Abstract
Background
Restless legs syndrome (RLS) is a common sleep disorder,. although controversial, growing evidence relates the presence of RLS to an increased risk of mortality, mainly due to cardiovascular events. The aim of this article was to review the role of RLS as a risk factor of mortality according to independent cohort studies.
Methods
We performed a literature review via PubMed database for articles relating RLS and mortality. We used the random-effects model to calculate the pooled effect estimates on mortality. Heterogeneity between studies was assessed using quantitative and qualitative analysis.
Results
Out of 100 articles identified, 13 were finally included. Although studies were heterogeneous (p = 0.001), no significant publication bias was found. When all cohort studies were considered, the random-effects model yielded a significantly increased risk of mortality in RLS versus non-RLS patients (13 studies, hazard ratio [HR] = 1.52, 95% confidence interval [CI] 1.28–1.80). However, this association was not statistically significant when only cohort studies using the international RLS diagnostic criteria were considered (5 studies, HR = 1.63, 95% CI 0.94–2.81).
Discussion
The results of this meta-analysis suggest that RLS seems to be a risk factor of mortality, although this association is conditioned by the diagnostic criteria used in the studies. Future long-term follow-up standardized mortality studies are needed to address this important question that carries potential impact on population global health.
Keywords: Restless legs syndrome, mortality, survival, epidemiology, cohorts, sleep disorder
Introduction
Restless legs syndrome (RLS) is a common sleep disorder, with an estimated prevalence of 5 to 10% of the adult population.1–3 RLS is defined as an urge to move the legs when resting or lying down, especially at night, that is relieved by activity.1 The pathophysiologic basis of RLS is poorly understood. Previous studies have identified a variety of both central and peripheral nervous system abnormalities in patients with RLS. Regarding central nervous system factors, the most consistent risk factor linked to RLS is reduced central iron stores, even in the setting of normal systemic iron studies, suggesting a perturbation in homeostatic mechanisms that regulate the iron influx and/or efflux from the cell.4 Likewise, alterations in dopaminergic systems, likely attributed to an increased dopamine turnover in RLS;5 and circadian disruption with increased circadian dopaminergic variation,6, 7 are also plausible mechanisms. Regarding peripheral nervous system, hyperalgesia characterized by elevated pinprick pain rating scores in the legs, has been associated with central sensitization to A-delta fiber high-threshold mechanoreceptor input, a characteristic sign of the hyperalgesic neuropathic pain present in RLS.4
The most common comorbid conditions associated with RLS include systemic iron deficiency (low serum ferritin levels <50 mcg/L and subsequent low central nervous system intracellular iron),8 renal failure with uremia, frequently associated with periodic limb movements of sleep indices,9 neuropathy,10 spinal cord pathology,11 pregnancy,12 multiple sclerosis,13 Parkinson´s disease and essential tremor.14,15 RLS is a treatable condition, that generally responds well to pharmacologic treatment including iron therapy and non-ergot dopamine agonists or alpha-2-delta calcium channel ligands, as the first line therapy, concurrent with avoidance of mental alerting activities, and stimulant substances like caffeine.
A growing body of evidence relates RLS to an increased risk of mortality attributable to both cerebrovascular and cardiovascular events,16,17 likely mediated by sympathetic hyperactivity,18 or linked to other chronic conditions (e.g., obesity, depression, and disability).19–22 However, this relationship has not been confirmed by others.23–25 Assessment and identification of RLS as a modifiable mortality risk factor carries important public health implications and suggests the need for RLS screening and monitoring. The aim of this study was to quantitatively review the association between RLS and global mortality according to the results of eligible independent cohort studies.
Methods
Search strategy and eligibility criteria
Search strategy and meta-analysis followed the PRISMA guidelines.26 We searched the PubMed database using the search terms “mortality,” “survival” and “RLS,” and “Willis–Ekbom disease.” The search was restricted to English and Spanish language articles involving human subjects, reviews, and meta-analysis, and prospective studies, in which these terms appeared in the title or abstract, and were published between January 1, 1950 and January 15, 2019. We also reviewed cross-references of selected publications for added perspective. In the meta-analysis, we only included prospective observational studies reporting regression analysis and hazard ratios (HR) for mortality with 95% confidence interval (CI). We excluded all case reports and observational studies not providing the aforementioned HR.
Data extraction
All relevant articles were independently evaluated by all of the authors, and disagreements were resolved by consensus. Data extracted from each study included first author, year of publication, population, number of participants, type of study design, criteria for RLS diagnosis, HR (95% CI) for mortality, possible confounding variables, advantages, and disadvantages.
Statistical analysis
HR for mortality of each selected study was recorded and presented in a forest plot graph. A pooled HR with a 95% CI was calculated based on data extracted from each study. A random-effects DerSimonian Laird model and the Galbraith plot were used to calculate the pooled effect estimates.27 We assessed heterogeneity between studies using the Cochran’s Q and I2 statistics, and statistical significance was considered with a p-value <0.001. For the qualitative interpretation of heterogeneity between studies, the funnel plot inspection28 and the Begg’s and Egger’s statistical tests29 were performed. Epidat version 3.1 software (Servicio Gallego de Salud, Spain) was used for statistical analysis.
