Abstract
Brain iron homeostasis is a dopamine-related mechanism that may be modified with long-term psychostimulant treatment in attention-deficit/hyperactivity disorder (ADHD). We previously reported that while medication-naïve youth with ADHD have reduced brain iron compared to controls and psychostimulant-medicated patients, no differences were detected between the latter groups. In this follow-up study, we examined whether the duration of psychostimulant treatment correlates with the degree of iron normalization.
Brain iron was indexed with MRI using an advanced method called magnetic field correlation (MFC) imaging and the conventional R2* proton transverse relaxation rate method. MFC was acquired in 30 psychostimulant-medicated youth with comorbid-free ADHD and 29 age-matched controls (all males). R2* was acquired in a subset of these individuals. Region-of-interest analyses for MFC and R2* group differences and within-group correlations with age and years of psychostimulant treatment were conducted in the globus pallidus (GP), putamen (PUT), caudate nucleus (CN), thalamus (THL) and red nucleus.
No significant MFC and R2* group differences were detected. However, while all regional MFC and R2* significantly increased with age in the control group, MFC and R2* increased in the GP, PUT, CN and THL with psychostimulant treatment duration in the ADHD group to a greater degree than with age. Our findings suggest that while youth with ADHD may have less prominent age-related brain iron increases than that seen in typical development, long-term use of psychostimulant medications may compensate through a normalizing effect on basal ganglia iron. Longitudinal studies following ADHD patients before and after long-term psychostimulant treatment are needed to confirm these findings.
Keywords: Brain iron, ADHD, Psychostimulants, Magnetic field correlation, MRI, R2*
Abbreviations: MFC, magnetic field correlation; GP, globus pallidus; PUT, putamen; CN, caudate nucleus; THL, thalamus; RN, red nucleus
Highlights
-
•
Brain iron does not differ between controls and psychostimulant-medicated ADHD.
-
•
Brain iron increases with age in controls.
-
•
Brain iron increases with psychostimulant use duration in ADHD more than with age.
-
•
Long-term psychostimulant treatment of ADHD normalizes brain iron levels.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is among the most prevalent neurodevelopmental disorders, with diagnosis rates capturing 11% of the US pediatric population (Anderson, 2018; Visser et al., 2014). Despite ongoing concerns of ADHD overdiagnosis (Adams, 2015; Bax et al., 2019; Layton et al., 2018; Storebø et al., 2018), psychostimulant medications remain the first-line treatment for ADHD and are increasingly prescribed in youth (6.6%) and adults (0.7%) (Anderson, 2018; Burcu et al., 2016; Visser et al., 2014). Psychostimulant medications act primarily on the striatal dopaminergic system and on the dopaminergic and noradrenergic systems in the frontal cortex (Faraone, 2018; Spencer et al., 2015; Volkow et al., 2005). A single-dose of the drug increases synaptic dopamine and noradrenaline levels by blocking catecholamine transporters. In ADHD, this acute increase in catecholamines corresponds to reduced symptoms (Wilens, 2008) and transient normalization of brain function as demonstrated by reduced resting-state functional connectivity (Silk et al., 2017), enhanced activation in cognitive control brain circuits during executive function tasks (Rubia et al., 2014) and reduced activation in reward circuits during reward response tasks (Rubia et al., 2009). As such, dopamine deficiency within frontostriatal circuits is suspected to play a central role in the pathophysiology of ADHD (Swanson et al., 2007).
Consistent with the lifetime chronicity of ADHD (40–65% persist into adulthood) (Barbaresi et al., 2013; Faraone et al., 2006; Geissler and Lesch, 2011), patients typically remain on psychostimulant medications for several years (Barbaresi, 2014), yet the effects of long-term psychostimulant treatment on the brain remain unclear. While limited and somewhat inconsistent (Castellanos et al., 2002; Hoogman et al., 2017; Norman et al., 2016; Sethi et al., 2017; Shaw et al., 2014), the literature implicates alterations in the dopaminergic system, brain structure and brain function, including increased striatal dopamine transporters (Fusar-Poli et al., 2012), basal ganglia volumes (Sobel et al., 2010; Nakao et al., 2011; Greven et al., 2015; Pretus et al., 2017), white matter microstructure (de Luis-García et al., 2015; Schweren et al., 2016), brain activation during inhibitory control tasks (Norman et al., 2016) and resting-state functional connectivity between the putamen and the default mode network (Battel et al., 2016). In a previous study, we detected the first account of a concordant psychostimulant medication effect on brain iron levels in ADHD (Adisetiyo et al., 2014).
Brain iron homeostasis is a critical dopamine-related mechanism, and its dysregulation has been implicated in ADHD (Adisetiyo et al., 2014; Cortese et al., 2012; Lozoff, 2011; Tseng et al. 2018; Wang et al., 2017; Yazici et al., 2019). Along with its many essential roles in the brain (Anderson and Frazer, 2017; Morris et al., 2018), iron is required for the synthesis and metabolism of dopamine, noradrenaline and myelin (Beard, 2003; Beard and Connor, 2003; Daubner et al., 2011; Ramsey et al., 1996). Indeed, age-related brain iron accumulation has been well documented in healthy brain development and is associated with cognitive and motor functioning (Hallgren and Sourander, 1958; Lozoff, 2011). Animal studies have shown that reduced iron in basal ganglia regions not only alters catecholamine systems within these regions but also modifies the effects of psychostimulants (Jenney et al., 2016; Jones et al., 2002; Mohamed et al., 2011; Morse et al., 1999). Using non-invasive magnetic resonance imaging (MRI) methods (Dusek et al., 2013), we along with others have demonstrated that striatal and thalamic iron levels are significantly reduced in medication-naïve children and adolescents with ADHD compared to age-matched typically developing controls (Adisetiyo et al., 2014; Cortese et al., 2012). Conversely, psychostimulant-medicated youth with ADHD have comparable brain iron levels as controls (Adisetiyo et al., 2014). However, because the details of medication history were not accounted for in these previous studies, we could not infer whether long-term psychostimulant use was correlated with the degree of iron normalization. Nonetheless, these findings are consistent with studies that demonstrate increased brain iron levels with methamphetamine and cocaine use (Adisetiyo et al., 2018; Ersche et al., 2017; Melega et al., 2007).
As an extension of our prior work (Adisetiyo et al., 2014), the goal of this follow-up study was to replicate the normalized brain iron findings observed in psychostimulant-medicated children and adolescents with ADHD compared to age-matched controls and examine whether brain iron levels increase with the duration of psychostimulant treatment. We utilized an advanced MRI method called magnetic field correlation (MFC) imaging (Jensen et al., 2009, 2006; Jensen and Helpern, 2014) along with the conventional R2* proton transverse relaxation rate method (Dusek et al., 2013) to index iron levels within dopamine and iron-rich basal ganglia regions that are known targets of psychostimulant medications (Faraone, 2018; Mink, 1996; Volkow et al., 2005). Multimodal imaging of brain iron was conducted to bolster the sensitivity and specificity for iron detection as the relationship between MRI metrics and brain tissue iron is complex and depends on numerous factors including the microscopic spatial distribution of iron and its molecular form (e.g., ferritin, hemosiderin) (Dusek et al., 2013). Because iron is strongly magnetic and has non-uniform microscopic spatial distributions in the brain (Connor et al., 1990; Iancu, 1992; Morris et al., 1992; Schenck and Zimmerman, 2004), it generates microscopic magnetic field inhomogeneities (MFIs) that significantly increase R2* (Brown et al., 2014). While R2* is highly sensitive to iron (Langkammer et al., 2010), it has limited specificity because it is affected by other dipolar interactions generated by tissue constituents unrelated to iron (Dusek et al., 2013). Compared to R2*, MFC has a more direct relationship to MFIs because MFC is independent of dipolar relaxation mechanisms (Jensen et al., 2009, 2006; Jensen and Helpern, 2014). Capitalizing on the strengths of both metrics to index iron, we hypothesized that while age will correlate with brain iron accumulation in control individuals (Adisetiyo et al., 2012; Hallgren and Sourander, 1958), psychostimulant treatment duration will be the driver of iron increases in youth with ADHD, particularly in the striatum and its primary output structure, the globus pallidus (Everitt and Robbins, 2005; Mink, 1996).
