Abstract
Recent evidence has advanced our understanding of the function of sleep to include removal of neurotoxic protein aggregates via the glymphatic system. However, most research on the glymphatic system utilizes animal models, and the function of waste clearance processes in humans remains unclear. Understanding glymphatic function offers new insight into the development of neurodegenerative diseases that result from toxic protein inclusions, particularly those characterized by neuropathological sleep dysfunction, like Parkinson’s disease (PD). In PD, we propose that glymphatic flow may be compromised due to the combined neurotoxic effects of alpha-synuclein protein aggregates and deteriorated dopaminergic neurons that are linked to altered REM sleep, circadian rhythms, and clock gene dysfunction. This review highlights the importance of understanding the functional role of glymphatic system disturbance in neurodegenerative disorders and the subsequent clinical and neuropathological effects on disease progression. Future research initiatives utilizing noninvasive brain imaging methods in human subjects with PD are warranted, as in vivo identification of functional biomarkers in glymphatic system functioning may improve clinical diagnosis and treatment of PD.
Keywords: Parkinson’s disease, REM-sleep behavior disorder, glymphatic system, alpha-synuclein, dopamine, clock genes
1. Introduction
Sleep is a prominent feature of human behavior, comprising around one-fourth to one-third of our daily lives. While sleep is lauded as important for overall health, its specific function has generally been unclear. However, the recent discovery of the glymphatic system provides new insight into the role of sleep as a method for macroscopic waste clearance in the central nervous system. The glymphatic system operates naturally during sleep (Jessen et al., 2015) and promotes effective clearance of accumulated waste in the brain through the flow of interstitial and cerebrospinal fluid via perivascular pathways (Iliff et al., 2012). Recent evidence suggests that the glymphatic system also clears neurotoxic protein aggregations (e.g., amyloid-beta) that are implicated in the development of various neurodegenerative disorders (Kress et al., 2014; Tarasoff-Conway et al., 2015; Verheggen et al., 2018).
The discovery of the glymphatic system provides important implications for the consequences of comorbid sleep disturbances that accompany many medical, psychiatric, and neurological conditions. Among these is Parkinson’s disease (PD), the second most common neurodegenerative disorder, affecting approximately 3% of individuals over 75 years of age (de Lau and Breteler, 2006; Tanner and Aston, 2000). Sleep disturbances are not only a common comorbidity in PD, but often precede the onset of classic motor symptoms (Barber and Dashtipour, 2012). REM-sleep behavior disorder (RBD) is especially prevalent in PD and leads to changes in cortical activity during sleep (Fantini et al., 2003), along with reduced sleep quality (Gjerstad et al., 2008). Furthermore, PD patients exhibit altered expression of clock genes, which regulate circadian rhythms (Breen et al., 2014; Cai et al., 2010). The amalgamation of sleep disturbances and circadian rhythm dysfunction in disorders such as PD can consequently disrupt the natural function of the glymphatic system.
Aggregated alpha-synuclein deposits, a protein believed to modulate synaptic neurotransmitter release and autophagic activity, underlie the neuropathology of PD (Bobela et al., 2015; Kalaitzakis et al., 2013; Stefanis, 2012). Similar to other proteins (e.g., amyloid-beta in Alzheimer’s disease [AD]), abnormal levels of alpha-synuclein are common to several neurodegenerative diseases (e.g., Lewy body disease, multiple system atrophy), suggesting that aggregation of this protein is particularly neurotoxic and exacerbates neuronal degeneration (Taylor, 2002). Noxious alpha-synuclein accumulation also damages pathways (Fahn and Sulzer, 2004; Kovacs et al., 2008) associated with both motor (de Lau and Breteler, 2006; Suchowersky et al., 2006) and non-motor (Chung et al., 2017; Narayanan et al., 2013; Rinne et al., 2000) symptoms in PD. It is possible that neuroprotective mechanisms such as the glymphatic system could organically abate the progression of PD pathology through the clearance of alpha-synuclein during sleep.
This review will discuss the clinical implications of the newly discovered glymphatic system and its relation to clock gene expression, alpha-synuclein accrual, and dopaminergic dysfunction in PD. We aim to establish a framework for understanding the mechanisms believed to underpin PD development by highlighting the relationship between neuropathological mechanisms and clinical presentation. We propose that glymphatic system disruption furthers the PD degenerative process via sleep-related dysfunction (e.g., RBD, disruption in clock gene expression). Understanding the role of sleep disturbances in the pathology of PD can provide significant implications for future research and clinical treatments.
2. Clinical and Neuropathological Profiles of Parkinson’s Disease
PD is characterized by a distinct cluster of clinical symptoms, including both motor and non-motor impairments (Jankovic, 2008; Maetzler et al., 2009; Moustafa et al., 2016). Motor deficits primarily include bradykinesia, in combination with resting tremor, muscular rigidity, or postural instability (de Lau and Breteler, 2006; Jankovic, 2008; Siderowf and Stern, 2003), while non-motor symptoms commonly include cognitive dysfunction and sleep disturbances that can be present in all stages of the disease process. In fact, one observational cross-sectional study assessing the prevalence of non-motor symptoms in 545 international patients with PD found that patients on average endorsed nine to twelve nonmotor symptoms, including cognitive dysfunction, depression, anxiety, sleep disorders, autonomic dysfunction, gastrointestinal problems, pain, and olfactory disturbances (Chaudhuri and Schapira, 2009; Martinez-Martin et al., 2007). Many of these non-motor symptoms manifest in early disease stages before the onset of hallmark motor symptoms (Aarsland et al., 2010; Janvin et al., 2003). Indeed, deterioration of the substantia nigra—the neural structure primarily implicated in the development of motor symptoms—does not occur until midway through the disease course (Braak et al., 2003; Hely et al., 2008, 2005).
Though the cardinal motor symptoms in PD are typified by dopaminergic loss within the nigrostriatal pathway, the depletion of dopamine within other pathways contributes to many of the comorbid symptoms that characterize PD (Gratwicke et al., 2015). One of the most common nonmotor symptoms in PD is RBD, a parasomnia characterized by a lack of muscle atonia that results in abnormal body movements during REM-sleep (Aygun et al., 2012; Barber and Dashtipour, 2012). Idiopathic RBD is considered a prodromal predictor of PD and other synucleinopathies (Iranzo et al., 2006), although increasing evidence implicates RBD as a part of the PD disease process (Boeve, 2013; Doppler et al., 2017; Iranzo et al., 2006). In fact, the estimated risk of phenoconversion to a Lewy body disease—including PD—after the diagnosis of idiopathic RBD increases with time, where 12% converted after three years, 20% after five years, 33% after seven years, and over 50% after ten years (Miyamoto and Miyamoto, 2018). Approximately 81% of individuals with idiopathic RBD will develop a synucleinopathic disease (i.e., dementia with Lewy bodies, PD, multiple system atrophy) within roughly 14 years (Schenck et al., 2013). Further, RBD appears to be exacerbated by dopaminergic dysfunction, particularly within the mesocortical and mesolimbic pathways (Fantini and Ferini-Strambi, 2007; Rye, 2004). Given that dopaminergic contributions promote alertness and wake-state activity (Isaac and Berridge, 2003; Murillo-Rodríguez et al., 2009), it is perhaps unsurprising that converging evidence indicates that sleep dysfunction in PD results from the deterioration of dopaminergic neurons within similar pathways as RBD (Dauer and Przedborski, 2003; Dickson et al., 2009; Grinberg et al., 2010; Jellinger, 2003; Kalaitzakis and Pearce, 2009; Thanvi et al., 2003) and implicates co-occurring mechanisms in both syndromes (Kim et al., 2010).
While parasomnias and other sleep disturbances are generally common to neurodegenerative diseases (e.g., AD), RBD in particular appears to occur mainly in synucleinopathies (e.g., Lewy body disease, PD, and multiple system atrophy; Boeve et al., 2003). RBD can occur in tauopathic diseases, such as progressive supranuclear palsy, as well (Gagnon et al., 2006); however, the incidence is much lower among these disorders as compared to synucleinopathies (Compta et al., 2009; Gagnon et al., 2006), suggesting a specific contribution of alpha-synuclein accumulation in RBD. Furthering support for the relationship between alpha-synuclein pathology and RBD comes from a study by Kalaitzakis and colleagues (2013) examining neuroanatomical correlates of disturbed sleep in PD. Utilizing post-mortem brain tissue samples from PD patients, those with RBD or other sleep disturbances demonstrated increased alpha-synuclein burden in several brainstem, hypothalamic, and limbic structures as compared to those without sleep disturbances (Kalaitzakis et al., 2013). Indeed, RBD is associated with deterioration of lower brainstem nuclei, such as the pedunculopontine nucleus (Chaudhuri and Schapira, 2009), which projects to both the ventral tegmental area and the substantia nigra (Boeve et al., 2007; French and Muthusamy, 2018) and subsequently influences dopaminergic projections that extend from both of these regions. Thus, deterioration of the pedunculopontine nucleus as part of the progression of PD would thereby exacerbate sleep dysfunction throughout the disease course.
Increasingly, the progression of alpha-synuclein pathology is also thought to be the primary source of cerebral dysfunction in PD (Ahlskog, 2007; Braak et al., 2003; Ferrer, 2011). Neural regions with high concentrations of dopaminergic projections—including the substantia nigra and, to a lesser extent, the ventral tegmental area—show increased vulnerability to alpha-synuclein accrual, suggesting characteristic dopaminergic pathway dysfunction in PD results from the accumulation of these protein inclusions (Alberico et al., 2015; Fahn and Sulzer, 2004). To this end, a study of 27 post-mortem patients with synucleinopathic diseases found correlations between decreased dopamine levels in the striatum and increased alpha-synuclein burden in the substantia nigra (Kovacs et al.,2008), supporting the notion of alpha-synuclein toxicity to dopaminergic neurons. Interestingly, alpha-synuclein accumulates similarly within the context of healthy aging. Healthy aged individuals demonstrate up to 600% higher alpha-synuclein levels within the substantia nigra than younger adults (Chu and Kordower, 2007) and experience age-related declines of alpha-synuclein in blood plasma levels (Koehler et al., 2015), bringing forth the question of PD as a manifestation of an accelerated aging process (Bobela et al., 2015; Reeve et al., 2014). However, differential processes between healthy aging and PD implicate that the alpha-synuclein accrual and dopaminergic dysfunction are affected above and beyond what is expected of healthy aging. RBD serves as a prime example of this distinctive process, as “idiopathic” RBD is now largely thought to represent a preclinical synucleinopathic disease (Boeve, 2013; Doppler et al., 2017; Iranzo et al., 2006). Despite age-related alpha-synuclein aggregation and subsequent deterioration of the substantia nigra, motor symptoms resulting from dopaminergic loss also appear to be confined to the pathological process of PD, with the onset of motor symptoms correlated with the percentage of dopaminergic loss. While conservative estimates suggest the occurrence of motor symptoms after a 50-70% loss of dopaminergic neurons (Cheng et al., 2010), other recent studies have demonstrated that motor symptoms can become apparent with dopaminergic neuronal loss as low as 29% (Greffard et al., 2006). Motor symptom severity is also associated with worsening sleep symptoms (Maetzler et al., 2009). These findings are especially relevant in the context of dopaminergic dysfunction and alpha-synuclein deposits occurring beyond the nigrostriatal system that defines classical PD motor symptoms and contributing to sleep-wake disturbances (Fantini and Ferini-Strambi, 2007).
