Abstract
Allergic contact dermatitis (ACD) is a common skin disease that results in significant cost and morbidity. Despite its high prevalence, therapeutic options are limited. Allergic contact dermatitis is regulated primarily by T cells within the adaptive immune system, but also by natural killer and innate lymphoid cells within the innate immune system. The chemokine receptor system, consisting of chemokine peptides and chemokine G protein–coupled receptors, is a critical regulator of inflammatory processes such as ACD. Specific chemokine signaling pathways are selectively up-regulated in ACD, most prominently CXCR3 and its endogenous chemokines CXCL9, CXCL10, and CXCL11. Recent research demonstrates that these 3 chemokines are not redundant and indeed activate distinct intracellular signaling profiles such as those activated by heterotrimeric G proteins and β-arrestin adapter proteins. Such differential signaling provides an attractive therapeutic target for novel ACD therapies and other inflammatory diseases.
Allergic contact dermatitis (ACD) is a common skin condition that is associated with lost productivity and significant medical cost.1 This review discusses the pathophysiology of ACD and describes the role of chemokines and their receptors in ACD. Improved understanding and appreciation for the role of chemokines in ACD combined with advances in our understanding of chemokine receptor (CKR) signaling highlight the chemokine system as an attractive therapeutic target for treating ACD.
Allergic contact dermatitis is mediated by many cell types within the immune system. Major cell types that propagate inflammation in ACD include dendritic cells, TH1 and TH17 cells, whereas regulatory T cells (Tregs) act to suppress inflammation.2–5 Effector and memory T lymphocytes are the key adaptive immune mediators of ACD.6 This differs from irritant contact dermatitis (ICD), which is a more rapid and nonspecific inflammatory dermatitis brought about by activation of the innate immune system by the proinflammatory properties of chemicals or other small molecules.7 Natural killer (NK), NK variant, and innate lymphoid cells of the innate immune system are also recognized to contribute to ACD.8,9
In ACD, dendritic cells, including epidermal Langerhans cells and other dermal dendritic cell populations, are critical to sensitizing individuals to foreign antigens. Dendritic cells are responsible for antigen capture, transport of antigens to draining lymph nodes, and activation of naive T cells. Naive T cells are then expanded into effector and/or memory T-cell populations, which are subdivided into 4 main categories: TH1, TH2, TH17, and Tregs. Interferon γ signaling10 polarizes T cells toward the TH1 phenotype, which is responsible for targeting and destroying intracellular pathogens. It is thought that the TH1 phenotype of T cells is the primary mediator of ACD inflammatory responses, but evidence suggests that certain haptens, such as fluorescein isothiocyanate and certain metals, also activate TH2 pathways that regulate the pathophysiology of ACD.11,12 TH9 cytokines can also be identified in positive patch test reaction sites and are thought to regulate allergic TH1 responses.13 TH1-polarized T cells can be distinguished from other T-cell populations by distinct surface markers and receptors, including distinct CKRs.14
HAPTEN ACTIVATION OF THE ADAPTIVE IMMUNE SYSTEM INITIATES ACD
Development of ACD requires the activation of T cells that have antigen-specific acquired immunity to small molecules known as haptens or contact allergens. Haptens are common in many household and workplace materials, as well as in personal care products and jewelry. Haptens are most commonly less than 500 d,15,16 more than 100-fold smaller than the receptor-protein complexes that recognize them to activate an allergic response. Hapten is derived from Greek “to fasten,” and in order to stimulate an allergic response, these small molecules must be covalently bound to a protein.17 Exceptions to the requirement of a hapten-protein covalent bond include metallic salts, such as cobalt and nickel, which instead form an ionic complex with a protein. After a hapten-protein association, this complex can then be presented to T-cell receptors via the major histocompatibility complex (MHC) encoded by human leukocyte antigen genes. Peptides presented on MHC class I are recognized by CD8+ cytotoxic T cells, whereas peptides presented on MHC class II are recognized by CD4+ T helper cells. Because haptens can bind to peptides that are presented either in the context of MHC class I, MHC class II, or both, they have the potential to activate both cytotoxic and helper subsets of T cells.
Although both patient-specific immune responses and hapten-specific features contribute to ACD, it remains unclear why some individuals, but not others, become sensitized to specific allergens. Wide genetic variation in human leukocyte antigen haplotypes contributes to differential allergic responses between individuals through differing affinities of the MHC for specific hapten-peptide pairings.18 The amount of antigen necessary for sensitization varies considerably between different haptens and is dependent on a variety of factors inherent to the molecule, including epidermal permeability, chemical stability, and reactivity. For example, loss-of-function mutations in the filaggrin gene that disrupt epidermal integrity increase a patient’s risk of nickel contact allergy.19 It is important to note that hapten-peptide presentation in the context of MHC to a T-cell receptor (signal 1) is not usually sufficient to activate T cells and drive the clonal expansion necessary for allergy development. A costimulatory signal (signal 2), such as activation of Toll-like receptor(s) (TLR), is also necessary.20 Costimulatory signals, including CD80 and CD86 (also known as B7–1 and B7–2, which are the ligands for CD28 and CTLA-4) and CD40 (the receptor for CD40L found on T cells), regulate a complex cross-talk between antigen-presenting cells and T cells. The combination of hapten presentation on an MHC of an antigen-presenting cell and costimulatory signal(s) can be sufficient for T-cell activation, expansion, and development of an allergic response. Toll-like receptor 4 in humans binds nickel, the most common contact allergen worldwide. Interestingly, mice do not develop an allergy to nickel, which may be due to differences in nonconserved histidine residues in the mouse Tlr4 sequence that impairs nickel binding relative to the human sequence.20 The necessity of a costimulatory signal, such as those caused by hapten-induced activation of TLRs, explains why not all small molecules that are presented to T cells in the context of the MHC induce an allergic response.
