Abstract
The basement membrane is a thin but dense, sheet-like specialized type of extracellular matrix that has remarkably diverse functions tailored to individual tissues and organs. Tightly controlled spatial and temporal changes in its composition and structure contribute to the diversity of basement membrane functions. These different basement membranes undergo dynamic transformations throughout animal life, most notably during development. Numerous developmental mechanisms are regulated or mediated by basement membranes, often by a combination of molecular and mechanical processes. A particularly important process involves cell transmigration through a basement membrane because of its link to cell invasion in disease. While developmental and disease processes share some similarities, what clearly distinguishes the two is dysregulation of cells and extracellular matrices in disease. With its relevance to many developmental and disease processes, the basement membrane is a vitally important area of research that may provide novel insights into biological mechanisms and development of innovative therapeutic approaches. Here we present a review of developmental and disease dynamics of basement membranes in Caenorhabditis elegans, Drosophila melanogaster, and vertebrates.
Keywords: basement membrane, development, morphogenesis, extracellular matrix, cell migration, invasion, proliferation, cancer, basement membrane pores
Introduction
The extracellular matrix (ECM) is a non-cellular component of multicellular organisms that plays essential roles in animal development and throughout life (Naba et al., 2016). Constantly undergoing remodeling, the ECM provides highly dynamic microenvironments. Two types of ECM—interstitial connective tissue and basement membrane—have both overlapping and distinct biological functions through their bidirectional interactions with cells and tissues. Both interstitial connective tissue and basement membrane ECMs can modulate cell proliferation, differentiation, angiogenesis, branching morphogenesis, tissue repair, and homeostasis (Bonnans et al., 2014; Rozario and DeSimone, 2010). Through binding and sequestering of soluble growth factors in the presence of appropriate cell-mediated forces or proteolytic degradation, both types of ECM can also enable spatial-temporal regulation of receptor-ligand interactions. ECMs provide adhesive scaffolds and sometimes even concentration gradients for migratory cells. Furthermore, ECMs can generate and transduce mechanical signals. Through their interactions with cell-surface receptors, ECMs modulate a remarkably wide range of signaling processes (DeSimone and Mecham, 2013; Hynes and Yamada, 2012).
On the other hand, interstitial connective tissue and basement membrane ECMs have many unique characteristics. Interstitial connective tissue ECMs can range from a gel-like scaffold comprised of collagen I, fibronectin, and/or cartilage proteoglycans to tough, dense sheets or tendons (DeSimone and Mecham, 2013; Jayadev and Sherwood, 2017). Other components can include a wide variety of glycoproteins, proteoglycans, other types of collagen except type IV, and integrins (transmembrane heterodimeric receptors). The interstitial connective tissue ECM provides structure to spaces between cells and modulates intercellular and inter-tissue interactions via integrins and other cell surface ECM receptors.
In contrast, the basement membrane is an ultrathin, dense, sheet-like ECM that is associated with virtually all organized cells (Yurchenco and Patton, 2009). The basement membrane underlies epithelial and endothelial cells and surrounds muscle, fat, and Schwann cells. Its primary structural elements consist of two polymeric networks comprised of laminin and type IV collagen, which are interconnected with nidogen, perlecan, and other molecules (Yurchenco, 2011).
The basement membrane is essential for animal development. It provides tissue integrity, elasticity, and biochemical and mechanical signaling, while facilitating intracellular and intercellular interactions. Mutations in basement membrane components lead to a variety of detrimental conditions affecting multiple organs and structures across embryonic and postnatal stages. During development, the basement membrane displays the opposing but complementary traits of rigidity and plasticity—it defines tissue boundaries and protects tissues from mechanical damage while exhibiting a high degree of plasticity that permits growth, development, and cellular and tissue interactions (McClatchey et al., 2016). The basement membrane achieves these seemingly contradictory tasks through its physical pliability and versatility in tissue- and time-specific structures and functions. The focus of this review will be on basement membranes in development: their functions, spatial and temporal variations in biochemical and biophysical properties, mechanisms by which they coordinate tissue morphogenesis, and the relevance of such developmental mechanisms to pathological conditions, including cancer invasion and congenital or acquired disorders.
Functions
The basement membrane provides tissues with a wide array of functions that include tissue separation, barrier, provision of an adhesive substrate and signaling platform for migration, polarization, differentiation, tissue shaping, and growth.
Tissue Separation and Barrier
While the basement membrane comprises a mere fraction of total ECM mass, the stable interlocking lattice of cross-linked type IV collagen and laminin forms a major structural barrier to transmigration of most cells (except leukocytes) in normal physiology and pathological conditions (Kalluri, 2003).
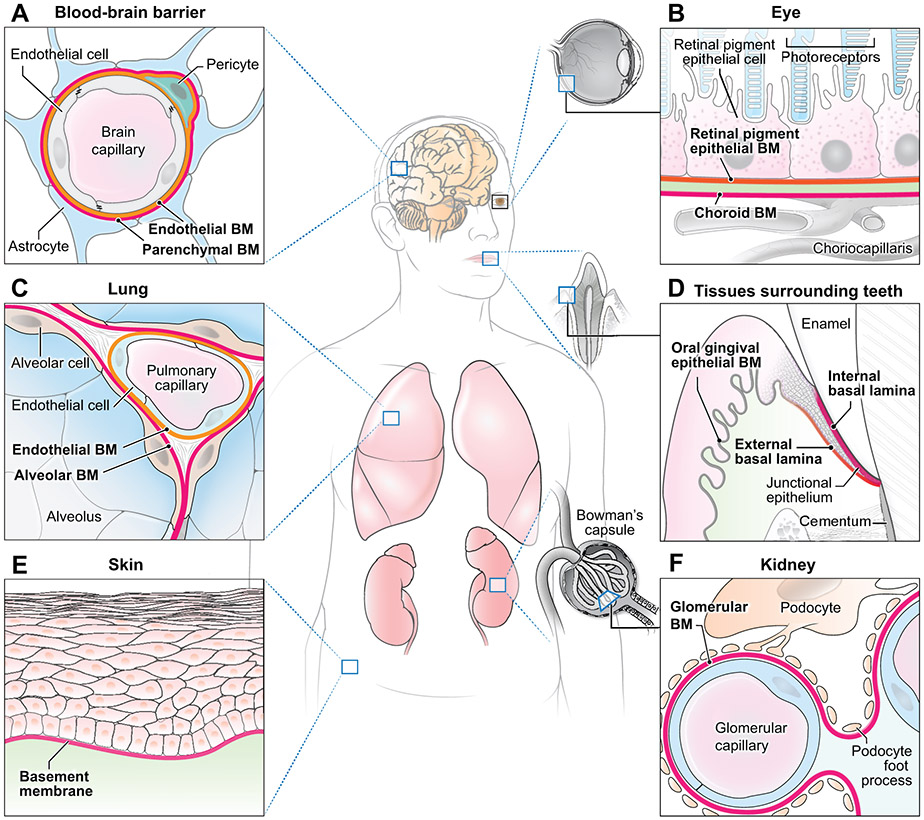
Basement membrane permeability varies greatly throughout the body. In the developing kidney, two basement membranes—one associated with the vascular endothelium and another with the podocyte epithelium—merge to form the glomerular basement membrane (Figure 1F) (Deen, 2004). The glomerular basement membrane serves as the kidney’s filtration barrier with selective molecular permeability. With its controlled porosity based on size and charge, the glomerular basement membrane is impermeable to plasma proteins while permitting low-molecular weight substances to pass readily. Lung alveolar development also involves a merger of alveolar and capillary basement membranes, which establishes a gas-exchange surface (Figure 1C) (Rodeck and Whittle, 2009).
Figure 1. Schematics of basement membranes (BMs) in various tissues.
(A) Blood-brain barrier: endothelial and parenchymal BMs surround the brain capillary. (B) Eye: retinal pigment epithelium BM, choroid BM, and an intermediate collagenous zone comprise the Bruch’s membrane. (C) Lung: endothelial and alveolar BMs merge to provide a site for gas exchange. (D) Tissues surrounding teeth: junctional epithelium adheres to the enamel via the internal basal lamina and to the mesenchyme via the external basal lamina. The oral gingival epithelium BM is adjacent to the oral epithelium. (E) Skin: cutaneous BM underlies basal epithelial cells. (F) Kidney: during development, endothelial and podocyte-derived epithelial BMs merge to form the glomerular BM. Figures are not drawn to scale.
In brain development, the blood-brain barrier is formed at the interface between the vascular system and the brain by merging capillary endothelial and parenchymal basement membranes (Figure 1A). The blood-brain barrier has particularly restrictive permeability and strictly controls the exchange and transport of molecules between the two tissues (Engelhardt et al., 2017). Integrity of the embryonic and neonatal blood-brain barrier is largely regulated by laminin α4 in the endothelial basement membrane, and genetic deletion of laminin α4 leads to hemorrhage (Thyboll et al., 2002). Cellular sources of laminin for regulating the blood-brain barrier vary across time. Barrier integrity is mainly regulated by pericyte-derived laminin during development, but by astrocytic laminin in adulthood (Yao et al., 2014). Mice defective in pericytic laminin occasionally manifest hydrocephalus and blood-brain barrier breakdown accompanied by reductions in pericytes, AQP4, and tight junction proteins (Gautam et al., 2016). In adulthood, pathological conditions such as stroke and Alzheimer’s disease can cause disruption of blood-brain barrier integrity. Basement membrane proteins are reduced in stroke whereas in Alzheimer’s disease, basement membrane thickening is accompanied by increased collagen IV deposition and amyloid-β accumulation (Thomsen et al., 2017).
The basement membrane separates tissues through adhesions mediated by hemidesmosomes at the epithelial-mesenchymal interface. Laminin, a key component of hemidesmosomes, contributes to cell-matrix adhesion by interacting with cell-surface integrins and anchoring fibrils predominantly comprised of collagen VII (Hohenester and Yurchenco, 2013). The formation of a stable adhesion complex between the two tissue layers protects tissues from destabilizing shear forces. The epidermal basement membrane and associated molecules collectively build a protective skin barrier (Figure 1E). For example, collagen VII anchoring fibrils connect the epidermal basement membrane to the dermis (Rousselle et al., 1997). Collagen VII originates within the basement membrane where it binds to laminin, projecting into the papillary dermis where anchoring plaques interweave with collagen I and III fibrils (Breitkreutz et al., 2013). Collagen VII mutations result in recessive dystrophic epidermolysis bullosa characterized by blistering and infection owing to a perturbed basement membrane barrier and impaired wound healing (Guerra et al., 2017). Sadly, the majority of those affected by the severe generalized subtype also develop lethal cutaneous squamous cell carcinoma (Fine et al., 2014).
Basement membranes serve as an important anatomical landmark for the clinical diagnostic classification of tumors. Conditions associated with more favorable prognoses, i.e., dysplasia and carcinoma in situ, represent confinement of the primary neoplasm by an intact basement membrane. Once neoplastic cells breach the basement membrane, however, they are considered to be invasive and have the capacity to metastasize (Moasser, 2013). Therefore, basement membranes have important barrier functions in both development and disease.
Cell Adhesion and Migration
Basement membranes provide an adhesive substrate for cells, and they are linked functionally to the actin cytoskeleton via integrins or other ECM receptors to mediate cell attachment and migration, as well as modulating intracellular signaling pathways. Adhesion to basement membranes via cell surface receptors allows cells to mechano-sense local stiffness, stiffness gradients, and other physical cues, which ultimately affect cellular behavior (Hynes, 1992). During Drosophila development, the receptor-ligand interplay between integrins and laminins in the basement membrane regulates follicle cell migration. Whereas integrin levels in follicle cells remain relatively stable, laminin expression in the basement membrane increases over time. The onset and speed of follicle cell migration are determined by this balance between integrin and laminin levels. Laminin-mutant eggs display altered cell migration and disrupted tissue shaping of developing follicles (Diaz de la Loza et al., 2017). Similarly, peripheral nerve establishment in mice requires laminin α5-dependent migration of neural crest cells, which differentiate into the peripheral nervous system and glial cells as they complete migration (Coles et al., 2006).
An in vitro model of cell migration demonstrates the profound effects of ECM dimensionality (2D vs. 3D) on cellular behavior (Hakkinen et al., 2011). Human foreskin fibroblasts in a 3D basement membrane extract (Matrigel) lose directionality and fail to migrate, yet the same substrate in a 2D configuration analogous to a basement membrane sheet allows highly efficient migration. These changes in cell migration patterns can be attributed to differences in ECM dimensionality. Even though useful for cell culture of epithelial cells, immersion of cells in a 3D basement membrane extract does not accurately simulate the basement membrane in vivo. A better representation of an in vivo environment is the 2D basement membrane extract model, which allows efficient cell migration. Indeed, cells in vivo migrate efficiently adjacent to the basement membrane. In the mouse submandibular gland, outer bud epithelial cells adjacent to the basement membrane show the highest rates of motility (Daley et al., 2017; Hsu et al., 2013). Motility of the outer bud cells is myosin II- and integrin α6β1-dependent, which suggests cell-ECM interaction. These findings highlight the importance of interactions between basement membranes, cell surface receptors, and cellular processes in regulating cell migratory behavior.
Polarity
During early morphogenesis, the basement membrane coordinates epithelial tissue organization by modulating apical polarity. Epithelial polarity is established through organizing polarity proteins and lipids at the plasma membrane as well as utilizing adhesion molecules as positional cues in interactions with other epithelial cells and the adjacent basement membrane (Tanos and Rodriguez-Boulan, 2008). Loss of epithelial polarity is observed in pathological conditions such as cancer, and the extent of loss often correlates with tumor aggressiveness. Evidence exists for a potential role of myoepithelial cell-derived laminin α chains in regulation of neoplastic mammary gland epithelial polarity (Slade et al., 1999).
Laminin is a key molecule for establishing apical polarity during tooth development (Fukumoto et al., 2006). Laminin α5 is a subunit of the major laminins in the tooth germ basement membrane, LM-511 and LM-521. Laminin α5-null mice display altered localization patterns of integrin α6β4 (the laminin α5 receptor) and hypoplastic tooth germs with reduced proliferation of the dental epithelium and loss of basal cell polarity. Importantly, the enamel knot (the signaling center for tooth morphogenesis) is defective in these mutant mice, with reduced sonic hedgehog (SHH) and fibroblast growth factor 4 (FGF4) (Fukumoto et al., 2006). Disruption in polarity formation is also evident in mammary epithelial cells when basement membrane stability is perturbed by loss of collagen IV or cell-basement membrane adhesions (Plachot et al., 2009).
Polarity formation is mediated through bidirectional interactions between the basement membrane and epithelial cells. Basal epithelial cells interact reciprocally with the basement membrane, providing positional cues for establishing spatially-restricted organization of the basement membrane (Gervais et al., 2016). The basal epithelial cells synthesize basement membrane proteins and organize their basal deposition, which requires expression of the polarity protein, PAR-1b. In the embryonic mouse submandibular salivary gland, establishment of basement membrane organization relies on basal expression of PAR-1b in the epithelium (Daley et al., 2012). Through reciprocal interactions with the epithelium, the basement membrane contributes to the establishment of apical polarity, cellular organization, and coherent tissue architecture.
