Key Points
Question
What is the long-term survival associated with tamoxifen therapy for postmenopausal patients with luminal A or luminal B subtype tumors?
Findings
This secondary analysis of the Stockholm Tamoxifen (STO-3) trial of 462 postmenopausal patients with lymph node-negative breast cancer found that patients with luminal A or luminal B tumor subtypes had a long-term risk of distant metastatic breast cancer and benefited from tamoxifen therapy for 15 years and 5 years after diagnosis, respectively.
Meaning
Patients with luminal A tumor subtype appeared to have a long-term benefit from tamoxifen therapy, and patients with luminal B subtype appeared to have an early benefit from therapy, when the risk of distant metastatic disease was high.
This secondary analysis of the Stockholm Tamoxifen (STO-3) clinical trial, which was conducted from 1976 to 1990, assessed the long-term survival associated with tamoxifen therapy in postmenopausal patients with luminal A or B breast cancer tumor subtypes.
Abstract
Importance
Patients with estrogen receptor (ER)–positive breast cancer have a long-term risk for fatal disease. However, the tumor biological factors that influence the long-term risk and the benefit associated with endocrine therapy are not well understood.
Objective
To compare the long-term survival from tamoxifen therapy for patients with luminal A or luminal B tumor subtype.
Design, Setting, and Participants
Secondary analysis of patients from the Stockholm Tamoxifen (STO-3) trial conducted from 1976 to 1990, which randomized postmenopausal patients with lymph node–negative breast cancer to receive adjuvant tamoxifen or no endocrine therapy. Tumor tissue sections were assessed in 2014 using immunohistochemistry and Agilent microarrays. Only patients with luminal A or B subtype tumors were evaluated. Complete long-term follow-up data up to the end of the STO-3 trial on December 31, 2012, were obtained from the Swedish National registers. Data analysis for the secondary analysis was conducted in 2017 and 2018.
Interventions
Patients were randomized to receive at least 2 years of tamoxifen therapy or no endocrine therapy; patients without recurrence who reconsented were further randomized to 3 additional years of tamoxifen therapy or no endocrine therapy.
Main Outcomes and Measures
Distant recurrence-free interval (DRFI) by luminal A and luminal B subtype and trial arm was assessed by Kaplan-Meier analyses and time-dependent flexible parametric models to estimate time-varying hazard ratios (HRs) that were adjusted for patient and tumor characteristics.
Results
In the STO-3 treated trial arm, 183 patients had luminal A tumors and 64 patients had luminal B tumors. In the untreated arm, 153 patients had luminal A tumors and 62 had luminal B tumors. Age at diagnosis ranged from 45 to 73 years. A statistically significant difference in DRFI by trial arm was observed (log rank, P < .001 [luminal A subtype, n = 336], P = .04 [luminal B subtype, n = 126]): the 25-year DRFI for luminal A vs luminal B subtypes was 87% (95% CI, 82%-93%) vs 67% (95% CI, 56%-82%) for treated patients, and 70% (95% CI, 62%-79%) vs 54% (95% CI, 42%-70%) for untreated patients, respectively. Patients with luminal A tumors significantly benefited from tamoxifen therapy for 15 years after diagnosis (HR, 0.57; 95% CI, 0.35-0.94), and those with luminal B tumors benefited from tamoxifen therapy for 5 years (HR, 0.38; 95% CI, 0.24-0.59).
Conclusions and Relevance
Patients with luminal A subtype tumors had a long-term risk of distant metastatic disease, which was reduced by tamoxifen treatment, whereas patients with luminal B tumors had an early risk of distant metastatic disease, and tamoxifen benefit attenuated over time.
Introduction
Breast tumors can be classified into 5 intrinsic subtypes as measured by mRNA expression,1 with most estrogen receptor (ER)–positive tumors classified as luminal A or luminal B subtype. In general, luminal A tumors have higher ER-expression and lower tumor cell proliferation than luminal B tumors.2,3 Approximately 1 in 4 patients with ER-positive breast cancer will develop distant metastasis and eventually die from the disease,4,5 and it is currently not possible to accurately predict a patient’s long-term risk or the benefit of endocrine therapy.