2. Material and methods
2.1. Participants
Individuals with ADHD and typically developing controls (males, 8–18 years old) were recruited through our medical center and the local community. Parental informed consent and child's assent were obtained as approved by the Institutional Review Board. Inclusion required an estimated full-scale IQ ≥ 79 on the Kaufman Brief Intelligence Test, Second Edition (Kaufman and Kaufman, 1990), right-handedness on the Edinburgh Handedness Inventory (Oldfield, 1971) and absence of neurological, cognitive and chronic medical disorders. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition/Text Revision (DSM-IV-TR) diagnoses (American Psychiatric Association, 2000) were assessed by licensed clinicians or supervised trainees using the Schedule of Affective Disorders and Schizophrenia for Children – Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997) administered to the parent and child; for a subset of participants recruited from a related study (n = 19), the Mini-International Neuropsychiatric Interview for Children and Adolescents (Sheehan et al., 1998) was used in lieu of the K-SADS-PL to reduce assessment burden. Diagnosis of psychotic, major depressive, conduct, tic, anxiety, pervasive developmental disorders, history of traumatic head injury or contraindications to MRI were exclusionary. Medical and medication histories were reviewed and executive function (off all medications) was assessed using the Behavioral Rating Inventory of Executive Function – Parent and Teacher Versions (Gioia et al., 2000). For individuals with ADHD, inclusion required a current diagnosis of ADHD (with childhood diagnosis of ADHD combined subtype at 12 years old or younger) and a history of taking psychostimulant medications (i.e., methylphenidate, dexmethylphenidate, dextroamphetamine/amphetamine, l-lysine-D-amphetamine). Prior to the study visit, parents were instructed to complete a medication form using medical records to document the start and end dates for each psychostimulant medication taken by their child; the form was reviewed during the study visit and, if necessary, follow-up calls were conducted to ensure discrepancies were resolved. The cumulative years of psychostimulant treatment duration were derived by summating the durations of each non-overlapping psychostimulant medication taken by the participant (Harstad et al., 2014); non-psychostimulant medications were not examined. For controls, regular medications and DSM-IV-TR diagnoses (current/past) were exclusionary. All participants were instructed to be off all medications on the day of the MRI scan.
2.2. Image acquisition and processing
An MRI brain scan was conducted using a 3T Siemens TIM Trio scanner (Siemens Healthineers, Erlangen, Germany). Whole-brain structural T1-weighted magnetization-prepared rapid acquisition gradient-echo (MPRAGE) images were acquired for tissue segmentation with the following parameters: repetition time (TR) = 1900 ms, echo time (TE) = 2.26 ms, voxel size = 1.0 × 1.0 × 1.0 mm3, matrix = 256 × 256 × 192, field of view (FOV) = 256 × 256 × 192 mm3, flip angle = 9° and acquisition time = 4 min 26 s. For MFC estimation, asymmetric spin-echo (ASE) images were acquired with the segmented echo-planar imaging (EPI) approach, which minimizes EPI distortion (Porter and Heidemann, 2009): TR = 5550 ms, TE = 40 ms, voxel size = 1.7 × 1.7 × 1.7 mm3, matrix = 128 × 128, FOV = 220 × 220 mm2, number of slices = 40, flip angle = 90°, bandwidth = 1346 Hz/pixel, EPI factor = 33, refocusing pulse time shifts = 0, −4, and −16 ms, no gaps, averages = 4, acquisition time = 6 min 40 s. Prior to MFC calculation, the ASE images were corrected for Gibbs-ringing artifacts (Kellner et al., 2016) and motion-corrected. To correct for motion, the ASE 0-shift images (time shift = 0 ms) from each average were linearly co-registered to the first average's 0-shift image using SPM8 (Statistical Parametric Mapping, University College London, UK); the rigid-body transformation obtained was applied to all the ASE images corresponding to each average. The co-registered ASE images were averaged, and the MFC parametric map was then calculated on a voxel-by-voxel basis (Jensen et al., 2009, 2006). For R2* estimation, T2* gradient echo images were acquired with TR = 4380 ms, TEs = 4.92, 9.84, 14.76, 19.68, 24.60, 29.52, 34.44, 39.36, 44.28, 49.20 ms, voxel = 1.7 × 1.7 × 1.5 mm3, matrix = 128 × 128, FOV = 220 × 220 mm2, number of slices = 82, flip angle = 20°, bandwidth = 260 Hz/pixel, no gaps, average = 1, and acquisition time = 5 min 34 s. R2* parametric maps were generated as previously described (Adisetiyo et al., 2014). Structural scans were screened by two experienced raters for gross abnormalities and referred to a neuroradiologist when warranted. Images with incidental findings of clinical significance or severe artifacts (i.e., blurred images, signal loss) were excluded.
2.3. Region-of-interest analyses
Region-of-interest (ROI) analyses were conducted on the globus pallidus (GP), putamen (PUT), caudate nucleus (CN), and thalamus (THL) because these brain regions have the highest concentrations of dopamine and iron (Beard and Connor, 2003; Hallgren and Sourander, 1958), are targets of psychostimulants (Faraone, 2018; Volkow et al., 2005) and have been previously studied in ADHD (Adisetiyo et al., 2014; Cortese et al., 2012). Additionally, MFC and R2* in these brain regions have been shown to significantly correlate with putative postmortem iron concentrations in normal aging (Adisetiyo et al., 2012; Langkammer et al., 2010). The red nucleus (RN) was examined as a control ROI because it has high iron content but is not a target of psychostimulants (Faraone, 2018; Hallgren and Sourander, 1958; Volkow et al., 2005). To optimize anatomical accuracy, automated ROI and cerebrospinal fluid (CSF) segmentation was conducted on each participant's high-resolution MPRAGE using the Freesurfer software (http://surfer.nmr.mgh.harvard.edu). Every participant's ROIs, CSF mask, 0-shift ASE image and MFC map were normalized (resliced and resampled) to the MNI152 standard space (1 mm3) using the non-linear registration algorithm from the Automatic Registration Toolbox software (Ardekani et al., 2005).
To exclude voxels with partial volume effects, CSF was removed from all parametric maps and ROIs were constrained with a consensus mask. The consensus masks for the GP, PUT, CN and THL were defined as ROI voxels with 100% overlap among all participants (i.e., 59 participants for MFC; 46 participants for R2*). Due to the unavailability of automatic RN segmentation, the RN consensus ROI was drawn on a normalized group average 0-shift ASE image with its boundary defined well within the clearly distinguishable anatomical boundary. For each participant, anatomical accuracy of the consensus ROIs was visually verified (Fig. 1) and applied to the corresponding parametric map to extract ROI means.
Fig. 1.
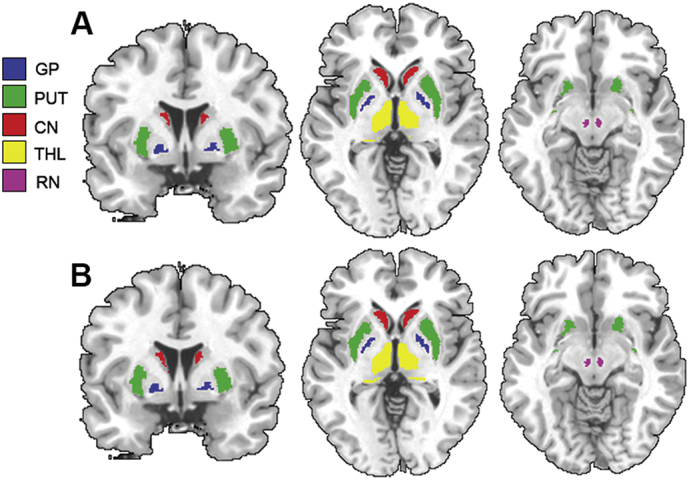
Brain iron indices in deep gray matter regions.
