Abstract
Motor neuron circuitry is one of the most elaborate circuitries in our body, which ensures voluntary and skilled movement that requires cognitive input. Therefore, both the cortex and the spinal cord are involved. The cortex has special importance for motor neuron diseases, in which initiation and modulation of voluntary movement is affected. Amyotrophic lateral sclerosis (ALS) is defined by the progressive degeneration of both the upper and lower motor neurons, whereas hereditary spastic paraplegia (HSP) and primary lateral sclerosis (PLS) are characterized mainly by the loss of upper motor neurons. In an effort to reveal the cellular and molecular basis of neuronal degeneration, numerous model systems are generated, and mouse models are no exception. However, there are many different levels of complexities that need to be considered when developing mouse models. Here, we focus our attention to the upper motor neurons, which are one of the most challenging neuron populations to study. Since mice and human differ greatly at a species level, but the cells/neurons in mice and human share many common aspects of cell biology, we offer a solution by focusing our attention to the affected neurons to reveal the complexities of diseases at a cellular level and to improve translational efforts.
Keywords: upper motor neurons, ALS, disease models, reporter lines
1. Complexity of the Motor Neuron Circuitry
Movement is one of the most complicated tasks the human body performs. It involves many different neuron and cell types located both in the cerebral cortex and the spinal cord. In addition, the circuitry extends towards muscle, recruiting them as the output of the motor function. Therefore, movement of our muscles is due to an orchestrated and highly controlled set of events that are executed by many different neurons and cells that work together in harmony within the motor neuron circuitry.
The cerebral cortex is the heart of movement as all voluntary movement is initiated in the motor cortex of the brain. The upper motor neurons, which are referred to as corticospinal motor neurons (CSMN) in mice and Betz cells in humans, have a unique importance. They receive many different levels and types of cortical input from long distance projection neurons such as thalamocortical neurons, callosal projection neurons, as well as local circuitry neurons [1,2]. In addition, they are inhibited by an array of different types of inhibitory neurons located both in layer 5 and in layer 2/3 of the cortex. The amount of information the upper motor neurons parallel process within femtoseconds is beyond the capacity of many different neurons in our brain. The upper motor neurons have a long apical dendrite, extending towards the top layers of the brain, and this is the main site of cortical integration. Therefore, the spine density along the apical dendrite and the primary and secondary branches of the apical dendrite, located especially within layer 2/3 and layer 1 is remarkable. Once the upper motor neuron integrates all input coming from many different sources, it may tilt the balance towards generating an action potential, which will be carried long distances towards the spinal cord targets.
Even though upper motor neurons are mostly considered as one neuron type, they can actually be grouped based on their target innervation patterns in the spinal cord. In fact, there is a very precise mapping and target recognition pattern that is developed early postnatally both in mice and humans. The upper motor neurons that innervate the cervical spinal cord are different from the ones that innervate the lumbar spinal cord. The molecular determinants of this targeted precise innervation are beginning to emerge but we still do not know the molecular signature of events that lead to the decision an upper motor neuron makes to exit the corticospinal tract at a precise location and reach out for its targets within the spinal cord. However, this is exceptionally important as it forms the connection between the cortex and the spinal cord and allows the brain to have a direct input to the spinal motor neurons. Even though in humans the cortex–spinal cord connectivity is mostly via direct monosynaptic connections, this is not the case for rodents, especially mice [3]. Many studies have revealed the connectivity patterns between cortex and spinal cord among different species, including cats, dogs, and monkeys [3,4,5]. Interestingly, different species have different types of connectivity patterns and unlike humans they have to rely more on interneurons and interneuron-mediated connections.
The spinal motor neurons also come in many different flavors. They can be grouped based on their size, excitation profile, and the types of muscles they innervate. Therefore, similar to upper motor neurons, not all spinal motor neurons are the same [6]. There is an immense variation among spinal motor neurons as well, and not all degenerate to the same degree and extent in motor neuron diseases, adding one more level of complexity. Interestingly, in some patients, a distinct set of spinal motor neuron may be more vulnerable than others, whereas in other patients, a different group of spinal motor neuron may show initial vulnerability and degeneration. Usually the alpha motor neurons are the ones that are mostly affected and the medium size spiny gamma neurons are spared in ALS patients [6]. The muscle innervation patterns of spinal motor neurons are also of great interest. Not all muscles are innervated with equal distribution of different spinal motor neurons and there is no muscle that is innervated by only one type of spinal motor neuron. There is a plethora of innervation spectrum of muscle fibers by different spinal motor neurons and this variety also adds complexity to the motor neuron circuitry and in part explains the heterogeneity observed in motor neuron disease patients.
Even though the neuronal component is complicated with upper motor neurons, spinal motor neurons, interneurons, and all other excitatory neurons located near and far, there is also a non-neuronal component of the motor neuron circuitry, which includes cells that are not neurons but are as important as neurons for the circuitry function. These cells are mainly astrocytes, microglia, and oligodendrocytes. They have long been considered to have assistive role for proper neuron function. In fact, one of the FDA-approved drugs, Riluzole, acts upon astrocytes and not on motor neurons, to improve the health of the motor neurons. Therefore, improving the health of non-neuronal cells is also critically important for the overall goal of improving the function of motor neuron circuitry. The intricate balance between the neuron–astrocyte interaction and the very many complexities when this interaction is perturbed adds many layers of complexity to the motor neuron circuitry.
Therefore, the motor neuron circuitry with the involvement of numerous different types of neurons in the brain that converge onto upper motor neurons, with non-neuronal cells that play significant roles in synapse formation and maintenance, with the very many different types of spinal motor neurons and the high-level complexity of muscle innervation patterns is one of the most complex systems in our bodies.
2. Developing Mouse Models to Study Upper Motor Neurons
The progressive degeneration of upper motor neurons is accepted as one of the major characteristics of neurodegenerative diseases affecting voluntary movement that require cortical input to the motor neuron circuitry. For example, hereditary spastic paraplegia (HSP) is best characterized by the progressive degeneration of upper motor neurons [7]. The disease manifests itself with stiffness in the legs, paralysis, and motor function defects. Primary lateral sclerosis (PLS) is also characterized by upper motor neuron death and CST (corticospinal tract) degeneration. However, in amyotrophic lateral sclerosis (ALS), both the spinal and corticospinal motor neurons progressively degenerate [8,9,10], adding complexity to the disease [11,12].
Discoveries of the genetic causes of diseases that are characterized by the progressive degeneration of upper motor neurons are emerging with a fast speed. Even though a handful of genes were known about ten years ago, today over 60 genes are identified in association with HSP and PLS [13,14,15,16,17], and 147 genes for ALS [18,19,20,21]. When a new mutation is identified in patients, one of the initial modes of action is to generate mouse models that either overexpresses that very human mutation, or a mouse model that lacks the mouse homolog of the gene of interest. In the knockout (KO) model, the overall impact of the protein product that is coded by that mutated gene is investigated on different organs, but mostly on the central nervous system and the motor neuron circuitry. In the overexpression model, the goal is to investigate the impact of the mutated protein on the health and function of cells/neurons, circuitries, and overall survival.
To date, hundreds of different mouse models are generated to investigate different aspects of the disease with the expectation that the mouse model would mimic disease pathology observed in patients. Especially for the motor neuron diseases, the results have been frustrating. With the exception of a few select cases, most of the mouse models did not develop motor function defects; they were able to walk comparable to control cases—albeit some developed gait defects—had life expectancies similar to healthy controls, their brain structure, thickness, and even overall neuron numbers appeared unchanged. This created an unprecedented frustration in the field. Some even chose to blame the mouse as a model system.
The hSOD1G93A mouse [22] was one of the first models developed and its ability to mimic some of the key features of ALS set the expectation for other mouse models so high that it was expected for a model to display a behavioral outcome. However, we have now come to realize that the SOD1 model was a special case. SOD1 protein is at the heart of many canonical pathways that are important for the health and function of motor neurons, such as oxidative stress, mitochondrial function, axon transport, and the unfolded protein response, all of which are responsible for motor neuron degeneration. Therefore, in the case of SOD1, not only one but many related cellular events and canonical pathways were cumulatively affected, leading to developing an output function that can be measured by behavioral assays and that progressively worsens with age. Unfortunately, not all mouse models were as “lucky”.
Even though the mutated gene in a patient is of great importance for a unique function in motor neurons, its absence or lack of function may not be strong enough to elicit a defect that is easy to measure with behavioral tests in mice. Therefore, the defect remains undetected and it is thus considered “nonexistent” [23,24,25,26,27]. Unfortunately, many of the good models that truly mimic the perturbed biology in affected neurons were considered a “failure” and were put aside without further investigation due to lack of proper outcome measures that could detect true cellular pathology [28,29,30,31]. This has been one of the major limitations in the field.
3. Mouse Models Developed with Genetic Linkage to Upper Motor Neuron Diseases
To date, 60 different genes are identified to cause HSP [13,14,15,16,17]. Mouse models of spastic paraplegia with autosomal dominant [32] and autosomal recessive [33] inheritance patterns have recently been reviewed. Here, we focus on motor neuron diseases with upper motor neuron involvement, and availability of mouse models with special emphasis in upper motor neuron defects (Table 1).
Table 1.
Genes for motor neuron disease with upper motor neuron involvement, the mouse models generated, and the investigation of the cortical component of motor neuron circuitry.
| Disease | Gene | Mouse Model Available | Motor Cortex Involvement |
|---|---|---|---|
| SPG5A | CYP7B1 [34,35] | Y [36,37] | |
| SPG7 | PARAPLEGIN [38,39,40,41,42] | Y [43,44,45] | |
| SPG11 | SPATACSIN [46] | Y [47,48] | Y [47,48] |
| SPG15 | ZFYVE26 (SPASTIZIN) [49] | Y [50] | Y [50] |
| SPG20 | SPARTIN [51,52] | Y [53] | |
| SPG21 | MASPARDIN [54] | Y [25] | N [25] |
| SPG26 | B4GALNT1 (GM1, GALNACT) [55] | Y [23] | N [23] |
| SPG28 | DDHD1 (PAPLA) [56] | Y [57,58] | |
| SPG30 | KIF1A [59] | Y [60,61,62] | |
| SPG35 | FA2H [63] | Y [64,65] | |
| SPG39 | PNPLA6 (NTE) [66] | Y [26,67] | N [26] |
| SPG44 | GJC2 (CX47) [68] | Y [69,70,71,72] | |
| SPG45 | NT5C2 [73] | Y [74] | |
| SPG46 | GBA2 [75,76] | Y [77,78,79,80] | |
| SPG47 | AP-4 [81] | Y [82] | |
| SPG48 | KIAA0415 (AP-5Z1) [83] | Y [84] | Y [84] |
| SPG54 | DDHD2 (KIAA0725P, IPLA1γ) [85] | Y [86,87] | |
| SPG63 | AMPD2 [88] | Y [89,90,91] | |
| SPG64 | ENTPD1 (CD39) [88] | Y [92,93,94] | |
| SPG75 | MAG [88,95] | Y [96,97,98,99,100,101,102,103] | |
| SPG76 | CAPN1 [104] | Y [105,106,107,108] | |
| SPG78 | ATP13A2 [109,110] | Y [111,112,113,114,115,116,117,118] | |
| SPG79 | UCHL1 [119] | Y [120,121,122,123,124,125,126,127] | Y [120,124,125,128,129] |
| SPG3A | ATL1 [130,131,132] | Y [133,134] | |
| SPG4 | SPAST [135,136] | Y [137,138,139,140] | Y [140] |
| SPG6 | NIPA1 (CXFIP1) [141] | Y [142] | |
| SPG8 | KIAA0196 (WASH C5, STRUMPELLIN, RTSC1) [143,144,145] | Y [146] | |
| SPG10 | KIF5A [147] | Y [24,148] | N [24] |
| SPG12 | RTN2 (NSPL1) [149] | Y [150] | |
| SPG13 | SSPD1 (HSP60, HSPD1) [151] | Y [152,153,154,155] | Y [153,155] |
| SPG17 | BSCL2/SEIPIN [156,157] | Y [27,158,159,160] | N [27] |
| SPG31 | REEP1 [161,162] | Y [163,164,165] | Y [163,165] |
| SPG42 | SLC33A1 [166,167] | Y [168] | |
| SPG73 | CPT1C [169] | Y [170,171] | Y [170] |
| SPG1 | L1CAM [172] | Y [173,174,175] | Y [173,174] |
| SPG2 | PLP1 [176,177,178,179] | Y [176,180,181,182,183,184,185,186,187] | |
| PLS | ALS2 [188,189,190,191,192] | Y [28,193,194,195,196,197] | Y [28] |
Genes that are linked to upper motor neuron dysfunction are emerging. For example, mutations in ALDH18A1 [198,199], ERLIN2 [13,16,85,200], TECPR2 [201], C12ORF65 [202], TFG [203], ERLIN1 [88], REEP2 [204], IBA57 [205], FARS2 [206,207], ZFYVE27 (PROTRUDIN) [208], PLSA1 [209,210] genes were detected in a broad spectrum of patients with upper motor neuron involvement. However, mouse models have not yet been generated.
Interestingly, some mouse models were generated even prior to the identification of genes in relation to upper motor neuron dysfunction (Table 1, highlighted green). These models thus were used to investigate pathologies that are not related to motor neuron diseases, such as detection of protein levels in the liver and kidney [37], observation of bile acid synthetic enzyme expression [36], investigating of wavy hair phenotype [57], lung injury [91], understanding prolonged bleeding times [94], colitis [92], investigating vascular and immune abnormalities [211], and diabetic nephropathy [93]. The cortical component was not investigated in detail, even though some of them, such as Gjc2 showed a motor function defect and a phenotype suggesting upper motor neuron involvement. Therefore, we suggest that these mouse models may offer insight to further reveal the underlying causes of upper motor neuron degeneration.
Upon identification of genes that lead to motor neuron diseases when mutated, a number of mouse models have been generated using different mouse genetics. One of the first lines of investigation is performed via behavioral analyses, using a battery of tests including rotarod, beam-walking, clasping response test, extension reflex test, wire hang test, horizontal pole test, treadmill walking, and measuring gait angles and step sequences. Most of the mouse models failed to display a motor dysfunction and were comparable to control groups, (i.e., Cy7b1, L1cam, Ddhd1, Kif1a, Fa2h, Nt5c2, Gba2 Ap4, Ampd2, Entpd1, Atl1, Spast, Kiaa0196, Rtn2, Reep1 mouse models) (Table 1, highlighted in blue).