Sleep disturbances and poorer sleep quality are also highly correlated with decreased cognitive performance in PD, particularly in predicting declines on tasks of attention and executive functioning (Stavitsky et al., 2012). The overlapping pathology between the development of cognitive impairments and RBD in PD implicates a common mechanism that likely results from advancing disease progression (Muslimović et al., 2009; Owen, 2004; Verbaan et al., 2007; Wakamori et al., 2014), particularly dopaminergic reductions in corticostriatal projections (Albin et al., 1989) and greater Lewv body densities in frontal gyri associated with PD-specific cognitive decline (Mattila et al., 2000). The effects of reduced sleep quality additionally appear to be multiplicative on the neurodegenerative process in PD. For example, one study assessing cognitive functioning of PD patients with and without RBD found that only individuals with concomitant PD and RBD demonstrated significant cognitive impairment, suggesting RBD may be predictive of cognitive decline (Vendette et al., 2007). Beyond PD, sleep disturbances are implicated more broadly in development of other neurodegenerative disorders, such as AD (Bombois et al., 2010; Bonanni et al., 2005; Liguori et al., 2014; Lim et al., 2013; Osorio et al., 2015), highlighting a specific role for sleep in the maintenance of cognitive functioning.
3. Clock Gene Expression in Parkinson’s Disease
Central to understanding the process of sleep and sleep dysfunctions is the circadian expression of sleep-wake states. The suprachiasmatic nucleus of the anterior hypothalamus regulates rhythmic behaviors and physiological functions across the 24-hour day cycle, including sleep (Ono et al., 2018). At the core of these circadian patterns are clock genes, a series of genes whose expression is dependent upon the 24-hour day cycle (Hastings et al., 2008; Kyriacou and Hastings, 2010). Several clock genes demonstrate altered expression in PD, including Bmal1 (Breen et al., 2014; Cai et al., 2010) and Bmal2, a functional paralog of Bmal1 (Ding et al., 2011; Shi et al., 2010). Irregular epigenetic regulation of the protein NPAS2, which indirectly influences the expression of Bmal1, has also been found (Lin et al., 2012). Decreased Bmal1 expression reduces the production of the BMAL1 protein and causes alterations in the transcription and translation of Per and Cry genes, resulting in additional behavioral and circadian mechanistic interferences (Buhr and Takahashi, 2013; Bunger et al., 2000).
Dopaminergic dysfunction appears to significantly contribute to circadian disruption, as dopaminergic transmission demonstrates circadian fluctuates. Clock genes are also most notably expressed in areas of the mesocortical and mesolimbic pathways (McClung, 2007), the same pathways implicated in the development of RBD (Fantini and Ferini-Strambi, 2007; Rye, 2004). Altered timing of Bmal1 expression is influenced by dopamine, affecting the BMAL1:CLOCK protein heterodimer in patients with PD that regulates circadian-based processes (Cai et al., 2010). Further, CLOCK function in the ventral tegmental area plays a central role in regulating dopamine-modulated behavioral responses (Roybal et al., 2007) and exerts a modulatory effect on dopaminergic production (McClung et al., 2005). Evidence insinuates that the relationship between dopamine and clock genes works both ways, with clock genes modulating dopamine production (Albrecht, 2013; Chung et al., 2014; McClung et al., 2005), and dopamine likewise influencing clock gene expression (Imbesi et al., 2009; Yujnovsky et al., 2006).
The considerable amount of interactions between the dopaminergic and circadian systems offers genuine evidence for a specific contribution of clock gene dysfunction to sleep disturbances that then perpetuate the development of PD. In support of this, Breen and colleagues (2014) expanded the current understanding of sleep disturbances and clock gene expression in PD using polysomnography studies and serum analysis to evaluate sleep quality and architecture, as well as peripheral clock gene expression, in patients with newly diagnosed PD. These patients experienced significant differences in sleep quality, including reduced REM sleep, compared to controls, which coincides with previous research on sleep in PD. However, the authors’ hallmark finding was the alteration in Bmal1 expression in this cohort compared to the pattern demonstrated by controls. Whereas controls experienced fluctuations in expression dependent on the time of day, Bmal1 expression in PD patients was relatively consistent across time. In a separate study, Bmal1 expression was also associated with PD symptom severity, as measured by the Unified Parkinson’s Disease Rating Scale (Cai et al., 2010). These results highlight the potential contribution of clock gene expression in relation to PD-specific sleep problems. Further, altered clock gene expression in PD can disturb the 24-hour day cycle with consequences to nighttime sleep, thus compromising glymphatic system functioning and thereby perpetuating disease progression.
Whether alpha-synuclein neurotoxicity additionally contributes to clock gene dysregulation in PD is still a matter of speculation. Insights into the role of alpha-synuclein and its relationship to circadian timing in general, however, offer initially compelling evidence in support of a contribution to PD pathology. Broadly, the peripheral role that alpha-synuclein plays in autophagic processes responsible for clearing cellular waste, including protein aggregates, that are also under circadian control (Bobela et al.,2015; Hastings and Goedert, 2013; Panda et al., 2002; Winslow et al., 2010) implicates one avenue by which alpha-synuclein is modulated by clock gene functioning. More directly, the expression of non-pathological alpha-synuclein and synuclein-related proteins demonstrates a rhythmic pattern following the circadian cycle (Hastings and Goedert, 2013). Correspondingly, animal studies suggest a strong relationship between pathological alpha-synuclein accumulation and circadian disruptions. In support of this, one study explored the influence of mice expressing human alpha-synuclein on the circadian system and found overexpression of this alpha-synuclein reduced excitability of mouse suprachiasmatic nucleus neurons during daytime (Kudo et al., 2011). Furthermore, in an electroencephalogram (EEG)-based study, mice overexpressing human alpha-synuclein experienced more non-REM sleep, less REM sleep during quiescent periods, and a shift in EEG power spectra towards lower frequencies and decreased gamma power (McDowell et al., 2014)—all alterations that resemble the clinical presentation of humans with PD. While there is a dearth of human research examining associations between circadian disruptions and alpha-synuclein pathology, one pilot study discovered more frequent methylation changes on Per and Cry genes in individuals with dementia with Lewy bodies (Liu et al., 2008), offering a potential mechanism for clock gene disruption in the context of pathological alpha-synuclein accrual. Given the common processes underlying Lewy body diseases, which includes PD, PD with dementia, and dementia with Lewy bodies, this study offers encouraging results thus far for human PD research.
The relationship between circadian-mediated non-pathological alpha-synuclein expression and cellular autophagic processes highlights a specific role for alpha-synuclein in the aging process. Indeed, as discussed above, healthy aging is also hallmarked by alpha-synuclein-related dopaminergic deterioration that may underlie age-related alterations in sleep (Crowley, 2011), Nevertheless, sleep disorders (e.g., RBD) appear to be predominately restricted to disease processes (Bombois et al., 2010) and suggest supplementary influences in the development of RBD and other sleep dysfunctions beyond age-related declines. Currently, the role of alpha-synuclein accumulation in circadian disruption and the initial development of sleep disturbances, both in healthy aging and neurodegenerative disease, is not well characterized and emphasizes the need for future examinations in these domains.
4. Glymphatic System and Neural Protective Functioning
Sleep is an integral facet for multiple protective brain functions, especially waste clearance. Recent evidence highlights the glymphatic system in particular as the primary source for clearing toxins from the brain via cerebrospinal fluid (CSF), an essential mechanism in restoring brain function in healthy individuals (Iliff et al., 2012). As interstitial space expands during sleep, the flow of both CSF and interstitial fluid (ISF) through the brain parenchyma clears the brain of naturally accumulating waste by flushing toxins and other gross particles (Iliff et al., 2012; Mendelsohn and Larrick, 2013).
The process of glymphatic system functioning during sleep is marked by doubling of the CSF clearance rate, alongside a 60% increase in the interstitial space in the brain during non-wake states as compared to wake states (Jessen et al., 2015; Xie et al., 2013). Interestingly, increased efficiency of the glymphatic system has not only been demonstrated in natural sleep but also during other sleep-like states (Xie et al., 2013). For example, EEG brain activity during general anesthesia appears markedly similar to activity patterns that occur across stages of both non-REM (Brown et al., 2010) and REM (Leslie et al., 2009) sleep. As a cautionary note, drug-induced sleep may not represent the same functional state as natural sleep despite similarities in some EEG states (Murphy et al., 2011). Nevertheless, based on these and other animal studies that reported enhanced glymphatic system activity during anesthesia (e.g., Benveniste et al., 2017; Gakuba et al., 2018; Ratner et al., 2017; von Holstein-Rathlou et al., 2018; but see Gakuba et al., 2018), it is currently postulated that glymphatic operation is mediated by sleep-like brain activity more so than the act of sleep itself. This further implies that sleep factors, such as clock gene expression, do not directly impact glymphatic function but instead alter the amount of natural opportunities for glymphatic operation. Consequently, factors that cause disturbances in sleep architecture, such as those observed in sleep-wake disorders (e.g., RBD), may further promote glymphatic disruption.
