation by Hydrophobic Association
Despite general success in maintaining the viability and secretory capacity of islets, it has been speculated that covalent PEGylation may have a damaging effect on surface membrane structures [351, 352]. Covalent PEGylation also may not be applicable for long-term encapsulation of porcine islets as they have a diffuse capsular ECM [344], and many islet membrane proteins are not permanently available structures as they eventually turnover [353]. This has prompted the study of alternative strategies to adhere nano-thin PEG coatings to the islet surface.
Lipid-conjugated PEG (lipid-PEG) chains can spontaneously associate with the cell phospholipid bilayer membrane via hydrophobic interactions of lipid alkyl tails [354], whereby they diffuse laterally to create a coating [355]. Teramura, Iwata, and colleagues developed a versatile platform based on this approach and have demonstrated it to have a minimal effect on islet survival or secretagogue-responsive insulin secretion [352, 356–363]. This group leveraged this encapsulation method to permit coating with additional layers of polymers such as poly(vinyl alcohol) (PVA) [360], alginate [352], or other PEG derivatives [363]. Further, a variety of factors were added to this LBL platform to improve insulin secretion, lessen fibrosis, or mitigate the IBMIR [356, 359, 361, 364]. Short-term graft success was reported following an intraportal infusion of xenogeneic islets coated with lipid-PEG via this method [357]. Additional characterization is necessary to evaluate the clinical potential of such coatings.
4.1.4. Perspectives on PEGylation
The use of PEG has been shown in numerous studies to be an effective polymer for micro- or nano-thin islet coatings. However, a few concerns and limitations must be noted. Again, covalent PEGylation may damage peripheral membrane proteins and impact islet health [351, 352, 365]. On the other hand, PEG coatings achieved via hydrophobic interactions have been shown to gradually dissociate from the cell surface [362, 366], which limits their viability as a long-term immunoisolating membrane. In general, these coatings may suffer from weak mechanical properties similar to many hydrogels and require further characterization. Additionally, the thickness of single-layer PEG coatings has been suggested to be too thin to offer full coverage of cell surface antigens, with mechanical properties potentially too weak for long-term immunoisolation [360]. Islet-surface PEG matrices are also noted to lack well-defined pore arrangements and primarily depend on the effects of steric exclusion to act as an immune barrier [367]. Confirming some of these concerns, rigorous characterization has occasionally revealed gaps in the nanocoatings [349].
It is worth mentioning that some of the suggested benefits of PEG, discussed at the beginning of Section 4.1, are not always observed [368]. For example, in contradiction with the prevailing notion, several studies have shown that PEGylation can increase nonspecific protein adsorption [369–371], which may hasten the onset of the FBR. Beyond fibrotic concerns, chronic PEG presentation can induce the generation of anti-PEG antibodies, which may precipitate an immune cascade against the graft [372–376]. At the very least, it may be concluded that materials other than PEG are worth considering for host-interfacing applications.
4.2. Layer-by-Layer Assembly
Motivated by the potential limitations of PEGylation, and in the interest of broadening the polymers which may be applied to islet nanoencapsulation, the LBL assembly method emerged, wherein alternating nano-thin films are formed on the islet surface. A technique for producing nanoscale bilayers on a charged substrate (such as that of a cell surface) was first reported in the early 1990’s [377]. Many early alginate-based microencapsulation systems applied this technique and incorporated a thin polyelectrolyte (often PLL or poly-l-ornithine) layer, and often another alginate coating [67, 378–381]. Applying polymer matrices directly on the islet surface was thus a logical progression of this practice.
An advantage of the LBL approach is the ability to tune the encapsulation structure at the nanoscale through the coordination of materials and deposition methods and investigate the consequences thereof. For example, Zhi et al. found that during in vitro culture with anti-islet antibodies, the deposition of one 1.5 nm chitosan/alginate bilayer was insufficient to prevent antibody penetration, whereas two- and three-bilayer encapsulation with these polymers provided a much greater isolation effect [382]. In vivo allogeneic transplantation in the kidney capsule in a follow-up study by this group revealed that 8 bilayers conferred substantially improved immune protection compared to 4 bilayers, suggesting a direct relationship between coating thickness and immunoisolation [383]. Expectedly, the optimal number of layers varies according to material selection and fabrication method [109, 384]. The LBL approach provides a versatile platform to study these relationships.
LBL assembly methods predominantly make use of electrostatic complexation, although several studies show the efficacy of leveraging hydrophobic interactions, hydrogen bonding, or covalent bonding to fabricate multilayer coatings. These strategies are discussed below.
4.2.1. Layer-by-Layer Assembly by Electrostatic Interaction
The islet surface is negatively charged [385], making it an available site for polycation deposition. The incorporation of additional polymer layers can be accomplished by polyelectrolyte complexation of oppositely charged polymers upon this basal layer. This is possible because the surface charge is effectively reversed with the deposition of each polyelectrolyte layer [377]. For the most part, this complexation reaction is self-limited (i.e. the bilayers are naturally restricted to nanoscale sizes) and can be achieved by simple incubation with alternating polymers [386].
Numerous studies have investigated the production of stable, nano-thin cell and cell-cluster coatings via electrostatic LBL assembly showing success in a laboratory setting with a wide variety of materials. Studies by the Pickup group show that polysaccharides such as chitosan and alginate can be complexed in nano-bilayers on the islet surface [382, 383]. In another investigation, this group also produced natural/synthetic bilayers featuring PLL/alginate, PLL/PC-heparin, and PLL-condroitin-4-sulfate with success [109]. The authors cited a modest increase in packed tissue volume of coated islets in comparison to free islets as evidence that the nanocoatings improved the mechanical strength of the clusters as well [383]. Miura et al. combined the previously described lipid-PEG membrane integration approach with LBL assembly using PLL as the alternating polymer; the PEG chain was terminated with amino groups to provide a positive charge for polyion complexation [352]. Recently, Syed et al. tested and characterized a 9-bilayer chitosan/poly(styrene-sulfonic acid, sodium salt) (PSS) human islet coating in a xenogeneic mouse transplantation model [387]. This coating method conferred robust protection against proinflammatory agents in vitro, and significantly reduced BG levels were reported for one month following the xenotransplant in the kidney capsule site [387]. To the authors’ knowledge, studies with multilayer nanoencapsulated islets have not been pursued in larger animal models.
A serious consideration for electrostatic LBL assembly design is the cytotoxicity of the polymer. It has been widely observed that polycations impair cell viability [388–391], although this may be mitigated by certain pairings with other polymers. Illustrating this point, it is widely known that PLL/alginate encapsulation has poor biocompatibility due to PLL incorporation [17, 392], whereas PLL/heparin and PLL/PEG pairings have been implemented for cell nanoencapsulation with a minimal impact on cell viability [352, 384]. It has also been suggested that the net surface charge of the coating should closely resemble that of an uncoated cell; Bhaiji et al., for example, ensured that the outermost layer was negatively charged [109]. Nonetheless, these potential limitations inspired the investigation of alterative LBL approaches.
4.2.2. Layer-by-Layer Assembly by Alternative Approaches
Concerns of cytotoxicity associated with polycation deposition may be addressed by utilizing hydrophobic interactions, hydrogen bonding, or covalent bonding. Totani et al. introduced alkyl chains in a PVA-based polymer, whereby the alkyl chains spontaneously integrated within the cell phospholipid bilayer by hydrophobic interactions, creating a thin coating on the surface of human embryonic kidney cells (HEK293) [361]. Cholesterol also spontaneously organizes itself within the cell membrane by hydrophobic interactions [393]. Our group exploited a functionalized cholesterol to covalently attach dextran-based drug eluting nanoparticles on the surface of individual cells [394]. Kozlovaskaya et al. created an LBL conformal coating technique by incorporating tannic acid, a natural polyphenol with antioxidative and immunomodulatory properties [395], and poly(N-vinylpyrrolidone) (PVPON) via hydrogen bonding [396]. The authors reported total nanoscale coating of several different mammalian islets, preserved islet function under cytokine co-culture, and in vitro stability under simulated physiological conditions for at least one week [396]. Islets coated via this method restored euglycemia in an allogeneic mouse transplant model for over 40 days, and characterization revealed that inflammatory macrophage activity was significantly depressed in nanoencapsulated islets in comparison to naked islet controls [397].
PEG can be introduced in LBL schemes to improve the biocompatibility, cytotoxicity or opsonization potential of the coating. Teramura et al. functionalized a lipid-PEG polymer with maleimide moieties to facilitate covalent crosslinking to a thiolated PVA by thiol-disulfide exchange reactions, which they used to create a conformal bilayer on islet surfaces [360]. In 2017, Yang et al. deposited a cationic and anionic gelatin bilayer on the surface of HeLa cells and utilized thiol-maleimide click chemistry to facilitate covalent conjugation to an external layer of thiol-functionalized PEG [398]. Over a series of papers, the Chaikoff group utilized a PLL-g-PEG copolymer for generating nano-thin multilayer coatings (Fig. 6C) [302, 399, 400]. One of these studies leveraged biotin-streptavidin binding, among the strongest noncovalent interactions found in nature [401], to adhere the bilayers [302]. In another study, biotinylated mPEG was covalently linked to the surface of an islet via amine reactions [103]. A PLGA nanoparticle surface coating was added via biotin-avidin interactions as a biodegradable drug delivery platform [103]. In summary, researchers have utilized a wide variety of materials and binding chemistries to assemble nano-thin multilayer coatings by the LBL approach.
4.3. Nanocoating Functionalization
The mechanical rigidity, charge distribution, topography, and hydrophobicity of nanocoatings are parameters that influence islet function and host response following transplantation [402]. As previous text has foreshadowed, thin polymer coatings are also highly versatile templates for chemical and biological functionalization and drug delivery. The scale of such coatings ensures that certain agents may be localized within nanometers of the islet, which can be especially useful when proximity is critical to impart functions such as ECM mimicry or insulinotropic properties. For example, the peptide sequence RGD, discussed in more detail in Section 2.2.2, mediates cell-matrix interactions in the native ECM [403], promotes isolated islet survival via integrin interactions in vitro [404], and has been incorporated in LBL assembly schemes to confer cell adhesion [405]. GLP-1 incorporation within the basal layer of a PEG-based LBL islet coating scheme increased insulin secretion in comparison to naked islets [406]. Similar results were obtained for insulin-secreting cells encapsulated in a GLP-1-mimetic peptide amphiphile nanogel [407]. Localized presentation at the islet surface, which can be accomplished by nano-thin coatings, may improve its efficacy as GLP-1 interacts with the GLP-1 receptor on the islet cell surface to impart function [191].
Alternatively, components may be presented at the outer surface of the coating to manipulate the host response. Islets injected into the liver, for example, interface directly with the bloodstream, potentially precipitating thrombotic reactions and the IBMIR. Functionalized nanostructures have been developed to mitigate this response. In the studies discussed in Section 4.2.1, the Pickup group incorporated a PC-modified chondroitin-4-sulfate surface layer to provide an anticoagulatory effect [382, 383]. The zwitterionic PC moiety is also theorized to reduce protein adsorption and cellular recognition [408]. Several investigators have achieved success with the surface presentation of thrombomodulin, an upstream enzyme implicated in anticoagulation pathways [409, 410], grafted to nanocoatings to abrogate this reaction at the islet-host interface [351, 356, 411]. Urokinase is another enzyme which activates fibrinolytic pathways [412]. Engraftment to the surface of lipid-PEG islet coatings degraded a fibrin gel in an in vitro assay and improved outcomes in a STZ-induced diabetic syngeneic mouse model following intraportal transplantation [358]. In addition to its role as an ECM glycosaminoglycan, heparin is well known for its role in anticoagulation [413]. Conjugation of heparin to nano-thin islet coatings enhanced vascularization [348] and mitigated thrombotic reactions [109, 348, 357, 364]. Soluble complement receptor 1 (sCR1) mitigates complement pathway activation during the IBMIR [414]. Integration of sCR1 within islet nanocoatings has been realized [415] and has been suggested to address the dual mechanisms of the IMBIR when co-presented with heparin [364]. Importantly, in the absence of sustained release mechanisms, the bioactivity of conjugated factors may be limited to a few days [358]. Further research is required to understand if this short therapeutic window is sufficient to overcome obstacles in long-term islet transplantation.
Cell surface engineering approaches have been applied to graft accessory cells to the islet surface. Cellular co-delivery may have advantages over ligand and factor incorporation as they perform more diverse functions at the graft site and have extended and potent bioactivity. Chondrocyte monolayer coatings were originally explored as cellular islet capsules due to their immune-modulating functions [416, 417]; however, these coatings were achieved solely by strategic coculture and thus the chondrocytes were not chemically adhered to the islet surface, nor was the system viable over a physiologically meaningful time. In 2009, HEK293 cells were conjugated to the islet surface via biotin-streptavidin interactions [418]. In a follow-up study, complementary single-strand DNA-functionalized lipid-PEG conjugates were incorporated into surface islet cell membranes and HEK293 cell membranes; HEK293 cells were immobilized to the islet surface by the binding of complementary DNA strands [419]. Importantly, the HEK293 cells proliferated while maintaining their attachment to the islet surface, creating a more complete coating [419]. Sertoli cells, which play a role in establishing immune tolerance in the testis [420], were immobilized to islet surfaces via a similar nucleotide-based strategy by this same group [421]. Sertoli cell surface localization was maintained when following transplantation in mice via intraportal infusion, suggesting robust conjugation of the cell line to the islet surface [421].
Following this early work, carefully chosen cell lines were selected to impart immune tolerance and mediate the IBMIR. Tregs have been delivered in islet transplantations as a means of establishing immune tolerance to the graft [44, 422]. Islet coating with islet-antigen specific Tregs has been realized to impart local tolerance [423, 424], though, to the authors’ knowledge, in vivo studies with this technology have not yet been pursued. Various endothelial cell types have been used to coat islets as well via simple coculture [425, 426], because of their noted resistance to reactive oxygen species [427], anticoagulatory properties [428], and role in vascular recruitment [429]. One study showed that endothelial colony-forming cell-coated islets greatly minimized the effect of the IBMIR and suggested this cell source to be more appropriate than mature endothelial cells [425]. Similarly, islet coating with neural crest stem cells (again via coculture) was shown to improve graft innervation and vascularization in a diabetic mouse model [430].
The practice of functionalizing the islet surface with accessory cells has the potential to be even more powerful in future islet transplantation. It has been suggested that one could decorate islets with the patients’ own cells, comprising an immune interface of “self” biological matter [431]. The potential for these methods to significantly improve islet transplantation graft outcomes, as evidenced by these select studies, warrants their further exploration.
4.4. Prospects of Nanoencapsulation
The broad survey of islet nanoencapsulation methods presented herein illustrates that this technology holds tremendous promise as a platform for islet replacement therapy. Ongoing research will certainly refine this practice and many of the method-specific problems above will be resolved. It is, nevertheless, worth discussing some potential broadly-relevant limitations of nanoencapsulation.
Despite the theoretical diffusional advantage of thinner hydrogel structures, several seminal studies spanning multiple decades have reported improved outcomes in larger capsule transplants [71, 72, 79, 303, 432]. Within the intraperitoneal space, larger (800 μm) hydrogel capsules remain free-floating in the peritoneal fluid while smaller (500 μm) capsules aggregate and adhere to peritoneal organs [303]. It has also been shown that decreasing spherical capsule size from 1.5 mm to 300 μm induces a progressively stronger fibrotic response [79]. Projecting this trend to the diameter of a nanoencapsulated islet (roughly 150 μm), a high degree of aggregation and a substantial fibrotic response can be predicted. Moreover, it has also been posited that such thin membranes may not provide adequate immune isolation [432]. Lastly, nanoencapsulation may be incompatible with stem cell-derived β-cells as it may be very difficult to prevent the migration of undifferentiated cells out of such coatings. These outstanding uncertainties can be addressed by future research.
5. Concluding Remarks
Many biological processes happen at the nanoscale where molecules interact and carry out their physiological functions. Nanotechnology allows scientists and engineers to manipulate processes at this scale. Such control in cell encapsulation and delivery systems has enabled fundamental advances in moderating the immune response, achieving adequate mass transfer, and designing devices across a broad range of length scales.
Nanotechnology enhances precision in material design, drug delivery, tethered factor presentation, deterrence of adverse protein adsorption, delivery or generation of oxygen, and specification of molecular weight cut-offs, all of which are pivotal considerations for cell replacement therapies. Likewise, delay of a fibrotic reaction is critical to the function of metabolic cell encapsulation devices and may be achieved through nanoscale chemical and biological material modification. Factors that can be delivered range from small synthetic molecules to large antibodies, the choice of which dictates the appropriate delivery method and nanomaterial characteristics.
