Abstract
Benzodiazepines are important therapeutic drugs, but they are often abused and co-abused with opioids. Clinical evidence suggests that benzodiazepines can inhibit respiration, and when combined with the respiratory-depressive effects of opioids, may increase likelihood of death. In this study we used oxygen sensors coupled with high-speed amperometry and multi-site thermorecording to examine how intravenous (iv) midazolam, a potent benzodiazepine, modulates the brain hypoxic and temperature effects of iv heroin in freely-moving rats. Oxygen levels and brain temperature were assessed with high temporal resolution in the nucleus accumbens (NAc), an important structure in the motivational-reinforcement circuit. When administered alone, midazolam (2 mg/kg) modestly decreased NAc temperature but had no evident effects on oxygen levels in this structure. In contrast, heroin (0.4 mg/kg) induced a strong decrease in NAc oxygen that was followed by a weaker, rebound-like oxygen increase. Midazolam pretreatment did not affect heroin-induced brain hypoxia but potentiated the initial hypothermia induced by heroin. However, co-administration of these drugs potentiated the heroin-induced oxygen decrease and enhanced heroin-induced brain hypothermia. Co-administration of heroin and midazolam also resulted in enhanced locomotor inhibition and loss of motor control. This effect caused some rats to collapse, resulting in nose and mouth occlusion, which caused a secondary hypoxic phase. These results could have important implications for human drug users, as the combined use of benzodiazepines with potent opioids not only results in sustained brain hypoxia but creates conditions of loss of motor control which could result in asphyxia and death.
Keywords: midazolam, opiates, brain hypoxia, vasoconstriction/vasodilation, nucleus accumbens, rats
1. Introduction
Opioids are important therapeutic drugs that alleviate pain of different origins, but these drugs have strong addictive properties and their abuse can result in serious health complications and death (Compton et al., 2016; McLaughlin, 2017). While opioids induce multiple physiological effects, respiratory depression, which results in brain hypoxia, appears to be the most dangerous one (Baud, 2009; Jaffe et al., 1997; Simon, 1997). This effect is minor and can be controlled following therapeutic use of opioid analgesics, but it can be robust and life-threatening when highly potent opioids such as heroin or fentanyl are administered intravenously at higher doses (Solis et al., 2017b, 2018). Heroin remains the most commonly used opioid drug of abuse, and it is often contaminated by other, more potent opioids such as fentanyl or carfentanil. Even a minor addition of these substances to heroin greatly potentiates its hypoxic effects and makes this drug more lethal (Solis et al., 2017c). Drug users also often combine opioids with other psychoactive substances such as alcohol or benzodiazepines, and since these drugs share CNS-inhibiting effects, it is possible that the polydrug use of these substances could result in enhancement of the physiological effects of opioids, including respiratory depression.
Benzodiazepines in particular are important drugs used to treat anxiety and insomnia, but they are also widely abused and can be co-abused with opioids (Gudin et al., 2013). Post-mortem blood samples obtained from heroin users are often found to contain varying amounts of benzodiazepine drugs (Minett et al., 2010). Benzodiazepines are also frequently involved in opioid-related deaths, and this trend has been increasing consistently since the late 1990s (Jones and McAninch, 2015; Calcaterra et al., 2013; Gudin et al. 2013). Clinical and pre-clinical data suggest that benzodiazepines upon acute intravenous (iv) administration decrease cerebral blood flow and brain oxygen consumption, and that they can inhibit respiration (Forster et al., 1982; Olkkola and Ahonen, 2008). While respiratory depression is a well-established effect of opioids, it is unclear whether relatively small changes in O2 and CO2 induced by benzodiazepines reflect true respiratory depression or if these changes are a consequence of the drugs’ sedative effects. Since opioids also induce sedative effects, while inducing respiratory depression, it is important to examine how benzodiazepines may modulate these potentially hazardous effects of opioids.
In this study, we examined how midazolam, a potent benzodiazepine, affects the respiratory-depressive, temperature, and behavioral effects of intravenous heroin in freely-moving rats. To characterize drug-induced respiratory depression, we examined changes in nucleus accumbens (NAc) oxygen levels as detected by chronically implanted oxygen sensors coupled with fixed-potential amperometry. While the decreases in oxygen levels resulting from respiratory depression are a global brain phenomenon dependent on the vascularization of brain tissue, we chose the NAc, an important structure involved in sensorimotor integration and mechanisms of natural and drug reinforcement (Wise and Bozarth, 1987; Mogenson et al., 1980; Di Chiara, 2002), as a representative structure for our recordings.
By using chronically implanted thermocouple sensors, we also examined drug-induced changes in temperature in the NAc, temporal muscle, and subcutaneous space. While oxygen monitoring in brain tissue allowed us to directly assess a functional outcome of drug-induced changes in respiratory activity, our three-point thermorecording technique allowed us to assess drug-induced changes in intracerebral heat production due to changes in brain metabolic activity (reflected in NAc-muscle differentials) and heat loss due to changes in skin vascular tone (vasodilation/vasoconstriction; reflected in skin-muscle differentials; Kiyatkin, 2010). Our thermorecording was also combined with the monitoring of locomotion, which allowed us to assess the effects of drugs on general behavioral activity.
2. Materials and Methods
2.1. Subjects
25 adult male Long-Evans rats (Charles River Laboratories) weighing 460±40 g at the time of surgery were used in this study. Rats were individually housed in a climate-controlled animal colony maintained on a 12–12 light-dark cycle with food and water available ad libitum. All procedures were approved by the NIDA-IRP Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865–23). Maximal care was taken to minimize the number of experimental animals and any possible discomfort or suffering at all stages of the study.
2.2. Overview of the study
This study describes the results of four experiments conducted in freely-moving rats. In the first two experiments, we examined changes in NAc oxygen or temperature parameters induced by midazolam alone and heroin delivered after midazolam. In the other two experiments we examined changes in NAc oxygen or temperature parameters following co-administration of midazolam and heroin.
