Abstract
Background
Chronic kidney disease (CKD) is associated with high morbidity and death, which increases as CKD progresses to end‐stage kidney disease (ESKD). There has been increasing interest in developing innovative, effective and cost‐efficient methods to engage with patient populations and improve health behaviours and outcomes. Worldwide there has been a tremendous increase in the use of technologies, with increasing interest in using eHealth interventions to improve patient access to relevant health information, enhance the quality of healthcare and encourage the adoption of healthy behaviours.
Objectives
This review aims to evaluate the benefits and harms of using eHealth interventions to change health behaviours in people with CKD.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 14 January 2019 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs using an eHealth intervention to promote behaviour change in people with CKD were included. There were no restrictions on outcomes, language or publication type.
Data collection and analysis
Two authors independently assessed trial eligibility, extracted data and assessed the risk of bias. The certainty of the evidence was assessed using GRADE.
Main results
We included 43 studies with 6617 participants that evaluated the impact of an eHealth intervention in people with CKD. Included studies were heterogeneous in terms of eHealth modalities employed, type of intervention, CKD population studied and outcomes assessed. The majority of studies (39 studies) were conducted in an adult population, with 16 studies (37%) conducted in those on dialysis, 11 studies (26%) in the pre‐dialysis population, 15 studies (35%) in transplant recipients and 1 studies (2%) in transplant candidates We identified six different eHealth modalities including: Telehealth; mobile or tablet application; text or email messages; electronic monitors; internet/websites; and video or DVD. Three studies used a combination of eHealth interventions. Interventions were categorised into six types: educational; reminder systems; self‐monitoring; behavioural counselling; clinical decision‐aid; and mixed intervention types. We identified 98 outcomes, which were categorised into nine domains: blood pressure (9 studies); biochemical parameters (6 studies); clinical end‐points (16 studies); dietary intake (3 studies); quality of life (9 studies); medication adherence (10 studies); behaviour (7 studies); physical activity (1 study); and cost‐effectiveness (7 studies).
Only three outcomes could be meta‐analysed as there was substantial heterogeneity with respect to study population and eHealth modalities utilised. There was found to be a reduction in interdialytic weight gain of 0.13kg (4 studies, 335 participants: MD ‐0.13, 95% CI ‐0.28 to 0.01; I2 = 0%) and a reduction in dietary sodium intake of 197 mg/day (2 studies, 181 participants: MD ‐197, 95% CI ‐540.7 to 146.8; I2 = 0%). Both dietary sodium and fluid management outcomes were graded as being of low evidence due to high or unclear risk of bias and indirectness (interdialytic weight gain) and high or unclear risk of bias and imprecision (dietary sodium intake). Three studies reported death (2799 participants, 146 events), with 45 deaths/1000 cases compared to standard care of 61 deaths/1000 cases (RR 0.74, CI 0.53 to 1.03; P = 0.08). We are uncertain whether using eHealth interventions, in addition to usual care, impact on the number of deaths as the certainty of this evidence was graded as low due to high or unclear risk of bias, indirectness and imprecision.
Authors' conclusions
eHealth interventions may improve the management of dietary sodium intake and fluid management. However, overall these data suggest that current evidence for the use of eHealth interventions in the CKD population is of low quality, with uncertain effects due to methodological limitations and heterogeneity of eHealth modalities and intervention types. Our review has highlighted the need for robust, high quality research that reports a core (minimum) data set to enable meaningful evaluation of the literature.
Plain language summary
eHealth interventions for people with chronic kidney disease
What is the issue?
Chronic kidney disease (CKD) is a condition where kidneys have reduced function over a period of time. To remain well people with CKD need to follow complex diet, lifestyle and medication advice and often need to use several specialist medical services. Some people with advanced CKD may need dialysis or treatment with a kidney transplant. Enabling patients to manage this condition by themselves improves quality and length of life and reduces healthcare costs. Electronic health (eHealth) interventions may improve patients’ ability to look after themselves and improve care provided by healthcare services. eHealth interventions refer to "health services and information delivered or enhanced through the Internet and related technologies". However, there is little research evaluating the impact of eHealth interventions in CKD.
What did we do?
We focused on randomised controlled trials (RCT), which enrolled people with CKD (including pre‐dialysis, dialysis or kidney transplant), and compared eHealth interventions to usual care.
What did we find?
We found 43 studies involving 6617 people who had CKD that examined if eHealth interventions improve patient care and health outcomes. eHealth interventions used different modes of technology, such as Telehealth, electronic monitors, mobile or tablet applications, text message or emails, websites, and DVDs or videos. Interventions were classified by their intention: educational, reminder systems, self‐monitoring, behavioural counselling, clinical decision‐aids and mixed interventions. We categorised outcomes into nine domains: dietary intake, quality of life, blood pressure control, medication adherence, results of blood tests, cost‐analysis, behaviour, physical activity and clinical end‐points such as death. We found that it was uncertain whether using an eHealth interventions improved clinical and patient‐centred outcomes compared with usual care. The quality of the included studies was low, meaning we could not be sure that future studies would find similar results.
Conclusions
We are uncertain whether using eHealth interventions improves outcomes for people with CKD. We need large and good quality research studies to help understand the impact of eHealth on the health of people with CKD.
Summary of findings
Summary of findings for the main comparison. EHealth interventions compared to standard care in chronic kidney disease populations.
| EHealth interventions compared to standard care in chronic kidney disease populations | |||||
| Patient or population: chronic kidney disease populations Setting: Intervention: eHealth interventions Comparison: standard care | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with standard care | Risk with eHealth interventions | ||||
| Mortality follow up: mean 12 months | Study population | RR 0.74 (0.53 to 1.03) | 2906 (3 RCTs) 4 | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| 61 per 1,000 | 45 per 1,000 (32 to 62) | ||||
| Interdialytic weight gain follow up: range 6 weeks to 16 weeks | MD 0.13 lower (0.27 lower to 0.01 higher) | ‐ | 335 (4 RCTs) 5 | ⊕⊕⊝⊝ LOW 1 2 | |
| Dietary sodium intake follow up: mean 4 months | MD 197 mg lower (540.7 lower to 146.8 higher) | ‐ | 181 (2 RCTs) 6 | ⊕⊕⊝⊝ LOW 1 3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level for uncertain or high risk of bias (allocation, blinding, outcome reporting, other biases)
2 Downgraded one level for inconsistency (different eHealth interventions used, different study lengths)
3 Downgraded one level for imprecision (small sample size or small number of events, confidence intervals overlap)
4 Behavioural counselling intervention (Ishani 2016), Clinical Decision Aid (Cooney 2015), Self‐monitoring and Education (Navaneethan 2017)
5 Behavioural counselling intervention (BalanceWise‐HD 2013), Self‐monitoring intervention (Schulz 2007, Welch 2013, Williams 2017)
6 Behavioural counselling intervention (BalanceWise‐HD 2013), Self‐monitoring intervention (Koprucki 2010)
Background
Description of the condition
Chronic Kidney Disease (CKD) is associated with high morbidity and death, which increases as CKD progresses to end‐stage kidney disease (ESKD). Complications of CKD include cardiovascular disease, premature death, cancer, cognitive decline, anaemia, bone and mineral disorders and bone fractures, and hospitalisation, all associated with high health care usage (Stevens 2013; Jha 2013). Enhancing patient engagement and self‐management are the cornerstones of optimal chronic disease management (Tong 2007). Self‐management programs can improve patient knowledge, health‐related quality of life, delay the need for dialysis, improve clinical outcomes (e.g. blood pressure), improve treatment adherence and improve survival (Bonner 2014; Chen 2011; Devins 2005). The prevention of CKD, and delaying its progression to ESKD, requires complex care because it involves both specific CKD management, as well as management of other prevalent co‐morbidities (Lopez‐Vargas 2014). Interventions should focus on effective, cost‐efficient methods to improve modifiable risk factors such as weight, blood glucose control, blood pressure (BP) control and poor dietary intake that can improve morbidity and death (Couser 2011).
Description of the intervention
With rates of CKD and renal replacement therapy rising, there is a need to find innovative and efficient ways to engage with people with CKD and improve health behaviours and outcomes. Worldwide there is a tremendous increase in the use of technologies with up to 94% of people in developed countries accessing the internet or owning a smartphone (Pew Research Center 2016). In healthcare there is increasing interest in utilising technology‐based interventions, commonly referred to as eHealth, to improve patient engagement and enhance care. eHealth refers to"health services and information delivered or enhanced through the Internet and related technologies" (Eysenbach 2001), with these interventions being used to replace standard care or used as an adjunct to standard care. There is a variety of different eHealth modalities reported in the literature, including: Telehealth, mobile phone (including text messaging and the use of applications on mobile phones), internet and computer, electronic monitors and wireless and Bluetooth enabled devices. Within these eHealth interventions there is wide use of these tools, which are categorised as patient self‐management interventions or clinician decision support tools.
With more people using technology, the development, adoption and implementation of eHealth holds tremendous promise to improve consumer access to relevant health information, enhance the quality of care and encourage the adoption of healthy behaviours. However, there is currently no published systematic review of data regarding the optimal type, intensity and duration of eHealth strategies to most effectively elicit knowledge and behaviour change. Additionally, there is currently no systematic review of data regarding the impact of eHealth interventions to improve patient‐centred and clinical outcomes in the CKD population.
How the intervention might work
There are promising outcomes of using eHealth interventions, when used in addition to traditional counselling techniques, for improving disease management in chronic disease populations. Systematic reviews evaluating the impact of various eHealth interventions compared to standard care report similar or improved results regarding glycaemic control (Kitsiou 2017), CVD clinical outcomes (e.g. hospitalisations, myocardial infarction, stroke) and CVD risk factors (e.g. body mass index, blood pressure, cholesterol) (Widmer 2015), weight loss maintenance (Sorgente 2017), dietary intake (Cotter 2014; Kelly 2016) and exercise behaviours (Cotter 2014). However, to date poor study methodologies and insufficient reporting limit the determination of mechanisms that have prompted behaviour change and resulted in the success or failure of interventions. (Kitsiou 2017; Widmer 2015). Further research is needed to ascertain the most effective eHealth intervention/s to promote behaviour change in different contexts and diseases. In addition, evaluation of the level of consumer personalisation, frequency of interaction and duration (e.g. number of weeks, months or years) of interventions is needed. Similar to traditional interventions (e.g. in‐person counselling, paper‐based education), eHealth interventions that are designed with a theoretical basis incorporating content that is adaptive to individuals’ needs and utilises interactive components such as self‐monitoring, personalised feedback, bidirectional communication and group or peer support may result in better clinical and patient‐centred outcomes (Cotter 2014; Kitsiou 2017; Widmer 2015). To date economic evaluations of eHealth interventions has been sparse and highlights an important area for further research (Kitsiou 2017; Sanyal 2018).
The use of eHealth interventions in chronic diseases, such as diabetes and CVD, have shown eHealth interventions can improve or provide similar outcomes to traditional interventions (Kitsiou 2017; Widmer 2015). Given the current literature showing positive trends for the use of eHealth in chronic disease management and health behaviour change, it is foreseeable that the CKD population will benefit from the use of eHealth interventions and further review of the literature in CKD is warranted.
Why it is important to do this review
It is important to conduct this review, as strategies for improving patient engagement and enhancing outcomes are vital to reduce morbidity and death associated with all stages of CKD. Additionally, eHealth holds much promise for enhancing the delivery of healthcare in CKD and it is vital to determine which strategies are effective at promoting behaviour change and improve outcomes in CKD.
Objectives
This review aimed to evaluate the benefits and harms of using eHealth interventions to change health behaviours in people with CKD.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alteration, use of alternate medical records, date of birth or other predictable methods) will be included.
Types of participants
Adults and children who have been diagnosed with CKD (i.e. pre‐dialysis, dialysis and kidney transplant recipients) were included.
Diagnosis of CKD is defined by estimated GFR (eGFR) < 60 mL/min or, eGFR < 90 mL/min with albuminuria or haematuria, for at least three months or as defined using other clinically indicated criteria.
Types of interventions
Any interventions that the authors report to be using eHealth technologies to promote behaviour change in CKD. eHealth technologies include:
Telephone and Telehealth
Mobile phone (including applications available on these devices)
Computers and tablets (including applications available on these devices)
Personal Digital Assistants
Internet (including e‐mail)
Electronic transmission (e.g. using technologies such as Bluetooth)
Social Media
Electronic decision support tools.
The comparisons were as follows.
eHealth intervention versus non‐eHealth intervention
eHealth intervention versus alternate eHealth intervention
eHealth intervention versus no intervention or usual care
Meta‐analyses were conducted by analysing similar interventions of the same classifications (e.g. educational versus reminder systems) together for analysis.
Types of outcome measures
Time intervals at which outcome assessment takes place may affect the effect of the intervention programs. We considered all time frames used by authors.
1. Clinical parameters
Electrolyte management (measured using biochemical measurements)
Kidney function (measured using eGFR and/or serum creatinine)
Fluid management (measured using interdialytic weight gain (IDWG))
Co‐morbidity management (measured using BP control, dyslipidaemia, HbA1c, fasting and random blood glucose readings, anthropometry)
Hospitalisation rates
Death (all causes)
2. Patient‐centred parameters
Dietary intake and behaviours (measured using self‐reported data and qualitative and quantitative surveys)
Physical activity behaviours (using validated tools, quantitative and qualitative surveys, self‐reported data)
Adherence to medications (using validated or self‐reported data)
Adherence to appointments (using validated or self‐reported data)
Quality of life (measured using global or disease‐specific validated tools)
Nutritional status (measured using validated tools)
Self‐management and self‐efficacy
Satisfaction with interventions.
3. Cost effectiveness
Incremental cost‐effectiveness ratios (defined as the cost per quality‐adjusted life year gained)
Cost per Disability Adjusted Life Years (DALY)
Costs associated with eHealth intervention.
4. Potential harms
Additional patient or health professional time associated with the use of eHealth intervention
Anxiety due to frequent monitoring
Accidents or accidental deaths associated with using the eHealth intervention (e.g. reading text message while driving).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 14 January 2019 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
We used the search strategy described to obtain titles and abstracts of studies relevant to the review. Two authors screened the titles and abstracts independently, studies that are not applicable were discarded. However, studies and reviews thought to include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts, and when necessary the full text, of these studies to determine studies that satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by the same authors using standard data extraction forms. Studies reported in non‐English language were translated before assessment. Where more than one publication of a study was found, only the publication with the most complete data was included, however when relevant outcomes were only published in earlier versions these data were used. Further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review. Disagreements were resolved in consultation with a third author.
Assessment of risk of bias in included studies
The following items were assessed independently by two authors using the risk of bias assessment tool (Higgins 2011) (seeAppendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. incidence of ESKD, death) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. quality of life, body weight), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales have been used, also reporting 95% confidence intervals (CI).
Unit of analysis issues
For studies with multiple treatment groups we combined all relevant experimental intervention groups of the study into a single group and combined all relevant control intervention groups into a single group to enable single pairwise comparison.
Dealing with missing data
Any further information required from the original authors was requested by email correspondence and relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population was carefully performed. Attrition rates, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) was critically appraised (Higgins 2011).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. Heterogeneity was then analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). A guide to the interpretation of I2 values was as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a confidence interval for I2) (Higgins 2011).
Assessment of reporting biases
Due to the small number of studies we were unable to assess for the existence of small study bias using funnel plots.
Data synthesis
We classified our studies by target of intervention (educational, reminder system, educational plus reminders, behavioural counselling, self‐monitoring and clinical decision aid). Treatment estimates for specified outcomes (those that were reported by two or more studies) were summarised within groups of intervention types and treatment effects were summarised using random‐effects meta‐analysis. The eHealth interventions and associated implementation strategies were described using the "Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide" (Hoffmann 2014) and tabulated in the review.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity. In our protocol we stated we would conduct subgroup analysis based on technology (e.g. mobile phone, internet). However, classifying interventions using technology type did not explain heterogeneity between interventions. Additionally, classification of studies by the World Health Organization's framework of interventions for clients (Appendix 3) did not provide sufficient subgroup differentiation as the majority of studies could be classified into two types of interventions: targeted communication to clients and personal health tracking. We determined that heterogeneity between eHealth interventions was better explained by the target of the intervention (e.g. educational versus self‐monitoring) and therefore we used these classifications when conducting subgroup analyses. There were insufficient extractable data to conduct subgroup and univariate meta‐regression analysis to explore the following variables as possible sources of heterogeneity: mean study age, mean proportion of men, adequacy of allocation concealment, sample size, and duration of follow up (< 12 months versus ≥ 12 months).
Sensitivity analysis
There were insufficient extractable data to perform the following sensitivity analyses in order to explore the influence of the following factors on effect size:
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' table includes an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b).
The key outcomes presented in the Summary of findings table 1 include:
Death
Fluid management
Dietary intake (sodium).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
We searched the Specialised Register and identified 132 records. After screening titles and abstracts and full‐text review, 43 studies (93 records) were included and nine studies (27 records) were excluded. Twelve ongoing studies were identified (CONNECT 2017; eNEPHRO 2017; Jung 2017; KARE 2015; Kosaka 2017; MAGIC 2016; NCT00394576; NCT02097550; NCT02610946; TELEGRAFT 2015; Waterman 2015; WISHED 2016), These 12 studies and will be assessed in a future update of this review (Figure 1).
1.
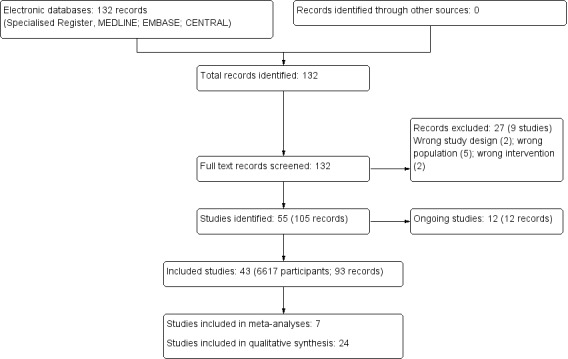
Study flow diagram.
Included studies
We included 43 studies (93 reports; 6617 participants) in this review. The included studies were conducted between 1999 and 2017, with the majority published since 2010 (38 of 43 studies, 88%). Nine studies (Durand 2000; Halleck 2017; Han 2016; Hardstaff 2002; Jammalamadaka 2015; Ong 2017; Potter 2016; SUBLIME 2016; White 2010) (23%) had only abstracts from conference proceedings or short reports available. All studies were published in English. The majority of studies were conducted in an adult population (39 studies), and the majority of studies were conducted in North America (26 studies). Eleven studies enrolled 4315 participants with pre‐dialysis CKD, 10 studies enrolled 681 participants on haemodialysis (HD), six studies enrolled 281 participants on peritoneal dialysis, 15 studies enrolled 1281 kidney transplant recipients, and one study enrolled 288 transplant candidates. Participant numbers ranged from 6 to 2199 (mean study population, 153; median study population, 75), with study durations varying from one clinic appointment to 24 months (median follow‐up period was 16 weeks). Most (20 studies) compared an eHealth intervention to usual care involving traditional methods (e.g. face‐to‐face counselling), 11 studies did not adequately describe the control group and 12 studies compared an active eHealth intervention to a passive, control eHealth intervention. Studies used various eHealth technologies including: Telehealth (e.g. phone calls, video monitoring, teleconferencing) (10 studies), mobile phone or tablet applications (11 studies), mobile phone text messaging or emails (2 studies), electronic monitors (11 studies), internet or website (4 studies), video or DVD (2 studies), or mixed methods, where more than one eHealth technology was used (3 studies). Table 2 provides an overview of the characteristics of included studies.
1. Overview of characteristics of included studies.
| Total studies (participants) | 43 (6617) | |
| No. studies | % studies | |
| Country | ||
| Australia | 1 | 2% |
| North America | 26 | 60% |
| UK | 5 | 12% |
| Europe | 6 | 14% |
| Middle East | 3 | 7% |
| Asia | 2 | 5% |
| Number of participants | ||
| 0‐50 | 17 | 40% |
| 51‐100 | 10 | 23% |
| 101‐200 | 10 | 23% |
| 201‐300 | 3 | 7% |
| 300+ | 3 | 7% |
| Length of intervention | ||
| ≤ 1 week | 4 | 9% |
| 1‐3 months | 16 | 37% |
| 4‐6 months | 9 | 21% |
| > 6 months | 13 | 30% |
| unclear | 1 | 2% |
| Participant age | ||
| Paediatric (including carers) | 4 | (9% |
| Adult (≥ 18 years) | 39 | (91% |
| Stage of CKD | ||
| CKD stage 1‐5 | 11 | 26% |
| Haemodialysis | 10 | 23% |
| Peritoneal dialysis | 6 | 14% |
| Transplant candidates | 1 | 2% |
| Transplant recipient | 15 | 35% |
| eHealth modality | ||
| Telehealth | 10 | 23% |
| Mobile or tablet app | 11 | 26% |
| Mobile phone text message | 2 | 5% |
| Electronic monitoring | 11 | 26% |
| Internet website | 4 | 9% |
| Video or DVD | 2 | 5% |
| Mixed methods | 3 | 7% |
| eHealth intervention category | ||
| Education | 4 | 9% |
| Reminders | 5 | 12% |
| Self‐monitoring | 9 | 21% |
| Behavioural counselling | 16 | 37% |
| Clinical decision‐aid Mixed interventions Unclear |
4 4 1 |
9% 9% 2% |
| Publication type | ||
| Abstract or short report | 10 | 23% |
| Journal article | 33 | 77% |
CKD ‐ chronic kidney disease
Our study classifications were as follows:
Educational (four studies: Baraz 2014; Diamantidis 2015; Giacoma 1999; InformMe 2017)
Reminders (6 studies: Halleck 2017; Han 2016; Henriksson 2016; Jammalamadaka 2015; McGillicuddy 2013; Potter 2016)
Self‐monitoring (9 studies: BALANCEWise‐HD 2011; BALANCEWise‐PD 2011; Koprucki 2010; Kullgren 2015; Ong 2017; Rifkin 2013; Schulz 2007; Welch 2013; Williams 2017)
Behavioural counselling (16 studies: BalanceWise‐HD 2013; BRIGHT 2013; Cargill 2003; iDiD 2016; Ishani 2016; Kargar Jahromi 2016; Li 2014b; MESMI 2010; Poorgholami 2016a; Reilly‐Spong 2015; Russell 2011; Schmid 2016; Swallow 2016; TAKE‐IT 2014; White 2010)
Clinical decision‐aids (4 studies: Cooney 2015; Durand 2000; Hardstaff 2002; iChoose 2016)
Mixed interventions (4 studies: Navaneethan 2017; Reese 2017; Robinson 2014a; Robinson 2015)
Of the 43 studies, seven studies reported outcome data used in quantitative analyses, while data from 24 studies could only be presented descriptively. Eleven studies could not be included in qualitative analyses due to insufficient reporting of outcome data (Cargill 2003; Diamantidis 2015; Giacoma 1999; Halleck 2017; Han 2016; Ong 2017; SUBLIME 2016; White 2010) or data was only being available for the intervention group (BALANCEWise‐HD 2011, BALANCEWise‐PD 2011; Swallow 2016). Reported outcomes were broadly categorised as:
Clinical parameters
Blood pressure control (9 studies)
Biochemical parameters (6 studies)
Clinical end‐points (16 studies)
Patient‐centred parameters
Dietary intake (3 studies)
Quality of life (9 studies)
Medication adherence (10 studies)
Behaviour (7 studies)
Physical activity (1 study)
Cost‐effectiveness
Cost‐analysis (7 studies)
We identified 98 outcomes within these domains. However, 65 outcomes (66%) were only reported by single studies. Additionally, due to the heterogeneity of interventions only three outcomes (dietary sodium intake, IDWG and death) were able to be quantitatively analysed. Tables 2 to 7 (Table 3; Table 4; Table 5; Table 6; Table 7; Table 8) contain descriptive analyses for reported outcomes. Figure 2 depicts a bubble plot of reported outcomes.
