Abstract
Curcumin shows antiglycemic effects in animals. Curcumin is chemically unstable at physiological pH, and its oxidative degradation products were shown to contribute to its anti-inflammatory effects. Since the degradation products may also contribute to other effects, we analyzed their role in the antiglycemic activity of curcumin. We quantified curcumin-induced release of glucagon-like peptide 1 (GLP-1) from mouse STC-1 cells that represent enteroendocrine L-cells as a major source of this anti-diabetic hormone. Curcumin induced secretion of GLP-1 in a dose-dependent manner. Two chemically stable analogues of curcumin that do not readily undergo degradation, were less active while two unstable analogues were active secretagogues. Chromatographically isolated spiroepoxide, an unstable oxidative metabolite of curcumin with anti-inflammatory activity, also induced secretion of GLP-1. Stable compounds like the final oxidative metabolite bicyclopentadione, and the major plasma metabolite, curcumin-glucuronide, were inactive. GLP-1 secretion induced by curcumin and its oxidative degradation products was associated with activation of PKC, ERK, and CaM kinase II. Since activity largely correlated with instability of curcumin and the analogs we tested the extent of covalent binding to proteins in STC-1 cells and found it occurred with similar affinity as N-ethylmaleimide, indicating covalent binding occurred with nucleophilic cysteine residues. These results suggest that oxidative metabolites of curcumin are involved in the antiglycemic effects of curcumin. Our findings support the hypothesis that curcumin functions as a pro-drug requiring oxidative activation to reveal its bioactive metabolites that act by binding to target proteins thereby causing a change in function.
Keywords: turmeric, polyphenol, antiglycemic, L cell, oxidative transformation
1. Introduction
Curcumin, a component of the turmeric spice, has been used in traditional Asian medicine for centuries and is increasingly recognized as a potential therapeutic agent. Turmeric and curcumin are best recognized for anti-inflammatory activities in vitro and in vivo but there are also reports characterizing metabolic and anti-hyperglycemic activities (1–4). Several targets and physiological systems involved in glucose control are regulated by curcumin including adenosine monophosphate kinase which may mediate its insulin sensitizing effects (5,6). Curcumin also induces secretion of glucagon-like peptide-1 (GLP-1) (7,8). GLP-1 and glucose-dependent insulinotropic peptide (GIP) are incretin hormones that are released in response to oral nutrient ingestion as potent inducers of insulin secretion (9). Incretins also protect pancreatic beta cells from damage, and GLP-1 may induce beta cell regeneration (9). Their effects on glucose homeostasis make the incretins potential therapeutic targets for the management of diabetes mellitus (10). GLP-1 targeting drugs act as GLP-1 receptor agonists or inhibitors of the GLP-1 degrading enzyme, dipeptidylpeptidase IV (11). In addition, there is increasing interest in the development of agents that can induce endogenous GLP-1 secretion (12). Curcumin may function as a potential GLP-1 secretagogue since it induced GLP-1 secretion in an in vitro cell line and in rats resulting in improved glucose control (13).
Curcumin is unstable under cell culture conditions and in buffer of physiological pH, suggesting that biologic effects may be mediated by the parent compound and/or its degradation products (14). The degradation of curcumin is an oxidation reaction that can occur spontaneously or catalyzed by peroxidase enzymes (15). Either reaction results in the removal of a phenolic hydrogen that leaves a curcumin radical that undergoes ring formation and oxygen incorporation to yield a bicyclopentadione (BCP) product in which the 7 carbons of the heptadienedione chain have formed a bicyclic moiety (16). The formation of the BCP proceeds through reactive electrophilic intermediates, and these have been suggested to exert biological effects by reaction with protein nucleophiles (14,17). Specifically, the anti-inflammatory effect of curcumin mediated by inhibition of NF-κB involves covalent binding of curcumin-derived electrophiles to the upstream kinase, IKKβ (18). Binding of small-molecule electrophiles to a redox-sensitive cysteine residue of IKKβ inhibits kinase activity resulting in inhibition of NF-κB (19,20). Using synthetic derivatives of curcumin that have different stability (i.e., different propensity to form electrophilic metabolites) a correlation was observed between the rate of degradation and the ability to inhibit NF-κB (18). A similar mechanistic approach was used to show that curcumin-derived electrophiles mediate the inhibition of human topoisomerase IIα (21).
The aim of this study was to analyze molecular mechanisms underlying the anti-hyperglycemic effects of curcumin. We wanted to test whether products resulting from degradation of curcumin are involved in the regulation of GLP-1 secretion. This was accomplished by comparing effects on GLP-1 secretion of analogs of curcumin that are either resistant or susceptible to degradation. Our findings support the concept that curcumin acts as a pro-drug forming degradation products that mediate, at least in part, the anti-hyperglycemic effects of the parent compound.
2. Materials and Methods
2.1. Materials
Curcumin, synthetic analogues, BCP, curcumin-glucuronide, and hexadeuterated standards were synthesized as described (16,18,22). Compounds were prepared as stock solutions in DMSO at 10 mM.
2.2. Preparation of spiroepoxide
The spiroepoxide reaction intermediate was isolated from an autoxidation reaction of curcumin. Curcumin (50 μM) was added to 50 mL of 20 mM NH4OAc at pH 8.0, and the reaction was performed in parallel in 10 50 mL tubes. The reaction was allowed to proceed for 25 min and terminated by extraction using 500 mg Waters HLB cartridges preconditioned with methanol followed by water and 50 mM ammonium acetate pH 8. The products were eluted with 500 μl of acetonitrile into a vial cooled on dry ice. The solution was evaporated using nitrogen gas while keeping the vial on dry ice. The spiroepoxide was isolated using RP-HPLC on a Waters Symmetry C18 5-mm column (250 × 46 mm). The mobile phase used a mixture of ammonium acetate (85%) and acetonitrile (15%) eluted at 1 ml/min flow rate. The spiroepoxide eluted at 3.7 min and was collected into glass tube on dry ice. Acetonitrile was evaporated using nitrogen gas and the remainder was diluted with ammonium acetate. The solution was extracted using HLB cartridges and eluted with 100% acetonitrile. An aliquot of the recovered eluate was quantified to determine the concentration of spiroepoxide. For spiroepoxide quantification, 50 μL of extracted sample was added to 450 μL of 50 mM ammonium acetate and scanned in a spectrophotometer. An absorbance at 263 nm of 0.5 AU is approximately equal to 3.8 μg/μl of spiroepoxide. A final spiroepoxide solution was prepared in DMSO at 40 mM concentration.
