Abstract
Atherosclerosis is a lipid-driven inflammatory disease of the arterial intima in which the balance of pro-inflammatory and inflammation-resolving mechanisms dictates the final clinical outcome. Intimal infiltration and modification of plasma-derived lipoproteins and their uptake mainly by macrophages, with ensuing formation of lipid-filled foam cells, initiate atherosclerotic lesion formation, and deficient efferocytotic removal of apoptotic cells and foam cells sustains lesion progression. Defective efferocytosis, as a sign of inadequate inflammation resolution, leads to accumulation of secondarily necrotic macrophages and foam cells and the formation of an advanced lesion with a necrotic lipid core, indicative of plaque vulnerability. Resolution of inflammation is mediated by specialized pro-resolving lipid mediators derived from omega-3 fatty acids or arachidonic acid and by relevant proteins and signalling gaseous molecules. One of the major effects of inflammation resolution mediators is phenotypic conversion of pro-inflammatory macrophages into macrophages that suppress inflammation and promote healing. In advanced atherosclerotic lesions, the ratio between specialized pro-resolving mediators and pro-inflammatory lipids (in particular leukotrienes) is strikingly low, providing a molecular explanation for the defective inflammation resolution features of these lesions. In this Review, we discuss the mechanisms of the formation of clinically dangerous atherosclerotic lesions and the potential of pro-resolving mediator therapy to inhibit this process.
Atherosclerosis is a disease characterized by low-grade, chronic inflammation of the arterial wall and is triggered by the subendothelial retention of plasma-derived, apolipoprotein B (apoB)-containing lipoproteins in the affected inner layer of the arterial wall, the intima1–3. The hallmarks of chronic atheroinflammation are a gradual accumulation of lipids and inflammatory cells, notably of various subsets of monocyte-derived macrophages, T lymphocytes and mast cells, in the developing atherosclerotic lesions4–8. The blood-derived cells of myeloid origin that infiltrate the developing atherosclerotic lesions also include monocyte-derived dendritic cells9-10, various subsets of B lymphocytes and, in the very advanced, often thrombotic lesions, neutrophils11–14. Circulating eosinophils and basophils can also contribute to the progression of coronary artery lesions but, unlike mast cells, these allergy-related inflammatory cells are usually not found in the developing atherosclerotic lesions15.
Extracellular modifications of the infiltrated plasma lipoproteins initiate the local innate and adaptive immune responses in the intima16. Indeed, the modification of lipoproteins seems to be a very early event in the pathogenesis of atherosclerosis given that oxidative modification of lipoproteins has been observed in arterial samples from human fetuses even before signs of inflammation, such as macrophage infiltration, are present17. The modified lipids then activate the inflammatory cells present in the intima, which respond by secreting pro-inflammatory chemokines and cytokines that in turn activate circulating leukocytes and the local endothelial cells, thereby promoting further recruitment of inflammatory cells of myeloid origin, particularly a specific subset of circulating monocytes with inflammatory potential, as shown in both mice and humans18–20. The intimal monocyte-derived macrophages also secrete lipoprotein-modifying enzymes and agents and, reciprocally, the modified forms of the lipoproteins can then stimulate the cells to secrete increasing amounts of enzymes that can further modify the lipoproteins. Therefore, a pro-atherogenic spiral of atherosclerotic plaque development involving both lipids and inflammation is generated16.
Another important link between lipid accumulation and inflammation in the arterial intima is the continued uptake by macrophages of the modified, cholesteryl ester-rich lipoproteins from the extracellular space of the intima. Understandably, uptake and ensuing lysosomal hydrolytic degradation of these pro-atherogenic lipoproteins by macrophages lowers the concentration of the pro-inflammatory lipoproteins in the extracellular fluid21. In the macrophages, the lysosomal hydrolysis of cholesteryl ester generates unesterified cholesterol molecules, which then exit the lysosomes and become re-esterified in the cytoplasm, with ensuing formation of cytoplasmic cholesteryl ester droplets that leads to foam cell formation, a hallmark of atherosclerosis. Given that any cellular excess of unes-terified cholesterol is toxic to macrophages, efficient esterification and storage of cholesterol as cholesteryl ester droplets can be considered to be a protective response22,23. However, the continual hydrolysis and re-esterification of droplet cholesterol constitutes a futile cycle that wastes ATP and thereby contributes to the premature death of the foam cells in poorly oxygenated regions of atherosclerotic lesions24,25. Therefore, whereas foam cell formation is a beneficial process in the early atherosclerotic lesion, in advanced lesions the foam cells die easily and release their contents to the extracellular space, thereby worsening the inflammatory status and the structural stability of the plaque26.
The inflammatory response in any given tissue of the body, including the atherosclerotic arterial intima, is linked to a resolution phase that repairs the collateral damage27,28. The resolution response is a specific, active process rather than a simple waning of the inflammatory response. In particular, the resolution of inflammation is mediated by a wide variety of molecules, including small lipids, proteins and signalling gases that begin to appear even in the earliest stages of the inflammatory response, that is, ‘in preparation’ for the ensuing critical resolution phase. Therefore, for example, although pus and oedema fluid are rich sources of resolution mediators, these pro-resolving mediators are also found in physiological body fluids such as tears and human milk, perhaps suggesting a role of these molecules in basal or preventive resolution of inflammation27. By activating cell-surface receptors, pro-resolving mediators block inflammatory cell influx and promote the egress of inflammatory cells, modulate pro-inflammatory T cell responses and promote clearance of both pathogens and dead cells (efferocytosis), with all these functions serving to limit tissue damage and to enable the repair of injured tissue29,30.
Many chronic inflammatory diseases, including advanced atherosclerosis, are characterized by an imbalance between pro-resolving and pro-inflammatory mediators, such as leukotrienes, leading to defective resolution of inflammation, tissue injury and damage-associated molecular pattern (DAMP)-mediated inflammation31–33. In particular, clinically dangerous atherosclerotic plaques are characterized by hallmarks of impaired resolution of inflammation, notably defective efferocytosis, DAMP-mediated inflammation, development of a necrotic lipid core and thinning of the protective collagen cap31,32,34,35 (FIG. 1 ). Moreover, the ratio of pro-resolving lipid mediators to leukotrienes is low in advanced versus early atherosclerotic plaques in humans36, and a low resolvin D1 (RvD1):leukotriene B4 ratio in saliva can predict carotid intimal thickness in humans37. Similarly, in mice, the advanced atherosclerotic lesions are deficient in pro-resolving lipid mediators37,38.
Fig. 1 |. Ligands and receptors transducing pro–resolving signalling in macrophages.
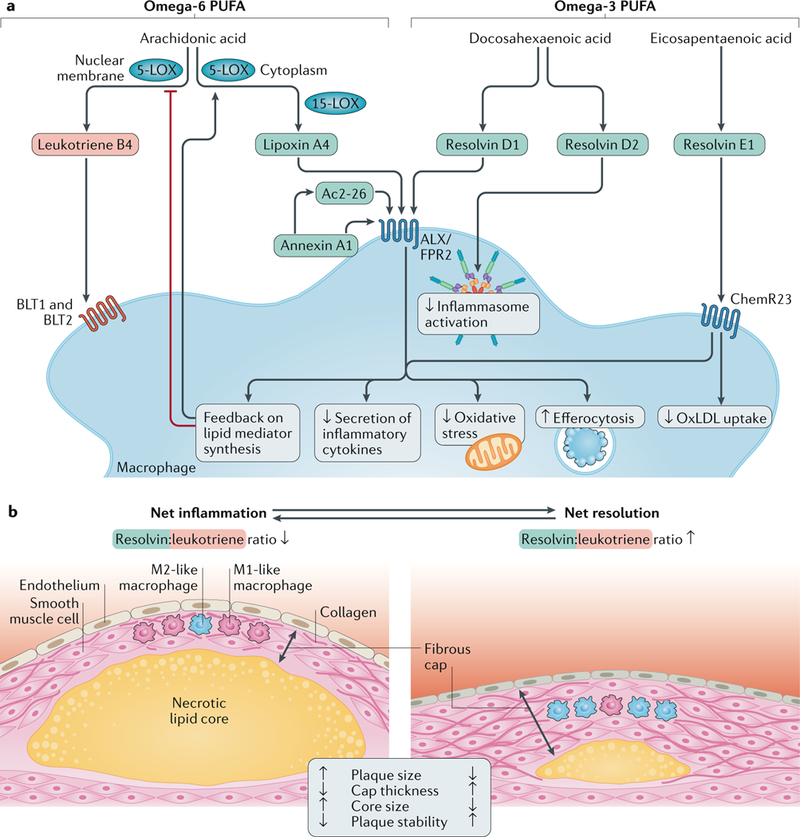
a |Specialized pro-resolving lipid mediators (in blue) include lipoxin A4, derived from the metabolism of the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid, and D-series and E-series resolvins formed from the metabolism of the omega-3 PUFAs docosahexaenoic acid and eicosapentaenoic acid, respectively. The protein pro-resolving mediator annexin A1 and the derivative peptide Ac2–26, as well as lipoxin A4 and resolvin D1, signal through N-formyl peptide receptor 2 (ALX/FPR2), whereas resolvin E1 transduces its effects through the ChemR23 receptor. These ligand-receptor interactions result in various effects, such as decreased secretion of inflammatory cytokines, reduced oxidative stress and improved efferocytosis. Resolvin E1 signalling through ChemR23 inhibits uptake of oxidized LDL (oxLDL), and resolvin D2 inhibits inflammasome activation. The ratio between pro-resolving and pro-inflammatory mediators (exemplified in the Figure by leukotriene B4 and its receptors BLT1 and BLT2) determines the macrophage phenotype. A disturbed balance in favour of pro-inflammatory pathways might serve as a marker of non-resolving inflammation in atherosclerosis. A pro-resolving amplification loop inhibits nuclear membrane localization of 5-lipoxygenase (5-LOX) and thereby further favours the formation of pro-resolving over pro-inflammatory mediators. b | Atherosclerotic plaques with net inflammation (left) and with net resolution of inflammation (right) are shown. Both atherosclerotic plaques are characterized by ongoing inflammation in which both pro-inflammatory and pro-resolving processes are concurrently active. However, the levels of these opposing processes are different in the two plaques: the resolvin:leukotriene ratio is low in the plaque with net inflammation and high in the plaque with net resolution. The plaque with net inflammation is larger and has a thin fibrous cap (with mainly pro-inflammatory M1-like macrophages, few smooth muscle cells and cleaved collagen) and a large necrotic lipid core. The plaque with net resolution is smaller and has a thicker fibrous cap (with mainly anti-inflammatory M2-like macrophages, abundant smooth muscle cells and intact collagen) and a smaller necrotic lipid core. The plaque with net inflammation is unstable and susceptible to rupture, whereas the plaque with net resolution is stable and not susceptible to rupture. During the long-lasting period of plaque development, the relative magnitude of pro-inflammatory and pro-resolving processes can vary over time. Therefore, during periods of net inflammation, plaque size tends to increase, and during periods of net resolution, plaque size tends to decrease — that is, plaque regression takes place.
Taken together, infiltration and accumulation of plasma lipoproteins and leukocyte subsets is a driving force of atherosclerotic lesion growth. Although powerful tools already exist to lower the plasma concentration of atherogenic lipoproteins, notably reducing the numbers of circulating LDL particles to very low levels, and thereby slow the progression or even induce regression of atherosclerotic lesions, a residual risk of cardiovascular events still exists in these patients39. Therefore, targeting inflammation by inhibiting pro-inflammatory cytokines has emerged as a novel promising mode of therapy to improve and complement the current lipid-lowering approaches40.
In this Review, we describe specific causes of inflammation and the mechanisms of inflammation resolution that become impaired in atherosclerosis and that are potentially amenable to therapeutic intervention by resolution-mediator therapy. In particular, we high-light the roles of different types of endogenous resolution mediator in suppressing atherogenic processes and how these mediators might be used as a therapy to prevent atherosclerotic cardiovascular disease.
Lipid-driven inflammation
In general, both the cellular and humoral components of the chronic, low-degree inflammation in an atherosclerotic arterial wall are the same as those in other tissues in the body. However, in sharp contrast to other tissues, both the initiation and progression of atherosclerosis result from the accumulation of lipoproteins in the arterial intima. Lipoproteins <70 nm in diameter (that is, all HDL, LDL and intermediate-density lipoprotein (IDL) particles, most VLDL remnants and the smallest chylo-micron remnants) can pass the endothelial barrier and enter the arterial intima from the circulation41,42. Furthermore, the interaction of apoB-containing lipoproteins (the non-HDL lipoproteins) with intimal extracellular proteoglycans (referred to as retention)1,43,44 inhibits the movement of the lipoproteins, thereby resulting in much higher subendothelial concentrations of these lipoproteins in the intima than in any other tissue45.