Ensuring cell survival, resistance to hypoxia and graft function is equally important and may be enabled by materials engineering on the nanoscale. Appropriately tailored emulsions improve islet oxygenation during culture and encapsulation. Certain approaches have been developed to produce oxygen locally as well. Additionally, factors may be incorporated within the encapsulating polymer matrix that aid in oxygen transport, reduce islet apoptosis, stimulate β-cell proliferation, provide ECM mimicking signals, and specifically adsorb or neutralize known detrimental factors. Such technologies may also leverage nanoscale manipulation to induce a mature, specialized and functional blood vessel network within or near the graft.
Many technologies surveyed herein may also be applicable to the broader practice of tissue engineering, regenerative medicine, and synthetic implants as similar problems plague these fields. Efforts to minimize the FBR or vascularize cell-laden grafts are challenges in many transplantation applications [433, 434]. The fibrotic response also degenerates the function of closed-loop artificial pancreas systems as the build-up of collagenous deposits impedes glucose-sensing and insulin delivery [435]. Improved oxygen delivery strategies may be applicable in wound healing [436]. Nanotechnology also fundamentally enables other modalities of diabetes treatment not covered in this text, such as long circulating insulin [437], wound healing [438], controlled insulin delivery, and glucose sensing [315, 439, 440].
As the field of cellular replacement therapy progresses, nanotechnology will continue to play an invaluable role in the improvement of these technologies. Macroencapsulation devices provide ease of monitoring and assurance of retrieval at the end of the graft life cycle. The nanoscale features of encapsulation membranes have been recognized from the earliest observations of molecular exclusion. Recent approaches to achieve fine nanoscale control have been developed to further tune this selective permeability and bestow devices with a variety of other desirable properties. Finally, nanoencapsulation is a promising example of nanotechnology which addresses critical areas of concern with traditional approaches, such as transplant volume and diffusional limitations. The bio-functionalization of thin polymer films holds potential to enable their clinical translation. Promising data on cellular replacement therapies in the lab and limited success in the clinic spur the search for continuous design improvements in which the nanoscale must be considered.
6. Acknowledgments
The work is partially supported by Juvenile Diabetes Research Foundation, National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK105967–01A1), the Hartwell Foundation and the Novo Nordisk Company. The sources of funding had no role in the decision to write this manuscript. The authors are grateful to Alan Chiu and Stephanie Fuchs for critical feedback on this manuscript.
Abbreviation Definition
- 3D
3-dimensional
- BG
blood glucose
- CsA
cyclosporine-A
- ECM
extracellular matrix
- FasL
Fas ligand
- FBR
foreign body reaction
- FGF
fibroblast growth factor
- GLP-1
glucagon-like peptide-1
- GSIS
glucose-stimulated insulin secretion
- HEK
human embryonic kidney
- IBMIR
immediate blood-mediated immune reaction
- IFN
interferon
- IGF
insulin-like growth factor
- IL
interleukin
- ivGTT
intravenous glucose tolerance test
- JAG-1
Jagged-1
- LBL
layer-by-layer
- LIF
leukemia inhibitory factor
- lipid-PEG
lipid-conjugated PEG
- M1
inflammatory macrophage phenotype
- M2
anti-inflammatory macrophage phenotype
- mAb
monoclonal antibody
- MCP
monocyte chemotactic protein
- MHC
major histocompatibility complex
- mPEG
monomethoxy poly(ethylene glycol)
- MR-1
anti-CD154 monoclonal antibody
- NEED
Nanofiber-Enabled Encapsulation Device
- NET
neutrophil extracellular traps
- NHS
N-hydroxysuccinimide
- NOD
non-obese diabetic
- PC
phosphorylcholine
- PCBMA
poly(carboxybetaine methacrylate)
- PCL
polycaprolactone
- PDGF
platelet-derived growth factor
- PDMS
polydimethylsiloxane
- PEG
poly(ethylene glycol)
- PFC
perfluorocarbon
- PHEMA
poly(2-hydroxyethyl methacrylate)
- PLA
poly(lactic acid)
- PLG
poly(lactide-co-glycolide)
- PLGA
poly(lactic-co-glycolic acid)
- PLL
poly-l-lysine
- PMMA
poly(methyl methacrylate)
- PSS
poly(styrene-sulfonic acid, sodium salt)
- PTFE
polytetrafluoroethylene
- PVA
poly(vinyl alcohol)
- PVPON
poly(N-vinylpyrrolidone)
- sCR1
soluble complement receptor 1
- starPEG
star-shaped PEG
- STZ
streptozotocin
- T1D
type 1 diabetes
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- TRAFFIC
Thread-Reinforced Alginate Fiber for Islets Encapsulation
- Tregs
regulatory T-cells
- VEGF
vascular endothelial growth factor
- XIAP
X-linked inhibitor of apoptosis
- Z1-Y15
triazole-modified alginate derivative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- [1].Atkinson MA, Eisenbarth GS, Michels AW, Type 1 diabetes, Lancet, 383 (2014) 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].DiMeglio LA, Evans-Molina C, Oram RA, Type 1 diabetes, Lancet, 391 (2018) 2449–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, Maahs DM, Tamborlane WV, Current state of type 1 diabetes treatment in the U.S.: Updated data from the T1D exchange clinic registry, Diabetes Care, 38 (2015) 971–978. [DOI] [PubMed] [Google Scholar]
- [4].Peña AS, Couper JJ, Harrington J, Gent R, Fairchild J, Tham E, Baghurst P, Hypoglycemia, but not glucose variability, relates to vascular function in children with type 1 diabetes, Diabetes Technol. Ther, 14 (2012) 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brownlee M, Biochemistry and molecular cell biology of diabetic complications, Nature, 414 (2001) 813–820. [DOI] [PubMed] [Google Scholar]
- [6].Ly TT, Gallego PH, Davis EA, Jones TW, Impaired awareness of hypoglycemia in a population-based sample of children and adolescents with type 1 diabetes, Diabetes Care, 32 (2009) 1802–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, Kaufman FR, Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes, JAMA, 316 (2016) 1407–1408. [DOI] [PubMed] [Google Scholar]
- [8].Valdes-Gonzalez R, Rodriguez-Ventura AL, White DJG, Bracho-Blanchet E, Castillo A, Ramírez-González B, López-Santos MG, León-Mancilla BH, Dorantes LM, Long-term follow-up of patients with type 1 diabetes transplanted with neonatal pig islets, Clin. Exp. Immunol, 162 (2010) 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV, Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen, New Engl. J. Med, 343 (2000) 230–238. [DOI] [PubMed] [Google Scholar]
- [10].Odorico J, Markmann J, Melton D, Greenstein J, Hwa A, Nostro C, Rezania A, Oberholzer J, Pipeleers D, Yang L, Cowan C, Huangfu D, Egli D, Ben-David U, Vallier L, Grey ST, Tang Q, Roep B, Ricordi C, Naji A, Orlando G, Anderson DG, Poznansky M, Ludwig B, Tomei A, Greiner DL, Graham M, Carpenter M, Migliaccio G, D’Amour K, Hering B, Piemonti L, Berney T, Rickels M, Kay T, Adams A, Report of the key opinion leaders meeting on stem cell-derived beta cells, Transplantation, 102 (2018) 1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shapiro AMJ, Pokrywczynska M, Ricordi C, Clinical pancreatic islet transplantation, Nat. Rev. Endocrinol, 13 (2017) 268–277. [DOI] [PubMed] [Google Scholar]
- [12].Scharp DW, Marchetti P, Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution, Adv. Drug Del. Rev, 67–68 (2014) 35–73. [DOI] [PubMed] [Google Scholar]
- [13].Ernst AU, Wang L-H, Ma M, Islet encapsulation, J. Mater. Chem. B, 6 (2018) 6705–6722. [DOI] [PubMed] [Google Scholar]
- [14].Desai T, Shea LD, Advances in islet encapsulation technologies, Nat. Rev. Drug Discov, 16 (2017) 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Robles L, Storrs R, Lamb M, Alexander M, Lakey JRT, Current status of islet encapsulation, Cell Transplant, 23 (2014) 1321–1348. [DOI] [PubMed] [Google Scholar]
- [16].Omami M, McGarrigle JJ, Reedy M, Isa D, Ghani S, Marchese E, Bochenek MA, Longi M, Xing Y, Joshi I, Wang Y, Oberholzer J, Islet microencapsulation: Strategies and clinical status in diabetes, Curr. Diab. Rep, 17 (2017) 47. [DOI] [PubMed] [Google Scholar]
- [17].Vaithilingam V, Tuch BE, Islet transplantation and encapsulation: An update on recent developments, Rev. Diabet. Stud, 8 (2011) 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nostro MC, Keller G, Generation of beta cells from human pluripotent stem cells: Potential for regenerative medicine, Semin. Cell Dev. Biol, 23 (2012) 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Korsgren O, Islet encapsulation: Physiological possibilities and limitations, Diabetes, 66 (2017) 1748–1754. [DOI] [PubMed] [Google Scholar]
- [20].Colton CK, Oxygen supply to encapsulated therapeutic cells, Adv. Drug Del. Rev, 67–68 (2014) 93–110. [DOI] [PubMed] [Google Scholar]
- [21].Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E, Omami M, Doloff JC, Mendoza-Elias J, Nourmohammadzadeh M, Khan A, Yeh C-C, Xing Y, Isa D, Ghani S, Li J, Landry C, Bader AR, Olejnik K, Chen M, Hollister-Lock J, Wang Y, Greiner DL, Weir GC, Strand BL, Rokstad AMA, Lacik I, Langer R, Anderson DG, Oberholzer J, Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques, Nat. Biomed. Eng, 2 (2018) 810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Avgoustiniatos ES, Dionne KE, Wilson DF, Yarmush ML, Colton CK, Measurements of the effective diffusion coefficient of oxygen in pancreatic islets, Ind. Eng. Chem. Res, 46 (2007) 6157–6163. [Google Scholar]
- [23].Dionne KE, Colton CK, Yarmush ML, Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans, Diabetes, 42 (1993) 12–21. [DOI] [PubMed] [Google Scholar]
- [24].Dionne KE, Colton CK, Yarmush ML, Effect of oxygen on isolated pancreatic tissue, ASAIO Trans., 35 (1989) 739–741. [DOI] [PubMed] [Google Scholar]
- [25].Brange J, Owens DR, Kang S, Vølund A, Monomeric insulins and their experimental and clinical implications, Diabetes Care, 13 (1990) 923–954. [DOI] [PubMed] [Google Scholar]
- [26].Hosoya O, Chono S, Saso Y, Juni K, Morimoto K, Seki T, Determination of diffusion coefficients of peptides and prediction of permeability through a porous membrane, J. Pharm. Pharmacol, 56 (2004) 1501–1507. [DOI] [PubMed] [Google Scholar]
- [27].Anderson JM, Rodriguez A, Chang DT, Foreign body reaction to biomaterials, Semin. Immunol, 20 (2008) 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Christo SN, Diener KR, Bachhuka A, Vasilev K, Hayball JD, Innate immunity and biomaterials at the nexus: Friends or foes, Biomed. Res. Int, 2015 (2015) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rogers TH, Babensee JE, Altered adherent leukocyte profile on biomaterials in Toll-like receptor 4 deficient mice, Biomaterials, 31 (2010) 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee J, Jackman JG, Kwun J, Manook M, Moreno A, Elster EA, Kirk AD, Leong KW, Sullenger BA, Nucleic acid scavenging microfiber mesh inhibits trauma-induced inflammation and thrombosis, Biomaterials, 120 (2017) 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sadtler K, Wolf MT, Ganguly S, Moad CA, Chung L, Majumdar S, Housseau F, Pardoll DM, Elisseeff JH, Divergent immune responses to synthetic and biological scaffolds, Biomaterials, 192 (2019) 405–415. [DOI] [PubMed] [Google Scholar]
- [32].Bryers JD, Giachelli CM, Ratner BD, Engineering biomaterials to integrate and heal: The biocompatibility paradigm shifts, Biotechnol. Bioeng, 109 (2012) 1898–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, Bader A, Chiu A, Sadraei A, Aresta-Dasilva S, Griffin M, Jhunjhunwala S, Webber M, Siebert S, Tang K, Chen M, Langan E, Dholokia N, Thakrar R, Qi M, Oberholzer J, Greiner DL, Langer R, Anderson DG, Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates, Nat. Mater, 16 (2017) 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mantovani A, Sica A, Locati M, New vistas on macrophage differentiation and activation, Eur. J. Immunol, 37 (2007) 14–16. [DOI] [PubMed] [Google Scholar]
- [35].Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M, The chemokine system in diverse forms of macrophage activation and polarization, Trends Immunol, 25 (2004) 677–686. [DOI] [PubMed] [Google Scholar]
- [36].Gordon S, Martinez FO, Alternative activation of macrophages: Mechanism and functions, Immunity, 32 (2010) 593–604. [DOI] [PubMed] [Google Scholar]
- [37].van Putten SM, Ploeger DTA, Popa ER, Bank RA, Macrophage phenotypes in the collagen-induced foreign body reaction in rats, Acta Biomater, 9 (2013) 6502–6510. [DOI] [PubMed] [Google Scholar]
- [38].Mosser DM, Edwards JP, Exploring the full spectrum of macrophage activation, Nat. Rev. Immunol, 8 (2008) 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, Schultze JL, Transcriptome-based network analysis reveals a spectrum model of human macrophage activation, Immunity, 40 (2014) 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Alvarez MM, Liu JC, Trujillo-de Santiago G, Cha B-H, Vishwakarma A, Ghaemmaghami AM, Khademhosseini A, Delivery strategies to control inflammatory response: Modulating M1–M2 polarization in tissue engineering applications, J. Controlled Release, 240 (2016) 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jhunjhunwala S, Aresta-DaSilva S, Tang K, Alvarez D, Webber MJ, Tang BC, Lavin DM, Veiseh O, Doloff JC, Bose S, Vegas A, Ma M, Sahay G, Chiu A, Bader A, Langan E, Siebert S, Li J, Greiner DL, Newburger PE, von Andrian UH, Langer R, Anderson DG, Neutrophil responses to sterile implant materials, PLoS One, 10 (2015) e0137550–e0137550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yipp BG, Kubes P, Netosis: How vital is it?, Blood, 122 (2013) 2784–2793. [DOI] [PubMed] [Google Scholar]
- [43].Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A, Neutrophil extracellular traps kill bacteria, Science, 303 (2004) 1532–1535. [DOI] [PubMed] [Google Scholar]
- [44].Krzystyniak A, Gołąb K, Witkowski P, Trzonkowski P, Islet cell transplant and the incorporation of Tregs, Curr. Opin. Organ Transplant, 19 (2014) 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sackett SD, Rodriguez A, Odorico JS, The nexus of stem cell-derived beta-cells and genome engineering, Rev. Diabet. Stud, 14 (2017) 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Niu D, Wei H-J, Lin L, George H, Wang T, Lee I-H, Zhao H-Y, Wang Y, Kan Y, Shrock E, Lesha E, Wang G, Luo Y, Qing Y, Jiao D, Zhao H, Zhou X, Wang S, Wei H, Güell M, Church GM, Yang L, Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9, Science, 357 (2017) 1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang L, Güell M, Niu D, George H, Lesha E, Grishin D, Aach J, Shrock E, Xu W, Poci J, Cortazio R, Wilkinson RA, Fishman JA, Church G, Genome-wide inactivation of porcine endogenous retroviruses (PERVs), Science, 350 (2015) 1101–1104. [DOI] [PubMed] [Google Scholar]
- [48].Millman JR, Xie C, Van Dervort A, Gürtler M, Pagliuca FW, Melton DA, Generation of stem cell-derived β-cells from patients with type 1 diabetes, Nat. Commun, 7 (2016) 11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ar’Rajab A, Dawidson I, Harris R, Immune privilege of the testis for islet xenotransplantation (rat to mouse), Cell Transplant., 3 (1994) 493–498. [DOI] [PubMed] [Google Scholar]