Given the necessity for a hapten to be attached to a carrier protein to elicit an allergic response, it is not surprising that low molecular weight is a common property of haptens. Many haptens also have lipophilic and electrophilic residues that increase affinity for covalently binding to proteins.21 Some molecules require oxidation or other modifications before they are directly reactive. “Pre haptens” form protein-reactive products through spontaneous ambient air oxidation, whereas “pro haptens” are oxidized via metabolic processes. For example, urushiol, the allergic component of poison ivy, is a “pro hapten” and does not stimulate an allergic response. However, once urushiol comes in contact with the skin, urushiol is oxidized and can stimulate an immune response. Hapten stability can influence patch testing, as the active form of the allergen must be present at a sufficient concentration to elicit a measureable reaction.22
T CELLS ARE CRITICAL REGULATORS OF ACD
Allergic contact dermatitis is divided into 3 main phases: sensitization, elicitation, and resolution.23 All of these phases are regulated in part by different populations of T cells. In the sensitization phase, haptens are collected by resident dendritic cells in the skin. Dendritic cells then migrate to the regional lymph nodes and present a hapten-peptide antigen complex to naive T cells. Presentation of an antigen (signal 1) and costimulation (signal 2) activate and clonally expand antigen-specific CD4+ and CD8+ T cells. This results in the maturation and expansion of T-cell populations that specifically recognize the hapten-protein complex in the context of MHC. Reexposure to the relevant hapten initiates transmigration of the expanded effector memory T-cell population to the dermis and epidermis, resulting in a clinical manifestation of ACD at the sites of hapten challenge. Finally, resolution of inflammation occurs, which is mediated in part by Treg secretion of interleukin 10, which prevents extravasation of circulating effector T cells into inflamed skin.24
In ACD, T cells cause injury to the skin via 3 major mechanisms: (1) producing cytokines that induce inflammation, (2) directly killing cells, and (3) activating other immune cell populations, such as NK cells and macrophages, which potentiate T-cell–driven inflammatory signals and promote tissue destruction. These 3 pathways require the presence of effector T cells in the skin.
Following differentiation, T cells acquire the capacity to produce effector cytokines and exit the lymph nodes. Egress of T cells from the lymphatic system into the skin occurs in 4 main steps: (1) rolling along the vessel endothelium, (2) activation of integrins on the endothelium that slow T-cell migration, (3) firm adhesion of a T cell to the endothelium (stopping the cell), and (4) transmigration, or extravasation, of a T cell from the vessel and into the skin. Reducing T-cell activation and infiltration improves symptoms and reduces inflammation. This can be achieved by blocking T-cell costimulatory signals or by enhancing T-cell coinhibitory signals.25 Although monoclonal antibodies that reduce T-cell signaling can blunt T cell–mediated responses, they are not commonly used because of cost, burden of infusion, and potential adverse effects. Topical or oral steroids, the most widely used treatments of ACD, broadly inhibit T-cell function, including T-cell chemotaxis.26,27 However, corticosteroids are associated with a plethora of adverse effects when used for an extended duration. A theoretically more attractive treatment method for ACD is selectively inhibiting the egress of activated T cells at the site of inflammation. Notably, chemokines mediate the 4 steps critical for T-cell egress from the vasculature to the skin. Specifically targeting T-cell signals that promote activation and extravasation has the potential to treat ACD without the adverse effects of nonspecific T-cell inhibitors.
THE CKR SYSTEM AS A THERAPEUTIC TARGET FOR ACD
The chemokine system is necessary for all aspects of ACD, from trafficking of antigen-presenting cells to the lymph node to the recruitment and polarization of cells such as Tregs and macrophages to attenuate the inflammatory response. Indeed, chemokines and CKRs play a central role in inflammatory diseases, including ACD. Chemokines are small proteins that range from approximately 8 to 13 kd in mass. Chemokines are partitioned into 4 main families based on the relative location of cysteine within their primary structure. These families are termed XC, CC, CXC, and CX3C, respectively, where “C” denotes a cysteine and “X” refers to any amino acid.28 Chemokines bind to and activate the CKR subclass of G protein–coupled receptors (GPCRs).29 For many CKRs, activation by an endogenous chemokine leads to integrin expression, which is necessary for appropriate lymphocyte trafficking. In addition, a chemokine gradient is necessary for the transmigration of T cells from vessels and into the skin. This transendothelial migration relies on a complex process of signaling factors and protein intermediaries promoting chemokine attraction, adhesion, and motility. Chemokines regulate a variety of transcription factors, including NF-κB (nuclear factor κ/light-chain enhancer of activated B cells)30 and NFAT (nuclear factor of activated T cells),31 which are key to T-cell activation and polarization.
As GPCRs, CKRs are attractive drug targets. More than 30% of US Food and Drug Administration (FDA)–approved drugs target GPCRs, which have both extracellular and intracellular binding surfaces that can interact with small molecules.32 Despite the therapeutic potential of CKRs, currently only 3 FDA-approved medications target the CKR family: maraviroc (Selzentry or Celsentri) for human immunodeficiency virus (targeting CCR5), plerixafor (Mozobil) for hematopoietic stem cell mobilization (CXCR4), and the recently approved mogamulizumab (Poteligeo) for mycosis fungoides and Sézary syndrome (CCR4). It is interesting to note that, despite the role of chemokines and CKRs in nearly every inflammatory process, none of the CKR drugs currently approved by the FDA is for a primary inflammatory indication. One common theory about the exceptional challenge of therapeutically targeting CKRs relative to other GPCRs is the promiscuity and redundancy of the CKR system. In the CKR system, nearly 50 chemokine peptides bind to more than 20 CKRs.33 Many CKRs bind multiple endogenous chemokines with similar affinities.34 For example, the CKR CXCR3, which is highly expressed in T cells found in ACD skin lesions, binds 3 endogenous ligands, CXCL9, CXCL10, and CXCL11. All 3 of these chemokines are sufficient to induce chemotaxis of CXCR3-expressing T cells.35 Binding of multiple endogenous ligands to the same GPCR is uncommon; most GPCRs display high-affinity binding to a single endogenous ligand. Adding to the complexity of CKR signaling, distinct contact allergens can activate different cellular responses that result in differential chemokine signaling patterns.