Tissue Shaping
The basement membrane mediates tissue remodeling through molecular and mechanical processes. Unlike localized biochemical intercellular signaling, mechanical stress can exert global tissue-shaping forces due to the transmission of tension through interconnected cells (Legoff et al., 2013). Involvement of the basement membrane in tissue shaping is best exemplified in Drosophila wing and egg chamber development. Collagen IV knockdown attenuates Dpp signaling (the Drosophila homolog of BMP) and reduces Drosophila wing size (Ma et al., 2017). During egg chamber development, the initially spherical egg chamber elongates in response to constrictive forces applied by the surrounding basement membrane. This constricting collagen IV, along with laminin and perlecan, is organized in a robust circumferential fibril-like orientation (Haigo and Bilder, 2011; Isabella and Horne-Badovinac, 2016). Secreted in site-specific fashion by Rab10 into the pericellular space between egg follicle cells, this fibril-like “molecular corset” of basement membrane proteins has been thought to arise from the collective rotation of follicle cells in a direction that coincides with the orientation of deposited collagen IV protein (Haigo and Bilder, 2011; Isabella and Horne-Badovinac, 2016). However, more recent findings suggest the involvement of an additional tissue-shaping mechanism independent of collective tissue rotation. Mutation of the atypical cadherin Fat2 results in egg chambers that fail to rotate; nonetheless, mutant eggs still display aligned collagen IV and proper egg chamber elongation (Aurich and Dahmann, 2016).
Though these findings demonstrate the role of basement membranes in tissue shaping, some important questions remain to be answered: what is the biological significance of collective epithelial migration; what establishes and regulates the fibril-like circumferential collagen IV alignment; how does fibril-like collagen IV biophysically compare to collagen I fibrils; and how do these findings in Drosophila translate to mammalian systems?
Signaling
Although many developmental mechanisms are known to be regulated by molecular signals, a combination of mechanical and regulatory gene processes can often provide a more complete mechanistic blueprint. For instance, pattern formation of avian feather follicles is initiated by mechanical compression of the epidermis by the contracting dermis, followed by β-catenin translocation to the nucleus in the compressed epithelial cells. New follicles emerge as the primordium basement membrane buckles increasingly (Shyer et al., 2017). Downstream genes associated with follicle formation are activated upon creation of cell aggregates by such dermal contraction. Since tissue morphogenesis is influenced by the level of tension in cells and the basement membrane (Moore et al., 2005), basement membrane buckling itself may also be involved in this mechanical crosstalk that regulates pattern formation of skin follicles.
During development and throughout animal life, basement membranes can function as signaling platforms by sequestering growth factors and other ligands. Perlecan, agrin, and collagen XVIII bind to many growth factors via heparan sulfate glycosaminoglycan chains. By signaling through cell surface receptors, growth factors such as FGFs, bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and transforming growth factor-β (TGF-β) regulate stem cell maintenance, cell migration, proliferation, and survival (Jayadev and Sherwood, 2017). Further, stem cell maintenance can be achieved directly by laminin; a combination of laminin and E-cadherin is capable of maintaining clonal survival and self-renewal of human embryonic stem cells (Rodin et al., 2014).
In addition to growth factors, a large number of ECM-modifying enzymes that mediate proteolysis, sulfation, and glycosylation act at basement membranes to modulate signaling (Yoshizaki and Yamada, 2013). Protease-mediated degradation of basement membranes can activate signaling pathways, not only by releasing bound growth factors, but also by releasing or exposing cryptic fragments of basement membrane proteins with signaling functions. Mouse submandibular salivary gland branching morphogenesis is enhanced by membrane-type 2 matrix metalloprotease (MT2-MMP) which releases bioactive collagen IV noncollagenous 1 (NC1) domains (Rebustini et al., 2009). MMP-9 also cleaves collagen IV, releasing a collagen IV fragment that regulates angiogenesis (Hamano et al., 2003). MMP-2 cleaves LM-111 (laminin-1), exposing a laminin fragment that mediates the epithelial-to-mesenchymal transition (EMT) in embryonic stem cells (Horejs et al., 2014).
Moreover, intact laminin and collagen IV can directly activate signaling pathways. Collagen IV modulates avian lung development through regulation of lung epithelial differentiation, myofibroblast proliferation, differentiation, and migration (Loscertales et al., 2016). By interacting with dystroglycan and integrin family receptors, laminin regulates cell survival, migration, differentiation, and proliferation (Bonnans et al., 2014). Laminin-integrin interactions create dynamic cell-ECM links that involve signaling pathway induction and intracellular cytoskeleton organization (Berrier and Yamada, 2007; DeSimone and Mecham, 2013; Hynes, 1999; Kim et al., 2011; Yurchenco, 2011). The signaling activated by laminin-cell membrane receptor binding can control gene transcription and chromatin remodeling of gene promoters (Domogatskaya et al., 2012). In mammary epithelia, functional differentiation leading to milk production is determined by growth hormones and interaction with neighboring cells as well as the basement membrane. Induction of β-casein expression in single cells depends on the types of substrate in which they are embedded (Streuli et al., 1991). Single cells in a collagen I substrate are unable to express β-casein unless they are permitted cell-cell contact. In contrast, single cells in a laminin-rich basement membrane substrate are capable of expressing β-casein. The binding of LM-111 to dystroglycan leads to growth hormone-induced activation of STAT5 and increases in β-casein and insulin growth factor-1 (IGF-1) expression (Leonoudakis et al., 2010). These findings show that the basement membrane-cell surface receptor interaction modulates growth hormone induction and functional differentiation of mammary epithelia.
Basement membrane receptors are spatially and temporally regulated throughout development. For example, isoforms of syndecan, a non-integrin ECM receptor, switch their binding specificity to growth factors. Through its heparan- and chondroitin-sulfate glycosaminoglycan side chains, syndecan binds to a variety of growth factors that include FGF, VEGF, epidermal growth factor, hepatocyte growth factor, and platelet-derived growth factor (PDGF), (Carey, 1997). In the embryonic mouse neuroepithelium, syndecan-1 switches its binding ligand from bFGF to aFGF in a stage-dependent manner (Nurcombe et al., 1993).
Basement membrane-mediated signaling can modulate tissue morphogenesis by sensitization of cells to growth factors. During Drosophila renal tubule development, targeted deposition of the basement membrane that ensheaths renal tubules is achieved by hemocytes (Bunt et al., 2010). Deposited collagen IV enhances sensitivity of the tubule cells to localized Dpp guidance signals, thereby guiding directional morphogenesis of renal tubules. Collagen IV mediates activation of this Dpp signaling pathway in a subset of tubule cells, which enables stereotypic positioning and outgrowth of Drosophila renal tubules (Bunt et al., 2010). More recently, migrating macrophages have been identified as major contributors to de novo basement membrane formation in the Drosophila embryo (Matsubayashi et al., 2017). The basement membrane of the Drosophila embryo exhibits a hierarchical pattern of deposition of laminin, followed by collagen IV, and finally perlecan. Absence of laminin severely impairs subsequent collagen IV and perlecan incorporation (Matsubayashi et al., 2017), which suggests that environmental cues from initial laminin deposition are likely necessary for matrix formation.
The basement membrane also contributes to neuronal extensions in Drosophila by enhancing the response of extending axons to environmental signals that guide their growth (Isabella and Horne-Badovinac, 2015). For example, perturbation of laminin results in axon guidance defects (Garcia-Alonso et al., 1996). Syndecan, as a coreceptor for Slit (Johnson et al., 2004), also provides guidance cues for developing axons. Slit is an extracellular protein that serves as a repulsive signal for axons that express the Robo receptor (Brose et al., 1999). Syndecan binding to Slit stabilizes the Slit-Robo interaction (Hussain et al., 2006). Taken together, basement membranes have diverse signaling functions through interactions with cell receptors, release of cryptic fragments upon proteolytic breakdown, and mechanical processes combined with molecular signaling.
Composition
Present in nearly all human tissues, basement membranes are spatially and temporally customized, with associated proteins that display time- and tissue-specific expression patterns. Databases of ECM molecules are provided by the Matrixome Project (Manabe et al., 2008), the Matrisome Project (Hynes and Naba, 2012), and the Human Protein Atlas (Uhlen et al., 2015). The Matrixome Project offers a high-resolution atlas that focuses on spatial and temporal localization of mouse basement membrane proteins. Based on immunohistochemical analyses of proteins following large-scale in silico and in vitro screening, this project has compiled a comprehensive atlas of more than 40 basement membrane proteins at http://www.matrixome.com/bm (Manabe et al., 2008). The Matrisome Project lists databases that contain the ECM compositions of 14 types of tissue and tumor at http://matrisomeproject.mit.edu (Hynes and Naba, 2012; Naba et al., 2016). The Human Protein Atlas provides a map of the human tissue proteome based on transcriptomics combined with immunohistochemistry (Uhlen et al., 2015).
Basement membranes emerged in the evolution of animal multicellularity. Basement membrane proteins are highly conserved among mammals (Ozbek et al., 2010). Particularly ancient molecules are the core basement membrane proteins collagen IV, laminin, nidogen, and perlecan (Yoshizaki and Yamada, 2013). These ECM proteins are commonly comprised of repeated domains and evolved by exon shuffling (Engel, 1996; Patthy, 1999). The small number of core basement membrane molecules is disproportionate to the diversity of their functions. This complexity of functional diversity stems from the addition of other proteins, post-translational modifications, protein-protein interactions, RNA splicing variants, alternative promoters, and spatiotemporal expression of, or functional changes in, basement membrane components and membrane receptors (Jayadev and Sherwood, 2017; Yoshizaki and Yamada, 2013). The combination of these mechanisms generates biochemically and biophysically unique basement membrane structures. This allows specialized biological functions tailored to individual tissues and organs.
Collagen IV
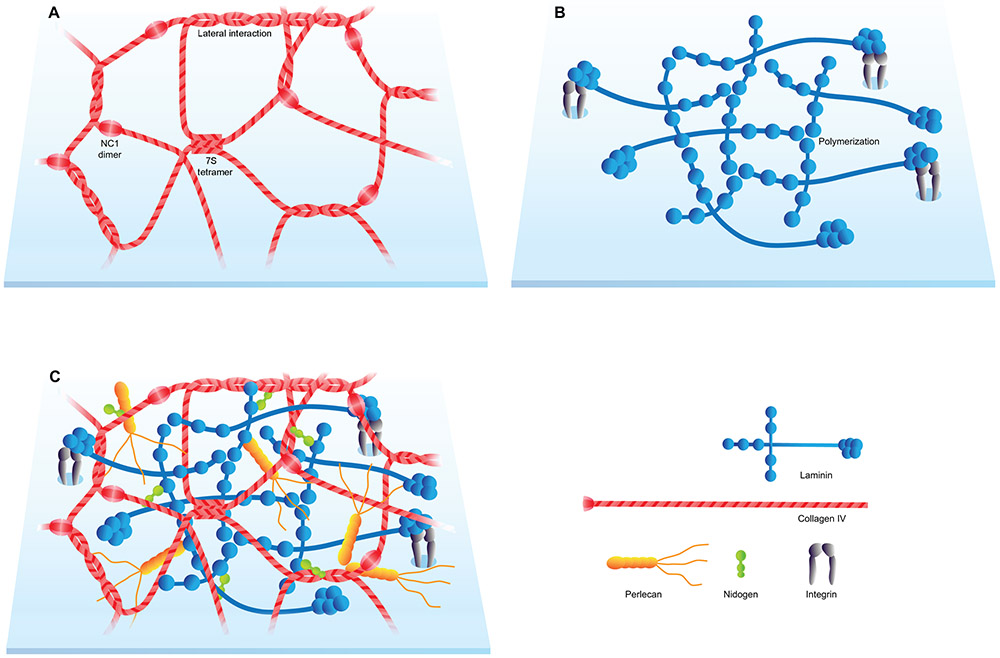
Collagen IV builds a network of triple-helical molecules, which most commonly consists of one α2 and two α1 subunits (Yurchenco, 2011). This network binds integrins, provides surrounding tissues with tensile strength, and tethers laminins, proteoglycans, and growth factors (Fidler et al., 2017). Collagen IV and laminin generate two independent but interconnected polymeric networks that serve as the foundation for basement membranes (Figure 2C). The collagen IV network is assembled in response to an extracellular Cl− ion signal, which triggers a conformational switch in NC1 domains (Cummings et al., 2016). The network assembly process of collagen IV trimers involves three modes of interactions: NC1 dimerization, N-terminal (7S) tetramerization, and lateral associations (Figure 2A) (Timpl et al., 1981; Yurchenco and Ruben, 1987). Found primarily in basement membranes, collagen IV has unique characteristics that distinguish it from other types of collagen. Due to the absence of a glycine at every third residue, collagen IV is unable to form a tight collagen helical structure (Abreu-Velez and Howard, 2012). Furthermore, its NC1 domain remains attached at the C-terminus. This restricts NC1 domain interactions, permitting only head-to-head fiber connections with the flat NC1 surfaces facing each other. As a result, collagen IV networks are structurally flatter and more pliable (Abreu-Velez and Howard, 2012). Mutations in collagen IV α3, α4, and α5 give rise to the kidney disease Alport’s syndrome and, in some cases, hemorrhagic stroke (Hudson et al., 2003).
Figure 2. Basic structure of the BM.
(A) Collagen IV network. (B) Laminin network. (C) Schematic representation of the BM with its major components.
Laminin
Laminins are a family of large heterotrimeric multidomain proteins that consist of three chains, α, β, and γ (Aumailley et al., 2005; Miner and Yurchenco, 2004). At least 16 different isoforms are present in vertebrates (Yurchenco, 2011). The first ECM proteins identified in the mammalian preimplantation embryo are the laminins subunits α1, α5, β1, and γ1 (Miner et al., 2004). While laminin β1 and γ1 subunits are nearly ubiquitous and shared by many laminin isoforms, laminin α subunits have more specific spatiotemporal distribution patterns (Pickering et al., 2017). Laminins generate honeycomb-like networks connected in a spot-welding-like manner by adhesive aggregates containing perlecan (Behrens et al., 2012).
Laminins assume a cross or Y shape. The short arms are comprised of α, β, or γ chains, with LN domains located at the end of each arm. LN domains provide a site for laminin polymerization (Figure 2B). LG domains at the end of the long arm (coiled-coil) bind to a cell surface through sulfated glycolipids, integrins, and α-dystroglycan (Yurchenco, 2011). The LG domain-cell surface receptor binding promotes laminin polymerization and therefore enhances network assembly. The LG1-3 cluster provides the principal binding sites for a variety of integrins such as α6β1, α6β4, α7β1, and α3β1 (Nishiuchi et al., 2006a). Laminin-integrin interactions create dynamic cell-ECM links that involve signaling pathway induction and intracellular cytoskeleton organization (Berrier and Yamada, 2007; DeSimone and Mecham, 2013; Hynes, 1999; Kim et al., 2011; Yurchenco, 2011).
Laminins have various cell type-specific expression patterns, and laminin mutations can cause embryonic lethality or severe diseases affecting multiple organs (Domogatskaya et al., 2012). LM-211 (laminin-2) is prominent in pancreas, muscle, and the nervous system, whereas LM-311 (laminin-6) and −321 (laminin-7) are found in lung and skin (Mecham, 2011). LM-332 (laminin-5) is the most abundant laminin expressed by keratinocytes (Botta et al., 2012). LM-332 binds tightly to the noncollagenous domain of collagen VII of anchoring fibrils (Rousselle et al., 1997) and the laminin-specific integrin α6β4 (Nishiuchi et al., 2006b). Integrin α6β4 binds to intracellular plectin, another important component of hemidesmosomes (Litjens et al., 2006). Disruption of this cell-matrix adhesion complex comprised of laminin, integrin, and anchoring fibrils causes epidermolysis bullosa (Pulkkinen and Uitto, 1999). LM-221 (laminin-4), −421 (laminin-9), and −521 (laminin-11) are found in the neuromuscular junction, where lack of laminin α4 leads to failure of innervation due to mismatched nerve-muscle connections of the active zones at the axon terminus and the junctional folds of the muscle endplate (Patton et al., 2001). LM-511 (laminin-10) is widely expressed in skin, intestine, lung, kidney, and salivary gland (Mecham, 2011). Laminin α5 regulates transformation of the neural tube into the emerging central nervous system during early embryonic mouse neurulation (Miner et al., 1998).