Results
Study selection and description
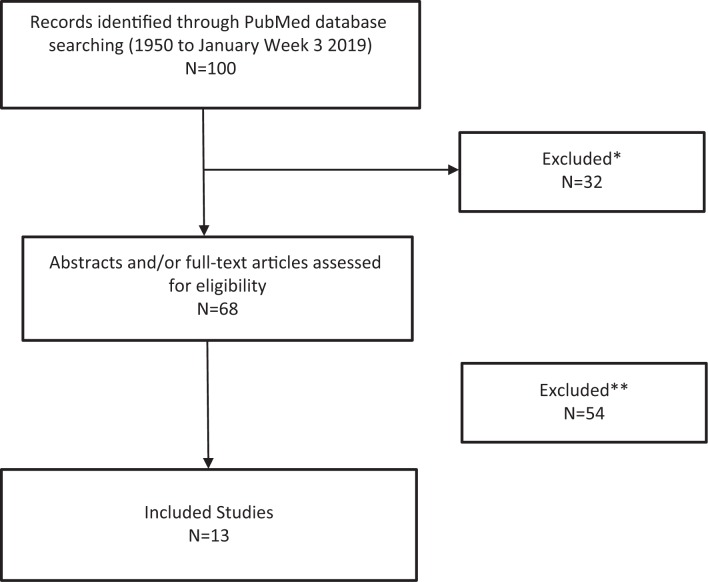
An initial screening of the papers yielded 68 articles eligible for analysis. After excluding non-longitudinal studies and those not matching the inclusion criteria, a total of 13 studies were finally selected and included in the meta-analysis (Figure 1). Table 1 shows the main features of the studies including the sample size; population type including general community versus end-stage renal disease, gender; RLS assessment criteria; HR (95% CI) for mortality as the primary outcome measure; covariates included in each multivariate Cox regression analysis; and the strengths and weaknesses of each study. Figure 2 shows the Forest Plot graph of eligible studies. The diagnosis of RLS was established using the International Restless Legs Syndrome Study Group (IRLSSG) diagnostic criteria30 in five studies (35.7%).31–35 In nine studies (64.3%), the diagnosis of RLS was based on self-administered questionnaires,4,36–38 ICD-9 codes,5,39 and other sleep quesionnaires.40,41 The results of 11 studies (78.6%)4,5,31–34,36,37,40,41 supported RLS as a risk factor for increased mortality. The opposite conclusion was found in only three studies (21.4%).35,38,39
Figure 1.
Prisma Flow Diagram for Identification of Relevant Studies. Reasons for exclusion: *=non-longitudinal studies; **=abstracts, non-matched inclusion criteria studies, language (non-English or Spanish).
Table 1.
Quality Assessment of Included Studies for Mortality in RLS
| Study | Population (n) | Follow-up (years) | RLS assessment | Outcome hazard ratio (95% CI) | Confounding factors adjustment | Disadvantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| Pollak, et al.38 | Elderly community-based population (1,885) | 3.5 | Self-administered sleep questionnaire | Females:1.36 (0.94–1.96) Males: 0.92 (0.60–1.42). | Age, Activities of daily living (ADL) problems, self-assessed health, income, cognitive impairment, depression, living alone, insomnia | The first prospective study analyzing survival in RLS urban-based community | Limited RLS diagnostic accuracy |
| Winkelman et al.36 | End-stage renal disease-based population (204) | 2.5 | Self-administered sleep questionnaire | 1.85 (1.12–3.07) | Age, sex, number of years of dialysis | Large sample and novel information (independent association of RLS with discontinuation of dialysis) | Clinic-based sample, limited RLS diagnostic accuracy |
| Unruh et al.37 | Dialysis-based population (894) | 3 | Self-administered sleep questionnaire | 1.39 (1.08–1.79) | Age, race, sex, dialysis mode, insulin-requiring diabetes, comorbidity and Karnofsky indexes, and center | Inclusion of patients with other types of dialysis, ethnic populations, and treatments information | Limited diagnostic accuracy |
| Molnar et al.31 | Kidney-transplantation recipients-based population (804) | 4 | RLS questionnaires | 2.02 (1.03–3.95) | Diabetes, arterial hypertension, transplantation vintage, glomerular filtration rate, serum albumin, hemoglobin, C-reactive protein | Large sample size, novel information (association of RLS with kidney transplant recipients), | Limited information on cardiovascular confounders, selection bias due to high proportion of missing data (25%) |
| Mallon et al.40 | Community-based population (5,102) | 20 | Upsala Sleep Inventory | Females:1.85 (1.20–2.85) Males:0.81 (0.51–1.28) | Age, short nigh sleep time, lifestyle factors (living alone), medical conditions such as smoking, habitual snoring, BMI ≥ 30, hypertension, heart disease, diabetes, asthma, and depression | Community-based population, large sample size, high response rate | Limited diagnostic accuracy, lack of information on treatments |
| La Manna et al.32 | End-stage renal disease patients on dialysis-based population (100) | 1.5 | RLS Study Group diagnostic criteria | 6.29 (1.74–22.79) | Age, gender, BMI, comorbidity index, albumin, residual diuresis | Novel information (need of phenotyping intermittent vs. continuous RLS and inflammatory markers) | Limited follow-up |
