Abstract
Purpose of review:
Hyperoxaluria can cause kidney disease through multiple mechanisms, including tubular obstruction from calcium oxalate crystals, sterile inflammation, and tubular epithelial cell injury. Hyperoxaluria is also observed in individuals with diabetes mellitus and obesity, which are in turn risk factors for chronic kidney disease. Whether hyperoxaluria is a potential mediator of increased risk of chronic kidney disease in diabetes mellitus and obesity is unknown.
Recent Findings:
Individuals with diabetes have increased levels of plasma glyoxal (a protein glycation product) and glyoxylate, both of which are precursors for oxalate. Increased gut absorption of oxalate in obesity may be due to obesity-associated inflammation. A recent study in individuals with chronic kidney disease found that higher 24h urinary oxalate excretion was independently associated with increased risk of kidney disease progression, especially in individuals with diabetes and obesity.
Summary:
Both diabetes mellitus and obesity are associated with higher urinary oxalate excretion through distinct mechanisms. Hyperoxaluria could be a mechanism by which kidney disease develops in individuals with diabetes mellitus or obesity and could also contribute to progressive loss of renal function. Future research on pharmacologic or dietary measures to limit oxalate absorption or generation are required to test whether lowering urinary oxalate excretion is beneficial in preventing kidney disease development and progression in diabetes mellitus and obesity.
Keywords: hyperoxaluria, diabetes, metabolic syndrome, chronic kidney disease, oxalate nephropathy
Introduction
Diabetes mellitus and obesity are massive public health burdens in the United States and increasingly around the world. Both conditions lead to premature mortality and accelerated morbidities, including kidney disease. Diabetes is the most common cause of chronic kidney disease (CKD) and end stage renal disease in the world, and obesity is likely a contributor to CKD development and progression.1 Both diabetes and obesity share another kidney-specific manifestation: nephrolithiasis. The most common constituent of kidney stones is calcium oxalate. Interestingly, both diabetes and obesity are independent risk factors for higher urinary oxalate excretion, which may account for the increased risk of nephrolithiasis observed in the two conditions.2,3 Recently, evidence from animal studies and a prospective cohort study have linked oxalate to the development of chronic kidney disease.4,5 In this review, we describe the evidence that could implicate hyperoxaluria as a novel mediator of CKD pathogenesis and progression in diabetes and obesity (Figure 1).
Figure 1.
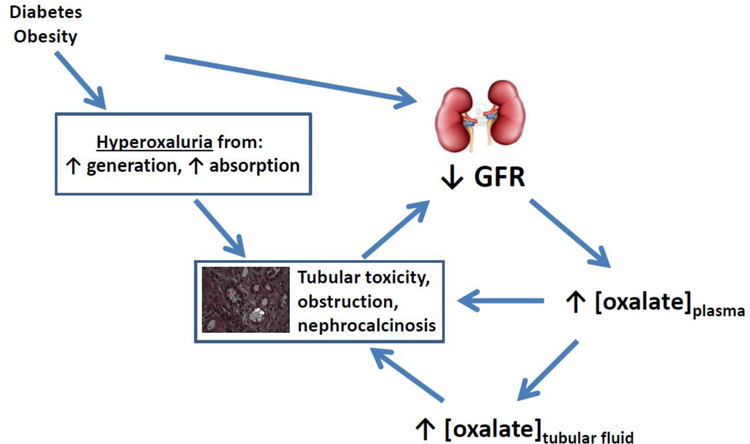
A schematic depicting how higher levels of oxalate absorption or generation in diabetes or obesity could contribute to the development or progression of chronic kidney disease.