Region-of-interest (ROI) analyses of magnetic field correlation (MFC) and the R2* relaxation rate brain iron indices were conducted on the globus pallidus (GP), putamen (PUT), caudate nucleus (CN), thalamus (THL) and red nucleus (RN). A. Consensus ROIs from the cohort with MFC data (n = 59). B. Consensus ROIs from the cohort with MFC and R2* data (n = 46).
2.4. Statistical analyses
Statistical analyses were performed with the SPSS software package (v24.0; IBM, Armonk, NY). Data distributions were tested for normality by using the Shapiro-Wilk test. Group comparisons were conducted with two-tailed Student's t-test for normally distributed measures (Cohen's d for effect size), the Mann-Whitney U test for non-normally distributed measures (rank biserial correlation [rrb] for effect size) and the Fisher's exact test for nominal measures. A sequential Bonferroni-type false discovery rate (FDR) correction method was conducted to correct for multiple comparisons wherein an FDR corrected p-value significance threshold was calculated (Benjamini and Hochberg, 1995). The FDR approach was applied over the entire set of p-values from each analysis type (i.e., between group comparison, within group correlations).
To test whether brain iron indices increased with age (Hallgren and Sourander, 1958), ROI MFC and R2* correlations with age were conducted within each group using Pearson's correlation (r) for normally distributed measures and Spearman's correlation (rs) for non-normally distributed measures; all one-tailed. To test whether brain iron indices increased with the years taking psychostimulant medication in the ADHD group (Adisetiyo et al., 2014), ROI MFC and R2* correlations with psychostimulant medication duration were similarly conducted. Additionally, as age and medication duration were collinearly related in the ADHD group, ROI MFC and R2* partial correlations with years of psychostimulant duration (controlling for age), and vice versa, were conducted.
3. Results
3.1. Demographics
MFC maps were successfully acquired from 59 participants (controls n = 29, ADHD n = 30); R2* maps were successfully acquired from a subset of these same participants (controls n = 23; ADHD n = 23). For the participants with MFC data (Table 1) and those with R2* data (Supplementary Table S1), the control and ADHD groups did not significantly differ in age, ethnicity distribution and IQ; the ADHD group had significantly higher parent and teacher ratings of executive function deficits (all parent ratings reflected behavior off medication while teacher ratings reflected behaviors off or on medication).
Table 1.
Demographics (MFC data).
| Control group |
ADHD group |
Group comparison |
||
|---|---|---|---|---|
| (n = 29) | (n = 30) | t | p-Value | |
| Age (years, mean ± SD) | 13.9 ± 3.5 | 14.0 ± 2.5 | a414 | 0.8 |
| Age range (years) | 8.2–18.6 | 8.5–18.8 | – | – |
| Ethnicity (C:AA:O) | 23:5:1 | 23:7:0 | b1.3 | 0.7 |
| KBIT-2 | ||||
| Verbal IQ | 111.0 ± 10.9 | 106.5 ± 13.1 | −1.4 | 0.2 |
| Nonverbal IQ | 105.9 ± 11.8 | 107.5 ± 14.1 | 0.5 | 0.6 |
| Composite IQ | 110.1 ± 11.1 | 108.5 ± 13.7 | −0.5 | 0.6 |
| ADHD subtype | – | |||
| Combined | – | 22 | – | – |
| Inattentive | – | 7 | – | – |
| Hyperactive | – | 1 | – | – |
| BRIEF-Parent | ||||
| Behavioral regulation index | 46.9 ± 7.7 | 61.5 ± 9.9 | a97 | <0.001 |
| Metacognition index | 47.8 ± 9.3 | 69.4 ± 6.7 | 10.3 | <0.001 |
| Global executive composite | 48.0 ± 9.3 | 68.3 ± 7.2 | 9.4 | <0.001 |
| BRIEF-Teacher | ||||
| Behavioral regulation index | 48.4 ± 4.5 | 61.7 ± 15.1 | a125 | <0.001 |
| Metacognition index | 50.7 ± 8.5 | 69.9 ± 16.4 | 5.1 | <0.001 |
| Global executive composite | 49.8 ± 6.6 | 68.5 ± 16.8 | 5.1 | <0.001 |
All participants, male, right-handed, comorbid-free, positive history of psychostimulant medication; MFC: magnetic field correlation; C: Caucasian; AA: African American; O: other; KBIT-2: Kaufman Brief Intelligence Test, second edition; BRIEF-Parent: Behavioral Rating Inventory of Executive Function-Parent version (T-scores); BRIEF-Teacher: Teacher version (T-scores, Controls = 25, ADHD = 24); t: Student's t-test (two-tailed); SD: standard deviation; df: degrees of freedom; − not applicable.
Mann-Whitney U test (Exact Sig. two-tailed).
Fisher's Exact Test (Exact Sig. two-sided).
All participants with ADHD were comorbid-free and had a history of taking psychostimulant medication; 22 of these participants (73.3%) were prescribed >1 type of psychostimulant medication over time and 1 individual had limited psychostimulant exposure (<1 week). For participants with MFC data (Table 1), the ADHD group consisted of 30 individuals that averaged 5.9 years (SD = 2.8, range = 0–12.6 years) on psychostimulant medication, with the average age of first treatment being 7.8 years old (SD = 2.8); 10 of these individuals also had a history of taking non-psychostimulant medication. For participants with R2* data (Supplementary Table S1), the ADHD group consisted of 23 individuals that averaged 5.1 years (SD = 2.6, range = 0–10.1 years) on psychostimulant medication, with a similar age of treatment onset as the MFC cohort; 8 of these individuals also had a history of taking non-psychostimulant medication.
3.2. Brain iron indices as a function of age and psychostimulant medication duration
There were no significant group differences in MFC and R2* brain iron indices between the control and ADHD groups (Table 2), even when age was controlled as a covariate (Supplementary Table S2A–B). These results were further demonstrated by supplementary multiple regression analysis wherein group effects and group by age interaction effects on MFC and R2* were not significant (Supplementary Table S3).
Table 2.
Group comparisons.
| A. MFC (s-2) |
Control group (n = 29) |
ADHD group (n = 30) |
Group comparison |
||
|---|---|---|---|---|---|
| Region | Mean (SD) | Mean (SD) | Statistic | p-Value | Effect size |
| GP | 579.4 (204.6) | 546.7 (159.2) | t = 0.7 | 0.495 | d = 0.2; small |
| PUT | 242.9 (92.7) | 244.3 (74.8) | t = −0.1 | 0.950 | d = 0.0; small |
| CN | 284.1 (88.8) | 285.1 (88.2) | U = 422 | 0.851 | rrb = 0.0; small |
| THL | 143.5 (36.8) | 153.6 (36.4) | U = 342 | 0.162 | rrb = 0.2; small |
| RN | 230.9 (77.6) | 243.1 (121.1) | U = 423 | 0.863 | rrb = 0.0; small |
| B. R2* (s-1) |
Control group (n = 23) |
ADHD group (n = 23) |
Group comparison |
||
|---|---|---|---|---|---|
| Region | Mean (SD) | Mean (SD) | Statistic | p-Value | Effect size |
| GP | 31.0 (3.6) | 31.0 (3.3) | t = 0.1 | 0.957 | d = 0.0; small |
| PUT | 19.5 (2.3) | 19.9 (1.7) | t = −0.6 | 0.550 | d = 0.2; small |
| CN | 18.4 (1.6) | 19.4 (2.7) | U = 214 | 0.275 | rrb = 0.2; small |
| THL | 18.7 (1.3) | 18.7 (1.3) | U = 241 | 0.617 | rrb = 0.1; small |
| RN | 23.8 (4.0) | 23.5(3.3) | U = 248 | 0.728 | rrb = 0.1; small |
GP: globus pallidus; PUT: putamen; CN: caudate nucleus; THL: thalamus; RN: red nucleus; t: Student's t-test (two-tailed); U: Mann-Whitney U test (Exact Sig. two-tailed); SD: standard deviation; d: Cohen's d; rrb: rank biserial correlation.