Lipoprotein modification
The retained lipoproteins (that is, those bound to the extracellular matrix of the arterial intima) are susceptible to modification by intimal oxidizing agents, proteases and lipases43,46,47. Indeed, blocking of LDL retention with antibodies generated against the sulfated glycosamino-glycans of proteoglycans decreases LDL modification and atherosclerosis48. Oxidative lipoprotein modifications lead to the generation of oxidized phospholipids (oxPLs) and other lipid mediators that have the potential to influence inflammation and atherogenesis in the arterial intima by inducing leukocyte recruitment and leukocyte activation49,50. The importance of oxidized LDL (oxLDL) in atherosclerosis was confirmed in a study published in 2018, in which macrophages of transgenic mice expressing a single-chain variable fragment of an oxPL-recognizing natural antibody showed decreased uptake of oxLDL compared with macrophages from control mice51. Moreover, these mice had decreased oxPL-induced inflammation and reduced atherosclerosis compared with the control mice. Notably, the oxPLs present in oxLDL activate endothelial cells and control the endothelial barrier permeability52,53, and oxPLs released from dying cells are captured by the CD14 receptor on dendritic cells, with ensuing inflammasome-dependent phagocyte hyperactivation54.
Oxidative, proteolytic and lipolytic modifications induce LDL aggregation46, and the aggregation susceptibility of LDL was shown to be associated with the risk of cardiovascular death55. Multiply modified, aggregated, apoB-containing lipoproteins are found in atherosclerotic lesions, and these particles were shown to embed cholesterol crystals56. Indeed, modification of plasma lipoproteins in vitro can induce the formation of cholesterol crystals56,57. Uptake of oxidized, proteolysed or lipolysed lipoproteins or of cholesterol crystals by macrophages, dendritic cells and vascular smooth muscle cells leads to accumulation of cytoplasmic lipid droplets, making the cells ‘foamy’10,58, thereby presenting the typical histological hallmarks of atherogenesis. Interestingly, cholesterol crystals can also be formed intracellularly after uptake of modified LDL, particularly oxLDL59.
NLRP3 inflammasome activation
Accumulation of lipoprotein-derived lipids in macrophages or dendritic cells readily induces activation of a multiprotein complex termed the inflammasome56,60 (FIG. 2). Of the various inflammasomes, NLRP3 is the most widely studied. Human atherosclerotic lesions show increased expression of the major components of NLRP3 (REF 61), and inhibition of the NLRP3 inflammasome reduces atherosclerosis in Apoe−/− mice62. The functional regulation of an active NLRP3 inflammasome in a cell is a ‘two-hit’ process, in which signal 1 primes the transcription of the inflammasome and signal 2, either by the same and/or additional stimuli, activates the inflammasome and induces secretion of two pro-inflammatory cytokines, IL-1β and IL-18 (REFS63,64). The priming step is induced, for example, by ligands of Toll-like receptors (TLRs), such as bacterial lipopoly-saccharide (LPS), with both oral and intestinal pathogens providing potential sources of LPS in the arterial intima65,66. In addition to microbial components, endogenous factors can also perform the priming step of the inflammasome activation, with these stimuli probably dominating the NLRP3 priming in sterile inflammatory diseases such as atherosclerosis63.
Fig. 2 |. Modified lipoproteins and cholesterol crystals induce inflammasome activation.
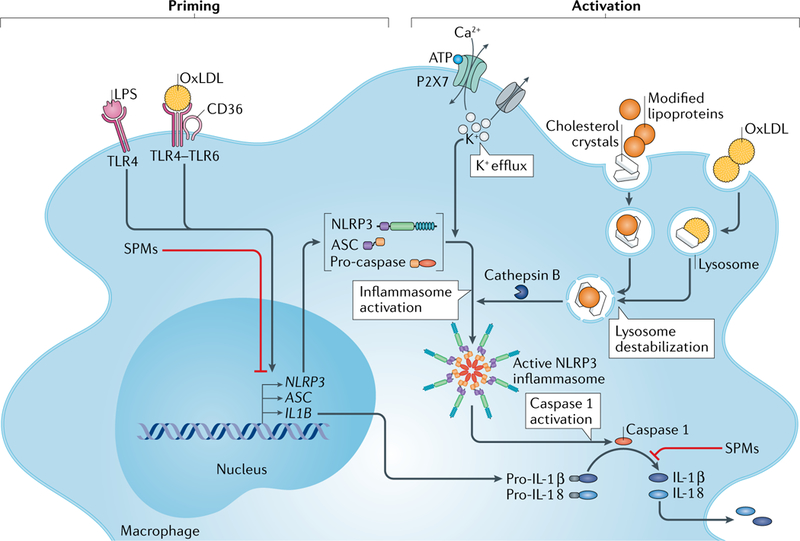
Activation of the NLRP3 inflammasome requires two steps: priming and activation. The priming step induces the expression of NLRP3 components and pro-IL-1β and can be triggered by lipopolysaccharide (LPS) and oxidized LDL (oxLDL). The activation step induces the activation of the NLRP3 inflammasome. Extracellular ATP activates the P2X7 receptor, which leads to K+ efflux with ensuing decreased intracellular K+ concentration and NLRP3 inflammasome activation. The activation step can also be triggered by cellular uptake of oxLDL. OxLDL uptake can lead to formation of cholesterol crystals in the lysosomes, with ensuing lysosomal destabilization and release of cathepsin B from disrupted lysosomes. The release of unesterified cholesterol from the disrupted lysosomes causes an increase in the content of unesterified cholesterol in intracellular membranes and can thereby cause NLRP3 activation. Moreover, uptake of cholesterol crystals and other modified lipoproteins (such as electronegative LDL, lipolysed LDL and VLDL and apolipoprotein B-containing lipid particles from atherosclerotic lesions) or immune complexes of oxLDL can induce activation of the NLRP3 inflammasome. Regardless of the activation mechanism, formation of the NLRP3 complex induces autocleavage and activation of caspase 1. Active caspase 1 can then cleave pro-IL-1β and the constitutively expressed pro-IL-18 to their mature, secretable forms. Specialized pro-resolving mediators (SPMs) can inhibit both the priming and the activation of the NLRP3 inflammasome. TLR, Toll-like receptor.
In macrophages in vitro, the LPS-primed NLRP3 inflammasome can be activated by adding ATP (the classical activation model) or palmitate (the lipotoxic activation model) to the cell culture system67. Palmitate can also activate the NLRP3 inflammasome in endothelial cells and thereby increase endothelial permeability68. These model systems are relevant to NLRP3 inflammasome activation in human atherosclerosis because necrotic cells release various DAMPs, among them ATP, and the concentration of circulating albumin-bound palmitate is elevated in states of insulin resistance and type 2 diabetes mellitus69,70.
OxLDL has been shown both to prime and to activate the NLRP3 inflammasome in macrophages in mouse models of atherosclerosis: first, oxLDL primes the NLRP3 inflammasome by binding to the CD36–TLR4–TLR6 signalling complex on the macrophage surface and, then, after having been transported to lysosomes, the oxLDL activates the NLRP3 inflammasome via induction of cholesterol crystallization and ensuing lysosomal damage in the macrophage59,71,72 (FIG. 2). In myeloid cells in which cholesterol efflux pathways have been genetically suppressed, accumulation of unesterified cholesterol in the cellular membranes without cholesterol crystal formation or lysosomal damage contributes to both priming and activation of the NLRP3 inflammasome73. Conversely, induction of cholesterol efflux from myeloid cells inhibits NLRP3 inflammasome activation73. NLRP3 inflammasome activation can also be induced by uptake of extracellular cholesterol crystals, leading to lysosomal damage and accumulation of cholesterol in cellular membranes58,71,73, and by uptake of oxLDL immune complexes74. Moreover, oxLDL, lipolysed LDL, electronegative LDL and modified lipoproteins isolated from human atherosclerotic lesions can activate the NLRP3 inflammasome56,59,74,75. Taken together, these findings show that extracellular and intracellular accumulation of cholesterol incites NLRP3 inflammasome-dependent intimal inflammation, whereas HDL-mediated cholesterol removal quenches the inflammation.
Resolution of inflammation
The host defence response against pathogens and tissue damage can be divided into two phases of inflammation: initiation and resolution. If functioning in a proper, temporally coordinated manner, acute inflammation is a protective process76. Although the resolution of inflammation was previously thought to be a passive response associated with catabolism of pro-inflammatory mediators and diffusion of chemotactic gradients, we now know that the resolution programme is a highly coordinated, active process76. Inflammation resolution is directed by the following mediators: proteins such as IL-10 and annexin A1; specialized pro-resolving mediators (SPMs), including resolvins, lipoxins, maresins and protectins; the endogenous gases nitric oxide (NO), hydrogen sulfide (H2S) and carbon monoxide (CO)77; the vagus nerve78; and regulatory T cells79. When sucscessfully executed, inflammation resolution decreases leukocyte recruitment, stimulates efferocytosis, repairs tissue damage and replenishes blood and lymphatic vessels. In the setting of infection, inflammation resolution also contributes to pathogen clearance76. However, when a defect in the resolution programme occurs, inflammation persists and chronic tissue damage ensues. Failure to stimulate inflammation resolution is now understood to promote many chronic inflammatory diseases, including atherosclerosis28,76,77.
Pro-resolving mediators
Lipid mediators of inflammation resolution.
Fatty acids can contribute in several different ways to both pro-inflammatory and pro-resolving signalling in atherosclerosis. For example, omega-3 fatty acids themselves can directly activate specific free fatty acid receptors (FFARs), of which FFAR4 has been associated with protection against the inflammatory response to vascular injury80. Conversely, fatty-acid-derived peroxidation products, such as the lipid aldehyde 4-hydroxy 2-nonenal (HNE), are vasculotoxic81. The generation of bioactive lipid mediators, such as prostaglandins, thromboxane and leukotrienes, has a major regulatory role in the inflammatory and thrombotic responses in atherosclerosis.
A unique class of fatty-acid-derived bioactive lipids has been termed SPMs27,82. The SPMs derived from the omega-3 fatty acids eicosapentaenoic acid (EPA), docosa-hexaenoic acid (DHA) and docosapentaenoic acid (DPA) include molecules such as E-series and D-series resolvins (generated from EPA and DHA, respectively), maresins and protectins. The SPMs derived from the omega-6 fatty acid arachidonic acid are included in the lipoxin family. Aspirin acetylation of the enzyme cyclooxygenase 2 blocks the formation of prostaglandins and modifies the metabolism of SPM precursors. The resulting R-epimers, referred to as aspirin-triggered resolvins and lipoxins, have similar biological activity but are more metabolically stable than the S-epimers76.
In response to an inflammatory response, the bioactive lipid mediators mentioned above are synthesized by a wide variety of cells, with neutrophils and macrophages serving as primary sources27. The main enzymes involved in the biosynthesis of lipid mediators include 5-lipoxygenase (5-LOX), 12-LOX and 15-LOX. The regsulation of 5-LOX is particularly interesting: when this enzyme is located on the nuclear membrane in neutrophils, mast cells and macrophages, it promotes the conversion of arachidonic acid into the pro-inflammatory leukotriene B4; by contrast, cytoplasmic 5-LOX favours the synthesis of the pro-resolving mediator lipoxin A4 (LXA4) from arachidonic acid in macrophages83–85. The nuclear (pro-inflammatory) location of 5-LOX is mediated by its phosphorylation by the enzyme MK2 (also known as MAPKAPK2)86. This pro-inflammatory MK2 pathway can be triggered by the calcium-activated kinase CaMKII and inhibited by SPMs, as part of a pro-resolving amplification loop, and by activators of the efferocytosis receptor tyrosine-protein kinase MER (MERTK)84,87,88. These regulatory pathways have essential roles in atherosclerosis (FIG. 1).
Importantly, the SPMs have been shown to inhibit the priming step and to promote the deactivation of the NLRP3 inflammasome in mouse macrophages67. RvD2 potently inhibited the secretion of mature IL-1β by mouse macrophages in vitro, and by the same mechanism also facilitated the inflammation resolution process in a mouse model of peritonitis, with self-resolution and limited inflammation in vivo67. These findings are of great interest because IL-1β can initiate an inflammatory cascade resembling the inflammatory response to infection or injury and can ultimately lead to a ‘cytokine storm’89,90, a systemic event mimicked on a microscale inside the progressing atherosclerotic plaque.
As the research field on resolution of inflammation has evolved, some questions have emerged on whether the physiological concentrations of pro-resolving lipid mediators in body fluids are high enough to trigger the types of cellular effector process that have been demonstrated by adding exogenous mediators to cultured cells. However, new work in this area using targeted, validated, high-precision mass spectrometry has begun to alleviate this concern91,92, particularly considering that the local release of resolution mediators in a confined tissue area with a low volume of extracellular fluid can lead to very high concentrations of these mediators in the immediate microenvironments of the activated cells. Moreover, as discussed below in the context of atherosclerosis, studies in mice with targeted deletions of genes encoding some of the most specific receptors for inflammation resolution mediators have shown defects in inflammation resolution processes in vivo, which further supports a physiological role for the endogenously generated resolution mediators93.
