ABSTRACT
Sesamoids bones are small intra‐tendinous (or ligamentous) ossifications found near joints and are often variable between individuals. Related bones, lunulae, are found within the menisci of certain joints. Several studies have described sesamoids and lunulae in lizards and their close relatives (Squamata) as potentially useful characters in phylogenetic analysis, but their status in the extant outgroup to Squamata, tuatara (Sphenodon), remains unclear. Sphenodon is the only living rhynchocephalian, but museum specimens are valuable and difficult to replace. Here, we use non‐destructive X‐ray microtomography to investigate the distribution of sesamoids and lunulae in 19 Sphenodon specimens and trace the evolution of these bones in Lepidosauria (Rhynchocephalia + Squamata). We find adult Sphenodon to possess a sesamoid and lunula complement different from any known squamate, but also some variation within Sphenodon specimens. The penultimate phalangeal sesamoids and tibial lunula appear to mineralize prior to skeletal maturity, followed by mineralization of a sesamoid between metatarsal I and the astragalocalcaneum (MTI‐AC), the palmar sesamoids, and tibiofemoral lunulae around attainment of skeletal maturity. The tibial patella, ulnar, and plantar sesamoids mineralize late in maturity or variably. Ancestral state reconstruction indicates that the ulnar patella and tibiofemoral lunulae are synapomophies of Squamata, and the palmar sesamoid, tibial patella, tibial lunula, and MTI‐AC may be synapomorphies of Lepidosauria. J. Morphol. 278:62–72, 2017. ©© 2016 Wiley Periodicals,Inc.
Keywords: lunula, ossicle, Rhynchocephalia, osteology, non‐destructive
INTRODUCTION
The tuatara of New Zealand, Sphenodon, is the only living member of the Rhynchocephalia and therefore an important taxon for understanding amniote morphology and evolution. It is the closest living relative to lizards and snakes (Squamata; Gauthier et al., 2012; Jones et al., 2013; Reeder et al., 2015), and therefore, the best available outgroup for inferring the evolutionary polarity of characters not preserved in the fossil record (e.g., Schwenk, 1986; Jones et al., 2009; Regnault et al., 2016). Although Sphenodon is generally considered to have been well‐surveyed anatomically (e.g., Robb, 1977), new discoveries related to its anatomy are still being made (e.g., Jones et al., 2009, 2011, 2012; Johnston, 2010; Kieser et al., 2011; Rheubert et al., 2013; Sanger et al., 2015; Regnault et al., 2016).
Using non‐destructive imaging techniques (e.g., Bever et al., 2005; Gignac et al., 2016), we can discover, document and explicitly record (and share; see https://osf.io/bds35/) the detailed 3D anatomy of organisms like Sphenodon that were previously considered thoroughly explored. In particular, these techniques are valuable in documenting small mineralized skeletal elements entrenched in soft tissues, such as sesamoid bones (ossifications in tendons/ligaments near to joints) and lunulae (ossifications within the meniscus of certain joints). These elements are easily missed in dissection or radiographs, and may be lost or destroyed in skeletal preparation. A recent study by Gauthier et al. (2012) used computed tomography (CT) to describe anatomical characters (primarily of the head) in over 190 species. However, for many taxa including Sphenodon, a number of postcranial characters (including sesamoid bones) remain uncoded (or have codings based on a single individual). As the only extant outgroup for the enormously diverse Squamata, knowledge of these characters in Sphenodon has phylogenetic applications; a need clearly identified, for example, by Maisano (2002a).
Sesamoid bones often exhibit variability between individuals (e.g., Vickaryous and Olson, 2007; Jerez et al., 2010; Gauthier et al., 2012; Regnault et al., 2016), necessitating examination of multiple specimens. Several studies have investigated sesamoid bone presence in squamates through clearing and staining (e.g., Maisano, 2002a; Jerez et al., 2010; Otero and Hoyos, 2013), however, this technique results in modification or partial destruction of specimens, is time‐consuming, and may have variable success depending on tissue thickness and other factors. Although both methods have their advantages, the rapid, non‐destructive nature of CT is ideal for investigation of small mineralized skeletal elements throughout the bodies of many specimens, especially for those such as Sphenodon that are considered precious and difficult to replace.
Here, we describe the sesamoid bones and lunulae in the head and limbs of 19 scanned Sphenodon specimens. It must be noted that the term “sesamoid” may refer to any organized, intratendinous/intraligamentous structure including those composed of fibrocartilage (e.g., the cartilago transiliens; see Tsai and Holliday, 2011). Here, we investigate only the mineralized sesamoids visible through radiographic imaging. We use “mineralized” and “bone” interchangeably here although there is greater complexity than this and some “bones” may actually be “metaplastic” calcified tissues (e.g., the patella as shown in Haines, 1969 and Regnault et al., 2016). More histological examination of this issue is required for Sphenodon and other lepidosaurs but see our initial findings for the patella in Regnault et al. (2016). Sesamoid bones tend to be found in animals with epiphyseal secondary centres of ossification, suggesting that the evolution of both traits is related (Haines, 1969; Carter et al., 1998). As suggested by other researchers (e.g., Maisano, 2002a), we hypothesize that Sphenodon (which also has secondary epiphyseal centres) will have many sesamoid bones, and that some sesamoid bones and lunulae thought previously to be synapomorphies of Squamata are in fact synapomorphies of Lepidosauria (the clade including squamates and rhynchocephalians). We anticipate that our findings will help to clarify the presence and evolution of sesamoid bones and lunulae in lepidosaurs through phylogenetic analyses and, following the work of Gauthier et al. (2012), provide an illustrated framework for future surveys of mineralized sesamoids using X‐ray CT. We also explore the relationship between sesamoid bone presence and skeletal maturity to propose a mineralization sequence for sesamoid bones in tuatara. Such data might eventually be useful in shedding more light on these elements themselves, which are not well understood (especially in non‐mammals): why do they ossify in some individuals but not others (age, genetics, environmental stimuli); and why do they form at some joints but not others (i.e., what are the consequences of their presence/absence)?
MATERIALS AND METHODS
We examined X‐ray microtomography (µCT) scans of 19 tuatara (Sphenodon punctatus Gray, 1842) from Regnault et al. (2016), taken using an XT H 225 ST CT system (Nikon Metrology, Brighton, MI, USA) with settings between 70–150 kV, 235–790 µA, 1000–1415 ms, and voxel size 0.093–0.125 mm (full settings available as supplementary online material to open‐access paper Regnault et al., 2016). We looked for evidence of the following sesamoids: basipterygoid‐pterygoid sesamoid, ulnar patella, palmar sesamoid, metacarpal and metatarsal sesamoids, penultimate phalangeal sesamoids (manus and pes), tibial patella, tibial lunula, fibular lunula, tibiofemoral lunulae, and the sesamoid between metatarsal I‐astragalocalcaneum. These represent sesamoid characters investigated by Gauthier et al. (2012). We also investigated the “ulnar sesamoid” figured by Howes and Swinnerton (1901, pp. 86–87, figure 19) (located between the ulna, ulnare and intermedium), and the plantar sesamoid mentioned in previous studies of lizard sesamoids (Maisano, 2002a; Jerez et al., 2010; Otero and Hoyos, 2013). Scans were viewed and 3D‐models for figures were created using Mimics software (version 16.0, Materialize NV, Leuven, Belgium). Specimens are listed in Table 1. Institution abbreviations used in tables and figures: University Museum of Zoology Cambridge (UMZC), the Natural History Museum London (BMNH), the Horniman Museum London (NH), or no official specimen abbreviation (S).