BIASED SIGNALING WITHIN THE CHEMOKINE SYSTEM
Previously, the importance of immune cell migration was thought to account for the putative ligand redundancy within the chemokine system.36 Although the concernment of correct and controlled trafficking has remained unchallenged, it is now appreciated that different chemokine ligands for the same receptor can activate divergent intracellular signaling events.37,38 Such divergent intracellular signaling events at the same receptor suggest distinct chemokine roles. This phenomenon of different ligands binding to the same receptor but activating distinct signaling pathways is referred to as biased agonism.39 At GPCRs, a peptide or small molecule may preferentially signal through a heterotrimeric G protein signaling pathway, whereas a different peptide or small molecule may preferentially signal through a β-arrestin signaling pathway. Differential activation of these G protein and β-arrestin pathways can lead to distinct physiological events. Biased signaling, or functional selectivity,40 not only offers an alternative explanation for the “redundancy” within the chemokine system but also offers a therapeutically attractive method to more selectively target promiscuous GPCRs while reducing adverse effects.
CXCR3 AS A THERAPEUTIC TARGET IN ACD
The CXCR3 signaling pathway, mediated by its endogenous chemokines CXCL9 (also known as monokine induced by interferon γ), CXCL10 (also known as interferon-induced protein 10), and CXCL11 (interferon-inducible T-cell α chemoattractant), is the most consistently up-regulated chemokine signaling pathway in ACD. In both human and mouse models, CXCR3 is up-regulated in ACD, but not ICD.10,41–44 Interferon γ, the classic TH1-polarizing cytokine, increases expression of CXCR3 and its ligands CXCL9, CXCL10, and CXCL11. This is in contrast to other chemokines, such as CCL2, where application of either an irritant or an allergen increases transcript levels. One study examining skin specimens after patch testing found that CXCL10 was most abundant and predominantly expressed by epidermal cells (mostly keratinocytes), whereas CXCL9 was expressed in both the epidermis and the dermis.41 This study demonstrated that more than 50% of infiltrating T cells in ACD expressed CXCR3, whereas only 20% of T cells expressed CXCR3 in ICD. Both mouse models and humans patch tested with allergens show that CXCL9 and CXCL10 are selectively up-regulated in a hapten-specific manner (Table 1). A separate study demonstrated that CXCL9, CXCL10, and CXCL11 were time dependently induced in 7 of 8 nickel-sensitized patients upon nickel sulfate exposure, with peak chemokine expression noted 48 hours after nickel elicitation.49
TABLE 1.
Chemokines and Cognate CKRs Implicated in the Pathophysiology of ACD
| Chemokine | CKR | Specificity for ACD | Time Noted | Reference |
|---|---|---|---|---|
| CCL1 | CCR8 | Nonspecific | 6 h45 | 45,46 |
| CCL2 | CCR2 | Nonspecific | 6–48 h10,47,48 | 48–50 |
| CCL3 | CCR1/CCR4/CCR5 | Nonspecific | 4–24 h48,51,52 | 48,52 |
| CCL4 | CCR5 | Nonspecific | 24 h48 | 48 |
| CCL5 | CCR1/CCR5 | Nonspecific | 12–48 h10 | 46,49,53,54 |
| CCL11 | CCR3/CCR5 | Nonspecific | 24 h48 | 48 |
| CCL20 | CCR6 | Nonspecific | 2–20 h49 | 49 |
| CCL22 | CCR4 | Nonspecific | 12–48 h10 | 49,55 |
| CCL17 | CCR4 | Unclear | 12–48 h10 | 49,50,53,55–57 |
| CXCL9 | CXCR3 | Specific | 48–96 h10,41,47,49 | 43,46,47,49,58 |
| CXCI 10 | CXCR3 | Specific | 48–96 h10,41,47 | 43,46,47,49,58 |
Chemokines that are also similarly up-regulated in irritant dermatitis are labeled “nonspecific” for ACD, whereas chemokines consistently increased in ACD relative to ICD are labeled “specific” for ACD.
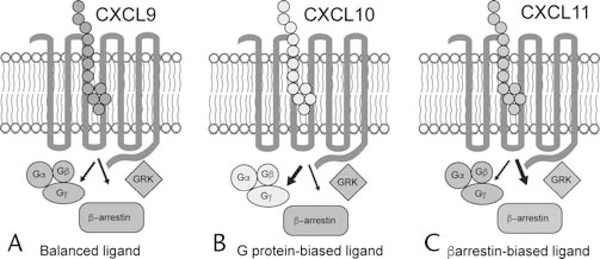
CXCL9, CXCL10, and CXCL11 have been shown to activate distinct signaling pathways at CXCR3 (Fig. 1). For example, CXCL9 acts as a partial agonist at both G protein and β-arrestin signaling pathways, the 2 appreciated major signaling pathways regulated by GPCRs.59 In contrast, CXCL10 acts as a full agonist at the G protein pathway but as a partial agonist at the β-arrestin pathway. CXCL11 is a full agonist for both pathways. These relative differences in signaling efficacy between the endogenous ligands for CXCR3 demonstrate that CXCL11 acts as a β-arrestin–biased ligand relative to CXCL9 and CXCL10.60–62
Figure 1.