2.3. Cell culture
STC-1 cells were obtained from ATCC (Manassas, VA) and maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 25 mM glucose, 10% fetal bovine serum (FBS), 100 units/ml penicillin G, and 100 μg/ml streptomycin. Cultures were passaged when cells were 70–90% confluent (about twice a week) while maintained at 37°C in a humidified incubator supplied with 5% CO2. Cells were harvested by trypsinization and plated at a density of 1.2–1.5 × 106 cells per well in 6-well plates and grown for 48 h, reaching 80–90% confluence on the day of the experiment. For treatment cells were washed twice with Hank’s Balanced Salt Solution (HBSS) and incubated with compounds (curcumin and analogues) or vehicle at the indicated concentration diluted in HBSS containing HEPES (20 mM, pH 7.4) and 0.1% BSA for 2 h. Cell supernatants were centrifuged (1,000 × g at 4°C for 5 min) and stored at −80°C until GLP-1 determination by ELISA.
2.4. MTT assay
Viability of treated STC-1 cells was determined using the 3-(4,5-dimethylthiazol-3-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich). Cells were seeded in poly-D-lysine coated 96-well plates and treated for 2–4 h with medium containing 0.5% DMSO (control) or compounds at various concentration, after which the MTT reaction was carried out. Absorbance was measured at 570 nm using a plate reader.
2.5. GLP-1 secretion assay
GLP-1 was quantified using a GLP-1 Total ELISA kit (Cat. No EZGLP1T-36K; EMD Millipore, USA) according to the manufacturer’s instruction. The ELISA kit measures both the active (residues 7–36) and inactive (residues 9–36) forms of GLP-1. Results are shown as the mean ± SD (standard deviation) of three independent assays (n=3). Each treatment was analyzed in duplicates. Data were evaluated for statistical significance by ANOVA. A p-value of <0.05 was considered to be statistically significant.
2.6. Western blot analysis
STC-1 cells (2 × 106) were lysed in 100 μl of RIPA buffer containing a protease and phosphatase inhibitor cocktail (Roche). Cell lysates were quantified, and an aliquot was resolved using 10% SDS-PAGE gels. Western blot analyses were performed using antibodies raised against the respective total and phospho-proteins, namely, for Protein kinase C, calcium calmodulin kinase ii (CaMKii) (PKC) (total anti-CaMKIIα, 6G9, catalog-# MA1–048, Thermo Fisher; phospho anti-pT286-CaMKII catalog-# sc-12886-R, Santa Cruz Biotechnology), for extracellular-regulated kinase (ERK)(total catalog-# 4695, phospho catalog-# 4376, both from Cell Signaling Technology), and a phospho-(Ser) PKC substrate antibody (catalog-# 2261 from Cell Signaling Technology). Protein expression was normalized by β-actin (catalog-# 3700, Cell Signaling Technology) or GAPDH (catalog-# ab8245, Abcam). Enhanced chemiluminescent reagents (GE Healthcare) were used to detect antigens after electrophoretic transfer of proteins resolved by SDS-PAGE to PVDF or nitrocellulose membranes, respectively.
2.7. LC-MS analysis of transformation products
LC-MS analyses were performed as described (18). Briefly, media samples of STC-1 cells treated with compounds (20 μM) for 2 h were extracted using Waters HLB cartridges and eluted with methanol. LC-MS analyses were performed in the negative ion mode using a ThermoScientific Vantage LC-MS system.
2.8. Curcumin protein adduction
STC-1 cells were treated with curcumin or N-ethylmaleimide in HBSS in 6-well plates for 2 h. Cells were lysed using 100 μl of cell lysis buffer containing a protease inhibitor cocktail (Roche) followed by centrifugation at 13,000 × g at 4°C for 10 min. For the detection of free thiol residues, 30 μg of protein (1 mg/ml) was incubated with 3 μl of tetramethylrhodamine-5-(and-6) C2 maleimide (1 mM in DMSO; AnaSpec) and 3 μl of SDS (1%) at 4°C in the dark for 30 min. Excess reagents were removed by precipitation with acetone at −20°C overnight. Samples were centrifuged at 13,000 × g for 10 min, acetone was removed, and samples were left to air dry for 10 min. Urea (8 M; 3 μl) was added to solubilize the protein at 4°C for 1 h followed by addition of 27 μL of SDS-PAGE sample buffer containing β-mercaptoethanol. Samples were resolved using a 7% SDS-PAGE gel. Derivatized protein was visualized using the Gel Doc EZ system (Bio-Rad) with setting for detection of rhodamine. The gel was then washed three times with water and stained with coomassie brilliant blue.
2.9. Statistical analysis
All data are presented as means ± S.D. Statistical analysis was performed using GraphPad Prism software v 7. To compare more than two groups, 1-way ANOVA followed by Dunnett’s post hoc was used. P < 0.05 values were considered statistically significant.
3. Results
3.1. Induction of GLP-1 secretion by curcumin and analogues
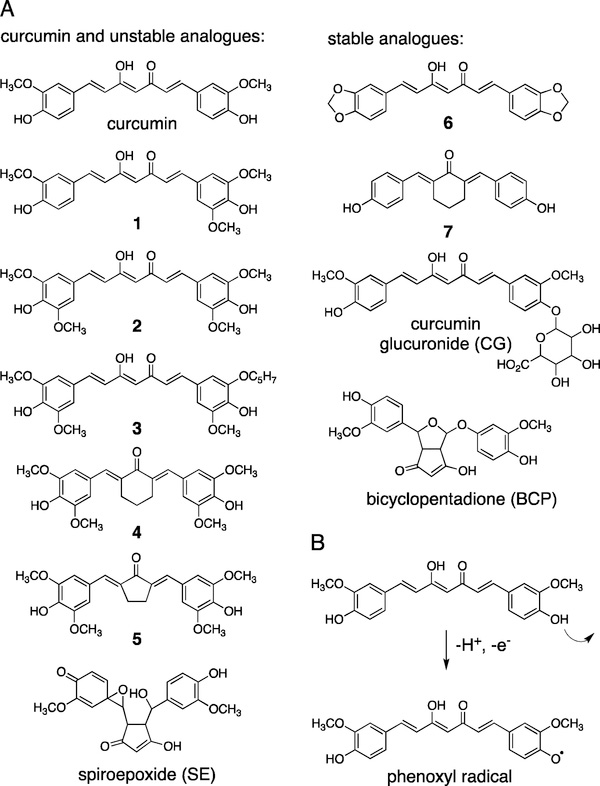
The chemical structures of curcumin, synthetic analogues, and metabolites used in this study are shown in Fig. 1A. The compounds are categorized as unstable (including curcumin) or stable. The term “stable”, however, is not an absolute but means that the compounds are less prone to undergo spontaneous degradation in buffer at physiological pH (18). Degradation of curcumin is initiated by removal of a hydrogen from the phenolic group (Fig. 1B) (14). Therefore, blocking one or both of the phenolic groups by chemical modification increases stability. Methoxy groups in the ortho position to the phenolic group decrease stability, while in their absence stability is increased (23). Thus, compounds 1–5 are unstable analogues, while relatively stable analogues are 6, 7, the major plasma metabolite curcumin-glucuronide, and BCP, the final product of curcumin degradation (Fig. 1A) (18). The distinction between stable and unstable analogues, however, is not absolute, and metabolic transformations in cells, e.g., dealkylation at the phenolic group or enzymatic H-abstraction, may convert stable into unstable analogues (15,18).