Interestingly, a small percentage of mouse models did indeed display gait abnormalities, motor function defects that emerge at later ages, and defects that suggest upper motor neuron involvement (i.e., Paraplegin, Spatacsin, Zfyve26, Spartin, Maspardin, B4galnt1, Pnpla6, Gjc2, Ap5, Ddhd2, Mag, Capn1, Atp13a2, Uchl1, Nipa1, Sspd1, Bscl2, Lsc33a1, Cpt1c mouse models, Table 1). Because Kif5a null mutants die immediately after birth, a Synapsin-promoter Cre-recombinase transgene was used for selective inactivation of Kif5a in neurons postnatally. Three fourths of mutant mice exhibited seizures and death at around 3 weeks of age. Nuclear area was found significantly smaller in Kif5a−/− spinal motor neurons in comparison to Kif5a+/+ controls. Kif5a−/− spinal motor neurons, as identified by morphology by anti-Islet, anti-Chat, anti-Map2, and anti-phospho-tau staining, showed reduced survival [24]. Paraplegin-deficient mice were affected by a distal axonopathy of spinal and peripheral axons, characterized by axonal swelling and degeneration. Mitochondrial morphologic abnormalities occurred in synaptic terminals and in distal regions of axons long before the first signs of swelling, and correlated with onset of motor impairment and degeneration. Axonal swellings occurred through massive accumulation of organelles and neurofilaments, suggesting impairment of anterograde axonal transport, while retrograde axonal transport was delayed in symptomatic mice [43]. In addition, an early-onset severe neurologic phenotype in Spg7-null/Afg3l2+/− mice characterized by loss of balance, tremor, and ataxia were detected. These mice displayed acceleration and worsening of the axonopathy as observed in Spg7-null mice [45]. Seipin KO mice displayed anxiety and depression-like symptoms. Neuron-specific Seipin KO mice also showed reduced mRNA and protein levels of Pparg in hippocampus and cortex [160]. Investig.igation of age-related motor dysfunction in Atp13a2 null model revealed gliosis, accumulation of ubiquitinated protein aggregates, lipofuscin, and endolysosomal abnormalities in cortex [113]. Ddhd2−/− mice had shorter stride lengths in gait measurement assays and this locomotor defect was observed both front and hind paws. In addition, it showed significant reduction in rearing behavior and rotarod balance was shortened [87]. The Spartin mouse model generated by targeted disruption of Spg20 gene shows significant gait phenotype and, interestingly, cerebral cortical neurons cultured from Spg20−/− mice exhibited increased axonal branching [53].
To date, four different mouse models for Spast have been generated. To understand the involvement of Spastin in synapse elimination and microtubule destabilization, a Spastin knock out mouse was generated via the “knockout-first” approach. In this mouse, Spastin deletion caused no obvious phenotype in young animals [138,140]. In the SpastKO mice, exons 5–7 of the Spast gene were deleted, introducing an early stop codon. Homozygous mutant mice developed a mild and late onset motor defects at 22 months [137,140]. Axonal swellings, impaired microtubule disassembly and reduced microtubule plus ends were identified, only in homozygous mutant mice. Deletion of mouse Spast gene, generating a premature stop codon, is responsible for axonal degeneration, restricted to the central nervous system, leading to late and mild motor defect [140]. The second SpastKO model was generated by deletion of exon 7 of the Spast gene [138]. Similar to the previous model, axon swellings were present and homozygous mutant mice developed slight gait abnormalities, detected as early as 7 months. In both models, anterograde axonal transport of mitochondria was prominently impaired, while retrograde transport remained relatively intact. The third model is the SpastN386K knock in model, in which N386K was introduced into the endogenous Spast locus within its AAA domain [212]. Only homozygous mutant mice showed abnormalities in gait parameters and axonal swelling were present in cultured cortical neurons. The fourth mouse model is the SPASTC448Y transgenic mice, which is generated by the insertion of the human full-length SPAST harboring C448Y into the Rosa26 locus [139]. Both heterozygous and homozygous mice show severe gait impairment, and male mice display a more severe phenotype.
Zfyve26 deficient mice generated by deleting exon 15 of the Zfyve26 gene. Young Zyfve26 KO mice did not show any obvious abnormalities or altered body weight compared to wild type littermates up to 8 months of age. At 16 months of age, the body weight of the knock out mice was reduced. At 12 months of age, KO animals showed progressive gait disorder and motor deficits. These are quantified by measuring foot base angle at toe off positions of the hind paws. Disruption of Zfyve26 caused severe neuron loss in the motor cortex and cerebellum [47].
The spatacsin mouse model was generated by disruption of Spg11 gene in mice via inserting stop codons in exon 32. It developed early-onset motor impairment and cognitive deficits. The behavioral deficits were associated with progressive brain atrophy with the loss of neurons in the primary motor cortex, cerebellum, and hippocampus as well as accumulation of dystrophic axons in the corticospinal tract. Spinal motor neurons also degenerate [47].
Cpt1c-deficient mice develop early onset of progressive motor disturbances, including impaired gait and coordination, severe muscle weakness and reduced locomotor activity. Cerebellar, striatum, and motor cortex extracts from Cpt1c-KO mice show reduced levels of ceramide and its derivative, sphingosine, mainly during fasting state, compared to wild type mice. Mice were assessed neurologically and behaviorally, and results showed impaired coordination, hypoactivity, and reduced muscle strength. Cpt1c KO mice also showed reduced levels of ceramide and sphingosine in the cerebellum, striatum, and motor cortex detected by Western blot analysis [170].
Heterozygous mice for a KO allele of the Hspd1 gene, encoding Hsp60 (Sspd1), demonstrate that Hspd1 haploinsufficiency is sufficient to cause a late disease onset in mice. These mice were tested behaviorally and analyzed for mitochondrial ATP production. They displayed a marked and progressive deterioration in performance of all motor tests performed compared to wild type littermate control mice [155]. A transgenic mouse in which exon 2 of Reep1 was removed and immunoblot studies with an antibody recognizing a C-terminal epitope showed that full-length Reep1 protein is absent in mice homozygous for the mutant transgene [213]. Behavioral examination of Reep1-null mice that were less than one-year of age did not reveal any obvious motor deficits. Older mice showed changes in hind limb function but more rigorous quantitative analysis revealed the onset of motor deficits at an earlier time point; a change in the foot/base angle during ambulation of 4 to 5-month-old Reep1 KO mice. Evaluation of 13-month old Reep1−/− mice did not reveal decrease in the motor cortex; however, ultrastructural studies of 7.5-month-old mice uncovered axonal deficits in the corticospinal tract. Careful EM studies of layer 5 pyramidal cells in the motor cortex of one-year-old mice showed a Reep1 dose-dependent increase in the average length and decrease in the number of individual ER structures. Loss of Reep1 decreases ER curvature, resulting in a reduction in the apparent number but an increase in the length of ER tubules [163].
Quantitative dendritic tree analysis was performed on layer 3 and layer 5 pyramidal neurons in the primary motor cortex of B4galnt1 null mice. The layer 5 neurons were observed as more mature, having larger cell bodies and more branched dendritic trunks at P3 [23]. However, Calbindin immunohistochemistry was performed to quantify Purkinje cell numbers in Ap5 mouse model [84], and the motor cortex was not investigated in detail. These mice accumulate autofluorescent material in neurons and develop late onset progressive gait abnormalities, recapitulating the human phenotype. In agreement with a role of Ap5 for the retrieval from late endosomes to the trans-Golgi network, several Golgi-related proteins were enriched in lysosomal fractions of KO mouse embryonic fibroblasts.
Since upper motor neurons make up of less than one percent of the motor cortex neurons and cells, it is challenging to reveal the extent of their degeneration, and thus most of these studies concluded no prominent upper motor neuron loss. (i.e., B4galnt1, Pnpla6, Kif5a, Bscl2 mouse models). One example is the Alsin KO mice, which was developed after mutations in the ALSIN gene were detected in juvenile ALS cases [214]. Four different groups generated mouse models for Alsin, but the mice did not have a profound motor function defect, even though it displayed gait abnormalities when aged. Immunocytochemistry analysis using neuronal markers such as Neun, did not reveal neuronal loss, and thus it was concluded at the time that the cortical neurons were unaltered in these mouse models. With the identification of molecular markers that are more specific to upper motor neurons in the motor cortex, such as Ctip2 [215], more cell-type-specific degeneration patterns of upper motor neurons were investigated. For example, in Spatacsin [48] and Uchl1 [120] mouse models, Ctip2 immunocytochemistry suggested a progressive degeneration of upper motor neurons with age, albeit different mouse models displayed different rates and extent of upper motor loss. Similarly, in the Alsin KO mice, the Ctip2 immunocytochemistry revealed upper motor neuron loss that could not be detected by Neun expression [28]. These studies further suggested the importance of visualization and direct cellular assessment of upper motor neurons for revealing the underlying mechanisms that are responsible for their vulnerability and degeneration.
4. Visualization of CSMN
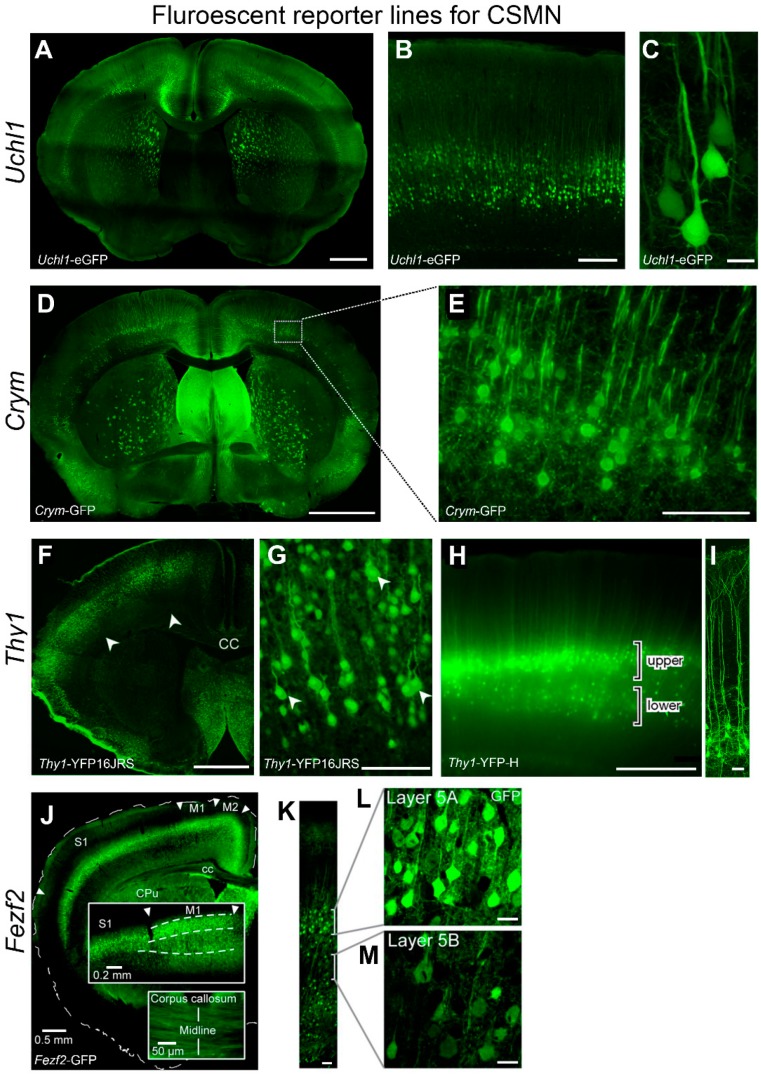
Recently, numerous novel techniques and approaches have been developed to help identify and visualize CSMN within the complex structure of the cerebral cortex. Retrograde labeling surgery, AAV-mediated gene delivery, and novel reporter lines now have the potential to change the future of CSMN investigations [2,120,216,217]. Reporter lines have been very informative on marking the cells and neurons of interest with fluorescence. To date, numerous reporter lines are generated which genetically labels upper motor neurons with fluorescence (Figure 1). Even though the labeling may not be cell-type specific in all of the reporter lines, presence of fluorescence allows detailed cellular analyses. Mu-crystallin (Crym)-GFP reporter is a high-fidelity marker of the CST [218]. CST labeling with Crym-GFP is ten times more efficient compared with BDA; however, low-level expression requires significant amplification of the GFP signal using immunofluorescence techniques. Thy1-YFP mice have extensively been used to study motor systems, not only SMN in mouse models of ALS [219,220], but also CSMN [221,222,223] and their axons in spinal cord injury [224] and an ALS mouse model [225]. Fezf2-GFP labels a heterogenous population of neurons that include corticospinal projection neurons and corticothalamic projection neurons in layer 5A and crossed corticostriatal projection neurons and crossed-corticocortical projection neurons in layer 5B of the mature motor cortex [226].
Figure 1.
Reporter mouse lines available for visualizing CSMN. (A–C) Uchl1-eGFP reporter mouse line. Low magnification image of coronal section through primary motor cortex (A), GFP labeled neurons in layer 5A and 5B of primary motor cortex (B), and high magnification image of CSMN (C). (D,E) Crym-GFP reporter mouse line. Low magnification image of coronal section through primary motor cortex (D), GFP labeled neurons in layer 5 of primary motor cortex (E). (F–I) Thy1-YFP reporter mouse line. Low magnification image of coronal section through primary motor cortex (F), and YFP labeled neurons in layer 5 of primary motor cortex (G) in the Thy1-YFP16JRS mouse. Low magnification image of a section through primary motor cortex (H), and YFP labeled neurons in layer 5 of primary motor cortex (I) in the Thy1-YFP-H mouse. (J–M). Fezf2-GFP reporter mouse line. Low magnification image of coronal section through primary motor cortex (J), GFP labeled neurons in layer 5A and 5B of primary motor cortex (K), and high magnification image of layer 5A (L) and layer 5B (M). Scale bars: 1 mm in (A), 250 μm in (B), 20 μm in (C), 1 mm in (D), 100 μm in (E), 1 mm in (F), 100 μm in (G), 500 μm in (H), 50 μm in (I), 0.5 mm in (J), 50 μm in (K), 20 μm in (L–M).
UCHL1 Offers a Unique Opportunity to Study Upper Motor Neuron Biology
Uchl1-eGFP mice in which the Uchl1 gene promoter is used to drive eGFP expression has been invaluable in selectively labeling CSMN in mice [227]. CSMN identity of eGFP+ neurons was confirmed by retrograde labeling, molecular marker expression profile, electrophysiology, cortical circuit mapping, and mouse genetics studies. CSMN in the motor cortex and their projections were genetically and stably labeled by GFP expression from P0 to P800. In the spinal cord, almost all ChAT+ SMN were eGFP+ at birth but, by P30 eGFP expression, became mostly restricted to a mixture of small α- and γ-SMN that are resistant to generation in motor neuron diseases, such as ALS. Crossing this reporter mouse with hSOD1G93A ALS mouse model [22] generated hSOD1G93A-UeGFP mice, which allowed detailed study of CSMN health with respect to mSOD1 mediated ALS. We observed a progressive degeneration of eGFP+ CSMN, as previously reported [225], with apical dendrite vacuolation and presence of autophagosomes, suggesting an ongoing intrinsic cellular degeneration.
Ubiquitin C-terminal hydrolase ligase 1 (UCHL1) is one of the most abundant proteins in the brain [228,229]. It is an important component of the ubiquitin–proteasome system (UPS) and can either add or remove ubiquitin to polyubiquitin chains [228,229,230]. Inhibition of Uchl1 results in a 50% reduction of free ubiquitin in vitro [231,232]. Absence of Uchl1 function in vivo leads to accumulation of ubiquitinylated proteins in motor cortex and increased ER stress in CSMN [120], enhanced neuronal protein synthesis and proteasomal protein degradation, with endoplasmic reticulum stress, and energy depletion, leading to proteasomal impairment and an accumulation of nondegraded ubiquitinated protein [124]. Increased protein turnover is associated with enhanced mTORC1 activity restricted to the postnatal period in Uchl1-deficient brains [124]. Uchl1 also regulates the balance between mTOR complexes by disrupting mTORC1 and promoting mTORC2 assembly [233]. Overexpression of Uchl1, on the other hand, leads to cancer [234,235]. The active site of Uchl1 required for its hydrolase activity contains a triad of Cys90, His161, and Asp176 [228,236]. Catalytically inactive Uchl1TgC90A mice indicate that its catalytic activity is essential for the oncogenic effects of Uchl1 in mice [233].
