Abstract
Environmental exposure to polychlorinated biphenyls (PCBs) is associated with an increased risk of incidence of metabolic disease, however the molecular mechanisms underlying this phenomenon are not fully understood. Our study provides new insights into molecular interactions between PCBs and retinoids (vitamin A and its metabolites) by defining a role for constitutive androstane receptor (CAR) in the disruption of retinoid homeostasis by non-coplanar 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB153). Administration of four weekly 50 mg/kg doses of PCB153 to C57BL/6 male mice resulted in a significant decline in the tissue concentrations of retinyl esters, retinol and all-trans-retinoic acid (atRA), while no decline in hepatic and adipose tissue retinoid levels were detected in Car-null littermates. Our data imply that disrupted retinoid homeostasis occurs as a consequence of PCB153-induced activation of CAR, and raise the possibility that CAR signaling can affect atRA homeostasis in vivo. A strong correlation between the changes in retinoid metabolism and extensive upregulation of hepatic CAR-driven Cyp2b10 expression implicates this CYP isoform as contributing to retinoid homeostasis disruption via atRA oxidation during PCB153 exposure. In response to PCB153-induced CAR activation and disruption of retinoid homeostasis, expression of hepatic Pepck, Cd36 and adipose tissue Pparγ, Cd36, Adipoq, and Rbp4 were altered; however, this was reversed by administration of exogenous dietary retinoids (300 IU daily for 4 weeks). Our study establishes that PCB153 exposure enables a significant disruption of retinoid homeostasis in a CAR-dependent manner. We propose that this contributes to the obesogenic properties of PCB153 and may contribute to the predisposition to the metabolic disease.
Keywords: Vitamin A, environmental pollutant, metabolic disruption, cytochrome P450, transcriptional regulation
INTRODUCTION
Polychlorinated biphenyls (PCBs) are human-made persistent environmental pollutants that are a global concern due to their bio-accumulation and adverse human health effects [1-3]. Environmental and occupational exposures to PCBs have been associated with liver, kidney, endocrine, and neurodevelopmental diseases [4]. Numerous meta-analyses and systemic reviews provide quantitative evidence supporting the conclusion that exposure to these organochlorine pollutants is also associated with an increased risk for incidence of obesity and type 2 diabetes [4-18]. The literature establishes that the majority of PCB species found in human tissues are the highly chlorinated non-coplanar congeners (substituted with more than two para and ortho substituents), with PCB153 having the highest concentration in tissues of non-acutely exposed humans [19-21]. Epidemiological surveys reveal that blood and tissue PCB153 concentrations reach into the μM range [19, 22-24], suggesting wide spread human environmental exposure. The highly lipophilic nature of non-coplanar PCBs allows them to be absorbed in high amounts [25], however the complexity of their metabolism owing to their high degree of chlorination makes them less likely to biodegrade [26-28]. In humans, hepatic cytochrome P450 2B isoform (CYP2B6) can metabolize PCB153 to 3-hydroxy-2,2’,4,4’,5,5’-hexachlorobiphenyl [29]. However, the low content of this CYP isoform in human liver (maximum 1-2% of total hepatic CYPs [30]) and its low catalytic activity towards PCB153 [29, 31], underlie the metabolic resistance and long retention of this PCB congener in human tissues. A number of cross-sectional biomonitoring studies have identified that intrinsic human elimination half-life of PCB153 (accounting for ongoing exposure and changes in body weight) is 8-15 years [32, 33]. Taken together these chemical and metabolic factors drive relatively extensive bioaccumulation of PCB153 within the body, enabling life-long chronic exposure [27, 34, 35].
The physiological importance of non-coplanar PCB accumulation in the body was established with the demonstration that these congeners interact with the xenobiotic nuclear receptors, including the constitutive androstane receptor (CAR) and pregnane X receptor (PXR), affecting transcription of target genes in a substitution- and tissue-dependent manner [36-39]. However, while several highly chlorinated non-coplanar PCB congeners are able to activate rodent PXR, they possess antagonistic properties towards its human ortholog SXR (human steroid and xenobiotic receptor), blunting induction of its target genes in human cells [40]. With regards to CAR, both the human and the rodent receptors have been shown to be activated by non-coplanar PCBs [38, 39, 41]. The congener-specific transactivation is achieved at non-coplanar PCB concentrations in the low μM range (1-10 μM), concentrations that are found in human tissues [19-24]. Based on this and by analogy to the action of phenobarbital (PB), a prototypical inducer of the canonical CAR target CYP2B, non-coplanar PCB congeners have been classified as “phenobarbital-like inducers”, as oppose to “3-methylcholantrene-type inducers”, which include coplanar PCBs that act through their interaction with the aryl hydrocarbon receptor (AhR) [42]. Investigation of CAR activation by PB has elucidated an indirect mechanism of activation through epidermal growth factor receptor (EGFR) inhibition leading to CAR dephosphorylation and thus activation [43]. It has been recently demonstrated that the same mechanism involving EGFR inhibition is applicable to CAR activation by PCB153 and related compounds that were previously characterized as CAR activators [44, 45]. Taken together, these recent findings have added complexity to our understanding of PCB-CAR interactions, suggesting that disruption of multiple signaling pathways contributes to the development of metabolic alterations resulting from PCB exposure [46].
The literature points to the possibility that PCB-induced metabolic disease, including obesity, decreased insulin sensitivity, and hepatic steatosis arise from alterations in expression of genes involved in energy metabolism in the liver and adipose tissue [47-55]. Many of these genes are known to be direct targets for retinoic acid signaling [56], suggesting that some of the adverse effects of exposure may arise from PCB-induced disruptions of normal retinoid (vitamin A and its metabolites) homeostasis and signaling. The literature provides compelling evidence of molecular interactions between PCBs and retinoids [57]. These interactions primarily involve the actions of the retinoic acid nuclear receptors (both the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs)) in regulating gene expression. The all-trans- and 9-cis-isomers of retinoic acid (atRA and 9cRA), the most bioactive retinoid species, regulate transcription upon binding to one of six cognate nuclear receptors (RARα, RARβ, RARγ, RXRα, RXRβ, or RXRγ) [58, 59]. RXRs are common heterodimerization partners for most xenobiotic receptors, including CAR [60-62]. Consequently, the RARs and RXRs have been shown to function as master regulators for genes encoding enzymes and transporters involved in xenobiotic metabolism [63-67]. For instance, studies involving the hepatocyte-specific disruption of the mouse Rxrα gene revealed decreased basal expression of a number of phase I enzymes, including CAR-driven CYP2B [62, 64], and suggested that CYP2B is among RXRα target genes in vivo [65, 68]. Addition of atRA or 9cRA together with CAR agonist TCPOBOP was found to further enhance the induction of responsive genes through a process mediated by RXRα/CAR and RXRγ/CAR heterodimers [63]. Consistent with these findings, the treatment of rats with the RXR selective agonist, bexarotene (LGD1069/Targretin), results in increased hepatic protein levels of CYP2B1/2 [69]. Moreover, PCBs and retinoids undergo extensive metabolic cross-talk, since key enzymatic biotransformations of both xenobiotics and retinoids are catalyzed by the hepatic monooxygenase system, predominantly by members of cytochrome P450 superfamily (CYPs), but also involving enzymes that catalyze phase II conjugation reactions [57]. Indeed, underscoring this bidirectional relationship, PCB exposure has been shown to cause marked alterations in retinoid metabolism, resulting in a very rapid loss of tissue retinoids as well as disrupted retinoid signaling, leading to developmental, metabolic and other functional impairments [70-72]. Despite the importance of PCB-retinoid interactions, these have not been extensively studied in the context of the metabolic consequences arising from them. The present study provides new insights into molecular interactions between PCBs and retinoids defining a role for constitutive androstane receptor (CAR) in the disruption of retinoid homeostasis by non-coplanar PCBs.
METHODS
2.1. Animal husbandry
All animal experiments were carried out with the approval of the Institutional Animal Care and Use Committee of Columbia University according to criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences [73]. Groups of ten- to twelve-week-old male Car-null mice on the C57BL/6 genetic background (model # 9103 obtained through a licensing agreement with Taconic Biosciences Inc., Rensselaer, NY, USA) and age-matched littermates (wild type) on the C57BL/6 genetic background were administered corn oil containing 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB153, Agilent, North Kingstown, RI) by oral gavage at a dose 50 mg/kg weekly. Control groups of animals received the same amount of corn oil alone. Separate groups of PCB153- and vehicle-treated animals additionally received 300 IU of vitamin A in the form of retinyl acetate. This is approximately 10-times higher amount than a mouse acquires daily from consumption of a chow diet (30 IU). Routinely, 6 animals per each group were employed. The mice were euthanized four weeks after receiving four weekly doses of 50 mg/kg of PCB153 each (cumulative dose 200 mg/kg). At the time of sacrifice, plasma, liver and perigonadal white adipose tissue (WAT) were collected for analyses. The dissected tissues were quickly weighed and frozen in liquid N2 and stored at −80 °C continuously without thaw until analysis.
2.2. HPLC separation and analyses for retinyl esters and retinol
Retinyl esters and retinol were extracted from plasma, liver and adipose tissue under a dim yellow safety light. Briefly, livers and adipose tissues were homogenized in 10 vol of PBS (10 mM sodium phosphate, pH 7.2, 150 mM sodium chloride) using 1.3 mm chrome steel beads on a Biospec Mini-BeadBeater-1 homogenizer (BioSpec Products, Inc., Bartlesville, OK, USA). An aliquot of plasma or tissue homogenate (200 μl) was then treated with an equal volume of absolute ethanol containing a known amount of retinyl acetate as an internal standard. The retinoids present in either plasma or tissue homogenates were extracted into hexane. The hexane extract was dried under a stream of N2 and redissolved in benzene. For determination of tissue and plasma levels of retinol and retinyl esters, a reverse-phase HPLC method employing a 4.6 × 250 mm Symmetry C18 Column (Waters, Milford, MA, USA) and a running solvent consisting of acetonitrile/methanol/methylene chloride (70:15:15 v/v) flowing at 1.8 ml/min was used. Retinol and retinyl esters were detected at 325 nm. Quantitation was based on comparisons of the area under the peaks and spectra for unknown samples to those of known amounts of standards. The recovery of internal standard was employed to correct for loss during extraction. For this analysis, HPLC grade solvents were purchased from Thermo Fisher Scientific (Pittsburgh, PA).