Biased signaling at CXCR3. The 3 endogenous ligands for CXCR3–CXCL9, CXCL10, and CXCL11–differentially signal through heterotrimeric G protein and β-arrestin pathways. G protein and β-arrestin pathways activate distinct signaling cascades that produce different physiological effects. In this example, CXCL9 (A) is taken as the reference agonist and signals equivalently through G proteins and β-arrestins. B, Relative to CXCL9, CXCL10 exhibits greater G protein signaling but equivalent β-arrestin signaling. C, CXCL11 exhibits greater β-arrestin signaling relative to both CXCL9 and CXCL10. Although β-arrestin signaling appears necessary for full-efficacy T-cell chemotaxis, the biological consequences of biased signaling are at early stages of investigation and remain to be fully elucidated. GRK indicates G protein receptor kinase.
This divergence between G protein and β-arrestin signaling induced by different CXCR3 ligands may have important physiological consequences in ACD. First, patients patch tested for contact allergy show coexpression of CXCR3 and β-arrestin in T cells in patch test–positive, but not negative, skin (Smith et al, personal communication). Unlike the endogenous peptide ligands, which are too large to penetrate the skin, such small molecules can diffuse through the epidermis and allow for a dissection of the signaling pathways critical to ACD. A recent study used small-molecule compounds with an identical affinity but divergent G protein and β-arrestin efficacy to study the physiological effects of G protein and β-arrestin signaling in ACD. In a mouse model of ACD, topical application of a small-molecule CXCR3 β-arrestin–biased agonist, but not a CXCR3 G protein–biased agonist, potentiated inflammation after elicitation of the allergic response (Smith et al, personal communication). The β-arrestin–biased agonist induced greater chemotaxis of effector/memory T cells relative to the G protein–biased agonist. Differences in chemotaxis effects were lost in T cells isolated from β-arrestin knockout mice, and chemotaxis was eliminated in T cells isolated from CXCR3 knockout mice, demonstrating the role of β-arrestin signaling in CXCR3-mediated chemotaxis. β-Arrestin activation of Akt (a key signaling kinase that mediates many cellular functions) downstream of CXCR3 is critical to the regulation of T cells, and ongoing preclinical work is examining this pathway as a possible therapeutic target for ACD.
Differences in the chemotactic response of CD4+ and CD8+ T-cell populations to CXCR3 chemokines have also been noted in ACD models, suggesting that different signaling transducers could tune the T migratory response of specific T-cell populations. For example, CXCL10 is nearly 2-fold more potent in attracting nickel-specific CD8+ T cells relative to CD4+ T cells,47 suggesting that the signaling transducers downstream of CXCR3 expressed in cytotoxic and helper T-cell populations may also differ. Whereas CXCL9 and CXCL10 appear necessary for the recruitment of CXCR3-expressing effector memory T cells that mediate the inflammatory response in ACD, the role of CXCL11 is less clear. CXCL11 has been shown to drive Treg activity in other disease processes,63,64 and mouse models suggest that CXCR3 expression on Tregs is essential for the resolution of inflammation.65 Further studies are necessary to dissect the physiological roles of biased signaling at CXCR3 and the function of T-cell subsets in ACD. Taken as a whole, these data highlight nonredundant roles for different CXCR3 endogenous chemokines with distinct signaling pathways and provide compelling evidence that biased agonists can impact the clinical utility of drugs targeting CXCR3 and likely other CKRs.
CCR4 SIGNALING IN ACD
CCR4 is expressed on essentially all skin-homing memory T cells and is highly correlated with the cutaneous inflammatory T-cell response. CCR4 binds 2 chemokines implicated in ACD, CCL17 and CCL22. CCL17 is also known as thymus and activation-regulated chemokine. It is expressed constitutively in the thymus and is also produced by keratinocytes, cutaneous venules, fibroblasts, and dendritic cells. CCL17 promotes the arrest and migration of skin-homing memory T cells and is associated with ACD.66 In a human study comparing nickel-induced inflammation with nickel-sensitive normal skin, a 50-fold increase in CCL17 protein and a 6-fold increase in CCR4 expression were observed,67 highlighting the role of this chemokine signaling axis. In addition, CCL17 genes were up-regulated in the epidermis of ACD lesions, but not in control patients.56 CCR4 is expressed to a greater degree on TH2 cells relative to TH1 T cells,68 and the involvement of CCR4 expressing cells in an ACD lesion likely depends on the populations of T cells activated by the specific hapten, which depends on whether it is presented by the MHC class I or II pathway. Notably, the canonical TH1-polarizing cytokine interferon γ can increase CCL17 expression,69 which sensitizes dendritic cells for CCR4-dependent migration to lymph nodes under inflammatory conditions. Thus, in addition to mediating T-cell migration, CCL17 plays an important role in cutaneous dendritic cell migration.70 Of note, serum CCL17 levels are also associated with disease activity in atopic dermatitis.71 Interestingly, the role for CCR4 in mouse models of ACD appears to differ from that in human samples. Global CCR4 knockout mice show a paradoxical increase in inflammation,72 similar to a paradoxical increase observed in global CXCR3 knockout mice.65 Compensatory changes in both immune polarization, as well as the expression of these receptors on multiple cell types (such as Tregs that promote resolution of inflammation), confound interpretation but nevertheless implicate these receptors in mediating ACD.