Mutations in the UCHL1 gene cause autosomal recessive spastic paraplegia-79 (SPG79) (MIM Number: #615491) [14,119,237,238]. The UCHL1GLU7ALA missense mutation identified in a Turkish family lies within the ubiquitin binding domain of UCHL1 protein and leads to near complete loss of hydrolase function [119]. All three siblings homozygous for the mutation have spasticity with upper motor neuron dysfunction, accompanied by early onset blindness, cerebellar ataxia, nystagmus, and dorsal column dysfunction. Two other missense mutations in the UCHL1 gene were identified in a Norwegian family [238]. Three siblings with compound heterozygous mutations UCHL1ARG178GLN and UCHL1ALA216ASP developed spasticity and ataxia following child onset blindness. Whereas UCHL1ALA216ASP was reported to be insoluble and therefore nonfunctional, UCHL1ARG178GLN mutation affects a rate-controlling residue in catalysis leading to a four-fold increase in hydrolytic activity of the UCHL1 protein. Recently, a third family from India was reported with two siblings carrying a deleterious homozygous splice-site variant predicted to cause splicing aberrations [237]. Both siblings have spasticity and child onset optic atrophy. Clinical features of all eight patients from three families are comparable and include spasticity, indicating upper motor neuron involvement.
There are several mouse models of Uchl1 available, some have spontaneous deletions within the Uchl1 region, and others with targeted deletions to generate Uchl1 KO mice. Uchl1nm3419 mice arose as a spontaneous deletion in the BL6 colony of Jackson laboratories displaying motor defects, and later were identified to carry a 795 base-pair intragenic deletion that results in the removal of 24 base-pairs of exon 6 and 771 base-pairs of intron 6 [122]. The Uchl1nm3419 mice, which lack all Uchl1 function display motor function defects as revealed by rotarod and grip test analysis [120]. By using known molecular markers of CSMN such as Ctip2 [215] or using retrograde labeling surgery [2], we were able to show progressive CSMN loss and cellular degeneration that is revealed by vacuolated apical dendrites, spine loss, and increased ER stress [120]. Interestingly, the SMN are also affected, even though they do not undergo massive cell loss as observed in CSMN. Muscular atrophy and distal degeneration of SMN axons is observed in which the neuromuscular junctions (NMJ) are denervated and lose their integrity [128].
Gracile axonal dystrophy (Gad) mice also have a spontaneous deletion but include the exons 7 and 8 encoding a truncated Uchl1 lacking a segment of 42 amino acids and containing a catalytic residue instead [121,126]. Gad mice develop sensory and motor ataxia, hindlimb paralysis, and degeneration of distal motor axons [121,129,239]. Unfortunately, to our knowledge, the motor cortex of these mice has never been studied and the CSMN degeneration remains to be investigated.
A Uchl1 KO mouse model has been generated with targeted deletion of a region containing exons 6 through 8 and the first six base pairs of exon 9 of Uchl1 [125]. Similar to spontaneous deletions of Uchl1, these animals also develop an ataxic phenotype with progressive motor defects leading to paralysis and degeneration of motor axons at the NMJs. Motor cortex and CSMN involvement has not been investigated. Recently, a floxed Uchl1 mouse line has been generated in which the exons 1–3 of the Uchl1 gene are flanked by loxP sites [124]. By crossing the floxed Uchl1 mice with constitutive deleter Cre mice, constitutive Uchl1 deficient (Uchl1d/d) mice have been generated, which developed progressive motor defects similar to other Uchl1 mouse models, including reduced performance in accelerating rotarod and open field tests and reduced forelimb strength. In whole brain lysates, levels of polyubiquitinated proteins were drastically decreased in 3-week-old Uchl1d/d mice and remained lower compared with Uchl1+/+ mice. Uchl1 deficiency resulted in decreased levels of polyubiquitinated proteins in juvenile mice, followed by an abnormal accumulation of polyubiquitinated proteins in old adult mice, resulting in an upregulation of proteasomal levels and ER stress. Data suggests Uchl1 is involved in regulation of protein synthesis in neurons before the first symptoms are observed and Uchl1 deficiency enhances mTOR activation. Although increased ER stress in the absence of Uchl1 is in line with our findings in the Uchl1nm3419 mice, CSMN loss and degeneration has not been investigated in this mouse model.
Although several Uchl1 mouse models exist, CSMN degeneration has been extensively studied mainly in the Uchl1nm3419 mice [120]. Thus, the Uchl1nm3419 mouse model remains the best characterized motor neuron disease model in terms of upper motor neuron involvement [120]. The Uchl1 null mice reveals very important knowledge on the cellular events that are responsible for upper motor neuron degeneration.
5. Shifting Focus from Mice to Neurons Generates Translational Outcomes
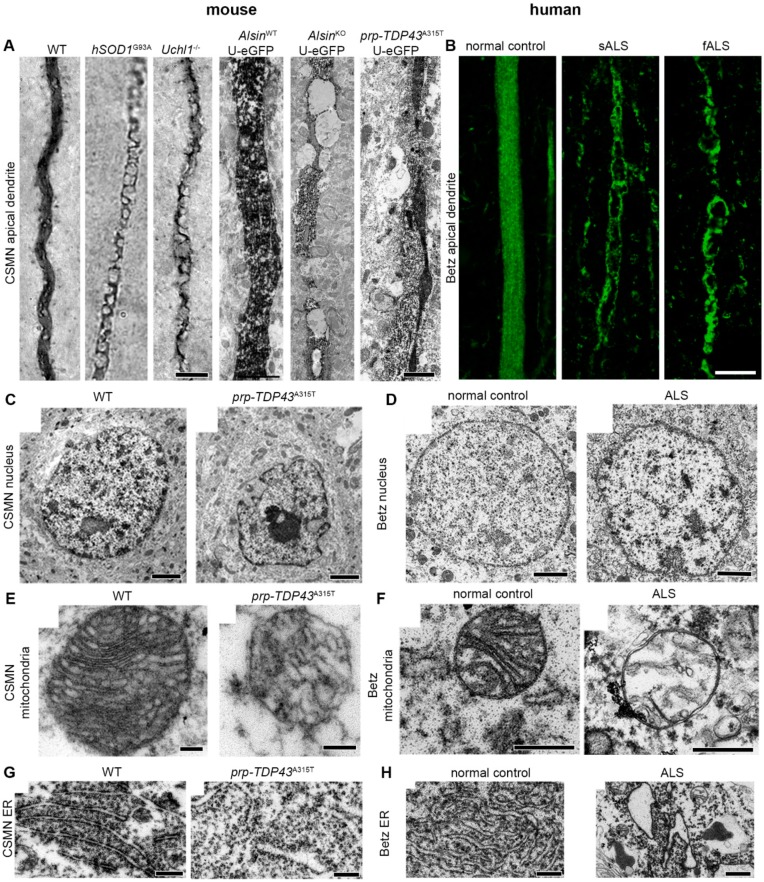
Compounds that extended the life-span of mouse models failed to improve the life-span of patients and this was considered as a “failure” for translational efforts [240,241,242,243,244,245,246,247,248]. However, mouse models remained one of the most essential components of preclinical investigations [248]. Therefore, it is important to choose the model that best represents the biology of interest. In addition, being able to visualize and cellularly assess the direct response of diseased neurons to treatment is of great importance. Many mouse neurons are similar and almost identical to the neurons in humans. Their birth, differentiation, maturation, target recognition, and circuitry integration patterns appear to be very similar. We also found that the cellular basis of upper motor neuron degeneration is almost identical between mice and humans. For example, the apical dendrite degeneration detected in many different mouse models of ALS was also detected in a broad spectrum of ALS patients, including sALS, fALS, and ALS/FTLD cases. The nucleocytoplasmic transport defects reporter in patients with TDP-43 pathology was also observed in CSMN of mice with Tdp-43 pathology. The mitochondrial defects, problems with ER were all comparable and similar between two species, when the vulnerable neurons were investigated at a cellular level (Figure 2) [249,250]. This very close correlation and direct recapitulation of cellular events in two different species led us to believe that the translation will be at a cellular level and that focusing our attention to the vulnerable and diseased neurons of well-defined mouse models of the diseases will inform us on the vulnerable and diseased neurons of patients, and this information forms the foundation for all translational research [251,252].
Figure 2.
Betz cell pathology is similar between mouse and human at a cellular level. (A) Apical dendrites of CSMN displaying vacuoles in brains of various mouse models of motor neuron disease. (B) Apical dendrites of Betz cells displaying vacuoles in brains of patients with sporadic or familial ALS. (C–H) Electron microscope images showing pathology of various organelles in CSMN of prp-TDP43A315T mouse and Betz cells of ALS patients. Observe similar nuclear membrane defects (C,D), mitochondria defects (E,F), and endoplasmic reticulum defects (G,H). Scale bars: 5 μm (brightfield, left), and 1 μm (E.M., right) in (A), 10 μm in (B), 2 μm in (C), 500 nm in (D), 200 nm in (E), 500 nm in (F), 1 μm in (G–H).
Taking advantage of the existing mouse models for the numerous genes that are known to cause motor neurons disease, we now can cross them with Uchl1-eGFP mice or other well-defined GFP reporter lines, retrogradely label CSMN surgically using fluorescent markers or AAV containing GFP expression vectors to study them at a cellular level. This will not only allow us to visualize the CSMN in these animal models, but also to isolate and purify them for further omics approaches that would shed light on the mechanism of disease onset and progression.
In some cases, more detailed functional assays are required to assess the importance of the identified gene or pathway in upper motor neuron health and survival. Crossing genes of interest that are floxed with Rbp4cre mice that express Cre recombinase, and the control of the Rbp4 promoter targeting subcerebral projection neurons that lie in layer 5, including the CSMN in the primary motor cortex, [253,254,255,256] would allow us to investigate the impact of genetic alterations selectively in CSMN, without affecting other cells and neurons in the circuitry. This approach is also very powerful to determine whether genes of interest would be potential drug targets in patients.
In neurodegenerative diseases, not all neurons are affected to the same extent. While some show initial vulnerability, many others remain unaffected until the end-stages of the diseases. Therefore, it is critically important to understand why a particular neuron population begins to suffer much earlier than others. This line of investigation is only possible when we bring clarity and transparency to cellular analyses. Since the cerebral cortex is very heterogenous and complex, this has been challenging. However, thanks to current developments, it is now possible to visualize and study distinct neuron populations with cellular precision that was not possible before. Such studies reveal very strong correlation between the upper motor neurons in mice and humans, reinforcing the idea that the upper motor neurons in two different species are almost identical at a cellular level. Focusing our attention on the neurons that display early vulnerability and undergo progressive degeneration in diseases will be translational and transformative. At the end of the day, our goal is not to cure the mice, but to improve the health of the neurons that degenerate. Shifting our focus from mice to neurons will help generate the translational information needed for building effective treatment strategies for patients. We now have the appropriate tools to shed light onto upper motor neurons and to build effective treatment strategies for diseases in which voluntary movement is affected.
Acknowledgments
We thank members of the Ozdinler lab for constructive criticism and edits of the manuscript.
Abbreviations
| ALS | Amyotrophic Lateral Sclerosis |
| PLS | Primary Lateral Sclerosis |
| HSP | Hereditary Spastic Paraplegia |
| CSMN | corticospinal motor neuron(s) |
| CST | corticospinal tract |
| EM | electron microscopy |
| ER | endoplasmic reticulum |
| UCHL1 | ubiquitin C-terminal hydrolase ligase 1 |
| UMN | upper motor neuron |
| GFP | green fluorescent protein |
| KO | green fluorescent protein |
Author Contributions
B.G., O.G., and P.H.O. contributed to writing the manuscript. B.G. and O.G. prepared the table. B.G. prepared the figures.