| Li et al.33 | Community-based population (18,425) | 8 | RLS diagnostic index | Men:1.92 (1.03–3.56) | Age, ethnicity, body mass index, life style factors (smoking, alcohol, and physical activity), chronic conditions (cancer, hypertension, cardiovascular disease and other comorbidities), sleep disorders and duration | Large sample size and exclusion of RLS mimics | Selection bias (relatively healthy Caucasian male population with access to health care) |
| Lin et al.34 | End-stage renal disease patients-based population (1,093) | 3 | RLS diagnostic index | 1.53 (95% CI 1.07–2.18) | Age, sex, duration of dialysis, comorbidity of diabetes mellitus, comorbidity of hypertension, serum hemoglobin level, transferrin saturation, serum iron level, and the numbers of cardio-/cerebrovascular events | Large survey of RLS in dialysis patients, inclusion of clinical laboratory data, and medical record review | |
| Stefanidis et al.35 | End-stage renal disease-based population (579) | 3 | RLS Study Group diagnostic criteria | 0.66 (0.41–1.06) | Age, sex, diabetic nephropathy, duration of dialysis, dialysis mode, body mass index, serum urea before hemodialysis (HD), urea reduction ratio, Kt/V, β2-microglobulin, C-reactive protein, albumin, hemoglobin, serum iron, ferritin, transferrin, transferrin saturation, calcium, phosphorus, parathyroid hormone | Large sample size, RLS diagnostic accuracy | Lack of information on mortality ascertainment and kidney transplantation during follow-up |
| Molnar et al.5 | Community-based population (3,000,000) | 8 | ICD-9 code | 1.88 (1.70–2.08) | Age, gender, race/ethnicity, income, marital status, baseline estimated glomerular filtration rate, comorbidities at baseline (diabetes, hypertension, cardiovascular disease, heart failure, cerebrovascular disease, peripheral vascular disease, lung disease, dementia, rheumatic disease, malignancy, HIV/AIDS, depression, presence of obstructive sleep apnea and presence of periodic limb movements in sleep, and BMI | Large sample size, and event numbers | Limited RLS diagnostic accuracy Use of propensity score method |
| Ricardo et al.41 | Non-institutionalized community-based population (1,470) | 3 | Sleep Hear Health Study Habits Questionnaire and Functional Outcomes of Sleep Questionnaire | 1.69 (1.04–2.75) | Diabetes, hypertension, tobacco, hypnotic consumption, congestive heart failure, depression, BMI, age, sex, race, ethnicity, income, glomerular filtration, and albuminuria | Large sample size and follow-up | Limited diagnostic accuracy, exclusion of subjects with missing creatinine/urine albumin data |
| DeFerio et al.39 | End-stage renal disease patients on dialysis-based population (1,456,114) | 2 | ICD-9 code 333.94 | 1.16 (0.88–1.44) | Age, sex, race, ethnicity, vascular access type, BMI, serum albumin level, coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, hypertension, diabetes, cancer, major depressive disorder, dysthymic disorder, anxiety, and tobacco dependence | Large sample size | Limited diagnostic accuracy and number of deaths during follow-up |
| Li et al.4 | Community-based population (57,417) | 10 | Single RLS item question | Women: Total mortality 1.15 (0.98–1.34) Cardiovascular mortality 1.43 (1.02–2.00) | Age, race, smoking status, BMI, physical activity, alcohol consumption, sleep duration, and snoring frequency; history of major chronic diseases (arthritis, diabetes mellitus, hypertension, hypercholesterolemia, Parkinson’s disease, and use of vitamin supplements, aspirin, antidepressant drugs, and antihypertensives) | Large sample size and follow-up | Selection bias (exclusion of not ever diagnosed with RLS by physicians, recall bias), and lack of renal and neuropathy information |
Abbreviations: ADL; BMI, Body Mass Index; CI, Confidence Interval; HD.
Figure 2.
