Abstract
Background:
Antibody drug conjugates (ADC) offer the potential of maximizing efficacy while minimizing systemic toxicity. ASG-5ME, an SLC44A4-targeting antibody carrying auristatin E (MMAE), a microtubule-disrupting agent, was investigated in men with metastatic castration resistant prostate cancer.
Methods:
The primary objective of this phase I study was to determine maximum tolerated dose (MTD) and recommended phase II dose. Secondary objectives were safety, antitumor activity, pharmacokinetic properties, immunogenicity, and the detection of SLC44A4 on circulating tumor cells. Patients (pts) were treated among 7 dose levels every 21 days. A dose expansion phase enrolled 20 additional pts at the MTD.
Results:
Twenty-six and 20 pts were treated in dose escalation and dose expansion cohorts respectively. The MTD was 2.7mg/kg. Dose-limiting toxicities occurred in 4 pts: grade 3 fatigue (n=1); grade 3 abdominal pain, diarrhea and fatigue (n=1); grade 4 neutropenia and hyponatremia and grade 3 maculopapular rash, constipation and hypoxia (n=1); grade 3 troponin elevation without cardiac sequelae (n=1). Fatigue and diarrhea were the most prevalent adverse events (AEs) across all cycles. Two grade 5 AEs occurred in the dose expansion cohort, each after 1 dose: 1 pt developed grade 3 hyperglycemia, renal insufficiency and leukopenia; 1 pt developed grade 3 hyperglycemia complicated by bacteremia. Free MMAE levels did not accumulate with repeat dosing. Of evaluable pts, 52% had either stable disease or a partial response.
Conclusions:
Further development of ASG-5ME is not being pursued due to its narrow therapeutic index. Some toxicities were potentially related to on-target effects on normal tissue expressing the SLC44A4 protein. However, other toxicities were consistent with studies of previous MMAE-containing ADCs. Unconjugated MMAE is a less likely etiology based on prior data.
Keywords: Prostate Cancer, Castration-Resistant, Antibody Drug Conjugate
Introduction
Docetaxel and cabazitaxel prolong life in men with metastatic castration-resistant prostate cancer (mCRPC), but are associated with cumulative side effects including neuropathy, fatigue and diarrhea [1, 2]. Antibody drug conjugates (ADC) hold the promise of targeted delivery of cytotoxic agents while limiting exposure to healthy tissue. In this study, we tested an ADC composed of ASG-5ME, a protein- specific monoclonal antibody, linked via a dipeptide molecule to auristatin E (MMAE).
Solute carrier family 44 member 4 (SLC44A4) is a transmembrane protein identified using a murine (M5–131.121) monoclonal antibody against its extracellular domain. [3, 4]. SLC44A4 was expressed in 87%of metastatic prostate cancer samples as well asnormal tissue, including bronchial epithelium, the gastrointestinal mucosa and hepatic bile ducts. In tumor samples SLC44A4 is adjacent to the intravascular space. By contrast, in normal tissue SLC44A4 expression is found on the apical surface, with the extracellular portion facing toward the lumen [5]. The expression level and location differential of SLC44A4 suggested it would make a reasonable target for an ADC.
AGS-5M2 is a fully human IgG2k monoclonal antibody that targets the first extracellular domain of SLC44A4. Using a valine-citrulline (vc) maleimidocaproyl linker, AGS-5M2 was conjugated to the microtubule disrupting agent, MMAE. MMAE is estimated to be 100–1000 times more potent than doxorubicin, but shows no degradation in plasma, in human liver lysosomal environment or by the action of proteases. The intact ADC is termed ASG-5ME [5]. Once bound to SLC44A4, proteolytic cleavage via cathepsin B of the vc linker occurs through the endocytic pathway and MMAE is released. Unconjugated MMAE disrupts tubulin polymerization, causing G2/M-phase cell cycle arrest and apoptosis [6].
In a preclinical in vitro model, PC3 cell lines engineered to express SLC44A4 were exposed to ASG-5ME, resulting in dose-dependent cell death. These effects were also observed in SLC44A4 prostate cancer xenograft models [5]. Owing to these promising preclinical data, a phase I study of ASG-5ME was initiated in men with CRPC.
Materials and Methods
Ethics
Written informed consent was granted by all study participants. This was an open-label, multi-center dose escalation phase I study with an expansion cohort in men with CRPC. This registered study (ClinicalTrials.gov Identifier: NCT01228760) was conducted through the Prostate Cancer Clinical Trial Consortium and received approval from the participating centers’ institutional review boards.
Trial Design
Patient demography is described in Table 1. Patients 18 years or older with histologically confirmed, CRPC, metastatic or non-metastatic and adequate hematologic and biochemical function were eligible. Key exclusion criteria included recent exposure (< 4 weeks) to cytotoxic chemotherapy or radiation therapy, daily use of prednisone (or equivalent corticosteroids) greater than 20 mg a day, antiandrogen therapy within 6 weeks of study enrollment, and monoclonal antibody therapy exposure within 3 months of enrollment, except denosumab.
Table 1.
