Abstract
Cyclic nucleotide-gated (CNG) ion channels are crucial for phototransduction in rod photoreceptors. Light triggers a biochemical cascade that reduces the concentration of cGMP in rods, closing CNG channels, which leads to membrane potential hyperpolarization and a decrease in the concentration of intracellular Ca2+. During light adaptation, the sensitivity of CNG channels to cGMP is decreased by Ca2+, which in conjunction with calmodulin (CaM), binds directly to CNG channels. The cGMP sensitivity of rod CNG channels is also reduced by phosphorylation of specific tyrosine residues in the three CNGA1 subunits and one CNGB1 subunit that comprise the rod channel. Here we show that phosphorylation prevents Ca2+/CaM inhibition. Experiments on native channels in rod outer segments and expressed channels in Xenopus oocytes show that Ca2+/CaM inhibition can be toggled off or on by promoting phosphorylation or dephosphorylation, respectively. Experiments in which the crucial tyrosine phosphorylation sites in CNGA1 and CNGB1 are replaced with phenylalanines show that residue Y498 in CNGA1 is the phosphorylation site responsible for regulating Ca2+/CaM inhibition. Ca2+/CaM inhibits the rod channel by binding to the N terminus of the CNGB1 subunit, causing it to uncouple from the C terminus of CNGA1. We propose that phosphorylation of CNGA1Y498, on the C terminus of CNGA1, triggers an equivalent uncoupling from the C terminus of CNGB1, thereby curtailing Ca2+/CaM inhibition. The control of CaM inhibition by CNG channel phosphorylation may be important for light adaptation and the regulation of phototransduction by IGF-1, a retinal paracrine factor that alters the tyrosine phosphorylation state of rod CNG channels.
Keywords: rod, photoreceptor, cGMP, channel, phosphorylation, calmodulin
Introduction
Cyclic-nucleotide gated (CNG) channels are crucial for generating the light response in rod photoreceptors (for review, see Yau and Baylor, 1989). Photoisomerization of rhodopsin leads to a decrease in the concentration of cGMP in rod outer segments (ROS), resulting in the closure of CNG channels. This causes a decrease of the inward “dark current,” resulting in hyperpolarization of the membrane potential. In addition to this electrical signal, closure of CNG channels produces a chemical signal. Ca2+ enters through CNG channels in the dark, so illumination decreases Ca2+ influx. The maintained operation of the Na+/Ca2+/K+ transporter results in a decrease in the cytoplasmic Ca2+ concentration (Yau and Nakatani, 1984; Gray-Keller and Detwiler, 1994). The drop in Ca2+ acts as the crucial signal for light adaptation in rods.
Intracellular Ca2+ regulates several steps in the phototransduction cascade, including the CNG channel itself (for review, see Burns and Baylor, 2001). Calmodulin (CaM), along with Ca2+ as a cofactor (Ca2+/CaM), binds directly to the rod CNG channel and inhibits its activity by decreasing the apparent affinity for cGMP (Hsu and Molday, 1993, 1994; Chen et al., 1994). The rod CNG channel is a heterotetramer consisting of three CNGA1 subunits and one CNGB1 subunit (Zheng et al., 2002; Zhong et al., 2002; Weitz et al., 2002). Ca2+/CaM binds with high affinity to the cytoplasmic N teminus of the CNGB1 subunit and weakens an intramolecular interaction between the N and C termini of the CNGB1 and CNGA1 channel subunits respectively, thereby impairing the ability of the channel to open in response to cGMP (Trudeau and Zagotta, 2002, 2003).
CNG channels are also modulated by phosphorylation, which also leads to changes in light response (Savchenko et al., 2001). Phosphorylation of tyrosine Y498 in the CNGA1 subunit and Y1097 in the CNGB1 subunit decreases the apparent affinity for cGMP (Molokanova et al., 1997, 1999, 2003). Subunits that contain these tyrosines can be labeled with [32P]ATP, but mutant channels in which they are replaced with phenylalanines do not exhibit P32 labeling. These results suggest that CNGA1Y498 and CNGB1Y1097 are the principal phosphorylation sites in the rod CNG channels (Molokanova et al., 2003). Insulin-like growth factor-1 (IGF-1), a paracrine factor in the retina, triggers a signaling cascade in rods that results in dephosphorylation of these crucial tyrosine residues, increasing the sensitivity of CNG channels and increasing the magnitude and speeding the kinetics of the light response. IGF-1 is synthesized and secreted by retinal pigment epithelial (RPE) cells (Waldbillig et al., 1991), which are adjacent to ROS, but how IGF-1 secretion is regulated is not known. Modulation of the rod CNG by IGF-1 and changes in tyrosine phosphorylation raises the possibility that these events contribute to light adaptation or nonadaptive changes in light sensitivity, for example, by circadian rhythms.
Thus, Ca2+/CaM and tyrosine phosphorylation each regulate the sensitivity of rod CNG channels to cGMP. In this paper, we explore whether these regulatory processes act independently or whether they occur through a common mechanism.
Materials and Methods
Materials
cDNA clones expressing the coding region for CNGA1 (Kaupp et al., 1989), CNGB1 (Chen et al., 1993), and mutants of CNGA1 and CNGB1 (Molokanova et al., 1999, 2003, respectively) were subcloned into the expression vector pGEM-HE (Liman et al., 1992) under the T7 promoter and were used for the production of cRNA using the mMessage Machine T7 kit (Ambion, Austin, TX) and Ribomax (Promega, Madison, WI). Xenopus laveis frogs were obtained from Nasco (Fort Atkinson, WI). Care and use of Xenopus frogs in this study have been approved by the University of California, Berkeley and the University of Miami Animal Research Committees and meets the guidelines of the National Institutes of Health. All other reagents were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant bovine calmodulin was a kind gift from Aldrin Gomes (University of Miami).
Rod CNG channel subunit expression
Adult female Xenopus laveis frogs were anesthetized by immersion in 0.1% 3-aminobenzoic acid ethyl ester for 1 hr, and the oocytes were surgically removed. Follicle cells were removed by treatment with collagenase B for 2 hr at room temperature. To ensure that all expressed CNG channels contain a CNGB1 subunit, CNGA1 and CNGB1 cRNA were injected in a 1:8 molar ratio using a Nanoject II injector (Drummond Scientific, Broomall, PA). Injected oocytes were incubated at 18°C for 2-7 d in regular Barth's solution before experiments [88 mm NaCl, 1 mm KCl, 2.4 mm NaHCO3, 0.3 mm Ca(NO3)2, 0.41 mm CaCl2, 0.82 mm MgSO4, 100 μg/ml gentamicin, and 15 mm HEPES, pH 7.6].