Perivascular pathways, which are essential to the circulatory exchange of CSF and ISF, acutely affect flow and waste clearance of extracellular fluid in CSF volume transmission—a communication process for extracellular transmission of chemical signals (e.g., neurotransmitters) to achieve flow and interaction within the CNS (Agnati et al., 2005). Perivascular pathways are, in part, arbitrated by astrocytic endfeet expression in aquaporin-4 (AQP4) channels that promote transmission of CSF flow out of the perivascular space and into the interstitial space (Iliff et al., 2012; Nedergaard, 2013; Tarasoff-Conway et al., 2015). There is some evidence that AQP4 channels imperatively help fluid to propagate a convective CSF-ISF flow. Arterial pulsations are primarily responsible for circulating this flow, allowing CSF to channel out of the perivascular spaces and connect with ISF to carry out accumulated waste products as it moves through the brain parenchyma (Iliff et al., 2013, 2012; Mestre et al., 2018). However, AQP4 has also been found to be independent of glymphatic function (Smith et al., 2017). These discrepant findings may be a result of varying experimental designs diversely affecting solute transport (Brinker et al., 2014; Hladky and Barrand, 2014; Slynko and Nekhlopochin, 2018), Hence, further empirical analyses targeting the role of AQP4 in the glymphatic hypothesis, particularly in relation to differences between animal and human brain parenchyma, are warranted.
Notably, protein accruals such as amyloid-beta and tau aggregates are found within this interstitial space of the brain, accumulating in extracellular spaces (Clavaguera et al., 2009; Gómez-Ramos et al., 2006; Mendelsohn and Larrick, 2013; Meyer-Luehmann et al., 2003; Yamada et al., 2011). Though alpha-synuclein conformation change and accumulation were traditionally thought to occur in the neuron itself, recent evidence supports that alpha-synuclein is excreted into extracellular spaces (Emmanouilidou et al., 2011) and may even be responsible for the transfer of alpha-synuclein aggregates between neurons (Hansen et al., 2011). Thus, the neuropathological underpinnings of CSF-ISF flow in the pathogenesis of neurodegenerative disease processes (Mendelsohn and Larrick, 2013) is of high interest, given the accumulation of proteinaceous waste and pervasive metabolic fluctuations inherent to degenerative pathology.
Current research has utilized animal models to explore these pathological associations, particularly within the context of AD and the glymphatic system. AD is characterized by the accumulation of amyloid-beta protein and neurofibrillary tau tangles (Goedert et al., 2006; Irwin et al., 2013), both of which are associated with sleep disturbances (Cedernaes et al., 2016; Ju et al., 2014; Peter-Derex et al., 2015). Patients with AD not only exhibit diminished total sleep time, but also experience loss of day-night variation and decreased periods of REM sleep compared with age-matched controls (Musiek et al., 2015), similar to the alterations in sleep architecture observed within synucleinopathies. Interestingly, a recent study exploring glymphatic system clearance in mouse models of AD found that not only was glymphatic system transport suppressed within this disease, but suppression occurred prior to the extensive accumulation of amyloid-beta (Peng et al., 2016). Thus, while failure of the glymphatic system to clear amyloid-beta may exacerbate the pathological progression of AD (Kyrtsos and Baras, 2015; Tarasoff-Conway et al., 2015; Yulug et al., 2017), systemic dysfunction could also be an early biomarker of disease pathology.
While the connection between amyloid-beta accumulation and disruption of the foundational CSF-ISF dynamic implicates one mechanism of glymphatic system dysfunction (Jessen et al., 2015; Tarasoff-Conway et al., 2015), AQP4 channels that mediate glymphatic system flow (Iliff et al., 2012) appear to be an additional key mechanism in this process. Astrocytes and microglia produce proteases that aid in amyloid-beta degradation (Ries and Sastre, 2016), with damaged glial cells reducing glymphatic clearance and potentiating amyloid-beta burden. One recent study found that AQP4 depletion resulted in impaired clearance of amyloid-beta in AD mouse models and led to increased protein levels, despite consistent amyloid-beta expression (Xu et al., 2015). Just as amyloid-beta aggregations are linked to sleep-wake disturbances and glymphatic system dysfunction in AD, we argue that similar processes occur in PD.
In PD, the impact of dopaminergic deterioration may be crucial in the disruption of sleep and CSF-ISF flow in the glymphatic system, given the significant involvement of dopaminergic contributions to both the disease and waste clearance system (Gratwicke et al., 2015; Jennings and Rusakov, 2016). AQP4 deficiency is a key factor in proliferating the sensitivity of dopaminergic neurons in PD mouse models administered 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; Fan et al., 2008), a prodrug that induces simulated PD-related dopaminergic deterioration to the substantia nigra (Blesa and Przedborski, 2014). While AQP4 mediates CSF flow, dopamine and noradrenaline receptors appear to modulate the influx and outflow of ISF flow, which escalates volume transmission signaling (Fuxe et al., 2014). Dopamine and noradrenaline increases occur primarily during wake states (España et al., 2016; Isaac and Berridge, 2003; Murillo-Rodríguez et al., 2009), as does amplified extrasynaptic activity in both dopamine (via D1 and D2 receptors) and catecholamine volume transmission signaling (Fuxe et al., 2015). Dopamine volume transmission has specifically been linked to dopaminergic D1 and D2 receptors on glial cells (Fuxe et al., 2015; Jennings and Rusakov, 2016) and decreased catecholamine volume transmission signaling is also associated with greater interstitial volume resulting from natural sleep (Xie et al., 2013). Furthermore, dopamine has been shown to reduce the proliferation of striatal glial cells, as well as the expression of AQP4 within these cells (Küppers et al., 2008). Additionally. AQP4 deficiency has been associated with aggravated dopaminergic degeneration and, in particular, enhanced susceptibility to insult of the dopaminergic neurons between the substantia nigra and ventral tegmental area (Zhang et al., 2016), Therefore, as dopaminergic neurons appear to modulate AQP4 function and AQP4 deficiency can exacerbate dopaminergic neuronal loss, the amalgamation of both processes may be impairing glymphatic system functioning and subsequently result in suboptimal clearance of alpha-synuclein.
It is important to note that while ineffective glymphatic system functioning in neurodegenerative diseases is linked to both dopaminergic dysfunction and protein accumulations, the directionality and degree to which these neuropathological dysfunctions occur and are affected within the glymphatic system remains unclear. Accordingly, the development and application of tools uncovering glymphatic system biomarkers would be of high clinical value. Glymphatic system biomarkers could allow for an early diagnosis of PD and inform treatment strategies by implementing sleep and glymphatic system-enhancing treatments to slow disease progression and provide interventions early in the course of the disease.
5. Methods for Examining Glymphatic System Functioning
In support of pathological links between CSF and PD pathology to measure glymphatic system functioning, one immunoassay study found a 13% decrease of alpha-synuclein in CSF in PD patients compared to healthy controls (Van Dijk et al., 2014). CSF alpha-synuclein levels alone demonstrate only 71% sensitivity and 53% specificity as a biomarker for PD, although positive predictive value for a diagnosis of any synucleinopathy was approximately 91% (Mollenhauer et al., 2011). Recent evidence also supports lower CSF alpha-synuclein concentrations in PD as compared to AD patients, but similar concentrations among Lewy body diseases, indicating a relationship between glymphatic system functioning and alpha-synuclein pathology overall rather than providing an indicator of PD-specific processes (Gao et al., 2015). Given the substantial overlap between synucleinopathies and low statistical utility of CSF biomarkers for PD expressly, examinations utilizing CSF alpha-synuclein levels in relation to sleep and glymphatic system activation as biomarkers for PD progression are warranted.
Further research into examining the CSF-ISF dynamics that underpin solute clearance mechanisms in the human brain is challenged by the limitations of current methods for successfully measuring glymphatic operation. Much of our understanding of how the glymphatic system operates—along with recent exploratory methods by which to measure it—is derived from animal models, which have thus far offered valuable experimental insight. Although emphasis for future studies should be placed on transitioning from animal to human models to explore more reliable and valid measurements of glymphatic functioning, current animal studies offer some insight how these methods may be applied.
One initial technique using in vivo 2-photon microscopy and ex vivo fluorescence imaging of intracisternally-infused fluorescent CSF tracers in anesthetized mice evidenced the transport of tagged amyloid-beta peptides through perivascular pathways (Iliff et al., 2012). Interestingly, the AQP4-deficient mice demonstrated slowed CSF influx and a 70% reduction in interstitial solute clearance, with suppressed clearance of amyloid-beta. While promising, associated complications between fluorescent-based imaging and intracisternal administration of infusions (i.e., directly to the CSF) in humans (Keane, 1973) may prevent the clinical application of this approach. In contrast, a follow-up study by Iliff et al. (2013) utilized contrast-enhanced magnetic resonance imaging (MRI) to successfully visualize brain-wide subarachnoid CSF-ISF exchange in rat brains from intrathecal paramagnetic contrast agent administration (i.e., into the subarachnoid space to reach CSF). These results evidence a more simplified method of analyzing similar functions in the human brain without the difficulties resulting from intracisternal administration. Likewise, time-sequenced ex vivo fluorescence imaging using lumbar intrathecal administrations of CSF tracers demonstrated similar glymphatic pathway functions to intracisternal methods in rat brains, suggesting a promising approach in humans with neurodegenerative diseases (Yang et al., 2013). Interestingly, the presence of glymphatic function was also found when applying this approach to the human brain for the first time, highlighting the potential for intrathecal gadolinium contrast medium to study CSF-ISF flow in humans (Eide and Ringstad, 2015). Furthermore, perivascular spaces in healthy subjects show increased sensitivity to heavily T2-weighted 3D-FLAIR images obtained approximately four hours after using a gadolinium-based contrast agent, particularly within the basal ganglia (Naganawa et al., 2017).
While current evidence supports a mechanistic understanding of utilizing in vivo and ex vivo imaging to measure glymphatic functioning, the risks, reliability, and feasibility of utilizing these measures in a clinical setting should be further evaluated. Despite several limitations, advances in MRI sequences and modern image analysis algorithms that account for motion artifacts have been successfully applied in the clinical setting, e.g., for differential diagnostic purposes in PD (for a review, see Heim et al., 2017). Thus, future studies utilizing less invasive MRI techniques to explore neuropathological correlates of neurodegenerative diseases in the human glymphatic system are warranted, and we refer to other neuroimaging methods, such as diffusion tensor imaging (DTI), that offer promising outcomes. In particular, non-invasive techniques would allow testing of glymphatic function in the human brain and, in turn, its role in pathology. Accordingly, Komlosh and colleagues (2018) recently introduced a novel human MRI flow phantom, which combines regions that mimic CSF-filled ventricles and brain interstitial space. Their data indicate that under certain flow conditions high- and low-q space diffusion MRI and DTI acquisitions can be used to map interstitial glymphatic flows.