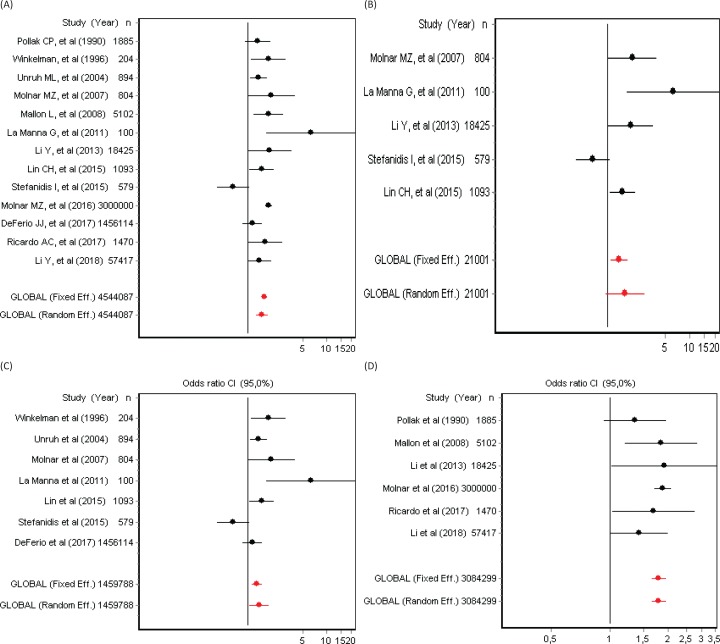
(A) Forest Plots on the Adjusted Hazard Ratio (95% Confidence Intervals) for Mortality in Patients Diagnosed with Restless Legs Syndrome in 13 RLS Cohort Studies. (B) Forest plots on the adjusted hazard ratio (95% confidence intervals) in five RLS cohort studies using the IRLSSG diagnostic criteria. (C) Forest plots on the adjusted hazard ratio (95% confidence intervals) in seven RLS end-stage renal disease cohort studies. (D) Forest plots on the adjusted hazard ratio (95% confidence intervals) in six RLS general community cohort studies.
Meta-analysis
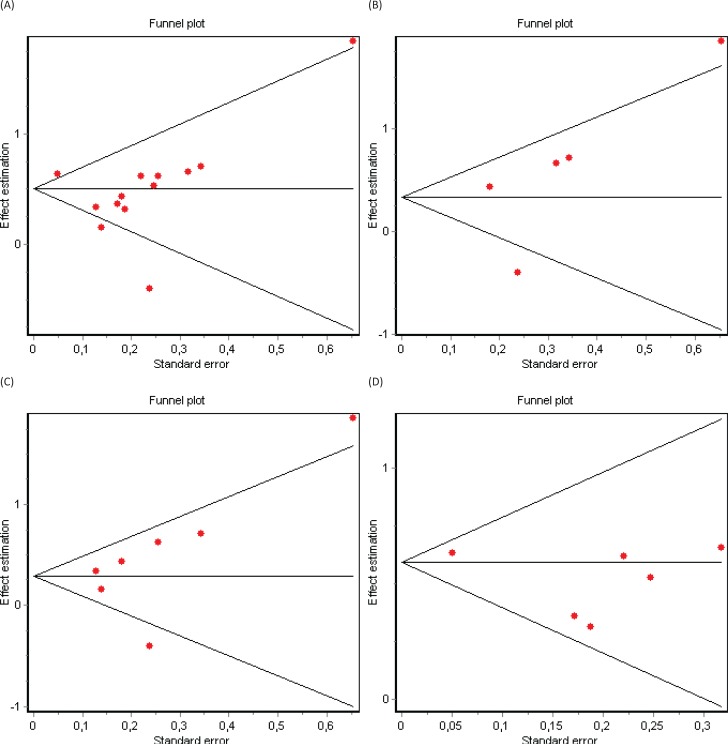
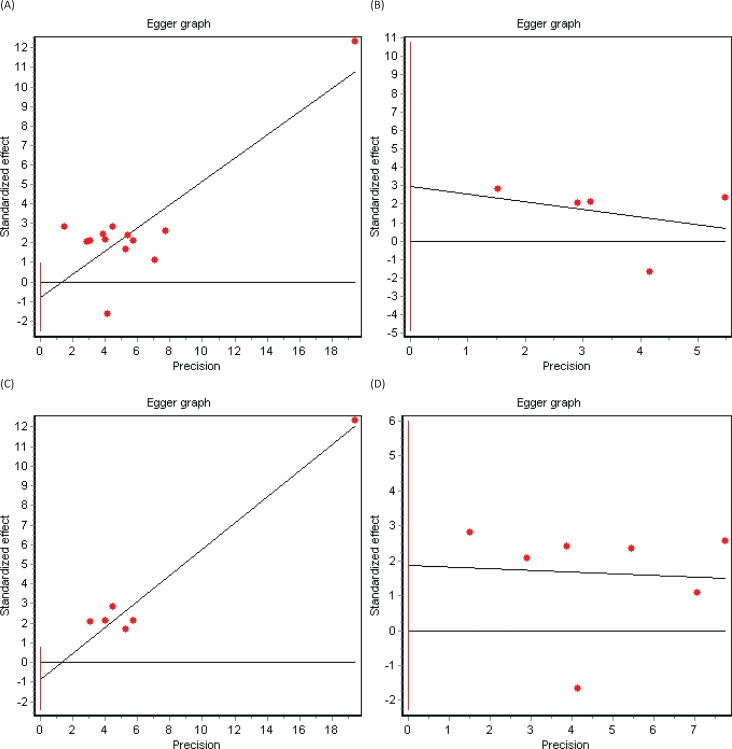
Significant heterogeneity between studies was present and confirmed with the Cochran’s Q test (p = 0.0003). According to the random-effects model including the 13 selected cohort studies, the pooled HR for mortality in patients with RLS compared to non-RLS was HR = 1.52 (95% CI 1.28–1.80) (Figure 2A). Three subgroup analyses were subsequently conducted. The first subgroup analysis included only studies using the IRLSSG diagnostic criteria (n = 5).30 Among this subset of studies, heterogeneity was present (Cochran’s Q test, p = 0.001), and the pooled HR of mortality favored the association between RLS and mortality, however not reaching statistical significance (HR = 1.63, 95% CI 0.94–2.81) (Figure 2B). The second subgroup analysis included end-stage renal disease-based studies (n = 7). Heterogeneity was also present (Cochran’s Q test, p = 0.004), and the pooled HR confirmed a statistically significant association between RLS and mortality: HR = 1.41, 95% CI 1.05–1.87 (Figure 2C). The third subgroup analysis included community-based studies. However, in this case, heterogeneity was not present (Cochran’s Q test, p = 0.45), and the pooled HR confirmed the association between RLS and mortality: HR = 1.80, 95% CI 1.65–1.97 (Figure 2D). Visual inspection of the funnel plots (Figures 3A, 3B, 3C, and 3D) and Egger graphs (Figures 4A, 4B, 4C, and 4D) showed no evidence of significant publication bias regarding both the total group and subgroup analysis (Begg’s test, p = 0.07, 0.08, 0.13, 0.10; Egger’s test, p = 0.35, 0.32, 0.30, 0.21, respectively).