Electrophysiology
All recordings were done on an Axopatch 200A patch clamp (Axon Instruments, Union City, CA).
Oocyte experiments and data analysis. Membrane potential was clamped at -80 mV. Currents were filtered at 1 kHz, recorded on a personal computer, and analyzed using PClamp 6.0 (Axon Instruments). Electrodes were used that had resistances of 1-3 MΩ. cGMP and other drugs were applied to the patch using gravity flow application. The vitelline membrane was mechanically removed using forceps, and the oocyte was placed into the recording chamber. After a gigaohm seal was established, an inside-out patch was formed and rapidly placed into the perfusion opening (<20 sec). Recording solutions consisted of the following (in mm): 115 Na-gluconate, 5 KCl, 2 nitrilotriacetic acid, and 10 HEPES, adjusted to pH 7.6 with NaOH. When necessary, 704 μm CaCl2 was added to give a free Ca2+ concentration of 50 μm. cGMP was added to the recording solution to final concentrations of 5-2000 μm. The pipette contained recording solution plus 500 μm niflumic acid to block endogenous Ca2+-activated chloride channels. Currents were measured from expressing CNG channels with increasing concentrations of cGMP, recorded and normalized to the saturating response using 2000 μm cGMP, and fit to the Hill equation as follows: I/Imax = 1/(1 + (K1/2/[A])n), where I is measured current, A is cGMP concentration, and n is the Hill coefficient, using nonlinear regression analysis (Prism 3.1; GraphPad Software, San Diego, CA). Variability in the results is presented as ±SEM. One hundred micromolar vanadate was added to recording solutions in experiments that required blockage of endogenous protein tyrosine phosphatases (PTPs) to prevent changes in the tyrosine phosphorylation state of expressing CNG channels. To block PTPs in intact oocytes, pervanadate was prepared immediately before oocyte incubation by adding 500 mm H2O2 to 10 mm sodium orthovanadate. Oocytes were incubated in this solution for 30 min to block endogenous PTPs present in the oocyte and to maximize the phosphorylation state of the expressing CNG channels before patch excision.
Rod photoreceptor experiments. Rod photoreceptors were obtained from retina of dark-adapted larval tiger salamanders (Ambystoma tigrinum) as described previously (Molokanova et al., 1997). Borosilicate glass pipettes (3-5 MΩ) were filled with a solution consisting of the following (in mm): 115 NaCl, 5 EGTA, 1 EDTA, and 10 HEPES, adjusted to pH 7.5 with NaOH. This solution also served as the standard bath and superfusion solution. Membrane potential was held at -50 mV. The patch pipette was placed in the outlet of a 1-mm-diameter tube connected to a 15-channel perfusion manifold. Current responses were digitized, stored, and later analyzed.
Results
Tyrosine phosphorylation and Ca2+/CaM binding cause similar changes in CNG channel gating
The phosphorylation state of rod CNG channels expressed in Xenopus oocytes can be regulated by constitutively active protein tyrosine kinases (PTKs) and PTPs endogenous to the oocyte (Molokanova et al., 1997). The activity of these enzymes, and therefore the effects of phosphorylation and dephosphorylation on CNG channels, can be monitored in excised patches taken from Xenopus oocytes. When inside-out patches containing rod CNG channels are exposed to saline lacking Mg2+-ATP, PTPs in the oocyte membrane spontaneously dephosphorylate the channels over 5-10 min, resulting in a twofold to threefold increase in sensitivity to cGMP. Subsequent application of Mg2+-ATP provides a substrate for PTKs that phosphorylate the channels, decreasing cGMP sensitivity by twofold to threefold. The PTP and PTK activity persists for at least 30 min after patch excision, suggesting that these enzymes are either integral or tightly membrane-associated proteins.
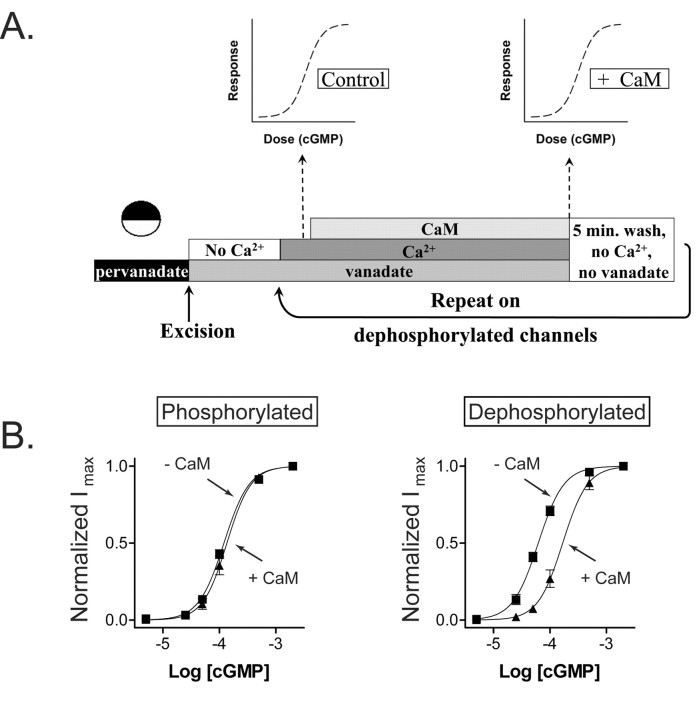
Figure 1A shows the change in cGMP sensitivity that occurs after patch excision. Oocytes were pretreated with 100 μm pervanadate, a membrane-permeant form of the PTP inhibitor vanadate, to prevent dephosphorylation and ensure the CNG channels were maximally phosphorylated. The cGMP sensitivity was assessed by applying 5-2000 μm cGMP immediately after patch excision. To ensure that the channels remained phosphorylated after patch excision, 100 μm vanadate was present in all superfusion solutions. The patch was then washed free of vanadate for 5 min before examining cGMP sensitivity a second time. Previous work demonstrates that a 5 min wash in vanadate-free solution allows complete dephosphorylation of the rod CNG channel expressed in oocytes (Molokanova et al., 2003). The resulting dose-response curves were fit with the Hill equation. The K1/2 value for channel activation decreased from 115 ± 4.5 to 54.2 ± 3.1 μm (n = 6) during dephosphorylation, a 2.1-fold change.