Non-lipid mediators of inflammation resolution.
In addition to lipids, other types of molecule have a role in the resolution of inflammation response. Examples of non-lipid mediators of resolution include proteins such as IL-10 and annexin A1 and the gasotransmitters H2S and NO (see below)30,94. Evidence for an atheroprotective role of IL-10 is extensive95, and available data support a similar protective role for the SPMs, annexin A1 and the gasotransmitters96–99 (see below). Furthermore, regulatory T cells can be activated by resolution mediators100, and these cells can potently suppress the progression of atherosclerosis101.
Pro-resolving mediators also include systemic hormones, such as α-melanocyte stimulating hormone (αMSH), a 13-amino-acid peptide derived from adrenocorticotropic hormone (ACTH), and the full-length ACTH, both of which can be carried to the inflammation site through plasma extravasation or can even be produced locally at an inflamed tissue site102. αMSH was originally identified as a skin-darkening hormone, is produced not only by melanocytes but also by macrophages and can potently modulate immune cell activity and immune processes. New knowledge in the field of melanocortins as mimetics of resolution can revitalize the use of ACTH in immuno-inflammatory diseases such as multiple sclerosis and systemic lupus erythematosus, and support the development of novel melanocortin-based drugs102. Whether these successes in melanocortin biology can be applied for therapeutic purposes in atherosclerosis remains to be seen.
Pro-resolving receptor signalling
Lipid and peptide pro-resolving mediators signal through specific G protein-coupled receptors (GPCRs), of which N-formyl peptide receptor 2 (ALX/FPR2; also known as FPR2 or ALX) and ChemR23 (also known as chemokine-like receptor 1 (CMKLR1)) have been evaluated in experimental models of atherosclerosis. ALX/FPR2 is the receptor for lipoxins, the DHA-derived RvD1 and for annexin A1 and its derivatives, whereas the EPA-derived resolvins signal their pro-resolving effects through the ChemR23 receptor103. These receptors are expressed in human aortic, coronary and carotid atherosclerotic lesions104–106, indicating that their pro-resolving ligands exert local effects within the vascular wall (TABLE 1).
Table 1 |.
Studies on specialized pro-resolving mediators in experimental models of atherosclerosis
| SPM target | Method | Model | Results | Refs |
| ALX/FPR2 pathways | ||||
| Resolvin D1 | Intraperitoneal injections | Ldlr −/− mice fed a Western diet | ↓ Atherosclerosis ↑ Plaque stability |
36 |
| Elastase perfusion in mice | ↓ Aortic aneurysms | 217 | ||
| 15-epi lipoxin A4 | Osmotic pump | Apoe−/− mice | ↓ Atherosclerosis | 99 |
| Carotid ligation in mice | ↓ Intimal hyperplasia | 110 | ||
| Ac2–26 (annexin A1-derived peptide) | Nanoparticle delivery | Ldlr−/− mice fed a Western diet | ↑ Plaque stability | 109 |
| Intraperitoneal injections | Apoe−/− mice | ↓ Atherosclerosis | 108 | |
| ALX/FPR2 systemic deficiency | Fpr2 knockout | Fpr2−/−Ldlr−/− mice | ↓ Atherosclerosis ↓ Plaque stability |
104 |
| Fpr2−/−Apoe−/− mice | ↓ Atherosclerosis | 99 | ||
| Fpr2−/−Apoe−/− mice | ↑ Atherosclerosis | 108 | ||
| Angiotensin II infusion in Fpr−/−Apoe−/− mice | ↑ Aortic aneurysms | 218 | ||
| ALX/FRP2 deficiency | Bone marrow transplantation | Transplantation of Fpr2−/− | ↓Atherosclerosis | 104 |
| in bone marrow cells | bone marrow cells into Ldlr−/− mice | ↔ Atherosclerosis | 109 | |
| ChemR23 pathways | ||||
| Resoivin E1 | Topical application in the oral cavity | Rabbits with periodontitis fed a high-fat diet | ↓ Atherosclerosis | 118 |
| Oral gavage | APOE*3-Leiden mice fed a Western diet | ↓ Atherosclerosis | 119 | |
| Intraperitoneal injections | Femoral wire injury in mice | ↓ Intimai hyperplasia | 219 | |
| ChemR23 systemic deficiency | Cmklrl knockout | Cmklr1−/−Apoe−/− mice | ↑ Atherosclerosis | 105 |
| ChemR23 deficiency in bone marrow cells | Bone marrow transplantation | Transplantation of Cmklr1−/− bone marrow cells into Ldlr−/− mice | ↑ Atherosclerosis | 105 |
ALX/FPR2, N-formyl peptide receptor 2; SPM, specialized pro-resolving mediator.
ALX/FPR2, the receptor for RvD1, LXA4 and annexin A1.
The ratio of 5-LOX-derived SPMs, notably RvD1, to pro-inflammatory leukotrienes is lower in advanced versus early atherosclerotic lesions in both humans and hypercholesterolaemic mice36. Plasma levels of another 5-LOX-derived SPM, LXA4, are decreased in patients with peripheral artery disease compared with healthy controls107. Given that both SPMs signal through ALX/ FPR2, their decreased levels in these patients suggest that the pro-resolving signalling through this receptor might be perturbed in advanced atherosclerosis, consistent with a failure in the resolution of inflammation. ALX/FPR2 is also activated by several other agonists, with partly opposing effects. For example, whereas SPMs and annexin-derived peptides promote M2-like macrophage polarization and pro-resolving signalling through ALX/FPR2, the same receptor elicits pro-inflammatory signalling when activated by acute phase, bacterial or mitochondrial peptides103. This dual role of ALX/FPR2 means that in the atherosclerotic state of non-resolving inflammation, an absence of pro-resolving ligands and an excess of pro-inflammatory ligands might skew signalling through this receptor towards a pro-inflammatory response. A study on genetic targeting of the murine ALX/FPR2 homologue in hyperlipidaemic mice provided arguments in favour of this hypothesis by showing that ALX/FPR2 deficiency reduced atherosclerosis consistently in different mouse models99,104.
Importantly, the paucity of pro-resolving mediators in advanced atherosclerotic lesions can be reversed by exogenous administration. Notably, intraperitoneal injections of RvD1 to Western-diet-fed Ldlr-knockout mice with mid-stage atherosclerotic lesions restore RvD1 levels in the lesions, increase efferocytosis, reduce oxidative stress in the lesions and induce a more stable plaque phenotype, thereby suppressing the progression of the mid-stage lesions to more advanced lesions36. Likewise, LXA4 administration with osmotic pumps to Apoe-knockout mice reduces atherosclerotic lesion size and inflammation, whereas this treatment does not alter the lesional phenotype of Apoe−/−Fpr2−/− mice99. Finally, the increased atherosclerosis burden observed in Apoe−/−Anxa1−/− mice compared with Apoe−/− mice can be reduced by treatment with the annexin A1 mimetic peptide Ac2–26, whereas this treatment does not affect atherosclerotic lesion size in Apoe−/− mice lacking ALX/FPR2 (REF108). Taken together, these observations emphasize the obligatory role of intact ALX/FPR2 signalling to functionally restore the signalling of annexin A1 and SPM pathways towards inflammation resolution in atherosclerosis.
One therapeutic challenge for stimulating the resolution of inflammation in atherosclerosis is how to restore the levels of pro-resolving mediators locally in the atherosclerotic lesion. One option tested experimentally is to use collagen-type-IV-targeted nanoparticles directed to release their content at the lesion109. Use of these nanoparticles to target the pro-resolving ALX/FPR2 agonist Ac2–26 to established atherosclerotic lesions in Ldlr-knockout mice fed a Western diet results in increased lesional Ac2–26 levels and a more stable plaque phenotype compared with mice receiving Ac2–26 nanoparticles without the collagen-type-IV-targeting peptide109. Ldlr-knockout mice that received ALX/FPR2-deficient haematopoietic cells, including macrophages, through bone marrow transplantation are mostly resistant to the atheroprotective effects of these collagen-type-IV-targeted Ac2–26 nanoparticles109. Although the above data suggest that lesional macrophages are the principal target of Ac2–26 in these studies, SPMs might also interact with lesional smooth muscle cells and endothelial cells107,110. For example, SPMs can reduce intimal hyperplasia after vascular injury110–112, and in one of the studies, the beneficial effects of the SPM LXA4 were reduced in ALX/FPR2-deficient mice110.
ChemR23, the receptor for EPA-derived resolvins.
The EPA-derived SPM RvE1 limits neutrophil-mediated inflammation by promoting neutrophil apoptosis and efferocytosis113. The levels of the RvE1 receptor ChemR23 become elevated during the differentiation of monocytes to macrophages in vitro114, and signalling of RvE1 via ChemR23 increases phagocytosis in human macrophages in vitro115,116. Consequently, murine macrophages derived from ChemR23-deficient mice have increased production of pro-inflammatory cytokines105,117 and a lack of RvE1-induced effects on phagocytosis compared with wild-type macrophages105.
In line with observations on arachidonic-acid-derived and DHA-derived SPMs, exogenous administration of RvE1 reduces experimental atherosclerosis118,119. Importantly, the RvE1 precursor 18-hydroxyeicosa-pentaenoic acid (18-HEPE) was identified as a major metabolite after EPA supplementation to hyperlipidae-mic mice105, suggesting that SPM levels can be restored by increasing substrate availability. This observation has important implications for dietary supplementation with omega-3 fatty acids, which are further discussed below.
The adipokine chemerin is another ChemR23 ligand116 that is present in human atherosclerotic lesions and correlates with disease severity106. Chemerin is a chemotactic molecule for M1-like macrophages114, exerts pro-inflammatory effects and increases the production of reactive oxygen species in vascular cells120. Chemerin also shows vasoconstrictor activity in vitro and increases blood pressure in mice121. However, ChemR23 deficiency in mice has different effects depending on the experimental model. For example, some models show reduced cerebral inflammation but exacerbated pulmonary inflammation116. These findings are in line with the results mentioned above for ALX/FPR2; that is, ChemR23 can transduce both pro-inflammatory and pro-resolving effects under different conditions and in response to different agonists. Of note, chemerin-derived peptides have been reported to induce similar pro-resolving actions to those of RvE1 through ChemR23 (REF116).
ChemR23 deficiency in Apoe-knockout mice increases atherosclerotic lesion size and macrophage content105. Similar findings along with an increased necrotic core size were also observed in chimeric animals receiving ChemR23-deficient bone marrow transplantation105. These findings, together with observations that ChemR23 localizes to a subset of lesional macrophages near the necrotic core105, point to a specific role for RvE1-ChemR23 signalling in macrophages to limit necrotic core formation. Intriguingly, RvE1 directly decreases the uptake of oxLDL by macrophages in vitro105. Furthermore, macrophages from ChemR23-deficient mice show a prolonged and continuous increase in oxLDL uptake in vitro, and when compared with aortas of wild-type mice, the atherosclerotic aortas of the ChemR23-deficient mice showed increased expression of genes related to lipid metabolism and lipoprotein uptake105. The importance of these findings has been further reinforced by transcriptomic profiling of human atherosclerotic lesions revealing that the expression levels of CMKLR1, which encodes ChemR23, were positively associated with markers of phagocytosis (which is consistent with pro-resolving signalling) and negatively associated with VLDLR and SORT1 expression levels105. Taken together, these observations indicate that the RvE1-ChemR23 pathway can directly act to limit oxLDL uptake and metabolism in immune cells. The therapeutic implications of these findings are also illustrated by the increased CMKLR1 mRNA levels in atherosclerotic lesions from patients receiving statin treatment105.
Omega-3 fatty acid supplementation could be anticipated to increase pro-resolving signalling through ALX/FPR2 and/or ChemR23 by increasing the biosynthesis of the SPM ligands for these receptors. However, a meta-analysis including data from ten randomized, controlled clinical trials did not detect any significant effects of omega-3 fatty acid supplements on coronary and cerebrovascular events overall or in any subgroups, including subgroups of patients with previous coronary heart disease or diabetes, those with lipid levels greater than a given cut-off level or those who were receiving statin therapy122. In addition, a subsequently reported randomized, controlled trial in patients with diabetes without evidence of atherosclerotic cardiovascular disease further supported that fish oil dietary supplementation does not alter the risk of serious cardiovascular events when used as a primary prevention strategy, at least in this group of patients123. By contrast, higher doses of pure EPA ethyl ester in patients with diabetes and hypertriglyceridaemia (≥150 mg/dl or 1.7 mmol/l) and with one additional risk factor124 significantly reduced major cardiovascular events compared with placebo125.
