Abstract
The results of many studies in a variety of species have significantly advanced our understanding of the role of visual experience and the mechanisms of postnatal eye growth, and the development of myopia. This paper surveys and reviews the major contributions that experimental studies using animal models have made to our thinking about emmetropization and development of myopia. These studies established important concepts informing our knowledge of the visual regulation of eye growth and refractive development and have transformed treatment strategies for myopia. Several major findings have come from studies of experimental animal models. These include the eye's ability to detect the sign of retinal defocus and undergo compensatory growth, the local retinal control of eye growth, regulatory changes in choroidal thickness, and the identification of components in the biochemistry of eye growth leading to the characterization of signal cascades regulating eye growth and refractive state. Several of these findings provided the proofs of concepts that form the scientific basis of new and effective clinical treatments for controlling myopia progression in humans. Experimental animal models continue to provide new insights into the cellular and molecular mechanisms of eye growth control, including the identification of potential new targets for drug development and future treatments needed to stem the increasing prevalence of myopia and the vision-threatening conditions associated with this disease.
Keywords: myopia, emmetropization, animal models, visual regulation, eye growth
1. Introduction
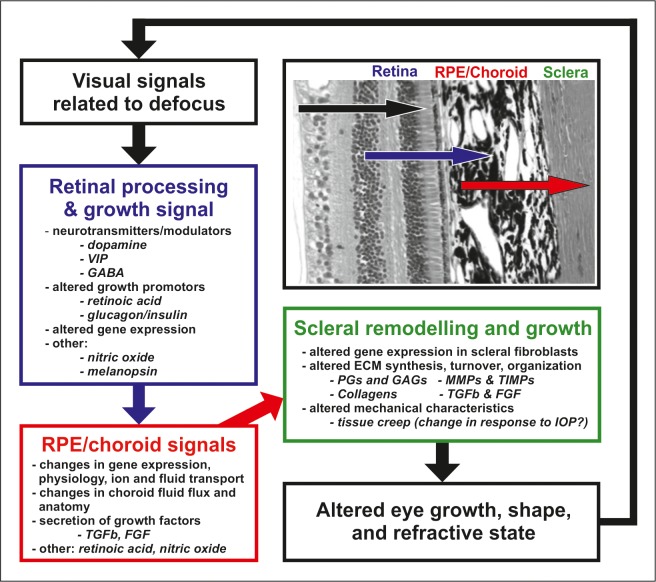
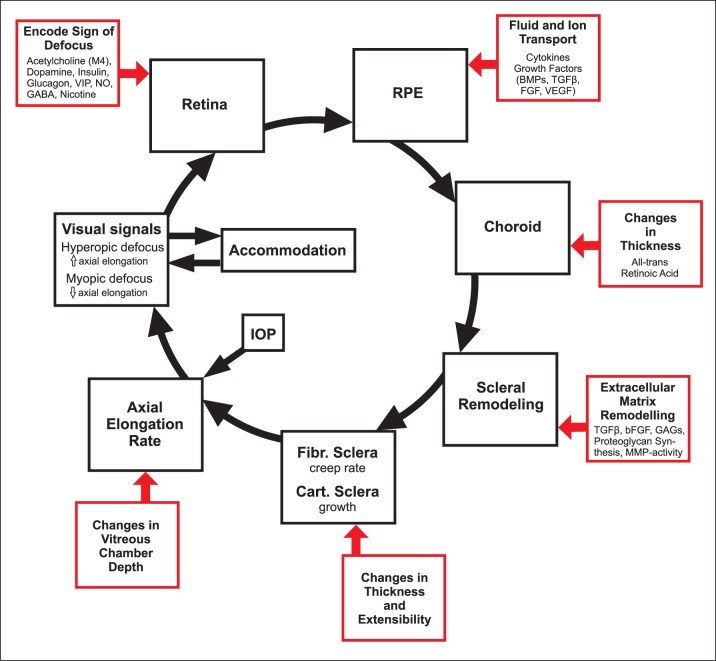
Emmetropization refers to the developmental process that matches the eye's optical power to its axial length so that the unaccommodated eye is focused at distance. Investigations using animal models have informed our understanding of the role of vision in postnatal eye growth, the mechanisms and operating characteristics of emmetropization, and the development of refractive errors (myopia, where the eye is typically too long for its optical power; and hyperopia, where the eye is too short for its optical power). Animal models have established the existence of visual regulation of eye growth and refractive development as well as local retinal control of eye growth. They have also revealed biochemical signaling cascades that transduce visual stimuli related to the sign of defocus into cellular and biochemical changes in the retina, which, in turn, signal changes in the retinal pigment epithelium (RPE), choroid, and eventually sclera, leading to altered eye growth and changes in refractive state. These studies provide a framework for the development of optical and pharmacologic treatments that can be used to effectively reduce the prevalence and progression of myopia, which has become a major public health concern.1
In this paper, the findings of investigations using experimental animal models to study emmetropization and myopia development are reviewed. The contributions that studies with experimental animal models have made to understanding the mechanisms of emmetropization, the development of myopia, and new treatments to reduce myopia progression are summarized. Current models of eye growth control, areas of investigation and major findings, and frameworks for the development of new and effective treatments for myopia are described.
2. Animal Models Commonly Used in Studies of Emmetropization and Myopia
Experimental models of myopia and the visual regulation of eye growth have been demonstrated in a wide variety of species from primates to invertebrates, including macaque and marmoset monkeys, tree shrews, guinea pigs, mice, chickens, fish, and squid. All of these species (with the exception of squid) have been shown to develop myopia in response to visual form deprivation (see Section 3.2), compensate for optically imposed myopic or hyperopic defocus by regulating axial length (see Section 3.4), and recover from the induced refractive error when form deprivation or optical defocus is removed (see Section 3.3). Even though the squid model is the least well-characterized, squid eye growth responds to improve focus under imposed visual conditions.2 Considering that all these varied species possess visually guided eye growth despite differences in ecology, ocular anatomy, visual function, and visual acuity, these results suggest that visual regulation of eye growth is a fundamental property of the camera-type eye, that it may have evolved more than once, and the mechanisms in vertebrates are evolutionarily conserved. From an experimental perspective, each species provides unique advantages to study the mechanisms of visually guided eye growth and key signaling pathways that regulate refractive eye development across species; however, anatomical and physiological differences must be taken into account when interpreting and translating results to humans.
General retinal cellular organization and neural signaling circuitry are highly conserved among vertebrate species3,4; however, there are significant variations between species. Diurnal primates, like humans, have a single fovea for high acuity, whereas other species may be multifoveal, or have an area centralis or visual streak, which are retinal areas with higher photoreceptor and ganglion cell density. The visual photopigment types underlying color vision also vary between species, as does retinal vascular anatomy. Table 1 summarizes structural similarities and differences between the retinas of the most commonly used experimental species.
Table 1.
Retinal Differences in Species Used for Myopia Models
|
Species |
Inner Retinal Blood Supply |
High Cell Density Region |
Photoreceptor Types and Peak Sensitivities |
Central Retinal Thickness |
Optic Nerve Head and Lamina Cribrosa |
| Chick | Avascular (Pecten) | Area centralis (24,000 ganglion cells/mm2)83 | Rods, S1 (415 nm, S2 (455 nm), M (508 nm), L (571 nm)781 | 295–350 μm at area centralis84,782 | Sparse glial and connective tissue19,783 |
| Zebrafish | Vascular | Area centralis (37,000 ganglion cells/mm2)112 | Rods (503 nm), UV (361 nm), S (411 nm), M (482 nm), L (565 nm) cones784 | 191 μm785 | Glial120 |
| Mouse | Vascular | Visual streak (6000 ganglion cells/mm2)786 | Rods, UV (370 nm) and M (505 nm) cones63 | 202 μm787 | Glial788 |
| Guinea pig | Avascular | Visual streak (2272 cells/mm2)39 | Rods, S (429 nm) and M (529 nm) cones475 | 150 μm789 | Collagenous41 |
| Tree shrew | Vascular | Area centralis27 | Rods, S (428 nm) and L (555 nm) cones32 | 213 μm790 | Collagenous33 |
| Marmoset | Vascular | Fovea12 | Rods, M/L (543, 556, 563 nm) cones791 | 230 μm12 | Collagenous19 |
| Rhesus | Vascular | Fovea (33,000 ganglion cells/mm2)792 | Rods, S 440 nm, M (536 nm), L (565 nm) cones16,793 | 207 μm794 | Collagenous795 |
| Human | Vascular | Fovea (38,000 ganglion cells/mm2)796 | Rods, S (419 nm), M (531 nm), L (558 nm) cones797 | 182 μm at fovea798 | Collagenous799 |
S, short wavelength; M, medium wavelength; L, long wavelength.
There are also significant species differences in the mechanisms and amount of accommodation, which regulates the dioptric power of the eye and may be indirectly involved in myopia development through its effects on retinal defocus. In many species, including human, accommodation is achieved by changing the power of the crystalline lens by contraction of the ciliary muscle, whereas in other species it is achieved by moving the lens.5 Changes in corneal power have also been observed in some species.6–8
For another recent review of different species used for experimental studies of emmetropization and myopia, see Schaeffel and Feldkaemper.9
2.1 Comparative Ocular Anatomy and Visual Physiology of Animal Models
2.1.1 Nonhuman Primates
Macaque monkeys were used in the original studies showing form-deprivation myopia (FDM) and visual influences on eye growth.10,11 Since then, both Old World (rhesus macaque – Macaca mulatta) and New World (common marmoset – Callithrix jacchus) monkeys have been used for myopia research. Both species have foveal retinas, eyes that are optically scaled down versions of human eyes, and visual physiology which is essentially identical to that of humans.12–15 The rhesus monkey retina is most similar to the human. It is rod-dominated (rod to cone ratio ∼20:1) with a cone-dominated fovea and possesses three cone types, with short-, middle- and long-wavelength sensitivities, in addition to rods.16 The fovea provides visual acuity of approximately 44 cyc/deg.13,14 The marmoset retina is cone-dominated with a well-developed fovea.12,15 The marmoset retina contains rods as well as cones, which exhibit a polymorphism of visual pigments, in which three photopigments are in the middle- to long-wavelength range, with peak sensitivities at 543, 556, and 563 nm.17 With this polymorphism, some animals are dichromatic (males and some females) while others are trichromatic (females). Visual acuity in marmosets is approximately 30 cyc/deg.12,18 Both rhesus and marmoset monkeys have vascular inner retinas with a foveal avascular zone. In rhesus monkeys, the optic nerve head contains a collagenous lamina cribrosa, closely resembling that in humans. In marmosets, the optic nerve also has a collagenous lamina cribrosa with characteristic sieve-like structure.19
The accommodative system in rhesus monkeys and marmosets is closely related to that in humans and other primates.20,21 The ciliary muscle and its pharmacology are similar to those of humans allowing cycloplegia (paralysis of accommodation) to be produced with muscarinic antagonists as in humans. Juvenile macaques and marmosets have an accommodative response of at least 20 diopters (D).22,23 In previous studies, accommodation was successfully stimulated in awake-behaving marmosets and measured with photorefraction, showing stimulus response slopes similar to humans.22 Additionally, rhesus monkeys have been shown to develop presbyopia at a similar rate as humans, once corrected for life span.20,24
Low availability due to low reproduction rate in macaques is a challenge, and the eyes and visual systems in macaques develop more slowly than in other species commonly used for myopia research. Marmosets give birth to twins or triplets approximately twice a year and are sexually mature at approximately 18 to 24 months.25
2.1.2 Tree Shrew
Tree shrews belong to the order of Scandentia, which are closely related to primates. They are among the first species shown to develop FDM26 and have since been used by several laboratories for myopia research. Tree shrews have a cone-dominated retina with rods comprising approximately 14% of the photoreceptor population.27 Tree shrews do not have foveas, but the retina has an area centralis,27–29 which provides a visual acuity of approximately 2.4 cyc/deg.30,31 Tree shrews are dichromatic, with short- and long-wavelength sensitive cones.32 The tree shrew inner retina is vascular. The optic nerve contains a collagenous lamina cribrosa with radially oriented laminar beams.33
Tree shrew eyes have relatively large crystalline lenses and relatively small vitreous chambers compared with primates. They do not appear to exhibit substantial accommodation31,34; however, when stimulated with carbachol, tree shrews can accommodate up to 8 D.35
Tree shrews typically give birth to two small litters a year.
2.1.3 Guinea Pig
Guinea pigs are diurnal rodents, which have been increasingly used as a model for myopia research. Guinea pigs develop FDM and can compensate appropriately for both imposed myopic and hyperopic defocus.36,37 Guinea pigs are dichromatic. In addition to rods, the retinas of guinea pigs include middle- and short-wavelength-sensitive cones, which occupy superior and inferior areas of the retina, respectively, while the transition zone contains both cone types and cells with both pigments.38 Guinea pigs do not have a fovea; however, the retinas have a visual streak,39 which provides a visual acuity of approximately 2.7 cyc/deg. The guinea pig retina is avascular, having the retinal blood supply provided solely by the choroidal circulation. Because retinal nutrients must diffuse from the choroid, the retina is typically thinner than in animals possessing inner retinal vasculature.40 The optic nerve contains a collagenous lamina cribrosa with connective tissue beams.41
Guinea pig eyes have relatively large crystalline lenses and relatively small vitreous chambers compared with primates.42 Guinea pigs do not appear to have an active accommodative response43; however, approximately 5 D of accommodation can be elicited pharmacologically in juvenile animals.44
Guinea pigs are able to breed year-round and grow rapidly, which allows large-scale studies.
2.1.4 Mouse
Mice are nocturnal rodents, which have been increasingly used for myopia research in recent years.45–50 Although mice are classified as nocturnal animals, they are also active during the day,51–53 photopic visual input plays an important role in their refractive development,54 and behavioral and functional studies suggest that vision is critical for accurate spatial navigation.55–58 Mice develop FDM and respond appropriately to imposed hyperopic and, to some extent, myopic defocus.46,59 Mouse myopia is axial in nature and has features of human myopia.46 Mice are dichromatic and the organization of the mouse retina is similar to that of other mammals.3,4,60 Similar to guinea pigs, the mouse retina includes middle- and short-wavelength–sensitive cones, which occupy superior and inferior areas of the retina, respectively, while high levels of both photopigments are expressed in the transition zone.61–63 The mouse retina does not possess a fovea, but a visual streak has been located just temporal of the optic disc,3,64,65 which provides an upper limit for visual acuity of approximately 1.4 cyc/deg.66 The mouse eye possesses an inner retinal vasculature with radially oriented vessels. The optic nerve contains a lamina cribrosa composed of glial cells.67
Mouse eyes have large crystalline lenses and relatively small vitreous chambers compared with primates.68,69 Mice are not known to possess lenticular accommodation.70,71
Mice breed year-round, produce large litters, and grow rapidly. Because of the availability of a large number of inbred and gene-targeted strains and well-established techniques for genome manipulation, the mouse has become a popular model for advanced genetic and molecular genetics studies of gene–environment interaction in refractive development and myopia.
2.1.5 Chicken
Studies with chicks were among the first to show that visual experience can modulate eye growth and refractive development.72 Since then, chicks have been used extensively because they are easy to obtain, are visually precocial, and develop rapidly. Most of the chick studies are performed on different strains of White Leghorn chicks. Breed and strain differences have been found, indicating genetic differences in the susceptibility to visual experience in eye growth control,73,74 which have been confirmed with selective breeding.75 Chicks develop FDM and rapidly compensate for both imposed myopic and hyperopic defocus (see Section 3 below).
The chick retina contains rods, four single cone photoreceptors, and one double cone photoreceptor.76,77 The cones contain oil droplets, which act as long-wavelength pass filters cutting off shorter wavelengths.78 Chick photoreceptors are present in a 3:2 cone-to-rod ratio with the majority of rods located in the inferior region of the retina and the majority of blue and violet cones in the superior retina.76,77 Chick retinas do not have a fovea, but have a largely rod-free area centralis that provides a visual acuity of approximately 7 cyc/deg.79–83 Optical coherence tomography (OCT) imaging shows that the retina is thickest in the region of the area centralis.84 The chick inner retina is avascular and is supplied with oxygen and nutrients by the pecten oculi, which is a vascular structure continuous with the choroid and projecting into the vitreous chamber. The pecten extends from the optic nerve head and oscillates in the vitreous with saccadic eye movements to facilitate ocular perfusion.85 The optic nerve possesses a poorly formed lamina cribrosa with sparse, longitudinally oriented connective tissue bundles.19 Chick retina, unlike mammalian retina, receives efferent input from the brain (centrifugal inputs) and unique axon-bearing amacrine cells not found in mammals.86
Chick eyes have small crystalline lenses and relatively large vitreous chambers.87 The chick possesses an active accommodative system with approximately 25 D of amplitude.88 Accommodation is achieved through changes in both corneal and lens surface curvatures, with the cornea being responsible for roughly 40% and the lens for 60% of the dioptric change.6,8,89 The ciliary muscle is responsible for both corneal and lenticular changes during accommodation.8 Unlike mammals, the chick ciliary muscle is striated and contains nicotinic acetylcholine receptors.90 Therefore, cycloplegia in chicks requires nicotinic antagonists.
Chicks, like other birds and most vertebrates (except most mammals), have a cartilaginous and fibrous sclera (see Section 3.5.4) with scleral ossicles associated with the cartilaginous sclera in the anterior segment of the eye.5
The circadian regulatory system in chicks is highly developed and possesses a number of differences from that of mammals,91–94 which may make refractive development of the chick eye more sensitive to changes in light cycle, such as constant light.95–99 For more on light cycles and circadian rhythms see Section 4 below.
2.1.6 Fish
Fish eyes grow throughout life, and have been shown in several species to be affected by changes in the visual environment.100–104 Teleost fish develop FDM and compensate for imposed hyperopic and myopic defocus.101,103 Fish from several species also compensate for defocus due to chromatic aberrations and effectively recover from induced refractive errors when visual form deprivation or imposed defocus is discontinued.100,101,103,104 Methods for accurately measuring zebrafish eyes and vision have been developed102,105–109
Zebrafish have tretrachromatic vision, with UV, short-, middle-, and long-wavelength–sensitive cones.110 Retinal morphology and stratification are similar to the mammalian retina.111 Ganglion cell counts show a region of higher density at the area centralis, which provides a visual acuity of approximately 0.7 cyc/deg.108,109,112 The zebrafish eye possesses an inner retinal vasculature that branches from the optic artery.111,113 The optic nerve head is comprised of an astroglial lamina cribrosa.114
In zebrafish, as in other aquatic animals, the relative refractive power of the lens is higher than that of terrestrial animals because corneal power is neutralized in water. The zebrafish crystalline lens is spherical and is not known to accommodate115; however, other teleost fish are known to accommodate by moving the lens.116,117 The zebrafish is a promising model for studies of visually guided eye growth because of its fast development and the availability of well-established protocols for genome manipulation and large repository of gene-targeted mutants.118–120
2.2 Schematic Eyes
Paraxial schematic eyes have been developed for the following species used for experimental myopia research: chick,81,87 mouse,69 guinea pig,42 tree shrew,31 marmoset,12 and macaque.121,122 For reviews of the comparative optics of eyes of vertebrates see several papers by Hughes.123–125 For a recent human schematic eye, see Atchison and Thibos.126
2.3 Relative Ocular Maturation Rates
In many respects, the emmetropization process is essentially completed in a relatively short period of time in all species (see Section 3.1). On average, marmosets and macaques exhibit relatively stable refractive errors at approximately 2 and 5 months of age, respectively. In tree shrews, guinea pigs, mice, and chicks, refractive state stabilizes after approximately a few weeks of visual experience. However, the vision-dependent mechanisms responsible for emmetropization remain active well into early adult life127–130 and help to maintain the optimal refractive error and ensure that an animal remains isometropic.
Because the time required to achieve the target refractive state for a given animal depends in part on the magnitude of its initial ametropia, the relative rates of ocular axial elongation provides a reasonable interspecies metric for comparing the time course of emmetropization and refractive development. The top plot in Figure 1 illustrates axial length plotted as a function of age for individual rhesus monkeys. The solid red line, which is the best-fitting five-parameter, double exponential function that rises to a maximum value, provides a reasonable description of ocular elongation for individual macaque eyes (thin lines). The vertical dashed line indicates the age at which the “normal” eye completed half of its total axial growth.
Figure 1.
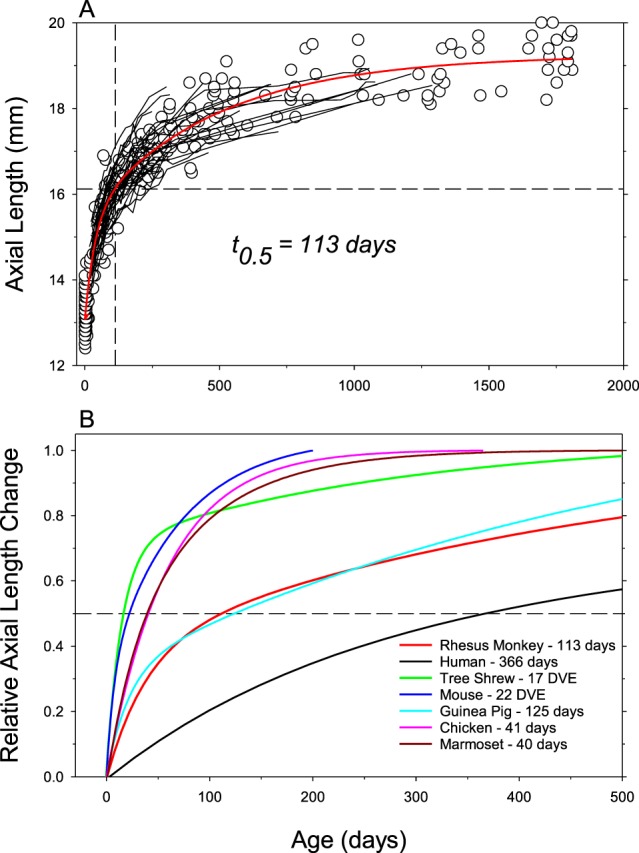
Eye growth in experimental animal models. (A) Axial length plotted as a function of age for individual rhesus monkeys.14,342 The symbols represent cross-sectional data; the thin black lines represent longitudinal data for individual monkeys. The solid red line shows the best-fitting double exponential function. The horizontal and vertical dashed lines show half-maximum axial length and the age when it was obtained, respectively. (B) Relative axial length changes for different species. The same double exponential function was used to fit the data for each species (humans, black line; rhesus monkey14,342,468 red line; tree shrew31,129,467 green line; mouse59,69,742,811–813 blue line; guinea pigs42,466,814,815 cyan line; chicks130 410,481,816,817 pink line; marmoset153,202,343 dark red line) and the functions were normalized to the total change in axial length that occurred from birth or eye opening and adulthood. For mice and tree shrews the abscissa represents days of visual experience.
The bottom plot in Figure 1 compares the time course for axial elongation between humans (black line) and the experimental species commonly used in refractive error research. The same double exponential functions were fit to the axial growth data for each species. The functions were normalized to indicate the relative change in axial elongation as a function of age. The age at which half the total axial growth (t0.5 values) is obtained encompasses the period of most rapid growth in most species (i.e., the period of rapid emmetropization) and appears to be a reasonable measure of the relative rates of ocular growth between species. Accordingly, tree shews (t0.5 = 17 days of visual experience, green line) and mice (t0.5 = 22 days, blue line) exhibit the fastest relative rates of axial elongation (note: the eyes of tree shrews and mice do not open until ∼20 and 14 days of age, respectively; consequently, for tree shrews the abscissa in Figure 1 represents days of visual experience). The t0.5 values for chicks (41 days, pink line) and marmosets (40 days, dark red line) are approximately twice as long as those for mice and tree shrews. The t0.5 values for guinea pigs (cyan line) and rhesus monkeys (red line) are approximately six times longer than mice and tree shrews and approximately one-third the rate calculated for humans. The similarity of the time constants for guinea pigs and rhesus monkeys is somewhat surprising and due in large part to the fact that guinea pig eyes continue to increase in axial length at a relatively fast rate well into adult life after a stable refractive state error has been achieved.
3. Visual Regulation of Eye Growth
It was once thought that the normal growth of the eye and the development of refractive errors were largely regulated by genetics.131–133 However, primarily as a result of research involving animal models, it is now widely accepted that both genetic and environmental (visual) factors are involved in refractive development and particularly in the genesis of common refractive errors, such as juvenile-onset myopia. Consequently, controlling the visual conditions that affect eye growth offers both noninvasive and economic means to reduce myopia progression. In this respect, probably the most fundamental discovery from animal studies is that ocular growth and refractive development are regulated by visual feedback associated with the eye's effective refractive state. In particular, experimental studies over more than 40 years, using a variety of animal models, including nonhuman primates, leave little doubt that retinal defocus carries specific visual information used to regulate the growth and refractive state of the eye. This idea is supported by the following four primary observations described below: (1) emmetropization, (2) the phenomenon of FDM, (3) the recovery from FDM, and (4) compensation for optically imposed defocus.
3.1 Evidence for Visual Regulation of Eye Growth: Emmetropization
At birth, or at the onset of visual experience, the eyes of the majority of animals used in refractive error research exhibit significant refractive errors and substantial individual differences in refractive error. These refractive errors diminish during early postnatal development as both eyes of individual animals grow in a coordinated fashion toward what is presumed to be the ideal refractive state for a given species through a process called emmetropization.
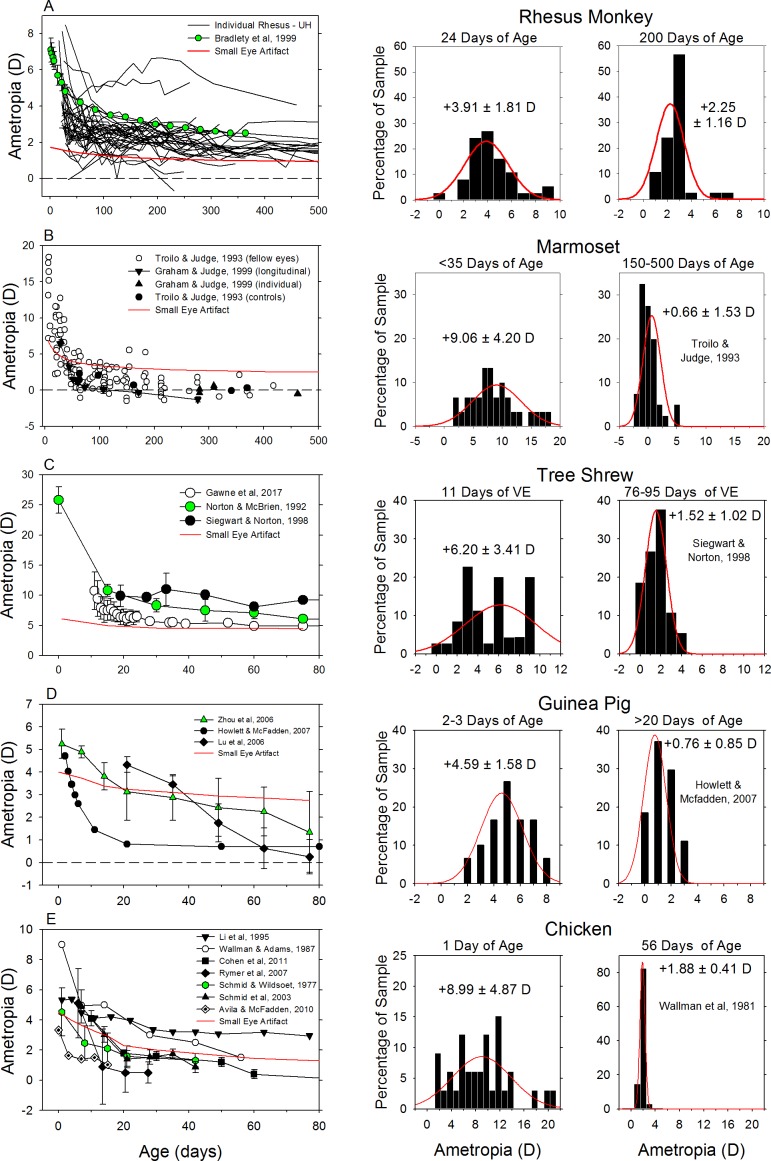
Emmetropization proceeds in a qualitatively similar manner in most of the commonly used laboratory species. For example, as illustrated in Figure 2, which shows data for rhesus monkeys (top row), marmosets, tree shews, guinea pigs, and chicks (bottom row), neonates typically, but not always, exhibit substantial hyperopic errors that exceed the potential measurement artifacts associated with small eyes (red lines)134 and over time these eyes grow in a manner that reduces the degree of hyperopia. The fact that some neonates are myopic and exhibit relative hyperopic shifts during emmetropization emphasizes that the observed refractive changes are not simply a consequence of changes in the magnitude of the small eye hyperopic artifact that takes place as the eye grows.
Figure 2.
Emmetropization in experimental animal models. The left column shows refractive errors for (A) rhesus monkeys,342,468 (B) marmosets,153,343 (C) tree shews,31,129,467 (D) guinea pigs,42,814,815 and (E) chicks175,410,481,816,818 plotted as a function of age (or days of visual experience for tree shrews). Longitudinal data from individual animals are shown as solid lines without symbols. Cross-sectional data for individual animals are represented by individual data points. Symbols connected by lines show mean data (typically cross-sectional) from a given study. The solid red lines represent the small eye artifact associated with common measurement techniques like retinoscopy. The middle and right columns contain refractive error frequency distributions obtained near birth/hatching and at ages when refractive development was relatively stable, respectively. The red lines in the histograms show the Gaussian distributions calculated using the mean and standard deviations of the data.
A hallmark of emmetropization is the systematic reduction over time of the intersubject differences in refractive error.135 The histograms in the middle and right columns of Figure 2, show, respectively, the distributions of refractive errors obtained early in the emmetropization process and at ages when the average refractive errors have stabilized. For all five of the represented animal species, the average refractive errors obtained later in life were less hyperopic than those obtained early during the emmetropization process, and the standard deviations of the means were substantially smaller. The optimization of refractive errors and the decrease in the between-subject variability is evidence that early ocular growth is regulated by visual feedback in a way that eliminates these early refractive errors. The fact that the course of ocular growth and refractive development become unpredictable when animals are reared in the dark 24 hours a day indicates that vision is important in the regulation of normal refractive development.54,136–138
Emmetropization is often thought of as the visual regulation of eye growth and not necessarily growth toward emmetropia. The target refractive state, or set point, for emmetropization, varies between experimental species. Like in humans, the eyes of rhesus monkeys, tree shrews, and chicks grow toward low amounts of hyperopia. On the other hand, the eyes of marmosets and guinea pigs develop low amounts of myopia. While these differences may reflect interspecies differences in the operational properties of the emmetropization process, it is well known that domesticated animals often exhibit less hyperopic/more myopic ametropias than their feral counterparts.139 In this respect, the low degrees of myopia in marmosets and guinea pigs may reflect an adaptation to their caged environments.
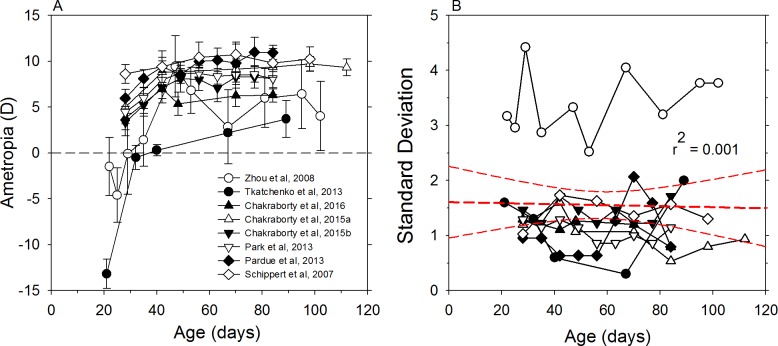
Mice also appear to undergo emmetropization, although the pattern appears to be different from that exhibited by the five species included in Figure 2. As shown in Figure 3, near the onset of visual experience C57BL mice, a strain commonly used in studies of eye development, are myopic or exhibit low to moderate degrees of hyperopia and become relatively more hyperopic until approximately 50 days of age. However, it should be noted that technical difficulties measuring refractive errors in the small eyes of juvenile mice just after eye opening (at 12–14 days of age) prevents direct comparisons with other species.
Figure 3.
The mouse model of FDM. (A) Mean (±SD) refractive errors plotted as a function of age for C57BL mice.50,54,543,555,742,811–813 (B) standard deviations of the mean refractive errors from the left panel are plotted as a function of age. The dashed red line represents the best-fitting linear regression and its 95% CIs.
The small size of mouse eyes makes determination of refractive state difficult. It is not certain how much the small eye hyperopic artifact contributes to the measured hyperopia. Using retinoscopy, Glicksten and Millodot134 estimated that the hyperopic error was on the order of +14 to +16 D. Calculations based on the focal length of paraxial schematic eye models suggest that the artifact could be over +30 D and that these estimates suggest that the artifact should become more hyperopic with age.69 On the other hand, comparisons of refractive errors obtained by retinoscopy in rodents to those obtained using cortical visual-evoked potentials suggest that the small eye artifact is much smaller or nonexistent,140 possibly because the primary retinal structures contributing to the light reflection are deeper in the retina than the vitreoretinal interface. Estimates of refractive error in the mouse eye are complicated by the large amount of high-order aberrations (particularly spherical aberration) and the mouse eye's large depth of focus.57 The estimated depth of focus of the mouse eye can vary between subjects in a given study (1.7–11 D57) and between studies, with estimates ranging to over 20 D.48
Perhaps due to refractive error measurements starting later in development, mice do not seem to exhibit an obvious reduction in the intersubject variability in refractive errors from 20 to 100 days of age. In the right plot in Figure 3, the standard deviations of the average measures are plotted as a function of age. Linear regression analysis indicated that the intersubject variability was essentially constant during early development, perhaps reflecting the small diopter range during the emmetropization process in this development period.
3.2 Evidence for Visual Regulation of Eye Growth: Form-Deprivation Myopia
During the course of their investigations of the effects of abnormal visual experience on brain development, Hubel et al.141–143 observed that surgical eyelid closure, a procedure employed to deprive an animal of spatial vision, produced axial myopia in infant monkeys. This serendipitous, but fundamental, discovery led to the development of the first truly useful animal model of myopia.10,11,144,145 Subsequently, the phenomenon of FDM has been studied in a wide range of animal species, and investigations of FDM have helped establish the role of vision in refractive development, define the operating characteristics of the vision-dependent mechanisms that influence ocular growth, define the ocular anatomic changes associated with vision-induced changes in refractive state, and identify functional changes in the retina, choroid, and sclera leading to our current understanding and theories of the cellular and biochemical mechanisms of eye growth control.
3.2.1 Form Deprivation Myopia: The Basic Phenomenon
In many respects, the phenomenon of FDM has been the most useful experimental animal model of myopia. Many studies have shown that depriving the retina of patterned visual stimulation by suturing the eyelids closed, or more recently by securing a translucent diffuser over the eye, consistently produces axial myopia relative to untreated eyes. These observations provided powerful scientific proof that alterations in vision can produce robust myopic changes. In this respect, the form-deprivation paradigm eliminated potentially confounding issues related to evolutionary pressures and self-selection that had limited many previous animal and human studies on the effects of vision on refractive development. In addition, the fact that monocular form deprivation produces axial myopic anisometropia, which demonstrated that the effects of vision are largely independent in the two eyes, provided an in-animal control for many other environmental factors and, most importantly, potentially confounding genetic factors that could mask the effects of vision on refractive development.
FDM has been observed in several experimental models (see Fig. 4) as well as in humans.146–149 It is primarily the result of increased axial elongation, mainly vitreous chamber, along with thinning of the choroid and the fibrous sclera.36,46,128,137,150–159 Only a few studies have reported changes in corneal curvature (see Section 3.5.5) and lens thickness with form deprivation.159–163 The diversity of species exhibiting FDM is impressive, ranging from fish,101,103 to birds, to mammals,36,46,164,165 and to primates,10,153,166–168 including humans (for another recent review see Schaeffel and Feldkaemper9). There are differences between species in the magnitude of FDM produced and rate of axial elongation, which in large part, reflect species differences in eye size and relative maturation rates. However, it is difficult to directly compare the quantitative differences between individual studies and animal models because of the differences in experimental paradigms, duration of imposed deprivation, degree of image degradation (e.g., variable reductions in image contrast through diffusers), normal pattern of emmetropization, inherent ocular anatomic variations, and/or differences in susceptibility to environmental myopia. The small numbers of qualitative, between-study inconsistencies in the effects of form deprivation that exist in the literature appear to reflect unintended side effects of the treatment strategies that may have masked axial myopic changes. For example, eyelid closure and some continuous contact lens–wearing strategies have been shown to alter the shape and power of the cornea masking potential axial myopic changes. Nonetheless, the fact that FDM occurs in such a wide variety of animals suggests that the vision-dependent mechanisms responsible for FDM are fundamental from an evolutionary point of view and have been conserved across species. Consequently, insights into the mechanisms that mediate FDM obtained in one species are likely to apply to other species, at least qualitatively.
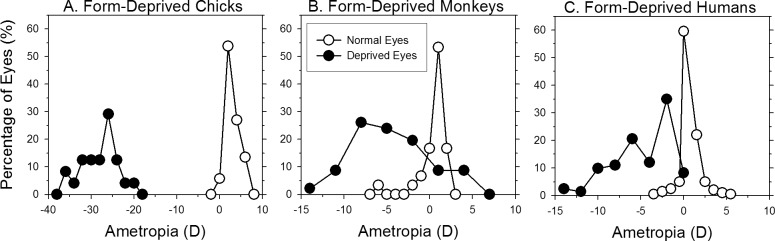
Figure 4.
Examples of FDM in animal models and humans. Refractive error frequency distributions for normal (open symbols) and form-deprived eyes (filled symbols) from chicks137 (A), rhesus monkeys166,168,819 (B), and humans146 (C). Form deprivation was produced in chicks using diffuser lenses; the data were obtained after either 28 or 42 days of age. Form deprivation was produced by surgical eyelid closure in monkeys; the data were obtained over a range of ages and durations of deprivations. Form deprivation in children occurred as a result of conditions (hemangioma and eyelid ptosis) that interfered with a clear retinal image.
With respect to the role of vision in the regulation of ocular growth and refractive development, as first proposed by Schaeffel et al.,169 form deprivation is an open-loop condition that prevents the vision feedback that normally coordinates ocular growth and emmetropization. In particular, form deprivation, especially that associated with strong diffusers or eye lid closure, virtually eliminates meaningful visual feedback regarding the eye's refractive status. When viewing through a strong diffuser, the eye cannot determine if it is emmetropic, myopic, or hyperopic and, consequently, the eye elongates in an unregulated or undamped manner.
The diffusers that are typically employed in form-deprivation experiments produce dramatic reductions in retinal image contrast, alterations in vision that would rarely be encountered during normal development. However, it is important to note that FDM is a graded phenomenon and that the degree of axial myopia is positively correlated with the degree of image degradation.157,170,171 Even relatively mild diffusers that reduce vision by amounts equivalent to small degrees of optical defocus can produce FDM, albeit smaller in magnitude than that produced by stronger diffusers. As a consequence, it is possible that the mechanisms responsible for FDM come into play during normal viewing conditions. More importantly, these results emphasize that the potential for a clear, high-contrast, retinal image is essential for normal emmetropization.
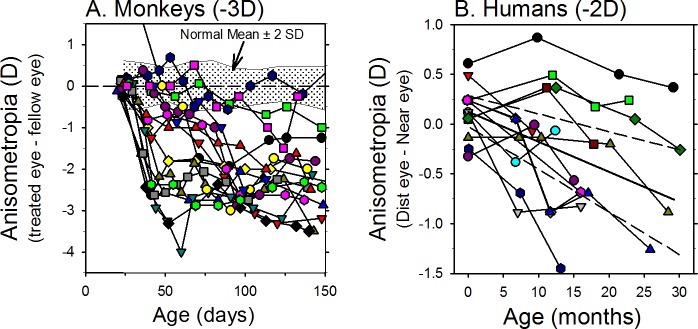
In a given species, the degree of FDM depends on both environmental and genetic factors. For example, it is well established that the magnitude of the changes in eye growth and myopia are also correlated with age of onset and the duration of the period of deprivation.168 In general, the degree of FDM is larger for earlier and longer periods of form deprivation. However, there are also substantial individual differences in the susceptibility to FDM. For example, Schaeffel and Howland169 showed that in response to equivalent periods of binocular form deprivation the between-subject differences in FDM were much larger than the interocular differences found in individual animals. Individual differences in susceptibility to environmental influences are also probably responsible for the large range of myopic anisometropias produced by form deprivation. As illustrated in Figure 5, equivalent periods of form deprivation produced by identical diffuser lenses can result in a substantial range of relative myopic errors in infant monkeys.
Figure 5.
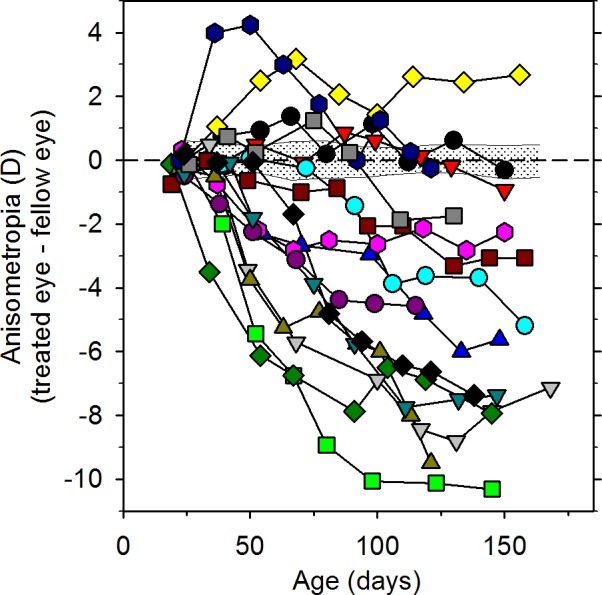
The effects of form deprivation are variable. Interocular differences in refractive error (treated eye − fellow eye) plotted as a function of age for individual rhesus macaque monkeys reared with monocular diffuser lenses. The first symbol of each plot represents the onset of form deprivation. The shaded area in each plot represents ±2 SDs of the mean anisometropia for normal control monkeys (adapted from Hung et al.470).
Constant darkness also deprives the eye of form vision. In chicks, constant darkness results in eye enlargement as it does in form deprivation; however, refraction becomes hyperopic because of significant corneal flattening induced by the constant darkness.137,172,173 This corneal effect appears to be related to the loss of circadian cues because similar effects were observed in constant light rearing as well.95 Raising macaque monkeys in constant darkness prevented emmetropization, leaving the monkeys generally more hyperopic than age-matched controls.136 In tree shrews, however, dark-rearing produced significantly more myopia than in control animals.174 The difference in response is unexplained, but taken together the results from all species generally support the importance of visual experience in emmetropization.
3.3 Evidence for Visual Regulation of Eye Growth: Recovery From Form-Deprivation Myopia
Although the phenomenon of FDM clearly demonstrates that visual experience can influence ocular growth and refractive development, form-deprivation paradigms provide little about the nature of the visual signals that influence early ocular growth or the process of emmetropization. One of the first clear indications that ocular growth and refractive development are regulated by signals associated with the eye's refractive state came from studies of recovery from FDM. In a variety of species, upon removing the diffuser lenses used to produce monocular form deprivation, young animals showed rapid and systematic reductions in the experimentally induced myopic anisometropias, principally due to a decrease in the myopia in the originally deprived eye.36,101,129,175–177 While nonvisual mechanisms that are sensitive to the overall shape of the eye173 may contribute to recovery from FDM, the fact that correcting the myopia induced by form deprivation with negative lenses prevents recovery confirms that vision-dependent mechanisms related to the eye's refractive state regulates eye growth and emmetropization.178,179
The recovery from FDM comes about primarily as a result of changes in vitreous chamber elongation rates. Removing the diffusers from a young animal with monocular FDM, results in myopic defocus in the treated eye and produces a dramatic reduction in the deprived eye's vitreous chamber growth rate. The abnormal axial elongation produced in FDM virtually comes to a halt while the fellow eye continues to grow at a more normal rate (see Fig. 6). At the same time, the cornea and crystalline lens continue to follow their normal developmental course and become flatter in both eyes (i.e., the normal reductions in corneal and lenticular refractive power are not altered by the recovery process). The concomitant increase in the eye's focal length results in a systematic reduction of the myopia in the formerly deprived eye. Once the vitreous chamber depth of the fellow control eye catches up to that of the formerly deprived eye, the refractive errors in the two eyes are reasonably matched. Subsequently, the formerly deprived eye begins to grow again and both eyes adopt similar vitreous chamber growth rates. The anatomic changes are in large respect qualitatively similar in all species, although it is likely that rapid choroidal thickness changes play a larger role in the early refractive error changes in chicks151 than in mammalian species (see Section 3.5.3).155,180
Figure 6.
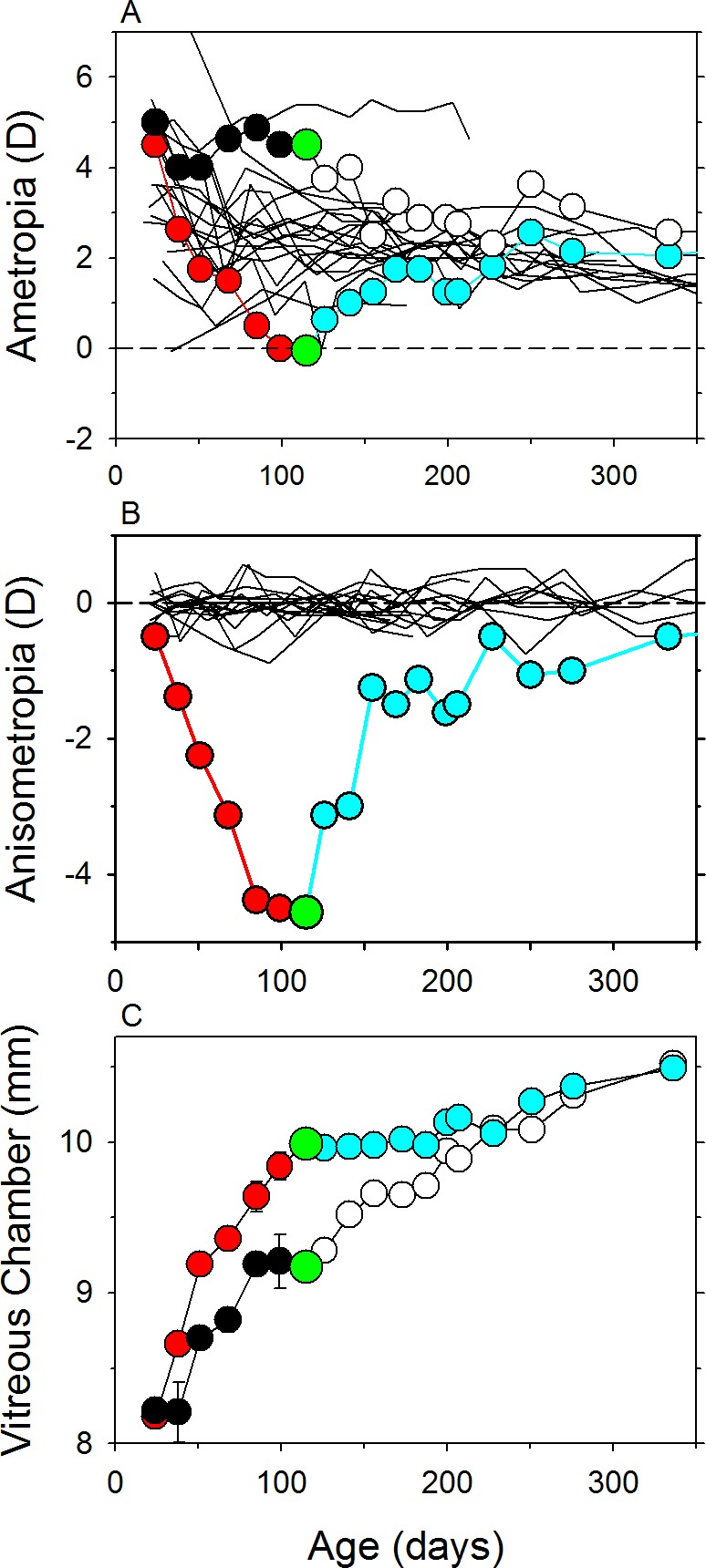
Example of recovery from FDM in rhesus macaques. (A) Spherical-equivalent refractive error plotted as a function of age for the treated (red and cyan symbols) and fellow control eyes (black and white symbols). (B) Interocular differences in refractive error for the same animal plotted as a function of age. (C) Vitreous chamber depth plotted as a function of age for the treated (red and cyan symbols) and fellow control eyes (black and white symbols). The first symbols represent the onset of treatment. The red and black symbols indicate the treatment period. The large green symbols represent the onset of the recovery period. The open and cyan symbols indicate the recovery period. The solid black lines in the top and middle panels are data from untreated control monkeys.