2. Descriptive analyses of reported outcomes for educational interventions.
| Outcome Study ID | Outcome measure | Study population (No. of participants); study duration | Results |
| Behavioural | |||
| Knowledge InformMe 2017 |
31‐item multiple choice test | Adults, kidney transplant candidates (28); 1 week | Intervention: mean 17.94 (SD 6.06) Control: mean 14.7 (SD 5) P = 0.001 |
| Willingness to perform a behaviour InformMe 2017 |
Willingness to accept an Increased Risk Donor Kidney Lower scores indicate more willingness |
Adults, kidney transplant candidates (188); 1 week | Intervention: mean 2.54 (SD 1.45) Control: mean 2.81 (SD 1.2) P = 0.09 |
| Quality of Life | |||
| Fatigue Baraz 2014 |
SF‐36 Higher scores indicate better QoL |
Adults, HD (90); 6 months | Intervention: mean 48.9 (SD 15) Control: mean 56.1 (SD 20.6) P = 0.034 |
| General health perception Baraz 2014 |
SF‐36 Higher scores indicate better QoL |
Adults, HD (90); 6 months | Intervention: mean 41.01 (SD 16.87) Control: mean 48.38 (SD 18.18) P = 0.94 |
| Mental health Baraz 2014 |
SF‐36 Higher scores indicate better QoL |
Adults, HD; (90); 6 months | Intervention: mean 49.84 (SD 18.84) Control: mean 55.07 (SD 27.9) P < 0.001 |
| Pain Baraz 2014 |
SF‐36 Higher scores indicate higher QoL |
Adults, HD (90); 6 months | Intervention: mean 55.45 (SD 29.14), Control: mean 53.22 (SD 32.34) P = NS |
| Physical functioning Baraz 2014 |
SF‐36 Higher scores indicate better QoL |
Adults, HD (90); 6 months | Intervention: mean 70.15 (SD 13.4) Control: mean 68.63 (SD 22.82) P = 0.021 |
| Role (emotional) Baraz 2014 |
SF‐36 Higher scores indicate better QoL |
Adults, HD (90); 6 months | Intervention: mean 50.53 (SD 21.92) Control: mean 44.76 (SD 19.7) P = 0.26 |
| Role (physical) Baraz 2014 |
SF‐36 Higher scores indicate better QoL |
Adults, HD (90); 6 months | Intervention: mean 50.51 (SD 18.9) Control: mean 60.48 (SD 22.14) P = 0.031 |
| Social functioning Baraz 2014 |
SF‐36 Higher scores indicate better QoL |
Adults, HD (90); 6 months | Intervention: mean 67.74 (SD 20.09) Control: mean 64.06 (SD 19.24) P < 0.001 |
CI ‐ confidence interval; HD ‐ haemodialysis; QoL ‐ quality of life; RR ‐ risk ratio; SD ‐ standard deviation
3. Descriptive analyses of reported outcomes for reminder interventions.
| Outcome Study ID | Outcome measure | Study population (No. of participants); study duration | Results |
| Biochemical parameters | |||
| Phosphate Jammalamadaka 2015 |
Serum phosphate | Adults, HD (27); 7 days | Intervention: mean 6.00 (SD 1.2) Control: mean 6.19 (SD 0.76) P = 0.76 |
| Blood pressure | |||
| Blood pressure within guideline recommendations McGillicuddy 2013 |
Blood pressure within pre‐specified goals | Adults, kidney transplant recipients (17); 3 months | RR 4.50 (95% CI 0.63, 32.38) P = 0.13 |
| Systolic blood pressure McGillicuddy 2013 |
Higher readings indicate poorer control | Adults, kidney transplant recipients (17); 3 months | Intervention: mean 131 (SD 10.5) Control: 155.3 (SD 15) P = 0.004 |
| Clinical end‐points | |||
| Hospitalisations Henriksson 2016 |
Unplanned admission rates to hospital or emergency department | Adults, kidney transplant recipients (80); 12 months | RR 0.71 (95% CI 0.48 to 1.05) Intervention: 22/53 events Control: 31/53 events |
| Rejection episodes Henriksson 2016 |
Number of rejection episodes | Adults, kidney transplant recipients (80); 12 months | Intervention: 6 rejections in 4 participants Control: 27 rejections in 13 participants) |
| Rejection episodes Potter 2016 |
Number of rejection episodes | Adults, kidney transplant recipients (46); 1 year | Intervention: 0/20 Control: 9/26 |
| Medication adherence | |||
| Medication adherence McGillicuddy 2013 |
Measured using electronic medication tray openings | Adults, kidney transplant recipients (19); 3 months | Intervention: mean 0.945 (SD 0.11) Control: mean 0.574 (SD 0.11) |
HD ‐ haemodialysis; RR ‐ risk ratio; SD ‐ standard deviation
4. Descriptive analyses of reported outcomes for self‐monitoring interventions.
| Outcome Study ID | Outcome measure | Study population (No. of participants); study duration | Results | |
| Behavioural | ||||
| Attitudes towards performing a behaviour Welch 2013 |
Perceived benefits of fluid adherence Higher score indicates more perceived benefits |
Adults, HD (33); 6 weeks | Intervention: mean 39.8 (SD 4.5) Control: mean 40.1 (SD 4.9) P = 0.28 |
|
| Perceived benefits of sodium adherence Welch 2013 |
Benefits of sodium adherence Higher score indicates higher perceived benefits |
Adults, HD (35); 6 weeks | Intervention: mean 29.9 (SD 4.4) Control: mean 30.3 (SD 4.2) P = 0.77 |
|
| Perceived control Welch 2013 |
7‐item mastery scale Higher score indicates higher perceived control |
Adults, HD (35); 6 weeks | Intervention: mean 28.5 (SD 4.9) Control: mean 23.6 (SD 14.3) P > 0.1 |
|
| Self‐efficacy (diet) Welch 2013 |
Cardiac diet self‐efficacy instrument Higher score indicates higher self‐efficacy |
Adults, HD (35); 6 weeks | Intervention: mean 32.7 (SD 10.1) Control: mean 31.1 (SD 10.2) P = 0.4 |
|
| Self‐efficacy (fluid) Welch 2013 |
Fluid Self‐Efficacy Scale Higher score indicates higher self‐efficacy |
Adults, HD (36); 6 weeks | Intervention: mean 41.4 (SD 5.8) Control: mean 43.9 (SD 6.4) P = 0.21 |
|
| Biochemical parameters | ||||
| Kidney function Kullgren 2015 |
Serum creatinine | Children, kidney transplant recipients (31); 4 weeks | Intervention: mean 16.8 (SD 21.2) Control: mean 11 (SD 15.2) P = 0.53 |
|
| Kidney function Rifkin 2013 |
Serum creatinine | CKD stage 3 or greater (43); 6 months | Intervention: mean 2.17 (SD 0.76) Control: mean 2.32 (SD 0.84) P = 0.12 |
|
| Serum sodium Kullgren 2015 |
% change in serum sodium | Children, kidney transplant recipients (31); 4 weeks | Intervention: median 0 (range ‐4.86 to 1.45) Control: median ‐0.72 (range ‐3.52 to 2.19) P = 0.29 |
|
| Urea Nitrogen Kullgren 2015 |
% change in blood urea nitrogen | Children, kidney transplant recipients (31); 4 weeks | Intervention: median ‐2.38 (range ‐36.84 to 61.54) Control: median 4.56 (range ‐31.25 to 107.33) P = 0.78 |
|
| Blood pressure | ||||
| Blood pressure control Rifkin 2013 |
Mean arterial pressure | CKD stage 3 or greater (43); 6 months | Intervention: mean 93.9 (SD 8.6) Control: mean 95.2 (SD 11.7) P = 0.67 |
|
| Diastolic blood pressure Rifkin 2013 |
Higher readings indicate poorer control | CKD stage 3 or greater (43); 6 months | Intervention: mean 73 (SD 10.3) Control: mean 73 (SD 12.6) P = 0.93 |
|
| Diastolic blood pressure Schulz 2007 |
Higher readings indicate poorer control | Adults, HD (101); 3 months | Intervention: mean 66.9 (SD 8.7) Control: mean 65 (SD 8.8) P < 0.05 |
|
| Management of hypertension Rifkin 2013 |
Number of anti‐hypertensive medications | CKD stage 3 or greater (43); 6 months | Intervention: mean 4 (SD 1.2) Control: mean 3.9 (SD 1.3) P = 0.61 |
|
| Systolic blood pressure Rifkin 2013 |
Higher readings indicate poorer control | CKD stage 3 or greater (43); 6 months | Intervention: mean 136 (SD 15.6) Control: mean 140 (SD 14.4) P = 0.48 |
|
| Systolic blood pressure Schulz 2007 |
Higher readings indicate poorer control | Adults, HD (101); 3 months | Intervention: mean 116 (SD 17) Control: mean 17.9 (SD 19.5) P = NS |
|
| Clinical end‐points | ||||
| Hospitalisations Schulz 2007 |
Unplanned admission rates to hospital or ED | Adults, HD (101); 3 months | Intervention: mean 2.2 (SD 5.5) Control: mean 3.31 (SD 7.3) |
|
| Medication usage Rifkin 2013 |
Total number of medications | CKD stage 3 or greater (43); 6 months | Intervention: mean 12 (SD 4.6) Control: mean 12.8 (SD 5.1) P = 0.62 |
|
| Sleep duration (minutes) Williams 2017 |
Fitbit Flex activity tracker | Adults, HD (29); 5 weeks | Intervention: mean 389.9 (SD 69.6) Control: mean 349.8 (SD 80.0) P = NS |
|
| Sleep efficiency (%) Williams 2017 |
Fitbit Flex activity tracker | Adults, HD (29); 5 weeks | Intervention: mean 86.1 (SD 4.6) Control: mean 80.3 (SD 7.1) P < 0.05 |
|
| Ultrafiltration Schulz 2007 |
mL/hour during dialysis, weekly average | Adults, HD (101); 3 months | Intervention: mean 621.6 (SD 169.7 mL/hour) Control: mean 652.5 (SD 198.6 mL/hour) P = 0.712 |
|
| Dietary intake | ||||
| Fluid intake Kullgren 2015 |
3‐day fluid log through electronic water bottle | Children, kidney transplant recipients (32); 4 weeks | Unadjusted OR 12.25 (95% CI 1.08 to 138.99) P = 0.043 Intervention group significantly improved. |
|
| Medication adherence | ||||
| Medication adherence Rifkin 2013 |
Morisky Medication Adherence Scale Higher scores indicate better adherence |
CKD stage 3 or greater (43); 6 months | Intervention: mean 7 (SD 1.2) Control: mean 7.2 (SD 1.4) P = 0.58 |
|
| Physical activity | ||||
| Physical activity, distance (km) Williams 2017 |
FitBit Flex activity tracker | Adults, HD (29); 5 weeks | Intervention: mean 2.3 (SD 1.2) Control: mean 2.2 (SD 0.8) P = NS |
|
| Physical activity (steps) Williams 2017 |
FitBit Flex activity tracker | Adults, HD (29); 5 weeks | Intervention: mean 5365 (SD 2765) Control: mean 5211 (SD 2010) P = NS |
|
CKD ‐ chronic kidney disease; HD ‐ haemodialysis; NS ‐ not significant; OR ‐ Odds ratio; SD ‐ standard deviation
5. Descriptive analyses of reported outcomes for behavioural counselling interventions.
|
Outcome Study ID |
Outcome measure | Study population (No. of participants); study duration | Results |
| Behavioural | |||
| Illness perception iDiD 2016 |
Brief Illness Perception Questionnaire Higher score indicates more negative perception of ESKD |
Adults, HD (25); 3 months | Intervention: mean 44.2 (SD 12.09) Control: mean 41.2 (SD 10.28) |
| Knowledge BRIGHT 2013 |
Modified Morisky’s Medication Adherence Scale Higher score indicates higher medication knowledge |
Adults, ≥ CKD stage 3 ± proteinuria (366); 6 months | Intervention: mean 2.6 (SD 0.6) Control: mean 2.6 (SD 0.6) P = 0.331 |
| Self‐care behaviours BRIGHT 2013 |
Summary of Diabetes Self Care Activities Higher sore indicates higher self‐care |
Adults, ≥ CKD stage 3 ± proteinuria (374); 6 months | Intervention: mean 4.5 (SD 1.2) Control: mean 4.2 (SD 1.2) P = 0.019 |
| Biochemical parameters | |||
| Kidney function MESMI 2010 |
Serum creatinine | Adults, CKD, eGFR < 60 mL/min + diabetes (75); 6 months | Intervention: mean 128 (SD 6.4) Control: mean 130 (SD 15.5) P = NS |
| Kidney function TAKE‐IT 2014 |
Annualised change in eGFR | Adolescents, kidney transplant recipients (169); 12 months | Intervention: median ‐2.3 (95% CI ‐10.6 to 10.3) Control: median ‐3.3 (95% CI ‐7.7 to 3.7) P = 0.5 |
| Blood pressure | |||
| Blood pressure within guideline recommendations BRIGHT 2013 |
‐‐ | Adults, ≥ CKD stage 3 ± proteinuria (403); 6 months | RR 1.22 (95% CI 1.04 to 1.43) P = 0.002 Favouring eHealth intervention |
| Blood pressure within guideline recommendation Ishani 2016 |
‐‐ | Adults, CKD, eGFR < 60 mL/min (76); 12 months | RR 1.04 (95% CI 0.74 to 1.47) P = 0.8 |
| Diastolic blood pressure MESMI 2010 |
Higher readings indicate poorer control | Adults, CKD, < eGFR 60 mL/min + diabetes (75); 6 months | Intervention: mean reduction 2.25 (SD 8.7) Control: mean reduction 3.1 (SD 8.6) P = 0.681 |
| Systolic blood pressure MESMI 2010 |
Higher readings indicate poorer control | Adults, CKD, < eGFR 60 mL/min + diabetes (75); 6 months | Intervention: mean reduction 6.9 (SD 20.5) Control: mean reduction 3 (SD 16.7) P = 0.371 |
| Clinical end‐points | |||
| Adverse events TAKE‐IT 2014 |
including post‐transplant lymphoproliferative disorder, Epstein‐Barr virus infection, CMV, BK virus infection, influenza, other infection, vomiting/diarrhoea, surgery/procedure, other, hospitalisations | Adolescents, kidney transplant recipients (169); 12 months | Intervention: 12.9 Control: 12.7 P = 0.9 |
| Cholesterol control Ishani 2016 |
Serum LDL‐C < 100 mg/dL | Adults, CKD, eGFR < 60mL/min (76); 12 months | Intervention: 31/61 (51%) Control: 8/15 (53%) P = 0.9 |
| Composite end point Ishani 2016 |
Death, ED admissions, hospitalisations and admission to skilled nursing facility | Adults, CKD, eGFR < 60 mL/min (600); 12 months | HR 0.98 (95% CI 0.75 to 1.29), P = 0.9 Intervention: 208/450 (46.2%) Control: 70/150 (46.7%) |
| Diabetes control MESMI 2010 |
Serum HbA1c | Adults, CKD, eGFR < 60 mL/min + diabetes (75); 6 months | Intervention: median 7.5 (IQR 7 to 8.5) Control: median 7 (IQR 6 to 9) |
| Diabetes control Ishani 2016 |
Serum HbA1c < 8% | Adults, CKD, eGFR < 60mL/min (48); 12 months | Intervention: 14/33 (42%) Control: 3/33 (15%) P = 0.6 |
| Graft failure TAKE‐IT 2014 |
‐‐ | Adolescents, kidney transplant recipients (169); 12 months | Intervention: 0 Control: 0 |
| Graft rejection, acute TAKE‐IT 2014 |
‐‐ | Adolescents, kidney transplant recipients (169); 12 months | Intervention: 1.06 Control: 1.69 P = 0.3 |
| Healthcare utilisation BRIGHT 2013 |
Service use (Primary health care services, community health, social care, secondary healthcare services, out‐of‐pocket expenses, costs of loss of productivity) | Adults, ≥ CKD stage 3 ± proteinuria (374); 6 months | Intervention: mean 6.1 (SD 5.5) Control: mean 6.5 (SD 4.7) P = 0.455 |
| Healthcare utilisation Ishani 2016 |
Admission to skilled nursing facility | Adults, CKD, eGFR < 60mL/min (600); 12 months | HR 3.07 (95% CI 0.71 to 13.24) Intervention: 18/450 (4%) Control: 2/150 (1.3%) |
| Healthcare utilisation Li 2014b |
Clinic visits, 3 or more visits | Adults, PD (135); 12 weeks | Intervention: 3/69 (4.4%) Control: 5/66 (7.6%) P = 0.039 |
| Hospitalisations Schmid 2016 |
Unplanned admission rates to hospital or ED | Adults, kidney transplant recipients (26); 12 months | Intervention: mean 0 (SD 0.74) Control: mean 2 (SD 1.48) |
| Hospitalisations Ishani 2016 |
Unplanned admission rates to hospital or ED | Adults, CKD, eGFR < 60 mL/min (600); 12 months | RR 1.12 (95% CI 0.83 to 1.51) Intervention: 134/450 events Control: 40/150 events |
| Hospitalisations Li 2014b |
Unplanned admission rates to hospital or ED | Adults, PD (135); 12 weeks | RR 0.60 (95% CI 0.21 to 1.73) Intervention: 5/69 Control: 8/66 |
| Hospitalisations TAKE‐IT 2014 |
‐‐ | Adolescents, kidney transplant recipients (169); 12 months | Intervention: 4.96
Control: 5.38 P = 0.7 |
| Kidney function Ishani 2016 |
Initiation on dialysis | Adults, CKD, eGFR < 60 mL/min (600); 12 months | HR 1.86 (95%CI 0.41 to 8.39), P = NS Intervention: 11/450 (2.4%) Control: 2/150 (1.3%) |
| Rejection episodes Schmid 2016 |
Number of rejection episodes | Adults, kidney transplant recipients (46); 12 months |
Intervention: 1/23 Control: 2/23 |
| Smoking status Ishani 2016 |
Number participants quit smoking | Adults, CKD, eGFR < 60 mL/min (52); 12 months | Intervention: 9/40 (23%) Control: 5/12 (42%) P = 0.3 |
| Medication adherence | |||
| Medication adherence Schmid 2016 |
% compliant according to composite adherence score | Adults, kidney transplant recipients (26); 12 months | RR 1.90 (95% CI 1.15 to 3.14), P = 0.013 Intervention: 19/23 Control: 10/23 |
| Medication adherence MESMI 2010 |
Pill counts to determine a score | Adults, CKD, eGFR < 6o mL/min + diabetes (75); 6 months | Intervention: mean 58.4 (SD 24.3) control: mean 66.6 (SD 22.2) P = 0.162 |
| Medication adherence Russell 2011 |
Medication Event Monitoring System used to record opening of bottles | Adults, kidney transplant recipients (13); 6 months |
Intervention: mean 0.88 (SD 0.09) Control: mean 0.77 (SD 0.06) P = 0.0396 |
| Medication adherence TAKE‐IT 2014 |
Perfect taking adherence was defined as taking all prescribed daily doses | Adolescents, kidney transplant recipients (169); 12 months | OR 1.50 (95% CI 1.06 to 2.12) In favour of eHealth intervention |
| Medication adherence TAKE‐IT 2014 |
Self‐reported using the Medical Adherence Measure Medication Module (MAM‐MM) | Adolescents, kidney transplant recipients (169) 12 months |
Taking adherence Intervention: 98.3 (SD 4.5) Control: 97.1 (SD 6.0) P = 0.2 Timing adherence Intervention: 95 (SD 7.9) Control: 92.9 (SD 9.3) P = 0.2 |
| Medication adherence TAKE‐IT 2014 |
Standard deviation of tacrolimus trough concentrations during intervention interval | Adolescents, kidney transplant recipients (169); 12 months | Intervention: 1.6 (CI 0.9 to 2.5) Control: 1.4 (CI 0.9 to 2.1) P = 0.5 |
| Medication motivation BRIGHT 2013 |
Modified Morisky’s Medication Adherence Scale Higher score indicates higher medication motivation |
Adults, ≥ CKD stage 3 ± proteinuria (369); 6 months | Intervention: mean 2.7 (SD 0.6) Control: mean 2.7 (SD 0.5) P = 0.568 |
| Dietary intake | |||
| PD dietary problems Koprucki 2010 |
Self‐reported questionnaire Unclear whether higher or lower scores represent an improvement in dietary problems |
Adults, PD (19); 4 months | Intervention: mean ‐10.5 points (SD 16.2) Control: mean +0.5 points (SD 20.1) P = 0.194 |
| Quality of Life | |||
| Anxiety BRIGHT 2013 |
Hospital Anxiety and Depression Scale (HADS‐A) Higher score indicate more anxiety |
Adults, ≥ CKD stage 3 ± proteinuria (345); 6 months | Intervention: mean 4.6 (SD 3.7) Control: mean 5.2 (SD 4.1) P = 0.06 |
| Anxiety iDiD 2016 |
Generalised Anxiety Disorder questionnaire Higher score indicate more anxiety |
Adults, HD (25); 3 months | Intervention: mean 4.4 (SD 4.1) Control: mean 3.9 (SD 3.6) |
| Anxiety Kargar Jahromi 2016 |
Depression Anxiety Stress Scales (DASS) Higher scores indicate worse anxiety |
Adults, HD (54); 1 month | Intervention: mean 8.68 (SD 0.9) Control: mean 16.72 (SD 1.98) P = 0.01 |
| Anxiety Reilly‐Spong 2015 |
State‐Trait Anxiety Inventory Higher scores indicate worse anxiety |
Adults, kidney transplant recipients (42); 2 months |
Intervention: mean 41.2 (SD 15.3) Control: mean 38.1 (SD 11.6) P = 0.55 |
| Burden Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 12 weeks | Intervention: mean 21.5 (SD 11.7) Control: mean 21.1 (SD 12.2) P = 0.86 |
| Cognitive function Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 12 weeks | Intervention: mean 74.2 (SD 15.7) Control: mean 76.8 (SD 16.5) P = 0.35 |
| Depression iDiD 2016 |
Patient Health Questionnaire – 9 Higher scores indicate more depressive symptoms |
Adults, HD (23); 3 months | Intervention: mean 7.5 (SD 5.4) Control: mean 7.6 (SD 4.7) |
| Depression Kargar Jahromi 2016 |
Depression Anxiety Stress Scales (DASS) Higher scores indicate worse anxiety |
Adults, HD (54); 1 month | Intervention: mean 8.96 (SD .17) Control: mean 16.2 (SD 1.6) |
| Depression Reilly‐Spong 2015 |
Center for Epidemiologic Studies Depression Scale Higher score indicate more symptoms |
Adults, kidney transplant recipients (51) 2 months |
Intervention: mean 14.7 (SD 9.4) Control: mean 9.1 (SD 5.8) P = 0.05 |
| Effects Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 12 weeks | Intervention: mean 63.2 (SD 14.2) Control: mean 62.1 (SD 14.3) P = 0.63 |
| Emotional well‐being BRIGHT 2013 |
heiQ Higher score indicates higher negative affect |
Adults, ≥ CKD stage 3 ± proteinuria (374); 6 months | Intervention: mean 31.4 (SD 22.2) Control: mean 34 (SD 22.2) P = 0.329 |
| Fatigue BRIGHT 2013 |
Medical Outcomes Survey, energy and vitality Higher score indicates more energy and vitality |
Adults, ≥ CKD stage 3 ± proteinuria (373); 6 months | Intervention: mean 52.4 (SD 22) Control: mean 50.8 (SD 21.8) P = 0.082 |
| Fatigue Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 6 weeks | Intervention: mean 48.4 (SD 17.7) Control: mean 43.3 (SD 18.9) P = 0.02 |
| Fatigue Reilly‐Spong 2015 |
Patient‐Reported Outcomes Measurement Information System – Fatigue Higher score indicate more symptoms |
Adults, kidney transplant recipients (38); 2 months | Intervention: mean 57 (SD 6.3) Control: mean 56.6 (SD 8.4) P = 0.65 |
| General health perception BRIGHT 2013 |
SF‐36 Higher scores indicate higher QoL |
Adults, ≥ CKD stage 3 ± proteinuria (372); 6 months | Intervention: mean 2.8 (SD 1.0) Control: 2.8 (SD 0.9) P = 0.832 |
| General health perception Li 2014b |
KDQoL‐SF Higher scores indicate higher QoL |
Adults, PD (135); 12 weeks | Intervention: mean 38.2 (SD 17.5) Control: mean 35.7 (SD 17.7) P = 0.41 |
| Health services navigation BRIGHT 2013 |
Health Education Impact Questionnaire | Adults, ≥ CKD stage 3 ± proteinuria (372); 6 months | Intervention: mean 70.5 (SD 16.2) Control: mean 69.4 (SD 15.9) P = 0.226 |
| Hope Poorgholami 2016a |
Miller’s questionnaire of hope Higher score indicates greater hopefulness |
Adults, HD (75); 2 months | Intervention: mean 187.0 (SD 11.46) Control 1: mean 170.96 (SD 7.99) Control 2: mean 91.16 (SD 11.06) P < 0.05 Significant improvement in the intervention group compared to both control groups |
| Loneliness BRIGHT 2013 |
UCLA Loneliness Scale Higher score indicates lower loneliness |
Adults, ≥ CKD stage 3 ± proteinuria (369); 6 months | Intervention: mean 30.3 (SD 5.3) Control: mean 31 (SD 4.4) P = 0.861 |
| Mental component score Reilly‐Spong 2015 |
SF‐12 Higher score indicates higher quality of life |
Adults, kidney transplant recipients (63); 2 months | Intervention: mean 49.7 (SD 10) Control: mean 46.7 (SD 9.8) P = 0.01 |
| Mental health BRIGHT 2013 |
Medical Outcomes Survey, psychological well being Higher score indicates higher psychological well being |
Adults, ≥ CKD stage 3 ± proteinuria (372); 6 months | Intervention: mean 74.7 (SD 18.8) Control: mean 74 (SD 19.9) P = 0.286 |
| Mental health Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 6 weeks | Intervention: mean 65.4 (SD 17.2) Control: mean 63.5 (SD 18.6) P = 0.77 |
| Mobility iDiD 2016 |
EQ‐5D Higher score indicates reduced mobility |
Adults, HD (25); 3 months | Intervention: mean 1.5 (SD 0.8) Control: mean 2.4 (SD 1.5) P = NS |
| Mood iDiD 2016 |
EQ‐5D Higher score indicates lower mood |
Adults, HD (25); 3 months | Intervention: mean 1.5 (SD 0.8) Control: mean 2.0 (SD 1.0) |
| Quality of life (global score) BRIGHT 2013 |
EQ‐5D Higher scored indicates reduced quality of life |
Adults, CKD ≥ stage 3 ± proteinuria (372); 6 months | Intervention: mean 0.71 (SD 0.28) Control: mean 0.67 (SD 0.29) P = 0.027 |
| Physical component score Reilly‐Spong 2015 |
SF‐12 Higher score indicates higher quality of life |
Adults, kidney transplant recipients (63); 2 months | Intervention: mean 33.2 (SD 9.8) Control: mean 38.5 (SD 10.4) P =0.96 |
| Physical functioning Li 2014b |
KDQoL‐SF Higher scores indicate higher QoL |
Adults, PD (135); 12 weeks | Intervention: mean 53.9 (SD 12.9) Control: mean 51.5 (SD 12.5) P = 0.28 |
| Pain iDiD 2016 |
EurQoL EQ‐5D Higher scores indicate more pain |
Adults, HD (18); 3 months | Intervention: mean 1.6 (SD 0.8) Control: mean 2.6 (SD 1.3) P = NS |
| Pain Li 2014b |
KDQoL‐SF Higher scores indicate less pain |
Adults, PD (135); 12 weeks | Intervention: mean 64.2 (SD 18.2) Control: mean 59.7 (SD 18.9) P = 0.16 |
| Pain Reilly‐Spong 2015 |
SF‐12 Higher scores indicate less pain |
Adults, kidney transplant recipients (38); 2 months | Intervention: mean 39.9 (SD 13.9) Control: 44.7 (SD 10.4) P = 0.94 |
| Patient satisfaction Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 12 weeks | Intervention: mean 75.9, SD 13.8 Control: mean 71.3 (SD 12.3) P = 0.04 |
| Positive and active engagement in life BRIGHT 2013 |
heiQ Higher score indicates higher engagement with life |
Adults, ≥ CKD stage 3 ± proteinuria (374); 6 months | Intervention: mean 66.4 (SD 19.7) Control: mean 66.5 (SD 17.6) P = 0.999 |
| Quality social interaction Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 12 weeks | Intervention: mean 73.2 (SD 15.1) Control: mean 71.7 (SD 14.1) P = 0.56 |
| Role, emotional Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 6 weeks | Intervention: mean 56.3 (SD 14.8) Control: mean 56.6 (SD 16.5) P = 0.77 |
| Role, physical Li 2014b |
KDQoL‐SF Higher scores indicate higher QoL |
Adults, PD (135); 12 weeks | Intervention: mean 20.8 (SD 16.9) Control: mean 20.4 (SD 15.1) P = 0.91 |
| Self‐monitoring and insight BRIGHT 2013 |
heiQ Higher score indicates higher self‐monitoring and insight |
Adults, ≥ CKD stage 3 ± proteinuria (374); 6 months | Intervention: mean 70.7 (SD 12.2) Control: mean 70.7 (SD 11.5) P = 0.644 |
| Sexual function Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 12 weeks | Intervention: mean 83.7 (SD 16.4) Control: mean 78.4 (SD 15.5) P = 0.05 |
| Side effects from corticosteroids, cardiac and kidney dysfunction Schmid 2016 |
End‐stage renal disease symptom checklist (ESRD‐SCL) Higher score indicate improved quality of life |
Adults, kidney transplant recipients (46); 12 months | Intervention: median 0 (IQR 0.2) Control: median 0.4 (IQR 0.6) P = 0.004 |
| Skills and technique acquisition BRIGHT 2013 |
heiQ Higher score indicates higher skills and technique acquisition |
Adults, ≥ CKD stage 3 ± proteinuria (369); 6 months | Intervention: mean 65.4 (SD 14.6) Control: mean 65.0 (SD 13.1) P = 0.218 |
| Social network (illness) BRIGHT 2013 |
heiQ Higher score = greater help with illness from social network |
Adults, ≥ CKD stage 3 ± proteinuria (342); 6 months | Intervention: mean 10.3 (SD 8.4) Control: mean 11.5 (SD 9) P = 0.208 |
| Social network (practical) BRIGHT 2013 |
heiQ Higher score = greater help with practical work from social network |
Adults, ≥ CKD stage 3 ± proteinuria (342); 6 months | Intervention: mean 6.2 (SD 6.2) Control: mean 8.1 (SD 7.1) P = 0.017 |
| Social support Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 12 weeks | Intervention: mean 74.1 (SD 14.7) Control: mean 73.2 (SD 15.1) P = 0.73 |
| Self‐care iDiD 2016 |
EQ‐5D Higher score indicates reduced self‐care |
Adults, HD (25); 3 months | Intervention: mean 1.2 (SD 0.6) Control: mean 1.4 (SD 0.9) P = NS |
| Sleep Li 2014b |
KDQoL‐SF Higher score indicates better sleep |
Adults, PD (160); 6 weeks | Intervention: mean 61.1 (SD 20.6) Control: mean 54.3 (SD 18.1) P = 0.1 |
| Sleep Reilly‐Spong 2015 |
Pittsburgh Sleep Quality Index Lower score indicates better sleep quality |
Adults, kidney transplant recipients (63); 2 months | Intervention: mean 7.3 (SD 4.7) Control: 6.1 (SD 3.4) P = 0.65 |
| Social capital BRIGHT 2013 |
heiQ Higher score indicates increased satisfaction with opportunities to participate in the community |
Adults, ≥ CKD stage 3 ± proteinuria (366); 6 months | Intervention: mean 3.7 (SD 0.8) Control: mean 3.6 (SD 0.8) P = 0.325 |
| Social integration BRIGHT 2013 |
heiQ Higher score indicates higher social integration |
Adults, ≥ CKD stage 3 ± proteinuria (371); 6 months | Intervention: mean 69.6 (SD 20.3) Control: mean 69.4 (SD 15.6) P = 0.537 |
| Social network (emotional) BRIGHT 2013 |
heiQ Higher score indicates greater help with emotional work from social network |
Adults, ≥ CKD stage 3 ± proteinuria (345); 6 months | Intervention: mean 13.4 (SD 10.4) Control: mean 14.9 (SD 11.4) P = 0.463 |
| Social functioning BRIGHT 2013 |
Medical Outcomes Survey, social/role activities limitations Higher score indicates lower social limitation |
Adults, ≥ CKD stage 3 ± proteinuria (371(; 6 months | Intervention: mean 73.2 (SD 28.2) Control: mean 68.7 (SD 30.5) P = 0.492 |
| Social functioning Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 6 weeks | Intervention: mean 42.5 (SD 19.3) Control: mean 43.4 (SD 18.8) P = 0.43 |
| Staff encouragement Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 12 weeks | Intervention: mean 87.3 (SD 12.8) Control: mean 81.2 (SD 15.1) P = 0.01 |
| Stress Kargar Jahromi 2016 |
Depression Anxiety Stress Scales (DASS) Higher score indicates higher stress |
Adults, HD (54); 1 month | Intervention: mean 8.36 (SD 1.03) Control: mean 13.76 (SD 1.44) P = 0.001 |
| Symptoms/problems Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 12 weeks | Intervention: mean 72.8 (SD 15) Control: mean 68.6 (SD 6.2) P = 0.08 |
| Usual activities iDiD 2016 |
EQ‐5D Higher scores indicate reduced ability to complete usual activities |
Adults, HD (25); 3 months | Intervention: mean 1.5 (SD 0.8) Control: mean 2.8 (SD 1.3) P = NS |
| Work status Li 2014b |
KDQoL Higher scores indicate improved quality of life |
Adults, PD (135); 12 weeks | Intervention: mean 17.3 (SD 11.6) Control: mean 14.8 (SD 9.9) P = 0.19 |
CI ‐ confidence interval; CKD ‐ chronic kidney disease; eGFR ‐ estimated glomerular filtration rate; HD ‐ haemodialysis; HR ‐ hazard ratio; IQR ‐ interquartile range; NS ‐ not significant; PD ‐ peritoneal dialysis; RR ‐ risk ratio; SD ‐ standard deviation
