Abstract
The receptors for the brain and gastrointestinal peptide, cholecystokinin, can be classified into CCKA and CCKB subtypes. Having recently cloned the rat CCKB receptor, we used it’s cDNA to isolate the human CCKB receptor homologue from brain and stomach which encodes a 447 amino acid protein with 90% identity to both rat CCKB and canine gastrin receptors. Northern hybridization identifies transcipts from stomach, pancreas, brain and gallbladder. The CCKB receptor gene maps to chromosome 11. Expression of the receptor cDNA in COS-7 cells was characteristic of a CCKB receptor subtype pharmacology. These data confirm that we have cloned a novel gene for the human brain and stomach CCKB receptor.
The cholecystokinin (CCK) family of peptides and their receptors are widely distributed throughout the central nervous system (CNS) and gastrointestinal tract (1,2). The receptors for cholecystokinin can be divided into two subtypes on the basis of their affinity for nonsulfated analogues of CCK. CCKA receptors, having a high affinity only for sulfated CCK-8, are found principally in the gastrointestinal tract and select areas of the CNS while CCKB (gastrin) receptors, having a high affinity for both sulfated and nonsulfated CCK analogues, are found principally in the CNS and select areas of the gastrointestinal tract (3,4). Recently, highly selective nonpeptide antagonists have been developed which support this subtype classification. Two of the most potent and selective antagonists are L-364,718 for CCKA receptors (5) and L-365,260, for CCKB receptors(4). Although the physiological and behavioral role of CNS CCKB receptors is not well understood, they have been shown to regulate anxiety, arousal, neuroleptic activity (6,7) and opiate induced analgesia (8,9). Outside the CNS, CCKB receptors regulate gastric acid secretion (10,11) and may play a role in gastrointestinal motility (12,13) and growth of normal and neoplastic gastrointestinal tissue (14).
Little is known about the structure and pharmacology of the human CCKB receptor because of either the difficulty in obtaining fresh tissue or long term culture of normal tissue expressing CCKB receptors. Tumor cell lines from human small cell carcinoma (14), and lymphoblastic T-cells (JURKAT) (15) have been shown to express CCK receptors with a CCKB receptor subtype similar to rat, however, some gastric carcinomas (16), leiomyomas (17) and colonic carcinomas (18) appear to have a unique “CCKB-like” pharmacology and structure. Having recently cloned the rat CCKB receptor cDNA (19), we used this cDNA to isolate the human CCKB receptor cDNA and analyze the receptor structure and functional expression.
MATERIALS AND METHODS
TISSUE PROCUREMENT, RNA ISOLATION, AND cDNA SYNTHESIS:
Human gastric fundus was obtained as a fresh surgical specimen and immediately frozen in liquid nitrogen. Total RNA was isolated using a low temperature guanidine isothiocyanate method (20) and poly (A)+ RNA was isolated using oligo d(T) cellulose chromatography. Oligo d(T) primed cDNA was synthesized using Superscript reverse transcriptase (BRL, Gaithersburg, MD) from one microgram poly (A)+ RNA.
ISOLATION OF cDNA CLONES:
A human frontal cortex cDNA library in the Lambda ZAP II vector (Stratagene, LaJolla, CA) was screened using a [32P] random primed probe derived from the rat CCKB receptor cDNA. Approximately 7.5 × 105 clones were screened at low stringency (3 × 20′ washes at 37°C, 2X SSC/0.l% SDS [1X SSC= .15 M NaCl; 15mM sodium citrate). Positively hybridizing clones were plaque purified.
PCR CLONING:
To obtain a full length human CCKB receptor cDNA, approximately 5 ng of. single stranded human stomach fundus cDNA and the following primers were used in the polymerase chain reaction: a 64 fold degenerate 5′ sense primer: 5′-GGA(G/C)(C/T)TC(A/G)(G/C)(A/T)GG(A/G)GCCATGGA-3′ was derived from a highly conserved 5′ flanking nucleotide sequence of the rat (19), and guinea pig (unpublished data) CCKB receptor cDNA. The 3′ antisense primer (nucleotides 1431 to 1452; Fig. 1) were derived from the 3′ noncoding region of the clone isolated from the human frontal cortex cDNA library described above. Each primer contained an additional 5′ 9 bp cap and Xba I site (ACTGACTAGTCTA) necessary for subsequent ligation into the vector, pCDL-SRα at the Xba-1 restriction enzyme site.