Baseline Patient Demographic and Clinical Characteristics (n = 46)
| Characteristic | n (%) |
|---|---|
| Age, years | |
| Median (range) | 69.5 (53, 87) |
| Ethnicity | |
| Hispanic or Latino | 1 (2) |
| Caucasian | 45 (98) |
| ECOG | |
| 0 | 30 (65) |
| 1 | 14 (31) |
| 2 | 2 (4) |
| Gleason score at diagnosis | |
| ≤ 7 | 22 (48) |
| 8 | 7 (15) |
| 9 | 14 (30) |
| 10 | 3 (7) |
| Extent of Disease | |
| Local recurrence | 2 |
| Bone only | 17 |
| Lymph node only | 5 |
| Bone and lymph node only | 14 |
| Non-metastatic biochemical relapse | 2 |
| Visceral involvement* | 8 |
| Number of prior chemotherapy regimens | |
| 0 | 19 (41) |
| 1 | 15 (33) |
| ≥2 | 12 (26) |
| CTC (Veridex) (n = 43) | |
| < 5 | 24 (56) |
| ≥5 | 19 (44) |
| Laboratory Values | Median (range) |
| PSA, ug/ml | 105.6 (2.4, 4340.5) |
| Hemoglobin | 12.1 (9.2, 15.6) |
| LDH | 243.0 (146, 791) |
| Alkaline Phosphatase | 99.5 (34, 1492) |
Brain, liver, and lung
Treatment Schedule
The dose escalation group comprised 7 cohorts: 0.3 mg/kg, 0.6 mg/kg, 1.2 mg/kg, 1.8 mg/kg, 2.4 mg/kg, 2.7 mg/kg and 3.0 mg/kg with 3, 3, 3, 3, 6, 6 and 2 subjects in each cohort, respectively. The dose expansion group comprised 2 cohorts, 2.4 mg/kg and 2.7 mg/kg, with 11 and 9 subjects, respectively.
Treatment and Dose Escalation
Patients received a 30-minute infusion of ASG-5ME every 3 weeks until disease progression, unacceptable toxicity, or withdrawal of consent. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria (NCI CTCAE) Version 4.0 [7]. The study’s dose limiting toxicity (DLT) window was defined as cycle 1, days 1–21 in the dose escalation cohort. DLT was defined as any non-laboratory adverse event (AE) grade ≥ 3 and non-hematologic laboratory grade ≥ 3. Hematologic DLT was defined as: grade 4 neutropenia lasting 5 days; grade 4 thrombocytopenia or anemia not related to underlying disease; and grade 3 thrombocytopenia with bleeding or platelet-transfusion requirement. Given the occurrence of vision changes in this trial and prior reports of visual changes with other ADCs, the protocol was amended to add complete eye exams (9).
Pharmacokinetic analysis
Blood samples for pharmacokinetic (PK) analysis were collected from the dose escalation cohort within 2 hours prior to cycles 1and 4 and at the following time points after cycles 1 and 4: 2, 4, 7, 24, 48, 72, 168 and 336 hours after treatment and within 2 minutes after the end of the infusion. Pretreatment samples were also taken prior to cycles 2, 3, 5–9 and beyond cycle 9 every 12 weeks (+/− 5 days).
PK parameters assessed included maximum observed plasma concentration (Cmax), time of maximum observed plasma concentration (Tmax), partial area under the concentration-time curve (AUC), terminal elimination half-life (t1/2), and volume of distribution (Vz). Additionally, PK analysis measured serum total antibody (Tab: serum free antibody + serum antibody drug conjugate), serum ADC, and serum MMAE. PK parameters were analyzed using noncompartmental methods (Phoenix™ WinNonlin® Build 6.2.1.51).
Antitumor effects
Disease assessment was performed every 4 cycles and included history, physical exam PSA levels and radiological imaging. Imaging outcomes were assessed by Prostate Cancer Working Group 2 (PCWG2) and RECIST criteria (version 1.1) [8].
Immunogenicity
Patient serum samples collected prior to cycles 1 – 9 and every 12 weeks thereafter were evaluated for human anti-ASG-5ME antibodies by a screening assay with a cut-point titration method. Biotinylated and (Ru+)-labeled ASG-5ME enabled the capture and detection of antibodies via electrochemiluminescence in a bridging ELISA. Both positive and negative controls were included.
Tissue and CTC Target Evaluation
SLC44A4 expression using immunohistochemical staining was performed on primary tumor specimens at the Agensys, Inc. research facility. CTCs were collected for enumeration during cycles 1 to 4 and prior to each dose thereafter. CTCs were assessed using the CellSearch System (Veridex, Raritan, NJ) [9]. Results were reported as the number of CTCs per 7.5ml of blood and divided into favorable versus unfavorable groups based on their previously defined prognostic significance [10]. A subset of CTC samples was assessed for the epithelial marker EPCAM and AGS-5ME expression with fluorescence-activated cell sorting (FACS).
Statistical Analysis
The primary objective of the study was to define the MTD and the subsequent phase II dose. Dose escalation followed a 3+3 design. As this is a first in-human study of ASG-5ME, there is no prior estimate of underlying DLT rate. Based on preclinical toxicology data and a < 5% DLT rate assumption, we predicted a 3% chance that the dose escalation phase would be halted in a given cohort of the study. If the preclinical toxicology data was inaccurate and a 50% DLT rate was assumed, there was an 83% chance that the dose escalation phase would be halted in a given cohort. An expansion cohort of 20 patients was enrolled to collect additional safety data and explore the antitumor effect of the drug.