6. Descriptive analyses of reported outcomes for clinical‐decision aid interventions.
| Outcome Study ID | Outcome measure | Study population (No. of participants); study duration | Results |
| Behavioural | |||
| Knowledge iChoose 2016 |
9 item scale, unvalidated | Adults, ESKD (443); 1 clinic appointment | Intervention: mean 6.11 (SD 1.91) Control: mean 5.48 (SD 1.87) P < 0.001 |
| Biochemical parameters | |||
| Serum parathyroid hormone Cooney 2015 |
‐‐ | Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (2199); 12 months | Intervention: 502/1070 (46.9%) Control: 182/1129 (16.1%) P < 0.001 |
| Serum phosphate Cooney 2015 |
‐‐ | Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (2199); 12 months | Intervention: 680/1070 (63.6%) Control: 527/1129 (46.7%) P < 0.001 |
| Blood pressure | |||
| Blood pressure within guideline recommendations Cooney 2015 |
‐‐ | Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (947); 12 months | RR 1.02 (95% CI 0.87 to 1.19) P = 0.84 |
| Management of hypertension Cooney 2015 |
Number of anti‐hypertensive medications | Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (2199); 12 months |
0 medications Intervention: 37 (7.8%), control: 65 (13.7%) 1 medication Intervention 52 (11%), control: 63 (13.3%) 2 medications Intervention 128 (27%), control: 105 (22.2%) 3 medications Intervention: 135 (28.5%), control: 121 (25.6%) 4+ medications Intervention: 122 (25.7%), control: 119 (25.2%) |
| Systolic blood pressure Cooney 2015 |
Higher readings indicate poorer control | Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (947); 12 months | Intervention: mean 135.1 (SD 17.4) Control: mean 134.4 (SD 17.6) P = 0.57 |
| Clinical end‐points | |||
| Access to kidney transplantation iChoose 2016 |
Composite score of transplant access (at least one of following outcomes: wait‐list, deceased, deceased or living donor transplant, 1 living donor inquiry) |
Adults, ESKD (443); 1 clinic appointment | Intervention: 168/226 (74.3%) Control: 155/216 (71.4%) |
| Healthcare utilisation Durand 2000 |
Frequency of planned medical visits | Adults, PD (30); intervention 9.5 months, control 7.8 months | Intervention: 1/41 days Control: 1/33 days |
| Hospitalisations Durand 2000 |
‐‐ | Adults, PD (30); intervention 9.5 months, control 7.8 months | RR 0.67 (95% CI 0.34 to 1.29) Intervention: 14/365 events Control: 21/365 events |
| Kidney function Cooney 2015 | Urine albumin creatinine ratio | Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (2199); 12 months | Intervention: 602/1070 (56.3%) Control: 435/1129 (38.5%) P < 0.001 |
| Kidney function Cooney 2015 |
Progression to ESKD (dialysis or transplantation) | Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (2199); 12 months | Intervention: 26/1070 (2.4%) Control: 20/1129 (1.8%); P =0.28 |
| Medication usage Cooney 2015 |
Prescribed appropriate medications | Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (2199); 12 months |
ACEI/ARB Intervention: 309/481 (64.2%) Control: 298/483 (61.7%) P = 0.41 Phosphate binder Intervention: 24/107 (22.4%) Control: 19/81 (23.5%) P = 0.87 Vitamin D Intervention: 310/501 (61.9%) Control: 218/416 (52.4%) P = 0.004 Bicarbonate Intervention: 31/132 (24%) Control: 18/137 (13%) P = 0.03 |
| Medication adherence | |||
| Medication adherence Cooney 2015 |
Morisky Medication Adherence Scale Higher scores indicate better adherence |
Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (2199); 12 months | Intervention: mean 6.7 (SD 1.2) Control: mean 6.8 (SD 1.2) P = 0.7 |
| Medication adherence Hardstaff 2002 |
Pill counts | Adults, kidney transplant recipients (91) | RR 0.72 (95% CI 0.42 to 1.15) Intervention: 26/67 Control: 13/24 |
| Quality of Life | |||
| Burden Cooney 2015 |
KDQoL Higher scores indicate improved quality of life |
Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (2199); 12 months | Intervention: mean 89.7 (SD 20.5) Control: mean 89.4 (SD 19.6) P = 0.93 |
| Effects Cooney 2015 |
KDQoL Higher scores indicate improved quality of life |
Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (2199); 12 months | Intervention: mean 94.2 (SD 11.9) Control: mean 94.4 (SD 14) P = 0.92 |
| Mental component score Cooney 2015 |
SF‐12 Higher score indicates higher quality of life |
Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (2199); 12 months | Intervention: mean 52 (SD 10.6) Control: mean 52.1 (SD 9.6) P = 0.9 |
| Physical component score Cooney 2015 |
SF‐12 Higher score indicates higher quality of life |
Adults, eGFR < 45 mL/min or eGFR < 60 mL/min in past 90 days to 2 years (2199); 12 months | Intervention: mean 39.3 (SD 9.8) Control: mean 36.8 (SD 10.3) P = 0.15 |
ACEi/ARB ‐ angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; eGFR ‐ estimated glomerular filtration rate; ESKD ‐ end‐stage kidney disease; PD ‐ peritoneal dialysis; RR ‐ risk ratio; SD ‐ standard deviation
7. Descriptive analyses of reported outcomes for mixed interventions.
| Outcome Study ID | Outcome measure |
Study population (no. of Participants); study duration |
Results |
| Behavioural | |||
| Attitudes towards performing a behaviour Robinson 2014a |
Attitude: importance of sun protection Higher score indicates higher importance |
Adults, kidney transplant recipients (101); 6 weeks |
Intervention: mean 7 (SD 12) Control: mean 0 (SD 8.5) P = 0.003 |
| Attitudes towards performing a behaviour Robinson 2015 |
Attitude: importance of sun protection Higher score indicates higher importance |
Adults, kidney transplant recipients (170); 6 weeks | Intervention: mean 6.59 (SD 3.87) Control: mean 1.07 (SD 0.705) P < 0.05 |
| Knowledge Robinson 2014a |
Knowledge of skin cancer and sun protection (self‐reported, validated tool | Adults, kidney transplant recipients (103); 6 weeks | Intervention: mean 9 (SD 6.75) Control: mean 0 (SD 6.75) P = 0.015 |
| Knowledge Robinson 2015 |
Knowledge of skin cancer and sun protection (self‐reported, validated tool) | Adults, kidney transplant recipients (170); 6 weeks | Intervention: mean 6.66 (SD 2.57) Control: mean 3.67 (SD 1.73) P = 0.04 |
| Self‐care behaviours Robinson 2014a |
Sun protection performed Higher score indicates more sun protection behaviours performed |
Adults, kidney transplant recipients (101); 6 weeks | Intervention: mean 12.5 (SD 19.6) Control: mean 2.5 (SD 17.5) P = 0.013 |
| Self‐care behaviours Robinson 2015 |
Sun protection performed Higher score indicates more sun protection behaviours performed |
Adults, kidney transplant recipients (170); 6 weeks | Intervention: mean 57.7 (SD 13.08) Control: mean 31.1 (SD 4.87) P = 0.013 |
| Willingness to perform a behaviour Robinson 2014a |
Willingness to use sun protection Higher scores indicate more willingness |
Adults, kidney transplant recipients (101); 6 weeks | Intervention: mean 8 (SD 25) Control: mean 0 (SD 34.5) P = 0.137 |
| Willingness to perform a behaviour Robinson 2015 |
Willingness to use sun protection Higher scores indicate more willingness |
Adults, kidney transplant recipients (170); 6 weeks | Intervention: mean 74.64 (SD 21.4) Control: mean 22.64 (SD 1.65) P = 0.09 |
| Biochemical parameters | |||
| Kidney function Navaneethan 2017 |
Measurement of serum creatinine | Adults, CKD, eGFR 15 to 45 mL/min (209); 24 months | Intervention: 42/50 (84%) Control: 57/57 (100%) P = 0.001 |
| Serum parathyroid hormone Navaneethan 2017 |
‐‐ | Adults, CKD, eGFR 15 to 45 mL/min (209); 24 months | Intervention: 22/50 (44%) Control: 33/57 (58%) P = 0.34 |
| Serum phosphate Navaneethan 2017 |
‐‐ | Adults, CKD, eGFR 15 to 45 mL/min (209); 24 months | Intervention: 28/50 (56%) Control: 39/57 (68%) P = 0.52 |
| Measurement of 25‐hydroxy Vitamin D Navaneethan 2017 |
‐‐ | Adults, CKD, eGFR 15 to 45 mL/min (209); 24 months | Intervention: 28/50 (56%) Control: 37/57 (65%) P = 0.31 |
| Blood pressure | |||
| Blood pressure within guideline recommendations Navaneethan 2017 | ‐‐ | Adults, CKD, eGFR 15 to 45 mL/min (209); 24 months | RR 0.97 (95% CI 0.86 to 1.09) P = 0.98 |
| Clinical end‐points | |||
| Cholesterol control Navaneethan 2017 |
Measurement of serum LDL‐C | Adults, CKD, eGFR 15 to 45 mL/min (209); 24 months | Intervention: 39/50 (78%) Control: 48/57 (84%) P = 0.36 |
| Diabetes control Navaneethan 2017 |
Measurement of serum HbA1c | Adults, CKD, eGFR 15 to 45 mL/min (209); 24 months | Intervention: 19/29 (79%) Control: 29/29 (100%) P = 0.02 |
| Hospitalisations Navaneethan 2017 |
Unplanned admission rates to hospital or emergency department | Adults, CKD, eGFR 15 to 45 mL/min (209); 24 months | Intervention: mean 4.06 (SD 14.11) Control: mean 2.29 (SD 9.09) P = 0.24 |
| Kidney function Navaneethan 2017 |
Urine albumin creatinine ratio | Adults, CKD, eGFR 15 to 45 mL/min (209); 24 months | Intervention: 19/50 (38%) Control: 25/57 (44%) P = 0.13 |
| Kidney function Navaneethan 2017 |
Progression to ESKD (dialysis or transplantation) | Adults, CKD, eGFR 15 to 45 mL/min (209); 24 months | Intervention: 4/50 (844%) Control: 1/57 (1.8%) P = 0.36 |
| Melanin index Robinson 2014a |
Spectrophotometry, right upper arm with sun protection | Adults, kidney transplant recipients (101); 6 weeks | Intervention: median ‐0.8 (range: ‐110 to 186) Control: median 5 (range: ‐193 to 108) P = 0.497 |
| Melanin index Robinson 2014a |
Spectrophotometry, right forearm with sun exposure | Adults, kidney transplant recipients (101); 6 weeks | Intervention: median 16.3 (range ‐113 to 132) Control: median 44 (range ‐56 to 317) P = 0.036 |
| Melanin index Robinson 2014a |
Spectrophotometry, cheek with sun exposure | Adults, kidney transplant recipients (101); 6 weeks | Intervention: median ‐1 (range: ‐59 to 240) Control: median 15 (range: ‐63 to 246) P = 0.114 |
| Sun damage Robinson 2014a |
Personnel assessment, right forearm | Adults, kidney transplant recipients (101); 6 weeks | Intervention: median 0 (range: ‐4 to 2) Control: median 2 (range: ‐5 to 8) P = 0.031 |
| Medication adherence | |||
| Medication adherence Reese 2017 |
Serum tacrolimus | Adults, kidney transplant recipients (117); 6 months | Intervention 1: mean 8.7 (SD 2.7) Intervention 2: mean 8.08 (SD 1.56) Control: mean 8.38 (SD 1.67) P = 0.4 |
| Medication adherence Reese 2017 |
Co‐efficient of variation for tacrolimus levels | Adults, kidney transplant recipients (117); 6 months | Intervention 1: mean 0.23 (SD 0.18) Intervention 2: mean 0.21 (SD 0.15) Control: mean 0.24 (SD 0.15) P = 0.7 |
| Medication adherence Reese 2017 |
% tacrolimus levels within range | Adults, kidney transplant recipients (117); 6 months | Intervention 1: mean 0.35 (SD 0.32) Intervention2: mean 0.37 (SD 0.26) Control: mean 0.42 (SD 0.3) P = 0.6 |
| Medication adherence Reese 2017 |
% of days bottles opened at correct times | Adults, kidney transplant recipients (117); 6 months | RR 1.41 (95% CI 1.21 to 1.65); P < 0.00 Intervention 1: 140/180 Intervention 2: 158/180 Control: 99/180 |
CI ‐ confidence interval; CKD ‐ chronic kidney disease; eGFR ‐ estimated glomerular filtration rate; ESKD ‐ end‐stage kidney disease; RR ‐ risk ratio; SD ‐ standard deviation
2.
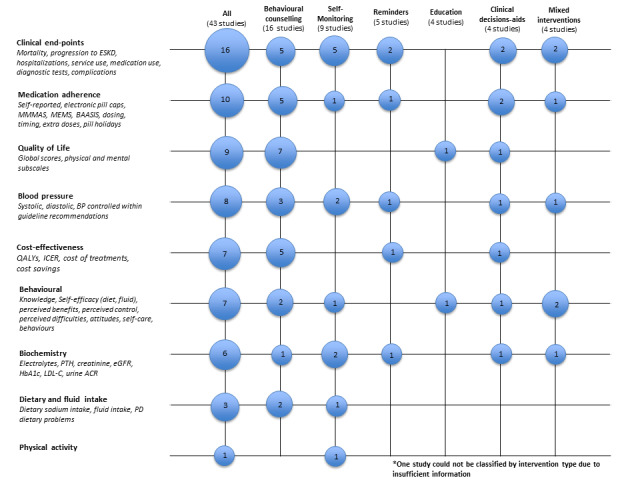
Bubble plot of reported outcomes by intervention type
Excluded studies
Nine studies (27 reports) were excluded during title and full text screening. The reasons for exclusion were study population did not have CKD (Abdel‐Kader 2011; Korus 2017; RaDIANT 2014; Roberto 2009; Wilson 2014), interventions did not include eHealth (Chen 2011e; SMILE 2010) and the wrong study design (Morales‐Barria 2016; Warren 2009).
Risk of bias in included studies
Figure 3 provides a summary of the risk of bias for the included studies with the study‐level data provided in Figure 4. Methodological quality varied considerably, with many studies providing insufficient information to accurately assess the risk of bias.
3.
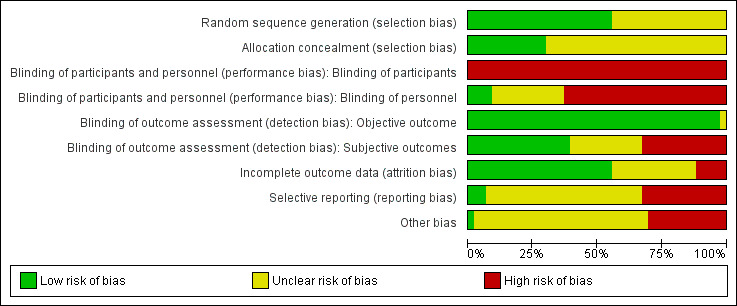
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
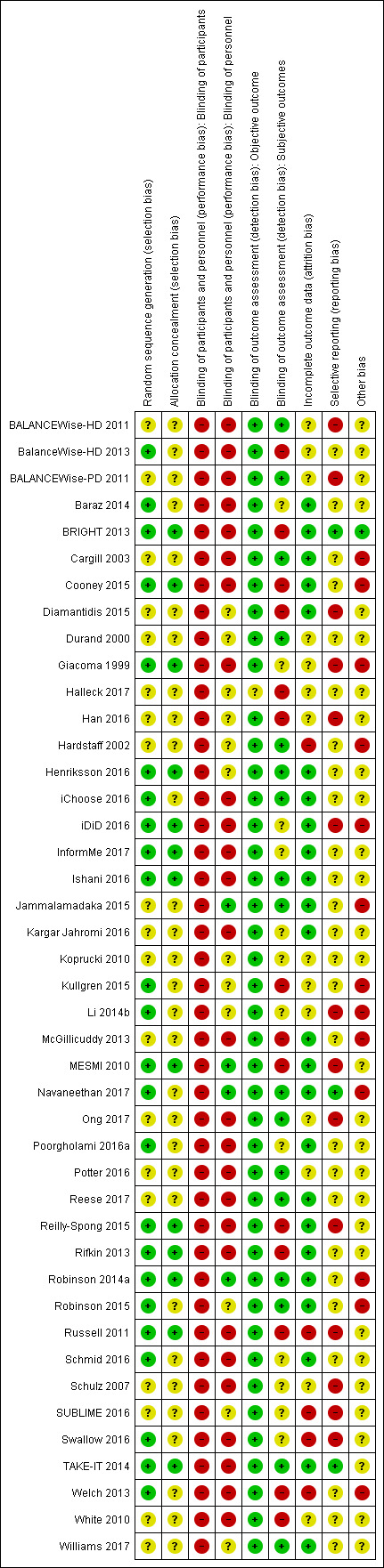
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Random sequence generation was assessed as low risk of bias in 24 studies (BalanceWise‐HD 2013; Baraz 2014; BRIGHT 2013; Cooney 2015; Giacoma 1999; Henriksson 2016; iChoose 2016; iDiD 2016; InformMe 2017; Ishani 2016; Kullgren 2015; Li 2014b; MESMI 2010; Navaneethan 2017; Poorgholami 2016a; Reilly‐Spong 2015; Rifkin 2013; Robinson 2014a; Robinson 2015; Russell 2011; Schmid 2016; Swallow 2016; TAKE‐IT 2014; Welch 2013), and unclear in the remaining 19 studies.
Allocation concealment
Allocation concealment was assessed at low risk of bias in 13 studies (BRIGHT 2013; Cooney 2015; Giacoma 1999; Henriksson 2016; iDiD 2016; InformMe 2017; Ishani 2016; MESMI 2010; Reilly‐Spong 2015; Rifkin 2013; Robinson 2014a; Russell 2011; TAKE‐IT 2014), and unclear in the remaining 30 studies with insufficient information to permit judgment.
Blinding
Performance bias
Performance bias (participants) was assessed as being at high or unclear risk of bias in all studies.
In four studies (Jammalamadaka 2015; MESMI 2010; Navaneethan 2017; Robinson 2014a) performance bias (personnel) was assessed to be at low risk of bias. Twenty‐seven studies were assessed to be at high risk of bias (BALANCEWise‐HD 2011; BalanceWise‐HD 2013; BALANCEWise‐PD 2011; Baraz 2014; BRIGHT 2013; Cargill 2003; Cooney 2015; Giacoma 1999; iChoose 2016; iDiD 2016; InformMe 2017; Ishani 2016; Kargar Jahromi 2016; McGillicuddy 2013; Ong 2017; Poorgholami 2016a; Potter 2016; Reese 2017; Reilly‐Spong 2015; Rifkin 2013; Russell 2011; Schmid 2016; Schulz 2007; Swallow 2016; TAKE‐IT 2014; Welch 2013; White 2010) and unclear in the remaining 12 studies.
Detection bias
Detection bias (objective outcomes) was assessed to be at low risk of bias in 42 studies, and unclear in one study (Halleck 2017).
