Abstract
Background:
Decreased activity of forkhead transcription factor class O (FoxO)3A, a negative regulator of NF-κB-mediated chemokine expression, is implicated in the pathogenesis of chronic obstructive pulmonary disease (COPD). Previously, we showed that quercetin reduces lung inflammation in a murine model of COPD. Here, we examined the mechanisms underlying decreased FoxO3A activation and its modulation by quercetin in COPD human airway epithelial cells and in a COPD mouse model.
Methods:
Primary COPD and normal human airway epithelial cells were treated with quercetin, LY294002 or erlotinib for two weeks. IL-8 was measured by ELISA. FoxO3A, Akt, and EGF receptor (EGFR) phosphorylation and nuclear FoxO3A levels were determined by Western blot analysis. Effects of quercetin on lung chemokine expression, nuclear FoxO3A levels and phosphorylation of EGFR and Akt were determined in COPD mouse model.
Results:
Compared to normal, COPD cells showed significantly increased IL-8, which negatively correlated with nuclear FoxO3A levels. COPD bronchial biopsies also showed reduced nuclear FoxO3A. Decreased FoxO3A in COPD cells was associated with increased phosphorylation of EGFR, Akt and FoxO3A and treatment with quercetin, LY294002 or erlotinib increased nuclear FoxO3A and decreased IL-8 and phosphorylation of Akt, EGFR and FoxO3A, Compared to control, elastase/LPS-exposed mice showed decreased nuclear FoxO3A, increased chemokines and phosphorylation of EGFR and Akt. Treatment with quercetin partially reversed these changes.
Conclusions:
In COPD airways, aberrant EGFR activity increases PI 3-kinase/Akt-mediated phosphorylation of FoxO3A, thereby decreasing nuclear FoxO3A and increasing chemokine expression. Quercetin restores nuclear FoxO3A and reduces chemokine expression partly by modulating EGFR/PI 3-kinase/Akt activity.
Keywords: Akt, inflammation, quercetin, IL-8, PI-3 kinase
INTRODUCTION
Airways inflammation is one of the central features of chronic obstructive pulmonary disease (COPD). Airway epithelial cells (AEC) significantly contribute to inflammation by producing cytokines and chemokines in response to various stimuli, including cigarette smoke (CS). This in turn leads to recruitment of inflammatory cells to the airways. Under normal conditions, the expression of cytokines is tightly regulated by negative regulatory molecules, thereby limiting the influx of inflammatory cells and restoring the tissue homeostasis. Therefore, inactivation or reduced expression of negative regulatory molecules may result in sustained cytokine expression and development of airways inflammation. Previously, we demonstrated that AEC isolated from COPD patients express significantly more C-X-C chemokines than normal cells 1, suggesting that COPD cells are defective in regulating cytokine expression.
The forkhead transcription factor (Fox)O3A negatively regulates pro-inflammatory cytokine expression 2, in addition to playing a critical role in a wide variety of cellular processes, including immune regulation, and promoting resistance to oxidative stress 3,4. FoxO3A inhibits IL-8 expression in cells under oxidative stress by sequestering the RelA/p65 component of NF-κB, a transcription factor that stimulates IL-8 promoter activity 2. Recently, FoxO3A deficiency was shown to increase susceptibility to CS-induced lung inflammation in mice, but the mechanisms are not well understood 5.
The regulation of FoxO3A activity is complex and primarily depends on post-translation modifications, such as phosphorylation, acetylation and ubiquitination, which affect its stability and subcellular localization (nuclear versus cytoplasmic) 6. Akt, a downstream target of PI-3 kinase, phosphorylates and promotes cytoplasmic accumulation of FoxO3A, ultimately leading to ubiquitination and degradation 7. FoxO3A that has been phosphorylated by Akt also binds to the 14–3-3 chaperone and remains in the cytoplasm 8. Phosphorylation of FoxO3A by Akt is also promoted by the acetylation of nuclear FoxO3A by CREB binding protein combined with decreased SIRT1 (a type II histone deacetylase), which results in exclusion of FoxO3A from the nucleus 9,10. These observations suggest a critical role for Akt in regulation of FoxO3A nuclear localization, but that role has not been established in AEC.
Increased Akt phosphorylation is seen in bronchial tissue of smokers 11 and CS activates PI-3 kinase activity via EGFR activation 12,13. Phosphorylation of both EGFR and Akt is increased by oxidative stress, which has been implicated in the pathogenesis of COPD 14,15. Endotoxin, a cell wall component of gram-negative bacteria that is present in appreciable amounts in CS 16,17 , also increases Akt phosphorylation via EGFR activation 18. Based on these observations, we hypothesized that activation of the EGFR/PI 3-kinase/Akt signaling pathway decreases nuclear FoxO3A levels in COPD AEC, thereby increasing expression of pro-inflammatory cytokines such as IL-8.
Quercetin (3,3’,4’,5,7-pentahydroxyflavone), a plant flavanoid is a potent antioxidant. Quercetin also modulates activities of several lipid kinases, tyrosine kinases and serine/threonine kinases 19–22. Previously, we demonstrated that quercetin decreases chemokine levels and overall lung inflammation, and prevents progression of emphysema in mice displaying typical pathological and physiological features of COPD 23,24, but the mechanisms are not understood. The present study examines the mechanisms underlying the decreased nuclear FoxO3A levels in COPD AEC and whether quercetin decreases chemokine expression by restoring nuclear FoxO3A levels. In this study, we also examined whether quercetin reduces chemokine expression in vivo by modulating nuclear FoxO3A levels.
METHODS (Details provided in on-line supplement)
Primary cells and bronchial sections.
This study was approved by the University of Michigan Investigational Review Board. All bronchial segments were obtained from intact and healthy tissue of healthy non-smokers, smokers without COPD or from COPD subjects during lung transplantation. Characteristics of COPD and normal donors are provided in Supplemental Table 1 and 2. AEC were isolated and cultured at air-liquid interface to promote differentiation into mucociliary phenotype as described 1. In some experiments, COPD mucociliary-differentiated AEC were treated with 1 μM erlotinib, LY294002 or quercetin for 2 weeks.
Bronchial tissue from healthy non-smokers, COPD or CF subjects obtained during lung transplantation was fixed in formalin and embedded in paraffin.
ELISA.
Conditioned basolateral media or lung homogenate supernatants were used to quantify chemokine levels by ELISA 1,23.
Transfection of 16HBE14o- cells.
Immortalized 16HBE14o- AECs were reverse transfected with non-targeting (NT)- or FoxO3A-siRNA and incubated for 2 days 25. Cell culture medium was used for IL-8 determination and cells for expression of FoxO3A protein.
Western blot analysis of nuclear, cytoplasmic and whole cell extracts.
Nuclear and cytoplasmic protein extracts and total cell extracts were prepared as described previously 25,26. Aliquots of whole cell, cytoplasmic or nuclear extracts containing equal amounts of total proteins were subjected to Western blot analysis and specific bands quantified by densitometry 23,25.