Figure 3.
(A) Funnel Plot in 13 RLS Cohort Studies. (B) Funnel plot in five RLS cohort studies using the IRLSSG diagnostic criteria. (C) Funnel plot in seven RLS end-stage renal disease cohort studies. (D) Funnel plot in six RLS general community cohort studies.
Figure 4.
(A) Egger Graph in 13 RLS Cohort Studies. (B) Egger graph in five RLS cohort studies using the IRLSSG diagnostic criteria. (C) Egger graph in seven RLS end-stage renal disease cohort studies. (D) Egger graph in six RLS general community cohort studies.
Discussion
The real factors that determine survival in RLS are not well established, and confounding factors might influence the true relationship between RLS and mortality. Previous cohort studies are conditioned by several factors, such as a limited number of patients, the characteristics of the study population (either from the general population or belonging to end-stage renal subsets of patients), and the relatively short follow-up duration, resulting in rather few observed deaths. In this meta-analysis including 13 cohort studies, using different RLS diagnostic criteria, we observed a higher risk of mortality among patients presenting RLS, likely associated with cardiovascular diseases and especially in women, compared to non-RLS population. In contrast, mortality was not significantly different when the meta-analysis was restricted to RLS studies that used the IRLSSG diagnostic criteria.30 A thorough review of the literature provides relevant information about the factors affecting this relationship.
Methodological issues
Although the results of the cohort studies included in this review were controlled for several known risk factors, these observational studies were not primarily designed to confirm causality. To assess the presence of RLS, earlier studies used mailed self-reporting questionnaires, a well-known and relatively inexpensive diagnostic screening method that is limited by diagnostic accuracy.7 In fact, one study showed that the four main criteria for the diagnosis of RLS applied to the general population, and not undergoing adequate differential diagnosis, wrongly classify 16% of healthy participants as having RLS.42 Similarly, the diagnostic accuracy of ICD-9 RLS codes is yet unknown.
Interview-based diagnosis using accepted international RLS diagnostic criteria30,43 is key to provide standardized RLS assessment across populations. Some years ago, the members of the Movement Disorder Society Committee on Rating Scales published a review article about the diagnostic instruments for RLS and provided recommendations based on the available evidence at that time.44 In this regard, an RLS survival study4 used a single question, included in the original diagnostic criteria for RLS, that was primarily developed for rapid screening of patients in a clinical setting.44,45 Based on its excellent sensitivity (100%) and specificity (96.8%),45 this single question may be considered a preliminary reliable screener for the diagnosis of RLS, needing subsequent confirmation by routine clinical interview.44 To improve the diagnostic accuracy, other studies33,34 have included the RLS diagnostic index with 10 items, which was developed to test the possibility that ancillary non-essential features may help to perform a more accurate diagnosis within the clinical setting, with a sensitivity of 93% and a specificity of 98.9%.46 Other diagnostic tests, recommended for clinical use (Cambridge Hopkins diagnostic questionnaire),30 and also for the general population (Hening Telephone Diagnostic Interview),47 exhibit excellent diagnostic attributes, although they have not been used in RLS survival studies.
Selection bias, like increased mortality found only in women, may be attributed to the fact that overall mortality in men generally exceeds that of women, and any factor influencing mortality in men needs to be more pronounced.40 On the contrary, as RLS is a commonly underdiagnosed condition,48 there is a tendency to include more severely affected patients, which might overestimate the true effect of RLS on global mortality. Moreover, the exact cause of death and the follow-up duration are not always clearly reported.
Regarding statistical methodology, the results of a multivariate Cox regression and propensity score method analysis can only account for the effects of known confounders. Therefore, there is a need for well-designed mortality studies analyzing age–sex standardized mortality ratios (SMR),49 in which observed deaths within RLS cohorts compared to expected deaths in the general population are reported.
