Abstract
Symptoms of posttraumatic stress disorder include hyperarousal, avoidance of trauma-related stimuli, re-experiencing of trauma, and mood changes. This review focuses on the frontal cortical areas that form crucial links in circuitry pertinent to posttraumatic stress disorder symptomatology: (1) the conditioned fear extinction circuit, (2) the salience circuit, and (3) the mood circuit. These frontal areas include the ventromedial prefrontal cortex (conditioned fear extinction), the dorsal anterior cingulate and insular cortices (salience), and the lateral orbitofrontal and subgenual cingulate cortices (mood). Frontal lobe structural abnormalities in posttraumatic stress disorder, including volumetric reductions in the cingulate cortices, impact all three circuits. Functional analyses of frontal cortices in posttraumatic stress disorder show abnormal activation in all three according to task demand and emotional valence. Network analyses reveal altered amygdalo-frontal connectivity and failure to suppress the default mode network during cognitive engagement. Spine shape alterations also have been detected in the medial orbitofrontal cortex in posttraumatic stress disorder postmortem brains, suggesting reduced synaptic plasticity. Importantly, frontal lobe abnormalities in posttraumatic stress disorder extend beyond emotion-related circuits to include the lateral prefrontal cortices that mediate executive functions. In conclusion, widespread frontal lobe dysfunction in posttraumatic stress disorder provides a neurobiologic basis for the core symptomatology of the disorder, as well as for executive function impairment.
Keywords: conditioned fear extinction, salience, mood, magnetic resonance imaging, executive functioning, functional connectivity, dendritic spines
Posttraumatic stress disorder (PTSD) afflicts approximately 3.5% of the general population of the United States.1 By definition, PTSD is a disorder that occurs in individuals who have been exposed to a traumatic experience. A wide range of events, e.g., natural disasters, terrorist attacks, and sexual abuse, can trigger the onset of PTSD, and not surprisingly, the disorder is particularly prevalent (∼20%) in combat-exposed veterans.2–5 Approximately one third of people diagnosed with PTSD have a severe form of the disorder1 and experience debilitating symptoms that disrupt family dynamics, other social interactions, and workplace functioning.6,7 Frequently, PTSD occurs in conjunction with depressive and anxiety symptomatology and/or substance abuse.1 In addition, increased suicidal ideation and behaviors occur in association with PTSD,8 and the risk for suicide is significantly higher in military veterans with PTSD relative to the general population.9
Neurobiologic studies of PTSD have largely focused on the amygdala and hippocampus as reviewed in detail elsewhere.10,11 Among other functions, the amygdala mediates emotion-related processing, including fear conditioning and extinction, while the hippocampus plays an important role in providing contextual memory for emotion-related processes.12–15 Major PTSD-related alterations have been found in these regions, including hyperactivity of the amygdala in response to trauma- and non-trauma-related, emotionally charged stimuli,10,11,16–18 although the findings are not entirely consistent. Smaller hippocampal volume in association with PTSD is perhaps the most replicated pathologic correlate of the disease,19–21 whereas both hippocampal activation and deactivation have been reported in PTSD during exposure to emotion-laden stimuli or during performance of memory tasks.10,22
To examine the role of frontal lobe in PTSD, an internet search was conducted using the terms “PTSD” and “MRI” and encompassing the years 2000–2018. Note that a few seminal articles published earlier are included as these were referenced multiple times in the more recent literature. Studies were included if they reported a structural or functional comparison between individuals diagnosed with PTSD and a comparison group of either trauma-exposed or trauma-naïve participants. Studies of PTSD in which participants were children or adolescents were excluded. In addition, only functional connectivity studies that shed further light on the structural and functional magnetic resonance imaging (MRI) findings in the frontal lobe have been included.
This review spotlights the role of the frontal cortex in PTSD in order to further explore the neurobiologic substrates of PTSD dysfunction. We focus on three frontal lobe circuits that have been identified as important to understand the PTSD symptomatology17,23,24: (1) the conditioned fear extinction circuit, (2) the salience circuit, and (3) the mood circuit—and review the literature on morphologic and functional alterations in the frontal lobe nodes of these circuits. Our goal is to forge a link between the burgeoning literature on neuroimaging findings in PTSD and known functional circuits in the frontal lobe to better understand the neurobiologic underpinnings of the disease.
Fear Conditioned Extinction Circuitry
It has been hypothesized that the circuitry of fear conditioning and fear conditioned extinction are intimately involved in the pathology of PTSD.25,26 Fear conditioning, which is the pairing of a negative, unconditioned stimulus (UC) to a neutral, conditioned stimulus (CS), is critically mediated by the basolateral amygdala.12 In PTSD, the unconditioned negative stimulus is the traumatic event, and the conditioned stimuli are the sights, sounds, and other sensory experiences that occur concurrently with the event. In normal subjects, physiological responses, such as visceral, cardiac, visual, and auditory sensations, that are associated with fear re-experiencing are gradually “extinguished” by an active re-learning process. In PTSD, a failure to extinguish these fear responses contributes to the persistent physical and cognitive symptoms of re-experiencing the trauma, including increased autonomic arousal and phobic behaviors.25 Importantly, extinction of conditioned fear is achieved by the formation of new memories, not by erasure of the initial fear conditioned association.27,28 Furthermore, although initial extinction is a function of the basolateral amygdala,29 memory or recall of extinction is modulated by a circuit involving the ventromedial prefrontal cortex (vmPFC), a cortical region equivalent to the infralimbic cortex in rodents.14,17,28,30 The human vmPFC encompasses the subgenual anterior cingulate cortex (sgACC, BA25), the rostral anterior cingulate cortex (rACC, BA32), the medial prefrontal cortex (mPFC, BA10), and the medial orbitofrontal cortex (mOFC, BA11). In particular, the sgACC has been implicated in human studies of retention of fear extinction.30
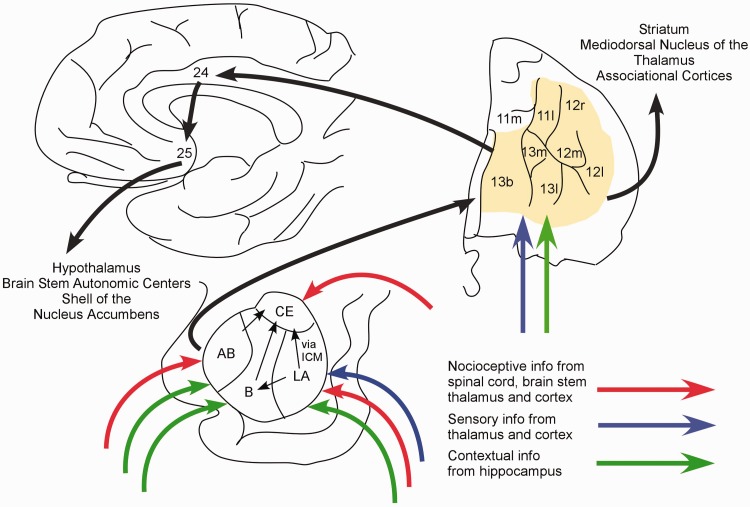
The amygdalar circuitry involved in fear conditioning is located primarily in the basolateral amygdala (Figure 1).12,31,32 Prominently involved regions include the lateral (LA), basal (B), accessory basal (AB), and central (CE) nuclei as well as the intercalated cell masses (ICM).32 Nocioceptive information reaches LA, AB, and CE through the spinal cord, brain stem, thalamus, and cortex. Neutral sensory information, e.g., from the auditory thalamus and cortex, is conveyed mainly to LA. Contextual information from the hippocampus is transmitted to LA, as well as to B and AB. Thus, the convergence of UC (nocioceptive input) with CS (other sensory information) and integration of UC with contextual information involves LA, B, and AB. During fear conditioning, LA disinhibits the output of CE neurons, although LA projects only indirectly to CE via ICM or B.33 As the major output nucleus of the basolateral amygdala, CE projections mediate the entire panoply of fear behaviors. CE stimulates hypothalamic and brain stem regions that govern blood pressure control, respiration, freezing behavior, and the release of adrenal stress hormones and projects to monoaminergic nuclei that may alter perception and cognition via thalamic and cortical modulation.12,31
Figure 1.