- [50].Xin Z-L, Ge S-L, Wu X-K, Jia Y-J, Hu H-T, Intracerebral xenotransplantation of semipermeable membrane-encapsuled pancreatic islets, World J. Gastroenterol, 11 (2005) 5714–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Posselt AM, Naji A, Roark JH, Markmann JF, Barker CF, Intrathymic islet transplantation in the spontaneously diabetic BB rat, Ann. Surg, 214 (1991) 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Posselt AM, Barker CF, Tomaszewski JE, Markmann JF, Choti MA, Naji A, Induction of donor-specific unresponsiveness by intrathymic islet transplantation, Science, 249 (1990) 1293–1295. [DOI] [PubMed] [Google Scholar]
- [53].Perez VL, Caicedo A, Berman DM, Arrieta E, Abdulreda MH, Rodriguez-Diaz R, Pileggi A, Hernandez E, Dubovy SR, Parel JM, Ricordi C, Kenyon NM, Kenyon NS, Berggren PO, The anterior chamber of the eye as a clinical transplantation site for the treatment of diabetes: A study in a baboon model of diabetes, Diabetologia, 54 (2011) 1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Woodward KB, Wang F, Zhao H, Yolcu ES, Shirwan H, Novel technologies to engineer graft for tolerance induction, Curr. Opin. Organ Transplant, 21 (2016) 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Klueh U, Liu Z, Ouyang T, Cho B, Feldman B, Henning TP, Kreutzer D, Blood-induced interference of glucose sensor function in vitro: Implications for in vivo sensor function, J. Diabetes Sci. Technol, 1 (2007) 842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP, The cellular and molecular biology of periprosthetic osteolysis, Clin. Orthop. Relat. Res, (2007) 251–261. [DOI] [PubMed] [Google Scholar]
- [57].Williams DF, On the mechanisms of biocompatibility, Biomaterials, 29 (2008) 2941–2953. [DOI] [PubMed] [Google Scholar]
- [58].Latour RA, Biomaterials: Protein-surface interactions, in: Wnek GE, Bowlin GL (Eds.) Encyclopedia of biomaterials and biomedical engineering, Taylor & Francis; 2005, pp. 1–15. [Google Scholar]
- [59].Wilson C, Clegg R, Leavesley D, Pearcy M, Mediation of biomaterial-cell interactions by adsorbed proteins: A review, Tissue Eng, 11 (2005) 1–18. [DOI] [PubMed] [Google Scholar]
- [60].Franz S, Rammelt S, Scharnweber D, Simon JC, Immune responses to implants – a review of the implications for the design of immunomodulatory biomaterials, Biomaterials, 32 (2011) 6692–6709. [DOI] [PubMed] [Google Scholar]
- [61].Blaszykowski C, Sheikh S, Thompson M, Biocompatibility and antifouling: Is there really a link, Trends Biotechnol, 32 (2014) 61–62. [DOI] [PubMed] [Google Scholar]
- [62].Blaszykowski C, Sheikh S, Thompson M, Surface chemistry to minimize fouling from blood-based fluids, Chem. Soc. Rev, 41 (2012) 5599–5612. [DOI] [PubMed] [Google Scholar]
- [63].Hillel A, Shah P, Elisseeff J, Hydrogels in cell encapsulation and tissue engineering, in: Jenkins M (Ed.) Biomedical polymers, Woodhead Publishing; 2007, pp. 57–82. [Google Scholar]
- [64].Fenoradosoa TA, Ali G, Delattre C, Laroche C, Petit E, Wadouachi A, Michaud P, Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow, J. Appl. Phycol, 22 (2010) 131–137. [Google Scholar]
- [65].Orive G, Hernández RM, Gascón AR, Pedraz JL, Encapsulation of cells in alginate gels, in: Guisan JM (Ed.) Immobilization of enzymes and cells, Humana Press; 2006, pp. 345–355. [Google Scholar]
- [66].Grassi M, Sandolo C, Perin D, Coviello T, Lapasin R, Grassi G, Structural characterization of calcium alginate matrices by means of mechanical and release tests, Molecules, 14 (2009) 3003–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Strand BL, Gåserød O, Kulseng B, Espevik T, Skjåk-Bræk G, Alginate-polylysine-alginate microcapsules: Effect of size reduction on capsule properties, J. Microencaps, 19 (2002) 615–630. [DOI] [PubMed] [Google Scholar]
- [68].Kulseng B, Thu B, Espevik T, Skjåk-Bræk G, Alginate polylysine microcapsules as immune barrier: Permeability of cytokines and immunoglobulins over the capsule membrane, Cell Transplant, 6 (1997) 387–394. [DOI] [PubMed] [Google Scholar]
- [69].de Vos P, Faas MM, Strand B, Calafiore R, Alginate-based microcapsules for immunoisolation of pancreatic islets, Biomaterials, 27 (2006) 5603–5617. [DOI] [PubMed] [Google Scholar]
- [70].Yang D, Jones KS, Effect of alginate on innate immune activation of macrophages, J. Biomed. Mater. Res. A, 90A (2009) 411–418. [DOI] [PubMed] [Google Scholar]
- [71].Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, Kang JW, Hollister-Locke J, Bochenek MA, Chiu A, Siebert S, Tang K, Jhunjhunwala S, Aresta-Dasilva S, Dholakia N, Thakrar R, Vietti T, Chen M, Coehn J, Siniakowicz K, Qi M, McGarrigle J, Lyle S, Harlan DM, Greiner DL, Oberholzer J, Weir GC, Langer R, Anderson DG, Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates, Nat. Biotechnol, 34 (2016) 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Vegas AJ, Veiseh O, Gütler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG, Long term glycemic control using polymer encapsulated, human stem-cell derived β-cells in immune competent mice, Nat. Med, 22 (2016) 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Neoh KG, Li M, Kang E-T, Chiong E, Tambyah PA, Surface modification strategies for combating catheter-related complications: Recent advances and challenges, J. Mater. Chem. B, 5 (2017) 2045–2067. [DOI] [PubMed] [Google Scholar]
- [74].Chen Y, Thayumanavan S, Amphiphilicity in homopolymer surfaces reduces nonspecific protein adsorption, Langmuir, 25 (2009) 13795–13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chen S, Li L, Zhao C, Zheng J, Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials, Polymer, 51 (2010) 5283–5293. [Google Scholar]
- [76].Zhang L, Cao Z, Bai T, Carr L, Ella-Menye J-R, Irvin C, Ratner B, Jiang S, Zwitterionic hydrogels implanted in mice resist the foreign-body reaction, Nat. Biotechnol, 31 (2013) 553–556. [DOI] [PubMed] [Google Scholar]
- [77].Ishihara K, Ziats NP, Tierney BP, Nakabayashi N, Anderson JM, Protein adsorption from human plasma is reduced on phospholipid polymers, J. Biomed. Mater. Res, 25 (1991) 1397–1407. [DOI] [PubMed] [Google Scholar]
- [78].Hayward JA, Chapman D, Biomembrane surfaces as models for polymer design: The potential for haemocompatibility, Biomaterials, 5 (1984) 135–142. [DOI] [PubMed] [Google Scholar]
- [79].Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader Andrew R., Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacík I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG, Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates, Nat. Mater, 14 (2015) 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Matlaga BF, Yasenchak LP, Salthouse TN, Tissue response to implanted polymers: The significance of sample shape, J. Biomed. Mater. Res, 10 (1976) 391–397. [DOI] [PubMed] [Google Scholar]
- [81].Salthouse TN, Some aspects of macrophage behavior at the implant interface, J. Biomed. Mater. Res, 18 (1984) 395–401. [DOI] [PubMed] [Google Scholar]
- [82].Kusaka T, Nakayama M, Nakamura K, Ishimiya M, Furusawa E, Ogasawara K, Effect of silica particle size on macrophage inflammatory responses, PLoS One, 9 (2014) e92634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jansen LE, Amer LD, Chen EYT, Nguyen TV, Saleh LS, Emrick T, Liu WF, Bryant SJ, Peyton SR, Zwitterionic PEG-PC hydrogels modulate the foreign body response in a modulus-dependent manner, Biomacromolecules, 19 (2018) 2880–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].van Sandwijk MS, Bemelman FJ, ten Berge IGM, Immunosuppressive drugs after solid organ transplantation, Neth. J. Med, 71 (2013) 281–289. [PubMed] [Google Scholar]
- [85].Colombo D, Ammirati E, Cyclosporine in transplantation—a history of converging timelines, J. Biol. Regul. Homeost. Agents, 25 (2011) 493–504. [PubMed] [Google Scholar]
- [86].Azzi J, Tang L, Moore R, Tong R, El Haddad N, Akiyoshi T, Mfarrej B, Yang S, Jurewicz M, Ichimura T, Lindeman N, Cheng J, Abdi R, Polylactide-cyclosporin A nanoparticles for targeted immunosuppression, FASEB J, 24 (2010) 3927–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C, Luca G, Basta G, Calafiore R, Preparation and in vitro and in vivo characterization of composite microcapsules for cell encapsulation, Int. J. Pharm, 324 (2006) 27–36. [DOI] [PubMed] [Google Scholar]
- [88].Shapiro AMJ, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems J-A, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DER, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JRT, International trial of the Edmonton protocol for islet transplantation, New Engl. J. Med, 355 (2006) 1318–1330. [DOI] [PubMed] [Google Scholar]
- [89].Jeong J-H, Hong SW, Hong S, Yook S, Jung Y, Park J-B, Khue CD, Im B-H, Seo J, Lee H, Ahn C-H, Lee DY, Byun Y, Surface camouflage of pancreatic islets using 6-arm-PEG-catechol in combined therapy with tacrolimus and anti-CD154 monoclonal antibody for xenotransplantation, Biomaterials, 32 (2011) 7961–7970. [DOI] [PubMed] [Google Scholar]
- [90].Haque MR, Jeong J-H, Byun Y, Combination strategy of multi-layered surface camouflage using hyperbranched polyethylene glycol and immunosuppressive drugs for the prevention of immune reactions against transplanted porcine islets, Biomaterials, 84 (2016) 144–156. [DOI] [PubMed] [Google Scholar]
- [91].Haque MR, Kim J, Park H, Lee HS, Lee KW, Al-Hilal TA, Jeong J-H, Ahn C-H, Lee DS, Kim SJ, Byun Y, Xenotransplantation of layer-by-layer encapsulated non-human primate islets with a specified immunosuppressive drug protocol, J. Controlled Release, 258 (2017) 10–21. [DOI] [PubMed] [Google Scholar]
- [92].Azadi SA, Vasheghani-Farahani E, Hashemi-Najafbabadi S, Godini A, Co-encapsulation of pancreatic islets and pentoxifylline in alginate-based microcapsules with enhanced immunosuppressive effects, Prog. Biomater, 5 (2016) 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dang TT, Thai AV, Cohen J, Slosberg JE, Siniakowicz K, Doloff JC, Ma M, Hollister-Lock J, Tang KM, Gu Z, Cheng H, Weir GC, Langer R, Anderson DG, Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug, Biomaterials, 34 (2013) 5792–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jiang K, Weaver JD, Li Y, Chen X, Liang J, Stabler CL, Local release of dexamethasone from macroporous scaffolds accelerates islet transplant engraftment by promotion of anti-inflammatory M2 macrophages, Biomaterials, 114 (2017) 71–81. [DOI] [PubMed] [Google Scholar]
- [95].Rahimi HR, Mohammadpour AH, Dastani M, Jaafari MR, Abnous K, Ghayour Mobarhan M, Kazemi Oskuee R, The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial, Avicenna J. Phytomed, 6 (2016) 567–577. [PMC free article] [PubMed] [Google Scholar]
- [96].Oskouie MN, Aghili Moghaddam NS, Butler AE, Zamani P, Sahebkar A, Therapeutic use of curcumin-encapsulated and curcumin-primed exosomes, J. Cell. Physiol, (2018). 10.1002/jcp.27615 [DOI] [PubMed] [Google Scholar]
- [97].Lin C-C, Boyer PD, Aimetti AA, Anseth KS, Regulating MCP-1 diffusion in affinity hydrogels for enhancing immuno-isolation, J. Controlled Release, 142 (2010) 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lin C-C, Metters AT, Anseth KS, Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFα, Biomaterials, 30 (2009) 4907–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Su J, Hu B-H, Lowe WL, Kaufman DB, Messersmith PB, Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation, Biomaterials, 31 (2010) 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Fisher JD, Acharya AP, Little SR, Micro and nanoparticle drug delivery systems for preventing allotransplant rejection, Clin. Immunol, 160 (2015) 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Gao W, Thompson L, Zhou Q, Putheti P, Fahmy TM, Strom TB, Metcalfe SM, Treg versus TH17 lymphocyte lineages are cross-regulated by LIF versus IL-6, Cell Cycle, 8 (2009) 1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Park J, Gao W, Whiston R, Strom TB, Metcalfe S, Fahmy TM, Modulation of CD4+ T lymphocyte lineage outcomes with targeted, nanoparticle-mediated cytokine delivery, Mol. Pharm, 8 (2011) 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Dong H, Fahmy TM, Metcalfe SM, Morton SL, Dong X, Inverardi L, Adams DB, Gao W, Wang H, Immuno-isolation of pancreatic islet allografts using pegylated nanotherapy leads to long-term normoglycemia in full MHC mismatch recipient mice, PLoS One, 7 (2012) e50265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Jhunjhunwala S, Balmert SC, Raimondi G, Dons E, Nichols EE, Thomson AW, Little SR, Controlled release formulations of IL-2, TGF-β1 and rapamycin for the induction of regulatory T cells, J. Controlled Release, 159 (2012) 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lu L, Zhou X, Wang J, Zheng S, Horwitz D, Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-β and retinoic acid, PLoS One, 6 (2010) e15150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Graham JG, Zhang X, Goodman A, Pothoven K, Houlihan J, Wang S, Gower RM, Luo X, Shea LD, PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes, Tissue Eng. Part A, 19 (2013) 1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Dufour JM, Lord SJ, Kin T, Rayat GR, Dixon DE, Bleackley RC, Korbutt GS, Rajotte RV, Comparison of successful and unsuccessful islet/sertoli cell cotransplant grafts in streptozotocin-induced diabetic mice, Cell Transplant, 16 (2007) 1029–1038. [DOI] [PubMed] [Google Scholar]
- [108].Hayward JA, Ellis CE, Seeberger K, Lee T, Salama B, Mulet-Sierra A, Kuppan P, Adesida A, Korbutt GS, Co-transplantation of mesenchymal stem cells with neonatal porcine islets improve graft function in diabetic mice, Diabetes, 66 (2017) 1312–1321. [DOI] [PubMed] [Google Scholar]
- [109].Bhaiji T, Zhi Z-L, Pickup JC, Improving cellular function and immune protection via layer-by-layer nanocoating of pancreatic islet β-cell spheroids cocultured with mesenchymal stem cells, J. Biomed. Mater. Res. A, 100A (2012) 1628–1636. [DOI] [PubMed] [Google Scholar]
- [110].Izadi Z, Hajizadeh-Saffar E, Hadjati J, Habibi-Anbouhi M, Ghanian MH, Sadeghi-Abandansari H, Ashtiani MK, Samsonchi Z, Raoufi M, Moazenchi M, Izadi M, Nejad ASSH, Namdari H, Tahamtani Y, Ostad SN, Akbari-Javar H, Baharvand H, Tolerance induction by surface immobilization of Jagged-1 for immunoprotection of pancreatic islets, Biomaterials, 182 (2018) 191–201. [DOI] [PubMed] [Google Scholar]
- [111].Waring P, Müllbacher A, Cell death induced by the Fas/Fas ligand pathway and its role in pathology, Immunol. Cell Biol, 77 (1999) 312–317. [DOI] [PubMed] [Google Scholar]
- [112].Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA, A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1), J. Exp. Med, 184 (1996) 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Papeta N, Chen T, Vianello F, Gererty L, Malik A, Mok Y-T, Tharp WG, Bagley J, Zhao G, Stevceva L, Yoon V, Sykes M, Sachs D, Iacomini J, Poznansky MC, Long-term survival of transplanted allogeneic cells engineered to express a T cell chemorepellent, Transplantation, 83 (2007) 174–183. [DOI] [PubMed] [Google Scholar]