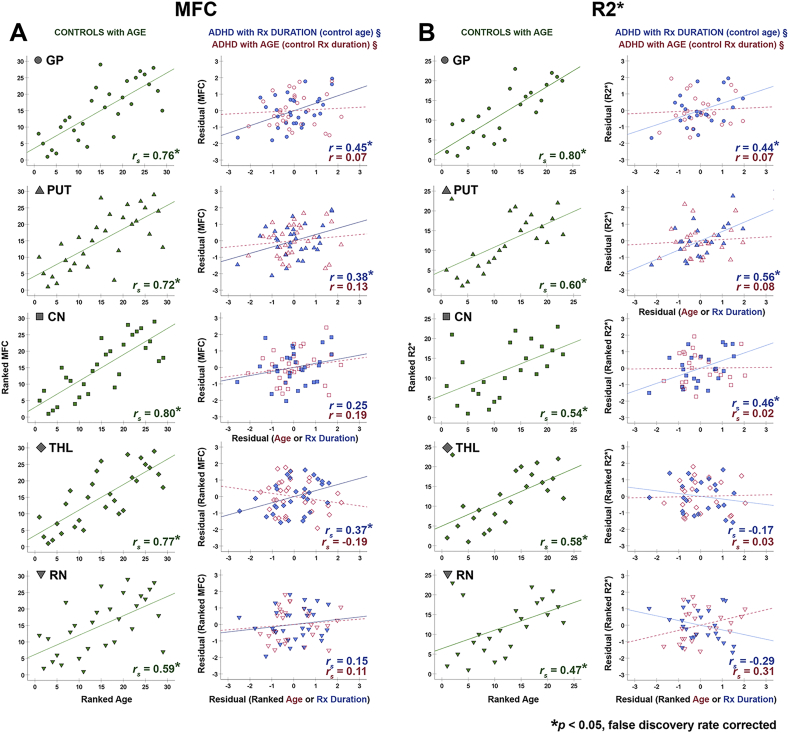
In the control group, MFC and R2* indices significantly increased with age in the GP, PUT, CN, THL and RN with large effect sizes (Table 3; Fig. 2). In the ADHD group, simple correlation analysis with age revealed significant positive correlations with MFC (moderate effect sizes) in the GP, PUT and CN, with trends for increased R2* with age in these regions. Alternatively, simple correlation analysis with psychostimulant treatment duration detected significant positive correlations with MFC and R2* (large effect sizes) in the GP, PUT and CN and with MFC in the THL (Table 3; Supplementary Fig. S1). All significant findings survived FDR correction.
Table 3.
Correlations with age and psychostimulant medication duration.
| Iron index | Control group |
ADHD group |
|||
|---|---|---|---|---|---|
| vs. Age | vs. Rx Duration | vs. Age | vs. Rx Duration (control for Age)a | vs. Age (control for Rx Duration)a | |
| A. MFC (s-2) | (n = 29) | (n = 30) | |||
| Region | rs (p-value) | r (p-value) | r (p-value) | r (p-value) | r (p-value) |
| GP | 0.76 (<0.001)⁎ | 0.60 (<0.001)⁎ | 0.44 (0.007)⁎ | 0.45 (0.007)⁎ | 0.07 (0.362) |
| PUT | 0.72 (<0.001)⁎ | 0.55 (0.001)⁎ | 0.45 (0.006)⁎ | 0.38 (0.021)⁎ | 0.13 (0.257) |
| CN | 0.80 (<0.001)⁎ | 0.45 (0.006)⁎ | 0.43 (0.009)⁎ | 0.25 (0.098) | 0.19 (0.167) |
| THL | 0.77 (<0.001)⁎ | rs = 0.33 (0.040)⁎ | rs = 0.05 (0.398) | rs = 0.37 (0.025)⁎ | rs = −0.19 (0.163) |
| RN | 0.59 (<0.001)⁎ | rs = 0.26 (0.083) | rs = 0.24 (0.103) | rs = 0.15 (0.216) | rs = 0.11 (0.290) |
| B. R2⁎ (s-1) | (n = 23) | (n = 23) | |||
| Region | rs (p-value) | r (p-value) | r (p-value) | r (p-value) | r (p-value) |
| GP | 0.80 (<0.001)⁎ | 0.54 (0.004)⁎ | 0.36 (0.048)# | 0.44 (0.018)⁎ | 0.07 (0.385) |
| PUT | 0.60 (0.001)⁎ | 0.66 (<0.001)⁎ | 0.43 (0.021)# | 0.56 (0.003)⁎ | 0.08 (0.365) |
| CN | 0.54 (0.004)⁎ | rs = 0.54 (0.004)⁎ | rs = 0.32 (0.071) | rs = 0.46 (0.015)⁎ | rs = 0.02 (0.473) |
| THL | 0.58 (0.002)⁎ | rs = −0.18 (0.203) | rs = −0.08 (0.360) | rs = −0.17 (0.229) | rs = 0.03 (0.448) |
| RN | 0.47 (0.012)⁎ | rs = −0.13 (0.283) | rs = 0.18 (0.201) | rs = −0.29 (0.100) | rs = 0.31 (0.078) |
GP: globus pallidus; PUT: putamen; CN: caudate nucleus; THL: thalamus; RN: red nucleus; Rx: psychostimulant medication; r: Pearson's correlation; rs: Spearman's correlation.
Partial correlations.
p < .05, one-tailed (false discovery rate corrected).
p < .05, one-tailed.
Fig. 2.
Brain iron indices increased with age in controls but increased with medication duration in individuals with ADHD.
A. In the control group (green), magnetic field correlation (MFC) indices of brain iron significantly increased with age in the globus pallidus (GP), putamen (PUT), caudate nucleus (CN), thalamus (THL) and red nucleus (RN); Conversely, in the ADHD group, MFC significantly increased with psychostimulant medication (Rx) duration when controlling for age in the GP, PUT and THL (blue). This correlation was not observed with age when controlling for Rx duration (red). B. Similar results were found with R2* relaxation rate indices of brain iron. r: Pearson's correlation, rs: Spearman's correlation (ranked values plotted), §: partial correlations (residual values plotted). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In the ADHD group, age and psychostimulant treatment duration were significantly correlated (n = 30 with MFC: r = 0.67, p < .001; n = 23 with R2*: r = 0.58, p = .004). Accounting for the collinearity between age and treatment duration, partial correlation analyses of MFC and R2* with medication duration (controlling for age) revealed significant positive correlations in the GP and PUT for both metrics, in the CN for R2* and in the THL for MFC. Conversely, partial correlation analyses of MFC and R2* with age (controlling for medication duration) found no significant correlations in any of the ROIs (Table 3; Fig. 2). All significant findings had moderate to large effect sizes and survived FDR correction. Similar findings were replicated in supplementary MFC analyses of the cohort with R2* data (Supplementary Tables S2C, S3C, S4 and S5) and in supplementary analyses excluding individuals with additional non-psychostimulant medication history (Supplementary Tables S6 and S7).
4. Discussion
Psychostimulant medications are the most common treatment for ADHD and reduce inattentive and hyperactive/impulsive symptoms in approximately 70% of the population (Wilens, 2008). The acute therapeutic effects of psychostimulants have a rapid onset (30 min) and last up to 12 h. Consequently, management of ADHD symptoms involves long-term use of psychostimulant medications, often lasting years (Barbaresi, 2014). Although limited, ADHD studies examining the effects of long-term psychostimulant treatment on the brain report alterations in the dopaminergic system (Fusar-Poli et al., 2012), GABAergic system (Solleveld et al., 2017), brain function (Battel et al., 2016), brain structure (Sobel et al., 2010; Nakao et al., 2011; Greven et al., 2015; Pretus et al., 2017; de Luis-García et al., 2015; Schweren et al., 2016) and brain metabolites (Benamor, 2014). Likewise, we previously reported the first account of a medication effect on brain iron levels in children and adolescents with ADHD (Adisetiyo et al., 2014). Whereas medication-naïve individuals with ADHD had significantly reduced MFC brain iron indices in the PUT, CN and THL, individuals with a history of psychostimulant treatment had comparable MFC in basal ganglia regions as age-matched control individuals. In this follow-up study, we replicated our findings in another independent cohort, once again demonstrating that psychostimulant-medicated children and adolescents with ADHD have comparable MFC and R2* indices of iron in basal ganglia regions as control individuals. Moreover, we found that the duration of psychostimulant treatment had a stronger statistical relationship to increased MFC and R2* in the ADHD group than age. These findings suggest that while youth with ADHD appear to have less prominent age-related brain iron increases than that seen in typical development, normalization of brain iron levels may result from long-term psychostimulant treatment of ADHD.