Fig. 1.
(A) The chemical structures of curcumin, synthetic analogues, and metabolites used in this study. Compounds are grouped into chemically unstable and stable depending on their propensity to undergo spontaneous degradation in buffer (15,18). (B) Degradation of curcumin is initiated by loss of a hydrogen from the phenolic hydroxyl group. The resulting phenoxyl radical undergoes cyclization and oxygenation reactions that result in formation of a spiroepoxide intermediate en route to bicyclopentadione as the major stable degradation product (16).
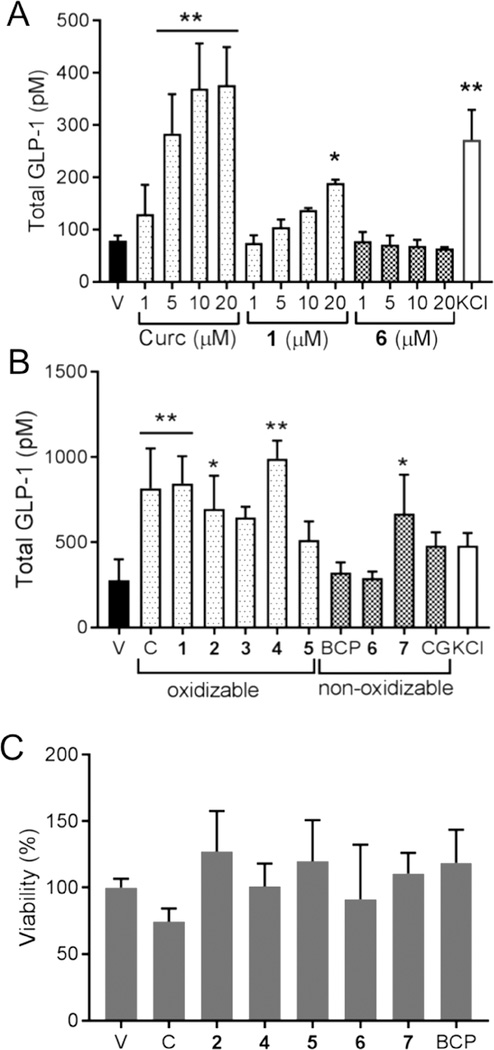
Treatment of mouse enteroendocrine STC-1 cells (24) with curcumin caused a dose-dependent increase of GLP-1 secretion with an EC50 of 4.5 μM (Fig. 2A). The highest release of GLP-1 induced by curcumin was comparable to the level achieved by the positive control, KCl (50 mM). Unstable analogue 1 dose-dependently increased GLP-1 secretion whereas stable analogue 6 did not (Fig. 2A). Additional analogues of curcumin were tested at a fixed concentration (20 μM), and 4 out of 6 unstable compounds, including curcumin, significantly induced secretion of GLP-1 from STC-1 cells while the increase induced by 3 and 5 was not statistically significant (Fig. 2B).
Fig. 2.
Induction of GLP-1 secretion from STC-1 cells. (A) Cells were treated with different concentrations of curcumin, unstable analogue 1, stable analogue 6, and KCl as positive control, and release of GLP-1 to the supernatant was quantified by ELISA. (B) Cells were treated with unstable analogues (1–5) of curcumin (C) and stable analogues (BCP, 6, 7) at 20 μM concentration, and the release of GLP-1 was quantified by ELISA. (C) An MTT assay was performed to test viability of STC-1 cells treated with curcumin and analogs for 2 h. Data represent the mean ± S.D. of 3 independent experiments. *p < 0.05 or **p < 0.005 versus control (Dunnett test).
The major oxidative and conjugate metabolites of curcumin, namely BCP and curcumin-glucuronide, respectively, did not elicit GLP-1 secretion. This was in accordance with their stability towards oxidative transformation and reported inactivity in different assays (21,25–28). Analogue 7 induced GLP-1 secretion modestly (about 2-fold) (Fig. 2B) although it was stable toward degradation. This compound also exhibited greater inhibition of NF-κB than predicted based on its stability (18). Curcumin or the test compounds did not negatively affect viability of STC-1 cells during the treatment time (Fig. 2C).
3.2. GLP-1 secretion is induced by the spiroepoxide reaction intermediate
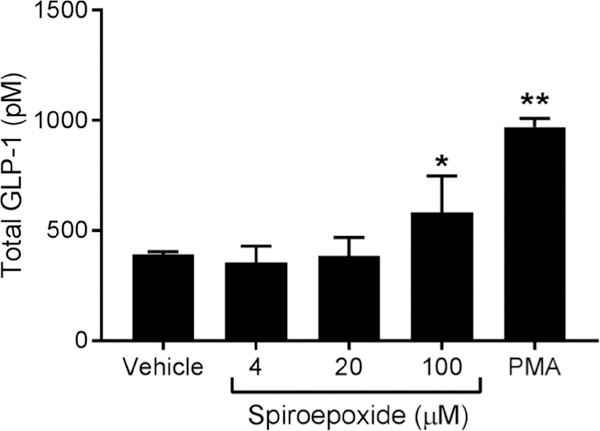
We next tested whether the spiroepoxide intermediate of oxidative transformation of curcumin was able to induce GLP-1 secretion by STC-1 cells. The spiroepoxide was isolated from autoxidation reactions of curcumin using HPLC conditions optimized to prevent further transformation (16). The spiroepoxide induced significant secretion of GLP-1 compared to vehicle control (Fig. 3). The higher concentration required to obtain a response (100 μM) is in agreement with results on its anti-inflammatory activity compared to curcumin and can be explained by its greater reactivity compared to curcumin when used in a cellular assay (18). Only a fraction of spiroepoxide added to the cell media is predicted to reach the cell cytoplasm intact while avoiding to be scavenged by soluble cellular nucleophiles like GSH (17).
