Abstract
Epidemiological research reveals that insufficient sleep in children has negative cognitive and emotional consequences; however, the physiological underpinnings of these observations remain understudied. We tested the hypothesis that the topographical distribution of deep sleep slow wave activity during the childhood predicts brain white matter microstructure (myelin) 3.5 y later. Healthy children underwent sleep high-density EEG at baseline (n=13; ages 2.4-8.0 y) and followup (n=14; ages 5.5-12.2 y). At follow-up, myelin (myelin water fraction) and cortical morphology were also quantified. Our investigation revealed 3 main findings. (1) The Frontal/Occipital (F/O)-ratio at baseline strongly predicted whole brain myelin at follow-up. (2) At follow-up, the F/O-ratio was only minimally (negatively) linked to brain myelin. (3) Cortical morphology was not related to the F/O-ratio, neither at baseline nor at follow-up. Our results support the hypothesis that during child development EEG markers during sleep longitudinally predict brain myelin content. Data extend previous findings reporting a link between EEG markers of sleep need and cortical morphology, by supporting the hypothesis that sleep is a necessary component to underlying processes of brain, and specifically myelin, maturation. In line with the overarching theory that sleep contributes to neurodevelopmental processes, it remains to be investigated whether chronic sleep loss negatively affects white matter myelin microstructure growth during sensitive periods of development.
Keywords: High density EEG, slow wave activity, development, myelin, early marker, topography
INTRODUCTION
The sleep electroencephalogram (EEG) objectively quantifies the need for recovery of neuronal networks (Achermann and Borbely, 2011). The sleep EEG can distinguish healthy from disordered brains in adults (Plante et al., 2012) and children (Bolsterli Heinzle et al., 2014). Recent advances have resulted in identification of a sleep EEG signature that reflects processes of brain maturation i.e., the ratio of Frontal/Occipital slow wave activity (F/O-ratio) (Kurth et al., 2010). This marker quantifies, in a simplified way, the regional maturation of slow wave activity topography in NREM sleep. Slow wave activity distribution is increased in scalp regions showing structural and behavioral maturation (Kurth et al., 2012). Interestingly, slow wave activity maturation not just temporally accompanies measures of brain structural and behavioral maturation, but precedes the maturation of gray matter thickness as well as the maturation of motor skills by ~3.7 years (Kurth et al., 2012). While the F/O-ratio may be a relatively simple measure of slow wave activity topography maturation that can also be applied to low spatial resolution EEG, its potential to predict neuromorphological maturation remains yet poorly understood.
EEG sleep markers as a measure of neurodevelopment may be relevant in identifying processes of atypical brain maturation. For example, children with attention-deficit/hyperactivity disorder show a less mature slow wave activity distribution in comparison to healthy children of the same age and sex (Ringli et al., 2013). Epidemiological studies further reveal temporal relationships between early sleep problems and later behavioral problems (Mindell et al., 2017; Sivertsen et al., 2015). Observations of bi-directional interactions between sleep disturbances and symptoms of psychiatric illness contribute to the growing evidence of a close relationship of slow waves and brain plasticity (Tesler et al., 2013). EEG sleep markers have thus the potential to improve early diagnosis of developmental disorders, and may ultimately support the reduction of social and economic burden (Olesen et al., 2012). To date, however, investigations that quantify the prognostic potential of sleep EEG markers for development of brain morphology years later are scarce.
The discovery of key maturational transitions in children’s sleep EEG was facilitated by data from studies using high-spatial resolution EEG (over 120 electrode channels). The results of these relatively recent studies indicate that (1) developmental transitions are most prominently manifested in a re-distribution of EEG power (i.e. topography) in slow wave activity of non-rapid eye movement (NREM) sleep (Kurth et al., 2010); (2) slow-wave activity topography is a brain marker for maturational processes in health and disease (Ringli et al., 2013; Tesler et al., 2013); and (3) slow wave activity topography reflects processes associated with behavioral learning (Wilhelm et al., 2014) and (4) has potential to foretell the maturation of behavioral skills by several years (Kurth et al., 2012). Thus, we propose that slow wave activity topography (simplified as the F/O-ratio) represents an early marker of behavioral and brain development, even before morphological maturation is observed (Kurth et al., 2012).
Although a large body of research has targeted the development of cortical gray matter morphology, white matter microstructure remains understudied. Novel magnetic resonance imaging (MRI) methodologies allows for the quantification of brain white matter microstructure across childhood (Deoni et al., 2012; Deoni et al., 2008; Lebel and Deoni, 2018). Moreover, it has been recently suggested that milestones in neuromorphological maturation originate from microstructural tissue growth instead of tissue loss (Natu et al., 2018; Sowell et al., 2004), suggesting that white matter microstructure is a novel focus. The central component of white matter microstructure is myelin; its growth is a principal characteristic of brain maturation (Deoni et al., 2012) and fundamental to the development of cognition (Fields, 2008; Johnson and Munakata, 2005). As a lipid layer, myelin surrounds axons and increases the speed of action potential propagation, and is thus critical to normative brain function.
Evidence is emerging that the myelin machinery is linked to neuronal activity specific to sleep. For example, rodents exhibit differential gene expression specific to the behavioral states of sleep or wake (Bellesi et al., 2018; Bellesi et al., 2013; Cirelli, 2005). By pooling the transcripts from all brain cells, one investigation uncovered that a multitude of genes expressed during sleep are involved in membrane synthesis, including that for myelin (Cirelli, 2005). A subsequent study targeted genome-wide profiling of oligodendrocytes – the precursor cells that are involved in new myelin formation (Bellesi et al., 2013). Results confirmed the differential expression in sleep and wake of the transcripts in oligodendrocytes: such that genes involved in phospholipid synthesis and myelination or else genes promoting oligodendrocyte proliferation were transcribed preferentially during sleep. Most recent findings indicate that sleep is specifically related to myelin thickness (Bellesi et al., 2018), which implies that chronic sleep loss may negatively affect myelin development. Additionally, our recent investigations in children indicate that myelin is an integrative component of local sleep need (Kurth et al., 2016) and the propagation dynamics of slow waves across the scalp (Kurth et al., 2017). Thus, overall evidence is increasing that slow wave activity during sleep is connected to myelin growth. Here we test the earlier proposed concept (Tarokh et al., 2010) that the regional distribution of slow wave activity across the scalp is a signature with which to monitor myelin connectivity in developing humans.
In summary, slow wave activity topography mirrors activity-dependent plastic changes (Huber et al., 2006; Huber et al., 2004), and is also linked to brain myelin development (Kurth et al., 2016; Kurth et al., 2017). Based on the underlying hypothesis that sleep contributes to neurodevelopmental processes, we tested the hypothesis that the F/O-ratio sleep marker will predict myelin content in children. We hypothesized that more “frontalized“ slow wave activity topography at baseline relates to increased myelin 3.5y later. We examined whether the F/O-ratio is a prognostic marker for brain myelin development that will longitudinally predict myelin content (myelin water fraction; MWF) as measured in whole brain and in identified regions of interest. Additionally, we investigated whether the F/O-ratio during sleep was predictive of established measures of cortical morphology from baseline to follow-up 3.5 y later.