It has been suggested that patients with ER-positive breast cancer have a long-term risk for fatal breast cancer,5 but little is known about the tumor biological factors that influence this long-term risk. Therefore, we investigated the long-term survival and benefit of endocrine therapy (tamoxifen) for patients with luminal A or luminal B tumor subtype using data from the Stockholm Tamoxifen (STO-3) trial, including complete long-term follow-up of patients randomized to receive adjuvant tamoxifen vs no endocrine therapy.
Methods
The STO-3 Trial
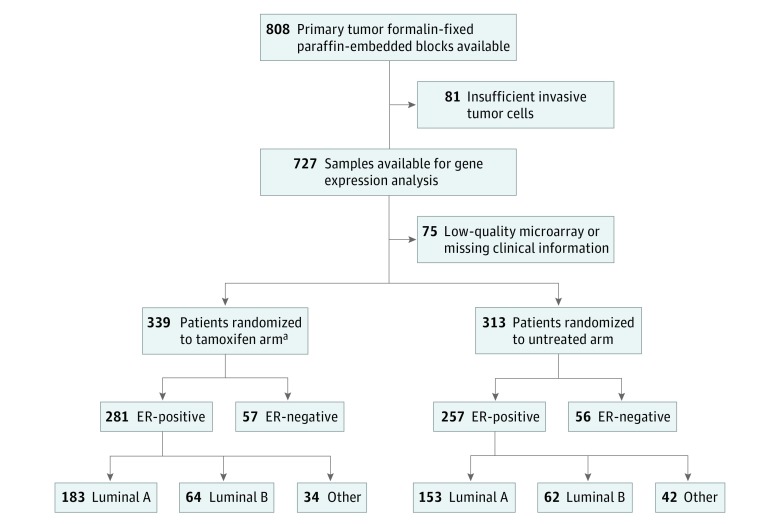
The Stockholm breast cancer study group conducted randomized trials from 1976 until 1990.6,7 The STO-3 trial enrolled postmenopausal patients with lymph node–negative breast cancer with tumors less than or equal to 30 mm in diameter. Patients were randomized to receive adjuvant tamoxifen (40 mg daily) for 2 years vs no adjuvant treatment (Figure 1). No other adjuvant therapy was given. In 1983, patients who reconsented and were relapse-free after 2 years of tamoxifen treatment were randomized to 3 additional years of tamoxifen therapy or no further therapy (see the eMethods in the Supplement).
Figure 1. Consort Diagram for the Stockholm Tamoxifen (STO-3) Trial.
aEstrogen receptor (ER) status was missing for 1 patient.
All residents in Sweden have a unique national registration number that enables automatic linkage of various records of personal information from high-quality national and regional registers and essentially complete coverage. Cancer registration has a legal basis in Sweden, and the Swedish Cancer Registry covers more than 96% of breast cancer cases according to validation studies.8 Information on metastatic disease was obtained from the regional Stockholm Breast Cancer Quality Registry as held by the Regional Cancer Center in Stockholm.9 Detailed patient and clinical information and complete long-term follow-up until December 31, 2012 was available for all patients included in the STO-3 randomized trial.
The STO-3 trial, which was conducted at the Regional Cancer Center Stockholm-Gotland in Stockholm, began in 1976, well before trial registration started in Sweden; therefore, information on trial number is not available. The STO-3 trial was approved by the ethical vetting board at Karolinska Institutet. At the time when the trial was approved and started, written consent was not considered ethically acceptable by the ethical boards in Sweden because it was thought to disturb the trust of the patient-doctor relationship; thus, only oral consent was accepted, and patients were orally informed.