Immunodetection of FoxO3A and IL-8.
Paraffin sections were deparaffinized, and subjected to immunofluorescence staining using antibody to FoxO3A as described previously 24 and/or IL-8 (R & D Systems, Minneapolis, MN) and sections were visualized under confocal microscopy (Carl Zeiss, Thornwood, NJ).
Animals and treatment.
Normal 8–10 week-old C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were exposed to elastase and LPS for four consecutive weeks and treated with quercetin or propylene glycol (vehicle), as described previously 23,24. Mice were exposed to cigarette smoke or room air for 6 weeks and treated with quercetin or vehicle during the last two weeks. Mice were then euthanized, and lung homogenates were used to prepare nuclear, cytoplasmic or whole cell extracts. All experiments described herein were approved by the Animal Care and Use Committee of the University of Michigan.
Statistical analysis.
Results are expressed as means ± SD. Data were analyzed using SigmaStat statistical software (Systat Software, Inc., San Jose, CA). To compare two groups, an unpaired t test with Welch’s correction was used. One-way analysis of variance (ANOVA) with Tukey-Kramer post-hoc analysis was performed to compare more than two groups. A ‘p’ value <0.05 was considered significant.
RESULTS
Reduced Nuclear FoxO3A correlates with elevated IL-8 expression in AEC.
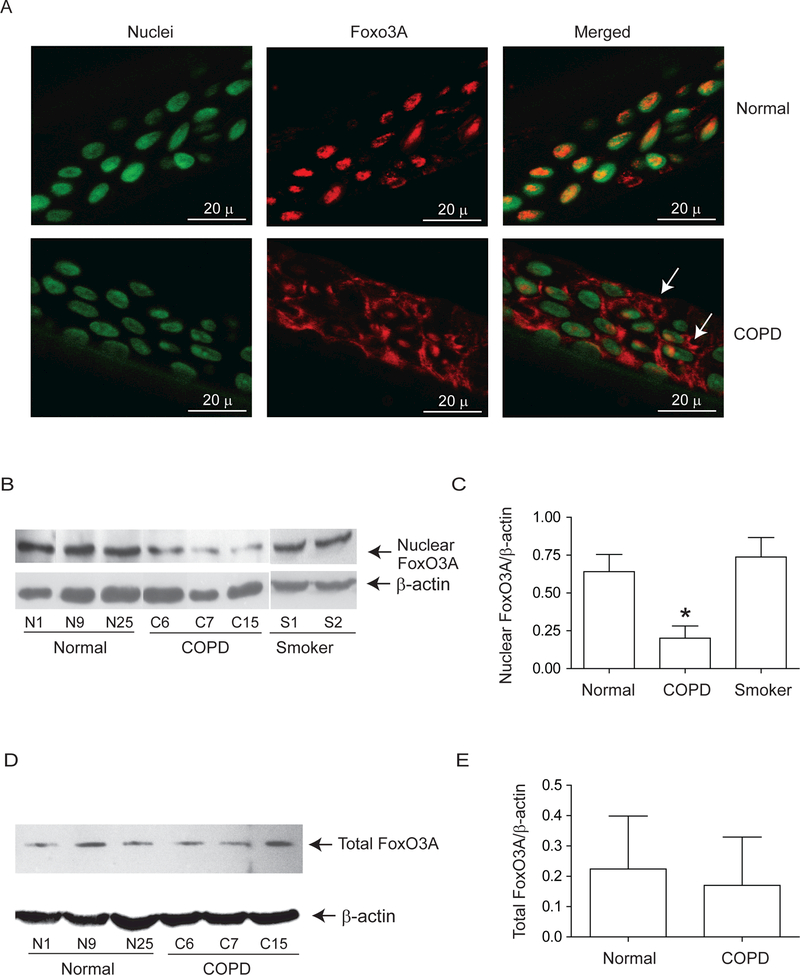
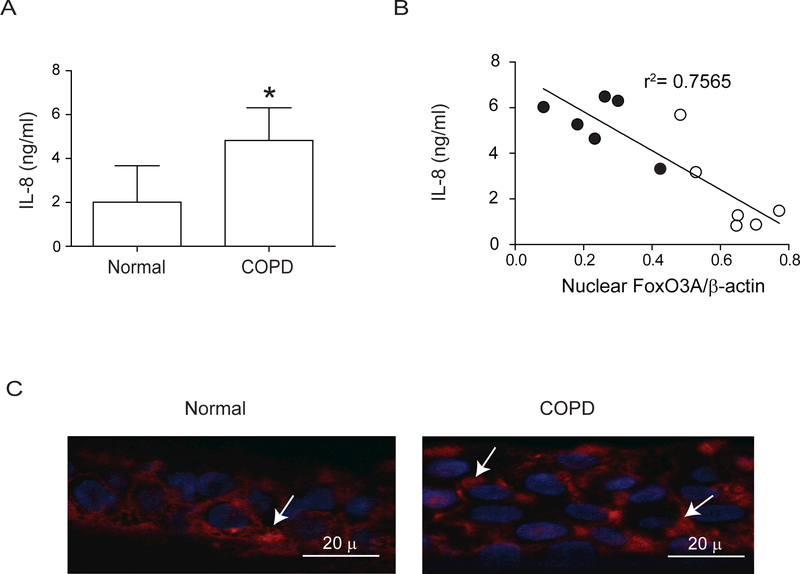
Both normal and COPD AEC grown at air/liquid interface showed mucocilliary differentiation (Supplemental Figure 1A) and transepithelial resistance of 400 to 600 Ω.cm2. Compared to normal, COPD cultures also showed more PAS positive cells (Supplemental Figure 1B). Immunofluorescence microscopy indicated that while normal cells show FoxO3A primarily in the nucleus, COPD AEC show majority of FoxO3A in the cytoplasm (Figure 1A). Consistent with this, Western blot analysis showed significantly less nuclear FoxO3A in COPD AEC than in normal (Figure 1B and1C). In contrast, there was no difference in nuclear FoxO3A levels between AEC from healthy smokers and normal, therefore subsequent experiments were performed with only normal and COPD cells. Despite reduced nuclear FoxO3A, COPD AEC showed total FOXO3A levels similar to normal AEC (Figure 1D and1E). In addition, COPD AEC showed increased IL-8 protein compared to normal AEC as observed previously1 (Figure 2A). Importantly, nuclear FoxO3A levels showed a strong negative correlation with IL-8 protein between individual subjects (Figure 2B). By immunofluorescence microscopy, IL-8 protein was observed in almost all cells in the cultures indicating that IL-8 expression is not specific to one cell type (Figure 2C), however COPD AEC showed comparatively more IL-8 protein than normal AEC.
Figure 1.