Funding
This research received funding from Les Turner ALS Foundation (P.H.O.) and NIH R21NS093557 (P.H.O.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Shepherd G.M. Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 2013;14:278–291. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jara J.H., Genc B., Klessner J.L., Ozdinler P.H. Retrograde labeling, transduction, and genetic targeting allow cellular analysis of corticospinal motor neurons: Implications in health and disease. Front. Neuroanat. 2014;8:16. doi: 10.3389/fnana.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemon R.N. Descending pathways in motor control. Annu. Rev. Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 4.Lemon R. Recent advances in our understanding of the primate corticospinal system. F1000Research. 2019;8 doi: 10.12688/f1000research.17445.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oudega M., Perez M.A. Corticospinal reorganization after spinal cord injury. J. Physiol. 2012;590:3647–3663. doi: 10.1113/jphysiol.2012.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanning K.C., Kaplan A., Henderson C.E. Motor neuron diversity in development and disease. Annu. Rev. Neurosci. 2010;33:409–440. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- 7.Fink J.K. Progressive spastic paraparesis: Hereditary spastic paraplegia and its relation to primary and amyotrophic lateral sclerosis. Semin. Neurol. 2001;21:199–207. doi: 10.1055/s-2001-15265. [DOI] [PubMed] [Google Scholar]
- 8.Udaka F., Kameyama M., Tomonaga M. Degeneration of Betz cells in motor neuron disease. A Golgi study. Acta Neuropathol. 1986;70:289–295. doi: 10.1007/BF00686086. [DOI] [PubMed] [Google Scholar]
- 9.Brown R.H., Jr., Robberecht W. Amyotrophic lateral sclerosis: Pathogenesis. Semin. Neurol. 2001;21:131–139. doi: 10.1055/s-2001-15260. [DOI] [PubMed] [Google Scholar]
- 10.Baker M.R. ALS—dying forward, backward or outward? Nat. Rev. Neurosci. 2014;10:660. doi: 10.1038/nrneurol.2013.221-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravits J., Paul P., Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007;68:1571–1575. doi: 10.1212/01.wnl.0000260965.20021.47. [DOI] [PubMed] [Google Scholar]
- 12.Eisen A., Weber M. The motor cortex and amyotrophic lateral sclerosis. Muscle Nerve. 2001;24:564–573. doi: 10.1002/mus.1042. [DOI] [PubMed] [Google Scholar]
- 13.Fink J.K. Hereditary spastic paraplegia: Clinico-pathologic features and emerging molecular mechanisms. Acta Neuropathol. 2013;126:307–328. doi: 10.1007/s00401-013-1115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackstone C. Converging cellular themes for the hereditary spastic paraplegias. Curr. Opin. Neuropathol. 2018;51:139–146. doi: 10.1016/j.conb.2018.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackstone C. Cellular pathways of hereditary spastic paraplegia. Annu. Rev. Neurosci. 2012;35:25–47. doi: 10.1146/annurev-neuro-062111-150400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boutry M., Morais S., Stevanin G. Update on the Genetics of Spastic Paraplegias. Curr. Neurol. Neurosci. Rep. 2019;19:18. doi: 10.1007/s11910-019-0930-2. [DOI] [PubMed] [Google Scholar]
- 17.Bis-Brewer D.M., Zuchner S. Perspectives on the Genomics of HSP Beyond Mendelian Inheritance. Front. Neurol. 2018;9:958. doi: 10.3389/fneur.2018.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dervishi I., Gozutok O., Murnan K., Gautam M., Heller D., Bigio E., Ozdinler P.H. Protein-protein interactions reveal key canonical pathways, upstream regulators, interactome domains, and novel targets in ALS. Sci. Rep. 2018;8:14732. doi: 10.1038/s41598-018-32902-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chia R., Chio A., Traynor B.J. Novel genes associated with amyotrophic lateral sclerosis: Diagnostic and clinical implications. Lancet Neurol. 2018;17:94–102. doi: 10.1016/S1474-4422(17)30401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghasemi M., Brown R.H., Jr. Genetics of Amyotrophic Lateral Sclerosis. Cold Spring Harb. Perspect. Med. 2018;8 doi: 10.1101/cshperspect.a024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor J.P., Brown R.H., Jr., Cleveland D.W. Decoding ALS: From genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D., Caliendo J., Hentati A., Kwon Y.W., Deng H.X., et al. Motor neuron degeneration in mice that express a human CuZn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 23.Dobrovic B., Curic G., Petanjek Z., Heffer M. Dendritic morphology and spine density is not altered in motor cortex and dentate granular cells in mice lacking the ganglioside biosynthetic gene B4galnt1—A quantitative Golgi cox study. Coll. Antropol. 2011;35:25–30. [PubMed] [Google Scholar]
- 24.Karle K.N., Mockel D., Reid E., Schols L. Axonal transport deficit in a KIF5A(-/-) mouse model. Neurogenetics. 2012;13:169–179. doi: 10.1007/s10048-012-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soderblom C., Stadler J., Jupille H., Blackstone C., Shupliakov O., Hanna M.C. Targeted disruption of the Mast syndrome gene SPG21 in mice impairs hind limb function and alters axon branching in cultured cortical neurons. Neurogenetics. 2010;11:369–378. doi: 10.1007/s10048-010-0252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winrow C.J., Hemming M.L., Allen D.M., Quistad G.B., Casida J.E., Barlow C. Loss of neuropathy target esterase in mice links organophosphate exposure to hyperactivity. Nat. Genet. 2003;33:477–485. doi: 10.1038/ng1131. [DOI] [PubMed] [Google Scholar]
- 27.Yagi T., Ito D., Nihei Y., Ishihara T., Suzuki N. N88S seipin mutant transgenic mice develop features of seipinopathy/BSCL2-related motor neuron disease via endoplasmic reticulum stress. Hum. Mol. Genet. 2011;20:3831–3840. doi: 10.1093/hmg/ddr304. [DOI] [PubMed] [Google Scholar]
- 28.Gautam M., Jara J.H., Sekerkova G., Yasvoina M.V., Martina M., Ozdinler P.H. Absence of alsin function leads to corticospinal motor neuron vulnerability via novel disease mechanisms. Hum. Mol. Genet. 2016;25:1074–1087. doi: 10.1093/hmg/ddv631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Of men, not mice. Nat. Med. 2013;19:379. doi: 10.1038/nm.3163. [DOI] [PubMed] [Google Scholar]
- 30.Janus C., Welzl H. Mouse models of neurodegenerative diseases: Criteria and general methodology. Methods Mol. Boil. 2010;602:323–345. doi: 10.1007/978-1-60761-058-8_19. [DOI] [PubMed] [Google Scholar]
- 31.Ransohoff R.M. All (animal) models (of neurodegeneration) are wrong. Are they also useful? J. Exp. Med. 2018;215:2955–2958. doi: 10.1084/jem.20182042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fassier C., Hazan J., Melki J. Movement Disorders: Genetics and Models. 2nd ed. Academic Press; Cambridge, MA, USA: 2015. Mouse Models of Autosomal Dominant Spastic Paraplegia; pp. 1073–1086. [Google Scholar]
- 33.Blackstone C. Movement Disorders: Genetics and Models. 2nd ed. Academic Press; Cambridge, MA, USA: 2015. Murine Models of Autosomal Recessive Hereditary Spastic Paraplegia; pp. 1087–1093. [Google Scholar]
- 34.Hentati A., Pericak-Vance M.A., Hung W.Y., Belal S., Laing N., Boustany R.M., Hentati F., Ben Hamida M., Siddique T. Linkage of ‘pure’ autosomal recessive familial spastic paraplegia to chromosome 8 markers and evidence of genetic locus heterogeneity. Hum. Mol. Genet. 1994;3:1263–1267. doi: 10.1093/hmg/3.8.1263. [DOI] [PubMed] [Google Scholar]
- 35.Tsaousidou M.K., Ouahchi K., Warner T.T., Yang Y., Simpson M.A., Laing N.G., Wilkinson P.A., Madrid R.E., Patel H., Hentati F., et al. Sequence alterations within CYP7B1 implicate defective cholesterol homeostasis in motor-neuron degeneration. Am. J. Hum. Genet. 2008;82:510–515. doi: 10.1016/j.ajhg.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biddinger S.B., Haas J.T., Yu B.B., Bezy O., Jing E., Zhang W., Unterman T.G., Carey M.C., Kahn C.R. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med. 2008;14:778–782. doi: 10.1038/nm1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li-Hawkins J., Lund E.G., Turley S.D., Russell D.W. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J. Biol. Chem. 2000;275:16536–16542. doi: 10.1074/jbc.M001811200. [DOI] [PubMed] [Google Scholar]
- 38.Garner C.C., Garner A., Huber G., Kozak C., Matus A. Molecular cloning of microtubule-associated protein 1 (MAP1A) and microtubule-associated protein 5 (MAP1B): Identification of distinct genes and their differential expression in developing brain. J. Neurochem. 1990;55:146–154. doi: 10.1111/j.1471-4159.1990.tb08832.x. [DOI] [PubMed] [Google Scholar]
- 39.De Michele G., De Fusco M., Cavalcanti F., Filla A., Marconi R., Volpe G., Monticelli A., Ballabio A., Casari G., Cocozza S. A new locus for autosomal recessive hereditary spastic paraplegia maps to chromosome 16q24.3. Am. J. Hum. Genet. 1998;63:135–139. doi: 10.1086/301930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casari G., De Fusco M., Ciarmatori S., Zeviani M., Mora M., Fernandez P., De Michele G., Filla A., Cocozza S., Marconi R., et al. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell. 1998;93:973–983. doi: 10.1016/S0092-8674(00)81203-9. [DOI] [PubMed] [Google Scholar]
- 41.Koyama K., Emi M., Nakamura Y. The cell adhesion regulator (CAR) gene, TaqI and insertion/deletion polymorphisms, and regional assignment to the peritelomeric region of 16q by linkage analysis. Genomics. 1993;16:264–265. doi: 10.1006/geno.1993.1173. [DOI] [PubMed] [Google Scholar]
- 42.Settasatian C., Whitmore S.A., Crawford J., Bilton R.L., Cleton-Jansen A.M., Sutherland G.R., Callen D.F. Genomic structure and expression analysis of the spastic paraplegia gene, SPG7. Hum. Genet. 1999;105:139–144. doi: 10.1007/s004399900087. [DOI] [PubMed] [Google Scholar]
- 43.Ferreirinha F., Quattrini A., Pirozzi M., Valsecchi V., Dina G., Broccoli V., Auricchio A., Piemonte F., Tozzi G., Gaeta L., et al. Axonal degeneration in paraplegin-deficient mice is associated with abnormal mitochondria and impairment of axonal transport. J. Clin. Investig. 2004;113:231–242. doi: 10.1172/JCI200420138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pirozzi M., Quattrini A., Andolfi G., Dina G., Malaguti M.C., Auricchio A., Rugarli E.I. Intramuscular viral delivery of paraplegin rescues peripheral axonopathy in a model of hereditary spastic paraplegia. J. Clin. Investig. 2006;116:202–208. doi: 10.1172/JCI26210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinelli P., La Mattina V., Bernacchia A., Magnoni R., Cerri F., Cox G., Quattrini A., Casari G., Rugarli E.I. Genetic interaction between the m-AAA protease isoenzymes reveals novel roles in cerebellar degeneration. Hum. Mol. Genet. 2009;18:2001–2013. doi: 10.1093/hmg/ddp124. [DOI] [PubMed] [Google Scholar]
- 46.Martinez Murillo F., Kobayashi H., Pegoraro E., Galluzzi G., Creel G., Mariani C., Farina E., Ricci E., Alfonso G., Pauli R.M., et al. Genetic localization of a new locus for recessive familial spastic paraparesis to 15q13-15. Neurology. 1999;53:50–56. doi: 10.1212/WNL.53.1.50. [DOI] [PubMed] [Google Scholar]
- 47.Branchu J., Boutry M., Sourd L., Depp M., Leone C., Corriger A., Vallucci M., Esteves T., Matusiak R., Dumont M., et al. Loss of spatacsin function alters lysosomal lipid clearance leading to upper and lower motor neuron degeneration. Neurobiol. Dis. 2017;102:21–37. doi: 10.1016/j.nbd.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varga R.E., Khundadze M., Damme M., Nietzsche S., Hoffmann B., Stauber T., Koch N., Hennings J.C., Franzka P., Huebner A.K., et al. In Vivo Evidence for Lysosome Depletion and Impaired Autophagic Clearance in Hereditary Spastic Paraplegia Type SPG11. PLoS Genet. 2015;11:e1005454. doi: 10.1371/journal.pgen.1005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes C.A., Byrne P.C., Webb S., McMonagle P., Patterson V., Hutchinson M., Parfrey N.A. SPG15, a new locus for autosomal recessive complicated HSP on chromosome 14q. Neurology. 2001;56:1230–1233. doi: 10.1212/WNL.56.9.1230. [DOI] [PubMed] [Google Scholar]
- 50.Khundadze M., Kollmann K., Koch N., Biskup C., Nietzsche S., Zimmer G., Hennings J.C., Huebner A.K., Symmank J., Jahic A., et al. A hereditary spastic paraplegia mouse model supports a role of ZFYVE26/SPASTIZIN for the endolysosomal system. PLoS Genet. 2013;9:e1003988. doi: 10.1371/journal.pgen.1003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel H., Cross H., Proukakis C., Hershberger R., Bork P., Ciccarelli F.D., Patton M.A., McKusick V.A., Crosby A.H. SPG20 is mutated in Troyer syndrome, an hereditary spastic paraplegia. Nat. Genet. 2002;31:347–348. doi: 10.1038/ng937. [DOI] [PubMed] [Google Scholar]
- 52.Cross H.E., McKusick V.A. The Troyer syndrome. A recessive form of spastic paraplegia with distal muscle wasting. Arch. Neurol. 1967;16:473–485. doi: 10.1001/archneur.1967.00470230025003. [DOI] [PubMed] [Google Scholar]
- 53.Renvoise B., Stadler J., Singh R., Bakowska J.C., Blackstone C. Spg20-/-mice reveal multimodal functions for Troyer syndrome protein spartin in lipid droplet maintenance, cytokinesis and BMP signaling. Hum. Mol. Genet. 2012;21:3604–3618. doi: 10.1093/hmg/dds191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simpson M.A., Cross H., Proukakis C., Pryde A., Hershberger R., Chatonnet A., Patton M.A., Crosby A.H. Maspardin is mutated in mast syndrome, a complicated form of hereditary spastic paraplegia associated with dementia. Am. J. Hum. Genet. 2003;73:1147–1156. doi: 10.1086/379522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkinson P.A., Simpson M.A., Bastaki L., Patel H., Reed J.A., Kalidas K., Samilchuk E., Khan R., Warner T.T., Crosby A.H. A new locus for autosomal recessive complicated hereditary spastic paraplegia (SPG26) maps to chromosome 12p11.1-12q14. J. Med. Gene. 2005;42:80–82. doi: 10.1136/jmg.2004.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouslam N., Benomar A., Azzedine H., Bouhouche A., Namekawa M., Klebe S., Charon C., Durr A., Ruberg M., Brice A., et al. Mapping of a new form of pure autosomal recessive spastic paraplegia (SPG28) Ann. Neurol. 2005;57:567–571. doi: 10.1002/ana.20416. [DOI] [PubMed] [Google Scholar]