Table 1.
Status of mineralized sesamoids and lunulae amongst 19 specimens of Sphenodon (Rhynchocephalia)
| Specimen | Mature | SVL (cm) | B‐P | Ulnar patella | Palmar | MC | PP (manus) | Tibial patella | Tibial lunula | Fibular lunula | Tibiofemoral lunulae | MTI‐AC | MT | PP (pes) | Ulnar | Plantar |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UMZC R2602 | No | 16.0 | n/a | A | A | A | A | A | A | A | A | A | A | A | A | A |
| S15 | No | 14.0 | A | A | A | A | A | A | A | A | A | A | A | A | A | A |
| S16 | No | 18.0 | A | A | A | A | P | A | A | A | A | A | A | A | A | A |
| UMZC R2603 | No | ? | n/a | A | P | A | P | A | P | A | A | A | A | P | A | A |
| UMZC R2596 | No | 19.0 | A | A | A | A | P | A | P | A | V | A | A | P | A | A |
| UMZC R2598 | No | ? | n/a | A | A | A | P | A | P | A | A | A | A | P | A | A |
| NH.3.116 | No | 21.0 | n/a | n/a | A | A | P | A | P | A | A | A | A | P | A | A |
| UMZC R2616 | Almost | 26.0 | n/a | n/a | n/a | n/a | n/a | A | P | A | A | A | A | A | n/a | A |
| UMZC R2609 | Yes | 22.5 | n/a | n/a | n/a | n/a | n/a | A | P | A | V (left only) | P (left only) | A | P | n/a | A |
| UMZC R2615 | Yes | 24.0 | n/a | n/a | n/a | n/a | P | A | P | A | A | P | A | P | n/a | A |
| UMZC R2609 | Yes | ? | A | A | A | A | P | A | P | A | V | A | A | P | P | A |
| UMZC R2605 | Yes | 22.0 | A | A | A | A | P | A | P | A | V | A | A | P | P | A |
| UMZC R2607 | Yes | 22.0 | n/a | A | P | A | P | A | P | A | A | P | A | P | A | A |
| UMZC R2595 | Yes | 25.0 | A | A | P | A | P | A | P | A | A | P | A | P | A | P |
| BMNH 1969.2204 | Yes | 24.5 | n/a | n/a | P | A | P | A | P | A |
D (right) V (left) |
P | A | P | A | A |
| S1/MEHJ#1 | Yes | 21.0 | A | A | P | A | P | P | P | A | A | P | A | P | A | P |
| UMZC R2604 | Yes | 18.5 | A | A | P | A | P | P | P | A | V | P | A | P | P (left only) | A |
| NH.84.19 | Yes | 21.0 | A | A | P | A | P | P | P | A | V | P | A | P | A | A |
| BMNH 1935.12.6.1 | Yes | 22.5 | n/a | n/a | P | A | P | P | P | A | D (left only) | P | A | P | A | P |
A= absent; D = dorsal; P = present; V = ventral; SVL = snout‐vent length; B‐P = Basipterygoid‐pterygoid; MC = metacarpal; PP = penultimate phalangeal; MTI‐AC = metatarsal I‐astragalocalcaneal; MT = metatarsal; n/a = not assessed (incomplete specimen or scan);? = unknown.
Not all scans included the full body (due to incomplete specimens and/or partial scans taken), therefore sesamoid presence is reported in terms of visible structures. Because we were using µCT in fixed specimens, our survey could only assess the presence or absence of sesamoid mineralization. Unfortunately we do not know the age of each specimen, but we used terminal fusion of long bones to estimate skeletal maturity (Maisano, 2002b). Against this standard, 11 of the 19 specimens were considered skeletally mature. Snout‐vent length (SVL) was recorded for comparison, but given that adult body size of tuatara is variable (e.g., Tracy, 1997; Tyrrell et al., 2000), we do not expect size to be a reliable indicator of age. We used binary logistic regression to ascertain whether SVL could predict sesamoid mineralization. Statistical analyses were performed in IBM SPSS Statistics for Windows (Version 20.0), and significance was set at P = 0.05.
To estimate the ancestral condition for Lepidosauria, we combined the new data from Sphenodon with the previous codings of Gauthier et al. (2012). To explore the effects of tree topology and reconstruction method on ancestral states, we performed ancestral state reconstructions with MESQUITE software (Maddison and Maddison, 2015), using phylogenies based on morphological data (parsimony‐based reconstruction using the Maximum parsimony strict consensus tree of Gauthier et al., 2012) and based on time‐calibrated molecular data (parsimony and maximum likelihood reconstructions using the tree of Zheng and Wiens, 2016). We performed maximum likelihood analysis with an MK1 model, assuming equal probability of character gain and loss. We must note that in the tree topology presented in Zheng and Wiens (2016), Dibamidae is the least nested member of Squamata; however, it has highly reduced limbs and is coded unknown for many sesamoid bones and lunulae of the distal limbs. Therefore, likelihoods cannot be calculated for Squamata sensu stricto but can calculated be for Squamata excluding Dibamidae.
RESULTS
The presence or absence of mineralized sesamoids in the µCT‐scanned Sphenodon punctatus specimens was variable, with different sesamoid bones exhibiting a range of states from universally present to universally absent (Table 1). Ancestral state reconstructions are available as supplementary online material. Scans are available from the Open Science Framework (https://osf.io/bds35/).
The basipterygoid‐pterygoid sesamoid bone is located ventral to the basipterygoid‐pterygoid articulation where the braincase meets the palate (see Gauthier et al., 2012: character 335). This sesamoid bone was absent in all Sphenodon specimens (Fig. 1) that included the head on scans (nine, of which seven were skeletally mature, see Table 1). Regardless of tree topology or method, the basipterygoid‐pterygoid sesamoid bone is reconstructed as absent in the most recent common ancestor of both lepidosaurs and crown squamates (likelihood 0.99 for both; see Table 2).
Figure 1.