2.3. Surgical preparations
Surgical procedures for the electrochemical experiments with oxygen monitoring have been described in detail elsewhere (Solis et al., 2017a). In brief, under general anesthesia (Equithesin, a mixture of sodium pentobarbital and chloral hydrate), each rat had a commercially produced Pt-Ir oxygen sensor (Model 7002–02; Pinnacle Technology, Inc., Lawrence, KS, USA) unilaterally implanted into the NAc. Target coordinates of the recordings in the right NAc shell were: AP +1.2 mm, ML ±0.8 mm, and DV +7.6 mm from the skull surface, according to coordinates of the rat brain atlas (Paxinos and Watson, 1998). The sensor was secured with dental acrylic to three stainless steel screws threaded into the skull. During the same surgical procedure, rats were implanted with a chronic jugular catheter, which ran subcutaneously to the head mount and was secured to the same head assembly. The top end of the catheter was connected to a miniature injection port (fixed to the same head assembly), and during our experiments this port was connected to a catheter extension, which extended to the outside of the recording chamber and was used for drug administration. Rats were allowed a minimum of 5 days of post-operative recovery and at least 3 daily habituation sessions (~6 h each) to the recording environment; jugular catheters were flushed daily with 0.2 ml heparinized saline to maintain patency.
Surgical procedures for the thermorecording experiments have been described in detail elsewhere (Kiyatkin et al., 2013; Bola and Kiyatkin, 2017). In brief, under the same general anesthesia protocol, rats were implanted with a jugular catheter and three copper-constantan thermocouple electrodes in the NAc shell, temporal muscle, and subcutaneously along the nasal ridge with the tip ~15 mm anterior to bregma. The probes and the injection port were secured with dental cement to the three stainless steel screws threaded into the skull.
2.4. Electrochemical detection of oxygen
Pinnacle oxygen sensors consist of an epoxy-sheathed disc electrode that is ground to a fine surface using a diamond-lapping disc. These sensors are prepared from Pt-Ir wire 180 μm in diameter, with a sensing area of 0.025 mm2 at the tip. The active electrode is incorporated with an integrated Ag/AgCl reference electrode. Dissolved oxygen is reduced on the active surface of these sensors, which is held at a stable potential of −0.6 V vs. the reference electrode, producing an amperometric current. The current from the sensor is relayed to a computer via a potentiostat (Model 3104, Pinnacle Technology) and recorded at 1-s intervals, using the PAL software utility (Version 1.5.0, Pinnacle Technology).
Oxygen sensors were calibrated in vitro at 37°C by the manufacturer (Pinnacle Technology) according to a standard protocol described elsewhere (Bolger et al., 2011). The sensors produced incremental current changes with increases in oxygen concentrations within the wide range of previously reported brain oxygen concentrations (0–40 μM). Substrate sensitivity of oxygen sensors varied from 0.96 to 1.52 nA/1μM (mean=1.27 nA/1 μM). Oxygen sensors were also tested by the manufacturer for their selectivity toward other electroactive substances, including dopamine (0.4 μM) and ascorbate (250 μM), none of which had significant effects on reduction currents.
2.5. Experimental procedures
All electrochemical recordings were performed in an electrically insulated chamber (38 × 47 × 47 cm) located in a larger open-faced cabinet (60 × 56 × 70 cm); the chamber was illuminated continuously with a dim red light bulb (15 W). Ambient temperature was maintained between 22–23°C and a continuously running air filtration fan provided background white noise. Recordings were conducted during the day (9:00–18:00) and the duration of recording sessions varied from 7 to 9 hrs.
At the beginning of each experimental session, rats were lightly anesthetized (<2 min) with isoflurane and the sensor was connected via an electrically shielded flexible cable and a multi-channel electrical swivel to the potentiostat (Model 3104, Pinnacle Technology). The injection port of the jugular catheter on the head mount was connected to a plastic catheter extension that allowed for stress- and cue-free drug delivery from outside of the cage. Since some rats received two different drugs during the same recording session, two catheter extensions mounted on the recording cable were used to minimize any contamination of one drug by another. This issue of possible contamination is only relevant for the first two experiments, in which heroin was injected 1 hour after midazolam; as estimated based on the volume of dead space of the catheter and injection port, the degree of contamination of heroin by midazolam could be within 10%. Testing began a minimum of 120 min after connecting the sensor to the potentiostat, when baseline currents were stabilized. A similar protocol was utilized for the thermorecording experiments, during which we also measured locomotor activity using 4 infrared motion detectors (Med Associates) as previously described (Brown and Kiyatkin, 2004).
In the first two experiments (n=7 rats for electrochemical and 6 for temperature recordings), we examined changes in NAc oxygen, temperature, and locomotion induced by midazolam alone (Midazolam Injection, Akorn; 2 mg/kg) and heroin (diamorphine HCl, NIDA-IRP Pharmacy; 0.4 mg/kg) delivered one hour after midazolam or saline. While the dose of heroin (0.4 mg/kg) used in this study is larger than the typical doses maintaining self-administration behavior in rats (0.075–0.1 mg/kg; Gerber and Wise, 1989), this dose is less than 2% of the accepted LD50 for iv administration in rats (Strandberg et al., 2006; Gable, 2004) and it is also well within the range used by habitual heroin users (see erowid.org). As shown previously, heroin at this dose induces a relatively strong decrease in NAc oxygen (Solis et al., 2017b). The dose of midazolam we used (2 mg/kg) exceeds the typical doses used in human clinical practice (70–80 μg/kg for memory block and 0.1–0.3 mg/kg for anesthesia), but this dose is only <3% of the LD50 for iv administration in rats (Schlappi, 1983). Each rat in these experiments was exposed to three recording sessions, in which we examined the effects of midazolam alone, heroin after midazolam, and heroin after saline. The drugs or saline were delivered intravenously in 0.3–0.5 ml volumes with a slow injection rate (0.1 ml/10 s). All of the injections were administered when the rats were in a quietly resting state and, because of the use of a catheter extension and manual drug administration from outside of the recording chamber, they were cue- and stress-free. At the end of each recording session, rats were lightly anesthetized with iv Equithesin (0.6–0.8 ml), disconnected from the recording instrument, and returned to the animal facilities. In the next two experiments (n=7 rats for electrochemical and 5 for temperature), we examined changes in NAc oxygen, temperature, and locomotion following administration of heroin alone (0.4 mg/kg) and a mixture of midazolam (2 mg/kg) and heroin (0.4 mg/kg). All experimental sessions were counter-balanced. In temperature-recording experiments, rats were tested with midazolam + heroin mixture during two daily sessions; one free day was allowed between these sessions. Following completion of the experiments, rats were deeply anesthetized, decapitated, and brains were removed and stored in 10% formalin for future sectioning and confirmation of sensor locations/assessment of tissue damage.