FIG. 1.
Nucleotide and deduced amino acid sequences of the human brain and stomach CCKB receptor cDNA clone. The solid lines labelled with Roman numerals I-VII delineate the putative transmembrane domains predicted by the Kyte-Doolittle criteria (31) and homology with the rat CCKB as well as other G-protein-coupled receptor superfamily members. The solid triangles indicate three potential sites for N-linked glycosylation. The solid underlines indicate potential sites for serine and threonine phosphorylation (25). The AATAAA cleavage and polyadenyalation sequence is underlined. Solid circles indicate cysteine residues which are potential sites for either disulfide bridge formation (26,27,28) (residues 127 and 205) or palmitoylation (29,30) (residue 408).
DNA SEQUENCING:
Both strands of the cDNA clones isolated from the human frontal cortex cDNA library and the product from PCR cloning from the human stomach cDNA were sequenced using the dsDNA Cycle Sequencing System (Bethesda Research Laboratory), Gaithersburg, MD).
DNA AND PROTEIN SEQUENCE ANALYSIS:
The nucleotide and deduced amino acid sequences were analyzed by the Wisconsin Genetics Computer Group software package using the “Pileup” program (21).
NORTHERN BLOT ANALYSIS OF mRNAs:
Approximately 2 μg of poly (A)+ RNA isolated by oligo dT cellulose chromatography were loaded in each lane and were separated electrophoretically on a 1.5% agarose/formaldehyde denaturing gel and blotted onto Nytran (Schleicher and Schucll, Keene, NH). The blot was hybridized with a [32P] random prime labelled probe derived from the coding region of the human CCKB receptor cDNA, and washed at high stringency (3 × 20′ washes with 0.1 X SSC/0.l% SDS @ 42°C). The blot was exposed for 48 hours and processed using a phosphorimager (Molecular Dynamics).
EXPRESSION of HUMAN CCKB RECEPTOR cDNA IN MAMMALIAN CELLS:
The product cloned by PCR amplification from human stomach fundus cDNA described above was digested with Xba I and ligated into the vector pCDL-SRα (22) at the Xba I site in the sense orientation. Two micrograms of vector plus insert were transfected into COS-7 cells (≈1×106 cells per 100 mm tissue culture plate) using the DEAE/dextran method as described (23). Approximately 48 hours after transfection, the cells were washed in phosphate buffered saline (PBS; pH 7.4) containing bovine serum albumin (BSA) 1mg/ml at 4 ° C, scraped from the culture plate and suspended in Dulbecco’s Modified Eagle Medium (DMEM) containing BSA 1 mg/ml, centrifiged (400X g), and suspended in the same medium at 4°C(≈300,000 cells/ml). Suspended cells (500 μl) were incubated for 60 minutes at 37° C with 50 pM of [125 I]Bolton-Hunter-CCK-8 (2200 Ci/mmol) either with or without the indicated concentrations of unlabelled agonist or antagonist. Cells were subsequently washed three times at 4° C with 2 ml PBS containing BSA 1 mg/ml at 4 ° C and filtered on glass fibers filters (Wbatman GF/A) using a suction manifold (Millipore, Bedford, MA). The filters were subsequently assayed for gamma radioactivity (Packard, Auto-Gamma).
CHROMOSOMAL MAPPING:
Human chromosomal localization was Performed using a somatic cell hybrid panel of human-hamster DNAs (BIOS Laboratories, New Haven, CT). Southern hybridization filters were generated from 25 specific hybrid DNA’s (5μg each) digested with the restriction endonuclease Bam Hl. The CCKB receptor probe was then hybridized to these filters using a standard protocol at 65° C with 3 X SSC. The final wash stringency was at 65° C with 1 X SSC.