2.3. LC/MS/MS separation and analyses for all-trans-retinoic acid and 4-hydroxy retinoic acid.
All solvents employed for sample extractions and liquid chromatography were LC/MS or LC grade and were purchased from Thermo Fisher Scientific (Pittsburgh, PA). All measurements were carried out on a Waters Xevo TQ MS ACQUITY UPLC system (Waters, Milford, MA). The system was controlled by MassLynx software version 4. 1 (Waters, Milford, MA).
All-trans-retinoic acid (atRA) was extracted using a two-step, acid-base extraction [74], with minor modifications. Briefly, 0.5 ml of 0.025 M KOH in ethanol was added to 250 μl of tissue homogenate, which contained 50 mg of wet tissue. Five nanograms of pentadeuterated all-trans-retinoic acid (atRA-d5) dissolved in absolute ethanol was added to each extract as an internal standard. The aqueous phase was extracted with 5 ml of hexane. The organic phase containing nonpolar retinoids (retinol and retinyl esters) was removed. Thirty microliters of 4 M HCl was then added to the aqueous phase, and polar retinoids, including retinoic acid, were removed by extraction into 5 ml hexane. The hexane was removed under N2. Extracts were resuspended in 70 μl of acetonitrile and transferred to amber LC/MS vials (Waters, Milford, MA). Only glass containers, pipettes, and calibrated syringes were used to handle and process retinoic acid. Samples were maintained at 4°C in the autosampler, and 5 μl was loaded onto a Waters ACQUITY UPLC HSS C18 column (2.1 mm inner diameter × 100 mm with 1.8 μm particles), preceded by a 2.1 × 5 mm guard column with the same packing material (Waters). The column was maintained at 40°C by a column heater. The flow rate was 300 μl/min in binary gradient mode with the following mobile phase gradient: initiated with 32% phase A [H2O, containing 0.1% formic acid] and 68% mobile phase B [acetonitrile, containing 0.1% formic acid]; the gradient was maintained for 6.3 min. The acetonitrile content of the solvent was increased linearly to 85% over 6.4 min and maintained until 9.5 min, increased to 100% to wash the column for 2 min, and then decreased acetonitrile to 68%. All-trans-retinoic acid (atRA) eluted between 8.2 and 8.4 min. Positive ESI-MS/MS mass spectrometry was performed using the following parameters: capillary voltage 3.8 kV, source temperature 150°C, desolvation temperature 500°C, desolvation gas flow 800 l/h, collision gas flow 0.15 ml/min. Optimized cone voltage was 16 V, collision energy for multiple reaction monitoring mode (MRM) was 18 eV, and the following transitions were used: atRA for quantification, m/z 301.16 → 123.00; atRA for verification, m/z 301.16 → 205.03; and atRA-d5, m/z 306.15 → 127.03.
4-Hydroxy all-trans-retinoic acid (4-HO atRA) was extracted using chloroform/methanol. Briefly, 500 μL of tissue homogenate containing 100 mg of liver tissue were mixed with 2 ml chloroform and 1 ml methanol containing 5 ng of atRA-d5 as an internal standard. The mixture was vortexed well and centrifuged at 3,000 g for 10 minutes. The lower organic phase was transferred to another clean glass tube using a Pasteur pipette. Two ml of chloroform was added to the residual aqueous phase, followed by vortex mixing and centrifugation again at 3,000 g for 10 min, to extract any remaining lipids. The lower organic phases were pooled and evaporated under nitrogen. The extracted lipids were reconstituted in 30 μL of acetonitrile/methanol (vol:vol = 1:1) and transferred to LC/MS autosampler vials (Waters, Milford, MA) for injection. The sample was maintained at 4°C in the autosampler and a volume of 5 μl was loaded onto a Waters ACQUITY UPLC HHS C18 column (2.1 mm inner diameter × 100 mm with 1.8 μm particles, Waters, P/N 186003533), and a 2.1 × 5 mm guard column with the same packing material (Waters, P/N 186003981). The column was maintained at 40°C. The flow rate was 300 μl/min in binary gradient mode with the following mobile phase gradient: initiated with 50% phase A (H2O containing 0.1% formic acid) and 50% mobile phase B (acetonitrile, containing 0.1% formic acid). Acetonitrile content was linearly increased to 100% over 5 min and maintained till 10 min. Then the content of acetonitrile was reduced to 50% and maintained for 2 min before the next injection. Species of interest were eluted between 8.2 and 8.4 min. Positive ESI-MS/MS with multiple reaction monitoring (MRM) mode was performed using the following parameters: capillary voltage, 4 kV; source temperature, 150 °C; desolvation temperature, 500 °C; desolvation gas flow, 1000 L/hr; and collision gas flow, 0.18 mL/min. Optimized cone voltage was 20 V, collision energy for multiple reaction monitoring mode (MRM) was 20 eV. The following transitions were used: 4-HO atRA, m/z 299.3 → 95.0; and atRA-d5, m/z 306.15 → 127.03.
2.4. HRMS analysis for PCB153
Tissue concentrations of 2,2',4,4',5,5'-hexachlorobiphenyl (PCB153) were determined by isotope dilution and internal standard high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) according to EPA developed method 1668C [75]. Analysis was performed by Pacific Rim Laboratories Inc. (Surrey, BC, Canada).
2.5. Liver histology and function test
Alanine aminotransferase (ALT) enzymatic activity was determined in mouse plasma using a kit from Genzyme Diagnostics (Genzyme Diagnostics P.E.I. Inc, Canada) according to the manufacturer’s protocol.
For paraffin sections, livers were first fixed in neutral buffered formalin and then processed into paraffin blocks according to standard protocols [76]. The embedded tissues were cut into 6 μm slices, mounted on charged adhesive slides, and dried overnight at 50 °C. Slides were then deparaffinized in xylene and rehydrated in graded alcohol and distilled water. Representative histological sections of each specimen were stained with hematoxylin/eosin (H&E) according to standard staining methods.
2.6. RNA preparation and quantitative real time PCR (qRT-PCR)
Total RNA was extracted from frozen tissues employing TRIzol Reagent (Ambion, Foster City, CA, USA) and isolated using the E.Z.N.A Total RNA Kit II (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer’s protocol. RNA was quantitated at 260 nm using a Nanodrop spectrophotometer. cDNA synthesis was performed using 0.5 μg of total RNA (in a final volume of 20 μl) and was carried out for 10 min at 25 °C followed by 120 min at 37 °C employing reverse transcriptase (Applied Biosystems, Foster City, CA, USA). The reaction was stopped at 85 °C for 5 min, using a thermal cycler (Eppendorf, Westbury, NY, USA). The primers employed for qRT-PCR analyses of target gene expression are provided in our previous publications [77, 78]. 18S RNA was employed as the reference housekeeping gene used to normalize mRNA expression.
qRT-PCR was performed in a total volume of 20 μl, including 40 ng of cDNA template, forward and reverse primers (100 nM each), and PerfeCTa SYBR Green FastMix (Quantabio, Beverly, MA, USA) using a LightCycler 480 instrument (Roche, Branchburg, NJ, USA). After initial denaturation and enzyme activation (95 °C for 10 min), 40 cycles (94 °C for 10 sec, 55 °C for 30 sec, 72 °C for 30 sec) were performed for the annealing/extension steps, and fluorescence was measured. A dissociation curve program was performed after each cycle. Expression levels of target genes were calculated based on the efficiency of each reaction and the crossing point deviation of each sample versus a control and are expressed as fold differences in comparison with the reference gene.
2.7. Western blotting
Hepatic and adipose tissue proteins were extracted using RIPA lysis and extraction buffer (Thermo Scientific, Rockford, IL) containing protease inhibitor cocktail (Millipore Sigma, Burlington, MA). For all tissues, 20 μg of total homogenate protein was analyzed. For plasma protein analysis, 2 μl of plasma was employed. The proteins were subjected to SDS PAGE in 12% gels followed by a transfer onto PVDF membrane. Immunoblotting was performed using rabbit polyclonal antibodies directed against mouse cytochrome P450 isoform 2B10 (CYP2B10, 1:5000, EMD Millipore, Temecula, CA), cytochrome P450 isoform 26A1 (CYP26A1, 1:1000, Bioss, Woburn, MA), retinol-binding protein 4 (RBP4, 1:3000), and peroxisome proliferator-activated receptor gamma (PPARγ, 1:1000, Santa Cruz Biotechnology, CA). Protein loading and relative quantification of protein expression were normalized using β-actin as a reference protein (1:10,000; Millipore Sigma, St. Louis, MO). Protein bands were visualized using Super Signal West Pico PLUS chemiluminescence system (Thermo Scientific, Rockford, IL), films were digitally scanned and analyzed using ImageJ (National Institutes of Health) to generate quantitative relative protein expression data [79].
2.8. Statistical analysis.
All data are presented as means ± S.D. To analyze differences between genotypes and treatment groups one-way ANOVA was employed first, followed by multiple comparisons employing Tukey's HSD post hoc test. P values less than 0.05 were considered to be statistically significant.
RESULTS
PCB153 administration results in hepatic Cyp2b10 upregulation in vivo
In order to make our study consistent with both experimental and translational data reported in the literature, we set out to approximate tissue concentrations of PCB153 to those levels reported in this literature. To this end, we treated mice with 4 weekly per os doses of 50 mg/kg of PCB153 (200 mg/kg cumulative dose). HRMS analysis of hepatic tissue isolated from PCB153 exposed wild type mice revealed that at the end of the four-week period the hepatic concentration of 2,2’,4,4’,5,5’-hexachlorobiphenyl was at a level of 11.57 ± 2.77 μg/g liver weight (578.7±138.1 ng/g lipid or 32.06 ± 7.65 μM). This PCB153 treatment was not associated with the development of signs of acute PCB-toxicity. Histological analysis of H&E stained tissue sections prepared from the livers of PCB153-treated mice did not provide evidence of tissue damage (Suppl. Fig. 1A) and plasma ALT activities were not significantly different between PCB153-treated and vehicle-treated animals (Suppl. Fig. 1B). Hepatic mRNA expression of glutamate-cysteine ligase catalytic subunit (Gclc), a gene known to be upregulated in response to the oxidative stress, was not affected by PCB153 administration to the experimental mice (Suppl. Fig. 1C).