Due to the manner in which recovery from experimentally induced myopia is achieved, the ability of a given animal to recover will greatly depend on the degree of myopia and the age at which unrestricted vision is restored.176,181 For example, it is not likely that an animal could recover fully from FDM if unrestricted vision was restored after the age at which the cornea and lens had stopped flattening, or if the initial degree of axial elongation exceeded normal adult eye lengths. Because it does not appear that vision-dependent mechanisms can result in a significant absolute reduction in axial length (at least in primates182) or in compensating corneal or lens growth, stopping abnormal axial elongation in an optically mature eye would only stabilize myopia if the eye's optical power could be decreased in some other way. Wallman183 suggested that this age-dependent limitation in the ability of the eye to recover from myopia may explain why common forms of myopia that develop in adolescent or adult humans persist. In children, corneal power reaches adult levels by 18 to 24 months of age, and after 8 to 10 years of age, when most myopia is typically diagnosed, the changes in lens power are small.184 Therefore, whereas human infants with myopia shortly after birth usually show some emmetropization, children who become myopic after their corneas and lenses become optically mature are unlikely to recover.185
3.4 Evidence for Visual Regulation of Eye Growth: Compensation for Lens-Imposed Defocus
The most rigorous and clinically relevant test for the hypothesis that ocular growth and refractive development are actively regulated by defocus was provided by studies that employed lenses to alter the eye's effective refractive state. The original study by Schaeffel et al.186 was first to show that the eyes of young chicks wearing positive or negative spectacle lenses compensated appropriately for the imposed defocus, essentially emmetropizing through the defocus imposed by the lens treatment. Specifically, placing a negative lens in front of an emmetropic eye optically simulated hyperopia and to compensate for the lens (i.e., to re-establish emmetropia when viewing through the lens), the chick eye grew until it developed a degree of myopia equivalent to the power of the lens. On the other hand, a positive lens produced myopic defocus on the retina, which led to inhibition of eye growth, resulting in the eye becoming more hyperopic in order to re-establish an emmetropic refractive state through the lens. The fact that chicks exhibit appropriate compensating eye growth for equivalent degrees of hyperopic and myopic defocus, even when accommodation and other behavioral cues to the sign of the effective refractive error are excluded, demonstrates that the eye can detect the sign of defocus and alter its growth in the appropriate direction to eliminate both myopic and hyperopic defocus.187,188
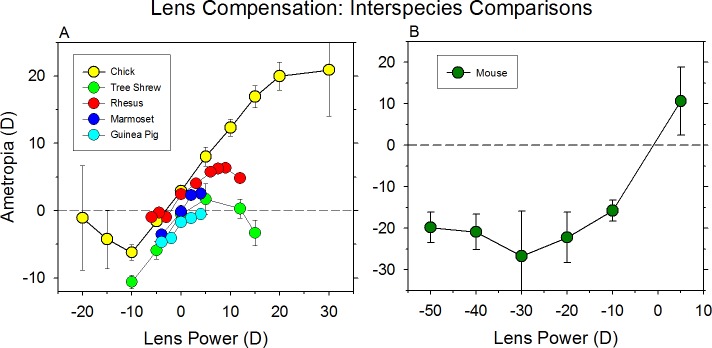
Although some early primate studies189–192 that employed contact lens regimens that produced unwanted corneal alterations failed to confirm the original findings of Schaeffel et al.,186 compensation for lens-imposed defocus (commonly referred to as “lens compensation”) has been replicated many times in chicks,193–196 and reported in many other species, including primates,191,197–199 tree shrews,200,201 guinea pigs,37 and mice.46,47,50,59 As illustrated in Figure 7, the effective operating range of the compensation process differs between species. For instance, in chicks, complete compensation has been shown for a range of spectacle lens powers between −10 and +20 D.194 Based on the available data, the ranges of compensating responses for other species is variable, but all species that have been studied in a systematic fashion exhibit compensating refractive changes for both negative and positive lenses as follows: macaque, −2 to +8 D198; marmosets, −8 to +5 D199,202; tree shrew, −5 to +5 D200,201; guinea pig, −4 to +4 D37; mice, −30 to +5 D46,47,59; fish (Tilapia), −8 to +8 D.103
Figure 7.
Compensation for lens-imposed retinal defocus occurs in a variety of species (A) chicks,398 tree shrews,159 marmosets,191 rhesus macaques,198 and guinea pigs,37 and (B) mice.46,47,59 The mean ametropia obtained at the end of the lens-rearing period is plotted as a function of the power of the treatment lenses.
The observed differences in the effective operating ranges of the emmetropization processes in these different species are likely to reflect several factors. In particular, when expressed in terms of diopters, shorter eyes and eyes with lower spatial frequency response properties would be expected to exhibit larger lens-compensation ranges.203 Interspecies differences in the average refractive errors found in normal neonates would influence the effective degree of defocus produced by a given powered lens and, thus, the effective lens compensation range. Natural and imposed differences in the set point target refractive error for emmetropization are also likely to influence the observed compensation ranges. For example, housing animals in cages that significantly restrict viewing distances may shift the compensation range in the myopic direction.204,205 In addition, behavioral issues are also likely to influence the lens-compensation range. For instance, it is reasonable to expect that animals with large accommodative amplitudes would exhibit greater ranges of compensation for negative than for positive lenses. However, in animals, such as primates, with well-developed binocular vision, issues relevant to accommodative convergence and efforts to maintain binocular vision at the expense of a clear retinal image, could mask this predicted asymmetry. Moreover, although the eye's refractive state is defined for distance viewing, animals with imposed myopia may simply prefer to fixate near objects, effectively eliminating the need to compensate for the imposed lens power.198
It is interesting that at the limits of the operating range for lens compensation, high degrees of either natural or imposed hyperopic defocus do not produce myopia. As illustrated in Figure 7 for chicks, mice, and primates, increasing negative lens powers beyond a species-specific value results in less compensating myopia or little or no changes in refractive error. It is not a simple limitation on the ability of the eye to increase its axial length because form deprivation and rearing strategies in which defocusing lens powers are increased gradually over time have been shown to produce much larger myopic errors.198 Why imposed hyperopic defocus beyond the operating limits of lens compensation often fails to consistently produce myopia is unclear. One possible explanation is that the higher degrees of optical defocus, especially with monocular treatment regimens, cause other visual system changes (e.g., accommodative vergence interactions and possibly amblyopia), which somehow interfere with the effects of chronic defocus on ocular growth. This, however, is not a particularly satisfying explanation because monocular form deprivation, which produces profound sensory deficits in young monkeys, consistently results in exaggerated ocular growth and high degrees of myopic anisometropia.192 Moreover, monkeys with severe form deprivation–induced amblyopia consistently exhibit recovery from FDM.176
Although there has, until recently, been a paucity of evidence for lens compensation in humans, when comparable optical conditions are produced in humans who successfully underwent emmetropization early in life, the resulting changes in refractive error are qualitatively similar to those in laboratory animals.206 Figure 8 shows the compensating refractive error changes produced by optically imposed anisometropia in monkeys and humans. In humans, the compensating change produced by an imposed anisometropia may be more apparent because regardless of viewing distance or which eye is used for fixation, the optical treatment consistently imposes an anisometropia. As illustrated in Figure 8, individual monkeys and humans consistently exhibit compensatory anisometropic changes that are in the appropriate direction to compensate for the imposed optical imbalance. In addition, recent human studies have documented small, short-term bidirectional changes in axial length and choroidal thickness in response to 1 to 2 hours of myopic and hyperopic defocus in young adult subjects,207–210 which suggests that the human eye can also detect the sign of imposed optical defocus and undergo appropriate compensatory changes in axial length.
Figure 8.
Examples of anisometropic compensation in individual infant rhesus macaque monkeys ([A] adapted from Hung L-F, Arumugam B, She Z, Ostrin L, Smith EL III. Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Exp Eye Res. 2018;176:147–160. Copyright © 2018 Elsevier Ltd.)470 and adolescent humans (age of onset 11 years) ([B] adapted from Phillips JR. Monovision slows juvenile myopia progression unilaterally. Br J Ophthalmol. 2005;89:1196–200. Copyright © 2005 British Journal of Ophthalmology).206 The first symbol in each plot represents the onset of treatment. The monkeys were reared with a −3 D lens in front of their treated eyes and a plano lens in front of their fellow eyes. The human subjects were corrected using a monovision contact lens strategy. The dominant eyes were corrected for distance; the fellow eyes were uncorrected by <2 D.
There is still much to learn about the phenomenon of lens compensation, but the results from animal studies have clearly demonstrated that something as simple as a spectacle lens can predictably alter ocular growth. These results provide a solid scientific foundation for optical treatment strategies to reduce the progression of juvenile-onset myopia in children (see accompanying International Myopia Institute reports in this issue211–213).
3.5 Ocular Anatomic Changes Associated with Experimentally Induced Refractive Errors
Experimentally induced changes in refractive state are associated with several anatomic changes to ocular components related to changes in eye shape and size, principally in the depth and shape of the vitreous chamber. These vision-dependent alterations are associated with a number of changes in the retina, RPE, choroid, and sclera. Anterior segment changes have been observed in eyes with experimentally induced ametropias, but have not been found to be related directly to the visual regulation of refractive state.214–216
3.5.1 Retina
The retina is the primary tissue where information about optical defocus is converted into molecular signals, which are then transmitted through the RPE and choroid to the sclera and translated into the structural changes in the sclera underlying development of myopia (see Section 5). Both visual form deprivation and lens-imposed defocus have been shown to cause large-scale changes in gene expression in the retina (see Section 6). Changes in gene expression induced by visual form deprivation have also been shown to result in increased proliferation of the retinal progenitors at the retinal periphery of monkeys resulting in increased neurogenesis and increased growth of the retina.217
3.5.2 Retinal Pigment Epithelium
The RPE also shows distinct morphologic changes during the development of myopia in humans and animals.218–224 In animal models, enlargement of the eye during the development of experimental myopia is associated with an increase in the overall surface area of the RPE through the expansion of individual RPE cells across the entire epithelium,218–220 although less pronounced in the temporal region.220 Such expansion may be due to either passive stretch or active growth of these cells. Like in many other ocular tissues, there also appears to be active changes in fluid dynamics within the RPE during periods of altered growth. In response to recovery from FDM, following diffuser removal, Liang et al.221 reported increased fluid retention and edema within the retina, RPE, and choroid, as well as ultrastructural reorganization of the RPE basal lamina. The authors hypothesized that this represented active changes in fluid movement across the RPE whose tight junctions act as a barrier that allows the regulated exchange of ions and water between the subretinal space and the choroid through modulation of its ionic channels. The role that any such fluid movement plays in the regulation of ocular growth is yet to be fully elucidated. Crewther et al.225–227 suggested that such ionically driven fluid exchange across the RPE between the subretinal space and choroid may, in fact, underlie the significant choroidal thickness changes observed during periods of altered eye growth. Specifically, the authors suggest that accumulation of ions within the subretinal space during the development of FDM may inhibit fluid movement from the vitreous to choroid, leading to vitreous chamber swelling and thinning of the choroidal lacunae in chicks. In contrast, during periods of reduced ocular growth associated with diffuser removal, reverse changes in the ionic state within the subretinal space may induce fluid movement from the vitreous chamber across the RPE causing swelling of the choroidal lacunae. Supporting this hypothesis, ion levels within freeze-dried preparations of the retina, RPE, and choroid have been reported to be significantly modulated during the development and recovery from FDM,221,226 while potassium and phosphate levels are reported to be reduced, and chloride levels increased in the vitreous chambers of form-deprived chicks.228 Furthermore, pharmacologic inhibition of ion movement has been shown to disrupt the compensatory response to lens wear in chicks.227 Together, these findings support the possibility that the choroidal thickness changes observed during alterations in the rate of ocular growth could be associated with adjustments in ionic fluid movement across the RPE. However, choroidal swelling may also be explained by exchanges of fluid between the choroidal vasculature and the neighboring suprachoroidea. In support of this, Liang et al.221 noted that the concentration of Na+ and Cl− ions in the choroidal lymphatics rises steeply over the first 72 hours of recovery from FDM, during which the choroid rapidly swells. The most likely source of these accumulating ions is the choroidal vasculature.
3.5.3 Choroid
The choroid is a highly vascular layer of connective tissue positioned between the RPE and sclera. Together with the ciliary body and iris, the choroid forms the uveal tract.
The past hundred years or so have yielded episodic but compelling pieces of evidence that the functions of the choroid are substantially more than supplying blood to the outer retina.229 For instance, work by van Alphen230 indicated that the choroid, and not the sclera, might be a major determinant of the size and shape of the eye, because when the sclera was removed from the posterior pole, and pressure corresponding to normal IOP applied, the exposed choroid did not balloon out, but maintained its curvature while being displaced posteriorly. Moreover, mysterious neurons currently known as intrinsic choroidal neurons were reported in human choroid as long ago as 1859,231 and their functions are still largely unknown.232 Nonvascular smooth muscle is located in the choroid, which has been verified in various species (birds,5,150,233–235 primates,236–238 rabbits239). Finally, the existence of large lacunae, possibly lymphatic vessels, in most species,5,235,240–242 including humans,238 indicate diverse functions unrelated to blood flow. Today, largely because of the finding, first in birds,150,151 then extended to pri-mates,155,180 that the thickness of the choroid changed in response to retinal defocus, thus acting as a means of positioning the image plane on the retina, it is widely accepted that the choroid is “multifunctional” and involved in numerous aspects of ocular/visual health.
The first evidence for the compensatory choroidal thickness changes in experimental myopia research came from observations of gross changes in the appearance/consistency of choroids from dissected myopic chick eyes, which led to the critical findings that myopic defocus caused large increases in choroidal thickness, and form deprivation or hyperopic defocus caused choroidal thinning.150,151 The subsequent use of higher-frequency ultrasound allowed finer resolution, and demonstrated that the choroidal responses were rapid (within hours), bidirectional, and highly precise. In chicks, the compensatory changes in choroidal thickness are symmetric and linear over a range of imposed defocus from approximately −15 to +15 D.151 The speed of this choroidal compensation is intermediate between that of (fast) lenticular accommodation and the (slow) changes in scleral extracellular matrix (ECM) synthesis that alter eye size, and so these choroidal responses may function as a mechanism to sustain focus on the retina until the eye length “catches up” to the front optics. Subsequently, the choroid returns to normal at a pace in concert with the changing size of the globe. This process of the scleral changes altering globe size together with the choroidal thickness changes altering the image plane create an association between faster-growing (large) eyes and thinner choroids, versus slower-growing (small) eyes and thicker choroids. This phenomenon has since been observed in all other species tested, including marmosets,180 rhesus macaques,155 guinea pigs,37 and humans.208 The responses in mammals are, however, much smaller in magnitude than those in birds.
Whether the thickness of the choroid influences the rate of scleral growth, perhaps by the secretion of regulatory molecules (see Section 5.3.1), has been a question of interest for some time because of its translational implications. If there were a causal relationship, for instance, then perhaps choroidal thickness in humans might be a “risk factor” for the development of myopia, which would make it a potentially valuable tool in deciding on treatment therapies for “at-risk” children.243
Two studies using the chick model have addressed the question of whether choroidal thickness is a predictor of ocular growth rate. The first was a heritability analysis on nearly 900, 4-day-old chicks,75,244 which showed approximately 50% of the variation in choroidal thickness was determined by genetics. Furthermore, initial choroidal thickness was not related to initial eye size nor to subsequent growth rates. In an extension of this study, a cohort of 500 chicks were deprived of form vision for 4 days to induce myopia, and initial choroidal thickness did not predict the growth response to the deprivation.243 A smaller study from a different lab, however, reported a significant association between initial choroidal thickness and subsequent growth rates such that eyes with thinner choroids grew faster than those with thicker ones, perhaps supporting the association of thicker choroids with greater secretion of a growth inhibitor.245 The discrepancy between these two studies may reflect differences in the age at the onset of the experiments, as the first study used younger chicks. The latter study also reported a negative correlation between initial choroidal thickness and subsequent changes in thickness; the thinner choroids of faster-growing eyes showed greater subsequent thickening. By contrast, initial choroidal thickness was not predictive of ocular growth rates in eyes wearing either positive lenses (slowing elongation), or negative lenses or diffusers (stimulating elongation). Neither were the magnitudes of choroidal thickness changes in response to defocus predictive of ocular growth rates. These differences between untreated eyes, in which thickness was predictive of growth, and experimentally manipulated eyes in which it was not predictive, might reflect a decoupling of the “choroidal system” from the “growth system” under experimental visual conditions. Together, these findings weaken the hypothesis that the magnitude of choroidal thickening is related to its “potency” for either a secreted signal molecule, or as a mechanical barrier to such a signal molecule, supporting separate mechanisms for the choroidal thickness and scleral responses.
Several other lines of evidence support separate mechanisms for the choroidal thickness and scleral responses to visual signals. First, a detailed study of the temporal integration characteristics of the two responses reported dissociations between choroidal thickness and scleral responses. If eyes were exposed to brief and infrequent episodes of defocus (7 minutes/4 times per day), in the case of positive lenses, only inhibition of axial elongation was observed, and not choroid thickening, while in the case of negative lenses, only the choroid thinning response was found, and not stimulation of axial elongation.246 Second, eyes with lesions of both ocular parasympathetic pathways (ciliary and pterygopalatine ganglia), did not respond to form deprivation with the usual development of myopia, but instead exhibited reduced axial growth.247 Surprisingly, however, choroids of the form-deprived eyes thinned, showing the usual compensatory response to form deprivation. This thinning of the choroid in these aberrantly slow-growing, form-deprived, lesioned eyes suggests a pathological response to form deprivation, as suggested by electron microscopy showing abnormalities in the photoreceptor outer segments and RPE in form-deprived eyes.221 Finally, a study in chicks found that oxotremorine, a muscarinic acetylcholine agonist, stimulated ocular growth, and thinned the choroid; however, two other agonists that were ineffective at stimulating growth also caused choroidal thinning.248,249 These three distinct lines of investigation show that choroidal thickness changes can be dissociated from axial growth suggesting that the former is not a necessary precursor for, or indicator of, the latter.
3.5.3.1 Mechanisms Underlying Changes in Choroidal Thickness
In chicks, the main anatomic changes accounting for the large increases in thickness in response to myopic defocus occurred in the choroidal stroma, where the presence of large, fluid-filled lacunae suggested potential underlying mechanisms.150 In addition, α-actin–positive nonvascular smooth muscle cells were identified in the stroma,232,234,250 and are also present in other species, including humans.238,239,251
The potential underlying mechanisms can be broadly divided into two categories as follows: those related to fluid-flux changes, and those related to smooth muscle activity (as reviewed by Nickla and Wallman229). The fluid-flux hypothesis posits that the rapidity (within hours) and magnitude (up to quadrupling) of the thickness changes favor a redistribution in fluid compartments as the main mechanism. This is supported by several lines of study. First, thicker choroids from eyes responding to myopic defocus synthesized more proteoglycans than thinner ones,252 suggesting that these hydrophilic matrix molecules play a role in the changing thickness of the stroma. However, the relatively small differences in synthesis rates between the two extremes in thicknesses weaken this hypothesis. Second, the permeability of the choroidal capillaries may increase, allowing movement of proteins from the lumen to the stromal matrix and/or lymphatics, followed by passive fluid flux.242 Several findings support this latter hypothesis, as follows: (1) form-deprived chick eyes had fewer fenestrations in its choriocapillaris253; (2) the protein content in suprachoroidal fluid was higher than normal in experimentally thickening choroids, and lower in experimentally thinned ones254; and (3) thicker choroids had higher amounts of fluorescein-dextran than thin ones after intravenous dextran injection,254 and these also had higher amounts of albumen.255 Third, because the anterior uvea (iris and ciliary body) is physically connected to the choroid, changes in the amount of aqueous flowing via the uveoscleral pathway might play a role. Finally, increased fluid flowing from the retina across the RPE might account for an increased amount of fluid in the stromal lacunae.256
It is possible that choroidal thickening and thinning occur via different mechanisms. The fluid-flux mechanism is probably too slow to account for the finding that choroids can thin by approximately 50 μm within an hour.257 A more likely possibility involves smooth muscle contraction. In fact, the choroidal stroma in birds and primates, including humans, contains actin-positive nonvascular smooth muscle cells that are innervated by the parasympathetic and sympathetic systems.232,234,238 The axon terminals contacting the smooth muscle are positive for Nicotinamide adenine dinucleotide phosphate–diaphorase, indicating the presence of nitric oxide (NO), and for vasoactive intestinal peptide (VIP), which are both parasympathetic transmitters. Notably, stimulation of ciliary axons innervating explant choroids caused a contraction of the tissue, which was blocked by atropine, suggesting muscarinic cholinergic parasympathetic innervation.235 Further support for a muscle contraction–mediated thinning is the finding that muscarinic agonists thin chick choroids both in the intact eye and in vitro.235
In summary, the choroid is a multifunctional structure, containing various tissue/cell types whose functions are as yet unknown. Many lines of study in animal models conclude that it plays important roles in the visual regulation of ocular growth. The existence in human choroids of similar features and physiological responses suggest a conservation of function among species. Recent studies on choroidal thickness changes in various ocular pathologies, including myopia and glaucoma, will help uncover its potential impact for the development of treatment therapies for vision health.
3.5.4 Sclera
The sclera is a dense connective tissue that forms the outer coating of the eye and defines the eye's size and shape. The anatomy of the sclera varies among vertebrates.5 In most vertebrates it is composed of two layers—an inner layer of hyaline cartilage and an outer layer of dense fibrous connective tissue (Fig. 9A). The two layers vary in their relative thicknesses in different regions of the ocular globe, the fibrous and cartilaginous layers are approximately equal in thickness at the posterior pole, but the fibrous layer progressively thins in equatorial and anterior ocular regions. Scleral ossicles, rings of bone in the sclera found in the anterior segment, are also found in all vertebrates except for eutherian mammals and crocodiles.5 The sclera in humans258 and other eutherian mammals (such as macaque monkeys, marmosets, tree shrews, guinea pigs, and mice) is composed of only a fibrous layer156,259,260 (see Figs. 9B, 9C), which is made primarily of collagen type I with smaller amounts of types III and V collagen, and held together with elastin and proteoglycans. However, ECM molecules previously believed unique to cartilage, such as aggrecan,261–263 proline arginine-rich and leucine-rich repeat protein,264 and cartilage olimeric matrix protein,265 are also present in the mammalian fibrous sclera, suggesting that cartilaginous components have been retained in the sclera through evolution and serve important biochemical and biomechanical functions.
Figure 9.
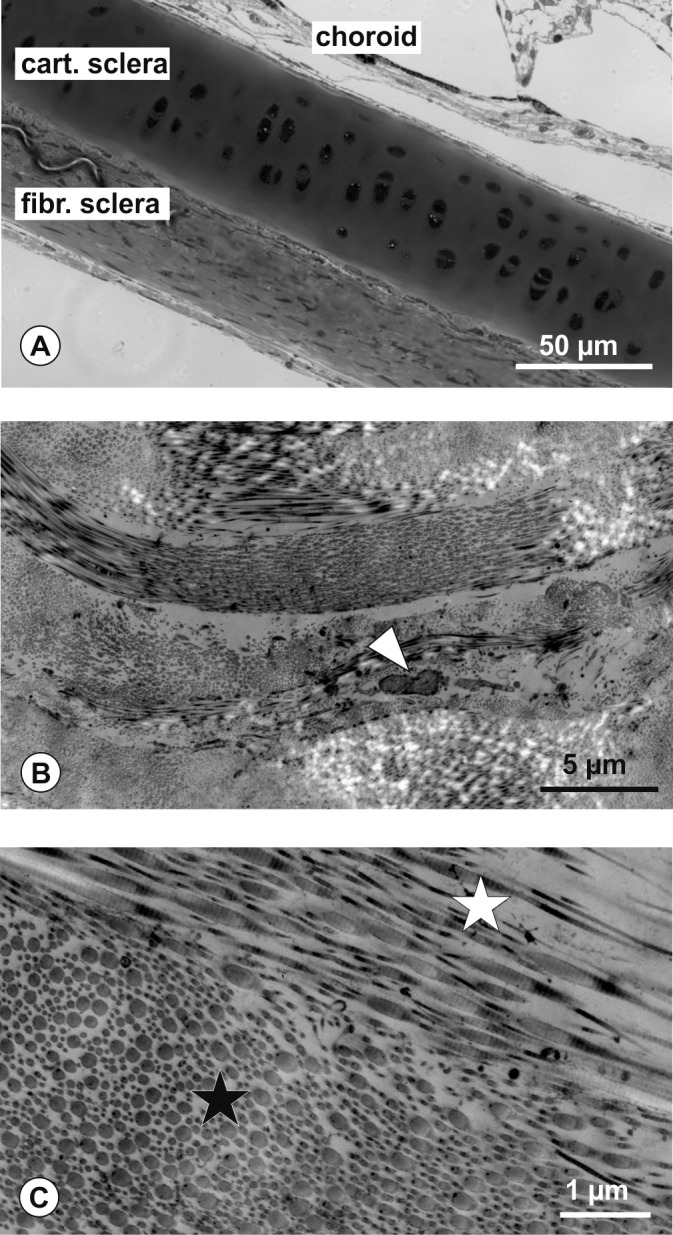
(A) Chick scleral cross section. The cartilaginous part (cart. sclera) facing the choroid and the fibrous part (fibr. sclera) forming the outer shell can be easily distinguished in this Toluidine blue stained semithin section. (B, C) Electron micrographs of marmoset sclera. (B) Layers of collagen fibers with various orientation are detectable. White arrowhead indicates the cell body of a fibroblast embedded between ECM layers. (C) Higher magnification showing longitudinally (white arrow) and cross-sectional (black arrow) collagen fiber bundles.
Significant changes in scleral ECM synthesis, accumulation, and turnover are associated with visually induced changes in eye size and refraction in a variety of animal models.159,260,266–268 Despite the differences in scleral anatomy, the fibrous sclera of mammals and the fibrous layer of the avian sclera appear to change in a similar manner in experimentally induced myopia. When ocular elongation accelerates during myopia development, the fibrous sclera thins in mammals259,260 and birds.154,269 Thinning of the fibrous sclera in chicks is similar to what is seen in the fibrous mammalian sclera.156,270,271 The cartilaginous sclera of birds, however, demonstrates increased growth as the eye elongates, which is accompanied by an increase in synthesis and accumulation of proteoglycans and an increased dry weight.266,272 All vertebrates appear to use similar signaling mechanisms to control the structure of the sclera and do so by controlling growth in the cartilage, where it is present, and by controlling remodeling in the fibrous sclera.
The scleral changes in experimental myopia development in primates, tree shrews, guinea pigs, and mice are similar to those associated with high myopia in humans. There is a restructuring of the ECM, a loss of ECM and scleral thinning.259,271,273–275 These alterations are associated with several changes in the mechanical properties of the sclera. Specifically, there are increases in the viscoelasticity and creep rate of the sclera,276,277 which make the tissue more extensible so that normal IOP may produce an enlargement of the vitreous chamber. A recent study also suggested that the crimp angle of tree shrew scleral collagen fibril bundles increases during the development of myopia, which could decrease the stiffness of the sclera. Decreases in crimp angle were observed during recovery from myopia.278
In contrast, myopia development in chicks is associated with active scleral growth due to increased ECM synthesis and the accumulation of proteoglycans in the cartilaginous layers of the sclera.266,279 The biochemical changes in the sclera and control of scleral growth during eye growth and myopia development will be discussed in Section 5.3.
In humans, mammals, and chicks, scleral changes associated with myopia development are most pronounced at the posterior pole.259,260,280 The preferential involvement of the posterior sclera in myopia may be related to regional differences in the growth states of the scleral cells, differences in scleral tensile stresses at the posterior pole, or it may reflect the distribution and density of retinal, choroidal thickness, and scleral components in the vision-dependent cascade that regulates ocular growth.281
3.5.5 Corneal and Anterior Segment Changes
While most of the vision-induced changes in the refractive state of the eye observed in experimental models and common refractive errors in humans can be explained by changes in the axial growth of the eye, changes in corneal curvature and anterior chamber depth have also been observed in some animal studies. Figure 10 shows the changes in corneal curvature and anterior chamber depth that have been described in experimentally induced myopia in several species. Overall, the largest changes in corneal curvature and anterior chamber depth were found in chicks, where high amounts of induced myopia were associated with steeper corneas and deeper anterior chambers. Smaller, but significant changes were found in other species. Nearly all of the significant changes for both corneal power and anterior chamber depth were observed in form-deprived animals, possibly reflecting the generally larger myopic errors obtained with form deprivation. However, steeper corneas were also correlated with increasing myopia produced by either diffusers or negative lenses in monkeys.163 Note that studies employing a lid-sutured paradigm, despite its significant myopia-induction effects, were not included in Figure 10 because corneal flattening is often a side effect of surgical lid closure.34,153,282–284
Figure 10.
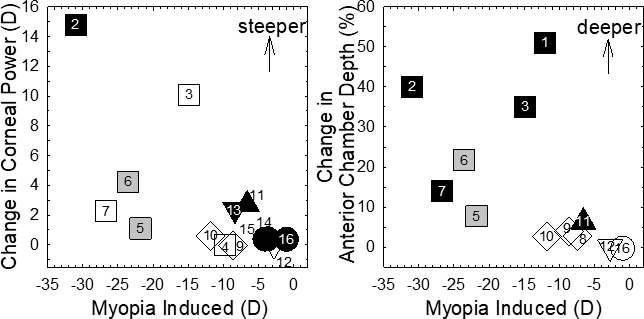
Changes in corneal power and anterior chamber depth found in different animal models with experimentally induced myopia. The x- and y-axis parameters represent either interocular difference (treated eye − fellow control eye) or intergroup differences (treated group − normal group). The filled and open symbols represent statistically significant and insignificant changes, respectively. Gray symbols indicate studies that did not perform statistical tests. Numbers inside or near each symbol represent different studies. █ Chicks: (1) Wallman et al.,72 diffusers; (2) Gottlieb et al.,137 diffusers; (3) Hayes et al.,820 diffusers; (4) Irving et al.,194 lenses; (5) Troilo et al.,160 diffusers; (6) Napper et al.,162 diffusers; (7) Napper et al.,556 diffusers; ⧫ tree shrews: (8) Guggenheim et al.,802 diffusers; (9) Siegwart et al.,129 diffusers; (10) McBrien et al.,271 lid-suture; ▴ guinea pigs: (11) Howlett et al.,36 diffusers; ▾ marmosets: (12) Graham and Judge,202 negative lenses; (13) Troilo and Nickla,347 diffusers; • rhesus monkeys: (14) Smith and Hung,157 diffusers; (15) Qiao-Grider et al.,176 diffusers; and (16) Qiao-Grider et al.,163 diffusers and negative lenses, induced myopia was not available, myopic anisometropia of more than −1.0 D was used. For chicks, the corneal radius of curvature values was converted to corneal powers using a refractive index of n' = 1.369.821
Although it is not clear how vision-dependent mechanisms could alter corneal power and anterior chamber depth during refractive development, some data suggest that the anterior segment changes are an epiphenomenon or side effect associated with changes in the posterior segment of the eye. For example, in chicks reared with hemiretinal form deprivation (i.e., diffusers that affected half of the retina), the nature of corneal changes (the direction of astigmatism in particular) varied with the location of the imposed deprivation (e.g., superior hemiretina versus temporal hemiretina285). Similarly, in monkeys both negative and positive spherical lens-rearing strategies, which elicited, respectively, either compensating increases or decreases in vitreous chamber elongation, produced similar corneal astigmatic errors.286 However, substantial vision-induced changes in vitreous chamber depth and refractive error can be produced in monkeys without concomitant changes in the anterior segment, suggesting that the anterior and posterior segments of the eye are independently regulated.163 In this respect, several manipulations have been shown to decouple anterior and posterior chamber alterations. For example, administration of a variety of neurotoxins can produce contrasting anterior and posterior segment changes.97,287–289 However, this effect might be specific to birds reared under constant light (see Section 4.1). Nevertheless, the evidence is strong that the growth of the cornea and anterior segment is largely programmed growth, while emmetropization acts through visually guided changes of scleral growth and vitreous chamber size and shape changes.
3.6 Key Operating Characteristics of Experimental Emmetropization
Understanding the functional operating characteristics of the vision-dependent mechanisms that regulate eye growth and emmetropization is critical for translating the concepts developed through animal research to human refractive development.
3.6.1 Local Retinal Mechanisms
Investigations into the neural circuits mediating emmetropization have employed reduction strategies in efforts to identify critical components in the process that transforms visual signals into molecular signals that influence eye growth. Most typically, diffusers or powered lenses have been employed to induce changes in refractive development in combination with manipulations that were intended to eliminate or isolate potential circuit components. It was assumed that if a visual system component was essential for emmetropization, then inactivating or eliminating that component should prevent or alter the effects of the optical treatment regimens on refractive development. This series of investigations, which involved multiple labs and several species of experimental animals, led to one of the most interesting discoveries related to emmetropization, specifically, that the dominant vision-dependent mechanisms that regulate eye growth and refractive development are located entirely within the eye and operate in a local, regionally selective manner.
The following experimental manipulations failed to prevent visually mediated changes in refractive error: (1) bilateral surgical removal of the striate cortices in form-deprived monkeys (i.e., resulting in perceptual blindness and eliminating a potential role for the visual cortex),145 (2) surgical transection of the optic nerve in form-deprived monkeys,145 in form-deprived and negative and positive lens–reared chicks,151,158,173,290 and in chicks recovering from FDM,291 (3) pharmacologic blockade of action potentials in retinal ganglion cells by tetrodotoxin in form-deprived tree shrews,292 and (4) sensory deafferentation by sectioning the trigeminal nerve in form-deprived monkeys.145 While many of these surgical manipulations can directly alter refractive development in isolation (e.g., optic nerve section generally produces hyperopic shifts in control eyes158,293), when these side effects are taken into account, the vision-induced changes in refractive development are comparable to those observed in lens- and diffuser-reared control animals. Consequently, these finding demonstrate that the visual signals (and other potential sensory signals) associated with form deprivation or optical defocus do not have to reach the central visual system or leave the eye for vision-dependent growth regulating mechanisms to function.
In addition, eliminating the primary parasympathetic and sympathetic neural inputs to the eye (and their associated physiological processes, such as accommodation) does not eliminate vision-induced changes in refractive development. In particular, surgically disrupting the ciliary nerves (chicks),151,293–295 the ciliary ganglion (monkeys),145 the Edinger-Westphal nucleus (chicks),187 or the superior cervical ganglion (monkeys)145 does not prevent FDM, lens-induced myopia (LIM), or lens-induced hyperopia (LIH). In addition, pharmacologic paralysis of accommodation does not prevent FDM or lens compensation in chicks296 and pharmacologic stimulation of accommodation does not prevent FDM in monkeys.145 Together these observations demonstrate that the act of accommodating, specifically ciliary muscle activity, is not essential for the visual regulation of ocular growth. Double ocular parasympathectomy (lesions of the ciliary and the pterygopalatine ganglion) affects FDM but not compensation for lens-induced defocus. This is further evidence of the existence of different mechanisms for FDM and LIM.
Overall, the results described above demonstrate that neural mechanisms in the retina can detect the presence of defocus and generate signals that alter axial growth in a manner that eliminates the optical errors. Another key feature associated with this emmetropizing process is that the underlying mechanisms operate in a local, regionally selective manner across the retina. The strongest evidence to support this idea comes from experiments in which form deprivation or optical defocus were imposed over only half of the visual field. The use of hemiretinal manipulations was pioneered by Wallman et al.,281 who first showed that hemiretinal form deprivation in chicks produced localized axial elongation and myopia that was restricted to the treated hemiretina. These findings were subsequently replicated in mammals, including primates,159,297 and extended to apply to lens compensation for imposed hemifield hyperopic and myopic defocus.188,298,299 Figure 11 shows magnetic resonance images (MRIs) of macaque eyes with full and partial visual field deprivation. Monocular full-field form deprivation increases vitreous chamber elongation in the treated eye. The increases are greatest near the optic axis and decrease with eccentricity along the horizontal meridian in a relatively symmetrical manner (i.e., the treated eye becomes more prolate in shape). As a result, the degree of FDM is greatest near the optic axis and decreases with eccentricity, again in a relatively symmetrical manner (i.e., the changes in eye shape produced central myopia and relative peripheral hyperopia). In contrast, with nasal-field form deprivation, vitreous chamber elongation is restricted to the temporal hemiretina. The horizontal MRIs reveal an obvious change in the curvature of the posterior globe of the treated eye at the border between the deprived and nondeprived hemiretinas. As with full-field form deprivation, the anterior segment of the eye was not affected by hemifield form deprivation. As a consequence, the nasal field diffusers produce myopia that was restricted to the nasal visual field.
Figure 11.
MRIs obtained in the horizontal plane for the treated (left) and control eyes (right) of rhesus macaque monkeys reared with monocular full-field form deprivation (A) and monocular nasal-field form deprivation (B) (adapted from Smith EL III. Prentice Award Lecture 2010: a case for peripheral optical treatment strategies for myopia. Optom Vis Sci. 2011;88:1029–44. Copyright © 2011 American Academy of Optometry).326 The nasal and temporal retinas are designated as N and T, respectively. In the middle panels, the outlines for the treated (red) and fellow eyes (blue) have been superimposed after rotating the fellow eye images around the optic axes so that the nasal retinas (N) are shown to the right for both eyes. The superimposed images were aligned using the lines that connected the equatorial poles of the crystalline lenses as a reference (the red lines shown in the treated- and fellow-eye images in the left and middle columns).
The fact that the vision-dependent mechanisms that dominate refractive development operate in a regionally selective fashion and can produce changes in eye shape suggests that it is unlikely that some central neural mechanisms play a primary role in refractive development. For example, it has often been speculated that the act of accommodation contributes to the development of myopia. It is difficult to imagine how the act of accommodation or mechanical changes associated with accommodation (e.g., a potential increase in IOP) could produce the regional changes in eye shape and refractive error shown in Figure 11.
The local nature of the vision-dependent emmetropization mechanisms may have evolved to optimize the eye's refractive state across the visual field (i.e., to promote optimal panoramic vision). It is likely that these local retinal mechanisms are also responsible for the relative lower-field myopia observed in many species300 and for the eccentricity-dependent changes in the pattern of refractive errors produced by rearing conditions that restrict viewing distance in a selective direction.301
3.6.2 Temporal Integration of Visual Signals
Navigating in a three-dimensional world requires the eyes to scan and fixate different points in the environment, and depending on accommodative state, the sign and magnitude of defocus that the eye experiences varies over time. How competing visual signals are integrated over time presumably determines the eye's visually regulated growth. In this regard, eye growth does not appear to be regulated by the simple time-averaged level of defocus. Instead, evidence suggests that the temporal integration properties of vision-dependent eye growth are nonlinear and normally reduce the likelihood that the eye will become myopic.302
Several nonlinear aspects of temporal integration of the visual signals for eye growth control have been identified. First, visual signals that slow ocular growth appear to have a greater effect on refractive development than signals that normally result in excessive ocular growth. For example, chick eyes exposed to successive, equal duration periods of myopic and hyperopic defocus exhibit increases in choroidal thickness, reduced axial elongation, and hyperopic shifts in refractive error.246,303–305 Even when the duration of exposure to imposed hyperopic defocus was substantially longer than that for the myopic defocus, the signals generated by myopic defocus still dominated refractive development.246,304 By themselves, short daily periods of imposed myopic defocus were also sufficient to produce hyperopic shifts in animals who experienced unrestricted vision most of the day.295 Similarly, in chicks,162,295 tree shews,306 and monkeys,307,308 brief daily periods of unrestricted vision greatly reduced the axial myopia produced by imposed hyperopic defocus or form deprivation that was maintained for the rest of the day. Interestingly, the quantitative relationship between the daily duration of unrestricted vision and the relative reduction in myopia was very similar in these three species with only 2 hours of unrestricted vision, reducing the degree of FDM or LIM by approximately 80%.308
The overall effects of both hyperopic and myopic defocus signals on refractive development depend on both the frequency and duration of daily exposure and not the total duration of exposure in a given day.246,309,310 When chicks are exposed to multiple brief periods of defocus throughout the day, with dark intervals between exposures, the compensating refractive changes were greater than those produced by a single-exposure period of the same total duration.246 Moreover, shorter duration but more frequent exposures were more effective than longer, less frequent exposures, as long as the total exposure duration was the same and the duration of each individual exposure period exceeded a critical duration.
It has been argued that these nonlinearities come about because the compensating signals produced by defocus have relatively rapid rise times to saturation levels and that these signals decay more slowly between exposures.181,302,311 In a detailed study of the rise and fall times for individual exposures, Zhu and Wallman311 found that both myopic and hyperopic defocus produced near maximal choroidal thickness changes with exposure durations on the order of 5 to 7 minutes. The decay times for the defocus-induced changes in choroidal thickness were slower than the rise times, with the time required for the signals to decay to 50% of the maximum response being approximately twice as long for myopic versus hyperopic defocus (6.7 vs. 3.2 hours). On the other hand, temporal dynamics for axial growth changes were very different for myopic and hyperopic defocus, with response decay to myopic defocus being dramatically slower and more enduring than those to hyperopic defocus. The decay for hyperopic defocus was approximately 50 times faster than that for myopic defocus. These results indicated the existence of different signals for hyperopic versus myopic defocus and for compensating axial elongation versus choroidal thickness, and the results provide an explanation for the dominating effects of myopic over hyperopic defocus.
The nonlinear temporal integration characteristics of the emmetropization process have important implications for efforts to determine the role of visual experience in the genesis of common refractive errors, such as juvenile-onset myopia. Specifically, the observed nonlinearities complicate the assessment of visual activities that may increase ocular growth and these nonlinearities may contribute to the inconsistencies related to the potential impact of near work on myopia.312 For instance, it is well-established that chronic hyperopic defocus promotes axial myopia in animal models, and it has been hypothesized that hyperopic defocus associated with underaccommodation during near work may promote the development of myopia in children,313,314 but there has been some disagreement in studies with children.315 Commonly used metrics of average near work in human subjects, such as the “diopter hour” (dioptric demand multiplied by hours spent at the near task),316 are only weakly correlated with myopia in children. These weak correlations may reflect the fact that these metrics do not take into consideration the manner in which different types of visual experience are integrated over time. Figure 12 considers the effects of providing infant monkeys reared with 3 D of imposed hyperopic defocus with four, 15-minute periods of unrestricted vision each day. With continuous lens wear, the average refractive error in animals reared with −3 D lenses is approximately 3 D more myopic than normal monkeys. Four daily 15-minute periods of unrestricted vision virtually eliminated this predictable compensation such that at the end of the treatment period, the average ametropia was not different from normal (although clearly, the pattern of refractive development in this group was different from normal). Using the control animals as a reference (i.e., 0-D hours), the monkeys that wore the −3-D lenses continuously and the lens-reared monkeys that had a total of 1 hour of unrestricted vision over the 12-hour daily lights-on cycle experienced, respectively, 36- and 33 D-hr/d of viewing conditions that would promote myopic growth. Considering the different outcomes for these two experimental groups, it is clear that diopter-hour units did not capture the critical aspects of visual experience that contributed to myopia. This is also supported by the very consistent protective effects reported for time outdoors against myopia.317,318 It likely reflects the fact that vision-dependent mechanisms that regulate refractive development are more sensitive, or more responsive, to stimuli that normally slow axial growth, making it easier to detect their influence on refractive development.
Figure 12.
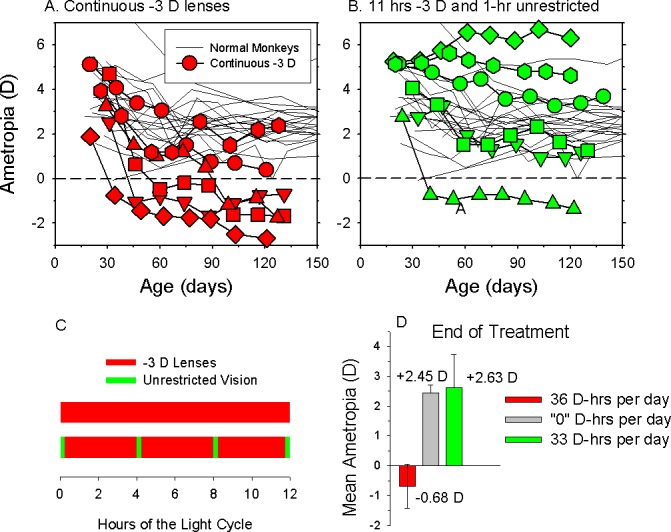
Longitudinal changes in spherical equivalent refractive errors for the right eyes of infant rhesus macaque monkeys reared with binocular −3 D lenses. The monkeys represented in panel A wore the lenses continuously throughout the daily 12-hour lights-on cycle. For the monkeys represented in panel B, the −3 D lenses were removed for four 15-minute periods during the daily 12-hour lights-on cycle. The black lines in the upper plots show data from normal infant monkeys. The schematic in the lower left (C) shows the times when these animals were allowed unrestricted vision. The lower right plot (D) compares plotted as mean end-of-treatment ametropias for normal monkeys and the two experimental groups of monkeys (adapted from Kee C-S, Hung L-F, Qiao-Grider Y, et al. Temporal constraints on experimental emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2007;48:957–962. Copyright © 2007 The Association for Research in Vision and Ophthalmology, Inc.).307
3.6.3 Effects of Simultaneous Competing Defocus Signals
Competing myopic and hyperopic defocus can occur simultaneously for superimposed objects. More importantly, virtually all current optical treatment strategies for myopia produce simultaneous competing defocus signals. In particular, multifocal lenses (especially contact lenses) and corneal reshaping therapy or orthokeratology frequently produce spatially superimposed, simultaneous competing image planes across all or a large proportion of the retina. How these visual signals, which compete to increase and decrease axial growth, are integrated determines the overall direction of refractive development and the effectiveness of any optical treatment strategy. To study the effects of simultaneous, competing defocus signals on emmetropization, chicks,319 guinea pigs,320 marmosets,321 and rhesus monkeys322,323 have been reared wearing lenses with concentric annular zones with alternating refracting powers. These dual-focus lenses established two competing image planes across the entire retina.
In chicks and guinea pigs, as illustrated in Figure 13, the compensation mechanisms of dual-focus lenses appear to direct refractive development toward either the average imposed defocus or to a refractive state slightly more hyperopic than the average. These results suggest that the vision-dependent mechanisms that regulate refractive development identify the effective sign and magnitude of defocus associated with each focal plane. They either average these signals in a linear manner (shown in guinea pigs320) or preferentially weight the image plane associated with the more positive powered lens component (shown in chicks319). This strategy is somewhat counterintuitive because the highest effective image contrasts would occur at the two secondary focal points associated with the lenses' two power zones, not at the dioptric midpoint. In marmosets reared with dual-focus contact lenses (+5-/−5-D power zones), the treated eyes developed a degree of hyperopia equivalent to that produce by +5-D single-vision lenses although, the degree of hyperopia did not completely compensate for the imposed myopic defocus.321 When the eyes of infant macaques were presented with two, approximately equally distinct focal planes, refractive development was directed toward the more myopic/less hyperopic focal plane and completely compensated for the more anterior foci.322 In all four species, the observed changes in refractive error were also associated with alterations in vitreous chamber elongation rate. There were, however, a number of methodologic issues that could explain the apparent differences between chicks, guinea pigs, and primates.322
Figure 13.
Effects of multifocal lens rearing. (A) Comparisons of the effects of dual focus, Fresnel-like lenses (50:50 area ratios) on refractive error development in rhesus macaques,322 chicks,319 marmosets,321 and guinea pigs.320 The left scale indicates the relative percentage change in ametropias at the end of treatment. For binocularly treated animals (rhesus monkeys), the ametropias for the right eyes are represented relative to that for control animals. For monocularly treated animals (all other species), the ametropias for the treated eyes are expressed relative to that of the fellow eye. Values of 0% and 100% indicate complete compensation for the most hyperopic and myopic image planes, respectively. Values of 50% indicate that the animals compensated for the average power of the dual focus treatment lenses (adapted from Arumugam B, Hung L-F, To C-H, Holden B, Smith EL III. The effects of simultaneous dual focus lenses on refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2014;55:7423–7432. Copyright © 2014 The Association for Research in Vision and Ophthalmology, Inc.).322 (B) The average ametropias for infant rhesus monkeys reared with dual focus Fresnel lenses (either +3 D and plano or −3 D and plano) plotted as a function of the percentage of surface areas that was devoted to the powered portions of the treatment lenses. Control monkeys reared with unrestricted vision are represented at the 0 point on the abscissa. Control monkeys reared with −3 and +3 D single-vision lenses are represented at the “100% −3 D” and “100% +3 D” positions, respectively. The dual-focus groups are positioned according to the proportion of lens surface areas devoted to the −3 and +3 D power zones (adapted from Arumugam B, Hung L-F, To C-H, Sankaridurg P, Smith EL III. The effects of the relative strength of simultaneous competing defocus signals on emmetropization in infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2016;57:3949–3960. Licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.).323
Dual-focus lenses complicate refractive development because both convergent and divergent rays are associated with both focal planes (i.e., both positive and negative defocus signals bracket both focal planes and to a lesser degree the dioptric midpoint between the two focal planes). The fact that the emmetropization mechanisms target the more anterior focal plane (or a point in front of the dioptric midpoint), has value from an evolutionary prospective because it reduces the likelihood that the eye will become myopic. In this respect, the eye responds to simultaneous competing defocus signals in a manner that is qualitatively similar to its responses to sequential competing defocus signals and the two focal planes associated with astigmatism (see Section 3.7.2). Moreover, this pattern of results obtained with dual-focus lenses indicates that multifocal lenses or correction strategies that impose simultaneous relative myopic defocus over a large part of the retina would be effective in slowing axial growth and reducing myopia progression in children.