- [114].Yolcu ES, Zhao H, Bandura-Morgan L, Lacelle C, Woodward KB, Askenasy N, Shirwan H, Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing regulatory T cells in mice, J. Immunol, 187 (2011) 5901–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Headen DM, Woodward KB, Coronel MM, Shrestha P, Weaver JD, Zhao H, Tan M, Hunckler MD, Bowen WS, Johnson CT, Shea L, Yolcu ES, García AJ, Shirwan H, Local immunomodulation with Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance, Nat. Mater, 17 (2018) 732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wang W, Shahzad KA, Li M, Zhang A, Zhang L, Xu T, Wan X, Shen C, An antigen-presenting and apoptosis-inducing polymer microparticle prolongs alloskin graft survival by selectively and markedly depleting alloreactive CD8+ T cells, Front. Immunol, 8 (2017) 657–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Cheung CY, Anseth KS, Synthesis of immunoisolation barriers that provide localized immunosuppression for encapsulated pancreatic islets, Bioconj. Chem, 17 (2006) 1036–1042. [DOI] [PubMed] [Google Scholar]
- [118].Obach M, Hosseini-Tabatabaei A, Montane J, Wind K, Soukhatcheva G, Dai D, Priatel JJ, Orban PC, Verchere CB, Prevention of autoimmune diabetes and islet allograft rejection by beta cell expression of XIAP: Insight into possible mechanisms of local immunomodulation, Mol. Cell. Endocrinol, 477 (2018) 48–56. [DOI] [PubMed] [Google Scholar]
- [119].Avgoustiniatos ES, Colton CK, Effect of external oxygen mass transfer resistances on viability of immunoisolated tissue, Ann. N. Y. Acad. Sci, 831 (1997) 145–166. [DOI] [PubMed] [Google Scholar]
- [120].Gorden DL, Mandriota SJ, Montesano R, Orci L, Pepper MS, Vascular endothelial growth factor is increased in devascularized rat islets of Langerhans in vitro, Transplantation, 63 (1997) 436–443. [DOI] [PubMed] [Google Scholar]
- [121].Vasir B, Aiello LP, Yoon KH, Quickel RR, Bonner-Weir S, Weir GC, Hypoxia induces vascular endothelial growth factor gene and protein expression in cultured rat islet cells, Diabetes, 47 (1998) 1894–1903. [DOI] [PubMed] [Google Scholar]
- [122].de Vos P, van Straaten JF, Nieuwenhuizen AG, de Groot M, Ploeg RJ, de Haan BJ, van Schilfgaarde R, Why do microencapsulated islet grafts fail in the absence of fibrotic overgrowth?, Diabetes, 48 (1999) 1381–1388. [DOI] [PubMed] [Google Scholar]
- [123].Barkai U, Rotem A, de Vos P, Survival of encapsulated islets: More than a membrane story, World J. Transplant, 6 (2016) 69–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Dulong J-J, Legallais C, A theoretical study of oxygen transfer including necrosis for the design of a bioartificial pancreas, Biotechnol. Bioeng, 96 (2006) 990–998. [DOI] [PubMed] [Google Scholar]
- [125].Lewis AS, Eliminating oxygen supply limitations for transplanted microencapsulated islets in the treatment of type 1 diabetes, Department of Chemical Engineering, Massachusetts Institute of Technology, 2008, pp. 234. [Google Scholar]
- [126].Mehmetoglu Ü, Ateş S, Berber R, Oxygen diffusivity in calcium alginate gel beads containing Gluconobacter suboxydans, Artif. Cells Blood Substit. Immobil. Biotechnol, 24 (1996) 91–106. [DOI] [PubMed] [Google Scholar]
- [127].Zhao W, Zhang Y, Liu Y, Tan M, Yu W, Xie H, Ma Y, Sun G, Lv G, Zhao S, Ma X, Oxygen diffusivity in alginate/chitosan microcapsules, J. Chem. Technol. Biotechnol, 88 (2012) 449–455. [Google Scholar]
- [128].Carlsson P-O, Palm F, Andersson A, Liss P, Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site, Diabetes, 50 (2001) 489–495. [DOI] [PubMed] [Google Scholar]
- [129].Buchwald P, Tamayo-Garcia A, Manzoli V, Tomei AA, Stabler CL, Glucose-stimulated insulin release: Parallel perifusion studies of free and hydrogel encapsulated human pancreatic islets, Biotechnol. Bioeng, 115 (2018) 232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Waxman K, Perfluorocarbons as blood substitutes, Ann. Emergency Med, 15 (1986) 1423–1424. [DOI] [PubMed] [Google Scholar]
- [131].Zekorn T, Siebers U, Bretzel RG, Heller S, Meder U, Ruttkay H, Zimmermann U, Federlin K, Impact of the perfluorochemical FC43 on function of isolated islets, Horm. Metab. Res, 23 (1991) 302–303. [DOI] [PubMed] [Google Scholar]
- [132].Maillard E, Juszczak MT, Langlois A, Kleiss C, Sencier MC, Bietiger W, Sanchez-Dominguez M, Krafft MP, Johnson PRV, Pinget M, Sigrist S, Perfluorocarbon emulsions prevent hypoxia of pancreatic β-cells, Cell Transplant, 21 (2012) 657–669. [DOI] [PubMed] [Google Scholar]
- [133].Johnson AS, O’Sullivan E, D’Aoust LN, Omer A, Bonner-Weir S, Fisher RJ, Weir GC, Colton CK, Quantitative assessment of islets of Langerhans encapsulated in alginate, Tissue Eng. Part C, 17 (2011) 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hrostowski HJ, Kaiser RH, The solubility of oxygen in silicon, J. Phys. Chem. Solids, 9 (1959) 214–216. [Google Scholar]
- [135].Henstock JR, Canham LT, Anderson SI, Silicon: The evolution of its use in biomaterials, Acta Biomater., 11 (2015) 17–26. [DOI] [PubMed] [Google Scholar]
- [136].Erdodi G, Kang J, Yalcin B, Cakmak M, Rosenthal KS, Grundfest-Broniatowski S, Kennedy JP, A novel macroencapsulating immunoisolatory device: The preparation and properties of nanomat-reinforced amphiphilic co-networks deposited on perforated metal scaffold, Biomed. Microdevices, 11 (2008) 297–312. [DOI] [PubMed] [Google Scholar]
- [137].Grundfest-Broniatowski SF, Tellioglu G, Rosenthal KS, Kang J, Erdodi G, Yalcin B, Cakmak M, Drazba J, Bennett A, Lu L, Kennedy JP, A new bioartificial pancreas utilizing amphiphilic membranes for the immunoisolation of porcine islets, ASAIO J, 55 (2009) 400–405. [DOI] [PubMed] [Google Scholar]
- [138].Chae SY, Kim YY, Kim SW, Bae YH, Prolonged glucose normalization of streptozotocin-induced diabetic mice by transplantation of rat islets coencapsulated with crosslinked hemoglobin, Transplant. Proc, 78 (2004) 392–397. [DOI] [PubMed] [Google Scholar]
- [139].Nadithe V, Mishra D, Bae YH, Poly(ethylene glycol) cross-linked hemoglobin with antioxidant enzymes protects pancreatic islets from hypoxic and free radical stress and extends islet functionality, Biotechnol. Bioeng, 109 (2012) 2392–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Devineau S, Kiger L, Galacteros F, Baudin-Creuza V, Marden M, Renault JP, Pin S, Manipulating hemoglobin oxygenation using silica nanoparticles: A novel prospect for artificial oxygen carriers, Blood Advances, 2 (2018) 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Piras AM, Dessy A, Chiellini F, Chiellini E, Farina C, Ramelli M, Della Valle E, Polymeric nanoparticles for hemoglobin-based oxygen carriers, Biochim. Biophys. Acta, 1784 (2008) 1454–1461. [DOI] [PubMed] [Google Scholar]
- [142].Gholipourmalekabadi M, Zhao S, Harrison BS, Mozafari M, Seifalian AM, Oxygen-generating biomaterials: A new, viable paradigm for tissue engineering?, Trends Biotechnol, 34 (2016) 1010–1021. [DOI] [PubMed] [Google Scholar]
- [143].Tootoonchi MH, Hashempour M, Blackwelder PL, Fraker CA, Manganese oxide particles as cytoprotective, oxygen generating agents, Acta Biomater, 59 (2017) 327–337. [DOI] [PubMed] [Google Scholar]
- [144].McQuilling JP, Arenas-Herrera J, Childers C, Pareta RA, Khanna O, Jiang B, Brey EM, Farney AC, Opara EC, New alginate microcapsule system for angiogenic protein delivery and immunoisolation of islets for transplantation in the rat omentum pouch, Transplant. Proc, 43 (2011) 3262–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL, Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated oxygen-generating biomaterials, Proc. Natl. Acad. Sci. U.S.A, 109 (2012) 4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Coronel MM, Geusz R, Stabler CL, Mitigating hypoxic stress on pancreatic islets via in situ oxygen generation, Biomaterials, 129 (2017) 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Yeh C-S, Wang R, Chang W-C, Shih Y.-h., Synthesis and characterization of stabilized oxygen-releasing CaO2 nanoparticles for bioremediation, J. Environ. Manage, 212 (2018) 17–22. [DOI] [PubMed] [Google Scholar]
- [148].Schlog W, Development of advanced biomaterials for bone tissue engineering, Fakultat fur Chemie und Pharmazie, Ludwig-Maximilians-Universitat Munchen, Munchen, 2012. [Google Scholar]
- [149].Wu H, Avgoustiniatos ES, Swette L, Bonner-Weir S, Weir GC, Colton CK, In situ electrochemical oxygen generation with an immunoisolation device, Ann. N. Y. Acad. Sci, 875 (1999) 105–125. [DOI] [PubMed] [Google Scholar]
- [150].Klein D, Ribeiro MM, Mendoza V, Jayaraman S, Kenyon NS, Pileggi A, Molano RD, Inverardi L, Ricordi C, Pastori RL, Delivery of Bcl-XL or its BH4 domain by protein transduction inhibits apoptosis in human islets, Biochem. Biophys. Res. Commun, 323 (2004) 473–478. [DOI] [PubMed] [Google Scholar]
- [151].Mendoza V, Klein D, Ichii H, Ribeiro MM, Ricordi C, Hankeln T, Burmester T, Pastori RL, Protection of islets in culture by delivery of oxygen binding neuroglobin via protein transduction, Transplant. Proc, 37 (2005) 237–240. [DOI] [PubMed] [Google Scholar]
- [152].Stagner JI, Seelan RS, Parthasarathy RN, White K, Reduction of ischemic cell death in cultured islets of Langerhans by the induction of cytoglobin, Islets, 1 (2009) 50–54. [DOI] [PubMed] [Google Scholar]
- [153].Lo JF, Wang Y, Blake A, Yu G, Harvat T, Jeon H, Oberholzer J, Eddington DT, Islet preconditioning via multimodal microfluidic modulation of intermittent hypoxia, Anal. Chem, 84 (2012) 1987–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Bloch K, Bloch O, Tarasenko I, Lazard D, Rapoport M, Vardi P, A strategy for the engineering of insulin producing cells with a broad spectrum of defense properties, Biomaterials, 32 (2011) 1816–1825. [DOI] [PubMed] [Google Scholar]
- [155].Bloch K, Papismedov E, Yavriyants K, Vorobeychik M, Beer S, Vardi P, Photosynthetic oxygen generator for bioartificial pancreas, Tissue Eng, 12 (2006) 337–344. [DOI] [PubMed] [Google Scholar]
- [156].Bloch K, Papismedov E, Yavriyants K, Vorobeychik M, Beer S, Vardi P, Immobilized microalgal cells as an oxygen supply system for encapsulated pancreatic islets: A feasibility study, Artif. Organs, 30 (2006) 715–718. [DOI] [PubMed] [Google Scholar]
- [157].Barkai U, Weir GC, Colton CK, Ludwig B, Bornstein SR, Brendel MD, Neufeld T, Bremer C, Leon A, Evron Y, Yavriyants K, Azarov D, Zimermann B, Maimon S, Shabtay N, Balyura M, Rozenshtein T, Vardi P, Bloch K, de Vos P, Rotem A, Enhanced oxygen supply improves islet viability in a new bioartificial pancreas, Cell Transplant., 22 (2013) 1463–1476. [DOI] [PubMed] [Google Scholar]
- [158].Lazard D, Vardi P, Bloch K, Induction of beta-cell resistance to hypoxia and technologies for oxygen delivery to transplanted pancreatic islets, Diabetes Metab. Res. Rev, 28 (2012) 475–484. [DOI] [PubMed] [Google Scholar]
- [159].Rickels MR, Schutta MH, Markmann JF, Barker CF, Naji A, Teff KL, β-cell function following human islet transplantation for type 1 diabetes, Diabetes, 54 (2005) 100–106. [DOI] [PubMed] [Google Scholar]
- [160].Lavallard V, Armanet M, Parnaud G, Meyer J, Barbieux C, Montanari E, Meier R, Morel P, Berney T, Bosco D, Cell rearrangement in transplanted human islets, FASEB J, 30 (2016) 748–760. [DOI] [PubMed] [Google Scholar]
- [161].Olaniru OE, Pingitore A, Giera S, Piao X, Castanera Gonzalez R, Jones PM, Persaud SJ, The adhesion receptor GPR56 is activated by extracellular matrix collagen III to improve β-cell function, Cell. Mol. Life Sci, 75 (2018) 4007–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Guruswamy Damodaran R, Vermette P, Decellularized pancreas as a native extracellular matrix scaffold for pancreatic islet seeding and culture, J. Tissue Eng. Regen. Med, 12 (2018) 1230–1237. [DOI] [PubMed] [Google Scholar]
- [163].Napierala H, Hillebrandt KH, Haep N, Tang P, Tintemann M, Gassner J, Noesser M, Everwien H, Seiffert N, Kluge M, Teegen E, Polenz D, Lippert S, Geisel D, Reutzel Selke A, Raschzok N, Andreou A, Pratschke J, Sauer IM, Struecker B, Engineering an endocrine neo-pancreas by repopulation of a decellularized rat pancreas with islets of Langerhans, Sci. Rep, 7 (2017) 41777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Wu D, Wan J, Huang Y, Guo Y, Xu T, Zhu M, Fan X, Zhu S, Ling C, Li X, Lu J, Zhu H, Zhou P, Lu Y, Wang Z, 3D culture of MIN-6 cells on decellularized pancreatic scaffold: in vitro and in vivo study, Biomed. Res. Int, 2015 (2015) 432645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, Balasubramani M, Johnson SA, Sicari BM, Kollar E, Badylak SF, Banerjee I, Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering, Biomaterials, 34 (2013) 6760–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Mirmalek-Sani SH, Orlando G, McQuilling JP, Pareta R, Mack DL, Salvatori M, Farney AC, Stratta RJ, Atala A, Opara EC, Soker S, Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering, Biomaterials, 34 (2013) 5488–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Narayanan K, Lim VY, Shen J, Tan ZW, Rajendran D, Luo SC, Gao S, Wan AC, Ying JY, Extracellular matrix-mediated differentiation of human embryonic stem cells: Differentiation to insulin-secreting beta cells, Tissue Eng. Part A, 20 (2014) 424–433. [DOI] [PubMed] [Google Scholar]
- [168].Sackett SD, Tremmel DM, Ma F, Feeney AK, Maguire RM, Brown ME, Zhou Y, Li X, O’Brien C, Li L, Burlingham WJ, Odorico JS, Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas, Sci. Rep, 8 (2018) 10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Jiang K, Chaimov D, Patel SN, Liang JP, Wiggins SC, Samojlik MM, Rubiano A, Simmons CS, Stabler CL, 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture, Biomaterials, (2018). 10.1016/j.biomaterials.2018.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Xu T, Zhu M, Guo Y, Wu D, Huang Y, Fan X, Zhu S, Lin C, Li X, Lu J, Zhu H, Zhou P, Lu Y, Wang Z, Three-dimensional culture of mouse pancreatic islet on a liver-derived perfusiondecellularized bioscaffold for potential clinical application, J. Biomater. Appl, 30 (2015) 379–387. [DOI] [PubMed] [Google Scholar]
- [171].De C, Pancreatic acellular matrix supports islet survival and function in a synthetic tubular device: in vitro and in vivo studies, Int. J. Mol. Med, 25 (2009) 195–202. [PubMed] [Google Scholar]
- [172].Wang X, Wang K, Zhang W, Qiang M, Luo Y, A bilaminated decellularized scaffold for islet transplantation: Structure, properties and functions in diabetic mice, Biomaterials, 138 (2017) 80–90. [DOI] [PubMed] [Google Scholar]
- [173].Peloso A, Urbani L, Cravedi P, Katari R, Maghsoudlou P, Fallas ME, Sordi V, Citro A, Purroy C, Niu G, McQuilling JP, Sittadjody S, Farney AC, Iskandar SS, Zambon JP, Rogers J, Stratta RJ, Opara EC, Piemonti L, Furdui CM, Soker S, De Coppi P, Orlando G, The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas, Ann. Surg, 264 (2016) 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Chaimov D, Baruch L, Krishtul S, Meivar-Levy I, Ferber S, Machluf M, Innovative encapsulation platform based on pancreatic extracellular matrix achieve substantial insulin delivery, J. Controlled Release, 257 (2017) 91–101. [DOI] [PubMed] [Google Scholar]