Nuclear FoxO3A is decreased in COPD AEC under basal conditions. Primary AEC from COPD or normal subjects were cultured in transwells at air/liquid interface to promote differentiation into mucociliary phenotype. (A) Cultures were fixed and embedded in paraffin. Five micron thick sections were deparaffinized, and incubated with antibody to FoxO3A. Bound antibody was detected by second antibody conjugated with Alexafluor-598 (red) and nuclei were counterstained with DAPI (green). Co-localization of FoxO3A with nuclei appears yellow and arrows point to cytoplasmic FoxO3A. Images are representative of AEC obtained from 3 COPD and 3 normal subjects. (B to C) Nuclear extracts from well-differentiated AEC cultures from healthy non-smokers, healthy smokers and patients with COPD were examined for FoxO3A by Western blot analysis and band densities were quantified and expressed as fold increase over β-actin. (D and E) Total proteins from healthy non-smokers or COPD AEC were subjected to Western blot analysis with antibody to FoxO3A and levels of FoxO3A was normalized to β-actin. Data in C to E represents mean ±SD calculated from cells isolated from three to six subjects per group; *, different from normal cells, p≤0.05, ANOVA.
Figure 2.
IL-8 levels negatively correlates with nuclear FoxO3A. (A) Basolateral media from unstimulated COPD and normal AEC differentiated into mucociliary phenotype was used to determine IL-8 by ELISA. Data represents mean ±SD calculated from cells isolated from six subjects per group; *, different from normal cells, p≤0.05; unpaired t test. (B) Correlation between nuclear FoxO3A (from Figure 1C) and IL-8 was assessed by linear regression analysis (○ and ● represent normal and COPD AEC respectively). (C) Sections of COPD and normal AEC were probed with antibody to IL-8, bound antibody was detected by antimouse IgG labeled with Alexafluor-598 and sections were counterstained with DAPI to visualize nuclei. Arrows represents IL-8. Images are representative of 3 normal and 3 COPD cultures.
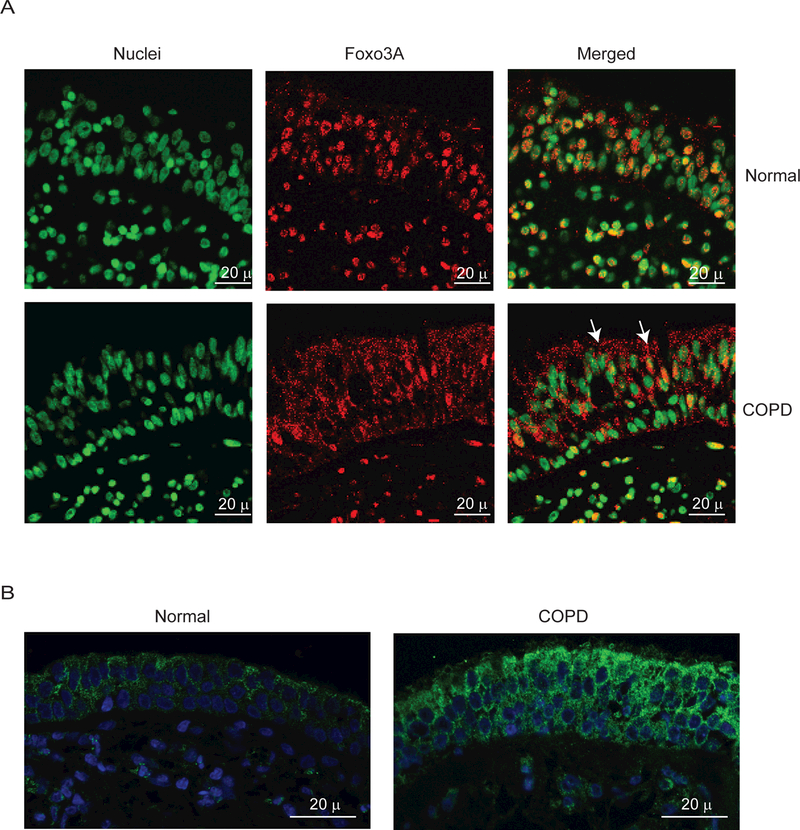
To exclude the possibility that the observed difference between normal and COPD AEC in nuclear FoxO3A was simply due to in vitro culturing conditions, we also immune-localized FoxO3A in bronchial sections obtained from COPD or normal subjects. In normal bronchial sections, majority of FoxO3A was found to co-localize with the nucleus of bronchial epithelial cells (Figure 3A), but in COPD sections, FoxO3A was mostly found in the cytoplasm and rarely in the nucleus. In both COPD and normal bronchial biopsies, IL-8 protein was observed in all most all the cells of bronchial epithelium, but the intensity was higher in COPD (Figure 3B). These results indicate that cultured AEC truly represents the changes in FoxO3A distribution and IL-8 expression observed in vivo.
Figure 3.
COPD bronchial epithelium shows decreased nuclear FoxO3A and increased IL-8 expression. Paraffin sections of bronchial tissue from COPD or normal subjects were deparaffinized, blocked and incubated with antibody to FoxO3A or IL-8. Bound antibodies were detected by antimouse or antirabbit IgG conjugated with Alexafluor-594 and nuclei were counterstained with DAPI. (A) FoxO3A and nuclei are represented by red and green respectively; co-localization of FoxO3A with nuclei appears yellow; arrows show FoxO3A in the cytoplasm. (B) IL-8 and nuclei are represented by green and blue respectively. Images are representative of bronchial sections from three COPD and two normal subjects.
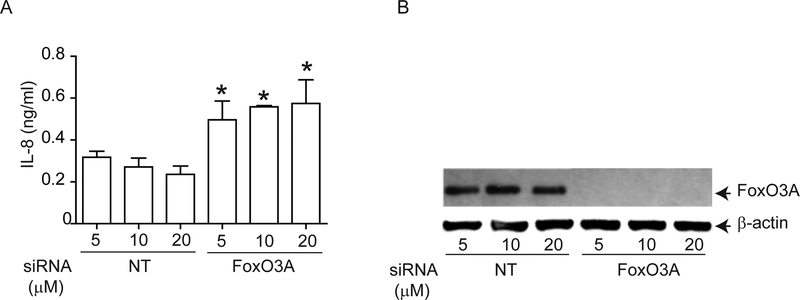
To determine whether FoxO3A regulates IL-8 expression in AEC, we knocked-down FoxO3A using siRNA and measured basal IL-8 expression in immortalize AEC, because primary AEC are not readily amenable to transfection. Compared to NT siRNA-, FoxO3A siRNA-transfected cells showed significantly increased levels of IL-8 (Figure 4A). FoxO3A siRNA-, but not NT siRNA-transfected cells showed complete knockdown of FoxO3A expression (Figure 4B). These results confirmed that FoxO3A regulates basal IL-8 expression in human AEC.
Figure 4.