In terms of managing myopia, multifocal treatment strategies have some disadvantages. In particular with dual-focus lenses both power zones typically cover a portion of the pupil producing chronic retinal image degradation. This is potentially significant because even mild degrees of image degradation can produce FDM (see Section 3.2.1). However, it is important to note that the results from all four of the animal species that have been reared with dual-focus lenses (rhesus macaque, common marmoset, guinea pig, chick) revealed no signs that the resulting reduction in image contrast produced axial growth or a myopic shift. Nevertheless, depending on a number of lens parameters, dual-focus lenses can reduce the best-corrected visual acuity relative to traditional single-vision lenses, although it may be possible to reduce the saliency of the imposed myopic defocus and, thus, the impact of the imposed defocus on vision without losing the ability to control axial growth. Manipulating the relative surface area devoted to the two power zones of a dual-focus lens can alter the relative saliency of the two-image planes without altering the dioptric interval between them. In chicks319 and guinea pigs,320 the ability of the more positive-powered component of a dual-focus lens to control refractive development is influenced by the relative surface areas of the treatment lenses that are devoted to the two power zones (i.e., the relative amount of light contributing to each image plane). Specifically, decreasing the surface area devoted to the more positive-powered lens component shifts the target for emmetropization in favor of the more negative-powered component. However, the degree of relative myopia was always less than that produced by single-vision lenses of the same negative power. In guinea pigs and marmosets, the relative effectiveness of the two power zones in controlling axial growth appeared to be linearly related to the relative surface area of the lens associated with each power component.320,324 That does not appear to be the case in rhesus monkeys,323 although there were several methodologic differences between the studies, including binocular treatment in the rhesus study, which has important implications for human treatment of myopia.
As illustrated in Figure 13, in infant macaques the surface area of a dual-focus lens devoted to the more positive-powered component can be reduced to one-fifth of a dual-focus lens' surface area without decreasing the ability of the more positive-powered component to reduce axial growth and produce relative hyperopic ametropias. Even when the saliency of the more posterior focal plane was much greater than for the more anterior focal plane, refractive development was still dominated by the relatively more myopic focal plane. From a lens-design perspective these results suggest that it may be possible to control myopia progression by imposing myopic defocus through a relatively small area of multifocal lenses, which should result in an overall improvement in central vision. In addition, this pattern of results indicates that as long as the imposed myopic defocus reaches threshold strength, the full treatment effect will prevail. If the strength of the myopic defocus signal, which is likely to be dependent on the magnitude of defocus and the amount of the lens' surface area devoted to the positive-powered component, does not reach this critical threshold, then there will be little or no treatment effects. In other words, the treatment effects will not be graded on an individual basis, but will likely follow an all-or-none rule.323
3.6.4 Spatial Integration of Visual Signals Across the Retina
The existence of vision-dependent growth-regulating mechanisms that function in a regionally selective manner has important implications for refractive development, especially in primates with a foveal retina specialized for central vision. Because the refractive state at the fovea depends on ocular changes at the posterior pole and in the periphery (e.g., tangential scleral expansion in the periphery promotes central axial elongation), peripheral visual signals could, depending on the relative ability of mechanisms across the retina to alter scleral elongation, influence eye shape and central refractive development in a manner that is independent of central vision.325,326
Little is known about the spatial integration properties of local growth regulating mechanisms. It would be valuable to know the size and effective sensitivity of the summation areas of these mechanisms and whether these properties change with eccentricity. Because cone photoreceptor density and resolution acuity are highest at the fovea, the fovea is the part of the retina that is most sensitive to optical defocus, and visual signals from the fovea largely control accommodation, it has historically been assumed that visual signals from the fovea would dominate axial growth and refractive development.327 However, several lines of evidence contradict this assumption.
If visual signals from the fovea dominated refractive development, then eliminating these signals should alter visually directed ocular growth. But this does not seem to be the case because eliminating visual signals from the fovea by laser ablation of the central 8° to 10° of the retina in infant monkeys does not alter the course of emmetropization, the development of FDM, the ability of the eye to recover from experimentally induced ametropias, or compensation for imposed hyperopic defocus.328,329 It is likely, however, that foveal signals normally influence ocular growth (possibly in proportion to the absolute number of critical cascade elements in the fovea), but these results indicate that foveal signals are not unique or essential for many aspects of vision-dependent ocular growth and that the periphery, in isolation, can detect the presence of a refractive error and alter eye growth to eliminate the error.
Moreover, when there are competing visual signals in the central versus the peripheral retina, experiments in chicks,330,331 marmosets,324 and macaques297,329 demonstrate that peripheral signals can dominate axial ocular growth and central refractive development. Figure 14 illustrates the effects of peripheral form deprivation and peripheral optical defocus on central refractive development.297,329 In all three subject groups, animals were viewing through treatment lenses with central apertures that allowed unrestricted central vision, but produced either form deprivation or optical defocus in the periphery. When viewing through these lenses, the central retina received visual signals that should have supported normal emmetropization, while the periphery experienced signals that normally result in either axial myopia (Fig. 14, panels A and B) or axial hyperopia (Fig. 14, panel C). Both peripheral form deprivation and peripheral hyperopic defocus produced central axial myopia; the range and average myopic errors were similar to those produced by full-field treatment lenses. Imposed peripheral myopic defocus slowed axial elongation producing central hyperopia and, interestingly, the degree of hyperopia was larger than that produced by full-field positive lenses. Presumably these higher degrees of hyperopia came about because, when viewing through the aperture lenses, the central retina controlled accommodation, which overcame the central compensating hyperopia while maintaining the peripheral myopic defocus (i.e., the peripheral signal to slow growth did not decrease as the eye developed central hyperopia). In contrast, with full-field positive lenses, the degree of myopic defocus in both the central and peripheral retina decreased as the eye developed compensating hyperopia. The ability of peripheral visual signals to override signals from the central retina can probably be attributed to the greater potential for spatial summation in the periphery. As suggested by Wallman and Winawer,181 although the density of many retinal neurons is highest in the central retina, the absolute numbers of neurons are higher in the periphery, simply because the peripheral retina is very large in comparison to the fovea. In addition, because of the geometry of the globe, small tangential expansions of the peripheral sclera would have a large effect on the axial position of the posterior retina.325,326
Figure 14.
The effects of imposing defocus on the peripheral retina. (A, B) Spherical equivalent refractive corrections obtained at ages corresponding to the end of the lens-rearing period for control monkeys (open diamonds) and monkeys reared with either diffusers ([A] adapted from Smith EL III, Kee C-S, Ramamirtham R, Qiao-Grider Y, Hung L-F. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–3972. Copyright © 2005 The Association for Research in Vision and Ophthalmology, Inc.)329 or −3 D lenses ([B] adapted from Smith EL III, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vis Res. 2009;49:2386–2392. Copyright © 2009 Elsevier Ltd.).822 The solid green and red symbols represent monkeys that worn treatment lenses that had central apertures that provided unrestricted vision for the central 24° to 32°. For comparison purposes, the half-filled diamonds represent monkeys that were reared with intact diffusers or −3 D lenses that altered vision across the entire field. The horizontal dashed line represents the average refractive error for the control monkeys; the solid lines denote ±1 SD from the control mean. (C) Changes in refractive error produced by rearing chicks with +5 D treatment lenses that had varying diameter central apertures that allowed unrestricted central vision (adapted from Liu Y, Wildsoet C. The effect of two-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Invest Ophthalmol Vis Sci. 2011;52:1078–1086. Copyright © 2011 Association for Research in Vision and Ophthalmology).330
Understanding the effects of peripheral vision on central refractive development is important because the eye's refractive state varies with eccentricity332–334 (i.e., the signal for ocular growth varies with eccentricity). It has been known for some time that myopic eyes, due to their relatively prolate shape, exhibit less myopia (relative peripheral hyperopia) in the periphery, but whether this is a cause or an effect of axial myopia is unclear. Studies of eye shape in form-deprived monkeys indicate that relative peripheral hyperopia can be a consequence of vision-induced axial myopia.335 Several recent studies have not found peripheral refractive state to be a useful predictor for either myopia onset or progression,336,337 suggesting it is not a major factor in myopia development. However, none of those studies have looked at refraction beyond 30° off-axis, and cannot rule out the possibility that integration of the defocus signals off-axis may be involved in the progression of myopia. The apparently weak predictive value of peripheral refraction inside of 30° for myopia onset does not exclude a role for peripheral defocus signals in the control of eye growth, which can be exploited as a treatment strategy. Experimental and clinical studies both support this approach.326,338 Whether or not peripheral refractive state is a factor in the onset, or progression, of myopia, the fact that imposed defocus in the retinal periphery can affect axial refractive state is useful for myopia control and an important consideration in optical correction strategies for myopia.
3.6.5 Age-Related Changes in Susceptibility to Visual Experience and Sensitive Periods for Myopia
In cold-blooded vertebrates, such as teleost fish, the eye continues to grow throughout their lifespan,339 and myopia can also be induced experimentally throughout the lifetime in these species.100,101 However, in warm-blooded vertebrates, the ability of visual experience to alter ocular growth and refractive state declines with age. In this respect, emmetropization can be considered to proceed in two phases. The “initial infantile phase” occurs during infancy and is characterized by a reduction in refractive error and a decrease in the variability of refractive state. As described in Section 3.1 and illustrated in Figure 15, many young animals are born with refractive errors; the emmetropization process rapidly reduces the refractive error and moves the eye toward a near emmetropic refractive state. This has been observed in humans,340,341 rhesus monkeys,14,342 marmosets,153,343 tree shrews,31 guinea pigs,42 mice,46,344 and chicks.345 As shown in Figure 15, for example, tree shrews initially have variable, hyperopic refractions that become less hyperopic as the eye grows rapidly during the initial infantile phase of emmetropization. The refractive changes during this period of rapid eye growth are known to be due, in part, to the visual regulation of eye growth, and passive optical scaling of refractive error in growing eyes.134,175 Following the infantile phase of emmetropization, there is a much longer “juvenile phase” of emmetropization, where refractions are relatively stable at or near emmetropia, while the eye is still growing. Experimental studies with animals have demonstrated that the stability of refraction during this phase is achieved by visually guided feedback, and the eye remains able to respond to imposed defocus as shown in Figure 15. Form deprivation can also produce myopia in older chickens,130,175,346 and monkeys127,128,347 even when their eyes have reached adult or near-adult size. During the juvenile phase, the rate of response and the magnitude of the changes in refractive error produced by visual experience decline with age. In addition, due to the nature of the ocular component changes produced by visual experience, the ability to compensate for positive power lenses declines much more rapidly with age.200 It is unknown, however, whether the visual control of eye growth is ever fully lost.
Figure 15.
Emmetropization and experimentally altered refractive state in tree shrew. (Top) Refraction as a function of days after eye opening (days of visual experience, or DVE). Each line is for an individual animal. Data for untreated animals and red light are shown as the average of both eyes; data for −5 D lens animals are for the treated eye only. Untreated tree shrews were raised under fluorescent colony lighting (data from Gawne et al.467), red light animals were exposed to ambient narrow-band red light stating at 95 DVE,467 and −5 D lens animals wore a monocular −5 D lens over one eye.823 Binocular +5 D lenses were worn starting at either 11 or 24 DVE.200 (Bottom) Axial length of the eyes as a function of time for the normal animals shown in the top panel.
3.7 Sign of Defocus and Nature of the Optical Signal
Animal studies have demonstrated convincingly that the fine-tuning of postnatal ocular growth to achieve and maintain emmetropia is actively controlled by visual signals related to defocus (see Sections 3.2.2 and 3.2.3 above). This regulation is primarily performed locally at the level of the retina acting on adjacent regions of sclera without much (if any) direct contribution from the central nervous system.158 The discovery that appropriate compensating growth occurs for equivalent degrees of imposed hyperopic and myopic defocus even when accommodation and all obvious behavioral cues are excluded,348 and that local bidirectional compensation occurs when the defocus is imposed over only half of the retina,188 indicates that the local retinal emmetropizing mechanisms can correctly identify the sign of defocus (i.e., whether the defocus is myopic or hyperopic). From an operational perspective, signals encoding the sign of defocus are ideal for regulating emmetropization.
However, it has been difficult to determine precisely what visual cues are used to determine the appropriate direction for the emmetropization response. In part, this is due to the fact that there are a surprisingly large number of visual cues that could potentially be used. Also, the emmetropization mechanism might use multiple visual cues, and integrate these cues in complex nonlinear ways. Understanding how the emmetropization process encodes the sign of defocus is critical for understanding the role of vision in the genesis of common refractive errors and for optimizing treatment strategies.
3.7.1 Longitudinal Chromatic Aberration
Experimental data suggest that signals derived from longitudinal chromatic aberration (LCA) provide directional cues for accommodation,349–353 and there is increasing evidence that the same is true for emmetropization. LCA occurs because refractive index varies inversely with the wavelength of light; short-wavelength blue light is refracted more strongly than long-wavelength red light. Consequently, in polychromatic lighting color fringes occur around retinal images that change with the eye's refractive state, providing a chromatic signal that can be used to identify whether defocus is hyperopic or myopic.354 Specifically, when the eye is hyperopically defocused the red components of the retinal image will be more blurred than the blue components. When the eye is myopically defocused, the blue components of the retinal image will be more blurred than the red components. LCA is robust and consistent across individuals and species, it is relatively constant as a function of eccentricity, and the magnitude of LCA in diopters is unaffected by changes in pupil size or accommodation, making it a useful signal for guiding emmetropization.
Experiments in chicks have identified several strategies involving LCA that can be used by emmetropization.352–355 These sign-detecting strategies are based on contrast signals and are potentially more robust than strategies based on simple comparisons of relative cone-excitation levels as contrast signals are independent of the color of the illuminant. Rucker and Wallman352 analyzed the impact of simulations of chromatic contrast signals on emmetropization, which were similar to those that have previously been shown to drive reflex accommodation in the appropriate direction.350,356,357 Chicks were exposed to grating patterns in which the spatial contrast of the red and blue components of a printed image of black and white sine-wave gratings (3 and 5 cyc/deg) were modified to simulate myopic and hyperopic defocus. The results showed that eyes exposed to these grating simulations produced the predicted sign-dependent growth responses. When the blue component of a black/white bar pattern was blurred, and the red component was clear, indicating the eye was too long, the rate of axial growth was reduced. Conversely, when the printed bar pattern had the red component blurred, and the blue component clear, the rate of axial growth increased.
Rucker and Wallman353 also revealed that changes in the eye's focus over time produced differences in the pattern of luminance and color contrasts (providing a temporal signal). Specifically, they showed that when the degree of hyperopic defocus decreases over time (as would occur during emmetropization), luminance contrast increases in conjunction with increases in the contrast in the M- and L-cone mechanisms. However, depending on the level of defocus, the contrast signals in the S-cones will decrease (i.e., decreasing hyperopic defocus produces an increase in luminance contrast and in the balance of chromatic contrast for the S-cone versus the M- and/or L-cone contrast mechanisms). In the case of increasing myopic defocus over time, the L- and M-cone luminance contrast signals decrease, but now the reductions in contrast for the S-cones and M- and L-cones are similar (i.e., the balance of chromatic contrast between S-cone and M- and/or L-cone components does not change over time). This analysis showed that the eye could theoretically detect the sign of defocus by detecting the presence or absence of a temporal chromatic signal across cone channels. A key feature of this idea is that as the eye grows toward emmetropia, the temporal chromatic signal will diminish until a point is reached when the contrast for all three cone types is approximately equal. Growth beyond this point would result in diminished luminance contrast of the retinal image without change in color contrast. Most importantly, flickering stimuli that simulate these two different scenarios produce predictable changes in ocular growth in young chicks.353 Specifically, to test this hypothesis, chicks were exposed to light that was modulated to produce changes in color or luminance contrast.353 The red, green, and blue components of a light-emitting diode (LED) were modulated in-phase to produce changes in luminance and in counterphase to produce red/green or blue/yellow changes in color. This experiment was performed at 2 Hz, in the middle of the range of temporal sensitivity of the chick,358 and with close to 100% contrast. The results showed that after 3 days of exposure to these lighting conditions, luminance flicker produced hyperopic shifts in refraction, while color flicker produced myopic shifts in refraction. These results support the hypothesis that the eye can use temporal signals associated with LCA for emmetropization.
Luminance contrast modulation alone could signal when the eye is in focus because in most natural scenes an in-focus image would produce high temporal frequency luminance modulations, while blurred retinal images would produce low-temporal frequency luminance modulations.359,360 To test this idea, Rucker et al.361 exposed chicks to LEDs that produced 80%, white-light luminance modulation. Eye growth was reduced at 10 compared with 0.2 Hz. The experiment was repeated in yellow light (to simulate a “warm white” indoor illuminant). In this case, chick eyes exposed to 5- and 10-Hz stimuli grew less, as in white light, while chick eyes that were exposed to lower temporal frequencies (0.2, 1, and 2 Hz) grew more. These results suggest that the eye responds to rapid changes in luminance contrast by slowing growth, regardless of the color of the light. High temporal frequency stimulation indicates that the eye is in focus, halting growth. The results also indicated that yellow light promotes increased eye growth at low frequency temporal stimulation, when sensitivity to luminance modulation is reduced. At low temporal frequencies the eye seems to be able to detect the myopic wavelength defocus of the blue component of a white light source, thus reducing eye growth.
Supporting the hypothesis that contrast is a critical variable underlying the effects of luminance modulation on eye growth, Rucker et al.361 found that absolute temporal contrast had a significant influence on the ability of temporally modulated stimuli to alter eye growth. At high contrast (>70%), high-temporal frequency stimulation slowed eye growth, but at lower contrast levels eye growth increased regardless of the temporal frequency or color. In other words, high-temporal contrasts, arising from an in-focus retinal image, are necessary for luminance modulation to slow eye growth. Other viewing conditions that induce high-contrast stimulation of the retina, such as high frequency, high contrast, stroboscopic, or sinusoidal flicker reduce eye growth in the chick.137,310,353,359 In fact, experiments overwhelmingly show that high temporal137,310,359,361,362 and spatial contrasts157,170,363–366 are required for the eye to slow its growth and prevent myopia.
Nevertheless, there is currently no consensus as to exactly how LCA cues are used for emmetropization, and indeed experiments using different wavelengths of light in different species have reported different results that are difficult to reconcile (see Section 4.2). Still, it is clear from many experiments across several species that changing the visible wavelength content of the environment can have significant effects on eye growth and refractive state, and it seems likely that chromatic cues are important for emmetropization.
3.7.2 Higher-Order Monochromatic Aberrations
While spherical optical power and astigmatism (see Section 3.7.3) dominate the optical characteristics of the eye, the optical quality of the retinal image is influenced by a number of higher-order monochromatic aberrations (HOAs), which are related to the shape and configuration of the eye's optical components. All eyes have HOAs; however, there are large interindividual differences in the magnitude and specific characteristics of HOAs. Spherical aberration, coma, and trefoil are the most commonly studied individual HOAs in visual optics. The overall effect of all HOAs taken together is often considered for an optical system. Animal studies have provided important insights into the changes in HOAs that take place during emmetropization and during or the development of vision-induced refractive errors, the potential role for vision in improving the eye's aberrations, and the potential role of HOAs in the genesis of myopia.
During the rapid postnatal infantile phase of ocular growth and emmetropization, there are substantial changes in the eye's optical and axial components that could influence the type and magnitude of HOAs. In particular, changes in the curvature of the cornea and lens and in the refractive index and thickness of the lens not only influence the eye's refractive status, but also alter the characteristics and magnitude of HOAs. Because HOAs influence retinal image quality and thus potentially the set-point and efficacy of emmetropization, it is important to understand the developmental changes that take place in HOAs. Cross-sectional studies show that, on average, HOAs are 20% to 50% greater in children than in adults.367,368 Longitudinal studies in chicks,369–372 marmosets,373 and rhesus monkeys374 have confirmed that HOAs are greater in neonates and decrease in magnitude in a monotonic fashion during emmetropization. Although eye growth models can account for age-dependent improvements, the observed improvements in HOAs appear to exceed predictions based on a geometric increase in the overall scale of the eye associated with the normal increases in axial length. In each of these species the resulting optical quality of adult eyes is nearly diffraction limited. In infant monkeys, age-dependent improvement in the modulation transfer function associated with this decrease in HOAs play a limited role in the improvement in the spatial contrast sensitivity of infant monkeys; HOAs have a much smaller impact on behavioral performance than spherical and astigmatic defocus.374
When results for different species are calculated for constant numeric apertures, the magnitude of HOAs in young animals is similar in chicks, marmosets, and rhesus monkeys.374 However, the characteristics of HOAs in these species are different, presumably reflecting interspecies differences in eye shape. For example, whereas the majority of humans and infant rhesus monkeys exhibit positive spherical aberration, marmosets exhibit negative spherical aberration and young chicks exhibit little or no spherical aberration.
Several observations suggest that there is a link between myopia and HOAs. Many,375–378 but not all studies,379,380 have reported that myopic humans have higher HOAs than nonmyopes. Because emmetropization is a vision-dependent process, it has been hypothesized that HOAs could promote the development of myopia in several ways. First, the chronic blur associated with HOAs could degrade the retinal image sufficiently to produce FDM.375,377,378 It is well established that chronic retinal image degradation promotes myopia in a graded manner. Even though retinal image degradation due to HOAs is usually small, the magnitude of HOAs is relatively constant over time, which increases the likelihood that a myopiagenic stimulus could produce axial elongation. HOAs could, by interacting with the eye's spherical ametropia, also alter the effective end point of emmetropization,381 and by increasing the eye's depth of focus, HOAs could result in greater variability in refractive errors.382
With respect to the relationship between refractive errors and HOAs, studies in chicks,369–372 marmosets,373 and rhesus monkeys,383 have demonstrated that viewing conditions that promote myopic growth, both form deprivation and optically imposed hyperopic defocus, also promote the development of larger than normal amounts of HOAs. The pattern of HOAs in ametropic eyes varies some between species; whereas ametropic rhesus macaque eyes showed larger amounts of positive spherical aberration and chicks and marmosets showed more negative spherical aberration. The alterations in HOAs observed in rhesus monkeys374 with experimentally induced ametropia were comparable to those observed in myopic humans.376,377
The ocular changes responsible for the elevated levels of HOAs in eyes with experimentally induced ametropias are not well understood. Priolo et al.384 found larger than normal amounts of spherical aberration in the isolated crystalline lenses from chick eyes with FDM, which were attributed to changes in refractive indices of the lens. However, many of the observed changes in HOAs probably reflect changes in the shapes and relative positions of the eye's optical components. While vision-induced spherical refractive errors are primarily the result of alterations in vitreous chamber elongation rate, the expansion of the globe is not symmetric in either humans or monkeys. In particular, nasotemporal asymmetries are common in myopic eyes and could affect the shape of the crystalline lens or its alignment with respect to the cornea.335 Changes in lens alignment and tilt could explain the alterations in coma and trefoil observed in ametropic monkeys.383
Several observations in animals with experimentally induced refractive errors suggest that changes in HOAs are a consequence rather than a cause of myopia. In rhesus monkeys, increased HOAs were found in both myopic and hyperopic monkeys and the patterns of HOAs were similar to those described in human ametropias.383 Every monkey eye that had elevated HOAs also had significant spherical and/or astigmatic refractive errors and the amount of HOAs were positively correlated with degree of axial ametropia (both myopic and hyperopic). Elevated HOAs did not prevent recovery from experimentally induced refractive errors, indicating that higher levels of HOAs do not prevent the eye from responding to the defocus signal.176,198 This is probably not surprising because when expressed in terms of equivalent spherical defocus, the magnitudes of HOAs observed in ametropic monkeys represent increases in defocus of less than 0.17 D.383
There is little support for the hypothesis that the age-dependent reductions in HOAs observed during postnatal emmetropization are mediated by vision-dependent mechanisms. In chicks and primates with experimentally induced refractive errors there were concomitant decreases in the total HOAs over the treatment period.370,372,373,383 Although these reductions in HOAs were smaller than those observed in untreated eyes, it is clear that a significant part of the early decrease in HOAs occurs passively and is independent of the visual experience. In this respect, there are also numerous examples of treated marmoset373 and rhesus macaque eyes383 that experienced substantial defocus or form deprivation, but showed HOA patterns that did not differ from controls. So, if there are vision-dependent mechanisms that optimize HOAs, they can function normally in the presence of highly degraded retinal images. It is more likely that the decrease in HOAs associated with emmetropization or in experimentally treated animals over time reflect passive changes associated with growth, such as those described by Artal et al.385
3.7.3 Astigmatism
Astigmatism is a type of refractive error that results from irregular curvature of the cornea or lens, or from the way the optics of these elements are combined. In children, the prevalence and degree of astigmatism is high during early infancy and generally decreases to adult levels before school age.386–388 However, astigmatism is frequently associated with spherical ametropias; both children and adults with high amounts of myopia or hyperopia also frequently exhibit high amounts of astigmatism.389–392 While studies of astigmatism in animals have been somewhat limited (for a review see Kee393), the results do provide insight into the causes of astigmatism and the question of whether astigmatism interferes with emmetropization.
As in humans, studies with chicks show the magnitude of astigmatism is higher at birth/hatching and decreases with age.394–396 The magnitude and axis of astigmatism found in chicks varies between studies (possibly reflecting strain differences), with Schmid and Wildoset395 reporting the largest astigmatic errors of approximately 8 D at hatching. The amount of refractive astigmatism found in chicks and the observed decrease with age are correlated with changes in the direction and magnitude of corneal astigmatism.395,396 Significant astigmatism is much less prevalent in infant macaques but, as in chicks and humans, when it exists it is primarily due to corneal toricity.397 In chicks, the fact that visual manipulations that enhanced corneal growth resulted in less astigmatism, but those that inhibited corneal development produced more, suggests astigmatism early in life is linked to anterior chamber development.395
Studies involving chicks and monkeys investigated the possibility that astigmatism is regulated in a vision-dependent manner like emmetropization. There is some evidence chicks can compensate for imposed astigmatic errors. Irving et al.398 and Chu and Kee399 found partial compensation for astigmatism in chicks reared with cylinder lenses. The magnitude of compensation varied with the axis of the cylinder lenses (and possibly the power); however, there was disagreement between these studies in terms of the axis of the cylinder lenses that produced the largest compensating changes. The compensating astigmatic errors were attributed, in part, to alterations in corneal toricity, but Chu and Kee399 also reported significant correlations with a variety of eye-shape parameters. On the other hand, Schmid and Wildsoet395 found no evidence that chicks were able to compensate for imposed astigmatic focusing errors. Rearing rhesus macaques with cylinder lenses can produce significant amounts of astigmatism that is corneal in nature. However, regardless of the axis of the imposed astigmatism, the axis of the ocular astigmatism in monkeys was always oblique and in most cases was not in the appropriate direction to compensate for the imposed error and in some cases actually compounded the imposed astigmatic errors.400 Thus, while visual experience can alter corneal shape and produce astigmatic errors in monkeys, there is little evidence for a visually guided mechanism that minimizes astigmatic errors.
Can the presence of astigmatism influence emmetropization? It has been hypothesized that astigmatism could disrupt emmetropization in a manner analogous to form deprivation.391,401 Like form deprivation, astigmatism degrades the retinal image and cannot be eliminated by either changing viewing distances or via accommodation. Studies in both chicks and monkeys do indicate that astigmatism can alter the course of emmetropization; however, there is little evidence that astigmatism promotes myopia. In chicks reared with optically imposed astigmatism, spherical emmetropization appears to target either the circle of least confusion,398 a point slightly in front of the dioptric midpoint,402 or the more myopic principal meridian.395 In macaque monkeys reared with optically imposed astigmatism, regardless of the axis, emmetropization was not directed toward the circle of least confusion, but toward one of two focal planes associated with the astigmatic principal meridians, most commonly the more anterior focal plane (i.e., astigmatism usually promoted relative hyperopic shifts).403 The pattern of results in monkeys suggests that the emmetropization process is insensitive to stimulus orientation and was targeting the image planes that contained the maximum effective contrasts.
There is evidence from animal models that visual manipulations that produce axial hyperopia or myopia also produce significant astigmatic errors. For example, in chicks, lens compensation to either positive or negative lenses is frequently accompanied by astigmatism.194,370,396 The astigmatism is due to changes in corneal toricity. The axis has been reported to be either predominately against-the-rule396 or oblique,370 and the magnitude of astigmatism is significantly correlated with the degree of spherical ametropia.396 In rhesus monkeys, astigmatism has also been observed to accompany FDM and both lens-induced hyperopia and myopia. These astigmatic errors, which were more frequently associated with large ametropias, especially high hyperopia, were corneal in nature, oblique in axis, bilaterally mirror-symmetric in binocularly lens-reared animals, and reversible.286 The results from both chicks and macaques suggest that these induced astigmatic errors are the passive consequence of altered axial growth, possibly as a result of vision-dependent changes in the shape of the globe that take place during axial elongation.393 The association of astigmatism with spherical ametropias observed in humans could reflect a similar process.
4. Effects of Ocular Circadian Rhythms and Light on Eye Growth and Myopia
Experimental data suggest that the emmetropization process is influenced by the lighting parameters in which animals are reared. Specifically, the duration,95 rhythmicity,404 spectral composition,354 and intensity of the ambient lighting405–407 can alter ocular growth and refractive development. Each of these areas have been comprehensively reviewed recently,354,404–407 and some key points are addressed below.
4.1 Diurnal Light Cycles and Ocular Circadian Rhythms
Raising chicks in constant light or constant darkness cause excessive ocular elongation and flattening of the cornea, which combine to alter refractive state.95,99,137,173,408–415 These findings were the impetus for the working hypothesis that the diurnal Zeitgeber (time-giver) of light and darkness influences emmetropization, and that altering this Zeitgeber leads to changes in eye growth that produce ametropias. Weiss and Schaeffel416 found that eyes of chicks grew in a rhythmic manner, elongating more during the day than at night. Notably, however, the increased eye growth associated with the FDM was a result of an increase in eye growth at night only, which was, in essence, a change in the diurnal rhythm in ocular elongation, suggesting that form deprivation influenced the Zeitgeber in a manner similar to that of constant light or dark in chicks.408,411,412
Subsequent studies in chicks characterized the rhythms in ocular dimensions in greater detail. The use of more frequent ultrasound measurements at 6-hour intervals enabled the resolution of the acrophase (peak) and shape of the rhythm in axial length; it peaked in the afternoon and oscillated sinusoidally with a period of approximately 24 hours.417,418 In addition, the better resolution afforded by higher frequency ultrasound417,418 and noncontact laser interferometry346 allowed the discovery of a 24-hour sinusoidal rhythm in choroidal thickness, which had an acrophase at approximately midnight, in approximate antiphase (9 hours apart) to the rhythm in axial length. This oscillation in choroidal thickness accounted for at least part of the eye “shrinkage” reported by Weiss and Schaeffel.416 Both of these rhythms persisted in constant darkness for at least three cycles, defining them as endogenous rhythms that are driven by an internal clock.419 Perhaps more importantly, while it was true that the deprived eyes were indeed growing faster at night as reported earlier,416 the rhythm in axial length was, in fact, not abolished, but rather phase-shifted several hours, bringing this rhythm into exact antiphase with the rhythm in choroidal thickness.417,418 The apparent discrepancy between the studies is explained by the different frequencies of measurement (6 vs. 12 hours) and selection of sample times used. Notably, a similar antiphase relationship was found in the rhythms in fast-growing eyes responding to negative lens–induced hyperopic defocus.417
The similarities between the phase-shifts in the rhythms in both FDM and LIM suggested that alterations in growth rates might be causally related to altered phases. Further support for this idea was the finding that the two rhythms shifted into phase with one another in eyes growing slower than normal in response to imposed myopic defocus. In fact, there was a significant positive correlation between growth rate and the phase difference between the two rhythms.420 However, recent evidence has weakened this hypothesis.421 Rhythms in axial length and choroidal thickness have been found in all animal models examined, including primates.422 Similar to chicks, the two rhythms in eyes of juvenile marmosets were in approximate antiphase, with the axial rhythm peaking in the day and the choroidal thickness rhythm peaking at night. However, in older marmosets, the acrophase of the axial length rhythm was at night instead of day, resulting in the two rhythms being in phase. This difference between the two age groups is possibly related to the differing ocular growth rates, because in slow-growing chick eyes responding to myopic defocus, the rhythms were in-phase, similar to that of the older, slower-growing marmoset eyes. Finally, by virtue of the development of noncontact techniques, such as OCT and interferometry, both of these rhythms have been documented in humans, with the axial length423–426 and choroidal thickness rhythms showing approximate antiphase relationships.423 In this regard, however, there are important species differences between the circadian systems of birds and mammals that should be considered (see Section 4.1.3).
4.1.1 Scleral Rhythms in Proteoglycan Synthesis in Chicks
Because eye growth (and axial length) in chicks is determined by the rate of synthesis of scleral ECM proteoglycans,252,266,279,280 a rhythm in scleral matrix synthesis might underlie the rhythm in axial length. Two different studies addressed this, using scleral explant cultures to measure the incorporation of radiolabeled sulfur into scleral glycosaminoglycans as an index of scleral growth. When scleras from control (untreated) eyes were dissected at different times of the day, scleral proteoglycan synthesis was found to be highest in the late night to early morning compared with afternoon or night.427,428 Using an automated perifusion culture system, it was found that scleras from control eyes exhibited an endogenous diurnal (24 hour) rhythm in proteoglycan synthesis that persisted for 3 days. However, scleras from FDM eyes showed the major frequency component at 1.875 cyc/d, with a secondary component at the diurnal (1 cyc/d) frequency. Because the phase was strongly reset by the culture conditions, this precluded a determination of possible phase differences between eyes growing at different rates. Size fractionation showed the secreted molecule to be chondroitin-6-sulfate. Together, these results suggest that an endogenous circadian rhythm in scleral ECM synthesis underlies the oscillations in eye length. It is possible that the ultradian oscillations in myopic scleras play a role in the higher rates of eye growth.
4.1.2 Rhythms in IOP and Their Relationship to Changes in the Rhythm in Axial Length
IOP shows diurnal oscillations in all species studied, but the phases and amplitudes differ between them. In rabbits, IOP is lower during the day than at night, and this rhythm continues in constant darkness.429–431 The rhythms in rats432,433 and mice434 are similar to that of rabbits, increasing during the course of the day, and remaining high at night. By contrast, guinea pigs41 and monkeys,435 show a peak in the early morning and decrease over the course of the day. In chicks, the IOP rhythm is sinusoidal, with an acrophase at midday, and persists for several cycles in constant darkness, defining it as an endogenous circadian rhythm.418 The acrophase and sinusoidal shape of the rhythm in IOP is similar to that of axial length, suggesting that the rhythm in IOP may influence the axial rhythm by mechanically inflating and deflating the eye. In support of this, ocular compliance (change in length per change in millimeter of mercury pressure) was consistent with IOP fluctuations accounting for the amplitude of the rhythm in axial length (8 μm/mm Hg × 8 mm Hg = 64 μm). However, the IOP rhythms in form-deprived eyes became desynchronized from the light/dark cycle, exhibiting variable acrophases (secondary arrhythmicity),418 yet there was no change in the acrophases of the axial length rhythms, weakening a role for IOP a principal driver of the changes eye size. Similarly, sympathectomy (lesioning the superior cervical ganglion) significantly reduced the amplitude of the rhythm in IOP but had no effect on the parameters of the rhythm in axial length,436 further weakening the idea of an inflationary role for IOP in driving the diurnal fluctuations in axial length. It is possible, however, that the changing forces exerted on the sclera by the changes in IOP lead to alternations in scleral matrix (proteoglycan) synthesis,428 because the application of mechanical force changes the synthesis of ECM molecules in connective tissues.437,438 It is also possible that the IOP rhythm has varying effects on scleral ECM production depending on the phase of cell cycle, which could, in turn, influence scleral compliance and hence eye size.
4.1.3 Species Differences in Circadian Rhythm Systems and the Response to Constant Light
Exposure to constant light suppresses form-deprivation and lens-induced myopia in chicks, but is dependent on the intensity of light.414,415,439,440 Rearing of chicks under high-intensity constant light (1000–3000 lux) resulted in the complete suppression of FDM,439,440 whereas low-intensity constant light (70–140 lux) resulted in only a slight reduction in FDM.414 The effect of constant light or constant dark on chick eye refractions is hyperopia due to corneal flattening.173,410,441,442 The effects of constant light appear to be unique to chicks, however, because constant light of comparable intensities (230–640 lux) did not have any significant effect on the refractive development in infant rhesus monkeys.443,444 Mice reared in constant light did not develop hyperopia or flattening of the cornea.54 Moreover, rearing mice in constant light did not alter eye growth or refractive state, alter emmetropization, or suppress FDM.54 These species differences in response to constant light may result from important physiological differences between avian and mammalian circadian systems.91–94
The circadian system in all vertebrates is comprised of the following three main components: retina, suprachiasmatic nucleus (SCN), and pineal gland.445–447 In mammals, the SCN plays the role of master circadian pacemaker, which controls circadian rhythms and production of melatonin by the pineal gland. The SCN circadian clock is entrained by the visual input from the retina.446,448,449 Organization of the avian circadian system differs from that in mammals in several ways.91,92,447 The most important difference is that the pineal gland in birds can function autonomously because it contains light-sensitive photoreceptors and an endogenous circadian clock that serves as an extraocular circadian regulator.92 Production of melatonin by the pineal gland in birds can be controlled directly by the endogenous pineal gland pacemaker that can be entrained and activated by the environmental light in the absence of retinal input.450–452 The anterior chamber depth and corneal radius of curvature in the chick appear to be controlled by the pineal gland–derived melatonin98 and is apparently vision-independent, because eliminating retinal input does not prevent characteristic ocular changes in the chicks reared in constant light.96,97 It is known that the pineal gland–mediated extraocular mechanism is critical for the regulation of the anterior chamber growth in chicks because hoods, which shield the pineal gland from extraocular light, prevent the anterior segment changes in chicks reared in constant light.98 Conversely, extraocular light does not influence plasma melatonin levels or entrain the circadian clock in mammals.93,94,449,453–455
4.2 Spectral Composition of Ambient Lighting
Experimental evidence from multiple species supports the hypothesis that the spectral composition of light can affect the growth and refractive development of the eye and is an important element in emmetropization. There are two broadly different aspects to this topic and how it might be involved in emmetropization. First, the role of shifts in the broad-band spectrum of ambient lighting, such as the differences between daylight and indoor lighting or the differences produced by filtering out a small part of the visible spectrum, is unclear. There has been little research in this area, but it has potential for important application for human myopia development. For example, it has been proposed that a lack of UV light (wavelength <400 nm) may be a factor in myopia development in humans.456 This idea is controversial; however, because the ocular media of humans is almost completely opaque to light at these wavelengths.457 Nevertheless, recent experimental evidence suggests that blue light protects against experimental myopia in chicks,361,458 and short-wavelength cone sensitivity has recently been reported to be reduced in human myopes.459 Second, many experiments have used narrow-band (‘quasimonochromatic') lighting to probe the importance of chromatic signals for emmetropization. Considerably more research has been done in this area.
The spectral composition of ambient lighting can potentially influence the operation of the defocus-driven emmetropization cascade in a variety of ways. First, as described in Section 3.7.1, experimental evidence suggests that the emmetropization process can use chromatic cues from LCA to encode the sign of defocus and to regulate appropriate compensating eye growth.352–355 However, while the chromatic signal from LCA is independent of the spectral composition of ambient lighting (although broad-band light is required), it is reasonable to expect that the spectral composition of ambient light would influence emmetropization through the detection of specific wavelength defocus in order to maximize luminance contrast.
Research involving several species supports the idea that the spectral composition of light influences normal eye growth and the development of refractive state. Several experimental species including fish,100 chicks,169,351,460–462 guinea pigs,463–466 tree shrews,467 and rhesus macaques,468–470 were exposed to either relatively short- or long-wavelength, quasimonochromatic lighting. Although there were differences between studies in the intensity and spectral characteristics of the light, as well as the age of onset and duration of the rearing period, all but one of these studies reported significant changes in normal refractive development for at least one of their quasimonochromatic lighting regimens. The Rohrer et al.460 study involving chicks, which employed wavelengths from the extreme ends of the spectrum (near UV <420 nm and deep red >650 nm) at low lighting levels, was the only study that did not find any significant alterations in emmetropization. The negative results in this particular study may reflect the fact that the spatial resolution of UV photoreceptors in chicks may be too low to mediate emmetropization and that chicks are unable to detect the sign of defocus in near UV at low-light levels.460,471
Considering that spectrally narrow-band lighting greatly reduces the potential chromatic signals associated with LCA, changes in refractive development observed in animals reared under quasimonochromatic lighting support the idea that the emmetropization process uses wavelength-specific defocus signals, in addition to chromatic signals from LCA, to guide ocular growth.352,354,355 If the eye uses myopic wavelength defocus arising from short-wavelength light to guide emmetropization, reduced growth with outdoor activity would be expected because of the enhanced blue component of outdoor light. In fish,100 chicks,351,460–462 and guinea pigs463–466 short-wavelength lighting consistently produced relative hyperopic shifts in refraction while long-wavelength lighting produced relative myopic shifts. This pattern of results was found over a wide range of lighting intensities and treatment durations. In studies that employed short-duration treatments (<30 days),351,355,465 the magnitude of the changes in refractive error were comparable to the amount of LCA associated with the peak wavelengths of the ambient lighting. This quantitative agreement suggests that under quasimonochromatic lighting, the emmetropization process alters growth to maximize luminance contrast associated with the different focal points associated with LCA, and that chromatic signals are not essential for normal emmetropization. With longer observation periods, however, the wavelength-dependent shifts in refractive error continued to increase beyond predictions based solely on the LCA.461,463,466 A recent study in tree shrews demonstrated that narrow-band blue light produced neither hyperopia nor myopia, but disrupted emmetropization resulting in instability in refractive development.472
In contrast to the pattern of results observed in chicks and guinea pigs, narrow-band lighting in tree shrews and monkeys either failed to alter emmetropization or induced refractive error changes that were in the opposite direction. For example, rearing tree shews under short-wavelength lighting resulted in axial myopia, and long-wavelength lighting consistently produced axial hyperopia.467,473 In two subsequent studies in rhesus macaques, monkeys raised with long-wavelength pass filters468 or under narrow-band, long-wavelength lighting470 for extended periods consistently produced axial hyperopia. In a different study using macaques, two of nine monkeys became myopic in red light,469 but there was substantial individual variability in refractive development in their long wavelength–reared animals and there were no significant alterations in vitreous chamber depth. In general, the refractive changes in tree shrews and monkeys were, like those in chicks and guinea pigs treated for long durations, progressive in nature suggesting that in many species quasimonochromatic lighting produces anomalous direction cues and disrupts emmetropization.
Interestingly, the spectral composition of ambient lighting could also affect ocular component changes that underlie vision-induced changes in refractive error.355,361 For example, in chicks reared under dim short-wavelength ambient lighting that preferentially stimulates the short-wavelength and UV-sensitive cones, compensation for imposed defocus by lenses is associated with changes in overall eye length but without accompanying changes in choroidal thickness.355 On the other hand, in chicks raised under dim long-wavelength ambient lighting, that selectively stimulates the long-wavelength and double cones, lens compensation is mediated by changes in choroidal thickness with little change in overall eye length.355 These results suggest that the two compensating ocular responses are regulated by different cones types and potentially influenced differently by the spectral composition of the ambient light.
The effects of narrow-band ambient lighting on lens compensation also vary between species. Although more complete compensating ocular growth occurs in broad-spectrum white light,355 young chicks typically exhibit lens compensation for either imposed myopic and hyperopic defocus when reared under narrow-band lighting,169,294,351,355 although chicks reared under UV lighting were reported to fail to exhibit lens compensation460 except at higher light intensities.471 The results from tree shrews are qualitatively similar to those from chicks. Specifically, when reared with monocular hyperopic defocus under red ambient lighting, tree shrews, which are largely insensitive to red light,467 develop relative myopic anisometropias.474 However, both the treated and fellow eyes of these tree shrews exhibited hyperopic shifts. In contrast, compensation to imposed hyperopic defocus was not prevented in guinea pigs reared under short-wavelength lighting, while long-wavelength lighting suppressed compensation for myopic and hyperopic defocus.463 Both the treated and fellow eyes of guinea pigs developed axial hyperopia in short-wavelength lighting and axial myopia in long-wavelength light when light was restricted to 600 nm, a wavelength to which the guinea pig retina is fairly insensitive.475 The results obtained in infant rhesus macaques reared with imposed defocus under narrow-wavelength lighting appear to be qualitatively different from the other species studied to date.470 Infant rhesus macaques reared with monocular diffusers or negative lenses and exposed to long-wavelength lighting did not develop relative myopia in their treated eyes as expected. Instead, these animals exhibited hyperopic shifts in both their treated and fellow eyes. Rhesus monkeys reared with monocular-imposed myopic defocus from positive lenses also showed relative hyperopic shifts in both eyes, but in addition they consistently developed compensating hyperopic anisometropias. Thus, in monkeys prolonged exposure to long-wavelength lighting effectively blocks defocus-induced myopia (and FDM), but not defocus-induced hyperopia.
Based on the available experimental data, there is no obvious explanation for the different responses in different species to monochromatic conditions. By removing wavelength cues, the emmetropization control system may use other visual stimuli, which may vary idiosyncratically with different species and experimental designs. This might explain some of the apparent inconsistencies observed between studies. Species differences in the cone types and wavelength sensitivities (see Table 1) may be part of the explanation and must be considered when interpreting the use of specific wavelengths experimentally as color signals. Color vision in primates is thought to have evolved differently from most eutherian mammals, and very differently from that of birds.476 In particular, primates are the only eutherian mammals that have evolved a third cone photopigment and, possibly more importantly, a midget cell system in the retina that supports an antagonistic combination of inputs from these unique M- and L-cones.476 Therefore, the emmetropization process may have developed ways to use chromatic cues differently in different species, which might help explain why alterations in the spectral composition of ambient lighting produced qualitatively different results in rhesus macaques468,470 (but see Ref. 463) with three cone types, versus guinea pigs463 with two cone types and dichromacy, or chicks351 with four types and tetrachromacy. Tree shrews are dichromats with S- and M-cones, and thought to be closely related to primates. In this respect, emmetropization mechanisms in tree shrews may have evolved in a manner more similar to that in primates. Other factors that could contribute to the different experimental results in different species are the effects of different wavelength light on circadian rhythms,465 hormone production and release,477 or any number of other unknown physiological effects.
4.3 Ambient Light Intensity
A topic of significant recent interest is the role of light intensity in the regulation of ocular growth. This has been driven by epidemiologic reports showing that time spent outdoors is protective against the development of myopia in children (for a review see Ref. 478), which has recently been supported by the positive findings from two separate clinical trials.479,480
In chicks, emmetropization is sensitive to illuminance, with the mean refraction of animals shifting more hyperopic when reared under brighter light levels. Specifically, Cohen et al.481 demonstrated that raising chicks under diurnal bright light (10,000 lux) maintains animals in a hyperopic state (+1.1 D) relative to that seen under control indoor light levels (500 lux, +0.03 D). In contrast, animals kept under low light (50 lux) show a myopic shift (−2.41 D), an observation also reported by earlier studies.482
Light levels have also been shown to significantly alter the development of experimental myopia (for reviews see Refs. 405, 406). Exposing chicks to 6 hours of bright indoor light per day significantly reduced the development of FDM compared with FDM seen under control indoor lighting.483 Recent studies in chicks also showed a strong negative logarithmic correlation (R2 = 0.95) between the development of FDM and the intensity of light exposure; FDM was almost completely abolished under illuminance of 40,000 lux.484 Additionally, high-light intensity not only prevented the onset of FDM, it also halted further progression in already myopic eyes.484 Raising chicks outdoors also provides a small, transient reduction in the development of myopia in response to continuous diffuser wear.485
Similar protection against the development of FDM has been observed in rhesus monkeys,338 tree shrews407 and mice.486 In species other than chicks, high light not only protects animals from the development of myopia, it also induces a relative hyperopic shift in the untreated eyes.407,483,487,488 In chicks, 5 hours of elevated light each day prevented the development of FDM, but 2 hours was ineffective suggesting a threshold effect.489,490 The effectiveness of bright light may also be influenced by the time of day of the exposure. Nickla et al.421 reported greater reductions of growth in response to myopic defocus if light was applied in the afternoon or evening, and found less efficacy if applied in the morning.