Results
Between October 2010 and February 2013, 26 patients in 7 dose escalation cohorts and 20 patients in 2 dose expansion cohorts (table 1). The median number of cycles completed for the entire study population was 3.5 (range 1–12) and the median time patients remained on study was 11.1 weeks (range: 1.6 – 39.1).
Sixteen patients (35%) had immunohistochemical staining for SLC44A4 performed on the primary tumor. Of these, 10 (63%) had high SLC44A4 expression (defined as 201–300), 4 (25%) had moderate expression (defined as 101–200) and 1 (6%) had low expression (defined as 1–100). Of the 10 patients with CTCs evaluated by FACS, 8 had > 82% SLC44A4 expression (table 5).
Table 5.
Circulating Tumor Cells (CTCs) Evaluated by FACS (n = 10)
| Subject | Total # of CTCs (Veridex) | Total # of CTCs (FACS) | Total # of ASG positive CTCs (FACS) (%) | Total # of EPCAM positive CTCs (FACS) (%) | Baseline PSA (ug/L) | # doses | Duration on study (days) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 195 | 180 (92) | 31 (16) | 2.35 | 1 | 21 |
| 2 | 0 | 127 | 127 (100) | 94 (74) | 24.2 | 1 | 23 |
| 3 | 71 | 186 | 59 (32) | 171 (92) | 1219.8 | 1 | 43 |
| 4 | 139 | 646 | 40 (6) | 636 (98) | 371.62 | 2 | 57 |
| 5 | 2 | 623 | 610 (98) | 29 (5) | 234.84 | 3 | 77 |
| 6 | 1 | 10 | 9 (90) | 9 (90) | 114 | 3 | 78 |
| 7 | 61 | 138 | 113 (82) | 30 (22) | 2475.7 | 4 | 72 |
| 8 | 6 | 113 | 101 (89) | 38 (34) | 214 | 4 | 85 |
| 9 | 0 | 117 | 105(90) | 24 (21) | 74.5 | 4 | 85 |
| 10 | 0 | 61 | 61 (100) | 3(5) | 57.4 | 4 | 106 |
Of the 44 patients evaluable for PSA change, 15 (34%) responded (defined as a > 25% decline in PSA from baseline), with 11 of these patients having a >50% decline. Twenty (44%) of 46 patients had measurable disease. Of the evaluable patients (n=16), two (12%) patients had a partial response, 7 (41%) had stable disease and 7 (41%) had progressive disease. Forty-two (91%) baseline CTC samples were received out of the 46 subjects treated, with 23 (55%) subjects having favorable CTC counts. Of the thirty-two (70%) patients with baseline and week 4 CTC samples, 16 (50%) patients had unfavorable CTC counts at baseline, with 4 subjects (25%) converting to a favorable CTC count at week 4.
Ten (22%) patients were taken off study for AE’s, 16 (35%) for radiographic progression, 9 (20%) for clinical progression, 9 (20%) at investigator discretion, and 2 (4%) for withdrawn consent. AE’s reported during cycle 1 in the dose escalation cohort are detailed in Table 2. Fatigue, the most common AE, occurred in 27% of subjects during cycle 1. DLTs occurred in 4 patients: 1 at 2.4 mg/kg (grade 3 fatigue and asthenia), 1 at 2.7 mg/kg (grade 3 abdominal pain, diarrhea, and constipation), and 2 at 3 mg/kg. For the latter 2 patients, one experienced grade 4 neutropenia and hyponatremia, grade 3 maculo-papular rash, constipation and hypoxia. The other patient experienced a grade 3 troponin elevation without findings on cardiac catheterization. Across all cycles, fatigue and diarrhea were the most prevalent AEs, seen in 46% and 24% of patients, respectively (table 4). Grade 3–4 AEs were observed in 55% and 46% of subjects in the dose expansion and dose escalation cohorts respectively. The higher number of grade 3–4 AEs led to a decrease in the initial dose chosen for the dose expansion cohort, from 2.7mg/kg to 2.4 mg/kg. Two deaths occurred during in the dose expansion cohort, triggering an amendment to cap the dose for patients weighing greater than 120 kg. The first on-study death occurred in a 64-year-old treated in the 2.7mg/kg group (305 mg at weight 114 kg) who had received 3 prior chemotherapy regimens for mCRPC. After one cycle, the patient developed grade 3 hyperglycemia, renal insufficiency, and leukopenia. Two weeks following treatment, he developed an ileus, profound hypotension, and renal failure requiring dialysis. He died 26 days after his first treatment of multi-organ failure thought to be related to the ADC. The second on-study death occurred in a 53-year-old chemotherapy-naïve patient who received ASG-5ME at 2.4 mg/kg (384 mg at weight 160 kg). One week after his first treatment, the patient developed grade 3 hyperglycemia, necessitating inpatient admission for insulin and oral hypoglycemics. Three days later and in the setting of streptococcus viridans bacteremia, the patient’s symptoms worsened, and he required intubation. In the setting of worsening sepsis and multi-organ failure, the patient died.
Table 2.