Nidogen
Nidogens are monomeric glycoproteins that contribute to a linkage of polymeric networks with stable affinity to the laminin and collagen IV (Figure 2C) (Fox et al., 1991; Mayer et al., 1993; Yurchenco, 2011). Expressed mainly by mesenchymal cells, nidogen 1 and nidogen 2 are ubiquitous basement membrane proteins (Nischt et al., 2007). Both nidogen 1 and nidogen 2 promote basement membrane stability by contributing, at least in part, to the connection of laminin and collagen IV networks (Has and Nyström, 2015). Nidogens consist of three globular domains termed G1-G3 (4-5 nm in diameter). The domains are interconnected by a flexible segment between G1 and G2 and a stiff rod between G2 and G3. G2 (collagen and proteoglycan-binding domain) and G3 (laminin-binding domain) are separated by 15 nm, which permits various orientations and interactions with other basement membrane proteins (Fox et al., 1991).
Nidogen-deficient mice generally manifest mild phenotypes in skin without effects on formation of the basement membrane. In contrast, a loss of endothelial basement membrane integrity results from substantial reductions in collagen IV, laminin-411 (laminin-8), and perlecan (Mokkapati et al., 2008). Mice lacking both nidogen-1 and −2 can have cardiac and pulmonary abnormalities associated with basement membrane assembly defects (Bader et al., 2005) and in some cases, altered FGF distribution, syndactyly (digit fusion), and failure to form the limb bud ectodermal basement membrane (Bose et al., 2006). Strikingly, a severe phenotype results from specific ablation of the nidogen-binding site in laminin γ1 (Willem et al., 2002). These mice exhibit renal agenesis, impaired lung development, and neonatal lethality, suggesting the importance of laminin-nidogen interaction in development and the potential role of the nidogen-binding site of laminin γ1 in other developmental mechanisms. Laminin γ1-nidogen interaction is also known to regulate pial development (Halfter et al., 2002). In addition to the nidogens, other binding molecules such as perlecan likely have compensatory and redundant functions that are independent of each other, since the basement membrane has many essential life functions.
Heparan Sulfate Proteoglycans
Heparan sulfate proteoglycans are glycoproteins with one or more heparan sulfate chains, which are a type of glycosaminoglycan (GAG) chain (Domogatskaya et al., 2012). The heparan sulfate proteoglycans present in the basement membrane are perlecan, agrin, and collagen XVIII. These glycated proteins define basement membrane structure in collaboration with other matrix proteins. Due to the hygroscopic properties of glycosaminoglycans, perlecan and agrin increase the volume of matrix (Domogatskaya et al., 2012). Collagen XVIII is a hybrid collagen-proteoglycan expressed at the initiation of lung and kidney organogenesis (Patel et al., 2017). Inhibition of collagen XVIII disrupts lung and kidney branching morphogenesis (Karihaloo et al., 2001; Lin et al., 2001).
Heparan sulfate binds to many growth factors including FGF with high affinity. Association and dissociation of heparan sulfate-ligand interactions can create a gradient of FGF ligand through mass action involving adjacent binding sites. In addition, heparan sulfate can approximate two adjacent proteins, facilitating growth factor-receptor tyrosine kinase interaction, such as for FGF and FGFR (Sarrazin et al., 2011). FGF10-FGFR2b signaling is essential for early embryonic development of multiple organs, including the lung, trachea (Min et al., 1998), mammary gland (Mailleux et al., 2002), lacrimal gland (Entesarian et al., 2005), and salivary gland (De Moerlooze et al., 2000; Ohuchi et al., 2000). Heparan sulfate alters the level of FGF10-FGFR2b affinity: when present in a ternary complex with heparan sulfate, the affinity between the receptor and the ligand increases (Kan et al., 1999). Proteolysis by heparanase (an endoglucuronidase that cleaves heparan sulfate) releases the growth factor, which in turn promotes neural differentiation of murine embryonic stem cells (Xiong et al., 2017) and enhances branching morphogenesis of the mouse salivary gland (Patel et al., 2007). Notably, heparanase promotes not only development but also tumor progression in the mammary gland (Boyango et al., 2017). This illustrates an example of the same mechanism mediating both development and disease.
Perlecan
Perlecan is a secreted heparan sulfate proteoglycan that is a potential linker of laminin and collagen IV networks (Figure 2C). Perlecan is a large (> 200 nm), evolutionarily old (> 550 million years) ECM protein (Farach-Carson et al., 2014). Both epidermal keratinocytes and dermal fibroblasts express perlecan (Sher et al., 2006). Perlecan binds multiple growth factors and basement membrane components including laminin and collagen IV (Battaglia et al., 1992). By sequestering growth factors such as FGF7, perlecan promotes keratinocyte survival and differentiation (Sher et al., 2006). Furthermore, perlecan interacts with integrin α2β1, α-dystroglycan, and fibrillin-1; fibrillin-1 generates microfibrils that anchor the epidermal basement membrane to the papillary matrix (Herzog et al., 2004; Woodall et al., 2008). Perlecan and agrin establish collateral associations between basement membrane proteins and cell surface receptors by linking nidogen and laminin to integrins, dystroglycan, and sulfated glycolipids. The chain of bound proteins involving dystroglycan is termed a dystrophin-glycoprotein complex that extends from basement membrane proteins to F-actin through dystroglycan and dystrophin/utrophin (Yurchenco, 2011).
Perlecan is indispensable for formation of the epidermal basement membrane (Costell et al., 1999). Perlecan is especially important in corneal epithelial development (Inomata et al., 2012) and maintenance of basement membrane integrity in regions with high mechanical stress, including the contracting myocardium and expanding brain vesicles (Costell et al., 1999). Moreover, perlecan has a distinct role in tissue development independent of other core basement membrane proteins. Though the basement membrane is absent in cartilage, perlecan is expressed in cartilage and helps to mediate cartilage development (Yoshizaki and Yamada, 2013).
FRAS/FREM
Located in the sublamina densa, FRAS1, FREM1, and FREM2 form a ternary complex known as the Fraser complex (Petrou et al., 2008). This protein complex controls stabilization of cell-matrix adhesions during embryonic development. Deficiency in FRAS1, FREM1, or FREM2 is the etiological origin of Fraser syndrome, which manifests as hidden eyes (cryptophthalmos), syndactyly, embryonic skin blistering, and renal agenesis or dysplasia with water transport deficits. Loss of Fras1 in mice leads to arrest of ureteric bud growth owing to severe deficiency of glial cell line-derived neutrophic factor (GDNF)—the inducer of the budding of ureteric buds from the Wolffian duct (Pitera et al., 2008).
The importance of this protein complex in embryonic development is linked to the spatially and temporally restricted expression of the basement membrane protein nephronectin, which is a ligand for integrin α8β1 (Sato et al., 2009). The Fraser complex anchors nephronectin to the ureteric bud basement membrane and therefore stabilizes its binding to integrin α8β1 (Kiyozumi et al., 2012). Nephronectin-null mice frequently display renal agenesis or hypoplasia (Linton et al., 2007) whereas α8-integrin mutations in humans can cause renal agenesis associated with compromised epithelial-mesenchymal interaction (Humbert et al., 2014). These studies reveal bound basement membrane and transmembrane receptor proteins that act in concert to direct spatiotemporal orchestration of mammalian organogenesis.
Spatial and Temporal Variations in Basement Membrane Thickness and Composition
Conventional transmission electron microscopy measurements made with desiccated tissues estimate basement membrane thickness to be less than 100 nm (Bluemink et al., 1976; Cutler, 1977; Lehtonen, 1975). These measurements are most likely gross underestimations. More realistic measurements by atomic force microscopy on hydrated chick, mouse, and human basement membranes identify their thickness to be at least twice as thick (Jayadev and Sherwood, 2017). The basement membrane requires sufficient thickness to allow network formation by laminin molecules (80 nm in length) and collagen IV molecules (400 nm in length). Variations in basement membrane thickness most likely result from differences in tissue hydration levels and inherent tissue and species differences (Candiello et al., 2010; Halfter et al., 2015).
Basement membrane density and thickness vary across time and space. Fibril-like aggregation of collagen IV in the embryonic mouse salivary gland becomes increasingly denser and thicker over time [see (Harunaga et al., 2014)]. Even though electron microscopy measurements on desiccated tissues likely underestimate the thickness of basement membranes, findings from classical studies consistently demonstrate region-specific variations. In developing lung (Bluemink et al., 1976), kidney (Lehtonen, 1975), mammary gland (Paine and Lewis, 2017), and submandibular salivary gland (Bernfield and Banerjee, 1982; Cutler, 1977), the basement membrane adjacent to regions of active growth and tissue expansion is thinner and discontinuous compared to the relatively static regions away from the bud tip.
Interestingly, the thinner basement membrane at the actively growing regions of the salivary gland exhibits micro-perforations through which epithelial bleb-like protrusions commonly extend (Harunaga et al., 2014). This observation is consistent with classical electron microscopy studies of the embryonic lung (Bluemink et al., 1976), kidney (Lehtonen, 1975), tooth (Burgess and Katchburian, 1982; Slavkin and Bringas, 1976), and salivary gland (Bernfield and Banerjee, 1982). These studies capture direct epithelial-mesenchymal contact through perforations, with cytoplasmic protrusions extending from epithelial or mesenchymal cells. As discussed below, the spatial specificity of this observation of cell interactions associated with active growth supports the idea that direct cellular contact may be a mode of epithelial-mesenchymal interaction.
This regional difference in basement membrane thickness coincides with the level of ECM turnover in the embryonic mouse submandibular gland. The basement membrane at active branching regions is subject to extensive remodeling. Glycosaminoglycans are degraded much more rapidly at the distal end bud region than in the interlobular clefts (Bernfield and Banerjee, 1982). Local alterations in the mechanical compliance of the basement membrane may also account for the regional differences in its morphological appearance. Tissue morphogenesis and patterning are controlled by both molecular signals and alterations in the mechanical compliance of the basement membrane as they respond to cellular forces (Mammoto and Ingber, 2010). Changes in cytoskeletal tension mediated by Rho-signaling through Rho-associated kinase have a profound impact on embryonic lung development. Inhibiting cellular tension results in disruption of the basement membrane, blood vessels, and bud formation, whereas increasing cell tension enhances lung branching and capillary development (Moore et al., 2005). Thus, increased mechanical compliance and distensibility associated with enhanced degradation and the outward expansive force from the growing organ may underlie thinning of the basement membrane at regions of active growth.
Mammary Gland Basement Membrane Composition and Density
Basement membrane composition and density vary within the developing mammary gland. During puberty, the terminal end bud emerges at the tip of the mammary duct (Sternlicht, 2006). The terminal end bud is the regulatory control point for proliferation, branching, angiogenesis, and pubertal branching morphogenesis (Huebner et al., 2014; Paine and Lewis, 2017). The tip of the terminal end bud represents the active site of branching morphogenesis, whereas the neck of the terminal end bud is the site of ductal morphogenesis and tissue stabilization (Silberstein and Daniel, 1982). Cells in the terminal end bud show spatially limited and reversible reductions in intercellular adhesion and polarity (Ewald et al., 2012), which correlate with a high degree of morphological plasticity in the region. Basement membrane accumulation towards the neck of terminal end buds contributes to sculpting of the elongating ducts by constriction (Jayadev and Sherwood, 2017). The basement membrane at the bulbous tip of the terminal end bud is very thin (104 nm), while it is thicker in the neck region of the bud (1.4 μm) (Paine and Lewis, 2017). The thin basement membrane at the tip of the terminal end buds consists primarily of laminin, collagen IV, and hyaluronic acid. In contrast, the basement membrane at the neck of the terminal end bud is thicker, forming a more defined meshwork of LM-111 and LM-332, collagen IV, and heparan sulfate proteoglycans (Fata et al., 2004; Keely et al., 1995). The variations in basement membrane composition and thickness of the terminal end bud likely facilitate epithelial-mesenchymal interaction and morphogenesis.
Laminin Expression in Development and Sjogren’s Syndrome
Present in skin, kidney, lung, thymus, brain, gastrointestinal tract, and lung, LM-332 (laminin-5) displays uneven spatial and temporal distribution throughout the epithelial basement membrane. LM-332 is enriched within hemidesmosomes, but its expression decreases during hair morphogenesis (Nanba et al., 2000). Time-dependent expression of different types of laminin is also observed in the subendothelial basement membrane. The subendothelial basement membrane provides a substrate for β-cells in pancreatic islets, podocytes in renal glomeruli, and alveolar epithelial cells in the lung (Hallmann et al., 2005). Two main laminin isoforms, LM-411 and LM-511 (laminin-10), are commonly present in the subendothelial basement membrane. When the basement membrane undergoes active remodeling during embryonic angiogenesis, LM-411 is the predominant laminin expressed in the vascular endothelial basement membrane. LM-511 emerges postnatally when the subendothelial basement membrane is stabilized (Hallmann et al., 2005). These time- and space-specific expression patterns of laminin suggest distinct functions for each isoform during development.
Laminin expression in the embryonic mouse submandibular salivary gland varies temporally and spatially. The salivary gland at embryonic day 13, which marks the onset of active branching, shows expression of laminin α1 and α5 surrounding the distal end bud and α1, α3, and α5 chains along the duct (Kadoya and Yamashina, 2005). This laminin distribution changes later in embryonic development, when α2 and α4 emerge adjacent to the end bud, while α3 increases around myoepithelial cells that surround acini, the saliva-secreting units.
The basement membrane of labial salivary glands in individuals with Sjogren’s syndrome shows structural disorganization and altered expression of collagen IV and laminin (Molina et al., 2006). The epithelial acinar basement membrane of Sjogren’s syndrome patients can display reduced laminins (LM-111 and LM-211), accompanied by decreased integrin (α1β1 and α2β1) receptors that bind to laminin (Laine et al., 2008). Basement membrane disorganization may be associated with loss of structural integrity and atrophy of salivary acini, ductal cell hyperplasia, and impaired secretory function. Lack of basement membrane integrity makes the salivary gland more susceptible to lymphocytic invasion in Sjogren’s syndrome patients (Hayashi, 2011; Molina et al., 2006). Thus, basement membrane structure and composition are greatly altered in disease, suggesting that the basement membrane plays an important role beyond development.
Glomerular and Retinal Capillary Basement Membrane Thickness
Two basement membranes (retinal pigment epithelial and choroid) in Bruch’s membrane maintain integrity of the retinal pigment epithelium, which separates the retina from the choriocapillaris (Figure 1B) (Strauss, 2005). Retinal pigment epithelium supports photoreceptor health, and disruption of its integrity is often a disease manifestation. Age-dependent thickening of the basement membrane is associated with pathology in the retina, but not in the kidney. In the kidney, thickening of the glomerular basement membrane during aging does not affect hydraulic permeability of the glomerular capillary wall (Neumann et al., 2004). In contrast, age-related thickening of retinal capillary basement membranes is likely linked to senile retinopathies (Nagata et al., 1986). Retinal vascular basement membrane thickening is associated with human microaneurysms during aging—a hallmark of retinal vascular disease. Small microaneurysms show basement membrane thickening and up-regulation of collagen IV, laminin, fibronectin, nidogen, and perlecan. On the other hand, large microaneurysms display loss of basement membrane integrity and increases in MMP-9 and plasminogen activator inhibitor-1 (Lopez-Luppo et al., 2017).
Other conditions linked to ocular basement membrane thickening are diabetes mellitus and drusen accumulation. Diabetic retinopathy is associated with basement membrane thickening, stiffening, and compositional changes (To et al., 2013). With aging, progressive accumulation of drusen comprised of cellular debris can overwhelm removal and result in thickened basement membrane (Salvi et al., 2006). Basal laminar drusen primarily accumulates in the macular area. With progressive accumulation, drusen is associated with the development of age-related macular degeneration.