MATERIAL AND METHODS
Participants
Healthy children were assessed repeatedly 3.5 years apart: baseline (T1; n=13; ages 2.4-8.0 y; mean 5.1± 1.8 y, 7 females) and follow-up (T2; n=14; ages 5.5-12.2y; mean 9.0 ± 2.0 y, 7 females), for subsamples of this cohort see studies (Doucette et al., 2015; Kurth et al., 2016; Kurth et al., 2010). One child entered the study at T2, resulting in 13 participants included at baseline and 14 participants at follow-up. Screening ensured that participants were in excellent health and had no personal or family history of psychopathology, chronic diseases, or sleep disorders and did not currently use medications affecting sleep or daytime arousal. Children were excluded for travel beyond 2 time zones within 2 months before assessments, and for caffeine use, daily/nightly cosleeping, physical or developmental disabilities, chronic medical conditions, head injury, preterm or post-term delivery, or low birth weight. Written parental consent and child assent was obtained, and the Institutional Review Board (Brown University) approved all procedures performed according to the Declaration of Helsinki.
Study procedures
During the 5 days leading up to the assessments, subjects followed an individualized, sleep/wakefulness schedule. This stabilization period provided minimization of sleep restriction and entrainment of the circadian system. Adherence to the sleep schedule was verified with wrist actigraphy and sleep diaries, as well as daily calls or emails to the laboratory. No naps were allowed during the 24h preceding the EEG assessment. However, children who regularly napped were allowed to nap on the day of assessment so as not to introduce heightened sleep pressure. EEG recordings were scheduled according to individual reported bedtimes and habitual patterns. At T1 and T2, participants underwent all-night sleep high-density EEG (hdEEG) in the natural environment of their homes to generate the F/O-ratio sleep EEG marker. At T2 and within 2 weeks of the sleep assessment, maps of the myelin water fraction (MWF), an established measure of myelin content, were quantified using the multicomponent driven equilibrium single pulse observation of T1 and T2 (mcDESPOT) MRI technique at Brown University (Deoni et al., 2008). At T2, we assessed socioeconomic context with the Four Factor Index of Social Status (Hollingshead, 1975). This index takes into account social status as a multidimensional concept, and integrates the factors of education, occupation, sex and marital status.
Sleep assessed with high-density EEG (hdEEG)
All-night hdEEG assessments were scheduled according to habitual bedtimes (128 channels, Electrical Geodesics Inc., Sensor Net, Portable System, Eugene, Oregon, US). Signals were referenced to the vertex for direct visualization (NetStation 4.5.1). Nets of different sizes were selected for each child according to head circumference, and adjusted to vertex and mastoids. Electrodes were filled with electrolyte gel (ECI, Electro Gel). Recordings were assessed with 500 Hz (0.01 – 200 Hz) sampling rate and impedances below 50 kΩ.
Myelin-specific MRI
Children were imaged using a 3-Tesla Siemens Trio Scanner, equipped with a 12-channel head RF array while watching a movie. The mcDESPOT protocol, which consists of a series of spoiled gradient recalled echo (SPGR) images and fully balanced steady-state free precession (bSSFP) images measured over a range of flip angles, were acquired (Deoni et al., 2012). Inversion-prepared (IR-) SPGR data were additionally acquired to correct for transmit magnetic field (B1) inhomogeneities, while the bSSFP data were acquired with two phase-cycling patterns to allow for correction for main magnetic field (B0) inhomogeneities. The field of view and imaging matrix were fitted for age and head size, while keeping a constant voxel size (1.8×1.8×1.8 mm3). Additional mcDESPOT scanning parameters are provided in the Supplemental Material (Table S1). To decrease acoustic noise, maximum imaging gradient slew rates and peak values were reduced, and passive measures were used (sound-insulating boreliner, MiniMuff ear pads, soundattenuating ear protection)(Dean et al., 2014a; Deoni et al., 2012). Acquisition of the mcDESPOT protocol was ~30 min for each child.
Analysis
Neurodevelopmental marker of the sleep EEG
Standard EEG preprocessing was applied (Kurth et al., 2010), including off-line data filtering (bandpass 0.5-50 Hz), down sampling to 128 Hz, re-referencing to the average across all channels and sleep stage scoring (Iber et al., 2007). Semi-automated artifact rejection was performed on a 20-s basis (Kurth et al., 2010) and poor quality channels were excluded. Power spectral analysis was performed with a Fast Fourier transform routine (20-s epochs, average of five 4-s epochs, Hanning window, no overlap, pwelch from MATLAB signal processing toolbox, Mathworks). Data obtained had a frequency resolution of 0.25 Hz. Artifact-free sleep was included (skipping epochs with artifacts). We computed the maturational status of slow wave activity topography with the F/O-ratio of slow wave activity, as previously published (1-4.5 Hz; Figure 1b) (Kurth et al., 2010; Lustenberger et al., 2017).
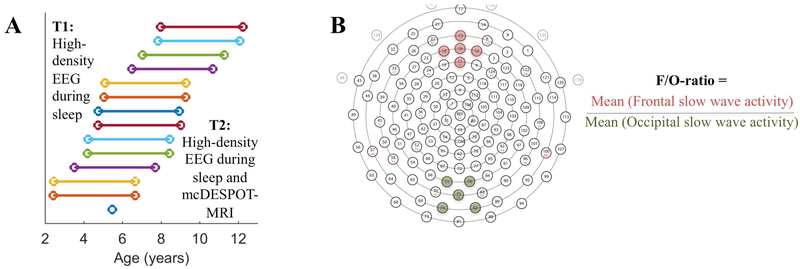
Figure 1.
A. Assessment. Children completed sleep high-density EEG (hdEEG) at the Baseline (T1) assessment and sleep hdEEG and Magnetic Resonance Imaging (MRI) 3.5y later at follow-Up (T2). Each subject is represented with a different color: 13 subjects were assessed twice (one subject entered the study at T2). B. Frontal/Occipital (F/O)-ratio. Slow wave activity (0.5-4.5 Hz, first 60 min of NREM sleep) within a cluster of 5 electrodes in the frontal region averaged and divided by the value of 5 averaged occipital electrodes. Clusters of electrodes are illustrated with colors, i.e., channels 10, 11 (Fz), 15, 16, 18 in the frontal cluster and 71, 74, 75 (Oz), 76, 82 in the occipital cluster (Electrical Geodesics Inc., 128-channel Sensor Net).
Myelin marker of brain morphology
Linear co-registration was performed for each subject’s raw SPGR, IR-SPGR, and bSSFP images to account for subtle intra-scan motion and removal of non-brain signal (Jenkinson et al., 2002; Smith, 2002). B0 and B1 field maps were computed, subsequently followed by estimation of MWF using a constrained and iterative fitting approach to a 3-pool tissue model. As formally shown, this approach provides stable estimations of MWF (Deoni and Kolind, 2015; Deoni et al., 2013b). Individual MWF maps were non-linearly co-registered to a common standardized space using the high flip angle T1-weighted SPGR image as previously described (Deoni et al., 2012). The resulting transformation matrix was applied to each individual’s MWF maps to align the MWF map to the standardized space. Maps were smoothed with a 4 mm full-width-at-half maximum 3D Gaussian kernel within a white and gray matter mask.
Brain myelin was quantified for whole brain and 3 additional brain regions as obtained from morphologic masks (Deoni et al., 2012). The brain regions of interest represented core fiber tracts that were previously linked to children’s sleep, i.e., superior longitudinal fasciculus, corpus callosum and optic radiation (Kurth et al., 2016; Kurth et al., 2017). The superior longitudinal fasciculus is the largest anterior-posterior, intra-hemispheric white matter connection and has been associated with the maturation of slow wave propagation, pertaining to the speed of propagation and the range of expanse on the scalp (Kurth et al., 2017). The corpus callosum is the primary inter-hemispheric pathway and largest white matter structure in the brain, and myelin in this region of interest has been linked to wave propagation distance in children (Kurth et al., 2017). The optic radiation is a multi-sensory integration circuit that connects the lateral geniculate body and the posterior thalamus with the primary visual cortex and has been previously related to increased sleep pressure in school-age children (Kurth et al., 2016). Mean MWF was calculated within the standardized space, from morphological masks of both the left and right hemispheres, where appropriate. Individual MWF values measured from standard space strongly show strong concordance with native space MWF values (Dean et al., 2014b).