2.6. Data analysis
Electrochemical data were sampled at 1 Hz (i.e. mean current over 1 s) using the PAL software and averaged to produce mean values for 2-min time bins. Electrochemical data were first analyzed as raw currents. Because each individual sensor differed slightly in background current and substrate sensitivity in vitro, currents were converted to concentrations and represented as relative changes, with the pre-stimulus baseline set to 100%. One-way repeated measures ANOVAs (followed by Fisher’s LSD post-hoc tests) were used to evaluate statistical significance of drug-induced oxygen changes, and two-way repeated measures ANOVAs were used to analyze between-treatment differences. We also assessed between-treatment differences in the area under the curve corresponding to the phases of heroin-induced oxygen decreases and subsequent increases, and we assessed these differences between the control heroin groups vs groups administered midazolam either before or with heroin. Similar procedures were used to analyze temperature data. Drug-induced changes in temperature parameters are shown as changes relative to the pre-injection baseline (set at 0°C).
3. Results
Data presented in this study were obtained from 25 rats with sensor locations in the NAc verified histologically. Based on the calibration of sensitivities of our oxygen sensors, basal values of oxygen in the NAc fluctuated between 9 and 24 μM (mean=16.25±1.64 μM, SD=4.91μM), and there were no significant differences between treatment groups. Absolute temperature values in rats habituated to the recording environment and in quiet resting conditions were: 37.00±0.09°C (SD=0.24°C) for the NAc, 35.97±0.15°C (SD=0.43°C) for the temporal muscle, and 34.93±0.17°C (SD=0.49°C) for the skin. These values and between-site differences were relatively stable in each group of rats.
3.1. NAc oxygen responses induced by midazolam, heroin, and heroin after midazolam
As shown in Figure 1A, midazolam (2 mg/kg) intravenously delivered to freely-moving rats under quiet resting conditions did not affect NAc oxygen levels; mean values for oxygen in the midazolam and saline groups were identical, showing a rapid and small (<10% above the pre-injection baseline) increase within the first minute following both injection types. In contrast, when heroin was injected 60 min after saline or midazolam, it induced a biphasic change in NAc oxygen which did not statistically differ depending on pretreatment (Fig. 1B). In both pretreatment groups, oxygen levels rapidly and strongly decreased within the first four minutes after heroin injection, reached their nadir (~40% of baseline) at 4–6 min, and then slowly returned to baseline (F5,455=4.98 and F6,546=5,49, p<0.001 for heroin delivered after saline or midazolam, respectively). The only difference in rats pretreated with midazolam was the absence of a rebound-like oxygen increase, an effect which was evident for rats in the control heroin group. Figure 1C shows mean values of the areas under the curve corresponding to the initial decreasing (0–16 min) and subsequent increasing (16–40 min) phases of the heroin-induced oxygen response. As shown, pretreatment with midazolam had no significant effect on the heroin-induced oxygen decreases, but slightly attenuated the subsequent oxygen increase. However, the latter change did not reach statistical significance in terms of between-treatment differences. All rats exposed to midazolam or heroin alone tolerated these treatments well, and they showed full restoration of pre-injection values of NAc oxygen and temperature parameters on the next observation day.
Figure 1.
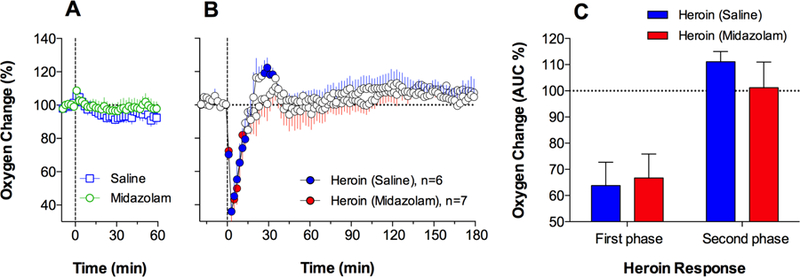
Mean (±SEM) changes in NAc oxygen levels (in percent vs. pre-injection baseline=100%) induced by iv administration of midazolam or saline (A) and heroin injected one hour after midazolam or saline (B). Filled symbols indicate values significantly different from pre-injection baseline (time=0) shown as black horizontal dotted lines. Vertical black lines indicate the moment of injection. C shows differences in the initial oxygen decreases (0–16 min) and subsequent increases (16–40 min) induced by heroin in the two treatment groups. These data were calculated as mean (±SEM) area under the curve in each treatment group and compared using Student’s t-test.
3.2. Temperature and locomotion responses induced by midazolam, heroin, and heroin after midazolam
Midazolam (2 mg/kg) intravenously injected in freely-moving rats significantly decreased temperature in each of the three recording locations (F5,755=4.19, 1.77, and 3.21 for the NAc, muscle, and skin, respectively; p<0.05). While temperature responses in the NAc and temporal muscle were monophasic and similar to each other, skin temperature initially transiently increased but then decreased below baseline (Fig. 2A). The decrease in brain and muscle temperature was modest (~1.0°C), and it was temporally paired with a robust but transient increase in the skin-muscle differential (F5,755=1.79, p<0.05), indicative of skin vasodilation (Fig. 2B). Midazolam also slightly decreased the brain-muscle differential during the first hour post-injection, but then this parameter slightly increased above its pre-injection baseline (F5,755=4.20, p<0.05). In contrast, the skin-muscle differential remained increased vs. baseline values for the entire 5 hours post-injection, indicative of persistent peripheral vasodilation. Midazolam also modestly decreased locomotor activity, and this effect was evident for ~2 hours post-injection (Fig. 2C).
Figure 2.