- 57.Inoue A., Arima N., Ishiguro J., Prestwich G.D., Arai H., Aoki J. LPA-producing enzyme PA-PLA(1)alpha regulates hair follicle development by modulating EGFR signalling. EMBO J. 2011;30:4248–4260. doi: 10.1038/emboj.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baba T., Kashiwagi Y., Arimitsu N., Kogure T., Edo A., Maruyama T., Nakao K., Nakanishi H., Kinoshita M., Frohman M.A., et al. Phosphatidic acid (PA)-preferring phospholipase A1 regulates mitochondrial dynamics. J. Biol. Chem. 2014;289:11497–11511. doi: 10.1074/jbc.M113.531921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klebe S., Azzedine H., Durr A., Bastien P., Bouslam N., Elleuch N., Forlani S., Charon C., Koenig M., Melki J., et al. Autosomal recessive spastic paraplegia (SPG30) with mild ataxia and sensory neuropathy maps to chromosome 2q37.3. Brain. 2006;129:1456–1462. doi: 10.1093/brain/awl012. [DOI] [PubMed] [Google Scholar]
- 60.Kondo M., Takei Y., Hirokawa N. Motor protein KIF1A is essential for hippocampal synaptogenesis and learning enhancement in an enriched environment. Neuron. 2012;73:743–757. doi: 10.1016/j.neuron.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 61.Yonekawa Y., Harada A., Okada Y., Funakoshi T., Kanai Y., Takei Y., Terada S., Noda T., Hirokawa N. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J. Cell Biol. 1998;141:431–441. doi: 10.1083/jcb.141.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meixner M., Jungnickel J., Grothe C., Gieselmann V., Eckhardt M. Myelination in the absence of UDP-galactose:ceramide galactosyl-transferase and fatty acid 2-hydroxylase. BMC Neurosci. 2011;12:22. doi: 10.1186/1471-2202-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dick K.J., Al-Mjeni R., Baskir W., Koul R., Simpson M.A., Patton M.A., Raeburn S., Crosby A.H. A novel locus for an autosomal recessive hereditary spastic paraplegia (SPG35) maps to 16q21-q23. Neurology. 2008;71:248–252. doi: 10.1212/01.wnl.0000319610.29522.8a. [DOI] [PubMed] [Google Scholar]
- 64.Potter K.A., Kern M.J., Fullbright G., Bielawski J., Scherer S.S., Yum S.W., Li J.J., Cheng H., Han X., Venkata J.K., et al. Central nervous system dysfunction in a mouse model of FA2H deficiency. Glia. 2011;59:1009–1021. doi: 10.1002/glia.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zoller I., Meixner M., Hartmann D., Bussow H., Meyer R., Gieselmann V., Eckhardt M. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J. Neurosci. 2008;28:9741–9754. doi: 10.1523/JNEUROSCI.0458-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rainier S., Bui M., Mark E., Thomas D., Tokarz D., Ming L., Delaney C., Richardson R.J., Albers J.W., Matsunami N., et al. Neuropathy target esterase gene mutations cause motor neuron disease. Am. J. Hum. Genet. 2008;82:780–785. doi: 10.1016/j.ajhg.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moser M., Li Y., Vaupel K., Kretzschmar D., Kluge R., Glynn P., Buettner R. Placental failure and impaired vasculogenesis result in embryonic lethality for neuropathy target esterase-deficient mice. Mol. Cell Biol. 2004;24:1667–1679. doi: 10.1128/MCB.24.4.1667-1679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orthmann-Murphy J.L., Salsano E., Abrams C.K., Bizzi A., Uziel G., Freidin M.M., Lamantea E., Zeviani M., Scherer S.S., Pareyson D. Hereditary spastic paraplegia is a novel phenotype for GJA12/GJC2 mutations. Brain. 2009;132:426–438. doi: 10.1093/brain/awn328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Georgiou E., Sidiropoulou K., Richter J., Papaneophytou C., Sargiannidou I., Kagiava A., von Jonquieres G., Christodoulou C., Klugmann M., Kleopa K.A. Gene therapy targeting oligodendrocytes provides therapeutic benefit in a leukodystrophy model. Brain. 2017;140:599–616. doi: 10.1093/brain/aww351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tress O., Maglione M., Zlomuzica A., May D., Dicke N., Degen J., Dere E., Kettenmann H., Hartmann D., Willecke K. Pathologic and phenotypic alterations in a mouse expressing a connexin47 missense mutation that causes Pelizaeus-Merzbacher-like disease in humans. PLoS Genet. 2011;7:e1002146. doi: 10.1371/journal.pgen.1002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelles E., Butzler C., Jung D., Temme A., Gabriel H.D., Dahl U., Traub O., Stumpel F., Jungermann K., Zielasek J., et al. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc. Natl. Acad. Sci. USA. 1996;93:9565–9570. doi: 10.1073/pnas.93.18.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Odermatt B., Wellershaus K., Wallraff A., Seifert G., Degen J., Euwens C., Fuss B., Bussow H., Schilling K., Steinhauser C., et al. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J. Neurosci. 2003;23:4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dursun U., Koroglu C., Kocasoy Orhan E., Ugur S.A., Tolun A. Autosomal recessive spastic paraplegia (SPG45) with mental retardation maps to 10q24.3–q25.1. Neurogenetics. 2009;10:325–331. doi: 10.1007/s10048-009-0191-3. [DOI] [PubMed] [Google Scholar]
- 74.Kviklyte S., Vertommen D., Yerna X., Andersen H., Xu X., Gailly P., Bohlooly Y.M., Oscarsson J., Rider M.H. Effects of genetic deletion of soluble 5’-nucleotidases NT5C1A and NT5C2 on AMPK activation and nucleotide levels in contracting mouse skeletal muscles. Am. J. Physiol. Endocrinol. Metab. 2017;313:E48–E62. doi: 10.1152/ajpendo.00304.2016. [DOI] [PubMed] [Google Scholar]
- 75.Boukhris A., Feki I., Elleuch N., Miladi M.I., Boland-Auge A., Truchetto J., Mundwiller E., Jezequel N., Zelenika D., Mhiri C., et al. A new locus (SPG46) maps to 9p21.2–q21.12 in a Tunisian family with a complicated autosomal recessive hereditary spastic paraplegia with mental impairment and thin corpus callosum. Neurogenetics. 2010;11:441–448. doi: 10.1007/s10048-010-0249-2. [DOI] [PubMed] [Google Scholar]
- 76.Matern H., Boermans H., Lottspeich F., Matern S. Molecular cloning and expression of human bile acid beta-glucosidase. J. Biol. Chem. 2001;276:37929–37933. doi: 10.1074/jbc.M104290200. [DOI] [PubMed] [Google Scholar]
- 77.Massimo A., Maura S., Nicoletta L., Giulia M., Valentina M., Elena C., Alessandro P., Rosaria B., Sandro S. Current and Novel Aspects on the Non-lysosomal beta-Glucosylceramidase GBA2. Neurochem. Res. 2016;41:210–220. doi: 10.1007/s11064-015-1763-2. [DOI] [PubMed] [Google Scholar]
- 78.Sultana S., Reichbauer J., Schule R., Mochel F., Synofzik M., van der Spoel A.C. Lack of enzyme activity in GBA2 mutants associated with hereditary spastic paraplegia/cerebellar ataxia (SPG46) Biochem. Biophys. Res. Commun. 2015;465:35–40. doi: 10.1016/j.bbrc.2015.07.112. [DOI] [PubMed] [Google Scholar]
- 79.Yildiz Y., Matern H., Thompson B., Allegood J.C., Warren R.L., Ramirez D.M., Hammer R.E., Hamra F.K., Matern S., Russell D.W. Mutation of beta-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J. Clin. Investig. 2006;116:2985–2994. doi: 10.1172/JCI29224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin E., Schule R., Smets K., Rastetter A., Boukhris A., Loureiro J.L., Gonzalez M.A., Mundwiller E., Deconinck T., Wessner M., et al. Loss of function of glucocerebrosidase GBA2 is responsible for motor neuron defects in hereditary spastic paraplegia. Am. J. Hum. Genet. 2013;92:238–244. doi: 10.1016/j.ajhg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blumkin L., Lerman-Sagie T., Lev D., Yosovich K., Leshinsky-Silver E. A new locus (SPG47) maps to 1p13.2-1p12 in an Arabic family with complicated autosomal recessive hereditary spastic paraplegia and thin corpus callosum. J. Neurol. Sci. 2011;305:67–70. doi: 10.1016/j.jns.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 82.Matsuda S., Miura E., Matsuda K., Kakegawa W., Kohda K., Watanabe M., Yuzaki M. Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron. 2008;57:730–745. doi: 10.1016/j.neuron.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 83.Slabicki M., Theis M., Krastev D.B., Samsonov S., Mundwiller E., Junqueira M., Paszkowski-Rogacz M., Teyra J., Heninger A.K., Poser I., et al. A genome-scale DNA repair RNAi screen identifies SPG48 as a novel gene associated with hereditary spastic paraplegia. PLoS Biol. 2010;8:e1000408. doi: 10.1371/journal.pbio.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khundadze M., Ribaudo F., Hussain A., Rosentreter J., Nietzsche S., Thelen M., Winter D., Hoffmann B., Afzal M.A., Hermann T., et al. A mouse model for SPG48 reveals a block of autophagic flux upon disruption of adaptor protein complex five. Neurobiol. Dis. 2019;127:419–431. doi: 10.1016/j.nbd.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 85.Al-Yahyaee S., Al-Gazali L.I., De Jonghe P., Al-Barwany H., Al-Kindi M., De Vriendt E., Chand P., Koul R., Jacob P.C., Gururaj A., et al. A novel locus for hereditary spastic paraplegia with thin corpus callosum and epilepsy. Neurology. 2006;66:1230–1234. doi: 10.1212/01.wnl.0000208501.52849.dd. [DOI] [PubMed] [Google Scholar]
- 86.Inloes J.M., Kiosses W.B., Wang H., Walther T.C., Farese R.V., Jr., Cravatt B.F. Functional Contribution of the Spastic Paraplegia-Related Triglyceride Hydrolase DDHD2 to the Formation and Content of Lipid Droplets. Biochemistry. 2018;57:827–838. doi: 10.1021/acs.biochem.7b01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inloes J.M., Hsu K.L., Dix M.M., Viader A., Masuda K., Takei T., Wood M.R., Cravatt B.F. The hereditary spastic paraplegia-related enzyme DDHD2 is a principal brain triglyceride lipase. Proc. Natl. Acad. Sci. USA. 2014;111:14924–14929. doi: 10.1073/pnas.1413706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Novarino G., Fenstermaker A.G., Zaki M.S., Hofree M., Silhavy J.L., Heiberg A.D., Abdellateef M., Rosti B., Scott E., Mansour L., et al. Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders. Science. 2014;343:506–511. doi: 10.1126/science.1247363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Helmering J., Juan T., Li C.M., Chhoa M., Baron W., Gyuris T., Richards W.G., Turk J.R., Lawrence J., Cosgrove P.A., et al. A mutation in Ampd2 is associated with nephrotic syndrome and hypercholesterolemia in mice. Lipids Health Dis. 2014;13:167. doi: 10.1186/1476-511X-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akizu N., Cantagrel V., Schroth J., Cai N., Vaux K., McCloskey D., Naviaux R.K., Van Vleet J., Fenstermaker A.G., Silhavy J.L., et al. AMPD2 regulates GTP synthesis and is mutated in a potentially treatable neurodegenerative brainstem disorder. Cell. 2013;154:505–517. doi: 10.1016/j.cell.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li P., Ogino K., Hoshikawa Y., Morisaki H., Toyama K., Morisaki T., Morikawa K., Ninomiya H., Yoshida A., Hashimoto K., et al. AMP deaminase 3 plays a critical role in remote reperfusion lung injury. Biochem. Biophys. Res. Commun. 2013;434:131–136. doi: 10.1016/j.bbrc.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 92.Friedman D.J., Kunzli B.M., Yi A.R., Sevigny J., Berberat P.O., Enjyoji K., Csizmadia E., Friess H., Robson S.C. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc. Natl. Acad. Sci. USA. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Friedman D.J., Rennke H.G., Csizmadia E., Enjyoji K., Robson S.C. The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy. Diabetes. 2007;56:2371–2379. doi: 10.2337/db06-1593. [DOI] [PubMed] [Google Scholar]
- 94.Enjyoji K., Sevigny J., Lin Y., Frenette P.S., Christie P.D., Esch J.S., 2nd, Imai M., Edelberg J.M., Rayburn H., Lech M., et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 95.Lossos A., Elazar N., Lerer I., Schueler-Furman O., Fellig Y., Glick B., Zimmerman B.E., Azulay H., Dotan S., Goldberg S., et al. Myelin-associated glycoprotein gene mutation causes Pelizaeus-Merzbacher disease-like disorder. Brain. 2015;138:2521–2536. doi: 10.1093/brain/awv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartsch S., Montag D., Schachner M., Bartsch U. Increased number of unmyelinated axons in optic nerves of adult mice deficient in the myelin-associated glycoprotein (MAG) Brain Res. 1997;762:231–234. doi: 10.1016/S0006-8993(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 97.Cafferty W.B., Duffy P., Huebner E., Strittmatter S.M. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones M.V., Nguyen T.T., Ewaleifoh O., Lebson L., Whartenby K.A., Griffin J.W., Calabresi P.A. Accelerated axon loss in MOG35-55 experimental autoimmune encephalomyelitis (EAE) in myelin-associated glycoprotein-deficient (MAGKO) mice. J. Neuroimmunol. 2013;262:53–61. doi: 10.1016/j.jneuroim.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 99.Li M., Shibata A., Li C., Braun P.E., McKerracher L., Roder J., Kater S.B., David S. Myelin-associated glycoprotein inhibits neurite/axon growth and causes growth cone collapse. J. Neurosci. Res. 1996;46:404–414. doi: 10.1002/(SICI)1097-4547(19961115)46:4<404::AID-JNR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 100.Lopez P.H., Ahmad A.S., Mehta N.R., Toner M., Rowland E.A., Zhang J., Dore S., Schnaar R.L. Myelin-associated glycoprotein protects neurons from excitotoxicity. J. Neurochem. 2011;116:900–908. doi: 10.1111/j.1471-4159.2010.07069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marcus J., Dupree J.L., Popko B. Myelin-associated glycoprotein and myelin galactolipids stabilize developing axo-glial interactions. J. Cell Biol. 2002;156:567–577. doi: 10.1083/jcb.200111047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Montag D., Giese K.P., Bartsch U., Martini R., Lang Y., Bluthmann H., Karthigasan J., Kirschner D.A., Wintergerst E.S., Nave K.A., et al. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron. 1994;13:229–246. doi: 10.1016/0896-6273(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 103.Pan B., Fromholt S.E., Hess E.J., Crawford T.O., Griffin J.W., Sheikh K.A., Schnaar R.L. Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: Neuropathology and behavioral deficits in single- and double-null mice. Exp. Neurol. 2005;195:208–217. doi: 10.1016/j.expneurol.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gan-Or Z., Bouslam N., Birouk N., Lissouba A., Chambers D.B., Veriepe J., Androschuk A., Laurent S.B., Rochefort D., Spiegelman D., et al. Mutations in CAPN1 Cause Autosomal-Recessive Hereditary Spastic Paraplegia. Am. J. Hum. Genet. 2016;98:1038–1046. doi: 10.1016/j.ajhg.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stifanese R., Averna M., De Tullio R., Pedrazzi M., Milanese M., Bonifacino T., Bonanno G., Salamino F., Pontremoli S., Melloni E. Role of calpain-1 in the early phase of experimental ALS. Arch. Biochem. Biophys. 