End-stage renal disease versus community-based RLS populations
Due to the heterogeneity present among studies, we conducted several subgroup analyses. Among end-stage renal and community-based populations, RLS was associated with a significantly increased mortality. According to some studies, the association between RLS and mortality seems to be influenced by advanced age, the presence of cardiovascular risk factors, end-stage renal disease, and other sleep-related conditions. However, the results provided by studies based on end-stage renal disease subjects may not be comparable to other relatively healthier populations. In community-based mortality studies, the mechanisms affecting survival in the RLS subpopulation are not well understood. Maintenance of the normal rest/activity circadian rhythm appears to be of particular importance for the preservation of general health.50 Accordingly, an increased risk of total mortality and coronary artery disease mortality has been found in RLS men presenting insomnia.38,40 Possible mechanisms linking insomnia and impaired global health include activation of inflammatory cytokines such as serum C-reactive,51 and changes of circulating levels of leptin and ghrelin proteins, which promote the development of obesity, diabetes, and cardiovascular disease.52,53 RLS patients exhibit dopamine disturbances that correlate with decreased function of the A11 diencephalospinal pathway, resulting in disinhibition of somatosensory and sympathetic pathways of the spinal cord. This may lead to an increased sympathetic activation, causing hypertension, cardiovascular disease, and stroke.6,51 Other studies relate the presence of periodic limb movements of sleep in RLS with nocturnal hypertension without a dipping pattern, which strongly correlates with left ventricular hypertrophy.54,55
Strengths and limitations
In this review, we followed the PRISMA guidelines,26 applied to the search strategy, and used a well-validated meta-analytic methodology. Although cohort studies showed differences in their sampling procedures, sample characteristics, and adjustment for confounding factors, no significant publication bias was found, allowing comparability between studies. However, several limitations have to be considered when interpreting our results, including the lack of well-defined diagnostic criteria of RLS in some studies, the inclusion of mixed primary and secondary RLS studies, the possibility of having included patients with conditions mimicking RLS like diabetic neuropathy, and the small number of studies included in the subgroup analyses, which limit the extrapolation of our findings.
Conclusions
RLS is currently an under-recognized condition, especially by primary care physicians. The association between RLS and increased mortality has potential impact on population global health. Currently, there is a need for well-designed mortality studies addressing the SMR and the influence of any RLS-specific therapy on mortality and comorbidity. The results of this review suggest an association between RLS and increased mortality, although the existence of this association depends on the diagnostic criteria of the studies included in the analysis. Increasing awareness of RLS and future long-term follow-up standardized mortality studies is needed to confirm these findings.
Footnotes
Citation: Cubo E, Gallego-Nieto C, Elizari-Roncal M, Barroso-Pérez T, Collazo C, Calvo S, et al. Is restless legs syndrome associated with an increased risk of mortality? A meta-analysis of cohort studies. Tremor Other Hyperkinet Mov. 2019; 9. doi: 10.7916/tohm.v0.650
Editor: Elan D. Louis, Yale University, USA
Funding: None.
Financial Disclosures: This work was supported by a grant from the Castilla y Leon Health Department (GRS 1764/A/18).
Conflicts of Interest: The authors report no conflicts of interest.
Ethics Statement: Not applicable for this category of article.
References
- 1.Ekbom K, Ulfberg J. Restless legs syndrome. J Intern Med. 2009;266:419–431. doi: 10.1111/j.1365-2796.2009.02159.x [DOI] [PubMed] [Google Scholar]
- 2.Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623–634. doi: 10.1016/j.sleep.2010.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath. 2012;16:987–1007. doi: 10.1007/s11325-011-0606-x [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Li Y, Winkelman JW, Han J, Hu FB, Gao X. Prospective study of restless legs syndrome and total and cardiovascular mortality among women. Neurology. 2018;90:e135–e141. doi: 10.1212/WNL.000000000000004814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molnar MZ, Lu JL, Kalantar-Zadeh K, Kovesdy CP. Association of incident restless legs syndrome with outcomes in a large cohort of US veterans. J Sleep Res. 2016;25:47–56. doi: 10.1111/jsr.12335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–597. doi: 10.1093/sleep/32.5.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trenkwalder C, Allen R, Hogl B, Paulus W, Winkelmann J. Restless legs syndrome associated with major diseases: a systematic review and new concept. Neurology. 