Detection bias (subjective outcomes) was assessed as being at low risk of bias in 17 studies (BALANCEWise‐HD 2011; BALANCEWise‐PD 2011; Cargill 2003; Durand 2000; Hardstaff 2002; Henriksson 2016; iChoose 2016; Ishani 2016; Jammalamadaka 2015; Navaneethan 2017; Ong 2017; Potter 2016; Reese 2017; Robinson 2014a; Robinson 2015; TAKE‐IT 2014; Williams 2017), high risk of bias in 14 studies (BalanceWise‐HD 2013; BRIGHT 2013; Cooney 2015; Diamantidis 2015; Halleck 2017; Han 2016; Kullgren 2015; McGillicuddy 2013; MESMI 2010; Reilly‐Spong 2015; Rifkin 2013; Russell 2011; Welch 2013; White 2010), and unclear in the remaining 12 studies.
Incomplete outcome data
Twenty‐four studies were considered to be low risk of attrition bias (Baraz 2014; BRIGHT 2013; Cargill 2003; Cooney 2015; Diamantidis 2015; Henriksson 2016; iChoose 2016; iDiD 2016; InformMe 2017; Ishani 2016; Jammalamadaka 2015; Kargar Jahromi 2016; McGillicuddy 2013; MESMI 2010; Navaneethan 2017; Poorgholami 2016a; Reese 2017; Reilly‐Spong 2015; Rifkin 2013; Robinson 2014a; Robinson 2015; Schmid 2016; TAKE‐IT 2014; Williams 2017). Five studies (Hardstaff 2002; Russell 2011; SUBLIME 2016; Swallow 2016; Welch 2013) were assessed to be at high risk of bias as more than 20% of participants were lost to follow‐up; the remaining 14 studies were unclear due to insufficient information.
Selective reporting
Studies were considered to be at high risk of bias if data were provided in a format which could not be entered into the meta‐analyses or if stated outcomes were not reported. We assessed three studies (BRIGHT 2013; Navaneethan 2017; TAKE‐IT 2014) to be at low risk of reporting bias. Fourteen studies were assessed at high risk of reporting bias (BALANCEWise‐HD 2011; BALANCEWise‐PD 2011; Diamantidis 2015; Giacoma 1999; Han 2016; iDiD 2016; Li 2014b; MESMI 2010; Ong 2017; Reilly‐Spong 2015; Russell 2011; Schulz 2007; SUBLIME 2016; Swallow 2016), and the remaining 26 studies were unclear due to insufficient information. Ten studies only had abstracts or short reports available, limiting our ability to accurately assess reporting bias.
Other potential sources of bias
One study was assessed to be at low risk of other potential bias due to transparent reporting and following protocol (BRIGHT 2013). Thirteen studies were assessed to be at high risk of bias (Cargill 2003; Cooney 2015; Giacoma 1999; Hardstaff 2002; iDiD 2016; Jammalamadaka 2015; Kullgren 2015; Li 2014b; McGillicuddy 2013; Navaneethan 2017; Robinson 2014a; Robinson 2015; Welch 2013), and the remaining 29 studies were assessed to have unclear risk due to insufficient information.
Effects of interventions
See: Table 1
Because of considerable heterogeneity in the population, interventions, and outcomes, we were unable to generate meaningful summary estimates with the exception of death, self‐management for IDWG and dietary sodium intake. The remainder of the studies are grouped by six categories of interventions and the results summarized descriptively.
Death (all causes)
Three studies conducted in pre‐dialysis CKD populations using behavioural counselling (Ishani 2016), education (Navaneethan 2017), and clinical decision‐aid (Cooney 2015) interventions reported death (Figure 5). The certainty of evidence was considered to be very low due to high or uncertain risk of bias, imprecision and indirectness. We are uncertain whether employing various eHealth interventions reduces the number of deaths (Analysis 1.1 (3 studies, 2906 participants): RR 0.74, 95% CI 0.53 to 1.03; I2 = 0%).
5.
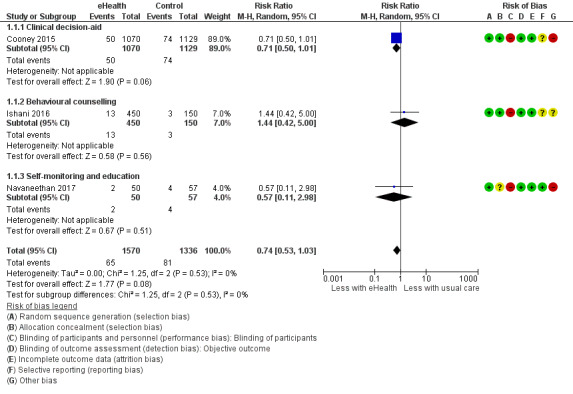
Forest plot of comparison: 1 Death, outcome: 1.1 Death.
1.1. Analysis.
Comparison 1 Death, Outcome 1 Death.
Interdialytic weight gain
Four studies conducted in HD‐dependent populations using self‐management (BalanceWise‐HD 2013) and self‐monitoring interventions (Schulz 2007; Welch 2013; Williams 2017) reported IDWG (Figure 6). The certainty of evidence was considered to be low due to high or uncertain risk of bias and indirectness. Participants using electronic self‐monitoring devices (e.g. personal digital assistants, Fitbit Flex or wireless body weight scales) reduced their average IDWG by 0.13 kg. Using an eHealth intervention to enhance patient self‐monitoring may lead to slightly improved IDWG when compared to a non‐eHealth intervention usual care group (Analysis 2.1 (4 studies, 335 participants): MD ‐0.13 kg, 95% CI ‐0.27 to 0.01; I2 = 0%).
6.
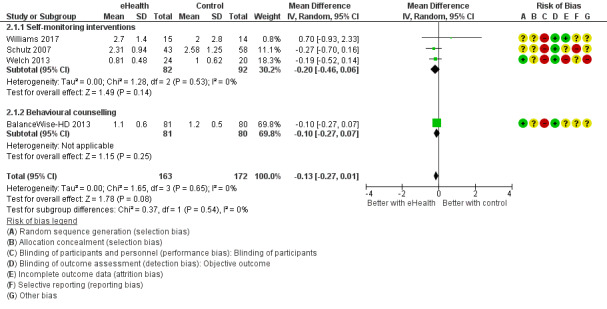
Forest plot of comparison: Interdialytic weight gain
2.1. Analysis.
Comparison 2 Interdialytic weight gains, Outcome 1 Interdialytic weight gain.
Dietary sodium intake
Two studies using behavioural counselling (BalanceWise‐HD 2013; SUBLIME 2016) and one using self‐monitoring interventions (Koprucki 2010) reported dietary sodium intake. Two were able to be combined due to similarities in target population (dialysis‐dependent populations), study length, and eHealth intervention used (BalanceWise‐HD 2013; Koprucki 2010) (Figure 7). The certainty of evidence was considered to be low due to high or uncertain risk of bias and imprecision (small sample size). Participants using an electronic dietary monitoring application consumed 197 mg less sodium/day. Self‐monitoring interventions with additional counselling from a clinician (e.g. use of personal digital assistants to track dietary intake with dietetic consultation) may lead to slightly improved dietary sodium intakes in a dialysis‐dependent population (Analysis 3.1 (2 studies, 181 participants): MD ‐196.97, 95% CI ‐540.76 to 146.83; I2 = 0%). SUBLIME 2016 did not provide sufficient detail to be included in the meta‐analysis, however they reported a statistically significant improvement in dietary sodium intake following a three‐month internet‐based self‐management intervention in CKD population when compared to a non‐eHealth control group.
7.
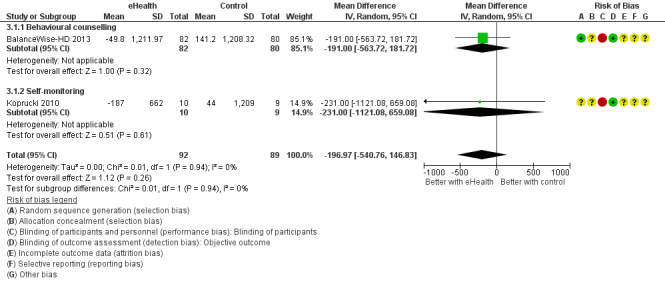
Forest plot of comparison: 5 Dietary sodium, outcome: 5.1 Dietary sodium intake.
3.1. Analysis.
Comparison 3 Dietary sodium, Outcome 1 Dietary sodium intake.
Educational interventions
Educational interventions were defined as interventions aimed at improving knowledge and skills that can be acquired by learning and instruction.
Four studies (Baraz 2014; Diamantidis 2015; Giacoma 1999; InformMe 2017) involving 457 participants evaluated educational interventions. Studies were conducted in various populations, including CKD (20 participants) (Diamantidis 2015), HD (90) (Baraz 2014), kidney transplant candidates (288) (InformMe 2017), and kidney transplant recipients (59) (Giacoma 1999).
A range of technologies were used, including iPad application (Diamantidis 2015; InformMe 2017), mobile phone text messaging (Diamantidis 2015), and video (Baraz 2014, Giacoma 1999). Three studies (Giacoma 1999, Baraz 2014, InformMe 2017) compared the eHealth intervention to usual in‐person education, while Diamantidis 2015 compared two eHealth interventions.
Knowledge was measured by Giacoma 1999 and InformMe 2017. Knowledge improved in the iPad education group compared to usual care (Analysis 8.1.1: SMD 0.59, 95% CI 0.35, 0.82; P < 0.001) (InformMe 2017) and post video‐based education (t = 4.9; P < 0.0001) (Giacoma 1999) (Table 3). InformMe 2017 evaluated participants willingness to accept a high risk donor kidney (Table 3), however there was no significant difference between the participants receiving education modules on an iPad app and those receiving usual care (Analysis 10.3.1: MD ‐0.20, 95% CI ‐0.44 to 0.03; P = 0.09). Baraz 2014 evaluated a number of quality of life domains using the SF‐36 (Table 3). There was no significant difference between oral or video education in any domains of physical or emotional quality of life. Diamantidis 2015 evaluated usability of text messaging and iPad applications and reported low rate of errors in both the text messaging and iPad application groups, however did not provide sufficient information for analysis. All outcomes reported in educational interventions are outlined in Table 3.
8.1. Analysis.
Comparison 8 Knowledge, Outcome 1 Change in knowledge (continuous).
10.3. Analysis.
Comparison 10 Behavioural outcomes, Outcome 3 Willingness to perform behaviour.
Reminder interventions
Reminder interventions were defined as systems used to prompt or aid the memory. The systems can be audible or visual alarms, computerized reminders or phone calls or messaging.
Five studies (Han 2016; Henriksson 2016; Jammalamadaka 2015; McGillicuddy 2013; Potter 2016) involving 311participants evaluated a reminder intervention. Studies were conducted in kidney transplant recipients (271 participants) (Han 2016; Henriksson 2016; McGillicuddy 2013; Potter 2016) and HD (40) (Jammalamadaka 2015). Wireless or electronic medication trays with audible and/or visual alarms (Henriksson 2016; McGillicuddy 2013; Potter 2016), mobile phone application (Han 2016), and mobile phone text message reminders (Jammalamadaka 2015) were evaluated. All five studies compared the use of an eHealth intervention to usual care.
Adherence was evaluated by three studies (Han 2016; Henriksson 2016; McGillicuddy 2013). McGillicuddy 2013 reported an improvement in medication adherence at three months (Analysis 6.2.3: MD 3.22, 95% CI 1.76 to 4.68). Henriksson 2016 only evaluated adherence in the intervention group and reported 97.9% compliance with immunosuppressive treatment at three months and 96% at 10 to 12 months. Han 2016 reported no difference in adherence between the intervention and control groups (74.1% versus 66.1%, P = 0.36). Both Potter 2016 and Henriksson 2016 reported number of biopsies performed, with Potter 2016 reporting less biopsies in the intervention group (4 versus 9). Conversely, Henriksson 2016 reported a higher rate of biopsies performed in the intervention group, with 32 biopsies needed in 17 participants, compared to 60 biopsies needed in 38 control participants. All outcomes reported in reminder interventions are detailed in Table 4.
6.2. Analysis.
Comparison 6 Medication adherence, Outcome 2 Medication adherence (continuous).
Self‐monitoring interventions
Self‐monitoring interventions were defined as interventions that are aimed at measuring one’s target behaviour and comparing to an external standard or goal that can result in lasting improvements in behaviour.
Nine studies (BALANCEWise‐HD 2011; BALANCEWise‐PD 2011; Koprucki 2010; Kullgren 2015; Ong 2017; Rifkin 2013l Schulz 2007; Welch 2013; Williams 2017) involving 498 participants utilised a self‐monitoring intervention. Studies were conducted in HD (215 participants) (BALANCEWise‐HD 2011; Schulz 2007; Welch 2013; Williams 2017), peritoneal dialysis (45) (BALANCEWise‐PD 2011; Koprucki 2010), CKD (206) (Ong 2017; Rifkin 2013), and paediatric kidney transplant recipients (32) (Kullgren 2015). Personal digital assistant (BALANCEWise‐HD 2011, BALANCEWise‐PD 2011; Koprucki 2010; Welch 2013), telemetric bodyweight machine (Schulz 2007), an interactive water bottle (Kullgren 2015), Fitbit Flex physical activity tracker (Williams 2017), and wireless transmission of clinical data to a healthcare team (Rifkin 2013) were evaluated. One study compared an interactive dietary monitoring application to a passive physical activity log (Welch 2013). Williams 2017 compared the use of a Fitbit Flex tracker with feedback regarding physical activity and sleep to no feedback. Koprucki 2010 compared an interactive dietary monitoring application plus computer‐based education module versus computer‐based education module alone. The remaining four studies (BALANCEWise‐HD 2011; Kullgren 2015; Rifkin 2013; Schulz 2007) compared an eHealth intervention to usual care.
Systolic and diastolic blood pressure was reported by three studies (Ong 2017; Rifkin 2013; Schulz 2007). Rifkin 2013 found no significant change in systolic or diastolic blood pressure between eHealth and usual care groups. Schulz 2007 found no significant chance in systolic blood pressure, however did report a significant improvement in diastolic blood pressure with the use of telemetric body weight scales compared to usual care. Ong 2017 reported a significant reduction in systolic and diastolic blood pressure with the use of a blood pressure self‐monitoring application that provided feedback, compared to a passive self‐monitoring application (MD ‐5 mmHg and ‐3.5 mmHg respectively).
Williams 2017 was the only study to report physical activity, and reported no difference in physical activity with the use of a Fitbit Flex with feedback on progress or no feedback on progress. Kullgren 2015 reported a significantly higher fluid intake in the intervention group using an interactive water bottle compared to those in the control group, however there were no differences in serum sodium, urea, or creatinine.
No data from BALANCEWise‐HD 2011 or BALANCEWise‐PD 2011 could be reported as only intervention group data was reported.
All outcomes reported in self‐monitoring interventions are outlined in Table 5.
Behavioural counselling interventions
Behavioural counselling interventions were defined as interventions aimed at enabling patients to assume responsibility for managing their condition through the systematic provision of education and supportive interventions to increase skills and confidence in managing health problems, and included regular assessment and/or progress, goal setting and problem solving support.
Sixteen studies (BalanceWise‐HD 2013; BRIGHT 2013; Cargill 2003; iDiD 2016; Ishani 2016; Kargar Jahromi 2016; Li 2014b; MESMI 2010; Poorgholami 2016a; Reilly‐Spong 2015; Russell 2011; Schmid 2016; SUBLIME 2016; Swallow 2016; TAKE‐IT 2014; White 2010) involving 2069 participants utilised a behavioural counselling intervention. Studies were conducted in CKD (1240 participants) (BRIGHT 2013; Ishani 2016; MESMI 2010; SUBLIME 2016; Swallow 2016), HD (339) (BalanceWise‐HD 2013; iDiD 2016; Kargar Jahromi 2016; Poorgholami 2016a), peritoneal dialysis (206) (Cargill 2003; Li 2014b; White 2010), kidney transplant recipients (124) (Schmid 2016; Reilly‐Spong 2015; Russell 2011), and adolescent kidney transplant recipients (169) (TAKE‐IT 2014). Telephone (Kargar Jahromi 2016; Li 2014b; Poorgholami 2016a), telephone plus website (BRIGHT 2013; iDiD 2016), telephone plus DVD education (MESMI 2010), videoconferencing support (Cargill 2003; Schmid 2016; Reilly‐Spong 2015; White 2010), Telehealth support with wireless transmission of clinical data (Ishani 2016), websites (SUBLIME 2016; Swallow 2016), personal digital assistants (BalanceWise‐HD 2013), and electronic medication monitors with clinician support (Russell 2011; TAKE‐IT 2014) were evaluated. One study compared a videoconferencing to telephone support (Reilly‐Spong 2015), All other studies compared eHealth to non‐eHealth usual care.
Fatigue was evaluated by three studies (BRIGHT 2013; Li 2014b; Reilly‐Spong 2015), with no differences detected between eHealth intervention and control groups in any studies. Four studies (Schmid 2016; MESMI 2010; Russell 2011; TAKE‐IT 2014) evaluated medication adherence.
Three studies (Russell 2011; Schmid 2016; TAKE‐IT 2014) reported significant improvements in medication adherence when using electronic monitoring plus clinician counselling. Russell 2011 reported a significant improvement in medication adherence using electronic medication monitoring with nurse education (SMD 1.27, 95% CI 0.01 to 2.53; P = 0.039). Similarly Schmid 2016 reported a significant improvement in medication adherence utilising video monitoring support with a multidisciplinary team (RR 1.90, 95% CI 1.15 to 3.14; P = 0.013). TAKE‐IT 2014 reported a significant improvement in both medication taking adherence (OR 1.66, CI 1.15 to 2.39) and timing adherence (OR 1.74, CI 1.21 to 2.50) using personalised coaching with electronic medication reminders. There was no difference in medication adherence in eHealth intervention or control groups reported by MESMI 2010.
Anxiety was evaluated by four studies (BRIGHT 2013; iDiD 2016; Kargar Jahromi 2016; Reilly‐Spong 2015). Kargar Jahromi 2016 reported a significant reduction in anxiety following a one month telephone follow‐up intervention (MD ‐5.15, 95% CI ‐6.29 to ‐4.01; P = 0.01), however BRIGHT 2013, iDiD 2016 and Reilly‐Spong 2015 found no difference in anxiety levels between eHealth intervention and control groups.
Depression was evaluated by three studies (iDiD 2016; Kargar Jahromi 2016; Reilly‐Spong 2015). Whilst Kargar Jahromi 2016 reported significantly less depression in the telephone follow‐up group (MD ‐5.09, 95% CI ‐6.22 to ‐3.96; P = 0.05), Reilly‐Spong 2015 reported higher levels in the eHealth intervention group receiving group teleconference support when compared to those a one‐on‐one telephone support (MD 0.72, 95% CI 0.15 to 1.28; P = 0.05). iDiD 2016 reported no difference in levels of depression when comparing an online CBT intervention versus online CBT with telephone support.
Three studies evaluated blood pressure (BRIGHT 2013; Ishani 2016; MESMI 2010). BRIGHT 2013 reported a significant improvement in blood pressure control when utilising a multi‐modal eHealth intervention (telephone follow‐up and website) compared to usual care. Ishani 2016 and MESMI 2010 reported no difference in blood pressure control.
Two studies (Ishani 2016; Li 2014b) evaluated hospital readmission rates, however no difference was found between eHealth intervention and control groups.
All outcomes reported by behavioural counselling interventions are outlined in Table 6.
Clinical decision‐aid interventions
Clinical decision‐aids provided clinicians or patients with knowledge and person‐specific information presented at times to enhance decision‐making.
Four studies (Cooney 2015; Durand 2000; Hardstaff 2002; iChoose 2016) involving 2543 participants utilised a clinical decision‐aid intervention. Studies were conducted in various populations, including CKD (2642 participants) (Cooney 2015; iChoose 2016), kidney transplant recipients (100) (Hardstaff 2002), and peritoneal dialysis (30) (Durand 2000). Telephone follow‐up (Cooney 2015), an online risk calculator (iChoose 2016), blue‐tooth transmission of clinical data to clinicians (Durand 2000), and Smartcap medication caps (Hardstaff 2002) were evaluated. All four studies compared an eHealth intervention to usual care.
Medication adherence was evaluated by two studies (Cooney 2015; Hardstaff 2002). Hardstaff 2002 reported an improvement in medication adherence in the eHealth group compared to usual care (RR 1.9, 95% CI 1.15 to 3.14). Cooney 2015 reported no significant difference in medication adherence between those receiving telephone follow‐up and those who did not (MD ‐0.08, 95% CI ‐0.17 to 0.00); however 51.5% of the intervention group did not receive the intervention. Cooney 2015 reported a lower rate of death in the intervention group, however this did not reach statistical significance (RR 0.71, 95% CI 0.5 to 1.01; P = 0.06).
All outcomes reported in clinical decision‐aid interventions are outlined in Table 7.
Mixed interventions
Four studies (Navaneethan 2017; Reese 2017; Robinson 2014a; Robinson 2015) involving 602 participants employed interventions with multiple strategies. Three studies were conducted in kidney transplant recipients (Reese 2017; Robinson 2014a; Robinson 2015) and one study in CKD (Navaneethan 2017). Reese compared usual care to a reminder intervention and a reminder plus education intervention. Robinson 2014a compared a paper based education module electronic reminders to usual care; Robinson 2015 compared an iPad education module with electronic reminders top usual care; and Navaneethan 2017 compared usual care (electronic self‐monitoring) to usual care plus education (direction to an educational website).
Knowledge, self‐care behaviours, attitudes towards performing behaviour and willingness to perform behaviour were evaluated by two studies (Robinson 2014a; Robinson 2015). There was a significant improvement in knowledge, self‐care behaviours, attitudes towards performing behaviour and willingness to perform behaviour in the eHealth intervention groups of both studies.
Reese 2017 reported a significant improvement in medication adherence from three to six months of the study with 55% adherence in usual care versus 78% in the reminders group and 88% in the reminders plus education group (P < 0.001).
Navaneethan 2017 reported no significant difference in rate of kidney function decline, rate of hospitalisations, dialysis initiation or transplantation and death during the two year study period between usual care and the additional educational intervention group.
All outcomes reported in mixed intervention studies are detailed in Table 8.
Cost‐analysis
Seven of 43 studies described costs associated with delivery of the eHealth intervention. Five studies (BRIGHT 2013; Durand 2000; Henriksson 2016; Schmid 2016; SUBLIME 2016) reported cost‐savings associated with the use of eHealth interventions. Positive cost‐analyses were based on cost of unexpected treatments (e.g. rejections, unplanned hospital admissions, increased specialist consultant visits) being higher in control groups or intervention groups having lower cost of treatment due to improved disease control (reduced blood pressure, reduced sodium intake). Cargill 2003 reported significantly higher costs due to set up of videophones and internet lines and ongoing phone charges, and one study (iDiD 2016) reported increased costs due to the increased rate of inpatient hospital admissions, that the authors attributed to the unevenly distributed allocation to the intervention arm.
Acceptability and feasibility
Eighteen studies measured acceptability (e.g. satisfaction, ease of use) and feasibility (e.g. intervention adherence and uptake). Studies reported participant satisfaction due to ease of use, low burden of eHealth intervention, informative and enjoyment of increased interactions with healthcare staff. eHealth interventions were reported as feasible due to high uptake and high levels of participant satisfaction. However, technical issues (e.g. poor internet connection or device failure) were reported to limit intervention uptake (Cargill 2003; McGillicuddy 2013).
Harms
Only Henriksson 2016 reported that six participants had prematurely withdrawn from the electronic medication monitoring trial due to feeling overly monitored. Other potential harms were not reported by any studies.
Discussion
Summary of main results
We identified 43 studies (93 reports, 6617 participants) that were conducted using a variety of eHealth technologies to replace or enhance standard care in CKD. eHealth interventions were evaluated for a mean of 12 weeks (ranging from one clinic appointment to 12 months), with the majority of studies (27; 63%) enrolling less than 100 participants. Interventions were classified as either educational, reminders, self‐monitoring, behavioural counselling clinical decision aids or mixed interventions, and were either compared to traditional methods (e.g. face‐to‐face counselling) (20) or to a different eHealth intervention (12); in 11 studies the control group was not described. The studies included in this review involved people with CKD stage 1‐5, dialysis‐dependent populations, transplant recipients and transplant candidates; the majority of studies were conducted in an adult population (40 studies).
There was considerable heterogeneity between eHealth intervention designs and eHealth technologies used. The multiplicity of outcomes reported limited our ability to conduct meaningful meta‐analyses. Only three outcomes could be meta‐analysed (dietary sodium intake, IDWG, death) due to substantial variation between eHealth intervention, study population and study length. Clinical end‐point outcomes were the most frequently reported, with 16 studies reporting 25 different clinical end‐points, 19 of which were only reported by one study. Additionally, there was a substantial number of behavioural, biochemical, and quality of life outcomes reported by only one study, limiting our ability to synthesize the data and formulate conclusions. Also, high or unclear risk of bias in many of the included studies, combined with imprecision in effect measurements, indirectness of interventions and study populations and poor reporting of study results led to low confidence in results. No studies in this review reported on outcomes related to physical activity or nutritional status.
Overall, these data suggest that current evidence for eHealth interventions in the CKD population is of low quality and is insufficient to guide clinical practice. However, possible benefits may be reduced costs relating to patient care. The increasing use of technology in people’s lifestyles, and the high levels of participant satisfaction and acceptability reported by studies, suggest that eHealth interventions may offer an adjunct to usual care in CKD. However, due to the low and very low quality of evidence it is unclear whether eHealth interventions alone alter health related behaviours in CKD population. Additionally, it remains unclear whether eHealth interventions offer a cost‐effective alternative to current treatment models.