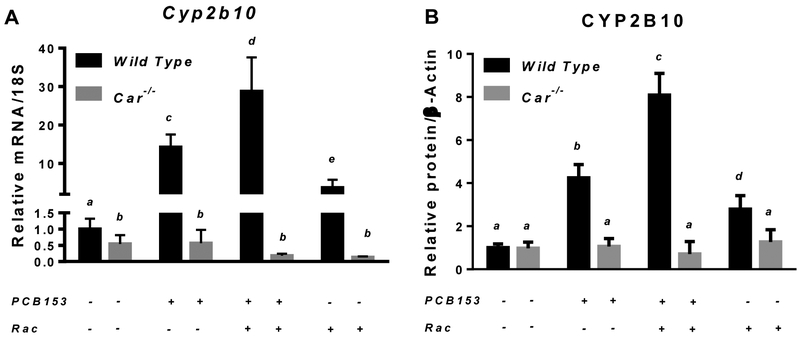
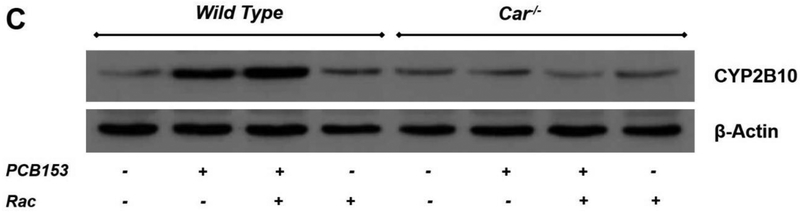
After four weeks of treatment, among the hepatic genes encoding xenobiotic-metabolizing CYPs, the most pronounced PCB153-induced induction of mRNA expression, by approximately 15 fold, was observed for the CAR-driven Cyp2b10 (Fig. 1A). In PCB153 treated mice, hepatic mRNA expression of Cyp3a11 was moderately, but significantly elevated by 1.5-fold (Suppl. Fig. 2A), Cyp4a10 expression was markedly downregulated by PCB153 administration (Suppl. Fig. 2B), and the transcript level for Cyp1a1 remained unchanged (Suppl. Fig. 2C). Cyp2b10 transcriptional upregulation was further confirmed to result in significantly higher protein levels of this cytochrome P450 isoform in the livers of PCB153-treated mice compared to vehicle-treated controls (Fig. 1B and 1C). No induction of either mRNA or protein expression of hepatic Cyp2b10 was observed in mice carrying the constitutive androstane receptor-null mutation (Car−/− mice), suggesting a congener specific pattern of nuclear receptor-mediated upregulation of cytochrome expression resulted from PCB153 administration (Fig. 1). Thus, these data confirmed that our in vivo PCB153 treatments allowed for achievement of a CAR-driven transcriptional response with no interfering non-specific responses, including oxidative stress and overt tissue damage.
Figure 1. mRNA and protein expression of Cyp2b10 in livers of wild type and Car−/− mice after PCB153 administration.
Panel A – Gene expression values (normalized to 18S rRNA levels) were determined by qRT-PCR for RNA obtained from livers of mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration. Panel B – Quantitative expression data (normalized to β-actin levels) generated by the analysis of digitally scanned immunoblots of specific protein expression in livers of wild type and Car−/− mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration. Panel C – Representative immunoblots of specific protein expression in livers of wild type and Car−/− mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration; Values marked with different letters (a, b, c, d) are statistically different, P < 0.05. All values are given as the mean ± 1 S.D., n = 6 for each group.
PCB153 administration results in a progressive CAR-mediated disruption of retinoid homeostasis
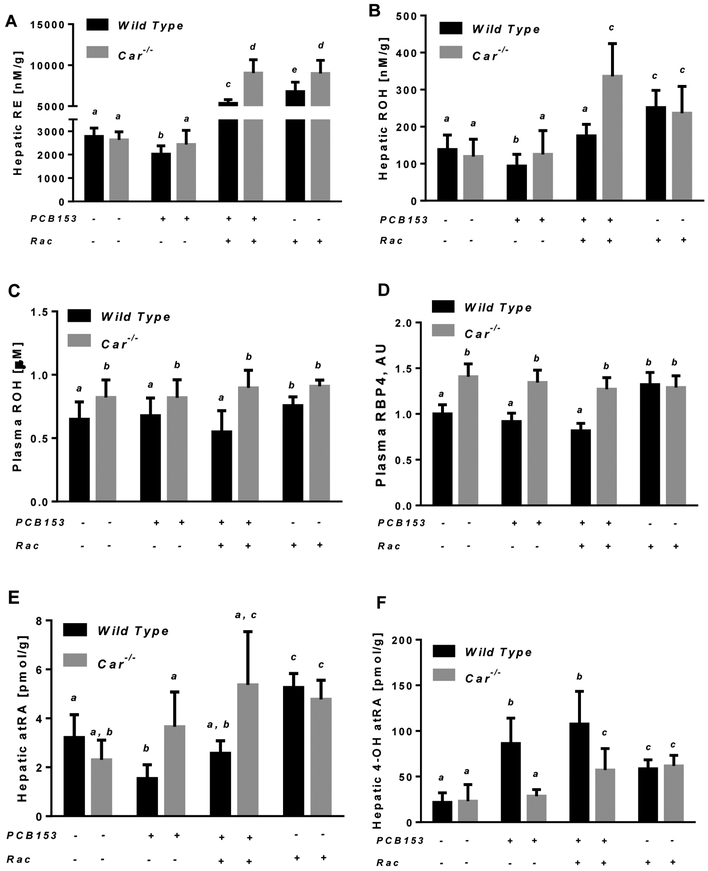
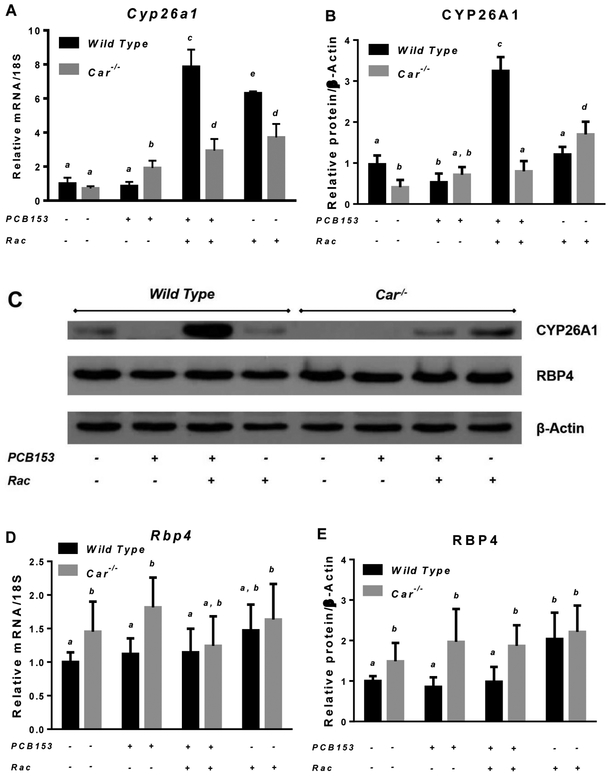
PCB153 administration to wild type mice resulted in a significant decrease of hepatic retinoid levels. After four weeks of treatment with PCB153, a significant decline in the concentrations of retinol and retinyl esters was detected, but only for wild type mice (Fig. 2). The concentrations of both hepatic retinyl esters and retinol declined by 27% and 33% respectively in wild type mice treated with four weekly doses of PCB153 compared to vehicle-treated animals (Fig. 2A and 2B). The observed drop in hepatic retinoid concentrations was not associated with impairments in retinol esterification as evidenced by no changes in mRNA expression of the lecithin:retinol acyltransferase (Lrat) gene (Suppl. Fig. 2D). No differences in plasma retinol concentrations or plasma retinol binding protein 4 (RBP4) were identified following PCB153 administration (Fig. 2C and 2D). This suggests that retinol being formed from retinyl ester hydrolysis is unlikely to undergo extrahepatic redistribution. Rather, it is being utilized within the liver by downstream pathways providing the formation all-trans-retinoic acid (atRA), a transcriptionally active retinoid species that is synthesized through retinol oxidation [80]. However, the hepatic concentration of atRA in PCB153-treated wild type animals was lower by more than 50% compared to atRA concentration in vehicle-treated mice (Fig. 2E). At the same time, in the livers of PCB153-treated wild type animals the concentration of 4-hydroxy-all-trans-retinoic acid (4-OH atRA), a catabolic product of atRA oxidation, was elevated for more than 4-fold (Fig. 2F). Interestingly, the elevation in 4-OH atRA concentrations in livers of wild type animals administered with PCB153 was not associated with an upregulation of hepatic Cyp26a1 (Fig. 3A-3C) a gene encoding a canonical atRA 4-hydroxylase [81]. Taken together, these data imply extensive hepatic retinol utilization during PCB153 exposure presumably aimed at providing sufficient amounts of atRA within the liver which is being progressively lost via its oxidation to 4-OH atRA.
Figure. 2. Hepatic and plasma retinoid concentrations in wild type and Car−/− mice after PCB153 administration.
Panels A and B – Retinyl ester (RE) and retinol (ROH) concentrations determined by HPLC in wild type and Car−/− livers of mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration; Panel C – Plasma retinol concentrations determined by HPLC in wild type and Car−/− mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration; Panel D – Relative levels of plasma RBP4 in wild type and Car−/− mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration; Panel E – all-trans-Retinoic acid (atRA) concentrations were determined by UPLC/MS/MS in wild type and Car−/− livers of mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration; Panel F – 4-hydroxy-all-trans-Retinoic acid (4-OH-atRA) concentrations were determined by UPLC/MS/MS for wild type and Car−/− livers of mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration. Values marked with different letters (a, b, c, d) are statistically different, P < 0.05. All values are given as the mean ± 1 S.D., n = 6 for each group.
Figure 3. mRNA and protein expression of Cyp26a1 and Rbp4 in livers of wild type and Car−/− mice after PCB153 administration.
Panels A and D – Gene expression values (normalized to 18S rRNA levels) were determined by qRT-PCR for RNA obtained from livers of mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration. Panels B and E – Quantitative expression data (normalized to β-actin levels) generated by the analysis of digitally scanned immunoblots of specific protein expression in livers of wild type and Car−/− mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration. Panel C – Representative immunoblots of specific protein expression in livers of wild type and Car−/− mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration. Values marked with different letters (a, b, c, d) are statistically different, P < 0.05. All values are given as the mean ± 1 S.D., n = 6 for each group.