CCR4 binds another chemokine, CCL22, which is implicated sporadically in ACD. CCL22 is also known as macrophage-derived chemokine and is a TH2 response–associated chemokine. Transcripts of CCL22 are increased in nickel-induced allergy relative to irritant dermatitis. Although ACD is classically considered a TH1 T-cell response, nickel allergy in particular can induce a TH2 response,73 and responding T cells up-regulate CCR4, CXCR3, and CCR10.74 Similar findings of increased CCL22 and subsequent induction of a TH2-type response were observed in mice responding to natural rubber latex challenge.48 Such results further highlight that certain haptens can induce allergen-specific chemokine expression patterns. CCL17 signals through both G proteins and β-arrestins; however, the degree of biased signaling that exists between CCL17 and CCL22 (if any) is currently unknown.
OTHER CHEMOKINES IMPLICATED IN THE PATHOGENESIS OF ACD
Additional chemokines and CKRs noted consistently throughout the literature to be correlated or causative with the pathogenesis of ACD are shown in Table 1. We highlight 2 additional chemokines that are often up-regulated in ACD, CCL5 and CCL11. However, this expression is not specific to ACD per se, as inflammatory dermatoses induce CCL5 and CCL11 transcription to similar degrees. CCL5, also known as RANTES (regulated on activation, normal T-cell expressed and secreted signaling), is implicated in both ICD75 and ACD.53 CCL5 and CXCL8 are induced by the TH2-promoting cytokine tumor necrosis factor α and play a role in the initial chemokine response to an allergen.46 A mouse model that uses systemic treatment with a modified CCL5 antagonist at both CCR1 and CCR5 showed reduced inflammation in both irritant and allergic models of cutaneous inflammation.54 These findings support the human data suggesting that CCL5-mediated CCR1/CCR5 signaling is important in the pathogenesis of ACD but that this signaling pathway is not specific for contact allergy. CCL5 induces both β-arrestin and G protein signaling at CCR5.76,77
CCL11, also known as eotaxin-1, is a promiscuous chemokine with endogenous agonist activity for the CKRs CCR3 and CCR5. Similar to CCL17 and CCL22, the expression is regulated by type 2 effector cytokines such as interleukin 4. CCL11 was originally named for its ability to stimulate the chemotaxis of eosinophils and basophils through CCR3 and CCR5.78 CCL11 has also been found to signal through CCR3 expressed by TH2-polarized T cells.79 Although some studies have correlated CCL11 expression with ACD inflammation,48,49 others have found minimal expression of CCL11 in ACD lesions.10 Inflammation induced by different haptens and/or the timing of sample acquisition may explain the differences noted between these studies.
TARGETING CKR SIGNALING USING BIASED AGONISTS
By preferentially activating beneficial signaling cascades while reducing activity at deleterious pathways, biased agonists are promising therapeutic tools that may not only improve clinical efficacy but also reduce adverse effects. The clinical benefit of biased agonists has been recently established at other GPCRs. For example, at the μ-opioid GPCR, G protein–biased agonists show comparable analgesic efficacy to morphine, but with a reduced on-target effect of respiratory depression.80,81 Chemokine receptors are particularly attractive GPCRs to target with biased agonists because endogenous chemokines activate distinct signaling pathways, such as G proteins and β-arrestins. Endogenous bias at CKRs suggests an evolutionarily conserved mechanism to preferentially signal through certain signaling cascades, although many of the distinct physiological functions regulated by specific signaling pathways remain unknown. With many CKRs binding multiple endogenous ligands, traditional antagonists that inactivate all receptor signaling may have deleterious effects. For example, broad pathway antagonism may silence beneficial cell signaling events in addition to pathologically activated pathways. Precisely targeted drugs, such as negative allosteric modulators of the β-arrestin pathway (which would theoretically reduce CXCR3-mediated chemotaxis while preserving G protein signaling), might be more useful therapeutic tools within the CKR pathway. However, more research is needed to test these hypotheses.
CONCLUDING REMARKS
T cells are the primary mediators of inflammation in ACD. The CKR system is a critical regulator of T-cell movement, and many different chemokines and CKRs are involved in the complex interplay of hapten sensitization, allergy elicitation, and inflammatory resolution. Because of the essential role of the CKR system in ACD, it offers an attractive therapeutic target. Chemokine signaling through CXCR3 in particular appears to play an important role in the pathophysiology of ACD, although a number of different chemokines and receptors are necessary to regulate T cell–mediated inflammation. The specific chemokines involved in ACD appear to be both hapten dependent and, in some cases, patient specific. The recent discoveries that different chemokines can activate distinct signaling pathways through the same receptor suggest that therapeutic blockade or activation of specific signaling pathways may offer efficacious treatments for a system that has been notoriously difficult to therapeutically target. Chemokine receptor–biased agonists offer an attractive opportunity for treating ACD. Given the complex interplay of chemokines in directing immune cell movement and function, systemic administration of either small molecules or peptides (such as monoclonal antibodies) targeting CKRs is likely to produce undesirable clinical effects. Topically applied, small topical small-molecule CKR antagonists, or biased agonists that antagonize certain signaling pathways while maintaining activity at other pathways, offer attractive options for treating ACD.
ACKNOWLEDGMENTS
The authors thank A.J. Wisdom for discussion and critical evaluation of the article.
Supported by T32GM7171 (to J.S.S.), 1R01GM122798-01A1 (to S.R.), K08HL114643-01A1 (to S.R.), Burroughs Wellcome Career Award for Medical Scientists (to S.R.), and the Duke Pinnell Center for Investigative Dermatology (to J.S.S., A.R.A., and S.R.).