Conditioned fear extinction circuitry. Fear conditioning is mediated in the basolateral amygdala where nocioceptive information is integrated with auditory and visual sensory information, as well as contextual information from the hippocampus. Fear conditioned responses are enacted via efferents to the hypothalamus and brain stem. Extinction of fear conditioning involves new learning in the vmPFC (shaded area), including cingulate ((Brodmann area (BA)25, BA32)) and orbitofrontal cortices (BA10 and BA11), and projections of the vmPFC to the amygdala to curtail the fear conditioned output of the amygdala. Frontal area designations according to Ongur and Price.38 AB: accessory basal nucleus; B: basal nucleus; CE: central nucleus; ICM: intercalated cell masses; LA: lateral nucleus.
Although the acquisition of the initial stages of conditioned fear extinction occurs within the basolateral amygdala with minimal requirement for additional input, the long-term retention of fear conditioned extinction requires participation of vmPFC-amygdala connections in humans (the infralimbic cortex in rodents).28,34 The vmPFC receives glutaminergic input from the mediodorsal nucleus of the thalamus, the hippocampus, the basolateral amygdala, and the auditory association cortex. It is hypothesized that exposure to the CS without the US activates glutaminergic inputs to the vmPFC, resulting in long-term potentiation at one or more of these synapses.28 In turn, the vmPFC projects to the amygdala resulting in depressed CE output, perhaps through the activation of ICM inhibitory neurons.28
Salience Circuitry
Salience circuitry (Figure 2) tags sensory information with emotional valence, directs attention to salient stimuli, and governs motor and visceral responses to such stimuli.35–37 Functional imaging analyses in human subjects have identified the dorsal anterior cingulate cortex (dACC, BA24) and fronto-insular cortex, as designated by Ongur and Price,38 as important links in the “salience network” as defined by functional analyses.39 These same areas appear to be involved in visceral self-awareness, such as the perception of heart rate, suggesting that visceral awareness contributes to evaluation of salience.36 Visceral afferents are relayed through the fronto-insular cortex to the dACC.38 Hyperactivity of the dACC and its associated salience network in PTSD has been postulated to contribute to hypervigilance and inappropriate reactivity to neutral stimuli in PTSD subjects.40
Figure 2.
Salience circuitry. Emotional tagging of sensory information occurs in part in the basolateral amygdala where sensory inputs from the thalamus and cortex are integrated with visceral afferents from autonomic centers. The amygdala relays information to the dorsolateral anterior cingulate cortex (dACC, BA24, shaded area) directly and indirectly through the thalamus and fronto-insular cortices (BA13, Iam, Iapm, Ial; shaded area); the insular cortex, especially Ia, also receives direct visceral input. Widespread efferents from the dACC then imbue salience to cognitive and motor circuitry through output projections to the striatum, thalamus, and associational cortices. Endocrine and visceral responses are conveyed through reciprocal projections to the amygdala and to subgenual anterior cingulate cortex (sgACC, BA25), an area with projections to the hypothalamus and brain stem. Frontal area designations according to Ongur and Price.38 AB: accessory basal nucleus; B: basal nucleus; CE: central nucleus; ICM: intercalated cell masses; LA: lateral nucleus.
Classically, the dACC has been considered part of the Papez circuit, linking medial temporal, thalamic, and cortical areas in a network for emotional processing.41 The dACC has both direct and indirect amygdala afferents, with the latter relayed through the anterior medial nucleus of the thalamus,42,43 establishing the dACC as an important structure in the mediation of emotion-guided behavior. The emotional tagging of inputs likely occurs in the amygdala through the integration of somatic sensory input with visceral input from the autonomic system.44 Nuclei in the basolateral amygdala, in particular B and AB, relay this viscerally tagged sensory input to the dACC.42 The dACC, in turn, is uniquely positioned to influence somatic motor, endocrine, and visceral autonomic output through projections to cortical and striatal areas, to the amygdala, and to the periaqueductal gray matter and the sgACC.45–47 The dACC itself may impart emotional salience on higher order cognitive processing via links to the mediodorsal nucleus of the thalamus, as well as connections with the dorsolateral prefrontal cortex (dlPFC) and posterior cingulate and parietal cortices.45,48,49
Mood Circuitry
Altered mood is an important aspect of PTSD that may be mediated in part by orbitofrontal and medial cortical networks (Figure 3). Postmortem and neuroimaging studies of major depressive disorder have identified the OFC, dlPFC, and cingulate cortices as pathologic sites in depression.50,51 Emphasis has been placed on the sgACC as a critical node and a target for deep brain stimulation treatment of the disorder.51 The lateral OFC is important in integrating multi-sensory inputs related to food intake with emotional tagging from the amygdala.38,52 Thus, decision-making that is guided by emotion and the reward properties of a stimulus is thought to be a core function of orbital areas. In accordance with this functional role, the OFC projects to a ventromedial diagonal swath of the rostral caudate and putamen, notably the same striatal region that receives input from the dACC and a striatal domain that has been associated with reward and motivation.47,53,54 Although the sgACC receives limited direct projections from the dlPFC, OFC, and medial frontal cortices,55 robust connectivity between the sgACC and dACC, an area that is densely reciprocally connected with the dlPFC and OFC, may facilitate the integration of emotion-driven sensory experiences with higher order cognitive processing. The sgACC is a major hub in the control of autonomic and endocrine function via projections to the brain stem and hypothalamus.45,46 In addition, the sgACC projects into the ventral striatopallidal loop, specifically the shell of the nucleus accumbens, which is important for goal-directed behavior, behavioral sensitization, and altered affective states.47 Notably, the shell has extensive projections to midbrain dopamine neurons through which the sgACC can indirectly influence multiple cortical and striatal systems via dopaminergic modulation.54
Figure 3.
Mood circuitry. The lateral orbital frontal cortex (OFC, shaded area) receives input from virtually all senses, including those related to food intake such as gustatory and olfactory information. These sensory inputs are integrated with limbic input from the amygdala and hippocampus that impart emotional and contextual importance. The OFC influences cognitive and motor activity through outputs to the striatum, thalamus, and associational cortices as well as reciprocal connections with the amygdala and hippocampal formation. The OFC also relays information via dorsal anterior cingulate cortex (dACC, BA24) to the subgenual anterior cingulate cortex (sgACC, BA25), a major output node of the mood network that influences the autonomic system via the hypothalamus and brain stem and the reward system via the ventral striatum. Frontal area designations according to Ongur and Price.38 AB: accessory basal nucleus; B: basal nucleus; CE: central nucleus; ICM: intercalated cell masses; LA: lateral nucleus.
Structural and Functional Abnormalities in PTSD
MRI has been used to examine structural and functional changes in the frontal lobe associated with PTSD relative to trauma-exposed and trauma-naïve comparison groups (Tables 1 to 3). The frontal lobe circuitry that has been theoretically implicated in conditioned fear extinction features prominently in PTSD neuroimaging findings. For example, ample evidence implicates the vmPFC, the frontal region critical to extinction, in abnormalities associated with PTSD. Smaller volume and reduced cortical thickness of rACC and sgACC have been observed in PTSD.56–67 The altered activity patterns observed in the vmPFC and dmPFC in PTSD during exposure to negative imagery, which include both increases and decreases in activation, suggest that medial prefrontal cortical regions are also functionally impaired.16,40,68–82 Furthermore, when participants with PTSD engage in cognitive tasks in the presence of negative distractors, cognitive conflict, or attentional demands, abnormalities in functional activation of vmPFC emerge.78,83–92 Most notably, the vmPFC is overactive in PTSD during fear conditioning93 yet under-active during fear conditioned extinction.94 Increased functional connectivity between the amygdala and the vmPFC has been observed in the resting state in PTSD.95,96 During exposure to fearful emotional faces, personal trauma scripts, or triggered intense autobiographical memories, increased functional connectivity between the amygdala and the vmPFC has been reported in PTSD.97–99 These findings may indicate greater amygdalar influence over the frontal cortex in mediation of fear extinction in PTSD. Note, however, that one study found decreased functional connectivity between the amygdala and the vmPFC when participants with PTSD viewed emotional faces.100
Table 1.