Summary of Adverse Events (AEs) during Cycle 1 in the Dose Escalation Cohort (n = 26)
| Escalation (n = 26) AE Grades |
||||
|---|---|---|---|---|
| G1&2 | G3 | G4 | G5 | |
| General disorder | ||||
| Fatigue | 6 (23.1%) | 1 (3.8%) | 0 | 0 |
| Hematologic disorder | ||||
| Neutropenia | 0 | 0 | 2 (7.7%) | 0 |
| Cardiac disorders | ||||
| Hypertension | 0 | 1 (3.8%) | 0 | 0 |
| Troponin increased | 0 | 1 (3.8%) | 0 | 0 |
| Gastrointestinal disorders | ||||
| Abdominal pain | 0 | 1 (3.8%) | 0 | 0 |
| Constipation | 0 | 2 (7.7%) | 0 | 0 |
| Diarrhea | 4 (15.4%) | 1 (3.8%) | 0 | 0 |
| Metabolic disorder | ||||
| Hypophosphatemia | 0 | 1 (3.8%) | 0 | 0 |
| Inappropriate antidiuretic hormone secretion | 0 | 0 | 1 (3.8%) | 0 |
| Pulmonary disorder | ||||
| Hypoxia | 0 | 1 (3.8%) | 0 | 0 |
| Miscellaneous | ||||
| Maculopapular rash | 0 | 1 (3.8%) | 0 | 0 |
| Musculoskeletal pain | 0 | 1 (3.8%) | 0 | 0 |
Subjects with AEs in multiple severity ratings are counted once under the maximum severity.
The table summarizes grade 1 and grade 2 AEs occurring in >=20% of subjects and all grade 3 or higher AEs.
Table 4.
Summary of all Adverse Events on Study (n = 46)
| Total (n=46) |
||||
|---|---|---|---|---|
| G1&2 | G3 | G4 | G5 | |
| General disorder | ||||
| Decreased appetite | 19 (41.3%) | 0 | 0 | 0 |
| Fatigue | 26 (56.5%) | 7 (15.2%) | 0 | 0 |
| Peripheral edema | 0 | 1 (2.2%) | 0 | 0 |
| Hematologic disorder | ||||
| Anemia | 0 | 1 (2.2%) | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 1 (2.2%) | 0 |
| Leukopenia | 0 | 2 (4.3%) | 0 | 0 |
| Neutropenia | 0 | 1 (2.2%) | 2 (4.3%) | 0 |
| Cardiac disorders | ||||
| Angina pectoris | 0 | 1 (2.2%) | 0 | 0 |
| Cardiac failure | 0 | 1 (2.2%) | 0 | 0 |
| Electrocardiogram abnormalities | 0 | 2 (4.3%) | 0 | 0 |
| Hypertension | 0 | 2 (4.3%) | 0 | 0 |
| Hypotension | 0 | 0 | 1 (2.2%) | 0 |
| Troponin increased | 0 | 1 (2.2%) | 0 | 0 |
| Ophthalmic disorders | ||||
| Blurry vision | 0 | 1 (2.2%) | 0 | 0 |
| Cataracts | 0 | 2 (4.3%) | 0 | 0 |
| Gastrointestinal disorders | ||||
| Abdominal pain | 0 | 1 (2.2%) | 0 | 0 |
| Constipation | 16 (34.8%) | 2 (4.3%) | 0 | 0 |
| Diarrhea | 13 (28.3%) | 2 (4.3%) | 0 | 0 |
| Enterovesical fistula | 0 | 1 (2.2%) | 0 | 0 |
| Gastrointestinal hemorrhage | 0 | 1 (2.2%) | 0 | 0 |
| Increased transaminases | 0 | 1 (2.2%) | 0 | 0 |
| Nausea | 14 (30.4%) | 1 (2.2%) | 0 | 0 |
| Small bowel obstruction | 0 | 1 (2.2%) | 0 | 0 |
| Infectious disorders | ||||
| Miscellaneous infections | 0 | 2 (4.3%) | 0 | 0 |
| Sepsis | 0 | 0 | 0 | 1 (2.2%) |
| Metabolic disorder | ||||
| Hyperglycemia | 0 | 3 (6.5%) | 1 (2.2%) | 0 |
| Hyponatremia | 0 | 0 | 1 (2.2%) | 0 |
| Hypophosphatemia | 0 | 1 (2.2%) | 0 | 0 |
| Inappropriate antidiuretic hormone secretion | 0 | 1 (2.2%) | 1 (2.2%) | 0 |
| Nervous System disorder | ||||
| Motor | 0 | 1 (2.2%) | 1 (2.2%) | 0 |
| Sensory | 14 (30.4%) | 2 (4.3%) | 0 | 0 |
| Renal disorder | ||||
| Acute renal failure | 0 | 0 | 2 (4.3%) | 0 |
| Pulmonary disorder | ||||
| Dyspnea | 14 (30.4%) | 1 (2.2%) | 0 | 0 |
| Hypoxia | 0 | 1 (2.2%) | 0 | 0 |
| Pulmonary embolism | 0 | 0 | 1 (2.2%) | 0 |
| Miscellaneous | ||||
| Depression | 0 | 1 (2.2%) | 0 | 0 |
| Maculopapular rash | 0 | 1 (2.2%) | 0 | 0 |
| Multi-organ failure | 0 | 0 | 0 | 2 (4.3%) |
| Musculoskeletal pain | 0 | 4 (8.7%) | 0 | 0 |
Subjects with AEs in multiple severity ratings are counted once under the maximum severity.