RESULTS AND DISCUSSION
Screening approximately 7.5 X 10 5 cDNA clones from a human frontal cortex cDNA library in the Lambda ZAP II vector resulted in the isolation of 44 clones that hybridized under low stringency to the rat CCKB receptor probe. The longest plaque purified clone, 2.1 Kb, contained a nucleotide sequence that was 85% homologous to the rat CCKB receptor cDNA. However, the clone contained an intron at the 5′ end and did not contain the first 82 bases of the 5′ cooing region. The remaining sequence was obtained by PCR cloning from the human stomach fundus cDNA. Using the rat (19) and guinea pig (unpublished data) CCKB receptor cDNA sequences a highly homologous 5′ flanking sequence was identified for the design of a 64-fold degenerate sense primer with the following sequence: 5′-GGA(G/C)(C/T)TC(A/G)(G/C)(A/T)GG(A/G)GCCATGGA-3′. The 3′ antisense primer, (5′-CAGGAAACCAACACCCAAAGC-3′) was obtained from the 3′ noncoding region of the clone isolated from the human frontal cortex cDNA library. PCR cloning from the human stomach fundus cDNA resulted in an ≈ 1.45 Kb product with an identical nucleotide sequence to the coding region of the clone isolated from the human frontal cortex cDNA and an additional 82 bp comprising the 5′ coding sequence. This nucleotide sequence plus the 3′ noncoding region of the human frontal cortex clone is 1969 bp in length (Fig. 1). The coding region sequence has approximately an 85% and 87% homology with the rat (19) CCKB receptors and the canine parietal cell gastrin receptor (24) nucleotide coding region, respectively. This high degree of homology is in the expected range for interspecies variation in the same gene. This high degree of sequence homology between the rat and human CCKB receptor and the canine gastrin receptor and the fact that the same CCKB receptor cDNA has been cloned from human brain and stomach as well as the dog brain (unpublished data) and parietal cell (24) suggest that the CCKB receptor and gastrin receptor are identical.
The nucleotide sequence contains a single long open reading frame which encodes for a unique 447 amino acid protein with a calculated molecular mass of ≈48.5 kDa. The receptor is 5 and 6 amino acids less than the rat CCK receptor and canine parietal cell gastrin receptor (24) respectively, principally, because of a loss of a block of 5 amino acids in the third intracellular loop (Fig. 2). The sequence allows for three potential N-linked glycosylation sites, all in the amino terminus (Fig. 1). The number of potential N-linked glycosylation sites is similar to that published for dog (24) and is less than that reported for the rat (19). There are two potential sites for protein kinase C phosphorylation (25) on serines in the first and third intracellular loop (residues 82 and 300, Fig. 1) and three potential sites for protein kinase A phosphorylation on serines in the second intracellular loop and amino terminus (residues 154 and 437) and on threonine in the third intracellular loop (residue 321; Fig. 1) (25). A comparison of the amino acid sequence of the human CCKB receptor with rat CCKB receptor (19) and canine gastrin receptor (24) shows an ≈ 90% identity, with the highest degree of homology in the transmembrane domains and the least degree of homology in the amino and carboxy termini and the third intracellular loop (Fig. 2). Similar to the rat CCKn receptor (19) and the canine gastrin receptor (24), there are conserved cysteines in the first and second extracellular loops which may form a disulfide bridge (residues 107, 157; Fig. 2) required for stabilization of the tertiary structure as demonstrated for rhodopsin(26), ß-adrenergic (27), and muscarinic receptors (28). A cysteine in the carboxy-terminus (residue 408; Fig. 2) may be a membrane anchoring palmitoylation site similar to rhodopsin and the ß-adrenergic receptors (29,30).
FIG. 2.
Alignment of the human CCKB receptor (HUCCKBR), rat CCKB receptor (RATCCKBR) and canine gastrin receptor (CANGASR) deduced protein sequences. Using the “Pileup” program sequence analysis package of the Genetics Computer Group (21) the human CCKB receptor was aligned for maximal homology with the rat CCKB receptor and the canine gastrin receptor. Shown here using amino acid acid letter symbols is the result of this alignment with solid lines indicating putative transmembrane domains and boxed letters indicating amino acids from rat and dog not conserved in the human receptor sequence.
A hydropathy plot (data not shown) using the criteria of Kyte and Doolittle (31) and homology with other G-protein-coupled receptor superfamily members identifies seven regions of hydrophobic residues corresponding to putative transmembrane domains expected for members of the G-protein-coupled superfamily of receptors (32,33).