Although the addition of EPA to a high-fat diet increases the plasma concentration of the RvE1 precursor 18-monohydroxy EPA and is associated with increased tissue concentration of RvE1 in experimental atherosclerosis105, whether increased SPM formation can be achieved with the clinically tested omega-3 fatty acid supplements and at the doses used remains controversial. For example, aspirin-triggered, pro-resolving omega-3 fatty acid derivatives can be detected in patients with diabetes and coronary artery disease who were treated with a mixture of DHA and EPA concomitantly with aspirin and statins126. However, the dose of the omega-3 fatty acids used in this study126 was threefold higher than in most of the previous studies122,123. By contrast, another study did not detect consistent plasma levels of SPMs after fish oil dietary supplementation in healthy volunteers127. Studies have also shown that in mice fed a diet enriched in omega-3 polyunsaturated fatty acids, endogenous arachidonic acid and EPA production through desaturation of the upstream precursor omega-6 and omega-3 fatty acids, respectively, are needed to favour SPM formation128. Of note, hyperlip-idaemic mice with knockdown of the gene encoding fatty acid desaturase 1, an enzyme involved in the biosynthesis of arachidonic acid and EPA, have an increased atherosclerosis burden associated with a pronounced decrease in the SPM:leukotriene ratio128. This finding illustrates the need for further optimization of the formulation and doses for omega-3 fatty acid supplementation to stimulate SPM formation adequately. The target population to study is also important, especially with regard to primary versus secondary prevention, lipid profiles, comorbidities and medication use. As pointed out above, the concomitant use of aspirin with omega-3 fatty acid supplementation leads to the formation of the more stable, aspirin-triggered SPM epimers. Likewise, statin use increases CMKLR1 mRNA levels in macrophages129 and atherosclerotic lesions105, which might further increase local pro-resolving signalling.
Pro-resolving gaseous transmitters
Gaseous signalling can also promote inflammation resolution. Examples of gaseous signalling molecules include NO, H2S and CO, which are dissolved in extracellular fluids and can diffuse through cell membranes to affect cellular function directly130. The most widely studied of these molecules is NO, which is well characterized as an endothelium-derived relaxing factor derived from arginine metabolism. NO signals by increasing soluble guanylate cyclase activity and intracellular cGMP levels. NO can also promote the apoptosis of inflammatory cells and act through S-nitrosylation to prevent inflammasome assembly in macrophages130.
Another endogenous vasorelaxant, H2S, affects cellular signalling by altering intracellular calcium and cAMP levels, affecting ion channels and promoting post-translational protein sulfhydration130. H2S production in macrophages reduces LPS-induced TNF levels and stimulates efferocytosis of apoptotic granulocytes131. The H2S donor sodium hydrosulfide reduces atherosclerosis in Apoe -knockout mice132, whereas genetic targeting of the H2S synthesizing enzyme cystathionine γ-lyase exacerbates atherosclerosis in this model98. A decrease in H2S levels in atherosclerosis132 might, therefore, lead to a failure in the resolution of the atherosclerotic inflammation process, raising the possibility that H2S donors could have a therapeutic role in cardiovascular disease prevention.
Pro-resolving-type macrophages are characterized by expression of the enzyme haem oxygenase 1 (HO1), which produces CO during haem degradation. CO might contribute to the anti-inflammatory functions of HO1 by limiting leukocyte infiltration into inflammatory sites and suppressing cytokine production130. In the murine zymosan-induced peritonitis model, inhaled CO increases macrophage phagocytosis and reduces granulocyte numbers in the exudate, thereby shortening the time to achieve complete resolution of inflammation133. Furthermore, CO inhalation also increased the levels of SPMs133, indicating that the different pro-resolving signalling pathways are tightly intertwined.
Macrophage life cycle in atherosclerosis
The resolution of local inflammation and, most importantly, the regression of an atherosclerotic lesion strongly depend on macrophages; therefore, a better understanding of the physiological and pathophysiological control of the checkpoints of the macrophage life cycle in atherosclerotic lesions is vital.
Both human and mouse atherosclerotic plaques are populated mostly by macrophages134,135. Because infiltration and accumulation of the atherogenic plasma lipoproteins is the driving force of atherogenesis, removal of these lipoproteins in the developing lesions through their uptake and degradation is the primary function of the intimal macrophages. As infiltration and accumulation of the atherogenic plasma lipoproteins is the driving force of atherogenesis, the internalization and degradation of subendothelially retained lipoproteins by intimal macrophages can, at least initially, represent a process that delays lesion progression. After lysosomal hydrolysis of the cholesteryl esters from lipoproteins by macrophages, the unesterified cholesterol emerging from lysosomes can have three possible fates: esterification to fatty acids in the endoplasmic reticulum, which leads to foam cell formation; efflux from the macrophage; and conversion into oxysterols such as 25-hydroxy-cholesterol, which can have regulatory functions in macrophages136. Three mechanisms are available for cholesterol removal from the atherosclerotic plaque (FIG. 3). First, cholesterol can be effluxed from the macrophages to HDL particles for return transport to the blood137. However, as the macrophages keep accumulating cholesteryl ester droplets, the initiation of the reverse cholesterol transport pathway is not optimal, being the initial cause of the dysfunction in the cholesterol-donating foam cell or in the cholesterol-accepting HDL particle. The second mechanism is that the macrophage foam cells themselves leave the lesion and thus carry their cholesterol cargo back to the blood138. Third, an old macrophage foam cell can be efferocytosed by a cholesterol efflux-competent macrophage, which could then leave the atherosclerotic lesion.
Fig. 3 |. Macrophage life cycle and cholesterol round trip in atherosclerosis.
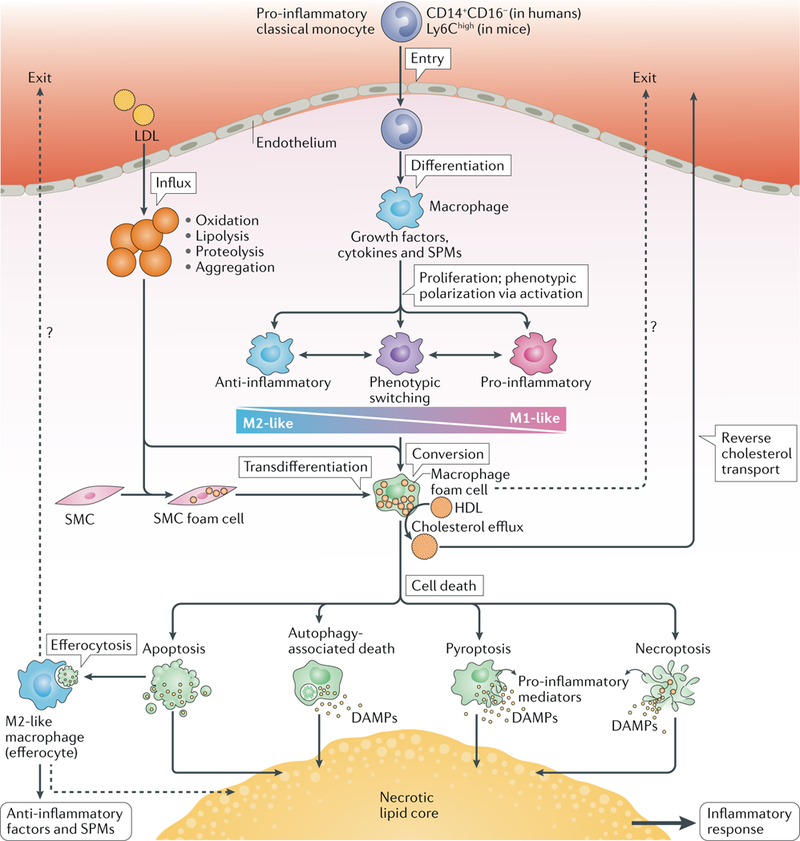
The pro-inflammatory subclass of circulating monocytes, the ‘classical monocytes’, are highly responsive to inflammatory signals and invade inflamed sites, such as atherosclerosis-susceptible areas of the arterial intima in which the cholesterol of locally modified LDL particles accumulates. In the atherosclerotic intima, monocytes differentiate into macrophages, which are then exposed to growth factors, cytokines and specialized pro-resolving mediators (SPMs). Different combinations of these factors determine the macrophage proliferation rate and their polarization into anti-inflammatory M2-like or pro-inflammatory M1-like phenotypes. Macrophages have high phenotypic plasticity, enabling phenotypic switching to provide the continuum of intermediate phenotypes that are often seen in advanced atherosclerotic lesions. A primary function of macrophages is to ingest modified LDL particles, which leads to accumulation of cytoplasmic cholesteryl ester droplets in the macrophages (that is, to foam cell formation). Smooth muscle cells (SMCs) can also form foam cells and then transdifferentiate into macrophage-like cells. HDL particles can induce cholesterol efflux from the foam cells, thereby initiating the process of reverse cholesterol transport for removal of cholesterol from the lesion. Some studies have provided evidence of egress of lesional macrophages during plaque regression (dotted lines). Thus, migration of foam cells to the blood might be an additional mechanism for cholesterol removal. Ultimately, old foam cells die via apoptotic or non-apoptotic forms of regulated cell death. Apoptosis is non-inflammatory and can even induce an anti-inflammatory response when the apoptotic cell is phagocytosed by an M2-like macrophage (efferocytosis) with ensuing release of anti-inflammatory factors and SPMs. The autophagic process itself is anti-inflammatory, but stressed cells undergoing extensive autophagy might die and can release pro-inflammatory mediators. Pyroptosis and necroptosis are pro-inflammatory via release of pro-inflammatory mediators, such as damage-associated molecular patterns (DAMPs). In advanced atherosclerotic lesions, influx of LDL particles and monocytes and formation of foam cells occur continually and, inevitably, some apoptotic foam cells escape efferocytosis and thereby contribute to the formation of a necrotic lipid core. Cholesterol-containing efferocytes can also die and contribute to the formation and growth of the necrotic lipid core. Therefore, unless foam cells (either of macrophage or SMC origin) and the macrophages that have ingested them return to the blood (dotted lines), all cholesteryl-ester-loaded cells, irrespective of their origin or mode of death, contribute to the generation and growth of a pro-inflammatory necrotic lipid core.
Macrophage origin and subsets
Macrophages in atherosclerotic lesions mainly originate from circulating monocytes, which migrate into atherosclerosis-susceptible sites of the arterial wall and into the growing atherosclerotic lesions139,140. Local proliferation of macrophages and transdifferentiation of intimal smooth muscle cells into macrophage-like cells are two additional sources of macrophages in the lesions141. The possibility of macrophages originating from smooth muscle cells has received much attention. Intimal smooth muscle cells not only accumulate cholesterol and turn into foam cells but the cholesterol accumulation in the cell can also drive their switching into pro-inflammatory, macrophage-like cells142,143. In addition, the normal arterial intima of mice and humans, like any tissue of the mammalian body, contains tissue-resident macrophages, which arise from embryonic precursors and from bone-marrow-derived circulating monocytes immediately after birth144. These macrophages self-renew and fulfil homeostatic and physiological functions in the tissue concerned144.
In adult mice under physiological, steady-state conditions, the circulating, nonclassical Ly6Clow monocytes remain in the circulation and patrol the vessels of the microcirculation, where these monocytes are critical for endothelial homeostasis and inflammation145. However, a 2017 study in mice revealed that these nonclassical monocytes also patrol the endothelium of large arteries, and this type of arterial patrolling is highly upregulated in hyperlipidaemia and atherosclerosis, thereby allowing improved protection of the arterial endothelium in the inflamed, high-shear stress and pulsatile environment of a developing atherosclerotic lesion146. Similarly, in humans, the nonclassical CD14+CD16+ monocytes normally remain in the blood circulation, where they patrol the endothelium to scan the integrity of endothelial cells147. Only after an inciting pathology, such as the initiating atherogenesis, do tissue-infiltrating macrophages appear in the arterial intima. In atherosclerotic mice, these infiltrating macrophages are derived from circulating pro-inflammatory, classical Ly6Chigh monocytes148. In humans, the macrophages in the arterial intima derive from the classical CD14+CD16- monocytes, which predominate in the circulating blood and are preferentially recruited into inflammatory sites, including the atherosclerotic plaques148.
In the arterial intima, as in other tissues, monocytes differentiate into macrophages with different phenotypes depending on the presence of growth factors, chemokines and cytokines in various combinations and ratios148. In addition, microenvironmental conditions, such as hypoxia, acidic pH and lipoprotein-derived lipids accumulating in the arterial intima, define the phenotype of macrophages in atherosclerotic lesions26. Two colony-stimulating factors, granulocyte-macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF), are widely expressed in the vascular wall149. These factors regulate not only monocyte-to-macrophage differentiation but also the number and function of mature macrophages150. In cell culture systems, both GM-CSF and M-CSF induce monocyte-to-macrophage differentiation, but with partially opposite responses in the differentiated macrophages. GM-CSF polarizes macrophages towards a pro-inflammatory M1-like phenotype whereas M-CSF polarizes macrophages to an anti-inflammatory M2-like phenotype. The antiinflammatory, M-CSF-differentiated macrophages can be subjected to either ‘classical activation, with IFNγ and LPS for polarization into a pro-inflammatory phenotype, or to ‘alternative activation’, with IL-4 for polarization into an anti-inflammatory phenotype. Interestingly, conversion of human M-CSF-differentiated macrophages into foam cells with acetylated LDL reduces their responses to classical pro-inflammatory activating stimuli151. The concept that overloading macrophages with cytoplasmic cholesteryl ester droplets (that is, their transformation into foam cells) shifts the macrophage phenotype from highly pro-inflammatory to fat-storing with less pro-inflammatory potential has received strong support in the past year by elegant results obtained in murine models of atherosclerosis152. In the context of atherosclerotic lesion progression, these fat-storing, less-pro-inflammatory-macrophage foam cells can ultimately be strongly pro-inflammatory when they become necrotic and release their contents into the plaque extracellular space.