Fig. 3.
Induction of GLP-1 secretion by the spiroepoxide reaction intermediate formed during degradation of curcumin. STC-1 cells were treated with different concentrations of HPLC-isolated spiroepoxide or PMA for 2 h. GLP-1 was quantified in the cell supernatant by ELISA. Data represent the mean ± S.D. of 3 independent experiments. *p < 0.05 or **p < 0.005 versus control (Dunnett test).
3.3. Activation of signaling pathways
We tested whether synthetic analogues and metabolites induced similar intracellular signaling pathways as curcumin in the regulation of GLP-1 secretion (7). Since calcium is involved in curcumin-induced GLP-1 secretion (7), activation of protein kinase C (PKC) and calcium calmodulin-dependent kinase II (CaMKII) were tested in STC-1 cells incubated with curcumin and analogues.
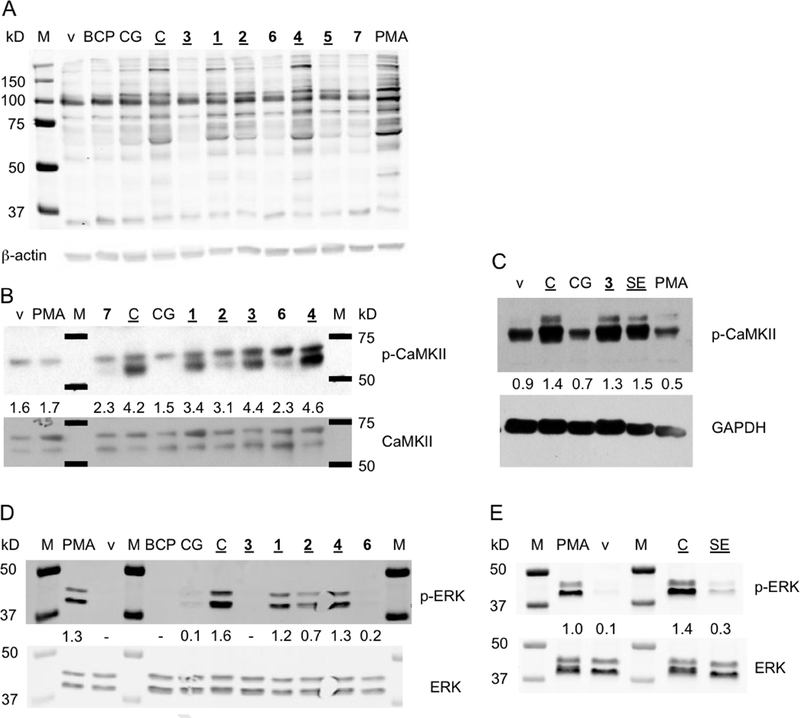
Activation of PKC was analyzed using an antibody that recognizes a PKC-specific phosphorylated sequence in target proteins (Fig. 4A). STC-1 cells treated with curcumin and unstable analogs 1, 2, and 4 showed a phosphorylation pattern resembling PMA treatment which was used as positive control. Although a similar pattern was also observed with stable analogues (6, 7) and stable metabolites (BCP, curcumin-glucuronide), the intensity of phosphorylation was much less. Unstable analogues 3 and 5, however, induced less than expected phosphorylation that was comparable to the stable analogues.
Fig. 4.
Activation of signaling molecules in STC-1 cells treated with curcumin, its synthetic analogues and metabolites (all at 20 μM concentration) or phorbol ester (PMA). Treated cells were harvested and protein was resolved by SDS-PAGE followed by blotting on nitrocellulose membrane. (A) The membrane was probed with a primary antibody that recognized a PKC-specific phosphorylated sequence in target proteins. (B, C) Immunoblot detection of phosphorylated and total CaMKii. (D,E) Immunoblot detection of phosphorylated and total ERK. Compounds considered unstable are underlined. The numbers between the phosphorylated and corresponding total or loading control bands indicate their relative intensity. BCP, bicyclopentadione, CG, curcumin-glucuronide, C, curcumin, SE, spiroepoxide, v, vehicle, M, molecular weight marker.
Curcumin and unstable analogues 1, 2, 3, and 4 induced phosphorylation of CaMKII at Thr286 whereas stable analogue 7 and the metabolites BCP and curcumin-glucuronide did not (Fig. 4B). Unexpectedly, treatment with stable analogue 6 also resulted in CaMKII phosphorylation. The HPLC-isolated spiroepoxide metabolite induced phosphorylation of CaMKII similar to what was achieved by curcumin and unstable analogue 3 (Fig. 4C).
Extracellular regulated kinase (ERK) is downstream of PKC and CaMKII and involved in GLP-secretion (29,30). ERK was activated by treatment of STC-1 cells with curcumin and unstable analogues 1, 2, and 4 while unstable 3 was inactive (Fig. 4D). The stable metabolites BCP and curcumin-glucuronide as well as stable analogue 6 did not induce ERK phosphorylation. HPLC-isolated spiroepoxide also induced ERK phosphorylation (Fig. 4E). Together, the analysis of signaling events largely supported the hypothesis that oxidative metabolism plays a role in the regulation of GLP-1 secretion by curcumin.
3.4. Metabolism of curcumin and analogues
Metabolic transformation of curcumin and its analogs was analyzed in order to determine the potential for formation of electrophilic metabolites in STC-1 cells compared to in vitro degradation (18). Oxidative transformation was assessed using LC-MS analyses by scanning for products with an increase in m/z of 32, equivalent to the incorporation of O2 as occurs during formation of the BCP metabolite of curcumin (31). Detection of oxygenated products was taken as an indication of formation of electrophilic products since these are mechanistic intermediates in oxidative transformation of curcumin (16).