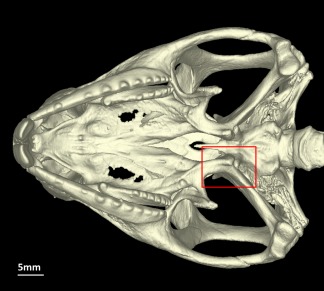
Sphenodon punctatus (UMZC R2604), ventral view of the skull (without the lower jaws), lacking the basipterygoid‐pterygoid sesamoid bone (red box). [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
Mineralized sesamoids and lunulae present in Sphenodon, and the results of ancestral state reconstructions for the lepidosaur and squamate ancestors using a morphology‐based topology (from Gauthier et al. 2012) or a molecular tree (from Zheng and Wiens, 2016)
| Sesamoid | Sphenodon state | Morphological tree + parsimony | Molecular tree + parsimony | Molecular tree + maximum likelihood | |||
|---|---|---|---|---|---|---|---|
| Lepidosauria | Squamata | Lepidosauria | Squamata | Lepidosauria | Squamata | ||
| B‐P | A | A | A | A | A | A (0.99) | A (>0.99) |
| Ulnar patella | A | A/P | P | A/P | A/P | P (0.99) | P (0.99) |
| Palmar | Sometimes P (77%) | P | P | P | P | P (0.99) | P (0.99) |
| MC | A | A | A | A | A | A (0.95) | A (0.95) |
| PP (manus) | P | P | P | P | P | P (>0.99) | P (>0.99) |
| Tibial patella | Sometimes P (36%) | A/P | P | A/P | A/P | P (0.86) | P (0.95) |
| Tibial lunula | P | P | P | P | P | P (0.99) | P (0.99) |
| Fibular lunula | A | A | A | A | A | P? (0.69) | P (0.87) |
| Tibiofemoral lunulae | Mostly V only (55%) or A (36%) | Both/V/A | Both | A | A |
P and separate? (0.65) (V = 0.10, A = 0.17, P and fused = 0.04, D only = 0.04) |
P and separate? (0.91) (V > 0.01, A = 0.09, P and fused > 0.01, D only > 0.01) |
| MTI‐AC | Sometimes P (82%) | P | P | P | P | A (0.87) | A (0.88) |
| MT | A | A | A | A | A | A (0.99) | A (0.99) |
| PP (pes) | P | P | P | P | P | P (>0.99) | P (>0.99) |
| Ulnar | Sometimes P (36%) | n/a | n/a | n/a | n/a | n/a | n/a |
| Plantar | Sometimes P (27%) | n/a | n/a | n/a | n/a | n/a | n/a |
A= absent; D = dorsal; P = present; V = ventral; SVL = snout‐vent length; B‐P = Basipterygoid‐pterygoid; MC = metacarpal; PP = penultimate phalangeal; MTI‐AC = metatarsal I‐astragalocalcaneal; MT = metatarsal; n/a = not analyzed.
The ulnar patella is located on the caudal aspect of the elbow (see Jerez et al., 2010: fig. 1A; Gauthier et al., 2012: character 531; Otero and Hoyos, 2013: fig. 1D). This sesamoid bone was absent in all Sphenodon specimens (Fig. 2) that included the elbow on scans (13, of which seven were skeletally mature). Reconstructed ancestral states varied with tree topology and method. Parsimony reconstruction over a morphological tree results in equivocal (equally likely to be present or absent) sesamoid bone presence in the lepidosaurian common ancestor, and presence in the ancestor of crown‐group squamates. Parsimony reconstruction over a molecular tree gives an equivocal result at both nodes. Maximum likelihood over a molecular tree suggests presence at both nodes, with likelihoods of 0.99 for the lepidosaurian ancestor and >0.99 for the squamate ancestor (Table 2).
Figure 2.
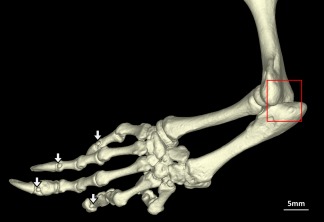
Sphenodon punctatus (UMZC R2604), lateral view of left forelimb lacking the ulnar patella (box) and showing presence of mineralized penultimate phalangeal sesamoids (arrows). [Color figure can be viewed at wileyonlinelibrary.com]
The ulnar sesamoid bone is located distally to the head of the ulna, between the pisiform, ulnare and intermedium (Fig. 3; see also Howes and Swinnerton, 1901, pp. 86–87 and fig. 19). This sesamoid bone was present in four Sphenodon specimens (all skeletally mature): it was present bilaterally (in both manus) in two specimens, unilateral in one specimen (left manus only), and present in the only scanned manus in the final specimen. The ulnar sesamoid was completely absent in the other 12 Sphenodon specimens. This sesamoid bone remains uncoded for many squamates (e.g., Gauthier et al., 2012), so it did not undergo ancestral state reconstruction.
Figure 3.
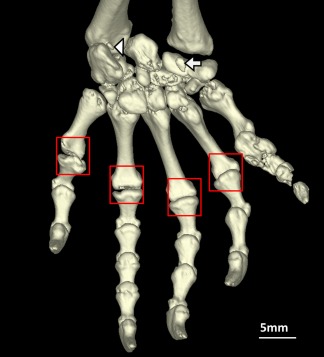
Sphenodon punctatus (BMNH 1935.12.6.1), palmar view of left manus lacking metacarpal sesamoids (boxes), and showing presence of the palmar sesamoid (arrow) and mineralized ulnar sesamoid (arrowhead). [Color figure can be viewed at wileyonlinelibrary.com]
The palmar sesamoid bone is located on the palmar surface of the carpus (Fig. 3; see also Jerez et al., 2010: fig. 1C; Gauthier et al., 2012: character 539; Otero and Hoyos, 2013: “Sesamoideum palmaria”) and is associated with the flexor plate of the hand (Abdala et al., 2009, 2015; Jerez et al., 2010). A small sesamoid bone was present here bilaterally in seven (out of nine) skeletally mature Sphenodon specimens, and absent in seven other specimens (two of which were skeletally mature). Considering that a palmar sesamoid was present in 77% of mature specimens, we chose to code the palmar sesamoid bone as “present” for phylogenetic analysis. Both morphological and molecular trees as well as parsimony and maximum likelihood methods suggest that the sesamoid bone was present in the common ancestor of lepidosaurs and crown squamates (likelihoods of 0.99 for both).
The metacarpal sesamoid bones are located on the palmar surface of the distal metacarpals (see Gauthier et al., 2012: character 541; Otero and Hoyos, 2013: “Sesamoidea metacarpale”). These sesamoid bones were absent in all Sphenodon specimens (Fig. 3) that included the manus on scans (16, of which nine were skeletally mature). Both morphological and molecular trees as well as parsimony and maximum likelihood methods suggest that these sesamoid bones were ancestrally absent in Lepidosauria and crown squamates (likelihoods of 0.95 for both), only evolving later within Squamata.