Unlike in wild type animals, PCB153 administration to mice lacking functional constitutive androstane receptor (Car−/− mice) did not display a significant loss of hepatic retinoids. The concentration of total retinyl esters, retinol and all-trans-retinoic acid remained at the level of vehicle-treated Car−/− mice (Fig. 2). Likewise the concentration of hepatic 4-OH atRA remained unchanged after PCB153 treatment of Car−/− mice (Fig. 2F). Thus, these data confirmed that the alterations in hepatic retinoid homeostasis that are detected in PCB153 exposed wild type mice are CAR-dependent.
Interestingly, ablation of the Car gene itself had a significant impact on several retinoid-related parameters. While we did not detect significant changes in hepatic retinyl ester and retinol concentrations in 10-12-week old mice, plasma retinol and RBP4 levels in Car−/− mice were significantly higher by more than 20% (Fig. 2C and 2D). This was associated with upregulated hepatic Rbp4 mRNA and protein expression (Fig. 3C-3E). While no significant differences were identified for hepatic Cyp26a1 mRNA expression between Car−/− and wild mice, protein levels of this CYP isoform were lower in the livers of Car-null animals (Fig. 3A-3C).
In order to replenish hepatic retinoid stores being lost as the result of PCB153 exposure, separate groups of PCB153-treated mice of both genotypes received daily retinoid supplementation in the form of retinyl acetate at a dose 300 IU (which is approximately 10-times higher than a mouse acquires daily from a chow diet). Additionally, separate groups of vehicle treated wild type and Car−/− animals received the same dose of retinyl acetate in order to allow for assessment of any side effects that might develop from retinoid supplementation. Although retinoid supplementation alone was associated with a moderate upregulation of hepatic Gclc mRNA expression (Suppl. Fig. 1C), it did not appear to induce hepatic tissue damage as evidenced by no changes in plasma ALT activity and liver histological analysis (Suppl. Fig. 1A and 1B). In wild type animals retinoid supplementation resulted in an elevation of hepatic retinyl ester, retinol and atRA concentrations 2.5-, 1.8- and 1.6-fold higher than the ones measured for vehicle-treated animals with no retinoid supplementation (Fig. 2). Likewise dietary retinoid administration was associated with significantly higher plasma retinol and RBP4 levels (Fig. 2C and 2D). However, for PCB153-treated mice, the extent to which dietary retinoids were able to replenish hepatic concentrations was significantly different (Fig. 2). While retinoid supplementation restored hepatic retinol and atRA concentrations diminished by PCB153 exposure, it failed to bring about the same level of hepatic retinyl esters as those observed for retinoid-supplemented vehicle-treated mice (Fig. 2A). Hepatic concentrations of retinyl esters, retinol and atRA in retinoid-supplemented PCB153-treated mice when compared to the ones in retinoid-supplemented vehicle-treated mice were lower by 21%, 30% and 52% respectively (Fig. 2). It is worth noting that retinoid supplementation of PCB153-treated mice was associated with a significant upregulation of Cyp26a1 mRNA and protein expression (Fig. 3A-3C); however, PCB153 treatment by itself was associated with a decline in Cyp26a1 protein and retinyl acetate treatment alone had a little impact on Cyp26a1 expression.
Retinoid supplementation also affected the expression of xenobiotic metabolizing CYPs. Among the studied CYPs implicated in xenobiotic biotransformation, retinoid supplementation of vehicle-treated mice resulted in a significant upregulation of Cyp2b10 and Cyp1a1 mRNA expression (Fig. 1, Suppl. Fig. 2C). However, in PCB153-exposed animals, retinoid supplementation affected only Cyp2b10 expression, which was greatly amplified at both the mRNA and protein levels (Fig. 1).
In Car−/− mice retinoid supplementation resulted in the accumulation of significantly higher amounts of retinyl esters and reinol as well as elevated plasma retinol levels compared to wild type retinoid-supplemented mice (Fig. 2). Moreover, the same high level of hepatic and plasma retinoids was observed in both vehicle- and PCB153-treated Car-null mice. Taken together, these data indicate that the absence of functional CAR significantly affects the organism’s ability to process excessive retinoids and suggests a role for CAR and CAR-driven pathways in retinoid metabolism.
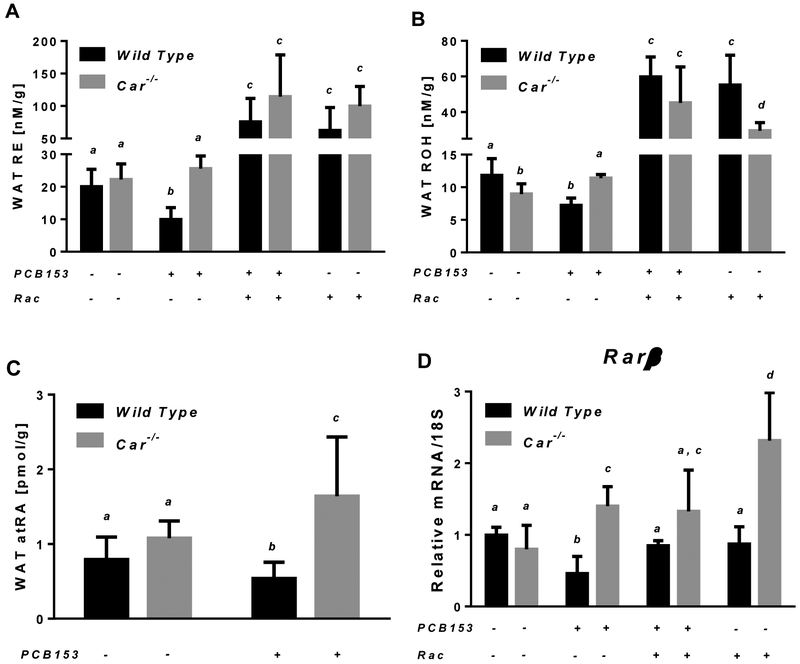
A similar pattern of CAR-dependent PCB153-induced disruption of retinoid homeostasis was observed in perigonadal white adipose, which is the second major site for retinoid storage and metabolism in the body. As a result of PCB153 administration to wild type mice, total retinyl ester, retinol and atRA concentrations in WAT were lower by 50%, 40% and 32% respectively (Fig. 4). The changes of retinoid concentrations in white adipose tissue triggered by PCB153 exposure were also associated with altered retinoid signaling. mRNA expression of retinoic acid responsive-gene encoding the β isoform of retinoic acid receptor (Rarβ) was significantly downregulated in the adipose tissue of PCB153-treated wild type mice compared to vehicle-treated controls (Fig. 4D). PCB153 administration to Car-null mice brought about a significant elevation of retinol and atRA concentrations in WAT, while retinyl ester concentrations remained unaffected. Yet, the higher levels of atRA in the adipose tissue of PCB153-treated Car-null mice were associated with enhanced Rarβ transcription (Fig. 4). Retinyl acetate supplementation to mice resulted in an elevation of WAT retinyl ester and retinol concentrations with no differences between the two genotypes (Fig. 4). Meanwhile WAT Rarβ transcript levels in retinoid supplemented PCB153-treated wild type mice were restored to the level of vehicle-treated animals (Fig. 4D).
Figure. 4. Retinoid concentrations and Rarβ mRNA expression in white adipose tissue of wild type and Car−/− mice after PCB153 administration.
Panels A and B – Retinyl ester (RE) and retinol (ROH) concentrations determined by HPLC in wild type and Car−/− adipose tissues of mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration; Panel C – all-trans-Retinoic acid (atRA) concentrations were determined by UPLC/MS/MS in wild type and Car−/− adipose tissues of mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks); Panel D – Gene expression values (normalized to 18S rRNA levels) were determined by qRT-PCR for RNA obtained from adipose tissues of mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration. Values marked with different letters (a, b, c, d) are statistically different, P < 0.05. All values are given as the mean ± 1 S.D., n = 6 for each group.