Frontal lobe structural MRI findings in PTSD.
| References | PTSD | Controls | Gender | Trauma | Methods | Findings | BA |
|---|---|---|---|---|---|---|---|
| Rauch et al.56 | 9 | 9 TE | F | Combat nursing | ROI | Smaller vol rACC, sgACC | 32, 25 |
| Yamasue et al.104 | 9 | 16 TE | M, F | Subway sarin | VBM/SPM | Smaller dACC | 24 |
| Corbo et al.57 | 14 acute | 14 TN | M, F | Unspecified civilian | VBM and ROI | VBM: smaller vol rACC, dACC, insula ROI: no difference in rACC, dACC shape changes explain discrepancy | 32, 24 |
| Chen et al.105 | 12 | 12 TE | M, F | Fire | VBM/SPM | Smaller dACC, insula | 24 |
| Kitayama et al.58 | 8 | 13 TN | M, F | Abuse | ROI | Smaller rACC + dACC no difference dACC alone | 32/24 |
| Hakamata et al.101 | 14 | 100 TE 70 TN | F | Breast cancer | VBM | vs. TN, TE, smaller mOFC | 11 |
| Bryant et al.59 | 13 | 13 TE 13 TN | M, F | Mixed civilian | VBM | Smaller vol rACC in PTSD non-responsive to CBT | 32 |
| Geuze et al.114 | 25 | 25 TE | M | Combat | ROI | Reduced cortical thickness, dlPFC, vlPFC | 9, 8, 46, 45, 44, 47 |
| Kasai et al.60 | 18 | 18 TN twins 23 TE 23 TN twins of TE | M | Combat | VBM/SPM | vs. all controls, smaller vol rACC vs. TE, smaller anterior and middle insula | 32 |
| Felmingham et al.61 | 21 | 17 TE | M, F | Mixed civilian | VBM/SPM | Smaller rACC, dACC, dmPFC, OFC | 32, 24 NS |
| Eckart et al.106 | 20 | 19 TE 13 TN | M | Refugee | ROI | vs. TN, smaller dACC | 24 |
| Chen et al.107 | 10 acute | 10 TE 20 TN | M,F | Coal mine flood | VBM/SPM | Smaller dACC | 24 |
| Herringa et al.62 | 13 | 15 TE | M | Combat | VBM | Smaller sgACC, insula, dmPFC | 25, 10 |
| Rocha-Rego et al.63 | 16 | 16 TE | M, F | Urban violence | VBM | Smaller rACC, dlPFC | 32, 6 |
| Bing et al.64 | 20 | 20 TN | M, F | Motor vehicle | FreeSurfer | Reduced cortical thickness rACC, vmPFC, vlPFC | 32, 10, 45 |
| Chao et al.108 | 34 vets | 43 TE 75 TN 34 rPTSD | M, F | Combat | FreeSurfer | vs. TN, rPTSD: smaller caudal dACC, insula vs. TE, smaller caudal dACC | 24 |
| Nardo et al.65 | 15 | 17 TE | M, F | Occupational | VBM | Smaller rACC, dlPFC | 32, 46 |
| Baldacara et al.109 | 32 | 32 TE | M, F | Urban violence | FreeSurfer | Smaller rACC, dACC | 32, 24 |
| Chalavi et al.110 | 16 | 28 TN | F | Interpersonal | FreeSurfer | Smaller frontal lobe, insula | |
| Mueller et al.66 | 40 | 45 TE | M | Combat | FreeSurfer | Reduced cortical thickness rACC, | 32 |
| Sussman et al.115 | 23 | 24 TE | M | Combat | Lobe-based and vertex-based | Reduced cortical thickness dlPFC vlPFC | NS |
| O'Doherty et al.67 | 25 | 25 TN 25 TE | M,F | Unspecified | VBM | Smaller dACC, rACC, mOFC vmPFC, dlPFC, insula | 24, 32, 11 10, 9, 46, 8 13, 14 |
F: female; M: male; NS: not specified; OFC: orbitofrontal cortex; TE: trauma-exposed control; TN: trauma-naïve control; ROI: region of interest; rPTSD: remitted PTSD; SPM: statistical parametric mapping; VBM: voxel-based morphology; PTSD: posttraumatic stress disorder; MRI: magnetic resonance imaging.
Table 3.
Frontal lobe functional connectivity findings in PTSD.
| References | PTSD | Controls | Gender | Trauma | Task | Method/seed regions | Functional connectivity (seed to target) |
|---|---|---|---|---|---|---|---|
| Gilboa et al.97 | 10 | 10 TE | M,F | Road or work accidents | Personal neutral and trauma scripts | PET/amyg/BA10 | For neutral scripts, incr corr BA10 to BA24, BA25 For trauma scripts, incr corr amyg to BA24,BA25 |
| Simmons et al.113 | 15 | 15 TN | F | Intimate partner | Cued anticipation of negative images | fMRI/ant insula/ant and middle insula | decr corr ant insula to amyg decr corr ant and middle insula to amyg |
| Bluhm et al.122 | 17 | 15 TN | F | Early life | Resting state | fMRI/BA31- precuneus/BA32 | decr corr PCC-precuneus to BA10, BA11 BA6,BA8, BA9 decr corr BA32 to PCC, mPFC (BA NS) |
| Daniels et al.102 | 12 | 12 TN | NS | NS | N-back working memory | fMRI/BA32/BA31 | For working memory vs. control condition, incr corr PCC to BA10, BA19, BA37 incr corr BA32 to hippo, BA20, BA35 decr corr PCC to BA8, BA9, BA10, BA22, BA31, BA23, BA30 decr corr BA32 to BA6, BA11 |
| Fonzo et al.98 | 12 | 12 TN | F | Intimate partner violence | Emotional face matching | fMRI/amyg BA24/insula | For fearful relative to happy faces, incr corr amyg to BA25 decr corr amyg to insula decr corr BA24 to ant insula incr corr BA24 to post insula decr corr insula to amyg |
| St. Jacques et al.99 | 15 | 14 TN | M,F | NS | Auditory cue- triggered AM | fMRI/vmPFC | For intense positive AMs, decr corr vmPFC (BA NS) to amyg For intense negative AMs, incr corr vmPFC (BA NS) to amyg |
| Sripada et al.95 | 15 | 14 TE | M | Combat | Resting state | fMRI/amyg | incr corr amyg to insula, BA32 decr corr amyg to BA47 |
| Chen and Etkin123 | 17 | 38 TN 39 GAD | M,F | NS | resting state | fMRI/ ant hippo/ post hippo | vs. TN, decr corr ant hippo to BA24, BA6 decr corr post hippo to BA32 vs. GAD, decr corr post hippo to BA32 |
| Stevens et al.100 | 20 | 20 TE | F | Civilian | Emotional faces | fMRI/amyg | For fearful vs. neutral faces, decr corr amyg to BA25, BA11 incr corr amyg to BA9 |
| Brown et al.96 | 20 | 22 TE | M,F | Combat | Resting state | fMRI/BLA/CMA | incr corr BLA to BA32, BA8, BA10, BA24 decr corr BLA to vlPFC (BA NS) |
| Zhu et al.103 | 27 21 coMDD | 37 TE | M,F | Mixed civilian | Resting state | fMIR/BLA/CMA/NAcc | For PTSD + PTSD-coMCC vs. TE, decr corr BLA to OFC (BA NS) decr corr CMA to thal For PTSD-coMDD vs. TE, decr corr BLA to OFC (BA NS) decr corr NAcc to thal, hippo |
act: activity; amyg: amygdala; ant: anterior; anti-corr: anti-correlation compared to controls; BA: Brodmann area; BLA: basolateral amygdala; CMA: centromedial amygdala; coMDD: comorbid for major depressive disorder; corr: correlation compared to controls; CVA: canonical variates analysis; decr: decreased; F: female; fMRI: functional magnetic resonance imaging; GAD: generalized anxiety disorder; hippo: hippocampus; incr: increased correlation compared to controls; M: male; rACC: rostral anterior cingulate cortex; NAcc: nucleus accumbens; NS: not specified; SCR: skin conductance response; PCC: posterior cingulate cortex; PET: photon emission tomography; TE: trauma-exposed controls; thal: thalamus; vlPFC: ventrolateral prefrontal cortex; vmPFC: ventromedial prefrontal cortex; vs.: versus.
Structural and functional changes in the mOFC have also been reported in PTSD. In women who developed PTSD symptomatology after breast cancer surgery, the BA11 region of the OFC was smaller than that of trauma-naive controls or resilient cancer patients, and the deficits were directly correlated to the greater elevation of PTSD symptoms.101 Because cancer- and surgery-related PTSD is a less common and sometimes controversial form of PTSD, these findings require replication in another PTSD cohort. Diminished activity in the mOFC in PTSD has been observed during trauma-related and emotionally valenced exposures.40,74,76 The mOFC is also under-activated during the performance of a memory task when emotionally laden stimuli are employed.84 Moreover, altered functional connectivity of the mOFC with other frontal regions during executive task performance has been associated with PTSD.124,102 Resting state functional connectivity between the mOFC and the basolateral amygdala is decreased by 50% in PTSD, and connectivity is inversely associated with PTSD symptoms.103 Functional connectivity between the amygdala and the mOFC is also decreased during viewing of emotion-evoking faces.100 Finally, a postmortem analysis of spine density in the mOFC uncovered a shift in spine populations, resulting in a greater density of stubby spines in brains from individuals with a diagnosis of PTSD subjects compared to those without.125 These findings suggest that neuroplasticity may be altered in frontal circuitry related to emotion processing in PTSD.