Cortical brain markers
In adults and adolescents EEG markers of sleep need are linked to cortical gray matter morphology. For example, gray matter volume correlates positively with slow wave activity in a region-specific manner (Buchmann et al., 2011). Further, the intense practice of a task induces an increase in gray matter and white matter subcortical volume. These changes are reversed after sleep indicating an interaction of sleep with cortical microstructure (Bernardi et al., 2016). Moreover, sleep deprivation impairs memory function, which is in part determined by cortical structure (Saletin et al., 2016). Thus, in order to extend the existing knowledge on sleep-cortical morphology to younger age, we also included cortical brain markers in the current analysis.
For cortical reconstruction, we included standard image pre-processing correction of low frequency signal intensity variation (RF coil bias field) using the Advanced Normalization Tools (ANTs) nonparametric non-uniform normalization (N3) (Sled et al., 1998). Cortical surface area, thickness, mean curvature, and gray matter volume were measured using the surface mesh-based cortical modeling software package, Freesurfer (Dale et al., 1999; Fischl et al., 1999). Images were visually inspected at each step in the processing protocol, and, when required, manually edited using gcut (http://freesurfer.net/fswiki/FsTutorial/SkullStripFix_freeview). This approach corrects improper skull stripping and removes non-brain tissue including dura and eye signal.
Statistics
Spearman correlations were performed between the F/O-ratio of slow wave activity at T1 and T2 with myelin content at T2 (i.e., MWF in whole brain, superior longitudinal fasciculus, corpus callosum, optic radiation), and with cortical markers at T2 (i.e., surface area, thickness, mean curvature, gray matter volume, MATLAB corr). Partial correlations (the above correlations extended with factor ‘age at T1 or T2’) were performed to control for effects of age (MATLAB partialcorr).
Wilcoxon Signed Rank Test (MATLAB signrank) was used for two-sided comparison of paired data. All results are reported at the significance threshold of α =0.05. P-values were corrected for multiple testing using False Discovery Rate (FDR) test (Benjamini and Hochberg, 1995). Selected maturational markers for the sleep EEG and brain morphology were incorporated, e.g., F/O-ratio, whole brain MWF, cortical thickness etc. This restriction of variables limited the number of statistical tests and thus reduced the likelihood of a type I error. Outliers were statistically classified using Grubb’s criteria (5% level). Analyses were performed for the full data whenever available (n=13 at baseline, n=14 at follow-up), while results are also presented for the restricted sample (both assessments n=13). Signal analysis and statistics were analyzed with MATLAB (Mathworks, Natick, Massachusetts, United States, version R2012a) and the statistics toolbox (Mathworks).
RESULTS
F/O-ratio of slow wave activity has state- and trait-like properties
Sleep hdEEG assessments showed sleep quality consistent with earlier at-home reports (Mason et al., 2008); subjects obtained 8.3-9.2 h sleep duration and nearly 90% sleep efficiency (Table 1). As expected, time in bed, sleep duration, N1, and REM sleep decreased with increasing age, while slow wave sleep and sleep cycle duration increased from T1 to T2 (Ohayon et al., 2004; Roffwarg et al., 1966). The F/O-ratio was computed for the first 60 min of artifact-free NREM sleep. This approach was taken in order to maximize comparability with existing research using the same time window, and to facilitate comparability across participants of different age, and accordingly, variability in homeostatic sleep pressure. The composition of sleep stages in the selected time window was comparable between T1 and T2 (Stage N2: 18.6±10.5% at T1 vs. 22.7±11.5% at T2, p=0.30. Stage N3: 81.4±10.5% at T1 vs. 77.4± 11.5% at T2, p=0.30, M±SD; Wilcoxon Signed Rank Test, n=13).
Table 1. Sleep EEG, brain myelin (MWF), and cortical markers (n=13 at T1, n=14 at T2).
Sleep variables included time in bed (lights off to lights on), sleep duration (stages N1, N2, N3, REM scored in 20-s epochs), sleep efficiency (total sleep time expressed as a % of time in bed), sleep latency (latency to the first epoch of stage N2 sleep), wake after sleep onset (WASO; duration of wake after first occurrence of stage N2 sleep), sleep stages (% of total sleep time), SWS (slow-wave sleep as stage N3), and REM sleep (rapid eye movement sleep). Sleep cycle duration includes only the first four sleep cycles. Because subjects were awakened in the morning, the last sleep cycle could not always be completed. Thus, we applied 2 additional criteria for the computation of cycle duration as in (Kurth et al., 2010): in subjects with only 4 cycles but with a missing REM sleep episode in the last cycle, the last cycle was excluded from the comparison (2 participants at T2). Further, only sleep cycles containing a minimum of 75 epochs of NREM sleep were included. Subjects with <4.5h of recorded sleep time were excluded for the computation of time in bed, WASO, N1, N2, SWS, REM sleep, and cycle duration. Wilcoxon Signed Rank Tests were performed between baseline and follow-up. The subject assessed only at T2 was included in the presented descriptive data, yet excluded for the statistical comparison of developmental change (outermost right column).
| T1 | T2 | Developmental change p (T1 vs. T2) |
|||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Sleep EEG | |||||
| Time in bed (min) | 625.38 | 58.02 | 568.05 | 36.66 | 0.006 |
| Total sleep duration (hh:min) | 09:14 | 00:44 | 08:23 | 00:57 | 0.02 |
| Sleep efficiency (%) | 88.71 | 4.47 | 88.46 | 7.27 | Ns (0.79) |
| Sleep latency (min) | 23.05 | 58.02 | 15.88 | 8.87 | Ns (0.37) |
| WASO (min) | 50.79 | 33.46 | 51.17 | 38.42 | Ns (0.99) |
| N1 (%) | 4.11 | 1.36 | 1.90 | 1.19 | 0.01 |
| N2 (%) | 49.51 | 5.72 | 50.34 | 4.94 | Ns (0.31) |
| SWS (%) | 20.19 | 2.89 | 26.00 | 6.76 | 0.006 |
| REM (%) | 26.19 | 3.42 | 21.76 | 6.00 | 0.006 |
| Cycle duration (%) | 90.05 | 8.68 | 113.96 | 23.91 | 0.02 |
| Myelin (MWF) Variables | |||||
| Whole brain | 0.1140 | 0.0129 | |||
| Superior longitudinal fasciculus | 0.1494 | 0.0185 | |||
| Corpus callosum | 0.1891 | 0.0189 | |||
| Optic radiation | 0.1746 | 0.0216 | |||
| Cortex Markers | |||||
| Surface area (mm2) | 4.99*10Λ4 | 9.69*10Λ3 | |||
| Gray matter volume (mm3) | 1.42*10Λ5 | 2.67*10Λ4 | |||
| Cortical thickness (mm) | 2.107 | 0.094 | |||
| Curvature | 0.1356 | 0.0051 | |||
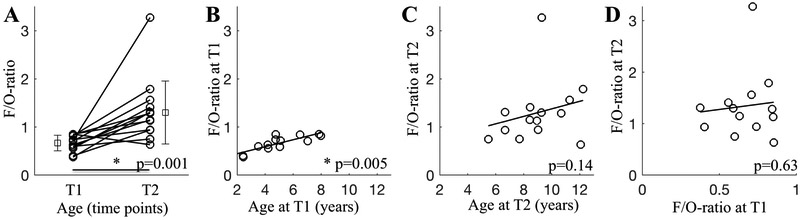
Next, the comparison of T1 and T2 revealed an increase in the F/O-ratio of slow wave activity with increasing age, indicating more “frontalized“ slow wave activity with increasing age (p=0.001; Figure 2A). This observation suggests that the F/O-ratio is a marker for maturational state of brain activity in sleep. We also investigated age as a continuous variable, due to the fact that the participants’ ages were represented across the 2-12 y age range (Figure 1). At T1, the F/O-ratio of slow wave activity was strongly correlated with age (Rho=0.75, p=0.005; Figure 2B), in contrast to T2 (Rho=0.41 p=0.14; Figure 2C). This approach uncovered large individual variability in the F/O-ratio beyond the effect of age, demonstrating a trait-like property of the F/O-ratio. We then measured the stability of the F/O-ratio within participants by determining whether a linear relationship existed between the F/O-ratio at T1 and the F/O-ratio at T2 (Figure 2D). The association between the F/O-ratio at T1 and T2 reached only significance when controlling for age (Rho= −0.15, p=0.63; partial correlation controlling for ‘age at T2’ Rho= −0.60, p=0.04), demonstrating that the F/O-ratio is not a universally stable trait throughout development. Moreover, the F/O-ratio of slow wave activity also indicates individual dynamics in its maturation.