The penultimate phalangeal sesamoid bones (manus) are located on the dorsal, distal aspect of the penultimate phalanges (Fig. 3; see also Conrad, 2006; Gauthier et al., 2012: character 457; Otero and Hoyos, 2013: “Sesamoidea digitorum manus”), and are associated with the extensor tendons of the manual digits. These sesamoid bones were present in almost all Sphenodon specimens that included the distal manus on scans (15, of which 10 were skeletally mature). In three other Sphenodon (all immature), the penultimate phalangeal sesamoid bones were completely absent in two specimens, and occasionally absent from some digits in the other specimen. As the penultimate phalangeal sesamoid bones (manus) were present in 100% of mature specimens, we coded them as “present” for phylogenetic analysis. Hence these sesamoids were most likely present in ancestral Lepidosauria (likelihood >0.99).
The tibial patella is located on the cranial aspect of the distal femur (Fig. 4; see also Gauthier et al., 2012: character 551; Regnault et al., 2016). This sesamoid bone is present in four (of 11) skeletally mature Sphenodon specimens, and absent in 15 other specimens (seven of which were skeletally mature). Parsimony reconstruction has been reported in a previous paper (Regnault et al., 2016); reanalysis with the more recent molecular phylogeny (using maximum likelihood, with a coding of “present/absent” or “0/1” using Gauthier et al.'s (2012) scheme) suggests that this sesamoid bone is ancestral for all Lepidosauria (likelihood 0.86) as well as Squamata (likelihood 0.95).
Figure 4.
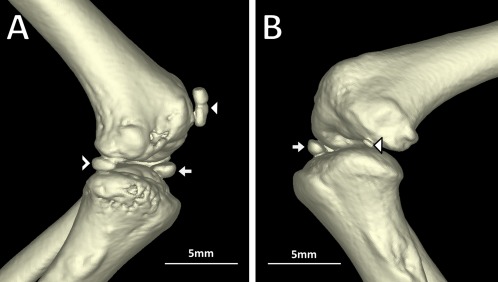
(A) Sphenodon punctatus (NH.84.19), lateral (postaxial) view of the left knee showing presence of the tibial patella (arrowhead), tibial lunula (arrow), and ventral tibiofemoral lunula (unfilled arrowhead); (B) Sphenodon punctatus (BMNH 1969.2204), lateral (postaxial) view of right knee showing presence of the tibial lunula (arrow) and dorsal tibiofemoral lunula (arrowhead). [Color figure can be viewed at wileyonlinelibrary.com]
The tibial lunula is located on the cranial aspect of the knee joint, between the femur and tibia (Fig. 4; see also Haines, 1942; Gauthier et al., 2012: character 552; Regnault et al., 2016: Fig. 1; Otero and Hoyos, 2013: “Lunula”). This lunula was present in almost all Sphenodon specimens (16, of which 11 were skeletally mature). In three other Sphenodon (all immature), the tibial lunula was absent or not clearly present. Again, with presence in 100% of mature specimens, we coded the tibial lunula as “present” for phylogenetic analysis and found it to most likely have been present in the ancestor of both lepidosaurs and crown squamates (likelihoods of 0.99 for both).
The fibular lunula is located on the cranial aspect of the knee joint, between the femur, tibia and fibula (see Gauthier et al., 2012: character 553). This lunula was absent in all Sphenodon specimens (19). The reconstructed ancestral state of the fibular lunula depends on method. parsimony reconstructions over morphological and molecular phylogenies suggests absence in the ancestor of both lepidosaurs and crown squamates, whereas maximum likelihood over the molecular tree suggests presence at both nodes (likelihoods of 0.69 and 0.87).
The dorsal and ventral tibiofemoral lunulae are located on the lateral aspect of the knee joint between the femur and tibia, cranially and caudally (Fig. 4; see also Gauthier et al. 2012: character 554). These lunulae were present in some form in eight Sphenodon specimens (seven of which were skeletally mature): four specimens had bilateral ventral tibiofemoral lunulae, one specimen had the ventral lunula in one knee but not the other, two specimens had the dorsal lunula in one knee but either the ventral or no tibiofemoral lunulae in the other. The dorsal and ventral tibiofemoral lunulae were absent in the remaining 11 Sphenodon specimens (four of which were skeletally mature). The ventral lunula was present in at least one leg in 55% of mature specimens, the dorsal lunula was present in at least one leg in 18%, and both were completely absent from both legs in 36%. Considering this variability, we chose to code the tibiofemoral lunulae as “absent/ventral only” for phylogenetic analysis (a polymorphic coding corresponding to a state of “1/2” using Gauthier et al.'s (2012) scheme). Parsimony reconstruction over a morphological tree shows the ancestral state for lepidosaurs as equally parsimonious between “both present and separate,” “ventral only,” and “absent” tibiofemoral lunulae; in the squamate ancestor, equivocal between “both present and separate” and “absent.” Parsimony reconstruction over a molecular tree results in absence in both ancestors. Maximum likelihood over the molecular tree suggests that the tibiofemoral lunulae are “both present and separate” in the ancestral lepidosaur and squamate (likelihoods 0.65 and 0.91; see Table 2). If the tibiofemoral lunulae for Sphenodon are coded “ventral only” instead of polymorphic, the reconstructed states are equally parsimonious between “both present and separate,” “ventral only,” and “absent” in both ancestors using either tree. The maximum likelihood over the molecular tree is virtually unchanged with this coding (likelihoods of 0.67 and 0.94)
The metatarsal I‐astragalocalcaneum (MTI‐AC) sesamoid bone, also called the meniscus tarsale, is located between the first metatarsal and the astragalocalcaneum (Fig. 5; see also Gauthier et al., 2012: character 562; Howes and Swinnerton, 1901: “meniscus tarsale”). This sesamoid bone was present in nine (out of 11) skeletally mature Sphenodon specimens, and absent in nine other specimens (two of which were skeletally mature). We chose to code the MTI‐AC sesamoid bone as “present” for phylogenetic analysis because it was present in 82% of mature specimens. Parsimony reconstructions over both trees show it present in the ancestor of both lepidosaurs and crown squamates, whereas reconstruction with maximum likelihood over a molecular tree suggest absence in both ancestral lepidosaurs and squamates (likelihoods of 0.87 and 0.88).
Figure 5.