ER, PR, ERBB2, and Ki-67 Immunohistochemistry
In 2014, formalin-fixed paraffin-embedded tumor tissue sections from 727 STO-3 trial participants underwent immunohistochemistry (IHC) analysis at the University of California Davis Medical Center laboratory to detect ER, progesterone receptor (PR), ERBB2 (formerly human epidermal growth factor receptor 2), and Ki-67. Gene expression in tissue sections from 652 patients was measured using Agilent microarrays. Additional details are described in the eMethods in the Supplement. Breast cancer pathologists at the University of California, who were part of the ATHENA Breast Health Network, scored the percentage of cancer cells positive for ER, PR, ERBB2, and Ki-67 (on whole-tumor sections with microscopes). A threshold of 10% or greater was used to define ER and PR positivity (according to the Swedish national guidelines), ERBB2 positivity was defined as a score of ≥3 on immunohistochemistry, and the Ki-67 threshold for positivity was 15% or greater.10
Tumor Grade and Intrinsic Subtypes
Tumor grade was retrospectively assessed according to the Nottingham system according to Elston-Ellis grading method.11,12 Tumors were assigned to 1 of 5 molecular subtypes (luminal A, luminal B, ERBB2-enriched, basal-like, normal-like) using the PAM50 gene expression classification as described by Parker et al13 (see the e Methods in the Supplement for details).
Statistical Analyses
Analyses of long-term (25 years) distant recurrence-free interval (DRFI) were performed for luminal A and luminal B tumor subtypes by STO-3 trial arm, according to the definition by Hudis et al.14 The event was distant breast cancer recurrence. Only patients with ER-positive breast cancer were included in the analyses because we wanted to investigate the benefit of endocrine therapy. Patient follow-up started at the date of primary breast cancer diagnosis and ended at the date of distant metastatic disease, death, emigration from Sweden (only 5 women emigrated), or the end of study follow-up (December 31, 2012).
Kaplan-Meier analyses were performed for patients with luminal A or luminal B subtype by STO-3 trial arm (treated vs untreated). Statistical significance was assessed using the log-rank test.
Time-varying analyses were performed using flexible parametric models, where the logarithm of the baseline hazard function was modeled as a natural cubic spline function of log time.15,16 We modeled this relationship using a 2 degrees-of-freedom spline, adjusting for the classical patient and tumor characteristics, namely age and calendar period of breast cancer diagnosis, PR status, Ki-67 status, tumor grade, and tumor size. We allowed for the effects of both tamoxifen and luminal subtype to change over the follow-up time (nonproportional hazards) by including interaction with time, using natural cubic splines (with 1 degree of freedom). The time scale in all analyses was the number of years since breast cancer diagnosis. For model selection, we assessed the goodness of fit using the Akaike Information Criterion (AIC) and included a penalty for the number of estimated parameters.
Data analysis was done from 2017 to 2018 using SAS version 9.4 (SAS Institute). Survival analyses and visual representations were performed using R version 3.4.3, including the survival and survminer packages17 (see the eAppendix in the Supplement). The flexible parametric survival modeling was performed using the R package rstpm2.16,18 All statistical tests were 2-sided, and P < .05 was considered statistically significant.
Results
In the STO-3 treated trial arm, 183 patients had luminal A tumors and 64 patients had luminal B tumors. In the untreated arm, 153 patients had luminal A tumors and 62 had luminal B tumors. Age at diagnosis ranged from 45 to 73 years. The eResults and eTable 1 in the Supplement outline the patient and tumor characteristics in the STO-3 trial. Patient characteristics such as age and calendar period of primary breast cancer diagnosis and trial arm distribution did not differ significantly between patients with luminal A and luminal B subtype. However as would be expected, most tumor characteristics were significantly different when contrasting luminal A and luminal B tumors.