Similar diagnostic techniques in the form of MRI biomarkers, including CSF/free-water (FW) volume fraction or water diffusivity within the parenchyma, offer promising results for exploring human glymphatic system functioning. For example, Thomas et al. (2018) studied human glymphatic function using a dual compartment tensor model and found diurnal fluctuations in water diffusion were due to an increase in the volume fraction of CSF-like FW. With the intent of applying these techniques to humans with neurodegenerative conditions, Taoka and colleagues (2017) used DTI analysis along the perivascular space in human cases of AD. They found that lower diffusivity within the perivascular space suggestive of decreased glymphatic system functioning was associated with lower Mini-Mental Status Exam scores. Another MR neuroimaging method using FW compensated DTI in combination with functional MRI (fMRI) found preliminary evidence for an association between lower FW fraction and decreased nighttime sleep in PD patients, which was subsequently correlated with frontal hypoactivation during procedural memory task performance (Müller-Oehring et al., 2018). Taken together, novel advances in noninvasive MR neuroimaging methods examining the circadian fluctuation of water diffusion in the brain may supplement standard MRI techniques to mechanistically elucidate the impact of the role of the human glymphatic system on PD pathology.
While MRI-DTI techniques have shown potential in evaluating glymphatic function, one primary limitation of these methods is the possible contributions of other pulsation mechanisms (Purdon and Weisskoff, 1998) interfering with the arterial pulsation that propels CSF-ISF flow (Iliff et al., 2013). These added pulsations can subsequently induce motion in both structural (Poncelet et al., 1992) and functional (Beall and Lowe, 2014) neuroimaging and require difficult methodological corrections. One recent study monitored physiological pulsations in brain tissue using a novel multimodal ultra-fast magnetic resonance encephalography (MREG) technology to minimize aliasing and spin-history artifacts that may confound CSF-ISF flow determinations (Kiviniemi et al., 2016). The researchers found that non-invasive MREG brain scans have increased statistical power to examine CSF-ISF flow dynamics of glymphatic system activity in humans by distinctly separating cardiac, respiratory, and vasomotor pulsation patterns that co-exist within the glymphatic system, offering further promising avenues for the development of non-invasive MRI methods (and image preprocessing algorithms that correct for cardiac, respiratory, and vasomotor pulsation image artifacts similar to those used in functional MRI analyses), such as this to examine glymphatic functioning.
6. Conclusion and Future Directions
Sleep promotes neuroprotection in healthy individuals. A fundamental physiological process during sleep is the activation of the glymphatic system, which promotes the clearance of accumulated toxic metabolites during sleep through the flow of ISF and CSF (Iliff et al., 2012) that can otherwise trigger irreversible neuronal injury (Xie et al., 2013).
Waste clearance systems that protect against toxic protein buildup appear to be disrupted in neuropathological diseases with altered sleep structure and dysregulated clock gene expressions, such as PD. This raises the question of whether, and to what degree, waste clearance systems are mechanistically altered due to sleep disturbances in neurodegenerative diseases. Thus, the co-occurrence of sleep disturbances, hallmark pathogenic alpha-synuclein aggregates, and dopaminergic dysfunction in PD may be linked through glymphatic system dysfunction. Recent research provides compelling evidence that amyloid-beta inclusions are removed via the glymphatic system (Tarasoff-Conway et al., 2015). These studies provide promising insight into the question of whether alpha-synuclein deposits, which are found in the same interstitial space of the brain as amyloid-beta, are similarly removed by the CSF during sleep to protect the brain from neurodegenerative decline. They also highlight the importance of understanding the role of glymphatic system dysfunction in PD, as sleep disturbances often precede motor symptoms that traditionally warrant the diagnosis of PD and are increasingly experienced throughout disease progression.
To our knowledge, research has not yet examined the degree to which the glymphatic system plays a role in counteracting the neurotoxic effects of PD pathology. Prior examinations of glymphatic system functioning and sleep have primarily utilized animal models (Iliff et al., 2013), with animal studies examining glymphatic system functioning in AD offering promising insights. In particular, recent developments in non-invasive specific diffusion-weighted MRI sequences assessing perivascular fluid movement could enable a more detailed understanding of glymphatic function in the human brain (e.g., Komlosh et al., 2018). The circadian clock likely plays a vital role in this context, given the general purpose of clock genes in managing and coordinating daily cycles in most, if not all, physiological and biochemical processes across the organism (Albrecht and Ripperger, 2018). Disruption of these mechanisms can lead to sleep disturbances and perpetuate protein accumulation, which subsequently initiates the development of neurodegenerative disorders (e.g., amyloid-beta accumulation in AD, alpha-synuclein in PD; Verheggen et al., 2018).
In human studies, recent advances in noninvasive brain imaging methods may provide alternative means for accurately testing neural network function in relation to disease progression and protective factors of sleep, including identification of discrete in vivo imaging biomarkers of glymphatic system function. Current research utilizing DTI offers promising results as a way to measure both general and disease-specific human glymphatic function (Komlosh et al., 2018; Müller-Oehring, et al., 2018; Taoka et al., 2017; Thomas et al., 2018). Novel non-invasive neuroimaging avenues for glymphatic system research in humans would also benefit from adopting clinical measurements. For example, overnight polysomnography of sleep quality and REM and non-REM sleep disturbances could be employed together with measures of glymphatic system flow and related to neurofunctional abnormalities and disease progression. Recent evidence in animal models demonstrates that glymphatic flow in mice positively correlated with slow-wave sleep and lower heart rate (Hablitz et al., 2019). Coupled with recent findings that interruption of slow-wave sleep in humans is associated with profound increases in CSF beta-amyloid levels (Ju et al., 2017), one promising direction for future research may explore whether interventions focused on increasing slow delta wave stages have a protective effect on people at risk for synucleinopathy-related diseases. Furthermore, since dopaminergic function is intricately coupled with the circadian system, characterization of circadian system changes over the course of PD alongside imaging biomarkers of glymphatic operation may provide a new avenue for understanding the role of sleep in counteracting neurocognitive decline and slowing progression of symptoms in PD (Videnovic and Golombek, 2017).
Successful identification of biomarkers could be utilized towards improving clinical diagnostic techniques, monitoring disease progression, and providing tools for treatment response in neurodegenerative diseases. Likewise, interventions targeting sleep disturbances may also improve the efficiency of the glymphatic function and intrinsic neuroprotective functions in degenerative conditions. The combined properties of glymphatic system activity, clock genes, and sleep for neuroprotection could provide the basis for developing holistic clinical rehabilitation techniques that are conducive to the management of concurrent sleep disturbances, and age-related changes in PD disease pathology.
Figure 1. Neuroprotective mechanisms of sleep.
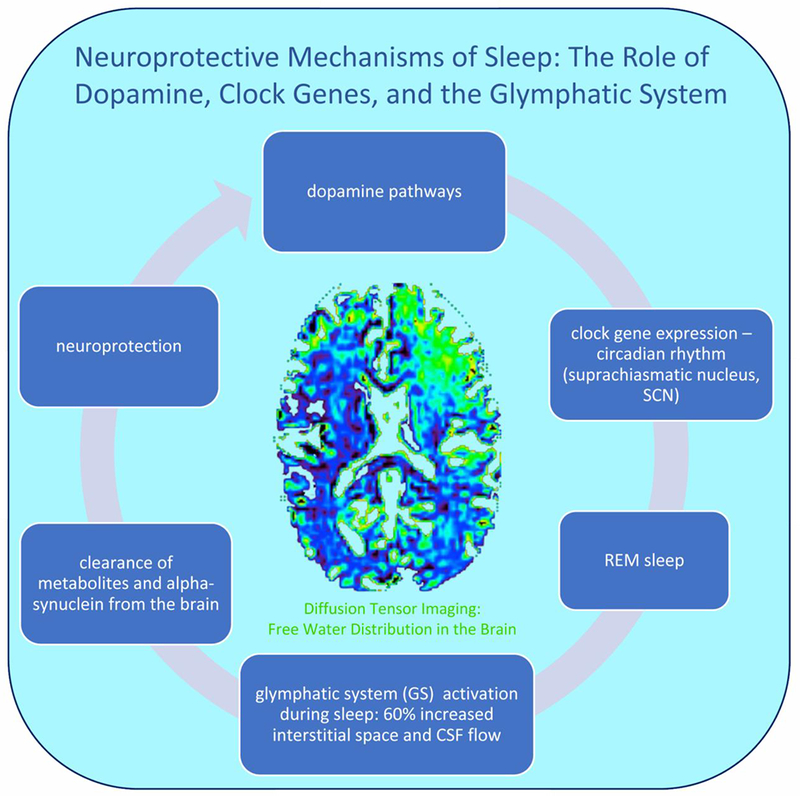
Midbrain dopamine neurons innervate corticolimbic and nigrostriatal systems involved in the control of sleep and wake states, including the SCN (circadian rhythm) (Monti and Monti, 2007) and striatal clock gene expression (Verwey et al., 2016) that promote healthy sleep. During sleep, glymphatic system activity is characterized by a 60% increase in the interstitial space and CSF flow (Jessen et al., 2015; Xie et al., 2013), and clearance of alpha-synuclein, along with other protein accumulations, from the brain (Iliff et al., 2012). Non-invasive free-water diffusion tensor imaging (FW-DTI) can be utilized in human subjects to detect the percent of free water in the tissue during sleep and wake states (Thomas et al., 2018).
Figure 2. Proposed model of prodromal factors in the development of Parkinson’s disease.