4.1. Disrupted iron homeostasis in ADHD
Iron homeostasis is a critical biological mechanism that is tightly regulated under physiological conditions (Anderson and Frazer, 2017; Duck and Connor, 2016). While required for virtually all basic cellular processes, iron is lethal to cells when unbound, especially in excess (Morris et al., 2018). Accordingly, intricate regulatory mechanisms for iron uptake, transport, usage and storage exist to ensure that iron supply meets the body's vast demands while preventing cell death (Morris et al., 2018). In the brain, iron is required for important neural processes implicated in ADHD including dopamine, noradrenaline and myelin synthesis and regulation (Beard and Connor, 2003). Maintaining iron homeostasis is essential for healthy brain development as low brain iron impairs catecholamine and myelin metabolism (Beard, 2003; Earley et al., 2014; Lozoff, 2011; Unger et al., 2007) and has been associated with developmental delay and cognitive deficits consistent with ADHD symptoms (McCann and Ames, 2007; Sidrak et al., 2014).
Indeed, growing evidence suggests that iron homeostasis may be disrupted in ADHD. Although the underlying mechanism are not understood, increased serum hepcidin (a master regulator of iron metabolism) (Yazici et al., 2019) and reduced brain iron levels (Adisetiyo et al., 2014; Cortese et al., 2012) have been detected in medication-naïve children with ADHD; variations in the hemochromatosis (HFE) gene (encodes for the iron uptake regulating HFE protein) (Nigg et al., 2016) and reduced serum ferritin (iron storage protein) (Tseng et al. 2018; Wang et al., 2017) have also been detected in studies of predominantly psychostimulant-medicated individuals with ADHD. Moreover, iron status in toddlerhood has been shown to predict sensitivity to psychostimulants in children with ADHD, with severe iron deficiency linked to higher medication dosage within the first year of treatment (Turner et al., 2012). Worthy of note is the inconsistency of peripheral iron markers in ADHD (Donfrancesco et al., 2013; Tseng et al. 2018; Wang et al., 2017). Discrepant findings may reflect the indirect and complex relationship between iron levels in the periphery and brain (Duck and Connor, 2016), the sensitivity of serum measures to confounds (e.g., inflammation, appetite suppression) (Dignass et al., 2018) as well as genetic differences influencing brain iron uptake which have been observed in animal studies (Morse et al., 1999).
4.2. Psychostimulants increase brain iron levels
Concordant with the literature on normal brain development (Beard and Connor, 2003; Hallgren and Sourander, 1958), we demonstrated that MFC and R2* brain iron indices significantly increased with age in all regions examined in the control group. In the ADHD group, simple age correlations detected a similar positive correlation with MFC and R2* in the GP, PUT and CN but to a lesser extent than simple correlations with psychostimulant medication duration, which detected positive correlations with MFC and R2* in more regions (GP, PUT, CN and THL) and with greater statistical significance. However, as all the participants with ADHD had a history of psychostimulant treatment, interpretation of these simple correlation findings is limited due to the significant collinearity between age and psychostimulant treatment duration. After accounting for this collinearity, we confirmed that MFC and R2* significantly increased with the duration of psychostimulant treatment in the GP, PUT, CN and THL even after controlling for age effects, whereas age no longer correlated with MFC or R2* when controlling for treatment duration. While we cannot definitively conclude that brain iron indices do not vary with age in the ADHD group, these results support a stronger relationship between psychostimulant treatment duration and increased MFC and R2*. Moreover, this psychostimulant medication effect was not detected in the RN control region, supporting the regional specificity of these results.
Along with reported changes in brain volume (Sobel et al., 2010; Nakao et al., 2011; Greven et al., 2015; Pretus et al., 2017), our findings add brain iron homeostasis to the list of neural processes likely altered by long-term psychostimulant treatment in ADHD. However, our supplementary volumetric analysis suggests that the long-term psychostimulant treatment effects on brain iron levels in basal ganglia regions are unrelated to regional brain volume. Specifically, we found no significant correlations between regional brain volume with MFC or R2* in the GP, PUT, CN and THL in either group (Supplementary Table S8). Additionally, regional volumes did not mediate the significant relationship between age and brain iron indices (MFC, R2*) within these regions in controls (Supplementary Table S9). These supplementary findings, along with those from (Ersche et al., 2017), implicate potentially distinct mechanisms underlying changes in brain iron and volume during typical development and from long-term psychostimulant use.
Our findings are consistent with the growing literature that implicates elevated brain iron within basal ganglia regions with prolonged psychostimulant exposure to cocaine (Ersche et al., 2017; Adisetiyo et al., 2018) and methamphetamines (Melega et al., 2007). Given that psychostimulants primarily modulate the dopaminergic system (Faraone, 2018; Spencer et al., 2015; Volkow et al., 2005), we speculate their long-term use may alter brain iron metabolism in basal ganglia regions via mechanisms linked to iron's role as a required cofactor for catecholamine synthesis and regulation (Daubner et al., 2011; Ramsey et al., 1996). Validation studies in animals and humans examining iron homeostasis and the dopaminergic system before and after long-term psychostimulant exposure are needed to elucidate the underlying mechanisms. Nonetheless, our findings suggest that long-term psychostimulant treatment may compensate for diminished age-related brain iron accumulation in ADHD that is observed in healthy brain development (Beard and Connor, 2003; Hallgren and Sourander, 1958).
4.3. Strengths, limitations and future studies
To our knowledge, this is the first study to examine the effects of psychostimulant treatment duration on brain iron levels in children and adolescents with ADHD. There is one R2* study of iron in the left ventral striatum of adults with ADHD (18–65 years old) that reported increased R2* correlating with age but not medication duration (Sethi et al., 2017). However, this study was limited to a brain region with low MRI reliability for iron detection (proximity to nasal-tissue interface can introduce artifacts) and its focus on an adult ADHD population may capture a distinct window of disease trajectory, wherein brain iron accumulation are at the highest levels and reaches a plateau (Hallgren and Sourander, 1958). Reduced specificity of the R2* metric for iron could also contribute to discrepant findings (Dusek et al., 2013). A strength of our study is that we conducted a multimodal brain iron assessment using the iron-sensitive R2* metric and the more iron-specific MFC metric that is independent of some of the non‑iron mechanisms affecting R2* (Jensen et al., 2009, 2006; Jensen and Helpern, 2014). While both metrics correlate with putative postmortem iron concentration in healthy brain (Adisetiyo et al., 2012; Langkammer et al., 2010), variance in their sensitivity and specificity for iron in different brain regions have been demonstrated (Adisetiyo et al., 2018) and are reflected in our findings (Table 3). Confounds such as tissue microstructure, calcification and water and protein content affect R2* and MFC differently (Brown et al., 2014; Dusek et al., 2013; Jensen and Helpern, 2014) which underscores the advantage of multimodal assessment of brain iron in that this approach capitalizes on the strengths of each modality for improved brain iron detection.
Study limitations are noted. Despite the moderate to large effect sizes of our findings, the limited sample size may have reduced statistical power. This may be reflected in our supplementary analysis of parental BRIEF ratings in the ADHD group, wherein correlations between high Behavioral Regulation Index scores and low MFC in the PUT and CN did not survive FDR correction (Supplementary Table S10). While this trend suggests a potential link between greater inhibitory control deficits and low striatal brain iron, future studies with larger cohorts and extensive measures of symptom severity are needed to confirm if brain iron and behavioral relationships exist. Also, as medication dose and non-psychostimulant medications were not systematically accounted for, their effects remain unclear. Nonetheless, potential non-psychostimulant medications effects were not confounding the results as similar findings were reproduced when ADHD patients with additional non-psychostimulant medication history were excluded in the supplementary analyses (Supplementary Tables S6 and S7). Additionally, while our study controlled for gender and comorbidity effects in the inclusion criteria, our findings are restricted to comorbid-free males with ADHD and thus have limited generalizability to the broader ADHD population. Lastly, the cross-sectional design of this study prevents causal inferences about normalized brain iron in the ADHD group and given that all participants with ADHD had a history of psychostimulant treatment, the extent of aberrant age-related iron accumulation trajectories in ADHD and the impact of baseline brain iron levels prior to taking medication remain unknown.