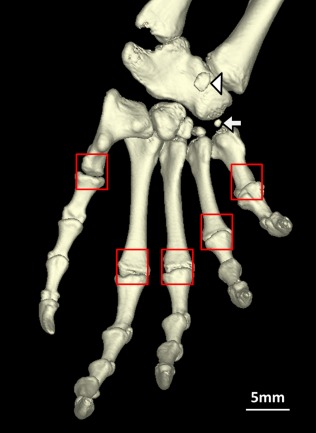
Sphenodon punctatus (BMNH 1935.12.6.1), plantar view of left pes lacking the metatarsal sesamoids (boxes), and showing presence of a mineralized plantar sesamoid (arrowhead) and mineralized metatarsal I‐astragalocalcaneum sesamoid (arrow). [Color figure can be viewed at wileyonlinelibrary.com]
Similar to the manus, the metatarsal sesamoid bones are located on the plantar surface of the distal metatarsals (Fig. 5; see also Gauthier et al., 2012: character 565; Otero and Hoyos, 2013: “Sesamoidea metatarsale”). These sesamoid bones were absent in all Sphenodon specimens (19, of which 11 were skeletally mature). Using parsimony and likelihood reconstructions, this sesamoid bone was most likely absent in the ancestors of both lepidosaurs and crown squamates (likelihoods of 0.99 for both), only evolving later within Squamata.
Again, as in the manus, the penultimate phalangeal sesamoid bones (pes) are located on the dorsal, distal aspects of the penultimate phalanges (see Conrad, 2006; Gauthier et al., 2012: character 569; Otero and Hoyos, 2013: “Sesamoidea digitorum pes”), and are associated with the extensor tendons of the pedal digits. These sesamoids were present in almost all Sphenodon specimens (15, of which 11 were skeletally mature). In four other Sphenodon (all immature), the penultimate phalangeal sesamoid bones were absent. We coded the penultimate phalangeal sesamoid bones (pes) as “present” for phylogenetic analysis due to their ubiquity in mature tuatara. These sesamoid bones are reconstructed as present in the ancestor of both lepidosaurs and crown squamates using both methods and trees (likelihoods of >0.99).
The plantar sesamoid bone is located on the plantar aspect of the tarsus (Fig. 5; see also Jerez et al., 2010: fig. 2C; Otero and Hoyos, 2013: “Sesamoideum plantaria”). This sesamoid bone was present in three skeletally mature Sphenodon specimens (out of 11), on the plantar aspect of the astragalocalcaneum (Fig. 5). This character was not coded by Gauthier et al. (2012) so it did not undergo phylogenetic analysis.
Logistic regression found that SVL did not approach significance as a predictor of sesamoid bone presence for the bones tested: ulnar sesamoid (P = 0.96), palmar sesamoid (P = 0.97), penultimate phalangeal sesamoids (manus) (P > 0.99), tibial patella (P = 0.18), tibial lunula (P = 0.99), tibiofemoral lunulae (P = 0.67), MTI‐AC sesamoid (P = 0.86), penultimate phalangeal sesamoids (pes) (P = 0.66), and plantar sesamoid (P = 0.31).
DISCUSSION
Here, we provide the most complete description of the sesamoid bones in Sphenodon to date. Despite the utility of these data (highlighted by Maisano, 2002a), modern comprehensive studies have left many of these character states unknown (e.g., Gauthier et al., 2012). Previous anatomical studies of Sphenodon have generally examined fewer than three specimens (and are often restricted to just one; e.g., Bayer, 1884; Haines, 1940; Gans and Weaver, 1976; Gorniak et al., 1982; Schwenk, 1986). Use of µCT allowed us to non‐destructively examine a relatively large sample (up to 19) of scientifically valuable Sphenodon specimens in clear, high resolution, three‐dimensional anatomical detail.
Adult Sphenodon appear to possess a complement of mineralized sesamoids and lunulae different from any known squamate thus far (see Gauthier et al., 2012), although the possibility of unmineralized sesamoids and variability may affect this conclusion. We report mineralized palmar and plantar sesamoids and tibiofemoral lunulae in adult Sphenodon for the first time, and observe that the metapodial sesamoids and fibular lunulae appear universally absent. We also confirm sesamoid status from some previous studies: absence of the basipterygoid‐pterygoid sesamoid bone and presence of penultimate phalangeal sesamoids (Gauthier et al., 2012), absence of the ulnar patella (Haines, 1940), presence of the tibial lunula (Haines, 1942; Regnault et al., 2016), and presence of the meniscus tarsale (MTI‐AC sesamoid) and the distal ulnar sesamoid of Howes and Swinnerton (1901).
Functional interpretations of sesamoid bones are problematic. Certain sesamoids may indicate particular functions or lifestyles. For example, palmar sesamoid reduction/absence has been found experimentally amongst traits associated with grasping ability in lizards, and may be related to exploration of narrow branch niches; see Abdala et al. (2009), Sustaita et al. (2013), and Fontanarrosa and Abdala (2016). Tuatara are known to dig (Reischek, 1885; Newman, 1987) and have been pictured climbing (Parkinson, 2002), and the palmar sesamoid in Sphenodon also appears extremely small. However, we find lizards with a postcranial sesamoid complement most similar to Sphenodon seem to be teiids such as Callopistes maculatus and Aspidoscelis tigris. They lack the MTI‐AC sesamoid bone but otherwise exhibit similar states. This group of taxa includes small fast‐moving terrestrial lizards (White and Anderson, 1994) with no obvious functional similarity to Sphenodon. Sesamoid complement alone is probably not informative, but may be useful when considered alongside other morphological and functional traits (Fontanarrosa and Abdala, 2016).
Sesamoid bones are notorious for their variability, and an advantage of scanning multiple specimens is the opportunity to identify which sesamoids are reliably present or absent versus those that are variable between (or even within) individuals. We found the following sesamoid bones (and lunulae) to be variable in adult Sphenodon: MTI‐AC sesamoid (present in 82% of adults), palmar sesamoid (77%), tibial patella (36%), plantar sesamoid (27%), ulnar sesamoid (33%) and tibiofemoral lunulae (ventral in 54% and dorsal in 18%). In particular, the latter two were variable even within individuals: the ulnar sesamoid was always much smaller or absent in one side of the specimen, and the presence and type (ventral or dorsal) of tibiofemoral lunulae could also vary between the specimen's right and left sides.
Scans from numerous specimens of varying maturity also allow us to begin inferring mineralization sequence. Our data suggest a consistent mineralization sequence in Sphenodon, although they are imperfect in the absence of known specimen ages and could be confused by issues of sesamoid variability (i.e., are they late to mineralize, or variable?). We hoped that SVL would be of use in this regard, but it proved to be unhelpful as a predictor of sesamoid mineralization. This may be due to the difficulty in accurately measuring curled‐up fixed specimens, and/or apparent variation in adult body size between different island populations (Tracy, 1997; Tyrrell et al., 2000). The latter has yet to be fully documented, and is something we were unable to test because specimen provenance was frequently unknown.