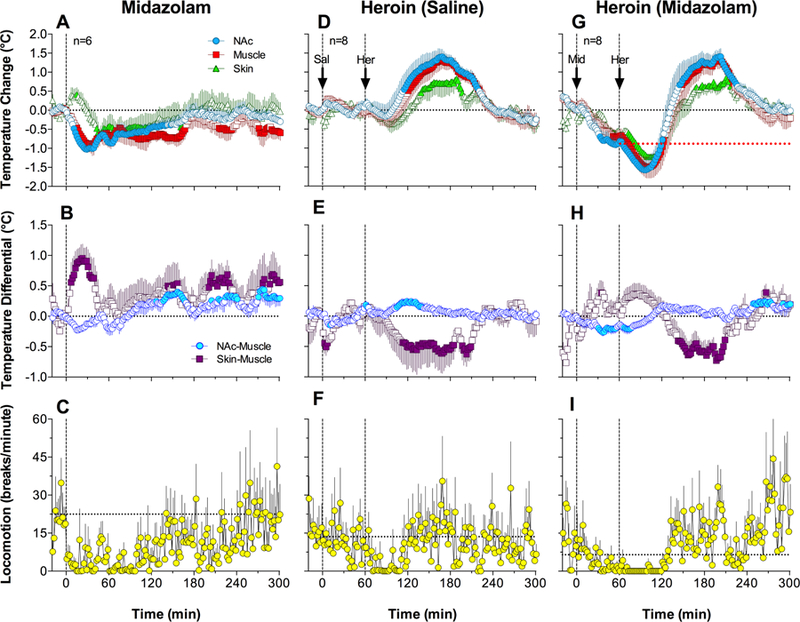
Mean (±SEM) changes in temperature, temperature differentials, and locomotion induced by iv administration of midazolam (A-C) and heroin injected one hour after saline (D-F) or midazolam pretreatment (G-I). Filled symbols indicate values significantly different from pre-injection baseline (time=0), shown as black horizontal dotted lines. Vertical black lines indicate the moment of injection. Red dotted line (G) shows the pre-heroin baseline established after midazolam injection. Horizontal dotted lines in the locomotion graphs show mean locomotion for 10 min preceding drug injection.
Saline injection did not induce significant temperature changes in any recording location; only weak increases in NAc and muscle temperature and transient decreases in skin temperature were seen within 5–10 min post-injection (Fig. 2D). The saline injection also did not affect locomotor activity (Fig. 2F). Consistent with our previous studies (Bola and Kiyatkin, 2017), heroin (0.4 mg/kg) intravenously injected in freely-moving rats induced a biphasic temperature response which was significant in each recording location (Fig. 2D; F7,1057=8.73, 8.21, and 4.30 for the NAc, muscle, and skin, respectively; p<0.001). Temperatures in each of these three locations transiently and slightly decreased after heroin injection (~0.3°C for ~30 min), but then strongly increased (~1.5°C; ~180 min). The increases in brain and muscle temperature were temporally coupled with a significant increase in the brain-muscle differential and a prolonged decrease in the skin-muscle differential (Fig. 2E; F7,1057=2.87 and 2.74, respectively; p<0.01). Heroin also decreased locomotor activity (Fig. 2F); this effect was slightly stronger but shorter in duration than that induced by midazolam. Unlike midazolam, heroin caused locomotor inhibition, which was coupled with stiffness of the body and tail erection.
Midazolam delivered before heroin injection decreased all temperatures to a new baseline, when heroin was injected (Fig. 2G; F7,210=16.55, 10.47, and 1.80 for the NAc, muscle, and skin, respectively; p<0.05). The temperature effect of heroin following midazolam injection was similar to that occurring after saline injection, but both the initial decrease and subsequent increase were stronger than in the latter case (F7,1057=14.44, 11.67, and 7.26 for the NAc, muscle, and skin, respectively; p<0.001). Due to the preceding temperature-decreasing effect of midazolam, the subsequent effect of heroin began at a ~1°C lower baseline, and the overall decrease in brain and muscle temperature was ~1.6°C compared to ~0.3°C in the control. In contrast to the decrease in the skin-muscle differential induced by heroin after saline, heroin delivered after midazolam induced a biphasic change in the skin-muscle differential (Fig. 2H; F7,210=5.48, p<0.001), which consisted of a rapid increase followed by a decrease. Heroin delivered after midazolam also inhibited locomotor activity (Fig. 2I), and this effect was stronger and more prolonged than those induced by control midazolam and heroin injections. As noted observationally, the behavioral effects differed as well: the rats exhibited locomotor immobility, similarly as with heroin alone, but unlike in the latter case, the rats’ bodies and tails were limp and the rats often collapsed.
3.3. Temperature responses induced by co-administration of heroin and midazolam
The temperature responses elicited by co-administration of heroin and midazolam (0.4 mg/kg + 2.0 mg/kg, respectively) drastically differed from those induced by heroin alone (Fig. 3). Temperature in each location strongly decreased for ~90 min after the injection but then increased above the pre-injection baseline (F4,576=10.19, 11.16 and 3.35 for NAc, muscle and skin, respectively; p<0.001). In contrast to the very small, ~0.3°C drop in the NAc and muscle temperatures seen after the control heroin injection (Fig. 2), co-administration of heroin and midazolam decreased these temperatures drastically (~1.7°C), with absolute decreases in individual cases up to 33.7°C. Co-administration of the drugs strongly decreased NAc-muscle differentials and strongly increased skin-muscle differentials (F4,576=2.16 and 6.43, respectively; p<0.001), and these changes were also much longer in duration (~90 min) than those induced by heroin or midazolam alone. In addition, co-administration induced locomotor inhibition, and this effect was stronger and more prolonged than that induced by either drug alone.
Figure 3.