2014;562:1–8. doi: 10.1016/j.abb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y., Hersheson J., Lopez D., Hammer M., Liu Y., Lee K.H., Pinto V., Seinfeld J., Wiethoff S., Sun J., et al. Defects in the CAPN1 Gene Result in Alterations in Cerebellar Development and Cerebellar Ataxia in Mice and Humans. Cell Rep. 2016;16:79–91. doi: 10.1016/j.celrep.2016.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu C.G., Li Y., Raza K., Yu X.X., Ghoshal S., Geddes J.W. Calpain 1 knockdown improves tissue sparing and functional outcomes after spinal cord injury in rats. J. Neurotrauma. 2013;30:427–433. doi: 10.1089/neu.2012.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arthur J.S., Elce J.S., Hegadorn C., Williams K., Greer P.A. Disruption of the murine calpain small subunit gene, Capn4: Calpain is essential for embryonic development but not for cell growth and division. Mol. Cell. Biol. 2000;20:4474–4481. doi: 10.1128/MCB.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kara E., Tucci A., Manzoni C., Lynch D.S., Elpidorou M., Bettencourt C., Chelban V., Manole A., Hamed S.A., Haridy N.A., et al. Genetic and phenotypic characterization of complex hereditary spastic paraplegia. Brain. 2016;139:1904–1918. doi: 10.1093/brain/aww111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Estrada-Cuzcano A., Martin S., Chamova T., Synofzik M., Timmann D., Holemans T., Andreeva A., Reichbauer J., De Rycke R., Chang D.I., et al. Loss-of-function mutations in the ATP13A2/PARK9 gene cause complicated hereditary spastic paraplegia (SPG78) Brain. 2017;140:287–305. doi: 10.1093/brain/aww307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fleming S.M., Santiago N.A., Mullin E.J., Pamphile S., Karkare S., Lemkuhl A., Ekhator O.R., Linn S.C., Holden J.G., Aga D.S., et al. The effect of manganese exposure in Atp13a2-deficient mice. Neurotoxicology. 2018;64:256–266. doi: 10.1016/j.neuro.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gusdon A.M., Zhu J., Van Houten B., Chu C.T. ATP13A2 regulates mitochondrial bioenergetics through macroautophagy. Neurobiol. Dis. 2012;45:962–972. doi: 10.1016/j.nbd.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kett L.R., Stiller B., Bernath M.M., Tasset I., Blesa J., Jackson-Lewis V., Chan R.B., Zhou B., Di Paolo G., Przedborski S., et al. alpha-Synuclein-independent histopathological and motor deficits in mice lacking the endolysosomal Parkinsonism protein Atp13a2. J. Neurosci. 2015;35:5724–5742. doi: 10.1523/JNEUROSCI.0632-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qiao C., Yin N., Gu H.Y., Zhu J.L., Ding J.H., Lu M., Hu G. Atp13a2 Deficiency Aggravates Astrocyte-Mediated Neuroinflammation via NLRP3 Inflammasome Activation. CNS Neurosci. Ther. 2016;22:451–460. doi: 10.1111/cns.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sato S., Koike M., Funayama M., Ezaki J., Fukuda T., Ueno T., Uchiyama Y., Hattori N. Lysosomal Storage of Subunit c of Mitochondrial ATP Synthase in Brain-Specific Atp13a2-Deficient Mice. Am. J. Pathol. 2016;186:3074–3082. doi: 10.1016/j.ajpath.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 116.Schultheis P.J., Fleming S.M., Clippinger A.K., Lewis J., Tsunemi T., Giasson B., Dickson D.W., Mazzulli J.R., Bardgett M.E., Haik K.L., et al. Atp13a2-deficient mice exhibit neuronal ceroid lipofuscinosis, limited alpha-synuclein accumulation and age-dependent sensorimotor deficits. Hum. Mol. Genet. 2013;22:2067–2082. doi: 10.1093/hmg/ddt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsunemi T., Hamada K., Krainc D. ATP13A2/PARK9 regulates secretion of exosomes and alpha-synuclein. J. Neurosci. 2014;34:15281–15287. doi: 10.1523/JNEUROSCI.1629-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Usenovic M., Tresse E., Mazzulli J.R., Taylor J.P., Krainc D. Deficiency of ATP13A2 leads to lysosomal dysfunction, alpha-synuclein accumulation, and neurotoxicity. J. Neurosci. 2012;32:4240–4246. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bilguvar K., Tyagi N.K., Ozkara C., Tuysuz B., Bakircioglu M., Choi M., Delil S., Caglayan A.O., Baranoski J.F., Erturk O., et al. Recessive loss of function of the neuronal ubiquitin hydrolase UCHL1 leads to early-onset progressive neurodegeneration. Proc. Natl. Acad. Sci. USA. 2013;110:3489–3494. doi: 10.1073/pnas.1222732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jara J.H., Genc B., Cox G.A., Bohn M.C., Roos R.P., Macklis J.D., Ulupinar E., Ozdinler P.H. Corticospinal Motor Neurons Are Susceptible to Increased ER Stress and Display Profound Degeneration in the Absence of UCHL1 Function. Cereb. Cortex. 2015;25:4259–4272. doi: 10.1093/cercor/bhu318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saigoh K., Wang Y.L., Suh J.G., Yamanishi T., Sakai Y., Kiyosawa H., Harada T., Ichihara N., Wakana S., Kikuchi T., et al. Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat. Genet. 1999;23:47–51. doi: 10.1038/12647. [DOI] [PubMed] [Google Scholar]
- 122.Walters B.J., Campbell S.L., Chen P.C., Taylor A.P., Schroeder D.G., Dobrunz L.E., Artavanis-Tsakonas K., Ploegh H.L., Wilson J.A., Cox G.A., et al. Differential effects of Usp14 and Uch-L1 on the ubiquitin proteasome system and synaptic activity. Mol. Cell. Neurosci. 2008;39:539–548. doi: 10.1016/j.mcn.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coulombe J., Gamage P., Gray M.T., Zhang M., Tang M.Y., Woulfe J., Saffrey M.J., Gray D.A. Loss of UCHL1 promotes age-related degenerative changes in the enteric nervous system. Front. Aging Neurosci. 2014;6:129. doi: 10.3389/fnagi.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reinicke A.T., Laban K., Sachs M., Kraus V., Walden M., Damme M., Sachs W., Reichelt J., Schweizer M., Janiesch P.C., et al. Ubiquitin C-terminal hydrolase L1 (UCH-L1) loss causes neurodegeneration by altering protein turnover in the first postnatal weeks. Proc. Natl. Acad. Sci. USA. 2019;116:7963–7972. doi: 10.1073/pnas.1812413116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen F., Sugiura Y., Myers K.G., Liu Y., Lin W. Ubiquitin carboxyl-terminal hydrolase L1 is required for maintaining the structure and function of the neuromuscular junction. Proc. Natl. Acad. Sci. USA. 2010;107:1636–1641. doi: 10.1073/pnas.0911516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamazaki K., Wakasugi N., Tomita T., Kikuchi T., Mukoyama M., Ando K. Gracile axonal dystrophy (GAD), a new neurological mutant in the mouse. Proc. Soc. Exp. Biol. Med. 1988;187:209–215. doi: 10.3181/00379727-187-42656. [DOI] [PubMed] [Google Scholar]
- 127.Suh J.G., Yamanishi T., Matsui K., Tanaka K., Wada K. Mapping of the gracile axonal dystrophy (gad) gene to a region between D5Mit197 and D5Mit113 on proximal mouse chromosome 5. Genomics. 1995;27:549–551. doi: 10.1006/geno.1995.1091. [DOI] [PubMed] [Google Scholar]
- 128.Genc B., Jara J.H., Schultz M.C., Manuel M., Stanford M.J., Gautam M., Klessner J.L., Sekerkova G., Heller D.B., Cox G.A., et al. Absence of UCHL 1 function leads to selective motor neuropathy. Ann. Clin. Transl. Neurol. 2016;3:331–345. doi: 10.1002/acn3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miura H., Oda K., Endo C., Yamazaki K., Shibasaki H., Kikuchi T. Progressive degeneration of motor nerve terminals in GAD mutant mouse with hereditary sensory axonopathy. Neuropathol. Appl. Neurobiol. 1993;19:41–51. doi: 10.1111/j.1365-2990.1993.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 130.Hazan J., Lamy C., Melki J., Munnich A., de Recondo J., Weissenbach J. Autosomal dominant familial spastic paraplegia is genetically heterogeneous and one locus maps to chromosome 14q. Nat. Genet. 1993;5:163–167. doi: 10.1038/ng1093-163. [DOI] [PubMed] [Google Scholar]
- 131.Boustany R.M., Fleischnick E., Alper C.A., Marazita M.L., Spence M.A., Martin J.B., Kolodny E.H. The autosomal dominant form of “pure” familial spastic paraplegia: Clinical findings and linkage analysis of a large pedigree. Neurology. 1987;37:910–915. doi: 10.1212/WNL.37.6.910. [DOI] [PubMed] [Google Scholar]
- 132.Zhao X., Alvarado D., Rainier S., Lemons R., Hedera P., Weber C.H., Tukel T., Apak M., Heiman-Patterson T., Ming L., et al. Mutations in a newly identified GTPase gene cause autosomal dominant hereditary spastic paraplegia. Nat. Genet. 2001;29:326–331. doi: 10.1038/ng758. [DOI] [PubMed] [Google Scholar]
- 133.Gao Y., Jiang T., Qu C., Tao H., Cao H., Zhao Y., Wang Y., Qu J., Chen J.G. Atlastin-1 regulates dendritic morphogenesis in mouse cerebral cortex. Neurosci. Res. 2013;77:137–142. doi: 10.1016/j.neures.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 134.Shih Y.T., Hsueh Y.P. VCP and ATL1 regulate endoplasmic reticulum and protein synthesis for dendritic spine formation. Nat. Commun. 2016;7:11020. doi: 10.1038/ncomms11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hazan J., Fontaine B., Bruyn R.P., Lamy C., van Deutekom J.C., Rime C.S., Durr A., Melki J., Lyon-Caen O., Agid Y., et al. Linkage of a new locus for autosomal dominant familial spastic paraplegia to chromosome 2p. Hum. Mol. Genet. 1994;3:1569–1573. doi: 10.1093/hmg/3.9.1569. [DOI] [PubMed] [Google Scholar]
- 136.Hentati A., Pericak-Vance M.A., Lennon F., Wasserman B., Hentati F., Juneja T., Angrist M.H., Hung W.Y., Boustany R.M., Bohlega S., et al. Linkage of a locus for autosomal dominant familial spastic paraplegia to chromosome 2p markers. Hum. Mol. Genet. 1994;3:1867–1871. doi: 10.1093/hmg/3.10.1867. [DOI] [PubMed] [Google Scholar]
- 137.Fassier C., Tarrade A., Peris L., Courageot S., Mailly P., Dalard C., Delga S., Roblot N., Lefevre J., Job D., et al. Microtubule-targeting drugs rescue axonal swellings in cortical neurons from spastin knockout mice. Dis. Models Mech. 2013;6:72–83. doi: 10.1242/dmm.008946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kasher P.R., De Vos K.J., Wharton S.B., Manser C., Bennett E.J., Bingley M., Wood J.D., Milner R., McDermott C.J., Miller C.C., et al. Direct evidence for axonal transport defects in a novel mouse model of mutant spastin-induced hereditary spastic paraplegia (HSP) and human HSP patients. J. Neurochem. 2009;110:34–44. doi: 10.1111/j.1471-4159.2009.06104.x. [DOI] [PubMed] [Google Scholar]
- 139.Qiang L., Piermarini E., Muralidharan H., Yu W., Leo L., Hennessy L.E., Fernandes S., Connors T., Yates P.L., Swift M., et al. Hereditary spastic paraplegia: Gain-of-function mechanisms revealed by new transgenic mouse. Hum. Mol. Genet. 2019;28:1136–1152. doi: 10.1093/hmg/ddy419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tarrade A., Fassier C., Courageot S., Charvin D., Vitte J., Peris L., Thorel A., Mouisel E., Fonknechten N., Roblot N., et al. A mutation of spastin is responsible for swellings and impairment of transport in a region of axon characterized by changes in microtubule composition. Hum. Mol. Genet. 2006;15:3544–3558. doi: 10.1093/hmg/ddl431. [DOI] [PubMed] [Google Scholar]
- 141.Fink J.K., Sharp G.B., Lange B.M., Wu C.B., Haley T., Otterud B., Peacock M., Leppert M. Autosomal dominant, familial spastic paraplegia, type I: Clinical and genetic analysis of a large North American family. Neurology. 1995;45:325–331. doi: 10.1212/WNL.45.2.325. [DOI] [PubMed] [Google Scholar]
- 142.Watanabe F., Arnold W.D., Hammer R.E., Ghodsizadeh O., Moti H., Schumer M., Hashmi A., Hernandez A., Sneh A., Sahenk Z., et al. Pathogenesis of autosomal dominant hereditary spastic paraplegia (SPG6) revealed by a rat model. J. Neuropathol. Exp. Neurol. 2013;72:1016–1028. doi: 10.1097/NEN.0000000000000000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hedera P., DiMauro S., Bonilla E., Wald J., Eldevik O.P., Fink J.K. Phenotypic analysis of autosomal dominant hereditary spastic paraplegia linked to chromosome 8q. Neurology. 1999;53:44–50. doi: 10.1212/WNL.53.1.44. [DOI] [PubMed] [Google Scholar]
- 144.Hedera P., Rainier S., Alvarado D., Zhao X., Williamson J., Otterud B., Leppert M., Fink J.K. Novel locus for autosomal dominant hereditary spastic paraplegia, on chromosome 8q. Am. J. Hum. Genet. 1999;64:563–569. doi: 10.1086/302258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rocco P., Vainzof M., Froehner S.C., Peters M.F., Marie S.K., Passos-Bueno M.R., Zatz M. Brazilian family with pure autosomal dominant spastic paraplegia maps to 8q: Analysis of muscle beta 1 syntrophin. Am. J. Med. Genet. 2000;92:122–127. doi: 10.1002/(SICI)1096-8628(20000515)92:2<122::AID-AJMG8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 146.Jahic A., Khundadze M., Jaenisch N., Schule R., Klimpe S., Klebe S., Frahm C., Kassubek J., Stevanin G., Schols L., et al. The spectrum of KIAA0196 variants, and characterization of a murine knockout: Implications for the mutational mechanism in hereditary spastic paraplegia type SPG8. Orphanet J. Rare Dis. 2015;10:147. doi: 10.1186/s13023-015-0359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reid E., Dearlove A.M., Rhodes M., Rubinsztein D.C. A new locus for autosomal dominant “pure” hereditary spastic paraplegia mapping to chromosome 12q13, and evidence for further genetic heterogeneity. Am. J. Hum. Genet. 1999;65:757–763. doi: 10.1086/302555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xia C.H., Roberts E.A., Her L.S., Liu X., Williams D.S., Cleveland D.W., Goldstein L.S. Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J. Cell Biol. 2003;161:55–66. doi: 10.1083/jcb.200301026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reid E., Dearlove A.M., Osborn O., Rogers M.T., Rubinsztein D.C. A locus for autosomal dominant “pure” hereditary spastic paraplegia maps to chromosome 19q13. Am. J. Hum. Genet. 2000;66:728–732. doi: 10.1086/302783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ikemoto T., Hosoya T., Takata K., Aoyama H., Hiramatsu T., Onoe H., Suzuki M., Endo M. Functional role of neuroendocrine-specific protein-like 1 in membrane translocation of GLUT4. Diabetes. 2009;58:2802–2812. doi: 10.2337/db09-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fontaine B., Davoine C.S., Durr A., Paternotte C., Feki I., Weissenbach J., Hazan J., Brice A. A new locus for autosomal dominant pure spastic paraplegia, on chromosome 2q24-q34. Am. J. Hum. Genet. 2000;66:702–707. doi: 10.1086/302776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Blackstone C., O’Kane C.J., Reid E. Hereditary spastic paraplegias: Membrane traffic and the motor pathway. Nat. Rev. Neurosci. 2011;12:31–42. doi: 10.1038/nrn2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bross P., Magnoni R., Bie A.S. Molecular chaperone disorders: Defective Hsp60 in neurodegeneration. Curr. Top. Med. Chem. 2012;12:2491–2503. doi: 10.2174/1568026611212220005. [DOI] [PubMed] [Google Scholar]