4.4. Conclusion
In conclusion, our results demonstrate that brain iron levels increase with the duration of psychostimulant treatment more prominently than with age in ADHD. While the results of this study are preliminary, they are consistent with the ADHD literature on long-term psychostimulant treatment effects on the brain that report alterations in the dopaminergic system (Fusar-Poli et al., 2012), GABAergic system (Solleveld et al., 2017), brain function (Battel et al., 2016), brain structure (Sobel et al., 2010; Nakao et al., 2011; Greven et al., 2015; Pretus et al., 2017; de Luis-García et al., 2015; Schweren et al., 2016) and brain metabolites (Benamor, 2014). Together, these studies demonstrate that long-term use of psychostimulant medications alter the brain in lasting ways beyond the acute single-dose effects. Elucidating the clinical significance of these long-term brain changes is imperative. If the degree of brain iron normalization corresponds with improved treatment outcomes, such evidence would inform clinicians to which patients are responding to treatment or could be used to monitor treatment adherence (Adler and Nierenberg, 2010; Rashid et al., 2018). Future longitudinal studies of medication-naïve individuals with ADHD before and after long-term psychostimulant treatment are needed to confirm this speculation, with consideration to medication onset (i.e., childhood versus adulthood), heterogeneity of ADHD profiles and treatment response. More critically, future studies are needed not only to clarify whether alterations in brain iron from long-term psychostimulant treatment are associated with improved outcomes but also whether such changes are detrimental in cases of misdiagnosis and improper prescription of psychostimulants.
Acknowledgements
This work was funded by The Klingenstein Third Generation Foundation (VA) and The Litwin Foundation (JAH). Drs. Adisetiyo and Gray reported no biomedical financial interests or potential conflicts of interest. Drs. Jensen and Helpern are inventors for patents related to MFC imaging that are owned by New York University and licensed to Siemens Healthcare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101993.
Appendix A. Supplementary data
Supplementary material
References
- Adams J.G. Psychostimulants: concerns over long-term adverse side effects. J. Miss. State Med. Assoc. 2015;56:346–347. [PubMed] [Google Scholar]
- Adisetiyo V., Jensen J.H., Ramani A., Tabesh A., Di Martino A., Fieremans E., Castellanos F.X., Helpern J.A. In vivo assessment of age-related brain Iron differences by magnetic field correlation imaging. J. Magn. Reson. Imaging. 2012;36:322–331. doi: 10.1002/jmri.23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adisetiyo V., Jensen J.H., Tabesh A., Deardorff R.L., Fieremans E., Di Martino A., Gray K.M., Castellanos F.X., Helpern J.A. Multimodal MR imaging of brain Iron in attention deficit hyperactivity disorder: a noninvasive biomarker that responds to psychostimulant treatment? Radiology. 2014;272:524–532. doi: 10.1148/radiol.14140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adisetiyo V., McGill C.E., DeVries W.H., Jensen J.H., Hanlon C.A., Helpern J.A. Elevated brain Iron in cocaine use disorder as indexed by magnetic field correlation imaging. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2018 doi: 10.1016/j.bpsc.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Adler L.D., Nierenberg A.A. Review of medication adherence in children and adults with ADHD. Postgrad. Med. 2010;122:184–191. doi: 10.3810/pgm.2010.01.2112. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, editor. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. 4th ed. American Psychiatric Association; Washington, D.C: 2000. [Google Scholar]
- Anderson J. Statistical Brief (Medical Expenditure Panel Survey (US)) Agency for Healthcare Research and Quality (US); Rockville (MD): 2018. Reported diagnosis and prescription utilization related to attention deficit hyperactivity disorder in children ages 5-17, 2008-2015. [PubMed] [Google Scholar]
- Anderson G.J., Frazer D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017;106:1559S–1566S. doi: 10.3945/ajcn.117.155804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani B.A., Guckemus S., Bachman A., Hoptman M.J., Wojtaszek M., Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J. Neurosci. Methods. 2005;142:67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Barbaresi W.J. Update on long-term stimulant medication treatment of attention-deficit hyperactivity disorder. J. Dev. Behav. Pediatr. 2014;35:446–447. doi: 10.1097/DBP.0000000000000084. [DOI] [PubMed] [Google Scholar]
- Barbaresi W.J., Colligan R.C., Weaver A.L., Voigt R.G., Killian J.M., Katusic S.K. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131:637–644. doi: 10.1542/peds.2012-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battel L., Kieling R.R., Kieling C., Anés M., Aurich N.K., da Costa J.C., Rohde L.A., Franco A.R. Intrinsic brain connectivity following long-term treatment with methylphenidate in children with attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2016;26:555–561. doi: 10.1089/cap.2015.0221. [DOI] [PubMed] [Google Scholar]
- Bax A., Bard D., Cuffe S., McKeown R., Wolraich M. The association between race/ethnicity and socioeconomic factors and the diagnosis and treatment of children with attention-deficit hyperactivity disorder. J. Dev. Behav. Pediatr. 2019;40:81–91. doi: 10.1097/DBP.0000000000000626. [DOI] [PubMed] [Google Scholar]
- Beard J. Iron deficiency alters brain development and functioning. J. Nutr. 2003;133:1468S–1472S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- Beard J.L., Connor J.R. Iron status and neural functioning. Annu. Rev. Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Benamor L. (1)H-magnetic resonance spectroscopy study of stimulant medication effect on brain metabolites in French Canadian children with attention deficit hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2014;10:47–54. doi: 10.2147/NDT.S52338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Brown R.W., Cheng Y.N., Haacke E.M., Thompson M.R., Venkatesan R. Magnetic Resonance Imaging. John Wiley & Sons, Ltd; 2014. Chapter 20 - Magnetic field inhomogeneity effects and T2* dephasing; pp. 569–617. [Google Scholar]
- Burcu M., Zito J.M., Metcalfe L., Underwood H., Safer D.J. Trends in stimulant medication use in commercially insured youths and adults, 2010-2014. JAMA Psychiatry. 2016;73:992–993. doi: 10.1001/jamapsychiatry.2016.1182. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Lee P.P., Sharp W., Jeffries N.O., Greenstein D.K., Clasen L.S., Blumenthal J.D., James R.S., Ebens C.L., Walter J.M., Zijdenbos A., Evans A.C., Giedd J.N., Rapoport J.L. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Connor J.R., Menzies S.L., St Martin S.M., Mufson E.J. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J. Neurosci. Res. 1990;27:595–611. doi: 10.1002/jnr.490270421. [DOI] [PubMed] [Google Scholar]
- Cortese S., Azoulay R., Castellanos F.X., Chalard F., Lecendreux M., Chechin D., Delorme R., Sebag G., Sbarbati A., Mouren M.-C., Bernardina B.D., Konofal E. Brain iron levels in attention-deficit/hyperactivity disorder: a pilot MRI study. World J. Biol. Psychiatry. 2012;13:223–231. doi: 10.3109/15622975.2011.570376. [DOI] [PubMed] [Google Scholar]