PCB153-induced alterations in retinoid homeostasis correlate with the expression of genes involved in lipid and carbohydrate metabolism
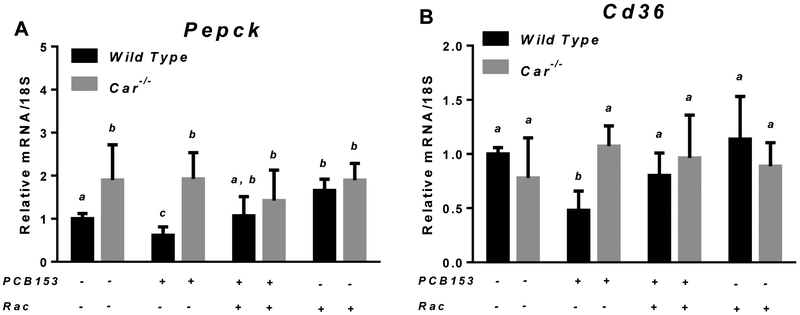
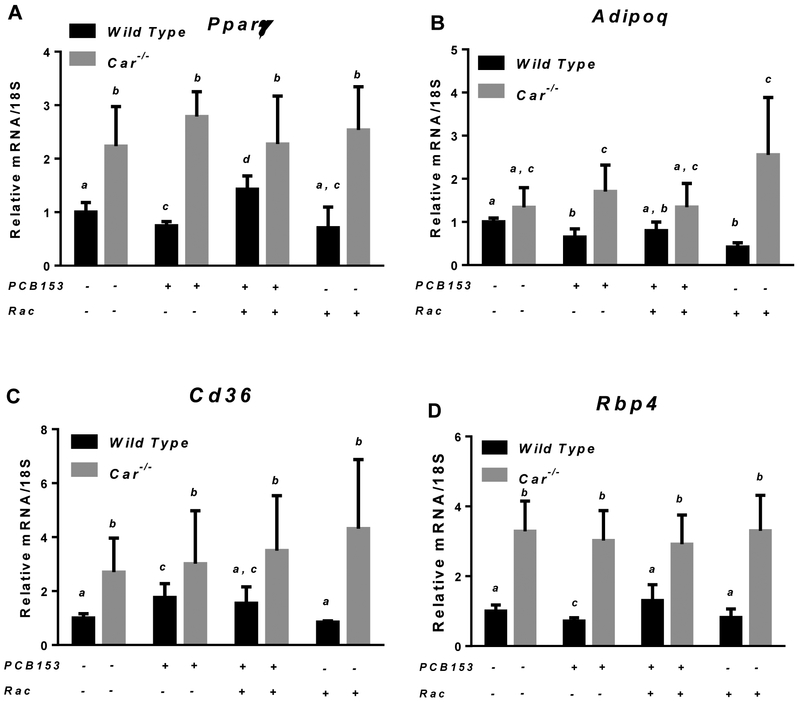
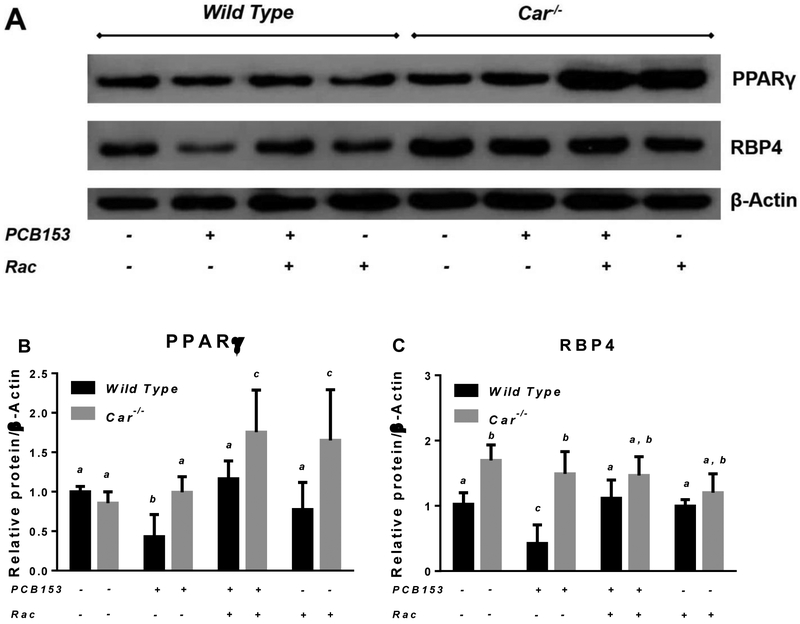
After the four-week period of treatment with PCB153, significant changes in the expression of genes involved in lipid and carbohydrate metabolism were identified in the liver and perigonadal WAT of wild type mice (Fig. 5 and 6). While mRNA expression of hepatic genes encoding the gluconeogenic enzyme Pepck and fatty acid transporter Cd36 was significantly downregulated in PCB153-treated wild type mice, in retinoid supplemented animals exposed to PCB153, hepatic Cd36 and Pepck mRNA expression were at the same level as for untreated controls (Fig. 5). A similar pattern of disrupted expression of the genes involved in lipid metabolism was observed in perigonadal WAT from PCB153-treated wild type mice. Diminished mRNA expression upon PCB153 exposure was seen for genes encoding a key regulator of adipocyte differentiation (Pparγ) and an adipokine involved in the regulation of fat metabolism and insulin sensitivity (Adipoq), whereas adipose tissue transcription of Cd36, a cell surface fatty acid transporter, was upregulated (Fig. 6 and 7). We note that adipose tissue expression of RBP4, which is an adipokine, was significantly affected by PCB153 exposure both at the mRNA and protein levels (Fig. 6D, 7A and 7C). Dietary administration of retinoids improved expression of adipose tissue genes involved in lipid metabolism, bringing about diminished by PCB153 administration message and protein levels to be equal to the untreated controls (Fig. 5-7).
Figure 5. mRNA expression of Pepck and Cd36 in livers of wild type and Car−/− mice after PCB153 administration.
Gene expression values (normalized to 18S rRNA levels) were determined by qRT-PCR for RNA obtained from livers of mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration. Values marked with different letters (a, b, c) are statistically different, P < 0.05. All values are given as the mean ± 1 S.D., n = 6 for each group.
Figure 6. mRNA expression of Pparγ, Adipoq, Cd36 and Rbp4 in white adipose tissues of wild type and Car−/− mice after PCB153 administration.
Gene expression values (normalized to 18S rRNA levels) were determined by qRT-PCR for RNA obtained from adipose tissues of mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration. Values marked with different letters (a, b, c) are statistically different, P < 0.05. All values are given as the mean ± 1 S.D., n = 6 for each group.
Figure 7. Protein expression of PPARγ and RBP4 in adipose tissues of wild type and Car−/− mice after PCB153 administration.
Panel A – Representative immunoblots of specific protein expression in adipose tissues of wild type and Car−/− mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration; Panels B and C – Quantitative expression data (normalized to β-actin levels) generated by the analysis of digitally scanned immunoblots of specific protein expression in adipose tissues of wild type and Car−/− mice after administration of PCB153 (50 mg/kg body weight weekly for 4 weeks), either with or without oral retinyl acetate (Rac, 300 IU daily for 4 weeks) administration. Values marked with different letters (a, b, c, d) are statistically different, P < 0.05. All values are given as the mean ± 1 S.D., n = 6 for each group.
While in wild type mice, the expression of hepatic and adipose tissue genes involved in energy metabolism was significantly affected by PCB153 administration and retinoid supplementation, in Car−/− animals it was almost completely unresponsive to such treatments. However, ablation of the Car gene enabled significantly higher baseline levels for the lipid-related genes evaluated (Fig. 5-7).
DISCUSSION
A number of important conclusions can be drawn from our study. First, our study reveals that significant in vivo alterations in retinoid homeostasis resulting from PCB153 exposure involve the actions of CAR. Our data lead to the conclusion that CAR and its downstream targets have roles in regulating retinoid metabolism and availability. Despite the absence of significant changes in retinoid-related parameters between intact wild type and Car-null mice, these changes become apparent when the mice are challenged either through CAR activation or retinoid excess. While PCB153-induced CAR activation leads to a substantial decrease in retinoid tissue levels, ablation of a Car gene brings about very substantial tissue retinoid accumulation upon dietary consumption of elevated retinoid levels. Second, our data support the conclusion that PCB153-induced CAR-driven alterations in retinoid homeostasis negatively affect the transcription of hepatic and adipose tissue genes involved in energy metabolism. Our data suggest that tissue accumulation of micromolar concentrations of PCB153 per se does not automatically lead to the development of dyslipidemia or loss of insulin responsiveness. However, this tissue burden enables a critical disruption of retinoid homeostasis linking these alterations with PCB153 obesogenic properties that, we propose, may contribute to a predisposition to the metabolic disease.
We employed a PCB153-dosing protocol described in the literature and widely used in animal toxicology studies [82, 83]. This allowed us to obtain hepatic PCB153 concentration at a level of 578.7±138.1 ng/g lipid (32.1 ± 7.7 μM). The literature suggests that this concentration can be detected in human tissues and reflects a moderate-to-high level of PCB153 environmental exposure in adult humans [19-21, 84-86]. Moreover, micromolar PCB153 concentrations, like ones achieved in the current study, have been reported to induce CAR transactivation in a number of in vitro studies [36, 37]. Consistent with the literature, our data indicate that the PCB153 treatment and resultant PCB153 tissue accumulation enable a robust CAR-driven upregulation of hepatic Cyp2b10 expression in vivo. In addition, our data established for our studies that PCB153 exposure is not associated with the development of significant adverse effects in the liver, such as oxidative stress and associated tissue damage. Taken together, these data allowed us to consider PCB153-CAR interactions and resultant transcriptional responses to be primary in mediating the effects of PCB153 exposure that we observed in the current study.
Our study revealed that one of the significant consequences of PCB153-CAR interactions is a decline of hepatic and adipose tissue retinoids, including retinyl ester, retinol and all-trans-retinoic acid (Fig. 3 and 4). Although the phenomenon of tissue retinoid loss and disrupted retinoid signaling during xenobiotic exposure has been extensively described in the literature [42, 57, 71, 72, 87-89], our study expands current understanding of the molecular mechanisms of retinoid homeostasis disruption by non-coplanar PCBs. No decline in hepatic and adipose tissue retinoid levels was observed in Car-null mice upon PCB153 exposure, providing experimental evidence that the disruption of retinoid metabolism observed in vivo is mediated by CAR and downstream genes controlled by this nuclear receptor.
So why and how do retinoids become a target for metabolic disruption by non-coplanar PCB153? Our earlier published studies have underscored a physiological need for retinoid-mediated transcription in order to respond to acute xenobiotic exposure through activation of detoxification pathways [90, 91]. Yet chronic xenobiotic exposure followed by permanent activation of detoxification pathways has been implicated in tissue retinoid loss [57]. In the current study, the loss of tissue retinoids took place only in mice with a functional CAR, while the mice with an ablated Car gene continued to maintain atRA, retinol and retinyl ester tissue concentrations upon PCB153 exposure (Fig. 2). Our data imply that the loss of hepatic retinoids as a result of PCB153 treatment is associated with neither tissue retinoid redistribution nor upregulation of Cyp26a1 (Fig. 3), a canonical atRA 4-hydroxylase [81] that is often associated with a decline in retinoid concentrations. The strong correlation between the changes in retinoid metabolism and extensive upregulation of hepatic CAR-driven CYP2B10 expression (Fig. 1 and 2) probably implicates this CYP isoform as contributing to retinoid oxidation during PCB153 exposure. While CYP2B enzymes are predominantly known for their involvement in metabolism of drugs and environmental chemicals [92], including PCBs [28, 93], several studies have proposed the members of CYP2B subfamily can catalyze the oxidation of retinoids. Roberts et al. reported that purified rabbit phenobarbital-inducible CYP2B4 shows high activity for catalyzing the 4-hydroxylation of retinoic acid, retinol and retinal [94]. In mice, increased hepatic microsomal atRA metabolism resulting in the formation of 4-OH- and 4-oxo-atRA species has been observed during administration of conazoles [95]. CYP2B along with CYP3A and CYP26A1 proteins were identified as being responsible for more than 50 % of the decrease in hepatic levels of atRA during this in vivo study [95]. Other studies, making use of phenotyped human liver microsomes, allowed for the identification of CYP2B6 as being able to catalyze the formation of both 4-OH and 4-oxo metabolites of atRA [96] and 9cRA [97], as well as contributing to 18-OH-atRA and 5,6-epoxy-atRA formation [96]. Our current work suggests that CYP2B10 contributes to retinoic acid oxidation in vivo following PCB153 exposure. Our data imply that the signaling triggered by PCB153 exposure activates pathways aimed at providing sufficient atRA concentrations by utilizing retinol mobilized from endogenous retinyl ester stores. Such extensive CAR-mediated retinoid utilization resulting from PCB153 exposure will also give rise to excessive amounts of atRA when additional exogenous retinoids are provided. Indeed, the administration of dietary retinoids to PCB153-treated wild type animals was associated with the huge upregulation of Cyp26a1 (Fig. 3), a retinoid responsive CYP aimed at counteracting the excessive amounts of atRA. This upregulation of Cyp26a1 was not seen in mice both receiving dietary retinoid and not exposed to PCB153. Thus, our data imply that PCB153-induced CAR-mediated signaling can affect atRA homeostasis in vivo, and raise the possibility that disrupted retinoid homeostasis occurs as a consequence of xenobiotic-induced activation of CAR.