Overall completeness and applicability of evidence
The strengths of this review include comprehensive systematic searching for eligible studies, rigid inclusion criteria for RCTs and data extraction and analysis by two independent investigators, which limited the risk of errors in determining study eligibility, data extraction, risk of bias assessment, and data synthesis. We aimed to evaluate the effectiveness of eHealth interventions to improve a range of important outcomes for people with CKD. We could not robustly assess the effect of eHealth as there were few studies of sufficient size and duration with adequate reporting of methods and outcomes to examine clinical or patient outcomes. The variability in outcome measures and measurement tools used limited our ability to synthesize the data, and the use of standardised outcomes would be helpful in the future.
Quality of the evidence
We assessed the quality of evidence using GRADE methodology. Full‐length journals were available for 33 studies, whilst 10 studies had only abstracts or short reports available. Included studies were commonly reported incompletely and were of poor methodological quality. The majority of studies were assessed to be at high risk or uncertain risk of bias relating to selection bias, performance bias, detection bias (subjective outcomes), reporting bias, and other biases. The high level of uncertain risk of bias assessment was due to poor methodological and outcome reporting of studies.
The overall certainty of evidence using GRADE was assessed as low for dietary sodium intake and IDWG. Our ability to conduct meta‐analyses was limited due to small, heterogeneous study populations, substantial variability of eHealth technologies used and the multiplicity of reported outcomes.
Potential biases in the review process
Potential biases in this review relate to the data availability in the individual studies. Firstly, the small number of data observations limited our ability to conduct robust statistical estimates of heterogeneity and meant we could not assess for potential publication bias due to the small number of studies. Secondly, studies were frequently at high risk of bias but poorer quality studies could not be excluded from sensitivity analyses due to the limited number of data observations. Thirdly, adverse event reporting in the available studies was inconsistent and infrequent. Finally, whilst a comprehensive search of the Cochrane Kidney and Transplant Specialised Register was performed for this review, reducing the possibility that potential eligible studies were omitted from the review, eligible studies published after the last search date or published in congress proceedings not routinely searched could have been missed.
Agreements and disagreements with other studies or reviews
Systematic reviews have evaluated the impact of various eHealth interventions such as telephone or mobile phone text message reminders (Beratarrechea 2014; Hamine 2015), electronic reminders (Tao 2015; Vervloet 2012) and electronic medication packaging (Checchi 2014) on treatment and medication adherence in non‐CKD, chronic disease populations. These reviews have reported positive improvements in medication adherence and appointment adherence, however similar to our review, authors highlighted poor methodological quality limiting the results of these interventions. Tao 2015 evaluated 22 RCTs (3152 participants) reported a 29% improvement in medication adherence with the use of electronic reminders (95% CI 0.18 to 0.41; P = 0.00). Similar to our findings, the authors highlight that the small number of studies and high heterogeneity of interventions limited results and any robust conclusions.
We were unable to conduct sensitivity analyses due to the small number of studies included in our meta‐analyses; we could not form any conclusions about the impact of type of technology used, behaviour change techniques and intensity of intervention. However in previous literature, it has been reported that eHealth interventions that were individualised and incorporated strategies such as self‐monitoring, personalised feedback and group or peer support resulted in significantly better outcomes, such as weight loss and diet and physical activity behaviours (Cotter 2014; Raajimakers 2015). It has also been reported that web, mobile phone text messaging and telemedicine technologies were more effective at improving CVD outcomes, than email, mobile phone, applications and monitoring sensors (Widmer 2015). Similar to our review, other systematic reviews evaluating eHealth interventions have been limited by small number of studies of low methodological quality. A previous systematic review of mobile technology interventions reported that overall usability, feasibility and acceptability were high among end‐users, and resulted in increased self‐management and knowledge (Hamine 2015). Our review also indicates that participant satisfaction was high for eHealth interventions (including video monitoring, Telehealth, dietary monitoring applications and websites). Similar to previous reviews (Kitsiou 2017; Sanyal 2018), economic evaluation of interventions in our review was lacking and insufficient to evaluate the cost‐effectiveness of these interventions.
Authors' conclusions
Implications for practice.
Overall, these data suggest that current evidence for the use of eHealth interventions in the CKD population is of low quality and insufficient to make a recommendation regarding their use to improve clinical care. Further cost‐analysis data is needed to ascertain whether eHealth interventions offer a cost‐effective alternative to standard practice. However, eHealth interventions appear to be acceptable to patients and feasible if technical issues are managed. Our findings indicate that eHealth interventions utilising behavioural counselling or self‐monitoring may help to improve fluid management and dietary sodium intake in dialysis patients, however further evaluation is needed. This has been supported by studies conducted in other chronic diseases (Cotter 2014; Raajimakers 2015) that found interventions using self‐monitoring, personalized feedback and peer group support improved outcomes. Current evidence from our review was insufficient to make recommendations for incorporation of specific eHealth strategies to enhance current care. Utilizing self‐monitoring techniques, providing personalized feedback and facilitating peer group support may enhance future practice and should be further evaluated in the CKD population.
Implications for research.
Questions remain about the impact of eHealth interventions on clinical end‐points and patient‐centred outcomes in the CKD population, with additional studies in CKD required to evaluate the impact of eHealth interventions to patient care. Future research should focus on larger scale trials to allow for meaningful interpretation of results. Additionally, evaluation and reporting of trials should be based on established frameworks that maintain methodological quality. Our review has highlighted the need for robust, high quality research that reports core (minimum) data set as outlined by the SONG collaboration (SONG 2017), including both clinical and patient‐centred outcomes, to enable meaningful evaluation of literature. Further, cost‐effectiveness, process and qualitative evaluations of interventions are needed to ensure robust assessment of the impact of these interventions.
Evidence of the use of established frameworks to design and evaluate the interventions included in this review, such as the Behaviour Change Wheel, CONSORT‐EHEALTH, RE‐AIM, was lacking. Future studies would benefit from drawing on frameworks that require theoretical modelling between processes and outcomes and a process evaluation of the study (Craig 2008; Michie 2013). All studies should provide greater description of intervention and standard models of care being assessed (Hoffmann 2014; Warner 2017) and include process evaluations of how they are being implemented (Moore 2013), using reporting guidelines for complex interventions.
In diabetic populations the use of these alert systems has improved medication adherence (Tao 2015) and highlights an important area in CKD that warrants further evaluation. Our systematic review reported on five studies using electronic alerts however due to small sample sizes and poor methodological quality we have been unable to provide recommendations for the use of these alerts. Based on our findings and previous literature (Cotter 2014; Raajimakers 2015) interventions incorporating self‐monitoring and personalised counselling should be further pursued.
Acknowledgements
We wish to thank the referees for their comments and feedback during the preparation of this manuscript. We thank the staff of Cochrane Kidney and Transplant (Fiona Russell, Gail Higgins and Narelle Willis) for their assistance with this review. We are grateful to corresponding authors who provided additional information regarding study design.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 3. World Health Organization digital health intervention classifications
| Type of intervention | Example of intervention | Studies |
| 1. Targeted client communication |
|
Baraz 2014; Cargill 2003; Cooney 2015; Giacoma 1999; Han 2016; Henriksson 2016; iChoose 2016; iDiD 2016; InformMe 2017; Jammalamadaka 2015; Kargar Jahromi 2016; Li 2014b; McGillicuddy 2013; Poorgholami 2016a; Potter 2016; Reese 2017; Reilly‐Spong 2015; Robinson 2014a; Robinson 2015; SUBLIME 2016; TAKE‐IT 2014 |
| 2. Untargeted client communication |
|
‐‐ |
| 3. Client‐to‐client information |
|
‐‐ |
| 4. Personal health tracking |
|
BALANCEWise‐HD 2011; BALANCEWise‐PD 2011; Durand 2000; Hardstaff 2002; Koprucki 2010; Kullgren 2015; Ong 2017; Rifkin 2013; Schulz 2007; Welch 2013; Williams 2017 |
| 5. Citizen based reporting |
|
‐‐ |
| 6. On‐demand information services to clients |
|
Diamantidis 2015 |
| 7. Client financial transactions |
|
‐ |
| One study could not be classified (Halleck 2017) Eight studies used multiple strategies (e.g. targeted client communication and personal health tracking) (BalanceWise‐HD 2013; BRIGHT 2013; Ishani 2016; MESMI 2010; Navaneethan 2017; Russell 2011; Schmid 2016; Swallow 2016; White 2010) | ||
Data and analyses
Comparison 1. Death.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 3 | 2906 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.03] |
| 1.1 Clinical decision‐aid | 1 | 2199 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.50, 1.01] |
| 1.2 Behavioural counselling | 1 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.42, 5.00] |
| 1.3 Self‐monitoring and education | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.11, 2.98] |
Comparison 2. Interdialytic weight gains.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interdialytic weight gain | 4 | 335 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.27, 0.01] |
| 1.1 Self‐monitoring interventions | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.46, 0.06] |
| 1.2 Behavioural counselling | 1 | 161 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.27, 0.07] |
Comparison 3. Dietary sodium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dietary sodium intake | 2 | 181 | Mean Difference (IV, Random, 95% CI) | ‐196.97 [‐540.76, 146.83] |
| 1.1 Behavioural counselling | 1 | 162 | Mean Difference (IV, Random, 95% CI) | ‐191.0 [‐563.72, 181.72] |
| 1.2 Self‐monitoring | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐231.0 [‐1121.08, 659.08] |
Comparison 4. Quality of Life (physical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 General health perception | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Educational intervention | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.83, 0.00] |
| 1.2 Behavioural counselling | 2 | 507 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.14, 0.21] |
| 2 Physical functioning | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Educational intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Behavioural counselling | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Role‐physical | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Educational intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Behavioural counselling | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pain | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Educational intervention | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.34, 0.49] |
| 4.2 Behavioural counselling | 3 | 191 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.42, 0.77] |
| 5 Physical Component Score (PCS) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Behavioural counselling | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Clinical decision‐aid | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Burden (KDQoL) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Behavioural counselling | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Clinical decision‐aid | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Effects (KDQoL) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Behavioural counselling | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Clinical decision‐aid | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
Comparison 4 Quality of Life (physical), Outcome 1 General health perception.
4.2. Analysis.
Comparison 4 Quality of Life (physical), Outcome 2 Physical functioning.
4.3. Analysis.
Comparison 4 Quality of Life (physical), Outcome 3 Role‐physical.
4.4. Analysis.
Comparison 4 Quality of Life (physical), Outcome 4 Pain.
4.5. Analysis.
Comparison 4 Quality of Life (physical), Outcome 5 Physical Component Score (PCS).
4.6. Analysis.
Comparison 4 Quality of Life (physical), Outcome 6 Burden (KDQoL).
4.7. Analysis.
Comparison 4 Quality of Life (physical), Outcome 7 Effects (KDQoL).
Comparison 5. Quality of Life (mental).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental Health (SF‐36) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Educational | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐5.23 [‐15.07, 4.61] |
| 1.2 Behavioural counselling | 2 | 507 | Mean Difference (IV, Random, 95% CI) | 1.06 [‐2.24, 4.35] |
| 2 Social functioning (SF‐36) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Educational | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 3.68 [‐4.45, 11.81] |
| 2.2 Behavioural counselling | 2 | 506 | Mean Difference (IV, Random, 95% CI) | 1.94 [‐3.35, 7.22] |
| 3 Fatigue | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Educational | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.81, 0.02] |
| 3.2 Behavioural counselling | 3 | 546 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.05, 0.28] |
| 4 Anxiety | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Behavioural counselling | 4 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Depression | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Behavioural counselling | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Sleep | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Behavioural counselling | 2 | 186 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.55, 0.69] |
| 7 Role‐emotional | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Education | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Behavioural counselling | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Mental Component Score (MCS) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Behavioural counselling | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Clinical decision‐aid | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
Comparison 5 Quality of Life (mental), Outcome 1 Mental Health (SF‐36).
5.2. Analysis.
Comparison 5 Quality of Life (mental), Outcome 2 Social functioning (SF‐36).
5.3. Analysis.
Comparison 5 Quality of Life (mental), Outcome 3 Fatigue.
5.4. Analysis.
Comparison 5 Quality of Life (mental), Outcome 4 Anxiety.
5.5. Analysis.
Comparison 5 Quality of Life (mental), Outcome 5 Depression.
5.6. Analysis.
Comparison 5 Quality of Life (mental), Outcome 6 Sleep.
5.7. Analysis.
Comparison 5 Quality of Life (mental), Outcome 7 Role‐emotional.
5.8. Analysis.
Comparison 5 Quality of Life (mental), Outcome 8 Mental Component Score (MCS).
Comparison 6. Medication adherence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Medication adherence (dichotomous) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Reminders | 1 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.21, 1.65] |
| 1.2 Behavioral counselling | 2 | 776 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.86, 2.24] |
| 1.3 Clinical decision aid | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.45, 1.15] |
| 1.4 Education plus reminders | 1 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.38, 1.84] |
| 2 Medication adherence (continuous) | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Behavioural counselling | 3 | 248 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.51, 0.57] |
| 2.2 Self‐monitoring intervention | 1 | 43 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.78, 0.47] |
| 2.3 Reminders | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | 3.22 [1.76, 4.68] |
| 2.4 Clinical decision aid | 1 | 2199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.17, 0.00] |
6.1. Analysis.
Comparison 6 Medication adherence, Outcome 1 Medication adherence (dichotomous).
Comparison 7. Change in serum creatinine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in serum creatinine | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Self‐monitoring interventions | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.65, 0.37] |
| 1.2 Behavioural counselling | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐7.29, 3.29] |
7.1. Analysis.
Comparison 7 Change in serum creatinine, Outcome 1 Change in serum creatinine.
Comparison 8. Knowledge.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in knowledge (continuous) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Education interventions | 1 | 288 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.35, 0.82] |
| 1.2 Education plus reminders | 2 | 271 | Std. Mean Difference (IV, Random, 95% CI) | 1.35 [1.08, 1.61] |
| 1.3 Behavioural counselling | 1 | 366 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.21, 0.21] |
| 1.4 Clinical decision aid | 1 | 443 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.15, 0.52] |
Comparison 9. Hospitalisation rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospitalisation rate (dichotomous) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Behavioural counselling | 2 | 735 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.65, 1.58] |
| 1.2 Clinical decision aid | 1 | 730 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.29] |
| 1.3 Reminders | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.48, 1.05] |
| 2 Hospitalisations (continuous) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Self‐monitoring interventions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Education | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Behavioural counselling | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
Comparison 9 Hospitalisation rate, Outcome 1 Hospitalisation rate (dichotomous).
9.2. Analysis.
Comparison 9 Hospitalisation rate, Outcome 2 Hospitalisations (continuous).
Comparison 10. Behavioural outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Self‐care behaviours | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Behavioural counselling | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Education plus reminders | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Attitudes towards performing a behaviour | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Education plus reminders | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Self‐monitoring intervention | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Willingness to perform behaviour | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Educational intervention | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Education plus reminders | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.
Comparison 10 Behavioural outcomes, Outcome 1 Self‐care behaviours.
10.2. Analysis.
Comparison 10 Behavioural outcomes, Outcome 2 Attitudes towards performing a behaviour.
Comparison 11. Blood pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic blood pressure | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Self‐monitoring intervention | 2 | 144 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐8.34, 2.99] |
| 1.2 Behavioural counselling | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐3.90 [‐12.40, 4.60] |
| 1.3 Reminder systems | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐24.20 [‐36.41, ‐11.99] |
| 1.4 Clinical decision aids | 1 | 947 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.53, 2.93] |
| 2 Diastolic blood pressure | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Self‐monitoring intervention | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 1.56 [‐1.56, 4.69] |
| 2.2 Behavioural counselling | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 0.85 [‐3.07, 4.77] |
| 3 BP within guideline recommendations | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Behavioural counselling | 2 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.37] |
| 3.2 Clinical decision‐aid | 1 | 870 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.19] |
| 3.3 Reminder systems | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 4.5 [0.63, 32.38] |
| 3.4 Education | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.09] |
11.1. Analysis.
Comparison 11 Blood pressure, Outcome 1 Systolic blood pressure.
11.2. Analysis.
Comparison 11 Blood pressure, Outcome 2 Diastolic blood pressure.
11.3. Analysis.
Comparison 11 Blood pressure, Outcome 3 BP within guideline recommendations.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
BALANCEWise‐HD 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Adherence to diet self‐monitoring (intervention group only)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Participants could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | The intervention and attention control activities were conducted by study staff as an addition to, but not as a replacement for, standard care |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Number of meals entered was an objective measure |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were measured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Primary outcomes were not reported |
| Other bias | Unclear risk | Inadequate sample size to meet power calculation |
BalanceWise‐HD 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a permuted block algorithm developed by the study statistician |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Participants could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | The intervention and attention control activities were conducted by study staff |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Use of objective measures (IDWG, adherence) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self‐reported dietary sodium intake and perceived difficulties questionnaire are subjective |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall 89.4% completion rate ‐ reasons for drop out reported but no mention if significantly different |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
BALANCEWise‐PD 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Participants could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | The intervention and attention control activities were conducted by study staff |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Number of meals entered was an objective measure |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were measured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Primary outcomes were not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Baraz 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Outcome measured at baseline and 6 months post intervention
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation was performed by using the random computer‐generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded due to the nature of the intervention |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Principle investigator delivered the intervention |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | No objective outcomes were measured |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Validated measure, however QoL is subjective and conducted in unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% to 8% loss‐to‐follow‐up in both groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
BRIGHT 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcomes measured at baseline and 6 months
Secondary outcomes measured at baseline and 6 months
Intervention uptake and evaluation measured at 6 months
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patient will be allocated to a trial arm via a minimization algorithm (incorporating a random component) |
| Allocation concealment (selection bias) | Low risk | Allocation adequately concealed using central allocation |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "Neither researchers or participants were blinded" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "Neither researchers or participants were blinded" |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective measures at low risk of bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Subjective measures self‐report questionnaires filled out by unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention Self‐Report: 16.2% loss to follow‐up Intervention BP: 9.4% loss to follow‐up; intention‐to‐treat analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the protocol were reported |
| Other bias | Low risk | No other biases detected |
Cargill 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised using sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomised using sealed envelopes" |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | No mention of blinding but likely this would have been broken |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective measures (hospitalisations, ward visits and cost of intervention) less likely to be biased |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants had outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Very low uptake of intervention; small sample size |
Cooney 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
Primary clinical outcome
Primary process of care outcome
Secondary clinical outcomes
Secondary process of care outcomes
Acceptability
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blinded computer‐generated randomisation list and a 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Blinded computer‐generated randomisation list |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Personnel responsible for data collection and analysis were blinded to study group assignment, however study pharmacists conducted phone surveys and reviews so blinding would have been broken There were no study‐related clinic visits for this pragmatic trial |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective measures at low risk of bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | "study pharmacists" phone surveys assessed QoL, med adherence HL and acceptability "The phone surveys assessed health related quality of life (SF‐12), medication adherence using the Morisky medication scale, Kidney Disease Quality of Life (KDQOL) Short form, health literacy, and the acceptability of the intervention" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used intention‐to‐treat analyses |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | No standardised methods for measuring BP, limited ability for the pharmacist to intervene, only 23% seen by Nephrologist, therefore medication doses etc would not have been changed, Only 518 patients in intervention group actually received intervention so this may have diluted the benefits |
Diamantidis 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
SMS text
PDA
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | No objective outcomes were measured |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants had to record what responses came out which may have resulted in some inaccurate answers being recorded by accident, satisfaction survey ‐ no mention of whether validated or how it was administered but could be at risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | High risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Trial patients not using their own medications or prescriptions, cash incentives small population ‐ not necessarily representative. This was a usability trial |
Durand 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Unlikely could have been blinded as transmitting information |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Unlikely personnel could have been blinded due to receiving information from patients, no mention of blinding |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | All outcome measures are objective |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were measured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Giacoma 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Outcomes measured at baseline and day 7 of admission
Outcomes measured day of admission, day of surgery, days 1, 2, 3, 7, 10 post surgery and day of discharge
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes were randomly picked by participants |
| Allocation concealment (selection bias) | Low risk | Sealed envelope draw with non replacement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Unblinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | No mention of whether personnel were blinded. Nurse gave knowledge questionnaire and then provided video ‐ so unlikely impossible to blind person giving intervention |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Blinding would not affect outcome as objective |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Nurse administering, non‐validated questionnaire who was aware of allocation. No mention of whether this nurse was blinded to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Insufficient information to permit judgement, reported outcomes (i.e. long term knowledge retention) was not originally stated in methods. |
| Other bias | High risk | Small sample size and not powered; use of non‐validated knowledge questionnaire |
Halleck 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Participants could not be blinded given the nature of this intervention |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Objective outcome | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Unclear how knowledge will be assessed, MMAS is a self‐reported measure of adherence so at high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Han 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcome is medication adherence
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Unlikely could be blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | objective adherence measurement, MEMS |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self‐reported medication adherence |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Preliminary data only |
| Selective reporting (reporting bias) | High risk | Insufficient information to permit judgement, preliminary data |
| Other bias | Unclear risk | Insufficient information to permit judgement, preliminary data |
Hardstaff 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcome
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Intervention Feedback group received feedback at first outpatient appointment, therefore could not have been blinded |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Adherence downloaded from smart top lid ‐ objective |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were measured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10% loss to follow‐up at 3 months, 36% loss to follow‐up at 12 months |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | This study was performed on willing volunteers who most likely represented our more compliant patients. The data available included patients who, on the whole, remembered to bring the bottles to clinic and also returned the bottles at the end of the study. The outstanding data are on the remaining patients who have not returned the bottles because they kept forgetting to bring them and so are likely to represent the less compliant patients in this cohort |
Henriksson 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Outcomes measured at baseline and 10 clinic visits over 12 months Primary outcome
Secondary outcomes (obtained from patient charts)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "patients were randomized to intervention or control using prenumbered, sealed, and opaque envelopes in four batches (20 per batch)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Each envelope randomly contained a note allocating the patient to either control or intervention. The randomization envelopes were assigned to the enrolled patients in consecutive order (1‐80)" |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Participants could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | No mention of blinding, other than statistician was blinded |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | The data were obtained from patient charts and the web‐based software according to the study plan, over 10 visits in 1 year, by 2 of the investigators. All outcomes were objective. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of all scheduled outpatient follow‐up visits during the 1‐year period (22 visits/patient), 6 participants missed a total of 11 visits (1%). There was no significant difference between the intervention and control groups. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
iChoose 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcome
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "research assistants obtained informed consent and randomized patients 1:1 with a random number generator application via iPad to receive center‐specific standard of care education about kidney transplant with (intervention) or without (control) supplemental use of iChoose Kidney" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "via iPad" |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "neither patients nor providers were blinded to the study group assignment" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "neither patients nor providers were blinded to the study group assignment" |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | No objective outcomes were measured |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Transplant knowledge was measured using a nine‐item scale developed by a multidisciplinary group of transplant nephrologists, surgeons, behavioral scientists, and patients that was included in the patient baseline and follow‐ up surveys" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nil loss to follow‐up; follow‐up is only 1 clinic appointment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
iDiD 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcome
Secondary outcomes (baseline, 12 weeks)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated random number generator with a 1:1 ratio was used |
| Allocation concealment (selection bias) | Low risk | The patient was informed of their group allocation via the online CBT program. The allocation sequence remained concealed from the trial coordinator and psychological therapists/supervisors |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Participants were informed of their allocation |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Blinding likely would have been broken for some participants as it was necessary for the research team to complete follow‐up measures with some patients. |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Measures of feasibility were objective and less likely to be biased |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Validated tools to measure self‐reported depression and anxiety used. Participants were asked to complete themselves, however some participants required assistance from research personnel which may have led to bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For primary outcome analyses 92% of participants completed follow‐up data, no detail as to which group had loss to follow up. |
| Selective reporting (reporting bias) | High risk | Satisfaction, serious adverse events and treatments for depression and anxiety were not reported |
| Other bias | High risk | Did not meet sample size requirement (66), randomisation of 1:1 was not achieved with no explanation why deviated from this |
InformMe 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes concealed until study arm was assigned |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Trial was single blinded; research team members assessing outcomes were blinded to assignments to the intervention |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | No objective outcomes were measured |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Outcomes were subjective and administered by research personnel who could have been made aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 people dropped out with no significant differences between them and those who did not drop out but data not shown |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Provided with financial incentives, higher drop‐out/refusal in intervention group; met sample size goal |
Ishani 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcome (measured at 12 months)
Secondary outcomes (measured at 12 months)
Intermediate study outcomes (measured at 12 months)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly assigned to receive the intervention or usual care using a centralized computer‐generated randomization scheme using permuted block sizes of 2, 4, or 6. Randomization was stratified by eGFR (<30 vs >30 mL/min/1.73 m2), presence of diabetes, and occurrence of a hospitalization in the past year" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization occurred over the telephone by an individual blinded to patient identity" |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Likely blinding would have been broken |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Outcome assessors were blinded. all outcomes were objective |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant (of 601) withdrew consent; used intention‐to‐treat analyses |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | baseline characteristics between groups similar, limited generalisability possible due to high proportion of men, met sample size calculation for power |