Salience circuitry is also prominently impacted in PTSD. For starters, there is near uniform consensus that dACC volume is diminished in PTSD.57,61,104–109 Meta-analyses have confirmed findings of smaller dACC volume in people diagnosed with PTSD.19,126–128 Reduced volume of the insular cortex has also been observed in PTSD.57,60,62,105,108,110 Functionally, altered activation of the dACC and insular cortex in PTSD occurs during exposure to negative imagery.40,71,75,76,79–82 During executive task performance, participants with PTSD exhibit abnormal activity in the dACC,85,88,89,94,111 as well as in the insula.90,112 In the resting state, increased connectivity of the dACC and the insula with the amygdala has been observed in PTSD,95,96 perhaps indicating an increased relay of emotionally valenced information to frontal nodes of the salience circuit. Likewise, amygdala connectivity with the dACC is enhanced in PTSD participants when listening to trauma scripts.97 However, during performance of emotion-laden tasks, diminished connectivity among nodes of the salience circuit, i.e., the amygdala, the dACC, and the anterior insula, has been observed.98,113 Thus, the opposite directionality in findings may indicate that, in PTSD, salience circuitry is overactive at rest and underactive during some emotionally charged situations.
With regard to mood circuitry, the sgACC is implicated in PTSD pathology by findings of decreased volume of the sgACC56,62 and altered activation of the sgACC during exposure to negative imagery.68,70,71 During performance of cognitive tasks that include emotionally valenced stimuli, decreased activity has been observed in the sgACC in PTSD participants84,85,89; in contrast, increased sgACC activity has been found during fear conditioning in PTSD.93 When PTSD participants listen to personal trauma scripts, increased functional connectivity between the amygdala and the sgACC has been described.97 When fearful faces are viewed, decreased connectivity between the amygdala and the sgACC has been reported.100 However, in a matching task that utilizes emotional faces, increased amygdala connectivity with the sgACC was found.98 From a more general perspective, alterations in the structural integrity of the cingulum bundle, the uncinate fasciculus, and other frontal tracts129–135 lend further support for the premise that impoverished connectivity in frontal lobe circuitry may underlie important aspects of behavioral disturbance in PTSD.136
Although the preponderance of evidence supports a role of the emotion-related circuitry in the frontal lobe in PTSD, some findings suggest that PTSD impacts the frontal regions associated with cognitive and executive functioning. For example, structural neuroimaging findings largely implicate the cingulate cortices and insula (Table 1); however, several studies have found smaller volume or reduced cortical thickness in individuals diagnosed with PTSD in the lateral prefrontal cortices.63,65,67,114,115 Likewise, functional neuroimaging studies have found changes in activity in the dlPFC and vlPFC in response to negative stimuli68,71,72,75,76,78,116; altered activity has even been reported in the motor cortex.126,117 Perhaps not surprisingly, abnormal activity patterns in the dlPFC and vlPFC are most often observed in participants with PTSD when they engage in cognitive tasks, particularly those involving working memory.78,84–86,88–91,118–121 One particularly enlightening study found that participants with PTSD fail to activate portions of the executive network, including the dlPFC, during a task requiring working memory.88 At the same time, these same participants show a pattern of hyperactivity in the executive network while performing a memory-independent task that normally does not activate the dlPFC.88 Thus, growing evidence suggests that frontal lobe disturbances in PTSD extend beyond those circuits most directly linked to emotion processing. It is well established that optimal cognitive performance is dependent on emotional status, including low stress levels, appropriate attentional focus, and emotional well-being. Indeed, the interdependence of cognition and emotional processing reflects the degree of connectivity of emotion-processing and cognitive circuits; an example is the central role that the dACC plays in salience and cognitive processing by virtue of dACC connections with the dlPFC and other higher order cortical areas.45,47–49 This relationship is further illustrated by the finding that poor neuropsychological performance in participants with PTSD has been associated with reduced functional connectivity between emotion circuitry (rACC and dmPFC) and the executive network (vlPFC).137 The implication of this observation is that disturbances in emotion-laden processing will have ripple effects that result in the deterioration of high-level cognitive processing.
Overall, the findings of altered activity in PTSD highlight the role of frontal lobe circuitry in PTSD dysfunction. However, there are notable inconsistencies among studies that may be due to methodologic factors. Certainly, parameters vary greatly among PTSD studies in terms of the cohorts examined, specifically in the type and recency of trauma exposure (Tables 1 to 3). Beyond simply the trauma exposure category, there are important differences among studies in cohort subtypes, emotional stimulus protocols, and the composition of the comparison group. With regard to cohort subtype, Lanius et al.74–76 examined the activity profile of PTSD participants associated with trauma-driven scripts and found decreased activity in the rACC and medial OFC in PTSD participants of non-specified subtype, whereas a subsequent study found enhanced activity in widespread regions in those with the dissociative subtype of PTSD (Table 2). These studies suggest that hyperarousal and dissociative subtypes of PTSD may have different underlying abnormalities in functional activation, thus in part accounting for the discrepancies among various studies. The results of a meta-analysis point to the possibility that the personal significance of the negative stimuli may also impact brain activation patterns in PTSD. Specifically, participants with PTSD were found to have greater activity in the dACC and decreased activity in widespread areas (BA11, BA10, BA46, and BA44) in response to trauma-related exposure, while negative stimuli that were not related to trauma evoked hyperactivity in the dlPFC (BA6 and BA8) and hypoactivity in the rACC and dmPFC (BA9).40 Finally, whether participants in the comparison group are trauma-exposed or trauma-naive could contribute to the diverse findings, and indeed, some studies found differential activation patterns in these two control groups.119,138 The issue highlighted here is that trauma-exposed individuals who do not develop PTSD are resilient individuals, whereas it is unclear whether trauma-naïve controls are or are not predisposed to developing PTSD because they have not experienced the triggering trauma that elicited symptoms in the PTSD group. Moreover, recent findings suggest that exposure to combat trauma has structural impacts on the brain regardless of a positive diagnosis for PTSD,139 providing further confirmation that trauma-exposed and trauma-naive groups are not equivalent.
Table 2.
Frontal lobe functional MRI findings in PTSD.