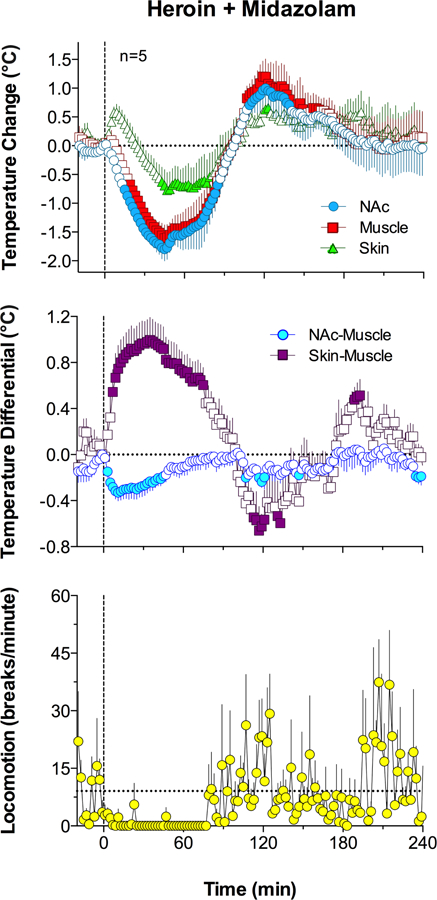
Mean (±SEM) changes in temperature, temperature differentials, and locomotion induced by co-administration of midazolam and heroin. Filled symbols indicate values significantly different from pre-injection baseline (time=0) shown as black horizontal dotted lines. Horizontal dotted line in bottom graph shows mean locomotion for 10 min preceding drug injection.
3.4. NAc oxygen responses induced by co-administration of heroin and midazolam
In contrast to the clearly biphasic NAc oxygen response induced by heroin alone, co-administration of midazolam and heroin induced a monophasic oxygen decrease (Fig. 4A; F8,728=4.57; p<0.001), consisting of a rapid and strong drop occurring 10–15 min post-injection and a more prolonged decrease thereafter. The amplitude of the initial oxygen drop (~50% of baseline) was similar in both groups, but significant between-group differences were seen at a later time following the injection. As shown by calculating mean areas under the curve (Fig. 4B), there were no significant between-group differences in the initial oxygen decreases, but the midazolam + heroin group significantly differed from the heroin-alone group in subsequent oxygen changes. In contrast to the increases seen in the heroin-alone group, oxygen slightly decreased in the co-administration group (109.6±4.9% vs. 91.4±5.8%; t=2.40, p<0.05).
Figure 4.
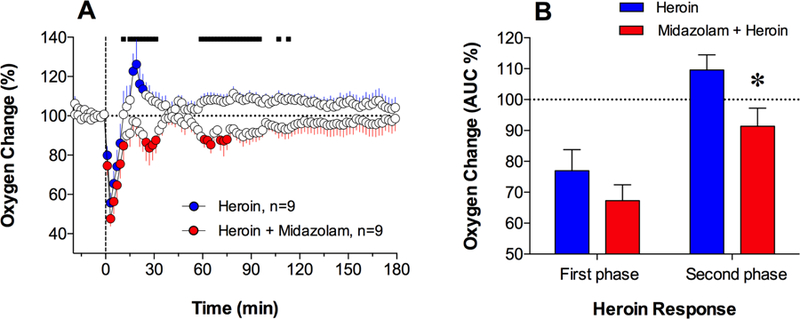
Mean (±SEM) changes in NAc oxygen levels (in percent vs. pre-injection baseline=100%) induced by iv administration of heroin and its co-administration with midazolam (A). Filled symbols indicate values significantly different from the pre-injection baseline and the bold horizontal line shows significant between-treatment differences. C shows differences in the initial oxygen decreases (0–12 min) and subsequent increases (12–40 min) induced by heroin in the two treatment groups. These data were calculated as mean (±SEM) area under the curve in each treatment group and compared using Student’s t-test. Asterisk shows significant between-treatment differences assessed in the areas under the curves (p<0.05).
In some individual tests with heroin + midazolam co-administration we observed unusual oxygen responses, which consisted of spontaneous oxygen decreases that occurred at a time later than the initial oxygen drops following injection (Fig. 5A, B). Such responses were observed in 4 of 10 rats that received the midazolam + heroin mixture, and they may be responsible for the significant between group differences in response curves found during the ~60–120 min following injection, as indicated by our post-hoc analyses (see bold black horizontal lines in Fig. 4A). While the exact reasons for these delayed oxygen decreases remain unclear, our observation of drug-induced behavior revealed that these additional decreases occurred when the rat’s nose was pressed against the chamber’s walls, which hindered breathing and thus induced transient asphyxiation. These unusual, secondary decreases varied in magnitude and they usually spontaneously disappeared when the rat moved its head slightly. However, in one recording session, which was not included in the final statistical analyses (rat identified as DP2, in Fig. 5), the rat’s body was lightly touched by the experimenter when oxygen dropped again at ~40–50 min post-injection (see grey arrow in Fig. 5C). In this case, the rat slightly moved its head and oxygen levels began to increase. Possible asphyxiation could explain one case of death observed following midazolam + heroin co-administration; this case was also not included in the final statistical analyses. In this case, temperature decreased during the ~25 min post-injection but then began to rapidly drop, and death occurred at ~30 min (Fig. 5D). In this case, both the brain-muscle and skin-muscle differentials showed a terminal rise (Fig. 5E) – a change typical to asphyxiation-induced death (Kiyatkin et al., 2014).
Figure 5.
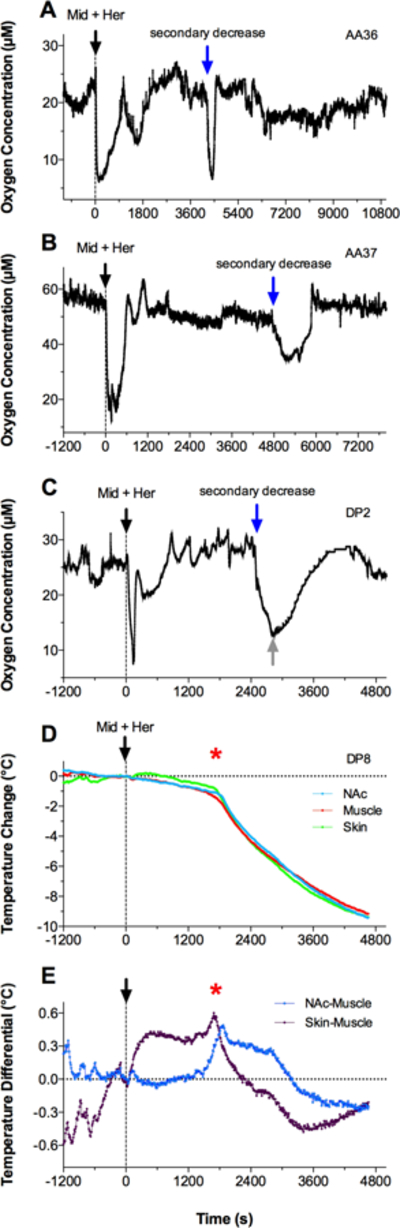
Original records of changes in Nac oxygen levels (A-C; shown in μM) and temperature parameters (D, E) induced by co-administration of midazolam and heroin (black arrow). A and B show two examples of oxygen changes, with the appearance of spontaneous, secondary oxygen decreases (blue arrow). C shows a similar example, when the rat was manually touched (grey arrow) when oxygen levels spontaneously decreased to very low levels. D and E show changes in temperatures in three recording locations and changes in brain-muscle and skin-muscle temperature differentials in the rat (DP8), which was injected with midazolam + heroin and died during the recording. Red asterisk in D and E shows the moment of full cessation of breathing.