Genetic inhibition of FoxO3A reduces IL-8 expression in normal airway epithelial cells. 16HBE14o- cells were reverse transfected with non-targeting (NT)- or FoxO3A-specific siRNA. Cells were incubated for 48 h, then media was changed and incubated for a further 24 h before culture supernatants were harvested and total cellular protein was isolated. (A) IL-8 levels measured by ELISA. Data represent mean and average from three independent experiments (*, different from cells transfected with NT siRNA, p≤0.05, unpaired t test). (B) FoxO3A protein levels by Western blot analysis. Representative of three independent experiments.
Akt phosphorylation is elevated in COPD AEC.
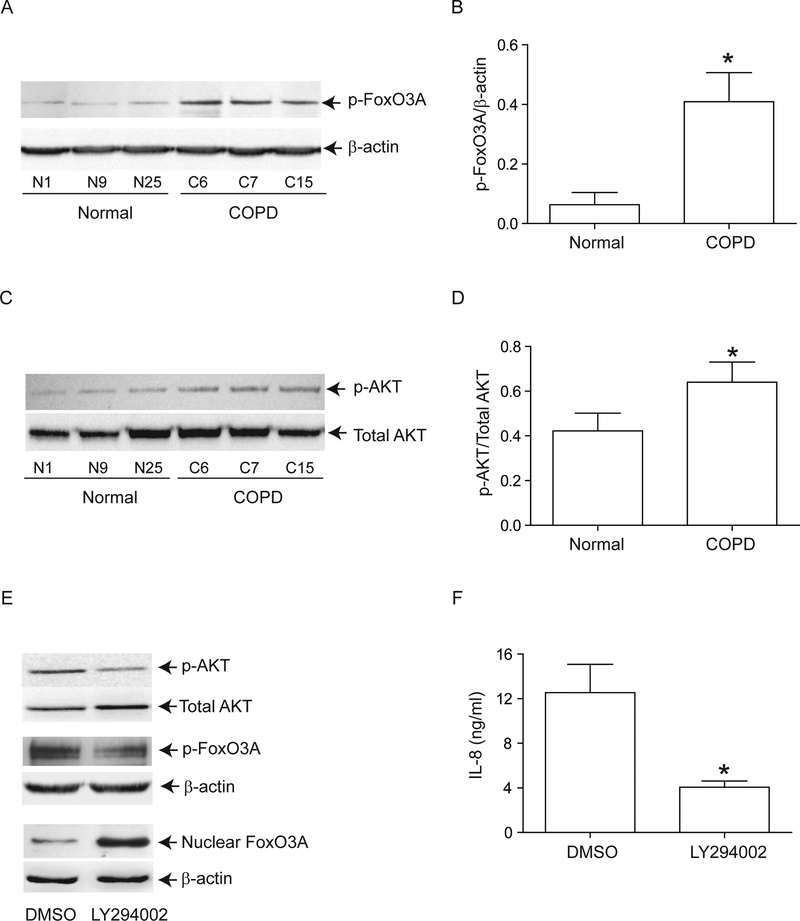
Next, we examined whether reduced nuclear FoxO3A in unstimulated COPD AEC is due to increased phosphorylation. Cytoplasmic extracts from COPD AEC showed significantly increased phosphorylated FoxO3A compared to normal AEC (Figure 5A and5B). Because Akt, a downstream target of PI-3 kinase phosphorylates FoxO3A, we examined Akt phosphorylation and the effects of PI-3 kinase inhibitor, LY294002 on nuclear FoxO3A. Compared to normal, COPD AEC showed increased Akt phosphorylation (Figure 5C and5D) and treatement with LY294002 decreased phosphorylation of both Akt and FoxO3A, increased nuclear FoxO3A (Figure 5E), and decreased IL-8 expression in COPD AEC (Figure 5F). These observations imply that increased PI-3 Kinase/Akt activation is largely responsible for the observed nuclear FoxO3A reduction in COPD AEC.
Figure 5.
Inhibition of PI-3 kinase increases nuclear FoxO3A and decreased IL-8 in COPD AEC. Primary AEC from COPD subjects or normal volunteers were cultured as described in Figure 1, then (A, B) cytoplasmic extracts were subjected to Western blot analysis with antibody to phospho-FoxO3A; (C, D) total cell lysates were subjected to Western blot analysis with antibodies to phospho-Akt and total Akt and band densitites were quantified by NIH image J. Data in B and D represents AEC from 6 subjects per group. (E) Mucociliary-differentiated COPD AEC were treated with DMSO (vehicle control) or 1 μM LY294002 for 2 weeks. Total proteins (for Akt), nuclear (for FoxO3A) or cytoplasmic (for phospho-FoxO3A) extracts were isolated from cells and subjected to Western blot analysis with relevant antibodies. Images are representative of 3 independent experiments. (F) IL-8 levels in the basolateral medium of the cells determined by ELISA; Mean ± SD from three independent experiments, performed in duplicates with cells obtained from three donors (*, different from normal airway epithelial cells, p≤0.05, unpaired t test).
Increased EGFR activity correlates with Akt phosphorylation in COPD AEC.
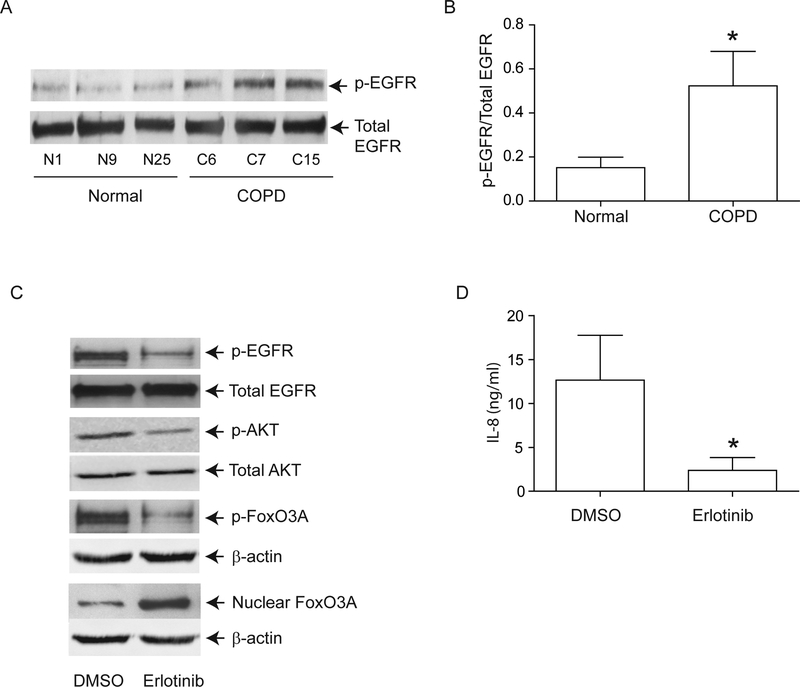
PI-3 kinase/Akt signaling pathway can be activated via tyrosine kinases including growth factor receptors 27. Since EGFR activity is upregulated in COPD patients 28, and cigarette smoke increases EGFR activity 12,13, we next looked for evidence of elevated EGFR activity in COPD AEC. EGFR activity, as assessed by EGFR phosphorylation on tyrosine residue 1173, was higher in COPD than in normal AEC (Figure 6A and6B). Treatment with erlotinib, an inhibitor of EGFR decreased EGFR, Akt and FoxO3A phosphorylation, increased nuclear FoxO3A (Figure 6C) and reduced IL-8 protein (Figure 6D) in COPD AEC, implying that upregulated EGFR may be responsible for PI-3 kinase/Akt activation and subsequent decrease in nuclear FoxO3A and increased basal IL-8 levels in COPD AEC.