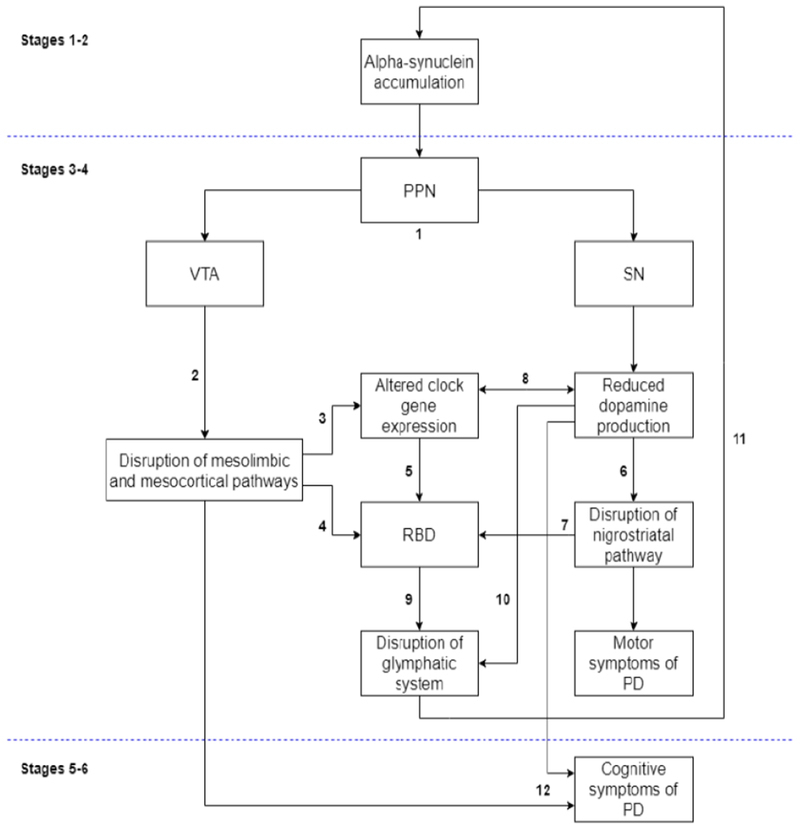
A pathological model for understanding the neurodegenerative progression of PD has been proposed by Braak and colleagues (2003, 2004) based on post-mortem analysis of brain tissue samples. Briefly, stages 1 and 2 of their model are characterized by the early development of alpha-synuclein rich Lewy bodies within lower brainstem regions and the olfactory bulb and represent a “preclinical” phase of the disease. Stages 3 and 4 mark the onset of clinical symptoms in PD due to spreading pathology to several key mid- and forebrain regions largely implicated in PD. including the substantia nigra (SN) and pedunculopontine nucleus (PPN), among others. Stages 5 and 6 are thought to represent the end stages of disease progression as Lewy bodies spread diffusely up through the cortex. We thus propose that the clinical and other biological manifestations of PD occur within the context of the Braak staging model. (1) As noted, alpha-synuclein accrual in prodromal PD occurs in dopaminergic-rich brainstem regions including the SN and PPN, the latter of which projects to both the ventral tegmental area (VTA) and the SN (Braak et al., 2003; French and Muthusamy, 2018). (2) Deterioration of the VTA leads to the disruption of mesolimbic and mesocortical dopaminergic pathways (Le Moal and Simon, 1991; Westerink and Kwint, 1996), which in turn (3) alter circadian functioning (McClung, 2007) and (4) promote disturbances in sleep through REM-sleep behavior disorder (RBD; Chaudhuri and Schapira, 2009; Fantini and Ferini-Strambi, 2007; Kalaitzakis et al., 2013; Rye, 2004). (5) Altered clock gene expression is highly implicated in the development of several forms of sleep disturbances, including RBD (Turek et al., 2001). (6) Concurrent deterioration of the SN leads to disruption of the nigrostriatal pathway via dopaminergic depletion, and (7) is implicated in RBD (Kim et al., 2010). (8) Altered clock gene expression decreases dopamine production (Albrecht, 2013), and reduced dopamine levels in turn blunt daily rhythms in clock gene expression (Cai et al., 2010). (9) We propose that disrupted sleep via RBD that is perpetuated by dysregulated clock gene expression, (10) along with dopaminergic dysfunction that promote effective waste clearance (Gratwicke et al., 2015; Jennings and Rusakov, 2016), reduces opportunities for glymphatic system functioning and (11) contributes to alpha-synuclein buildup in perivascular pathways, thereby exacerbating neurodegeneration and emerging disease symptoms (Musiek and Holtzman, 2016). Continued mechanistic disturbances in dopaminergic pathways, sleep, and glymphatic clearance of alpha-synuclein over time perpetuate the course of PD development, (12) leading to later clinical signs of PD, such as cognitive decline.
Highlights:
During sleep, glymphatic system (GS) activation clears toxins from the brain
Striatal dopamine modulates clock gene expression and sleep-wake rhythm
Dopaminergic dysfunction and alpha-synuclein aggregations characterize PD
Disrupted sleep in PD may decrease metabolic clearance with alpha-synuclein buildup
Non-invasive neuroimaging of the GS may improve diagnostic and interventional tools
Acknowledgments
We thank Dr. Fiona Baker for comments on the manuscript.
Funding
This work was supported by National Institutes of Health (NIH)/National Institute on Alcohol Abuse and Alcoholism (NIAAA) [grant numbers R01 AA023165 (TS), U01 AA017347 (AP), K05 AA017168 (EVS)], NIH/National Institute of Neurological Disorders and Stroke (NINDS) [grant number K23 NS075097 (KLP)], and Michael J. Fox Foundation for Parkinson’s Research (KLP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interests
None.
References
- Aarsland Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, Bum D, Barone P, Pagonabarraga J, Allcock L, Santangelo G, Foltynie T, Janvin C, Larsen JP, Barker RA, Emre M, 2010. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 75, 1062–1069. 10.1212/WNL.0b013e3181f39d0e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati LF, Genedani S, Lenzi PL, Leo G, Mora F, Ferre S, Fuxe K, 2005. Energy gradients for the homeostatic control of brain ECF composition and for VT signal migration: Introduction of the tide hypothesis. J. Neural Transm. 112, 45–63. 10.1007/s00702-004-0180-5 [DOI] [PubMed] [Google Scholar]
- Ahlskog JE, 2007. Beating a dead horse: Dopamine and Parkinson disease. Neurology 69, 1701–1711. 10.1212/01.wnl.0000296942.14309.4a [DOI] [PubMed] [Google Scholar]
- Alberico SL, Cassell MD, Narayanan NS, 2015. The vulnerable ventral tegmental area in Parkinson’s disease. Basal Ganglia 5, 51–55. 10.1016/j.baga.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB, 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375. 10.1016/0166-2236(89)90074-X [DOI] [PubMed] [Google Scholar]
- Albrecht U, 2013. Circadian clocks and mood-related behaviors, in: Kramer A, Merrow M (Eds.), Circadian Clocks. Springer, Heidelberg, Berlin, pp. 227–239. 10.1007/978-3-642-25950-0_9 [DOI] [Google Scholar]
- Albrecht U., Ripperger JA, 2018. Circadian clocks and sleep: Impact of rhythmic metabolism and waste clearance on the brain. Trends Neurosci. 41, 677–688. 10.1016/J.TINS.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Aygun D, Turkel Y, Akkurt A, Onar MK, 2012. The effect of REM sleep behaviour disorder on clinical severity in Parkinson’s disease. Turkish J. Med. Sci. 42, 1033–1038. 10.3906/sag-1105-35 [DOI] [Google Scholar]
- Barber A, Dashtipour K, 2012. Sleep sisturbances in Parkinson’s disease with emphasis on rapid eye movement sleep behavior disorder. Int. J. Neurosci 122, 407–412. 10.3109/00207454.2012.677882 [DOI] [PubMed] [Google Scholar]
- Beall EB, Lowe MJ, 2014. SimPACE: Generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: A new, highly effective slicewise motion correction. Neuroimage 101, 21–34. 10.1016/j.neuroimage.2014.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, Probst S, Nedergaard M, Stein EA, Lu H, 2017. Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with high-dose isoflurane. Anesthesiology 127, 976–988. 10.1097/ALN.0000000000001888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesa J, Przedborski S, 2014. Parkinson’s disease: Animal models and dopaminergic cell vulnerability. Front. Neuroanat 8, 155 10.3389/fnana.2014.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobela W, Aebischer P, Biomolecules B.S. -, 2015, U., 2015. Alpha-synuclein as a mediator in the interplay between aging and Parkinson’s disease, mdpi.com 5, 2675–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, 2013. Idiopathic REM sleep behaviour disorder in the development of Parkinson’s disease. Lancet Neurol. 12, 469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Parisi JE, Dickson DW, Ferman TJ, Benarroch EE, Schmeichel AM, Smith GE, Petersen RC, Ahlskog JE, Matsumoto JY, Knopman DS, Schenck CH, Mahowald MW, 2003. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 61, 40–45. 10.1212/01.WNL.0000073619.94467.B0 [DOI] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, Benarroch E, Ahlskog JE, Smith GE, Caselli RC, Tippman-Peikert M, Olson EJ, Lin SC, Young T, Wszolek Z, Schenck CH, Mahowald MW, Castillo PR, Del Tredici K, Braak H, 2007. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 130, 2770–2788. 10.1093/brain/awm056 [DOI] [PubMed] [Google Scholar]
- Bombois S, Derambure P, Pasquier F, Monaca C, 2010. Sleep disorders in aging and dementia. J. Nutr. Heal. Aging 14, 212–217. 10.1007/s12603-010-0052-7 [DOI] [PubMed] [Google Scholar]
- Bonanni E, Maestri M, Tognoni G, Fabbrini M, Nucciarone B, Manca ML, Gori S, Iudice A, Murri L, 2005. Daytime sleepiness in mild and moderate Alzheimer’s disease and its relationship with cognitive impairment. J. Sleep Res. 14, 311–317. 10.1111/j.1365-2869.2005.00462.x [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E, 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211. 10.1016/SO197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K, 2004. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 318, 121–134. 10.1007/s00441-004-0956-9 [DOI] [PubMed] [Google Scholar]
- Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, Barker RA, 2014. Sleep and circadian rhythm regulation in early Parkinson’s disease. JAMA Neurol. 71, 589 10.1001/jamaneurol.2014.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker T, Stopa E, Morrison J, Klinge P, 2014. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11, 10 10.1186/2045-8118-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Lydic R, Schiff ND, 2010. General anesthesia, sleep, and coma. N. Engl. J. Med 363, 2638–2650. 10.1056/NEJMra0808281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Takahashi JS, 2013. Molecular components of the mammalian circadian clock. Handb. Exp. Pharmacol 217, 3–27. 10.1007/978-3-642-25950-0-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA, 2000. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017. 10.1016/S0092-8674(00)00205-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Liu S, Sothern RB, Xu S, Chan P, 2010. Expression of clock genes Perl and Bmal1 in total leukocytes in health and Parkinson’s disease. Eur. J. Neurol 17, 550–554. 10.1111/j.1468-1331.2009.02848.x [DOI] [PubMed] [Google Scholar]
- Cedernaes J, Osorio RS, Varga AW, Kam K, Schiöth HB, Benedict C, 2016. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease. Sleep Med. Rev. 31, 102–111. 10.1016/j.smrv.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AH, 2009. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 8, 464–474. 10.1016/S1474-4422(09)70068-7 [DOI] [PubMed] [Google Scholar]
- Cheng HC, Ulane CM, Burke RE, 2010. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol 67, 715–725. 10.1002/ana.21995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Kordower JH, 2007. Age-associated increases of α-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol. Dis 25, 134–149. 10.1016/j.nbd.2006.08.021 [DOI] [PubMed] [Google Scholar]