2016;86:1336–1343. doi: 10.1212/WNL.0000000000002542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao X, Schwarzschild MA, Wang H, Ascherio A. Obesity and restless legs syndrome in men and women. Neurology. 2009;72:1255–1261. doi: 10.1212/01.wnl.0000345673.35676.1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Mirzaei F, O'Reilly EJ, Winkelman J, Malhotra A, Okereke OI, et al. Prospective study of restless legs syndrome and risk of depression in women. Am J Epidemiol. 2012;176:279–288. doi: 10.1093/aje/kws016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Li Y, Malhotra A, Ning Y, Gao X. Restless legs syndrome status as a predictor for lower physical function. Neurology. 2014;82:1212–1218. doi: 10.1212/WNL.0000000000000284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szentkiralyi A, Winter AC, Schurks M, Völzke H, Hoffmann W, E Buring J, et al. Restless legs syndrome and all-cause mortality in four prospective cohort studies. BMJ Open. 2012;2. doi: 10.1136/bmjopen-2012-001652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakkas GK, Giannaki CD, Karatzaferi C, Maridaki M, Koutedakis Y, Hadjigeorgiou GM, et al. Current trends in the management of uremic restless legs syndrome: a systematic review on aspects related to quality of life, cardiovascular mortality and survival. Sleep Med Rev. 2015;21:39–49. doi: 10.1016/j.smrv.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 13.Van Den Eeden SK, Albers KB, Davidson JE, Kushida CA, Leimpeter AD, Nelson LM, et al. Risk of cardiovascular disease associated with a restless legs syndrome diagnosis in a retrospective cohort study from Kaiser Permanente Northern California. Sleep. 2015;38:1009–1015. doi: 10.5665/sleep.4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ondo WG. Clinical features and diagnosis of restless legs syndrome and periodic limb movement disorder in adults. UpToDate; 2019. [accessed January, 18th, 2019]. [Google Scholar]
- 15.Earley CJ, Kuwabara H, Wong DF, et al. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep. 2013;36:51–57. doi: 10.5665/sleep.2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Borreguero D, Larrosa O, Granizo JJ, de la Llave Y, Hening WA. Circadian variation in neuroendocrine response to L-dopa in patients with restless legs syndrome. Sleep. 2004;27:669–673. doi: 10.1016/j.sleep.2004.01.007 [DOI] [PubMed] [Google Scholar]
- 17.Earley CJ, Hyland K, Allen RP. Circadian changes in CSF dopaminergic measures in restless legs syndrome. Sleep Med. 2006;7:263–268. doi: 10.1016/j.sleep.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 18.Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54:1698–1700. doi: 10.1212/WNL.54.8.1698 [DOI] [PubMed] [Google Scholar]
- 19.Giannaki CD, Hadjigeorgiou GM, Karatzaferi C, Pantzaris MC, Stefanidis I, Sakkas GK. Epidemiology, impact, and treatment options of restless legs syndrome in end-stage renal disease patients: an evidence-based review. Kidney Int. 2014;85:1275–1282. doi: 10.1038/ki.2013.394 [DOI] [PubMed] [Google Scholar]
- 20.Ondo W, Jankovic J. Restless legs syndrome: clinicoetiologic correlates. Neurology. 1996;47:1435–1441. doi: 10.1212/WNL.47.6.1435 [DOI] [PubMed] [Google Scholar]
- 21.Bara-Jimenez W, Aksu M, Graham B, Sato S, Hallett M. Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology. 2000;54:1609–1616. doi: 10.1212/WNL.54.8.1609 [DOI] [PubMed] [Google Scholar]
- 22.Dunietz GL, Lisabeth LD, Shedden K, Shamim-Uzzaman QA, Bullough AS, Chames MC, et al. Restless legs syndrome and sleep-wake disturbances in pregnancy. J Clin Sleep Med. 2017;13:863–870. doi: 10.5664/jcsm.6654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schurks M, Bussfeld P. Multiple sclerosis and restless legs syndrome: a systematic review and meta-analysis. Eur J Neurol. 2013;20:605–615. doi: 10.1111/j.1468-1331.2012.03873.x [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Esteban JC, Zarranz JJ, Tijero B, Velasco F, Barcena J, Rouco I, et al. Restless legs syndrome in Parkinson's disease. Mov Disord. 2007;22:1912–1916. doi: 10.1002/mds.21624 [DOI] [PubMed] [Google Scholar]
- 25.Ondo WG, Lai D. Association between restless legs syndrome and essential tremor. Mov Disord. 2006;21:515–518. doi: 10.1002/mds.20746 [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: a simulation study. Stat Methods Med Res. 2012;21:409–426. doi: 10.1177/0962280210392008 [DOI] [PubMed] [Google Scholar]
- 28.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/S1389-9457(03)00010-8 [DOI] [PubMed] [Google Scholar]
- 31.Molnar MZ, Szentkiralyi A, Lindner A, Czira ME, Szeifert L, Kovacs AZ, et al. Restless legs syndrome and mortality in kidney transplant recipients. Am J Kidney Dis. 2007;50:813–820. doi: 10.1053/j.ajkd.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 32.La Manna G, Pizza F, Persici E, Baraldi O, Comai G, Cappuccilli ML, et al. Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol Dial Transplant. 2011;26:1976–1983. doi: 10.1093/ndt/gfq681 [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Wang W, Winkelman JW, Malhotra A, Ma J, Gao X. Prospective study of restless legs syndrome and mortality among men. Neurology. 2013;81:52–59. doi: 10.1212/WNL.0b013e318297eee0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CH, Sy HN, Chang HW, Liou HH, Lin CY, Wu VC, et al. Restless legs syndrome is associated with cardio/cerebrovascular events and mortality in end-stage renal disease. Eur J Neurol. 2015;22:142–149. doi: 10.1111/ene.12545 [DOI] [PubMed] [Google Scholar]