Figure 6.
EGFR phosphorylation is increased in COPD cells and inhibition of EGFR decreases IL-8 and increases nuclear FoxO3A in these cells. Primary human AEC from COPD subjects or normal volunteers were cultured as described in Figure 1. (A, B) Total cell lysates were prepared and subjected to Western blot analysis using antibodies to phospho-EGFR and total EGFR. A, Representative western blot; B, phospho-EGFR/total EGFR ratios, n = 6 per group. (C) mucociliary-differentiated COPD cells were treated with DMSO (vehicle control) or 1 μM erlotinib for 2 weeks. Total proteins (for EGFR), nuclear (for FoxO3A) or cytoplasmic (for phospho-FoxO3A) extracts were isolated from cells and subjected to Western blot analysis with relevant antibodies. Blots are representative of three independent experiments. (D) IL-8 levels in the basolateral medium were determined by ELISA. Data represents mean and SD calculated from 3 independent experiments performed in duplicates with cells obtained from three donors (* different from normal airway epithelial cells, p≤0.05, unpaired t test).
Quercetin treatment decreases IL-8 expression by inhibiting EGFR and Akt activation in COPD cells.
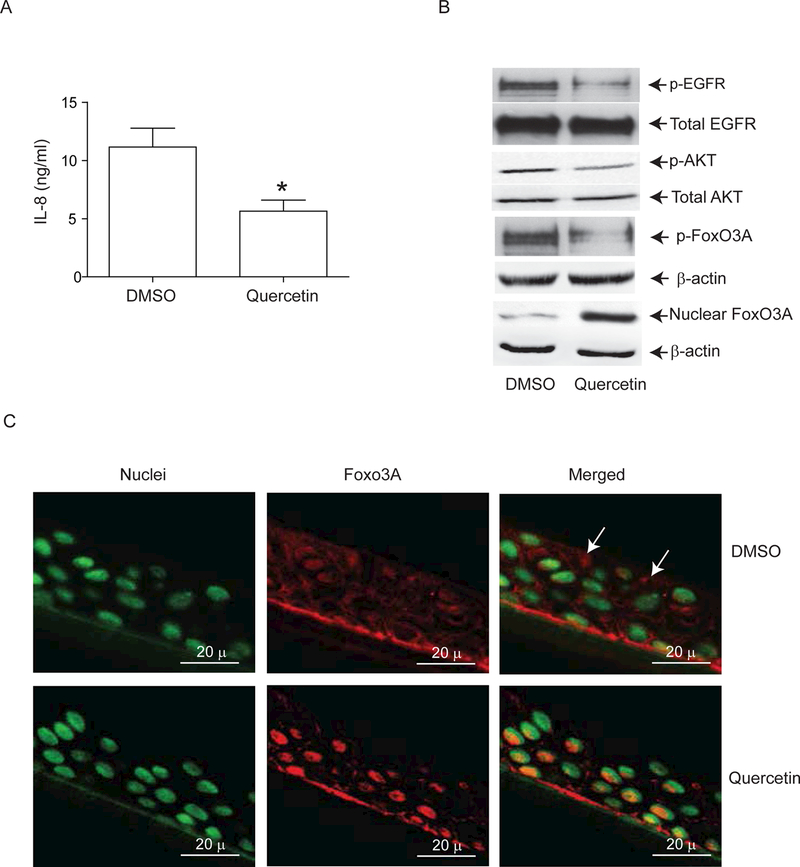
One of the ways by which the anti-inflammatory nutriceutical quercetin appears to work is by blocking both tyrosine and threonine/serine protein kinases 19–22. We examined whether quercetin decreases IL-8 secretion in COPD AEC by reducing phosphorylation of EGFR, Akt and FoxO3A. Compared to DMSO-treated cells, COPD AEC treated with quercetin showed decreased IL-8 levels (Figure 7A), reduced phosphorylation of EGFR and Akt, and increased nuclear FoxO3A (Figure 7B). Increased nuclear FoxO3A in quercetin treated COPD AEC was further confirmed by immunolocalization (Figure 7C). Thus quercetin decreases IL-8 levels in COPD AEC in association with decreased activities of both EGFR and PI-3 kinase.
Figure 7.
Quercetin treatment decreases phosphorylation of EGFR, Akt and FoxO3A and IL-8 levels in COPD airway epithelial cells. Primary airway epithelial cells from COPD subjects were cultured as described in Figure 1 and treated with DMSO (vehicle control) or 1 μM quercetin for 2 weeks. (A) IL-8 levels in the basolateral medium were determined by ELISA. Data represent mean and SD calculated from 3 independent experiments performed in duplicates or triplicates. (B) Total proteins (for EGFR and Akt), nuclear (for FoxO3A) or cytoplasmic (for phospho FoxO3A) extracts were isolated from cells and subjected to Western blot analysis with relevant antibodies. Western blots are representative of 3 independent experiments conducted with cells obtained from three donors (* different from normal airway epithelial cells, p≤0.05, unpaired t test). (C) Some cultures were fixed and embedded in paraffin. Five micron thick sections were used for immune-localization of FoxO3A by confocal immunofluorescence microscopy. Co-localization of FoxO3A with nucleus appears yellow and arrows point to cytoplasmic FoxO3A. Images are representative of 3 independent experiments.
Quercetin treatment increases nuclear FoxO3A levels in elastase/LPS-exposed mice.