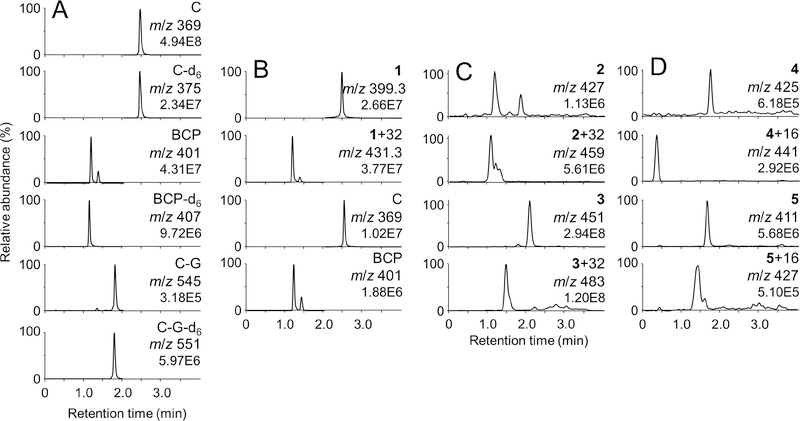
Curcumin and its oxidation product BCP were identified in supernatants of STC-1 cells by co-elution with deuterated authentic standards (Fig. 5A). Curcumin-glucuronide was also detected, indicating the ability of the cells to form phase II conjugate metabolites. Unstable compound 1 underwent efficient transformation to a dioxygenated metabolite (M + 32) and in addition, but to a lesser extent, experienced loss of a methoxy group to convert to curcumin and its corresponding BCP oxidation product (Fig 5B). A dioxygenation product (M + 32) was prominent in cells incubated with unstable 2 and 3 indicating that analogues 1–3 form the expected electrophilic metabolites in STC-1 cells. Unstable compounds 4 and 5 gave products that incorporated only a single atom of oxygen (M + 16), indicating that their degradation proceeds via a divergent and unknown mechanism (Fig 5C). Mono- or dioxygenated products were not detected upon incubation of analogues 6 and 7 with STC-1 cells, indicating that these analogues are stable towards oxidative transformation not only in buffer but also in the STC-1 cells.
Fig. 5.
Transformation of curcumin and analogs 1–5 in STC-1 cells. Cells were incubated with compounds (20 μM) for 2 h, extracted, and analyzed by LC-ESI-MS. (A) Selected reaction monitoring chromatograms of curcumin (C), bicyclopentadione (BCP), curcumin-glucuronide (C-G) and their respective hexadeuterated (d6) standards. The ion transitions monitored are listed in Materials and Methods. (B) Extracted ion chromatograms for 1, dioxygenated 1 (1 + 32), as well as curcumin and BCP derived from transformation of 1 in STC-1 cells. (C) Extracted ion chromatograms for 2, 3, and their respective dioxygenated transformation products (M + 32). (D) Extracted ion chromatograms for 4, 5, and their respective monooxygenated transformation products (M + 16).
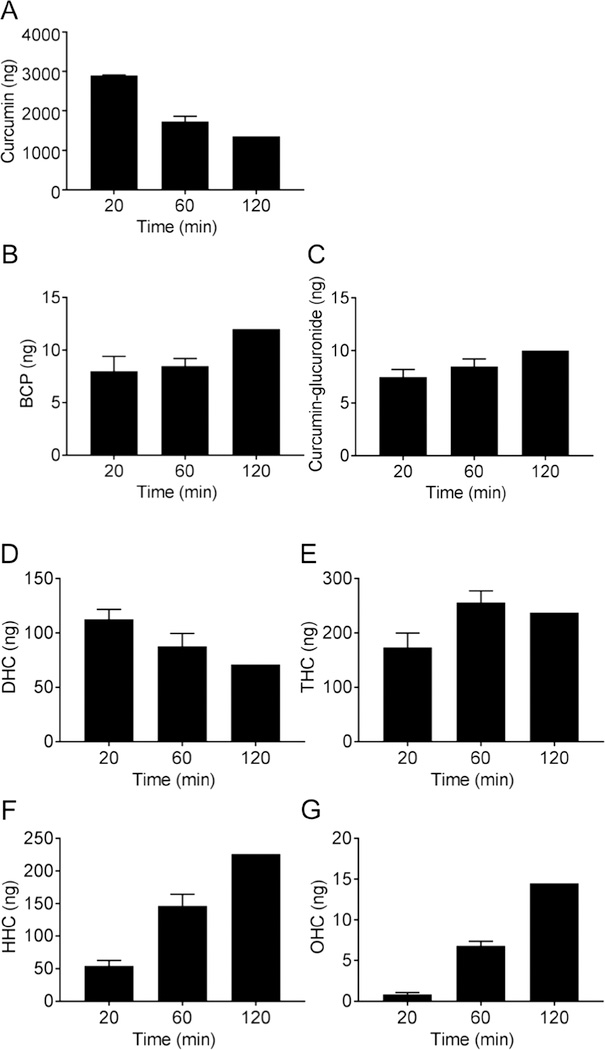
We also analyzed whether curcumin undergoes reductive metabolism in STC-1 cells since reduction of the double bonds of curcumin is a major route of in vivo metabolism, often associated with a loss in bioactivity (7,32). Cell supernatants were collected 20, 60, and 120 min after addition of curcumin, extracted, and quantified by LC-MS using d6-curcumin as internal standard. After 2 h incubation curcumin was decreased to less than half the value at 20 min (Fig. 6). Dihydrocurcumin was highest at 20 min, tetrahydrocurcumin peaked at 1 h, and hexa- and octahydrocurcumin were highest at 2 h. The time course of appearance of reduced metabolites was compatible with consecutive enzymatic saturation of the 4 double bonds present in the heptadienedione chain of curcumin (33). The BCP oxidation products and phase II metabolite curcumin-glucuronide also increased over time.
Fig. 6.
Metabolism of curcumin by STC-1 cells. STC-1 cells were treated with curcumin, and cell media were collected after 20, 60, and 120 min. Media were extracted and (A) curcumin and its metabolites (B) bicyclopentadione (BCP), (C) curcumin-glucuronide, (D) dihydrocurcumin (DHC), (E) tetrahydrocurcumin (THC), (F) hexahydrocurcumin (HHC), and (G) octahydrocurcumin (OHC) were quantified using LC-MS.