Figure 2. State-like and trait-like properties of the F/O-ratio of slow wave activity (n=13 for T1, n=14 for T2).
A. F/O-ratio comparison between T1 and T2 (p=0.001, Wilcoxon Signed Rank Test). Mean and Standard Deviation are presented for both time points, next to individual data. B. F/O-ratio illustrated for effective age at T1 (Rho=0.75, p=0.005). C. F/O-ratio was not related to effective age at T2 (Rho=0.41 p=0.14). D. Linear association between F/O-ratio at T1 with T2 reached significance only with inclusion of ‘age’ as control variable (Rho= −0.15, p=0.63; partial correlation factor ‘age at T2’: Rho= −0.60, p=0.04). Results were consistent with exclusion of the subject with T2 assessment only (n=13: p=0.001 for panel A; Rho=0.32 p=0.28 for panel C; no change in panels B and D).
One data point at T2 with maximal F/O-ratio was identified as an outlier (Figure 2C). Yet, analyses with exclusion of this point revealed very similar results (F/O-ratio increase from T1 to T2: p=0.002, Wilcoxon Signed Rank Test, and weak correlation between F/O-ratio at T1 and T2: Rho= −0.21, p=0.51, partial correlation factor ‘age at T2’: R= −0.65, p=0.03). Thus, we utilized non-parametric statistics and included this data point in subsequent analyses for the following reasons: (1) this maturation period entails large inter-individual variability of the F/O-ratio slow wave activity marker (Kurth and Huber, 2012) and (2) this participant was not an outlier on any other measure (F/O-ratio at T1; MWF in whole brain, superior longitudinal fasciculus, corpus callosum or optic radiation; cortical reconstruction as surface area, gray matter volume, curvature or gray matter thickness). One outlier in cortical thickness was retained in analyses for the same reasons.
Prognostic potential: slow wave activity topography is a predictor of global brain myelin development
Next, we analyzed brain myelin content (MWF) to test whether slow wave activity topography (F/O-ratio) predicts MWF development 3.5y later. Brain myelin was quantified for selected morphological masks (Deoni et al., 2012): myelin brain regions included whole brain and 3 core fiber tracts previously linked to children’s sleep, i.e., superior longitudinal fasciculus, corpus callosum and optic radiation (Kurth et al., 2016; Kurth et al., 2017). Further, because previous data in adolescents and adults indicate links between EEG markers of sleep need and cortical morphology (Bernardi et al., 2016; Buchmann et al., 2011; Saletin et al., 2016), we also included 4 cortical markers in the current approach. For cortical reconstruction, we included standard image pre-processing correction of low frequency signal intensity variation (Sled et al., 1998).
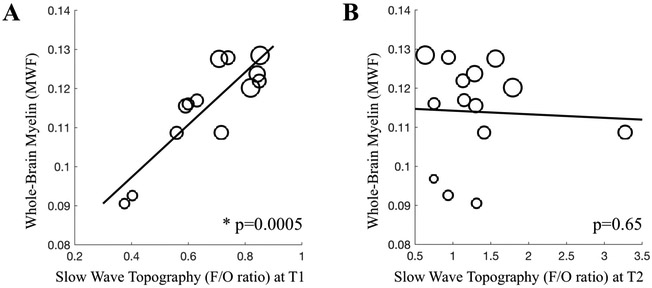
Our results revealed that MWF and cortical measures were comparable with previously published data in children (Dean et al., 2014b) (Table 1). In alignment with our hypothesis, slow wave activity maps were predictive of myelin development, in that the F/O-ratio at T1 was associated with MWF at T2 (Table 2, Figure 3). This effect was particularly strong for whole brain MWF (Spearman correlation Rho=0.84, p=0.0005, pFDR=0.009; correction for ‘age at T1’ with partial correlation: Rho=0.70, p=0.01, pFDR=0.06). To a lesser degree, the F/O-ratio at T1 also predicted MWF in the superior longitudinal fasciculus at T2 (Rho=0.62, p=0.03, pFDR=0.09; correction for ‘age at T1’ with partial correlation: Rho=0.50, p=0.10, pFDR=0.17). Nonetheless, effects were substantial, as reflected in 38-71% of MWF variance explained by the F/O-ratio at T1 (i.e., Rho2).
Table 2. Predictions of brain myelin by slow wave activity topography in children.
Associations between Frontal/Occipital ratio (F/O-ratio) of slow wave activity and brain myelin content as myelin water fraction (MWF) were calculated with Spearman and partial correlations controlling for ‘age at T1 or T2’ (n=13 for T1, n=14 for T2). To reduce the chance of falsepositive findings, we additionally performed false discovery rate (FDR) analysis. Significant associations (p<0.05) are presented in bold. With exclusion of the one subject with the T2 assessment only, results were overall consistent (n=13). Relationships with F/O-ratio at T2: Whole brain: Spearman correlation Rho= −0.29, p=0.33, pFDR=0.38; partial correlation: Rho= −0.74, p=0.006, pFDR=0.04. Superior longitudinal fasciculus: Spearman correlation Rho= −0.46, p=0.12, pFDR=0.17; partial correlation: Rho= −0.72, p=0.008, pFDR=0.04. Corpus callosum: Spearman correlation Rho= −0.35, p=0.24, pFDR=0.29; partial correlation: Rho= −0.49, p=0.11, pFDR=0.17. Optic radiation: Spearman correlation Rho= −0.18, p=0.57, pFDR=0.57; partial correlation: Rho= −0.37, p=0.23, pFDR=0.29.
| Myelin (MWF) at T2 | Whole brain | Superior longitudinal fasciculus |
Corpus callosum | Optic radiation | |||||
|---|---|---|---|---|---|---|---|---|---|
| Spearman correlation |
Partial correlation |
Spearman correlation |
Partial correlation |
Spearman correlation |
Partial correlation |
Spearman correlation |
Partial correlation |
||
| Slow wave activity topography | F/O-ratio at T1 |
Rho=0.84 p=0.0005 pFDR=0.009 |
Rho=0.70 p=0.01 pFDR=0.06 |
Rho=0.62 p=0.03 pFDR=0.09 |
Rho=0.50 p=0.10 pFDR=0.17 |
Rho=0.56 p=0.05 pFDR=0.13 |
Rho=0.51 p=0.09 pFDR=0.17 |
Rho=0.53 p=0.07 pFDR=0.15 |
Rho=0.29 p=0.36 pFDR=0.45 |
| F/O-ratio at T2 | Rho=−0.13 p=0.65 pFDR=0.69 |
Rho=−0.69 p=0.009 pFDR=0.06 |
Rho=−0.26 p=0.37 pFDR=0.45 |
Rho=−0.64 p=0.02 pFDR=0.07 |
Rho=−0.18 p=0.53 pFDR=0.61 |
Rho=−0.40 p=0.18 pFDR=0.28 |
Rho=−0.05 p=0.86 pFDR=0.86 |
Rho=−0.33 p=0.27 pFDR=0.39 |
|
Figure 3. Prediction of whole brain myelin content by slow wave activity topography.