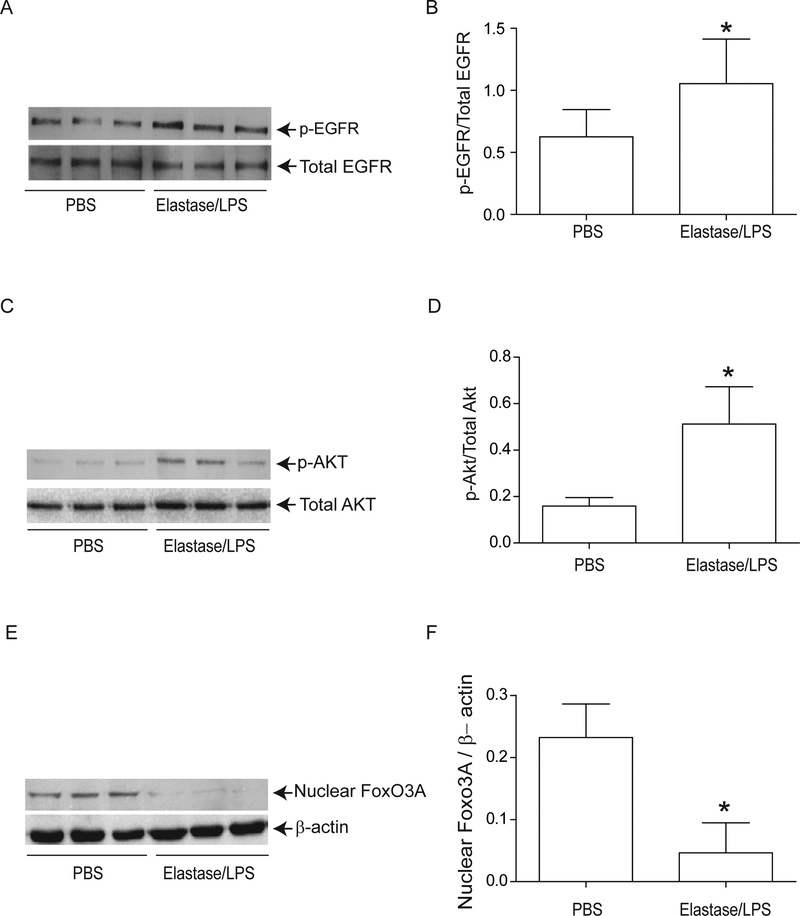
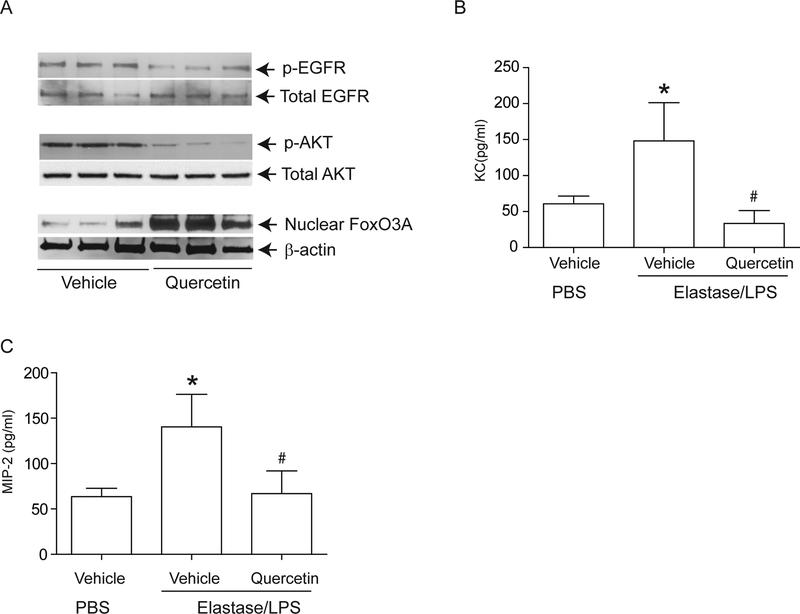
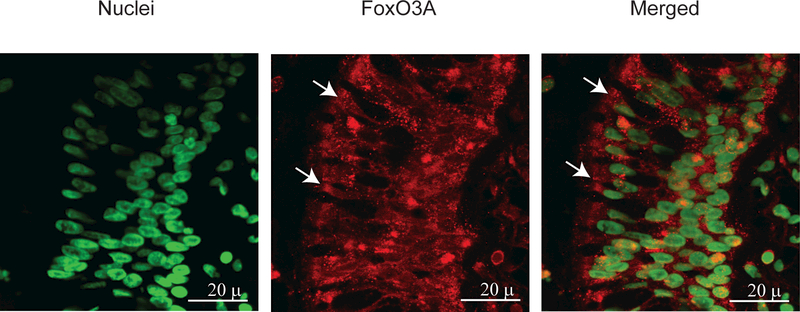
To determine whether these findings were relevant in vivo, we examined our established murine model 23,24,26 for changes in nuclear FoxO3A, and for the phosphorylation state of EGFR and Akt. Compared to PBS-exposed mice, elastase/LPS-exposed mice showed a significantly increased phosphorylation of both EGFR (Figure 8A and8B) and Akt (Figure 8C and8D) and reduced nuclear FoxO3A (Figure 8E and8F). Due to the unavailability of suitable antibody to mouse phospho-FoxO3A, we could not examine FoxO3A phosphorylation. Quercetin treatment significantly reduced phosphorylation of EGFR and Akt and increased nuclear FoxO3A in elastase/LPS-exposed mice (Figure 9A) and also decreased the KC and MIP-2 to the levels that observe in PBS-exposed mice(Figure 9B and9C).
Figure 8.
Elastase/LPS-exposed mice show decreased nuclear FoxO3A levels and increased phosphorylation of EGFR and Akt. Mice were exposed to elastase/LPS or PBS for 4 weeks. Lungs were excised, homogenized in PBS and aliquots of homogenates were used for either total protein (for EGFR and Akt) or nuclear protein (for FoxO3A) extraction. Aliquots equivalent to equal protein was subjected to Western blot analysis. Western blot images in A, C, and E show samples analyzed from 3 individual animals. Panels B, D and E represent ratios of p-EGFR/total EGFR, p-Akt/total Akt and FoxO3A/β-actin respectively. Data represents mean and SD calculated from 3 independent experiments (n=6 mice * different from PBS-exposed animals, p≤0.05, unpaired t test).
Figure 9.
Quercetin treatment reduced chemokine expression in Elastase/LPS-exposed mice correlates with increased nuclear FoxO3A levels. Mice were exposed to elastase/LPS for 4 weeks and then treated with quercetin or vehicle for 2 weeks. PBS-exposed animals treated with vehicle were used as controls in some experiments. Lungs were excised, homogenized in PBS and aliquots of homogenates were used for either nuclear (for FoxO3A), or total protein (for EGFR and Akt) extraction. Aliquots equivalent to equal protein was subjected to Western blot analysis. Western blot images in A, B, and C show samples analyzed from 3 individual mice. (B,C) Lung homogenate supernatants were used for determination of chemokines by ELISA. Data represents mean and SD calculated from 3 independent experiments (n=6–9 * different from PBS-exposed animals treated with vehicle, p≤0.05; #different from elastase/LPS exposed mice treated with vehicle p≤0.05, ANOVA with Tukey-Kramer post hoc analysis).