- [175].Vlahos AE, Cober N, Sefton MV, Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets, Proc. Natl. Acad. Sci. U.S.A, 114 (2017) 9337–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Yap WT, Salvay DM, Silliman MA, Zhang X, Bannon ZG, Kaufman DB, Lowe WL Jr., Shea LD, Collagen IV-modified scaffolds improve islet survival and function and reduce time to euglycemia, Tissue Eng. Part A, 19 (2013) 2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Salvay DM, Rives CB, Zhang X, Chen F, Kaufman DB, Lowe WL Jr., Shea LD, Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation, Transplantation, 85 (2008) 1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Krishnamurthy M, Li J, Fellows GF, Rosenberg L, Goodyer CG, Wang R, Integrin alpha3, but not beta1, regulates islet cell survival and function via PI3K/Akt signaling pathways, Endocrinology, 152 (2011) 424–435. [DOI] [PubMed] [Google Scholar]
- [179].Kaido T, Yebra M, Cirulli V, Montgomery AM, Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1, J. Biol. Chem, 279 (2004) 53762–53769. [DOI] [PubMed] [Google Scholar]
- [180].Marchioli G, van Gurp L, van Krieken PP, Stamatialis D, Engelse M, van Blitterswijk CA, Karperien MB, de Koning E, Alblas J, Moroni L, van Apeldoorn AA, Fabrication of three-dimensional bioplotted hydrogel scaffolds for islets of Langerhans transplantation, Biofabrication, 7 (2015) 025009. [DOI] [PubMed] [Google Scholar]
- [181].Phelps EA, Cianciaruso C, Santo-Domingo J, Pasquier M, Galliverti G, Piemonti L, Berishvili E, Burri O, Wiederkehr A, Hubbell JA, Baekkeskov S, Advances in pancreatic islet monolayer culture on glass surfaces enable super-resolution microscopy and insights into beta cell ciliogenesis and proliferation, Sci. Rep, 7 (2017) 45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Stephens CH, Orr KS, Acton AJ, Tersey SA, Mirmira RG, Considine RV, Voytik-Harbin SL, In situ type I oligomeric collagen macroencapsulation promotes islet longevity and function in vitro and in vivo, American Journal of Physiology-Endocrinology and Metabolism, 315 (2018) E650–E661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Bernard AB, Chapman RZ, Anseth KS, Controlled local presentation of matrix proteins in microparticle-laden cell aggregates, Biotechnol. Bioeng, 111 (2014) 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Lim DJ, Antipenko SV, Vines JB, Andukuri A, Hwang PT, Hadley NT, Rahman SM, Corbett JA, Jun HW, Improved MIN6 beta-cell function on self-assembled peptide amphiphile nanomatrix inscribed with extracellular matrix-derived cell adhesive ligands, Macromol. Biosci, 13 (2013) 1404–1412. [DOI] [PubMed] [Google Scholar]
- [185].Lin CC, Anseth KS, Cell-cell communication mimicry with poly(ethylene glycol) hydrogels for enhancing beta-cell function, Proc. Natl. Acad. Sci. U.S.A, 108 (2011) 6380–6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Li W, Lee S, Ma M, Kim SM, Guye P, Pancoast JR, Anderson DG, Weiss R, Lee RT, Hammond PT, Microbead-based biomimetic synthetic neighbors enhance survival and function of rat pancreatic beta-cells, Sci. Rep, 3 (2013) 2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Chen W, Zhang Q, Luk BT, Fang RH, Liu Y, Gao W, Zhang L, Coating nanofiber scaffolds with beta cell membrane to promote cell proliferation and function, Nanoscale, 8 (2016) 10364–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Zhang Y, Jalili RB, Warnock GL, Ao Z, Marzban L, Ghahary A, Three-dimensional scaffolds reduce islet amyloid formation and enhance survival and function of cultured human islets, American Journal Pathology, 181 (2012) 1296–1305. [DOI] [PubMed] [Google Scholar]
- [189].Beenken-Rothkopf LN, Karfeld-Sulzer LS, Davis NE, Forster R, Barron AE, Fontaine MJ, The incorporation of extracellular matrix proteins in protein polymer hydrogels to improve encapsulated beta-cell function, Ann. Clin. Lab. Sci, 43 (2013) 111–121. [PubMed] [Google Scholar]
- [190].MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB, The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion, Diabetes, 51 Suppl 3 (2002) S434–442. [DOI] [PubMed] [Google Scholar]
- [191].Marathe CS, Rayner CK, Jones KL, Horowitz M, Glucagon-like peptides 1 and 2 in health and disease: A review, Peptides, 44 (2013) 75–86. [DOI] [PubMed] [Google Scholar]
- [192].Jones B, Bloom SR, Buenaventura T, Tomas A, Rutter GA, Control of insulin secretion by GLP-1, Peptides, 100 (2018) 75–84. [DOI] [PubMed] [Google Scholar]
- [193].Vilsbøll T, The effects of glucagon-like peptide-1 on the beta cell, Diabetes Obes. Metab, 11 (2009) 11–18. [DOI] [PubMed] [Google Scholar]
- [194].Urusova IA, Farilla L, Hui H, D’Amico E, Perfetti R, GLP-1 inhibition of pancreatic islet cell apoptosis, Trends Endocrinol. Metab, 15 (2004) 27–33. [DOI] [PubMed] [Google Scholar]
- [195].Lin CC, Anseth KS, Glucagon-like peptide-1 functionalized PEG hydrogels promote survival and function of encapsulated pancreatic beta-cells, Biomacromolecules, 10 (2009) 2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Petrik J, Arany E, McDonald TJ, Hill DJ, Apoptosis in the pancreatic islet cells of the neonatal rat is associated with a reduced expression of insulin-like growth factor II that may act as a survival factor, Endocrinology, 139 (1998) 2994–3004. [DOI] [PubMed] [Google Scholar]
- [197].Phillips BW, Hentze H, Rust WL, Chen QP, Chipperfield H, Tan EK, Abraham S, Sadasivam A, Soong PL, Wang ST, Lim R, Sun W, Colman A, Dunn NR, Directed differentiation of human embryonic stem cells into the pancreatic endocrine lineage, Stem Cells Dev., 16 (2007) 561–578. [DOI] [PubMed] [Google Scholar]
- [198].Forget A, Waibel M, Rojas-Canales DM, Chen S, Kawazoe N, Harding FJ, Loudovaris T, Coates PTH, Blencowe A, Chen G, Voelcker NH, IGF-2 coated porous collagen microwells for the culture of pancreatic islets, J. Mater. Chem. B, 5 (2017) 220–225. [DOI] [PubMed] [Google Scholar]
- [199].Bal T, Nazli C, Okcu A, Duruksu G, Karaoz E, Kizilel S, Mesenchymal stem cells and ligand incorporation in biomimetic poly(ethylene glycol) hydrogels significantly improve insulin secretion from pancreatic islets, J. Tissue Eng. Regen. Med, 11 (2017) 694–703. [DOI] [PubMed] [Google Scholar]
- [200].Ludwig B, Ziegler CG, Schally AV, Richter C, Steffen A, Jabs N, Funk RH, Brendel MD, Block NL, Ehrhart-Bornstein M, Bornstein SR, Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets, Proc. Natl. Acad. Sci. U.S.A, 107 (2010) 12623–12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Sophia Fox AJ, Bedi A, Rodeo SA, The basic science of articular cartilage: Structure, composition, and function, Sports Health, 1 (2009) 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Banik BL, Bowers DT, Fattahi P, Brown JL, Bio-instructive scaffolds for musculoskeletal interfaces, in: Brown JL, Kumbar SG, Banik BL (Eds.) Bio-instructive scaffolds for musculoskeletal tissue engineering and regenerative medicine, Academic Press; 2017, pp. 203–233. [Google Scholar]
- [203].Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E, The vascular basement membrane: A niche for insulin gene expression and beta cell proliferation, Dev. Cell, 10 (2006) 397–405. [DOI] [PubMed] [Google Scholar]
- [204].Fowler MJ, Microvascular and macrovascular complications of diabetes, Clin. Diabetes, 26 (2008) 77–82. [Google Scholar]
- [205].Bowers DT, Botchwey EA, Brayman KL, Advances in local drug release and scaffolding design to enhance cell therapy for diabetes, Tissue Eng. Part B Rev, 21 (2015) 491–503. [DOI] [PubMed] [Google Scholar]
- [206].Oliver Cassell CS, Stefan Hofer OP, Morrison WA, Knight KR, Vascularisation of tissue-engineered grafts: The regulation of angiogenesis in reconstructive surgery and in disease states, Br. J. Plast. Surg, 55 (2002) 603–610. [DOI] [PubMed] [Google Scholar]
- [207].Briquez PS, Clegg LE, Martino MM, Gabhann FM, Hubbell JA, Design principles for therapeutic angiogenic materials, Nat. Rev. Mater, 1 (2016) 1–15. [Google Scholar]
- [208].Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, Torio-Padron N, Schramm R, Rucker M, Junker D, Haufel JM, Carvalho C, Heberer M, Germann G, Vollmar B, Menger MD, Angiogenesis in tissue engineering: Breathing life into constructed tissue substitutes, Tissue Eng, 12 (2006) 2093–2104. [DOI] [PubMed] [Google Scholar]
- [209].Phelps EA, Garcia AJ, Engineering more than a cell: Vascularization strategies in tissue engineering, Curr. Opin. Biotechnol, 21 (2010) 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Xu C, Yang F, Wang S, Ramakrishna S, In vitro study of human vascular endothelial cell function on materials with various surface roughness, Journal of Biomedical Research Part A, 71 (2004) 154–161. [DOI] [PubMed] [Google Scholar]
- [211].Chong DS, Turner LA, Gadegaard N, Seifalian AM, Dalby MJ, Hamilton G, Nanotopography and plasma treatment: Redesigning the surface for vascular graft endothelialisation, Eur. J. Vasc. Endovasc. Surg, 49 (2015) 335–343. [DOI] [PubMed] [Google Scholar]
- [212].Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC, Neovascularization of synthetic membranes directed by membrane microarchitecture, J. Biomed. Mater. Res, 29 (1995) 1517–1524. [DOI] [PubMed] [Google Scholar]
- [213].Pedraza E, Brady AC, Fraker CA, Molano RD, Sukert S, Berman DM, Kenyon NS, Pileggi A, Ricordi C, Stabler CL, Macroporous three-dimensional PDMS scaffolds for extrahepatic islet transplantation, Cell Transplant., 22 (2013) 1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Cabric S, Sanchez J, Johansson U, Larsson R, Nilsson B, Korsgren O, Magnusson PU, Anchoring of vascular endothelial growth factor to surface-immobilized heparin on pancreatic islets: Implications for stimulating islet angiogenesis, Tissue Eng. Part A, 16 (2010) 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Phelps EA, Templeman KL, Thule PM, Garcia AJ, Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization, Drug Deliv. Transl. Res, 5 (2015) 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Weaver JD, Headen DM, Aquart J, Johnson CT, Shea LD, Shirwan H, Garcia AJ, Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites, Science Advances, 3 (2017) e1700184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA, Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth, FASEB J, 17 (2003) 2260–2262. [DOI] [PubMed] [Google Scholar]
- [218].Vernon RB, Preisinger A, Gooden MD, D’Amico LA, Yue BB, Bollyky PL, Kuhr CS, Hefty TR, Nepom GT, Gebe JA, Reversal of diabetes in mice with a bioengineered islet implant incorporating a type I collagen hydrogel and sustained release of vascular endothelial growth factor, Cell Transplant., 21 (2012) 2099–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Sheridan MH, Shea LD, Peters MC, Mooney DJ, Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery, J. Controlled Release, 64 (2000) 91–102. [DOI] [PubMed] [Google Scholar]
- [220].Linn T, Erb D, Schneider D, Kidszun A, Elcin AE, Bretzel RG, Elcin YM, Polymers for induction of revascularization in the rat fascial flap: Application of vascular endothelial growth factor and pancreatic islet cells, Cell Transplant, 12 (2003) 769–778. [DOI] [PubMed] [Google Scholar]
- [221].Trivedi N, Steil GM, Colton CK, Bonner-Weir S, Weir GC, Improved vascularization of planar membrane diffusion devices following continuous infusion of vascular endothelial growth factor, Cell Transplant, 9 (2000) 115–124. [DOI] [PubMed] [Google Scholar]
- [222].Brady AC, Martino MM, Pedraza E, Sukert S, Pileggi A, Ricordi C, Hubbell JA, Stabler CL, Proangiogenic hydrogels within macroporous scaffolds enhance islet engraftment in an extrahepatic site, Tissue Eng. Part A, 19 (2013) 2544–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Balamurugan AN, Gu Y, Tabata Y, Miyamoto M, Cui W, Hori H, Satake A, Nagata N, Wang W, Inoue K, Bioartificial pancreas transplantation at prevascularized intermuscular space: Effect of angiogenesis induction on islet survival, Pancreas, 26 (2003) 279–285. [DOI] [PubMed] [Google Scholar]
- [224].Chow LW, Wang LJ, Kaufman DB, Stupp SI, Self-assembling nanostructures to deliver angiogenic factors to pancreatic islets, Biomaterials, 31 (2010) 6154–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Hwa AJ, Weir GC, Transplantation of macroencapsulated insulin-producing cells, Curr. Diab. Rep, 18 (2018) 50. [DOI] [PubMed] [Google Scholar]
- [226].Yi L, Wang X, Dhumpa R, Schrell AM, Mukhitov N, Roper MG, Integrated perfusion and separation systems for entrainment of insulin secretion from islets of Langerhans, Lab Chip, 15 (2015) 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Geron E, Boura-Halfon S, Schejter ED, Shilo BZ, The edges of pancreatic islet beta cells constitute adhesive and signaling microdomains, Cell Rep, 10 (2015) 317–325. [DOI] [PubMed] [Google Scholar]
- [228].de Vargas LM, Sobolewski J, Siegel R, Moss LG, Individual beta cells within the intact islet differentially respond to glucose, J. Biol. Chem, 272 (1997) 26573–26577. [DOI] [PubMed] [Google Scholar]
- [229].Benninger RK, Head WS, Zhang M, Satin LS, Piston DW, Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet, J. Physiol. (Lond.), 589 (2011) 5453–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Benninger RKP, Hodson DJ, New understanding of beta-cell heterogeneity and in situ islet function, Diabetes, 67 (2018) 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Bisceglie VV, Uber die antineoplastiche immunitat, Krebsforsch, 40 (1933) 141–158. [Google Scholar]
- [232].Algire GH, Legallais FY, Recent developments in the transparent-chamber technique as adapted to the mouse, J. Natl. Cancer Inst, 10 (1949) 225–253. [PubMed] [Google Scholar]
- [233].Algire GH, Weaver JM, Prehn RT, Growth of cells in vivo in diffusion chambers. I. Survival of homografts in immunized mice, J. Natl. Cancer Inst, 15 (1954) 493–507. [PubMed] [Google Scholar]
- [234].Algire GH, Weaver JM, Prehn RT, Studies on tissue homotransplantation in mice, using diffusion-chamber methods, Ann. N. Y. Acad. Sci, 64 (1957) 1009–1013. [DOI] [PubMed] [Google Scholar]
- [235].Prehn RT, Weaver JM, Algire GH, The diffusion-chamber technique applied to a study of the nature of homograft resistance, J. Natl. Cancer Inst, 15 (1954) 509–517. [PubMed] [Google Scholar]
- [236].Ben-David U, Benvenisty N, The tumorigenicity of human embryonic and induced pluripotent stem cells, Nat. Rev. Cancer, 11 (2011) 268. [DOI] [PubMed] [Google Scholar]
- [237].Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR, Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies, Stem Cell Res., 2 (2009) 198–210. [DOI] [PubMed] [Google Scholar]
- [238].Suzuki K, Bonner-Weir S, Trivedi N, Yoon K-H, Hollister-Lock J, Colton CK, Weir GC, Function and survival of macroencapsulated syngeneic islets transplanted into streptozocin-diabetic mice, Transplantation, 66 (1998) 21–28. [DOI] [PubMed] [Google Scholar]
- [239].Rafael E, Wu GS, Hultenby K, Tibell A, Wernerson A, Improved survival of macroencapsulated islets of Langerhans by preimplantation of the immunoisolating device: A morphometric study, 12 (2003) 407–412. [DOI] [PubMed] [Google Scholar]
- [240].Sorenby AK, Kumagai-Braesch M, Sharma A, Hultenby KR, Wernerson AM, Tibell AB, Preimplantation of an immunoprotective device can lower the curative dose of islets to that of free islet transplantation: Studies in a rodent model, Transplantation, 86 (2008) 364–366. [DOI] [PubMed] [Google Scholar]
- [241].Rafael E, Gazelius B, Wu GS, Tibell A, Longitudinal studies on the microcirculation around the Theracyte™ immunoisolation device, using the laser doppler technique, Cell Transplant, 9 (2000) 107–113. [DOI] [PubMed] [Google Scholar]