- Daubner S.C., Le T., Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011;508:1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luis-García R., Cabús-Piñol G., Imaz-Roncero C., Argibay-Quiñones D., Barrio-Arranz G., Aja-Fernández S., Alberola-López C. Attention deficit/hyperactivity disorder and medication with stimulants in young children: a DTI study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;57:176–184. doi: 10.1016/j.pnpbp.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Dignass A., Farrag K., Stein J. Limitations of serum ferritin in diagnosing Iron deficiency in inflammatory conditions. Int. J. Chronic Dis. 2018;2018 doi: 10.1155/2018/9394060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donfrancesco R., Parisi P., Vanacore N., Martines F., Sargentini V., Cortese S. Iron and ADHD: time to move beyond serum ferritin levels. J. Atten. Disord. 2013;17:347–357. doi: 10.1177/1087054711430712. [DOI] [PubMed] [Google Scholar]
- Duck K.A., Connor J.R. Iron uptake and transport across physiological barriers. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2016;29:573–591. doi: 10.1007/s10534-016-9952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek P., Dezortova M., Wuerfel J. Chapter nine - imaging of iron. In: Bhatia K.P., Schneider S.A., editors. International Review of Neurobiology, Metal Related Neurodegenerative Disease. Academic Press; 2013. pp. 195–239. [Google Scholar]
- Earley C.J., Connor J., Garcia-Borreguero D., Jenner P., Winkelman J., Zee P.C., Allen R. Altered brain iron homeostasis and dopaminergic function in restless legs syndrome (Willis-Ekbom disease) Sleep Med. 2014;15:1288–1301. doi: 10.1016/j.sleep.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Ersche K.D., Acosta-Cabronero J., Jones P.S., Ziauddeen H., van Swelm R.P., Laarakkers C.M., Raha-Chowdhury R., Williams G.B. Disrupted iron regulation in the brain and periphery in cocaine addiction. Transl. Psychiatry. 2017;7:e1040. doi: 10.1038/tp.2016.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Faraone S.V. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 2018;87:255–270. doi: 10.1016/j.neubiorev.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone S.V., Biederman J., Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol. Med. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Rubia K., Rossi G., Sartori G., Balottin U. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am. J. Psychiatry. 2012;169:264–272. doi: 10.1176/appi.ajp.2011.11060940. [DOI] [PubMed] [Google Scholar]
- Geissler J., Lesch K.-P. A lifetime of attention-deficit/hyperactivity disorder: diagnostic challenges, treatment and neurobiological mechanisms. Expert. Rev. Neurother. 2011;11:1467–1484. doi: 10.1586/ern.11.136. [DOI] [PubMed] [Google Scholar]
- Gioia G.A., Isquith P.K., Guy S.C., Kenworthy L. Test review behavior rating inventory of executive function. Child Neuropsychol. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Greven C.U., Bralten J., Mennes M., O’Dwyer L., van Hulzen K.J.E., Rommelse N., Schweren L.J.S., Hoekstra P.J., Hartman C.A., Heslenfeld D., Oosterlaan J., Faraone S.V., Franke B., Zwiers M.P., Arias-Vasquez A., Buitelaar J.K. Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatry. 2015;72:490–499. doi: 10.1001/jamapsychiatry.2014.3162. [DOI] [PubMed] [Google Scholar]
- Hallgren B., Sourander P. The effect of age on the non-haemin Iron in the human brain. J. Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Harstad E.B., Weaver A.L., Katusic S.K., Colligan R.C., Kumar S., Chan E., Voigt R.G., Barbaresi W.J. ADHD, stimulant treatment, and growth: a longitudinal study. Pediatrics. 2014;134:e935–e944. doi: 10.1542/peds.2014-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M., Bralten J., Hibar D.P., Mennes M., Zwiers M.P., Schweren L.S.J., van Hulzen K.J.E., Medland S.E., Shumskaya E., Jahanshad N., Zeeuw P. de, Szekely E., Sudre G., Wolfers T., Onnink A.M.H., Dammers J.T., Mostert J.C., Vives-Gilabert Y., Kohls G., Oberwelland E., Seitz J., Schulte-Rüther M., Ambrosino S., Doyle A.E., Høvik M.F., Dramsdahl M., Tamm L., van Erp T.G.M., Dale A., Schork A., Conzelmann A., Zierhut K., Baur R., McCarthy H., Yoncheva Y.N., Cubillo A., Chantiluke K., Mehta M.A., Paloyelis Y., Hohmann S., Baumeister S., Bramati I., Mattos P., Tovar-Moll F., Douglas P., Banaschewski T., Brandeis D., Kuntsi J., Asherson P., Rubia K., Kelly C., Martino A.D., Milham M.P., Castellanos F.X., Frodl T., Zentis M., Lesch K.-P., Reif A., Pauli P., Jernigan T.L., Haavik J., Plessen K.J., Lundervold A.J., Hugdahl K., Seidman L.J., Biederman J., Rommelse N., Heslenfeld D.J., Hartman C.A., Hoekstra P.J., Oosterlaan J., Polier G. von, Konrad K., Vilarroya O., Ramos-Quiroga J.A., Soliva J.C., Durston S., Buitelaar J.K., Faraone S.V., Shaw P., Thompson P.M., Franke B. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry. 2017;4:310–319. doi: 10.1016/S2215-0366(17)30049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iancu T.C. Ferritin and hemosiderin in pathological tissues. Electron Microsc. Rev. 1992;5:209–229. doi: 10.1016/0892-0354(92)90011-e. [DOI] [PubMed] [Google Scholar]
- Jenney C.B., Alexander D.N., Jones B.C., Unger E.L., Grigson P.S. Preweaning iron deficiency increases non-contingent responding during cocaine self-administration in rats. Physiol. Behav. 2016;167:282–288. doi: 10.1016/j.physbeh.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.H., Helpern J.A. In vivo characterization of brain iron with magnetic field correlation imaging. Future Neurol. 2014;9:247–250. doi: 10.2217/fnl.14.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.H., Chandra R., Ramani A., Lu H., Johnson G., Lee S.-P., Kaczynski K., Helpern J.A. Magnetic field correlation imaging. Magn. Reson. Med. 2006;55:1350–1361. doi: 10.1002/mrm.20907. [DOI] [PubMed] [Google Scholar]
- Jensen J.H., Szulc K., Hu C., Ramani A., Lu H., Xuan L., Falangola M.F., Chandra R., Knopp E.A., Schenck J., Zimmerman E.A., Helpren J.A. Magnetic field correlation as a measure of iron-generated magnetic field inhomogeneities in the brain. Magn. Reson. Med. 2009;61:481–485. doi: 10.1002/mrm.21823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.C., Wheeler D.S., Beard J.L., Grigson P.S. Iron deficiency in rats decreases acquisition of and suppresses responding for cocaine. Pharmacol. Biochem. Behav. 2002;73:813–819. doi: 10.1016/s0091-3057(02)00906-1. [DOI] [PubMed] [Google Scholar]
- Kaufman A., Kaufman N.L. American Guidance Service; Circle Pines, MN: 1990. Kaufman Brief Intelligence Test. [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kellner E., Dhital B., Kiselev V.G., Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 2016;76:1574–1581. doi: 10.1002/mrm.26054. [DOI] [PubMed] [Google Scholar]
- Langkammer C., Krebs N., Goessler W., Scheurer E., Ebner F., Yen K., Fazekas F., Ropele S. Quantitative MR imaging of brain Iron: a postmortem validation study. Radiology. 2010;257:455–462. doi: 10.1148/radiol.10100495. [DOI] [PubMed] [Google Scholar]
- Layton T.J., Barnett M.L., Hicks T.R., Jena A.B. Attention deficit–hyperactivity disorder and month of school enrollment. N. Engl. J. Med. 2018;379:2122–2130. doi: 10.1056/NEJMoa1806828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J. Nutr. 2011;141:740S–746S. doi: 10.3945/jn.110.131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J.C., Ames B.N. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am. J. Clin. Nutr. 2007;85:931–945. doi: 10.1093/ajcn/85.4.931. [DOI] [PubMed] [Google Scholar]
- Melega W.P., Lacan G., Harvey D.C., Way B.M. Methamphetamine increases basal ganglia iron to levels observed in aging. Neuroreport. 2007;18:1741–1745. doi: 10.1097/WNR.0b013e3282f0d4f4. [DOI] [PubMed] [Google Scholar]
- Mink J.W. The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mohamed W.M.Y., Unger E.L., Kambhampati S.K., Jones B.C. Methylphenidate improves cognitive deficits produced by infantile iron deficiency in rats. Behav. Brain Res. 2011;216:146–152. doi: 10.1016/j.bbr.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Morris C.M., Candy J.M., Oakley A.E., Bloxham C.A., Edwardson J.A. Histochemical distribution of non-haem iron in the human brain. Cells Tissues Organs. 1992;144:235–257. doi: 10.1159/000147312. [DOI] [PubMed] [Google Scholar]