In chicks,487 tree shrews,406 and guinea pigs,491 elevated light exposure for periods between 5 and 7.5 hr/d, also showed the response to imposed hyperopic defocus by negative lens wear. In each of these species, compensatory growth to the imposed hyperopic defocus still occurred under higher light levels, but at a significantly slower rate to that under normal laboratory lighting levels. In chicks, compensation to −10-D lenses occurred within 5 days under normal laboratory light (500 lux). In contrast, compensation is delayed by 24 hours in chicks exposed to 15,000 lux for 5 hr/d.487 However, the rate of compensation to monocular −3-D lenses in infant macaque monkeys is unaffected by daily exposure to 25,000 lux for 6 hr/d, compared with control light conditions.492
Unlike FDM, elevated light levels do not prevent the development of LIM, but rather reduce the rate of progression, with full compensation still occurring. This suggests possible mechanistic differences between FDM and LIM.493–495 This may be related to the fact that FDM is an ‘open-loop' condition without a feedback signal, whereas LIM is a ‘closed-loop' condition in which the visual feedback related to the imposed defocus guides growth for emmetropization while the lens is in place. Therefore, although there are many similarities in the biological pathways and structural changes observed in response to FDM and LIM, the differential effect of light also illustrates possible differences in the underlying visual conditions or mechanisms.496 It will be important to evaluate the role of pupil size in the LIM experiments.
Several mechanisms have been suggested to explain bright-light effects on eye growth, including chromatic cues, UV-induced changes in vitamin D levels, increased physical activity, faster local retinal luminance changes, pupil responses, greater depth of focus, and higher effective contrast.405,407,483,484,487 A role for light-induced changes in dopamine (DA) release, as discussed in Section 5.1.1, has been implicated and supported by several studies in a variety of species suggesting that retinal DA and dopaminergic pathways are involved in experimental changes in ocular growth.474,497–509
The hypothesis that light-induced increases in retinal DA levels may underlie the protective effects of time outdoors, first postulated by Rose et al.,318 is, in part, supported by findings from animal studies. Both animal and human studies support the hypothesis that reduced exposure to light with reduced outdoor activity may be part of the explanation for the increase in myopia prevalence worldwide.1
5. Biochemistry of Emmetropization and Myopia
Current thinking about the mechanisms controlling eye growth have been influenced by studies showing that vision-dependent regulation of eye growth can be restricted to local regions of the retina,159,188,281,297–299 and occur without input to the brain,158,290,292,293 supporting the idea that the retina can sense and respond to the sign of defocus by initiating signals that result in altered eye growth and adaptive changes in refractive state (see Section 3.6.1). The molecular and biochemical changes observed in the retina, and in many other studies showing changes in the RPE, choroid, and sclera under different experimental visual conditions, have given rise to a “signaling cascade theory” of eye growth and refractive state control (see Fig. 16).
Figure 16.
Does a biochemical signal cascade beginning in retina and ending with changes in sclera extracellular matrix control eye growth and refractive state? Biochemical retinal signal(s) in response to myopic or hyperopic defocus conditions, may initiate a signal pathway cascade from retina to RPE, the choroid, and eventually sclera, controlling the remodeling and synthesis of scleral extracellular matrix and eye growth directly or in response to IOP effects. A general list of substances and functions implicated at different steps in experimentally induced changes in eye growth and refractive state are indicated. PG, proteoglycan; GAG, glycosaminoglycans.
The putative signal cascade for emmetropization is initiated in the retina in response to visual experience and is thought to bring about a change in the rate of scleral growth through a biochemical signal pathway or pathways that involve intermediate steps within the RPE and choroid. In the following sections, substances implicated in the mechanisms underlying emmetropization and myopia will be reviewed.
5.1 Retinal Signals Associated with Visually Regulated Eye Growth and Myopia
The retina can sense hyperopic and myopic defocus and initiate different growth responses through apparently different independent molecular mechanisms.510,511 Earlier studies have identified several genes (see Section 6) and retinal substances that possibly play a role in the regulation of ocular growth. These include the following: DA,499 the immediate early genes early growth response-1 (Egr-1)512 and FBJ osteosarcoma oncogene (cFos),512 glucagon,513,514 insulin,515,516 VIP,10,517 retinoic acid (RA),518 NO,519 and sonic hedgehog gene expression (Shh).520 Several other key growth factors have also been shown to influence axial elongation, including FGF521,522 and TGF-β523–526; however, it is unclear if their effects originate from the retina. The list of possible growth modulators of visually guided growth has significantly expanded in recent years through transcriptome, proteome, and more recently microRNAome studies, which through enrichment analysis have highlighted new biochemical signaling pathways that may underlie the regulation of ocular growth.510,511,527–538
5.1.1 Retinal Dopamine
The dopaminergic system has been implicated in the regulation of ocular growth. Retinal DA is downregulated during increased ocular growth in chicks,499 rhesus monkeys,498 guinea pigs,539 and tree shrews.540 However, there are inconsistencies in studies with mice.541–543
DA is the major catecholamine found in the retina. It is synthesized and released from the dopaminergic amacrine and interplexiform cells. These cells make up less than 1% of the amacrine cell population; however, through an extensive network of axon-like processes, dopaminergic amacrine cells cover the retina.544 DA release is strongly affected by light levels and has a diurnal rhythm, with high release during the day and low release at night.545–547 In the chick, this rhythm is primarily light driven, but possesses also a minor circadian component.548 DA is considered to have a neuromodulatory role in light adaptation, by controlling cell coupling, and mediating retinal diurnal rhythms.
The role of DA in the regulation of ocular growth has been suggested by the changes seen in DA pathway metabolites during experimentally induced changes in eye growth and refractive state and the effects of dopaminergic drugs on experimentally induced myopia.497,500 A role for DA was first suggested by two early studies that reported a reduction in retinal levels of DA and its primary metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) during the development of FDM in chicks499 and rhesus monkeys,498 and later in tree shrews540 and guinea pigs.539 In chicks, reduced DA release is associated with complete inhibition of the normal diurnal rise in retinal DA levels observed with FDM.499 Similar reductions in retinal DA levels are seen in response to lens-induced myopia,549 although not as consistently as that reported for form deprivation.439
Pharmacologic studies support a role for DA in the regulation of ocular growth. Seminal work by Stone et al.499 showed that daily subconjunctival injection of the nonselective DA receptor agonist apomorphine in chicks, retards FDM in a dose-dependent manner. This effect was abolished by coadministration of the DA antagonist, haloperidol, further supporting involvement of dopaminergic pathways. Since then, various dopaminergic agonists have been shown to slow the development of experimental myopia in a variety of species. Specifically, using a number of agonists shown to be effective at preventing the development of experimental myopia in chicks,502,503,505 similar findings have been reported in the rhesus monkey,501 guinea pigs,550 and mice.551 Administration of synthetic DA in rabbits,507,508 or its precursor levodopa (L-DOPA) in guinea pigs,509 also effectively reduces experimental myopia.
Recent studies have suggested that light-induced increases in retinal DA release,458,486,487,552 driven by ON-bipolar cell activity,486 underlie the ability of higher illumination levels to retard the development of experimentally induced myopia.407,483,486–488,491 It has been speculated that light-induced changes in DA release may underlie the reduction of myopia incidence in children with more time spent outdoors.318,487 This hypothesis is supported by correlations between retinal DA release, illumination, and less myopia in chicks.481,482,553 More evidence comes from the observation that daily intravitreal injections of the D2 receptor antagonist spiperone in chicks,487 and the D1 receptor antagonist SCH39166 in mice,486 abolished the protective effects of bright-light exposure against the development of FDM. Furthermore, in mice, form-deprived eyes exposed to bright light display increased ON-bipolar cell activity, which drives DA release,486 consistent with previous reports that ocular growth rate is affected by ON-pathway manipulations.542,554,555
Pharmacologic evidence also indicates that changes in retinal DA levels may underlie the protective effects of short periods of unrestricted vision, which blocks the development of FDM.162,483,503,556 Specifically, intravitreal injection of the D2 receptor antagonist spiperone, but not the D1 receptor antagonist SCH-23390, abolished the effects of brief periods of vision on FDM.503 If animals were placed in darkness instead of given a period of vision, the deprivation effect persisted, but could be prevented by intravitreal injection of the non-specific DA receptor agonist (+/−)-2-amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene (ADTN) or the specific D2 receptor agonist quinpirole, but not by the D1 receptor agonist SKF-38393.503 What visual signal might drive this increased dopaminergic activity during unrestricted vision is still unclear and may represent either a response to myopic defocus or an enrichment of the visual image. It is unclear whether DA plays a similar role in the ability of brief periods of normal vision to retard the development of LIM.495,505
As suggested by the above paragraphs, the relationship between retinal DA release and eye growth is not a simple one. There may be differences in the role(s) of DA in deprivation-induced versus negative lens–induced myopia, for instance.495 There are inconsistencies in the effect of positive lens–induced myopic defocus on retinal DA release.549,557 There is also pharmacologic evidence that is inconsistent with the hypothesis relating DA signaling to ocular growth inhibition. Specifically, the use of reserpine557,558 to suppress DA release, or the use of the neurotoxin 6-OHDA,416,559 which kills dopaminergic amacrine cells, both resulted in the inhibition of FDM, the opposite of what would be expected if DA inhibited eye growth. Whether these drugs had secondary effects, unrelated to DA, which mediate the inhibition of myopia, cannot be ruled out.
It is also possible that the effects of DA are not related to the effects of retinal DA concentration, per se, but rather to the amplitude of DA's diurnal rhythm. This view is suggested by the finding that retinal DA levels in FDM are lower only during the day, when levels are normally high, but not at night.499 Further evidence that the DA rhythm is involved in emmetropization is the finding than rearing chicks in low daytime illuminance results in lower daytime DA release (measured as vitreal DOPAC) and more myopia than rearing chicks in high-daytime illuminance.481,553 Finally, photoperiod alterations, which have varying effects on the retinal DA rhythm,439,560 have long been known to affect eye growth.95,136,408,410,411,440
5.1.2 Other Monoamines
Monoamines, such as melatonin, serotonin, and epinephrine, have been implicated in modulating eye growth. Melatonin is synthesized by pinealocytes, retinal photoreceptors, and epithelial cells of the ciliary body and exhibit a circadian rhythm with peak levels occurring at night.561–563 The exact role of melatonin in the regulation of ocular growth is yet to be elucidated, although intravitreal injections show, even though variable, an effect against the development of FDM across different studies in chicks.558,564,565 Serotonin, as the natural precursor of melatonin, has also been implicated in the regulation of ocular growth as administration of 5,7-dihydroxytryptamine (5,7-DHT) enhances the development of FDM in chicks.558 In line with this, serotonergic antagonists have been shown to inhibit the development of LIM in chicks.566
Though not thoroughly studied in animal models, a role for epinephrine in refractive development has been suggested following clinical studies using timolol, a β-adrenergic receptor antagonist, which exhibited a small inhibitory effect against myopia development in children.567,568 In cynomolgus monkeys topical administration of epinephrine did not alter refractive development, although administration of timolol resulted in monkeys significantly developing myopia in otherwise untreated eyes.569 However, the administration of timolol did not influence the development of FDM and LIM in chicks.570
5.1.3 Vasoactive Intestinal Peptide (VIP)
VIP is a 28-amino acid (neuro)regulatory peptide that is influenced by form deprivation in chick and primate.10,517 At the peptide level, VIP expression is elevated during periods of form deprivation–induced ocular growth.10,517 Administration of a porcine VIP analogue is capable of preventing FDM in chicks,571,572 although this result is confounded by the fact that VIP antagonists are also observed to prevent FDM.571 More recently, a genome-wide meta-analysis has reported an association between VIP receptor 2 (VIPR2) and high myopia in Chinese populations.573 VIP has several links to other key modulators postulated to play a role in growth regulation. VIP belongs to glucagon/secretin superfamily, a family with several members already shown to modulate ocular growth.514 VIP is also known to exert a synergistic effect on retinal cAMP levels with DA.574 There are suggestions that VIP concentration is altered during the day in the choroid of chicks,575 but it is not clear if this is also the case in the retina.
5.1.4 Melanopsin
Melanopsin is a G-protein coupled opsin encoded by the OPN4 gene in vertebrates. Unlike other opsins, melanopsin is not involved in phototransduction by photoreceptors in the outer retina but is sensitive to light.576,577 Recently, ganglion cells in the inner retina, characterized as intrinsically photosensitive retinal ganglion cells (ipRGCs), have been found to contain melanopsin and are directly sensitive to light.578 The ipRGCs are primarily involved in nonimage forming functions, including circadian rhythm entrainment and regulation of pupil size. Axons of the ipRGCs project directly to the suprachiasmatic nucleus,579 the olivary pretectal nucleus,580,581 and other midbrain centers. The ipRGCs are most sensitive to short-wavelength light with a peak sensitivity at approximately 482 nm.582 Unlike rod and cone photoreceptors that hyperpolarize to light, ipRGCs depolarize. Single-cell recordings in isolated retinas from rhesus monkeys show that direct stimulation of ipRGCs results in a unique firing pattern that has a longer latency than rod and cone photoreceptors, with sustained depolarization after cessation of the stimulus.578 The ipRGCs have been shown to synapse with dopaminergic amacrine cells,583 with reciprocal synapses between cells.584 With a potential role of DA in refractive development, it is possible that ipRGCs and melanopsin are also involved.
The role of melanopsin in refractive development has been investigated in guinea pigs.465 Animals were raised in either monochromatic short-wavelength (480 nm, peak sensitivity of melanopsin ganglion cells) or medium-wavelength (530 nm, peak sensitivity of the guinea pig medium wavelength cone) light. Animals that were raised in short-wavelength light were 2 D less myopic than those raised in medium-wavelength light. Additionally, animals raised in short-wavelength light were found to have increased melanopsin-immunolabeled cells, melanopsin RNA, and melanopsin protein. These results suggest an association between melanopsin activation and refractive development. However, further investigations are required to understand the role of melanopsin in relation to DA and the effects of short wavelength light on myopia development.
Another recent study investigated the contribution of melanopsin to normal refractive development and FDM using melanopsin knockout mice (Opn4−/−).585 The authors showed that Opn4−/− mice raised under normal conditions were significantly more myopic than wild-type mice after 4 weeks, but became more hyperopic after 16 weeks. Opn4−/− mice undergoing form deprivation became more myopic than wild-type mice. These results suggest that melanopsin signaling pathways contribute to both normal refractive development and FDM in mice.
5.1.5 Glucagon and Insulin
A role for glucagon in the regulation of ocular growth was suggested by reports that the number of glucagonergic amacrine cells positively labelled for the immediate early gene Egr-1 was modulated bidirectionally to opposing growth stimuli.512 Glucagon is a 29 amino acid–long peptide produced from the proteolytic cleavage of the precursor molecule preproglucagon (PPG), which also gives rise to the bioactive peptides miniglucagon, oxyntomodulin, glucagon-like peptide-1 (GLP-1), and glucagon-like peptide-2 (GLP-2),586 a number of which have also been postulated to play a role in ocular growth.514 PPG is part of the superfamily of secretin-glucagon peptides, which includes VIP, that act through G-protein coupled receptors. Glucagon, originally isolated as a pancreatic hormone released in response to hypoglycemia, has been identified as a possible neurotransmitter in the central nervous system.587,588
The role of glucagon in the retina and eye growth is unclear. In chicks, glucagon shows a similar bidirectional response to opposite visual growth stimuli, similar, although time shifted, to that seen with Egr-1 expression. Specifically, glucagon and mRNA levels in the retina are reduced during periods of increased ocular growth,528,589,590 while glucagon levels in the choroid,590 and mRNA levels in the retina,591 are elevated during periods of reduced growth. Importantly, in chicks, administration of glucagon or agonist Lys17,18,Glu21-glucagon inhibits experimentally induced myopia in a dose-dependent manner,513,515,590 while the glucagon antagonist des-His1-Glu1-glucagon-amide inhibits compensation to positive lenses or recovery from FDM.513,515,590 This suggests that retinal glucagon acts as a growth inhibitor in the avian eye; however, its role in mammal eyes is unclear. Although glucagononergic cells have not been detected in the mouse592 or primate,593 PPG and glucagon receptor genes,589 as well as glucagon-related peptides have been observed to be present in mouse retina. These peptides include VIP,517 as well as peptide histidine isoleucine (PHI), pituitary adenylate cyclase-activating polypeptide (PACAP), and glucose-dependent insulinotropic polypeptide (GIP).592
As with the opposite roles that glucagon and insulin have in regulating blood glucose levels, insulin appears to oppose the actions of glucagon in eye growth by stimulating ocular growth.515,516,594 In chicks, intravitreal injections of insulin or insulin-like growth factor 1 (IGF1) induce myopic refractive shifts in otherwise untreated eyes, enhance the axial eye growth associated with imposing hyperopic defocus with negative lenses, and block the development of hyperopia in response to imposing myopic defocus with positive lenses. Administration of insulin-like growth factor 2 antisense oligonucleotides (IGF2), which reduces IGF2 mRNA levels in the retina, inhibits the development of FDM in the guinea pig.595 In contrast, injection of recombinant human IGF2 induced greater levels of myopia in diffuser-treated guinea pigs.596
In contrast to glucagon, intravitreal administration of insulin is a potent growth stimulator, inducing a myopic shift in otherwise untreated eyes,516 while inducing overcompensation to negative lenses,515,516,594 and preventing compensation to positive lens-imposed defocus.515,516,594 Insulin primarily acts through two cell signaling pathways, a mitogen-activated protein kinase (MEK) pathway and the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway. Co-administration of the PI3K inhibitor Ly294002 prevented the growth enhancing effects of insulin.594 In contrast, coadministration of the MEK inhibitor U0126 had no effect on insulin's action, further defining the mechanism of action by which insulin may enhance ocular growth rates.597,598 Insulin and IGF1 receptor mRNA levels in the RPE and choroid are upregulated in response to imposed hyperopic defocus and downregulated in response to imposed myopic defocus.599
5.2 Biochemistry of RPE in Visually Regulated Eye Growth and Myopia
Because of its location between retina and choroid, the RPE may relay growth signals emanating from the retina.256,600 Supporting this view, receptors for a number of retinal molecules that are postulated to play a critical role in the regulation of ocular growth, as discussed above, are found within the RPE. These include DA,359,601,602 acetylcholine,603–607 VIP,608 glucagon,589,608,609 RA,610,611 insulin,594,598 and serotonin.612,613 The RPE also synthesizes and releases, along with the retina, several growth factors and cytokines that have been implicated in growth regulation including the following: IGF-1, TGF-β, FGF, VEGF, and bone morphogenetic protein (BMP).525,614–618 Of particular interest, several BMP family members, including BMP-2, -4, and -7, have been observed to show a rapid bidirectional response to opposing growth stimuli.614,615 In chicks, all three forms are upregulated in response to plus lens wear and downregulated in response to negative lens wear, with only slight differences in their time courses.
In cultured human RPE cell lines (ARPE-19), BMP-2 mRNA expression can be enhanced by incubation with DA, indicating a possible pathway by which the RPE may relay growth signals from the retina.619 Another interesting RPE candidate is TGF-β2, a member of the same superfamily of growth factors as that of BMPs. TGF-β2 mRNA expression, which has previously been implicated in the signaling cascade between retina and sclera,525,620–624 is upregulated within the RPE in response to plus lens wear.616 Importantly, TGF-β2 release from RPE cells can be directly modulated by the administration of cholinergic agents known to alter ocular growth625 suggesting a pathway by which cholinergic signals from the retina may be relayed through the RPE. The list of signaling molecules emanating from the RPE continues to expand as transcriptome and proteome analyses are undertaken,531,617,618,626 although, due to the use of combined retinal–RPE or RPE–choroid extracts, it is sometimes difficult to isolate those changes associated directly with the RPE.525,531,617,626–628
Tissue culture experiments support an important role for RPE in eye growth. The presence of RPE is critical for proliferation of scleral chondrocytes and fibroblasts.629,630 The ability of apomorphine, a nonspecific DA agonist known to inhibit experimental myopia, to inhibit the proliferation of scleral chondrocytes only occurs in the presence of RPE cells.631 In eye cup preparations, insulin-induced choroidal thinning requires the presence of the RPE,598 and the influence of gamma-aminobutyric acid (GABA)ergic agents that inhibit experimental myopia, on scleral DNA content and glycosaminoglycan synthesis appears to require, at least to some extent, the presence of the RPE.632
5.3 Biochemistry of Sclera in Visually Regulated Eye Growth and Myopia
The sclera is likely to be the final destination in the signaling cascade for controlling eye size and shape. This dense connective tissue defines the size and shape of the eye and is known to be a dynamic tissue that undergoes constant remodeling throughout life. Results from research over the last 25 years have established that scleral remodeling is regulated by genetic and environmental influences, which can have profound effects on ocular size and refraction.
Studies from avian and mammalian models indicate that scleral remodeling during myopia development is accomplished by selective modulation of scleral protein expression (see Table 2). In chicks, myopia is associated with an increased synthesis of the sulfated proteoglycans aggrecan, and to a lesser extent, decorin, in the cartilaginous layer of the posterior sclera, decreased proteoglycan synthesis269,280 and overall thinning of the posterior fibrous sclera.154 Because the cartilaginous sclera contributes to the vast majority of proteoglycan synthesis (∼90% of newly synthesized sulfated proteoglycans), measurements of proteoglycan synthesis in the intact chick sclera are largely a reflection of proteoglycan synthesis in cartilaginous sclera. Similar to the chick fibrous sclera, the mammalian sclera demonstrates decreased sulfated proteoglycan synthesis during myopia development259,260 as well as decreased collagen content in the posterior sclera.259,273 Additionally, changes in scleral crosslinking have been suggested to play a role controlling scleral viscoelasticity and ocular elongation in tree shrews,633 guinea pigs,267,634,635 and rabbits.636
Table 2.
Biochemical Changes in the Sclera During Myopia Development
|
Protein |
Animal Model |
Effect |
| Sulfated proteoglycans | Chick – FDM | Increased266 |
| Chick – LIM | Increased252 | |
| Tree shrew – FDM | Decreased152,259 | |
| Marmoset – FDM | Decreased260 | |
| Collagen synthesis | Tree shrew – FDM | Decreased259,273 |
| Collagen type I | Tree shrew – LIM | Decreased800 |
| Guinea pig – LIM | Decreased801 | |
| Integrin α2β1 | Guinea pig – LIM | Decreased801 |
| MMP-2 | Chick – FDM | Increased637 |
| Tree shrew – FDM | Increased802 | |
| TIMP-2 | Tree shrew – FDM | Decreased640 |
| BMP-2 | Guinea pig – LIM | Decreased803 |
| Guinea pig – FDM | Decreased642 | |
| BMP-5 | Guinea pig – FDM | Decreased642 |
Changes confirmed in experimental animal models of myopia at the protein level by at least two methods.
Several types of matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPs) that regulate them, have been implicated in the process of matrix remodeling in the sclera of myopic eyes. In the sclera of tree shrews and the fibrous scleral layer of chicks, myopia is associated with increased expression of MMP-2 and decreased expression of TIMP-2,637–640 most likely promoting accelerated scleral collagen degradation and thinning of the fibrous sclera. In support of this, adding TIMP-2 in vivo inhibited the rate of collagen degradation and reduced myopia progression, possibly through suppression of MMP-2 activation and activity.640
These changes in scleral protein expression may be regulated by changes in the expression of several endogenous cytokines and growth factors, including BMP-2, BMP-5,641,642 TGF-β,624 and cyclic AMP,643 as well as by diffusible factors originating from the retina, RPE, and choroid.497,515,598,599,632,644
The sclera in myopia undergoes a sequence of biochemical changes ranging from the loss of scleral dry weight, reduced collagen accumulation, lower hyaluron and sulfated glycosaminoglycan levels, upregulated enzymatic degradation, downregulated aggrecan, and downregulated collagen type I synthesis.259,271 Previous studies suggested that these biochemical alterations could make it easier for collagen fibrils to slide across each other,565 consequently it causes collagen fibril bundles to increase crimp angle during myopia development.278 Moreover, the genes encoding signaling receptors, degradative enzymes and inhibitors, and ECM proteins in sclera could change their gene expression rates in response to myopia growth signals (see Fig. 17).263
Figure 17.
Information flow from myopiagenic stimuli signals that would produce gene expression changes related to signaling, degradative enzymes, and inhibitors and ECM proteins (adapted from Guo L, Frost MR, He L, Siegwart JT Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci. 2013;54:6806–6819. Copyright © 2013 Association for Research in Vision and Ophthalmology).263
5.3.1 Choroidal Regulation of Scleral Remodeling
Because of its proximity to the sclera, the choroid has been implicated in the regulation of scleral metabolism during visually guided ocular growth via the synthesis and secretion of specific growth factors.150,269,645 Most notably, choroidal synthesis of all-trans-retinoic acid (RA) and its synthesizing enzyme, retinaldehyde dehydrogenase 2 (RALDH2), cause changes in scleral proteoglycan synthesis and are affected by visual stimuli that alter the refractive state of the eye.
5.3.2 Retinoic acid
RA is a lipid soluble derivative of vitamin A. RA has multiple effects on cell proliferation and differentiation in early development throughout the body, including the eye.518 RA synthesis rate is driven by changes in retinaldehyde dehydrogenase 2 (RALDH2)646 and secreted RA is associate most heavily with the RA-binding protein apolipoprotein A1.647 Changes in RA levels have been observed in both the retina and choroid during experimentally induced changes in ocular growth.
RA and RALDH2 levels in the retina are elevated in experimental myopia in chicks,648,649 guinea pigs,650 and marmosets.651 Retinal RA levels are suppressed during periods of reduced ocular growth.650 In chicks, mRNA levels of retinal retinoic acid receptors (RARs) are elevated in response to myopic defocus.649,652
The choroid normally produces significantly more RA than the retina.653 Choroidal RA synthesis in chicks653 and guinea pigs650,654 decreases in experimentally induced eye growth and increases when eye growth is reduced, opposite of that seen in the retina of these species. In marmosets, the choroidal RA synthesis was higher in myopic eyes,651 which may be related to the differences in the histology of the avian and mammalian scleras and their function as a target tissues in eye growth control (see Section 3.5.4). Why the same visual stimuli cause opposite changes in choroidal RA in guinea pigs and marmosets is unknown, but it may be related to differences in RA degradation rates, modulation of RARs, or the presence of additional regulatory steps in the cascade between the retina and choroid that differ between chicks, guinea pigs, and primates.
At the sclera, RA binding suppresses glycosaminoglycan synthesis651,653 and the proliferation of scleral chondrocytes and fibroblasts.518 Furthermore, mRNA levels for RARbeta in the sclera are elevated in form-deprived eyes.518 Interestingly, feeding RA to chicks or guinea pigs induces longer eyes with thinner lenses, although with no net change in refractive state.650,655 Furthermore, dietary RA does not appear to affect compensation for lens imposed defocus, although it does increase eye growth significantly.655
Taken together, the changes in retinal and choroidal RA synthesis and the effects of RA on scleral growth, suggest that RA has an important role in eye growth regulation. It appears to be both part of the signal cascade from retina to sclera, and possibly the effector of scleral extracellular changes. Additional study is necessary to elucidate the molecular mechanisms involved in the regulation of retinal and choroidal RA synthesis and the modulation of its action on the sclera, which may lead to the development of new therapeutic approaches for the control of myopia through modulation of retinoid signaling in the choroid or sclera.
5.4 Pharmacologic Clues to the Mechanisms of Emmetropization and Myopia
Testing the effects of various drugs on visually regulated emmetropization and experimental models of myopia provide clues to and a means to test hypotheses about pathways controlling eye growth and regulating refractive state.
The two classes of drugs that have been most widely studied and that inhibit the development of experimentally induced myopia are DA agonists497 (see Section 5.1.1) and cholinergic (muscarinic) antagonists.35,656–659 Several other drugs have shown effects on experimental models and interact with dopaminergic and cholinergic pathways and may provide benefits for understanding eye growth control and possibly have potential therapeutic value (see also Section 5.1 and Table 3 for a summary of drug studies on emmetropization).
Table 3.
Specific Drug Effects on Experimental Myopia
|
Drug Category |
Drug Treatment |
Effects |
Species |
| Dopamine agonists | Dopamine (nonspecific) | Inhibits FDM | Rabbit507,508 |
| Apomorphine (nonspecific) | Inhibits FDM and LIM, enhances LIH, biphasic effect on spontaneous myopia in guinea pig | Chick499,504-506 | |
| Guinea pig550,804 | |||
| Macaque501 | |||
| Mouse551 | |||
| ADTN (nonspecific) | Inhibits FDM | Chick503 | |
| Levodopa (nonspecific) | Inhibits FDM | Guinea pig509,805 | |
| SKF-38393 (D1 specific) | Did not affect FDM or LIM, inhibits spontaneous myopia in guinea pig | Chick503,505 | |
| Guinea pig804 | |||
| Tree shrew474 | |||
| Quinpirole (D2 specific) | Inhibits FDM and LIM, enhanced spontaneous myopia in guinea pig | Chick503,505 | |
| Guinea pig804 | |||
| Tree shrew474 | |||
| PD168077 (D4 specific) | Inhibits FDM | Tree shrew474 | |
| Dopamine antagonists | Sulpiride (D2 specific) | Enhances FDM in chicks, inhibits FDM in mice | Chick558 |
| Mouse779 | |||
| SCH-23390 (D1 specific) | Does not antagonize antimyopia effects of apomorphine or diisopropylfluorophosphate (DFP) in FDM, of unrestricted vision in FDM and LIM (LIM varies), can enhance FDM | Chick495,503–505,558,684 | |
| Tree shrew474 | |||
| Haloperidol (D2 specific) | Antagonizes antimyopia effect of apomorphine in FDM | Chick499 | |
| Spiperone (D2 specific) | Antagonizes the antimyopia effects on both FDM and LIM, but does not affect FDM alone, inhibits FDM in tree shrew | Chick495,503–505,684,685 | |
| Tree shrew474 | |||
| PD168568 (D4 specific) | No effect on FDM | Tree shrew474 | |
| Muscarinic-cholinergic antagonists | Atropine (nonspecific) | Inhibits FDM and LIM | Chick656–658,666 |
| Macaque662 | |||
| Mouse59,687 | |||
| Pirenzepine (m1 specific) | Inhibits FDM, LIM, and inhibits spontaneous myopia in guinea pigs | Chick656,658,675,676,806 | |
| Macaque662 | |||
| Tree shrew35 | |||
| Guinea pig807 | |||
| Scopolamine (nonspecific) | Inhibits FDM | Chick658 | |
| Tropicamide (nonspecific) | Inhibits FDM | Chick658 | |
| Dexetimide (nonspecific) | Inhibits FDM | Chick658 | |
| Oxyphenonium (nonspecific) | Inhibits FDM | Chick658 | |
| Propanetheline (nonspecific) | Inhibits FDM | Chick658 | |
| Benztropine (m1 specific) | Inhibits FDM | Chick658 | |
| Heahydro-siladifenidol (m1, m3, and m4 specific) | Inhibits FDM | Chick658 | |
| p-fluorohexahydrosila-diphenidol (m3 specific) | Inhibits FDM | Chick658 | |
| AFDX-116 (m2 and m4 specific) | Inhibits FDM | Chick658 | |
| Quinuclidinyl benzilate (nonspecific) | Inhibits FDM | Chick658 | |
| Muscarinic toxin 3 (m4 specific) | Inhibits FDM | Tree shrew681 | |
| Muscarinic toxin 7 (m1 specific) | Inhibits FDM and LIM | Tree shrew681 | |
| GABA antagonists | TPMPA (A0r specific) | Inhibits FDM | Chick697,698 |
| Guinea pig808 | |||
| 3-ACPBPA (A0r specific) | Inhibits FDM and LIM | Chick697,708 | |
| SCH50911 (B specific) | Inhibits FDM | Chick698 | |
| 2-Hydroxysaclofen (B specific) | Inhibits FDM | Chick698 | |
| SR95531 (A specific) | Inhibits FDM | Chick698 | |
| Bicuculline (A specific) | Inhibits FDM | Chick697,698,709 | |
| CGP46381 (B specific) | Inhibits FDM | Guinea pig809 | |
| Monoamines | Melatonin (nonspecific) | Variable influence on FDM | Chick558,564 |
| Mianserin (5-HT2 antagonist) | Inhibits LIM | Chick566 | |
| Ethiothepin maleate, RS 23597-190 hydrochloride, and 5-3-(1-methyl-1H-indol-3-yl)-1,2,4-oxadiazole (combination of 5-HT antagonists) | Inhibits LIM | Chick566 | |
| Timolol (β-adrenergic receptor antagonist) | Leads to myopia development in monkey, did not influence experimental myopia in chicks | Chick570 | |
| Macaque569 | |||
| Epinephrine (nonspecific) | Did not alter refractive development | Macaque569 | |
| Neuropeptides | Porcine VIP (nonspecific) | Inhibits FDM | Chick571 |
| RA | RA (nonspecific) | Increases normal eye growth | Chick518,655 |
| Disulfiram (inhibits RA synthesis) | Inhibits FDM, but not LIM | Chick649 | |
| Enkephalin targets | Naloxone (NMDA agonist) | Inhibits FDM | Chick710,711 |
| nor-binaltorphimine (κ-specific antagonist) | Inhibits FDM | Chick710 | |
| U50488 (κ-specific agonist) | Inhibits FDM | Chick710 | |
| Dextromethorphan (NMDA antagonist) | Inhibits FDM | Chick711 | |
| MK801 (NMDA antagonist) | Inhibits FDM | Chick711 | |
| AP5 (NMDA antagonist) | Inhibits FDM | Chick711 | |
| Nitric oxide donors | L-arginine (nonspecific) | Inhibits FDM | Chick686 |
| Sodium nitroprusside (nonspecific) | Inhibits FDM | Chick686 | |
| Nitric oxide synthase inhibitors | L-NIO (nonspecific) | Inhibits antimyopia effect of atropine and quinpirole | Chick249,686 |
| L-NMMA (nonspecific) | Inhibits antimyopia effect of atropine and quinpirole | Chick249,686 | |
| L-NAME (nonspecific) | Inhibits FDM and LIM recovery and compensation to LIH, inhibits antimyopia effect of atropine and quinpirole | Chick249,519,686,690–692 | |
| Glucagonergic agonists | Glucagon (nonspecific) | Inhibits FDM and LIM | Chick513,515,590,810 |
| Lys,Glu-glucagon (nonspecific) | Inhibits FDM and LIM | Chick513,590 | |
| Glucagonergic antagonists | des-His1-Glu9-glucagon-amide | Inhibits LIH | Chick590 |
| Insulin | Insulin (nonspecific) | Exacerbates LIM, suppresses LIH, induces myopic shift in otherwise untreated eyes | Chick515,516,594 |
| U0126 (MEK inhibitor) | Does not alter insulin's effects | Chick594 | |
| Ly294002 (PI3K inhibitor) | Blocks exacerbated growth from insulin | Chick594 | |
| Nicotinic-cholinergic antagonists | Chlorisondamine (nonspecific) | Inhibits FDM | Chick688 |
| Mecamylamine (nonspecific) | Inhibits FDM | Chick688 | |
| Dihydro-β-erythroidine (α3 and α4 specific) | Inhibits FDM | Chick688 | |
| Methyllycaconitine (α7 specific) | Inhibits FDM | Chick688 | |
| Adenosine antagonists | 7-methylxanthine (nonspecific) | Inhibits FDM and LIM | Rabbit713 |
| Guinea pig274 | |||
| Primate714 |
5.4.1 Cholinergic Drugs
The ability of muscarinic-cholinergic agents to prevent the development of myopia is one of the most consistent and well-documented findings in both animal and clinical studies. Acetylcholine was one of the first neurotransmitters to be implicated in ocular growth after the nonspecific muscarinic cholinergic antagonist atropine was shown to inhibit the development of myopia.145,656,657,660–664 Muscarinic receptors are a group of G-protein coupled acetylcholine receptors that can be broken down into the subtypes m1 to m5 in mammals. Chicks lack the receptor homolog of the mammalian m1 but express four muscarinic receptor subtypes corresponding to the other mammalian subtypes.665 In chicks, atropine inhibits the development of both FDM502,657,658,666,667 and LIM248,506,666 by inducing choroidal thickening248 and reduced scleral proteoglycan synthesis and growth in a dose-dependent fashion.668,669 Similar effects were observed in rhesus macaques,145,662,670 tree shrews,671 guinea pigs,672 and mice.59,661,673 Myopia is similarly inhibited in chicks by oxyphenonium, which is also classed as a nonselective muscarinic-cholinergic antagonist, although a number of other related compounds have been found to be ineffective at preventing myopia.658
Originally, it was assumed atropine retarded the progression of myopia due to its cycloplegic effect on smooth muscle fibers in the ciliary muscle, which blocked the accommodative function of the eye, due to the predominant theory at the time that myopia was associated with excessive accommodation. It is now known, however, that atropine works through a nonaccommodative mechanism as it can inhibit the development of myopia in chicks,656 in which accommodation and light-induced pupillary constriction are mediated by nicotinic rather than muscarinic receptors, while also inhibiting the development of myopia in nonaccommodating mammals.657 This has led to a search for other sites of action with evidence for both retinal and nonretinal locations.659 However, as muscarinic acetylcholine receptors are so widely distributed,665 it has made identifying a site of action difficult. Several lines of evidence have also pointed toward a nonmuscarinic mode of action as follows: (1) the generally high dose of atropine required to prevent myopia in animal models; (2) its continued effect following ablation of cholinergic amacrine cells674; and (3) its effectiveness in inhibiting proteoglycan synthesis in isolated scleral cells.668,669 For a more complete review see McBrien et al.659
Various specific muscarinic antagonists have been tested to further define the role of muscarinic cholinergic receptors in myopia. Pirenzepine, the partially selective m1/m4 muscarinic antagonist, has been shown to slow the development of both FDM and LIM in chicks,656,675–677 guinea pigs,678 tree shrews,35 and rhesus monkeys.662 Although pirenzepine is a partially selective muscarinic antagonist, there is evidence that it also cross-reacts with the m3 receptor, inducing increased pupil size in tree shrews35 and rhesus monkeys.679,680 The importance of m1/m4 receptors was confirmed by using the selective m4 antagonist muscarinic toxin 3 (MT3)681,682 and the m2/m4-specific antagonist himbacine683 to reduce the development of experimental myopia in chicks and tree shrews. The selective m1 antagonist muscarinic toxin 7 (MT7) also inhibits myopia in tree shrews,365,681 but not chicks, which lack the m1 receptor. However, as with pirenzepine, there is evidence of cross reactivity of these more selective compounds with adrenergic (and possibly other) receptors.656,658,684
Drug interactions provide some evidence to the mechanisms of eye growth control. There is evidence for interactions between the cholinergic system with other key neuromodulators postulated to play a role in growth regulation, including DA, NO, and GABA. Specifically, in chicks, atropine stimulates the synthesis and release of DA in the retina when injected into form-deprived eyes,667 while coadministration of spiperone, a DA D2 receptor antagonist, prevents the protective effects of the muscarinic receptor antagonists MT-3 against the development of FDM.681,685 Similarly, the effects of atropine against FDM are lost when coadministered with NO synthase inhibitors.686 More recently, proteomic analysis has suggested that atropine modifies GABAergic signaling when injected into negative lens-treated eyes.687 Finally, injection of dopaminergic, GABAergic, and cholinergic drugs known to inhibit myopia reverses the downregulation in Egr-1 mRNA expression normally associated with the development of form deprivation,502 suggesting a common retinal target for each of these systems. The effect of NO compounds on Egr-1 expression has not been investigated.
There is some evidence that nicotinic cholinergic receptors may also be involved in regulatory pathways for eye growth.688 Nicotinic receptors consist of a large and diverse group of acetylcholine-gated nonselective cation channels usually associated with multiple subunits.689 Several nicotinic receptor antagonists were found to inhibit FDM in chicks.688 Nonselective antagonists were found to have the highest level of efficacy; however, other antagonists demonstrated a multiphasic dose response.688 Less work has been done on nicotinic cholinergic receptors compared with muscarinic receptors because of the large diversity of receptors and complex nature of their responses.660
5.4.2 Drugs Affecting Nitric Oxide
NO is a gaseous neuromodulator expressed throughout the eye in all vertebrates. Several animal studies have supported a role for NO in the regulation of ocular growth. The first studies in chicks reported that injections of the nitric oxide synthase (NOS) inhibitor N-omega-nitro-L-arginine methyl ester (L-NAME), which reduces the synthesis of NO, inhibited the development of FDM519 and LIM,690 suggesting that NO was part of the signal cascade mediating growth stimulation and myopia development. However, subsequent studies, also on chicks, demonstrated the opposite. NOS inhibitors resulted in disinhibition of ocular growth in eyes recovering from myopia, or compensating for positive lens defocus,691,692 both of which slow growth. NOS inhibitors also prevented the inhibitory effects of daily periods of vision on FDM and in eyes recovering from FDM, suggesting that NO is part of the signal cascade mediating growth inhibition.693 In all these cases, the growth disinhibition was associated with an inhibition of the choroidal thickening in response to lens-imposed myopic defocus (see Section 3.5.3). Furthermore, increasing NO levels by administration of L-arginine, a NOS substrate, inhibited the development of FDM in a dose-dependent manner, and coadministration of the NOS inhibitor L-NNMA prevented this protective effect.686 Finally, the NO donor sodium nitroprusside was protective against FDM.686 These studies support a role for NO in the compensatory choroidal thickening in response to myopic defocus, and in the associated ocular growth inhibition. The discrepancy between the results of the earlier and later studies is difficult to reconcile, but perhaps the effects of L-NAME depend on the state of ocular growth and visual input.
The mechanisms by which NO regulates choroidal thickness and/or ocular (scleral) growth are as yet unknown. Because NO is readily diffusible, it is difficult to determine where the critical changes are occurring, as evidenced by the finding that changes in NOS activity were observed in all layers of the eye during the development of FDM in guinea pigs.694 However, it is known that eNOS is released by the endothelium of blood vessels, and nNOS is released from parasympathetic neurons, both of which influence choroidal blood flow,695 suggesting a role for changes in blood flow in the choroidal compensatory responses. In addition, NOS-positive axon terminals synapse onto choroidal nonvascular smooth muscle in birds and primates,238,696 supporting a role for smooth muscle tonus in the response. The underlying mechanisms warrant further study.
Studies in chicks have given us some hints as to the position of NO with regard to other putative signaling molecules in the cascade from retina to sclera. For instance, the growth-inhibitory effects of the DA receptor agonist quinpirole in eyes responding to negative lenses or diffusers are abolished if coinjected with NOS inhibitors.249 Similarly, the growth-inhibitory effect of the cholinergic muscarinic antagonist atropine in chick FDM is abolished if coinjected with NOS inhibitors.686 Both these studies provide strong evidence that the growth-inhibitory actions of dopaminergic and cholinergic drugs are mediated by NO; that is, NO acts downstream of both. The current interest in the therapeutic potential of increasing the time children spend out-of-doors to prevent myopia makes studies of the potential interaction between DA (which mediates light-adaptive processes) and NO timely and relevant.
5.4.3 GABAergic Drugs
GABA is the most prominent inhibitory neurotransmitter found in the body.697,698 Based on its colocalization and functional interactions with retinal DA699,700 and acetylcholine701,702 in retina, the role of GABA in ocular development is of interest.698
GABAergic receptors can be broken into three broad groups698 GABAA receptors are ionotrophic receptors found in cone photoreceptors, bipolar cells, and ganglion cells and are thought to mediate synaptic feedback between cone photoreceptors and horizontal cells.697,703 GABAB receptors are G-protein coupled receptors located on bipolar cells, photoreceptor terminals, and ganglion cells and are believed to regulate intracellular messengers and neuronal function.697,703 GABAA0r (formerly GABAC) receptors are ionotrophic receptors found in horizontal cells and bipolar cell axon terminals 703 and may be involved in mediating GABAergic synaptic functions in both the inner and outer plexiform layers.697 Furthermore, GABAA0r receptors have been found on chick sclera fibroblasts and chondrocytes,704 suggesting a potential pathway for GABA to directly influence scleral remodeling and eye growth.704
Experimental studies have revealed that several GABA receptor antagonists can retard the development of experimental myopia.697,698,705–709 In FDM, antagonists against all three receptor subtypes can retard axial elongation to some extent in chicks and guinea pigs,698,709 while GABAA0r antagonists are effective at preventing the development of LIM in chicks.708 GABAA- and GABAB-specific antagonists have not been tested in LIM.
The administration of certain GABA agonists has been shown to enhance the development of experimental myopia in chicks,697,698 while the protective effects of brief periods of normal vision against the development of FDM is abolished by the administration of the GABAA/A0r agonist muscimol and the GABAB agonist baclofen, with their effect enhanced by dopaminergic antagonists and inhibited by dopaminergic agonists.697 This may suggest that interactions between the GABAergic and dopaminergic systems underlie the protective effects of brief periods of normal vision, with both systems alone shown to be able to influence the response.
5.4.4 Drugs Affecting Neuropeptides
Antagonists for the retinal neuropeptides VIP and enkephalin have been found to inhibit FDM.514,517,571,710 In primates, as discussed in Section 5.1.3, FDM was associated with increased immunoreactivity for VIP,517 and antagonists for VIP have been shown to inhibit the development of FDM in a dose-dependent manner in chicks,571 and porcine VIP slightly reduced FDM.571
In chicks, enkephalin, which is expressed by the enkephalin, neurotensin, and somatostatin-like immunoreactive (ENSLI) amacrine cells of the retina, forms a reciprocal inhibitory circuit with DA. Nonselective (naloxone710,711 and U50488711) and selective (nor-binaltorphimine,711 dextromethorphan,710 MK801,710 AP5710) opioid antagonists inhibit FDM in chicks. However, retinal levels of proenkephalin are unaffected by FDM, with the opioid agonist morphine showing no effect on FDM development.710 Thus, it is unclear what role enkephalins plays in ocular development.
5.4.5 Adenosine Antagonists
In a recent clinical trial, children receiving 7-methylxanthine for a period of 24 months exhibited a small reduction in the progression of myopia.712 7-methylxanthine, a metabolite of caffeine, works as an adenosine receptor antagonist and has been demonstrated to significantly reduce the development of FDM in rabbits713 guinea pigs,274 and macaques714 when administered orally. Furthermore, administration of 7-methylxanthine leads to increased collagen concentration and diameter of collagen fibrils in the posterior sclera of rabbits.715 Oral administration of 7-mthylxanthine in rhesus macaques also reduces the axial myopia produced by hyperopic defocus, facilitates hyperopic shifts produced by imposed myopic defocus, and induces hyperopia in otherwise untreated eyes.714 This pattern of results suggests that 7-methylxanthine has therapeutic potential for treating myopia.
6. Molecular Biology of Emmetropization and Myopia: Gene–Environment Interactions
The relative roles of genes and environment in the development of refractive error, particularly myopia, have been the subject of much debate over many years.716 Experimental studies with animal models together with genetic analysis in humans show that the phenotypic expression of eye growth and refractive state is controlled by complex interactions between genetic and environmental factors. Experimental models are also yielding many insights into the gene–environment interaction underlying refractive development of the eye and the signaling pathways controlling visually guided eye growth.
Several studies in humans provide evidence that the development of myopia is controlled by an interaction of environmental and genetic factors.717–720 Twin and family studies suggest that the contribution of genetic factors for refractive error development may be as high as 50% to 80%.721–726 Genetic mapping studies have identified over 100 chromosomal loci linked to human myopia718,727–735 and revealed interactions between specific gene variants and environmental factors, such as near work and the level of education. For example, a recent study found five genetic variants that showed evidence of interaction with refractive error and near work.736 A study by the CREAM consortium also identified three additional chromosomal loci, which exhibited significant association with refractive error and level of education.736 Evidence in support of a gene–environment interaction in refractive error development was reported in a study showing that children who carried a low-frequency variant in the promoter region of a myopia susceptibility gene APLP2, were approximately five times more likely to develop myopia if they spent more than 2 hours reading per day compared with children, who did not carry the gene variant.737 Moreover, this study also demonstrated that lack of APLP2 protein (a modulator of glucose and insulin homeostasis) has a dose-dependent suppressive effect on susceptibility to FDM in mice, thus, confirming gene–environment interaction between APLP2 and visual input.
Detecting changes in gene expression associated with experimentally induced changes in eye growth and refractive state is a powerful approach to provide clues to the biochemical pathways controlling eye growth. Experimental studies in several species show that the development of visually induced myopia is associated with large-scale changes in gene expression in all ocular tissues so far examined.510,511,528,529,531–533,535,617 These studies will help identify components of the regulatory pathways underlying eye growth and involved in the development of myopia, providing potential new targets for drug development and future treatments.