- 35.Stefanidis I, Vainas A, Giannaki CD, Dardiotis E, Spanoulis A, Sounidaki M, et al. Restless legs syndrome does not affect 3-year mortality in hemodialysis patients. Sleep Med. 2015;16:1131–1138. doi: 10.1016/j.sleep.2015.04.023 [DOI] [PubMed] [Google Scholar]
- 36.Winkelman JW, Chertow GM, Lazarus JM. Restless legs syndrome in end-stage renal disease. Am J Kidney Dis. 1996;28:372–378. doi: 10.12669/pjms.306.5691 [DOI] [PubMed] [Google Scholar]
- 37.Unruh ML, Levey AS, D'Ambrosio C, Fink NE, Powe NR, Meyer KB, et al. Restless legs symptoms among incident dialysis patients: association with lower quality of life and shorter survival. Am J Kidney Dis. 2004;43:900–909. doi: 10.1053/j.ajkd.2004.01.013 [DOI] [PubMed] [Google Scholar]
- 38.Pollak CP, Perlick D, Linsner JP, Wenston J, Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health. 1990;15:123–135. doi: 10.1007/BF01321316 [DOI] [PubMed] [Google Scholar]
- 39.DeFerio JJ, Govindarajulu U, Brar A, Cukor D, Lee KG, Salifu MO. Association of restless legs syndrome and mortality in end-stage renal disease: an analysis of the United States Renal Data System (USRDS). BMC Nephrol. 2017;18:258. doi: 10.1186/s12882-017-0660-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallon L, Broman JE, Hetta J. Restless legs symptoms with sleepiness in relation to mortality: 20-year follow-up study of a middle-aged Swedish population. Psychiatry Clin Neurosci. 2008;62:457–463. doi: 10.1111/j.1440-1819.2008.01831.x [DOI] [PubMed] [Google Scholar]
- 41.Ricardo AC, Goh V, Chen J, Cedillo-Couvert E, Kapella M, Prasad B, et al. Association of sleep duration, symptoms, and disorders with mortality in adults with chronic kidney disease. Kidney Int Rep. 2017;2:866–873. doi: 10.1016/j.ekir.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hening WA, Allen RP, Washburn M, Lesage SR, Earley CJ. The four diagnostic criteria for Restless Legs Syndrome are unable to exclude confounding conditions ("mimics"). Sleep Med. 2009;10:976–981. doi: 10.1016/j.sleep.2008.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014;15:860–873. doi: 10.1016/j.sleep.2014.03.025 [DOI] [PubMed] [Google Scholar]
- 44.Walters AS, Frauscher B, Allen R, Benes H4, Chaudhuri KR5, Garcia-Borreguero D, et al. Review of diagnostic instruments for the restless legs syndrome/Willis-Ekbom Disease (RLS/WED): critique and recommendations. J Clin Sleep Med. 2014;10:1343–1349. doi: 10.5664/jcsm.4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferri R, Lanuzza B, Cosentino FI, Iero I, Tripodi M, Spada RS, et al. A single question for the rapid screening of restless legs syndrome in the neurological clinical practice. Eur J Neurol. 2007;14:1016–1021. doi: 10.1111/j.1468-1331.2007.01862.x [DOI] [PubMed] [Google Scholar]
- 46.Benes H, Kohnen R. Validation of an algorithm for the diagnosis of Restless Legs Syndrome: the Restless Legs Syndrome-Diagnostic Index (RLS-DI). Sleep Med. 2009;10:515–523. doi: 10.1016/j.sleep.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 47.Hening WA, Allen RP, Thanner S, Washburn T, Heckler D, Walters AS, et al. The Johns Hopkins telephone diagnostic interview for the restless legs syndrome: preliminary investigation for validation in a multi-center patient and control population. Sleep Med. 2003;4:137–141. doi: 10.1016/S1389-9457(03)00006-6 [DOI] [PubMed] [Google Scholar]
- 48.Gupta R, Lahan V, Goel D. Restless Legs Syndrome: a common disorder, but rarely diagnosed and barely treated--an Indian experience. Sleep Med. 2012;13:838–841. doi: 10.1016/j.sleep.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 49.Tsai SP, Wen CP. A review of methodological issues of the standardized mortality ratio (SMR) in occupational cohort studies. Int J Epidemiol. 1986;15:8–21. doi: 10.1093/ije/15.1.8 [DOI] [PubMed] [Google Scholar]
- 50.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0 [DOI] [PubMed] [Google Scholar]
- 51.Ferini-Strambi L, Walters AS, Sica D. The relationship among restless legs syndrome (Willis-Ekbom Disease), hypertension, cardiovascular disease, and cerebrovascular disease. J Neurol. 2014;261:1051–1068. doi: 10.1007/s00415-013-7065-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008 [DOI] [PubMed] [Google Scholar]
- 53.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22:575–580. doi: 10.1093/sleep/22.5.575 [DOI] [PubMed] [Google Scholar]
- 55.Mirza M, Shen WK, Sofi A, Jahangir A, Mori N, Tajik AJ, et al. Frequent periodic leg movement during sleep is associated with left ventricular hypertrophy and adverse cardiovascular outcomes. J Am Soc Echocardiogr. 2013;26:783–790. doi: 10.1016/j.echo.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]