Overview of oxalate metabolism
Oxalate (C2O42−, a dicarboxylic acid) is a terminal metabolite in humans. Oxalate in the blood and urine is derived endogenously through metabolism and also exogenously from the diet6–8 Endogenous synthesis occurs primarily in the liver, where the most common metabolic pathways converge on oxalate’s immediate precursor, glyoxylate, which has recently been identified as a potential metabolite marker of type 2 diabetes mellitus.9 Glyoxylate production in vivo occurs from the metabolism of amino acids (hydroxyproline, serine, glycine, tryptophan), glucose, fructose, ascorbic acid, and glycolate.10–13 Another precursor of oxalate is glyoxal, which can be a product of cellular peroxidation and protein glycation; glyoxal can be directly or indirectly converted into glyoxylate, which is then metabolized into oxalate.11,14
Many foods contain oxalate, which is absorbed primarily in the small intestine but also in the stomach and large intestine.15 Foods containing high amounts of oxalate include spinach, almonds, potatoes, and cocoa. Average daily oxalate intake and absorption varies across individuals due to inter-individual differences in absorption and regional differences in oxalate content of foods. A major determinant of oxalate absorption is the availability of free calcium in the gut: calcium readily and rapidly precipitates with oxalate as CaOx complexes, which are absorbed much less than free oxalate.16 Intestinal oxalate absorption occurs passively and transcellularly, with variable amounts of absorption vs. secretion occurring along the gastrointestinal tract.15,17,18 SLC26 anion exchangers mediate transcellular transport and are expressed on both apical and basolateral membranes of the human small intestine and colon. Evidence suggests that inflammation-induced changes in intestinal oxalate transport could account for obesity-induced hyperoxaluria due to suppression of active intestinal oxalate secretion or increased absorption, as described below.19
Oxalate degradation in the colon can occur through oxalate-metabolizing gut flora including Oxalobacter formigenes and other bacterial species20,21. Fecal excretion accounts for <10% of oxalate excretion.18
Circulating oxalate is freely filtered at the glomerulus, reabsorbed, and secreted by the proximal tubule.22,23 The 24h urinary excretion of oxalate is a reflection of dietary oxalate intake, net intestinal absorption (accounting for fecal excretion and colonic degradation), and endogenous oxalate synthesis from the liver. Hyperoxaluria therefore can occur from multiple causes. In human studies that have examined predictors of 24h urinary oxalate excretion in stone formers, clinical variables that have been linked to higher urinary oxalate excretion include age, higher BMI, diabetes, and higher fructose and oxalate intake.2,24 In a study involving individuals with CKD, factors independently associated with higher urinary oxalate excretion included diabetes, white vs. black race, thiazide diuretic use, and lower urinary calcium excretion and lower serum calcium.4 Hyperoxaluria is also observed in the primary hyperoxalurias, autosomal recessive disorders due to mutations in enzymes involved in oxalate metabolism, and as a secondary condition in enteric hyperoxaluria, due to increased intestinal absorption of oxalate.
Diabetes Mellitus and Urinary Oxalate Excretion
24h urine excretion of oxalate has been found to be higher in individuals with vs. without diabetes mellitus in multiple studies. Diabetes mellitus is associated with an increased risk for kidney stone formation, and calcium oxalate stones are the most common type of kidney stones.25–27 In a study of 3,123 individuals with established CKD, diabetes mellitus was independently associated with higher urinary oxalate excretion: after adjustment for a number of variables including medications, body mass index, age, race, sex, and laboratory tests, individuals with diabetes mellitus had 11% higher 24h urinary oxalate excretion than those without diabetes.4
Glyoxylate, the immediate precursor of oxalate, has been identified through metabolomic profiling of human plasma to be a potential metabolite marker of diabetes mellitus.9,28,29 A retrospective analysis of long-term blood donors found that elevated serum glyoxylate levels pre-dated the diagnosis of diabetes mellitus by up to 3 years, in analyses performed with matching for age, gender, and BMI.9 In a mouse model of diabetes (C57BLKS/J-Lepr−/−), glyoxylate levels were 6-fold higher in diabetic mice than control mice.9
Another potential precursor of oxalate is glyoxal, an alpha-oxoaldehyde which can be generated from the glycation of proteins or from lipid peroxidation from hyperglycemia in diabetes.28,30 Glyoxal has been hypothesized to be an important source of endogenous oxalate synthesis in humans and a source of oxidative stress.11,31. In a small study, glyoxal was found in a HPLC-UV screen of alpha-dicarbonyl compounds to be elevated in the plasma of individuals with diabetes compared with healthy subjects, and to correlate with HBA1C, fasting glucose, and microalbuminuria.31 Another related alpha-oxoaldehyde, methylglyoxal, was also found to be associated with incident cardiovascular disease and mortality in prospective studies of 1,003 type 2 and 159 type 1 diabetic patients.32,33 Baseline and six-year longitudinal methylglyoxal levels were inversely correlated eGFR in 1481 screen-detected type 2 diabetic patients.34 In a prospective three-year observational study of 150 individuals with CKD stages 3–5, higher methylgloxal levels (tertiles 2 and 3 compared with tertile 1) were associated with a >2-fold and > 6-fold increased risk for progression to ESRD, respectively.35
Obesity and Urinary Oxalate Excretion
Higher BMI was independently linked to higher urinary oxalate excretion in the Health Professionals Follow-Up Study, Nurses’ Health Study, and Nurses’ Health Study II.3 In the Chronic Renal Insufficiency Cohort (CRIC) study, higher BMI was associated with higher urinary oxalate excretion in unadjusted analyses, but not after multivariable adjustment.4 Obesity is known risk factor for nephrolithiasis,36 which is most commonly due to calcium oxalate containing stones.