Ocular basement membranes have sidedness. The inner epithelial face of the human adult ocular basement membrane is two-fold stiffer than the outer stromal side (Halfter et al., 2013). Laminin is enriched on the stiffer inner epithelial-facing side, whereas collagen IV is enriched on the stromal side, with a distinct distribution of the C and N-terminal domains in the basement membrane. This unique sidedness of basement membrane structure may lead to spatial and temporal variations in levels of susceptibility to basement membrane thickening and degradation. Biological implications of basement membrane sidedness in development and aging remain to be identified.
Glomerular Basement Membrane Composition
Along with their adjacent organs, basement membranes undergo dramatic transformation throughout development, with altered composition and structure. For instance, laminin and collagen isoforms switch during progression from embryonic development to adult stages in the human glomerular basement membrane. The basement membrane during kidney development is formed by the stage-specific assembly of a common laminin γ1 and different α and β subunits (Skorecki et al., 2015). Components of the fetal glomerular basement membrane include collagen α1 and α2(IV) and laminin α1 and β1, which are gradually replaced by collagen α3-α5(IV) and laminin α5 and β2 subunits (Abrahamson et al., 2013; Harvey et al., 1998; Miner et al., 1997). This developmental switch in collagen and laminin isoforms is crucial for kidney development and maturation. Laminin α5-mutant mice show glomerular basement membrane breakdown and renal agenesis or defective glomerulogenesis because of a failed transition from laminin α1 to α5 (Miner and Li, 2000). A laminin γ1 loss-of-function mutation in the murine ureteric bud causes renal agenesis as a result of failure of basement membrane formation and compromised integrin-based and growth factor-based (FGF2, WNT11, and GDNF/RET) signaling pathways (Yang et al., 2011). Laminin β2 mutation is the etiological cause of Pierson syndrome, which is characterized by neurological, ocular, and renal deficits (Miner et al., 2006).
Dental Basement Membrane Composition
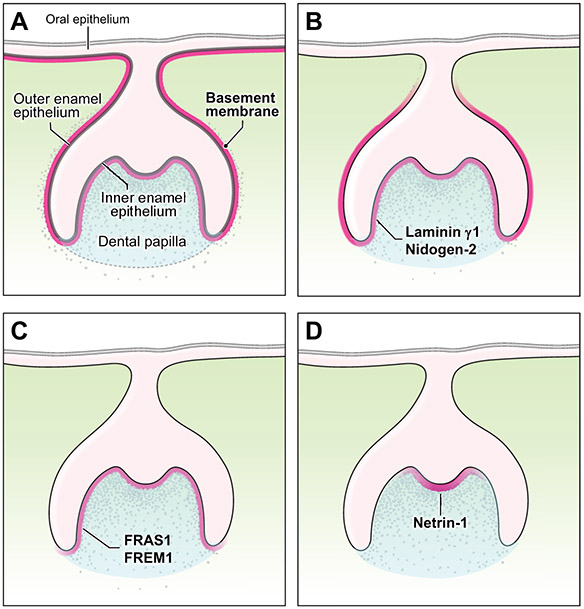
During tooth morphogenesis, the dental basement membrane undergoes dynamic structural and chemical transformation involving degradation and calcification. Epithelial and mesenchymal cells in the tooth bud differentiate into their respective secretory cells (ameloblasts and odontoblasts) along the basement membrane (He et al., 2010). Many basement membrane proteins show site-specific expression in the embryonic day 16.5 mouse tooth bud. Laminin subunits α5 and β1, collagens IV and XVIII, nidogen-1, perlecan, agrin, and other proteins are widely expressed throughout the basement membranes of the tooth bud and oral epithelium (Figure 3A) (Manabe et al., 2008). Laminin γ1 and nidogen-2 are expressed specifically around the entire tooth bud (Figure 3B). FRAS1 and FREM1 are localized to the basement membrane subjacent to the inner enamel epithelium (Figure 3C). Other proteins such as netrin-1 show a gradient expression pattern along the inner enamel epithelium; the expression level of netrin-1 is highest adjacent to the primary enamel knot, from which it gradually declines laterally (Figure 3D) (Manabe et al., 2008).
Figure 3. The BM of embryonic mouse tooth bud.
(A) The basement membrane underlies the oral epithelium and surrounds inner and outer enamel epithelia of the enamel organ. The dental papilla lies beneath the inner enamel epithelium and gives rise to the dentin and pulp. (B) Laminin γ1 and nidogen-2 are expressed around the tooth bud. (C) FRAS1 and FREM1 show a spatially specific expression pattern along the inner enamel epithelium. (D) A gradient expression pattern of netrin-1 is found subjacent to the primary enamel knot.
A similar gradient pattern is found in odontoblast differentiation. Odontoblast differentiation follows a spatial gradient that begins adjacent to the enamel knot and proceeds in a direction away from the enamel knot (Bloch-Zupan et al., 2012). Gradients of SHH and BMP are thought to regulate cell proliferation and differentiation (Li et al., 2015; Seppala et al., 2017). By binding to heparan sulfate proteoglycans, netrin-1 may modulate morphogen gradients (Manabe et al., 2008).
As ameloblasts and odontoblasts secrete enamel and dentin matrices, respectively, the basement membrane is degraded and replaced with interdigitations of calcified enamel and dentin (Sahlberg et al., 1992; Thesleff et al., 1981). This interface forms the dentin-enamel junction, which joins two unique matrices that have different biomechanical properties (Chun et al., 2014). Composed of mineralized connective tissue, dentin is similar to bone in its composition and nanostructure. On the other hand, enamel primarily consists of hydroxyapatite and represents the hardest tissue in the body (Gibson et al., 2001). Accordingly, the dentin-enamel junction serves as a crack-arrest barrier for the brittle enamel, preventing the two distinct matrices from dislodging (Imbeni et al., 2005). However, how the dentin-enamel junction, which is devoid of a basement membrane, establishes the effective adhesion needed to withstand enormous occlusal loads is not completely understood. The extent of basement membrane degradation in teeth—whether it is complete or partial—has been questioned because presence of collagen IV and VII at the dentin-enamel junction has been reported in adult teeth (McGuire et al., 2014a; McGuire et al., 2014b). The dentin-enamel junction becomes a vulnerable site for enamel delamination in irradiated teeth of head and neck cancer patients, where radiation likely affects biochemical and structural integrity at the dentin-enamel junction. Further research is required to identify detailed mechanisms maintaining the integrity of the dentin-enamel junction in health and post-irradiation.
Basement Membranes in Tissues Surrounding Teeth
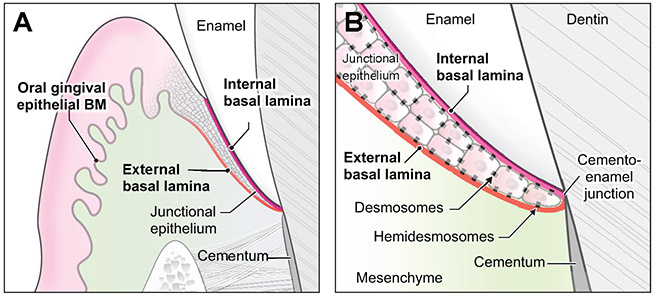
A tooth is surrounded by supporting dento-gingival tissues that consist of junctional epithelium, basement membranes, and gingival fibers (Newman et al., 2011). Oral gingival and junctional epithelia surrounding the tooth are associated with three distinct basal laminae (Figure 1D; 4A). The oral gingival epithelium basement membrane consists of typical basement membrane components, including collagen IV, nidogen, LM-332 (laminin-5), LM-311 (laminin-6), LM-321 (laminin-7), LM-511, and LM-521 (laminin-11) (Oksonen et al., 2001). Located adjacent to the tooth surface, the junctional epithelium is sandwiched between two unique basement membranes: the internal and external basal laminae. The internal basal lamina mediates attachment to the tooth surface, whereas the external basal lamina attaches to the gingival connective tissue (Figure 4B) (Newman et al., 2011). Compared to the oral gingival epithelium, the junctional epithelium contains relatively few desmosomes and less E-cadherin, with wide intercellular spaces permissive for leukocyte migration (Hatakeyama et al., 2006). The internal and external basal laminae undergo continuous rapid remodeling because the adjacent junctional epithelium displays high turnover as a means of defense against bacterial infection (Dabija-Wolter et al., 2013). Forming an epithelial attachment to the tooth surface through basal laminae, the junctional epithelium serves as the first line of defense against bacterial plaque; its disruption and detachment below the cemento-enamel junction signify initiation of periodontitis (Kinane, 2001; Marks et al., 1994). The composition of the external basal lamina—collagen IV, LM-332, LM-311, and LM511, and nidogen-1—is similar to that of the oral gingival epithelium (Oksonen et al., 2001), whereas the internal basal lamina is primarily comprised of LM-332 and collagen VIII (Larjava et al., 2011). These differences in composition suggest that the two types of basal lamina may derive in part from different cell populations and may have evolved along different pathways to function in their unique local microenvironments.
Figure 4. Basic structure of dental supporting basal laminae.
(A) Schematic illustration of basal laminae around a tooth. (B) Desmosomes mediate intercellular adhesions. Hemidesmosomes mediate attachment of internal and external basal laminae to enamel and mesenchyme, respectively.
Basement Membrane Micro-Perforations
Although composed of a structurally dense sheet of interwoven networks, basement membranes in some tissues have micro-perforations. A combination of covalent and noncovalent forces coalesce the interconnected laminin and collagen IV meshwork into a molecular sieve with pores (Kalluri, 2003). Pores or micro-perforations in the basement membrane exist in a wide range of shapes and sizes (5 nm-8 μm in diameter) (Hironaka et al., 1993; Takeuchi and Gonda, 2004). The size and form appear to be spatially and temporally determined. Proper sizes of basement membrane pores or micro-perforations are crucial for regulation of permeability and intercellular interactions – and in certain tissues, permissibility for cell migration.
Alterations in basement membrane pore size can be a pathological manifestation. For instance, disruption/enlargement of the extremely tiny (~5-10 nm in diameter) (Hironaka et al., 1993) pores of the glomerular basement membrane is the underlying cause of proteinuria in the kidney disease Alport’s syndrome, which results from collagen IV mutations (Barker et al., 1990; Longo et al., 2002).
Basement membrane micro-perforations are normal physiological findings in bronchial airway (0.75-3.85 μm in diameter) (Howat et al., 2001) and in the intestinal villi and lymph nodules of ileal Peyer’s patches (~1-8 μm in diameter) (Takeuchi and Gonda, 2004). Basement membrane micro-perforations in these tissues facilitate lymphocyte migration, especially in the small intestine, where the abundance of micro-perforations corresponds to the degree of lymphocytic infiltration.
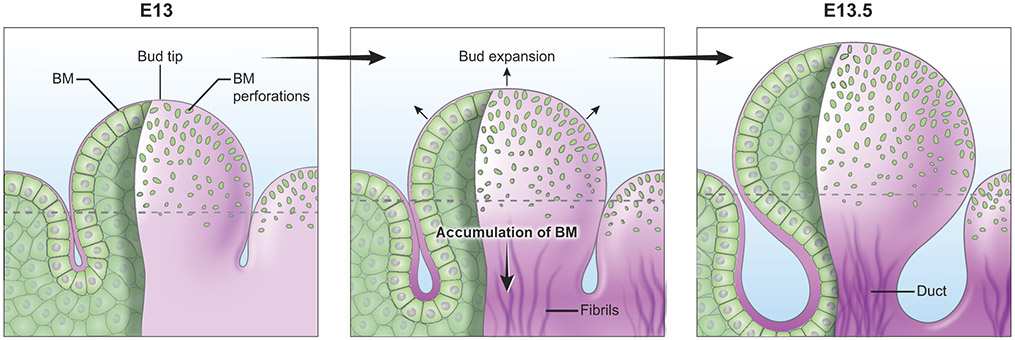
The transient presence of basement membrane micro-perforations in the embryonic mouse submandibular salivary gland correlates with the time and location of maximal epithelial expansion (Harunaga et al., 2014). Micro-perforations are most prominent at the beginning of the active branching phase, when cell proliferation and repetitive clefting and branching substantially increase the organ surface area (Figure 5). The tip of the end bud has both the largest size and density of micro-perforations. The largest micro-perforations (4 μm in diameter) are still smaller than the size of a cell. With 90% of the micro-perforations being even smaller (<2.5 μm in diameter), they are not permissive to cell transmigration. Both the size and density of micro-perforations dramatically decrease further away from the tip. Micro-perforations eventually disappear towards the equator of the bud, which is a site of basement membrane accumulation (Figure 5). Protease and myosin II-dependent global translocation of the basement membrane occurs rearward toward the duct, in a direction opposite to epithelial outgrowth. The long axis of the oblong micro-perforations is oriented parallel to the presumptive pulling force that drives global basement membrane translocation. Fibril-like basement membrane aggregates have varying thickness and direction, but their general orientation also runs parallel to the long axis of the end bud. Thus, the general orientation of fibril-like basement membranes also coincides with the axis of the basement membrane translocation (Harunaga et al., 2014), which suggests a possible link between pore shape, orientation of fibril-like deposition, and global basement membrane translocation.
Figure 5. BM micro-perforations in embryonic mouse submandibular salivary gland.
Micro-perforations are prominent at the tip of epithelial buds. These perforations gradually disappear towards the equator (dotted line). As the growing buds expand rapidly, the number of perforations peaks at embryonic day (E) 13.5. Basement membrane translocation away from the bud tip is accompanied by accumulation of fibril-like basement membrane proteins at the forming secondary duct.
Consistent with findings of classical electron microscopy studies on epithelial-mesenchymal inductive interactions (Bluemink et al., 1976; Cutler, 1977; Lehtonen, 1975), cellular protrusions from basal epithelial cells are observed exclusively at the tip of the end bud in the embryonic mouse lung and submandibular gland. The protrusions appear to expand the basement membrane micro-perforations as they protrude as far as 5 μm beyond the basement membrane (Harunaga et al. 2014). Individual micro-perforations undergo local dilation and contraction, a process dependent on actomyosin-mediated rapid physical distortion of subjacent cells and protease activity. Unlike C. elegans anchor (uterine) cell invasion, which involves physical widening of a gap by the cell and laminin accumulation at the edge of the gap (Hagedorn et al., 2013; Matus et al., 2014), the mouse submandibular salivary gland does not exhibit basement membrane displacement or accumulation around the periphery of the micro-perforation. Micro-perforations in the salivary gland basement membrane are not enlarged to a size sufficient to permit cell crossing. Rather, the salivary gland basement membrane as a whole becomes distensible in response to cellular forces; the amount of distensibility and the size of perforations are just sufficient for extension of cellular processes (Harunaga et al., 2014). Similarly, epithelial-mesenchymal cell contact is found during tooth morphogenesis. Differentiating ameloblasts lining the inner enamel epithelium extend cellular processes through the basement membrane, which penetrate into developing uncalcified dentin (Burgess and Katchburian, 1982). Cytoplasmic processes from odontoblasts in the tooth bud mesenchyme subsequently penetrate the basement membrane to contact preameloblasts (Slavkin and Bringas, 1976). A series of reciprocal inductions between the two apposed tissues is essential for sequential differentiation of the epithelial and mesenchymal cells into their respective matrix-secreting cells (Simmer et al., 2010). In addition to growth factors secreted by the oral epithelium (Tucker and Sharpe, 2004), this cellular contact may be another mechanism of cross-tissue interactions. The implications of direct cell-cell contact, the detailed mechanisms of micro-perforation formation in mammalian organs, the function(s) of micro-perforations, and the source of global basement membrane translocation are important research questions to be explored in future studies.