Our data establish that CAR activation affects retinoid homeostasis and that this is associated with altered expression of genes involved in carbohydrate and lipid metabolism in PCB153 exposed animals. CAR, which was initially discovered and referred to as a xenobiotic receptor, has received much research attention regarding its role in normal physiology and endobiotic metabolism [98, 99]. Indeed, several studies have implicated CAR as an important modulator of lipid metabolism, including having a role in regulating serum triglyceride levels, fatty acid oxidation and bile acids homeostasis [100-103]. In our current study, we provide data that extend the role of CAR to modulating tissue retinoid levels and that this action can become important under conditions of metabolic stress, linking PCB153-induced CAR activation with a predisposition for development of metabolic disease. Although over the 4-week period of our study, we did not detect changes in body weight and glucose tolerance among the animals from different experimental groups (data not shown), our study establishes that the expression of hepatic Pepck and Cd36 and adipose tissue Pparγ, Cd36, Adipoq, and Rbp4 are significantly altered by PCB153 exposure in a CAR-dependent manner (Fig. 5-7). While this observation is consistent with the reports from both animal studies involving exposure to PCB153 or a mixture of non-coplanar PCB congeners [39, 49, 51, 83] and human epidemiological studies [104, 105], our new data underscore the association of these alterations with CAR-mediated disruption of retinoid homeostasis and signaling (Fig. 3 and 4). We consider this finding as a step towards future investigations aimed at advancing understanding of the physiological and pathological importance of retinoid signaling as a molecular target for mediating the obesogenic effects of PCB exposure.
CONCLUTIONS
In conclusion, our data establish that the pathways involved in maintaining retinoid homeostasis in plasma, liver and WAT are targets for the CAR-mediated disruptive actions of PCB153. Although our study provides considerable insight into how PCB153-induced CAR activation alters retinoid homeostasis, significant gaps regarding tissue-specific effects of both CAR activation and disrupted retinoid homeostasis need to be addressed in future systematic studies.
Supplementary Material
HIGHLIGHTS.
PCB153 exposure disrupts retinoid homeostasis in a CAR-dependent manner;
CYP2b10 contributes to retinoic acid oxidation during PCB153 exposure;
Dietary retinoids improve PCB153-altered metabolic genes expression.
ACKNOWLEDGEMENTS
This work was supported by grants R01 DK068437 (WSB) and R01 DK101251 (WSB) from the US Public Health Services, National Institutes of Health. The authors also acknowledge support from the Columbia University NIEHS Center Pilot Project Award (3P30 ES009089).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.NTP toxicology and carcinogenesis studies of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) (CAS No. 57465–28-8) in female Harlan Sprague-Dawley rats (Gavage Studies). Natl Toxicol Program Tech Rep Ser, 2006(520): p. 4–246. [PubMed] [Google Scholar]
- 2.NTP technical report on the toxicology and carcinogenesis studies of 2,2',4,4',5,5'-hexachlorobiphenyl (PCB 153) (CAS No. 35065–27-1) in female Harlan Sprague-Dawley rats (Gavage studies). Natl Toxicol Program Tech Rep Ser, 2006(529): p. 4–168. [PubMed] [Google Scholar]
- 3.Toxicology and carcinogenesis studies of a binary mixture of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) (Cas No. 57465-28-8) and 2,2',4,4',5,5'-hexachiorobiphenyl (PCB 153) (CAS No. 35065–27-1) in female Harlan Sprague-Dawley rats (gavage studies). Natl Toxicol Program Tech Rep Ser, 2006(530): p. 1–258. [PubMed] [Google Scholar]
- 4.Faroon O and Ruiz P, Polychlorinated biphenyls: New evidence from the last decade. Toxicol Ind Health, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang M, Chen K, Yang F, and Liu W, Exposure to organochlorine pollutants and type 2 diabetes: a systematic review and meta-analysis. PLoS One, 2014. 9(10): p. e85556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, Jacobs D, Kohrle J, Lee DH, Rylander L, Rignell-Hydbom A, Tornero-Velez R, Turyk ME, Boyles AL, Thayer KA, and Lind L, Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect, 2013. 121(7): p. 774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh S, Murinova L, Trnovec T, Loffredo CA, Washington K, Mitra PS, and Dutta SK, Biomarkers linking PCB exposure and obesity. Curr Pharm Biotechnol, 2014. 15(11): p. 1058–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Y, Chou EL, Baecker A, You NC, Song Y, Sun Q, and Liu S, Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: A systematic review and meta-analysis. J Diabetes, 2016. 8(4): p. 516–32. [DOI] [PubMed] [Google Scholar]
- 9.Neel BA and Sargis RM, The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes, 2011. 60(7): p. 1838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aminov Z, Haase R, and Carpenter DO, Diabetes in Native Americans: elevated risk as a result of exposure to polychlorinated biphenyls (PCBs). Rev Environ Health, 2016. 31(1): p. 115–9. [DOI] [PubMed] [Google Scholar]
- 11.Aminov Z, Haase R, Rej R, Schymura MJ, Santiago-Rivera A, Morse G, DeCaprio A, Carpenter DO, and Akwesasne E Task Force on the, Diabetes Prevalence in Relation to Serum Concentrations of Polychlorinated Biphenyl (PCB) Congener Groups and Three Chlorinated Pesticides in a Native American Population. Environ Health Perspect, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uemura H, Arisawa K, Hiyoshi M, Kitayama A, Takami H, Sawachika F, Dakeshita S, Nii K, Satoh H, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, and Suzuki T, Prevalence of metabolic syndrome associated with body burden levels of dioxin and related compounds among Japan's general population. Environ Health Perspect, 2009. 117(4): p. 568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esser A, Schettgen T, Gube M, Koch A, and Kraus T, Association between polychlorinated biphenyls and diabetes mellitus in the German HELPcB cohort. Int J Hyg Environ Health, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Suarez-Lopez JR, Lee DH, Porta M, Steffes MW, and Jacobs DR Jr., Persistent organic pollutants in young adults and changes in glucose related metabolism over a 23-year follow-up. Environ Res, 2015. 137: p. 485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rylander L, Rignell-Hydbom A, and Hagmar L, A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environ Health, 2005. 4: p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang SL, Tsai PC, Yang CY, and Guo YL, Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care, 2008. 31(8): p. 1574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Airaksinen R, Rantakokko P, Eriksson JG, Blomstedt P, Kajantie E, and Kiviranta H, Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care, 2011. 34(9): p. 1972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casas M, Nieuwenhuijsen M, Martinez D, Ballester F, Basagana X, Basterrechea M, Chatzi L, Chevrier C, Eggesbo M, Fernandez MF, Govarts E, Guxens M, Grimalt JO, Hertz-Picciotto I, Iszatt N, Kasper-Sonnenberg M, Kiviranta H, Kogevinas M, Palkovicova L, Ranft U, Schoeters G, Patelarou E, Petersen MS, Torrent M, Trnovec T, Valvi D, Toft GV, Weihe P, Weisglas-Kuperus N, Wilhelm M, Wittsiepe J, Vrijheid M, and Bonde JP, Prenatal exposure to PCB-153, p,p'-DDE and birth outcomes in 9000 mother-child pairs: exposure-response relationship and effect modifiers. Environ Int, 2015. 74: p. 23–31. [DOI] [PubMed] [Google Scholar]
- 19.CDC, Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, February 2015. 2015.