The table summarizes grade 1 and grade 2 AEs occurring in > 20% of subjects and all grade 3 or higher AEs.
The Cmax and AUC increased linearly without significant accumulation for ASG-5ME, free MMAE and Tab (figure 1a). Tab was higher overall compared to ADC concentration alone, implying that some amount of free antibody was administered (Table 3 and figure 1c). Only 1 of the 40 evaluable subjects evaluable developed anti-ASG-5ME antibodies and did so after cycle 4 (0.6mg/kg cohort).
Figure 1a.
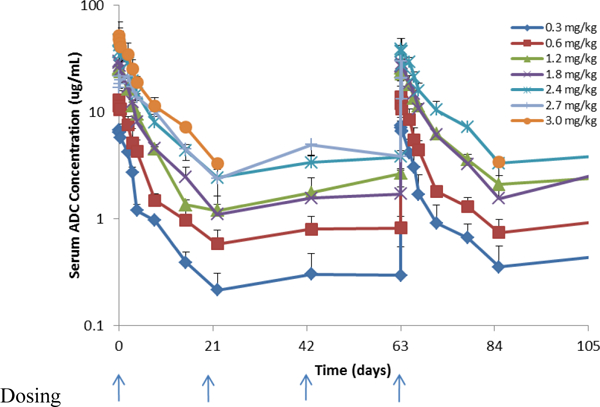
Serum concentration time profiles for ASG-5ME antibody drug conjugate (ADC)
Table 3.
Pharmacokinetic Properties (n = 46): [No. pts, value +/−SD]
| 0.3 mg/kg (N=3) | 0.6 mg/kg (N=3) | 1.2 mg/kg (N=3) | 1.8 mg/kg (N=3) | 2.4 mg/kg (N=17) | 2.7 mg/kg (N=15) | 3.0 mg/kg (N=2) | |
|---|---|---|---|---|---|---|---|
| ADC | |||||||
| Study Week 1 | |||||||
| AUC(d0–21): day*mg/mL | 3, 26.9 +/− 2.06 | 3, 55.3 +/− 12.54 | 3, 122.3 +/− 4.93 | 3, 131.7 +/− 10.02 | 3, 239.0 +/− 28.00 | 4, 213.3 +/− 34.80 | - |
| Cmax: mg/mL | 3, 7.1 +/− 0.44 | 3, 13.1 +/− 3.38 | 3, 28.1 +/− 1.78 | 3, 30.9 +/− 2.75 | 16, 52.3 +/− 9.63 | 15, 48.9 +/− 21.60 | 2, 52.1 +/− 2.26 |
| T1/2: days | 3, 6.59 +/− 2.015 | 3, 11.21 +/− 4.643 | 3, 5.51 +/− 1.373 | 3, 6.75 +/− 0.718 | 14, 7.03 +/− 2.142 | 11, 37.00 +/− 98.515 | 2, 6.54 +/− 1.810 |
| Tmax: days | 3, 0.04 +/− 0.036 | 3, 0.02 +/− 0.000 | 3, 0.09 +/− 0.073 | 3, 0.09 +/− 0.073 | 16, 0.05 +/− 0.075 | 15, 0.35 +/− 0.678 | 2, 0.02 +/− 0.000 |
| Vz: mL/kg | 3, 33.6 +/− 8.84 | 3, 51.9 +/− 24.25 | 3, 40.5 +/− 40.45 | 3, 60.2 +/− 38.15 | 4, 40.8 +/− 14.15 | 5, 140.1 +/− 122.61 | 2, 82.1 +/− 16.12 |
| Study Week 4 | |||||||
| Cmax: mg/mL | 3, 7.5 +/− 1.43 | 3, 13.8 +/− 3.66 | 2, 26.6 +/− 3.32 | 2, 29.1 +/− 6.29 | 7, 79.8 +/− 48.42 | 6, 63.0 +/− 47.68 | - |
| T1/2: days | 3, 5.8 +/− 3.26 | 3, 8.1 +/− 1.85 | 2, 10.0 +/− 0.70 | 2, 8.3 +/− 3.68 | 5, 6.7 +/− 1.62 | 5, 7.4 +/− 1.74 | - |
| TAb | |||||||
| Study Week 1 | |||||||
| AUC(d0–21): day*mg/mL |
3, 41.6 +/− 5.06 | 3, 83.4 +/− 14.35 | 3, 158.3 +/− 6.43 | 3, 209.7 +/− 39.55 | 3, 333.7 +/− 32.47 | 4, 270.3 +/− 27.18 | - |
| Cmax: mg/mL | 3, 7.7 +/− 0.56 | 3, 15.1 +/− 3.76 | 3, 29.1 +/− 1.50 | 3, 32.9 +/− 2.72 | 16, 60.2 +/− 9.80 | 15, 51.2 +/− 19.02 | 2, 64.3 +/− 7.78 |
| T1/2: days | 3, 10.16 +/− 3.781 | 3, 9.96 +/− 1.283 | 3, 6.84 +/− 1.820 | 3, 9.23 +/− 2.185 | 14, 8.77 +/− 1.892 | 11, 9.14 +/− 2.064 | 2, 9.97 +/− 3.295 |