- Chung S, Lee Y, Lee JJ, Lee PH, Sohn YH, 2017. Rapid eye movement sleep behaviour disorder and striatal dopamine depletion in patients with Parkinson’s disease. Eur. J. Neurol 24, 1314–1319. 10.1111/ene.13388 [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M, 2009. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11, 909–913. 10.1038/ncb 1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y, Marti MJ, Rey MJ, Ezquerra M, 2009. Parkinsonism, dysautonomia, REM behaviour disorder and visual hallucinations mimicking synucleinopathy in a patient with progressive supranuclear palsy. J. Neurol. Neurosurg. Psychiatry 80, 578–579. 10.1136/jnnp.2007.142810 [DOI] [PubMed] [Google Scholar]
- Crowley K, 2011. Sleep and sleep disorders in older adults. Neuropsychol. Rev 21, 41–53. 10.1002/9781118772034.ch27 [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S, 2003. Parkinson’s disease: Mechanisms and models. Neuron 39, 889–909. 10.1016/S0896-6273(03)00568-3 [DOI] [PubMed] [Google Scholar]
- de Lau LML, Breteler MMB, 2006. Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–35. 10.1016/S1474-4422(06)70471-9 [DOI] [PubMed] [Google Scholar]
- Dickson DW, Fujishiro H, Orr C, DelleDonne A, Josephs KA, Frigerio R, Burnett M, Parisi JE, Klos KJ, Ahlskog JE, 2009. Neuropathology of non-motor features of Parkinson disease. Park. Relat. Disord 15, S1–S5. 10.1016/S1353-8020(09)70769-2 [DOI] [PubMed] [Google Scholar]
- Ding H, Liu S, Yuan Y, Lin Q, Chan P, Cai Y, 2011. Decreased expression of Bmal2 in patients with Parkinson’s disease. Neurosci. Lett 499, 186–188. 10.1016/j.neulet.2011.05.058 [DOI] [PubMed] [Google Scholar]
- Doppler K, Jentschke HM, Schulmeyer L, Vadasz D, Janzen A, Luster M, Höffken FL, Mayer G, Brumberg J, Booij J, Musacchio T, Klebe S, Sittig-Wiegand E, Volkmann J, Sommer C, Oertel WH, 2017. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol. 133, 535–545. 10.1007/s00401-017-1684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide PK, Ringstad G, 2015. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol. Short Reports 4, 205846011560963. 10.1177/2058460115609635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Elenis D, Papasilekas T, Stranjalis G, Gerozissis K, Ioannou PC, Vekrellis K, 2011. Assessment of α-synuclein secretion in mouse and human brain parenchyma. PLoS One 6, e22225 10.1371/journal.pone.0022225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Schmeichel BE, Berridge CW, 2016. Norepinephrine at the nexus of arousal, motivation and relapse. Brain Res. 1641, 207–216. 10.1016/j.brainres.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Sulzer D, 2004. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx 1, 139–154. 10.1602/neurorx.1.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Kong H, Shi X, Sun X, Ding J, Wu J, Hu G, 2008. Hypersensitivity of aquaporin 4-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine and astrocytic modulation. Neurobiol. Aging 29, 1226–1236. 10.1016/j.neurobiolaging.2007.02.015 [DOI] [PubMed] [Google Scholar]
- Fantini ML, Ferini-Strambi L, 2007. Idiopathic rapid eye movement sleep behaviour disorder. Neurol. Sci 28, S15–S20. 10.1007/s10072-007-0734-z [DOI] [PubMed] [Google Scholar]
- Fantini ML, Gagnon J-F, Petit D, Rompré S, Décary A, Carrier J, Montplaisir J, 2003. Slowing of electroencephalogram in rapid eye movement sleep behavior disorder. Ann. Neurol 53, 774–780. 10.1002/ana.10547 [DOI] [PubMed] [Google Scholar]
- Ferrer I, 2011. Neuropathology and Neurochemistry of Nonmotor Symptoms in Parkinson’s Disease. Parkinsons. Dis 2011, 1–13. 10.4061/2011/708404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French IT, Muthusamy KA, 2018. A Review of the Pedunculopontine Nucleus in Parkinson’s Disease. Front. Aging Neurosci. 10 10.3389/fnagi.2018.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Marcoli M, Borroto-Escuela DO, 2015. Volume transmission in central dopamine and noradrenaline neurons and its astroglial targets. Neurochem. Res 40, 2600–2614. 10.1007/s11064-015-1574-5., [DOI] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Palkovits M, Tarakanov AO, Ciruela F, Agnati LF, 2014. Moonlighting proteins and protein-protein interactions as neurotherapeutic targets in the G protein-coupled receptor field. Neuropsychopharmacology 39, 131–155. 10.1038/npp.2013.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon JF, Postuma RB, Mazza S, 2006. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet … 5, 424–432. 10.1016/S1474-4422(06)70441-0 [DOI] [PubMed] [Google Scholar]
- Gakuba C, Gaberel T, Goursaud S, Bourges J, D Palma C, Quenault A, de Lizarrondo SM, Vivien D, Gauberti M, 2018. General anesthesia inhibits the activity of the “glymphatic system.” Theranostics 8, 710–722. 10.7150/thno.19154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Tang H, Nie K, Wang L, Zhao J, Gan R, Huang J, Zhu R, Feng S, Duan Z, Zhang Y, Wang L, 2015. Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson’s disease diagnosis: A systematic review and meta-analysis. Int. J. Neurosci 125, 645–654. 10.3109/00207454.2014.961454 [DOI] [PubMed] [Google Scholar]
- Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP, 2008. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson’s disease overtime. J. Neurol. Neurosurg. Psychiatry 79, 387–391. 10.1136/jnnp.2007.116830 [DOI] [PubMed] [Google Scholar]
- Goedert M, Klug A, Crowther RA, 2006. Tau protein, the paired helical filament and Alzheimer’s disease. J. Alzheimer’s Dis. 9, 195–207. 10.1016/S0361-9230(99)00138-0 [DOI] [PubMed] [Google Scholar]
- Gómez-Ramos A, Díaz-Hemández M, Cuadros R, Hernández F, Avila J, 2006. Extracellular tau is toxic to neuronal cells. FEBS Lett. 580, 4842–4850. https://doi.Org/10.1016/j.febslet.2006.07.078 [DOI] [PubMed] [Google Scholar]
- Gratwicke J, Jahanshahi M, Foltynie T, 2015. Parkinson’s disease dementia: A neural networks perspective. Brain 138, 1454–1476. 10.1093/brain/awv104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greffard S, Verny M, Bonnet AM, Beinis JY, Gallinari C, Meaume S, Piette F, Hauw JJ, Duyckaerts C, 2006. Motor score of the unified Parkinson disease rating scale as a good predictor of lewy body-associated neuronal loss in the substantia nigra. Arch. Neurol 63, 584–588. 10.1001/archneur.63.4.584 [DOI] [PubMed] [Google Scholar]
- Grinberg LT, Rueb U, Alho AT di L, Heinsen FL, 2010. Brainstem pathology and nonmotor symptoms in PD. J. Neurol. Sci 289, 81–88. 10.1016/jjns.2009.08.021 [DOI] [PubMed] [Google Scholar]
- Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN, Lilius TO, Nedergaard M, 2019. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv 5, eaav5447 10.1126/sciadv.aav5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergström AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P, 2011. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest 121, 715–725. 10.1172/JCI43366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Goedert M, 2013. Circadian clocks and neurodegenerative diseases: Time to aggregate? Curr. Opin. Neurobiol 23, 880–887. 10.1016/j.conb.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Reddy AB, 2008. Two decades of circadian time. J. Neuroendocrinol 20, 812–819. 10.1111/j.1365-2826.2008.01715.x [DOI] [PubMed] [Google Scholar]
- Heim B, Krismer F, De Marzi R, Seppi K, 2017. Magnetic resonance imaging for the diagnosis of Parkinson’s disease. J. Neural Transm. 124, 915–964. 10.1007/s00702-017-1717-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely MA, Morris JGL, Reid WGJ, Trafficante R, 2005. Sydney Multicenter Study of Parkinson’s disease: Non-L-dopa-responsive problems dominate at 15 years. Mov. Disord 20, 190–199. 10.1002/mds.20324 [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL, 2008. The Sydney Multicenter Study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord 23, 837–844. 10.1002/mds.21956 [DOI] [PubMed] [Google Scholar]
- Hladky SB, Barrand MA, 2014. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 11, 26 10.1186/2045-8118-11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H, 2013. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest 123, 1299–1309. 10.1172/JCI67677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M, 2012. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid. Sci. Transl. Med 4, 147ra111–147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbesi M, Yildiz S, Dirim Arslan A, Sharma R, Manev H, Uz T, 2009. Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience 158, 537–544. 10.1016/j.neuroscience.2008.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranzo A, Molinuevo JL, Santamaria J, Serradell M, Martí MJ, Valldeoriola F, Tolosa E 2006. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 5, 572–577. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Lee VM-Y, Trojanowski JQ, 2013. Amyloid beta-peptide and the dementia of Parkinson’s disease. Nat Rev Neurosci. 14, 626–636. 10.1038/nm3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac SO, Berridge CW, 2003. Wake-promoting actions of dopamine D1 and D2 receptor stimulation. J. Pharmacol. Exp. Ther 307, 386–394. 10.1124/jpet.103.053918 [DOI] [PubMed] [Google Scholar]
- Jankovic J, 2008. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 79, 368–76. 10.1136/jnnp.2007.131045 [DOI] [PubMed] [Google Scholar]
- Janvin C, Aarsland D, Larsen JP, Hugdahl K, 2003. Neuropsychological profile of patients with Parkinson’s disease without dementia. Dement. Geriatr. Cogn. Disord 15, 126–131. 10.1159/000068483 [DOI] [PubMed] [Google Scholar]
- Jellinger KA, 2003. α-Synuclein pathology in Parkinson’s and Alzheimer’s disease brain: Incidence and topographic distribution—a pilot study. Acta Neuropathol. 106, 191–202. 10.1007/s00401-003-0725-y [DOI] [PubMed] [Google Scholar]
- Jennings A, Rusakov DA, 2016. Do astrocytes respond to dopamine? Opera Medica Physiol. 2, 34–43. 10.20388/OMP2016.001.0017 [DOI] [Google Scholar]