6.1 Changes in Retinal Gene Expression
The immediate early gene Egr-1, also known as ZENK, ZIF268, NGFI-A, or Krox-24 in chicks, was one of the first genes found to be affected by the visual input producing experimental myopia, and is one of the earliest observable molecular changes associated with experimentally induced retinal defocus.512 Egr-1 is a transcription factor that encodes a short-lived nuclear protein with a zinc finger-binding domain. Its expression is normally induced rapidly and transiently by extracellular stimuli, although its expression can be delayed by hours to days and remain altered for prolonged periods.738 Egr-1 shows bidirectional expression in glucagonergic amacrine cells of chicks; it is upregulated in response to imposed myopic defocus and downregulated in response to imposed hyperopic defocus, suggesting it may be an initiator of the eye growth signal cascade.502,512,528,532,739 Consistent with the observation that elevated expression of Egr-1 is associated with growth suppression, administration of pharmacologic agents that block the development of experimental myopia, reverse the downregulation of Egr-1 normally observed in response to FDM or LIM.500
Similar to chicks, bidirectional changes in Egr-1 expression have been observed in guinea pigs740 and rhesus macaques.593 The changes in Egr-1 expression in these mammalian retina appear to be associated with a subpopulation of GABAergic amacrine cells and a subpopulation of ON-bipolar cells.593 In mice, Egr-1 expression has only been investigated during a period of increased ocular growth, where, as with the other species investigated, its transcript levels are reduced after 1 hour of form deprivation.741 Consistent with these results, Egr-1 knockout mice have more myopia compared with wild-type control animals.742
Increased expression of Egr-1 is associated with positive lens–induced defocus in rhesus macaques,743 although changes in Egr-1 levels were also observed to some degree in the contralateral control eyes. Despite all evidence to date indicating that Egr-1 expression is elevated with imposed myopic defocus, two studies unexpectedly found that Egr-1 mRNA transcript levels were downregulated in response to imposed myopic defocus using positive power lenses.528,532
cFos is a light-driven immediate early gene that belongs to the same family of nuclear transcription factors as Egr-1. cFos-immunoreactivity in the chick retina is elevated following 2 hours of diffuser removal when the retina is experiencing myopic defocus, and is reduced when the diffuser is removed under low illumination.512 Transcriptome studies have reported a significant downregulation in cFos mRNA levels during imposed hyperopic defocus.529 However, it should be noted that cFos-immunoreactivity does not appear to be altered in response to imposed myopic defocus from positive lens wear.512 Inhibition of cFos expression in the retina of the guinea pig using a cFos antisense oligonucleotide, induced significant levels of myopia.744
Another early gene implicated in the development of myopia is Shh. In chicks, retinal expression of Shh mRNA and protein levels are significantly elevated during the development of experimental myopia.520,745 Similar increases in Shh mRNA and protein expression are observed in mice, with injection of cyclopamine, an inhibitor of the hedgehog pathway, stimulating greater MMP-2 levels in the sclera and inhibiting the development of FDM in mice and guinea pigs.746,747 Injection of a Shh amino-terminal peptide induces myopic growth in otherwise untreated eyes or greater amounts of myopia in diffuser-treated eyes in mice.746
Several studies have performed extensive gene expression profiling, involving hundreds of genes, to explore the changes in retinal gene expression at different time points during the development of experimental myopia in chicks, mice, and primates.510,511,528,529,531,532,535–537 These studies aim to understand the complexities of the retinal signaling and the components of the regulatory pathways underlying the visual control of eye growth. New insights into the control of eye growth have been gained through this approach. In a recent study in marmosets,511 it was shown that the primate retina distinguishes between hyperopic and myopic defocus by generating distinct bidirectional signaling pathways. The group of genes differentially expressed in response to imposed hyperopic defocus were largely different from those differentially expressed in response to imposed myopic defocus, contrary to the hypothesis that myopic and hyperopic defocus trigger opposite changes in the same set of genes. There was also a transition from one set of differentially expressed genes after the first 10 days of imposed defocus to a different set of differentially expressed genes after 5 weeks of defocus (when the eye had begun to compensate) suggesting a change in the regulatory pathways as the eyes detect and then compensate for the imposed defocus. Interestingly, many of the genes identified in this study localized within identified human myopia quantitative trait loci (QTL)733 suggesting functional overlaps with myopia in humans.
6.2 Gene Expression Changes in Other Ocular Tissues
Rada and Wiechmann533 analyzed changes in gene expression in the retina/RPE/choroid complex during the recovery from FDM in chicks, and identified 12 differentially expressed genes. The majority of changes were small (≤3.7-fold); however, one gene, avian thymic hormone, was highly upregulated in recovering eyes (+12.3-fold). Shelton et al.617 used human genome oligonucleotide–based microarrays to analyze differential gene expression in the choroid/RPE of marmoset monkeys after 92 days of binocular lens treatment with lenses of opposite sign (±5 D). These authors reported the identification of 204 differentially expressed genes. In a recent RNA-seq study, Riddell et al.510 also identified a number of differentially expressed genes in the retina/RPE/choroid in the chick model of myopia.
Analysis of myopia-associated genes in these studies suggests that multiple biological processes are involved in refractive eye development, including ECM remodeling, visual cycle, neuronal development, eye growth, ion transport, retinal cell development, and neural signaling.510,626,720,731–733,748–755 Importantly, genes differentially expressed in animal models of myopia were often found to be localized within QTLs linked to human myopia.626
Increasing evidence also suggests that miRNAs are involved in the development of myopia.735,756 Chen et al.757 reported that single nucleotide polymorphism (SNP) rs662702 in the 3′ untranslated region (UTR) of the eye development homeobox gene PAX6 was significantly associated with extreme myopia (although it should be noted that genetic studies linking PAX6 to myopia have produced conflicting evidence758). This SNP was located in a miR-328 binding site and the risk allele was shown to reduce expression of PAX6. Presence of the risk allele was also associated with changes in expression of several other myopia-associated genes and proteins, such as TGF-β3, MMP-2, collagen 1, and integrin B1. Moreover, RA, which is implicated in myopia development,518,648–651,759 increased miR-328 expression in a dose-dependent fashion that, in turn, suppressed PAX6 expression.757 FDM in mice is associated with large-scale changes in miRNA expression in the retina.756
In experimental species the genetic background of individual animals and breeds are known to affect eye size, refraction, and the response to visual signals. Several studies have indicated that genetic background affects eye size and refractive eye development in primates760 and mice.542,543,742,761–767 It appears that even small variations in genetic background, such as differences between individual strains of mice, can substantially affect eye size766,767 as well as refractive eye development and susceptibility to experimentally induced myopia.48,543 Studies in chicks also observed the degree of FDM induced in individual birds to vary widely, suggesting that genetic differences might underlie this variability.73,160,440,768 Subsequent work in chicks with normal visual experience confirmed that emmetropization was fully effective in offspring from crosses between ‘broiler' and ‘layer' chicks despite very large differences in eye size between the progenitor broiler and layer lines.769 This implies, at least in chicks, that genetic predisposition to a long axial length or a steep corneal curvature can be compensated by regulating the rate of eye growth via visually guided feedback. Other studies in chicks suggested that individual animals have their own natural ‘set-point' toward which emmetropization aims770 and that the level of FDM induced by two successive episodes of visual deprivation is correlated within individual animals,74 strongly arguing that susceptibility to induced myopia in chicks is genetically determined. More recent work in guinea pigs,771 mice,48 and dogs772–775 has confirmed that specific strains or breeds have a higher prevalence of spontaneous myopia or are more susceptible to FDM. This is further evidence that among the genetic differences that define strains there are naturally occurring polymorphisms capable of altering refractive development.
Chen et al.75 tested the hypothesis that susceptibility to FDM in the chick is genetically determined. Starting with a genetically diverse (outbred) population, they form deprived a large number of individuals and mated males and females that both developed a high degree of FDM or that both developed a low degree of FDM. After two rounds of selective breeding, chicks from parental birds with high susceptibility to FDM were themselves more susceptible to FDM, while conversely chicks from parental birds with low susceptibility to FDM were themselves less susceptible. Results of this selective breeding indicated that approximately 50% of the variability in the degree of FDM was attributed to genetic inheritance,244 with the mode of inheritance being more consistent with a polygenic model than dominant or recessive inheritance of a single major susceptibility gene. Interestingly, chicks selected for high susceptibility to FDM were also more susceptible to lens-induced myopia, but not to lens-induced hyperopia.75,244 This is strong evidence that at least some of the molecular pathways that determine susceptibility to FDM and lens-induced myopia are shared (unlike some of evidence from pharmacologic studies, in which the possibility of off-target drug effects make such findings inconclusive496).
Genetically modified mice have also been used to explore the contributions of different retinal pathways to refractive eye development. Mice with mutations in proteins involved in the retinal ON pathway transmission (Nyx and mGluR6) have shown increased susceptibility to FDM.542,585 In contrast, mice with a genetic defect in the retinal OFF pathway (Vsx1) show the same response to visual form deprivation as wild-type mice.543 These results suggest that ON pathway transmission may have greater contribution to refractive eye development and myopia than OFF pathway signaling,555 a result predicted by earlier work by Crewther and Crewther.554 Other studies showed that photoreceptors are essential for refractive eye development as Gnat1−/− mice with a rod transducin mutation do not develop FDM.776
Genetic knockouts support the hypothesis that DA signaling is implicated in refractive eye development.497,777 Mice with diminished retinal DA because of the genetic deletion of tyrosine hydroxylase become more myopic than wild-type mice at all ages; although their response to form deprivation was similar to wild-type mice.778 Examination of the role of different DA receptors using gene knockouts has shown that DA D2 receptor (D2R) knockout mice have normal refractive development, but reduced susceptibility to FDM compared with wild-type mice.779
Perhaps the most compelling evidence supporting similarities between human and mouse signaling pathways underlying visually guided eye growth and development of refractive state comes from recent studies that analyzed the effect of targeted deletion of four candidate genes for human myopia on refractive eye development in mice. Nonsense mutations in the gene encoding a transmembrane protein (SLITRK6) was found to cause the development of high myopia in three families of distinct ethnic origin, while targeted deletion of SLITRK6 causes abnormal eye enlargement consistent with myopia in mice.765 It was also found that a candidate gene, SCO2, localized within a human myopia locus on chromosome 22q13.33 is highly differentially expressed in the lens-induced mouse myopia model.750 The APLP2 gene was associated with refractive error development in nonhuman primates, human children, and mice.535,737 Genetic deletion of lumican (LUM), a candidate gene for human myopia, results in excessive axial elongation of the eye in mice.780
7. Conclusions
Experimental models have established the importance of visual feedback in eye and refractive state development. Studies with several well-established experimental myopia paradigms in a variety of species have helped create a framework for understanding the interactions of visual experience, environment, and genetics, as well as the pathways controlling postnatal eye growth, emmetropization, and the development of myopia. Experimental models have led to speculation that myopia may develop initially as an adaptation to environmental visual conditions through mechanisms of emmetropization, but may progress due to a combination of visual conditions and genetic factors that alter the operation of visually guided eye growth control mechanisms. The main features of a basic model of visually controlled eye growth and refractive state, based on the findings of experimental animal models, are summarized in Figure 18.
Figure 18.
A heuristic model of the visually regulated control of eye growth and refractive state.
Much has been learned about emmetropization and myopia development from the study of experimental animal models. Over a span of more than 40 years, some of this work has led to new and effective optical treatments for myopia in humans. Experimental models continue to inform the refinement of those treatments. As research with experimental models achieves a more complete understanding of the mechanisms of emmetropization, including the neural circuits, cellular, and molecular biology involved, the development of new, and even more effective, treatments are possible.
8. Summary Points
Results from experimental studies using animal models have shifted thinking about the control of eye growth and the development of refractive state. The following list of summary points highlight the main contributions from research using experimental models to understanding the mechanisms of emmetropization, and the development and control of myopia.
Visual signals relating to retinal defocus control eye growth and guide emmetropization, and the refractive development of the eye. Imposing hyperopic or myopic defocus in animal models results in compensatory changes in eye growth that reduces the imposed refractive error. Visually regulated changes in eye growth produce the largest effects in the eyes of younger animals, but can produce compensatory changes in the eyes of older animals as well.
Visual signals guiding eye growth are processed locally within the eye. Optic nerve section does not prevent compensation for defocus and restricting defocus to local retinal regions results in local changes in eye growth. Visual signals in large areas of peripheral retina produce growth changes that can affect axial length and central refractive state.
The choroid is an active component in the visual control of eye growth and refraction. Choroidal thickness changes are part of the compensatory response to imposed defocus and may act as an accommodative response that modulates emmetropization and eye growth.
The eye growth response to visual signals involves changes to sclera ECM synthesis and biomechanical properties.
Light intensity and the spectral composition of light affect eye growth in complex ways that interact with ocular circadian rhythms and the temporal characteristics of visual signals.
Atropine affects eye growth and prevents experimentally imposed myopia through cellular mechanisms that do not involve accommodation or ciliary muscle activity, and may act through muscarinic and nonmuscarinic actions.
Experimental studies have identified several biochemical compounds, most notably retinal DA, RA, and NO that are involved in the modulation of eye growth. Various changes in the retina, RPE, choroid, and sclera suggest the existence of a cascade of cell signals arising from the retina that modulates scleral biochemistry and regulates eye growth.
Molecular changes in gene expression in retina, RPE, choroid, and sclera support the signal cascade hypothesis and suggest that the retina signals hyperopic defocus and myopic defocus for eye growth through different pathways. Identifying the components of these pathways may offer specific targets for the development of novel drug treatments for controlling eye growth and myopia progression.
Acknowledgments
The authors acknowledge the contributions of Baskar Arumugam, Ranjay Chakraborty, Marita Feldkaemper, Adriana Iglesias Gonzalez, Jeremy Guggenheim, Toshihide Kurihara, Sally McFadden, Frances Rucker, and Frank Schaeffel. The authors also thank Kate Thomson, Cindy Karuta, the members of the International Myopia Institute white paper committees for their reviews and suggestions, and Monica Jong for her assistance throughout this project.
Supported by grants from the International Myopia Institute (Sydney, NSW, Australia). The publication costs of the International Myopia Institute reports were supported by donations from the Brien Holden Vision Institute, Carl Zeiss Vision, Coopervision, Essilor, Alcon, and Vision Impact Institute.
Disclosure: D. Troilo, Alcon (C), Essilor (C), Johnson & Johnson (C), P; E.L. Smith III, Brien Holden Vision Institute (F), SightGlass Vision (C), Tree House Eyes (C), P; D.L. Nickla, None; R. Ashby, P; A.V. Tkatchenko, P; L.A. Ostrin, None; T.J. Gawne, None; M.T. Pardue, None; J.A. Summers, P; C. Kee, None; F. Schroedl, None; S. Wahl, Carl Zeiss Vision International (E); L. Jones, Alcon (F, C, R), Allergan (F), Contamac (F), CooperVision (F, C, R), Essilor (F), GL Chemtec (F), Inflamax Research (F), Johnson & Johnson Vision (F, C, R), Menicon (F), Nature's Way (F), Novartis (F, C, R), Ophtecs (C, R), Safilens (F), Santen (F), Shire (F), SightGlass (F), TearLab (F), TearScience (F), Visioneering (F)
References
- 1.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Turnbull PR, Backhouse S, Phillips JR. Visually guided eye growth in the squid. Curr Biol. 2015;25:R791–R792. doi: 10.1016/j.cub.2015.07.073. [DOI] [PubMed] [Google Scholar]
- 3.Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu SM. From retinal circuitry to eye diseases – in memory of Henk Spekreijse. Vision Res. 2009;49:992–995. doi: 10.1016/j.visres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walls GL. The Vertebrate Eye and its Adaptive Radiations Hafner Publishing 1970 ed. Bloomfield Hills: Cranbrook Institute of Science;; 1942. [Google Scholar]
- 6.Troilo D, Wallman J. Changes in corneal curvature during accommodation in chicks. Vision Res. 1987;27:241–247. doi: 10.1016/0042-6989(87)90186-6. [DOI] [PubMed] [Google Scholar]
- 7.Schaeffel F, Howland HC. Corneal accommodation in chick and pigeon. J Comp Physiol A. 1987;160:375–384. doi: 10.1007/BF00613027. [DOI] [PubMed] [Google Scholar]
- 8.Glasser A, Troilo D, Howland HC. The mechanism of corneal accommodation in chicks. Vision Res. 1994;34:1549–1566. doi: 10.1016/0042-6989(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 9.Schaeffel F, Feldkaemper M. Animal models in myopia research. Clin Exp Optom. 2015;98:507–517. doi: 10.1111/cxo.12312. [DOI] [PubMed] [Google Scholar]
- 10.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 11.Wiesel TN, Raviola E. Increase in axial length of the macaque monkey eye after corneal opacification. Invest Ophthalmol Vis Sci. 1979;18:1232–1236. [PubMed] [Google Scholar]
- 12.Troilo D, Howland HC, Judge SJ. Visual optics and retinal cone topography in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1301–1310. doi: 10.1016/0042-6989(93)90038-x. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein B, Grether WF. A comparison of visual acuity in the rhesus monkey and man. J Comp Physiol A. 1940;30:187–195. [Google Scholar]
- 14.Qiao-Grider Y, Hung L-F, Kee CS, Ramamirtham R, Smith EL., III. Normal ocular development in young rhesus monkeys (Macaca mulatta) Vision Res. 2007;47:1424–1444. doi: 10.1016/j.visres.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendrickson AE, Troilo D, Possin DE, Springer A. Development of neural retina and vasculature in the marmoset Callithrix jacchus. J Comp Neurol. 2006;497:270–286. doi: 10.1002/cne.20996. [DOI] [PubMed] [Google Scholar]
- 16.Bowmaker JK, Dartnall HJ, Lythgoe JN, Mollon JD. The visual pigments of rods and cones in the rhesus monkey, Macaca mulatta. J Physiol. 1978;274:329–348. doi: 10.1113/jphysiol.1978.sp012151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Travis DS, Bowmaker JK, Mollon JD. Polymorphism of visual pigments in a Callitrichid monkey. Vision Res. 1988;28:481–490. doi: 10.1016/0042-6989(88)90170-8. [DOI] [PubMed] [Google Scholar]
- 18.Nummela SU, Coop SH, Cloherty SL, Boisvert CJ, Leblanc M, Mitchell JF. Psychophysical measurement of marmoset acuity and myopia. Dev Neurobiol. 2016;77:300–313. doi: 10.1002/dneu.22467. [DOI] [PubMed] [Google Scholar]
- 19.Morcos Y, Chan-Ling T. Concentration of astrocytic filaments at the retinal optic nerve junction is coincident with the absence of intra-retinal myelination: comparative and developmental evidence. J Neurocytol. 2000;29:665–678. doi: 10.1023/a:1010835404754. [DOI] [PubMed] [Google Scholar]
- 20.Croft MA, Kaufman PL, Crawford KS, Neider MW, Glasser A, Bito LZ. Accommodation dynamics in aging rhesus monkeys. Am J Physiol. 1998;44:R1885–R1897. doi: 10.1152/ajpregu.1998.275.6.R1885. [DOI] [PubMed] [Google Scholar]
- 21.Ostrin LA, Glasser A. Autonomic drugs and the accommodative system in rhesus monkeys. Exp Eye Res. 2010;90:104–112. doi: 10.1016/j.exer.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troilo D, Quinn N, Baker K. Accommodation and induced myopia in marmosets. Vision Res. 2007;47:1228–1244. doi: 10.1016/j.visres.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith EL, III,, Harwerth RS. Behavioral measurements of accommodative amplitude in rhesus monkeys. Vision Res. 1984;24:1821–1827. doi: 10.1016/0042-6989(84)90013-0. [DOI] [PubMed] [Google Scholar]
- 24.Bito LZ, DeRousseau CJ, Kaufman PL, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Invest Ophthalmol Vis Sci. 1982;23:23–31. [PubMed] [Google Scholar]
- 25.Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus) Comp Med. 2003;53:364–368. [PubMed] [Google Scholar]
- 26.Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124:154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- 27.Müller B, Peichl L. Topography of cones and rods in the tree shrew retina. J Comp Neurol. 1989;282:581–594. doi: 10.1002/cne.902820409. [DOI] [PubMed] [Google Scholar]
- 28.Samorajski T, Ordy JM, Keefe JR. Structural organization of the retina in the tree shrew (Tupaia glis) J Cell Biol. 1966;28:489–504. doi: 10.1083/jcb.28.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petry HM, Erichsen JT, Szel A. Immunocytochemical identification of photoreceptor populations in the tree shrew retina. Brain Res. 1993;616:344–350. doi: 10.1016/0006-8993(93)90230-k. [DOI] [PubMed] [Google Scholar]
- 30.Petry HM, Fox R, Casagrande VA. Spatial contrast sensitivity of the tree shrew. Vision Res. 1984;24:1037–1042. doi: 10.1016/0042-6989(84)90080-4. [DOI] [PubMed] [Google Scholar]
- 31.Norton TT, McBrien NA. Normal development of the refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32:833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 32.Petry HM, Harosi FI. Visual pigments of the tree shrew (Tupaia belangeri) and greater galago (Galago crassicaudatus) a microspectrophotometric investigation. Vision Res. 1990;30:839–851. doi: 10.1016/0042-6989(90)90053-n. [DOI] [PubMed] [Google Scholar]
- 33.Albon J, Farrant S, Akhtar S, et al. Connective tissue structure of the tree shrew optic nerve and associated ageing changes. Invest Ophthalmol Vis Sci. 2007;48:2134–2144. doi: 10.1167/iovs.06-0084. [DOI] [PubMed] [Google Scholar]
- 34.McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Res. 1992;32:843–852. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- 35.Cottriall CL, McBrien NA. The M1 muscarinic antagonist pirenzepine reduces myopia and eye enlargement in the tree shrew. Invest Ophthalmol Vis Sci. 1996;37:1368–1379. [PubMed] [Google Scholar]
- 36.Howlett MH, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus) Vision Res. 2006;46:267–283. doi: 10.1016/j.visres.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 37.Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–227. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Rohlich P, van Veen T, Szel A. Two different visual pigments in one retinal cone cell. Neuron. 1994;13:1159–1166. doi: 10.1016/0896-6273(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 39.Do-Nascimento JL, Do-Nascimento RS, Damasceno BA, Silveira LC. The neurons of the retinal ganglion cell layer of the guinea pig: quantitative analysis of their distribution and size. Braz J Med Biol Res. 1991;24:199–214. [PubMed] [Google Scholar]
- 40.Buttery RG, Hinrichsen CFL, Weller WL, Haight JR. How thick should a retina be? A comparative study of mammalian species with an without intraretinal vasculature. Vision Res. 1991;31:169–187. doi: 10.1016/0042-6989(91)90110-q. [DOI] [PubMed] [Google Scholar]
- 41.Ostrin LA, Wildsoet CF. Optic nerve head and intraocular pressure in the guinea pig eye. Exp Eye Res. 2016;146:7–16. doi: 10.1016/j.exer.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howlett MH, McFadden SA. Emmetropization and schematic eye models in developing pigmented guinea pigs. Vision Res. 2007;47:1178–1190. doi: 10.1016/j.visres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Jiang L, Schaeffel F, Zhou X, et al. Spontaneous axial myopia and emmetropization in a strain of wild-type guinea pig (Cavia porcellus) Invest Ophthalmol Vis Sci. 2009;50:1013–1019. doi: 10.1167/iovs.08-2463. [DOI] [PubMed] [Google Scholar]
- 44.Ostrin LA, Garcia MB, Choh V, Wildsoet CF. Pharmacologically stimulated pupil and accommodative changes in Guinea pigs. Invest Ophthalmol Vis Sci. 2014;55:5456–5465. doi: 10.1167/iovs.14-14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tejedor J, de la Villa P. Refractive changes induced by form deprivation the mouse eye. Invest Ophthalmol Vis Sci. 2003;44:32–36. doi: 10.1167/iovs.01-1171. [DOI] [PubMed] [Google Scholar]
- 46.Tkatchenko TV, Shen Y, Tkatchenko AV. Mouse experimental myopia has features of primate myopia. Invest Ophthalmol Vis Sci. 2010;51:1297–1303. doi: 10.1167/iovs.09-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barathi VA, Boopathi VG, Yap EP, Beuerman RW. Two models of experimental myopia in the mouse. Vision Res. 2008;48:904–916. doi: 10.1016/j.visres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Schaeffel F, Burkhardt E, Howland HC, Williams RW. Measurement of refractive state and deprivation myopia in two strains of mice. Optom Vis Sci. 2004;81:99–110. doi: 10.1097/00006324-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Zhou G, Williams RW. Mouse models for the analysis of myopia: an analysis of variation in eye size of adult mice. Optom Vis Sci. 1999;76:408–418. doi: 10.1097/00006324-199906000-00021. [DOI] [PubMed] [Google Scholar]
- 50.Pardue MT, Stone RA, Iuvone PM. Investigating mechanisms of myopia in mice. Exp Eye Res. 2013;114:96–105. doi: 10.1016/j.exer.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flores AE, Flores JE, Deshpande H, et al. Pattern recognition of sleep in rodents using piezoelectric signals generated by gross body movements. IEEE Trans Biomed Eng. 2007;54:225–233. doi: 10.1109/TBME.2006.886938. [DOI] [PubMed] [Google Scholar]
- 52.Yang HS, Vitaterna MH, Laposky AD, Shimomura K, Turek FW. Genetic analysis of daily physical activity using a mouse chromosome substitution strain. Physiol Genomics. 2009;39:47–55. doi: 10.1152/physiolgenomics.00066.2009. [DOI] [PubMed] [Google Scholar]
- 53.Jacobs GH. Losses of functional opsin genes, short-wavelength cone photopigments, and color vision-A significant trend in the evolution of mammalian vision. Vis Neurosci. 2013;30:39–53. doi: 10.1017/S0952523812000429. [DOI] [PubMed] [Google Scholar]
- 54.Tkatchenko TV, Shen Y, Braun RD, et al. Photopic visual input is necessary for emmetropization in mice. Exp Eye Res. 2013;115:87–95. doi: 10.1016/j.exer.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buhot MC, Dubayle D, Malleret G, Javerzat S, Segu L. Exploration, anxiety, and spatial memory in transgenic anophthalmic mice. Behav Neurosci. 2001;115:455–467. [PubMed] [Google Scholar]
- 56.Cook MN, Williams RW, Flaherty L. Anxiety-related behaviors in the elevated zero-maze are affected by genetic factors and retinal degeneration. Behav Neurosci. 2001;115 [PubMed] [Google Scholar]
- 57.de la Cera EG, Rodriguez G, Llorente L, Schaeffel F, Marcos S. Optical aberrations in the mouse eye. Vision Res. 2006;46:2546–2553. doi: 10.1016/j.visres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 58.Faulstich BM, Onori KA, du Lac S. Comparison of plasticity and development of mouse optokinetic and vestibulo-ocular reflexes suggests differential gain control mechanisms. Vision Res. 2004;44:3419–3427. doi: 10.1016/j.visres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Jiang X, Kurihara T, Kunimi H, et al. A highly efficient murine model of experimental myopia. Sci Rep. 2018;8:2026. doi: 10.1038/s41598-018-20272-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chalupa LM, Williams RW. Eye, Retina, and Visual System of the Mouse. Cambridge: MIT Press;; 2008. [Google Scholar]
- 61.Szel A, Rohlich P, Mieziewska K, Aguirre G, van Veen T. Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: a developmental study. J Comp Neurol. 1993;331:564–577. doi: 10.1002/cne.903310411. [DOI] [PubMed] [Google Scholar]
- 62.Szel A, Rohlich P, Caffe AR, Juliusson B, Aguirre G, Van Veen T. Unique topographic separation of two spectral classes of cones in the mouse retina. J Comp Neurol. 1992;325:327–342. doi: 10.1002/cne.903250302. [DOI] [PubMed] [Google Scholar]
- 63.Applebury ML, Antoch MP, Baxter LC, et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 64.Drager UC, Olsen JF. Ganglion cell distribution in the retina of the mouse. Invest Ophthalmol Vis Sci. 1981;20:285–293. [PubMed] [Google Scholar]
- 65.Leamey CA, Protti DA, Dreher B. Comparative survey of the mammalian visual system with reference to the mouse. In: Chalupa LM, Williams RW, editors. Eye, Retina, and Visual System of the Mouse. Cambridge, MA: MIT Press;; 2008. pp. 35–60. [Google Scholar]
- 66.Pettigrew JD, Dreher B, Hopkins CS, McCall MJ, Brown M. Peak density and distribution of ganglion cells in the retinae of microchiropteran bats: implications for visual acuity. Brain Behav Evol. 1988;32:39–56. doi: 10.1159/000116531. [DOI] [PubMed] [Google Scholar]
- 67.Sun M, Lye-Barthel M, Mastand RH, Jakobs TC. The morphology and spatial arrangement of astrocytes in the optic nerve head of the mouse. J Comp Neurol. 2009;516:1–19. doi: 10.1002/cne.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Remtulla S, Hallett PE. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 1985;25:21–31. doi: 10.1016/0042-6989(85)90076-8. [DOI] [PubMed] [Google Scholar]
- 69.Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 2004;44:1857–1867. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 70.Kuszak JR, Mazurkiewicz M, Jison L, Madurski A, Ngando A, Zoltoski RK. Quantitative analysis of animal model lens anatomy: accommodative range is related to fiber structure and organization. Vet Ophthalmol. 2006;9:266–280. doi: 10.1111/j.1463-5224.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 71.Ott M. Visual accommodation in vertebrates: mechanisms, physiological response and stimuli. J Comp Physiol A. 2006;192:97–111. doi: 10.1007/s00359-005-0049-6. [DOI] [PubMed] [Google Scholar]
- 72.Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- 73.Schmid K, Wildsoet C. Breed- and gender-dependent differences in eye growth and form deprivation responses in chick. J Comp Physiol A. 1996;178:551–561. doi: 10.1007/BF00190185. [DOI] [PubMed] [Google Scholar]
- 74.Saltarelli D, Wildsoet C, Nickla D, Troilo D. Susceptibility to form deprivation myopia in chicks is not altered by an early experience of myopia. Optom Vis Sci. 2004;81:119–126. doi: 10.1097/00006324-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Chen YP, Hocking PM, Wang L, et al. Selective breeding for susceptibility to myopia reveals a gene-environment interaction. Invest Ophthalmol Vis Sci. 2011;52:4003–4011. doi: 10.1167/iovs.10-7044. [DOI] [PubMed] [Google Scholar]
- 76.Bruhn SL, Cepko CL. Development of the pattern of photoreceptors in the chick retina. J Neurosci. 1996;16:1430–1439. doi: 10.1523/JNEUROSCI.16-04-01430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kram YA, Mantey S, Corbo JC. Avian cone photoreceptors tile the retina as five independent, self-organizing mosaics. PLoS One. 2010;5:e8992. doi: 10.1371/journal.pone.0008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bowmaker JK, Heat LA, Wilkie SE, Hunt DM. Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vision Res. 1997;37:2183–2194. doi: 10.1016/s0042-6989(97)00026-6. [DOI] [PubMed] [Google Scholar]
- 79.Schmid KL, Wildsoet CF. Assessment of visual acuity and contrast sensitivity in the chick using an optokinetic nystagmus paradigm. Vision Res. 1998;38:2629–2634. doi: 10.1016/s0042-6989(97)00446-x. [DOI] [PubMed] [Google Scholar]
- 80.Jarvis JR, Abeyesinghe SM, McMahon CE, Wathes CM. Measuring and modelling the spatial contrast sensitivity of the chicken (Gallus g. domesticus) Vision Res. 2009;49:1448–1454. doi: 10.1016/j.visres.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 81.Irving EL, Sivak JG, Curry TA, Callender MG. Chick eye optics: zero to fourteen days. J Comp Physiol A. 1996;179:185–194. doi: 10.1007/BF00222785. [DOI] [PubMed] [Google Scholar]
- 82.Morris VB. An afoveate area centralis in the chick retina. J Comp Neurol. 1982;210:198–203. doi: 10.1002/cne.902100210. [DOI] [PubMed] [Google Scholar]
- 83.Straznicky C, Chehade M. The formation of the area centralis of the retinal ganglion cell layer in the chick. Development. 1987;100:411–420. doi: 10.1242/dev.100.3.411. [DOI] [PubMed] [Google Scholar]
- 84.Nava DR, Antony B, Zhang LI, Abramoff MD, Wildsoet CF. Novel method using 3-dimensional segmentation in spectral domain-optical coherence tomography imaging in the chick reveals defocus-induced regional and time-sensitive asymmetries in the choroidal thickness. Vis Neurosci. 2016;33:E010. doi: 10.1017/S0952523816000067. [DOI] [PubMed] [Google Scholar]
- 85.Pettigrew JD, Wallman J, Wildsoet CF. Saccadic oscillations facilitate ocular perfusion from the avian pecten. Nature. 1990;343:362–363. doi: 10.1038/343362a0. [DOI] [PubMed] [Google Scholar]
- 86.Weller C, Lindstrom SH, De Grip WJ, Wilson M. The area centralis in the chicken retina contains efferent target amacrine cells. Vis Neurosci. 2009;26:249–254. doi: 10.1017/S0952523808080917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schaeffel F, Howland HC. Visual optics in normal and ametropic chickens. Clin Vis Sci. 1988;3:83–98. [Google Scholar]
- 88.Ostrin LA, Liu Y, Choh V, Wildsoet CF. The role of the iris in chick accommodation. Invest Ophthalmol Vis Sci. 2011;52:4710–4716. doi: 10.1167/iovs.10-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Glasser A, Murphy CJ, Troilo D, Howland HC. The mechanism of lenticular accommodation in chicks. Vision Res. 1995;35:1525–1540. doi: 10.1016/0042-6989(94)00211-4. [DOI] [PubMed] [Google Scholar]
- 90.Pilar G, Nuñez R, McLennan IS, Meriney SD. Muscarinic and nicotinic synaptic activation of the developing chicken iris. J Neurosci. 1987;7:3813–3826. doi: 10.1523/JNEUROSCI.07-12-03813.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Underwood H, Steele CT, Zivkovic B. Circadian organization and the role of the pineal in birds. Microsc Res Tech. 2001;53:48–62. doi: 10.1002/jemt.1068. [DOI] [PubMed] [Google Scholar]
- 92.Natesan A, Geetha L, Zatz M. Rhythm and soul in the avian pineal. Cell Tiss Res. 2002;309:35–45. doi: 10.1007/s00441-002-0571-6. [DOI] [PubMed] [Google Scholar]
- 93.Yamazaki S, Goto M, Menaker M. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. J Biol Rhythms. 1999;14:197–201. doi: 10.1177/074873099129000605. [DOI] [PubMed] [Google Scholar]
- 94.Meijer JH, Thio B, Albus H, Schaap J, Ruijs AC. Functional absence of extraocular photoreception in hamster circadian rhythm entrainment. Brain Res. 1999;831:337–339. doi: 10.1016/s0006-8993(99)01509-7. [DOI] [PubMed] [Google Scholar]
- 95.Li T, Troilo D, Howland HC. Diurnal illumination patterns affect the development of the chick eye. Vision Res. 2000;40:2387–2393. doi: 10.1016/s0042-6989(00)00098-5. [DOI] [PubMed] [Google Scholar]
- 96.Lauber JK, Oishi T. Ocular responses of genetically blind chicks to the light environment and to lid suture. Curr Eye Res. 1989;8:757–764. doi: 10.3109/02713688909000865. [DOI] [PubMed] [Google Scholar]
- 97.Lauber JK, Oishi T. Kainic acid and formoguanamine effects on environmentally-induced eye lesions in chicks. J Ocul Pharmacol. 1990;6:151–156. doi: 10.1089/jop.1990.6.151. [DOI] [PubMed] [Google Scholar]
- 98.Li T, Howland HC. Role of the pineal gland in ocular development of the chick in normal and constant light conditions. Invest Ophthalmol Vis Sci. 2006;47:5132–5136. doi: 10.1167/iovs.05-0671. [DOI] [PubMed] [Google Scholar]
- 99.Li T, Howland HC. Modulation of constant light effects on the eye by ciliary ganglionectomy and optic nerve section. Vision Res. 2000;40:2249–2256. doi: 10.1016/s0042-6989(00)00097-3. [DOI] [PubMed] [Google Scholar]
- 100.Kröger RHH, Wagner HJ. The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. J Comp Physiol A. 1996;179:837–842. doi: 10.1007/BF00207362. [DOI] [PubMed] [Google Scholar]
- 101.Shen W, Vijayan M, Sivak JG. Inducing form-deprivation myopia in fish. Invest Ophthalmol Vis Sci. 2005;46:1797–1803. doi: 10.1167/iovs.04-1318. [DOI] [PubMed] [Google Scholar]
- 102.Collery RF, Veth KN, Dubis AM, Carroll J, Link BA. Rapid, accurate, and non-invasive measurement of zebrafish axial length and other eye dimensions using SD-OCT allows longitudinal analysis of myopia and emmetropization. PLoS One. 2014;9:e110699. doi: 10.1371/journal.pone.0110699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol Vis Sci. 2007;48:4829–4837. doi: 10.1167/iovs.06-1273. [DOI] [PubMed] [Google Scholar]
- 104.Timucin OB, Arabaci M, Cuce F, et al. The effects of light sources with different spectral structures on ocular axial length in rainbow trout (Oncorhynchus mykiss) Exp Eye Res. 2016;151:212–221. doi: 10.1016/j.exer.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 105.Rinner O, Rick JM, Neuhauss SC. Contrast sensitivity, spatial and temporal tuning of the larval zebrafish optokinetic response. Invest Ophthalmol Vis Sci. 2005;46:137–142. doi: 10.1167/iovs.04-0682. [DOI] [PubMed] [Google Scholar]
- 106.Haug MF, Biehlmaier O, Mueller KP, Neuhauss SC. Visual acuity in larval zebrafish: behavior and histology. Front Zool. 2010;7:8. doi: 10.1186/1742-9994-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mueller KP, Schnaedelbach OD, Russig HD, Neuhauss SC. VisioTracker, an innovative automated approach to oculomotor analysis. J Vis Exp. 2011;56:3556. doi: 10.3791/3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tappeiner C, Gerber S, Enzmann V, Balmer J, Jazwinska A, Tschopp M. Visual acuity and contrast sensitivity of adult zebrafish. Front Zool. 2012;9:10. doi: 10.1186/1742-9994-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cameron DJ, Rassamdana F, Tam P, et al. The optokinetic response as a quantitative measure of visual acuity in zebrafish. J Vis Exp. 2013;80:50832. doi: 10.3791/50832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Allison WT, Haimberger TJ, Hawryshyn CW, Temple SE. Visual pigment composition in zebrafish: evidence for a rhodopsin-porphyropsin interchange system. Vis Neurosci. 2004;21:945–952. doi: 10.1017/S0952523804216145. [DOI] [PubMed] [Google Scholar]
- 111.Richardson R, Tracey-White D, Webster A, Moosajee M. The zebrafish eye-a paradigm for investigating human ocular genetics. Eye. 2017;31:68–86. doi: 10.1038/eye.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mangrum WI, Dowling JE, Cohen ED. A morphological classification of ganglion cells in the zebrafish retina. Vis Neurosci. 2002;19:767–779. doi: 10.1017/s0952523802196076. [DOI] [PubMed] [Google Scholar]
- 113.Alvarez Y, Cederlund ML, Cottell DC, et al. Genetic determinants of hyaloid and retinal vasculature in zebrafish. BMC Dev Biol. 2007;7:114. doi: 10.1186/1471-213X-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koke JR, Mosier AL, Garcia DM. Intermediate filaments of zebrafish retinal and optic nerve astrocytes and Muller glia: differential distribution of cytokeratin and GFAP. BMC Res Notes. 2010;3:50. doi: 10.1186/1756-0500-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Easter SS, Nicola GN. The development of vision in the zebrafish (Danio rerio) Dev Biol. 1996;180:646–663. doi: 10.1006/dbio.1996.0335. [DOI] [PubMed] [Google Scholar]
- 116.Fernald RD, Wright SE. Growth of the visual system in the African cichlid fish, Haplochromis burtoni Accommodation. Vision Res. 1985;25:163–170. doi: 10.1016/0042-6989(85)90109-9. [DOI] [PubMed] [Google Scholar]
- 117.Somiya H. Dynamic mechanism of visual accommodation in teleosts: structure of the lens muscle and its nerve control. Proc R Soc Lond B Biol Sci. 1987;230:77–91. doi: 10.1098/rspb.1987.0010. [DOI] [PubMed] [Google Scholar]
- 118.Fadool JM, Dowling JE. Zebrafish: a model system for the study of eye genetics. Prog Ret Eye Res. 2007;27:89–110. doi: 10.1016/j.preteyeres.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malicki JJ, Pujic Z, Thisse C, Thisse B, Wei XY. Forward and reverse genetic approaches to the analysis of eye development in zebrafish. Vision Res. 2002;42:527–533. doi: 10.1016/s0042-6989(01)00262-0. [DOI] [PubMed] [Google Scholar]
- 120.Veth KN, Willer JR, Collery RF, et al. Mutations in zebrafish lrp2 result in adult-onset ocular pathogenesis that models myopia and other risk factors for glaucoma. PLoS Genet. 2011;7:e1001310. doi: 10.1371/journal.pgen.1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Greene PR. Optical constants and dimensions for the myopic, hyperopic and normal rhesus eye. Exp Eye Res. 1990;51:351–360. doi: 10.1016/0014-4835(90)90148-n. [DOI] [PubMed] [Google Scholar]
- 122.Lapuerta P, Schein SJ. A four-surface schematic eye of Macaque monkey obtained by an optical method. Vision Res. 1995;35:2245–2254. doi: 10.1016/0042-6989(94)00320-l. [DOI] [PubMed] [Google Scholar]
- 123.Hughes A. The topography of vision in mammals of contrasting life style: comparative optics and retinal organisation. In: Crescitelli F, editor. The Visual System in Vertebrates. Berlin, Germany: Springer-Verlag;; 1977. pp. 613–756. [Google Scholar]
- 124.Hughes A. A useful table of reduced schematic eyes for vertebrates which includes computed longitudinal chromatic aberrations. Vision Res. 1979;19:1273–1275. doi: 10.1016/0042-6989(79)90195-0. [DOI] [PubMed] [Google Scholar]
- 125.Hughes A. The schematic eye comes of age. In: Pettigrew JD, KJ Sanderson, Levick WR, editors. Visual Neuroscience. Cambridge, UK: Cambridge University Press;; 1986. pp. 60–89. [Google Scholar]
- 126.Atchison DA, Thibos LN. Optical models of the human eye. Clin Exp Optom. 2016;99:99–106. doi: 10.1111/cxo.12352. [DOI] [PubMed] [Google Scholar]
- 127.Smith EL, III,, Bradley DV, Fernandes A, Boothe RG. Form deprivation myopia in adolescent monkeys. Optom Vis Sci. 1999;76:428–431. doi: 10.1097/00006324-199906000-00023. [DOI] [PubMed] [Google Scholar]
- 128.Troilo D, Nickla DL, Wildsoet CF. Form deprivation myopia in mature common marmosets (Callithrix jacchus) Invest Ophthalmol Vis Sci. 2000;41:2043–2049. [PubMed] [Google Scholar]
- 129.Siegwart JT, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- 130.Papastergiou GI, Schmid GF, Laties AM, Pendrak K, Lin T, Stone RA. Induction of axial eye elongation and myopic refractive error shift in one-year-old chickens. Vision Res. 1998;38:1883–1888. doi: 10.1016/s0042-6989(97)00347-7. [DOI] [PubMed] [Google Scholar]
- 131.Sorsby A, Benjamin B, Sheridan M, Stone J, Leary GA. Refraction and its components during the growth of the eye from the age of three. Memo Med Res Counc. 1961;301(Special):1–67. [PubMed] [Google Scholar]
- 132.Sorsby A, Leary GA, Richards MJ. Correlation ametropia and component ametropia. Vision Res. 1961;2:309–313. [Google Scholar]
- 133.Sorsby A, Leary GA, Fraser GR. Family studies on ocular refraction and its components. J Med Genet. 1966;3:269–273. doi: 10.1136/jmg.3.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–606. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- 135.Wallman J, Adams J, Trachtman JN. The eyes of young chickens grow toward emmetropia. Invest Ophthalmol Vis Sci. 1981;20:557–561. [PubMed] [Google Scholar]
- 136.Guyton DL, Greene PR, Scholz RT. Dark-rearing interference with emmetropization in the rhesus monkey. Invest Ophthalmol Vis Sci. 1989;30:761–764. [PubMed] [Google Scholar]
- 137.Gottlieb MD, Fugate-Wentzek LA, Wallman J. Different visual deprivations produce different ametropias and different eye shapes. Invest Ophthalmol Vis Sci. 1987;28:1225–1235. [PubMed] [Google Scholar]
- 138.Yinon U. Myopia induction in animals following alteration of the visual input during development: a review. Curr Eye Res. 1984;3:677–690. doi: 10.3109/02713688409003072. [DOI] [PubMed] [Google Scholar]
- 139.Smith EL., III. Environmentally induced refractive errors in animals. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Oxford, UK: Butterworth Heinemann;; 1998. pp. 57–90. [Google Scholar]
- 140.Mutti DO, Ver Hoeve JN, Zadnik K, Murphy CJ. The artifact of retinoscopy revisited: comparison of refractive error measured by retinoscopy and visual evoked potential in the rat. Optom Vis Sci. 1997;74:483–488. doi: 10.1097/00006324-199707000-00014. [DOI] [PubMed] [Google Scholar]
- 141.Hubel DH, Wiesel TN, LeVay S. Functional architecture of area 17 in normal and monocularly deprived macaque monkeys. Cold Spring Harb Symp Quant Biol. 1976;40:581–589. doi: 10.1101/sqb.1976.040.01.054. [DOI] [PubMed] [Google Scholar]
- 142.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol London. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Raviola E, Wiesel TN. Effect of dark-rearing on experimental myopia in monkeys. Invest Ophthalmol Vis Sci. 1978;17:485–488. [PubMed] [Google Scholar]
- 145.Raviola E, Wiesel TN. An animal model of myopia. New Eng J Med. 1985;312:1609–1615. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- 146.Rabin J, Van Sluyters RC, Malach R. Emmetropization: a vision-dependent phenomenon. Invest Ophthalmol Vis Sci. 1981;20:561–564. [PubMed] [Google Scholar]