4. Discussion
Benzodiazepines when used non-medically are often combined with the use of opioid drugs. Since benzodiazepines have sedative and hypnotic properties, they could potentiate the CNS-inhibiting effects of opioids. While controversial, some studies suggest that benzodiazepines inhibit respiration and that these effects may synergize with the known respiratory-depressive effects of opioids, possibly resulting in coma or death. In this study, we utilized oxygen sensors coupled with amperometry to examine how midazolam, a representative benzodiazepine drug, affects changes in NAc oxygen levels induced by iv heroin in freely-moving rats. Our electrochemical measurements were supplemented with temperature measurements from the NAc, temporal muscle, and skin, which provided information on how midazolam, heroin, and their mixture affect brain temperature and which clarified possible mechanisms underlying changes in brain oxygen and temperature.
4.1. Midazolam
Midazolam is a highly potent benzodiazepine drug, with prominent sedative, hypnotic, and muscle relaxant effects. Consistent with this profile, we found that midazolam delivered at a relatively low dose (2 mg/kg, or <3% of the LD50; Schlappi, 1983) modestly decreased locomotor activity and temperatures in the brain and temporal muscle. Following iv delivery, these effects developed rapidly, with major changes occurring 30–40 min post-injection and a slow restoration to baseline occurring thereafter. Consistent with the known muscle relaxant effects of benzodiazepines, midazolam had the strongest and most prolonged effect on muscle temperature, which persisted until the end of the 5-hour analysis interval. Midazolam also increased skin-muscle differentials, suggesting peripheral vasodilation and enhanced heat loss to the external environment as the primary cause of its brain hypothermic effects. While a slight decrease in brain-muscle differentials indicates decreased intracerebral heat production (as a consequence of brain metabolic inhibition), this effect was weak and its contribution to hypothermia appears to be minimal. This pattern of temperature responses was qualitatively similar to that induced by general anesthetics (Kiyatkin and Brown, 2005), but the changes were much weaker and more transient.
Clinical and preclinical evidence indicate that midazolam has respiratory depressant effects (Forster et al., 1980; Gueye et al., 2002; Megarbane et al., 2005) that could lead to brain and body hypoxia due to decreased blood oxygen levels. However, iv midazolam did not affect NAc oxygen levels within a one-hour time interval, when changes in temperature and locomotion were maximal. This lack of oxygen changes could be related to the relatively low drug dose; previous studies assessing respiration in rats used this drug at much higher doses (160 mg/kg; Gueye et al., 2002; Megarbane et al., 2005). Future studies should examine the hypoxic effects of higher doses of midazolam alone and in combination with opioids.
4.2. Heroin
Unlike midazolam, heroin induced a strong decrease in NAc oxygen levels with a transient rebound of similar duration. As shown previously and confirmed by the monitoring of oxygen in the subcutaneous space (Solis et al., 2017; Kiyatkin, 2018), this biphasic brain oxygen response reflects the interaction of two opposing and relatively independent mechanisms. While the initial drop in oxygen results from decreases in blood levels due to respiratory depression, subsequent oxygen increases reflect cerebral vasodilation that enhances oxygen entry into brain tissue. In contrast to midazolam, heroin modestly increased brain temperature (1.0–1.5°C) and, as shown by calculating skin-muscle differentials, this effect occurred due to peripheral vasoconstriction and diminished heat loss to the external environment. Heroin also slightly increased intracerebral heat production (Fig. 2D, E), but this effect and its contribution to hyperthermia appear minor. Heroin-induced hyperthermia was preceded by weak temperature decreases immediately after the injection, but this effect was minor and not statistically significant with the modest heroin dose used in this study. As shown previously, this initial hypothermic effect of heroin becomes stronger and more prolonged with increases in drug dose, whereas the subsequent hyperthermic effect remains relatively stable (Bola and Kiyatkin, 2018). Thus, despite common inhibiting effects on locomotor activity, heroin and midazolam have opposite effects on temperature parameters and only heroin decreases brain oxygen levels, due to respiratory depression.
4.3. Interactions of midazolam and heroin
Since heroin users may inject this drug following the consumption of benzodiazepines, and they can also use these substances simultaneously, we utilized two strategies to examine how midazolam modulates the effects of heroin. When heroin was injected one hour after midazolam, when brain and body temperatures were ~0.7°C lower than baseline, it induced a similar biphasic temperature response as in the control. However, the initial decrease was stronger and more prolonged than in the control, with a total temperature decrease of 1.5–2.0°C vs. the baseline. Then temperature slowly increased above baseline, and this hyperthermic phase was similar to that in the control in terms of response magnitude. The potentiated post-injection temperature decrease was coupled with a rise in skin-muscle differentials, suggesting peripheral vasodilation with enhanced heat loss as its primary cause. Increased skin-muscle differentials and hypothermia were also evident when the effects of heroin were assessed vs. the new post-midazolam temperature baselines, suggesting that midazolam has a delayed potentiating effect on the initial hypothermic effects of heroin, which were evident to a minimal extent when the drug was injected alone.