Figure 1.
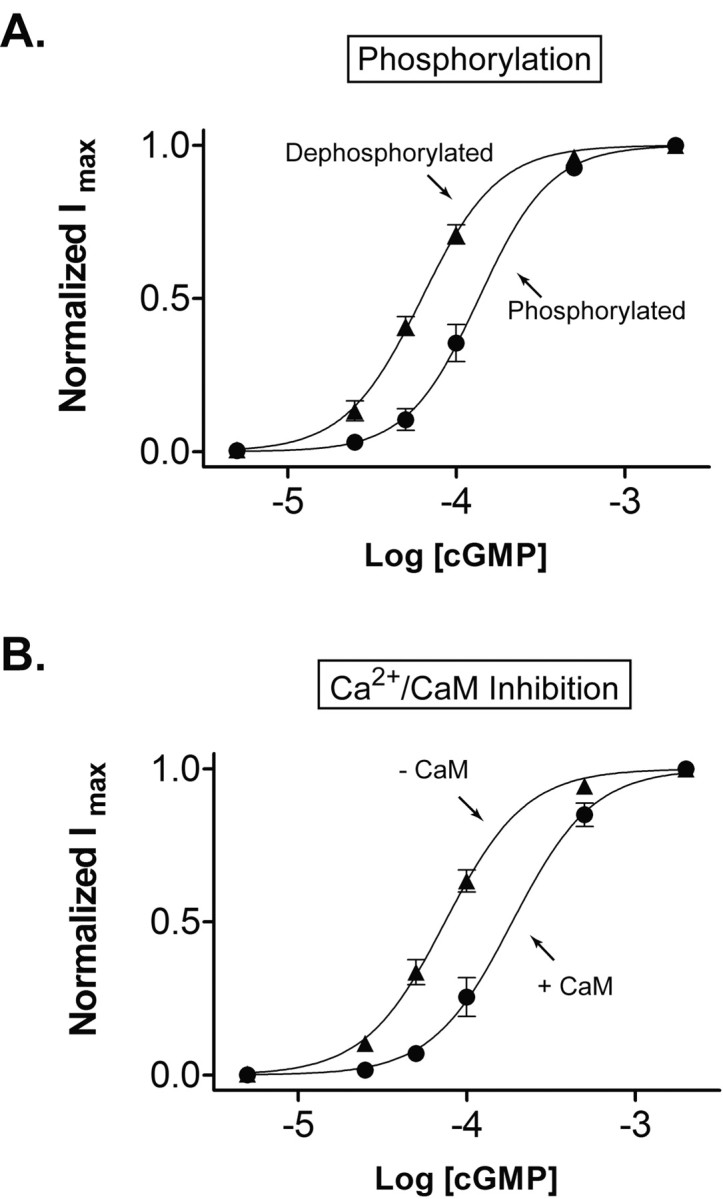
Similar extent of modulation of CNG channels by tyrosine phosphorylation and Ca2+/CaM. A, cGMP dose-response curves of rod CNG channels under conditions that promote phosphorylation or dephosphorylation. To maximize phosphorylation (circles), intact oocytes were preincubated with pervanadate for 30 min, and patches were continuously exposed to vanadate after excision. To maximize dephosphorylation (triangles), patches were exposed to vanadate-free solution for 5 min. Continuous curves show fits to the Hill equation. B, cGMP dose-response curves obtained before (triangles) and during (circles) application of Ca2+/CaM. For this experiment, CNG channels were first allowed to dephosphorylate for 5 min in vanadate-free saline before cGMP was applied.
A very similar change in sensitivity can be elicited by applying Ca2+/CaM on rod CNG channels (Fig. 1B). Channels were first allowed to dephosphorylate for 5 min after excision, and dose-response data were obtained before and after addition 250 nm CaM. The K1/2 for channel activation by cGMP increased from 65.7 ± 3.8 μm in the absence of Ca2+/CaM to 150.2 ± 5.6 μm in the presence of Ca2+/CaM, a 2.3-fold change (n = 4). In both experiments, 50 μm Ca2+ was present to control for possible CaM-independent effects. Previous work showed that 50 μm Ca2+ plus 250 nm CaM is sufficient to saturate modulation of rod CNG channels (Grunwald et al., 1998).
Phosphorylation of expressed rod channels prevents Ca2+/CaM inhibition
The similar magnitude of the phosphorylation and Ca2+/CaM effects would be consistent with the two modulators acting through a common molecular mechanism. To test this idea, we examined the effect of Ca2+/CaM on channels that were already phosphorylated. To promote complete phosphorylation, excised patches were treated with ATP-γ-S. Previous work on rod CNG channels (Molokanova et al., 1997) and other proteins (Hoar et al., 1979) show that ATP-γ-S can replace ATP in phosphorylation reactions, but the thio-phosphorylated substrate is resistant to dephosphorylation by most phosphatases. Hence ATP-γ-S permanently decreases the sensitivity of rod CNG channels to cGMP such that no recovery occurs, even after it is washed away for several minutes (Fig. 2A). Surprisingly, application of Ca2+/CaM on thio-phosphorylated channels has no affect on cGMP sensitivity (Fig. 2B). Hence, the effects of phosphorylation appear to prevent Ca2+/CaM inhibition of rod CNG channels.
Figure 2.