Basement Membrane Transmigration and Invasion
Basement Membrane Transmigration
Basement membranes in many tissues are a permissive substrate for certain migrating cells. In tissues where leukocyte trafficking is prevalent, such as in lymph nodes, the basement membrane has pre-existing gaps at specific locations that facilitate routine cell transmigration (Nourshargh and Alon, 2014). Neutrophils can migrate between the circulation and the subendothelial space in response to injury. Upon sterile thermal hepatic injury, neutrophils migrate from the vasculature into the subendothelial space and then later reverse-migrate back into the circulation (Wang et al., 2017). However, the basement membranes of many other tissues contain pores that are substantially smaller than the diameters of cells in physiological conditions. Consequently, enlargement of basement membrane pores is required for cells to traverse the barrier, by means of expansion, disassembling, stretching, proteolytic breakdown, or finding regions with reduced density and cross-linking (Rowe and Weiss, 2008; Wang et al., 2006). Nonetheless, many cells frequently transmigrate through basement membranes during embryonic development, such as during gastrulation (Nakaya et al., 2008), and disease, as in inflammatory disease (Sorokin, 2010) and metastatic cancer (Steeg, 2003).
Transmigration through mammalian basement membranes is often not solely a simple mechanical process. Rather, it may evoke molecular signaling that alters gene transcription in transmigrating cells. During leukocyte trafficking, leukocytes traverse beyond the endothelium and through the vascular basement membrane (Nourshargh and Alon, 2014). Their vascular wall breaching involves extensive alterations of both the migrating leukocytes and the matrix components through reciprocal signaling. Breaching of the vascular wall and basement membrane prime the transmigrating leukocytes for clearing pathogens and neoplastic cells with enhanced survival and increased effector functions (Nourshargh et al., 2010). Though the mechanistic role of the basement membrane in the priming process has yet to be elucidated, cell transmigration through the basement membrane is most likely a combined molecular and mechanical process that is tightly regulated during development and leukocyte transmigration, which becomes dysregulated in disease.
Basement Membrane Invasion during Tumor Progression
Normal developmental processes are often hijacked during tumor progression. Reduced epithelial cell polarity, EMT, and attenuation of intercellular and cell-matrix adhesion during tumor progression partially recapitulate developmental processes (Ewald et al., 2012; Tanos and Rodriguez-Boulan, 2008). While tumor progression and normal morphogenetic processes may share some regulatory mechanisms, what clearly differentiates tumor progression from developmental processes is transcriptional dysregulation. Because the basement membrane serves as a barrier against malignant cell dissemination, basement membrane breaching and dysregulation are hallmarks of many malignant tumors (Nguyen-Ngoc et al., 2012). Tumor progression involves alterations of ECM along with transcriptional and post-transcriptional acquisition of invasive cellular machinery by tumor cells (Ozanne et al., 2006).
Invadopodia are membrane-associated F-actin based structures that can help mediate cell invasion through surface expression of proteolytic enzymes that locally degrade ECM (Gimona et al., 2008). Cellular manifestation of invadopodia can be a part of normal morphogenetic processes such as axon guidance (Santiago-Medina et al., 2015) and can also be induced in non-malignant cells by a dense fibrillar collagen matrix (Artym et al., 2015). However, acquisition of invadopodia by tumor cells reflects a transcriptional and signaling switch. Malignant cells often insert invadopodia into the basement membrane for local degradation as they establish adhesion to the underlying stroma (Friedl and Wolf, 2003). Transcriptional and signaling switches confer on tumor cells the ability to extend invadopodia, perforate, and invade through the basement membrane. The invasive cells can destroy the basement membrane as they invade with local protease activity, consistent with a prominent characteristic of tumor histology: a reduction or complete absence of the basement membrane (Tosios et al., 1998). The presence of such basement membrane-invasive cells portends a poorer prognosis (Hanahan and Weinberg, 2000).
This invasive behavior of neoplastic cells may initially be attenuated or blocked by basement membranes. The barrier function of basement membranes derives from its tightly cross-linked matrix and niche formation that prevents carcinoma cell dissemination. Both normal and malignant mammary epithelia initially form cellular protrusions and disseminate in a collagen I substrate (Nguyen-Ngoc et al., 2012). However, a striking difference arises in subsequent behavior of these cells. Malignant mammary epithelium continues to disseminate without organizing structured basement membranes. On the other hand, normal mammary epithelium ceases to disseminate as it progressively organizes basement membranes. Deletion of P-cadherin, an adhesion gene that is downregulated in breast carcinoma cells, induces epithelial cell dissemination into collagen I but not in Matrigel. These findings demonstrate that the malignant epithelium preferentially disseminates in collagen I and suggest that breaks in basement membranes may induce invasion (Nguyen-Ngoc et al., 2012).
Normal non-malignant epithelial cells depend on pro-survival signaling from laminin that defines their niche (Chiarugi and Giannoni, 2008). Leaving their ECM niche can result in apoptosis due to lack of survival signaling. Thus, remaining in contact with their natural niche is crucial for basal epithelial cell survival. However, tumor cells that undergo malignant transformation can survive outside of their niche by actively altering their transcription pattern and basement membrane composition. Malignantly transformed cells can overexpress the basement membrane protein they depend on or alter their intrinsic integrin signaling to adjust to a foreign ECM (Chiarugi and Giannoni, 2008). For example, a mechanism commonly employed by carcinoma cells is overexpression of LM-332—a mechanism observed in esophageal, colon, and squamous cell carcinomas (Marinkovich, 2007). Survival signals from LM-332 can enable carcinoma cells to leave their natural niche and metastasize. Furthermore, angiogenesis is stimulated by laminin and perlecan, providing essential nutrients and oxygen for survival of disseminated cancer cells.
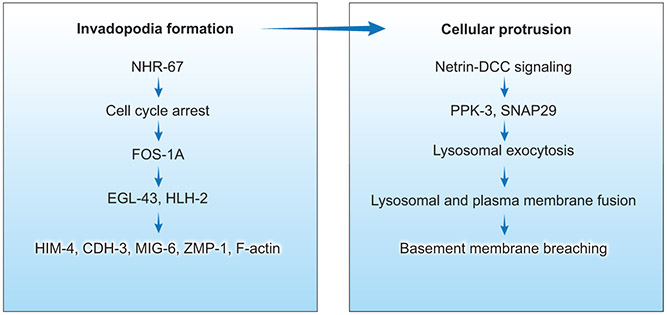
While invasive tumor cells may have enhanced survival, invasion and proliferation can be mutually incompatible cell states as demonstrated in mice (Gil-Henn et al., 2013) and C. elegans (Sherwood and Sternberg, 2003). The C. elegans model of basement membrane invasion utilizes a hermaphrodite developmental process in which anchor cells transmigrate through juxtaposed uterine and ventral epidermal basement membranes to initiate uterine-vulval connection (Sherwood and Sternberg, 2003). Transcription factor nhr-67/tlx-directed G1 cell-cycle arrest is required for invadopodia formation, pro-invasive gene transcription, and basement membrane invasion (Figure 6) (Matus et al., 2015). G1 cell-cycle arrest promotes the expression of FOS-1A (the vertebrate FOS proto-oncogene) in C. elegans. FOS-1A in turn triggers a transcriptional cascade; through transcription factors HLH-2 (E proteins) (Schindler and Sherwood, 2011) and EGL-43 (EVII) (Rimann and Hajnal, 2007), FOS-1A regulates pro-invasive genes, including CDH-3 (protocadherin), HIM-4 (hemicentin), ZMP-1 (MMP) (Sherwood et al., 2005), MIG-6 (papilin), and F-actin (Schindler and Sherwood, 2011). This requirement for cell-cycle arrest is partially echoed in a developmental process underlying mammalian tooth morphogenesis. Throughout incisor placode and bud morphogenesis, a subset of non-proliferative epithelial cells remains in the G1 phase (Ahtiainen et al., 2016). These cells localize to the emerging enamel knot that exhibits restricted expression of SHH, BMP, FGF, and cyclin-dependent kinase inhibitor P21. The neighboring epithelial cells surrounding this non-proliferating emerging enamel knot contribute to the growth of the tooth bud (Ahtiainen et al., 2016).
Figure 6. Factors involved in C. elegans BM transmigration.
The first stage is initiated by cell cycle arrest and activation of a transcriptional cascade that lead to invadopodia formation. The second stage involves netrin-DCC signaling that directs growth of a single large cellular protrusion, allowing the anchor cell to breach the juxtaposed uterine and vulval basement membranes.
C. elegans anchor cell transmigration takes place in two stages: first, the generation of initially tiny basement membrane perforations by small invadopodia, and second, subsequent local gap enlargement by proteolysis and physical force from a large cellular protrusion (Figure 6). Deciphering the mechanism of the second stage that clears a path for tissue entry is important because of its similarity to tumor cell transmigration and invasion during cancer metastasis. The first stage involving invadopodia formation is dependent on integrin, which promotes netrin receptor (DCC) localization to the anchor cell membrane in contact with the basement membrane (Hagedorn et al., 2009 ; Hagedorn et al., 2013). Netrin-DCC signaling directs the second stage via creation of a single large cellular protrusion through the exocyst and SNARE-mediated fusion of lysosomal membranes to fuel local plasma membrane expansion (Naegeli et al., 2017). This outward expansion of the invasive protrusion is anchored and promoted by F-actin and dystroglycan, which form a diffusion barrier at the neck of the protrusion. This transient large anchor cell protrusion and MMP protease activity expand the hole by a combination of local basement membrane displacement and degradation, enabling the cell to transmigrate (Naegeli et al., 2017).
Cell invasion in development and tumors can correlate with EMT (Micalizzi et al., 2010; Thiery et al., 2009). EMT is promoted by the transcription factors SNAIL, SLUG, TWIST, and ZEB1/2 (Hanahan and Weinberg, 2011). EMT in development is well-exemplified by neural crest cell migration. First induced in the ectodermal germ layer during gastrulation, neural crest cells undergo EMT involving SNAI2, ZEB2, and TWIST after neural tube closure (Simões-Costa and Bronner, 2015). Upon acquisition of migratory behavior and transformation into multipotent progenitors, neural crest cells leave the central nervous system and migrate through the basement membrane along highly stereotypical paths. Along with EMT, degradation of the basement membrane by proteases is crucial for neural crest cell migration (Theveneau and Mayor, 2011). However, transcription factors alone do not specify invasive cellular behavior, since their effects on protease activity and invadopodia formation are indirect (Eckert et al., 2011; Jorda et al., 2005). In addition, EMT and invadopodia are not essential for all instances of basement membrane breaching. During development and metastatic carcinoma, cells can collectively migrate or invade through the basement membrane without undergoing complete EMT (Chui, 2013). Thus, additional mechanisms may as well regulate carcinoma cell invasion.
During tumor progression, dysregulation of proteases such as MMPs facilitate basement membrane degradation and invasion (Wiseman and Werb, 2002). However, clinical trials of MMP inhibitors have been largely unsuccessful. Some of the potential reasons relate to issues with drug specificity, side effects that limited amounts that could be tolerated, study design of clinical trials with respect to the stage of cancer, as well as study design of many initial in vitro preclinical studies. The initial in vitro studies using cancer cell lines that overexpress certain MMP family members may not accurately reflect in vivo dynamics, where the primary source of proteases in carcinoma is apparently non-neoplastic stromal cells (Madsen and Bugge, 2015; Nielsen et al., 2001). The cellular source of proteases indeed matters because the activity of released enzymes significantly differs between cell types (Kessenbrock et al., 2010).
The basement membrane initially physically separates neoplastic epithelial cells from nonmalignant stromal cells. The likelihood that the stromal cells are the major source of proteases in carcinomas raises a fundamental question: how do neoplastic epithelial cells initiate basement membrane breaching? One possibility is activation of stromal cells by inflammatory signals such as interleukins-6 and −8 from neoplastic cells. Another potential mechanism is localized loss of the adhesion receptor dystroglycan. Loss of dystroglycan in basal epiblast cells during chick gastrulation allows epiblast cells undergoing EMT to cross through the basement membrane into the developing embryo (Nakaya et al., 2011). Additionally, mechanical forces may mediate basement membrane breaching. During post-implantation embryonic mouse development, localized constriction at the distal tip of the developing embryo by the surrounding maternal uterus appears to rupture the basement membrane. Basement membrane rupture in turn permits epiblast cell transmigration and initiation of establishment of the anterior-posterior axis (Hiramatsu et al., 2013). Similarly, tumor cell invasion may involve a combination of molecular signaling, degradation of basement membrane components, global mechanical hydrostatic force from the growing tumor, and local breaching force exerted by an invasive cellular protrusion. The mechanism of this initial basement membrane breaching remains a crucial gap in our understanding of tumor progression.
Perspectives and Future Directions
Advances in high-throughput omics and bioinformatics techniques have enabled the identification of novel ECM genes and proteins at unprecedented speed. However, the relevance of many of these newly discovered genes to development and disease remains to be unraveled. Accordingly, time-course proteomic and functional analyses of basement membrane proteins from embryonic development through adulthood and disease will help identify their roles in both development and disease.
In carcinoma progression, the basement membrane serves as an important barrier that spatially confines malignant epithelial cells prior to invasion. Identification of mechanisms underlying basement membrane invasion will provide valuable insight into cancer progression. One of the important topics that require further investigation is the source of specific ECM-degrading proteases and their contributions in initiation of basement membrane breaching. Also not clear is whether initial protease-induced breaks in the basement membrane are enlarged by tumor cell protrusions. For example, do tumor cells use cellular extensions that expand rapidly due to focal exocytosis of lysosomes to enlarge initially tiny basement membrane holes analogous to the mechanism used by C. elegans anchor cells? Another challenge will be the full elucidation of the biophysical, transcriptional, and post-translational changes affecting the basement membrane at different stages of cancer.
Identification of complex developmental and disease mechanisms will require a better understanding of not only molecular signaling but also biomechanical processes. Further investigations are needed to characterize the biophysical properties of the different basement membranes in relation to development and disease. If technically feasible, measurement of local stiffness versus tension within the basement membranes of embryonic tissues will provide valuable insight, which might be achieved by atomic force microscopy (Casuso et al., 2011) or fluorescence-resonance energy transfer measurements of tension sensors (Grashoff et al., 2010). The levels of stiffness or tension may well vary across developmental stages, throughout the body, and in different regions of the basement membrane within the same organ. Variations in tension, stiffness, and patterns of micro-perforation may alter the levels of cell adhesion, the extent of epithelial-mesenchymal interactions, patterns of cell migration, and/or the mode of tissue remodeling and morphogenesis. Live imaging of tissue explants will be valuable for structural and functional characterization of basement membrane constituents in developing, mature, and pathological or genetically perturbed tissues. Temporally and spatially controlled gene perturbation will be crucial because many developmental and disease mechanisms are precisely controlled at the single-cell level.
The ultimate goal of future research will be to develop effective therapeutic approaches, e.g., to help prevent basement membrane breaching in cancer. Additional future therapeutic approaches could benefit individuals afflicted by conditions in which the basement membrane plays a pivotal role in pathogenesis and disease progression. For example, gene therapy for laminin α2-deficient congenital muscular dystrophy (Qiao et al., 2005) and genetically modified epidermal stem cell therapy for epidermolysis bullosa (Hirsch et al., 2017; Mavilio et al., 2006) represent potentially promising future directions for the treatment of congenital disorders. Another recent advance in regenerative medicine research is laminin-guided human pluripotent stem cell differentiation into endothelial progenitor cells, e.g., using a fragment of LM-411 (Ohta et al., 2016). LM-411 found predominantly in the vascular endothelial basement membrane seems to instruct cell fate determination and differentiation into endothelial cells. Because some laminin isoforms are cell type-specific, this method may provide a means of guiding differentiation toward specific cell types when combined with chemical differentiation protocols. With its relevance to many developmental and disease processes, the basement membrane provides an exciting and vitally important area for further research.