Kaplan-Meier Analysis of DRFI Associated With Tamoxifen Therapy
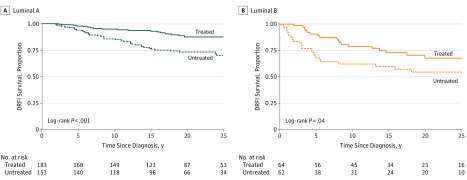
Kaplan-Meier analyses by trial arm were performed for patients with luminal A or luminal B tumors (Figure 2). A statistically significant difference in DRFI (25 years) by trial arm was seen in both luminal A (log rank, P < .001) and luminal B (log rank, P = .04) subtypes. The DRFI for luminal A vs B subtype was 87% (95% CI, 82%-93%) vs 67% (95% CI, 56%-82%) for treated patients, and 70% (95% CI, 62%-79%) vs 54% (95% CI, 42%-70%) for untreated patients, respectively. For patients with luminal A subtype in both the tamoxifen treated and untreated arm, a risk for distant metastasis was observed throughout 25 years of follow-up (Figure 2A). The long-term risk was also seen for patients with luminal B tumors treated with tamoxifen, in contrast to untreated patients with luminal B tumors whose level of risk was high during the first 5 years and then declined (Figure 2B).
Figure 2. Kaplan-Meier Plots of Distant Recurrence-Free Interval .
Kaplan-Meier analyses of distant recurrence-free survival interval by Stockholm Tamoxifen (STO-3) trial arm (tamoxifen treated vs untreated arm) for patients with (A) luminal A and (B) luminal B tumor subtypes. The P value is based on a 2-sided log-rank test. DRFI indicates distant recurrence-free interval.
Time-Varying Multivariable Analysis of Long-term Distant Recurrence-Free Survival Associated With Tamoxifen
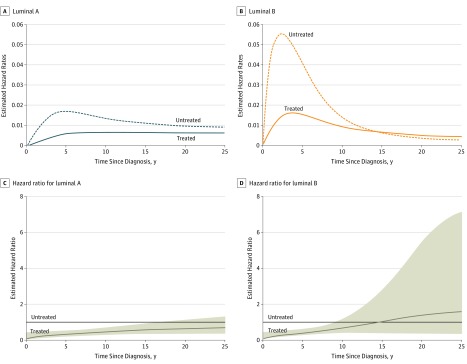
We used flexible parametric modeling to perform a time-varying analysis of the long-term benefit of tamoxifen therapy for patients with luminal A or luminal B tumors, adjusting for classic patient and tumor characteristics (age, calendar period of breast cancer diagnosis, PR status, Ki-67 status, tumor grade, and tumor size) in addition to crude estimates (only adjusting for age and calendar period of breast cancer diagnosis). Crude and adjusted analysis yielded similar results (eTable 2 in the Supplement). Consistent with the Kaplan-Meier analysis, a risk of distant recurrence was observed throughout 25 years of follow-up in patients with luminal A tumors (Figure 3). The estimated hazard rate for patients with luminal A tumors reached a peak hazard rate at around 5 years after diagnosis and later stabilized throughout the 25-year follow-up (Figure 3A). Furthermore, the findings from the time-varying hazard ratio (HR) analysis (Figure 3C; eTable 2 in the Supplement) suggested that patients with luminal A subtype in the treated arm had a significant benefit from tamoxifen for 15 years (HR at 15 years, 0.57; 95% CI, 0.35-0.94) compared with patients in the untreated arm (see eTable 2 in the Supplement).
Figure 3. Estimated Time-Dependent Hazard Rates and Relative Hazard Ratios.
Estimated time-dependent hazard rates of distant recurrence for treated vs untreated patients with luminal A (A) or luminal B (B) subtypes. Estimated hazard ratio by tamoxifen treated vs untreated trial arm for patients with luminal A (C) or luminal B (D) subtypes. The untreated group is the reference. Shading indicates the 95% CI.
Treated patients with luminal B tumors had a lower early risk compared with that of untreated luminal B patients; however, the ongoing risk (estimated hazard rate) of distant recurrence for treated and untreated patients with luminal B tumors converged after 10 to 15 years (Figure 3B). Untreated patients with luminal B tumors had a high early risk during the first 5 to 10 years after diagnosis, but the risk declined steeply and reached the risk level of untreated patients with luminal A tumors approximately 10 years after diagnosis (Figure 3B). Treated patients with luminal B tumors had a risk of distant metastasis similar to that of untreated patients with luminal A tumors, which peaked at around 5 years after diagnosis, and remained the same throughout the 25-year follow-up. Finally, findings from the time-varying HR analysis suggested that patients in the treated arm with luminal B subtype benefited from tamoxifen (Figure 3D; eTable 2 in the Supplement) for 5 years (HR at 5 years, 0.38; 95% CI, 0.24-0.59) compared with patients in the untreated arm (see eTable 2 in the Supplement).