3.5. Adduction of curcumin to protein
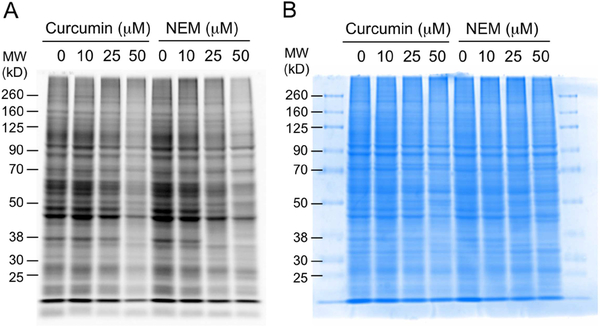
In order to further explore a mechanism of action of curcumin we analyzed the extent of covalent adduction of curcumin derived electrophiles to proteins in STC-1 cells. Curcumin and its electrophilic oxidation products react with nucleophilic thiols in a Michael-type addition forming a covalent adduct (17). In order to detect protein binding by curcumin, free cysteine residues remaining after treatment with curcumin were probed by derivatization with rhodamine-maleimide, a cysteine reactive fluorescent dye (34). Fluorescent bands on an SDS-PAGE gel indicate protein containing a free cysteine that was derivatized by rhodamine-maleimide. Protein cysteine residues targeted by curcumin are blocked from reaction with rhodamine-maleimide. Thus, the more protein cysteine residues are bound by curcumin the less fluorescence intensity is expected. Treatment with cysteine-reactive N-ethylmaleimide (NEM) was used as positive control for cysteine residues available for binding.
Treatment of STC-1 cells with curcumin resulted in a dose-dependent decrease of fluorescence intensity similar to the decrease observed with NEM (Fig. 7). Proteins across a wide range of molecular weights were adducted by curcumin, and the bands that were decreased in intensity matched those in the NEM positive control. This indicated that curcumin covalently binds to a large number of proteins in STC-1 cells. The molecular targets are proteins with accessible nucleophilic cysteine residues.
Fig. 7.
Adduction of curcumin to protein in STC-1 cells. Cells were incubated with curcumin or N-ethylmaleimide (NEM) for 2 h in HBSS, harvested, and derivatized with rhodamine-maleimide. Protein samples were resolved using SDS-PAGE, and the gel was imaged (A) by fluorescence and (B) after staining with coomassie brilliant blue.
4. Discussion
Curcumin is chemically unstable and readily degrades under cell culture conditions (31,35). We sought to test whether the degradation products are involved in the regulation of GLP-1 secretion by curcumin. In order to test this hypothesis we compared the effects of curcumin with synthetic analogues and natural metabolites that have different stability towards spontaneous degradation. The unstable analogues largely resembled curcumin in the secretion of GLP-1 and in the activation of signaling pathways that are involved in its regulation (36). Stable analogues, like 6 that does not undergo degradation because its phenolic groups are blocked by derivatization, the stable BCP oxidation product, and the phase II conjugate curcumin-glucuronide were less or inactive in inducing secretion of GLP-1 and activation of signaling events in STC-1 cells. These findings suggest that curcumin serves as a pro-drug that undergoes oxidative transformation (degradation) to form the ultimately active products.
Based on previous mechanistic studies (16–18,21,37) we propose that the active metabolites of curcumin are electrophilic reaction intermediates formed during oxidative transformation of curcumin to the final BCP oxidation product. LC-MS analyses of STC-1 cells identified oxygenated metabolites that indicated formation of electrophilic intermediates as mechanistic precursors to the final products (16). We hypothesize that the electrophilic reaction intermediates exert biological effects by binding to nucleophilic sites on targets proteins, affecting the function of adducted proteins. Among candidate metabolites of curcumin degradation the spiroepoxide electrophile was shown to react with nucleophilic small molecule thiols, and this suggested that similar adduct formation may occur with the nucleophilic thiol moiety (-SH) of cysteine residues in redox-regulated proteins (17). In addition, curcumin itself appears to be electrophilic via its enone moieties, and the same is true for the quinone methide intermediates that result from hydrogen removal at the phenolic hydroxyl (16). These electrophiles have been implicated in formation of Michael-type mono- and di-adducts of glutathione with curcumin (38). It has been difficult to decide, however, which is the reactive electrophile, i.e., the enone or the quinone methide or both since the resulting adducts are identical in structure (17).
Our hypothesis invokes that one or more proteins that control secretion of GLP-1 are redox-regulated, and thus prone to modulation by curcumin derived electrophiles. Protein redox-regulation involves reversible and irreversible oxidation of cysteine residues resulting in a change in function when oxidatively modified. Oxidative modification of cysteine can occur, depending on the type and strength of the oxidant, as oxidation of the thiol to sulfenic, sulfinic, and sulfonic acids, formation of disulfides with GSH and other nucleophilic thiols, or Michael-type covalent adduction with small molecule electrophiles (39–41). The latter type of functional regulation is well described for lipid-derived electrophiles like 4-hydroxy-nonenal and 15deoxy-prostaglandin J2 (42,43). In the context of GLP-1 secretion, redox-dependent changes in activity have been described for CaMKii and ERK. CaMKii oxidation results in activation of kinase activity. Redox activation is achieved by oxidation of neighboring methionine residues Met281 and Met282 of CaMKii resulting in persistent and Ca2+/calmodulin independent activation (44). Indirect redox mechanisms may also play a role through oxidation and inactivation of cellular phosphatases (45) or calmodulin (46). ERK signaling is fine-tuned by redox regulation through cysteine oxidation, including sulfenylation (47). Oxidation of ERK reduced phosphotransfer activity by 80–90%, thus identifying redox processes as important regulators of ERK signal transduction (47). Interestingly, ERK activation has been described to occur both upstream and downstream of GLP-1 (29,48). It should be noted that Takikawa and colleagues ruled out a role for ERK in regulating curcumin-induced GLP-1 secretion using GLUTag cells (7), and neither was functional relevance of ERK signaling established in our studies using STC-1 cells.
Nevertheless, our studies revealed effects of curcumin and its unstable analogues on both CaMKii and ERK phosphorylation such that the overall outcome was stimulation of GLP-1 secretion. Identification of the ultimate and functionally relevant targets of curcumin-derived electrophiles in this pathway will require advanced proteomics studies.
Further evidence that curcumin or its metabolites adduct to cellular protein in STC-1 cells came from our preliminary analyses using rhodamine-maleimide, a fluorescent dye that covalently binds to protein thiols. SDS-PAGE analysis following reaction with rhodamine-maleimide revealed a pattern of proteins with derivatizable cysteine residues in STC-1 cells. Treatment of the cells with curcumin dose-dependently decreased the intensity of the rhodamine staining, indicating that a large number of cysteine-containing proteins are adducted by curcumin or a curcumin-derived electrophile.