- 147.O'Leary DJ, Millodot M. Eyelid closure causes myopia in humans. Experientia. 1979;35:1478–1479. doi: 10.1007/BF01962795. [DOI] [PubMed] [Google Scholar]
- 148.von Noorden GK, Lewis RA. Ocular axial length in unilateral congenital cataracts and blepharoptosis. Invest Ophthalmol Vis Sci. 1987;28:750–752. [PubMed] [Google Scholar]
- 149.Huo L, Cui D, Yang X, et al. A restrospective study: form-deprivation myopia in unilateral congenital ptosis. Clin Exp Optom. 2012;95:404–409. doi: 10.1111/j.1444-0938.2012.00716.x. [DOI] [PubMed] [Google Scholar]
- 150.Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35:37–70. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 151.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 152.McBrien NA, Lawlor P, Gentle A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Invest Ophthalmol Vis Sci. 2000;41:3713–3719. [PubMed] [Google Scholar]
- 153.Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1311–1324. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- 154.Gottlieb MD, Joshi HB, Nickla DL. Scleral changes in chicks with form-deprivation myopia. Curr Eye Res. 1990;9:1157–1165. doi: 10.3109/02713689009003472. [DOI] [PubMed] [Google Scholar]
- 155.Hung LF, Wallman J, Smith EL., III. Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci. 2000;41:1259–1269. [PubMed] [Google Scholar]
- 156.Funata M, Tokoro T. Scleral changes in experimentally myopic monkeys. Graefes Arch Clin Exp Ophthalmol. 1990;228:174–179. doi: 10.1007/BF00935729. [DOI] [PubMed] [Google Scholar]
- 157.Smith EL, III,, Hung LF. Form-deprivation myopia in monkeys is a graded phenomenon. Vision Res. 2000;40:371–381. doi: 10.1016/s0042-6989(99)00184-4. [DOI] [PubMed] [Google Scholar]
- 158.Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res. 1987;6:993–999. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- 159.Norton TT, Siegwart JT. Animal models of emmetropization: matching axial length to the focal plane. Basic Sci. 1995;66:405–414. [PubMed] [Google Scholar]
- 160.Troilo D, Li T, Glasser A, Howland HC. Differences in eye growth and the response to visual deprivation in different strains of chicken. Vision Res. 1995;35:1211–1216. doi: 10.1016/0042-6989(94)00230-j. [DOI] [PubMed] [Google Scholar]
- 161.McKanna JA, Casagrande VA. Reduced lens development in lid-suture myopia. Exp Eye Res. 1978;26:715–723. doi: 10.1016/0014-4835(78)90106-9. [DOI] [PubMed] [Google Scholar]
- 162.Napper GA, Brennan NA, Barrington M, Squires MA, Vessey GA, Vingrys AJ. The duration of normal visual exposure necessary to prevent form deprivation myopia in chicks. Vision Res. 1995;35:1337–1344. doi: 10.1016/0042-6989(94)00226-c. [DOI] [PubMed] [Google Scholar]
- 163.Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., III. Nature of the refractive errors in rhesus monkeys (Macaca mulatta) with experimentally induced ametropias. Vision Res. 2010;50:1867–1881. doi: 10.1016/j.visres.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lu F, Zhou X, Zhao H, et al. Axial myopia induced by a monocularly-deprived facemask in guinea pigs: a non-invasive and effective model. Exp Eye Res. 2006;82:628–636. doi: 10.1016/j.exer.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 165.Jiang L, Long K, Schaeffel F, et al. Disruption of emmetropization and high susceptibility to deprivation myopia in albino guinea pigs. Invest Ophthalmol Vis Sci. 2011;52:6124–6132. doi: 10.1167/iovs.10-7088. [DOI] [PubMed] [Google Scholar]
- 166.von Noorden GK, Crawford ML. Lid closure and refractive error in macaque monkeys. Nature. 1978;272:53–54. doi: 10.1038/272053a0. [DOI] [PubMed] [Google Scholar]
- 167.Thorn F, Doty RW, Gramiak R. Effect of eyelid suture on the development of ocular dimensions in macaques. Curr Eye Res. 1981/1982;1:727–733. doi: 10.3109/02713688108998372. [DOI] [PubMed] [Google Scholar]
- 168.Smith EL, III,, Harwerth RS, Crawford MLJ, von Noorden GK. Observations on the effects of form deprivation on the refractive status of the monkey. Invest Ophthalmol Vis Sci. 1987;28:1236–1245. [PubMed] [Google Scholar]
- 169.Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31:717–734. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- 170.Tran N, Chiu S, Tian Y, Wildsoet CF. The significance of retinal image contrast and spatial frequency composition for eye growth modulation in young chicks. Vision Res. 2008;48:1655–1662. doi: 10.1016/j.visres.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bowrey HE, Metse AP, Leotta AJ, Zeng G, McFadden SA. The relationship between image degradation and myopia in the mammalian eye. Clin Exp Optom. 2015;998:555–563. doi: 10.1111/cxo.12316. [DOI] [PubMed] [Google Scholar]
- 172.Yinon U, Koslowe KC. Hypermetropia in dark reared chicks and the effect of lid suture. Vision Res. 1986;26:999–1005. doi: 10.1016/0042-6989(86)90156-2. [DOI] [PubMed] [Google Scholar]
- 173.Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–1250. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- 174.Norton TT, Amedo AO, Siegwart JT., Jr. Darkness causes myopia in visually experienced tree shrews. Invest Ophthalmol Vis Sci. 2006;47:4700–4707. doi: 10.1167/iovs.05-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 176.Qiao-Grider Y, Hung L-F, Kee CS, Ramamirtham R, Smith EL., III. Recovery from form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3361–3372. doi: 10.1167/iovs.04-0080. [DOI] [PubMed] [Google Scholar]
- 177.Ji FT, Li Q, Zhu YL, et al. Form deprivation myopia in C57BL/6 mice [in Chinese] Zhonghua Yan Ke Za Zhi. 2009;45:1020–1026. [PubMed] [Google Scholar]
- 178.Amedo AO, Norton TT. Visual guidance of recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Ophthalmic Physiol Opt. 2012;32:89–99. doi: 10.1111/j.1475-1313.2011.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wildsoet CF, Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Res. 2000;40:3273–3282. doi: 10.1016/s0042-6989(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 180.Troilo D, Nickla DL, Wildsoet CW. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci. 2000;41:1249–1258. [PubMed] [Google Scholar]
- 181.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 182.Zhu X, McBrien NA, Smith EL, III,, Troilo D, Wallman J. Eyes in various species can shorten to compensate for myopic defocus. Invest Ophthalmol Vis Sci. 2013;54:2634–2644. doi: 10.1167/iovs.12-10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wallman J. Retinal control of eye growth and refraction. Prog Ret Res. 1993;12:133–153. [Google Scholar]
- 184.Mutti DO, Mitchell GL, Jones LA, et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074–3080. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- 185.Gwiazda J, Thorn F, Bauer J, Held R. Emmetropization and the progression of manifest refraction in children followed from infancy to puberty. Clin Vis Sci. 1993;8:337–344. [Google Scholar]
- 186.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 187.Schaeffel F, Troilo D, Wallman J, Howland HC. Developing eyes that lack accommodation grow to compensate for imposed defocus. Vis Neurosci. 1990;4:177–183. doi: 10.1017/s0952523800002327. [DOI] [PubMed] [Google Scholar]
- 188.Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997;37:659–668. doi: 10.1016/s0042-6989(96)00224-6. [DOI] [PubMed] [Google Scholar]
- 189.Smith EL, III,, Hung LF, Harwerth RS. Effects of optically induced blur on the refractive status of young monkeys. Vision Res. 1994;34:293–301. doi: 10.1016/0042-6989(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 190.Crewther SG, Nathan J, Kiely PM, Brennan NA, Crewther DP. The effect of defocussing contact lenses on refraction in cynomolgus monkeys. Clin Vision Sci. 1988;3:221–228. [Google Scholar]
- 191.Whatham AR, Judge SJ. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001;41:267–273. doi: 10.1016/s0042-6989(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 192.Smith EL, III,, Hung LF, Arumugam B, Wensveen JM, Chino YM, Harwerth RS. Observations on the relationship between anisometropia, amblyopia and strabismus. Vision Res. 2017;134:26–42. doi: 10.1016/j.visres.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Irving EL, Callender MG, Sivak JG. Inducing myopia, hyperopia, and astigmatism in chicks. Optom Vis Sci. 1991;68:364–368. doi: 10.1097/00006324-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 194.Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12:448–456. [PubMed] [Google Scholar]
- 195.Park TW, Winawer J, Wallman J. Further evidence that chick eyes use the sign of blur in spectacle lens compensation. Vision Res. 2003;43:1519–1531. doi: 10.1016/s0042-6989(03)00180-9. [DOI] [PubMed] [Google Scholar]
- 196.Hammond DS, Wallman J, Wildsoet CF. Dynamics of active emmetropisation in young chicks - influence of sign and magnitude of imposed defocus. Ophthalmic Physiol Opt. 2013;33:215–226. doi: 10.1111/opo.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hung L-F, Crawford MLJ, Smith EL., III. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 198.Smith EL, III,, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 199.Troilo D, Totonelly K, Harb E. Imposed anisometropia, accommodation, and regulation of refractive state. Optom Vis Sci. 2009;86:31–39. doi: 10.1097/OPX.0b013e318194072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Siegwart JT, Jr,, Norton TT. Binocular lens treatment in tree shrews: effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Exp Eye Res. 2010;91:660–669. doi: 10.1016/j.exer.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008;8(3):1. doi: 10.1167/8.3.1. [DOI] [PubMed] [Google Scholar]
- 202.Graham B, Judge SJ. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus) Vision Res. 1999;39:189–206. doi: 10.1016/s0042-6989(98)00189-8. [DOI] [PubMed] [Google Scholar]
- 203.Flitcroft DI. The lens paradigm in experimental myopia: oculomotor, optical and neurophysiological considerations. Ophthalmic Physiol Opt. 1999;19:103–111. doi: 10.1046/j.1475-1313.1999.00432.x. [DOI] [PubMed] [Google Scholar]
- 204.Young FA. The effect of restricted space on the primate eye. Am J Ophthalmol. 1961;52:799–806. doi: 10.1016/0002-9394(61)90904-7. [DOI] [PubMed] [Google Scholar]
- 205.Young FA. The effect of restricted visual space on the refractive error of the young monkey eye. Invest Ophthalmol. 1963;2:571–577. [PubMed] [Google Scholar]
- 206.Phillips JR. Monovision slows juvenile myopia progression unilaterally. Br J Ophthalmol. 2005;89:1196–1200. doi: 10.1136/bjo.2004.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang D, Chun RK, Liu M, et al. Optical defocus rapidly changes choroidal thickness in schoolchildren. PLoS One. 2016;11:e0161535. doi: 10.1371/journal.pone.0161535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chakraborty R, Read SA, Collins MJ. Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Exp Eye Res. 2012;103:47–54. doi: 10.1016/j.exer.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 209.Chakraborty R, Read SA, Collins MJ. Hyperopic defocus and diurnal changes in human choroid and axial length. Optom Vis Sci. 2013;90:1187–1198. doi: 10.1097/OPX.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 210.Read SA, Collins MJ, Sander BP. Human optical axial length and defocus. Invest Ophthalmol Vis Sci. 2010;51:6262–6269. doi: 10.1167/iovs.10-5457. [DOI] [PubMed] [Google Scholar]
- 211.Gifford KL, Richdale K, Kang P, et al. IMI – Clinical Management Guidelines Report. Invest Ophthalmol Vis Sci. 2019;60:M184–M203. doi: 10.1167/iovs.18-25977. [DOI] [PubMed] [Google Scholar]
- 212.Wildsoet CF, Chia A, Cho P, et al. IMI – Interventions for Controlling Myopia Onset and Progression Report. Invest Ophthalmol Visiual Sci. 2019;60:M106–M131. doi: 10.1167/iovs.18-25958. [DOI] [PubMed] [Google Scholar]
- 213.Wolffsohn JS, Kollbaum PS, Berntsen DA, et al. IMI – Clinical Myopia Control Trials and Instrumentation Report. Invest Ophthalmol Vis Sci. 2019;60:M132–M160. doi: 10.1167/iovs.18-25955. [DOI] [PubMed] [Google Scholar]
- 214.Bailey MD, Sinnott LT, Mutti DO. Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci. 2008;49:4353–4360. doi: 10.1167/iovs.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pucker AD, Jackson AR, Morris HJ, Fischer AJ, McHugh KM, Mutti DO. Ciliary muscle cell changes during guinea pig development. Invest Ophthalmol Vis Sci. 2015;56:7691–7696. doi: 10.1167/iovs.15-17927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pucker AD, Sinnott LT, Kao CY, Bailey MD. Region-specific relationship between refractive error and ciliary muscle thickness in children. Invest Ophthalmol Vis Sci. 2013;54:4710–4716. doi: 10.1167/iovs.13-11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tkatchenko AV. Whole-mount BrdU staining of proliferating cells by DNase treatment: application to postnatal mammalian retina. Biotechniques. 2006;40:29–30. doi: 10.2144/000112094. 32. [DOI] [PubMed] [Google Scholar]
- 218.Fleming PA, Harman AM, Beazley LD. Changing topography of the RPE resulting from experimentally induced rapid eye growth. Vis Neurosci. 1997;14:449–461. doi: 10.1017/s0952523800012128. [DOI] [PubMed] [Google Scholar]
- 219.Harman AM, Hoskins R, Beazley LD. Experimental eye enlargement in mature animals changes the retinal pigment epithelium. Vis Neurosci. 1999;16:619–628. doi: 10.1017/s0952523899164022. [DOI] [PubMed] [Google Scholar]
- 220.Lin T, Grimes PA, Stone RA. Expansion of the retinal pigment epithelium in experimental myopia. Vision Res. 1993;33:1881–1885. doi: 10.1016/0042-6989(93)90015-o. [DOI] [PubMed] [Google Scholar]
- 221.Liang H, Crewther SG, Crewther DP, Junghans BM. Structural and elemental evidence for edema in the retina, retinal pigment epithelium, and choroid during recovery from experimentally induced myopia. Invest Ophthalmol Vis Sci. 2004;45:2463–2474. doi: 10.1167/iovs.03-1009. [DOI] [PubMed] [Google Scholar]
- 222.Jonas JB, Ohno-Matsui K, Holbach L, Panda-Jonas S. Retinal pigment epithelium cell density in relationship to axial length in human eyes. Acta Ophthalmol. 2017;95:e22–28. doi: 10.1111/aos.13188. [DOI] [PubMed] [Google Scholar]
- 223.Bai HX, Mao Y, Shen L, et al. Bruch's membrane thickness in relationship to axial length. PLoS One. 2017;12:e0182080. doi: 10.1371/journal.pone.0182080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jonas JB, Ohno-Matsui K, Jiang WJ, Panda-Jonas S. Bruch's membrane and the mchanism of myopization: a new theory. Retina. 2017;37:1428–1440. doi: 10.1097/IAE.0000000000001464. [DOI] [PubMed] [Google Scholar]
- 225.Crewther DP. The role of photoreceptors in the control of refractive state. Prog Retin Eye Res. 2000;19:421–457. doi: 10.1016/s1350-9462(00)00004-5. [DOI] [PubMed] [Google Scholar]
- 226.Crewther SG, Liang H, Junghans BM, Crewther DP. Ionic control of ocular growth and refractive change. Proc Natl Acad Sci U S A. 2006;103:15663–15668. doi: 10.1073/pnas.0607241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Crewther SG, Murphy MJ, Crewther DP. Potassium channel and NKCC cotransporter involvement in ocular refractive control mechanisms. PLoS One. 2008;3:e2839. doi: 10.1371/journal.pone.0002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Seko Y, Shimokawa H, Pang J, Tokoro T. Disturbance of electrolyte balance in vitreous of chicks with form-deprivation myopia. Jpn J Ophthalmol. 2000;44:15–19. doi: 10.1016/s0021-5155(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 229.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.van Alphen GWHM. Choroidal stress and emmetropization. Vision Res. 1986;26:723–734. doi: 10.1016/0042-6989(86)90086-6. [DOI] [PubMed] [Google Scholar]
- 231.Muller H. Ueber glatte Muskeln und Nervengeflechte der chorioidea im menschlichen Auge [in German] Vehr physik-med Ges Wurzburg. 1859;10:179–192. [Google Scholar]
- 232.Stubinger K, Brehmer A, Neuhuber WL, Reitsamer H, Nickla D, Schrodl F. Intrinsic choroidal neurons in the chicken eye: chemical coding and synaptic input. Histochem Cell Biol. 2010;134:145–157. doi: 10.1007/s00418-010-0723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kajikawa J. Beiträge zur Anatomie und Physiologie des Vogelauges [in German] Albrecht von Graefes Arch Ophthalmol. 1923;112:260–346. [Google Scholar]
- 234.Guglielmone R, Cantino D. Autonomic innervation of the ocular choroid membrane in the chicken. Cell Tissue Res. 1982;222:417–431. doi: 10.1007/BF00213222. [DOI] [PubMed] [Google Scholar]
- 235.Meriney SD, Pilar G. Cholinergic innervation of the smooth muscle cells in the choroid coat of the chick eye and its development. J Neurosci. 1987;7:3827–3839. doi: 10.1523/JNEUROSCI.07-12-03827.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Krebs W, Krebs I. Primate Retina and Choroid: Atlas of Fine Structure in Man and Monkey. New York: Springer-Verlag;; 1991. [Google Scholar]
- 237.Flugel-Koch C, May A, Lutjen-Drecoll E. Presence of a contractile cell network in the human choroid. Ophthalmologica. 1996;210:296–302. doi: 10.1159/000310728. [DOI] [PubMed] [Google Scholar]
- 238.Poukens V, Glasgow BJ, Demer JL. Nonvascular contractile cells in sclera and choroid of humans and monkeys. Invest Ophthalmol Vis Sci. 1998;39:1765–1774. [PubMed] [Google Scholar]
- 239.Haddad A, Laicine EM, Tripathi BJ, Tripathi RC. An extensive system of extravascular smooth muscle cells exists in the choroid of the rabbit eye. Exp Eye Res. 2001;73:345–353. doi: 10.1006/exer.2001.1042. [DOI] [PubMed] [Google Scholar]
- 240.De Stefano ME, Mugnaini E. Fine structure of the choroidal coat of the avian eye. Lymphatic vessels. Invest Ophthalmol Vis Sci. 1997;38:1241–1260. [PubMed] [Google Scholar]
- 241.Krebs W, Krebs IP. Ultrastructural evidence for lymphatic capillaries in the primate retina. Arch Ophthalmol. 1988;106:1615–1616. doi: 10.1001/archopht.1988.01060140783055. [DOI] [PubMed] [Google Scholar]
- 242.Junghans BM, Crewther SG, Liang H, Crewther DP. A role for choroidal lymphatics during recovery from form deprivation myopia? Optom Vis Sci. 1999;76:796–803. doi: 10.1097/00006324-199911000-00028. [DOI] [PubMed] [Google Scholar]
- 243.Guggenheim JA, Chen YP, Yip E, et al. Pre-treatment choroidal thickness is not predictive of susceptibility to form-deprivation myopia in chickens. Ophthalmic Physiol Opt. 2011;31:516–528. doi: 10.1111/j.1475-1313.2011.00827.x. [DOI] [PubMed] [Google Scholar]
- 244.Chen YP, Prashar A, Erichsen JT, To CH, Hocking PM, Guggenheim JA. Heritability of ocular component dimensions in chickens: genetic variants controlling susceptibility to experimentally induced myopia and pretreatment eye size are distinct. Invest Ophthalmol Vis Sci. 2011;52:4012–4020. doi: 10.1167/iovs.10-7045. [DOI] [PubMed] [Google Scholar]
- 245.Nickla DL, Totonelly K. Choroidal thickness predicts ocular growth in normal chicks but not in eyes with experimentally altered growth. Clin Exp Optom. 2015;98:564–570. doi: 10.1111/cxo.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Res. 2002;42:2651–2668. doi: 10.1016/s0042-6989(02)00300-0. [DOI] [PubMed] [Google Scholar]
- 247.Nickla DL, Schroedl F. Parasympathetic influences on emmetropization in chicks: evidence for different mechanisms in form deprivation vs negative lens-induced myopia. Exp Eye Res. 2012;102:93–103. doi: 10.1016/j.exer.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Nickla DL, Zhu X, Wallman J. Effects of muscarinic agents on chick choroids in intact eyes and eyecups: evidence for a muscarinic mechanism in choroidal thinning. Ophthalmic Physiol Opt. 2013;33:245–256. doi: 10.1111/opo.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nickla DL, Lee L, Totonelly K. Nitric oxide synthase inhibitors prevent the growth-inhibiting effects of quinpirole. Optom Vis Sci. 2013;90:1167–1175. doi: 10.1097/OPX.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.De Stefano ME, Mugnaini E. Fine structure of the choroidal coat of the avian eye. Vascularization, supporting tissue and innervation. Anat Embryol. 1997;195:393–418. doi: 10.1007/s004290050060. [DOI] [PubMed] [Google Scholar]
- 251.Bellairs R, Harkness ML, Harkness RD. The structure of the tapetum of the eye of the sheep. Cell Tiss Res. 1975;157:73–91. doi: 10.1007/BF00223231. [DOI] [PubMed] [Google Scholar]
- 252.Nickla DL, Wildsoet C, Wallman J. Compensation for spectacle lenses involves changes in proteoglycan synthesis in both the sclera and choroid. Curr Eye Res. 1997;16:320–326. doi: 10.1076/ceyr.16.4.320.10697. [DOI] [PubMed] [Google Scholar]
- 253.Hirata A, Negi A. Morphological changes of choriocapillaris in experimentally induced chick myopa. Graefes Arch Clin Exp Ophthalmol. 1998;236:132–137. doi: 10.1007/s004170050053. [DOI] [PubMed] [Google Scholar]
- 254.Pendrak K, Papastergiou GI, Lin T, Laties AM, Stone RA. Choroidal vascular permeability in visually regulated eye growth. Exp Eye Res. 2000;70:629–637. doi: 10.1006/exer.2000.0825. [DOI] [PubMed] [Google Scholar]
- 255.Summers Rada JA, Palmer L. Choroidal regulation of scleral glycosaminoglycan synthesis dring recovery from induced myopia. Invest Ophthalmol Vis Sci. 2007;48:2957–2966. doi: 10.1167/iovs.06-1051. [DOI] [PubMed] [Google Scholar]
- 256.Rymer J, Wildsoet CF. The role of the retinal pigment epithelium in eye growth regulation and myopia: a review. Vis Neurosci. 2005;22:251–261. doi: 10.1017/S0952523805223015. [DOI] [PubMed] [Google Scholar]
- 257.Zhu X, Park TW, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005;46:2238–2241. doi: 10.1167/iovs.04-0956. [DOI] [PubMed] [Google Scholar]
- 258.Curtin BJ, Iwamoto T, Renaldo DP. Normal and staphylomatous sclera in high myopia. Arch Ophthalmol. 1979;97:912–921. doi: 10.1001/archopht.1979.01020010470017. [DOI] [PubMed] [Google Scholar]
- 259.Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–1282. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- 260.Rada JA, Nickla DL, Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000;41:2050–2058. [PubMed] [Google Scholar]
- 261.Coster L, Rosenberg LC, van der Rest M, Poole AR. The dermatan sulfate proteoglycans of bovine sclera and their relationship to those of articular cartilage. An immunological and biochemical study. J Biol Chem. 1987;262:3809–3812. [PubMed] [Google Scholar]
- 262.Rada JA, Achen VR, Perry CA, Fox PW. Proteoglycans in the human sclera. Invest Ophthalmol Vis Sci. 1997;38:1740–1751. [PubMed] [Google Scholar]
- 263.Guo L, Frost MR, He L, Siegwart JT, Jr,, Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci. 2013;54:6806–6819. doi: 10.1167/iovs.13-12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Johnson JM, Young TL, Summers Rada JA. Small leucine rich repeat proteoglycans (SLRPs) in the human sclera: identification of abundant levels of PRELP. Mol Vis. 2006;12:1057–1066. [PubMed] [Google Scholar]
- 265.Young TL, Scavello GS, Paluru PC, Choi JD, Rappaport EF, Rada JA. Microarray analysis of gene expression in human donor sclera. Mol Vis. 2004;10:163–176. [PubMed] [Google Scholar]
- 266.Rada JA, Thoft RA, Hassell JR. Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Dev Biol. 1991;147:303–312. doi: 10.1016/0012-1606(91)90288-e. [DOI] [PubMed] [Google Scholar]
- 267.Yuan Y, Li M, Chen Q, et al. Crosslinking enzyme lysyl oxidase modulates scleral remodeling in form-deprivation myopia. Curr Eye Res. 2018;43:200–207. doi: 10.1080/02713683.2017.1390770. [DOI] [PubMed] [Google Scholar]
- 268.McBrien N. Role of the sclera in the development and pathological complications of myopia. Prog Ret Eye Res. 2003;22:307–338. doi: 10.1016/s1350-9462(02)00063-0. [DOI] [PubMed] [Google Scholar]
- 269.Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest Ophthalmol Vis Sci. 1997;38:1726–1739. [PubMed] [Google Scholar]
- 270.Young FA. The nature and control of myopia. J Am Optom Assoc. 1977;48:451–457. [PubMed] [Google Scholar]
- 271.McBrien NA, Cornell LM, Gentle A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci. 2001;42:2179–2187. [PubMed] [Google Scholar]
- 272.Christensen AM, Wallman J. Evidence that scleral growth underlies visual deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 1991;32:2143–2150. [PubMed] [Google Scholar]
- 273.Gentle A, Liu YY, Martin JE, Conti GL, McBrien NA. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem. 2003;278:16587–16594. doi: 10.1074/jbc.M300970200. [DOI] [PubMed] [Google Scholar]
- 274.Cui D, Trier K, Zeng J, et al. Effects of 7-methylxanthine on the sclera in form deprivation myopia in guinea pigs. Acta Ophthalmol. 2011;89:328–334. doi: 10.1111/j.1755-3768.2009.01688.x. [DOI] [PubMed] [Google Scholar]
- 275.Wu H, Chen W, Zhao F, et al. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci U S A. 2018;115:E7091–E7100. doi: 10.1073/pnas.1721443115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Siegwart JT, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 277.Phillips JR, Khalaj M, McBrien NA. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthalmol Vis Sci. 2000;41:2028–2034. [PubMed] [Google Scholar]
- 278.Grytz R, Siegwart JT., Jr. Changing material properties of the tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2015;56:2065–2078. doi: 10.1167/iovs.14-15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rada JA, Mcfarland AL, Cornuet PK, Hassell JR. Proteoglycan synthesis by scleral chondrocytes is modulated by a vision dependent mechanism. Curr Eye Res. 1992;11:767–782. doi: 10.3109/02713689209000750. [DOI] [PubMed] [Google Scholar]
- 280.Rada JA, Matthews AL, Brenza H. Regional proteoglycan synthesis in the sclera of experimentally myopic chicks. Exp Eye Res. 1994;59:747–760. doi: 10.1006/exer.1994.1161. [DOI] [PubMed] [Google Scholar]
- 281.Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek L. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- 282.McBrien NA, Gentle A. The role of visual information in the control of scleral matrix biology in myopia. Curr Eye Res. 2001;23:313–319. doi: 10.1076/ceyr.23.5.313.5440. [DOI] [PubMed] [Google Scholar]
- 283.Marsh-Tootle WL, Norton TT. Refractive and structural measures of lid-suture myopia in tree shrew. Invest Ophthalmol Vis Sci. 1989;30:2245–2257. [PubMed] [Google Scholar]
- 284.Yinon U, Koslowe KC, Lobel D, Landshman N, Barishak YR. Lid suture myopia in developing chicks: optical and structural considerations. Curr Eye Res. 1982;83:877–882. doi: 10.3109/02713688209020025. [DOI] [PubMed] [Google Scholar]
- 285.Chu CH, Deng L, Kee CS. Effects of hemiretinal form deprivation on central refractive development and posterior eye shape in chicks. Vision Res. 2012;55:24–31. doi: 10.1016/j.visres.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 286.Kee CS, Hung LF, Qiao-Grider Y, Ramamirtham R, Smith EL., III. Astigmatism in monkeys with experimentally induced myopia or hyperopia. Optom Vis Sci. 2005;82:248–260. doi: 10.1097/01.opx.0000159357.61498.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wildsoet CF, Pettigrew JD. Kainic acid-induced eye enlargement in chickens: differential effects on anterior and posterior segments. Invest Ophthalmol Vis Sci. 1988;29:311–319. [PubMed] [Google Scholar]
- 288.Fischer AJ, Morgan IG, Stell WK. Colchicine causes excessive ocular growth and myopia in chicks. Vision Res. 1999;39:685–697. doi: 10.1016/s0042-6989(98)00178-3. [DOI] [PubMed] [Google Scholar]
- 289.Ehrlich D, Sattayasai J, Zappia J, Barrington M. Effects of selective neurotoxins on eye growth in the young chick. In: Bock G, Widdows K, editors. Myopia and the Control of Eye Growth. Chichester, UK: Wiley;; 1990. pp. 63–88. [DOI] [PubMed] [Google Scholar]
- 290.Wildsoet CF, Pettigrew JD. Experimental myopia and anomalous eye growth patterns unaffected by optic nerve section in chickens: evidence for local control of eye growth. Clin Vision Sci. 1988;3:99–107. [Google Scholar]
- 291.Troilo D, Francis E, Yi G. Vision Science and Its Applications. Santa Fe, NM: Optical Society of America;; 1997. The temporal characteristics of eye growth control mechanisms differ following optic nerve section; pp. 134–137. [Google Scholar]
- 292.Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis Neurosci. 1994;11:143–153. doi: 10.1017/s0952523800011184. [DOI] [PubMed] [Google Scholar]
- 293.Wildsoet CE. Neural pathways subserving negative lens-induced emmetropization in chicks - insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res. 2003;27:371–385. doi: 10.1076/ceyr.27.6.371.18188. [DOI] [PubMed] [Google Scholar]
- 294.Wildsoet CF, Howland HC, Falconer S, Dick K. Chromatic aberration and accommodation: their role in emmetropization in the chick. Vision Res. 1993;33:1593–1604. doi: 10.1016/0042-6989(93)90026-s. [DOI] [PubMed] [Google Scholar]
- 295.Schmid KL, Wildsoet CF. Effects on compensatory reponses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 296.Schwahn HN, Schaeffel F. Chick eyes under cycloplegia compensate for spectacle lenses despite 6-hydroxy dopamine treatment. Invest Ophthalmol Vis Sci. 1994;35:3516–3524. [PubMed] [Google Scholar]
- 297.Smith EL, III,, Huang J, Hung L-F, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009;50:5057–5069. doi: 10.1167/iovs.08-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Smith EL, III,, Hung LF, Arumugam B. Visual regulation of refractive development: insights from animal studies. Eye (Lond) 2014;28:180–188. doi: 10.1038/eye.2013.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Smith EL, III,, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Optical defocus influences refractive development in monkeys via local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010;51:3864–3873. doi: 10.1167/iovs.09-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Smith EL., III. Spectacle lenses and emmetropization: the role of optical defocus in regulating ocular development. Optom Vis Sci. 1998;75:388–398. doi: 10.1097/00006324-199806000-00023. [DOI] [PubMed] [Google Scholar]
- 301.Miles FA, Wallman J. Local ocular compensation for imposed local refractive error. Vision Res. 1990;30:339–349. doi: 10.1016/0042-6989(90)90076-w. [DOI] [PubMed] [Google Scholar]
- 302.Zhu X. Temporal integration of visual signals in lens compensation (a review) Exp Eye Res. 2013;114:69–76. doi: 10.1016/j.exer.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Winawer J, Zhu X, Choi J, Wallman J. Ocular compensation for alternating myopic and hyperopic defocus. Vision Res. 2005;45:1667–1677. doi: 10.1016/j.visres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 304.Zhu XY, Winawer JA, Wallman J. Potency of myopic defocus in spectacle lens compensation. Invest Ophthalmol Vis Sci. 2003;44:2818–2827. doi: 10.1167/iovs.02-0606. [DOI] [PubMed] [Google Scholar]
- 305.Nickla DL. Transient increases in choroidal thickness are consistently associated with brief daily visual stimuli that inhibit ocular growth in chicks. Exp Eye Res. 2007;84:951–959. doi: 10.1016/j.exer.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 306.Shaikh AW, Siegwart JT, Norton TT. Effect of interrupted lens wear on compensation for minus lens in tree shrews. Optom Vis Sci. 1999;76:308–315. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 307.Kee CS, Hung LF, Qiao-Grider Y, et al. Temporal constraints on experimental emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2007;48:957–962. doi: 10.1167/iovs.06-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Smith EL, III,, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Invest Ophthalmol Vis Sci. 2002;43:291–299. [PubMed] [Google Scholar]
- 309.Crewther SG, Barutchu A, Murphy MJ, Crewther DP. Low frequency temporal modulation of light promotes a myopic shift in refractive compensation to all spectacle lenses. Exp Eye Res. 2006;83:322–328. doi: 10.1016/j.exer.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 310.Schwahn HN, Schaeffel F. Flicker parameters are different for supression of myopia and hyperopia. Vision Res. 1997;37:2661–2674. doi: 10.1016/s0042-6989(97)00114-4. [DOI] [PubMed] [Google Scholar]
- 311.Zhu X, Wallman J. Temporal properties of compensation for positive and negative spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50:37–46. doi: 10.1167/iovs.08-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123:697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 313.Gwiazda J, Bauer J, Thorn F, Held R. Shifts in tonic accommodation after near work are related to refractive errors in children. Ophthalmic Physiol Opt. 1995;15:93–97. [PubMed] [Google Scholar]
- 314.Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34:690–694. [PubMed] [Google Scholar]
- 315.Mutti DO, Mitchell GL, Hayes JR, et al. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006;47:837–846. doi: 10.1167/iovs.05-0888. [DOI] [PubMed] [Google Scholar]
- 316.Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children's refractive error. Invest Ophthalmol Vis Sci. 2002;43:3633–3640. [PubMed] [Google Scholar]
- 317.Jones LA, Sinnott LT, Muti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48:3524–3532. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 319.Tse DY, Lam CS, Guggenheim JA, et al. Simultaneous defocus integration during refractive development. Invest Ophthalmol Vis Sci. 2007;48:5352–5359. doi: 10.1167/iovs.07-0383. [DOI] [PubMed] [Google Scholar]
- 320.McFadden SA, Tse DY, Bowrey HE, et al. Integration of defocus by dual power fresnel lenses inhibits myopia in the mammalian eye. Invest Ophthalmol Vis Sci. 2014;55:908–917. doi: 10.1167/iovs.13-11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Benavente-Perez A, Nour A, Troilo D. The effect of simultaneous negative and positive defocus on eye growth and development of refractive state in marmosets. Invest Ophthalmol Vis Sci. 2012;53:6479–6487. doi: 10.1167/iovs.12-9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Arumugam B, Hung L-F, To CH, Holden B, Smith EL., III. The effects of simultaneous dual focus lenses on refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2014;55:7423–7432. doi: 10.1167/iovs.14-14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Arumugam B, Hung LF, To CH, Sankaridurg P, Smith EL., III. The effects of the relative strength of simultaneous competing defocus signals on emmetropization in infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2016;57:3949–3960. doi: 10.1167/iovs.16-19704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Benavente-Perez A, Nour A, Troilo D. Axial eye growth and refractive error development can be modified by exposing the peripheral retina to relative myopic or hyperopic defocus. Invest Ophthalmol Vis Sci. 2014;55:6765–6773. doi: 10.1167/iovs.14-14524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Seidemann A, Schaeffel F, Guirao A, Lopez-Gil N, Artal P. Peripheral refractive errors in myopic, emmetropic, and hyperopic young subjects. J Opt Soc Am A Opt Image Sci Vis. 2002;19:2363–2373. doi: 10.1364/josaa.19.002363. [DOI] [PubMed] [Google Scholar]
- 326.Smith EL., III. Prentice Award Lecture 2010: a case for peripheral optical treatment strategies for myopia. Optom Vis Sci. 2011;88:1029–1044. doi: 10.1097/OPX.0b013e3182279cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Stone RA, Flitcroft DI. Ocular shape and myopia. Ann Acad Med Singapore. 2004;33:7–15. [PubMed] [Google Scholar]
- 328.Smith EL, III,, Ramamirtham R, Qiao-Grider Y, et al. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007;48:3914–3922. doi: 10.1167/iovs.06-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Smith EL, III,, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–3972. doi: 10.1167/iovs.05-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Liu Y, Wildsoet C. The effect of two-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Invest Ophthalmol Vis Sci. 2011;52:1078–1086. doi: 10.1167/iovs.10-5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Liu Y, Wildsoet C. The effective add inherent in 2-zone negative lenses inhibits eye growth in myopic young chicks. Invest Ophthalmol Vis Sci. 2012;53:5085–5093. doi: 10.1167/iovs.12-9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Millodot M. Effect of ametropia on peripheral refraction. Am J Optom Physiol Opt. 1981;58:691–695. doi: 10.1097/00006324-198109000-00001. [DOI] [PubMed] [Google Scholar]
- 333.Millodot M, Lamont A. Refraction in the periphery of the eye. J Opt Soc Am. 1974;64:110–111. doi: 10.1364/josa.64.000110. [DOI] [PubMed] [Google Scholar]
- 334.Mutti DO, Sholtz RI, Friedman NE, Zadnik K. Peripheral refraction and ocular shape in children. Invest Ophthalmol Vis Sci. 2000;41:1022–1030. [PubMed] [Google Scholar]
- 335.Huang J, Hung LF, Ramamirtham R, et al. Effects of form deprivation alters peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta) Invest Ophthalmol Vis Sci. 2009;50:4033–4044. doi: 10.1167/iovs.08-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Mutti DO, Sinnott LT, Mitchell GL, et al. Relative peripheral refractive error and the risk of onset and progression of myopia in children. Invest Ophthalmol Vis Sci. 2011;52:199–205. doi: 10.1167/iovs.09-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Atchison DA, Li SM, Li H, et al. Relative peripheral hyperopia does not predict development and progression of myopia in children. Invest Ophthalmol Vis Sci. 2015;56:6162–6170. doi: 10.1167/iovs.15-17200. [DOI] [PubMed] [Google Scholar]
- 338.Smith EL., III. Optical treatment strategies to slow myopia progression: effects of the visual extent of the optical treatment zone. Exp Eye Res. 2013;114:77–88. doi: 10.1016/j.exer.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Reh TA, Levine EM. Multipotential stem cells and progenitors in the vertebrate retina. J Neurobiol. 1998;36:206–220. [PubMed] [Google Scholar]
- 340.Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Am J Ophthalmol. 1951;34:1407–1413. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- 341.Chen J, Xie A, Hou L, Su Y, Lu F, Cycloplegic Thorn F. and noncycloplegic refractions of Chinese neonatal infants. Invest Ophthalmol Vis Sci. 2011;52:2456–2461. doi: 10.1167/iovs.10-5441. [DOI] [PubMed] [Google Scholar]
- 342.Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta) birth to young adulthood. Invest Ophthalmol Vis Sci. 1999;40:214–229. [PubMed] [Google Scholar]
- 343.Graham B, Judge SJ. Normal development of refractive state and ocular component dimensions in the marmoset (Callithrix jacchus) Vision Res. 1999;39:177–187. doi: 10.1016/s0042-6989(98)00188-6. [DOI] [PubMed] [Google Scholar]
- 344.Tkatchenko TV, Shen Y, Tkatchenko AV. Analysis of postnatal eye development in the mouse with high-resolution small animal magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2010;51:21–27. doi: 10.1167/iovs.08-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Pickett-Seltner RL, Sivak JG, Pasternak JJ. Experimentally induced myopia in chicks: morphometric and biochemical analysis during the first 14 days after hatching. Vision Res. 1988;28:323–328. doi: 10.1016/0042-6989(88)90160-5. [DOI] [PubMed] [Google Scholar]
- 346.Papastergiou GI, Schmid GF, Riva CE, Mendel MJ, Stone RA, Laties AM. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp Eye Res. 1998;66:195–205. doi: 10.1006/exer.1997.0421. [DOI] [PubMed] [Google Scholar]
- 347.Troilo D, Nickla DL. The response to visual form deprivation differs with age in marmosets. Invest Ophthalmol Vis Sci. 2005;46:1873–1881. doi: 10.1167/iovs.04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Feldkaemper M, Diether S, Schwahn H, Schaeffel F. Are experimental myopia and hyperopia based on similar retinal processing. Invest Ophthalmol Vis Sci. 1997;38:S542. [Google Scholar]
- 349.Kruger PB, Mathews S, Aggarwala KR, Sanchez N. Chromatic aberration and ocular focus - Fincham revisited. Vision Res. 1993;33:1397–1411. doi: 10.1016/0042-6989(93)90046-y. [DOI] [PubMed] [Google Scholar]
- 350.Kruger PB, Matthews S, Aggarwala KR, Yager D, Kruger ES. Accommodation responds to changing contrast of long, middle and short spectral-waveband components of the retinal image. Vision Res. 1995;35:2415–2429. doi: 10.1016/0042-6989(94)00316-5. [DOI] [PubMed] [Google Scholar]
- 351.Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002;42:2409–2417. doi: 10.1016/s0042-6989(02)00262-6. [DOI] [PubMed] [Google Scholar]
- 352.Rucker FJ, Wallman J. Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: evidence that the eye uses longitudinal chromatic aberration to guide eye-growth. Vision Res. 2009;49:1775–1783. doi: 10.1016/j.visres.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Rucker FJ, Wallman J. Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus. J Vis. 2012;12(6):23. doi: 10.1167/12.6.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Rucker FJ. The role of luminance and chromatic cues in emmetropisation. Ophthalmic Physiol Opt. 2013;33:196–214. doi: 10.1111/opo.12050. [DOI] [PubMed] [Google Scholar]
- 355.Rucker FJ, Wallman J. Cone signals for spectacle-lens compensation: differential responses to short and long wavelengths. Vision Res. 2008;48:1980–1991. doi: 10.1016/j.visres.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Stark LR, Lee RS, Kruger PB, Rucker FJ, Fan HY. Accommodation to simulations of defocus and chromatic aberration in the presence of chromatic misalignment. Vision Res. 2002;42:1485–1498. doi: 10.1016/s0042-6989(02)00074-3. [DOI] [PubMed] [Google Scholar]
- 357.Rucker FJ, Kruger PB. The role of short-wavelength sensitive cones and chromatic aberration in the response to stationary and step accommodation stimuli. Vision Res. 2004;44:197–208. doi: 10.1016/j.visres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 358.Jarvis JR, Taylor NR, Prescott NB, Meeks I, Wathes CM. Measuring and modelling the photopic flicker sensitivity of the chicken (Gallus g. domesticus) Vision Res. 2002;42:99–106. doi: 10.1016/s0042-6989(01)00268-1. [DOI] [PubMed] [Google Scholar]
- 359.Rohrer B, Iuvone PM, Stell WK. Stimulation of dopaminergic amacrine cells by stroboscopic illumination or fibroblast growth factor (bFGF, FGF-2) injections: possible roles in prevention of form-deprivation myopia in the chick. Brain Res. 1995;686:169–181. doi: 10.1016/0006-8993(95)00370-6. [DOI] [PubMed] [Google Scholar]
- 360.Gawne TJ, Siegwart JT, Jr,, Ward AH, Norton TT. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 2016;155:75–84. doi: 10.1016/j.exer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Rucker F, Britton S, Spatcher M, Hanowsky S. Blue light protects against temporal frequency sensitive refractive changes. Invest Ophthalmol Vis Sci. 2015;56:6121–6131. doi: 10.1167/iovs.15-17238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Rucker F, Henriksen M, Yanase T, Taylor C. The role of temporal contrast and blue light in emmetropization. Vision Res. 2018;151:78–87. doi: 10.1016/j.visres.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Schmid K, Wildsoet C. Contrast and spatial-frequency reuirements for emmetropization in chicks. Vision Res. 1997;37:2011–2021. doi: 10.1016/s0042-6989(97)00014-x. [DOI] [PubMed] [Google Scholar]
- 364.Schmid KL, Brinkworth DR, Wallace KM, Hess R. The effect of manipulations to target contrast on emmetropization in chick. Vision Res. 2006;46:1099–1107. doi: 10.1016/j.visres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 365.Diether S, Wildsoet CF. Stimulus requirements for the decoding of myopic and hyperopic defocus under single and competing defocus conditions in the chicken. Invest Ophthalmol Vis Sci. 2005;46:2242–2252. doi: 10.1167/iovs.04-1200. [DOI] [PubMed] [Google Scholar]
- 366.Hess RF, Schmid KL, Dumoulin SO, Field DJ, Brinkworth DR. What image properties regulate eye growth? Curr Biol. 2006;16:687–691. doi: 10.1016/j.cub.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 367.Brunette I, Bueno JM, Parent M, Hamam H, Simonet P. Monochromatic aberrations as a function of age, from childhood to. Invest Ophthalmol Vis Sci. 2003;44:5438–5446. doi: 10.1167/iovs.02-1042. [DOI] [PubMed] [Google Scholar]
- 368.Wang J, Candy TR. Higher order monochromatic aberrations of the human infant eye. J Vis. 2005;5(6):543–555. doi: 10.1167/5.6.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Coletta NJ, Marcos S, Wildsoet C, Troilo D. Double-pass measurement of retinal image quality in the chicken eye. Optom Vis Sci. 2003;80:50–57. doi: 10.1097/00006324-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 370.Kisilak ML, Campbell MC, Hunter JJ, Irving EL, Huang L. Aberrations of chick eyes during normal growth and lens induction of myopia. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:845–855. doi: 10.1007/s00359-006-0122-9. [DOI] [PubMed] [Google Scholar]
- 371.Tian Y, Wildsoet CF. Diurnal fluctuations and developmental changes in ocular dimensions and optical aberrations in young chicks. Invest Ophthalmol Vis Sci. 2006;47:4168–4178. doi: 10.1167/iovs.05-1211. [DOI] [PubMed] [Google Scholar]
- 372.Garcia de la Cera E, Rodriguez G, Marcos S. Longitudinal changes of optical aberrations in normal and form-deprived myopic chick eyes. Vision Res. 2006;46:579–589. doi: 10.1016/j.visres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 373.Coletta NJ, Marcos S, Troilo D. Ocular wavefront aberrations in the common marmoset Callithrix jacchus: effects of age and refractive error. Vision Res. 2010;50:2515–2529. doi: 10.1016/j.visres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ramamirtham R, Kee CS, Hung LF, Qiao-Grider Y, Roorda A, Smith EL., III. Monochromatic ocular wave aberrations in young monkeys. Vision Res. 2006;46:3616–3633. doi: 10.1016/j.visres.2006.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.He JC, Sun P, Held R, Thorn F, Sun XR, Gwiazda JE. Wavefront aberrations in eyes of emmetropic and moderately myopic school children and young adults. Vision Res. 2002;42:1063–1070. doi: 10.1016/s0042-6989(02)00035-4. [DOI] [PubMed] [Google Scholar]
- 376.Llorente L, Barbero S, Cano D, Dorronsoro C, Marcos S. Myopic versus hyperopic eyes: axial length, corneal shape and optical aberrations. J Vis. 2004;4(4):288–298. doi: 10.1167/4.4.5. [DOI] [PubMed] [Google Scholar]
- 377.Paquin M-P, Hamam H, Simonet P. Objective measurement of optical aberrations in myopic eyes. Optom Vis Sci. 2002;79:285–291. doi: 10.1097/00006324-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 378.Collins MJ, Wildsoet CF, Atchison DA. Monochromatic aberrations and myopia. Vision Res. 1995;35:1157–1163. doi: 10.1016/0042-6989(94)00236-f. [DOI] [PubMed] [Google Scholar]
- 379.Carkeet A, Luo HD, Tong L, Saw SM, Tan DTH. Refractive error and monochromatic aberrations in Singaporean children. Vision Res. 2002;42:1809–1824. doi: 10.1016/s0042-6989(02)00114-1. [DOI] [PubMed] [Google Scholar]
- 380.Cheng X, Bradley A, Hong X, Thibos L. Relationship between refractive error and monochromatic aberrations of the eye. Optom Vis Sci. 2003;80:43–49. doi: 10.1097/00006324-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 381.Thibos LN, Bradley A, Liu T, Lopez-Gil N. Spherical aberration and the sign of defocus. Optom Vis Sci. 2013;90:1284–1291. doi: 10.1097/OPX.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 382.Charman WN. Aberrations and myopia. Ophthalm Physiol Opt. 2005;25:285–301. doi: 10.1111/j.1475-1313.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 383.Ramamirtham R, Kee CS, Hung LF, et al. Wave aberrations in rhesus monkeys with vision-induced ametropias. Vision Res. 2007;47:2751–2766. doi: 10.1016/j.visres.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Priolo S, Sivak JG, Kuszak JR, Irving EL. Effects of experimentally induced ametropia on the morphology and optical quality of the avian crystalline lens. Invest Ophthalmol Vis Sci. 2000;41:3516–3522. [PubMed] [Google Scholar]
- 385.Artal P, Guirao A, Berrio E, Williams DR. Compensation of corneal aberrations by the internal optics in the human eye. J Vis. 2001;1(1):1. doi: 10.1167/1.1.1. [DOI] [PubMed] [Google Scholar]