Two recent studies identified mechanisms of hyperoxaluria in obesity, highlighting the role of inflammation.37,38 Amin et al. found evidence using the obese ob/ob mouse model to support reduced active intestinal oxalate secretion from local and systemic inflammation as a cause for a reduction in fecal excretion of oxalate.38 Bashir et al. from the same laboratory also found evidence for increased paracellular absorption of oxalate all along the gastrointestinal tract.37 They also found that proinflammatory cytokines and oxidative stress, which are elevated in obesity, significantly enhanced paracellular intestinal absorption of oxalate in vitro and ex vivo. These studies highlight an important role for increased gut absorption and decreased gut secretion – both of which are enhanced by inflammation – as a cause of obesity-associated hyperoxaluria.
Evidence on the association between urinary oxalate excretion and CKD
In 3,123 CRIC participants,4 higher levels of urinary oxalate excretion (≥ 40th percentile compared with < 40th percentile) were found to be associated with a 32% higher risk of kidney disease progression and 37% higher risk of ESRD in multivariable-adjusted analyses. Cross-sectionally, higher urinary oxalate excretion was observed in those with lower eGFR and greater albuminuria. In prospective analyses of kidney function decline, the strongest signals were observed in those with higher BMI (45% higher risk of ESRD) and those with diabetes mellitus (44% higher risk of ESRD). A review of renal biopsy cases described the association of oxalate nephropathy as a cause of progressive CKD and ESRD.39 The mechanisms by which oxalate can cause kidney injury have also been explored in several animal studies5,40,41. Sterile inflammation from the intracellular nucleotide-binding domain, leucine-rich repeat–containing receptor, pyrin domain–containing-3 (NLRP3) inflammasome activation has been reviewed as a key mechanism underlying the observation.5,41 CKD models from oxalate feeding have also been introduced as a reproducible model of chronic kidney disease that recapitulates the clinical manifestations of CKD in humans.5,42 Recently, Saenz et al. found that metabolic syndrome contributes to hyperoxaluria-induced renal injury in a murine model of nephrolithiasis, consistent with the observation in CRIC of a stronger signal of the oxalate association with kidney dysfunction in those with higher BMI.43
Potential for future therapeutics
If the association between hyperoxaluria and CKD in the setting of diabetes and/or obesity is indeed causal, then therapeutic strategies aimed at lowering urinary oxalate excretion may prove fruitful to prevent CKD or slow its progression. Reducing oxalate absorption from the gastrointestinal tract may be accomplished by dietary modifications (e.g., avoiding high oxalate-containing foods; supplemental calcium), medications to bind oxalate in the gut (e.g., calcium or non-calcium-containing phosphorous binders44), or medications to enzymatically degrade in the gastrointestinal tract.45 Reducing oxalate generation by the liver is being explored for the treatment of primary hyperoxaluria using gene-targeting technologies to inhibit enzymes involved in oxalate metabolism.46,47 Recently, Le Dudal et al. showed promising results with stiripentol, an antiepileptic drug that inhibits lactate dehydrogenase 5 isoenzyme (the last step of hepatic oxalate production).48 Stiripentol reduced oxalate generation in vitro and in rat models protected kidneys from oxalate-induced injury from ethylene glycol intoxication and chronic calcium oxalate nephropathy. The authors also found lower oxalate excretion in a small number of patients treated with stiripentol and used the drug to reduce urinary oxalate excretion in a young girl with severe type 1 hyperoxaluria.