We also examined the effect of quercetin in CS-induced changes in FoxO3A distribution in mice. Interestingly, CS-exposed mice showed nuclear FoxO3A, and KC and MIP-2 levels similar to room air-exposed mice (Supplemental Figure 2A to 2D) and not surprisingly quercetin did not affect nuclear FoxO3A, KC or MIP-2 levels. In contrast, compared to room air-exposed, CS-exposed mice showed significant increase in MCP-1 levels and quercetin treatment reduced MCP-1 levels in CS-exposed mice (Supplemental Figure 2C), indicating that quercetin as expected negatively regulate chemokines whose expression is NF-κB independent.
Nuclear FoxO3A levels are decreased in bronchial epithelium of CF patients.
We determined the FoxO3A distribution in sections of bronchial biopsies from CF patients (who also show chronic lung inflammation) by immune-localization. Bronchial epithelium showed FoxO3A primarily in cytoplasm and rarely in the nucleus (Figure 10) similar to that observed in COPD bronchial biopsies.
Figure 10.
Nuclear FoxO3A is decreased in CF bronchial epithelium. Paraffin section of bronchial tissue from CF patients was deparaffinized, blocked and incubated with antibody to FoxO3A. Bound antibody was detected by antirabbit IgG conjugated with Alexafluor-594 and nuclei were counterstained with DAPI. (A) FoxO3A and nuclei are represented by red and green respectively; co-localization of FoxO3A with nuclei appears yellow; arrows show FoxO3A in the cytoplasm. Images are representative of bronchial sections from three CF patients.
DISCUSSION
The principal findings of this study support a biochemical network in AECs of subjects with COPD by which persistent activation of the receptor tyrosine kinase EGFR mediates increased airway inflammation via activation of PI-3 kinase and Akt, consequent cytoplasmic retention of the anti-inflammatory transcription factor FoxO3A and increased basal levels of important neutrophil chemo-attractant IL-8. Further, we show that low concentrations of quercetin, a non-toxic plant flavanol with anti-oxidant and kinase-inhibiting properties, block this pathway both in differentiated COPD AEC in vitro and in a murine model 23,24,26 that faithfully mimics many features of COPD pathology and physiology. These novel findings provide a mechanistic explanation for our previous observation of excessive C-X-C chemokine elaboration in COPD 1, and motivate additional study of a quercetin as a therapy to mitigate the development or progression of oxidative stress-induced lung damage.
These results complement and extend recent evidence for an important regulatory role for FoxO3A in COPD pathogenesis in humans 5, as it has in other chronic inflammatory diseases such as inflammatory bowel disease and rheumatoid arthritis 29,30. FoxO3A-deficient mice develop spontaneous, multi-systemic inflammatory syndrome, accompanied by NF-κB hyperactivity and increased pro-inflammatory cytokines 2. FoxO3A-deficient mice are also more susceptible to CS-induced lung inflammation and airspace enlargement 5. We extend the previous finding that Foxo3A expression is downregulated in lung epithelial cells of COPD patients 5, by showing reduced nuclear FoxO3A localization, which we confirmed in bronchial epithelium of COPD subjects in situ, and by demonstrating increased phosphorylated cytoplasmic FoxO3A in AEC from COPD patients. Interestingly, we found a similar decrease in nuclear FoxO3A in elastase/LPS-exposed mice, in which we have previously shown pathological changes typical of COPD including emphysema, extensive inflammation, remodeling of small airways, goblet cell hyperplasia, and susceptibility to viral and bacterial infections 23,24,26. In contrast, CS-exposed mice showed no changes in nuclear FoxO3A similar to that observed in AEC from healthy smokers, indicating the requirement for other endogenous/exogenous factors in addition to CS to reduce nuclear FoxO3A levels. These findings may partly explain why not all smokers develop COPD. Because FoxO3A interacts with RelA/p65 component of NF-κB in the nucleus, thereby inhibiting NF-κB binding to IL-8 promoter 5, it is not surprising that we observed increased IL-8 expression in COPD AEC. Increased IL-8 secretion has also been noted in CF airways and the observation that nuclear FoxO3A is decreased in bronchial epithelium of these patients indicate that reduction in nuclear FoxO3A is not specific to COPD, but rather associated with chronic inflammatory conditions. Additional studies will be needed to define the possible consequences of altering nuclear FoxO3A levels on elaboration of other inflammatory mediators in the resting state and in response to relevant pro-inflammatory stimuli or infection. Further, clinical studies are required to establish the direct relationship of reduced nuclear FoxO3A or FoxO3A polymorphisms with disease severity in COPD patients.
Given the importance of FoxO3A shuttling between the nucleus and cytoplasm based on its phosphorylation state 10, identification of Akt and PI-3 kinase as mediators of FoxO3A regulation in AEC is important to a greater understanding of smoking or other environmental factors-induced lung disease. Akt phosphorylation is increased in the lungs and peripheral blood monocytes of COPD patients compared to normal subjects 31 and also increased by CS in both mice and in human AEC 11,32. Although CS can increase PI-3 kinase activity either by decreasing the expression of PTEN (phosphatase and tensin homologue deleted on chromosome 10) 33 or via increasing the activation of EGFR, we believe that the latter may be more relevant mechanism, because there was no decrease in the expression of PTEN in COPD AEC compared to normal (data not shown). In contrast, our finding of significantly increased EGFR phosphorylation with unchanged total EGFR levels in COPD cells compared to normal, and results using erlotinib, which inhibits EGFR specifically, both implicate EGFR activity for increased PI-3 kinase activity in COPD cells. Consistent with our observations, EGFR activity is increased in COPD patients 28 and CS stimulates PI-3 kinase/Akt activity via EGFR activation in vitro 12,13. Moreover CS also increases LPS-stimulated EGFR activity in normal AEC 34. Although the epigenetic changes that maintains elevated EGFR activation in COPD AECs are not known at present, this study indicate the suitability of COPD AECs for understanding the biochemical mechanisms that mediates increased airway inflammation in COPD.
Quercetin has a broad spectrum of anti-inflammatory actions, dependent at least in part on its ability to block the ATP-binding sites of multiple lipid and protein kinases. Previously, we have demonstrated that quercetin decreases TNF-α-stimulated IL-8 production by blocking PI-3 kinase activity in human airway epithelial cells 35 and significantly reduces chemokine expression and lung inflammation in elastase/LPS-exposed mice 23,24. We interpret the current data on the effect of quercetin on nuclear FoxO3A localization as further support for a EGFR-PI-3 kinase-Akt pathway that ultimately regulates production of inflammatory mediators. However, one cannot rule out the possibility that quercetin may also increase nuclear FoxO3A levels by increasing SIRT1 levels, as we and others have shown that quercetin increases expression of SIRT1 23,36. SIRT1 deacetylates nuclear FoxO3A, particularly in cells undergoing oxidative stress, which show increased nuclear accumulation of protein acetylases, including CBP/ p300-associated factor 9. Deacetylation by SIRT1 prevents phosphorylation of FoxO3A by Akt and subsequent export of FoxO3A from nucleus to cytoplasm.