Our sample suggests that the penultimate phalangeal sesamoids and tibial lunula mineralize first, prior to terminal fusion of long bones/skeletal maturity; followed by the MTI‐AC sesamoid, palmar sesamoid, and tibiofemoral lunulae, likely around or after skeletal maturity; finally the tibial patella, ulnar and plantar sesamoids may mineralize in skeletally mature individuals. The sequence in Sphenodon appears different from those reported in lizards such as Mabuya (where the penultimate phalangeal sesamoids and tibial lunula mineralize later, possibly after the tibial patella, despite early formation of their cartilage anlages; Jerez et al., 2010). However, ossification sequences seem to evolve relatively quickly compared to discrete (binary presence/absence) characters (Maisano, 2002a), and so it would not be surprising if these sequences were different between Sphenodon and squamates.
Despite the limitation of Sphenodon as the only rhynchocephalian for which clear sesamoid data exist, many of the character states we have found are similar to those of the topologically least nested extant squamate species in the sample (e.g., Pholidobolus montium, Strophurus ciliaris); see supplementary online material. The basipterygoid‐pterygoid sesamoid bone is absent in Sphenodon; it is also absent in most squamates surveyed (but present in teiid lizards such as Tupinambis and Ameiva (Gauthier et al., 2012), the gymnophthalmid Colobosaura modesta and the scincid Sphenomorphus solomonis (Gauthier et al., 2012). The palmar sesamoid is present in Sphenodon; most squamates examined appear to possess this sesamoid (or multiple palmar sesamoids; Jerez et al., 2010) but exceptions include some Anolis (Otero and Hoyos, 2013), chameleons and most gekkotans except Strophurus ciliaris (Gauthier et al., 2012). Three patterns of palmar structures, related to the palmar sesamoid, have been described in lizards (Moro and Abdala, 2004; Abdala et al., 2009; Sustaita et al., 2013) and the palmar sesamoid in Sphenodon appears consistent with the P‐pattern, although soft tissue traits could not be evaluated in this study. The metacarpal and metatarsal sesamoids are absent in Sphenodon; similarly, they tend to be absent in topologically least nested squamates, becoming variably present within some squamate clades [e.g., the iguanians Liolaemus belli (Gauthier et al., 2012) and some Anolis (Otero and Hoyos, 2013); variable in gekkotans and scincoids (Gauthier et al., 2012)]. The penultimate phalangeal sesamoids (manus and pes) are present in Sphenodon; they are also found in most squamates except chameleons and occasionally other species (Gauthier et al., 2012, Otero and Hoyos, 2013). The tibial lunula is present in Sphenodon; it is also present in almost all squamates surveyed that possess reasonably well‐developed hind limbs (Gauthier et al., 2012), with rare exceptions; for example, Anolis aequatorialis and A. transversalis (Otero and Hoyos, 2013).
In contrast to the above, there are sesamoids for which Sphenodon appears to differ from many Squamata and/or the reconstructed ancestral state of squamates. The ulnar patella is absent in Sphenodon; this sesamoid appears to have been present ancestrally in squamates although it was later lost in some lineages (e.g., chameleons, teiids such as Tupinambis teguixin, xantusiids such as Lepidophyma flavimaculatum, anguimorphans such as Elgaria multicarinata and Varanus acanthurus, and gymnophthalmids such as Leposoma and Prionodactylus (Gauthier et al., 2012; Otero and Hoyos, 2013). Because of this difference between Sphenodon and many of the least‐nested squamates, the parsimony‐reconstructed ancestral state for lepidosaurs is unclear; likelihood suggests ancestral presence, however this must be viewed critically given the long, lone‐branch Sphenodon represents. The fibular lunula is absent in Sphenodon; this bone is found widely amongst Squamata with well‐developed hind limbs but it is absent from almost all iguanians surveyed, and appears absent in dibamids (Gauthier et al., 2012). Trees that place iguanians or dibamids outside all other squamate clades suggest ancestral absence of the fibular lunula is most parsimonious; by contrast likelihood again suggests presence. Mineralized tibiofemoral lunulae were variable in Sphenodon, with the most common states being the ventral lunula only or absence of both, or occasionally the dorsal lunula only. Limbed squamates tend to have both dorsal and ventral elements, however some have neither (e.g., chameleons), the ventral lunula only (e.g., teiids, scincoids), or fused lunulae (e.g., the gecko Gonatodes albogularis, the anguimorphan Xenosaurus grandis; Gauthier et al., 2012). The most likely ancestral state for Squamata is presence of both lunulae as separate elements (or potentially both absent), but the very different and variable configuration of these bones in Sphenodon makes the ancestral state for Lepidosauria unclear. The MTI‐AC sesamoid is present in Sphenodon; in squamates it is variable. It appears to be present in gekkotans, but absent from many iguanians, lacertoids, and scinocoids, as well as all anguimorphans surveyed to date (Gauthier et al., 2012). Ancestral states vary with the method used. The MTI‐AC sesamoid bone may have been ancestrally absent for lepidosaurs (and acquired separately by Sphenodon, gekkonids, phrynosomatids, and so forth, as suggested by our likelihood reconstruction), or alternatively been ancestrally present (but lost within Squamata after the divergence of total group Gekkota from other squamates, and later reappeared in some lineages, as suggested by our parsimony reconstructions). Scattered presence of particular sesamoid bones or lunulae across many lepidosaurian taxa may be due to a high rate of evolution of these bones (Baum and Smith, 2013); multiple loss or gain of bones being obscured by subsequent state changes, making evolutionary reconstruction challenging.
Our ancestral state reconstructions support the hypothesis that the ulnar patella and tibiofemoral lunulae (dorsal and ventral) are synapomophies of Squamata (Maisano, 2002a). However (as anticipated by Maisano), presence in Sphenodon of the palmar sesamoid bone, tibial patella, and tibial lunula (and potentially the presence of the MT‐AC sesamoid bone) suggest that these features are not synapomorphies of Squamata but of Lepidosauria generally. The plantar sesamoid bone also appears widely distributed in squamates (Maisano, 2002a; Jerez et al., 2010; Otero and Hoyos, 2013), and so may be also be a synapomorphy of lepidosaurs. We have found Sphenodon to possess a similar number of sesamoid bones to squamate species, further supporting our hypothesis and that of Maisano that lepidosaurs possess a large number of sesamoids.
Further careful inspections (and new discoveries) of articulated lepidosaur or stem‐lepidosaur material from the Middle Triassic would greatly aid our understanding of the early lepidosaur phenotype (Jones et al., 2013). Rhynchocephalia originated over 240 million years ago (Jones et al., 2013), and was extremely diverse in size, body proportions, and lifestyle (e.g., Cocude‐Michel, 1963; Reynoso, 2000; Rauhut et al., 2012; Apesteguía et al., 2014). Sphenodon is the only extant species, and cannot be assumed to possess a sesamoid complement representative of the entire clade. Future data from fossils will be important for resolving the evolution of sesamoid bones in Lepidosauria. Such data may require similar micro‐imaging methods and scrutiny to uncover previously overlooked sesamoid bones or distinguish apparent from actual absence. Many lepidosauromorph fossils are disarticulated, and when rare‐articulated postcranial material is available, sesamoid bones appear absent (e.g., the gliding kuehneosaur Icarosaurus siefkeri from Colbert, 1966; rhynchocephalian lagerstätte deposits such as Solnhofen, reported by Cocude‐Michel, 1963) or unclear (e.g., small ovoid structures figured near the knee joint of Ankylosphenodon from Reynoso, 2000; a rounded element near the elbow joint of Huehuecuetzpalli mixtecus from Reynoso, 1998). However, there are many issues inferring sesamoid bone absence from fossilized material, as discussed by Regnault et al. (2016).