Conclusion
Both diabetes and obesity are associated with increased absorption or generation of oxalate, which in turn may increase the risk of kidney injury. Whether targeting oxalate generation or absorption could be protective in diabetes or obesity will require additional investigation.
Key points:
Diabetes mellitus and obesity are both associated with higher urinary oxalate excretion and increased risk of kidney stones.
Diabetes mellitus and obesity are also risk factors for chronic kidney disease
Oxalate can cause kidney injury through multiple mechanisms, including obstruction and sterile inflammation
The association of diabetes mellitus and obesity with chronic kidney disease raises the question whether oxalate may be a mediator
Further study is required to evaluate whether targeting oxalate absorption or generation could be protective in diabetes mellitus or obesity
Acknowledgment:
Financial support and sponsorship: This work was supported by funding R01DK103784
Footnotes
Conflicts of interest: Dr. Waikar has served as an advisor for Allena Pharmaceuticals.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet (London, England) 2013;382(9888):260–272. [DOI] [PubMed] [Google Scholar]
- 2.Taylor EN, Curhan GC. Determinants of 24-hour urinary oxalate excretion. Clinical journal of the American Society of Nephrology : CJASN 2008;3(5):1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor EN, Curhan GC. Body size and 24-hour urine composition. American journal of kidney diseases : the official journal of the National Kidney Foundation 2006;48(6):905–915. [DOI] [PubMed] [Google Scholar]
- ** 4.Waikar SS, Srivastava A, Palsson R, et al. Association of Urinary Oxalate Excretion With the Risk of Chronic Kidney Disease Progression. JAMA internal medicine 2019.This is a prospective cohort study involving over 3,000 individuals with CKD who had measurements of 24h urinary oxalate excretion. The authors found that higher urinary oxalate excretion was associated with an increased risk of kidney failure in a heterogeneous group of individuals with various forms of chronic kidney disease.
- 5.Knauf F, Asplin JR, Granja I, et al. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney international 2013. [DOI] [PMC free article] [PubMed]
- 6.Williams HE, Wandzilak TR. Oxalate synthesis, transport and the hyperoxaluric syndromes. The Journal of urology 1989;141(3 Pt 2):742–749. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa Y, Miyazato T, Hatano T. Oxalate and urinary stones. World journal of surgery 2000;24(10):1154–1159. [DOI] [PubMed] [Google Scholar]
- 8.Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney international 2001;59(1):270–276. [DOI] [PubMed] [Google Scholar]
- 9.Nikiforova VJ, Giesbertz P, Wiemer J, et al. Glyoxylate, a new marker metabolite of type 2 diabetes. Journal of diabetes research 2014;2014:685204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight J, Jiang J, Assimos DG, Holmes RP. Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney international 2006;70(11):1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange JN, Wood KD, Knight J, Assimos DG, Holmes RP. Glyoxal formation and its role in endogenous oxalate synthesis. Advances in urology 2012;2012:819202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knight J, Assimos DG, Callahan MF, Holmes RP. Metabolism of primed, constant infusions of [1,2-(1)(3)C(2)] glycine and [1-(1)(3)C(1)] phenylalanine to urinary oxalate. Metabolism: clinical and experimental 2011;60(7):950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight J, Assimos DG, Easter L, Holmes RP. Metabolism of fructose to oxalate and glycolate. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 2010;42(12):868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry 1995;34(11):3702–3709. [DOI] [PubMed] [Google Scholar]
- 15.Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urological research 2005;33(1):1–16. [DOI] [PubMed] [Google Scholar]
- 16.von Unruh GE, Voss S, Sauerbruch T, Hesse A. Dependence of oxalate absorption on the daily calcium intake. Journal of the American Society of Nephrology : JASN 2004;15(6):1567–1573. [DOI] [PubMed] [Google Scholar]