In conclusion, our studies provide an insight into one of the mechanisms by which IL-8 expression is upregulated in COPD airways in the stable state. We demonstrate that COPD AEC cultured in vitro retain their pro-inflammatory phenotype, with sustained increases in activity of EGFR and Akt, which in turn modulate nuclear FoxO3A levels. We show that low dose of quercetin modulates the activities of EGFR and Akt, and inhibits chemokine expression both in vitro and in vivo. These results together with our previous studies 23 suggest that treatment with quercetin may reduce inflammation and improve lung function in COPD patients.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge National Disease Research Interchange, Philadelphia, PA for providing tracheobronchial segments from healthy non-smokers and COPD subjects. We thank Marisa Linn for her assistance with processing tissues for immunofluorescence, and Drs. M. Hershenson and J. Curtis for their valuable critiques while carrying out this work and also for critical reading of the manuscript.
Supported by NIH grants AT004793, HL089772 (U.S.)
REFERENCES
- 1.Schneider D, Ganesan S, Comstock AT, Meldrum CA, Mahidhara R, Goldsmith AM, et al. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;182(3):332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 2004;21(2):203–13. [DOI] [PubMed] [Google Scholar]
- 3.Dejean AS, Beisner DR, Ch’en IL, Kerdiles YM, Babour A, Arden KC, et al. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol 2009;10(5):504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storz P Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal 2011;14(4):593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang JW, Rajendrasozhan S, Yao H, Chung S, Sundar IK, Huyck HL, et al. FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J Immunol 2011;187(2):987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calnan DR, Brunet A. The FoxO code. Oncogene 2008;27(16):2276–88. [DOI] [PubMed] [Google Scholar]
- 7.Dejean AS, Hedrick SM, Kerdiles YM. Highly specialized role of Forkhead box O transcription factors in the immune system. Antioxid Redox Signal 2011;14(4):663–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shukla S, Shukla M, Maclennan GT, Fu P, Gupta S. Deregulation of FOXO3A during prostate cancer progression. Int J Oncol 2009;34(6):1613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004;303(5666):2011–5. [DOI] [PubMed] [Google Scholar]
- 10.van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem Sci 2005;30(2):81–6. [DOI] [PubMed] [Google Scholar]
- 11.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 2003;111(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gensch E, Gallup M, Sucher A, Li D, Gebremichael A, Lemjabbar H, et al. Tobacco smoke control of mucin production in lung cells requires oxygen radicals AP-1 and JNK. J Biol Chem 2004;279(37):39085–93. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Li Q, Kolosov VP, Perelman JM, Zhou X. Regulation of cigarette smoke-mediated mucin expression by hypoxia-inducible factor-1alpha via epidermal growth factor receptor-mediated signaling pathways. J Appl Toxicol 2011. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem 2000;275(19):14624–31. [DOI] [PubMed] [Google Scholar]
- 15.MacNee W Oxidants/antioxidants and COPD. Chest 2000;117(5 Suppl 1):303S–17S. [DOI] [PubMed] [Google Scholar]
- 16.Sebastian A, Pehrson C, Larsson L. Elevated concentrations of endotoxin in indoor air due to cigarette smoking. J Environ Monit 2006;8(5):519–22. [DOI] [PubMed] [Google Scholar]
- 17.Hasday JD, Bascom R, Costa JJ, Fitzgerald T, Dubin W. Bacterial endotoxin is an active component of cigarette smoke. Chest 1999;115(3):829–35. [DOI] [PubMed] [Google Scholar]
- 18.Binker MG, Binker-Cosen AA, Richards D, Oliver B, Cosen-Binker LI. LPS-stimulated MUC5AC production involves Rac1-dependent MMP-9 secretion and activation in NCIH292 cells. Biochem Biophys Res Commun 2009;386(1):124–9. [DOI] [PubMed] [Google Scholar]
- 19.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000;351(Pt 1):95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Remesy C, Chap H, et al. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol 1997;53(11):1649–57. [DOI] [PubMed] [Google Scholar]
- 21.Huang YT, Hwang JJ, Lee PP, Ke FC, Huang JH, Huang CJ, et al. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol 1999;128(5):999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peet GW, Li J. IkappaB kinases alpha and beta show a random sequential kinetic mechanism and are inhibited by staurosporine and quercetin. J Biol Chem 1999;274(46):32655–61. [DOI] [PubMed] [Google Scholar]
- 23.Ganesan S, Faris AN, Comstock AT, Chattoraj SS, Chattoraj A, Burgess JR, et al. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir Res 2010;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sajjan U, Ganesan S, Comstock AT, Shim J, Wang Q, Nagarkar DR, et al. Elastase- and LPS-exposed mice display altered responses to rhinovirus infection. Am J Physiol Lung Cell Mol Physiol 2009;297(5):L931–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comstock AT, Ganesan S, Chattoraj A, Faris AN, Margolis BL, Hershenson MB, et al. Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by NADPH oxidase 1. J Virol 2011;85(13):6795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganesan S, Faris AN, Comstock AT, Sonstein J, Curtis JL, Sajjan US. Elastase/LPS-Exposed Mice Exhibit Impaired Innate Immune Responses to Bacterial Challenge Role of Scavenger Receptor A. Am J Pathol 2012;180(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem 2009;284(16):10334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodruff PG. Novel outcomes and end points: biomarkers in chronic obstructive pulmonary disease clinical trials. Proc Am Thorac Soc 2011;8(4):350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludikhuize J, de Launay D, Groot D, Smeets TJ, Vinkenoog M, Sanders ME, et al. Inhibition of forkhead box class O family member transcription factors in rheumatoid synovial tissue. Arthritis Rheum 2007;56(7):2180–91. [DOI] [PubMed] [Google Scholar]
- 30.Snoeks L, Weber CR, Wasland K, Turner JR, Vainder C, Qi W, et al. Tumor suppressor FOXO3 participates in the regulation of intestinal inflammation. Lab Invest 2009;89(9):1053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.To Y, Ito K, Kizawa Y, Failla M, Ito M, Kusama T, et al. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;182(7):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakayama H, Numakawa T, Ikeuchi T. Nicotine-induced phosphorylation of Akt through epidermal growth factor receptor and Src in PC12h cells. J Neurochem 2002;83(6):1372–9. [DOI] [PubMed] [Google Scholar]
- 33.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A 1999;96(8):4240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baginski TK, Dabbagh K, Satjawatcharaphong C, Swinney DC. Cigarette smoke synergistically enhances respiratory mucin induction by proinflammatory stimuli. Am J Respir Cell Mol Biol 2006;35(2):165–74. [DOI] [PubMed] [Google Scholar]
- 35.Nanua S, Zick SM, Andrade JE, Sajjan US, Burgess JR, Lukacs NW, et al. Quercetin blocks airway epithelial cell chemokine expression. Am J Respir Cell Mol Biol 2006;35(5):602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, et al. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem Biophys Res Commun 2009;378(4):836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.