Regarding the hypothesis that oxidative activation of curcumin mediates GLP-1 secretion, there was not a clear-cut distinction between unstable analogues being active and stable ones being inactive. Not all observed effects aligned with the “oxidative activation” hypothesis, and the hypothesis should not be taken as an exclusive mechanism to explain the biological activity of curcumin. Some but not all discrepancies may be explained by cellular metabolism and enzymatic activation of analogues that are otherwise stable in vitro. The instances that did not align with the general hypothesis indicated a contribution to GLP-secretion by other mechanisms and maybe other metabolites. For example, in one study, ferulic acid but not curcumin resulted in release of GLP-1 from Caco2 cells (49). Ferulic acid does not appear to be an in vitro degradation product of curcumin (50) but it may be formed as an in vivo metabolite (51). The reductive (and un-oxidizable) metabolite tetrahydrocurcumin, however, did not possess any GLP-1 secreting effects (7). Thus, while oxidative metabolism appears to play a critical role in GLP-1 secretion, other metabolites and mechanisms likely contribute to the stimulatory effect of curcumin on GLP-1 secretion.
Highlights.
Degradation products of curcumin contribute to the regulation of GLP-1 secretion
Curcumin analogs that undergo degradation induce secretion of GLP-1
Curcumin covalently adducts to protein in STC-1 cells
Protein binding by curcumin may contribute to the regulation of GLP-1 secretion
5. Acknowledgement
This work was supported by National Institutes of Health grants R01AT006896 to C.S. A.-M.A.-O. was supported by a Fulbright Scholarship (E056609). J.A.G.-B. was supported by a postdoctoral award from the American Heart Association (16POST30690001). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- BCP
bicyclopentadione
- C-G
curcumin-glucuronide
- GLP-1
glucagon-like peptide-1
- m/z
mass to charge ratio
- NEM
N-ethylmaleimide
Footnotes
Conflict of interest
The authors declare that no conflict of interest exists with the content of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- 1.Nabavi SF, Thiagarajan R, Rastrelli L, Daglia M, Sobarzo-Sanchez E, Alinezhad H, and Nabavi SM (2015) Curcumin: a natural product for diabetes and its complications. Curr. Top. Med. Chem 15, 2445–2455 [DOI] [PubMed] [Google Scholar]
- 2.Ghosh S, Banerjee S, and Sil PC (2015) The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol 83, 111–124 [DOI] [PubMed] [Google Scholar]
- 3.Rivera-Mancia S, Lozada-Garcia MC, and Pedraza-Chaverri J (2015) Experimental evidence for curcumin and its analogs for management of diabetes mellitus and its associated complications. Eur. J. Pharmacol 756, 30–37 [DOI] [PubMed] [Google Scholar]
- 4.Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, and Jirawatnotai S (2012) Curcumin extract for prevention of type 2 diabetes. Diabetes care 35, 2121–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghorbani Z, Hekmatdoost A, and Mirmiran P (2014) Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int. J. Endocrinol. Metab 12, e18081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez-Flores LM, Lopez-Briones S, Macias-Cervantes MH, Ramirez-Emiliano J, and Perez-Vazquez V (2014) A PPARgamma, NF-kappaB and AMPK-dependent mechanism may be involved in the beneficial effects of curcumin in the diabetic db/db mice liver. Molecules 19, 8289–8302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takikawa M, Kurimoto Y, and Tsuda T (2013) Curcumin stimulates glucagon-like peptide-1 secretion in GLUTag cells via Ca2+/calmodulin-dependent kinase II activation. Biochem. Biophys. Res. Commun 435, 165–170 [DOI] [PubMed] [Google Scholar]
- 8.Tsuda T (2015) Possible abilities of dietary factors to prevent and treat diabetes via the stimulation of glucagon-like peptide-1 secretion. Mol. Nutr. Food Res 59, 1264–1273 [DOI] [PubMed] [Google Scholar]
- 9.Campbell JE, and Drucker DJ (2013) Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 17, 819–837 [DOI] [PubMed] [Google Scholar]
- 10.Amori RE, Lau J, and Pittas AG (2007) Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 298, 194–206 [DOI] [PubMed] [Google Scholar]
- 11.Lovshin JA, and Drucker DJ (2009) Incretin-based therapies for type 2 diabetes mellitus. Nat. Rev. Endocrinol 5, 262–269 [DOI] [PubMed] [Google Scholar]
- 12.Lim GE, and Brubaker PL (2006) Glucagon-like Peptide 1 secretion by the L-cell. The view from within. Diabetes 55 (Supplement), S70–S77 [Google Scholar]
- 13.Kato M, Nishikawa S, Ikehata A, Dochi K, Tani T, Takahashi T, Imaizumi A, and Tsuda T (2017) Curcumin improves glucose tolerance via stimulation of glucagon-like peptide-1 secretion. Mol. Nutr. Food Res 61 [DOI] [PubMed] [Google Scholar]
- 14.Schneider C, Gordon ON, Edwards RL, and Luis PB (2015) Degradation of curcumin: From mechanism to biological implications. J. Agric. Food Chem 63, 7606–7614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luis PB, Gordon ON, Nakashima F, Joseph AI, Shibata T, Uchida K, and Schneider C (2017) Oxidative metabolism of curcumin-glucuronide by peroxidases and isolated human leukocytes. Biochem. Pharmacol 132, 143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon ON, Luis PB, Sintim HO, and Schneider C (2015) Unraveling curcumin degradation. Autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. J. Biol. Chem 290, 4817–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luis PB, Boeglin WE, and Schneider C (2018) Thiol reactivity of curcumin and its oxidation products. Chem. Res. Toxicol 31, 269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards RL, Luis PB, Varuzza PV, Joseph AI, Presley SH, Chaturvedi R, and Schneider C (2017) The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem 292, 21243–21252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li N, and Karin M (1999) Is NF-kappaB the sensor of oxidative stress? FASEB J. 13, 1137–1143 [PubMed] [Google Scholar]