Our new data for Sphenodon are useful in that they permit a preliminary estimation of the ancestral condition for lepidosaurs. However, in the absence of fossil data, this condition must be viewed with caution. As the only rhynchocephalian with clear character data, Sphenodon exerts a strong influence on the parsimony‐reconstructed state of the ancestral lepidosaur. Likelihood reconstructions must also not be interpreted without the context of the available data; the long branch between Sphenodon and the ancestral lepidosaur allows plenty of time for state changes, and as a result, and converse to parsimony‐reconstructions, Sphenodon has little impact on the ancestral lepidosaurian state using this method. Another limitation of our study is that, despite its many advantages, µCT cannot discern unmineralized (cartilaginous) sesamoids in fixed specimens and (as noted in the Introduction) typically cannot distinguish how sesamoid “bones” mineralized—that is, via “typical” endochondral, intramembranous, or metaplastic modes of formation. When possible, contrast‐enhanced staining (e.g., Tsai and Holliday, 2011) may be even better for documenting sesamoids and their relationship to other anatomical structures in extant taxa. Clearing and staining as well as tissue histology, classical dissection and micro‐imaging also remain extremely important methods of investigation (Jerez et al., 2010; Otero and Hoyos, 2013) and we advocate use of multiple approaches (e.g., Regnault et al., 2016) to yield maximum information whenever possible.
Author Contributions
The study was conceived and designed by MEHJ and SR. Data were acquired by SR and JRH, and interpreted by SR, JRH and MEHJ. The manuscript was drafted by MEHJ and SR, with critical revisions by JRH.
Supporting information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
For access to specimens, we thank Susan Evans and University College London, Moya Meredith Smith and King's College London, Steven Le Comber and Queen Mary University of London, Nicolas Di‐poi and the University of Helsinki, Patrick Campbell and Natural History Museum London, Paolo Viscardi and the Horniman Museum. We thank Keturah Smithson, Matthew Lowe and the University Museum of Zoology Cambridge for specimen access and µCT scanning. We also thank two anonymous reviewers and Virginia Abdala for their helpful comments on a previous version of this manuscript.
LITERATURE CITED
- Abdala V, Manzano AS, Tulli MJ, Herrel A. 2009. The tendinous patterns in the palmar surface of the lizard manus: Functional consequences for grasping ability. Anat Rec 292:842–853. [DOI] [PubMed] [Google Scholar]
- Abdala V, Gizante MB, Diogo R, Molnar J, Kohlsdorf T. 2015. Musculoskeletal anatomical changes that accompany limb reduction in lizards. J Morphol 276:1290–1310. [DOI] [PubMed] [Google Scholar]
- Apesteguía S, Gómez RO, Rougier GW. 2014. The youngest South American rhynchocephalian, a survivor of the K/Pg extinction. Proc R Soc B 281:20140811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum DA, Smith SD. 2013. Using trees to study character evolution In: Tree Thinking: An Introduction to Phylogenetic Biology. Colorado: Roberts and Company Publishers; pp 305–348. [Google Scholar]
- Bayer F. 1884. Über die Extremitäten einer jungen Hatteria . Sitzb d k Akad d Wiss 90:237–245. + Tafel. [Google Scholar]
- Bever GS, Bell CJ, Maisano JA. 2005. The ossified braincase and cephalic osteoderms of Shinisaurus crocodilurus (Squamata, Shinisauridae). Palaeontol Electronica 8( 4A):1–36. [Google Scholar]
- Carter DR, Mikić B, Padian K. 1998. Epigenetic mechanical factors in the evolution of long bone epiphyses. Zool J Linn Soc 123:163–178. [Google Scholar]
- Cocude‐Michel M. 1963. Les rhynchocephales et les sauriens de calcaires lithographiques (Jurassique supérieur) d'Europe occidentale. Nouv Arch Mus Hist Nat 7:1–187. [Google Scholar]
- Colbert EH. 1966. A gliding reptile from the Triassic of New Jersey. Novitates 2246:1–23. [Google Scholar]
- Conrad JL. 2006. Postcranial skeleton of Shinisaurus crocodilurus (Squamata: Anguimorpha). J Morphol 267:759–775. [DOI] [PubMed] [Google Scholar]
- Fontanarrosa G, Abdala V. 2016. Bone indicators of grasping hands in lizards. PeerJ 4:e1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans C, Weaver EG. 1976. Ear and hearing in Sphenodon punctatus . Proc Natl Acad Sci USA 73:4244–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier JA, Kearney M, Maisano JA, Rieppel O, Behlke ADB. 2012. Assembling the squamate tree of life: Perspectives from the phenotype and the fossil record. Bull Peabody Mus Nat Hist 53:3–308. [Google Scholar]
- Gignac PM, Kley NJ, Clarke JA, Colbert MW, Morhardt AC, Cerio D, Cost IN, Cox PG, Daza JD, Early CM, Echols MS, Henkelman M, Herdina AN, Holliday CM, Zhiheng L, Mahlow K, Merchant S, Müller J, Orsbon CP, Paluh DJ, Thies ML, Tsai HP, Witmer LM. 2016. Diffusible iodine‐based contrast‐enhanced computed tomography (diceCT): An emerging tool for rapid, high‐resolution, 3‐D imaging of metazoan soft tissues. J Anat 228:889–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorniak GC, Rosenberg HI, Gans C. 1982. Mastication in the Tuatara, Sphenodon punctatus (Reptilia: Rhynchocephalia): Structure and activity of the motor system. J Morphol 171:321–353. [DOI] [PubMed] [Google Scholar]
- Gray JE. 1842. Description of two hitherto unrecorded species of reptiles from New Zealand; presented to the British Museum by Dr Dieffenbach. London, Zoological Miscellany; pp 1–72. [Google Scholar]
- Haines RW. 1940. Note on the independence of sesamoid and epiphysial centres of ossification. J Anat 75:101–105. [PMC free article] [PubMed] [Google Scholar]
- Haines RW. 1942. The tetrapod knee joint. J Anat 17:270–301. [PMC free article] [PubMed] [Google Scholar]
- Haines RW. 1969. Epiphyses and sesamoids In: Gans C, Editor. Biology of the Reptilia. New York: Academic Press; pp 81–115. [Google Scholar]