- 17.Whittamore JM, Hatch M. The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man. Urolithiasis 2017;45(1):89–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knauf F, Ko N, Jiang Z, et al. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. Journal of the American Society of Nephrology : JASN 2011;22(12):2247–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakhaee K Unraveling the mechanisms of obesity-induced hyperoxaluria. Kidney international 2018;93(5):1038–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney international 2006;69(4):691–698. [DOI] [PubMed] [Google Scholar]
- 21.Liebman M, Al-Wahsh IA. Probiotics and other key determinants of dietary oxalate absorption. Advances in nutrition 2011;2(3):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatch M, Freel RW. Renal and intestinal handling of oxalate following oxalate loading in rats. American journal of nephrology 2003;23(1):18–26. [DOI] [PubMed] [Google Scholar]
- 23.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proceedings of the National Academy of Sciences of the United States of America 2001;98(16):9425–9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otto BJ, Bozorgmehri S, Kuo J, Canales M, Bird VG, Canales B. Age, Body Mass Index, and Gender Predict 24-Hour Urine Parameters in Recurrent Idiopathic Calcium Oxalate Stone Formers. Journal of endourology / Endourological Society 2017;31(12):1335–1341. [DOI] [PubMed] [Google Scholar]
- 25.Meydan N, Barutca S, Caliskan S, Camsari T. Urinary stone disease in diabetes mellitus. Scandinavian journal of urology and nephrology 2003;37(1):64–70. [DOI] [PubMed] [Google Scholar]
- 26.Lieske JC, de la Vega LS, Gettman MT, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. American journal of kidney diseases : the official journal of the National Kidney Foundation 2006;48(6):897–904. [DOI] [PubMed] [Google Scholar]
- 27.Chung SD, Chen YK, Lin HC. Increased risk of diabetes in patients with urinary calculi: a 5-year followup study. The Journal of urology 2011;186(5):1888–1893. [DOI] [PubMed] [Google Scholar]
- 28.Lapolla A, Flamini R, Dalla Vedova A, et al. Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method. Clinical chemistry and laboratory medicine 2003;41(9):1166–1173. [DOI] [PubMed] [Google Scholar]
- 29.Padberg I, Peter E, Gonzalez-Maldonado S, et al. A new metabolomic signature in type-2 diabetes mellitus and its pathophysiology. PloS one 2014;9(1):e85082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. The Biochemical journal 1999;344 Pt 1:109–116. [PMC free article] [PubMed] [Google Scholar]
- * 31.Wang XJ, Ma SB, Liu ZF, Li H, Gao WY. Elevated levels of alpha-dicarbonyl compounds in the plasma of type II diabetics and their relevance with diabetic nephropathy. Journal of chromatography B, Analytical technologies in the biomedical and life sciences 2019;1106–1107:19–25.Alpha-dicarbonyl compounds include glyoxal and methylglyoxal, which may be precursors to oxalate. The finding of elevated plasma levels of these alpha-dicarbonyl compounds in type 2 diabetes may contribute to diabetes-associated hyperoxaluria.
- 32.Hanssen NMJ, Scheijen J, Jorsal A, et al. Higher Plasma Methylglyoxal Levels Are Associated With Incident Cardiovascular Disease in Individuals With Type 1 Diabetes: A 12-Year Follow-up Study. Diabetes 2017;66(8):2278–2283. [DOI] [PubMed] [Google Scholar]
- 33.Hanssen NMJ, Westerink J, Scheijen J, van der Graaf Y, Stehouwer CDA, Schalkwijk CG. Higher Plasma Methylglyoxal Levels Are Associated With Incident Cardiovascular Disease and Mortality in Individuals With Type 2 Diabetes. Diabetes care 2018;41(8):1689–1695. [DOI] [PubMed] [Google Scholar]
- 34.Jensen TM, Vistisen D, Fleming T, et al. Methylglyoxal is associated with changes in kidney function among individuals with screen-detected Type 2 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association 2016;33(12):1625–1631. [DOI] [PubMed] [Google Scholar]
- 35.Tezuka Y, Nakaya I, Nakayama K, Nakayama M, Yahata M, Soma J. Methylglyoxal as a Prognostic Factor in Patients with Chronic Kidney Disease. Nephrology (Carlton, Vic) 2018. [DOI] [PubMed]
- 36.Carbone A, Al Salhi Y, Tasca A, et al. Obesity and kidney stone disease: a systematic review. Minerva urologica e nefrologica = The Italian journal of urology and nephrology 2018;70(4):393–400. [DOI] [PubMed] [Google Scholar]
- * 37.Bashir M, Meddings J, Alshaikh A, et al. Enhanced gastrointestinal passive paracellular permeability contributes to the obesity-associated hyperoxaluria. American journal of physiology Gastrointestinal and liver physiology 2019;316(1):G1–g14.This study found evidence for inflammation-induced increased passive absorption of oxalate along the gastrointestinal tract as a factor for obesity-associated hyperoxaluria.