- Morris G., Berk M., Carvalho A.F., Maes M., Walker A.J., Puri B.K. Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav. Brain Res. 2018;341:154–175. doi: 10.1016/j.bbr.2017.12.036. [DOI] [PubMed] [Google Scholar]
- Morse A.C., Beard J.L., Azar M.R., Jones B.C. Sex and genetics are important cofactors in assessing the impact of Iron deficiency on the developing mouse brain. Nutr. Neurosci. 1999;2:323–335. doi: 10.1080/1028415X.1999.11747287. [DOI] [PubMed] [Google Scholar]
- Nakao T., Radua J., Rubia K., Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am. J. Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Nigg J.T., Elmore A.L., Natarajan N., Friderici K.H., Nikolas M.A. Variation in an iron metabolism gene moderates the association between blood lead levels and attention-deficit/hyperactivity disorder in children. Psychol. Sci. 2016;27:257–269. doi: 10.1177/0956797615618365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman L.J., Carlisi C., Lukito S., Hart H., Mataix-Cols D., Radua J., Rubia K. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry. 2016;73:815–825. doi: 10.1001/jamapsychiatry.2016.0700. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Porter D.A., Heidemann R.M. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn. Reson. Med. 2009;62:468–475. doi: 10.1002/mrm.22024. [DOI] [PubMed] [Google Scholar]
- Pretus C., Ramos-Quiroga J.A., Richarte V., Corrales M., Picado M., Carmona S., Vilarroya Ó. Time and psychostimulants: opposing long-term structural effects in the adult ADHD brain. A longitudinal MR study. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2017;27:1238–1247. doi: 10.1016/j.euroneuro.2017.10.035. [DOI] [PubMed] [Google Scholar]
- Ramsey A.J., Hillas P.J., Fitzpatrick P.F. Characterization of the active site iron in tyrosine hydroxylase Redox states of the iron. J. Biol. Chem. 1996;271:24395–24400. doi: 10.1074/jbc.271.40.24395. [DOI] [PubMed] [Google Scholar]
- Rashid M.A., Lovick S., Llanwarne N.R. Medication-taking experiences in attention deficit hyperactivity disorder: a systematic review. Fam. Pract. 2018;35:142–150. doi: 10.1093/fampra/cmx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A.-M., Brammer M., Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rubia K., Alegria A.A., Cubillo A.I., Smith A.B., Brammer M.J., Radua J. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol. Psychiatry. 2014;76:616–628. doi: 10.1016/j.biopsych.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck J.F., Zimmerman E.A. High-field magnetic resonance imaging of brain iron: birth of a biomarker? NMR Biomed. 2004;17:433–445. doi: 10.1002/nbm.922. [DOI] [PubMed] [Google Scholar]
- Schweren L.J.S., Hartman C.A., Zwiers M.P., Heslenfeld D.J., Franke B., Oosterlaan J., Buitelaar J.K., Hoekstra P.J. Stimulant treatment history predicts frontal-striatal structural connectivity in adolescents with attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2016;26:674–683. doi: 10.1016/j.euroneuro.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Sethi A., Evelyn-Rahr E., Dowell N., Jain S., Voon V., Critchley H.D., Harrison N.A., Cercignani M. Magnetization transfer imaging identifies basal ganglia abnormalities in adult ADHD that are invisible to conventional T1 weighted voxel-based morphometry. NeuroImage Clin. 2017;15:8–14. doi: 10.1016/j.nicl.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., De Rossi P., Watson B., Wharton A., Greenstein D., Raznahan A., Sharp W., Lerch J.P., Chakravarty M.M. Mapping the development of the basal ganglia in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:780–789.e11. doi: 10.1016/j.jaac.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. (quiz 34-57) [PubMed] [Google Scholar]
- Sidrak S., Yoong T., Woolfenden S. Iron deficiency in children with global developmental delay and autism spectrum disorder. J. Paediatr. Child Health. 2014;50:356–361. doi: 10.1111/jpc.12483. [DOI] [PubMed] [Google Scholar]
- Silk T.J., Malpas C., Vance A., Bellgrove M.A. The effect of single-dose methylphenidate on resting-state network functional connectivity in ADHD. Brain Imaging Behav. 2017;11:1422–1431. doi: 10.1007/s11682-016-9620-8. [DOI] [PubMed] [Google Scholar]
- Sobel L.J., Bansal R., Maia T.V., Sanchez J., Mazzone L., Durkin K., Liu J., Hao X., Ivanov I., Miller A., Greenhill L.L., Peterson B.S. Basal ganglia surface morphology and the effects of stimulant medications in youth with attention deficit hyperactivity disorder. Am. J. Psychiatry. 2010;167:977–986. doi: 10.1176/appi.ajp.2010.09091259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solleveld M.M., Schrantee A., Puts N.A.J., Reneman L., Lucassen P.J. Age-dependent, lasting effects of methylphenidate on the GABAergic system of ADHD patients. NeuroImage Clin. 2017;15:812–818. doi: 10.1016/j.nicl.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R.C., Devilbiss D.M., Berridge C.W. The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biol. Psychiatry. 2015;77:940–950. doi: 10.1016/j.biopsych.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storebø O.J., Pedersen N., Ramstad E., Kielsholm M.L., Nielsen S.S., Krogh H.B., Moreira-Maia C.R., Magnusson F.L., Holmskov M., Gerner T., Skoog M., Rosendal S., Groth C., Gillies D., Buch Rasmussen K., Gauci D., Zwi M., Kirubakaran R., Håkonsen S.J., Aagaard L., Simonsen E., Gluud C. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents - assessment of adverse events in non-randomised studies. Cochrane Database Syst. Rev. 2018;5 doi: 10.1002/14651858.CD012069.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J.M., Kinsbourne M., Nigg J., Lanphear B., Stefanatos G.A., Volkow N., Taylor E., Casey B.J., Castellanos F.X., Wadhwa P.D. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol. Rev. 2007;17:39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- Tseng P.-T., Cheng Y.-S., Yen C.-F., Chen Y.-W., Stubbs B., Whiteley P., Carvalho A.F., Li D.-J., Chen T.-Y., Yang Wei-Cheng, Tang C.-H., Chu C.-S., Yang Wei-Chieh, Liang H.-Y., Wu C.-K., Lin P.-Y. Peripheral iron levels in children with attention-deficit hyperactivity disorder: a systematic review and meta-analysis. Sci. Rep. 2018;8:788. doi: 10.1038/s41598-017-19096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.A., Xie D., Zimmerman B.M., Calarge C.A. Iron status in toddlerhood predicts sensitivity to psychostimulants in children. J. Atten. Disord. 2012;16:295–303. doi: 10.1177/1087054710385067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger E.L., Paul T., Murray-Kolb L.E., Felt B., Jones B.C., Beard J.L. Early iron deficiency alters sensorimotor development and brain monoamines in rats. J. Nutr. 2007;137:118–124. doi: 10.1093/jn/137.1.118. [DOI] [PubMed] [Google Scholar]
- Visser S.N., Danielson M.L., Bitsko R.H., Holbrook J.R., Kogan M.D., Ghandour R.M., Perou R., Blumberg S.J. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:34–46.e2. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Fowler J.S., Ding Y.-S. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Wang Y., Huang L., Zhang L., Qu Y., Mu D. Iron status in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0169145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens T.E. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 2008;28:S46–S53. doi: 10.1097/JCP.0b013e318173312f. [DOI] [PubMed] [Google Scholar]
- Yazici K.U., Yazici I.P., Ustundag B. Increased serum Hepcidin levels in children and adolescents with attention deficit hyperactivity disorder. Clin. Psychopharmacol. Neurosci. 2019;17:105–112. doi: 10.9758/cpn.2019.17.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material