Importantly, the pre-foam-cell macrophages have strong phenotypic plasticity when activated, with a spectrum ranging from pro-inflammatory to wound-healing phenotypes. The dichotomous classification of macrophages into pro-inflammatory and anti-inflammatory phenotypes (that is, into M1-like and M2-like macrophages) represents extreme opposite ends of a wide spectrum of macrophage phenotypes but provides a useful concept and framework when discussing the role of macrophages in inflammation and its resolution in atherosclerotic plaques151. In both cell culture systems and experimental animals (such as mice), macrophages have a high degree of plasticity to adopt various phenotypes, exemplified by the extended classification of the macrophages into a number of subgroups153. Indeed, findings on unique macrophage subsets (such as TREM2+ macrophages) on the basis of single-cell analyses have uncovered unprecedented phenotypic heterogeneity of macrophages and monocyte-derived dendritic cells in atherosclerotic lesions and have defined a novel atlas of the immune cell repertoire in atherosclerosis135,154.
The phenotypic heterogeneity of macrophage subtypes allows macrophages to have various combinations of both pathogenic and protective functions in humans. Moreover, individual macrophages hold the potential for a dynamic phenotype switch, which affects the functional properties ofthe macrophage155,156. Of note, in human atherosclerotic plaques, pro-inflammatory macrophages and less-inflammatory macrophages are localized in distinct areas, a finding that probably reflects, among other things, differences in the composition of macrophage-polarizing growth factors and cytokines in the respective microenvironments within the plaque157.
Annexin A1, which is expressed in tissue-resident effector cells including macrophages and mast cells158, and several members of the SPM family, such as LXA4, RvD1 and RvE1, stimulate macrophage polarization towards an M2-like or intermediate phenotype114,159,160. In addition, SPM biosynthesis is higher in M2-like macrophages than in M1-like macrophages and increases further in response to efferocytosis161. This increase in SPM biosynthesis with efferocytosis might be due, in part, to activation of the efferocytosis receptor MERTK by apoptotic cells87,88,162.
Macrophage accumulation
Two interesting observations in atherosclerotic mice have been made in the past decade with regard to the accumulation of macrophages in the growing lesions. First, if only limited or no influx of monocytes into the lesions occurs, the proliferation of the already existing lesional macrophages can explain the macrophage replenishment that is observed in the lesions and possibly also the increase in macrophage numbers seen in advanced atherosclerotic plaques163. Second, continuous influx of Ly6Chigh monocytes and their differentiation into macrophages in the absence of macrophage egress from the atherosclerotic lesion leads to an ongoing increase in the total mass of macrophages, with the oldest macrophages in the deep layers and the youngest ones in the superficial layers of the lesion140. The dominance of either mechanism of macrophage population maintenance and growth depends on the mouse model used and on the type and age of the atherosclerotic lesion. In human plaques, both monocyte influx and local macrophage proliferation are likely to coexist but in various ratios depending on the lesion stage. Interestingly, however, the magnitude of macrophage proliferation in advanced coronary lesions in humans seems to be very low (<1%)164. This finding correlates with the notion that two competing pathways can lead to a local increase in macrophage mass in advanced lesions: migration of monocytes from the bloodstream into the plaque and proliferation of macrophages within the plaque164, with the former pathway apparently being dominant in humans.
Macrophage death
Cells in the atherosclerotic lesions can die in several ways165, and the inflammatory response to each form of cell death is highly variable. In contrast to the highly pro-inflammatory necrotic types of cell death, such as necroptosis and pyroptosis, apoptosis and autophagy do not tend to trigger an inflammatory response166. Apoptosis is characterized by a shrunken nucleus, degraded cellular contents and an intact plasma membrane with the generation of apoptotic bodies. Apoptosis is triggered by caspase 8, and when this protease is inhibited, the kinases RIP1 and RIP3 become phosphoryl-ated and a complex called the necrosome is formed165. Components of the dying cells are packaged into these apoptotic bodies, thereby minimizing the release of intracellular DAMPs from the dying cells. Furthermore, ‘eat-me’ and ‘find-me’ signals on the apoptotic cells promote their efferocytosis. Autophagy is considered to be a pro-survival stress response, but extensive autophagy is also associated with cell death167. During autophagy, cellular macromolecules are targeted to autophagosomes, which fuse with lysosomes to form autolysosomes, in which the macromolecules are hydrolysed. Interestingly, autophagy is induced in macrophage foam cells generated in vitro by the uptake of oxLDL, aggregated LDL or VLDL and promotes cholesterol efflux to HDL168.
Both pyroptosis and necroptosis are pro-inflamma-tory166. Pyroptosis is a form of cell death associated with inflammasome activation and requires the activity of caspase 1 (REF72). Pyroptotic cell death is highly pro-inflammatory because it leads to the release of intracellular contents to the extracellular fluid but also to the release of cytokines such as IL-1β and IL-18. Necroptosis, a programmed form of necrosis, shares many factors with apoptosis, but, in contrast to apoptosis, necroptosis and necrosis do not involve caspase activation. Necroptosis leads to disruption of the plasma membrane and release of the cellular contents and various DAMPs. Necroptosis has been shown to have a role in atherosclerosis and, importantly, this form of cell death has been shown to be active in unstable atherosclerotic lesions in humans169. In this study, a radiotracer developed with the small-molecule inhibitor of necroptosis, necrostatin 1, localized specifically to atherosclerotic plaques in Apoe- /- mice, and treatment of Apoe- /- mice with necro-statin 1 reduced atherosclerotic lesion size and markers of plaque instability, such as necrotic core formation169.
Uptake of modified lipoproteins, such as oxLDL, has been shown in cultured macrophages to induce autophagy168, apoptosis170, pyroptosis (via induction of NRLP3 inflammasome activation72) and necroptosis169. Conversely, SPMs improve cell survival171,172 and decrease NLRP3 inflammasome activation in macro-phages67. Therefore, the balance of pro-inflammatory and anti-inflammatory molecules in the atherosclerotic lesion probably influences the form of cell death. In human atherosclerotic plaques, both apoptotic and oncotic (necrotic) modes of cell death have been described173. Interestingly, analysis of human carotid plaques removed during carotid endarterectomy showed that lipid-lowering treatment with pravastatin for only 3 months reduced the magnitude of cell death (mostly of smooth muscle cells) in the carotid plaques, possibly as a consequence of reduced LDL oxidation in the lesions174.
Regulation of efferocytosis
Billions of cells throughout the body die daily but are rapidly and safely removed by an evolutionarily conserved programme for clearing cell debris termed efferocytosis175. In early atherogenesis, observations in hyperlipidaemic mice support the idea that efficient efferocytosis of apoptotic foam cells reduces the development of atherosclerotic lesions176. Efferocytosis involves a panoply of molecules that include ligands present on the apoptotic cells (such as phosphatidylserine and calretic-ulin), ligand-receptor bridging molecules (for example, GAS6, protein S and lactadherin (MFGE8)), ‘find-me’ and ‘don’t-eat-me’ signals on the apoptotic cells (such as nucleotides and CD47, respectively), cell-surface receptors on the efferocytes (such as MERTK, T cell immunoglobulin mucin receptor (TIM) proteins and LDL receptor-related protein 1 (LRP1)) and enzymes involved in phagolysosomal degradation of apoptotic cells (vacuolar ATPases and DNase II)177 (FIG. 4).
Fig. 4 |. Defective efferocytosis drives necrotic core formation in atherosclerosis.
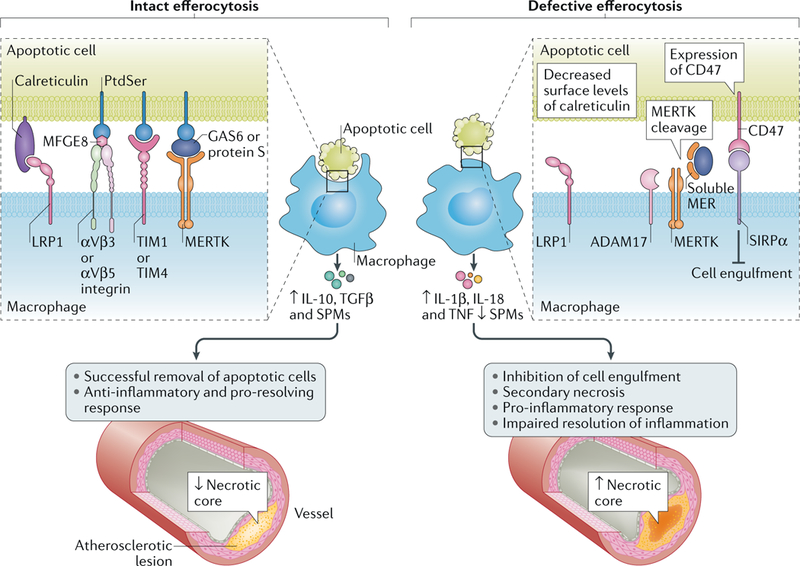
Intact efferocytosis by macrophages prevents necrotic core development in atherosclerotic lesions. However, when efferocytosis becomes defective, such as through cleavage of the tyrosine-protein kinase receptor MERTK by the protease ADAM17 on the macrophage, through inappropriate expression of CD47 on apoptotic cells resulting in CD47-SIRPα interaction or through reduced surface levels of calreticulin on apoptotic cells, atherosclerosis advances and necrotic core formation occurs. LRP1, LDL receptor-related protein 1; MFGE8, lactadherin; PtdSer, phosphatidylserine; SPM, specialized pro-resolving mediator; TIM, T cell immunoglobulin mucin receptor.
By clearing apoptotic cells, organogenesis and development can occur normally178, anti-inflammatory and antitumour responses are preserved179 and homeostasis is maintained180. Accordingly, efferocytosis, particularly by macrophages, is a critically important cellular effector arm of the inflammation resolution response. Both SPMs and IL-10 promote efferocytosis36,93,181–185, and new work indicates that a cellular pathway of the inflammation resolution response — mediated by regulatory T cells — also promotes efferocytosis79. The mechanism of the regulatory T cell response involves secretion of IL-13, which in turn stimulates macrophages to secrete and then be activated by the pro-efferocytic cytokine IL-10 (REF79). Interestingly, IL-13 promotes other resolving processes during atherosclerosis, including increasing lesional collagen production, reducing monocyte recruitment and skewing macrophages towards a pro-resolving phenotype186. Moreover, as part of a positive-feedback amplification cycle, engagement of efferocyte receptors by their ligands often stimulates the production of pro-resolving and anti-inflammatory molecules, such as RvD1, IL-10 and TGFβ77.
Not surprisingly, when efferocytosis becomes defective or when apoptotic cell receptors become dysfunctional (predominantly during non-resolving inflammation), autoimmunity, tissue necrosis and pathological inflammation ensue187. The inability to clear apoptotic cells efficiently drives many diseases, but defective effero-cytosis has a particularly prominent role in atheroscle-rosis188–190. As discussed above, in early atherosclerosis, apoB-containing lipoproteins are retained in the subendothelial matrix of medium-to-large arteries and stimulate a robust inflammatory response, which includes the release of chemotactic factors that drive leukocyte recruitment49. With advancing atherogenesis, this inflammatory response fails to resolve properly, which further sustains leukocyte recruitment. Moreover, both external factors (for example, cytokines) and intracellular processes (such as endoplasmic reticulum stress) trigger apoptosis of cells in the atherosclerotic lesion191,192. Although the predominant cell type in developing atherosclerotic plaques is phagocytic monocyte-derived macrophages, the capacity of these cells to execute efferocytosis is impaired during atherosclerosis progression177.
Failure to clear dead cells in the atherosclerotic lesion leads to the accumulation of secondarily necrotic cells and the formation of a highly inflammatory necrotic core188,189. Interestingly, the macrophage-like cells derived from vascular smooth muscle cells are distinct from classical monocyte-derived macrophages and dendritic cells193. Therefore, as a result of their reduced capacity to phagocytose apoptotic cells, these macrophage-like cells might contribute to the formation of the necrotic core.
The necrotic core is the primary feature of atherosclerotic plaque vulnerability, which renders lesions susceptible to rupture and lumenal thrombosis, the primary causes of clinical complications of atherosclerotic cardiovascular diseases. Although why efferocytosis fails in atherosclerosis is uncertain, efforts to define the mechanisms of defective efferocytosis during atherosclerosis have identified dysfunction in a number of the cellular and molecular steps required for apoptotic cell engulfment.