- Howes GB, Swinnerton HH. 1901. On the development of the skeleton of the tuatara, Sphenodon punctatus; with remarks on the egg, on the hatching, and on the hatched young. Trans Zool Soc Lond 16:1–84. [Google Scholar]
- Jerez A, Mangione S, Abdala V. 2010. Occurrence and distribution of sesamoid bones in squamates: A comparative approach. Acta Zool 91:295–305. [Google Scholar]
- Johnston P. 2010. The constrictor dorsalis musculature and basipterygoid articulation in Sphenodon . J Morphol 271:280–292. [DOI] [PubMed] [Google Scholar]
- Jones MEH, Curtis N, O'Higgins P, Fagan M, Evans SE. 2009. The head and neck muscles associated with feeding in Sphenodon (Reptilia: Lepidsauria: Rhynchocephalia). Palaeontol Electronica 12(2, 7A):1–56. [Google Scholar]
- Jones MEH, Curtis N, Fagan MJ, O'Higgins P, Evans SE. 2011. Hard tissue anatomy of the cranial joints in Sphenodon (Rhynchocephalia): Sutures, kinesis, and skull mechanics. Palaeontol Electronica 14(17A):1–92. [Google Scholar]
- Jones MEH, O'Higgins P, Fagan M, Evans SE, Curtis N. 2012. Shearing mechanics and the influence of a flexible symphysis during oral food processing in Sphenodon (Lepidosauria: Rhynchocephalia). Anat Rec 295:1075–1091. [DOI] [PubMed] [Google Scholar]
- Jones MEH, Anderson CL, Hipsley CA, Evans SE, Schoch R. 2013. Integration of molecules and new fossils supports a Triassic origin for Lepidosauria (lizards, snakes, and tuatara). BMC Evol Biol 13:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser JA, He L‐H, Dean MC, Jones MEH, Duncan WJ, Swain MV, Nelson NJ. 2011. Structure and compositional characteristics of caniniform dental enamel in the tuatara Sphenodon punctatus (Lepidosauria: Rhynchocephalia). New Zealand Dent J 107:44–50. [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. 2015. Mesquite: A modular system for evolutionary analysis. Version 2.75. Available at: http://mesquiteproject.org
- Maisano JA. 2002a. The potential utility of postnatal skeletal developmental patterns in squamate phylogenetics. Zool J Linn Soc 136:277–313. [Google Scholar]
- Maisano JA. 2002b. Terminal fusions of skeletal elements as indicators of maturity in squamates. J Vertebr Paleontol 22:268–275. [Google Scholar]
- Moro S, Abdala V. 2004. Analisis descriptivo de la miologia flexora y extensora del miembro anterior de Polychrus acutirostris (Squamata, Polychrotidae). Pap Avulsos Zool 44:81–89. [Google Scholar]
- Newman DG. 1987. Use and population densities of tuatara (Sphenodon punctatus) and how they are influenced by fairy prions (Pachyptila turtur) on Stephens Island, New Zealand. Herpetologica 43:336–344. [Google Scholar]
- Otero T, Hoyos JM. 2013. Sesamoid elements in lizards. Herpetol J 23:105–114. [Google Scholar]
- Parkinson, B. 2002. The Tuatara, New Zealand Wild Series, 2nd ed. New Zealand: Reed Publishing. [Google Scholar]
- Rauhut OWM, Heyng AM, López‐Arbarello A, Hecker A. 2012. A new rhynchocephalian from the Late Jurassic of Germany with a dentition that is unique amongst tetrapods. PLoS One 7:e46839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder TW, Townsend TM, Mulcahy DG, Noonan BP, Wood PL Jr, Sites JW Jr, Wiens JJ. 2015. Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLoS One 10:e0118199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault S, Jones MEH, Pitsillides AA, Hutchinson JR. 2016. Anatomy, morphology and evolution of the patella in squamate lizards and tuatara (Sphenodon punctatus). J Anat 228:864–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischek A. 1885. Observations on Sphenodon punctatum, fringe‐back lizard (tuatara). Trans Proc NZ Inst 18:108–110. [Google Scholar]
- Reynoso V‐H. 1998. Huehuecuetzpalli mixtecus gen. et sp. nov: A basal squamate (Reptilia) from the Early Cretaceous of Tepexi de Rodríguez, Central México. Phil Trans R Soc Lond B 353:477–500. [Google Scholar]
- Reynoso V‐H. 2000. An unusual aquatic sphenodontian (Reptilia: Diapsida) from the Tlayua Formation (Albian), central Mexico. J Paleontol 74:133–148. [Google Scholar]
- Rheubert JL, Cree A, Downes M, Sever DM. 2013. Reproductive morphology of the male tuatara, Sphenodon punctatus . Acta Zool 94:454–461. [Google Scholar]
- Robb J. 1977. The Tuatara. Durham: Meadowfield Press Limited; pp 1–64. [Google Scholar]
- Sanger TJ, Gredler ML, Cohn MJ. 2015. Resurrecting embryos of the tuatara, Sphenodon punctatus, to resolve vertebrate phallus evolution. Biol Lett 11:20150694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk K. 1986. Morphology of the tongue in the tuatara, Sphenodon punctatus (Reptilia: Lepidosauria), with comments on function and phylogeny. J Morphol 188:129–156. [DOI] [PubMed] [Google Scholar]
- Sustaita D, Pouydebat E, Manzano A, Abdala V, Hertel F, Herrel A. 2013. Getting a grip on tetrapod grasping: form, function, and evolution. Biol Rev 88:380–405. [DOI] [PubMed] [Google Scholar]
- Tracy MR. 1997. Size variation in tuatara (Sphenodon). New Zealand J Zool 24:330. [Google Scholar]
- Tsai HP, Holliday CM. 2011. Ontogeny of the alligator cartilago transiliens and its significance for sauropsid jaw muscle evolution. PLoS One 6:e24935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell CL, Cree A, Towns DR. 2000. Variation in reproduction and condition of northern tuatara (Sphenodon punctatus punctatus) in the presence and absence of kiore. Science for Conservation 153. Wellington, New Zealand: Department of Conservation; pp 1–42. [Google Scholar]
- Vickaryous MK, Olson WM. 2007. Sesamoids and ossicles in the appendicular skeleton In: Hall BK, editor. Fins into Limbs: Evolution, Development and Transformation. Chicago: University of Chicago Press; pp 323–341. [Google Scholar]
- White TD, Anderson RA. 1994. Locomotor patterns and costs as related to body size and form in teiid lizards. J Zool 233:107–128. [Google Scholar]
- Zheng Y, Wiens JJ. 2016. Combining phylogenomic and supermatrix approaches, and a time‐calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol Phylogenet Evol 4:537–547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