- [242].Kumagai-Braesch M, Jacobson S, Mori H, Jia X, Takahashi T, Wernerson A, Flodstrom-Tullberg M, Tibell A, The Theracyte device protects against islet allograft rejection in immunized hosts, Cell Transplant, 22 (2013) 1137–1146. [DOI] [PubMed] [Google Scholar]
- [243].Bruin JE, Rezania A, Xu J, Narayan K, Fox JK, O’Neil JJ, Kieffer TJ, Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice, Diabetologia, 56 (2013) 1987–1998. [DOI] [PubMed] [Google Scholar]
- [244].Gabr MM, Zakaria MM, Refaie AF, Ismail AM, Khater SM, Ashamallah SA, Azzam MM, Ghoneim MA, Insulin-producing cells from adult human bone marrow mesenchymal stromal cells could control chemically induced diabetes in dogs: A preliminary study, Cell Transplant., 27 (2018) 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Farina M, Alexander JF, Thekkedath U, Ferrari M, Grattoni A, Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond, Adv. Drug Del. Rev, (2018). [DOI] [PubMed] [Google Scholar]
- [246].Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, Kelly JR, Haakmeester C, Srijemac R, Wilson AZ, Kerr J, Frazier MA, Kroon EJ, D’Amour KA, Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo, Stem Cells Translational Medicine, 4 (2015) 1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Schweicher J, Nyitray C, Desai TA, Membranes to achieve immunoprotection of transplanted islets, Fronteirs in Bioscience, 19 (2014) 49–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Song S, Roy S, Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: Cells, biomaterials, and devices, Biotechnol Bioeng, 113 (2016) 1381–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Chang R, Faleo G, Russ HA, Parent AV, Elledge SK, Bernards DA, Allen JL, Villanueva K, Hebrok M, Tang Q, Desai TA, Nanoporous immunoprotective device for stem-cell derived beta-cell replacement therapy, ACS Nano, 11 (2017) 7747–7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Greene LE, Law M, Tan DH, Montano M, Goldberger J, Somorjai G, Yang P, General route to vertical ZnO nanowire arrays using textured ZnO seeds, Nano Lett, 5 (2005) 1231–1236. [DOI] [PubMed] [Google Scholar]
- [251].Nyitray CE, Chang R, Faleo G, Lance KD, Bernards DA, Tang Q, Desai TA, Polycaprolactone thin-film micro-and nanoporous cell-encapsulation devices, ACS Nano, 9 (2015) 5675–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, Brendel MD, Neufeld T, Block NL, Yavriyants K, Steffen A, Ludwig S, Chavakis T, Reichel A, Azarov D, Zimermann B, Maimon S, Balyura M, Rozenshtein T, Shabtay N, Vardi P, Bloch K, de Vos P, Schally AV, Bornstein SR, Barkai U, Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist, Proc. Natl. Acad. Sci. U.S.A, 109 (2012) 5022–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Carlsson PO, Espes D, Sedigh A, Rotem A, Zimerman B, Grinberg H, Goldman T, Barkai U, Avni Y, Westermark GT, Carlbom L, Ahlstrom H, Eriksson O, Olerud J, Korsgren O, Transplantation of macroencapsulated human islets within the bioartificial pancreas βair to patients with type 1 diabetes mellitus, Am. J. Transplantation, 18 (2018) 1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, Philips R, Safety and viability of microencapsulated human islets transplanted into diabetic humans, Diabetes Care, 32 (2009) 1887–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Weir GC, Islet encapsulation: Advances and obstacles, Diabetologia, 56 (2013) 1458–1461. [DOI] [PubMed] [Google Scholar]
- [256].Storrs R, Dorian R, King SR, Lakey JRT, Rilo H, Preclinical development of the islet sheet, Ann. N. Y. Acad. Sci, 944 (2001) 252–266. [DOI] [PubMed] [Google Scholar]
- [257].Lacy PE, Hegre OD, Gerasimidi-Vazeou A, Gentile FT, Dionne KE, Maintenance of normoglycemia in diabetic mice by subcutaneous xenografts of encapsulated islets, Science, 254 (1991) 1782–1784. [DOI] [PubMed] [Google Scholar]
- [258].An D, Chiu A, Flanders JA, Song W, Shou D, Lu YC, Grunnet LG, Winkel L, Ingvorsen C, Christophersen NS, Fels JJ, Sand FW, Ji Y, Qi L, Pardo Y, Luo D, Silberstein M, Fan J, Ma M, Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes, Proc. Natl. Acad. Sci. U.S.A, 115 (2018) E263–E272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Zheng Y, Bai H, Huang Z, Tian X, Nie FQ, Zhao Y, Zhai J, Jiang L, Directional water collection on wetted spider silk, Nature, 463 (2010) 640–643. [DOI] [PubMed] [Google Scholar]
- [260].Washburn EW, The dynamics of capillary flow, Phys. Rev, 17 (1921) 273–283. [Google Scholar]
- [261].Mei Y, Liu J, Wu R, Xia R, Capillarity-induced mechanical behaviors of a polymer microtube surrounded by a droplet, AIP Adv., 4 (2014) 127128 10.1063/1.4904364 [DOI] [Google Scholar]
- [262].An D, Ji Y, Chiu A, Lu YC, Song W, Zhai L, Qi L, Luo D, Ma M, Developing robust, hydrogel-based, nanofiber-enabled encapsulation devices (NEEDs) for cell therapies, Biomaterials, 37 (2015) 40–48. [DOI] [PubMed] [Google Scholar]
- [263].Senthamizhan A, Balusamy B, Uyar T, 1 - Electrospinning: A versatile processing technology for producing nanofibrous materials for biomedical and tissue-engineering applications, in: Uyar T, Kny E (Eds.) Electrospun materials for tissue engineering and biomedical applications, Woodhead Publishing; 2017, pp. 3–41. [Google Scholar]
- [264].Braghirolli DI, Steffens D, Pranke P, Electrospinning for regenerative medicine: A review of the main topics, Drug Discov. Today, 19 (2014) 743–753. [DOI] [PubMed] [Google Scholar]
- [265].Boudriot U, Dersch R, Greiner A, Wendorff JH, Electrospinning approaches toward scaffold engineering—a brief overview, Artif. Organs, 30 (2006) 785–792. [DOI] [PubMed] [Google Scholar]
- [266].Fine D, Grattoni A, Hosali S, Ziemys A, De Rosa E, Gill J, Medema R, Hudson L, Kojic M, Milosevic M, Brousseau Iii L, Goodall R, Ferrari M, Liu X, A robust nanofluidic membrane with tunable zero-order release for implantable dose specific drug delivery, Lab Chip, 10 (2010) 3074–3083. [DOI] [PubMed] [Google Scholar]
- [267].Sabek OM, Ferrati S, Fraga DW, Sih J, Zabre EV, Fine DH, Ferrari M, Gaber AO, Grattoni A, Characterization of a nanogland for the autotransplantation of human pancreatic islets, Lab Chip, 13 (2013) 3675–3688. [DOI] [PubMed] [Google Scholar]
- [268].Song S, Faleo G, Yeung R, Kant R, Posselt AM, Desai TA, Tang Q, Roy S, Silicon nanopore membrane (SNM) for islet encapsulation and immunoisolation under convective transport, Sci Rep, 6 (2016) 23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Ulery BD, Nair LS, Laurencin CT, Biomedical applications of biodegradable polymers, J. Polym. Sci., Part B: Polym. Phys, 49 (2011) 832–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Piconi C, Porporati AA, Bioinert ceramics: Zirconia and alumina, in: Antoniac IV (Ed.) Handbook of bioceramics and biocomposites, Springer International Publishing, Cham, 2016, pp. 59–89. [Google Scholar]
- [271].Flamme KEL, Mor G, Gong D, Tempa TL, Fusaro VA, Grimes CA, Desai TA, Nanoporous alumina capsules for cellular macroencapsulation: Transport and biocompatibility, Diabetes Technol. Ther, 7 (2005) 684–694. [DOI] [PubMed] [Google Scholar]
- [272].Mendelsohn A, Desai T, Inorganic nanoporous membranes for immunoisolated cell-based drug delivery, Therapeutic applications of cell microencapsulation, Springer; 2010, pp. 104–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Randall CL, Kalinin YV, Jamal M, Shah A, Gracias DH, Self-folding immunoprotective cell encapsulation devices, Nanomedicine, 7 (2011) 686–689. [DOI] [PubMed] [Google Scholar]
- [274].Kriz J, Vilk G, Mazzuca DM, Toleikis PM, Foster PJ, White DJG, A novel technique for the transplantation of pancreatic islets within a vascularized device into the greater omentum to achieve insulin independence, Am. J. Surg, 203 (2012) 793–797. [DOI] [PubMed] [Google Scholar]
- [275].Pepper AR, Pawlick R, Gala-Lopez B, MacGillivary A, Mazzuca DM, White DJ, Toleikis PM, Shapiro AM, Diabetes is reversed in a murine model by marginal mass syngeneic islet transplantation using a subcutaneous cell pouch device, Transplantation, 99 (2015) 2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Gala-Lopez B, Pepper A, Dinyari P, Malcolm AJ, Kin T, Pawlick LR, Senior PA, Shapiro AMJ, Subcutaneous clinical islet transplantation in a prevascularized subcutaneous pouch—preliminary experience, CellR4, 4 (2016) e2132. [Google Scholar]
- [277].Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM, A prevascularized subcutaneous device-less site for islet and cellular transplantation, Nat. Biotechnol, 33 (2015) 518–523. [DOI] [PubMed] [Google Scholar]
- [278].Lee KY, Mooney DJ, Alginate: Properties and biomedical applications, Prog. Polym. Sci, 37 (2012) 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Elliott RB, Escobar L, Tan PL, Garkavenko O, Calafiore R, Basta P, Vasconcellos AV, Emerich DF, Thanos C, Bambra C, Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates, Transplant. Proc, 37 (2005) 3505–3508. [DOI] [PubMed] [Google Scholar]
- [280].Tam SK, Dusseault J, Bilodeau S, Langlois G, Halle JP, Yahia L, Factors influencing alginate gel biocompatibility, Journal of Biomedical Research Part A, 98 (2011) 40–52. [DOI] [PubMed] [Google Scholar]
- [281].Lamb M, Storrs R, Li S, Liang O, Laugenour K, Dorian R, Chapman D, Ichii H, Imagawa D, Foster C 3rd, King S, Lakey JRT, Function and viability of human islets encapsulated in alginate sheets: in vitro and in vivo culture, Transplant. Proc, 43 (2011) 3265–3266. [DOI] [PubMed] [Google Scholar]
- [282].Omer A, Duvivier-Kali VF, Trivedi N, Wilmot K, Bonner-Weir S, Weir GC, Survival and maturation of microencapsulated porcine neonatal pancreatic cell clusters transplanted into immunocompetent diabetic mice, Diabetes, 52 (2003) 69. [DOI] [PubMed] [Google Scholar]
- [283].Buitinga M, Assen F, Hanegraaf M, Wieringa P, Hilderink J, Moroni L, Truckenmuller R, van Blitterswijk C, Romer GW, Carlotti F, de Koning E, Karperien M, van Apeldoorn A, Microfabricated scaffolds lead to efficient remission of diabetes in mice, Biomaterials, 135 (2017) 10–22. [DOI] [PubMed] [Google Scholar]
- [284].Sabek OM, Farina M, Fraga DW, Afshar S, Ballerini A, Filgueira CS, Thekkedath UR, Grattoni A, Gaber AO, Three-dimensional printed polymeric system to encapsulate human mesenchymal stem cells differentiated into islet-like insulin-producing aggregates for diabetes treatment, J Tissue Eng, 7 (2016) 2041731416638198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Xia Y, Boey F, Venkatraman SS, Surface modification of poly (L-lactic acid) with biomolecules to promote endothelialization, Biointerphases, 5 (2010) FA32–FA40. [DOI] [PubMed] [Google Scholar]
- [286].Rasal RM, Janorkar AV, Hirt DE, Poly (lactic acid) modifications, Prog. Polym. Sci, 35 (2010) 338–356. [Google Scholar]
- [287].Cheng Y, Deng S, Chen P, Ruan R, Polylactic acid (PLA) synthesis and modifications: A review, Frontiers of Chemistry in China, 4 (2009) 259–264. [Google Scholar]
- [288].Farina M, Ballerini A, Fraga DW, Nicolov E, Hogan M, Demarchi D, Scaglione F, Sabek OM, Horner P, Thekkedath U, Gaber OA, Grattoni A, 3D printed vascularized device for subcutaneous transplantation of human islets, Biotechnol. J, 12 (2017) 1700169. [DOI] [PubMed] [Google Scholar]
- [289].Kim BS, Mooney DJ, Development of biocompatible synthetic extracellular matrices for tissue engineering, Trends Biotechnol, 16 (1998) 224–230. [DOI] [PubMed] [Google Scholar]
- [290].Fu S, Thacker A, Sperger DM, Boni RL, Velankar S, Munson EJ, Block LH, Rheological evaluation of inter-grade and inter-batch variability of sodium alginate, AAPS PharmSciTech, 11 (2010) 1662–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Tonnesen HH, Karlsen J, Alginate in drug delivery systems, Drug Dev. Ind. Pharm, 28 (2002) 621–630. [DOI] [PubMed] [Google Scholar]
- [292].Tze WJ, Wong FC, Chen LM, Implantable artificial capillary unit for pancreatic islet allograft and xenograft, Diabetologia, 16 (1979) 247–252. [DOI] [PubMed] [Google Scholar]
- [293].Monaco AP, Maki T, Ozato H, Carretta M, Sullivan SJ, Borland KM, Mahoney MD, Chick WL, Muller TE, Wolfrum J, Transplantation of islet allografts and xenografts in totally pancreatectomized diabetic dogs using the hybrid artificial pancreas, Ann. Surg, 214 (1991) 339–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Qi M, Transplantation of encapsulated pancreatic islets as a treatment for patients with type 1 diabetes mellitus, Adv. Med, 2014 (2014) 429710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Prochorov A, Tretjak S, Goranov V, Glinnik A, Goltsev M, Treatment of insulin dependent diabetes mellitus with intravascular transplantation of pancreatic islet cells without immunosuppressive therapy, Adv. Med. Sci, 53 (2008) 240–244. [DOI] [PubMed] [Google Scholar]
- [296].Song S, Blaha C, Moses W, Park J, Wright N, Groszek J, Fissell W, Vartanian S, Posselt AM, Roy S, An intravascular bioartificial device (iBAP) with silicon nanopore membranes (SNM) for islet encapsulation under convective mass transport, Lab Chip, 17 (2017) 1778–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Calafiore R, Basta G, Clinical application of microencapsulated islets: Actual prospectives on progress and challenges, Adv. Drug Del. Rev, 67–68 (2014) 84–92. [DOI] [PubMed] [Google Scholar]
- [298].Iwata H, Arima Y, Tsutsui Y, Design of bioartificial pancreas from the standpoint of oxygen supply, Artif. Organs, 42 (2018) E168–E185. [DOI] [PubMed] [Google Scholar]
- [299].Chicheportiche D, Reach G, In vitro kinetics of insulin release by microencapsulated rat islets: Effect of the size of the microcapsules, Diabetologia, 31 (1988) 54–57. [DOI] [PubMed] [Google Scholar]
- [300].Wilson JT, Chaikof EL, Challenges and emerging technologies in the immunoisolation of cells and tissues, Adv. Drug Del. Rev, 60 (2008) 124–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Schneider S, Mach M.-A. Von, Kraus O, Kann P, Johannes Feilen P., Intraportal transplantation of allogenic pancreatic islets encapsulated in barium alginate beads in diabetic rats, Artif. Organs, 27 (2003) 1053–1056. [DOI] [PubMed] [Google Scholar]
- [302].Wilson JT, Cui W, Chaikof EL, Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation, Nano Lett, 8 (2008) 1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].de Vos P, de Haan B, Pater J, van Schilfgaarde R, Association between capsule diameter, adequacy of encapsulation, and survival of microencapsulated rat islet allografts, Transplantation, 62 (1996) 893–899. [DOI] [PubMed] [Google Scholar]
- [304].de Vos P, de Haan B, Wolters GH, van Schilfgaarde R, Factors influencing the adequacy of microencapsulation of rat pancreatic islets, Transplantation, 62 (1996) 888–893. [DOI] [PubMed] [Google Scholar]
- [305].Ma M, Chiu A, Sahay G, Doloff JC, Dholakia N, Thakrar R, Cohen J, Vegas A, Chen D, Bratlie KM, Dang T, York RL, Hollister-Lock J, Weir GC, Anderson DG, Core–shell hydrogel microcapsules for improved islets encapsulation, Adv. Healthc. Mater, 2 (2012) 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Onoe H, Okitsu T, Itou A, Kato-Negishi M, Gojo R, Kiriya D, Sato K, Miura S, Iwanaga S, Kuribayashi-Shigetomi K, Matsunaga YT, Shimoyama Y, Takeuchi S, Metre-long cell-laden microfibres exhibit tissue morphologies and functions, Nat. Mater, 12 (2013) 584–590. [DOI] [PubMed] [Google Scholar]