A. Frontal/Occipital ratio (F/O-ratio) of slow wave activity at T1 (Rho=0.84 p=0.0005; with partial correlation Rho=0.70, p=0.01, n=13). B. F/O-ratio of slow wave activity at T2 (p=0.65, n=14; and p=0.33 with n=13). Marker size refers to age, with larger circles indicating older age.
At follow-up (i.e., T2), we observed variability in the manner of associations between the F/O-ratio and MWF. In contrast to T1, the F/O-ratio at T2 revealed only weak negative associations with whole brain (Spearman correlation Rho= −0.13, p=0.65, pFDR=0.69; correction for ‘age at T2’ with partial correlation: Rho= −0.69, p=0.009, pFDR=0.06) as well as with superior longitudinal fasciculus (Spearman correlation Rho= −0.26, p=0.37, pFDR=0.45; correction for ‘age at T2’ with partial correlation: Rho= −0.64, p=0.02, pFDR=0.07) or else no association with MWF (Table 2, Figure 3). These data demonstrate that the F/O-ratio assessed at T1 is a strong and specific marker of observed brain MWF 3.5y later.
Unlike myelin, cortical development is not predicted by the F/O-ratio of slow wave activity
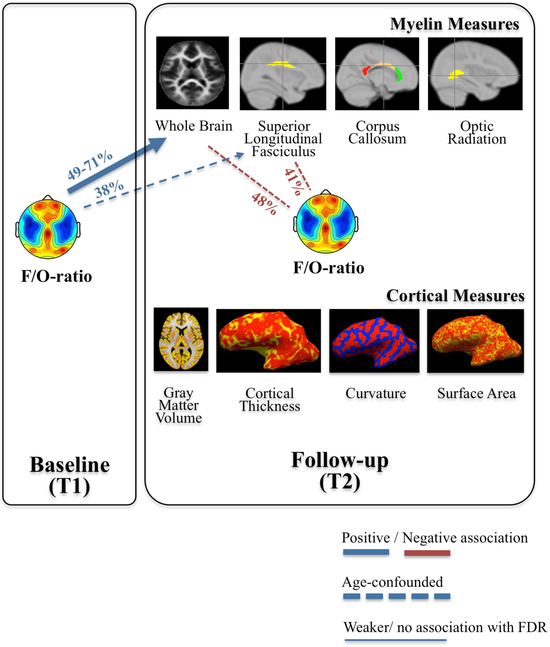
In contrast to the link with myelin, we found no strong associations between the F/O-ratio and global cortical morphology measures, neither at T1 nor at T2. The F/O-ratio at T1 nearly reached significance in explaining inter-individual variability in cortical curvature at T2; however, this association was influenced by age-effects (Rho=0.55, p=0.05, pFDR=0.61, partial correlation Rho=0.27, p=0.39, pFDR=0.61; Table 3). In sum, brain mapping using mcDESPOT and Freesurfer overall indicate that the topographical distribution of slow wave activity (F/O-ratio) is an early sleep marker that predicts brain myelin development 3.5y later, whereas global measures of cortical morphology are negligibly predicted by the F/O-ratio in childhood (Figure 4).
Table 3. Cortical morphology and slow wave activity topography.
Associations between Frontal/Occipital ratio (F/O-ratio) of slow wave activity and cortical morphological measures were calculated with Spearman correlations and partial correlations controlling for ‘age at T1 or T2’ (n=13 for T1, n=14 for T2). False discovery rate (FDR) analysis was performed. Significant associations (p<0.05) are presented in bold. With exclusion of the one subject with the T2 assessment only, results were overall consistent (n=13). Relationships with F/O-ratio at T2: Surface area: Spearman correlation Rho= 0.09, p=0.78, pFDR=0.89; partial correlation: Rho= −0.01, p=0.97, pFDR=0.97. Gray matter volume: Spearman correlation Rho=0.24, p=0.44, pFDR=0.78; partial correlation: Rho=0.21, p=0.52, pFDR=0.84. Cortical thickness: Spearman correlation Rho= 0.36, p=0.22, pFDR=0.66; partial correlation: Rho=0.41, p=0.18, pFDR=0.66. Curvature: Spearman correlation Rho= −0.14, p=0.64, pFDR=0.85; partial correlation: Rho= −0.38, p=0.22, pFDR=0.66.
| Cortical markers at T2 | Surface area (mm2) | Gray matter volume (mm3) | Cortical thickness (mm) | Curvature | |||||
|---|---|---|---|---|---|---|---|---|---|
| Spearman correlation |
Partial correlation |
Spearman correlation |
Partial correlation |
Spearman correlation |
Partial correlation |
Spearman correlation |
Partial correlation |
||
| Slow wave activity topography | F/O-ratio at T1 | Rho=0.30 p=0.32 pFDR=0.61 |
Rho=0.15 p=0.64 pFDR=0.85 |
Rho=0.12 p=0.70 pFDR=0.86 |
Rho=0.05 p=0.87 pFDR=0.93 |
Rho=−0.35 p=0.25 pFDR=0.61 |
Rho=−0.44 p=0.16 pFDR=0.61 |
Rho=0.55 p=0.05 pFDR=0.61 |
Rho=0.27 p=0.39 pFDR=0.61 |
| F/O-ratio at T2 | Rho=0.24 p=0.42 pFDR=0.61 |
Rho=0.07 p=0.82 pFDR=0.93 |
Rho=0.37 p=0.20 pFDR=0.61 |
Rho=0.28 p=0.35 pFDR=0.61 |
Rho=0.27 p=0.35 pFDR=0.61 |
Rho=0.36 p=0.23 pFDR=0.61 |
Rho=0.02 p=0.93 pFDR=0.93 |
Rho=−0.31 p=0.31 pFDR=0.61 |
|
Figure 4. Summarized relationships between slow wave activity topography (F/O-ratio), brain myelin and cortical markers of brain maturation.
Associations are coded for corrections and represent associations between parameters: dashed lines are used when statistical significance of relationships no longer existed when controlling for “age”, and thin lines when correlations were no longer significant when corrected for multiple comparisons. Numbers (%) refer to Rho2. Data are presented in Figure 3, and Tables 2 and 3.
Finally, we evaluated whether socioeconomic status confounded the three main findings. Overall, results were consistent with including the correction for socioeconomic status (Supplemental Tables S2, S3). First, the F/O-ratio at T1 predicted whole brain MWF at T2 (partial correlation with factor ‘socioeconomic status’, Rho=0.84, p=0.006, pFDR=0.005; Supplemental Table S2). In comparison with uncorrected data, this association is even stronger (significance with FDR correction). An additional association was observed between the F/O-ratio at T1 and MWF in the corpus callosum, which did yet not survive FDR control (partial correlation with factor ‘socioeconomic status’, Rho=0.59, p=0.04, pFDR=0.13; Supplemental Table S2). Second, the F/O-ratio at T2 was not linked to MWF at T2 when accounting for effects of socioeconomic status (Supplemental Table S2). The relationship between F/O-ratio at T2 and MWF at T2 entirely disappeared in all regions. Third, the F/O-ratio was not related to cortical morphology at T2 when controlling for socioeconomic status (Supplemental Table S3). In line with uncorrected findings, correction for socioeconomic status did not reveal any significant associations, neither at T1, nor at T2.