Footnotes
The authors have no conflicts of interest to declare.
references
- 1.Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol 2017;76:958–972. [DOI] [PubMed] [Google Scholar]
- 2.Verhagen J, Akdis M, Traidl-Hoffmann C, et al. Absence of T-regulatory cell expression and function in atopic dermatitis skin. J Allergy Clin Immunol 2006;117:176–183. [DOI] [PubMed] [Google Scholar]
- 3.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol 2005;6: 1182–1190. [DOI] [PubMed] [Google Scholar]
- 4.Homey B, Steinhoff M, Ruzicka T, et al. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol 2006;118: 178–189. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi Y, Staquet MJ, Dezutter-Dambuyant C, et al. Development of motility of Langerhans cell through extracellular matrix by in vitro hapten contact. Eur J Immunol 1994;24:2254–2257. [DOI] [PubMed] [Google Scholar]
- 6.Cruz PD. What accounts for contact allergy? Dermatitis 1994;5:189–193. [Google Scholar]
- 7.Nosbaum A, Vocanson M, Rozieres A, et al. Allergic and irritant contact dermatitis. Eur J Dermatol 2009;19:325–332. [DOI] [PubMed] [Google Scholar]
- 8.Campos RA, Szczepanik M, Lisbonne M, et al. Invariant NKT cells rapidly activated via immunization with diverse contact antigens collaborate in vitro with B-1 cells to initiate contact sensitivity. J Immunol 2006;177:3686–3694. [DOI] [PubMed] [Google Scholar]
- 9.Deniz G, van de Veen W, Akdis M. Natural killer cells in patients with allergic diseases. J Allergy Clin Immunol 2013;132:527–535. [DOI] [PubMed] [Google Scholar]
- 10.Goebeler M, Trautmann A, Voss A, et al. Differential and sequential expression of multiple chemokines during elicitation of allergic contact hypersensitivity. Am J Pathol 2001;158:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dearman RJ, Kimber I. Role of CD4(+) T helper 2-type cells in cutaneous inflammatory responses induced by fluorescein isothiocyanate. Immunology 2000;101:442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minang JT, Arestrom I, Troye-Blomberg M, et al. Nickel, cobalt, chromium, palladium and gold induce a mixed TH1- and TH2-type cytokine response in vitro in subjects with contact allergy to the respective metals. Clin Exp Immunol 2006;146:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Harberts E, Tammaro A, et al. IL-9 regulates allergen-specific TH1 responses in allergic contact dermatitis. J Invest Dermatol 2014;134: 1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (TH1s) and TH2s . J Exp Med 1998;187:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin SF. T lymphocyte-mediated immune responses to chemical haptens and metal ions: implications for allergic and autoimmune disease. Int Arch Allergy Immunol 2004;134:186–198. [DOI] [PubMed] [Google Scholar]
- 16.Cherniakov IN, Maksimov IV, Glazkova VA. Prevention of altitude decompression sickness during short flights in a depressurized cabin at high altitudes [in Russian]. Kosm Biol Aviakosm Med 1977;11:63–67. [PubMed] [Google Scholar]
- 17.Weltzien HU, Moulon C, Martin S, et al. T cell immune responses to haptens. Structural models for allergic and autoimmune reactions. Toxicology 1996; 107:141–151. [DOI] [PubMed] [Google Scholar]
- 18.von Bonin A, Plaga S, Ruh H, et al. Analysis of major histocompatibility complex class I-restricted hapten recognition by mutation of the V-J joining of T cell receptor alpha chains. Eur J Immunol 1996;26:179–186. [DOI] [PubMed] [Google Scholar]
- 19.Novak N, Baurecht H, Schafer T, et al. Loss-of-function mutations in the filaggrin gene and allergic contact sensitization to nickel. J Invest Dermatol 2008;128:1430–1435. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M, Raghavan B, Muller V, et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol 2010;11:814–819. [DOI] [PubMed] [Google Scholar]
- 21.Basketter D, Dooms-Goossens A, Karlberg AT, et al. The chemistry of contact allergy: why is a molecule allergenic? Contact Dermatitis 1995; 32:65–73. [DOI] [PubMed] [Google Scholar]
- 22.Joy NM, Rice KR, Atwater AR. Stability of patch test allergens. Dermatitis 2013;24:227–236. [DOI] [PubMed] [Google Scholar]
- 23.Vocanson M, Hennino A, Rozieres A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy 2009;64:1699–1714. [DOI] [PubMed] [Google Scholar]
- 24.Ring S, Schafer SC, Mahnke K, et al. CD4+ CD25+ regulatory T cells suppress contact hypersensitivity reactions by blocking influx of effector T cells into inflamed tissue. Eur J Immunol 2006;36:2981–2992. [DOI] [PubMed] [Google Scholar]
- 25.Das S, Ariizumi K, Cruz PD Jr. T-cell inhibitors: a bench-to-bedside review. Dermatitis 2012;23:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wendt E, White GE, Ferry H, et al. Glucocorticoids suppress CCR9-mediated chemotaxis, calcium flux, and adhesion to MAdCAM-1 in human T cells. J Immunol 2016;196:3910–3919. [DOI] [PubMed] [Google Scholar]
- 27.Belgi G, Friedmann PS. Traditional therapies: glucocorticoids, azathioprine, methotrexate, hydroxyurea. Clin Exp Dermatol 2002;27:546–554. [DOI] [PubMed] [Google Scholar]
- 28.Bachelerie F, Ben-Baruch A, Burkhardt AM, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev 2014;66:1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tensen CP, Flier J, Van Der Raaij-Helmer EM, et al. Human IP-9: a keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3). J Invest Dermatol 1999;112:716–722. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Sanchez N, Riol-Blanco L, de la Rosa G, et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood 2004;104:619–625. [DOI] [PubMed] [Google Scholar]