- 386.Mohindra I, Held R, Gwiazda J, Brill S. Astigmatism in infants. Science. 1978;202:329–331. doi: 10.1126/science.694539. [DOI] [PubMed] [Google Scholar]
- 387.Atkinson J. O. B, French J. Infant astigmatism: its disappearance with age. Vision Res. 1980;20:891–893. doi: 10.1016/0042-6989(80)90070-x. [DOI] [PubMed] [Google Scholar]
- 388.Gwiazda J, Scheiman M, Mohindra I, Held R. Astigmatism in children: changes in axis and amount from birth to six years. Invest Ophthalmol Vis Sci. 1984;25:88–92. [PubMed] [Google Scholar]
- 389.Kaye SB, Patterson A. Association between total astigmatism and myopia. J Cataract Refract Surg. 1997;23:1496–1502. doi: 10.1016/s0886-3350(97)80020-x. [DOI] [PubMed] [Google Scholar]
- 390.Pärssinen O. Astigmatism and school myopia. Acta Ophthalmol. 1991;69:786–790. doi: 10.1111/j.1755-3768.1991.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 391.Fulton AB, Hansen RM, Petersen RA. The relation of myopia and astigmatism in developing eyes. Ophthalmology. 1982;89:298–302. doi: 10.1016/s0161-6420(82)34788-0. [DOI] [PubMed] [Google Scholar]
- 392.Farbrother JE, Welsby JW, Guggenheim JA. Astigmatic axis is related to the level of spherical ametropia. Optom Vis Sci. 2004;81:18–26. doi: 10.1097/00006324-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 393.Kee CS. Astigmatism and its role in emmetropization. Exp Eye Res. 2013;114:89–95. doi: 10.1016/j.exer.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 394.Schaeffel F, Hagel G, Eikermann J, Collett T. Lower-field myopia and astigmatism in amphibians and chickens. J Opt Soc Am. 1994;11:487–495. doi: 10.1364/josaa.11.000487. [DOI] [PubMed] [Google Scholar]
- 395.Schmid KL, Wildsoet CF. Natural and imposed astigmatism and their relation to emmetropization in the chick. Exp Eye Res. 1997;64:837–847. doi: 10.1006/exer.1997.0282. [DOI] [PubMed] [Google Scholar]
- 396.Kee CS, Deng L. Astigmatism associated with experimentally induced myopia or hyperopia in chickens. Invest Ophthalmol Vis Sci. 2008;49:858–867. doi: 10.1167/iovs.06-1370. [DOI] [PubMed] [Google Scholar]
- 397.Kee CS, Hung LF, Qiao Y, Habib A, Smith EL. Prevalence of astigmatism in infant monkeys. Vision Res. 2002;42:1349–1359. doi: 10.1016/s0042-6989(02)00060-3. [DOI] [PubMed] [Google Scholar]
- 398.Irving EL, Callender MG, Sivak JG. Inducing ametropias in hatchling chicks by defocus – aperture effects and cyclindrical lenses. Vision Res. 1995;36:1165–1174. doi: 10.1016/0042-6989(94)00235-e. [DOI] [PubMed] [Google Scholar]
- 399.Chu CH, Kee CS. Effects of optically imposed astigmatism on early eye growth in chicks. PLoS One. 2015;10:e0117729. doi: 10.1371/journal.pone.0117729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Kee CS, Hung LF, Qiao Y, Smith EL., III. Astigmatism in infant monkeys reared with cylindrical lenses. Vision Res. 2003;43:2721–2739. doi: 10.1016/s0042-6989(03)00469-3. [DOI] [PubMed] [Google Scholar]
- 401.Gwiazda J, Grice K, Held R, McLellan J, Astigmatism Thorn F. and the development of myopia in children. Vision Res. 2000;40:1019–1026. doi: 10.1016/s0042-6989(99)00237-0. [DOI] [PubMed] [Google Scholar]
- 402.McLean RC, Wallman J. Severe astigmatic blur does not interfere with spectacle lens compensation. Invest Ophthalmol Vis Sci. 2003;44:449–457. doi: 10.1167/iovs.01-0670. [DOI] [PubMed] [Google Scholar]
- 403.Kee CS, Hung LF, Qiao-Grider Y, Roorda A, Smith EL., III. Effects of optically imposed astigmatism on emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2004;45:1647–1659. doi: 10.1167/iovs.03-0841. [DOI] [PubMed] [Google Scholar]
- 404.Nickla DL. Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber. Exp Eye Res. 2013;114:25–34. doi: 10.1016/j.exer.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Ashby R. Animal studies and the mechanism of myopia – protection by light? Optom Vis Sci. 2016;93:1052–1054. doi: 10.1097/OPX.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 406.Norton TT. What do animal studies tell us about the mechanism of myopia-protection by light? Optom Vis Sci. 2016;93:1049–1051. doi: 10.1097/OPX.0000000000000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Norton TT, Siegwart JT., Jr. Light levels, refractive development, and myopia – a speculative review. Exp Eye Res. 2013;114:48–57. doi: 10.1016/j.exer.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Jensen LS, Matson WE. Enlargement of avian eye by subjecting chicks to continuous incandescent illumination. Science. 1957;125:741. doi: 10.1126/science.125.3251.741. [DOI] [PubMed] [Google Scholar]
- 409.Li T, Howland HC. The effects of constant and diurnal illumination of the pineal gland and the eyes on ocular growth in chicks. Invest Ophthalmol Vis Sci. 2003;44:3692–3697. doi: 10.1167/iovs.02-0990. [DOI] [PubMed] [Google Scholar]
- 410.Li T, Troilo D, Glasser A, Howland HC. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Res. 1995;35:1203–1209. doi: 10.1016/0042-6989(94)00231-a. [DOI] [PubMed] [Google Scholar]
- 411.Lauber JK, McGinnis J. Eye lesions in domestic fowl reared under continuous light. Vision Res. 1966;6:619–626. doi: 10.1016/0042-6989(66)90073-3. [DOI] [PubMed] [Google Scholar]
- 412.Lauber JK, Shutze JV, McGinnis J. Effects of exposure to continuous light on the eye of the growing chick. Proc Soc Exp Biol Med. 1961;106:871–872. doi: 10.3181/00379727-106-26505. [DOI] [PubMed] [Google Scholar]
- 413.Oishi T, Lauber JK. Light, experimental avian myopia and the role of the suprarenals. J Ocul Pharmacol. 1986;2:139–146. doi: 10.1089/jop.1986.2.139. [DOI] [PubMed] [Google Scholar]
- 414.Guo SS, Sivak JG, Callender MG, Herbert KL. Effects of continuous light on experimental refractive errors in chicks. Ophthalm Physiol Opt. 1996;16:486–490. [PubMed] [Google Scholar]
- 415.Padmanabhan V, Shih J, Wildsoet CF. Constant light rearing disrupts compensation to imposed- but not induced- hyperopia and facilitates compensation to imposed myopia in chicks. Vision Res. 2007;47:1855–1868. doi: 10.1016/j.visres.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Weiss S, Schaeffel F. Diurnal growth rhythms in the chicken eye – relation to myopia development and retinal dopamine levels. J Comp Physiol. 1993;172:263–270. doi: 10.1007/BF00216608. [DOI] [PubMed] [Google Scholar]
- 417.Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- 418.Nickla DL, Wildsoet C, Wallman J. The circadian rhythm in intraocular pressure and its relation to diurnal ocular growth changes in chicks. Exp Eye Res. 1998;66:183–193. doi: 10.1006/exer.1997.0425. [DOI] [PubMed] [Google Scholar]
- 419.Nickla DL, Wildsoet CF, Troilo D. Endogenous rhythms in axial length and choroidal thickness in chicks: implications for ocular growth regulation. Invest Ophthalmol Vis Sci. 2001;42:584–588. [PubMed] [Google Scholar]
- 420.Nickla DL. The phase relationships between the diurnal rhythms in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:399–407. doi: 10.1007/s00359-005-0077-2. [DOI] [PubMed] [Google Scholar]
- 421.Nickla DL, Thai P, Zanzerkia Trahan R, Totonelly K. Myopic defocus in the evening is more effective at inhibiting eye growth than defocus in the morning: effects on rhythms in axial length and choroid thickness in chicks. Exp Eye Res. 2017;154:104–115. doi: 10.1016/j.exer.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Nickla DL, Wildsoet CF, Troilo D. Diurnal rhythms in intraocular pressure, axial length, and choroidal thickness in a primate model of eye growth, the common marmoset. Invest Ophthalmol Vis Sci. 2002;43:2519–2528. [PubMed] [Google Scholar]
- 423.Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52:5121–5129. doi: 10.1167/iovs.11-7364. [DOI] [PubMed] [Google Scholar]
- 424.Stone RA, Quinn GE, Francis EL, et al. Diurnal axial length fluctuations in human eyes. Invest Ophthalmol Vis Sci. 2004;45:63–70. doi: 10.1167/iovs.03-0294. [DOI] [PubMed] [Google Scholar]
- 425.Read SA, Collins MJ, Iskander DR. Diurnal variation in axial length, intraocular pressure and anterior eye biometrics. Invest Ophthalmol Vis Sci. 2008;49:2911–2918. doi: 10.1167/iovs.08-1833. [DOI] [PubMed] [Google Scholar]
- 426.Wilson LB, Quinn GE, Ying G, et al. The relation of axial length and intraocular pressure fluctuations in human eyes. Invest Ophthalmol Vis Sci. 2006;47:1778–1784. doi: 10.1167/iovs.05-0869. [DOI] [PubMed] [Google Scholar]
- 427.Devadas M, Morgan I. Light controls scleral precursor synthesis. Neuroreport. 1996;7:2010–2012. doi: 10.1097/00001756-199608120-00031. [DOI] [PubMed] [Google Scholar]
- 428.Nickla DL, Rada JA, Wallman J. Isolated chick sclera shows a circadian rhythm in proteoglycan synthesis perhaps associated with the rhythm in ocular elongation. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;185:81–90. doi: 10.1007/s003590050368. [DOI] [PubMed] [Google Scholar]
- 429.Lee TC, Kiuchi Y, Gregory DS. Light exposure decreases IOP in rabbits during the night. Curr Eye Res. 1995;14:443–448. doi: 10.3109/02713689509003754. [DOI] [PubMed] [Google Scholar]
- 430.Rowland JM, Potter DE, Reiter RJ. Circadian rhythm in intraocular pressure: a rabbit model. Curr Eye Res. 1981;1:169–173. doi: 10.3109/02713688109001822. [DOI] [PubMed] [Google Scholar]
- 431.McLaren JW, Brubaker RF, FitzSimon JS. Continuous measurement of intraocular pressure in rabbits by telemetry. Invest Ophthalmol Vis Sci. 1996;37:966–975. [PubMed] [Google Scholar]
- 432.Moore CG, Johnson EC, Morrison JC. Circadian rhythm of intraocular pressure in the rat. Curr Eye Res. 1996;15:185–191. doi: 10.3109/02713689608997412. [DOI] [PubMed] [Google Scholar]
- 433.Krishna R, Mermoud A, Baerveldt G, Minckler DS. Circadian rhythm of intraocular pressure: a rat model. Ophthalmic Res. 1995;27:163–167. doi: 10.1159/000267660. [DOI] [PubMed] [Google Scholar]
- 434.Dalvin LA, Fautsch MP. Analysis of circadian rhythm gene expression with reference to diurnal pattern of intraocular pressure in mice. Invest Ophthalmol Vis Sci. 2015;56:2657–2663. doi: 10.1167/iovs.15-16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Bito LZ, Merritt SQ, DeRousseau CJ. Intraocular pressure of rhesus monkeys (Macaca mulatta) I. An initial survey of two free-breeding colonies. Invest Ophthalmol Vis Sci. 1979;18:785–793. [PubMed] [Google Scholar]
- 436.Schmid GF, Papastergiou GI, Lin T, Riva CE, Laties AM, Stone RA. Autonomic denervations influence ocular dimensions and intraocular pressure in chicks. Exp Eye Res. 1999;68:573–581. doi: 10.1006/exer.1998.0649. [DOI] [PubMed] [Google Scholar]
- 437.van Kampen GPJ, van de Stadt RJ. Cartilage and chondrocyte responses to mechanical loading in vitro. In: Helminen HJ, Kiviranta I, Tammi M, Saamanen AM, Paukkonen K, Jurvelin J, editors. Joint Loading: Biology and Health of Articular Structures. Kent, England: Butterworth & Co; 1987. pp. 112–125. Ltd.; [Google Scholar]
- 438.Takano-Yamamoto T, Soma S, Nakagawa K, Kobayashi Y, Kawakami M, Sakuda M. Comparison of the effects of hydrostatic compressive forces on glycosaminoglycan synthesis and proliferation in rabbit chondrocytes from mandibular condylar cartilage, nasal septum, and spheno-occipital synchondrosis in vitro. Am J Orthod Dentofacial Orthop. 1991;99:448–455. doi: 10.1016/S0889-5406(05)81578-1. [DOI] [PubMed] [Google Scholar]
- 439.Bartmann M, Schaeffel F, Hagel G, Zrenner E. Constant light affects retinal dopamine levels and blocks deprivation myopia but not lens-induced refractive errors in chickens. Vis Neurosci. 1994;11:199–208. doi: 10.1017/s0952523800001565. [DOI] [PubMed] [Google Scholar]
- 440.Stone RA, Lin T, Desai D, Capehart C. Photoperiod, early post-natal eye growth, and visual deprivation. Vision Res. 1995;35:1195–1202. doi: 10.1016/0042-6989(94)00232-b. [DOI] [PubMed] [Google Scholar]
- 441.Oishi T, Lauber JK, Vriend J. Experimental myopia and glaucoma in chicks. J Zool Sci. 1987;4:455–464. [Google Scholar]
- 442.Liu J, Pendrak K, Capehart C, Sugimoto R, Schmid GF, Stone RA. Emmetropisation under continuous but non-constant light in chicks. Exp Eye Res. 2004;79:719–728. doi: 10.1016/j.exer.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 443.Smith EL, III,, Hung LF, Kee CS, Qiao-Grider Y, Ramamirtham R. Continuous ambient lighting and lens compensation in infant monkeys. Optom Vis Sci. 2003;80:374–382. doi: 10.1097/00006324-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 444.Smith EL, III,, Bradley DV, Fernandes A, Hung LF, Boothe RG. Continuous ambient lighting and eye growth in primates. Invest Ophthalmol Vis Sci. 2001;42:1146–1152. [PubMed] [Google Scholar]
- 445.Aronson BD, Bellpedersen D, Block GD, et al. Circadian rhythms. Brain Res Rev. 1993;18:315–333. doi: 10.1016/0165-0173(93)90015-r. [DOI] [PubMed] [Google Scholar]
- 446.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 447.Brandstatter R. Encoding time of day and time of year by the avian circadian system. J Neuroendocrinol. 2003;15:398–404. doi: 10.1046/j.1365-2826.2003.01003.x. [DOI] [PubMed] [Google Scholar]
- 448.Challet E. Minireview: entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–5655. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- 449.Yamazaki S, Alones V, Menaker M. Interaction of the retina with suprachiasmatic pacemakers in the control of circadian behavior. J Biol Rhythms. 2002;17:315–329. doi: 10.1177/074873040201700405. [DOI] [PubMed] [Google Scholar]
- 450.Deguchi T. A circadian oscillator in cultured cells of chicken pineal gland. Nature. 1979;282:94–96. doi: 10.1038/282094a0. [DOI] [PubMed] [Google Scholar]
- 451.Kasal CA, Menaker M, Perez-Polo JR. Circadian clock in culture: N-acetyltransferase activity of chick pineal glands oscillates in vitro. Science. 1979;203:656–658. doi: 10.1126/science.569904. [DOI] [PubMed] [Google Scholar]
- 452.Takahashi JS, Murakami N, Nikaido SS, Pratt BL, Robertson LM. The avian pineal, a vertebrate model system of the circadian oscillator: cellular regulation of circadian rhythms by light, second messengers, and macromolecular synthesis. Recent Prog Horm Res. 1989;45:279–348. doi: 10.1016/b978-0-12-571145-6.50010-8. [DOI] [PubMed] [Google Scholar]
- 453.Lockley SW, Skene DJ, Thapan K, et al. Extraocular light exposure does not suppress plasma melatonin in humans. J Clin Endocrinol Metab. 1998;83:3369–3372. doi: 10.1210/jcem.83.9.5244. [DOI] [PubMed] [Google Scholar]
- 454.Lupi D, Cooper HM, Froehlich A, Standford L, McCall MA, Foster RG. Transgenic ablation of rod photoreceptors alters the circadian phenotype of mice. Neuroscience. 1999;89:363–374. doi: 10.1016/s0306-4522(98)00353-4. [DOI] [PubMed] [Google Scholar]
- 455.Lee HS, Nelms JL, Nguyen M, Silver R, Lehman MN. The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nat Neurosci. 2003;6:111–112. doi: 10.1038/nn1006. [DOI] [PubMed] [Google Scholar]
- 456.Torii H, Ohnuma K, Kurihara T, Tsubota K, Negishi K. Violet light transmission is related to myopia progression in adult high myopia. Sci Rep. 2017;7:14523. doi: 10.1038/s41598-017-09388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Schaeffel F, Smith EL., III. Inhibiting myopia by (nearly) invisible light? EBioMedicine. 2017;16:27–28. doi: 10.1016/j.ebiom.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Wang M, Schaeffel F, Jiang B, Feldkaemper M. Effects of light of different spectral composition on refractive development and retinal dopamine in chicks. Invest Ophthalmol Vis Sci. 2018;59:4413–4424. doi: 10.1167/iovs.18-23880. [DOI] [PubMed] [Google Scholar]
- 459.Taylor CP, Shepard TG, Rucker FJ, Eskew RT. Sensitivity to S-cone stimuli and the development of myopia. Invest Ophthalmol Vis Sci. 2018;59:4622–4630. doi: 10.1167/iovs.18-24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Rohrer B, Schaeffel F, Zrenner E. Longitudinal chromatic aberration and emmetropization: results from the chicken eye. J Physiol (London) 1992;449:363–376. doi: 10.1113/jphysiol.1992.sp019090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Foulds WS, Barathi VA, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Invest Ophthalmol Vis Sci. 2013;54:8004–8012. doi: 10.1167/iovs.13-12476. [DOI] [PubMed] [Google Scholar]
- 462.Torii H, Kurihara T, Seko Y, et al. Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine. 2017;15:210–219. doi: 10.1016/j.ebiom.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Liu R, Qian YF, He JC, et al. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res. 2011;92:447–453. doi: 10.1016/j.exer.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 464.Jiang L, Zhang S, Schaeffel F, et al. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus) Vision Res. 2014;94:24–32. doi: 10.1016/j.visres.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 465.Wang F, Zhou J, Lu Y, Chu R. Effects of 530 nm green light on refractive status, melatonin, MT1 receptor, and melanopsin in the guinea pig. Curr Eye Res. 2011;36:103–111. doi: 10.3109/02713683.2010.526750. [DOI] [PubMed] [Google Scholar]
- 466.Long Q, Chen DH, Chu RY. Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutan Ocul Toxicol. 2009;28:176–180. doi: 10.3109/15569520903178364. [DOI] [PubMed] [Google Scholar]
- 467.Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res. 2017;140:55–65. doi: 10.1016/j.visres.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Smith EL, III,, Hung LF, Arumugam B, Holden BA, Neitz M, Neitz J. Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2015;56:6490–6500. doi: 10.1167/iovs.15-17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Liu R, Hu M, He JC, et al. The effects of monochromatic illumination on early eye development in rhesus monkeys. Invest Ophthalmol Vis Sci. 2014;55:1901–1909. doi: 10.1167/iovs.13-12276. [DOI] [PubMed] [Google Scholar]
- 470.Hung L-F, Arumugam B, She Z, Ostrin L, Smith EL., III. Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Exp Eye Res. 2018;176:147–160. doi: 10.1016/j.exer.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Hammond DS, Wildsoet CF. Compensation to positive as well as negative lenses can occur in chicks reared in bright UV lighting. Vision Res. 2012;67:44–50. doi: 10.1016/j.visres.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Gawne TJ, Ward AH, Norton TT. Juvenile tress shrews do not maintain emmetropia in narrow-band blue light. Optom Vis Sci. 2018;95:911–920. doi: 10.1097/OPX.0000000000001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Gawne TJ, Siegwart JT, Jr,, Ward AH, Norton TT. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 2017;155:75–84. doi: 10.1016/j.exer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Ward AH, Siegwart JT, Frost MR, Norton TT. Intravitreally-administered dopamine D2-like (and D4), but not D1-like, receptor agonists reduce form-deprivation myopia in tree shrews. Vis Neurosci. 2017;34:E003. doi: 10.1017/S0952523816000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Jacobs GH, Deegan JF. Spectral sensitivity, photopigments, and color vision in the guinea pig (Cavia porcellus) Behav Neurosci. 1994;108:993–1004. doi: 10.1037//0735-7044.108.5.993. [DOI] [PubMed] [Google Scholar]
- 476.Jacobs GH, Nathans J. The evolution of Primate color vision. Sci Am. 2009;300:56–63. doi: 10.1038/scientificamerican0409-56. [DOI] [PubMed] [Google Scholar]
- 477.Lewis P, Poultry Morris T. and coloured light. Worlds Poult Sci J. 2000;56:189–207. [Google Scholar]
- 478.French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013;114:58–68. doi: 10.1016/j.exer.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 479.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. J Am Med Assoc. 2015;314:1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 480.Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120:1080–1085. doi: 10.1016/j.ophtha.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 481.Cohen Y, Belkin M, Yehezkel O, Solomon AS, Polat U. Dependency between light intensity and refractive development under lightdark cycles. Exp Eye Res. 2011;92:40–46. doi: 10.1016/j.exer.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 482.Feldkaemper M, Diether S, Kleine G, Schaeffel F. Interactions of spatial and luminance information in the retina of chickens during myopia. Exp Eye Res. 1999;68:105–115. doi: 10.1006/exer.1998.0590. [DOI] [PubMed] [Google Scholar]
- 483.Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:5348–5354. doi: 10.1167/iovs.09-3419. [DOI] [PubMed] [Google Scholar]
- 484.Karouta C, Ashby RS. Correlation between light levels and the development of deprivation myopia. Invest Ophthalmol Vis Sci. 2014;56:299–309. doi: 10.1167/iovs.14-15499. [DOI] [PubMed] [Google Scholar]
- 485.Stone RA, Cohen Y, McGlinn AM, et al. Development of experimental myopia in chicks in a natural environment. Invest Ophthalmol Vis Sci. 2016;57:4779–4789. doi: 10.1167/iovs.16-19310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Chen S, Zhi Z, Ruan Q, et al. Bright light supresses form-deprivation myopia development with activation of dopamine D1 receptor signalling in the ON pathway in retina. Invest Ophthalmol Vis Sci. 2017;58:2306–2316. doi: 10.1167/iovs.16-20402. [DOI] [PubMed] [Google Scholar]
- 487.Ashby RS, Schaeffel F. The effect of bright light on lens-compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51:5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- 488.Smith EL, III,, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53:421–428. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Lan W, Feldkaemper M, Schaeffel F. Intermittent episodes of bright light suppress myopia in the chicken more than continuous bright light. PLoS One. 2014;9:e110906. doi: 10.1371/journal.pone.0110906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Backhouse S, Collins AV, Phillips JR. Influence of periodic vs continuous daily bright light exposure on development of experimental myopia in the chick. Ophthalmic Physiol Optics. 2013;33:1–10. doi: 10.1111/opo.12069. [DOI] [PubMed] [Google Scholar]
- 491.Li W, Lan W, Yang S, et al. The effect of spectral property and intensity of light on natural refractive development and compensation to negative lenses in guinea pigs. Invest Ophthalmol Vis Sci. 2014;55:6324–6332. doi: 10.1167/iovs.13-13802. [DOI] [PubMed] [Google Scholar]
- 492.Smith EL, III,, Hung LF, Arumugam B, Huang J. Negative lens-induced myopia in infant monkeys: effects of high ambient lighting. Invest Ophthalmol Vis Sci. 2013;54:2959–2969. doi: 10.1167/iovs.13-11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Kee CS, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001;42:575–583. [PubMed] [Google Scholar]
- 494.Choh V, Lew MY, Nadel MW, Wildsoet CF. Effects of interchanging hyperopic defocus and form deprivation stimuli in normal and optic nerve-sectioned chicks. Vision Res. 2006;46:1070–1079. doi: 10.1016/j.visres.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 495.Nickla DL, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res. 2011;93:782–785. doi: 10.1016/j.exer.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Morgan IG. The biological basis of myopic refractive error. Clin Exp Optom. 2013;86:276–288. doi: 10.1111/j.1444-0938.2003.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 497.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013;114:106–119. doi: 10.1016/j.exer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 498.Iuvone PM, Tigges M, Fernandes A, Tigges J. Dopamine synthesis and metabolism in rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Visual Neurosci. 1989;2:465–471. doi: 10.1017/s0952523800012360. [DOI] [PubMed] [Google Scholar]
- 499.Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 501.Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991;32:1674–1677. [PubMed] [Google Scholar]
- 502.Ashby R, McCarthy CS, Maleszka R, Megaw P, Morgan IG. A muscarinic cholinergic antagonist and a dopamine agonist rapidly increase ZENK mRNA expression in the form-deprived chicken retina. Exp Eye Res. 2007;85:15–22. doi: 10.1016/j.exer.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 503.McCarthy CS, Megaw P, Devadas M, Morgan IG. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res. 2007;84:100–107. doi: 10.1016/j.exer.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 504.Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993;10:447–453. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- 505.Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res. 2010;91:715–720. doi: 10.1016/j.exer.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Schmid KL, Wildsoet CF. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci. 2004;81:137–147. doi: 10.1097/00006324-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 507.Gao Q, Liu Q, Ma P, Zhong X, Wu J, Ge J. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch Clin Exp Ophthalmol. 2006;244:1329–1335. doi: 10.1007/s00417-006-0254-1. [DOI] [PubMed] [Google Scholar]
- 508.Lin Z, Chen X, Ge J, et al. Effects of direct intravitreal dopamine injection on sclera and retina in form-deprived myopic rabbits. J Ocul Pharmacol Ther. 2008;24:543–550. doi: 10.1089/jop.2008.0041. [DOI] [PubMed] [Google Scholar]
- 509.Mao J, Liu S, Qin W, Li F, Wu X, Tan Q. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Optom Vis Sci. 2010;87:53–60. doi: 10.1097/OPX.0b013e3181c12b3d. [DOI] [PubMed] [Google Scholar]
- 510.Riddell N, Giummarra L, Hall NE, Crewther SG. Bidirectional expression of metabolic, structural, and immune pathways in early myopia and hyperopia. Front Neurosci. 2016;10:390. doi: 10.3389/fnins.2016.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Tkatchenko TV, Troilo D, Benavente-Perez A, Tkatchenko AV. Gene expression in response to optical defocus of opposite signs reveals bidirectional mechanism of visually guided eye growth. PLoS Biol. 2018;16:e2006021. doi: 10.1371/journal.pbio.2006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Fischer AJ, McGuire JJ, Schaeffel F, Stell WK. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci. 1999;2:706–712. doi: 10.1038/11167. [DOI] [PubMed] [Google Scholar]
- 513.Vessey KA, Lencses KA, Rushforth DA, Hruby VJ, Stell WK. Glucagon receptor agonists and antagonists affect the growth of the chick eye: a role for glucagonergic regulation of emmetropization? Invest Ophthalmol Vis Sci. 2005;46:3922–3931. doi: 10.1167/iovs.04-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Vessey KA, Rushforth DA, Stell WK. Glucagon- and secretin-related peptides differentially alter ocular growth and the development of form-deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2005;46:3932–3942. doi: 10.1167/iovs.04-1027. [DOI] [PubMed] [Google Scholar]
- 515.Zhu X, Wallman J. Opposite effects of glucagon and insulin on compensation for spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50:24–36. doi: 10.1167/iovs.08-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Feldkaemper MP, Neacsu I, Schaeffel F. Insulin acts as a powerful stimulator of axial myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:13–23. doi: 10.1167/iovs.08-1702. [DOI] [PubMed] [Google Scholar]
- 517.Stone RA, Laties AM, Raviola E, Wiesel TN. Increase in retinal vasoactive intestinal polypeptide after eyelid fusion in primates. Proc Natl Acad Sci U S A. 1988;85:257–260. doi: 10.1073/pnas.85.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Seko Y, Shimokawa H, Tokoro T. In vivo and in vitro association of retinoic acid with form-deprivation myopia in the chick. Exp Eye Res. 1996;63:443–452. doi: 10.1006/exer.1996.0134. [DOI] [PubMed] [Google Scholar]
- 519.Fujikado T, Kawasaki Y, Fujii J, et al. The effect of nitric oxide synthase inhibitor on form-deprivation myopia. Curr Eye Res. 1997;16:992–996. doi: 10.1076/ceyr.16.10.992.9021. [DOI] [PubMed] [Google Scholar]
- 520.Escano MFT, Fujii S, Sekiya Y, Yamamoto M, Negi A. Expression of sonic hedgehog and retinal opsin genes in experimentally-induced myopic chick eyes. Exp Eye Res. 2000;71:459–467. doi: 10.1006/exer.2000.0898. [DOI] [PubMed] [Google Scholar]
- 521.An J, Hsi E, Zhou X, Tao Y, Juo SH, Liang CL. The FGF2 gene in a myopia animal model and human subjects. Mol Vis. 2012;18:471–478. [PMC free article] [PubMed] [Google Scholar]
- 522.Jobling AI, Wan R, Gentle A, Bui BV, McBrien NA. Retinal and choroidal TGF-beta in the tree shrew model of myopia: isoform expression, activation and effects on function. Exp Eye Res. 2009;88:458–466. doi: 10.1016/j.exer.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 523.Rohrer B, Stell WK. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-beta) act as stop and go signals to modulate postnatal ocular growth in the chick. Exp Eye Res. 1994;58:553–561. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- 524.Rohrer B, Tao J, Stell WK. Basic fibroblast growth factor, its high- and low-affinity receptors, and their relationship to form-deprivation myopia in the chick. Neuroscience. 1997;79:775–787. doi: 10.1016/s0306-4522(97)00042-0. [DOI] [PubMed] [Google Scholar]
- 525.Seko Y, Shimokawa H, Tokoro T. Expression of bFGF and TGF-b2 in experimental myopia in chicks. Invest Ophthalmol Vis Sci. 1995;36:1183–1187. [PubMed] [Google Scholar]
- 526.Mei F, Wang J, Chen Z, Yuan Z. Potentially important micrornas in form-deprivation myopia revealed by bioinformatics analysis of MicroRNA profiling. Ophthalmic Res. 2017;57:186–193. doi: 10.1159/000452421. [DOI] [PubMed] [Google Scholar]
- 527.Ishibashi K, Fujii S, Escano MFT, Sekiya Y, Yamamoto M. Up-regulation of crystallin mRNAs in form-deprived chick eyes. Exp Eye Res. 2000;70:153–158. doi: 10.1006/exer.1999.0765. [DOI] [PubMed] [Google Scholar]
- 528.Ashby RS, Feldkaemper MP. Gene expression within the amacrine cell layer of chicks after myopic and hyperopic defocus. Invest Ophthalmol Vis Sci. 2010;51:3726–3735. doi: 10.1167/iovs.09-4615. [DOI] [PubMed] [Google Scholar]
- 529.Brand C, Schaeffel F, Feldkaemper MP. A microarray analysis of retinal transcripts that are controlled by image contrast in mice. Mol Vis. 2007;13:920–932. [PMC free article] [PubMed] [Google Scholar]
- 530.Lam DSC, Lee WS, Leung YF, et al. TGF beta-induced factor: a candidate gene for high myopia. Invest Ophthalmol Vis Sci. 2003;44:1012–1015. doi: 10.1167/iovs.02-0058. [DOI] [PubMed] [Google Scholar]
- 531.McGlinn AM, Baldwin DA, Tobias JW, Budak MT, Khurana TS, Stone RA. Form-deprivation myopia in chick induces limited changes in retinal gene expression. Invest Ophthalmol Vis Sci. 2007;48:3430–3436. doi: 10.1167/iovs.06-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Schippert R, Schaeffel F, Feldkaemper MP. Microarray analysis of retinal gene expression in chicks during imposed myopic defocus. Mol Vis. 2008;14:1589–1599. [PMC free article] [PubMed] [Google Scholar]
- 533.Rada JA, Wiechmann AF. Ocular expression of avian thymic hormone: changes during the recovery from induced myopia. Mol Vis. 2009;15:778–792. [PMC free article] [PubMed] [Google Scholar]
- 534.Bertrand E, Fritsch C, Diether S, et al. Identification of apolipoprotein A1 as a “STOP” signal for myopia. Mol Cell Proteomics. 2006;5:2158–2166. doi: 10.1074/mcp.M600073-MCP200. [DOI] [PubMed] [Google Scholar]
- 535.Tkatchenko AV, Walsh PA, Tkatchenko TV, Gustincich S, Raviola E. Form deprivation modulates retinal neurogenesis in primate experimental myopia. Proc Natl Acad Sci U S A. 2006;103:4681–4686. doi: 10.1073/pnas.0600589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Stone RA, Khurana TS. Gene profiling in experimental models of eye growth: clues to myopia pathogenesis. Vision Res. 2010;50:2322–2333. doi: 10.1016/j.visres.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Stone RA, McGlinn AM, Baldwin DA, Tobias JW, Iuvone PM, Khurana TS. Image defocus and altered retinal gene expression in chick: clues to the pathogenesis of ametropia. Invest Ophthalmol Vis Sci. 2011;52:5765–5777. doi: 10.1167/iovs.10-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Li Q, Wojciechowski R, Simpson CL, et al. for the CREAM Consortium. Genome-wide association study for refractive astigmatism reveals genetic co-determination with spherical equivalent refractive error: the CREAM consortium. Hum Genet. 2015;134:131–146. doi: 10.1007/s00439-014-1500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Mao J, Liu S, Qin W, Xiang Q, Wu X. Exogenous levodopa increases the neuro retinal dopamine of guinea pig myopic eyes in vitro. Eye Sci. 2011;26:211–216. doi: 10.3969/j.issn.1000-4432.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 540.McBrien NA, Cottriall CL, Annies R. Retinal acetylcholine content in normal and myopic eyes: a role in ocular growth control? Vis Neurosci. 2001;18:571–580. doi: 10.1017/s0952523801184075. [DOI] [PubMed] [Google Scholar]
- 541.Wu XH, Li YY, Zhang PP, et al. Unaltered retinal dopamine levels in a C57BL/6 mouse model of form-deprivation myopia. Invest Ophthalmol Vis Sci. 2015;56:967–977. doi: 10.1167/iovs.13-13362. [DOI] [PubMed] [Google Scholar]
- 542.Pardue MT, Faulkner AE, Fernandes A, et al. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008;49:706–712. doi: 10.1167/iovs.07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Chakraborty R, Park H, Aung MH, et al. Comparison of refractive development and retinal dopamine in OFF pathway mutant and C57BL/6J wild-type mice. Mol Vis. 2014;20:1318–1327. [PMC free article] [PubMed] [Google Scholar]
- 544.Witkovsky P, Dearry A. Functional roles of dopamine in the vertebrate retina. Prog Retin Res. 1992;11:247–292. [Google Scholar]
- 545.Ribelayga C, Wang Y, Mangel SC. Dopamine mediates circadian clock regulation of rod and cone input to fish retinal horizontal cells. J Physiol. 2002;544:801–816. doi: 10.1113/jphysiol.2002.023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Adachi A, Nogi T, Ebihara S. Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain Res. 1998;792:361–369. doi: 10.1016/s0006-8993(98)00206-6. [DOI] [PubMed] [Google Scholar]
- 547.Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- 548.Megaw PL, Boelen MG, Morgan IG, Boelen MK. Diurnal patterns of dopamine releasein chicken retina. Neurochem Int. 2006;48:17–23. doi: 10.1016/j.neuint.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 549.Guo SS, Sivak JG, Callender MG, Diehl-Jones B. Retinal dopamine and lens-induced refractive errors in chicks. Curr Eye Res. 1995;14:385–389. doi: 10.3109/02713689508999936. [DOI] [PubMed] [Google Scholar]
- 550.Dong F, Zhi Z, Pan M, et al. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011;17:2824. [PMC free article] [PubMed] [Google Scholar]
- 551.Yan T, Xiong W, Huang F, et al. Daily injection but not continuous infusion of apomorphine inhibits form-deprivation myopia in mice. Invest Ophthalmol Vis Sci. 2015;56:2475–2485. doi: 10.1167/iovs.13-12361. [DOI] [PubMed] [Google Scholar]
- 552.Lan W, Yang Z, Feldkaemper M, Schaeffel F. Changes in dopamine and ZENK during suppression of myopia in chicks by intense illuminance. Exp Eye Res. 2016;145:118–124. doi: 10.1016/j.exer.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 553.Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012;103:33–40. doi: 10.1016/j.exer.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 554.Crewther SG, Crewther DP. Inhibition of retinal ON/OFF systems differentially affects refractive compensation to defocus. Neuroreport. 2003;14:1233–1237. doi: 10.1097/00001756-200307010-00009. [DOI] [PubMed] [Google Scholar]
- 555.Chakraborty R, Park HN, Hanif AM, Sidhu CS, Iuvone PM, Pardue MT. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp Eye Res. 2015;137:79–83. doi: 10.1016/j.exer.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Napper GA, Brennan NA, Barrington M, Squires MA, Vessey GA, Vingrys AJ. The effect of an interrupted daily period of normal visual stimulation on form deprivation myopia in chicks. Vision Res. 1997;37:1557–1564. doi: 10.1016/s0042-6989(96)00269-6. [DOI] [PubMed] [Google Scholar]
- 557.Ohngemach S, Hagel G, Schaeffel F. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Vis Neurosci. 1997;14:493–505. doi: 10.1017/s0952523800012153. [DOI] [PubMed] [Google Scholar]
- 558.Schaeffel F, Bartmann M, Hagel G, Zrenner E. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res. 1995;35:1247–1264. doi: 10.1016/0042-6989(94)00221-7. [DOI] [PubMed] [Google Scholar]
- 559.Li XX, Schaeffel F, Kohler K, Zrenner E. Dose-dependent effects of 6-hydroxydopamine on deprivation myopia, electroretinograms, and dopaminergic amacrine cells in chickens. Vis Neurosci. 1992;9:483–492. doi: 10.1017/s0952523800011287. [DOI] [PubMed] [Google Scholar]
- 560.Iuvone PM. Regulation of retinal dopamine biosynthesis and tyrosine hydroxylase activity by light. Fed Proc. 1984;43:2709–2713. [PubMed] [Google Scholar]
- 561.Cahill GM, Besharse JC. Light-sensitive melatonin synthesis by xenopus photoreceptors after destruction of the inner retina. Vis Neurosci. 1992;8:487–490. doi: 10.1017/s0952523800005009. [DOI] [PubMed] [Google Scholar]
- 562.Cahill GM, Grace MS, Besharse JC. Rhythmic regulation of retinal melatonin: metabolic pathways, neurochemical mechanisms, and the ocular circadian clock. Cell Mol Neurobiol. 1991;11:529–560. doi: 10.1007/BF00734814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Martin XD, Malina HZ, Brennan MC, Hendrickson PH, Lichter PR. The ciliary body – the third organ found to synthesize indoleamines in humans. Eur J Ophthalmol. 1992;2:67–72. doi: 10.1177/112067219200200203. [DOI] [PubMed] [Google Scholar]
- 564.Hoffmann M, Schaeffel F. Melatonin and deprivation myopia in chickens. Neurochem Int. 1996;28:95–107. doi: 10.1016/0197-0186(95)00050-i. [DOI] [PubMed] [Google Scholar]
- 565.Rada JA, Wiechmann AF. Melatonin receptors in chick ocular tissues: implications for a role of melatonin in ocular growth regulation. Invest Ophthalmol Vis Sci. 2006;47:25–33. doi: 10.1167/iovs.05-0195. [DOI] [PubMed] [Google Scholar]
- 566.George A, Schmid KL, Pow DV. Retinal serotonin, eye growth and myopia development in chick. Exp Eye Res. 2005;81:616–625. doi: 10.1016/j.exer.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 567.Jensen H. Myopia progression in young school children. A prospective study of myopia progression and the effect of a trial with bifocal lenses and beta blocker eye drops. Acta Ophthalmol Suppl. 1991;200:1–79. [PubMed] [Google Scholar]
- 568.Goldschmidt E, Jensen H, Marushak D, Østergaard E. Can timolol maleate reduce the progression of myopia? Acta Opthalmol. 1985;63:90. [Google Scholar]
- 569.Lutjen-Drecoll E, Kaufman PL, Eichhorn M. Long-term timolol and epinephrine in monkeys I. Functional morphology of the ciliary processes. Trans Ophthalmol Soc U K. 1986;105:180–195. [PubMed] [Google Scholar]
- 570.Schmid KL, Abbott M, Humphries M, Pyne K, Wildsoet CF. Timolol lowers intraocular pressure but does not inhibit the development of experimental myopia in chick. Exp Eye Res. 2000;70:659–666. doi: 10.1006/exer.2000.0834. [DOI] [PubMed] [Google Scholar]
- 571.Pickett-Seltner RL, Stell WK. The effect of vasoactive intestinal peptide on development of fom deprivation myopia in the chick: a pharmacological and immunocytochemical study. Vision Res. 1995;35:1265–1270. doi: 10.1016/0042-6989(94)00244-g. [DOI] [PubMed] [Google Scholar]
- 572.Cakmak AI, Basmak H, Gursoy H, et al. Vasoactive intestinal peptide, a promising agent for myopia? Int J Ophthalmol. 2017;10:211–216. doi: 10.18240/ijo.2017.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Yiu WC1 YM, Fung WY, Ng PW, Yip SP. Genetic susceptibility to refractive error: association of vasoactive intestinal peptide receptor 2 (VIPR2) with high myopia in Chinese. PLoS One. 2013;8:e61805. doi: 10.1371/journal.pone.0061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Makman MH, Brown JH, Mishra RK. Cyclic AMP in retina and caudate nucleus: influence of dopamine and other agents. Adv Cyclic Nucleotide Res. 1975;5:661–679. [PubMed] [Google Scholar]
- 575.Hohberger B, Jessberger C, Hermann F, et al. VIP changes during daytime in chicken intrinsic choroidal neurons. Exp Eye Res. 2018;170:8–12. doi: 10.1016/j.exer.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 576.Diaz NM, Morera LP, Guido ME. Melanopsin and the non-visual photochemistry in the inner retina of vertebrates. Photochem Photobiol. 2016;92:29–44. doi: 10.1111/php.12545. [DOI] [PubMed] [Google Scholar]
- 577.Leung NY, Montell C. Unconventional roles of opsins. Annu Rev Cell Dev Biol. 2017;33:241–264. doi: 10.1146/annurev-cellbio-100616-060432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 579.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 580.Baver SB, Pickard GE, Sollars PJ. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 581.Dacey DM, Peterson BB, Robinson FR, Gamlin PDR. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- 582.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 583.Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, Tosini G. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci. 2005;22:3129–3136. doi: 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- 585.Chakraborty R, Pardue MT. Molecular and biochemical aspects of the retina on refraction. Prog Mol Biol Transl Sci. 2015;134:249–267. doi: 10.1016/bs.pmbts.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Hasegawa S, Terazono K, Nata K, Takada T, Yamamoto H, Okamoto H. Nucleotide sequence determination of chicken glucagon precursor cDNA. Chicken preproglucagon does not contain glucagon-like peptide II. FEBS Letters. 1990;264:117–120. doi: 10.1016/0014-5793(90)80779-i. [DOI] [PubMed] [Google Scholar]
- 587.Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. J Biol Chem. 1988;263:13475–13478. [PubMed] [Google Scholar]
- 588.Larsen PJ, Holst JJ. Glucagon-related peptide 1 (GLP-1): hormone and neurotransmitter. Regul Pept. 2005;128:97–107. doi: 10.1016/j.regpep.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 589.Buck C, Schaeffel F, Simon P, Feldkaemper M. Effects of positive and negative lens treatment on retinal and choroidal glucagon and glucagon receptor mRNA levels in the chicken. Invest Ophthalmol Vis Sci. 2004;45:402–409. doi: 10.1167/iovs.03-0789. [DOI] [PubMed] [Google Scholar]
- 590.Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis Neurosci. 2002;19:755–766. doi: 10.1017/s0952523802196064. [DOI] [PubMed] [Google Scholar]
- 591.Feldkaemper MP, Wang HY, Schaeffel F. Changes in retinal and choroidal gene expression during development of refractive errors in chicks. Invest Ophthalmol Vis Sci. 2000;41:1623–1628. [PubMed] [Google Scholar]
- 592.Mathis U, Schaeffel F. Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefes Arch Clin Exp Ophthalmol. 2006;245:267–275. doi: 10.1007/s00417-006-0282-x. [DOI] [PubMed] [Google Scholar]
- 593.Zhong X, Ge J, Smith EL, Stell WK. Image defocus modulates activity of bipolar and amacrine cells in macaque retina. Invest Ophthalmol Vis Sci. 2004;45:2065–2074. doi: 10.1167/iovs.03-1046. [DOI] [PubMed] [Google Scholar]
- 594.Penha AM, Burkhardt E, Schaeffel F, Feldkaemper MP. Effects of intravitreal insulin and insulin signaling cascade inhibitors on emmetropization in the chick. Mol Vis. 2012;18:2608–2622. [PMC free article] [PubMed] [Google Scholar]
- 595.Tang RH, Tan J, Deng ZH, Zhao SZ, Miao YB, Zhang WJ. Insulin-like growth factor-2 antisense oligonucleotides inhibits myopia by expression blocking of retinal insulin-like growth factor-2 in guinea pig. Clin Exp Ophthalmol. 2012;40:503–511. doi: 10.1111/j.1442-9071.2011.02683.x. [DOI] [PubMed] [Google Scholar]
- 596.Deng ZH, Tan J, Liu SZ, Zhao SZ, Wang JT. The correlation between the regulation of recombinant human IGF-2 on eye growth and form-deprivation in guinea pig. Graefes Arch Clin Exp Ophthalmol. 2010;248:519–525. doi: 10.1007/s00417-009-1287-z. [DOI] [PubMed] [Google Scholar]
- 597.Kusakari T, Sato T, Tokoro T. Visual deprivation stimulates the exchange of the fibrous sclera into the cartilaginous sclera in chicks. Exp Eye Res. 2001;73:533–546. doi: 10.1006/exer.2001.1064. [DOI] [PubMed] [Google Scholar]
- 598.Sheng C, Zhu X, Wallman J. In vitro effects of insulin and RPE on choroidal and scleral components of eye growth in chicks. Exp Eye Res. 2013;116:439–448. doi: 10.1016/j.exer.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 599.Marcha Penha A, Burkhardt E, Schaeffel F, Feldkaemper MP. Effects of intravitreal insulin and insulin signaling cascade inhibitors on emmetropization in the chck. Mol Vis. 2011;18:2608–2622. [PMC free article] [PubMed] [Google Scholar]
- 600.Zhang Y, Wildsoet CF. RPE. and choroid mechanisms underlying ocular growth and myopia. Prog Mol Biol Transl Sci. 2015;134:221–240. doi: 10.1016/bs.pmbts.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Baba K, DeBruyne JP, Tosini G. Dopamine 2 receptor activation entrains circadian clocks in mouse retinal pigment epithelium. Sci Rep. 2017;7:5103. doi: 10.1038/s41598-017-05394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Versaux-Botteri C, Gibert JM, Nguyen-Legros J, Vernier P. Molecular identification of a dopamine D1b receptor in bovine retinal pigment epithelium. Neurosci Lett. 1997;237:9–12. doi: 10.1016/s0304-3940(97)00783-0. [DOI] [PubMed] [Google Scholar]
- 603.Maneu V, Gerona G, Fernandez L, Cuenca N, Lax P. Evidence of alpha 7 nicotinic acetylcholine receptor expression in retinal pigment epithelial cells. Vis Neurosci. 2010;27:139–147. doi: 10.1017/S0952523810000246. [DOI] [PubMed] [Google Scholar]
- 604.Nuckels RJ, Forstner MR, Capalbo-Pitts EL, Garcia DM. Developmental expression of muscarinic receptors in the eyes of zebrafish. Brain Res. 2011;1405:85–94. doi: 10.1016/j.brainres.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 605.Salceda R. Muscarinic receptors binding in retinal pigment epithelium during rat development. Neurochem Res. 1994;19:1207–1210. doi: 10.1007/BF00965157. [DOI] [PubMed] [Google Scholar]
- 606.Crook RB, Song MK, Tong LP, Yabu JM, Polansky JR, Lui GM. Stimulation of inositol phosphate formation in cultured human retinal pigment epithelium. Brain Res. 1992;583:23–30. doi: 10.1016/s0006-8993(10)80006-x. [DOI] [PubMed] [Google Scholar]
- 607.Friedman Z, Hackett SF, Campochiaro PA. Human retinal pigment epithelial cells possess muscarinic receptors coupled to calcium mobilization. Brain Res. 1988;446:11–16. doi: 10.1016/0006-8993(88)91291-7. [DOI] [PubMed] [Google Scholar]
- 608.Koh SW, Chader GJ. Elevation of intracellular cyclic AMP and stimulation of adenylate cyclase activity by vasoactive intestinal peptide and glucagon in the retinal pigment epithelium. J Neurochem. 1984;43:1522–1526. doi: 10.1111/j.1471-4159.1984.tb06072.x. [DOI] [PubMed] [Google Scholar]
- 609.Puddu A, Sanguineti R, Montecucco F, Viviani GL. Retinal pigment epithelial cells express a functional receptor for glucagon-like peptide-1 (GLP-1) Mediators Inflamm. 2013. 975032. [DOI] [PMC free article] [PubMed]
- 610.Mao J, Liu S. Regulation of RPE barrier function by all-trans retinoic acid in myopia. Neurosci Lett. 2014;568:17–22. doi: 10.1016/j.neulet.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 611.Wang S, Liu S, Mao J, Wen D. Effect of retinoic acid on the tight junctions of the retinal pigment epithelium-choroid complex of guinea pigs with lens-induced myopia in vivo. Int J Mol Med. 2014;33:825–832. doi: 10.3892/ijmm.2014.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Li B, Luo X, Li T, et al. Effects of constant flickering light on refractive status, 5-HT and 5-HT2A receptor in guinea pigs. PloS One. 2016;11:e0167902. doi: 10.1371/journal.pone.0167902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Nash M, Flanigan T, Leslie R, Osborne N. Serotonin-2A receptor mRNA expression in rat retinal pigment epithelial cells. Ophthalmic Res. 1999;31:1–4. doi: 10.1159/000055506. [DOI] [PubMed] [Google Scholar]