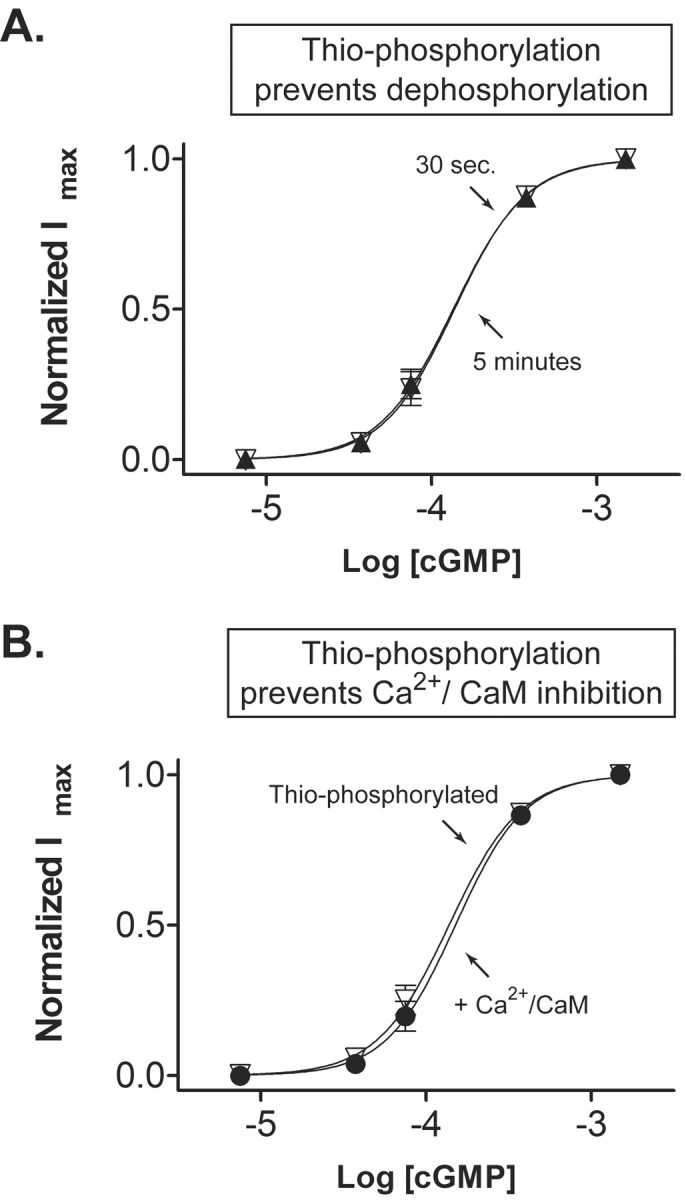
Thio-phosphorylation of rod CNG channels prevents Ca2+/CaM inhibition. A, Excised patches containing rod CNG channels were incubated with 250 μm Mg2+ ATP-γ-S for 5 min. The patch was then washed free of ATP-γ-S, and cGMP dose-response curves were obtained at 30 sec and 5 min after treatment. Note that ATP-γ-S prevents the spontaneous increase in cGMP sensitivity that occurs after excision (see Fig. 1 A), indicating that the CNG channels are persistently thio-phosphorylated. B, cGMP dose-response curves obtained before (triangles) and during (circles) application of Ca2+/CaM. Note that thio-phosphorylation prevents Ca2+/CaM inhibition.
To further explore the effects of phosphorylation on Ca2+/CaM inhibition, we used the experimental protocol illustrated in Figure 3A. Oocytes were first treated with pervanadate to ensure maximal phosphorylation of rod CNG channels. Patches were excised into a Ca2+-free solution to remove any endogenous Ca2+-binding proteins that might be associated with CNG channels in intact cells. Dose-response data for cGMP activation were obtained in the presence of 50 μm Ca2+, but before and after addition of 250 nm CaM, to allow determination of Ca2+/CaM inhibition. Figure 3B shows that Ca2+/CaM had no effect on channels that had undergone the above treatment to maximize phosphorylation. We next examined these same channels in their dephosphorylated state by removing vanadate, thereby allowing the PTPs to be active for a period of 5 min. Now Ca2+/CaM effectively inhibited the channels, increasing the K1/2 for cGMP from 59.2 ± 1.0 to 162.2 ± 5.0 μm (n = 6) (Fig. 3B). Hence, channels that were phosphorylated and unaffected by Ca2+/CaM indeed become susceptible to Ca2+/CaM inhibition once they are dephosphorylated.
Figure 3.
Dephosphorylation of rod CNG channels restores calmodulin inhibition. A, Experimental paradigm for studying the effect of tyrosine phosphorylation on Ca2+/CaM inhibition. Xenopus oocytes expressing rod CNG channels are preincubated with 100 μm pervanadate to block endogenous PTPs and maximize channel phosphorylation. An inside-out patch is excised into Ca2+-free solution to remove endogenous Ca2+-binding proteins. After superfusing with 50 μm Ca2+ plus vanadate, channel sensitivity in the absence of CaM is assessed by obtaining cGMP dose-response data. CaM at 250 nm is then added, and a second dose-response relationship is obtained. Finally, vanadate and Ca2+ are washed away for 5 min to allow dephosphorylation, and the above protocol is repeated. B, Dose-response relationships of cGMP activation measured with and without Ca2+/CaM under conditions that promote phosphorylation (left) and dephosphorylation (right) of rod CNG channels using the above paradigm.
Where is the phosphorylation site that interferes with Ca2+/CaM inhibition?
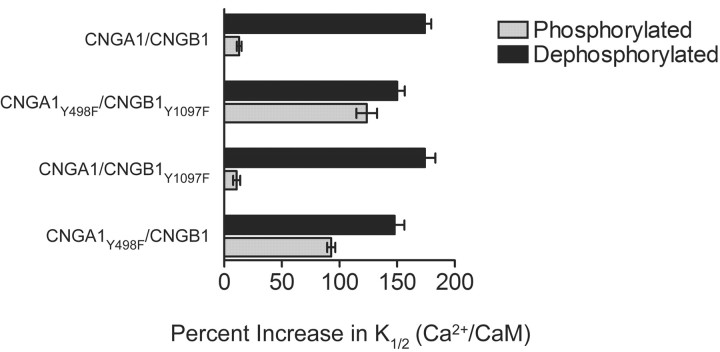
Both the CNGA1 and CNGB1 subunits contain tyrosine residues (Y498 and Y1097, respectively) that are substrates for PTKs and PTPs. The phosphorylation states of these two residues have been identified as the primary sites that regulate the cGMP sensitivity of the rod channel (Molokanova et al., 1997, 1999, 2003). We tested whether phosphorylation of one or both of these residues is responsible for suppressing Ca2+/CaM inhibition, using the paradigm illustrated in Figure 3A. We used channels with one phosphorylation site removed (CNGA1/CNGB1Y1097F and CNGA1Y498F/CNGB1) or both sites removed (CNGA1Y498F/CNGB1Y1097F) and compared these results to those obtained from wild-type channels with both sites present (CNGA1/CNGB1) (Fig. 3B). Table 1 shows the K1/2 values for cGMP activation of these channels with and without Ca2+/CaM and under conditions promoting phosphorylation or dephosphorylation.
Table 1.