High stringency northern blot analysis of 2 μg of organ specific polyadenylated mRNA reveals that the CCKB receptor cDNA full-coding-region probe hybridizes to predominantly a 2.8 Kb transcript and to a lesser degree to a 3.3 Kb transcript in stomach fundus, pancreas, and gallbladder. However, in the brain, the probe hybridizes equally to both transcripts (Fig. 3). This transcript size is similar in size to the 2.7 Kb size reported in rats (19), and is larger than the canine gastrin transcript (approximately 2.1 Kb) (24). Both bands were present in all of the tissues expressing transcripts after high stringency washing suggesting that they are probably transcripts from the same gene. The presence of a strongly hybridizing band in human gallbladder is not suprising given that the guinea pig gallbladder has been shown previously to possess CCKB receptors (12).
FIG. 3.
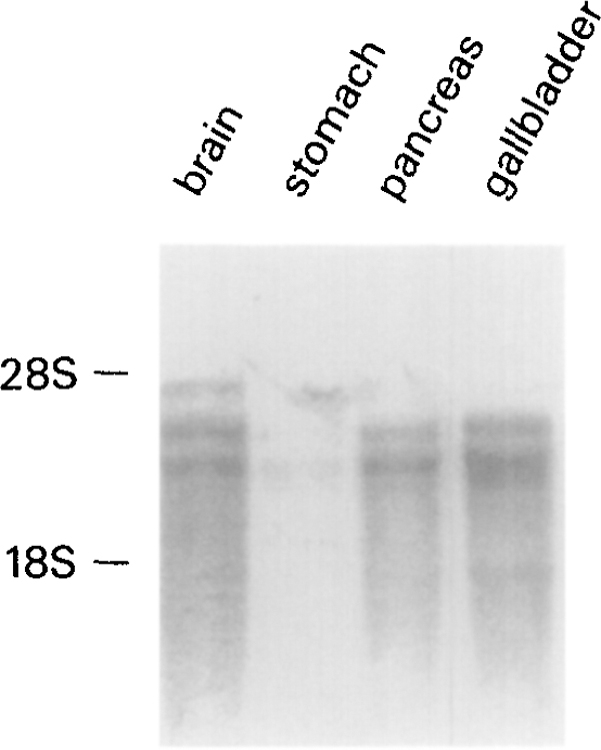
Northern blot analysis of poly (A)+ RNA from the human organs. Two micrograms of poly (A)+ RNA from human brain, stomach, pancreas, and gallbladder were separated on a 1.5% denaturing/formaldehyde agarose gel and probed under conditions of high stringency with the coding region of the CCKB receptor cDNA labelled with [32P] by random primer extension. The blot was exposed for 48 hours and scanned with a phosphorimager (Molecular Dynamics). The lines on the left correspond to the migration Positions of the 28S and 18S ribosomal RNA.
We used the CCKB receptor cDNA as a [32P]random prime labelled probe to hybridize to a blot of human-hamster chromosomal hybrid DNA’s cut with Bam Hl. The presence of an 11 Kb hybridizing fragment in both the hybrid 1049 and the parental human control indicates that the CCKB receptor maps to chromosome 11. Further localization studies will allow more specific mapping of the CCKB receptor gene on chromosome 11.