| References | PTSD | Controls | Gender | Trauma | Task | Method | Activation changes | BA |
|---|---|---|---|---|---|---|---|---|
| Negative stimuli exposure | ||||||||
| Shin et al.68 | 7 | 7 TN | M | Combat | Combat images | PET | incr sgACC decr vlPFC | 25 44, 45 |
| Bremmer et al.70 | 10 | 10 TE | M | Combat | Combat images | PET | decr sgACC | 25 |
| Bremner et al.71 | 10 | 12 TE | F | Childhood sexual abuse | Personalized trauma scripts | PET | incr dlPFC decr sgACC, dACC | 9, 6 25, 24 |
| Shin et al.72 | 8 | 8 TE | F | Childhood sexual abuse | Personalized trauma scripts | PET | incr vlPFC decr dlPFC, dmPFC | 47 46, 8, 9, 10 |
| Zubieta et al.73 | 12 | 11 TE 12 TN | M | Combat | Combat sounds | SPECT | incr mPFC | 9/10 |
| Lanius et al.74 | 9 | 9 TE | M, F | Sexual abuse/motor vehicle | Trauma-related images | fMRI | decr rACC, vmPFC | 32, 10/11 |
| Lanius et al.75 | 7 Dis | 10 TN | F (1 M TN) | Sexual abuse | Trauma-related images | fMRI | incr vlPFC, vmPFC, dmPFC, rACC, dACC | 47, 10, 9, 32, 24 |
| Lanius et al.76 | 10 | 10 TE | NS | Childhood sexual abuse or assault/adult motor vehicle | Trauma-related or neg. (sad, anxious) images | fMRI | for trauma-related, decr rACC, dACC, dmPFC, vlPFC for sad, decr rACC, dACC for anxious, decr dACC, dlPFC, vlPFC | 32, 24, 10, 11, 47 32, 24 24, 9, 46, 47 |
| Shin et al.16 | 17 | 19 TE | M, F | Combat/combat nursing | Trauma-related images | PET | decr mPFC, rACC | 10, 32 |
| Britton et al.77 | 16 | 15 TE, 14 TN | M | Combat | Emotional auto- biographical images | PET | decr rACC | 32 |
| Shin et al.69 | 13 | 13 TE | M | Combat/firefighting | Fearful faces | fMRI | decr rACC, vmPFC, dmPFC | 32, 10, 9 |
| Hou et al.78 | 10 acute | 7 TE | M | Coal mining accident | Trauma-related images | fMRI | decr rACC, dmPFC, vlPFC | 32, 10, 45 |
| Bryant et al.79 | 15 | 15 TN | M, F | Motor vehicle/assault | Masked fearful faces | fMRI | incr dmPFC, dACC | 9, 24 |
| Aupperle et al.116 | 37 | 34 TN | F | intimate partner violence | Anticipation of neg. images | fMRI | incr dlPFC, pre-central gyrus decr dlPFC, vlPFC | 44, 4, 6 46, 47 |
| Mazza et al.117 | 10 | 10 TE (all F) | M, F | Earthquake | Neg. images | fMRI | incr precentral gyrus | 4 |
| Ke et al.80 | 24 acute | 14 TE | M | Coal mining accident | Trauma-related images | fMRI | decr dmPFC, dACC | 9, 24 |
| Dahlgren et al.81 | 12 | 12 TN (twin) 14 TE 14 TN (twin of TE) | M | Combat | Non-combat trauma scripts | fMRI | vs. all controls, decr rACC dACC, mPFC | 32, 24, 8 |
| Hall et al.82 | 21 | 21 TE | M,F | Mixed civilian | Involuntary trauma recall | fMRI | incr vmPFC, dACC | 24, 10 |
| Cognitive performance | ||||||||
| Shin et al.83 | 8 | 8 TE | M | Combat | Counting Stroop with trauma-related vs. negative images | fMRI | decr rACC | 32 |
| Bremner et al.84 | 10 | 11 TN | F | Childhood sexual abuse | Recall of neg. word vs. neutral word | PET | decr vlPFC, dmPFC, sgACC, rACC | 47/11, 9, 25, 32 |
| Bryant et al.85 | 14 | 14 TN | M, F | Interpersonal assault/motor vehicle accident | Auditory oddball | fMRI | incr dACC, rACC ventral dlPFC, ventral dmPFC decr sgACC, dorsal dlPFC dorsal dmPFC | 24, 32 6, 10, 8, 9 25, 9 25, 8, 9 |
| Hou et al.78 | 10 acute | 7 TE | M | Coal mining accident | Short-term recall trauma-related images | fMRI | decr rACC, dmPFC, dlPFC | 32, 10, 45 |
| Falconer et al.111 | 23 | 23 TN 17 TE | M, F | Mixed civilian | Go/ No Go inhibition | fMRI | vs. TN, decr vlPFC, dlPFC, dACC vs. TE, decr vlPFC, precentral gyrus, dlPFC | 10, 47, 9, 24 47, 4, 6 |
| Geuze et al.86 | 12 | 12 TE | M | Combat | Paired word recall | fMRI | In encoding phase, decr dlPFC, vmPFC precentral gyrus In encoding phase, incr dlPFC In retrieval phase, decr vlPFC, precentral gyrus | 46, 10, 6 9 45, 6 |
| Kim et al.87 | 12 | 12 TN | M, F | Subway fire | Same/difference judgment with emotional conflict | fMRI | decr rACC | 32 |
| Moores et al.88 | 13 | 12 TN | M, F | mixed civilian | WM vs. memory for fixed target | fMRI | decr dlPFC, precentral gyrus, dACC, rACC | 9/46, 6, 24, 32 |
| Felmingham et al.89 | 11 | 11 TN | M, F | Assault/motor vehicle accident | Auditory oddball targets without or with SCR | fMRI | Without SCR, incr rACC, dlPFC, vlPFC with SCR, decr sgACC, vlPFC, incr dlPFC, dACC | 32, 8, 6, 45 25, 44 8, 9, 24 |
| Morey et al.118 | 22 | 20 TE | M,F | Combat | WM with trauma-related distractors | fMRI | During delay, incr dlPFC | 47 |
| New et al.119 | 14 | 14 TE | F | Sexual abuse | Effortful modulation of emotion to neg. images | fMRI | decr dlPFC vs. TN in diminish condition vs. TN, TE in enhance condition | 6, 9 |
| Whalley et al.112 | 16 | 16 TE 16 MDD | M,F | Mixed civilian/combat | Recall of objects on neg., pos., or neutral images | fMRI | Across all conditions, decr dlPFC for neg. vs. neutral images, incr dlPFC for pos. vs. neutral images, incr precentral gyrus, insula | 46 86 4 |
| Bruce et al.90 | 32 | 21 TN | F | Interpersonal | Attention with fearful faces as distractors | fMRI | incr insula, rACC, dmPFC, dlPFC | 13, 32, 10, 9, 8 |
| Fani et al.91 | 18 | 19 TE | F | Mixed civilian | Attention bias with fearful faces | fMRI | incr dlPFC, vmPFC, | 46, 10 |
| Blair et al.120 | 14 | 15 TE 19 TN | M,F | Mixed civilian | Stroop with neg., pos., or neutral distractors | fMRI | For neg. and pos. distractors, decr dlPFC | 9 |
| Offringa et al.92 | 17 | 18 TE | M, F | Mixed civilian | Stroop with fearful faces | fMRI | decr rACC | 32 |
| Rabinak et al.121 | 21 | 21 TE | M | combat | Regulation of neg. emotion | fMRI | decr dlPFC | 46 |
| Fear conditioning/ extinction | ||||||||
| Milad et al.94 | 16 | 15 TE | M,F | Mixed combat and civilian | Fear conditioned extinction | fMRI | In extinction recall phase, decr vmPFC incr dACC | 10 24 |
| Grupe et al.93 | 16 | 17 TE | M | Combat | Fear conditioning | fMRI | incr sgACC, vmPFC | 25, 10 |
BA: Brodmann area; BOLD: blood oxygenation level-dependent; dACC: decr, decreased compared to controls; Dis: dissociative subtype; dACC: dorsal anterior cingulate cortex; dmPFC: dorsomedial prefrontal cortex; dlPFC: dorsolateral prefrontal cortex; vlPFC: ventrolateral prefrontal cortex; F: female; fMRI: functional magnetic resonance imaging; incr: increased compared to controls; M: male; MDD: major depressive disorder; neg.: negative; NS: not specified either in text or illustrations; pos.: positive; rACC: rostral anterior cingulate cortex; SCR: skin conductance response; SPECT: single photon emission tomography; TE: trauma-exposed controls; TN: trauma-naïve controls; vs.: versus; WM: working memory.
Default Mode Network Connectivity in PTSD
In recent years, the role of large-scale networks in normative cerebral function and in the dysfunction associated with psychiatric illness has come to the forefront.140,141 One such network is the default mode network, which includes prominently the posterior cingulate, parietal, and temporal cortices, and like the salience and central executive networks, the default mode network also encompasses frontal regions (BA10, sgACC, rACC, BA9, and dACC).142 The default mode network was originally described as a “ground state” of brain oscillations such that connectivity within the default mode network needed to be overridden before other circuits were activated.142 More recent analyses indicate that the default mode network is engaged in internal mentation, self-referential, and social processes.143
A “triple network hypothesis” has been advanced to suggest that aberrant functioning of the default mode, salience network, and central executive network may constitute a common pathologic mechanism underlying a wide range of neuropsychological disorders.140 With regard to PTSD, some have suggested that activity in the default mode and executive networks is suppressed by overactivity in the salience network.144,145 Several studies of functional connectivity indicate that the default mode network may be compromised in PTSD, and in particular anterior frontal portions of the network show a weakening that correlates with increased PTSD symptom severity.122,123,146,147 Furthermore, altered connectivity between the amygdala and nodes of the default mode network (rACC) and the salience network (dACC) has been described in PTSD.95 Taken together, these studies suggest that processes related to non-task-specific mentation may be abnormal in PTSD.
Limitations
This review provides an overview of structural and functional abnormalities associated with PTSD in the context of frontal lobe circuitry that has been implicated in the disorder. One limitation of the study is that the literature reviewed may not be comprehensive. Although we attempted to include all relevant studies published between the years 2000 and 2018, it is possible that pertinent studies were missed in the literature search. In addition, this review does not assign greater weight to studies that are methodologically stronger, as for instance studies having large numbers of participants. Finally, only the functional connectivity studies most pertinent to the discussion of frontal lobe structural and functional neuro-imaging findings are included in this review. Nonetheless, we have made every effort to include literature that is representative of the field.