- 614.Zhang Y, Liu Y, Wildsoet CF. Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2012;53:6072–6080. doi: 10.1167/iovs.12-9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Zhang Y, Liu Y, Ho C, Wildsoet CF. Effects of imposed defocus of opposite sign on temporal gene expression patterns of BMP4 and BMP7 in chick RPE. Exp Eye Res. 2013;109:98–106. doi: 10.1016/j.exer.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Zhang Y, Raychaudhuri S, Wildsoet CF. Imposed optical defocus induces isoform-specific up-regulation of tgfbeta gene expression in chick retinal pigment epithelium and choroid but not neural retina. PLoS One. 2016;11:e0155356. doi: 10.1371/journal.pone.0155356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Shelton L, Troilo D, Lerner M, Gusev Y, Brackett DJ, Rada JS. Microarray analysis of choroid/RPE gene expression in marmoset eyes undergoing changes in ocular growth and refraction. Mol Vis. 2008;14:1465–1479. [PMC free article] [PubMed] [Google Scholar]
- 618.He L, Frost MR, Siegwart JT, Jr,, Norton TT. Altered gene expression in tree shrew retina and retinal pigment epithelium produced by short periods of minus-lens wear. Exp Eye Res. 2018;168:77–88. doi: 10.1016/j.exer.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Li HH, Sun YL, Cui DM, Wu J, Zeng JW. Effect of dopamine on bone morphogenesis protein-2 expression in human retinal pigment epithelium. Int J Ophthalmol. 2017;10:1370–1373. doi: 10.18240/ijo.2017.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Chen BY, Wang CY, Chen WY, Ma JX. Altered TGF-beta2 and bFGF expression in scleral desmocytes from an experimentally-induced myopia guinea pig model. Graefes Arch Clin Exp Ophthalmol. 2013;251:1133–1144. doi: 10.1007/s00417-013-2269-8. [DOI] [PubMed] [Google Scholar]
- 621.Jia Y, Yue Y, Hu DN, Chen JL, Zhou JB. Human aqueous humor levels of transforming growth factor-beta2: association with matrix metalloproteinases/tissue inhibitors of matrix metalloproteinases. Biomed Rep. 2017;7:573–578. doi: 10.3892/br.2017.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Schippert R, Brand C, Schaeffel F, Feldkaemper MP. Changes in scleral MMP-2, TIMP-2 and TGFbeta-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Exp Eye Res. 2006;82:710–719. doi: 10.1016/j.exer.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 623.Siegwart JT, Norton TT. The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery. Invest Ophthalmol Vis Sci. 2002;43:2067–2075. [PMC free article] [PubMed] [Google Scholar]
- 624.Jobling AI, Nguyen M, Gentle A, McBrien NA. Isoform-specific changes in scleral transforming growth factor-beta expression and the regulation of collagen synthesis during myopia progression. J Biol Chem. 2004;279:18121–18126. doi: 10.1074/jbc.M400381200. [DOI] [PubMed] [Google Scholar]
- 625.Tan J, Deng ZH, Liu SZ, Wang JT, Huang C. TGF-beta2 in human retinal pigment epithelial cells: expression and secretion regulated by cholinergic signals in vitro. Curr Eye Res. 2010;35:37–44. doi: 10.3109/02713680903374190. [DOI] [PubMed] [Google Scholar]
- 626.Riddell N, Crewther SG. Novel evidence for complement system activation in chick myopia and hyperopia models: a meta-analysis of transcriptome datasets. Sci Rep. 2017;7:9719. doi: 10.1038/s41598-017-10277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Fujii S, Honda S, Sekiya Y, Yamasaki M, Yamamoto M, Saijoh K. Differential expression of nitric oxide synthase isoforms in form-deprived chick eyes. Curr Eye Res. 1998;17:586–593. [PubMed] [Google Scholar]
- 628.Fujii S, Escano MFT, Ishibashi K, et al. Differential expression of neuroendocrine-specific protein in form-deprived chick eyes. Invest Ophthalmol Vis Sci. 2000;41:1533–1541. [PubMed] [Google Scholar]
- 629.Seko Y, Tanaka Y, Tokoro T. Scleral cell growth is influenced by retinal pigment epithelium in vitro. Graefes Arch Clin Exp Ophthalmol. 1994;232:545–552. doi: 10.1007/BF00181998. [DOI] [PubMed] [Google Scholar]
- 630.Wang IJ, Shih YF, Sung YS, Yu MJ, Lin LL, Hung PT. Influence of destruction of retina-RPE complex on the proliferation of scleral chondrocytes in chicks. J Ocul Pharmacol Ther. 1998;14:429–436. doi: 10.1089/jop.1998.14.429. [DOI] [PubMed] [Google Scholar]
- 631.Seko Y, Tanaka Y, Tokoro T. Apomorphine inhibits the growth-stimulating effect of retinal pigment epithelium on scleral cells in vitro. Cell Biochem Funct. 1997;15:191–196. doi: 10.1002/(SICI)1099-0844(199709)15:3<191::AID-CBF738>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 632.Christian PG, Harkin DG, Schmid KL. GABAergic agents modify the response of chick scleral fibroblasts to myopic and hyperopic eye cup tissues. Curr Eye Res. 2014;39:172–187. doi: 10.3109/02713683.2013.834941. [DOI] [PubMed] [Google Scholar]
- 633.McBrien NA, Norton TT. Prevention of collagen crosslinking increases form-deprivation myopia in tree shrew. Exp Eye Res. 1994;59:475–486. doi: 10.1006/exer.1994.1133. [DOI] [PubMed] [Google Scholar]
- 634.Li X, Wu M, Zhang L, Liu H, Zhang L, He J. Riboflavin and ultraviolet A irradiation for the prevention of progressive myopia in a guinea pig model. Exp Eye Res. 2017;165:1–6. doi: 10.1016/j.exer.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 635.Liu S, Li S, Wang B, et al. Scleral cross-linking using riboflavin UVA irradiation for the prevention of myopia progression in a guinea pig model: blocked axial extension and altered scleral microstructure. PLoS One. 2016;11:e0165792. doi: 10.1371/journal.pone.0165792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Dotan A, Kremer I, Gal-Or O, et al. Scleral cross-linking using riboflavin and ultraviolet-a radiation for prevention of axial myopia in a rabbit model. J Vis Exp. 2016;110:e53201. doi: 10.3791/53201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Rada JA, Brenza HL. Increased latent gelatinase activity in the sclera of visually deprived chicks. Invest Ophthalmol Vis Sci. 1995;36:1555–1565. [PubMed] [Google Scholar]
- 638.Rada JA, Perry CA, Slover ML, Achen VR. Gelatinase A and TIMP-2 expression in the fibrous sclera of myopic and recovering chick eyes. Invest Ophthalmol Vis Sci. 1999;40:3091–3099. [PubMed] [Google Scholar]
- 639.Siegwart JT, Jr,, Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2005;46:3484–3492. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Liu HH, Kenning MS, Jobling AI, McBrien NA, Gentle A. Reduced scleral TIMP-2 expression is associated with myopia development: TIMP-2 supplementation stabilizes scleral biomarkers of myopia and limits myopia development. Invest Ophthalmol Vis Sci. 2017;58:1971–1981. doi: 10.1167/iovs.16-21181. [DOI] [PubMed] [Google Scholar]
- 641.Li H, Cui D, Zhao F, Huo L, Hu J, Zeng J. BMP-2 Is involved in scleral remodeling in myopia development. PLoS One. 2015;10:e0125219. doi: 10.1371/journal.pone.0125219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Wang Q, Xue ML, Zhao GQ, Liu MG, Ma YN, Ma Y. Form-deprivation myopia induces decreased expression of bone morphogenetic protein-2, 5 in guinea pig sclera. Int J Ophthalmol. 2015;8:39–45. doi: 10.3980/j.issn.2222-3959.2015.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Tao Y, Pan M, Liu S, et al. cAMP level modulates scleral collagen remodeling, a critical step in the development of myopia. PLoS One. 2013;8:e71441. doi: 10.1371/journal.pone.0071441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Feldkaemper MP, Burkhardt E, Schaeffel F. Localization and regulation of glucagon receptors in the chick eye and preproglucagon and glucagon receptor expression in the mouse eye. Exp Eye Res. 2004;79:321–329. doi: 10.1016/j.exer.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 645.Rada JA, Palmer L. Choroidal regulation of scleral glycosaminoglycan synthesis during recovery from induced myopia. Invest Ophthalmol Vis Sci. 2007;48:2957–2966. doi: 10.1167/iovs.06-1051. [DOI] [PubMed] [Google Scholar]
- 646.Summers Rada JA, Hollaway LR, Lam W, Li N, Napoli JL. Identification of RALDH2 as a visually regulated retinoic acid synthesizing enzyme in the chick choroid. Invest Ophthalmol Vis Sci. 2012;53:1649–1662. doi: 10.1167/iovs.11-8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Summers JA, Harper AR, Feasley CL, et al. Identification of apolipoprotein A-I as a retinoic acid-binding protein in the eye. J Biol Chem. 2016;291:18991–19005. doi: 10.1074/jbc.M116.725523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Seko Y, Shimizu M, Tokoro T. Retinoic acid increases in the retina of the chick with form deprivation myopia. Ophthalmic Res. 1998;30:361–367. doi: 10.1159/000055496. [DOI] [PubMed] [Google Scholar]
- 649.Bitzer M, Feldkaemper M, Schaeffel F. Visually induced changes in components of the retinoic acid system in fundal layers of the chick. Exp Eye Res. 2000;70:97–106. doi: 10.1006/exer.1999.0762. [DOI] [PubMed] [Google Scholar]
- 650.McFadden SA, Howlett MHC, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44:643–653. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 651.Troilo D, Nickla DL, Mertz JR, Rada JS. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest Ophthalmol Vis Sci. 2006;47:1768–1777. doi: 10.1167/iovs.05-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Morgan I, Megaw P. Using natural STOP growth signals to prevent excessive axial elongation and the development of myopia. Ann Acad Med Singapore. 2004;33:16–20. [PubMed] [Google Scholar]
- 653.Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000;70:519–527. doi: 10.1006/exer.1999.0813. [DOI] [PubMed] [Google Scholar]
- 654.Mao JF, Liu SZ, Dou XQ. Retinoic acid metabolic change in retina and choroid of the guinea pig with lens-induced myopia. Int J Ophthalmol. 2012;5:670–674. doi: 10.3980/j.issn.2222-3959.2012.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.McFadden SA, Howlett MH, Mertz JR, Wallman J. Acute effects of dietary retinoic acid on ocular components in the growing chick. Exp Eye Res. 2006;83:949–961. doi: 10.1016/j.exer.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 656.Stone RA, Lin T, Laties AM. Muscarinic antagonist effects on experimental chick myopia. Exp Eye Res. 1991;52:755–758. doi: 10.1016/0014-4835(91)90027-c. [DOI] [PubMed] [Google Scholar]
- 657.McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci. 1993;34:205–215. [PubMed] [Google Scholar]
- 658.Luft WA, Ming Y, Stell WK. Variable effects of previously untested muscarinic receptor antagonists on experimental myopia. Invest Ophthalmol Vis Sci. 2003;44:1330–1338. doi: 10.1167/iovs.02-0796. [DOI] [PubMed] [Google Scholar]
- 659.McBrien N, Stell WK, Carr B. How does atropine exert its anti-myopia effects? Ophthalmic Physiol Opt. 2013;33:373–377. doi: 10.1111/opo.12052. [DOI] [PubMed] [Google Scholar]
- 660.Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47. doi: 10.1016/j.exer.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Barathi VA, Beuerman RW, Schaeffel F. Effects of unilateral topical atropine on binocular pupil responses and eye growth in mice. Vision Res. 2009;49:383–387. doi: 10.1016/j.visres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 662.Tigges M, Iuvone PM, Fernandes A, et al. Effects of muscarinic cholinergic receptor antagonists on postnatal eye growth of rhesus monkeys. Optom Vis Sci. 1999;76:397–407. doi: 10.1097/00006324-199906000-00020. [DOI] [PubMed] [Google Scholar]
- 663.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285–2291. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 664.Song YY, Wang H, Wang BS, Qi H, Rong ZX, Chen HZ. Atropine in ameliorating the progression of myopia in children with mild to moderate myopia: a meta-analysis of controlled clinical trials. J Ocul Pharmacol Ther. 2011;27:361–368. doi: 10.1089/jop.2011.0017. [DOI] [PubMed] [Google Scholar]
- 665.Fischer AJ, McKinnon LA, Nathanson NM, Stell WK. Identification and localization of muscarinic acetylcholine receptors in the ocular tissues of the chick. J Comp Neurol. 1998;392:273–284. [PubMed] [Google Scholar]
- 666.Diether S, Schaeffel F, Lambrou GN, Fritsch C, Trendelenburg AU. Effects of intravitreally and intraperitonally injected atropine on two types of experimental myopia in chicken. Exp Eye Res. 2006;84:266–274. doi: 10.1016/j.exer.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 667.Schwahn HN, Kaymak H, Schaeffel F. Effects of atropine on refractive development, dopamine release, and slow retinal potentials in the chick. Vis Neurosci. 2000;17:165–176. doi: 10.1017/s0952523800171184. [DOI] [PubMed] [Google Scholar]
- 668.Lind GJ, Chew SJ, Marzani D, Wallman J. Muscarinic acetylcholine receptor antagonists inhibit chick scleral chondrocytes. Invest Ophthalmol Vis Sci. 1998;39:2217–2231. [PubMed] [Google Scholar]
- 669.Wang IJ, Shih YF, Tseng HS, Huang SH, Lin LLK, Hung FT. The effect of intravitreal injection of atropine on the proliferation of scleral chondrocyte in vivo. J Ocul Pharmacol Ther. 1998;14:337–343. doi: 10.1089/jop.1998.14.337. [DOI] [PubMed] [Google Scholar]
- 670.Crawford KS, Kaufman PL, Bito LZ. The role of the iris in accommodation of the rhesus monkey. Invest Ophthalmol Vis Sci. 1990;31:2185–2190. [PubMed] [Google Scholar]
- 671.McBrien NA, Jobling AI, Truong HT, Cottriall CL, Gentle A. Expression of muscarinic receptor subtypes in tree shrew ocular tissues and their regulation during the development of myopia. Mol Vis. 2009;15:464–475. [PMC free article] [PubMed] [Google Scholar]
- 672.Zou L, Liu R, Zhang X, et al. Upregulation of regulator of G-protein signaling 2 in the sclera of a form deprivation myopic animal model. Mol Vis. 2014;20:977–987. [PMC free article] [PubMed] [Google Scholar]
- 673.Barathi VA, Beuerman RW, Schaeffel F. Effects of unilateral topical atropine on binocular pupil responses and eye growth in mice. Vision Res. 2009;49:383–387. doi: 10.1016/j.visres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 674.Fischer AJ, Miethke P, Morgan IG, Stell WK. Cholinergic amacrine cells are not required for the progression and atropine-mediated supression of form-deprivation myopia. Brain Res. 1998;794:48–60. doi: 10.1016/s0006-8993(98)00188-7. [DOI] [PubMed] [Google Scholar]
- 675.Leech EM, Cottriall CL, McBrien NA. Pirenzepine prevents form deprivation myopia in a dose dependent manner. Ophthalmic Physiol Opt. 1995;15:351–356. [PubMed] [Google Scholar]
- 676.Rickers M, Schaeffel F. Dose-dependent effects of intravitreal pirenzepine on deprivation myoia and lens-induced refractive errors in chickens. Exp Eye Res. 1995;61:509–516. doi: 10.1016/s0014-4835(05)80147-2. [DOI] [PubMed] [Google Scholar]
- 677.Metlapally S, McBrien NA. The effect of pirenzepine on positive- and negative-lens-induced refractive error and ocular growth in chicks. Invest Ophthalmol Vis Sci. 2010;51:5438–5444. doi: 10.1167/iovs.09-4431. [DOI] [PubMed] [Google Scholar]
- 678.Le QH, Cheng NN, Wu W, Chu RY. Effect of pirenzepine ophthalmic solution on form-deprivation myopia in the guinea pigs. Chin Med J. 2005;118:561–566. [PubMed] [Google Scholar]
- 679.Ostrin LA, Frishman LJ, Glasser A. Th effects of pirenzepine on pupil size and accommodation in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3620–3628. doi: 10.1167/iovs.04-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Siatkowski RM, Cotter SA, Crockett RS, et al. Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J Amer Assoc Ped Ophthalmol Strab. 2008;12:332–329. doi: 10.1016/j.jaapos.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 681.Arumugam B, McBrien NA. Muscarinic antagonist control of myopia: evidence for M4 and M1 receptor-based pathways in the inhibition of experimentally-induced axial myopia in the tree shrew. Invest Ophthalmol Vis Sci. 2012;53:5827–5837. doi: 10.1167/iovs.12-9943. [DOI] [PubMed] [Google Scholar]
- 682.McBrien NA, Arumugam B, Gentle A, Chow A, Sahebjada S. The M4 muscarinic antagonist MT-3 inhibits myopia in chick: evidence for site of action. Ophthalmic Physiol Opt. 2011;31:529–539. doi: 10.1111/j.1475-1313.2011.00841.x. [DOI] [PubMed] [Google Scholar]
- 683.Cottriall CL, Truong HT, McBrien NA. Inhibition of myopia development in chicks using himbacine: a role for M-4 receptors? Neuroreport. 2001;12:2453–2456. doi: 10.1097/00001756-200108080-00033. [DOI] [PubMed] [Google Scholar]
- 684.Cottriall CL, Brew J, Vessey KA, McBrien NA. Diisopropylfluorophosphate alters retinal neurotransmitter levels and reduces experimentally-induced myopia. Naunyn Schmiedebergs Arch Pharm. 2001;364:372–382. doi: 10.1007/s002100100460. [DOI] [PubMed] [Google Scholar]
- 685.Arumugam B, McBrien N. The D2 antagonist spiperone prevents muscarinic antagonist control of experimentally-induced myopia in chick. Invest Ophthalmol Vis Sci. 2010;51 doi: 10.1167/iovs.12-9943. ARVO E-Abstract 1195. [DOI] [PubMed] [Google Scholar]
- 686.Carr BJ, Stell WK. Nitric oxide (NO) mediates the inhibition of form-deprivation myopia by atropine in chicks. Sci Rep. 2016;6:9. doi: 10.1038/s41598-016-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Barathi VA, Chaurasia SS, Poidinger M, et al. Involvement of GABA transporters in atropine-treated myopic retina as revealed by iTRAQ quantitative proteomics. J Proteome Res. 2014;13:4647–4658. doi: 10.1021/pr500558y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Stone RA, Sugimoto R, Gill AS, Liu J, Capehart C, Lindstrom JM. Effects of nicotinic antagonists on ocular growth and experimental myopia. Invest Ophthalmol Vis Sci. 2001;42:557–565. [PubMed] [Google Scholar]
- 689.Wu J, Lukas RJ. Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem Pharm. 2011;82:800–807. doi: 10.1016/j.bcp.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Fujikado T, Tsujikawa K, Tamura M, Hosohata J, Kawasaki Y, Tano Y. Effect of a nitric oxide synthase inhibitor on lens-induced myopia. Ophthalmic Res. 2001;33:75–79. doi: 10.1159/000055647. [DOI] [PubMed] [Google Scholar]
- 691.Nickla DL, Wildsoet CF. The effect of the nonspecific nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on the choroidal compensatory response to myopic defocus in chickens. Optom Vis Sci. 2004;81:111–118. doi: 10.1097/00006324-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 692.Nickla DL, Damyanova P, Lytle G. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor L-NAME. Exp Eye Res. 2009;88:1092–1099. doi: 10.1016/j.exer.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Nickla DL, Wilken E, Lytle G, Yom S, Mertz JR. Inhibiting the transient choroidal thickening response using nitric oxide synthase inhibitor L-NAME prevents the ameliorative effects of visual experience on ocular growth in two different visual paradigms. Exp Eye Res. 2006;83:456–464. doi: 10.1016/j.exer.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 694.Wu J, Liu Q, Yang X, Yang H, Wang XM, Zeng JW. Time-course of changes to nitric oxide signaling pathways in form-deprivation myopia in guinea pigs. Brain Res. 2007;1186:155–163. doi: 10.1016/j.brainres.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 695.Nilsson SF. Nitric oxide as a mediator of parasympathetic vasodilation in ocular and extraocular tissues in the rabbit. Invest Ophthalmol Vis Sci. 1996;37:2110–2109. [PubMed] [Google Scholar]
- 696.Cuthbertson S, Jackson B, Toledo C, et al. Innervation of orbital and choroidal blood vessels by the pterygopalatine ganglion in pigeons. J Comp Neurol. 1997;386:422–442. [PubMed] [Google Scholar]
- 697.Schmid KL, Strasberg G, Rayner CL, Hartfield PJ. The effects and interactions of GABAergic and dopaminergic agents in the prevention of form deprivation myopia by brief periods of normal vision. Exp Eye Res. 2013;110:88–95. doi: 10.1016/j.exer.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 698.Stone RA, Liu J, Sugimoto R, Capehart C, Zhu XS, Pendrak K. GABA. experimental myopia, and ocular growth in chick. Invest Ophthalmol Vis Sci. 2003;44:3933–3946. doi: 10.1167/iovs.02-0774. [DOI] [PubMed] [Google Scholar]
- 699.Nguyen-Legros J, Versaux-Botteri C, Vernier P. Dopamine receptor localization in the mammalian retina. Mole Neurobiol. 1999;19:181–204. doi: 10.1007/BF02821713. [DOI] [PubMed] [Google Scholar]
- 700.Kazula A, Nowak JZ, Iuvone PM. Regulation of melatonin and dopamine biosynthesis in chick retina: the role of GABA. Vis Neurosci. 1993;10:621–629. doi: 10.1017/s0952523800005320. [DOI] [PubMed] [Google Scholar]
- 701.Agardh E, Bruun A, Ehinger B, Storm-Mathisen J. GABA immunoreactivity in the retina. Invest Ophthalmol Vis Sci. 1986;27:674–678. [PubMed] [Google Scholar]
- 702.Santos PF, Carvalho AL, Carvalho AP, Duarte CB. Differential acetylcholine and GABA release from cultured chick retina cells. Eur J Neurosci. 1998;10:2723–2730. doi: 10.1046/j.1460-9568.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 703.Popova E. GABAergic neurotransmission and retinal ganglion cell function. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015;201:261–283. doi: 10.1007/s00359-015-0981-z. [DOI] [PubMed] [Google Scholar]
- 704.Cheng ZY, Chebib M, Schmid KL. rho1 GABAC receptors are expressed in fibrous and cartilaginous layers of chick sclera and located on sclera fibroblasts and chondrocytes. J Neurochem. 2011;118:281–287. doi: 10.1111/j.1471-4159.2011.07300.x. [DOI] [PubMed] [Google Scholar]
- 705.Cheng ZY, Wang XP, Schmid KL, et al. GABAB receptor antagonist CGP46381 inhibits form-deprivation myopia development in guinea pigs. Biomed Res Int. 2015;2015:207312. doi: 10.1155/2015/207312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Cheng ZY, Wang XP, Schmid KL, Han XG. Inhibition of form-deprivation myopia by a GABAAOr receptor antagonist, (1,2,5,6-tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA), in guinea pigs. Graefes Arch Clin Exp Ophthalmol. 2014;252:1939–1946. doi: 10.1007/s00417-014-2765-5. [DOI] [PubMed] [Google Scholar]
- 707.Johnston GA, Chebib M, Hanrahan JR, Mewett KN. Neurochemicals for the investigation of GABA(C) receptors. Neurochem Res. 2010;35:1970–1977. doi: 10.1007/s11064-010-0271-7. [DOI] [PubMed] [Google Scholar]
- 708.Chebib M, Hinton T, Schmid KL, et al. Novel, potent, and selective GABAC antagonists inhibit myopia development and facilitate learning and memory. J Pharmacol Exp Ther. 2009;328:448–457. doi: 10.1124/jpet.108.146464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Leung CKS, Yeung CK, Chiang SWY, Chan KP, Pang CP, Lam DSC. GABAA. and GABAC (GABAA0r) receptors affect ocular growth and form-deprivation myopia. Cutan Ocul Toxicol. 2005;24:187–196. [Google Scholar]
- 710.Pickett Seltner RL, Rohrer B, Grant V, Stell WK. Endogenous opiates in the chick retina and their role in form-deprivation myopia. Vis Neurosci. 1997;14:801–809. doi: 10.1017/s0952523800011548. [DOI] [PubMed] [Google Scholar]
- 711.Fischer AJ, Seltner RLP, Stell WK. Opiate and N-methyl-D-aspartate receptors in form deprivation myopia. Vis Neurosci. 1998;15:1089–1096. doi: 10.1017/s0952523898156080. [DOI] [PubMed] [Google Scholar]
- 712.Trier K, Munk Ribel-Madsen S, Cui D, Brogger Christensen S. Systemic 7-methylxanthine in retarding axial eye growth and myopia progression: a 36-month pilot study. J Ocul Biol Dis Infor. 2008;1:85–93. doi: 10.1007/s12177-008-9013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Nie HH, Huo LJ, Yang X, et al. Effects of 7-methylxanthine on form-deprivation myopia in pigmented rabbits. Int J Ophthalmol. 2012;5:133–137. doi: 10.3980/j.issn.2222-3959.2012.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Hung L-F, Arumugam B, Ostrin L, et al. The adenosine receptor antagonist, 7-methylxanthine, alters emmetropizing responses in infant monkeys. Invest Ophthalmol Vis Sci. 2018;59:472–486. doi: 10.1167/iovs.17-22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Trier K, Olsen EB, Kobayashi T, Ribel-Madsen SM. Biochemical and ultrastructural changes in rabbit sclera after treatment with 7-methylxanthine, theobromine, acetazolamide, or L-ornithine. Br J Ophthalmol. 1999;83:1370–1375. doi: 10.1136/bjo.83.12.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Morgan I, Rose K. How genetic is school myopia? Prog Ret Eye Res. 2005;24:1–38. doi: 10.1016/j.preteyeres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 717.Morgan I. The biological basis of myopic refractive error. Clin Exp Optom. 2003;86:276–288. doi: 10.1111/j.1444-0938.2003.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 718.Young TL. Molecular genetics of human myopia: an update. Optom Vis Sci. 2009;86:E8–E22. doi: 10.1097/OPX.0b013e3181940655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Baird PN, Schache M, Dirani M. The Genes in Myopia (GEM) study in understanding the aetiology of refractive errors. Prog Ret Eye Res. 2010;29:520–542. doi: 10.1016/j.preteyeres.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 720.Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet. 2011;79:301–320. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Peet JA, Cotch MF, Wojciechowski R, Bailey-Wilson JE, Stambolian D. Heritability and familial aggregation of refractive error in the Old Order Amish. Invest Ophthalmol Vis Sci. 2007;48:4002–4006. doi: 10.1167/iovs.06-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Lyhne N, Sjolie AK, Kyvik KO, Green A. The importance of genes and environment for ocular refraction and its determiners: a population based study among 20–45 year old twins. Br J Ophthalmol. 2001;85:1470–1476. doi: 10.1136/bjo.85.12.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: the twin eye study. Invest Ophthalmol Vis Sci. 2001;42:1232–1236. [PubMed] [Google Scholar]
- 724.Teikari JM, Kaprio J, Koskenvuo MK, Vannas A. Heritability estimate for refractive errors – a population-based sample of adult twins. Genet Epidemiol. 1988;5:171–181. doi: 10.1002/gepi.1370050304. [DOI] [PubMed] [Google Scholar]
- 725.Dirani M, Chamberlain M, Shekar SN, et al. Heritability of refractive error and ocular biometrics: the Genes in Myopia (GEM) twin study. Invest Ophthalmol Vis Sci. 2006;47:4756–4761. doi: 10.1167/iovs.06-0270. [DOI] [PubMed] [Google Scholar]
- 726.Lopes MC, Andrew T, Carbonaro F, Spector TD, Hammond CJ. Estimating heritability and shared environmental effects for refractive error in twin and family studies. Invest Ophthalmol Vis Sci. 2009;50:126–131. doi: 10.1167/iovs.08-2385. [DOI] [PubMed] [Google Scholar]
- 727.Solouki AM, Verhoeven VJ, van Duijn CM, et al. A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat Genet. 2010;42:897–901. doi: 10.1038/ng.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Hysi PG, Young TL, Mackey DA, et al. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet. 2010;42:902–905. doi: 10.1038/ng.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Li YJ, Goh L, Khor CC, et al. Genome-wide association studies reveal genetic variants in CTNND2 for high myopia in Singapore Chinese. Ophthalmology. 2011;118:368–375. doi: 10.1016/j.ophtha.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Li Z, Qu J, Xu X, et al. A genome-wide association study reveals association between common variants in an intergenic region of 4q25 and high-grade myopia in the Chinese Han population. Hum Mol Genet. 2011;20:2861–2868. doi: 10.1093/hmg/ddr169. [DOI] [PubMed] [Google Scholar]
- 731.Shi Y, Qu J, Zhang D, et al. Genetic variants at 13q12.12 are associated with high myopia in the han chinese population. Am J Hum Genet. 2011;88:805–813. doi: 10.1016/j.ajhg.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Shi Y, Li Y, Zhang D, et al. Exome sequencing identifies ZNF644 mutations in high myopia. PLoS Genet. 2011;7:e1002084. doi: 10.1371/journal.pgen.1002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Verhoeven VJ, Hysi PG, Wojciechowski R, et al. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet. 2013;45:314–318. doi: 10.1038/ng.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Flitcroft DI, Loughman J, Wildsoet CF, Williams C, Guggenheim JA; for the CREAM Consortium. Novel myopia genes and pathways identified from syndromic forms of myopia. Invest Ophthalmol Vis Sci. 2018;59:338–348. doi: 10.1167/iovs.17-22173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Tedja MS, Wojciechowski R, Hysi PG, et al. Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat Genet. 2018;50:834–848. doi: 10.1038/s41588-018-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Fan Q, Guo X, Tideman JW, et al. Childhood gene-environment interactions and age-dependent effects of genetic variants associated with refractive error and myopia: the CREAM Consortium. Sci Rep. 2016;6:25853. doi: 10.1038/srep25853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Tkatchenko AV, Tkatchenko TV, Guggenheim JA, et al. APLP2 regulates refractive error and myopia development in mice and humans. PLoS Genet. 2015;11:e1005432. doi: 10.1371/journal.pgen.1005432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Caputto BL, Guido ME. Immediate early gene expression within the visual system: light and circadian regulation in the retina and the suprachiasmatic nucleus. Neurochem Res. 2000;25:153–162. doi: 10.1023/a:1007508020173. [DOI] [PubMed] [Google Scholar]
- 739.Bitzer M, Schaeffel F. Defocus-induced changes in ZENK expression in the chicken retina. Invest Ophthalmol Vis Sci. 2002;43:246–252. [PubMed] [Google Scholar]
- 740.Ashby RS, Zeng G, Leotta AJ, Tse DY, McFadden SA. Egr-1 mRNA expression is a marker for the direction of mammalian ocular growth. Invest Ophthalmol Vis Sci. 2014;55:5911–5921. doi: 10.1167/iovs.13-11708. [DOI] [PubMed] [Google Scholar]
- 741.Brand C, Burkhardt E, Schaeffel F, Choi JW, Feldkaemper MP. Regulation of Egr-1, VIP, and Shh mRNA and Egr-1 protein in the mouse retina by light and image quality. Mol Vis. 2005;11:309–320. [PubMed] [Google Scholar]
- 742.Schippert R, Burkhardt E, Feldkaemper M, Schaeffel F. Relative axial myopia in Egr-1 (ZENK) knockout mice. Invest Ophthalmol Vis Sci. 2007;48:11–17. doi: 10.1167/iovs.06-0851. [DOI] [PubMed] [Google Scholar]
- 743.Zhong X. Expression of pax-6 in rhesus monkey of optical defocus induced myopia and form deprivation myopia. Chin Med J. 2004;117:722–725. [PubMed] [Google Scholar]
- 744.Liu Q, Wu J, Wang X, Zeng J. Changes in muscarinic acetylcholine receptor expression in form deprivation myopia in guinea pigs. Mol Vis. 2007;13:1234–1244. [PubMed] [Google Scholar]
- 745.Akamatsu S, Fujii S, Escaño MF, Ishibashi K, Sekiya Y, Yamamoto M. Altered expression of genes in experimentally induced myopic chick eyes. Jpn J Ophthalmol. 2001;45:137–143. doi: 10.1016/s0021-5155(00)00360-9. [DOI] [PubMed] [Google Scholar]
- 746.Qian YS, Chu RY, He JC, et al. Incidence of myopia in high school students with and without red-green color vision deficiency. Invest Ophthalmol Vis Sci. 2009;50:1598–1605. doi: 10.1167/iovs.07-1362. [DOI] [PubMed] [Google Scholar]
- 747.Chen M, Qian Y, Dai J, Chu R. The sonic hedgehog signaling pathway induces myopic development by activating matrix metalloproteinase (MMP)-2 in guinea pigs. PLoS One. 2014;9:e96952. doi: 10.1371/journal.pone.0096952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Tran-Viet KN, St Germain E, Soler V, et al. Study of a US cohort supports the role of ZNF644 and high-grade myopia susceptibility. Mol Vis. 2012;18:937–944. [PMC free article] [PubMed] [Google Scholar]
- 749.Tran-Viet KN, Soler V, Quiette V, et al. Mutation in collagen II alpha 1 isoforms delineates Stickler and Wagner syndrome phenotypes. Mol Vis. 2013;19:759–766. [PMC free article] [PubMed] [Google Scholar]
- 750.Tran-Viet KN, Powell C, Barathi T, et al. Mutations in SCO2 are associated with autosomal-dominant high-grade myopia. Am J Hum Genet. 2013;92:820–826. doi: 10.1016/j.ajhg.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Mordechai SL, Gradstein A, Pasanen R, et al. High myopia caused by a mutation in LEPREL1, encoding prolyl 3-hydroxylase 2. Am J Hum Genet. 2011;89:438–445. doi: 10.1016/j.ajhg.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Zhao F, Wu J, Xue A, et al. Exome sequencing reveals CCDC111 mutation associated with high myopia. Hum Genet. 2013;132:913–921. doi: 10.1007/s00439-013-1303-6. [DOI] [PubMed] [Google Scholar]
- 753.Wojciechowski R, Hysi PG. Focusing in on the complex genetics of myopia. PLoS Genet. 2013;9:e1003442. doi: 10.1371/journal.pgen.1003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Hawthorne FA, Young TL. Genetic contributions to myopic refractive error: Insights from human studies and supporting evidence from animal models. Exp Eye Res. 2013;114:141–149. doi: 10.1016/j.exer.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 755.Kiefer AK, Tung JY, Do JD, et al. Genome-wide analysis points to roles for extracellular matrix remodeling, the visual cycle, and neuronal development in myopia. PLoS Genet. 2013;9:e1003299. doi: 10.1371/journal.pgen.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.Tkatchenko AV, Luo X, Tkatchenko TV, et al. Large-scale microRNA expression profiling identifies putative retinal miRNA-mRNA signaling pathways underlying form-deprivation myopia in mice. PLoS One. 2016;11:e0162541. doi: 10.1371/journal.pone.0162541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Chen KC, Hsi E, Hu CY, Chou WW, Liang CL, Juo SH. MicroRNA-328 may influence myopia development by mediating the PAX6 gene. Invest Ophthalmol Vis Sci. 2012;53:2732–2739. doi: 10.1167/iovs.11-9272. [DOI] [PubMed] [Google Scholar]
- 758.Tang SM, Rong SS, Young AL, Tam PO, Pang CP, Chen LJ. PAX6 gene associated with high myopia: a meta-analysis. Optom Vis Sci. 2014;91:419–429. doi: 10.1097/OPX.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 759.Ding Y, Chen X, Yan D, et al. Association analysis of retinoic acid receptor beta (RARbeta) gene with high myopia in Chinese subjects. Mol Vis. 2010;16:855–861. [PMC free article] [PubMed] [Google Scholar]
- 760.Qiao-Grider Y, Hung L-F, Kee CS, Ramamirtham R, Smith EL., III. A comparison of refractive development between two subspecies of infant rhesus monkeys (Macaca mulatta) Vision Res. 2007;47:1424–1444. doi: 10.1016/j.visres.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Wisard J, Faulkner A, Chrenek MA, et al. Exaggerated eye growth in IRBP-deficient mice in early development. Invest Ophthalmol Vis Sci. 2011;52:5804–5811. doi: 10.1167/iovs.10-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Zhou X, Huang Q, An J, et al. Genetic deletion of the adenosine A2A receptor confers postnatal development of relative myopia in mice. Invest Ophthalmol Vis Sci. 2010;51:4362–4370. doi: 10.1167/iovs.09-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Zhou GM, Strom RC, Giguere V, Williams RW. Modulation of retinal cell populations and eye size in retinoic acid receptor knockout mice. Mol Vis. 2001;7:253–260. [PubMed] [Google Scholar]
- 764.Barathi VA, Kwan JL, Tan QS, et al. Muscarinic cholinergic receptor (M2) plays a crucial role in the development of myopia in mice. Dis Model Mech. 2013;6:1146–1158. doi: 10.1242/dmm.010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Tekin M, Chioza BA, Matsumoto Y, et al. SLITRK6 mutations cause myopia and deafness in humans and mice. J Clin Invest. 2013;123:2094–2102. doi: 10.1172/JCI65853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Zhou G, Williams RW. Eye1 and Eye2: gene loci that modulate eye size, lens weight, and retinal area in the mouse. Invest Ophthalmol Vis Sci. 1999;40:817–825. [PubMed] [Google Scholar]
- 767.Puk O, Dalke C, Favor J, de Angelis MH, Graw J. Variations of eye size parameters among different strains of mice. Mamm Genome. 2006;17:851–857. doi: 10.1007/s00335-006-0019-5. [DOI] [PubMed] [Google Scholar]
- 768.Sivak JG, Barrie DL, Weerheim JA. Bilateral experimental myopia in chicks. Optom Vis Sci. 1989;66:854–858. doi: 10.1097/00006324-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 769.Prashar A, Hocking PM, Erichsen JT, Fan Q, Saw SM, Guggenheim JA. Common determinants of body size and eye size in chickens from an advanced intercross line. Exp Eye Res. 2009;89:42–48. doi: 10.1016/j.exer.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 770.Tepelus TC, Schaeffel F. Individual set-point and gain of emmetropization in chickens. Vision Res. 2010;50:57–64. doi: 10.1016/j.visres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 771.Ren Y, Xie R, Zhou M, Pan X, Lu F. Spontaneous high myopia in one eye will affect the development of form deprivation myopia in the fellow eye. Curr Eye Res. 2011;36:513–521. doi: 10.3109/02713683.2011.568660. [DOI] [PubMed] [Google Scholar]
- 772.Kubai MA, Labelle AL, Hamor RE, Mutti DO, Famula TR, Murphy CJ. Heritability of lenticular myopia in English Springer spaniels. Invest Ophthalmol Vis Sci. 2013;54:7324–7328. doi: 10.1167/iovs.12-10993. [DOI] [PubMed] [Google Scholar]
- 773.Kubai MA, Bentley E, Miller PE, Mutti DO, Murphy CJ. Refractive states of eyes and association between ametropia and breed in dogs. Am J Vet Res. 2008;69:946–951. doi: 10.2460/ajvr.69.7.946. [DOI] [PubMed] [Google Scholar]
- 774.Black J, Browning SR, Collins AV, Phillips JR. A canine model of inherited myopia: familial aggregation of refractive error in labrador retrievers. Invest Ophthalmol Vis Sci. 2008;49:4784–4789. doi: 10.1167/iovs.08-1828. [DOI] [PubMed] [Google Scholar]
- 775.Williams LA, Kubai MA, Murphy CJ, Mutti DO. Ocular components in three breeds of dogs with high prevalence of myopia. Optom Vis Sci. 2011;88:269–274. doi: 10.1097/OPX.0b013e3182058ff0. [DOI] [PubMed] [Google Scholar]
- 776.Park H, Jabbar SB, Tan CC, et al. Visually-driven ocular growth in mice requires functional rod photoreceptors. Invest Ophthalmol Vis Sci. 2014;55:6272–6279. doi: 10.1167/iovs.14-14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777.Zhou X, Pardue MT, Iuvone PM, Qu J. Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res. 2017. [DOI] [PMC free article] [PubMed]
- 778.Bergen MA, Park HN, Chakraborty R, et al. Altered refractive development in mice with reduced levels of retinal dopamine. Invest Ophthalmol Vis Sci. 2016;57:4412–4419. doi: 10.1167/iovs.15-17784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Huang F, Yan T, Shi F, et al. Activation of dopamine D2 receptor is critical for the development of form-deprivation myopia in the C57BL/6 mouse. Invest Ophthalmol Vis Sci. 2014;55:5537–5544. doi: 10.1167/iovs.13-13211. [DOI] [PubMed] [Google Scholar]
- 780.Song Y, Zhang F, Zhao Y, et al. Enlargement of the axial length and altered ultrastructural features of the sclera in a mutant lumican transgenic mouse model. PLoS One. 2016;11:e0163165. doi: 10.1371/journal.pone.0163165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Okano T, Kojima D, Fukada Y, Shichida Y, Yoshizawa T. Primary structures of chicken cone visual pigments: vertebrate rhodopsins have evolved out of cone visual pigments. Proc Natl Acad Sci U S A. 1992;89:5932–5936. doi: 10.1073/pnas.89.13.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Ostrin LA, Choh V, Wildsoet CF. The pattern ERG in chicks – stimulus dependence and optic nerve section. Vision Res. 2016;128:45–52. doi: 10.1016/j.visres.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Fujita Y, Imagawa T, Uehara M. Fine structure of the retino-optic nerve junction in the chicken. Tissue Cell. 2001;33 doi: 10.1054/tice.2000.0152. [DOI] [PubMed] [Google Scholar]
- 784.Allison WT, Haimberger TJ, Hawryshyn CW, Temple SE. Visual pigment composition in zebrafish: Evidence for a rhodopsin-porphyropsin interchange system. Vis Neurosci. 2004;21:945–952. doi: 10.1017/S0952523804216145. [DOI] [PubMed] [Google Scholar]
- 785.Bailey TJ, Davis DH, Vance JE, Hyde DR. Spectral-domain optical coherence tomography as a noninvasive method to assess damaged and regenerating adult zebrafish retinas. Invest Ophthalmol Vis Sci. 2012;53:3126–3138. doi: 10.1167/iovs.11-8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Salinas-Navarro M, Jimenez-Lopez M, Valiente-Soriano VF, et al. Retinal ganglion cell population in adult albino and pigmented mice: a computerized analysis of the entire population and its spatial distribution. Vision Res. 2009;49:637–647. doi: 10.1016/j.visres.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 787.Ruggeri M, Wehbe H, Jiao S, et al. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:1808–1814. doi: 10.1167/iovs.06-0815. [DOI] [PubMed] [Google Scholar]
- 788.May CA, Lutjen-Drecoll E. Morphology of the murine optic nerve. Invest Ophthalmol Vis Sci. 2002;43:2206–2212. [PubMed] [Google Scholar]
- 789.Rodriguez-Ramos Fernandez J, Dubielzig RR. Ocular comparative anatomy of the family Rodentia. Vet Ophthalmol. 2013;16:94–99. doi: 10.1111/vop.12070. [DOI] [PubMed] [Google Scholar]
- 790.Abbott CJ, Grunert U, Pianta MJ, McBrien NA. Retinal thinning in tree shrews with induced high myopia: optical coherence tomography and histological assessment. Vision Res. 2011;51:376–385. doi: 10.1016/j.visres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 791.Williams AJ, Hunt DM, Bowmaker JK, Mollon JD. The polymorphic photopigments of the marmoset: spectral tuning and genetic basis. EMBO J. 1992;11:2039–2045. doi: 10.1002/j.1460-2075.1992.tb05261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Perry VH, Cowey A. The ganglion cell and cone distributions in the monkey's retina: Implications for central magnification factors. Vision Res. 1985;25:1795–1810. doi: 10.1016/0042-6989(85)90004-5. [DOI] [PubMed] [Google Scholar]
- 793.Marks WB, Dobelle WH, Macnichol EF. Visual pigments of single primate cones. Science. 1964;143:1181–1183. doi: 10.1126/science.143.3611.1181. [DOI] [PubMed] [Google Scholar]
- 794.Patel NB, Hung LF, Harwerth RS. Postnatal maturation of the fovea in Macaca mulatta using optical coherence tomography. Exp Eye Res. 2017;164:8–21. doi: 10.1016/j.exer.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Ivers KM, Sredar N, Patel NB, et al. In vivo changes in lamina cribrosa microarchitecture and optic nerve head structure in early experimental glaucoma. PloS One. 2015;10:e0134223. doi: 10.1371/journal.pone.0134223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- 797.Dartnall HJ, Bowmaker JK, Mollon JD. Human visual pigments: microspectrophotometric results from the eyes of seven persons. Proc R Soc Lond B Biol Sci. 1983;220:115–130. doi: 10.1098/rspb.1983.0091. [DOI] [PubMed] [Google Scholar]
- 798.Chan A, Duker JS, Ko TH, Fujimoto JG, Schuman JS. Normal macular thickness measurements in healthy eyes using stratus optical coherence tomography. Arch Ophthalmol. 2006;124:193–198. doi: 10.1001/archopht.124.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Jonas JB, Mardin CY, Schlorzer-Schrehardr U, Naumann GOH. Morphometry of the human lamina cribrosa surface. Invest Ophthalmol Vis Sci. 1991;32:401–405. [PubMed] [Google Scholar]
- 800.Frost MR, Norton TT. Alterations in protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Invest Ophthalmol Vis Sci. 2012;53:322–336. doi: 10.1167/iovs.11-8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Tian XD, Cheng YX, Liu GB, et al. Expressions of type I collagen, alpha2 integrin and beta1 integrin in sclera of guinea pig with defocus myopia and inhibitory effects of bFGF on the formation of myopia. Int J Ophthalmol. 2013;6:54–58. doi: 10.3980/j.issn.2222-3959.2013.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Guggenheim JA, McBrien NA. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996;37:1380–1395. [PubMed] [Google Scholar]
- 803.Li H, Cui D, Zhao F, Huo L, Hu J, Zeng J. BMP-2 is involved in scleral remodeling in myopia development. PLoS One. 2015;10:e0125219. doi: 10.1371/journal.pone.0125219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804.Jiang L, Long K, Zhou X, Qu J. Reciprocal activities of two type dopamine receptors determine the myopia development. Invest Ophthalmol Vis Sci. 2012;53:ARVO E-Abstract 3437. [Google Scholar]
- 805.Mao J, Liu S. Different roles of retinal dopamine in albino Guinea pig myopia. Neurosci Lett. 2017;639:94–97. doi: 10.1016/j.neulet.2016.12.061. [DOI] [PubMed] [Google Scholar]
- 806.Cottriall CL, McBrien NA, Annies R, Leech EM. Prevention of form-deprivation myopia with pirenzepine: a study of drug delivery and distribution. Ophthalmic Physiol Opt. 1999;19:327–335. [PubMed] [Google Scholar]
- 807.Qian L, Zhao H, Li X, et al. Pirenzepine inhibits myopia in guinea pig model by regulating the balance of MMP-2 and TIMP-2 expression and increased tyrosine hydroxylase levels. Cell Biochem Biophys. 2015;71:1373–1378. doi: 10.1007/s12013-014-0359-9. [DOI] [PubMed] [Google Scholar]
- 808.Cheng ZY, Wang XP, Schmid KL, Han XG. Inhibition of form-deprivation myopia by a GABAAOr receptor antagonist, (1,2,5,6-tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA), in guinea pigs. Graefes Arch Clin Exp Ophthalmol. 2014;252:1939–1946. doi: 10.1007/s00417-014-2765-5. [DOI] [PubMed] [Google Scholar]
- 809.Cheng ZY, Wang XP, Schmid KL, et al. GABAB receptor antagonist CGP46381 inhibits form-deprivation myopia development in guinea pigs. Biomed Res Int. 2015. 207312. [DOI] [PMC free article] [PubMed]
- 810.Vessey KA, Rushforth DA, Stell WK. Glucagon- and secretin-related peptides differentially alter ocular growth and the development of form-deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2005;46:3932–3942. doi: 10.1167/iovs.04-1027. [DOI] [PubMed] [Google Scholar]
- 811.Zhou X, Shen M, Xie J, et al. The development of the refractive status and ocular growth in C57BL/6 mice. Invest Ophthalmol Vis Sci. 2008;49:5208–5214. doi: 10.1167/iovs.07-1545. [DOI] [PubMed] [Google Scholar]
- 812.Chakraborty R, Hn Park, Tan CC, Weiss P, Prunty MC, Pardue MT. Association of body length with ocular parameters in mice. Optom Vis Sci. 2017;94 doi: 10.1097/OPX.0000000000001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813.Park HN, Tan CC, Faulkner A, et al. Retinal degeration increase susceptibility to myopia in mice. Mol Vis. 2013;19:2068–2079. [PMC free article] [PubMed] [Google Scholar]
- 814.Zhou X, Qu J, Xie R, et al. Normal development of refractive state and ocular dimensions in guinea pigs. Vision Res. 2006;46:2815–2823. doi: 10.1016/j.visres.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 815.Lu F, Zhou X, Zhao H, et al. Axial myopia induced by a monocularly-deprived facemask in guinea pigs: a non-invasive and effective model. Exp Eye Res. 2006;82:628–636. doi: 10.1016/j.exer.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 816.Schmid KL, Hills T, Abbott M, Humphries M, Pyne K, Wildsoet CF. Relationship between intraocular pressure and eye growth in chick. Ophthalm Physiol Opt. 2003;23:25–33. doi: 10.1046/j.1475-1313.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 817.Avila MV, McFadden SA. A detailed paraxial schematic eye for the white leghorn chick. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196:825–840. doi: 10.1007/s00359-010-0562-0. [DOI] [PubMed] [Google Scholar]
- 818.Rymer J, Choh V, Bharadwaj S, et al. The albino chick as a model for studying ocular developmental anomalies, including refractive errors, associated with albinism. Exp Eye Res. 2007;85:431–442. doi: 10.1016/j.exer.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.Raviola E, Wiesel TN. Neural control of eye growth and experimental myopia in primates. In: Bock G, Widdows K, editors. Myopia and the Control of Eye Growth. Chichester, UK: Wiley;; 1990. pp. 22–44. [DOI] [PubMed] [Google Scholar]
- 820.Hayes BP, Fitzke FW, Hodos W, Holden AL. A morphological analysis of experimental myopia in young chickens. Invest Ophthalmol Vis Sci. 1986;27:981–991. [PubMed] [Google Scholar]
- 821.Mandelman T, Sivak JG. Longitudinal chromatic aberration of the vertebrate eye. Vision Res. 1983;23:1555–1559. doi: 10.1016/0042-6989(83)90169-4. [DOI] [PubMed] [Google Scholar]
- 822.Smith EL, III,, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49:2386–2392. doi: 10.1016/j.visres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823.Norton TT, Amedo AO, Siegwart JT. The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Res. 2010;50:564–576. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]