DISCUSSION
Our work reveals that a simple marker in the sleep EEG predicts the development of whole brain brain white matter microstructure, i.e., myelin, in healthy children. We used the F/O-ratio – an established index for EEG slow wave activity topography (Kurth et al., 2010) – with state- and trait-like properties. Our results indicate 3 primary findings. First, the F/O-ratio in children strongly predicts global brain myelin development at a follow-up evaluation 3.5 years later. Second, in contrast, the sleep marker F/O-ratio assessed at follow-up was only minimally and negatively linked to brain myelin measured at follow-up. Third, the F/O-ratio in childhood is not a predictor of the most commonly used cortical developmental markers (e.g., gray matter volume, cortical thickness). These novel findings extend work first performed in rodents to children; that is, they support the hypothesis that slow wave activity measured non-invasively during sleep not only reflects, but also strongly predicts brain myelin development in a specific age timeframe, supporting its use as a maturational marker. This concept strongly promotes sleep-related translational avenues of mental health by means of 2 fundamental components: the predictive role of slow wave activity topography for brain development (Kurth et al., 2010), and its possibility to be modified non-invasively, e.g., actively with behavioral tasks (Huber et al., 2004; Wilhelm et al., 2014) or passively with auditory stimulation during sleep (Fattinger et al., 2017).
These results confirm our previous work showing that the F/O-ratio increases with age (Kurth et al., 2010). As expected, we found that the F/O-ratio indicates a maturational state as well as an individual trait. This observation suggests that even though the F/O-ratio increases with age in a longitudinal manner, it shows individual variability and distinguishes neurophysiological characteristics of individual participants. We further tested and confirmed the prognostic potential of the individual differences in the F/O-ratio for predicting brain morphology development beyond maturational effects. We used the F/O-ratio as our sleep EEG marker for 2 reasons. First, brain maturation is a region-specific process (Dean et al., 2015; Giedd, 2004; Shaw et al., 2008), and the spatial maturation is observable in the sleep EEG, most prominently in the slow wave activity frequency band (Kurth et al., 2010). The F/O-ratio is a simplified and global index that previously showed stable estimations of slow wave activity topography maturation across childhood (Kurth et al., 2010). Secondly, the F/O-ratio can be computed universally in already existing datasets with fewer EEG derivations (frontal and occipital derivations required). The quantification of key neurodevelopmental transitions using this simple index will support the next translational step to test its prognostic use in pediatric developmental disorders.
Here, we demonstrate for the first time that the F/O-ratio of slow wave activity in children predicts whole brain myelin content assessed 3.5 years later. This new discovery extends existing research by promoting a prognostic connection of sleep neurophysiology with brain myelin during a sensitive period of development. Previous understanding of links between sleep and brain maturation was dominated by data from adolescents and adults proposing that cortical gray matter structure is a primary generator of slow wave activity appearance; however, recent advancements in myelin quantification have provided investigation of the association between myelin and slow wave activity. Our results and other accumulating evidence introduces the novel concept that myelin is a key cornerstone of sleep neurophysiology – particularly in children undergoing rapid developmental change.
Novel lines of research are currently promoting the rethinking of cortical gray and white matter developmental trajectories (Natu et al., 2018). Across child development, microstructural tissue growth (instead of tissue loss) is now proposed, contesting the hitherto determined maturational trajectories of cortical gray matter (Natu et al., 2018; Sowell et al., 2004). Consequently, white matter microstructure is becoming a novel focus (Deoni et al., 2012; Lebel and Deoni, 2018), yet, to date, little is known about myelin development within the context of sleep. In adults, data indicate that the sleep/wakefulness cycle affects white matter with regard to cortical mean diffusivity and subcortical volume (Bernardi et al., 2016). In adolescents, high variability in sleep duration is linked to reduced white matter integrity assessed one year later (Telzer et al., 2015). These authors speculate that highly variable sleep duration in adolescents may result in long-term brain impairments. Additionally, our previous studies with children revealed regional relationships of sleep need and slow wave propagation patterns with brain myelin (Kurth et al., 2016; Kurth et al., 2017). Animal data further streamline these observations by proposing that neuronal activity specific to sleep promotes the brain myelin machinery (Bellesi et al., 2013; Cirelli, 2005). Our current results support this concept with a prospective approach.
Although slow wave activity undergoes age-related maturation (Kurth et al., 2010), it is at the same time modified by experiences (Huber et al., 2006; Huber et al., 2004; Wilhelm et al., 2014). This dualism in the factors that regulate slow wave activity obscures the effects of genetics (preprogrammed maturation processes) and the environment (experience-induced changes). In the present approach, we considered this phenomenon by presenting results controlling and not controlling for age. This allowed distinguishing the associations that were merely driven by age from those that were robust after controlling for age. The latter findings revealed that individual differences in slow wave activity topography indeed account for variability in myelin content, thereby illuminating the predictive value of individual traits in slow wave activity topography for myelin maturation. Explicitly, associations between F/O-ratio with whole brain MWF were robust, signifying that the myelin outcome is predicted by individual differences in the sleep marker F/O-ratio. In contrast, callosal effects and those observed in MWF of superior longitudinal fasciculus were primarily driven by maturation (i.e., age). In the process of brain maturation, sleep EEG profiles experience the influence of nature in one brain region, and nurture in another (Rusterholz et al., 2018). This dynamic may influence the state- and trait-like characteristics of the F/O-ratio.
The central role of sleep in developmental processes is increasingly recognized (Volk and Huber, 2015). Brain activity during sleep itself may actively contribute to neurodevelopment (Kurth et al., 2013; Kurth et al., 2012; Olini and Huber, 2014; Schoch et al., 2018). Age-specific properties in the regulation of slow oscillations were suggested as a fundament of such relationships: slow oscillations reflect burst firing (Steriade et al., 1993a; Steriade et al., 1993b), and firing mode specific to sleep may alter the functionality of glutamatergic synapses by changing AMPA receptor availability (Birtoli and Ulrich, 2004; Lante et al., 2011) (for detailed discussion see (Kurth et al., 2012)). In the context of the current investigation, neuronal elements underlying slow wave activity topographical distribution may be targets for interventions to modify brain myelin.
Links between behavioral learning and white matter plasticity remain understudied (Fields, 2011; Kukley et al., 2007; Stevens et al., 2002). Cellular and molecular mechanisms underlying the association of white matter and learning may involve changes in myelin formation / remodeling, fiber crossing, axon diameter, astrocyte morphology or angiogenesis (Zatorre et al., 2012). Given these factors, it not surprising that our results uncovered a global relationship of MWF and our simple sleep EEG marker. This finding aligns with our recent research (Kurth et al., 2017) that supports the finding that slow wave activity topography is linked to global myelin connectivity in children. However, it remains possible that slow wave activity considered in a more regional (less global) manner also reflects properties of cortical measures in children, as concluded from research with adults (Buchmann et al., 2011).
Implications from this investigation open a sleep-centered window of opportunity: the promotion of “good” sleep may foster healthy myelin development. Particularly, at-risk groups may benefit from diagnostic and prevention advances, and sleep-centered interventions may promote healthy neurodevelopment with non-invasive behavioral (Werner et al., 2015) or auditory strategies (Fattinger et al., 2017). We recognize other predictors of myelin development, including genetic and nutritional factors (Deoni et al., 2013a). Yet, here we demonstrate that the most established marker of sleep depth (slow wave activity) is conserved in neuronal network development. Although experimental studies in developing humans are limited, only longitudinal data can identify patterns with important functional roles in the establishment and maintenance of human brain connectivity. Thus, these findings mark an initial and important step towards the goal of ultimately linking specific sleep patterns to their etiology in neurodevelopmental and neuropsychiatric disorders.