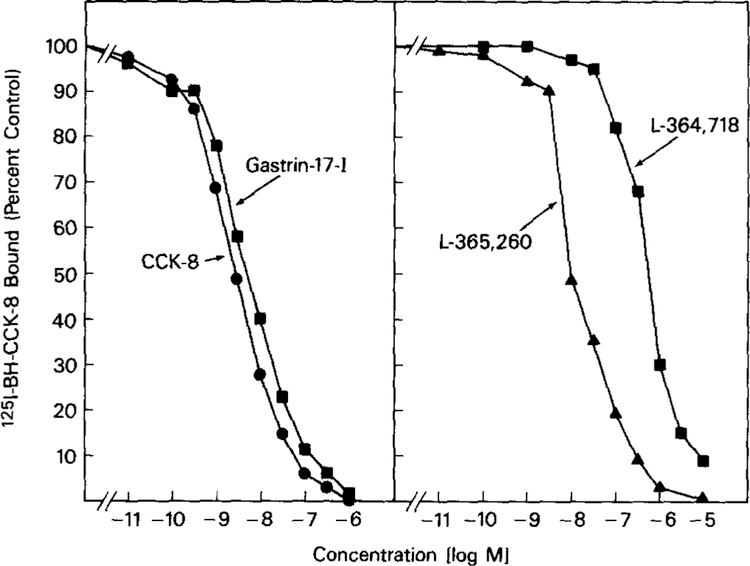
To confirm that the CCKB receptor clone isolated from both human brain and stomach encodes for a functional CCKB receptor, and to demonstrate for the first time, the precise pharmacology of a pure human CCKB receptor, ligand binding dose-inhibition studies were performed. The human CCKB receptor cDNA obtained from PCR cloning (1.45 Kb insert) was cloned into the Xba I site of the mammalian expression vector, pCDL-SRα, and transfected into COS-7 cells using DEAE/dextran. Radioligand binding studies using [125I]-BH-CCK-8 alone or in the presence of increasing concentrations of either unlabelled CCK receptor agonists or antagonists were performed. These studies showed that [125I]-BH-CCK-8 binding inhibition by CCK-8 (EC50 = 3 × 10−9 nM) was 2-fold more potent than gastrin-17-I (EC50 = 6.4 × 10−9 nM) and inhibition by the CCKB receptor antagonist, L-365,260 (EC50 =l × 10−8 nM) was 50-fold more potent than the CCKB receptor specific antagonist L-364,718 (EC50 =5 × 10−7 nM)(Fig. 4). These findings are similar to that reported previously for native rat CCKB receptors (34), in the transformed human T-lymphocyte, JURKAT cells (15), and in a human small cell carcinoma cell line (14). These results differ significantly from the results reported for the canine gastrin receptor (24). The canine parietal cell gastrin receptor has almost a 7-fold greater affinity for the CCKA receptor antagonist L-364,718 than for the gastrin receptor antagonist L-365,260 (24). This divergence in canine gastrin receptor reversal in affinity for the antagonist L-364,718 and L-365,260 (6).
FIG. 4.
Ability of CCK receptor agonists and antagonists to inhibit binding of [125I]BH-CCK-8 to COS-7 cells expressing the human CCKB receptor. COS-7 cells were transfected with the mammalian expression vector, pCDL-SRα, containing the human CCKB receptor cDNA. Transfected COS-7 cells were incubated with either the tracer alone or increasing concentrations of agonists CCK-8 or gastrin-17-I (left panel) or antagonists L-365,260 and L-364,718 (right panel). Data are presented as percent saturable binding (total binding in the presence of labelled hormone alone minus binding in the presence of 1 μM CCK-8).
In the present study we have, using the previously cloned rat CCKB receptor DNA sequence as a probe, cloned from both a human brain cDNA library and from human gastric fundus the human CCKB receptor DNA. The DNA sequence is 90 percent homologous to the rat and dog sequences. Expression of the cloned DNA gives binding characteristics similar to the rat CCKB but different to the dog. Furthermore, we show that the brain and stomach CCKB receptors are identical. These results will enhance our understanding of the central nervous system and gastrointestinal CCKB receptor and permit the introduction of specific agonists and antagonists to this receptor. This will be useful for the elucidation of the mechanisms of various neuropsychiatric diseases and will hasten the treatment of disorders such as anxiety, and panic disorders as well as schizophrenia.
Footnotes
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under Accession No. L04473.