Acknowledgments
We thank Thomas Bugge for expert advice on carcinoma cell invasion and Shaohe Wang for valuable assistance with the artwork. Work in the authors’ laboratory is supported by the Intramural Research Program of the NIH, NIDCR.
References
- Abrahamson DR, St John PL, Stroganova L, Zelenchuk A, Steenhard BM, 2013. Laminin and type IV collagen isoform substitutions occur in temporally and spatially distinct patterns in developing kidney glomerular basement membranes. J Histochem Cytochem 61, 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu-Velez AM, Howard MS, 2012. Collagen IV in Normal Skin and in Pathological Processes. N Am J Med Sci 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahtiainen L, Uski I, Thesleff I, Mikkola ML, 2016. Early epithelial signaling center governs tooth budding morphogenesis. J. Cell Biol. 214, 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artym VV, Swatkoski S, Matsumoto K, Campbell CB, Petrie RJ, Dimitriadis EK, Li X, Mueller SC, Bugge TH, Gucek M, Yamada KM, 2015. Dense fibrillar collagen is a potent inducer of invadopodia via a specific signaling network. J. Cell Biol. 208, 331–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD, 2005. A simplified laminin nomenclature. Matrix Biol 24, 326–332. [DOI] [PubMed] [Google Scholar]
- Aurich F, Dahmann C, 2016. A Mutation in fat2 Uncouples Tissue Elongation from Global Tissue Rotation. Cell Rep 14, 2503–2510. [DOI] [PubMed] [Google Scholar]
- Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, Murshed M, Nischt R, 2005. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol. Cell. Biol. 25, 6846–6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K, 1990. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248, 1224–1227. [DOI] [PubMed] [Google Scholar]
- Battaglia C, Mayer U, Aumailley M, Timpl R, 1992. Basement-membrane heparan sulfate proteoglycan binds to laminin by its heparan sulfate chains and to nidogen by sites in the protein core. Eur. J. Biochem. 208, 359–366. [DOI] [PubMed] [Google Scholar]
- Behrens DT, Villone D, Koch M, Brunner G, Sorokin L, Robenek H, Bruckner-Tuderman L, Bruckner P, Hansen U, 2012. The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J. Biol. Chem. 287, 18700–18709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Banerjee SD, 1982. The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev. Biol. 90, 291–305. [DOI] [PubMed] [Google Scholar]
- Berrier AL, Yamada KM, 2007. Cell–matrix adhesion. Journal of Cellular Physiology 213, 565–573. [DOI] [PubMed] [Google Scholar]
- Bloch-Zupan A, Sedano HO, Scully C, 2012. Dento/Oro/Craniofacial Anomalies and Genetics. Elsevier. [Google Scholar]
- Bluemink JG, Van Maurik P, Lawson KA, 1976. Intimate cell contacts at the epithelial/mesenchymal interface in embryonic mouse lung. J Ultrastruct Res 55, 257–270. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Chou J, Werb Z, 2014. Remodelling the extracellular matrix in development and disease. Nature reviews. Molecular cell biology 15, 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose K, Nischt R, Page A, Bader BL, Paulsson M, Smyth N, 2006. Loss of nidogen-1 and −2 results in syndactyly and changes in limb development. J. Biol. Chem. 281, 39620–39629. [DOI] [PubMed] [Google Scholar]
- Botta A, Delteil F, Mettouchi A, Vieira A, Estrach S, Négroni L, Stefani C, Lemichez E, Meneguzzi G, Gagnoux-Palacios L, 2012. Confluence switch signaling regulates ECM composition and the plasmin proteolytic cascade in keratinocytes. J. Cell Sci. 125, 4241–4252. [DOI] [PubMed] [Google Scholar]
- Boyango I, Barash U, Fux L, Naroditsky I, Ilan N, Vlodavsky I, 2017. Targeting heparanase to the mammary epithelium enhances mammary gland development and promotes tumor growth and metastasis. Matrix Biol. [DOI] [PubMed] [Google Scholar]
- Breitkreutz D, Koxholt I, Thiemann K, Nischt R, 2013. Skin basement membrane: the foundation of epidermal integrity--BM functions and diverse roles of bridging molecules nidogen and perlecan. Biomed Res Int 2013, 179784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T, 1999. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96, 795–806. [DOI] [PubMed] [Google Scholar]
- Bunt S, Hooley C, Hu N, Scahill C, Weavers H, Skaer H, 2010. Hemocyte-Secreted Type IV Collagen Enhances BMP Signaling to Guide Renal Tubule Morphogenesis in Drosophila. Dev. Cell 19, 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess AM, Katchburian E, 1982. Morphological types of epithelial-mesenchymal cell contacts in odontogenesis. J. Anat. 135, 577–584. [PMC free article] [PubMed] [Google Scholar]
- Candiello J, Cole GJ, Halfter W, 2010. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol 29, 402–410. [DOI] [PubMed] [Google Scholar]
- Carey DJ, 1997. Syndecans: multifunctional cell-surface co-receptors. Biochem. J. 327 ( Pt 1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casuso I, Rico F, Scheuring S, 2011. Biological AFM: where we come from--where we are--where we may go. J Mol Recognit 24, 406–413. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Giannoni E, 2008. Anoikis: a necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 76, 1352–1364. [DOI] [PubMed] [Google Scholar]
- Chui MH, 2013. Insights into cancer metastasis from a clinicopathologic perspective: Epithelial-Mesenchymal Transition is not a necessary step. Int. J. Cancer 132, 1487–1495. [DOI] [PubMed] [Google Scholar]
- Chun KJ, Choi HH, Lee JY, 2014. Comparison of mechanical property and role between enamel and dentin in the human teeth. Journal of Dental Biomechanics 5, 1758736014520809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles EG, Gammill LS, Miner JH, Bronner-Fraser M, 2006. Abnormalities in neural crest cell migration in laminin α5 mutant mice. Dev. Biol. 289, 218–228. [DOI] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fassler R, 1999. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 147, 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CF, Pedchenko V, Brown KL, Colon S, Rafi M, Jones-Paris C, Pokydeshava E, Liu M, Pastor-Pareja JC, Stothers C, Ero-Tolliver IA, McCall AS, Vanacore R, Bhave G, Santoro S, Blackwell TS, Zent R, Pozzi A, Hudson BG, 2016. Extracellular chloride signals collagen IV network assembly during basement membrane formation. J. Cell Biol. 213, 479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler LS, 1977. Intercellular contacts at the epithelial-mesenchymal interface of the developing rat submandibular gland in vitro. Journal of embryology and experimental morphology 39, 71–77. [PubMed] [Google Scholar]
- Dabija-Wolter G, Bakken V, Cimpan MR, Johannessen AC, Costea DE, 2013. In vitro reconstruction of human junctional and sulcular epithelium. Journal of Oral Pathology & Medicine 42, 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Gervais EM, Centanni SW, Gulfo KM, Nelson DA, Larsen M, 2012. ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Development 139, 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Matsumoto K, Doyle AD, Wang S, DuChez BJ, Holmbeck K, Yamada KM, 2017. Btbd7 is essential for region-specific epithelial cell dynamics and branching morphogenesis in vivo. Development 144, 2200–2211. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C, 2000. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127, 483–492. [DOI] [PubMed] [Google Scholar]
- Deen WM, 2004. What determines glomerular capillary permeability? J. Clin. Invest. 114, 1412–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone DW, Mecham R, 2013. Extracellular Matrix in Development. Springer Berlin; Heidelberg. [Google Scholar]
- Diaz de la Loza MC, Diaz-Torres A, Zurita F, Rosales-Nieves AE, Moeendarbary E, Franze K, Martin-Bermudo MD, Gonzalez-Reyes A, 2017. Laminin Levels Regulate Tissue Migration and Anterior-Posterior Polarity during Egg Morphogenesis in Drosophila. Cell Rep 20, 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domogatskaya A, Rodin S, Tryggvason K, 2012. Functional diversity of laminins. Annu. Rev. Cell. Dev. Biol. 28, 523–553. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J, 2011. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell 19, 372–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, 1996. Domain organizations of modular extracellular matrix proteins and their evolution. Matrix Biol 15, 295–299. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Vajkoczy P, Weller RO, 2017. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 18, 123–131. [DOI] [PubMed] [Google Scholar]
- Entesarian M, Matsson H, Klar J, Bergendal B, Olson L, Arakaki R, Hayashi Y, Ohuchi H, Falahat B, Bolstad AI, Jonsson R, Wahren-Herlenius M, Dahl N, 2005. Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat. Genet. 37, 125–127. [DOI] [PubMed] [Google Scholar]
- Ewald AJ, Huebner RJ, Palsdottir H, Lee JK, Perez MJ, Jorgens DM, Tauscher AN, Cheung KJ, Werb Z, Auer M, 2012. Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. Journal of cell science 125, 2638–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farach-Carson MC, Warren CR, Harrington DA, Carson DD, 2014. Border patrol: insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol 34, 64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ, 2004. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast cancer research : BCR 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler AL, Darris CE, Chetyrkin SV, Pedchenko VK, Boudko SP, Brown KL, Gray Jerome W, Hudson JK, Rokas A, Hudson BG, 2017. Collagen IV and basement membrane at the evolutionary dawn of metazoan tissues. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine JD, Bruckner-Tuderman L, Eady RA, Bauer EA, Bauer JW, Has C, Heagerty A, Hintner H, Hovnanian A, Jonkman MF, Leigh I, Marinkovich MP, Martinez AE, McGrath JA, Mellerio JE, Moss C, Murrell DF, Shimizu H, Uitto J, Woodley D, Zambruno G, 2014. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol 70, 1103–1126. [DOI] [PubMed] [Google Scholar]
- Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J, 1991. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. The EMBO Journal 10, 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Wolf K, 2003. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer 3, 362–374. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Miner JH, Ida H, Fukumoto E, Yuasa K, Miyazaki H, Hoffman MP, Yamada Y, 2006. Laminin alpha5 is required for dental epithelium growth and polarity and the development of tooth bud and shape. J. Biol. Chem. 281, 5008–5016. [DOI] [PubMed] [Google Scholar]
- Garcia-Alonso L, Fetter RD, Goodman CS, 1996. Genetic analysis of Laminin A in Drosophila: extracellular matrix containing laminin A is required for ocellar axon pathfinding. Development 122, 2611–2621. [DOI] [PubMed] [Google Scholar]
- Gautam J, Zhang X, Yao Y, 2016. The role of pericytic laminin in blood brain barrier integrity maintenance. Scientific Reports 6, 36450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais EM, Sequeira SJ, Wang W, Abraham S, Kim JH, Leonard D, DeSantis KA, Larsen M, 2016. Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development. Organogenesis 12, 194–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CW, Yuan ZA, Hall B, Longenecker G, Chen E, Thyagarajan T, Sreenath T, Wright JT, Decker S, Piddington R, Harrison G, Kulkarni AB, 2001. Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J. Biol. Chem. 276, 31871–31875. [DOI] [PubMed] [Google Scholar]
- Gil-Henn H, Patsialou A, Wang Y, Warren MS, Condeelis JS, Koleske AJ, 2013. Arg/Abl2 promotes invasion and attenuates proliferation of breast cancer in vivo. Oncogene 32, 2622–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M, Buccione R, Courtneidge SA, Linder S, 2008. Assembly and biological role of podosomes and invadopodia. Curr. Opin. Cell Biol. 20, 235–241. [DOI] [PubMed] [Google Scholar]
- Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA, 2010. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra L, Odorisio T, Zambruno G, Castiglia D, 2017. Stromal microenvironment in type VII collagen-deficient skin: The ground for squamous cell carcinoma development. Matrix Biol 63, 1–10. [DOI] [PubMed] [Google Scholar]
- Hagedorn EJ, Yashiro H, Ziel JW, Ihara S, Wang Z, Sherwood DR, 2009. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell’s invasive membrane in C. elegans. Dev. Cell 17, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn EJ, Ziel JW, Morrissey MA, Linden LM, Wang Z, Chi Q, Johnson SA, Sherwood DR, 2013. The netrin receptor DCC focuses invadopodia-driven basement membrane transmigration in vivo. The Journal of Cell Biology 201, 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo SL, Bilder D, 2011. Global Tissue Revolutions in a Morphogenetic Movement Controlling Elongation. Science 331, 1071–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkinen KM, Harunaga JS, Doyle AD, Yamada KM, 2011. Direct Comparisons of the Morphology, Migration, Cell Adhesions, and Actin Cytoskeleton of Fibroblasts in Four Different Three-Dimensional Extracellular Matrices. Tissue Engineering. Part A 17, 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Dong S, Yip YP, Willem M, Mayer U, 2002. A critical function of the pial basement membrane in cortical histogenesis. J. Neurosci. 22, 6029–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Monnier C, Muller D, Oertle P, Uechi G, Balasubramani M, Safi F, Lim R, Loparic M, Henrich PB, 2013. The bi-functional organization of human basement membranes. PLoS One 8, e67660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Oertle P, Monnier CA, Camenzind L, Reyes-Lua M, Hu H, Candiello J, Labilloy A, Balasubramani M, Henrich PB, Plodinec M, 2015. New concepts in basement membrane biology. FEBS J. 282, 4466–4479. [DOI] [PubMed] [Google Scholar]
- Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM, 2005. Expression and Function of Laminins in the Embryonic and Mature Vasculature. Physiol. Rev. 85, 979–1000. [DOI] [PubMed] [Google Scholar]
- Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R, 2003. Physiological levels of tumstatin, a fragment of collagen IV α3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via αVβ3 integrin. Cancer Cell 3, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA, 2000. The hallmarks of cancer. Cell 100, 57–70. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA, 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Harunaga JS, Doyle AD, Yamada KM, 2014. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Dev. Biol. 394, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SJ, Zheng K, Sado Y, Naito I, Ninomiya Y, Jacobs RM, Hudson BG, Thorner PS, 1998. Role of distinct type IV collagen networks in glomerular development and function. Kidney International 54, 1857–1866. [DOI] [PubMed] [Google Scholar]
- Has C, Nyström A, 2015. Chapter Four - Epidermal Basement Membrane in Health and Disease, in: Miner JH (Ed.), Current Topics in Membranes. Academic Press, pp. 117–170. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Yaegashi T, Oikawa Y, Fujiwara H, Mikami T, Takeda Y, Satoh M, 2006. Expression pattern of adhesion molecules in junctional epithelium differs from that in other gingival epithelia. J Periodontal Res 41, 322–328. [DOI] [PubMed] [Google Scholar]
- Hayashi T, 2011. Dysfunction of Lacrimal and Salivary Glands in Sjögren’s Syndrome: Nonimmunologic Injury in Preinflammatory Phase and Mouse Model. J. Biomed. Biotechnol. 2011, 407031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Zhang Y, Kim SO, Radlanski RJ, Butcher K, Schneider RA, DenBesten PK, 2010. Ameloblast differentiation in the human developing tooth: effects of extracellular matrices. Matrix Biol 29, 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog C, Has C, Franzke CW, Echtermeyer FG, Schlotzer-Schrehardt U, Kroger S, Gustafsson E, Fassler R, Bruckner-Tuderman L, 2004. Dystroglycan in skin and cutaneous cells: beta-subunit is shed from the cell surface. J Invest Dermatol 122, 1372–1380. [DOI] [PubMed] [Google Scholar]