- * 38.Amin R, Asplin J, Jung D, et al. Reduced active transcellular intestinal oxalate secretion contributes to the pathogenesis of obesity-associated hyperoxaluria. Kidney international 2018;93(5):1098–1107.This paper found evidence for inflammation-induced reductions in active transcellular intestinal oxalate secretion as a factor for obesity-associated hyperoxaluria.
- ** 39.Lumlertgul N, Siribamrungwong M, Jaber BL, Susantitaphong P. Secondary Oxalate Nephropathy: A Systematic Review. Kidney international reports 2018;3(6):1363–1372.This paper summarizes case reports and case series to describe clinical characteristics and outcomes of 108 patients with secondary hyperoxaluria, which was most commonly from fat malabsorption.
- 40.Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clinical and experimental nephrology 2004;8(2):75–88. [DOI] [PubMed] [Google Scholar]
- 41.Mulay SR, Kulkarni OP, Rupanagudi KV, et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. The Journal of clinical investigation 2013;123(1):236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulay SR, Eberhard JN, Pfann V, et al. Oxalate-induced chronic kidney disease with its uremic and cardiovascular complications in C57BL/6 mice. American journal of physiology Renal physiology 2016;310(8):F785–f795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 43.Saenz-Medina J, Jorge E, Corbacho C, et al. Metabolic syndrome contributes to renal injury mediated by hyperoxaluria in a murine model of nephrolithiasis. Urolithiasis 2018;46(2):179–186.This study provides animal model evidence of a causal link between hyperoxaluria from metabolic syndrome with kidney injury.
- 44.Lieske JC, Regnier C, Dillon JJ. Use of sevelamer hydrochloride as an oxalate binder. J Urol 2008. April;179(4):1407–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lingeman JE, Pareek G, Easter L, Pease R, Grujic D, Brettman L, Langman CB. ALLN-177, oral enzyme therapy for hyperoxaluria. Int Urol Nephrol 2019. April;51(4):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zabaleta N, Barberia M, Martin-Higueras C, Zapata-Linares N, Betancor I, Rodriguez S, Martinez-Turrillas R, Torella L, Vales A, Olagüe C, Vilas-Zornoza A, Castro-Labrador L, Lara-Astiaso D, Prosper F, Salido E, Gonzalez-Aseguinolaza G, Rodriguez-Madoz JR. CRISPR/Cas9-mediated glycolate oxidase disruption is an efficacious and safe treatment for primary hyperoxaluria type I. Nat Commun 2018. December 21;9(1):5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liebow A, Li X, Racie T, Hettinger J, Bettencourt BR, Najafian N, Haslett P, Fitzgerald K, Holmes RP, Erbe D, Querbes W, Knight J. An Investigational RNAi Therapeutic Targeting Glycolate Oxidase Reduces Oxalate Production in Models of Primary Hyperoxaluria. J Am Soc Nephrol 2017. February;28(2):494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 48.Dudal M, Huguet L, Perez J, Vandermeersch S, Bouderlique E, Tang E, Martori C, Chemaly N, Nabbout R, Haymann JP, Frochot V, Baud L, Deschênes G, Daudon M, Letavernier E. Stiripentol protects against calcium oxalate nephrolithiasis and ethylene glycol poisoning. J Clin Invest 2019. April 4;130.This study found that an antiepileptic drug used in children affected by Dravet syndrome may reduce oxalate generation by the liver by inhibiting hepatic as well as neuronal lactate dehydrogenase 5 isoenzyme, the enzyme involved in the last step in hepatic oxalate production.