- 154.Christensen J.H., Nielsen M.N., Hansen J., Fuchtbauer A., Fuchtbauer E.M., West M., Corydon T.J., Gregersen N., Bross P. Inactivation of the hereditary spastic paraplegia-associated Hspd1 gene encoding the Hsp60 chaperone results in early embryonic lethality in mice. Cell Stress Chaperones. 2010;15:851–863. doi: 10.1007/s12192-010-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Magnoni R., Palmfeldt J., Christensen J.H., Sand M., Maltecca F., Corydon T.J., West M., Casari G., Bross P. Late onset motoneuron disorder caused by mitochondrial Hsp60 chaperone deficiency in mice. Neurobiol. Dis. 2013;54:12–23. doi: 10.1016/j.nbd.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 156.Patel H., Hart P.E., Warner T.T., Houlston R.S., Patton M.A., Jeffery S., Crosby A.H. The Silver syndrome variant of hereditary spastic paraplegia maps to chromosome 11q12-q14, with evidence for genetic heterogeneity within this subtype. Am. J. Hum. Genet. 2001;69:209–215. doi: 10.1086/321267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Silver J.R. Familial spastic paraplegia with amyotrophy of the hands. Ann. Hum. Genet. 1966;30:69–75. doi: 10.1111/j.1469-1809.1966.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 158.Guo J., Qiu W., Soh S.L., Wei S., Radda G.K., Ong W.Y., Pang Z.P., Han W. Motor neuron degeneration in a mouse model of seipinopathy. Cell Death Dis. 2013;4:e535. doi: 10.1038/cddis.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cui X., Wang Y., Tang Y., Liu Y., Zhao L., Deng J., Xu G., Peng X., Ju S., Liu G., et al. Seipin ablation in mice results in severe generalized lipodystrophy. Hum. Mol. Genet. 2011;20:3022–3030. doi: 10.1093/hmg/ddr205. [DOI] [PubMed] [Google Scholar]
- 160.Zhou L., Yin J., Wang C., Liao J., Liu G., Chen L. Lack of seipin in neurons results in anxiety- and depression-like behaviors via down regulation of PPARgamma. Hum. Mol. Genet. 2014;23:4094–4102. doi: 10.1093/hmg/ddu126. [DOI] [PubMed] [Google Scholar]
- 161.Zuchner S., Kail M.E., Nance M.A., Gaskell P.C., Svenson I.K., Marchuk D.A., Pericak-Vance M.A., Ashley-Koch A.E. A new locus for dominant hereditary spastic paraplegia maps to chromosome 2p12. Neurogenetics. 2006;7:127–129. doi: 10.1007/s10048-006-0029-1. [DOI] [PubMed] [Google Scholar]
- 162.Zuchner S., Wang G., Tran-Viet K.N., Nance M.A., Gaskell P.C., Vance J.M., Ashley-Koch A.E., Pericak-Vance M.A. Mutations in the novel mitochondrial protein REEP1 cause hereditary spastic paraplegia type 31. Am. J. Hum. Genet. 2006;79:365–369. doi: 10.1086/505361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Deutch A.Y., Hedera P., Colbran R.J. REEPing the benefits of an animal model of hereditary spastic paraplegia. J. Clin. Investig. 2013;123:4134–4136. doi: 10.1172/JCI72324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lim Y., Cho I.T., Schoel L.J., Cho G., Golden J.A. Hereditary spastic paraplegia-linked REEP1 modulates endoplasmic reticulum/mitochondria contacts. Ann. Neurol. 2015;78:679–696. doi: 10.1002/ana.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Renvoise B., Malone B., Falgairolle M., Munasinghe J., Stadler J., Sibilla C., Park S.H., Blackstone C. Reep1 null mice reveal a converging role for hereditary spastic paraplegia proteins in lipid droplet regulation. Hum. Mol. Genet. 2016;25:5111–5125. doi: 10.1093/hmg/ddw315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lin P., Li J., Liu Q., Mao F., Li J., Qiu R., Hu H., Song Y., Yang Y., Gao G., et al. A missense mutation in SLC33A1, which encodes the acetyl-CoA transporter, causes autosomal-dominant spastic paraplegia (SPG42) Am. J. Hum. Genet. 2008;83:752–759. doi: 10.1016/j.ajhg.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lin P., Mao F., Liu Q., Shao C., Yan C., Gong Y. Prenatal diagnosis of autosomal dominant hereditary spastic paraplegia (SPG42) caused by SLC33A1 mutation in a Chinese kindred. Prenat. Diagn. 2010;30:485–486. doi: 10.1002/pd.2485. [DOI] [PubMed] [Google Scholar]
- 168.Liu P., Jiang B., Ma J., Lin P., Zhang Y., Shao C., Sun W., Gong Y. S113R mutation in SLC33A1 leads to neurodegeneration and augmented BMP signaling in a mouse model. Dis. Model Mech. 2017;10:53–62. doi: 10.1242/dmm.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rinaldi C., Schmidt T., Situ A.J., Johnson J.O., Lee P.R., Chen K.L., Bott L.C., Fado R., Harmison G.H., Parodi S., et al. Mutation in CPT1C Associated With Pure Autosomal Dominant Spastic Paraplegia. JAMA Neurol. 2015;72:561–570. doi: 10.1001/jamaneurol.2014.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Carrasco P., Jacas J., Sahun I., Muley H., Ramirez S., Puisac B., Mezquita P., Pie J., Dierssen M., Casals N. Carnitine palmitoyltransferase 1C deficiency causes motor impairment and hypoactivity. Behav. Brain Res. 2013;256:291–297. doi: 10.1016/j.bbr.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 171.Casals N., Zammit V., Herrero L., Fado R., Rodriguez-Rodriguez R., Serra D. Carnitine palmitoyltransferase 1C: From cognition to cancer. Prog. Lipid Res. 2016;61:134–148. doi: 10.1016/j.plipres.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 172.Jouet M., Rosenthal A., Armstrong G., MacFarlane J., Stevenson R., Paterson J., Metzenberg A., Ionasescu V., Temple K., Kenwrick S. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat. Genet. 1994;7:402–407. doi: 10.1038/ng0794-402. [DOI] [PubMed] [Google Scholar]
- 173.Demyanenko G.P., Tsai A.Y., Maness P.F. Abnormalities in neuronal process extension, hippocampal development, and the ventricular system of L1 knockout mice. J. Neurosci. 1999;19:4907–4920. doi: 10.1523/JNEUROSCI.19-12-04907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guseva D., Angelov D.N., Irintchev A., Schachner M. Ablation of adhesion molecule L1 in mice favours Schwann cell proliferation and functional recovery after peripheral nerve injury. Brain. 2009;132:2180–2195. doi: 10.1093/brain/awp160. [DOI] [PubMed] [Google Scholar]
- 175.Tapanes-Castillo A., Weaver E.J., Smith R.P., Kamei Y., Caspary T., Hamilton-Nelson K.L., Slifer S.H., Martin E.R., Bixby J.L., Lemmon V.P. A modifier locus on chromosome 5 contributes to L1 cell adhesion molecule X-linked hydrocephalus in mice. Neurogenetics. 2010;11:53–71. doi: 10.1007/s10048-009-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kobayashi H., Hoffman E.P., Marks H.G. The rumpshaker mutation in spastic paraplegia. Nat. Genet. 1994;7:351–352. doi: 10.1038/ng0794-351. [DOI] [PubMed] [Google Scholar]
- 177.Saugier-Veber P., Munnich A., Bonneau D., Rozet J.M., Le Merrer M., Gil R., Boespflug-Tanguy O. X-linked spastic paraplegia and Pelizaeus-Merzbacher disease are allelic disorders at the proteolipid protein locus. Nat. Genet. 1994;6:257–262. doi: 10.1038/ng0394-257. [DOI] [PubMed] [Google Scholar]
- 178.Cambi F., Tang X.M., Cordray P., Fain P.R., Keppen L.D., Barker D.F. Refined genetic mapping and proteolipid protein mutation analysis in X-linked pure hereditary spastic paraplegia. Neurology. 1996;46:1112–1117. doi: 10.1212/WNL.46.4.1112. [DOI] [PubMed] [Google Scholar]
- 179.Bonneau D., Rozet J.M., Bulteau C., Berthier M., Mettey R., Gil R., Munnich A., Le Merrer M. X linked spastic paraplegia (SPG2): Clinical heterogeneity at a single gene locus. J. Med. Genet. 1993;30:381–384. doi: 10.1136/jmg.30.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Al-Saktawi K., McLaughlin M., Klugmann M., Schneider A., Barrie J.A., McCulloch M.C., Montague P., Kirkham D., Nave K.A., Griffiths I.R. Genetic background determines phenotypic severity of the Plp rumpshaker mutation. J. Neurosci. Res. 2003;72:12–24. doi: 10.1002/jnr.10561. [DOI] [PubMed] [Google Scholar]
- 181.Edgar J.M., McLaughlin M., Barrie J.A., McCulloch M.C., Garbern J., Griffiths I.R. Age-related axonal and myelin changes in the rumpshaker mutation of the Plp gene. Acta Neuropathol. 2004;107:331–335. doi: 10.1007/s00401-003-0808-9. [DOI] [PubMed] [Google Scholar]
- 182.Griffiths I., Klugmann M., Anderson T., Yool D., Thomson C., Schwab M.H., Schneider A., Zimmermann F., McCulloch M., Nadon N., et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- 183.Klugmann M., Schwab M.H., Puhlhofer A., Schneider A., Zimmermann F., Griffiths I.R., Nave K.A. Assembly of CNS myelin in the absence of proteolipid protein. Neuron. 1997;18:59–70. doi: 10.1016/S0896-6273(01)80046-5. [DOI] [PubMed] [Google Scholar]
- 184.Luders K.A., Patzig J., Simons M., Nave K.A., Werner H.B. Genetic dissection of oligodendroglial and neuronal Plp1 function in a novel mouse model of spastic paraplegia type 2. Glia. 2017;65:1762–1776. doi: 10.1002/glia.23193. [DOI] [PubMed] [Google Scholar]
- 185.Yool D.A., Klugmann M., McLaughlin M., Vouyiouklis D.A., Dimou L., Barrie J.A., McCulloch M.C., Nave K.A., Griffiths I.R. Myelin proteolipid proteins promote the interaction of oligodendrocytes and axons. J. Neurosci. Res. 2001;63:151–164. doi: 10.1002/1097-4547(20010115)63:2<151::AID-JNR1007>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 186.Nixon C.W., Connelly M.E. Hind-leg paralysis: A new sex-linked mutation in the Syrian hamster. J. Hered. 1968;59:276–278. doi: 10.1093/oxfordjournals.jhered.a107718. [DOI] [PubMed] [Google Scholar]
- 187.Schneider A., Montague P., Griffiths I., Fanarraga M., Kennedy P., Brophy P., Nave K.A. Uncoupling of hypomyelination and glial cell death by a mutation in the proteolipid protein gene. Nature. 1992;358:758–761. doi: 10.1038/358758a0. [DOI] [PubMed] [Google Scholar]
- 188.Eymard-Pierre E., Yamanaka K., Haeussler M., Kress W., Gauthier-Barichard F., Combes P., Cleveland D.W., Boespflug-Tanguy O. Novel missense mutation in ALS2 gene results in infantile ascending hereditary spastic paralysis. Ann. Neurol. 2006;59:976–980. doi: 10.1002/ana.20879. [DOI] [PubMed] [Google Scholar]
- 189.Gros-Louis F., Meijer I.A., Hand C.K., Dube M.P., MacGregor D.L., Seni M.H., Devon R.S., Hayden M.R., Andermann F., Andermann E., et al. An ALS2 gene mutation causes hereditary spastic paraplegia in a Pakistani kindred. Ann. Neurol. 2003;53:144–145. doi: 10.1002/ana.10422. [DOI] [PubMed] [Google Scholar]
- 190.Herzfeld T., Wolf N., Winter P., Hackstein H., Vater D., Muller U. Maternal uniparental heterodisomy with partial isodisomy of a chromosome 2 carrying a splice acceptor site mutation (IVS9-2A>T) in ALS2 causes infantile-onset ascending spastic paralysis (IAHSP) Neurogenetics. 2009;10:59–64. doi: 10.1007/s10048-008-0148-y. [DOI] [PubMed] [Google Scholar]
- 191.Ben Hamida M., Hentati F., Ben Hamida C. Hereditary motor system diseases (chronic juvenile amyotrophic lateral sclerosis). Conditions combining a bilateral pyramidal syndrome with limb and bulbar amyotrophy. Brain. 1990;113:347–363. doi: 10.1093/brain/113.2.347. [DOI] [PubMed] [Google Scholar]
- 192.Hentati A., Bejaoui K., Pericak-Vance M.A., Hentati F., Speer M.C., Hung W.Y., Figlewicz D.A., Haines J., Rimmler J., Ben Hamida C., et al. Linkage of recessive familial amyotrophic lateral sclerosis to chromosome 2q33-q35. Nat. Genet. 1994;7:425–428. doi: 10.1038/ng0794-425. [DOI] [PubMed] [Google Scholar]
- 193.Deng H.X., Zhai H., Fu R., Shi Y., Gorrie G.H., Yang Y., Liu E., Dal Canto M.C., Mugnaini E., Siddique T. Distal axonopathy in an alsin-deficient mouse model. Hum. Mol. Genet. 2007;16:2911–2920. doi: 10.1093/hmg/ddm251. [DOI] [PubMed] [Google Scholar]
- 194.Devon R.S., Orban P.C., Gerrow K., Barbieri M.A., Schwab C., Cao L.P., Helm J.R., Bissada N., Cruz-Aguado R., Davidson T.L., et al. Als2-deficient mice exhibit disturbances in endosome trafficking associated with motor behavioral abnormalities. Proc. Natl. Acad. Sci. USA. 2006;103:9595–9600. doi: 10.1073/pnas.0510197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yamanaka K., Miller T.M., McAlonis-Downes M., Chun S.J., Cleveland D.W. Progressive spinal axonal degeneration and slowness in ALS2-deficient mice. Ann. Neurol. 2006;60:95–104. doi: 10.1002/ana.20888. [DOI] [PubMed] [Google Scholar]
- 196.Hadano S., Benn S.C., Kakuta S., Otomo A., Sudo K., Kunita R., Suzuki-Utsunomiya K., Mizumura H., Shefner J.M., Cox G.A., et al. Mice deficient in the Rab5 guanine nucleotide exchange factor ALS2/alsin exhibit age-dependent neurological deficits and altered endosome trafficking. Hum. Mol. Genet. 2006;15:233–250. doi: 10.1093/hmg/ddi440. [DOI] [PubMed] [Google Scholar]
- 197.Jacquier A., Bellouze S., Blanchard S., Bohl D., Haase G. Astrocytic protection of spinal motor neurons but not cortical neurons against loss of Als2/alsin function. Hum. Mol. Genet. 2009;18:2127–2139. doi: 10.1093/hmg/ddp136. [DOI] [PubMed] [Google Scholar]
- 198.Coutelier M., Goizet C., Durr A., Habarou F., Morais S., Dionne-Laporte A., Tao F., Konop J., Stoll M., Charles P., et al. Alteration of ornithine metabolism leads to dominant and recessive hereditary spastic paraplegia. Brain. 2015;138:2191–2205. doi: 10.1093/brain/awv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Slavotinek A.M., Pike M., Mills K., Hurst J.A. Cataracts, motor system disorder, short stature, learning difficulties, and skeletal abnormalities: A new syndrome? Am. J. Med. Genet. 1996;62:42–47. doi: 10.1002/(SICI)1096-8628(19960301)62:1<42::AID-AJMG9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 200.Yildirim Y., Orhan E.K., Iseri S.A., Serdaroglu-Oflazer P., Kara B., Solakoglu S., Tolun A. A frameshift mutation of ERLIN2 in recessive intellectual disability, motor dysfunction and multiple joint contractures. Hum. Mol. Genet. 2011;20:1886–1892. doi: 10.1093/hmg/ddr070. [DOI] [PubMed] [Google Scholar]
- 201.Oz-Levi D., Ben-Zeev B., Ruzzo E.K., Hitomi Y., Gelman A., Pelak K., Anikster Y., Reznik-Wolf H., Bar-Joseph I., Olender T., et al. Mutation in TECPR2 reveals a role for autophagy in hereditary spastic paraparesis. Am. J. Hum. Genet. 2012;91:1065–1072. doi: 10.1016/j.ajhg.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shimazaki H., Takiyama Y., Ishiura H., Sakai C., Matsushima Y., Hatakeyama H., Honda J., Sakoe K., Naoi T., Namekawa M., et al. A homozygous mutation of C12orf65 causes spastic paraplegia with optic atrophy and neuropathy (SPG55) J. Med. Genet. 2012;49:777–784. doi: 10.1136/jmedgenet-2012-101212. [DOI] [PubMed] [Google Scholar]