- 31.Escribano C, Delgado-Martin C, Rodriguez-Fernandez JL. CCR7-dependent stimulation of survival in dendritic cells involves inhibition of GSK3beta. J Immunol 2009;183:6282–6295. [DOI] [PubMed] [Google Scholar]
- 32.Santos R, Ursu O, Gaulton A, et al. A comprehensive map of molecular drug targets. Nat Rev DrugDiscov 2017;16:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koelink PJ, Overbeek SA, Braber S, et al. Targeting chemokine receptors in chronic inflammatory diseases: an extensive review. Pharmacol Ther 2012; 133:1–18. [DOI] [PubMed] [Google Scholar]
- 34.White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation—therapeutic opportunities and pharmacological challenges. Pharmacol Rev 2013;65:47–89. [DOI] [PubMed] [Google Scholar]
- 35.Colvin RA, Campanella GS, Sun J, et al. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem 2004; 279:30219–30227. [DOI] [PubMed] [Google Scholar]
- 36.Proudfoot AE. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol 2002;2:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohout TA, Nicholas SL, Perry SJ, et al. Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J Biol Chem 2004;279:23214–23222. [DOI] [PubMed] [Google Scholar]
- 38.Zidar DA, Violin JD, Whalen EJ, et al. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci USA 2009;106:9649–9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith JS, Lefkowitz RJ, Rajagopal S. Biased signalling: from simple switches to allosteric microprocessors. Nat Rev Drug Discov 2018;17:243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urban JD, Clarke WP, von Zastrow M, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 2007;320:1–13. [DOI] [PubMed] [Google Scholar]
- 41.Flier J, Boorsma DM, Bruynzeel DP, et al. The CXCR3 activating chemokines IP-10, Mig, and IP-9 are expressed in allergic but not in irritant patch test reactions. J Invest Dermatol 1999;113:574–578. [DOI] [PubMed] [Google Scholar]
- 42.Ku HO, Jeong SH, Kang HG, et al. Gene expression profiles and pathways in skin inflammation induced by three different sensitizers and an irritant. Toxicol Lett 2009;190:231–237. [DOI] [PubMed] [Google Scholar]
- 43.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci U S A 1992;89:1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carbone T, Nasorri F, Pennino D, et al. CD56highCD16−CD62L− NK cells accumulate in allergic contact dermatitis and contribute to the expression of allergic responses. J Immunol 2010;184:1102–1110. [DOI] [PubMed] [Google Scholar]
- 45.Kish DD, Gorbachev AV, Parameswaran N, et al. Neutrophil expression of Fas ligand and perforin directs effector CD8 T cell infiltration into antigen-challenged skin. J Immunol 2012;189:2191–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sebastiani S, Albanesi C, De PO, et al. The role of chemokines in allergic contact dermatitis. Arch Dermatol Res 2002;293:552–559. [DOI] [PubMed] [Google Scholar]
- 47.Sebastiani S, Albanesi C, Nasorri F, et al. Nickel-specific CD4(+) and CD8(+) T cells display distinct migratory responses to chemokines produced during allergic contact dermatitis. J Invest Dermatol 2002;118:1052–1058. [DOI] [PubMed] [Google Scholar]
- 48.Lehto M, Koivuluhta M, Wang G, et al. Epicutaneous natural rubber latex sensitization induces T helper 2-type dermatitis and strong prohevein-specific IgE response. J Invest Dermatol 2003;120:633–640. [DOI] [PubMed] [Google Scholar]
- 49.Meller S, Lauerma AI, Kopp FM, et al. Chemokine responses distinguish chemical-induced allergic from irritant skin inflammation: memory T cells make the difference. J Allergy Clin Immunol 2007;119:1470–1480. [DOI] [PubMed] [Google Scholar]
- 50.Martin AP, Gagliardi J, Baena-Cagnani CE, et al. Expression of CS-1 fibronectin precedes monocyte chemoattractant protein-1 production during elicitation of allergic contact dermatitis. Clin Exp Allergy 2003;33:1118–1124. [DOI] [PubMed] [Google Scholar]
- 51.Wang HW, Tedla N, Lloyd AR, et al. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J Clin Invest 1998;102:1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ying S, Meng Q, Barata LT, et al. Macrophage inflammatory protein-1alpha and C-C chemokine receptor-1 in allergen-induced skin late-phase reactions: relationship to macrophages, neutrophils, basophils, eosinophils and T lymphocytes. Clin Exp Allergy 2001;31:1724–1731. [DOI] [PubMed] [Google Scholar]
- 53.Baumer W, Seegers U, Braun M, et al. TARC and RANTES, but not CTACK, are induced in two models of allergic contact dermatitis. Effects of cilomilast and diflorasone diacetate on T-cell-attracting chemokines. Br J Dermatol 2004;151:823–830. [DOI] [PubMed] [Google Scholar]
- 54.Canavese M, Altruda F, Silengo L. Therapeutic efficacy and immunological response of CCL5 antagonists in models of contact skin reaction. PLoS One 2010;5:e8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kusumoto M, Xu B, Shi M, et al. Expression of chemokine receptor CCR4 and its ligands (CCL17 and CCL22) in murine contact hypersensitivity. J Interferon Cytokine Res 2007;27:901–910. [DOI] [PubMed] [Google Scholar]