In contrast to the strong effects of midazolam pretreatment on temperature responses induced by heroin, pretreatment did not affect heroin-induced NAc oxygen decreases, which had similar latencies and magnitudes as those induced by heroin administered alone. Therefore, it appears that midazolam pretreatment does not affect acute heroin-induced brain hypoxia, suggesting such hypoxic effects as being independent from and not influenced by the sedative effects of midazolam. However, in rats that received heroin after midazolam pretreatment, the subsequent oxygen increase normally induced by heroin-alone was reduced, making the overall oxygen response monophasic. Since this secondary, increasing phase of the oxygen response normally occurs due to heroin-induced cerebral vasodilation/increased cerebral blood flow (Solis et al., 2017b), it is reasonable to assume that attenuation of this phase in rats pretreated with midazolam results from decreased cerebral blood flow due to prolonged skin vasodilation and redistribution of circulating blood to the periphery. As shown by calculated skin-muscle differentials, midazolam administered alone induced skin vasodilation, and this effect was prominent for ~60 min following heroin injection after midazolam pretreatment (see Fig. 2).
While pretreatment with midazolam had minimal effects on NAc oxygen responses induced by heroin, changes were more prominent when these drugs were co-administered. The addition of midazolam to heroin greatly potentiated the initial hypothermic effect of heroin, resulting in a dramatic decrease in brain temperature (up to 33.7°C – levels below the physiological range; Kiyatkin, 2010). In contrast to the clearly biphasic oxygen response induced by heroin-alone, the response elicited by co-administration of these drugs was monophasic, with an initial robust decrease followed by a slow return to baseline. The amplitude of the initial rapid decrease was of similar magnitude to that induced by heroin-alone, but the total duration of decrease was significantly prolonged due to the absence of the rebound-like oxygen increase. This change in the pattern of oxygen response was similar to that seen with the midazolam pretreatment group, but it was clearly stronger, statistically significant, and between-group differences in response curves were maintained for a longer time. Similarly as described for midazolam pretreatment, this prolongation of the heroin-induced oxygen decrease appears to be related to peripheral vasodilation (as evidenced by changes in skin-muscle differentials) and thus decreased cerebral blood flow. This mechanism is consistent with multiple human studies that showed that midazolam decreases cerebral blood flow due to increased cerebral vascular resistance (i.e., cerebral vasoconstriction) and modest decreases in arterial blood pressure (Cheng et al., 1993; Hoffman et al., 1986; Ogawa et al., 2010). Such effect counteracts the enhanced intracerebral oxygen entry that is induced by heroin following the initial, rapid oxygen decreases that are caused by respiratory depression and subsequent decreases in blood oxygen levels. Thus, it appears that midazolam co-administered with heroin disables this compensatory/adaptive mechanism, which normally allows for oxygen to rebound, thus resulting in more prolonged brain hypoxia. While these differences are not so large, they can be functionally important because the brain can tolerate strong brain hypoxia if it is transient, but neuronal damage increases exponentially when hypoxia becomes more prolonged (Hossmann, 1999).
Unexpectedly, we found that co-administration of heroin and midazolam also induced unusual oxygen responses, which consisted of spontaneous and relatively strong, secondary oxygen decreases occurring to a different extent in 4 out of the 10 tested rats. Due to these secondary decreases, the total duration of the NAc oxygen decrease in the midazolam + heroin group was drastically longer than in the heroin-alone group (~120 min vs. 12 min; see Fig. 4A). Our observations of animal behavior during the electrochemical recordings suggested that at least in some cases, the secondary hypoxic events could be caused by breathing impairment due to the rats collapsing into the plastic cage wall and their inability to remove their nose from the surface. In support of this cause, a light touch of the rat (which induces arousal) resulted in it moving its nose, which rapidly eliminated the oxygen decreases. Therefore, it appears that a combination of the behavior-inhibiting effects of both drugs and the hypnogenic effects of benzodiazepines induce a loss of motor control and falling, and that if this results in mouth and nose occlusion, asphyxia/suffocation could occur. Mechanistically, these behavioral effects resulting in secondary hypoxic episodes could result from diminished or blocked responsiveness to internal/external stimuli, such as internal cues to move one’s nose if occluded. This mechanism could also be responsible for the death of one rat, which was administered the midazolam + heroin mixture in our temperature experiments. This fatality was unexpected because the doses of each drug in the midazolam + heroin mixture were within 2–3% of their LD50s. While these observations can be viewed as anecdotal, they could be highly relevant for real-life human cases and they highlight the exaggerated dangers of the co-abuse of opioids and benzodiazepines, which appears to be much more dangerous than the use of each drug alone.
4.4. Functional implications
We show that midazolam co-administered with heroin is able to induce powerful brain and body hypothermia and potentiate brain hypoxia. While opioids used alone induce brain hypoxia and co-administration with benzodiazepines can prolong this dangerous effect, benzodiazepines also inhibit behavioral responses to external and internal stimuli, creating conditions for asphyxia that could result in death. Previously, a similar mechanism was implied to explain the dangers of fentanyl and fentanyl-heroin co-administration, as their ability to inhibit voluntary control over one’s body could result in suffocation (Solis et al., 2017c). Possibly, a similar mechanism could also explain the dangers of the co-use of opioids and alcohol, the latter which also inhibits responsiveness to external and internal stimuli and induces loss of behavioral control.