| Tmax: days | 3, 0.1 +/− 0.07 | 3, 0.0 +/− 0.00 | 3, 0.1 +/− 0.07 | 3, 0.1 +/− 0.07 | 16, 0.1 +/− 0.11 | 15, 0.4 +/− 0.87 | 2, 0.1 +/− 0.00 |
| Vz: mL/kg | 3, 22.2 +/− 10.86 | 3, 21.8 +/− 11.26 | 3, 31.3 +/− 34.86 | 3, 42.6 +/− 33.16 | 4, 27.8 +/− 8.54 | 5, 55.3 +/− 26.74 | 2, 65.7 +/− 8.98 |
| Study Week 4 | |||||||
| Cmax: mg/mL | 3, 9.4 +/− 2.22 | 3, 16.8 +/− 3.80 | 2, 32.6 +/− 0.64 | 2, 36.9 +/− 6.93 | 7, 55.5 +/− 16.47 | 6, 49.0 +/− 10.34 | - |
| T1/2: days | 3, 8.06 +/− 4.178 | 3, 11.54 +/− 1.577 | 2, 8.44 +/− 1.266 | 2, 8.71 +/− 1.259 | 5, 8.53 +/− 1.753 | 4, 10.60 +/− 2.717 | - |
| MMAE | |||||||
| Study Week 1 | |||||||
| AUC(d0–21): day*mg/mL |
2, 9835.0 +/−5465.94 | 3, 10390.0 +/−2493.81 | 3, 24733.3 +/−8894.01 | 2, 35350.0 +/−12798.63 | 4, 34575.0 +/−1497.50 | 4, 51675.0 +/−24000.47 | 1, 122000 +/− - |
| Cmax: mg/mL | 3, 0.7 +/− 0.35 | 3, 1.1 +/− 0.36 | 3, 2.2 +/− 0.93 | 3, 2.7 +/− 1.50 | 16, 5.3 +/− 3.46 | 15, 4.8 +/− 2.02 | 2, 6.1 +/− 4.77 |
| T1/2: days | 3, 4.20 +/− 0.441 | 3, 4.79 +/− 0.534 | 3, 4.53 +/− 0.099 | 3, 5.85 +/− 1.584 | 4, 4.06 +/− 0.281 | 4, 4.39 +/− 0.976 | - |
| Tmax: days | 3, 3.3 +/− 0.58 | 3, 2.7 +/− 0.58 | 3, 1.4 +/− 2.26 | 3, 3.0 +/− 1.00 | 5, 3.6 +/− 0.55 | 6, 4.3 +/− 1.86 | 2, 6.0 +/− 2.83 |
| Study Week 4 | |||||||
| Cmax: mg/mL | 3, 0.9 +/− 0.72 | 3, 1.0 +/− 0.09 | 2, 2.1 +/− 1.11 | 2, 2.4 +/− 1.47 | 7, 4.3 +/− 1.34 | 5, 3.7 +/− 1.12 | - |
Figure 1c.
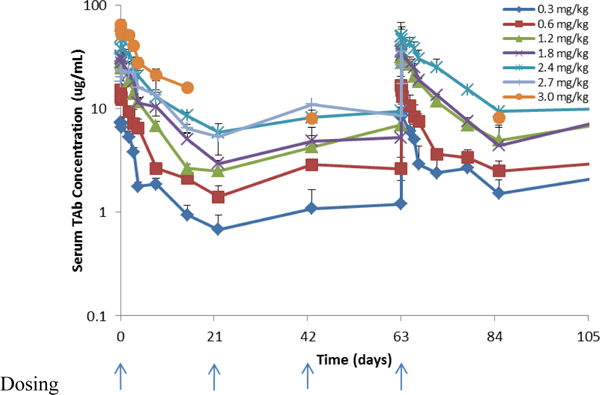
Serum concentration time profiles for ASG-5ME total antibody (Tab)
Discussion
This is the first trial of ASG-5ME in prostate cancer. Although ADCs are intended to increase agent efficacy while decreasing off-target side effects [11, 12], ASG-5ME was associated with significant toxicities, including 2 probable drug-related deaths. Limited antitumor activity was seen. This high toxicity-to-response ratio was observed despite the presence of the target on primary tumor tissue specimens and CTCs.
The reasons for the observed toxicities are not entirely clear. The PK properties of the agent demonstrate no cumulative increase at trough levels to suggest drug accumulation over cycles. Indeed, the two patient deaths occurred after only a single dose. Free MMAE is produced upon ADC internalization into cells and was measurable in the systemic circulation. The MMAE Cmax values obtained in this study were similar to those of an earlier report on MMAE levels in a weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies, yet similar toxicities were not observed in that study [13]. Hence, deconjugated free MMAE seems an unlikely culprit.