- Hiramatsu R, Matsuoka T, Kimura-Yoshida C, Han SW, Mochida K, Adachi T, Takayama S, Matsuo I, 2013. External mechanical cues trigger the establishment of the anterior-posterior axis in early mouse embryos. Dev. Cell 27, 131–144. [DOI] [PubMed] [Google Scholar]
- Hironaka K, Makino H, Yamasaki Y, Ota Z, 1993. Renal basement membranes by ultrahigh resolution scanning electron microscopy. Kidney Int 43, 334–345. [DOI] [PubMed] [Google Scholar]
- Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, Scaglione D, Reichelt J, Klausegger A, Kneisz D, Romano O, Secone Seconetti A, Contin R, Enzo E, Jurman I, Carulli S, Jacobsen F, Luecke T, Lehnhardt M, Fischer M, Kueckelhaus M, Quaglino D, Morgante M, Bicciato S, Bondanza S, De Luca M, 2017. Regeneration of the entire human epidermis using transgenic stem cells. Nature 551, 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester E, Yurchenco PD, 2013. Laminins in basement membrane assembly. Cell Adh Migr 7, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horejs CM, Serio A, Purvis A, Gormley AJ, Bertazzo S, Poliniewicz A, Wang AJ, DiMaggio P, Hohenester E, Stevens MM, 2014. Biologically-active laminin-111 fragment that modulates the epithelial-to-mesenchymal transition in embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America 111, 5908–5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howat WJ, Holmes JA, Holgate ST, Lackie PM, 2001. Basement Membrane Pores in Human Bronchial Epithelium : A Conduit for Infiltrating Cells? The American Journal of Pathology 158, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JC, Koo H, Harunaga JS, Matsumoto K, Doyle AD, Yamada KM, 2013. Region-specific epithelial cell dynamics during branching morphogenesis. Dev. Dyn. 242, 1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG, 2003. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348, 2543–2556. [DOI] [PubMed] [Google Scholar]
- Huebner RJ, Lechler T, Ewald AJ, 2014. Developmental stratification of the mammary epithelium occurs through symmetry-breaking vertical divisions of apically positioned luminal cells. Development 141, 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert C, Silbermann F, Morar B, Parisot M, Zarhrate M, Masson C, Tores F, Blanchet P, Perez MJ, Petrov Y, Khau Van Kien P, Roume J, Leroy B, Gribouval O, Kalaydjieva L, Heidet L, Salomon R, Antignac C, Benmerah A, Saunier S, Jeanpierre C, 2014. Integrin alpha 8 recessive mutations are responsible for bilateral renal agenesis in humans. Am. J. Hum. Genet. 94, 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SA, Piper M, Fukuhara N, Strochlic L, Cho G, Howitt JA, Ahmed Y, Powell AK, Turnbull JE, Holt CE, Hohenester E, 2006. A molecular mechanism for the heparan sulfate dependence of slit-robo signaling. J. Biol. Chem. 281, 39693–39698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25. [DOI] [PubMed] [Google Scholar]
- Hynes RO, 1999. Cell adhesion: old and new questions. Trends Cell Biol. 9, M33–37. [PubMed] [Google Scholar]
- Hynes RO, Naba A, 2012. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 4, a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Yamada KM, 2012. Extracellular Matrix Biology. Cold Spring Harbor Laboratory Press. [Google Scholar]
- Imbeni V, Kruzic JJ, Marshall GW, Marshall SJ, Ritchie RO, 2005. The dentin-enamel junction and the fracture of human teeth. Nat Mater 4, 229–232. [DOI] [PubMed] [Google Scholar]
- Inomata T, Ebihara N, Funaki T, Matsuda A, Watanabe Y, Ning L, Xu Z, Murakami A, Arikawa-Hirasawa E, 2012. Perlecan-deficient mutation impairs corneal epithelial structure. Invest Ophthalmol Vis Sci 53, 1277–1284. [DOI] [PubMed] [Google Scholar]
- Isabella AJ, Horne-Badovinac S, 2015. Building from the ground up: basement membranes in Drosophila development. Current topics in membranes 76, 305–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabella AJ, Horne-Badovinac S, 2016. Rab10-mediated secretion synergizes with tissue movement to build a polarized basement membrane architecture for organ morphogenesis. Dev. Cell 38, 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev R, Sherwood DR, 2017. Basement membranes. Curr. Biol. 27, R207–r211. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Ghose A, Epstein E, Lincecum J, O’Connor MB, Van Vactor D, 2004. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr. Biol. 14, 499–504. [DOI] [PubMed] [Google Scholar]
- Jorda M, Olmeda D, Vinyals A, Valero E, Cubillo E, Llorens A, Cano A, Fabra A, 2005. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J. Cell Sci. 118, 3371–3385. [DOI] [PubMed] [Google Scholar]
- Kadoya Y, Yamashina S, 2005. Salivary gland morphogenesis and basement membranes. Anat Sci Int 80, 71–79. [DOI] [PubMed] [Google Scholar]
- Kalluri R, 2003. Basement membranes: structure, assembly and role in tumour angiogenesis. Nature reviews. Cancer 3, 422–433. [DOI] [PubMed] [Google Scholar]
- Kan M, Wu X, Wang F, McKeehan WL, 1999. Specificity for fibroblast growth factors determined by heparan sulfate in a binary complex with the receptor kinase. J. Biol. Chem. 274, 15947–15952. [DOI] [PubMed] [Google Scholar]
- Karihaloo A, Karumanchi SA, Barasch J, Jha V, Nickel CH, Yang J, Grisaru S, Bush KT, Nigam S, Rosenblum ND, Sukhatme VP, Cantley LG, 2001. Endostatin regulates branching morphogenesis of renal epithelial cells and ureteric bud. Proc Natl Acad Sci U S A 98, 12509–12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely PJ, Wu JE, Santoro SA, 1995. The spatial and temporal expression of the alpha 2 beta 1 integrin and its ligands, collagen I, collagen IV, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation; research in biological diversity 59, 1–13. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z, 2010. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 141, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Turnbull J, Guimond S, 2011. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 209, 139–151. [DOI] [PubMed] [Google Scholar]
- Kinane DF, 2001. Causation and pathogenesis of periodontal disease. Periodontol 2000 25, 8–20. [DOI] [PubMed] [Google Scholar]
- Kiyozumi D, Takeichi M, Nakano I, Sato Y, Fukuda T, Sekiguchi K, 2012. Basement membrane assembly of the integrin alpha8beta1 ligand nephronectin requires Fraser syndrome-associated proteins. J. Cell Biol. 197, 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine M, Virtanen I, Porola P, Rotar Z, Rozman B, Poduval P, Konttinen YT, 2008. Acinar epithelial cell laminin-receptors in labial salivary glands in Sjogren’s syndrome. Clin Exp Rheumatol 26, 807–813. [PubMed] [Google Scholar]
- Larjava H, Koivisto L, Hakkinen L, Heino J, 2011. Epithelial integrins with special reference to oral epithelia. J. Dent. Res. 90, 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legoff L, Rouault H, Lecuit T, 2013. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development 140, 4051–4059. [DOI] [PubMed] [Google Scholar]
- Lehtonen E, 1975. Epithelio-mesenchymal interface during mouse kidney tubule induction in vivo. J Embryol Exp Morphol 34, 695–705. [PubMed] [Google Scholar]
- Leonoudakis D, Singh M, Mohajer R, Mohajer P, Fata JE, Campbell KP, Muschler JL, 2010. Dystroglycan controls signaling of multiple hormones through modulation of STAT5 activity. J. Cell Sci. 123, 3683–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Feng J, Liu Y, Ho TV, Grimes W, Ho HA, Park S, Wang S, Chai Y, 2015. BMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the developing tooth. Dev. Cell 33, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhang S, Rehn M, Itaranta P, Tuukkanen J, Heljasvaara R, Peltoketo H, Pihlajaniemi T, Vainio S, 2001. Induced repatterning of type XVIII collagen expression in ureter bud from kidney to lung type: association with sonic hedgehog and ectopic surfactant protein C. Development 128, 1573–1585. [DOI] [PubMed] [Google Scholar]
- Linton JM, Martin GR, Reichardt LF, 2007. The ECM protein nephronectin promotes kidney development via integrin alpha8beta1-mediated stimulation of Gdnf expression. Development 134, 2501–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litjens SHM, de Pereda JM, Sonnenberg A, 2006. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 16, 376–383. [DOI] [PubMed] [Google Scholar]
- Longo I, Porcedda P, Mari F, Giachino D, Meloni I, Deplano C, Brusco A, Bosio M, Massella L, Lavoratti G, Roccatello D, Frasca G, Mazzucco G, Muda AO, Conti M, Fasciolo F, Arrondel C, Heidet L, Renieri A, De Marchi M, 2002. COL4A3/COL4A4 mutations: from familial hematuria to autosomal-dominant or recessive Alport syndrome. Kidney Int 61, 1947–1956. [DOI] [PubMed] [Google Scholar]
- Lopez-Luppo M, Nacher V, Ramos D, Catita J, Navarro M, Carretero A, Rodriguez-Baeza A, Mendes-Jorge L, Ruberte J, 2017. Blood Vessel Basement Membrane Alterations in Human Retinal Microaneurysms During Aging. Invest Ophthalmol Vis Sci 58, 1116–1131. [DOI] [PubMed] [Google Scholar]
- Loscertales M, Nicolaou F, Jeanne M, Longoni M, Gould DB, Sun Y, Maalouf FI, Nagy N, Donahoe PK, 2016. Type IV collagen drives alveolar epithelial-endothelial association and the morphogenetic movements of septation. BMC Biol. 14, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Cao X, Dai J, Pastor-Pareja JC, 2017. Basement Membrane Manipulation in Drosophila Wing Discs Affects Dpp Retention but Not Growth Mechanoregulation. Dev. Cell 42, 97–106.e104. [DOI] [PubMed] [Google Scholar]
- Madsen DH, Bugge TH, 2015. The source of matrix-degrading enzymes in human cancer: Problems of research reproducibility and possible solutions. J. Cell Biol. 209, 195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, Kato S, Dickson C, Thiery JP, Bellusci S, 2002. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development 129, 53–60. [DOI] [PubMed] [Google Scholar]
- Mammoto T, Ingber DE, 2010. Mechanical control of tissue and organ development. Development (Cambridge, England) 137, 1407–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe R, Tsutsui K, Yamada T, Kimura M, Nakano I, Shimono C, Sanzen N, Furutani Y, Fukuda T, Oguri Y, Shimamoto K, Kiyozumi D, Sato Y, Sado Y, Senoo H, Yamashina S, Fukuda S, Kawai J, Sugiura N, Kimata K, Hayashizaki Y, Sekiguchi K, 2008. Transcriptome-based systematic identification of extracellular matrix proteins. Proc Natl Acad Sci U S A 105, 12849–12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich MP, 2007. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat. Rev. Cancer 7, 370–380. [DOI] [PubMed] [Google Scholar]
- Marks SC Jr., McKee MD, Zalzal S, Nanci A, 1994. The epithelial attachment and the dental junctional epithelium: ultrastructural features in porcine molars. Anat Rec 238, 1–14. [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y, Louani A, Dragu A, Sanchez-Sanchez BJ, Serna-Morales E, Yolland L, Gyoergy A, Vizcay G, Fleck RA, Heddleston JM, Chew TL, Siekhaus DE, Stramer BM, 2017. A Moving Source of Matrix Components Is Essential for De Novo Basement Membrane Formation. Curr. Biol. 27, 3526–3534.e3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus DQ, Chang E, Makohon-Moore SC, Hagedorn MA, Chi Q, Sherwood DR, 2014. Cell division and targeted cell cycle arrest opens and stabilizes basement membrane gaps. Nat Commun 5, 4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus DQ, Lohmer LL, Kelley LC, Schindler AJ, Kohrman AQ, Barkoulas M, Zhang W, Chi Q, Sherwood DR, 2015. Invasive Cell Fate Requires G1 Cell-Cycle Arrest and Histone Deacetylase-Mediated Changes in Gene Expression. Developmental cell 35, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A, Maruggi G, Ferrari G, Provasi E, Bonini C, Capurro S, Conti A, Magnoni C, Giannetti A, De Luca M, 2006. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat. Med. 12, 1397–1402. [DOI] [PubMed] [Google Scholar]
- Mayer U, Nischt R, Poschl E, Mann K, Fukuda K, Gerl M, Yamada Y, Timpl R, 1993. A single EGF-like motif of laminin is responsible for high affinity nidogen binding. EMBO J. 12, 1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey ST, Wang Z, Linden LM, Hastie EL, Wang L, Shen W, Chen A, Chi Q, Sherwood DR, 2016. Boundary cells restrict dystroglycan trafficking to control basement membrane sliding during tissue remodeling. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JD, Gorski JP, Dusevich V, Wang Y, Walker MP, 2014a. Type IV collagen is a novel DEJ biomarker that is reduced by radiotherapy. J. Dent. Res. 93, 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JD, Walker MP, Dusevich V, Wang Y, Gorski JP, 2014b. Enamel organic matrix: potential structural role in enamel and relationship to residual basement membrane constituents at the dentin enamel junction. Connect. Tissue Res. 55, 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecham R, 2011. The Extracellular Matrix: an Overview. Springer Berlin; Heidelberg. [Google Scholar]
- Micalizzi DS, Farabaugh SM, Ford HL, 2010. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 15, 117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS, 1998. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 12, 3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Cunningham J, Sanes JR, 1998. Roles for Laminin in Embryogenesis: Exencephaly, Syndactyly, and Placentopathy in Mice Lacking the Laminin α5 Chain. The Journal of Cell Biology 143, 1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Go G, Cunningham J, Patton BL, Jarad G, 2006. Transgenic isolation of skeletal muscle and kidney defects in laminin beta2 mutant mice: implications for Pierson syndrome. Development 133, 967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Li C, 2000. Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Developmental biology 217, 278–289. [DOI] [PubMed] [Google Scholar]
- Miner JH, Li C, Mudd JL, Go G, Sutherland AE, 2004. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development 131, 2247–2256. [DOI] [PubMed] [Google Scholar]
- Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR, 1997. The Laminin α Chains: Expression, Developmental Transitions, and Chromosomal Locations of α1-5, Identification of Heterotrimeric Laminins 8–11, and Cloning of a Novel α3 Isoform. The Journal of Cell Biology 137, 685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD, 2004. LAMININ FUNCTIONS IN TISSUE MORPHOGENESIS. Annu. Rev. Cell. Dev. Biol. 20, 255–284. [DOI] [PubMed] [Google Scholar]
- Moasser MM, 2013. Neoplasia, in: Hammer GD, McPhee SJ (Eds.), Pathophysiology of Disease: An Introduction to Clinical Medicine, 7e McGraw-Hill Education, New York, NY. [Google Scholar]
- Mokkapati S, Baranowsky A, Mirancea N, Smyth N, Breitkreutz D, Nischt R, 2008. Basement membranes in skin are differently affected by lack of nidogen 1 and 2. J Invest Dermatol 128, 2259–2267. [DOI] [PubMed] [Google Scholar]
- Molina C, Alliende C, Aguilera S, Kwon YJ, Leyton L, Martinez B, Leyton C, Perez P, Gonzalez MJ, 2006. Basal lamina disorganisation of the acini and ducts of labial salivary glands from patients with Sjogren’s syndrome: association with mononuclear cell infiltration. Ann. Rheum. Dis. 65, 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE, 2005. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev. Dyn. 232, 268–281. [DOI] [PubMed] [Google Scholar]
- Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO, 2016. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biology 49, 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naegeli K, Hastie E, Garde A, Wang Z, Keeley D, Gordon KL, Pani AM, Kelley LC, Morrissey MA, Chi Q, Goldstein B, Sherwood DR, 2017. Cell invasion in vivo via rapid exocytosis of a transient lysosome-derived membrane domain. Dev. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata M, Katz ML, Robison WG Jr., 1986. Age-related thickening of retinal capillary basement membranes. Invest Ophthalmol Vis Sci 27, 437–440. [PubMed] [Google Scholar]