Socioeconomic context is intertwined with neurodevelopment (Hackman and Farah, 2009) and with sleep quality in children (El-Sheikh et al., 2010). Yet, while age is a key confounder in this study, we found that socioeconomic status plays no principal role in our main effects. The observation that interactions of sleep with myelin or cortical measures are independent from the social context may be important when developing healthy interventions targeting sleep.
Of note, variability in sleep architecture was rather low in our data. This may be surprising given the well-known maturation of slow wave sleep and REM sleep (Roffwarg et al., 1966). Yet, our within-subject statistics confirm the developmental effects as expected (Kurth et al., 2010; Ohayon et al., 2004). While the nature of the data (small sample size, wide age range) mask developmental effects, moderator variables known affect sleep architecture (pathologies, sleep problems) were controlled in our thoroughly screened healthy cohort. Also, homeostatic effects originating from variable sleep pressure were also carefully controlled via adherence to regular bedtimes during several days preceding assessment. Further, the small sample size of this study, and relatedly, confined statistical power should be considered with caution. Yet, we reduced the likelihood of a type I error to support the predictive value by enclosing, i.e., mean MWF values for a restricted number of brain regions, the focus on one sleep marker, and statistical control for age-effects. This restriction did not allow for investigating regional association of sleep and brain morphology more closely. Further, this study design was framed previously and thus restricted, i.e., wide-age range of participants and MRI only at T2. The availability of MWF at baseline would allow to test whether the F/O-ratio is related to MWF already at T1. It is known that myelin grows rapidly across the first 3 years of life, and keeps maturing until leveling off in adolescence/ young adulthood (Lebel and Deoni, 2018). We anticipated myelin growth in all participants of our sample from T1 to T2, because follow-up assessments were spaced to encompass the age when myelin growth occurs rapidly in healthy children (Dean et al., 2015). Examining the relationship between MWF and slow wave activity at T1 was not crucial to the current goal of this study: our aims were to identify an early physiological marker that was simple to measure and that predicted later brain structure. For these reasons we selected a sleep EEG marker that can be applied to low spatial resolution EEG. Finally, future systematic investigations in children within a more similar age at each assessment time point would be important to identifying the confounding effects of socioeconomic context and psychosocial status.
In conclusion, the F/O-ratio in the sleep EEG is a simple index and a promising prognostic physiological tool that predicts global brain myelin development in children. Findings complement animal data (Bellesi et al., 2013; Cirelli, 2005) and support the theory that neuronal activity specific to sleep promotes the brain myelin machinery. Based on our current understanding, we cannot identify whether specific sleep behaviors in children represent a risk for brain development; however, it should soon be possible to examine whether chronically shortened or fragmented sleep in sensitive periods of development alter brain myelin growth leading to compromised health outcomes.
Supplementary Material
Highlights:
This study extends to children a concept developed initially in a rodent model
A sleep EEG marker predicts brain myelin morphology in a childhood period
Deep sleep (slow wave activity) is a strong prognostic marker for myelin maturation
Sleep is a new translational avenue for the developmental process of mental health
Slow wave activity may mediate benefits of sleep at the systems and cellular level
Acknowledgements
This work was supported by the Clinical Research Priority Program Sleep and Health of the University of Zurich, the Swiss National Science Foundation (PBZHP3-138801, PBZHP3-147180; PP00A-114923), the National Institutes of Health (R01-MH086566), Jacob’s Foundation, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54 HD090256), National Institutes of Mental Health (K99-MH11059) and seed funding from the Center for Innovation and Creativity at the University of Colorado Boulder. We thank the anonymous reviewers for their contribution to the peer review of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The authors declare no competing financial interests.
REFERENCES
- Achermann P, Borbély AA, 2011. Sleep homeostasis and models of sleep regulation In: Kryger MH, Roth T, Dement WC (Eds.), Principles and Practice of Sleep Medicine. Elsevier Saunders, Missouri, pp. 431–444. [Google Scholar]
- Bellesi M, Haswell JD, de Vivo L, Marshall W, Roseboom PH, Tononi G, Cirelli C, 2018. Myelin modifications after chronic sleep loss in adolescent mice. Sleep 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C, 2013. Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci 33, 14288–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R STAT SOC 57, 289–300. [Google Scholar]
- Bernardi G, Cecchetti L, Siclari F, Buchmann A, Yu X, Handjaras G, Bellesi M, Ricciardi E, Kecskemeti SR, Riedner BA, Alexander AL, Benca RM, Ghilardi MF, Pietrini P, Cirelli C, Tononi G, 2016. Sleep reverts changes in human gray and white matter caused by wake-dependent training. Neuroimage 129, 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtoli B, Ulrich D, 2004. Firing mode-dependent synaptic plasticity in rat neocortical pyramidal neurons. J Neurosci 24, 4935–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolsterli Heinzle BK, Fattinger S, Kurth S, Lebourgeois MK, Ringli M, Bast T, Critelli H, Schmitt B, Huber R, 2014. Spike wave location and density disturb sleep slow waves in patients with CSWS (continuous spike waves during sleep). Epilepsia 55, 584–591. [DOI] [PubMed] [Google Scholar]
- Buchmann A, Ringli M, Kurth S, Schaerer M, Geiger A, Jenni OG, Huber R, 2011. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex 21, 607–615. [DOI] [PubMed] [Google Scholar]
- Cirelli C, 2005. A molecular window on sleep: changes in gene expression between sleep and wakefulness. Neuroscientist 11, 63–74. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Dean DC 3rd, Dirks H, O’Muircheartaigh J, Walker L, Jerskey BA, Lehman K, Han M, Waskiewicz N, Deoni SC, 2014a. Pediatric neuroimaging using magnetic resonance imaging during non-sedated sleep. Pediatr Radiol 44, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC 3rd, O’Muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, Walker L, Han M, Deoni SC, 2014b. Modeling healthy male white matter and myelin development: 3 through 60months of age. Neuroimage 84, 742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, O’Muircheartaigh J, Dirks H, Waskiewicz N, Walker L, Doernberg E, Piryatinsky I, Deoni SC, 2015. Characterizing longitudinal white matter development during early childhood. Brain Struct Funct 220, 1921–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S, Dean DC, Piryatinsky I, O’Muircheartaigh J, Waskiewicz N, Lehman K, Han M, Dirks H, 2013a. Breastfeeding and early white matter development: A cross-sectional study. Neuroimage 82, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Dean DC 3rd, O’Muircheartaigh J, Dirks H, Jerskey BA, 2012. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage 63, 1038–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Kolind SH, 2015. Investigating the stability of mcDESPOT myelin water fraction values derived using a stochastic region contraction approach. Magn Reson Med 73, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Matthews L, Kolind SH, 2013b. One component? Two components? Three? The effect of including a nonexchanging “free” water component in multicomponent driven equilibrium single pulse observation of T1 and T2. Magn Reson Med 70, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Rutt BK, Arun T, Pierpaoli C, Jones DK, 2008. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med 60, 1372–1387. [DOI] [PubMed] [Google Scholar]
- Doucette MR, Kurth S, Chevalier N, Munakata Y, LeBourgeois MK, 2015. Topography of Slow Sigma Power during Sleep is Associated with Processing Speed in Preschool Children. Brain Sci 5, 494–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Kelly RJ, Buckhalt JA, Benjamin Hinnant J, 2010. Children’s sleep and adjustment over time: the role of socioeconomic context. Child Dev 81, 870–883. [DOI] [PubMed] [Google Scholar]