- 20.Dirinck E, Jorens PG, Covaci A, Geens T, Roosens L, Neels H, Mertens I, and Van Gaal L, Obesity and persistent organic pollutants: possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obesity (Silver Spring), 2011. 19(4): p. 709–14. [DOI] [PubMed] [Google Scholar]
- 21.Dirinck EL, Dirtu AC, Govindan M, Covaci A, Jorens PG, and Van Gaal LF, Endocrine-disrupting polychlorinated biphenyls in metabolically healthy and unhealthy obese subjects before and after weight loss: difference at the start but not at the finish. Am J Clin Nutr, 2016. 103(4): p. 989–98. [DOI] [PubMed] [Google Scholar]
- 22.Cave M, Appana S, Patel M, Falkner KC, McClain CJ, and Brock G, Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect, 2010. 118(12): p. 1735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehead TP, Crispo Smith S, Park JS, Petreas MX, Rappaport SM, and Metayer C, Concentrations of persistent organic pollutants in California women's serum and residential dust. Environ Res, 2015. 136: p. 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinete N, Schettgen T, Bertram J, and Kraus T, Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: a review. Environ Sci Pollut Res Int, 2014. 21(20): p. 11951–72. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, De S, Chen Y, Sutton DC, Ayorinde FO, and Dutta SK, Polychlorinated biphenyls (PCB-153) and (PCB-77) absorption in human liver (HepG2) and kidney (HK2) cells in vitro: PCB levels and cell death. Environ Int, 2010. 36(8): p. 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyer A and Biziuk M, Environmental fate and global distribution of polychlorinated biphenyls. Rev Environ Contam Toxicol, 2009. 201: p. 137–58. [DOI] [PubMed] [Google Scholar]
- 27.Matthews HB and Dedrick RL, Pharmacokinetics of PCBs. Annu Rev Pharmacol Toxicol, 1984. 24: p. 85–103. [DOI] [PubMed] [Google Scholar]
- 28.Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A, and Robertson LW, Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol, 2015. 45(3): p. 245–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ariyoshi N, Oguri K, Koga N, Yoshimura H, and Funae Y, Metabolism of highly persistent PCB congener, 2,4,5,2',4',5'-hexachlorobiphenyl, by human CYP2B6. Biochem Biophys Res Commun, 1995. 212(2): p. 455–60. [DOI] [PubMed] [Google Scholar]
- 30.Hanna IH, Reed JR, Guengerich FP, and Hollenberg PF, Expression of Human Cytochrome P450 2B6 in Escherichia coli: Characterization of Catalytic Activity and Expression Levels in Human Liver. Archives of Biochemistry and Biophysics, 2000. 376(1): p. 206–216. [DOI] [PubMed] [Google Scholar]
- 31.Schnellmann RG, Putnam CW, and Sipes IG, Metabolism of 2,2',3,3',6,6'-hexachlorobiphenyl and 2,2',4,4',5,5'-hexachlorobiphenyl by human hepatic microsomes. Biochem Pharmacol, 1983. 32(21): p. 3233–9. [DOI] [PubMed] [Google Scholar]
- 32.Ritter R, Scheringer M, MacLeod M, Moeckel C, Jones KC, and Hungerbuhler K, Intrinsic human elimination half-lives of polychlorinated biphenyls derived from the temporal evolution of cross-sectional biomonitoring data from the United Kingdom. Environ Health Perspect, 2011. 119(2): p. 225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grandjean P, Budtz-Jorgensen E, Barr DB, Needham LL, Weihe P, and Heinzow B, Elimination half-lives of polychlorinated biphenyl congeners in children. Environ Sci Technol, 2008. 42(18): p. 6991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SK, Ou YC, and Yang RS, Comparison of pharmacokinetic interactions and physiologically based pharmacokinetic modeling of PCB 153 and PCB 126 in nonpregnant mice, lactating mice, and suckling pups. Toxicol Sci, 2002. 65(1): p. 26–34. [DOI] [PubMed] [Google Scholar]
- 35.Oberg M, Sjodin A, Casabona H, Nordgren I, Klasson-Wehler E, and Hakansson H, Tissue distribution and half-lives of individual polychlorinated biphenyls and serum levels of 4-hydroxy-2,3,3',4',5-pentachlorobiphenyl in the rat. Toxicol Sci, 2002. 70(2): p. 171–82. [DOI] [PubMed] [Google Scholar]
- 36.Al-Salman F and Plant N, Non-coplanar polychlorinated biphenyls (PCBs) are direct agonists for the human pregnane-X receptor and constitutive androstane receptor, and activate target gene expression in a tissue-specific manner. Toxicol Appl Pharmacol, 2012. 263(1): p. 7–13. [DOI] [PubMed] [Google Scholar]
- 37.Gahrs M, Roos R, Andersson PL, and Schrenk D, Role of the nuclear xenobiotic receptors CAR and PXR in induction of cytochromes P450 by non-dioxinlike polychlorinated biphenyls in cultured rat hepatocytes. Toxicol Appl Pharmacol, 2013. 272(1): p. 77–85. [DOI] [PubMed] [Google Scholar]
- 38.Wahlang B, Falkner KC, Clair HB, Al-Eryani L, Prough RA, States JC, Coslo DM, Omiecinski CJ, and Cave MC, Human receptor activation by aroclor 1260, a polychlorinated biphenyl mixture. Toxicol Sci, 2014. 140(2): p. 283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopec AK, Burgoon LD, Ibrahim-Aibo D, Mets BD, Tashiro C, Potter D, Sharratt B, Harkema JR, and Zacharewski TR, PCB153-elicited hepatic responses in the immature, ovariectomized C57BL/6 mice: comparative toxicogenomic effects of dioxin and non-dioxin-like ligands. Toxicol Appl Pharmacol, 2010. 243(3): p. 359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabb MM, Kholodovych V, Grun F, Zhou C, Welsh WJ, and Blumberg B, Highly chlorinated PCBs inhibit the human xenobiotic response mediated by the steroid and xenobiotic receptor (SXR). Environ Health Perspect, 2004. 112(2): p. 163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahlang B, Song M, Beier JI, Cameron Falkner K, Al-Eryani L, Clair HB, Prough RA, Osborne TS, Malarkey DE, Christopher States J, and Cave MC, Evaluation of Aroclor 1260 exposure in a mouse model of diet-induced obesity and non-alcoholic fatty liver disease. Toxicol Appl Pharmacol, 2014. 279(3): p. 380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safe SH, Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol, 1994. 24(2): p. 87–149. [DOI] [PubMed] [Google Scholar]
- 43.Mutoh S, Sobhany M, Moore R, Perera L, Pedersen L, Sueyoshi T, and Negishi M, Phenobarbital indirectly activates the constitutive active androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci Signal, 2013. 6(274): p. ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardesty JE, Wahlang B, Falkner KC, Clair HB, Clark BJ, Ceresa BP, Prough RA, and Cave MC, Polychlorinated biphenyls disrupt hepatic epidermal growth factor receptor signaling. Xenobiotica; the fate of foreign compounds in biological systems, 2017. 47(9): p. 807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardesty JE, Al-Eryani L, Wahlang B, Falkner KC, Shi H, Jin J, Vivace BJ, Ceresa BP, Prough RA, and Cave MC, Epidermal Growth Factor Receptor Signaling Disruption by Endocrine and Metabolic Disrupting Chemicals. Toxicological sciences : an official journal of the Society of Toxicology, 2018. 162(2): p. 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernandez JP, Mota LC, and Baldwin WS, Activation of CAR and PXR by Dietary, Environmental and Occupational Chemicals Alters Drug Metabolism, Intermediary Metabolism, and Cell Proliferation. Curr Pharmacogenomics Person Med, 2009. 7(2): p. 81–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ibrahim MM, Fjaere E, Lock EJ, Naville D, Amlund H, Meugnier E, Le Magueresse Battistoni B, Froyland L, Madsen L, Jessen N, Lund S, Vidal H, and Ruzzin J, Chronic consumption of farmed salmon containing persistent organic pollutants causes insulin resistance and obesity in mice. PLoS One, 2011. 6(9): p. e25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boucher MP, Lefebvre C, and Chapados NA, The effects of PCB126 on intra-hepatic mechanisms associated with non alcoholic fatty liver disease. J Diabetes Metab Disord, 2015. 14: p. 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wahlang B, Prough RA, Falkner KC, Hardesty JE, Song M, Clair HB, Clark BJ, States JC, Arteel GE, and Cave MC, Polychlorinated Biphenyl-Xenobiotic Nuclear Receptor Interactions Regulate Energy Metabolism, Behavior, and Inflammation in Non-alcoholic-Steatohepatitis. Toxicol Sci, 2016. 149(2): p. 396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gray SL, Shaw AC, Gagne AX, and Chan HM, Chronic exposure to PCBs (Aroclor 1254) exacerbates obesity-induced insulin resistance and hyperinsulinemia in mice. J Toxicol Environ Health A, 2013. 76(12): p. 701–15. [DOI] [PubMed] [Google Scholar]
- 51.Mesnier A, Champion S, Louis L, Sauzet C, May P, Portugal H, Benbrahim K, Abraldes J, Alessi MC, Amiot-Carlin MJ, Peiretti F, Piccerelle P, Nalbone G, and Villard PH, The Transcriptional Effects of PCB118 and PCB153 on the Liver, Adipose Tissue, Muscle and Colon of Mice: Highlighting of Glut4 and Lipin1 as Main Target Genes for PCB Induced Metabolic Disorders. PLoS One, 2015. 10(6): p. e0128847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrante MC, Amero P, Santoro A, Monnolo A, Simeoli R, Di Guida F, Mattace Raso G, and Meli R, Polychlorinated biphenyls (PCB 101, PCB 153 and PCB 180) alter leptin signaling and lipid metabolism in differentiated 3T3-L1 adipocytes. Toxicol Appl Pharmacol, 2014. 279(3): p. 401–8. [DOI] [PubMed] [Google Scholar]
- 53.Gadupudi GS, Klaren WD, Olivier AK, Klingelhutz AJ, and Robertson LW, PCB126-Induced Disruption in Gluconeogenesis and Fatty Acid Oxidation Precedes Fatty Liver in Male Rats. Toxicol Sci, 2016. 149(1): p. 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker NA, Shoemaker R, English V, Larian N, Sunkara M, Morris AJ, Walker M, Yiannikouris F, and Cassis LA, Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ Health Perspect, 2015. 123(10): p. 944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arsenescu V, Arsenescu RI, King V, Swanson H, and Cassis LA, Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect, 2008. 116(6): p. 761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balmer JE and Blomhoff R, Gene expression regulation by retinoic acid. J Lipid Res, 2002. 43(11): p. 1773–808. [DOI] [PubMed] [Google Scholar]
- 57.Shmarakov IO, Retinoid-xenobiotic interactions: the Ying and the Yang. Hepatobiliary Surg Nutr, 2015. 4(4): p. 243–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chawla A, Repa JJ, Evans RM, and Mangelsdorf DJ, Nuclear receptors and lipid physiology: opening the X-files. Science, 2001. 294(5548): p. 1866–70. [DOI] [PubMed] [Google Scholar]
- 59.Duong V and Rochette-Egly C, The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochim Biophys Acta, 2011. 1812(8): p. 1023–31. [DOI] [PubMed] [Google Scholar]
- 60.Woods CG, Heuvel JP, and Rusyn I, Genomic profiling in nuclear receptor-mediated toxicity. Toxicol Pathol, 2007. 35(4): p. 474–94. [DOI] [PubMed] [Google Scholar]
- 61.Aleksunes LM and Klaassen CD, Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug Metab Dispos, 2012. 40(7): p. 1366–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wan YJ, An D, Cai Y, Repa JJ, Hung-Po Chen T, Flores M, Postic C, Magnuson MA, Chen J, Chien KR, French S, Mangelsdorf DJ, and Sucov HM, Hepatocyte-specific mutation establishes retinoid X receptor alpha as a heterodimeric integrator of multiple physiological processes in the liver. Mol Cell Biol, 2000. 20(12): p. 4436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen S, Wang K, and Wan YJ, Retinoids activate RXR/CAR-mediated pathway and induce CYP3A. Biochem Pharmacol, 2010. 79(2): p. 270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai Y, Dai T, Ao Y, Konishi T, Chuang KH, Lue Y, Chang C, and Wan YJ, Cytochrome P450 genes are differentially expressed in female and male hepatocyte retinoid X receptor alpha-deficient mice. Endocrinology, 2003. 144(6): p. 2311–8. [DOI] [PubMed] [Google Scholar]
- 65.Cai Y, Konishi T, Han G, Campwala KH, French SW, and Wan YJ, The role of hepatocyte RXR alpha in xenobiotic-sensing nuclear receptor-mediated pathways. Eur J Pharm Sci, 2002. 15(1): p. 89–96. [DOI] [PubMed] [Google Scholar]
- 66.Yamada H, Yamaguchi T, and Oguri K, Suppression of the expression of the CYP2B1/2 gene by retinoic acids. Biochem Biophys Res Commun, 2000. 277(1): p. 66–71. [DOI] [PubMed] [Google Scholar]
- 67.Saito K, Kobayashi K, Mizuno Y, Furihata T, and Chiba K, Constitutive androstane/active receptor is a target of retinoic acid receptor in humans. Biochem Pharmacol, 2010. 80(1): p. 129–35. [DOI] [PubMed] [Google Scholar]
- 68.Honkakoski P, Zelko I, Sueyoshi T, and Negishi M, The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Molecular and cellular biology, 1998. 18(10): p. 5652–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howell SR, Shirley MA, and Ulm EH, Effects of retinoid treatment of rats on hepatic microsomal metabolism and cytochromes P450. Correlation between retinoic acid receptor/retinoid × receptor selectivity and effects on metabolic enzymes. Drug Metab Dispos, 1998. 26(3): p. 234–9. [PubMed] [Google Scholar]
- 70.Brouwer A and van den Berg KJ, Early and differential decrease in natural retinoid levels in C57BL/Rij and DBA/2 mice by 3,4,3',4'-tetrachlorobiphenyl. Toxicol Appl Pharmacol, 1984. 73(2): p. 204–9. [DOI] [PubMed] [Google Scholar]
- 71.Roos R, Andersson PL, Halldin K, Hakansson H, Westerholm E, Hamers T, Hamscher G, Heikkinen P, Korkalainen M, Leslie HA, Niittynen M, Sankari S, Schmitz HJ, van der Ven LT, Viluksela M, and Schrenk D, Hepatic effects of a highly purified 2,2',3,4,4',5,5'-heptachlorbiphenyl (PCB 180) in male and female rats. Toxicology, 2011. 284(1–3): p. 42–53. [DOI] [PubMed] [Google Scholar]
- 72.Chen LC, Berberian I, Koch B, Mercier M, Azais-Braesco V, Glauert HP, Chow CK, and Robertson LW, Polychlorinated and polybrominated biphenyl congeners and retinoid levels in rat tissues: structure-activity relationships. Toxicol Appl Pharmacol, 1992. 114(1): p. 47–55. [DOI] [PubMed] [Google Scholar]
- 73.Council NR, Guide for the Care and Use of Laboratory Animals: Eighth Edition. 2011, Washington, DC: The National Academies Press; 246. [Google Scholar]
- 74.Kane MA, Folias AE, Wang C, and Napoli JL, Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem, 2008. 80(5): p. 1702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agency, U.S.E.P., Method 1668C Chlorinated Biphenyl Congeners in Water, Soil, Sediment, Biosolids, and Tissue by HRGC/HRMS 2010.