Pre-clinical IHC studies identified that SLC44A4 was found to be expressed on normal tissue including lung, fallopian tube, bladder, gastrointestinal tract, kidney, uterus, and liver [5]. Unlike other in- development ADCs in prostate cancer that target tumor-specific molecules such as prostate-specific membrane antigen (PSMA), the degree of SLC44A4’s expression on normal tissue could potentially explain the toxicities seen with this drug [15, 16]. It is also worth noting that little is known about the endogenous function of SLC44A4. It appears to play no role in tumorigenesis, supported by the lack of antitumor effect when targeted by AGS-5M2 alone. Toxicities may therefore have related either to cytotoxic drug delivery to normal tissues, or interference with normal cellular processes by targeting SLC44A4. Another possibility relates to intracellular drug localization. Tight junctions assure an epithelial barrier between systemic circulation and luminal spaces and greatly reduce diffusion of large macromolecules, thus limiting non-tumor cell effects[17]. It is now known that the neonatal FC receptor (FcRn) can transcytose IgGs across the epithelial barrier into the lumen. [18–22]. It is possible that ASG- 5ME can be transported across the epithelial barrier and contribute to significant toxicity observed.
However, these potential etiologies are undercut by the fact that this drug has also been tested in a pancreatic cancer population. In that phase I study, an MTD of 1.2 mg/kg was given weekly for 3 weeks of 4-week cycles. The most frequent grade 3 or 4 AEs reported in the MTD cohort were fatigue (28%), abdominal pain (22%), vomiting (17%) and neutropenia (17%) [14]. Hyperglycemia events were not reported, in contrast to our study, and no study-related deaths were reported. Keeping in mind that these are different patient populations, the weekly versus every 3-week dosing schedule of ASG-5ME could potentially play a role in its overall tolerability and merits further evaluation with a similar CRPC population. It is probable that the observed side effects in this trial reflect a combination of both on-target and off-target effects, and factors that may also relate to dose and schedule.
ASG-5ME did show signs of antitumor activity when measured by PSA response, specifically in the higher dosing cohorts. Furthermore, 52% and 41% of the evaluable patients had stable disease or a partial response respectively, suggesting the relevance of the target in this disease setting. Despite these antitumor effects, the narrow therapeutic index and observed toxicities precludes further development within these dosing cohorts. As a result of this study, ASG-5ME for men with CPRC will not be further pursued. Investigation of other ADCs with payloads such as tubulysin B hydrazide or targets such as PSMA continues [16, 23].
Figure 1b.
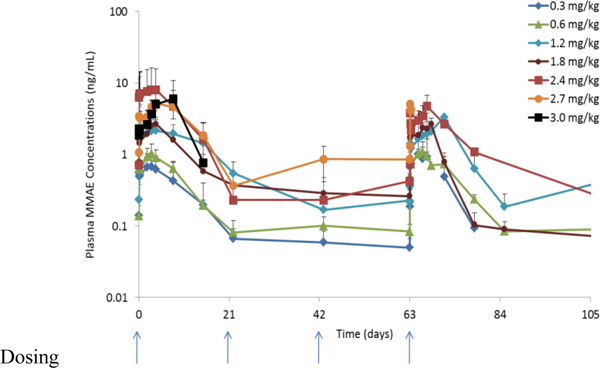
Serum concentration time profiles for ASG-5ME free MMAE (MMAE)
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Deaglan McHugh: No disclosures
Emmanuel S. Antonarakis: Honoraria - Astellas Pharma; Dendreon; ESSA; Janssen Biotech; Medivation; Sanofi. Consulting or Advisory Role - Astellas Pharma; Dendreon; ESSA; Janssen Biotech; Medivation; Sanofi. Research Funding - Aragon Pharmaceuticals (Inst); Astellas Pharma (Inst); Dendreon (Inst); Exelixis (Inst); Genentech (Inst); Janssen Biotech (Inst); Johnson & Johnson (Inst); Millennium (Inst); Novartis (Inst); Sanofi (Inst); Tokai Pharmaceuticals (Inst). Travel, Accommodations, Expenses - Dendreon; Medivation; Sanof
Mario A. Eisenberger: Honoraria - pfizer; Sanofi. Consulting or Advisory Role - Astellas Pharma; Bayer; Ipsen; pfizer; Sanofi; Sanofi. Research Funding - Genentech; Sanofi; Tokai Pharmaceuticals. Travel, Accommodations, Expenses - Astellas Pharma; Bayer; Pfizer; Sanofi
Elisabeth Heath: Honoraria - Bayer, Sanofi, Dendreon. Consulting or Advisory Role – Agensys.
Speaker’s Bureau – Sanofi.
Justine Bruce: No disclosures
Daniel C. Danila: Honoraria - Angle; Astellas Scientific and Medical Affairs Inc; Bayer; Xian-Janssen Pharmaceutical. Consulting or Advisory Role - Angle; Bayer. Research Funding - Genentech; Janssen Research & Development (Inst); Prostate Cancer Foundation. Patents, Royalties, Other Intellectual Property - GENE EXPRESSION PROFILE ASSOCIATED WITH PROSTATE CANCER. Travel, Accommodations, Expenses - American Austrian Open Medical Institute; Angle; Bayer; Cambridge Healthtech Institute; Global Technology Community; Oncology Education; Prostate Cancer Foundation; Xian-Janssen Pharmaceutical
Dana Rathkopf: Consulting or Advisory Role - Janssen Oncology. Research Funding - AstraZeneca (Inst); Celgene (Inst); Ferring (Inst); Genentech/Roche (Inst); Janssen Oncology (Inst); Medivation (Inst); Millennium (Inst); Novartis (Inst); Taiho Pharmaceutical (Inst); Takeda (Inst); TRACON Pharma (Inst) Jarett Feldman: No disclosures
Susan Slovin: Consulting or Advisory Role – Bayer.