K½ values for cGMP activation
|
|
Phosphorylated |
Dephosphorylated |
||||
|---|---|---|---|---|---|---|
|
|
Control
|
250 nm CaM
|
Control
|
250 nm CaM
|
||
| CNGA1/CNGB1 (n = 6) | 120.6 ± 2.2 μm, nH = 1.9 ± 0.1 | 136.3 ± 3.7 μm, nH = 2.0 ± 0.1 | 59.2 ± 1.0 μm, nH = 1.9 ± 0.1 | 162.2 ± 5.0 μm, nH = 2.0 ± 0.1 | ||
| CNGA1Y498F/CNGB1Y1097F (n = 4) | 75.1 ± 5.3 μm, nH = 2.1 ± 0.1 | 169.0 ± 7.5 μm, nH = 2.1 ± 0.1 | 62.2 ± 1.5 μm, nH = 1.9 ± 0.1 | 155.5 ± 5.9 μm, nH = 2.0 ± 0.1 | ||
| CNGA1/CNGB1Y1097F (n = 13) | 112.2 ± 2.1 μm, nH = 2.1 ± 0.1 | 124.3 ± 5.4 μm, nH = 2.0 ± 0.1 | 56.4 ± 2.6 μm, nH = 1.9 ± 0.2 | 154.3 ± 5.1 μm, nH = 1.9 ± 0.1 | ||
| CNGA1Y498F/CNGB1 (n = 9)
|
132.5 ± 3.4 μm, nH = 1.9 ± 0.1
|
255.3 ± 4.9 μm, nH = 2.0 ± 0.1
|
68.4 ± 3.3 μm, nH = 1.8 ± 0.2
|
169.1 ± 5.8 μm, nH = 1.8 ± 0.2
|
||
In wild-type channels with both phosphorylation sites intact, Ca2+/CaM increased the K1/2 by only 13% when conditions promoted phosphorylation. However, when conditions promoted dephosphorylation, Ca2+/CaM increased the K1/2 by 174% (Fig. 4). In channels with neither phosphorylation site intact, Ca2+/CaM had a similar effect under both conditions (125 and 155%, respectively). However, when the CNGA1 subunit contained the only phosphorylation site, Ca2+/CaM increased the K1/2 by 11% when conditions promoted phosphorylation and 174% when conditions promoted dephosphorylation. In contrast, in CNG channels that have an intact phosphorylation site only on CNGB1, Ca2+/CaM caused a 93% increase when phosphorylation was promoted and a 148% increase when dephosphorylation was promoted. These results suggest that the phosphorylation state of residue Y498 on CNGA1 is the major determinant of the effectiveness of Ca2+/CaM inhibition, with Y1097 on CNGB1 playing only a minor role.
Figure 4.
Phosphorylation of CNGA1 but not CNGB1 prevents inhibition of rod CNG channels by Ca2+/CaM. The percentage increase in cGMP K1/2 values by Ca2+/CaM was calculated for each channel type based on the results in Table 1. Ca2+/CaM had little effect on channels containing wild-type CNGA1 subunits (CNGA1/CNGB1 and CNGA1/CNGB1Y1097F) when conditions promoted phosphorylation but greatly increased the K1/2 for cGMP activation when conditions promoted dephosphorylation. In contrast, Ca2+/CaM had similar effects on channels containing CNGA1Y498F channels (CNGA1Y498F/CNGB1 and CNGA1Y498F/CNGB1Y1097F) when either phosphorylation or dephosphorylation were promoted. Note that there was little difference in the phosphorylation versus dephosphorylation dependence of Ca2+/CaM modulation in channels in which the CNGB1 subunit contains the only phosphorylation site (CNGA1Y498F/CNGB1).
Phosphorylation of native rod CNG channels prevents Ca2+/CaM inhibition
We next investigated whether tyrosine phosphorylation of native CNG channels from retinal rod outer segments could also prevent Ca2+/CaM inhibition. Unlike in oocytes, excised patches from rods do not seem to have spontaneously active PTPs or PTKs that remain associated with the excised patch. However, pretreatment of intact rods from tiger salamander with PTP or PTK inhibitors does result in a twofold to threefold change in cGMP sensitivity of CNG channels in subsequently excised patches (Molokanova et al., 1997), suggesting that these enzymes are present and can control the sensitivity of the channels.
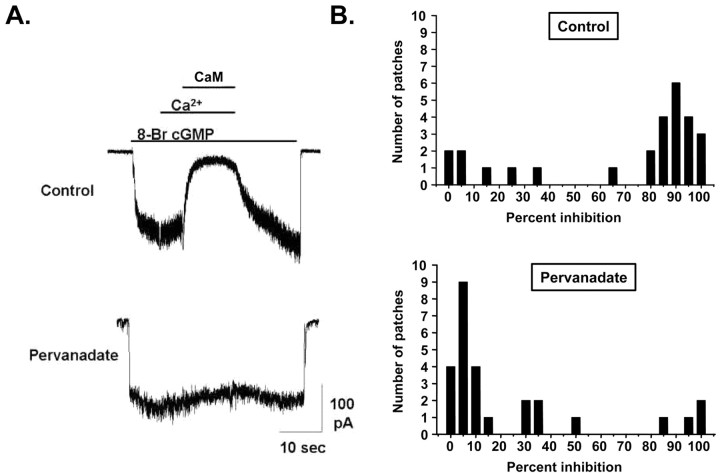
To examine the effect of channel phosphorylation on Ca2+/CaM inhibition on native CNG channels from ROS, intact retina from tiger salamander were treated with normal saline or saline containing 100 μm pervanadate for 1 hr before ROS isolation and patch excision. Application of 250 nm CaM in the presence of 50 μm Ca2+ strongly inhibited CNG channels that were activated with 2 μm 8-Br-cGMP (Fig. 5A, top), below the K1/2 for channel activation (∼5 μm). In contrast, Ca2+/CaM had no effect when the activation of the CNG channel current was elicited with 100 μm 8-Br-cGMP, consistent with previous results showing that CaM does not affect the saturating current through rod CNG channels (Hsu and Molday, 1993; Gordon et al., 1995). When retinas were pretreated with pervanadate, Ca2+/CaM had little or no effect on the subsaturating CNG current (Fig. 5A, bottom). The effects of Ca2+/CaM on 27 control and 27 pervanadate-treated rods are shown in Figure 5B. In 73% of control rods, Ca2+/CaM reduced the subsaturating CNG current by at least 60%. However, only 15% of pervanadate-treated rods exhibited Ca2+/CaM inhibition of >60%. Hence, pervanadate pretreatment significantly reduced the number of rods whose CNG channels were inhibited by Ca2+/CaM (two-tailed t test; p < 0.001).
Figure 5.