- 76.Fischer AH, Jacobson KA, Rose J, and Zeller R, Paraffin embedding tissue samples for sectioning. CSH Protoc, 2008. 2008: p. pdb prot4989. [DOI] [PubMed] [Google Scholar]
- 77.Shmarakov IO, Jiang H, Yang KJ, Goldberg IJ, and Blaner WS, Hepatic retinoid stores are required for normal liver regeneration. J Lipid Res, 2013. 54(4): p. 893–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clugston RD, Jiang H, Lee MX, Piantedosi R, Yuen JJ, Ramakrishnan R, Lewis MJ, Gottesman ME, Huang LS, Goldberg IJ, Berk PD, and Blaner WS, Altered hepatic lipid metabolism in C57BL/6 mice fed alcohol: a targeted lipidomic and gene expression study. J Lipid Res, 2011. 52(11): p. 2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schneider CA, Rasband WS, and Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 2012. 9: p. 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kedishvili NY, Retinoic Acid Synthesis and Degradation, in The Biochemistry of Retinoid Signaling II: The Physiology of Vitamin A - Uptake, Transport, Metabolism and Signaling, Asson-Batres MA and Rochette-Egly C, Editors. 2016, Springer Netherlands: Dordrecht, p. 127–161. [Google Scholar]
- 81.Thatcher JE and Isoherranen N, The role of CYP26 enzymes in retinoic acid clearance. Expert Opin Drug Metab Toxicol, 2009. 5(8): p. 875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hardesty JE, Wahlang B, Falkner KC, Shi H, Jin J, Wilkey D, Merchant M, Watson C, Prough RA, and Cave MC, Hepatic signalling disruption by pollutant Polychlorinated biphenyls in steatohepatitis. Cell Signal, 2018. 53: p. 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wahlang B, Falkner KC, Gregory B, Ansert D, Young D, Conklin DJ, Bhatnagar A, McClain CJ, and Cave M, Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J Nutr Biochem, 2013. 24(9): p. 1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guvenius DM, Hassanzadeh P, Bergman A, and Norent K, Metabolites of polychlorinated biphenyls in human liver and adipose tissue. Environmental Toxicology and Chemistry, 2002. 21(11): p. 2264–2269. [PubMed] [Google Scholar]
- 85.Polychlorinated Biphenyls and Polybrominated Biphenyls. Exposure data, in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2016, IARC Working Group on the Evaluation of Carcinogenic Risk to Humans Lyon (FR): International Agency for Research on Cancer. [Google Scholar]
- 86.Fång J, Nyberg E, Winnberg U, Bignert A, and Bergman Å, Spatial and temporal trends of the Stockholm Convention POPs in mothers’ milk — a global review. Environmental Science and Pollution Research, 2015. 22(12): p. 8989–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chu I, Lecavalier P, Hakansson H, Yagminas A, Valli VE, Poon P, and Feeley M, Mixture effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin and polychlorinated biphenyl congeners in rats. Chemosphere, 2001. 43(4–7): p. 807–14. [DOI] [PubMed] [Google Scholar]
- 88.van der Plas SA, Lutkeschipholt I, Spenkelink B, and Brouwer A, Effects of Subchronic Exposure to Complex Mixtures of Dioxin-like and Non-Dioxin-like Polyhalogenated Aromatic Compounds on Thyroid Hormone and Vitamin A Levels in Female Sprague-Dawley Rats. Toxicological Sciences, 2001. 59(1): p. 92–100. [DOI] [PubMed] [Google Scholar]
- 89.Håkansson H, Manzoor E, Trossvik C, Ahlborg UG, Chu I, and Villenueve D, Effect on tissue vitamin A levels in the rat following subchronic exposure to four individual PCB congeners (IUPAC 77, 118, 126, and 153). Chemosphere, 1994. 29(9): p. 2309–2313. [DOI] [PubMed] [Google Scholar]
- 90.Shmarakov IO, Borschovetska VL, and Blaner WS, Hepatic Detoxification of Bisphenol A is Retinoid-Dependent. Toxicol Sci, 2017. 157(1): p. 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shmarakov IO, Borschovetska VL, Marchenko MM, and Blaner WS, Retinoids modulate thioacetamide-induced acute hepatotoxicity. Toxicol Sci, 2014. 139(2): p. 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang H and Tompkins LM, CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab, 2008. 9(7): p. 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu Z, Kania-Korwel I, Lehmler HJ, and Wong CS, Stereoselective formation of mono- and dihydroxylated polychlorinated biphenyls by rat cytochrome P450 2B1. Environ Sci Technol, 2013. 47(21): p. 12184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roberts ES, Vaz AD, and Coon MJ, Role of isozymes of rabbit microsomal cytochrome P-450 in the metabolism of retinoic acid, retinol, and retinal. Mol Pharmacol, 1992. 41(2): p. 427–33. [PubMed] [Google Scholar]
- 95.Chen PJ, Padgett WT, Moore T, Winnik W, Lambert GR, Thai SF, Hester SD, and Nesnow S, Three conazoles increase hepatic microsomal retinoic acid metabolism and decrease mouse hepatic retinoic acid levels in vivo. Toxicol Appl Pharmacol, 2009. 234(2): p. 143–55. [DOI] [PubMed] [Google Scholar]
- 96.Marill J, Cresteil T, Lanotte M, and Chabot GG, Identification of human cytochrome P450s involved in the formation of all-trans-retinoic acid principal metabolites. Mol Pharmacol, 2000. 58(6): p. 1341–8. [DOI] [PubMed] [Google Scholar]
- 97.Marill J, Capron CC, Idres N, and Chabot GG, Human cytochrome P450s involved in the metabolism of 9-cis- and 13-cis-retinoic acids. Biochem Pharmacol, 2002. 63(5): p. 933–43. [DOI] [PubMed] [Google Scholar]
- 98.Moreau A, Vilarem MJ, Maurel P, and Pascussi JM, Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm, 2008. 5(1): p. 35–41. [DOI] [PubMed] [Google Scholar]
- 99.Wada T, Gao J, and Xie W, PXR and CAR in energy metabolism. Trends Endocrinol Metab, 2009. 20(6): p. 273–9. [DOI] [PubMed] [Google Scholar]
- 100.Maglich JM, Lobe DC, and Moore JT, The nuclear receptor CAR (NR1I3) regulates serum triglyceride levels under conditions of metabolic stress. J Lipid Res, 2009. 50(3): p. 439–45. [DOI] [PubMed] [Google Scholar]
- 101.Roth A, Looser R, Kaufmann M, Blattler SM, Rencurel F, Huang W, Moore DD, and Meyer UA, Regulatory cross-talk between drug metabolism and lipid homeostasis: constitutive androstane receptor and pregnane X receptor increase Insig-1 expression. Mol Pharmacol, 2008. 73(4): p. 1282–9. [DOI] [PubMed] [Google Scholar]
- 102.Gao J, He J, Zhai Y, Wada T, and Xie W, The constitutive androstane receptor is an antiobesity nuclear receptor that improves insulin sensitivity. J Biol Chem, 2009. 284(38): p. 25984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lickteig AJ, Csanaky IL, Pratt-Hyatt M, and Klaassen CD, Activation of Constitutive Androstane Receptor (CAR) in Mice Results in Maintained Biliary Excretion of Bile Acids Despite a Marked Decrease of Bile Acids in Liver. Toxicol Sci, 2016. 151(2): p. 403–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mullerova D, Kopecky J, Matejkova D, Muller L, Rosmus J, Racek J, Sefrna F, Opatrna S, Kuda O, and Matejovic M, Negative association between plasma levels of adiponectin and polychlorinated biphenyl 153 in obese women under non-energy-restrictive regime. Int J Obes (Lond), 2008. 32(12): p. 1875–8. [DOI] [PubMed] [Google Scholar]
- 105.Lim JE and Jee SH, Association between serum levels of adiponectin and polychlorinated biphenyls in Korean men and women. Endocrine, 2015. 48(1): p. 211–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.