- Jessen NA, Munk ASF, Lundgaard I, Nedergaard M, 2015. The glymphatic system: A beginner’s guide. Neurochem. Res 40, 2583–2599. 10.1007/s11064-015-1581-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YES, Lucey BP, Holtzman DM, 2014. Sleep and Alzheimer disease pathology-a bidirectional relationship. Nat. Rev. Neurol 10.1038/nrneurol.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YES, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, Fagan AM, Mignot E, Zempel JM, Claassen JAHR, Holtzman DM, 2017. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain 140, 2104–2111. 10.1093/brain/awx148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzakis ME, Gentleman SM, Pearce RKB, 2013. Disturbed sleep in Parkinson’s disease: Anatomical and pathological correlates. Neuropathol. Appl. Neurobiol 39, 644–653. 10.1111/nan.12024 [DOI] [PubMed] [Google Scholar]
- Kalaitzakis ME, Pearce RKB, 2009. The morbid anatomy of dementia in Parkinson’s disease. Acta Neuropathol. 118, 587–598. 10.1007/s00401-009-0597-x [DOI] [PubMed] [Google Scholar]
- Keane JR, 1973. Cisternal puncture complications. Treatment of coccidioidal meningitis with amphotericin B. Calif Med 119, 10–15. [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Yoon IY, Kim JM, Jeong SH, Kim KW, Shin YK, Kim BS, Kim SE, 2010. The implication of nigrostriatal dopaminergic degeneration in the pathogenesis of REM sleep behavior disorder. Eur. J. Neurol 17, 487–492. 10.1111/j.1468-1331.2009.02854.x [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Wang X, Korhonen V, Keinänen T, Tuovinen T, Autio J, Levan P, Keilholz S, Zang YF, Hennig J, Nedergaard M, 2016. Ultra-fast magnetic resonance encephalography of physiological brain activity-glymphatic pulsation mechanisms? J. Cereb. Blood Flow Metab. 36, 1033–1045. 10.1177/0271678X15622047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler NKU, Stransky E, Meyer M, Gaertner S, Shing M, Schnaidt M, Celej MS, Jovin TM, Leyhe T, Laske C, Batra A, Buchkremer G, Fallgatter AJ, Wernet D, Richartz-Salzburger E, 2015. Alpha-synuclein levels in blood plasma decline with healthy aging. PLoS One 10, e0123444 10.1371/journal.pone.0123444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komlosh ME, Benjamini D, Williamson NW, Horkay F, Hutchinson EB, Basser PJ, 2018. A novel MRI phantom to study interstitial fluid transport in the glymphatic system. Magn. Reson. Imaging. 10.1016/j.mri.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Milenkovic IJ, Preusser M, Budka H, 2008. Nigral burden of α-synuclein correlates with striatal dopamine deficit. Mov. Disord 23, 1608–1612. 10.1002/mds.22207 [DOI] [PubMed] [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew J, Plog BA, Ding F, Deane R, Nedergaard M, 2014. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol 76, 845–861. 10.1002/ana.24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Loh DH, Truong D, Wu Y, Colwell CS, 2011. Circadian dysfunction in a mouse model of Parkinson’s disease. Exp. Neurol 232, 66–75. 10.1016/j.expneurol.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Küppers E, Gleiser C, Brito V, Wachter B, Pauly T, Hirt B, Grissmer S, 2008. AQP4 expression in striatal primary cultures is regulated by dopamine - Implications for proliferation of astrocytes. Eur. J. Neurosci 28, 2173–2182. 10.1111/j.1460-9568.2008.06531.x [DOI] [PubMed] [Google Scholar]
- Kyriacou CP, Hastings MH, 2010. Circadian clocks: Genes, sleep, and cognition. Trends Cogn. Sci. 14, 259–267. 10.1016/j.tics.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Kyrtsos CR, Baras JS, 2015. Modeling the role of the glymphatic pathway and cerebral blood vessel properties in Alzheimer’s disease pathogenesis. PLoS One 10, e0139574 10.1371/journal.pone.0139574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Simon H, 1991. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol. Rev 71, 155–234. 10.1152/physrev.1991.71.1.155 [DOI] [PubMed] [Google Scholar]
- Leslie K, Sleigh J, Paech MJ, Voss L, Lim CW, Sleigh C, 2009. Dreaming and electroencephalographic changes during anesthesia maintained with propofol or desflurane. Anesthesiology 111, 547–555. 10.1097/ALN.0b013e3181adf768 [DOI] [PubMed] [Google Scholar]
- Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, Albanese M, Mercuri NB, Izzi F, Bernardini S, Nitti A, Sancesario GM, Sica F, Marciani MG, Placidi F, 2014. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 71, 1498–1505. 10.1001/jamaneurol.2014.2510 [DOI] [PubMed] [Google Scholar]
- Lim ASP, Kowgier M, Yu L, Buchman AS, Bennett DA, 2013. Sleep rragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep 36, 1027–1032. 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Ding FL, Zheng Z, Gu Z, Ma J, Chen L, Chan P, Cai Y, 2012. Promoter methylation analysis of seven clock genes in Parkinson’s disease. Neurosci. Lett 507, 147–150. 10.1016/j.neulet.2011.12.007 [DOI] [PubMed] [Google Scholar]
- Liu HC, Hu CJ, Tang YC, Chang JG, 2008. A pilot study for circadian gene disturbance in dementia patients. Neurosci. Lett 435, 229–233. 10.1016/j.neulet.2008.02.041 [DOI] [PubMed] [Google Scholar]
- Maetzler W, Liepelt I, Berg D, 2009. Progression of Parkinson’s disease in the clinical phase: potential markers. Lancet Neurol. 8, 1158–1171. 10.1016/S1474-4422(09)70291-1 [DOI] [PubMed] [Google Scholar]
- Martinez-Martin P, Schapira AHV, Stocchi F, Sethi K, Odin P, MacPhee G, Brown RG, Naidu Y, Clayton L, Abe K, Tsuboi Y, MacMahon D, Barone P, Rabey M, Bonuccelli U, Forbes A, Breen K, Tluk S, Olanow CW, Thomas S, Rye D, Hand A, Williams AJ, Ondo W, Chaudhuri KR, 2007. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; Study using nonmotor symptoms questionnaire in 545 patients. Mov. Disord 22, 1623–1629. 10.1002/mds.21586 [DOI] [PubMed] [Google Scholar]
- Mattila PM, Rinne JO, Helenius H, Dickson DW, Röyttä M, 2000. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol. 100, 285–290. 10.1007/s004019900168 [DOI] [PubMed] [Google Scholar]
- McClung CA, 2007. Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. Scientific World Journal. 10.1100/tsw.2007.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitatema M, Takahashi JS, White FJ, Cooper DC, Nestler EJ, 2005. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc. Natl. Acad. Sci. U. S. A 102, 9377–9381. 10.1073/pnas.0503584102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell KA, Shin D, Roos KP, Chesselet MF, 2014. Sleep dysfunction and EEG alterations in mice overexpressing alpha-synuclein. J. Parkinsons. Dis 4, 531–539. 10.3233/JPD-140374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn AR, Larrick JW, 2013. Sleep facilitates clearance of metabolites from the brain: Glymphatic function in aging and neurodegenerative diseases. Rejuvenation Res. 16, 518–523. 10.1089/rej.2013.1530 [DOI] [PubMed] [Google Scholar]
- Mestre H, Hablitz L, Xavier A, Feng W, ELife WZ -, 2018, U., 2018. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7 10.7554/eLife.40070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Stalder M, Herzig MC, Kaeser SA, Kohler E, Pfeifer M, Boncristiano S, Mathews PM, Mercken M, Abramowski D, Staufenbiel M, Jucker M, 2003. Extracellular amyloid formation and associated pathology in neural grafts. Nat. Neurosci 6, 370–377. 10.1038/nn1022 [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Miyamoto M, 2018. Phenoconversion from idiopathic rapid eye movement sleep behavior disorder to Lewy body disease. Mov. Disord. Clin. Pract 5, 506–511. 10.1002/mdc3.12647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG, 2011. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: A cohort study. Lancet Neurol. 10, 230–240. 10.1016/S1474-4422(11)70014-X [DOI] [PubMed] [Google Scholar]
- Monti JM, Monti D, 2007. The involvement of dopamine in the modulation of sleep and waking. Sleep Med. Rev. 10.1016/j.smrv.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Chakravarthy S, Phillips JR, Gupta A, Keri S, Polner B, Frank MJ, Jahanshahi M, 2016. Motor symptoms in Parkinson’s disease: A unified framework. Neurosci. Biobehav. Rev 68, 727–740. 10.1016/j.neubiorev.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Müller-Oehring EM, Peterson E, Hong JY, Poston KL, Brontë-Stewart HM, Schulte T, 2018. Neuroimaging of the glymphatic system in relation to motor skill in Parkinson’s disease, in: The Annual Meeting of the Society for Neuroscience The Annual Meeting of the Society for Neuroscience, San Diego, CA. [Google Scholar]
- Murillo-Rodríguez E, Arias-Carrión O, Sanguino-Rodríguez K, González-Arias M, Haro R, 2009. Mechanisms of sleep-wake cycle modulation. CNS Neurol. Disord. - Drug Targets 8, 245–253. 10.2174/187152709788921654 [DOI] [PubMed] [Google Scholar]
- Murphy M, Bruno MA, Riedner BA, Boveroux P, Noirhomme Q, Landsness EC, Brichant JF, Phillips C, Massimini M, Laureys S, Tononi G, Boly M, 2011. Propofol anesthesia and sleep: A high-density EEG study. Sleep 34, 283–291. 10.1093/sleep/34.3.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM, 2016. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science (80-. ). 354, 1004–1008. 10.1126/science.aah4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Xiong DD, Holtzman DM, 2015. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med 10.1038/emm.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimović D, Post B, Speelman JD, De Haan RJ, Schmand B, 2009. Cognitive decline in Parkinson’s disease: A prospective longitudinal study. J. Int. Neuropsychol. Soc 15, 426–437. 10.1017/S1355617709090614 [DOI] [PubMed] [Google Scholar]