- 20.Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, and Glass CK (2000) 15-Deoxy-delta12,14-prostaglandin J2 inhibits multiple steps in the NF-kappaB signaling pathway. Proc. Natl. Acad. Sci. U.S.A 97, 4844–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ketron AC, Gordon ON, Schneider C, and Osheroff N (2013) Oxidative metabolites of curcumin poison human type II topoisomerases. Biochemistry 52, 221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon ON, Graham LA, and Schneider C (2013) Facile synthesis of deuterated and [(14) C]labeled analogs of vanillin and curcumin for use as mechanistic and analytical tools. J. Labelled Comp. Radiopharm 56, 696–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon ON, Luis PB, Ashley RE, Osheroff N, and Schneider C (2015) Oxidative transformation of demethoxy- and bisdemethoxycurcumin: Products, mechanism of formation, and poisoning of human topoisomerase IIalpha. Chem. Res. Toxicol 28, 989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy T, Green BD, Calderwood D, Gillespie A, Cryan JF, and Giblin L (2015) STC-1 cells in The Impact of Food Bioactives on Health (Verhoeckx K. e. a. ed.), Springer Cham; pp 211–220 [Google Scholar]
- 25.Pal A, Sung B, Bhanu Prasad BA, Schuber PT Jr., Prasad S, Aggarwal BB, and Bornmann WG (2014) Curcumin glucuronides: Assessing the proliferative activity against human cell lines. Bioorg. Med. Chem 22, 435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoji M, Nakagawa K, Watanabe A, Tsuduki T, Yamada T, Kuwahara S, Kimura F, and Miyazawa T (2014) Comparison of the effects of curcumin and curcumin glucuronide in human hepatocellular carcinoma HepG2 cells. Food Chem. 151, 126–132 [DOI] [PubMed] [Google Scholar]
- 27.Sanidad KZ, Zhu J, Wang W, Du Z, and Zhang G (2016) Effects of stable degradation products of curcumin on cancer cell proliferation and inflammation. J. Agric. Food Chem 64, 9189–9195 [DOI] [PubMed] [Google Scholar]
- 28.Kunihiro AG, Luis PB, Brickey JA, Frye JB, Chow HS, Schneider C, and Funk JL (2019) Beta-Glucuronidase Catalyzes Deconjugation and Activation of Curcumin-Glucuronide in Bone. J. Nat. Prod [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reimer RA (2006) Meat hydrolysate and essential amino acid-induced glucagon-like peptide-1 secretion, in the human NCI-H716 enteroendocrine cell line, is regulated by extracellular signal-regulated kinase1/2 and p38 mitogen-activated protein kinases. J. Endocrinol 191, 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim GE, Huang GJ, Flora N, LeRoith D, Rhodes CJ, and Brubaker PL (2009) Insulin regulates glucagon-like peptide-1 secretion from the enteroendocrine L cell. Endocrinology 150, 580–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griesser M, Pistis V, Suzuki T, Tejera N, Pratt DA, and Schneider C (2011) Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J. Biol. Chem 286, 1114–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, and Gescher A (2001) Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 61, 1058–1064 [PubMed] [Google Scholar]
- 33.Hassaninasab A, Hashimoto Y, Tomita-Yokotani K, and Kobayashi M (2011) Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. U.S.A 108, 6615–6620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiraki T, Kamiya N, Shiki S, Kodama TS, Kakizuka A, and Jingami H (2005) Alpha,beta-unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor gamma. J. Biol. Chem 280, 14145–14153 [DOI] [PubMed] [Google Scholar]
- 35.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, and Lin JK (1997) Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal 15, 1867–1876 [DOI] [PubMed] [Google Scholar]
- 36.Drucker DJ (2018) The ascending GLP-1 road from clinical safety to reduction of cardiovascular complications. Diabetes 67, 1710–1719 [DOI] [PubMed] [Google Scholar]
- 37.Joseph AI, Edwards RL, Luis PB, Presley SH, Porter NA, and Schneider C (2018) Stability and anti-inflammatory activity of the reduction-resistant curcumin analog, 2,6-dimethyl-curcumin. Org. Biomol. Chem 16, 3271–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathews S, and Rao MNA (1991) Interaction of Curcumin with Glutathione. Int. J. Pharm 76, 257–259 [Google Scholar]
- 39.Leonard SE, and Carroll KS (2011) Chemical ‘omics’ approaches for understanding protein cysteine oxidation in biology. Curr. Opin. Chem. Biol 15, 88–102 [DOI] [PubMed] [Google Scholar]
- 40.Lo Conte M, and Carroll KS (2013) The redox biochemistry of protein sulfenylation and sulfinylation. J. Biol. Chem 288, 26480–26488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poole LB (2015) The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med 80, 148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchida K (2003) 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Progress in lipid research 42, 318–343 [DOI] [PubMed] [Google Scholar]
- 43.Uchida K, and Shibata T (2008) 15-Deoxy-Delta(12,14)-prostaglandin J2: an electrophilic trigger of cellular responses. Chem. Res. Toxicol 21, 138–144 [DOI] [PubMed] [Google Scholar]
- 44.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, and Anderson ME (2008) A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133, 462–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howe CJ, Lahair MM, McCubrey JA, and Franklin RA (2004) Redox regulation of the calcium/calmodulin-dependent protein kinases. J. Biol. Chem 279, 44573–44581 [DOI] [PubMed] [Google Scholar]
- 46.Robison AJ, Winder DG, Colbran RJ, and Bartlett RK (2007) Oxidation of calmodulin alters activation and regulation of CaMKII. Biochem. Biophys. Res. Commun 356, 97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keyes JD, Parsonage D, Yammani RD, Rogers LC, Kesty C, Furdui CM, Nelson KJ, and Poole LB (2017) Endogenous, regulatory cysteine sulfenylation of ERK kinases in response to proliferative signals. Free Radic. Biol. Med 112, 534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, and Olefsky JM (2008) Beta-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc. Natl. Acad. Sci. U.S.A 105, 6614–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song WY, Aihara Y, Hashimoto T, Kanazawa K, and Mizuno M (2015) (−)-Epigallocatechin-3-gallate induces secretion of anorexigenic gut hormones. J. Clin. Biochem. Nutr 57, 164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon ON, and Schneider C (2012) Vanillin and ferulic acid: not the major degradation products of curcumin. Trends Mol. Med 18, 361–363; author reply 363–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vitaglione P, Barone Lumaga R, Ferracane R, Radetsky I, Mennella I, Schettino R, Koder S, Shimoni E, and Fogliano V (2012) Curcumin bioavailability from enriched bread: the effect of microencapsulated ingredients. J. Agric. Food Chem 60, 3357–3366 [DOI] [PubMed] [Google Scholar]