- Nakaya Y, Sukowati EW, Alev C, Nakazawa F, Sheng G, 2011. Involvement of dystroglycan in epithelial-mesenchymal transition during chick gastrulation. Cells Tissues Organs 193, 64–73. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Sukowati EW, Wu Y, Sheng G, 2008. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat. Cell Biol. 10, 765–775. [DOI] [PubMed] [Google Scholar]
- Nanba D, Hieda Y, Nakanishi Y, 2000. Remodeling of Desmosomal and Hemidesmosomal Adhesion Systems During Early Morphogenesis of Mouse Pelage Hair Follicles. Journal of Investigative Dermatology 114, 171–177. [DOI] [PubMed] [Google Scholar]
- Neumann KH, Kellner C, Kuhn K, Stolte H, Schurek HJ, 2004. Age-dependent thickening of glomerular basement membrane has no major effect on glomerular hydraulic conductivity. Nephrol. Dial. Transplant. 19, 805–811. [DOI] [PubMed] [Google Scholar]
- Newman MG, Takei H, Klokkevold PR, Carranza FA, 2011. Carranza’s Clinical Periodontology. Elsevier Health Sciences. [Google Scholar]
- Nguyen-Ngoc KV, Cheung KJ, Brenot A, Shamir ER, Gray RS, Hines WC, Yaswen P, Werb Z, Ewald AJ, 2012. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc Natl Acad Sci U S A 109, E2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen BS, Sehested M, Duun S, Rank F, Timshel S, Rygaard J, Johnsen M, Dano K, 2001. Urokinase plasminogen activator is localized in stromal cells in ductal breast cancer. Lab. Invest. 81, 1485–1501. [DOI] [PubMed] [Google Scholar]
- Nischt R, Schmidt C, Mirancea N, Baranowsky A, Mokkapati S, Smyth N, Woenne EC, Stark HJ, Boukamp P, Breitkreutz D, 2007. Lack of nidogen-1 and −2 prevents basement membrane assembly in skin-organotypic coculture. J Invest Dermatol 127, 545–554. [DOI] [PubMed] [Google Scholar]
- Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, Tsuji T, Yamada M, Sekiguchi K, 2006a. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol 25, 189–197. [DOI] [PubMed] [Google Scholar]
- Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, Tsuji T, Yamada M, Sekiguchi K, 2006b. Ligand-binding specificities of laminin-binding integrins: A comprehensive survey of laminin–integrin interactions using recombinant α3β1, α6β1, α7β1 and α6β4 integrins. Matrix Biology 25, 189–197. [DOI] [PubMed] [Google Scholar]
- Nourshargh S, Alon R, 2014. Leukocyte migration into inflamed tissues. Immunity 41, 694–707. [DOI] [PubMed] [Google Scholar]
- Nourshargh S, Hordijk PL, Sixt M, 2010. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 11, 366–378. [DOI] [PubMed] [Google Scholar]
- Nurcombe V, Ford MD, Wildschut JA, Bartlett PF, 1993. Developmental regulation of neural response to FGF-1 and FGF-2 by heparan sulfate proteoglycan. Science 260, 103–106. [DOI] [PubMed] [Google Scholar]
- Ohta R, Niwa A, Taniguchi Y, Suzuki NM, Toga J, Yagi E, Saiki N, Nishinaka-Arai Y, Okada C, Watanabe A, Nakahata T, Sekiguchi K, Saito MK, 2016. Laminin-guided highly efficient endothelial commitment from human pluripotent stem cells. Sci Rep 6, 35680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N, 2000. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem. Biophys. Res. Commun. 277, 643–649. [DOI] [PubMed] [Google Scholar]
- Oksonen J, Sorokin LM, Virtanen, Hormia M, 2001. The junctional epithelium around murine teeth differs from gingival epithelium in its basement membrane composition. J. Dent. Res. 80, 2093–2097. [DOI] [PubMed] [Google Scholar]
- Ozanne BW, Spence HJ, McGarry LC, Hennigan RF, 2006. Invasion is a genetic program regulated by transcription factors. Curr. Opin. Genet. Dev. 16, 65–70. [DOI] [PubMed] [Google Scholar]
- Ozbek S, Balasubramanian PG, Chiquet-Ehrismann R, Tucker RP, Adams JC, 2010. The evolution of extracellular matrix. Mol Biol Cell 21, 4300–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine IS, Lewis MT, 2017. The Terminal End Bud: the Little Engine that Could. J Mammary Gland Biol Neoplasia 22, 93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VN, Knox SM, Likar KM, Lathrop CA, Hossain R, Eftekhari S, Whitelock JM, Elkin M, Vlodavsky I, Hoffman MP, 2007. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development 134, 4177–4186. [DOI] [PubMed] [Google Scholar]
- Patel VN, Pineda DL, Hoffman MP, 2017. The function of heparan sulfate during branching morphogenesis. Matrix Biol 57-58, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L, 1999. Genome evolution and the evolution of exon-shuffling--a review. Gene 238, 103–114. [DOI] [PubMed] [Google Scholar]
- Patton BL, Cunningham JM, Thyboll J, Kortesmaa J, Westerblad H, Edstrom L, Tryggvason K, Sanes JR, 2001. Properly formed but improperly localized synaptic specializations in the absence of laminin [alpha]4. Nat. Neurosci. 4, 597–604. [DOI] [PubMed] [Google Scholar]
- Petrou P, Makrygiannis AK, Chalepakis G, 2008. The Fras1/Frem family of extracellular matrix proteins: structure, function, and association with Fraser syndrome and the mouse bleb phenotype. Connect. Tissue Res. 49, 277–282. [DOI] [PubMed] [Google Scholar]
- Pickering J, Cunliffe VT, Van Eeden F, Borycki AG, 2017. Hedgehog signalling acts upstream of Laminin alpha1 transcription in the zebrafish paraxial mesoderm. Matrix Biol 62, 58–74. [DOI] [PubMed] [Google Scholar]
- Pitera JE, Scambler PJ, Woolf AS, 2008. Fras1, a basement membrane-associated protein mutated in Fraser syndrome, mediates both the initiation of the mammalian kidney and the integrity of renal glomeruli. Human molecular genetics 17, 3953–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachot C, Chaboub LS, Adissu HA, Wang L, Urazaev A, Sturgis J, Asem EK, Lelièvre SA, 2009. Factors necessary to produce basoapical polarity in human glandular epithelium formed in conventional and high-throughput three-dimensional culture: example of the breast epithelium. BMC Biol. 7, 77–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen L, Uitto J, 1999. Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biology 18, 29–42. [DOI] [PubMed] [Google Scholar]
- Qiao C, Li J, Zhu T, Draviam R, Watkins S, Ye X, Chen C, Li J, Xiao X, 2005. Amelioration of laminin-alpha2-deficient congenital muscular dystrophy by somatic gene transfer of miniagrin. Proc Natl Acad Sci U S A 102, 11999–12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebustini IT, Myers C, Lassiter KS, Surmak A, Szabova L, Holmbeck K, Pedchenko V, Hudson BG, Hoffman MP, 2009. MT2-MMP-dependent release of collagen IV NC1 domains regulates submandibular gland branching morphogenesis. Dev. Cell 17, 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimann I, Hajnal A, 2007. Regulation of anchor cell invasion and uterine cell fates by the egl-43 Evi-1 proto-oncogene in Caenorhabditis elegans. Dev. Biol. 308, 187–195. [DOI] [PubMed] [Google Scholar]
- Rodeck CH, Whittle MJ, 2009. Fetal Medicine: Basic Science and Clinical Practice. Churchill Livingstone. [Google Scholar]
- Rodin S, Antonsson L, Niaudet C, Simonson OE, Salmela E, Hansson EM, Domogatskaya A, Xiao Z, Damdimopoulou P, Sheikhi M, Inzunza J, Nilsson AS, Baker D, Kuiper R, Sun Y, Blennow E, Nordenskjold M, Grinnemo KH, Kere J, Betsholtz C, Hovatta O, Tryggvason K, 2014. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun 5, 3195. [DOI] [PubMed] [Google Scholar]
- Rousselle P, Keene DR, Ruggiero F, Champliaud M-F, Rest M.v.d., Burgeson RE, 1997. Laminin 5 Binds the NC-1 Domain of Type VII Collagen. The Journal of Cell Biology 138, 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RG, Weiss SJ, 2008. Breaching the basement membrane: who, when and how? Trends Cell Biol. 18, 560–574. [DOI] [PubMed] [Google Scholar]
- Rozario T, DeSimone DW, 2010. The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 341, 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlberg C, Reponen P, Tryggvason K, Thesleff I, 1992. Association between the expression of murine 72 kDa type IV collagenase by odontoblasts and basement membrane degradation during mouse tooth development. Arch Oral Biol 37, 1021–1030. [DOI] [PubMed] [Google Scholar]
- Salvi SM, Akhtar S, Currie Z, 2006. Ageing changes in the eye. Postgrad. Med. J. 82, 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Medina M, Gregus KA, Nichol RH, O’Toole SM, Gomez TM, 2015. Regulation of ECM degradation and axon guidance by growth cone invadosomes. Development 142, 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S, Lamanna WC, Esko JD, 2011. Heparan Sulfate Proteoglycans. Cold Spring Harbor Perspectives in Biology 3, a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Uemura T, Morimitsu K, Sato-Nishiuchi R, Manabe R, Takagi J, Yamada M, Sekiguchi K, 2009. Molecular basis of the recognition of nephronectin by integrin alpha8beta1. J. Biol. Chem. 284, 14524–14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AJ, Sherwood DR, 2011. The transcription factor HLH-2/E/Daughterless regulates anchor cell invasion across basement membrane in C. elegans. Dev. Biol. 357, 380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppala M, Fraser G, Birjandi A, Xavier G, Cobourne M, 2017. Sonic Hedgehog Signaling and Development of the Dentition. Journal of Developmental Biology 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher I, Zisman-Rozen S, Eliahu L, Whitelock JM, Maas-Szabowski N, Yamada Y, Breitkreutz D, Fusenig NE, Arikawa-Hirasawa E, Iozzo RV, Bergman R, Ron D, 2006. Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J. Biol. Chem. 281, 5178–5187. [DOI] [PubMed] [Google Scholar]
- Sherwood DR, Butler JA, Kramer JM, Sternberg PW, 2005. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell 121, 951–962. [DOI] [PubMed] [Google Scholar]
- Sherwood DR, Sternberg PW, 2003. Anchor cell invasion into the vulval epithelium in C. elegans. Dev. Cell 5, 21–31. [DOI] [PubMed] [Google Scholar]
- Shyer AE, Rodrigues AR, Schroeder GG, Kassianidou E, Kumar S, Harland RM, 2017. Emergent cellular self-organization and mechanosensation initiate follicle pattern in the avian skin. Science 357, 811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW, 1982. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev. Biol. 90, 215–222. [DOI] [PubMed] [Google Scholar]
- Simmer JP, Papagerakis P, Smith CE, Fisher DC, Rountrey AN, Zheng L, Hu JC, 2010. Regulation of dental enamel shape and hardness. Journal of dental research 89, 1024–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões-Costa M, Bronner ME, 2015. Establishing neural crest identity: a gene regulatory recipe. Development (Cambridge, England) 142, 242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu ASL, Luyckx V, 2015. Brenner and Rector’s The Kidney E-Book. Elsevier Health Sciences. [Google Scholar]
- Slade MJ, Coope RC, Gomm JJ, Coombes RC, 1999. The human mammary gland basement membrane is integral to the polarity of luminal epithelial cells. Exp. Cell Res. 247, 267–278. [DOI] [PubMed] [Google Scholar]
- Slavkin HC, Bringas P Jr., 1976. Epithelial-mesenchyme interactions during odontogenesis. IV. Morphological evidence for direct heterotypic cell-cell contacts. Dev. Biol. 50, 428–442. [DOI] [PubMed] [Google Scholar]
- Sorokin L, 2010. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10, 712–723. [DOI] [PubMed] [Google Scholar]
- Steeg PS, 2003. Metastasis suppressors alter the signal transduction of cancer cells. Nat. Rev. Cancer 3, 55–63. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, 2006. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast cancer research : BCR 8, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O, 2005. The retinal pigment epithelium in visual function. Physiol. Rev. 85, 845–881. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ, 1991. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J. Cell Biol. 115, 1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Gonda T, 2004. Distribution of the pores of epithelial basement membrane in the rat small intestine. J. Vet. Med. Sci. 66, 695–700. [DOI] [PubMed] [Google Scholar]
- Tanos B, Rodriguez-Boulan E, 2008. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene 27, 6939–6957. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Barrach HJ, Foidart JM, Vaheri A, Pratt RM, Martin GR, 1981. Changes in the distribution of type IV collagen, laminin, proteoglycan, and fibronectin during mouse tooth development. Dev. Biol. 81, 182–192. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA, 2009. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Routhe LJ, Moos T, 2017. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab 37, 3300–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K, 2002. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol. Cell. Biol. 22, 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kuhn K, 1981. A network model for the organization of type IV collagen molecules in basement membranes. Eur. J. Biochem. 120, 203–211. [DOI] [PubMed] [Google Scholar]
- To M, Goz A, Camenzind L, Oertle P, Candiello J, Sullivan M, Henrich PB, Loparic M, Safi F, Eller A, Halfter W, 2013. Diabetes-induced morphological, biomechanical, and compositional changes in ocular basement membranes. Exp Eye Res 116, 298–307. [DOI] [PubMed] [Google Scholar]
- Tosios KI, Kapranos N, Papanicolaou SI, 1998. Loss of basement membrane components laminin and type IV collagen parallels the progression of oral epithelial neoplasia. Histopathology 33, 261–268. [DOI] [PubMed] [Google Scholar]
- Tucker A, Sharpe P, 2004. The cutting-edge of mammalian development; how the embryo makes teeth. Nature reviews. Genetics 5, 499–508. [DOI] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F, 2015. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419. [DOI] [PubMed] [Google Scholar]
- Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P, 2017. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358, 111–116. [DOI] [PubMed] [Google Scholar]
- Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S, 2006. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J. Exp. Med. 203, 1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem M, Miosge N, Halfter W, Smyth N, Jannetti I, Burghart E, Timpl R, Mayer U, 2002. Specific ablation of the nidogen-binding site in the laminin gamma1 chain interferes with kidney and lung development. Development 129, 2711–2722. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z, 2002. Stromal Effects on Mammary Gland Development and Breast Cancer. Science 296, 1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall BP, Nystrom A, Iozzo RA, Eble JA, Niland S, Krieg T, Eckes B, Pozzi A, Iozzo RV, 2008. Integrin alpha2beta1 is the required receptor for endorepellin angiostatic activity. J. Biol. Chem. 283, 2335–2343. [DOI] [PubMed] [Google Scholar]
- Xiong A, Kundu S, Forsberg M, Xiong Y, Bergstrom T, Paavilainen T, Kjellen L, Li JP, Forsberg-Nilsson K, 2017. Heparanase confers a growth advantage to differentiating murine embryonic stem cells, and enhances oligodendrocyte formation. Matrix Biol 62, 92–104. [DOI] [PubMed] [Google Scholar]
- Yang DH, McKee KK, Chen ZL, Mernaugh G, Strickland S, Zent R, Yurchenco PD, 2011. Renal collecting system growth and function depend upon embryonic gamma1 laminin expression. Development 138, 4535–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Chen ZL, Norris EH, Strickland S, 2014. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun 5, 3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K, Yamada Y, 2013. Gene evolution and functions of extracellular matrix proteins in teeth Orthodontic waves (English ed.) 72, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, 2011. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Patton BL, 2009. Developmental and Pathogenic Mechanisms of Basement Membrane Assembly. Curr. Pharm. Des. 15, 1277–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Ruben GC, 1987. Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J. Cell Biol. 105, 2559–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]