MERTK, a cell-surface tyrosine kinase receptor, binds one of two phosphatidylserine-binding bridging molecules, GAS6 or protein S, to drive adhesion and internalization of apoptotic cells194 (FIG. 4). As atherosclerosis progresses, inflammatory stimuli drive ADAM17-mediated MERTK cleavage from the cell surface195. Levels of the cleaved product, soluble MER, increase in advancing atherosclerosis in both mice and humans and can compete with MERTK for binding of GAS6 and protein S and thereby compromise efferocytosis in the lesion162. Athero-prone mice genetically engineered to express cleavage-resistant MERTK show a decreased propensity to develop necrotic cores compared with control mice162. Conversely, mice lacking MERTK or expressing a constitutively inactive form of MERTK show larger plaque size and increased necrotic core area compared with control mice196,197. Another apoptotic cell receptor, LRP1, is similarly downregulated by athero-relevant mediators and can be rendered inactive by ADAM17-mediated proteolytic cleavage198. Consistent with the role of defective efferocytosis in contributing to atherosclerosis progression, loss of LRP1 in haematopoietic cells increases atherosclerotic lesion area and necrotic core size in athero-prone mice fed a high-fat diet199,200. TIM proteins are another class of apoptotic cell receptors. Treatment with blocking antibodies against TIM1 or TIM4 promotes the progression of atherosclerosis in mice by preventing efferocytosis in the atherosclerotic lesion, leading to the accumulation of secondarily necrotic cells and the synthesis of pro-inflammatory cytokines201. In addition, mice treated with an anti-TIM1 antibody show robust reductions in the number of regulatory T cells in the blood201, which have been shown to stimulate lesional efferocytosis in an IL-10-dependent manner79. Another phosphatidylserine receptor, scavenger receptor class B member 1 (SRB1), has been demonstrated to promote efferocytosis via the SRC-PI3K-RAC1 pathway202. In addition, deletion of Scarb1 (encoding SRB1) in macrophages results in defective efferocytosis in vitro and increased plaque size, necrotic core area and inflammation in vivo202.
The inability to clear dead cells within atherosclerotic lesions is driven by defects in the phagocytes, but apoptotic cells in mouse and human atherosclerotic lesions also have variations in ‘eat-me’ and ‘don’t-eat-me’ signals that render them resistant to efferocytosis. For example, Apoe−/− mice with deletion of Cdkn2b had lower levels of calreticulin than control Apoe−/− mice, and Cdkn2b−/−Apoe−/− mice fed a Western diet had increased plaque size and larger necrotic core area than control Apoe−/− mice, which was attributed to intralesional apoptotic cells being resistant to phagocytosis203. Interestingly, atherosclerotic plaques from patients carrying a genetic variation at the 9p21 locus (which includes the CDKN2B gene), which has been associated with atherosclerosis in humans204,205, have reduced levels of calreticulin compared with atherosclerotic plaques from non-carriers203, further supporting a role for defective efferocytosis in cardiovascular disease206. Supporting the concept that apoptotic cells can inappropriately disrupt efferocytosis, apoptotic cells in atherosclerotic plaques from patients with symptomatic cerebrovascular disease have increased levels of the TNF-dependent, ‘don’t-eat-me’ signal CD47 on the cell surface compared with non-atherosclerotic vascular tissue207. By binding to its cognate receptor SIRPa (also known as SHPS1) on the phagocyte, CD47 inhibits cytoskeletal rearrangement to prevent cell engulfment208. Accordingly, delivery of CD47-blocking antibodies to Apoe−/− mice fed a high-fat diet increases efferocytosis in the atherosclerotic lesion and reduces necrotic core formation207.
Together, these studies demonstrate that defective efferocytosis transforms benign lesions into clinically relevant atheromas in both mice and humans (FIG. 4). These atheromas are particularly sensitive to the accumulation of necrotic cells and to the loss of anti-inflammatory and pro-resolving responses that, as mentioned above, are inherent to the efferocytosis programme. These findings raise the possibility that therapeutic strategies that increase efferocytosis in the atherosclerotic lesion might reduce the risk of plaque rupture.
Conclusions
In atherogenesis, lipid accumulation in the arterial intima is the root cause of the formation of inflammatory atherosclerotic lesions. If this pro-inflammatory source is reduced, resolution of the inflammation can ensue, just as the removal of a splinter from an inflamed finger will allow the initiation of inflammation resolution and healing. Indeed, lipid-lowering interventions in atherosclerotic mice seem both to attenuate lipoprotein modification in the arterial wall and to promote a pro-resolving phenotype in lesional macrophages, all of which precede plaque regression209. Most importantly, these studies demonstrate that in an already inflamed advanced atherosclerotic plaque, an improvement in the balance of pro-inflammatory and anti-inflammatory processes allows the resolution of the inflammation (FIG. 5). This concept is likely to be relevant to humans because dramatic reductions in plasma LDL-cholesterol levels lead to the regression of even advanced atherosclerotic lesions, and plaque regression is accompanied by lower rates of atherosclerotic cardiovascular disease events210,211.
Fig. 5 |. Resolution versus chronic inflammation in atherosclerosis.
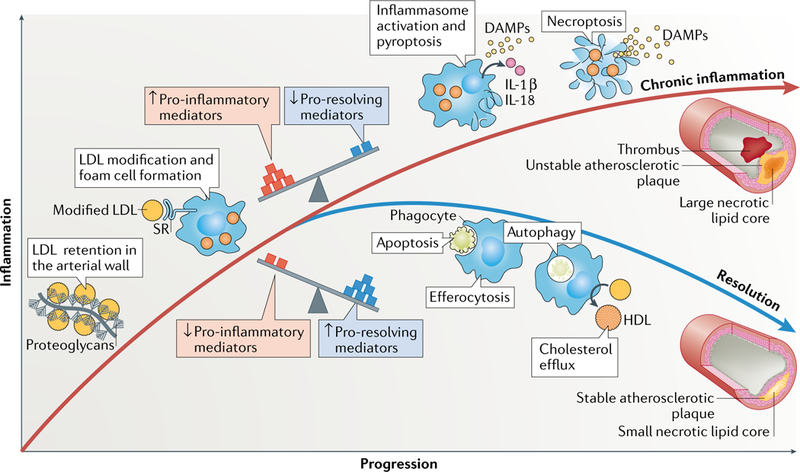
The balance of pro-inflammatory and antiinflammatory processes controls the resolution of the lipid-driven inflammation in atherosclerotic lesions. Retention of LDL particles by arterial wall proteoglycans and subsequent modification of the retained LDL induce inflammation in the arterial wall. Macrophages ingest the modified LDL particles via scavenger receptor (SR)-mediated endocytosis and become foam cells. If the balance between pro-inflammatory and pro-resolving mediators is tilted towards inflammation, the resolving mechanisms fail. Under these conditions, pyroptosis (mediated by inflammasome activation) or necroptosis can ensue. These pro-inflammatory forms of cell death further promote inflammation and generation of a large necrotic lipid core. These unstable atherosclerotic plaques might ultimately lead to plaque rupture and a local occluding arterial thrombus. Conversely, if the balance between the mediators is tilted towards pro-resolving mediators, apoptosis and autophagy-associated cell death and cholesterol efflux from the lesions are favoured, and efferocytosis of the dead cells can lead to resolution of inflammation. These processes promote the formation of a stable plaque with a small necrotic lipid core. DAMPs, damage-associated molecular patterns.
The anti-inflammatory effect achieved by lowering LDL-cholesterol alone has now been complemented by targeting inflammation with a pure anti-inflammatory therapy — that is, by inhibiting IL-1β with a monoclonal antibody in the CANTOS trial212. The results of this study in patients with advanced coronary atherosclerosis who were receiving maximal LDL-lowering therapy showed an additional benefit in the reduction of the rate of recurrent cardiovascular events when IL-1β was inhibited. Unfortunately, the anti-IL-1β antibody therapy was associated with a small but significant increase in certain fatal infections212, which can be explained by the host defence-compromising potential of a pure anti-inflammatory therapy33.
From a translational perspective, SPMs suppress inflammation but, unlike anti-inflammatory drugs, these pro-resolving mediators promote both repair and continued pathogen clearance33,213–215. Therefore, SPMs might not suppress host defence to the same extent as anti-inflammatory drugs, and SPMs are being tested in clinical trials for certain inflammatory diseases33,216. As summarized in this Review, treatment of Western-diet-fed Ldlr−/− mice with athero-targeted nanoparticles packaged with a pro-resolving peptide derived from annexin A1 (Ac2–26) or with SPMs markedly suppressed the formation of necrotic, thin-capped plaques36,38,99,109,118. These preclinical findings, together with the landmark trial of IL-1β inhibition, raise the prospect for a future trial designed to determine the efficacy and safety of pro-resolving therapies for atherothrombotic vascular disease. If proved to be valid, these therapies would advance the inflammation-targeted therapeutic strategy from fighting the bad to promoting the good.
Key points.
Modified lipoproteins and cholesterol crystals accumulate in the arterial intima and induce foam cell formation and inflammation.
Defective efferocytosis of apoptotic foam cells leads to necrotic core formation.
Defective efferocytosis is a sign of failure in the resolution of inflammation.
Inflammation resolution is mediated by specialized pro-resolving lipid mediators, proteins and signalling gases.
Improvement of the balance between pro-inflammatory and pro-resolving processes enables the resolution of inflammation.
Pro-resolving mediator therapy could be a novel approach to suppressing the formation of clinically dangerous atherosclerotic lesions.
Acknowledgements
M.B.’s research is supported by grants from the Swedish Research Council (2014-2312), the Swedish Heart and Lung Foundation (20180571) and the Marianne and Marcus Wallenberg Foundation (2015.0104). A.Y.’s research is supported by the NIH (T32 HL007343-28 and K99 HL145131). I.T.’s research is supported by the NIH (R01 HL075662, R01 HL127464 and R01 HL132412). K.O.’s research is supported by the Academy of Finland (315568), the Aarne Koskelo Foundation and the Finnish Foundation for Cardiovascular Research. The Wihuri Research Institute is maintained by the Jenny and Antti Wihuri Foundation.