REFERENCES
- 1.Hill DR, Campbell NJ, Shaw TM, and Woodruff GN (1987) J. Neurosci 7, 2967–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen RT, Wank SA, Rowley WH, Sato S, and Gardner JD (1989) Trends Pharmacol. Sci 10, 418–423. [DOI] [PubMed] [Google Scholar]
- 3.Saito A, Sankaran H, Goldfine ID, and Williams JA (1980) Science 1155–l156. [DOI] [PubMed]
- 4.Moran TH, Robinson PH, Goldrich MS, and McHugh PR (1975) Brain Research 362, 986–989. [DOI] [PubMed] [Google Scholar]
- 5.Chang RSL, and Lotti VJ (1986) Proc. Natl. Acad. Sci 834923–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lotti VJ, and Chang RSL (1989) Eur. J. Pharm 162, 273–280. [DOI] [PubMed] [Google Scholar]
- 7.Crawley JN (1991) Trends Pharm. Sci 12, 232–236. [DOI] [PubMed] [Google Scholar]
- 8.Singh L, et al. (1991) Proc. Natl. Acad. Sci (1991) 88, 1130–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisenfeld-Hullin Z, et al. (1990) Proc. Natl. Acad. Sci 87, 7105–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandvik AK, and Waldum HL (1991) Am. J. Physiol 260, G925–G928. [DOI] [PubMed] [Google Scholar]
- 11.Lin CW,et al. (1992) Am. J. Physiol 262, G1113–G1120. [DOI] [PubMed] [Google Scholar]
- 12.Grider JR, and Makhlouf GM (1990) Am. J. Physiol 259, G184–G190. [DOI] [PubMed] [Google Scholar]
- 13.Singh P, et al. (1991) Am. J. Physiol 249, G761–G769. [DOI] [PubMed] [Google Scholar]
- 14.Staley J, Jensen RT, and Moody TW (1990) Peptides 11, 1033–6. [DOI] [PubMed] [Google Scholar]
- 15.Lignon M, Bernad N, and Martinez J (1991) Mol. Pharm 39, 615–620. [PubMed] [Google Scholar]
- 16.Weinstock J, and Baldwin GS (1988) Cancer Research 48, 932–937. [PubMed] [Google Scholar]
- 17.Pearson RK, Hadac M, and Miller LJ (1989) Am. J. Physiol 256, G1005–1010. [DOI] [PubMed] [Google Scholar]
- 18.Chicone l., Narayan S, Townsend CM, and Singh P (1989) Biochem. Biophys. Res. Comm 164, 512–519. [DOI] [PubMed] [Google Scholar]
- 19.Wank SA, Pisegna JR, and de Weerth A (1992) Proc. Natl. Acad. Sci.USA 89, 8691–8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han JH, Stratowa C, and Rutter WJ (1987) Biochem 26, 1617–25. [DOI] [PubMed] [Google Scholar]
- 21.Devereaux J, Haebrli P, and Smithies O (1984) Nuc. Acids Res 12, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takebe Y, et al. (1988) Mol. Cell. Biol 8, 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullen BR (1987) Methods Enzymol 152, 684–704. [DOI] [PubMed] [Google Scholar]
- 24.Kopin AS, et al. (1992) Proc. Natl. Acad. Sci. USA 89, 3605–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennelly PJ, and Krebs EG (1991) J. Biol. Chem 15555–15558. [PubMed]
- 26.Karnik SS, et al. (1988) Proc. Natl. Acad. Sci. USA 85, 8459–8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon RA, et al. (1987) EMBO. J 6, 3269–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulme EC, Birdsall NJ, and Buckley NJ (1990) Ann. Rev. Pharmacol
- 29.O’Dowd B, et al. (1989) J. Biol. Chem 264, 7564–7569. [PubMed] [Google Scholar]
- 30.Ovchinikov YA, Abdulaev NG, and Bogachuk AS(1988) FEBS Lett 230, l–5. [DOI] [PubMed] [Google Scholar]
- 31.Kyte J, and Doolittle RR (1982) J. Mol. Biol 157, 105–132. [DOI] [PubMed] [Google Scholar]
- 32.Lambert M, Bui ND, and Christophe J (1991) Reg. Pep 322,151–167. [DOI] [PubMed] [Google Scholar]
- 33.Merrit JE, Taylor CW, Rubin RP, and Putney JW (1986) Biochem. J 236, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen RT, Huang SC., von Schrenck T, Wank SA, and Gardner JD (1990) in GI Endocrinology: Receptors and Post-Receptor Mechanisms) (Thompson JT, Townsend CM, Greeley GA, Rayford PL Jr., Cooper CW,Singh PO, and Rubin N, Ed.) pp. 95–113. Academic Press, New York, N.Y. [Google Scholar]