Discussion
In this study, we used data from the STO-3 trial of patients randomized to receive adjuvant tamoxifen vs no endocrine therapy to investigate long-term survival and benefit associated with tamoxifen therapy for patients with lymph node–negative, ER-positive, luminal A or luminal B subtype tumors. To our knowledge, this study is the first to show that patients with luminal A subtype tumors had a risk of distant metastatic disease throughout a 25-year follow-up period, and the findings from the time-varying analysis suggested that patients in the treated arm had a long-term benefit from tamoxifen therapy that attenuated by 15 years. In addition, for untreated patients with luminal B tumors, less than half experienced the risk of distant metastatic disease in the first 5 years after diagnosis, and the risk stabilized and precipitously declined 5 years after diagnosis. Interestingly, patients with luminal B subtype in the treated arm had a risk of distant metastatic disease throughout the follow-up period, similar to untreated patients with luminal A subtype. The findings from the time-varying analysis also suggested that benefit from tamoxifen in patients with luminal B subtype was greatest in the first 5 years and then attenuated; by 10 years, the risk in treated and untreated patients was the same.
The long-term benefit of tamoxifen therapy for patients with luminal A or luminal B subtype tumors has been unknown up to now. However, it has been suggested that patients with luminal B tumor subtype may benefit less from endocrine therapy compared with patients with luminal A subtype,2 supporting our current findings of long-term benefit for patients with luminal A subtype and short-term benefit for patients with luminal B subtype. There is a biological rationale for the suggested differences in benefit. For instance, luminal A tumors in general have lower proliferation rates and are more likely to be purely ER-driven; therefore, patients with this tumor subtype can potentially benefit longer from tamoxifen therapy.19,20 In contrast, luminal B tumors often have higher proliferation rates that, in addition to the ER-signaling pathway, may be driven by multiple active oncogenic pathways; therefore, these tumors might be less amendable to endocrine therapy.1,19,20,21,22 Patients with luminal A or luminal B subtype tumors should be offered adjuvant endocrine therapy. In patients with luminal B subtype, up-front chemotherapy should be discussed and endocrine therapy potentially extended for up to 10 years, particularly in those in the higher risk strata according to other tumor characteristics.
Limitations
The present secondary analysis of the STO-3 trial assessed long-term survival in the trial cohort from 1976 until 1990. There are several limitations to our study. As with most long-term follow up studies, clinical recommendations for disease management and treatment have changed since the initiation of the trial. The STO-3 trial was performed before aromatase inhibitors became one of the recommended treatment options for ER-positive breast cancer and when the duration of tamoxifen treatment was shorter than current recommendations. In this population-based trial of patients with lymph-node negative breast cancer, the number of patients with luminal B subtype tumors was lower than the number of patients with luminal A tumors due to tumor biology; therefore, analyses of patients with luminal B subtype are limited in statistical power. In addition, for patients with luminal B tumors, current treatment recommendations generally include chemotherapy.
Conclusions
We conclude that tamoxifen appears to confer a long-term benefit for patients with lymph node–negative, ER-positive, luminal A subtype tumors, and a short-term benefit for patients with luminal B subtype tumors. Given that the risk of distant metastatic disease is low for patients with the luminal A subtype but persists in the long term, whereas the risk for patients with luminal B subtype is higher initially but decreases after 5 years, tamoxifen treatment is beneficial for patients with luminal A or luminal B subtype tumors.
eMethods.
eResults.
eAppendix. R Program Log File.
eReferences.