Footnotes
Reviewer information
Nature Reviews Cardiology thanks C. J. Binder, K. Ley, and the other anonymous reviewer(s), for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Publisher's Disclaimer: Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Williams KJ & Tabas I The response-to-retention hypothesis of early atherogenesis. Arteriosder. Thromb. Vasc. Biol. 15, 551–561 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KJ & Tabas I The response-to-retention hypothesis of atherogenesis reinforced. Curr. Opin. Lipidol. 9, 471–474 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Tabas I, Williams KJ & Boren J Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116, 1832–1844 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Galkina E & Ley K Immune and inflammatory mechanisms of atherosclerosis*. Annu. Rev. Immunol. 27, 165–197 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansson GK Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Hansson GK, Libby P & Tabas I Inflammation and plaque vulnerability. J. Intern. Med. 278, 483–493 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P, Ridker PM & Maseri A Inflammation and atherosclerosis. Circulation 105, 1135–1143 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Shi GP, Bot I & Kovanen PT Mast cells in human and experimental cardiometabolic diseases. Nat. Rev. Cardiol. 12, 643–658 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Zernecke A Dendritic cells in atherosclerosis: evidence in mice and humans. Arterioscler. Thromb. Vasc. Biol.35 763–770 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Paulson KE et al. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ. Res. 106, 383–390 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Major AS, Fazio S & Linton MF B-Lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 22, 1892–1898 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Douna H & Kuiper J Novel B cell subsets in atherosclerosis. Curr. Opin. Lipidol 27, 493–498 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Ketelhuth DFJ & Hansson GK Adaptive response of T and B cells in atherosclerosis. Circ. Res. 118, 668–678 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Doring Y, Soehnlein O & Weber C Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ. Res. 120, 736–743 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Niccoli G, Montone RA, Sabato V & Crea F Role of allergic inflammatory cells in coronary artery disease. Circulation 138, 1736–1748 (20l8). [DOI] [PubMed] [Google Scholar]
- 16.Pentikäinen MO, Öörni K, Ala-Korpela M & Kovanen PT Modified LDL -trigger of atherosclerosis and inflammation in the arterial intima. J. Intern. Med. 247, 359–370 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Napoli C et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J. Clin. Invest. 100, 2680–2690 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber C & Noels H Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 17, 1410–1422 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Berg KE et al. Elevated CD14++CD16-monocytes predict cardiovascular events. Circ. Cardiovasc. Genet.5, 122–131 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Schiopu A et al. Associations between macrophage colony-stimulating factor and monocyte chemotactic protein 1 in plasma and first-time coronary events: a nested case-control study. J. Am. Heart Assoc. 5, e002851 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown MS & Goldstein JL Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu. Rev. Biochem. 52, 223–261 (1983). [DOI] [PubMed] [Google Scholar]
- 22.Sorci-Thomas MG & Thomas MJ Microdomains, inflammation, and atherosclerosis. Circ. Res. 118, 679–691 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li AC & Glass CK The macrophage foam cell as a target for therapeutic intervention. Nat. Med. 8, 1235–1242 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Brown MS, Ho YK & Goldstein JL The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J. Biol. Chem. 255, 9344–9352 (1980). [PubMed] [Google Scholar]
- 25.Öörni K et al. Acidification of the intimal fluid: the perfect storm for atherogenesis. J. Lipid Res. 56, 203–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabas I & Bornfeldt KE Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 118, 653–667 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serhan CN et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21, 325–332 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan C & Ding A Nonresolving inflammation. Cell 140, 871–882 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Serhan CN Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol. 177, 1576–1591 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perretti M & D’Acquisto F Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 9, 62–70 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Tabas I Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merched AJ, Ko K, Gotlinger KH, Serhan CN & Chan L Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 22, 3595–3606 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabas I & Glass CK Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 339, 166–172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolodgie FD et al. Pathologic assessment of the vulnerable human coronary plaque. Heart 90, 1385–1391 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kojima Y, Weissman IL & Leeper NJ The role of efferocytosis in atherosclerosis. Circulation 135, 476–489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fredman G et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun. 7, 12859 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thul S, Labat C, Temmar M, Benetos A & Back M Low salivary resolvin D1 to leukotriene B4 ratio predicts carotid intima media thickness: a novel biomarker of non-resolving vascular inflammation. Eur. J. Prev. Cardiol. 24, 903–906 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Viola JR et al. Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ. Res. 119, 1030–1038 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Packard CJ LDL cholesterol: how low to go? Trends Cardiovasc. Med. 28, 348–354 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Weber C & von Hundelshausen P CANTOS Trial validates the inflammatory pathogenesis of atherosclerosis: setting the stage for a new chapter in therapeutic targeting. Circ. Res. 121, 1119–1121 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Nordestgaard BG, Wootton R & Lewis B Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler. Thromb. Vasc. Biol. 15, 534–542 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Shaikh M et al. Quantitative studies of transfer in vivo of low density, Sf 12–60, and Sf 60–400 lipoproteins between plasma and arterial intima in humans. Arterioscler. Thromb. 11, 569–577 (1991). [DOI] [PubMed] [Google Scholar]
- 43.Borén J & Williams KJ The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr. Opin. Lipidol. 27, 473–483 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Skalen K et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417, 750–754 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Smith EB, Keen GA & Grant A Factors influencing the accumulation in fibrous plaques of lipid derived from low density lipoprotein. I. Relation between fibrin and immobilization of apo B-containing lipoprotein. Atherosclerosis 84, 165–171 (1990). [DOI] [PubMed] [Google Scholar]
- 46.Öörni K, Pentikäinen MO, Ala-Korpela M & Kovanen PT Aggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions. J. Lipid Res. 41, 1703–1714 (2000). [PubMed] [Google Scholar]
- 47.Houde M & Van Eck M Escaping the atherogenic trap: preventing LDL fusion and binding in the intima. Atherosclerosis 275, 376–378 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Soto Y et al. Antiatherosclerotic effect of an antibody that binds to extracellular matrix glycosaminoglycans. Arterioscler. Thromb. Vasc. Biol. 32, 595–60 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Yurdagul A Jr., Finney AC, Woolard MD & Orr AW The arterial microenvironment: the where and why of atherosclerosis. Biochem. J. 473, 1281–1295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adamson S & Leitinger N Phenotypic modulation of macrophages in response to plaque lipids. Curr. Opin. Lipidol. 22, 335–342 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Que X et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 558, 301–306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watson AD et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/ endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 272, 13597–13607 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Fu P & Birukov KG Oxidized phospholipids in control of inflammation and endothelial barrier. Transl Res. 153, 166–176 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanoni I, Tan Y, Di Gioia M, Springstead JR & Kagan JC By capturing inflammatory lipids released from dying cells, the receptor CD14 induces inflammasome-dependent phagocyte hyperactivation. Immunity 47, 697–709 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruuth M et al. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Ear. Heart J. 39, 2562–2573 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lehti S et al. Extracellular lipid accumulates in human carotid arteries as distinct three-dimensional structures with proinflammatory properties. Am. J. Pathol. 188, 525–538 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Guarino AJ, Tulenko TN & Wrenn SP Cholesterol crystal nucleation from enzymatically modified low-density lipoproteins: combined effect of sphingomyelinase and cholesterol esterase. Biochemistry 43, 1685–1693 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Rajamäki K et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLOS ONE 5, e11765 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheedy FJ et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westerterp M et al. Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. CellMetab. 25, 1294–1304 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajamäki K et al. P38δ MAPK: a novel regulator of NLRP3 inflammasome activation with increased expression in coronary atherogenesis. Arterioscler. Thromb. Vasc. Biol. 36, 1937–1946 (2016). [DOI] [PubMed] [Google Scholar]
- 62.van der Heijden T et al. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice -brief report. Arterioscler. Thromb. Vasc. Biol. 37, 1457–1461 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Patel MN et al. Inflammasome priming in sterile inflammatory disease. Trends Mol. Med. 23, 165–180 (2017). [DOI] [PubMed] [Google Scholar]
- 64.He Y, Hara H & Nunez G Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41, 1012–1021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA Jr & Progulske-Fox, A. Human atherosclerotic plaque contains viable invasive Actinobacillas actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 25, e17–e18 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Caesar R, Fak F & Backhed F Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J. Intern. Med. 268, 320–328 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Lopategi A et al. Frontline science: specialized proresolving lipid mediators inhibit the priming and activation of the macrophage NLRP3 inflammasome. J. Leukoc. Biol. 105, 25–36 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Wang L, Chen Y, Li X, Zhang Y & Gulbins E Enhancement of endothelial permeability by free fatty acid through lysosomal cathepsin B-mediated Nlrp3 inflammasome activation. Oncotarget 7, 73229–73241 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schroder K, Zhou R & Tschopp J The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Wen H et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duewell P & Latz E Assessment and quantification of crystal-induced lysosomal damage. Methods Mol. Biol. 1040, 19–27 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Grebe A, Hoss F & Latz E NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ. Res. 122, 1722–1740 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Westerterp M et al. Cholesterol efflux pathways suppress inflammasome activation, NETosis and atherogenesis. Circulation 138, 898–912 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rhoads JP et al. Oxidized low-density lipoprotein immune complex priming of the Nlrp3 inflammasome involves TLR and FcyR cooperation and is dependent on CARD9. J. Immunol. 198, 2105–2114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Estruch M et al. Electronegative LDL induces priming and inflammasome activation leading to IL-1beta release in human monocytes and macrophages. Biochim. Biophys. Acta 1851, 1442–1449 (2015). [DOI] [PubMed] [Google Scholar]
- 76.Serhan CN Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fredman G & Tabas I Boosting inflammation resolution in atherosclerosis: the next frontier for therapy. Am. J. Pathol. 187, 1211–1221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mirakaj V, Dalli J, Granja T, Rosenberger P & Serhan CN Vagus nerve controls resolution and pro-resolving mediators of inflammation. J. Exp. Med. 211, 1037–1048 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Proto JD et al. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity 49, 666–677 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X et al. Endogenously generated omega-3 fatty acids attenuate vascular inflammation and neointimal hyperplasia by interaction with free fatty acid receptor 4 in mice. J. Am. Heart Assoc. 4, e001856 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Breitzig M, Bhimineni C, Lockey R & Kolliputi N 4-hydroxy-2-nonenal: a critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 311, C537–C543 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serhan CN, Krishnamoorthy S, Recchiuti A & Chiang N Novel anti-inflammatory—pro-resolving mediators and their receptors. Curr. Top. Med. Chem. 11, 629–647 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radmark O & Samuelsson B Regulation of 5-lipoxygenase enzyme activity. Biochem. Biophys. Res. Commun. 338, 102–110 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Fredman G et al. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc. Natl Acad. Sci. USA 111, 14530–14535 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dichlberger A, Kovanen PT & Schneider WJ Mast cells: from lipid droplets to lipid mediators. Clin. Sci. 125, 121–130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Werz O, Klemm J, Samuelsson B & Radmark O 5-Lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc. Natl Acad. Sci. USA 97, 5261–5266 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cai B et al. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc. Natl Acad. Sci. USA 113, 6526–6531 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai B et al. MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci. Signal. 11, eaar3721 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dinarello CA Interleukin-1 β and the autoinflammatory diseases. N. Engl. J. Med. 360, 2467–2470 (2009). [DOI] [PubMed] [Google Scholar]
- 90.D’Elia RV, Harrison K, Oyston PC, Lukaszewski RA & Clark GC Targeting the “cytokine storm” for therapeutic benefit. Clin. Vaccine Immunol. 20, 319–327 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.English JT, Norris PC, Hodges RR, Dartt DA & Serhan CN Identification and profiling of specialized pro-resolving mediators in human tears by lipid mediator metabolomics. Prostaglandins Leukot. Essent. Fatty Acids 117, 17–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arnardottir H, Orr SK, Dalli J & Serhan CN Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 9, 757–766 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kasikara C, Doran AC, Cai B & Tabas I The role of non-resolving inflammation in atherosclerosis. J. Clin. Invest. 128, 2713–2723 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wallace JL, Vong L, McKnight W, Dicay M & Martin GR Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 137, 569–578 (2009). [DOI] [PubMed] [Google Scholar]
- 95.Han X & Boisvert WA Interleukin-10 protects against atherosclerosis by modulating multiple atherogenic macrophage function. Thromb. Haemost. 113, 505–512 (2015). [DOI] [PubMed] [Google Scholar]
- 96.de Jong RJ et al. Protective aptitude of annexin A1 in arterial neointima formation in atherosclerosis-prone mice -brief report. Arterioscler. Thromb. Vasc. Biol. 37, 312–315 (2017). [DOI] [PubMed] [Google Scholar]
- 97.Zhang R et al. Hydrogen sulfide inhibits L-type calcium currents depending upon the protein sulfhydryl state in rat cardiomyocytes. PLOS ONE 7, e37073 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mani S et al. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 127, 2523–2534 (2013). [DOI] [PubMed] [Google Scholar]