Conditions that promote phosphorylation of native rod CNG channels prevent Ca2+/CaM inhibition. A, CNG currents from inside-out patches excised from ROS in response to 2 μm 8-Br-cGMP. In an ROS patch excised from a rod treated with control saline (top), Ca2+/CaM (50 μm Ca2+ plus 250 nm CaM) inhibits CNG current by ∼90% (top). In an ROS patch excised from a rod treated with 100 μm pervanadate for 1 hr, Ca2+/CaM had little effect (bottom). B, Population histograms of Ca2+/CaM inhibition of patches excised from ROS. Most patches from ROS exposed to control saline had CNG channels that were greatly inhibited by Ca2+/CaM (top), whereas the majority of patches from pervanadate-treated ROS had channels that were only slightly inhibited (bottom).
These results strongly suggest that promoting phosphorylation of native rod channels prevents Ca2+/CaM inhibition. It should be noted that both the control and pervanadate-treated groups had “outlier” cells that behaved differently from the majority of the population. Hence, some control rods (∼15%) had CNG channels that did not exhibit Ca2+/CaM inhibition, and some pervanadate-treated rods (∼15%) exhibited nearly full Ca2+/CaM inhibition. It is possible that the minority of control rods that did not exhibit Ca2+/CaM inhibition had unusually active PTKs (or inactive PTPs), resulting in channels that were already phosphorylated. Likewise, the subset of pervandate-treated rods that did exhibit Ca2+/CaM inhibition may have had unusually inactive PTKs, such that even with PTPs blocked, the channels remained dephosphorylated.
Discussion
Modulation by tyrosine phosphorylation and Ca2+/CaM may involve a similar mechanism
The gating of CNG channels is governed by intramolecular interactions within (Varnum and Zagotta, 1996) and between subunits. Studies on CNGA1 channels show that the N terminus can bind to the C terminus of an adjacent subunit (Gordon et al., 1997). This interaction produces an excitatory effect on channel gating, allowing them to activate more completely at lower concentrations of cyclic nucleotides (Sunderman and Zagotta, 1999a,b). Rod CNG channels also exhibit a similar intramolecular interaction. The high-affinity Ca2+/CaM binding site is located on the N terminus of the CNGB1 subunit (Grunwald et al., 1998, 1999; Weitz et al., 1998), which interacts with the C terminus of the CNGA1 subunit (Trudeau and Zagotta, 2002, 2003). Biochemical studies on polypeptides derived from these subunits show that Ca2+/CaM binding interrupts their interaction, presumably the mechanism whereby Ca2+/CaM inhibits channel gating (Trudeau and Zagotta, 2002). Additional studies using fluorescence resonance energy transfer (FRET) on intact rod CNG channels containing fluorescently tagged subunits have shown that Ca2+/CaM disrupts the N and C termini interaction over the same time course as inhibition of channel gating (Trudeau and Zagotta, 2003). Because the CNGA1:CNGB1 stoichiometry of native rod CNG channels (Weitz et al., 2002), as well as channels expressed in Xenopus (Zheng et al., 2002; Zhong et al., 2002), has been determined to be 3:1, disruption of a single intrasubunit interface between the N terminus of the CNGB1 subunit and the C terminus of one of the CNGA1 subunits appears to be responsible for Ca2+/CaM inhibition.
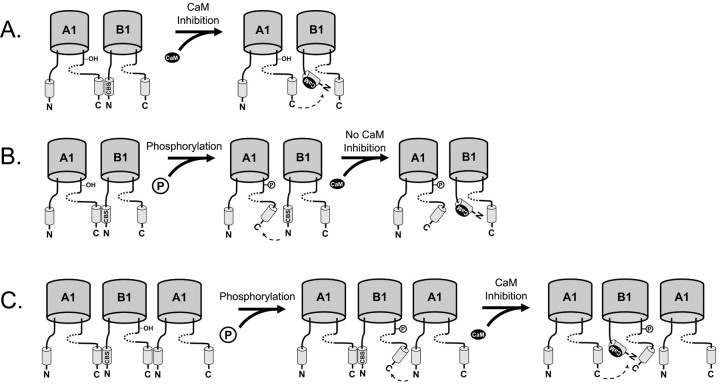
Our studies show that the phosphorylation of Y498 on the CNGA1 subunit prevents Ca2+/CaM inhibition. Phosphorylation of this site causes inhibition similar in magnitude to that produced by Ca2+/CaM binding. The effects of phosphorylation and Ca2+/CaM inhibition are nonadditive when CNGA1 contains the only phosphorylation site. These observations suggest that, although the relevant phosphorylation site and Ca2+/CaM binding site are on different subunits, inhibition caused by both processes may share a common mechanism. Thus, it is possible that phosphorylation and Ca2+/CaM disrupt the same intramolecular interaction but from opposite sides, as illustrated in Figure 6. Ca2+/CaM binding to the N terminus of CNGB1 can disrupt the interaction between the C terminus of CNGA1 and the N terminus of CNGB1, as shown in Figure 6A (Trudeau and Zagotta, 2003). We propose that phosphorylation of Y498 in the CNGA1 subunit also disrupts the interaction between the C terminus of CNGA1 and the N terminus of CNGB1, preventing Ca2+/CaM from having any additional effect (Fig. 6B). In contrast, although CNGB1 also has a modulatory phosphorylation site (Y1097), phosphorylation of the CNGB1 subunit on the C terminus does not alter the ability of Ca2+/CaM to disrupt the interaction mediated by the N terminus of CNGB1 with the CNGA1 subunit (Fig. 6C). Hence, the effect of phosphorylating the CNGB1 subunit is additive with respect to Ca2+/CaM inhibition. This model assumes that interface between the C terminal of CNGB1 and the N terminal of CNGA1 does not play a role in Ca2+/CaM inhibition, although it might play a role in gating.
Figure 6.
Proposed mechanism for suppression of Ca2+/CaM inhibition of rod CNG channels by phosphorylation of CNGA1Y498. A, Ca2+/CaM inhibits rod CNG channels by binding to a Ca2+/CaM binding-site (CBS) on CNGB1 (B1) and disrupting its interaction with the C terminus of CNGA1 (A1) (Trudeau and Zagotta, 2002). B, Phosphorylation of CNGA1Y498 also disrupts the interaction between the C terminus (C) of CNGA1 and the N terminus (N) of CNGB1. Thus, when Ca2+/CaM binds the CBS, there is no additional effect on the intersubunit interaction. C, Phosphorylation of CNGB1Y1097 may disrupt a similar interface between the C-terminus region of CNGB1 and the N-terminus region of CNGA1. Binding of Ca2+/CaM to the Ca2+/CaM binding-site can still disrupt the interface between the C terminus of CNGA1 and the N terminus of CNGB1. Hence, phosphorylation of CNGB1Y1097 has no effect on Ca2+/CaM inhibition. The cyclic-nucleotide binding domain is shown as a dotted line.