eTable 1. Patient and tumor characteristics by Luminal A and Luminal B subtypes as identified by PAM50 gene expression analysis
eTable 2. Time-dependent relative hazard ratio by trial arm (tamoxifen treated versus untreated) for luminal A and luminal B subtype using flexible parametric survival models
References
- 1.Sørlie T, Tibshirani R, Parker J, et al. . Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100(14):8418-8423. doi: 10.1073/pnas.0932692100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ades F, Zardavas D, Bozovic-Spasojevic I, et al. . Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. J Clin Oncol. 2014;32(25):2794-2803. doi: 10.1200/JCO.2013.54.1870 [DOI] [PubMed] [Google Scholar]
- 3.Harbeck N, Thomssen C, Gnant M. St. Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care (Basel). 2013;8(2):102-109. doi: 10.1159/000351193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colleoni M, Sun Z, Price KN, et al. . Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group trials I to V. J Clin Oncol. 2016;34(9):927-935. doi: 10.1200/JCO.2015.62.3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan H, Gray R, Braybrooke J, et al. ; EBCTCG . 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 Years. N Engl J Med. 2017;377(19):1836-1846. doi: 10.1056/NEJMoa1701830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fornander T, Rutqvist LE, Cedermark B, et al. . Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet. 1989;1(8630):117-120. doi: 10.1016/S0140-6736(89)91141-0 [DOI] [PubMed] [Google Scholar]
- 7.Rutqvist LE, Johansson H; Stockholm Breast Cancer Study Group . Long-term follow-up of the randomized Stockholm trial on adjuvant tamoxifen among postmenopausal patients with early stage breast cancer. Acta Oncol. 2007;46(2):133-145. doi: 10.1080/02841860601034834 [DOI] [PubMed] [Google Scholar]
- 8.Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27-33. doi: 10.1080/02841860802247664 [DOI] [PubMed] [Google Scholar]
- 9.Emilsson L, Lindahl B, Köster M, Lambe M, Ludvigsson JF. Review of 103 Swedish healthcare quality registries. J Intern Med. 2015;277(1):94-136. doi: 10.1111/joim.12303 [DOI] [PubMed] [Google Scholar]
- 10.Lindström LS, Yau C, Czene K, et al. ; STO Trialists Group . Intratumor heterogeneity of the estrogen receptor and the long-term risk of fatal breast cancer. J Natl Cancer Inst. 2018;110(7):726-733. doi: 10.1093/jnci/djx270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerevall PL, Ma XJ, Li H, et al. . Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer. 2011;104(11):1762-1769. doi: 10.1038/bjc.2011.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. the value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403-410. doi: 10.1111/j.1365-2559.1991.tb00229.x [DOI] [PubMed] [Google Scholar]
- 13.Parker JS, Mullins M, Cheang MC, et al. . Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160-1167. doi: 10.1200/JCO.2008.18.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudis CA, Barlow WE, Costantino JP, et al. . Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127-2132. doi: 10.1200/JCO.2006.10.3523 [DOI] [PubMed] [Google Scholar]
- 15.Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9(2):265-290. doi: 10.1177/1536867X0900900206 [DOI] [Google Scholar]
- 16.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175-2197. doi: 10.1002/sim.1203 [DOI] [PubMed] [Google Scholar]
- 17.Therneau TM. Survminer packages in R statistical program [computer program]. 2015.
- 18.Clements M, Xing-Rong L Rstpm2 package: generalized survival models in R statistical program [computer program]. 2017.
- 19.Prat A, Cheang MCU, Martín M, et al. . Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31(2):203-209. doi: 10.1200/JCO.2012.43.4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sørlie T, Perou CM, Tibshirani R, et al. . Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869-10874. doi: 10.1073/pnas.191367098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23(29):7350-7360. doi: 10.1200/JCO.2005.03.3845 [DOI] [PubMed] [Google Scholar]
- 22.Perou CM, Jeffrey SS, van de Rijn M, et al. . Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci U S A. 1999;96(16):9212-9217. doi: 10.1073/pnas.96.16.9212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eResults.
eAppendix. R Program Log File.
eReferences.
eTable 1. Patient and tumor characteristics by Luminal A and Luminal B subtypes as identified by PAM50 gene expression analysis
eTable 2. Time-dependent relative hazard ratio by trial arm (tamoxifen treated versus untreated) for luminal A and luminal B subtype using flexible parametric survival models