Jammalamadaka 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract stated "randomised", author contacted and said "we did not randomise" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Author contacted ‐ "both participants and personnel were blinded to the strategy" however participants would have known whether receiving text message reminders |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Author contacted ‐ "both participants and personnel were blinded to the strategy", as intervention only 1 week blinding may have been upheld |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Serum phosphate |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | author quote: "no loss to follow‐up" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Small sample size, short study duration and follow‐up, unlikely to change primary outcome in 7 days, intervention participants younger than control |
Kargar Jahromi 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | "double blind" however participants could not have been blinded to their allocation |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | "double blind" researchers conducted the intervention unlikely they could have been blinded to a participants allocation Quote: "All interventions are conducted by the researcher responsible for this trial" |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | No objective outcomes were measured |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | DASS completed before intervention was carried out and then after whilst self‐report this is a validated tool. No mention of whether research personnel present while people filling out. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up in both groups (10%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Small sample size limiting generalizability |
Koprucki 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Outcome measures taken at baseline and 4 months
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Unlikely could have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective measures low risk of bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient information to permit judgement on who administered subjective measures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Small sample size |
Kullgren 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Outcome measures assessed at baseline and 1 month
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Blinding not possible |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | No reporting of blinding of personnel |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Biochemical measures of creatinine, BUN and sodium are objective |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Self‐reported measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reported loss to follow up or incomplete diaries |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | small sample size ‐ population not generalisable, limited follow‐up time, control and intervention groups significantly different with respect to time since transplant, low uptake rate of the intervention |
Li 2014b.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Unable to be blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | No mention but probably not blinded because of nature of intervention |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Hospital records are objective |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No mention of whether blinded, some measures (QoL) used validated measures, while others (health service utilisation) was self‐reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout (13.7% to 17.5%), no mention of whether these drop outs significantly different; only those with full outcome data included in study; reasons for drop outs similar across both groups |
| Selective reporting (reporting bias) | High risk | Insufficient information to permit judgement |
| Other bias | High risk | Small sample size, short duration ‐ not generalisable under powered |
McGillicuddy 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Outcomes measured baseline, month 1, month 2, month 3
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | No mention of which personnel involved. non adherent messages etc were sent to the study coordinator |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective measures (SBP) are at low risk of bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants self reported this outcome and were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Before randomisation quite high dropout but after only one person dropped out of the intervention group because the clinic schedule was incompatible for the patient to continue. The researchers were aiming to get 20 participants and achieved this |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Small sample not likely generalisable randomisation of intervention and control results in sig diff in age and adherence which questions the validity of conclusions. could not participant in the study if they did not have strong cellular signal at their house. This may skew the data against rural participants, or those who are more time poor |
MESMI 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Outcomes measured at 0, 3, 6 and 9 months post intervention
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation |
| Allocation concealment (selection bias) | Low risk | Identity kept in locked cabinet and research assistant blinded to allocation |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Research assistant blinded and participants asked not to discuss their allocation with research assistant when measures taken |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Adherence (Morisky's, pill count, SF‐12 ‐ validated, serum levels, BP) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | QoL, self‐efficacy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 5% lost to follow up |
| Selective reporting (reporting bias) | High risk | No reporting of QoL (SF12), medication adherence self‐efficacy scale or health care utilisation in paper as were outlined in protocol |
| Other bias | Unclear risk | Study was under powered |
Navaneethan 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcome Change in eGFR Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme that was stratified by family health centre |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization allocation was concealed" however not detail on how this was achieved |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "Participants were aware of their assignment" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "Study personnel (study coordinator and the navigators) were aware of their assignment, but the outcome assessors were not aware of the study assignments". |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | All outcomes are objective and at low risk of bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective measures being used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All stated outcomes have been reported |
| Other bias | High risk | "We did not power the study specifically to estimate the interaction of the two interventions" |
Ong 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcomes (measured at baseline, 6 months, 12 months)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Insufficient information to permit judgement, however unlikely as providers are given real time alerts |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | BP is objective |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective measures reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 25 withdrew due to incomplete data or due to medical complications; unclear which study group withdrawals were from |
| Selective reporting (reporting bias) | High risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Poorgholami 2016a.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention groups
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Not possible due to nature of the intervention |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Follow‐up calls made by investigator or his assistant, likely blinding was not upheld |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | No objective outcomes were measured |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Completed in the dialysis ward, no mention of who gave out to patients. Valid questionnaire |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Potter 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention groups
Control groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Blinding of participants would not be possible with this intervention |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Study personnel notified if missed medication doses in intervention group 2 |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | All outcomes described are objective and less risk of bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only preliminary data is being reported |
| Selective reporting (reporting bias) | Unclear risk | Only preliminary data is being reported |
| Other bias | Unclear risk | Insufficient information to permit judgement as limited detail is able to be obtained from abstracts |
Reese 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group 1
Intervention group 2
Control group
|
|
| Outcomes | Primary outcome
Secondary outcomes
Post hoc analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded given the nature of the intervention |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Study coordinator contacted patients if adherence was below 90% in the feedback group, no mention of blinding of study coordinator for participants in other groups |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Post hoc analyses were conducted by blinded personnel, no mention of whether this also occurred for primary and secondary outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/120 dropped out (2.5%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Reilly‐Spong 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group 1
Intervention group 2
|
|
| Outcomes | Primary outcome (measured at baseline, 2 months, 6 months)
Secondary outcomes (measured at baseline, 2 months, 6 months)
Other outcome (measured at 2 months)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated using permuted blocks |
| Allocation concealment (selection bias) | Low risk | conducted by statistician who was masked. participants completed baseline assessments prior to randomisation |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "single blind" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Unlikely could have been blinded / blinding would have been broken |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective measures (salivary cortisol and sleep actigraphy) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Feasibility and acceptability measures taken with staff, QoL and anxiety measures are patient reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up (12.5% to 12.9%) |
| Selective reporting (reporting bias) | High risk | Salivary cortisol and sleep actigraphy and a number of emotional state outcomes were not reported in either paper |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Rifkin 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Outcomes measured at baseline and 6 months
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Odd/even is a simple randomisation technique which is considered to maintain randomness |
| Allocation concealment (selection bias) | Low risk | Used opaque envelopes |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Study personnel contacted intervention participants when BP too high. study physicians and pharmacist met weekly re: BP logs |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Not blinded but objective measures |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Questionnaires were collected by the treating physicians (not the study physicians) however likely participants could have broken blinding. Self‐report questionnaires about adherence by unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.5% loss to follow‐up (4 out of 47) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Small sample, short follow‐up |
Robinson 2014a.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcome measure (assessed at baseline and 6 weeks)
Secondary outcomes (assessed at baseline and 6 weeks)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using stratified random blocks using RCore Team (19), to assure equal allocation to groups over the accrual period, in total, as well as within ethnic/racial groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially blinded sealed envelopes were provided by the statistician to the study coordinator, to be opened by the participant after the baseline visit" |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Blinding not possible |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Biologic measures at baseline and 6 weeks assessed by research coordinator blinded to the study group |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective measures of pigmentation used |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective measure of pigmentation from RAs who trained by dermatologist, used validated self‐reported attitudes, knowledge and behaviour |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up (1) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Did not reach power calculation, small sample population; financial incentives provided |
Robinson 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Outcomes measured at baseline and 6 weeks
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified random blocks using R Core Team |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Participants could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Research co‐ordinators and dermatologist blinded, but may have been broken |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective measures of pigmentation used |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Validated self‐reported measures of knowledge, behaviours and attitudes. research personnel assessing skin pigmentation were trained by a clinical dermatologist for the study blinded however this blinding may have been broken and RAs not dermatologists which may question accuracy of their assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% loss to follow‐up (9/172) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Low participation rate ‐ may not be representative; higher participation rates among white people; monetary incentives |
Russell 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcome (measured daily and assessed at baseline and 6 months)
Secondary outcome (measured at 6 months)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation was conducted by a person independent of the research team to either the continuous self‐improvement intervention group or the attention control group. Person allocating was blinded |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Participants could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Principle Investigator conducted the home visits with the intervention group |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective: electronic monitoring records |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Subjective: adherence diaries and perception of burden ‐ not clear who was asking patients this but could have been influenced feasibility ‐ could of been influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 28% (2/7) had unusable data from Medication Event Monitoring System data |
| Selective reporting (reporting bias) | High risk | Number of outcomes outlined in the protocol were not reported |
| Other bias | Unclear risk | Small sample but only a feasibility study; received financial incentive |
Schmid 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Data reported at baseline, 3, 6 and 12 months. Used intention‐to‐treat analysis
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation schedule provided by the Institute of Medical Biometry and Medical Informatics |
| Allocation concealment (selection bias) | Unclear risk | Quote: "concealed allocation" but no further information |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Nurses delivering intervention could not have been blinded |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Hospital admissions, LOS, adherence |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Psychosocial measures were validated and assessed by psychologist ‐ no mention of whether psychologist blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used intention‐to‐treat analyses, low loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Small sample size |
Schulz 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcomes (assessed baseline and 3 months)
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Physicians received alarms from study participants |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | All outcomes are objective |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Stated outcomes in abstracts and papers have not been reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
SUBLIME 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Outcomes measured at baseline, 3, 6, 9 months
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective data such as BP, 24‐hour urine sodium, cost‐analysis |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No reporting of how well being and quality of life is measured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 28% drop out in intervention, 3.3% drop out in control ‐ no mention if these participants differed; no mention of whether used ITT analyses |
| Selective reporting (reporting bias) | High risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Swallow 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Outcome measures (assessed pre‐test and 20 weeks)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized block sizes in an allocation ratio of 1:1 stratified by CKD stages (3 versus 4/5) and ethnicity (White/Black versus South Asian) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Not possible due to the nature of the intervention |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Insufficient information to permit judgement but likely blinding would have been broken |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Google analytics for usage |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Qualitative interviews and validated questionnaires, unclear who conducted interviews. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22% to 24% loss to follow‐up, reasons given; no mention of whether these participants were different |
| Selective reporting (reporting bias) | High risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Only technologically savvy families could have participated. Lower recruitment rate from south Asian descent participants |
TAKE‐IT 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | Primary outcome (12 months)
Secondary outcomes (12 months)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation is maintained until 3 month visit |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Not blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | primary and secondary outcomes predominantly measured objectively |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective assessment of adherence used in addition to objective methods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% loss after randomisation in intervention group, groups were balanced with respect to age, time since transplant, gender. Analyses conducted using intention‐to‐treat and as‐treated |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Welch 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was blocked and stratified by dialysis unit |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Blinding not possible |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | No mention of blinding but likely would be broken |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective measures (IDWG) used |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participant data were collected by RAs during HD treatment. The RAs read questionnaire items for baseline and follow‐up data collections to each participant, who responded verbally to each item |
| Incomplete outcome data (attrition bias) All outcomes | High risk | overall attrition rate of 25% by the end of the 8‐week follow‐up. There were no statistically significant differences in age, gender, race, dialysis unit, or group between those who continued in the study and those who did not |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Under powered, small sample size only 2 dialysis units involved and not generalisable |
White 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Author replied to email stating neither participants or personnel were blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Author replied to email stating neither participants or personnel were blinded |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Objective measures (ED visits, hospitalisations) used |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Subjective measures using self‐report at high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Williams 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised, method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Participants could not have been blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Unclear who provided the feedback to participants |
| Blinding of outcome assessment (detection bias) Objective outcome | Low risk | Sleep and physical activity measured objectively |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective measures, other than patient experience. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants were not included in analyses as they died during the study period, no mention of which group they were allocated to, however low rate of missing data overall (n = 2; 6%). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
ACEi ‐ angiotensin‐converting enzyme inhibitor; ACR ‐ albumin:creatinine ratio; ARB ‐ angiotensin receptor blocker; BP ‐ blood pressure; BUN ‐ blood urea nitrogen; CBT ‐ cognitive behaviour therapy; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; CSA ‐ cyclosporin; DBP ‐ diastolic blood pressure; ED ‐ emergency department; eGFR ‐ estimated glomerular filtration rate; EMD ‐ electronic medication dispenser; ESKD ‐ end‐stage kidney disease; HbA1c ‐ haemoglobin A1c (glycated); HD ‐ haemodialysis; HEiQ ‐ Health Education Impact Questionnaire; HRQoL ‐ health‐related quality of life; IDWG ‐ interdialytic weight gain; LDL ‐ low density lipoprotein; MAP ‐ mean arterial pressure; MDRD ‐ Modified Diet in Renal Disease; MEMSI ‐Medication Self‐Management Intervention; PD ‐ peritoneal dialysis; PDA ‐ personal digital assistant; PHR ‐ personal health record; PTH ‐ parathyroid hormone; QoL ‐ quality of life; RCT ‐ randomised controlled trial; SBP ‐ systolic blood pressure; SBP ‐ systolic blood pressure; SCr ‐ serum creatinine; SMS ‐ short messaging service; TAC ‐ tacrolimus; UACR ‐ urine albumin:creatinine ratio; UF ‐ ultrafiltration; VAS ‐ visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel‐Kader 2011 | Wrong target population |
| Chen 2011e | Wrong intervention |
| Korus 2017 | Wrong target population |
| Morales‐Barria 2016 | Wrong study design |
| RaDIANT 2014 | Wrong target population |
| Roberto 2009 | Wrong target population |
| SMILE 2010 | Wrong intervention |
| Warren 2009 | Wrong study design |
| Wilson 2014 | Wrong target population |
Characteristics of ongoing studies [ordered by study ID]
CONNECT 2017.
| Trial name or title | Assessment of telehome monitoring in patients on peritoneal dialysis: a multicentre randomized controlled trial (CONNECT) |
| Methods | Parallel assignment, RCT |
| Participants | Adult patients on PD for at least 3 months, the patient or their primary care giver able to read and speak English, the patient or primary care giver cognitively and physically capable and willing to interact with a tablet computer and perform self‐measurements (e.g. taking weight) |
| Interventions | Interventions
Standard of care
|
| Outcomes | Primary outcome: composite of technique failure (switching to HD for ≥ 12 weeks), infections (peritonitis, exit‐site, tunnel) and hospital encounters (ER visits, hospitalisations) Secondary outcome: HRQoL (Kidney Disease Quality of Life‐36 (KDQOL‐36) Instrument and EQ‐5D to assess HRQoL), time spent communicating (measured through automated telephone logs and paper telephone logs that are documented by nurses), number of missed appointments, nurse overtime hours, number of clinic visits, hospitalisation days, nursing costs, healthcare utilisation costs, dialysis supply costs |
| Starting date | June 2016 |
| Contact information | Melissa Subnath melissa.subnath@lhsc.on.ca |
| Notes | Clinical trials last updated on 11 December 2017, recruitment is ongoing |
eNEPHRO 2017.
| Trial name or title | Medico‐economic evaluation of a telemedicine system for the management of chronic renal failure |
| Methods | Open, label, parallel group, RCT |
| Participants | Adult patients with CKD stage 3b‐4 (nephrology care < 2 years), ESKD on ambulatory dialysis, kidney transplantation (> 3 months and < 12 months), patients can use IT tool or having someone in entourage who knows how to use |
| Interventions | Usual Care eNephro Application Telemedicine system which is a collaborative and expert system, consisting of: A dynamic shared medical record for the collection of administrative, medical, biological and clinical data for each patient. All health professionals can access the folder and fill in the support. It is the same for patients treated at home. A secure messaging for communication between health professionals and between patients and health professionals Expert systems analyzing data from each patient A management tool of therapeutic education A compliance assistance: electronic pillbox and pharmaceutical care Patients included in this study are major patients, male and female who signed a consent form. These patients have a chronic renal failure moderate to end up being treated by ambulatory dialysis or kidney transplantation. The patients of each population will be randomly assigned in group 1 (traditional care) or in group 2 (traditional care added by telemedicine system) |
| Outcomes | Primary outcomes: combined endpoint achievement of target BP and proteinuria (measured at 1 year), cumulative duration of hospitalisations for 1 year, cumulative duration unplanned short stay for 1 year, survival at one year Secondary outcomes: compliance (baseline, 6 months, 12 months), QoL (baseline, 12 months), anxiety‐depression state (baseline, 12 months), change in eGFR (baseline, 12 months), anaemia control (12 months), consultations and hospitalisations unplanned (12 months), disease costs (12 months), intervention costs (12 months), acceptability (12 months) |
| Starting date | November 2015 |
| Contact information | Professor Michele Kessler m.kessler@chu‐nancy.fr |
| Notes | Clinical trials last updated April 2016, recruitment for the study is ongoing, estimated completion Dec 2016 |
Jung 2017.
| Trial name or title | The efficacy and stability of an information and communication technology‐based centralized monitoring system of adherence to immunosuppressive medication in kidney transplant recipients: study protocol for a randomized controlled trial |
| Methods | Multicentre, open‐label, prospective, RCT (1:1 randomisation). The planned follow‐up duration is 6 months. |
| Participants | Kidney transplant recipients, n = 114 |
| Interventions | Intervention
Control
|
| Outcomes | The primary outcome in this trial is adherence to medication, including dose‐taking compliance, dose‐frequency compliance, dose‐interval compliance, drug holidays, medication possession ratio Secondary outcomes: Both groups are to make six office visits after randomisation at 4, 8, 12, 16, 20, and 24 weeks. Each visit requires measurement of blood drug level, creatinine level, and estimated glomerular filtration rate (eGFR). Serum BK virus is assessed at 12 weeks and Panel reactive anti‐ body (PRA) at 24 weeks. At each visit, subjects go over the diary with investigators and fill out a questionnaire using the Modified Morisky Adherence Scale. The ICT‐based centralized clinical trial monitoring group completes a patient Satisfaction Questionnaire developed by the ICT Clinical Trial Support Center at 4 and 24 weeks. Cost‐effectiveness evaluation parameters include installation of the ICT‐based centralized monitoring system, additional hospitalisation due to non‐adherence, ambulatory tests, and trips for hospital visits. Process evaluation: The Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE‐AIM) framework will be used in order to evaluate translatability and feasibility of ICT‐ based centralized monitoring system |
| Starting date | January 2017 |
| Contact information | ylkim@knu.ac.kr Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, South Korea |
| Notes | Clinical trials registration: NCT03136588, registered on 20 April 2017 |
KARE 2015.
| Trial name or title | The Kidney Awareness Registry and Education (KARE) study: protocol of a randomized controlled trial to enhance provider and patient engagement with chronic kidney disease |
| Methods | Single blind, factorial assignment, RCT |
| Participants | CKD (eGFR < 60mL/min), speak Chinese, Spanish or Cantonese, have primary care provider |
| Interventions | Experimental: ATSM + Health Coach and CKD Registry ‐ primary care providers can access online CKD registry to identify patients, get notifications of CKD status and access guidelines and education materials + patients receive automated telephone self‐management which blends automated phone calls with live targeted call‐backs from a health coach. Patients will receive bi‐weekly automated calls for 52 weeks in their native language, consisting of pre‐recorded queries pertaining to CKD management, preventive services, and lifestyle changes. Patients will interact with the system using a touch‐tone keypad; Out‐of‐range values or invalid responses will prompt a live call‐back within 24‐48 hours by a health coach Active comparator: CKD registry only Active comparator: Automated telephone self‐management + health coaching Placebo comparator: usual care ‐ primary care providers will manage their patients with CKD as per usual |
| Outcomes | Primary outcome: change in BP (baseline, 12 months) Secondary outcomes: change in CKD awareness, functional status and symptoms (baseline, 12 months) |
| Starting date | April 2013 |
| Contact information | Dr Delphine Tuot delphine.tuot@ucsf.edu Alexandra Velasquez velasqueza@medsfgh.ucsf.edu |
| Notes | Clinical trials last verified October 2016, recruitment is ongoing, estimated completion December 2017 |
Kosaka 2017.
| Trial name or title | Assessment of efficacy of a CKD support decision making application and home blood pressure measurement system in patients with CKD: study protocol of a randomized, controlled trial |
| Methods | Clinical, prospective, RCT with balanced randomisation (1:1) |
| Participants | Inclusion criteria: patient at the kidney internal medicine outpatient clinics, age over 20 years old, provision of informed consent, to be assure by doctor, RRT not yet selected, and eGFR < 60 |
| Interventions | Intervention: will receive conventional care from the attending physician; the patient and physician will also be given a tablet equipped with the CKD‐SDM app and an automated sphygmomanometer for home blood pressure monitoring for 2 months. The CKD‐SDM app includes 61 items in three categories: "Let’s study CKD", "What’s about RRT?", and “Learn and consent of CKD”. Control: will receive conventional care and only the automated sphygmomanometer for 2 months |
| Outcomes | The primary outcome measure is change in home BP data from baseline. Secondary outcomes are renal function, spot urine test, self‐efficacy for chronic illness, disease burden, knowledge level of self‐management in CKD, and decision for RRT |
| Starting date | Recruitment began in March 2017 |
| Contact information | Shiho Kosaka skosaka‐tky@umin.ac.jp |
| Notes | UMIN clinical trials last updated on 25/07/2017 |
MAGIC 2016.
| Trial name or title | MAGIC Study: aims, design and methods using SystemCHANGE to improve immunosuppressive medication adherence in adult kidney transplant recipients |
| Methods | 4 year, two‐centre, RCT (single blind) |
| Participants | Adult kidney transplant recipients, prescribed at least 1 immunosuppressive medication taken twice daily, functioning kidney transplant, received kidney‐only transplant, transplant physician has agreed can participate, able to speak, hear and understand English, able to open electronic medication cap, self‐administering immunosuppressive medication, has telephone / access to telephone, no cognitive impairment, no other life‐shortening diagnoses, not currently hospitalised |
| Interventions | Intervention: SystemCHANGE ‐ initial home visit conducted, 2 weeks later phone review and then monthly phone calls over 6 month intervention. Phone reviews include reviewing electronic medication reports, goal setting, determining process owners, identifying lifestyle routines, identifying cyclical nature of routines, possible solutions for change and story boards for success. Research assistant encouraged patient to continue using electronic monitoring cap for an additional 6 months during maintenance phase. Attention control: home visit and monthly phone reviews. these patients receive educational materials that address healthy living after transplantation. if participant asks questions about medication they are directed back to their transplant team. encouraged to continue using electronic medication monitoring and diary for additional 6 months of maintenance phase |
| Outcomes | Primary outcome: medication adherence ‐ MEMS Cap, cost‐effectiveness (ICER) Secondary outcomes: Blood creatinine, BUN level, acute and chronic rejection, infection, health‐related QoL, death will be collected retrospectively from medical records |
| Starting date | June 2014 |
| Contact information | Dr Cynthia Russell RussellC@umkc.edu |
| Notes | clinical trials last updated October 2016, recruitment in study is ongoing, estimated completion date May 2018 |
NCT00394576.
| Trial name or title | Assessing novel methods of improving patient education of nutrition: ehealth, health literacy and chronic kidney disease |
| Methods | RCT |
| Participants | CKD stage 3, 4, 5, aged 18 to 90 years, ability to read English, adequate visual acuity |
| Interventions | Intervention: web‐based nutritional education intervention + usual care Usual care |
| Outcomes | Primary outcome: phosphorus knowledge, dietary phosphorus intake (as per serum phosphate, calcium, PTH, calcium phosphorus product), dietary phosphorus intake as per 24 hour recall diary Secondary outcomes: correlations between dietary phosphorus intake, serum phosphorus levels and CECs will be made |
| Starting date | November 2006 |
| Contact information | Dr Jonathan B Jaffery |
| Notes | Clinicaltrials.gov not updated in 2 years, previous estimated completion date June 2009) No published data has been found |
NCT02097550.
| Trial name or title | Primary care eHealth intervention for improved outcomes in chronic kidney disease (CKD eHealth) |
| Methods | Open label, parallel assignment, RCT |
| Participants | Adult CKD stage 3a (eGFR 45‐59) with poorly controlled risk factors for CKD progression and/or CVD morbidity / death and stage 3B (eGFR 30‐44) who have primary care provider, non‐pregnant, ability to use computer or smartphone, ability to understand English |
| Interventions | Experimental: eHealth Intervention ‐ Patients randomized to this arm will receive eHealth materials every 2‐4 weeks over the 12‐month intervention. However, the exact nature of timing, dose, and delivery channel will be informed by the formative research. Developing and testing an electronic health intervention (that will combine secure e‐mail, smartphone text message, and online video materials) to promote patient use of effective medications. Standard Care with physician |
| Outcomes | Primary: CKD metabolic control (12 months) ‐ consist of clinical and laboratory measurements that are routinely performed in primary care settings Secondary: new indicated medication prescriptions (12 months), adherence proxy measures (12 months) ‐ refills for prescriptions, patient and provider satisfaction (12 months), urine albumin (6 months), SBP (6 months), HbA1c (6 months), LDL‐C (6 months), CKD progression measured by eGFR (12 months), DBP (12 months) HDL‐C (12 months), total cholesterol (12 months) |
| Starting date | May 2016 |
| Contact information | Dr Veronica Yank |
| Notes | Clinical trials last verified September 2016, trial is ongoing but not recruiting, estimated completion may 2018 |
NCT02610946.
| Trial name or title | Do technology apps improve compliance in adolescent renal transplant recipients? |
| Methods | Open label, efficacy study, RCT |
| Participants | Adolescent (12‐18 years) kidney transplant recipients |
| Interventions | Intervention: Electronic application ‐ Use of electronic apps (iphone or iPad mini) to determine whether it can improve compliance with transplant care and readiness to transition to adult care Paper‐based calendars, reminders, medication list and BP, fluid intake tracking methods |
| Outcomes | Primary outcome: medication compliance (12 months) as assessed by presence or absence of antibody‐mediated rejection based on donor‐specific antibody levels Secondary outcome: readiness to transition (baseline, 12 months) ‐ knowledge of transplant care and readiness to transition to adult care assessed by questionnaire of disease knowledge |
| Starting date | April 2015 |
| Contact information | Dr Ha Tran hatran@stanford.edu Dr Priya Chandra priyac1@stanford.edu |
| Notes | Clinical trials last verified November 2015, recruitment ongoing |
TELEGRAFT 2015.