- 99.Petri MH et al. Aspirin-triggered lipoxin A4 inhibits atherosclerosis progression in apolipoprotein E−/− mice. Br. J. Pharmacol. 174, 4043–4054 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krishnamoorthy N et al. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J. Immunol. 194, 863–867 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Foks AC, Lichtman AH & Kuiper J Treating atherosclerosis with regulatory T cells. Arterioscler. Thromb. Vasc. Biol. 35, 280–287 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perretti M, Leroy X, Bland EJ & Montero-Melendez T Resolution pharmacology: opportunities for therapeutic innovation in inflammation. Trends Pharmacol. Sci. 36, 737–755 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Bäck M et al. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR review 7. Br. J. Pharmacol. 171, 3551–3574 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Petri MH et al. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc. Res. 105, 65–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laguna-Fernandez A et al. ERV1/ChemR23 signaling protects from atherosclerosis by modifying oxLDL uptake and phagocytosis in macrophages. Circulation 138, 1693–1705 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kostopoulos CG, Spiroglou SG, Varakis JN, Apostolakis E & Papadaki HH Chemerin and CMKLR1 expression in human arteries and periadventitial fat: a possible role for local chemerin in atherosclerosis? BMC Cardiovasc. Disord. 14, 56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ho KJ et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am. J. Pathol. 177, 2116–2123 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Drechsler M et al. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ. Res. 116, 827–835 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fredman G et al. Targeted nanoparticles containing the proresolving peptide Ac2–26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl Med. 7, 275ra220 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petri MH et al. Aspirin-triggered 15-epi-lipoxin A4 signals through FPR2/ALX in vascular smooth muscle cells and protects against intimal hyperplasia after carotid ligation. Int. J. Cardiol 179, 370–372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Akagi D, Chen M, Toy R, Chatterjee A & Conte MS Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 29, 2504–2513 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miyahara T et al. D-Series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 27, 2220–2232 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.El Kebir D, Gjorstrup P & Filep JG Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl Acad. Sci. USA 109, 14983–14988 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herova M, Schmid M, Gemperle C & Hersberger M ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J. Immunol. 194, 2330–2337 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Ohira T et al. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J. Biol. Chem. 285, 3451–3461 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kennedy AJ & Davenport AP International Union of Basic and Clinical Pharmacology CIII: chemerin receptors CMKLR1 (chemerin1) and GPR1 (chemerin2) nomenclature, pharmacology, and function. Pharmacol. Rev. 70, 174–196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lopez-Vicario C et al. Association of a variant in the gene encoding for ERV1/ChemR23 with reduced inflammation in visceral adipose tissue from morbidly obese individuals. Sci. Rep 7, 15724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hasturk H et al. Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arterioscler. Thromb. Vasc. Biol. 35, 1123–1133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salic K et al. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis 250, 158–165 (2016). [DOI] [PubMed] [Google Scholar]
- 120.Neves KB et al. Chemerin regulates crosstalk between adipocytes and vascular cells through Nox. Hypertension 66, 657–666 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Kunimoto H et al. Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am. J. Physiol. Heart Circ. Physiol. 309, H1017–H1028 (2015). [DOI] [PubMed] [Google Scholar]
- 122.Aung T et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 3, 225–234 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.The ASCEND Study Collaborative Group. Effects of n-3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 379, 1540–1550 (2018). [DOI] [PubMed] [Google Scholar]
- 124.Bhatt DL et al. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial. Clin. Cardiol. 40, 138–148 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bhatt DL et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22 (2019). [DOI] [PubMed] [Google Scholar]
- 126.Elajami TK et al. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 30, 2792–2801 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Skarke C et al. Bioactive products formed in humans from fish oils. J. Lipid Res. 56, 1808–1820 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gromovsky AD et al. Delta-5 fatty acid desaturase FADS1 impacts metabolic disease by balancing proinflammatory and proresolving lipid mediators. Arterioscler. Thromb. Vasc. Biol. 38, 218–231 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tuomisto TT et al. Simvastatin has an antiinflammatory effect on macrophages via upregulation of an atheroprotective transcription factor, Kruppel-like factor 2. Cardiovasc. Res. 78, 175–184 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Wallace JL, Ianaro A, Flannigan KL & Cirino G Gaseous mediators in resolution of inflammation. Semin. Immunol. 27, 227–233 (2015). [DOI] [PubMed] [Google Scholar]
- 131.Dufton N, Natividad J, Verdu EF & Wallace JL Hydrogen sulfide and resolution of acute inflammation: a comparative study utilizing a novel fluorescent probe. Sci. Rep. 2, 499 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Y et al. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 29, 173–179 (2009). [DOI] [PubMed] [Google Scholar]
- 133.Chiang N et al. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J. Immunol. 190, 6378–6388 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Winkels H, Ehinger E, Ghosheh Y, Wolf D & Ley K Atherosclerosis in the single-cell era. Curr. Opin. Lipidol 29, 389–396 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Winkels H et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ. Res. 122, 1675–1688 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shibata N et al. 25-Hydroxycholesterol activates the integrated stress response to reprogram transcription and translation in macrophages. J. Biol. Chem. 288, 35812–35823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Talbot CPJ, Plat J, Ritsch A & Mensink RP Determinants of cholesterol efflux capacity in humans. Prog. Lipid Res. 69, 21–32 (2018). [DOI] [PubMed] [Google Scholar]
- 138.van Gils JM et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat. Immunol. 13, 136–143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Swirski FK et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl Acad. Sci. USA 103, 10340–10345 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Williams JW et al. Limited macrophage positional dynamics in progressing or regressing murine atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 38, 1702–1710 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nagenborg J, Goossens P, Biessen EAL & Donners M Heterogeneity of atherosclerotic plaque macrophage origin, phenotype and functions: Implications for treatment. Eur. J. Pharmacol. 816, 14–24 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Allahverdian S, Chehroudi AC, McManus BM, Abraham T & Francis GA Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 129, 1551–1559 (2014). [DOI] [PubMed] [Google Scholar]
- 143.Bennett MR, Sinha S & Owens GK Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hashimoto D et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ensan S et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1+ precursors and circulating monocytes immediately after birth. Nat. Immunol. 17, 159–168 (2016). [DOI] [PubMed] [Google Scholar]
- 146.Quintar A et al. Endothelial protective monocyte patrolling in large arteries intensified by western diet and atherosclerosis. Circ. Res. 120, 1789–1799 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patel AA et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 214, 1913–1923 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gautier EL, Jakubzick C & Randolph GJ Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29, 1412–1418 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Di Gregoli K & Johnson JL Role of colony-stimulating factors in atherosclerosis. Curr. Opin. Lipidol. 23, 412–421 (2012). [DOI] [PubMed] [Google Scholar]
- 150.Ushach I & Zlotnik A Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J. Leukoc. Biol. 100, 481–489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.da Silva RF, Lappalainen J, Lee-Rueckert M & Kovanen PT Conversion of human M-CSF macrophages into foam cells reduces their proinflammatory responses to classical M1-polarizing activation. Atherosclerosis 248, 170–178 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Kim K et al. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ. Res. 123, 1127–1142 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Colin S, Chinetti-Gbaguidi G & Staels B Macrophage phenotypes in atherosclerosis. Immunol. Rev. 262, 153–166 (2014). [DOI] [PubMed] [Google Scholar]
- 154.Cochain C et al. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ. Res. 122, 1661–1674 (2018). [DOI] [PubMed] [Google Scholar]
- 155.Mosser DM & Edwards JP Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Italiani P & Boraschi D From monocytes to M1/M2 macrophages: phenotypical versus functional differentiation. Front. Immunol. 5, 514 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chinetti-Gbaguidi G et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARy and LXRa pathways. Circ. Res. 108, 985–995 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.de Jong R, Leoni G, Drechsler M & Soehnlein O The advantageous role of annexin A1 in cardiovascular disease. CellAdh. Migr. 11, 261–274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li Y et al. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene 30, 3887–3899 (2011). [DOI] [PubMed] [Google Scholar]
- 160.Titos E et al. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 187,5408–5418 (2011). [DOI] [PubMed] [Google Scholar]
- 161.Dalli J & Serhan C Macrophage proresolving mediators — the when and where. Microbiol. Spectr. 10.1128/microbiolspec.MCHD-0001-2014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cai B et al. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J. Clin. Invest. 127, 564–568 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Robbins CS et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gordon D, Reidy MA, Benditt EP & Schwartz SM Cell proliferation in human coronary arteries. Proc. Natl Acad. Sci. USA 87, 4600–4604 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kavurma MM, Rayner KJ & Karunakaran D The walking dead: macrophage inflammation and death in atherosclerosis. Curr. Opin. Lipidol. 28, 91–98 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tait SW, Ichim G & Green DR Die another way — non-apoptotic mechanisms of cell death. J. Cell Sci. 127, 2135–2144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Das G, Shravage BV & Baehrecke EH Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb. Perspect. Biol. 4, a008813 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ouimet M et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 13, 655–667 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Karunakaran D et al. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci. Adv. 2, e1600224 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Andres V, Pello OM & Silvestre-Roig C Macrophage proliferation and apoptosis in atherosclerosis. Curr. Opin. Lipidol. 23, 429–438 (2012). [DOI] [PubMed] [Google Scholar]
- 171.Mai J et al. The atheroprotective role of lipoxin A4 prevents oxLDL-induced apoptotic signaling in macrophages via JNK pathway. Atherosclerosis 278, 259–268 (2018). [DOI] [PubMed] [Google Scholar]
- 172.Prieto P et al. Activation of autophagy in macrophages by pro-resolving lipid mediators. Autophagy 11, 1729–1744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Crisby M et al. Cell death in human atherosclerotic plaques involves both oncosis and apoptosis. Atherosclerosis 130, 17–27 (1997). [DOI] [PubMed] [Google Scholar]
- 174.Crisby M et al. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 103, 926–933 (2001). [DOI] [PubMed] [Google Scholar]
- 175.Han CZ & Ravichandran KS Metabolic connections during apoptotic cell engulfment. Cell 147, 1442–1445 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hamada M et al. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat. Commun. 5, 3147 (2014). [DOI] [PubMed] [Google Scholar]
- 177.Yurdagul A et al. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front. Cardiovasc. Med. 4, 86 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Penaloza C, Lin L, Lockshin RA & Zakeri Z Cell death in development: shaping the embryo. Histochem. Cell Biol. 126, 149–158 (2006). [DOI] [PubMed] [Google Scholar]
- 179.Nagata S, Hanayama R & Kawane K Autoimmunity and the clearance of dead cells. Cell 140, 619–630 (2010). [DOI] [PubMed] [Google Scholar]
- 180.Elliott MR & Ravichandran KS The dynamics of apoptotic cell clearance. Dev. Cell 38, 147–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G & Serhan CN Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol. 180, 2018–2027 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Godson C et al. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164, 1663–1667 (2000). [DOI] [PubMed] [Google Scholar]
- 183.Mitchell S et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J. Am. Soc. Nephrol. 13, 2497–2507 (2002). [DOI] [PubMed] [Google Scholar]
- 184.Campana L et al. The STAT3-IL-10-IL-6 pathway is a novel regulator of macrophage efferocytosis and phenotypic conversion in sterile liver injury. J. Immunol. 200, 1169–1187 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ogden CA et al. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt’s lymphoma. J. Immunol. 174, 3015–3023 (2005). [DOI] [PubMed] [Google Scholar]
- 186.Cardilo-Reis L et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 4, 1072–1086 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Green DR, Oguin TH & Martinez J The clearance of dying cells: table for two. Cell Death Differ. 23, 915–926 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thorp E & Tabas I Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J. Leukoc. Biol. 86, 1089–1095 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Thorp E, Subramanian M & Tabas I The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. Eur. J. Immunol. 41, 2515–2518 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tajbakhsh A, Rezaee M, Kovanen PT & Sahebkar A Efferocytosis in atherosclerotic lesions: malfunctioning regulatory pathways and control mechanisms. Pharmacol. Ther. 188, 12–25 (2018). [DOI] [PubMed] [Google Scholar]
- 191.Tabas I The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ. Res. 107, 839–850 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tabas I Macrophage apoptosis in atherosclerosis: consequences on plaque progression and the role of endoplasmic reticulum stress. Antioxid. Redox Signal.11, 2333–2339 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vengrenyuk Y et al. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler. Thromb. Vasc. Biol. 35, 535–546 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Penberthy KK & Ravichandran KS Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 269, 44–59 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Thorp E et al. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase CS, and p38 mitogen-activated protein kinase (MAPK). J. Biol. Chem. 286, 33335–33344 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Thorp E, Cui D, Schrijvers DM, Kuriakose G & Tabas I Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb. Vasc. Biol. 28, 1421–1428 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ait-Oufella H et al. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 28, 1429–1431 (2008). [DOI] [PubMed] [Google Scholar]
- 198.Gorovoy M, Gaultier A, Campana WM, Firestein GS & Gonias SL Inflammatory mediators promote production of shed LRP1/CD91, which regulates cell signaling and cytokine expression by macrophages. J. Leukoc. Biol. 88, 769–778 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yancey PG et al. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler. Thromb. Vasc. Biol. 30, 787–795 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Overton CD, Yancey PG, Major AS, Linton MF & Fazio S Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ. Res. 100, 670–677 (2007). [DOI] [PubMed] [Google Scholar]
- 201.Foks AC et al. Blockade of Tim-1 and Tim-4 enhances atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 36, 456–465 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tao H et al. Macrophage SR-BI mediates efferocytosis via Src/PI3K/Rac1 signaling and reduces atherosclerotic lesion necrosis. J. Lipid Res. 56, 1449–1460 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kojima Y et al. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J. Clin. Invest. 124, 1083–1097 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Helgadottir A et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 316, 1491–1493 (2007). [DOI] [PubMed] [Google Scholar]
- 205.McPherson R et al. A common allele on chromosome 9 associated with coronary heart disease. Science 316, 1488–1491 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nanda V et al. CDKN2B regulates TGFp signaling and smooth muscle cell investment of hypoxic neovessels. Circ. Res. 118, 230–240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kojima Y et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536, 86–90 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tsai RK & Discher DE Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J. Cell Biol. 180, 989–1003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Feig JE et al. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLOS ONE 7, e39790 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tang X et al. The effect of statin therapy on plaque regression following acute coronary syndrome: a meta-analysis of prospective trials. Coron. Artery Dis. 27, 636–649 (2016). [DOI] [PubMed] [Google Scholar]
- 211.Rosenson RS, Hegele RA, Fazio S & Cannon CP The evolving future of PCSK9 inhibitors. J. Am. Coll. Cardiol. 72, 314–329 (2018). [DOI] [PubMed] [Google Scholar]
- 212.Ridker PM et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017). [DOI] [PubMed] [Google Scholar]
- 213.Dalli J & Serhan CN Identification and structure elucidation of the pro-resolving mediators provides novel leads for resolution pharmacology. Br. J. Pharmacol. 10.1111/bph.14336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chiang N et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Spite M et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461, 1287–1291 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Romano M, Cianci E, Simiele F & Recchiuti A Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 760, 49–63 (2015). [DOI] [PubMed] [Google Scholar]
- 217.Pope NH et al. D-Series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB J. 30, 4192–4201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Petri MH et al. Resolution of inflammation through the lipoxin and ALX/FPR2 receptor pathway protects against abdominal aortic aneurysms. JACC Basic TransiSci. 3, 719–727 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liu G et al. Resolvin E1 attenuates injury-induced vascular neointimal formation by inhibition of inflammatory responses and vascular smooth muscle cell migration. FASEB J. 32, 5413–5425 (2018). [DOI] [PubMed] [Google Scholar]