Our model suggests that phosphorylation prevents Ca2+/CaM from regulating rod CNG channel gating. However, it is also possible that phosphorylation simply prevents Ca2+/CaM from binding to the CNGB1 subunit. Although the relevant phosphorylation site (CNGA1Y498) is on a different subunit than the Ca2+/CaM binding site (on CNGB1), it is possible that its phosphorylation allosterically prevents Ca2+/CaM binding. However, it should be noted that fluorescently labeled CaM can bind to a peptide fragment of CNGB1 that contains the Ca2+/CaM binding site in the absence of CNGA1 (Trudeau and Zagotta, 2002), indicating that CNGA1 is not required for Ca2+/CaM to bind to its site on CNGB1.
Another possibility is that tyrosine phosphorylation and Ca2+/CaM inhibition cause distinct conformational changes in the channel, but these ultimately converge to have a similar effect on gating. Additional experiments, for example involving FRET measurements of fluorescently tagged subunits, are needed to directly determine whether phosphorylation of the C terminus of CNGA1 indeed does disrupt its interaction with the N terminus of CNGB1.
Physiological consequences for rod photoreceptor function
Regulation of CNG channel cGMP sensitivity is one of three Ca2+-regulated processes that are thought to contribute to rod light adaptation, each mediated by a distinct Ca2+-binding protein: (1) Ca2+, through Ca2+/CaM, regulates CNG channels, (2) Ca2+, through guanylyl cyclase-activating protein, regulates cGMP production by guanylyl cyclase (Mendez et al., 2001) and (3) Ca2+ indirectly regulates phosphodiesterase activity, probably by inhibiting rhodopsin phosphorylation by rhodopsin kinase (Burns and Baylor, 2001). In each case, the drop in internal Ca2+ concentration that occurs in the light opposes the light-driven cGMP-signaling cascade, thereby downregulating the cascade and resetting its sensitivity to subsequent illumination. The dark concentration of Ca2+ in ROS is ∼500 nm (Gray-Keller and Detwiler, 1996) and decreases to 53 nm when measured in saturating light (Gray-Keller and Detwiler, 1994). The K1/2 for Ca2+ regulation of CNG channel sensitivity is ∼50 nm (Hsu and Molday, 1993; Nakatani et al., 1995; Sagoo and Lagnado, 1996), therefore only bright light decreases internal Ca2+ by enough to substantially affect Ca2+/CaM inhibition.
Tyrosine phosphorylation of CNGA1 and CNGB1 subunits have different effects on Ca2+/CaM inhibition. Therefore, phosphorylation of the two subunits are expected to have different effects on light adaptation. Modulation of the rod channels through these two tyrosine phosphorylation sites allows regulation of cGMP sensitivity with or without regulation of Ca2+/CaM inhibition, increasing the flexibility of channel regulation.
One physiological factor that is thought to regulate the tyrosine phosphorylation state of rod CNG channels is IGF-1. IGF-1 increases the cGMP sensitivity of CNG channels by twofold to threefold, thereby increasing the amplitude of the response to both dim and saturating light. IGF-1 has no effect on homomeric CNGA1 channels lacking Y498 (Molokanova et al., 1999) or heteromeric channels lacking both phosphorylation sites (Molokanova et al., 2003). Related olfactory CNG channels, which have a phenylalanine in the position equivalent to Y498 on CNGA1 (Y477 in CNGA2), are unaffected by IGF-1 application, but if a tyrosine is introduced at this position, IGF-1 application now leads to an increase in cGMP sensitivity (Savchenko et al., 2001). IGF-1 is synthesized and released by the RPE (Waldbillig et al., 1991), but how its release is regulated is not understood. It is possible that changes in the neurotransmitter (Dearry and Burnside, 1989) or ionic concentrations (Dmitriev et al., 1999) in the subretinal space during light responses act as signals that regulate IGF-1 release. In this scenario, IGF-1 would be part of a negative feedback loop for light adaptation of rods (Kramer and Molokanova, 2001).
Our results suggest that tyrosine phosphorylation of native rod CNG channels prevents Ca2+/CaM inhibition. The converse interaction is also possible, with elevated Ca2+ affecting channel phosphorylation or dephosphorylation. Studies on excised patches from rod outer segments suggest that the cGMP sensitivity of native CNG channels can be regulated by a Ca2+-sensitive serine/threonine phosphatase (Gordon et al., 1992). It is not known whether the PTPs or PTKs that regulate the native rod channel are also Ca2+ sensitive. Although these enzymes can modulate channel activity in intact rods, they are either lost or inactive in excised patches from rods (Savchenko et al., 2001), making it difficult to assess how their activity might be regulated by regulatory factors such as Ca2+/CaM. It should be noted that Ca2+ sensitivity of phosphorylation or dephosphorylation could result from intrinsically Ca2+-sensitive enzymes, or from Ca2+/CaM binding to the channel substrate, allosterically effecting the phosphorylation or dephosphorylation reaction. Biochemical identification of the relevant PTPs and PTKs in rods may be necessary before their regulatory mechanisms can be determined.
Footnotes
This work was supported by National Institutes of Health Grants EY12608 (R.H.K.) and DA08102 (C.W.L.) and National Research Service Award Postdoctoral Fellowship Grant EY13506 (J.L.K.).