Banmeet Anand: No diclosures
Rong Chu: No discosures
Jacqueline Lackey: No disclosures
Leonard Reyno: No disclosures
Emmanuel S. Antonarakis: Honoraria - Astellas Pharma; Dendreon; ESSA; Janssen Biotech; Medivation; Sanofi. Consulting or Advisory Role - Astellas Pharma; Dendreon; ESSA; Janssen Biotech; Medivation; Sanofi. Research Funding - Aragon Pharmaceuticals (Inst); Astellas Pharma (Inst); Dendreon (Inst); Exelixis (Inst); Genentech (Inst); Janssen Biotech (Inst); Johnson & Johnson (Inst); Millennium (Inst); Novartis (Inst); Sanofi (Inst); Tokai Pharmaceuticals (Inst). Travel, Accommodations, Expenses - Dendreon; Medivation; Sanof
Michael Morris: Consulting or Advisory Role - Astellas Pharma; Bayer; Endocyte. Research Funding - Bayer (Inst); Endocyte (Inst); Progenics (Inst); Sanofi (Inst). Travel, Accommodations, Expenses - Bayer; Endocyte
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Tannock IF, et al. , Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med, 2004. 351(15): p. 1502–12. [DOI] [PubMed] [Google Scholar]
- 2.de Bono JS, et al. , Prednisone plus cabazitaxel or mitoxantrone for metastatic castration- resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet, 2010. 376(9747): p. 1147–54. [DOI] [PubMed] [Google Scholar]
- 3.O’Regan S, et al. , An electric lobe suppressor for a yeast choline transport mutation belongs to a new family of transporter-like proteins. Proc Natl Acad Sci U S A, 2000. 97(4): p. 1835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hediger MA, et al. , The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch, 2004. 447(5): p. 465–8. [DOI] [PubMed] [Google Scholar]
- 5.Mattie M, et al. , The Discovery and Preclinical Development of ASG-5ME, an Antibody-Drug Conjugate Targeting SLC44A4-Positive Epithelial Tumors Including Pancreatic and Prostate Cancer. Mol Cancer Ther, 2016. 15(11): p. 2679–2687. [DOI] [PubMed] [Google Scholar]
- 6.Pettit GR, The dolastatins. Fortschr Chem Org Naturst, 1997. 70: p. 1–79. [DOI] [PubMed] [Google Scholar]
- 7.NCI and NIH, Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. 2010.
- 8.Scher HI, et al. , Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol, 2008. 26(7): p. 1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danila DC, et al. , Circulating tumor cell number and prognosis in progressive castration- resistant prostate cancer. Clin Cancer Res, 2007. 13(23): p. 7053–8. [DOI] [PubMed] [Google Scholar]
- 10.de Bono JS, et al. , Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res, 2008. 14(19): p. 6302–9. [DOI] [PubMed] [Google Scholar]
- 11.Verma S, et al. , Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med, 2012. 367(19): p. 1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Younes A, et al. , Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med, 2010. 363(19): p. 1812–21. [DOI] [PubMed] [Google Scholar]
- 13.Fanale MA, et al. , A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res, 2012. 18(1): p. 248–55. [DOI] [PubMed] [Google Scholar]
- 14.Coveler AL, et al. , A phase 1 clinical trial of ASG-5ME, a novel drug-antibody conjugate targeting SLC44A4, in patients with advanced pancreatic and gastric cancers. Invest New Drugs, 2016. 34(3): p. 319–28. [DOI] [PubMed] [Google Scholar]
- 15.Wright GL Jr., et al. , Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol, 1995. 1(1): p. 18–28. [DOI] [PubMed] [Google Scholar]
- 16.Endocyte, Phase 1 of EC1169 In Patients With Recurrent MCRPC 2014.
- 17.Gumbiner B, Structure, biochemistry, and assembly of epithelial tight junctions. American Journal of Physiology-Cell Physiology, 1987. 253(6): p. C749–C758. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida M, et al. , Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity, 2004. 20(6): p. 769–783. [DOI] [PubMed] [Google Scholar]
- 19.Ghetie V, et al. , Abnormally short serum half‐lives of IgG in β2‐microglobulin‐deficient mice. European journal of immunology, 1996. 26(3): p. 690–696. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson BL, et al. , Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. Journal of Clinical Investigation, 1999. 104(7): p. 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiekermann GM, et al. , Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life. Journal of Experimental Medicine, 2002. 196(3): p. 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Israel E, et al. , Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology, 1997. 92(1): p. 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrylak DP, et al. , A phase II trial of prostate-specific membrane antigen antibody drug conjugate (PSMA ADC) in taxane-refractory metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology, 2014. 32(4): p. 5s. [Google Scholar]