Moreover, this review does not address the important question of whether alterations in frontal lobe structure and function are present before the trauma exposure that results in PTSD. If abnormalities appear before trauma exposure, these alterations may predispose individuals to develop PTSD, whereas abnormalities that appear after onset of the disorder reflect pathologic changes associated with the disorder. Additional studies that examine individuals before and after trauma exposure, e.g., soldiers before and after combat deployment, are needed to address this issue.
Clinical Implications
Greater understanding of the frontal circuitry impacted in PTSD could be informative for targeting treatment of the disorder with transcranial magnetic stimulation (TMS). For example, in participants with comorbid PTSD and major depressive disorder, the strength of functional connectivity between specific regions, i.e., sgACC and the default mode network, correlates with the response to TMS stimulation of the dlPFC.148,149 Network analyses have also been useful in predicting effective psychotherapies for PTSD, including cognitive remediation, mindfulness-based stress reduction, and biofeedback.149 The focus of this review on frontal lobe disturbances in activity and connectivity in PTSD may also facilitate development of new pharmacologic therapies to target disruptions in cortical connectivity, much as recent discovery of the novel antidepressant properties of ketamine have been validated by clinical study of glutamate signaling and prefrontal functional connectivity.150–152
Concluding Remarks
Without question, the neuroimaging of individuals diagnosed with PTSD has provided valuable insight into PTSD-related alterations in frontal lobe structural integrity and functional activity. Volumetric deficits and altered activity patterns have been observed in the emotion-processing circuitry related to fear conditioned extinction, salience, and mood in PTSD, as might be expected given the symptomatology of PTSD. Yet, it is important to note that structural and functional abnormalities extend to frontal regions that mediate cognitive and executive functioning, areas that generally have not been theoretically implicated in PTSD. We hypothesize that the disturbed functioning of emotional circuitry impacts executive networks that mediate critical functions, such as rational thinking, inhibitory control, and working memory. Moving forward, postmortem analyses of PTSD brains have the potential to probe the cellular bases of these abnormalities and deepen our understanding of PTSD pathophysiology and the mechanisms underlying this debilitating condition. The establishment of the National PTSD Brain Bank153 (https://www.research.va.gov/programs/tissue_banking/ptsd/default.cfm) has been instrumental in providing access to quality PTSD brains for ongoing research efforts in a number of laboratories, including our own. Our group is examining spine densities on neurons in the sgACC, mOFC, and dACC in PTSD and control brains and simultaneously assessing genetic and epigenetic signatures of these selected brain regions. This work and the work by others will lead to a clearer picture of the pathological substrates of PTSD and aid in developing strategies for preventing this disease, as well as novel therapies for treatment.
Acknowledgment
The authors thank Dr. Leisa Glantz for careful editing of this manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Defense and Veterans Affairs (5101CX001245). The funding source was not involved in interpretation of data or writing of the report. This manuscript is the result of work supported with resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT, Central Texas Veterans Health Care System, Temple, TX and the Durham VA Medical Center, Durham, NC. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the VA or the United States government.
References
- 1.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seal KH, Metzler TJ, Gima KS. Bertenthal D, Maguen S, Marmar CR. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2000–2008. Am J Public Health 2009; 99: 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson LC. A systematic review of probably posttraumatic stress disorder in first responders following man-made mass violence. Psychiatry Res 2015; 229: 21–26. [DOI] [PubMed] [Google Scholar]
- 4.Ramchand R, Rudavsky R, Grant S. Tanielian T, Jaycox L. Prevalence of, risk factors for, and consequences of posttraumatic stress disorder and other mental health problems in military populations deployed to Iraq and Afghanistan. Curr Psychiatry Rep 2015; 17: 37. [DOI] [PubMed] [Google Scholar]
- 5.Vasterling JJ, Aslan M, Proctor SP, et al. Longitudinal examination of posttraumatic stress disorder as a long-term outcome of Iraq war deployment. Am J Epidemiol 2016; 184: 796–805. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed Washington, DC: American Psychiatric Publishing, 2013. [Google Scholar]
- 7.Pai A, Suris AM, North CS. Posttraumatic stress disorder in the DSM-5: controversy, change, and conceptual considerations. Behav Sci 2017; 7: pii: E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krysinska D, Lester D. Post-traumatic stress disorder and suicide risk: a systematic review. Arch Suicide Res 2019; 14: 1–23. [DOI] [PubMed] [Google Scholar]
- 9.Bachynski KE, Canham-Chervak M, Black SA, Dad EO, Millikan AM, Jones BH Mental health risk factors for suicides in the US Army, 2007-8. Inj Prev 2012; 18: 405–412. [DOI] [PubMed] [Google Scholar]
- 10.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann NY Acad Sci 2006; 1071: 67–79. [DOI] [PubMed] [Google Scholar]
- 11.Hughes KC, Shin LM. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev Neurother 2011; 11: 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci 1992; 15: 353–375. [DOI] [PubMed] [Google Scholar]
- 13.Bouton ME, Westbrook RF, Corcoran KA. Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry 2006; 60: 352–360. [DOI] [PubMed] [Google Scholar]
- 14.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry 2007; 12: 120–150. [DOI] [PubMed] [Google Scholar]
- 15.Linquist KA, Wager TD, Kober H. Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci 2012; 35: 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 2004; 61: 168–176. [DOI] [PubMed] [Google Scholar]
- 17.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry 2006; 60: 376–382. [DOI] [PubMed] [Google Scholar]
- 18.Badura-Brack A, McDermott TJ, Heinrichs-Graham E, et al. Veterans with PTSD demonstrate amygdala hyperactivity while viewing threatening faces: a MEG study. Biol Psychol 2018; 132: 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 2006; 30: 1004–1031. [DOI] [PubMed] [Google Scholar]
- 20.O'Doherty DC, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res 2015; 232: 1–33. [DOI] [PubMed] [Google Scholar]
- 21.Logue MW, van Rooij SJH, Dennis EL, et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry 2018; 83: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dretsch MN, Wood KH, Daniel TA, et al. Exploring the neurocircuitry underpinning predictability of threat in soldiers with PTSD compared to deployment exposed controls. Open Neuroimag J 2016; 10: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron 2016; 92: 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shalev A, Liberzon I, Marmar C. Post-traumatic stress disorder. New Engl J Med 2017; 376: 2459–2469. [DOI] [PubMed] [Google Scholar]
- 25.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 2004; 161: 195–216. [DOI] [PubMed] [Google Scholar]
- 26.Jovanovic T, Norrholm SD, Fennell JE, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res 2009; 167: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 2002; 52: 976–986. [DOI] [PubMed] [Google Scholar]
- 28.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 2006; 60: 337–343. [DOI] [PubMed] [Google Scholar]
- 29.Barad M, Gean PW, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry 2006; 60: 322–328. [DOI] [PubMed] [Google Scholar]
- 30.Phelps EA, Delgado MR, Nearing KI, LeDoux JE Extinction learning in humans: role of the amygdala and vmPFC. Neuron 2004; 43: 897–905. [DOI] [PubMed] [Google Scholar]
- 31.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 2000; 23: 155–184. [DOI] [PubMed] [Google Scholar]
- 32.Maren S, Quirk GJ. Neuronal signaling of fear memory. Nat Rev Neurosci 2004; 5: 845–852. [DOI] [PubMed] [Google Scholar]
- 33.Pare D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol 2004; 92: 1–9. [DOI] [PubMed] [Google Scholar]
- 34.Do-Monte FH, Manzano-Nieves G, Quinones-Laracuente K, Ramos-Medina L, Quirk GL Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci 2015; 35: 3607–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Sci 1999; 354: 1325–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Critchley HD, Wiens S, Rotshtein P. Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7: 189–195. [DOI] [PubMed] [Google Scholar]
- 37.Rolls ET. Functions of the anterior insula in taste, autonomic, and related functions. Brain Cogn 2016; 110: 4–19. [DOI] [PubMed] [Google Scholar]
- 38.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rat, monkey, and humans. Cereb Cortex 2000; 10: 206–219. [DOI] [PubMed] [Google Scholar]
- 39.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord 2012; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papez JW. A proposed mechanism of emotion. J Neuropsychiatry Clin Neurosci 1995; 7: 103–112. [DOI] [PubMed] [Google Scholar]
- 42.Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol 1981; 198: 121–136. [DOI] [PubMed] [Google Scholar]