Correspondence should be addressed to Dr. Richard H. Kramer, 121 Life Sciences Addition, Department of Molecular and Cell Biology, University of California, Berkeley, CA 94720. E-mail: rhkramer@uclink4.berkeley.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2310100-07$15.00/0
References
- Burns ME, Baylor DA ( 2001) Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci 24: 779-805. [DOI] [PubMed] [Google Scholar]
- Chen TY, Peng YW, Dhallan RS, Ahamed B, Reed RR, Yau KW ( 1993) A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature 362: 764-767. [DOI] [PubMed] [Google Scholar]
- Chen TY, Illing M, Molday LL, Hsu YT, Yau KW, Molday RS ( 1994) Subunit 2 (or beta) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca2+-calmodulin modulation. Proc Natl Acad Sci USA 91: 11757-11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearry A, Burnside B ( 1989) Light-induced dopamine release from teleost retinas acts as a light-adaptive signal to the retinal pigment epithelium. J Neurochem 53: 870-878. [DOI] [PubMed] [Google Scholar]
- Dmitriev AV, Govardovskii VI, Schwahn HN, Steinberg RH ( 1999) Light-induced changes of extracellular ions and volume in the isolated chick retina-pigment epithelium preparation. Vis Neurosci 16: 1157-1167. [DOI] [PubMed] [Google Scholar]
- Gordon SE, Brautigan DL, Zimmerman AL ( 1992) Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron 9: 739-748. [DOI] [PubMed] [Google Scholar]
- Gordon SE, Downing-Park J, Zimmerman AL ( 1995) Modulation of the cGMP-gated ion channel in frog rods by calmodulin and an endogenous inhibitory factor. J Physiol (Lond) 486: 533-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Varnum MD, Zagotta WN ( 1997) Direct interaction between amino- and carboxyl-terminal domains of cyclic nucleotide-gated channels. Neuron 19: 431-441. [DOI] [PubMed] [Google Scholar]
- Gray-Keller MP, Detwiler PB ( 1994) The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron 13: 849-861. [DOI] [PubMed] [Google Scholar]
- Gray-Keller MP, Detwiler PB ( 1996) Ca2+ dependence of dark- and light-adapted flash responses in rod photoreceptors. Neuron 17: 323-331. [DOI] [PubMed] [Google Scholar]
- Grunwald ME, Yu WP, Yu HH, Yau KW ( 1998) Identification of a domain on the beta-subunit of the rod cGMP-gated cation channel that mediates inhibition by calcium-calmodulin. J Biol Chem 273: 9148-9157. [DOI] [PubMed] [Google Scholar]
- Grunwald ME, Zhong H, Lai J, Yau KW ( 1999) Molecular determinants of the modulation of cyclic nucleotide-activated channels by calmodulin. Proc Natl Acad Sci USA 96: 13444-13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoar PE, Kerrick WG, Cassidy PS ( 1979) Chicken gizzard: relation between calcium-activated phosphorylation and contraction. Science 204: 503-506. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Molday RS ( 1993) Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature 361: 76-79. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Molday RS ( 1994) Interaction of calmodulin with the cyclic GMP-gated channel of rod photoreceptor cells. Modulation of activity, affinity purification, and localization. J Biol Chem 269: 29765-29770. [PubMed] [Google Scholar]
- Kaupp UB, Niidome T, Tanabe T, Terada S, Bönigk W, Stühmer W, Cook NJ, Kangawa K, Matsuo H, Hirose, T, Miyata, T, Numa S ( 1989) Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature 342: 762-766. [DOI] [PubMed] [Google Scholar]
- Kramer RH, Molokanova E ( 2001) Modulation of cyclic-nucleotide-gated channels and regulation of vertebrate phototransduction. J Exp Biol 204: 2921-2931. [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P ( 1992) Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9: 861-871. [DOI] [PubMed] [Google Scholar]
- Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA, Chen J ( 2001) Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci USA 98: 9948-9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E, Trivedi B, Savchenko A, Kramer RH ( 1997) Modulation of rod photoreceptor cyclic nucleotide-gated channels by tyrosine phosphorylation. J Neurosci 17: 9068-9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E, Maddox F, Luetje CW, Kramer RH ( 1999) Activity-dependent modulation of rod photoreceptor cyclic nucleotide-gated channels mediated by phosphorylation of a specific tyrosine residue. J Neurosci 19: 4786-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E, Krajewski JL, Satpaev D, Luetje CW, Kramer RH ( 2003) Subunit contributions to phosphorylation-dependent modulation of rod cyclic-nucleotide gated channels. J Physiol (Lond) 552: 345-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K, Koutalos Y, Yau KW ( 1995) Ca2+ modulation of the cGMP-gated channel of bullfrog retinal rod photoreceptors. J Physiol (Lond) 484: 69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoo MS, Lagnado L ( 1996) The action of cytoplasmic calcium on the cGMP-activated channel in salamander rod photoreceptors. J Physiol (Lond) 497: 309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savchenko A, Kraft TW, Molokanova E, Kramer RH ( 2001) Growth factors regulate phototransduction in retinal rods by modulating cyclic nucleotide-gated channels through dephosphorylation of a specific tyrosine residue. Proc Natl Acad Sci USA 98: 5880-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderman ER, Zagotta WN ( 1999a) Mechanism of allosteric modulation of rod cyclic nucleotide-gated channels. J Gen Physiol 113: 601-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderman ER, Zagotta WN ( 1999b) Sequence of events underlying the allosteric transition of rod cyclic nucleotide-gated channels. J Gen Physiol 113: 621-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau MC, Zagotta WN ( 2002) Mechanism of calcium/calmodulin inhibition of rod cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 99: 8424-8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau MC, Zagotta WN ( 2003) Calcium/calmodulin modulation of olfactory and rod cyclic nucleotide-gated ion channels. J Biol Chem 278: 18705-18708. [DOI] [PubMed] [Google Scholar]
- Varnum MD, Zagotta WN ( 1996) Subunit interactions in the activation of cyclic nucleotide-gated ion channels. Biophys J 70: 2667-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldbillig RJ, Pfeffer BA, Schoen TJ, Adler AA, Shen-Orr Z, Scavo L, LeRoith D, Chader GJ ( 1991) Evidence for an insulin-like growth factor autocrine-paracrine system in the retinal photoreceptor-pigment epithelial cell complex. J Neurochem 57: 1522-1533. [DOI] [PubMed] [Google Scholar]
- Weitz D, Zoche M, Muller F, Beyermann M, Korschen HG, Kaupp UB, Koch KW ( 1998) Calmodulin controls the rod photoreceptor CNG channel through an unconventional binding site in the N-terminus of the beta-subunit. EMBO J 17: 2273-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB ( 2002) Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron 36: 881-889. [DOI] [PubMed] [Google Scholar]
- Yau KW, Baylor DA ( 1989) Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci 12: 289-327. [DOI] [PubMed] [Google Scholar]
- Yau KW, Nakatani K ( 1984) Electrogenic Na-Ca exchange in rod outer segments. Nature 311: 661-663. [DOI] [PubMed] [Google Scholar]
- Zheng J, Trudeau MC, Zagotta WN ( 2002) Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 36: 891-896. [DOI] [PubMed] [Google Scholar]
- Zhong H, Molday LL, Molday RS, Yau KW ( 2002) The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature 420: 193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]