- [307].Safley SA, Gordon KB, Barber GF, Holdcraft RW, Turgeon N, Larsen CP, Gazda L, Weber CJ, Human and porcine islet transplants in a new double capsule, J. Surg. Res, 179 (2013) 264–265. [Google Scholar]
- [308].Safley S, Barber GF, Duncanson SM, Holdcraft RW, Gazda LS, Poznansky MC, Sambanis A, Weber CJ, CXCL12-delivery by double microcapsules and dual costimulatory blockade synergistically prolong the survival of porcine islet xenografts in diabetic NOD mice, Frontiers in Bioengineering and Biotechnology Conference Abstract: 10th World Biomaterials Congress, (2016). [Google Scholar]
- [309].Lu Y-C, Song W, An D, Kim BJ, Schwartz R, Wu M, Ma M, Designing compartmentalized hydrogel microparticles for cell encapsulation and scalable 3D cell culture, J. Mater. Chem. B, 3 (2015) 353–360. [DOI] [PubMed] [Google Scholar]
- [310].Bennet W, Groth C-G, Larsson R, Nilsson B, Korsgren O, Isolated human islets trigger an instant blood mediated inflammatory reaction: Implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes, Ups. J. Med. Sci, 105 (2000) 125–133. [DOI] [PubMed] [Google Scholar]
- [311].Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF, Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation, Am. J. Transplantation, 14 (2014) 428–437. [DOI] [PubMed] [Google Scholar]
- [312].Gibly RF, Graham JG, Luo X, Lowe WL Jr., Hering BJ, Shea LD, Advancing islet transplantation: From engraftment to the immune response, Diabetologia, 54 (2011) 2494–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Barshes NR, Wyllie S, Goss JA, Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: Implications for intrahepatic grafts, J. Leukocyte Biol, 77 (2005) 587–597. [DOI] [PubMed] [Google Scholar]
- [314].Suszynski TM, Avgoustiniatos ES, Papas KK, Oxygenation of the intraportally transplanted pancreatic islet, J. Diabetes Res, 2016 (2016) 7625947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Zhi Z-L, Khan F, Pickup JC, Multilayer nanoencapsulation: A nanomedicine technology for diabetes research and management, Diabetes Res. Clin. Pract, 100 (2013) 162–169. [DOI] [PubMed] [Google Scholar]
- [316].Lim F, Sun AM, Microencapsulated islets as bioartifical endocrine pancreas, Science, 210 (1980) 908–910. [DOI] [PubMed] [Google Scholar]
- [317].An D, Warning A, Yancey KG, Chang C-T, Kern VR, Datta AK, Steen PH, Luo D, Ma M, Mass production of shaped particles through vortex ring freezing, Nat. Commun, 7 (2016) 12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Thanos CG, Bintz BE, Bell WJ, Qian H, Schneider PA, MacArthur DH, Emerich DF, Intraperitoneal stability of alginate-polyornithine microcapsules in rats: An FTIR and SEM analysis, Biomaterials, 27 (2006) 3570–3579. [DOI] [PubMed] [Google Scholar]
- [319].Hoesli CA, Raghuram K, Kiang RL, Mocinecová D, Hu X, Johnson JD, Lacík I, Kieffer TJ, Piret JM, Pancreatic cell immobilization in alginate beads produced by emulsion and internal gelation, Biotechnol. Bioeng, 108 (2010) 424–434. [DOI] [PubMed] [Google Scholar]
- [320].Markwick KE, Dussault MA, Bégin-Drolet A, Hoesli CA, Microchannel emulsification: A novel approach to cell encapsulation, XXIV International Conference on Bioencapsulation, Bioencapsulation Research Group , Lisbon, Portugal, 2016, pp. 12–13. [Google Scholar]
- [321].Sefton MV, Hwang JR, Babensee JE, Selected aspects of the microencapsulation of mammalian cells in HEMA-MAA, Ann. N. Y. Acad. Sci, 831 (2006) 260–270. [DOI] [PubMed] [Google Scholar]
- [322].Abraham S, Jeong EH, Arakawa T, Shoji S, Kim KC, Kim I, Go JS, Microfluidics assisted synthesis of well-defined spherical polymeric microcapsules and their utilization as potential encapsulants, Lab Chip, 6 (2006) 752–756. [DOI] [PubMed] [Google Scholar]
- [323].Sharma V, Hunckler M, Ramasubramanian MK, Opara EC, Katuri KC, Microfluidic approach to cell microencapsulation, in: Opara EC (Ed.) Cell microencapsulation: Methods and protocols, Springer New York, New York, NY, 2017, pp. 71–76. [DOI] [PubMed] [Google Scholar]
- [324].Zhu J, Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering, Biomaterials, 31 (2010) 4639–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Nguyen KT, West JL, Photopolymerizable hydrogels for tissue engineering applications, Biomaterials, 23 (2002) 4307–4314. [DOI] [PubMed] [Google Scholar]
- [326].D’souza AA, Shegokar R, Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications, Expert Opin. Drug Deliv, 13 (2016) 1257–1275. [DOI] [PubMed] [Google Scholar]
- [327].Nicodemus GD, Bryant SJ, Cell encapsulation in biodegradable hydrogels for tissue engineering applications, Tissue Eng. Part B Rev, 14 (2008) 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Kyburz KA, Anseth KS, Synthetic mimics of the extracellular matrix: How simple is complex enough?, Ann. Biomed. Eng, 43 (2015) 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Abbina S, Siren EMJ, Moon H, Kizhakkedathu JN, Surface engineering for cell-based therapies: Techniques for manipulating mammalian cell surfaces, ACS Biomater. Sci. Eng, 4 (2017) 3658–3677. [DOI] [PubMed] [Google Scholar]
- [330].Topchiyeva IN, Synthesis of biologically active polyethylene glycol derivatives. A review, Polym. Sci. USSR, 32 (1990) 833–851. [Google Scholar]
- [331].Suk JS, Xu Q, Kim N, Hanes J, Ensign LM, Pegylation as a strategy for improving nanoparticle-based drug and gene delivery, Adv. Drug Del. Rev, 99 (2016) 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Owens DE, Peppas NA, Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles, Int. J. Pharm, 307 (2006) 93–102. [DOI] [PubMed] [Google Scholar]
- [333].Sawhney AS, Pathak CP, Hubbell JA, Modification of islet of Langerhans surfaces with immunoprotective poly(ethylene glycol) coatings via interfacial photopolymerization, Biotechnol. Bioeng, 44 (1994) 383–386. [DOI] [PubMed] [Google Scholar]
- [334].Cruise GM, Hegre OD, Scharp DS, Hubbell JA, A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets, Biotechnol. Bioeng, 57 (1998) 655–665. [DOI] [PubMed] [Google Scholar]
- [335].Cruise GM, Scharp DS, Hubbell JA, Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels, Biomaterials, 19 (1998) 1287–1294. [DOI] [PubMed] [Google Scholar]
- [336].Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA, In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes, Cell Transplant., 8 (1999) 293–306. [DOI] [PubMed] [Google Scholar]
- [337].Scharp D, Yu X, Sheckler V, Hubbell J, Latta P, Clinically relevant results from encapsulated islet allografts implanted subcutaneously (SQ) in diabetic baboons without long term immunosuppression, Diabetes, Supplemental Abstract Book: 65th Scientific Sessions, 54 (2005) A48. [Google Scholar]
- [338].Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, Pileggi A, Molano RD, Ricordi C, Stabler CL, Hubbell JA, Device design and materials optimization of conformal coating for islets of Langerhans, Proc. Natl. Acad. Sci. U.S.A, 111 (2014) 10514–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Manzoli V, Villa C, Bayer AL, Morales LC, Molano RD, Torrente Y, Ricordi C, Hubbell JA, Tomei AA, Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol, Am. J. Transplantation, 18 (2018) 590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Stendahl JC, Kaufman DB, Stupp SI, Extracellular matrix in pancreatic islets: Relevance to scaffold design and transplantation, Cell Transplant., 18 (2009) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Wang RN, Rosenberg L, Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship, J. Endocrinol, 163 (1999) 191–190. [DOI] [PubMed] [Google Scholar]
- [342].Panza JL, Wagner WR, Rilo HL, Rao RH, Beckman EJ, Russell AJ, Treatment of rat pancreatic islets with reactive PEG, Biomaterials, 21 (2000) 1155–1164. [DOI] [PubMed] [Google Scholar]
- [343].Contreras JL, Xie D, Mays J, Smyth CA, Eckstein C, Rahemtulla FG, Young CJ, Anthony Thompson J, Bilbao G, Curiel DT, Eckhoff DE, A novel approach to xenotransplantation combining surface engineering and genetic modification of isolated adult porcine islets, Surgery, 136 (2004) 537–547. [DOI] [PubMed] [Google Scholar]
- [344].van Deijnen JHM, Hulstaert CE, Wolters GHJ, van Schilfgaarde R, Significance of the peri-insular extracellular matrix for islet isolation from the pancreas of rat, dog, pig, and man, Cell Tissue Res, 267 (1992) 139–146. [DOI] [PubMed] [Google Scholar]
- [345].Barani L, Vasheghani-Farahani E, Lazarjani HA, Hashemi-Najafabadi S, Atyabi F, Effect of molecular mass of methoxypoly(ethylene glycol) activated with succinimidyl carbonate on camouflaging pancreatic islets, Biotechnol. Appl. Biochem, 57 (2010) 25–30. [DOI] [PubMed] [Google Scholar]
- [346].Lazarjani HA, Vasheghani-Farahani E, Barani L, Hashemi-Najafabadi S, Shojaosadati SA, Effect of polymer concentration on camouflaging of pancreatic islets with mPEG-succinimidyl carbonate, Artif. Cells Blood Substit. Immobil. Biotechnol, 38 (2010) 250–258. [DOI] [PubMed] [Google Scholar]
- [347].Lee DY, Yang K, Lee S, Chae SY, Kim K-W, Lee MK, Han D-J, Byun Y, Optimization of monomethoxy-polyethylene glycol grafting on the pancreatic islet capsules, J. Biomed. Mater. Res, 62 (2002) 372–377. [DOI] [PubMed] [Google Scholar]
- [348].Lou S, Zhang X, Zhang J, Deng J, Kong D, Li C, Pancreatic islet surface bioengineering with a heparin-incorporated starPEG nanofilm, Mater. Sci. Eng, C, 78 (2017) 24–31. [DOI] [PubMed] [Google Scholar]
- [349].Rengifo HR, Giraldo JA, Labrada I, Stabler CL, Long-term survival of allograft murine islets coated via covalently stabilized polymers, Adv. Healthc. Mater, 3 (2014) 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Giraldo JA, Molano RD, Rengifo HR, Fotino C, Gattás-Asfura KM, Pileggi A, Stabler CL, The impact of cell surface PEGylation and short-course immunotherapy on islet graft survival in an allogeneic murine model, Acta Biomater., 49 (2017) 272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Wilson JT, Haller CA, Qu Z, Cui W, Urlam MK, Chaikof EL, Biomolecular surface engineering of pancreatic islets with thrombomodulin, Acta Biomater., 6 (2010) 1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Miura S, Teramura Y, Iwata H, Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)-phospholipids anchored to cell membrane, Biomaterials, 27 (2006) 5828–5835. [DOI] [PubMed] [Google Scholar]
- [353].Stützer I, Esterházy D, Stoffel M, The pancreatic beta cell surface proteome, Diabetologia, 55 (2012) 1877–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Kato K, Itoh C, Yasukouchi T, Nagamune T, Rapid protein anchoring into the membranes of mammalian cells using oleyl chain and poly(ethylene glycol), Biotechnol. Prog, 20 (2004) 897–904. [DOI] [PubMed] [Google Scholar]
- [355].Sou K, Endo T, Takeoka S, Tsuchida E, Poly(ethylene glycol)-modification of the phospholipid vesicles by using the spontaneous incorporation of poly(ethylene glycol)-lipid into the vesicles, Bioconj. Chem, 11 (2000) 372–379. [DOI] [PubMed] [Google Scholar]
- [356].Chen H, Teramura Y, Iwata H, Co-immobilization of urokinase and thrombomodulin on islet surfaces by poly(ethylene glycol)-conjugated phospholipid, J. Controlled Release, 150 (2011) 229–234. [DOI] [PubMed] [Google Scholar]
- [357].Teramura Y, Iwata H, Surface modification of islets with PEG-lipid for improvement of graft survival in intraportal transplantation, Transplantation, 88 (2009) 624–630. [DOI] [PubMed] [Google Scholar]
- [358].Teramura Y, Iwata H, Improvement of graft survival by surface modification with poly(ethylene glycol)-lipid and urokinase in intraportal islet transplantation, Transplantation, 91 (2011) 271–278. [DOI] [PubMed] [Google Scholar]
- [359].Teramura Y, Iwata H, Islets surface modification prevents blood-mediated inflammatory responses, Bioconj. Chem, 19 (2008) 1389–1395. [DOI] [PubMed] [Google Scholar]
- [360].Teramura Y, Kaneda Y, Iwata H, Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)–lipids in the cell membrane, Biomaterials, 28 (2007) 4818–4825. [DOI] [PubMed] [Google Scholar]
- [361].Totani T, Teramura Y, Iwata H, Immobilization of urokinase on the islet surface by amphiphilic poly(vinyl alcohol) that carries alkyl side chains, Biomaterials, 29 (2008) 2878–2883. [DOI] [PubMed] [Google Scholar]
- [362].Yamamoto T, Teramura Y, Itagaki T, Arima Y, Iwata H, Interaction of poly(ethylene glycol)-conjugated phospholipids with supported lipid membranes and their influence on protein adsorption, Sci. Technol. Adv. Mat, 17 (2016) 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Teramura Y, Oommen OP, Olerud J, Hilborn J, Nilsson B, Microencapsulation of cells, including islets, within stable ultra-thin membranes of maleimide-conjugated PEG-lipid with multifunctional crosslinkers, Biomaterials, 34 (2013) 2683–2693. [DOI] [PubMed] [Google Scholar]
- [364].Luan NM, Teramura Y, Iwata H, Layer-by-layer co-immobilization of soluble complement receptor 1 and heparin on islets, Biomaterials, 32 (2011) 6487–6492. [DOI] [PubMed] [Google Scholar]
- [365].Kellam B, de Bank PA, Shakesheff KM, Chemical modification of mammalian cell surfaces, Chem. Soc. Rev, 32 (2003) 327–337. [DOI] [PubMed] [Google Scholar]
- [366].Itagaki T, Arima Y, Kuwabara R, Kitamura N, Iwata H, Interaction between cells and poly(ethylene glycol)-lipid conjugates, Colloids Surf. B. Biointerfaces, 135 (2015) 765–773. [DOI] [PubMed] [Google Scholar]
- [367].Jang JY, Lee DY, Park SJ, Byun Y, Immune reactions of lymphocytes and macrophages against PEG-grafted pancreatic islets, Biomaterials, 25 (2004) 3663–3669. [DOI] [PubMed] [Google Scholar]
- [368].Verhoef JJF, Anchordoquy TJ, Questioning the use of PEGylation for drug delivery, Drug Deliv. Transl. Res, 3 (2013) 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].Betker JL, Gomez J, Anchordoquy TJ, The effects of lipoplex formulation variables on the protein corona and comparisons with in vitro transfection efficiency, J. Controlled Release, 171 (2013) 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Johnstone SA, Masin D, Mayer L, Bally MB, Surface-associated serum proteins inhibit the uptake of phosphatidylserine and poly(ethylene glycol) liposomes by mouse macrophages, Biochim. Biophys. Acta, 1513 (2001) 25–37. [DOI] [PubMed] [Google Scholar]
- [371].Dos Santos N, Allen C, Doppen A-M, Anantha M, Cox KAK, Gallagher RC, Karlsson G, Edwards K, Kenner G, Samuels L, Webb MS, Bally MB, Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: Relating plasma circulation lifetimes to protein binding, Biochim. Biophys. Acta, 1768 (2007) 1367–1377. [DOI] [PubMed] [Google Scholar]
- [372].Sroda K, Rydlewski J, Langner M, Kozubek A, Gryzbek M, Sikorski AF, Repeated injections of PEG-PE liposomes generate anti-PEG antibodies, Cell. Mol. Biol. Lett, 10 (2005) 37–47. [PubMed] [Google Scholar]
- [373].Zhang P, Sun F, Liu S, Jiang S, Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation, J. Controlled Release, 244 (2016) 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P, Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents, Expert Opin. Drug Deliv, 9 (2012) 1319–1323. [DOI] [PubMed] [Google Scholar]