- 43.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 1984; 230: 465–496. [DOI] [PubMed] [Google Scholar]
- 44.Benarroch EE. Basic Neurosciences With Clinical Applications, Philadelphia, PA: Butterworth Heinemann/Elsevier, 2006. [Google Scholar]
- 45.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 1992; 2: 435–443. [DOI] [PubMed] [Google Scholar]
- 46.Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol 2000; 421: 172–188. [PubMed] [Google Scholar]
- 47.Haber SN, Brucker JL. Cognitive and limbic circuits that are affected by deep brain stimulation. Front Biosci 2009; 14: 1823–1834. [DOI] [PubMed] [Google Scholar]
- 48.Baleydier C, Mauguiere F. The duality of the cingulate gyrus in monkey: neuroanatomical study and functional hypothesis. Brain 1980; 103: 525–554. [DOI] [PubMed] [Google Scholar]
- 49.Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 1988; 8: 4049–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 2013; 14: 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest 2009; 119: 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbas H. Connections underlying the synthesis of cognition, memory and emotion in primate prefrontal cortices. Brain Res Bull 2000; 52: 319–330. [DOI] [PubMed] [Google Scholar]
- 53.Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci 1985; 5: 776–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience 2014; 282: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M. The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex 2012; 48: 58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rauch SL, Shin LM, Segal E, et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport 2003; 14: 913–916. [DOI] [PubMed] [Google Scholar]
- 57.Corbo V, Clement MH, Armony JL, Pruessner JC, Brunet A. Size versus shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biol Psychiatry 2005; 58: 119–124. [DOI] [PubMed] [Google Scholar]
- 58.Kitayama N, Quinn S, Bremmer JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord 2006; 90: 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bryant RA, Felmingham K, Whitford TJ, et al. Rostral anterior cingulate volume predicts treatment response to cognitive-behavioral therapy for posttraumatic stress disorder. Rev Psychiatr Neurosci 2008; 33: 142–146. [PMC free article] [PubMed] [Google Scholar]
- 60.Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry 2008; 63: 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Felmingham K, Williams LM, Whitford TJ, et al. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport 2009; 20: 1402–1406. [DOI] [PubMed] [Google Scholar]
- 62.Herringa R, Phillips M, Almeida J, Insana S, Germain A. Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Res 2012; 203: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rocha-Rego V, Pereira MG, Oliveira L, et al. Decreased premotor cortex volume in victims of urban violence with posttraumatic stress disorder. PLoS One 2012; 7: e42560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bing X, Ming-Guo Q, Jing-Na Z, et al. Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Res 2013; 1490: 225–232. [DOI] [PubMed] [Google Scholar]
- 65.Nardo D, Hogberg G, Lanius RA, et al. Gray matter volume alterations related to trait dissociation in PTSD and traumatized controls. Acta Psychiatr Scand 2013; 128: 222–233. [DOI] [PubMed] [Google Scholar]
- 66.Mueller SG, Ng P, Neylan T, et al. Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatry Res 2015; 234: 194–201. [DOI] [PubMed] [Google Scholar]
- 67.O'Doherty DCM, Tickell A, Ryder W, et al. Frontal and subcortical grey matter reductions in PTSD. Psychiatry Res Neuroimag 2017; 266: 109. [DOI] [PubMed] [Google Scholar]
- 68.Shin LM, Kosslyn SM, McNally RJ, et al. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Arch Gen Psychiatry 1997; 54: 233–241. [DOI] [PubMed] [Google Scholar]
- 69.Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 2005; 62: 273–281. [DOI] [PubMed] [Google Scholar]
- 70.Bremmer JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry 1999; 45: 806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bremner JD, Narayan M, Staib LH. Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 1999; 156: 1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shin LM, McNally RJ, Kosslyn SM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry 1999; 156: 575–584. [DOI] [PubMed] [Google Scholar]
- 73.Zubieta JK, Chinitz JA, Lombardi U, Fig LM, Cameron OG, Liberzon I Medial frontal cortex involvement in PTSD symptom: a SPECT study. J Psychiatr Res 1999; 33: 259–264. [DOI] [PubMed] [Google Scholar]
- 74.Lanius RA, Williamson PC, Densmore M, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry 2001; 158: 1920–1922. [DOI] [PubMed] [Google Scholar]
- 75.Lanius RA, Williamson PC, Boksman K, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry 2002; 52: 305–311. [DOI] [PubMed] [Google Scholar]
- 76.Lanius RA, Williamson PC, Hopper J, et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry 2003; 53: 204–210. [DOI] [PubMed] [Google Scholar]
- 77.Britton JC, Phan KL, Taylor SF. Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry 2005; 57: 832–840. [DOI] [PubMed] [Google Scholar]
- 78.Hou C, Liu J, Wang K, et al. Brain responses to symptom provocation and trauma-related short-term memory recall in coal mining accident survivors with acute severe PTSD. Brain Res 2007; 1144: 165–174. [DOI] [PubMed] [Google Scholar]
- 79.Bryant RA, Kemp AH, Felmingham KL, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp 2008; 29: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ke J, Zhang L, Qi R, et al. A longitudinal fMRI investigation in acute posttraumatic stress disorder (PTSD). Acta Radiologica 2016; 57: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 81.Dahlgren MK, Laifer LM, VanElzakker MB, et al. Diminished medial prefrontal cortex activation during the recollection of stressful events is an acquired characteristic of PTSD. Psychol Med 2018; 48: 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hall SA, Brodar KE, LaBar KS. Berntsen D, Rubin DC. Neural responses to emotional involuntary memories in posttraumatic stress disorder: differences in timing and activity. Neuroimage Clin 2018; 19: 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shin LM, Whalen PJ, Pitman RK, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 2001; 50: 932–942. [DOI] [PubMed] [Google Scholar]
- 84.Bremner JD, Vythilingam M, Vermetten E, et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry 2003; 53: 879–889. [DOI] [PubMed] [Google Scholar]
- 85.Bryant RA, Felmingham KL, Kemp AH, et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry 2005; 58: 111–118. [DOI] [PubMed] [Google Scholar]
- 86.Geuze E, Vermetten E, Ruf M. de Kloet CS, Westenberg HG. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. J Psychiatr Res 2008; 42: 659–669. [DOI] [PubMed] [Google Scholar]
- 87.Kim MJ, Chey J, Chung A, et al. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. J Psychiatr Res 2008; 42: 268–277. [DOI] [PubMed] [Google Scholar]
- 88.Moores KA, Clark CR, McFarlane AC, Brown GC, Puge Am Taylor DJ Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Res 2008; 163: 156–170. [DOI] [PubMed] [Google Scholar]
- 89.Felmingham KL, Williams LM, Kemp AH. Rennie C, Gordon E, Bryant RA. Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in posttraumatic stress disorder. Psychiatry Res 2009; 173: 59–62. [DOI] [PubMed] [Google Scholar]
- 90.Bruce SE, Buchholz KR, Brown WJ, Yan L, Durbin A, Sheline YI Altered emotional interference processing in the amygdala and insula in women with Post-Traumatic Stress Disorder. Neuroimage Clin 2012; 2: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fani N, Jovanovic T, Ely TD, et al. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol 2012; 90: 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Offringa R, Handwerger Brohawn K, Staples LK, et al. Diminished rostral anterior cingulate cortex activation during trauma-unrelated emotional interference in PTSD. Biol Mood Anxiety Disord 2013; 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grupe DW, Wielgosz J, Davidson RJ, Nitschke JB Neurobiological correlates of distinct posttraumatic stress disorder symptom profiles during threat anticipation in combat veterans. Psychol Med 2016; 46: 1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009; 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sripada RK, King AP, Garfinkel SN, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci 2012; 37: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown VM, LaBar KS, Haswell CC, et al. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology 2014; 39: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gilboa A, Shalev AY, Laor L, et al. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry 2004; 55: 263–272. [DOI] [PubMed] [Google Scholar]
- 98.Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB Exaggerated and disconnected insular-amygdalar BOLD response to threat-related emotional faces in women with intimate-partner violence PTSD. Biol Psychiatry 2010; 68: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.St. Jacques PL, Botzung A, Miles A, Rubin DC Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. J Psychiatr Res 2011; 45: 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stevens JS, Jovanovic T, Fani N, et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 2013; 47: 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hakamata Y, Matsuoka Y, Inagaki M, et al. Structure of orbitofrontal cortex and its longitudinal course in cancer-related post-traumatic stress disorder. Neurosci Res 2007; 59: 383–389. [DOI] [PubMed] [Google Scholar]