- Naganawa S, Nakane T, Kawai H, Taoka T, 2017. Gd-based contrast enhancement of the perivascular spaces in the basal ganglia. Magn. Reson. Med. Sci 16, 61–65. 10.2463/mrms.mp.2016-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Rodnitzky RL, Uc EY, 2013. Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev. Neurosci 24, 267–278. 10.1515/revneuro-2012-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, 2013. Garbage truck of the brain. Science (80-. ). 340, 1529–1530. 10.1126/science.1240514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono D, Honma K. ichi, Yanagawa Y, Yamanaka A, Honma S, 2018. Role of GABA in the regulation of the central circadian clock of the suprachiasmatic nucleus. J. Physiol. Sci 68, 333–343. 10.1007/s12576-018-0604-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu S. -e., Lim J, Wohlleber ME, Ducca EL, Koushyk V, Glodzik L, Mosconi L, Ayappa I, Rapoport DM, de Leon MJ, Weiner MW, Aisen P, Petersen R, Jack C, Jagust W, Morris JC, Saykin AJ, Trojanowski JQ, Toga AW, Beckett L, 2015. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 84, 1964–1971. 10.1212/wnl.0000000000001566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, 2004. Cognitive dysfunction in Parkinson’s disease: The role of frontostriatal circuitry. Neurosci. 10, 525–37. 10.1177/1073858404266776 [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay S, Takahashi JS, Hogenesch JB, 2002. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320. [DOI] [PubMed] [Google Scholar]
- Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, Regan S, Kasper T, Peng S, Ding F, Benveniste H, Nedergaard M, Deane R, 2016. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis 93, 215–225. https://doi.Org/10.1016/j.nbd.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter-Derex L, Yammine P, Bastuji H, Croisile B, 2015. Sleep and Alzheimer’s disease. Sleep Med. Rev. 10.1016/j.smrv.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Poncelet BP, Wedeen VJ, Weisskoff RM, Cohen MS, 1992. Brain parenchyma motion: Measurement with cine echo-planar MR imaging. Radiology 185, 645–651. 10.1148/radiology.185.3.1438740 [DOI] [PubMed] [Google Scholar]
- Purdon PL, Weisskoff RM, 1998. Effect of temporal autocorrelation due to physiological noise and stimulus paradigm on voxel-level false-positive rates in fMRI. Hum. Brain Mapp. 6, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner V, Gao Y, Lee H, Elkin R, Nedergaard M, Benveniste H, Tannenbaum A, 2017. Cerebrospinal and interstitial fluid transport via the glymphatic pathway modeled by optimal mass transport. Neuroimage 152, 530–537. 10.1016/j.neuroimage.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve A, Simcox E, Turnbull D, 2014. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 14, 19–30. 10.1016/j.arr.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries M, Sastre M, 2016. Mechanisms of Aβ clearance and degradation by glial cells. Front. Aging Neurosci. 8, 160 10.3389/fnagi.2016.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Portin R, Ruottinen H, Nurmi E, Bergman J, Haaparanta M, Solin O, 2000. Cognitive impairment and the brain dopaminergic system in Parkinson disease. Arch. Neurol 57, 470 10.1001/archneur.57.4.470 [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Bimbaum S, Vitatema MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, McClung CA, 2007. Mania-like behavior induced by disruption of CLOCK. Proc. Natl. Acad. Sci 104, 6406–6411. 10.1073/pnas.0609625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye DB, 2004. Parkinson’s disease and RLS: The dopaminergic bridge. Sleep Med. 5, 317–328. 10.1016/j.sleep.2004.01.016 [DOI] [PubMed] [Google Scholar]
- Schenck CH, Boeve BF, Mahowald MW, 2013. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: A 16-year update on a previously reported series. Sleep Med. 14, 744–748. 10.1016/j.sleep.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH, 2010. Circadian clock gene Bmal1 is not essential; Functional replacement with its paralog, Bmal2. Curr. Biol 20, 316–321. 10.1016/j.cub.2009.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderowf A, Stern M, 2003. Update on Parkinson disease. Ann. Intern. Med 138, 651–658. 10.7326/0003-4819-138-8-200304150-00013 [DOI] [PubMed] [Google Scholar]
- Slynko IF, Nekhlopochin AS, 2018. Cerebrospinal fluid flow. Part 1. Classical theory and its evolution. Ukr. Neurosurg. J 0, 15–23. 10.25305/unj.134714 [DOI] [Google Scholar]
- Smith AJ, Yao X, Dix JA, Jin B-J, Verkman AS, 2017. Test of the “glymphatic” hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6 10.7554/elife.27679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavitsky K, Neargarder S, Bogdanova Y, McNamara P, Cronin-Golomb A, 2012. The impact of sleep quality on cognitive functioning in Parkinson’s disease. J. Int. Neuropsychol. Soc 18, 108–17. 10.1017/S1355617711001482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis L, 2012. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2, a009399 10.1101/cshperspect.a009399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner WJ, 2006. Practice parameter: Diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 66, 968–975. 10.1212/01.wnl.0000215437.80053.d0 [DOI] [PubMed] [Google Scholar]
- Tanner CM, Aston DA, 2000. Epidemiology of Parkinson’s disease and akinetic syndromes. Curr. Opin. Neurol 12, 427–430. 10.1097/00019052-200008000-00010 [DOI] [PubMed] [Google Scholar]
- Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, Kishimoto T, Naganawa S, 2017. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn. J. Radiol 35, 172–178. 10.1007/s11604-017-0617-z [DOI] [PubMed] [Google Scholar]
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Menard J, Zetterberg FL, Wisniewski T, de Leon MJ, 2015. Clearance systems in the brain—implications for Alzheimer disease. Nat. Rev. Neurol 11, 457–470. 10.1038/nrneurol.2015.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, 2002. Toxic proteins in neurodegenerative disease. Science (80-. ). 296, 1991–1995. 10.1126/science.1067122 [DOI] [PubMed] [Google Scholar]
- Thanvi BR, Munshi SK, Vijaykumar N, Lo TCN, 2003. Neuropsychiatric non-motor aspects of Parkinson’s disease. Post Grad. Med. J. 79, 561–565. 10.1136/pmj.79.936.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Sadeghi N, Nayak A, Trefler A, Sarils J, Baker CI, Pierpaoli C, 2018. Impact of time-of-day on diffusivity measures of brain tissue derived from diffusion tensor imaging. Neuroimage 173, 25–34. 10.1016/j.neuroimage.2018.02.026 [DOI] [PubMed] [Google Scholar]
- Turek FW, Dugovic C, Zee PC, 2001. Current understanding of the circadian clock and the clinical implications fo … Arch. Neurol 58, 1781–1787. 10.1001/archneur.58.11.1781 [DOI] [PubMed] [Google Scholar]
- Van Dijk KD, Bidinosti M, Weiss A, Raijmakers P, Berendse HW, van de Berg WDJ, 2014. Reduced α-synuclein levels in cerebrospinal fluid in Parkinson’s disease are unrelated to clinical and imaging measures of disease severity. Eur. J. Neurol 21, 388–394. 10.1111/ene.12176 [DOI] [PubMed] [Google Scholar]
- Vendette M, Gagnon J-F, Décary A, Massicotte-Marquez J, Postuma RB, Doyon J, Panisset M, Montplaisir J, 2007. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology 69, 1843–1849. 10.1212/01.wnl.0000278114.14096.74 [DOI] [PubMed] [Google Scholar]
- Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, Middelkoop HAM, van Hilten JJ, 2007. Cognitive impairment in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 78, 1182–1187. 10.1136/jnnp.2006.112367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheggen ICM, Van Boxtel MPJ, Verhey FRJ, Jansen JFA, Backes WH, 2018. Interaction between blood-brain barrier and glymphatic system in solute clearance. Neurosci. Biobehav. Rev 90, 26–33. 10.1016/J.NEUBIOREV.2018.03.028 [DOI] [PubMed] [Google Scholar]
- Verwey M, Dhir S, Amir S, 2016. Circadian influences on dopamine circuits of the brain: Regulation of striatal rhythms of clock gene expression and implications for psychopathology and disease. F1000Research 5, 2062 10.12688/f1000research.9180.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A, Golombek D, 2017. Circadian dysregulation in Parkinson’s disease. Neurobiol. Sleep Circadian Rhythm. 2, 53–58. 10.1016/J.NBSCR.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, Petersen NC, Nedergaard M, 2018. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci. Lett 662, 253–258. 10.1016/j.neulet.2017.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamori T, Agari T, Yasuhara T, Kameda M, Kondo A, Shinko A, Sasada S, Sasaki T, Furuta T, Date I, 2014. Cognitive functions in Parkinson’s disease: Relation to diseaseseverity and hallucination. Parkinsonism Relat. Disord. 20, 415–20. 10.1016/j.parkreldis.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Westerink B, Kwint H, 1996. The pharmacology of mesolimbic dopamine neurons: a dualprobe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J. Neurosci 16, 2605–2611. 10.1523/jneurosci.16-08-02605.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, Brown S, O’Kane CJ, Rubinsztein DC, 2010. α-Synuclein impairs macroautophagy: Implications for Parkinson’s disease. J. Cell Biol. 190, 1023–1037. 10.1083/jcb.201003122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M, 2013. Sleep drives metabolite clearance from the adult brain. Science (80-. ). 342, 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, Cai Z, Wu T, Hu G, Xiao M, 2015. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Αβ accumulation and memory deficits. Mol. Neurodegener 10, 58 10.1186/s13024-015-0056-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Cirrito JR, Stewart FR, Jiang H, Finn MB, Holmes BB, Binder LI, Mandelkow E-M, Diamond MI, Lee VM-Y, Holtzman DM, 2011. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J. Neurosci 31, 13110–13117. 10.1523/jneurosci.2569-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, Benveniste H, Iliff JJ, Nedergaard M, 2013. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J. Transl. Med 11, 107 10.1186/1479-5876-11-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P, 2006. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc. Natl. Acad. Sci 103, 6386–6391. 10.1073/pnas.0510691103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yulug B, Hanoglu L, Kilic E, 2017. Does sleep disturbance affect the amyloid clearance mechanisms in Alzheimer’s disease? Psychiatry Clin. Neurosci. 71, 673–677. 10.1111/pcn.12539 [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang B, Sun H, Zhou Y, Liu M, … D.-N J, 2016, U., 2016. Aquaporin-4 deficiency diminishes the differential degeneration of midbrain dopaminergic neurons in experimental Parkinson’s disease. Elsevier; 614, 7–15. [DOI] [PubMed] [Google Scholar]