Highlights:
Midazolam decreased brain and body temperatures due to skin vasodilation
Midazolam alone did not induce respiratory depression
Pretreatment with midazolam potentiated heroin-induced temperature decreases
Pretreatment with midazolam did not potentiate heroin-induced brain hypoxia
Midazolam and heroin co-administration potentiated heroin-induced oxygen decreases
ACKNOWLEDGEMENTS
The study was supported by the Intramural Research Program of the NIH, NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The Authors report no conflict of interest
References
- Baud FJ (2009). Mechanisms of opioid-induced overdose: experimental approach to clinical concerns. Ann. Pharm. Fr 67, 353–359. [DOI] [PubMed] [Google Scholar]
- Bola RA, and Kiyatkin EA (2017). Brain temperature effects of intravenous heroin: state dependency, environmental modulation, and the effects of dose. Neuropharmacology 126, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger FB, Bennett R, and Lowry JP (2011). An in vitro characterization comparing carbon paste and Pt microelectrodes for real-time detection of brain tissue oxygen. Analyst 136, 4028–4035. [DOI] [PubMed] [Google Scholar]
- Brown PL, and Kiyatkin EA (2004). Brain hyperthermia induced by MDMA (ecstasy): modulation by environmental conditions. Eur. J. Neurosci 20, 51–58. [DOI] [PubMed] [Google Scholar]
- Calcaterra S, Glanz J, and Binswanger IA (2013). National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug Alcohol Depend 131, 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MA, Hoffman WE, Baughman VL, and Albrecht RF (1993). The effects of midazolam and sufentanil sedation on middle cerebral artery flow velocity in awake patients. J. Neurosurg. Anesthesiol 5, 232–236. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, and Baldwin GT (2016). Relationship between nonmedical prescription-opioid use and heroin use. N. Engl. J. Med 374, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A, Gardaz JP, Suter PM, and Gemperie M (1980). Respiratory depression by midazolam and diazepam. Anesthesiology 53, 494–497. [DOI] [PubMed] [Google Scholar]
- Forster A, Juge O, and Morel D (1982). Effects of midazolam on cerebral blood flow in human volunteers. Anesthesiology 56, 453–455. [DOI] [PubMed] [Google Scholar]
- Gable RS (2004). Comparison of acute lethal toxicity of commonly abused psychoactive substances. Addiction 99, 686–696. [DOI] [PubMed] [Google Scholar]
- Gerber GJ, and Wise RA (1989). Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacol. Biochem. Behav 32, 527–531. [DOI] [PubMed] [Google Scholar]
- Gudin JA, Mogali S, Jones JD, and Comer SD (2013). Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad. Med 125, 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueye PN, Borron SW, Risede P, Monier C, Buneaux F, Debray M et al. (2002). Buprenorphine and midazolam act in combination to depress respiration in rats. Toxicol. Sci 65, 107–114. [DOI] [PubMed] [Google Scholar]
- Hoffman WE, Miletich DJ, and Albrecht RF (1986). The effects of midazolam on cerebral blood flow and oxygen consumption and its interaction with nitrous oxide. Anesth. Analg 65, 729–733. [PubMed] [Google Scholar]
- Hossman KA (1999). The hypoxic brain. Insights from ischemic research. Adv. Exp. Med. Biol 474, 155–169. [PubMed] [Google Scholar]
- Jaffe JH, Knapp CM, and Ciraulo DA (1997). Opiates: Clinical Aspects. In: Substance Abuse. comprehensive Textbook (3rd Ed) edited by Lowinson JH, Ruis P, Millman RB Langrod JG (pp. 158–165). Baltimore: et al. : Williams and Wilkins. [Google Scholar]
- Jones CM, and McAninch JK (2015). Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am. J. Prev. Med 49, 493–501. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA (2010). Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front. Biosci 15, 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA (2018). Central and peripheral mechanisms underlying physiological and drug-induced fluctuations in brain oxygen in freely-moving rats. Front. Integr. Neurosci 12, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, and Brown PL (2005). Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiol. Behav 84, 563–570. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann M, Shaham Y (2014). Critical role of peripheral vasoconstriction in fatal brain hyperthermia induced by MDMA (Ecstasy) under conditions that mimic human drug use. J. Neurosci 34, 7754–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Wakabayashi KT, and Lenoir M (2013). Physiological fluctuations in brain temperature as a factor affecting electrochemical evaluations of extracellular glutamate and glucose in behavioral experiments. ACS Chem. Neurosci 4:652–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K (2017). Deadly chemistry. Science 355, 1364–1366. [DOI] [PubMed] [Google Scholar]
- Megarbane B, Lesguillons N, Galliot-Guilley M, Borron SW, Trout H, Declèves X et al. (2005). Cerebral and plasma kinetics of a high dose of midazolam and correlations with its respiratory effects in rats. Toxicol. Lett 159, 22–31. [DOI] [PubMed] [Google Scholar]
- Minett WJ, Moore TL, Juhascik MP, Nields HM, and Hull MJ (2010). Concentrations of opiates and psychotropic agents in polydrug overdoses: a surprising correlation between morphine and antidepressants. J. Forensic Sci 55, 1319–1325. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Iwasaki K, Aoki K, Gokan D, Hirose N, Ogawa S (2010). The differential effects of midazolam and Propofol sedation on dynamic cerebral autoregulation. Anesth. Analg 111, 1279–1284. [DOI] [PubMed] [Google Scholar]
- Olkkola KT, and Ahonen J (2008). Midazolam and other benzodiazepines. Handb. Exp. Pharmacol 182, 335–360. [DOI] [PubMed] [Google Scholar]
- Paxinos G, and Watson C (1998). The rat brain in stereotaxic coordinates. 4th ed. Academic Press: San Diego, CA. [Google Scholar]
- Schlappi B (1983). Safety aspects of midazolam. Br. J. Clin. Pharmacol 16, 37S–41S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon EJ (1997). Opiates: Neurobiology. In: Substance Abuse, Third Edition (Lowinson JH, Ruiz P, Millman RB, Langrod JG, eds), pp 148–158. Baltimore: Williams & Wilkins. [Google Scholar]
- Solis E Jr., Cameron-Burr KT, and Kiyatkin EA (2017a). Rapid physiological fluctuations in nucleus accumbens oxygen levels Induced by arousing stimuli: relationships with changes in brain glucose and metabolic neural activation. Front. Integr. Neurosci 11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, Shaham Y, and Kiyatkin EA (2017b). Intravenous heroin induces rapid brain hypoxia and hyperglycemia that precede brain metabolic response. eNeuro [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, and Kiyatkin EA (2017c). Heroin contaminated with fentanyl dramatically enhances brain hypoxia and induces brain hypothermia. eNeuro [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, Shaham Y, and Kiyatkin EA (2018). Fentanyl-induced brain hypoxia triggers brain hyperglycemia and biphasic changes in brain temperature. Neuropsychopharmacology 43, 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg JJ, Kugelberg FC, Alkas SK, Gustavsson A, Zahlsen K, Spigset O et al. (2006). Toxicological analysis in rats subjected to heroin and morphine overdose. Toxicol. Lett 166, 11–18. [DOI] [PubMed] [Google Scholar]
- Ziegler WH, Schalch E, Leishman B, and Eckert M (1983). Comparison of the effects of intravenously administered midazolam, triazolam and their hydroxy metabolites. Br. J. Clin. Pharmacol 16, 63S–69S. [DOI] [PMC free article] [PubMed] [Google Scholar]