| Trial name or title | A personalized follow‐up of kidney transplant recipients using video conferencing based on a 1‐year scoring system predictive of long term graft failure (TELEGRAFT study): protocol for a randomized controlled trial |
| Methods | Phase 4, open level, randomised, multicentric and prospective study Randomised to novel eHealth program versus standard care 1:1 randomisation, stratified by centres and performed at 1 year post kidney transplant with patient participation planned for 2 years |
| Participants | 1 year post kidney transplant, access to high speed internet, without ongoing CMV or BKV infection, men and non‐pregnant women, without mental disorders and provide informed consent |
| Interventions | eHealth intervention: provided with a USB which allows collection of medication information before video conferencing. USB opens a secure internet connection via an intuitive interface specifically designed for non‐internet specialist patients. Also provided with tablet computer (e.g. iPad) devoted for video conferencing. Low risk patients will be interviewed 3 times with VC with pulse, weight, temperature and BP collected on USB, with only 1 in‐person complete checkup conducted per year. For high risk patients they will have in person 1 complete check up and 5 standard visits + 6 additional VCs to reinforce follow‐up. Standard care: patients classified as low risk of graft failure within first 8 years post‐transplantation will be scheduled 4 visits at the hospital per year, whilst high risk patients will be scheduled 6 visits. Standard visits include clinical examination of BP, weight, blood and urine monitoring and 1 visit encompassing a complete checkup of further biochemistry, morphologic exams and questionnaires related to QoL and psychological dimensions. |
| Outcomes | Primary outcome is composite and defined by absence of major complications until 2 years post randomisation (e.g. patient alive with functioning kidney, without acute rejection episodes, without decrease in eGFR higher than 25% and without cancer. Secondary outcomes: to evaluate efficiency of system ‐ incremental cost‐effectiveness ratios, transplant specific QoL, evolution of psychological dimensions related to stress and coping, anxiety/depression |
| Starting date | February 2012 |
| Contact information | aurelie.meurette@chu‐nantes.fr |
| Notes | clinical trials updated May 2016 ‐ recruitment ongoing, estimated completion date September 2020 |
Waterman 2015.
| Trial name or title | Explore Transplant at Home: a randomized control trial of an educational intervention to increase transplant knowledge for Black and White socioeconomically disadvantaged dialysis patients. |
| Methods | open label, parallel assignment, RCT |
| Participants | Dialysis patients who are aged 18‐74 years, self‐identify as African‐American or White, household income at or below 250% of the federal poverty line, be able to read and speak English |
| Interventions | Standard Care ‐ will not receive any educational materials and will only participate in surveys. dialysis providers will be asked to continue their current practices throughout study period without change. Experimental: Patient‐Guided ‐ Over an 8‐month period, patients in the Patient‐Guided intervention condition will receive four educational modules and twelve transplant education postcards in the mail. Modules will be mailed once every other month and consist of an introductory letter, a transplant video, and printed resources. Transplant education postcard will be mailed every two weeks following the mailing of each module, for a total of three postcards over the course of 6‐weeks. Experimental: Educator‐Guided ‐ Patients in the Educator‐Guided intervention condition will receive the same intervention components as those in the Patient‐Guided condition; however, the key difference in this condition is that Educator‐Guided patients will also receive telephonic support from an experienced clinical social worker in the role of a Transplant Educator to maximally facilitate learning. Telephonic meetings with the Transplant Educator will occur after the mailing of each study module, for a total of four calls, each lasting 20‐minutes, totaling 1 hour and 20 minutes. Finally, Patient‐Guided and Educator‐Guided patients will have the option of enrolling in an educational text messaging service designed to supplement the ET education they are receiving in the mail. |
| Outcomes | Primary outcome: DDKT and LDKT knowledge (9 months) Secondary outcomes: informed decision making (9 months), decisional balance (9months) |
| Starting date | July 2014 |
| Contact information | Dr Amy Waterman |
| Notes | Clinical trials last verified August 2016, study is ongoing but not recruiting patients, estimated completion august 2016 |
WISHED 2016.
| Trial name or title | The WISHED Trial: implementation of an interactive health communication application for patients with chronic kidney disease |
| Methods | Multi‐centre RCT comparing the use of a secured web‐based Interactive Health Communication Applications (IHCA) versus usual care in the promotion of home‐based dialysis therapies |
| Participants | recruited through CKD clinics |
| Interventions | Usual care: continue to be seen in CKD clinic IHCA: usual care + participants will log into website during randomisation visit and provided an orientation of session to familiarise with website. email reminders to log‐in are sent periodically and frequency of visits will be monitored. website provides easy navigation and provides content that encompasses informational and social support to reduce conflict and uncertainty in ESRD therapy decision‐making. Website includes "Frequently asked questions", demonstration videos and still photographs of equipment and pre‐recorded videos with local experts and existing patients. updated information will continue to be added by variety of content‐expert healthcare professionals. social support component of website will include video and text narratives of patients addressing benefits and challenges of home dialysis and a moderated forum for patients to discuss issues surrounding home dialysis with current home dialysis patients. Participants will also be able to email "experts" including nephrologists, nurses and existing patients with questions |
| Outcomes | Outcomes measured at baseline, 6 months, 12 months Primary outcome: proportion of patients who receive any dialysis using home based therapy (PD or HHD) within 3 months of dialysis initiation. Those who have not initiated or have had pre‐emptive transplant will be regarded as non‐home‐based dialysis outcomes. Secondary outcomes: proportion of patients intending to perform home‐based therapy at 1 year, dialysis knowledge measured using locally developed tool, decision conflict, level of social support |
| Starting date | March 2012 |
| Contact information | Dr Scott Brimble brimbles@mcmaster.ca Cathy Moreau cmoreau@stjoes.ca |
| Notes | Clinical trials updated April 2016, study recruitment is ongoing, estimated completion date June 2017 |
BP ‐ blood pressure; BUN ‐ blood urea nitrogen; CKD ‐ chronic kidney disease; CMV ‐ cytomegalovirus; CVD ‐ cardiovascular disease; eGFR ‐ estimated glomerular filtration rate; ER ‐ emergency room; ESKD ‐ end‐stage kidney disease; HD ‐ haemodialysis; HRQoL ‐ health‐related quality of life; PD ‐ peritoneal dialysis; PTH ‐ parathyroid hormone; QOL ‐ quality of life; RCT ‐ randomised controlled trial; RRT ‐ renal replacement therapy
Differences between protocol and review
An additional potential harm was added to "Types of outcome measures". "Anxiety due to frequent monitoring" was added to outcomes as this was reported by one study.
The important outcomes listed in the Summary of Findings Table have been changed. A number of the original outcomes listed were either not reported by any study (physical activity) or were too broad to be reported in this format (quality of life). We removed change in electrolyte management, physical activity, adherence to treatment and quality of life from the Summary of Findings table, and added in death as this has been an important outcome to consumers in both HD and transplantation as published by the Song Initiative (SONG 2017).
Contributions of authors
Draft the protocol: JS, JC, AW, KC, VL, CC
Study selection: JS, ZC
Extract data from studies: JS, ZC
Enter data into RevMan: JS, ZC
Carry out the analysis: JS, ZC
Interpret the analysis: JS, ZC
Draft the final review: JS, JC, AW, KC, VL, CC
Disagreement resolution: VL
Update the review: JS
Sources of support
Internal sources
Centre for Kidney Research, The Children's Hospital at Westmead, Australia.
External sources
-
BEAT‐CKD, Australia.
PhD Scholarship (through NHMRC project grant)
-
National Health and Medical Research Council (NHMRC), Australia.
PhD Scholarship
-
National Institute for Health Research (NIHR), UK.
This review is funded by the National Institute for Health Research (NIHR) Cochrane Incentive Scheme 2018 (NIHR128415). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Declarations of interest
None known
New
References
References to studies included in this review
BALANCEWise‐HD 2011 {published data only}
- Stark S, Snetselaar L, Piraino B, Stone RA, Kim S, Hall B, et al. Personal digital assistant‐based self‐monitoring adherence rates in 2 dialysis dietary intervention pilot studies: BalanceWise‐HD and BalanceWise‐PD. Journal of Renal Nutrition 2011;21(6):492‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
BalanceWise‐HD 2013 {published data only}
- Clark‐Cutaia MN, Ren D, Hoffman LA, Burke LE, Sevick MA. Adherence to hemodialysis dietary sodium recommendations: influence of patient characteristics, self‐efficacy, and perceived barriers. Journal of Renal Nutrition 2014;24(2):92‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark‐Cutaia MN, Ren D, Hoffman LA, Snetselaar L, Sevick MA. Psychometric validation of the self‐efficacy for restricting dietary salt in hemodialysis scale. Topics in Clinical Nutrition 2013;28(4):384‐91. [EMBASE: 2013726385] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevick MA, Piraino BM, St‐Jules DE, Hough LJ, Hanlon JT, Marcum ZA, et al. No difference in average interdialytic weight gain observed in a randomized trial with a technology‐supported behavioral intervention to reduce dietary sodium intake in adults undergoing maintenance hemodialysis in the United States: primary outcomes of the BalanceWise study. Journal of Renal Nutrition 2016;26(3):149‐58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- St‐Jules DE, Woolf K, Pompeii ML, Sevick MA. Exploring problems in following the hemodialysis diet and their relation to energy and nutrient intakes: the BalanceWise study. Journal of Renal Nutrition 2016;26(2):118‐24. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
BALANCEWise‐PD 2011 {published data only}
- Stark S, Snetselaar L, Piraino B, Stone RA, Kim S, Hall B, et al. Personal digital assistant‐based self‐monitoring adherence rates in 2 dialysis dietary intervention pilot studies: BalanceWise‐HD and BalanceWise‐PD. Journal of Renal Nutrition 2011;21(6):492‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baraz 2014 {published data only}
- Baraz S, Zarea K, Dashtbozorgi B. Comparing the effect of two educational programs on the quality of life of hemodialysis patients in Iran. Iranian Red Crescent Medical Journal 2014;16(8):e19368. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
BRIGHT 2013 {published data only}
- Blakeman T, Blickem C, Kennedy A, Reeves D, Bower P, Gaffney H, et al. Effect of information and telephone‐guided access to community support for people with chronic kidney disease: randomised controlled trial. PLoS ONE [Electronic Resource] 2014;9(10):e109135. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blickem C, Blakeman T, Kennedy A, Bower P, Reeves D, Gardner C, et al. The clinical and cost‐effectiveness of the BRinging Information and Guided Help Together (BRIGHT) intervention for the self‐management support of people with stage 3 chronic kidney disease in primary care: study protocol for a randomized controlled trial. Trials [Electronic Resource] 2013;14(1):28. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney H, Blakeman T, Blickem C, Kennedy A, Reeves D, Dawson S, et al. Predictors of patient self‐report of chronic kidney disease: baseline analysis of a randomised controlled trial. BMC Family Practice 2014;15:196. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cargill 2003 {published data only}
- Cargill A, Watson AR. An evaluation of telecare to support patients receiving chronic peritoneal dialysis at home [abstract]. Peritoneal Dialysis International 2002;22(1):136. [CENTRAL: CN‐00594560] [PubMed] [Google Scholar]
- Cargill A, Watson AR. Telecare support for patients undergoing chronic peritoneal dialysis. Peritoneal Dialysis International 2003;23(1):91‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Cooney 2015 {published data only}
- Cooney D, Moon H, Liu Y, Miller RT, Perzynski A, Watts B, et al. A pharmacist based intervention to improve the care of patients with CKD: a pragmatic, randomized, controlled trial. BMC Nephrology 2015;16:56. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diamantidis 2015 {published data only}
- Diamantidis CJ, Ginsberg JS, Yoffe M, Lucas L, Prakash D, Aggarwal S, et al. Remote usability testing and satisfaction with a mobile health medication inquiry system in CKD. Clinical Journal of the American Society of Nephrology: CJASN 2015;10(8):1364‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantidis CJ, Zuckerman M, Ginsberg JS, Lucas L, Fink JC. Remote usability testing of a mobile phone‐based medication inquiry system (MIS) in chronic kidney disease (CKD) [abstract no: PUB090]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):914‐5A. [Google Scholar]
Durand 2000 {published data only}
- Chanliau J, Durand PY, Vega L, Thomesse JP, Charpillet F, Kessler M. PD telemedicine by DIATELIC: preliminary results of the largest French pilot study [abstract]. 26th Annual Conference on Dialysis; 2006 Feb 26‐28; San Francisco, CA. 2006.
- Durand PY, Chanliau J, Kessler M, Romary L, Thomesse JP, Charpillet F, et al. Diatelic: a new 'intelligient' telemedine system to avoid hydration disorders in CAPD patients [abstract]. Peritoneal Dialysis International 2000;20(Suppl 1):S77. [Google Scholar]
- Durand PY, Chanliau J, Mariot A, Thomesse J, Romary L, Charpillet F, et al. Cost‐benefit assessment of a smart telemedicine system in patients undergoing CAPD: preliminary results [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A162. [CENTRAL: CN‐00445173] [Google Scholar]
Giacoma 1999 {published data only}
- Giacoma T, Ingersoll GL, Williams M. Teaching video effect on renal transplant patient outcomes. ANNA Journal 1999;26(1):29‐33, 81. [MEDLINE: ] [PubMed] [Google Scholar]
Halleck 2017 {published data only}
- Halleck F, Georgi S, Khadzhynov D, Lehner L, Schrezenmeier E, Durr M, et al. Prescribed medications, adherence and smartphone use in kidney transplant recipients: Evaluating the potential of a mobile app to enhance medication adherence [abstract no: OS178]. Transplant International 2017;30(Suppl 2):69. [EMBASE: 618770482] [Google Scholar]
- Halleck F, Georgi S, Khadzhynov D, Schmidt D, Schrezenmeier E, Lehner L, et al. A mobile app to improve medication adherence in kidney transplant recipients‐analysis of a prospective interventional study [abstract no: B202]. American Journal of Transplantation 2018;18(Suppl 4):699‐700. [EMBASE: 622279560] [Google Scholar]
- Staeck O, Georgi S, Khadzhynov D, Lehner L, Schrezenmeier E, Schmidt D, et al. Use of a mobile app to improve medication adherence in kidney transplant recipients‐A prospective interventional study [abstract no: SaO014]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i321. [EMBASE: 622605515] [Google Scholar]
Han 2016 {published data only}
- Han A, Cho MJ, Cho S, Lee CH, Choi C, Kim SY, et al. Use of mobile internet application to improve adherence to immunosuppressive medication adherence in kidney transplant recipients: preliminary results of the PRIMA (ImProving Adherence to Immunosuppressive Therapy by Mobile Internet application) study [abstract no: 420.4]. Transplantation 2016;100(7 Suppl 1):S161. [EMBASE: 613004382] [Google Scholar]
Hardstaff 2002 {published data only}
- Hardstaff R, Green K, Talbot D. Measurement of compliance posttransplantation‐‐the results of a 12‐month study using electronic monitoring. Transplantation Proceedings 2003;35(2):796‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hardstaff R, Green K, Talbot D. Noncompliance postrenal transplantation: measuring the extent of the problem using electronic surveillance and nurse practitioner interviews. Transplantation Proceedings 2002;34(5):1608. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Henriksson 2016 {published data only}
- Henriksson J, Tyden G, Hoijer J, Wadstrom J. A prospective randomized trial on the effect of using an electronic monitoring drug dispensing device to improve adherence and compliance. Transplantation 2016;100(1):203‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Henriksson J, Wadstrom J, Hoijer J, Tyden G. A prospective randomized trial on the effect of using an electronic monitoring drug dispensary device to improve adherence and non‐compliance [abstract no: BO220]. Transplant International 2015;28(Suppl 4):204. [EMBASE: 72111815] [DOI] [PubMed] [Google Scholar]
iChoose 2016 {published data only}
- Basu M, Pastan S, Mohan S, Wolf M, Friedewald J, Ladner D, et al. Provider attitudes toward shared mobile decision aid utilized among patients evaluated for kidney transplantation: iChoose kidney randomized trial [abstract no: 172]. American Journal of Transplantation 2016;16(Suppl 3):264. [EMBASE: 611699717] [Google Scholar]
- Basu M, Pastan SO, Mohan S, Friedewald JJ, Ladna DP, Patzer RE. Knowledge of treatment options in patients evaluated for kidney transplantation: iChoose kidney randomized trial [abstract no: SA‐PO1002]. Journal of the American Society of Nephrology 2015;26:864A. [CENTRAL: CN‐01658335] [Google Scholar]
- Patzer RE, Basu M, Mohan S, Smith KD, Wolf M, Ladner D, et al. A randomized controlled trial of a mobile clinical decision aid to improve access to kidney transplantation: iChoose kidney. KI Reports 2016;1(1):34‐42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer RE, McPherson L, Basu M, Mohan S, Wolf M, Chiles M, et al. Effect of the iChoose kidney decision aid in improving knowledge about treatment options among transplant candidates: a randomized controlled trial. American Journal of Transplantation 2018;18(8):1954‐65. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer RE, McPherson LJ, Basu M, Mohan S, Pastan SO. Effectiveness of iChoose kidney decision aid on kidney transplant knowledge [abstract no: TH‐PO981]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):359. [Google Scholar]
iDiD 2016 {published data only}
- Hudson JL, Moss‐Morris R, Game D, Carroll A, McCrone P, Hotopf M, et al. Improving distress in dialysis (iDiD): a feasibility two‐arm parallel randomised controlled trial of an online cognitive behavioural therapy intervention with and without therapist‐led telephone support for psychological distress in patients undergoing haemodialysis. BMJ Open 2016;6(4):e011286. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JL, Moss‐Morris R, Norton S, Picariello F, Game D, Carroll A, et al. Tailored online cognitive behavioural therapy with or without therapist support calls to target psychological distress in adults receiving haemodialysis: a feasibility randomised controlled trial. Journal of Psychosomatic Research 2017;102:61‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
InformMe 2017 {published data only}
- Gordon E, Ortiz K, Vera K, Martinez S, Sohn M, Chang C, et al. A decision aid for kidney transplant candidates about increased risk donor (IRD) kidneys: A randomized controlled trial of increased knowledge [abstract no: 1337]. Transplantation 2014;98(Suppl 1):165. [EMBASE: 71544041] [Google Scholar]
- Gordon EJ, Sohn MW, Chang CH, McNatt G, Vera K, Beauvais N, et al. Effect of a mobile web app on kidney transplant candidates' knowledge about increased risk donor kidneys: a randomized controlled trial. Transplantation 2017;10(6):1167‐76. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishani 2016 {published data only}
- Ishani A, Christopher J, Palmer D, Otterness S, Clothier B, Nugent S, et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. American Journal of Kidney Diseases 2016;68(1):41‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jammalamadaka 2015 {published data only}
- Jammalamadaka D, Nahman NS, White JJ. "Binder Reminders" for persistent hyperphosphatemia in hemodialysis patients: a fellow's quality improvement product [abstract no: PUB514]. Journal of the American Society of Nephrology 2015;26(Abstracts):1007A. [CENTRAL: CN‐01658469] [Google Scholar]
Kargar Jahromi 2016 {published data only}
- Kargar Jahromi M, Javadpour S, Taheri L, Poorgholami F. Effect of nurse‐led telephone follow ups (tele‐nursing) on depression, anxiety and stress in hemodialysis patients. Global Journal of Health Science 2016;8(3):168‐73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koprucki 2010 {published data only}
- Koprucki M, Piraino B, Bender F, Snetselaar L, Hall B, Stark S, et al. RCT of Personal Digital Assistant (PDA) supported dietary intervention to reduce sodium intake in PD [abstract no: 162]. American Journal of Kidney Diseases 2010;55(4):A72. [EMBASE: 70124819] [Google Scholar]
Kullgren 2015 {published data only}
- Kullgren KA, Scholl P, Kidwell KM, Hmiel SP. Using an interactive water bottle to target fluid adherence in pediatric kidney transplant recipients: a pilot study. Pediatric Transplantation 2015;19(1):35‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2014b {published data only}
- Li J, Wang H, Xie H, Mei G, Cai W, Ye J, et al. Effects of post‐discharge nurse‐led telephone supportive care for patients with chronic kidney disease undergoing peritoneal dialysis in China: a randomized controlled trial. Peritoneal Dialysis International 2014;34(3):278‐88. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGillicuddy 2013 {published data only}
- McGillicuddy JW. EHealth and transplantation [abstract no: PM‐4]. American Journal of Transplantation 2014;14(Suppl 2):54‐5. [EMBASE: 71388189] [Google Scholar]
- McGillicuddy JW, Gregoski MJ, Brunner‐Jackson BM, Weiland AK, Patel SK, Rock RA, et al. Facilitating medication adherence and eliminating therapeutic inertia using wireless technology: proof of concept findings with uncontrolled hypertensives and kidney transplant recipients. WH '12 Proceedings of the Conference on Wireless Health; 2012 Oct 23‐25; San Diego (CA). 2012. [CENTRAL: CN‐01658221]
- McGillicuddy JW, Gregoski MJ, Weiland AK, Rock RA, Brunner‐Jackson BM, Patel SK, et al. Mobile health medication adherence and blood pressure control in renal transplant recipients: a proof‐of‐concept randomized controlled trial. JMIR Research Protocols 2013;2(2):e32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy JW, Taber DJ, Mueller M, Patel S, Baliga PK, Chavin KD, et al. Sustainability of improvements in medication adherence through a mobile health intervention. Progress in Transplantation 2015;25(3):217‐23. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
MESMI 2010 {published data only}
- Williams A, Manias E, Walker R, Gorelik A. A multifactorial intervention to improve blood pressure control in co‐existing diabetes and kidney disease: a feasibility randomized controlled trial. Journal of Advanced Nursing 2012;68(11):2515‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Williams AF, Manias E, Walker RG. The devil is in the detail ‐ a multifactorial intervention to reduce blood pressure in co‐existing diabetes and chronic kidney disease: a single blind, randomized controlled trial. BMC Family Practice 2010;11:3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Navaneethan 2017 {published data only}
- Navaneethan SD, Jolly SE, Schold JD, Arrigain S, Nakhoul G, Konig V, et al. Pragmatic randomized, controlled trial of patient navigators and enhanced personal health records in CKD. Clinical Journal of the American Society of Nephrology: CJASN 2017;12(9):1418‐27. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ong 2017 {published data only}
- Ong SW, Jassal SV, Min K, Uddin A, Porter EC, Tomlinson G. Impact on blood pressure of mobile‐based application (eKidneyCare) in patients with CKD: a randomized, controlled trial [abstract no: SA‐OR011]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):74. [CENTRAL: CN‐01657425] [Google Scholar]
Poorgholami 2016a {published data only}
- Poorgholami F, Mansoori P, Montaseri Z, Najafi K. Effect of self care education with and without telephone follow‐up on the level of hope in renal dialysis patients: a single‐blind randomized controlled clinical trial. International Journal of Community Based Nursing & Midwifery 2016;4(3):256‐64. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Potter 2016 {published data only}
- Bodzin A, Becker Y, Josephson M, Witkowski P, Lockwood M, Potter L, et al. Randomized controlled study evaluating adherence monitoring with electronic feedback on reducing renal allograft rejection [abstract no: 281]. American Journal of Transplantation 2016;16(Suppl 3):302. [EMBASE: 611700054] [Google Scholar]
- Kane B, Lockwood M, Bodzin A, Potter L, Lourenco L, Millis JM. Randomized controlled study evaluating adherence monitoring with electronic feedback on reducing renal allograft rejection [abstract no: 626.2]. Transplantation 2016;100(7 Suppl 1):S430. [EMBASE: 613004919] [Google Scholar]
- Kane B, Lockwood M, Potter L, Lourenco L, Bodzin A, Covington D, et al. Prospective randomized controlled study evaluating the relationship between electronic adherence data and tacrolimus concentration data [abstract no: C73]. American Journal of Transplantation 2016;16(Suppl 3):626. [EMBASE: 611699014] [Google Scholar]
- Potter L, Kane B, Lockwood M, Bodzin A, Covington D, Mayer K, et al. Prospective randomised controlled study evaluating the relationship between electronic adherence data and tacrolimus concentration data [abstract no: 747.4]. Transplantation 2016;100(7 Suppl):S317. [EMBASE: 613005655] [Google Scholar]
Reese 2017 {published data only}
- Reese P, Bloom R, Trofe‐Clark J, Mussell A, Leidy D, Levsky S, et al. Customized reminders and provider notification to improve adherence to immunosuppression [abstract no: 1074]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953144] [Google Scholar]
- Reese PP, Bloom RD, Trofe‐Clark J, Mussell A, Leidy D, Levsky S, et al. Automated reminders and physician notification to promote immunosuppression adherence among kidney transplant recipients: a randomized trial. American Journal of Kidney Diseases 2017;69(3):400‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reilly‐Spong 2015 {published data only}
- Gross CR, Reilly‐Spong M, Park T, Zhao R, Gurvich O. Randomized clinical trial of telephone‐adapted mindfulness training on anxiety, symptom distress, and quality of life in patients with progressive renal disease [abstract no: P05.28]. Journal of Alternative & Complementary Medicine 2016;22(6):A77. [EMBASE: 611808428] [Google Scholar]
- Gross CR, Reilly‐Spong M, Park T, Zhao R, Gurvich OV, Ibrahim HN. Telephone‐adapted Mindfulness‐Based Stress Reduction (tMBSR) for patients awaiting kidney transplantation. Contemporary Clinical Trials 2017;57:37‐43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly‐Spong M, Reibel D, Pearson T, Koppa P, Gross CR. Telephone‐adapted Mindfulness‐Based Stress Reduction (tMBSR) for patients awaiting kidney transplantation: Trial design, rationale and feasibility. Contemporary Clinical Trials 2015;42:169‐84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rifkin 2013 {published data only}
- Rifkin DE, Abdelmalek JA, Miracle CM, Low C, Barsotti R, Rios P, et al. Linking clinic and home: a randomized, controlled clinical effectiveness trial of real‐time, wireless blood pressure monitoring for older patients with kidney disease and hypertension. Blood Pressure Monitoring 2013;18(1):8‐15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2014a {published data only}