- [375].Hershfield MS, Ganson NJ, Kelly SJ, Scarlett EL, Jaggers DA, Sundy JS, Induced and preexisting anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients, Arthrit. Res. Ther, 16 (2014) R63–R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Ganson NJ, Kelly SJ, Scarlett E, Sundy JS, Hershfield MS, Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase, Arthrit. Res. Ther, 8 (2006) R12–R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Decher G, Hong JD, Schmitt J, Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces, Thin Solid Films, 210/211 (1992) 831–835. [Google Scholar]
- [378].Safley SA, Cui H, Cauffiel S, Tucker-Burden C, Weber CJ, Biocompatibility and immune acceptance of adult porcine islets transplanted intraperitoneally in diabetic NOD mice in calcium alginate poly-L-lysine microcapsules versus barium alginate microcapsules without poly-L-lysine, J. Diabetes Sci. Technol, 2 (2008) 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].Omer A, Duvivier-Kali V, Fernandes J, Tchipashvili V, Colton CK, Weir GC, Long-term normoglycemia in rats receiving transplants with encapsulated islets, Transplantation, 79 (2005) 52–58. [DOI] [PubMed] [Google Scholar]
- [380].Sun Y, Ma X, Zhou D, Vacek I, Sun AM, Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression, J. Clin. Invest, 98 (1996) 1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Calafiore R, Basta G, Alginate/poly-L-ornithine microcapsules for pancreatic islet cell immunoprotection, in: Kühtreiber WM, Lanza RP, Chick WL (Eds.) Cell encapsulation technology and therapeutics, Birkhäuser Boston, Boston, MA, 1999, pp. 138–150. [Google Scholar]
- [382].Zhi Z.-l., Liu B, Jones PM, Pickup JC, Polysaccharide multilayer nanoencapsulation of insulin-producing β-cells grown as pseudoislets for potential cellular delivery of insulin, Biomacromolecules, 11 (2010) 610–616. [DOI] [PubMed] [Google Scholar]
- [383].Zhi Z.l., Kerby A, King AJF, Jones PM, Pickup JC, Nano-scale encapsulation enhances allograft survival and function of islets transplanted in a mouse model of diabetes, Diabetologia, 55 (2012) 1081–1090. [DOI] [PubMed] [Google Scholar]
- [384].Hwang J, Choi D, Choi M, Seo Y, Son J, Hong J, Choi J, Synthesis and characterization of functional nanofilm-coated live immune cells, ACS Appl. Mater. Interfaces, 10 (2018) 17685–17692. [DOI] [PubMed] [Google Scholar]
- [385].Dean PM, Surface electrostatic-charge measurements on islet and zymogen granules: Effect of calcium ions, Diabetologia, 10 (1974) 427–430. [DOI] [PubMed] [Google Scholar]
- [386].Krol S, del Guerra S, Grupillo M, Diaspro A, Gliozzi A, Marchetti P, Multilayer nanoencapsulation. New approach for immune protection of human pancreatic islets, Nano Lett, 6 (2006) 1933–1939. [DOI] [PubMed] [Google Scholar]
- [387].Syed F, Bugliani M, Novelli M, Olimpico F, Suleiman M, Marselli L, Boggi U, Filipponi F, Raffa V, Krol S, Campani D, Masiello P, De Tata V, Marchetti P, Conformal coating by multilayer nano-encapsulation for the protection of human pancreatic islets: in-vitro and in-vivo studies, Nanomed. Nanotechnol. Biol. Med, 14 (2018) 2191–2203. [DOI] [PubMed] [Google Scholar]
- [388].Chanana M, Gliozzi A, Diaspro A, Chodnevskaja I, Huewel S, Moskalenko V, Ulrichs K, Galla H-J, Krol S, Interaction of polyelectrolytes and their composites with living cells, Nano Lett, 5 (2005) 2605–2612. [DOI] [PubMed] [Google Scholar]
- [389].Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T, In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis, Biomaterials, 24 (2003) 1121–1131. [DOI] [PubMed] [Google Scholar]
- [390].Teramura Y, Iwata H, Cell surface modification with polymers for biomedical studies, Soft Matter, 6 (2010) 1081–1091. [Google Scholar]
- [391].Choksakulnimitr S, Masuda S, Tokuda H, Takakura Y, Hashida M, In vitro cytotoxicity of macromolecules in different cell culture systems, J. Controlled Release, 34 (1995) 233–241. [Google Scholar]
- [392].King A, Lau J, Nordin A, Sandler S, Andersson A, The effect of capsule composition in the reversal of hyperglycemia in diabetic mice transplanted with microencapsulated allogeneic cells, Diabetes Technol. Ther, 5 (2003) 653–663. [DOI] [PubMed] [Google Scholar]
- [393].Spector AA, Yorek MA, Membrane lipid composition and cellular function, J. Lipid Res, 26 (1985) 1015–1035. [PubMed] [Google Scholar]
- [394].Zhang Y, An D, Song W, Pardo Y, Ma M, Drug-eluting conformal coatings on individual cells, Cell. Mol. Bioeng, 9 (2016) 382–397. [Google Scholar]
- [395].Lopes GKB, Schulman HM, Hermes-Lima M, Polyphenol tannic acid inhibits hydroxyl radical formation from fenton reaction by complexing ferrous ions, Biochim. Biophys. Acta. Gen. Subj, 1472 (1999) 142–152. [DOI] [PubMed] [Google Scholar]
- [396].Kozlovskaya V, Zavgorodnya O, Chen Y, Ellis K, Tse Hubert M, Cui W, Thompson JA, Kharlampieva E, Ultrathin polymeric coatings based on hydrogen-bonded polyphenol for protection of pancreatic islet cells, Adv. Funct. Mater, 22 (2012) 3389–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [397].Pham-Hua D, Padgett LE, Xue B, Anderson B, Zeiger M, Barra JM, Bethea M, Hunter CS, Kozlovskaya V, Kharlampieva E, Tse HM, Islet encapsulation with polyphenol coatings decreases pro-inflammatory chemokine synthesis and T cell trafficking, Biomaterials, 128 (2017) 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [398].Yang J, Li J, Li X, Wang X, Yang Y, Kawazoe N, Chen G, Nanoencapsulation of individual mammalian cells with cytoprotective polymer shell, Biomaterials, 133 (2017) 253–262. [DOI] [PubMed] [Google Scholar]
- [399].Wilson JT, Krishnamurthy VR, Cui W, Qu Z, Chaikof EL, Noncovalent cell surface engineering with cationic graft copolymers, J. Am. Chem. Soc, 131 (2009) 18228–18229. [DOI] [PubMed] [Google Scholar]
- [400].Wilson JT, Cui W, Kozlovskaya V, Kharlampieva E, Pan D, Qu Z, Krishnamurthy VR, Mets J, Kumar V, Wen J, Song Y, Tsukruk VV, Chaikof EL, Cell surface engineering with polyelectrolyte multilayer thin films, J. Am. Chem. Soc, 133 (2011) 7054–7064. [DOI] [PubMed] [Google Scholar]
- [401].Weber PC, Ohlendorf DH, Wendoloski JJ, Salemme FR, Structural origins of high-affinity biotin binding to streptavidin, Science, 243 (1989) 85. [DOI] [PubMed] [Google Scholar]
- [402].Van Tassel PR, Nanotechnology in medicine: Nanofilm biomaterials, Yale J. Biol. Med, 86 (2013) 527–536. [PMC free article] [PubMed] [Google Scholar]
- [403].Ruoslahti E, Fibronectin and its receptors, Annu. Rev. Biochem, 57 (1988) 375–413. [DOI] [PubMed] [Google Scholar]
- [404].Pinske GGM, Bouwman WP, Jiawan-Lalai R, Terpstra OT, Bruijn JA, de Heer E, Integrin signaling via RGD peptides and anti-β1 antibodies confers resistance to apoptosis in islets of Langerhans, Diabetes, 55 (2006) 312–317. [DOI] [PubMed] [Google Scholar]
- [405].Picart C, Elkaim R, Richert L, Audoin F, Arntz Y, Da Silva Cardoso M, Schaaf P, Voegel JC, Frisch B, Primary cell adhesion on RGD-functionalized and covalently crosslinked thin polyelectrolyte multilayer films, Adv. Funct. Mater, 15 (2005) 83–94. [Google Scholar]
- [406].Kizilel S, Scavone A, Lieu X, Nothias J-M, Ostrega D, Witkowski P, Millis M, Encapsulation of pancreatic islets within nano-thin functional polyethylene glycol coatings for enhanced insulin secretion, Tissue Eng. Part A, 16 (2010) 2217–2228. [DOI] [PubMed] [Google Scholar]
- [407].Kahn SE, Alessio DA, Schwartz MW, Fujimoto WY, Ensinck JW, Taborsky GJ, Porte D, Evidence of cosecretion of islet amyloid polypeptide and insulin by β-cells, Diabetes, 39 (1990) 634. [DOI] [PubMed] [Google Scholar]
- [408].Harnett W, Harnett MM, Modulation of the host immune system by phosphorylcholine-containing glycoproteins secreted by parasitic filarial nematodes, Biochim. Biophys. Acta, 1539 (2001) 7–15. [DOI] [PubMed] [Google Scholar]
- [409].de Wouwer Marlies V, Collen D, Conway EM, Thrombomodulin-Protein C-EPCR system, Atertio. Thromb. Vasc. Biol, 24 (2004) 1374–1383. [DOI] [PubMed] [Google Scholar]
- [410].Suzuki K, Kusumoto H, Deyashiki Y, Nishioka J, Maruyama I, Zushi M, Kawahara S, Honda G, Yamamoto S, Horiguchi S, Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation, EMBO J, 6 (1987) 1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Stabler CL, Sun X-L, Cui W, Wilson JT, Haller CA, Chaikof EL, Surface re-engineering of pancreatic islets with recombinant azido-thrombomodulin, Bioconj. Chem, 18 (2007) 1713–1715. [DOI] [PubMed] [Google Scholar]
- [412].Ploug M, Gårdsvoll H, Jørgensen TJD, Hansen LL, Danø K, Structural analysis of the interaction between urokinase-type plasminogen activator and its receptor: A potential target for anti-invasive cancer therapy, Biochem. Soc. Trans, 30 (2002) 177. [DOI] [PubMed] [Google Scholar]
- [413].Oduah EI, Linhardt RJ, Sharfstein ST, Heparin: Past, present, and future, Pharmaceuticals (Basel), 9 (2016) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [414].Khera R, Das N, Complement receptor 1: Disease associations and therapeutic implications, Mol. Immunol, 46 (2009) 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [415].Luan NM, Teramura Y, Iwata H, Immobilization of soluble complement receptor 1 on islets, Biomaterials, 32 (2011) 4539–4545. [DOI] [PubMed] [Google Scholar]
- [416].Pollok JM, Lorenzen M, Kölln PA, Török É, Kaufmann PM, Kluth D, Bohuslavizki KH, Gundlach M, Rogiers X, In vitro function of islets of Langerhans encapsulated with a membrane of porcine chondrocytes for immunoisolation, Dig. Surg, 18 (2001) 204–210. [DOI] [PubMed] [Google Scholar]
- [417].Lee JI, Nishimura R, Sakai H, Sasaki N, Kenmochi T, A newly developed immunoisolated bioartificial pancreas with cell sheet engineering, Cell Transplant., 17 (2008) 51–59. [DOI] [PubMed] [Google Scholar]
- [418].Teramura Y, Iwata H, Islet encapsulation with living cells for improvement of biocompatibility, Biomaterials, 30 (2009) 2270–2275. [DOI] [PubMed] [Google Scholar]
- [419].Teramura Y, Minh LN, Kawamoto T, Iwata H, Microencapsulation of islets with living cells using polyDNA-PEG-lipid conjugate, Bioconj. Chem, 21 (2010) 792–796. [DOI] [PubMed] [Google Scholar]
- [420].Kaur G, Thompson LA, Dufour JM, Sertoli cells – immunological sentinels of spermatogenesis, Semin. Cell Dev. Biol, 30 (2014) 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [421].Takemoto N, Teramura Y, Iwata H, Immobilization of sertoli cells on islets of Langerhans, Biomater. Sci, 1 (2013) 315–321. [DOI] [PubMed] [Google Scholar]
- [422].Bayry J, Gautier J-F, Regulatory T cell immunotherapy for type 1 diabetes: A step closer to success?, Cell Metab., 23 (2016) 231–233. [DOI] [PubMed] [Google Scholar]
- [423].Gołab K, Kizilel S, Bal T, Hara M, Zielinski M, Grose R, Savari O, Wang X-J, Wang L-J, Tibudan M, Krzystyniak A, Marek-Trzonkowska N, Millis JM, Trzonkowski P, Witkowski P, Improved coating of pancreatic islets with regulatory T cells to create local immunosuppression by using the biotin-polyethylene glycol-succinimidyl valeric acid ester molecule, Transplant. Proc, 46 (2014) 1967–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [424].Marek N, Krzystyniak A, Ergenc I, Cochet O, Misawa R, Wang L-J, Gołab K, Wang X, Kilimnik G, Hara M, Kizilel S, Trzonkowski P, Millis JM, Witkowski P, Coating human pancreatic islets with CD4+CD25highCD127- regulatory T cells as a novel approach for the local immunoprotection, Ann. Surg, 254 (2011) 512–519. [DOI] [PubMed] [Google Scholar]
- [425].Kim JH, Oh BJ, Lee HN, Park HS, Park SG, Park KS, Endothelial colony-forming cell coating of pig islets prevents xenogeneic instant blood-mediated inflammatory reaction, Cell Transplant., 20 (2011) 1805–1815. [DOI] [PubMed] [Google Scholar]
- [426].Barba-Gutierrez DA, Daneri-Navarro A, Villagomez-Mendez JJA, Kanamune J, Robles-Murillo AK, Sanchez-Enriquez S, Villafan-Bernal JR, Rivas-Carrillo JD, Facilitated engraftment of isolated islets coated with expanded vascular endothelial cells for islet transplantation, Transplant. Proc, 48 (2016) 669–672. [DOI] [PubMed] [Google Scholar]
- [427].He T, Peterson E. T, Holmuhamedov L. E, Terzic A, Caplice M. N, Oberley W. L, Katusic ZS, Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase, Atertio. Thromb. Vasc. Biol, 24 (2004) 2021–2027. [DOI] [PubMed] [Google Scholar]
- [428].Abou-Saleh H, Yacoub D, Théorêt J-F, Gillis M-A, Neagoe P-E, Labarthe B, Théroux P, Sirois MG, Tabrizian M, Thorin E, Merhi Y, Endothelial progenitor cells bind and inhibit platelet function and thrombus formation, Circulation, 120 (2009) 2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [429].Basire A, Sabatier F, Ravet S, Lamy E, Mialhe A, Zabouo G, Paul P, Gurewich V, Sampol J, Dignat-George F, High urokinase expression contributes to the angiogenic properties of endothelial cells derived from circulating progenitors, Thrombosis Haemostasis, 95 (2006) 678–688. [PubMed] [Google Scholar]
- [430].Lau J, Vasylovska S, Kozlova EN, Carlsson P-O, Surface coating of pancreatic islets with neural crest stem cells improves engraftment and function after intraportal transplantation, Cell Transplant., 24 (2015) 2263–2272. [DOI] [PubMed] [Google Scholar]
- [431].Teramura Y, Ekdahl KN, Barbu A, A hybrid of cells and pancreatic islets toward a new bioartificial pancreas, Regen. Ther, 3 (2016) 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [432].Calafiore R, Basta G, Luca G, Boselli C, Bufalari A, Bufalari A, Cassarani MP, Giustozzi BM, Brunetti P, Transplantation of pancreatic islets contained in minimal volume microcapsules in diabetic high mammalians, Ann. N. Y. Acad. Sci, 875 (1999) 219–232. [DOI] [PubMed] [Google Scholar]
- [433].Chang WG, Niklason LE, A short discourse on vascular tissue engineering, Regen. Med, 2 (2017) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [434].Boehler RM, Graham JG, Shea LD, Tissue engineering tools for modulation of the immune response, BioTechniques, 51 (2011) 239–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [435].Koh A, Nichols SP, Schoenfisch MH, Glucose sensor membranes for mitigating the foreign body response, J. Diabetes Sci. Technol, 5 (2011) 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [436].Gottrup F, Oxygen in wound healing and infection, World J. Surg, 28 (2004) 312–315. [DOI] [PubMed] [Google Scholar]
- [437].Xie J, Lu Y, Wang W, Zhu H, Wang Z, Cao Z, Simple protein modification using zwitterionic polymer to mitigate the bioactivity loss of conjugated insulin, Adv. Healthc. Mater, 6 (2017) 1601428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [438].Xiao J, Chen S, Yi J, Zhang HF, Ameer GA, A cooperative copper metal–organic framework-hydrogel system improves wound healing in diabetes, Adv. Funct. Mater, 27 (2016) 1604872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [439].DiSanto RM, Subramanian V, Gu Z, Recent advances in nanotechnology for diabetes treatment, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol, 7 (2015) 548–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [440].Alvarez MM, Aizenberg J, Analoui M, Andrews AM, Bisker G, Boyden ES, Kamm RD, Karp JM, Mooney DJ, Oklu R, Peer D, Stolzoff M, Strano MS, Trujillo-de Santiago G, Webster TJ, Weiss PS, Khademhosseini A, Emerging trends in micro- and nanoscale technologies in medicine: From basic discoveries to translation, ACS Nano, 11 (2017) 5195–5214. [DOI] [PubMed] [Google Scholar]