- Fattinger S, de Beukelaar TT, Ruddy KL, Volk C, Heyse NC, Herbst JA, Hahnloser RHR, Wenderoth N, Huber R, 2017. Deep sleep maintains learning efficiency of the human brain. Nat Commun 8, 15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, 2008. White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, 2011. Imaging learning: the search for a memory trace. Neuroscientist 17, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM, 1999. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207. [DOI] [PubMed] [Google Scholar]
- Giedd JN, 2004. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci 1021, 77–85. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, 2009. Socioeconomic status and the developing brain. Trends Cogn Sci 13, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB, 1975. Four Factor Index of Social Status. Yale University Press, Hew Haven, CT. [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G, 2006. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci 9, 1169–1176. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G, 2004. Local sleep and learning. Nature 430, 78–81. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Quan SF (Eds.), 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1 st ed. American Academy of Sleep Medicine, Westchester, Illinois. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Munakata Y, 2005. Processes of change in brain and cognitive development. Trends Cogn Sci 9, 152–158. [DOI] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D, 2007. Vesicular glutamate release from axons in white matter. Nat Neurosci 10, 311–320. [DOI] [PubMed] [Google Scholar]
- Kurth S, Achermann P, Rusterholz T, Lebourgeois MK, 2013. Development of Brain EEG Connectivity across Early Childhood: Does Sleep Play a Role? Brain Sci 3, 1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Dean DC 3rd, Achermann P, O’Muircheartaigh J, Huber R, Deoni SC, LeBourgeois MK, 2016. Increased Sleep Depth in Developing Neural Networks: New Insights from Sleep Restriction in Children. Front Hum Neurosci 10, 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Huber R, 2012. Sleep Slow Oscillations and Cortical Maturation In: Frank M (Ed.), Sleep and Brain Activity. Elsevier. [Google Scholar]
- Kurth S, Riedner BA, Dean DC, O’Muircheartaigh J, Huber R, Jenni OG, Deoni S, LeBourgeois M, 2017. Traveling Slow Oscillations During Sleep: A Marker of Brain Connectivity in Childhood. Sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R, 2010. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci 30, 13211–13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Ringli M, Lebourgeois MK, Geiger A, Buchmann A, Jenni OG, Huber R, 2012. Mapping the electrophysiological marker of sleep depth reveals skill maturation in children and adolescents. Neuroimage 63, 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lante F, Toledo-Salas JC, Ondrejcak T, Rowan MJ, Ulrich D, 2011. Removal of synaptic Ca(2)+-permeable AMPA receptors during sleep. J Neurosci 31, 3953–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Deoni S, 2018. The development of brain white matter microstructure. Neuroimage 182, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustenberger C, Mouthon AL, Tesler N, Kurth S, Ringli M, Buchmann A, Jenni OG, Huber R, 2017. Developmental trajectories of EEG sleep slow wave activity as a marker for motor skill development during adolescence: a pilot study. Dev Psychobiol 59, 5–14. [DOI] [PubMed] [Google Scholar]
- Mason TB 2nd, Teoh L, Calabro K, Traylor J, Karamessinis L, Schultz B, Samuel J, Gallagher PR, Marcus CL, 2008. Rapid eye movement latency in children and adolescents. Pediatr Neurol 39, 162–169. [DOI] [PubMed] [Google Scholar]
- Mindell JA, Leichman ES, DuMond C, Sadeh A, 2017. Sleep and Social-Emotional Development in Infants and Toddlers. J Clin Child Adolesc Psychol 46, 236–246. [DOI] [PubMed] [Google Scholar]
- Natu VS, Gomez J, Barnett M, Jeska B, Kirilina E, Jaeger C, Zhen Z, Cox S, Weiner K, Weiskopf N, Grill-Spector K, 2018. Apparent thinning of visual cortex during childhood is associated with myelination, not pruning. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV, 2004. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27, 1255–1273. [DOI] [PubMed] [Google Scholar]
- Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B, group, C.s., European Brain, C., 2012. The economic cost of brain disorders in Europe. Eur J Neurol 19, 155–162. [DOI] [PubMed] [Google Scholar]
- Olini N, Huber R, 2014. Diurnal changes in electrocorticogram sleep slow-wave activity during development in rats. J Sleep Res. [DOI] [PubMed] [Google Scholar]
- Plante DT, Landsness EC, Peterson MJ, Goldstein MR, Wanger T, Guokas JJ, Tononi G, Benca RM, 2012. Altered slow wave activity in major depressive disorder with hypersomnia: a high density EEG pilot study. Psychiatry Res 201, 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli M, Souissi S, Kurth S, Brandeis D, Jenni OG, Huber R, 2013. Topography of sleep slow wave activity in children with attention-deficit/hyperactivity disorder. Cortex 49, 340–347. [DOI] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC, 1966. Ontogenetic development of the human sleep-dream cycle. Science 152, 604–619. [DOI] [PubMed] [Google Scholar]
- Rusterholz T, Hamann C, Markovic A, Schmidt SJ, Achermann P, Tarokh L, 2018. Nature and Nurture: Brain Region-Specific Inheritance of Sleep Neurophysiology in Adolescence. J Neurosci 38, 9275–9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletin JM, Goldstein-Piekarski AN, Greer SM, Stark S, Stark CE, Walker MP, 2016. Human Hippocampal Structure: A Novel Biomarker Predicting Mnemonic Vulnerability to, and Recovery from, Sleep Deprivation. J Neurosci 36, 2355–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch SF, Riedner BA, Deoni SC, Huber R, LeBourgeois MK, Kurth S, 2018. Across-night dynamics in traveling sleep slow waves throughout childhood. Sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP, 2008. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28, 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivertsen B, Harvey AG, Reichborn-Kjennerud T, Torgersen L, Ystrom E, Hysing M, 2015. Later emotional and behavioral problems associated with sleep problems in toddlers: a longitudinal study. JAMA Pediatr 169, 575–582. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC, 1998. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17, 87–97. [DOI] [PubMed] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction. Hum Brain Mapp 17, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW, 2004. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci 24, 8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nunez A, 1993a. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci 13, 3284–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ, 1993b. Thalamocortical oscillations in the sleeping and aroused brain. Science 262, 679–685. [DOI] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD, 2002. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36, 855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA, Achermann P, 2010. Developmental changes in brain connectivity assessed using the sleep EEG. Neuroscience 171, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Goldenberg D, Fuligni AJ, Lieberman MD, Galvan A, 2015. Sleep variability in adolescence is associated with altered brain development. Dev Cogn Neurosci 14, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesler N, Gerstenberg M, Huber R, 2013. Developmental changes in sleep and their relationships to psychiatric illnesses. Curr Opin Psychiatry 26, 572–579. [DOI] [PubMed] [Google Scholar]
- Volk C, Huber R, 2015. Sleep to grow smart? Arch Ital Biol 153, 99–109. [DOI] [PubMed] [Google Scholar]
- Werner H, Hunkeler P, Benz C, Molinari L, Guyer C, Hafliger F, Huber R, Jenni OG, 2015. The Zurich 3-step concept for the management of behavioral sleep disorders in children: a before-and-after study. J Clin Sleep Med 11, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Kurth S, Ringli M, Mouthon AL, Buchmann A, Geiger A, Jenni OG, Huber R, 2014. Sleep slow-wave activity reveals developmental changes in experience-dependent plasticity. J Neurosci 34, 12568–12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H, 2012. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15, 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.