- 102.Daniels JK, McFarlane AC, Bluhm RL, et al. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci 2010; 35: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu X, Helpman L, Papini S, et al. Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress Anxiety 2017; 34: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamasue H, Kasai K, Iwanami A, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A 2003; 100: 9039–9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen S, Xia W, Li L, et al. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometry study. Psychiatry Res 2006; 146: 65–72. [DOI] [PubMed] [Google Scholar]
- 106.Eckart C, Stoppel C, Kaufmann J, et al. Structural alterations in lateral prefrontal, parietal, and posterior midline regions of men with chronic posttraumatic stress disorder. J Psychiatry Neurosci 2011; 36: 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Y, Fu K, Feng C, et al. Different regional gray matter loss in recent onset PTSD and non PTSD after a single prolonged trauma exposure. PLoS One 2012; 7: e48298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chao L, Weiner M, Neylan T. Regional cerebral volumes in veterans with current versus remitted posttraumatic stress disorder. Psychiatry Res 2013; 213: 193–201. [DOI] [PubMed] [Google Scholar]
- 109.Baldacara L, Zugman A, Araujo C, et al. Reduction of anterior cingulate in adults with urban violence-related PTSD. J Affect Disord 2014; 168: 13–20. [DOI] [PubMed] [Google Scholar]
- 110.Chalavi S, Vissia EM, Giesen ME, et al. Similar cortical but not subcortical gray matter abnormalities in women with posttraumatic stress disorder with versus without dissociative identity disorder. Psychiatr Res Neuroimag 2015; 231: 308–319. [DOI] [PubMed] [Google Scholar]
- 111.Falconer E, Bryant R, Felmingham KL, et al. The neural networks of inhibitory control in posttraumatic stress disorder. J Psychiatry Neurosci 2008; 33: 413–422. [PMC free article] [PubMed] [Google Scholar]
- 112.Whalley MG, Rugg MD, Smith AP. Dolan RJ, Brewin CR. Incidental retrieval of emotional contexts in post-traumatic stress disorder and depression: an fMRI study. Brain Cogn 2009; 69: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simmons A, Paulus MP, Thorp SR. Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol Psychiatry 2008; 64: 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Geuze E, Westenberg HG, Heinecke A, de Kloet CS, Goebel R, Vermetten E Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage 2008; 41: 675–681. [DOI] [PubMed] [Google Scholar]
- 115.Sussman D, Pang EW, Jetly R. Dunkley BT, Taylor MJ. Neuroanatomical features in soldiers with post-traumatic stress disorder. BMC Neurosci 2016; 17: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aupperle RL, Allard CB, Grimes EM, et al. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry 2012; 69: 360–371. [DOI] [PubMed] [Google Scholar]
- 117.Mazza M, Tempesta D, Pino MC, Catalucci A, Gallucci M, Ferrara M Regional cerebral changes and functional connectivity during the observation of negative emotional stimuli in subjects with post-traumatic stress disorder. Eur Arch Psychiatry Clin Neurosci 2013; 263: 575–583. [DOI] [PubMed] [Google Scholar]
- 118.Morey RA, Dolcos F, Petty CM, et al. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J Psychiatr Res 2009; 43: 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.New AS, Fan J, Murrough JW, et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry 2009; 66: 656–664. [DOI] [PubMed] [Google Scholar]
- 120.Blair KS, Vythilingam M, Crowe SL, et al. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol Med 2013; 43: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rabinak CA, MacNamara A, Kennedy AE, et al. Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depress Anxiety 2014; 31: 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bluhm RL, Williamson PC, Osuch EA, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci 2009; 34: 187–194. [PMC free article] [PubMed] [Google Scholar]
- 123.Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology 2013; 38: 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shaw ME, Strother SC, McFarlane AC, et al. Abnormal functional connectivity in posttraumatic stress disorder. Neuroimage 2002; 15: 661–674. [DOI] [PubMed] [Google Scholar]
- 125.Young KA, Thompson PM, Cruz DA. Williamson DE, Selemon LD. BA11 FKBP5 expression levels correlate with dendritic spine density in postmortem PTSD and controls. Neurobiol Stress 2015; 2: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kuhn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry 2013; 73: 70–74. [DOI] [PubMed] [Google Scholar]
- 127.Meng Y, Qui C, Zhu H, et al. Anatomical deficits in adult posttraumatic stress disorder: a meta-analysis of voxel-based morphometry studies. Behav Brain Res 2014; 270: 307–315. [DOI] [PubMed] [Google Scholar]
- 128.Bromis K, Calem M, Reinders AATS. Williams SCR, Kempton MJ. Meta-analysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. Am J Psychiatry 2018; 175: 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abe O, Yamasue H, Kasai K, et al. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res 2006; 146: 231–242. [DOI] [PubMed] [Google Scholar]
- 130.Bierer LM, Ivanov I, Carpenter DM, et al. White matter abnormalities in Gulf War veterans with posttraumatic stress disorder: a pilot study. Psychoneuroendocrinology 2015; 51: 567–576. [DOI] [PubMed] [Google Scholar]
- 131.Fani N, King TZ, Shin J, et al. Structural and functional connectivity in posttraumatic stress disorder: associations with FKBP5. Depress Anxiety 2016; 33: 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim MJ, Lyoo IK, Kim SJ, et al. Disrupted white matter tract integrity of the anterior cingulate in trauma survivors. Neuroreport 2005; 16: 1049–1053. [DOI] [PubMed] [Google Scholar]
- 133.Kim SH, Jeong DU, Sim ME, et al. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology 2006; 54: 120–125. [DOI] [PubMed] [Google Scholar]
- 134.Schuff N, Zhang Y, Zhan W, et al. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: a MRI study. Neuroimage 2011; 54S1: S62–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sanjuan PM, Thoma R, Claus ED. Mays N, Caprihan A. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Res 2013; 214: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Krystal JH, Abdallah CG, Averill LA, et al. Synaptic loss and the pathophysiology of PTSD: implications for ketamine as a prototype novel therapeutic. Curr Psychiatry Rep 2017; 19: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Clausen AN, Francisco AJ, Thelen J, et al. PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depress Anxiety 2017; 34: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kennis M, Rademaker AR, van Rooij SJ. Kahn RS, Geuze E.Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post-traumatic stress disorder. Hum Brain Mapp 2015; 36: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wrocklage KM, Averill LA, Scott JC, et al. Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. Eur Neuropsychopharmacol 2017; 27: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 2011; 15: 483–506. [DOI] [PubMed] [Google Scholar]
- 141.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106: 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Raichle ME. The brain's default mode network. Ann Rev Neurosci 2015; 38: 433–447. [DOI] [PubMed] [Google Scholar]
- 143.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL Functional-anatomic fractionation of the brain's default network. Neuron 2010; 65: 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yehuda R, Hoge CW, McFarlane AC, et al. Post-traumatic stress disorder. Nat Rev Dis Primers 2015; 1: 15057. [DOI] [PubMed] [Google Scholar]
- 145.Akiki TJ, Averill CL, Abdallah CG. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr Psychiatry Rep 2017; 19: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lei D, Li K, Li L, et al. Disrupted functional brain connectome in patients with posttraumatic stress disorder. Radiology 2015; 276: 818–827. [DOI] [PubMed] [Google Scholar]
- 147.Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. NeuroImage 2018; 176: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Philip NS, Berredo J, van't Wout-Frank M. Tyrka AR, Price LH, Carpenter LL. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol Psychiatry 2018; 83: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lanius RA, Frewen PA, Tursich M, Jetly R, McKinnon MC Restoring large-scale brain networks in PTSD and related disorders: a proposal for neuroscientifically-informed treatment interventions. Eur J Psychotraumatol 2015; 6: 27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abdallah CG, Averill CL, Salas R, et al. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol Psychiatry Cogn Neurosci Neuroimaging 2017; 2: 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Abdallah CG, De Feyter HM, Averill LA, et al. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 2018; 43: 2154–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS Ketamine: a paradigm shift for depression research and treatment. Neuron 2019; 101: 774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Friedman MJ, Huber BR, Brady CB, et al. VA's national PTSD brain bank: a national resource for research. Curr Psychiatry Rep 2017; 19: 73. [DOI] [PubMed] [Google Scholar]