- 56.Kamsteeg M, Jansen PA, van Vlijmen-Willems IM, et al. Molecular diagnostics of psoriasis, atopic dermatitis, allergic contact dermatitis and irritant contact dermatitis. Br J Dermatol 2010;162:568–578. [DOI] [PubMed] [Google Scholar]
- 57.Tsunemi Y, Saeki H, Nakamura K, et al. CCL17 transgenic mice show an enhanced TH2-type response to both allergic and non-allergic stimuli. Eur J Immunol 2006;36:2116–2127. [DOI] [PubMed] [Google Scholar]
- 58.Tokuriki A, Seo N, Ito T, et al. Dominant expression of CXCR3 is associated with induced expression of IP-10 at hapten-challenged sites of murine contact hypersensitivity: a possible role for interferon-gamma-producing CD8(+) T cells in IP-10 expression. J Dermatol Sci 2002;28:234–241. [DOI] [PubMed] [Google Scholar]
- 59.Smith JS, Rajagopal S. The β-arrestins: multifunctional regulators of G protein–coupled receptors. J Biol Chem 2016;291:8969–8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajagopal S, Bassoni DL, Campbell JJ, et al. Biased agonism as a mechanism for differential signaling by chemokine receptors. J Biol Chem 2013;288: 35039–35048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith JS, Alagesan P, Desai NK, et al. C-X-C motif chemokine receptor 3 splice variants differentially activate beta-arrestins to regulate downstream signaling pathways. Mol Pharmacol 2017;92(2):136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berchiche YA, Sakmar TP. CXC chemokine receptor 3 alternative splice variants selectively activate different signaling pathways. Mol Pharmacol 2016; 90:483–495. [DOI] [PubMed] [Google Scholar]
- 63.Zohar Y, Wildbaum G, Novak R, et al. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Invest 2014;124:2009–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karin N, Wildbaum G, Thelen M. Biased signaling pathways via CXCR3 control the development and function of CD4+ T cell subsets. J Leukoc Biol 2016;99:857–862. [DOI] [PubMed] [Google Scholar]
- 65.Suga H, Sugaya M, Miyagaki T, et al. CXCR3 deficiency prolongs TH1-type contact hypersensitivity. J Immunol 2013;190:6059–6070. [DOI] [PubMed] [Google Scholar]
- 66.Campbell JJ, Haraldsen G, Pan J, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999;400:776–780. [DOI] [PubMed] [Google Scholar]
- 67.Riis JL, Johansen C, Vestergaard C, et al. Kinetics and differential expression of the skin-related chemokines CCL27 and CCL17 in psoriasis, atopic dermatitis and allergic contact dermatitis. Exp Dermatol 2011;20:789–794. [DOI] [PubMed] [Google Scholar]
- 68.Imai T, Nagira M, Takagi S, et al. Selective recruitment of CCR4-bearing TH2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol 1999;11:81–88. [DOI] [PubMed] [Google Scholar]
- 69.Ju SM, Song HY, Lee SJ, et al. Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 1,2,3,4,6-penta-O-galloyl-beta-D-glucose via blockade of NF-kappaB and STAT1 activation in the HaCaT cells. Biochem Biophys Res Commun 2009;387:115–120. [DOI] [PubMed] [Google Scholar]
- 70.Stutte S, Quast T, Gerbitzki N, et al. Requirement of CCL17 for CCR7- and CXCR4-dependent migration of cutaneous dendritic cells. Proc Natl Acad SciU SA 2010;107:8736–8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kakinuma T, Nakamura K, Wakugawa M, et al. Thymus and activation-regulated chemokine in atopic dermatitis: serum thymus and activation-regulated chemokine level is closely related with disease activity. JAllergy Clin Immunol 2001;107:535–541. [DOI] [PubMed] [Google Scholar]
- 72.Lehtimaki S, Tillander S, Puustinen A, et al. Absence of CCR4 exacerbates skin inflammation in an oxazolone-induced contact hypersensitivity model. J Invest Dermatol 2010;130:2743–2751. [DOI] [PubMed] [Google Scholar]
- 73.Werfel T, Hentschel M, Kapp A, et al. Dichotomy of blood- and skin-derived IL-4–producing allergen-specific T cells and restricted V beta repertoire in nickel-mediated contact dermatitis. J Immunol 1997;158:2500–2505. [PubMed] [Google Scholar]
- 74.Moed H, Boorsma DM, Stoof TJ, et al. Nickel-responding T cells are CD4+ CLA+ CD45RO+ and express chemokine receptors CXCR3, CCR4 and CCR10. Br J Dermatol 2004;151:32–41. [DOI] [PubMed] [Google Scholar]
- 75.Ouwehand K, Scheper RJ, de Gruijl TD, et al. Epidermis-to-dermis migration of immature Langerhans cells upon topical irritant exposure is dependent on CCL2 and CCL5. Eur JImmunol 2010;40:2026–2034. [DOI] [PubMed] [Google Scholar]
- 76.Huttenrauch F, Nitzki A, Lin FT, et al. Beta-arrestin binding to CC chemokine receptor 5 requires multiple C-terminal receptor phosphorylation sites and involves a conserved Asp-Arg-Tyr sequence motif. J Biol Chem 2002; 277:30769–30777. [DOI] [PubMed] [Google Scholar]
- 77.Corbisier J, Gales C, Huszagh A, et al. Biased signaling at chemokine receptors. J Biol Chem 2015;290:9542–9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogilvie P, Bardi G, Clark-Lewis I, et al. Eotaxin is a natural antagonist for CCR2 and an agonist for CCR5. Blood 2001;97:1920–1924. [DOI] [PubMed] [Google Scholar]
- 79.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science 1997;277:2005–2007. [DOI] [PubMed] [Google Scholar]
- 80.Schmid CL, Kennedy NM, Ross NC, et al. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 2017;171:1165. e13–1175.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soergel DG, Subach RA, Burnham N, et al. Biased agonism of the μ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 2014;155:1829–1835. [DOI] [PubMed] [Google Scholar]