- 203.Beetz C., Johnson A., Schuh A.L., Thakur S., Varga R.E., Fothergill T., Hertel N., Bomba-Warczak E., Thiele H., Nurnberg G., et al. Inhibition of TFG function causes hereditary axon degeneration by impairing endoplasmic reticulum structure. Proc. Natl. Acad. Sci. USA. 2013;110:5091–5096. doi: 10.1073/pnas.1217197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Esteves T., Durr A., Mundwiller E., Loureiro J.L., Boutry M., Gonzalez M.A., Gauthier J., El-Hachimi K.H., Depienne C., Muriel M.P., et al. Loss of association of REEP2 with membranes leads to hereditary spastic paraplegia. Am. J. Hum. Genet. 2014;94:268–277. doi: 10.1016/j.ajhg.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lossos A., Stumpfig C., Stevanin G., Gaussen M., Zimmerman B.E., Mundwiller E., Asulin M., Chamma L., Sheffer R., Misk A., et al. Fe/S protein assembly gene IBA57 mutation causes hereditary spastic paraplegia. Neurology. 2015;84:659–667. doi: 10.1212/WNL.0000000000001270. [DOI] [PubMed] [Google Scholar]
- 206.Vernon H.J., McClellan R., Batista D.A., Naidu S. Mutations in FARS2 and non-fatal mitochondrial dysfunction in two siblings. Am. J. Med. Genet. A. 2015;167A:1147–1151. doi: 10.1002/ajmg.a.36993. [DOI] [PubMed] [Google Scholar]
- 207.Yang Y., Liu W., Fang Z., Shi J., Che F., He C., Yao L., Wang E., Wu Y. A Newly Identified Missense Mutation in FARS2 Causes Autosomal-Recessive Spastic Paraplegia. Hum. Mutat. 2016;37:165–169. doi: 10.1002/humu.22930. [DOI] [PubMed] [Google Scholar]
- 208.Mannan A.U., Krawen P., Sauter S.M., Boehm J., Chronowska A., Paulus W., Neesen J., Engel W. ZFYVE27 (SPG33), a novel spastin-binding protein, is mutated in hereditary spastic paraplegia. Am. J. Hum. Genet. 2006;79:351–357. doi: 10.1086/504927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dupre N., Valdmanis P.N., Bouchard J.P., Rouleau G.A. Autosomal dominant primary lateral sclerosis. Neurology. 2007;68:1156–1157. doi: 10.1212/01.wnl.0000258678.58808.86. [DOI] [PubMed] [Google Scholar]
- 210.Valdmanis P.N., Dupre N., Rouleau G.A. A locus for primary lateral sclerosis on chromosome 4ptel-4p16.1. Arch. Neurol. 2008;65:383–386. doi: 10.1001/archneur.65.3.383. [DOI] [PubMed] [Google Scholar]
- 211.Enjyoji K., Kotani K., Thukral C., Blumel B., Sun X., Wu Y., Imai M., Friedman D., Csizmadia E., Bleibel W., et al. Deletion of cd39/entpd1 results in hepatic insulin resistance. Diabetes. 2008;57:2311–2320. doi: 10.2337/db07-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Connell J.W., Allison R., Reid E. Quantitative Gait Analysis Using a Motorized Treadmill System Sensitively Detects Motor Abnormalities in Mice Expressing ATPase Defective Spastin. PLoS ONE. 2016;11:e0152413. doi: 10.1371/journal.pone.0152413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Beetz C., Koch N., Khundadze M., Zimmer G., Nietzsche S., Hertel N., Huebner A.K., Mumtaz R., Schweizer M., Dirren E., et al. A spastic paraplegia mouse model reveals REEP1-dependent ER shaping. J. Clin. Investig. 2013;123:4273–4282. doi: 10.1172/JCI65665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yang Y., Hentati A., Deng H.X., Dabbagh O., Sasaki T., Hirano M., Hung W.Y., Ouahchi K., Yan J., Azim A.C., et al. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat. Genet. 2001;29:160–165. doi: 10.1038/ng1001-160. [DOI] [PubMed] [Google Scholar]
- 215.Arlotta P., Molyneaux B.J., Chen J., Inoue J., Kominami R., Macklis J.D. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 216.Jara J.H., Stanford M.J., Zhu Y., Tu M., Hauswirth W.W., Bohn M.C., DeVries S.H., Ozdinler P.H. Healthy and diseased corticospinal motor neurons are selectively transduced upon direct AAV2-2 injection into the motor cortex. Gene. Ther. 2016;23:272–282. doi: 10.1038/gt.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jara J.H., Villa S.R., Khan N.A., Bohn M.C., Ozdinler P.H. AAV2 mediated retrograde transduction of corticospinal motor neurons reveals initial and selective apical dendrite degeneration in ALS. Neurobiol. Dis. 2012;47:174–183. doi: 10.1016/j.nbd.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fink K.L., Strittmatter S.M., Cafferty W.B. Comprehensive Corticospinal Labeling with mu-crystallin Transgene Reveals Axon Regeneration after Spinal Cord Trauma in ngr1-/- Mice. J. Neurosci. 2015;35:15403–15418. doi: 10.1523/JNEUROSCI.3165-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schaefer A.M., Sanes J.R., Lichtman J.W. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J. Comp. Neurol. 2005;490:209–219. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
- 220.Wong F., Fan L., Wells S., Hartley R., Mackenzie F.E., Oyebode O., Brown R., Thomson D., Coleman M.P., Blanco G., et al. Axonal and neuromuscular synaptic phenotypes in Wld(S), SOD1(G93A) and ostes mutant mice identified by fiber-optic confocal microendoscopy. Mol. Cell. Neurosci. 2009;42:296–307. doi: 10.1016/j.mcn.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 221.Richter M.W., Roskams A.J. Corticospinal neurons respond differentially to neurotrophins and myelin-associated glycoprotein in vitro. J. Neurosci. Res. 2009;87:2222–2236. doi: 10.1002/jnr.22053. [DOI] [PubMed] [Google Scholar]
- 222.Feng G., Mellor R.H., Bernstein M., Keller-Peck C., Nguyen Q.T., Wallace M., Nerbonne J.M., Lichtman J.W., Sanes J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/S0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 223.Yu J., Anderson C.T., Kiritani T., Sheets P.L., Wokosin D.L., Wood L., Shepherd G.M. Local-Circuit Phenotypes of Layer 5 Neurons in Motor-Frontal Cortex of YFP-H Mice. Front. Neural Circuits. 2008;2:6. doi: 10.3389/neuro.04.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bareyre F.M., Kerschensteiner M., Misgeld T., Sanes J.R. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat. Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 225.Ozdinler P.H., Benn S., Yamamoto T.H., Guzel M., Brown R.H., Jr., Macklis J.D. Corticospinal motor neurons and related subcerebral projection neurons undergo early and specific neurodegeneration in hSOD1G93A transgenic ALS mice. J. Neurosci. 2011;31:4166–4177. doi: 10.1523/JNEUROSCI.4184-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tantirigama M.L., Oswald M.J., Clare A.J., Wicky H.E., Day R.C., Hughes S.M., Empson R.M. Fezf2 expression in layer 5 projection neurons of mature mouse motor cortex. J. Comp. Neurol. 2016;524:829–845. doi: 10.1002/cne.23875. [DOI] [PubMed] [Google Scholar]
- 227.Yasvoina M.V., Genc B., Jara J.H., Sheets P.L., Quinlan K.A., Milosevic A., Shepherd G.M., Heckman C.J., Ozdinler P.H. eGFP expression under UCHL1 promoter genetically labels corticospinal motor neurons and a subpopulation of degeneration-resistant spinal motor neurons in an ALS mouse model. J. Neurosci. 2013;33:7890–7904. doi: 10.1523/JNEUROSCI.2787-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bishop P., Rocca D., Henley J.M. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016;473:2453–2462. doi: 10.1042/BCJ20160082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Day I.N., Thompson R.J. UCHL1 (PGP 9.5): Neuronal biomarker and ubiquitin system protein. Prog. Neuropathol. 2010;90:327–362. doi: 10.1016/j.pneurobio.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 230.Liu Y., Fallon L., Lashuel H.A., Liu Z., Lansbury P.T., Jr. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002;111:209–218. doi: 10.1016/S0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 231.Cartier A.E., Djakovic S.N., Salehi A., Wilson S.M., Masliah E., Patrick G.N. Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1. J. Neurosci. 2009;29:7857–7868. doi: 10.1523/JNEUROSCI.1817-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Leroy E., Boyer R., Auburger G., Leube B., Ulm G., Mezey E., Harta G., Brownstein M.J., Jonnalagada S., Chernova T., et al. The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- 233.Hussain S., Bedekovics T., Liu Q., Hu W., Jeon H., Johnson S.H., Vasmatzis G., May D.G., Roux K.J., Galardy P.J. UCH-L1 bypasses mTOR to promote protein biosynthesis and is required for MYC-driven lymphomagenesis in mice. Blood. 2018;132:2564–2574. doi: 10.1182/blood-2018-05-848515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hussain S., Foreman O., Perkins S.L., Witzig T.E., Miles R.R., van Deursen J., Galardy P.J. The de-ubiquitinase UCH-L1 is an oncogene that drives the development of lymphoma in vivo by deregulating PHLPP1 and Akt signaling. Leukemia. 2010;24:1641–1655. doi: 10.1038/leu.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jara J.H., Frank D.D., Ozdinler P.H. Could dysregulation of UPS be a common underlying mechanism for cancer and neurodegeneration? Lessons from UCHL1. Cell Biochem. Biophys. 2013;67:45–53. doi: 10.1007/s12013-013-9631-7. [DOI] [PubMed] [Google Scholar]
- 236.Das C., Hoang Q.Q., Kreinbring C.A., Luchansky S.J., Meray R.K., Ray S.S., Lansbury P.T., Ringe D., Petsko G.A. Structural basis for conformational plasticity of the Parkinson’s disease-associated ubiquitin hydrolase UCH-L1. Proc. Natl. Acad. Sci. USA. 2006;103:4675–4680. doi: 10.1073/pnas.0510403103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Das Bhowmik A., Patil S.J., Deshpande D.V., Bhat V., Dalal A. Novel splice-site variant of UCHL1 in an Indian family with autosomal recessive spastic paraplegia-79. J. Hum. Genet. 2018;63:927–933. doi: 10.1038/s10038-018-0463-6. [DOI] [PubMed] [Google Scholar]
- 238.Rydning S.L., Backe P.H., Sousa M.M.L., Iqbal Z., Oye A.M., Sheng Y., Yang M., Lin X., Slupphaug G., Nordenmark T.H., et al. Novel UCHL1 mutations reveal new insights into ubiquitin processing. Hum. Mol. Genet. 2017;26:1217–1218. doi: 10.1093/hmg/ddx072. [DOI] [PubMed] [Google Scholar]
- 239.Mukoyama M., Yamazaki K., Kikuchi T., Tomita T. Neuropathology of gracile axonal dystrophy (GAD) mouse. An animal model of central distal axonopathy in primary sensory neurons. Acta Neuropathol. 1989;79:294–299. doi: 10.1007/BF00294664. [DOI] [PubMed] [Google Scholar]
- 240.Ittner L.M., Halliday G.M., Kril J.J., Gotz J., Hodges J.R., Kiernan M.C. FTD and ALS--translating mouse studies into clinical trials. Nat. Rev. Neurosci. 2015;11:360–366. doi: 10.1038/nrneurol.2015.65. [DOI] [PubMed] [Google Scholar]
- 241.Philips T., Rothstein J.D. Rodent Models of Amyotrophic Lateral Sclerosis. Curr. Protoc. Pharmacol. 2015;69:1–21. doi: 10.1002/0471141755.ph0567s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ahmed R.M., Irish M., van Eersel J., Ittner A., Ke Y.D., Volkerling A., van der Hoven J., Tanaka K., Karl T., Kassiou M., et al. Mouse models of frontotemporal dementia: A comparison of phenotypes with clinical symptomatology. Neurosci. Biobehav. Rev. 2017;74:126–138. doi: 10.1016/j.neubiorev.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 243.Picher-Martel V., Valdmanis P.N., Gould P.V., Julien J.P., Dupre N. From animal models to human disease: A genetic approach for personalized medicine in ALS. Acta Neuropathol. Commun. 2016;4:70. doi: 10.1186/s40478-016-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.De Giorgio F., Maduro C., Fisher E.M.C., Acevedo-Arozena A. Transgenic and physiological mouse models give insights into different aspects of amyotrophic lateral sclerosis. Dis. Models Mech. 2019;12 doi: 10.1242/dmm.037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Morrice J.R., Gregory-Evans C.Y., Shaw C.A. Animal models of amyotrophic lateral sclerosis: A comparison of model validity. Neural. Regen. Res. 2018;13:2050–2054. doi: 10.4103/1673-5374.241445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lutz C. Mouse models of ALS: Past, present and future. Brain Res. 2018;1693:1–10. doi: 10.1016/j.brainres.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 247.Batra R., Lee C.W. Mouse Models of C9orf72 Hexanucleotide Repeat Expansion in Amyotrophic Lateral Sclerosis/Frontotemporal Dementia. Front. Cell Neurosci. 2017;11:196. doi: 10.3389/fncel.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fisher E.M.C., Bannerman D.M. Mouse models of neurodegeneration: Know your question, know your mouse. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aaq1818. [DOI] [PubMed] [Google Scholar]
- 249.Geevasinga N., Menon P., Ozdinler P.H., Kiernan M.C., Vucic S. Pathophysiological and diagnostic implications of cortical dysfunction in ALS. Nat. Rev. Neurosci. 2016;12:651–661. doi: 10.1038/nrneurol.2016.140. [DOI] [PubMed] [Google Scholar]
- 250.Gautam M., Jara J.H., Kocak N., Rylaarsdam L.E., Kim K.D., Bigio E.H., Hande Ozdinler P. Mitochondria, ER, and nuclear membrane defects reveal early mechanisms for upper motor neuron vulnerability with respect to TDP-43 pathology. Acta Neuropathol. 2019;137:47–69. doi: 10.1007/s00401-018-1934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Genc B., Ozdinler P.H. Moving forward in clinical trials for ALS: Motor neurons lead the way please. Drug Discov. Today. 2014;19:441–449. doi: 10.1016/j.drudis.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Dervishi I., Ozdinler P.H. Incorporating upper motor neuron health in ALS drug discovery. Drug Discov. Today. 2018;23:696–703. doi: 10.1016/j.drudis.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gerfen C.R., Paletzki R., Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kim J., Hughes E.G., Shetty A.S., Arlotta P., Goff L.A., Bergles D.E., Brown S.P. Changes in the Excitability of Neocortical Neurons in a Mouse Model of Amyotrophic Lateral Sclerosis Are Not Specific to Corticospinal Neurons and Are Modulated by Advancing Disease. J. Neurosci. 2017;37:9037–9053. doi: 10.1523/JNEUROSCI.0811-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Leone D.P., Heavner W.E., Ferenczi E.A., Dobreva G., Huguenard J.R., Grosschedl R., McConnell S.K. Satb2 Regulates the Differentiation of Both Callosal and Subcerebral Projection Neurons in the Developing Cerebral Cortex. Cereb. Cortex. 2015;25:3406–3419. doi: 10.1093/cercor/bhu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Woodworth M.B., Greig L.C., Liu K.X., Ippolito G.C., Tucker H.O., Macklis J.D. Ctip1 Regulates the Balance between Specification of Distinct Projection Neuron Subtypes in Deep Cortical Layers. Cell Rep. 2016;15:999–1012. doi: 10.1016/j.celrep.2016.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]