- Robinson JK, Guevara Y, Gaber R, Clayman ML, Kwasny MJ, Friedewald JJ, et al. Efficacy of a sun protection workbook for kidney transplant recipients: a randomized controlled trial of a culturally sensitive educational intervention. American Journal of Transplantation 2014;14(12):2821‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2015 {published data only}
- Robinson JK, Friedewald JJ, Desai A, Gordon EJ. A randomized controlled trial of a mobile medical app for kidney transplant recipients: effect on use of sun protection. Transplantation Direct 2016;2(1):e51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JK, Friedewald JJ, Desai A, Gordon EJ. Response across the health‐literacy spectrum of kidney transplant recipients to a sun‐protection education program delivered on tablet computers: randomized controlled trial. JMIR Cancer 2015;1(2):e8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JK, Kwasny M, Friedewald J, Desai A, Gordon EJ. Sun protection education delivered by tablet personal computers: effective with kidney transplant recipients with inadequate health literacy [abstract no: 158]. Journal of Investigative Dermatology 2015;135(Suppl 1):S28. [EMBASE: 71874336] [Google Scholar]
Russell 2011 {published data only}
- Russell C, Conn V, Ashbaugh C, Madsen R, Wakefield M, Webb A, et al. Taking immunosuppressive medications effectively (TIMELink): a pilot randomized controlled trial in adult kidney transplant recipients. Clinical Transplantation 2011;25(6):864‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CL, Conn VS, Ashbaugh C, Coffey D, Wakefield M. A pilot RCT to increase medication adherence in transplantation [abstract no: 1168]. American Journal of Transplantation 2010;10(Suppl 4):376. [EMBASE: 70464544] [Google Scholar]
Schmid 2016 {published data only}
- Hils S, Schmid A, Bogatyreva L, Hauschke D, Kramer‐Zucker A, Pisarski P. Telemedical supported aftercare after living kidney transplantation‐a single center experience [abstract no: O179]. Transplant International 2015;28(Suppl 4):69. [EMBASE: 72111423] [Google Scholar]
- Hils S, Schmid A, Bogatyreva L, Hauschke D, Pisarski P. Telemedical supported aftercare as an innovative project‐study improves the quality of life after living kidney transplantation‐a single center experience [abstract no: D2730]. Transplantation 2014;98(Suppl 1):843. [EMBASE: 71546416] [Google Scholar]
- Kaier K, Hils S, Fetzer S, Hehn P, Schmid A, Hauschke D, et al. Results of a randomized controlled trial analyzing telemedically supported case management in the first year after living donor kidney transplantation ‐ a budget impact analysis from the healthcare perspective. Health Economics Review 2017;7(1):1. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarski P, Hils S, Schmid A, Kramer‐Zucker A, Janigen B. Telemedicin as an innovative project‐study in adherence improvement after living kideney transplantation at the transplantation center Freiburg [abstract no: O162]. Transplant International 2013;26(Suppl 2):73. [EMBASE: 71359226] [Google Scholar]
- Schmid A, Hils S, Kramer‐Zucker A, Bogatyreva L, Hauschke D, Geest S, et al. Telemedically‐supported case management of living‐donor renal transplant recipients to optimize routine evidence‐based aftercare: a single‐center randomized controlled trial. American Journal of Transplantation 2016;17(6):1594‐605. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schmid A, Pisarski P, Hils S, Hauschke D, Bogatyreva L. Telemedical supported after care in living kidney recipients‐an innovative project‐study at the transplantation center Freiburg interim results of a prospective ongoing study of 50 patients [abstract no: P84]. Transplant International 2013;26(Suppl 1):43. [EMBASE: 71356349] [Google Scholar]
Schulz 2007 {published data only}
- Neumann CL, Wagner F, Menne J, Brockes C, Schmidt‐Weitmann S, Rieken EM, et al. Body weight telemetry is useful to reduce interdialytic weight gain in patients with end‐stage renal failure on hemodialysis. Telemedicine Journal & E‐Health 2013;19(6):480‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schulz EG, Scheele K, Bargfeldt M, Fischer N, Korth U, Weber MH. Body weight telemonitoring in patients with endstage renal failure on hemodialysis and its implication for hemoglobin variability [abstract no: V34]. DMW 2006;131(Suppl 6):S156. [CENTRAL: CN‐01658333] [Google Scholar]
- Schulz EG, Wagner F, Fischer N, Schwarz A, Wolf A, Korth U, et al. Body weight telemetry in hemodialysis patients reduces systolic blood pressure, interdialytic weight gain and ultrafiltration [abstract no: 411]. Journal of Clinical Lipidology 2008;2(Suppl 5):S192. [CENTRAL: CN‐01658332] [Google Scholar]
- Schulz EG, Wagner F, Fischer N, Wolf A, Korth U, Weber MH. Body weight telemetry in patients with endstage renal failure on hemodialysis [abstract no: SaP408]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi372. [CENTRAL: CN‐01658354] [DOI] [PubMed] [Google Scholar]
- Schulz EG, Wagner F, Fischer N, Wolf A, Korth U, Weber MH. Body weight telemetry in patients with endstage renal failure on hemodialysis: preliminary data [Korpergewichts‐Telemetrie bei Hamodialysepatienten‐‐vorlaufige Daten]. Deutsche Medizinische Wochenschrift 2007;132(9):423‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schulz EG, Wagner F, Wolf A, Korth U, Weber MH. Body weight telemetry in hemodialysis patients educes systolic blood pressure, interdialytic weight gain and ultrafiltration [abstract no: PS/26/WED/64]. Journal of Hypertension 2016;26(Suppl 1):S385. [CENTRAL: CN‐01658337] [Google Scholar]
- Schulz EG, Wagner F, Wolf A, Korth U, Weber MH. Body weight telemetry in hemodialysis patients reduces systolic blood pressure, interdialytic weight gain and ultrafiltration [abstract no: P‐107]. Journal of Clinical Hypertension 2009;11(4 Suppl A):A60. [CENTRAL: CN‐01658338] [Google Scholar]
SUBLIME 2016 {published data only}
- Humalda JK, Klaassen G, Vries H, Meuleman Y, Laverman GD, Bos WJ, et al. The 'sublime' approach: Cost‐efficacy of a novel self‐management approach for dietary sodium restriction in CKD patients [abstract no: SP351]. Nephrology Dialysis Transplantation 2016;31(Suppl 1):i206‐7. [EMBASE: 72326453] [Google Scholar]
Swallow 2016 {published data only}
- Swallow V, Carolan I, Smith T, Webb NJ, Knafl K, Santacroce S, et al. A novel Interactive Health Communication Application (IHCA) for parents of children with long‐term conditions: development, implementation and feasibility assessment. Informatics for Health & Social Care 2016;41(1):20‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TAKE‐IT 2014 {published data only}
- Bignall O, Pai A, Amaral S, Furth S, Bergman N, Goebel J, et al. Differences in reported barriers to immunosuppression adherence among a cohort of pediatric renal transplant recipients from the United States and Canada [abstract no: D161]. American Journal of Transplantation 2016;16(Suppl 3):751. [EMBASE: 611700425] [Google Scholar]
- Boucquemont J, Foster B. Adherence patterns in adolescent and young adult kidney transplant recipients [abstract no: 229]. American Journal of Transplantation 2016;16(Suppl 3):284. [EMBASE: 611700136] [Google Scholar]
- Boucquemont J, Foster B, TAKE‐IT Investigators. Sex and age differences in medication adherence in adolescent and young adult kidney transplant recipients [abstract no: SA‐PO404]. Journal of the American Society of Nephrology 2016;27(Abstracts):721A. [CENTRAL: CN‐01657535] [Google Scholar]
- Foster B, Pai A, Furth S. The TAKE‐IT intervention improves medication adherence in adolescent kidney transplant recipients [abstract no: 359]. American Journal of Transplantation 2017;17(Suppl 3):332‐3. [EMBASE: 615704529] [Google Scholar]
- Foster B, Zhao H, Pai A, Zelikovsky N, Holly CD, Smith JM, et al. Medication adherence barrier burden predicts subsequent taking adherence in adolescent kidney transplant recipients in the TAKE‐IT trial [abstract no: SA‐PO1027]. Journal of the American Society of Nephrology 2015;26(Abstracts):870A. [CENTRAL: CN‐01657534] [Google Scholar]
- Foster BJ, Pai A, Zhao H, Furth S. The TAKE‐IT study: aims, design, and methods. BMC Nephrology 2014;15(1):139. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BJ, Pai AL, Zelikovsky N, Amaral S, Bell L, Dharnidharka VR, et al. A randomized trial of a multicomponent intervention to promote medication adherence: the teen adherence in kidney transplant effectiveness of intervention trial (TAKE‐IT). American Journal of Kidney Diseases 2018;72(1):30‐41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BJ, Zhao H, Pai A, Zelikovsky N, Holly CD, Smith JM, et al. Medication adherence barrier burden predicts subsequent adherence to dosing time schedule in adolescent kidney transplant recipients in the TAKE‐IT Trial [abstract no: SA‐PO1031]. Journal of the American Society of Nephrology 2015;26(Abstracts):871A. [CENTRAL: CN‐01657537] [Google Scholar]
Welch 2013 {published data only}
- Welch JL, Astroth KS, Perkins SM, Johnson CS, Connelly K, Siek KA, et al. Using a mobile application to self‐monitor diet and fluid intake among adults receiving hemodialysis. Research in Nursing & Health 2013;36(3):284‐98. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2010 {published data only}
- White S. Telemonitoring in the renal program: empowering patients and engaging providers [abstract]. Peritoneal Dialysis International 2011;31(Suppl 1):S29. [EMBASE: 70716162] [Google Scholar]
- White S, Bezaire D, McMurray S. Telemonitoring in the renal program: empowering patients and engaging providers [abstract]. Peritoneal Dialysis International 2010;30(Suppl 1):S14. [CENTRAL: CN‐01658223] [Google Scholar]
Williams 2017 {published data only}
- Williams S, Han M, Ye X, Zhang H, Meyring‐Wosten A, Bonner M, et al. Physical activity and sleep patterns in hemodialysis patients in a suburban environment. Blood Purification 2017;43(1‐3):235‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdel‐Kader 2011 {published data only}
- Abdel‐Kader K, Fischer GS, Li J, Moore CG, Hess R, Unruh ML. Automated clinical reminders for primary care providers in the care of CKD: a small cluster‐randomized controlled trial. American Journal of Kidney Diseases 2011;58(6):894‐902. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2011e {published data only}
- Chen SH, Tsai YF, Sun CY, Wu IW, Lee CC, Wu MS. The impact of self‐management support on the progression of chronic kidney disease‐‐a prospective randomized controlled trial. Nephrology Dialysis Transplantation 2011;26(11):3560‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Korus 2017 {published data only}
- Korus M, Cruchley E, Gold A, Stinson J, Parekh R, Anthony S. A self‐management website for teenagers who have undergone kidney or liver transplantation: a feasibility randomized controlled pilot trial [abstract no: 401.3]. Pediatric Transplantation 2017;21(Suppl 1):33‐4. [EMBASE: 616208183] [Google Scholar]
Morales‐Barria 2016 {published data only}
- Morales‐Barria J, Morales F, Celis B, Johansson P, Metcalfe C. "Preventing": A new application for smartphones to assess and improve immunosupressant's adherence in renal transplant recipients [abstract no: P.1955]. Transplantation 2016;100(7 Suppl 1):S885. [EMBASE: 613004354] [Google Scholar]
RaDIANT 2014 {published data only}
- Gander JC, Sauls L, Browne T, Plantinga L, McPherson LJ, Gibney EM, et al. Feasibility and sustainability of the RaDIANT community study among Georgia dialysis facillities [abstract no: SA‐PO1006]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):865A. [Google Scholar]
- Hamoda RE, Gander JC, McPherson LJ, Arriola KJ, Cobb L, Pastan SO, et al. Process evaluation of the RaDIANT community study: a dialysis facility‐level intervention to increase referral for kidney transplantation. BMC Nephrology 2018;19(1):13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer RE, Gander J, Sauls L, Amamoo MA, Krisher J, Mulloy LL, et al. The RaDIANT community study protocol: community‐based participatory research for reducing disparities in access to kidney transplantation. BMC Nephrology 2014;15(1):171. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer RE, Paul S, Plantinga L, Gander J, Sauls L, Krisher J, et al. A randomized trial to reduce disparities in referral for transplant evaluation. Journal of the American Society of Nephrology 2016;28(3):935‐42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer RE, Sauls L, Gander JC, Amamoo MA, Plantinga L, Evans DD, et al. A randomized multicomponent intervention to reduce disparities in transplant referral: interim results from the RaDIANT community study [abstract no: SA‐PO1092]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):B3. [Google Scholar]
- Patzer RE, Sauls L, Gander JC, Plantinga L, Paul S, Gibney EM, et al. A randomized multicomponent intervention to reduce disparities in transplant referral: results from the RaDIANT Community study [abstract no: FR‐PO1001]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):596A. [CENTRAL: CN‐01658334] [Google Scholar]
Roberto 2009 {published data only}
- Roberto AJ, Krieger JL, Beam MA. Enhancing web‐based kidney disease prevention messages for Hispanics using targeting and tailoring. Journal of Health Communication 2009;14(6):525‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SMILE 2010 {published data only}
- Belayev LY, Mor MK, Sevick MA, Shields AM, Rollman BL, Palevsky PM, et al. Longitudinal associations of depressive symptoms and pain with quality of life in patients receiving chronic hemodialysis. Hemodialysis International 2015;19(2):216‐24. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Mor MK, Shields AM, Sevick MA, Arnold RM, Palevsky PM, et al. Associations of health literacy with dialysis adherence and health resource utilization in patients receiving maintenance hemodialysis. American Journal of Kidney Diseases 2013;62(1):73‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Green JA, Mor MK, Shields AM, Sevick MA, Palevsky PM, Fine MJ, et al. Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(6):1354‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Mor MK, Shields AM, Sevik MA, Palevsky PM, Fine MJ, et al. Renal provider perceptions and practice patterns regarding the management of pain, sexual dysfunction, and depression in hemodialysis patients. Journal of Palliative Medicine 2012;15(2):163‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mor MK, Sevick MA, Shields AM, Green JA, Palevsky PM, Arnold RM, et al. Sexual function, activity, and satisfaction among women receiving maintenance hemodialysis. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(1):128‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena‐Polanco JE, Mor MK, Tohme FA, Fine MJ, Palevsky PM, Weisbord SD. Acceptance of antidepressant treatment by patients on hemodialysis and their renal providers. Clinical Journal of the American Society of Nephrology: CJASN 2017;12(2):298‐303. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbord SD, Mor MK, Green JA, Sevick MA, Shields AM, Zhao X, et al. Comparison of symptom management strategies for pain, erectile dysfunction, and depression in patients receiving chronic hemodialysis: a cluster randomized effectiveness trial. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(1):90‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbord SD, Mor MK, Sevick MA, Shields AM, Rollman BL, Palevsky PM, et al. Associations of depressive symptoms and pain with dialysis adherence, health resource utilization, and mortality in patients receiving chronic hemodialysis. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(9):1594‐602. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbord SD, Shields AM, Mor MK, Sevick MA, Homer M, Peternel J, et al. Methodology of a randomized clinical trial of symptom management strategies in patients receiving chronic hemodialysis: the SMILE study. Contemporary Clinical Trials 2010;31(5):491‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Warren 2009 {published data only}
- Warren G, Heisler M, Perry E, Ferriter M, Piette J, Magee J. Telephone peer mentoring: a new approach to improving access to kidney transplantation [abstract no: 223]. American Journal of Kidney Diseases 2009;53(4):A78. [EMBASE: 70141712] [Google Scholar]
Wilson 2014 {published data only}
- Biswas A, Parikh CR, Feldman HI, Garg AX, Latham S, Lin H, et al. Identification of patients expected to benefit from electronic alerts for acute kidney injury. Clinical Journal of the American Society of Nephrology: CJASN 2018;13(6):842‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Bia JR, Ubaid‐Ullah M, Testani JM, Wilson FP. Provider acceptance of an automated electronic alert for acute kidney injury. Clinical Kidney Journal 2016;9(4):567‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah MU, Gitelman Y, Borovskiy Y, Aqeel I, Negoianu D, Fuchs BD, et al. Provider acceptance of a short messaging system‐based, electronic alert for acute kidney injury [abstract no: SA‐PO021]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):637A. [Google Scholar]
- Wilson FP, Reese PP, Shashaty MG, Ellenberg SS, Gitelman Y, Bansal AD, et al. A trial of in‐hospital, electronic alerts for acute kidney injury: design and rationale. Clinical Trials 2014;11(5):521‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, et al. Automated, electronic alerts for acute kidney injury: a single‐blind, parallel‐group, randomised controlled trial. Lancet 2015;385(9981):1966‐74. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FP, Shashaty MG, Reese PP, Gitelman Y, Borovskiy Y, Feldman HI, et al. In‐hospital, electronic alerts for acuute kidney injury: a randomized controlled trial [abstract no: A‐PO1107]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):B7. [Google Scholar]
References to ongoing studies
CONNECT 2017 {published data only}
- Jeffs L, Jain AK, Man RH, Onabajo N, Desveaux L, Shaw J, et al. Exploring the utility and scalability of a telehomecare intervention for patients with chronic kidney disease undergoing peritoneal dialysis‐a study protocol. BMC Nephrology 2017;18(1):155. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
eNEPHRO 2017 {published data only}
- Thilly N, Chanliau J, Frimat L, Combe C, Merville P, Chauveau P, et al. Cost‐effectiveness of home telemonitoring in chronic kidney disease patients at different stages by a pragmatic randomized controlled trial (eNephro): rationale and study design. BMC Nephrology 2017;18(1):126. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2017 {published data only}
- Jung HY, Seong SJ, Choi JY, Cho JH, Park SH, Kim CD, et al. The efficacy and stability of an information and communication technology‐based centralized monitoring system of adherence to immunosuppressive medication in kidney transplant recipients: study protocol for a randomized controlled trial. Trials [Electronic Resource] 2017;18(1):480. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
KARE 2015 {published data only}
- Tuot DS, Velasquez A, McCulloch CE, Banerjee T, Zhu Y, Hsu CY, et al. The Kidney Awareness Registry and Education (KARE) study: protocol of a randomized controlled trial to enhance provider and patient engagement with chronic kidney disease. BMC Nephrology 2015;16:166. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kosaka 2017 {published data only}
- Kosaka S, Hoshino J, Yamanouchi M, Sekine A. Assessment of efficacy of a CKD support decision making application and home blood pressure measurement system in patients with CKD: study protocol of a randomized, controlled trial [abstract no: PUB188]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):1013. [CENTRAL: CN‐01657824] [Google Scholar]
MAGIC 2016 {published data only}
- Russell CL, Moore S, Hathaway D, Cheng AL, Chen G, Goggin K. MAGIC Study: aims, design and methods using SystemCHANGETM to improve immunosuppressive medication adherence in adult kidney transplant recipients. BMC Nephrology 2016;17(1):84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00394576 {published data only}
- Jaffery JB. Assessing novel methods of improving patient education of nutrition. www.clinicaltrials.gov/ct2/show/NCT00394576 (first received 1 November 2006).
NCT02097550 {published data only}
- Yank V. Primary care eHealth intervention for improved outcomes in chronic kidney disease (CKD eHealth). www.clinicaltrials.gov/ct2/show/NCT02097550 (first received 27 March 2014).
NCT02610946 {published data only}
- Tran H. Do technology Apps improve compliance in adolescent renal transplant recipients?. www.clinicaltrials.gov/ct2/show/NCT02610946 (first received 20 November 2015).
TELEGRAFT 2015 {published data only}
- Foucher Y, Meurette A, Daguin P, Bonnaud‐Antignac A, Hardouin JB, Chailan S, et al. A personalized follow‐up of kidney transplant recipients using video conferencing based on a 1‐year scoring system predictive of long term graft failure (TELEGRAFT study): protocol for a randomized controlled trial. BMC Nephrology 2015;16:6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waterman 2015 {published data only}
- Waterman AD, McSorley AM, Peipert JD, Goalby CJ, Peace LJ, Lutz PA, et al. Explore Transplant at Home: a randomized control trial of an educational intervention to increase transplant knowledge for black and white socioeconomically disadvantaged dialysis patients. BMC Nephrology 2015;16:150. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WISHED 2016 {published data only}
- Harvey A, Walsh M, Jain AK, Bosch E, Moreau C, Garland J, et al. The WISHED Trial: implementation of an interactive health communication application for patients with chronic kidney disease. Canadian Journal of Kidney Health & Disease 2016;3:29. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Beratarrechea 2014
- Beratarrechea A, Lee AG, Wilner JM, Jahanqir E, Ciapponi A, Rubenstein A. The impact of mobile health interventions on chronic disease outcomes in developing countries: a systematic review. Telemedicine Journal & E‐Health 2014;20(1):75‐82. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonner 2014
- Bonner A, Havas K, Douglas C, Thepha T, Bennet P, Clark R. Self‐management programs in stages 1‐4 chronic kidney disease: a literature review. Journal of Renal Care 2014;40(3):194‐204. [PUBMED: 24628848] [DOI] [PubMed] [Google Scholar]
Checchi 2014
- Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence: a systematic review. JAMA 2014;312(2):1237‐47. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2011
- Chen SH, Tsai YF, Sun CY, Wu IW, Lee CC, Wu MS. The impact of self‐management support on the progression of chronic kidney disease‐‐a prospective randomized controlled trial. Nephrology Dialysis Transplantation 2011;26(11):3560‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cotter 2014
- Cotter AP, Durant N, Agne AA, Cherrington AL. Internet interventions to support lifestyle modification for diabetes management: a systematic review of the evidence. Journal of Diabetes & its Complications 2014;28(2):243‐51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Couser 2011
- Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney International 2011;80(12):1258‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Devins 2005
- Devins GM, Mendelssohn DC, Barre PE, Taub K, Binik YM. Predialysis psychoeducational intervention extends survival in CKD: a 20‐year follow‐up. American Journal of Kidney Diseases 2005;46(6):1088‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eysenbach 2001
- Eysenbach G. What is e‐health?. Journal of Medical Internet Research 2001;3(2):E20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman A D, Akl E A, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hamine 2015
- Hamine S, Gerth‐Guyette E, Faulx D, Greeb BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. Journal of Medical Internet Research 2015;17(2):e52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jha 2013
- Jha V, Garcia‐Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives.[Erratum appears in Lancet. 2013 Jul 20;382(9888):208]. Lancet 2013;382(9888):260‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kelly 2016
- Kelly JT, Reidlinger DP, Hoffmann TC, Campbell KL. Telehealth methods to deliver dietary interventions in adults with chronic disease: a systematic review and meta‐analysis. American Journal of Clinical Nutrition 2016;104(6):1693‐702. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kitsiou 2017
- Kitsiou S, Pare G, Jaana M, Gerber B. Effectiveness of mHealth interventions for patients with diabetes: an overview of systematic reviews. PLoS ONE [Electronic Resource] 2017;12(3):e0173160. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez‐Vargas 2014
- Lopez‐Vargas PA, Tong A, Phoon RK, Chadban SJ, Shen Y, Craig JC. Knowledge deficit of patients with stage 1‐4 CKD: a focus group study. Nephrology 2014;19(4):234‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Michie 2013
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine 2013;46(1):81‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moore 2013
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Cooper C, et al. Process evaluation in complex public health intervention studies: the need for guidance. Journal of Epidemiology & Community Health 2013;68(2):101‐2. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pew Research Center 2016
- Pew Research Center. Smartphone ownership and internet usage continues to climb in emerging economies. 2016. www.pewglobal.org/2016/02/22/smartphone‐ownership‐and‐internet‐usage‐continues‐to‐climb‐in‐emerging‐economies/ (accessed 13 February 2019).
Raajimakers 2015
- Raaijmakers LC, Pouwels S, Berghuls SA, Nienhuijs SW. Technology‐based interventions in the treatment of overweight and obesity: a systematic review. Appetite 2015;95:138‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sanyal 2018
- Sanyal C, Stolee P, Juzwishin D, Husereau D. Economic evaluations of eHealth technologies: a systematic review. PLoS ONE [Electronic Resource] 2018;13(6):e0198112. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
SONG 2017
- SONG Initiative. The SONG Handbook Version 1.0. 2017. www.songinitiative.org/reports‐and‐publications/ (accessed 13 February 2019).
Sorgente 2017
- Sorgente A, Pietrabissa G, Manzoni GM, Re F, Simpson S, Perona S, et al. Web‐based interventions for weight loss or weight loss maintenance in overweight and obese people: a systematic review of systematic reviews. Journal of Medical Internet Research 2017;19(6):e229. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2013
- Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Annals of Internal Medicine 2013;158(11):825‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tao 2015
- Tao D, Xie L, Wang T, Wang T. A meta‐analysis of the use of electronic reminders for patient adherence to medication in chronic disease care. Journal of Telemedicine & Telecare 2015;21(1):3‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tong 2007
- Tong B, Stevenson C. Comorbidity of cardiovascular disease, diabetes and chronic kidney disease in Australia. www.aihw.gov.au/reports/heart‐stroke‐vascular‐disease/comorbidity‐of‐cardiovascular‐disease‐diabetes‐an/contents/table‐of‐contents (accessed 13 February 2019).
Vervloet 2012
- Vervloet M, Linn AJ, Weert JC, Bakker DH, Bouvy ML, Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. Journal of the American Medical Informatics Association 2012;19(5):696‐704. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warner 2017
- Warner MM, Kelly JT, Reidlinger DP, Hoffman TC, Campbell KL. Reporting of telehealth‐delivered dietary intervention trials in chronic disease: systematic review. Journal of Medical Internet Research 2017;19(12):e410. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Widmer 2015
- Widmer RJ, Collins NM, Collins CS, West CP, Lerman LO, Lerman A. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta‐analysis. Mayo Clinic Proceedings 2015;90(4):469‐80. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Stevenson 2016
- Stevenson JK, Campbell ZC, Webster AC, Chow CK, Campbell KL, Lee VW. eHealth interventions for people with chronic kidney disease. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012379] [DOI] [PMC free article] [PubMed] [Google Scholar]


